What is a sigmoidoscopy
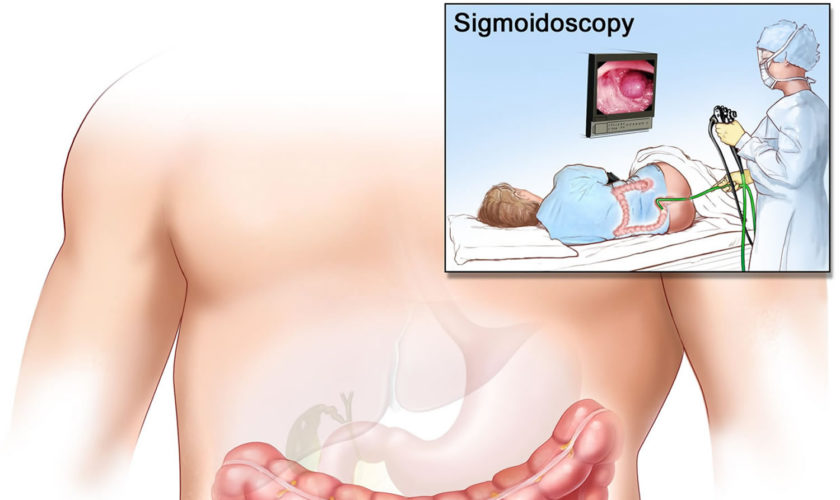
A sigmoidoscopy is a medical procedure that allows examination of the mucosal surface of the end-region of the colon (large intestine) – the rectum and sigmoid (lower) – for lesions including cancer or pre-cancerous polyps (growths). Using sigmoidoscopy to screen average-risk populations for colon cancer and rectal cancer has been associated with a 50-80% reduction in cancer death. A sigmoidoscope is a thin, tube-like instrument with a light and a camera for viewing. A sigmoidoscope may also have a tool to remove polyps or tissue samples, which are checked under a microscope for signs of cancer. The sigmoidoscope is inserted via the anus through the rectum into the sigmoid colon. The doctor is able to see any inflammation, internal bleeding or other things that may be abnormal. A sigmoidoscopy is a short procedure, lasting only about 15-25 minutes. Sigmoidoscopy doesn’t usually require sedation and people can go straight home after the procedure. Special training is required to carry out the procedure but it is performed routinely by a variety of practitioners including physicians, physician’s assistants, nurses and gastroenterologists.
There are two types of sigmoidoscopes:
- The rigid sigmoidoscope – This is about 25cm long and allows examination of up to about 20cm of the rectum and distal sigmoid colon, though it is best suited for rectal assessments only.
- The flexible sigmoidoscope – This is made of a flexible fiber optic tube and can be up to 60cm long. It is able to reach further than the rigid sigmoidoscope. Depending on its length it is also able to view the descending colon. The upper parts of the colon however, are out of its reach and require a colonoscopy.
A special camera on the sigmoidoscope allows the person carrying out the procedure to monitor the examination on a video screen.
Why is a sigmoidoscopy done?
A sigmoidoscopy is able to diagnose a variety of conditions including colorectal cancer or colonic polyps, inflammatory bowel disease (eg. ulcerative colitis, Crohns disease), bowel obstruction and causes of bleeding, abdominal pain or diarrhoea. As well as being able to see the rectum and lower colon, the sigmoidoscope is able to take samples of or even remove lesions or polyps of interest.
A sigmoidoscopy may be used to examine or diagnose certain conditions or structures in your lower colon, including the following:
- Polyps
- Tumors
- Ulcers (sores)
- Inflammation (redness and swelling)
- Hemorrhoids
- Diverticula (pouches on your colon wall)
- Strictures (narrowing of your lower colon)
Sigmoidoscopy can also be used to investigate the following:
- Changes in your bowel habits
- Lower belly pain
- Itching around your anus
- Blood or mucus in your stool
- Low iron levels
- Low blood counts
A tissue biopsy is usually required for the accurate diagnosis of colon or rectal cancers. The sigmnoidoscope is able to take a biopsy or even surgically remove a polyp in the therapeutic treatment of bowel cancer. A polyp may be removed, even if it is benign (non-cancerous) because it may be prone to becoming malignant (cancerous). Patients with a family history of colon or rectal cancer may be done regularly to check for the development of polyps. After surgery to remove any cancerous lesions, sigmoidoscopy may also be carried out regularly in order to monitor the disease.
