Aneurysmal bone cyst
Aneurysmal bone cyst is a benign, blood-filled lesion in the bone that tends to expand or grow and mostly diagnosed in children and adolescents. While it is referred to as a cyst, it is a true benign bone tumor surrounded by a thin wall of bone. Aneurysmal bone cyst is a type of osseous lesion characterized by a benign pseudocyst with fibrous connective tissue stroma and large spaces filled with blood and no endothelial lining 1. The World Health Organization (WHO) defines aneurysmal bone cyst as a benign tumorlike lesion that is described as “an expanding osteolytic lesion consisting of blood-filled spaces of variable size separated by connective tissue septa containing trabeculae or osteoid tissue and osteoclast giant cells” 2. Even though aneurysmal bone cysts are not cancerous, aneurysmal bone cysts tend to grow quickly, and treatment is recommended. If an aneurysmal bone cyst is not treated it can cause pain, fractures, disrupt growth and cause neurological symptoms.
Aneurysmal bone cysts can occur in any bones, but they are more common in the long bones, most commonly found around the knee, pelvis or spine with prevalence of only 2% in the jaws 3.
Aneurysmal bone cysts are typically eccentrically located in the metaphysis of long bones, adjacent to an unfused growth plate. Although they have been described in most bones, the most common locations are 4:
- Long bones: 50-60%, typically the metaphysis
- lower limb: 40%
- tibia and fibula: 24%, especially proximal tibia
- femur: 13%, especially proximally
- upper limb: 20%
- lower limb: 40%
- Spine and sacrum: 20-30%
- especially posterior elements, with extension into the vertebral body in 40% of cases 5
- Craniofacial: jaw, basisphenoid, and paranasal sinuses
- Epiphysis, epiphyseal equivalent, or apophysis: rare but important
Almost all aneurysmal bone cysts of the spine involve the posterior elements, and a high incidence of neurologic symptoms is observed, as well as more local aggressive behavior.
The pelvis accounts for approximately 50% of lesions occurring in the flat bones 6. Secondary lesions tend to have a predilection for the areas of the body in which the primary lesion typically arises.
Although the aneurysmal bone cyst can appear in persons of any age, it is generally a disease of the young (albeit a rare one in the very young). About 50-80% of aneurysmal bone cysts occur before age 20 7, with 70-86% occurring in patients between 10 and 20 years of age 3. The mean patient age at onset is 13-17.7 years and 13 years the median age of patients reported by many studies 8. Most studies have also found aneurysmal bone cysts occur slightly more frequently in females than males.
Aneurysmal bone cysts are generally considered rare, accounting for only 1-6% of all primary bony tumors 9. A group from Austria reported an annual incidence of 0.14 aneurysmal bone cysts per 100,000 people 10; however, the true incidence is difficult to calculate because of the existence of spontaneous regression and clinically silent cases.
