Childhood glaucoma
Childhood glaucoma also known as congenital glaucoma, pediatric glaucoma, infantile glaucoma is a rare childhood eye condition where high pressure builds up inside the eye during fetal development that is either present at birth or develops during very early childhood, potentially causing vision loss and even blindness if left untreated 1, 2, 3, 4, 5, 6, 7. Glaucoma can affect one eye or both. Children with childhood glaucoma have ocular hypertension (high pressure inside their eyes). The fluids in their eyes (aqueous humor) fail to drain normally, so they build up, raising the pressure inside. This puts stress on their optic nerves and can eventually cause structural changes to their eyes. Childhood glaucoma is typically diagnosed within the first few months of life commonly in children under 2 years of age caused by a developmental defect in the eye’s drainage system, preventing fluid from flowing out properly and is often suspected when there is eye enlargement at birth 4. Congenital glaucoma affects about 1 in 10,000 children under 2 years of age in the United States 8, 9.
Eye doctors often classify childhood glaucoma by the age when it first appears 6:
- Newborn (neonatal) onset (0-1 month)
- Infantile onset (>1-24 months)
- Late onset or late-recognized (>24 months)
- Spontaneously arrested primary congenital glaucoma (very rare), classic findings of eye stretching including Haab striae with normal intraocular pressure (IOP); must follow as glaucoma suspects.
Primary congenital glaucoma commonly presents between the ages of 3-9 months, but the most severe form is the newborn onset 6. Infantile glaucoma affects individuals between the ages of 1 and 36 months 10, while juvenile glaucoma is used to indicate individuals diagnosed with glaucoma between the ages of 3 and 40 years 11. In most cases, childhood glaucoma is diagnosed by the age of six months, with 80% diagnosed in the first year of life.
The elevated intraocular pressure (IOP) is associated with the classic “triad” of symptoms such as eyes are sensitive to light (photophobia), excessive tearing of the eye (epiphora) and uncontrollable muscle twitching that forces eyes closed (blepharospasm), which occurs due to rapid expansion of the child’s eye causing buphthalmos (“ox-eyed” in Greek), corneal enlargement, horizontal or oblique breaks in Descemet membrane (Haab striae) and subsequent corneal edema and opacification (see Figure 3 and 4 below). If Haab striae and buphthalmos are seen without elevated intraocular pressure (IOP), optic nerve cupping or corneal edema, then the patient has spontaneously arrested primary congenital glaucoma 2. It’s very important to recognize and treat childhood glaucoma as soon as possible to minimize the damage and vision loss it can cause in your child.
Due to the elasticity of the eye in young children, the 2013 International Classification System for Childhood Glaucoma defined childhood glaucoma as irreversible or reversible damage to the whole eye and not just the optic nerve as glaucoma is defined for adults 2. Therefore, additional important clinical signs in primary congenital glaucoma, besides elevated intraocular pressure (IOP) and optic nerve cupping, are corneal enlargement and clouding, Haab striae, and buphthalmos. Not all signs are always present, however, and other parts of the eye also stretch with elevated intraocular pressure (IOP). Diagnosis of childhood glaucoma can be delayed if corneas remain clear, despite being enlarged, and bilateral primary congenital glaucoma can be missed if signs and symptoms are mild in one eye. Irreversible vision loss results if elevated intraocular pressure (IOP) is untreated or uncontrolled in primary congenital glaucoma. Optic nerve damage occurs, and focal corneal edema overlying Haab striae, which can be single or multiple, can lead to permanent corneal scarring and opacification. This corneal scarring can obscure the visual axis or cause astigmatism (a common eye condition where the cornea or sometimes the lens doesn’t have a perfectly round spherical shape leading to blurry or distorted vision at all distances, this irregular shape causes light rays to focus at multiple points on the retina instead of a single point, resulting in a fuzzy or wavy image) with or without refractive amblyopia. Amblyopia may also develop due to optic nerve damage, anisometropia (a condition of asymmetric refraction between the two eyes), strabismus or a combination.
There are many causes of childhood glaucoma. It can be hereditary or it can be associated with other eye disorders.
- If childhood glaucoma cannot be attributed to any other cause, other noticeable eye defects or systemic problem, it is classified as primary congenital glaucoma. The cause of primary congenital glaucoma is not completely understood, though there is significant research to suggest that the trabecular meshwork is immature and compressed. Studies suggest that the normal posterior migration of embryonic neural crest cells destined to become the trabecular meshwork is abnormally halted 12. The drainage angle where the inside of the sclera (the white of your eye) and the outer edge of your iris meet of children with primary congenital glaucoma is described as immature, thick, and compressed. High intraocular pressures (IOP) are believed to be a consequence of increased resistance to aqueous outflow in this abnormal trabecular meshwork. Researchers have identified several gene mutations that can lead to primary congenital glaucoma. Mutations in the CYP1B1 (cytochrome P450 family 1 subfamily B member 1) gene are the predominant genetic anomalies linked to primary congenital glaucoma 13.
- If childhood glaucoma is a result of another eye disorder, eye injury, or other disease, it is classified as secondary childhood glaucoma.
- Associated with eye abnormalities e.g., Axenfeld-Rieger syndrome, aniridia (a rare genetic eye disorder characterized by the complete or partial absence of the iris, the colored part of the eye), iridotrabecular dysgenesis, Peter’s anomaly, sclerocornea (a rare, non-progressive, congenital condition where the cornea, normally transparent, becomes opaque and blends with the sclera, the white part of the eye), microcornea (a congenital condition where the cornea, the transparent front part of the eye, is smaller than normal, with a horizontal diameter of less than 10-11 mm), microphthalmos (a developmental disorder of the eye where one or both eyes are abnormally small and have anatomical malformations), ectopia lentis, persistent fetal vasculature, oculodermal melanocytosis, posterior polymorphous dystrophy,
- Associated with systemic abnormalities e.g., chromosomal disorders like trisomy 21 (Down syndrome), connective tissue disorders such as Marfan syndrome, Stickler syndrome (a group of genetic disorders that primarily affect connective tissues, particularly in the face, eyes, ears, and joints), phakomatoses (a group of genetic disorders also known as neurocutaneous syndromes or neuro-oculo-cutaneous syndromes characterized by systemic hamartomas, primarily affecting the central nervous system, eyes, and skin) common examples include neurofibromatosis (types 1 and 2), Sturge-Weber syndrome, tuberous sclerosis, Lowe syndrome and von Hippel-Lindau disease
- Glaucoma secondary to acquired causes e.g., retinopathy of prematurity, eye trauma, intraocular tumors, uveitis, eye inflammation, lens‑induced (with/without pupillary block), steroid-induced, intraocular infections, maternal rubella (congenital rubella syndrome), raised episcleral venous pressure
- Glaucoma after surgery for congenital cataract.
Childhood glaucoma commonly starts with a defect in the way your child’s eye develops. The most common defect is in the trabecular meshwork, the tissue that the eye fluids (aqueous humor) drain through. When the trabecular meshwork doesn’t develop right, the aqueous humor fluids don’t drain properly. The buildup of fluids (aqueous humor) causes pressure in your child’s eye, which damages their optic nerve. It can also cause their cornea to enlarge, stretch, tear and scar. This process is progressive. How fast it progresses depends on how severe the defect in your child’s eye is, how much fluid (aqueous humor) is building up and how high the pressure is inside the eye (intraocular pressure [IOP]). When glaucoma appears in young infants, it’s because these conditions were already progressing during fetal development. When symptoms appear later, it’s because these conditions were less severe at birth, so they took longer to build up.
To diagnose childhood glaucoma, your child’s eye doctor will ask about your child’s medical history and do a complete eye examination of your child.
Furthermore, your child’s eye doctor may perform diagnostic procedures such as:
- Visual acuity test – the common eye chart test (with letters and images), which measures vision ability at various distances.
- Pupil dilation – the pupil is widened with eyedrops to allow a close-up examination of the eye’s retina and optic nerve.
- Visual field – a test to measure a child’s side (peripheral) vision. Lost peripheral vision may be an indication of glaucoma.
- Tonometry – a standard test to determine the fluid pressure inside the eye.
Younger children may be examined with hand-held instruments, whereas older children are often examined with standard equipment that is used with adults. An eye examination can be difficult for a child. It is important that parents encourage cooperation. At times, the child may have to be examined under anesthesia, especially young children, in order to examine the eye and the fluid drainage system, and to determine the appropriate treatment.
Specific treatment for glaucoma will be determined by your child’s eye doctor based on:
- your child’s age, overall health, and medical history
- extent of the disease
- your child’s tolerance for specific medications, procedures, or therapies
- expectations for the course of the disease
- your opinion or preference
It is important for treatment of childhood glaucoma to start as early as possible. Treatment may include:
- Medications. Some medications cause the eye to produce less fluid, while others lower pressure by helping fluid drain from the eye.
- Surgery. The purpose of surgery is to create a new opening for fluid to leave the eye. Surgical procedures are performed by using microsurgery or lasers.
Both medications and surgery have been successfully used to treat childhood glaucoma. However, surgery is the primary treatment modality for primary congenital glaucoma. In managing secondary childhood glaucoma, medications is the first-line treatment 14.
Surgical procedures used to treat glaucoma in children include the following:
- Trabeculotomy and goniotomy.
- Trabeculotomy is a surgical procedure, primarily used in the treatment of childhood glaucoma, that creates a new drainage opening in the eye’s trabecular meshwork, improving the outflow of aqueous humor and reducing the intraocular pressure (IOP).
- Goniotomy is a microinvasive glaucoma surgery (MIGS) technique that improves fluid flow in the eye to lower intraocular pressure (IOP). A goniotomy involves making a small incision within the trabecular meshwork, the eye’s natural drainage system, to create a more efficient pathway for fluid outflow. This procedure can be used to treat conditions like childhood glaucoma.
- Trabeculectomy. Trabeculectomy is a surgical procedure that involves the removal of part of the trabecular meshwork drainage system, allowing the fluid to drain from the eye. Trabeculectomy works by creating a new drainage pathway for the fluid (aqueous humor) within the eye, allowing it to drain into a space beneath the outer layer of the eye (conjunctiva). This new pathway, called a bleb, helps reduce eye pressure and can slow or prevent further vision loss.
- Iridotomy. Iridotomy is a surgical procedure to treat or prevent angle-closure glaucoma, a condition where the iris (colored part of the eye) blocks the drainage angle, leading to increased eye pressure. The eye surgeon may use a laser to create this hole. Laser iridotomy involves using a laser to create a small hole in the iris, allowing fluid to flow freely and preventing or relieving pressure build-up.
- Cyclophotocoagulation. Cyclophotocoagulation is a laser procedure that uses a laser beam to freeze selected areas of the ciliary body – the part of the eye that produces aqueous humor – to reduce the production of fluid and reduce intraocular pressure (IOP). Cyclophotocoagulation is a type of cyclodestruction procedure, meaning it aims to reduce intraocular pressure (IOP) by damaging the ciliary body, a key part of the eye that produces aqueous humor. This type of surgery may be performed with severe cases of childhood glaucoma.
The primary treatment of primary congenital glaucoma is angle surgery, either goniotomy or trabeculotomy, to lower intraocular pressure (IOP) by improving aqueous outflow. If angle surgery is not successful, trabeculectomy enhanced with mitomycin C or glaucoma implant surgery with a Molteno, Baerveldt, or Ahmed implant can be performed. In refractory cases, cycloablation can be performed using an Nd:YAG laser, diode laser, or cryotherapy, with diode laser being the most widely used device. Medications, either topically or orally, is typically used as a temporizing measure prior to surgery and to help decrease corneal clouding to facilitate goniotomy, and to supplement intraocular pressure (IOP) control after surgery.
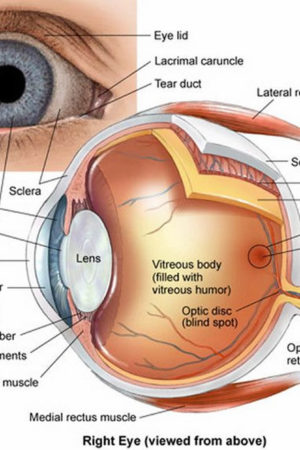
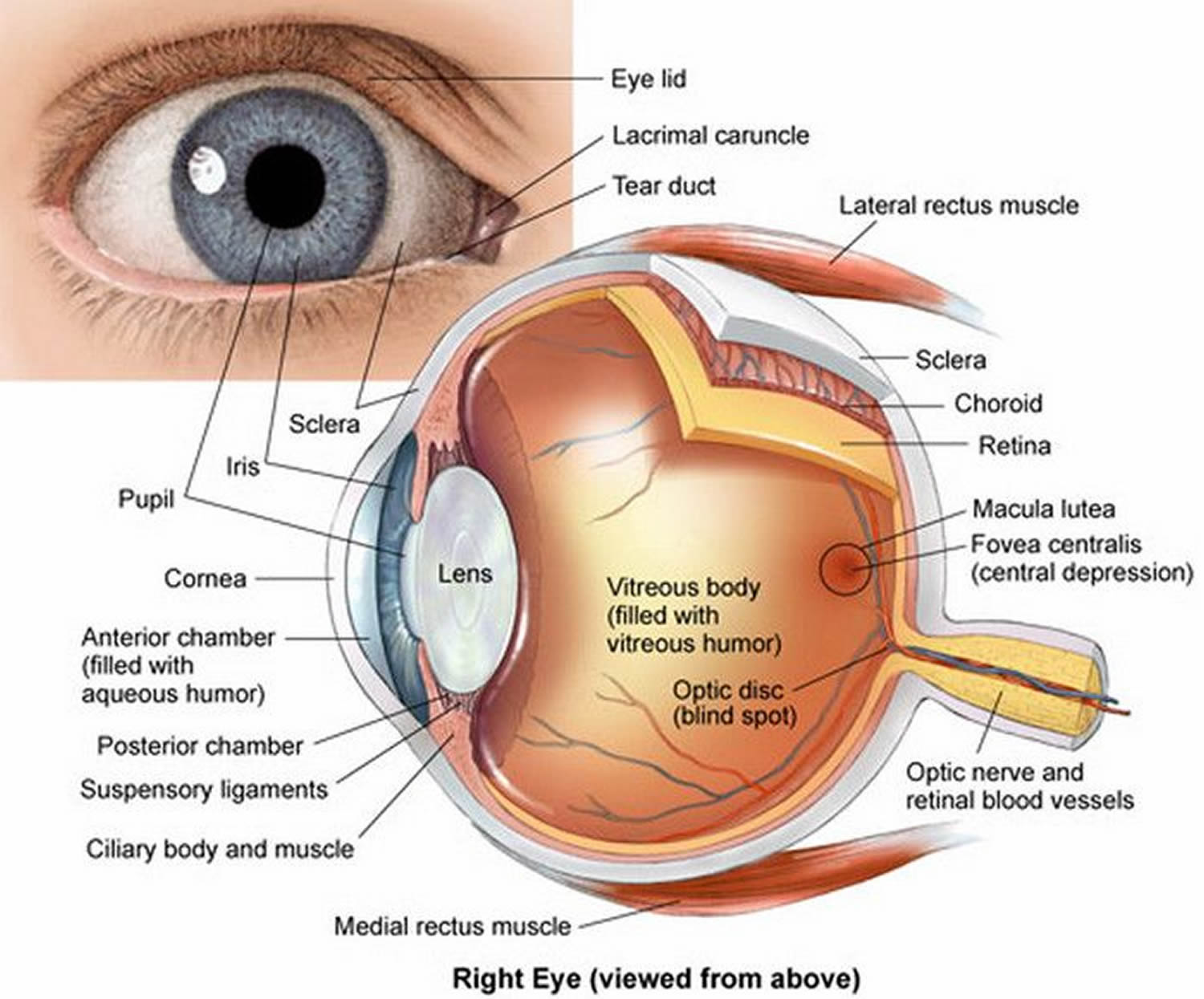
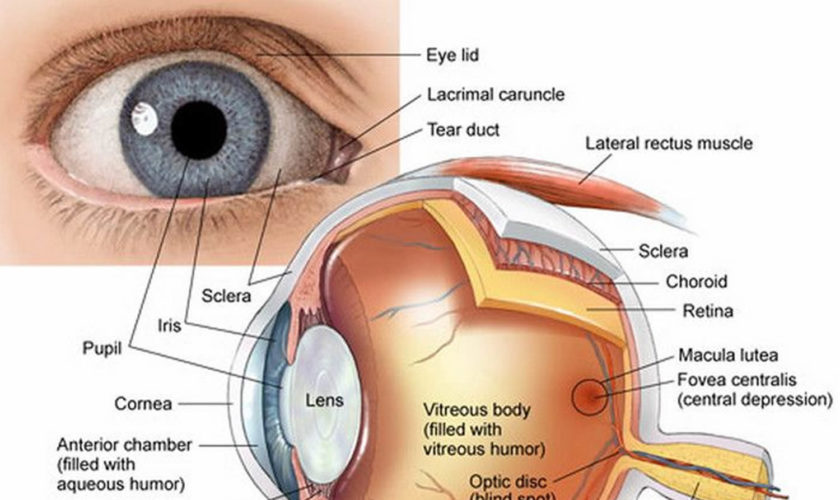
Figure 1. Eye anatomy
Figure 2. Congenital glaucoma
Footnote: Primary congenital glaucoma with cloudy corneas.
