What is human metapneumovirus
Human metapneumovirus can cause upper and lower respiratory disease in people of all ages, especially among young children, older adults, and people with weakened immune systems. Discovered in 2001, human metapneumovirus is in the paramyxovirus family along with respiratory syncytial virus (RSV) 1. Broader use of molecular diagnostic testing has increased identification and awareness of human metapneumovirus as an important cause of upper and lower respiratory infection. Retrospective serologic studies demonstrated the presence of human metapneumovirus antibodies in humans more than 50 years earlier. Although the human metapneumovirus was primarily known as causative agent of respiratory tract infections in children, human metapneumovirus is an important cause of respiratory infections in adults as well. Almost all children are infected by human metapneumovirus below the age of five; the repeated infections throughout life indicate transient immunity. Human metapneumovirus infections usually are mild and self-limiting, but in the frail elderly and the immunocompromised patients, the clinical course can be complicated. Since culturing the virus is relatively difficult, diagnosis is mostly based on a nucleic acid amplification test, such as reverse transcriptase polymerase chain reaction. To date, no vaccine is available and treatment is supportive. However, ongoing research shows encouraging results 1.
Human metapneumovirus is classified as the first human member of the metapneumovirus genus in the pneumovirinae subfamily within the paramyxoviridae family. Human metapneumovirus is an enveloped negative-sense single-stranded RNA virus. The RNA genome includes 8 genes coding for 9 different proteins. human metapneumovirus is identical in gene order to the avian pneumovirus, which also belongs to the metapneumovirus genus 2.
Phylogenetic analysis has identified two genotypes of human metapneumovirus, namely A and B 3. Both genotypes may co-circulate simultaneously, but during an epidemic, one genotype usually dominates 4. Within each of these subgroups two clades are designated A1, A2, B1 and B2 5. This classification is mainly based on the sequence variability of the attachment (G) and fusion (F) surface glycoproteins 3. The highly conserved F protein constitutes an antigenic determinant that mediates cross-lineage neutralization and protection 6. In 2006, two further subgroups, A2a and A2b, were described, but this further splitting was based on limited data and has not been confirmed by other groups 7. In addition, no clinical significance of these subgroups has yet been shown.
Since human metapneumovirus is a recently recognized respiratory virus, healthcare professionals may not routinely consider or test for human metapneumovirus. However, healthcare professionals should consider human metapneumovirus testing during winter and spring, especially when human metapneumovirus is commonly circulating.
