What are dental implants
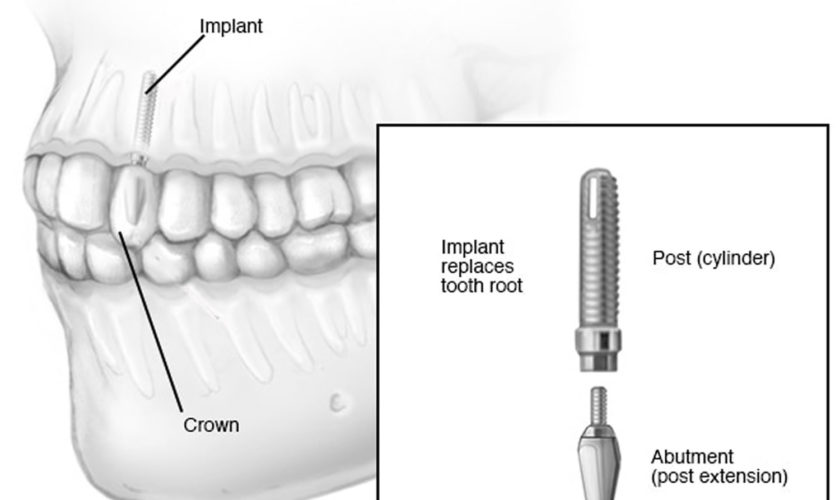
A dental implant is a dental procedure to replace a missing tooth, to support one or more false teeth (dentures). Unlike dentures, which are false teeth that you can easily remove, dental implants are placed in your jawbone in a series of minor operations. A dental implant is a piece of metal titanium that looks like a screw and is put into the jawbone that can replace the root of a tooth when it fails. Over time, bone grows around the dental implant, which helps to hold it in place. Once the dental implant integrates to your bone, a structure called an abutment is connected to the implant and then the artificial tooth/teeth are attached to the metal dental implant to fill in the gap left by the missing tooth. The crown is specially made to look like your other teeth in size, shape and color. Most dental implants look exactly like natural teeth. Dental implants can also be used to hold a dental bridge or dentures in place.
Titanium is “biocompatible”, meaning it is not rejected by the body and the metal will fuse with the surrounding living bone. Dental implants can last a lifetime and can improve your appearance, your confidence, and your ability to eat the foods you like, and participate in an active lifestyle, without worrying about your teeth. Dental implants are made of titanium and can never get dental decay.
If you have a single tooth missing, you will need an implant to support it. If you have a number of teeth missing, and these are next to each other, you could still have one implant for each tooth. Or you may find that, if you have two or more implants, they may be able to support more than one tooth each. Your dentist will talk to you about the best option for you.
There are 3 parts to an “implant tooth”: the dental implant itself, the abutment, and the artificial tooth. State-of-the-art technology makes it possible for these implant-supported replacement teeth to look, feel, and function like natural teeth. Dental implants are available privately but can be expensive – costing in the thousands of dollars, rather than hundreds of dollars. Make sure you know all the costs before you start. If you have private health insurance, ask the insurance company how much of your dental implant costs they’ll cover.
A dental implant is usually done in stages over a few months, with a series of minor operations and tests.
- You’ll need scans and x-rays to give the dentist a very clear picture of where to place the implant.
- You’ll have a minor operation to have the dental implant put in your jawbone. You will usually be given an anesthetic so that you don’t feel pain during the surgery.
- You wait for a few months while the bone grows around the dental implant.
- Once the implant is stable, your dentist will create a foundation for your new tooth.
- Some time later, your dentist will take a mould of your mouth to make the artificial tooth or teeth.
- Finally, the artificial tooth or teeth are screwed or cemented into the foundation.
It’s a slow process, with quite a few visits to the dentist and possibly some trips to have scans or x-rays done.
There are many types of dental implants and many ways to use dental implants to achieve your goals of a pleasing smile and good chewing function. Visit with your dentist or prosthodontist to find out how dental implants can improve your life and if you are a good candidate for dental implants. Be aware that not all general dentists and dental specialists perform dental implant therapy in their practice. Dental implants like all dental procedures require dental education and additional training. A prosthodontist is a specialist with an additional three years of training after dental school who focuses on the restoration and replacement of teeth, including dentures for even the most complex cases. A prosthodontist also will be able to help determine if another treatment option might be more suited to your particular situation.
In general, dental implants may be right for you if you:
- Have one or more missing teeth
- Have a jawbone that’s reached full growth
- Have adequate bone to secure the implants or are able to have a bone graft
- Have healthy oral tissues
- Don’t have health conditions that will affect bone healing
- Are unable or unwilling to wear dentures
- Ill-fitting and loose dentures
- Want to improve your speech
- Are willing to commit several months to the process
Most patients find that a dental implant is secure, stable and a good replacement for their own tooth.
In children dental implant is usually deferred until their jaw growth is complete. There are, however, some instances when a dental implant may be appropriate, such as when it is part of the child’s orthodontic treatment plan. Your family dentist or orthodontist can guide you in this instance.
Dental implants are usually successful. Complications are rare but include:
- infection
- injury or damage to surrounding teeth or blood vessels
- nerve damage
- sinus problems.