The sigmoidscope can be useful in the identification of the cause of bowel obstruction and may also be used to rectify the obstruction. Its ability to closely examine the wall of the rectum and lower colon allows the sigmoidoscope to identify any areas of inflammation, or sources of bleeding.
Sigmoidoscopy risks and limitations
The sigmoidoscopy procedure is considered safe and the risk of complication very low, when performed by a trained practitioner. During three sigmoidoscopy studies carried out in the UK, Italy and Norway the procedure showed a complication rate of 3 per 100 000 examination. Although the procedure is able to identify a large number of pathologies, it does not have the reach of a colonoscope, which is able to examine right to the end of the small intestine.
Sigmoidoscopy procedure
Before a sigmoidoscopy your doctor will inform you about everything that is involved and you will be asked to sign a written consent form. The sigmoidoscopy procedure is quite short with duration of 5-10 minutes. It can be carried out in a hospital or in a physician’s office. The sigmoidoscope is inserted through the anus to gain access to the rectum and distal colon. You will not need to be sedated for the procedure but you may feel some discomfort or a bloated feeling. As well as an onboard camera, the sigmoidoscope also has a number of tools that are used to take samples of anything the doctor may be interested in analysing.
Sigmoidoscopy prep
Before your procedure, be sure to tell your health care provider:
- If you are pregnant or think you may be pregnant
- If you are sensitive to or allergic to any medicines, latex, tape or anesthesia medicines (local and general)
- About all the medicines you take, including over-the-counter drugs, prescription medicines, vitamins, herbs and other supplements
- If you have a history of bleeding disorders
- If you are taking any blood-thinning medicines, aspirin, ibuprofen or other medicines that affect blood clotting; these drugs may need to be stopped before the procedure
Like most forms of endoscopy, for a sigmoidoscopy to work properly, it needs to have a clear view of the walls of the rectum and large intestine. It is therefore necessary that the colon is completely empty before the procedure. As preparation, your doctor will put you on a special diet to reduce the amount of solid food you eat in the days leading up to the procedure. You will be asked to take in only water on the day of the sigmoidoscopy and you may also be given laxatives in order to ensure that everything is cleared out of your system. On the morning of the examination you may also be given a saline enema to flush out the rectum.
Follow any other instructions your provider gives you to get ready for your sigmoidoscopy.
After the sigmoidoscopy procedure
Following a sigmoidoscopy some people experience a bloated feeling, this is a normal side effect of the procedure. A sigmoidoscopy is relatively non-invasive and usually doesn’t require any anaesthesia so you should be able to go straight home after the procedure. Your doctor will discuss the results with you once they have been analysed. If there was a biopsy taken during the procedure, some small amounts of blood may appear in the next stool. If you develop a fever or pain in your abdomen you should contact your doctor immediately.
What do the results mean?
Any samples can be analysed in a laboratory to see whether further examination or surgery is needed. A positive sigmoidoscopy result (cancerous polyps are found) will usually prompt examination of the full colon, requiring a colonoscopy. Patients with a family history of colorectal cancer or familial adenomatous polyposis (FAP) should be screened at regular intervals in order to monitor the onset or development of disease. If results suggest that you may have an irritable bowel disease such as Crohn’s disease, your doctor will inform you about the condition and treatments available.
What is a flexible sigmoidoscopy
Flexible sigmoidoscopy is a procedure in which a trained medical professional uses a flexible, narrow tube with a light and tiny camera on one end, called a sigmoidoscope or scope, to look inside your rectum and lower colon, also called the sigmoid colon and descending colon. Flexible sigmoidoscopy can show irritated or swollen tissue, ulcers, polyps, and cancer. However, on the negative side, flexible sigmoidoscopy doesn’t allow the doctor to see the entire colon. As a result, any cancers or polyps farther into the colon can’t be detected with flexible sigmoidoscopy alone.