[Source 6 ]Figure 3. Buphthalmos in primary congenital glaucoma
Footnotes: Buphthalmos is derived from “ox-eyed” in Greek. Buphthalmos describes the visible enlargement of the eyeball at birth or soon after due to increased intraocular pressure (IOP) 15. Buphthalmic eyes typically have corneal diameters exceeding 12 mm in newborns or 13 mm in children older than 1 year 15. The corneal diameters sometimes exceed 16 mm, with the globe appearing noticeably enlarged. Normal corneal diameters are 9.5 to 10.5 mm at birth and 11 to 12 mm by age 1 16. Primary congenital glaucoma (onset at birth) and primary infantile glaucoma (onset after birth to 3 years) are the most frequent causes of buphthalmos 17, 18. Corneal edema, increased corneal diameter, and optic disc cupping are the classical manifestations in patients with buphthalmos 19.
[Source 20 ]Figure 4. Haab striae in primary congenital glaucoma
Footnotes: Haab striae are curvilinear breaks in Descemet’s membrane, resulting acutely from stretching of the cornea in primary congenital glaucoma. Haab striae are typically oriented horizontally or concentric to the limbus in contrast to Descemet’s tears, resulting from birth trauma, that are usually vertical or obliquely oriented.
[Source 21 ]How would a parent know if a child is suffering from glaucoma?
In keeping with the rest of an infant the immature eye is floppy and somewhat elastic. Thus early in life (that is before the second birthday) raised intraocular pressure (IOP) will stretch the eye and actually cause it to increase in size that is the eyeball expands in all directions rather like a balloon being inflated. Early medical writers termed this buphthalmos (ox eye) as this increase in size of the eye was thought to make the infant’s eye look like an ox’s eye.
The stretching of the eye has a number of harmful effects on the eye. As the eye enlarges the cornea increases in size. One of the many layers of the cornea called the Descemet’s membrane does not have much give and rather than stretch it will split as the eye enlarges leading to “striae”, which are areas of bare stroma bordered by two separated edges of Descemet’s membrane that become ridges due to deposition of hyaline 22. These are called Haab striae (see Figure 4) and are associated with acute overlying focal corneal edema when the intraocular pressure (IOP) is high. Haab striae occur in about 25% of primary congenital glaucoma eyes presenting at birth, and more than 60% of primary congenital glaucoma eyes identified at 6 months of age 23. There may be single or multiple, and are oriented horizontally or obliquely. In addition, the Descemet’s membrane splitting results in the cornea losing some of its clarity and becoming cloudy. This cloudiness of the cornea is the result of fluid entering the cornea from the anterior chamber via the splits in Descemet’s membrane because the endothelial cell layer cannot pump fluid out of the cornea in an eye with elevated intraocular pressure (IOP). This cloudiness of the cornea is known as corneal edema. Corneal edema causes discomfort and sensitivity to light and increased tear production.
After normalization of intraocular pressure (IOP), corneal edema may clear; however, Haab striae remain and may be associated with corneal scarring. In poorly controlled cases of primary congenital glaucoma may end up with dense stromal opacification even after intraocular pressure (IOP) is controlled.
Therefore, the main features of childhood glaucoma that may be identified by a parent are photophobia (sensitivity to light), increased tearing with an enlarged and cloudy cornea.
What happens when glaucoma is suspected in a young child?
The child needs to be assessed by an ophthalmologist (eye specialist). Ideally the child should be referred to an ophthalmologist with experience in managing childhood glaucoma. Often an examination under anesthetic is required to adequately examine the child and confirm the diagnosis of glaucoma. The diagnosis is confirmed by the presence of typical corneal changes (enlargement, clouding and splits in Descemet’s membrane called Haab striae), raised intraocular pressure (IOP) and optic disc cupping.
Will my child’s vision be impaired?
Severe loss of vision due to infantile glaucoma is fortunately rare. However, if glaucoma is not appropriately treated there is a risk of progressive visual impairment. Rarely does childhood glaucoma result in severe visual impairment but life-long follow-up is needed for all children after a diagnosis of glaucoma is made. Vision impairment is particularly seen if the onset of the glaucoma is at or before birth. Glasses are commonly required for myopia (short sightedness). This is due to the overall length of the eyeball being increased by the raised intraocular pressure. Photophobia (sensitivity to light) may be a persistent problem if the splits in Descemet’s membrane are severe.
Therefore prompt recognition and timely treatment will improve the chance of a good vision outcome.
Will my child’s lifestyle need to alter in any way?
Most children with childhood glaucoma lead normal lives. Glasses may be required for focusing errors or photophobia (sensitivity to light). Adolescents often have difficulty accepting the need for long-term medication and regular medical review. Ensuring compliance with regular use of eye drops may be especially difficult. The small number of children with more severe visual impairment will require some degree of help at school.
Is childhood glaucoma hereditary?
Some types of childhood glaucoma are hereditary. About 10% of primary congenital glaucoma or infantile glaucoma cases are inherited. Recent research has identified some specific gene mutations linked to this disease; for which genetic testing and counseling for affected families is may be available.
Other conditions that cause secondary glaucoma can be inherited. For example, neurofibromatosis and aniridia are dominantly inherited and are passed on to the children of affected individuals approximately 50% of the time. The incidence of glaucoma that occurs in association with these conditions, however, is less predictable.
What are the chances of another baby of mine developing glaucoma?
The risk is not zero but it is quite low. Primary open angle glaucoma in adolescents may show a familial tendency just as in adult open angle glaucoma. Inherited juvenile open angle glaucoma is well recognized but very rare. This form of glaucoma is generally not detectable till the twenties rather than during later childhood.
Aqueous Humor Production and Physiology
The aqueous humor is a water-like fluid that is produced by the ciliary body epithelium that sits directly behind the iris (the colored part of your eye). Aqueous humor is produced at a rate of 2-3 microliters per minute (2-3 μL/minute) 24, 25. The aqueous humor is composed of organic and inorganic ions, carbon dioxide, amino acids, carbohydrates, glutathione, and water 24, 26. The aqueous humor fills the anterior chamber of your eye with continual production, secretion, and reabsorption 24. The production, circulation and reabsorption of aqueous humor are vital processes maintaining homeostasis of the eye. Imbalances between the production and secretion of aqueous humor may lead to increased intraocular pressure (IOP) and optic nerve damage such as in the setting of ocular hypertension or glaucoma.
Aqueous humor functions as a physical component allowing clear optics and filling the anterior chamber of the eye 24, 25. The aqueous humor is responsible for providing nourishment to the avascular components of the anterior chamber including the cornea and lens 24, 25. In addition, aqueous humor is responsible for removing waste products, blood, macrophages and other debris from the anterior chamber, including the trabecular meshwork 24, 25. The structure and function of the trabecular meshwork may become compromised by chronic oxidative stress from reactive oxygen species and insufficient antioxidant defense in the aqueous humor 24, 25, 27, 28. Decreased levels of antioxidants in aqueous humor are present in glaucomatous eyes versus normal eyes, consistent with the presence of increased oxidative stress and low-grade inflammation 27, 28.
The primary anatomic structures vital to the homeostasis of aqueous humor include the ciliary body as the site of principle production, and the trabecular meshwork and uveoscleral pathway as the sites of primary outflow 24, 29. Aqueous humor is produced by the ciliary body via a multistep process closely correlating with systemic vascular blood flow 24, 30, 31. Initially, blood enters the ciliary processes, which propels ultrafiltrate from the blood into the ciliary interstitial space via a pressure gradient 24, 30, 31. Next, the ciliary epithelium transports plasma components from the basal to the apical surface in order to synthesize aqueous humor and transport it into the posterior chamber 24, 30, 31. Passive diffusion and ultrafiltration are key in initial synthesis, and active secretion across a blood-aqueous barrier via aquaporins, Na-K-ATPase and carbonic anhydrase enzymes are necessary for final synthesis 24, 30, 31, 32. These active transport enzymes necessary for final synthesis are common pharmacologic targets in decreasing aqueous humor production. Although systemic blood flow via the ciliary artery is required for the initial production of ultrafiltrate, the production of aqueous humor is independent from systemic blood pressure due to a fixed rate of 4% filtration of plasma 31. Therefore, there is minimal association between systemic high blood pressure (hypertension) and elevated intraocular pressure (IOP). The estimated rate of aqueous humor production is approximately 2.4 microliters per minute (2.4 μL/minute), with diurnal variations leading to higher aqueous humor flow in the morning and lower flow in the evening 24, 30.
While aqueous humor production is well documented, the mechanism of drainage is still poorly understood.
There are 2 main drainage pathways for aqueous humor 24, 33, 30:
- The conventional pathway via trabecular meshwork, Schlemm’s canal, collector channels, and the episcleral venous system), and
- The unconventional pathway via uveoscleral, uveovortex, uveolymphatic.
The conventional pathway drainage pathways for aqueous humor involves passive drainage throughout the trabecular meshwork although the Schlemm’s canal has been documented with paracellular and intracellular pores 24, 33, 30. The trabecular meshwork is a triangular porous structure composed of a layer of connective tissue and endothelium with sympathetic innervation from superior sympathetic ganglion, and parasympathetic innervation from the ciliary ganglion 24, 33, 30. The trabecular meshwork may be divided into the uveal meshwork (iris root, ciliary body, peripheral cornea), corneoscleral meshwork (scleral spur), and juxtacanalicular meshwork (transition into Schlemm’s canal) 24, 33, 30. Schlemm’s canal is a structure with composition similar to venous vasculature, with fenestrated thin endothelium surrounded by connective tissue 24, 33, 30. After drainage through the trabecular meshwork and the Schlemm’s canal, aqueous humor continues through collector channels into the episcleral venous system which deposits into the main venous system 24, 33, 30.
Resistance to outflow through the trabecular meshwork and Schlemm’s canal has been documented although it is poorly understood, yet resistance remains an important factor in regulating intraocular pressure and the pathogenesis of glaucomatous processes. In humans, up to 75% of aqueous outflow resistance is contributed by the trabecular meshwork while the remaining 25% is due to resistance beyond Schlemm’s canal 24. The rate of outflow is directly influenced by iris and ciliary muscles which contract and relax based on cholinergic innervation and pharmacodynamics 24, 33, 30, 29, 34. In ciliary contraction, the trabecular meshwork and Schlemm’s canal dilate, decreasing resistance and increasing outflow 24, 33, 30, 29, 34. The rate of outflow is also influenced by intraocular pressure, with higher intraocular pressure altering the structure of endothelial lining in Schlemm’s canal to increase the number of porous vacuoles allowing increased outflow 24, 33, 30, 29, 34. However, it is still debated if this finding substantially contributes to increasing outflow in glaucomatous eyes 24, 33, 30, 29, 34.
The unconventional pathway involves drainage into the orbital vasculature, vortex veins and ciliary lymphatics, contributing up to 25-40% of total aqueous outflow in cynomolgus and vervet monkey models. The uveoscleral pathway involves diffusion into the sclera and episcleral through the orbital vasculature. The uveovortex pathway involves osmotic absorption of fluid through the choroid, passing into the vortex veins 33. Lastly, the uveolymphatic pathway involves drainage into lymphatic vessels within the ciliary body, although the extent of drainage under normal physiological conditions remains controversial 33. In addition, the unconventional pathway also includes corneal, iridial and retinal routes, albeit less clinically significant 35. Regardless of downflow pathway, all unconventional paths require drainage through the interstitial spaces of the ciliary muscle 33, 35. Resistance also exists within the unconventional pathway likely due to ciliary muscle tone, as seen with changes in outflow in the setting of pilocarpine, increasing ciliary tone and decreasing flow, and atropine, decreasing ciliary tone and increasing flow 33, 35. Therefore, the unconventional pathways are also clinically important in moderating intraocular pressure, and serve as a potential target in glaucoma therapy.
Figure 5. Normal aqueous outflow
Footnotes: The ciliary body is a structure that sits directly behind the iris (the colored part of your eye). One of ciliary body’s jobs is to create an important fluid called aqueous humor, a fluid that nourishes the cornea and lens. Aqueous humor flows through a specific route into the front of the eye (the anterior chamber). This route allows aqueous humor to send important nutrients and oxygen to other parts of the eye, such as the lens and cornea. The aqueous humor is produced behind the iris, flows into the anterior chamber through the pupil, and exits the eye between the iris and cornea via the trabecular meshwork, a specialized eye tissue located at the chamber angle of the eye next to the cornea 36. In a healthy eye, this is a constant process. The ciliary body is always producing aqueous humor, and 80%-90% aqueous humor is always draining through the trabecular meshwork. The trabecular meshwork is a specialized spongy tissue in the anterior chamber of the eye that regulates the outflow of aqueous humor 36. The trabecular meshwork acts as a filter, controlling how quickly aqueous humor drains out of the eye through a structure called Schlemm’s canal, ultimately maintaining intraocular pressure (IOP). The canal of Schlemm, also known as Schlemm’s canal or the scleral venous sinus, is a circular, lymphatic-like vessel in the eye that drains aqueous humor from the anterior chamber into the episcleral blood vessels. The canal of Schlemm and the trabecular meshwork (TM) play a crucial role in maintaining intraocular pressure (IOP) by facilitating the outflow of aqueous humor. Too much aqueous humor production or obstruction of its outflow causes a rise in intraocular pressure (IOP) that can lead to glaucoma.
[Source 37 ]Childhood Glaucoma types
According to the Childhood Glaucoma Research Network (CGRN) classification, childhood glaucoma is classified into primary glaucoma, secondary glaucoma and glaucoma suspect (Figure 6) 2.
- Primary childhood glaucoma encompasses primary congenital glaucoma (PCG) and juvenile open-angle glaucoma (JOAG)
- Primary congenital glaucoma (PCG) is further classified as:
- Neonatal onset (0–1 month of age),
- Infantile onset (1–24 months of age),
- Late onset or late recognition of disease (>2 years of age),
- Spontaneously arrested primary congenital glaucoma (PCG). Spontaneously arrested primary congenital glaucoma (PCG) was diagnosed in the presence of buphthalmos and Haab striae, with normal intraocular pressure (IOP), normal-appearing optic discs, and no corneal edema 38.
- Juvenile open-angle glaucoma (JOAG) is defined as a diagnosis of open-angle glaucoma between age 4 to less than 40 years of age, not exhibiting features of primary congenital glaucoma (PCG) (i.e., buphthalmos, Haab striae). Individuals were further reported to have normal-tension glaucoma (NTG, described as maximum recorded IOP ≤ 21 mmHg) or high-tension glaucoma (HTG, maximum recorded IOP > 21 mmHg) in the affected eye/s, where possible.
- Primary congenital glaucoma (PCG) is further classified as:
- Secondary childhood glaucoma is classified based on the underlying pathology. Secondary childhood glaucoma includes glaucoma associated with nonacquired ocular anomalies (e.g., Axenfeld-Rieger spectrum, iris hypoplasia, aniridia), glaucoma associated with nonacquired systemic disease (e.g., phacomatoses, Juvenile Idiopathic Arthritis [JIA]), and glaucoma associated with acquired conditions (e.g., uveitis, trauma, or intraocular surgery). Glaucoma following cataract surgery is classified separately 39.
- Glaucoma associated with acquired conditions in which glaucoma is secondary to a condition that is not present at birth.
- Glaucoma associated with nonacquired ocular anomalies in which glaucoma is secondary to a nonacquired condition that is predominantly ocular.
- Glaucoma associated with nonacquired systemic disease in which glaucoma develops in the presence of a disease that is predominantly systemic, with or without ocular manifestations.
