Central pain syndrome
Central pain syndrome is often referred to as neuropathic pain, is a rare neurological condition caused by damage to or dysfunction of the pain-conducting pathways of the central nervous system (CNS), which includes the brain, brainstem, and spinal cord 1. Many different names have been used for central pain syndrome, including Dejerine-Roussy syndrome, thalamic pain syndrome, central post-stroke syndrome and others. The current name central pain syndrome acknowledges that damage to various areas of the central nervous system can cause central pain, and that a stroke is not necessarily the cause. When central pain syndrome is due to a stroke, it may be referred to as the more specific term “central post-stroke pain” 2.
Central pain syndrome can be caused by stroke, multiple sclerosis, tumors, epilepsy, brain or spinal cord trauma, or Parkinson’s disease. The character of the pain associated with central pain syndrome differs widely among individuals partly because of the variety of potential causes. Central pain syndrome may affect a large portion of the body or may be more restricted to specific areas, such as hands or feet. The extent of pain is usually related to the cause of the CNS injury or damage. Primary symptoms are pain and loss of sensation, usually in the face, arms, and/or legs. Pain or discomfort may be felt after being touched, or even in the absence of a trigger. Pain is typically constant, may be moderate to severe in intensity, and is often made worse by touch, movement, emotions, and temperature changes, usually cold temperatures 2. Individuals experience one or more types of pain sensations, the most prominent being burning. Mingled with the burning may be sensations of “pins and needles;” pressing, lacerating, or aching pain; and brief, intolerable bursts of sharp pain similar to the pain caused by a dental probe on an exposed nerve. Individuals may have numbness in the areas affected by the pain. The burning and loss of touch sensations are usually most severe on the distant parts of the body, such as the feet or hands. Central pain syndrome often begins shortly after the causative injury or damage, but may be delayed by months or even years, especially if it is related to post-stroke pain.
Chronic widespread pain seen in centralized pain occurs in between 10 to 40 percent of patients with rheumatoid arthritis, psoriatic arthritis, osteoarthritis, spondyloarthritis, and lupus. Centralized pain occurs between five and 15 percent of the general population, the majority of which have fibromyalgia 3. The criteria for the diagnosis of fibromyalgia overlap considerably with centralized pain, with patients with fibromyalgia who report severe fatigue at a fivefold increase for widespread pain 4. The prevalence of the overlap of central pain syndrome and knee osteoarthritis is between 10 and 15 percent. The prevalence increases if knee pain was bilateral compared to only a single knee 5.
The reported incidence of centralized pain in rheumatoid arthritis is estimated to be between 13 to 40 percent. One concern with this patient population is overtreating their rheumatoid arthritis because of increased symptoms, which are actually due to centralized pain. Inflammatory markers are lower in patients with comorbid fibromyalgia and rheumatoid arthritis, while simultaneously, they reported decreased quality of life compared to those with rheumatoid arthritis alone 6.
Centralized pain syndrome appears in 10 to 30 percent of patients with spondyloarthritis. Separately, 13 to 20 percent of patients with ankylosing spondylitis meet the criteria for fibromyalgia 7. Patients with widespread pain are much more likely to have clinically significant fatigue with a comorbid mood disorder with moderate to severe symptoms. In one study, centralized pain was present in 53 percent of patients with psoriatic arthritis, but only five percent of the average population 8. Patients with centralized pain were also more likely to be lost to follow up after initiating treatment. Separately, centralized pain presents in 20 to 40 percent of patients with lupus or Sjogren syndrome. Furthermore, symptoms were more prevalent as chronic diseases progressed, as well as in patients with comorbid depression 9. Over a third of women with chronic back pain suffer from centralized pain. The daily impact of central pain is significant in patients with chronic back pain, including severe limitations in their activity level. Given the significant prevalence of widespread, centralized pain in women with chronic low back pain, it is reasonable to consider central pain syndrome as part of the differential diagnosis in this patient population 10.
