Entoptic phenomena
Entoptic phenomena is a phrase derived from the Greek words ‘inside’ and ‘light’ or ‘vision’ was introduced by Johann Benedict Listing (1808 – 1882), which describes the ability of an individual to perceive substances endogenous to their own eye, such as retinal vessels or vitreous opacities 1. Entoptic phenomena refer to positive visual phenomena generated from physiologic or pathologic processes within the eye 2. The most well-known entoptic phenomenon is Moore’s lightning streaks, which are seen when vitreous liquefaction produces retinal traction, resulting in photopsias often referred to as flashes of light 3. The entoptic phenomenon is usually brought about by shining a focused light such as from a penlight or a hand‐held ophthalmoscope on the sclera, or in front of the pupil, and then rapidly moving it back‐and‐forth 1. When coming from in front, this motion is not always necessary, and the light may also be replaced by a small opening in a black screen while looking at a bright white wall. Entoptic phenomena has been historically used to determine the photoreceptive layer of the retina and raised some intriguing questions about the nature of the visual process 1. The presence or absence of different entoptic phenomena can raise red flags for posterior and anterior abnormalities, and even refractive and convergence conditions—making them potential markers of disease presence and progression.
Entoptic phenomena were first described by Johann Purkinje in the early 1800s, to describe the fleeting, black afterimage of retinal vasculature, later coined the ‘Purkinje tree’ 4. Entoptic phenomena occurs due to the location and pattern of the branching retinal vascular ‘tree’ in front of the photoreceptor layer, casting a shadow that is only induced when the anterior segment of the eye is illuminated 4. It differs from a real image, particularly in that it does not track with eye or retinal movement due to the direct and constant relationship with the photoreceptor layer 5. In fact, it is this observation, which led to the conclusion that there must be a rapid mechanism of image creation and erasure as the foundation of normal visual processing 5.
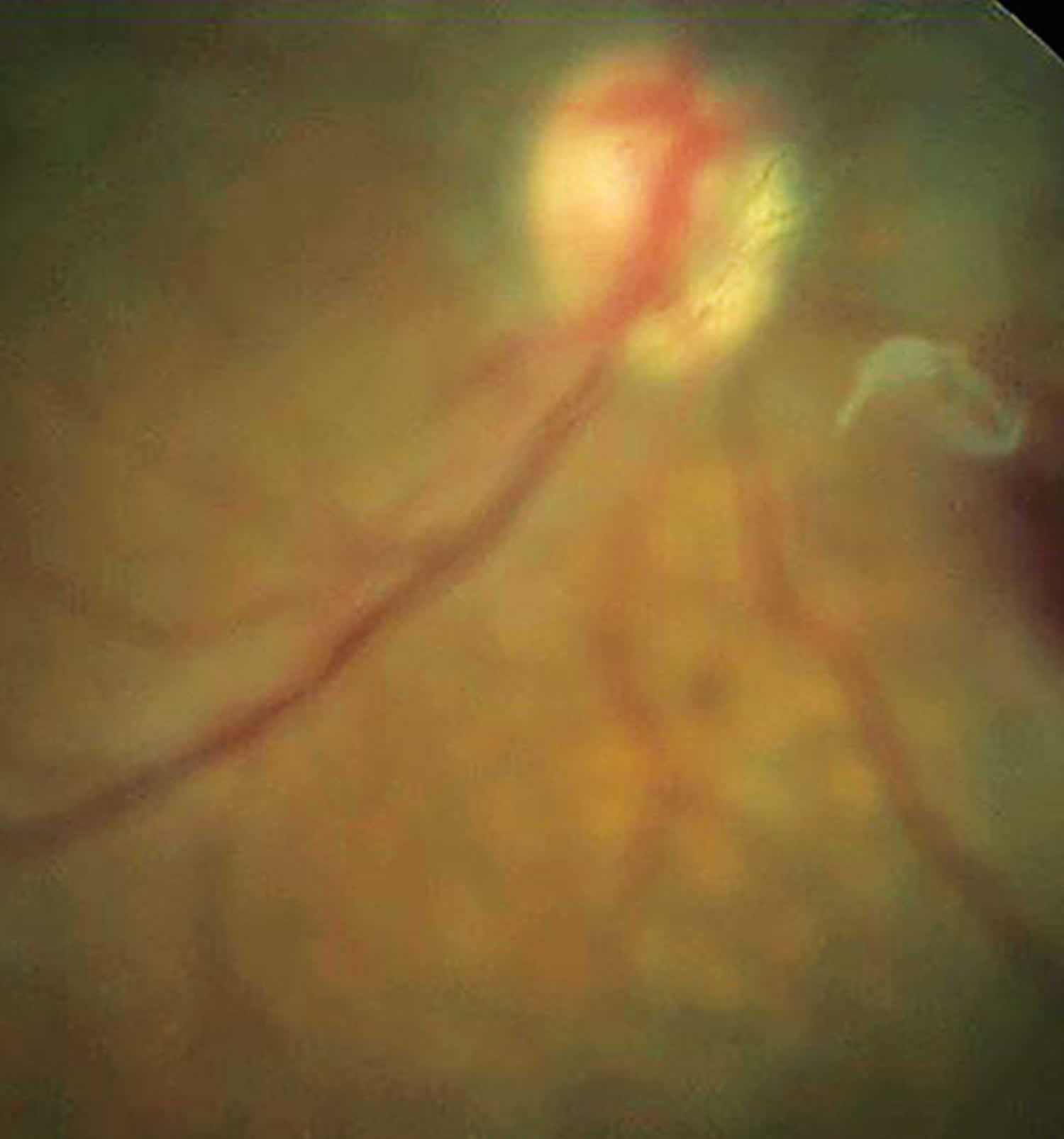
Figure 1. Entoptic phenomena
Footnote: Entoptic phenomenon is perceived as vitreous floater secondary to posterior vitreous detachment.
