Leukocyte adhesion deficiency
Leukocyte adhesion deficiency is a rare primary immunodeficiency caused by a defect of cellular adhesion molecules resulting in clinical syndromes. Leukocyte adhesion deficiency is a combined (B cell) and cellular (T cell) immunodeficiency disorder 1. Leukocyte adhesion deficiency syndromes are characterized by defects affecting how white blood cells (leukocytes) respond and travel to the site of a wound or infection.
Major immunologic features 2:
- There is an inability to form pus.
- There is a deficiency of various glycoproteins including LFA-1/Mac-1, glycoprotein 150/95.
- Leukocytes cannot migrate to infection sites to kill invading microorganisms due to mutations in the CD18 glycoprotein.
- Adhesion molecules deficiency results in abnormal inflammatory response and eventually recurrent bacterial infections.
Leukocyte adhesion deficiency is characterized by recurrent bacterial infections. The clinical picture is characterized by marked leukocytosis and localized bacterial infections that are difficult to detect until they have progressed to an extensive level secondary to lack of leukocyte recruitment at the site of infection. Thus the infections in patients with leukocyte adhesion deficiency act similarly as those observed in patients with neutropenia.
There are three different types of leukocyte adhesion deficiency syndromes 3. The specific symptoms and the severity of leukocyte adhesion deficiency syndromes vary from one person to another.
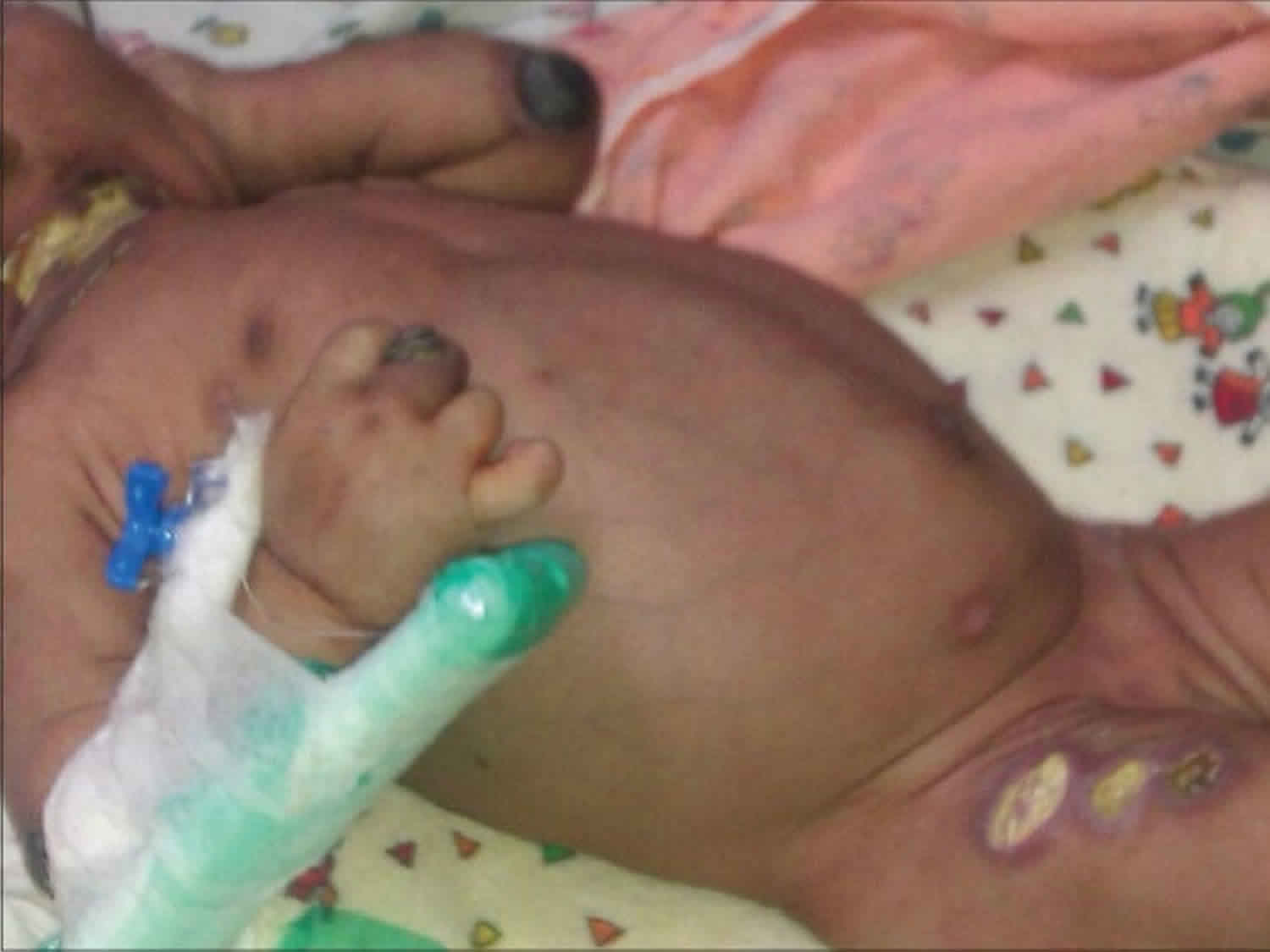
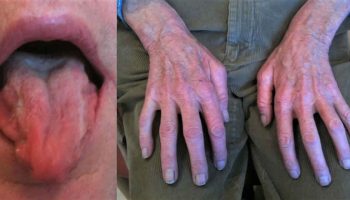
- Leukocyte adhesion deficiency type 1 – in which steady adhesion of leukocyte to endothelial surfaces is defective by mutations in CD18 gene resulting in defective or deficient beta-2 integrin. Leukocyte adhesion deficiency type 1 is estimated to occur in 1 per million people worldwide 4. At least 300 cases of this condition have been reported in the scientific literature. Usually the first signs occur in infancy or early childhood. Patients present recurrent, life-threatening bacterial infections of the skin, mouth, and respiratory tract. Delayed umbilical cord separation is common. Skin infections may evolve into large ulcers. Severe periodontitis is often present later in life and leads to early tooth loss. A lack of swelling, redness, heat, or pus is observed in the area of infection.