A tiny video camera at the tip of the flexible sigmoidoscope allows the doctor to view the inside of the rectum and most of the sigmoid colon — about the last two feet (61 centimeters) of the large intestine. If necessary, tissue samples (biopsies) can be taken through the scope during a flexible sigmoidoscopy exam.
Why do doctors use flexible sigmoidoscopy?
A flexible sigmoidoscopy can help a doctor find the cause of unexplained symptoms, such as:
- bleeding from your anus
- changes in your bowel activity such as diarrhea
- pain in your abdomen
- unexplained weight loss
Doctors also use flexible sigmoidoscopy as a screening tool for colon polyps and colon and rectal cancer. Screening may find diseases at an early stage, when a doctor has a better chance of curing the disease. Sigmoidoscopy is one option for colon cancer screening, but there are other options like colonoscopy that allow visualization of the whole colon. Talk with your doctor about your options.
Colorectal cancer is the second-leading cause of cancer death in the United States. In 2016, an estimated 134,000 persons will be diagnosed with the disease, and about 49,000 will die from it 1. Colorectal cancer is most frequently diagnosed among adults aged 65 to 74 years; the median age at death from colorectal cancer is 73 years 2.
Screening for colon and rectal cancer
Your doctor will recommend screening for colon and rectal cancer at age 50 if you don’t have health problems or other factors that make you more likely to develop colon cancer 1.
Factors that make you more likely to develop colorectal cancer include:
- someone in your family has had polyps or cancer of the colon or rectum
- a personal history of inflammatory bowel disease, such as ulcerative colitis or Crohn’s disease
- other factors, such as if you weigh too much or smoke cigarettes
If you are more likely to develop colorectal cancer, your doctor may recommend screening at a younger age, and you may need to be tested more often.
If you are older than age 75, talk with your doctor about whether you should be screened. The current colorectal cancer screening guidelines from the U.S. Preventive Services Task Force 1 are as follow:
- Adults aged 50 to 75 years: The U.S. Preventive Services Task Force 1 recommends screening for colorectal cancer starting at age 50 years and continuing until age 75 years. The risks and benefits of different screening methods vary.
- Adults aged 76 to 85 years: The decision to screen for colorectal cancer in adults aged 76 to 85 years should be an individual one, taking into account the patient’s overall health and prior screening history.
- Adults in this age group who have never been screened for colorectal cancer are more likely to benefit.
- Screening would be most appropriate among adults who 1) are healthy enough to undergo treatment if colorectal cancer is detected and 2) do not have comorbid conditions that would significantly limit their life expectancy.
Evidence from randomized clinical trials demonstrates that annual or every 2 years screening with guaiac-based fecal occult blood test (gFOBT) as well as 1-time and every 3- to 5-year flexible sigmoidoscopy reduces colorectal cancer deaths 3. The Cancer Intervention and Surveillance Modeling Network (CISNET) models found that several screening strategies were estimated to yield comparable life-years gained (i.e., life-years gained with the noncolonoscopy strategies were within 90% of those gained with the colonoscopy strategy) among adults aged 50 to 75 years and an efficient balance of benefits and harms 4. These screening strategies include 1) annual screening with fecal immunochemical test (FIT), 2) screening every 10 years with flexible sigmoidoscopy and annual screening with fecal immunochemical test (FIT), 3) screening every 10 years with colonoscopy, and 4) screening every 5 years with CT colonography. The findings for CT colonography depend on the proxy measure used for the burden of screening (number of lifetime colonoscopies or lifetime cathartic bowel preparations). Two of the 3 Cancer Intervention and Surveillance Modeling Network models found that fecal immunochemical test (FIT)-DNA screening every 3 years (as recommended by the manufacturer) was estimated to yield life-years gained less than 90% of the colonoscopy screening strategy (84% and 87%, respectively). Another way to conceptualize these findings is to note that Cancer Intervention and Surveillance Modeling Network (CISNET) modeling found that fecal immunochemical test (FIT)-DNA screening every 3 years was estimated to provide about the same amount of benefit as screening with flexible sigmoidoscopy alone every 5 years.
