Pseudoexfoliation syndrome
Pseudoexfoliation syndrome also known as Viking disease, is a complex and age-related systemic disorder characterized by the progressive accumulation and granular deposition of pseudoexfoliative material (PXM) in various intraocular and extraocular tissues 1. Pseudoexfoliative material is composed of amyloid, laminin, collagen, elastic fibers, and basement membrane, and the same material seen in ocular tissue has been shown in other parts of the body, such as the heart, liver, kidneys, lungs, cerebral meninges, vessel walls, and skin, indicating pseudoexfoliation syndrome is a diffuse disease with ocular and systemic manifestations 2. There is a higher prevalence of open angle glaucoma in about 50% of these patients 3. The diagnosis of pseudoexfoliation syndrome is so important because it is a major risk factor for complications during cataract surgery and the most frequent cause of secondary glaucoma. In addition to ocular complications, pseudoexfoliation syndrome is related with numerous systemic abnormalities, for which the list is growing steadily.
Pseudoexfoliation syndrome principally affects northern Europeans and especially Scandinavians, although it has been reported in all population types and races 4. In all populations, the prevalence of pseudoexfoliation syndrome increases markedly with aging 4. Occurrence is negligible in the middle-aged population (49–54 years), but it increases to 5% in Americans aged 75–85 years and to 6.25% for elderly Australian subjects aged 85 years or older 5.
Even though the exact cause and pathogenesis of pseudoexfoliation syndrome are not fully understood, scientists know that multiple factors play roles in its pathogenesis. Geographic and environmental factors together with genetic predisposition may explain the different prevalence of pseudoexfoliation syndrome worldwide. The pathological process in intraocular and extraocular tissues is characterized by the chronic and progressive accumulation of pseudoexfoliative material, which is either the result of excessive production and/or insufficient breakdown, and is regarded as pathognomonic of pseudoexfoliation syndrome, based on its unique light-microscopy and ultrastructural criteria 6. This is supported by common gene variants in LOX1 at locus 15q22 coding a pivotal enzyme that serves as both a cross-linking enzyme and a scaffolding element, which ensures spatially defined deposition of elastin 7. LOX1 also regulates the prompter of elastin 8. Therefore, it has been proposed that pseudoexfoliation syndrome is a kind of elastosis that results from the overproduction of elastic microfibrillar components, such as fibrillin 9.
In patients with pseudoexfoliation syndrome, almost all tissue of the anterior segment of the eye is involved, with important implications for patient management. Characteristic alterations in the anterior ocular segment may predispose the eye to a broad spectrum of intraocular complications, such as increased intraocular pressure (IOP), cataract formation, zonular instability, phacodonesis, blood–aqueous barrier dysfunction, melanin dispersion, posterior synechiae, and keratopathy 10. Also, these pathological alterations may explain the markedly increased intraoperative and postoperative complications in patients with pseudoexfoliation syndrome who undergo intraocular surgery. These ocular complications include posterior capsular rupture and vitreous loss, zonular dehiscence, intraocular hemorrhage, corneal edema and decompensation, severe postoperative inflammation, IOP spikes, synechiae, capsular phimosis, secondary cataract, and intraocular lens (IOL) subluxation 11.
Table 1. Possible clinical and surgical complications in patients with pseudoexfoliation syndrome
| Tissue involvement | Clinical complications | Surgical complications |
|---|---|---|
| Lens, ciliary body, zonules | Cataract Zonular instability Phacodonesis Lens subluxation/dislocation Angle-closure glaucoma (due to pupillary/ciliary block) | Posterior capsular rupture Vitreous loss Zonular dialysis Intraocular lens decentration Capsular phimosis Secondary cataract |
| Iris | Iris rigidity Poor mydriasis Melanin dispersion Blood–aqueous barrier defect (pseudouveitis) Capillary hemorrhage Posterior synechiae | Miosis Poor surgical access Intra/postoperative hyphema Severe postoperative inflammation Posterior synechiae Pupillary block |
| Trabecular meshwork | Intraocular hypertension Open-angle glaucoma | Postoperative intraocular pressure elevation |
| Cornea | Endothelial decompensation Endothelial migration/proliferation | Endothelial decompensation |
| Posterior segment | Retinal vein occlusion |
Pseudoexfoliation syndrome causes
The cause of pseudoexfoliation syndrome is unknown, but there is a gentic link to the gene LOXL1. In March 2008 of the American Glaucoma Society in Washington, D.C. Dr. Allingham. described a LOXL1 is a member of a family of enzymes that are active in the cross-linking of collagen and elastin in the extracellular matrix, he explained. “Because pseudoexfoliation syndrome is associated with abnormalities of the extracellular matrix and the basement membrane, this gene could reasonably play a role in the pathophysiology of the condition.”. Pseudoexfoliation syndrome may a generalized disorder involving abnormal production or turnover of extracellular matrix in the basement membrane.