- Leukocyte adhesion deficiency type 2 – in which there is an absence of Sialyl Lewis X of E-selectin
- Leukocyte adhesion deficiency type 3 – in which there is a defect in beta integrins 1, 2, and 3; this impairs the integrin activation cascade – specifically, a mutation in the kindlin-3 gene causes this type of leukocyte adhesion deficiency.
Leukocyte adhesion deficiency syndromes affect males and females in equal numbers. The exact incidence of these disorders in the general population is unknown. leukocyte adhesion deficiency type 1 is by far the more common one with several hundreds of patients reported in the medical literature from all over the world. leukocyte adhesion deficiency type 2 is very rare reported in less than 10 patients and leukocyte adhesion deficiency type 3 is also rare with 25 patients mainly from the Middle East region. These disorders often go unrecognized and may be misdiagnosed, making it difficult to determine their true frequency in the general population. Leukocyte adhesion deficiency type 1 was first described in the medical literature in 1979. Leukocyte adhesion deficiency type 2 was first reported in 1992. Leukocyte adhesion deficiency type 3 was first reported in 1997.
Leukocyte adhesion deficiency type 1
The symptoms of leukocyte adhesion deficiency type 1 can vary from one to person to another. Some individuals have a severe form of the disorder that can cause life-threatening complications; other individuals have a milder form. Leukocyte adhesion deficiency type 1 is usually characterized by recurrent, often severe, bacterial infections, and delayed detachment of the umbilical cord stump after birth. In newborns, the stump normally falls off within the first two weeks of life; but, in infants with leukocyte adhesion deficiency type 1, this separation usually occurs at three weeks or later. In addition, affected infants often have inflammation of the umbilical cord stump (omphalitis) due to a bacterial infection. Fungal infections are also common. Bacterial and fungal infections most often affect the skin and mucous membranes (mucosal surfaces). A hallmark of leukocyte adhesion deficiency type 1 is the lack of pus formation at the sites of infection. In people with this condition, wounds are slow to heal, which can lead to additional infection. Recurrent, bacterial infections usually develop shortly after birth in leukocyte adhesion deficiency type 1 and can cause life-threatening complications in many cases. Individuals with the milder form of leukocyte adhesion deficiency type 1 have fewer and less severe infections.
After infancy, affected children may develop progressive inflammation of the tissues that surround and support the teeth (periodontitis) and inflammation of the gums (gingivitis). Periodontitis can eventually cause tooth loss. Wounds either from surgery or trauma are slow to heal (delayed wound healing) and may be more likely to scar. Affected individuals may also develop sores in the area surrounding.
Life expectancy in individuals with leukocyte adhesion deficiency type 1 is often severely shortened. Due to repeat infections, affected individuals may not survive past infancy.
Mutations in the ITGB2 gene cause leukocyte adhesion deficiency type 1. Seven new mutations in the ITGB2 gene have been reported, which encode the beta2 integrin family including three frameshift deletions (Tyr382fsX9, Asn282fsX41, and Lys636fsX22), two splicing (IVS4-6C>A, IVS7+1G>A) and three missense (Asp128Tyr, Gly716Ala, and Ala239Thr) 5. This gene provides instructions for making one part (the β2 subunit) of at least four different proteins known as beta2 integrins. Integrins that contain the β2 subunit are found embedded in the membrane that surrounds white blood cells (leukocytes). These integrins help leukocytes gather at sites of infection or injury, where they contribute to the immune response. Beta2 integrins recognize signs of inflammation and attach (bind) to proteins called ligands on the lining of blood vessels. This binding leads to linkage (adhesion) of the leukocyte to the blood vessel wall. Signaling through the β2 integrins triggers the transport of the attached leukocyte across the blood vessel wall to the site of infection or injury.
