Hemostasis
Hemostasis is the body’s way of stopping injured blood vessels from bleeding 1. Hemostasis includes clotting of your blood. Hemostasis is the physiological process that stops bleeding at the site of an injury while maintaining normal blood flow elsewhere in the circulation 2. Hemostasis is a process that involves multiple interlinked steps. This cascade culminates into the formation of a “platelet plug” (a hemostatic plug) that closes up the damaged site of the blood vessel controlling the bleeding. Hemostasis begins with trauma to the lining of the blood vessel also known as the endothelium. The endothelium in blood vessels maintains an anticoagulant surface that serves to maintain blood in its fluid state, but if the blood vessel is damaged components of the subendothelial matrix are exposed to the blood 2. Several of these components activate the two main processes of hemostasis to initiate formation of a blood clot, composed primarily of platelets and fibrin. This process is tightly regulated such that it is activated within seconds of an injury but must remain localized to the site of injury.
Formation of a clot also involves activation of a sequence of blood clotting factors, which are proteins produced mainly by the liver. There are over a dozen blood clotting factors. They interact in a complicated series of chemical reactions that ultimately generate thrombin. Thrombin converts fibrinogen, a blood clotting factor that is normally dissolved in blood, into long strands of fibrin that radiate from the clumped platelets and form a net that entraps more platelets and blood cells. The fibrin strands add bulk to the developing clot and help hold it in place to keep the vessel wall plugged.
Severe liver disease (such as cirrhosis or liver failure) can reduce production of clotting factors and increase risk of excessive bleeding. Because the liver needs vitamin K to make some of the clotting factors, vitamin K deficiency can cause excessive bleeding.
There are various cellular components in the process of coagulation. Most notably are those processes associated with the endothelium, platelets, and hepatocytes.
- Endothelium. Clotting factors III and VIII originate from the endothelial cells while the clotting factor IV comes from the plasma 3. Factor III, IV, and VIII all undergo K dependent gamma-carboxylation of their glutamic acid residues, which allows for binding with calcium and other ions while in the coagulation pathway 4.
- Platelets. These are non-nucleated disc-like cells created from megakaryocytes that arise from the bone marrow. They are about 2 to 3 microns in size. Some of their unique structural elements include plasma membrane, open canalicular system, spectrin and actin cytoskeleton, microtubules, mitochondria, lysosomes, granules, and peroxisomes 5. These cells release proteins involved in clotting and platelet aggregation.
- Hepatocytes. The liver produces the majority of the proteins that function as clotting factors and as anticoagulants.
The mechanism of hemostasis can divide into four stages:
- Constriction of the blood vessel.
- Formation of a temporary “platelet plug.”
- Activation of the coagulation cascade.
- Formation of “fibrin plug” or the final clot.
Multiple anticoagulant mechanisms regulate and control these systems to maintain blood fluidity in the absence of injury and generate a clot that is proportional to the injury. The proper balance between procoagulant systems and anticoagulant systems is critical for proper hemostasis and the avoidance of pathological bleeding or thrombosis.
Too much clotting can block blood vessels that are not bleeding. Too little clotting can cause excessive bleeding from minor injury. Consequently, your body has control mechanisms to limit clotting and dissolve clots that are no longer needed. An abnormality in any part of the system that controls bleeding can lead to excessive bleeding or excessive clotting, both of which can be dangerous. When clotting is poor, even a slight injury to a blood vessel may lead to severe blood loss. When clotting is excessive, small blood vessels in critical places can become clogged with clots. Clogged vessels in the brain can cause strokes, and clogged vessels leading to the heart can cause heart attacks. Pieces of clots from veins in the legs, pelvis, or abdomen can travel through the bloodstream to the lungs and block major arteries there (pulmonary embolism).
Figure 1. Hemostasis
Hemostasis process
Hemostasis facilitates a series of enzymatic activations that lead to the formation of a clot with platelets and fibrin polymer 6. This clot seals the injured area, controls and prevents further bleeding while the tissue regeneration process takes place. Once the injury starts to heal, the plug slowly remodels, and it dissolves with the restoration of normal tissue at the site of the damage 6.
When an injury causes a blood vessel wall to break, platelets are activated. They change shape from round to spiny, stick to the broken vessel wall and each other, and begin to plug the break. They also interact with other blood proteins to form fibrin. Fibrin strands form a net that entraps more platelets and blood cells, producing a clot that plugs the break.
Hemostasis involves four major processes:
- Narrowing (constriction) of blood vessels (vasoconstriction)
- Primary hemostasis: Activity of cell-like blood particles that help in blood clotting (platelets)
- Secondary hemostasis: Activity of proteins found in blood that work with platelets to help the blood clot (clotting factors)
- Tertiary hemostasis: The clot attracts and stimulates the growth of fibroblasts and smooth muscle cells within the vessel wall, and begins the repair process which ultimately results in the dissolution of the clot (fibrinolysis).
Vasoconstriction
An injured blood vessel constricts so that blood flows out more slowly and clotting can start. At the same time, the accumulating pool of blood outside the blood vessel (a hematoma) presses against the vessel, helping prevent further bleeding.
Within about 30 minutes of damage/trauma to the blood vessels, vascular spasm ensues, which leads to vasoconstriction or narrowing (constriction) of blood vessels. At the site of the disrupted endothelial lining, the extracellular matrix (ECM) or collagen becomes exposed to the blood components 7.
Primary Hemostasis (Platelet factors)
Primary hemostasis refers to platelet aggregation and platelet plug formation. Platelets are activated in a multifaceted process (see below), and as a result they adhere to the site of injury and to each other, plugging the injury.
