What is PFAS
PFAS is also known as Perfluoroalkyl and Polyfluoroalkyl substances or “forever chemicals“, are a group of man-made chemicals that includes perfluorooctanoic acid (PFOA), perfluorooctane sulfonic acid (PFOS), perfluorononanoic acid (PFNA), perfluoroalkyl acids (PFAAs), perfluoroalkane sulfonic acids (PFSAs), perfluorohexane sulfonic acid (PFHxS), perfluoroalkyl carboxylic acids (PFCAs), polytetrafluoroethylene (PTFE), also known as Teflon and many others (over 7800 PFAS chemical structures identified to date) 1, 2, 3, 4, 5, 6, 7, 8. All PFAS contain at least one perfluoroalkyl moiety (CnF2n+1–) 9. Chemically, individual PFAS can be very different. However, they all have a carbon-fluorine bond, which is very strong and therefore, they do not degrade easily. Fully fluorinated aliphatic carbon chains are known as perfluoroalkyl substances while those with incomplete replacement of hydrogen atoms by fluorine are referred to as polyfluoroalkyl substances.
PFAS have been manufactured and used in the aerospace, automotive, construction, and electronics industries around the world since the 1940s as chemicals that resist grease, oil, water, and heat 10, 11. PFAS have been widely used for their water resistant (hydrophobic) and oil repellent (oleophobic) properties in consumer products such as disposable food packaging, cookware, outdoor gear, furniture, and carpet 12, 13, 14, 11. Certain PFAS are also authorized by the US Food and Drug Agency (FDA) for limited use in cookware, food packaging, and food processing equipment 5. PFAS are also one of the main components (1–5% w/w) of aqueous film forming foams (fire-fighting foams) used frequently at airports and military bases in firefighting foam for firefighting and training activities 15, 16, 17, 18. Aqueous film forming foams (fire-fighting foams) contamination of groundwater is a major source of drinking water contamination and has been identified as a nationally significant challenge in countries such as the United States and Sweden 19, 20. Releases of PFAS to the environment can occur next to chemical manufacturing locations, at industrial sites where PFAS are used, and at various stages of product use and disposal. The carbon-fluorine bond in these compounds is extremely strong and thus many PFAS are not appreciably degraded under environmental conditions 21. This has resulted in their accumulation in the environment since the onset of production in the late 1940s 22. In 2015, the Centers for Disease Control and Prevention (CDC) National Health and Nutrition Examination Survey (NHANES) reported that PFAS were found in the blood of nearly all Americans sampled 23. US companies no longer manufacture the two best-known PFAS, perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) 24. But these legacy PFAS persist in the environment, even as thousands of others remain in production.
People can be exposed to PFAS in different ways, for example through food. Food can become contaminated through contaminated soil and water used to grow the food, through the concentration of these substances in animals via feed and water, through food packaging containing PFAS, or equipment that contained PFAS during food processing. Exposure to PFAS may lead to adverse health effects 25, 26, 27, 28.
Concerns about the public health impact of PFAS have arisen for the following reasons 29:
- Widespread occurrence. Studies find PFAS in the blood and urine of people, and scientists want to know if they cause health problems.
- Numerous exposures. PFAS are used in hundreds of products globally, with many opportunities for human exposure.
- Growing numbers. PFAS are a group of nearly 15,000 synthetic chemicals, according to a chemicals database maintained by the U.S. Environmental Protection Agency (https://comptox.epa.gov/dashboard/chemical-lists/PFASSTRUCT).
- Persistent. PFAS remain in the environment for an unknown amount of time.
- Bioaccumulation. People may encounter different PFAS chemicals in various ways. Over time, people may take in more of the chemicals than they excrete, a process that leads to bioaccumulation in bodies.
Because there are many types of PFAS chemicals, which often occur in complex mixtures and in various everyday products, researchers face challenges in studying them. More research is needed to fully understand all sources of exposure, and if and how they may cause health problems. The research conducted to date reveals possible links between human exposures to PFAS and adverse health outcomes. These health effects include 30, 31, 32, 33, 2:
- altered metabolism,
- fertility,
- reduced fetal growth and increased risk of being overweight or obese,
- increased risk of some cancers,
- reduced ability of the immune system to fight infections.
Potential of PFAS to cause a wide range of negative health impacts depends of various factors, such as the conditions of exposure (dose/concentration, duration, route of exposure, etc.) and characteristics associated with the exposed target (e.g., age, sex, ethnicity, health status, and genetic predisposition) 34. Endocrine disruptive effects of PFAS have been reported to affect fertility, body weight control, thyroid and mammary gland function 35. Developmental effects have been observed in children such as alterations in the behavior or accelerated puberty but also in the newborns such as decreased birthweight 35. Increased risk of kidney, prostate and testicular cancer has been associated with long-term exposure to PFAS in the general population alongside with disturbances in the cholesterol metabolism or reduced efficiency of the immune system against infections 35.
Studies have shown that some PFAS are toxic for both animals and humans 36, 6, 7, 37, 38, 39, 40, 41. Certain PFAS such as perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS), perfluorononanoic acid (PFNA) and perfluorohexane sulfonic acid (PFHxS), don’t break down in the environment or in the human body, and can accumulate over time. Furthermore, the most frequently detected PFAS, perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA), are highly mobile once introduced to the aquatic environment 42 and are not removed by conventional wastewater treatment 43, 44. PFAS therefore pose a severe threat to clean water supply worldwide 45.
In light of the past and current evidence of PFAS toxic effects, there is an increasing awareness and a general agreement that PFAS substances need to be regulated at multiple levels to minimize their adverse effects on human health and the environment. Manufacturers have phased out production of certain PFAS and in some cases replaced them with new PFAS or chemical substitutes 46. For example in textile treatments, many polymers containing long perfluoroalkyl side chains (more than seven perfluorinated carbons) were replaced by analogs containing short perfluoroalkyl side chains (six or four perfluorinated carbons) or fluorine-free moieties (e.g., siloxanes and hydrocarbon polymers) 47. Between 2000 to 2002, the main global manufacturer of PFAS (3M Company) voluntarily discontinued manufacturing of the parent chemical perfluorooctane sulfonyl fluoride (POSF) used to produce perfluorooctane sulfonic acid (PFOS) and its precursors perfluorooctane sulfonamide-based chemistry 48, 49. United States introduced a variety of programs to curb use of the most abundant environmental PFAS, including the PFOA Stewardship Program enacted in 2006 to end production of the longest chained compounds by 2015 11. Perfluorooctane sulfonic acid (PFOS) was added to the Stockholm Convention’s list of globally restricted Persistent Organic Pollutants (POPs) in 2009 11. A proliferation of new PFAS have been reported in the environmental literature as industry has rapidly replaced PFOS and PFOA with shorter chain length PFAS and new chemicals that are difficult to detect using standard methods 16. Emerging evidence from animal experiments suggests some of these alternative PFAS can be equally hazardous 50. Environmental health scientists thus face a considerable challenge in understanding the relative importance of diverse exposure pathways to PFAS in different human populations and their potential effects on human health in a rapidly changing chemical landscape 11.
Despite the many regulations to limit the PFAS spread and the phasing out the main PFAS substances (expressed in PFOS and PFOA), other compounds including PFAAs and related substances are still widely used in various industries including fire-fighting foams, photographic, semiconductor and others 51. Therefore, the detection of PFAS in human bodies have not decreased 51, 52, 53, 54.
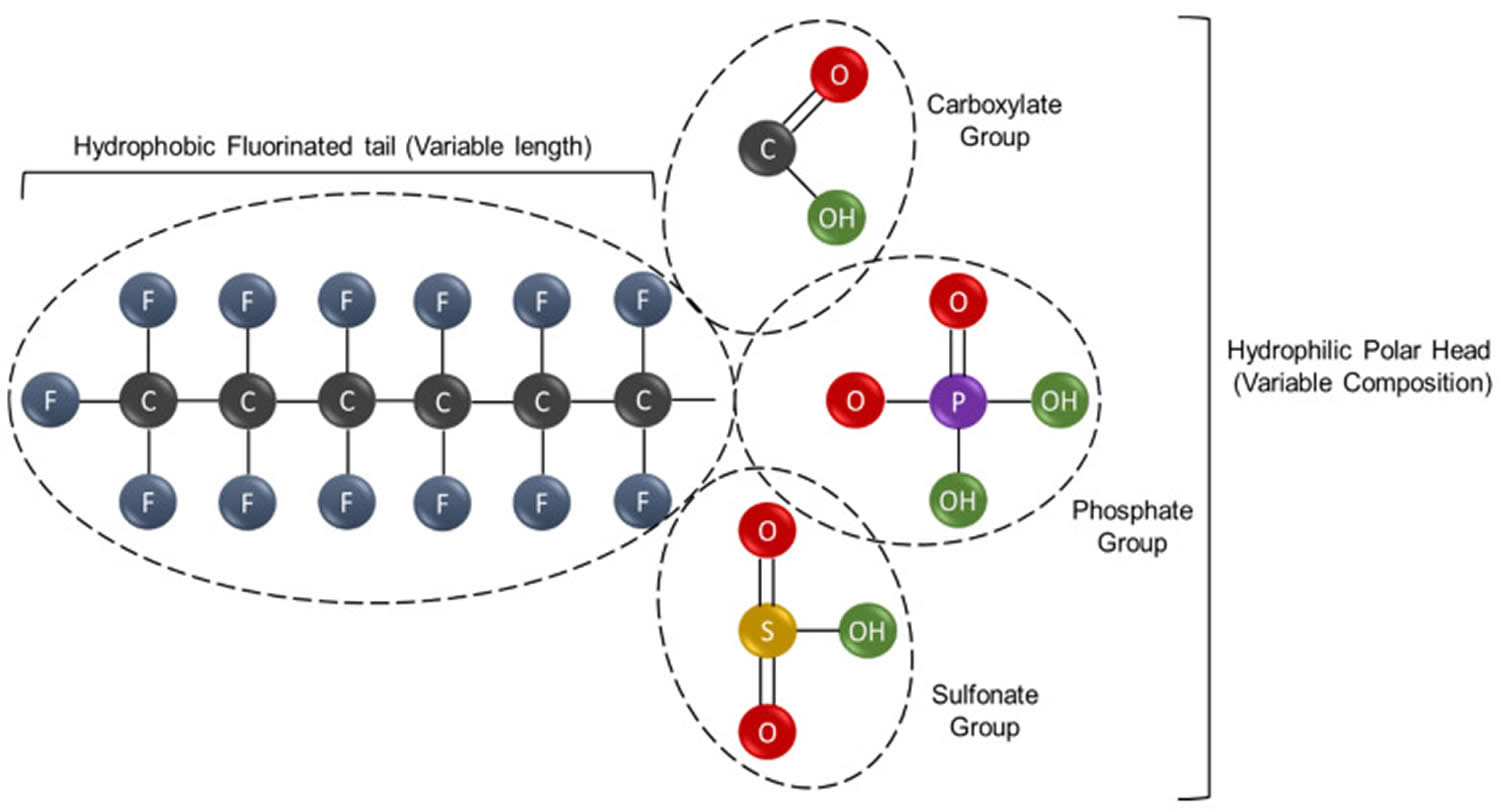
Figure 1. PFAS chemical structure
Footnote: A general structural formula for perfluoroalkyl substance (PFAS), containing a hydrophobic perfluorinated alkyl tail, and a hydrophilic functional (R) group outlined in a red box. The general structure of non-polymeric, perfluorinated PFAS substances. Non-polymeric PFAS are compounds of variable composition and physicochemical properties that however share two common features. These are represented by the hydrophobic tail, composed by a variable number of carbon atoms at different degree of fluorination, and the hydrophilic head, which contains polar groups. The specific combination of these chemical determinants, namely the carbon chain length, the type of functional groups and the number of fluoride atoms, generates an enormous number of different PFAS molecules with ample downstream applicability. Some of the most common polar groups, are shown.
[Source 35 ]Figure 2. PFAS classification
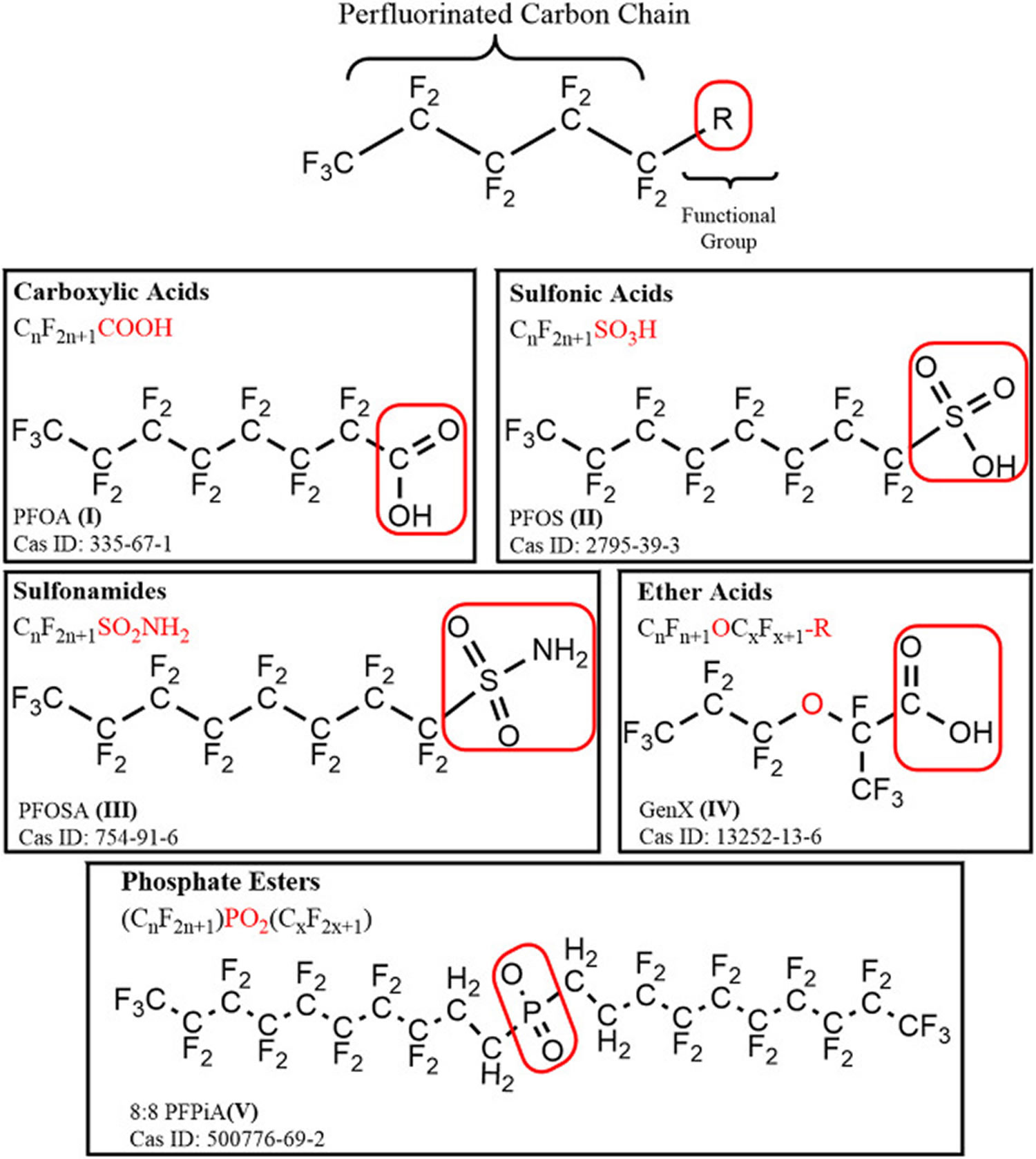
[Source 9 ]Figure 3. PFAS substances
Footnote: Examples of non-polymeric and polymeric PFAS substances. (A,B) Perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS), two well-known non-polymeric PFAS are shown. Both these compounds possess a relatively long tail containing eight fluorinated carbon atoms, but differ in the chemical composition of the polar head group, which is a carboxylic acid for PFOA and a sulfonic acid for PFOS. Under specific pH environmental conditions, these functional groups can dissociate into the respective anion forms (i.e., carboxylate and sulfonate). (C) Exemplary structure of Polytetrafluoroethylene (PTFE), also known as Teflon, a polymeric PFAS belonging to the subgroup of fluoropolymers. This type of compound is constituted by a moiety of (CF2-CF2)n atoms which is repeated up to thousands of times; (D) Exemplary structure of a lubrificant also known as Krytox, which is a polymeric PFAS belonging to the subgroup of perfluoropolyethers. In this case the (CF[CF3]−CF2−O)n moiety is repeated between 10–60 times.
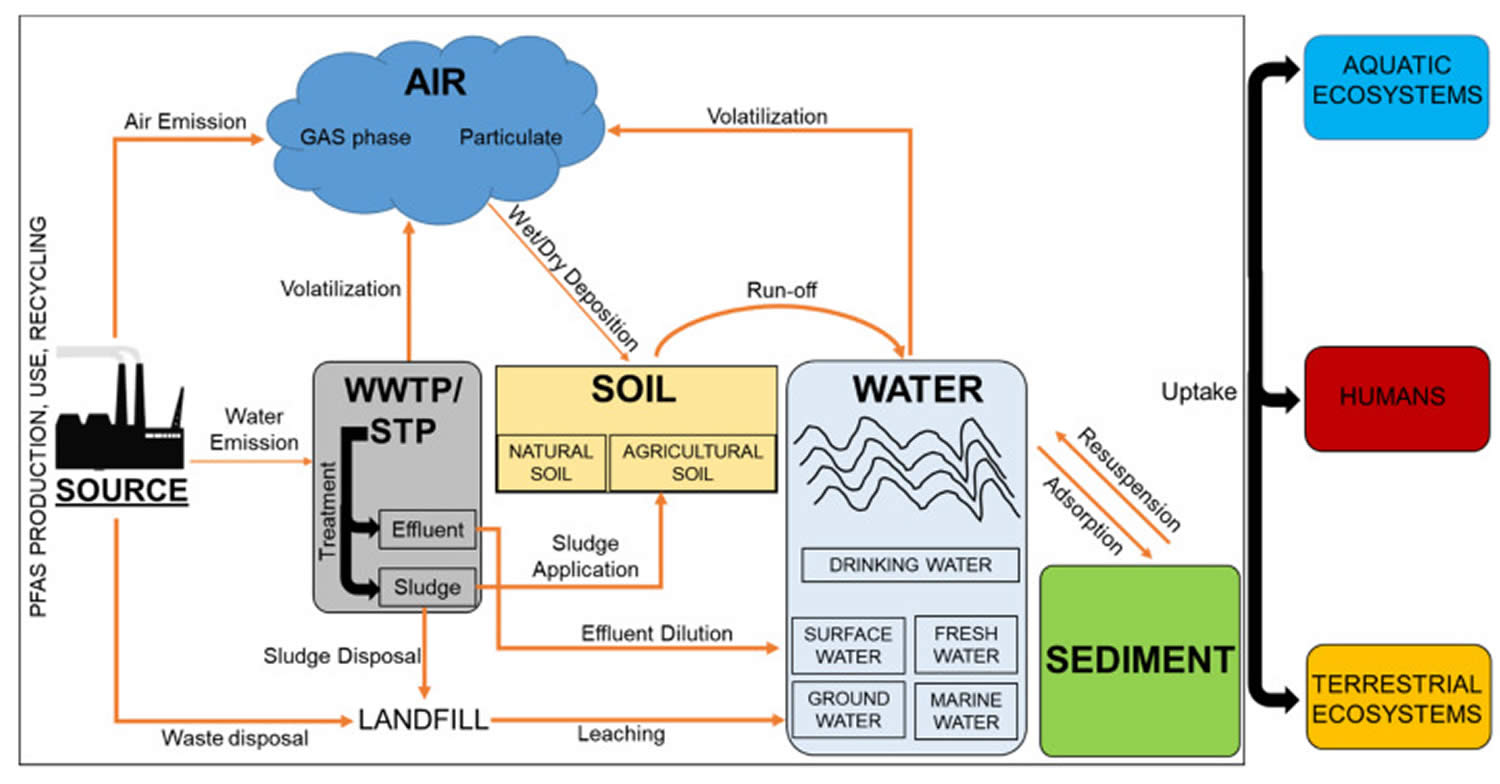
[Source 35 ]Figure 4. PFAS emission sources
Footnotes: Schematic illustration of the PFAS environmental distribution and exposure routes for humans and biota. The environmental distribution of PFAS substances involves multiple dispersion routes and exposure pathways that link the sources to the final receptors, represented by humans and wildlife. Industrial manufacture processes, industrial uses and recycling activities, represent primary sources of PFAS emissions. Other indirect sources are the landfilling or the application of contaminated sludge to agriculture land. Volatilization, deposition, leaching and run off processes regulate the redistribution of PFAS between air, soil, water and sediment compartments. Collectively, these pathways contribute to the short-term and long-term exposure of aquatic ecosystems, terrestrial ecosystems and humans to PFAS substances, that can also enter into the food chain through bioaccumulation and indirect human exposure via the ingestion of contaminated food sources.