Pros And Cons of Dental Implants
Every procedure has both pros and cons, and dental implants are no exception, although they’re completely safe. Here are the biggest upsides and downsides of getting an implant.
Pros
Unlike traditional dentures, dental implants are placed into and integrate with your jawbone. This gives them enough support for the artificial tooth to withstand the same amount of pressure as natural teeth and not slide or shift.
After your mouth has healed post-surgery, dental implants are usually more comfortable than standard dentures or bridges. Implants don’t require any surgery or removal of any teeth except the affected or damaged teeth, as is the case with your typical denture or teeth bridge.
On top of all this, having a dental implant can help preserve the bone in your jaw. When teeth are missing for a long time, the jawbone begins to deteriorate. This can deform your entire jaw and, in turn, your facial structure.
Cons
The biggest negative to getting an implant is the cost. Average cost of dental implant is about $1,000-3,000. And in many cases, dental insurance will not cover the procedure as they may categorize it as a “cosmetic” elective procedure.
Unfortunately, dental implants are not perfect. They can fail for a number of reasons, including smoking, an autoimmune disease, or infection. These can also cause the implant to become loose or painful, in either case it may need to be removed.
Dental implants results
Most dental implants are successful. Sometimes, however, the bone fails to fuse sufficiently to the metal implant. Smoking, for example, can contribute to implant failure and complications. Dental implants fail about 5% of the time. To prevent a dental implant failure, keep your mouth clean, visit your dentist regularly, and eat healthily.
According to research by Buser and colleagues 1, patients exposed to with irradiation (radiotherapy) before or after implantation, or patients with severe diabetes or heavy smoking habits have significantly increased risks of dental implant failure. It has been suggested that such conditions could impair implant survivability by increasing the susceptibility of the patient to other diseases or by interfering with the tissue healing process 2. Moreover, osteoporosis, with its high prevalence in the aged population, is also considered a relative contraindication for dental implant therapy 3; the alveolar ridge atrophy and low bone mineral density, caused by osteoporosis may impair bone quality and quantity at implant sites 4.
If the bone fails to fuse sufficiently, the implant is removed, the bone is cleaned up, and you can try the procedure again in two or three months.
You can help your dental work — and remaining natural teeth — last longer if you:
- Practice excellent oral hygiene. Just as with your natural teeth, keep implants, artificial teeth and gum tissue clean. Specially designed brushes, such as an interdental brush that slides between teeth, can help clean the nooks and crannies around teeth, gums and metal posts.
- See your dentist regularly. Schedule regular dental checkups to ensure the health and proper functioning of your implants.
- Avoid damaging habits. Don’t chew hard items, such as ice and hard candy, which can break your crowns — or your natural teeth. Avoid tooth-staining tobacco and caffeine products. Get treatment if you grind your teeth.
Figure 1. Dental implants
How long will a dental implant last?
With proper care, you can expect your dental implant to last as long as 25 years.
Can you drink alcohol after dental implant surgery?
This is a question that dental implant patients often ask. It’s obvious that smoking is not good for your implant (or your oral hygiene in general), but drinking alcohol is not as obvious.
For the same reason you should avoid mouthwash with alcohol in it – as well as any type of alcoholic beverage. The alcohol can restrict blood flow, which is not good for healing, and it can even increase your chance of infection.
You may want to check with your dentist on the specific instructions, but typically, dentists allow their implant patients to drink alcohol no sooner than 72 hours after the final stage.
When might a dental implant be needed?
If you have one or more teeth that are missing, having a dental implant might be an option for replacing the missing teeth. In general, a dental implant might be suitable if you:
- have a fully grown, healthy jawbone
- don’t have gum disease
- don’t have conditions that affect bone healing.
Your dentist will discuss with you whether a dental implant procedure is suitable for you.
What happens if the dental implant does not fuse with the bone?
This happens very rarely. If the dental implant becomes loose during the healing period, or just after, it is easily removed and your jaw will heal in the normal way. Once your jaw has healed, another dental implant can be placed there. Or the dentist can make a bridge, fitting it to the implanted false teeth that have been successful.
Are dental implants safe?
Dental implants are a safe, well-established treatment. It’s probably true to say that dental implants, much like natural teeth, will last for as long as you care for them.
How well you look after your dental implants and whether you go for your regular maintenance appointments – will have the biggest impact on how long they will last.
If you don’t look after your dental implants they will develop a coating similar to what you get on neglected natural teeth. Left untreated, this can lead to gum infection, bleeding, soreness and general discomfort. You could get all these problems with natural teeth.
If your dental implants are well looked after and if the bone they are fitted to is strong and healthy, you can expect them to last for many years. However, just as with other surgical implants (such as a hip replacement) there is no lifetime guarantee.
What are alternatives to dental implants?
The alternatives to dental implants are wearing dentures or a dental bridge.
Fixed bridges and removable dentures, however, are not the perfect solution and often bring with them a number of other problems. Removable dentures may slip or cause embarrassing clicking sounds while eating or speaking. A removable partial denture may also contribute to the loss of adjacent teeth. A dental bridge may require the cutting down of healthy, adjacent teeth that may or may not need to be restored in the future. Then there is the additional cost of possibly having to replace the bridge once, twice or more over the course of a lifetime. Of even greater concern, fixed bridges often affect adjacent healthy teeth, and denture wearers may suffer bone loss in the area where the tooth or teeth are missing. Recurrent decay, gum disease and wear and tear often doom fixed bridgework to early failure. For these reasons, fixed bridges and removable dentures usually need to be replaced every seven to 15 years. Studies show that within five to seven years there is a failure rate of up to 30% in teeth located next to a fixed bridge or removable partial denture.
