Low potassium diet
A low potassium diet is defined as a dietary potassium intake of 2–3 g/day (approximately 51–77 mmol/day) 1. Current dietary guidelines from the US Food and Nutrition Board of the Institute of Medicine recommend potassium intake of 3,400 mg/day in adult men and 2,600 mg/day in adult women with normal kidney function 2, dietary potassium restriction of usually less than 3 g per day is recommended in the management of patients with chronic kidney disease (CKD), especially those who tend to develop high blood potassium (also known as hyperkalemia). Your kidney is the major organ responsible for regulating potassium absorption and excretion in your body 3. In healthy people with normal kidney function, high dietary potassium intakes do not pose a health risk because the kidneys eliminate excess amounts in the urine 4. Healthy kidneys keep the right amount of potassium in your blood to keep your heart beating at a steady pace. In patients with advanced chronic kidney disease (CKD), the progressive decline in kidney function contributes to the development of high blood potassium (hyperkalemia), the definition of which varies between institutional guidelines and clinical studies and is generally considered to be a serum or plasma potassium level above the upper limits of normal, usually greater than 5.0 mEq/L to 5.5 mEq/L 5, 6, 7. Hyperkalemia risk increases as chronic kidney disease (CKD) progresses 6, with a reported prevalence of up to 10% in those with CKD without kidney replacement therapy, 16% in patients with kidney failure receiving hemodialysis, and 11% in those receiving continuous ambulatory peritoneal dialysis 7. Dietary potassium restriction is often recommended to prevent and treat hyperkalemia in patients with chronic kidney disease (CKD) 8, 9. Proper dietary counseling is imperative in chronic kidney disease (CKD) and end-stage renal disease (ESRD, which is the final, permanent stage of chronic kidney disease, where kidney function has declined to the point that the kidneys can no longer function on their own) patients with chronic or recurrent hyperkalemia or those at risk of hyperkalemia with sporadic hyperkalemic surges as renal failure progresses. In patients with non-dialysis dependent chronic kidney disease (CKD) stages 1–5, the National Kidney Foundation suggests an unrestricted potassium intake unless the serum potassium level is elevated. In hemodialysis patients, potassium intake should be up to 2.7–3.1 g/day and in peritoneal dialysis patients close to 3–4 g/day; in both cases, adjustments based on serum potassium levels are crucial 10. A recent comprehensive review paper on nutritional management of chronic kidney disease (CKD) by Kalantar-Zadeh and Fouque 11 has suggested an intake of 4.7 g/day in the early stages of CKD without risk of high blood potassium (also known as hyperkalemia), but a dietary potassium restriction of less than 3 g (less than 77 mmol) per day in CKD patients who tend to develop hyperkalemia (serum potassium levels >5.3 mEq/L).
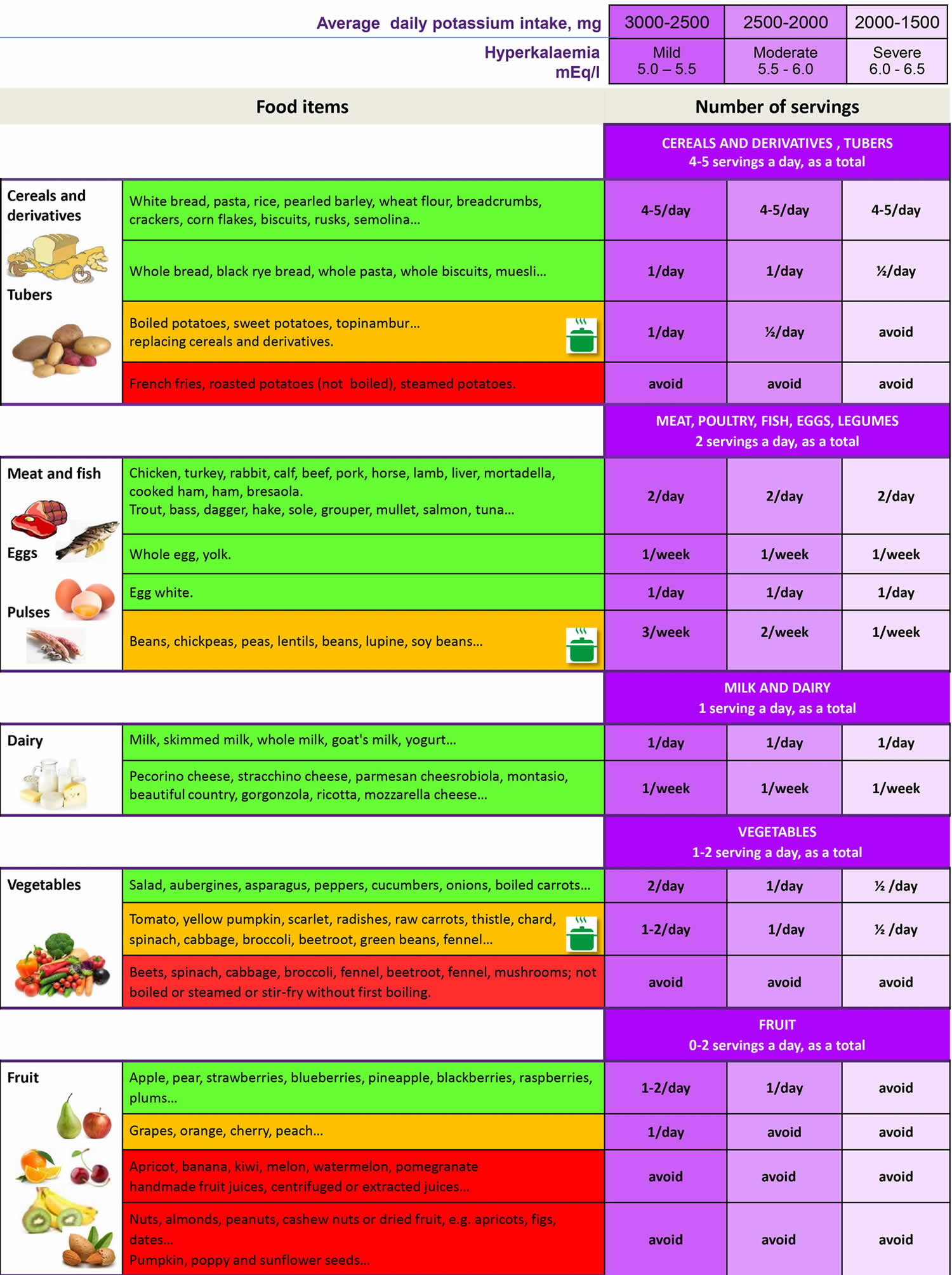
In fact, patients with chronic kidney disease (CKD) grades 3 and 4 usually receive instructions to restrict their dietary potassium intake in order to prevent development of hyperkalemia 12. The U.S. Department of Agriculture’s FoodData Central (https://fdc.nal.usda.gov) lists the nutrient content of many foods and provides a comprehensive list of foods containing potassium ordered by nutrient content (https://www.nal.usda.gov/sites/www.nal.usda.gov/files/potassium.pdf). The 2015–2020 Dietary Guidelines for Americans (https://health.gov/our-work/nutrition-physical-activity/dietary-guidelines/previous-dietary-guidelines/2015) also provides a list of foods containing potassium. To control potassium levels, limit potassium-rich foods such as avocados, bananas, kiwis, and dried fruit (see Table 2 below). Choose fruits and vegetables that are lower in potassium. Have very small portions of foods that are higher in potassium, such as one or two cherry tomatoes on a salad or a few raisins in your oatmeal. You can remove some of the potassium from potatoes by dicing or shredding them and then boiling them in a full pot of water. Your kidney dietitian will give you more specific information about the potassium content of foods.
It is estimated that the body absorbs about 85%–90% of dietary potassium 13. The forms of potassium in fruits and vegetables include potassium phosphate, sulfate, citrate, and others, but not potassium chloride (the form used in salt substitutes and some dietary supplements) 14.
Milk, coffee, tea, other nonalcoholic beverages, and potatoes are the top sources of potassium in the diets of U.S. adults 15. Among children in the United States, milk, fruit juice, potatoes, and fruit are the top sources 16.
Dietary potassium restrictions are often implemented during earlier stages of chronic kidney disease (CKD) when patients are still non-dialysis dependent and are reinforced in the majority of patients transitioning to end-stage renal disease (ESRD), particularly in hemodialysis patients 17. There are data suggesting that high-potassium intake increases the risk for hyperkalemia in some patients with chronic kidney disease (CKD) and current recommendations and guidelines severely restrict dietary potassium in those patients with chronic kidney disease (CKD), leading to a mismatch between overall health recommendations regarding the benefits of a plant-based/high-potassium diet and the type of diet recommended in patients with chronic kidney disease (CKD). Potassium levels can rise between hemodialysis sessions and affect your heartbeat. Eating too much potassium can be dangerous to your heart and may even cause death. A recent study by Noori et al. 18 examined the association between dietary potassium intake via Food Frequency Questionnaire 19 at the start of a 5-year cohort of 224 hemodialysis patients in Southern California, found that patients with higher potassium intake had greater dietary energy, protein, and phosphorus intake and higher pre-dialysis serum potassium and phosphorus levels. Greater dietary potassium intake was associated with increased mortality in survival models that were adjusted for nutritional factors including serum potassium and phosphorus levels, in that death risk of the 3 higher quartiles of dietary potassium intake were 1.4, 2.2 and 2.4 times higher than the lowest dietary potassium quartile 18. Hence, higher dietary potassium load per se, independent of serum potassium, is incrementally associated with higher mortality. These and other data have been used to justify strict dietary potassium restrictions in chronic kidney disease (CKD) and dialysis patients.