Platelets are small anuclear cell fragments that bud off from megakaryocytes, specialized large polyploid blood cells that originate in the bone marrow 8. Platelets are present at 150 to 400 million per milliliter of blood and circulate for about ten days 9.. In a healthy blood vessel, and under normal blood flow, platelets do not adhere to surfaces or aggregate with each other. However, in the event of injury platelets are exposed to subendothelial matrix, and adhesion and activation of platelets begins (Figure 2).
Multiple receptors on the surface of platelets are involved in these adhesive interactions, and these receptors are targeted by multiple adhesive proteins. The key for all of these receptors is that the adhesive interaction only takes place in the event of an injury to the blood vessel. This restriction is maintained in several different ways.
Platelet adhesion
The extracellular matrix (ECM) releases cytokines and inflammatory markers that lead to adhesion of the platelets and their aggregation at that site which leads to the formation of a platelet plug and sealing of the defect. The platelet adhesion is a complex process mediated by interactions between various receptors and proteins including tyrosine kinase receptors, glycoprotein receptors, other G-protein receptors as well as the von Willebrand Factor (vWF). The von Willebrand Factor functions via binding to the Gp 1b-9 within the platelets 7.
As soon as a blood vessel wall is damaged, a series of reactions activates platelets so that they stick to the injured area. The “glue” that holds platelets to the blood vessel wall is von Willebrand factor, a large protein produced by the cells of the vessel wall. The proteins collagen and thrombin act at the site of the injury to induce platelets to stick together. As platelets accumulate at the site, they form a mesh that plugs the injury. The platelets change shape from round to spiny, and they release proteins and other substances that entrap more platelets and clotting proteins in the enlarging plug that becomes a blood clot.
Platelet activation
The platelets that have adhered undergo very specific changes. They release their cytoplasmic granules that include ADP, thromboxane A2, serotonin, and multiple other activation factors. They also undergo a transformation of their shape into a pseudopodal shape which in-turn leads to release reactions of various chemokines. P2Y1 receptors help in the conformational changes in platelets 7.
Platelet aggregation
With the mechanisms mentioned above, various platelets are activated, adhered to each other and the damaged endothelial surface leading to the formation of a primary platelet plug.
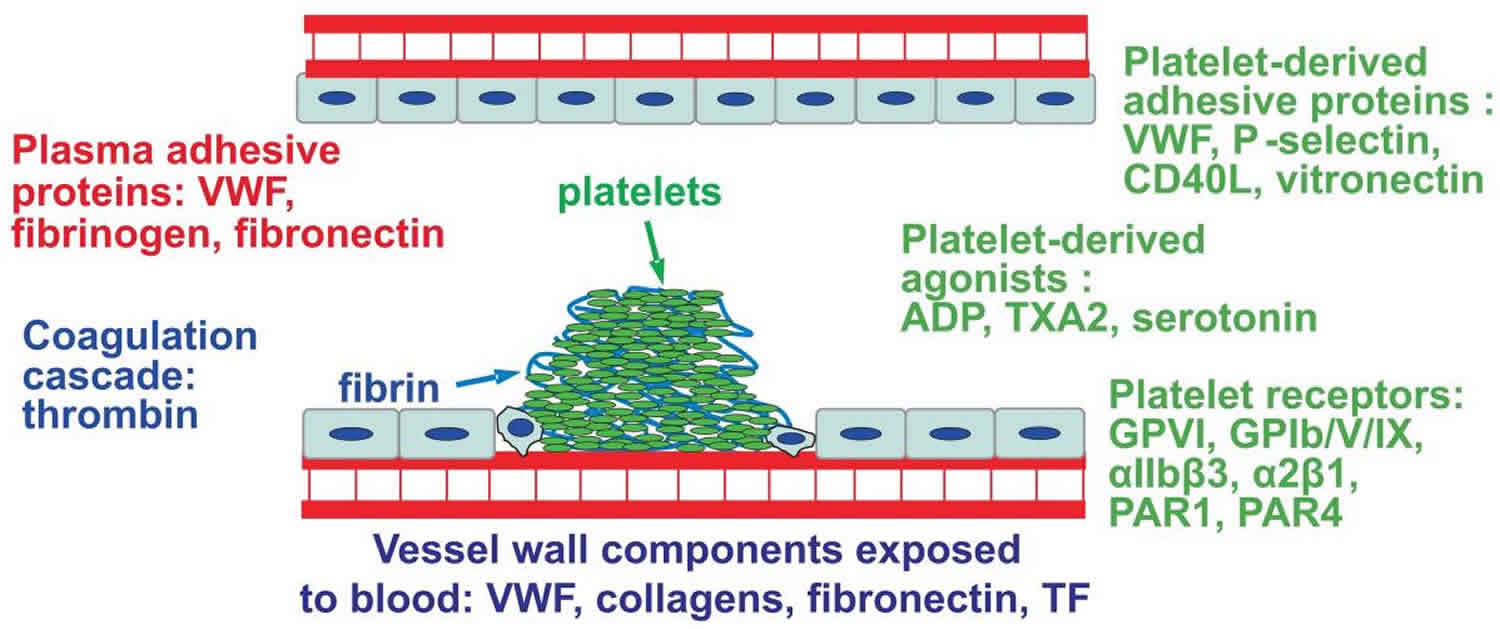
Figure 2. Primary hemostasis
Footnote: Primary hemostasis; the platelet response. Platelet aggregation at the site of injury is mediated by platelet receptors, platelet-derived agonists, platelet-derived adhesive proteins and plasma-derived adhesive proteins. Fibrin deposition around the resulting platelet plug is generated by the coagulation cascade.