[Source 35 ]Table 1. List of main PFAS categories and subgroups
| Non-Polymeric PFAS | |||
|---|---|---|---|
| Perfluorinated PFAS | Polyfluorinated PFAS | ||
| Subgroup | Examples | Subgroup | Examples |
| Perfluoroalkyl acids (PFAAs) Perfluoroalkane sulfonic acids & sulfonates (PFSAs) Perfluoroalkane sulfnic acids (PFSIAs) Perfluorocarboxylic acids & carboxylates (PFCAs) Perfluoroalkyl phosphonic acids (PFPAs) Perfluoroalkyl phosphinic acids (PFPIAs) | PFBS, PFHxS, PFOS PFOSI PFBA, PFHxA, PFOA C8-PFPA C8/C8-PFPiA | Fluorotelomer compounds (FT) | 6:2 FTO, 8:2 FTI |
| Perfluoroalkane sulfonamido compounds (Me/Et/Bu-FASAs) Miscellaneous | MeFOSA, FOSE 4,8-Dioxa-3H-perfluorononanoate | ||
| Perfluoroalkyl ether acids (PFEAs) | GenX, Adona, F-53B | ||
| Perfluoroalkane sulfonamides (FASA) | FOSA | ||
| Perfluoroalkane sulfonyl fluorides (PASFs) | PBSF, POSF | ||
| Perfluoroalkyl iodides (PFAIs) | PFHxI | ||
| Perfluoroalkanoyl fluorides (PAFs) | POF | ||
| Perfluoroalkyl aldehydes (PFALs) | PFNAL | ||
| Polymeric PFAS | |||
| Subgroup | Examples | ||
| Fluoropolymers | PVDF, FEP, PFA, ETFE, PTFE (Teflon) | ||
| Side-chain Fluorinated Polymers | Fluorinated urethane/acrylate/methacrylate/oxetane plolymers | ||
| Perfluoropolyethers (PFPEs) | PFPE-BP, Fluorolink-PFPE | ||
Table 2. List of PFAS extended chemical names and acronyms
| PFAS Chemical Name | PFAS Acronym | PFAS Chemical Name | PFAS Acronym |
|---|---|---|---|
| Perfluorooctanoic acid | PFOA | N-ethyl perfluorooctane sulfonamide | Et-FOSA |
| Perfluorooctane sulfonic acid | PFOS | N-methyl perfluorooctane sulfonamide | Me-FOSA |
| Perfluorooctanesulfonamide | PFOSA/FOSA | N-ethyl-perfluorooctane sulfonamido acetic acid | N-Et-FOSAA |
| Perfluorooctane sulfinic acid | PFOSI | N-methyl-perfluorooctane sulfonamido acetic acid | N-Me-FOSAA |
| Perfluorononanoic acid | PFNA | 2-(N-Methyl-perfluorooctane sulfonamido) acetic acid | Me-FOSAA/Me-PFOSA-AcOH) |
| Perfluorononanal | PFNAL | 2-(N-Ethyl-perfluorooctane sulfonamido) acetic acid | Et-FOSAA/Et-PFOSA-AcOH |
| Perfluorononane sulfonic acid | PFNS | perfluorooctane sulfonamido ethanol | FOSE |
| Perfluoroundecanoic acid | PFUnDA | N-ethyl perfluorooctane sulfonamido ethanol | Et-FOSE |
| Perfluoroundecanoate | PFUnA | perfluorohexane sulfonamide | FHxSA |
| Perfluorodecanoic acid | PFDA | bis(perfluorooctyl)phosphinic acid | C8/C8-PFPiA |
| Perfluorododecanoic acid | PFDoA | 6:2 Fluorotelomer olefin | 6:2 FTO |
| Perfluorodecane sulfonic acid | PFDS | 6:2,8:2,10:2 fluorotelomer alcohol | 6:2,8:2,10:2 FTOH |
| Perfluorobutanoic acid | PFBA | 6:2,8:2 fluorotelomer sulfonic acid | 6:2,8:2 FTSA |
| Perfluorobutane sulfonic acid | PFBS | 6:2 fluorotelomer thioether amido sulfonate | 6:2 FtTAoS |
| Perfluorophosphonic acid | PFPA | 8:2 fluorotelomer iodide | 8:2 FTI |
| Perfluoropentanoic acid | PFPeA | 8:2 fluorotelomer unsaturated carboxylic acid | 8:2 FTUCA |
| Perfluoropolyether-benzophenone | PFPE-BP | 9-chlorohexadecafluoro-3-oxanone-1-sulfonic acid | 6:2 Cl-PFESA (F-53B) |
| Perfluorohexanoic acid | PFHxA | 1-chloroeicosafluoro-3-oxaundecane-1-sulfonic acid | 8:2 Cl-PFESA |
| Perfluorohexyl iodide | PFHxI | Polyvinylidene fluoride | PVDF |
| Perfluorohexane sulfonic acid | PFHxS | Fluorinated ethylene propylene | FEP |
| Perfluoroheptanoic acid | PFHpA | Perfluoroalkoxy polymer | PFA |
| Perfluoroheptane sulfonic acid | PFHpS | Ethylene tetrafluoroethylene | ETFE |
| Perfluorotridecanoic acid | PFTrDA | Polytetrafluoroethylene | PTFE |
| Perfluorooctanoyl fluoride | POF | Hexafluoropropylene oxide dimer acid | HFPO-DA/GenX |
| Perfluorobutane sulfonyl fluoride | PBSF | 3H-perfluoro-3-[(3-methoxy-propoxy)] propanoic acid | ADONA |
| Perfluorooctane sulfonyl fluoride | POSF | Ammonium pentadecafluorooctanoate | APFO |
| Ammonium perfluoro(2-methyl-3-oxahexanoate) | PMOH |
PFAS Uses
The exceptional strength of the Carbon-Fluorine bond confers very high thermal and chemical stability to PFAS molecules while the presence of hydrophobic and hydrophilic properties along with the variability in the carbon chain length and chemical composition generates an enormous range of different molecules with useful physicochemical properties. For this reason, since 1940’s, PFAS substances have been frequently employed in a vast number of industrial or consumer’s products covering more than 100 sectors of use. Some of the most common PFAS applications include Teflon, pesticide formulation, firefighting foams, surfactants, lubricants, paints, waxes, cosmetics, aerospace, aviation, automotive, textiles coating, water and stain-repellent fabrics, oil production, medical products, food processing, building and construction, energy, paper and packaging, cables and wiring, electronic and semiconductors (see Table 3) 55, 56, 57.
Many PFASs are used as surfactants 9. Traditional surfactants comprise a water-soluble hydrophilic portion and a water-insoluble hydrophobic portion. Surfactants lower the surface tension of a liquid, or the interfacial tension between 2 liquids, or between a liquid and a solid. In fluorinated surfactants, the hydrophobic portion contains F bound to C, often as a perfluoroalkyl moiety. The extent of fluorination and location of the F atoms affect the surfactant properties. PFAS surfactants, often referred to as “fluorinated surfactants,” “fluorosurfactants,” “fluorinated tensides,” or “fluorotensides,” are superior in their aqueous surface tension reduction at very low concentrations and are useful as wetting and leveling agents, emulsifiers, foaming agents, or dispersants 13, 58, 14. The term “tenside” is encountered most frequently in publications of German origin, and the synonym “surfactant” is preferred in English.
Table 3. PFAS uses in industrial and consumers products
| SECTOR OF USE | TYPE OF USE |
|---|---|
| Non-Polymeric PFAS | |
| Fire prevention | Fire-fighting foams such as foams based on aqueous films (Acqueous Film-Forming Foams, AFFs) |
| Biocides | Active products in plants grow regulators (PGRs) Active or inert (emulsifiers, solvents, carriers, aerosol propellants) ingredients in pesticides |
| Electronic | Flame retardants |
| Aviation and Aerospace | Additives for hydraulic fluids |
| Metal plating | Humectants and anti-fog agents |
| Household Products | Surfactants in floor cleaning; treatment for textiles, leather, carpets; car waxes |
| Building and Construction | Additives in coatings and paints |
| Medical Products | Stain-resistant and water-repellent articles, X-ray film |
| Personal care products | Cosmetics, makeup, nail polish, shampoo |
| Metal plating | Wetting agent, anti-mist agents |
| Oil and mining production | Surfactants used in oil-well production and mining flotation |
| PFAS synthesis | Use as monomers for the synthesis of fluoropolymers with fluorinated side chain |
| Automotive | Treatment for external surfaces and internal leather coatings, textiles or carpets |
| Textiles and leather | Treatment aimed to create a coating with oil-water-stain-repellent properties |
| Semiconductors | Use in the production of semiconductor chips |
| Polymeric PFAS | |
| Fire prevention | Raw materials for firefighting equipment, protective clothes and fuel repellents |
| Electronic | Insulators and materials for welding |
| Aviation and Aerospace | Insulators, sleeves |
| Household Products | Non-stick coatings |
| Building and Construction | Coating of architectural materials, additives in paints, dyes, stains and sealants |
| Medical Products | Use in surgical patches, biocompatible human implants and medical prosthesis |
| Personal care products | Use in dental floss and lotions |
| Oil and mining production | Use in lining of gas pipes |
| Automotive | Mechanical components, seals and lubricants |
| Textiles, leather and clothing | Use in the manufacture of clothing and housewares as well as in coatings having oil-water-repellent properties |
| Semiconductors | Use as fluids in mechanical vacuum pumps |
| Energy | Film for solar panels |
| Paper and packaging | Use in water-oil-repellent materials, paperboard, and bags for food packaging |
| Cables and wiring | Coatings resistant to weathering, flame and soil |
| Food processing | Production of materials used for cooking (non-stick pans) and food storage (containers) |
How are humans exposed to PFAS?
The widespread use of PFAS and their persistence in the environment means that PFAS from past and current uses have resulted in increasing levels of contamination of the air, water, and soil. Over time, PFAS may leak into the soil, water, and air. Human exposure to PFAS is widespread and variable by geography and occupation. Because PFAS break down slowly, if at all, people and animals are repeatedly exposed to them, and blood levels of some PFAS can build up over time. People are most likely exposed to PFAS chemicals by consuming PFAS-contaminated water or food, using products made with PFAS, skin contact or breathing air and dust particulate containing PFAS 59. Despite the presence of some gaps in our understanding, it is generally accepted that the dietary intake and the consumption of drinking water represent major pathways for the general population 60, 61 while inhalation and dermal contact is far more relevant in case of occupational exposure 62, 63, 64. In total, 67 to 84 percent of exposure to perfluorooctanoic acid (PFOA) and 88–99% of exposure to perfluorooctyl sulfonic acid (PFOS) in humans come from food products 65. Furthermore, the home environment plays a crucial role in total exposure to PFASs. Home air was found to be responsible for 40% of exposure to PFOA in 25% of women who lived in environments with high dust concentrations 66.
PFAS food consumption rates vary by age, geography and culture but typical exposure factors are relatively well known 67. PFAS concentrations have been reported in milk, meat, vegetables, fruits, and bread in the sub- to low nanogram/g range, while the majority of food samples analyzed contained PFAS below detection limits 68, 69. In homogenized whole meals, a similar concentration range was reported, although the maximum concentration observed was 118 ng PFOA per gram of fresh food 70. Food can become contaminated through contaminated soil and water used to grow the food, through the concentration of these substances in animals via feed and water, through food packaging containing PFAS, or equipment that contained PFAS during food processing. The major exposure pathways for perfluorooctane sulfonic acid (PFOS) for the general population in Europe and North America are food and water ingestion, dust ingestion, and hand-to-mouth transfer from mill-treated carpets 71. For perfluorooctanoic acid (PFOA), major exposure pathways are oral exposure resulting from migration from paper packaging and wrapping into food, general food and water ingestion, inhalation from impregnated clothes, and dust ingestion 71. Exposure pathways for other PFAS such as perfluorohexane sulfonic acid (PFHxS) and perfluorononanoic acid (PFNA) are less extensively studied but expected to be similar to perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS). A study reported that PFAS can pass through the placenta to the fetus 72. A total of 123 pairs of samples of mothers’ blood and omphalic cord blood were obtained at the Oslo University Hospital. Maternal blood was collected at the 37th week of gestation for the detection of seven PFAS compounds. The level of PFAS measured in cord blood was 30–79% of that of the pregnant mother 72.
Since the 1960s, the US Food and Drug Agency (FDA) has authorized specific PFAS for use in specific food contact applications 73. Some PFAS are used in cookware, food packaging, and in food processing for their non-stick and grease, oil, and water-resistant properties. To ensure food contact substances are safe for their intended use, the FDA conducts a rigorous scientific review before they are authorized for the market 73.
PFAS that are authorized for use in contact with food generally fall into four application categories 73:
- Non-stick cookware: PFAS may be used as a coating to make cookware non-stick.
- Gaskets, O-Rings, and other parts used in food processing equipment: PFAS may be used as a resin in forming certain parts used in food processing equipment that require chemical and physical durability.
- Processing aids: PFAS may be used as processing aids for manufacturing other food contact polymers to reduce build-up on manufacturing equipment.
- Paper or paperboard food packaging: PFAS may be used as grease-proofing agents in fast-food wrappers, microwave popcorn bags, take-out paperboard containers, and pet food bags to prevent oil and grease from foods from leaking through the packaging.
The FDA reviews new scientific information on the authorized uses of food contact substances to ensure that these uses continue to be safe 73. When the FDA identifies potential safety concerns, the agency ensures that these concerns are addressed or that these substances are no longer used in food contact applications. The FDA can work with industry to reach voluntary market phase-out agreements for such food contact substances. The FDA can also revoke food contact authorizations when the agency determines that there is no longer a reasonable certainty of no harm from the authorized use of a food contact substance 73.
One report by the Centers for Disease Control and Prevention, using data from the National Health and Nutrition Examination Survey (NHANES), found PFAS in the blood of 97% of Americans 23. Another National Health and Nutrition Examination Survey (NHANES) report suggested blood levels of PFOS and PFOA in people have been reduced since those chemicals were removed from consumer products in the early 2000s. However, new PFAS chemicals have been created and exposure to them is difficult to assess. The US Food and Drug Agency (FDA) is undertaking a study of PFAS in food 74.
Dietary exposure to PFAS has primarily been estimated using the exposure factor approach by measuring PFAS concentrations in various foods and multiplying by food consumption rates for a given population or demographic group 46. A number of studies have estimated dietary exposure to PFAS using the exposure factor approach.
Several studies have used the epidemiologic approach to associate serum PFAS concentrations with different food sources. For example, one study found associations between serum concentrations of several PFAS and fish/seafood consumption in Norway 75. In a cohort of 941 American adults with blood sampled between 1996 and 1999, investigators reported positive associations of several PFAS in plasma with consumption of “meat/fish/shellfish (especially fried fish, and excluding omega-3 fatty acid rich fish), low-fiber and high-fat bread/cereal/rice/pasta, and coffee/tea,” but inverse associations with some other foods such as vegetables and fruit 76. Another study reported associations between serum perfluorooctanoic acid (PFOA) and perfluorononanoic acid (PFNA) and fast food consumption and take-out coffee in the USA using data from the US National Health and Nutrition Examination Survey (NHANES), suggesting a role for food contact materials 77. A different study also based on the US National Health and Nutrition Examination Survey (NHANES) data reported associations between serum PFAS concentrations and fast food restaurant meals as well as microwave popcorn 78. A small (n=61) but remarkable Norwegian study examined food consumption using several approaches but report few significant correlations with PFAS in blood 79. While diet is likely an important route of exposure for many people, it is difficult to estimate and thus uncertain 46. Statistically representative surveys of dietary exposure to PFAS are therefore needed as well as better data on the sources of PFAS found in food and links to those present in food contact materials 46.
Human exposures to perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) have been declining in western countries and Japan over the last decade due to these regulatory interventions because of their adverse effects on human health 80, 81, 82, 83, 84.
PFAS Health Effects
While the science surrounding potential health effects of PFAS is developing, exposure to some types of PFAS have been associated with serious health effects such as liver toxicity, reproductive disorders, neurotoxicity and immunotoxicity 85. PFAS are potentially capable of producing a wide range of adverse health effects depending on various factors, such as the conditions of exposure (dose/concentration, duration, route of exposure, etc.) and factors associated with the individuals exposed (e.g., age, sex, ethnicity, health status, and genetic predisposition) 34. Moreover, the mechanisms underlying toxic health effects attributed to PFAS in humans are not well understood 3. There is strong evidence that PFAS toxic effects that are observed in rodents include liver damage (hepatotoxicity), damage to the immune system (immunotoxicity) and developmental toxicity (e.g., increased fetal and/or neonatal mortality and reduction in fetal weight and/or postnatal growth), involve the activation of peroxisome proliferator-activated receptor-alpha (PPARα); however, humans and nonhuman primates are less responsive to peroxisome proliferator-activated receptor-alpha (PPARα) than rodents 86, 80. In addition, peroxisome proliferator-activated receptor-alpha (PPARα)-independent mechanisms are also involved, and it is not known if species differences exist for these mechanisms 4.
The available epidemiology studies suggest the following associations between PFAS exposure and several adverse health effects 6, 7, 37, 38, 39, 40, 41:
- Increased cholesterol levels (PFOA, PFOS, PFNA);
- Changes in liver enzymes (PFOA, PFOS, PFHxS);
- Decreased vaccine response in children (PFOA, PFOS, PFHxS);
- Increased risk of high blood pressure or preeclampsia in pregnant women (PFOA, PFOS); and
- Decreases in infant birth weights (<20 g (0.7 ounces) fall in birth weight per 1 ng/ml elevation in PFOA or PFOS in blood).
Selected human studies (published since 2010) investigating toxic effects of PFAS are presented in Table 4. Majority of the human studies explored the linkage between PFAS concentration and lipid status, mainly cholesterol level 87, 88, while a study was also conducted to assess the connection between PFAS and cholesterol at the gene expression level 89. Multiple studies find significant associations between PFAS exposure and adverse immune outcomes in children 11. Abnormally elevated cholesterol or fats in the blood (dyslipidemia) is the strongest metabolic outcome associated with PFAS exposure. Eriksen et al. 87 discovered substantial positive relationships between PFOS, PFAS, and total cholesterol in 753 individuals, while sex and prevalence of diabetes were suggested to influence the connection between these two substances and cholesterol. Fletcher et al. 89 observed an inverse relationship between serum PFOA levels and the expression level of genes involved in cholesterol transport in whole blood (NR1H2, NPC1 and ABCG1). A positive correlation was found between PFOS and a transcript involved in cholesterol mobilization (NCEH1), while a negative relationship was seen between PFOS and a transcript involved in cholesterol transport (NCEH2). Sex-specific effects were also noticed in Fletcher et al. study 89. On the other hand, in a study involving 815 participants ≤18 years of age, Geiger et al. 88 found that serum PFOA and PFOS were related with high total cholesterol and LDL-C levels, regardless of age, gender, race-ethnicity, body mass index, yearly family income, physical activity, or serum cotinine levels. PFOA and PFOS were not shown to be substantially linked with aberrant HDL-C and triglyceride levels.
Other studies explored the link between PFAS concentration and different hormones, such as thyroid 90, 91 and sex hormones 91, 92, 93, as well as development 94, 92. By assessing the connection between the levels of 14 PFAS in healthy men from the general population and different sex hormones and semen sample quality, Joensen et al. 95 found that only PFOS levels were negatively associated with testosterone, calculated free testosterone (FT), free androgen index (FAI) and ratios of T/LH, FAI/LH and FT/LH. Other PFAS were found at lower levels than PFOS and did not exhibit the same associations 95. Also, after measuring PFAS levels in 1682 males and females 12 to 80 years of age, Lewis et al. 90 found no significant relationships between any of the PFAS and testosterone. PFAS were suggested to be associated with increases in FT3, TT3, and FT4 among adult females. The authors concluded that, during the adolescence, PFAS may be related to increases in TSH among males and decreases in TSH among females, suggesting sex-specific effects 90. In contrast, Li et al. 91 discovered no associations between PFAS and thyroid hormones in adults and seniors in 3297 participants from Ronneby, a municipality with highly contaminated drinking water by PFAS (exposed group), with the exception of a positive association between PFAS and fT4 in males over 50. Thyroid hormone levels were observed to be higher in Ronneby preteen children compared to the control group. Weak evidence of a link between increasing PFAS levels and lower fT3 in preteen boys and lower TSH in adolescent men was found 91.
Lopez-Espinosa et al. 94 aimed to investigate whether PFOS and PFOA were linked to the markers of sexual development. Their study included 3076 boys and 2931 girls aged 8–18 years. For boys, there was a link between increased PFOS and a lower chance of reaching puberty. Higher PFOA or PFOS concentrations in girls were related to a lower risk of post menarche 94. The same group of researchers examined the link between PFAS levels and estradiol, total testosterone, and IGF-1 in 2292 children. In boys, PFOA concentrations were substantially related to testosterone levels; PFOS concentrations were related to estradiol, testosterone, and IGF-1, while PFNA concentrations were linked to IGF-1. Significant linkage was discovered in girls between PFOS and testosterone and IGF-1, as well as PFNA and IGF-1 96. Furthermore, Wang et al. 92 concluded that PFAS may affect estrogen homeostasis and foetal growth during pregnancy, and that estrogens may mediate the relationship between PFAS exposure and foetal growth after examining 424 mother-infant pairs.
Some of the studies also explored the linkage between the PFAS exposure and liver function 97, 98, 99. In 47,092 adult participants, Gallo et al. 100 found a positive association between PFOA and PFOS concentrations and serum ALT level. On the other hand, the relationship with bilirubin appeared to increase at low levels of PFOA and decrease at higher levels 100. In 1002 individuals from Sweden, Salihovic et al. 101 have also found a positive association of PFHpA, PFOA, PFNA, and PFOS concentrations and ALT activity, but also positive associations of PFHpA, PFOA, PFNA, PFDA, and PFUnDA with ALP. These authors noted that the changes of investigated PFAS concentrations were positively associated with gamma glutamyl transferase (GGT) levels and inversely associated with the changes in circulating bilirubin 101. On the other hand, in 2883 participants, (1801 non-obese and 1082 obese), Jain and Ducatman 102 investigated the connection between liver function alterations and various PFAS. They concluded that connections might only be observed in the obese participants: alanine aminotransferase (ALT) was positively associated with PFOA, PFHxS, and PFNA. On the other hand, PFOA and PFNA were associated with GGT 102. Epidemiological studies revealed a connection between PFAS and decrease in vaccination antibody production in early infants and children, especially having in mind that, if breastfed, they have a relatively high exposure and may be more susceptible as their immune system develops. Abraham et al. 93 found significant associations between the concentration of PFOA, but not PFOS, and adjusted levels of vaccine antibodies against Haemophilus influenza type b, tetanus and diphtheria for which no observed adverse effect concentrations (NOAECs) were 12.2, 16.9 and 16.2 µg/L, respectively. Furthermore, PFOA levels were shown to be inversely related to the interferon gamma (IFN-γ) production of ex-vivo lymphocytes after stimulation with tetanus and diphtheria toxoid 93. Furthermore, Budtz-Jorgensen E and Grandjean P 103 found an approximate BMDL of 1 ng/mL serum for both PFOS and PFOA for the serum concentrations of specific IgG antibodies against tetanus and diphtheria at ages 5 and 7 as outcome parameters. These authors proposed the reference concentration of about 0.1 ng/mL as the serum-based target, a level which is below the most reported human serum-PFAS concentrations 103. Grandjean et al. 104 discovered that prenatal exposure to PFAS had an inverse relationship with antibody concentrations five years later, while concentrations measured at 3 and 6 months of age had the highest inverse relationships with antibody concentrations at 5 years of age, especially for tetanus. The same authors have found that diphtheria antibody concentrations dropped at higher PFAS concentrations at 13 and 7 years after booster vaccinations at 5 years of age; the correlations were statistically significant for PFDA at 7 years and PFOA at 13 years, implying a 25% decrease for each doubling of exposure 98.
Evidence for cancer is limited to manufacturing locations with extremely high exposures and insufficient data are available to characterize impacts of PFAS exposures on neurodevelopment 11. The International Agency for Research on Cancer concluded that perfluorooctanoic acid (PFOA) is possibly carcinogenic to humans (Group 2B) and the United States Environmental Protection Agency indicated that there was suggestive evidence of cancer causing potential of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) in humans 105, 106, 107, 108. Increases in testicular and kidney cancer were noted in highly exposed humans 109, 110, 111.