However, it is important to appreciate that many potassium rich foods are considered “heart healthy” including fresh fruits and vegetables, fresh squeezed juices, legumes, and grains 18. A case-control study comparing dietary patterns of hemodialysis patients versus the general population suggested that dietary restriction in dialysis patients, including low potassium and phosphorus, leads to avoidance of most fresh fruits and vegetables and a more atherogenic diet with lower vitamin C, fiber, and carotenoid 20. Fibre intake has a major role in the modulation of intestinal microbiota, with high-fiber diets promoting the growth of bacteria with saccharolytic metabolism and lowering proteolytic-derived uremic toxins 21 and also leading to faster bowel transit time. Conversely, reduced bowel motility and constipation can induce dysbiosis of the intestinal microbiota, contributing to uremic intoxication and increase net absorption of potassium, leading to hyperkalemia 22. Therefore, a high fiber content in the diet should be preserved even when the potassium intake is to be lowered.
In a recent cross-sectional study by Koueiry et al. 23 using the “Dialysis Food Frequency Questionnaire” 19, 97% of hemodialysis patients had lower than the recommended dietary fiber intake of 25 g/day or more. The study concluded that most dialysis patients did not meet the dietary guidelines for reducing the risk of cardiovascular disease 23. Hence, a low potassium diet falls outside of what is generally recommended as a healthy diet and lifestyle, and such dietary restrictions on dialysis patients may contribute to atherosclerosis and increased cardiovascular disease and mortality in the chronic kidney disease (CKD) patient population according to several studies 20, 23.
The contribution of dietary intake to potassium balance may vary based on the carbohydrate content of the food 24. Although steady-state serum potassium concentrations are maintained by kidney potassium excretion 25, high-potassium foods that also contain high carbohydrate levels initially promote insulin secretion, which facilitates potassium uptake into cells and minimizes the increase in serum potassium levels 26. In contrast, high-potassium foods that are low in carbohydrates, such as meat, may result in greater increases in serum potassium levels despite having similar potassium content to high-carbohydrate foods 24. The processing of foods may also influence potassium content because there are hidden sources of potassium due to food additives and products such as low-sodium salt substitutes 1.
In patients with stage 4–5 non-dialysis dependent CKD and ESRD, dietary potassium management also has to be synchronized with additional nutritional goals, namely the amount of protein intake (restricted in non-dialysis dependent-CKD or increased in ESRD), high fiber intake, reduced net fixed acid production and cardiovascular effects and the favoring of a heart-healthy diet (typically consisting of fruits and vegetables) 27.
In clinical practice, a common dilemma in the management of advanced chronic kidney disease (CKD) patients with chronic hyperkalaemia is the patients’ deprivation of the beneficial effects of renin-angiotensin-aldosterone system (RAAS) inhibitors or of the favorable effects of vegetarian diets in order to control hyperkalaemia 28. A solution may be the use of intestinal potassium binders 29. The use of potassium binding resins for hyperkalemia control may also allow for more consumption of heart-healthy diets with less risk of hyperkalemia, although their intake can be limited by side effects and more studies are needed 30. Until recently, the only potassium binder available for hyperkalemia management was sodium polystyrene sulfonate; however, long-term sodium polystyrene sulfonate use is limited by the associated risk for gastrointestinal adverse events 31. Two newer potassium binders, patiromer and sodium zirconium cyclosilicate, are now available for hyperkalemia treatment in the United States and European Union. In clinical studies that included patients with chronic kidney disease (CKD) and those receiving RAAS-inhibitor therapy, patiromer and sodium zirconium cyclosilicate showed efficacy for the treatment of hyperkalemia 32. Of note, the sodium zirconium cyclosilicate studies did not impose dietary restrictions during potassium-binder therapy 32, indicating that sodium zirconium cyclosilicate may remain effective in patients who consume a heart-healthy plant-based diet. Both potassium binders were well tolerated in these studies, with no serious gastrointestinal adverse events. Long-term studies have indicated that these newer potassium binders can safely and effectively reduce the risk for recurrent hyperkalemia over 12 months 32.
Studies have indicated that the newer potassium binders may be used in hemodialysis patients with hyperkalemia. In patients with kidney failure receiving hemodialysis, sodium zirconium cyclosilicate on nondialysis days reduced the incidence of predialysis hyperkalemia, with treatment response (predialysis serum potassium levels of 4.0-5.0 mEq/L during ≥3 of 4 hemodialysis sessions) achieved in 41% of patients with sodium zirconium cyclosilicate versus 1% with placebo 33. In a real-world study of patients with kidney failure receiving hemodialysis, patiromer significantly reduced serum potassium levels during the three 30-day intervals following initiation, with an ∼50% relative reduction in the prevalence of severe hyperkalemia (potassium ≥ 6.0 mEq/L) after patiromer therapy initiation 34. An analysis of a phase 3 study showed that patiromer was associated with reductions in blood pressure and aldosterone levels, as well as lowering serum potassium levels, in patients with chronic kidney disease (CKD) and hyperkalemia who were receiving RAAS-inhibitor therapy, including those with hypertension and type 2 diabetes 35. Preliminary findings from the AMBER (Spironolactone With Patiromer in the Treatment of Resistant Hypertension in Chronic Kidney Disease) trial indicated that patiromer administration may allow for more patients with advanced chronic kidney disease (CKD) and resistant hypertension to continue spironolactone therapy 36.
Taken together, these data suggest that patiromer and sodium zirconium cyclosilicate are both viable adjunctive long-term therapy options that may allow for diet liberalization in patients with chronic kidney disease (CKD) and hyperkalemia 24. In addition, the newer potassium binders were safe and effective in patients receiving concomitant RAAS-inhibitor therapy. Therefore, patiromer and sodium zirconium cyclosilicate use may also enable clinicians to optimize RAAS-inhibitor therapy, providing patients with the associated cardiorenal benefits of RAAS inhibitors while minimizing the risk for recurrent hyperkalemia. Further research is needed to determine whether potassium binders in combination with a plant-based diet can improve RAAS-inhibitor use and reduce the risk for recurrent hyperkalemia among patients with chronic kidney disease (CKD). However, the capacity of intestinal potassium-binders to remove potassium is limited and they could be expensive in the long run. Therefore, a careful control of the dietary potassium load is in an important aspect of the management of chronic kidney disease (CKD) and heart failure patients with, or at risk of hyperkalemia.
Evidence to support stringent reductions in dietary sources of potassium are lacking for some patients 24. Accumulating evidence suggests that a high-potassium diet, which includes foods that are high in potassium as well as other vitamins, minerals, and fiber, is associated with several health benefits, including prevention of chronic kidney disease (CKD) progression, improvements in blood pressure and bone health, and decreased risks for cardiovascular disease and coronary artery disease (coronary heart disease), diabetes, kidney stone formation, and stroke 37, 38, 39. Moreover, many potassium-rich foods, including fresh fruits and vegetables, grains, and legumes, are considered “heart-healthy” (Table 2 and 3) 17.
A recent Kidney Disease: Improving Global Outcomes (KDIGO) consensus report recognized the validity of dietary potassium restriction as a strategy for managing acute hyperkalemia; however, this report also hypothesized that restriction of dietary potassium for the prevention of hyperkalemia in chronic kidney disease (CKD) may deprive patients of the benefits of a high-potassium diet 40. The KDIGO report acknowledged the lack of direct evidence supporting dietary potassium restriction in patients with chronic kidney disease (CKD), although they found no evidence confirming the safety of increased potassium intake in those with advanced chronic kidney disease (CKD) 40. The KDIGO report recommended developing educational materials that include information regarding the potassium content of foods that promote a plant-based low-potassium diet, to be used when a reduction in high-potassium foods is clinically indicated 40.
Most recommendations for a low-potassium diet assume similar bioavailability of the potassium in different foods 41. The data demonstrating the need for potassium restriction in patients with declining kidney function originated from a potassium balance study conducted in the 1940s, in which potassium tablet administration in individuals with normal kidney function resulted in a significant increase in serum potassium levels 42. Based on these findings and others, there is a long-held belief that potassium-containing foods increase serum potassium levels. However, there is limited evidence to suggest that plant-based diets are associated with increased risk for hyperkalemia in patients with chronic kidney disease (CKD), with 1 study reporting hyperkalemia associated with a plant-based diet in a patient with type 4 renal tubular acidosis 43. In an observational study of patients receiving long-term hemodialysis, an increase in dietary potassium intake was weakly correlated with predialysis serum potassium levels 18, whereas another study of hemodialysis patients found no correlation between serum potassium levels and absolute reported potassium intake or potassium density 44.