A biopsy-proven incidence study from the Netherlands showed that aneurysmal bone cysts were the second most common tumor or tumorlike lesion found in children 11.
Aneurysmal bone cysts may occur spontaneously, or be a secondary reaction to another bony growth elsewhere in the body. Research has revealed a high incidence of accompanying tumors specifically chondroblastoma and giant cell tumors in 23 to 32 percent of patients with an aneurysmal bone cyst.
There is no consensus in the literature regarding the best therapeutic method for treating aneurysmal bone cysts. The most commonly used treatment methods are resection, curettage, embolization and intracystic injections. The choice of treatment method varies greatly, especially in children 12. But, despite of the method chosen, the goal is always the success of the treatment, which consists of complete reossification, with new bone formation with normal volume and mineralization characteristics 13 and without recurrence of the cystic lesion 14. There is a 10-15 percent recurrence rate with treatment 15.
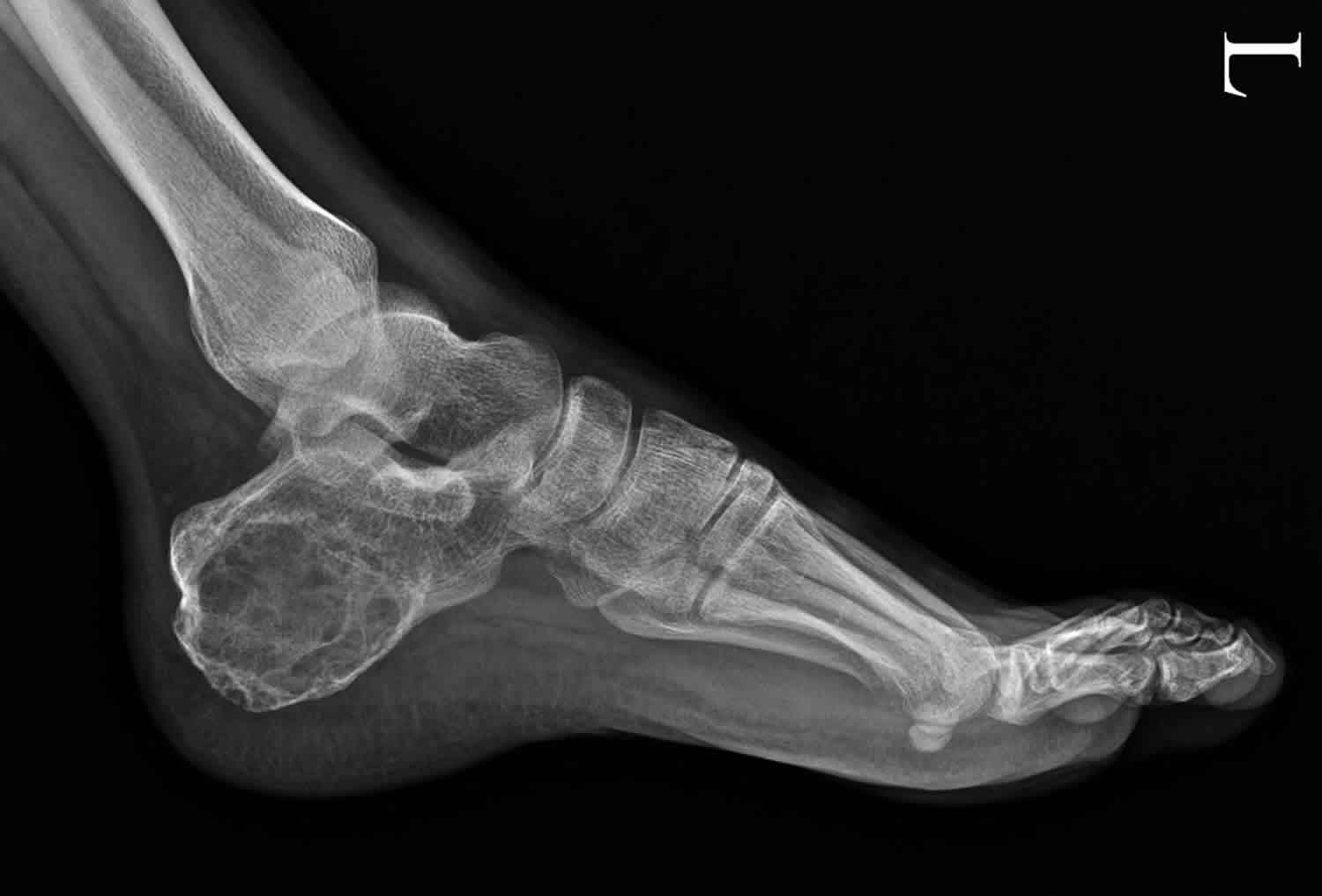
Figure 1. Aneurysmal bone cyst calcaneus
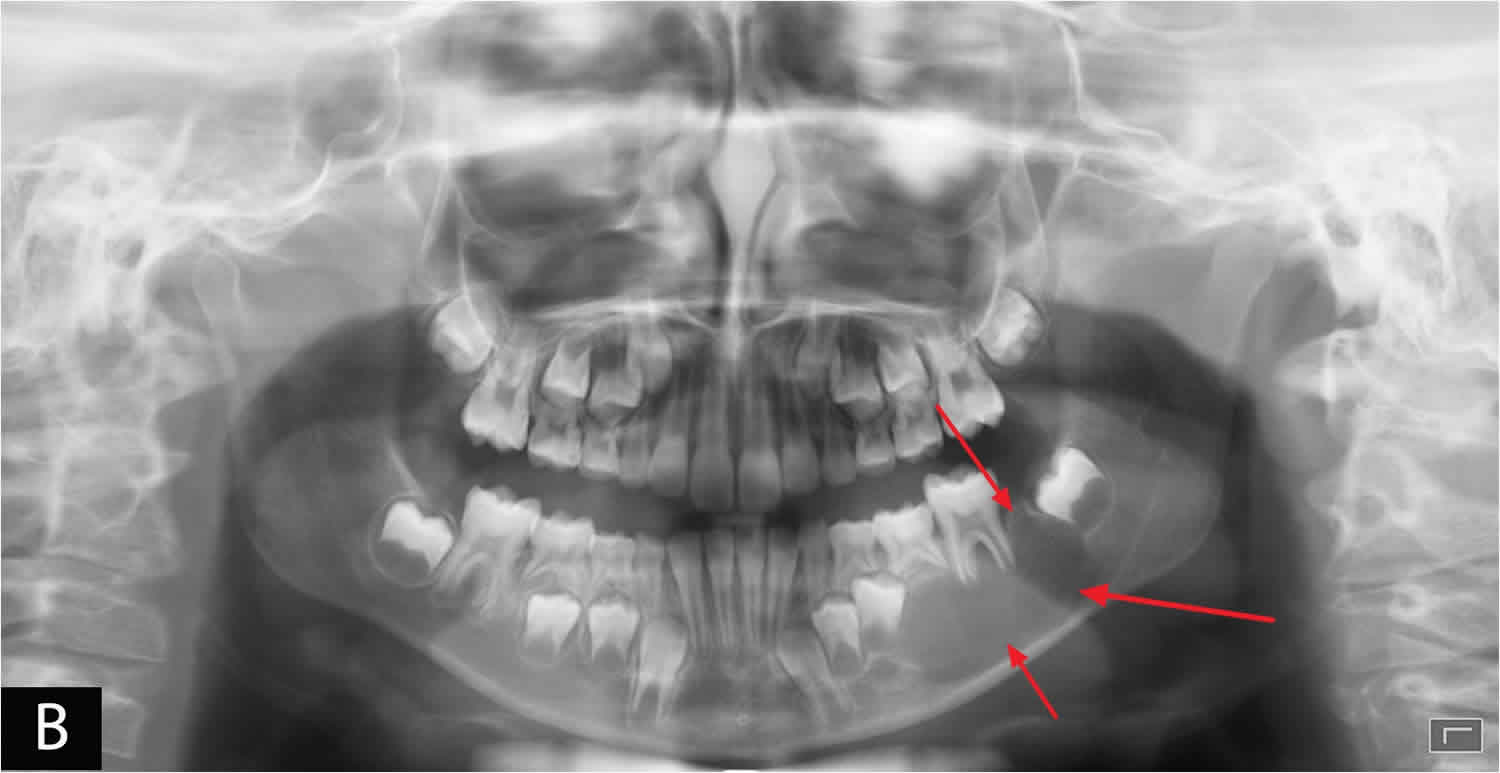
Figure 2. Aneurysmal bone cyst jaw
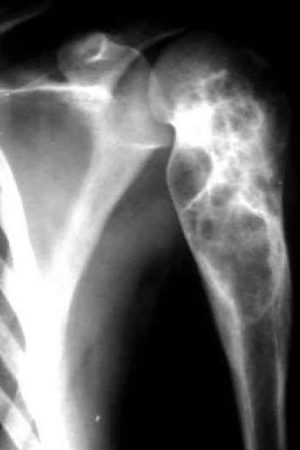
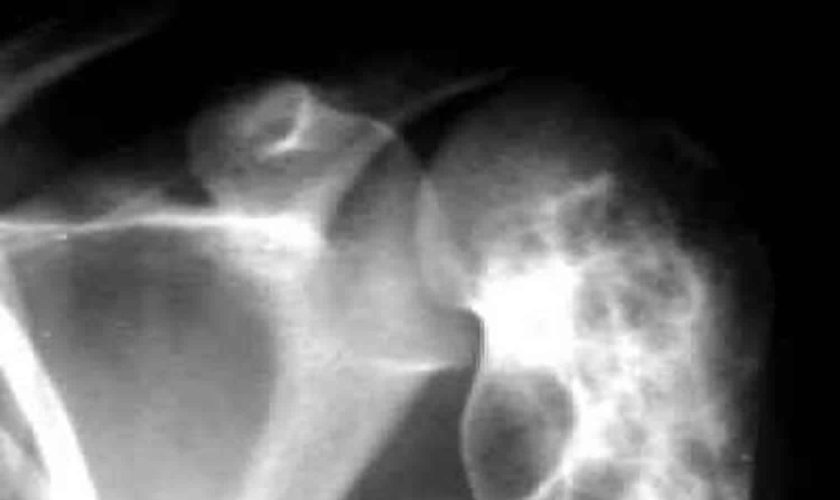
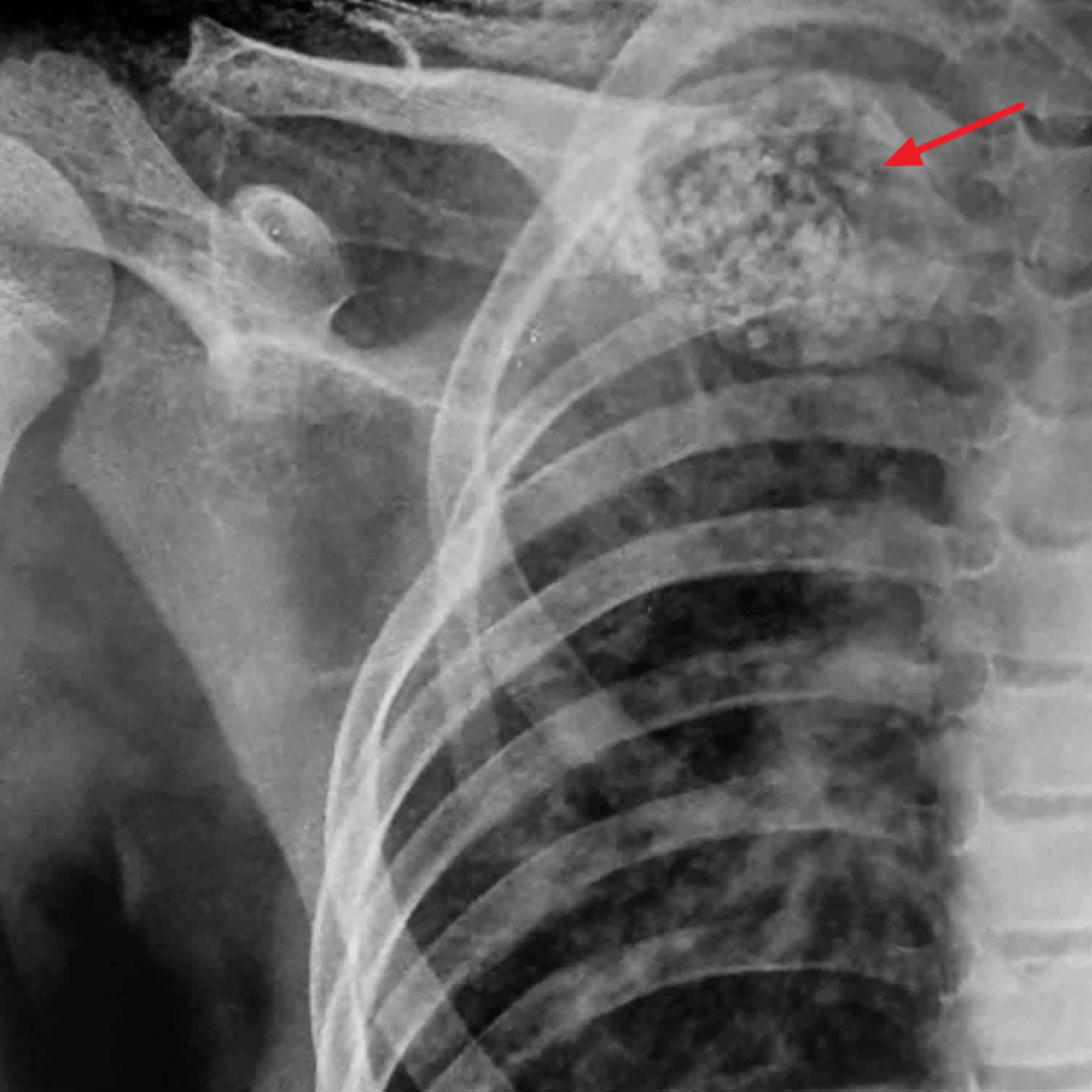
[Source 16 ]Figure 3. Aneurysmal bone cyst of the clavicle
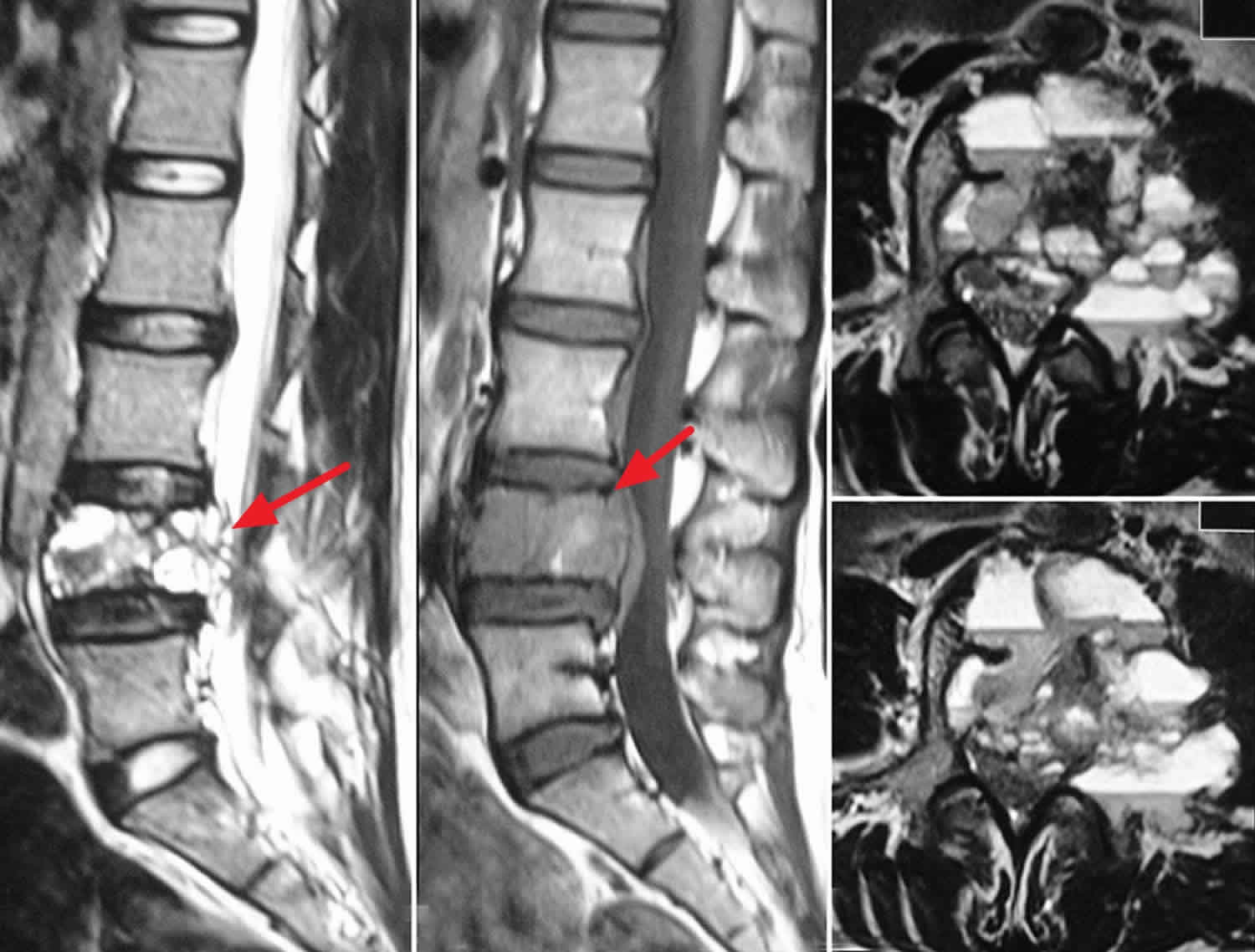
Figure 4. Aneurysmal bone cyst spine
Footnote: MRI scan of the lumbar spine sagittal T1 (a) and T2 (b) Weighted images and axial sections; (c and d) of T2 weighted images showing characteristic findings of aneurysmal bone cyst with multiple fluid-fluid levels.
[Source 17 ]Aneurysmal bone cyst causes
While the cause of aneurysmal bone cysts is currently unknown. Most investigators believe that aneurysmal bone cysts are the result of a vascular malformation within the bone; however, the ultimate cause of the malformation is a topic of controversy. Different theories about several vascular malformations exist; these include arteriovenous fistulas and venous blockage. The vascular lesions then cause increased pressure, expansion, erosion, and resorption of the surrounding bone. The malformation is also believed to cause local hemorrhage that initiates the formation of reactive osteolytic tissue. Findings from a study in which manometric pressures within the aneurysmal bone cysts were measured support the theory of altered hemodynamics.
Three commonly proposed theories are as follows:
- Aneurysmal bone cysts may be caused by a reaction secondary to another bony lesion – This theory has been proposed because of the high incidence of accompanying tumors in 23-32% of aneurysmal bone cysts; although giant cell tumors of bone are most commonly present, many other benign and malignant tumors are found, including fibrous dysplasia, osteoblastoma, chondromyxoid fibroma, nonossifying fibroma, chondroblastoma, osteosarcoma, chondrosarcoma, unicameral or solitary bone cyst, hemangioendothelioma, and metastatic carcinoma; aneurysmal bone cysts in the presence of another lesion are called secondary aneurysmal bone cysts, and treatment of these aneurysmal bone cysts is based on what is appropriate for the underlying tumor
