What is Guggul
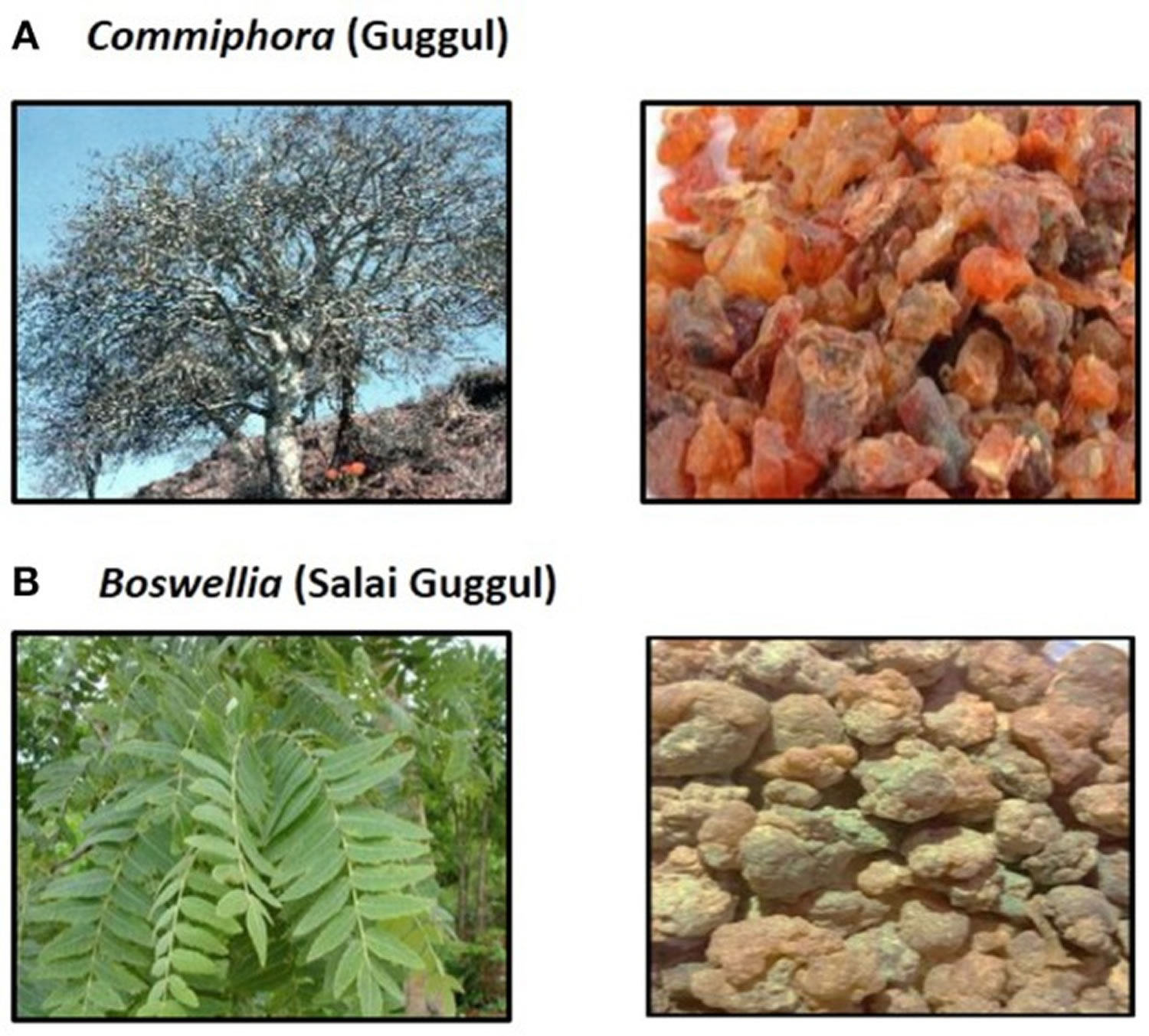
Guggul also known as guggulu or gugulipid, is the oleo-gum resin procured from the white sap of various plants used in Indian Ayurvedic medicine. Two plants, Commiphora wightii (Indian bdellium or myrrh tree) and Boswellia serrata (Salai or Sallaki guggul or Shallaki from Indian olibanum or Indian Frankincense tree) are commonly utilized to prepare guggul (see Figure 1) 1. Guggul is considered to have beneficial effects on multiple organ systems and is used to treat symptoms ranging from cholesterol-lowering, leg swelling and non-specific ulcers to diseases such as inflammatory bowel disease and aggressive malignancies 1. Ancient script on medicine and surgery; Sushrut Samhita, describes that guggul when taken orally can cure internal tumors, malignant sores, obesity, liver dysfunction, intestinal worms, leucoderma, sinus, and edema 2. Guggul has also been used as an Ayurvedic medicine for the prevention and treatment of various other diseases such as inflammatory bowel disease (IBD), ulcers, arthritis, cardiovascular diseases, diabetes etc 3. The main ingredients of guggul are guggulsterone, guggulsterol, boswellic acid and an ethyl-acetate soluble fraction called guggulipid consisting of highly bioactive phytochemicals 1. A large number of test tube and in vivo studies have shown that guggul and its bioactive components act on multiple molecular targets leading to anti-inflammatory, antioxidant, and anti-apoptotic activity. This has led to the use of guggul for conditions such as arthritis, in fat-burners, for dyslipidemia and cardiovascular health. However, studies on the safety and clinical efficacy of guggul or its specific bioactive components are non-existent in published literature 2. Grieco et al 4 described the case of a 63-year-old woman who consumed an over-the-counter lipid-lowering Ayurvedic product called ”Equisterol®” (containing guggul sterol, sitosterol, chlorogenic acid, policosanol, multivitamins and red yeast rice derived monacolin) for 6 months which was followed by the development of acute severe hepatitis. Liver biopsy revealed extensive necroinflammation with eosinophilic infiltration of hepatic lobules. Drug withdrawal and supportive care led to complete resolution of symptoms and normalization of liver tests within 10 days 4. The liver injury could have been due to the monacolin (with statins like activity) in red yeast, even though herb-yeast interaction was not ruled out 4. Yellapu et al 5 described a female bodybuilder who consumed a multi-ingredient fat burner supplement leading to acute liver failure. The supplements (Somalyz and Lipolyz, Species Nutrition, United States) contained usnic acid, L-carnitine, choline and ethanolamine, gamma-aminobutyric acid, vitamin E, green tea extract, guggulsterone Z, and guggulsterone E. Histopathology revealed massive hepatic necrosis and parenchymal collapse with a few areas of ductular regeneration. She underwent cadaveric liver transplantation and was discharged uneventfully. Even though various known plant-derived hepatotoxins (such as usnic acid, green tea extracts) were components of the supplement, the presence of guggul and its interactions were not ruled out 5. Guggul use has been implicated in the development of skin rash, diarrhea, headaches, nausea, and liver toxicity with high doses 6. As reported by Polavarapu and co-workers 6, a 44-year old male developed fatigue, malaise, and jaundice after consuming a fat-burner product (Lipo-6™ containing guggulsterones and green tea extract) for 1 month. Withdrawal of the herbal supplement resulted in complete clinical resolution after 1 month 6. Dalal et al 7 described a middle-aged woman who developed severe hepatocellular jaundice due to the intake of three different Ayurvedic herbal and Homeopathic medications (punarnaya mandur, extract from the Boerhavia diffusa and kanchnar guggulu, extract from Bauhinia variegate). Liver biopsy demonstrated mild portal chronic inflammation and interface activity with grade 3 bridging fibrosis, presence of ceroid-laden Kupffer cells, and conspicuous eosinophils suggestive of herbal-induced liver injury 7. Analysis of the retrieved herbal products and other medications did not reveal known hepatotoxic components, and the patient improved after a short follow-up 7. Guggul and its bioactive compounds have been implicated in possible and probable drug-induced liver injury with hepatocellular pattern of liver damage, which is usually self-limiting. However, there have been reports of acute liver failure requiring liver transplantation when guggul compounds have been part of multiherbal fat-burner products, a herb-herb interaction that remains unexplored 1.