Hyperkalemia risk factors may differ among patients with advanced chronic kidney disease (CKD) because these individuals are potentially more tolerant of higher serum potassium levels than those with normal kidney function. It is hypothesized that patients with advanced chronic kidney disease (CKD) sense and maintain potassium homeostasis by different mechanisms, with the gastrointestinal tract potentially playing a role in potassium homeostasis in addition to the kidneys 45. Gastrointestinal potassium excretion may increase to compensate for reduced kidney function. For example, in healthy adults with a “normal” diet, ∼10% of potassium intake is actively secreted by the colon and excreted in feces 46, whereas fecal potassium excretion appears to be approximately 3-fold higher in patients with kidney failure 47. However, it is unclear whether this increase in fecal potassium excretion plays a clinically significant role in maintaining potassium homeostasis 46. Furthermore, there are no guidelines regarding how much dietary potassium restriction is needed to prevent hyperkalemia in patients with advanced chronic kidney disease (CKD). This generally varies according to the patient’s age and comorbid conditions and further research is needed before recommendations can be made.
A recent cohort study over 3 years in 81,013 prevalent hemodialysis patients suggested that the best pre-hemodialysis serum potassium range associated with the greatest survival was 4.6 to 5.3 mEq/L, whereas potassium levels <4.0 or ≥5.6 mEq/L were associated with increased mortality 48. Another clinically relevant finding in this study was that serum potassium correlated closely with nutritional markers, in particular nPCR (nPNA) suggesting that patients who eat more protein also tend to have higher serum potassium level 48. In a more recent cohort study that examined the mortality of hyperkalemia in 111,651 hemodialysis and 10,468 peritoneal dialysis patients, the latter group was 3.3 times more likely to have lower serum potassium <4.0 mEq/L, and their death risk was 51% and 52% higher with serum potassium levels <3.5 mEq/L and ≥5.5 mEq/L 49. Hence, both hypokalemia (low blood potassium) and hyperkalemia (high blood potassium) appear to be harmful in dialysis patients irrespective of dialysis modality 17. It is important to note that the variability of serum potassium in dialysis patients is not the exclusive function of dietary potassium load and that several other factors such as potassium concentration in the dialysate bath or the dialysis treatment length and frequency play important roles 17.
Table 1. Recommended dietary potassium intake at different stages of chronic kidney disease in adults
| Normal kidney function (eGFR ≥ 60 *) and no proteinuria but at higher CKD risk, e.g., diabetes, hypertension, or solitary kidney | Mild to moderate CKD (eGFR 30 < 60 *) without substantial proteinuria (<0.3 g/day) | Advanced CKD (eGFR < 30 *) or any CKD with substantial proteinuria (>0.3 g/day) | Prevalent dialysis therapy, or any CKD stage with existing or imminent PEW | |
| Dietary Potassium (g/day) | Same as recommended for the general population (4.7 g/day). | Same as the general population unless frequent or severe hyperkalemia excursions. | <3 g/day if hyperkalemia occurs frequently while maintaining high fiber intake. | <3 g/day target high fiber intake |
Footnotes: * The unit for eGFR is mL/min/1.73 m2 body surface area (BSA).
Abbreviations: CKD = chronic kidney disease; d = per day (such as in g/kg/day); eGFR = estimated glomerular filtration rate in mL/min/1.73 m2, PEW = protein energy wasting.
[Source 11 ]Table 2. Potassium content of selected foods
| Food | Milligrams (mg) per serving |
| Apricots, dried, ½ cup | 1101 |
| Lentils, cooked, 1 cup | 731 |
| Prunes, dried, ½ cup | 699 |
| Squash, acorn, mashed, 1 cup | 644 |
| Raisins, ½ cup | 618 |
| Potato, baked, flesh only, 1 medium | 610 |
| Kidney beans, canned, 1 cup | 607 |
| Orange juice, 1 cup | 496 |
| Soybeans, mature seeds, boiled, ½ cup | 443 |
| Banana, 1 medium | 422 |
| Milk, 1%, 1 cup | 366 |
| Spinach, raw, 2 cups | 334 |
| Chicken breast, boneless, grilled, 3 ounces | 332 |
| Yogurt, fruit variety, nonfat, 6 ounces | 330 |
| Salmon, Atlantic, farmed, cooked, 3 ounces | 326 |
| Beef, top sirloin, grilled, 3 ounces | 315 |
| Molasses, 1 tablespoon | 308 |
| Tomato, raw, 1 medium | 292 |
| Soymilk, 1 cup | 287 |
| Yogurt, Greek, plain, nonfat, 6 ounces | 240 |
| Broccoli, cooked, chopped, ½ cup | 229 |
| Cantaloupe, cubed, ½ cup | 214 |
| Turkey breast, roasted, 3 ounces | 212 |
| Asparagus, cooked, ½ cup | 202 |
| Apple, with skin, 1 medium | 195 |
| Cashew nuts, 1 ounce | 187 |
| Rice, brown, medium-grain, cooked, 1 cup | 154 |
| Tuna, light, canned in water, drained, 3 ounces | 153 |
| Coffee, brewed, 1 cup | 116 |
| Lettuce, iceberg, shredded, 1 cup | 102 |
| Peanut butter, 1 tablespoon | 90 |
| Tea, black, brewed, 1 cup | 88 |
| Flaxseed, whole, 1 tablespoon | 84 |
| Bread, whole-wheat, 1 slice | 81 |
| Egg, 1 large | 69 |
| Rice, white, medium-grain, cooked, 1 cup | 54 |
| Bread, white, 1 slice | 37 |
| Cheese, mozzarella, part skim, 1½ ounces | 36 |
| Oil (olive, corn, canola, or soybean), 1 tablespoon | 0 |
Table 3. Potassium content of animal-origin food, beverages, sugar and sweets and fats
| Potassium (mg) | Potassium (mg) | ||||||
| 100 g | Serving | 100 kcal | 100 g | Serving | 100 kcal | ||
| Meat | Milk and dairy | ||||||
| Chicken breast | 370 | 370 | 370 | Milk | 150 | 188 | 234 |
| Chicken thigh | 355 | 355 | 332 | Yogurt | 150 | 188 | 170 |
| Duck | 290 | 290 | 182 | Brie | 100 | 50 | 31 |
| Lamb | 350 | 350 | 220 | Cheddar | 120 | 60 | 31 |
| Liver | 320 | 320 | 225 | Cottage cheese | 89 | 89 | 77 |
| Pork | 290 | 290 | 185 | Cream cheese | 150 | 150 | 84 |
| Rabbit | 360 | 360 | 261 | Emmenthal cheese | 107 | 54 | 27 |
| Turkey breast | 320 | 320 | 221 | Gouda cheese | 89 | 45 | 26 |
| Turkey thigh | 310 | 310 | 167 | Parmesan cheese | 120 | 60 | 30 |
| Beef | 330 | 330 | 206 | Pecorino cheese | 94 | 47 | 24 |
| Veal | 360 | 360 | 391 | Ricotta cheese | 119 | 119 | 82 |
| Preserved meat | Spreadable cheese | 108 | 54 | 35 | |||
| Bresaola | 505 | 253 | 334 | Stracchino cheese | 62 | 62 | 21 |
| Canned meat | 140 | 140 | 226 | Fats | |||
| Cooked ham | 227 | 114 | 106 | Butter | 15 | 2 | 2 |
| Ham | 454 | 227 | 203 | Cream | 91 | 9 | 44 |
| Mortadella | 130 | 65 | 41 | Margarine | 5 | 1 | 1 |
| Salami | 473 | 237 | 123 | Olive oil | 0 | 0 | 0 |
| Sausage | 130 | 65 | 33 | Sugar and Sweets sand | |||
| Wurstel | 140 | 70 | 52 | Dark chocolate | 300 | 30 | 55 |
| Fish | Fruit ice cream | 180 | 72 | 101 | |||
| Anchovies | 278 | 417 | 290 | Honey | 51 | 3 | 17 |
| Carpa | 286 | 429 | 204 | Marmalade | 100 | 5 | 45 |
| Hake | 320 | 480 | 451 | Milk chocolate | 420 | 42 | 74 |
| Herring | 320 | 480 | 148 | Milk ice cream | 110 | 44 | 46 |
| Mussel | 320 | 480 | 381 | Sugar | 2 | 0 | 1 |
| Salmon | 310 | 465 | 168 | Beverages | |||
| Shrimp | 266 | 399 | 375 | Beer | 35 | 116 | 78 |
| Sole | 280 | 420 | 326 | Cola | 1 | 3 | 3 |
| Trout | 429 | 644 | 364 | Orange juice | 150 | 300 | 417 |
| Egg | Red wine | 110 | 138 | 145 | |||
| Egg white | 135 | 95 | 314 | Tea | 0 | 0 | 0 |
| Whole egg | 133 | 67 | 104 | Wine | 61 | 76 | 86 |
Table 4. Potassium content of plant-based foods
| Potassium (mg) | Potassium (mg) | ||||||
| 100 g | Serving | 100 kcal | 100 g | Serving | 100 kcal | ||
| Cereals and tubers | Pulses | ||||||
| Barley | 120 | 60 | 38 | Beans | 650 | 650 | 625 |
| Buckwheat | 220 | 176 | 60 | Dry beans | 1445 | 723 | 465 |