- Glaucoma following cataract surgery in which cataract surgery precedes glaucoma onset regardless of any coexisting ocular or systemic abnormality.
As per the Childhood Glaucoma Research Network (CGRN) classification, individuals were classified as having glaucoma associated with nonacquired ocular anomalies, even in the presence of systemic disease, if the disorder was predominantly ocular 2. This includes individuals with Peters’ anomaly or Axenfeld-Rieger spectrum (ARS) 2. Peters anomaly is a rare congenital disorder characterized by central corneal opacity with a relatively clear peripheral cornea, often with iris and lens adhesions 40. Peters anomaly can have associated systemic abnormalities like cleft lip, cleft palate, short stature, abnormal ears, and intellectual disability 40. Individuals with only posterior embryotoxon and no systemic features were not considered to have Axenfeld-Rieger spectrum (ARS) as per the 9th Consensus Report of the World Glaucoma Association 2. When an individual had anterior segment dysgenesis (ASD) that did not fit a specific phenotype, experts used the term “unclassified ASD” as recommended by Idrees et al 41. Individuals with primary angle-closure glaucoma were classified as having glaucoma associated with nonacquired ocular anomalies because this entity is caused by anatomic disorders of the iris, lens, and retrolenticular structures 42.
Figure 6. Childhood Glaucoma types
Footnotes: Childhood Glaucoma Research Network and World Glaucoma Association algorithm for the classification of childhood glaucoma.
Abbreviations: AL = axial length; C/D = cup-disc; JOAG = juvenile open-angle glaucoma; ROP = retinopathy of prematurity; VF = visual field
[Source 2 ]What is Glaucoma Suspect?
Eye specialist (ophthalmologist) will refer to someone as a “glaucoma suspect” if they think the person might be showing early signs of glaucoma such as higher than normal eye pressure called ocular hypertension but have no signs of optic nerve damage. Glaucoma suspects have no symptoms to suggest eye disease. They are usually identified as glaucoma suspects during routine checks by their optometrist. Many people suspected of having glaucoma at this stage turn out not to have it at all, but some do develop it in time and it is these people who can benefit the most from timely treatment. Their ophthalmologist (eye specialist) may notice something different about their optic nerve. Most “glaucoma suspects” have no symptoms. That is why you need to be carefully monitored by your ophthalmologist if you are a glaucoma suspect. An ophthalmologist can check for any changes over time and begin treatment if needed.
If someone has a very high intraocular pressure (high IOP) or very advanced optic nerve damage then the diagnosis of glaucoma is usually straightforward. However sometimes it is not entirely clear whether someone has glaucoma or not. The early signs of glaucoma can be subtle, and many glaucoma patients have a normal pressure.
There is no single test that is 100% effective in confirming the diagnosis of glaucoma all the time. Sometimes the only way to be sure that someone has glaucoma is to arrange follow up eye examinations every 4-6 months or so to work out whether progressive damage is occurring to the optic nerve in one or both eyes. Features in the examination which might lead to a patient being classified as a ‘glaucoma suspect’ include (glaucoma suspect requires at least one criteria) 2:
- Intraocular pressure (IOP) >21 mm Hg on two separate occasions
- A ‘suspicious’ optic disc appearance on examination such as ‘cupping’ of the disc or thinning of the neuro-retinal rim or nerve fiber layers.
- Unusual or defective visual fields
- Increased corneal diameter or axial length in the setting of normal intraocular pressure (IOP)
These are changes that can be seen with glaucoma, but can also be seen in other conditions such as farsightedness (myopia) where it may be a variation of normal.
Other risk factors for glaucoma such as a strong family history of glaucoma but without definite changes to the optic nerve as yet. Generally speaking, “glaucoma suspects” will not show any visual field defects on testing, or may show some field defects which are not yet entirely convincing as evidence of glaucoma. If you are a ‘glaucoma suspect’, the most important treatment is good follow-up care.
It is very important that someone suspected of experiencing the early onset of glaucoma has regular eye checks to make sure there is no continuing damage to the optic nerve. Even though a person is not yet receiving any treatment for glaucoma, she or he may still risk losing their vision if in fact they do turn out to have glaucoma. Thus it is very important to maintain follow-up care. Typically for a low-risk glaucoma suspect, this may require visits every 6 to 12 months. At each follow-up visit your eye doctor will check your vision and eye pressure, and examine the front and back of your eye, paying careful attention to the appearance of your optic nerves.
To examine the structure of the optic nerve, your doctor will perform a careful examination in the office, obtain optic nerve imaging, and obtain a baseline set of optic nerve photographs. To examine the function of the optic nerve, an automated visual field test we be implemented with the help of a technician, who will instruct you on the correct way to perform the test. All of these tests may be repeated at yearly intervals (or more or less frequently, as determined by your eye doctor) to assess if there are changes or “progression” over time. The follow-up visits are crucial to maintaining optimal eye health.
Sometimes eye doctors are on the fence about whether to start treatment, and it is only through repeat follow-up visits that they get a sense of whether or not someone has glaucoma. Usually a person thought to be a “glaucoma suspect” will not be treated for the condition until the diagnosis is confirmed. Typically, glaucoma advances slowly so its progress can be tracked safely without treatment until the diagnosis is confirmed.
If you’re a “glaucoma suspect” and needed treatment, initial treatment options may include topical eye drops or laser treatment of the drainage angle to increase the amount of fluid draining from the eye, both of which can lower the eye pressure. The decision to treat is often not a cut-and-dry one; your ophthalmologist will assess all of your risk factors, your examination findings, and seek your input as to whether to treat or continue to observe your eyes over time. Some patients prefer to “watch and wait” or are worried about the side effects of treatment, while others may be more risk-averse and would rather begin treatment and have peace of mind. There are some glaucoma risk calculators available but most eye doctors would agree that these may aid in diagnosis and assessment, but will not replace your doctor’s clinical judgment.
Childhood glaucoma causes
There are many causes of childhood glaucoma. It can be hereditary or it can be associated with other eye disorders.
- If childhood glaucoma cannot be attributed to any other cause, other noticeable eye defects or systemic problem, it is classified as primary congenital glaucoma. The cause of primary congenital glaucoma is not completely understood, though there is significant research to suggest that the trabecular meshwork is immature and compressed. Studies suggest that the normal posterior migration of embryonic neural crest cells destined to become the trabecular meshwork is abnormally halted 12. The drainage angle where the inside of the sclera (the white of your eye) and the outer edge of your iris meet of children with primary congenital glaucoma is described as immature, thick, and compressed. High intraocular pressures (IOP) are believed to be a consequence of increased resistance to aqueous outflow in this abnormal trabecular meshwork. Researchers have identified several gene mutations that can lead to primary congenital glaucoma. Mutations in the CYP1B1 (cytochrome P450 family 1 subfamily B member 1) gene are the predominant genetic anomalies linked to primary congenital glaucoma 13.
- If childhood glaucoma is a result of another eye disorder, eye injury, or other disease, it is classified as secondary childhood glaucoma.
- Associated with eye abnormalities e.g., Axenfeld-Rieger syndrome, aniridia (a rare genetic eye disorder characterized by the complete or partial absence of the iris, the colored part of the eye), iridotrabecular dysgenesis, Peter’s anomaly, sclerocornea (a rare, non-progressive, congenital condition where the cornea, normally transparent, becomes opaque and blends with the sclera, the white part of the eye), microcornea (a congenital condition where the cornea, the transparent front part of the eye, is smaller than normal, with a horizontal diameter of less than 10-11 mm), microphthalmos (a developmental disorder of the eye where one or both eyes are abnormally small and have anatomical malformations), ectopia lentis, persistent fetal vasculature, oculodermal melanocytosis, posterior polymorphous dystrophy,
- Associated with systemic abnormalities e.g., chromosomal disorders like trisomy 21 (Down syndrome), connective tissue disorders such as Marfan syndrome, Stickler syndrome (a group of genetic disorders that primarily affect connective tissues, particularly in the face, eyes, ears, and joints), phakomatoses (a group of genetic disorders also known as neurocutaneous syndromes or neuro-oculo-cutaneous syndromes characterized by systemic hamartomas, primarily affecting the central nervous system, eyes, and skin) common examples include neurofibromatosis (types 1 and 2), Sturge-Weber syndrome, tuberous sclerosis, Lowe syndrome and von Hippel-Lindau disease
- Glaucoma secondary to acquired causes e.g., retinopathy of prematurity, eye trauma, intraocular tumors, uveitis, eye inflammation, lens‑induced (with/without pupillary block), steroid-induced, intraocular infections, maternal rubella (congenital rubella syndrome), raised episcleral venous pressure
- Glaucoma after surgery for congenital cataract.
Childhood glaucoma commonly starts with a defect in the way your child’s eye develops. The most common defect is in the trabecular meshwork, the tissue that the eye fluids (aqueous humor) drain through. When the trabecular meshwork doesn’t develop right, the aqueous humor fluids don’t drain properly. The buildup of fluids (aqueous humor) causes pressure in your child’s eye, which damages their optic nerve. It can also cause their cornea to enlarge, stretch, tear and scar. This process is progressive. How fast it progresses depends on how severe the defect in your child’s eye is, how much fluid (aqueous humor) is building up and how high the pressure is inside the eye (intraocular pressure [IOP]). When glaucoma appears in young infants, it’s because these conditions were already progressing during fetal development. When symptoms appear later, it’s because these conditions were less severe at birth, so they took longer to build up.
Primary congenital glaucoma
Most cases of primary congenital glaucoma are sporadic without a family history of the disease 4, 6, 1, 43. The significant risk factors for primary congenital glaucoma are consanguineous marriage also known as cousin marriage (a marriage between two individuals related by blood, typically first or second cousins, or closer), genetic predisposition, and first-degree relatives (including siblings) with glaucoma. Approximately 90% of cases belong to this category. About 10-40% are familial with an autosomal recessive inheritance pattern with incomplete penetrance ranging from 40% to 100% 2, 4. Autosomal dominant inheritance has also been reported 44.
Mutations in the CYP1B1 (cytochrome P450 family 1 subfamily B member 1) gene are the predominant genetic anomalies linked to primary congenital glaucoma 13. The CYP1B1 (cytochrome P450 family 1 subfamily B member 1) gene is essential for the formation of the trabecular meshwork and the anterior portion of the eye. The CYP1B1 gene codes for an enzyme that metabolizes compounds vital for the developing eye, such as fatty acids and vitamins 45, and is expressed in fetal and adult neuroepithelium and ciliary body 2, 46. Severe trabecular meshwork atrophy is seen in mouse models deficient of CYP1B1 47. In zebrafish, CYP1B1 has been found to indirectly affect neural crest migration to the anterior segment and angle by playing a role in ocular fissure closure 48. While the exact mechanism by which CYP1B1 mutations causes primary congenital glaucoma is unknown, scientists know that levels of a protein product of this gene are inadequate for appropriate embryogenic ocular development, resulting in goniodysgenesis. A twin study demonstrated that CYP1B1 gene activity may be implicated in a common pathway primary congenital glaucoma, juvenile open-angle glaucoma (JOAG), and primary open angle glaucoma (POAG). Recent studies propose that the CYP1B1 mutation may also interfere with the ability of retinal ganglion cells to respond to the stress generated by high intraocular pressure (IOP) and the resultant increase in reactive oxygen species 49, 50. CYP1B1 mutations are associated with 15-20% of primary congenital glaucoma cases in Japan and the United States, 75-100% of cases in Saudi Arabia, and all cases in Slovakia Roma 51, 52.
Additional implicated genes, including LTBP2 (latent transforming growth factor beta binding protein 2), are located next to the GLC3C locus 53, 54. These genetic mutations cause a dysfunctional trabecular meshwork, obstructing proper drainage of aqueous fluid and increasing intraocular pressure (IOP). Several gene loci have been linked to primary congenital glaucoma, which includes GLC3A, GLC3B, GLC3C, GLC3D, and GLC3E. Locus GLC3A has been linked to the CYP1B1 gene 55.
Mutations in CYP1B1 (cytochrome P450 family 1 subfamily B member 1) gene are most commonly responsible for autosomal recessively inherited cases 56. A recent systematic review reported that CYP1B1 was the most common gene mutation reported in the current literature and that the other gene variants related to childhood glaucoma included MYOC (myocilin), LTBP2, FOXC1 (forkhead box C1), PITX2 (paired-like homeodomain transcription factor 2), ANGPT1 (angiopoietin 1) and TEK (or receptor tyrosine kinase) 57.
Currently, the chance of identifying a genetic cause is 40% when genetic testing is done 58.
Studies from Western countries have reported primary congenital glaucoma incidences ranging from 1 per 10,000 to 1 per 30,000 live births 59. The incidence is reportedly as high as 1/2500 in countries like Saudi Arabia. Slovakian Roma have the greatest incidence at 1/1250 60. The higher incidence in particular countries and ethnic groups is related to the higher prevalence of consanguineous marriages, particularly in those with frequent cousin-cousin marriages.
Approximately 65% to 80% of cases of primary congenital glaucoma are bilateral 58. A male-to-female ratio of 3:2 has been reported in studies from the United States and Europe 22 A Japanese study quoted a male-to-female ratio of 6:5 in patients with CYP1BI mutation and 19:2 without the mutation 61. Several studies have reported that glaucoma accounted for 7% to 18% of children registered in blind schools 62, 63. Asia, India, and Saudi Arabia have a mean presentation age of 3 to 4 months compared to 11 months in Western countries 64. primary congenital glaucoma appears earlier in high-incidence ethnicities.
Risk factors for developing primary congenital glaucoma
The only known risk factors are genetic – consanguinity and affected siblings. Parents of primary congenital glaucoma patients should be aware that the chance of a second child with primary congenital glaucoma is a small but real risk that usually is no more than 3%. If two children have the disease, then the risk of subsequent children increases to as high as 25%, with the assumption of autosomal recessive inheritance 22. In 2018, Yu-Wai-Man et al 58 compiled the clinical utility gene card for primary congenital glaucoma which describes situations for which gene testing may be useful. Carriers of the CYP1B1 gene mutation and double null CYP1B1 alleles are, on average, more likely to have higher intraocular pressure (IOP) and require more surgeries 45.
Primary congenital glaucoma pathophysiology
The primary pathophysiologic process in primary congenital glaucoma is the defect in the development of the trabecular meshwork and the anterior chamber angle. This hampers the aqueous outflow through the anterior chamber and increases intraocular pressure (IOP). In 1955 and 1966, Barkan 65 and Worst 66 proposed that the presence of an imperforate membrane at the angle of the anterior chamber impeded the aqueous outflow; this was later disproved. The obstruction site is trabecular, as opposed to pretrabecular. The isolated maldevelopment of the trabecular meshwork, known as isolated trabeculodysgenesis, is the fundamental disease 67.
The formation of an immature angle is believed to stem from the developmental arrest of tissues originating from neural crest cells during the third trimester of gestation. The degree of angle abnormalities is contingent upon the point at which angle development is halted 68. The pathophysiology is believed to result from compacted thick trabecular sheets that merge and inhibit the posterior movement of the iris during the development of the anterior portion. The trabecular sheets position the iris more anteriorly, leading to the iris’ characteristic “high” insertion in children with primary congenital glaucoma.
Currently, the most accepted theory of the pathogenesis of primary congenital glaucoma proposed by Anderson states that excessive or premature accumulation of collagenous beams within the trabecular meshwork prevents normal insertion of the ciliary body and iris 69. This results in an anteriorly inserted iris root and ciliary muscle, which can obstruct the trabecular meshwork, and narrow or completely compress the Schlemm canal elevating intraocular pressure (IOP) 70, 69. Increased intraocular pressure (IOP) leads to the typical symptoms of buphthalmos (enlargement of the eye) and Haab striae (breaks in the Descemet membrane). Histopathological and electron microscopic studies of primary congenital glaucoma have demonstrated obstruction through the outflow pathway 70. Frequently, the ciliary muscle is inserted high on the trabecular meshwork. Moreover, a detailed framework analysis has shown an excessive amount of collagen in the trabecular meshwork. Other studies have demonstrated fibrillary collagen fibers, elastin fibers, and ground substances in the intervening trabecular meshwork and the canal of Schlemm 71. The microscopic observations explain the clinical manifestation of elevated intraocular pressure (IOP) and optic nerve impairment.
More recently, ultrasound biomicroscopy and anterior segment optical coherence tomography have been used to determine angle abnormalities in primary congenital glaucoma patients 72.