Abbreviations: ADP = adenosine diphosphate, TXA2 = thromboxane A2 and VWF = von Willebrand factor.
(Source 2 )
Secondary Hemostasis (Blood clotting factors)
Secondary hemostasis refers to the deposition of insoluble fibrin, which is generated by the cascade of coagulation serine proteases that culminates in cleavage of soluble fibrinogen by thrombin (Figure 3). Thrombin cleavage generates insoluble fibrin that forms a crosslinked fibrin mesh at the site of an injury. This insoluble fibrin forms a mesh that is incorporated into and around the platelet plug. This mesh serves to strengthen and stabilize the blood clot. These two processes happen simultaneously and are mechanistically intertwined. The fibrinolysis pathway also plays a significant role in hemostasis. Fibrin generation also occurs simultaneously to platelet aggregation 10. In intact and healthy blood vessels this cascade is not activated and several anticoagulant mechanisms prevent its activation. These include the presence of thrombomodulin and heparan sulfate proteoglycans on vascular endothelium. Thrombomodulin is a cofactor for thrombin that converts it from a procoagulant to an anticoagulant by stimulating activation of the anticoagulant serine protease protein C 11. Heparan sulfate proteoglycans stimulate the activation of the serine protease inhibitor (or serpin) antithrombin, which inactivates thrombin and factor Xa 12. Pathological thrombus formation, called thrombosis, or pathological bleeding can occur whenever this process is dis-regulated. The complexity of these systems has been increasingly appreciated in the last few decades.
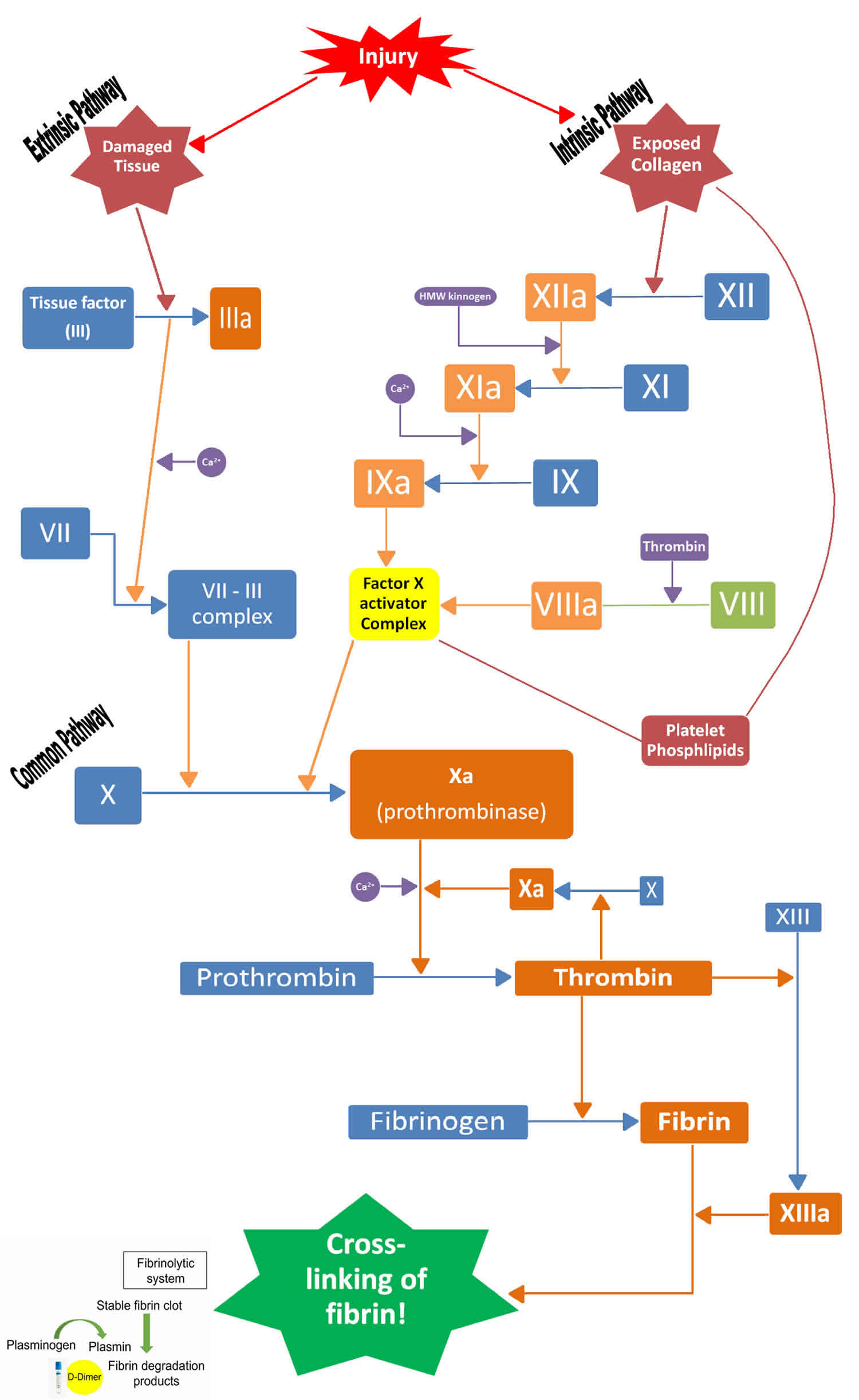
When the vascular system is injured, blood is exposed to extravascular tissues, which are rich in tissue factor, a cofactor for the serine protease factor VIIa 13.. The complex of tissue factor and factor VIIa activates factor X and factor IX. This activation pathway is historically termed the extrinsic pathway of coagulation. Factor IXa also activates factor X, in the presence of its cofactor factor VIIIa. Factor Xa, also in the presence of its cofactor factor Va, then activates prothrombin to generate thrombin 14.