Table 1. Characteristics of Colorectal Cancer Screening Strategies
| Screening Method | Frequencyb | Evidence of Efficacy | Other Considerations |
|---|---|---|---|
| Stool-Based Tests | |||
| gFOBT | Every year | RCTs with mortality end points: High-sensitivity versions (eg, Hemoccult SENSA) have superior test performance characteristics than older tests (eg, Hemoccult II) | Does not require bowel preparation, anesthesia, or transportation to and from the screening examination (test is performed at home) |
| FITc | Every year | Test characteristic studies: Improved accuracy compared with gFOBTCan be done with a single specimen | Does not require bowel preparation, anesthesia, or transportation to and from the screening examination (test is performed at home) |
| FIT-DNA | Every 1 or 3 yearsd | Test characteristic studies: Specificity is lower than for FIT, resulting in more false-positive results, more diagnostic colonoscopies, and more associated adverse events per screening testImproved sensitivity compared with FIT per single screening test | There is insufficient evidence about appropriate longitudinal follow-up of abnormal findings after a negative diagnostic colonoscopy; may potentially lead to overly intensive surveillance due to provider and patient concerns over the genetic component of the test |
| Direct Visualization Tests | |||
| Colonoscopyc | Every 10 years | Prospective cohort study with mortality end point | Requires less frequent screening. Screening and diagnostic followup of positive results can be performed during the same examination. |
| CT colonographye | Every 5 years | Test characteristic studies | There is insufficient evidence about the potential harms of associated extracolonic findings, which are common |
| Flexible sigmoidoscopy | Every 5 years | RCTs with mortality end points: Modeling suggests it provides less benefit than when combined with FIT or compared with other strategies | Test availability has declined in the United States |
| Flexible sigmoidoscopy with FITc | Flexible sigmoidoscopy every 10 years plus FIT every year | RCT with mortality end point (subgroup analysis) | Test availability has declined in the United States. Potentially attractive option for patients who want endoscopic screening but want to limit exposure to colonoscopy |
Abbreviations: FIT=fecal immunochemical test; FIT-DNA=multitargeted stool DNA test; gFOBT=guaiac-based fecal occult blood test; RCT=randomized clinical trial.
a Although a serology test to detect methylated SEPT9 DNA was included in the systematic evidence review, this screening method currently has limited evidence evaluating its use (a single published test characteristic study met inclusion criteria, which found it had a sensitivity to detect colorectal cancer of <50%). It is therefore not included in this table.
b Applies to persons with negative findings (including hyperplastic polyps) and is not intended for persons in surveillance programs. Evidence of efficacy is not informative of screening frequency, with the exception of gFOBT and flexible sigmoidoscopy alone.
c Strategy yields comparable life-years gained (ie, the life-years gained with the noncolonoscopy strategies were within 90% of those gained with the colonoscopy strategy) and an efficient balance of benefits and harms in CISNET modeling.
d Suggested by manufacturer.
e Strategy yields comparable life-years gained (ie, the life-years gained with the noncolonoscopy strategies were within 90% of those gained with the colonoscopy strategy) and an efficient balance of benefits and harms in CISNET modeling when lifetime number of colonoscopies is used as the proxy measure for the burden of screening, but not if lifetime number of cathartic bowel preparations is used as the proxy measure.
Several randomized clinical trials have shown that flexible sigmoidoscopy alone reduces deaths from colorectal cancer 3. Flexible sigmoidoscopy combined with fecal immunochemical test (FIT) has been studied in a single trial and was found to reduce the colorectal cancer–specific mortality rate more than flexible sigmoidoscopy alone 5. Modeling studies conducted by Cancer Intervention and Surveillance Modeling Network (CISNET) also consistently estimate that combined testing yields more life-years gained and colorectal cancer deaths averted compared with flexible sigmoidoscopy alone 4. Flexible sigmoidoscopy can result in direct harms, such as colonic perforations and bleeding, although the associated event rates are much lower than those observed with colonoscopy 3. Harms can also occur as a result of follow-up colonoscopy.