Entoptic phenomenon physiological application
The entoptic phenomena of the retinal vessels has many characteristics of an after‐image. It remains visible after the intermittent short stimulus has stopped; in this manner, it also has the nature of palinopsia. It remains visible when only one eye is stimulated while the other is open. In contrast to real images, the entoptic vascular tree does not move when the globe with its retina is passively moved, for instance by digital pressure or pull with forceps 6. Passive motion of the globe normally moves the perception of the external world because the final location of the ordinary real image of the world is in the cortex, which conceives it as stable, based on inputs from the extra‐ocular muscles and all other sensory organs (including the other, stable, eye). On the other hand, when one commands to oneself to intentionally look right or left, after‐images, and the entoptic vasculature, also move in the same direction 7. The extent of this movement is, however, not at all proportional to that of the globe, which may even remain completely at rest. The apparent size of the vascular tree, similar to the size of after‐images, depends on the distance judgment of the background unto which it is projected rather than the actual size on the retinal vasculature 8. And, above all, microelectric recordings from the retina (elctroretinograms) show no evidence of activity relating to after‐images, whereas recordings from the cortex (Electroencephalogram, visual evoked potentials) do so indeed 9.
That retinal vessels may throw a bit of shadow on the retina (relative scotoma) is demonstrated under very strict testing conditions by angioscotometry with minute object sizes (0.5–1.5 mm diameter) seen at close range (19 cm) through a collimating lens. One of the scotomas’ contrasts to the entoptic view is their enlargement by pressure on the globe, pressure on the other eye or compression of the jugular veins 10.
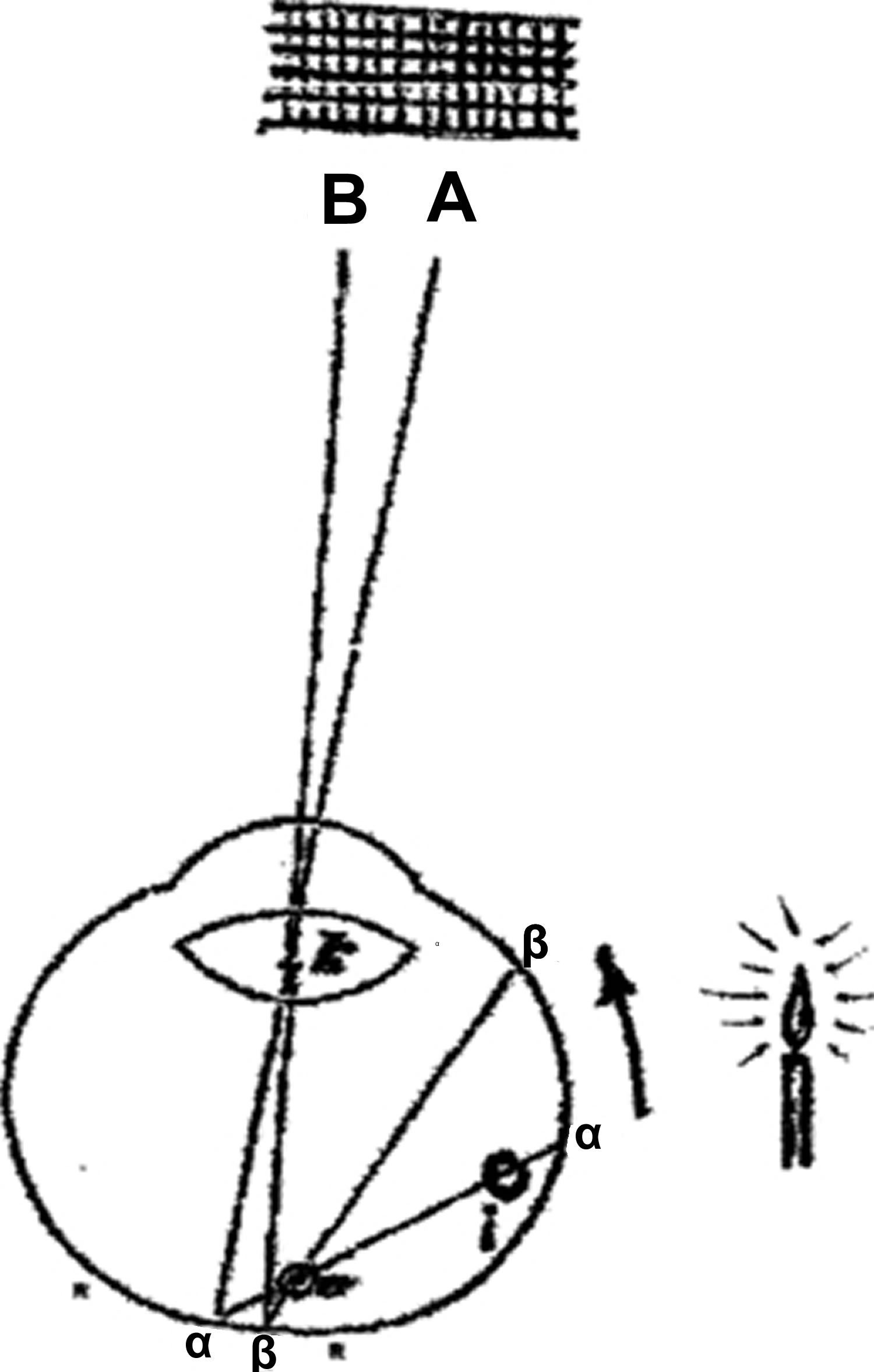
