What is Aicardi syndrome
Aicardi syndrome is an extremely rare genetic neurological disorder that occurs almost exclusively in female babies, first described by the French neurologist, Dr. Jean Aicardi, in 1965 1. Aicardi syndrome occurs in about 1 in 105,000 to 167,000 newborns in the United States, there are currently about 1,000 cases in the United States. Researchers estimate that there are approximately 4,000 affected individuals worldwide 2. The severity of Aicardi syndrome and the associated signs and symptoms vary from person to person. The three main features of Aicardi syndrome that occur together in most affected individuals are 3:
- Complete or partial absence of the nerve tissue that allows the right and left sides of the brain to communicate (corpus callosum)
- Seizures beginning in infancy (infantile spasms), that may become hard to control (refractory epilepsy)
- Defects or holes in the light sensitive tissue at the back of the eye (retina) known as chorioretinal lacunae
Other signs and symptoms may include 4:
- Developmental delay
- Intellectual disability that ranges from very mild to severe
- Characteristic facial features, such as a short distance between the nose and lips, a flat nose, large ears and thin eyebrows.
- Other brain malformations such as a very small head (microcephaly)
- Other eye defects, such as very small eyes (microphthalmia) or a defect of the nerve connecting the retina to the brain (optic nerve) known as coloboma.
People with Aicardi syndrome have absent or underdeveloped tissue connecting the left and right halves of the brain (agenesis or dysgenesis of the corpus callosum) 2. They have seizures beginning in infancy (infantile spasms), which tend to progress to recurrent seizures (epilepsy) that can be difficult to treat. Affected individuals also have chorioretinal lacunae, which are defects in the light-sensitive tissue at the back of the eye (retina).
People with Aicardi syndrome often have additional brain abnormalities, including asymmetry between the two sides of the brain, brain folds and grooves that are small in size or reduced in number, cysts, and enlargement of the fluid-filled cavities (ventricles) near the center of the brain. Some have an unusually small head (microcephaly). Most affected individuals have moderate to severe developmental delay and intellectual disability, although some people with this disorder have milder disability.
In addition to chorioretinal lacunae, people with Aicardi syndrome may have other eye abnormalities such as small or poorly developed eyes (microphthalmia) or a gap or hole (coloboma) in the optic nerve, a structure that carries information from the eye to the brain. These eye abnormalities may cause blindness in affected individuals.
Some people with Aicardi syndrome have unusual facial features including a short area between the upper lip and the nose (philtrum), a flat nose with an upturned tip, large ears, and sparse eyebrows. Other features of this condition include small hands, hand malformations, and spinal and rib abnormalities leading to progressive abnormal curvature of the spine (scoliosis). They often have gastrointestinal problems such as constipation or diarrhea, gastroesophageal reflux, and difficulty feeding.
The severity of Aicardi syndrome varies. Some people with this disorder have very severe epilepsy and may not survive past childhood. Less severely affected individuals may live into adulthood with milder signs and symptoms.
There is no cure for Aicardi syndrome nor is there a standard course of treatment. Treatment generally involves medical management of seizures and programs to help parents and children cope with developmental delays. Long-term management by a pediatric neurologist with expertise in the management of infantile spasms is recommended.
What is Aicardi Goutieres syndrome?
Aicardi-Goutieres syndrome also called familial infantile encephalopathy with calcification of basal ganglia and chronic cerebrospinal fluid lymphocytosis, is an inherited disorder that mainly affects the brain, the immune system and the skin, but can also involve the joints, liver, kidney, blood system, lung, and thyroid 5, 6, 7, 8, 9, 10, 11, 12, 13. Most newborns with Aicardi-Goutieres syndrome do not show any signs or symptoms of the disorder. However, about 20 percent are born with a combination of features that include an enlarged liver and spleen (hepatosplenomegaly), elevated blood levels of liver enzymes, a shortage of blood cells called platelets that are needed for normal blood clotting (thrombocytopenia), and neurological abnormalities. While this combination of signs and symptoms is typically associated with the immune system’s response to a viral infection that is present at birth (congenital), no actual infection is found in these infants. For this reason, Aicardi-Goutieres syndrome is sometimes referred to as a “mimic of congenital infection”. Aicardi-Goutieres syndrome is a rare disorder. More than 500 people with Aicardi-Goutières syndrome have been described in the scientific literature, though the exact prevalence of the condition is unknown 8. Studies suggest that Aicardi Goutieres syndrome is one of the most common genetic disorders affecting the white matter of the brain 10.