- Aneurysmal bone cysts may arise de novo; those that arise without evidence of another lesion are classified as primary aneurysmal bone cysts
- Aneurysmal bone cysts may arise in an area of previous trauma
Recently aneurysmal bone cysts have been linked to a mutation of the ubiquitin specific peptidase 6 (USP6) gene on chromosome 17. Researchers are currently working to better understand the genetic mutation, learn when it develops, and discover how it may affect a child’s development.
A certain percentage of primary aneurysmal bone cysts may be truly neoplastic—as opposed to vascular, developmental, or reactive—phenomena. It has been shown that as many as 69% of primary aneurysmal bone cysts demonstrate a characteristic clonal t(16;17) genetic translocation 18 leading to upregulation of the TRE17/USP6 oncogene 19, whereas no secondary aneurysmal bone cysts demonstrate this cytogenetic aberration.
Most primary aneurysmal bone cysts demonstrate a t(16;17)(q22;p13) fusion of the TRE17/CDH11-USP6 oncogene. This fusion leads to increased cellular cadherin-11 activity that seems to arrest osteoblastic maturation in a more primitive state 20. This process may be the neoplastic driving force behind primary aneurysmal bone cysts as opposed to secondary aneurysmal bone cysts, which seem to occur reactively as a result of another underlying disease process.
Aneurysmal bone cysts consist of blood-filled spaces of variable size that are separated by connective tissue containing trabeculae of bone or osteoid tissue and osteoclast giant cells. They are not lined by endothelium. A fine needle aspiration cytology is usually nondiagnostic, often dominated by fresh blood 21.
Although often primary, up to a third of aneurysmal bone cysts are secondary to an underlying lesion (e.g. fibrous dysplasia, osteosarcoma, giant cell tumor, chondromyxoid fibroma6, non-ossifying fibroma, chondroblastoma) 22, 23.
A variant of aneurysmal bone cysts is the giant cell reparative granuloma which is usually seen in the tubular bones of the hands and feet as well as in the craniofacial skeleton. Occasionally they are also seen in appendicular long bones where they are known as solid aneurysmal bone cysts. Histologically these two entities are identical 24.
Aneurysmal bone cyst symptoms
Patients with aneurysmal bone cyst may present with pain, which may be of insidious onset or abrupt due to pathological fracture, with a palpable lump or with restricted movement or a combination of these symptoms in the affected area. The symptoms are usually present for several weeks to months before the diagnosis is made, and the patient may also have a history of a rapidly enlarging mass. Neurologic symptoms associated with aneurysmal bone cysts may develop secondary to pressure or tenting of the nerve over the lesion, typically in the spine.