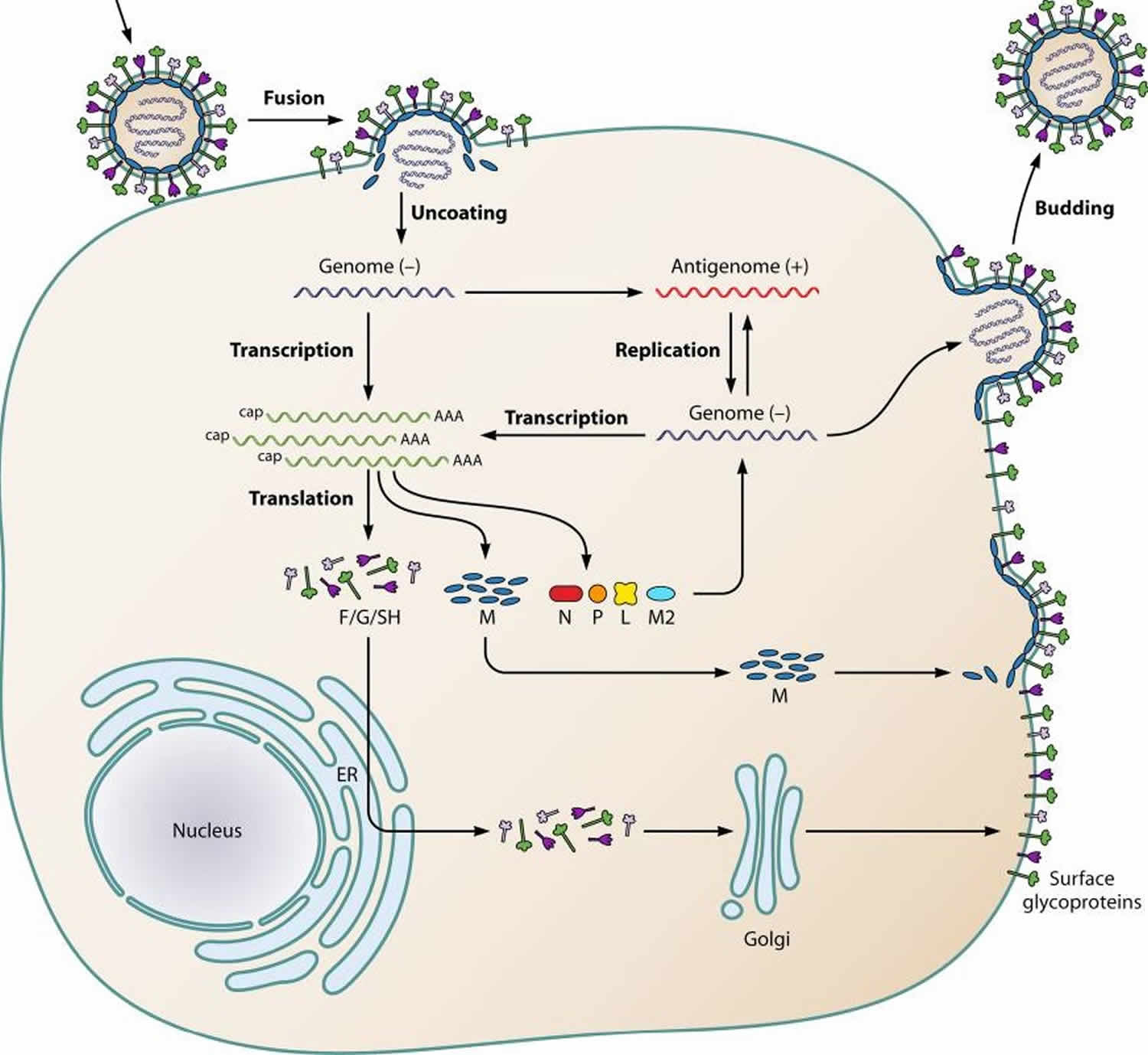
Figure 1. Human metapneumovirus life cycle
Footnote: Schematic representation of the human metapneumovirus life cycle. After attachment of the virion to the plasma membrane, the viral and plasma membranes fuse, resulting in uncoating of the virion and release of the RNP (containing the negative-sense viral RNA) into the cytoplasm. After primary transcription, the genome is replicated to produce the antigenome. The antigenome is used to synthesize genomic RNA, which is used to produce additional antigenomes for incorporation into progeny virions or as a template for secondary transcription. After translation, M proteins and RNPs are transported intracellularly to the plasma membrane and the viral glycoproteins F (fusion), G (glycoprotein), and SH (small hydrophobic) are transported from the endoplasmic reticulum (ER) to the Golgi apparatus and then the plasma membrane. Finally, new virions are assembled and are subsequently released from the plasma membrane by a budding process.
[Source 8 ]Human metapneumovirus in adults
Respiratory viruses are a common trigger for exacerbations of chronic obstructive pulmonary disease (COPD), and have been associated with respiratory failure in patients with cardiopulmonary disease such as COPD and congestive heart failure 9. Walsh et al. 10 performed a cohort-study during four winters to investigate the clinical outcome and incidence of human metapneumovirus infections. Serum samples were taken before and after the observation period (November 15 to April 15) each year. In case of respiratory symptoms a nasopharyngeal swab for human metapneumovirus RNA analysis and serum were sampled. They showed that 71% of infections with human metapneumovirus were asymptomatic in the healthy young adults (19–40 years) in contrast to 39% in the high risk adults (patients with symptomatic lung disease, chronic obstructive pulmonary disease (COPD), congestive heart failure). These patients were also more likely to use medical care service. Patients were ill for a mean of 10 days in the young adults versus 16 days in the high risk group. Johnstone et al. investigated the potential role of respiratory viruses in the natural history of community-acquired pneumonia. In 39% of the 193 patients who were admitted because of community-acquired pneumonia, a pathogen was identified. Of these pathogens, 39% were viruses and the easily transmissible viruses such as influenza, human metapneumovirus, and RSV were the most common (respectively 24, 24 and 17%). There were few clinically meaningful differences in presentation and no differences in outcomes according to the presence or absence of viral infection. The patients with viral infection were, compared with bacterial infection, significant older, more likely to have cardiac disease and more frail 11.