Risk factors for developing pseudoexfoliation syndrome
Risk factors for developing pseudoexfoliation syndrome:
- Advance age over 70
- Possible genetic prevalence
- Its prevalence in different human populations: high prevalence in Scandinavia.
Pseudoexfoliation syndrome prevention
- Routine annual eye exam by an ophthalmologist for the patients over ago 50.
- Biomicroscopic examination(Slit Lamp) used by an eye doctor to examine the anterior lens capsule, pupillary dilation, intraocular pressure and optic nerve.
- Treatment of elevated eye pressure with eye drops, laser or surgery.
- Special precaution and awareness is essential prior and during cataract surgery.
- Anterior chamber depth less than 2.5 mm centrally could be an indication of zonular instability
- Poor pupillary dilation
- Zonular dialysis
- Phacodonesis
All of the above may poses a significantly higher risk for intraoperative complications.
Pseudoexfoliation syndrome symptoms
No real symptom is associated with pseudoexfoliation syndrome, most of the finding are based on an eye exam. The intraocular eye pressure elevation may not have associated symptoms.
Signs
- Increased intraocular pressure
- Poor dilation with peripupillary transillumination defect
- Fibrillar white flaky deposits on the anterior lens capsule
- Deposits at pupillary border
Physical examination
Poor pupillary dilation, transillumination defect on the pupilary borders, white fluffy deposits on the anterior lens capsule.
Cataract
Pseudoexfoliation syndrome represents an independent additional hazard for the development of lens opacification and cataract progression, most commonly of a nuclear type 12. Moreover, cataract is the most common cause of patients with pseudoexfoliation syndrome requiring surgical intervention. The results of the Reykjavik Eye Study, which included 1,045 subjects who were followed up for 12 years, revealed that eyes with pseudoexfoliation syndrome at baseline were more likely to have cataract surgery during the 12 years 13. Another population-based 30-year follow-up study found that pseudoexfoliation syndrome was a strong predictor of cataract surgery, accounting for a 2.38-fold increased risk in multivariate analysis 14. Furthermore, it has been shown that lens-densitometry values of affected and even unaffected eyes of patients with clinically unilateral pseudoexfoliation syndrome are significantly higher than those of healthy eyes 15. However, the exact pathophysiology of the association between pseudoexfoliation syndrome and cataractogenesis is not clear yet. On the other hand, it has been emphasized that oxidative stress, ocular ischemia, aqueous hypoxia, increased growth-factor levels, and reduced protection against ultraviolet light by lower levels of ascorbic acid in aqueous humor can contribute to this association 16. Also, changes in the iris vasculature and blood–aqueous barrier in pseudoexfoliation syndrome may influence the composition of aqueous humor and subsequently may affect lens metabolism, resulting in cataract formation 17.
Pseudoexfoliation syndrome is not only associated with increased risk of cataractogenesis. It is also well known that patients with pseudoexfoliation syndrome are much more prone to higher risks of complications during and even after cataract extraction 4. These complications can occur from poor pupillary dilation, zonular fragility leading to intraoperative or postoperative IOL dislocation and vitreous loss, postoperative IOP spikes potentiating glaucomatous damage, capsular phimosis, prolonged and heightened intraocular inflammation, postoperative corneal decompensation, and secondary cataract 11. On the other hand, advances in techniques and instruments for cataract surgery have improved markedly, and operative management of cases with pseudoexfoliation syndrome and even overall outcomes for patients with pseudoexfoliation syndrome who undergo cataract surgery could be similar to those for non-pseudoexfoliation syndrome patients with the appropriate preoperative, intraoperative, and postoperative approaches 18.