A most thorough investigation of the entoptic phenomenon was carried out by Heinrich Müller 11, whose name is attached to retinal cells and an orbital muscle, published in a paper 37 pages long titled in translation: ‘On the entoptic awareness of the retinal vessels, especially as evidence for light’s perception by the retinal elements which lie in the rear’. With the help of two assistants, Müller shone light on his globe at location a (see Figure 2 below) and marked the projection of the visible blood vessel v’ on a screen in front of the eye (A). A second area (b) was then illuminated, and this projection marked (B). On a true diagram of the globe, about an inch in size, he drew the lines aα, αA, and bβ, βB through the nodal point. He thus geometrically deduced location v’ of the blood vessel and was able to calculated the distance between this point and the retina (R, where α and β are also located). This distance was then transposed on a fixated anatomical specimen of a globe and measured from a retinal blood vessel backwards. It ended at the cellular layer of the rods and cones. Therefore, Müller concluded, this area must be the photoreceptive one.
Müller’s judicious mode of reasoning and conclusion (which Helmholtz called theory) 10 seems to contain, nonetheless, several untenable presuppositions forming perhaps insurmountable obstacles to accepting their validity. First, the light source was strangely presupposed to emanate from a single point on the retina (a or b, Figure 2), not spreading out from its real origin. Even granted this assumption, the blood vessels on this side of the globe (right) (i) must cast their shadows across to the other side (left) (α), thus being superimposed on the vessels’ shadows on this side (v), for a shadow is never on the same side from whence light arrives. In reality, the projection of the total vascular tree (AB) does not appear this way.
Secondly, when the light is moved from point a to b, the vessel’s projection always moves in the same direction from A to B (right to left). The theory assumes that these distances ab and AB are directly proportional, and therefore, the position of point v (the vessel) may be geometrically calculated. In reality, although no direct fixed ratio exists, that is, small movements of the light (a–b) may cause large movements of the projection (A–B) or small ones, and large movements may result in small displacements of the image, or none at all, as Purkinje himself and Brewster already remarked. A variable ratio cannot simply be submitted to geometrical deductions.