This is in accordance with the results of Hamelin et al. 12 who found human metapneumovirus in 4.1% of patients with community-acquired pneumonia or exacerbation of chronic obstructive pulmonary disease. Martinello et al. 13 also showed that human metapneumovirus was frequently identified in patients hospitalized because of an exacerbation of chronic obstructive pulmonary disease (COPD). human metapneumovirus (both genotype A and B) was identified in nasopharyngeal specimens (by RT‑PCR) in 12% of these patients (6/50). RSV, influenza A and parainfluenza type 3 were identified in respectively 8%, 4% and 2%.
Along with these results, Williams and colleagues 14 showed that human metapneumovirus was detected (by RT-PCR of nasal wash specimens) in almost 7% (7/101) of the adults hospitalized for an acute asthma exacerbation, compared to 1.3% in follow-up patients. While none of these patients tested positive for human metapneumovirus three months after discharge, a direct etiologic role of the virus seems very likely 14.
Recently, we reported a case series of adult patients, including two patients known with COPD, with severe human metapneumovirus infections with respiratory insufficiency and the need of ICU admission 15.
Healthy elderly patients over 65 years
Since adults are not routinely screened for human metapneumovirus in the hospital and clinical course can be asymptomatic or mild, infections in the elderly are likely to be underreported. The reported yearly incidence in adults is between 4 and 11% and in adults aged over 50 years; hospitalization rates for human metapneumovirus were similar to those associated with influenza and RSV 16.
Walsh et al. showed that the risk for symptomatic severe human metapneumovirus infection was higher in the elderly. human metapneumovirus infection was asymptomatic in 44% of the healthy elderly in contrast to 71% of the healthy young adults. Thirty-eight % of the elderly with human metapneumovirus infection used medical care in contrast to 9% in the young adults 17. Rates for hospitalization in elderly patients over 65 years were also significantly higher for human metapneumovirus infection (22.1/10,000 residents) compared to influenza virus (12.3/10,000 residents), but similar to those of RSV infection (25.4/10,000 residents) 16. Antibody levels prior to infection were higher in elderly, suggesting possible immune dysregulation associated with decreased viral clearance in elderly 18.
Immunocompromised
Several case reports and case series concerning human metapneumovirus infections in immunocompromised patients have been published reporting varying morbidity and mortality 19. While immunocompromised patients, including patients with haematological malignancies and solid organ and hematopoietic stem cell transplant (HSCT) patients appear to acquire human metapneumovirus infection at the same frequency as immunocompetent individuals, they seem to be at risk for severe infections, probably due to poor viral clearance 20. Clinical course is prolonged and respiratory failure may develop 21. However, Debiaggi showed that hematopoietic stem cell transplant recipients may frequently develop symptomless human metapneumovirus infection 22.
Sumino et al. 23 examined a cohort of 688 patients who underwent a bronchoscopy. Of these patients, 72% were immunocompromised (mainly lung transplant patients) and 30% were patients without acute illness who underwent routine bronchoscopy for surveillance after lung transplantation or follow-up of rejection. Six cases of human metapneumovirus infection were identified using RT-PCR; four of these were immunocompromised hosts. In the asymptomatic individuals, no cases were identified.
Kamboj et al. 24 showed that human metapneumovirus is detected in 2.7% of cancer patients with respiratory disease. However, human metapneumovirus was associated with mild respiratory disease and RSV and influenza were more often found. In patients with hematologic malignancies human metapneumovirus was found more often.
Debur et al. 25 showed that human metapneumovirus was present in 2.5% of hematologic stem cell transplant recipients with respiratory disease. Most patients presented with upper respiratory tract infection, while 27% had a lower RTA. No patients died 25.
Englund et al. 26 performed a retrospective survey to demonstrate the importance of human metapneumovirus in hematopoietic stem-cell transplant recipients. In 3% of these patients who underwent a BAL because of LRTI was human metapneumovirus detected (by RT-PCR). Clinical course in this group was severe and 80% died with acute respiratory failure.
Williams et al. 27 showed that human metapneumovirus is found in the same frequency as RSV, influenza and parainfluenzavirus in hematologic malignancy patients with acute respiratory disease. All patients presented with an upper respiratory tract infection, but 41% progressed to a lower respiratory tract infection. One third (three patients) of these patients died, however in two of these patients potential bacterial pathogens were also found in their BAL fluid 27.
Cane et al. 28 published a case report about a HSCT recipient who succumbed to progressive respiratory failure following an upper respiratory prodrome and where human metapneumovirus was detected as the sole pathogen in the nasopharyngeal aspirate.
