Open-angle glaucoma
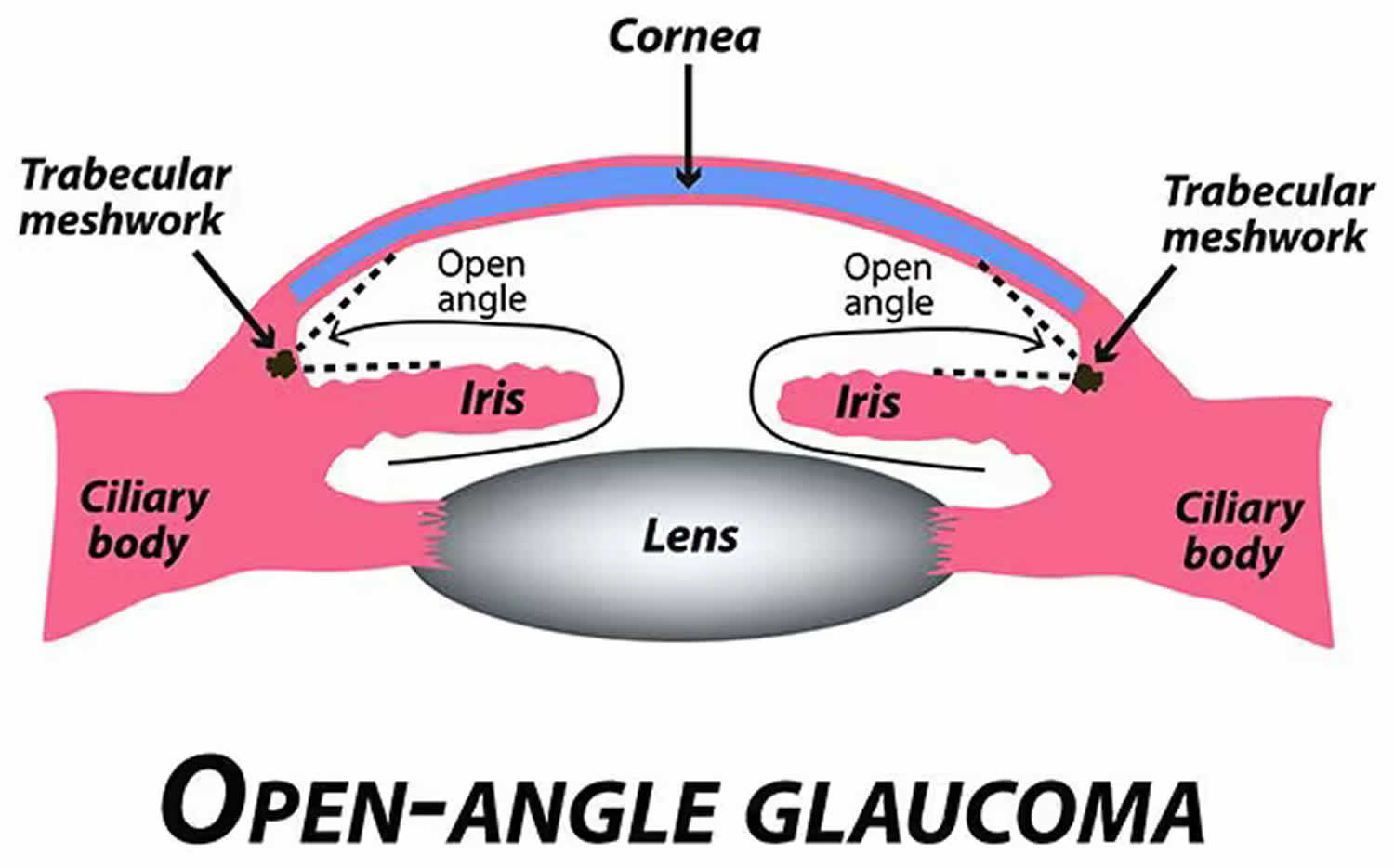
“Open-angle” means that the drainage angle, where the inside of the sclera (the white of your eye) and the outer edge of your iris meet called the irido-corneal angle is open wide. Open-angle glaucoma is the most common type of glaucoma where the drainage angle between the cornea and the iris is wide open and allows the aqueous fluid of your eye to make its way to the trabecular meshwork – the main site for fluid drainage from your eye 1, 2 , 3. In patients with open-angle glaucoma, non-visible abnormalities in the trabecular meshwork reduce the outflow of aqueous humor 1. Experts aren’t sure what causes open-angle glaucoma, but it may be caused by pressure building up in your eye called raised intraocular pressure (IOP) 4, 1. If the aqueous fluid in your eye can’t drain fast enough, it creates pressure that pushes on the optic nerve in the back of your eye. Over time, the raised intraocular pressure (IOP) damages the optic nerve, which affects your vision. This can eventually lead to blindness. In fact, open-angle glaucoma is the most common type of glaucoma in the United States, where 9 in 10 people with glaucoma have the open-angle type and open-angle glaucoma causes almost 2 in 10 cases of blindness in African Americans 5, 6. In the United States, open-angle glaucoma is what most people mean when they talk about glaucoma. People with high blood pressure (hypertension) or diabetes are at higher risk for open-angle glaucoma. Open-angle glaucoma is painless and causes no vision changes at first. Many people with open-angle glaucoma don’t have any symptoms until they start to lose their vision, and people may not notice vision loss right away.
There are 2 types of open-angle glaucoma that are classified depending on the cause:
- Primary open-angle glaucoma (POAG): If there is no identifiable factor causing the glaucoma (i.e., the cause of the glaucoma is unknown), this is referred to as primary open-angle glaucoma (POAG) or chronic open-angle glaucoma (COAG). Primary open-angle glaucoma (POAG) is the most common form of glaucoma in the U.S. Primary open-angle glaucoma (POAG) can be of 2 types. Primary open-angle glaucoma (POAG) with either elevated intraocular pressure (IOP) or normal intraocular pressure (IOP) also known as normal tension glaucoma (NTG) or low tension glaucoma.
- Primary open-angle glaucoma (POAG) with elevated intraocular pressure (IOP) and no other underlying disease. Primary open-angle glaucoma (POAG) accounts for approximately 75% of glaucoma cases in the United States 7, 8. Primary open-angle glaucoma (POAG) affects an estimated 2 to 3% of U.S. adults age 40 and older, and the prevalence is predicted to increase as the population skews older over time 9. The prevalence of primary open-angle glaucoma (POAG) in people of African and Hispanic descent in the United States is at least 3 times higher than in non-Latino White people 10, 11, 12, 13, 14. The reasons behind this association are unclear and complex, and warrant more study because there are also socioeconomic factors that influence access to care and the detection and treatment of glaucoma 15, 12, 16, 9.
- Normal tension glaucoma (NTG), also known as normal or low-pressure glaucoma, is a type of primary open-angle glaucoma (POAG) where the optic nerve damage and vision loss characteristic of glaucoma occur despite eye pressure (intraocular pressure [IOP]) remaining within the normal range and an open, normal appearing anterior chamber angle 17, 18, 19, 20, 21, 22. Normal tension glaucoma (NTG) has the same characteristics as primary open-angle glaucoma (POAG) except the intraocular pressure (IOP) is in the normal range, that is, less than 21 mmHg. One theory regarding the mechanism of injury in normal tension glaucoma (NTG) is insufficient blood flow leading to optic nerve damage. While some try to delineate normal tension glaucoma and primary open angle glaucoma (POAG) as two completely unique disease processes, it has also been suggested that primary open-angle glaucoma (POAG) and normal tension glaucoma (NTG) exist on a continuum with intraocular pressure (IOP) playing a larger role in primary open angle glaucoma (POAG), and vascular or mechanical factors as the root cause in normal tension glaucoma 23.
- Primary open-angle glaucoma (POAG) with elevated intraocular pressure (IOP) and no other underlying disease. Primary open-angle glaucoma (POAG) accounts for approximately 75% of glaucoma cases in the United States 7, 8. Primary open-angle glaucoma (POAG) affects an estimated 2 to 3% of U.S. adults age 40 and older, and the prevalence is predicted to increase as the population skews older over time 9. The prevalence of primary open-angle glaucoma (POAG) in people of African and Hispanic descent in the United States is at least 3 times higher than in non-Latino White people 10, 11, 12, 13, 14. The reasons behind this association are unclear and complex, and warrant more study because there are also socioeconomic factors that influence access to care and the detection and treatment of glaucoma 15, 12, 16, 9.
- Secondary open-angle glaucoma: Secondary glaucoma is when another condition or problem within the eye increases eye pressure such as eye injury, eye surgery or eye procedures, certain medications especially corticosteroids and cycloplegics or other eye diseases (e.g., pigmentary dispersion syndrome, pseudo-exfoliation syndrome, uveitis) causing the glaucoma. This is when another condition or event increases eye pressure, which leads to glaucoma.
Open-angle glaucoma is a diagnosis of exclusion, and other ocular emergencies such as acute closed-angle glaucoma must be ruled out 24. Diagnosis of open-angle glaucoma requires eye assessment by an eye specialist. This will include examination of the optic nerve where it leaves the eye (the optic disc), a check of the intraocular pressure and a field of vision test. Other tests may include measurement of the central corneal thickness and an examination of the drainage angle with a special lens placed on the eye.
Your eye specialist will conduct a thorough eye assessment to diagnose open-angle glaucoma:
- Intraocular pressure (IOP)
- Open- normal appearing anterior chamber angle
- Characteristics signs of optic disc damage
- Visual function loss on perimetry
Frequently, the diagnosis is not clear-cut and the patient may present with some risk factors and signs, but not others. In such cases, the patient may be labeled as a glaucoma suspect. These patients require repeat assessments at regular intervals with the frequency of visits dependent upon index of suspicion.
Glaucoma eyesight damage is permanent and it cannot be reversed. But medicine and surgery can help to stop further damage. To treat glaucoma, your ophthalmologist may use one or more of the following treatments.
- Medications. Glaucoma medications lower pressure inside your eye in different ways. Some of them cause your pupil to relax more, improving aqueous humor drainage. Others slow the production of aqueous humor.
- Glaucoma surgery. This approach usually aims to improve fluid flow and drainage. Examples of glaucoma surgeries that do this include laser trabeculoplasty, laser iridotomy, trabeculectomy (glaucoma filtration surgery), drainage tubes and minimally invasive glaucoma surgery (MIGS).
Figure 1. Eye anatomy
Figure 2. Retinal layers showing retinal ganglion cell
Footnotes: Schematic representation of the retina and the retinal cell layers. (A) Blood supply and (B) structure of the retina. The retina is a layered structure lining the back of the eye consisting of a pigmented layer called retinal pigment epithelium (RPE), and a multilayered neuroretina. The retinal pigment epithelium (RPE) is in close contact with the outer segments of the photosensitive rod and cone cells of the neuroretina. The connecting cilium connects the photoreceptor outer segments with the cell bodies, which constitute a layer known as the outer nuclear layer (ONL). The axons of the photoreceptors synapse with the neuronal (bipolar, amacrine, and horizontal) cells of the inner nuclear layer (INL) via the outer plexiform layer (OPL). The axons of the inner nuclear layer (INL) cells in turn synapse with the ganglion cell layer (GCL) via the inner plexiform layer (IPL). The axons of the ganglion cells converge to form the optic nerve. Approximately 1.2 million nerve fibers, or axons, make up each human optic nerve. A retinal ganglion cell (RGC) is a type of neuron located near the inner surface (ganglion cell layer [GCL]) of the retina of the eye. A retinal ganglion cell (RGC) receives visual information from photoreceptors via two intermediate neuron types: bipolar cells and retina amacrine cells. Retina amacrine cells, particularly narrow field cells, are important for creating functional subunits within the ganglion cell layer and making it so that ganglion cells can observe a small dot moving a small distance. Retinal ganglion cells collectively transmit image-forming and non-image forming visual information from the retina in the form of action potential to several regions in the thalamus, hypothalamus, and mesencephalon, or midbrain. Visual images from the retina travel through the optic nerve, optic tract, and eventually to the visual part of the brain (the occipital lobe). There the images are processed and interpreted by the brain. Any disease process which affects the optic nerve could disrupt this input, leading to visual loss.
Abbreviations: ILM: internal limiting membrane, NFL: nerve fiber layer, GCL: ganglion cell layer, IPL: inner plexiform layer, INL: inner nuclear layer, OPL: outer plexiform layer, ONL: outer nuclear layer, ELM: external limiting membrane, OS: photoreceptor outer segment, RPE: retinal pigment epithelium
[Source 25 ]Figure 3. Open-angle glaucoma
What is Glaucoma Suspect?
Eye specialist (ophthalmologist) will refer to someone as a “glaucoma suspect” if they think the person might be showing early signs of glaucoma such as higher than normal eye pressure called ocular hypertension (elevated intraocular pressure [IOP] greater than 21 mmHg) but have no signs of optic nerve damage. Glaucoma suspects have no symptoms to suggest eye disease. They are usually identified as glaucoma suspects during routine checks by their optometrist. Many people suspected of having glaucoma at this stage turn out not to have it at all, but some do develop it in time and it is these people who can benefit the most from timely treatment. Their ophthalmologist (eye specialist) may notice something different about their optic nerve. Most “glaucoma suspects” have no symptoms. That is why you need to be carefully monitored by your ophthalmologist if you are a glaucoma suspect. An ophthalmologist can check for any changes over time and begin treatment if needed.
If someone has a very high intraocular pressure (high IOP) or very advanced optic nerve damage then the diagnosis of glaucoma is usually straightforward. However sometimes it is not entirely clear whether someone has glaucoma or not. The early signs of glaucoma can be subtle, and many glaucoma patients have a normal pressure.
There is no single test that is 100% effective in confirming the diagnosis of glaucoma all the time. Sometimes the only way to be sure that someone has glaucoma is to arrange follow up eye examinations every 4-6 months or so to work out whether progressive damage is occurring to the optic nerve in one or both eyes. Features in the examination which might lead to a patient being classified as a ‘glaucoma suspect’ include:
- A high pressure within the eyeball (high IOP) but with no optic nerve damage yet this is also referred to as ocular hypertension.
- A ‘suspicious’ optic disc appearance on examination such as ‘cupping’ of the disc or thinning of the neuro-retinal rim or nerve fiber layers.
- Unusual or defective visual fields.
These are changes that can be seen with glaucoma, but can also be seen in other conditions such as farsightedness (myopia) where it may be a variation of normal.
Other risk factors for glaucoma such as a strong family history of glaucoma but without definite changes to the optic nerve as yet. Generally speaking, “glaucoma suspects” will not show any visual field defects on testing, or may show some field defects which are not yet entirely convincing as evidence of glaucoma. If you are a ‘glaucoma suspect’, the most important treatment is good follow-up care.
It is very important that someone suspected of experiencing the early onset of glaucoma has regular eye checks to make sure there is no continuing damage to the optic nerve. Even though a person is not yet receiving any treatment for glaucoma, she or he may still risk losing their vision if in fact they do turn out to have glaucoma. Thus it is very important to maintain follow-up care. Typically for a low-risk glaucoma suspect, this may require visits every 6 to 12 months. At each follow-up visit your eye doctor will check your vision and eye pressure, and examine the front and back of your eye, paying careful attention to the appearance of your optic nerves.
To examine the structure of the optic nerve, your doctor will perform a careful examination in the office, obtain optic nerve imaging, and obtain a baseline set of optic nerve photographs. To examine the function of the optic nerve, an automated visual field test we be implemented with the help of a technician, who will instruct you on the correct way to perform the test. All of these tests may be repeated at yearly intervals (or more or less frequently, as determined by your eye doctor) to assess if there are changes or “progression” over time. The follow-up visits are crucial to maintaining optimal eye health.
Sometimes eye doctors are on the fence about whether to start treatment, and it is only through repeat follow-up visits that they get a sense of whether or not someone has glaucoma. Usually a person thought to be a “glaucoma suspect” will not be treated for the condition until the diagnosis is confirmed. Typically, glaucoma advances slowly so its progress can be tracked safely without treatment until the diagnosis is confirmed.
If you’re a “glaucoma suspect” and needed treatment, initial treatment options may include topical eye drops or laser treatment of the drainage angle to increase the amount of fluid draining from the eye, both of which can lower the eye pressure. The decision to treat is often not a cut-and-dry one; your ophthalmologist will assess all of your risk factors, your examination findings, and seek your input as to whether to treat or continue to observe your eyes over time. Some patients prefer to “watch and wait” or are worried about the side effects of treatment, while others may be more risk-averse and would rather begin treatment and have peace of mind. There are some glaucoma risk calculators available but most eye doctors would agree that these may aid in diagnosis and assessment, but will not replace your doctor’s clinical judgment.