Systemic symptoms
The detection of pseudoexfoliative material in visceral organs, such as lungs, liver, kidneys, and gallbladder, and cerebral meninges has led to the hypothesis that pseudoexfoliation syndrome might be associated with systemic comorbidities or comortality 19. While several studies have reported that cardiovascular and cerebrovascular diseases, aortic aneurysms, and dementia are strongly associated with pseudoexfoliation syndrome 20, others have not supported this association 21. Until now, most studies addressing vascular dysfunction in pseudoexfoliation syndrome have been limited by several weaknesses: studies were frequently isolated; retrospective investigations conducted with a variety of methods on small patient populations from different geographic areas. Furthermore, systemic diseases associated with pseudoexfoliation syndrome are not specific to pseudoexfoliation syndrome. Therefore, their increased frequency in pseudoexfoliation syndrome might potentially be associated with certain systemic biochemical changes that contribute to their clinical manifestations 22.
The underlying mechanisms between pseudoexfoliation syndrome and cardiovascular/cerebrovascular diseases are not completely understood, but several possible biological mechanisms have been proposed. The accumulation of pseudoexfoliative material in various tissues seen with aging is one of the suggested causal mechanisms 23. Pericellular accumulation of pseudoexfoliative material can disturb the normal structure of the basement membrane and lead to endothelial dysfunction 24. Other possible mechanisms are overexpression of the bFGF, an imbalance in matrix metal-loproteinases (MMPs) and tissue inhibitors of MMPs, and increased serum antiphospholipid-antibody level 25. Additionally, increased serum oxidative stress and elevated serum homocysteine levels may play a role in the development of systemic vascular diseases in cases with pseudoexfoliation syndrome 26. There is evidence that an excess level of homocysteine can induce neural cell death and degradation of the elastic structures in the arterial wall 27. It has also been shown that the aqueous humor endothelin 1 concentrations of pseudoexfoliation syndrome patients are significantly higher than that of age-matched controls 28. This process might, in due course, result in weakened elasticity and contractility of vascular wall muscles and increased vascular resistance. However, the exact mechanisms of action and other potential causative biochemical changes require further investigations to elucidate potential pathways of the effects of pseudoexfoliation syndrome on vascular disease. Such studies need to be population-based and need to address quantitative relationships among potential biochemical changes and the severity of the corresponding vascular disease or dysfunction. Without this information, it is not possible to give a recommendation for the timing and frequency of cardiovascular/cerebrovascular evaluation of patients with pseudoexfoliation syndrome.
Pseudoexfoliation syndrome complications
Pseudoexfoliation syndrome complications may include:
- Glaucoma or optic nerve cupping
- Cataract surgery complications:
- Drop nucleus or lens fragment
- Zonular dialysis
- Diffuse zonular weakness with or without phacodonesis
- Lens subluxation, either the natural lens or intraocular lens (acutely or delayed presentation)
- Other systemic problems
Pseudoexfoliation syndrome diagnosis
Pseudoexfoliation syndrome diagnosis is done by an eye exam using slit lamp and intraocular pressure exam. On regular routine exam an eye doctor could see a white fluffy material deposit on the anterior lens casule, and pupillary margin. Gonioscopy may show increase pigment deposit on the trabecular meshwork.
Diagnostic procedures
- Slit lamp exam
- Intraocular pressure check
- Pupil check and transillumination exam
- Dilated eye exam to check optic nerve
- Gonioscopy to check for trabecular meshwork hyperpigmentation and open angle
Laboratory test
- Genetic test for a single mutation in the LOXL1
- Check for homocysteine level in tear film and plasma. Scientists believe that elevated levels of plasma homocysteine are a risk factor for cardiovascular disease, and two studies have found higher levels of plasma and tear fluids homocysteine level in psuedoexfoliation patients.
A definitive diagnosis can be made only by observing pseudoexfoliative material on the anterior lens surface with a dilated pupil. On the other hand, the classic biomicroscopy illustration of “target-like” lens depositions represents a late stage of the disease that is preceded by a long, chronic, and preclinical course. As the precapsular layer of the lens becomes thicker, the focal defects begin in the mid-peripheral zone with abrasive movements of the iris, starting often in the upper nasal quadrant (mini-pseudoexfoliation syndrome), which further extend and become confluent with the classic biomicroscopy illustration of manifest pseudoexfoliation syndrome 6. Although the recognition of this delicate layer requires an experienced controller, there are some clinical hints that help alert the ophthalmologist to the early stages of pseudoexfoliation syndrome. The main anterior-segment structures that can be affected in pseudoexfoliation syndrome and the clinical findings observed in these structures are outlined in the following sections.