In lung transplant patients, human metapneumovirus was found in 6% of adults with respiratory tract infection. This was significantly lower then the most frequently found viral cause, namely parainfluenza virus (17%) 29. RSV and influenza were found in 12% and 14% respectively. The rate of required hospitalization and length of stay of hospitalization were not different between human metapneumovirus and other respiratory viruses. In this study, viral respiratory tract infection was associated with acute graft rejection. However, this rate was significantly higher for RSV infection compared to human metapneumovirus infection.
Larcher et al. 30 found human metapneumovirus in 25% of BAL fluids from lung transplant patients. Not al of them had respiratory symptoms at the time of the lavage. In this study 30, human metapneumovirus infection seems associated with acute graft rejection, but not with the development of bronchiolitis obliterans. However, other studies suggest that viral respiratory tract infection is associated with the risk of the development of bronchiolitis obliterans 31.
Human metapneumovirus transmission
Human metapneumovirus is most likely spread from an infected person to others through:
- secretions from coughing and sneezing,
- close personal contact, such as touching or shaking hands, and
- touching objects or surfaces that have the viruses on them then touching the mouth, nose, or eyes.
In the U.S., human metapneumovirus circulates in distinct annual seasons. human metapneumovirus circulation begins in winter and lasts until or through spring. human metapneumovirus, respiratory syncytial virus (RSV), and influenza can circulate simultaneously during the respiratory virus season.
Human metapneumovirus is associated with severe infection in patients with pulmonary disease and chronic obstructive pulmonary disease (COPD). Studies on human metapneumovirus in BALB/c in mice and cotton rats show airway obstruction and hyperresponsiveness after infection. Initially human metapneumovirus infection in the lung is characterized by interstitial inflammation with alveolitis starting on day 3 with a peak on day 5 and subsequently decreasing inflammation 32. However, after 2–3 weeks this develops in a more prominent peribronchiolar and perivascular infiltrate. Hamelin et al. 33 also show airway obstruction in BALB/c mice after a single human metapneumovirus challenge with a peak on day 5, but still present until day 70. In addition, significant hyperresponsiveness after methacholine challenge was also shown until day 70, indicating long-term pulmonary inflammation after human metapneumovirus infection.
Darniot and colleagues 34 demonstrated in a mice model that susceptibility to human metapneumovirus infection is age related with aged mice showing severer illness and mortality compared to young mice. Aged mice showed greater virus replication in the lung; however, viral clearance was not delayed. In addition, lower levels of virus specific antibodies, neutralizing antibodies and interferon gamma with a significant increase in IL4 and CD4+ lymphocytes were observed in aged mice after human metapneumovirus infection. This suggests an important role for the cellular immune response in controlling human metapneumovirus infection 34. This hypothesis is partially confirmed by Ditt et al. 35, who found that human metapneumovirus infection in aged mice results in a diminished TNF-alfa expression resulting in low levels of NF-Kb compared to young mice.
Lüsebrink et al. 36 demonstrated that neutralizing antibodies seem to be present in all age groups in humans and that neutralizing capacities remain high, with a minor decrease for individuals over 69 years of age. Therefore, they hypothesized that the cellular response has a more important role in the clearing of human metapneumovirus infection than the neutralizing humoral immune response 36.
Sastre et al. 37 used a recombinant fusion protein-based enzyme linked immunosorbent assay (F‑ELISA) in the same set of sera. Their results support the hypothesis that it appears likely that neutralizing antibodies play a minor role in the control of human metapneumovirus infections in humans. In addition, Falsey et al. found higher serum antibodies at baseline, a greater response in binding antibody and a trend towards greater neutralizing antibody responses in older adults compared to younger adults with human metapneumovirus illness of the same severity suggesting immune dysregulation in aged patients with an human metapneumovirus infection 17.
Overall, neutralizing antibodies seem to play a minor role in controlling human metapneumovirus infections. Cellular immune responses seem to be more important for the susceptibility of human metapneumovirus infections in aged patients.
Human metapneumovirus symptoms
Symptoms commonly associated with human metapneumovirus include cough, fever, nasal congestion, and shortness of breath. Clinical symptoms of human metapneumovirus infection may progress to bronchitis or pneumonia and are similar to other viruses that cause upper and lower respiratory infections. The estimated incubation period is 3 to 6 days, and the median duration of illness can vary depending upon severity but is similar to other respiratory infections caused by viruses.