ITGB2 gene mutations that cause leukocyte adhesion deficiency type 1 lead to the production of a β2 subunit that cannot bind with other subunits to form β2 integrins. Leukocytes that lack these integrins cannot attach to the blood vessel wall or cross the vessel wall to contribute to the immune response. As a result, there is a decreased response to injury and foreign invaders, such as bacteria and fungi, resulting in frequent infections, delayed wound healing, and other signs and symptoms of this condition.
Leukocyte adhesion deficiency type 2
Infants with leukocyte adhesion deficiency type 2 develop recurrent, bacterial infections. However, the infections and their complications are usually milder than those seen in infants with leukocyte adhesion deficiency type 1. Pneumonia, chronic middle ear infections (otitis media), infection of the tissues that surround and support the teeth (periodontitis) and localized infection of the tissue underneath the surface of the skin (cellulitis) commonly occur in leukocyte adhesion deficiency type 2. The infections are usually not life-threatening and are often treated in an outpatient basis. No pus formation is seen at the site of infection. Generally, the frequency of infections in leukocyte adhesion deficiency type 2 decreases after affected individuals reach three years of age. As affected individuals grow older, severe periodontitis is the main infectious complication.
Unlike leukocyte adhesion deficiency type 1, infants with leukocyte adhesion deficiency type 2 do not experience a delay in the separation of the umbilical cord. Individuals with leukocyte adhesion deficiency type 2 do have additional complications not seen in leukocyte adhesion deficiency type 1 including a unique blood type called the Bombay (hh) blood type. Additional features that characterize leukocyte adhesion deficiency type 2 include diminished muscle tone resulting in floppiness (hypotonia), distinctive facial features, severe mental retardation and severe growth deficiencies resulting in short stature.
Leukocyte adhesion deficiency type 2 may also be known as congenital disorder of glycosylation type 2c due to the primary defect in fucose metabolism.
Leukocyte adhesion deficiency type 3
Individuals with leukocyte adhesion deficiency type 3 have recurrent bacterial and fungal infections that follow a similar course of infection as seen in individuals with leukocyte adhesion deficiency type 1. However, these affected individuals also have a bleeding tendency that can cause life-threatening complications. The bleeding complication of leukocyte adhesion deficiency type 3 resembles a rare disorder known as Glanzmann thrombasthenia, which is characterized by impaired function of blood cells required for clotting (platelets). Affected individuals have a tendency to bleed easily and profusely especially after surgical procedures. Other symptoms may include susceptibility to easy bruising, nosebleeds (epistaxis), bleeding from the gums (gingival), and/or large red or purple colored spots on the skin that are caused by bleeding under the skin (subcutaneous). The bleeding problem usually starts at birth.
Individuals who were once classified as having leukocyte adhesion deficiency type 1 variant (because of the similar disease expression) are now considered to have leukocyte adhesion deficiency type 3 because the underlying genetic cause of leukocyte adhesion deficiency type 3 is different from the underlying genetic cause of leukocyte adhesion deficiency type 1.
Leukocyte adhesion deficiency causes
Primarily in leukocyte adhesion deficiency, leukocytes cannot escape from the blood to tissues that have been attacked by microbes. Continuous surveillance of foreign antigens by leukocyte trafficking suffers disruption as well.
Leukocyte adhesion syndromes are rare, genetic disorders. leukocyte adhesion deficiency type 1 is caused by mutations of the ITGB2 gene. Leukocyte adhesion deficiency type 2 is caused by mutations of the SLC35C1 gene. The genetic defect in leukocyte adhesion deficiency type 3 is a mutation in the gene for Kindlin 3, a protein essential for all integrins activation. Lack of integrins activation affects the ability of leukocytes and platelets to bind to the endothelium. In some cases, there is also a mutation in the gene for CalDAGGEF1, another protein important in integrins activation.
Leukocyte adhesion deficiency syndromes are classified as a primary immunodeficiency disorders. The immune system protects the body from bacteria, viruses, parasites and other foreign, harmful substances. White blood cells (leukocytes) are part of the immune system. White blood cells continually look for signs of disease, infection or injury. Normally, white blood cells circulate in the bloodstream. When they detect an infection or foreign substance, white blood cells race to the site of infection or inflammation to protect the body. White blood cells may destroy foreign material by ingesting it themselves or producing unique antibodies that destroy harmful material. A specific type of white blood cell, called a neutrophil, is most often affected in leukocyte adhesion deficiency syndromes. The main role of neutrophils is to defend the body against bacteria and fungi.
White blood cells travel (migrate) to the site of inflammation or infection in the body through a complex process sometimes referred to as the adhesion cascade. This process requires several, precise steps. These steps include the tethering of white blood cells to the thin layer of cells that line the inside surface of blood vessels (endothelium), the rolling of white blood cells along the endothelium, leukocyte activation and the eventual attachment (adhesion) of white blood cells directly to the endothelium. Ultimately, white blood cells move from the endothelium through the blood vessel wall and into the surrounding tissue, eventually traveling to the site of an infection or inflammation to protect the body.