Pathologic fracture occurs in about 8% of aneurysmal bone cysts, but the incidence may be as high as 21% in aneurysmal bone cysts that have spinal involvement.
The symptoms of an aneurysmal bone cyst can include:
- Pain
- Swelling
- Stiffness
- Deformity in the area of the growth
- The feeling of warmth over the affected area
- Decreased range of motion, weakness or stiffness
- Reactive torticollis
- Occasionally, bruit over the affected area
- Warmth over the affected area
Aneurysmal bone cyst diagnosis
Diagnostic tests to diagnose aneurysmal bone cysts, including:
- X-rays, which produce images of bones.
- Magnetic resonance imaging (MRI), which uses a combination of large magnets, radiofrequencies and a computer to produce detailed images of organs, soft tissues, muscles, ligaments and other structures within the body. Your child is exposed to no radiation during an MRI.
- Computed tomography (CT) scan, which uses a combination of X-rays and computer technology to examine bones and produces cross-sectional images (“slices”) of the body.
- EOS imaging, an imaging technology that creates 3-dimensional models from two flat images. Unlike a CT scan, EOS images are taken while the child is in an upright or standing position, enabling improved diagnosis due to weight-bearing positioning.
- Angiography, a radiograph-type X-ray test which reveals the inside of blood vessels and organs.
- Needle biopsy, which is a procedure where a doctor places a small needle through the skin and into the lesion to withdraw a small sample of the abnormal tissue. The tissue is analyzed to confirm any findings.
In addition to diagnosing the specific type of growth your child may have, these tests will also help determine the size and location of the tumor. All of this information is crucial in determining the best treatment options for your child.
Aneurysmal bone cyst staging
The staging of benign musculoskeletal neoplasms was described by Enneking in 1986 25, who classified benign lesions into the following three broad categories:
- Stage 1: Latent (inactive)
- Stage 2: Active
- Stage 3: Aggressive
This system has been adopted by the Musculoskeletal Tumor Society (MSTS). Part of the Enneking classification contains the Lodwick radiographic grading system (see below) 26.
Lodwick radiographic grading with bone destruction
Lodwick grade 1A is characterized as follows 27:
- Mandatory geographic destruction
- Characteristic regular, lobulated, or multicentric edge
- No or partial cortex penetration
- Mandatory sclerotic rim
- Expanded shell optional, 1 cm or less
Lodwick grade 1B is characterized as follows:
- Mandatory geographic destruction
- Characteristic regular, lobulated, multicentric, or ragged or poorly defined edge
- No or partial cortex penetration
- Optional sclerotic rim
- If sclerotic rim present, expanded shell must be larger than 1 cm
Lodwick grade 1C is characterized as follows:
- Mandatory geographic destruction
- Edge characteristic is regular, lobulated, multicentric, ragged or poorly defined, or moth-eaten, 1 cm or smaller
- Mandatory total penetration of the cortex
- Optional sclerotic rim
- Optional expanded shell
Lodwick grade 2 is characterized as follows:
- Moth-eaten or geographic destruction – If geographic destruction, mandatory moth-eaten edge is larger than 1 cm
- By definition, total penetration of cortex
- Optional sclerotic rim, but unlikely
- Optional expanded shell, but unlikely
Lodwick grade 3 is characterized as follows:
- Mandatory permeated destruction
- Any edge
- By definition, total penetration of cortex
- Optional sclerotic rim, but unlikely
Latent or inactive musculoskeletal neoplasms
Latent (inactive) musculoskeletal neoplasms have the following characteristics:
- Asymptomatic
- Usually incidental findings
- Rare to have a pathologic fracture or other dysfunction
- May grow slowly, but almost always reach a steady state where they no longer grow
- Remain intracompartmental
- Do not deform the compartment
- If palpable, are small, movable, and nontender
- Radiography – Well marginated, with a mature shell of cortical-like reactive bone without deformation or expansion of the encasing bone; Lodwick 1A
- Isotope scan – Little or no increased uptake
- Angiography – No significant neoangiogenesis
- CT – Homogeneous density, good margination, no cortical broaching or cross-facial extension
- Histology – Low cell-to-matrix ratio; mature, well-differentiated matrices; benign cytologic characteristics; encapsulation by mature fibrous tissue or cortical bone; little or no reactive mesenchymal proliferation, inflammatory infiltrate, or neoangiogenesis about the lesions
Active musculoskeletal neoplasms
Active musculoskeletal neoplasms have the following characteristics:
- Mildly symptomatic
- Discovered because of patient discomfort or the presence of a pathologic fracture or mechanical dysfunction
- Grow steadily, continue to enlarge during observation
- Appear responsive to contact inhibition but not at normal levels
- Can expand by deformation of the overlying cortical bone, articular cartilage, or fascial septa
- Remain encapsulated
- Only a thin layer of filmy areolar tissue separates the reactive zone between the lesions and the surrounding normal tissue.
- If palpable, are small with moderate tenderness and movable (The increase in size can be felt on serial examinations.)
- Radiography – Well-defined, yet irregular margination; a mature cancellous ring margin, rather than a cortical shell; irregular or corrugated inner aspect, resulting in a septated appearance; expansion, bulging, deformation, or the combination of overlying cortex/reactive bone is frequently observed; Lodwick 1B
- Isotope scan – Increased isotope uptake only around the limits of the defect
- Angiography – Often, a reactive angiogenesis is observed around the lesion, almost never within.
- CT – Homogeneous density; irregular but intact reactive bone, expansion of the overlying cortex, and intracompartmental containment by bone or fascia
- Histology – Relatively balanced cell-to-matrix ratio; well-defined matrices; benign cytologic characteristics; intact capsule of mature fibrous tissue and/or cancellous bone; narrow zone of mesenchymal, inflammatory, and vascular reactive tissue between the capsule and the surrounding normal tissue; resorption of the preexisting bone by osteoclasts, rather than by neoplastic cells, as the mechanism of expansion; may have areas of intermittent resorption that produce an irregular, serrated, and sometimes corrugated interface between the capsule and the adjacent reactive bone
Aggressive musculoskeletal neoplasms
Aggressive musculoskeletal neoplasms have the following characteristics:
- Despite being benign, may act more like a low-grade malignancy
- Often symptomatic
- Discovered because of patient discomfort, a growing mass, or a pathologic fracture
- If palpable, are often large and tender; may feel rapid enlargement on serial physical examinations; may feel more fixed
- May have an inflammatory appearance
- Little contact inhibition
- Penetrate or permeate the natural barriers to tumor growth, which are cortical bone, fascial septa, and articular cartilage
- Penetrate the capsule with fingerlike projections directly into the surrounding zone
- Destroy or resorb the surrounding bone or fascia and permeate into adjacent tissues or compartments rather than expanding by concomitant endosteal resorption and subperiosteal apposition
- In unrestrained areas, may expand rapidly and may be preceded by a pseudocapsule
- Radiography – Ragged, permeative interface with adjacent bone; incomplete attempts at containment by reactive bone; cortical destruction; endosteal buttresses; periosteal Codman triangles; rapid soft-tissue expansion; Lodwick 1C
- Isotope scan – Increased uptake in the early vascular phase and the late bone phase, often beyond radiographic limits
- Angiography – Distinct reactive zone of neovasculature on the early arterial phase and an intralesional hypervascular blush on the late venous phase
- CT – Nonhomogeneous, mottled, attenuating areas with defects in attempts at reactive containment; early extracompartmental extension from bone; indistinct margins in soft tissues; possible neurovascular bundle involvement
- Histology – High cell-to-matrix ratio; clearly differentiated matrices of varying maturity; predominantly benign cytologic characteristics without anaplasia or pleomorphism, but with frequent hyperchromatic nuclei; mitosis occasionally encountered; possible vascular invasion; extensions are usually still continuous with the main mass but may have some satellite lesions; thick, succulent zone of reactive tissue between the penetrated capsule and the more peripheral normal tissue (zone or pseudocapsule encircles but does not inhibit growth of the aggressive tumor; however, it does inhibit tumor nodules from extending directly into normal tissue); destruction of surrounding bone via reactive osteoclasts, not by tumor cells; tumor fingers that may grow into the reactive bone
Aneurysmal bone cyst treatment
There are many treatment options available for bone and soft tissue tumors, and some children will need a combination of these therapies. Orthopaedic, oncology and other specialists collaborate to provide your child with individualized care and the best possible outcomes. Your child’s clinical team will recommend the best treatment for your child’s individual situation.