Rather than resting on the gum line like removable dentures, or using adjacent teeth as anchors like fixed bridges, dental implants are long-term replacements that your oral surgeon surgically places in the jawbone. Composed of titanium metal that “fuses” with the jawbone through a process called osseointegration,
dental implants never slip, make embarrassing noises or decay like teeth anchoring fixed bridges. Because dental implants fuse with the jawbone, bone loss is generally less of a problem.
Are the implant teeth difficult to clean?
No. But aftercare is important if you are going to have a long-lasting, successful implant. Your dental team should give you detailed advice on how to look after your dental implants. Cleaning around the teeth attached to the dental implants is no more difficult than cleaning natural teeth. However, there may be areas that are difficult to reach and you’ll be shown how to clean them. You may need to visit your hygienist more often but your dental team will be able to talk to you about this.
Can I take the teeth out if they are fixed to implants?
Most teeth attached to dental implants can only be fitted and removed by the dentist. However, if you have removable dentures attached to the implants, you’ll be able to take them out for cleaning.
Do dental implants hurt?
Placing a dental implant is often easier than taking a tooth out and is usually done using a simple local anaesthetic. You will not feel any pain at the time but, just like after an extraction, you may feel some discomfort during the week after the dental implant surgery.
Sometimes your dentist might give you a sedative if you are very nervous or if the case is a complicated one. General anaesthetics are rarely used for dental implant surgery and are generally only used for very complicated cases.
Can dental implants always be used to replace missing teeth?
It depends on the condition of the bone in your jaw. Your dentist will arrange for a number of special tests to find out the amount of bone still there. If there is not enough, or if it isn’t healthy enough, it may not be possible to place dental implants without grafting bone into the area first.
I have some of my own teeth. Can I still have dental implants?
Yes. You can have any number of teeth replaced with dental implants – from one single tooth to a complete set.
If I had gum disease when I had my own teeth, will I get it with the dental implants?
Yes, if you don’t care for them well enough. If you keep your dental implants clean and have them regularly checked by your dental team you should not have any problems. Smoking also affects the health of implants. So, if you smoke, you may need to look after your dental implants more carefully. Some dentists will not place dental implants in people who are smokers.
How long does the dental implant treatment take?
Your dental team will be able to give you a rough timetable before the dental implant treatment starts.
Some false teeth can now even be fitted at the same time as the dental implants (these are called ‘immediate implants’). Check with your dental team to see whether these are suitable for you. Usually the false teeth are fitted 3 to 4 months after the dental implants are put in. Sometimes treatment takes longer and your dental team will be able to talk to you about your treatment time.
Do dental implants last?
Unlike natural teeth, dental implants are not susceptible to dental disease such as decay; however, the health of the gums is vital to maintaining lasting implant success. Conscientious home care by the patient and regular professional cleanings and check-ups are essential elements for dental implant sustainability. Each patient is different, and success relies upon diagnosis and planning, medical history, and a variety of other factors.
What if I want “metal-free” implants?
There is no such thing as “metal-free” teeth. Almost all materials used for teeth replacements have some sort of metal ions in them. Dental implants are made of titanium alloy (similar to hip implants, shoulder implants, and knee implants), which is highly biocompatible, and an allergy to titanium is extremely rare! Currently, there are no proven alternatives to titanium dental implants.
How do dental implants help if I have full dentures or partial dentures?
Actually implants are most popularly used in patients who wear full dentures or partial dentures. Dental implants can be used to provide retention and support for a removable implant overdenture, which basically snaps on the implants! Therefore the partial or full denture does not move and reduces the dependency on denture adhesives/denture glue. Or, dental implants can be used for a fixed denture, where a patient’s dentures are fixed/bolted to the implants through titanium components. Only the dentist can remove this from the mouth.
Can dentures be made into implants?
Dentures cannot be “made into implants”. Implants are metal screws placed into the jawbone to help anchor and support artificial teeth (dentures). It may be possible to have implants placed beneath existing dentures to aid in the stabilization and support for those dentures. This could only be done if the current dentures were otherwise in excellent condition. You should consult with your prosthodontist to have your existing dentures carefully examined.
Are dental implants removable like dentures or do they stay in your mouth?
No, dental implants are fixed solidly in the bone and allow teeth to be replaced in a manner that is closest to natural teeth.
What do I do if the screws in the denture are loosening?
If you had implants inserted, it sounds like the implants were intended to help stabilize the denture, in which case, they should reduce the movement. If your dentures rub your cheeks or if you’re biting your cheeks, you should return to the dentist or prosthodontist who made the dentures to continue to have the fit and bite refined. You should visit your dentist or prosthodontist to remedy these difficulties.
What if I have an accident?
Dental implants and the teeth they support can be damaged by an accident in the same way that natural teeth can. So it is important that you wear a professionally made mouthguard if you play sports that involve contact or moving objects.
