What is fucoidan
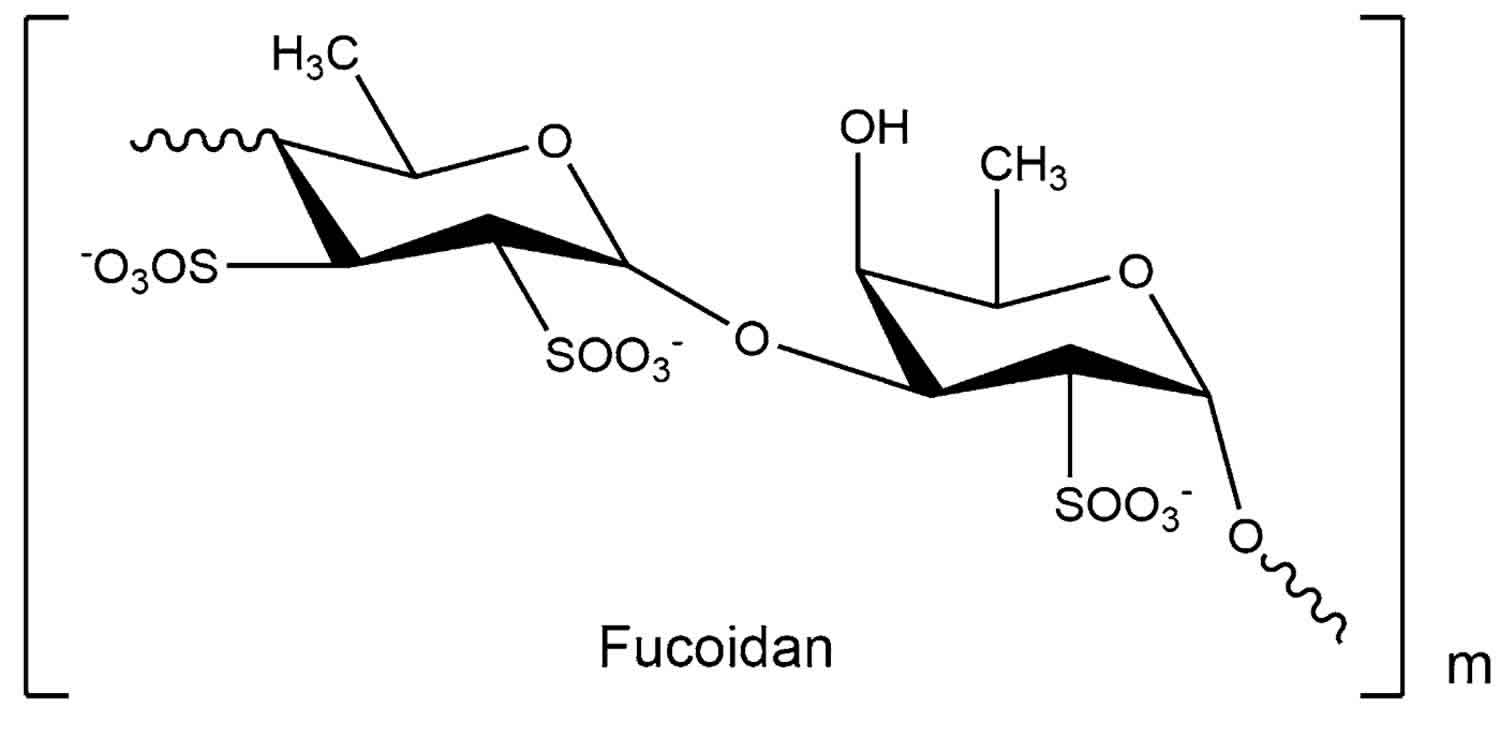
Fucoidan is a group of high molecular weight, fucose-based polysaccharides largely made up of l-fucose and sulfate groups that is recognized as a key component of particular brown marine algae species such as Laminaria digitata, Ascophyllum nodosum and Fucus vesiculosus 1 and more recently identified in seagrasses 2. The total content of fucoidan may vary between 20 and 30 % of algae dry weight and it depends on the type of seaweed 3. Fucoidan occurs in all brown algae in different ratio and it is in the intercellular tissues and is considered to be a substance used by the weeds to protect themselves against the effects of drying out when exposed. There are two different forms of fucoidan such as F-fucoidan, which is >95 % composed of sulphated esters of fucose, and U-fucoidan, which is approximately 20 % glucuronic acid 3. The sulphated polysaccharide in fucoidan consists mainly of L-fucose units; at the same time it can also contain minor amounts of sugars such as galactose, mannose, xylose, or uronic acid and sometimes proteins. This is varying between different species 3. Fucoidan exhibits many biological activities which include anti-inflammatory, anticell proliferation, antiadhesion, antiviral, anticoagulant, antitumor and antiviral activities 3. Fucoidan is absorbed through the gut epithelium into the systemic circulation, although its oral bioavailability is low 4. The modest benefits of fucoidan appear to be indirect, due to modulation of the intestinal environment where it is a good source of fiberand acts as a microbiota-assessible carbohydrate, rather than the direct activities seen in cell culture studies 5. Consequently, benefits may depend on an intestinal environment/microbiome that is capable of utilizing the fucoidan. Most clinical studieswith evidence of benefits, albeit minor ones, were conducted in Japan, where fucoidan rich seaweed is a normal part of the diet, thus people there may already have microbiome more conducive to extracting benefits from fucoidan.
The brown seaweeds containing fucoidan are widely consumed as part of the normal diet in East Asia, particularly in Japan, China, and Korea 6. Wakame blades (Undaria pinnatifida) are green when cooked and have a subtly sweet flavor and satiny texture. The blades are normally cut into small pieces as they tend to expand during cooking. In Japan and Europe, wakame (Undaria pinnatifida) is consumed either dried or salted. It is mainly used in soups (particularly miso soup) and salads (tofu salad), or simply used as a side dish. These dishes are typically dressed with soy sauce and vinegar/rice vinegar. In addition, Goma wakame, also known as seaweed salad, is a popular side dish at American and European sushi restaurants. Literally translated, it means “sesame seaweed”, as sesame seeds and oil are usually included in the recipe.
The bioactive properties of fucoidan preparations have been assessed in a range of in vitro and animal models 7 and include demonstrated antimicrobial 8, antiviral 9 and anticancer 10 effects. While evidence from animal models suggests fucoidans may also possess immune-modulating effects 11, the ability of fucoidan to act as potential modulators of mucosal health generally, and mucosal immune function appears underappreciated and requires further investigation given (i) the fucose-based structure of fucoidans, (ii) the role of fucose as a terminal sugar in human mucin glycoproteins 12, and (iii) evidence from ex vivo tissue preparations suggesting fucose may regulate gut motility 13.
In clinical and animal studies, dietary fucoidan have been shown to increase innate immunity and decrease inflammation 14. In a clinical study, ingestion of fucoidan was shown to restore a marker of gut innate immunity (lysozyme) to normal levels in athletes 15. As a known selectin blockade agent, fucoidan has been shown to act as an anti-inflammatory 16. In addition, dietary fucoidan has been shown to attenuate pulmonary damage in a model of acute viral infection 17.
Currently, fucoidans are available for use in cosmetics, functional foods, dietary supplements and for inclusion in pet, livestock and aquaculture feed supplements 18. Fucoidan has been consumed for a long time in Japan, China, and Korea as part of whole seaweed, and it is used as nutraceuticals in Australia and the United States. The fucoidan materials are also used in cosmetics because their ability to absorb directly by the human skin with the following different effects includes whitening, preserving moisture, removing freckles 3. A fucoidan preparation called ‘Haikun Shenxi capsules’ was approved in China in 2003, and its clinical use as a therapeutic for chronic renal failure 19. There is now regulatory approval in the US and Europe for the use of fucoidan in supplements and cosmetics 7. There have been new developments in assay techniques for measuring fucoidan and establishing bioavailability. The first clinical trial on intravenous delivery of a radiolabelled fucoidan has now been reported 20. This encouraging research focuses on the use of fucoidan to image thrombi and may become the first intravenous clinical application of fucoidan.
The effects of fucoidan on microbiome is an emerging area of focus. Global concern regarding the increase of drug-resistant superbugs and the lack of new antibiotics for treating human and animal diseases has led to a call for new approaches. In agriculture, there is an urgent need to develop strategies to replace antibiotics for food-producing animals, especially poultry and livestock. In human health, there is increasing awareness of a connection between the microbiome and disease conditions. Fucoidans have bacteria-inhibiting qualities against the ulcer-causing Helicobacter pylori 21 and modulate the growth and biofilm-forming properties of other types of bacteria 22. Additional antiviral activity and the anti-inflammatory nature of fucoidans 18 make them suitable for a wide range of digestive tract applications. In particular, fucoidan can attenuate inflammation generated by lipopolysaccharides produced by Gram-negative bacteria 23. New research demonstrates activity against norovirus, for which there are no current treatments 24. Perhaps much of the biological activity ascribed to fucoidans may be due their effects on modulating microbiome and inflammation from the oral cavity and throughout the length of the gut 7.
However, no fucoidan has been declared as a drug 14. The reason is that the structural diversity of fucoidan is extremely large. Fucoidan represents the family of fucose-containing homo- and heteropolysaccharides from polysaccharides with a high content of uronic acids and low fucose and sulfate contents, for example, from Hizikia fusiforme alga, for practically pure α-L-fucans with the main component of polysaccharide-fucose (brown alga Fucus evanescens). Except for fucose, these polysaccharides can contain minor amounts of other monosaccharides (galactose, mannose, xylose, glucose) and also sulfates, uronic acids, acetyl groups, and protein 25. In particular, fucoidan from Fucus evanescens has a similar l-fucosyl backbone of alternating α (1→4) and α (1→3) l-fucosyls with sulphate substitution at C2. An additional sulphate may occupy position 4 in some of the α (1→3)-linked fucosyls, and the remaining hydroxyl groups may be randomly acetylated 26.