Treatment for aneurysmal bone cysts may include:
- Intralesional curettage, which involves scraping out the bone to completely remove the tumor and all cyst lining
- Intraoperative adjuvants — such as cryotherapy (liquid nitrogen), phenol (a chemical) or cauterization (burning the tumor bed) — which are used to remove microscopic tumor cells
- Bone grafting, a surgical procedure to replace missing bone with artificial graft material or cadaver bone
Depending on the size and location of aneurysmal bone cyst removed, your child may be able to return home that day or may spend one night in the hospital.
Aneurysmal bone cysts generally are treated surgically usually with curettage and resection 28. The extent of the treatment depends on the localization of the cyst, its size, its clinical characteristics and the age of the patient 29. With the vascular type, bleeding may be intense 3, especially when the lesion is reached. Accordingly, preoperative embolization is commonly performed to minimize excessive bleeding during the curettage procedure.
Some anatomic locations may be difficult to access surgically. If this situation is encountered, other methods of treatment, such as intralesional injection, selective serial arterial embolization and sclerotherapy performed by an interventional radiologist, may be successful 12. Percutaneous embolization, or sclerotherapy, has been considered a treatment option for aneurysmal bone cysts as reported by some authors 12 primarily because, if performed by an experienced professional, it is an easy, safe, cost-effective and minimally invasive procedure compared to surgery. In addition, a success rate of over 90% with the use of sclerotherapy for treating aneurysmal bone cysts is reported 30.
It is possible to find different fibrosing agents in the literature, including Ethibloc®, Absolute Alcohol and Histoacryl® 14. However, the most commonly used agents are Ethibloc and Absolute Alcohol.
Histoacryl® consists of an acrylic resine (n-butyl-2-cyanoacrylate) that acts as a tissue glue, and in contact with blood, it is quickly polymerized, preventing bleeding 31. In order to prevent n-butyl-2-cyanoacrylate solidification from occurring too fast, mixing with lipiodol is required 31. After injection, the cystic lesion is filled with this solidifying mixture that is visualized as an opacified image 32. The Histoacryl® will then be reabsorbed, and the reossification process will take place in the region of the lesion 32. Many authors had demonstrated new bone formation occurring in different areas of the human body when sclerosis of bone lesions are performed with fibrosing agents 33.
The first use of n-butyl-2-cyanoacrylate in sclerotherapy was reported by Soehendra et al. in 1986 34, who used this tissue adhesive agent to treat bleeding gastric varices, reporting the success of the therapy in 3 patients. Since then, many authors have begun to use this fibrosing agent in sclerotherapy.
However, since aneurysmal bone cysts are most frequently found in the long bones, the therapeutic procedures most commonly reported also involve these skeletal regions. According to the literature, percutaneous embolization with Histoacryl® is generally used to treat aneurysmal bone cysts in long bones 14. In addition, this fibrosing agent is the only recommended to treat aneurysmal bone cyst in the skull and spine, due to the considerable inflammatory reaction caused by Ethibloc 14. Rossi et al. 35 reported 36 aneurysmal bone cysts (4 in the thoracic cage, 6 in the spine, 9 in the long bones and 17 in the pelvis) treated with n-butyl-2-cyanoacrylate injection. The authors reported a success rate of 94%, and in most lesions (61%) only one embolization was required. In a total of 55 procedures performed, complications were observed only in 3 of them (5%) 35.
The literature also reports the use of Histoacryl® in other facial lesions. Alaraj et al. 36 reported a series of 20 patients with cranial, facial, and neck tumors treated with n-butyl-2-cyanoacrylate embolization, either preoperatively or palliatively in cases of uncontrollable bleeding.
In the future, advances in osteoinductive materials (eg, genetically engineered bone morphogenic protein) may offer a less invasive treatment of aneurysmal bone cyst.
Impending pathologic fracture, especially a fracture of the hip, is a challenging problem and an indication for intervention, which often includes curettage, adjuvant treatment, and internal fixation.
Rarely, asymptomatic aneurysmal bone cysts may be seen in which there is clinically insignificant destruction of bone. In such cases, close monitoring alone of the lesion may be indicated because of the evidence that some aneurysmal bone cysts spontaneously resolve. When a patient is monitored in this manner, the diagnosis must be certain, and the lesion should not be increasing in size.
Surgical therapy
Extensive preoperative planning should be completed with the use of cross-sectional imaging. Embolization as a treatment or preoperative technique should be considered. When possible, a tourniquet should be used. Thought should also be given to what possible methods and materials may be needed to provide stability after aneurysmal bone cyst excision or resection.
Depending on the size and nature of the lesion, the patient’s fluid volume and blood loss may have to be monitored closely.
Curettage and excision
The unusual stage 1 aneurysmal bone cyst can be treated with intralesional curettage 37; the more common stage 2 aneurysmal bone cyst is treated by intralesional excision. The difference between curettage and excision is that excision involves wide unroofing of the lesion through a cortical window by careful abrasion of all the surfaces with a high-speed burr and, possibly, local adjuvants such as phenol, methylmethacrylate (MMA), or liquid nitrogen. These adjuvants are controversial because firm evidence that they are effective is lacking, and their use entails considerable risk.
En-bloc or wide excision is typically reserved for stage 3 aneurysmal bone cysts that are not amenable to intralesional excision (eg, extensive bony destruction); the recurrence rate after en-bloc excision is about 7%. Reconstructive options after wide excision include structural allografting and reconstruction with either endoprostheses or allograft-prosthetic composites.
In the past, intralesional excision was the mainstay of treatment. The aneurysmal bone cyst is accessed, a window is opened in the bony wall, and then the contents of the aneurysmal bone cyst are removed. Excision of the walls with curettes, rongeurs, or high-speed burrs has been described. The intralesional method leaves more bony structure intact than en-bloc or regional resection.
Intralesional excision may also be used around joints and other vital areas to try to preserve function. The defect may then be filled with bone chips, bone strut, or other supporting material to add strength and to enhance healing of the excised area.
Concerns for local resection include the following:
- The region must be expendable and not affect function (eg, spinous process, rib, clavicle, or fibula)
- Some investigators believe that elective arterial embolization should be tried first if it is not contraindicated
Concerns for en-bloc excision of a deep lesion include the following:
- Resection destabilizes the area; some surgeons use more than one third of the bone width
- Loss of function (eg, joint loss) is possible
- Some investigators believe that elective arterial embolization should be tried first if it is not contraindicated
Concerns for intralesional removal include the following:
- The area may be surgically inaccessible
- Some investigators believe that elective arterial embolization should be tried first if it is not contraindicated
Adjuvant therapy
The surgeon may also use adjuvant therapy, which extends the area of treatment beyond that which can be physically excised. The use of liquid nitrogen, phenol, argon beam gas plasma photocoagulation, and polymethylmethacrylate (PMMA) may achieve an extended area of treatment.
The adjuvants involve the use of chemical, freezing, or thermal means to cause bone necrosis and microvascular damage to the walls of the physically excised cyst, disrupting the possible etiology. Compared with en-bloc and regional resection, the use of adjuvants leaves more bone intact, and an increased area is treated compared with the area treated with intralesional resection alone.
Liquid nitrogen is the most popular adjuvant, and it is often described in the literature. After the aneurysmal bone cyst is exposed and a window is opened, liquid nitrogen may be applied by pouring it into the cyst through a funnel or by using a machine that is designed to spray the liquid onto the walls of the lesion. The surgeon should be sure to leave the window open, allowing the gas to escape.
A total of two or three cycles of freezing and thawing should be used to obtain maximum bone necrosis. The surrounding tissue, especially the neurovascular bundles, must be protected to ensure these structures are not damaged. Avoiding the use of a tourniquet with cryotherapy is suggested to keep the surrounding tissue vascularized, making it more resistant to freezing.