Childhood glaucoma prevention
There is no known way to prevent primary congenital glaucoma. Early detection and treatment are essential to maximize visual potential. A family history of glaucoma and a parental consanguineous marriage are essential elements to consider when considering a diagnosis of primary congenital glaucoma 73.
In the future, prenatal genetic screening may emerge as a preventative measure. It can be offered to parents in at-risk populations, such as those with family history or in consanguineous relationships in areas with higher primary congenital glaucoma prevalence (Slovakia, Saudi Arabia, China, etc.). Parents with unborn children who test positively for mutations in CYP1B1 on genetic screening can be alerted about the potential need for urgent surgical management soon after birth 45.
Childhood glaucoma signs and symptoms
Childhood glaucoma symptoms may not be as obvious in children. The following are the most common symptoms of childhood glaucoma. However, each child may experience symptoms differently. Symptoms may include:
- Excessive tearing (epiphora)
- Eye(s) that is sensitive to light (photophobia)
- Closure of one or both eyes in the light
- Cloudy, enlarged cornea (cloudy cornea)
- One eye may be larger than the other (bupthalmos)
- Vision loss
The classic triad of childhood glaucoma symptoms includes:
- Epiphora (watery eyes, tearing).
- Photophobia (light sensitivity).
- Blepharospasm (uncontrollable eyelid twitching).
Other signs of childhood glaucoma may include:
- Buphthalmos (enlarged eyeballs or ox-eye).
- Bluish discoloration of the eyeball.
- Whitening or clouding of the cornea.
You may or may not be able to tell that your child has vision issues, like:
- Blurry vision (astigmatism).
- Nearsightedness (myopia).
- Favoring one eye (anisometropia).
An eye exam might reveal further signs of glaucoma, like:
- Corneal edema (swelling).
- Tears in the cornea.
- Corneal scarring.
Children usually have signs and symptoms in both eyes. But sometimes, they appear only in one.
If the eye pressure increases rapidly, there may be pain and discomfort. Parents may notice that the child becomes irritable, fussy, and develops a poor appetite. Early detection and diagnosis is very important to prevent loss of vision. The symptoms of glaucoma may resemble other eye problems or medical conditions. Always consult your child’s doctor for a diagnosis.
Childhood glaucoma complications
Untreated intraocular pressure (IOP) or delayed treatment in an infant eye may lead to severe complications and significant visual impairment in addition to permanent optic nerve damage and glaucomatous visual field defects. High intraocular pressure (IOP) causes corneal edema and corneal stretching with development of Haab striae. With prolonged corneal edema, both diffuse and focal overlying Haab striae, the cornea can become permanently opacified. Buphthalmos with axial elongation, and Haab striae cause abnormally high refractive errors including myopia and astigmatism, that can impair vision both by blurring vision and causing refractive amblyopia, which can be exacerbated by anisometropia in unilateral cases. In severe buphthalmos, with continued stretching, the lens could dislocate, and risk of retinal complications increases (i.e. lacquer cracks and retinal detachments). Overcoming these complications can be difficult in severe cases. Corneal transplantation for corneal opacification is avoided if possible due to high risk of failure and complications in young children.
Childhood glaucoma diagnosis
The diagnosis of childhood glaucoma can often be made clinically via thorough and precise ophthalmologic assessment, even without an accurate measurement of intraocular pressure (IOP). The hallmark of primary congenital glaucoma, however, is an elevated intraocular pressure (IOP) and ocular stretching in the absence of other ocular and systemic conditions that can cause glaucoma, such as Axenfeld-Reiger syndrome, aniridia, or surgical removal of cataract in infancy (i.e. glaucoma following cataract surgery) 74 .
The clinical diagnosis of primary congenital glaucoma can be difficult, especially when a child does not cooperate with intraocular pressure (IOP) measurement. If a reliable intraocular pressure (IOP) measurement is elevated in the setting of other classic signs of ocular stretching, then the diagnosis of glaucoma is made, and if no other ocular or systemic developmental anomalies are seen, then primary congenital glaucoma is the diagnosis. The presence of Haab striae suggests congenital glaucoma, and if seen without ocular developmental anomalies or systemic syndromes, then primary congenital glaucoma is the diagnosis. If intraocular pressure (IOP) is normal with Haab striae, then one may have a case of spontaneously arrested primary congenital glaucoma, which still needs to be followed over time for elevated intraocular pressure (IOP) 6.
Medical History
Primary congenital glaucoma patients often present to the physician’s office due to abnormal appearance of the eyes such as a cloudiness or a blue tint to the eyes, or patient behavior such as eye rubbing or shying away from light. While there may be tearing, there is no ocular discharge and usually no eye redness. The patients are otherwise healthy. A positive family history is helpful but often is not present since most cases are sporadic.
Physical Examination
The clinical examination must include 75:
- Fixation of light: The patient’s ability to fixate and follow light should be tested with each eye separately. There may be exotropia (where one or both eyes turn outward, away from the nose) due to poor fixation and nystagmus in long-standing cases.
- Sclera: The sclera(e) may appear bluish in color because of high myopia, scleral thinning, and exposure to underlying uveal tissue 76.
- Cornea: Corneal examination might reveal signs of corneal enlargement or buphthalmos. Normal corneal size from birth to 6 months should be between 9.5 to 11.5 mm. A size of greater than 12 mm should raise the suspicion of glaucoma. A corneal diameter of more than 13 mm in any child older than 6 months indicates corneal enlargement. The slit-lamp examination may reveal horizontal or oblique tears and breaks in the Descemet membrane called Haab striae (see Figure 4). Another critical finding is corneal edema. This usually starts as epithelial edema and then gradually involves the deeper layers of the cornea, occasionally causing permanent opacities impairing vision profoundly 77.
- Anterior chamber: The anterior chamber is usually deep.
- Iris: Iridodonesis, ectropion uvea, hypoplasia, or any atrophic patches may be present 78.
- Pupil: The pupil may be oval, dilated, and ischemic.
- Lens: The clinician should evaluate for lenticular opacities or lens subluxation due to excessive stretching of zonules 79.
- Optic disc: This typically demonstrates reversible cupping in the early stages. Later stages may present with an enlarged cup-to-disc ratio or even atrophy 80, 81.
- Intraocular pressure (IOP): Intraocular pressure (IOP) is usually elevated at presentation and can be measured using a pneumotonometer in the outpatient setting 74.
Childhood glaucoma signs
The main clinical signs of childhood glaucoma include elevated intraocular pressure (IOP) >21 mmHg, corneal edema and/or enlargement of the eye with buphthalmos, and Haab striae. The intraocular pressure (IOP) at presentation is usually between 30-40 mmHg, though it can be outside this range 82. Intraocular pressure (IOP) in the low-20s mmHg is acceptable if the optic nerve is healthy and the patient’s eye growth is within normal limits, but may not be if there are other more severe signs of primary congenital glaucoma.
With intraocular pressure (IOP) in the 30-40s mmHg, the cornea becomes cloudy due to diffuse and/or focal edema. As in adult eyes, the endothelial cell layer cannot pump fluid out of the cornea in an eye with elevated intraocular pressure (IOP). In young children however, there is the additional insult of corneal stretching from the high intraocular pressure (IOP) causing not only enlargement of the cornea, but Descemet breaks, leading to “striae,” which are areas of bare stroma bordered by two separated edges of Descemet membrane that become ridges due to deposition of hyaline 22. These are called Haab striae and are associated with acute overlying focal corneal edema when the intraocular pressure (IOP) is high. They occur in about 25% of primary congenital glaucoma eyes presenting at birth, and more than 60% of primary congenital glaucoma eyes identified at 6 months of age 23. There may be single or multiple, and are oriented horizontally or obliquely. After normalization of intraocular pressure (IOP), corneal edema may clear; however, Haab striae remain and may be associated with corneal scarring. The poorly controlled cases of primary congenital glaucoma may end up with dense stromal opacification even after intraocular pressure (IOP) is controlled.
A newborn’s cornea is typically 9.5-10.5 mm in diameter and increases to 10.0-11.5 mm by age 1 83. Any diameter above 12.0 mm before 1 year of age suggests an abnormality, especially if there is asymmetry between the two eyes. If the diameter is greater than 13 mm at any age, glaucoma suspicion should be high. Along with corneal stretching in the setting of elevated IOP, there is stretching of the scleral wall and all tissues within the eye leading to buphthalmos. Corneal enlargement stops around age 3 years, while sclera can continue to stretch up to age 10 years of age 22.
Other signs related to the eye distension include abnormally deep anterior chamber, myopia (mainly due to elongation and enlargement of the eye), astigmatism (from Haab striae and corneal stretching), anisometropia (almost always present in unilateral primary congenital glaucoma), and optic nerve cupping.
The optic nerve cupping in very young children may be seen solely due to optic canal stretching and posterior bowing of the lamina cribrosa without a decrease in the neuroretinal rim 84, 85. When the IOP is normalized, there can be notable reversal of cupping. While cupping may resolve, retinal nerve fiber layer damage, if present, is permanent. In older children and those with advanced glaucoma, cupping occurs due to neuroretinal rim tissue loss, especially at the vertical disk poles 22.
Any asymmetry between eyes in the aforementioned signs should raise suspicion of glaucoma. Lastly, amblyopia, either deprivation or both, may also be present with the other signs mentioned above.
Diagnostic procedures
The main diagnostic test for primary congenital glaucoma is the measurement of the intraocular pressure (IOP), which should be done prior to instilling dilating drops. In a cooperative infant or young child, this measurement can be obtained in the clinic setting with a Perkins applanation tonometer, Tono-pen (a portable Mackay-Marg-type tonometer) and/or Icare rebound tonometer. In older patients, standard Goldmann applanation tonometry can be performed. A pneumotonometer may be useful to confirm intraocular pressure (IOP) during examination under anesthesia or in clinic if available, and may be less influenced by corneal abnormalities. A Schiötz indentation tonometry is not recommended in these patients due to under- or overestimation of intraocular pressure (IOP) in childhood glaucoma 86, 87. For the uncooperative child, an examination under anesthesia should be performed.
Of note, the Icare rebound tonometer has decreased the need for examinations under anesthesia as it does not require a topical anesthetic 88. Two models available in the United States (Icare TAO1i and Icare ic100) require the patient to be upright, while the newest model, recently approved in the US (Icare ic200), allows measurement in a supine patient. The IOP measured by Icare in cooperative, awake children with known or suspected glauacoma, has been shown to be within 3 mmHg of IOP obtained by Goldmann applanation tonometry in 63% and is higher than measured by Goldmann applanation tonometry in 75% of children 89. By contrast, Icare tonometry may under-measure the IOP compared to Tono-pen readings in the setting of corneal edema 90.
Because anesthetic agents variably alter the intraocular pressure (IOP), with most lowering intraocular pressure (IOP), measurements should be obtained as soon as possible after induction of anesthesia and before intubation. If the intraocular pressure (IOP) is actually elevated, it often remains greater than 20 mmHg under anesthesia, which suggests glaucoma. The normal intraocular pressure (IOP) is lower in infants and young children than adults. A newborn has an average intraocular pressure (IOP) of 10-12 mm Hg, increasing to 14 mm Hg by age 7 or 8 years of age. An asymmetric measurement or an elevated intraocular pressure (IOP) measurement in the presence of other clinical signs helps make the diagnosis of glaucoma.
Corneal diameter measurement is another key diagnostic procedure for primary congenital glaucoma. Some providers check horizontal diameters only, while some check horizontal and vertical diameters. If there is pannus or scarring obscuring the limbus, the measurement may not be accurate. In the office a millimeter ruler can be placed above the eyes and if the child is not cooperative, a close-up digital photograph can be taken with the ruler in place, and a measurement can be made from the photo. This is most amenable to horizontal corneal diameter measurement. While under anesthesia, calipers with the tips placed at the limbus 180 degrees apart are used across the widest diameter, and then measured with a graduated ruler to check the measurement. Ideally, the measurement can be estimated to the nearest 0.25 mm 2.
Examination for Haab striae is done with an oblique slit beam with a portable slit lamp if the patient is younger or under anesthesia, or on a regular slit lamp in the clinic if the patient is older. Retroillumination can also be used to identify Haab striae. . In older patients with treated primary congenital glaucoma, corneal endothelial protuberances and hyperproliferation of the Descemet membrane/pre-Descemet’s layer complex have been demonstrated with anterior segment OCT (ASOCT). These may demarcate areas in which the edges of the Descemet membrane have re-approximated during the healing process 77.
If a view through the cornea allows it, gonioscopy is done in clinic if tolerated, ideally a Sussman (or similar) indirect gonioscopy lens as it fits easily between a young child’s small palpebral fissure. Using a gonioscopy lens without a handle may be easier as it allows the examiner to hold open the eyelids while placing the lens. More commonly, for initial diagnosis of primary congenital glaucoma, gonioscopy is performed under under anesthesia with a Koeppe or similar direct gonioscopy lens and portable slit lamp. There are different sized Koeppe lenses to fit different corneal diameters. The Koeppe lens is best handled with a glove to avoid fingerprint smudges. The Koeppe lens cup is filled with balanced salt solution and placed quickly on the eye or placed on the eye and tilted with one edge abutting the sclera while filling the space between the lens and eye with solution. Then a binocular microscope such as the portable slit lamp is angled towards the angle of interest and the lens can be shifted slightly toward the angle to optimize the view.
Gonioscopy in these cases helps guide surgical planning in cases of primary congenital glaucoma, and may also identify other angle abnormalities which might identify other secondary glaucoma types, for example Axenfeld-Rieger anomaly (many irido-corneal attachments with anteriorly placed Schwalbe line). Infants with primary congenital glaucoma usually do not have a visible scleral spur because the peripheral iris inserts into the trabecular meshwork (in contrast to normal infants whose peripheral iris and ciliary body have recessed to the scleral spur or posterior to it). There may also be scalloped edges of the peripheral iris and pale peripheral iris stroma in front of the angle causing a “morning mist” appearance. If there are peripheral anterior synechiae, posterior embryotoxon, or other abnormalities, then the diagnosis is unlikely primary congenital glaucoma. Gonioscopy photographs can be taken by instilling the eye with coupling gel and angling the camera lens (i.e. RetCam) obliquely toward the angle of interest and adjusting the focus until the angle comes into clear view.
Axial length is measured with A-scan ultrasonography, ideally using the immersion and not contact method, either in clinic or under anesthesia. It is best done under anesthesia, during baseline examination to determine if the axial length is greater than normal for the patient’s age, and repeated approximately every 3-4 months to assess if the growth rate is greater than average. Of note, measuring axial length itself is not an indication for examination under anesthesia if a patient is otherwise doing well, and can be performed at intervals when examination under anesthesia is needed for clinical management. Sampaolesi and Kiskis provided linear regressions from data of normal children. Sampaolesi used immersion A-scans and found the normal axial length for a one-month-old lies between 17.25 mm (5th percentile) and 20.25 mm (95th percentile). Sampaolesi also recommended that axial length be measured after dilation with cycloplegic drops 91, 2.
Optic nerve evaluation is performed with either indirect or direct ophthalmoloscopy with attention to the cup-to-disc ratio. In the setting of a small pupil, a magnified view of the nerve can be obtained by using a direct ophthalmoscope through a Koeppe gonioscopy lens on the eye. Fundus photography is also recommended for comparisons between serial examinations. B-scan ultrasonography is recommended if the cornea does not allow fundus examination to rule out posterior disease. Severe optic nerve cupping may sometimes be noted on the posterior B-scan.
Pachymetry is used to measure central corneal thickness. The central cornea may be thicker due to corneal edema, and has also shown to be thinner in primary congenital glaucoma patients without corneal edema, likely due to stretching of their tissues 92. Other small studies have shown either no significant difference in central corneal thickness between normal eyes and eyes treated for primary congenital glaucoma, or the central corneal thickness was thicker in eyes treated for primary congenital glaucoma than in normal eyes 93, 94. Corneal hysteresis and corneal resistance factor have been found to be lower in eyes with primary congenital glaucoma compared to normal eyes 93, 94.
Perimetry can be attempted starting around age 7-8 years of age if the patient does not have nystagmus, cognitive impairment or severe vision loss. Quicker testing algorithms such as SITA-FAST may allow children to perform more reliably 95. Goldman perimetry can be very helpful in young children.