Figure 1. Guggul
Footnotes: (A) Commiphora and Gum guggul. (B) Boswellia and Salai guggul.
[Source 2 ]Chemical constituents of guggul
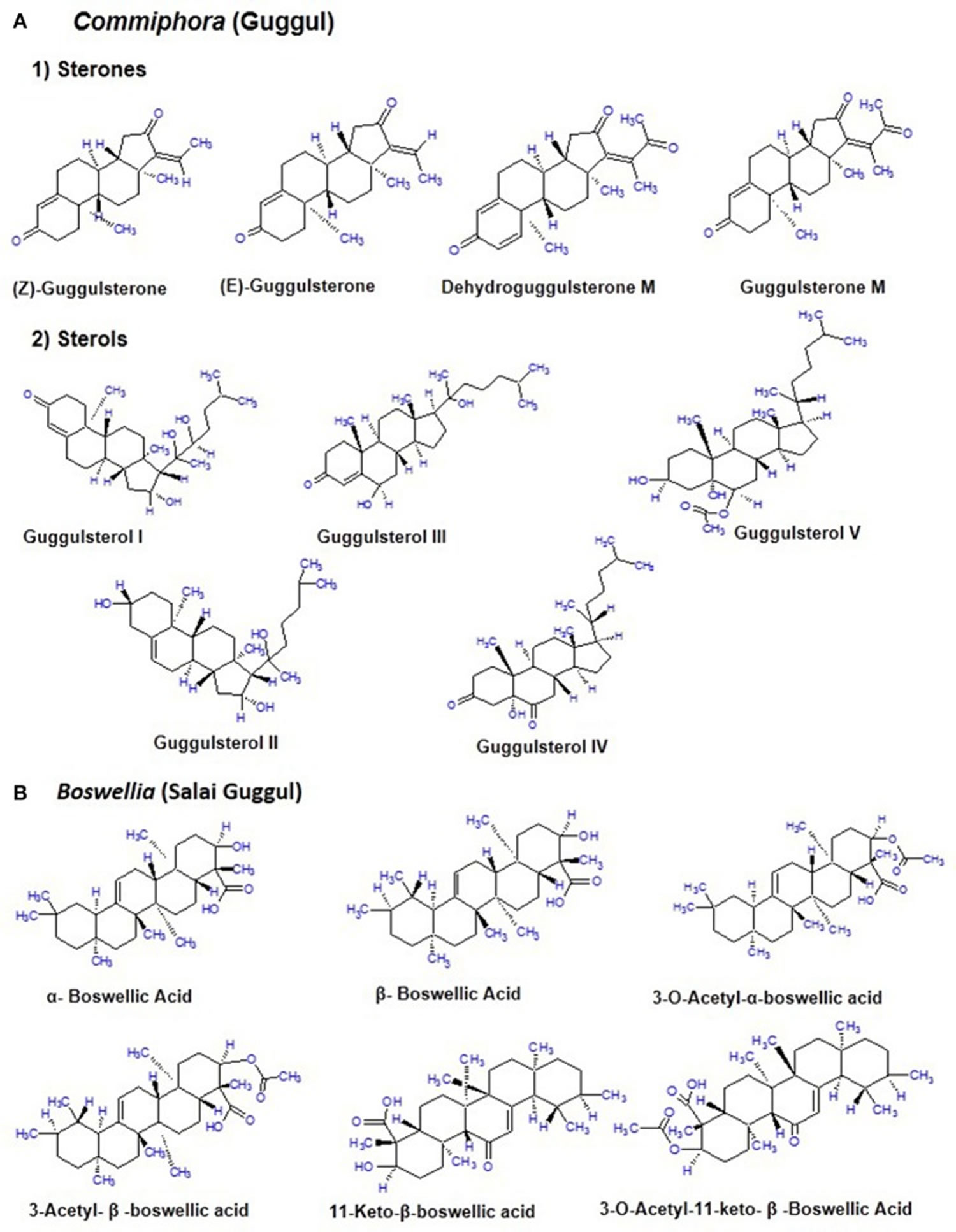
The guggul tree which belongs to the family Burseraceae, is mainly found in the dry regions of the Indian subcontinent mainly India, Pakistan and Bangladesh 2. The oleogum resin of guggul tree (Commiphora mukul) is a yellowish substance that is tapped during winter and ~700–900 g of resin is obtained from each tree 8. The guggul or balsam or the oleo gum resin is found in the balsam canals in the phloem of the large veins of leaf and base of the stem. It is a complicated mixture of minerals, gum, terpenes, sterols (Guggulsterol -I,-II,-III,-IV,-V), essential oils, sterones (Z-, E-, M-guggulsterone, and dehydroguggulsterone-M), ferrulates, lignans, and flavanones. The ethyl acetate soluble fraction also known as guggulipid, consists of various bioactive components like diterpenoids, triterpenoids, steroids, lignans, and fatty tetrol esters. Based on the pH gradient, further fractionation yields 95% neutral, 4% acidic, and 1% basic fractions. The neutral fraction when subjected to further fractionation produces 88% non-ketonic and 12% ketonic fractions. A large number of steroids including the two isomers E-(cis-) and Z-(trans-) guggulsterone [4, 17(20)-pregnadiene-3, 16-dione] were obtained from the ketonic fraction. Nearly 5% guggulipid and 2% gum guggul by weight is present in the guggulsterone (see Figure 2A) 9.