Central pain syndrome treatment typically includes pain medications, but complete relief of pain may not be possible. Tricyclic antidepressants such as nortriptyline or anticonvulsants such as neurontin (gabapentin) can sometimes be useful. Lowering stress levels appears to reduce pain 1.
Central pain syndrome key points
- Central pain syndrome occurs when the central nervous system becomes sensitized to pain, leading to a lower pain threshold.
- The prevalence of widespread pain or central pain syndrome is relatively common. It is present in up to 20% of patients with chronic pain from any cause.
- Common symptoms of central pain syndrome are: pain from nonpainful contact or pressure (allodynia), and increased pain from painful stimuli (hyperalgesia).
- Patients suffering from chronic rheumatological and musculoskeletal conditions for at least three months are at increased risk of developing central pain syndrome.
- There is both an environmental and genetic component to developing central pain syndrome.
- A patient can both have a chronic disease causing pain (peripheral pain) and centralized pain (central pain syndrome), and this risk increases over time.
- Functional MRI may be a useful diagnostic test in the diagnosis of various pain disorders.
- Without proper management, the morbidity from central pain syndrome is high.
- Central pain syndrome can have a significant negative impact on multiple chronic disease states, including osteoarthritis and rheumatoid arthritis.
- Central pain syndrome requires proper identification to receive appropriate treatment. It requires a different treatment strategy compared with peripheral or mechanical pain
- A pain medicine specialist alongside a primary care physician may be necessary to treat central pain syndrome.
- Treatment of a comorbid disease alongside centralized pain can improve a patient’s pain.
- First-line treatments for central pain syndrome include antidepressants and anticonvulsants.
Central pain syndrome causes
Historically, central pain syndrome was a psychiatric diagnosis, seen following a traumatic brain injury, or thought to be a diagnosis of exclusion. The theory behind centralized pain was that it was a dysfunction of the nervous system rather than an adaptive change seen with musculoskeletal (nociceptive) pain 11. For example, it is beneficial to withdraw one’s hand away from an open flame. The purpose of pain is protection – to limit harm. Central pain was thought to be the feeling of pain without the fire. Not only is it not protective, but it is also actually maladaptive.
Central pain syndrome develops following damage to the central nervous system – the brain, brainstem or spinal cord. Such damage is most often associated with a stroke, multiple sclerosis, spinal cord (but also brain) injury or brain tumors. Central pain syndrome can also develop after neurosurgical procedures involving the brain or spine.
Central pain syndrome results from damage to the pain-transmission pathway from the level of the spinal cord up to the cortex, the grey matter that covers the cerebral hemispheres.
Central pain syndrome is due to a disturbed communication between the sensory thalamus and the sensory cortex. The thalamus is a structure deep in the brain that acts as the key hub in sensory processing, in synchrony with the cortex. When this synchrony is disturbed by neural damage that unbalances the brain’s inhibitory tone, the cortex and some thalamic nuclei are “locked” together in an altered state of activity. central pain syndrome is thus due to a dysfunctional corticothalamic loop along the sensory pain-conducting pathways.
As multiple systemic syndromes overlap, sensory amplification and pain increase. There is an estimated 2% to 4% of the population that suffers from fibromyalgia, 1% from chronic fatigue, and 4% from somatoform disorders. There is considerable overlap between regional pain syndromes such as these and psychiatric disorders.
Central pain syndrome is a type of neuropathic pain in the central nervous system. It can be seen in patients following a stroke or in patients who have multiple sclerosis. It is seen in various chronic rheumatological and musculoskeletal disorders as well. When any acute pain becomes chronic, it can undergo centralization, putting patients at risk for developing central pain syndrome.
