Carotid body
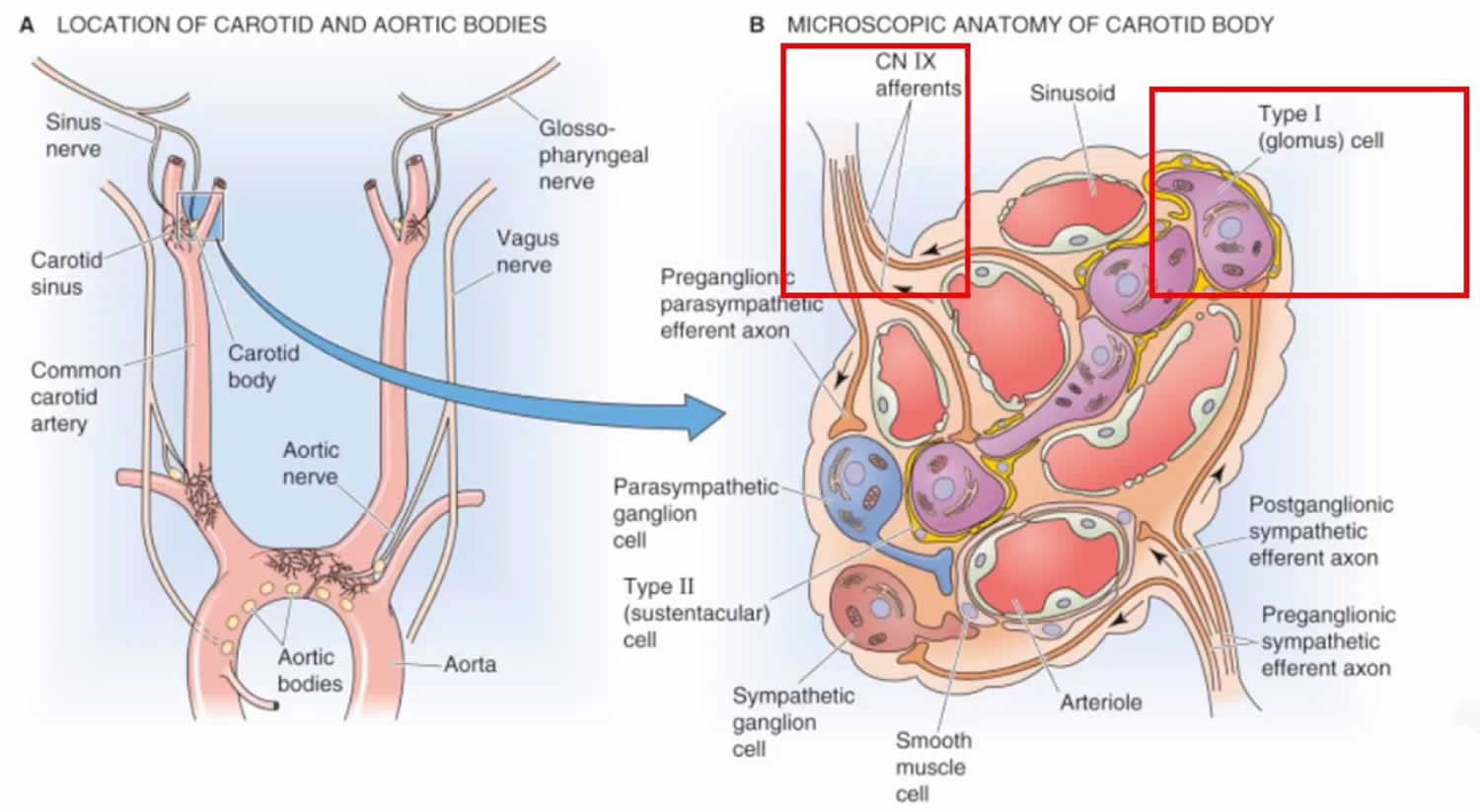
The carotid body also called carotid glomus, is a small cluster of chemoreceptors that measures around 2 x 3 x 5 millimeters and weighing 12 mg, located at the common carotid artery at its point of bifurcation into the external and internal carotid trunks, in the adventitia between the external and internal carotids. The carotid body contains chemoreceptors that are sensitive to hypoxia, hypercapnia and acidosis, and aims to maintain the body’s homeostasis and blood pressure.
Carotid body function
The carotid body measures changes in the composition of arterial blood flowing through it, including the partial pressures of oxygen and carbon dioxide and is also sensitive to changes in pH and temperature.
The carotid body is made up of two types of cell: type 1 (glomus) cells, and type 2 (sustentacular) cells. Glomus cells are derived from neural crest 1. They release a variety of neurotransmitters, including acetylcholine, ATP, and dopamine that trigger EPSP’s in synapsed neurons leading to the respiratory center.
Type 2 cells resemble glia and act as supporting cells.