Fucoidan chemical structure
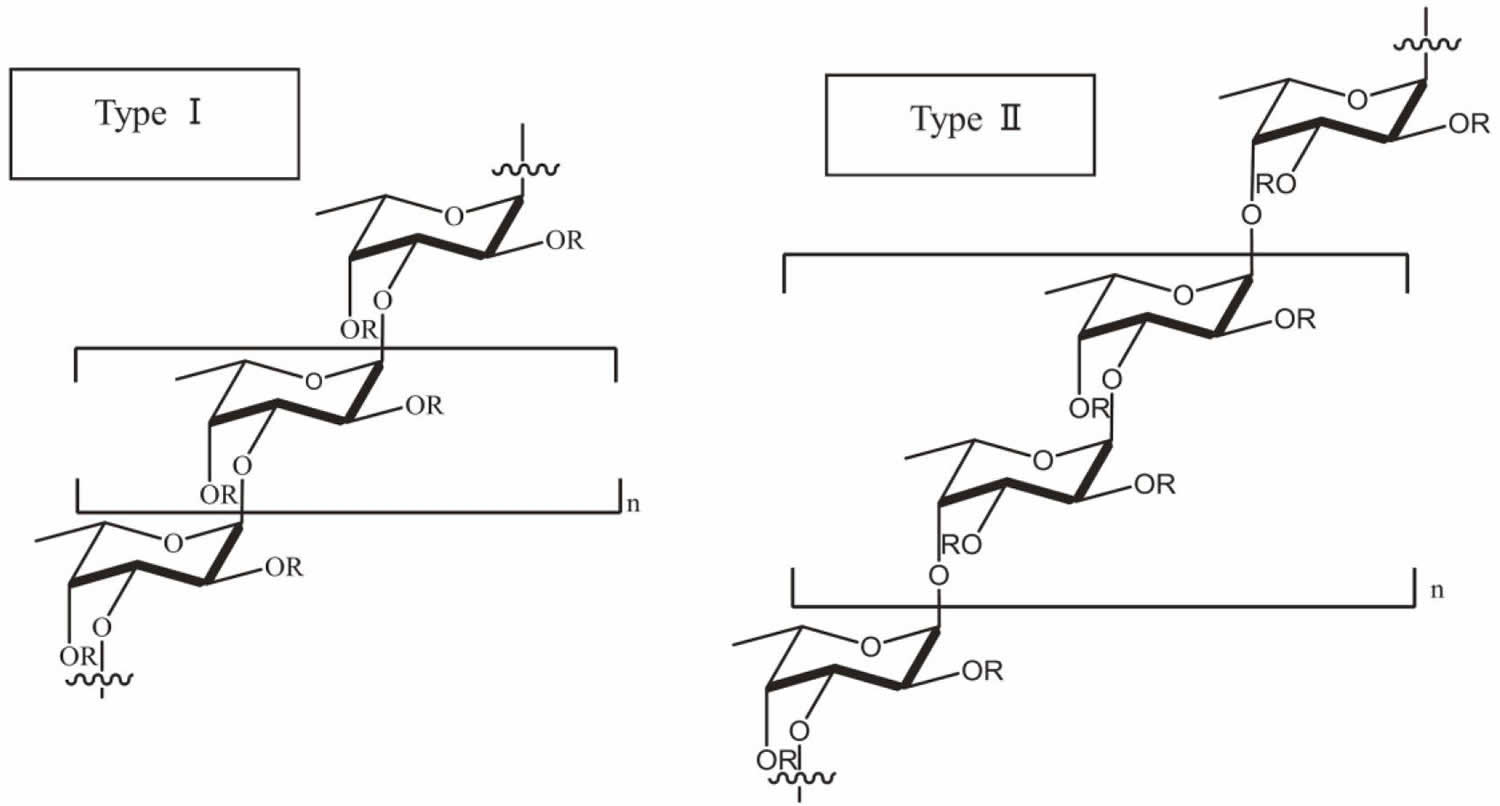
Fucoidan is made up of l-fucose, sulfate groups and one or more small proportions of xylose, mannose, galactose, rhamnose, arabinose, glucose, glucuronic acid and acetyl groups in a variety of brown algae 27. In a number of studies, researchers have also used galactofucan to represent a kind of fucoidan. Galactofucan is known as a monosaccharide and the composition of the monosaccharide is galactose accompanied by fucose, similar to rhamnofucan (rhamnose and fucose) and rhamnogalactofucan (rhamnose, galactose and fucose). In addition to the structure of fucoidan, there is also a variation amongst different seaweed types. Nevertheless, fucoidan normally has two types of homofucose (Figure 2). One type (I) encompasses repeated (1→3)-l-fucopyranose, and the other type (II) encompasses alternating and repeated (1→3)- and (1→4)-l-fucopyranose 28.
By a way of illustration, most of the fucoidans sourced from species belonging to the Fucales have an alternating linkage of (1→3)-α-l-fucose and (1→4)-α-l-fucose 29. Structures of Ascophyllum nodosum fucoidan 30 and Fucus vesiculosus fucoidan show a resemblance of one another, the difference is only significant based on sulfate patterns and the presence of glucuronic acid. A number of Fucales species, such as Fucus serratus, Fucus distichus and Pelvetia canaliculate, present similar fucoidan backbone, but show more diversity in the branching and the presence of different monosaccharides 31. However, exceptions do exist, for instance, fucoidans from the Bifurcaria bifucardia and Himanthalia elongate do not follow or ascribe to such a structural feature 32. Hence, identifying the structure of fucoidan based on the species they belong to presents a challenge.
Another important fact that deserves mentioning is that the structure of fucoidan is also highly dependent on the harvest season. This is based on the Undaria pinnafida fucoidan, which exhibited distinct characteristics and bioactivity, especially when harvested during different seasons 33. In addition, it should be indicated that the purification method also plays a critical role in the structure of fucoidan. To such an extent that new purification methods have led to the revelation that the fucoidan structure is comprised of multiple fractions 34. An investigation reported that the structure of crude fucoidan sourced from Ascophyllum nodosum showed a predominant repetition of [→(3)-α-l-Fuc(2SO3−) − (1→4)-α-Fuc(2,3diSO3−)-(1)]n 35. However, from the same species, a purified fraction comprised of primarily α-(1→3)-fucosyl residues with a sparse linkage of α-(1→4) and was found to be highly branched 36. Therefore, the employment of different extraction methods results in distinct structures. For example, a report states that one species produced two distinct fucoidan structures, particularly galactofuctans and uronofucoidans 37. Hence, it should be emphasized that purification techniques are one of the determining factors towards the structure and the associated bioactivities.
Figure 1. Fucoidan chemical structure
Footnote: Structure of fucoidan from Fucus evanescens.
Figure 2. Chemical structure of fucoidan
Footnotes: Type I and type II of common backbone chains in brown seaweed fucoidan. R can be fucopyranose, glucuronic acid and sulfate groups, while the location of galactose, mannose, xylose, rhamnose, arabinose and glucose in several kinds of seaweed species remains unknown.
[Source 1 ]Fucoidan sources
Fucoidan is a sulfated polysaccharide which can be found amongst a number of marine sources, including sea cucumbers 38 or brown algae 39. A great number of algae and invertebrates have been ascertained for their fucoidan contents inclusive of Fucus vesiculosus, Sargassum stenophyllum, Chorda filum, Ascophyllum nodosum, Dictyota menstrualis, Fucus evanescens, Fucus serratus, Fucus distichus, Caulerpa racemosa, Hizikia fusiforme, Padina gymnospora, Kjellmaniella crassifolia, Analipus japonicus and Laminaria hyperborea shown in Figure 3. In these sources, different types of fucoidan can be obtained and the methods of extraction employed are different, especially when they are reported in different studies 1.
Fucoidan is usually extracted from the sporophyll of Undaria pinnatifida 40. However, a key difference between fucoidan from Undaria pinnatifida and those from of other brown seaweed species such as Fucus vesiculosus lies in the composition of monosaccharides that form the backbone of the polysaccharide molecule 41. Fucoidan from Undaria pinnatifida is sulfated galactofucan 42. In contrast, fucoidan isolated from the vast majority of other brown seaweeds mainly consists of sulfated fucose 42. Literature shows that sulfate content, monosaccharide composition, and structural conformation of fucoidan affect its biological activity 43. As such, it is suggested that fucoidan from Undaria pinnatifida with different monosaccharide composition and structural conformation, would possess a wide range of biological activities, which offers itself as an attractive functional ingredient of health products 44.
The brown seaweed species Undaria pinnatifida is native to the cold temperate seas of China, Japan, and Korea, and has been introduced in many other places including the Europe Atlantic, French Mediterranean, Australia, and New Zealand 6. It is regarded as a highly invasive species with a high tolerance for light, temperature, and salinity 45. It is also highly fertile with high growth rate and large reproductive output, releasing spores all year round 46. It is farmed extensively in Japan, Korea, and Japan and as such, it is an abundant source from which fucoidan could be extracted and used.
Figure 3. Sources of fucoidan
Footnotes: 1. Fucus vesiculosus, 2. Laminaria digitata, 3. Fucus evanescens, 4. Fucus serratus, 5. Ascophyllum nodosum, 6. Pelvetia canaliculata, 7. Cladosiphon okamuranus, 8. Sargassum fusiforme, 9. Laminaria japonica, 10. Sargassum horneri, 11. Nemacystus decipiens, 12. Padina gymnospora, 13. Laminaria hyperborea.
[Source 1 ]Fucoidan supplement
In recent years, fucoidan extracts have attained regulatory approvals in a number of global jurisdictions for use in foods and dietary supplements. In particular, fucoidan extracts from Undaria pinnatifida and Fucus vesiculosus were filed as ‘Generally Recognised as Safe’ (GRAS) with the US FDA by the Australian manufacturer, Marinova 47. The FDA had no further questions in relation to the two GRAS determinations, which permit daily consumption of high-concentration fucoidan extracts from Undaria pinnatifida or Fucus vesiculosus at rates of up to 250 mg/day 7.
In the European Union, the same fucoidan extracts from Undaria pinnatifida and Fucus vesiculosus were assessed by the European Commission and found to be substantially equivalent to the parental seaweeds from which they are extracted, and hence were approved as novel foods under the Commission Implementing Regulation (EU) 2017/2470 of 20 December 2017, for consumption up to 250 mg/day 7.
In Canada and Australia, the respective agencies have approved a number of listed medicines containing fucoidan extracts. In Australia, fucoidan has been approved in a species-specific context for both Undaria pinnatifida and Fucus vesiculosus. They are recognised as listable components of their parental herbal (seaweed) source 7.
Fucoidan health benefits
The first clinical trial on intravenous delivery of a radiolabelled fucoidan has now been reported 20. The ability of fucoidan to bind to p-selectin groups on platelets assists in the imaging of blood flow-limiting thrombi. This application may become the first non-oral clinical application of fucoidan.
Reports of orally delivered clinical use of fucoidan have increased in the last few years. A recent paper outlines the use of fucoidan from Saccharina japonica to address kidney disease in China 48. This traditional Chinese medicine formulation shows promise for use in other parts of the world and is supported by studies in the literature detailing the use of fucoidan to treat kidney disease in a variety of animal models. Mesenchymal stem cells are a class of cells that show promise in regenerating diseased organs 49. Currently, the damage caused to mesenchymal stem cells by uremic toxins makes kidney mesenchymal stem cell transplantation unfeasible. However, fucoidan from Fucus vesiculosus reversed senescence caused by the uremic toxin p-cresol, indicating a potential support for both resident and transplanted mesenchymal stem cells 50.
Following on from an animal study showing inhibition of inflammation in ethanol-induced gastritis 51, a clinical study in young Chinese men showed a substantial—and no doubt welcome— decrease in gastritis (cause undisclosed) after ingestion of a blend of fucoidan and wheat peptides 52.
Additional studies have demonstrated fucoidan utility in reducing inflammation in cancer patients 53. No interactions or adverse effects were observed with the commonly used hormone therapies tamoxifen and letrozole in breast cancer patients. Reductions in joint pain were also noted following co-administration of fucoidan from Undaria pinnatifida 54.
Fucoidan exerts anti-inflammatory effects by reducing the production of proinflammatory cytokines 55. Orally administered fucoidan reduces the levels of proinflammatory cytokines, including interleukin 1β (IL-1β) and IL-6, in patients with advanced cancer 56. Similarly, orally administered fucoidan has been shown to reduce radiation-induced pneumonitis and lung fibrosis in a mouse model 57. Orally administered fucoidan also exerts antithrombotic effects by inducing biosynthesis of prostacyclin 58. The crude extract of the brown seaweed Cladosiphon okamuranus (mozuku) consistently exerted antithrombotic effects in rats after 8 weeks of oral administration, likely through the fucoidan in the extract 59. Another study shows that low-molecular-weight fucoidan prepared in the laboratory has greater antithrombotic activity and oral bioavailability than middle-molecular-weight fucoidan 60.