Standard tabletop optical coherence tomography (OCT) can be considered once a child can be examined at the regular slit lamp to evaluate the retinal nerve fiber layer and ganglion cell layer. It may be helpful especially if the child cannot perform perimetry. While devices currently do not carry normative data for children, studies have collected data on normal children 96, 97, 98, 99. Handheld and mounted spectral-domain OCT devices are emerging technologies that can be used during examination under anesthesia 100, 101.
Childhood glaucoma diagnostic criteria
Childhood glaucoma diagnostic criteria per Childhood Glaucoma Research Network definition 2:
Definition of childhood glaucoma required two or more of the main categories (1‑5)
- Intraocular pressure (IOP) >21 mm Hg (investigator discretion if examination under anaesthesia data alone)
- Optic disc cupping
- Progressive increase in cup‑disc ratio
- Cup‑disc asymmetry of ≥0.2 when disc sizes are similar
- Focal rim thinning
- Corneal findings
- Haab striae
- Diameter
- >11 mm in newborn
- >12 mm in child <1 year of age
- >13 mm any age
- Progressive myopia /myopic shift coupled with an increase in ocular dimensions out of keeping with normal growth
- Reproducible visual field defect that is consistent with glaucomatous optic neuropathy with no other observable reason for the visual field defect
Childhood glaucoma differential diagnosis
The differential diagnoses of childhood glaucoma can be remembered by the mnemonic STUMPED, which includes the following conditions:
- S: Sclerocornea, congenital hereditary stromal dystrophy 102. This uncommon disorder is characterized by corneal opacification and may be mistaken for primary congenital glaucoma. A flattened cornea with no concomitant elevation in IOP or optic nerve impairment defines Sclerocornea.
- T: Trauma, tears in Descemet membrane or endothelial (ie, from forceps).
- U: Ulcer caused by various factors, including viral, fungal, bacterial, neurotrophic, and pythium (a parasitic aquatic oomycete that causes vision-threatening keratitis) 103, 104, 105
- M: Metabolic disorders, eg, mucolipidoses, mucopolysaccharidosis, tyrisinosis
- P: Peters anomaly, an uncommon disorder characterized by central corneal opacity and adherence of the iris to the cornea, may manifest with glaucoma; the principal anomaly is the corneal-lenticular contact 106
- E: Endothelial dystrophy, congenital hereditary endothelial dystrophy, posterior polymorphous dystrophy, Fuchs dystrophy 107
- D: Dermoid 108
Other significant differentials which should be kept in mind include:
- Interstitial keratitis
- High myopia
- Megalocornea
- Corneal abrasion
- Messman dystrophy
- Reis-Buckler dystrophy
- Retinoblastoma
- Retinopathy of prematurity
- Persistent primary hyperplastic vitreous
- Traumatic glaucoma 109
- Congenital rubella syndrome
- Sturge-Weber syndrome
- Aniridia
- Optic disc pit
- Optic atrophy 110
- Coloboma
Childhood glaucoma treatment
It is important for treatment of childhood glaucoma to start as early as possible. The management of childhood glaucoma is directed toward lowering and controlling the intraocular pressure (IOP) and treating the secondary complications such as refractive change and amblyopia that develop during the course of the disease.
Childhood glaucoma treatment may include:
- Medications. Some medications cause the eye to produce less fluid, while others lower pressure by helping fluid drain from the eye.
- Surgery. The purpose of surgery is to create a new opening for fluid to leave the eye. Surgical procedures are performed by using microsurgery or lasers.
Both medications and surgery have been successfully used to treat childhood glaucoma. However, surgery is the primary treatment modality for primary congenital glaucoma. In managing secondary childhood glaucoma, medications is the first-line treatment 14.
Surgical procedures used to treat childhood glaucoma include the following:
- Trabeculotomy and goniotomy.
- Trabeculotomy is a surgical procedure, primarily used in the treatment of childhood glaucoma, that creates a new drainage opening in the eye’s trabecular meshwork, improving the outflow of aqueous humor and reducing the intraocular pressure (IOP).
- Goniotomy is a microinvasive glaucoma surgery (MIGS) technique that improves fluid flow in the eye to lower intraocular pressure (IOP). A goniotomy involves making a small incision within the trabecular meshwork, the eye’s natural drainage system, to create a more efficient pathway for fluid outflow. This procedure can be used to treat conditions like childhood glaucoma.
- Trabeculectomy. Trabeculectomy is a surgical procedure that involves the removal of part of the trabecular meshwork drainage system, allowing the fluid to drain from the eye. Trabeculectomy works by creating a new drainage pathway for the fluid (aqueous humor) within the eye, allowing it to drain into a space beneath the outer layer of the eye (conjunctiva). This new pathway, called a bleb, helps reduce eye pressure and can slow or prevent further vision loss.
- Iridotomy. Iridotomy is a surgical procedure to treat or prevent angle-closure glaucoma, a condition where the iris (colored part of the eye) blocks the drainage angle, leading to increased eye pressure. The eye surgeon may use a laser to create this hole. Laser iridotomy involves using a laser to create a small hole in the iris, allowing fluid to flow freely and preventing or relieving pressure build-up.
- Cyclophotocoagulation. Cyclophotocoagulation is a laser procedure that uses a laser beam to freeze selected areas of the ciliary body – the part of the eye that produces aqueous humor – to reduce the production of fluid and reduce intraocular pressure (IOP). Cyclophotocoagulation is a type of cyclodestruction procedure, meaning it aims to reduce intraocular pressure (IOP) by damaging the ciliary body, a key part of the eye that produces aqueous humor. This type of surgery may be performed with severe cases of childhood glaucoma.
The primary treatment of primary congenital glaucoma is angle surgery, either goniotomy or trabeculotomy, to lower intraocular pressure (IOP) by improving aqueous outflow. If angle surgery is not successful, trabeculectomy enhanced with mitomycin C or glaucoma implant surgery with a Molteno, Baerveldt, or Ahmed implant can be performed. In refractory cases, cycloablation can be performed using an Nd:YAG laser, diode laser, or cryotherapy, with diode laser being the most widely used device. Medications, either topically or orally, is typically used as a temporizing measure prior to surgery and to help decrease corneal clouding to facilitate goniotomy, and to supplement intraocular pressure (IOP) control after surgery.
Medications
Medications for primary congenital glaucoma is typically used as an adjunct (add-on) to surgery. Most medications in the United States have not been approved for children, however many studies have been performed that inform doctors on their safety and efficacy in children. Timolol (a non-selective beta blocker) is the first choice in pediatric glaucoma. In cases with insufficient reduction of the intraocular pressure (intraocular pressure (IOP)), the combination of timolol once a day and dorzolamide twice a day brings about a good control of the intraocular pressure (IOP). Both medications are effective and well tolerated. The alpha2-agonists have more and potentially serious adverse effects in children and are contraindicated for children younger than 2 years of age. Latanoprost tends to be less effective in lowering intraocular pressure (IOP) in children than in adults 111.
- Beta-blockers (beta-adrenergic antagonists): Topical beta-blockers play a large role in primary congenital glaucoma treatment and include timolol (non-selective beta-1 and beta-2 blocker, concentrations of 0.1% available in some countries, 0.25% and 0.5% solutions, and 0.25% and 0.5% gel-forming solution), and betaxolol (selective beta-1-blocker, concentrations of 0.25% and 0.5% solutions). Given potentially high plasma levels of the medication from topical instillation in small children, the lowest concentration available should be initiated first. The solution drops are approved for BID dosing though may be just as effective dosed once in the morning. The gel-forming solutions are approved for once daily dosing. Beta-blockers typically reduce intraocular pressure (IOP) by 20-30%. Side effects are mainly systemic and include respiratory distress, caused by apnea or bronchospasm (which may present as coughing instead of wheezing), and bradycardia. Beta-blockers should be avoided in patients with bradycardia, second- or third-degree atrioventricular block, and active asthma or “reactive airways.” Betaxolol may be less likely to cause pulmonary distress (e.g. asthma attacks) and cardiac side effects 112.
- Carbonic anhydrase inhibitors: Oral carbonic anhydrase inhibitors include acetazolamide (Diamox, dose 10-20 mg/kg/day divided into 3 or 4 doses) and methazolamide (Neptazane, dose < 2 mg/kg/day, divided into 2 doses) 113, 2. Acetazolamide can be prepared in a flavored syrup (have the pharmacist crush the tablets and suspend the powder in syrup) with a concentration of 50 mg/ml for ease of use. Children can also take the tablet crushed in applesauce or something similar. It reduces the intraocular pressure (IOP) about 20-35%. Side effects occur in >40% of patients and include lethargy, decreased appetite, weight loss, gastrointestinal discomfort, diarrhea and metabolic acidosis. Topical carbonic anhydrase inhibitors include dorzolamide 2% (Trusopt) and brinzolamide 1% (Azopt) drops twice a day (BID) or three times a day (TID). These medications may produce less reduction in intraocular pressure (IOP) (about 25%) than oral carbonic anhydrase inhibitors, but also appear to have fewer systemic side effects. Rarely, side effects can occur, particularly in premature infants, such as metabolic acidosis 114. Topical carbonic anhydrase inhibitors ideally should be avoided or used as a later option in the setting of compromised corneas, especially of a corneal transplant 2.
- Combination beta-blocker/carbonic anhydrase inhibitor: Timolol 0.5%-dorzolamide 2% (Cosopt) drop twice a day (BID) has been shown to be effective in reducing intraocular pressure (IOP) in children requiring more than one topical medication. It is approved for twice a day (BID) dosing, but cautious use in young children is warranted due to the higher concentration of timolol.
- Adrenergic agonists (avoid or use with caution in children younger than age 6 years or weight less than 20 kg): Apraclonidine 0.5% (Iopidine) and brimonidine (Alphagan, Alphagan P, 0.1%, 0.15%, 0.2%) are alpha-2 selective agonists and are dosed twice a day (BID) to three times a day (TID). Their effectiveness has not been studied specifically for primary congenital glaucoma. The side effects in children limit their use. Due to being highly lipophilic, brimonidine passes through the blood-brain barrier potentially causing severe sleepiness, respiratory depression, apnea and coma, especially in neonates and infants, thus it is strictly contraindicated in patients 2 years old or younger. It may also cause bradycardia, hypotension, hypotonia, and hypothermia. Apraclonidine is more hydrophilic which reduces its blood-brain barrier penetration and thus has fewer central nervous system side effects than brimonidine. It must still be used with caution and is best used for short- or intermediate-term intraocular pressure (IOP) lowering. Tachyphylaxis and ocular allergy limit its effectiveness long-term.
- Combination beta-blocker/alpha-2 adrenergic agonists: Timolol 0.5%-brimonidine 0.2% (Combigan) must not be prescribed to children if there is a contraindication to the individual components.
- Prostaglandin analogs: Latanoprost 0.005% (Xalatan), travoprost 0.004% (Travatan), bimatoprost 0.01% (Lumigan), and tafluprost (Zioptan, preservative-free) are dosed nightly. Latanoprost reduces the intraocular pressure (IOP) in primary congenital glaucoma 15-20% 115. While the FDA has not approved prostaglandin analogs in children, Europe has approved latanoprost for children. Side effects mainly include lash growth, conjunctival injection, and less commonly iris pigmentation alteration, allergy, uveitis and periocular hyperpigmentation. Side effects seem more prominent with use of travoprost and bimatoprost and less with latanoprost. Long-term side effects are still unknown in children. Prostaglandin-related periorbitopathy has been described in children 116. This class of medication is relatively contraindicated when active inflammation or uveitis is present.
- Combination beta-blocker/prostaglandin analog: Available in countries outside the United States.
- Miotic agents: These do not play much of a role in primary congenital glaucoma likely due to their immature angle anatomy and high ciliary muscle insertion. They include echothiophate, phospholine iodide (irreversible cholinesterase and pseudocholinesterase inhibitors) and pilocarpine (direct parasympathomimetic). Miotic are useful perioperatively for angle surgery. Pilocarpine (0.5-6%, most common 1-2%) is dosed once to four times a day, usually 2-3 times a day after angle surgery. Side effects include miosis, decreased heart rate, apnea, sweating, and hypersalivation, and theoretically may induce cataract and retinal detachment.
- Modified prostaglandin analogs and rho-associated protein kinase inhibitors: Latanoprostene bunod (Vyzulta) and netarsudil (Rhopressa) have not been studied in patients younger than 18 years of age.
Doctors can start with a either a carbonic anhydrase inhibitor, beta-blocker or prostaglandin analog, or a combination, and progressively add another medication class, keeping in mind medications are generally a temporizing measure prior to surgery. If prescribed before initial surgery, medications should not be used without fairly frequent follow-up, and ideally surgery performed within 2 weeks of primary congenital glaucoma diagnosis. Early discussion preparing family and caregivers for surgery is necessary. Medications should be continued until surgery, and may help maximize corneal clearing by reducing the intraocular pressure (IOP). After surgery, medications may still be needed as an adjunct and family and caregivers should be made aware of this. Compliance may be an issue when the medication regimen becomes complex and should be addressed. primary congenital glaucoma requires lifelong serial measurements of intraocular pressure (IOP), corneal diameter, axial length, refractive error, and optic nerve cupping. If an adequate assessment is not possible in the outpatient clinic, an examination under anesthesia should be performed.
Surgery
Surgery is the mainstay of treatment for patients with primary congenital glaucoma. The type of surgical procedure depends on the disease severity, cornea clarity, and surgeon’s choice and experience. There are 4 major surgical options for primary congenital glaucoma; however once the diagnosis of primary congenital glaucoma is established, angle surgery is the first procedure of choice to incise/open the trabecular meshwork with the hope of allowing aqueous flow from the anterior chamber directly into Schlemm canal. It is generally agreed that angle surgery is most successful in infantile-onset primary congenital glaucoma, and less so in newborn or late-recognized primary congenital glaucoma. Goniotomy is preferred by some surgeons when the cornea is clear enough to permit visualization of anterior segment structures (although some prefer trabeculotomy regardless of the corneal clarity, see below). An incision is made across the trabecular meshwork under direct gonioscopic visualization using a goniotomy knife (Swan knife, needle-knife, disposable 25-gauge needle on a syringe) and surgical goniotomy lens (i.e. Barkan or other goniotomy lens). Traditionally, it is first performed nasally, however modifications can be made to complete it temporally as well at one surgical session. If a surgeon is comfortable with devices and modified techniques such as using the Kahook dual blade, Trabectome, gonioscopy-assisted transluminal trabeculotomy with suture or lighted microcatheter, or Omni, these devices can be used safely to perform a goniotomy in children, however it is not recommended to use these devices in a pediatric eye prior to extensive experience in an adult eye. There is no data to suggest these modified techniques do better than traditional goniotomy or trabeculotomy. Complications include hyphema, anterior chamber shallowing, peripheral anterior synechiae, and rarely, iridodialysis, cyclodialysis, cataract, scleral perforation, epithelial ingrowth, and retinal detachment 2, 117.
When the cornea is not clear enough to permit visualization of the angle, or if preferred due to technical factors or surgeon experience or preference, trabeculotomy ab externo (“trabeculotomy”) is the procedure of choice. Access to Schlemm canal is obtained externally via a partial scleral flap to allow cannulation of Schlemm canal. The older technique opened ~90 degrees of Schlemm canal with a curved rigid pronged probe called a trabeculotome, which can then be rotated gently into the anterior chamber to incise through the trabecular meshwork. A trabeculotome curved in the opposite direction can then be used to cannulate another 90 degrees of Schlemm canal and complete 180 degrees of trabeculotomy. Alternatively, and preferred by many angle surgeons at this time, a 6-0 polypropylene (Prolene) suture or an illuminated microcatheter can be threaded into the entire Schlemm canal and pulled across the anterior chamber to complete a 360-degree trabeculotomy. Complications include hyphema, unintentional filtering blebs, choroidal detachment, cyclodialysis, iridodialysis, lens injury, and infection.
Traditional goniotomy and trabeculotomy ab externo (incising 2 quadrants) have success rates ranging for goniotomy 30-65% and for trabeculotomy 40-80%, with success reported as low as 10% to as high as 94% 2, 118.
Combined trabeculotomy and trabeculectomy (CTT) can be performed if Schlemm canal could not be cannulated or prior trabeculotomy failed, in which a trabeculectomy is added to the trabeculotomy by removing a block of tissue in the scleral flap bed followed by a surgical iridectomy as done in regular trabeculectomy. Mitomycin C may be used with care. Combined trabeculotomy and trabeculectomy (CTT) can be an initial surgical procedure, especially in Indian and Middle Eastern patients 119, 120.