While the central chemoreceptors in the brainstem are highly sensitive to CO2, the carotid body is a peripheral chemoreceptor that provides afferent input to the respiratory center that is highly O2 dependent.
The output of the carotid bodies is low at an oxygen partial pressure above about 100 mmHg (torr) (at normal physiological pH), but below this the activity of the type 1 glomus cells increases rapidly.
The peripheral chemoreceptor’s input is usually secondary to CO2 central chemoreceptors in healthy patients, but becomes the primary driver of ventilation in individuals who suffer from chronic hypercapnia (such as emphysema). Non-responsive hypercapnia can induce a tolerance mechanism within the cerebrospinal fluid, effectively negating carbon dioxide as a ventilation stimulus. In major cases this can prevent the use of general anaesthesia, as the carotid body is unable to communicate with the central nervous system sufficiently to stimulate breathing during recovery.
The feedback from the carotid body is sent to the respiratory centers in the medulla oblongata via the afferent branches of the glossopharyngeal nerve (IX). These centers, in turn, regulate breathing and blood pressure.
Figure 1. Carotid body
Carotid body tumor
Carotid body tumor also called chemodectoma or carotid body paraganglioma, is the most common (approximately 80% of the cases) head and neck paragangliomas arising at the bifurcation of carotid arteries 2. The incidence of carotid body tumor is greater in populations living at altitudes higher than 2000 meters above the sea level, due to the chronic hypoxia 3. In this regard, it has been proposed that environmental hypoxia modulates genetic predisposition to carotid body tumor 4. The literature mentions a carotid body tumor mortality rate of less than 2% 5. Carotid body paragangliomas comprise a set of rare, slow-growing neuroendocrine tumors arising in the anterolateral aspect of the upper neck 6. Paragangliomas are commonly associated with germline and somatic mutations involving at least one of more than thirty causative genes.
Carotid body tumors are highly vascularized tumors originating in the neural crest and are typically benign tumor arising from the paraganglia of carotid body hence, the name carotid paraganglioma. However, in 10–15% of cases the carotid body tumors can become malignant, characterized by multifocal growth, metastases, and relapse after surgical treatment 2. Typically, carotid body tumors are slow-growing tumors. Surgical treatment remains high-risk and extremely challenging due to the location of the carotid body tumor in close proximity of important nerves and blood vessels 5. Potential damage to carotid arteries can result in cerebral circulation dysfunction 7. One of the main difficulties in carotid body tumor management is the identification of tumors that are predisposed to be aggressive, since morphological criteria alone are not sufficiently informative 8. To create more specific treatment strategies, there is a growing need for identification of new biomarkers that can distinguish between indolent and aggressive metastatic forms of carotid body tumors. Many germline and somatic mutations are involved in progression of paragangliomas. Up to 30–40% of all head and neck paragangliomas are hereditary 9. The majority of these familial paraganglioma syndromes are due to mutations in genes encoding distinctive subunits of the mitochondrial succinate dehydrogenase complex 10. The succinate dehydrogenase complex participates in both the citric acid cycle and the electron transport chain, playing an essential role in energy metabolism. Besides, somatic mutations in more than 30 causative genes are described as drivers for paragangliomas 11.
Carotid body tumors are divided by means of the Shamblin classification 12, according to the involvement of the carotid wall and difficulty of surgical resection. Shamblin 1 was reserved for carotid body tumors that were relatively small and easy to resect, with minimal or no adherence to the carotid wall. Shamblin 2 consisted of carotid body tumors with partial involvement of the carotid wall, and Shamblin 3 completely involved the carotid bifurcation.