Furthermore, though patients with chronic pain syndrome consider their pain to be peripheral in origin, in reality, it is mostly centralized. The neural signal has become amplified, leading to hyperalgesia and allodynia. When a patient suffers from a peripheral pain state with nociceptive pain, such as rheumatoid arthritis, with time, this pain becomes centralized. The patient’s pain is then considered to be in a mixed state 12. Chronic back pain is such an example of peripheral pain becoming centralized 13. Risk factors for fibromyalgia parallel the risk factors for central pain syndrome. These risk factors include trauma, infection, chronic stress, obesity, and depression. Centralized sensitization occurs when there is minimal or no nociceptive input. Functional neuroimaging can aid in diagnosis as well.
In reality, centralized pain is not mutually exclusive to other types of pain. There is an overlap between pain states. Any central pain state can have a component of peripheral pain, such as in the case of peripheral neuropathy. Moreover, there is a significant family predisposition for centralized pain syndrome 14. Psychological stressors can also trigger worsening symptoms. In many cases, environmental factors cause a triggering event in patients with genetic susceptibility, leading to widespread, centralized pain 15. Thus environmental stressors must be managed. Early life trauma, infection, or emotional stress can lead to centralized pain to occur in 5% to 10% of patients 16.
There is an estimated 50% environmental component to developing centralized pain and a 50% genetic component. First degree relatives are at eight times greater risk of developing widespread pain compared to the average population. However, there is no significant difference based upon the sex of the patient or family member. The genetic association is more prominent in families with a history of mood disorders 17. There may be a genetic component to the widespread pain, but there has not been a single genetic polymorphism identified 18.
Central pain syndrome symptoms
Central pain syndrome often begins shortly after the injury or damage that caused it. However, it may be delayed by months or even years, especially if it is related to post-stroke pain. In most cases, central pain syndrome remains a lifelong condition. The characteristics of the pain associated with central pain syndrome differ widely, partly because of the variety of potential causes. It may affect a large portion of the body, or be restricted to specific areas such as the hands or feet.
The severity of pain is usually related to the cause of the central nervous system (CNS) injury or damage. Pain is typically constant, may be moderate to severe in intensity, and is often made worse by touch, movement, emotions, and temperature changes (usually cold temperatures).
People with central pain syndrome experience one or more types of pain sensations, the most prominent being burning. Mingled with the burning may be sensations of pins and needles, pressing, lacerating, aching, or brief, intolerable bursts of sharp pain. Some people also experience numbness. The burning and loss-of-touch sensations are usually most severe on the distal parts of the body, such as the feet or hands.
Central pain syndrome can be limited to a specific area of the body such as the hands or feet or may be widespread over a large portion of the body. Some areas of the body may be more intensely affected than other areas. Pain can fluctuate during the day and can be affected by several factors including touch, emotions such as stress, certain movements or overall level of activity, and temperature changes, especially cold temperatures. Rest and distraction may lessen symptoms.
Central pain syndrome is associated with mood changes, fatigue, cognitive disturbances, sleep changes, pain catastrophizing, and symptoms of neuropathic pain (burning, numbness, tingling, and paraesthesias). Generally, patients with central pain syndrome will have multifocal pain, memory complaints, and often comorbid major depressive disorder or generalized anxiety disorder 3. Noxious stimuli, such as extremes of temperature or loud sounds 19.
Central pain syndrome can vary greatly from one individual to another, in part, based upon the underlying cause of the condition. In some cases, symptoms of central pain syndrome can be vague and difficult to characterize. Many different pain sensations including burning, stabbing, lacerating, pressing, aching, prickling or tingling (a feeling of being on “pins and needles”) may occur. A constant burning sensation is in several cases the most prominent symptom. Pain can be constant and unrelenting or it may come and go (intermittent). In most cases, pain is constant and usually moderate or severe in intensity. In some cases, pain has been described as agonizing. Some affected individuals may experience short bursts of sharp, excruciating pain, which has been compared to the pain that occurs when a dental probe strikes an exposed nerve. In some cases, the constant pain associated with central pain syndrome can be debilitating and affect an individual’s ability to perform daily tasks and significantly impair quality of life.
