Multiple evanescent white dot syndrome
Multiple evanescent white dot syndrome or MEWDS, is a rare, unilateral, self-limited disease characterized by multiple small white dots that spontaneously resolve over a period of months 1. MEWDS produce transient small gray-white dots classically located at the retinal pigment epithelium (RPE) or outer retina with foveal granularityas and are found primarily in young adults 2. Other features include an edematous appearing optic nerve head and the presence of cells in the vitreous 3. No known racial or hereditary predilections have been reported 4. Associated clinical findings include a flu-like prodrome, predisposition to involve young females, blurred disc margins, and temporal scotomata 5. Although the precise pathogenesis remains unknown, a viral-like infection with a possible immune-mediated mechanism and genetic susceptibility is suspected 6. In all of the MEWDS variants, a predilection for inflammation seems to exist in the peripapillary circulation. In this location, a plentiful source of ciliary arteries communicates axially with the retinal circulation and accounts for the characteristic enlargement of the blind spot that is observed in patients with this disorder 7. MEWDS is a disease of the photoreceptors and is almost completely reversible. The vitreous, retinal pigment epithelium, and choroid are only secondarily and transiently involved. Therefore, MEWDS can be considered a “common cold” of the retina 3.
Symptoms of MEWDS include blurred vision, visual field loss, photopsias, and floaters. The various diagnoses are differentiated by history, appearance, laterality, and fluorescein angiogram findings. MEWDS affects females ranging from 14 to 47 years of age and is preceded by a flu-like illness in approximately half of cases. Patients present with complaint of a sudden decrease in vision, as low as 20/200, paracentral scotomata, including an enlarged blind spot, and shimmering temporal photopsias. Bilateral cases of multiple evanescent white dot syndrome with simultaneous or sequential onset are rare. Exam may reveal an afferent pupillary defect and typically shows a quiet anterior chamber, mild vitritis, vascular sheathing, and disc edema. The characteristic finding is the presence of multiple discrete grayishwhite spots at the level of the outer retina or retinal pigment epithelium (RPE) measuring 100 to 200 μm, typically concentrated in the macula (Figure 1). The lesions seem to spare fovea, which may have a granular appearance due to tiny white or yellowish-orange specks and a distorted or absent reflex. On fluorescein angiography the lesions show early punctate hyperfluorescence spots in wreath-like configuration that stain late, with disc and vascular leakage. Indocyanine green angiography reveals peripapillary hypofluorescence and more numerous hypofluorescent lesions than expected clinically. Electrophysiologic studies show abnormalities during the acute stage of the disease that normalize during the recovery phase, including decreased a-wave and early receptor potential amplitudes on electroretinogram (ERG), subnormal electro-oculogram (EOG), and an enlarged blind spot.
The clinical course of multiple evanescent white dot syndrome is short, with most patients experiencing nearly complete visual recovery over a period of 1 to 2 months, although photopsias and scotomata may last longer. The white dots spontaneously resolve, leaving only foveal granularity and mild pigmentary changes as evidence of prior disease. Recurrences are rare but a chronic form of bilateral multiple evanescent white dot syndrome with multiple recurrences can occur. The cause of multiple evanescent white dot syndrome is unknown. Although often preceded by a flu-like illness, there are no systemic manifestations. The diagnosis is clinical, and there is no diagnostic laboratory test. As multiple evanescent white dot syndrome is a selflimited disease with an excellent visual prognosis, no treatment is needed. The differential diagnosis includes acute macular neuroretinopathy, acute idiopathic enlarged blind spot syndrome, multifocal choroiditis and panuveitis, punctate inner choroidopathy, acute zonal occult outer retinopathy, and sarcoidosis. multiple evanescent white dot syndrome has been reported in association with both acute macular neuroretinopathy and multifocal choroiditis and panuveitis. Acute macular neuroretinopathy and acute idiopathic enlarged blind spot syndrome are rare conditions that primarily affect young women and result in central or paracentral scotomata. It has been suggested that acute macular neuroretinopathy (characterized by the presence of reddish flat or depressed circumscribed macular lesions) and acute idiopathic enlarged blind spot syndrome (shows no retinal, choroidal, or disc abnormalities) may represent atypical cases of multiple evanescent white dot syndrome.
