Retinal vasculitis
Retinal vasculitis can be an isolated condition or a complication of local or systemic inflammatory disorders characterized by inflammation of the retinal vessels 1. Retinal vasculitis is a sight-threatening condition associated with various infective, auto-immune, inflammatory or neoplastic disorders 1. Unfortunately, ophthalmologists and rheumatologists frequently miscommunicate in consulting on patients with retinal vasculitis 2. A rheumatologist classifies vasculitis on the basis of the size of the vessel affected, its location, and the associated histological changes 1. Unfortunately, ophthalmologists and rheumatologists frequently miscommunicate in consulting on patients with retinal vasculitis 2. Usually this classification requires a biopsy; occasionally the diagnosis is based on imaging such as an abdominal angiogram. A diagnosis of vasculits indicates documented or presumed damage of the vessel wall.
In contrast, an ophthalmologist diagnoses retinal vasculitis on the basis of perivascular infiltrates on a dilated eye examination and imaging usually in the form of a fluorescein angiogram 1. Unfortunately, ophthalmologists and rheumatologists frequently miscommunicate in consulting on patients with retinal vasculitis 2. Vasculitis can be suggested by a finding such as an intraretinal hemorrhage, which indicates an abnormality of a retinal vessel, or a cotton wool spot indicative of local retinal ischemia. A biopsy is a rarity due to the potential damage caused by a retinal biopsy. Angiography is consistent with retinal vasculitis if fluorescein stains the vessel wall or leaks beyond the vessel. This leakage demonstrates increased vascular permeability, which is a very different threshold for diagnosing vasculitis compared to vessel wall destruction on histopathology.
Retinal vasculitis can be classified into descriptive subsets 2. The location of the affected retinal vessels is a critical discriminator since a vasculitis affecting vessels near the macula often affects visual acuity while a vasculitis of peripheral retinal vessels can be asymptomatic 2. In addition, it can be classified into occlusive or non-occlusive forms. In the occlusive form, retinal ischemia can lead to neovascularization which predisposes to vitreous hemorrhage. Retinal vasculitis can also be categorized based on whether or not it is retinal arteriole- or venule-predominant (or involving both types of retinal vessels). This distinction can be helpful to determine its etiology (see Tables 1 to 3 below). Some causes of occlusive retinal vasculitis include the vasculopathy of Susac’s syndrome, Idiopathic Retinal Vasculitis, Aneurysms, and Neuroretinitis (IRVAN) 3, the vasculopathy associated with anti-phospholipid antibody syndrome and systemic lupus erythematosus. A common cause of predominant retinal phlebitis is sarcoidosis. The extent of retinal vascular leakage in retinal vasculitis can also vary, from diffuse involvement, to isolated peripheral vascular leakage; the extent and location of retinal vascular involvement can be associated with ocular complications that can affect the vision.
The annual incidence of retinal vasculitis is 1-2 per 10,000 4. In one series, approximately 55% patients with retinal vasculitis had associated systemic inflammatory disease 5. In another larger series including more than 1300 patients, retinal vasculitis was seen in approximately 15% patients with uveitis. In this series, systemic vasculitis was associated with retinal vasculitis in only 1.4% cases 6.