Phenol is much less often used as an adjuvant. Some authors have questioned the effectiveness of phenol because of its poor penetration of bony tissue compared with that of liquid nitrogen. However, phenol has had some success in certain studies, and it has the benefit of being easy to use. Phenol is simply applied to the mechanically removed walls by using soaked swabs. Any remaining phenol is removed with suction, and the cavity is filled with absolute alcohol. Finally, the cavity is irrigated with isotonic sodium chloride solution.
Polymethylmethacrylate (PMMA) may also be used, though the effectiveness of its thermal properties in causing bone necrosis has been questioned in the literature. However, polymethylmethacrylate (PMMA) does have the benefit of rendering a large lesion mechanically sound and making it easier to recognize a local recurrence. If polymethylmethacrylate (PMMA) is used in a subchondral location, the joint surface should be protected by placing cancellous grafts or Gelfoam (Pharmacia & Upjohn Co, Kalamazoo, MI) before cementation. It is not clear that removing the cement and replacing it with a bone graft is necessary.
Argon beam coagulation has also been used in several studies, with some promising results 38. One study noted that surgical treatment with curettage and adjuvant argon beam coagulation is an effective treatment option for aneurysmal bone cyst; the primary complication was postoperative fracture 38.
An additional study found that argon beam photocoagulation, while avoiding the toxic effects of phenol, yielded statistically equivalent recurrence rates, functional outcomes, and complication rates in the treatment of benign-aggressive bone tumors 39. However, the authors also noted an increased fracture rate in the argon beam photocoagulation cohort as compared with the phenol cohort.
Concerns for adjuvant intralesional therapy include the following:
- Substances such as liquid nitrogen and phenol could penetrate tissues and damage the surrounding structures, with neural and vascular tissues being at particularly high risk; for this reason, some investigators discourage the use of intralesional therapy in the spine
- Caution should be used in areas prone to fracture; liquid nitrogen and argon beam photocoagulation can make the surrounding bone stock more brittle and thus increase the likelihood of fracture
Additional considerations
NOTE: Special consideration is necessary in dealing with aneurysmal bone cysts that are near open physes. The reader is referred to the literature for general considerations when operating around physes. The reported rate of physeal injury is significant, and patients and their families must be made aware of this possibility. Furthermore, it has been shown that attempts to spare the adjacent physes by performing a less-than-aggressive curettage of aneurysmal bone cysts have resulted in increased risk of local recurrence in patients with open growth plates 40.
Spinal aneurysmal bone cysts usually cause neurologic symptoms and pose treatment challenges. The details of surgical excision can be found elsewhere. There is evidence to support an attempt at one or two trials of selective arterial embolization before surgical excision.
A group in Japan developed an endoscopic approach to the treatment of aneurysmal bone cyst 41. They successfully treated four patients with aneurysmal bone cysts that lacked the aneurysmal component. The technique was completed with a variety of curettes, ball forceps, Kirschner wires (K-wires), an arthroscope, and a drill. The method may leave a more stable structure and is minimally invasive.
Treatment for a secondary aneurysmal bone cyst is that which is appropriate for the underlying lesion.
Selective arterial embolization
Selective arterial embolization has shown much promise for aneurysmal bone cysts in small studies. However, the number of cases treated with this therapy is not large, both because aneurysmal bone cysts are rare and because selective arterial embolization has been available only since the 1980s 42.
With the use of angiography, an embolic agent is placed at a feeding artery to the aneurysmal bone cyst, cutting off the nutrient supply and altering the hemodynamics of the lesion. Various materials, such as springs and foam, have been used to create the emboli.
Selective arterial embolization has the advantage of being able to reach difficult locations, being able to save joint function when subchondral bone destruction is present, and making the complications that are associated with invasive surgery (eg, bleeding) less likely to occur. Selective arterial embolization may be performed within 48 hours before surgery to reduce the amount of intraoperative hemorrhage.
Some of the literature suggests that selective arterial embolization can be a primary treatment for aneurysmal bone cyst if the following conditions are met:
- Histologically confirmed tissue diagnosis of aneurysmal bone cyst
- Technical feasibility and safety
- Stability; no evidence of pathologic fracture or impeding fracture
- No neurologic involvement
Contraindications for selective arterial embolization include the following:
- Uncertain diagnosis; need to perform an open biopsy
- Structural instability; pathologic or impending fracture
- Neurologic symptoms
- Mechanical disruption
- Unsafe location to embolize with angiography or anatomically (eg, segmental arteries, certain cervical and thoracic areas that may lead to spinal cord ischemia, or subcutaneous bones [such as the clavicle or iliac crest] that may lead to adjacent skin necrosis and need for flap or skin graft coverage)
Intralesional injection
Only case evidence exists for intralesional injection, but the injection may be attempted for cases in which surgical access is difficult and for those in which other modalities are contraindicated 43.
Note: Do not use this approach if the patient has allergies to the injection components, a pathologic or impeding fracture, neurologic symptoms, or unbearable symptoms such as pain. Do not use intralesional injection if a more proven treatment is indicated.
There has also been case evidence for the use of calcitonin 44 and methylprednisolone injections in the regression of aneurysmal bone cysts. This is thought to combine the inhibitory angiostatic and fibroblastic effects of methylprednisolone with the osteoclastic inhibitory effect and the trabecular bone-stimulating properties of calcitonin. The injections are performed under computed tomography (CT) guidance and anesthesia. Growth of the aneurysmal bone cyst must be closely monitored, and the treatment may need to be repeated several times. Years may pass before the aneurysmal bone cyst resolves.
ETHIBLOC (Ethicon, Norderstedt, Germany) injection is also performed under CT guidance and anesthesia 45. The injected solution is a mixture of zein, oleum papaveris, and propylene glycol and acts as a fibrosing agent, and an inflammatory reaction may occur after its administration. Bony healing may take months to years. Side effects (eg, localized thrombosis, pulmonary embolus, osseocutaneous fistula formation, and severe surrounding tissue necrosis) make it a poor first-line choice in the absence of an obvious surgical contraindication 46.
Some case evidence also suggests healing improvement when systemic calcitonin treatment is used as an adjuvant to other treatment modalities.
An Australian study by Clayer 15 in 15 patients with pathologically confirmed aneurysmal bone cyst suggests that percutaneous aspiration and injection of aneurysmal bone cysts using an aqueous solution of calcium sulfate may have value. All patients except one who have reported pain before the procedure were completely without symptoms at 4 weeks post injection. The calcium sulfate was reabsorbed within 8 weeks.
During the minimum 2-year follow-up period, two patients developed local recurrence of the lesion, one of whom later developed a pathologic fracture. Two other patients sustained pathologic fractures at 12 and 22 months post injection, respectively. Clayer concluded that this procedure has “early clinical and radiological responses and a low complication rate in a consecutive group of patients with aneurysmal bone cyst” 15.
In several series, intralesional percutaneous injection of doxycycline has been reported to be beneficial in inducing stromal cell necrosis, reversing bone destruction, and preserving neighboring anatomy including physes and subchondral bone 47. The principal proposed mechanisms of action for the success seen include the following 48:
- Matrix metalloproteinase (MMP) and angiogenesis inhibition
- Osteoclast inhibition and apoptosis
- Enhanced osteoblastic bone healing
Contraindications for intralesional injection are as follows:
- Uncertain diagnosis; need to obtain an open biopsy
- Structural instability; pathologic or impending fracture
- Neurologic symptoms
- Mechanical disruption
- Allergy to injected substance
- Unbearable symptoms; lengthy time to resolution.
Treatment complications
Complications can vary with the location in which the aneurysmal bone cyst arises. Many of these are related to the proximity of the surrounding tissues.
Universal complications that have been described with surgery include the following:
- Recurrence
- Blood loss
- Wound infection
- Wound slough
- Wound hematoma
- Osteomyelitis
- Damage to the surrounding tissue
- Possible physis damage
- Pulmonary embolism
Additional complications that have been shown with spinal locations include the following:
- Tear of the dura
- Transient spastic paralysis from hematomas
- Tear in the vena cava
- Persistent back ache
- Deformity
- Neurologic symptoms
Complications that are associated with liquid nitrogen include the following:
- Rare gas embolism
- Rare late fracture
- Wound necrosis
- Damage to the surrounding tissue (eg, neurovascular bundles, physis)
A complication that is associated with phenol is necrosis of the surrounding tissue exposed to the phenol (eg, neurovascular bundles, physis).