There are several types of Aicardi-Goutieres syndrome, depending on the gene that causes the condition: TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR1, IFIH1, LSM11, and RNU7-1 14, 15, 16, 17, 18, 19, 20, 21, 22. Mutations in TREX1, RNASEH2B, RNASEH2C, RNASEH2A, SAMHD1, ADAR, IFIH1, LSM11, and RNU7‐1 genes can explain about 95% of patients with Aicardi-Goutieres syndrome 23, 6. All genes are involved in nucleic acid (a large biomolecules that carry information in cells and make up genetic material) metabolism signaling pathways, with mutations leading to persistent activation of type 1 interferon signaling. The term “type 1 interferonopathies” was proposed in 2011 to describe a group of monogenic phenotypes associated with a pathological upregulation of type 1 interferon signaling, including Aicardi-Goutieres syndrome 24. Most Aicardi-Goutieres syndrome cases are inherited in an autosomal recessive pattern, although rare autosomal dominant cases have been reported.
Aicardi-Goutières syndrome is often divided into 2 types, which are distinguished by the severity of features and the age at which they begin:
- Early-onset Aicardi-Goutières syndrome also called the Classic Aicardi-Goutières syndrome are the most common and are often associated with severe disease and poor prognosis 25.
- Individuals with the early-onset form of Aicardi-Goutières syndrome can experience severe brain dysfunction (encephalopathy) within the first months of life that usually results in severe intellectual and physical disability 11. This encephalopathic phase of the disorder can last for weeks or months. Affected infants stop developing new skills and begin losing skills they had already acquired (developmental regression). Infants with early-onset Aicardi-Goutières syndrome can have seizures. Medical imaging reveals loss of white matter in the brain (leukodystrophy) 26. White matter consists of nerve cells covered by myelin, which is a substance that protects nerves and allows them to rapidly transmit nerve impulses. Growth of the brain and skull slows down, resulting in an abnormally small head size (microcephaly). Affected individuals may have abnormal deposits of calcium (calcification) in the brain. As a result of this neurological damage, most people with Aicardi-Goutières syndrome have profound intellectual disabilities, developmental delay, developmental regression, dystonia, epilepsy and sleeping disorder. Additional symptoms may include epilepsy, painful itchy skin lesion (chilblains), vision problems, and joint and muscle stiffness (spasticity), involuntary muscle twisting and contractions (dystonia), and weak muscle tone (hypotonia) in the torso. Other signs and symptoms may include a very small head (microcephaly), presence of white blood cells and other sign of inflammation in the cerebrospinal fluid, which is the fluid that surrounds the brain and spinal cord (central nervous system). Symptoms usually progress over several months before the disease course stabilizes.
- Affected babies are usually extremely irritable and do not feed well. They also have muscle stiffness (spasticity), involuntary tensing of various muscles (dystonia), and weak muscle tone (hypotonia). They can have vision problems including vision loss and increased pressure in the eye (glaucoma).
- Some newborns have a combination of features that include an enlarged liver and spleen (hepatosplenomegaly), elevated blood levels of liver enzymes, and a shortage of blood cells called platelets that are needed for normal blood clotting (thrombocytopenia). They may develop intermittent fevers in the absence of infection (sterile pyrexias). While this combination of signs and symptoms is typically associated with the immune system’s response to a viral infection that is present at birth (congenital), no actual infection is found in these infants. For this reason, Aicardi-Goutières syndrome is sometimes referred to as a “mimic of congenital infection”.
- In some affected newborns, white blood cells, interferon proteins, and other immune system molecules can be detected in the cerebrospinal fluid, which is the fluid that surrounds the brain and spinal cord (central nervous system). These findings are consistent with inflammation and tissue damage in the central nervous system (brain and spinal cord).
- About 40 percent of people with the early-onset form of Aicardi-Goutières syndrome develop a skin problem called chilblains. Chilblains are painful, itchy skin lesions that are puffy and red, and they usually appear on the fingers, toes, nose, and ears. They are caused by inflammation of small blood vessels and may be brought on or made worse by exposure to cold temperatures.
- In about 20 percent of cases, the early-onset form of Aicardi-Goutières syndrome begins prenatally. Slow growth (intrauterine growth retardation) and brain abnormalities, especially brain calcification, may be seen on ultrasound imaging. These individuals have the most severe neurological problems and the highest risk for early death.