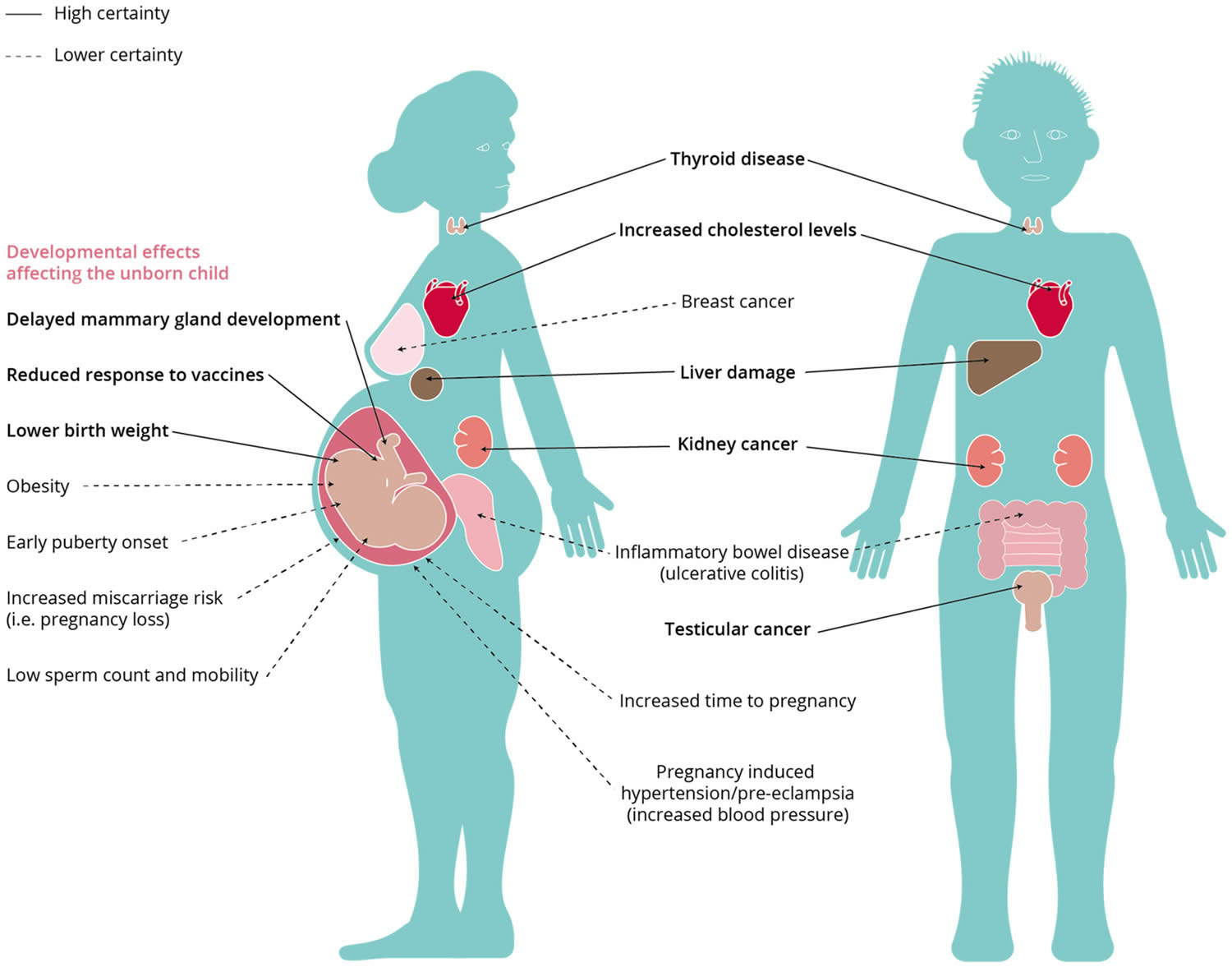
Figure 5. PFAS effects on human health
[Source 2 ]Table 4. Selected human studies exploring the toxicity of PFAS
| Substance | Population | Measured Parameters | Results | References |
|---|---|---|---|---|
| PFOS PFOA | middle-aged Danish population; 753 individuals (663 men and 90 women), 50–65 years of age, nested within a Danish cohort of 57,053 participants | serum levels of total cholesterol | Statistically significant positive associations between PFOS, PFAS and total cholesterol level Sex and prevalent diabetes modified the association between PFOA and PFOS and cholesterol | 87 |
| PFOS PFOA | 815 participants ≤18 years of age from the National Health and Nutrition Examination Survey 1999–2008 | dyslipidemia: total cholesterol >170 mg/dL, low-density lipoprotein cholesterol (LDL-C) >110 mg/dL, high-density lipoprotein cholesterol (HDL-C) <40 mg/dL or triglycerides >150 mg/dL. | Serum PFOA and PFOS-positively associated with high total cholesterol and LDL-C, independent of age, sex, race-ethnicity, body mass index, annual household income, physical activity and serum cotinine levels PFOA and PFOS-not significantly associated with abnormal HDL-C and triglyceride levels. | 88 |
| PFOS PFOA | 290 individuals (144 men + 146 women) exposed to background levels of PFOS and elevated concentrations of PFOA through drinking water, aged between 20 and 60 years | expression of genes involved in cholesterol metabolism | Inverse associations between serum PFOA levels and the whole blood expression level of genes involved in cholesterol transport (NR1H2, NPC1 and ABCG1) A positive association between PFOS and a transcript involved in cholesterol mobilisation (NCEH1), and a negative relationship with a transcript involved in cholesterol transport (NR1H3) Reductions in the levels of mRNAs involved in cholesterol transport were seen with PFOA in men (NPC1, ABCG1, and PPARA) and in women (NR1H2 expression) Increase in the levels of a cholesterol mobilisation transcript (NCEH1) in women. PFOS was positively associated with expression of genes involved in both cholesterol mobilisation and transport in women (NCEH1 and PPARA) | 89 |
| PFOA PFOS PFHxS PFNA PFDA | 2883 participants, (1801 non-obese and 1082 obese), aged more than or equal to 20 years old | liver function parameters: AST, ALT, GGT, ALP, and total bilirubin (TB) | Among obese participants only, alanine aminotransferase (ALT)-positively associated with PFOA, PFHxS, and PFNA PFOA and PFNA were associated with gamma GGT in obese participants | 102 |
| 14 PFCs | Healthy men from the general population, median age of 19 years | total testosterone (T), estradiol (E), sex hormone-binding globulin (SHBG), luteinizing hormone (LH), follicle-stimulating hormone (FSH) and inhibin-B and Semen samples analysis | PFOS levels-negatively associated with testosterone, calculated free testosterone (FT), free androgen index (FAI) and ratios of T/LH, FAI/LH and FT/LH Other PFCs were found at lower levels than PFOS and did not exhibit the same associations. PFC levels were not significantly associated with semen quality | 95 |
| PFOA PFOS PFHxS PFNA | 1682 males and females 12 to 80 years of age | testosterone (T), thyroid stimulating hormone (TSH), and free and total triiodothyronine (FT3, TT3) and thyroxine (FT4, TT4) | Exposure to PFAS may be associated with increases in FT3, TT3, and FT4 among adult females During adolescence, PFAS may be related to increases in TSH among males and decreases in TSH among females No significant relationships were observed between PFAS and T in any of the models | 90 |
| PFOS PFOA | 3076 boys and 2931 girls aged 8–18 years | subjects were classified as having reached puberty based on either hormone levels (total >50 ng/dL and free >5 pg/mL testosterone in boys and estradiol >20 pg/mL in girls) or onset of menarche | For boys, there was a relationship of reduced odds of reached puberty (raised testosterone) with increasing PFOS (delay of 190 days between the highest and lowest quartile) For girls, higher concentrations of PFOA or PFOS were associated with reduced odds of postmenarche (130 and 138 days of delay, respectively) | 94 |
| PFOS PFOA PFNA | 2292 children (6–9 years of age) | estradiol, total testosterone, and IGF-1 | In boys, PFOA concentrations were significantly associated with testosterone levels; PFOS with estradiol, testosterone, and IGF-1; and PFNA with IGF-1 In girls, significant associations were found between PFOS and testosterone and IGF-1; and PFNA and IGF-1 | 96 |
| PFOS PFOA | 424 mother-infant pairs | estrone (E1), b-estradiol (E2), and estriol (E3), infants: head circumference, body weight, body length | PFOS was positively related to E1 and E3, but negatively related to E2 Serum PFOA was positively related to serum E1 and negatively related to head circumference at birth Serum E2 was negatively related to head circumference, body weight, and body length at birth and serum E3 was positively related to body weight Serum E3 mediated the relationship between serum PFOS and body weight PFAS could affect estrogen homeostasis and fetal growth during pregnancy and estrogens might mediate the association between exposure to PFAS and fetal growth | 92 |
| PFOS PFOA | 47,092 adults | alanine transaminase (ALT), γ-glutamyltransferase (GGT), direct bilirubin | Positive association between PFOA and PFOS concentrations and serum ALT level, a marker of hepatocellular damage. The relationship with bilirubin appears to rise at low levels of PFOA and to fall again at higher levels. | 100 |
| PFHpA PFOA PFNA PFDA PFUnDA PFDoDA PFHxS PFOSA | 1002 individuals from Sweden (50% women) at ages 70, 75 and 80 | bilirubin and hepatic enzymes alanine aminotransferase (ALT), alkaline phosphatase (ALP), and γ-glutamyltransferase (GGT) | Positive associations of PFHpA, PFOA, PFNA, PFDA, and PFUnDA with ALP Concentrations of PFHpA, PFOA, PFNA, and PFOS were positively associated with the activity of ALT The changes in PFAS concentrations were positively associated with GGT and inversely associated with the changes in circulating bilirubin | 101 |
| PFOS PFOA PFHxS | 3297 participants from Ronneby, a municipality with drinking water highly contaminated by PFAS (exposed group) | thyroid hormone levels, with adjustments for age, sex and BMI | No associations between PFAS and thyroid hormones in adults and seniors except for a positive association between PFAS and fT4 in males over 50 Higher thyroid hormone levels in the preteen children from Ronneby compared to the reference group Weak evidence of associations between increased PFAS levels and decreased fT3 in preteen boys, and decreased TSH in teenage males | 112 |
| PFOA PFOS | 101 healthy 1-year-old children | Antibodies against haemophilus infuenza type b, tetanus and diphtheria, interferon gamma, cholesterol | Significant associations between PFOA, but not PFOS concentrations, and adjusted levels of vaccine antibodies against haemophilus influenza type b, tetanus and diphtheria PFOA levels inversely related to the interferon gamma (IFN) production of ex-vivo lymphocytes after stimulation with tetanus and diphtheria toxoid No infuence of PFOA and PFOS on infections and cholesterol level during the frst year of life | 93 |
| PFOA PFOS | 1146 children | serum concentrations of specific IgG antibodies against tetanus and diphtheria at ages 5 and 7 | Approximate BMDL of 1 ng/mL serum for both PFOS and PFOA for the serum concentrations of specific IgG antibodies against tetanus and diphtheria at ages 5 and 7 Proposed reference concentration of about 0.1 ng/mL as the serum-based target | 103 |
| PFHxS, PFOS, PFOA, PFDA, PFNA | 275 males and 349 females participated in clinical examinations and provided blood samples at ages 18 months and 5 years | serum concentrations of antibodies against tetanus and diphtheria vaccines determined at age 5 | Pre-natal exposure showed inverse associations with the antibody concentrations five years later, with decreases by up to about 20% for each two-fold higher exposure Associations for serum concentrations at 18 months and 5 years were weaker Concentrations estimated for ages 3 and 6 months showed the strongest inverse associations with antibody concentrations at age 5 years, particularly for tetanus Joint analyses showed statistically significant decreases in tetanus antibody concentrations by 19–29% at age 5 for each doubling of the PFAS exposure in early infancy | 104 |
| PFHxS, PFOA, PFOS, PFNA, PFDA. | 516 subjects | PFAS serum concentrations and concentration of antibodies against diphtheria and tetanus | Diphtheria antibody concentrations decreased at elevated PFAS concentrations at 13 y and 7 y; the associations were statistically significant for perfluorodecanoate (PFDA) at 7 y and for perfluorooctanoate (PFOA) at 13 y, both suggesting a decrease by ∼25% for each doubling of exposure Structural equation models showed that a doubling in PFAS exposure at 7 y was associated with losses in diphtheria antibody concentrations at 13 y of 10–30% for the five PFAS | 98 |
PFAS Cholesterol and Metabolic effects
Cross-sectional and longitudinal studies published between 1996–2020 based on human populations in Australia, Canada, China, several European countries, Japan, South Korea, Taiwan, UK and the U.S. found consistent evidence of modest positive associations between elevated serum PFAS concentrations and detrimental lipid profiles, such as elevated total cholesterol and low-density lipoprotein cholesterol (LDL-C), or reduced high-density lipoprotein cholesterol (HDL-C) in adults and children, although the magnitude of the cholesterol effect is inconsistent across different exposure levels 11, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131. Perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) exhibit the most consistent finding across studies. The effect size varies across studies, which can be a result of different exposure levels. Increases in serum PFOA and PFOS from the lowest to the highest quintiles among children in C8 health project was associated with 4.6 and 8.5 mg/dL total cholesterol (reference level for children is <170 mg/dL) 131. Among the National Health and Nutrition Examination Survey (NHANES) 2003–2004 participants, increases in serum PFOA and PFOS from the lowest to the highest quartiles were associated with 9.8 and 13.4 mg/dL total cholesterol (reference level for adults is <200 mg/dL) 114. Maisonet et al. 132 reported a non-linear relationship between prenatal PFOA concentrations and total cholesterol at ages 7 and 15 of the child.
Studies of large populations, featuring wide exposure ranges, demonstrate that serum lipids rapidly increase beginning at background PFAS (1–10 ng/mL) serum concentration and then are followed by attenuating (“plateaued”) cholesterol measurements as (log-transformed) exposures to long-chain PFAS increase 126, 113, 131. These findings suggest partially saturable mechanisms; thus, the cholesterol dose response at pharmacologic or acutely toxic doses should be viewed with caution; associations can be missed or may be misleading when an environmental range of exposure is absent. At background PFAS exposure levels, residual associations may be more detectable in obese participants 133, 134, a finding congruent with experimental PFAS outcomes in rodents fed “Western” or high-fat diets laced with PFAS 135, 136, 137. Human gene expression pathways provide support for an interaction of obesity and PFAS exposures and suggest possible sex differences 138. A pharmacokinetic model predicts that approximately half of the PFOS-exposed population would experience a >20% rise in serum cholesterol 139. Risk-assessment implications for low-PFAS dose increases in cholesterol have been noted 140, 126 and a review of population and toxicity data concluded that dyslipidemia is the strongest metabolic outcome of PFAS exposure 11.
Human PFAS lipid findings may be related to experimental findings of induced adipogenesis, impaired bile acid metabolism/synthesis, strongly decreased CYP7A1 enzyme activity, altered fatty acid transport, and intracellular lipid accumulation with steatosis, including in PPAR-α-null or PPAR-α-humanized animals 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151. Independent of PFAS exposure, similar alterations in metabolic pathways have been related to disrupted fatty acid beta-oxidation and increased free cholesterol in toxicology studies 152.
Emerging longitudinal and diabetes clinical trial data indicate that PFAS may increase human insulin resistance, associated with dysregulated lipogenesis activity 153, 127. Longitudinal studies of clinically diagnosed diabetes patients have sometimes associated PFAS exposures with diabetes 154 or with small changes in glycemic markers 155; however, diabetes associations to date are not consistent 156, 157, 155. Future studies should consider whether PFAS may instigate autoimmune diabetic outcomes in humans, as shown in experimental studies 158. Experimental data reveal that PFAS activate G protein-coupled receptor 40, a free fatty acid-regulated membrane receptor on islet ß cells, stimulating insulin secretion 159, 160.
There is some but much less consistent evidence of a modest positive correlation with metabolic diseases such as diabetes, overweight, obesity and heart diseases 11. The majority of studies are cross-sectional, which have limited causal interpretation 161. A few studies provided stronger evidence than observational studies, such as Diabetes Prevention Program Trial where at baseline, a doubling in plasma perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) concentrations was associated with small differences in markers of insulin secretion and β – cell function. However, there was limited evidence suggesting that PFAS concentrations are associated with diabetes incidence or changes in glycemic indicators during the follow-up period 155. And in the diet-induced weight-loss trial, higher baseline plasma PFAS concentrations were associated with a greater weight regain, especially in women 30.
An odds ratio (OR) represents the odds that an outcome will occur given a particular exposure, compared to the odds of the outcome occurring in the absence of that exposure 162:
- Odds Ratio (OR) = 1 Exposure does not affect odds of outcome
- Odds Ratio (OR) greater than 1 means Exposure is associated with higher odds of outcome
- Odds Ratio (OR) less than 1 means Exposure is associated with lower odds of outcome
Eighteen studies have examined the associations between PFAS exposures and glucose metabolism, insulin resistance and diabetes. Overall the results across different studies are inconclusive 11. Lin et al. 163 was the first to report a positive association between serum PFAS concentrations and glucose homeostasis among adults and adolescents in the National Health and Nutrition Examination Survey (NHANES). They reported a considerable effective size – doubling serum PFNA concentrations was associated with hyperglycemia odds ratio (OR) of 3.16 163. Later studies tend to report smaller effect sizes. Exposure during pregnancy may affect the mother and child during gestation and later in life. In a small pregnancy cohort in the U.S., each standard deviation of increase in PFOA was associated with a 1.87-fold increase of gestational diabetes risk 164. In a larger Spanish cohort 165, a null result was reported for PFOA, but PFOS, PFHxS and gestational diabetes had positive associations: Odds Ratio (OR) per log10-unit increase=1.99 and Odds Ratio (OR)=1.65, respectively.
Results for hypertension and other vascular diseases including stroke are also inconsistent 11. Two of the earliest studies examined the relationship between PFAS exposure and hypertension among NHANES and found different results for children and adults. Adjusted Odds Ratio (OR)=2.62 for hypertension comparing 80th vs. 20th percentiles serum PFOA among NHANES adults in the U.S. 166, while among children a null finding was reported 167. In some later cohort studies, null results and even protective effects associated with PFAS exposure and hypertension were reported 168, 169. A cross-sectional study on carotid artery intima-media thickness in adolescents reported increased risks with increase in plasma perfluorooctanesulfonic acid (PFOS) 170. However, a more recent study on artery stiffness found protective effects of PFOA and PFNA among children and adolescents enrolled in the World Trade Center Health Registry 124.
Other metabolic endpoints include thyroid disease (which could also be considered an endpoint for endocrine disruption), cardiovascular diseases, uric acid metabolism, and body weight. Except for uric acid metabolism, most results are inconclusive 11. An increase in hyperuricemia risks and PFOA exposure was observed in all four studies (two from NHANES and two from C8 Health Project) 11.
In summary, the strongest evidence for a relationship between PFAS exposure and metabolic outcome is in the area of dyslipidemia 11. Animal studies have found decreases in serum cholesterol levels associated with increased PFAS exposures, which contradicts epidemiological findings 11. The difference may lie in different levels of expression for nuclear receptors involved in the toxicological pathway, such as peroxisome proliferator-activated receptor (PPAR)-alpha. It may also be related to differences in exposure levels. Dietary factors can influence metabolic outcomes 171, introducing bias into observed relationships if not controlled for properly. Explanations for null findings include healthy worker effects and non-linear relationships, such as a decreasing slopes as exposure increases (log-linear relationships) 172.
PFAS Liver effects
The liver is a primary target organ for long-chain PFAS storage, and accompanying experimental evidence of toxicity includes hepatocyte fat infiltration, specific P450 (CYP) pathway induction, apoptosis, hepatocellular adenomas and carcinomas, and disrupted fatty acid trafficking that can be peroxisome proliferator-activated receptor alpha (PPARα)-dependent or -independent and present across species 173, 174, 175, 176, 177, 178, 143, 179.
Population studies demonstrate significant associations of long-chain PFAS (>6 fluorinated carbons) exposure to higher liver enzymes, such as alanine aminotransferase in adults and adolescents 180, 181, 182, 183, 184, 185, including in longitudinal studies 186, 187. Following low-dose exposures, these associations may be more evident in obese participants 172, 181, 188.
Based on experimental data, nonalcoholic fatty liver disease (NAFLD) has been investigated as a clinical outcome of PFAS exposure mediating consistent population PFAS-altered liver enzyme findings 189, 190, 191, 145. Studies with nonalcoholic fatty liver disease (NAFLD) cytokeratin C18 biomarkers have provided supportive evidence for PFAS inducing steatosis 192. Metabolomic studies have been directed at potentially explanatory human glycerophosphocholine and fatty acid profiles 193, 144, 194. Processes which favor steatosis promote advanced liver disease including liver cancer in humans 195. Associations of PFAS with advanced human liver disease and liver cancer are technically hard to study for reasons including (and not limited to) lethality, selection of comparison populations, and alterations of excretion mechanics associated with disease states. In a clinic-based study, mostly obese (85%) children aged 7 to 19 years with biopsy-proven NAFLD had more advanced disease associated with PFOS and PFHxS exposure as well as associations with lipid and amino acid pathways linked to NAFLD pathogenesis 196. However, an adult study reported that serum PFHxS was inversely associated with hepatic lobular inflammation in morbidly obese bariatric surgery patients 197. A study of heavily exposed workers (n = 462, geometric mean serum PFOA of 4048 ng/mL) detected significantly increased incident mortality for cirrhosis and liver cancer compared to a regional population 198, whereas no PFAS association to cancer or advanced liver disease was reported in a 3M worker cohort or in the C8 Health study population 199, 200, 201.
Emerging animal toxicology and histology and human population data provide mechanistic clues that PFAS disrupt hepatic metabolism, leading to increased bile acid reuptake and lipid accumulation in liver 202, 141. A review of NAFLD and toxicant exposure concluded that PFAS are associated with early steatosis (“fatty liver”), the preclinical stage of NAFLD 203.
PFAS Neurodevelopmental effects
In vitro studies suggest perfluorooctanesulfonic acid (PFOS) can trigger the “opening” of tight junction in brain endothelial cells and increase the permeability of the blood brain barrier 204. There has therefore been some interest in investigating the neurotoxic effects associated with PFAS exposures. In laboratory animals, it has been reported that PFOS, PFOA and PFHxS exposures during the peak time of rapid brain growth in mice resulted in an inability to habituate in unfamiliar environment 205. A few studies reported neurotoxicity of PFOS, PFHxS, and PFOA in cell culture systems 206, as well as altered behavioral responses 207 and deficits in learning and memory ability in rodents 208. In contrast, no significant developmental neurotoxic effects were seen from prenatal exposure to PFOS in US EPA guideline-based studies with rats 209.
Liew et al 210 reviewed 21 epidemiological studies in 2018 and concluded that evidence is mixed regarding neurodevelopmental effects of PFAS exposures. Health outcomes examined included developmental milestones in infancy, attention-deficit/hyperactivity disorder (ADHD) and behaviors in childhood, and neuropsychological functions such as IQ and other scales or scores 210. Neurodevelopmental trajectories are highly complicated and there is great heterogeneity in the instruments and methods to evaluate neurodevelopmental endpoints. Existing evidence suggests that PFAS can impact the nervous system, with particularly harmful effects from developmental exposures or exposures in sensitive populations 211. However, the limitations and inconsistencies in the current research make the severity of the neurotoxicological ramifications of PFAS exposure largely unknown 211. Additional research is needed to establish a link between neurodevelopmental outcomes and PFAS exposures.
PFAS Immune effects
Immunotoxicity of PFASs has been demonstrated in multiple animal models, including rodents, birds, reptiles and other mammalian and non-mammalian wildlife. Epidemiological data is relatively sparse but mounting evidence suggests that the immunotoxic effects in laboratory animal models occur at serum concentrations that are comparable to body burden of highly exposed humans and wildlife 212.
The health outcomes related to PFAS immunotoxicity include both molecular-level (i.e. antibody concentrations) and organ/system-level (i.e. infection of respiratory system). In general, more consistent results across different studies were reported for molecular-level health endpoints such as vaccine antibody or other immune markers such as immunoglobulin 11.
Five studies examined the association between PFAS exposure and suppression of antibody response to vaccination among children, adolescents or adults. Four out of the five found statistically significant associations between higher PFAS exposure and suppressed immune response 11. Grandjean et al. 213 was the first to link PFAS exposure in children to deficits in immune function. The authors reported a 2-fold increase in perfluorooctane sulfonate (PFOS) in child serum was associated with a −49% decline in tetanus and diphtheria antibody concentrations 213. Decreased immunological response persisted at age 13 years 214. This effect size is larger than later studies and can be attributed to different exposure levels, different vaccine strains, and different times elapsed since vaccination (peak antibodies vs residual antibodies). Adverse associations have also been found in PFAS exposure and other childhood vaccinations such as rubella, mumps, and Hemophilus influenza vaccinations in children 215, 216, 217 and adult influenza vaccination such as FluMist 218 and anti-H3N2 219. In a single study, modest down-regulation of C-reactive protein response, a marker of human systemic inflammation, was also reported to be associated with perfluorooctanoic acid (PFOA) blood levels 220.
Disease outcomes linked with immunosuppression such as clinician-recorded diagnoses of childhood infections have also been associated with prenatal exposures to PFOS and perfluorohexane sulfonate (PFHxS) 221. A pregnancy cohort study prospectively detected increased risk of airway and throat infections and diarrhea in children through age 10 yr, correlated with cord-blood PFAS measurements 222. A recent review concluded that exposure to PFAS in infancy and childhood resulted in an immunosuppressive effect characterized by an increased incidence of atopic dermatitis and lower respiratory tract infections 223. Some of the immunological effects were sex-specific, but the authors cautioned that there were inconsistencies across studies 223. Overall, available data provide strong evidence that PFAS exposure can suppress the human immune response.
Population studies of immune hyperreactive diseases have resulted in mixed findings. Studies on childhood allergy and asthma outcomes have shown no association with PFAS 224, 222, whereas others have found substantial effects, including provocative evidence that subgroups of individuals not adequately immunized may be at an increased risk for disease a priori 225, 226. For example, a case-control study of Taiwanese children compared the first and fourth quartiles of serum measurements for 11 PFAS with asthma and other immune markers and reported confidence intervals well above 1.0 for PFOA and others 225. However, review articles concerning PFAS and childhood allergy and asthma offer nuanced, age- and sex-specific interpretations and advise against firm conclusions 223.
