Vertebral artery
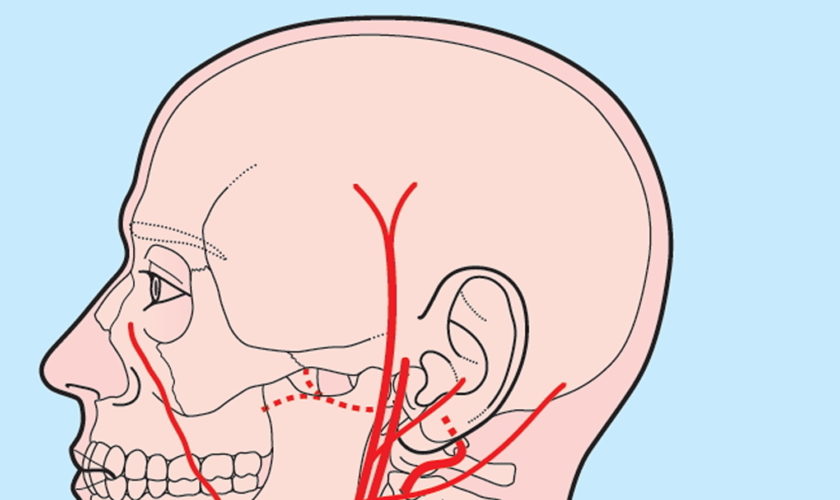
The brain receives its arterial supply from two pairs of vessels, the vertebral and internal carotid arteries (Figure 1), which are interconnected in the cranial cavity to produce a cerebral arterial circle (of Willis). The vertebral artery, a component of the vertebrobasilar artery system, supplies 20% of the blood to the brain (primarily the posterior cranial fossa), with the remaining 80% being supplied by the carotid system. The vertebral artery supply blood to the brainstem, spinal cord, and to the vertebrae and their associated ligaments and muscles.
In the cranial cavity, the vertebral arteries unite to form a single basilar artery. This vessel passes along the ventral brainstem and gives rise to branches leading to the pons, midbrain, and cerebellum. The basilar artery ends by dividing into two posterior cerebral arteries that supply parts of the occipital and temporal lobes of the cerebrum. The posterior cerebral arteries also help form the cerebral arterial circle (circle of Willis) at the base of the brain, which connects the vertebral artery and internal carotid artery systems (Figure 1). The union of these systems provides alternate pathways for blood to circumvent blockages and reach brain tissues. It also equalizes blood pressure in the brain’s blood supply.
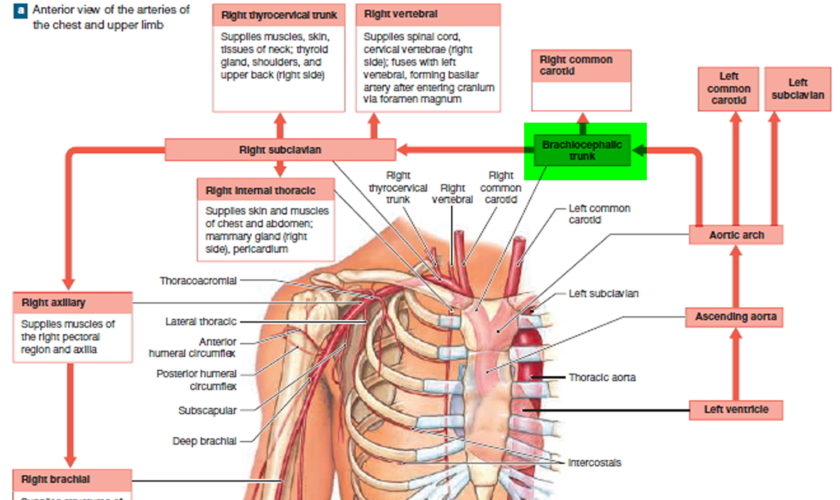
The vertebral artery usually originates from the posterior surface of the subclavian artery as the first branch of the subclavian artery, but it can also originate from the aortic arch and common carotid artery. Each vertebral artery arises from the first part of each subclavian artery in the lower part of the neck, and passes superiorly through the transverse foramina of the upper six cervical vertebrae (Figure 3 and 4). In approximately 88% of individuals, the artery enters the transverse foramen of C VI (cervical spine C6), but it has been shown to enter as far superior as the transverse foramen of C IV (cervical spine C4). Continuing to pass superiorly, the vertebral artery passes through the foramina of vertebrae C V (cervical spine C5) to C 1 (cervical spine C1 also known as the Atlas).
At the superior border of vertebra C I (cervical spine C1 or the Atlas), the artery turns medially and crosses the posterior arch of vertebra CI. From here it passes through the foramen magnum to enter the posterior cranial fossa.
On entering the cranial cavity through the foramen magnum each vertebral artery gives off a small meningeal branch. Continuing forward, the vertebral artery gives rise to three additional branches before joining with its companion vessel to form the basilar artery (Figure 1).
One branch joins with its companion from the other side to form the single anterior spinal artery, which then descends in the anterior median fissure of the spinal cord.
A second branch is the posterior spinal artery, which passes posteriorly around the medulla and then descends on the posterior surface of the spinal cord in the area of the attachment of the posterior roots, there are two posterior spinal arteries, one on each side (although the posterior spinal arteries can originate directly from the vertebral arteries, they more commonly branch from the posterior inferior cerebellar arteries).
Just before the two vertebral arteries join, each gives off a posterior inferior cerebellar artery.
The basilar artery travels in a rostral direction along the anterior aspect of the pons. Its branches in a caudal to rostral direction include the anterior inferior cerebellar arteries, several small pontine arteries, and the superior cerebellar arteries. The basilar artery ends as a bifurcation, giving rise to two posterior cerebral arteries.
Figure 1. Brain blood supply
Figure 2. Vertebral artery origin
Figure 3. Vertebral artery in the neck
Vertebral artery segments
The vertebral artery usually arises from the subclavian artery and is angiographically divided into five segments 1.