Filtering surgery is considered when one or more angle surgeries have failed and includes traditional trabeculectomy with or without mitomycin C and glaucoma drainage device implantation. Trabeculectomy may be best done using techniques of the Moorfields Safer Surgery System, including fornix-based conjunctival flaps, small radial cuts, mitomycin C under the sclera flap and subconjunctival tissue with wider spread to enhance posterior aqueous flow and reduce bleb-related complications. Use of an anterior chamber maintainer in all cases and releasable sutures are also recommended 121, 122, 123. EX-PRESS mini glaucoma shunts are not used commonly in primary congenital glaucoma as safety and efficacy have not been established long-term in young children. Severe complications of trabeculectomy include vitreous loss, ectasia, scleral collapse, retinal detachment, and phthisis. The child is also at lifelong risk of complications and infection including bleb leak, wound rupture, blebitis, and endophthalmitis.
Reported success rates for trabeculectomy performed for primary congenital glaucoma range between 50-87% 2. The risk of failure is 5.6 times higher in patients age 1 year or less 124. The higher risk of failure in advanced primary congenital glaucoma young patients is due to buphthalmos, lack of scleral rigidity, and highly active healing and scarring.
When trabeculectomy fails or is not a desirable option, then the other filtering option with glaucoma drainage device (GDD) surgery or cyclodestructive procedures are the next surgical choices. All models of glaucoma drainage devices (Molteno, Baerveldt, and Ahmed valve) can be used in primary congenital glaucoma patients, and glaucoma drainage devices can be implanted safely in neonates with attention to eye and implant parameters. Generally, it is advisable to use a fornix incision with conjunctival and Tenon capsule incision 8 mm posterior to the limbus, and double-layer closure with running 8-0 polyglactin (Vicryl) suture on a vascular needle. The flexible implants (Baerveldt and Ahmed) can be trimmed posteriorly so as to prevent plate-optic nerve touch. The amount to trim can be calculated with the online Freedman-Margeta GDD calculator (https://people.duke.edu/~freed003/GDDCalculator/) 125. Some surgeons place the first tube inferior nasal to preserve conjunctiva superiorly for possible trabeculectomy when the patient is older, however others prefer superior temporal placement of the first tube, for better efficacy, and are able to perform successful trabeculectomy superior nasal at a future time. Complications from glaucoma drainage devices are many and include those of trabeculectomy plus cornea-tube touch, tube erosion through the conjunctiva or cornea, implant migration, and cataract. Infection rates are low 126. The Ahmed valve may additionally fail due to fibrovascular ingrowth into the valve chamber 127.
Success rates vary widely for primary congenital glaucoma and childhood glaucoma. For the Molteno, the range is 56-95%, with slightly higher success with the double-plate implant compared to the one-plate implant 128, 129. The Baerveldt success rates range from 80-95% at 12 months, decreasing to < 50% by 60 months 130, 131. The Ahmed glaucoma valve has about a 55% success rate at 5 years 132, 133.
Cyclodestructive procedures are useful tools in managing refractory primary congenital glaucoma after all other options have been tried, to reduce aqueous production. Results are unpredictable and complications exist. Laser cyclophotocoagulation (CPC) has largely replaced cyclocryotherapy, and diode laser is preferred to Nd:YAG laser due to decreased adverse events such as sympathetic ophthalmia. Transscleral and endoscopic application of laser are both options, with endoscopic preferred if the eye anatomy allows. Transscleral Micropulse-cyclophotocoagulation may have less severe complications than traditional transscleral cyclophotocoagulation and be as effective in children 134, though further research is needed. The limbal anatomy may be distorted and blind application of transscleral cyclophotocoagulation may be better guided with ultrasound biomicroscopy 135. A general rule of thumb for all cyclodestructive procedures is to maintain 1-2 clock hours of untouched ciliary processes, even after repeated sessions, thus careful documentation of treated areas is recommended. Rare complications include hypotony, retinal detachment, visual loss, and phthisis.
Success for transscleral cyclophotocoagulation ranges from 30-79% with retreatment in ~70% of patients, and has been comparable to implanting a second glaucoma drainage device (GDD) in children 136, 137, 138, 139. Endoscopic cyclophotocoagulation (ECP) has been reported to be 64% successful at 1 year, and 16% by 5 years, with sequential endoscopic cyclophotocoagulation (ECP) bringing the rate up to 81% at 1 year, and 34% at 5 years 140, 141.
Surgical Complications
Surgical complications include:
- Hyphema
- Shallow anterior chamber 142
- Peripheral anterior synechiae
- Iridodialysis
- Cyclodialysis (a condition where the longitudinal ciliary muscle fibers separates from the scleral spur, the area where the muscle attaches to the eye’s wall) 143
- Cataract 144, 145
- Epithelial ingrowth
- Choroidal detachment
- Retinal detachment
- Phthisis bulbi
Filtering Procedure-Related Complications:
- Over or under-filtration
- Blebitis
- Vitreous loss
- Scleral collapse
- Scleral flap leak
- Tube lens touch
- Endothelial decompensation from tube cornea touch
- Tube erosion
- Implant migration
- Diplopia from implant-related restrictions
- Endophthalmitis
Cyclodestructive Procedure-Related Complications:
- Hypotony
- Retinal detachment 146
- Phthisis
Anesthesia-Related Complications:
- Oculocardiac reflex
- Anaphylaxis
- Malignant hyperthermia
- Cardiovascular collapse
- Hepatic porphyria
- Hypoxic brain injury.
Postoperative and Rehabilitation Care
The children undergoing surgery should be started on topical steroids, either prednisolone 1% or dexamethasone 0.1% for 1 week each using an 8/7/6/5/4/3/2/1 tapering dosage. In addition, a topical antibiotic in the form of tobramycin 0.3% or 0.3% moxifloxacin or gatifloxacin 4 times per day for 20 days should be supplemented to prevent secondary infection. These patients will need close follow-ups postsurgery to look for signs of hypotony, inflammation, or infection. Moreover, the IOP needs to be recorded every 3 to 4 months for at least 2 years postsurgery.
Cycloplegic refraction will be needed every 6 months for these patients. Moreover, lifelong regular follow-up every 6 months is needed for intraocular pressure (IOP) monitoring and early detection of any surgery-related complications. Cases of failing angle or filtration surgery should be counseled for the need for glaucoma drainage device (GDD) and the risk of subsequent failure, amblyopia, blindness, and phthisis bulbi (a shrunken, disfigured, and non-functional eye that has undergone significant damage).
Surgical follow up
In the short term, patients require frequent follow up to follow response to treatment and monitor for hypotony, infection, and excessive inflammation. For young patients, or patients with less than 2 years of intraocular pressure (IOP) control, follow-up is recommended at least every 3-4 months. Regular life-long follow-up is needed (at least every 6 months) because even if long-term intraocular pressure (IOP) control from a surgical intervention is achieved, asymptomatic relapse can occur at any time and will need to be managed with medications or further surgery. Additionally, vision-threatening complications may occur at any time, especially after filtering surgeries.
Glaucoma drainage device (GDD) patency can be assessed with B-scan ultrasonography in the clinic 147.
Childhood glaucoma prognosis
The prognosis for children with primary congenital glaucoma is quite variable, with some achieving good vision, while others go blind 6. While primary congenital glaucoma accounts for less than 0.01% of all patients with eye diseases, it has been blamed for 5% of childhood blindness worldwide 58. Vision loss is secondary to corneal scarring or optic nerve damage, and often amblyopia in asymmetric or unilateral cases. Surgical management is the primary treatment modality. If intraocular pressure (IOP) is controlled, vision in the better eye ultimately can be 20/60 or better 148, 149.
A study from the United States showed a lack of progression following adequate treatment in 90.3% at 1 year, 83.1% at 5 years, 70.8% at 10 years, and 58.3% at 34 years 150. Thus highlighting the importance of appropriate management and follow-ups for these patients. Another study showed that angle procedures were 90% successful among patients presenting between 2 months and 1 year of age, compared to 50% among those presenting either in infantile or late-onset or late-recognized cases 151.
Consideration must also be given to the burden on caregivers, whose actions likely will affect the patient’s prognosis. Recent studies have highlighted that one-third of primary congenital glaucoma caregivers could have moderate to severe depression, and quality of life is poorer for the caregiver if the patient is older and has had the disease longer 152, 153.
- Knight LSW, Ruddle JB, Taranath DA, et al. Childhood and Early Onset Glaucoma Classification and Genetic Profile in a Large Australasian Disease Registry. Ophthalmology. 2021 Nov;128(11):1549-1560. https://www.aaojournal.org/article/S0161-6420(21)00288-8/fulltext[↩][↩]
- Weinreb R.N., Grajewski A.L., Papadopoulos M., Grigg J.,Freedman S.F. Childhood glaucoma: the 9th consensus report of the World Glaucoma Association. Netherlands: Kugler Publications, 2013.[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Xia Q, Zhang D, Zhuang Y, Dai Y, Jia H, Du Q, Wen T, Jiang Y. Animal Model Contributions to Primary Congenital Glaucoma. J Ophthalmol. 2022 May 26;2022:6955461. doi: 10.1155/2022/6955461[↩]
- Kaur K, Zeppieri M, Gurnani B. Primary Congenital Glaucoma. [Updated 2024 Nov 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK574553[↩][↩][↩][↩]
- Abu-Amero KK, Edward DP. Primary Congenital Glaucoma. 2004 Sep 30 [Updated 2017 Aug 17]. In: Adam MP, Feldman J, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2025. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1135[↩]
- Primary Congenital Glaucoma. https://eyewiki.org/Primary_Congenital_Glaucoma[↩][↩][↩][↩][↩][↩][↩]
- Lingham G, Thakur S, Safi S, Gordon I, Evans JR, Keel S. A systematic review of clinical practice guidelines for childhood glaucoma. BMJ Open Ophthalmol. 2022 Jan 31;7(1):e000933. doi: 10.1136/bmjophth-2021-000933[↩]
- Congenital Glaucoma. https://glaucoma.org/types/congenital-glaucoma[↩]
- Glaucoma in Infants. https://www.rch.org.au/ophthal/patient_information/Glaucoma/[↩]
- Yeung HH, Kumar-Singh R, Walton DS. Infantile Aphakic Glaucoma: A Proposed Mechanism. J Pediatr Ophthalmol Strabismus. 2022 Jul-Aug;59(4):236-242. doi: 10.3928/01913913-20210929-02[↩]
- Jafer Chardoub AA, Zeppieri M, Blair K. Juvenile Glaucoma. [Updated 2024 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK562263[↩]
- Alward, W.L.M. Glaucoma: The Requisites in Ophthalmology. St. Louis: Mosby Inc; 2000.[↩][↩]
- Haddad A, Ait Boujmia OK, El Maaloum L, Dehbi H. Meta-analysis of CYP1B1 gene mutations in primary congenital glaucoma patients. Eur J Ophthalmol. 2021 Nov;31(6):2796-2807. doi: 10.1177/11206721211016308. Erratum in: Eur J Ophthalmol. 2024 Jul;34(4):NP71-NP72. doi: 10.1177/11206721231156301[↩][↩][↩]
- Greco, A. et al., 2022, ‘Childhood Glaucoma and Medical Treatment: An Up to Date’, in G. L. Giudice (ed.), Vision Correction and Eye Surgery, IntechOpen, London. 10.5772/intechopen.100579.[↩][↩]
- Feroze KB, Blair K, Patel BC. Buphthalmos. [Updated 2025 Jan 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430887[↩][↩]
- Beck AD. Diagnosis and management of pediatric glaucoma. Ophthalmol Clin North Am. 2001 Sep;14(3):501-12. doi: 10.1016/s0896-1549(05)70248-0[↩]
- Mark HH. Buphthalmos: early glaucoma history. Acta Ophthalmol. 2011;89:591–594. doi: 10.1111/j.1755-3768.2009.01783.x[↩]
- Bouhenni RA, Ricker I, Hertle RW. Prevalence and clinical characteristics of childhood Glaucoma at a tertiary care Children’s hospital. J Glaucoma. 2019;28:655–659. doi: 10.1097/IJG.0000000000001259[↩]
- Vasileiadis GT, Frangouli O. Unilateral congenital buphthalmos. BMJ Case Rep. 2015 Jun 3;2015:bcr2015210979. doi: 10.1136/bcr-2015-210979[↩]
- Kaushik S, Dhingra D, Joshi G, Bazgain K, Thattaruthody F, Pandav SS. Acute Hydrops in the Fellow Eye of Infants with Primary Congenital Glaucoma after Intraocular Pressure Reduction in 1 Eye. Ophthalmol Glaucoma. 2020 Jul-Aug;3(4):301-305. doi: 10.1016/j.ogla.2020.03.007[↩]
- Haab striae in primary congenital glaucoma. https://eyerounds.org/atlas/pages/Haab/index.htm#gsc.tab=0[↩]
- deLuise VP, Anderson DR. Primary infantile glaucoma (congenital glaucoma). Surv Ophthalmol. 1983 Jul-Aug;28(1):1-19. doi: 10.1016/0039-6257(83)90174-1[↩][↩][↩][↩][↩][↩]
- Morin JD, Bryars JH. Causes of loss of vision in congenital glaucoma. Arch Ophthalmol. 1980 Sep;98(9):1575-6. doi: 10.1001/archopht.1980.01020040427005[↩][↩]