Specific chemicals such as proteins are necessary for white blood cells to be able to tether, roll along and stick (adhere) to the endothelium. Individuals with leukocyte adhesion deficiency syndromes do not have sufficient levels of these proteins and their white blood cells cannot tether, roll along or fail to stick to the endothelium. Therefore, the white blood cells of individuals with leukocyte adhesion deficiency syndromes fail to reach the site of infection or inflammation.
The ITGB2 gene, which causes leukocyte adhesion deficiency type 1, contains instructions for creating (encoding) a protein known as CD18. CD18 is a subunit of integrin or a cell surface protein and is normally found on the surface of white blood cells. Mutations of the ITGB2 gene result in defective CD18 or deficient levels of CD18. Without sufficient levels of functional CD18, white blood cells cannot stick (adhere) to the endothelium. In rare cases, CD18 is expressed normally but because of a specific ITGB2 mutation, the protein is nonfunctional. These cases are referred to as leukocyte adhesion deficiency type 1 variant.
Specific proteins are also necessary for white blood cells to roll along the endothelium. Individuals with leukocyte adhesion deficiency type 2 do not have sufficient levels of these proteins. The SLC35C1 gene contains instructions for creating (encoding) an enzyme (GPD-fucose transporter) that is required to transport a specific sugar (fucose) in the body. Fucose is necessary for a carbohydrate structure known as CD15 (sialyl Lewis x antigen) to bind to a type of protein (glycoprotein) called selectin found on the endothelium. This process is required for white blood cells to be able to roll along the endothelium. Since white blood cells cannot roll along the endothelium in leukocyte adhesion deficiency type 2, they are unable to stick to the endothelium and fail to travel to the site of infection and inflammation.
Leukocyte adhesion deficiency inheritance pattern
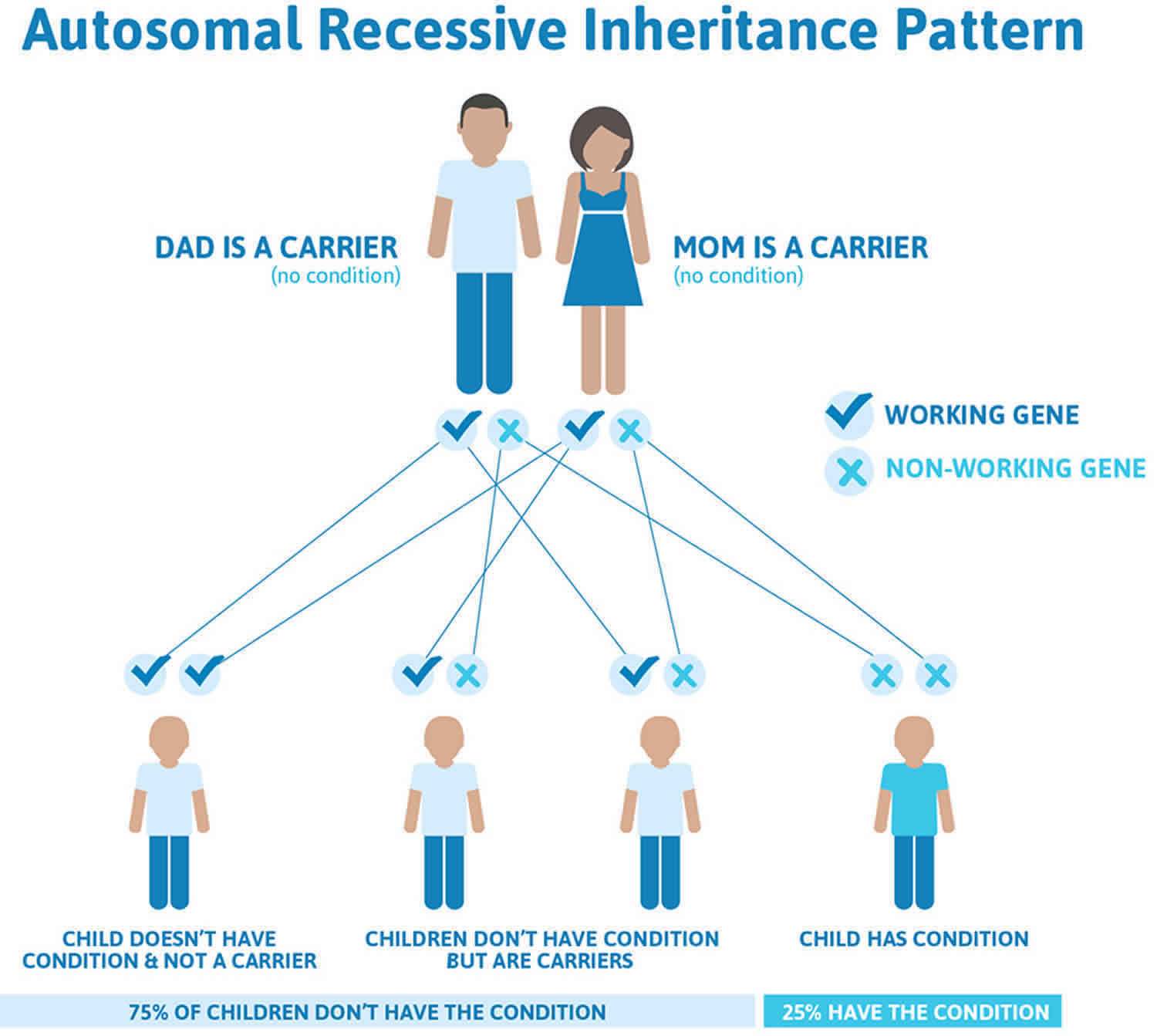
Leukocyte adhesion deficiency is inherited in an autosomal recessive pattern, which means both copies of the gene in each cell have mutations. The parents of an individual with an autosomal recessive condition each carry one copy of the mutated gene, but they typically do not show signs and symptoms of the condition.