| Corn flakes | 99 | 45 | 27 | Dry chickpeas | 800 | 400 | 239 |
| Pasta | 160 | 128 | 45 | Dry lentils | 980 | 490 | 302 |
| Rice | 110 | 88 | 30 | Dry soy beans | 1740 | 870 | 437 |
| Rye bread | 190 | 95 | 86 | Lupine | 351 | 351 | 308 |
| Toasted bread | 140 | 35 | 34 | Peas | 202 | 202 | 266 |
| White Bread | 176 | 88 | 64 | Fruits | |||
| Whole bread | 210 | 105 | 86 | Apple | 120 | 180 | 267 |
| Whole rice | 250 | 200 | 70 | Apricot | 320 | 480 | 1143 |
| Potatoes | 570 | 1140 | 671 | Banana | 350 | 525 | 530 |
| Sweet potatoes | 370 | 740 | 425 | Blackberry | 260 | 390 | 722 |
| Vegetables | Blueberry | 160 | 240 | 640 | |||
| Asparagus | 240 | 480 | 828 | Cherry | 229 | 344 | 603 |
| Basil | 300 | 600 | 769 | Fig | 270 | 405 | 574 |
| Beetroot | 300 | 600 | 1579 | Grape | 192 | 288 | 315 |
| Broccoli | 340 | 680 | 1259 | Grapefruit | 230 | 345 | 885 |
| Carrot | 220 | 440 | 667 | Kiwi | 400 | 600 | 909 |
| Cauliflower | 350 | 700 | 1400 | Lemon | 140 | 210 | 298 |
| Celery | 280 | 560 | 1400 | Mango | 250 | 375 | 472 |
| Chard | 286 | 572 | 1682 | Melon | 333 | 500 | 1009 |
| Cucumber | 140 | 280 | 1000 | Orange | 200 | 300 | 588 |
| Eggplant | 184 | 368 | 1227 | Peach | 260 | 390 | 963 |
| Fennel | 276 | 552 | 3067 | Pear | 130 | 195 | 325 |
| Green beans | 280 | 560 | 1556 | Pineapple | 250 | 375 | 625 |
| Leeks | 310 | 620 | 1069 | Pomegranate | 290 | 435 | 460 |
| Lettuce | 240 | 192 | 1263 | Raspberry | 220 | 330 | 647 |
| Mushrooms | 235 | 470 | 870 | Strawberry | 160 | 240 | 593 |
| Olives | 432 | 130 | 304 | Tangerine | 210 | 315 | 292 |
| Onions | 140 | 280 | 538 | Watermelon | 280 | 420 | 1867 |
| Peeled tomatoes | 230 | 460 | 1095 | Dried fruits and nuts | |||
| Pepperoni | 210 | 420 | 955 | Dried figs | 1010 | 303 | 395 |
| Pumpkin | 202 | 404 | 1122 | Dried plum | 824 | 247 | 375 |
| Red radish | 180 | 360 | 1385 | Almond | 860 | 258 | 159 |
| Rocket salad | 369 | 295 | 1476 | Cashew nuts | 565 | 170 | 104 |
| Spinach | 530 | 1060 | 1710 | Nuts | 368 | 110 | 56 |
| Zucchini | 210 | 420 | 1909 | Peanuts | 680 | 204 | 114 |
Table 5. Potassium removal by home-based cooking methods
| Food Group/Item | Type of Treatment or Food Processing | % Potassium Content Reduction |
| Vegetables (15 different varieties) | Each food was placed in 2 liters of hot tap water (100–110 °F), stirred vigorously for 15–20 s and allowed to stand for a predetermined time period. Ham and hot dogs (meat group) were placed in boiling water bath, stirred and allowed to boil for 3 min. Avocado and banana from the fruit group were placed in cold tap water, stirred gently and allowed to stand for the predetermined time period 51. | 59 ± 40 |
| Fruits (8 different varieties) | 43 ± 16 | |
| Legumes (5 different varieties) | 78.5 ± 20.5 | |
| Meats (7 different varieties) | 57 ± 41 | |
| Tuberous root vegetables * | Soaking 52 | 8.00% |
| Tuberous root vegetables * | Double cooking (boil, rinse and boil again) 52 | 46.00% |
| White Potato (Solanum tuberosum) | Leaching overnight after cubing 53 | 0–4% |
| White Potato (Solanum tuberosum) | Leaching overnight after shredding 53 | 2–17% |
| White Potato (Solanum tuberosum) | Boiling after cubing 53 | 50.00% |
| White Potato (Solanum tuberosum) | Boiling after shredding 53 | 69–75% |
| Banana (Matooke) | Soaking | No significant reduction |
| Banana (Matooke) | Boiling 60 min at 200 °C 54 | 37.00% |
| Chocolate | Soaking 55 | 16.00% |
| Potato | 16.00% | |
| Apple | 26.00% | |
| Tomato | 37.00% | |
| Banana | Soaking 55 | 41.00% |
Footnotes: * Fresh and sweet batata, cocomalanga, dasheen, eddo, black yam, white yam, yellow yam, yampi, malanga, red yautia, white yautia and yucca.
[Source 1 ]Table 6. Summary of heart-healthy foods that are restricted by a Low-Potassium Diet
| Recommended Food Groups | Examples of Heart-Healthy Foods | Restricted by Low-Potassium Diet? |
| Fruits and vegetables | Green vegetables (eg, spinach, kale, collard greens, broccoli, asparagus) | Yes (except kale) |
| Berries (eg, strawberries, blueberries, blackberries, raspberries) | No | |
| Avocadoes | Yes | |
| Potatoes and sweet potatoes | Yes | |
| Carrots | Yes (if raw) | |
| Peppers | No | |
| Pumpkin and squash | Yes | |
| Tomatoes | Yes | |
| Cantaloupes and papaya | Yes | |
| Citrus fruits (eg, oranges, grapefruits) | Yes | |
| Apples | No | |
| Garlic | No | |
| Soybeans | Yes | |
| Whole grains | Whole wheat | Yes (bran/bran products) |
| Brown rice | ||
| Oats | ||
| Rye | ||
| Barley | ||
| Buckwheat | ||
| Quinoa | ||
| Low-fat dairy products | Fat-free or low-fat milk, yogurt, and cheese | Yes |
| Skinless poultry and fish | Prepared without added saturated or trans fat | Yes |
| Fish high in omega-3 | ||
| Nuts and legumes | Walnuts | Yes |
| Almonds | Yes | |
| Seeds (eg, chia, flaxseed, hemp) | Yes | |
| Dried beans and lentils | Yes | |
| Nontropical vegetable oils | Extra-virgin olive oil | No |
Table 7. Dietary recommendations and restriction in dialysis patients and their implications
| Recommended range | Evidence | Observations | |
| Dietary protein recommendations | 1.2–1.4 g/kg/day | Epidemiologic studies show greatest survival with 1.2–1.4 g/kg/day. | Most dialysis patients eat <1.0 g/kg/day. |
| Dietary phosphorus restrictions | <800 mg/day | Mostly based on epidemiologic association between serum phosphorus and mortality. | Adhering to low phosphorus diet may result in inadequate protein intake. |
| Dietary potassium (K) restrictions | <3 g/day | Very recent data suggest an association between higher K load and death. | Most K rich foods are heart-healthy. |
| Dietary salt and fluid restrictions | <2.5 g/day | Salt data are mostly opinion based.There is more recent data on adverse outcomes from fluid retention. | Less fluid intake may be difficult to adhere to if patients are to eat larger amounts of protein and calorie. |
| Dietary carbohydrate and glycemic restrictions | Mostly for diabetic dialysis patients | Higher A1c >9% may be associated with higher death risk. | May aggravate burnt-out diabetes leading to poor outcomes, especially if A1c<6%. |
| Dietary lipid restrictions | e.g. DASH diet | Recent data show benefit of Omega-3 and unsaturated fatty acids. | Dietary fat restriction may aggravate calorie malnutrition. |
| Dietary vitamins and trace elements | Different data | Mixed data regarding vitamin D, and inadequate data for most others. | Some old data suggest certain vitamins such as A should be given with restriction. |
| Dietary calcium restrictions | <1200 mg/day | Data are mostly based on association between serum calcium and mortality. | Hypocalcemia may be aggravated, especially with calcimimetics. |
| Meals restrictions during hemodialysis treatment | Meals during hemodialysis | Anecdotal case reports regarding aspiration during hemodialysis while eating. | Hemodialysis induced hypoglycemia. |
Low potassium foods for kidney patients
Potassium is present in a large variety of foods, both from animal and plant sources and in beverages. Potassium is found mainly intracellularly in animals, where it has a crucial role in determining the electric potential of cell membranes and then the excitability of nervous system and muscle cells. Hence it is not surprising that food rich in cells, such as meats, poultry or fish, are relevant sources of potassium 1. Many fruits and vegetables are also excellent sources of potassium, as are some legumes (e.g., soybeans) and potatoes. Milk, yogurt, and nuts also contain potassium 56. Among starchy foods, whole-wheat flour and brown rice are much higher in potassium than their refined counterparts, white wheat flour and white rice.