If just the teeth are damaged, they can usually be removed from the implant and replaced.
However, if the titanium implant itself is damaged beyond repair, it can be safely left in the jaw if it is too difficult to remove. Another implant may be fitted alongside it to replace the damaged one.
What are the disadvantages of dental implants?
Like any minor oral surgical procedure, there is a risk of infection, inflammation, and pain, but your prosthodontist will discuss how these can be managed for your specific situation. If there is no available bone to place the implant, then bone and gum grafting procedures may be required, which can increase the cost of your treatment. However, the upfront investment can pay off in the long term.
How much do implants cost and does insurance cover the cost?
In general, the cost of replacing a single tooth with a dental implant is almost the same as replacing it with a regular fixed bridge. Dental implant treatment may qualify for some insurance coverage, but situations vary. The cost will vary by patient needs, bone quantity, and region. A prosthodontist will make an assessment based on your unique needs.
What is the success rate of dental implants?
It varies from individual to individual and with health and habits. For a healthy individual with good oral hygiene and good health, dental implants are predictably successful with reported success rates above 90-95 percent.
Types of dental implants
There are two main types of dental implants: endosteal and subperiosteal (whether it’s a mini dental implant or standard implant).
- Endosteal dental implants are inserted into the bone, fusing with it.
- Subperiosteal dental implants simply sit on top of the jawbone — these are rarely used anymore because of their poor long-term performance.
The type of dental implant that your dentist chooses will be specific to your situation (but it will either be a endosteal and subperiosteal implant). In either case, it will require a recovery period that could last several months.
Dental implants can also be classified by the following type :
- ‘Immediate’ implants are placed in dental sockets just after tooth extraction.
- ‘Immediate-delayed’ implants are those implants inserted after weeks up to about a couple of months to allow for soft tissue healing.
- ‘Delayed’ implants are those placed thereafter in partially or completely healed bone.
The potential advantages of immediate implants are that treatment time can be shortened and that bone volumes might be partially maintained thus possibly providing good aesthetic results. The potential disadvantages are an increased risk of infection and failures. After implant placement in postextractive sites, gaps can be present between the implant and the bony walls. A well conducted systematic review found insufficient evidence to determine possible advantages or disadvantages of immediate, immediate-delayed or delayed implants 5. There is currently too little evidence to draw any reliable conclusions, however, the aesthetic outcome could be slightly better when placing implants early after tooth extraction, though early placed implants might be at a higher risk of failure. There is not enough evidence supporting or refusing the need of bone augmentation when extracted teeth are immediately replaced with dental implants, nor it is known whether any augmentation procedure is better than the others. Bone substitutes (anorganic bovine bone) can be used instead of self generated (autogenous) bone graft 5.
Single-Tooth Implants
A single-tooth implant replaces the roots of one missing tooth. It doesn’t involve treating the surrounding teeth as some procedures do. This is a good option not just for aesthetics, but also because missing one tooth could change the sound of your speech, make it more difficult to chew, the natural teeth around it can shift over time, and you can even experience bone loss.
The single tooth implant replaces the missing tooth’s roots. A single tooth implant is a stand-alone unit and does not involve treating the teeth next to it.
Implant-Supported Bridges and Dentures
Dental implants can also help support a denture bridge when you have multiple teeth missing (even all of your teeth missing). Rather than getting support from nearby teeth, an implant-supported bridge uses implants as its support system.
A common type of denture implant is the All-On-4 (AO4) denture. With this prosthesis, the dentist inserts four or more implants to act as the anchors for the denture.
Dental implant procedure
Dental implants are metal screws made of titanium directly implanted (surgically placed) into the gum and jawbone where teeth are missing. Dental implants replace the roots of missing teeth and support single crowns, large bridges or dentures and replace missing teeth.
Dental implant process
There are generally three phases to getting a dental implant:
- First, the dentist surgically places the implant into the jawbone. Your dentist may recommend a diet of soft foods, cold foods and warm soup during the healing process.
- Next, the bone around the implant heals in a process called osseointegration. What makes an implant so strong is that the bone actually grows around it and holds it in place. Osseointegration means “combines with the bone” and takes time. Some patients might need to wait until the implant is completely integrated, up to several months, before replacement teeth can be attached to the implant. Other patients can have the implants and replacement teeth placed all in one visit.
- Finally, it’s time for the placement of the artificial tooth/teeth. For a single tooth implant, your dentist will customize a new tooth for you, called a dental crown. The crown will be based on size, shape, color and fit, and will be designed to blend in with your other teeth. If you are replacing more than a single tooth, custom-made bridges or dentures will be made to fit your mouth and your implants. (Note: The replacement teeth usually take some time to make. In the meantime, your dentist may give you a temporary crown, bridge or denture to help you eat and speak normally until the permanent replacement is ready.)
If you are interested in dental implants, it’s a good idea to discuss it carefully with your dentist first. If you are in good general health this treatment may be an option for you. In fact, your health is more of a factor than your age. You may be medically evaluated by a physician before any implant surgery is scheduled.
Chronic illnesses, such as diabetes or leukemia, may interfere with healing after surgery. Patients with these issues may not be good candidates for implants. Using tobacco can also slow healing.