Fucoidan extracted from Fucus vesiculosus has been known as an alpha-glucosidase inhibitor that is able to treat diabetes 61. Among other studies, fucoidan was mentioned to have an ability to attenuate diabetic retinopathy through inhibiting vascular endothelial growth factor (VEGF) signaling 62. Additionally, a report of a low molecular-weight fucoidan was noted to provide protection against diabetic associated symptoms in Goto-Kakizaki rats 63. Fucoidan also improves glucose tolerance by modulating AMP-activated protein kinase (AMPK) signaling and GLUT4 activity 64. Some studies mention that fucoidan from the sea cucumber Pearsonothuria graeffei with a molecular weight of 310 kDa can be used as a form of functional food to treat metabolic syndromes 65. Fucoidan from the sea cucumber Pearsonothuria graeffei enabled weight reduction in high fat diet-fed mice, it also reduced hyperlipidemia, and protected the liver from steatosis. Concurrently, fucoidan from the sea cucumber Pearsonothuria graeffei reduced the serum inflammatory cytokines combined with reduced macrophage infiltration into adipose tissue. Furthermore, it was declared that the treatment effect for metabolic syndrome was primarily related to the 4-O-sulfated structure of fucoidan, since it was identified as a tetrasaccharide repeating unit with a backbone of [→3Fuc (2S, 4S) α1→3Fucα1→3Fuc(4S) α1→3Fucα1→]n.
Fucoidan administration improved taste sensitivity in diabetics 66, whilst a study on obese, but non-diabetic, subjects using a fucoidan-polyphenol complex from Fucus vesiculosus showed no effects on glucose or insulin resistance 67.
Fucoidan is recognized as a prebiotic to regulate the intestinal ecosystem or microbiome 68. Fucoidan promotes the growth of beneficial bacteria which represents a mechanism inhibiting the development of metabolic syndromes 69. A report by Parnell et al. 70 showed that prebiotics containing fucoidan can regulate blood glucose and metabolism by providing a beneficial environment for the growth stimulation of probiotics. Cheng et al. 71 also demonstrated that Sargassum fusiforme fucoidan could modify gut microbiota during the alleviation of streptozotocin-induced hyperglycemia in mice. The yield of Sargassum fusiforme fucoidan was 6.02%., with sulfate content up to 14.55% and the average molecular weight of 205.8 kDa. This study was done with diabetic mice where after a 6-week administration, Sargassum fusiforme fucoidan impressively decreased the fasting blood glucose, diet and water intake. Additionally, Sargassum fusiforme fucoidan attenuated the pathological changes in the heart and liver tissues, hence, improving liver function. Also, Sargassum fusiforme fucoidan suppressed oxidative stress in streptozotocin-induced diabetic mice which are manifestations associated with metabolic syndromes. Concurrently, Sargassum fusiforme fucoidan significantly altered the gut microbiota in diabetic mice, what was noted is Sargassum fusiforme fucoidan decreased the relative abundances of the diabetes-related intestinal bacteria, which might be the potential mechanism for relieving the symptoms of diabetes 71.
A clinical topical study using a cream containing 4% fucoidan was found to be effective for treating oral herpes 72. This topical application is supported by a substantial body of research indicating excellent inhibitory activity against herpes viruses 73.
Fucoidan has also been shown to have antiviral activity against influenza A virus 74, hepatitis B virus 75, canine distemper virus 76 and human immunodeficiency virus (HIV), mainly in vitro 77. One study in human volunteers showed that serum/plasma concentrations of fucoidan following oral ingestion of a Cladosiphon okamuranus (mozuku) extract were sufficient to exert anti-HIV activity 78. Furthermore, a more recent study by Kwon et al 79 showed fucoidan extracted from Saccharina japonica (kombu) to have strong antiviral activity against Covid-19 (SARS-CoV-2) in vitro. The activity of fucoidan against SARS-CoV-2 was even greater than that of remdesivir. Thus, fucoidan in dietary brown seaweeds might exert antiviral effects against SARS-CoV-2, at least within the intestine.
Fucoidan and cancer
Observational studies indicate that daily intake of seaweed (30 g fresh or 2 g dried) is associated with overall reduced disease risk 80 and some types of cancer, including breast cancer 81. However, there is no clear evidence that this association is mediated by the anti-cancer effects of fucoidan.
The anti-cancer properties of fucoidan have been extensively studied in test tube and animal models, however, there have only been a few small clinical trials testing fucoidan supplementation in cancer patients, and the benefits have been minimal. Bioactive polysaccharides are attractive anti-tumor agents because they typically do not act on tumor cells directly, but rather, induce changes in the host’s immune system tumor microenvironment that reduces the viability of tumor cells 82. Since the polysaccharides themselves are not directly cytotoxic, they do not pose a risk to the viability of healthy host cells. Fucoidan shows anti-tumor effects against a variety of cell cancer lines in cell culture and xenograft models, though the anti-cancer effects are highly dependent on sulfate content. In cancer cell lines, fucoidan can induce apoptosis primarily through inhibition of P13K/Akt or ERK1/2 signaling 83. Due to fucoidan low oral bioavailability, its anti-cancer potency in vivois greatly diminished, and has primarily been tested for its ability to be used as an adjunct to traditional anti-cancer therapies. The clinical trials conducted thus far suggest that fucoidan may be beneficial for cancer patients receiving chemotherapy in terms of reducing side effects/improving tolerability of the chemotherapy. In advanced colorectal cancer patients (n=20, average age 70) undergoing chemotherapy [5-fluorouracil/leucovorin (FOLFOX) or 5-fluorouracil/leucovorin (FOLFIRI)], fucoidan (4.05 g/day in 150 ml from Cladosiphon okamuranus[Okinawamozuku], Marine Products Kimuraya Co., Ltd.) for 6 months reduced chemotherapy-associated fatigue (incidence 10% with fucoidan vs. 60% in controls) 84. The fucoidan treated patients were able to tolerate more cycles of chemotherapy (19.9 vs 10.8), and this resulted in a trend toward longer survival in this cohort. Critically, a separate open-label trial in breast cancer patients (n=20) found that fucoidan treatment (500 mg 2x daily Maritech extract from Undaria pinnatifida, Marinova) had no significant effects on the pharmacokinetics of the chemotherapeutic agents letrozole, tamoxifen, or their active metabolites 85, suggesting that fucoidan does not negatively interfere with the efficacy of the chemotherapeutics. In an open-label trial in advanced cancer patients with metastases (n=20), fucoidan (4 g/day in 400 ml extracted from Cladosiphon novae-caledoniae kylin [Mozuku], Power Fucoidan, Daiichi Sangyo) for at least 4 weeks was associated with a stabilization of quality of life scores and a reduction of proinflammatory cytokines (1L-1β, IL-6, TNFα). The reduction in IL-1βwithin the first two weeks was a prognostic factor for survival (Hazard ratio, HR: 0.08) 86. A double-blind, controlled RCT in metastatic colorectal cancer patients undergoing chemotherapy (n=54) found that fucoidan (4 g powder, molecular weight 0.8 kDa, from Sargassum hemiphyllum, Hi-Q Marine Biotech) for 6 months improved the disease control rate (92.8% vs 69.2%), but had no significant effects on overall response rate or survival 87.
These studies suggest that fucoidan supplementation is not harmful, and may improve treatment tolerability or survival in some cancer patients, but has minimal to no direct anti-cancer potency.
Fucoidan appears to act both directly on cancer cells and indirectly, by increasing immune clearance of cancer cells and preventing metastasis. For example, tumour metastasis and cachexia were attenuated via the inhibition of vascular endothelial growth factor (VEGF) and matrix metalloproteases in a Lewis lung cancer tumour model mice given oral fucoidan 88.
Fucoidan acts independently as checkpoint modulators and may serve as alternative complementary agents for the treatment of cancers with high PD-L1 expression 89. Apoptosis or cell cycle arrest appears as a common feature of fucoidan bioactivity directly on cancer cells. Uterine cancer cell lines were inhibited by fucoidans derived from Undaria pinnatifida, whilst normal cells were unaffected 90. Lower molecular weight fractions of Undaria fucoidan were highly effective inhibitors of human prostate cancer cells, when delivered orally in a xenografted mouse model 91. Head and neck cancers, which may be caused by human papilloma viruses (HPV), are particularly difficult to treat. Fucoidan from Fucus vesiculosus inhibited head and neck squamous cell lines, causing cell cycle arrest 92. It also enhanced the response to the anticancer drug cisplatin in HPV-affected lines. Preclinical evaluations of fucoidans from Fucus vesiculosus and Undaria pinnatifida in a mouse model showed activity as sole agents and additive activity with anticancer agents in some breast cancer and ovarian cancer tumours 93. It should be noted that fucoidans do not always act as inhibitors of cancer cell lines, and were ineffective against uveal melanoma cell lines 94, instead providing protective and angiogenic effects.
The mechanisms of these anticancer activities are multifaceted. They include induction 95 or even inhibition 92, of reactive oxygen species, destabilization of mitochondria, and the cleavage of caspases and PARP. Using a systematic screen of the entire set of 4733 haploid Saccharomyces cerevisiae gene deletion strains, an analysis of cell pathways revealed that multiple cellular pathways were affected by exposure to fucoidan extracts, and that cDNA damage and cell cycle arrest occurred in colon cancer cell lines. Normal fibroblasts were unaffected under the same conditions 96. There were global effects of the fucoidan studied on a wide range of eukaryotic cellular processes, including RNA metabolism, protein synthesis, sorting, targeting and transport, carbohydrate metabolism, mitochondrial maintenance, cell cycle regulation and DNA damage repair-related pathways. Thus, the mechanisms by which fucoidans may act on cancer cells is becoming clearer. The lack of effect on normal fibroblasts means that fucoidan extracts have potential utility as anticancer agents.
In a Russian study 97, fucoidan from Fucus evanescens was found to increase cancer susceptibility towards radiation. The growing amount of evidence described above suggests that fucoidans place stresses on multiple cellular pathways, thereby increasing cancer cell susceptibility to radiation or other agents. Conversely, fucoidans may confer radiation protection effects that help to prevent lung damage and subsequent fibrosis of healthy tissues 98.
The immunosuppression experienced after chemotherapy can lead to infectious diseases and reduced quality of life. Previous research has shown some increase in circulating stem cells after ingestion of a fucoidan extract 99. In a recent Russian study 100, semisynthetic fucoidan fractions were assessed in a cyclophosphamide immune-suppressed mouse model. A subcutaneously delivered fucoidan-derived octasaccharide was more effective in neutrophil regeneration than the gold standard peptide treatment, rG-CSF. Recent research is summarized in Table 1.
Table 1. In vitro and in vivo fucoidan cancer studies
| Source of Fucoidan | Aim of Study | Type of Study | Outcome | Reference |
| Undaria pinnatifida Fucus vesiculosus | Orthotopic cancers in mice | In vivo Mouse | Safety of fucoidan usage during breast cancer treatment and potential to improve tamoxifen activity. | 101, 93 |
| Semisynthetic fucoidan fraction | Cyclophosphamide-treated mice, haemopoiesis | In vivo mouse | Synthetic octasaccharide is identified as an effective stimulator of haematopoiesis. | 100 |
| Undaria pinnatifida | Cell cycle arrest in HCT116 and MOA yeast gene deletion study | In vitro | Global effects of fucoidan on a wide range of eukaryotic cellular processes and inhibitory effect on colon cancer cells. | 96 |
| Undaria pinnatifida | Uterine carcinoma and sarcoma cell lines | In vitro | Anticancer agent activity against endometrial stromal sarcoma and carcinosarcoma. | 90 |
| Fucus evanescens | Radio sensitisation human melanoma, breast adenocarcinoma, and colorectal carcinoma cell lines | In vitro | Increased the inhibitory effect of X-ray radiation on proliferation and colony formation-activating caspases, suppressed anti-apoptotic protein and enhanced fragmentation of DNA. | 97 |
| Fucus vesiculosus | Radiation-induced lung fibrosis | In vivo mouse | Fucoidan changed the expression patterns of inflammatory cytokines and attenuated radiation-induced lung fibrosis | 98 |
Anti-skin aging
Fucoidans have been incorporated into skin care products due to their anti-inflammatory and antioxidant properties. In hairless mice, topically applied fucoidan was shown to be protective against ultraviolet B (UVB) induced skin photoaging by reducing neutrophil recruitment and associated inflammatory cytokine production (IL-1β) and oxidative stress induction 102. In a company sponsored clinical study (n=20) in Australia, topically applied 3% w/w fucoidan cream (Undaria pinnatifida extract with 8% fucoidan [27.4% sulfate] or Fucus vesiculosus extract with 60% fucoidan [10.1% sulfate], 30% polyphenol from Marinova) was effective at reducing erythema and transepithelial water loss after UV exposure compared to placebo 103. Use of the Fucus vesiculosus extract for 60 days also led to trends for reduced skin spots (in 50% of participants), increased skin brightness (in 65%), and improved wrinkles (in 45%). In vitrostudies indicate that the extract can increase Sirt1 expression and has antioxidant properties, which may contribute to its protective effects. Another company sponsored placebo-controlled randomized controlled trial (n=94, average age 41) in Korea found that 400 mg of fucoidan containing capsules (MK-R7: Complex of 150 mg Cistanche tubulosa extract and 50 mg Laminaria japonica including echinacoside glycosides and fucoidan, Misuba RTech) increased hair density (23.29 n/cm2± 24.26 vs 10.35 n/cm2± 20.08) and hair diameter (0.018 mm ± 0.015 vs 0.003 mm ± 0.013) in people with mild to moderate patterned hair loss 104. However, these effects appear to have marginally reached statistical significance, and it is unclear if the improvements are clinically meaningful.