Completed trials of flexible sigmoidoscopy provide indirect evidence that colonoscopy—a similar endoscopic screening method—reduces colorectal cancer mortality. A prospective cohort study also found an association between patients who self-reported being screened with colonoscopy and a lower colorectal cancer mortality rate 6. Colonoscopy has both indirect and direct harms. Harms may be caused by bowel preparation prior to the procedure (e.g, dehydration and electrolyte imbalances), the sedation used during the procedure (eg, cardiovascular events), or the procedure itself (e.g, infection, colonic perforations, or bleeding).
Most doctors recommend colonoscopy to screen for colon cancer because colonoscopy shows the entire colon and can remove colon polyps. However, preparing for and performing a flexible sigmoidoscopy may take less time and you may not need anesthesia. Health care providers may combine flexible sigmoidoscopy with other tests.
If your doctor finds abnormal tissue or one or more polyps during a flexible sigmoidoscopy, you should have a colonoscopy to examine the rest of your colon.
Government health insurance plans, such as Medicare, and private health insurance plans sometimes change whether and how often they pay for cancer screening tests. Check with your insurance plan to find out how often your insurance will cover a screening flexible sigmoidoscopy.
Benefits of Colorectal Cancer Screening and Early Intervention
The U.S. Preventive Services Task Force 1 found convincing evidence that screening for colorectal cancer in adults aged 50 to 75 years reduces colorectal cancer mortality. The U.S. Preventive Services Task Force 1 found no head-to-head studies demonstrating that any of the screening strategies it considered are more effective than others, although the tests have varying levels of evidence supporting their effectiveness, as well as different strengths and limitations (see Table 1). About one-third of eligible adults in the United States have never been screened for colorectal cancer 7, and offering choice in colorectal cancer screening strategies may increase screening uptake 8. As such, the screening tests are not presented in any preferred or ranked order; rather, the goal is to maximize the total number of persons who are screened because that will have the largest effect on reducing colorectal cancer deaths.
The benefit of early detection of and intervention for colorectal cancer declines after age 75 years. Among older adults who have been previously screened for colorectal cancer, there is at best a moderate benefit to continuing screening during the ages of 76 to 85 years. However, adults in this age group who have never been screened for colorectal cancer are more likely to benefit than those who have been previously screened.
The time between detection and treatment of colorectal cancer and realization of a subsequent mortality benefit can be substantial. As such, the benefit of early detection of and intervention for colorectal cancer in adults 86 years and older is at most small.
To date, no method of screening for colorectal cancer has been shown to reduce all-cause mortality in any age group 9.
Harms of Colorectal Cancer Screening Screening and Early Intervention
The harms of screening for colorectal cancer in adults aged 50 to 75 years are small. The majority of harms result from the use of colonoscopy, either as the screening test or as follow-up for positive findings detected by other screening tests. The rate of serious adverse events from colorectal cancer screening increases with age 3. Thus, the harms of screening for colorectal cancer in adults 76 years and older are small to moderate.
Flexible sigmoidoscopy risks
A flexible sigmoidoscopy exam poses few risks. Rarely, complications of a flexible sigmoidoscopy exam may include:
- bleeding
- perforation of the colon or rectum wall
- severe pain in your abdomen
- death, although this risk is rare
Bleeding and perforation are the most common complications from flexible sigmoidoscopy. Most cases of bleeding occur in patients who have polyps removed. The doctor can treat bleeding that occurs during the flexible sigmoidoscopy right away. However, you may have delayed bleeding up to 2 weeks after the procedure. The doctor diagnoses and treats delayed bleeding with a colonoscopy or repeat flexible sigmoidoscopy. The doctor may need to treat perforation with surgery.