- Goel M, Picciani RG, Lee RK, Bhattacharya SK. Aqueous humor dynamics: a review. Open Ophthalmol J. Sep 3 2010;4:52-9. doi:10.2174/1874364101004010052[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Toris CB, Koepsell SA, Yablonski ME, Camras CB. Aqueous humor dynamics in ocular hypertensive patients. J Glaucoma. 2002 Jun;11(3):253-8. doi: 10.1097/00061198-200206000-00015[↩][↩][↩][↩][↩]
- Macknight AD, McLaughlin CW, Peart D, Purves RD, Carré DA, Civan MM. Formation of the aqueous humor. Clin Exp Pharmacol Physiol. 2000 Jan-Feb;27(1-2):100-6. doi: 10.1046/j.1440-1681.2000.03208.x[↩]
- Ferreira SM, Lerner SF, Brunzini R, Evelson PA, Llesuy SF. Oxidative stress markers in aqueous humor of glaucoma patients. Am J Ophthalmol. 2004 Jan;137(1):62-9. doi: 10.1016/s0002-9394(03)00788-8[↩][↩]
- Kaeslin MA, Killer HE, Fuhrer CA, Zeleny N, Huber AR, Neutzner A. Changes to the Aqueous Humor Proteome during Glaucoma. PLoS One. 2016 Oct 27;11(10):e0165314. doi: 10.1371/journal.pone.0165314[↩][↩]
- Brubaker RF. The flow of aqueous humor in the human eye. Trans Am Ophthalmol Soc. 1982;80:391-474. https://pmc.ncbi.nlm.nih.gov/articles/instance/1312276/pdf/taos00019-0414.pdf[↩][↩][↩][↩][↩]
- R TCYMT. Aqueous humor dynamics. In: DC CNL, ed. Atlas of Glaucoma. Second edition ed. Informa HK; 2007:13 – 28:chap 3.[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Kiel JW, Hollingsworth M, Rao R, Chen M, Reitsamer HA. Ciliary blood flow and aqueous humor production. Prog Retin Eye Res. Jan 2011;30(1):1-17. doi:10.1016/j.preteyeres.2010.08.001[↩][↩][↩][↩][↩]
- Macknight AD, McLaughlin CW, Peart D, Purves RD, Carre DA, Civan MM. Formation of the aqueous humor. Clin Exp Pharmacol Physiol. Jan-Feb 2000;27(1-2):100-6. doi:10.1046/j.1440-1681.2000.03208.x[↩]
- Johnson M, McLaren JW, Overby DR. Unconventional aqueous humor outflow: A review. Exp Eye Res. May 2017;158:94-111. doi:10.1016/j.exer.2016.01.017[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Roy Chowdhury U, Hann CR, Stamer WD, Fautsch MP. Aqueous humor outflow: dynamics and disease. Invest Ophthalmol Vis Sci. May 2015;56(5):2993-3003. doi:10.1167/iovs.15-16744[↩][↩][↩][↩]
- Fautsch MP, Johnson DH. Aqueous humor outflow: what do we know? Where will it lead us? Invest Ophthalmol Vis Sci. Oct 2006;47(10):4181-7. doi:10.1167/iovs.06-0830[↩][↩][↩]
- Trabecular Meshwork Research. https://ethier.gatech.edu/anterior-segmenttrabecular-meshwork-biomechanics[↩][↩]
- J. Buffault, A. Labbé, P. Hamard, F. Brignole-Baudouin, C. Baudouin. The trabecular meshwork: Structure, function and clinical implications. A review of the literature. Journal Français
d’Ophtalmologie, 2020, 43 (7), pp.e217-e230. https://hal.sorbonne-universite.fr/hal-02962228v1/document[↩] - Thau A, Lloyd M, Freedman S, Beck A, Grajewski A, Levin AV. New classification system for pediatric glaucoma: implications for clinical care and a research registry. Curr Opin Ophthalmol. 2018 Sep;29(5):385-394. doi: 10.1097/ICU.0000000000000516[↩]
- Kong L, et al. An update on progress and the changing epidemiology of causes of childhood blindness worldwide. J AAPOS. 2012;16(6):501–507. doi: 10.1016/j.jaapos.2012.09.004[↩]
- Jat NS, Tripathy K. Peters Anomaly. [Updated 2023 Aug 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK580540[↩][↩]
- Idrees F, Vaideanu D, Fraser SG, Sowden JC, Khaw PT. A review of anterior segment dysgeneses. Surv Ophthalmol. 2006 May-Jun;51(3):213-31. doi: 10.1016/j.survophthal.2006.02.006[↩]
- Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014 May 14;311(18):1901-11. doi: 10.1001/jama.2014.3192[↩]
- Wiggs JL, Pasquale LR. Genetics of glaucoma. Hum Mol Genet. 2017 Aug 1;26(R1):R21-R27. doi: 10.1093/hmg/ddx184[↩]
- Lewis CJ, Hedberg-Buenz A, DeLuca AP, Stone EM, Alward WLM, Fingert JH. Primary congenital and developmental glaucomas. Hum Mol Genet. 2017 Aug 1;26(R1):R28-R36. doi: 10.1093/hmg/ddx205[↩]
- Shah M, Bouhenni R, Benmerzouga I. Geographical Variability in CYP1B1 Mutations in Primary Congenital Glaucoma. J Clin Med. 2022 Apr 6;11(7):2048. doi: 10.3390/jcm11072048[↩][↩][↩]
- Bejjani BA, Xu L, Armstrong D, Lupski JR, Reneker LW. Expression patterns of cytochrome P4501B1 (Cyp1b1) in FVB/N mouse eyes. Exp Eye Res. 2002 Sep;75(3):249-57. https://doi.org/10.1006/exer.2002.2025[↩]
- Teixeira LB, Zhao Y, Dubielzig RR, Sorenson CM, Sheibani N. Ultrastructural abnormalities of the trabecular meshwork extracellular matrix in Cyp1b1-deficient mice. Vet Pathol. 2015 Mar;52(2):397-403. doi: 10.1177/0300985814535613[↩]
- Williams AL, Eason J, Chawla B, Bohnsack BL. Cyp1b1 Regulates Ocular Fissure Closure Through a Retinoic Acid-Independent Pathway. Invest Ophthalmol Vis Sci. 2017 Feb 1;58(2):1084-1097. doi: 10.1167/iovs.16-20235[↩]
- Suri F, Yazdani S, Narooie-Nejhad M, Zargar SJ, Paylakhi SH, Zeinali S, Pakravan M, Elahi E. Variable expressivity and high penetrance of CYP1B1 mutations associated with primary congenital glaucoma. Ophthalmology. 2009 Nov;116(11):2101-9. doi: 10.1016/j.ophtha.2009.04.045[↩]
- Alghamdi A, Aldossary W, Albahkali S, Alotaibi B, Alrfaei BM. The loss of microglia activities facilitates glaucoma progression in association with CYP1B1 gene mutation (p.Gly61Glu). PLoS One. 2020 Nov 10;15(11):e0241902. doi: 10.1371/journal.pone.0241902[↩]
- Schlötzer-Schrehardt U, Zenkel M, Küchle M, Sakai LY, Naumann GO. Role of transforming growth factor-beta1 and its latent form binding protein in pseudoexfoliation syndrome. Exp Eye Res. 2001 Dec;73(6):765-80. doi: 10.1006/exer.2001.1084[↩]
- Hyytiäinen M, Keski-Oja J. Latent TGF-beta binding protein LTBP-2 decreases fibroblast adhesion to fibronectin. J Cell Biol. 2003 Dec 22;163(6):1363-74. doi: 10.1083/jcb.200309105[↩]
- Ali M, McKibbin M, Booth A, et al. Null mutations in LTBP2 cause primary congenital glaucoma. Am J Hum Genet. 2009 May;84(5):664-71. doi: 10.1016/j.ajhg.2009.03.017[↩]
- Suri F, Yazdani S, Elahi E. LTBP2 knockdown and oxidative stress affect glaucoma features including TGFβ pathways, ECM genes expression and apoptosis in trabecular meshwork cells. Gene. 2018 Oct 5;673:70-81. doi: 10.1016/j.gene.2018.06.038[↩]
- Sarfarazi M, Akarsu AN, Hossain A, Turacli ME, Aktan SG, Barsoum-Homsy M, Chevrette L, Sayli BS. Assignment of a locus (GLC3A) for primary congenital glaucoma (Buphthalmos) to 2p21 and evidence for genetic heterogeneity. Genomics. 1995 Nov 20;30(2):171-7. doi: 10.1006/geno.1995.9888[↩]
- Fan BJ, Wiggs JL. Glaucoma: genes, phenotypes, and new directions for therapy. J Clin Invest. 2010 Sep;120(9):3064-72. doi: 10.1172/JCI43085[↩]
- Kumar A, Han Y, Oatts JT. Genetic changes and testing associated with childhood glaucoma: A systematic review. PLoS One. 2024 Feb 22;19(2):e0298883. doi: 10.1371/journal.pone.0298883[↩]
- Yu-Wai-Man C, Arno G, Brookes J, Garcia-Feijoo J, Khaw PT, Moosajee M. Primary congenital glaucoma including next-generation sequencing-based approaches: clinical utility gene card. Eur J Hum Genet. 2018 Nov;26(11):1713-1718. doi: 10.1038/s41431-018-0227-y[↩][↩][↩][↩]
- Tamçelik N, Atalay E, Bolukbasi S, Çapar O, Ozkok A. Demographic features of subjects with congenital glaucoma. Indian J Ophthalmol. 2014 May;62(5):565-9. doi: 10.4103/0301-4738.126988[↩]
- Genĉík A. Epidemiology and genetics of primary congenital glaucoma in Slovakia. Description of a form of primary congenital glaucoma in gypsies with autosomal-recessive inheritance and complete penetrance. Dev Ophthalmol. 1989;16:76-115.[↩]
- Ohtake Y, Tanino T, Suzuki Y, Miyata H, Taomoto M, Azuma N, Tanihara H, Araie M, Mashima Y. Phenotype of cytochrome P4501B1 gene (CYP1B1) mutations in Japanese patients with primary congenital glaucoma. Br J Ophthalmol. 2003 Mar;87(3):302-4. doi: 10.1136/bjo.87.3.302[↩]
- Alabdulwahhab KM, Ahmad MS. Visual Impairment and Blindness in Saudi Arabia’s School for the Blind: A Cross-Sectional Study. Clin Optom (Auckl). 2020 Oct 7;12:169-173. doi: 10.2147/OPTO.S265293[↩]
- Souma T, Tompson SW, Thomson BR, et al. Angiopoietin receptor TEK mutations underlie primary congenital glaucoma with variable expressivity. J Clin Invest. 2016 Jul 1;126(7):2575-87. doi: 10.1172/JCI85830[↩]
- Badawi AH, Al-Muhaylib AA, Al Owaifeer AM, Al-Essa RS, Al-Shahwan SA. Primary congenital glaucoma: An updated review. Saudi J Ophthalmol. 2019 Oct-Dec;33(4):382-388. doi: 10.1016/j.sjopt.2019.10.002[↩]
- Barkan O. Pathogenesis of congenital glaucoma: gonioscopic and anatomic observation of the angle of the anterior chamber in the normal eye and in congenital glaucoma. Am J Ophthalmol. 1955;40:1-11.[↩]
- Worst JGF. The pathogenesis of congential glaucoma. An embryological and goniosurgical study. Springfield, Ill: Charles C. Thomas; 1966.[↩]
- Mandal AK, Chakrabarti D. Update on congenital glaucoma. Indian J Ophthalmol. 2011 Jan;59 Suppl(Suppl1):S148-57. doi: 10.4103/0301-4738.73683[↩]
- Mandal AK, Chakrabarti D, Gothwal VK. Approach to primary congenital glaucoma: A perspective. Taiwan J Ophthalmol. 2023 Oct 19;13(4):451-460. doi: 10.4103/tjo.TJO-D-23-00104[↩]
- Anderson DR. The development of the trabecular meshwork and its abnormality in primary infantile glaucoma. Trans Am Ophthalmol Soc. 1981;79:458-85. https://pmc.ncbi.nlm.nih.gov/articles/instance/1312195/pdf/taos00020-0482.pdf[↩][↩]
- Tawara A, Inomata H. Developmental immaturity of the trabecular meshwork in congenital glaucoma. Am J Ophthalmol. 1981 Oct;92(4):508-25. doi: 10.1016/0002-9394(81)90644-9[↩][↩]
- García-Antón MT, Salazar JJ, de Hoz R, Rojas B, Ramírez AI, Triviño A, Aroca-Aguilar JD, García-Feijoo J, Escribano J, Ramírez JM. Goniodysgenesis variability and activity of CYP1B1 genotypes in primary congenital glaucoma. PLoS One. 2017 Apr 27;12(4):e0176386. doi: 10.1371/journal.pone.0176386[↩]
- Pilat AV, Proudlock FA, Shah S, Sheth V, Purohit R, Abbot J, Gottlob I. Assessment of the anterior segment of patients with primary congenital glaucoma using handheld optical coherence tomography. Eye (Lond). 2019 Aug;33(8):1232-1239. doi: 10.1038/s41433-019-0369-3[↩]
- Papadopoulos M, Cable N, Rahi J, Khaw PT; BIG Eye Study Investigators. The British Infantile and Childhood Glaucoma (BIG) Eye Study. Invest Ophthalmol Vis Sci. 2007 Sep;48(9):4100-6. doi: 10.1167/iovs.06-1350[↩]
- Sihota R, Sidhu T, Agarwal R, Sharma A, Gupta A, Sethi A, Dada T, Pandey V. Evaluating target intraocular pressures in primary congenital glaucoma. Indian J Ophthalmol. 2021 Aug;69(8):2082-2087. doi: 10.4103/ijo.IJO_3473_20[↩][↩]
- Mandal AK. Acute Corneal Hydrops in Children with Primary Infantile Glaucoma: A Report of 31 Cases over 23 Years at the LVPEI. PLoS One. 2016 Jun 1;11(6):e0156108. doi: 10.1371/journal.pone.0156108[↩]
- Drechsler J, Lee A, Maripudi S, Kueny L, Levin MR, Saeedi OJ, Bazemore M, Karwoski B, Birdsong R, Martinez C, Jaafar MS, Yousaf S, Ahmed ZM, Madigan WP, Alexander JL. Corneal Structural Changes in Congenital Glaucoma. Eye Contact Lens. 2022 Jan 1;48(1):27-32. doi: 10.1097/ICL.0000000000000844[↩]
- Gupta S, Mahalingam K, Singh A, Selvan H, Somarajan BI, Gupta V. Posterior corneal morphological changes in primary congenital glaucoma. Indian J Ophthalmol. 2022 Jul;70(7):2571-2577. doi: 10.4103/ijo.IJO_317_22[↩][↩]
- Sihota R, Mahalingam K, Maurya AK, Sharma A, Bukke AN, Dada T. Primary congenital glaucoma: An iridotrabeculodysgenesis? Indian J Ophthalmol. 2024 Mar 1;72(3):328-334. doi: 10.4103/IJO.IJO_370_23[↩]
- Walton DS. Chronic newborn primary congenital glaucoma with secondary lens subluxation. J Pediatr Ophthalmol Strabismus. 2009 Jul-Aug;46(4):200, 231. doi: 10.3928/01913913-20090706-02[↩]
- Snehi S, Singh AK, Kaushik S. Acquired Lens Zonular Loss with Bean Pot Optic Disc Cupping in Congenital Glaucoma. Ophthalmol Glaucoma. 2023 Mar-Apr;6(2):159. doi: 10.1016/j.ogla.2023.01.001[↩]
- Gupta V, James MK, Singh A, Kumar S, Gupta S, Sharma A, Sihota R, Kennedy DJ. Differences in Optic Disc Characteristics of Primary Congenital Glaucoma, Juvenile, and Adult Onset Open Angle Glaucoma Patients. J Glaucoma. 2016 Mar;25(3):239-43. doi: 10.1097/IJG.0000000000000154[↩]
- Walton DS. Diagnosis and treatment of glaucoma in childhood. In: Epstein DL, editor. Chandler and Grant’s Glaucoma. 3rd ed. Phila: Lea & Febinger; 1986.[↩]
- Kiskis AA, Markowitz SN, Morin JD. Corneal diameter and axial length in congenital glaucoma. Can J Ophthalmol. 1985 Apr;20(3):93-7.[↩]
- Kessing SV, Gregersen E. The distended disc in early stages of congenital glaucoma. Acta Ophthalmol (Copenh). 1977 Jun;55(3):431-5. doi: 10.1111/j.1755-3768.1977.tb06119.x[↩]
- Quigley HA. The pathogenesis of reversible cupping in congenital glaucoma. Am J Ophthalmol. 1977 Sep;84(3):358-70. doi: 10.1016/0002-9394(77)90680-8[↩]
- Fayed MA, Chen TC. Pediatric intraocular pressure measurements: Tonometers, central corneal thickness, and anesthesia. Surv Ophthalmol. 2019 Nov-Dec;64(6):810-825. doi: 10.1016/j.survophthal.2019.05.003[↩]
- Eisenberg DL, Sherman BG, McKeown CA, Schuman JS. Tonometry in adults and children. A manometric evaluation of pneumatonometry, applanation, and TonoPen in vitro and in vivo. Ophthalmology. 1998 Jul;105(7):1173-81. doi: 10.1016/S0161-6420(98)97016-6[↩]
- Grigorian F, Grigorian AP, Olitsky SE. The use of the iCare tonometer reduced the need for anesthesia to measure intraocular pressure in children. J AAPOS. 2012 Dec;16(6):508-10. doi: 10.1016/j.jaapos.2012.07.004[↩]
- Flemmons MS, Hsiao YC, Dzau J, Asrani S, Jones S, Freedman SF. Icare rebound tonometry in children with known and suspected glaucoma. J AAPOS. 2011 Apr;15(2):153-7. doi: 10.1016/j.jaapos.2010.11.022[↩]