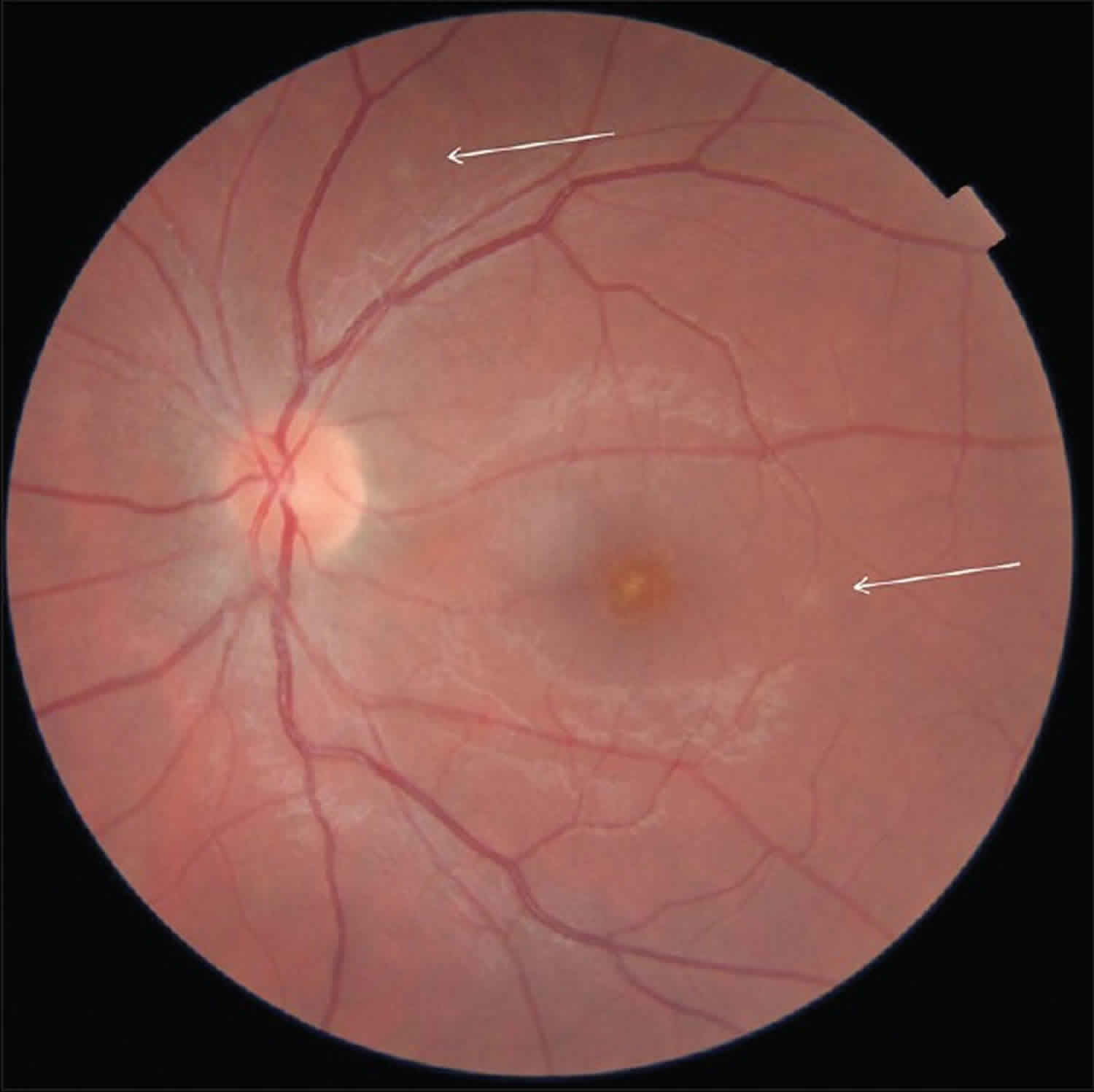
Figure 1. Multiple evanescent white dot syndrome
Footnote: Retinography of the left eye. Arrows indicate residual white dots.