Aqueous Humor Production and Physiology
The aqueous humor is a water-like fluid that is produced by the ciliary body that sits directly behind the iris (the colored part of your eye). Aqueous humor is produced at a rate of 2-3 microliters per minute (2-3 μL/minute) 26, 27. The aqueous humor is composed of organic and inorganic ions, carbon dioxide, amino acids, carbohydrates, glutathione, and water 26, 28. The aqueous humor fills the anterior chamber of your eye with continual production, secretion, and reabsorption 26. The cornea and the lens of your eye have no blood supply. They receive nourishment from nutrients in the aqueous fluid that fills your eye. The aqueous fluid flows between the iris and lens through the pupil and to the drainage angle at the junction of the iris and the cornea. Aqueous fluid exits the eye through a tissue called the trabecular meshwork in the drainage angle. As the aqueous fluid passes through the eye, it supplies the lens and cornea with nutrients and carries away waste products. The production, circulation and reabsorption of aqueous humor are vital processes maintaining homeostasis of the eye. The pressure of the fluid in your eye called the intraocular pressure (IOP) is determined by the amount of aqueous humor fluid entering the eye through the ciliary body and exiting the eye through the trabecular meshwork. In most people, the balance between the aqueous humor fluid coming in and going out of the eye results in an eye pressure between 10 and 21 mm of Hg. In patients with glaucoma, aqueous humor fluid drains from the eye through the trabecular meshwork at a slower rate causing the pressure in your eye to rise or increased intraocular pressure (ocular hypertension) resulting in optic nerve damage and glaucoma.
Aqueous humor functions as a physical component allowing clear optics and filling the anterior chamber of the eye 26, 27. The aqueous humor is responsible for providing nourishment to the avascular components of the anterior chamber including the cornea and lens 26, 27. In addition, aqueous humor is responsible for removing waste products, blood, macrophages and other debris from the anterior chamber, including the trabecular meshwork 26, 27. The structure and function of the trabecular meshwork may become compromised by chronic oxidative stress from reactive oxygen species and insufficient antioxidant defense in the aqueous humor 26, 27, 29, 30. Decreased levels of antioxidants in aqueous humor are present in glaucomatous eyes versus normal eyes, consistent with the presence of increased oxidative stress and low-grade inflammation 29, 30.
The primary anatomic structures vital to the homeostasis of aqueous humor include the ciliary body as the site of principle production, and the trabecular meshwork and uveoscleral pathway as the sites of primary outflow 26, 31. Aqueous humor is produced by the ciliary body via a multistep process closely correlating with systemic vascular blood flow 26, 32, 33. Initially, blood enters the ciliary processes, which propels ultrafiltrate from the blood into the ciliary interstitial space via a pressure gradient 26, 32, 33. Next, the ciliary epithelium transports plasma components from the basal to the apical surface in order to synthesize aqueous humor and transport it into the posterior chamber 26, 32, 33. Passive diffusion and ultrafiltration are key in initial synthesis, and active secretion across a blood-aqueous barrier via aquaporins, Na-K-ATPase and carbonic anhydrase enzymes are necessary for final synthesis 26, 32, 33, 34. These active transport enzymes necessary for final synthesis are common pharmacologic targets in decreasing aqueous humor production. Although systemic blood flow via the ciliary artery is required for the initial production of ultrafiltrate, the production of aqueous humor is independent from systemic blood pressure due to a fixed rate of 4% filtration of plasma 33. Therefore, there is minimal association between systemic high blood pressure (hypertension) and elevated intraocular pressure (IOP). The estimated rate of aqueous humor production is approximately 2.4 microliters per minute (2.4 μL/minute), with diurnal variations leading to higher aqueous humor flow in the morning and lower flow in the evening 26, 32.
While aqueous humor production is well documented, the mechanism of drainage is still poorly understood.
There are 2 main drainage pathways for aqueous humor 26, 35, 32:
- The conventional pathway via trabecular meshwork, Schlemm’s canal, collector channels, and the episcleral venous system), and
- The unconventional pathway via uveoscleral, uveovortex, uveolymphatic.
The conventional pathway drainage pathways for aqueous humor involves passive drainage throughout the trabecular meshwork although the Schlemm’s canal has been documented with paracellular and intracellular pores 26, 35, 32. The trabecular meshwork is a triangular porous structure composed of a layer of connective tissue and endothelium with sympathetic innervation from superior sympathetic ganglion, and parasympathetic innervation from the ciliary ganglion 26, 35, 32. The trabecular meshwork may be divided into the uveal meshwork (iris root, ciliary body, peripheral cornea), corneoscleral meshwork (scleral spur), and juxtacanalicular meshwork (transition into Schlemm’s canal) 26, 35, 32. Schlemm’s canal is a structure with composition similar to venous vasculature, with fenestrated thin endothelium surrounded by connective tissue 26, 35, 32. After drainage through the trabecular meshwork and the Schlemm’s canal, aqueous humor continues through collector channels into the episcleral venous system which deposits into the main venous system 26, 35, 32.
Resistance to outflow through the trabecular meshwork and Schlemm’s canal has been documented although it is poorly understood, yet resistance remains an important factor in regulating intraocular pressure and the pathogenesis of glaucomatous processes. In humans, up to 75% of aqueous outflow resistance is contributed by the trabecular meshwork while the remaining 25% is due to resistance beyond Schlemm’s canal 26. The rate of outflow is directly influenced by iris and ciliary muscles which contract and relax based on cholinergic innervation and pharmacodynamics 26, 35, 32, 31, 36. In ciliary contraction, the trabecular meshwork and Schlemm’s canal dilate, decreasing resistance and increasing outflow 26, 35, 32, 31, 36. The rate of outflow is also influenced by intraocular pressure, with higher intraocular pressure altering the structure of endothelial lining in Schlemm’s canal to increase the number of porous vacuoles allowing increased outflow 26, 35, 32, 31, 36. However, it is still debated if this finding substantially contributes to increasing outflow in glaucomatous eyes 26, 35, 32, 31, 36.
The unconventional pathway involves drainage into the orbital vasculature, vortex veins and ciliary lymphatics, contributing up to 25-40% of total aqueous outflow in cynomolgus and vervet monkey models. The uveoscleral pathway involves diffusion into the sclera and episcleral through the orbital vasculature. The uveovortex pathway involves osmotic absorption of fluid through the choroid, passing into the vortex veins 35. Lastly, the uveolymphatic pathway involves drainage into lymphatic vessels within the ciliary body, although the extent of drainage under normal physiological conditions remains controversial 35. In addition, the unconventional pathway also includes corneal, iridial and retinal routes, albeit less clinically significant 37. Regardless of downflow pathway, all unconventional paths require drainage through the interstitial spaces of the ciliary muscle 35, 37. Resistance also exists within the unconventional pathway likely due to ciliary muscle tone, as seen with changes in outflow in the setting of pilocarpine, increasing ciliary tone and decreasing flow, and atropine, decreasing ciliary tone and increasing flow 35, 37. Therefore, the unconventional pathways are also clinically important in moderating intraocular pressure, and serve as a potential target in glaucoma therapy.
Figure 4. Normal aqueous outflow
Footnotes: The ciliary body is a structure that sits directly behind the iris (the colored part of your eye). One of ciliary body’s jobs is to create an important fluid called aqueous humor, a fluid that nourishes the cornea and lens. Aqueous humor flows through a specific route into the front of the eye (the anterior chamber). This route allows aqueous humor to send important nutrients and oxygen to other parts of the eye, such as the lens and cornea. The aqueous humor is produced behind the iris, flows into the anterior chamber through the pupil, and exits the eye between the iris and cornea via the trabecular meshwork, a specialized eye tissue located at the chamber angle of the eye next to the cornea 38. In a healthy eye, this is a constant process. The ciliary body is always producing aqueous humor, and 80%-90% aqueous humor is always draining through the trabecular meshwork. The trabecular meshwork is a specialized spongy tissue in the anterior chamber of the eye that regulates the outflow of aqueous humor 38. The trabecular meshwork acts as a filter, controlling how quickly aqueous humor drains out of the eye through a structure called Schlemm’s canal, ultimately maintaining intraocular pressure (IOP). The canal of Schlemm, also known as Schlemm’s canal or the scleral venous sinus, is a circular, lymphatic-like vessel in the eye that drains aqueous humor from the anterior chamber into the episcleral blood vessels. The canal of Schlemm and the trabecular meshwork (TM) play a crucial role in maintaining intraocular pressure (IOP) by facilitating the outflow of aqueous humor. Too much aqueous humor production or obstruction of its outflow causes a rise in intraocular pressure (IOP) that can lead to glaucoma.
[Source 39 ]Figure 5. Drance hemorrhage (optic disc hemorrhage)
Footnotes: Optic disc hemorrhage or Drance hemorrhage indicating inadequate intraocular pressure control in a patient with normal tension glaucoma. Disc hemorrhages are more common in normal tension glaucoma than in primary open angle glaucoma (POAG). This patient also has peripapillary atrophy, visible as a pale ring around the optic nerve.
[Source 40 ]Open-angle glaucoma causes
Scientists aren’t sure what causes glaucoma, but the most common types usually happen when the fluid pressure inside your eye (IOP) slowly rises, damaging the optic nerve. As the optic nerve gradually gets worse, blind spots develop in your vision. Primary open-angle glaucoma (POAG) is the most common type of open-angle glaucoma that is characterized by raised intraocular pressure (IOP) caused by increased resistance to drainage in the trabecular meshwork, despite the drainage angle between the cornea and iris remains open 3. Due to this blockage in the trabecular meshwork, the pressure in the eye gradually increases, resulting in optic nerve damage and progressive visual loss. Many different abnormalities have been noted on histopathological examination of the drainage angle in patients with primary open-angle glaucoma (POAG). These include narrowed intertrabecular spaces, thickened basement membranes, fused trabecular beams, reduction in trabecular endothelial cells, reduction in actin filaments, narrowing of collector channels, foreign material accumulation, scleral spur thickening, and closure of Schlemm’s canal 2 .
Secondary open-angle glaucoma can have multiple causes but is far less common than primary open-angle glaucoma (POAG). Secondary glaucoma is when another condition or problem within the eye increases eye pressure such as eye injury, eye surgery or eye procedures, certain medications especially corticosteroids and cycloplegics or other eye diseases (e.g., pigmentary dispersion syndrome, pseudo-exfoliation syndrome, uveitis) causing the glaucoma.
Genetics
- Myocilin (MYOC) gene mutation on chromosome 1q24.3: The MYOC (myocilin) gene provides instructions for producing a protein called myocilin 41. Myocilin is found in certain structures of the eye, called the trabecular meshwork and the ciliary body, that regulate the pressure within the eye (intraocular pressure) 41. Myocilin is a cytoskeletal protein expressed in the trabecular meshwork and is also known as trabecular meshwork glucocorticoid-inducible response protein 42. Myocilin’s function is not well understood, but it may help to control the intraocular pressure through its action in the muscle tissue of the ciliary body. Researchers believe that myocilin functions together with other proteins in the eye as part of the extracellular matrix, which is an intricate lattice that forms in the space between cells and provides structural support 41. Myocilin may interact with a number of other proteins that also function in the extracellular matrix. The MYOC gene is implicated in cases of hereditary juvenile open-angle glaucoma and adult open-angle glaucoma 42.
- WD repeat domain 36 (WDR36) gene mutation on chromosome 5q22.1: The WDR36 (WD repeat domain 36) gene is a gene in humans that encodes a protein containing WD repeat domains that is involved in ribosomal ribonucleic acid (RNA) processing, p53 stress-pathway response, cell cycle progression, signal transduction, apoptosis, and gene regulation 43, 44, 45, 46, 47. WDR36 gene been identified as a causative gene for primary open-angle glaucoma (POAG) 48, 47, 49, 50, 51, 52. WDR36 protein is expressed in the lens, iris, sclera, ciliary muscles, ciliary body, trabecular meshwork, retina, and optic nerve in the eye 1. WDR36 protein is also expressed outside the eye in the human heart, placenta, liver, skeletal muscle, kidney, and pancreas. Four mutations in WDR36 at the GLC1G locus (N355S, A449T, R529Q, and D658G) have been identified, with a study implicating the gene in approximately 6% of patients with primary open-angle glaucoma (POAG) 1. However, results from a recent 2017 Chinese study repudiated this claim by showing that the association between WDR36 and primary open-angle glaucoma (POAG) is inconsistent across populations and calls for more data supporting the WDR36 protein’s role in primary open-angle glaucoma (POAG) 53, 54.
- CAV1/CAV2 genes mutation on chromosome 7q31:
- The CAV1 (caveolin-1) gene provides instructions for making a protein called caveolin-1. CAV1 (caveolin-1) protein appears to have diverse functions in cells and tissues throughout the body 55. Caveolin-1 (CAV1) is the major component of caveolae, which are small pouches in the membrane that surrounds cells. Caveolae have multiple functions, some of which are not well understood. They are known to be involved in the transport of molecules from the cell membrane to the interior of the cell (endocytosis), processing of molecules on their way into the cell, maintaining the cell structure, and regulating chemical signaling pathways. Studies suggest that caveolae are particularly numerous in adipocytes, which are cells that store fats for energy. Adipocytes make up most of the body’s fatty (adipose) tissue. In these cells, caveolae appear to be essential for the normal transport, processing, and storage of fats. Caveolin-1 (CAV1) is also found in many other parts of cells, where it regulates various chemical signaling pathways. Through these pathways, caveolin-1 is involved in regulating cell growth and division (proliferation), the process by which cells mature to perform specific functions (differentiation), cell survival and the self-destruction of cells (apoptosis), and cell movement. The functions of caveolin-1 likely differ depending on the type of cell and the part of the cell where the protein is found.
- The CAV2 (caveolin-2) gene encodes a protein that is a major component of caveolae, which are invaginations of the plasma membrane. It plays a role in various cellular processes like signal transduction, lipid metabolism, and cell growth. Caveolin-2 (CAV2) is co-expressed with CAV1 (caveolin-1), forming hetero-oligomers within caveolae. It also interacts with G-protein alpha subunits and can regulate their activity, as well as acting as a scaffolding protein.
- Caveolin-1 (CAV1) and Caveolin-2 (CAV2) are expressed in most human cell types, including tissues such as the scleral spur cells, trabecular meshwork, and retinal ganglion cells. In vitro studies of CAV1 showed consistent upregulation in the trabecular meshwork after one hour of increased intraocular pressure (IOP) 56, 57.
- CAV1/CAV2 genes are associated with primary open-angle glaucoma (POAG) susceptibility in populations of European and East Asian ancestry 1.
- Cyclin dependent kinase inhibitor 2B antisense noncoding RNA (CDKN2B-AS1) gene mutation on chromosome 9p21: CDKN2B-AS1 is a cyclin-dependent kinase inhibitor 2B antisense long non-coding ribonucleic acid (lncRNA) that regulates cyclin-dependent kinase inhibitor 2A and 2B in the cell cycle 58. A United States-based observational case study found that this region modifies optic nerve vulnerability to glaucomatous change. Single nucleotide polymorphism (SNP) in CDKN2B-AS1 gene is thought to be implicated in primary open-angle glaucoma (POAG) by causing retinal ganglion cells (RGCs) to undergo apoptosis during their quiescent post-mitotic state 59, 60.
- Optineurin (OPTN) gene on chromosome 10p13: Optineurin (OPTN) is the coiled-coil protein product implicated in adult-onset primary open-angle glaucoma (POAG) and normal-tension glaucoma (NTG) 1, 17, 61. Optineurin (OPTN) is involved in various cellular functions, including apoptosis, cellular morphogenesis, inflammation, vasoconstriction, membrane protein trafficking, vesicular trafficking, and transcription activation 62.
- Lysyl oxidase-like 1 (LOXL1) gene on chromosome 15q24.1: Lysyl oxidase-like 1 (LOXL1) gene codes for an extracellular copper-dependent amine oxidase enzyme that catalyzes the first step in crosslinking collagen and elastin in the extracellular matrix and is implicated in cases of pseudoexfoliation syndrome. Single nucleotide polymorphism (SNP) in the LOXL1 gene is associated with excessive levels of crosslinked amyloid-like fibrillar glycoproteins that deposit in the anterior segment and are more common in Scandinavian populations 63, 64. Single nucleotide polymorphism (SNP) in the LOXL1 gene can present as exfoliation glaucoma as the first signs of a more systemic severe condition that implicates multiple tissues with the expression of copper-dependent amine oxidase enzyme, including the liver, kidney, and gallbladder 65, 66.