How you prepare for dental implants
Because dental implants require one or more surgical procedures, you must have a thorough evaluation to prepare for the process, including a:
- Comprehensive dental exam. You may have dental X-rays taken and models made of your teeth and mouth.
- Treatment plan. Tailored to your situation, this plan takes into account factors such as how many teeth you need to have replaced and the condition of your jawbone. The planning process may involve a variety of dental specialists, including a doctor who specializes in conditions of the mouth, jaw and face (oral and maxillofacial surgeon), a dentist who works with the structures that support teeth (periodontist) and a dentist who will restore the implants with crowns, bridges or dentures.
Tell your doctor about any medical conditions and any medications you take, including prescription and over-the-counter drugs and supplements. If you have certain heart conditions or orthopedic implants, your doctor may prescribe antibiotics before surgery to help prevent infection.
To control pain, anesthesia options during surgery include local anesthesia, sedation or general anesthesia. Talk to your dental specialist about which option is best for you. Your dental care team will instruct you about eating and drinking before surgery, depending on what type of anesthesia you have. If you’re having general anesthesia, plan to have someone take you home after surgery and expect to rest for the remainder of the day.
Dental implant surgery
What you can expect during a dental implant
Dental implant surgery is usually an outpatient surgery performed in stages:
- Your damaged tooth is removed.
- Your jawbone is prepared for surgery, a process that may involve bone grafting.
- After your jawbone heals, your oral surgeon places the dental implant metal post in your jawbone.
- You go through a healing period that may last several months.
- Your oral surgeon places the abutment, which is an extension of the implant metal post. (In some cases, when the implant is very stable, this can be done at the same time that the implant is placed.)
- After the soft tissue heals, your dentist will make molds of your teeth and jawbone and later place the final tooth or teeth.
The entire process can take many months from start to finish. Much of that time is devoted to healing and waiting for the growth of new bone in your jaw.
When bone grafting is required
If your jawbone isn’t thick enough or is too soft, you may need bone grafting before you can have dental implant surgery. That’s because the powerful chewing action of your mouth exerts great pressure on your bone, and if it can’t support the implant, the surgery likely would fail. A bone graft can create a more solid base for the implant.
In bone grafting, a piece of bone is removed from another part of your jaw or your body — your hip, for example — and transplanted to your jawbone. Another option is to use artificial bone (bone commercially available) to place in these areas. It may take several months for the transplanted bone to grow enough new bone to support a dental implant.
In some cases, you may need only minor bone grafting, which can be done at the same time as the implant surgery. The condition of your jawbone determines how you proceed.
Placing the dental implant
During surgery to place the dental implant, your oral surgeon makes a cut to open your gum and expose the bone. Holes are drilled into the bone where the dental implant metal post will be placed. Since the post will serve as the tooth root, it’s implanted deep into the bone.
At this point, you’ll still have a gap where your tooth is missing. A type of partial, temporary denture can be placed for appearance, if needed. You can remove this denture for cleaning and while you sleep.
Waiting for bone growth
Once the metal implant post is placed in your jawbone, osseointegration begins. During this process, the jawbone grows into and unites with the surface of the dental implant. This process, which can take several months, helps provide a solid base for your new artificial tooth — just as roots do for your natural teeth. Once your mouth is healed, you’ll be ready for the next stage.
Placing the healing collar and/or temporary crown
After the implants have fused with your jawbone, you’re ready for the next stage, which is getting a healing collar and possibly a temporary crown.
The dentist will place the healing collar (also called a healing cap) on the head of the implant — this helps guide the gum tissue in the proper way to heal. It’s a round piece of metal that keeps the gums away from the implant. This collar will stay on for 10-14 days.
After this time, in which your tissue should have healed, the dentist will remove it and move onto the next step.
Placing the abutment
When osseointegration is complete, you may need additional surgery to place the abutment — which is the part that screws into the implant and will support the crown. This minor surgery is typically done with local anesthesia in an outpatient setting. Once the abutment is placed, your dentist will take another impression of the abutment for each replacement tooth.
To place the abutment:
- Your oral surgeon reopens your gum to expose the dental implant
- The abutment is attached to the dental implant
- The gum tissue is then closed around, but not over, the abutment
In some cases, the abutment is attached to the dental implant metal post when the post is implanted. That means you won’t need an extra surgical step. Because the abutment juts past the gumline, however, it’s visible when you open your mouth — and it will be that way until your dentist completes the tooth prosthesis. Some people don’t like that appearance and prefer to have the abutment placed in a separate procedure.
Choosing your new artificial teeth
After the abutment is placed, your gums must heal for one or two weeks before the artificial tooth (the crown) can be attached. Once your gums heal, you’ll have more impressions made of your mouth and remaining teeth. These impressions are used to make the crown — your realistic-looking artificial tooth. The crown can’t be placed until your jawbone is strong enough to support use of the new tooth.
You and your dental specialist can choose artificial teeth that are either removable, fixed or a combination of both.
- Removable crown. This type is similar to a conventional removable denture. It contains artificial white teeth surrounded by pink plastic gum. It’s mounted on a metal frame that’s attached to the implant abutment, and it snaps securely into place. It can be easily removed for repair or daily cleaning.