Fucoidan in imaging and anti-coagulant function
French researchers have developed an extensive body of work regarding the use of radiolabelled fucoidan as an imaging agent to locate thrombi and image early-stage inflammatory processes in autoimmune endocarditis 105. A clinical trial on the safety of the reagent was completed in 2019 20, clearing the way for commercialization and clinical use of this imaging tool.
This work has expanded to assist in the targeting of thrombi to deliver a clinically used thrombolytic agent, recombinant tissue plasminogen activator (rtPA). A fucoidan extract is incorporated into nanoparticles which are then loaded with the rtPA. This allows the specific targeting of thrombi at lower overall doses, and may avoid some of the haemorrhagic complications of using rtPA 106. Interestingly, fucoidans themselves are good inhibitors of the tPA-PASI1 complex, and act as thrombolytics in their own right. Combined Korean- and Russian-based research has demonstrated the utility of these fractions, building on earlier work that demonstrated anti-thrombotic activity without anticoagulant activity in a fraction from Undaria pinnatifida 107. The potential to use orally bioavailable thrombolytic approaches are attractive 108.
Dextran-coated superparamagnetic iron oxide nanoparticles or ‘SPIONs’ are in use as MRI contrast agents. The wider clinical potential of SPIONs is limited by their rapid removal from circulation via the reticuloendothelial system. Fucoidan ingestion appears to increase the retention time of SPIONs by preventing their uptake into the reticuloendothelial system 109. This useful activity expands on the applications for imaging of thrombi.
Gradually depolymerised fractions of Fucus vesiculosus fucoidans (without removing sulphates) were examined for bioactivity 110. Researchers concluded that the fractions gradually lost their anti-oxidative and anti-proliferative activities due to the removal of terpenoids and polyphenolics. It is likely that the terpenoid and polyphenolic components were co-extracted with the initial fucoidan and subsequently removed, rather than being incorporated within the structure of the fucoidan polymer itself. Interestingly, lower molecular weight fractions maintained an anti-inflammatory activity, whilst having a low anticoagulant activity. Studies are summarized in Table 2.
Table 2. Fucoidan imaging and anti-coagulation studies
| Fucoidan Source | Aim of Study | Type of Study | Outcome | Reference |
| Radiolabelled fucoidan source unspecified | Safety | Human clinical | Safe to use. Maximum activity in liver. Activity reduced to <5% after 24 h. | 20 |
| Undaria pinnatifida Fucus evanescens Saccharina cichorioides Costaria costata Fucus vesiculosus Eisenia bicyclis | Thrombolytic activity of fucoidan | In vivo mouse thrombosis model, iv | Fucoidans inhibit the tPA-PAI1 complex, indicating activation of plasma tissue-type plasminogen activator is a mechanism of fucoidan-mediated thrombolysis in a mouse thrombosis model | 107 |
| Unspecified | Thrombolytic therapy based on fucoidan nanoparticles with recombinant tissue plasminogen activator (rtPA) | In vivo mouse model with induced clotting, iv | Successful thrombolysis | 106 |
| Laminaria japonica | Anti-thrombotic | In vivo mouse model. Oral delivery | Lower molecular weight fucoidan was most effective | 108 |
| Fucus vesiculosus and 18 gradually depolymerised fractions | Degraded fucoidan fractions | In vitro | Anti-inflammatory activity, however only negligible anticoagulant activity and FXII-activating potency | 110 |
| Fucus vesiculosus Macrocystis pyrifera Undaria pinnatifida | Intravenous fucoidan administration prior to SPIONs | In vivo mouse model In vitro | Increased residence time of circulating superparamagnetic iron oxide nanoparticles (SPIONs) for imaging by blocking their uptake by reticuloendothelial uptake | 111 |
Neuroprotective benefit
Fucoidan can protect against onset of inflammatory and oxidative damage by mitigating immune cell activation in animal models. However, fucoidan is unlikely to significantly benefit after onset of neurological damage. Types of evidence: 2 clinical trials for seaweed extract for cognitive function (n=60, n=59) and numerous laboratory studies (various fucoidan formulations). Due to its high molecular weight and low bioavailability, orally supplemented fucoidan is not expected to be brain penetrant 112. However, the neuroprotective effects described in preclinical studies are largely indirect and primarily involve the ability of fucoidan to promote a neuroprotective environment through the reduction of inflammatory and oxidative stressors. Fucoidan appears to be most effective when used as a preventative, to mitigate tissue damaging immune cell infiltration and activation.
No studies have directly tested the specific effects of fucoidan supplementation, however, there have been a couple of placebo-controlled clinical trials examining the effects of brown seaweed extract supplementation on cognitive function. One study testing fermented extract from Laminaria japonicain healthy elderly Japanese adults (Treatment: n=28, age 72.35 ± 5.54; Placebo: n=32, age 74.57 ± 5.69) found that 6 weeks of supplementation (1.5 g/day) led to improvements on tests for cognitive function and memory [K-MMSE, numerical memory test (working memory), Raven’s test (visual and spatial reasoning), iconic memory test (visual spatial memory)] relative to placebo 113. Another study tested post-prandial cognitive function following consumption of a hot water extract derived from Ascophyllum nodosumand Fucus vesiculosus (InSea2® 500 mg, equivalent to 10 g dried seaweed) in healthy adults (Treatment n=30, age 33.1±14.6; Placebo n=29, age 37.9 ±16.9) and found improvements in accuracy on digit vigilance and choice reaction time tests 114. The fermented extract was characterized for GABA content (mean 54.5± 0.071 mg/g), while the hot water extract was characterized for polyphenol content (>20%). These studies suggest that brown seaweed contains compounds that can at least temporarily improve some aspects of cognitive function, however, the fucoidan content of these preparations was not characterized, thus it is not clear if any of these effects can be attributed to fucoidan.
Potential prevention of Alzheimer’s disease
Different preparations of fucoidan have been found to protect against beta amyloid (Aβ) induced cytotoxicity in cell culture and Caenorhabditis elegans (roundworm), and mitigate scopolamine, ethanol, sodium nitrate, D-galactose, or A40 induced cognitive impairment in rodents 115. The neuroprotective effects were generally seen when fucoidan was administered prior to or shortly after exposure to the cell stressor, and were associated with reduced production of reactive oxygen species (ROS). Fucoidan treatment was also associated with less caspase activation and apoptosis. Fucoidans are similar in structure to glycosaminoglycans, which are known to regulate oxidative stress induced apoptosis. The properties most important for neuroprotective activity have not been fully characterized, but likely include the degree of sulfation, molecular weight, and sugar composition. One study comparing fucoidans with different chemical compositions derived from Fucus vesiculosusor Undaria pinnatifidafound that the sample with the highest polyphenol content, lowest carbohydrates, lowest sulfate content, and highest molecular weight showed the lowest neuroprotection against beta amyloid (Aβ) in PC12 cells 115. Some of the fucoidans (molecular weight 33-56 kDa, sulfates 23-31%) enhanced neurite outgrowth, which has also been seen with bioactive polysaccharides from other species. In rat cognitive impairment models, a fucoidan isolated from Sargassum fusiforme, SFPS65A, with a sulfate content of 17.5% was protective, while a related isolate, SFPS65B, with a lower sulfate content of 2.7% was not neuroprotective 116.
Potential prevention of Parkinson’s disease
Fucoidan pretreatment has been found to be protective in models of MPTP, 6-OHDA, or rotenone mediated dopaminergic neurotoxicity 117. Fucoidan can reduce toxin induced behavioral deficits, mitochondrial dysfunction, and dopamine neuron cell death. The protective effects primarily involve the inhibition of microglial activation, and a reduction in levels of oxidative stress (ROS, protein carbonylation, lipid peroxidation). In these studies 118, 119, 120 benefits were seen with fucoidan derived from Laminaria japonicafrom Qingdao, China (48% total sugar, 28% fucose, 29% sulfate, fucose: galactose 1:0.24, MW 7kDa), a 198 kDa L-fucose-4-sulfate fucoidan from the China National Institute for Control of Pharm and Biological products (16121901), and a fucoidan from Turbinaria decurrenscollected in Yervadi, India (carbohydrate 59.62%, sulfate 26.52%, and uronic acid 6.3%, MW 234 kDa). Since the protective effects appear to stem largely from preventing the induction of pathological processes associated with the onset of neuronal damage, it is not clear whether fucoidan would benefit as a treatment for patients with established disease.
Potential benefit in stroke and traumatic brain injury
Fucoidan pretreatment has been found to be protective in rodent models of cerebral ischemia (stroke) 121, 122, LPS-induced neuroinflammation 123, 124, intracerebral hemorrhage 125 and traumatic brain injury 126. The vast majority of these studies used a fucoidan preparation supplied by Sigma which is derived from Fucus vesiculosusand contains 44% fucose and 26% sulfate 127. Fucoidan treated animals had reduced neurological deficit scores, reduced infarct/lesion volumes, decreased cell death near the lesion, reduced pro-inflammatory cytokines (IL-1β, IL-6, TNFα, MPO), and reduced oxidative stress markers (8-OHdG, 4-HNE, MDA). Effects were typically dose dependent, with lower doses (typically under 50 mg/kg) failing to show benefits. The neuroprotective effects may also be contingent on time of administration, as fucoidan was protective in a model of traumatic brain injury when given from 0-4 hours after injury, but failed to protect against neurological deficits when given 6 hours after injury 127. In this study, the protective effects were attributed to the induction of Sirt3. The time window effect may relate to its mechanism of action in inhibiting the entry of activated immune cells into the brain by blocking P-selectin mediated interactions, which can mitigate the damage. Therefore, fucoidan would not be expected to have a significant effect once immune cells have entered, and damage has occurred.
Immunosenescence
Immunosenescence refers to the gradual deterioration of your immune system as you get older. The ability to mount an effective immune response to a pathogen declines with age due to the rise of senescent immune cells. It involves your capacity to respond to infections and maintain your long-term immune memory that was acquired (usually in your early life) either by infection or vaccination. Consequently, elderly people tend to produce weaker responses to viral antigens in vaccines, and thus have reduced immunity. Adjuvants are often added to vaccines to boost immune responses in the elderly. In a placebo-controlled randomized controlled trial in elderly adults in Japan (n=70, average age 87, 91% female), treatment with 300 mg of Mekabu fucoidan (derived from Undaria pinnatifida, Riken) for 4 weeks prior to receiving the flu vaccine increased antibody titers for all 3 flu strains in the vaccine 128. The antibody titers were maintained for over 20 weeks after vaccination. Fucoidan treated patients also experienced a transient increase in natural killer (NK) cell activity, suggesting that fucoidan can help reverse some of the signs of aging-associated immunosenescence.