Several study results based on whole-exome sequencing using gene-based and single-variant analyses have revealed more than 40 new previously unreported genes associated with glaucoma phenotypes 67. Understanding open-angle glaucoma genetic and molecular mechanisms is crucial to developing new drug targets 68, 69.
Normal tension glaucoma causes
The cause and mechanism for the development of normal tension glaucoma is unknown and remains an area of active research and debate. Several theories have been proposed to explain the onset and progression of normal tension glaucoma (NTG). Whereas intraocular pressure (IOP) is the main driver of progressive visual loss in most patients with primary open angle glaucoma (POAG), normal tension glaucoma (NTG) likely represents a diverse and multifactorial group of causes with a common final pathway of retinal ganglion cell loss 17. Despite intraocular pressure (IOP) in the normal range of 10 to 21 mmHg, there is evidence that an intraocular pressure (IOP)-dependent mechanism plays a role in the cause in many eyes with normal tension glaucoma 70, 71. Proposed intraocular pressure (IOP)-independent mechanisms include vascular insufficiency (lower blood pressure or reduced ocular blood flow) at the optic nerve head, impaired cerebrospinal fluid (CSF) circulation resulting in low retrobulbar cerebrospinal fluid pressure causing stagnation and decreased optic nerve protection, failure of the glymphatic system in the optic nerve, metabolic and neurodegenerative disorders, oxidative stress, and structural anomalies including structural weakness of the lamina cribrosa 72, 73, 74, 61, 75, 76. All of these mechanisms need further research to better define the pathophysiology of the disease process 23.
It has been theorized that the disease process in normal tension glaucoma results from an enhanced sensitivity to what would otherwise be physiologic intraocular pressure (IOP), resulting in glaucomatous damage of the optic nerve. This enhanced sensitivity may be due to impaired optic nerve blood flow, a higher translaminar pressure gradient (intraocular pressure [IOP] minus intracranial pressure [ICP]) due to lower intracranial pressure (ICP), or a structurally abnormal lamina cribrosa, which cannot withstand a normal range of intraocular pressure (IOP) 23. This theory of enhanced sensitivity is useful, at least conceptually, to rationalize the impact of intraocular pressure (IOP) in a disease process that may have an intraocular pressure (IOP) independent underlying etiology 23. The evidence for a role of IOP contributing to normal tension glaucoma comes from the Collaborative Normal Tension Glaucoma Study, which showed a slowing of disease progression in patients achieving a 30% or more reduction of already normal intraocular pressure (IOP) 70, 77. While some try to delineate normal tension glaucoma and primary open angle glaucoma (POAG) as two completely unique disease processes, it has also been suggested that the diseases exist on a continuum with IOP playing a larger role in primary open angle glaucoma (POAG), and vascular or mechanical factors at the etiologic root in normal tension glaucoma 23.
Specific histological studies of eyes with normal tension glaucoma are scarse but in general mimic those changes seen in primary open angle glaucoma (POAG) 23. Histopathologic changes of the optic nerve head include disarrangement and posterior bowing of the lamina cribrosa along with loss of nerve fibers 78. Non-invasive imaging by OCT and scanning laser modalities have characterized thinning of the peripapillary choroid 79, as well as thinning of the ganglion cell layers in normal tension glaucoma patients compared to other primary open angle glaucoma (POAG) and normal patients 80. In Asian patients this thinning has been correlated with vascular narrowing in asymmetric normal tension glaucoma when compared to normal fellow eyes and primary open angle glaucoma (POAG) patients with elevated pressures 81, 82, 83.
Genetics is also known to play a role in normal tension glaucoma, because of the strong association with family history with 21% of patients reporting a family history of glaucoma and variation in prevalence in different ethnicities that persists after migration 17. Four major genes have been implicated in normal tension glaucoma: optineurin (OPTN), TANK binding kinase 1 (TBK1), methyltransferase-like 23 (METTL23), and myocilin (MYOC) 61. Optineurin (OPTN) gene mutations, particularly the E50K variant, have been strongly associated with normal tension glaucoma, causing early-onset disease, large cup-to-disc ratios, and retinal ganglion cell death 23. TANK binding kinase 1 (TBK1) copy number variations have also been linked to normal tension glaucoma, with duplications and triplications contributing to retinal ganglion cell (RGC) loss 23. Methyltransferase-like 23 (METTL23) gene mutations were recently identified in familial normal tension glaucoma cases, with evidence suggesting that these mutations impact histone arginine methylation, potentially leading to retinal ganglion cell (RGC) degeneration 23. Myocilin (MYOC) gene commonly associated with primary open angle glaucoma (POAG) has been implicated in some normal tension glaucoma cases, though its role remains less clear 23. Further genetic research is certainly needed to better understand the role of these genes in normal tension glaucoma 61.
Genes associated with normal tension glaucoma 17, 61:
- Optineurin (OPTN)
- TANK binding kinase (TBK1)
- Methyltransferase-like 23 (METTL23)
- Myocilin (MYOC)
Normal tension glaucoma is typically not considered to be a heritable disease, as approximately 2% of normal tension glaucoma cases are caused primarily by a mutation of a single gene and found to be transmitted by an autosomal dominant inheritance pattern 23. Nevertheless, individuals who carry one of the many autosomal dominant gene mutations may present with symptoms of normal tension glaucoma as early as 23 years old 84. Genetic and pedigree studies continue to further identify numerous new genes associated with the development of normal tension glaucoma, but further studies that demonstrate a higher incidence of disease are necessary before a clinical indication for genetic screening and counseling can be recommended 23.
Though the quantity of axons that compose optic nerves in humans remains a predictable constant between individuals, variability in surface area of optic discs is observed. It is unclear if certain optic nerve head parameters place an eye at increased risk of normal tension glaucoma 23. Optic nerves with a larger surface area and with thinner inferior/inferotemporal rims have been reported to be at an increased risk for developing normal tension glaucoma 85, 86. Other studies evaluating the optic nerve head by scanning laser ophthalmoscopy found no morphologic differences between high-tension and normal tension glaucoma patients 87.
Frequently an area of peripapillary atrophy in a crescent or halo configuration is observed in patients with normal tension glaucoma. While this pattern of atrophy can be a finding in eyes without normal tension glaucoma, in glaucomatous eyes, peripapillary atrophy often occurs adjacent to areas of greatest disc thinning and corresponding visual field loss 88. While thinning of the optic nerve rim is observed in all primary open angle glaucoma (POAG), focal thinning or ‘notching’ is more commonly observed in normal tension glaucoma 89.
Risk factors for developing open-angle glaucoma
Risk factors for developing open-angle glaucoma include 1:
- Older age (African American, 40+ years; Caucasians, 65+ years) 90
- Race (African-American, Afro-Caribbean, and West African patients have a 4-fold increased risk of developing open-angle glaucoma) 90
- Family history of glaucoma (eg, the Rotterdam Eye study found a 9.2 times higher risk of developing open-angle glaucoma if first-degree relatives had glaucoma) 91
- Elevated intraocular pressure (IOP) 92
- Myopia or nearsightedness (eg, results from studies have reported an increased risk of glaucoma of up to 20% for each diopter increase in myopia) 93, 10
- Increased cup-to-disc ratio 94
- Optic disc hemorrhage also called Drance hemorrhage 95
- Thin central corneal thickness 96, 10
- Low ocular perfusion pressure 97
- Low blood pressure (systolic and diastolic blood pressure) also known as hypotension 98
- High blood pressure also known as hypertension (systemic arterial hypertension has been associated with but is not a confirmed risk factor for open-angle glaucoma) 99
- Type 2 diabetes 100
- Hypothyroidism (underactive thyroid)
- Obstructive sleep apnea (OSA)
- Steroid use
- High pattern standard deviation on visual fields 101
- Migraine or vasospasm (a condition of sudden constriction of a blood vessel, reducing its diameter and flow rate) 102
- Low intracranial pressure or low cerebral spinal fluid (CSF) pressure 103
- Oral contraceptive pill or hormonal birth control pill 104
- Lifestyle risk factors include smoking, obesity, alcohol, anxiety, stress, and sleep apnea 105.
Intraocular pressure (IOP)
The most important risk factor for developing open-angle glaucoma is an elevated intraocular pressure (IOP) (i.e., ocular hypertension), usually defined as intraocular pressure (IOP) above 21 mm Hg 92. In patients with ocular hypertension (raised IOP but no signs of glaucomatous optic disc or visual field changes), higher intraocular pressure (IOP) is associated with a higher risk of developing open-angle glaucoma 92. Raised intraocular pressure (IOP) in animal models results in glaucomatous optic neuropathy. Population studies have shown increased prevalence of glaucoma with increasing intraocular pressure (IOP). Approximately 10% of patients with an elevated intraocular pressure (IOP) develop glaucoma after five years, and 30% at 20 years 90. Although the risk of glaucoma progression is directly related to the degree of elevation of intraocular pressure (IOP), up to 40% of patients with glaucoma have normal intraocular pressure (IOP) at initial diagnosis, and about 5% of people in the United States have ocular hypertension without glaucoma 10, 12, 106. Intraocular pressure (IOP) is also thought to be a risk factor for normal tension glaucoma, despite intraocular pressure (IOP) never being higher than the normal range.
Age
The prevalence of open-angle glaucoma increases with age, even after compensating for the association between age and intraocular pressure (IOP). Age was also found to be an important risk factor for the conversion of ocular hypertension to open-angle glaucoma in both Ocular Hypertension Treatment Study (OHTS) and the European Glaucoma Prevention Study (EGPS).
Race
Several studies have shown primary open-angle glaucoma (POAG) to be more prevalent in people of African-Caribbean descent compared with Caucasians 2. Not only is open-angle glaucoma more prevalent in black race, its onset is earlier, and disease progression has been shown to be faster and more refractory to treatment. Black patients with ocular hypertension have been found to be more likely to progress to open-angle glaucoma. The prevalence of primary open-angle glaucoma (POAG) in Hispanics is thought to be between that of African-Caribbean and Caucasian populations.
Refractive error
Myopia has been shown to be a risk factor for primary open-angle glaucoma (POAG) in several studies. However, it can be difficult to diagnose true open-angle glaucoma in myopic patients and controversy exists over whether it is real risk factor. Myopic optic discs are notoriously difficult to assess, and myopic patients may have visual field defects unrelated to any glaucomatous process.
Central corneal thickness
A thinner cornea has been shown to be a risk factor for ocular hypertension patients developing primary open-angle glaucoma (POAG). This may be in part due to IOP measurement error (IOP tends to be read lower in patients with thinner corneas), but there are also theories that a thinner cornea may indicate less rigid support structures around the optic nerve head, and a resultant increased propensity to damage 2.
Family history of glaucoma
A first-degree relative (i.e., parents, brother and sister and children) with primary open-angle glaucoma (POAG) is a risk factor for the development of open-angle glaucoma. This has been reported in several studies with the odds ratio varying from 3 to 13 2. The risk is thought to be higher still if the affected relative is a sibling (brother or sister) 2. Several genes associated with open-angle glaucoma have been identified, though these account for less than 5% of all open-angle glaucoma in the general population 2. It is therefore thought that the hereditary aspect of open-angle glaucoma is likely to be polygenic and that gene-environment interactions are important 2.
Other risk factors
A high prevalence of open-angle glaucoma has been found in diabetic patients, and a high prevalence of diabetes has been found in open-angle glaucoma patients. People with diabetes are 2 times more likely to get glaucoma than people without diabetes 90. However, controversy exists as to whether diabetes truly is a risk factor for open-angle glaucoma, as several large population studies have found no association 2 . The role of blood pressure in the development of open-angle glaucoma is complicated and there is no consensus 2. High blood pressure (hypertension) may predispose to glaucomatous damage via increased peripheral vascular resistance in small vessels, while a low blood pressure may reduce the perfusion pressure of the optic disc 2. There is a relative scarcity of data regarding the lifestyle and nutritional epidemiology of open-angle glaucoma 2. There is a suggestion that exercise and a diet high in green collards and with a high omega 6 to omega 3 fatty acid ratio are protective against open-angle glaucoma 2.
Open-angle glaucoma pathophysiology
A commonly held view is that glaucoma is a group of conditions with varying pathophysiological processes that share a common end-point of optic nerve head damage. There are multiple proposed mechanisms of damage, some of which are intraocular pressure (IOP)- dependent, and others are intraocular pressure (IOP)- independent.
Raised intraocular pressure (IOP) is thought to damage the optic nerve head via induced mechanical changes at the lamina cribrosa, or via vascular dysfunction and resultant ischemia. There are several postulated mechanisms as to the cause of elevated intraocular pressure (IOP), the majority of which are related to reduced aqueous outflow. Structural changes include:
- Trabecular meshwork obstruction by foreign material (e.g. glycosaminoglycans, pigment, red blood cells)
- Trabecular endothelial cell loss (resulting in trabecular beams fusing)
- Loss of trabecular endothelial cell phagocytic activity
- Loss of giant vacuoles from Schlemm’s canal endothelium
- Reduced pore size and density in the wall of Schlemm’s canal
It has been suggested that these changes may be brought on by altered endogenous corticosteroid metabolism with secondary trabecular meshwork changes or oxidative damage.
Intraocular pressure (IOP) independent mechanisms of damage
These include:
- Reduced ocular perfusion pressure (and hence the association with vascular diseases such as diabetes, hypertension and migraine)
- Excitotoxic damage from excessive glutamate
- Autoimmune-mediated nerve damage
- Loss of neurotrophic factors
- Failure of cellular repair mechanisms
- Abnormal autoregulation of retinal and choroidal vasculature
Open-angle glaucoma prevention
Glaucoma isn’t preventable, but early detection and treatment can manage pressure inside your eye and help prevent or delay vision loss. There are a few main ways that can happen:
- Regular eye exams. Pressure increases in your eyes are often detectable before they can cause glaucoma and its symptoms. Early detection lets you and your eye specialist try to prevent glaucoma from worsening or, at least, slow down its progress. It is recommended that people over 40 years of age should receive a baseline eye examination. The patient’s underlying characteristics and risk factors determine the frequency of follow-up visits.
- Managing ocular hypertension. If you have higher-than-normal pressure in your eyes, your eye care specialist can offer treatment options. Following their guidance can be crucial and help you avoid — or at least delay — vision loss.
- Knowing and managing your risk factors. Some of the conditions that you can try to manage or prevent include high blood pressure and diabetes. Using protective items like safety glasses and goggles can help prevent eye injuries.
- Eat a healthy diet. Eating a healthy diet can help you maintain your health, but it won’t prevent glaucoma from worsening. Several vitamins and nutrients are important to eye health, including zinc, copper, selenium, and antioxidant vitamins C, E and A.
- Exercise regularly. Regular exercise may reduce eye pressure.
- Limit your caffeine. Drinking beverages with large amounts of caffeine may increase your eye pressure.
- Take prescribed medicine. Using your eye drops or other medicines as prescribed can help you get the best possible result from your treatment. Be sure to use the eye drops exactly as prescribed. Otherwise, your optic nerve damage could get worse.
Open-angle glaucoma signs and symptoms
Most patients with primary open angle glaucoma (POAG) have no symptoms of the condition. There is no pain and vision seems normal. In its early stages, glaucoma may not cause any symptoms or have no warning signs. Most people with open-angle glaucoma do not notice any change in their vision until the damage is quite severe. This is why glaucoma is called the “silent thief of sight” and up to half of the people in the United States with glaucoma may not know they have it. And symptoms may not appear until glaucoma causes irreversible eyesight damage. As the disease progresses, blind spots develop in your peripheral (side) vision. Side vision also is called peripheral vision. In later stages, difficulty seeing things in your central vision.
It’s important to have regular eye exams that include measurements of your eye pressure. If glaucoma is found early, vision loss can be slowed or prevented. If you have glaucoma, you’ll need treatment or monitoring for the rest of your life.
Some of the more common glaucoma symptoms include:
- Eye pain or pressure
- Headaches
- Red or bloodshot eyes
- Double vision (diplopia)
- Blurred vision
- Gradually developing low vision
- Gradually developing blind spots (scotomas) or visual field defects like tunnel vision.