Figure 1. Large intestine (colon)
Figure 2. Flexible sigmoidoscopy
Flexible sigmoidoscopy prep
To prepare for a flexible sigmoidoscopy, you will need to talk with your doctor, change your diet, and clean out your bowel.
Before a flexible sigmoidoscopy exam, you’ll need to clean out (empty) your colon. Any residue in your colon may obscure the view of your colon and rectum during the exam.
Talk with your doctor
You should talk with your doctor about any medical conditions you have and all prescribed and over-the-counter medicines, vitamins, and supplements you take, including:
- arthritis medicines
- aspirin or medicines that contain aspirin
- blood thinners
- diabetes medicines
- nonsteroidal anti-inflammatory drugs, such as ibuprofen or naproxen
- vitamins that contain iron or iron supplements
Change your diet and clean out your bowel
A health care professional will give you written bowel prep instructions to follow at home before the procedure. A health care professional orders a bowel prep so that little or no stool is present in your intestine. A complete bowel prep lets you pass stool that is clear and liquid. Stool inside your colon can prevent your doctor from clearly seeing the lining of your intestine.
You may need to follow a clear liquid diet the day before the procedure. The instructions will provide specific direction about when to start and stop the clear liquid diet. In most cases, you may drink or eat the following:
- fat-free bouillon or broth
- gelatin in flavors such as lemon, lime, or orange
- plain coffee or tea, without cream or milk
- sports drinks in flavors such as lemon, lime, or orange
- strained fruit juice, such as apple or white grape—doctors recommend avoiding orange juice and red or purple liquids.
- water
Your doctor will tell you how long before the procedure you should have nothing by mouth.
- Typically, you won’t be able to eat the day before the exam. Drinks may be limited to clear liquids — plain water, broth, carbonated beverages, and tea and coffee without milk or cream. You may not be able to eat or drink anything after midnight the night before the exam.
- Take a laxative the night before the exam. The laxative will be in either pill or liquid form.
- Use an enema kit. In some cases, you may need to use an over-the-counter enema kit — either the night before the exam or a few hours before the exam — to empty your colon. Or you may be asked to take two enemas at home the morning of the procedure.
- Adjust your medications. Remind your doctor of your medications at least a week before the exam — especially if you have diabetes, if you take medications or supplements that contain iron, or if you take aspirin or other blood thinners. You may need to adjust your dosages or stop taking the medication temporarily.
A health care professional will ask you to follow the directions for a bowel prep before the procedure. The bowel prep will cause diarrhea, so you should stay close to a bathroom.
Different bowel preps may contain different combinations of laxatives—pills that you swallow or powders that you dissolve in water and other clear liquids—and enemas. Some people will need to drink a large amount, often a gallon, of liquid laxative over a scheduled amount of time—most often the night before the procedure.
You may find this part of the bowel prep difficult; however, completing the prep is very important. Your doctor will not be able to see your sigmoid colon clearly if the prep is incomplete.
Call a health care professional if you have side effects that prevent you from finishing the prep.
Flexible sigmoidoscopy procedure
A trained medical professional performs a flexible sigmoidoscopy during an office visit or at a hospital or an outpatient center. You typically do not need sedatives or anesthesia, and the procedure takes about 15 to 20 minutes. Flexible sigmoidoscopy may require slightly more time if biopsies are taken. Sedation and pain medications usually aren’t necessary. If a polyp is found, your doctor will likely recommend a full colonoscopy to look at your whole colon.
Wearing a gown, you’ll begin the exam lying on your side on the exam table, usually with your knees drawn toward your chest. For the procedure, you’ll be asked to lie on a table while the doctor inserts a sigmoidoscope into your anus and slowly guides it through your rectum and into your sigmoid colon. The scope pumps air into your large intestine to give the doctor a better view. The camera sends a video image of your intestinal lining to a monitor, allowing the doctor to examine the tissues lining your sigmoid colon and rectum. The doctor may ask you to move several times on the table to adjust the scope for better viewing. Once the scope has reached your transverse colon, the doctor slowly withdraws it and examines the lining of your sigmoid colon again.