Human metapneumovirus infections may be more severe in older patients or patients with underlying medical conditions. It is a significant cause of acute respiratory diseases in adults over 65 years and adults with comorbid diseases, such as COPD, asthma, cancer, immunocompromised status, including HIV or post transplantation.
Human metapneumovirus complications
Bacterial and fungal superinfections might complicate viral respiratory infections. To our knowledge no studies specific addressing this issue have been executed, although some studies report the presence of potential bacterial pathogens in the BAL fluid, sputum or blood cultures in those patients with sometimes lethal outcome 38.
In a mouse model that human metapneumovirus infection predisposes to severe bacterial infections 39. Higher levels of airway obstruction, pneumococcal replication and inflammatory cytokines and chemokines were observed in the lungs of superinfected mice, which were challenged with Streptococcus pneumoniae (S. pneumoniae) five days after human metapneumovirus infection. Inactivated human metapneumovirus did not result in these changes after a pneumococcal challenge, suggesting that human metapneumovirus replication rather than the host response to human metapneumovirus may be responsible for these effects. Mice infected with influenza A show long‑term impairment of Streptococcus pneumoniae lung clearance, but the mechanism producing these effects might be different. In contrast to these findings, Ludewick et al. showed that BALB/c mice infected with human metapneumovirus had a normal bacterial lung clearance when they were challenged with Streptococcus pneumoniae 14 days after human metapneumovirus infection 40.
Human metapneumovirus diagnosis
Infection with human metapneumovirus can be confirmed usually by:
- direct detection of viral genome by polymerase chain reaction (PCR) assays or nucleic acid amplification tests (NAAT) and
- direct detection of viral antigens in respiratory secretions using immunofluorescence or enzyme immunoassay (EIA).
Virus culture is relatively difficult, because human metapneumovirus grows slowly in conventional cell culture and has mild cytopathic effects. The rapid culture technique is known as shell vial amplification 41.
Detection of viral RNA by NAAT such as reverse transcriptase-PCR (RT-PCR) assay is the most sensitive method for diagnosis of human metapneumovirus infection 42.
Methods for detection of human metapneumovirus antigens, such as enzyme immuno-assay (EIA) and enzyme-linked immunosorbent assay (ELISA) are not commonly used. No commercial immunochromatographic assays are available. A direct immunofluorescent-antibody (IFA) test—which is a rapid test in which labeled antibodies to detect specific viral antigens in direct patients materials are used—could be useful for the diagnosis of human metapneumovirus infections in outbreaks. The test results are known within two hours. However, the sensitivity of IFA is lower than that of RT-PCR and needs to be validated before use.
Detection of the immune response against the virus by serologic testing is only used for epidemiologic studies. One of the disadvantages of serology is the fact that the interval between virus spreading and detection of human metapneumovirus-specific IgM and IgG antibodies is relatively long. However, a combined approach of serology and RT-PCR has added diagnostic value in the diagnosis of human metapneumovirus infections in the case of investigating the magnitude of an outbreak for instance in long term care facilities 42.
Human metapneumovirus treatment
Currently, there is no specific antiviral therapy to treat human metapneumovirus and no vaccine to prevent human metapneumovirus. Medical care is supportive. However, your patients can help prevent the spread of human metapneumovirus and other respiratory viruses by following these steps:
- Wash their hands often with soap and water
- Avoid touching their eyes, nose, or mouth with unwashed hands
- Avoid close contact with people who are sick
Patients who have cold-like symptoms should:
- Cover their mouth and nose when coughing and sneezing
- Wash their hands frequently and correctly (with soap and water for 20 seconds)
- Avoid sharing their cups and eating utensils with others
- Refrain from kissing others
- Stay at home when they are sick
In addition, cleaning possible contaminated surfaces (such as doorknobs and shared toys) may potentially help stop the spread of human metapneumovirus.
Till now a lot of experience on treatment of human metapneumovirus has been gained in individual cases and small case series 43. The combination of ribavirin with IVIG seems to be very promising, although this combination is expensive and has disadvantages. Several other treatment regimes have been investigated and proven to be effective in vitro and in animal studies. Both immunoglobulins (like mAb 338 and Fab DS7) and synthetic fusion inhibitors showed to be efficient against human metapneumovirus. The recently discovered approach of RNA interference (RNAi) could be the technique of the future. However, up till now, no treatment proven to be effective in large clinical trials is available and treatment of human metapneumovirus infection is mainly supportive 1.