Open-angle glaucoma signs
The International Society for Geographical and Epidemiological Ophthalmology has published a consensus definition for primary open-angle glaucoma (POAG). This definition does not include intraocular pressure (IOP) – i.e. primary open-angle glaucoma (POAG) is diagnosed based on signs of glaucomatous optic neuropathy regardless of the level of intraocular pressure (IOP). Patients can be classified as normal tension glaucoma (NTG) or high tension glaucoma (HTG) based on the intraocular pressure (IOP). Sometimes an intraocular pressure (IOP) spike may be missed in a clinical setting. In these cases, if high tension glaucoma (HTG) is suspected, measurement of intraocular pressure (IOP) at hourly intervals throughout the day, beginning in the early morning, may be indicated. This is termed phasing. If a patient has all the features of primary open-angle glaucoma but consistently normal intraocular pressure (IOP) (less than or equal to 21 mmHg), this is considered normal tension glaucoma (NTG).
Open-angle glaucoma complications
Complications of glaucoma include:
- Blindness: usually painless
- Painful blind eye or absolute glaucoma: Open-angle glaucoma predisposes to central retinal venous occlusion (CRVO), leading to neovascular glaucoma and painful blind eye 107.
Open-angle glaucoma diagnosis
The only sure way to diagnose glaucoma is with a complete eye exam. An eye specialist can diagnose glaucoma using an eye exam, including several tests that are part of routine eye exams. A comprehensive eye exam can detect glaucoma long before you have eye damage and the symptoms that follow. Many of these tests involve pupil dilation (mydriasis), so your eye doctor can get a better look inside your eye. Your eye care specialist examines your eyes using a special magnifying lens. This provides a clear view of important tissues at the back of your eye to check for glaucoma or other eye problems. For a few hours after the exam your vision may be blurry and sensitive to light, so you will need someone to take you home.
Some of the most helpful glaucoma tests include:
- Visual acuity testing. A visual acuity test assesses how clearly someone can see at a distance, typically using a Snellen chart or other standardized chart. The test is performed by an optometrist or ophthalmologist and involves reading progressively smaller letters or identifying shapes, with the results expressed as a fraction like 20/20 or 6/6, indicating the distance at which the person can see the letters or shapes
- Visual field testing also called perimetry. This check of your peripheral (side) vision allows your eye care provider to find out how well you can see objects off to the side of your vision without moving your eyes. This test measures the entire area the forward-looking eye sees to document straight-ahead (central) and side (peripheral) vision. It measures the dimmest light seen at each spot tested. Each time patients perceive a flash of light, they respond by pressing a button.
- Depth perception testing. A depth perception test assesses your ability to see the world in three dimensions (3D) and judge distances accurately. It checks if your eyes work together and if your brain processes the visual information correctly. These tests use 3D images or patterns like the Randot Stereo test to gauge how well your eyes coordinate to perceive depth. Some tests involve holding a finger in front of your eyes and focusing on a distant object, checking for double vision of the finger.
- Tonometry. This measures the pressure inside your eye. Increased eye pressure is the most important risk factor for glaucoma. There are several methods of measuring eye pressure. The most common method is known as applanation, in which a tiny instrument contacts the eye’s surface after it is numbed with an eye drop.
- Air-puff test. You’ll rest your chin on a machine and your eye specialist will blow a puff of air into your eye. This quick and painless test is used as part of a routine glaucoma screening. If the results show that your eye pressure is high, your eye specialist will do other eye-pressure tests to get a more accurate measurement.
- Applanation tonometry (Goldmann Applanation Tonometry). Your eye specialist will numb your eyes with drops before measuring your eye pressure using one of these methods:
- You’ll rest your chin on a special magnifying device called a slit lamp. Your eye care specialist will examine your eye through the slit lamp while gently pressing a special tool on your eye to test the pressure.
- Your eye care specialist will gently press a handheld device against your eye. The device measures your eye pressure.
- Pachymetry. Pachymetry is a simple, painless test that measures the thickness of the cornea, the clear front part of the eye. The eye doctor uses an ultrasonic wave instrument to help determine the thickness of the cornea and better evaluate eye pressure.
- Ophthalmoscopy. Your eye care specialist will do a dilated eye exam to look for damage to your optic nerve. This exam is part of a routine glaucoma check-up. You’ll be given eye drops that widen (dilate) your pupils (the openings that let light into your eyes). You’ll look straight ahead while your eye care specialist looks into your eye using a device with a light and magnifying lens.
- Slit lamp exam. A slit lamp exam is a common eye test that uses a microscope with a focused beam of light to examine the front of your eye and the back of your eye with the aid of special lenses.
- Gonioscopy. “Gonio” means angle. Gonioscopy is a specialized eye examination that allows an ophthalmologist to visualize the anterior chamber drainage angle, the space between the iris and the cornea where fluid drains out of the eye. Gonioscopy is a crucial part of diagnosing and monitoring glaucoma and other eye conditions. Eye doctors regularly examine the drainage angle to see if there is any visible obstruction to fluid leaving the eye through the trabecular meshwork. A special lens (gonioscopy lens) is needed to examine the trabecular meshwork. The gonioscopy lens is gently placed against the surface of the cornea and allows eye doctors to see the trabecular meshwork in the drainage angle. Gonioscopy determines whether the diagnosis is considered “open” or “closed” angle glaucoma. Gonioscopy is an acquired skill that allows the ophthalmologist to visualize the angle between the cornea and iris and determine if it is open. The angle between the iris and cornea should be 20° to 45° to be considered “open” so that aqueous humor can circumvent the posterior chamber to the anterior chamber 99.
If your eye specialist has a reason to suspect damage to your retina and/or optic nerve, they may also use additional types of eye imaging. These include:
- Optical coherence tomography (OCT). Optical Coherence Tomography (OCT) measures the reflection of low-coherence infrared light directed toward the back of the eye, and the path of scattered photons helps recreate an image of the retina. Optical coherence tomography (OCT) provides high-resolution cross-sectional imaging of the retina, optic nerve, and anterior segment. Using optical coherence tomography (OCT) a 3D reconstruction of the optic nerve can be created. Optical coherence tomography (OCT) is valuable for monitoring morphological changes in the optic nerve and retinal nerve fiber layer, especially in patients with ocular hypertension and early-to-moderate glaucoma 108. OCT is highly reproducible and is widely used as an adjunct in routine glaucoma patient management. The most recent advances of OCT include OCT-A or OCT-Angiography, whereby the blood flow to vessels surrounding the optic nerve and in the macula can be measured. This is still an active area of research, but scientists do know that some patients’ optic nerves are very vulnerable to changes in optic nerve blood flow, and this new measurement may be useful in evaluating these patients. Peripapillary retinal nerve fiber layer (RNFL) analysis can show thinning in this layer. As the most commonly used scanning protocol for glaucoma diagnosis, optical coherence tomography (OCT) samples retinal ganglion cells (RGCs) from the entire retina 109. Some of the drawbacks included variability in optic nerve head morphology from patient to patient. The macular thickness is proposed as a means of glaucoma detection, given that 50% of retinal ganglion cells (RGCs) are found in the macula, and retinal ganglion cell (RGC) bodies are thicker than their axons and potentially easier to detect 110. Retinal nerve fiber layer (RNFL), macular thickness measurements, and visual field results are key in managing cases.
- Heidelberg Retina Tomograph (HRT): Heidelberg Retina Tomograph (HRT) is also a laser that can produce a 3D representation of the optic nerve.
- Nerve Fiber Analyzer (GDx): Nerve Fiber Analyzer (GDx) uses laser light to measure the thickness of the nerve fiber layer.
- Fluorescein angiography. Fluorescein angiography is a diagnostic test used to examine the blood vessels in the retina and choroid of the eye. Fluorescein angiography involves injecting a fluorescent dye into the bloodstream and taking photographs of your retina and its blood vessels as the dye circulates, revealing potential blockages, leaks, or other abnormalities in the blood vessels. Fluorescein angiography is often recommended to find and diagnose eye disease including 111:
- macular edema (swelling in the retina that distorts vision)
- diabetic retinopathy (damaged or abnormal blood vessels in the eye caused by diabetes)
- macular degeneration
- blockage of veins inside the eye, called branch retinal vein occlusion (BRVO) or central retinal vein occlusion (CRVO)
- macular pucker (a wrinkle in the retina caused by a buildup of fluid behind it)
- ocular melanoma (a type of cancer affecting the eye)
- rack changes in eye disease over time
- target treatment areas
- Less commonly, ultrasound, computed tomography (CT) or magnetic resonance imaging (MRI).
The following triad is the cornerstone of Open Angle Glaucoma diagnosis 112:
- Optic disc or retinal nerve fiber layer changes
- Visual field changes
- Elevated intraocular pressure (IOP)
Figure 5. Goldmann Applanation Tonometry
Figure 6. Gonioscopy (the drainage angle is examined using a special lens)
Footnotes: Gonioscopy. (A) and (B) Gonioscopy lens. (C) The gonioscopy lens is gently held against the cornea. Eye doctors look through the gonioscopy lens to see the drainage angle.
[Source 113 ]Optic Nerve
The optic nerve should be evaluated using a slit lamp and 90D or 78D lens so that the three-dimensional features of the optic nerve are appreciated 1. The inferior neuroretinal rim (neuroretinal rim) is the thickest, followed by superior, nasal, and temporal neuroretinal rim; this is called the ISNT rule (inferior [I] ≥ superior [S] ≥ nasal [N] ≥ temporal [T] rule) 114, 115. The ISNT rule (inferior [I] ≥ superior [S] ≥ nasal [N] ≥ temporal [T]) refers to the characteristic pattern of neuroretinal rim width in a normal optic disc 114. In a healthy eye, the neuroretinal rim is typically broadest inferiorly, followed by superiorly, nasally, and thinnest temporally 114. This pattern, represented as inferior [I] ≥ superior [S] ≥ nasal [N] ≥ temporal [T], can be used as a reference point for evaluating optic disc rim changes, especially in the context of glaucoma diagnosis. In open-angle glaucoma, the superior and inferior neuroretinal rim are thinned, breaking the ISNT rule 1. The optic cup should be determined by its contour and not color. A recent Journal of the Americal Medical Association Rational Clinical Examination systematic review of primary open-angle glaucoma diagnosis found that the risk of glaucoma was highest when an examination revealed an increased cup-disk ratio (CDR), cup-disk ratio asymmetry, disc hemorrhage, or elevated intraocular pressure (IOP) 116.
Typical optic nerve head changes in open-angle glaucoma include 1:
- Diffuse or focal narrowing (notching/shelving) of the neuroretinal rim precisely at the superior or inferior part of the optic disc
- Symmetrically enlarged cup-to-disc ratios greater than 0.5
- Increased vertical cup-to-disc ratio and thinning of neuroretinal rim
- Asymmetry of cup-disk ratio (CDR) of 0.2 or more
- Hemorrhage at or around the optic disc
- Peripapillary atrophy
- Baring of circumlinear vessels (the gap between the superficial vessels and disc margin)
- Bayonetting of vessels (the vessel first goes back and then climbs along the wall of the deep cup and then angles again on the disc margin)
- Very deep (excavated) cup with bean-pot cupping and laminar dot sign
- Nasalization of optic disc vessels
- Diffuse or focal (arcuate) thinning/defect of the retinal nerve fiber layer contiguous with an area of neuroretinal rim-notch
- The neuroretinal rim is typically pink and not pale in open-angle glaucoma. The pallor of the neuroretinal rim usually denotes an atrophic optic nerve, as seen in primary angle closure glaucoma (POAG).
Figure 7. ISNT rule
Footnotes: ISNT rule for a normal optic nerve. The ISNT rule is that optic disc rim thickness shows a characteristic configuration of inferior (I) greater than or equal to superior (S) greater than or equal to nasal (N) greater than or equal to temporal (T) (or inferior [I] ≥ superior [S] ≥ nasal [N] ≥ temporal [T]).
[Source 114 ]Figure 8. Glaucomatous Optic Nerve
Footnotes: (A) Normal optic nerve. The pink area of neural tissue forms the neuroretinal rim, whereas the central empty space corresponds to the cup. (B) Glaucomatous optic nerve showing loss of superior neural retinal rim (thinning) and excavation with enlargement of the cup. The arrowheads point to an associated retinal nerve fiber layer defect, which appears as a wedge-shaped dark area emanating from the optic nerve head. The superior neural losses correspond to the inferior defect (black scotoma) seen on the visual field. There is also a small retinal nerve fiber layer defect inferiorly, but the corresponding hemifield of the visual field remains within normal limits. (C) More extensive neural tissue loss from glaucoma with severe neuroretinal rim loss, excavation, and enlargement of the cup. There is severe loss of visual field both in the superior as well as in the inferior hemifield.
[Source 42 ]Figure 9. Optical Coherence Tomography (OCT) of Glaucomatous Optic Nerve
Footnotes: (A) The arrowheads point to a retinal nerve fiber layer (RNFL) defect. (B) Areas of thicker retinal nerve fiber layer (RNFL) appear in yellow and red. Arrowheads point to the retinal nerve fiber layer (RNFL) defect. A deviation map compares the retinal nerve fiber layer (RNFL) thickness values with a normative database and highlights the defect. (E) Arrowheads point to a visual field defect.
[Source 42 ]Visual Field
Perimetry also known as visual field testing is an important diagnostic tool that maps out your visual field on a printout, making it helpful and necessary in diagnosing and managing open-angle glaucoma. Perimetry testing provides a baseline visual field for glaucoma suspects and confirmed open-angle glaucoma cases so clinicians can track disease progression 1.
To make a diagnosis of acquired glaucomatous visual field defect, Hoddap–Parrish–Anderson criteria are used 117:
- Glaucoma hemifield test outside normal limits on at least 2 fields.
- A cluster of 3 or more non-edge points in a location typical for glaucoma, all of which are depressed on the pattern deviation plot at a perimetry (P) <5% and one of which is depressed at a perimetry (P) <1% on 2 consecutive fields.
- A corrected pattern standard deviation that occurs in less than 5% of normal fields on 2 consecutive fields.
Static automated threshold perimetry is used with white stimulus on a white background. Most studies used the Humphrey Field Analyzer, but other perimeters like Octopus have also been used successfully. Non-conventional perimetry like SWAP (short-wavelength automated perimetry using blue stimulus on yellow background), pulsar perimetry, rarebit, Matrix and frequency-doubling technology have been proposed in earlier studies for the detection of early glaucoma visual field loss, however, tend not to be used in routine clinical practice 118, 119, 120, 121, 122, 123, 124.
The visual field must be reliable, and defects should be repeatable on at least 2 fields. When treating patients long-term, it is preferable to use the same machine, the same degree of field, and protocol (eg, 24-2, 30-3, or 10-2) to compare the fields to note for progression or stability. At least 40% to 50% ganglion cell loss is needed to reliably show visual field defects in threshold perimetry 125, 126.
Structural changes of the optic nerve and retinal nerve fiber layer (RNFL) tend to occur earlier than functional change (visual field loss) in most patients with open-angle glaucoma. This is relevant to the concept of preperimetric glaucoma, which is defined as ‘the presence of characteristic glaucomatous changes in the optic disc and increased vulnerability to damage in the retinal nerve fiber layer (RNFL), without the presence of visual field defects detectable with standard automated perimetry 127. For patients with risk factors, suspect optic discs, and/or ocular hypertension, periodic visual field and optical coherence tomography (OCT) testing are recommended to detect early visual field defects and changes to determine whether therapy is needed.
Typical visual field changes in open-angle glaucoma include 1:
- Increased variability of responses in an area that later developed field defects
- Asymmetry of the visual field between the eyes
- Paracentral scotoma- commonly superonasal
- Roenne’s nasal step- an area of depression above or below the horizontal meridian on the nasal side
- Temporal wedge
- Sickle-shaped (Seidel) scotoma
- Bjerrum scotoma or arcuate scotoma
- Annular or ring scotoma when arcuate scotoma is present on both above and below the horizontal meridian
- General constriction of peripheral field
- A temporal island of the visual field
Figure 10. Humphrey Field Analyzer
Figure 11. Hoddap–Parrish–Anderson criteria
Abbreviations: MD = mean deviation; dB = decibels (refers to the logarithmic scale used to measure visual sensitivity and it represents the intensity of light stimuli that a person can see at a specific location in their visual field)
[Source 117 ]Intraocular Pressure
Intraocular pressure (IOP) is measured with tonometry and several different tonometers are used 128, 129. The gold standard for intraocular pressure (IOP) measurement is Goldmann Applanation Tonometry (GAT) 130. However, Goldmann Applanation Tonometry is not available in all instances, and non-contact tonometry is also frequently used. In the UK, this is certainly true in the community where optometrists’ preferred method of IOP measurement is non-contact 2.