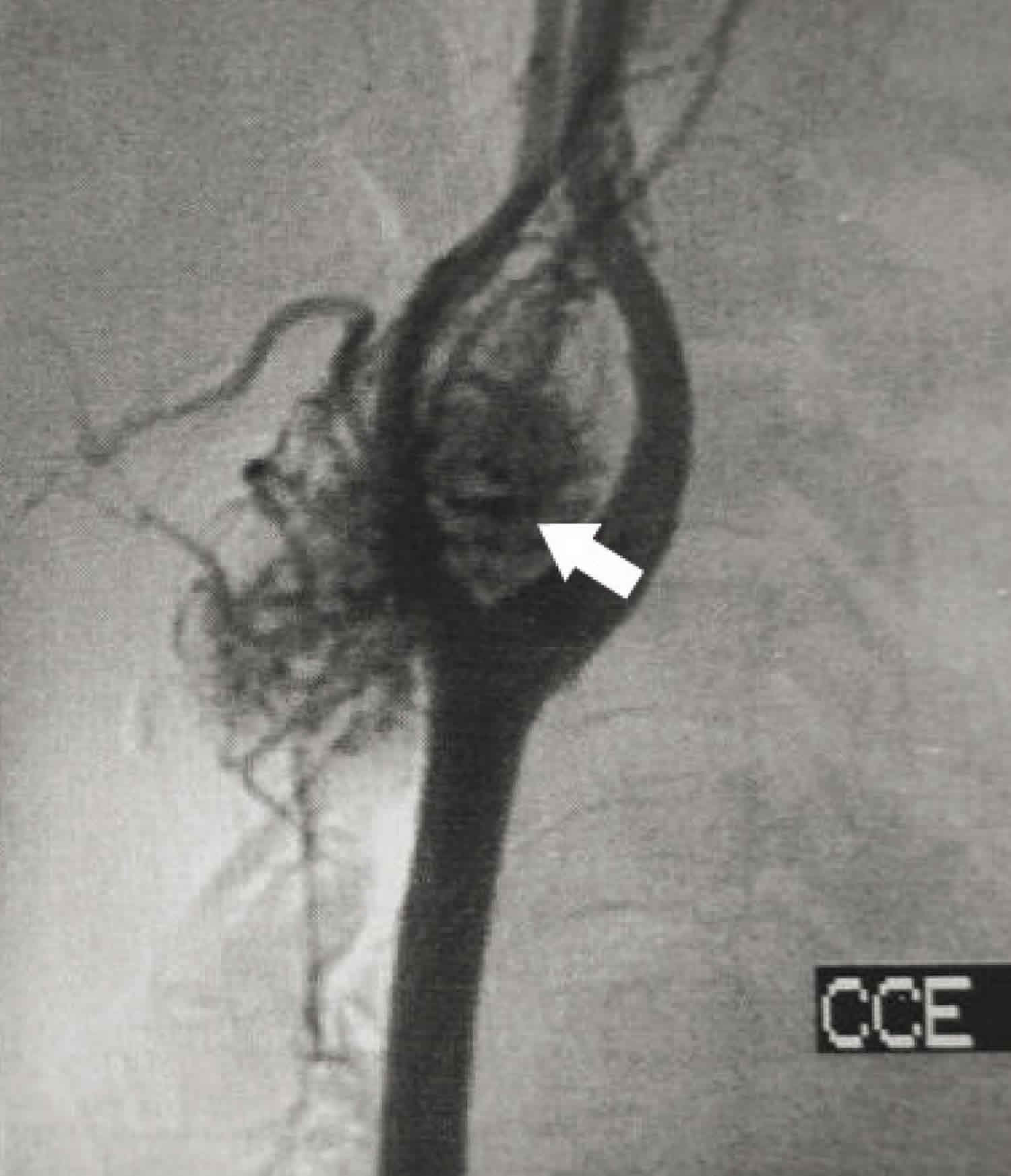
Figure 2. Carotid body tumor at the carotid bifurcation (angiogram)
Footnote: Angiography showing vascular mass at the carotid bifurcation (arrow)
[Source 13 ]Figure 3. Carotid body tumor
Carotid body paraganglioma causes
Carotid body paragangliomas can be classified into three different forms: sporadic (60%), familial (10–50%), and hyperplastic, that is, associated with carotid body hyperplasia due to chronic hypoxemia, as in subjects living at high altitudes or in patients affected with cardio-respiratory diseases 14. The latter form may occur in both familial and sporadic cases. Familial paragangliomas most commonly develop in the head and neck, usually in the carotid body, and up to 80% of familial paragangliomas are multifocal, compared to only 10–20% of sporadic paragangliomas 15.
Patients without family history present succinate dehydrogenase (SDH) mutation only in 11% of cases, while those with familial history carry succinate dehydrogenase (SDH) mutation in 83% of cases. Patients without succinate dehydrogenase (SDH) mutation reportedly present a mean age of 50.3 years at diagnosis, while those with SDH mutation report a mean age of 38 years at diagnosis. Genetic testing is advised in case of carotid body paragangliomas, familial history, multicentric presentation, and young age at diagnosis 16.
Paragangliomas, together with the related pheochromocytomas, are considered the tumors with the highest degree of hereditability in humans. Sporadic forms account for about 60% of the cases and familial (or hereditary) forms account for about 40% 17. The most frequently involved predisposition genes are those coding for the subunits of the SDH enzyme, a multiprotein complex composed of proteins encoded by the SDHA, SDHB, SDHC, and SDHD genes and by an assembly co-factor encoded by the SDHAF2 gene 18.
The hereditary paraganglioma pheochromocytoma syndrome is inherited in an autosomal dominant pattern and results from mutations in one of the four SDH genes. The SDH-related familial paragangliomas can be distinguished into four 18 or five 19 types: type 1, associated with SDHD gene, which is the gene most frequently mutated in head and neck paragangliomas; type 2, which is rare and associated with SDHAF2 mutations; type 3, which is associated with SDHC gene; and type 4, which is associated with SDHB gene 20. Furthermore, familial paragangliomas can be associated with germline mutations in other genes, including RET, NF1, VHL, HIF2A, FH, TMEM127, and MAX genes, some of which are related to syndromic entities in which paraganglioma may be linked to other neural crest tumors, such as multiple endocrine neoplasia (RET), von Hippel-Lindau (VHL) syndrome, neurofibromatosis type 1 (NF1), Carney–Stratakis syndrome, and the Carney triad 21.