- As a result of the severe neurological problems usually associated with Aicardi-Goutieres syndrome, most people with early-onset form of Aicardi-Goutières syndrome do not survive past childhood. However, some affected individuals who develop Aicardi-Goutieres syndrome later or have milder neurological problems live into into adolescence or adulthood.
- Later-onset Aicardi-Goutières syndrome.
- People with the later-onset form of Aicardi-Goutières syndrome typically have normal development in infancy. In these individuals, severe brain dysfunction (encephalopathy) typically occurs after 1 year of age. Similar to those with the early-onset form of Aicardi-Goutières syndrome, babies with the later-onset form of Aicardi-Goutières syndrome experience irritability, poor feeding, and sterile pyrexias (fever or elevated body temperature that occurs in the absence of an infection). Over time, affected individuals show developmental delays and regression. They may also have joint and muscle stiffness (spasticity) and weak muscle tone (hypotonia), and the growth of the brain and head may slow leading to microcephaly (very small head). The health and developmental problems in people with the later-onset form of Aicardi-Goutières syndrome are typically not as severe as those in individuals with the early-onset form of Aicardi-Goutières syndrome, though the severity can vary among affected individuals.
- Because the signs and symptoms of Aicardi-Goutières syndrome are caused in part by abnormal activation of interferon proteins, Aicardi-Goutières syndrome is also characterized as an “interferonopathy”, meaning that it is a disease related to dysregulated or dysfunctional interferons 27.
Within the first year of life, most individuals with Aicardi-Goutieres syndrome experience an episode of severe brain dysfunction (encephalopathy), typically lasting for several months. During this encephalopathic phase of the disorder, affected babies are usually extremely irritable and do not feed well. They may develop intermittent fevers in the absence of infection (sterile pyrexias) and may have seizures. They stop developing new skills and begin losing skills they had already acquired (developmental regression). Growth of the brain and skull slows down, resulting in an abnormally small head size (microcephaly). In this phase of the disorder, white blood cells and other immune system molecules associated with inflammation can be detected in the cerebrospinal fluid, which is the fluid that surrounds the brain and spinal cord (central nervous system). These abnormal findings are consistent with inflammation and tissue damage in the central nervous system.
The encephalopathic phase of Aicardi-Goutieres syndrome causes permanent neurological damage that is usually severe. Medical imaging reveals loss of white matter in the brain (leukodystrophy). White matter consists of nerve fibers covered by myelin, which is a substance that protects nerves and insures rapid transmission of nerve impulses. Affected individuals also have abnormal deposits of calcium (calcification) in the brain. As a result of this neurological damage, most people with Aicardi-Goutieres syndrome have profound intellectual disability. They also have muscle stiffness (spasticity); involuntary tensing of various muscles (dystonia), especially those in the arms; and weak muscle tone (hypotonia) in the torso.
Some people with Aicardi-Goutieres syndrome have features characteristic of autoimmune disorders, which occur when the immune system malfunctions and attacks the body’s own systems and organs. Some of these features overlap with those of another disorder called systemic lupus erythematosus (SLE). A feature of SLE that also occurs in about 40 percent of people with Aicardi-Goutieres syndrome is a skin problem called chilblains. Chilblains are painful, itchy skin lesions that are puffy and red, and usually appear on the fingers, toes, and ears. They are caused by inflammation of small blood vessels, and may be brought on or made worse by exposure to cold. Vision problems, joint stiffness, and mouth ulcers are other features that can occur in both disorders.
To diagnose Aicardi Goutieres syndrome, a doctor will do a physical exam and review the person’s medical history, including any symptoms they are experiencing. Diagnostic tests for Aicardi Goutieres syndrome may include the following:
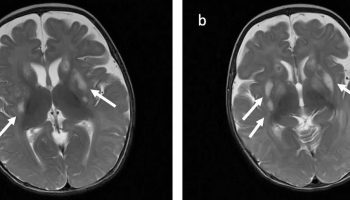
- MRI (magnetic resonance imaging) is key to diagnosing Aicardi Goutieres syndrome, as it can detect shrinking or small areas of the brain and calcium buildup (calcification) in the brain.
- Cerebrospinal fluid (CSF) testing can show an increase in immune system activity that appears in Aicardi Goutieres syndrome. This test is not used alone to diagnose Aicardi Goutieres syndrome, but if results are positive, a doctor can look for other signs of the disease.