Phenolics are common natural products found in plants and possess substantial antioxidant and anti-inflammatory effects. Various phenolic compounds such as hydroxybenzoic acid derivatives such as gallic acid, protocatechuic acid, gentisic acid, vanillic acid, p-hydroxy benzoic acid, syringic acid, ellagic acid, and cinnamic acid derivatives which include caffeic acid, chlorogenic acid, ferulic acid, sinapic acid, and p-coumaric acid are largely present in plants. These phenolic compounds are predominantly available in guggul as well, which in part contributes to its immense biological function against diverse human chronic diseases 10.
Guggulsterone is the only known antagonist of farnesoid X receptor (FXR). This farnesoid X receptor, also known as NR1H4 (nuclear receptor subfamily 1, group H, member 4), is a bile acid receptor 2. Bioinformatics studies (molecular docking simulation) revealed that guggulsterone binds to farnesoid X receptor (FXR) and nuclear factor-kappa B (NF-κB) and it docks into two non-canonical binding sites of farnesoid X receptor (FXR), helix 1-loop-helix 2 loop and parts of helix—helix 8 including helix 8-loop-helix 9 11. Different bile acids and chenodeoxycholic acids act as natural ligand for farnesoid X receptor, whose expression is elevated in the liver and intestine. When farnesoid X receptor binds to its ligand, it gets activated and reaches the cell nucleus, where it forms a heterodimer with RXR. This heterodimer binds to the hormone response elements on DNA and regulates various genes. Farnesoid X receptor activation downregulates cholesterol 7 alpha-hydroxylase (CYP7A1), the rate-limiting enzyme in bile acid synthesis from cholesterol by inducing the expression of small heterodimer partner which in turn inhibits the transcription of CYP7A1 gene. While obeticholic acid, fexaramine, cafestol, and chenodeoxycholic acid act as agonist of farnesoid X receptor; guggulsterone, from the gum resin of guggul has also been confirmed to inhibit pro-inflammatory signals, together with transcription factor NF-κB 8. Another study reported that the inhibitory activity of NF-κB is due to the binding of guggulsterone to the RH domain of NF-κB precursor protein p105 containing important sequences for DNA binding and dimerization 12.
Boswellia serrata, commonly known as salai guggul, Indian olibanum, loban, or kundur, belongs to the Burseraceae family and is found in dry mountainous regions of India, Northern Africa, and the Middle East. Burseraceae family includes 17 genera and 600 species of plants. The genus Boswellia has 25 different species distributed throughout the tropical regions. Boswellia serrata is one such medicinal plant which potential to combat various chronic disorders. The active pharmacological principle of the oleo gum resin from the trees of different Boswellia species is the boswellic acid 13. The gum resin of the Boswellia species mainly consists of mucus, resin acids, and volatile oil with different quantitative composition from species to species. The gum resin of salai guggul contains pentacyclic triterpenic acids, namely α-boswellic acids, β-boswellic acids, γ-boswellic acid, acetyl-β-boswellic acid, 11-keto-β-boswellic acid (KBA), acetyl-11-keto-β-boswellic acid (AKBA), and tetracyclic triterpenic acids like tirucallic acids viz 3-oxotirucallic acid, 3-hydroxytirucallic acid, and 3-acetoxytirucallic acid (Figure 2B). Other oleo gum resin compounds which display biological activities are: betulinic acid, lupenoic acid, epi-lupeol, isoincensole, isoincensole acetate and 1-ursene-2-diketone-incensole acetate along with few other terpenes that can be found in volatile oil 13.
Figure 2. Chemical constituents of Commiphora (guggul) and Boswellia (Salai guggul)
Footnotes: (A) Commiphora (guggul) (B) Boswellia (Salai guggul)
[Source 2 ]Guggul uses
Guggulu has a long history of use in Ayurveda. Guggul has been used to treat obesity, hyperlipidemia, atherosclerosis, osteoarthritis, rheumatoid arthritis, gout, facial paralysis, sciatica, constipation, hemorrhoids, liver disorders, inflammation, cyst, cervical lymphadenitis, coronary thrombosis, anemia, diabetes, urinary calculus, increased frequency and turbidity of urine, and skin diseases 14. Boswellia extract is also used as a perfume and aroma (frankincense). In the United States, gum guggul extracts are marketed as dietary supplements that will help control serum lipid and cholesterol concentrations and stimulate the thyroid gland 15.
Several clinical trials have been conducted to evaluate the effect of guggul from Commiphora and Boswellia on various chronic disorders. Human studies on guggul has been found to be effective against different diseases such as asthma, breast fibroadenoma, chronic kidney disease, colitis, Crohn’s disease, fascioliasis, hepatitis C, hypercholesterolaemia, hyperlipidemia, metabolic syndrome, nodulocystic acne, arthritis, schistosomiasis, stress urinary incontinence etc (see Table 1) 2. However, Boswellia serrata extracts have been not been approved for these uses in the United States.
The recommended daily intake of gum guggul extract formulations is highly variable among different manufacturers. The Indian Pharmacopeia bases its recommended daily intake levels of gum guggul extracts on the concentration of guggulsterones, such that consumption equates to 75 mg guggulsterones (25 mg, three times a day) 16. Assuming a daily intake of 75 mg of guggulsterones and an adult weight of 60 kg, average human daily consumption would equate to 1.25 mg guggulsterones/kg/day or 85.6 mg gum guggul extract formulation/kg/day based on the 1.46% guggulsterone content of the tested formulation. Boswellia serrata extracts are available over-the-counter in varying concentrations, the usual recommended dose being 250 to 500 mg two or three times daily. Boswellia serrata is also found in many multiingredient products advertised for joint health and gastrointestinal complaints.