Chronic autoimmune outcomes, including thyroid disease and inflammatory bowel disease (IBD), have also been considered. A study in contaminated communities (n = 32 254) detected an association between both prevalence and incidence of ulcerative colitis (UC) and PFOA exposure 227. A worker study (n = 3713) found a higher prevalence and incidence of ulcerative colitis (UC) with increasing log PFOA serum concentrations 169. A case-control study of children and young adults from a background exposure community in Atlanta, Georgia, USA, also found higher serum PFOA levels in patients with ulcerative colitis (UC) 228. In contrast to PFOA-related associations in US populations, a study of a contaminated community in Sweden (n = 63 074) did not show a consistent association of inflammatory bowel disease (IBD) with any PFAS exposure 229.
Recent, thorough reviews emphasize the following key concepts 230, 231, 232:
- There is concordance between animal studies and human epidemiological observations that PFAS modify the immune response, and
- There are noted complexities in assuming dose-response continuums, including possible differences in life-stage vulnerability.
- Authors of these reviews note uncertainty about which outcome will be of most importance but agree that immunotoxicity should be included among sensitive human PFAS toxicity endpoints.
PFAS and Cancer
Numerous studies have investigated PFAS carcinogenicity, mainly focusing on perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA). Perfluorohexanoic acid (PFHxA) is the only other PFAS that has been investigated in an animal study and null findings were reported 233. Human studies for PFOS and PFOA include chemical workers, communities with contaminated drinking water, and the general population. A 3.3-fold increase in prostate cancer mortality was reported for each month spent in the chemical division with PFOA production was observed among occupationally exposed workers, but the number of cases was small 234. Later data from this occupational cohort did not support an association between occupational exposure and cancer mortality or incidence 235. The strongest evidence for increased cancer risk has been reported by studies among community members whose drinking water was contaminated by PFOA. Barry et al 200 and Vieira et al 201 showed a positive association between PFOA levels and kidney and testicular cancers among participants in the C8 Health Project. These studies form the foundation of the overall conclusion from the C8 Health Project. Results among studies conducted in general population are inconsistent. Eriksen et al 236 was a the first to examine PFOA exposure and cancer in the general population and they did not find an association between plasma PFOA or PFOS concentration and prostate, bladder, pancreatic or liver cancer.
The International Agency for Research on Cancer (IARC) concluded that perfluorooctanoic acid (PFOA) is possibly carcinogenic to humans (Group 2B) and the United States Environmental Protection Agency indicated that there was suggestive evidence of cancer causing potential of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) in humans 105, 106, 107, 108. Increases in testicular and kidney cancer were noted in highly exposed humans 109, 110, 111.
PFAS in Kidney disease, uric acid, and kidney cancer
Extended human half-lives of long-chain PFAS are attributed to active renal tubular reabsorption. Legacy PFAS such as PFOA and PFOS are concentrated in renal tissues, and histopathologic, molecular, oxidative stress, and epigenetic studies provide evidence of potential nephrotoxicity 237, 238, 239, 240. In addition, the strong influence of kidney reabsorption on the extended half-lives of long-chain PFAS is consistent with both human protein binding and experimental PFAS excretion data.
Human studies have associated legacy PFAS exposure to diminished glomerular filtration and/or defined chronic kidney disease in adults and children 241, 242, 243, 244. However, this outcome may be due to reverse causation 245, 242. Some reviews of the available epidemiologic and toxicologic evidence suggest causative links between PFAS and diminished kidney function and chronic kidney disease 239, 246; these authors also note several knowledge gaps and uncertainty about which proposed mechanisms of action are most important. A propensity score approach to NHANES data 247, 248 and a study with repeated PFAS and health measures over an 18-yr period 244 recently concluded that PFAS exposure likely causes diminished renal glomerular filtration.
Uric acid, a biomarker of increased risk for renal disease 249, is also consistently associated with PFAS exposure in adults and children 250, 118, 183, 243, 251, 252, including a visible dose-response curve that begins at or near historic background levels in human populations 250, 252. Serum PFAS concentrations exhibit an inverted U-shaped pattern related to glomerular filtration, initially exhibiting a modest accumulation as glomerular filtration begins to decrease and then decreasing in advancing renal disease, likely due to failure of normal strong reabsorption mechanisms in moderate to severe kidney disease 247. This finding is more dramatic across stages of glomerular filtration when there is also albuminuria 253. Studies suggest that the association of PFAS to uric acid is not due to reverse causation and is underestimated because the failing kidney excretes long-chain PFAS but retains uric acid. An implication is that population outcomes that occur in the presence of either albuminuria or moderate to severe renal disease such as hypertension 254 increasing presence of and uric acid (a biomarker of renal disease) 255, 252 can be underestimated in cross-sectional studies; in other words, the link between these health outcomes and PFAS exposure is obscured in these studies because of enhanced PFAS excretion patterns in the presence of either albuminuria or moderate to severe kidney disease. Furthermore, the strong influence of renal reabsorption on the long half-lives of long chain PFAS is consistent with both human protein binding of PFAS and experimental PFAS excretion rates in high-dose rodent studies 256.
Kidney cancer diagnoses have been increasing since 1975, a finding that is partially independent of improved detection, with 5-year cancer-specific survival of approximately 80% 257. The C8 Health studies noted longitudinal (n = 32 254) increases of kidney cancer (hazard ratio = 1.10) and kidney cancer mortality 258, 200, 201. A review of 6 published studies found long-chain PFAS exposure associated with kidney cancer or kidney cancer mortality, with risks ranging from 1.07 to 12.8 239. Subsequent preliminary data from the heavily exposed Veneto, Italy, population also suggest a significant increase in kidney cancer mortality with PFAS exposure 259. Evidence is accumulating for PFAS as a cause of chronic disease and kidney cancer.
PFAS Thyroid effect
The C8 Science Panel concluded that there is a “probable link” of PFOA exposure to thyroid disease, with sex-specific outcomes in women (for hyperthyroid disease) versus men (hypothyroid disease) 260. Subsequent reviews drew attention to hypothyroid outcomes in women and children and to the possibility that populations with a priori circulating antithyroid peroxidase antibodies may be at additional risk 261. A broad childhood disease review noted “some evidence” that PFAS cause childhood hypothyroidism and characterized the number of studies as “limited” for childhood disease conclusions 262. A meta-analysis of 12 child and adult studies that excluded populations with higher exposures noted that PFAS exposure is negatively associated with serum total thyroxine levels and that “PFAS could induce thyroid dysfunction and disease” 263.
Human thyroid disease is mostly the result of an autoimmune response and is 5 to 10 times more prevalent in women than men 264. Concerning PFAS and clinically diagnosed outcomes, women in the highest quartile of PFOA exposure (>5.7 ng/mL) reported clinical hypothyroid disease (odds ratio 2.2) over 3 cycles of National Health and Nutrition Examination Survey (NHANES) data (1999–2006, n = 3974 adults), with similar findings in men 265. The C8 Science Panel studies (median serum PFOA 26.1 ng/mL) found thyroid disease hazard ratios of 1.00, 1.24, 1.27, 1.36, and 1.37 across cumulative exposure quintiles in women 266, with parallel hypothyroid findings in children aged 1 to 17 years 267. The Ronneby, Sweden, population experienced excess risk of thyroid disease in a discrete time period (1984–2005) among women (hazard ratio 1.29) that did not persist over time despite higher cumulative PFAS exposure 268. The authors did not link exposure to hypothyroid outcome, noting a nonmonotonic dose-response relationship 268.
Human population studies augment experimental data that PFAS interact with thyroid hormone binding proteins, one of several mechanisms by which PFAS can perturb feedback relationships between free thyroid hormone and the hypothalamic-pituitary-thyroid axis 269, 270, 271. Exposures to PFAS also interfere with thyroid peroxidase (TPO) enzyme activity in vitro 272. Several PFAS studies have pursued this putative mechanism, finding that maternal and neonatal thyroid hormone outcomes were more readily detected in those with a priori abnormally high circulating anti-TPO antibodies 273. One case-control study investigated congenital hypothyroidism, a rare condition 274. Serum concentrations of PFOA (5.40 vs 2.12 ng/mL), perfluorononanoic acid (PFNA; 1.93 vs 0.63 ng/mL), perfluorodecanoic acid (PFDA; 0.52 vs 0.30 ng/mL), and perfluoroundecanoic acid (0.98 vs 0.44 ng/mL) were higher in the newborns with congenital hypothyroidism; and levels of several PFAS, including PFOA and PFHxS, were correlated with thyroid autoantibodies 274.
Thyroid disease is not the only concern. Doctors are concerned about subclinically elevated thyroid-stimulating hormone (TSH) in early pregnancy because it may be associated with several possible adverse maternal and fetal outcomes 275. This general concern has prompted numerous PFAS-exposure evaluations of corresponding TSH in maternal serum, cord blood, and newborns. A review of maternal and child biomarkers with PFAS exposure noted that higher TSH has been reported in 4 second-trimester studies 276, but there are also conflicting findings. Studies measuring PFAS in the first trimester have also found associations between PFAS exposure and altered TSH levels in newborns, including nonmonotonic patterns of dose response that mirror the marked alterations of thyroid hormone levels during pregnancy 277.
From the available studies, PFAS definitively alter human thyroid hormones and potentially contribute to thyroid auto-immunity but do not so far appear to be a cause of thyroid cancer 200, 201. Also, thyroid cancer is usually survived; thus, morbidity rather than mortality studies are useful.
PFAS Reproductive and Developmental effects
Exposure to PFOA impairs human sperm motility and sperm penetration into viscous media 278, 279 and is longitudinally associated with lower sperm concentration and count and higher adjusted levels of luteinizing and follicle-stimulating hormones in young men 280, 281, 282. Serum concentrations of PFAS are also cross-sectionally associated with deleterious markers of semen quality 283, 284.
Legacy and emerging PFAS have been found in follicular fluid 285. They appear to alter endometrial regulation such as progesterone activity in young women 286 and possibly menstrual cycle length 287. Associations with menarche and menopause may be substantially due to reverse causation because menstruation is a route by which women eliminate PFAS 245, partially explaining why men have higher PFAS levels than women in the same communities. Women on birth control and who do not menstruate or with poor cyclicity because of age, activity level, or disease may have elevated PFAS levels in comparison with menstruating women. Exposure to PFAS has been associated with endometriosis in the United States and in China 288, 289, but the specific PFAS associated with this effect vary among studies.
Time-to-pregnancy (fecundity) studies provide indirect evidence of changes in fertility. Methodologic considerations include maternal and paternal age, parity (which in turn affects serum PFAS), and health status. Among 1240 women in the Danish National Birth Cohort, PFOS exposure was associated with decreased fecundity (median serum PFOS 35.5 ng/mL) 290. Reverse causation may explain this finding because it is duplicated in parous, but not among nonparous, women 291, 292. Prospective odds of actual infertility in the Maternal-Infant Research on Environmental Chemicals cohort (n = 1743) at low-dose exposures were associated with PFOA (geometric mean 1.66 ng/mL; odds ratio = 1.31) and PFHxS (odds ratio = 1.27) 293. The reported fertility rate improved following water filtration in a PFAS-contaminated community along with measures of birth weight 294.
Per- and polyfluoroalkyl substances (PFAS) reliably move across the placenta and enter breast milk 295, 296; serum PFAS levels in young children generally exceed maternal serum concentrations 297, 298, 299. Population studies provide evidence that breastfeeding duration and milk quantity are adversely affected by PFAS exposure 300, 301, 302.
A systematic review reported that PFOA exposure was associated with a small decrease in infant birthweight; the meta-analysis estimated that a 1-ng/mL increase in PFOA was associated with an approximately 19-g reduction in birth weight 303. The authors noted similarities in experimental studies 304, 305 and concluded that there was “sufficient” human and corroborative toxicology evidence of a detrimental effect of PFOA on birth weight 304, 305, 303. However, another meta-subpopulation analysis, focused on early pregnancy or the time shortly before conception, detected only a small and nonsignificant association, which was less subject to bias 306. Different approaches to the possible confounding role of shifting glomerular filtration rates in pregnancy can affect interpretations; evidence suggests this consideration can, at most, only partially explain associations of PFAS exposure to decreased birth weight 307, 308. A recent review of mostly prospective cohort studies (n = 24 studies) noted PFAS associated with altered fetal and postnatal growth measures, such as lower birth weight. Many (n = 22) of the relevant studies suggest developmental and childhood immunomodulatory effects, whereas 21 studies concerning neurodevelopment were inconclusive 210. The authors of the review noted methodologic challenges of developmental and newborn epidemiology, including consideration of critical exposure windows for developmental effects, the effects of breastfeeding and parity on maternal PFAS levels, and the variety of possible mechanistic explanations for growth outcomes, such as disruption of glucocorticoid and thyroid hormone metabolism in utero 210. Recent Faroe Island studies report that prenatal PFAS effects on thyroid hormone status do not support a causal relationship 309.
Review articles suggest that prenatal exposure to PFOA may increase risk of subsequent childhood adiposity, noting that steroid hormones, retinoid X receptor, and other pathways may be contributing to this effect 310, 311. Prospective evidence supports this relationship in adults with a high risk of diabetes 155. However, some well-performed community studies do not support this outcome in adults or children 312, 313.
Based on several preliminary findings, supported by longitudinal follow-up studies 314, 315, 316, 317, 318, the C8 Science Panel concluded that PFOA is probably linked to pregnancy-induced hypertension or preeclampsia. Population-level evidence implicating additional PFAS having pregnancy-induced hypertension (preeclampsia) has included studies with longitudinal designs 319, 320, 321. Experimental support includes PFAS effects on human trophoblast migration in vitro 322 and recent evidence of PFOA and GenX (or hexafluoropropylene oxide dimer acid) effects on mouse placenta, as well as excessive gestational weight gain 323. However, a recent longitudinal study did not find an association of PFAS with pregnancy-associated hypertension (preeclampsia) 324.
The possibility that circulating PFAS may reduce bone mineral density (BMD) has been investigated. Cross-sectional and practical trial associations have been found in adults 325, 326, 327 and there is emerging longitudinal evidence from a mother and child pair study indicating that children may also be affected 328.
Testicular cancer diagnoses are increasing steadily, a trend unrelated to improved detection 329, 330. Most patients diagnosed (>90%) will be cured and die of other causes; mortality studies therefore provide little help in understanding disease risk factors. The C8 Science Panel detected longitudinal evidence for increased testicular cancer risk (1.35, 95% CI 1.00–1.79) for cumulative PFOA exposure 200. There are ample supportive data of testicular damage following PFAS exposure, including strong evidence of endocrine disruption; but the cell-specific associations are different in humans (germ cell) than the outcomes in rodents (stromal) 2.
Per- and polyfluoroalkyl substances have deleterious effects on conception, pregnancy, and infant development 2. The underlying birth weight data are mostly supportive, although the subsequent growth and adiposity literature is mixed 2. The most sensitive reproductive and developmental outcomes are a topic of ongoing discussion.
- US EPA. 2020a. PFAS Master List of PFAS Substances. https://comptox.epa.gov/dashboard/chemical-lists/pfasmaster[
]
- Fenton SE, Ducatman A, Boobis A, DeWitt JC, Lau C, Ng C, Smith JS, Roberts SM. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ Toxicol Chem. 2021 Mar;40(3):606-630. doi: 10.1002/etc.4890[
][
][
][
][
][
]
- Mumtaz MM, Buser MC, Pohl HR. Per- and polyfluoroalkyl mixtures toxicity assessment “Proof-of-Concept” illustration for the hazard index approach. J Toxicol Environ Health A. 2021 Jul 3;84(13):553-567. doi: 10.1080/15287394.2021.1901251[
][
]
- Agency for Toxic Substances and Disease Registry (ATSDR). 2021. Toxicological profile for Perfluoroalkyls. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. https://www.atsdr.cdc.gov/ToxProfiles/tp200.pdf[
][
]
- Per- and Polyfluoroalkyl Substances (PFAS). https://www.fda.gov/food/environmental-contaminants-food/and-polyfluoroalkyl-substances-pfas[
][
]
- Mi X, Yang Y, Zeeshan M, Wang Z, Zeng X, Zhou Y, Yang A, Hu L, Yu H, Zeng X, et al. 2020. Serum levels of per- and polyfluoroalkyl substances alternatives and blood pressure by sex status: Isomers of C8 health project in China. Chemosphere 261:1–8. doi: 10.1016/j.chemosphere.2020.127691[
][
][
]
- NTP. 2016. NTP monograph on immunotoxicity associated with exposure to perfluorooctanoic acid (PFOA) or perfluorooctane sulfonate (PFOS). U.S. Department of Health and Human Services, National Institutes of Health, National Toxicology Program. https://ntp.niehs.nih.gov/sites/default/files/ntp/ohat/pfoa_pfos/pfoa_pfosmonograph_508.pdf[
][
][
]
- OECD (The Organisation for Economic Co-operation and Development). Toward a New Comprehensive Global Database of Per- and Polyfluoroalkyl Substances (PFASs): Summary Report on Updating the OECD 2007 List of Per and Polyfluoroalkyl Substances (PFASs)., 2018.[
]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SP. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag. 2011 Oct;7(4):513-41. doi: 10.1002/ieam.258[
][
][
]
- Jian JM, Chen D, Han FJ, Guo Y, Zeng L, Lu X, Wang F. A short review on human exposure to and tissue distribution of per- and polyfluoroalkyl substances (PFASs). Sci Total Environ. 2018 Sep 15;636:1058-1069. doi: 10.1016/j.scitotenv.2018.04.380[
]
- Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol. 2019 Mar;29(2):131-147. doi: 10.1038/s41370-018-0094-1[
][
][
][
][
][
][
][
][
][
][
][
][
][
][
][
][
][
]
- Johns K, Stead G. 2000. Fluoroproducts: the extremophiles. Journal of Fluorine Chemistry 104:5–18.[
]
- Kissa E. Fluorinated surfactants: Synthesis–Properties–Applications (Surfactant science series 50) New York (NY): Marcel Dekker; 1994. p. 469.[
][
]
- Kissa E. Fluorinated surfactants and repellents (2nd edition revised and expanded) (Surfactant science series 97) New York (NY): Marcel Dekker; 2001. p. 640.[
][
]
- Vecitis CD, Wang Y, Cheng J, Park H, Mader BT, Hoffmann MR. Sonochemical degradation of perfluorooctanesulfonate in aqueous film-forming foams. Environ Sci Technol. 2010 Jan 1;44(1):432-8. doi: 10.1021/es902444r[