A complication that is associated with selective arterial embolization is unintentional embolization of a vital area.
Finally, fracture risk may be elevated in those adjuvantly treated with argon beam photocoagulation, particularly in weightbearing bones.
Aneurysmal bone cyst recurrence
Aneurysmal bone cysts can recur in 10-15 percent of patients, so it is important for your child to continue to see your child’s surgeon after treatment. Recurrence usually happens within the first year after surgery, and almost all episodes occur within 2 years 49. However, patients should still be monitored on a regular basis for 5 years. It is beneficial to detect recurrence early when the lesion is still small and easier to treat. Children should be monitored until they have reached maturity to ensure that any possible recurrence does not cause deformity or interfere with their growth. Any patients who have received radiation should be monitored for life because of the risk of secondary sarcoma.
Your child will see the orthopaedic surgeon about one to two weeks after surgery, then again every three to four months for two years to monitor for possible recurrence of the growth.
During follow-up visits, X-rays and other diagnostic testing of the tumor site are recommended to closely monitor your child’s health, check the reconstruction, and make sure there is no recurrence.
If the aneurysmal bone cyst returns, surgeons will treat the recurrence with intralesional curettage, intraoperative adjuvants, and bone grafting.
In most cases, an aneurysmal bone cyst tumor will not recur more than two years after surgery.
In a published review of 897 cases of aneurysmal bone cyst, the following rates of occurrence were reported 50:
- Tibia – 17.5%
- Femur – 15.9%
- Vertebra – 11.2%
- Pelvis – 11.6%
- Humerus – 9.1%
- Fibula – 7.3%
- Foot – 6.3%
- Hand – 4.7%
- Ulna – 3.8%
- Radius – 3.1%
- Other – 9.2%
Spontaneous regression may occur, including following partial removal, but this is not the typical natural history 51.
Aneurysmal bone cyst prognosis
The prognosis for an aneurysmal bone cyst is generally excellent, though some patients need repeated treatments because of recurrence, which is the most common problem encountered when treating an aneurysmal bone cyst.
The overall cure rate is 90-95% 52. A younger age, open growth plates, and a metaphyseal location all have been associated with an increased risk of recurrence 52. The stage of the aneurysmal bone cyst has not been shown to influence the rate of recurrence; however, most clinicians believe that Enneking/Musculoskeletal Tumor Society (MSTS) stage 3 lesions have the highest recurrence rate, other factors being equal. Capanna morphologic types I and II recur more often than types III, IV, and V.
Reported primary recurrence rates have varied greatly. Small studies have shown a benefit to using selective arterial embolization, and some authors advocate it as a first-line treatment. Other authors argue that not enough data on selective embolization exist and that surgery is the first-line treatment. Intralesional excision has the most data to suggest that it is a safe and effective method.
Recurrence rates for different techniques have varied. Some studies have reported recurrence rates as high as 59% with intralesional excision 53 and as low as 0% with resection 50. In a summary of studies of different treatment methods, the following rates of recurrence were reported 50:
- Irradiation – 34 performed with 4 recurrences (11.8% recurrence rate)
- Irradiation and curettage – 35 performed with 5 recurrences (14.3% recurrence rate)
- Curettage and bone graft – 484 performed with 149 recurrences (30.8% recurrence rate)
- Curettage and cryobiopsy – 78 performed with 10 recurrences (12.8% recurrence rate)
- Marginal resection – 81 performed with 6 recurrences (7.4% recurrence rate)
- Wide resection – 59 performed with 0 recurrences (0% recurrence rate).
- Y. Zadik, A. Aktas, S. Drucker, D.W. Nitzan. Aneurysmal bone cyst of mandibular condyle: a case report and review of the literature. J Cranio-Maxillo-Fac Surg, 40 (2012), pp. e243-e248, 10.1016/j.jcms.2011.10.026[↩]
- Schajowicz F. Aneurysmal bone cyst. Histologic Typing of Bone Tumours. Berlin: Springer-Verlag; 1992. 37.[↩]
- M.H. Motamedi. Aneurysmal bone cysts of the jaws: clinicopathological features, radiographic evaluation and treatment analysis of 17 cases. J Cranio-Maxillofacial Surg, 26 (1998), pp. 56-62[↩][↩][↩]
- Meyers SP. MRI of bone and soft tissue tumors and tumorlike lesions, differential diagnosis and atlas. Thieme Publishing Group. (2008) ISBN:3131354216[↩]
- Blake MA. Imaging in oncology. Springer Verlag. (2008) ISBN:0387755861[↩]
- Kransdorf MJ, Sweet DE. Aneurysmal bone cyst: concept, controversy, clinical presentation, and imaging. AJR Am J Roentgenol. 1995 Mar. 164(3):573-80.[↩]
- M. Boubbou, K. Atarraf, S. Tizniti. Aneurysmal bone cyst primary. About eight pediatric cases: radiological aspects and review of the literature. Pan Afr Med J, 15 (2013), 10.11604/pamj.2013.15.111.2117[↩]
- A. Leithner, R. Windhager, S. Lang, O.A. Haas, F. Kainberger, R. Kotz. Aneurysmal bone cyst. A population based epidemiologic study and literature review. Clin Orthop Relat Res (1999), pp. 176-179[↩]
- Aneurysmal Bone Cyst. https://emedicine.medscape.com/article/1254784-overview#a7[↩]
- Leithner A, Windhager R, Lang S, et al. Aneurysmal bone cyst. A population based epidemiologic study and literature review. Clin Orthop Relat Res. 1999 Jun. (363):176-9.[↩]
- van den Berg H, Kroon HM, Slaar A, Hogendoorn P. Incidence of biopsy-proven bone tumors in children: a report based on the Dutch pathology registration “PALGA”. J Pediatr Orthop. 2008 Jan-Feb. 28(1):29-35.[↩]
- J. Cottalorda, S. Bourelle. Modern concepts of primary aneurysmal bone cyst. Arch Orthop Trauma Surg, 127 (2007), pp. 105-114, 10.1007/s00402-006-0223-5[↩][↩][↩]
- S. Rastogi, M.K. Varshney, V. Trikha, S.A. Khan, B. Choudhury, R. Safaya. Treatment of aneurysmal bone cysts with percutaneous sclerotherapy using polidocanol. J Bone Joint Surg Br (2006), 10.1302/0301-620X.88B9.17829[↩]
- J. Dubois, V. Chigot, G. Grimard, M. Isler, L. Garel. Sclerotherapy in aneurysmal bone cysts in children: a review of 17 cases. Pediatr Radiol, 33 (2003), pp. 365-372, 10.1007/s00247-003-0899-4[↩][↩][↩][↩]
- Clayer M. Injectable form of calcium sulphate as treatment of aneurysmal bone cysts. ANZ J Surg. 2008 May. 78(5):366-70.[↩][↩][↩]
- Percutaneous embolization of aneurysmal bone cyst of the mandible: A 3-year follow-up. Oral and Maxillofacial Surgery Cases Volume 5, Issue 4, December 2019, 100121 https://doi.org/10.1016/j.omsc.2019.100121[↩]
- Cugati G, Pande A, Jain PK, Symss NP, Ramamurthi R, Vasudevan CM. Aneurysmal bone cyst of the lumbar spine. Asian J Neurosurg. 2015;10:216–218.[↩]