Table 1. Clinical trials of guggul (Commiphora and Boswellia) against various chronic diseases
| Disease | Dose | Pts (#) | Clinical outcome | References |
| Healthy volunteer | 1 g b | 10 | Diminished efficacy | Dalvi et al 17 |
| 125 mg, 2 capsules d | 20 | Increased pain threshold & tolerance force, well -tolerated | Prabhavathi et al. 18 | |
| 140 mg e | 47 | Effective | Chilelli et al. 19 | |
| 2 × 250 mg d | 12 | High and quick absorption | Riva et al. 20 | |
| Asthma | 900 mg/day; 6 weeks d | 40 | Improved disease condition | Gupta et al. 21 |
| 500 mg/day d | 32 | Effective | Ferrara et al. 22 | |
| Breast fibroadenomas | –e | 64 | Reduction in fibroadenoma mass | Pasta et al. 23 |
| Chronic kidney disease (CKD) | 516 mg e | 16 | Safe and tolerable | Moreillon et al. 24 |
| –b | 60 | Effective | Shelmadine et al. 25 | |
| Colitis | 900 mg/day; 6 weeks d | 30 | Safe and effective | Gupta et al. 26 |
| 1050 mg/day; 6 weeks d | – | Effective | Gupta et al. 27 | |
| Crohn’s disease | –d | 102 | Safe and effective | Gerhardt et al. 28 |
| 2,400 mg/day; 52 weeks d | 108 | Well-tolerated | Holtmeier et al. 29 | |
| Fascioliasis | 12 mg/kg/day; 6 days f | 7 | Safe, well-tolerated and effective | Massoud et al. 30 |
| 600 mg/day; 6 days f | 1019 | Safe and effective | Abo-Madyan et al. 31 | |
| Hepatitis C | –a | 15 | Effective | Scholtes et al. 32 |
| Hypercholesterolemia | 1,500 mg/day; 12 weeks b | 205 | Effective | Nityanand et al. 33 |
| 100 mg/day; 24 weeks f | 61 | Mild side effects | Singh et al. 34 | |
| 1,000, 2,000 mg/day; 3 days b 103 | – | Well-tolerated, caused dermatologic hypersensitivity | Szapary et al. 35 | |
| 2,160 mg/day; 12 weeks b | 43 | Clinical magnitude is obscure | Nohr et al. 36 | |
| Hyperlipidemia | –f | – | – | Verma and Bordia 37 |
| 75 mg/day; 8 weeks a | – | Safe and effective | Beg et al. 38 | |
| 2 g/day; 8 weeks b | 59 | Effective | Vyas et al. 39 | |
| Metabolic syndrome | 2 pills/day; 4 months b | 78 | Effective | Patti et al. 40 |
| Nodulocystic acne | 50 mg/day; 3 months a | 20 | Reduced inflammatory lesions | Thappa and Dogra 41 |
| Osteoarthritis | 2 capsules, every 8 hours; 3 month-15 day wash-out-3 m e | 42 | – | Kulkarni et al. 42 |
| 500 mg b | 30 | Safe and effective | Singh et al. 43 | |
| 999 mg/day; 8 weeks d | 30 | Well-tolerated | Kimmatkar et al. 44 | |
| 100 or 250 mg/day; 90 days d | 75 | Safe and effective | Sengupta et al. 45 | |
| 1000 mg/day e | 30 | Safe and well-tolerated | Kizhakkedath 46 | |
| 6 capsules/day; 24 weeks e | 440 | Effective, improved knee function | Chopra et al. 47 | |
| Polyarthritis | 3600 mg/day d | 78 | No measurable efficacy | Sander et al. 48 |
| RT-related edema | 4200 mg/day d | 44 | Effective, reduced cerebral edema | Kirste et al. 49 |
| Schistosomiasis | 10 mg/kg/day; 3days f | 204 | Well-tolerated | Sheir et al. 50 |
| 600 mg/days; 6 days f | 1019 | Safe and effective | Abo-Madyan et al. 51 | |
| Skin damage in mammary carcinoma | Cream, twice/day d | 114 | Well-tolerated | Togni et al. 52 |
| Stress urinary incontinence | 4 g/day, 8 weeks e | 30 | Effective | Arkalgud Rangaswamy et al. 53 |
Footnotes: (a) Gugglusterone; (b) Guggul; (c) Formulation of guggul; (d) Boswellia; (e) Formulation of Boswellia; (f) Commiphora.
Guggul health benefits
Arthritis
Arthritis is mainly caused due to inflammation of joints, the tissues surrounding the joints and other connective tissues. Osteoarthritis is the most common form of arthritis which affects a wide range of people across all the places. As guggul has been reported to exhibit high affectivity against arthritis pre-clinically; hence, its effect was evaluated in the clinical setting as well. In one such study, 30 patients with arthritis were treated with gum guggul for 1 month which resulted in remarkable improvement in the total scores of Western Ontario and MacMaster Osteoarthritis Index and condition of the patients 43. Another study was conducted by Kimmatkar et al. 44, to check the safety, tolerability, and efficacy of Boswellia serrata (Salai or Sallaki guggul) extract in 30 patients with knee osteoarthritis. The patients receiving drug treatment reported a decrease in knee pain and swelling of the knee joint as well as increased knee flexion and walking distance.
Asthma
Asthma is a chronic multifactorial inflammatory disease of the respiratory tract and is one of the major health concern. Notably, Boswellia guggul has been found to be effective in the treatment of this disease. In a clinical study, 40 patients having 23 males, and 17 females in the age range of 18–75 years, suffering from bronchial asthma were treated with 300 mg of gum resin thrice daily for a period of 6 weeks 21. This led to improved prognosis in around 70% of the patients as various signs and symptoms of bronchial asthma like rhonchi, dyspnoea, and attacks disappeared upon treatment 21.
Breast fibroadenomas
Breast fibroadenoma accounts for the majority of breast lumps in young women. Boswellia serrata (Salai or Sallaki guggul) was found to exert beneficial effect against breast fibroadenomas as evidenced by a study conducted by Pasta and group 23. They showed that treatment with the combination of Boswellia, betaine, and myo-inositol resulted in decreased fibroadenoma dimension in young women without exerting any toxic effects. The combination also resulted in reduced fibroadenoma volume in 38.8% of the patients in the experimental group, whereas the same was observed only in 17.85% patients in the placebo group 23.
Cardiovascular diseases
Cardiovascular diseases is a group of diseases which involves the heart and the blood vessels is one of the most common causes of death across the globe. Notably, guggul presents a potent remedy for cardiovascular diseases. For example, Singh and group 54 conducted a study to evaluate the cardioprotective benefits of guggul by enrolling 200 patients suffering from ischemic heart disease. The patients were treated with the combination of gum guggul and Inula racemosa for 6 months which resulted in the reduced levels of total cholesterol, triglyceride, and total blood lipids in the patients. It also restored the normal electrocardiogram (ECG) in 26% of the patients, showed improvement of ECG in 59% of the patients and lessened the chest pain in 25% of the patients 54.
Chronic kidney disease
Chronic kidney disease (CKD) is a progressive disease where occurs due to enhanced inflammation and oxidative stress leading to reduced kidney function. Studies have indicated Boswellia serrata (Salai or Sallaki guggul) in combination with Curcuma longa as an effective regimen to obtain reduced inflammation in patients with CKD which functioned via modulation of prostaglandin E2 (PGE2) 25. Moreover, this regimen was found to be safe, well tolerated which also enhanced the levels of inflammatory cytokines in CKD patients 24.