Thrombin is the central serine protease in the coagulation cascade, and it executes several critical reactions 15. Thrombin critically cleaves fibrinogen to generate insoluble fibrin. Thrombin activates platelets via cleavage of PAR1 and PAR4 16. Thrombin is also responsible for positive feedback activation of coagulation that is critical for clot propagation. Thrombin activates factor XI, which then activates factor IX and thrombin activates cofactors VIII and V 15. This has historically been called the intrinsic pathway of coagulation, but it is more appropriate to think of it as a positive feedback loop 17.
The updated cell-based model of hemostasis focuses on the important fact that these reactions are controlled by their localization on different cellular surfaces. Coagulation is initiated by the cofactor tissue factor (the extrinsic pathway), which is a transmembrane protein present on fibroblasts and other extravascular tissues. The factor Xa generated here forms prothrombinase complex on these surfaces sufficient to generate only a small amount of thrombin. Then amplification and propagation of coagulation via the positive feedback loop occurs on the surface of platelets, which are activated near the site of injury by that trace thrombin and by adherence to extracellular matrix. Thus, the active coagulation complexes of this positive feedback loop form on the surface of activated platelets 18.
Ultimately thrombin also plays an important role in down regulation of the coagulation cascade by binding to thrombomodulin on endothelial cells and then activating protein C (APC) 11. The activated protein C anticoagulant system is important for the down regulation of the coagulation cascade. Activated protein C (APC) cleaves and inactivates the procoagulant cofactors VIIIa and Va 19. This reaction also requires a cofactor, protein S; in addition, factor V provides anticoagulant function as a cofactor for APC/protein S in the inactivation of factor VIIIa and factor Va 20. These complexes between proteases and cofactors (procoagulant and anticoagulant) form on negatively charged membrane surfaces that are provided by activated platelets 21. This localization of the coagulation cascade reactions is critical to restrict coagulation to the site of injury.
The coagulation cascade is also down-regulated by inactivation of all the serine proteases by serine protease inhibitors. Most of these inhibitors are in the serpin family of inhibitors 22. Antithrombin is arguably the most important of these 23. Antithrombin inhibits thrombin and factor Xa, as well as factor IXa and factor XIa in the presence of heparin or heparan sulfate 24. Other serpins that play roles in coagulation include heparin cofactor II (thrombin inhibitor), protein Z-dependent protease inhibitor (factor Xa inhibitor), protein C inhibitor (APC inhibitor) and C1-inhibitor (factor XIa inhibitor) 25. Two non-serpin inhibitors, tissue factor pathway inhibitor and alpha-2-macroglobulin, also play a significant role, inhibiting factor Xa and thrombin, respectively 26.
Extrinsic pathway
The tissue factor binds to factor VII and activates it. The activated factor VII (factor VIIa) further activates factor X and factor IX via proteolysis. Activated factor IX (factor IXa) binds with its cofactor – activated factor VIII (factor VIIIa), which leads to the activation of factor X (factor Xa). Factor Xa binds to activated factor V (factor Va) and calcium and generates a prothrombinase complex that cleaves the prothrombin into thrombin 4.
Intrinsic pathway
With thrombin production, there occurs conversion of factor XI to activated factor XI (factor XIa). Factor XIa with activated factor VII and tissue factor converts factor IX to activated factor IX (factor IXa). The activated factor IX combines with activated factor VIII (factor VIIIa) and activates factor X. Activated factor X (factor Xa) binds with activated factor V (factor Va) and converts prothrombin to thrombin. Thrombin acts as a cofactor and catalysis and enhances the bioactivity of many of the aforementioned proteolytic pathways 4.
Fibrin clot formation
The final steps in the coagulation cascade involve the conversion of fibrinogen to fibrin monomers which polymerizes and forms fibrin polymer mesh and result in a cross-linked fibrin clot. This reaction is catalyzed by activated factor XIII (factor XIIIa) that stimulates the lysine and the glutamic acid side chains causing cross-linking of the fibrin molecules and formation of a stabilized clot.
Figure 3. Secondary hemostasis
Footnote: Secondary hemostasis; the coagulation cascade. At the site of injury, tissue facto initiates the coagulation cascade that results in the formation of the serine protease thrombin. Thrombin performs multiple functions, including fibrin generation, platelet activation, positive feedback activation of the intrinsic pathway, and negative feedback activation of the activated protein C pathway. D-Dimer, fibrin degradation product from the fibrinolytic system.
Tertiary Hemostasis (Clot Resolution)
The reactions that result in the formation of a blood clot are balanced by other reactions that stop the clotting process and dissolve clots (fibrinolysis) after the blood vessel has healed. Without this control system, minor blood vessel injuries could trigger widespread clotting throughout the body—which actually happens in some diseases.
Activated platelets contract their internal actin and myosin fibrils in their cytoskeleton, which leads to shrinkage of the clot volume. Plasminogen then activates to plasmin, which promotes lysis of the fibrin clot; this restores the flow of blood in the damaged/obstructed blood vessels 4.
The role of the fibrinolytic system is to dissolve blood clots during the process of wound healing and to prevent blood clots in healthy blood vessels. The fibrinolytic system is composed primarily of three serine proteases that are present as zymogens (i.e., proenzymes) in the blood. Plasmin cleaves and breaks down fibrin. Plasmin is generated from the zymogen plasminogen by the proteases tissue-type plasminogen activator (tPA) and urokinase-type plasminogen activator (uPA). TPA and plasminogen come together on the surface of a fibrin clot, to which they both bind. TPA then activates plasminogen, which subsequently cleaves fibrin. UPA activates plasminogen in the presence of the uPA receptor, which is found on various cell types 27. All three of these serine proteases are down-regulated by serpins that are present in blood. Alpha-2-antiplasmin inhibits plasmin, and plasminogen activator inhibitors 1 and 2 inhibit tPA and uPA 22.