- The first segment (V1) begins at the origin of the subclavian artery and extends to the point where the artery enters the transverse foramen of the sixth cervical vertebra.
- The second segment (V2) begins from the level of the fifth or sixth cervical vertebra to the second cervical vertebra travelling through the transverse foramina at each vertebral level, with an alternating intra and inter-osseous course. On account of having such a unique anatomic environment, the V2 segment may be exposed to the extrinsic compression from spondylotic exostosis of the spine 2. This segment can be extremely tortuous, which can make the placement of a stent in the mid or distal extracranial vertebral artery difficult 3.
- The third segment (V3) traverses the transverse foramen of C2 and terminates as the artery pierces the posterior atlanto-occipital membrane.
- The fourth segment (V4) is demarcated by the atlanto-occipital membrane and where the artery finally enters the foramen magnum at the base of the skull.
- The fifth segment (V5) traverses the foramen magnum and courses along the anterior lateral surface of the medulla oblongata, before it finally unites with the opposite vertebral artery at the inferior border of the pons to form the basilar artery. V5 commonly gives rise to the ipsilateral posterior inferior cerebellar artery (PICA).
The branches of the vertebral artery can be classified as cervical and cranial. The cervical vertebral artery produces spinal and muscular branches. The muscular branches typically originate from the second (V2) and third (V3) segments of the vertebral artery. These branches typically supply the dorsal cervical musculature and are best visualized in the presence of the common carotid artery or vertebral artery occlusion. Small branches from the second segment (V2) may anastomose with spinal arteries. Branches of the V3 segment typically anastomose with a branch of the occipital artery. The cranial branches of the vertebral artery are meningeal, posterior spinal, anterior spinal, posterior inferior cerebellar artery (PICA) and medullary arteries. The posterior inferior cerebellar artery (PICA) is the largest branch of the vertebral artery, coursing backward to the inferior surface of the cerebellum. It is divided into medial and lateral branches and may anastomose with the anterior cerebellar artery and superior cerebellar artery of the basilar artery.
Figure 4. Vertebral artery segments
Figure 5. Vertebral artery segments (angiogram)

Note: The right subclavian artery with visualization of the vertebral artery origin and the V1 and V2 segments. The right internal mammary artery (RIMA) can also be seen, as can the thyrocervical trunk with an ascending cervical branch.
[Source 1]Figure 6. Vertebral artery intracranial segments (angiogram)

Note: Intracranial run-off of an injection of the vertebral artery in a cross-table lateral projection using digital subtraction. This shows a solitary posterior inferior cerebellar artery; two superimposed anterior inferior cerebellar arteries; two superimposed superior cerebellar arteries; and a solitary posterior cerebral artery with obvious branching. The blush of the cerebellum is faintly visualized.
[Source 1]Variants of the vertebral artery anatomy
Anatomic variants of vertebral artery are much more common than those of the carotid artery and most often involve the origin of vertebral artery from the aortic arch in V1 segment or the distal branches in V3 and V4 segments 2. The vertebral artery generally arises from the superior-posterior aspect of the first part of the subclavian artery. However, in approximately 5%-6% of cases, the left vertebral artery arises directly from the aortic arch between the left common carotid artery and the left subclavian artery 4. On rare occasions, the right vertebral artery may arise distal to the left subclavian artery or from the right common carotid artery (0.18%) 5. In 50% of individuals, the diameter of the left vertebral artery is larger than the right vertebral artery diameter 2. In 25% of individuals, the vertebral artery diameters are equal to each other. On the other hand, in approximately 10% of individuals one vertebral artery is prominently smaller in diameter than the other 2. In these kinds of cases, the smaller vertebral artery may terminate in the posterior inferior cerebellar artery (PICA) or have a hypoplastic segment between the posterior inferior cerebellar artery (PICA) and basilar artery that contributes little to basilar artery blood flow 2.
The posterior inferior cerebellar artery (PICA) generally originates from the intradural segment of the vertebral artery, but may alternatively originate from the extracranial segment. The posterior inferior cerebellar artery (PICA) may be absent unilaterally or bilaterally, in approximately 20% and 2% of individuals, respectively. In 1% of cases, the vertebral artery terminates in the posterior inferior cerebellar artery (PICA) 6.
In the literature, the incidence of normal entrance to the transverse foramina ranges from 90% to 93% 3. Additionally, it may enter into the transverse foramen at other levels than C6 7. The prevalence of vertebral artery entry at C4 level ranges from 0.5% to 1.3% and at C5 level ranges from 5% to 6.6%, which often makes the V1 segment longer 8. However, it may enter at the C7 level (0.8%-5.4%), which makes the V1 segment shorter.
Tortuosity of the vertebral artery from the origin to the transverse foramen is another variation. Matula et al. 9 reported 47.2% tortuosity of the V1 segment. It is important in endovascular procedures, because severe tortuosity of the proximal V1 segment combined with a stenosis can preclude safe stent placement. Moreover, it was also reported as an independent and significant predictor of in-stent restenosis owing to unnatural straightening of the tortuous segment 10.
These anatomic variations must be considered in clinical assessment and treatment.
Vertebral artery stenosis
Vertebral artery stenosis may occur either extra- or intra-cranially, but it is often localized at the origin of the vessel as it arises from the subclavian artery 11. In a large series which included 4748 patients with ischemic stroke, some degree of proximal extracranial vertebral artery stenosis was seen in 18% of cases on the right and 22.5% on the left 12. This is the second most common location of stenosis after internal carotid artery stenosis at the carotid bifurcation 13. About 25% of ischemic strokes occur in the vertebrobasilar territory 14. Around one fifth of posterior circulation strokes occur in the setting of extracranial vertebral artery stenosis 15, 16, 17, 18.
Extracranial vertebral artery stenosis
Atherosclerosis is the most common cause of extracranial vertebral artery stenosis 19. However, an atherosclerotic plaque situated at the vertebral artery origin is considered to be less prone to ulceration and smoother than that seen at the carotid system 20. The less common causes of extracranial vertebral artery stenosis are arterial dissection, extrinsic compression due to trauma, osteophytes, fibrous bands and vasculitis (most commonly in giant cell arteritis) 13.