Diabetes mellitus
A large population of the world is affected by diabetes mellitus or type 2 diabetes. Several preclinical studies have shown that the gum resin of commiphora and boswellia are highly effective against this disease. In a clinical study conducted by Ahangarpour et al. 55, it was observed that the treatment of patients with diabetes mellitus with Boswellia serrata (Salai or Sallaki guggul) gum resin (900 mg daily for 6 weeks orally) resulted in decreased risk factors associated with this disease. Further, the treatment also helped in maintaining fructosamine levels, hepatic enzyme activities, and to bring lipid profiles close to normal levels in the patients 55.
Eczema and psoriasis
Eczema also known as dermatitis and psoriasis are caused mainly due to inflammation of the skin. Boswellia serrata (Salai or Sallaki guggul) has been found to exert effectiveness against eczema and psoriasis. A group of scientists revealed that Boswellia-based cream lessens the use of topical corticosteroids and can diminish the grade of erythema and the skin superficial symptoms 52. Furthermore, in a double blind study, the efficacy of a novel formulation of boswellic acid (Bosexil®) containing Boswellia serrata (Salai or Sallaki guggul) resin extract and lecithin was evaluated against both psoriasis and eczema. Improvement in psoriasis, scales (70% of cases), and erythema (50% of cases) was observed with Bosexil® compared to placebo. In addition, when eczema patients were administrated with Bosexil® formulation, it showed improvement in both erythema (60% of cases) and itch (60% of cases) of the patients without any case of waning 52.
Fascioliasis
Fascioliasis is a parasitic worm infection caused by the common liver fluke Fasciola hepatica and Fasciola gigantica. The formulation of myrhh, the gum resisn of Commiphora molmol was reported to be safe, well tolerated, and effective for the management of fascioliasis 30. The formulated drug comprised of 8 parts of resin and 3.5 parts of volatile oils, all extracted from myrrh. They observed that 7 patients who were passing fasciola eggs in their stools displayed distinct improvement of the general condition, drop in the egg count, and improvement of all signs and symptoms with no adverse side effects after treatment with the drug 30.
Gingivitis
Gingivitis, the inflammation of gingiva is a very common form of gum disease. Frankincense extract has been found to exhibit efficacy against gingivitis. A double blinded randomized placebo controlled trial was conducted among 75 female patients aged between 15 and 18 years with moderate plaque-induced gingivitis 56. Six groups were randomly formed based on the administration of 0.1 g of frankincense extract, 0.2 g of its powder, placebo, and whether the patients have undergone scaling and root planning or not. Gingival index, plaque index, bleeding index, and probing pocket depth were measured on the 0, 7th, and 14th days of the study. Detailed analysis of the data revealed that scaling and root planning along with the application of frankincense extract or powder might cause significant decrease in inflammatory indices in comparison to the groups without drug therapy and scaling and root planning 56.
Inflammatory bowel disease
Different clinical studies with guggul have shown its efficacy against inflammatory bowel disease which include colitis and Crohn’s disease. For instance, the gum resin of Boswellia serrata (Salai or Sallaki guggul) was found to be effective in the treatment of chronic colitis with minimal side effects in a clinical study conducted by Gupta et al 26. In this study, the patients with chronic colitis were treated with gum resin from Boswellia serrata (Salai or Sallaki guggul) at a dose of 900 mg daily divided in three doses for 6 weeks. The treatment resulted in the improvement of stool properties, hemoglobin, serum iron, calcium, phosphorus, proteins, total leukocytes, and eosinophils in the patients 26. Further, a double-blind, placebo-controlled, randomized, parallel study on 82 patients with Crohn’s disease was conducted where patients were given a new Boswellia serrata (Salai or Sallaki guggul) extract; Boswelan. In this trial, remission was observed in 59.9% of the actively treated patients. Additionally, this study also confirmed better tolerability of Boswelan in long-term treatment of Crohn’s disease (Holtmeier et al., 2011). Furthermore, leukotrienes play an important role in inflammation of the colon in ulcerative colitis. Sallai guggul gum resin is known to be specific, non-redox, and non-competitive inhibitors of 5-LOX, a crucial enzyme of leukotriene biosynthesis. Patients with grade II and III ulcerative colitis were treated with Boswellia serrata (Salai or Sallaki guggul) gum resin at a dose of 350 mg thrice daily for 6 weeks. Stool properties, histopathology, and scan microscopy of rectal biopsies, blood parameters including hemoglobin, serum iron, calcium, phosphorus, proteins, total leukocytes, and eosinophils showed slightly better improvement in Boswellia treated patients 27.
Nodulocystic acne
Guggul is considered to be very effective in topical and oral complementary as well as an alternative medicine for the treatment of acne 57. In a clinical study conducted by Thappa and Dogra 41, patients with nodulocystic acne were given guggulipid equivalent to 25 mg guggulsterone for 3 months, which resulted in progressive reduction in lesions in majority of patients. However, patients with oily faces displayed better response to guggulipid.
Guggul side effects
Guggul side effects are few and largely mild and transient gastrointestinal symptoms of nausea, diarrhea or constipation 58. In most controlled studies, adverse events were no more frequent with Boswellia extracts than with placebo. In a previously conducted clinical trial, individuals were treated with a guggulipid formulation at doses of up to 150 mg guggulsterones/day for 8 weeks, with adverse reports limited to low incidences of diarrhea and the development of a hypersensitivity skin rash 59. Boswellia serrata extract has not been linked to serum enzyme elevations during therapy, although there have been few prospective studies in humans that have reported on its effects on laboratory test results in any detail. In small trials, Boswellia extracts have appeared to be well tolerated with only minor and few adverse effects which have been similar in frequency among persons receiving placebo 58. Despite wide scale use as an herbal supplement, Boswellia extract has not been convincingly linked to published instances of clinically apparent liver injury 58. Boswellia is often included in multi-ingredient dietary supplements some of which have been implicated in liver injury, but a specific contribution from Boswellia to the injury could not be established. The frequency of hypersensitivity reactions to Boswellia is also not known.