Disorders of hemostasis
Disorders of hemostasis can be roughly divided into platelet disorders, such as idiopathic thrombocytopenic purpura, and disorders of coagulation, such as hemophilia.
Hyper-coagulation
The hemostatic cascade is meant to control hemorrhage and be a protective mechanism. At times, this process is triggered inadvertently while the blood is within the lumen of the blood vessel and without any bleeding. This situation leads to a pathologic phenomenon of thrombosis, which can have catastrophic complications by obstructing blood flow leading to ischemia and even infarction of the tissues supplied by the occluded blood vessels. In this way, a physiologic process becomes a pathologic process leading to morbidity and/or mortality. Some of the examples include Antiphospholipid antibody syndrome, Factor 5 Leiden mutation, Protein C deficiency, protein S deficiency, Prothrombin gene mutation, etc.
Hypo-coagulation
When there is any defect in the functionality of any component of this hemostatic cascade, it can lead to ineffective hemostasis and inability to control hemorrhage; this can lead to severe blood loss, hemorrhage and also complications that can hence ensue due to the inhibited blood supply to vital organs. Some of the examples include Von Willebrand disease, hemophilia, disseminated intravascular coagulation, deficiency of the clotting factors, platelet disorders, collagen vascular disorders, etc.
Iatrogenic Coagulopathy
Medicine is currently in the era of widespread use of antiplatelet agents like aspirin, clopidogrel, ticagrelor and anticoagulants like warfarin, heparin, low molecular weight heparin, rivaroxaban, apixaban, dabigatran, fondaparinux amongst others for various commonly encountered clinical conditions like cardiac stenting/ percutaneous coronary intervention, atrial fibrillation, deep venous thrombosis, pulmonary embolism, and many more. The way these medications affect the functionality of the various components of clotting cascade can help patients with their clinical conditions. However, it can lead to bleeding/thrombosis in cases of inappropriate dosage, non-compliance, medication interactions, and result in significant morbidity and mortality.
Bleeding disorders
The main bleeding disorders are genetically inherited. Hemophilia results from defects in secondary hemostasis. Hemophilia A is due to deficiency of factor VIII, and hemophilia B is due to deficiency of factor IX. Activated factor VIII and activated factor IX together form the intrinsic factor Xase complex on activated membrane surfaces that is critical in the positive feedback loop of blood coagulation 14. Therefore, deficiency in either of these proteins causes a very similar bleeding phenotype characterized by excess bruising, spontaneous bleeding into joints, muscles, internal organs and the brain. Factor VIII and factor IX are both X-linked genes. Thus hemophilia is primarily expressed in males, with hemophilia A present in about 1 in 5000 males and hemophilia B present in about 1 in 20,000 males. However, multiple mutations in either factor VIII or factor IX have been identified, and not all of them cause complete loss of protein or protein function. In fact, almost half of hemophilia A sufferers have de novo mutations that were not inherited from their parents. Depending on the mutation, hemophilia can be severe (<1% function), moderate (1–5%) or mild (5–20%) 28.
Hemophilia A and hemophilia B are treated mainly by infusion of recombinant factor VIII or factor IX, respectively. Before factor VIII and factor IX were available for infusion, hemophiliacs had a greatly reduced life expectancy and quality of life. In the 1960’s and 1970’s partially pure and than highly purified factor VIII or IX from human plasma became the standard therapy. However, the advent of HIV virus in the early 1980’s resulted in a majority of hemophiliacs getting infected from factor preparations. As a result many hemophiliacs died, and many who did survive are infected with HIV. Virus inactivation procedures and restrictions on blood donations have now made plasma-derived protein preparations very safe 29. However, recombinant factor VIII and factor IX are ever more widely used out of a desire for even greater safety. This desire has largely led the development of improved 2nd and 3rd generation recombinant factor VIII products. 2nd generation factor VIII is formulated without any animal- or human-derived proteins in the final formulation, but it is still generated in tissue culture medium containing animal products. These products include Kogenate FS, Helixate FS and Refacto. Recently, 3rd generation recombinant factor VIII has come on to the market. These products have no exposure to human or animal products during tissue culture production or during purification and formulation 30. The third generation factor VIII’s are Advate and Xyntha.
Von Willebrand disease is a bleeding disorder caused by deficiency or defect in von Willebrand factor (VWF). von Willebrand factor (VWF) is involved in platelet aggregation and is also a carrier for factor VIII. Thus deficiency of VWF causes defects in platelet aggregation but also causes a deficiency of factor VIII 31. Von Willebrand disease can result from multiple different mutations in VWF. These different mutations cause various different forms of Von Willebrand disease. These have been grouped into three overall categories. Type 1 Von Willebrand disease is a partial quantitative defect, while type 3 Von Willebrand disease results from a complete absence of VWF. In type 2 Von Willebrand disease, a normal amount of VWF is present but it has functional defects. Type 2 Von Willebrand disease is broken down into several subcategories 32. von Willebrand factor (VWF) is an autosomal gene, so the disease is present equally in men and women. The severity of the different types of Von Willebrand disease varies, and various therapies are available and preferred for different forms of the disease.