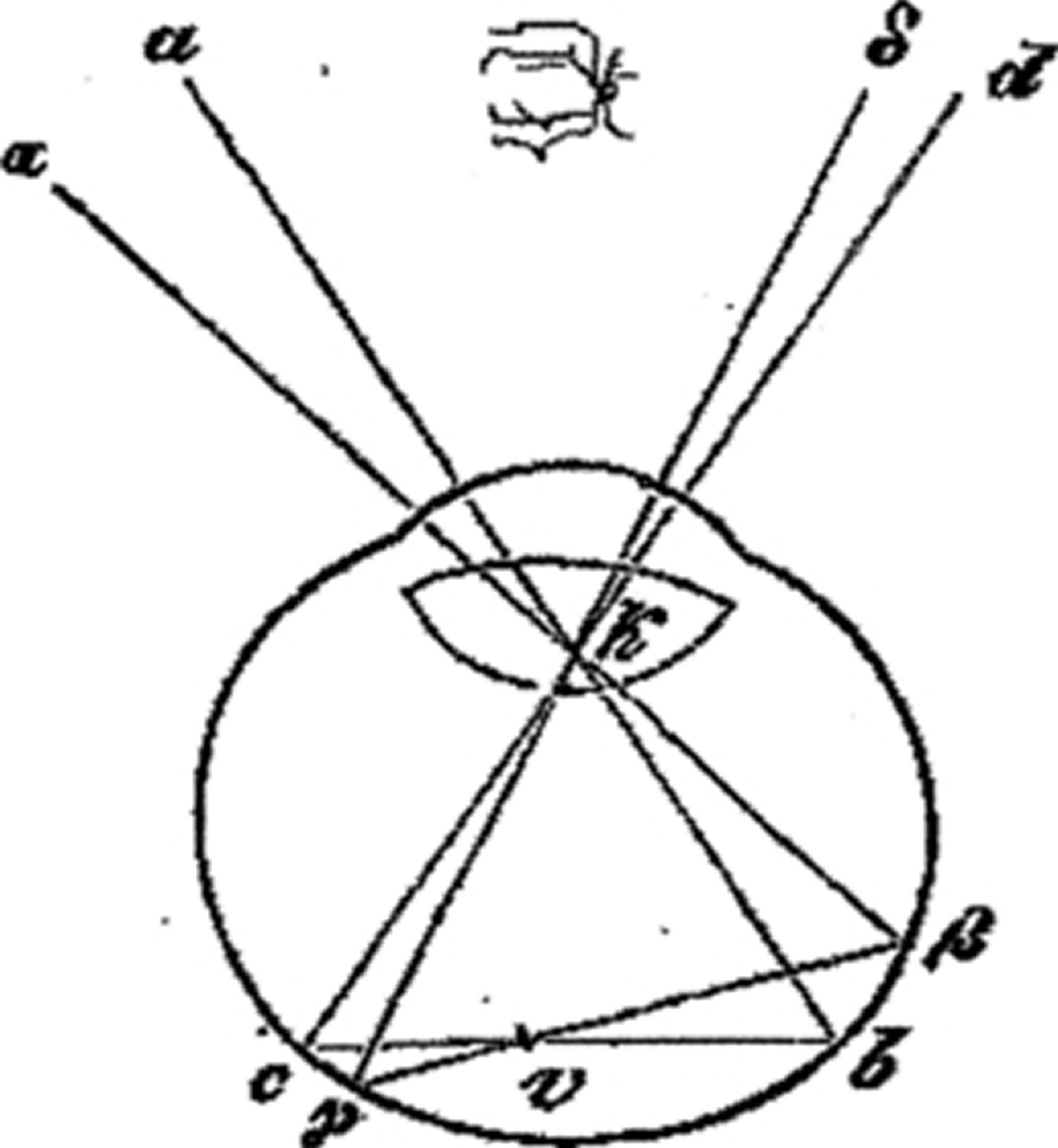
Thirdly, the seen projection of the vascular tree is always inverted (αβ is seen as AB, the nasally located blind spot, for instance, is seen laterally). When the light is shaken in place in front and to one side of the eye (at location A or at B), the projected image of the vessels moves in the same direction as the light, as noted above when light falls on the sclera, but surprisingly, when the light originates at A (aα Figure 3), the image is located across the midline at B (δd Figure 3), and vice versa, when the light source is at B, the image moves to A, that is, it moved in the opposite direction. When the light source is at αa (left Figure 3), the image of vessels is to the right of it, and when it moves to γd, the image moved to the left of it 12. Following Meissner’s hint 13. Müller fit his theory to accommodate this contradiction by suggesting that the light source does not directly illuminate the blood vessels (v) but is first reflected off the retina on the opposite side (bβ and cγ). In this way, when the light from αa projects the vessel to δd, and then moves to δd, the image of the vessel is projected to αa in the opposite direction, as observed (Figure 3).
Figure 2. Entoptic phenomenon
Footnote: The candle’s light is assumed to originate at a, the vessel’s image is projected to A.
[Source 14 ]Figure 3. Entoptic phenomenon
Footnote: Vessels’ image remains centred no matter whether light arrives from the right or left; it moves to the left when the light moved to the right – in opposite direction – unless light is assumed to reflect from the retina at b β.