- Fixed crown. In this type, an artificial tooth is permanently screwed or cemented onto an individual implant abutment. You can’t remove the tooth for cleaning or during sleep. If affordability isn’t a concern, you can opt to replace several missing teeth this way. Most of the time, each crown is attached to its own dental implant. However, because implants are exceptionally strong, several teeth can be replaced by one implant if they’re bridged together.
Dental implant recovery
Whether you have dental implant surgery in one stage or multiple stages, you may experience some of the typical discomforts associated with any type of dental surgery, such as:
- Swelling of your gums and face
- Bruising of your skin and gums
- Pain at the implant site
- Minor bleeding
You may also have some bleeding from the gums.
To help make your recovery speedier and the pain minimal, here are some dental implant recovery tips you should follow:
- Be careful not to disturb the surgical areas
- Brush your teeth the night of surgery with a gentle toothbrush
- After 24 hours, gently rinse with salt water 2-3 times a day
- Rest as much as possible
- Apply ice if needed
You might need to eat soft foods after each surgery to let the dental implant site heal.
No special care is required for a dental implant. You will probably be advised to keep your natural and artificial teeth clean by brushing and flossing, and have regular dental check-ups.
You may notice a greenish-yellowish discoloration or bruising around the surgical areas, but this is normal and should only last for 2-3 days after the surgery. After that, you can apply moist heat to the area to decrease the discoloration.
If you happen to feel a sharp protrusion or a edge of your tooth, this is also common. If it concerns you, feel free to contact your dentist.
You may also experience dry lips, a sore throat, and stiff jaw muscles, all expected signs of having gauze in your mouth. All of these things should go away after about 2-3 days.
Here are some general instructions for how to care for you implants in the days following the procedure to help your recovery time:
- Rinse your mouth with salt water, taking five minutes to use up an entire 8-ounce glass. You can do this as often as you’d like, at least twice a day.
- Gently brush your teeth as you normally would as soon as you can after the procedure. Because you may have soreness and swelling, you may not be able to (or want to) brush with regular vigor. But it’s important to have great oral hygiene during this recovery time.
Another thing you should consider during recovery is altering your diet.
If swelling, discomfort or any other problem gets worse in the days after surgery, contact your oral surgeon. You may need pain medications or antibiotics.
After each stage of surgery, you may need to eat soft foods while the surgical site heals. Typically, your surgeon will use stitches that dissolve on their own. If your stitches aren’t self-dissolving, your doctor removes them.
Dental implant recovery diet
It’s a smart idea to consider temporarily changing your diet to help your mouth heal quickly and properly. It doesn’t sound fun, but it can greatly help.
The best thing you can do regarding your diet is to avoid direct contact with the implant for as long as possible. This means no hard foods until the area is fully healed.
Foods you should (and shouldn’t) eat after dental implant surgery
Generally speaking, you should eat mostly soft foods, alternating which side of your mouth you’re chewing on.
Here are some ideas of foods that are good to eat following surgery:
- Avocado
- Baked beans
- Bananas
- Eggs
- Fish (don’t use citrus)
- Healthy juice or smoothies
- Oatmeal
- Noodles
- Rice
On the flip side, there are plenty of foods to AVOID right after surgery:
- Hard foods, like chips, nuts, or apples
- Acidic foods, like tomatoes, lemons, limes, orange juice
- Popcorn
- Spicy foods as they may cause discomfort
Dental implant recovery time
Typically, it will take 5-7 days for all the symptoms to subside. However, some patients may experience a longer recovery time due to some different factors.
Here are some of the reasons why your recovery time may vary.
How many implants you get
Depending on whether you get a single implant, multiple implants, or an All-On-4 implants, your recovery time can vary. Basically, the more implants mean a longer and more involved surgery, which means a bit longer of a recovery time.
Sinus lift
Recovery from a sinus lift dental implant can vary as well. A sinus lift (or a sinus augmentation) can also lengthen your recovery time. This is a surgery that adds bone to your upper jaw by your molars and premolars. The dentist will have to move the sinus membrane upward (or be lifted) in order for the additional bone to fit.
Bone grafting
Bone grafting is required when your jawbone is not thick enough to secure an implant. When you chew, it puts a lot of pressure on the bone and it needs to be as strong as possible.
If you need a bone graft, your dentist will take bone from somewhere else in your body (like your hip) and transplant it into your jawbone. They can also use artificial bone.
In either case, it can take several months for the healing process to take place. But if minimal bone grafting is needed, it may not take that long.
Osseointegration
Osseointegration is the process of a dental implant integrating with your jawbone. This takes time to happen successfully — and it may be different for each patient — so it may add to the recovery time.
Dental implants aftercare
Your dental team will give you instructions on how to look after your dental implant. They may give you some painkillers after the surgery – or make sure you have some at home – to take over the next few days if you need them.
After your dental implants have been placed, the bone in your jaw needs to grow onto them and fuse to them. This usually takes a few months. Sometimes the dental implants may be stable enough when they are placed for the false teeth to be fitted sooner than this.
If you are having one, two or three teeth replaced, you may have a temporary denture in the meantime. If you already have full dentures, you can keep wearing these while your dental implants are healing. Your dentures will need altering, to fit properly after the surgery, and a ‘healing cap’ will usually be placed onto the implant site to protect it.