A recent study showed that high protein intake in maintenance dialysis patients has direct correlation with hyperkalemia 18. In this study higher dietary potassium intake was associated with increased death risk in long-term hemodialysis patients, even after adjustments for serum potassium level; dietary protein; energy and phosphorus intake; and nutritional and inflammatory marker levels. Furthermore, potassium is almost ubiquitous, which makes it very difficult for patients to make dietary choices based on general guidance alone. Nutritional therapy in chronic kidney disease (CKD) is very complex, as it has to consider concomitantly the intake of protein, energy, sodium, phosphorus and potassium. Adding a low-potassium diet to already restricted diets required in comorbid conditions such as diabetes and heart disease can also increase the burden of dietary requirements 57. Skilled renal dietitians are required to properly select foods to individualize dietary programs with low potassium content and can help you add foods to your food list. This activity is time consuming and it is difficult to realize in the common clinical practice without the support of dedicated and qualified health professionals, which also look to improve patients’ lifestyle.
Dietary recommendations for patients receiving hemodialysis are considered to be among the most restrictive and may thus lead to reduced adherence in many patients 58, with nonadherence estimated at 25% to 86% 17. Patient-reported barriers to adherence include eating away from home, lack of appetite, craving salty foods, being too tired to cook, finding the diet bland and tasteless, difficulty tracking nutrient intake, feeling deprived, and lack of motivation to eat the right foods, each reported by more than half the participants 44. Onset of depression, stress, beliefs about loss of control, and the patient’s level of social support also contribute to nonadherence 59.
Potassium content also varies widely among different foods and is dependent on the method of food preparation, its carbohydrate content, and whether it is combined with other foods that influence potassium homeostasis 24. Additionally, potassium content does not always directly equate to the bioavailability of potassium from food, which may differ based on its preparation 60. Additional aspects useful to limit effective potassium intake is education about the use of cooking procedures (as soaking or boiling) in order to obtain food demineralization 55, 61: boiling is able to remove up to 60–80% of the potassium content of several raw foods (see Table 5). For example, the potassium content of fresh green beans is reduced by 15% with soaking, 33% with cooking, and 46% with soaking and cooking 62.
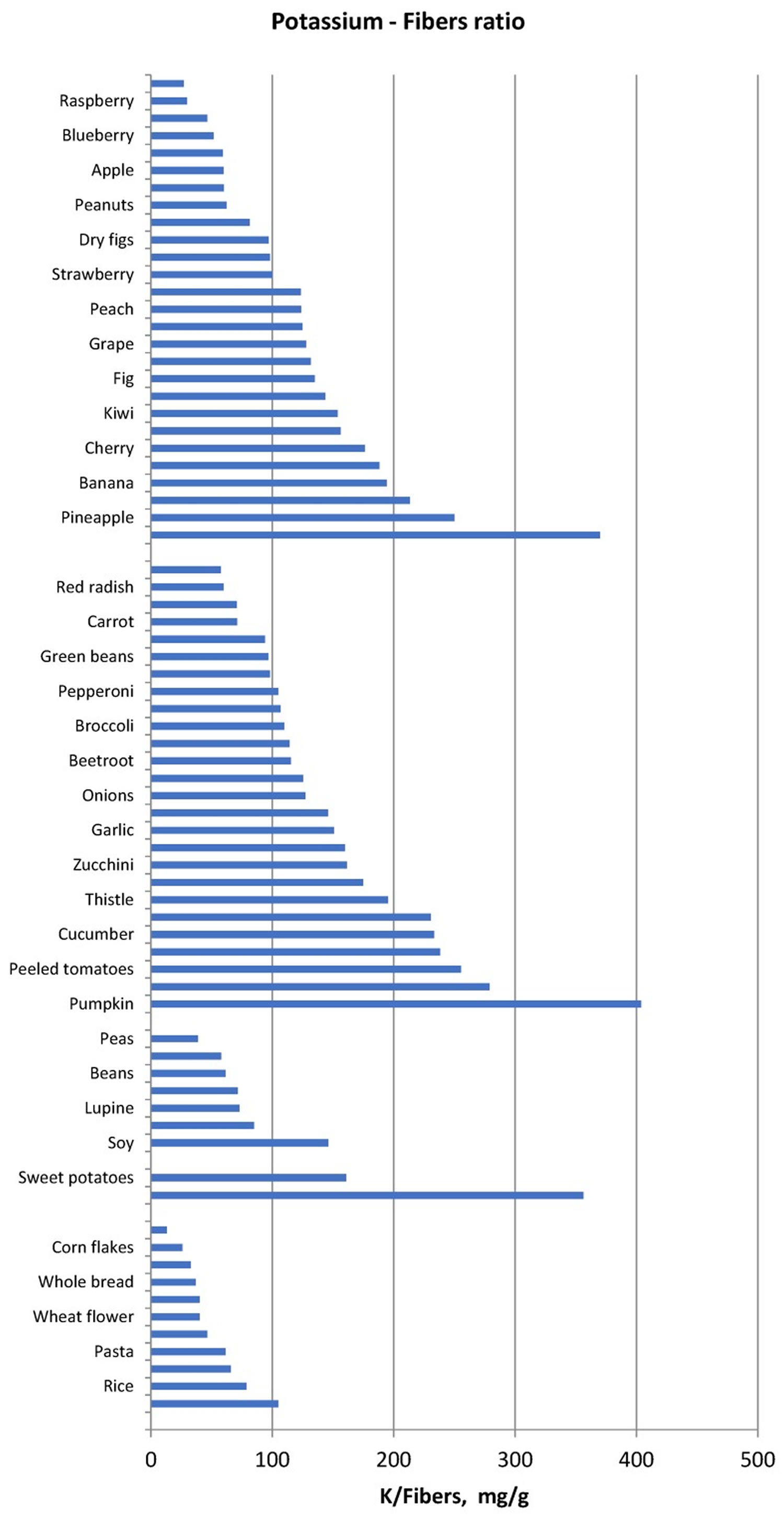
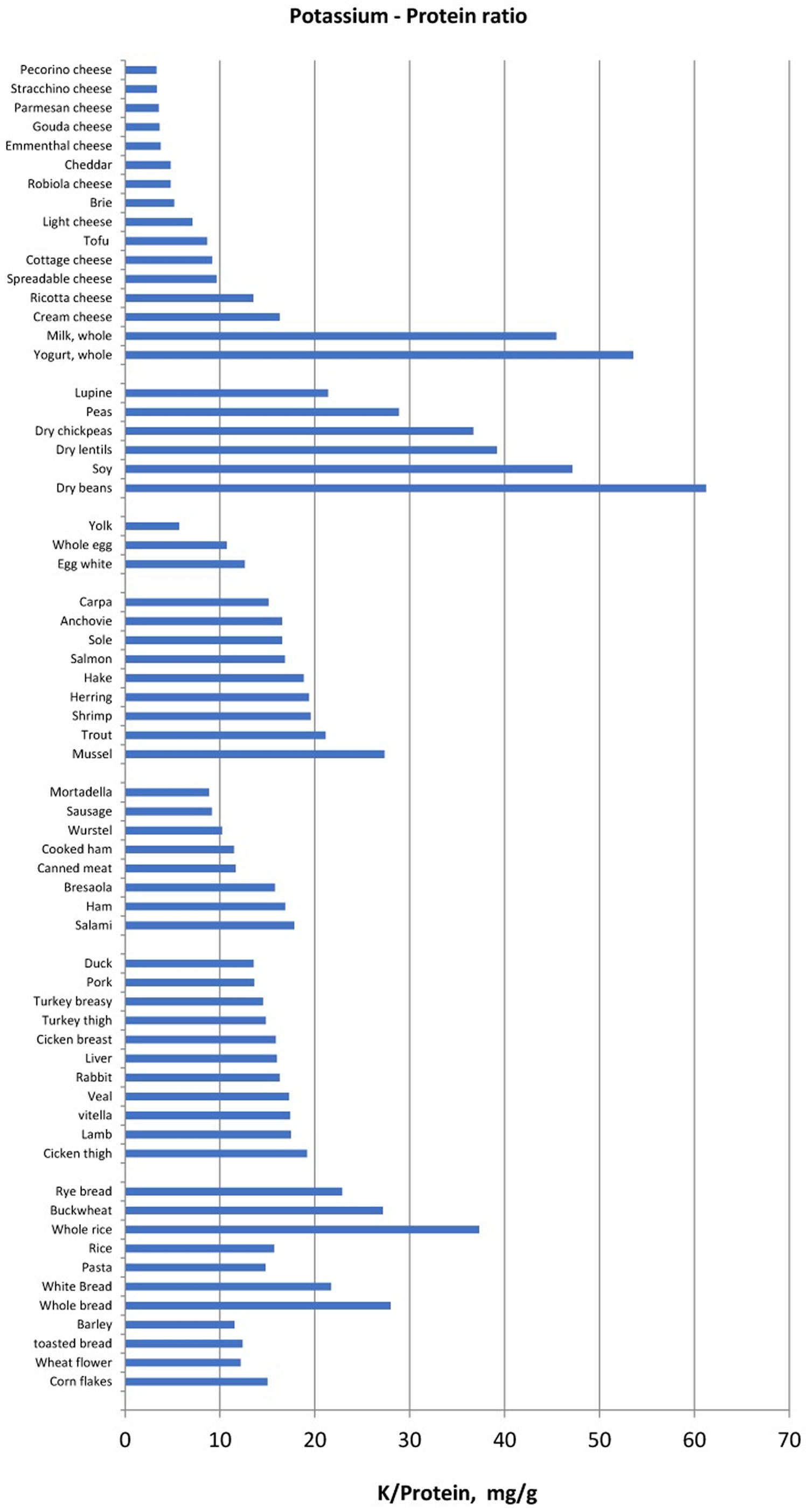
Another method of food selection may be based on the potassium content normalized for unit of fiber, namely the reporting of the potassium content of vegetables and fruits also as “mg per 1 g fibre”. Foods with low potassium to fibre ratio may be allowed whereas foods with very high potassium to fibre ratio should be avoided (Figure 1). Similarly, since protein intake must be increased in haemodialysis and peritoneal dialysis patients, food selection should be addressed to reduce potassium intake without reducing dietary protein intake. Hence, reporting potassium intake per unit (g) of protein may be another method that can make it easier to limit the intake of foods that supply more potassium for the same protein intake (Figure 2) in ESRD patients.