[Source 14 ]Entoptic phenomena clinical application
Entoptic phenomena of the vascular tree was sometimes used clinically to determine potential visual acuity, where nonperception of the vessels strongly suggested poor acuity 15. The test correctly identified 91% of eyes with good macular function, and if no vascular pattern was seen, it was probable that the eye had poor macular function 16. More than half the untrained patients with diabetes were able to visualize entoptically their own parafoveal retinopathy 17. Some advocated the method for regular examination of the retina 18.
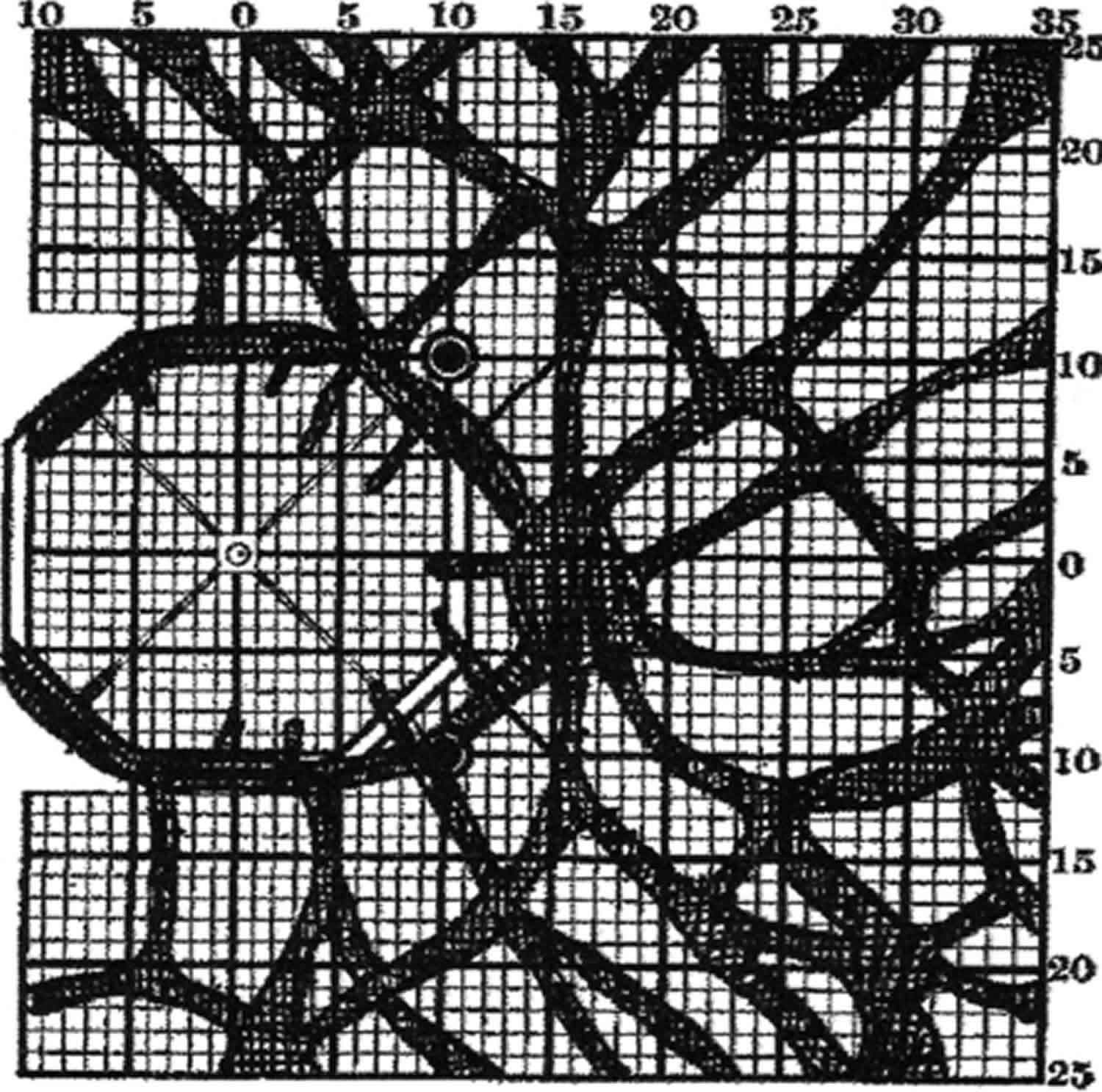
Under strictly controlled artificial circumstances, it is possible to show objectively that retinal blood vessels do in fact prevent some light from reaching the photoreceptive layer of the retina, and in this manner made to cast shadows. The method was variously termed skiaskotometry (Greek: ςκια‐ shadow; ςκóτος– darkness) (Figure 4), scotometry 19, angioscotometry 20. It seems of little clinical use 21 and Evans lamented ‘the erroneous impression that the mapping of retinal‐vessel shadows is advocated for clinical study’.