Fucoidan dosage
Fucoidan clinically beneficial dose has been not established. Fucoidan is derived from brown algae and sold OTC as a supplement. The biological activity of the fucoidan can vary greatly depending on a variety of factors including: the species of brown seaweed from which it was obtained, the location the seaweed was grown, the time of year the seaweed was harvested, the method of extraction, the sugar content of the fucan backbone, and the level of branching of the backbone 83. The fucoidan content in brown seaweed is maximal in late autumn and early winter, which is also the time when it has the highest degree of sulfation 129. The most important aspects for bioactivity appear to be the molecular weight and the degree of sulfation. High molecular weight fucoidans with high levels of sulfates tend to have the most bioactivity in vitro, however, low molecular weight fucoidans have much greater bioavailability in vivo 83. In many of the commonly used extraction methods, particularly acidic hydrolysis, branching and sulfates are eliminated, which reduces the bioactivity. Extraction via gamma irradiation or enzymatic degradation is better at preserving the bioactive sulfate groups. The optimal form of fucoidan for therapeutic use has not been established, and is likely to vary for different disease indications based on the properties necessary for therapeutic benefit 18. It is anticipated that in order to be a viable therapy, a low molecular weight formulation will need to be developed which contains a high proportion of the bioactive sulfate groups.
Due to the low oral bioavailability noted in various fucoidan preparations in clinical trials, there is no clear evidence that currently available preparations of fucoidan supplements can elicit the types of benefits seen in preclinical studies. Therefore, incorporating fucoidan rich brown seaweed, such as Kombu (Saccharina japonica), Wakame (Undaria pinnatifida), Hijiki (Sargassum fusiforme), Nori (Pyropia tenera), and Mozuku (Nemacystus decipiens), into your diet is likely the best currently available source of fucoidan. Brown seaweed is also rich in various minerals and other compounds with purported antioxidant and anti-inflammatory properties, which may act to increase the bioavailability of fucoidan and/or act synergistically. However, seaweed is rich in iodine, so very high consumption may increase the risk for thyroid cancer.
Fucoidan side effects
Fucoidan is well-tolerated with no adverse events in clinical trials, even at high doses. Possible risk for increased bleeding when used in combination with blood thinners.
Fucoidan intake, even at high doses, has been shown to be safe in clinical studies. Up to 4.05 g per day of fucoidan (in 150 ml from Cladosiphon okamuranus [Okinawamozuku], Marine Products Kimuraya Co., Ltd) has been tested inhealthy volunteers for 2 weeks 130 and in cancer patients for 6 months 84 with no evidence of adverse effects. In all clinical trials, fucoidan use was well-tolerated, not associated with an increase in adverse events, did not induce significant changes on blood tests or vital signs, and exhibited no evidence of cytotoxicity. As a rich source of water-soluble fiber, fucoidan can promote bowel movements, and in one study a subset of patients (4/13) developed diarrhea that resolved upon discontinuation of fucoidan 131. Due to fucoidan’s anti-clotting activity, although generally weak in vivo, it has drug interactions with blood thinners, such as warfarin and heparin 132. It is possible that the low incidence of adverse events is related to the low oral bioavailability, determined to be less than 0.6% in humans in a pharmacokinetic study 133 and that preparations with higher oral bioavailability may be associated with more side effects. Although fucoidan has not been tested as a supplement in clinical trials longer than 1-year, long-term intake of fucoidan rich brown algae is associated with health benefits (i.e. Okinawa diet). In Japan, the daily intake of fucoidan from brown algae and other dietary sources is estimated to be about 400 mg/capita 134.
- Luthuli, S., Wu, S., Cheng, Y., Zheng, X., Wu, M., & Tong, H. (2019). Therapeutic Effects of Fucoidan: A Review on Recent Studies. Marine drugs, 17(9), 487. https://doi.org/10.3390/md17090487[↩][↩][↩][↩]
- Kannan RR, Arumugam R, Anantharaman P. Pharmaceutical potential of a fucoidan-like sulphated polysaccharide isolated from Halodule pinifolia. Int J Biol Macromol. 2013 Nov;62:30-4. doi: 10.1016/j.ijbiomac.2013.08.005[↩]
- Anbu, RM & Valliappan, Karuppiah & Li, Zhi-Yong. (2015). Prospect of Marine Algae for Production of Industrially Important Chemicals. 10.1007/978-3-319-22813-6_9[↩][↩][↩][↩][↩]
- Kenichi Tamama, Potential benefits of dietary seaweeds as protection against COVID-19, Nutrition Reviews, 2020;, nuaa126, https://doi.org/10.1093/nutrit/nuaa126[↩]
- de Jesus Raposo MF, de Morais AM, de Morais RM. Emergent Sources of Prebiotics: Seaweeds and Microalgae. Mar Drugs. 2016 Jan 28;14(2):27. doi: 10.3390/md14020027[↩]
- Zhao, Y., Zheng, Y., Wang, J., Ma, S., Yu, Y., White, W. L., Yang, S., Yang, F., & Lu, J. (2018). Fucoidan Extracted from Undaria pinnatifida: Source for Nutraceuticals/Functional Foods. Marine drugs, 16(9), 321. https://doi.org/10.3390/md16090321[↩][↩]
- Fitton, H. J., Stringer, D. S., Park, A. Y., & Karpiniec, S. N. (2019). Therapies from Fucoidan: New Developments. Marine drugs, 17(10), 571. https://doi.org/10.3390/md17100571[↩][↩][↩][↩][↩][↩]
- Besednova NN, Zaporozhets TS, Somova LM, Kuznetsova TA. Review: prospects for the use of extracts and polysaccharides from marine algae to prevent and treat the diseases caused by Helicobacter pylori. Helicobacter. 2015 Apr;20(2):89-97. doi: 10.1111/hel.12177[↩]
- Ahmadi A, Zorofchian Moghadamtousi S, Abubakar S, Zandi K. Antiviral Potential of Algae Polysaccharides Isolated from Marine Sources: A Review. Biomed Res Int. 2015;2015:825203. doi: 10.1155/2015/825203[↩]
- Kwak J. Y. (2014). Fucoidan as a marine anticancer agent in preclinical development. Marine drugs, 12(2), 851–870. https://doi.org/10.3390/md12020851[↩]
- Tomori M, Nagamine T, Miyamoto T, Iha M. Evaluation of the Immunomodulatory Effects of Fucoidan Derived from Cladosiphon Okamuranus Tokida in Mice. Mar Drugs. 2019 Sep 24;17(10):547. doi: 10.3390/md17100547[↩]
- Chow WL, Lee YK. Free fucose is a danger signal to human intestinal epithelial cells. Br J Nutr. 2008 Mar;99(3):449-54. doi: 10.1017/S0007114507812062[↩]
- Bienenstock J, Buck RH, Linke H, Forsythe P, Stanisz AM, Kunze WA. Fucosylated but not sialylated milk oligosaccharides diminish colon motor contractions. PLoS One. 2013 Oct 2;8(10):e76236. doi: 10.1371/journal.pone.0076236[↩]
- Kuznetsova, T. A., Smolina, T. P., Makarenkova, I. D., Ivanushko, L. A., Persiyanova, E. V., Ermakova, S. P., Silchenko, A. S., Zaporozhets, T. S., Besednova, N. N., Fedyanina, L. N., & Kryzhanovsky, S. Р. (2020). Immunoadjuvant Activity of Fucoidans from the Brown Alga Fucus evanescens. Marine drugs, 18(3), 155. https://doi.org/10.3390/md18030155[↩][↩]
- Cox AJ, Cripps AW, Taylor PA, Fitton JH, West NP. Fucoidan Supplementation Restores Fecal Lysozyme Concentrations in High-Performance Athletes: A Pilot Study. Mar Drugs. 2020 Aug 4;18(8):412. doi: 10.3390/md18080412[↩]
- Fitton J.H., Stringer D.N., Karpiniec S.S., Park A.Y. The Manufacture, Characterization, and Uses of Fucoidans from Macroalgae. 8 April 2019 ed. CRC Press; Boca Raton, FL, USA: 2019. pp. 47–60.[↩]
- Fitton, J. H., Park, A. Y., Karpiniec, S. S., & Stringer, D. N. (2020). Fucoidan and Lung Function: Value in Viral Infection. Marine drugs, 19(1), 4. https://doi.org/10.3390/md19010004[↩]
- Fitton, J. H., Stringer, D. N., & Karpiniec, S. S. (2015). Therapies from Fucoidan: An Update. Marine drugs, 13(9), 5920–5946. https://doi.org/10.3390/md13095920[↩][↩][↩]
- Wang J, Geng L, Yue Y, Zhang Q. Use of fucoidan to treat renal diseases: A review of 15 years of clinic studies. Prog Mol Biol Transl Sci. 2019;163:95-111. doi: 10.1016/bs.pmbts.2019.03.011[↩]
- Zheng K.H., Kaiser Y., Poel E., Verberne H., Aerts J., Rouzel F., Stroes E., Letourneur D., Chauvierre C. 99Mtc-Fucoidan As Diagnostic Agent For P-Selectin Imaging: First-In-Human Evaluation (Phase I) Atherosclerosis. 2019;287:e143. doi: 10.1016/j.atherosclerosis.2019.06.425[↩][↩][↩][↩]
- Lin YH, Lu KY, Tseng CL, Wu JY, Chen CH, Mi FL. Development of genipin-crosslinked fucoidan/chitosan-N-arginine nanogels for preventing Helicobacter infection. Nanomedicine (Lond). 2017 Jun;12(12):1491-1510. doi: 10.2217/nnm-2017-0055[↩]
- Oka S, Okabe M, Tsubura S, Mikami M, Imai A. Properties of fucoidans beneficial to oral healthcare. Odontology. 2020 Jan;108(1):34-42. doi: 10.1007/s10266-019-00437-3[↩]
- Park J, Cha JD, Choi KM, Lee KY, Han KM, Jang YS. Fucoidan inhibits LPS-induced inflammation in vitro and during the acute response in vivo. Int Immunopharmacol. 2017 Feb;43:91-98. doi: 10.1016/j.intimp.2016.12.006[↩]
- Hanisch FG, Hansman GS, Morozov V, Kunz C, Schroten H. Avidity of α-fucose on human milk oligosaccharides and blood group-unrelated oligo/polyfucoses is essential for potent norovirus-binding targets. J Biol Chem. 2018 Jul 27;293(30):11955-11965. doi: 10.1074/jbc.RA117.001369[↩]