It is rare to see any history of autosomal recessive conditions within a family because if someone is a carrier for one of these conditions, they would have to have a child with someone who is also a carrier for the same condition. Autosomal recessive conditions are individually pretty rare, so the chance that you and your partner are carriers for the same recessive genetic condition are likely low. Even if both partners are a carrier for the same condition, there is only a 25% chance that they will both pass down the non-working copy of the gene to the baby, thus causing a genetic condition. This chance is the same with each pregnancy, no matter how many children they have with or without the condition.
- If both partners are carriers of the same abnormal gene, they may pass on either their normal gene or their abnormal gene to their child. This occurs randomly.
- Each child of parents who both carry the same abnormal gene therefore has a 25% (1 in 4) chance of inheriting a abnormal gene from both parents and being affected by the condition.
- This also means that there is a 75% ( 3 in 4) chance that a child will not be affected by the condition. This chance remains the same in every pregnancy and is the same for boys or girls.
- There is also a 50% (2 in 4) chance that the child will inherit just one copy of the abnormal gene from a parent. If this happens, then they will be healthy carriers like their parents.
- Lastly, there is a 25% (1 in 4) chance that the child will inherit both normal copies of the gene. In this case the child will not have the condition, and will not be a carrier.
These possible outcomes occur randomly. The chance remains the same in every pregnancy and is the same for boys and girls.
Figure 1 illustrates autosomal recessive inheritance. The example below shows what happens when both dad and mum is a carrier of the abnormal gene, there is only a 25% chance that they will both pass down the abnormal gene to the baby, thus causing a genetic condition.
Figure 1. Leukocyte adhesion deficiency autosomal recessive inheritance pattern
People with specific questions about genetic risks or genetic testing for themselves or family members should speak with a genetics professional.
Resources for locating a genetics professional in your community are available online:
- The National Society of Genetic Counselors (https://www.findageneticcounselor.com/) offers a searchable directory of genetic counselors in the United States and Canada. You can search by location, name, area of practice/specialization, and/or ZIP Code.
- The American Board of Genetic Counseling (https://www.abgc.net/about-genetic-counseling/find-a-certified-counselor/) provides a searchable directory of certified genetic counselors worldwide. You can search by practice area, name, organization, or location.
- The Canadian Association of Genetic Counselors (https://www.cagc-accg.ca/index.php?page=225) has a searchable directory of genetic counselors in Canada. You can search by name, distance from an address, province, or services.
- The American College of Medical Genetics and Genomics (http://www.acmg.net/ACMG/Genetic_Services_Directory_Search.aspx) has a searchable database of medical genetics clinic services in the United States.
Leukocyte adhesion deficiency symptoms
The classic presentation of leukocyte adhesion deficiency is recurrent bacterial infections, neutrophil adhesion defects, and umbilical cord sloughing delays. The adhesion defects result in poor leukocyte chemotaxis, particularly the neutrophil, with an inability to form pus and neutrophilia.
Individuals with leukocyte adhesion deficiency commonly suffer from bacterial infections beginning in the neonatal period. Infections such as omphalitis, pneumonia, gingivitis, and peritonitis are common and usually life-threatening due to the inability to destroy the invading pathogens. Individuals with leukocyte adhesion deficiency do not form abscesses because granulocytes cannot migrate to the sites of infection.
Characteristics of patients with leukocyte adhesion deficiency include the following 6:
Leukocyte adhesion deficiency type 1:
- Delayed separation of the umbilical cord
- Recurrent pyogenic infections, with onset in the first weeks of life
- Infections caused meanly by Staphylococcus aureus and Pseudomonas aeruginosa
- Absent pus formation
- Periodontitis
Leukocyte adhesion deficiency type 2:
- Recurrent skin infections
- Pneumonia
- Bronchiectasis
- Tuberculosis
- Denture abnormalities
- Infections are less severe and fewer as compared to leukocyte adhesion deficiency type 1
Leukocyte adhesion deficiency type 3:
- Omphalitis
- Osteoporosis like bone features
- Bleeding complications
- Hematological abnormalities, e.g., bone marrow failure
Other miscellaneous manifestations may include:
- Vaginitis
- Peritonitis
- Osteomyelitis
- Perianal abscesses
- Sinusitis
- Tracheobronchitis
- Necrotic soft tissue infections
- Otitis media
- Meningitis
- Graft versus host reaction
- Recurrent tonsillitis
- Conjunctivitis
- Granuloma
- Oral candidiasis
- Aphthous stomatitis
- Urinary tract infections
- Lymphocytic interstitial pneumonitis
- Glomerulonephritis
- Hemolytic-uremic syndrome
- Nail dystrophy
- Persistent Hyperinsulinemic hypoglycemia of infancy
- Pyoderma gangrenosum
- Megakaryocytic acute myeloid leukemia
Leukocyte adhesion deficiency complications
The most common complications are infectious diseases affecting the skin, respiratory system, gastrointestinal system, oral cavity, and some internal organs. Leukocyte adhesion deficiency has a high mortality rate.