The main drug used for treatment of type 1 (mild) and most type 2 Von Willebrand disease is desmopressin (DDAVP), which is a vasopressin analogue that stimulates release of von Willebrand factor (VWF) from endothelial cells and therefore temporarily increases the concentration of VWF in the blood. Type 3 Von Willebrand disease is more severe, and it is treated by infusion with pasteurized plasma-derived pure VWF/factor VIII concentrate (Humate-P). Both of these drugs can also be supplemented with the antifibrinolytic tranexamic acid. A significant risk for women with Von Willebrand disease is menorrhagia (abnormally heavy and prolonged menstrual period). This can often be treated with tranexamic acid or vasopressin, but another alternative is combined oral contraceptives to block menses 33.
There are several genetic platelet disorders that derive from defects in platelet receptors. Bernard-Soulier syndrome is due to a deficiency of GPIb-IX-V 34. As a consequence, these individuals are defective in platelet aggregation because their platelets do not bind VWF. Bernard-Soulier syndrome is also characterized by abnormally large platelets and thrombocytopenia 35. Glanzmann thrombasthenia is a deficiency of αIIbβ3 36. Since αIIbβ3 is also critical for platelet aggregation; and since it binds to fibrinogen, collagen, VWF, fibronectin and vitronectin; these individuals are also defective in platelet aggregation 35. However, they have normal platelet numbers. The most common therapy for these disorders is transfusion of fresh frozen platelets during bleeding episodes or prophylactically during surgery. Deficiencies in collagen receptors and disorders in platelet secretion also exist. These are rare and typically result in mild bleeding. Treatment is usually transfusion of platelets 35.
Hemostasis and thrombosis
Proper hemostasis is a function of balance between procoagulant systems (platelets, coagulation cascade) and anticoagulant systems (APC/protein S, fibrinolysis, serpins). If hemostasis is out of balance due to a defect in one of these systems, then either thrombosis (too much clotting) or bleeding (not enough clotting) may be the result.
Thrombosis
Arterial thrombi are composed largely of aggregated platelets, whereas venous thrombi are composed more of fibrin with red blood cells enmeshed. The composition of these different thrombi is dictated by the different conditions in the arterial circulation and the venous circulation. One important aspect of this is blood flow, with higher flow rates and therefore higher sheer forces in the arterial circulation 37. Classically, arterial thrombosis and venous thrombosis are thought to have different risk factors. However, recent studies have suggested that some of the classic risk factors for arterial thromboses, such as obesity and high cholesterol, are also risk factors for venous thrombosis 38. The classic risk factors for venous thrombosis cause a hypercoagulable state and result in an increased tendency for activation of the coagulation cascade. These include acquired risk factors such as cancer, surgery, immobilization, fractures and pregnancy. Genetic risk factors include multiple variants in the coagulation cascade. The most common are factor VLeiden and prothrombin G20210A. Others are protein C or protein S heterozygosity and mutations in antithrombin 39.
Arterial thrombosis is generally treated with drugs that inhibit platelet aggregation. Acetylsalicylic acid (aspirin) is a cyclo-oxygenase (COX) inhibitor and irreversibly inhibits COX-1 in the thromboxane A2 synthesis pathway 40. Clopidogrel (trade name Plavix) blocks adenosine diphosphate (ADP) activation of platelets by inhibiting the ADP receptor P2Y12 41. Prasugrel (trade name Effient) is a new P2Y12 inhibitor that was recently approved in the United States for certain indications 42. Several new P2Y12 receptor antagonists are in clinical trials, but none of them has been approved as of yet. Unlike clopidogrel, some of these are reversible inhibitors and thus might only temporarily inhibit platelet aggregation 43. These might be more appropriate for acute short-term treatment rather than for long-term prophylaxis. There are also several other drugs that inhibit platelet aggregation whose use is less widespread. There are three αIIbβ3 integrin inhibitors, abciximab, eptifibatide and tirofiban. These are all intravenous (IV) drugs that are used primarily to treat or prevent acute coronary events in hospital 44. Dipyridamole (Persantine) is an oral drug that inhibits adenosine reuptake and thromboxane synthesis and is used for secondary prevention of stroke and transient ischaemic attack 45.
Venous thrombosis is treated with drugs that inhibit the coagulation cascade. Historically this has included unfractionated heparin (UFH), which stimulates inhibition of coagulation serine proteases by antithrombin 46. Unfractionated heparin is only bioavailable via IV injection, which limits its utility to hospital care. Long-term prophylaxis has typically been maintained with one of several coumarine derivatives, which are vitamin K antagonists (e.g. warfarin, [trade name Coumadin], acenocoumarol [trade name Sintrom]). Vitamin K antagonists inhibit post-translational processing of the vitamin K-dependent coagulation serine proteases and therefore down-regulate the coagulation cascade overall 47.
More recently alternatives to these two classes of drugs have come onto the market, and many more are advancing through clinical trials. The first of these was low molecular weight heparin (LMWH), which is unfractionated heparin that is broken down into smaller fragments either chemically or enzymatically 48. Multiple versions of LMWH are on the market and they all function similarly but they are not identical. The advantages of these versus unfractionated heparin are that they are bio-available via subcutaneous injection and proper dosing is much less variable. The next generation of low molecular weight heparin was the synthetic low molecular weight heparin mimic, fondaparinux. Fondaparinux is a sulfated pentasaccharide that stimulates antithrombin inactivation of factor Xa but is too small to stimulate inactivation of thrombin 39. Idraparinux is very similar to fondaparinux, but it has a longer half-life. However, idraparinux is not yet approved for use, and the longer half-life is actually a concern since there is no reversal agent. A variant of idraparinux is under investigation. This variant contains a biotin moiety, which binds with high affinity to the egg white protein avidin. Avidin could then serve as a reversal agent 49.