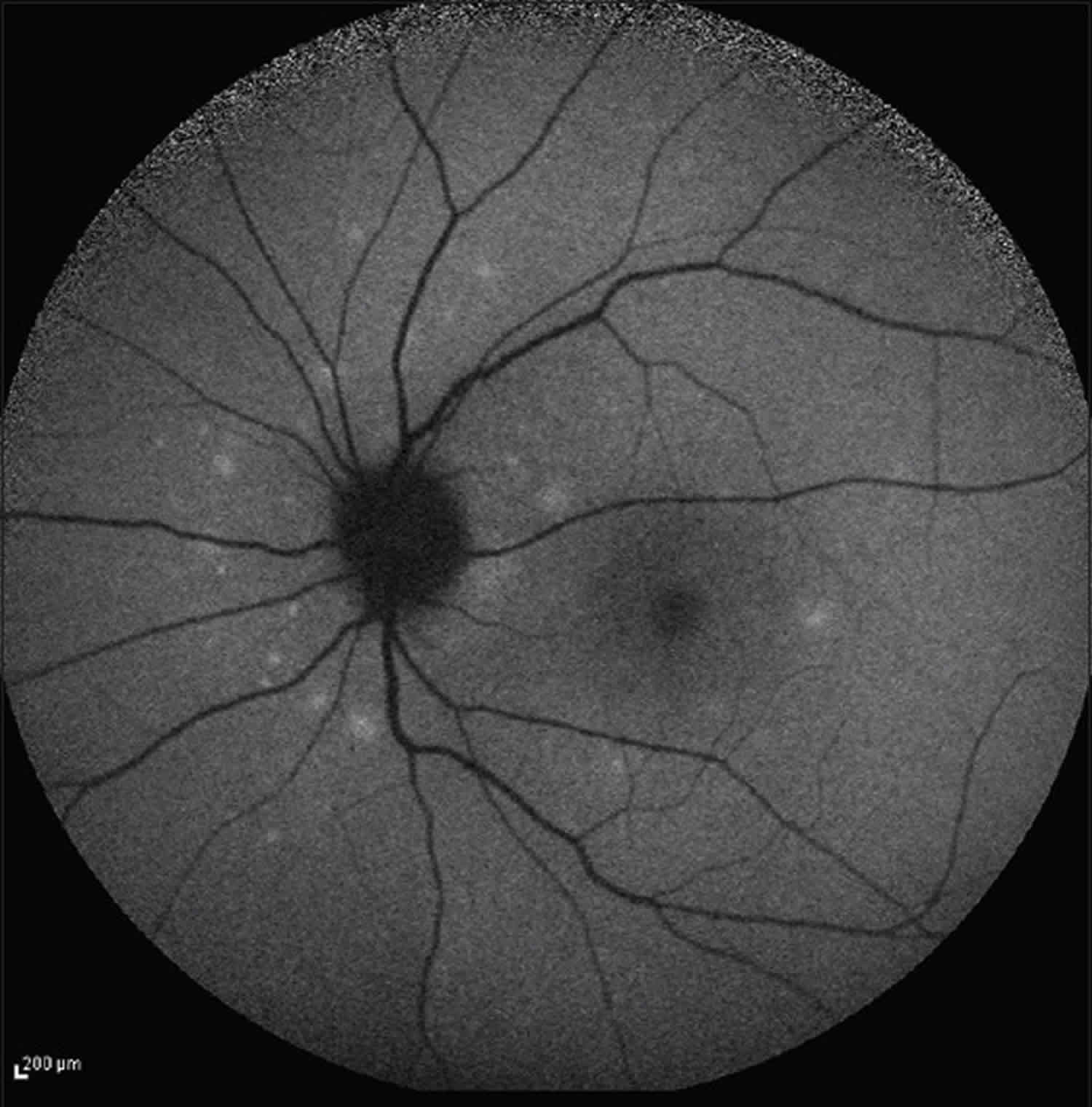
[Source 8 ]Figure 2. Multiple evanescent white dot syndrome
Footnote: Posterior pole autofluorescence outer segment shows multiple white dots concentrated in the peripapillary area.