Implant Care
Basically, you should care for your implant the same way you would care for your natural teeth. You should be brushing 2-3 times a day, flossing daily, and rinsing with non-alcoholic mouthwash. Also, it’s important to have a regular checkup with your dentist every six months.
X-Rays
Your dentist may take more X-rays during your follow-up visits. They will be looking at how the implant, abutment, and crown are fitting together and if there are any issues with their alignment.
Dental implant complications
Like any surgery, dental implant surgery poses some health risks. Problems are rare, though, and when they do occur they’re usually minor and easily treated. Risks include:
- Infection at the implant site
- Injury or damage to surrounding structures, such as other teeth or blood vessels
- Nerve damage, which can cause pain, numbness or tingling in your natural teeth, gums, lips or chin
- Sinus problems, when dental implants placed in the upper jaw protrude into one of your sinus cavities.
As with any surgery, there are possible risks. With dental implants, there’s the chance the implant could fail, whether it’s due to infection, the bite, clenching, or grinding of the teeth.
If you’re getting a replacement tooth on the lower jaw, there is a chance a nerve that runs under the lower jaw can be injured during the drilling or placement of the implant. It may cause numbness or tingling, however this may just be temporary until the nerve heals. But there is the chance that the nerve won’t heal.
When getting implants in the upper jaw, there’s a chance that the drilling may break through to the sinuses above the upper teeth, which may lead to an infection. To keep this from happening, the dentist will take X-rays before the surgery so they know where any nearby nerves or sinuses are located.
Dental implant failures
Despite the many benefits of dental implants, they do have the potential to fail. The rate at which dental implants fail is about 5% — a small number, but when you consider how many people get implants, you realize a lot of people suffer from this. And the rate of failure can be due to many things.
Reasons for dental implant failure
The factors for whether or not an implant(s) fails has a lot to do with how experienced the dentist is and how careful they were during the procedure.
But there are also many other reasons an implant could fail, and these reasons can cause either an early dental implant failure or late/long-term failure.
Early Dental Implant Failure
Early failure of dental implants usually happens within three to four months after the procedure during the process of the implant integrating with the jaw bone.
Here are some of the most common causes of early dental implant failure.
Autoimmune diseases
The dental implant failure rate with patients who have an autoimmune disease is higher than those without.
An autoimmune disease is basically illness that causes the body’s immune system to fight against itself, attacking and damaging the body’s tissues. This can lead to a high probability of the body rejecting an implant.
Types of autoimmune diseases include:
- Rheumatoid arthritis
- Lupus
- Multiple sclerosis
- Type 1 diabetes
- Celiac disease
Poor blood supply
Blood clots open wounds and carry nutrients needed for the body to heal. So if after getting implant you have a lack of blood rushing to the surgical area, the healing process will be slowed or even halted.
This poor blood supply could be from a blood disease, a blood clot elsewhere in the body, or an autoimmune disease.
Medications
Some medications can get in the way of the success of dental implants. That’s why it’s so important to be completely upfront with your dentist about the medications you take or have taken in the past.
One example is bisphosphonates, which is a type of medication used to treat osteoporosis.
Poor health
If you don’t take good care of your teeth, gums, and entire body, this can affect the success of dental implants. They will be less likely to integrate with an unhealthy body than with a healthy body.
Infections
Infections can destroy an implant’s chances of successfully integrating.
One of the most common infections that affects dental implants is peri-implantitis, a type of gum disease. This infection can begin during or after the surgery, whether it’s because there’s an open wound or because of the dental cement, which can leak under the crown and get stuck in the gums.
In most cases of peri-implantitis, it requires a dental implant failure replacement. Patients with an autoimmune disease, those who smoke, or those with bad oral hygiene are more likely to get this infection.
Overloading
In a typical implant procedure, the dentist will complete it in two stages: insertion of the implant and the placing of the abutment and crown. The time in between these two stages allows the implant to fuse to the jawbone before placing the abutment and crown.
But sometimes the dentist will do what’s called an “immediate loading procedure”. This is where both stages are done one right after the other. It makes the treatment time shorter, requires less surgical care afterwards, and can lead to a quicker recovery.
However, this way of doing the procedure can lead to overloading, an implant failure caused by excessive force or pressure on the abutment and crown. This can especially be a problem if the patient has poor bone density.
Rejection of the foreign body
As is the case with any organ transplant, the patient’s body can reject the implant. Although it’s rare, it’s still possible. Basically, the body sees the implant as a malignant foreign body and rejects its integration. This leads to a loose and/or failed implant.
Allergic reaction
Most dental implants are made of titanium alloy and can sometimes have small traces of nickel. This can cause an allergic inflammatory reaction in some patients. The reaction can be a tingling or burning feeling (more on symptoms below).
Nerve/Tissue Damage
If the nerves or tissue surrounding the implant are damaged, the patient can feel ongoing pain or numbness after the procedure. The sensations can spread from the area to the cheek, tongue, chin, and lips.
In most cases, nerve damage is due to an inexperienced dentist making placing the implant too close to a nerve. No matter how experienced the dentist, minimal tissue damage is expected. But if the damage is extreme, that can be due to the surgeon’s mistake.