The most common mechanism of stroke in patients with vertebral artery stenosis is intra-arterial embolism, rather than hemodynamic failure 21. Hemodynamic stroke, however, is less commonly caused by vertebral artery stenosis, because both vertebral arteries feed into one basilar artery 11. Also, in contrast to the internal carotid artery, the vertebral artery gives off numerous branches at the neck region, therefore facilitating a considerable collateral blood supply, which often reconstitutes the distal artery after occlusion at the origin 11.
Individuals with occlusive disease of proximal segments of the vertebral artery are at relatively high risk for posterior or vertebrobasilar circulation ischemia 21. Indeed, a systematic review suggested that patients with symptomatic vertebral artery stenosis may have a greater recurrent stroke risk in the first 7 d after symptoms onset than patients with recently symptomatic carotid stenosis 22. Nevertheless, the best medical therapy for these patients is unclear, and the precise role of invasive treatment remains uncertain 23.
The role of imaging in diagnosis of vertebral artery stenosis
The American Heart Association/American Stroke Association guideline recommends an evidence-based diagnostic approach for diagnosing vertebral artery disease 2. In this American Heart Association/American Stroke Association guideline, 11 studies about comparing noninvasive methods and digital subtraction angiography (DSA) for the detection of vertebral artery stenosis have been systematically reviewed. According to these studies, computed tomography angiography (CTA) and contrast-enhanced magnetic resonance angiography (MRA) were associated with higher sensitivity (94%) and specificity (95%) than Doppler ultrasonography (US) (sensitivity 70%) 2. Also, of these noninvasive imaging methods, computed tomography angiography (CTA) had slightly superior accuracy 2. Doppler US is relatively less suitable and technically difficult for detection of vertebral artery stenosis in related anatomic regions. Digital subtraction angiography (DSA) as a noninvasive method is typically required before revascularization for patients with symptomatic posterior cerebral ischemia, because of the fact that neither contrast-enhanced magnetic resonance angiography (MRA) nor computed tomography angiography (CTA) reliably delineates the origins of the vertebral arteries 24. Thus the gold standard for diagnosing vertebral artery stenosis remains digital subtraction angiography (DSA), although it has a small morbidity and associated mortality. The complications of digital subtraction angiography (DSA) associated with morbidity and mortality can be divided into two major groups: clinical and technical. The former includes groin hematoma, contrast medium reaction, transient neurological event and permanent neurological deficit; the latter includes carotid and vertebral artery dissection and femoral or iliac artery dissection 25. Although the overall neurological complication rate related to cerebral angiography is 1.5% 26, recent reports using diffusion-weighted imaging suggest that there is a much higher rate of subclinical neurological events 27, 28, 29.
How is vertebral artery stenosis treated ?
Although optimum management of patients with vertebral artery stenosis is not well established in the literature, treatment options fundamentally include medical, surgical and endovascular therapies. For all patients with vertebral artery stenosis, optimal medical therapy should include risk factor modifications, antiplatelet and statin therapies 2. In patients with ongoing symptoms despite optimal medical treatment, endovascular and surgical options should be considered.
Medical therapy
Aspirin (81-325 mg daily), clopidogrel (75 mg daily) or the combination of aspirin and extended-release dipyridamole (25 and 200 mg twice daily, respectively) are acceptable options. Combination antiplatelet regimens (e.g., aspirin and clopidogrel) are emerging as the mainstay of medical therapy for patients with vertebrobasilar insufficiency. A combination of aspirin and dipyridamole was shown to significantly reduce the rate of stroke in patients with vertebrobasilar insufficiency compared to placebo 30. Selection of an antiplatelet regimen should be individualized on the basis of patient risk factor profiles, cost, tolerance, resistance and other clinical characteristics 31.
Surgery
Surgery to this region of the vertebral artery is technically difficult due to poor access to the vessel origin, hence surgery is not considered in most centers. It may be the only viable treatment option in those patients who fail medical therapy but have lesions or anatomy that are unfavorable for angioplasty and/or stent therapy. In the study by Buerger et al. 32 that includes 369 consecutive extracranial vertebral artery reconstructions, stroke and death rates of the procedure were found to be low (5.1% in the 215 patients treated before 1991 and 1.9% in the 154 patients treated since 1991). The combined morbidity and mortality rates of surgical therapy for vertebral artery stenosis range from 10% to 20% and have dampened enthusiasm for this option 33, 34, 35. Horner’s syndrome and lymphocele are considerable postoperative complications of the surgery.
Prognosis (Outlook) of vertebral artery stenosis
In a review of 300 interventions for proximal vertebral artery stenosis, the risk of death was 0.3%, the risk of periprocedural neurological complications was 5.5%, and the risk of posterior system stroke was 0.7% at a mean follow-up of 14.2 mo. Restenosis occurred in 26% of cases (range: 0%-43%) after a mean of 12 mo (range: 3-25 mo), although restenosis was not consistently correlated with recurrent symptoms 36. In a Cochrane review of 313 cases of vertebral artery intervention, 173 cases which underwent a vertebral artery stenting procedure were identified from 20 studies 37. Analysis of these studies revealed a 30-d major stroke and death rate of 3.2% and a 30-d transient ischemic attack and non-disabling stroke rate of 3.2% 37. These analyses suggest that vertebral artery stenting is safe and effective.
In summary, although angioplasty and stenting of the vertebral vessels are technically feasible, there is insufficient evidence from randomized trials to demonstrate that endovascular management is superior to best medical management 19.
Vertebral artery dissection
The term dissection implies a tear in the wall of a major artery leading to the intrusion of blood within the layers of an arterial wall (intramural hematoma). An arterial wall consists of three layers-intima (the innermost layer), media (the middle muscular layer), and adventitia (the outermost layer). It is generally agreed that a tear in the wall of an artery leads to a collection of blood between the layers of the artery, leading to formation of an intramural hematoma. This causes stenosis of the lumen when blood collects between the intima and media or an aneurysmal dilatation of the artery when the hematoma predominantly involves the media and adventitia 38.