Pain sensations associated with central pain syndrome are generally spontaneous, which means they occur despite no apparent cause or trigger. People with this disorder may be extra-sensitive or have a heightened response to acute painful stimuli (hyperalgesia, hyperpathia), which means that pain that would normally be small or minimal is felt to a far greater degree. In addition, individuals may feel pain from stimuli that would normally not be painful (allodynia). For example, affected individuals may experience pain when touched, even when lightly touched. In severe cases, this can include pain caused by a strong breeze, the weight of a blanket, or even the clothes a person is wearing.
Some individuals with central pain syndrome may initially experience impairment or distortion of sensation, especially of touch (dysesthesia). Dysesthesia is described as vague, unpleasant sensations. Some individuals may experience a painful numbness, especially affecting the feet. Itching (pruritus) has also been reported in individuals with central pain syndrome.
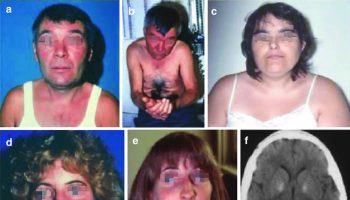
Some individuals with central post-stroke pain may experience painful symptoms on one side of the body (from head to foot); other notable, accompanying symptoms include loss of sensation (hemihypesthesia), partial or complete muscle weakness (hemiparesis, hemiplegia), and , on occasion, abnormal, involuntary, irregular jerky motions and slow, writhing movements (hemichoreoathetosis).
Central pain syndrome complications
The impact of central pain disorder affects multiple conditions.
In rheumatoid arthritis, central pain is associated with neuropathic symptoms, increase pain scores without changes to inflammatory markers, increased adverse outcomes, as well as reduced remission rates.
Central sensitization is associated with the increased use of opioids, as well as increased pain severity in patients with osteoarthritis and is related to poorer patient outcomes.
Patients with bilateral knee pain are at increased risk for joint pain in areas other than the knees at a one year follow up 5.
For spondyloarthritis, central sensitization correlates with worse outcomes and disease scores, as well as poorer results in treatment.
In chronic back pain, central pain syndrome causes more significant pain and mood changes, as well as increased adverse outcomes. In joint hypermobility syndrome it correlates with increased pain severity and poorer patient outcomes.
For chronic whiplash injuries, centralized pain correlates with cognitive disturbances, increased pain, and poorer outcomes.
In lupus, centralized pain carries associations with more significant sleep disturbances and mood changes and worse outcomes.
In patients with carpal tunnel syndrome, central pain syndrome is associated with poorer surgical outcomes, while in lateral epicondylitis, it is associated with more severe pain, increased duration of pain, and greater risk of failed treatment responses.
Preoperative increases in fibromyalgia pain scores correlated with the administration of more postoperative morphine equivalents and a reduced response of nonsteroidal anti-inflammatory drugs (NSAIDs) 20.
Central pain syndrome diagnosis
A diagnosis of central pain syndrome is based upon identification of characteristic symptoms, a detailed patient history, a thorough clinical exam and a variety of specialized tests. The diagnosis of centralized pain is symptoms of pain lasting at least three months, with widespread allodynia or hyperalgesia, without any apparent cause of pain 21. Central pain syndrome is suspected in individuals who complain of pain or other abnormal sensations following injury to the central nervous system. Other conditions that cause pain may need to be ruled out before a diagnosis of central pain syndrome is made. The clinical exam may include sensory testing to confirm and pinpoint the presence of sensory abnormalities, but also to rule out other causes of pain 22. Imaging tests such as a CT scan and MRI may be used to see tumors, infarcts, cerebral bleeding, and other lesions that may cause pain. MRI is the preferred technique when central pain syndrome is suspected 2.
Functional neuroimaging (fMRI) have helped shed light on central pain. An fMRI can help distinguish structural and functional brain abnormalities in patients with various chronic pain disorders. Patients with fibromyalgia have demonstrated unique brain patterns on fMRI. It can be a useful tool in not only diagnosis but also predicting patients at risk for developing various pain disorders 23. These abnormal findings on fMRI include decreased brain volume and cortical thickness, as well as an increase in the level of excitatory neurotransmitters 24.