However, the paraganglioma-associated SDH gene variants have incomplete penetrance: in the case of SDHA only 1.7%, for SDHB 22.0%, and for SDHC 8.3% 22. Furthermore, engineered mice mutated in sdhb, the homolog of human SDHB, do not develop any cancer 23, which suggests that SDH gene mutations per se might not be sufficient to cause paraganglioma.
Somatic mutations that often insist on pathways linked to paraganglioma predisposition are clearly involved in carotid body paraganglioma and vagal paraganglioma, as in other paragangliomas. Interestingly, at the somatic level, an exome analysis performed in 52 carotid body paragangliomas revealed in both hereditary and sporadic cases potential tumor-associated driver mutations in genes affecting metabolism and DNA repair, including, in order of frequency, SDHD (13.5%), IDH1 (7.7%), ARNT (5.8%), SDHC (5.8%), KMT2D (5.8%), TP53BP1 (5.8), etc. 24.
The hyperplastic form of carotid body paraganglioma has been mainly described in individuals living at high altitudes and in patients suffering from chronic obstructive pulmonary disease or cyanotic heart disease 14, which result in chronic exposure to low partial pressure of oxygen. This upregulates the physiological functions of the carotid body, which essentially monitors the fluctuating concentrations of oxygen, carbon dioxide, temperature, and pH in the arterial blood and hence regulates the cardiorespiratory centers in the medulla oblongata via the afferent branches of the glossopharyngeal nerve. Interestingly, there is an association between environmental hypoxia and gender: in fact, at sea level, the male to female carotid body paraganglioma ratio is 1:1.4, while at high altitude it decreases to 1:8.3, a phenomenon which is still unexplained 25. Finally, hypoxia has been proposed as a modulator of SDHB/D mutations penetrance 26.
Carotid body tumor classification
The classification proposed by Shamblin 12 has been widely accepted. However, Shamblin classification has its limitations. To truly assess a tumor, a clinical classification that helps in surgical planning and predicting outcomes is desirable, and this is where the Shamblin classification is found lacking 27. In fact, the Shamblin classification is essentially an anatomical and radiological classification that subdivides carotid body paragangliomas based on the encirclement of the internal and external carotid arteries. However, the involvement of the external carotid artery and its excision, if required, do not lead to any significant morbidity. Shamblin’s classification also does not predict true arterial infiltration and thereby preoperative intra-arterial management. Luna-Ortiz et al. 28 rightly pointed out that small tumors (Shamblin type 1) might also infiltrate the carotid arteries, thereby making excision difficult. These authors proposed a further distinction of Shamblin type 3 into types 3a and 3b, wherein small tumors infiltrating the carotid are included under type 3b. In an attempt to make the classification more predictive, Arya et al. 29 described Shamblin types 1, 2 and 3 tumors according to the radiological degree of involvement of the internal carotid artery as ≤180°, >180° but <270°, and ≥270°, respectively. However, this is at best an extension of the existing Shamblin classification and does not accord any additional benefit in terms of surgical management or prediction of outcome. The vertical growth of carotid body paragangliomas poses a specific surgical challenge when these tumors reach the infratemporal fossa (skull base) and involve the carotid canal or the jugular foramen, and also this is not addressed by the Shamblin classification. Considering this, we propose a modification to the Shamblin classification, as shown in Table 1, which allows complete and systematic assessment of carotid body paragangliomas and the surgical planning thereafter. This classification takes into account the involvement of the internal carotid artery and the application of intra-arterial stenting according to the infiltration of the artery. It also takes into account the compartmentalization of the parapharyngeal space into upper, middle, and lower compartments and the extent of tumor spread accordingly. The choice of surgical approach is determined by the extent of the spread according to these compartments.
Table 1. Shamblin classification of carotid body tumor (modified Shamblin classification)
[Source 27 ]Carotid body tumor symptoms
Carotid body tumors are difficult to diagnose because of their rarity and few manifested symptoms 13. The paramount clinical characteristic is a pulsatile neck mass. As reported in the literature, the diagnosis can be confused even with benign conditions, including congenital branchial cysts or other benign conditions 30. Thus, as pointed out in the literature, a pulsatile mass near the carotid bulb, in the absence of any suggestion of metastatic disease, should raise the possible diagnosis of carotid body tumor and should be followed by specific arterial imaging studies.