- Genetic testing from a blood sample can show changes in one of the genes associated with Aicardi Goutieres syndrome. Results from this test along with others can help diagnose a specific type of Aicardi Goutieres syndrome.
Aicardi Goutieres syndrome cannot be cured currently, and treatments primarily is symptomatic and supportive aiming to manage systemic and organ inflammation to reduce the progression of organ damage 13. This means that you can treat the symptoms, but there is no cure for the disease 28. The treatment for Aicardi Goutieres syndrome mainly targets type 1 interferon signaling pathways 29, 30.
Aicardi Goutieres syndrome prognosis depends mainly on the severity neurologic problems and in the age of onset of these problems 31.
Aicardi syndrome prognosis
The prognosis for girls with Aicardi syndrome varies according to the severity of their symptoms 2. Some people with Aicardi syndrome have very severe epilepsy and may not survive past childhood and adolescence. Less severely affected individuals may live into adulthood with milder signs and symptoms have been described.
Development and Long Term Outcomes
Most affected individuals have moderate to severe developmental and intellectual disability, although some children with Aicardi syndrome have a more mild form of disability. The severity of Aicardi syndrome can vary greatly. Affected children should enter the early intervention system as soon as possible although it is not clear how much of the disabilities can be attenuated through therapies or are pre-determined. Many children with Aicardi will learn to sit independently and feed themselves and many are able to walk. Some children can speak in short sentences but complex oral language is the exception. The majority of children with Aicardi syndrome communicate with gestures, sounds and other nonverbal means and receptive communication is more advanced than expressive. Chronic developmental regressions have been documented, mostly due to a change or increase in seizures.
Aicardi syndrome life expectancy
Aicardi goutieres syndrome life expectancy is highly variable depending on the number and severity of features present in a child with Aicardi syndrome.
Survival is highly variable and likely depends on the severity of seizures and other organ system involvement, particularly conditions that affect the respiratory system. Median survival from birth is more than 30 years, and as children age, their chance of survival increases. For example, the probability of surviving another 5 years is over 85% for an affected person that is 25 years old. The ages of highest mortality risk are in the first few years of life and in adolescence. Common causes of death include respiratory failure, systemic infections with difficult to treat pathogens, and sudden unexpected death in epilepsy (SUDEP).
Aicardi syndrome signs and symptoms
Aicardi syndrome signs and symptoms (are not present in all Aicardi syndrome cases):
- Vascular malformations or vascular malignancy;
- Microcephaly;
- Hypotonia;
- Spasticity or hypertonia;
- Scoliosis;
- Prominent premaxilla;
- Cleft lip or palate;
- Gastroesophageal reflux;
- Feeding problems;
- Small or malformed hands;
- Precocious or delayed puberty; and/or
- Global developmental disabilities.
Aicardi syndrome typically begins as involuntary muscle spasms between four months and four years of age. Other symptoms may include epilepsy, intellectual disability, profound muscle weakness (hypotonia), an abnormally small head (microcephaly), abnormally small eyes (microphthalmia), prominent premaxilla, upturned nasal tip, decreased angle of the nasal bridge, and sparse lateral eyebrows, an incomplete development of the retina and nerve in the back of the eye (colobomas), and/or abnormalities of the ribs and/or spinal column with marked scoliosis present in up to one third of affected individuals. Periventricular heterotopias (nerve cells migration disorder in the brain during early fetal development characterized by the presence of clumps of neurons near the brain’s ventricles), microgyria (poorly formed cerebral cortex), enlarged ventricles or porencephalic cysts, axial hypotonia, and appendicular hypertonia with spasticity may occur. Epilepsy that is refractory to medication develops over time, with a variety of seizure types (infantile spasms that can be asymmetric or unilateral, partial seizures) and ‘split’ brain electroencephalogram (EEG).
Children of all ages with Aicardi syndrome have significant delay in motor development and intellectual deficit. Aicardi syndrome can be life-threatening during childhood due to complications from upper respiratory infections.
Gastrointestinal disorders, vascular malformations, small hands, pigmentary skin lesions, and an increased incidence of tumors are rare.