The literature review of the clinical findings of patients with leukocyte adhesion deficiency type 1 reveals that recurrent infections (93.3%) and poor wound healing (86%) are the most prevalent clinical findings. A defect in CD18 (the beta subunit of the integrins) was present in all patients 7.
Mortality for severe leukocyte adhesion deficiency type 1 was reported as 75% by the age of 2 years (in an initial 1988 multicenter retrospective evaluation). Patients with moderate disease (2% to 30% CD18-expressing neutrophils) survive childhood, with multiple infections affecting the skin and mucosal surfaces; documented mortality exceeds 50% by the age of 40 years 8.
Leukocyte adhesion deficiency diagnosis
A diagnosis of a leukocyte adhesion deficiency syndrome is suspected based upon a thorough clinical evaluation, a detailed patient history, identification of characteristic findings and a variety of tests such as a complete blood count (CBC). A complete blood count can detect elevated levels of a type of white blood cell known as a neutrophil (neutrophilia) and also lymphocytes. A diagnosis of leukocyte adhesion deficiency type 1 should be ruled out in any infant with recurrent soft tissue infections and a very high white blood cell count (leukocytosis).
A diagnosis of leukocyte adhesion deficiency type 1 or leukocyte adhesion deficiency type 2 or type 3 can be confirmed through molecular genetic testing, which can reveal the characteristic mutations of the ITGB2, the SLC35C1 or the FERMT3 genes that cause these disorders. Molecular genetic testing is available on a clinical basis.
Diagnosis before birth (prenatal diagnosis) is possible in families where the exact molecular defect has already been identified. A test known as chorionic villi biopsy is performed. Chorionic villi are thin, hair-like structures found on the placenta. Chorionic villi cells contain the same genetic material found in the cells of the fetus. A sample of tissue is taken from the placenta and studied to detect the presence of the specific genetic mutation that has caused leukocyte adhesion deficiency type 1, leukocyte adhesion deficiency type 2 or leukocyte adhesion deficiency type 3 in that family. Leukocyte adhesion deficiency type 2 can also be detected before birth by identifying the characteristic blood type (Bombay blood phenotype) associated with the disorder. This is possible at approximately 20 weeks gestation.
Leukocyte adhesion deficiency test
The immunological investigation of a patient with leukocyte adhesion deficiency includes 9:
Flow Cytometry Analysis (definitive test):
- Demonstrates the absence of functional CD18 and the associated alpha subunit molecules on the surface of leukocytes using CD11 and CD18 monoclonal antibodies (leukocyte adhesion deficiency type 1)
- Demonstrates the absence of sialyl Lewis X expression (CD15a) using a monoclonal antibody directed against sialyl Lewis X ( leukocyte adhesion deficiency-type 2)
Sequence analysis using genetic testing.
- To define the exact molecular defect in the beta-2 subunit
Quantitative Serum Immunoglobulins.
- IgG
- IgM
- IgA
- IgE
Antibody Activity.