Lens
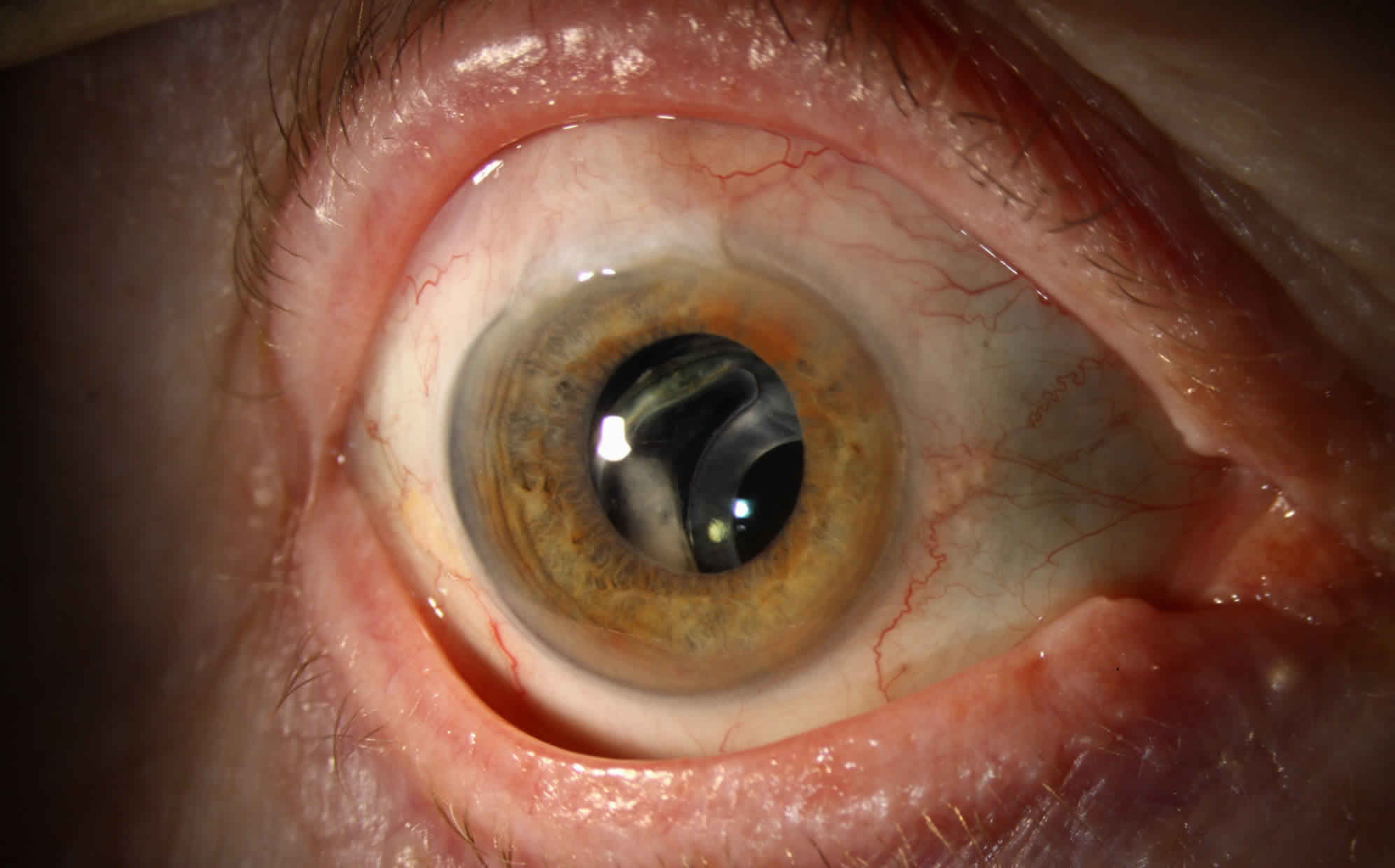
The deposition of whitish pseudoexfoliative material on the anterior lens surface is the most consistent and hallmark diagnostic feature of pseudoexfoliation syndrome. The classic pattern consists of three distinct zones of pseudoexfoliative material deposition that may become visible when the pupil is fully dilated: a relatively homogeneous central disk-shaped zone corresponding roughly to the pupil diameter, a granular, often layered, peripheral zone, and a clear intermediate zone 4. The clear intermediate zone results from the rubbing of the iris over the lens surface during pupillary movement.