Figure 4. Retinal vessel scotoma
Blue field entoptic phenomenon
Blue arc entoptic phenomena were first observed by Dr. Purkinje in the early 19th century after viewing the embers of a fire in the dark, consist of transient and varying shades of blue arches, but did not completely understand their significance 22. Subsequent investigation 23 indicated that the shape of the perceived blue arc images is strongly influenced by the anatomic topology of the nerve fiber layer. Researchers believe they arise in response to the stimulus of the blue-yellow wavelength systems, and follow the distinct anatomy of the nerve fiber layer; potential exists to use this to help practitioners diagnose and monitor very early stages of glaucoma 24. Other theories speculate that leukocytes, moving within one’s own retinal capillaries, mediate the blue field entoptic phenomenon 25. Blue arc entoptic phenomenon is inversely correlated with the degree of amblyopia, with studies concluding that its loss varies with the severity of the condition 26.
Various investigators reported on the optimum conditions for demonstrating the blue arc entoptic phenomenon. While stimuli of various shapes and sizes are capable of producing the blue arc entoptic phenomenon, vertical rectangular stimuli are best for eliciting the response 22. The arcs are best seen in the dark after a 2- to 3-minute period of exposure to room light. The response is transient, lasting only approximately 0.5 seconds, and occurs immediately upon stimulus presentation 22.
The mechanism for how the blue arc entoptic phenomenon is generated remains unknown. The arcs are always varying shades of blue, depending on the degree of dark adaptation. The optimum amount of dark adaptation (2 to 3 minutes) produces brilliant powder blue arcs, but these can fade to grey and ultimately white with longer periods of dark adaptation 27. Regardless of the color of the stimulating light, the entoptic arcs are always varying shades of blue, but red light seems to be the best for generating the blue arcs 28. Ingling and Drum 27 proposed that the blue color of the arcs is related to activation of the blue-yellow opponency system when presenting stimuli to partially dark-adapted retina. The short, medium, and long wavelength cones combine in antagonistic ways to produce two parallel color systems with connections to specified retinal ganglion cells: the blue-yellow system (where yellow results from a combination of long and medium wavelength cones that are opposed by short wavelength cones), which transmit via bistratified retinal ganglion cells 29 and the red-green system (where long wavelength cones are opposed by medium wavelength cones), which transmits via midget retinal ganglion cells 30.
Electrophysiological studies indicate that rods and short-wavelength cones (also known as blue cones) provide inhibitory input to the peripheral portion of the receptive field of blue/yellow color opponency ganglion cells, whereas red (long wavelength) and green cones (medium wavelength) contribute positive, central yellow-on neuronal activity 31. Immediately after shutting the lights off for a light-adapted subject, there is residual stimulation of the center of the field by red and green cones and peripheral inhibition by the rods. The simultaneous center-surround stimulation shuts off the red and green cones and activates the blue (short wavelength) cones. For stimulus presentation in the early dark adaptation period, rod inhibitory input accompanied by activation of short-wavelength cones and their specified ganglion cell axon elicits the blue arc entoptic phenomenon. Exactly how the blue cone–retinal ganglion cell axon complex is stimulated is unknown, but a bioluminescence phenomenon has been ruled out 32. As the eye continues to dark adapt under scoptic conditions, yellow-on activity subsides and the arcs seen on stimulus presentation turn gray and eventually diminish altogether. Extinction of the blue arc imagery occurs because the center-on portion of the receptive field no longer requires peripheral inhibitory input by short wavelength cones, as the latter become inactive.
Duke-Elder’s text mentions that the blue arcs are obliterated in patients with visual scotomas, but no investigator has explored the clinical utility of the blue arc entoptic phenomenon 33. Pasquale and Brusie 24 hypothesized that the blue arc entoptic phenomenon is depressed in glaucoma for two reasons. First, NFL dropout occurs in glaucoma, and the blue arc entoptic phenomenon is strongly influenced by the nerve fiber layer. Second, the blue arc entoptic phenomenon may depend on intact circuitry of the blue-yellow opponent system, and these circuits are defective in early glaucoma, as demonstrated by short-wavelength automated perimetry 34. In their study, the inability to perceive the blue arcs correlated with structural and functional features associated with glaucoma, although older age and media opacity were also predictors of this entoptic response 24.