Figure 7. Vertebral artery dissection
The overall incidence of vertebral artery dissection is approximately 1-1.5 per 100,000 39. Spontaneous dissections of the carotid and vertebral artery account for only about 2 percent of all ischemic strokes 40, but they are an important cause of ischemic stroke in patients under the age of 45 years and account for 10 to 25 percent of such cases.
Extracranial vertebral dissections account for about 15% of all cervicocerebral arterial dissections 38.
Spontaneous dissections of the vertebral arteries affect all age groups, including children, but there is a distinct peak in the fifth decade of life 41. Although there is no overall sex-based predilection, women are on average about five years younger than men at the time of the dissection 42.
Aetiopathogenesis of cervicocerebral arterial dissections is incompletely understood, though trauma, genetic factors, respiratory infections, and underlying arteriopathy are considered important. A typical picture of local pain, headache, and ipsilateral Horner’s syndrome followed after several hours by cerebral or retinal ischaemia is rare. A history of a minor precipitating event is frequently elicited in patients with a spontaneous dissection of the vertebral artery 43. Some precipitating events associated with hyperextension or rotation of the neck include practicing yoga, painting a ceiling, coughing, vomiting, sneezing, the receipt of anesthesia, and the act of resuscitation 44. Such neck movements, particularly when they are sudden, may injure the artery as a result of mechanical stretching.
Chiropractic manipulation of the neck has been associated with vertebral artery dissection 45. It has been estimated that as many as 1 in 20,000 spinal manipulations causes a stroke.
A recent history of a respiratory tract infection is a risk factor for spontaneous dissections of the vertebral artery 46. The possibility of an infectious trigger is supported by the finding of a seasonal variation in the incidence of spontaneous dissections of the vertebral arteries, with a peak incidence in the fall.
A potential link with common risk factors for vascular disease, such as tobacco use, hypertension, and the use of oral contraceptives, has not been systematically evaluated. One case-control study suggested migraine as a risk factor for dissection 47.
Patients with a spontaneous dissection of the vertebral artery are thought to have an underlying structural defect of the arterial wall, although the exact type of arteriopathy remains elusive in most cases 43. Foremost among the heritable connective tissue disorders that are associated with an increased risk of spontaneous dissections of the vertebral arteries is Ehlers-Danlos syndrome type IV 48. Others include Marfan’s syndrome, autosomal dominant polycystic kidney disease, and osteogenesis imperfecta type I 49. Although these well-characterized heritable connective tissue disorders have been identified in only 1 to 5 percent of patients with spontaneous dissection of the vertebral artery, one-fifth of patients have a clinically apparent but as yet unnamed connective tissue disorder 50.
Spontaneous vertebral artery dissection is mainly divided into two types 51:
- Ischemic type, which is manifest by ischemic symptoms and/or infarction of the vertebrobasilar circulation due to arterial narrowing and thromboembolism; and
- Hemorrhagic type, which presents as a subarachnoid hemorrhage caused by rupture of an intradural vertebral artery dissecting aneurysm.
More than a quarter of patients with stroke caused by cervical artery dissection develop relevant disability, while almost half report a decreased quality of life 52. The socio-economic consequences are significant, because patients with cervical artery dissection are on average 45 years of age and play an important role in private, business and social life 53. Brain imaging studies and detection of micro-embolic signals by transcranial ultrasound in patients with cervical artery dissection suggest that arterial embolism is the main mechanism of stroke 54, 55.
Vertebral artery dissection symptoms
The cervical arteries are innervated with pain-sensitive nerve fibers that may generate neck pain and headache when provoked. Several studies have shown that pain is typically the first symptom associated with cervical artery dissection 56 and a recent descriptive study involving 245 cervical artery dissection patients reported that 8% of them presented with head or neck pain as their only symptom 57. In this study, pain-only cervical artery dissection patients were mostly composed of vertebral artery or multiple artery dissection cases, with only 3 cases of internal carotid artery dissection. Pain related to cervical artery dissection frequently occurs suddenly and is of severe intensity, often described by patients as being different from any previous pain. Accordingly, the clinical manifestations of cervical artery dissection typically include severe head and neck pain that involves mostly the ipsilateral occipitocervical area when the vertebral artery is affected 58 or the periorbital, frontal, and upper cervical region when the internal carotid artery is involved 59. These symptoms may or may not be followed by ischemic involvement in the brain, cerebellum, or brain stem. The interval of time between the initial pain of cervical artery dissection and ischemic symptoms is quite variable, however, with reports ranging from almost immediately to several weeks.
Prognosis of vertebral artery dissection
Extracranial vertebral artery dissections generally carry a good prognosis. Most dissections of the vertebral arteries heal spontaneously and especially, extracranial vertebral artery dissections generally carry a good prognosis. However, intracranial vertebral artery dissections are usually associated with severe neurological deficits or subarachnoid hemorrhage and carry a poor prognosis, so an urgent surgical intervention may be required in patients presenting with hemorrhage. A literature review reports 50% of cases having no neurological deficit, 21% mild deficits only, and 25% moderate to severe deficits, the remaining 4% having died 38. In a recent study looking at the relation between recanalization rate and neurological outcome, no relation was found between these two variables 60, the neurological outcome was dependent on the lesion localization and the presence of good collaterals.
Intracranial dissections are usually associated with severe neurological deficits or subarachnoid hemorrhage and carry a poor prognosis. The risk of a recurrent dissection in an initially unaffected artery is about 2 percent during the first month but then decreases to a rate of only about 1 percent per year 42. However, the increased risk persists for at least a decade and possibly longer 50. The risk of a recurrence is higher in young patients with a heritable arteriopathy 61. Only rarely do dissections recur in the same artery 42.
Doppler ultrasound, MRI/MRA, and CT angiography are useful non-invasive diagnostic tests.
How is vertebral artery dissection treated ?