Grieco et al 4 described the case of a 63-year-old woman who consumed an over-the-counter lipid-lowering Ayurvedic product called ”Equisterol®” (containing guggul sterol, sitosterol, chlorogenic acid, policosanol, multivitamins and red yeast rice derived monacolin) for 6 months which was followed by the development of acute severe hepatitis. Liver biopsy revealed extensive necroinflammation with eosinophilic infiltration of hepatic lobules. Drug withdrawal and supportive care led to complete resolution of symptoms and normalization of liver tests within 10 days 4. The liver injury could have been due to the monacolin (with statins like activity) in red yeast, even though herb-yeast interaction was not ruled out 4. Yellapu et al 5 described a female bodybuilder who consumed a multi-ingredient fat burner supplement leading to acute liver failure. The supplements (Somalyz and Lipolyz, Species Nutrition, United States) contained usnic acid, L-carnitine, choline and ethanolamine, gamma-aminobutyric acid, vitamin E, green tea extract, guggulsterone Z, and guggulsterone E. Histopathology revealed massive hepatic necrosis and parenchymal collapse with a few areas of ductular regeneration. She underwent cadaveric liver transplantation and was discharged uneventfully. Even though various known plant-derived hepatotoxins (such as usnic acid, green tea extracts) were components of the supplement, the presence of guggul and its interactions were not ruled out 5. Guggul use has been implicated in the development of skin rash, diarrhea, headaches, nausea, and liver toxicity with high doses 6. As reported by Polavarapu and co-workers 6, a 44-year old male developed fatigue, malaise, and jaundice after consuming a fat-burner product (Lipo-6™ containing guggulsterones and green tea extract) for 1 month. Withdrawal of the herbal supplement resulted in complete clinical resolution after 1 month 6. Dalal et al 7 described a middle-aged woman who developed severe hepatocellular jaundice due to the intake of three different Ayurvedic herbal and Homeopathic medications (punarnaya mandur, extract from the Boerhavia diffusa and kanchnar guggulu, extract from Bauhinia variegate). Liver biopsy demonstrated mild portal chronic inflammation and interface activity with grade 3 bridging fibrosis, presence of ceroid-laden Kupffer cells, and conspicuous eosinophils suggestive of herbal-induced liver injury 7. Analysis of the retrieved herbal products and other medications did not reveal known hepatotoxic components, and the patient improved after a short follow-up 7. Guggul and its bioactive compounds have been implicated in possible and probable drug-induced liver injury with hepatocellular pattern of liver damage, which is usually self-limiting. However, there have been reports of acute liver failure requiring liver transplantation when guggul compounds have been part of multiherbal fat-burner products, a herb-herb interaction that remains unexplored 1.
- Philips, C. A., Ahamed, R., Rajesh, S., George, T., Mohanan, M., & Augustine, P. (2020). Comprehensive review of hepatotoxicity associated with traditional Indian Ayurvedic herbs. World journal of hepatology, 12(9), 574–595. https://doi.org/10.4254/wjh.v12.i9.574[↩][↩][↩][↩][↩]
- Kunnumakkara, A. B., Banik, K., Bordoloi, D., Harsha, C., Sailo, B. L., Padmavathi, G., Roy, N. K., Gupta, S. C., & Aggarwal, B. B. (2018). Googling the Guggul (Commiphora and Boswellia) for Prevention of Chronic Diseases. Frontiers in pharmacology, 9, 686. https://doi.org/10.3389/fphar.2018.00686[↩][↩][↩][↩][↩][↩][↩]
- Shishodia S, Harikumar KB, Dass S, Ramawat KG, Aggarwal BB. The guggul for chronic diseases: ancient medicine, modern targets. Anticancer Res. 2008 Nov-Dec;28(6A):3647-64.[↩]
- Grieco A, Miele L, Pompili M, Biolato M, Vecchio FM, Grattagliano I, Gasbarrini G. Acute hepatitis caused by a natural lipid-lowering product: when “alternative” medicine is no “alternative” at all. J Hepatol. 2009 Jun;50(6):1273-7. doi: 10.1016/j.jhep.2009.02.021[↩][↩][↩][↩][↩][↩]
- Yellapu RK, Mittal V, Grewal P, Fiel M, Schiano T. Acute liver failure caused by ‘fat burners’ and dietary supplements: a case report and literature review. Can J Gastroenterol. 2011 Mar;25(3):157-60. doi: 10.1155/2011/174978[↩][↩][↩][↩]
- Polavarapu AD, Daoud M, Philipose J, Deeb L. Fat burner causing liver injury [Abstract] Am J Gastro. 2017;112:S1571.[↩][↩][↩][↩][↩][↩]
- Dalal KK, Holdbrook T, Peikin SR. Ayurvedic drug induced liver injury. World J Hepatol. 2017 Nov 8;9(31):1205-1209. doi: 10.4254/wjh.v9.i31.1205[↩][↩][↩][↩][↩][↩]
- Yamada T, Sugimoto K. Guggulsterone and Its Role in Chronic Diseases. Adv Exp Med Biol. 2016;929:329-361. doi: 10.1007/978-3-319-41342-6_15[↩][↩]
- Sarup P, Bala S, Kamboj S. Pharmacology and Phytochemistry of Oleo-Gum Resin of Commiphora wightii (Guggulu). Scientifica (Cairo). 2015;2015:138039. doi: 10.1155/2015/138039[↩]
- Hazra A. K., Sur T. K., Chakraborty B., Seal T. (2018). HPLC analysis of phenolic acids and antioxidant activity of some classical ayurvedic guggulu formulations. Int. J. Res. Ayurveda Pharm. 9, 112–117. 10.7897/2277-4343.09122[↩]
- Yang L, Broderick D, Jiang Y, Hsu V, Maier CS. Conformational dynamics of human FXR-LBD ligand interactions studied by hydrogen/deuterium exchange mass spectrometry: insights into the antagonism of the hypolipidemic agent Z-guggulsterone. Biochim Biophys Acta. 2014 Sep;1844(9):1684-93. doi: 10.1016/j.bbapap.2014.06.007[↩]
- Khan, M. K., Ansari, I. A., & Khan, M. S. (2013). Dietary phytochemicals as potent chemotherapeutic agents against breast cancer: Inhibition of NF-κB pathway via molecular interactions in rel homology domain of its precursor protein p105. Pharmacognosy magazine, 9(33), 51–57. https://doi.org/10.4103/0973-1296.108140[↩]