[Source 8 ]MEWDS causes
An infectious etiology is thought to be involved in the pathogenesis of MEWDS since frequently there is a viral prodrome 9. However, the exact pathogenesis is unknown.
MEWDS symptoms
The typical patient with MEWDS is a healthy middle aged female age 15-50. There is a gender disparity as women are affected with MEWDS four times more often than men. Roughly 30% of patients have experienced an associated viral prodrome. Patients present with acute, painless, unilateral change in vision. They may notice photopsias, dyschromatopsia, or a temporal or paracentral scotoma. Majority of the cases have unilateral involvement, but bilateral MEWDS has been described and is usually asymmetric.
MEWDS diagnosis
On fundus exam, one sees flat, multifocal, grey-white lesions (100-200 microns), appearing to reach as deep as the retinal pigment epithelium. Typically lesions are found outside the fovea in the posterior pole. A characteristic finding in MEWDS is an orange-yellow fovea with granularity. One might also note optic disc edema, mild vitritis (usually posterior vitreous cells), mild anterior chamber flare, a relative afferent pupillary defect and an enlarged blind spot. Sheathing of retinal veins and superficial retinal hemorrhages are rarely seen.
Based on their clinical and angiographic evidences 2, the lesions are best characterized on fluorescein angiography as “wreath-like” punctate areas of early hyperfluorescence (window defects in the retinal pigment epithelium, retinal vascular abnormalities, or a combination of both) 7. On indocyanine green angiography (indocyanine green angiography), numerous hypocyanescent lesions significantly outnumber those visible on clinical examination and fluorescein angiography 10.
The MEWDS lesions of the fundus are variable in size, ranging from dots (small lesions approximately 100 μm) to spots (larger lesions 200 μm). Small lesions (dots) are anterior to the larger lesions (spots) 7. Dots are superficial to spots in a seemingly dual-layered pattern at the level of the middle retina and the deep retina and retinal pigment epithelium. Dots appear to be localized in the middle retina, and occur in larger numbers around the optic nerve head and the nasal retina. High magnification reveals that each dot is composed of many smaller lesions arranged in clusters. Occasionally, the dots align beneath retinal vessels, and patchy venous sheathing is observable 6. In contrast, spots are localized to the deep retina and retinal pigment epithelium and extend to the midperipheral fundus. Dots and spots seem to be absent in the foveal area in MEWDS, despite the foveal granularity typically seen on clinical examination. During the acute phase of the disorder, new dots and spots appear in a cluster as older lesions disappear, with spots resolving before dots. The combination of dots and spots produces a pattern on indocyanine green angiography that appears to be pathognomonic for MEWDS.
The indocyanine green angiography imaging modality most clearly demonstrates the multifocal spots and a zonal hypocyanescence in the peripapillary area. Such findings correspond to enlargement of the blind spot on visual field testing.
Ultra-high resolution optical coherence tomography (OCT), capable of 3-μm axial resolution, has clearly identified pathologic disruptions of the ellipsoid zone (ellipsoid zone) in cases of MEWDS, without evidence of retinal pigment epithelium disturbances or photoreceptor cell body loss 11.
At the fovea, optical coherence tomography shows disruption of the ellipsoid zone and accumulations of hyperreflective material of variable size and shape. These accumulations vary from dome shaped deposits over the retinal pigment epithelium, to linear and vertical aggregations, and other irregular formations centered at the ellipsoid zone.