Medical Treatment
The treatment of extracranial cervicocerebral arterial dissection is mainly medical using anticoagulants or antiplatelet agents although controlled studies to show their effectiveness are lacking. In a 2013 meta-analysis 62 comparing antiplatelets versus anticoagulants for the treatment of cervical artery dissection found antiplatelets should be given precedence over anticoagulants as a first line treatment in patients with cervical artery dissection unless results of an adequately powered randomised trial suggest the opposite. The prognosis of extracranial cervicocerebral arterial dissection is generally much better than that of the intracranial cervicocerebral arterial dissection. Recurrences are rare in cervicocerebral arterial dissection.
One approach is to obtain a magnetic resonance angiogram (MRA) after three months, continue anticoagulant therapy for three more months if luminal irregularities are found, and then repeat the magnetic resonance study and change to antiplatelet therapy if the luminal irregularities are still present. The rationale behind this approach is the high rate of recanalization within the first three months after the dissection and the observation that, after the discontinuation of anticoagulation, symptoms occasionally recur within three to six months after the onset of dissection but rarely after six months. The fear that anticoagulant therapy or intravenous thrombolysis with tissue-plasminogen activator will extend the dissection appears to be unfounded 43. Nevertheless, anticoagulation is not innocuous, and some patients are treated with antiplatelet therapy alone, particularly those who have no symptoms of ischemia.
Surgical Interventions
Most vertebral artery dissections heal spontaneously 43. However, an urgent surgical intervention may be required in patients presenting with subarachnoid hemorrhage. Symptomatic aneurysmal dilatation of the artery may also warrant surgery. Chronic vertebral artery dissections have also been treated by surgical reconstruction to prevent further ischemic or thromboembolic complications, if medical treatment with six month anticoagulation fails or if the dissecting aneurysms and/or high grade stenosis persist 38. Surgical interventions include endovascular treatment and the arterial repair.
Endovascular Therapy
Endovascular treatment has largely supplanted surgery as the initial therapy of choice once medical therapy fails or is contraindicated 63.
Endovascular occlusion of the parent artery
A saccular aneurysm will persist unless treated by surgical clipping or endovascular embolization. In contrast, reflecting the intrinsic mechanism of healing, a dissection can resolve spontaneously. The aims of treatment are, first, to reduce the “hemodynamic stress” on the vessel wall that could produce rebleeding and, second, to provide a suitable environment for healing. Both goals can be achieved by eliminating or reversing the flow within the dissection through sacrificing the parent artery close to or even far proximal to the dissection 64. If the dissection is not completely excluded from the antegrade arterial circulation following proximal occlusion, the potential for rebleeding still exists 65. Rebleeding can be anticipated when the dissection cavity increases in size after proximal occlusion 66. Rebleeding from a “caecum-like” dissection usually occurs within several hours after proximal occlusion, presumably as result of hemodynamic changes in its lumen.
Endovascular trapping
Because histopathology shows that the rupture point is in close proximity to the entrance into the dissection 67, an option in treatment is double catheterization and simultaneous embolization of the proximal and distal portions of the dissection 68. Endovascular trapping does not cross the dissected segment (which should be avoided). However, ischemia may occur in the territory of brainstem perforators arising from the healthy vessel distal to the dissected portion that will be occluded. Therefore, it is not likely that trapping is superior to occlusion of the proximal parent vessel 64. This is because the theoretical benefits (exclusion of the diseased vessel segment) are outweighed by the risk of occluding brain stem perforators in the occluded distal healthy vessel.
Intracranial stenting
Stent implantation has the advantage of preserving the patency of the parent vessel and remodeling blood flow. Nevertheless, there are unnecessary difficulties and hazards. These are that the stent has to be navigated through the dissection, inducing the risk of further dissecting the wall, that uncovered stents that do not fully exclude the dissection from the circulation may lead to early rebleeding, and that stent-assisted coiling of the dissection (as well as coiling of the dissection itself) might cause rupture of the dissection or lead to recanalization if the coil migrates through the dissected vessel wall, so not completely securing the situation 69. Finally, the use of stent grafts in the intracranial circulation is experimental, and likely to occlude perforators to the brain stem and even may induce a secondarily symptomatic excessive neointimal proliferation.
Surgery
Surgical treatment should be considered for patients with persistent ischemic symptoms refractory to optimal medical care who are not candidates for endovascular treatment 70. Arterial ligation can be a very safe and simple treatment, assuming the presence of adequate collateral blood flow has been established. However, delayed ischemia because of propagation of thrombus or embolization is a potential threat in these patients in the early postoperative period. Trapping of the dissected segment of vertebral artery with or without extracranial-intracranial arterial bypass also can be a curative treatment option.
Rebleeding after surgical proximal occlusion has been reported 71, whereas trapping excludes the dissection from the circulation thus eliminating the risk of rebleeding which has not been reported after surgical trapping for vertebral artery dissections. However, the location of dissection makes the surgical approach technically demanding with a high risk of cranial nerve damage. Rigorous comparison between surgical and endovascular treatment is difficult, because most reports of surgical treatment include less than 10 patients and their features are not comparable.