Finally, attention should be paid to hidden sources of potassium, such as salt substitutes and certain food additives. The former contain potassium instead of sodium and are usually recommended in hypertensive patients to reduce sodium intake and to increase potassium intake. Two categories of salt substitutes are available on the market: low-sodium salts and sodium-free salts. In the low-sodium salt the sodium chloride content must not exceed 35% (corresponding to a sodium content not exceeding 13.6 g%) and cannot be less than 20% (corresponding to a sodium content not less than 7.8%) with a potassium: sodium ratio of at least 1.5:1. In the sodium-free salts potassium content ranges between 20% and 30% and sodium content is fixed at a maximum value of 0.12%.
Many salt substitutes contain potassium chloride as a replacement for some or all of the sodium chloride in salt. The potassium content of these products varies widely, from about 440 mg to 2,800 mg potassium per teaspoon 4. Hence, for instance, 5–6 g of a current salt substitute can supply from 1000–1200 mg to 1500–1800 mg of extra-potassium. Some people, such as those with kidney disease or who are taking certain medications, should consult their healthcare provider before taking salt substitutes because of the risk of hyperkalemia posed by the high levels of potassium in these products.
Sherman and Mehta 63 found that potassium content in foods with additives varied widely and that uncooked, enhanced meat and poultry products had potassium levels up to threefold greater than similar unenhanced food products. The use of additives in packaged poultry, fish or meat foods can increase the effective dietary potassium load and of special concerns in patients with CKD, are sodium-reduced products 64. For instance, additive-free products had an average potassium content <387 mg/100 g, whereas five of the 25 products with additives analyzed in that investigation contained at least 692 mg/100 g with a maximum of 930 mg/100 g 63. Table 9 reports the most frequently used potassium-based additives and their acceptable daily intake (ADI) 63. A temporary acceptable daily intake (ADI) of 3 mg/kg is currently established, while an ADI of 25 mg/kg for potassium sorbate is under evaluation 65. Paying attention to the food labels may be useful although the quantity of potassium added as an additive is not generally available.
Figure 1. Potassium to fiber ratio (mg/g) in several food categories
[Source 1 ]Figure 2. Potassium to protein ratio (mg/g) in several food categories
[Source 1 ]Table 8. Low potassium foods chart
[Source 1 ]Table 9. Most frequently used potassium-based food additives and the corresponding acceptable daily intake (ADI)
| Categories | Chemical Name | E Number (Europe) | ADI | Where to Find Them |
| Preservatives | Potassium sorbate | E202 | 3 mg/kg | Pre-cooked or long-lasting foods, powder dressings, nuts, sauces, preserved meats, stuffed pasta (tortellini, ravioli), jellies, concentrated fruit juices, processed cheeses, wine, margarine |
| Potassium metabisulphite | E224 | 0.35 mg/kg | ||
| Potassium nitrate | E252 | 5 mg/kg | ||
| Antioxidants and acidity regulators | Potassium citrate | E332 | No limit | |
| Potassium tartrate | E336 | 30 mg/kg | ||
| Stabilizers, emulsifier, thickeners | Potassium alginate | E402 | 50 mg/kg | |
| Potassium diphosphate | E450 | 70 mg/kg | ||
| Potassium triphosphate | E451 | 70 mg/kg | ||
| Flavour enhancer | Potassium glutamate | E622 | Not defined | |
| Potassium guanylate | E628 | |||
| Potassium inosinate | E632 |
Summary of low potassium diet and foods
A low potassium diet is defined as a dietary potassium intake of 2–3 g/day (approximately 51–77 mmol/day) 1. Low potassium diet of usually less than 3 g per day is recommended in the management of patients with chronic kidney disease (CKD), especially those who tend to develop hyperkalemia. Special attention must be paid to avoid excess potassium intake, but together with maintenance of a high fiber intake and a low net fixed-acid load. This is an important point since constipation and metabolic acidosis are major risk factors for chronic hyperkalemia.
To achieve a reduction of potassium load without causing a decrease in alkali or fiber intake, experts recommend avoiding foods which contain excessive amount of potassium, favoring foods with low potassium content relative to fiber and protein content, providing education about the use of cooking procedures (especially boiling and soaking) in order to achieve demineralization and increasing attention to hidden sources of potassium (e.g., food additives and low-sodium salt substitutes).
However, the traditional low-potassium diet is complex and can counteract the benefits of a plant-based diet, with only modest evidence to support the rationale for these dietary restrictions. Furthermore, reduction of fruit and vegetable intake due to their high-potassium content results in a less heart-healthy diet and may have adverse effects on the patient’s acid-base balance and intestinal microbiota. There is increasing evidence to support the multiple health benefits of a plant-based diet in patients with chronic kidney disease (CKD), including improvements in blood pressure and reductions in the risk for cardiovascular disease, stroke, and chronic kidney disease (CKD) progression. Recent evidence suggests that adjunctive treatment with the newer potassium-binding agents may allow for diet liberalization and optimization of RAAS inhibitor therapy in patients with chronic kidney disease (CKD) and a history of hyperkalemia while minimizing the risk for recurrent hyperkalemia, although further studies are needed to confirm this.