- Haas LE, Thijsen SF, van Elden L, Heemstra KA. Human metapneumovirus in adults. Viruses. 2013;5(1):87–110. Published 2013 Jan 8. doi:10.3390/v5010087 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3564111/[↩][↩][↩]
- Genetic diversity between human metapneumovirus subgroups. Biacchesi S, Skiadopoulos MH, Boivin G, Hanson CT, Murphy BR, Collins PL, Buchholz UJ. Virology. 2003 Oct 10; 315(1):1-9.[↩]
- A newly discovered human pneumovirus isolated from young children with respiratory tract disease. van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, Osterhaus AD. Nat Med. 2001 Jun; 7(6):719-24.[↩][↩]
- Agapov E., Sumino K.C., Gaudreault-Keener M., Storch G.A., Holtzman M.J. Genetic variability of human metapneumovirus infection: Evidence of a shift in viral genotype without a change in illness. J. Infect. Dis. 2006;193:396–403. doi: 10.1086/499310[↩]
- Mackay I.M., Bialasiewicz S., Jacob K.C., McQueen E., Arden K.E., Nissen M.D., Sloots T.P. Genetic diversity of human metapneumovirus over 4 consecutive years in Australia. J. Infect. Dis. 2006;193:1630–1633. doi: 10.1086/504260[↩]
- Skiadopoulos M.H., Biacchesi S., Buchholz U.J., Riggs J.M., Surman S.R., Amaro-Carambot E., McAuliffe J.M., Elkins W.R., St Claire M., Collins P.L., et al. The two major human metapneumovirus genetic lineages are highly related antigenically, and the fusion (F) protein is a major contributor to this antigenic relatedness. J. Virol. 2004;78:6927–6937[↩]
- Huck B., Scharf G., Neumann-Haefelin D., Puppe W., Weigl J., Falcone V. Novel human metapneumovirus sublineage. Emerg. Infect. Dis. 2006;12:147–150. doi: 10.3201/eid1201.050772[↩]
- Schildgen V, van den Hoogen B, Fouchier R, et al. Human Metapneumovirus: lessons learned over the first decade. Clin Microbiol Rev. 2011;24(4):734–754. doi:10.1128/CMR.00015-11 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3194831[↩]
- Duncan C.B., Walsh E.E., Peterson D.R., Lee F.E., Falsey A.R. Risk factors for respiratory failure associated with respiratory syncytial virus infection in adults. J. Infect. Dis. 2009;200:1242–1246. doi: 10.1086/605948[↩]
- Walsh E.E., Falsey A.R., Hennessey P.A. Respiratory syncytial and other virus infections in persons with chronic cardiopulmonary disease. Am. J. Respir. Crit. Care Med. 1999;160:791–795[↩]
- Johnstone J., Majumdar S.R., Fox J.D., Marrie T.J. Viral Infection in Adults Hospitalized with Community-Acquired Pneumonia: Prevalence, Pathogens, and Presentation. Chest. 2008;134:1141–1148. doi: 10.1378/chest.08-0888[↩]
- Hamelin M.E., Boivin G. Human metapneumovirus: A ubiquitous and long-standing respiratory pathogen. J. Pediatr. Infect. Dis. 2005;24:S203–S207. doi: 10.1097/01.inf.0000188158.27840.7c [↩]
- Martinello R.A., Esper F., Weibel C., Ferguson D., Landry M.L., Kahn J.S. Human metapneumovirus and exacerbations of chronic obstructive pulmonary disease. J. Infect. 2006;53:248–254. doi: 10.1016/j.jinf.2005.11.010[↩]
- Williams J.V., Crowe J.E., Jr, Enriquez R., Minton P., Peebles R.S., Jr, Hamilton R.G., Higgins S., Griffin M., Hartert T.V. Human metapneumovirus infection plays an etiologic role in acute asthma exacerbations requiring hospitalization in adults. J. Infect. Dis. 2005;192:1149–1153. doi: 10.1086/444392[↩][↩]
- Haas L.E., de Rijk N.X., Thijsen S.F. Human metapneumovirus infections on the ICU: A report of three cases. Ann. Intensive Care. 2012;2:30. doi: 10.1186/2110-5820-2-30[↩]
- Widmer K., Zhu Y., Williams J.V., Griffin M.R., Edwards K.M., Talbot H.K. Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. J. Infect. Dis. 2012;206:56–62. doi: 10.1093/infdis/jis309[↩][↩]