References- Bajzer CT. Vertebral artery. In: Bhatt DL, editor. Guide to Peripheral and Cerebrovascular Intervention. London: Remedica; 2004. Available from: https://www.ncbi.nlm.nih.gov/books/NBK27430/
- Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, Cates CU, Creager MA, Fowler SB, Friday G, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Stroke. 2011;42:e464–e540. http://stroke.ahajournals.org/content/42/8/e464.long
- Wehman JC, Hanel RA, Guidot CA, Guterman LR, Hopkins LN. Atherosclerotic occlusive extracranial vertebral artery disease: indications for intervention, endovascular techniques, short-term and long-term results. J Interv Cardiol. 2004;17:219–232. https://www.ncbi.nlm.nih.gov/pubmed/15318894
- Lemke AJ, Benndorf G, Liebig T, Felix R. Anomalous origin of the right vertebral artery: review of the literature and case report of right vertebral artery origin distal to the left subclavian artery. AJNR Am J Neuroradiol. 1999;20:1318–1321. http://www.ajnr.org/content/20/7/1318.long
- Gluncic V, Ivkic G, Marin D, Percac S. Anomalous origin of both vertebral arteries. Clin Anat. 1999;12:281–284. https://www.ncbi.nlm.nih.gov/pubmed/10398389
- Wollschlaeger G, Wollschlaeger PB, Lucas FV, Lopez VF. Experience and result with postmortem cerebral angiography performed as routine procedure of the autopsy. Am J Roentgenol Radium Ther Nucl Med. 1967;101:68–87. https://www.ncbi.nlm.nih.gov/pubmed/6037344
- Lin YH, Hung CS, Tseng WY, Lee RK, Wang YC, Lin MS, Yeh MH, Chao CL, Ho YL, Jeng JS, et al. Safety and feasibility of drug-eluting stent implantation at vertebral artery origin: the first case series in Asians. J Formos Med Assoc. 2008;107:253–258. https://www.ncbi.nlm.nih.gov/pubmed/18400611
- Voetsch B, DeWitt LD, Pessin MS, Caplan LR. Basilar artery occlusive disease in the New England Medical Center Posterior Circulation Registry. Arch Neurol. 2004;61:496–504. https://www.ncbi.nlm.nih.gov/pubmed/15096396
- Matula C, Trattnig S, Tschabitscher M, Day JD, Koos WT. The course of the prevertebral segment of the vertebral artery: anatomy and clinical significance. Surg Neurol. 1997;48:125–131. https://www.ncbi.nlm.nih.gov/pubmed/9242236
- Zhou Z, Yin Q, Xu G, Yue X, Zhang R, Zhu W, Fan X, Ma M, Liu X. Influence of vessel size and tortuosity on in-stent restenosis after stent implantation in the vertebral artery ostium. Cardiovasc Intervent Radiol. 2011;34:481–487. https://www.ncbi.nlm.nih.gov/pubmed/20683721
- Caplan L. Posterior circulation ischemia: then, now, and tomorrow. The Thomas Willis Lecture-2000. Stroke. 2000;31:2011–2023. http://stroke.ahajournals.org/content/31/8/2011.long
- Hass WK, Fields WS, North RR, Kircheff II, Chase NE, Bauer RB. Joint study of extracranial arterial occlusion. II. Arteriography, techniques, sites, and complications. JAMA. 1968;203:961–968. https://www.ncbi.nlm.nih.gov/pubmed/5694318
- Cloud GC, Markus HS. Diagnosis and management of vertebral artery stenosis. QJM. 2003;96:27–54. https://www.ncbi.nlm.nih.gov/pubmed/12509646
- Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337:1521–1526. https://www.ncbi.nlm.nih.gov/pubmed/1675378
- Caplan LR, Amarenco P, Rosengart A, Lafranchise EF, Teal PA, Belkin M, DeWitt LD, Pessin MS. Embolism from vertebral artery origin occlusive disease. Neurology. 1992;42:1505–1512. https://www.ncbi.nlm.nih.gov/pubmed/1641144
- Koroshetz WJ, Ropper AH. Artery-to-artery embolism causing stroke in the posterior circulation. Neurology. 1987;37:292–295. https://www.ncbi.nlm.nih.gov/pubmed/3808311
- Pessin MS, Daneault N, Kwan ES, Eisengart MA, Caplan LR. Local embolism from vertebral artery occlusion. Stroke. 1988;19:112–115. http://stroke.ahajournals.org/content/19/1/112.long
- Wityk RJ, Chang HM, Rosengart A, Han W, DeWitt LD, Pessin MS, Caplan LR. Proximal Extracranial Vertebral Artery Disease in the New England Medical Center Posterior Circulation Registry. Arch Neurol. 1998;55(4):470–478. doi:10.1001/archneur.55.4.470 http://jamanetwork.com/journals/jamaneurology/fullarticle/773717
- Kocak B, Korkmazer B, Islak C, Kocer N, Kizilkilic O. Endovascular treatment of extracranial vertebral artery stenosis. World Journal of Radiology. 2012;4(9):391-400. doi:10.4329/wjr.v4.i9.391. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3460226/
- Caplan L. Stroke: A Clinical Approach. 3rd ed. Stoneham, MA: Butterworth-Heinemann; 2000.
- Wityk RJ, Chang HM, Rosengart A, Han WC, DeWitt LD, Pessin MS, Caplan LR. Proximal extracranial vertebral artery disease in the New England Medical Center Posterior Circulation Registry. Arch Neurol. 1998;55:470–478. https://www.ncbi.nlm.nih.gov/pubmed/9561974
- Flossmann E, Rothwell PM. Prognosis of vertebrobasilar transient ischaemic attack and minor stroke. Brain. 2003;126:1940–1954. https://www.ncbi.nlm.nih.gov/pubmed/12847074
- Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB, Gorelick P, Halperin J, Harbaugh R, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:577–617. http://stroke.ahajournals.org/content/37/2/577.long
- Long A, Lepoutre A, Corbillon E, Branchereau A. Critical review of non- or minimally invasive methods (duplex ultrasonography, MR- and CT-angiography) for evaluating stenosis of the proximal internal carotid artery. Eur J Vasc Endovasc Surg. 2002;24:43–52. https://www.ncbi.nlm.nih.gov/pubmed/12127847