Retinal vasculitis may be more common in individuals under the age of 40, with a slight preponderance in females 5. The mean age of diagnosis of retinal vasculitis is 34 without any gender differences 7. Retinal vasculitis is usually bilateral and is visual threatening. As many as one-third patients may suffer from severe visual loss (<20/200) as a result of retinal vasculitis and its complications 8.
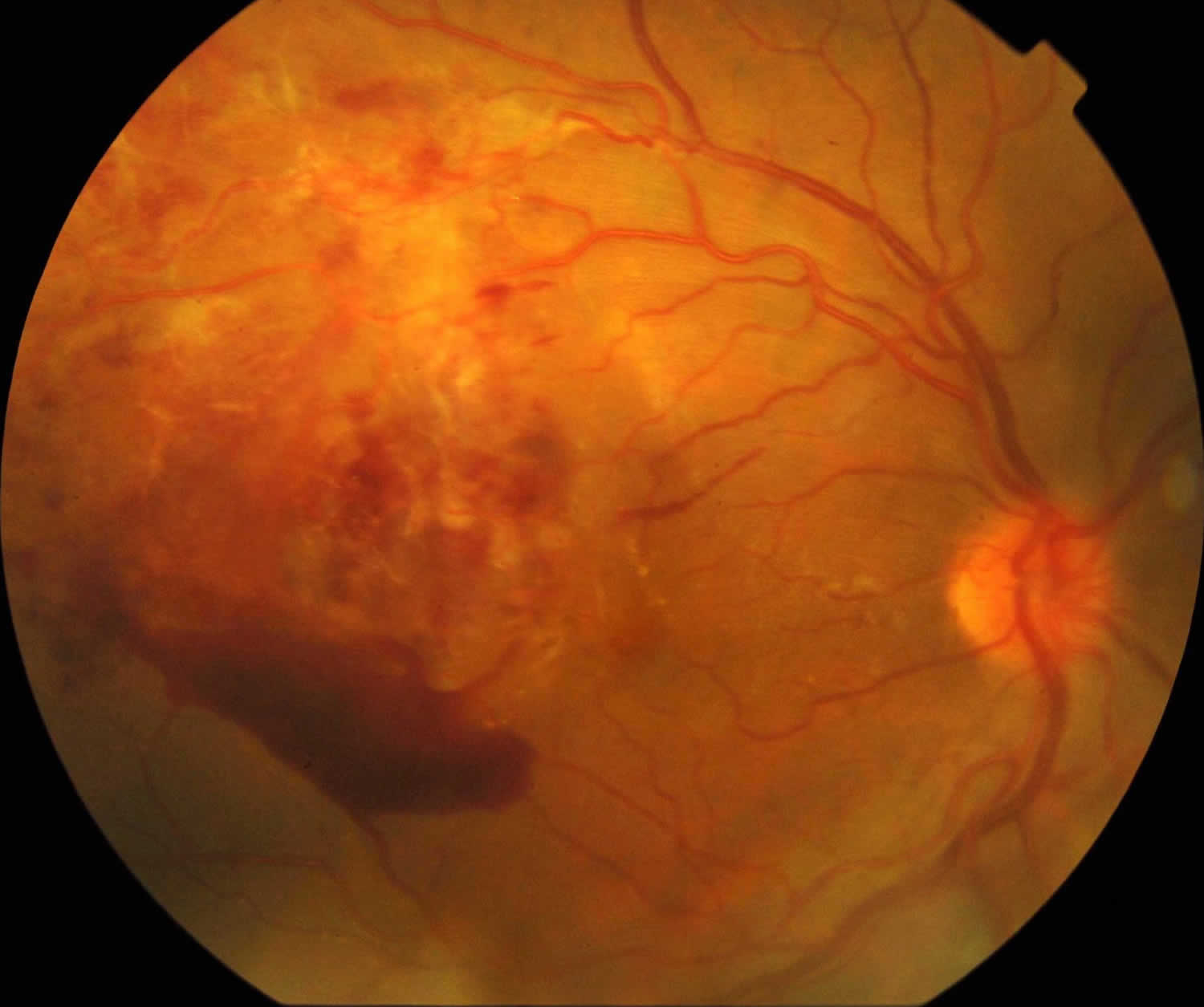
Figure 1. Retinal vasculitis
Footnote: Right eye there are retinal and preretinal haemorrhages, cotton-wool spots and vascular sheathing.
Retinal vasculitis classification
Systemic vascultis has been classified by the 2012 International Chapel Hill Consensus Conference on the Nomenclature of Vasculitides 9. The details of this classification can be found at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4029362/pdf/nihms528151.pdf.
The retinal vasculitis may be divided by the:
- Predominantly involved vessels
- Artery- acute retinal necrosis (ARN), idiopathic retinal vasculitis, aneurysm and neuroretinitis (IRVAN), systemic lupus erythematosus (SLE), polyarteritis nodosa (PAN), Syphilis, progressive outer retinal necrosis (PORN) and Churg Strauss Syndome
- Vein- Eales’ disease, intermediate uveitis, sarcoidosis, multiple sclerosis, tuberculosis, Birdshot chorioretinitis, HIV paraviral syndrome
- Both artery and vein- Frosted branch angiitis, toxoplasmosis, relapsing polychondritis, granulomatosis with polyangiitis (formerly Wegener’s), Chron’s disease
- Involvement of peripheral vessels or vessels around the posterior pole.
Retinal vasculitis stages
The retinal vasculitis has been classically divided into:
- Stage of inflammation: This denotes active inflammation and is clinically denoted by perivascular segmental whitish infiltrates with fuzzy borders (cuffing), retinal edema, retinal hemorrhages, cystoid macular edema, in some cases snow balls in the vitreous, inflammatory vascular occlusions (the blockage of veins may not is at the area of active vasculitis which may not correspond to the arteriovenous junction). Active choroiditis (eg. Birdshot chorioretinitis), retinitis (eg. Cytomegaloviral retinitis, ), intermediate uveitis, and anterior uveitis (panuveitis) may also be seen. The ocular disease or systemic disease causing the retinal vascultiis should be treated as per protocol. Infectious causes shouid be ruled out and treated as necessary. Unilateral noninfectious retinal vasculitis involving posterior pole, causing cystoid macular edema or visual decline is treated with periocular steroid (posterior subtenon triamcinolone or intravitreal triamcinolone) after ruling out glaucoma or steroid response . Oral steroid also remains an option in cases where periocular steroid is contraindicated. Role of systemic or local steroid in peripheral noninfectious vasculitis not involving macula is controversial. Bilateral active noninfectious retinal vasculitis involving macula may be treated with oral steroid (after ruling out infection) or sequential posterior subtenon triamcinolone.
- Stage of ischemia: It is clinically characterized by sclerosed vessels and tortuous collaterals. Healed paravascular pigmented choroiditic patches may be seen. Fluorescein angiogram shows retinal capillary non perfusion though neovascularization is not seen. Most ophthalmologists will follow up such cases. The collaterals should not be lasered as it may denote a healing response to revascularize the area which suffered ischemia due to inflammatory retinal venous occlusion.