A new class of anticoagulant drugs is small molecules that directly inhibit factor Xa or thrombin. Many of these are being developed as oral drugs, which could be a significant advantage over low molecular weight heparin or fondaparinux. Rivaroxaban is the only direct factor Xa inhibitor that is currently approved, but it is still not yet approved in the United States. Apixaban, betrixaban and edoxiban are similar drugs that are still in clinical trials. Initially these are in trials for relatively short-term prophylaxis during/after orthopedic surgery, but the hope is that they would ultimately prove safe for long-term prophylaxis.
Direct thrombin inhibitors include argatroban, which is approved in the US. However, argatroban is administered via IV. Hirudin, another direct thrombin inhibitor, is a polypeptide produced in the saliva of leeches. Several recombinant hirudin derivatives are approved for IV administration. These hirudin derivatives are primarily used during acute percutaneous interventions in patients in which heparin is contra-indicated. Dabigatran is the only oral direct thrombin inhibitor that is currently approved, but it is not yet approved in the United States. If oral direct thrombin or direct factor Xa inhibitors ultimately prove safe for long-term prophylaxis, they could potentially replace warfarin, which is less than ideal because of variability in dosing that requires regular monitoring to avoid over or under-dosing, which increases risk for bleeding and thrombosis 50.
- LaPelusa A, Dave HD. Physiology, Hemostasis. [Updated 2019 Oct 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK545263[↩]
- Gale AJ. Continuing education course #2: current understanding of hemostasis. Toxicol Pathol. 2011;39(1):273–280. doi:10.1177/0192623310389474 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3126677[↩][↩][↩]
- Holthenrich A, Gerke V. Regulation of von-Willebrand Factor Secretion from Endothelial Cells by the Annexin A2-S100A10 Complex. Int J Mol Sci. 2018 Jun 13;19(6).[↩]
- Palta S, Saroa R, Palta A. Overview of the coagulation system. Indian J Anaesth. 2014 Sep;58(5):515-23.[↩][↩][↩][↩]
- Thon JN, Italiano JE. Platelets: production, morphology and ultrastructure. Handb Exp Pharmacol. 2012;(210):3-22.[↩]
- Smith SA, Travers RJ, Morrissey JH. How it all starts: Initiation of the clotting cascade. Crit. Rev. Biochem. Mol. Biol. 2015;50(4):326-36.[↩][↩]
- Periayah MH, Halim AS, Mat Saad AZ. Mechanism Action of Platelets and Crucial Blood Coagulation Pathways in Hemostasis. Int J Hematol Oncol Stem Cell Res. 2017 Oct 01;11(4):319-327[↩][↩][↩]
- Mechanisms of thrombopoiesis. Schulze H, Shivdasani RA. J Thromb Haemost. 2005 Aug; 3(8):1717-24.[↩]
- Zucker-Franklin D. Megakaryocyte and platelet structure. In: Hoffman R, Benz EJ, Shattil SJ, Furie B, Silberstein LE, McGlave P, editors. Hematology Basic Principles and Practice. 3. Churchill Livingstone; New York: 2000. pp. 1730–1740.[↩]
- Pathogenesis of thrombosis. Furie B. Hematology Am Soc Hematol Educ Program. 2009; ():255-8.[↩]
- Esmon CT, Owen WG. Identification of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. Proc Natl Acad Sci USA. 1981;78:2249–2252.[↩][↩]
- Shimada K, Kobayashi M, Kimura S, Nishinaga M, Takeuchi K, Ozawa T. Anticoagulant heparin-like glycosaminoglycans on endothelial cell surface. Jpn Circ J. 1991;55:1016–1021.[↩]
- Kirchhofer D, Nemerson Y. Initiation of blood coagulation: the tissue factor/factor VIIa complex. Curr Opin Biotechnol. 1996;7:386–391.[↩]
- Dahlback B. Blood coagulation. Lancet. 2000;355:1627–1632.[↩][↩]
- Lane DA, Philippou H, Huntington JA. Directing thrombin. Blood. 2005;106:2605–2612.[↩][↩]
- Kahn ML, Zheng YW, Huang W, Bigornia V, Zeng D, Moff S, Farese RV, Jr, Tam C, Coughlin SR. A dual thrombin receptor system for platelet activation. Nature. 1998;394:690–694.[↩]
- Bouma BN, von dem Borne PA, Meijers JC. Factor XI and protection of the fibrin clot against lysis–a role for the intrinsic pathway of coagulation in fibrinolysis. Thromb Haemost. 1998;80:24–27.[↩]
- Hoffman M, Monroe DM., III A cell-based model of hemostasis. Thromb Haemost. 2001;85:958–965.[↩]
- Fulcher CA, Gardiner JE, Griffin JH, Zimmerman TS. Proteolytic inactivation of activated human factor VIII procoagulant protein by activated protein C and its analogy to factor V. Blood. 1984;63:486–489.[↩]
- Cramer TJ, Griffin JH, Gale AJ. Factor V Is an Anticoagulant Cofactor for Activated Protein C during Inactivation of Factor Va. Pathophysiol Haemost Thromb. 2010;37:17–23.[↩]
- Mann KG, Nesheim ME, Church WR, Haley P, Krishnaswamy S. Surface-dependent reactions of the vitamin K-dependent enzyme complexes. Blood. 1990;76:1–16.[↩]