- Dawkins AA, Evans AL, Wattam J, Romanowski CA, Connolly DJ, Hodgson TJ, Coley SC. Complications of cerebral angiography: a prospective analysis of 2,924 consecutive procedures. Neuroradiology. 2007;49:753–759. https://www.ncbi.nlm.nih.gov/pubmed/17594083
- Willinsky RA, Taylor SM, TerBrugge K, Farb RI, Tomlinson G, Montanera W. Neurologic complications of cerebral angiography: prospective analysis of 2,899 procedures and review of the literature. Radiology. 2003;227:522–528. https://www.ncbi.nlm.nih.gov/pubmed/12637677
- Chuah KC, Stuckey SL, Berman IG. Silent embolism in diagnostic cerebral angiography: detection with diffusion-weighted imaging. Australas Radiol. 2004;48:133–138. https://www.ncbi.nlm.nih.gov/pubmed/15230745
- Kato K, Tomura N, Takahashi S, Sakuma I, Watarai J. Ischemic lesions related to cerebral angiography: Evaluation by diffusion weighted MR imaging. Neuroradiology. 2003;45:39–43. https://www.ncbi.nlm.nih.gov/pubmed/12525953
- Bendszus M, Koltzenburg M, Bartsch AJ, Goldbrunner R, Günthner-Lengsfeld T, Weilbach FX, Roosen K, Toyka KV, Solymosi L. Heparin and air filters reduce embolic events caused by intra-arterial cerebral angiography: a prospective, randomized trial. Circulation. 2004;110:2210–2215. http://circ.ahajournals.org/content/110/15/2210.long
- Sivenius J, Riekkinen PJ, Smets P, Laakso M, Lowenthal A. The European Stroke Prevention Study (ESPS): results by arterial distribution. Ann Neurol. 1991;29:596–600. https://www.ncbi.nlm.nih.gov/pubmed/1892362
- Sacco RL, Diener H-C, Yusuf S, et al. Aspirin and Extended-Release Dipyridamole versus Clopidogrel for Recurrent Stroke. The New England journal of medicine. 2008;359(12):1238-1251. doi:10.1056/NEJMoa0805002. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2714259/
- Berguer R, Flynn LM, Kline RA, Caplan L. Surgical reconstruction of the extracranial vertebral artery: management and outcome. J Vasc Surg. 2000;31:9–18. https://www.ncbi.nlm.nih.gov/pubmed/10642704
- Bhatt DL, Kapadia SR, Bajzer CT, Chew DP, Ziada KM, Mukherjee D, Roffi M, Topol EJ, Yadav JS. Dual antiplatelet therapy with clopidogrel and aspirin after carotid artery stenting. J Invasive Cardiol. 2001;13:767–771. https://www.ncbi.nlm.nih.gov/pubmed/11731685
- Koskas F, Kieffer E, Rancurel G, Bahnini A, Ruotolo C, Illuminati G. Direct transposition of the distal cervical vertebral artery into the internal carotid artery. Ann Vasc Surg. 1995;9:515–524. https://www.ncbi.nlm.nih.gov/pubmed/8746828
- Berguer R. Long-term results of vertebral artery reconstruction. In: Yao JST, Pearce WH, et al., editors. Long-term results in vascular surgery. Norwalk, CT: Appleton and Lange; 1993. p. 69.
- Eberhardt O, Naegele T, Raygrotzki S, Weller M, Ernemann U. Stenting of vertebrobasilar arteries in symptomatic atherosclerotic disease and acute occlusion: case series and review of the literature. J Vasc Surg. 2006;43:1145–1154. https://www.ncbi.nlm.nih.gov/pubmed/16765230
- Coward L, Featherstone R, Brown MM. Percutaneous transluminal angioplasty and stenting for vertebral artery stenosis. Cochrane Database of Systematic Reviews 2005, Issue 2. Art. No.: CD000516. DOI: 10.1002/14651858.CD000516.pub2. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD000516.pub2/full
- Thanvi B, Munshi S, Dawson S, Robinson T. Carotid and vertebral artery dissection syndromes. Postgraduate Medical Journal. 2005;81(956):383-388. doi:10.1136/pgmj.2003.016774.
- Bogousslavsky J, Regli F. Ischemic stroke in adults younger than 30 years of age. Cause and prognosis. Arch Neurol. 1987;44:479–482. https://www.ncbi.nlm.nih.gov/pubmed/3579657
- Bassetti C, Carruzzo A, Sturzenegger M, Tuncdogan E. Recurrence of cervical artery dissection. A prospective study of 81 patients. Stroke. 1996;27:1804–1807. http://stroke.ahajournals.org/content/27/10/1804.long
- Schievink WI, Mokri B, Piepgras DG. Spontaneous dissections of cervicocephalic arteries in childhood and adolescence. Neurology. 1994;44:1607–1612. https://www.ncbi.nlm.nih.gov/pubmed/7936283
- Schievink WI, Mokri B, O’Fallon WM. Recurrent spontaneous cervical-artery dissection. N Engl J Med. 1994;330:393–397. http://www.nejm.org/doi/full/10.1056/NEJM199402103300604
- Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344:898–906. http://www.nejm.org/doi/full/10.1056/NEJM200103223441206
- Fisher CM, Ojemann RG, Roberson GH. Spontaneous dissection of cervico-cerebral arteries. Can J Neurol Sci. 1978;5:9–19. https://www.ncbi.nlm.nih.gov/pubmed/647502
- Hufnagel A, Hammers A, Schonle PW, Bohm KD, Leonhardt G. Stroke following chiropractic manipulation of the cervical spine. J Neurol. 1999;246:683–688. https://www.ncbi.nlm.nih.gov/pubmed/10460445
- Grau AJ, Brandt T, Buggle F, Orberk E, Mytilineos J, Werle E, et al. Association of cervical artery dissection with recent infection. Arch Neurol. 1999;56:851–856. http://jamanetwork.com/journals/jamaneurology/fullarticle/775214
- D’Anglejan-Chatillon J, Ribeiro V, Mas JL, Youl BD, Bousser MG. Migraine–a risk factor for dissection of cervical arteries. Headache. 1989;29:560–561. https://www.ncbi.nlm.nih.gov/pubmed/2583992
- Schievink WI, Michels VV, Piepgras DG. Neurovascular manifestations of heritable connective tissue disorders. A review. Stroke. 1994;25:889–903. https://www.ncbi.nlm.nih.gov/pubmed/8160237
- Schievink WI, Bjornsson J, Piepgras DG. Coexistence of fibromuscular dysplasia and cystic medial necrosis in a patient with Marfan’s syndrome and bilateral carotid artery dissections. Stroke. 1994;25:2492–2496. https://www.ncbi.nlm.nih.gov/pubmed/7974595