Jansen et al. 31 determined that the average growth rate of carotid body paragangliomas was 0.83 mm per year, about the same as that of other head and neck paragangliomas. In obese patients, these tumors may go unnoticed and thereby present in advanced stages. Both set of tumors are characteristically diagnosed by their pulsatile nature and their limited mobility in their supero-inferior axis when compared to the lateral axis (Fontaine’s sign) 32. They are only rarely associated with catecholamine hypersecretion and, hence, urinary catecholamine screening is often performed only in the presence of symptoms like tachycardia and/or hypertension or in case of a family history of paraganglioma. Preoperative cranial nerve paralysis is a feature of advanced lesions. Medially growing tumors may cause swelling of the oropharynx, leading to hoarseness, dysphagia, or foreign body sensation that are symptoms of the compression of cranial nerves IX, X, and XII. Furthermore, these tumors can also reach the skull base and extend intracranially 33 . Involvement of the sympathetic chain can lead to symptoms characteristic of Horner’s syndrome 34.
Carotid body tumor diagnosis
The carotid body tumor diagnosis is based on a combination of clinical findings, fine needle aspiration (FNA) and imaging methods, such as cervical ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI) and angiography. Contrast CT and MRI are complementary and are the investigations of choice, since they are able to identify the anatomical location and vascularity of these tumors. They help to differentiate paraganglioma from other prestyloid or poststyloid tumors and from tumors that originate from the deep lobe of the parotid. They also provide vital information on potential intracranial and/or intradural spread. Specific imaging characteristics, like salt-and-pepper appearance in contrast MRI, are crucial to differentiate carotid body paragangliomas from other tumors of the parapharyngeal space and dictate the need for further workup, as well as choice of surgical approach 35.
Fine needle aspiration (FNA) was helpful for the diagnosis in some patients, however, this procedure may be dangerous and performing it is controversial because of the risk of bleeding 36.
Ultrasonography and CT scans can be very helpful for the preoperative carotid body tumor diagnosis and may show enlargement of the bifurcation and hypervascularized tumors. Some authors have reported that ultrasonography with Doppler flowmetry can help surgeons in the initial approach 37. However, CT scans or magnetic resonance imaging (MRI) are better for identifying the dimensions and anatomical correlations of the carotid body tumor 38. CT is indicated for tumors invading the skull base, to better delineate the details of the bony erosion and the extension. MRI is indicated in most cases and is complementary to CT.
The main diagnostic tool is arterial studies such as contrast angiography, computed angiotomography or magnetic resonance angiography (MRA), thereby confirming or not confirming the suspected diagnosis. Digital subtraction angiography is the gold standard to study internal carotid artery infiltration, which will be seen as stenosis of the arterial lumen. It is also useful to detect the feeding vessels supplying the tumor, to check the collateral circulation through the circle of Willis and to determine the status of the venous drainage of the brain 39. It is usually performed 24– 48 hours before surgery to enable embolization of the feeding vessels. However, the MRA seems to be preferable nowadays 40.
Carotid body tumor treatment
The factors to be considered in the treatment of carotid body paragangliomas are age, lower cranial nerve paralysis, internal carotid artery involvement, and multicentricity. As a general rule, in young patients, a total tumor resection must be attempted, as they usually tolerate the loss of lower cranial nerve function; on the contrary, a wait-and-scan policy or radiotherapy may be adopted for older patients or patients who are not fit for surgery 27.
In the literature, the best treatment for carotid body tumor is considered to be surgical, with dissection of the tumor in the sub-adventitial avascular plane of the carotid artery 41 and this is reinforced by the fact that 6–12.5% of them have a malignant potential 42. Large tumors (Shamblin 2 and Shamblin 3) may require vascular procedures, including repairs, sutures and resections of the arterial segments 43. At times, it may be necessary to sacrifice the external carotid artery, perform anastomosis between the internal and common carotid arteries or undertake vascular reconstruction with grafts 44. Some authors have described use of a stent inserted into the internal carotid artery before the surgery, in cases presenting as Shamblin 3 with complete involvement of this artery 45. Occurrences of technical complications in anastomoses between the common and internal carotid arteries after resection of the carotid body have also been reported in the literature. These cases evolved with several hematomas and, in some cases, with stroke caused by deficits of cerebral irrigation 46.
The following surgical approaches are used based on the compartmentalization of the parapharyngeal space into upper, middle, and lower parapharyngeal space 27:
- Transcervical approach for tumors of the lower parapharyngeal space—In this approach, the posterior belly of the digastric muscle is resected, the extra-temporal facial nerve is identified, and the styloid process is transected to allow larger and safer access to the parapharyngeal space.
- Transcervical-transparotid approach for tumors of the middle parapharyngeal space—In addition to the transcervical approach, this procedure includes parotidectomy with preservation of the facial nerve.