Late or Long-Term Failure
If a dental implant fails anywhere from one to 10 years after the procedure, this is considered a late/long-term failure. There are several factors that can contribute to a late dental implant failure.
“Under-Engineered” Procedure
An under-engineered procedure means that there are too few implants to support the replacement teeth. This leads to too much stress on the implants. This can be because the dentist was not knowledgeable or experienced enough to know how many implants to use.
Smoking
Smokers who need dental implants are at a higher risk of experiencing a failed implant, anywhere between 6.5% to 20%, according to the Journal of International Society of Preventive & Community Dentistry
So smoking is definitely a risk factor in failed dental implants, but it can still be done. Your smoking history is something your dentist will as about before scheduling the procedure. And for overall dental health, smokers should try to stop (as their dentists have surely recommended already).
Bruxism
Bruxism is a fancy word for repetitive teeth grinding or clenching, and it’s actually common.
It can happen for many reasons, like stress, misaligned teeth, or missing teeth. This grinding or clenching puts extra pressure on the teeth and causes them to wear down or be damaged. Usually, bruxism happens overnight while a person is sleeping.
Bruxism can make it difficult for an implant to integrate with the jaw bone because excessive pressure on the teeth and implant and hurt the process. If it persists, it can lead to a failed implant.
Poor oral hygiene
If a dental implant patient doesn’t take care of their gums and teeth, this can lead to gum disease and infections. And as we mentioned earlier, those things can cause implants to fail.
Lack of gum tissue
The amount of gum tissue determines the quality of the seal around the implants — the more tissue, the better the seal. So if a patient lacks the adequate amount of gum tissue, this can cause the implants to loosen and eventually fail.
Head and neck radiation
If a patient has had head and/or neck cancer (including and especially mouth cancer) and has been treated with radiation therapy, this can increase the risk of implant failure.
Lack of bone
Patients who don’t have sufficient bone to support an implant, bacteria can get in there and infect the site. It’s difficult for the body to rid this small and crowded area of bacteria, and the dentist may need to replace the implant.
Infections
Just as with early dental implant failures, infections can affect implants in the long run. Bacteria can get under the gums and cause an infection, slowly eating away at the implant cement and the surrounding areas.
Mechanical damages
Obviously, if an implant is damaged (from an injury to the mouth/teeth), that can cause it to fail. If it’s left uncared for, the dentist may eventually have to replace the implant.
Dental implant failure prevention
Before you start experiencing symptoms, the best way to avoid problems is to properly maintain your dental implants, helping you to avoid implant failure.
First, you can do this by keeping up good oral hygiene, brushing and flossing twice a day and using an alcohol-free antibacterial mouthwash.
Second, scheduling regular visits to your dentist is crucial to the health of not only your dental implant but your overall mouth health. It’s best to schedule an appointment every three to six months.
Third, you may want to consider updating your diet to eat more bone- and gum-healthy foods, like milk, eggs, and fish. And you should also avoid hard foods and candies to prevent damage to the implant.
Dental implant failure symptoms
Here are the main symptoms and signs of late and early dental implant failure you should look out for:
- Severe pain or discomfort
- A loose or shifting implant
- Swelling or inflammation of the gums
- Gum recession around dental implant
- Difficulty chewing and biting
Andy pain, swelling, or shifting are common signs of a failing dental implant. If you experience these symptoms, contact your dentist.
Dental implant failure treatment
How you take care of the potential failure depends on the cause. But no matter what the cause is, the first thing you should do is contact your dentist.
If the implant area is infected, try to gently clean the area on a regular basis until you can meet with your dentist. Good oral hygiene is also very important in these cases. The sooner you notice an infection and get it taken care of, the better.
On the other hand, if the implant is failing because of too much stress or damage, your dentist may need to perform and bone graft in order to make it more secure.
References- Buser D, von Arx T, ten Bruggenkate CM, Weingart D (2000) Basic surgical principles with ITI implants. Clin Oral Implants Res 11 Suppl.: 59–68. https://www.ncbi.nlm.nih.gov/pubmed/11168257
- Klokkevold PR, Han TJ (2007) How do smoking, diabetes, and periodontitis affect outcomes of implant treatment? Int J Oral Maxillofac Implants 22 Suppl.: 173–202. https://www.ncbi.nlm.nih.gov/pubmed/18437796
- Gaetti-Jardim EC, Santiago-Junior JF, Goiato MC, Pellizer EP, Mafro-Filho O, et al. (2011) Dental implants in patients with osteoporosis: a clinical reality? J Craniofac Surg 22: 1111–1113 https://www.ncbi.nlm.nih.gov/pubmed/21586959
- Moedano DE, Irigoyen ME, Borges-Yáñez A, Flores-Sánchez I, Rotter RC (2011) Osteoporosis, the risk of vertebral fracture, and periodontal disease in an elderly group. Gerodontology 28: 19–27. https://www.ncbi.nlm.nih.gov/pubmed/19863666
- Esposito M, Grusovin MG, Polyzos IP, Felice P, Worthington HV. Interventions for replacing missing teeth: dental implants in fresh extraction sockets (immediate, immediate-delayed and delayed implants). Cochrane Database of Systematic Reviews 2010, Issue 9. Art. No.: CD005968. DOI: 10.1002/14651858.CD005968.pub3 http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD005968.pub3/full