- McKee EC, Ely AL, Duncan JE, Dosunmu EO, Freedman SF. A comparison of Icare PRO and Tono-Pen XL tonometers in anesthetized children. J AAPOS. 2015 Aug;19(4):332-7. doi: 10.1016/j.jaapos.2015.04.004[↩]
- Sampaolesi R, Caruso R. Ocular echometry in the diagnosis of congenital glaucoma. Arch Ophthalmol. 1982 Apr;100(4):574-7. doi: 10.1001/archopht.1982.01030030576003[↩]
- Henriques MJ, Vessani RM, Reis FA, de Almeida GV, Betinjane AJ, Susanna R Jr. Corneal thickness in congenital glaucoma. J Glaucoma. 2004 Jun;13(3):185-8. doi: 10.1097/00061198-200406000-00002[↩]
- Doozandeh A, Yazdani S, Ansari S, Pakravan M, Motevasseli T, Hosseini B, Yasseri M. Corneal profile in primary congenital glaucoma. Acta Ophthalmol. 2017 Nov;95(7):e575-e581. doi: 10.1111/aos.13357[↩][↩]
- Zareei A, Razeghinejad MR, Salouti R. Corneal Biomechanical Properties and Thickness in Primary Congenital Glaucoma and Normal Eyes: A Comparative Study. Med Hypothesis Discov Innov Ophthalmol. 2018 Summer;7(2):68-72. https://pmc.ncbi.nlm.nih.gov/articles/PMC6146241[↩][↩]
- Donahue SP, Porter A. SITA visual field testing in children. J AAPOS. 2001 Apr;5(2):114-7. doi: 10.1067/mpa.2001.113840[↩]
- Salchow DJ, Oleynikov YS, Chiang MF, Kennedy-Salchow SE, Langton K, Tsai JC, Al-Aswad LA. Retinal nerve fiber layer thickness in normal children measured with optical coherence tomography. Ophthalmology. 2006 May;113(5):786-91. doi: 10.1016/j.ophtha.2006.01.036[↩]
- Ahn HC, Son HW, Kim JS, Lee JH. Quantitative analysis of retinal nerve fiber layer thickness of normal children and adolescents. Korean J Ophthalmol. 2005 Sep;19(3):195-200. doi: 10.3341/kjo.2005.19.3.195[↩]
- Hess DB, Asrani SG, Bhide MG, Enyedi LB, Stinnett SS, Freedman SF. Macular and retinal nerve fiber layer analysis of normal and glaucomatous eyes in children using optical coherence tomography. Am J Ophthalmol. 2005 Mar;139(3):509-17. doi: 10.1016/j.ajo.2004.10.047[↩]
- Rao A, Sahoo B, Kumar M, Varshney G, Kumar R. Retinal nerve fiber layer thickness in children <18 years by spectral-domain optical coherence tomography. Semin Ophthalmol. 2013 Mar;28(2):97-102. doi: 10.3109/08820538.2012.760626[↩]
- Rotruck JC, House RJ, Freedman SF, Kelly MP, Enyedi LB, Prakalapakorn SG, Lim ME, El-Dairi MA. Optical Coherence Tomography Normative Peripapillary Retinal Nerve Fiber Layer and Macular Data in Children 0-5 Years of Age. Am J Ophthalmol. 2019 Dec;208:323-330. doi: 10.1016/j.ajo.2019.06.025[↩]
- Hsu ST, Chen X, Ngo HT, House RJ, Kelly MP, Enyedi LB, Materin MA, El-Dairi MA, Freedman SF, Toth CA, Vajzovic L. Imaging Infant Retinal Vasculature with OCT Angiography. Ophthalmol Retina. 2019 Jan;3(1):95-96. doi: 10.1016/j.oret.2018.06.017[↩]
- Gouider D, Choura R, Mekni M, Sayadi J, Malek I, Nacef L. Sclerocornea: A rare ocular condition. J Fr Ophtalmol. 2021 Sep;44(7):1089-1091. doi: 10.1016/j.jfo.2021.05.001[↩]
- Gurnani B, Christy J, Narayana S, Rajkumar P, Kaur K, Gubert J. Retrospective multifactorial analysis of Pythium keratitis and review of literature. Indian J Ophthalmol. 2021 May;69(5):1095-1101. doi: 10.4103/ijo.IJO_1808_20[↩]
- Gurnani B, Narayana S, Christy J, Rajkumar P, Kaur K, Gubert J. Successful management of pediatric pythium insidiosum keratitis with cyanoacrylate glue, linezolid, and azithromycin: Rare case report. Eur J Ophthalmol. 2022 Sep;32(5):NP87-NP91. doi: 10.1177/11206721211006564[↩]
- Gurnani B, Kaur K. Pythium Keratitis. [Updated 2023 Jul 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK573072[↩]
- Raven ML, Rodriguez ME, Potter HD. Corneal Leukoma with Features of Both Sclerocornea and Peter’s Anomaly. Ophthalmology. 2016 Sep;123(9):1988. doi: 10.1016/j.ophtha.2016.05.011[↩]
- Altamirano F, Ortiz-Morales G, O’Connor-Cordova MA, Sancén-Herrera JP, Zavala J, Valdez-Garcia JE. Fuchs endothelial corneal dystrophy: an updated review. Int Ophthalmol. 2024 Feb 12;44(1):61. doi: 10.1007/s10792-024-02994-1[↩]
- Nusbaum L, Paul M, Maharshak I. [DEEP ORBITAL DERMOID CYSTS]. Harefuah. 2023 Feb;162(2):98-102. Hebrew.[↩]
- Balamurugan S, Gurnani B, Kaur K, Gireesh P, Narayana S. Traumatic intralenticular abscess-What is so different? Indian J Radiol Imaging. 2020 Jan-Mar;30(1):92-94. doi: 10.4103/ijri.IJRI_369_19[↩]
- Kaur K, Gurnani B, Devy N. Atypical optic neuritis – a case with a new surprise every visit. GMS Ophthalmol Cases. 2020 Feb 27;10:Doc11. doi: 10.3205/oc000138[↩]
- Coppens G, Stalmans I, Zeyen T, Casteels I. The safety and efficacy of glaucoma medication in the pediatric population. J Pediatr Ophthalmol Strabismus. 2009 Jan-Feb;46(1):12-8. doi: 10.3928/01913913-20090101-05[↩]
- Buckley MM, Goa KL, Clissold SP. Ocular betaxolol. A review of its pharmacological properties, and therapeutic efficacy in glaucoma and ocular hypertension. Drugs. 1990 Jul;40(1):75-90. doi: 10.2165/00003495-199040010-00005[↩]
- Maren TH, Haywood JR, Chapman SK, Zimmerman TJ. The pharmacology of methazolamide in relation to the treatment of glaucoma. Invest Ophthalmol Vis Sci. 1977 Aug;16(8):730-42.[↩]
- Capino AC, Dannaway DC, Miller JL. Metabolic Acidosis with Ophthalmic Dorzolamide in a Neonate. J Pediatr Pharmacol Ther. 2016 May-Jun;21(3):256-9. doi: 10.5863/1551-6776-21.3.256[↩]
- Maeda-Chubachi T, Chi-Burris K, Simons BD, Freedman SF, Khaw PT, Wirostko B, Yan E; A6111137 Study Group. Comparison of latanoprost and timolol in pediatric glaucoma: a phase 3, 12-week, randomized, double-masked multicenter study. Ophthalmology. 2011 Oct;118(10):2014-21. doi: 10.1016/j.ophtha.2011.03.010[↩]
- Kim JS, Blizzard S, Woodward JA, Leyngold IM, Liss J, Freedman SF. Prostaglandin-Associated Periorbitopathy in Children and Young Adults with Glaucoma. Ophthalmol Glaucoma. 2020 Jul-Aug;3(4):288-294. doi: 10.1016/j.ogla.2020.03.009[↩]
- El Sayed Y, Esmael A, Mettias N, El Sanabary Z, Gawdat G. Factors influencing the outcome of goniotomy and trabeculotomy in primary congenital glaucoma. Br J Ophthalmol. 2021 Sep;105(9):1250-1255. doi: 10.1136/bjophthalmol-2018-313387[↩]
- Huang H, Bao WJ, Yamamoto T, Kawase K, Sawada A. Postoperative outcome of three different procedures for childhood glaucoma. Clin Ophthalmol. 2018 Dec 17;13:1-7. doi: 10.2147/OPTH.S186929[↩]
- Mandal AK, Gothwal VK, Nutheti R. Surgical outcome of primary developmental glaucoma: a single surgeon’s long-term experience from a tertiary eye care centre in India. Eye (Lond). 2007 Jun;21(6):764-74. doi: 10.1038/sj.eye.6702324[↩]
- Khalil DH, Abdelhakim MA. Primary trabeculotomy compared to combined trabeculectomy-trabeculotomy in congenital glaucoma: 3-year study. Acta Ophthalmol. 2016 Nov;94(7):e550-e554. doi: 10.1111/aos.13018[↩]
- Jayaram H, Scawn R, Pooley F, Chiang M, Bunce C, Strouthidis NG, Khaw PT, Papadopoulos M. Long-Term Outcomes of Trabeculectomy Augmented with Mitomycin C Undertaken within the First 2 Years of Life. Ophthalmology. 2015 Nov;122(11):2216-22. doi: 10.1016/j.ophtha.2015.07.028[↩]
- Khaw PT, Chiang M, Shah P, Sii F, Lockwood A, Khalili A. Enhanced trabeculectomy: the Moorfields Safer Surgery System. Dev Ophthalmol. 2012;50:1-28. doi: 10.1159/000334776[↩]
- Low S, Hamada S, Nischal KK. Antimetabolite and releasable suture augmented filtration surgery in refractory pediatric glaucomas. J AAPOS. 2008 Apr;12(2):166-72. doi: 10.1016/j.jaapos.2007.09.012[↩]
- Beck AD, Wilson WR, Lynch MG, Lynn MJ, Noe R. Trabeculectomy with adjunctive mitomycin C in pediatric glaucoma. Am J Ophthalmol. 1998 Nov;126(5):648-57. doi: 10.1016/s0002-9394(98)00227-x[↩]
- Margeta MA, Kuo AN, Proia AD, Freedman SF. Staying away from the optic nerve: a formula for modifying glaucoma drainage device surgery in pediatric and other small eyes. J AAPOS. 2017 Feb;21(1):39-43.e1. doi: 10.1016/j.jaapos.2016.09.027[↩]
- Ishida K, Mandal AK, Netland PA. Glaucoma drainage implants in pediatric patients. Ophthalmol Clin North Am. 2005 Sep;18(3):431-42, vii. doi: 10.1016/j.ohc.2005.05.009[↩]
- Tung I, Marcus I, Thiamthat W, Freedman SF. Second glaucoma drainage devices in refractory pediatric glaucoma: failure by fibrovascular ingrowth. Am J Ophthalmol. 2014 Jul;158(1):113-7. doi: 10.1016/j.ajo.2014.03.017[↩]
- Cunliffe IA, Molteno AC. Long-term follow-up of Molteno drains used in the treatment of glaucoma presenting in childhood. Eye (Lond). 1998;12 ( Pt 3a):379-85. doi: 10.1038/eye.1998.90[↩]
- Beck AD, Freedman S, Kammer J, Jin J. Aqueous shunt devices compared with trabeculectomy with Mitomycin-C for children in the first two years of life. Am J Ophthalmol. 2003 Dec;136(6):994-1000. doi: 10.1016/s0002-9394(03)00714-1[↩]
- Tai AX, Song JC. Surgical outcomes of Baerveldt implants in pediatric glaucoma patients. J AAPOS. 2014 Dec;18(6):550-3. doi: 10.1016/j.jaapos.2014.08.003[↩]
- Mandalos A, Tailor R, Parmar T, Sung V. The Long-term Outcomes of Glaucoma Drainage Device in Pediatric Glaucoma. J Glaucoma. 2016 Mar;25(3):e189-95. doi: 10.1097/IJG.0000000000000164[↩]
- Razeghinejad MR, Kaffashan S, Nowroozzadeh MH. Results of Ahmed glaucoma valve implantation in primary congenital glaucoma. J AAPOS. 2014 Dec;18(6):590-5. doi: 10.1016/j.jaapos.2014.08.008[↩]
- Pakravan M, Esfandiari H, Yazdani S, Doozandeh A, Dastborhan Z, Gerami E, Kheiri B, Pakravan P, Yaseri M, Hassanpour K. Clinical outcomes of Ahmed glaucoma valve implantation in pediatric glaucoma. Eur J Ophthalmol. 2019 Jan;29(1):44-51. doi: 10.1177/1120672118761332[↩]
- Abdelrahman AM, El Sayed YM. Micropulse Versus Continuous Wave Transscleral Cyclophotocoagulation in Refractory Pediatric Glaucoma. J Glaucoma. 2018 Oct;27(10):900-905. doi: 10.1097/IJG.0000000000001053[↩]
- Way AL, Nischal KK. High-frequency ultrasound-guided transscleral diode laser cyclophotocoagulation. Br J Ophthalmol. 2014 Jul;98(7):992-4. doi: 10.1136/bjophthalmol-2014-305163[↩]
- Bock CJ, Freedman SF, Buckley EG, Shields MB. Transscleral diode laser cyclophotocoagulation for refractory pediatric glaucomas. J Pediatr Ophthalmol Strabismus. 1997 Jul-Aug;34(4):235-9. doi: 10.3928/0191-3913-19970701-11[↩]
- Kirwan JF, Shah P, Khaw PT. Diode laser cyclophotocoagulation: role in the management of refractory pediatric glaucomas. Ophthalmology. 2002 Feb;109(2):316-23. doi: 10.1016/s0161-6420(01)00898-3[↩]
- Autrata R, Rehurek J. Long-term results of transscleral cyclophotocoagulation in refractory pediatric glaucoma patients. Ophthalmologica. 2003 Nov-Dec;217(6):393-400. doi: 10.1159/000073068[↩]
- Sood S, Beck AD. Cyclophotocoagulation versus sequential tube shunt as a secondary intervention following primary tube shunt failure in pediatric glaucoma. J AAPOS. 2009 Aug;13(4):379-83. doi: 10.1016/j.jaapos.2009.05.006[↩]
- Neely DE, Plager DA. Endocyclophotocoagulation for management of difficult pediatric glaucomas. J AAPOS. 2001 Aug;5(4):221-9. doi: 10.1067/mpa.2001.116868[↩]
- Glaser TS, Mulvihill MS, Freedman SF. Endoscopic cyclophotocoagulation (ECP) for childhood glaucoma: a large single-center cohort experience. J AAPOS. 2019 Apr;23(2):84.e1-84.e7. doi: 10.1016/j.jaapos.2018.10.014[↩]
- Christy J, Jain N, Gurnani B, Kaur K. Twinkling Eye -A Rare Presentation in Neovascular Glaucoma. J Glaucoma. 2019 May 23. doi: 10.1097/IJG.0000000000001287[↩]
- Gurnani B, Kaur K, Sekaran S. First case of coloboma, lens neovascularization, traumatic cataract, and retinal detachment in a young Asian female. Clin Case Rep. 2021 Aug 30;9(9):e04743. doi: 10.1002/ccr3.4743[↩]
- Gurnani B, Kaur K, Gireesh P. A rare presentation of anterior dislocation of calcified capsular bag in a spontaneously absorbed cataractous eye. Oman J Ophthalmol. 2021 Jun 28;14(2):120-121. doi: 10.4103/ojo.OJO_65_2019[↩]
- Gurnani B, Kaur K, Gireesh P. Rare Coexistence of Bilateral Congenital Sutural and Cortical Blue Dot Cataracts. J Pediatr Ophthalmol Strabismus. 2020 Jan 1;57(1):68. doi: 10.3928/01913913-20191011-01[↩]
- Kaur K, Gurnani B, Kannusamy V, Yadalla D. A tale of orbital cellulitis and retinopathy of prematurity in an infant: First case report. Eur J Ophthalmol. 2022 Nov;32(6):NP20-NP23. doi: 10.1177/11206721211026098[↩]
- Baig NB, Lin AA, Freedman SF. Ultrasound evaluation of glaucoma drainage devices in children. J AAPOS. 2015 Jun;19(3):281-4. doi: 10.1016/j.jaapos.2015.02.001[↩]
- Neustein RF, Bruce BB, Beck AD. Primary Congenital Glaucoma Versus Glaucoma Following Congenital Cataract Surgery: Comparative Clinical Features and Long-term Outcomes. Am J Ophthalmol. 2016 Oct;170:214-222. doi: 10.1016/j.ajo.2016.08.012[↩]
- Biglan AW, Hiles DA. The visual results following infantile glaucoma surgery. J Pediatr Ophthalmol Strabismus. 1979 Nov-Dec;16(6):377-81. doi: 10.3928/0191-3913-19791101-10[↩]
- de Silva DJ, Khaw PT, Brookes JL. Long-term outcome of primary congenital glaucoma. J AAPOS. 2011 Apr;15(2):148-52. doi: 10.1016/j.jaapos.2010.11.025[↩]
- Shaffer RN. Prognosis of goniotomy in primary infantile glaucoma (trabeculodysgenesis). Trans Am Ophthalmol Soc. 1982;80:321-5. https://pmc.ncbi.nlm.nih.gov/articles/instance/1312272/pdf/taos00019-0346.pdf[↩]
- Kantipuly A, Pillai MR, Shroff S, Khatiwala R, Raman GV, Krishnadas SR, Lee Robin A, Ehrlich JR. Caregiver Burden in Primary Congenital Glaucoma. Am J Ophthalmol. 2019 Sep;205:106-114. doi: 10.1016/j.ajo.2019.05.003[↩]
- McLaughlin DE, Semrov A, Munshi H, Patel AJ, Rahi J, Grajewski AL; Balkan CGRN Study Group. The impact of childhood glaucoma on psychosocial functioning and quality of life: a review of the literature. Eye (Lond). 2023 Oct;37(15):3157-3173. doi: 10.1038/s41433-023-02492-1[↩]