When determining the intraocular pressure (IOP) of a patient using tonometry, certain variables must be considered. Tonometry measurements can, for example, vary between examiners by approximately 10% per individual, translating to a difference in intraocular pressure (IOP) measurement of 1 mm Hg to 2 mm Hg 131.
An individual’s corneal thickness measured with pachymetry or diurnal variations of intraocular pressure (IOP) (eg, higher intraocular pressure (IOP) in early morning hours or other times of the day or variability in the time of day of maximal intraocular pressure (IOP) between patients) can also have a tremendous effect on the accuracy of intraocular pressure (IOP) measurements. Study results have shown that intraocular pressure (IOP) is overestimated in individuals with thick corneas or central corneal thickness (CCT) while underestimated in those with thinner corneas 132, 133. Newer methods of IOP measurement aim to overcome variations in corneal biomechanics and give a more accurate estimate of true IOP. These include the Reichert Ocular Response Analyzer (ORA) and the Pascal Dynamic Contour Tonometer (DCT). The ORA is a non-contact tonometer that measures a biomechanical attribute of the cornea termed hysteresis. The DCT uses uses principle of contour matching instead of applanation to reduce the effect of corneal biomechanics. Furthermore, multiple measurements should be taken and correlated with optic nerve and visual field examinations when a patient is suspected of elevated intraocular pressure (IOP).
If previous tonometry measurements are available, they should be reviewed and compared to the most recent ones. Also, the intraocular pressure (IOP) may be different on different days, and different instruments may capture different values of intraocular pressure (IOP). If a difference of 3 mm Hg or more is noted between the 2 eyes, there is an increased suspicion of glaucoma 1. Clinicians should expect approximately 10% variation between individual and repeat measurements over 2 to 3 occasions before deciding on the treatment 1.
Elevated intraocular pressure (IOP) is an important and modifiable risk factor; however, it is not a diagnostic factor for open-angle glaucoma. An ophthalmologist should check the patient’s intraocular pressure (IOP) using applanation tonometry, remaining aware that the applanation tonometry test causes patients to squeeze their eyes and elevate their pressure readings. Normal intraocular pressure (IOP) should range between 12 mm Hg and 21 mm Hg 1. Approximately 75% of patients with elevated intraocular pressure (IOP) never develop glaucomatous optic nerve atrophy or visual field deficits. When a patient has recorded a reliably high intraocular pressure (IOP) reading above 21 mm Hg, they are deemed patients with glaucoma or patients with ocular hypertension 99, 134.
Corneal Photokeratoscopy
Corneal photokeratoscopy or corneal topography, is a potential biological marker to monitor patients with primary open-angle glaucoma (POAG). Preliminary results have shown a forward shift of the posterior and anterior corneal surfaces; this is correlated with more advanced stages of functional damage, indicating a link between corneal structural changes and the duration and intensity of elevated IOP. Further studies are needed to validate this marker in patients with primary open-angle glaucoma (POAG) 135.
Glaucoma Stages
The Americal Academy of Ophthalmology’s classifies the severity of glaucomatous damage into different categories 136:
- Mild: Definite optic disc or retinal nerve fiber layer (RNFL) abnormalities consistent with glaucoma as detailed above and a normal visual field tested with standard automated perimetry (SAP) are seen.
- Moderate: Definite optic disc or retinal nerve fiber layer (RNFL) abnormalities consistent with glaucoma, as detailed above, are seen, and visual field abnormalities in one hemifield are not within 5° of fixation as tested with standard automated perimetry (SAP).
- Severe: Definite optic disc or retinal nerve fiber layer (RNFL) abnormalities consistent with glaucoma as detailed above are seen, and visual field abnormalities in both hemifields or loss within 5° of fixation in at least one hemifield as tested with standard automated perimetry (SAP).
- Indeterminate: Definite optic disc or retinal nerve fiber layer (RNFL) abnormalities consistent with glaucoma, as detailed above, are seen, and the patient cannot perform visual field testing, has unreliable or uninterpretable visual field test results, or has not performed visual fields yet.
Several other different staging systems are recognized based on visual field damage, optic disc cupping, and retinal nerve fiber layer (RNFL) defects with optical coherence tomograpy (OCT), some of which are applicable in a routine clinical setting or clinical trials 137, 138, 139, 140, 141, 142.
Open-angle glaucoma differential diagnoses
Open angle glaucoma differential diagnoses should include 1:
- Optic nerve head diseases
- Physiological cup: Deep cup with healthy neuroretinal rim is seen with normal retinal nerve fiber layer thickness and no visual field defect; disc size may be large
- Optic disc drusen
- Optic disc coloboma
- Anomalous optic disc
- Tilted disc
- Ischemic optic neuropathy
- Retinal diseases causing similar visual field defects
- Branch retinal vein occlusion (BRVO)
- Branch retinal artery occlusion (BRAO)
- Retinitis pigmentosa
- Panretinal photocoagulation
- Central nervous system diseases
- Pituitary tumor: Neuroretinal rim is typically pale, pallor more than cupping, and bitemporal hemianopia exists, which respects the vertical line passing through the fixation (contrary to glaucoma, whose visual field respects the horizontal meridian)
- Intracranial hypotension due to cerebrospinal fluid shunts or other neurologic pathologies
- Foster Kennedy Syndrome is a rare neurological condition characterized by optic atrophy in one eye and papilledema (swelling of the optic disc) in the other eye, often accompanied by anosmia (loss of smell). Foster Kennedy Syndrome is usually caused by a mass lesion compressing the optic nerve on one side, leading to optic atrophy, and increased intracranial pressure causing swelling of the optic disc on the other side.
- Cerebrovascular pathologies or traumas
- Multiple sclerosis
Open-angle glaucoma treatment
The main goal of treating glaucoma is to keep it from getting worse by lowering the pressure inside your eye (IOP) 2, 143. Below a certain upper limit of intraocular pressure (IOP), it is estimated that the visual field, the optic nerve head, and retinal nerve fiber layer (RNFL) parameters will not deteriorate. This helps to maintain a patient’s quality of life.
Some of the most likely treatments for this include:
- Medications. This mainly involves medications that lower pressure inside your eye. They can prevent glaucoma from developing if you have higher-than-normal intraocular pressure (ocular hypertension) or keep it from worsening enough to cause damage and symptoms.
- Glaucoma surgeries. These mainly focus on improving the drainage of aqueous humor fluid to lower pressure inside your eye. Surgery options include trabeculectomy, tube shunts, laser therapy and minimally invasive glaucoma surgeries (MIGS).
There is no strong evidence supporting which of medication, laser or surgical therapy should be given initially. Your eye specialist can tell you more about your treatment options and help you choose one that fits your needs best.
The Collaborative Initial Glaucoma Treatment Study (CIGTS) showed no significant difference in outcome between patients randomized to either medical therapy or trabeculectomy 70, 77. Commonly, patients are started on medical therapy with possible add-on (adjunctive) laser therapy, and only if these measures fail surgery considered.
Unfortunately, some primary open-angle glaucoma (POAG) patients continue to progress despite lowering of intraocular pressure (IOP). This provides some evidence that there are intraocular pressure (IOP)-independent mechanisms at play 2. Therefore, searching for other reversible risk factors for primary open-angle glaucoma is essential 2. For example, this may come from epidemiological study of primary open-angle glaucoma and identifying lifestyle or nutritional risk factors associated with primary open-angle glaucoma 2.
Table 1. Glaucoma medications
| Intraocular pressure lowering agents | ||||||
|---|---|---|---|---|---|---|
| Drug Class | Example | Mechanism | IOP Lowering | Dosing | Side Effects | Notes |
| Prostaglandin F2a analogues | Latanoprost (Xalatan) Travoprost (Travatan) Bimatoprost (Lumigan) Tafluprost (Zioptan; preservative free) Latanoprostene bunod (Vyzulta) | Increase outflow via uveoscleral pathway; decrease outflow resistance; mechanism unclear | Latanaprost and Travoprost: 25-32% Bimatoprost: 27-33% Tafluprost: 27-31% Latanoprostene: As a class, most potent | Once daily, usually in evening | Ocular: Irritation, increased pigmentation of iris, lashes, and skin, hypertrichosis, prostaglandin associated periorbitopathy, loss of orbital fat over time | Often preferred as first line; highly effective at IOP lowering, with minimal systemic side effects. Among preservative containing drops, side effects tend to be greatest with bimatoprost, and least with latanoprost. Preservative free tafluprost may reduce irritation and ocular side effects and improve compliance in some patients.2 |
| Systemic: flu-like symptoms, myalgias and arthralgias, nasal congestion; generally very well tolerated | ||||||
| Beta blockers (beta-adrenergic antagonists) | Non-Selective: Timolol maleate (Timoptic; Timoptic occudose = preservative free)Timolol hemihydrate (Betimol)Levobunolol (Betagan)MetipranololCarteolol HCl (partial alpha agonist) | Decrease aqueous production | 20-30% | 1-2 times daily OR Once daily in morning; more effective in AM | Ocular: Blurring, irritation, punctate keratitis; metipranolol associated with uveitis | Generally well tolerated from the perspective of ocular side effects. History of asthma or other airway disease is a strong contraindication. Suspect side effects of therapy in a patient with new onset depression, lethargy, or sexual dysfunction. Carteolol may have less detrimental effect on lipid profile in some patients.3 Betaxolol may be slightly less likely to cause asthma or lung disease exacerbation, but still carries significant risk.4 |
| Systemic: symptoms of b-blockade: heart block and bradycardia, decreased exercise tolerance, asthma and lung disease exacerbation, decreased symptoms of hypoglycemia in diabetes, depression, sexual dysfunction, lipid profile changes | ||||||
| Selective b 1: Betaxalol | Same | 15-20% | 2 times daily | |||
| Alpha agonists (Alpha2-adrenergic agonists) | Apraclonidine HCl (Iopidine) Brimonidine tartrate (Alphagan) | Decreases aqueous production, increases uveoscleral outflow to lesser extent | 20-30% | 2-3 times daily | Ocular: Irritation, allergy, pruritis, dry eye | As a class, most likely to cause ocular irritation. High risk of apnea and CNS depression in infants with brimonidine. If CNS depression is a particular concern in any patient, apraclonidine does not cross blood-brain barrier and may be better choice.5,6 |
| Systemic: Lethargy, hypotension, vasovagal attack, headache, dry mouth and nose, insomnia, anxiety; risk of apnea and CNS depression in infants with brimonidine, apraclonidine will not cross blood-brain barrier | ||||||
| Carbonic anhydrase inhibitors | Oral: Acetazolamide (Diamox)Methazolamide (Neptazane) | Decreases aqueous production | 15-20% | Acetazolamide: 500-1000mg per day in 2-4 doses Methazolamide: 25-50mg 2-3 times per day IV Acetazolamide: 5-10mg/kg q6-8 hours | Ocular: None | Sulfa allergies are often cited as a contraindication to use of these drugs, but there is little structural overlap with antibiotic sulfa drugs, and thus most patients allergic to sulfa drugs will not be affected by carbonic anhydrase inhibitors. 7 |
| Systemic: hypokalemia, poor tolerance of carbonated beverages, acidosis, paresthesias, blood dyscrasias, lethargy, nephrolithiasis, others
| ||||||
| Topical: Dorzolamide (Trusopt)Brinzolamide (Azopt) | Same | Generally not as effective as oral acetazolamide | 2 times daily (only FDA approved for 3 times daily) | Ocular: Induced myopia, blurred vision, stinging (less with Brinzolamide), allergic conjunctivitis, keratopathy | Generally very safe, often used in infants and pregnant women. | |
| Systemic: Less than with oral agents; bad taste in mouth | ||||||
| Parasympathomimetic agents | Pilocarpine HCl Echothiophate iodide (Phospholine iodide) | Miotics; increase trabecular outflow; ciliary muscles contract, put traction on scleral spur, open trabecular meshwork | 15-25% | Pilocarpine: 2-4 times daily, usually 4 times daily Echothiopentate: 1-2 times daily, usually 2 times daily | Ocular: Posterior synechiae, intense miosis, keratitis, cataract, retinal detachment, angle closure, epiphora, induced myopia | Consider echothiophate in patients with a complicated anterior segment, e.g. aphakia, PK, ACIOL, etc., with inadequate pressure control. In certain patients it can be the sole effective drug.8 |
| Systemic: increased salivation, increased gastric secretion, abdominal cramps | ||||||
| Rho kinase inhibitors | Netarsudil (Rhopressa) | Increases trabecular outflow, decreases episcleral venous pressure9 | Reduces IOP by ~5.5-6 mmHg regardless of baseline IOP10 | FDA approved for once daily, more efficacious when used twice daily in studies | Ocular: conjunctival hyperemia and hemorrhage, corneal deposits (verticillata), blurry vision, epiphora, pruritis, punctate keratitis, eyelid erythema, conjunctival edema, foreign body sensation | Though not as effective at IOP reduction as other classes, may have benefit in patients with lower baseline IOP (IOP reduction less dependent on baseline IOP than other classes), and as an adjunct to other drugs, as mechanism of action (increases trabecular outflow rather than uveoscleral) is different from the most commonly used classes.10 |
| Systemic: None achieving statistical significance in recent studies10 | ||||||
| Hyperosmotic agents | Mannitol 20% (parenteral) Glycerol 50% (oral) | Creates osmotic gradient, dehydrates vitreous | Mannitol: 0.5-2.0 g/kg Glycerol: 1-1.5 g/kg | Ocular: IOP rebound, aqueous flare | ||
| Systemic: urinary retention, headache, nausea, vomiting, diarrhea, electrolyte disturbances, cardiac complications, contraindicated in renal failure | ||||||
Eye drops
Glaucoma treatment often starts with prescription eye drops. Some may decrease eye pressure by improving how fluid drains from your eye. Others decrease the amount of fluid your eye makes. Depending on how low your eye pressure needs to be, more than one eye drop may be prescribed.
Prescription eye drop medicines include:
- Prostaglandins. These increase the outflow of the fluid in your eye, helping to reduce eye pressure (IOP). Medicines in this category include latanoprost (Xalatan), travoprost (Travatan Z), tafluprost (Zioptan), bimatoprost (Lumigan) and latanoprostene bunod (Vyzulta). Possible side effects include mild reddening and stinging of the eyes, darkening of the iris, darkening of the pigment of the eyelashes or eyelid skin, and blurred vision. This class of medicine is prescribed for once-a-day use.
- Beta blockers. These reduce the production of fluid in your eye, helping to lower eye pressure. Examples include timolol (Betimol, Istalol, Timoptic) and betaxolol (Betoptic S). Possible side effects include difficulty breathing, slowed heart rate, lower blood pressure, impotence and fatigue. This class of medicine can be prescribed for once- or twice-daily use depending on your condition.
- Alpha-adrenergic agonists. These reduce the production of the fluid that flows throughout the inside of your eye. They also increase the outflow of fluid in the eye. Examples include apraclonidine (Iopidine) and brimonidine (Alphagan P, Qoliana). Possible side effects include irregular heart rate; high blood pressure; fatigue; red, itchy or swollen eyes; and dry mouth. This class of medicine is usually prescribed for twice-daily use but sometimes can be prescribed for use three times a day.
- Carbonic anhydrase inhibitors. These medicines reduce the production of fluid in your eye. Examples include dorzolamide and brinzolamide (Azopt). Possible side effects include a metallic taste, frequent urination, and tingling in the fingers and toes. This class of medicine is usually prescribed for twice-daily use but sometimes can be prescribed for use three times a day.
- Rho kinase inhibitor. This medicine lowers eye pressure by suppressing the rho kinase enzymes responsible for fluid increase. It is available as netarsudil (Rhopressa) and is prescribed for once-a-day use. Possible side effects include eye redness and eye discomfort.
- Miotic or cholinergic agents. These increase the outflow of fluid from your eye. An example is pilocarpine (Isopto Carpine). Side effects include headache, eye pain, smaller pupils, possible blurred or dim vision, and nearsightedness. This class of medicine is usually prescribed to be used up to four times a day. Because of potential side effects and the need for frequent daily use, these medicines are not prescribed very often anymore.