- Hahn T., Lang S., Ulber R., Muffler K. Novel procedures for the extraction of fucoidan from brown algae. Process Biochem. 2012;47:1691–1698. doi: 10.1016/j.procbio.2012.06.016[↩]
- Zvyagintseva T.N., Shevchenko N.M., Chizhov A.O., Krupnova T.N., Sundukova E.V., Isakov V.V. Water-soluble polysaccharides of some brown seaweeds. Distribution, structure and dependence on the developmental conditions. J. Exp. Mar. Biol. Ecol. 2003;294:1–13. doi: 10.1016/S0022-0981(03)00244-2[↩]
- Koh H.S.A., Lu J., Zhou W. Structure characterization and antioxidant activity of fucoidan isolated from Undaria pinnatifida grown in New Zealand. Carbohydr. Polym. 2019;212:178–185. doi: 10.1016/j.carbpol.2019.02.040[↩]
- Usoltseva R.V., Shevchenko N.M., Malyarenko O.S., Anastyuk S.D., Kasprik A.E., Zvyagintsev N.V., Ermakova S.P. Fucoidans from brown algae Laminaria longipes and Saccharina cichorioides: Structural characteristics, anticancer and radio-sensitizing activity in vitro. Carbohydr. Polym. 2019;221:157–165. doi: 10.1016/j.carbpol.2019.05.079[↩]
- Lahrsen E., Liewert I., Alban S. Gradual degradation of fucoidan from Fucus vesiculosus and its effect on structure, antioxidant and antiproliferative activities. Carbohydr. Polym. 2018;192:208–216. doi: 10.1016/j.carbpol.2018.03.056[↩]
- Foley S.A., Szegezdi E., Mulloy B., Samali A., Tuohy M.G. An unfractionated fucoidan from Ascophyllum nodosum: Extraction, characterization, and apoptotic effects in vitro. J. Nat. Prod. 2012;75:1674. doi: 10.1021/np300601m[↩]
- Descamps V., Colin S., Lahaye M., Jam M., Richard C., Potin P., Barbeyron T., Yvin J.C., Kloareg B. Isolation and culture of a marine bacterium degrading the sulfated fucans from marine brown algae. Mar. Biotechnol. 2006;8:27–39. doi: 10.1007/s10126-005-5107-0[↩]
- Jabbar Mian A., Percival E. Carbohydrates of the brown seaweeds Himanthalia lorea and Bifurcaria bifurcata: Part II. structural studies of the “fucans” Carbohydr. Res. 1973;26:147–161. doi: 10.1016/S0008-6215(00)85031-4[↩]
- Fletcher H.R., Biller P., Ross A.B., Adams J.M.M. The seasonal variation of fucoidan within three species of brown macroalgae. Algal Res. 2017;22:79–86. doi: 10.1016/j.algal.2016.10.015[↩]
- Garcia-Vaquero M., Rajauria G., O’Doherty J.V., Sweeney T. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Res. Int. 2017;99:1011–1020. doi: 10.1016/j.foodres.2016.11.016[↩]
- Chevolot L., Foucault A., Chaubet F., Kervarec N., Sinquin C., Fisher A.M., Boisson-Vidal C. Further data on the structure of brown seaweed fucans: Relationships with anticoagulant activity. Carbohydr. Res. 1999;319:154–165. doi: 10.1016/S0008-6215(99)00127-5[↩]
- Marais M.F., Joseleau J.P. A fucoidan fraction from Ascophyllum nodosum. Carbohydr. Res. 2001;336:155–159. doi: 10.1016/S0008-6215(01)00257-9[↩]
- Ponce N.M.A., Pujol C.A., Damonte E.B., Flores M.L., Stortz C.A. Fucoidans from the brown seaweed Adenocystis utricularis: Extraction methods, antiviral activity and structural studies. Carbohydr. Res. 2003;338:153–165. doi: 10.1016/S0008-6215(02)00403-2[↩]
- Mansour MB, Balti R, Yacoubi L, Ollivier V, Chaubet F, Maaroufi RM. Primary structure and anticoagulant activity of fucoidan from the sea cucumber Holothuria polii. Int J Biol Macromol. 2019 Jan;121:1145-1153. doi: 10.1016/j.ijbiomac.2018.10.129[↩]
- Zhao Y, Zheng Y, Wang J, Ma S, Yu Y, White WL, Yang S, Yang F, Lu J. Fucoidan Extracted from Undaria pinnatifida: Source for Nutraceuticals/Functional Foods. Mar Drugs. 2018 Sep 9;16(9):321. doi: 10.3390/md16090321[↩]
- Helen F.J. Method and composition for the treatment of a viral infection. Arch. Oral. Biol. 2006;41:299–305.[↩]
- Kalimuthu S., Kim S.K. Fucoidan, A Sulfated Polysaccharides from Brown Algae as Therapeutic Target for Cancer. Springer International Publishing; Cham, Switzerland: 2015. pp. 145–164.[↩]
- Kylin H. Biochemistry of sea algae. HZ Physiol. Chem. 1913;83:171–197. doi: 10.1515/bchm2.1913.83.3.171[↩][↩]
- Yang C., Chung D., Shina I., Lee H., Kim J., Lee Y., You S. Effects of molecular weight and hydrolysis conditions on anticancer activity of fucoidans from sporophyll of Undaria pinnatifida. Int. J. Biol. Macromol. 2008;43:433–437. doi: 10.1016/j.ijbiomac.2008.08.006[↩]
- Vishchuk O.S., Ermakova S.P., Zvyagintseva T.N. Sulfated polysaccharides from brown seaweeds Saccharina japonica and Undaria pinnatifida: Isolation, structural characteristics, and antitumor activity. Carbohydr. Res. 2011;346:2769–2776. doi: 10.1016/j.carres.2011.09.034[↩]
- Hewitt C.L., Campbell M.L., Mcennulty F., Moore K.M., Murfet N.B., Robertson B., Schaffelke B. Efficacy of physical removal of a marine pest: The introduced kelp Undaria pinnatifida in a Tasmanian Marine Reserve. Biol. Invasions. 2005;7:251–263. doi: 10.1007/s10530-004-0739-y[↩]
- Wallentinus I. Alien Species Alert: Undaria pinnatifida:(Wakame or Japanese Kelp) The Council; Göteborg, Sweden: 2007.[↩]
- https://www.fda.gov/media/104562/download[↩]
- Wang J., Geng L., Yue Y., Zhang Q. Use of fucoidan to treat renal diseases: A review of 15 years of clinic studies. Prog. Mol. Biol. Transl. Sci. 2019;163:95–111. doi: 10.1016/bs.pmbts.2019.03.011[↩]
- Masterson C.H., Curley G.F., Laffey J.G. Modulating the distribution and fate of exogenously delivered MSCs to enhance therapeutic potential: knowns and unknowns. Intensive Care Med. Exp. 2019;7:41. doi: 10.1186/s40635-019-0235-4[↩]
- Lee J.H., Yun C.W., Hur J., Lee S.H. Fucoidan Rescues p-Cresol-Induced Cellular Senescence in Mesenchymal Stem Cells via FAK-Akt-TWIST Axis. Mar. Drugs. 2018;16:121. doi: 10.3390/md16040121[↩]
- Kan J., Hood M., Burns C., Scholten J., Chuang J., Tian F., Pan X., Du J., Gui M. A Novel Combination of Wheat Peptides and Fucoidan Attenuates Ethanol-Induced Gastric Mucosal Damage through Anti-Oxidant, Anti-Inflammatory, and Pro-Survival Mechanisms. Nutrients. 2017;9:978. doi: 10.3390/nu9090978[↩]
- Kan J., Cheng J., Xu L., Hood M., Zhong D., Cheng M., Liu Y., Chen L., Du J. The combination of wheat peptides and fucoidan protects against chronic superficial gastritis and alters gut microbiota: a double-blinded, placebo-controlled study. Eur. J. Nutr. 2019 doi: 10.1007/s00394-019-02020-6[↩]
- Takahashi H., Kawaguchi M., Kitamura K., Narumiya S., Kawamura M., Tengan I., Nishimoto S., Hanamure Y., Majima Y., Tsubura S., et al. An Exploratory Study on the Anti-inflammatory Effects of Fucoidan in Relation to Quality of Life in Advanced Cancer Patients. Integr. Cancer Ther. 2018;17:282–291. doi: 10.1177/1534735417692097[↩]
- Tocaciu S., Oliver L., Lowenthal R., Peterson G.M., Patel R., Shastri M., McGuinness G., Olesen I., Fitton J.H. The effect of Undaria pinnatifida fucoidan on the pharmacokinetics of letrozole and tamoxifen in patients with breast cancer. Integr. Cancer Ther. 2016;2016:99–105. doi: 10.1177/1534735416684014[↩]
- Asanka Sanjeewa KK , Jayawardena TU , Kim H-S , et al. Fucoidan isolated from Padina commersonii inhibit LPS-induced inflammation in macrophages blocking TLR/NF-κB signal pathway. Carbohydr Polym. 2019;224:115195.[↩]
- Takahashi H , Kawaguchi M , Kitamura K , et al. An exploratory study on the anti-inflammatory effects of fucoidan in relation to quality of life in advanced cancer patients. Integr Cancer Ther. 2018;17:282–291.[↩]
- Yu H-H , Chengchuan Ko E , Chang C-L , et al. Fucoidan inhibits radiation-induced pneumonitis and lung fibrosis by reducing inflammatory cytokine expression in lung tissues. Mar Drugs. 2018;16:392, [10.3390/md16100392][↩]
- Ren R , Azuma Y , Ojima T , et al. Modulation of platelet aggregation-related eicosanoid production by dietary F-fucoidan from brown alga Laminaria japonica in human subjects. Br J Nutr. 2013;110:880–890.[↩]
- Yasuzawa T , Mima A , Ueshima S. Antithrombotic effect of oral administration of mozuku (Cladosiphon okamuranus, brown seaweed) extract in rat. J Nutr Sci Vitaminol (Tokyo). 2019;65:171–176.[↩]
- Zhao X , Guo F , Hu J , et al. Antithrombotic activity of oral administered low molecular weight fucoidan from Laminaria Japonica. Thromb Res. 2016;144:46–52.[↩]
- Shan X, Liu X, Hao J, Cai C, Fan F, Dun Y, Zhao X, Liu X, Li C, Yu G. In vitro and in vivo hypoglycemic effects of brown algal fucoidans. Int J Biol Macromol. 2016 Jan;82:249-55. doi: 10.1016/j.ijbiomac.2015.11.036[↩]
- Yang W, Yu X, Zhang Q, Lu Q, Wang J, Cui W, Zheng Y, Wang X, Luo D. Attenuation of streptozotocin-induced diabetic retinopathy with low-molecular-weight fucoidan via inhibition of vascular endothelial growth factor. Exp Eye Res. 2013 Oct;115:96-105. doi: 10.1016/j.exer.2013.06.011[↩]
- Cui W, Zheng Y, Zhang Q, Wang J, Wang L, Yang W, Guo C, Gao W, Wang X, Luo D. Low-molecular-weight fucoidan protects endothelial function and ameliorates basal hypertension in diabetic Goto-Kakizaki rats. Lab Invest. 2014 Apr;94(4):382-93. doi: 10.1038/labinvest.2014.12[↩]
- Jeong YT, Kim YD, Jung YM, Park DC, Lee DS, Ku SK, Li X, Lu Y, Chao GH, Kim KJ, Lee JY, Baek MC, Kang W, Hwang SL, Chang HW. Low molecular weight fucoidan improves endoplasmic reticulum stress-reduced insulin sensitivity through AMP-activated protein kinase activation in L6 myotubes and restores lipid homeostasis in a mouse model of type 2 diabetes. Mol Pharmacol. 2013 Jul;84(1):147-57. doi: 10.1124/mol.113.085100[↩]