The accumulations of hyperreflective material resting on the retinal pigment epithelium extend inward through the interdigitation zone, ellipsoid zone, and outer nuclear layer towards the inner retina. Irregularities of the retinal pigment epithelium are also observed.
External to the fovea, optical coherence tomography imaging also reveals the presence of discontinuities or disruptions centered on the ellipsoid zone, including the interdigitation zone and, occasionally, the outer nuclear layer. The spots observed with fluorescein angiography and indocyanine green angiography correlate with these optical coherence tomography findings. Vertical extension of the ellipsoid abnormalities could be correlated to the dots on fluorescein angiography and indocyanine green angiography 12. Peripapillary subretinal fluid and pigment epithelial detachment can be seen on optical coherence tomography 13.
En face optical coherence tomography imaging demonstrates an unremarkable choroid and choriocapillaris and unaffected retinal pigment epithelium layer 11. The ellipsoid zone and interdigitation zone are disrupted in areas corresponding to spots as well as dots. The en face optical coherence tomography of the outer portion of the outer nuclear layer shows accumulations of hyperreflective material, which correspond to the dots visible on fluorescein angiography.
Enhanced depth imaging optical coherence tomography (EDI-OCT) measurements suggest a transient choroidal thickening; however, the changes are not statistically significant 12.
Short-wavelength fundus autofluorescence of the foveal area reveals hyporeflective dots and hyperreflective areas that correlate to spots observed on fluorescein angiography and indocyanine green angiography, as well as to areas of disruption of the ellipsoid zone on optical coherence tomography 12.
There is no evidence of choroidopathy; however, nonspecific choroidal thickening, common in many chorioretinal inflammatory diseases, is seen in some patients. In spite of some nonspecific secondary mottling and proliferation of the retinal pigment epithelium, there is no evidence of primary retinal pigment epithelium disease either.
The classic “wreath-like” retinal lesion best seen on fluorescein angiography may be due to middle or deep retinal capillary hyperfluorescence, perhaps from dilation of the retinal microcirculation by inflammation. Inflammatory mediators may extend from the outer and inner layers to the middle retina to affect the deep retinal capillaries. Another explanation for the early hyperfluorescence of these lesions on fluorescein angiography may relate to the excitation of microglia which have been implicated in immune mediated inflammatory processes of the retina. The stellate configuration of microglial cells in the retina resembles the wreath-like lesions seen on fluorescein angiography in MEWDS. Activation of microglia by inflammation, in conjunction with dilation of the deeper retinal circulation, may contribute to the presenting signs of MEWDS 14. Similar observations have been made in retinal microglia of transgenic mice expressing the green fluorescent protein. The green fluorescent protein from the jellyfish Aequorea victoria is the most widely used fluorescent reporter in biological research. Transgenic mice and zebra fish expressing green fluorescent protein in specific cell lineages or whole tissues have been extensively used for in vivo microscopy experiments over the past few decades 15.
In the adult retina, microglial cells are distributed primarily in the middle to inner retina: the plexiform layers, ganglion cell layer, and nerve fiber layer. They display highly motile protrusions in all directions, unaccompanied by soma migration. This suggests that the process dynamics may also serve to exchange signals between neighboring microglia, and may help to explain laminar retinal microglia distribution. Interestingly, in the adult retina, microglial cells have varying morphologies throughout the different layers. Activation of microglial cells plays a key role in the initiation and perpetuation of the retinal inflammatory response. Activated microglial cells can proliferate and migrate to the site of injury, where morphological alterations are usually accompanied by changes in signaling and gene expression 16.