- Rau JC, Beaulieu LM, Huntington JA, Church FC. Serpins in thrombosis, hemostasis and fibrinolysis. J Thromb Haemost. 2007;5(Suppl 1):102–115.[↩][↩]
- Egeberg O. Inherited antithrombin deficiency causing thrombophilia. Thromb Diath Haemorrh. 1965;13:516–530.[↩]
- Quinsey NS, Greedy AL, Bottomley SP, Whisstock JC, Pike RN. Antithrombin: in control of coagulation. Int J Biochem Cell Biol. 2004;36:386–389.[↩]
- Han X, Fiehler R, Broze GJ., Jr Characterization of the protein Z-dependent protease inhibitor. Blood. 2000;96:3049–3055.[↩]
- Broze GJ, Jr, Warren LA, Novotny WF, Higuchi DA, Girard JJ, Miletich JP. The lipoprotein-associated coagulation inhibitor that inhibits the factor VII-tissue factor complex also inhibits factor Xa: insight into its possible mechanism of action. Blood. 1988;71:335–343.[↩]
- Lijnen HR, Collen D. Molecular and cellular basis of fibrinolysis. In: Hoffman R, Benz EJ, Shattil SJ, Furie B, Silberstein LE, McGlave P, editors. Hematology Basic Principles and Practice. 3. Churchill Livingstone; New York: 2000. pp. 1804–1814.[↩]
- Mannucci PM, Tuddenham EG. The hemophilias–from royal genes to gene therapy. N Engl J Med. 2001;344:1773–1779.[↩]
- Mannucci PM. Back to the future: a recent history of haemophilia treatment. Haemophilia. 2008;14(Suppl 3):10–18.[↩]
- Pipe SW. Recombinant clotting factors. Thromb Haemost. 2008;99:840–850.[↩]
- Terraube V, O’Donnell JS, Jenkins PV. Factor VIII and von Willebrand factor interaction: biological, clinical and therapeutic importance. Haemophilia. 2010;16:3–13.[↩]
- Federici AB. Prophylaxis of bleeding episodes in patients with von Willebrand’s disease. Blood Transfus. 2008;6(Suppl 2):s26–s32.[↩]
- Rodeghiero F, Castaman G, Tosetto A. How I treat von Willebrand disease. Blood. 2009;114:1158–1165.[↩]
- Hagen I, Nurden A, Bjerrum OJ, Solum NO, Caen J. Immunochemical evidence for protein abnormalities in platelets from patients with Glanzmann’s thrombasthenia and Bernard-Soulier syndrome. J Clin Invest. 1980;65:722–731.[↩]
- Bennett JS. Hereditary disorders of platelet function. In: Hoffman R, Benz EJ, Shattil SJ, Furie B, Silberstein LE, McGlave P, editors. Hematology Basic Principles and Practice. 3. Churchill Livingstone; New York: 2000. pp. 2154–2172.[↩][↩][↩]
- Phillips DR, Agin PP. Platelet membrane defects in Glanzmann’s thrombasthenia. Evidence for decreased amounts of two major glycoproteins. J Clin Invest. 1977;60:535–545.[↩]
- Tangelder GJ, Slaaf DW, Arts T, Reneman RS. Wall shear rate in arterioles in vivo: least estimates from platelet velocity profiles. Am J Physiol. 1988;254:H1059–H1064.[↩]
- Franchini M, Mannucci PM. Venous and arterial thrombosis: different sides of the same coin? Eur J Intern Med. 2008;19:476–481.[↩]
- Bauer KA. Fondaparinux sodium: a selective inhibitor of factor Xa. Am J Health Syst Pharm. 2001;58(Suppl 2):S14–S17.[↩][↩]
- Burch JW, Stanford N, Majerus PW. Inhibition of platelet prostaglandin synthetase by oral aspirin. J Clin Invest. 1978;61:314–319.[↩]
- Mills DC, Puri R, Hu CJ, Minniti C, Grana G, Freedman MD, Colman RF, Colman RW. Clopidogrel inhibits the binding of ADP analogues to the receptor mediating inhibition of platelet adenylate cyclase. Arterioscler Thromb. 1992;12:430–436.[↩]
- Sugidachi A, Asai F, Yoneda K, Iwamura R, Ogawa T, Otsuguro K, Koike H. Antiplatelet action of R-99224, an active metabolite of a novel thienopyridine-type G(i)-linked P2T antagonist, CS-747. Br J Pharmacol. 2001;132:47–54.[↩]
- Giossi A, Pezzini A, Del ZE, Volonghi I, Costa P, Ferrari D, Padovani A. Advances in antiplatelet therapy for stroke prevention: the new P2Y12 antagonists. Curr Drug Targets. 2010;11:380–391.[↩]
- Cohen M. Antiplatelet therapy in percutaneous coronary intervention: a critical review of the 2007 AHA/ACC/SCAI guidelines and beyond. Catheter Cardiovasc Interv. 2009;74:579–597.[↩]
- Weber R, Weimar C, Diener HC. Medical prevention of stroke and stroke recurrence in patients with TIA and minor stroke. Expert Opin Pharmacother. 2009;10:1883–1894.[↩]
- Damus PS, Hicks M, Rosenberg RD. Anticoagulant action of heparin. Nature. 1973;246:355–357.[↩]
- Furie B. Pathogenesis of thrombosis. Hematology Am Soc Hematol Educ Program. 2009:255–258.[↩]
- Weitz JI. Low-molecular-weight heparins. N Engl J Med. 1997;337:688–698.[↩]
- Harenberg J. Development of idraparinux and idrabiotaparinux for anticoagulant therapy. Thromb Haemost. 2009;102:811–815.[↩]
- Laux V, Perzborn E, Heitmeier S, von DG, Dittrich-Wengenroth E, Buchmuller A, Gerdes C, Misselwitz F. Direct inhibitors of coagulation proteins – the end of the heparin and low-molecular-weight heparin era for anticoagulant therapy? Thromb Haemost. 2009;102:892–899.[↩]