Furthermore, fMRI is useful in helping to determine the connectivity between multiple regions of the brain. The extent of alteration correlates with the scope of the patient’s pain. A functional MRI could be an objective measure of the severity of fibromyalgia 25. The pain signals seen on fMRI in patients diagnosed with fibromyalgia are different than the average population. Functional MRI is a promising test that may be beneficial in the future for the diagnosis of multiple pain disorders 26.
Increased pain responses have been seen in patients with central pain syndrome on positron emission tomography as well as electroencephalography 27.
Screening instruments for central pain are available. These tools included the central sensitization inventory (CSI), as well as the painDETECT measure. These tools can serve to assess neuropathic pain and centralized pain syndromes such as fibromyalgia, respectively 28. It is challenging to differentiate the origin of pain, whether it is central or peripheral. The painDETECT instrument cannot distinguish between peripheral neuropathy secondary to a central or peripheral source 28.
Clinical testing and workup
Magnetic resonance imaging (MRI) is the technique of choice to visualize tumors, infarcts, cerebral hemorrhage, demyelinating plaques and other causal lesions. An MRI uses a magnetic field and radio waves to produce cross-sectional images of particular organs and bodily tissues. Recent permutations of this technique (such as magnetic resonance spectroscopy or default-state MRI) have only research interest.
Laser evoked potentials are rarely needed to confirm damage to pain conducting pathways and are not available on a routine basis.
Screening tools (in the form of questionnaires) have been developed, but miss 12-45% of cases and as such are not recommended for finalizing a diagnosis.
Central pain syndrome treatment
Treatment of central pain syndrome is known to be challenging 29. Treatment often focuses on treating the underlying chronic disease state associated with centralized pain. The method of treatment may vary depending on the cause of the neurological damage. Pain medications (analgesics) often provide only some relief of pain 29. Treatment of a comorbid condition associated with centralized pain is beneficial in the relief of a patient’s pain 30. For example, in knee osteoarthritis, neuroimaging changes associated with central pain syndromes improved with joint replacement. Central pain disorders often respond to neuromodulators, antiepileptics, or antidepressants rather than peripheral pain pharmacological agents such as nonsteroidal anti-inflammatory drugs (NSAIDs) or opioid analgesics 12.
In general, first-line management includes the use of tricyclic antidepressants such as nortriptyline, serotonin and norepinephrine reuptake inhibitors (SNRIs) such as duloxetine or venlafaxine, as well as the anticonvulsants pregabalin and gabapentin or topical lidocaine. Second-line management involves the use of opioid analgesics such as tramadol, along with first-line medication. Third-line management may include other antidepressant or anticonvulsant medications 31. There is strong evidence for the use of tricyclic antidepressants such as amitriptyline, serotonin and norepinephrine reuptake inhibitors such as duloxetine or venlafaxine. There is moderate evidence for the use of tramadol or an selective serotonin reuptake inhibitors (SSRIs), and weak evidence for using S-adenosyl-L-methionine (SAMe).
Lowering stress levels appears to reduce pain 1. Other treatment alternatives have included the administration of a sympathetic blockade (a type of nerve block) and a guanethidine block, as well as psychological evaluation and treatment. Rarely, surgery is necessary 32. Stereotactic radiosurgery of the pituitary has been used with some success. Other forms of potential treatments that have been discussed in the literature include transcutaneous electrical nerve stimulation (TENS); deep brain stimulation; and motor cortex stimulation 33.
Motor cortex stimulation as well as deep brain stimulation are effective treatment modalities for patients with refractory pain, centralized pain, and peripheral neuropathy 34.
Nonpharmacological interventions are also primary treatment for patients with centralized pain, including cognitive-behavioral therapy. A holistic approach is necessary for the treatment of centralized pain. There often can be a structural, immunologic, or inflammatory component to the underlying disease.