Iris
Iris changes are an early and well-recognized clinical feature in pseudoexfoliation syndrome. Pseudoexfoliative material is most prominent at the pupillary border, next to the lens. The deposition of pseudoexfoliative material at the pupillary margin and on the iris sphincter with pigment loss in pupillary ruff is frequent and one of the hallmark signs of pseudoexfoliation syndrome 29. Loss of iris pigment and deposition throughout the anterior segment are reflected in iris sphincter–region transillumination, loss of the pupillary ruff, pigment dispersion in the anterior chamber after pupillary dilation, pigment accumulation, and increased trabecular meshwork pigmentation 30. Iris vascular abnormalities are also characteristics in pseudoexfoliation syndrome that are often narrowed and may become obliterated, and cells of vessel walls can become completely degenerated in advanced stages of pseudoexfoliation syndrome 4.
Cornea
Scattered flakes of pseudoexfoliative material can be present on the endothelial surface on the cornea. Pigment accumulation on the cornea may cause diffuse and aspecific pigmentation on the central endothelium, rarely having the pattern of a Krukenberg spindle. More frequently, pigment is deposited on Schwalbe’s line, with one or several undulating pigmented lines (Sampaolesi line) observed in the peripheral cornea anterior to Schwalbe’s line, and this is an early sign of pseudoexfoliation syndrome 29.
Other tissue
Pseudoexfoliative material can be detected earliest on the ciliary processes and zonules, which are often frayed and broken. The deposition of pseudoexfoliative material on the zonules leads to weakening of the zonules and increased incidence of zonular dialysis and spontaneous subluxation or dislocation of the lens in advanced cases with pseudoexfoliation syndrome.
The trabecular meshwork shows moderate–excessive pigmentation in pseudoexfoliation syndrome. Increased trabecular pigmentation is a prominent sign of pseudoexfoliation syndrome, and occurs in almost all cases with clinically evident disease 29. Furthermore, pseudoexfoliative material can be found on the vitreous face, on vitreous strands when the face is ruptured, on the posterior lens capsule, and on IOLs after cataract extraction.
Pseudoexfoliation syndrome treatment
Pseudoexfoliation syndrome management
- Routine regular eye exam
- Glaucoma treatment if the patient develops it
- Special surgical precaution for cataract surgery pre-operatively, intra-operatively and post-operatively
Medical therapy
- Drops if the patient has glaucoma
- Use of antioxidants
- Lower homocysteine if the level is high in plasma or tear film
Medical follow up
Routine annual eye screening exam with dilation, if the patient has glaucoma then he/she needs 3-4 time per year eye exam.
- Tekin K, Inanc M, Elgin U. Monitoring and management of the patient with pseudoexfoliation syndrome: current perspectives. Clin Ophthalmol. 2019;13:453–464. Published 2019 Mar 1. doi:10.2147/OPTH.S181444 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6402616[
]
- Ritch R. Systemic associations of exfoliation syndrome. Asia Pac J Ophthalmol. 2016;5(1):45–50.[
]
- Pseudoexfoliation Syndrome. https://eyewiki.org/Pseudoexfoliation_Syndrome[
]
- Ritch R, Schlötzer-Schrehardt U. Exfoliation syndrome. Surv Ophthalmol. 2001;45(4):265–315.[
][
][
][
][
]
- Kanthan GL, Mitchell P, Burlutsky G, Rochtchina E, Wang JJ. Pseudoexfoliation syndrome and the long-term incidence of cataract surgery: the Blue Mountains Eye study. Am J Ophthalmol. 2013;155(1):83–88.[
]
- Schlötzer-Schrehardt U, Naumann GOH. Ocular and systemic pseudoexfoliation syndrome. Am J Ophthalmol. 2006;141(5):921–937.[
][
]