In acute MEWDS, the ellipsoid zone is disrupted but is restored during recovery. No abnormalities are seen in the outer nuclear layer and the retinal pigment epithelium during follow-up. These optical coherence tomography findings suggest that there is disruption in outer segments and ellipsoid zone but photoreceptor cell bodies are intact, which may contribute to almost complete recovery of the photoreceptor outer segments. The reduced full field electroretinography (ffERG) and multifocal electroretinography (mfERG) amplitudes during the acute stage, which recover with restoration of the ellipsoid zone, support the presumption that the main lesion of MEWDS involves damage to the photoreceptor outer segments and the ellipsoid zone, but not the retinal pigment epithelium or choriocapillaris 11.
Protrusion of the hyperreflective material from the ellipsoid zone towards the outer nuclear layer corresponds to the location of dots visualized with photography, indocyanine green angiography, and fluorescein angiography. The presence of a plaque of outer retinal involvement from confluent spots appears to be pathognomonic for MEWDS, differentiating it from idiopathic multifocal choroiditis. MEWDS is predominantly a disease of the outer retina, centered on the ellipsoid zone, but also involving the interdigitation zone and the outer nuclear layer. Minor changes in the acute and healed stages also occur in the retinal pigment epithelium and choroid 12.
Testing
Fluorescein angiography
Fluorescein angiography reveals early punctate hyperfluorescence in a wreath-like pattern and late staining, in areas corresponding to the white dots. Retinal vascular sheathing and optic nerve staining may be seen in some patients with MEWDS. This early hyperfluorescence and late staining is in contrast to acute posterior multifocal placoid pigment epitheliopathy where early hypofluorescence is seen followed by late hyperfluorescence.
Fundus autofluorescence
Fundus autofluorescence is a quick and non-invasive method of establishing a diagnosis. Hyperautofluorescent dots correlate with the white dots seen on fundoscopy. Even in the absence of white dots on clinical examination, fundus autofluorescence can show characteristic hyperautofluorescent lesions. Recent reports suggest that fundus autofluorescence may be the most sensitive and practical ancillary test to detect MEWDS.
Optical coherence tomography
Discontinuities in inner segment-outer segment junction 17 and mild attenuation of external limiting membrane 17 have been reported in acute phase. Recurrent episodes may result in thinning of outer nuclear layer 18.
Visual field
An enlarged blind spot is typically seen. Other features include a central or paracentral scotoma. Acute Idiopathic Blind Spot Enlargement Syndrome may represent a form of the same disease process.
Indocyanine green angiography
Hypocyanescent spots, typically outnumbering the clinical lesions are seen.
Electroretinogram
Changes are reversible. A reduced a-wave amplitude may be seen and early receptor potential may be prolonged.
Electroculogram
A reversible decrease in Arden’s ratio may be noted.
Visual evoked responses may show a reversible decrease in amplitude and prolongation of latency.
MEWDS treatment
Since MEWDS is a self-limited disease, with almost all patients regaining good visual acuity within 3-9 weeks, no treatment is recommended. Photopsias and scotomata gradually resolve and the lesions will disappear and may be replaced by mild pigment mottling or chorioretinal scarring. MEWDS typically is a self-limited disease, however, patients with MEWDS may have persistent blind spot enlargement 9. While it is uncommon, 10% of patients may also experience a recurrence. That being said, the prognosis is quite good for these patients 9.
MEWDS prognosis
Though the onset of MEWDS is acute, the disease is typically self limiting and recurrences are rare. Visual prognosis is excellent.