Blue field entoptic phenomenon causes
Blue field entoptic phenomena are different from optical illusions, which are perceptual effects occurring within the brain. Most entoptical phenomena have a direct, known physical cause. However, like optical illusions or hallucinations, the observer of an entoptical effect cannot give others a direct view of what he or she observes. Helmholtz 35 commented on entoptic phenomena which could be seen easily by some observers, but could not be seen at all by others. This variance is not surprising because the specific aspects of the eye that produce these images are unique to each individual. Because of the variation between individuals, and the inability for two observers to share a nearly identical stimulus, these phenomena are unlike most visual sensations. They are also unlike most optical illusions which are produced by viewing a common stimulus. Yet, there is enough commonality between the main entoptic phenomena that their physical origin is now well understood.
Some examples of entoptical effects include:
- Floaters or muscae volitantes are slowly drifting transparent blobs of varying size and shape, which are particularly noticeable when lying on the ground looking up at the sky. They are caused by imperfections in the fluid of the eye.
- Moore’s lightning streaks. These are also a commonly encountered photopsia, described as the flash of light that many patients experience in cases of anomalous posterior vitreous detachment, where vitreous liquefaction precedes the weakening of vitreoretinal adhesions, resulting in instances of retinal traction 24.
- The blue field entoptic phenomenon has the appearance of tiny bright dots moving rapidly along squiggly lines in the visual field. It is much more noticeable when viewed against a field of pure blue light and is caused by white blood cells moving in the capillaries in front of the retina.
- Glaucoma 24
- Haidinger’s brush is a very subtle yellow-and-blue pattern that is seen when viewing a field of light that is polarized.
- Purkinje Tree is an image of the retinal blood vessels in one’s own eye. It can be seen by shining a bright, moving light like a penlight onto the sclera (the white of the eye) in a darkened room. Normally the image of the retinal blood vessels is invisible because of adaptation. The unusual angle casts the image onto unadapted portions of the retina. Unless the light moves, the image disappears within a second or so. If the light is moved at about 1 Hz, adaptation is defeated, and a clear image can be seen indefinitely. The vascular figure is often seen by patients during an ophthalmic examination when the doctor is using an ophthalmoscope. In the process of aligning the instrument so that the doctor can view the blood vessels through the pupil, the light from the instrument often falls briefly on the sclera, so that the patient gets a quick glimpse of the vascular figure.
- A phosphene is the perception of light without light actually entering the eye, for instance caused by pressure applied to the closed eyes.
- Purkinje images are the reflections of objects from within the eye. For example, a small spot of light in an otherwise dark room can be seen directly and also as a dim reflection, probably from light reflecting from the anterior surface of the lens then back into the eye from the rear of the surface of the cornea. Sometime an even dimmer reflection can be seen, probably from light reflected from the posterior surface of the lens then reflected back into the eye from the cornea or from the anterior surface of the lens.
A phenomenon that could be entoptical if the eyelashes are considered to be part of the eye is seeing light diffracted through the eyelashes. The phenomenon appears as one or more light disks crossed by dark blurry lines (the shadows of the lashes) each having fringes of spectral colour. The disk shape is given by the circular aperture of the pupil.
The most commonly observed pathological phenomenon is the shadow cast by vitreous floaters, appearing as either a black spot, in the case of posterior vitreous detachment with Weiss ring, or colorless ellipses thought to be due to embryonic remnants or proteins in the vitreous. These constituents cast a shadow on the retina and elicit the symptomatic presentation.
Blue field entoptic phenomenon treatment
Blue field entoptic phenomenon treatment involves treating the underlying cause.
- Mark HH. The entoptic view of the retinal vessels. Acta Ophthalmologica. 2014;92(3):e237-40. https://doi.org/10.1111/aos.12192[↩][↩][↩]