Central pain syndrome prognosis
Central pain syndrome is not a fatal disorder, but the syndrome causes disabling chronic pain and suffering among the majority of individuals who have it. Prognosis is better in patients when the underlying disease state, which initially caused the pain, can be cured, corrected, or managed, as in the case of a shoulder replacement in osteoarthritis. Patients with osteoarthritis and centralized pain report pain reduction with pharmacological therapy aimed at the osteoarthritis, as well as an increase in adverse pain outcomes following joint replacement surgery 35.
Prognostically, when osteoarthritis is comorbid with central pain syndrome, the severity of patients’ pain does not correlate with the radiographic severity of osteoarthritis. Furthermore, these patients with radiological evidence of osteoarthritis in a single joint were at increased risk of having multiple painful joints 5. Furthermore, this comorbid population has a higher degree of synovitis and effusion in knee osteoarthritis 36.
Central sensitization also plays a role in inflammatory arthritis. It occurs in a significant subset of the patient population 37. Furthermore, patients with rheumatoid arthritis and comorbid fibromyalgia have worse reported pain, poorer mental health, take more medications for pain, including prednisone, while simultaneously have lower levels of inflammatory markers 38. Patients with inflammatory arthritis and central pain syndrome also have poorer outcomes 39. Patients with centralized pain and comorbid rheumatoid arthritis suffer from hyperalgesia at nonarticular sites in addition to articular surfaces 40.
- Central Pain Syndrome Information Page. https://www.ninds.nih.gov/Disorders/All-Disorders/Central-Pain-Syndrome-Information-Page[↩][↩][↩]
- Central Pain Syndrome. https://rarediseases.org/rare-diseases/central-pain-syndrome[↩][↩][↩]
- McBeth J, Jones K. Epidemiology of chronic musculoskeletal pain. Best Pract Res Clin Rheumatol. 2007 Jun;21(3):403-25.[↩][↩]
- Dean LE, Arnold L, Crofford L, Bennett R, Goldenberg D, Fitzcharles MA, Paiva ES, Staud R, Clauw D, Sarzi-Puttini P, Jones GT, Ayorinde A, Flüß E, Beasley M, Macfarlane GJ. Impact of Moving From a Widespread to Multisite Pain Definition on Other Fibromyalgia Symptoms. Arthritis Care Res (Hoboken). 2017 Dec;69(12):1878-1886.[↩]
- Felson DT, Niu J, Quinn EK, Neogi T, Lewis CL, Lewis CE, Frey Law L, McCulloch C, Nevitt M, LaValley M. Multiple Nonspecific Sites of Joint Pain Outside the Knees Develop in Persons With Knee Pain. Arthritis & rheumatology (Hoboken, N.J.). 2017 Feb;69(2):335-342.[↩][↩][↩]
- Andersson ML, Svensson B, Bergman S. Chronic widespread pain in patients with rheumatoid arthritis and the relation between pain and disease activity measures over the first 5 years. J. Rheumatol. 2013 Dec;40(12):1977-85.[↩]
- Baraliakos X, Regel A, Kiltz U, Menne HJ, Dybowski F, Igelmann M, Kalthoff L, Krause D, Saracbasi-Zender E, Schmitz-Bortz E, Braun J. Patients with fibromyalgia rarely fulfil classification criteria for axial spondyloarthritis. Rheumatology (Oxford). 2018 Sep 01;57(9):1541-1547.[↩]
- Magrey MN, Antonelli M, James N, Khan MA. High frequency of fibromyalgia in patients with psoriatic arthritis: a pilot study. Arthritis. 2013;2013:762921.[↩]