- Panoutsakopoulos G, Pandis N, Kyriazoglou I, Gustafson P, Mertens F, Mandahl N. Recurrent t(16;17)(q22;p13) in aneurysmal bone cysts. Genes Chromosomes Cancer. 1999 Nov. 26(3):265-6.[↩]
- Guseva NV, Jaber O, Tanas MR, Stence AA, Sompallae R, Schade J, et al. Anchored multiplex PCR for targeted next-generation sequencing reveals recurrent and novel USP6 fusions and upregulation of USP6 expression in aneurysmal bone cyst. Genes Chromosomes Cancer. 2017 Apr. 56 (4):266-277.[↩]
- Lau AW, Pringle LM, Quick L, Riquelme DN, Ye Y, Oliveira AM, et al. TRE17/ubiquitin-specific protease 6 (USP6) oncogene translocated in aneurysmal bone cyst blocks osteoblastic maturation via an autocrine mechanism involving bone morphogenetic protein dysregulation. J Biol Chem. 2010 Nov 19. 285(47):37111-20.[↩]
- Layfield LJ. Cytopathology of bone and soft tissue tumors. Oxford University Press, USA. (2002) ISBN:019513236X[↩]
- Subach BR, Copay AG, Martin MM, Schuler TC, Romero-Gutierrez M. An unusual occurrence of chondromyxoid fibroma with secondary aneurysmal bone cyst in the cervical spine. Spine J. 2010;10(2):e5-e9. doi:10.1016/j.spinee.2009.11.016[↩]
- Tomoyuki K, Susa M, Nakayama R, et al. Secondary aneurysmal bone cyst following chondroblastoma of the patella. Rare Tumors. 2013;5(3):e43. Published 2013 Sep 4. doi:10.4081/rt.2013.e43[↩]
- Ilaslan H, Sundaram M, Unni KK. Solid variant of aneurysmal bone cysts in long tubular bones: giant cell reparative granuloma. AJR Am J Roentgenol. 2003;180(6):1681-1687. doi:10.2214/ajr.180.6.1801681[↩]
- Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res. 1986 Mar. 204:9-24.[↩]
- Lodwick GS. The bones and joints. Hodes PJ, ed. Atlas of Tumor Radiology. Chicago: Year Book; 1971.[↩]
- Lodwick GS. Radiographic diagnosis and grading of bone tumors with comments on computer evaluation. Presented at the Proceedings of the Fifth National Cancer Conference, Philadelphia. September 17-19, 1964.[↩]
- Park HY, Yang SK, Sheppard WL, Hegde V, Zoller SD, Nelson SD, et al. Current management of aneurysmal bone cysts. Curr Rev Musculoskelet Med. 2016 Dec. 9 (4):435-444.[↩]
- Z.J. Sun, H.L. Sun, R.L. Yang, R.A. Zwahlen, Y.F. Zhao. Aneurysmal bone cysts of the jaws. Int J Surg Pathol, 17 (2009), pp. 311-322, 10.1177/1066896909332115[↩]
- P. Tsagozis, O. Brosjö. Current strategies for the treatment of aneurysmal bone cysts. Orthop Rev, 7 (2015), p. 6182, 10.4081/or.2015.6182[↩]
- S. Seewald, T.L. Ang, H. Imazu, M. Naga, S. Omar, S. Groth, et al. A standardized injection technique and regimen ensures success and safety of N-butyl-2-cyanoacrylate injection for the treatment of gastric fundal varices (with videos). Gastrointest Endosc, 68 (2008), pp. 447-454, 10.1016/j.gie.2008.02.050[↩][↩]
- D. Liu, X. Ma, F. Zhao, J. Zhang. Intraosseous embolotherapy of central arteriovenous malformations in the jaw: long-term experience with 8 cases. J Oral Maxillofac Surg, 67 (2009), pp. 2380-2387, 10.1016/j.joms.2009.04.088[↩][↩]
- J.T.K. Woon, D. Hoon, A. Graydon, M. Flint, A.J. Doyle. Aneurysmal bone cyst treated with percutaneous doxycycline: is a single treatment sufficient? Skelet Radiol, 48 (2019), pp. 765-771, 10.1007/s00256-019-03188-y[↩]
- N. Soehendra, V.C. Nam, H. Grimm, I. Kempeneers. Endoscopic obliteration of large esophagogastric varices with bucrylate. Endoscopy, 18 (1986), pp. 25-26, 10.1055/s-2007-1013014[↩]
- G. Rossi, E. Rimondi, T. Bartalena, A. Gerardi, M. Alberghini, E.L. Staals, et al. Selective arterial embolization of 36 aneurysmal bone cysts of the skeleton with N-2-butyl cyanoacrylate. Skelet Radiol, 39 (2010), pp. 161-167, 10.1007/s00256-009-0757-z[↩][↩]
- A. Alaraj, R. Dashti, N.K. Mehta, S. Goodin, F.T. Charbel, V.A. Aletich. Augmentation of N-butyl cyanoacrylate embolization of cranial, head, and neck tumors by simultaneous infusion of 5% dextrose solution. J Neurointerventional Surg, 7 (2015), pp. 463-470, 10.1136/neurintsurg-2013-011071[↩]
- Chowdhry M, Chandrasekar CR, Mohammed R, Grimer RJ. Curettage of aneurysmal bone cysts of the feet. Foot Ankle Int. 2010 Feb. 31(2):131-5.[↩]
- Steffner RJ, Liao C, Stacy G, et al. Factors associated with recurrence of primary aneurysmal bone cysts: is argon beam coagulation an effective adjuvant treatment?. J Bone Joint Surg Am. 2011 Nov 2. 93(21):e1221-9.[↩][↩]
- Benevenia J, Patterson FR, Beebe KS, Abdelshahed MM, Uglialoro AD. Comparison of phenol and argon beam coagulation as adjuvant therapies in the treatment of stage 2 and 3 benign-aggressive bone tumors. Orthopedics. 2012 Mar 7. 35(3):e371-8.[↩]
- Erol B, Topkar MO, Caliskan E, Erbolukbas R. Surgical treatment of active or aggressive aneurysmal bone cysts in children. J Pediatr Orthop B. 2015 Sep. 24 (5):461-8.[↩]
- Otsuka T, Kobayashi M, Sekiya I, et al. A new treatment of aneurysmal bone cyst by endoscopic curettage without bone grafting. Arthroscopy. 2001 Sep. 17(7):E28.[↩]
- Rossi G, Mavrogenis AF, Facchini G, Bartalena T, Rimondi E, Renzulli M, et al. How effective is embolization with N-2-butyl-cyanoacrylate for aneurysmal bone cysts?. Int Orthop. 2016 Dec 8.[↩]
- Bush CH, Adler Z, Drane WE, Tamurian R, Scarborough MT, Gibbs CP. Percutaneous radionuclide ablation of axial aneurysmal bone cysts. AJR Am J Roentgenol. 2010 Jan. 194(1):W84-90.[↩]
- Chang CY, Kattapuram SV, Huang AJ, Simeone FJ, Torriani M, Bredella MA. Treatment of aneurysmal bone cysts by percutaneous CT-guided injection of calcitonin and steroid. Skeletal Radiol. 2017 Jan. 46 (1):35-40.[↩]
- Adamsbaum C, Kalifa G, Seringe R, Dubousset J. Direct Ethibloc injection in benign bone cysts: preliminary report on four patients. Skeletal Radiol. 1993. 22(5):317-20.[↩]
- Rapp TB, Ward JP, Alaia MJ. Aneurysmal bone cyst. J Am Acad Orthop Surg. 2012 Apr. 20(4):233-41.[↩]
- Woon JTK, Hoon D, Graydon A, Flint M, Doyle AJ. Aneurysmal bone cyst treated with percutaneous doxycycline: is a single treatment sufficient?. Skeletal Radiol. 2019 May. 48 (5):765-771.[↩]
- Shiels WE 2nd, Mayerson JL. Percutaneous doxycycline treatment of aneurysmal bone cysts with low recurrence rate: a preliminary report. Clin Orthop Relat Res. 2013 Aug. 471 (8):2675-83.[↩]
- Brosjö O, Pechon P, Hesla A, Tsagozis P, Bauer H. Sclerotherapy with polidocanol for treatment of aneurysmal bone cysts. Acta Orthop. 2013 Oct. 84(5):502-5.[↩]
- Schreuder HW, Veth RP, Pruszczynski M, et al. Aneurysmal bone cysts treated by curettage, cryotherapy and bone grafting. J Bone Joint Surg Br. 1997 Jan. 79(1):20-5.[↩][↩][↩]
- Bocklage TJM, Quinn RHM, Schmit BPM et-al. Bone and Soft Tissue Tumours: A Multidisciplinary Review With Case Presentations. JP MEDICAL PUBLISHERS. ISBN:1907816224[↩]
- Gibbs CP Jr, Hefele MC, Peabody TD, et al. Aneurysmal bone cyst of the extremities. Factors related to local recurrence after curettage with a high-speed burr. J Bone Joint Surg Am. 1999 Dec. 81(12):1671-8.[↩][↩]
- Marcove RC, Sheth DS, Takemoto S, Healey JH. The treatment of aneurysmal bone cyst. Clin Orthop Relat Res. 1995 Feb. 311:157-63.[↩]