Seizures
Seizures usually begin in the first few months of life, but may occur in utero or immediately at birth. The seizures are difficult to treat and refractory epilepsy is almost universal. Seizure onset almost always begins with infantile spasms which eventually evolve into a number of other difficult to control seizure types. A pediatric neurologist with expertise in the management of infantile spasms and medically refractory epilepsy is important for the long-term management of the seizures. Finding a balance between minimizing the frequency and severity of seizures, the adverse effects of the anti-epileptic drugs, and optimizing the quality of life for child and family is essential.
Aicardi syndrome causes
The cause of Aicardi syndrome is unknown. Because Aicardi syndrome occurs almost exclusively in females, researchers believe that it is probably the result of a mutation in a gene on the X chromosome. People normally have 46 chromosomes in each cell. Two of the 46 chromosomes, known as X and Y, are called sex chromosomes because they help determine whether a person will develop male or female sex characteristics. Genes on these chromosomes are also involved in other functions in the body. Females typically have two X chromosomes (46,XX), and males have one X chromosome and one Y chromosome (46,XY).
Early in embryonic development in females, one of the two X chromosomes is permanently inactivated in somatic cells (cells other than egg and sperm cells). X-inactivation ensures that females, like males, have only one active copy of the X chromosome in each body cell. Usually X-inactivation occurs randomly, so that each X chromosome is active in about half the body’s cells. Sometimes X-inactivation is not random, and one X chromosome is active in more than half of cells. When X-inactivation does not occur randomly, it is called skewed X-inactivation.
Skewed X-inactivation sometimes occurs when there is a severe gene mutation in one of the X chromosomes in each cell. Because the cells where this chromosome is active will not be able to survive as well, X-inactivation will appear to be skewed. Skewed X-inactivation has been identified in girls with Aicardi syndrome, further supporting the idea that the disorder is caused by a mutation in a gene on the X chromosome. However, this gene has not been identified, and it is unknown how the genetic change that causes Aicardi syndrome results in the various signs and symptoms of this disorder.
Inheritance pattern
Nearly all known cases of Aicardi syndrome are sporadic, which means that they are not passed down through generations and occur in people with no history of the disorder in their family. Aicardi syndrome is believed to result from new gene mutations.
Aicardi syndrome is classified as an X-linked dominant condition. While the gene associated with this disorder is not known, it is believed to be located on the X chromosome. In females (who have two X chromosomes), a mutation in one of the two copies of the gene in each cell is sufficient to cause the disorder. In males (who have only one X chromosome), a mutation in the only copy of the gene in each cell is nearly always lethal very early in development, so almost all babies with Aicardi syndrome are female. However, a few affected males with an extra copy of the X chromosome in each cell (47,XXY) have been identified. Males with a 47,XXY chromosome pattern also have a condition called Klinefelter syndrome.
Aicardi syndrome diagnosis
It is usual to have an magnetic resonance imaging (MRI) of the brain. This study makes pictures of the brain to look for a small or missing corpus callosum and other problems with the formation of the brain. Individuals with Aicardi syndrome should have a test to look at the brain waves (EEG) to diagnose and treat seizures. An ophthalmologist should look into the eyes at the retina. In Aicardi syndrome, this almost always reveals small cream-colored cavities (lucunae) within the retina.
Aicardi syndrome is classically defined in over 90% of cases by three cardinal features 32:
- Agenesis of the corpus callosum;
- Chorioretinal lacunae; and
- Infantile spasms.
In 1999, the diagnostic spectrum of Aicardi syndrome was broadened to include patients with present, but usually abnormal, corpus callosum or absence of infantile spasms or lacunae, if other typical brain abnormalities are present. Specifically, the revised criteria were expanded to include two classic features plus at least two other major or supporting features. Retinal lacunae and seizures are present in all, or almost all, of the cases. Major and supporting features include:
Major Features
- Cortical malformations (mostly polymicrogyria);
- Periventricular and subcortical heterotopia;
- Cysts around third cerebral ventricle and/or choroid plexus;
- Papillomas of choroid plexuses; and/or
- Optic disc/nerve coloboma.
Supporting Features
- Vertebral and costal abnormalities;
- Microphthalmia or other eye abnormalities;
- “Split-brain” EEG; and/or
- Gross cerebral hemispheric asymmetry.
Aicardi syndrome treatment
Medications may be used to suppress the seizures caused by Aicardi syndrome. The seizures are often hard to treat. The doctor may need to try a number of medicines to see which medication works best. Studies have shown that there is not one medicine that works for everyone with Aicardi syndrome.