- Schievink WI, Wijdicks EF, Michels VV, Vockley J, Godfrey M. Heritable connective tissue disorders in cervical artery dissections : a prospective study. Neurology. 1998;50:1166–1169. https://www.ncbi.nlm.nih.gov/pubmed/9566419
- Park K-W, Park J-S, Hwang S-C, Im S-B, Shin W-H, Kim B-T. Vertebral Artery Dissection: Natural History, Clinical Features and Therapeutic Considerations. Journal of Korean Neurosurgical Society. 2008;44(3):109-115. doi:10.3340/jkns.2008.44.3.109. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2588305/
- Fischer U, Ledermann I, Nedeltchev K, Meier N, Gralla J, et al. (2009) Quality of life in survivors after cervical artery dissection. J Neurol 256: 443–449. https://www.ncbi.nlm.nih.gov/pubmed/19319463
- Arnold M, Kappeler L, Georgiadis D, Berthet K, Keserue B, et al. (2006) Gender differences in spontaneous cervical artery dissection. Neurology 67: 1050–1052. https://www.ncbi.nlm.nih.gov/pubmed/17000975
- Benninger DH, Georgiadis D, Kremer C, Studer A, Nedeltchev K, et al. (2004) Mechanism of ischemic infarct in spontaneous carotid dissection. Stroke 35: 482–485. http://stroke.ahajournals.org/content/35/2/482.long
- Srinivasan J, Newell DW, Sturzenegger M, Mayberg MR, Winn HR (1996) Transcranial Doppler in the evaluation of internal carotid artery dissection. Stroke 27: 1226–1230. http://stroke.ahajournals.org/content/27/7/1226.long
- De Bray JM, Penisson-Besnier I, Dubas F, Emile J. Extracranial and intracranial vertebrobasilar dissections: diagnosis and prognosis. Journal of Neurology, Neurosurgery, and Psychiatry. 1997;63(1):46-51. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2169649/
- Arnold M, Cumurciuc R, Stapf C, Favrole P, Berthet K, Bousser M. Pain as the only symptom of cervical artery dissection. Journal of Neurology, Neurosurgery, and Psychiatry. 2006;77(9):1021-1024. doi:10.1136/jnnp.2006.094359. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2077740/
- Triano J., Kawchuk G.N., editors. Current concepts in spinal manipulation and cervical arterial incidents. NCMIC Group Inc; West Des Moines (Iowa): 2005.
- Haneline MT, Lewkovich G. Identification of internal carotid artery dissection in chiropractic practice. The Journal of the Canadian Chiropractic Association. 2004;48(3):206-210. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1769453/
- Caso V, Paciaroni M, Corea F, Hamam M, Milia P, Pelliccioli GP, et al. Recanalization of cervical artery dissection : influencing factors and role in neurological outcome. Cerebrovasc Dis. 2004;17:93–97. https://www.karger.com/Article/Abstract/75775
- Schievink WI, Mokri B, Piepgras DG, Kuiper JD. Recurrent spontaneous arterial dissections : risk in familial versus nonfamilial disease. Stroke. 1996;27:622–624. http://stroke.ahajournals.org/content/27/4/622.long
- Sarikaya H, da Costa BR, Baumgartner RW, et al. Antiplatelets versus Anticoagulants for the Treatment of Cervical Artery Dissection: Bayesian Meta-Analysis. Schlachetzki F, ed. PLoS ONE. 2013;8(9):e72697. doi:10.1371/journal.pone.0072697. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3764185/
- Schievink WI. The treatment of spontaneous carotid and vertebral artery dissections. Curr Opin Cardiol. 2000;15:316–321. https://www.ncbi.nlm.nih.gov/pubmed/11128183
- Zhao WY, Krings T, Alvarez H, Ozanne A, Holmin S, Lasjaunias P. Management of spontaneous haemorrhagic intracranial vertebrobasilar dissection: review of 21 consecutive cases. Acta Neurochir(Wien) 2007;149:585–596. discussion 596. https://www.ncbi.nlm.nih.gov/pubmed/17514349
- Yasui T, Komiyama M, Nishikawa M, Nakajima H. Subarachnoid hemorrhage from vertebral artery dissecting aneurysms involving the origin of the posteroinferior cerebellar artery : report of two cases and review of the literature. Neurosurgery. 2000;46:196–200. discussion 200-201. https://www.ncbi.nlm.nih.gov/pubmed/10626950
- Aoki N, Sakai T. Rebleeding from intracranial dissecting aneurysm in the vertebral artery. Stroke. 1990;21:1628–1631. http://stroke.ahajournals.org/content/21/11/1628.long
- Mizutani T, Kojima H, Asamoto S, Miki Y. Pathological mechanism and three-dimensional structure of cerebral dissecting aneurysms. J Neurosurg. 2001;94:712–717. https://www.ncbi.nlm.nih.gov/pubmed/11354401
- Kai Y, Hamada JI, Morioka M, Todaka T, Mizuno T, Ushio Y. Endovascular coil trapping for ruptured vertebral artery dissecting aneurysms by using double microcatheters technique in the acute stage. Acta Neurochir(Wien) 2003;145:447–451. discussion 451. https://www.ncbi.nlm.nih.gov/pubmed/12836068
- MacKay CI, Han PP, Albuquerque FC, McDougall CG. Recurrence of a vertebral artery dissecting pseudoaneurysm after successful stentsupported coil embolization : case report. Neurosurgery. 2003;53:754–759. discussion 760-761. https://www.ncbi.nlm.nih.gov/pubmed/12943592
- Schievink WI, Piepgras DG, McCaffrey TV, Mokri B. Surgical treatment of extracranial internal carotid artery dissecting aneurysms. Neurosurgery. 1994;35:809–815. discussion 815-816. https://www.ncbi.nlm.nih.gov/pubmed/7838327
- Kawamata T, Tanikawa T, Takeshita M, Onda H, Takakura K, Toyoda C. Rebleeding of intracranial dissecting aneurysm in the vertebral artery following proximal clipping. Neurol Res. 1994;16:141–144. https://www.ncbi.nlm.nih.gov/pubmed/7914000