- Jampol, Lee M. “Multiple Evanescent White Dot Syndrome: I. Clinical Findings.” Archives of Ophthalmology 102, no. 5 (May 1, 1984): 671. doi:10.1001/archopht.1984.01040030527008[↩]
- Sieving PA, Fishman GA, Jampol LM, Pugh D. Multiple evanescent white dot syndrome, II: Electrophysiology of the photoreceptors during retinal pigment epithelial disease. Arch Ophthalmol. 1984;102:675–679.[↩][↩]
- Tavallali A, Yannuzzi LA. MEWDS, Common Cold of the Retina. J Ophthalmic Vis Res. 2017;12(2):132–134. doi:10.4103/jovr.jovr_241_16 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5423364[↩][↩]
- Borruat FX, Herbort CP, Spertini F, Desarnaulds AB. HLA typing in patients with multiple evanescent white dot syndrome (MEWDS) Ocul Immunol Inflamm. 1998;6:39–41.[↩]
- Jampol LM, Sieving PA, Pugh D, Fishman GA, Gilbert H. Multiple evanescent white dot syndrome, I: Clinical findings. Arch Ophthalmol. 1984;102:671–674.[↩]
- Dell’Omo R, Pavesio CE. Multiple evanescent white dot syndrome (MEWDS) Int Ophthalmol Clin. 2012;52:221–228.[↩][↩]
- Gross NE, Yannuzzi LA, Freund KB, Spaide RF, Amato GP, Sigal R. Multiple evanescent white dot syndrome. Arch Ophthalmol. 2006;124:493–500.[↩][↩][↩]
- Pellegrini F, Interlandi E. A case of multiple evanescent white dot syndrome misdiagnosed as optic neuritis: Differential diagnosis for the neurologist. J Neurosci Rural Pract. 2016;7(2):283–285. doi:10.4103/0976-3147.178658 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4821940[↩][↩]
- Multiple Evanescent White Dot Syndrome. https://eyewiki.aao.org/Multiple_Evanescent_White_Dot_Syndrome[↩][↩][↩]
- Hua R, Chen K, Liu LM, Liu NN, Chen L, Teng WP. Multi-modality imaging on multiple evanescent white dot syndrome-A Spectralis Study. Int J Ophthalmol. 2012;5:644–647.[↩]
- De Bats F, Wolff B, Vasseur V, Affortit A, Kodjikian L, Sahel JA, et al. “En-face” spectral-domain optical coherence tomography findings in multiple evanescent white dot syndrome. J Ophthalmol. 2014;2014:928028[↩][↩][↩]
- Marsiglia M, Gallego-Pinazo R, Souza EC, Munk MR, Yu S, Mrejen S, et al. Expanded spectrum of multiple evanescent white dot syndrome with multimodal imaging. Retina. 2016;36:64–74.[↩][↩][↩][↩]
- Chao DL, Marsiglia M, Ahmad B, Sridhar J, Shah GK, de Souza EC, et al. Peripapillary serous detachment in multiple evanescent white dot syndrome. Retina. 2015;35:521–524.[↩]
- Wang M, Wang X, Zhao L, Ma W, Rodriguez IR, Fariss RN, et al. Macroglia-Microglia Interactions via TSPO Signaling Regulates Microglial Activation in the Mouse Retina. J Neurosci. 2014;34:3793–3806.[↩]
- Progatzky F, Dallman MJ, Lo Celso C. From seeing to believing: Labelling strategies for in vivo cell-tracking experiments. Interface Focus. 2013;3:20130001[↩]
- Madeira MH, Boia R, Santos PF, Ambrósio AF, Santiago AR. Contribution of microglia-mediated neuroinflammation to retinal degenerative diseases. Mediators Inflamm. 2015;2015:673090[↩]
- Lavigne, Luciana Castro, David Leonardo Cruvinel Isaac, José Osório Duarte Júnior, and Marcos Pereira de Avila. “Transient Spectral Domain Optical Coherence Tomography Findings in Classic MEWDS: A Case Report.” Arquivos Brasileiros De Oftalmologia 77, no. 3 (June 2014): 185–87.[↩][↩]
- Nguyen, My Hanh T., Andre J. Witkin, Elias Reichel, Tony H. Ko, James G. Fujimoto, Joel S. Schuman, and Jay S. Duker. “Microstructural Abnormalities in MEWDS Demonstrated by Ultrahigh Resolution Optical Coherence Tomography.” Retina (Philadelphia, Pa.) 27, no. 4 (May 2007): 414–18. doi:10.1097/01.iae.0000246676.88033.25[↩]