]
- Wang Z, DeWitt JC, Higgins CP, Cousins IT. A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)? Environ Sci Technol. 2017 Mar 7;51(5):2508-2518. doi: 10.1021/acs.est.6b04806[
][
]
- Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH. Sources, fate and transport of perfluorocarboxylates. Environmental Science and Technology. 2006;40:32–44. doi: 10.1021/es0512475[
]
- Wang Z, Cousins IT, Scheringer M, Buck RC, Hungerbühler K. Global emission inventories for C4–C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, part I: Production and emissions from quantifiable sources. Environment International. 2014;70:62–75. doi: 10.1016/j.envint.2014.04.013[
]
- Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, Lohmann R, Carignan CC, Blum A, Balan SA, Higgins CP, Sunderland EM. Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environ Sci Technol Lett. 2016 Oct 11;3(10):344-350. doi: 10.1021/acs.estlett.6b00260[
]
- Banzhaf S, Filipovic M, Lewis J, Sparrenbom CJ, Barthel R. A review of contamination of surface-, ground-, and drinking water in Sweden by perfluoroalkyl and polyfluoroalkyl substances (PFASs). Ambio. 2017 Apr;46(3):335-346. doi: 10.1007/s13280-016-0848-8[
]
- Wang Z, Cousins IT, Scheringer M, Hungerbuehler K. Hazard assessment of fluorinated alternatives to long-chain perfluoroalkyl acids (PFAAs) and their precursors: status quo, ongoing challenges and possible solutions. Environ Int. 2015 Feb;75:172-9. doi: 10.1016/j.envint.2014.11.013[
]
- Armitage J, Cousins IT, Buck RC, Prevedouros K, Russell MH, MacLeod M, Korzeniowski SH. Modeling global-scale fate and transport of perfluorooctanoate emitted from direct sources. Environ Sci Technol. 2006 Nov 15;40(22):6969-75. doi: 10.1021/es0614870[
]
- Lewis RC, Johns LE, Meeker JD. Serum Biomarkers of Exposure to Perfluoroalkyl Substances in Relation to Serum Testosterone and Measures of Thyroid Function among Adults and Adolescents from NHANES 2011-2012. Int J Environ Res Public Health. 2015 May 29;12(6):6098-114. doi: 10.3390/ijerph120606098[
][
]
- Beans C. News Feature: How “forever chemicals” might impair the immune system. Proc Natl Acad Sci U S A. 2021 Apr 13;118(15):e2105018118. doi: 10.1073/pnas.2105018118[
]
- Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999-2008. Environ Sci Technol. 2011 Oct 1;45(19):8037-45. doi: 10.1021/es1043613[
]
- Khalil N, Chen A, Lee M, Czerwinski SA, Ebert JR, DeWitt JC, Kannan K. Association of Perfluoroalkyl Substances, Bone Mineral Density, and Osteoporosis in the U.S. Population in NHANES 2009-2010. Environ Health Perspect. 2016 Jan;124(1):81-7. doi: 10.1289/ehp.1307909[
]
- Stubleski J, Salihovic S, Lind L, Lind PM, van Bavel B, Kärrman A. Changes in serum levels of perfluoroalkyl substances during a 10-year follow-up period in a large population-based cohort. Environ Int. 2016 Oct;95:86-92. doi: 10.1016/j.envint.2016.08.002[
]
- Jian JM, Chen D, Han FJ, Guo Y, Zeng L, Lu X, Wang F. A short review on human exposure to and tissue distribution of per- and polyfluoroalkyl substances (PFASs). Sci Total Environ. 2018 Sep 15;636:1058-1069. https://doi.org/10.1016/j.scitotenv.2018.04.380[
]
- Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS). https://www.niehs.nih.gov/health/topics/agents/pfc/index.cfm[
]
- Liu G, Dhana K, Furtado JD, Rood J, Zong G, Liang L, Qi L, Bray GA, DeJonge L, Coull B, Grandjean P, Sun Q. Perfluoroalkyl substances and changes in body weight and resting metabolic rate in response to weight-loss diets: A prospective study. PLoS Med. 2018 Feb 13;15(2):e1002502. doi: 10.1371/journal.pmed.1002502[
][
]
- Bach CC, Vested A, Jørgensen KT, Bonde JP, Henriksen TB, Toft G. Perfluoroalkyl and polyfluoroalkyl substances and measures of human fertility: a systematic review. Crit Rev Toxicol. 2016 Oct;46(9):735-55. doi: 10.1080/10408444.2016.1182117[
]
- Braun JM. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol. 2017 Mar;13(3):161-173. doi: 10.1038/nrendo.2016.186[
]
- Kielsen K, Shamim Z, Ryder LP, Nielsen F, Grandjean P, Budtz-Jørgensen E, Heilmann C. Antibody response to booster vaccination with tetanus and diphtheria in adults exposed to perfluorinated alkylates. J Immunotoxicol. 2016;13(2):270-3. doi: 10.3109/1547691X.2015.1067259[
]
- Fenton S.E., Ducatman A., Boobis A., DeWitt J.C., Lau C., Ng C., Smith J.S., Roberts S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2021;40:606–630. doi: 10.1002/etc.4890[
][
]
- Panieri E, Baralic K, Djukic-Cosic D, Buha Djordjevic A, Saso L. PFAS Molecules: A Major Concern for the Human Health and the Environment. Toxics. 2022 Jan 18;10(2):44. doi: 10.3390/toxics10020044[
][
][
][
][
][
][
][
][
][
]
- Borg D, Lund BO, Lindquist NG, Håkansson H. Cumulative health risk assessment of 17 perfluoroalkylated and polyfluoroalkylated substances (PFASs) in the Swedish population. Environment International. 2013;59:112–123. doi: 10.1016/j.envint.2013.05.009[
]
- Winquist A, and Steenland K. 2014. Modeled PFOA exposure and coronary artery disease, hypertension, and high cholesterol in community and worker cohorts. Environ. Health Perspect 122 (12):1299–305. doi: 10.1289/ehp.1307943[
][
]
- Grandjean P, Andersen EW, Budtz-Jørgensen E, Nielsen F, Mølbak K, Weihe P, and Heilmann C. 2012. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. J. Am. Med. Assoc 307 (4):391–97. doi: 10.1001/jama.2011.2034[
][
]
- Grandjean P, Heilmann C, Weihe P, Nielsen F, Mogensen UB, and Budtz-Jørgensen E. 2017. Serum vaccine antibody concentrations in adolescents exposed to perfluorinated comopounds. Environ. Health Perspect 125 (1–7):077018. doi: 10.1289/EHP275[
][
]
- Lenters V, Portengen L, Rignell-Hydbom A, Jönsson BA, Lindh CH, Piersma AH, Toft G, Bonde JP, Heederik D, Rylander L, & Vermeulen R (2016). Prenatal Phthalate, Perfluoroalkyl Acid, and Organochlorine Exposures and Term Birth Weight in Three Birth Cohorts: Multi-Pollutant Models Based on Elastic Net Regression. Environ. Health Perspect 124(3), 365–372. 10.1289/ehp.1408933[
][
]
- Olsen GW, Burris JM, Burlew MM & Mandel JH 2000. Plasma cholecystokinin and hepatic enzymes, cholesterol and lipoproteins in ammonium perfluorooctanoate production workers. Drag Chem Toxicol. 23(4):603–20. doi: 10.1081/DCT-100101973[
][
]
- Fujii S, Polprasert C, Tanaka S, Lien NPH, Qiu Y. New POPs in the water environment: Distribution, bioaccumulation and treatment of perfluorinated compounds—A review paper. Journal of Water Supply: Research and Technology—AQUA. 2007;56:313–326. doi: 10.2166/aqua.2007.005[
]
- Arvaniti OS, Stasinakis AS. Review on the occurrence, fate and removal of perfluorinated compounds during wastewater treatment. Science of the Total Environment. 2015;524–525:81–92. doi: 10.1016/j.scitotenv.2015.04.023[
]
- Filipovic M, Berger U. Are perfluoroalkyl acids in waste water treatment plant effluents the result of primary emissions from the technosphere or of environmental recirculation? Chemosphere. 2015;129:74–80. doi: 10.1016/j.chemosphere.2014.07.082[
]
- Yan H, Cousins IT, Zhang C, Zhou Q. Perfluoroalkyl acids in municipal landfill leachates from China: Occurrence, fate during leachate treatment and potential impact on groundwater. Science of the Total Environment. 2015;524–525:23–31. doi: 10.1016/j.scitotenv.2015.03.111[
]
- De Silva AO, Armitage JM, Bruton TA, Dassuncao C, Heiger-Bernays W, Hu XC, Kärrman A, Kelly B, Ng C, Robuck A, Sun M, Webster TF, Sunderland EM. PFAS Exposure Pathways for Humans and Wildlife: A Synthesis of Current Knowledge and Key Gaps in Understanding. Environ Toxicol Chem. 2021 Mar;40(3):631-657. doi: 10.1002/etc.4935[
][
][
][
]
- Schellenberger S, Hill PJ, Levenstam O, Gillgardd P, Cousins IT, Taylor M, Blackburn RS. 2019a. Highly fluorinated chemicals in functional textiles can be replaced by re-evaluating liquid repellency and end-user requirements. Journal of Cleaner Production 217:134–143.[
]
- 3M Company. 1999. Fluorochemical Use, Distribution and Release Overview. US EPA Public Docket AR226-0550, 3M Company: St Paul, MN.[
]
- Land M, Wit CAd, Cousins IT, Herzke D, Johansson J, Martin JW What is the effect of phasing out long-chain per- and polyfluoroalkyl substances on the concentrations of perfluoroalkyl acids and their precursors in the environment? A systematic review protocol. Environ Evidence 2015; 4: 3.[
]
- Gomis MI, Vestergren R, Borg D, Cousins IT. Comparing the toxic potency in vivo of long-chain perfluoroalkyl acids and fluorinated alternatives. Environ Int. 2018 Apr;113:1-9. doi: 10.1016/j.envint.2018.01.011[
]
- Wang T, Wang P, Meng J, Liu S, Lu Y, Khim JS, Giesy JP. A review of sources, multimedia distribution and health risks of perfluoroalkyl acids (PFAAs) in China. Chemosphere. 2015 Jun;129:87-99. doi: 10.1016/j.chemosphere.2014.09.021[
][
]
- Sznajder-katarzy, K.; Surma, M.; Cie, I. A Review of Perfluoroalkyl Acids (PFAAs) in terms of Sources, Applications, Human Exposure, Dietary Intake, Toxicity, Legal Regulation, and Methods of Determination. J. Chem. 2019.[
]
- Uebelacker, L.A. A Review of the Pathways of Human Exposure to Poly- and Perfluoroalkyl Substances (PFASs) and Present Understanding of Health Effects Elsie. Physiol. Behav. 2017, 176, 139–148.[
]
- Lindstrom, A.B.; Strynar, M.J.; Libelo, E.L. Polyfluorinated compounds: Past, present, and future. Environ. Sci. Technol. 2011, 45, 7954–7961.[
]
- Gluge J., Scheringer M., Cousins I.T., DeWitt J.C., Goldenman G., Herzke D., Lohmann R., Ng C.A., Trier X., Wang Z. An overview of the uses of per- and polyfluoroalkyl substances (PFAS) Environ. Sci. Process. Impacts. 2020;22:2345–2373. doi: 10.1039/D0EM00291G[
]
- Bach C., Dauchy X., Boiteux V., Colin A., Hemard J., Sagres V., Rosin C., Munoz J.F. The impact of two fluoropolymer manufacturing facilities on downstream contamination of a river and drinking water resources with per- and polyfluoroalkyl substances. Environ. Sci. Pollut. Res. Int. 2017;24:4916–4925. doi: 10.1007/s11356-016-8243-3[
]
- Adamson D.T., Nickerson A., Kulkarni P.R., Higgins C.P., Popovic J., Field J., Rodowa A., Newell C., DeBlanc P., Kornuc J.J. Mass-Based, Field-Scale Demonstration of PFAS Retention within AFFF-Associated Source Areas. Environ. Sci. Technol. 2020;54:15768–15777. doi: 10.1021/acs.est.0c04472[
]
- Taylor CK. Fluorinated surfactants in practice. In: Karsa D, editor. Design and selection of performance surfactants: Annual surfactants review. New York (NY): John Wiley & Sons; 1999. pp. 271–316.[
]
- De Silva A.O., Armitage J.M., Bruton T.A., Dassuncao C., Heiger-Bernays W., Hu X.C., Karrman A., Kelly B., Ng C., Robuck A., et al. PFAS Exposure Pathways for Humans and Wildlife: A Synthesis of Current Knowledge and Key Gaps in Understanding. Environ. Toxicol. Chem. 2021;40:631–657. doi: 10.1002/etc.4935[
]
- Post G.B., Cohn P.D., Cooper K.R. Perfluorooctanoic acid (PFOA), an emerging drinking water contaminant: A critical review of recent literature. Environ. Res. 2012;116:93–117. doi: 10.1016/j.envres.2012.03.007[
]
- Augustsson A., Lennqvist T., Osbeck C.M.G., Tibblin P., Glynn A., Nguyen M.A., Westberg E., Vestergren R. Consumption of freshwater fish: A variable but significant risk factor for PFOS exposure. Environ. Res. 2021;192:110284. doi: 10.1016/j.envres.2020.110284[
]
- Kaiser M.A., Dawson B.J., Barton C.A., Botelho M.A. Understanding potential exposure sources of perfluorinated carboxylic acids in the workplace. Ann. Occup. Hyg. 2010;54:915–922. doi: 10.1093/annhyg/meq066[
]
- Nilsson H., Karrman A., Rotander A., van Bavel B., Lindstrom G., Westberg H. Professional ski waxers’ exposure to PFAS and aerosol concentrations in gas phase and different particle size fractions. Environ. Sci. Process Impacts. 2013;15:814–822. doi: 10.1039/c3em30739e[
]
- Franko J., Meade B.J., Frasch H.F., Barbero A.M., Anderson S.E. Dermal penetration potential of perfluorooctanoic acid (PFOA) in human and mouse skin. J. Toxicol. Environ. Health A. 2012;75:50–62. doi: 10.1080/15287394.2011.615108[
]
- Anastasiadis X, Matsas A, Panoskaltsis T, Bakas P, Papadimitriou DT, Christopoulos P. Impact of Chemicals on the Age of Menarche: A Literature Review. Children (Basel). 2023 Jul 17;10(7):1234. doi: 10.3390/children10071234[
]
- Haug L.S., Huber S., Becher G., Thomsen C. Characterisation of human exposure pathways to perfluorinated compounds—Comparing exposure estimates with biomarkers of exposure. Environ. Int. 2011;37:687–693. doi: 10.1016/j.envint.2011.01.011[
]
- US EPA. 2011. Exposure Factors Handbook: 2011 Edition. US EPA Office of Research and Development, Washington DC. EPA/600/R-090/052F. 1466 pp.[
]
- Ericson I, Gómez M, Nadal M, van Bavel B, Lindström G, Domingo JL. 2007. Perfluorinated chemicals in blood of residents in Catalonia (Spain) in relation to age and gender: A pilot study. Environment International 33:616–623. DOI: 10.1016/j.envint.2007.01.003[
]
- Tittlemier SA, Pepper K, Seymour C, Moisey J, Bronson R, Cao X-L, Dabeka RW. 2007. Dietary Exposure of Canadians to Perfluorinated Carboxylates and Perfluorooctane Sulfonate via Consumption of Meat, Fish, Fast Foods, and Food Items Prepared in Their Packaging. Journal of Agricultural and Food Chemistry 55:3203–3210. DOI: 10.1021/jf0634045[
]
- Fromme H, Schlummer M, Möller A, Gruber L, Wolz G, Ungewiss J, Böhmer S, Dekant W, Mayer R, Liebl B, Twardella D. 2007. Exposure of an Adult Population to Perfluorinated Substances Using Duplicate Diet Portions and Biomonitoring Data. Environmental Science & Technology 41:7928–7933. DOI: 10.1021/es071244n[
]
- Trudel D, Horowitz L, Wormuth M, Scheringer M, Cousins IT, and Hungerbühler K. 2008. Estimating consumer exposure to PFOS and PFOA. Risk Anal. 28 (2):251–69. doi: 10.1111/j.1539-6924.2008.01017.x[
][
]
- Gützkow K.B., Haug L.S., Thomsen C., Sabaredzovic A., Becher G., Brunborg G. Placental transfer of perfluorinated compounds is selective—A Norwegian Mother and Child sub-cohort study. Int. J. Hyg. Environ. Health. 2012;215:216–219. doi: 10.1016/j.ijheh.2011.08.011[
][
]
- Authorized Uses of PFAS in Food Contact Applications. https://www.fda.gov/food/process-contaminants-food/authorized-uses-pfas-food-contact-applications[
][
][
][
][
]
- FDA Makes Available Testing Method for PFAS in Foods and Final Results from Recent Surveys. https://www.fda.gov/food/cfsan-constituent-updates/fda-makes-available-testing-method-pfas-foods-and-final-results-recent-surveys[
]
- Haug LS, Thomsen C, Brantsaeter AL, Kvalem HE, Haugen M, Becher G, Alexander J, Meltzer HM, Knutsen HK. 2010. Diet and particularly seafood are major sources of perfluorinated compounds in humans. Environment International 36:772–778. DOI: 10.1016/j.envint.2010.05.016[
]
- Lin PID, Cardenas A, Hauser R, Gold DR, Kleinman KP, Hivert MF, Fleisch AF, Calafat AM, Sanchez-Guerra M, Osorio-Yanez C, Webster TF, Horton ES, Oken E. 2020. Dietary characteristics associated with plasma concentrations of per- and polyfluoroalkyl substances among adults with pre-diabetes: Cross-sectional results from the Diabetes Prevention Program Trial. Environment International 137:10. DOI: 10.1016/j.envint.2019.105217[
]
- Nelson JW, Hatch EE, Webster TF. 2010. Exposure to Polyfluoroalkyl Chemicals and Cholesterol, Body Weight, and Insulin Resistance in the General US Population. Environmental Health Perspectives 118:197–202. DOI: 10.1289/ehp.0901165[
]
- Susmann HP, Schaider LA, Rodgers KM, Rudel R. 2019. Dietary Habits Related to Food Packaging and Population Exposure to PFASs. Environmental Health Perspectives 127:10. DOI: 10.1289/ehp4092[
]
- Poothong S, Papadopoulou E, Padilla-Sanchez JA, Thomsen C, Haug LS. 2020. Multiple pathways of human exposure to poly- and perfluoroalkyl substances (PFASs): From external exposure to human blood. Environment International 134:9. DOI: 10.1016/j.envint.2019.105244[
]
- EFSA Panel on Contaminants in the Food Chain (EFSA CONTAM Panel); Schrenk D, Bignami M, Bodin L, Chipman JK, Del Mazo J, Grasl-Kraupp B, Hogstrand C, Hoogenboom LR, Leblanc JC, Nebbia CS, Nielsen E, Ntzani E, Petersen A, Sand S, Vleminckx C, Wallace H, Barregård L, Ceccatelli S, Cravedi JP, Halldorsson TI, Haug LS, Johansson N, Knutsen HK, Rose M, Roudot AC, Van Loveren H, Vollmer G, Mackay K, Riolo F, Schwerdtle T. Risk to human health related to the presence of perfluoroalkyl substances in food. EFSA J. 2020 Sep 17;18(9):e06223. doi: 10.2903/j.efsa.2020.6223[
][
]
- Gomis MI, Vestergren R, MacLeod M, Mueller JF, Cousins IT. Historical human exposure to perfluoroalkyl acids in the United States and Australia reconstructed from biomonitoring data using population-based pharmacokinetic modelling. Environ Int. 2017 Nov;108:92-102. doi: 10.1016/j.envint.2017.08.002[
]
- Okada E, Kashino I, Matsuura H, Sasaki S, Miyashita C, Yamamoto J, Ikeno T, Ito YM, Matsumura T, Tamakoshi A, Kishi R. Temporal trends of perfluoroalkyl acids in plasma samples of pregnant women in Hokkaido, Japan, 2003-2011. Environ Int. 2013 Oct;60:89-96. doi: 10.1016/j.envint.2013.07.013[
]
- Nøst TH, Vestergren R, Berg V, Nieboer E, Odland JØ, Sandanger TM. Repeated measurements of per- and polyfluoroalkyl substances (PFASs) from 1979 to 2007 in males from Northern Norway: assessing time trends, compound correlations and relations to age/birth cohort. Environ Int. 2014 Jun;67:43-53. doi: 10.1016/j.envint.2014.02.011[
]
- Ritscher A, Wang Z, Scheringer M, Boucher JM, Ahrens L, Berger U, Bintein S, Bopp SK, Borg D, Buser AM, Cousins I, DeWitt J, Fletcher T, Green C, Herzke D, Higgins C, Huang J, Hung H, Knepper T, Lau CS, Leinala E, Lindstrom AB, Liu J, Miller M, Ohno K, Perkola N, Shi Y, Småstuen Haug L, Trier X, Valsecchi S, van der Jagt K, Vierke L. Zürich Statement on Future Actions on Per- and Polyfluoroalkyl Substances (PFASs). Environ Health Perspect. 2018 Aug;126(8):84502. doi: 10.1289/EHP4158[
]
- Mokra K. Endocrine Disruptor Potential of Short- and Long-Chain Perfluoroalkyl Substances (PFASs)-A Synthesis of Current Knowledge with Proposal of Molecular Mechanism. Int J Mol Sci. 2021 Feb 21;22(4):2148. doi: 10.3390/ijms22042148[
]
- Corton JC, Cunningham ML, Hummer BT, Lau C, Meek B, Peters JM, Popp JA, Rhomberg L, Seed J, Klaunig JE. Mode of action framework analysis for receptor-mediated toxicity: The peroxisome proliferator-activated receptor alpha (PPARα) as a case study. Crit Rev Toxicol. 2014 Jan;44(1):1-49. doi: 10.3109/10408444.2013.835784[
]
- Eriksen K.T., Raaschou-Nielsen O., McLaughlin J.K., Lipworth L., Tjonneland A., Overvad K., Sorensen M. Association between plasma PFOA and PFOS levels and total cholesterol in a middle-aged Danish population. PLoS One. 2013;8:e56969. doi: 10.1371/journal.pone.0056969[
][
][
]
- Geiger S.D., Xiao J., Ducatman A., Frisbee S., Innes K., Shankar A. The association between PFOA, PFOS and serum lipid levels in adolescents. Chemosphere. 2014;98:78–83. doi: 10.1016/j.chemosphere.2013.10.005[
][
][
]
- Fletcher T., Galloway T.S., Melzer D., Holcroft P., Cipelli R., Pilling L.C., Mondal D., Luster M., Harries L.W. Associations between PFOA, PFOS and changes in the expression of genes involved in cholesterol metabolism in humans. Environ. Int. 2013;57–58:2–10. doi: 10.1016/j.envint.2013.03.008[
][
][
][
]
- Lewis R.C., Johns L.E., Meeker J.D. Serum Biomarkers of Exposure to Perfluoroalkyl Substances in Relation to Serum Testosterone and Measures of Thyroid Function among Adults and Adolescents from NHANES 2011-2012. Int. J. Environ. Res. Public Health. 2015;12:6098–6114. doi: 10.3390/ijerph120606098[
][
][
][
]
- Li Y., Xu Y., Fletcher T., Scott K., Nielsen C., Pineda D., Lindh C.H., Olsson D.S., Andersson E.M., Jakobsson K. Associations between perfluoroalkyl substances and thyroid hormones after high exposure through drinking water. Environ. Res. 2021;194:110647. doi: 10.1016/j.envres.2020.110647[
][
][
][
]
- Wang H., Du H., Yang J., Jiang H., O K., Xu L., Liu S., Yi J., Qian X., Chen Y., et al. PFOS, PFOA, estrogen homeostasis, and birth size in Chinese infants. Chemosphere. 2019;221:349–355. doi: 10.1016/j.chemosphere.2019.01.061[
][
][
][
]
- Abraham K., Mielke H., Fromme H., Volkel W., Menzel J., Peiser M., Zepp F., Willich S.N., Weikert C. Internal exposure to perfluoroalkyl substances (PFASs) and biological markers in 101 healthy 1-year-old children: Associations between levels of perfluorooctanoic acid (PFOA) and vaccine response. Arch. Toxicol. 2020;94:2131–2147. doi: 10.1007/s00204-020-02715-4[
][
][
][
]
- Lopez-Espinosa M.J., Fletcher T., Armstrong B., Genser B., Dhatariya K., Mondal D., Ducatman A., Leonardi G. Association of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) with age of puberty among children living near a chemical plant. Environ. Sci. Technol. 2011;45:8160–8166. doi: 10.1021/es1038694[
][
][