- Torrente-Segarra V, Salman-Monte TC, Rúa-Figueroa Í, Pérez-Vicente S, López-Longo FJ, Galindo-Izquierdo M, Calvo-Alén J, Olivé-Marqués A, Ibañez-Ruán J, Horcada L, Sánchez-Atrio A, Montilla C, Rodríguez-Gómez M, Díez-Álvarez E, Martinez-Taboada V, Andreu JL, Fernández-Berrizbeitia O, Hernández-Beriain JA, Gantes M, Hernández-Cruz B, Pecondón-Español Á, Marras C, Bonilla G, Pego-Reigosa JM., RELESSER Study Group of the Spanish Society of Rheumatology (SER). Study Group of Systemic Autoimmune Diseases of the SER (EAS-SER). Fibromyalgia prevalence and related factors in a large registry of patients with systemic lupus erythematosus. Clin. Exp. Rheumatol. 2016 Mar-Apr;34(2 Suppl 96):S40-7.[↩]
- Nordeman L, Gunnarsson R, Mannerkorpi K. Prevalence and characteristics of widespread pain in female primary health care patients with chronic low back pain. Clin J Pain. 2012 Jan;28(1):65-72.[↩]
- Clauw DJ, Hassett AL. The role of centralised pain in osteoarthritis. Clin. Exp. Rheumatol. 2017 Sep-Oct;35 Suppl 107(5):79-84.[↩]
- Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011 Mar;152(3 Suppl):S2-15.[↩][↩]
- Mutso AA, Petre B, Huang L, Baliki MN, Torbey S, Herrmann KM, Schnitzer TJ, Apkarian AV. Reorganization of hippocampal functional connectivity with transition to chronic back pain. J. Neurophysiol. 2014 Mar;111(5):1065-76.[↩]
- Buskila D. Genetics of chronic pain states. Best Pract Res Clin Rheumatol. 2007 Jun;21(3):535-47.[↩]
- Williams DA, Clauw DJ. Understanding fibromyalgia: lessons from the broader pain research community. J Pain. 2009 Aug;10(8):777-91.[↩]
- McLean SA, Clauw DJ. Predicting chronic symptoms after an acute “stressor”–lessons learned from 3 medical conditions. Med. Hypotheses. 2004;63(4):653-8.[↩]
- Kato K, Sullivan PF, Evengård B, Pedersen NL. Importance of genetic influences on chronic widespread pain. Arthritis Rheum. 2006 May;54(5):1682-6.[↩]
- Arnold LM, Fan J, Russell IJ, Yunus MB, Khan MA, Kushner I, Olson JM, Iyengar SK. The fibromyalgia family study: a genome-wide linkage scan study. Arthritis Rheum. 2013 Apr;65(4):1122-8.[↩]
- Staud R, Robinson ME, Price DD. Temporal summation of second pain and its maintenance are useful for characterizing widespread central sensitization of fibromyalgia patients. J Pain. 2007 Nov;8(11):893-901.[↩]
- Edwards RR, Dolman AJ, Martel MO, Finan PH, Lazaridou A, Cornelius M, Wasan AD. Variability in conditioned pain modulation predicts response to NSAID treatment in patients with knee osteoarthritis. BMC Musculoskelet Disord. 2016 Jul 13;17:284.[↩]
- Dydyk AM, Givler A. Central Pain Syndrome. [Updated 2020 Jan 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK553027[↩]
- Klit H, Finnerup NB, Jensen TS. Central post-stroke pain: clinical characteristics, pathophysiology, and management. Lancet Neurology. 2009; 8:859-868. https://www.ncbi.nlm.nih.gov/pubmed/19679277[↩]
- Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. N. Engl. J. Med. 2013 Apr 11;368(15):1388-97.[↩]
- Jensen KB, Srinivasan P, Spaeth R, Tan Y, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Vitton O, Gracely R, Ingvar M, Kong J. Overlapping structural and functional brain changes in patients with long-term exposure to fibromyalgia pain. Arthritis Rheum. 2013 Dec;65(12):3293-303.[↩]