The treatment for Aicardi syndrome is based on the clinical and developmental manifestations and may include a number of medical and therapeutic specialties such as pediatric neurology or epileptology, neurosurgery, ophthalmology, orthopedics, gastroenterology, physical therapy, speech therapy and occupational therapy. Treatment of seizures is initiated at the time of onset and often includes ACTH and vigabatrin for the treatment of infantile spasms. As refractory epilepsy continues, placement of a vagus nerve stimulator or brain surgery is often considered. Most children are not candidates for brain surgery due to multiple areas of epileptogenesis. Brain surgery, such as hemispherectomy or cortical resection, has been performed in many children with Aicardi syndrome although virtually all continue to have seizures. However, surgery may have provided better seizure control or a temporary period of seizure-freedom, allowing progress in development and/or improved quality of life.
- Aicardi J, Lefebvre J, Lerique-Koechlin A. A new syndrome: spasm in flexion, callosal agenesis, ocular abnormalities. Electroencephalogr Clin Neurophysiol. 1965. 19:609-10.[↩]
- Aicardi syndrome. https://ghr.nlm.nih.gov/condition/aicardi-syndrome[↩][↩][↩]
- Aicardi Syndrome. https://emedicine.medscape.com/article/941426-overview[↩]
- Sutton VR, Van den Veyver IB. Aicardi Syndrome. 2006 Jun 30 [Updated 2014 Nov 6]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2018. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1381[↩]
- Aicardi J, Goutières F. A progressive familial encephalopathy in infancy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis. Ann Neurol. 1984;15:49‐54. DOI: 10.1002/ana.410150109[↩]
- Crow YJ, Chase DS, Lowenstein Schmidt J, Szynkiewicz M, Forte GM, Gornall HL, et al. Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1 . Am J Med Genet A. 2015;167A:296‐312. DOI: 10.1002/ajmg.a.36887[↩][↩]
- Crow YJ, Manel N. Aicardi‐Goutières syndrome and the type I interferonopathies. Nat Rev Immunol. 2015;15:429‐440. DOI: 10.1038/nri3850[↩]
- Aicardi-Goutières syndrome. https://medlineplus.gov/genetics/condition/aicardi-goutieres-syndrome[↩][↩]
- Aicardi-Goutières Syndrome. https://www.ninds.nih.gov/health-information/disorders/aicardi-goutieres-syndrome[↩]
- Aicardi-Goutières Syndrome. https://rarediseases.org/rare-diseases/aicardi-goutieres-syndrome/[↩][↩]
- Crow YJ. Aicardi-Goutières Syndrome. 2005 Jun 29 [Updated 2016 Nov 22]. In: Adam MP, Feldman J, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2025. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1475[↩][↩]
- Aicardi-Goutieres syndrome. https://ghr.nlm.nih.gov/condition/aicardi-goutieres-syndrome[↩]
- Dell’Isola GB, Dini G, Culpepper KL, Portwood KE, Ferrara P, Di Cara G, Verrotti A, Lodolo M. Clinical spectrum and currently available treatment of type I interferonopathy Aicardi-Goutières syndrome. World J Pediatr. 2023 Jul;19(7):635-643. doi: 10.1007/s12519-022-00679-2[↩][↩]
- Aicardi J, Goutières F. A Progressive familial encephalopathy in infancy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis. Ann Neurol. 1984;15:49–54. doi: 10.1002/ana.410150109[↩]
- Crow YJ, Chase DS, Lowenstein Schmidt J, Szynkiewicz M, Forte GMA, Gornall HL, et al. Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1. Am J Med Genet Part A. 2015;167A:296–312. doi: 10.1002/ajmg.a.36887[↩]
- Crow YJ, Hayward BE, Parmar R, Robins P, Leitch A, Ali M, et al. Mutations in the gene encoding the 3’‐5′ DNA exonuclease TREX1 cause Aicardi‐Goutières syndrome at the AGS1 locus. Nat Genet. 2006;38:917‐920. DOI: 10.1038/ng1845[↩]