During the procedure, your doctor may remove polyps and send them to a lab for testing. Colon polyps are common in adults and are harmless in most cases. However, most colon cancer begins as a polyp, so removing polyps early is an effective way to prevent cancer.
If your doctor finds abnormal tissue, he or she may perform a biopsy. You won’t feel the biopsy.
If your doctor found polyps or other abnormal tissue during a flexible sigmoidoscopy, your doctor may suggest you return for a colonoscopy.
After a flexible sigmoidoscopy
After a flexible sigmoidoscopy, you can expect the following:
- You may have cramping in your abdomen or bloating during the first hour after the procedure. You may feel bloated or pass gas for a few hours as you clear the air from your colon. Walking may help relieve any discomfort.
- You can resume regular activities right away after the procedure.
- You can return to a normal diet.
- You may also notice a small amount of blood with your first bowel movement after the exam, which usually isn’t cause for alarm. Consult your doctor if you continue to pass blood or blood clots or if you have persistent abdominal pain or a fever of 100 °F (37.8 °C) or higher.
A health care professional will give you written instructions on how to take care of yourself after the procedure and will review them with you. You should follow all instructions.
If the doctor removed polyps or performed a biopsy, you may have light bleeding from your anus. This bleeding is normal. Some results from a flexible sigmoidoscopy are available right after the procedure, and your doctor will share these results with you. A pathologist will examine the biopsy tissue. Biopsy results take a few days or longer to come back.
Seek care right away
If you have any of the following symptoms after a flexible sigmoidoscopy, seek care right away:
- severe pain in your abdomen
- fever
- continued bloody bowel movements or continued bleeding from your anus
- dizziness
- weakness
Flexible sigmoidoscopy results
Your doctor will review the results of the flexible sigmoidoscopy exam and then share the results with you.
- Negative result. A flexible sigmoidoscopy exam is considered negative if the doctor doesn’t find any abnormalities in the colon. If you’re at average risk of colon cancer — you have no colon cancer risk factors other than age — your doctor may recommend waiting five years and then repeating the exam.
- Positive result. A flexible sigmoidoscopy exam is considered positive if the doctor finds polyps or abnormal tissue in the colon. Depending on the findings, you may need additional testing — such as a colonoscopy — so that any abnormalities can be examined more thoroughly, biopsied or removed. During colonoscopy, your doctor can also screen the entire colon for other abnormalities.
How much of your colon and rectum can be viewed during a flexible sigmoidoscopy depends on the experience of the doctor doing your exam and the success of the colon preparation. If your doctor is concerned about the quality of the view through the scope, he or she may recommend a repeat flexible sigmoidoscopy exam or another screening test.
References- Final Recommendation Statement. Colorectal Cancer: Screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/colorectal-cancer-screening2
- Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975–2015. National Cancer Institute. https://seer.cancer.gov/csr/1975_2015/
- Lin JS, Piper M, Perdue LA, et al. Screening for Colorectal Cancer: A Systematic Review for the U.S. Preventive Services Task Force. Evidence Synthesis No. 135. AHRQ Publication No. 14-05203-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2016.
- Zauber A, Knudsen A, Rutter CM, Lansdorp-Vogelaar I, Kuntz KM. Evaluating the Benefits and Harms of Colorectal Cancer Screening Strategies: A Collaborative Modeling Approach. AHRQ Publication No. 14-05203-EF-2. Rockville, MD: Agency for Healthcare Research and Quality; 2015.
- Holme Ø, Løberg M, Kalager M, et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial. JAMA. 2014;312(6):606-15.
- Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369(12):1095-105.
- Shapiro JA, Klabunde CN, Thompson TD, Nadel MR, Seeff LC, White A. Patterns of colorectal cancer test use, including CT colonography, in the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2012;21(6):895-904.
- Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012;172(7):575-82.
- Lin JS, Piper M, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the U.S. Preventive Services Task Force. JAMA. doi:10.1001/jama.2016.3332