Combination drugs:
- Timolol/Brinzolamide (Azarga-not available in the US)
- Timolol/Dorzolamide (Cosopt)
- Timolol/Latanoprost (Xalacom-not available in the US)
- Timolol/Bimatoprost (Ganfort-not available in the US)
- Timolol/Brimonidine (Combigan)
- Brinzolamide/Brimonidine (Simbrinza)
Because some of the eye drop medicine is absorbed into the bloodstream, you may experience some systemic side effects unrelated to your eyes. To minimize this absorption, close your eyes for 1 to 2 minutes after putting the drops in. You also may press lightly at the corner of your eyes near your nose to close the tear duct for 1 to 2 minutes. Wipe off any unused drops from your eyelid.
You may be prescribed multiple eye drops or need to use artificial tears. Make sure you wait at least five minutes in between using different drops.
Never change or stop taking your glaucoma medications without talking to your ophthalmologist. If you are about to run out of your medication, ask your ophthalmologist if you should have your prescription refilled.
Alpha agonists for glaucoma
Alpha agonists also called alpha2-adrenergic agonists work by reducing the amount of fluid your eye produces. Alpha agonists also increase the amount of fluid that drains out of the eyes. This helps lower eye pressure and, hopefully, saves your vision.
Possible side effects of alpha agonists include:
- red, stinging or painful eyes after using drops
- blurry vision
- allergy (redness, itching, tearing and swelling of the eye)
- a large (dilated) pupil
- headaches
- dry mouth
- feeling tired, weak or dizzy
- decreased blood pressure
- a fast or irregular heartbeat
- feeling nervous
Blurry vision, stinging, and redness may improve with time. But if the side effects still bother you, call your ophthalmologist. They may be able to lower your dose or change your medicine. Most side effects go away when the medicine is stopped. If you feel you need to stop taking this medication due to side effects or other concerns, please discuss with your doctor.
Beta-blockers for glaucoma
Beta-blockers also called beta-adrenergic antagonists work by reducing the amount of fluid your eye produces. This helps lower pressure in your eye and, hopefully, saves your vision.
Possible side effects of beta-blockers include:
- red, stinging or painful eyes after using drops.
- blurry vision
- breathing problems in people with asthma, emphysema, or chronic obstructive pulmonary disease (COPD)
- a slow or irregular heartbeat
- feeling tired
- depression
- dizziness
- a change in sex drive or sexual function
- getting overly tired during exercise
- in people with diabetes, low blood sugar symptoms becoming difficult to notice
Blurry vision, stinging, and redness may improve with time. But if the side effects still bother you, call your ophthalmologist. They may be able to lower your dose or change your medicine. Most side effects go away when the medication is stopped. Never suddenly quit taking your medicine unless your doctor tells you to.
Carbonic anhydrase inhibitors for glaucoma
Carbonic anhydrase inhibitors work by reducing the amount of fluid your eye produces. This helps lower eye pressure and, hopefully, saves your vision. Your ophthalmologist may have you take this medicine as an eye drop or by mouth as a pill.
Possible side effects of carbonic anhydrase inhibitors include:
- stinging eyes after putting drops in.
- red eyes
- blurry vision
- a skin rash (especially in people who are allergic to sulfa drugs)
- changes in how things taste to you (especially with carbonated drinks)
- bad taste or upset stomach (nausea)
- feeling tired
- decreased energy
- increase in urination (with the pills)
- tingling around the mouth and fingertips (with the pills)
- lower potassium (with the pills)
Blurry vision, stinging, and redness may improve with time. But if the side effects still bother you, call your ophthalmologist. They may be able to lower your dose or change your medicine. Most side effects go away when the medication is stopped. Never suddenly quit taking your medicine unless your doctor tells you to.
Miotics for glaucoma
Miotics also called cholinergic agents make your pupil constrict (get smaller or miosis), increasing the amount of fluid that drains out of the eye. This helps lower eye pressure and, hopefully, helps protect your vision.
Possible side effects of miotics include:
- blurred vision
- nearsightedness (trouble focusing on distant objects)
- dim vision with difficulty seeing in the dark or at night
- headache or brow ache (aching around eye)
While very rare, there is the possibility that your retina could detach. This is when the light-sensitive tissue lining the back of the eye pulls away. You would suddenly notice dark specks or spots (floaters) or flashing lights in your vision. If you have these symptoms, see your ophthalmologist immediately.
Side effects may go away after you take the medicine for a while. But if the side effects still bother you, call your ophthalmologist. They may be able to lower your dose or change your medicine. If you feel you need to stop taking this medication due to side effects or other concerns, please discuss with your doctor.
Prostaglandin analogs for glaucoma
Prostaglandin analogs also called prostaglandin F2a analogues work by increasing the drainage of fluid out of your eye. This helps lower eye pressure and, hopefully, saves your vision.
Possible side effects of prostaglandin analogs include:
- red, stinging or painful eyes after using drops.
- feeling like something is in your eye
- blurry vision
- a permanent change in your eye color (occurs mostly in hazel eyes)
- an increase in thickness, number and length of eyelashes
- darkening of the eyelid
- worsening of existing angina and asthma
- joint aches
- light sensitivity
- eyes gradually sinking deeper into their sockets, keeping eyelids from working properly
Blurry vision, stinging, and redness may improve with time. But if the side effects still bother you, call your ophthalmologist. They may be able to lower your dose or change your medicine. Most side effects go away when the medication is stopped. Never suddenly quit taking your medicine unless your doctor tells you to.
Oral medicines
Eye drops alone may not bring eye pressure down to the desired level. So an eye doctor also may prescribe oral medicine. This medicine is usually a carbonic anhydrase inhibitor. Possible side effects include frequent urination, tingling in the fingers and toes, depression, stomach upset, and kidney stones.
Medical follow-up
Once the diagnosis of primary open-angle glaucoma (POAG) has been established, it is important you understand about the natural history of glaucoma with the potential for irreversible visual loss in cases which are not treated or under treated. When starting a topical medication, your need to know that it’s a life-long therapy with potential side-effects and the need to use the drops every day, and the fact the treatment is preventative not curative.
It is popular practice to define a target intraocular pressure (IOP) for intraocular pressure (IOP) lowering. This would be based on current evidence, the stage of your primary open-angle glaucoma and other risk factors for progression. A reasonable initial target is for at least 20%-30% reduction in intraocular pressure (IOP). A larger reduction may well be needed for patients presenting with advanced disease. Advanced Glaucoma Intervention Study showed reduced risk of progression for patients who’s intraocular pressure (IOP) were consistently below 18 or largely below 15.
First review after commencing a new therapy should occur within 6-8 weeks, though the patient should understand to present sooner if they develop any side-effects. Thereafter, 3-4 monthly review is sufficient if the patient remains stable. At each review, the patient needs the following assessed 2:
- Any subjective change in vision or any side-effects
- Intraocular pressure (IOP) – is it within target?
- Signs of progression – standard automated perimetry, optic disc assessment (clinical +/- HRT, OCT)
If the patient has no side effects, the intraocular pressure (IOP) is within target level and there are no signs of progression, then a routine 3-4 month review can be arranged. If the initial therapy was not at all effective, it should be swapped for an alternative class of drop. If the initial therapy significantly reduced intraocular pressure (IOP), but not to target level, then an additional drop should be prescribed (or a combination drop used). If there are signs of progression despite target intraocular pressure (IOP), the target should be lowered further, and the patient more closely followed. If target intraocular pressure (IOP) is not reached despite fully tolerated topical medications, laser or surgical therapy should be considered.
Surgery, laser and other therapies
Other treatment options include laser therapy and surgery. The following techniques may help to drain fluid within the eye and lower eye pressure:
- Laser trabeculoplasty is an option if eye drops can’t be tolerated. Laser trabeculoplasty also may be used if medicine hasn’t slowed the progression of the glaucoma. An eye doctor also may recommend laser surgery before using eye drops. It’s done in the eye doctor’s office. An eye doctor uses a small laser to improve the drainage of the tissue located at the angle where the iris and cornea meet. It may take a few weeks before the full effect of this procedure becomes apparent.
- Laser iridotomy. Laser iridotomy is for people who have angle-closure glaucoma. The ophthalmologist uses a laser to create a tiny hole in the iris. This hole helps fluid flow to the drainage angle.
- Glaucoma filtration surgery also called a trabeculectomy. The eye doctor (ophthalmologist) creates an opening in the white of your eye, which also is known as the sclera. This is where your eye surgeon creates a tiny flap in the sclera. They will also create a bubble like a pocket in the conjunctiva called a filtration bleb. It is usually hidden under the upper eyelid and cannot be seen. Aqueous humor will be able to drain out of the eye through the flap and into the bleb, lowering eye pressure. In the bleb, the fluid is absorbed by tissue around your eye.
- Drainage tubes. In this procedure, the eye surgeon inserts a small drainage tube in your eye to drain excess fluid to lower eye pressure. The glaucoma drainage implant sends the fluid to a collection area called a reservoir. Your eye surgeon creates this reservoir beneath the conjunctiva. The fluid is then absorbed into nearby blood vessels.
- Minimally invasive glaucoma surgery (MIGS). An eye doctor may suggest a minimally invasive glaucoma surgery (MIGS) procedure to lower eye pressure. This procedure generally require less immediate postoperative care and have less risk than trabeculectomy or using a drainage device. A minimally invasive glaucoma surgery (MIGS) procedure is often combined with cataract surgery. There are a number of minimally invasive glaucoma surgery (MIGS) techniques available.
- Cataract surgery. For some people with narrow angles, removing the eye’s natural lens can lower eye pressure. With narrow angles, the iris and the cornea are too close together. This can cover (block) the eye’s drainage channel. Removing the eye’s lens with cataract surgery creates more space for fluid to leave the eye. This can lower eye pressure.
After your procedure, you’ll need to see your eye doctor for follow-up exams. You can expect to visit your ophthalmologist about every 3 to 6 months. However, this can vary depending on your treatment needs. And you may eventually need to undergo additional procedures if your eye pressure begins to rise or other changes happen in your eye.
Open-angle glaucoma prognosis
Advanced open-angle glaucoma may cause optic atrophy and no perception of light, though most open-angle glaucoma patients will not lose vision in their lifetime 1. One study in St Lucia found that 35% of untreated eyes progressed to end-stage disease over 10 years 145. There is strong evidence that lowering intraocular pressure (IOP) can reduce the rate of progression significantly. During 6 years of the Early Manifest Glaucoma Trial, 53% progressed, though treatment reduced the rate of progression by half, and for each mmHg of intraocular pressure (IOP) lowering achieved, the rate was reduced by 10%. However, the rate of progression and risk of blindness may be hard to predict in individual patients 2.
Risk factors for progression of open-angle glaucoma include 136:
- Old age
- Elevated intraocular pressure (IOP)
- Increased cup-to-disc ratio or small optic rim area
- Beta peripapillary atrophy
- Disc hemorrhage
- Thin central corneal thickness
- Reduced corneal hysteresis
- Low ocular perfusion pressure
- Poor compliance with therapy
- Pseudoexfoliation.
- Mahabadi N, Zeppieri M, Tripathy K. Open Angle Glaucoma. [Updated 2024 Mar 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441887[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Primary Open-Angle Glaucoma. https://eyewiki.org/Primary_Open-Angle_Glaucoma[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Veeraraghavan N. Adult Eye Conditions: Primary Open-Angle Glaucoma and Cataract. FP Essent. 2022 Aug;519:19-23.[↩][↩]
- Dietze J, Blair K, Zeppieri M, et al. Glaucoma. [Updated 2024 Mar 16]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK538217[↩]
- Glaucoma. https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/glaucoma[↩]
- Types of Glaucoma. https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/glaucoma/types-glaucoma[↩]
- Yanoff M, Duker JS. Ophthalmology. 5th ed. Elsevier; 2018.[↩]
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014 Nov;121(11):2081-90. doi: 10.1016/j.ophtha.2014.05.013[↩]
- Glaucoma in the African American and Hispanic Communities: What We Know. https://www.brightfocus.org/resource/glaucoma-in-the-african-american-and-hispanic-communities-what-we-know/[↩][↩]
- Kang JM, Tanna AP. Glaucoma. Med Clin North Am. 2021 May;105(3):493-510. doi: 10.1016/j.mcna.2021.01.004[↩][↩][↩][↩]