- Cupisti, A., Kovesdy, C. P., D’Alessandro, C., & Kalantar-Zadeh, K. (2018). Dietary Approach to Recurrent or Chronic Hyperkalaemia in Patients with Decreased Kidney Function. Nutrients, 10(3), 261. https://doi.org/10.3390/nu10030261[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Dietary Reference Intakes for Sodium and Potassium (2019). Consensus Study Report. https://www.nap.edu/read/25353/chapter/1[↩]
- DuBose TD Jr. Regulation of Potassium Homeostasis in CKD. Adv Chronic Kidney Dis. 2017 Sep;24(5):305-314. doi: 10.1053/j.ackd.2017.06.002[↩]
- Institute of Medicine. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. Washington, DC; 2005.[↩][↩]
- Simon LV, Hashmi MF, Farrell MW. Hyperkalemia. [Updated 2021 Aug 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470284[↩]
- Rosano GMC, Tamargo J, Kjeldsen KP, Lainscak M, Agewall S, Anker SD, Ceconi C, Coats AJS, Drexel H, Filippatos G, Kaski JC, Lund L, Niessner A, Ponikowski P, Savarese G, Schmidt TA, Seferovic P, Wassmann S, Walther T, Lewis BS. Expert consensus document on the management of hyperkalaemia in patients with cardiovascular disease treated with renin angiotensin aldosterone system inhibitors: coordinated by the Working Group on Cardiovascular Pharmacotherapy of the European Society of Cardiology. Eur Heart J Cardiovasc Pharmacother. 2018 Jul 1;4(3):180-188. doi: 10.1093/ehjcvp/pvy015[↩][↩]
- Belmar Vega L, Galabia ER, Bada da Silva J, Bentanachs González M, Fernández Fresnedo G, Piñera Haces C, Palomar Fontanet R, Ruiz San Millán JC, de Francisco ÁLM. Epidemiology of hyperkalemia in chronic kidney disease. Nefrologia (Engl Ed). 2019 May-Jun;39(3):277-286. English, Spanish. doi: 10.1016/j.nefro.2018.11.011[↩][↩]
- KDIGO KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):S1–S150.[↩]
- KDOQI Chronic kidney disease: major recommendations. https://www.andeal.org/vault/pqnew95.pdf[↩]
- Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis. 2000 Jun;35(6 Suppl 2):S17-S104. doi: 10.1053/ajkd.2000.v35.aajkd03517. Erratum in: Am J Kidney Dis 2001 Oct;38(4):917.[↩]
- Kalantar-Zadeh K, Fouque D. Nutritional Management of Chronic Kidney Disease. N Engl J Med. 2017 Nov 2;377(18):1765-1776. doi: 10.1056/NEJMra1700312[↩][↩]
- Hassan K. (2015). Association of low potassium diet and folic acid deficiency in patients with CKD. Therapeutics and clinical risk management, 11, 821–827. https://doi.org/10.2147/TCRM.S83751[↩]
- Stone, M. S., Martyn, L., & Weaver, C. M. (2016). Potassium Intake, Bioavailability, Hypertension, and Glucose Control. Nutrients, 8(7), 444. https://doi.org/10.3390/nu8070444[↩]
- He, F.J. and MacGregor, G.A. (2008), Beneficial effects of potassium on human health. Physiologia Plantarum, 133: 725-735. https://doi.org/10.1111/j.1399-3054.2007.01033.x[↩]
- O’Neil, C. E., Keast, D. R., Fulgoni, V. L., & Nicklas, T. A. (2012). Food sources of energy and nutrients among adults in the US: NHANES 2003–2006. Nutrients, 4(12), 2097–2120. https://doi.org/10.3390/nu4122097[↩]
- Keast, D. R., Fulgoni, V. L., 3rd, Nicklas, T. A., & O’Neil, C. E. (2013). Food sources of energy and nutrients among children in the United States: National Health and Nutrition Examination Survey 2003–2006. Nutrients, 5(1), 283–301. https://doi.org/10.3390/nu5010283[↩]
- Kalantar-Zadeh, K., Tortorici, A. R., Chen, J. L., Kamgar, M., Lau, W. L., Moradi, H., Rhee, C. M., Streja, E., & Kovesdy, C. P. (2015). Dietary restrictions in dialysis patients: is there anything left to eat?. Seminars in dialysis, 28(2), 159–168. https://doi.org/10.1111/sdi.12348[↩][↩][↩][↩][↩][↩]
- Noori, N., Kalantar-Zadeh, K., Kovesdy, C. P., Murali, S. B., Bross, R., Nissenson, A. R., & Kopple, J. D. (2010). Dietary potassium intake and mortality in long-term hemodialysis patients. American journal of kidney diseases : the official journal of the National Kidney Foundation, 56(2), 338–347. https://doi.org/10.1053/j.ajkd.2010.03.022[↩][↩][↩][↩][↩]
- Kalantar-Zadeh, K., Kovesdy, C. P., Bross, R., Benner, D., Noori, N., Murali, S. B., Block, T., Norris, J., Kopple, J. D., & Block, G. (2011). Design and development of a dialysis food frequency questionnaire. Journal of renal nutrition : the official journal of the Council on Renal Nutrition of the National Kidney Foundation, 21(3), 257–262. https://doi.org/10.1053/j.jrn.2010.05.013[↩][↩]
- Kalantar-Zadeh K, Kopple JD, Deepak S, Block D, Block G. Food intake characteristics of hemodialysis patients as obtained by food frequency questionnaire. J Ren Nutr. 2002 Jan;12(1):17-31. doi: 10.1053/jren.2002.29598[↩][↩]
- Cosola, C., De Angelis, M., Rocchetti, M. T., Montemurno, E., Maranzano, V., Dalfino, G., Manno, C., Zito, A., Gesualdo, M., Ciccone, M. M., Gobbetti, M., & Gesualdo, L. (2017). Beta-Glucans Supplementation Associates with Reduction in P-Cresyl Sulfate Levels and Improved Endothelial Vascular Reactivity in Healthy Individuals. PloS one, 12(1), e0169635. https://doi.org/10.1371/journal.pone.0169635[↩]
- St-Jules D.E., Goldfarb D.S., Sevick M.A. Nutrient Non-equivalence: Does Restricting High-Potassium Plant Foods Help to Prevent Hyperkalemia in Hemodialysis Patients? J. Ren. Nutr. 2016;26:282–287. doi: 10.1053/j.jrn.2016.02.005[↩]
- Khoueiry G, Waked A, Goldman M, El-Charabaty E, Dunne E, Smith M, Kleiner M, Lafferty J, Kalantar-Zadeh K, El-Sayegh S. Dietary intake in hemodialysis patients does not reflect a heart healthy diet. J Ren Nutr. 2011 Nov;21(6):438-47. doi: 10.1053/j.jrn.2010.09.001[↩][↩][↩]
- Clegg, D. J., Headley, S. A., & Germain, M. J. (2020). Impact of Dietary Potassium Restrictions in CKD on Clinical Outcomes: Benefits of a Plant-Based Diet. Kidney medicine, 2(4), 476–487. https://doi.org/10.1016/j.xkme.2020.04.007[↩][↩][↩][↩][↩][↩]
- Palmer B. F. (2015). Regulation of Potassium Homeostasis. Clinical journal of the American Society of Nephrology : CJASN, 10(6), 1050–1060. https://doi.org/10.2215/CJN.08580813[↩]
- Palmer BF, Clegg DJ. Physiology and Pathophysiology of Potassium Homeostasis: Core Curriculum 2019. Am J Kidney Dis. 2019 Nov;74(5):682-695. doi: 10.1053/j.ajkd.2019.03.427[↩]
- Bellizzi, V., Cupisti, A., Locatelli, F., Bolasco, P., Brunori, G., Cancarini, G., Caria, S., De Nicola, L., Di Iorio, B. R., Di Micco, L., Fiaccadori, E., Garibotto, G., Mandreoli, M., Minutolo, R., Oldrizzi, L., Piccoli, G. B., Quintaliani, G., Santoro, D., Torraca, S., Viola, B. F., … “Conservative Treatment of CKD” study group of the Italian Society of Nephrology (2016). Low-protein diets for chronic kidney disease patients: the Italian experience. BMC nephrology, 17(1), 77. https://doi.org/10.1186/s12882-016-0280-0[↩]
- Goraya, N., Simoni, J., Jo, C. H., & Wesson, D. E. (2013). A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clinical journal of the American Society of Nephrology : CJASN, 8(3), 371–381. https://doi.org/10.2215/CJN.02430312[↩]
- Weir MR, Bakris GL, Bushinsky DA, Mayo MR, Garza D, Stasiv Y, Wittes J, Christ-Schmidt H, Berman L, Pitt B; OPAL-HK Investigators. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015 Jan 15;372(3):211-21. doi: 10.1056/NEJMoa1410853[↩]
- Pitt, B., Anker, S. D., Bushinsky, D. A., Kitzman, D. W., Zannad, F., Huang, I. Z., & PEARL-HF Investigators (2011). Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. European heart journal, 32(7), 820–828. https://doi.org/10.1093/eurheartj/ehq502[↩]
- Laureati P, Xu Y, Trevisan M, Schalin L, Mariani I, Bellocco R, Sood MM, Barany P, Sjölander A, Evans M, Carrero JJ. Initiation of sodium polystyrene sulphonate and the risk of gastrointestinal adverse events in advanced chronic kidney disease: a nationwide study. Nephrol Dial Transplant. 2020 Sep 1;35(9):1518-1526. doi: 10.1093/ndt/gfz150[↩]
- Spinowitz, B. S., Fishbane, S., Pergola, P. E., Roger, S. D., Lerma, E. V., Butler, J., von Haehling, S., Adler, S. H., Zhao, J., Singh, B., Lavin, P. T., McCullough, P. A., Kosiborod, M., Packham, D. K., & ZS-005 Study Investigators (2019). Sodium Zirconium Cyclosilicate among Individuals with Hyperkalemia: A 12-Month Phase 3 Study. Clinical journal of the American Society of Nephrology : CJASN, 14(6), 798–809. https://doi.org/10.2215/CJN.12651018[↩][↩][↩]