- Roy NK, Deka A, Bordoloi D, Mishra S, Kumar AP, Sethi G, Kunnumakkara AB. The potential role of boswellic acids in cancer prevention and treatment. Cancer Lett. 2016 Jul 10;377(1):74-86. doi: 10.1016/j.canlet.2016.04.017[↩][↩]
- Anurekha J., Gupta V. B. Chemistry and pharmacological profile of guggulu—a review. Indian Journal of Traditional Knowledge. 2006;5:478–483.[↩]
- National Toxicology Program. NTP Technical Report on the Toxicity Studies of a Gum Guggul Extract Formulation Administered by Gavage to Sprague Dawley (Hsd:Sprague Dawley® SD®) Rats and B6C3F1/N Mice: Toxicity Report 99 [Internet]. Research Triangle Park (NC): National Toxicology Program; 2020 Jun. Discussion. Available from: https://www.ncbi.nlm.nih.gov/books/NBK561194[↩]
- Indian Ministry of Health and Family Welfare. Indian pharmacopoeia. Vol 2. New Delhi, India: Delhi: Controller of Publications; 1996. p. 357-358.[↩]
- Dalvi S. S., Nayak V. K., Pohujani S. M., Desai N. K., Kshirsagar N. A., Gupta K. C. (1994). Effect of gugulipid on bioavailability of diltiazem and propranolol. J. Assoc. Physicians India 42, 454–455.[↩]
- Prabhavathi K., Chandra U. S., Soanker R., Rani P. U. (2014). A randomized, double blind, placebo controlled, cross over study to evaluate the analgesic activity of Boswellia serrata in healthy volunteers using mechanical pain model. Indian J. Pharmacol. 46, 475–479. 10.4103/0253-7613.140570[↩]
- Chilelli N. C., Ragazzi E., Valentini R., Cosma C., Ferraresso S., Lapolla A., Sartore G. (2016). Curcumin and Boswellia serrata modulate the glyco-oxidative status and lipo-oxidation in master athletes. Nutrients 8:E745. 10.3390/nu8110745[↩]
- Riva A., Morazzoni P., Artariam C., Allegrinim P., Meinsm J., Savio Appendino G., et al. . (2016). A single-dose, randomized, cross-over, two-way, open-label study for comparing the absorption of boswellic acids and its lecithin formulation. Phytomedicine 23, 1375–1382. 10.1016/j.phymed.2016.07.009[↩]
- Gupta I., Gupta V., Parihar A., Gupta S., Lüdtke R., Safayhi H., et al. (1998). Effects of B. serrata gum resin in patients with bronchial asthma: results of a double-blind, placebo-controlled, 6-week clinical study. Eur. J. Med. Res. 3, 511–514.[↩][↩][↩]
- Ferrara T., De Vincentiis G., Di Pierro F. (2015). Functional study on Boswellia phytosome as complementary intervention in asthmatic patients. Eur. Rev. Med. Pharmacol. Sci. 19, 3757–3762.[↩]
- Pasta V., Dinicola S., Giuliani A., Harrath A. H., Alwasel S. H., Tartaglia F., et al. . (2016). A randomized trial of Boswellia in association with betaine and myo-inositol in the management of breast fibroadenomas. Eur. Rev. Med. Pharmacol. Sci. 20, 1860–1865.[↩][↩][↩]
- Moreillon J. J., Bowden R. G., Deike E., Griggs J., Wilson R., Shelmadine B., et al. . (2013). The use of an anti-inflammatory supplement in patients with chronic kidney disease. J. Complement Integr. Med. 10, 143–152. 10.1515/jcim-2012-0011[↩][↩]
- Shelmadine B. D., Bowden R. G., Moreillon J. J., Cooke M. B., Yang P., Deike E., et al. . (2017). A pilot study to examine the effects of an anti-inflammatory supplement on eicosanoid derivatives in patients with chronic kidney disease. J. Altern. Complement. Med. 23, 632–638. 10.1089/acm.2016.0007[↩][↩]
- Gupta I., Parihar A., Malhotra P., Gupta S., Lüdtke R., Safayhi H., et al. (2001). Effects of gum resin of B. serrata in patients with chronic colitis. Planta Med. 67, 391–395. 10.1055/s-2001-15802[↩][↩][↩]
- Gupta I., Parihar A., Malhotra P., Singh G. B., Lüdtke R., Safayhi H., et al. (1997). Effects of B. serrata gum resin in patients with ulcerative colitis. Eur. J. Med. Res. 2, 37–43.[↩][↩]
- Gerhardt H., Seifert F., Buvari P., Vogelsang H., Repges R. (2001). Therapy of active Crohn disease with Boswellia serrata extract H 15. Z. Gastroenterol. 39, 11–17. 10.1055/s-2001-10708[↩]
- Holtmeier W., Zeuzem S., Preiss J., Kruis W., Böhm S., Maaser C., et al. (2011). Randomized, placebo-controlled, double- blind trial of B. serrata in maintaining remission of Crohn’s disease: good safety profile but lack of efficacy. Inflamm. Bowel Dis. 17, 573–582. 10.1002/ibd.21345[↩]
- Massoud A., El Sisi S., Salama O., Massoud A. (2001). Preliminary study of therapeutic efficacy of a new fasciolicidal drug derived from Commiphora molmol (myrrh). Am. J. Trop. Med. Hyg. 65, 96–99. 10.4269/ajtmh.2001.65.96[↩][↩][↩]
- Abo-Madyan A. A., Morsy T. A., Motawea S. M., Morsy A. T. (2004b). Clinical trial of Mirazid in treatment of human fascioliasis, Ezbet El-Bakly (Tamyia Center) Al-Fayoum Governorate. J. Egypt. Soc. Parasitol. 34, 807–818.[↩]
- Scholtes C., André P., Trépo C., Cornu C., Remontet L., Ecochard R., et al. . (2012). Farnesoid X receptor targeting for hepatitis C: study protocol for a proof-of-concept trial. Therapie 67, 423–427. 10.2515/therapie/2012058[↩]
- Nityanand S., Srivastava J. S., Asthana O. P. (1989). Clinical trials with gugulipid. A new hypolipidaemic agent. J. Assoc. Physicians India 37, 323–328.[↩]
- Singh R. B., Niaz M. A., Ghosh S. (1994). Hypolipidemic and antioxidant effects of Commiphora mukul as an adjunct to dietary therapy in patients with hypercholesterolemia. Cardiovasc. Drugs Ther. 8, 659–664. 10.1007/BF00877420[↩]
- Szapary P. O., Wolfe M. L., Bloedon L. T., Cucchiara A. J., DerMarderosian A. H., Cirigliano M. D., et al. . (2003). Guggulipid for the treatment of hypercholesterolemia: a randomized controlled trial. JAMA 290, 765–772. 10.1001/jama.290.6.765[↩]
- Nohr L. A., Rasmussen L. B., Straand J. (2009). Resin from the mukul myrrh tree, guggul, can it be used for treating hypercholesterolemia? A randomized, controlled study. Complement. Ther. Med. 17, 16–22. 10.1016/j.ctim.2008.07.001[↩]