- Stein JD, Khawaja AP, Weizer JS. Glaucoma in Adults-Screening, Diagnosis, and Management: A Review. JAMA. 2021 Jan 12;325(2):164-174. doi: 10.1001/jama.2020.21899[↩]
- Gedde SJ, Vinod K, Wright MM, Muir KW, Lind JT, Chen PP, Li T, Mansberger SL; American Academy of Ophthalmology Preferred Practice Pattern Glaucoma Panel. Primary Open-Angle Glaucoma Preferred Practice Pattern®. Ophthalmology. 2021 Jan;128(1):P71-P150. doi: 10.1016/j.ophtha.2020.10.022[↩][↩][↩]
- Allison K, Patel D, Alabi O. Epidemiology of Glaucoma: The Past, Present, and Predictions for the Future. Cureus. 2020 Nov 24;12(11):e11686. doi: 10.7759/cureus.11686[↩]
- About Glaucoma. https://www.cdc.gov/vision-health/about-eye-disorders/glaucoma.html[↩]
- Skaat A, De Moraes CG, Bowd C, Sample PA, Girkin CA, Medeiros FA, Ritch R, Weinreb RN, Zangwill LM, Liebmann JM; Diagnostic Innovations in Glaucoma Study and African Descent and Glaucoma Evaluation Study Groups. African Descent and Glaucoma Evaluation Study (ADAGES): Racial Differences in Optic Disc Hemorrhage and Beta-Zone Parapapillary Atrophy. Ophthalmology. 2016 Jul;123(7):1476-83. doi: 10.1016/j.ophtha.2016.03.025[↩]
- Allison K, Patel DG, Greene L. Racial and Ethnic Disparities in Primary Open-Angle Glaucoma Clinical Trials: A Systematic Review and Meta-analysis. JAMA Netw Open. 2021 May 3;4(5):e218348. doi: 10.1001/jamanetworkopen.2021.8348[↩]
- Gosling D, Meyer JJ. Normal Tension Glaucoma. [Updated 2022 Dec 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK576377[↩][↩][↩][↩][↩]
- Salvetat ML, Pellegrini F, Spadea L, Salati C, Zeppieri M. Pharmaceutical Approaches to Normal Tension Glaucoma. Pharmaceuticals (Basel). 2023 Aug 17;16(8):1172. doi: 10.3390/ph16081172[↩]
- Shields MB. Normal-tension glaucoma: is it different from primary open-angle glaucoma? Curr Opin Ophthalmol. 2008 Mar;19(2):85-8. doi: 10.1097/ICU.0b013e3282f3919b[↩]
- Lee BL, Bathija R, Weinreb RN. The definition of normal-tension glaucoma. J Glaucoma. 1998 Dec;7(6):366-71.[↩]
- Van Buskirk EM. The tale of normal-tension glaucoma. J Glaucoma. 1998 Dec;7(6):363-5.[↩]
- Sowka J. New thoughts on normal tension glaucoma. Optometry. 2005 Oct;76(10):600-8. doi: 10.1016/j.optm.2005.08.020[↩]
- Normal Tension Glaucoma. https://eyewiki.org/Normal_Tension_Glaucoma[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Khazaeni B, Zeppieri M, Khazaeni L. Acute Angle-Closure Glaucoma. [Updated 2023 Nov 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430857[↩]
- Shahror, R.A., Morris, C.A., Mohammed, A.A. et al. Role of myeloid cells in ischemic retinopathies: recent advances and unanswered questions. J Neuroinflammation 21, 65 (2024). https://doi.org/10.1186/s12974-024-03058-y[↩]
- Goel M, Picciani RG, Lee RK, Bhattacharya SK. Aqueous humor dynamics: a review. Open Ophthalmol J. Sep 3 2010;4:52-9. doi:10.2174/1874364101004010052[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Toris CB, Koepsell SA, Yablonski ME, Camras CB. Aqueous humor dynamics in ocular hypertensive patients. J Glaucoma. 2002 Jun;11(3):253-8. doi: 10.1097/00061198-200206000-00015[↩][↩][↩][↩][↩]
- Macknight AD, McLaughlin CW, Peart D, Purves RD, Carré DA, Civan MM. Formation of the aqueous humor. Clin Exp Pharmacol Physiol. 2000 Jan-Feb;27(1-2):100-6. doi: 10.1046/j.1440-1681.2000.03208.x[↩]
- Ferreira SM, Lerner SF, Brunzini R, Evelson PA, Llesuy SF. Oxidative stress markers in aqueous humor of glaucoma patients. Am J Ophthalmol. 2004 Jan;137(1):62-9. doi: 10.1016/s0002-9394(03)00788-8[↩][↩]
- Kaeslin MA, Killer HE, Fuhrer CA, Zeleny N, Huber AR, Neutzner A. Changes to the Aqueous Humor Proteome during Glaucoma. PLoS One. 2016 Oct 27;11(10):e0165314. doi: 10.1371/journal.pone.0165314[↩][↩]
- Brubaker RF. The flow of aqueous humor in the human eye. Trans Am Ophthalmol Soc. 1982;80:391-474. https://pmc.ncbi.nlm.nih.gov/articles/instance/1312276/pdf/taos00019-0414.pdf[↩][↩][↩][↩][↩]
- R TCYMT. Aqueous humor dynamics. In: DC CNL, ed. Atlas of Glaucoma. Second edition ed. Informa HK; 2007:13 – 28:chap 3.[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Kiel JW, Hollingsworth M, Rao R, Chen M, Reitsamer HA. Ciliary blood flow and aqueous humor production. Prog Retin Eye Res. Jan 2011;30(1):1-17. doi:10.1016/j.preteyeres.2010.08.001[↩][↩][↩][↩][↩]
- Macknight AD, McLaughlin CW, Peart D, Purves RD, Carre DA, Civan MM. Formation of the aqueous humor. Clin Exp Pharmacol Physiol. Jan-Feb 2000;27(1-2):100-6. doi:10.1046/j.1440-1681.2000.03208.x[↩]
- Johnson M, McLaren JW, Overby DR. Unconventional aqueous humor outflow: A review. Exp Eye Res. May 2017;158:94-111. doi:10.1016/j.exer.2016.01.017[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Roy Chowdhury U, Hann CR, Stamer WD, Fautsch MP. Aqueous humor outflow: dynamics and disease. Invest Ophthalmol Vis Sci. May 2015;56(5):2993-3003. doi:10.1167/iovs.15-16744[↩][↩][↩][↩]
- Fautsch MP, Johnson DH. Aqueous humor outflow: what do we know? Where will it lead us? Invest Ophthalmol Vis Sci. Oct 2006;47(10):4181-7. doi:10.1167/iovs.06-0830[↩][↩][↩]
- Trabecular Meshwork Research. https://ethier.gatech.edu/anterior-segmenttrabecular-meshwork-biomechanics[↩][↩]
- J. Buffault, A. Labbé, P. Hamard, F. Brignole-Baudouin, C. Baudouin. The trabecular meshwork: Structure, function and clinical implications. A review of the literature. Journal Français
d’Ophtalmologie, 2020, 43 (7), pp.e217-e230. https://hal.sorbonne-universite.fr/hal-02962228v1/document[↩] - Optic disc hemorrhage in normal tension glaucoma. https://eyerounds.org/atlas/pages/Optic-Disc-Hemorrhage-in-NTG.htm#gsc.tab=0[↩]
- MYOC gene. https://medlineplus.gov/genetics/gene/myoc[↩][↩][↩]
- Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014 May 14;311(18):1901-11. doi: 10.1001/jama.2014.3192[↩][↩][↩][↩]
- Skarie J.M., Link B.A. The primary open-angle glaucoma gene WDR36 functions in ribosomal RNA processing and interacts with the p53 stress-response pathway. Hum. Mol. Genet. 2008;17:2474–2485. doi: 10.1093/hmg/ddn147[↩]
- Dores G.M., Curtis R.E., Toro J.R., Devesa S.S., Fraumeni J.F., Jr. Incidence of cutaneous sebaceous carcinoma and risk of associated neoplasms: Insight into Muir-Torre syndrome. Cancer. 2008;113:3372–3381. doi: 10.1002/cncr.23963[↩]
- Fuse N. Genetic bases for glaucoma. Tohoku J. Exp. Med. 2010;221:1–10. doi: 10.1620/tjem.221.1[↩]
- Hauser M.A., Allingham R.R., Linkroum K., Wang J., RaRocque-Abramson K., Figueiredo D., Santiago-Turla C., del Bono E.A., Haines J.L., Pericak-Vance M.A., et al. Distribution of WDR36 DNA sequence variants in patients with primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2006;47:2542–2546. doi: 10.1167/iovs.05-1476[↩]
- Fan BJ, Wang DY, Cheng CY, Ko WC, Lam SC, Pang CP. Different WDR36 mutation pattern in Chinese patients with primary open-angle glaucoma. Mol Vis. 2009;15:646-53. https://pmc.ncbi.nlm.nih.gov/articles/PMC2664842[↩][↩]
- Meer E, Aleman TS, Ross AG. WDR36-Associated Neurodegeneration: A Case Report Highlights Possible Mechanisms of Normal Tension Glaucoma. Genes (Basel). 2021 Oct 15;12(10):1624. doi: 10.3390/genes12101624[↩]
- Su HA, Li SY, Yang JJ, Yen YC. An Application of NGS for WDR36 Gene in Taiwanese Patients with Juvenile-Onset Open-Angle Glaucoma. Int J Med Sci. 2017 Sep 20;14(12):1251-1256. doi: 10.7150/ijms.20729[↩]
- Frezzotti P, Pescucci C, Papa FT, Iester M, Mittica V, Motolese I, Peruzzi S, Artuso R, Longo I, Mencarelli MA, Mittica P, Motolese E, Renieri A. Association between primary open-angle glaucoma (POAG) and WDR36 sequence variance in Italian families affected by POAG. Br J Ophthalmol. 2011 May;95(5):624-6. doi: 10.1136/bjo.2009.167494[↩]
- Pasutto F, Mardin CY, Michels-Rautenstrauss K, Weber BH, Sticht H, Chavarria-Soley G, Rautenstrauss B, Kruse F, Reis A. Profiling of WDR36 missense variants in German patients with glaucoma. Invest Ophthalmol Vis Sci. 2008 Jan;49(1):270-4. doi: 10.1167/iovs.07-0500[↩]
- Weisschuh N, Wolf C, Wissinger B, Gramer E. Variations in the WDR36 gene in German patients with normal tension glaucoma. Mol Vis. 2007 May 16;13:724-9. https://pmc.ncbi.nlm.nih.gov/articles/PMC2765470[↩]
- Liu K, He W, Zhao J, Zeng Y, Cheng H. Association of WDR36 polymorphisms with primary open angle glaucoma: A systematic review and meta-analysis. Medicine (Baltimore). 2017 Jun;96(26):e7291. doi: 10.1097/MD.0000000000007291[↩]
- Monemi S, Spaeth G, DaSilva A, Popinchalk S, Ilitchev E, Liebmann J, Ritch R, Héon E, Crick RP, Child A, Sarfarazi M. Identification of a novel adult-onset primary open-angle glaucoma (POAG) gene on 5q22.1. Hum Mol Genet. 2005 Mar 15;14(6):725-33. doi: 10.1093/hmg/ddi068[↩]
- CAV1 gene. https://medlineplus.gov/genetics/gene/cav1[↩]
- Thorleifsson G, Walters GB, Hewitt AW, et al. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nat Genet. 2010 Oct;42(10):906-9. doi: 10.1038/ng.661[↩]
- Borrás T. Gene expression in the trabecular meshwork and the influence of intraocular pressure. Prog Retin Eye Res. 2003 Jul;22(4):435-63. doi: 10.1016/s1350-9462(03)00018-1[↩]
- Pasquale LR, Loomis SJ, Kang JH, et al. CDKN2B-AS1 genotype-glaucoma feature correlations in primary open-angle glaucoma patients from the United States. Am J Ophthalmol. 2013 Feb;155(2):342-353.e5. doi: 10.1016/j.ajo.2012.07.023[↩]
- Nunes HF, Ananina G, Costa VP, Zanchin NIT, de Vasconcellos JPC, de Melo MB. Investigation of CAV1/CAV2 rs4236601 and CDKN2B-AS1 rs2157719 in primary open-angle glaucoma patients from Brazil. Ophthalmic Genet. 2018 Apr;39(2):194-199. doi: 10.1080/13816810.2017.1393830[↩]
- Burdon KP, Macgregor S, Hewitt AW, Sharma S, Chidlow G, Mills RA, Danoy P, Casson R, Viswanathan AC, Liu JZ, Landers J, Henders AK, Wood J, Souzeau E, Crawford A, Leo P, Wang JJ, Rochtchina E, Nyholt DR, Martin NG, Montgomery GW, Mitchell P, Brown MA, Mackey DA, Craig JE. Genome-wide association study identifies susceptibility loci for open angle glaucoma at TMCO1 and CDKN2B-AS1. Nat Genet. 2011 Jun;43(6):574-8. doi: 10.1038/ng.824[↩]
- Pan Y, Iwata T. Molecular genetics of inherited normal tension glaucoma. Indian J Ophthalmol. 2024 May 1;72(Suppl 3):S335-S344. doi: 10.4103/IJO.IJO_3204_23[↩][↩][↩][↩][↩]
- Shim MS, Kim KY, Noh M, Ko JY, Ahn S, An MA, Iwata T, Perkins GA, Weinreb RN, Ju WK. Optineurin E50K triggers BDNF deficiency-mediated mitochondrial dysfunction in retinal photoreceptor cell line. Biochem Biophys Res Commun. 2018 Sep 18;503(4):2690-2697. doi: 10.1016/j.bbrc.2018.08.025[↩]
- Ma L, Zeng Y, Wei J, Yang D, Ding G, Liu J, Shang J, Kang Y, Ji X. Knockdown of LOXL1 inhibits TGF-β1-induced proliferation and fibrogenesis of hepatic stellate cells by inhibition of Smad2/3 phosphorylation. Biomed Pharmacother. 2018 Nov;107:1728-1735. doi: 10.1016/j.biopha.2018.08.156[↩]
- Berner D, Zenkel M, Pasutto F, Hoja U, Liravi P, Gusek-Schneider GC, Kruse FE, Schödel J, Reis A, Schlötzer-Schrehardt U. Posttranscriptional Regulation of LOXL1 Expression Via Alternative Splicing and Nonsense-Mediated mRNA Decay as an Adaptive Stress Response. Invest Ophthalmol Vis Sci. 2017 Nov 1;58(13):5930-5940. doi: 10.1167/iovs.17-22963[↩]
- Guven Yilmaz S, Palamar M, Onay H, Ilim O, Aykut A, Ozkinay FF, Yagci A. Association of Lysyl Oxidase-like 1 Gene Polymorphism in Turkish Patients With Pseudoexfoliation Syndrome and Pseudoexfoliation Glaucoma. J Glaucoma. 2017 Jul;26(7):684-685. doi: 10.1097/IJG.0000000000000672[↩]
- Sharma S, Martin S, Sykes MJ, Dave A, Hewitt AW, Burdon KP, Ronci M, Voelcker NH, Craig JE. Biological effect of LOXL1 coding variants associated with pseudoexfoliation syndrome. Exp Eye Res. 2016 May;146:212-223. doi: 10.1016/j.exer.2016.03.013[↩]
- Gao XR, Chiariglione M, Arch AJ. Whole-exome sequencing study identifies rare variants and genes associated with intraocular pressure and glaucoma. Nat Commun. 2022 Nov 30;13(1):7376. doi: 10.1038/s41467-022-35188-3[↩]
- Mabuchi F, Mabuchi N, Sakurada Y, et al. Genetic variants associated with glaucomatous visual field loss in primary open-angle glaucoma. Sci Rep. 2022 Dec 1;12(1):20744. doi: 10.1038/s41598-022-24915-x[↩]
- Zukerman R, Harris A, Oddone F, Siesky B, Verticchio Vercellin A, Ciulla TA. Glaucoma Heritability: Molecular Mechanisms of Disease. Genes (Basel). 2021 Jul 27;12(8):1135. doi: 10.3390/genes12081135[↩]
- The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998 Oct;126(4):498-505. doi: 10.1016/s0002-9394(98)00272-4[↩][↩][↩]
- Heijl A, Leske MC, Bengtsson B, et al. Reduction of Intraocular Pressure and Glaucoma Progression: Results From the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268–1279. doi:10.1001/archopht.120.10.1268[↩]
- Mozaffarieh M, Flammer J. New insights in the pathogenesis and treatment of normal tension glaucoma. Curr Opin Pharmacol. 2013 Feb;13(1):43-9. doi: 10.1016/j.coph.2012.10.001[↩]
- Crawford Downs J, Roberts MD, Sigal IA. Glaucomatous cupping of the lamina cribrosa: a review of the evidence for active progressive remodeling as a mechanism. Exp Eye Res. 2011 Aug;93(2):133-40. doi: 10.1016/j.exer.2010.08.004[↩]
- Wostyn P, Killer HE. Normal-Tension Glaucoma: A Glymphopathy? Eye Brain. 2023 Apr 6;15:37-44. doi: 10.2147/EB.S401306[↩]
- Adeghate J, Rahmatnejad K, Waisbourd M, Katz LJ. Intraocular pressure-independent management of normal tension glaucoma. Surv Ophthalmol. 2019 Jan-Feb;64(1):101-110. doi: 10.1016/j.survophthal.2018.08.005[↩]
- Leung DYL, Tham CC. Normal-tension glaucoma: Current concepts and approaches-A review. Clin Exp Ophthalmol. 2022 Mar;50(2):247-259. doi: 10.1111/ceo.14043[↩]
- Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998 Oct;126(4):487-97. doi: 10.1016/s0002-9394(98)00223-2. Erratum in: Am J Ophthalmol 1999 Jan;127(1):120.[↩][↩]
- Holländer R. Der Einfluss der Membranpermeabilität auf die TMPD-Oxydase-Aktivität bei Bakterien [The Influence of the Permeability of the Cell Membrane on TMPD Oxydase Activity (author’s transl)]. Zentralbl Bakteriol Orig A. 1977;237(2-3):351-7. German.[↩]
- Hirooka K, Tenkumo K, Fujiwara A, Baba T, Sato S, Shiraga F. Evaluation of peripapillary choroidal thickness in patients with normal-tension glaucoma. BMC Ophthalmol. 2012 Jul 28;12:29. doi: 10.1186/1471-2415-12-29[↩]
- Firat PG, Ozsoy E, Demirel S, Cumurcu T, Gunduz A. Evaluation of peripapillary retinal nerve fiber layer, macula and ganglion cell thickness in amblyopia using spectral optical coherence tomography. Int J Ophthalmol. 2013;6(1):90-4. doi: 10.3980/j.issn.2222-3959.2013.01.19[↩]