- Oleggini R, Gastaldo N, di Donato A. Regulation of elastin promoter by lysyl oxidase and growth factors: cross control of lysyl oxidase on TGF-β1 effects. Matrix Biol. 2007;26(6):494–505.[
]
- Liu X, Zhao Y, Gao J, et al. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet. 2004;36(2):178–182.[
]
- Ritch R, Schlötzer-Schrehardt U. Exfoliation (pseudoexfoliation) syndrome: toward a new understanding. Proceedings of the first international think tank. Acta Ophthalmol Scand. 2001;79(2):213–217.[
]
- Conway RM, Schlötzer-Schrehardt U, Küchle M, Naumann GOH. Pseudoexfoliation syndrome: pathological manifestations of relevance to intraocular surgery. Clin Exp Ophthalmol. 2004;32(2):199–210.[
]
- Sangal N, Chen TC. Cataract surgery in pseudoexfoliation syndrome. Semin Ophthalmol. 2014;29(5–6):403–408.[
][
]
- Nazarali S, Damji F, Damji KF. What have we learned about exfoliation syndrome since its discovery by John Lindberg 100 years ago? Br J Ophthalmol. 2018;102(10):1342–1350.[
]
- Arnarsson A, Sasaki H, Jonasson F. Twelve-year incidence of exfoliation syndrome in the Reykjavik eye study. Acta Ophthalmol. 2013;91(2):157–162.[
]
- Ekström C, Botling Taube A. Pseudoexfoliation and cataract surgery: a population-based 30-year follow-up study. Acta Ophthalmol. 2015;93(8):774–777.[
]
- Durukan I. Evaluation of corneal and lens clarity in unilateral pseudo-exfoliation syndrome: a densitometric analysis. Clin Exp Optom. 2018;101(6):740–746.[
]
- Napora KJ, Obuchowska I, Mariak Z. The influence of pseudoexfoliation syndrome on cataract development. Klin Oczna. 2008;110(1–3):98–101.[
]
- Kanthan GL, Mitchell P, Burlutsky G, et al. Pseudoexfoliation syndrome and the long-term incidence of cataract surgery: the Blue Mountains Eye study. Am J Ophthalmol. 2013;155(1):83–88.[
]
- Shingleton BJ, Nguyen BK, Eagan EF, Nagao K, O’Donoghue MW. Outcomes of phacoemulsification in fellow eyes of patients with unilateral pseudoexfoliation: single-surgeon series. J Cataract Refract Surg. 2008;34(2):274–279.[
]
- Schlötzer-Schrehardt UM, Koca MR, Naumann GO, Volkholz H. Pseudoexfoliation syndrome. Ocular manifestation of a systemic disorder? Arch Ophthalmol. 1992;110(12):1752–1756.[
]
- Cumurcu T, Dorak F, Cumurcu BE, Erbay LG, Ozsoy E. Is there any relation between pseudoexfoliation syndrome and Alzheimer’s type dementia? Semin Ophthalmol. 2013;28(4):224–229.[
]
- Ekström C, Kilander L. Pseudoexfoliation and Alzheimer’s disease: a population-based 30-year follow-up study. Acta Ophthalmol. 2014;92(4):355–358.[
]
- Holló G. Vascular dysfunction in exfoliation syndrome. J Glaucoma. 2018;27(1):72–74.[
]
- Ulus T, Nadir A, Yaz YA, et al. Cardiovascular involvement in patients with pseudoexfoliation syndrome. J Cardiovasc Med. 2013;14(8):587–592.[
]
- Oltulu R, Satirtav G, Kayitmazbatir ET, Bitirgen G, Ozkagnici A, Karaibrahimoglu A. Characteristics of the cornea in patients with pseudoexfoliation syndrome. Arq Bras Oftalmol. 2015;78(6):348–351.[
]
- Wang W, He M, Zhou M, Zhang X. Ocular pseudoexfoliation syndrome and vascular disease: a systematic review and meta-analysis. PLoS One. 2014;9(3):e92767.[
]
- Cumurcu T, Gunduz A, Ozyurt H, Nurcin H, Atis O, Egri M. Increased oxidative stress in patients with pseudoexfoliation syndrome. Ophthalmic Res. 2010;43(4):169–172.[
]
- Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015;14(1):6.[
]
- Koliakos GG, Konstas AG, Schlötzer-Schrehardt U, et al. Endothelin-1 concentration is increased in the aqueous humour of patients with exfoliation syndrome. Br J Ophthalmol. 2004;88(4):523–527.[
]
- Ariga M, Nivean M, Utkarsha P. Pseudoexfoliation syndrome. J Curr Glaucoma Pract. 2013;7(3):118–120.[
][
][
]
- Prince AM, Ritch R. Clinical signs of the pseudoexfoliation syndrome. Ophthalmology. 1986;93(6):803–807.[
]