- Napadow V, Kim J, Clauw DJ, Harris RE. Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis Rheum. 2012 Jul;64(7):2398-403.[↩]
- López-Solà M, Woo CW, Pujol J, Deus J, Harrison BJ, Monfort J, Wager TD. Towards a neurophysiological signature for fibromyalgia. Pain. 2017 Jan;158(1):34-47.[↩]
- Lee U, Kim M, Lee K, Kaplan CM, Clauw DJ, Kim S, Mashour GA, Harris RE. Functional Brain Network Mechanism of Hypersensitivity in Chronic Pain. Sci Rep. 2018 Jan 10;8(1):243.[↩]
- Timmerman H, Wolff AP, Bronkhorst EM, Wilder-Smith OHG, Schenkels MJ, van Dasselaar NT, Huygen FJPM, Steegers MAH, Vissers KCP. Avoiding Catch-22: validating the PainDETECT in a in a population of patients with chronic pain. BMC Neurol. 2018 Jun 29;18(1):91.[↩][↩]
- YiLi Zhou. CHAPTER 48 – Principles of Pain Management. Bradley: Neurology in Clinical Practice, 5th ed.[Electronic version]. Deutschland: Butterworth-Heinemann; 2008 ; 905.[↩][↩]
- Brummett CM, Urquhart AG, Hassett AL, Tsodikov A, Hallstrom BR, Wood NI, Williams DA, Clauw DJ. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis & rheumatology (Hoboken, N.J.). 2015 May;67(5):1386-94.[↩]
- Classic Central Pain Syndromes: Review of Neurologic Causes of Pain. https://www.practicalpainmanagement.com/classic-central-pain-syndromes-review-neurologic-causes-pain[↩]
- Shoulder Pain in Hemiplegia. https://emedicine.medscape.com/article/328793-overview[↩]
- Schott GD. From thalamic syndrome to central poststroke pain. Journalof Neurology, Neurosurgery, and Psychiatry. December, 1996; 61(6):560-564. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC486645/pdf/jnnpsyc00012-0002.pdf[↩]
- Moore NZ, Lempka SF, Machado A. Central neuromodulation for refractory pain. Neurosurg. Clin. N. Am. 2014 Jan;25(1):77-83.[↩]
- Finan PH, Buenaver LF, Bounds SC, Hussain S, Park RJ, Haque UJ, Campbell CM, Haythornthwaite JA, Edwards RR, Smith MT. Discordance between pain and radiographic severity in knee osteoarthritis: findings from quantitative sensory testing of central sensitization. Arthritis Rheum. 2013 Feb;65(2):363-72.[↩]
- Neogi T, Guermazi A, Roemer F, Nevitt MC, Scholz J, Arendt-Nielsen L, Woolf C, Niu J, Bradley LA, Quinn E, Law LF. Association of Joint Inflammation With Pain Sensitization in Knee Osteoarthritis: The Multicenter Osteoarthritis Study. Arthritis & rheumatology (Hoboken, N.J.). 2016 Mar;68(3):654-61.[↩]
- Pollard LC, Kingsley GH, Choy EH, Scott DL. Fibromyalgic rheumatoid arthritis and disease assessment. Rheumatology (Oxford). 2010 May;49(5):924-8.[↩]
- Chakr RMDS, Brenol C, Ranzolin A, Bernardes A, Dalosto AP, Ferrari G, Scalco S, Olszewski V, Kohem C, Monticielo O, Brenol JCT, Xavier RM. Rheumatoid arthritis seems to have DMARD treatment decision influenced by fibromyalgia. Rev Bras Reumatol Engl Ed. 2017 Sep – Oct;57(5):403-411.[↩]
- Koop SM, ten Klooster PM, Vonkeman HE, Steunebrink LM, van de Laar MA. Neuropathic-like pain features and cross-sectional associations in rheumatoid arthritis. Arthritis Res. Ther. 2015 Sep 03;17:237.[↩]
- Duffield SJ, Miller N, Zhao S, Goodson NJ. Concomitant fibromyalgia complicating chronic inflammatory arthritis: a systematic review and meta-analysis. Rheumatology (Oxford). 2018 Aug 01;57(8):1453-1460.[↩]