IgG antibodies (post-immunization)
- Tetanus toxoid
- Diphtheria toxoid
- Pneumococcal polysaccharide
- Polio
IgG antibodies (post-exposure)
- Rubella
- Measles
- Varicella Zoster
Detection of isohemagglutinins (IgM)
- Anti-type A blood
- Anti-type B blood
Other assays
- Test for heterophile antibody
- Anti-streptolysin O titer
- Immunodiagnosis of infectious diseases (HIV, hepatitis B, and C, HTLV and dengue)
- Serum protein electrophoresis
Blood lymphocyte subpopulations
- Total lymphocyte count
- T lymphocytes (CD3, CD4, and CD8)
- B lymphocytes (CD19 and CD20)
- CD4/CD8 ratio
Lymphocyte stimulation assays
- Phorbol ester and ionophore
- Phytohemagglutinin
- Antiserum to CD3
- Chemotaxis of human lymphocytes
Phagocytic function
Nitroblue tetrazolium (NBT) test (before and after stimulation with endotoxin)
- Unstimulated
- Stimulated
Neutrophil mobility
- In medium alone
- In the presence of chemoattractant
- In vivo and in vitro chemotaxis of granulocytes
Complement System Evaluation
Measurement of individuals components by immunoprecipitation tests, ELISA, or Western blotting
- C3 serum levels
- C4 serum levels
- Factor B serum levels
- C1 inhibitor serum levels
Hemolytic assays
- CH50
- CH100
Complement system functional studies
- Classical pathway assay (using IgM on a microtiter plate)
- Alternative pathway assay (using LPS on a microtiter plate)
- Mannose pathway assay (using mannose on a microtiter plate)
Microbiological studies
- Nasopharyngeal swab (testing for Rhinovirus)
- Stool (testing for viral, bacterial or parasitic infection)
- Sputum (bacterial culture and pneumocystis PCR)
- Blood (bacterial culture, HIV by PCR, HTLV testing)
- Urine (testing for cytomegalovirus and proteinuria)
- Cerebrospinal fluid (culture, chemistry, and histopathology)
Other investigations of immunodeficiency disorders
- Bone marrow biopsy
- Complete blood cell count
- Blood chemistry
- Histopathological studies
- Tumoral markers
- Levels of cytokines
- Chest x-ray
- Diagnostic ultrasound
- Liver function test
Leukocyte adhesion deficiency treatment
The treatment of leukocyte adhesion deficiency syndromes are directed toward the specific symptoms that are apparent in each individual. The main aspect of treatment is antibiotic therapy to treat the repeated, characteristic infections associated with the leukocyte adhesion deficiency syndrome disorders. Prompt antibiotic therapy is essential during acute infectious episodes. Individuals with moderate or mild forms of leukocyte adhesion deficiency type 1 or leukocyte adhesion deficiency type 1 usually respond to conservative therapy and prompt treatment for acute episodes. Preventive (prophylactic) antibiotic therapy may be necessary for some individuals with more serious forms of leukocyte adhesion deficiency type 1.
The only curative therapy for individuals with leukocyte adhesion deficiency syndromes is a bone marrow transplant (hematopoietic stem cell transplant) 10. A bone marrow transplant may also be known as a stem cell transplant. Hematopoietic stem cells are special cells found in bone marrow that manufacture different types of blood cells (e.g., red blood cells, white blood cells, platelets). In allogeneic stem cell transplantation, stem cells are donated from another person, usually from a closely matched family member. Stem cell transplants have the potential to correct the inherent, genetic defect of the white blood cells of individuals with leukocyte adhesion deficiency syndromes. However, because stem cell transplants can cause severe, even life-threatening complications, they are usually reserved for individuals with severe complications or individuals who have no other viable treatment options. The initial results of bone marrow transplants for individuals with the severe form of leukocyte adhesion deficiency type 1 have been very encouraging. In a recent publication from several main centers in the world the overall survival of individuals who have had a bone marrow transplant for leukocyte adhesion deficiency type 1 is almost 75%. By the age of 2 years, leukocyte adhesion deficiency type 1 is fatal in severe cases without hematopoietic stem cell transplant 8.
In the absence of tissue neutrophils in patients with leukocyte adhesion deficiency type 1, inhibition of the IL-23/IL-17 axis is deficient, resulting in an unregulated hyperinflammatory response, which leads to chronic inflammation. In patients with leukocyte adhesion deficiency type 1, this process is particularly important in the gingivae but may also be involved in poorly healing cutaneous wounds that often affect these patients. A case report described in vivo beneficial effects of ustekinumab, a monoclonal antibody that binds the p40 subunit shared by IL-12 and IL-23, in a patient with leukocyte adhesion deficiency type 1, with dramatic improvement of periodontitis and of sacral wound 11.
Recombinant human interferon-gamma treatment has been used in leukocyte adhesion deficiency type 1 12.
Leukocyte adhesion deficiency type 2 does not require prophylactic antibiosis. A trial of fucose supplementation is recommended in all patients diagnosed with leukocyte adhesion deficiency type 2 13. Fucose replacement administered orally or intravenously has variable effectiveness in improving phagocytic functions.
In some cases, white blood cell (granulocyte) transfusions may be required to treat life-threatening infectious complications. Because of the possibility of adverse side effects, white blood cell transfusions are rarely used and only in severe cases when all other therapeutic options have failed. Blood transfusions are required for individuals with leukocyte adhesion deficiency type 3 who experience severe bleeding episodes.
Recombinant factor VIIa is considered effective in treating and preventing severe bleeding in a child patient with leukocyte adhesion deficiency type 3 14.
Use of prophylactic immunoglobulin therapy was successful in two patients with the severe form of leukocyte adhesion deficiency 15.
Researchers are studying gene therapy as a potential treatment for individuals with leukocyte adhesion deficiency syndromes. Gene therapy is an experimental therapy that involves replacing mutated genes with healthy copies, inactivating mutated genes, or introducing a new gene into the body that helps the body fight disease. Gene therapy with insertion of the CD18 subunit is currently under investigation. Because patients with decreased expression of CD18 (1-30%) have a milder disease, partial reconstitution is anticipated to provide clinical benefit.
Genetic counseling may be of benefit for affected individuals and their families. Other treatment is symptomatic and supportive.