- Westheimer G. Entoptic Phenomena. In: Kaufman PL, Alm A, editors. Adler’s Physiology of the Eye: Clinical Applications. 10th ed. St Louis: Mosby; 2003. pp. 441–452.[↩]
- Moore RF. Subjective lightning streaks. Br J Ophthalmol. 1935;19(10):545–547.[↩]
- Purkinje J (1819): Beiträge zur kenntniss des sehens in subjectiver Hinsicht. Prag: J.G Calve.[↩][↩]
- Cappola D, Purves D. The extraordinary disappearance of entoptic images. Proc Natl Acad Sci USA. 1996;93(4):8001-4.[↩][↩]
- vom Hofe K (1941): Untersuchungen über das Verhalten eines zentrales optischen Nachbildes bei und nach unwillkürlichen Bewegungen sowie mechanischen Verlagerungen des Auges. Albrecht Von Graefe Arch Klin Exp Ophthalmol144: 164–169.[↩]
- Göthlin GF (1927): Die Bewegungen und die physiologischen Kosequenzen der Bewegung eines zentralen optischen Nachbildes. Zentralbl f d ges Ophth19: 289–290.[↩]
- Dwyer J, Ashton R & Broese J (1990): Emmert’s law in the Ames room. Perception19: 35–41.[↩]
- Fishman GA, Birch DG, Holder GE et al. (2001): Electrophysiologic testing. Am Acad Ophthalm2001: 2–10.[↩]
- Duke‐Elder S (1962b): System of ophthalmology, Vol. 7. St Louis, MO: CV Mosby 454–455.[↩][↩]
- Müller H (1855) Über die entoptische wahrnehmung der netzhautgefässe, insbesondere als beweismittel für die lichtperception durch die nach hinten gelegenen netzhautelemente. Würzburg: Stahel.[↩]
- Duke‐Elder S (1968): System of ophthalmology, Vol. 4. St Louis, MO: CV Mosby 441, 663, 671–672.[↩]
- Meissner G (1854): Beiträge zur physiologie des sehorgans. Leipzig: Engelmann 76–84.[↩]
- Mark, H. H. (2014), The entoptic view of the retinal vessels. Acta Ophthalmologica, 92: e237-e240. doi:10.1111/aos.12192 https://onlinelibrary.wiley.com/doi/full/10.1111/aos.12192[↩][↩]
- Murillo‐Lopez F, Maumenee AE & Guyton DL (2000): Perception of Purkinje vessel shadows and foveal granular pattern as measure of potential visual acuity. J Cataract Refract Surg26: 260–265.[↩]
- Talbot EM, Murdoch JR & Keating D (1992): The Purkinje vascular entopic test. Eye6: 322–325.[↩]
- Applegate RA, Bradley A, Van Huven WA et al. (1997): Entopic evaluation of diabetic retinopathy. Invest Ophthalmol Vis Sci38: 783–791.[↩]
- Eber SI (1922): Autoophthalmoscopy, subjective examination of the retina. Am J Ophthalmol5: 973–974.[↩]
- Evans JN (1938): Clinical scotometry. London: Oxford University Press.[↩]
- Goldmann H (1947): Beitrag zur Angioskotometrie. Ophthalmologica114: 147–158.[↩]
- Harms H, Aulhorne E (1959): Comparative studies on the value of quantitative perimetry, skiaskotometry and fusion frequency for the recognition of beginning visual field disorders in glaucoma. Doc Ophthalmol13: 303–356.[↩]
- Moreland JD. On demonstrating the blue arcs phenomenon. Vision Res. 1968;8(8):99–107.[↩][↩][↩]
- Dolecek RL, DeLaunay J. Entoptic mapping of the Purkinje blue arcs. J Opt Soc Am. 1945;35:676–680.[↩]
- Pasquale LR, Brusie S. The blue arc entoptic phenomenon in glaucoma (an American ophthalmological thesis). Trans Am Ophthalmol Soc. 2013;111:46–55. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3797830[↩][↩][↩][↩][↩]
- Grunwald J, Sinclair S, Crandall A, Riva C. Blue field entoptic phenomenon in amblyopia. Ophthalmology. 1981 Oct;88(10):1054-7.[↩]
- Applegate R, Bradley A, van Heuven W, et al. Entoptic evaluation of diabetic retinopathy. Invest Opthalmol Vis Sci. 1997;38(5):783-91.[↩]
- Ingling CR, Drum BA. Why the blue arcs of the retina are blue. Vision Res. 1977;17(3):498–500.[↩][↩]
- Moreland JD. The blue arcs of the retina. In: Richter M, editor. Proceedings of the International Colour Meeting Lucerne 1965. Vol. 1. Göttingen: Musterschmidt; 1966. pp. 173–186.[↩]
- Dacey DM, Lee BB. The ‘blue-on’ opponent pathway in primate retina originates from a distinct bistratified retinal ganglion cell type. Nature. 1994;367(6465):731–735.[↩]
- Leventhal AG, Rodieck RW, Dreher B. Retinal ganglion cell classes in the Old World Monkey: morphology and central projections. Science. 1981;13(4512):1139–1142.[↩]
- Calkins DJ. Seeing with S cones. Prog Retin Eye Res. 2001;20(3):255–287.[↩]
- Moreland JD. Threshold measurements of the blue arcs phenomenon. Vision Res. 1968;8(8):1093–1106.[↩]
- Duke-Elder WS. Textbook of Ophthalmology. Vol. 1. St Louis: CV Mosby; 1940. Entoptic phenomena associated with retinal structures; pp. 816–817.[↩]
- Johnson CA, Adams AJ, Casson EJ, Brandt JD. Blue-on-yellow perimetry can predict the development of glaucomatous visual field loss. Arch Ophthalmol. 1993;111(5):645–650.[↩]
- Helmholtz H (1896): Handbuch der physiologischen optik. Hamburg & Leipzig: Voss 192– 202.[↩]