- Hu S.W., Xia G.H., Wang J.F., Wang Y.M., Li Z.J., Xue C.H. Fucoidan from sea cucumber protects against high-fat high-sucrose diet-induced hyperglycaemia and insulin resistance in mice. J. Funct. Foods. 2014;10:128–138. doi: 10.1016/j.jff.2014.05.012[↩]
- Sakai C., Abe S., Kouzuki M., Shimohiro H., Ota Y., Sakinada H., Takeuchi T., Okura T., Kasagi T., Hanaki K. A Randomized Placebo-controlled Trial of an Oral Preparation of High Molecular Weight Fucoidan in Patients with Type 2 Diabetes with Evaluation of Taste Sensitivity. Yonago Acta Med. 2019;62:14–23. doi: 10.33160/yam.2019.03.003[↩]
- Wright C.M., Bezabhe W., Fitton J.H., Stringer D.N., Bereznicki L.R.E., Peterson G.M. Effect of a Fucoidan Extract on Insulin Resistance and Cardiometabolic Markers in Obese, Nondiabetic Subjects: A Randomized, Controlled Trial. J. Altern. Complement. Med. 2019;25:346–352. doi: 10.1089/acm.2018.0189[↩]
- Chen Q, Liu M, Zhang P, Fan S, Huang J, Yu S, Zhang C, Li H. Fucoidan and galactooligosaccharides ameliorate high-fat diet-induced dyslipidemia in rats by modulating the gut microbiota and bile acid metabolism. Nutrition. 2019 Sep;65:50-59. doi: 10.1016/j.nut.2019.03.001[↩]
- Li S , Li J , Mao G , Wu T , Hu Y , Ye X , Tian D , Linhardt RJ , Chen S . A fucoidan from sea cucumber Pearsonothuria graeffei with well-repeated structure alleviates gut microbiota dysbiosis and metabolic syndromes in HFD-fed mice. Food Funct. 2018 Oct 17;9(10):5371-5380. doi: 10.1039/c8fo01174e[↩]
- Parnell JA, Reimer RA. Prebiotic fiber modulation of the gut microbiota improves risk factors for obesity and the metabolic syndrome. Gut Microbes. 2012 Jan-Feb;3(1):29-34. doi: 10.4161/gmic.19246[↩]
- Cheng Y, Sibusiso L, Hou L, Jiang H, Chen P, Zhang X, Wu M, Tong H. Sargassum fusiforme fucoidan modifies the gut microbiota during alleviation of streptozotocin-induced hyperglycemia in mice. Int J Biol Macromol. 2019 Jun 15;131:1162-1170. doi: 10.1016/j.ijbiomac.2019.04.040[↩][↩]
- Tsubura S.S.A. Case report using 4% fucoidan cream for recurrent oral herpes labialis: Patient symptoms markedly improved in terms of time to healing and time to loss of discomfort. Dent. Open, J. 2017;4:19–23. doi: 10.17140/DOJ-5-135[↩]
- Zayed A., Muffler K., Hahn T., Rupp S., Finkelmeier D., Burger-Kentischer A., Ulber R. Physicochemical and Biological Characterization of Fucoidan from Fucus vesiculosus Purified by Dye Affinity Chromatography. Mar. Drugs. 2016;14:79. doi: 10.3390/md14040079[↩]
- Wang W, Wu J, Zhang X, Hao C, Zhao X, Jiao G, Shan X, Tai W, Yu G. Inhibition of Influenza A Virus Infection by Fucoidan Targeting Viral Neuraminidase and Cellular EGFR Pathway. Sci Rep. 2017 Jan 17;7:40760. doi: 10.1038/srep40760[↩]
- Li H, Li J, Tang Y, Lin L, Xie Z, Zhou J, Zhang L, Zhang X, Zhao X, Chen Z, Zuo D. Fucoidan from Fucus vesiculosus suppresses hepatitis B virus replication by enhancing extracellular signal-regulated Kinase activation. Virol J. 2017 Sep 16;14(1):178. doi: 10.1186/s12985-017-0848-8[↩]
- Trejo-Avila LM, Morales-Martínez ME, Ricque-Marie D, Cruz-Suarez LE, Zapata-Benavides P, Morán-Santibañez K, Rodríguez-Padilla C. In vitro anti-canine distemper virus activity of fucoidan extracted from the brown alga Cladosiphon okamuranus. Virusdisease. 2014 Dec;25(4):474-80. doi: 10.1007/s13337-014-0228-6[↩]
- Thuy TT, Ly BM, Van TT, Quang NV, Tu HC, Zheng Y, Seguin-Devaux C, Mi B, Ai U. Anti-HIV activity of fucoidans from three brown seaweed species. Carbohydr Polym. 2015 Jan 22;115:122-8. doi: 10.1016/j.carbpol.2014.08.068[↩]
- Prokofjeva MM , Imbs TI , Shevchenko NM , et al. Fucoidans as potential inhibitors of HIV-1. Mar Drugs. 2013;11:3000–3014.[↩]
- Kwon PS , Oh H, Kwon S-J , et al. Sulfated polysaccharides effectively inhibit SARS-CoV-2 in vitro. Cell Discov. 2020;6:50.[↩]
- Brownlee I, Fairclough A, Hall Aet al.(2012) The potential health benefits of seaweed and seaweed extract. InMarine Biology : EarthSciences in the 21st Century, pp. 119-136 [VH Pomin, editor]. Hauppauge, New York: Nova Science Publishers.[↩]
- Teas, J., Vena, S., Cone, D. L., & Irhimeh, M. (2013). The consumption of seaweed as a protective factor in the etiology of breast cancer: proof of principle. Journal of applied phycology, 25(3), 771–779. https://doi.org/10.1007/s10811-012-9931-0[↩]
- Sanjeewa KKA, Lee J-S, Kim W-Set al.(2017) The potential of brown-algae polysaccharides for the development of anticancer agents: An update on anticancer effects reported for fucoidan and laminaran. Carbohydrate Polymers177, 451-459. https://doi.org/10.1016/j.carbpol.2017.09.005[↩]
- Van Weelden G, Bobiński M, Okła K, Van Weelden WJ, Romano A, Pijnenborg JMA. Fucoidan Structure and Activity in Relation to Anti-Cancer Mechanisms. Marine Drugs. 2019; 17(1):32. https://doi.org/10.3390/md17010032[↩][↩][↩]
- Ikeguchi, M., Yamamoto, M., Arai, Y., Maeta, Y., Ashida, K., Katano, K., Miki, Y., & Kimura, T. (2011). Fucoidan reduces the toxicities of chemotherapy for patients with unresectable advanced or recurrent colorectal cancer. Oncology letters, 2(2), 319–322. https://doi.org/10.3892/ol.2011.254[↩][↩]
- Tocaciu, S., Oliver, L. J., Lowenthal, R. M., Peterson, G. M., Patel, R., Shastri, M., McGuinness, G., Olesen, I., & Fitton, J. H. (2018). The Effect of Undaria pinnatifida Fucoidan on the Pharmacokinetics of Letrozole and Tamoxifen in Patients With Breast Cancer. Integrative cancer therapies, 17(1), 99–105. https://doi.org/10.1177/1534735416684014[↩]
- Takahashi, H., Kawaguchi, M., Kitamura, K., Narumiya, S., Kawamura, M., Tengan, I., Nishimoto, S., Hanamure, Y., Majima, Y., Tsubura, S., Teruya, K., & Shirahata, S. (2018). An Exploratory Study on the Anti-inflammatory Effects of Fucoidan in Relation to Quality of Life in Advanced Cancer Patients. Integrative cancer therapies, 17(2), 282–291. https://doi.org/10.1177/1534735417692097[↩]
- Tsai, H. L., Tai, C. J., Huang, C. W., Chang, F. R., & Wang, J. Y. (2017). Efficacy of Low-Molecular-Weight Fucoidan as a Supplemental Therapy in Metastatic Colorectal Cancer Patients: A Double-Blind Randomized Controlled Trial. Marine drugs, 15(4), 122. https://doi.org/10.3390/md15040122[↩]
- Huang T.H., Chiu Y.H., Chan Y.L., Chiu Y.H., Wang H., Huang K.C., Li T.L., Hsu K.H., Wu C.J. Prophylactic Administration of Fucoidan Represses Cancer Metastasis by Inhibiting Vascular Endothelial Growth Factor (VEGF) and Matrix Metalloproteinases (MMPs) in Lewis Tumor-Bearing Mice. Mar. Drugs. 2015;13:1882–1900. doi: 10.3390/md13041882[↩]
- Teruya K., Kusumoto Y., Eto H., Nakamichi N., Shirahata S. Selective Suppression of Cell Growth and Programmed Cell Death-Ligand 1 Expression in HT1080 Fibrosarcoma Cells by Low Molecular Weight Fucoidan Extract. Mar. Drugs. 2019;17:421. doi: 10.3390/md17070421[↩]
- Bobinski M., Okla K., Bednarek W., Wawruszak A., Dmoszynska-Graniczka M., Garcia-Sanz P., Wertel I., Kotarski J. The Effect of Fucoidan, a Potential New, Natural, Anti-Neoplastic Agent on Uterine Sarcomas and Carcinosarcoma Cell Lines: ENITEC Collaborative Study. Arch. Immunol. Ther. Exp. 2019;67:125–131. doi: 10.1007/s00005-019-00534-9[↩][↩]
- Lee J., Lee S., Synytsya A., Capek P., Lee C.W., Choi J.W., Cho S., Kim W.J., Park Y.I. Low Molecular Weight Mannogalactofucans Derived from Undaria pinnatifida Induce Apoptotic Death of Human Prostate Cancer Cells In Vitro and In Vivo. Mar. Biotechnol. 2018;20:813–828. doi: 10.1007/s10126-018-9851-3[↩]
- Blaszczak W., Lach M.S., Barczak W., Suchorska W.M. Fucoidan Exerts Anticancer Effects Against Head and Neck Squamous Cell Carcinoma In Vitro. Molecules. 2018;23:302. doi: 10.3390/molecules23123302[↩][↩]
- Mathew L.B.M., Gaikwad A., Nyshadham P., Nugent E.K., Gonzalez A., Smith J.A. Preclinical Evaluation of Safety of Fucoidan Extracts From Undaria pinnatifida and Fucus vesiculosus for Use in Cancer Treatment. Integr. Cancer Ther. 2016;1-13 doi: 10.1177/1534735416680744[↩][↩]
- Dithmer M., Kirsch A.M., Richert E., Fuchs S., Wang F., Schmidt H., Coupland S.E., Roider J., Klettner A. Fucoidan Does Not Exert Anti-Tumorigenic Effects on Uveal Melanoma Cell Lines. Mar. Drugs. 2017;15:193. doi: 10.3390/md15070193[↩]
- Hsu H.-Y., Lin T.-Y., Hu C.-H., Shu D.T.F., Lu M.-K. Fucoidan upregulates TLR4/CHOP-mediated caspase-3 and PARP activation to enhance cisplatin-induced cytotoxicity in human lung cancer cells. Cancer Lett. 2018;432:112–120. doi: 10.1016/j.canlet.2018.05.006[↩]
- Corban M., Ambrose M., Pagnon J., Stringer D., Karpiniec S., Park A., Eri R., Fitton J.H., Gueven N. Pathway Analysis of Fucoidan Activity Using a Yeast Gene Deletion Library Screen. Mar. Drugs. 2019;17:54. doi: 10.3390/md17010054[↩][↩]
- Malyarenko O.S., Zdobnova E.V., Silchenko A.S., Kusaykin M.I., Ermakova S.P. Radiosensitizing effect of the fucoidan from brown alga Fucus evanescens and its derivative in human cancer cells. Carbohydr. Polym. 2019;205:465–471. doi: 10.1016/j.carbpol.2018.10.083[↩][↩]
- Yu H.H., Chengchuan Ko E., Chang C.L., Yuan K.S., Wu A.T.H., Shan Y.S., Wu S.Y. Fucoidan Inhibits Radiation-Induced Pneumonitis and Lung Fibrosis by Reducing Inflammatory Cytokine Expression in Lung Tissues. Mar. Drugs. 2018;16:392. doi: 10.3390/md16100392[↩][↩]
- Irhimeh M.R., Fitton J.H., Lowenthal R.M. Fucoidan ingestion increases the expression of CXCR4 on human CD34+ cells. Exp. Hematol. 2007;35:989–994. doi: 10.1016/j.exphem.2007.02.009[↩]