- Transcervical-transmastoid approach for tumors of the upper parapharyngeal space with a posterior extension—In this approach, the transcervical approach is extended to the postauricular region, with a view to open the lateral skull base. In this procedure, the mastoid tip is removed, leaving the VII nerve in its canal. This is followed by infralabyrinthine dissection to expose the sigmoid sinus and the jugular bulb in order to control the uppermost part of the tumor.
- Infratemporal fossa approach-type A for tumors of the upper parapharyngeal space with extension to the vertical tract of the internal carotid artery and the jugular bulb—In this approach, a permanent anterior transposition of the facial nerve is performed to provide optimal exposure of the uppermost parapharyngeal internal carotid artery, the vertical portions of the petrous internal carotid artery and the jugular foramen.
In patients with bilateral carotid body tumors, obviously, the procedures should not be done at the same time, but spaced out one from another, especially because of possible vascular or cranial nerve lesions 47. Conservative treatment should be reserved for patients who are not suitable for surgery, such as clinically unstable patients, extremely old patients or those with the certainty of stroke. Some authors have suggested that radiotherapy could be used, but this is not recommended by most institutions. Furthermore, studies have shown that this therapeutic method is ineffective in these tumors 48.
Embolization is another therapeutic method, usually done prior to the surgical procedure, as an attempt to decrease intraoperative bleeding 44. However, this demands specialized skill and also has the risk of possible significant vascular complications and cranial nerve deficits. In the literature, it is mentioned that vascular complications and cranial nerve deficits can occur in about 33% of the patients 5. A famous study at the Mayo Clinic evaluated patients’ complications compared between three periods of that institution’s surgical history. It was concluded that, despite the decreases in vascular complications and mortality over the course of time, through better diagnostic methods and surgical technologies or skills, the rate of neurological injuries was not statistically different between the three periods studied, spanning a total of fifty years 49. Power et al. 50 demonstrated that preoperative embolization may have some benefits for the surgical approach, especially relating to surgical bleeding, but the patients that they followed up did not present any differences in definitive neurological complications.
It is important to mention that the malignant potential has been reported to be around 2-6 % in the literature, most frequently with metastasis to regional lymph nodes 46. Some authors have prescribed chemotherapy for carotid body tumor metastasized cases 51.
- Gonzalez, C., Almaraz, L., Obeso, A. and Rigual, R. Carotid Body Chemoreceptors: From Natural Stimuli to Sensory Discharges. Physiol. Rev. 1994, Oct. 74(4): 829:98. [↩]
- Exome analysis of carotid body tumor. BMC Med Genomics. 2018; 11(Suppl 1): 17. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5836820[↩][↩]
- Rodríguez-Cuevas S, López-Garza J, Labastida-Almendaro S. Carotid body tumors in inhabitants of altitudes higher than 2000 meters above sea level. Head Neck. 1998 Aug;20(5):374–8.[↩]
- Astrom K, Cohen JE, Willett-Brozick J, Aston CE, Baysal BE. Altitude is a phenotypic modifier in hereditary paraganglioma type 1: Evidence for an oxygen-sensing defect. Hum Genet. 2003 Aug;113(3):228–37.[↩]
- Wieneke JA, Smith A. Paraganglioma: carotid body tumor. Head Neck Pathol. 2009;3(4):303-6.[↩][↩][↩]
- Prasad SC, Paties CT, Pantalone MR, et al. Carotid Body and Vagal Paragangliomas: Epidemiology, Genetics, Clinicopathological Features, Imaging, and Surgical Management. In: Mariani-Costantini R, editor. Paraganglioma: A Multidisciplinary Approach [Internet]. Brisbane (AU): Codon Publications; 2019 Jul 2. Chapter 5. Available from: https://www.ncbi.nlm.nih.gov/books/NBK543230/ doi: 10.15586/paraganglioma.2019.ch5[↩]