- Crow YJ, Leitch A, Hayward BE, Garner A, Parmar R, Griffith E, et al. Mutations in genes encoding ribonuclease H2 subunits cause Aicardi‐Goutières syndrome and mimic congenital viral brain infection. Nat Genet. 2006;38:910‐916. DOI: 10.1038/ng1842[↩]
- Rice GI, Bond J, Asipu A, Brunette RL, Manfield IW, Carr IM, et al. Mutations involved in Aicardi‐Goutières syndrome implicate SAMHD1 as regulator of the innate immune response. Nat Genet. 2009;41:829‐832. DOI: 10.1038/ng.373[↩]
- Rice GI, Kasher PR, Forte GM, Mannion NM, Greenwood SM, Szynkiewicz M, et al. Mutations in ADAR1 cause Aicardi‐Goutières syndrome associated with a type I interferon signature. Nat Genet. 2012;44:1243‐1248. DOI: 10.1038/ng.2414[↩]
- Rice GI, Del Toro Duany Y, Jenkinson EM, Forte GM, Anderson BH, Ariaudo G, et al. Gain‐of‐function mutations in IFIH1 cause a spectrum of human disease phenotypes associated with upregulated type I interferon signaling. Nat Genet. 2014;46:503‐509. DOI: 10.1038/ng.2933[↩]
- Uggenti C, Lepelley A, Depp M, Badrock AP, Rodero MP, El‐Daher MT, et al. cGAS‐mediated induction of type I interferon due to inborn errors of histone pre‐mRNA processing. Nat Genet. 2020;52:1364‐1372. DOI: 10.1038/s41588-020-00737-3[↩]
- AICARDI-GOUTIERES SYNDROME 1; AGS1. https://omim.org/entry/225750[↩]
- Rice G, Patrick T, Parmar R, Taylor CF, Aeby A, Aicardi J, et al. Clinical and molecular phenotype of Aicardi‐Goutières syndrome. Am J Hum Genet. 2007;81:713‐725. DOI: 10.1086/521373[↩]
- Crow YJ. Type I interferonopathies: a novel set of inborn errors of immunity. Ann N Y Acad Sci. 2011;1238:91‐98. DOI: 10.1111/j.1749-6632.2011.06220.x[↩]
- Zhang S, Zhang W, Ding C, Ren X, Fang F, Wu Y. Neurophenotype and genetic analysis of children with Aicardi-Goutières syndrome in China. Pediatr Investig. 2024 May 30;8(3):193-200. doi: 10.1002/ped4.12428[↩]
- Livingston JH, Crow YJ. Neurologic phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR1, and IFIH1: Aicardi‐Goutières syndrome and beyond. Neuropediatrics. 2016;47:355‐360. DOI: 10.1055/s-0036-1592307[↩]
- Cetin Gedik K, Lamot L, Romano M, Demirkaya E, Piskin D, Torreggiani S, Adang LA, Armangue T, Barchus K, Cordova DR, Crow YJ, Dale RC, Durrant KL, Eleftheriou D, Fazzi EM, Gattorno M, Gavazzi F, Hanson EP, Lee-Kirsch MA, Montealegre Sanchez GA, Neven B, Orcesi S, Ozen S, Poli MC, Schumacher E, Tonduti D, Uss K, Aletaha D, Feldman BM, Vanderver A, Brogan PA, Goldbach-Mansky R. The 2021 European Alliance of Associations for Rheumatology/American College of Rheumatology points to consider for diagnosis and management of autoinflammatory type I interferonopathies: CANDLE/PRAAS, SAVI and AGS. Ann Rheum Dis. 2022 May;81(5):601-613. doi: 10.1136/annrheumdis-2021-221814[↩]
- Aicardi-Goutieres Syndrome. https://ulf.org/leukodystrophies/aicardi-goutieres-syndrome[↩]
- Cetin Gedik K, Lamot L, Romano M, Demirkaya E, Piskin D, Torreggiani S, et al. The 2021 European Alliance of Associations for Rheumatology/American College of Rheumatology points to consider for diagnosis and management of autoinflammatory type I interferonopathies: CANDLE/PRAAS, SAVI and AGS. Ann Rheum Dis. 2022;81:601‐613. DOI: 10.1136/annrheumdis-2021-221814[↩]
- Jiang J, Zhao M, Chang C, Wu H, Lu Q. Type I interferons in the pathogenesis and treatment of autoimmune diseases. Clin Rev Allergy Immunol. 2020;59:248‐272. DOI: 10.1007/s12016-020-08798-2[↩]
- Crow YJ. Aicardi-Goutières Syndrome. 2005 Jun 29 [Updated 2016 Nov 22]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1475[↩]
- What is Aicardi Syndrome. https://aicardisyndromefoundation.org/aicardi-syndrome/[↩]