- Verma S. K., Bordia A. (1988). Effect of Commiphora mukul (gum guggulu) in patients of hyperlipidemia with special reference to HDL-cholesterol. Indian J. Med. Res. 87, 356–360.[↩]
- Beg M., Singhal K. C., Afzaal S. (1996). A study of effect of guggulsterone on hyperlipidemia of secondary glomerulopathy. Indian J. Physiol. Pharmacol. 40, 237–240.[↩]
- Vyas K. Y., Bedarkar P., Galib R., Prajapati P. K. (2015). Comparative Anti-hyperlipidaemic activity of Navina (fresh) and Purāṇa (old) Guggulu. Anc. Sci. Life. 35, 101–109. 10.4103/0257-7941.171672[↩]
- Patti A. M., Al-Rasadi K., Katsiki N., Banerjee Y., Nikolic D., Vanella L., et al. . (2015). Effect of a natural supplement containing Curcuma longa, guggul, and chlorogenic acid in patients with metabolic syndrome. Angiology 66, 856–861. 10.1177/0003319714568792[↩]
- Thappa D. M., Dogra J. (1994). Nodulocystic acne: oral gugulipid versus tetracycline. J. Dermatol. 21, 729–731. 10.1111/j.1346-8138.1994.tb03277.x[↩][↩]
- Kulkarni R. R., Patki P. S., Jog V. P., Gandage S. G., Patwardhan B. (1991). Treatment of osteoarthritis with a herbomineral formulation: a double-blind, placebo-controlled, cross-over study. J. Ethnopharmacol. 33, 91–95. 10.1016/0378-8741(91)90167-C[↩]
- Singh B. B., Mishra L. C., Vinjamury S. P., Aquilina N., Singh V. J., Shepard N. (2003). The effectiveness of C. mukul for osteoarthritis of the knee: an outcomes study. Altern. Ther. Health Med. 9, 74–79.[↩][↩]
- Kimmatkar N., Thawani V., Hingorani L., Khiyani R. (2003). Efficacy and tolerability of B. serrata extract in treatment of osteoarthritis of knee–a randomized double blind placebo controlled trial. Phytomedicine 10, 3–7. 10.1078/094471103321648593[↩][↩]
- Sengupta K., Alluri K. V., Satish A. R., Mishra S., Golakoti T., Sarma K. V., et al. . (2008). A double blind, randomized, placebo controlled study of the efficacy and safety of 5-Loxin for treatment of osteoarthritis of the knee. Arthritis Res. Ther. 10:R85. 10.1186/ar2461[↩]
- Kizhakkedath R. (2013). Clinical evaluation of a formulation containing Curcuma longa and Boswellia serrata extracts in the management of knee osteoarthritis. Mol. Med. Rep. 8, 1542–1548. 10.3892/mmr.2013.1661[↩]
- Chopra A., Saluja M., Tillu G., Sarmukkaddam S., Venugopalan A., Narsimulu G., et al. . (2013). Ayurvedic medicine offers a good alternative to glucosamine and celecoxib in the treatment of symptomatic knee osteoarthritis: a randomized, double-blind, controlled equivalence drug trial. Rheumatology 52, 1408–1417. 10.1093/rheumatology/kes414[↩]
- Sander O., Herborn G., Rau R. (1998). Is H15 (resin extract of Boswellia serrata, “incense”) a useful supplement to established drug therapy of chronic polyarthritis? Results of a double-blind pilot study. Z. Rheumatol. 57, 11–16. 10.1007/s003930050051[↩]
- Kirste S., Treier M., Wehrle S. J., Becker G., Abdel-Tawab M., Gerbeth K., et al. . (2011). Boswellia serrata acts on cerebral edema in patients irradiated for brain tumors: a prospective, randomized, placebo-controlled, double-blind pilot trial. Cancer 117, 3788–3795. 10.1002/cncr.25945[↩]
- Sheir Z., Nasr A. A., Massoud A., Salama O., Badra G. A., El-Shennawy H., et al. . (2001). A safe, effective, herbal antischistosomal therapy derived from myrrh. Am. J. Trop. Med. Hyg. 65, 700–704. 10.4269/ajtmh.2001.65.700[↩]
- Abo-Madyan A. A., Morsy T. A., Motawea S. M. (2004a). Efficacy of Myrrh in the treatment of schistosomiasis (haematobium and mansoni) in Ezbet El-Bakly, Tamyia Center, El-Fayoum Governorate, Egypt. J. Egypt Soc. Parasitol. 34, 423–446.[↩]
- Togni S., Maramaldi G., Bonetta A., Giacomelli L., Di Pierro F. (2015). Clinical evaluation of safety and efficacy of Boswellia-based cream for prevention of adjuvant radiotherapy skin damage in mammary carcinoma: a randomized placebo controlled trial. Eur. Rev. Med. Pharmacol. Sci. 19, 1338–1344.[↩][↩][↩]
- Arkalgud Rangaswamy P., Sultana A., Rahman K., Nagapattinam S. (2014). Efficacy of Boswellia serrata L. and Cyperus scariosus L. plus pelvic floor muscle training in stress incontinence in women of reproductive age. Complement. Ther. Clin. Pract. 20, 230–236. 10.1016/j.ctcp.2014.08.003[↩]
- Singh R. P., Singh R., Ram P., Batliwala P. G. (1993). Use of Pushkar- Guggul, an indigenous antiischemic combination, in the management of ischemic heart disease. Int. J. Pharmacol. 31, 147–160. 10.3109/13880209309082932[↩][↩]
- Ahangarpour A, Heidari H, Fatemeh RA, Pakmehr M, Shahbazian H, Ahmadi I, Mombeini Z, Mehrangiz BH. Effect of Boswellia serrata supplementation on blood lipid, hepatic enzymes and fructosamine levels in type2 diabetic patients. J Diabetes Metab Disord. 2014 Feb 4;13(1):29. doi: 10.1186/2251-6581-13-29[↩][↩]
- Khosravi Samani M, Mahmoodian H, Moghadamnia A, Poorsattar Bejeh Mir A, Chitsazan M. The effect of Frankincense in the treatment of moderate plaque-induced gingivitis: a double blinded randomized clinical trial. Daru. 2011;19(4):288-94.[↩][↩]
- Magin PJ, Adams J, Pond CD, Smith W. Topical and oral CAM in acne: a review of the empirical evidence and a consideration of its context. Complement Ther Med. 2006 Mar;14(1):62-76. doi: 10.1016/j.ctim.2005.10.007[↩]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Boswellia Serrata. [Updated 2020 Nov 4]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK563692[↩][↩][↩]
- Szapary PO, Wolfe ML, Bloedon LT, Cucchiara AJ, DerMarderosian AH, Cirigliano MD, Rader DJ. Guggulipid for the treatment of hypercholesterolemia: A randomized controlled trial. JAMA. 2003;290(6):765–772. 10.1001/jama.290.6.765[↩]