- Falsey A.R., Hennessey P.A., Formica M.A., Criddle M.M., Biear J.M., Walsh E.E. Humoral immunity to human metapneumovirus infection in adults. Vaccine. 2010;28:1477–1480[↩][↩]
- Miller R.A. The aging immune system: Primer and prospectus. Science. 1996;273:70–74.[↩]
- Egli A., Bucher C., Dumoulin A., Stern M., Buser A., Bubendorf L., Gregor M., Servida P., Sommer G., Bremerich J., et al. Human metapneumovirus infection after allogeneic hematopoietic stem cell transplantation. Infection. 2012;40:677–684. doi: 10.1007/s15010-012-0279-9[↩]
- Peck A.J., Englund J.A., Kuypers J., Guthrie K.A., Corey L., Morrow R., Hackman R.C., Cent A., Boeckh M. Respiratory virus infection among hematopoietic cell transplant recipients: Evidence for asymptomatic parainfluenza virus infection. Blood. 2007;110:1681–1688[↩]
- Huck B., Egger M., Bertz H., Peyerl-Hoffman G., Kern W.V., Neumann-Haefelin D., Falcone V. Human metapneumovirus infection in a hematopoietic stem cell transplant recipient with relapsed multiple myeloma and rapidly progressing lung cancer. J. Clin. Microbiol. 2006;44:2300–2303[↩]
- Debiaggi M., Canducci F., Sampaolo M., Marinozzi M.C., Parea M., Terulla C., Colombo A.A., Alessandrino E.P., Bragotti L.Z., Arghittu M., et al. Persistent symptomless human metapneumovirus infection in hematopoietic stem cell transplant recipients. J. Infect. Dis. 2006;194:474–478. doi: 10.1086/505881[↩]
- Sumino K.C., Agapov E., Pierce R.A., Trulock E.P., Pfeifer J.D., Ritter J.H., Gaudreault-Keener M., Storch G.A., Holtzman M.J. Detection of severe human metapneumovirus infection by real-time polymerase chain reaction and histopathological assessment. J. Infect. Dis. 2005;192:1052–1060. doi: 10.1086/432728[↩]
- Kamboj M., Gerbin M., Huang C.K., Brennan C., Stiles J., Balashov S., Park S., Kiehn T.E., Perlin D.S., Pamer E.G., et al. Clinical characterization of human metapneumovirus infection among patients with cancer. J. Infect. 2008;57:464–471. doi: 10.1016/j.jinf.2008.10.003[↩]
- Debur M.C., Vidal L.R., Stroparo E., Nogueira M.B., Almeida S.M., Takahashi G.A., Rotta I., Pereira L.A., Silveira C.S., Delfraro A., et al. Impact of human metapneumovirus infection on in and outpatients for the years 2006-2008 in Southern Brazil. Memorias do Instituto Oswaldo Cruz. 2010;105:1010–1018. doi: 10.1590/S0074-02762010000800010[↩][↩]
- Englund J.A., Boeckh M., Kuypers J., Nichols W.G., Hackman R.C., Morrow R.A., Fredricks D.N., Corey L. Brief communication: Fatal human metapneumovirus infection in stem-cell transplant recipients. Ann. Intern. Med. 2006;144:344–349[↩]
- Williams J.V., Martino R., Rabella N., Otegui M., Parody R., Heck J.M., Crowe J.E., Jr. A prospective study comparing human metapneumovirus with other respiratory viruses in adults with hematologic malignancies and respiratory tract infections. J. Infect. Dis. 2005;192:1061–1065. doi: 10.1086/432732[↩][↩]
- Cane P.A., van den Hoogen B.G., Chakrabarti S., Fegan C.D., Osterhaus A.D. Human metapneumovirus in a haematopoietic stem cell transplant recipient with fatal lower respiratory tract disease. Bone Marrow Transplant. 2003;31:309–310. doi: 10.1038/sj.bmt.1703849[↩]
- Weinberg A., Lyu D.M., Li S., Marquesen J., Zamora M.R. Incidence and morbidity of human metapneumovirus and other community-acquired respiratory viruses in lung transplant recipients. Transpl. Infect. Dis. 2010;12:330–335. doi: 10.1111/j.1399-3062.2010.00509.x[↩]