- Zheng Y, Cheung N, Aung T, Mitchell P, He M, Wong TY. Relationship of retinal vascular caliber with retinal nerve fiber layer thickness: the singapore malay eye study. Invest Ophthalmol Vis Sci. 2009 Sep;50(9):4091-6. doi: 10.1167/iovs.09-3444[↩]
- Kim JM, Sae Kim M, Ju Jang H, Ho Park K, Caprioli J. The association between retinal vessel diameter and retinal nerve fiber layer thickness in asymmetric normal tension glaucoma patients. Invest Ophthalmol Vis Sci. 2012 Aug 17;53(9):5609-14. doi: 10.1167/iovs.12-9783[↩]
- Lee JY, Yoo C, Park JH, Kim YY. Retinal vessel diameter in young patients with open-angle glaucoma: comparison between high-tension and normal-tension glaucoma. Acta Ophthalmol. 2012 Nov;90(7):e570-1. doi: 10.1111/j.1755-3768.2011.02371.x[↩]
- Fox AR, Fingert JH. Familial normal tension glaucoma genetics. Prog Retin Eye Res. 2023 Sep;96:101191. doi: 10.1016/j.preteyeres.2023.101191[↩]
- Burk RO, Rohrschneider K, Noack H, Völcker HE. Are large optic nerve heads susceptible to glaucomatous damage at normal intraocular pressure? A three-dimensional study by laser scanning tomography. Graefes Arch Clin Exp Ophthalmol. 1992;230(6):552-60. doi: 10.1007/BF00181778[↩]
- Tuulonen A, Takamoto T, Wu DC, Schwartz B. Optic disk cupping and pallor measurements of patients with a disk hemorrhage. Am J Ophthalmol. 1987 Apr 15;103(4):505-11. doi: 10.1016/s0002-9394(14)74272-2[↩]
- Vogel W, Dietze O, Judmaier G, Then P, Schmid T, Margreiter R. Delayed clearance of HBsAG after transplantation for fulminant delta-hepatitis. Lancet. 1988 Jan 2-9;1(8575-6):52. doi: 10.1016/s0140-6736(88)91025-2[↩]
- Buus DR, Anderson DR. Peripapillary crescents and halos in normal-tension glaucoma and ocular hypertension. Ophthalmology. 1989 Jan;96(1):16-9. doi: 10.1016/s0161-6420(89)32930-7[↩]
- Yamazaki Y, Hayamizu F, Miyamoto S, Nakagami T, Tanaka C, Inui S. Optic disc findings in normal tension glaucoma. Jpn J Ophthalmol. 1997 Jul-Aug;41(4):260-7. doi: 10.1016/s0021-5155(97)00052-x[↩]
- Michels TC, Ivan O. Glaucoma: Diagnosis and Management. Am Fam Physician. 2023 Mar;107(3):253-262. https://www.aafp.org/pubs/afp/issues/2023/0300/glaucoma.html[↩][↩][↩][↩]
- Wolfs RC, Klaver CC, Ramrattan RS, van Duijn CM, Hofman A, de Jong PT. Genetic risk of primary open-angle glaucoma. Population-based familial aggregation study. Arch Ophthalmol. 1998 Dec;116(12):1640-5. doi: 10.1001/archopht.116.12.1640[↩]
- Birhanu G, Tegegne AS. Predictors for elevation of Intraocular Pressure (IOP) on glaucoma patients; a retrospective cohort study design. BMC Ophthalmol. 2022 Jun 7;22(1):254. doi: 10.1186/s12886-022-02431-w[↩][↩][↩]
- Ha A, Kim CY, Shim SR, Chang IB, Kim YK. Degree of Myopia and Glaucoma Risk: A Dose-Response Meta-analysis. Am J Ophthalmol. 2022 Apr;236:107-119. doi: 10.1016/j.ajo.2021.10.007[↩]
- Ikeda Y, Mori K, Maruyama Y, Ueno M, Yoshii K, Yamamoto Y, Imai K, Omi N, Sato R, Sato F, Nakano M, Hamuro J, Tashiro K, Sotozono C, Kinoshita S. Novel Vertical Cup-to-Disc Classification to Identify Normal Eyes That Maintain Non-Glaucoma Status: A 10-Year Longitudinal Study. J Glaucoma. 2023 Feb 1;32(2):127-132. doi: 10.1097/IJG.0000000000002109[↩]
- Lee EJ, Kee HJ, Han JC, Kee C. Evidence-based understanding of disc hemorrhage in glaucoma. Surv Ophthalmol. 2021 May-Jun;66(3):412-422. doi: 10.1016/j.survophthal.2020.09.001[↩]
- Zeppieri M, Brusini P, Miglior S. Corneal thickness and functional damage in patients with ocular hypertension. Eur J Ophthalmol. 2005 Mar-Apr;15(2):196-201. doi: 10.1177/112067210501500203[↩]
- Kim KE, Oh S, Baek SU, Ahn SJ, Park KH, Jeoung JW. Ocular Perfusion Pressure and the Risk of Open-Angle Glaucoma: Systematic Review and Meta-analysis. Sci Rep. 2020 Jun 22;10(1):10056. doi: 10.1038/s41598-020-66914-w[↩]
- Leske MC, Wu SY, Hennis A, Honkanen R, Nemesure B; BESs Study Group. Risk factors for incident open-angle glaucoma: the Barbados Eye Studies. Ophthalmology. 2008 Jan;115(1):85-93. doi: 10.1016/j.ophtha.2007.03.017[↩]
- Prum BE Jr, Rosenberg LF, Gedde SJ, Mansberger SL, Stein JD, Moroi SE, Herndon LW Jr, Lim MC, Williams RD. Primary Open-Angle Glaucoma Preferred Practice Pattern(®) Guidelines. Ophthalmology. 2016 Jan;123(1):P41-P111. doi: 10.1016/j.ophtha.2015.10.053. Epub 2015 Nov 12. Erratum in: Ophthalmology. 2018 Jun;125(6):949. doi: 10.1016/j.ophtha.2018.03.048[↩][↩][↩]
- Li Y, Mitchell W, Elze T, Zebardast N. Association Between Diabetes, Diabetic Retinopathy, and Glaucoma. Curr Diab Rep. 2021 Sep 8;21(10):38. doi: 10.1007/s11892-021-01404-5[↩]
- Matsuda A, Hara T, Miyata K, Matsuo H, Murata H, Mayama C, Asaoka R. Do pattern deviation values accurately estimate glaucomatous visual field damage in eyes with glaucoma and cataract? Br J Ophthalmol. 2015 Sep;99(9):1240-4. doi: 10.1136/bjophthalmol-2014-306019[↩]
- Gramer G, Weber BH, Gramer E. Migraine and Vasospasm in Glaucoma: Age-Related Evaluation of 2027 Patients With Glaucoma or Ocular Hypertension. Invest Ophthalmol Vis Sci. 2015 Dec;56(13):7999-8007. doi: 10.1167/iovs.15-17274[↩]
- Baneke AJ, Aubry J, Viswanathan AC, Plant GT. The role of intracranial pressure in glaucoma and therapeutic implications. Eye (Lond). 2020 Jan;34(1):178-191. doi: 10.1038/s41433-019-0681-y[↩]
- Hogden K, Mikelberg F, Sodhi M, Khosrow-Khavar F, Mansournia MA, Kezouh A, Etminan M. The association between hormonal contraceptive use and glaucoma in women of reproductive age. Br J Clin Pharmacol. 2021 Dec;87(12):4780-4785. doi: 10.1111/bcp.14920[↩]
- Moreno-Montañés J, Gándara E, Gutierrez-Ruiz I, Moreno-Galarraga L, Ruiz-Canela M, Bes-Rastrollo M, Martínez-González MÁ, Fernandez-Montero A. Healthy Lifestyle Score and Incidence of Glaucoma: The Sun Project. Nutrients. 2022 Feb 12;14(4):779. doi: 10.3390/nu14040779[↩]
- Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK 2nd, Wilson MR, Gordon MO. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002 Jun;120(6):701-13; discussion 829-30. doi: 10.1001/archopht.120.6.701[↩]
- Mokhles P, Schouten JS, Beckers HJ, Azuara-Blanco A, Tuulonen A, Webers CA. Glaucoma blindness at the end of life. Acta Ophthalmol. 2017 Feb;95(1):10-11. doi: 10.1111/aos.12933[↩]
- Mahmoudinezhad G, Moghimi S, Proudfoot JA, Brye N, Nishida T, Yarmohammadi A, Kamalipour A, Zangwill LM, Weinreb RN. Effect of Testing Frequency on the Time to Detect Glaucoma Progression With Optical Coherence Tomography (OCT) and OCT Angiography. Am J Ophthalmol. 2023 Jan;245:184-192. doi: 10.1016/j.ajo.2022.08.030[↩]
- Bhat KS, Reddy MV, Pai V. Correlation of retinal nerve fiber layer thickness with perimetric staging in primary open-angle glaucoma – A cross-sectional study. Oman J Ophthalmol. 2022 Mar 2;15(1):36-42. doi: 10.4103/ojo.ojo_345_20[↩]
- Kansal V, Armstrong JJ, Pintwala R, Hutnik C. Optical coherence tomography for glaucoma diagnosis: An evidence based meta-analysis. PLoS One. 2018 Jan 4;13(1):e0190621. doi: 10.1371/journal.pone.0190621[↩]
- What Is Fluorescein Angiography? https://www.aao.org/eye-health/treatments/what-is-fluorescein-angiography[↩]
- Tripathy K, Sharma YR, Chawla R, Basu K, Vohra R, Venkatesh P. Triads in Ophthalmology: A Comprehensive Review. Semin Ophthalmol. 2017;32(2):237-250. doi: 10.3109/08820538.2015.1045150[↩]
- Chapter 3. The Glaucoma Eye. https://eyerounds.org/books/glaucoma_guide/chapter3.html#gsc.tab=0[↩]
- Harizman N, Oliveira C, Chiang A, et al. The ISNT Rule and Differentiation of Normal From Glaucomatous Eyes. Arch Ophthalmol. 2006;124(11):1579–1583. doi:10.1001/archopht.124.11.1579[↩][↩][↩][↩]
- Maupin E, Baudin F, Arnould L, Seydou A, Binquet C, Bron AM, Creuzot-Garcher CP. Accuracy of the ISNT rule and its variants for differentiating glaucomatous from normal eyes in a population-based study. Br J Ophthalmol. 2020 Oct;104(10):1412-1417. doi: 10.1136/bjophthalmol-2019-315554[↩]
- Hollands H, Johnson D, Hollands S, Simel DL, Jinapriya D, Sharma S. Do findings on routine examination identify patients at risk for primary open-angle glaucoma? The rational clinical examination systematic review. JAMA. 2013 May 15;309(19):2035-42. doi: 10.1001/jama.2013.5099[↩]
- Susanna R Jr, Vessani RM. Staging glaucoma patient: why and how? Open Ophthalmol J. 2009 Sep 17;3:59-64. doi: 10.2174/1874364100903020059[↩][↩]
- Kalyani VK, Bharucha KM, Goyal N, Deshpande MM. Comparison of diagnostic ability of standard automated perimetry, short wavelength automated perimetry, retinal nerve fiber layer thickness analysis and ganglion cell layer thickness analysis in early detection of glaucoma. Indian J Ophthalmol. 2021 May;69(5):1108-1112. doi: 10.4103/ijo.IJO_2409_20[↩]
- Zeppieri M, Brusini P, Parisi L, Johnson CA, Sampaolesi R, Salvetat ML. Pulsar perimetry in the diagnosis of early glaucoma. Am J Ophthalmol. 2010 Jan;149(1):102-12. doi: 10.1016/j.ajo.2009.07.020[↩]
- Salvetat ML, Zeppieri M, Parisi L, Johnson CA, Sampaolesi R, Brusini P. Learning effect and test-retest variability of pulsar perimetry. J Glaucoma. 2013 Mar;22(3):230-7. doi: 10.1097/IJG.0b013e318237bfe7[↩]
- Salvetat ML, Zeppieri M, Parisi L, Brusini P. Rarebit perimetry in normal subjects: test-retest variability, learning effect, normative range, influence of optical defocus, and cataract extraction. Invest Ophthalmol Vis Sci. 2007 Nov;48(11):5320-31. doi: 10.1167/iovs.06-1495[↩]
- Brusini P, Salvetat ML, Parisi L, Zeppieri M. Probing glaucoma visual damage by rarebit perimetry. Br J Ophthalmol. 2005 Feb;89(2):180-4. doi: 10.1136/bjo.2003.041178[↩]
- Brusini P, Salvetat ML, Zeppieri M, Tosoni C, Parisi L, Felletti M. Visual field testing with the new Humphrey Matrix: a comparison between the FDT N-30 and Matrix N-30-F tests. Acta Ophthalmol Scand. 2006 Jun;84(3):351-6. doi: 10.1111/j.1600-0420.2006.00637.x[↩]
- Mastropasqua L, Brusini P, Carpineto P, Ciancaglini M, Di Antonio L, Zeppieri MW, Parisi L. Humphrey matrix frequency doubling technology perimetry and optical coherence tomography measurement of the retinal nerve fiber layer thickness in both normal and ocular hypertensive subjects. J Glaucoma. 2006 Aug;15(4):328-35. doi: 10.1097/01.ijg.0000212230.65545.d3[↩]
- Harwerth RS, Quigley HA. Visual field defects and retinal ganglion cell losses in patients with glaucoma. Arch Ophthalmol. 2006 Jun;124(6):853-9. doi: 10.1001/archopht.124.6.853[↩]
- Harwerth RS, Carter-Dawson L, Shen F, Smith EL 3rd, Crawford ML. Ganglion cell losses underlying visual field defects from experimental glaucoma. Invest Ophthalmol Vis Sci. 1999 Sep;40(10):2242-50.[↩]
- Shiga Y, Aizawa N, Tsuda S, Yokoyama Y, Omodaka K, Kunikata H, Yasui T, Kato K, Kurashima H, Miyamoto E, Hashimoto M, Nakazawa T. Preperimetric Glaucoma Prospective Study (PPGPS): Predicting Visual Field Progression With Basal Optic Nerve Head Blood Flow in Normotensive PPG Eyes. Transl Vis Sci Technol. 2018 Jan 23;7(1):11. doi: 10.1167/tvst.7.1.11[↩]
- Brusini P, Salvetat ML, Zeppieri M. How to Measure Intraocular Pressure: An Updated Review of Various Tonometers. J Clin Med. 2021 Aug 27;10(17):3860. doi: 10.3390/jcm10173860[↩]
- Bader J, Zeppieri M, Havens SJ. Tonometry. [Updated 2023 Dec 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK493225[↩]
- Zeppieri M, Gurnani B. Applanation Tonometry. [Updated 2023 Jun 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK582132[↩]
- Campbell P, Edgar DF, Shah R. Inter-optometrist variability of IOP measurement for modern tonometers and their agreement with Goldmann Applanation Tonometry. Clin Exp Optom. 2021 Jul;104(5):602-610. doi: 10.1080/08164622.2021.1878831[↩]
- Biswas S, Biswas P. Relationship between Diurnal Variation in Intraocular Pressure and Central Corneal Power. Optom Vis Sci. 2023 Jan 1;100(1):96-104. doi: 10.1097/OPX.0000000000001974[↩]
- Belovay GW, Goldberg I. The thick and thin of the central corneal thickness in glaucoma. Eye (Lond). 2018 May;32(5):915-923. doi: 10.1038/s41433-018-0033-3[↩]
- Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002 Feb;86(2):238-42. doi: 10.1136/bjo.86.2.238[↩]
- Gil P, Pires J, Matos R, Cardoso MS, Lopes N, Matias J, Mariano M. Corneal Elevation Topography in Primary Open Angle Glaucoma. J Glaucoma. 2017 Feb;26(2):e41-e45. doi: 10.1097/IJG.0000000000000535[↩]
- Primary Open-Angle Glaucoma Preferred Practice Pattern® Guidelines. Prum, Bruce E. et al. Ophthalmology, Volume 123, Issue 1, P41 – P111. https://www.aaojournal.org/article/S0161-6420(15)01276-2/fulltext[↩][↩]
- Brusini P, Zeppieri M, Tosoni C, Parisi L, Salvetat ML. Optic disc damage staging system. J Glaucoma. 2010 Sep;19(7):442-9. doi: 10.1097/IJG.0b013e3181ca7303[↩]
- Spaeth GL, Shields MB. The stages of glaucoma. Am J Ophthalmol. 2006 Jan;141(1):147-8. doi: 10.1016/j.ajo.2005.08.026[↩]
- Brusini P. Estimating glaucomatous anatomical damage by computerized automated perimetry. Acta Ophthalmol Scand Suppl. 1997;(224):28-9. doi: 10.1111/j.1600-0420.1997.tb00461.x[↩]
- Spaeth GL, Lopes JF, Junk AK, Grigorian AP, Henderer J. Systems for staging the amount of optic nerve damage in glaucoma: a critical review and new material. Surv Ophthalmol. 2006 Jul-Aug;51(4):293-315. doi: 10.1016/j.survophthal.2006.04.008[↩]
- Brusini P. OCT Glaucoma Staging System: a new method for retinal nerve fiber layer damage classification using spectral-domain OCT. Eye (Lond). 2018 Jan;32(1):113-119. doi: 10.1038/eye.2017.159[↩]
- Ng M, Sample PA, Pascual JP, Zangwill LM, Girkin CA, Liebmann JM, Weinreb RN, Racette L. Comparison of visual field severity classification systems for glaucoma. J Glaucoma. 2012 Oct-Nov;21(8):551-61. doi: 10.1097/IJG.0b013e31821dac66[↩]
- Sihota R, Angmo D, Ramaswamy D, Dada T. Simplifying “target” intraocular pressure for different stages of primary open-angle glaucoma and primary angle-closure glaucoma. Indian J Ophthalmol. 2018 Apr;66(4):495-505. doi: 10.4103/ijo.IJO_1130_17[↩]
- Medical Management of Glaucoma. https://morancore.utah.edu/section-10-glaucoma/medical-management-of-glaucoma[↩]
- Wilson MR. Progression of visual field loss in untreated glaucoma patients and suspects in St Lucia, West Indies. Trans Am Ophthalmol Soc. 2002;100:365-410. https://pmc.ncbi.nlm.nih.gov/articles/instance/1358971/pdf/12545702.pdf[↩]