- Fishbane, S., Ford, M., Fukagawa, M., McCafferty, K., Rastogi, A., Spinowitz, B., Staroselskiy, K., Vishnevskiy, K., Lisovskaja, V., Al-Shurbaji, A., Guzman, N., & Bhandari, S. (2019). A Phase 3b, Randomized, Double-Blind, Placebo-Controlled Study of Sodium Zirconium Cyclosilicate for Reducing the Incidence of Predialysis Hyperkalemia. Journal of the American Society of Nephrology : JASN, 30(9), 1723–1733. https://doi.org/10.1681/ASN.2019050450[↩]
- Kovesdy, C. P., Rowan, C. G., Conrad, A., Spiegel, D. M., Fogli, J., Oestreicher, N., Connaire, J. J., & Winkelmayer, W. C. (2018). Real-World Evaluation of Patiromer for the Treatment of Hyperkalemia in Hemodialysis Patients. Kidney international reports, 4(2), 301–309. https://doi.org/10.1016/j.ekir.2018.10.020[↩]
- Weir MR, Bakris GL, Gross C, Mayo MR, Garza D, Stasiv Y, Yuan J, Berman L, Williams GH. Treatment with patiromer decreases aldosterone in patients with chronic kidney disease and hyperkalemia on renin-angiotensin system inhibitors. Kidney Int. 2016 Sep;90(3):696-704. doi: 10.1016/j.kint.2016.04.019[↩]
- Agarwal R, Rossignol P, Mayo MR, et al. Patiromer vs. placebo to enable spironolactone in patients with resistant hypertension and CKD according to baseline kidney function (AMBER trial). Presented at: American Society of Nephrology: Kidney Week, 2019; November 5-10, 2019; Washington, DC.[↩]
- Talaei, M., Koh, W. P., Yuan, J. M., & van Dam, R. M. (2019). DASH Dietary Pattern, Mediation by Mineral Intakes, and the Risk of Coronary Artery Disease and Stroke Mortality. Journal of the American Heart Association, 8(5), e011054. https://doi.org/10.1161/JAHA.118.011054[↩]
- Chiavaroli, L., Viguiliouk, E., Nishi, S. K., Blanco Mejia, S., Rahelić, D., Kahleová, H., Salas-Salvadó, J., Kendall, C. W., & Sievenpiper, J. L. (2019). DASH Dietary Pattern and Cardiometabolic Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Nutrients, 11(2), 338. https://doi.org/10.3390/nu11020338[↩]
- Ferraro, P. M., Taylor, E. N., Gambaro, G., & Curhan, G. C. (2017). Dietary and Lifestyle Risk Factors Associated with Incident Kidney Stones in Men and Women. The Journal of urology, 198(4), 858–863. https://doi.org/10.1016/j.juro.2017.03.124[↩]
- Clase CM, Carrero JJ, Ellison DH, Grams ME, Hemmelgarn BR, Jardine MJ, Kovesdy CP, Kline GA, Lindner G, Obrador GT, Palmer BF, Cheung M, Wheeler DC, Winkelmayer WC, Pecoits-Filho R; Conference Participants. Potassium homeostasis and management of dyskalemia in kidney diseases: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2020 Jan;97(1):42-61. doi: 10.1016/j.kint.2019.09.018[↩][↩][↩]
- St-Jules DE, Goldfarb DS, Sevick MA. Nutrient Non-equivalence: Does Restricting High-Potassium Plant Foods Help to Prevent Hyperkalemia in Hemodialysis Patients? J Ren Nutr. 2016 Sep;26(5):282-7. doi: 10.1053/j.jrn.2016.02.005. Epub 2016 Mar 12. Erratum in: J Ren Nutr. 2016 Nov;26(6):416.[↩]
- Keith N.M., Osterberg A.E., Burchell H.B. Some effects of potassium salts in man. Ann Intern Med. 1942;16(5):879–892.[↩]
- Moorthi, R. N., Armstrong, C. L., Janda, K., Ponsler-Sipes, K., Asplin, J. R., & Moe, S. M. (2014). The effect of a diet containing 70% protein from plants on mineral metabolism and musculoskeletal health in chronic kidney disease. American journal of nephrology, 40(6), 582–591. https://doi.org/10.1159/000371498[↩]
- St-Jules, D. E., Woolf, K., Pompeii, M. L., & Sevick, M. A. (2016). Exploring Problems in Following the Hemodialysis Diet and Their Relation to Energy and Nutrient Intakes: The BalanceWise Study. Journal of renal nutrition : the official journal of the Council on Renal Nutrition of the National Kidney Foundation, 26(2), 118–124. https://doi.org/10.1053/j.jrn.2015.10.002[↩][↩]
- Batlle, D., Boobés, K., & Manjee, K. G. (2015). The Colon as the Potassium Target: Entering the Colonic Age of Hyperkalemia Treatment?. EBioMedicine, 2(11), 1562–1563. https://doi.org/10.1016/j.ebiom.2015.10.027[↩]
- Agarwal R, Afzalpurkar R, Fordtran JS. Pathophysiology of potassium absorption and secretion by the human intestine. Gastroenterology. 1994 Aug;107(2):548-71. doi: 10.1016/0016-5085(94)90184-8[↩][↩]
- Mathialahan T, Maclennan KA, Sandle LN, Verbeke C, Sandle GI. Enhanced large intestinal potassium permeability in end-stage renal disease. J Pathol. 2005 May;206(1):46-51. doi: 10.1002/path.1750[↩]
- Kovesdy CP, Regidor DL, Mehrotra R, Jing J, McAllister CJ, Greenland S, Kopple JD, Kalantar-Zadeh K. Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin J Am Soc Nephrol. 2007 Sep;2(5):999-1007. doi: 10.2215/CJN.04451206[↩][↩]
- Torlén, K., Kalantar-Zadeh, K., Molnar, M. Z., Vashistha, T., & Mehrotra, R. (2012). Serum potassium and cause-specific mortality in a large peritoneal dialysis cohort. Clinical journal of the American Society of Nephrology : CJASN, 7(8), 1272–1284. https://doi.org/10.2215/CJN.00960112[↩]
- U.S. Department of Agriculture, Agricultural Research Service. FoodData Central. https://fdc.nal.usda.gov[↩]
- Jones WL. Demineralization of a wide variety of foods for the renal patient. J Ren Nutr. 2001 Apr;11(2):90-6. doi: 10.1016/s1051-2276(01)38751-4[↩]
- Burrowes JD, Ramer NJ. Removal of potassium from tuberous root vegetables by leaching. J Ren Nutr. 2006 Oct;16(4):304-11. doi: 10.1053/j.jrn.2006.07.012[↩][↩]
- Bethke PC, Jansky SH. The effects of boiling and leaching on the content of potassium and other minerals in potatoes. J Food Sci. 2008 Jun;73(5):H80-5. doi: 10.1111/j.1750-3841.2008.00782.x[↩][↩][↩][↩]
- Asiimwe J, Sembajwe LF, Senoga A, Bakiika E, Muwonge H, Kalyesubula R. Overnight soaking or boiling of “Matooke” to reduce potassium content for patients with chronic kidney disease: does it really work? Afr Health Sci. 2013 Sep;13(3):546-50. doi: 10.4314/ahs.v13i3.2[↩]
- Picq C, Asplanato M, Bernillon N, Fabre C, Roubeix M, Ricort JM. Effects of water soaking and/or sodium polystyrene sulfonate addition on potassium content of foods. Int J Food Sci Nutr. 2014 Sep;65(6):673-7. doi: 10.3109/09637486.2014.908172[↩][↩][↩]
- Bailey JL, Sands JM, Franch HA. Water, electrolytes, and acid-based metabolism. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2014:102-32.[↩]
- Bataille S, Landrier JF, Astier J, Cado S, Sallette J, Giaime P, Sampol J, Sichez H, Ollier J, Gugliotta J, Serveaux M, Cohen J, Darmon P. Haemodialysis patients with diabetes eat less than those without: A plea for a permissive diet. Nephrology (Carlton). 2017 Sep;22(9):712-719. doi: 10.1111/nep.12837[↩]
- Oquendo LG, Asencio JMM, de Las Nieves CB. Contributing factors for therapeutic diet adherence in patients receiving haemodialysis treatment: an integrative review. J Clin Nurs. 2017 Dec;26(23-24):3893-3905. doi: 10.1111/jocn.13804[↩]
- Lambert, K., Mullan, J., & Mansfield, K. (2017). An integrative review of the methodology and findings regarding dietary adherence in end stage kidney disease. BMC nephrology, 18(1), 318. https://doi.org/10.1186/s12882-017-0734-z[↩]
- Macdonald-Clarke CJ, Martin BR, McCabe LD, McCabe GP, Lachcik PJ, Wastney M, Weaver CM. Bioavailability of potassium from potatoes and potassium gluconate: a randomized dose response trial. Am J Clin Nutr. 2016 Aug;104(2):346-53. doi: 10.3945/ajcn.115.127225[↩]
- Asiimwe, J., Sembajwe, L. F., Senoga, A., Bakiika, E., Muwonge, H., & Kalyesubula, R. (2013). Overnight soaking or boiling of “Matooke” to reduce potassium content for patients with chronic kidney disease: does it really work?. African health sciences, 13(3), 546–550. https://doi.org/10.4314/ahs.v13i3.2[↩]
- Martínez-Pineda M, Yagüe-Ruiz C, Caverni-Muñoz A, Vercet-Tormo A. Reduction of potassium content of green bean pods and chard by culinary processing. Tools for chronic kidney disease. Nefrologia. 2016 Jul-Aug;36(4):427-32. English, Spanish. doi: 10.1016/j.nefro.2016.03.022[↩]
- Sherman RA, Mehta O. Phosphorus and potassium content of enhanced meat and poultry products: implications for patients who receive dialysis. Clin J Am Soc Nephrol. 2009 Aug;4(8):1370-3. doi: 10.2215/CJN.02830409[↩][↩][↩]
- Parpia AS, L’Abbé M, Goldstein M, Arcand J, Magnuson B, Darling PB. The Impact of Additives on the Phosphorus, Potassium, and Sodium Content of Commonly Consumed Meat, Poultry, and Fish Products Among Patients With Chronic Kidney Disease. J Ren Nutr. 2018 Mar;28(2):83-90. doi: 10.1053/j.jrn.2017.08.013[↩]
- Dietary Guidelines Advisory Committee . The Report of the Dietary Guidelines Advisory Committee on Dietary Guidelines for Americans. Department of Health and Human Services and Department of Agriculture; Washington, DC, USA: 2005.[↩]