][
]
- Joensen U.N., Veyrand B., Antignac J.P., Blomberg Jensen M., Petersen J.H., Marchand P., Skakkebaek N.E., Andersson A.M., Le Bizec B., Jorgensen N. PFOS (perfluorooctanesulfonate) in serum is negatively associated with testosterone levels, but not with semen quality, in healthy men. Hum. Reprod. 2013;28:599–608. doi: 10.1093/humrep/des425[
][
][
]
- Lopez-Espinosa M.J., Mondal D., Armstrong B.G., Eskenazi B., Fletcher T. Perfluoroalkyl Substances, Sex Hormones, and Insulin-like Growth Factor-1 at 6–9 Years of Age: A Cross-Sectional Analysis within the C8 Health Project. Environ. Health Perspect. 2016;124:1269–1275. doi: 10.1289/ehp.1509869[
][
]
- Stratakis N., David V.C., Jin R., Margetaki K., Valvi D., Siskos A.P., Maitre L., Garcia E., Varo N., Zhao Y., et al. Prenatal Exposure to Perfluoroalkyl Substances Associated With Increased Susceptibility to Liver Injury in Children. Hepatology. 2020;72:1758–1770. doi: 10.1002/hep.31483[
]
- Grandjean P., Heilmann C., Weihe P., Nielsen F., Mogensen U.B., Budtz-Jorgensen E. Serum Vaccine Antibody Concentrations in Adolescents Exposed to Perfluorinated Compounds. Environ. Health Perspect. 2017;125:077018. doi: 10.1289/EHP275[
][
][
]
- US-EPA. 3M Phase-out Plan for POSF-based Products. https://www.epa.gov/archive/epapages/newsroom_archive/newsreleases/33aa946e6cb11f35852568e1005246b4.html[
]
- Gallo V., Leonardi G., Genser B., Lopez-Espinosa M.J., Frisbee S.J., Karlsson L., Ducatman A.M., Fletcher T. Serum perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) concentrations and liver function biomarkers in a population with elevated PFOA exposure. Environ. Health Perspect. 2012;120:655–660. doi: 10.1289/ehp.1104436[
][
][
]
- Salihovic S., Stubleski J., Karrman A., Larsson A., Fall T., Lind L., Lind P.M. Changes in markers of liver function in relation to changes in perfluoroalkyl substances – A longitudinal study. Environ. Int. 2018;117:196–203. doi: 10.1016/j.envint.2018.04.052[
][
][
]
- Jain R.B., Ducatman A. Selective Associations of Recent Low Concentrations of Perfluoroalkyl Substances With Liver Function Biomarkers: NHANES 2011 to 2014 Data on US Adults Aged >/=20 Years. J. Occup. Environ. Med. 2019;61:293–302. doi: 10.1097/JOM.0000000000001532[
][
][
]
- Budtz-Jorgensen E., Grandjean P. Application of benchmark analysis for mixed contaminant exposures: Mutual adjustment of perfluoroalkylate substances associated with immunotoxicity. PLoS One. 2018;13:e0205388. doi: 10.1371/journal.pone.0205388[
][
][
]
- Grandjean P., Heilmann C., Weihe P., Nielsen F., Mogensen U.B., Timmermann A., Budtz-Jorgensen E. Estimated exposures to perfluorinated compounds in infancy predict attenuated vaccine antibody concentrations at age 5-years. J. Immunotoxicol. 2017;14:188–195. doi: 10.1080/1547691X.2017.1360968[
][
]
- IARC. 2017. Agents classified by the IARC Monographs, Volumes 1–117. Lyon, France: International Agency for Research on Cancer.[
][
]
- Benbrahim-Tallaa L, Lauby-Secretan B, Loomis D, Guyton KZ, Grosse Y, El Ghissassi F, Bouvard V, Guha N, Mattock H, Straif K; International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of perfluorooctanoic acid, tetrafluoroethylene, dichloromethane, 1,2-dichloropropane, and 1,3-propane sultone. Lancet Oncol. 2014 Aug;15(9):924-5. https://doi.org/10.1016/S1470-2045(14)70316-X[
][
]
- EPA. 2016b. Drinking water health advisory for perfluorooctanoic acid (PFOA). U.S. Environmental Protection Agency. https://www.epa.gov/sites/default/files/2016-05/documents/pfoa_health_advisory_final-plain.pdf[
][
]
- EPA. 2016c. Drinking water health advisory for perfluorooctane sulfonate (PFOS). U.S. Environmental Protection Agency. https://www.epa.gov/sites/default/files/2016-05/documents/pfos_health_advisory_final-plain.pdf[
][
]
- Barry V, Winquist A, and Steenland K. 2013. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ. Health Perspect. 121 (11–12):1313–18. doi: 10.1289/ehp.1306615[
][
]
- Shearer JJ, Callahan CL, Calafat AM, Huang WY, Jones RR, Sabbisetti VS, Freedman ND, Sampson JN, Silverman DT, Purdue MP, et al. 2020. Serum concentrations of per- and polyfluoroalkyl substances and risk of renal cell carcinoma. J. Natl. Cancer Inst Epub ahead of print. doi: 10.1093/jnci/djaa143[
][
]
- Vieira VM, Hoffman K, Shin HM, Weinberg JM, Webster TF, and Fletcher T. 2013. Perfluorooctanoic acid exposure and cancer outcomes in a contaminated community: A geographic analysis. Environ. Health Perspect 121 (3):318–23. doi: 10.1289/ehp.1205829[
][
]
- Li Y., Xu Y., Fletcher T., Scott K., Nielsen C., Pineda D., Lindh C.H., Olsson D.S., Andersson E.M., Jakobsson K. Associations between perfluoroalkyl substances and thyroid hormones after high exposure through drinking water. Environ. Res. 2021;194:110647. doi: 10.1016/j.envres.2020.11064[
]
- Steenland K, Tinker S, Frisbee S, Ducatman A, Vaccarino V. Association of perfluorooctanoic acid and perfluorooctane sulfonate with serum lipids among adults living near a chemical plant. Am J Epidemiol. 2009 Nov 15;170(10):1268-78. doi: 10.1093/aje/kwp279[
][
]
- Nelson JW, Hatch EE, Webster TF. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ Health Perspect. 2010 Feb;118(2):197-202. doi: 10.1289/ehp.0901165[
][
]
- Eriksen KT, Raaschou-Nielsen O, McLaughlin JK, Lipworth L, Tjønneland A, Overvad K, Sørensen M. Association between plasma PFOA and PFOS levels and total cholesterol in a middle-aged Danish population. PLoS One. 2013;8(2):e56969. doi: 10.1371/journal.pone.0056969[
]
- Fitz-Simon N, Fletcher T, Luster MI, Steenland K, Calafat AM, Kato K, Armstrong B. Reductions in serum lipids with a 4-year decline in serum perfluorooctanoic acid and perfluorooctanesulfonic acid. Epidemiology. 2013 Jul;24(4):569-76. doi: 10.1097/EDE.0b013e31829443ee. Erratum in: Epidemiology. 2013 Nov;24(6):941.[
]
- Fisher M, Arbuckle TE, Wade M, Haines DA. Do perfluoroalkyl substances affect metabolic function and plasma lipids?–Analysis of the 2007-2009, Canadian Health Measures Survey (CHMS) Cycle 1. Environ Res. 2013 Feb;121:95-103. doi: 10.1016/j.envres.2012.11.006. Epub 2012 Dec 22. Erratum in: Environ Res. 2013 Oct;126:221.[
]
- Geiger SD, Xiao J, Shankar A. Positive association between perfluoroalkyl chemicals and hyperuricemia in children. Am J Epidemiol. 2013 Jun 1;177(11):1255-62. doi: 10.1093/aje/kws392[
][
]
- Fu Y, Wang T, Fu Q, Wang P, Lu Y. Associations between serum concentrations of perfluoroalkyl acids and serum lipid levels in a Chinese population. Ecotoxicol Environ Saf. 2014 Aug;106:246-52. doi: 10.1016/j.ecoenv.2014.04.039[
]
- Starling AP, Engel SM, Whitworth KW, Richardson DB, Stuebe AM, Daniels JL, Haug LS, Eggesbø M, Becher G, Sabaredzovic A, Thomsen C, Wilson RE, Travlos GS, Hoppin JA, Baird DD, Longnecker MP. Perfluoroalkyl substances and lipid concentrations in plasma during pregnancy among women in the Norwegian Mother and Child Cohort Study. Environ Int. 2014 Jan;62:104-12. doi: 10.1016/j.envint.2013.10.004[
]
- Winquist A, Steenland K. Modeled PFOA exposure and coronary artery disease, hypertension, and high cholesterol in community and worker cohorts. Environ Health Perspect. 2014 Dec;122(12):1299-305. doi: 10.1289/ehp.1307943[
]
- Skuladottir M, Ramel A, Rytter D, Haug LS, Sabaredzovic A, Bech BH, Henriksen TB, Olsen SF, Halldorsson TI. Examining confounding by diet in the association between perfluoroalkyl acids and serum cholesterol in pregnancy. Environ Res. 2015 Nov;143(Pt A):33-8. doi: 10.1016/j.envres.2015.09.001[
]
- Zeng XW, Qian Z, Emo B, Vaughn M, Bao J, Qin XD, Zhu Y, Li J, Lee YL, Dong GH. Association of polyfluoroalkyl chemical exposure with serum lipids in children. Sci Total Environ. 2015 Apr 15;512-513:364-370. doi: 10.1016/j.scitotenv.2015.01.042[
]
- Koshy TT, Attina TM, Ghassabian A, Gilbert J, Burdine LK, Marmor M, Honda M, Chu DB, Han X, Shao Y, Kannan K, Urbina EM, Trasande L. Serum perfluoroalkyl substances and cardiometabolic consequences in adolescents exposed to the World Trade Center disaster and a matched comparison group. Environ Int. 2017 Dec;109:128-135. doi: 10.1016/j.envint.2017.08.003[
][
]
- Liu G, Zhang B, Hu Y, Rood J, Liang L, Qi L, Bray GA, DeJonge L, Coull B, Grandjean P, Furtado JD, Sun Q. Associations of Perfluoroalkyl substances with blood lipids and Apolipoproteins in lipoprotein subspecies: the POUNDS-lost study. Environ Health. 2020 Jan 13;19(1):5. doi: 10.1186/s12940-020-0561-8[
]
- Li Y, Barregard L, Xu Y, Scott K, Pineda D, Lindh CH, Jakobsson K, Fletcher T. Associations between perfluoroalkyl substances and serum lipids in a Swedish adult population with contaminated drinking water. Environ Health. 2020 Mar 14;19(1):33. doi: 10.1186/s12940-020-00588-9[
][
][
]
- Lin PD, Cardenas A, Hauser R, Gold DR, Kleinman KP, Hivert MF, Fleisch AF, Calafat AM, Webster TF, Horton ES, Oken E. Per- and polyfluoroalkyl substances and blood lipid levels in pre-diabetic adults-longitudinal analysis of the diabetes prevention program outcomes study. Environ Int. 2019 Aug;129:343-353. doi: 10.1016/j.envint.2019.05.027[
][
]
- Dong Z, Wang H, Yu YY, Li YB, Naidu R, Liu Y. Using 2003-2014 U.S. NHANES data to determine the associations between per- and polyfluoroalkyl substances and cholesterol: Trend and implications. Ecotoxicol Environ Saf. 2019 May 30;173:461-468. doi: 10.1016/j.ecoenv.2019.02.061[
]
- He X, Liu Y, Xu B, Gu L, Tang W. PFOA is associated with diabetes and metabolic alteration in US men: National Health and Nutrition Examination Survey 2003-2012. Sci Total Environ. 2018 Jun 1;625:566-574. doi: 10.1016/j.scitotenv.2017.12.186[
]
- Convertino M, Church TR, Olsen GW, Liu Y, Doyle E, Elcombe CR, Barnett AL, Samuel LM, MacPherson IR, Evans TRJ. Stochastic Pharmacokinetic-Pharmacodynamic Modeling for Assessing the Systemic Health Risk of Perfluorooctanoate (PFOA). Toxicol Sci. 2018 May 1;163(1):293-306. doi: 10.1093/toxsci/kfy035[
]
- Frisbee SJ, Shankar A, Knox SS, Steenland K, Savitz DA, Fletcher T, Ducatman AM. Perfluorooctanoic acid, perfluorooctanesulfonate, and serum lipids in children and adolescents: results from the C8 Health Project. Arch Pediatr Adolesc Med. 2010 Sep;164(9):860-9. doi: 10.1001/archpediatrics.2010.163[
][
][
]
- Maisonet M, Näyhä S, Lawlor DA, Marcus M. Prenatal exposures to perfluoroalkyl acids and serum lipids at ages 7 and 15 in females. Environ Int. 2015 Sep;82:49-60. doi: 10.1016/j.envint.2015.05.001[
]
- Timmermann CA, Rossing LI, Grøntved A, Ried-Larsen M, Dalgård C, Andersen LB, Grandjean P, Nielsen F, Svendsen KD, Scheike T, Jensen TK. Adiposity and glycemic control in children exposed to perfluorinated compounds. J Clin Endocrinol Metab. 2014 Apr;99(4):E608-14. doi: 10.1210/jc.2013-3460[
]
- Jain RB, Ducatman A. Roles of gender and obesity in defining correlations between perfluoroalkyl substances and lipid/lipoproteins. Sci Total Environ. 2019 Feb 25;653:74-81. doi: 10.1016/j.scitotenv.2018.10.362[
]
- Tan X, Xie G, Sun X, Li Q, Zhong W, Qiao P, Sun X, Jia W, Zhou Z. High fat diet feeding exaggerates perfluorooctanoic acid-induced liver injury in mice via modulating multiple metabolic pathways. PLoS One. 2013 Apr 23;8(4):e61409. doi: 10.1371/journal.pone.0061409[
]
- Quist EM, Filgo AJ, Cummings CA, Kissling GE, Hoenerhoff MJ, Fenton SE. Hepatic Mitochondrial Alteration in CD-1 Mice Associated with Prenatal Exposures to Low Doses of Perfluorooctanoic Acid (PFOA). Toxicol Pathol. 2015 Jun;43(4):546-57. doi: 10.1177/0192623314551841[
]
- Rebholz SL, Jones T, Herrick RL, Xie C, Calafat AM, Pinney SM, Woollett LA. Hypercholesterolemia with consumption of PFOA-laced Western diets is dependent on strain and sex of mice. Toxicol Rep. 2016;3:46-54. doi: 10.1016/j.toxrep.2015.11.004[
]
- Fletcher T, Galloway TS, Melzer D, Holcroft P, Cipelli R, Pilling LC, Mondal D, Luster M, Harries LW. Associations between PFOA, PFOS and changes in the expression of genes involved in cholesterol metabolism in humans. Environ Int. 2013 Jul;57-58:2-10. doi: 10.1016/j.envint.2013.03.008[
]
- Chou WC, Lin Z. Probabilistic human health risk assessment of perfluorooctane sulfonate (PFOS) by integrating in vitro, in vivo toxicity, and human epidemiological studies using a Bayesian-based dose-response assessment coupled with physiologically based pharmacokinetic (PBPK) modeling approach. Environ Int. 2020 Apr;137:105581. doi: 10.1016/j.envint.2020.105581[
]
- New Jersey Drinking Water Quality Institute Health Effects Subcommittee. 2017. Health-based maximum contaminant level support document: Perfluorooctanoic acid (PFOA). Trenton, NJ, USA. https://www.state.nj.us/dep/watersupply/pdf/pfoa-appendixa.pdf[
]
- Schlezinger JJ, Puckett H, Oliver J, Nielsen G, Heiger-Bernays W, Webster TF. Perfluorooctanoic acid activates multiple nuclear receptor pathways and skews expression of genes regulating cholesterol homeostasis in liver of humanized PPARα mice fed an American diet. Toxicol Appl Pharmacol. 2020 Oct 15;405:115204. doi: 10.1016/j.taap.2020.115204[
][
]
- Behr AC, Kwiatkowski A, Ståhlman M, Schmidt FF, Luckert C, Braeuning A, Buhrke T. Impairment of bile acid metabolism by perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in human HepaRG hepatoma cells. Arch Toxicol. 2020 May;94(5):1673-1686. doi: 10.1007/s00204-020-02732-3. Epub 2020 Apr 6. Erratum in: Arch Toxicol. 2021 Aug;95(8):2891.[
]
- Zhang H, He J, Li N, Gao N, Du Q, Chen B, Chen F, Shan X, Ding Y, Zhu W, Wu Y, Tang J, Jia X. Lipid accumulation responses in the liver of Rana nigromaculata induced by perfluorooctanoic acid (PFOA). Ecotoxicol Environ Saf. 2019 Jan 15;167:29-35. doi: 10.1016/j.ecoenv.2018.09.120[
][
]
- Salihovic S, Fall T, Ganna A, Broeckling CD, Prenni JE, Hyötyläinen T, Kärrman A, Lind PM, Ingelsson E, Lind L. Identification of metabolic profiles associated with human exposure to perfluoroalkyl substances. J Expo Sci Environ Epidemiol. 2019 Mar;29(2):196-205. doi: 10.1038/s41370-018-0060-y[
][
]
- Das KP, Wood CR, Lin MT, Starkov AA, Lau C, Wallace KB, Corton JC, Abbott BD. Perfluoroalkyl acids-induced liver steatosis: Effects on genes controlling lipid homeostasis. Toxicology. 2017 Mar 1;378:37-52. doi: 10.1016/j.tox.2016.12.007[
][
]
- Filgo AJ, Quist EM, Hoenerhoff MJ, Brix AE, Kissling GE, Fenton SE. Perfluorooctanoic Acid (PFOA)-induced Liver Lesions in Two Strains of Mice Following Developmental Exposures: PPARα Is Not Required. Toxicol Pathol. 2015 Jun;43(4):558-68. doi: 10.1177/0192623314558463[
]
- Wang L, Wang Y, Liang Y, Li J, Liu Y, Zhang J, Zhang A, Fu J, Jiang G. PFOS induced lipid metabolism disturbances in BALB/c mice through inhibition of low density lipoproteins excretion. Sci Rep. 2014 Apr 3;4:4582. doi: 10.1038/srep04582[
]
- Bjork JA, Butenhoff JL, Wallace KB. Multiplicity of nuclear receptor activation by PFOA and PFOS in primary human and rodent hepatocytes. Toxicology. 2011 Oct 9;288(1-3):8-17. doi: 10.1016/j.tox.2011.06.012[
]
- Bijland S, Rensen PC, Pieterman EJ, Maas AC, van der Hoorn JW, van Erk MJ, Havekes LM, Willems van Dijk K, Chang SC, Ehresman DJ, Butenhoff JL, Princen HM. Perfluoroalkyl sulfonates cause alkyl chain length-dependent hepatic steatosis and hypolipidemia mainly by impairing lipoprotein production in APOE*3-Leiden CETP mice. Toxicol Sci. 2011 Sep;123(1):290-303. doi: 10.1093/toxsci/kfr142[
]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci. 2007 Oct;99(2):366-94. doi: 10.1093/toxsci/kfm128[
]
- Guruge KS, Yeung LW, Yamanaka N, Miyazaki S, Lam PK, Giesy JP, Jones PD, Yamashita N. Gene expression profiles in rat liver treated with perfluorooctanoic acid (PFOA). Toxicol Sci. 2006 Jan;89(1):93-107. doi: 10.1093/toxsci/kfj011[
]
- Perla FM, Prelati M, Lavorato M, Visicchio D, Anania C. The Role of Lipid and Lipoprotein Metabolism in Non-Alcoholic Fatty Liver Disease. Children (Basel). 2017 Jun 6;4(6):46. doi: 10.3390/children4060046[
]
- Alderete TL, Jin R, Walker DI, Valvi D, Chen Z, Jones DP, Peng C, Gilliland FD, Berhane K, Conti DV, Goran MI, Chatzi L. Perfluoroalkyl substances, metabolomic profiling, and alterations in glucose homeostasis among overweight and obese Hispanic children: A proof-of-concept analysis. Environ Int. 2019 May;126:445-453. doi: 10.1016/j.envint.2019.02.047[
]
- Sun Q, Zong G, Valvi D, Nielsen F, Coull B, Grandjean P. Plasma Concentrations of Perfluoroalkyl Substances and Risk of Type 2 Diabetes: A Prospective Investigation among U.S. Women. Environ Health Perspect. 2018 Mar 1;126(3):037001. doi: 10.1289/EHP2619[
]
- Cardenas A, Gold DR, Hauser R, Kleinman KP, Hivert MF, Calafat AM, Ye X, Webster TF, Horton ES, Oken E. Plasma Concentrations of Per- and Polyfluoroalkyl Substances at Baseline and Associations with Glycemic Indicators and Diabetes Incidence among High-Risk Adults in the Diabetes Prevention Program Trial. Environ Health Perspect. 2017 Oct 2;125(10):107001. doi: 10.1289/EHP1612[
][
][
][
]
- Karnes C, Winquist A, Steenland K. Incidence of type II diabetes in a cohort with substantial exposure to perfluorooctanoic acid. Environ Res. 2014 Jan;128:78-83. doi: 10.1016/j.envres.2013.11.003[
]
- Donat-Vargas C, Bergdahl IA, Tornevi A, Wennberg M, Sommar J, Kiviranta H, Koponen J, Rolandsson O, Åkesson A. Perfluoroalkyl substances and risk of type II diabetes: A prospective nested case-control study. Environ Int. 2019 Feb;123:390-398. doi: 10.1016/j.envint.2018.12.026[
]
- Bodin J, Groeng EC, Andreassen M, Dirven H, Nygaard UC. Exposure to perfluoroundecanoic acid (PFUnDA) accelerates insulitis development in a mouse model of type 1 diabetes. Toxicol Rep. 2016 Aug 29;3:664-672. doi: 10.1016/j.toxrep.2016.08.009[
]
- Qin WP, Cao LY, Li CH, Guo LH, Colbourne J, Ren XM. Perfluoroalkyl Substances Stimulate Insulin Secretion by Islet β Cells via G Protein-Coupled Receptor 40. Environ Sci Technol. 2020 Mar 17;54(6):3428-3436. doi: 10.1021/acs.est.9b07295[
]
- Zhang L, Duan X, Sun W, Sun H. Perfluorooctane sulfonate acute exposure stimulates insulin secretion via GPR40 pathway. Sci Total Environ. 2020 Jul 15;726:138498. doi: 10.1016/j.scitotenv.2020.138498[
]
- Steenland K, Fletcher T, Savitz DA. Epidemiologic evidence on the health effects of perfluorooctanoic acid (PFOA). Environ Health Perspect. 2010 Aug;118(8):1100-8. doi: 10.1289/ehp.0901827[
]
- Szumilas M. Explaining odds ratios. J Can Acad Child Adolesc Psychiatry. 2010 Aug;19(3):227-9. Erratum in: J Can Acad Child Adolesc Psychiatry. 2015 Winter;24(1):58. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2938757[
]
- Lin CY, Chen PC, Lin YC, Lin LY. Association among serum perfluoroalkyl chemicals, glucose homeostasis, and metabolic syndrome in adolescents and adults. Diabetes Care. 2009 Apr;32(4):702-7. doi: 10.2337/dc08-1816[
][
]
- Zhang C, Sundaram R, Maisog J, Calafat AM, Barr DB, Buck Louis GM. A prospective study of prepregnancy serum concentrations of perfluorochemicals and the risk of gestational diabetes. Fertil Steril. 2015 Jan;103(1):184-9. doi: 10.1016/j.fertnstert.2014.10.001[
]
- Matilla-Santander N, Valvi D, Lopez-Espinosa MJ, Manzano-Salgado CB, Ballester F, Ibarluzea J, Santa-Marina L, Schettgen T, Guxens M, Sunyer J, Vrijheid M. Exposure to Perfluoroalkyl Substances and Metabolic Outcomes in Pregnant Women: Evidence from the Spanish INMA Birth Cohorts. Environ Health Perspect. 2017 Nov 13;125(11):117004. doi: 10.1289/EHP1062[
]
- Min JY, Lee KJ, Park JB, Min KB. Perfluorooctanoic acid exposure is associated with elevated homocysteine and hypertension in US adults. Occup Environ Med. 2012 Sep;69(9):658-62. doi: 10.1136/oemed-2011-100288[
]
- Geiger SD, Xiao J, Shankar A. No association between perfluoroalkyl chemicals and hypertension in children. Integr Blood Press Control. 2014 Jan 13;7:1-7. doi: 10.2147/IBPC.S47660[
]
- Conway B, Costacou T Perfluoroalkyl acids and stroke risk in persons with and without diabetes: A salutatory effect of high oxygen carrying capacity environmental contaminants. Circulation 2016; 133.[
]
- Steenland K, Zhao L, Winquist A. A cohort incidence study of workers exposed to perfluorooctanoic acid (PFOA). Occup Environ Med. 2015 May;72(5):373-80. doi: 10.1136/oemed-2014-102364[
][
]
- Lin CY, Lin LY, Wen TW, Lien GW, Chien KL, Hsu SH, Liao CC, Sung FC, Chen PC, Su TC. Association between levels of serum perfluorooctane sulfate and carotid artery intima-media thickness in adolescents and young adults. Int J Cardiol. 2013 Oct 9;168(4):3309-16. doi: 10.1016/j.ijcard.2013.04.042[
]
- Olsen GW, Ehresman DJ, Buehrer BD, Gibson BA, Butenhoff JL, Zobel LR. Longitudinal assessment of lipid and hepatic clinical parameters in workers involved with the demolition of perfluoroalkyl manufacturing facilities. J Occup Environ Med. 2012 Aug;54(8):974-83. doi: 10.1097/JOM.0b013e31825461d2[
]