- Amato B, Bianco T, Compagna R, Siano M, Esposito G, Buffone G, Serra R, de Franciscis S. Surgical resection of carotid body paragangliomas: 10 years of experience. Am J Surg. 2014;207(2):293–298. doi: 10.1016/j.amjsurg.2013.06.002[↩]
- Zhikrivetskaya SO, Snezhkina AV, Zaretsky AR, Alekseev BY, Pokrovsky AV, Golovyuk AL, Melnikova NV, Stepanov OA, Kalinin DV, Moskalev AA, et al. Molecular markers of paragangliomas/pheochromocytomas. Oncotarget. 2017;8(15):25756–25782. doi: 10.18632/oncotarget.15201[↩]
- Gimenez-Roqueplo AP, Dahia PL, Robledo M. An update on the genetics of paraganglioma, pheochromocytoma, and associated hereditary syndromes. Horm Metab Res. 2012;44(5):328–333. doi: 10.1055/s-0031-1301302[↩]
- Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A, van der Mey A, Taschner PE, Rubinstein WS, Myers EN, et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287(5454):848–851. doi: 10.1126/science.287.5454.848.[↩]
- Toledo RA, Qin Y, Srikantan S, Morales NP, Li Q, Deng Y, Kim SW, Pereira MA, Toledo SP, Su X, et al. In vivo and in vitro oncogenic effects of HIF2A mutations in pheochromocytomas and paragangliomas. Endocr Relat Cancer. 2013;20(3):349–359. doi: 10.1530/ERC-13-0101[↩]
- Shamblin WR, ReMine WH, Sheps SG, Harrison EG Jr. Carotid body tumor (chemodectoma). Clinicopathologic analysis of ninety cases. Am J Surg. 1971;122:732–9.[↩][↩]
- Casarim, André Luís Maion, Tincani, Alfio José, Del Negro, André, Aguiar, Camila Guimarães, Fanni, Renato Ventura, & Martins, Antonio Santos. (2014). Carotid body tumor: retrospective analysis on 22 patients. Sao Paulo Medical Journal, 132(3), 133-139. Epub April 14, 2014.https://dx.doi.org/10.1590/1516-3180.2014.1323452[↩][↩]
- Sajid MS, Hamilton G, Baker DM., Joint Vascular Research Group. A multicenter review of carotid body tumour management. Eur J Vasc Endovasc Surg. 2007 Aug;34(2):127–30.[↩][↩]
- Lee JH, Barich F, Karnell LH, Robinson RA, Zhen WK, Gantz BJ, et al. National cancer data base report on malignant paragangliomas of the head and neck. Cancer. 2002;94:730–7.[↩]
- Davila VJ, Chang JM, Stone WM, Fowl RJ, Bower TC, Hinni ML, et al. Current surgical management of carotid body tumors. J Vasc Surg. 2016;64:1703–10.[↩]
- Dahia PL. Pheochromocytoma and paraganglioma pathogenesis: Learning from genetic heterogeneity. Nat Rev Cancer. 2014;14:108–19.[↩]
- Williams MD. Paragangliomas of the head and neck: An overview from diagnosis to genetics. Head Neck Pathol. 2017;11:278–87.[↩][↩]
- Pacak K, Wimalawansa SJ. Pheochromocytoma and paraganglioma. Endocr Pract. 2015;21:406–12.[↩]
- Benn DE, Robinson BG, Clifton-Bligh RJ. 15 years of paraganglioma: Clinical manifestations of paraganglioma syndromes types 1–5. Endocr Relat Cancer. 2015;22:T91–103.[↩]
- Petr EJ, Else T. Genetic predisposition to endocrine tumors: Diagnosis, surveillance and challenges in care. Semin Oncol. 2016;43(5):582–90.[↩]
- Benn DE, Zhu Y, Andrews KA, Wilding M, Duncan EL, Dwight T, et al. Bayesian approach to determining penetrance of pathogenic SDH variants. J Med Genet. 2018;55:729–34.[↩]
- Piruat JI, Millán-Uclés Á. Genetically modeled mice with mutations in mitochondrial metabolic enzymes for the study of cancer. Front Oncol. 2014;4:200.[↩]
- Snezhkina AV, Lukyanova EN, Kalinin DV, Pokrovsky AV, Dmitriev AA, Koroban NV, et al. Exome analysis of carotid body tumor. BMC Med Genomics. 2018;11 Suppl 1:17.[↩]
- Barnes L, Tse LLY, Hunt JL. Carotid body paraganglioma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics of head and neck tumours. Lyon, France: IARC Press; 2005. pp. 362–3.[↩]
- Her YF, Nelson-Holte M, Maher LJ. Oxygen concentration controls epigenetic effects in models of familial paraganglioma. Clin Cancer Res. 2010 Aug 15;16(16):4148–54.[↩]
- Prasad SC, Paties CT, Pantalone MR, et al. Carotid Body and Vagal Paragangliomas: Epidemiology, Genetics, Clinicopathological Features, Imaging, and Surgical Management. In: Mariani-Costantini R, editor. Paraganglioma: A Multidisciplinary Approach [Internet]. Brisbane (AU): Codon Publications; 2019 Jul 2. Chapter 5. Available from: https://www.ncbi.nlm.nih.gov/books/NBK543230[↩][↩][↩][↩]