- Larcher C., Geltner C., Fischer H., Nachbaur D., Muller L.C., Huemer H.P. Human metapneumovirus infection in lung transplant recipients: clinical presentation and epidemiology. J. Heart Lung Transplant. 2005;24:1891–1901. doi: 10.1016/j.healun.2005.02.014[↩][↩]
- Kumar D., Erdman D., Keshavjee S., Peret T., Tellier R., Hadjiliadis D., Johnson G., Ayers M., Siegal D., Humar A. Clinical impact of community-acquired respiratory viruses on bronchiolitis obliterans after lung transplant. Am. J. Transplant. 2005;5:2031–2036. doi: 10.1111/j.1600-6143.2005.00971.x[↩]
- Hamelin M.E., Yim K., Kuhn K.H., Cragin R.P., Boukhvalova M., Blanco J.C., Prince G.A., Boivin G. Pathogenesis of human metapneumovirus lung infection in BALB/c mice and cotton rats. J. Virol. 2005;79:8894–8903. doi: 10.1128/JVI.79.14.8894-8903.2005[↩]
- Hamelin M.E., Prince G.A., Gomez A.M., Kinkead R., Boivin G. Human metapneumovirus infection induces long-term pulmonary inflammation associated with airway obstruction and hyperresponsiveness in mice. J. Infect. Dis. 2006;193:1634–1642. doi: 10.1086/504262[↩]
- Darniot M., Pitoiset C., Petrella T., Aho S., Pothier P., Manoha C. Age-associated aggravation of clinical disease after primary metapneumovirus infection of BALB/c mice. J. Virol. 2009;83:3323–3332. doi: 10.1128/JVI.02198-08[↩][↩]
- Ditt V., Lusebrink J., Tillmann R.L., Schildgen V., Schildgen O. Respiratory infections by HMPV and RSV are clinically indistinguishable but induce different host response in aged individuals. PLoS One. 2011;6:e16314[↩]
- Lusebrink J., Wiese C., Thiel A., Tillmann R.L., Ditt V., Muller A., Schildgen O., Schildgen V. High seroprevalence of neutralizing capacity against human metapneumovirus in all age groups studied in Bonn, Germany. Clin. Vaccine Immunol. 2010;17:481–484. doi: 10.1128/CVI.00398-09[↩][↩]
- Sastre P., Ruiz T., Schildgen O., Schildgen V., Vela C., Rueda P. Seroprevalence of human respiratory syncytial virus and human metapneumovirus in healthy population analyzed by recombinant fusion protein-based enzyme linked immunosorbent assay. Virol. J. 2012;9:130-422X-9-130. doi: 10.1186/1743-422X-9-130[↩]
- Walsh E.E., Peterson D.R., Falsey A.R. Human metapneumovirus infections in adults: Another piece of the puzzle. Ann. Intern. Med. 2008;168:2489–2496. doi: 10.1001/archinte.168.22.2489[↩]
- Kukavica-Ibrulj I., Hamelin M.E., Prince G.A., Gagnon C., Bergeron Y., Bergeron M.G., Boivin G. Infection with human metapneumovirus predisposes mice to severe pneumococcal pneumonia. J. Virol. 2009;83:1341–1349.[↩]
- Ludewick H.P., Aerts L., Hamelin M.E., Boivin G. Long-term impairment of streptococcus pneumoniae lung clearance is observed after initial infection with influenza a virus but not human metapneumovirus in mice. J. Gen. Virol. 2011;92:1662–1665. doi: 10.1099/vir.0.030825-0[↩]
- Hamelin M.E., Boivin G. Development and validation of an enzyme-linked immunosorbent assay for human metapneumovirus serology based on a recombinant viral protein. Clin. Diagn. Lab. Immunol. 2005;12:249–253[↩]
- Chiu C.Y., Alizadeh A.A., Rouskin S., Merker J.D., Yeh E., Yagi S., Schnurr D., Patterson B.K., Ganem D., DeRisi J.L. Diagnosis of a critical respiratory illness caused by human metapneumovirus by use of a pan-virus microarray. J. Clin. Microbiol. 2007;45:2340–2343[↩][↩]
- Raza K., Ismailjee S.B., Crespo M., Studer S.M., Sanghavi S., Paterson D.L., Kwak E.J., Rinaldo C.R., Jr, Pilewski J.M., McCurry K.R., et al. Successful outcome of human metapneumovirus (hMPV) pneumonia in a lung transplant recipient treated with intravenous ribavirin. J. Heart Lung Transplant. 2007;26:862–864 [↩]