- Lin CY, Lin LY, Chiang CK, Wang WJ, Su YN, Hung KY, Chen PC. Investigation of the associations between low-dose serum perfluorinated chemicals and liver enzymes in US adults. Am J Gastroenterol. 2010 Jun;105(6):1354-63. doi: 10.1038/ajg.2009.707[
][
]
- National Toxicology Program. 2020a. NTP technical report on the toxicology and carcinogenesis studies of perfluorooctanoic acid (CAS no. 335-67-1) administered in feed to Sprague Dawley (Hsd:Sprague Dawley® SD®) rats. Technical Report 598. US Department of Health and Human Services, Research Triangle Park, NC. https://ntp.niehs.nih.gov/sites/default/files/ntp/about_ntp/trpanel/2019/december/tr598draft.pdf[
]
- Hui Z, Li R, Chen L. The impact of exposure to environmental contaminant on hepatocellular lipid metabolism. Gene. 2017 Jul 30;622:67-71. doi: 10.1016/j.gene.2017.04.024[
]
- Li K, Sun J, Yang J, Roberts SM, Zhang X, Cui X, Wei S, Ma LQ. Molecular Mechanisms of Perfluorooctanoate-Induced Hepatocyte Apoptosis in Mice Using Proteomic Techniques. Environ Sci Technol. 2017 Oct 3;51(19):11380-11389. doi: 10.1021/acs.est.7b02690[
]
- Guillette TC, McCord J, Guillette M, Polera ME, Rachels KT, Morgeson C, Kotlarz N, Knappe DRU, Reading BJ, Strynar M, Belcher SM. Elevated levels of per- and polyfluoroalkyl substances in Cape Fear River Striped Bass (Morone saxatilis) are associated with biomarkers of altered immune and liver function. Environ Int. 2020 Mar;136:105358. doi: 10.1016/j.envint.2019.105358[
]
- Xu J, Shimpi P, Armstrong L, Salter D, Slitt AL. PFOS induces adipogenesis and glucose uptake in association with activation of Nrf2 signaling pathway. Toxicol Appl Pharmacol. 2016 Jan 1;290:21-30. doi: 10.1016/j.taap.2015.11.002[
]
- Yao X, Sha S, Wang Y, Sun X, Cao J, Kang J, Jiang L, Chen M, Ma Y. Perfluorooctane Sulfonate Induces Autophagy-Dependent Apoptosis through Spinster 1-Mediated lysosomal-Mitochondrial Axis and Impaired Mitophagy. Toxicol Sci. 2016 Sep;153(1):198-211. doi: 10.1093/toxsci/kfw118[
]
- Zhang L, Krishnan P, Ehresman DJ, Smith PB, Dutta M, Bagley BD, Chang SC, Butenhoff JL, Patterson AD, Peters JM. Editor’s Highlight: Perfluorooctane Sulfonate-Choline Ion Pair Formation: A Potential Mechanism Modulating Hepatic Steatosis and Oxidative Stress in Mice. Toxicol Sci. 2016 Sep;153(1):186-97. doi: 10.1093/toxsci/kfw120[
]
- Sakr CJ, Kreckmann KH, Green JW, Gillies PJ, Reynolds JL, Leonard RC. Cross-sectional study of lipids and liver enzymes related to a serum biomarker of exposure (ammonium perfluorooctanoate or APFO) as part of a general health survey in a cohort of occupationally exposed workers. J Occup Environ Med. 2007 Oct;49(10):1086-96. doi: 10.1097/JOM.0b013e318156eca3[
]
- Gallo V, Leonardi G, Genser B, Lopez-Espinosa MJ, Frisbee SJ, Karlsson L, Ducatman AM, Fletcher T. Serum perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) concentrations and liver function biomarkers in a population with elevated PFOA exposure. Environ Health Perspect. 2012 May;120(5):655-60. doi: 10.1289/ehp.1104436[
][
]
- Yamaguchi M, Arisawa K, Uemura H, Katsuura-Kamano S, Takami H, Sawachika F, Nakamoto M, Juta T, Toda E, Mori K, Hasegawa M, Tanto M, Shima M, Sumiyoshi Y, Morinaga K, Kodama K, Suzuki T, Nagai M, Satoh H. Consumption of seafood, serum liver enzymes, and blood levels of PFOS and PFOA in the Japanese population. J Occup Health. 2013;55(3):184-94. doi: 10.1539/joh.12-0264-oa[
]
- Gleason JA, Post GB, Fagliano JA. Associations of perfluorinated chemical serum concentrations and biomarkers of liver function and uric acid in the US population (NHANES), 2007-2010. Environ Res. 2015 Jan;136:8-14. doi: 10.1016/j.envres.2014.10.004[
][
]
- Attanasio R. Sex differences in the association between perfluoroalkyl acids and liver function in US adolescents: Analyses of NHANES 2013-2016. Environ Pollut. 2019 Nov;254(Pt B):113061. doi: 10.1016/j.envpol.2019.113061[
]
- Nian M, Li QQ, Bloom M, Qian ZM, Syberg KM, Vaughn MG, Wang SQ, Wei Q, Zeeshan M, Gurram N, Chu C, Wang J, Tian YP, Hu LW, Liu KK, Yang BY, Liu RQ, Feng D, Zeng XW, Dong GH. Liver function biomarkers disorder is associated with exposure to perfluoroalkyl acids in adults: Isomers of C8 Health Project in China. Environ Res. 2019 May;172:81-88. doi: 10.1016/j.envres.2019.02.013[
]
- Sakr CJ, Leonard RC, Kreckmann KH, Slade MD, Cullen MR. Longitudinal study of serum lipids and liver enzymes in workers with occupational exposure to ammonium perfluorooctanoate. J Occup Environ Med. 2007 Aug;49(8):872-9. doi: 10.1097/JOM.0b013e318124a93f. Erratum in: J Occup Environ Med. 2007 Nov;49(11):1294.[
]
- Darrow LA, Groth AC, Winquist A, Shin HM, Bartell SM, Steenland K. Modeled Perfluorooctanoic Acid (PFOA) Exposure and Liver Function in a Mid-Ohio Valley Community. Environ Health Perspect. 2016 Aug;124(8):1227-33. doi: 10.1289/ehp.1510391[
]
- Jain RB, Ducatman A. Selective Associations of Recent Low Concentrations of Perfluoroalkyl Substances With Liver Function Biomarkers: NHANES 2011 to 2014 Data on US Adults Aged ≥20 Years. J Occup Environ Med. 2019 Apr;61(4):293-302. doi: 10.1097/JOM.0000000000001532[
]
- Martin MT, Brennan RJ, Hu W, Ayanoglu E, Lau C, Ren H, Wood CR, Corton JC, Kavlock RJ, Dix DJ. Toxicogenomic study of triazole fungicides and perfluoroalkyl acids in rat livers predicts toxicity and categorizes chemicals based on mechanisms of toxicity. Toxicol Sci. 2007 Jun;97(2):595-613. doi: 10.1093/toxsci/kfm065[
]
- Wan HT, Zhao YG, Wei X, Hui KY, Giesy JP, Wong CK. PFOS-induced hepatic steatosis, the mechanistic actions on β-oxidation and lipid transport. Biochim Biophys Acta. 2012 Jul;1820(7):1092-101. doi: 10.1016/j.bbagen.2012.03.010[
]
- Wang L, Wang Y, Liang Y, Li J, Liu Y, Zhang J, Zhang A, Fu J, Jiang G. Specific accumulation of lipid droplets in hepatocyte nuclei of PFOA-exposed BALB/c mice. Sci Rep. 2013;3:2174. doi: 10.1038/srep02174[
]
- Bassler J, Ducatman A, Elliott M, Wen S, Wahlang B, Barnett J, Cave MC. Environmental perfluoroalkyl acid exposures are associated with liver disease characterized by apoptosis and altered serum adipocytokines. Environ Pollut. 2019 Apr;247:1055-1063. doi: 10.1016/j.envpol.2019.01.064[
]
- Kingsley SL, Walker DI, Calafat AM, Chen A, Papandonatos GD, Xu Y, Jones DP, Lanphear BP, Pennell KD, Braun JM. Metabolomics of childhood exposure to perfluoroalkyl substances: a cross-sectional study. Metabolomics. 2019 Jun 21;15(7):95. doi: 10.1007/s11306-019-1560-z[
]
- Wahlang B, Jin J, Beier JI, Hardesty JE, Daly EF, Schnegelberger RD, Falkner KC, Prough RA, Kirpich IA, Cave MC. Mechanisms of Environmental Contributions to Fatty Liver Disease. Curr Environ Health Rep. 2019 Sep;6(3):80-94. doi: 10.1007/s40572-019-00232-w[
]
- Massoud O, Charlton M. Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis and Hepatocellular Carcinoma. Clin Liver Dis. 2018 Feb;22(1):201-211. doi: 10.1016/j.cld.2017.08.014[
]
- Jin R, McConnell R, Catherine C, Xu S, Walker DI, Stratakis N, Jones DP, Miller GW, Peng C, Conti DV, Vos MB, Chatzi L. Perfluoroalkyl substances and severity of nonalcoholic fatty liver in Children: An untargeted metabolomics approach. Environ Int. 2020 Jan;134:105220. doi: 10.1016/j.envint.2019.105220[
]
- Rantakokko P, Männistö V, Airaksinen R, Koponen J, Viluksela M, Kiviranta H, Pihlajamäki J. Persistent organic pollutants and non-alcoholic fatty liver disease in morbidly obese patients: a cohort study. Environ Health. 2015 Sep 29;14:79. doi: 10.1186/s12940-015-0066-z[
]
- Girardi P, Merler E. A mortality study on male subjects exposed to polyfluoroalkyl acids with high internal dose of perfluorooctanoic acid. Environ Res. 2019 Dec;179(Pt A):108743. doi: 10.1016/j.envres.2019.108743[
]
- Lundin JI, Alexander BH, Olsen GW, Church TR. Ammonium perfluorooctanoate production and occupational mortality. Epidemiology. 2009 Nov;20(6):921-8. doi: 10.1097/EDE.0b013e3181b5f395[
]
- Barry V, Winquist A, Steenland K. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ Health Perspect. 2013 Nov-Dec;121(11-12):1313-8. doi: 10.1289/ehp.1306615[
][
][
][
][
]
- Vieira VM, Hoffman K, Shin HM, Weinberg JM, Webster TF, Fletcher T. Perfluorooctanoic acid exposure and cancer outcomes in a contaminated community: a geographic analysis. Environ Health Perspect. 2013 Mar;121(3):318-23. doi: 10.1289/ehp.1205829[
][
][
][
]
- Salihović S, Dickens AM, Schoultz I, Fart F, Sinisalu L, Lindeman T, Halfvarson J, Orešič M, Hyötyläinen T. Simultaneous determination of perfluoroalkyl substances and bile acids in human serum using ultra-high-performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2020 Apr;412(10):2251-2259. doi: 10.1007/s00216-019-02263-6[
]
- Armstrong LE, Guo GL. Understanding Environmental Contaminants’ Direct Effects on Non-alcoholic Fatty Liver Disease Progression. Curr Environ Health Rep. 2019 Sep;6(3):95-104. doi: 10.1007/s40572-019-00231-x[
]
- Wang X, Li B, Zhao WD, Liu YJ, Shang DS, Fang WG, Chen YH. Perfluorooctane sulfonate triggers tight junction “opening” in brain endothelial cells via phosphatidylinositol 3-kinase. Biochem Biophys Res Commun. 2011 Jul 1;410(2):258-63. doi: 10.1016/j.bbrc.2011.05.128[
]
- Johansson N, Fredriksson A, Eriksson P. Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice. Neurotoxicology. 2008 Jan;29(1):160-9. doi: 10.1016/j.neuro.2007.10.008[
]
- Slotkin TA, MacKillop EA, Melnick RL, Thayer KA, Seidler FJ. Developmental neurotoxicity of perfluorinated chemicals modeled in vitro. Environ Health Perspect. 2008 Jun;116(6):716-22. doi: 10.1289/ehp.11253[
]
- Goulding DR, White SS, McBride SJ, Fenton SE, Harry GJ. Gestational exposure to perfluorooctanoic acid (PFOA): Alterations in motor related behaviors. Neurotoxicology. 2017 Jan;58:110-119. doi: 10.1016/j.neuro.2016.11.008[
]
- Viberg H, Lee I, Eriksson P. Adult dose-dependent behavioral and cognitive disturbances after a single neonatal PFHxS dose. Toxicology. 2013 Feb 8;304:185-91. doi: 10.1016/j.tox.2012.12.013[
]
- Butenhoff JL, Ehresman DJ, Chang SC, Parker GA, Stump DG. Gestational and lactational exposure to potassium perfluorooctanesulfonate (K+PFOS) in rats: developmental neurotoxicity. Reprod Toxicol. 2009 Jun;27(3-4):319-330. https://doi.org/10.1016/j.reprotox.2008.12.010[
]
- Liew Z, Goudarzi H, Oulhote Y. Developmental Exposures to Perfluoroalkyl Substances (PFASs): An Update of Associated Health Outcomes. Curr Environ Health Rep. 2018 Mar;5(1):1-19. doi: 10.1007/s40572-018-0173-4[
][
][
][
]
- Starnes HM, Rock KD, Jackson TW, Belcher SM. A Critical Review and Meta-Analysis of Impacts of Per- and Polyfluorinated Substances on the Brain and Behavior. Front Toxicol. 2022 Apr 11;4:881584. doi: 10.3389/ftox.2022.881584[
][
]
- DeWitt JC, Peden-Adams MM, Keller JM, Germolec DR. Immunotoxicity of perfluorinated compounds: recent developments. Toxicol Pathol. 2012;40(2):300-11. doi: 10.1177/0192623311428473[
]
- Grandjean P, Andersen EW, Budtz-Jørgensen E, Nielsen F, Mølbak K, Weihe P, Heilmann C. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA. 2012 Jan 25;307(4):391-7. doi: 10.1001/jama.2011.2034. Erratum in: JAMA. 2012 Mar 21;307(11):1142.[
][
]
- Grandjean P, Heilmann C, Weihe P, Nielsen F, Mogensen UB, Budtz-Jørgensen E. Serum Vaccine Antibody Concentrations in Adolescents Exposed to Perfluorinated Compounds. Environ Health Perspect. 2017 Jul 26;125(7):077018. doi: 10.1289/EHP275[
]
- Abraham K, Mielke H, Fromme H, Völkel W, Menzel J, Peiser M, Zepp F, Willich SN, Weikert C. Internal exposure to perfluoroalkyl substances (PFASs) and biological markers in 101 healthy 1-year-old children: associations between levels of perfluorooctanoic acid (PFOA) and vaccine response. Arch Toxicol. 2020 Jun;94(6):2131-2147. doi: 10.1007/s00204-020-02715-4[
]
- Stein CR, McGovern KJ, Pajak AM, Maglione PJ, Wolff MS. Perfluoroalkyl and polyfluoroalkyl substances and indicators of immune function in children aged 12-19 y: National Health and Nutrition Examination Survey. Pediatr Res. 2016 Feb;79(2):348-57. doi: 10.1038/pr.2015.213[
]
- Granum B, Haug LS, Namork E, Stølevik SB, Thomsen C, Aaberge IS, van Loveren H, Løvik M, Nygaard UC. Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J Immunotoxicol. 2013 Oct-Dec;10(4):373-9. doi: 10.3109/1547691X.2012.755580[
]
- Stein CR, Ge Y, Wolff MS, Ye X, Calafat AM, Kraus T, Moran TM. Perfluoroalkyl substance serum concentrations and immune response to FluMist vaccination among healthy adults. Environ Res. 2016 Aug;149:171-178. doi: 10.1016/j.envres.2016.05.020[
]
- Looker C, Luster MI, Calafat AM, Johnson VJ, Burleson GR, Burleson FG, Fletcher T. Influenza vaccine response in adults exposed to perfluorooctanoate and perfluorooctanesulfonate. Toxicol Sci. 2014 Mar;138(1):76-88. doi: 10.1093/toxsci/kft269[
]
- Genser B, Teles CA, Barreto ML, Fischer JE. Within- and between-group regression for improving the robustness of causal claims in cross-sectional analysis. Environ Health. 2015 Jul 10;14:60. doi: 10.1186/s12940-015-0047-2[
]
- Goudarzi H, Miyashita C, Okada E, Kashino I, Chen CJ, Ito S, Araki A, Kobayashi S, Matsuura H, Kishi R. Prenatal exposure to perfluoroalkyl acids and prevalence of infectious diseases up to 4years of age. Environ Int. 2017 Jul;104:132-138. doi: 10.1016/j.envint.2017.01.024[
]
- Impinen A, Longnecker MP, Nygaard UC, London SJ, Ferguson KK, Haug LS, Granum B. Maternal levels of perfluoroalkyl substances (PFASs) during pregnancy and childhood allergy and asthma related outcomes and infections in the Norwegian Mother and Child (MoBa) cohort. Environ Int. 2019 Mar;124:462-472. doi: 10.1016/j.envint.2018.12.041[
][
]
- Kvalem HE, Nygaard UC, Lødrup Carlsen KC, Carlsen KH, Haug LS, Granum B. Perfluoroalkyl substances, airways infections, allergy and asthma related health outcomes – implications of gender, exposure period and study design. Environ Int. 2020 Jan;134:105259. doi: 10.1016/j.envint.2019.105259[
][
][
]
- Impinen A, Nygaard UC, Lødrup Carlsen KC, Mowinckel P, Carlsen KH, Haug LS, Granum B. Prenatal exposure to perfluoralkyl substances (PFASs) associated with respiratory tract infections but not allergy- and asthma-related health outcomes in childhood. Environ Res. 2018 Jan;160:518-523. doi: 10.1016/j.envres.2017.10.012[
]
- Qin XD, Qian ZM, Dharmage SC, Perret J, Geiger SD, Rigdon SE, Howard S, Zeng XW, Hu LW, Yang BY, Zhou Y, Li M, Xu SL, Bao WW, Zhang YZ, Yuan P, Wang J, Zhang C, Tian YP, Nian M, Xiao X, Chen W, Lee YL, Dong GH. Association of perfluoroalkyl substances exposure with impaired lung function in children. Environ Res. 2017 May;155:15-21. doi: 10.1016/j.envres.2017.01.025[
][
]
- Timmermann CA, Budtz-Jørgensen E, Jensen TK, Osuna CE, Petersen MS, Steuerwald U, Nielsen F, Poulsen LK, Weihe P, Grandjean P. Association between perfluoroalkyl substance exposure and asthma and allergic disease in children as modified by MMR vaccination. J Immunotoxicol. 2017 Dec;14(1):39-49. doi: 10.1080/1547691X.2016.1254306[
]
- Steenland K, Zhao L, Winquist A, Parks C. Ulcerative colitis and perfluorooctanoic acid (PFOA) in a highly exposed population of community residents and workers in the mid-Ohio valley. Environ Health Perspect. 2013 Aug;121(8):900-5. doi: 10.1289/ehp.1206449[
]
- Steenland K, Kugathasan S, Barr DB. PFOA and ulcerative colitis. Environ Res. 2018 Aug;165:317-321. doi: 10.1016/j.envres.2018.05.007[
]
- Xu Y, Li Y, Scott K, Lindh CH, Jakobsson K, Fletcher T, Ohlsson B, Andersson EM. Inflammatory bowel disease and biomarkers of gut inflammation and permeability in a community with high exposure to perfluoroalkyl substances through drinking water. Environ Res. 2020 Feb;181:108923. doi: 10.1016/j.envres.2019.108923[
]
- National Toxicology Program. 2016. Immunotoxicity associated with exposure to perfluorooctanoic acid (PFOA) or perfluorooctane sulfonate (PFOS). US Department of Health and Human Services, Research Triangle Park, NC. https://ntp.niehs.nih.gov/sites/default/files/ntp/ohat/pfoa_pfos/pfoa_pfosmonograph_508.pdf[
]
- DeWitt JC, Blossom SJ, Schaider LA. Exposure to per-fluoroalkyl and polyfluoroalkyl substances leads to immunotoxicity: epidemiological and toxicological evidence. J Expo Sci Environ Epidemiol. 2019 Mar;29(2):148-156. doi: 10.1038/s41370-018-0097-y[
]
- Pachkowski B, Post GB, Stern AH. The derivation of a Reference Dose (RfD) for perfluorooctane sulfonate (PFOS) based on immune suppression. Environ Res. 2019 Apr;171:452-469. doi: 10.1016/j.envres.2018.08.004[
]
- Klaunig JE, Shinohara M, Iwai H, Chengelis CP, Kirkpatrick JB, Wang Z, Bruner RH. Evaluation of the chronic toxicity and carcinogenicity of perfluorohexanoic acid (PFHxA) in Sprague-Dawley rats. Toxicol Pathol. 2015 Feb;43(2):209-20. doi: 10.1177/0192623314530532[
]
- Gilliland FD, Mandel JS. Mortality among employees of a perfluorooctanoic acid production plant. J Occup Med. 1993 Sep;35(9):950-4. doi: 10.1097/00043764-199309000-00020[
]
- Raleigh KK, Alexander BH, Olsen GW, Ramachandran G, Morey SZ, Church TR, Logan PW, Scott LL, Allen EM. Mortality and cancer incidence in ammonium perfluorooctanoate production workers. Occup Environ Med. 2014 Jul;71(7):500-6. doi: 10.1136/oemed-2014-102109[
]
- Eriksen KT, Sørensen M, McLaughlin JK, Lipworth L, Tjønneland A, Overvad K, Raaschou-Nielsen O. Perfluorooctanoate and perfluorooctanesulfonate plasma levels and risk of cancer in the general Danish population. J Natl Cancer Inst. 2009 Apr 15;101(8):605-9. doi: 10.1093/jnci/djp041[
]
- Rashid F, Ramakrishnan A, Fields C, Irudayaraj J. Acute PFOA exposure promotes epigenomic alterations in mouse kidney tissues. Toxicol Rep. 2020 Jan 2;7:125-132. doi: 10.1016/j.toxrep.2019.12.010[
]
- Sakuma A, Wasada Ochi H, Yoshioka M, Yamanaka N, Ikezawa M, Guruge KS. Changes in hepato-renal gene expression in microminipigs following a single exposure to a mixture of perfluoroalkyl acids. PLoS One. 2019 Jan 4;14(1):e0210110. doi: 10.1371/journal.pone.0210110[
]
- Stanifer JW, Stapleton HM, Souma T, Wittmer A, Zhao X, Boulware LE. Perfluorinated Chemicals as Emerging Environmental Threats to Kidney Health: A Scoping Review. Clin J Am Soc Nephrol. 2018 Oct 8;13(10):1479-1492. doi: 10.2215/CJN.04670418[
][
][
]
- Wen LL, Lin CY, Chou HC, Chang CC, Lo HY, Juan SH. Perfluorooctanesulfonate Mediates Renal Tubular Cell Apoptosis through PPARgamma Inactivation. PLoS One. 2016 May 12;11(5):e0155190. doi: 10.1371/journal.pone.0155190[
]
- Shankar A, Xiao J, Ducatman A. Perfluoroalkyl chemicals and chronic kidney disease in US adults. Am J Epidemiol. 2011 Oct 15;174(8):893-900. doi: 10.1093/aje/kwr171[
]
- Watkins DJ, Josson J, Elston B, Bartell SM, Shin HM, Vieira VM, Savitz DA, Fletcher T, Wellenius GA. Exposure to perfluoroalkyl acids and markers of kidney function among children and adolescents living near a chemical plant. Environ Health Perspect. 2013 May;121(5):625-30. doi: 10.1289/ehp.1205838[
][
]
- Kataria A, Trachtman H, Malaga-Dieguez L, Trasande L. Association between perfluoroalkyl acids and kidney function in a cross-sectional study of adolescents. Environ Health. 2015 Nov 21;14:89. doi: 10.1186/s12940-015-0077-9[
][
]
- Blake BE, Pinney SM, Hines EP, Fenton SE, Ferguson KK. Associations between longitudinal serum perfluoroalkyl substance (PFAS) levels and measures of thyroid hormone, kidney function, and body mass index in the Fernald Community Cohort. Environ Pollut. 2018 Nov;242(Pt A):894-904. doi: 10.1016/j.envpol.2018.07.042[
][
]
- Dhingra R, Winquist A, Darrow LA, Klein M, Steenland K. A Study of Reverse Causation: Examining the Associations of Perfluorooctanoic Acid Serum Levels with Two Outcomes. Environ Health Perspect. 2017 Mar;125(3):416-421. doi: 10.1289/EHP273[
][
]
- Ferrari F, Orlando A, Ricci Z, Ronco C. Persistent pollutants: focus on perfluorinated compounds and kidney. Curr Opin Crit Care. 2019 Dec;25(6):539-549. doi: 10.1097/MCC.0000000000000658[
]
- Jain RB, Ducatman A. Perfluoroalkyl substances follow inverted U-shaped distributions across various stages of glomerular function: Implications for future research. Environ Res. 2019 Feb;169:476-482. doi: 10.1016/j.envres.2018.11.033[
][
]
- Zhao J, Hinton P, Chen J, Jiang J. Causal inference for the effect of environmental chemicals on chronic kidney disease. Comput Struct Biotechnol J. 2019 Dec 17;18:93-99. doi: 10.1016/j.csbj.2019.12.001[
]
- Obermayr RP, Temml C, Gutjahr G, Knechtelsdorfer M, Oberbauer R, Klauser-Braun R. Elevated uric acid increases the risk for kidney disease. J Am Soc Nephrol. 2008 Dec;19(12):2407-13. doi: 10.1681/ASN.2008010080[
]
- Steenland K, Tinker S, Shankar A, Ducatman A. Association of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) with uric acid among adults with elevated community exposure to PFOA. Environ Health Perspect. 2010 Feb;118(2):229-33. doi: 10.1289/ehp.0900940[
][
]