- Luna-Ortiz K, Rascon-Ortiz M, Villavicencio-Valencia V, Granados-Garcia M, Herrera-Gomez A. Carotid body tumors: Review of a 20-year experience. Oral Oncol. 2005;41:56–61.[↩]
- Arya S, Rao V, Juvekar S, Dcruz AK. Carotid body tumors: Objective criteria to predict the Shamblin group on MR imaging. AJNR Am J Neuroradiol. 2008;29:1349–54.[↩]
- Williams MD, Phillips MJ, Nelson WR, Rainer WG. Carotid body tumor. Arch Surg. 1992;127(8):963-7; discussion 967-8.[↩]
- Jansen TTG, Marres HAM, Kaanders J, Kunst HPM. A meta-analysis on the surgical management of paraganglioma of the carotid body per Shamblin class. Clin Otolaryngol. 2018.[↩]
- Del Guercio L, Narese D, Ferrara D, Butrico L, Padricelli A, Porcellini M. Carotid and vagal body paragangliomas. Transl Med Unisa. 2013;6:11–15.[↩]
- Pellitteri PK, Rinaldo A, Myssiorek D, Jacksond CG, Bradleye PJ, Devaney KO, et al. Paragangliomas of the head and neck. Oral Oncol. 2004;40:563–75.[↩]
- Suarez C, Rodrigo JP, Mendenhall WM, Hamoir M, Silver CE, Grégoire V, et al. Carotid body paragangliomas: A systematic study on management with surgery and radiotherapy. Eur Arch Otorhinolaryngol. 2014;271:23–34.[↩]
- Zanoletti E, Mazzoni A. Vagal paraganglioma. Skull Base. 2006;16:161–7.[↩]
- Nora JD, Hallett JW Jr, O’Brien PC, et al. Surgical resection of carotid body tumors: long-term survival, recurrence, and metastasis. Mayo Clin Proc. 1988;63(4):348-52.[↩]
- França LHG, Bredt CG, Vedolin A, Back LA, Stahlke Junior HJ. Tratamento cirúrgico do tumor de corpo carotídeo: experiência de 30 anos do Hospital de Clínicas da UFPR [Surgical treatment of the carotid body tumor: a 30-year experience]. J Vasc Br. 2003;2(3):171-6.[↩]
- Demattè S, Di Sarra D, Schiavi F, Casadei A, Opocher G. Role of ultrasound and color Doppler imaging in the detection of carotid paragangliomas. J Ultrasound. 2012;15(3):158-63.[↩]
- Sniezek JC, Netterville JL, Sabri AN. Vagal paragangliomas. Otolaryngol Clin North Am. 2001;34:925–39. vi.[↩]
- Arya S, Rao V, Juvekar S, Dcruz AK. Carotid body tumors: objective criteria to predict the Shamblin group on MR imaging. AJNR Am J Neuroradiol. 2008;29(7):1349-54.[↩]
- Anand VK, Alemar GO, Sanders TS. Management of the internal carotid artery during carotid body tumor surgery. Laryngoscope. 1995;105(3 Pt 1):231-5.[↩]
- Zhang WC, Cheng JP, Li Q, Zhang L, Wang XD, Anniko M. Clinical and pathological analysis of malignant carotid body tumour: A report of nine cases. Acta Otolaryngol. 2009;129:1320–5.[↩]
- Lim JY, Kim J, Kim SH, et al. Surgical treatment of carotid body paragangliomas: outcomes and complications according to the shamblin classification. Clin Exp Otorhinolaryngol. 2010;3(2):91-5.[↩]
- Crespo Rodríguez AM, Hernández Delgado G, Barrena Caballo MR, Guelbenzu Morte S. Paragangliomas de cabeza y cuello: diagnóstico por imagen y embolización [Head and neck paragangliomas: imaging diagnosis and embolization]. Acta Otorrinolaringol Esp. 2007;58(3):83-93.[↩][↩]
- McDougall CM, Liu R, Chow M. Covered carotid stents as an adjunct in the surgical treatment of carotid body tumors: a report of 2 cases and a review of the literature. Neurosurgery. 2012;71(1 Suppl Operative):182-4; discussion 185.[↩]
- Mitchell RO, Richardson JD, Lambert GE. Characteristics, surgical management, and outcome in 17 carotid body tumors. Am Surg. 1996;62(12):1034-7.[↩][↩]
- Arias-Stella J, Valcarcel J. The human carotid body at high altitudes. Pathol Microbiol (Basel). 1973;39(3):292-7.[↩]
- Kasper GC, Welling RE, Wladis AR, et al. A multidisciplinary approach to carotid paragangliomas. Vasc Endovascular Surg. 2007;40(6):467-74.[↩]
- Hallett JW Jr, Nora JD, Hollier LH, Cherry KJ Jr, Pairolero PC. Trends in neurovascular complications of surgical management for carotid body and cervical paragangliomas: a fifty-year experience with 153 tumors. J Vasc Surg. 1988;7(2):284-91.[↩]
- Power AH, Bower TC, Kasperbauer J, et al. Impact of preoperative embolization on outcomes of carotid body tumor resections. J Vasc Surg. 2012;56(4):979-89.[↩]
- Lázaro B, Klemz M, Flores MS, Landeiro JA. Malignant paraganglioma swith vertebral metastasis: case report. Arq Neuropsiquiatr. 2003;61(2B):463-7.[↩]