- Anisimova N.Y., Ustyuzhanina N.E., Bilan M.I., Donenko F.V., Ushakova N.A., Usov A.I., Kiselevskiy M.V., Nifantiev N.E. Influence of Modified Fucoidan and Related Sulfated Oligosaccharides on Hematopoiesis in Cyclophosphamide-Induced Mice. Mar. Drugs. 2018;16:333. doi: 10.3390/md16090333[↩][↩]
- Burney M., Mathew L., Gaikwad A., Nugent E.K., Gonzalez A.O., Smith J.A. Evaluation Fucoidan Extracts From Undaria pinnatifida and Fucus vesiculosus in Combination With Anticancer Drugs in Human Cancer Orthotopic Mouse Models. Integr. Cancer Ther. 2018;17:755–761. doi: 10.1177/1534735417740631[↩]
- Kim, Y. I., Oh, W. S., Song, P. H., Yun, S., Kwon, Y. S., Lee, Y. J., Ku, S. K., Song, C. H., & Oh, T. H. (2018). Anti-Photoaging Effects of Low Molecular-Weight Fucoidan on Ultraviolet B-Irradiated Mice. Marine drugs, 16(8), 286. https://doi.org/10.3390/md16080286[↩]
- Fitton JH, Dell’Acqua G, Gardiner V-A, Karpiniec SS, Stringer DN, Davis E. Topical Benefits of Two Fucoidan-Rich Extracts from Marine Macroalgae. Cosmetics. 2015; 2(2):66-81. https://doi.org/10.3390/cosmetics2020066[↩]
- Seok, J., Kim, T. S., Kwon, H. J., Lee, S. P., Kang, M. H., Kim, B. J., & Kim, M. N. (2015). Efficacy of Cistanche Tubulosa and Laminaria Japonica Extracts (MK-R7) Supplement in Preventing Patterned Hair Loss and Promoting Scalp Health. Clinical nutrition research, 4(2), 124–131. https://doi.org/10.7762/cnr.2015.4.2.124[↩]
- Vigne J, Cognet T, Guedj K, Morvan M, Merceron O, Louedec L, Choqueux C, Nicoletti A, Escoubet B, Chaubet F, Michel JB, Rouzet F. Early Detection of Localized Immunity in Experimental Autoimmune Myocarditis Using [99mTc]Fucoidan SPECT. Mol Imaging Biol. 2020 Jun;22(3):643-652. doi: 10.1007/s11307-019-01420-8[↩]
- Juenet M, Aid-Launais R, Li B, Berger A, Aerts J, Ollivier V, Nicoletti A, Letourneur D, Chauvierre C. Thrombolytic therapy based on fucoidan-functionalized polymer nanoparticles targeting P-selectin. Biomaterials. 2018 Feb;156:204-216. doi: 10.1016/j.biomaterials.2017.11.047[↩][↩]
- Choi Y, Min SK, Usoltseva R, Silchenko A, Zvyagintseva T, Ermakova S, Kim JK. Thrombolytic fucoidans inhibit the tPA-PAI1 complex, indicating activation of plasma tissue-type plasminogen activator is a mechanism of fucoidan-mediated thrombolysis in a mouse thrombosis model. Thromb Res. 2018 Jan;161:22-25. doi: 10.1016/j.thromres.2017.11.015[↩][↩]
- Zhao X, Guo F, Hu J, Zhang L, Xue C, Zhang Z, Li B. Antithrombotic activity of oral administered low molecular weight fucoidan from Laminaria Japonica. Thromb Res. 2016 Aug;144:46-52. doi: 10.1016/j.thromres.2016.03.008[↩][↩]
- Lahrsen E, Liewert I, Alban S. Gradual degradation of fucoidan from Fucus vesiculosus and its effect on structure, antioxidant and antiproliferative activities. Carbohydr Polym. 2018 Jul 15;192:208-216. doi: 10.1016/j.carbpol.2018.03.056[↩]
- Lahrsen E, Schoenfeld AK, Alban S. Size-dependent pharmacological activities of differently degraded fucoidan fractions from Fucus vesiculosus. Carbohydr Polym. 2018 Jun 1;189:162-168. doi: 10.1016/j.carbpol.2018.02.035[↩][↩]
- Abdollah MRA, Carter TJ, Jones C, Kalber TL, Rajkumar V, Tolner B, Gruettner C, Zaw-Thin M, Baguña Torres J, Ellis M, Robson M, Pedley RB, Mulholland P, T M de Rosales R, Chester KA. Fucoidan Prolongs the Circulation Time of Dextran-Coated Iron Oxide Nanoparticles. ACS Nano. 2018 Feb 27;12(2):1156-1169. doi: 10.1021/acsnano.7b06734[↩]
- Fitton J. H. (2011). Therapies from fucoidan; multifunctional marine polymers. Marine drugs, 9(10), 1731–1760. https://doi.org/10.3390/md9101731[↩]
- Reid, S., Ryu, J. K., Kim, Y., & Jeon, B. H. (2018). The Effects of Fermented Laminaria japonica on Short-Term Working Memory and Physical Fitness in the Elderly. Evidence-based complementary and alternative medicine : eCAM, 2018, 8109621. https://doi.org/10.1155/2018/8109621[↩]
- Haskell-Ramsay, C. F., Jackson, P. A., Dodd, F. L., Forster, J. S., Bérubé, J., Levinton, C., & Kennedy, D. O. (2018). Acute Post-Prandial Cognitive Effects of Brown Seaweed Extract in Humans. Nutrients, 10(1), 85. https://doi.org/10.3390/nu10010085[↩]
- Alghazwi M, Smid S, Karpiniec Set al.(2019) Comparative study on neuroprotective activities of fucoidans from Fucus vesiculosus and Undaria pinnatifida. International Journal of Biological Macromolecules122, 255-264. https://doi.org/10.1016/j.ijbiomac.2018.10.168[↩][↩]
- Hu P, Li Z, Chen Met al.(2016) Structural elucidation and protective role of a polysaccharide from Sargassum fusiforme on ameliorating learning and memory deficiencies in mice. Carbohydrate Polymers139, 150-158. https://doi.org/10.1016/j.carbpol.2015.12.019[↩]
- Liang, Z., Liu, Z., Sun, X., Tao, M., Xiao, X., Yu, G., & Wang, X. (2019). The Effect of Fucoidan on Cellular Oxidative Stress and the CatD-Bax Signaling Axis in MN9D Cells Damaged by 1-Methyl-4-Phenypyridinium. Frontiers in aging neuroscience, 10, 429. https://doi.org/10.3389/fnagi.2018.00429[↩]
- Zhang, F.‐L., He, Y., Zheng, Y., Zhang, W.‐J., Wang, Q., Jia, Y.‐J., Song, H.‐L., An, H.‐T., Zhang, H.‐B., Qian, Y.‐J., Tong, Y.‐L., Dong, L. and Wang, X.‐M. (2014), Therapeutic Effects of Fucoidan in 6‐Hydroxydopamine‐Lesioned Rat Model of Parkinson’s disease: Role of NADPH oxidase‐1. CNS Neurosci Ther, 20: 1036-1044. https://doi.org/10.1111/cns.12340[↩]
- Zhang, L., Hao, J., Zheng, Y., Su, R., Liao, Y., Gong, X., Liu, L., & Wang, X. (2018). Fucoidan Protects Dopaminergic Neurons by Enhancing the Mitochondrial Function in a Rotenone-induced Rat Model of Parkinson’s Disease. Aging and disease, 9(4), 590–604. https://doi.org/10.14336/AD.2017.0831[↩]
- Luo D, Zhang Q, Wang Het al.(2009) Fucoidan protects against dopaminergic neuron death in vivo and in vitro. European Journal of Pharmacology617, 33-40. https://doi.org/10.1016/j.ejphar.2009.06.015[↩]
- Ahn, J. H., Shin, M. C., Kim, D. W., Kim, H., Song, M., Lee, T. K., Lee, J. C., Kim, H., Cho, J. H., Kim, Y. M., Kim, J. D., Choi, S. Y., Won, M. H., & Park, J. H. (2019). Antioxidant Properties of Fucoidan Alleviate Acceleration and Exacerbation of Hippocampal Neuronal Death Following Transient Global Cerebral Ischemia in High-Fat Diet-Induced Obese Gerbils. International journal of molecular sciences, 20(3), 554. https://doi.org/10.3390/ijms20030554[↩]
- Kim H, Ahn JH, Song Met al.(2019) Pretreated fucoidan confers neuroprotection against transient global cerebral ischemic injury in the gerbil hippocampal CA1 area via reducing of glial cell activation and oxidative stress. Biomedicine & Pharmacotherapy109, 1718-1727. https://doi.org/10.1016/j.biopha.2018.11.015[↩]
- Kang GH, Yan BC, Cho G-Set al.(2012) Neuroprotective effect of fucoidin on lipopolysaccharide accelerated cerebral ischemic injury through inhibition of cytokine expression and neutrophil infiltration. Journal of the Neurological Sciences318, 25-30. https://doi.org/10.1016/j.jns.2012.04.013[↩]
- Cui, Y.‐Q., Jia, Y.‐J., Zhang, T., Zhang, Q.‐B. and Wang, X.‐M. (2012), Fucoidan Protects against Lipopolysaccharide‐Induced Rat Neuronal Damage and Inhibits the Production of Proinflammatory Mediators in Primary Microglia. CNS Neurosci Ther, 18: 827-833. https://doi.org/10.1111/j.1755-5949.2012.00372.x[↩]
- Del Bigio MR (1999) Effect of fucoidan treatment on collagenase-induced intracerebral hemorrhage in rats. Neurological research (New York)21, 415-419.[↩]
- Wang, T., Zhu, M. & He, ZZ. Low-Molecular-Weight Fucoidan Attenuates Mitochondrial Dysfunction and Improves Neurological Outcome After Traumatic Brain Injury in Aged Mice: Involvement of Sirt3. Cell Mol Neurobiol 36, 1257–1268 (2016). https://doi.org/10.1007/s10571-015-0323-2[↩]
- Li, B., Lu, F., Wei, X., & Zhao, R. (2008). Fucoidan: structure and bioactivity. Molecules (Basel, Switzerland), 13(8), 1671–1695. https://doi.org/10.3390/molecules13081671[↩][↩]
- Negishi, H., Mori, M., Mori, H., & Yamori, Y. (2013). Supplementation of elderly Japanese men and women with fucoidan from seaweed increases immune responses to seasonal influenza vaccination. The Journal of nutrition, 143(11), 1794–1798. https://doi.org/10.3945/jn.113.179036[↩]
- Fletcher HR, Biller P, Ross ABet al.(2017) The seasonal variation of fucoidan within three species of brown macroalgae. Algal Research22, 79-86. https://doi.org/10.1016/j.algal.2016.10.015[↩]
- Abe, S., Hiramatsu, K., Ichikawa, O., Kawamoto, H., Kasagi, T., Miki, Y., Kimura, T. and Ikeda, T. (2013), Safety Evaluation of Excessive Ingestion of Mozuku Fucoidan in Human. Journal of Food Science, 78: T648-T651. https://doi.org/10.1111/j.1750-3841.2012.02966.x[↩]
- Araya N (2011) Fucoidan therapy decreases the proviral load in patients with human T-lymphotropic virus type-1-associated neurological disease. Antiviral therapy16, 89-98.[↩]
- Fucoidan. https://www.mskcc.org/cancer-care/integrative-medicine/herbs/fucoidan[↩]
- Irhimeh MR (2005) A quantitative method to detect fucoidan in human plasma using a novel antibody. Methods and findings in experimental and clinical pharmacology27, 705-710.[↩]
- Ren R, Azuma Y, Ojima Tet al.(2013) Modulation of platelet aggregation-related eicosanoid production by dietary F-fucoidan from brown alga Laminaria japonica in human subjects. British Journal of Nutrition 110, 880-890.[↩]