- Qin XD, Qian Z, Vaughn MG, Huang J, Ward P, Zeng XW, Zhou Y, Zhu Y, Yuan P, Li M, Bai Z, Paul G, Hao YT, Chen W, Chen PC, Dong GH, Lee YL. Positive associations of serum perfluoroalkyl substances with uric acid and hyperuricemia in children from Taiwan. Environ Pollut. 2016 May;212:519-524. doi: 10.1016/j.envpol.2016.02.050[
]
- Zeng XW, Lodge CJ, Dharmage SC, Bloom MS, Yu Y, Yang M, Chu C, Li QQ, Hu LW, Liu KK, Yang BY, Dong GH. Isomers of per- and polyfluoroalkyl substances and uric acid in adults: Isomers of C8 Health Project in China. Environ Int. 2019 Dec;133(Pt A):105160. doi: 10.1016/j.envint.2019.105160[
][
][
]
- Jain RB, Ducatman A. Perfluoroalkyl acids serum concentrations and their relationship to biomarkers of renal failure: Serum and urine albumin, creatinine, and albumin creatinine ratios across the spectrum of glomerular function among US adults. Environ Res. 2019 Jul;174:143-151. doi: 10.1016/j.envres.2019.04.034[
]
- Jain RB. Variabilities in concentrations of selected perfluoroalkyl acids among normotensives and hypertensives across various stages of glomerular function. Arch Environ Occup Health. 2021;76(1):12-22. doi: 10.1080/19338244.2020.1732856[
]
- Jain RB, Ducatman A. Dynamics of associations between perfluoroalkyl substances and uric acid across the various stages of glomerular function. Environ Sci Pollut Res Int. 2019 Apr;26(12):12425-12434. doi: 10.1007/s11356-019-04666-5[
]
- Cheng W, Ng CA. A Permeability-Limited Physiologically Based Pharmacokinetic (PBPK) Model for Perfluorooctanoic acid (PFOA) in Male Rats. Environ Sci Technol. 2017 Sep 5;51(17):9930-9939. doi: 10.1021/acs.est.7b02602[
]
- Gandaglia G, Ravi P, Abdollah F, Abd-El-Barr AE, Becker A, Popa I, Briganti A, Karakiewicz PI, Trinh QD, Jewett MA, Sun M. Contemporary incidence and mortality rates of kidney cancer in the United States. Can Urol Assoc J. 2014 Jul;8(7-8):247-52. doi: 10.5489/cuaj.1760[
]
- Steenland K, Woskie S. Cohort mortality study of workers exposed to perfluorooctanoic acid. Am J Epidemiol. 2012 Nov 15;176(10):909-17. doi: 10.1093/aje/kws171[
]
- Mastrantonio M, Bai E, Uccelli R, Cordiano V, Screpanti A, Crosignani P. Drinking water contamination from perfluoroalkyl substances (PFAS): an ecological mortality study in the Veneto Region, Italy. Eur J Public Health. 2018 Feb 1;28(1):180-185. doi: 10.1093/eurpub/ckx066. Erratum in: Eur J Public Health. 2023 Aug 14[
]
- C8 Science Panel. 2012. Probable Link Evaluation of Thyroid disease. http://www.c8sciencepanel.org/pdfs/Probable_Link_C8_Thyroid_30Jul2012.pdf[
]
- Coperchini F, Awwad O, Rotondi M, Santini F, Imbriani M, Chiovato L. Thyroid disruption by perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA). J Endocrinol Invest. 2017 Feb;40(2):105-121. doi: 10.1007/s40618-016-0572-z[
]
- Rappazzo KM, Coffman E, Hines EP. Exposure to Perfluorinated Alkyl Substances and Health Outcomes in Children: A Systematic Review of the Epidemiologic Literature. Int J Environ Res Public Health. 2017 Jun 27;14(7):691. doi: 10.3390/ijerph14070691[
]
- Lee JE, Choi K. Perfluoroalkyl substances exposure and thyroid hormones in humans: epidemiological observations and implications. Ann Pediatr Endocrinol Metab. 2017 Mar;22(1):6-14. doi: 10.6065/apem.2017.22.1.6[
]
- Tadic M, Cuspidi C, Vasic D, Kerkhof PLM. Cardiovascular Implications of Diabetes, Metabolic Syndrome, Thyroid Disease, and Cardio-Oncology in Women. Adv Exp Med Biol. 2018;1065:471-488. doi: 10.1007/978-3-319-77932-4_29[
]
- Melzer D, Rice N, Depledge MH, Henley WE, Galloway TS. Association between serum perfluorooctanoic acid (PFOA) and thyroid disease in the U.S. National Health and Nutrition Examination Survey. Environ Health Perspect. 2010 May;118(5):686-92. doi: 10.1289/ehp.0901584[
]
- Winquist A, Steenland K. Perfluorooctanoic acid exposure and thyroid disease in community and worker cohorts. Epidemiology. 2014 Mar;25(2):255-64. doi: 10.1097/EDE.0000000000000040[
]
- Lopez-Espinosa MJ, Mondal D, Armstrong B, Bloom MS, Fletcher T. Thyroid function and perfluoroalkyl acids in children living near a chemical plant. Environ Health Perspect. 2012 Jul;120(7):1036-41. doi: 10.1289/ehp.1104370[
]
- Andersson EM, Scott K, Xu Y, Li Y, Olsson DS, Fletcher T, Jakobsson K. High exposure to perfluorinated compounds in drinking water and thyroid disease. A cohort study from Ronneby, Sweden. Environ Res. 2019 Sep;176:108540. doi: 10.1016/j.envres.2019.108540[
][
]
- Berg V, Nøst TH, Hansen S, Elverland A, Veyhe AS, Jorde R, Odland JØ, Sandanger TM. Assessing the relationship between perfluoroalkyl substances, thyroid hormones and binding proteins in pregnant women; a longitudinal mixed effects approach. Environ Int. 2015 Apr;77:63-9. doi: 10.1016/j.envint.2015.01.007[
]
- Ren XM, Qin WP, Cao LY, Zhang J, Yang Y, Wan B, Guo LH. Binding interactions of perfluoroalkyl substances with thyroid hormone transport proteins and potential toxicological implications. Toxicology. 2016 Jul 29;366-367:32-42. doi: 10.1016/j.tox.2016.08.011[
]
- Zhang J, Begum A, Brännström K, Grundström C, Iakovleva I, Olofsson A, Sauer-Eriksson AE, Andersson PL. Structure-Based Virtual Screening Protocol for in Silico Identification of Potential Thyroid Disrupting Chemicals Targeting Transthyretin. Environ Sci Technol. 2016 Nov 1;50(21):11984-11993. doi: 10.1021/acs.est.6b02771[
]
- Song M, Kim YJ, Park YK, Ryu JC. Changes in thyroid peroxidase activity in response to various chemicals. J Environ Monit. 2012 Aug;14(8):2121-6. doi: 10.1039/c2em30106g[
]
- Webster GM, Rauch SA, Marie NS, Mattman A, Lanphear BP, Venners SA. Cross-Sectional Associations of Serum Perfluoroalkyl Acids and Thyroid Hormones in U.S. Adults: Variation According to TPOAb and Iodine Status (NHANES 2007-2008). Environ Health Perspect. 2016 Jul;124(7):935-42. doi: 10.1289/ehp.1409589[
]
- Kim DH, Kim UJ, Kim HY, Choi SD, Oh JE. Perfluoroalkyl substances in serum from South Korean infants with congenital hypothyroidism and healthy infants–Its relationship with thyroid hormones. Environ Res. 2016 May;147:399-404. doi: 10.1016/j.envres.2016.02.037[
][
]
- Forhead AJ, Fowden AL. Thyroid hormones in fetal growth and prepartum maturation. J Endocrinol. 2014 Jun;221(3):R87-R103. doi: 10.1530/JOE-14-0025[
]
- Ballesteros V, Costa O, Iñiguez C, Fletcher T, Ballester F, Lopez-Espinosa MJ. Exposure to perfluoroalkyl substances and thyroid function in pregnant women and children: A systematic review of epidemiologic studies. Environ Int. 2017 Feb;99:15-28. doi: 10.1016/j.envint.2016.10.015[
]
- Inoue K, Ritz B, Andersen SL, Ramlau-Hansen CH, Høyer BB, Bech BH, Henriksen TB, Bonefeld-Jørgensen EC, Olsen J, Liew Z. Perfluoroalkyl Substances and Maternal Thyroid Hormones in Early Pregnancy; Findings in the Danish National Birth Cohort. Environ Health Perspect. 2019 Nov;127(11):117002. doi: 10.1289/EHP5482[
]
- Šabović I, Cosci I, De Toni L, Ferramosca A, Stornaiuolo M, Di Nisio A, Dall’Acqua S, Garolla A, Foresta C. Perfluoro-octanoic acid impairs sperm motility through the alteration of plasma membrane. J Endocrinol Invest. 2020 May;43(5):641-652. doi: 10.1007/s40618-019-01152-0[
]
- Yuan Y, Ding X, Cheng Y, Kang H, Luo T, Zhang X, Kuang H, Chen Y, Zeng X, Zhang D. PFOA evokes extracellular Ca2+ influx and compromises progesterone-induced response in human sperm. Chemosphere. 2020 Feb;241:125074. doi: 10.1016/j.chemosphere.2019.125074[
]
- Joensen UN, Bossi R, Leffers H, Jensen AA, Skakkebaek NE, Jørgensen N. Do perfluoroalkyl compounds impair human semen quality? Environ Health Perspect. 2009 Jun;117(6):923-7. doi: 10.1289/ehp.0800517[
]
- Vested A, Ramlau-Hansen CH, Olsen SF, Bonde JP, Kristensen SL, Halldorsson TI, Becher G, Haug LS, Ernst EH, Toft G. Associations of in utero exposure to perfluorinated alkyl acids with human semen quality and reproductive hormones in adult men. Environ Health Perspect. 2013 Apr;121(4):453-8. doi: 10.1289/ehp.1205118[
]
- Song X, Tang S, Zhu H, Chen Z, Zang Z, Zhang Y, Niu X, Wang X, Yin H, Zeng F, He C. Biomonitoring PFAAs in blood and semen samples: Investigation of a potential link between PFAAs exposure and semen mobility in China. Environ Int. 2018 Apr;113:50-54. doi: 10.1016/j.envint.2018.01.010[
]
- Louis GM, Chen Z, Schisterman EF, Kim S, Sweeney AM, Sundaram R, Lynch CD, Gore-Langton RE, Barr DB. Perfluorochemicals and human semen quality: the LIFE study. Environ Health Perspect. 2015 Jan;123(1):57-63. doi: 10.1289/ehp.1307621[
]
- Pan Y, Cui Q, Wang J, Sheng N, Jing J, Yao B, Dai J. Profiles of Emerging and Legacy Per-/Polyfluoroalkyl Substances in Matched Serum and Semen Samples: New Implications for Human Semen Quality. Environ Health Perspect. 2019 Dec;127(12):127005. doi: 10.1289/EHP4431[
]
- Kang Q, Gao F, Zhang X, Wang L, Liu J, Fu M, Zhang S, Wan Y, Shen H, Hu J. Nontargeted identification of per- and polyfluoroalkyl substances in human follicular fluid and their blood-follicle transfer. Environ Int. 2020 Jun;139:105686. doi: 10.1016/j.envint.2020.105686[
]
- Di Nisio A, Rocca MS, Sabovic I, De Rocco Ponce M, Corsini C, Guidolin D, Zanon C, Acquasaliente L, Carosso AR, De Toni L, Foresta C. Perfluorooctanoic acid alters progesterone activity in human endometrial cells and induces reproductive alterations in young women. Chemosphere. 2020 Mar;242:125208. doi: 10.1016/j.chemosphere.2019.125208[
]
- Lum KJ, Sundaram R, Barr DB, Louis TA, Buck Louis GM. Perfluoroalkyl Chemicals, Menstrual Cycle Length, and Fecundity: Findings from a Prospective Pregnancy Study. Epidemiology. 2017 Jan;28(1):90-98. doi: 10.1097/EDE.0000000000000552[
]
- Louis GM, Peterson CM, Chen Z, Hediger ML, Croughan MS, Sundaram R, Stanford JB, Fujimoto VY, Varner MW, Giudice LC, Kennedy A, Sun L, Wu Q, Kannan K. Perfluorochemicals and endometriosis: the ENDO study. Epidemiology. 2012 Nov;23(6):799-805. doi: 10.1097/EDE.0b013e31826cc0cf[
]
- Campbell S, Raza M, Pollack AZ. Perfluoroalkyl substances and endometriosis in US women in NHANES 2003-2006. Reprod Toxicol. 2016 Oct;65:230-235. doi: 10.1016/j.reprotox.2016.08.009[
]
- Fei C, McLaughlin JK, Lipworth L, Olsen J. Maternal levels of perfluorinated chemicals and subfecundity. Hum Reprod. 2009 May;24(5):1200-5. doi: 10.1093/humrep/den490[
]
- Whitworth KW, Haug LS, Baird DD, Becher G, Hoppin JA, Skjaerven R, Thomsen C, Eggesbo M, Travlos G, Wilson R, Longnecker MP. Perfluorinated compounds and subfecundity in pregnant women. Epidemiology. 2012 Mar;23(2):257-63. doi: 10.1097/EDE.0b013e31823b5031[
]
- Bach CC, Liew Z, Bech BH, Nohr EA, Fei C, Bonefeld-Jorgensen EC, Henriksen TB, Olsen J. Perfluoroalkyl acids and time to pregnancy revisited: An update from the Danish National Birth Cohort. Environ Health. 2015 Jul 7;14:59. doi: 10.1186/s12940-015-0040-9[
]
- Vélez MP, Arbuckle TE, Fraser WD. Maternal exposure to perfluorinated chemicals and reduced fecundity: the MIREC study. Hum Reprod. 2015 Mar;30(3):701-9. doi: 10.1093/humrep/deu350[
]
- Waterfield G, Rogers M, Grandjean P, Auffhammer M, Sunding D. Reducing exposure to high levels of perfluorinated compounds in drinking water improves reproductive outcomes: evidence from an intervention in Minnesota. Environ Health. 2020 Apr 22;19(1):42. doi: 10.1186/s12940-020-00591-0[
]
- Gyllenhammar I, Benskin JP, Sandblom O, Berger U, Ahrens L, Lignell S, Wiberg K, Glynn A. Perfluoroalkyl Acids (PFAAs) in Serum from 2-4-Month-Old Infants: Influence of Maternal Serum Concentration, Gestational Age, Breast-Feeding, and Contaminated Drinking Water. Environ Sci Technol. 2018 Jun 19;52(12):7101-7110. doi: 10.1021/acs.est.8b00770[
]
- VanNoy BN, Lam J, Zota AR. Breastfeeding as a Predictor of Serum Concentrations of Per- and Polyfluorinated Alkyl Substances in Reproductive-Aged Women and Young Children: A Rapid Systematic Review. Curr Environ Health Rep. 2018 Jun;5(2):213-224. doi: 10.1007/s40572-018-0194-z[
]
- Fromme H, Mosch C, Morovitz M, Alba-Alejandre I, Boehmer S, Kiranoglu M, Faber F, Hannibal I, Genzel-Boroviczény O, Koletzko B, Völkel W. Pre- and postnatal exposure to perfluorinated compounds (PFCs). Environ Sci Technol. 2010 Sep 15;44(18):7123-9. doi: 10.1021/es101184f[
]
- Papadopoulou E, Sabaredzovic A, Namork E, Nygaard UC, Granum B, Haug LS. Exposure of Norwegian toddlers to perfluoroalkyl substances (PFAS): The association with breastfeeding and maternal PFAS concentrations. Environ Int. 2016 Sep;94:687-694. doi: 10.1016/j.envint.2016.07.006[
]
- Eryasa B, Grandjean P, Nielsen F, Valvi D, Zmirou-Navier D, Sunderland E, Weihe P, Oulhote Y. Physico-chemical properties and gestational diabetes predict transplacental transfer and partitioning of perfluoroalkyl substances. Environ Int. 2019 Sep;130:104874. doi: 10.1016/j.envint.2019.05.068[
]
- Romano ME, Xu Y, Calafat AM, Yolton K, Chen A, Webster GM, Eliot MN, Howard CR, Lanphear BP, Braun JM. Maternal serum perfluoroalkyl substances during pregnancy and duration of breastfeeding. Environ Res. 2016 Aug;149:239-246. doi: 10.1016/j.envres.2016.04.034[
]
- Timmermann CAG, Budtz-Jørgensen E, Petersen MS, Weihe P, Steuerwald U, Nielsen F, Jensen TK, Grandjean P. Shorter duration of breastfeeding at elevated exposures to perfluoroalkyl substances. Reprod Toxicol. 2017 Mar;68:164-170. doi: 10.1016/j.reprotox.2016.07.010[
]
- Rosen EM, Brantsæter AL, Carroll R, Haug L, Singer AB, Zhao S, Ferguson KK. Maternal Plasma Concentrations of Per- and polyfluoroalkyl Substances and Breastfeeding Duration in the Norwegian Mother and Child Cohort. Environ Epidemiol. 2018 Sep;2(3):e027. doi: 10.1097/EE9.0000000000000027[
]
- Lam J, Koustas E, Sutton P, Johnson PI, Atchley DS, Sen S, Robinson KA, Axelrad DA, Woodruff TJ. The Navigation Guide – evidence-based medicine meets environmental health: integration of animal and human evidence for PFOA effects on fetal growth. Environ Health Perspect. 2014 Oct;122(10):1040-51. doi: 10.1289/ehp.1307923[
][
]
- Johnson PI, Sutton P, Atchley DS, Koustas E, Lam J, Sen S, Robinson KA, Axelrad DA, Woodruff TJ. The Navigation Guide – evidence-based medicine meets environmental health: systematic review of human evidence for PFOA effects on fetal growth. Environ Health Perspect. 2014 Oct;122(10):1028-39. doi: 10.1289/ehp.1307893[
][
]
- Koustas E, Lam J, Sutton P, Johnson PI, Atchley DS, Sen S, Robinson KA, Axelrad DA, Woodruff TJ. The Navigation Guide – evidence-based medicine meets environmental health: systematic review of nonhuman evidence for PFOA effects on fetal growth. Environ Health Perspect. 2014 Oct;122(10):1015-27. doi: 10.1289/ehp.1307177[
][
]
- Steenland K, Barry V, Savitz D. Serum Perfluorooctanoic Acid and Birthweight: An Updated Meta-analysis With Bias Analysis. Epidemiology. 2018 Nov;29(6):765-776. doi: 10.1097/EDE.0000000000000903[
]
- Interstate Technology and Regulatory Council. 2020. Chemistry, terminology and acronyms. https://pfas-1.itrcweb.org/2-2-chemistry-terminology-and-acronyms[
]
- Wikström S, Lin PI, Lindh CH, Shu H, Bornehag CG. Maternal serum levels of perfluoroalkyl substances in early pregnancy and offspring birth weight. Pediatr Res. 2020 May;87(6):1093-1099. doi: 10.1038/s41390-019-0720-1[
]
- Xiao C, Grandjean P, Valvi D, Nielsen F, Jensen TK, Weihe P, Oulhote Y. Associations of Exposure to Perfluoroalkyl Substances With Thyroid Hormone Concentrations and Birth Size. J Clin Endocrinol Metab. 2020 Mar 1;105(3):735–45. doi: 10.1210/clinem/dgz147[
]
- Hall JM, Greco CW. Perturbation of Nuclear Hormone Receptors by Endocrine Disrupting Chemicals: Mechanisms and Pathological Consequences of Exposure. Cells. 2019 Dec 19;9(1):13. doi: 10.3390/cells9010013[
]
- Halldorsson TI, Rytter D, Haug LS, Bech BH, Danielsen I, Becher G, Henriksen TB, Olsen SF. Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: a prospective cohort study. Environ Health Perspect. 2012 May;120(5):668-73. doi: 10.1289/ehp.1104034[
]
- Barry V, Darrow LA, Klein M, Winquist A, Steenland K. Early life perfluorooctanoic acid (PFOA) exposure and overweight and obesity risk in adulthood in a community with elevated exposure. Environ Res. 2014 Jul;132:62-9. doi: 10.1016/j.envres.2014.03.025[
]
- Martinsson M, Nielsen C, Björk J, Rylander L, Malmqvist E, Lindh C, Rignell-Hydbom A. Intrauterine exposure to perfluorinated compounds and overweight at age 4: A case-control study. PLoS One. 2020 Mar 16;15(3):e0230137. doi: 10.1371/journal.pone.0230137[
]
- Avanasi R, Shin HM, Vieira VM, Savitz DA, Bartell SM. Impact of Exposure Uncertainty on the Association between Perfluorooctanoate and Preeclampsia in the C8 Health Project Population. Environ Health Perspect. 2016 Jan;124(1):126-32. doi: 10.1289/ehp.1409044[
]
- Avanasi R, Shin HM, Vieira VM, Bartell SM. Variability and epistemic uncertainty in water ingestion rates and pharmacokinetic parameters, and impact on the association between perfluorooctanoate and preeclampsia in the C8 Health Project population. Environ Res. 2016 Apr;146:299-307. doi: 10.1016/j.envres.2016.01.011[
]
- Darrow LA, Stein CR, Steenland K. Serum perfluorooctanoic acid and perfluorooctane sulfonate concentrations in relation to birth outcomes in the Mid-Ohio Valley, 2005-2010. Environ Health Perspect. 2013 Oct;121(10):1207-13. doi: 10.1289/ehp.1206372[
]
- Savitz DA, Stein CR, Bartell SM, Elston B, Gong J, Shin HM, Wellenius GA. Perfluorooctanoic acid exposure and pregnancy outcome in a highly exposed community. Epidemiology. 2012 May;23(3):386-92. doi: 10.1097/EDE.0b013e31824cb93b[
]
- Stein CR, Savitz DA, Dougan M. Serum levels of perfluorooctanoic acid and perfluorooctane sulfonate and pregnancy outcome. Am J Epidemiol. 2009 Oct 1;170(7):837-46. doi: 10.1093/aje/kwp212[
]
- Huang R, Chen Q, Zhang L, Luo K, Chen L, Zhao S, Feng L, Zhang J. Prenatal exposure to perfluoroalkyl and polyfluoroalkyl substances and the risk of hypertensive disorders of pregnancy. Environ Health. 2019 Jan 9;18(1):5. doi: 10.1186/s12940-018-0445-3[
]
- Wikström S, Lindh CH, Shu H, Bornehag CG. Early pregnancy serum levels of perfluoroalkyl substances and risk of preeclampsia in Swedish women. Sci Rep. 2019 Jun 24;9(1):9179. doi: 10.1038/s41598-019-45483-7[
]
- Borghese MM, Walker M, Helewa ME, Fraser WD, Arbuckle TE. Association of perfluoroalkyl substances with gestational hypertension and preeclampsia in the MIREC study. Environ Int. 2020 Aug;141:105789. doi: 10.1016/j.envint.2020.105789[
]
- Szilagyi JT, Freedman AN, Kepper SL, Keshava AM, Bangma JT, Fry RC. Per- and Polyfluoroalkyl Substances Differentially Inhibit Placental Trophoblast Migration and Invasion In Vitro. Toxicol Sci. 2020 Jun 1;175(2):210-219. doi: 10.1093/toxsci/kfaa043[
]
- Blake BE, Cope HA, Hall SM, Keys RD, Mahler BW, McCord J, Scott B, Stapleton HM, Strynar MJ, Elmore SA, Fenton SE. Evaluation of Maternal, Embryo, and Placental Effects in CD-1 Mice following Gestational Exposure to Perfluorooctanoic Acid (PFOA) or Hexafluoropropylene Oxide Dimer Acid (HFPO-DA or GenX). Environ Health Perspect. 2020 Feb;128(2):27006. doi: 10.1289/EHP6233[
]
- Huo X, Huang R, Gan Y, Luo K, Aimuzi R, Nian M, Ao J, Feng L, Tian Y, Wang W, Ye W, Zhang J; Shanghai Birth Cohort. Perfluoroalkyl substances in early pregnancy and risk of hypertensive disorders of pregnancy: A prospective cohort study. Environ Int. 2020 May;138:105656. doi: 10.1016/j.envint.2020.105656[
]
- Di Nisio A, De Rocco Ponce M, Giadone A, Rocca MS, Guidolin D, Foresta C. Perfluoroalkyl substances and bone health in young men: a pilot study. Endocrine. 2020 Mar;67(3):678-684. doi: 10.1007/s12020-019-02096-4[
]
- Hu Y, Liu G, Rood J, Liang L, Bray GA, de Jonge L, Coull B, Furtado JD, Qi L, Grandjean P, Sun Q. Perfluoroalkyl substances and changes in bone mineral density: A prospective analysis in the POUNDS-LOST study. Environ Res. 2019 Dec;179(Pt A):108775. doi: 10.1016/j.envres.2019.108775[
]
- Lin LY, Wen LL, Su TC, Chen PC, Lin CY. Negative association between serum perfluorooctane sulfate concentration and bone mineral density in US premenopausal women: NHANES, 2005-2008. J Clin Endocrinol Metab. 2014 Jun;99(6):2173-80. doi: 10.1210/jc.2013-3409[
]
- Cluett R, Seshasayee SM, Rokoff LB, Rifas-Shiman SL, Ye X, Calafat AM, Gold DR, Coull B, Gordon CM, Rosen CJ, Oken E, Sagiv SK, Fleisch AF. Per- and Polyfluoroalkyl Substance Plasma Concentrations and Bone Mineral Density in Midchildhood: A Cross-Sectional Study (Project Viva, United States). Environ Health Perspect. 2019 Aug;127(8):87006. doi: 10.1289/EHP4918[
]
- Cheng L, Albers P, Berney DM, Feldman DR, Daugaard G, Gilligan T, Looijenga LHJ. Testicular cancer. Nat Rev Dis Primers. 2018 Oct 5;4(1):29. doi: 10.1038/s41572-018-0029-0[
]
- Park JS, Kim J, Elghiaty A, Ham WS. Recent global trends in testicular cancer incidence and mortality. Medicine (Baltimore). 2018 Sep;97(37):e12390. doi: 10.1097/MD.0000000000012390[
]