Leukocyte adhesion deficiency prognosis
The leukocyte adhesion deficiency prognosis varies depending on the severity of the disease; it is usually fatal before one year of age. Moderate leukocyte adhesion deficiency cases can live longer than the third decade of life with appropriate antimicrobial therapy. Those patients with successful allogeneic hematopoietic stem cell transplant can have a better quality of life.
- Justiz Vaillant AA, Ahmad F. Leukocyte Adhesion Deficiency. [Updated 2019 Jun 18]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539770[↩]
- Hanna S, Etzioni A. Leukocyte adhesion deficiencies. Ann. N. Y. Acad. Sci. 2012 Feb;1250:50-5.[↩]
- Stepensky PY, Wolach B, Gavrieli R, Rousso S, Ben Ami T, Goldman V, Rozovsky K, Hanna S, Etzioni A, Weintraub M. Leukocyte adhesion deficiency type III: clinical features and treatment with stem cell transplantation. J. Pediatr. Hematol. Oncol. 2015 May;37(4):264-8.[↩]
- Cox DP, Weathers DR. Leukocyte adhesion deficiency type 1: an important consideration in the clinical differential diagnosis of prepubertal periodontitis. A case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008 Jan;105(1):86-90.[↩]
- Parvaneh N, Mamishi S, Rezaei A, Rezaei N, Tamizifar B, Parvaneh L, Sherkat R, Ghalehbaghi B, Kashef S, Chavoshzadeh Z, Isaeian A, Ashrafi F, Aghamohammadi A. Characterization of 11 new cases of leukocyte adhesion deficiency type 1 with seven novel mutations in the ITGB2 gene. J. Clin. Immunol. 2010 Sep;30(5):756-60.[↩]
- Justiz Vaillant AA, Qurie A. Immunodeficiency. [Updated 2019 Jun 18]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK500027[↩]
- Movahedi M, Entezari N, Pourpak Z, Mamishi S, Chavoshzadeh Z, Gharagozlou M, Mir-Saeeid-Ghazi B, Fazlollahi MR, Zandieh F, Bemanian MH, Farhoudi A, Aghamohammadi A. Clinical and laboratory findings in Iranian patients with leukocyte adhesion deficiency (study of 15 cases). J. Clin. Immunol. 2007 May;27(3):302-7.[↩]
- Almarza Novoa E, Kasbekar S, Thrasher AJ, Kohn DB, Sevilla J, Nguyen T, Schwartz JD, Bueren JA. Leukocyte adhesion deficiency-I: A comprehensive review of all published cases. J Allergy Clin Immunol Pract. 2018 Jul – Aug;6(4):1418-1420.e10.[↩][↩]
- Simpson AM, Chen K, Bohnsack JF, Lamont MN, Siddiqi FA, Gociman B. Pyoderma Gangrenosum-like Wounds in Leukocyte Adhesion Deficiency: Case Report and Review of Literature. Plast Reconstr Surg Glob Open. 2018 Aug;6(8):e1886.[↩]
- Stepensky PY, Wolach B, Gavrieli R, Rousso S, Ben Ami T, Goldman V, et al. Leukocyte Adhesion Deficiency Type III: Clinical Features and Treatment With Stem Cell Transplantation. J Pediatr Hematol Oncol. 2014 Jul 28.[↩]
- Moutsopoulos NM, Zerbe CS, Wild T, Dutzan N, Brenchley L, DiPasquale G, Uzel G, Axelrod KC, Lisco A, Notarangelo LD, Hajishengallis G, Notarangelo LD, Holland SM. Interleukin-12 and Interleukin-23 Blockade in Leukocyte Adhesion Deficiency Type 1. N. Engl. J. Med. 2017 Mar 23;376(12):1141-1146.[↩]
- Weening RS, Bredius RG, Vomberg PP, van der Schoot CE, Hoogerwerf M, Roos D. Recombinant human interferon-gamma treatment in severe leucocyte adhesion deficiency. Eur. J. Pediatr. 1992 Feb;151(2):103-7.[↩]
- Marquardt T, Lühn K, Srikrishna G, Freeze HH, Harms E, Vestweber D. Correction of leukocyte adhesion deficiency type II with oral fucose. Blood. 1999 Dec 15;94(12):3976-85.[↩]
- Saultier P, Szepetowski S, Canault M, Falaise C, Poggi M, Suchon P, Barlogis V, Michel G, Loyau S, Jandrot-Perrus M, Bordet JC, Alessi MC, Chambost H. Long-term management of leukocyte adhesion deficiency type III without hematopoietic stem cell transplantation. Haematologica. 2018 Jun;103(6):e264-e267[↩]
- Yamazaki-Nakashimada M, Maravillas-Montero JL, Berrón-Ruiz L, López-Ortega O, Ramírez-Alejo N, Acevedo-Ochoa E, Rivas-Larrauri F, Llamas-Guillén B, Blancas-Galicia L, Scheffler-Mendoza S, Olaya-Vargas A, Santos-Argumedo L. Successful adjunctive immunoglobulin treatment in patients affected by leukocyte adhesion deficiency type 1 (LAD-1). Immunol. Res. 2015 Mar;61(3):260-8.[↩]