- Stage of neovascularization: This stage most commonly present with vitreous hemorrhage. Laser of the retinal capillary non perfusion area as denoted by the fluorescein angiogram is the treatment of choice.
- Stage of complications: Complication are nonresolving vitreous hemorrhage , tractional retinal detachment, combined rhegmatogenous and tractional retinal detachment, epiretinal membrane, neovascular glaucoma, neovascularization of the iris and others. Managemnt options include vitrectomy, filtration surgery and others.
Retinal vasculitis causes
Retinal vasculitis may be associated with a variety of clinical conditions. Typically, a retinal vasculitis would occur as a part of an ocular or systemic disease. Rarely, it may be isolated, idiopathic condition, termed as idiopathic retinal vasculitis. It may also present as an initial manifestation of an underlying disorder. Isolated retinal vasculitis is seen in approximately 3% of the cases diagnosed with uveitis 10.
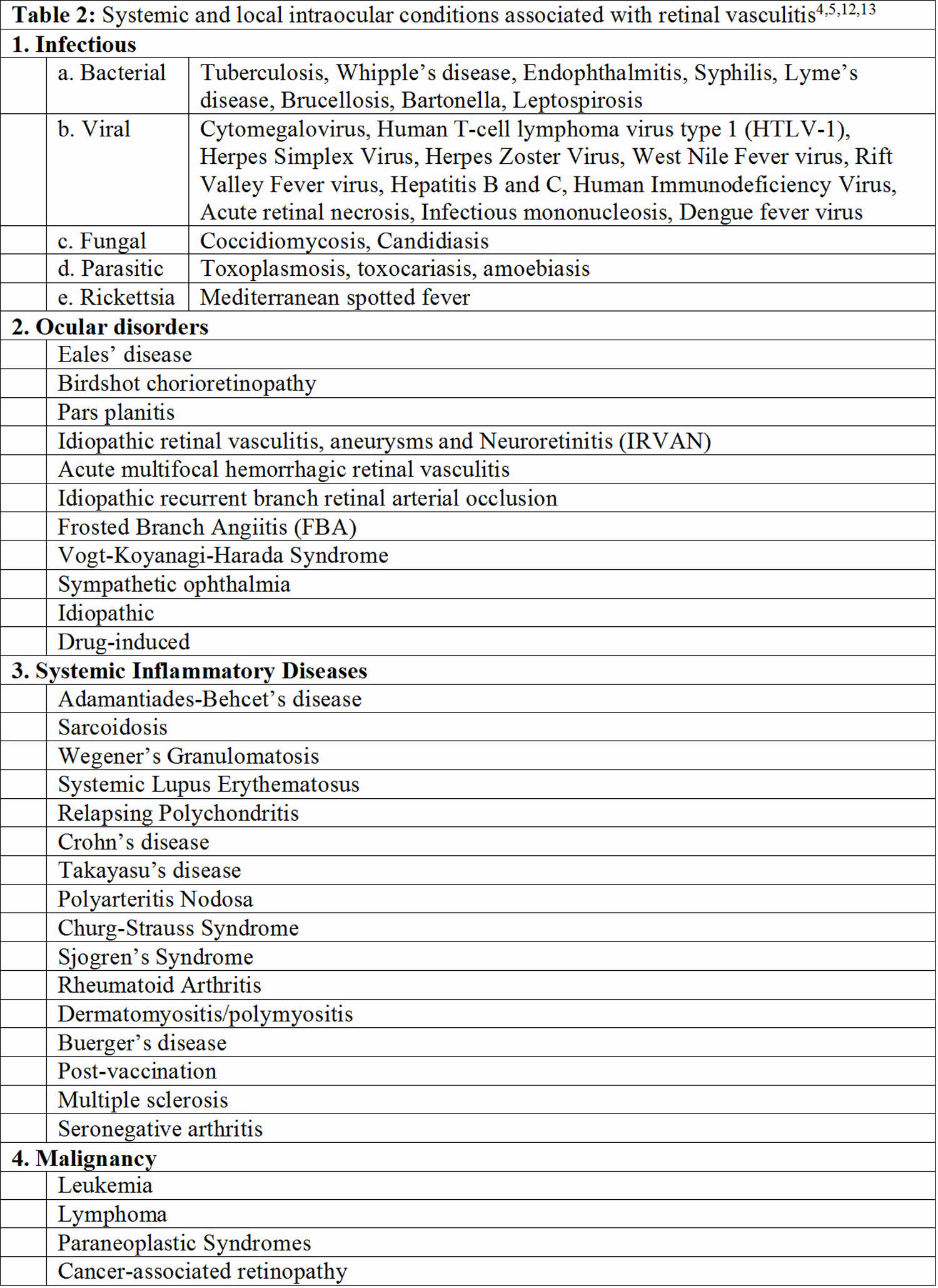
Retinal vasculitis may be associated with a number of systemic and local diseases (Tables 1 to 3) 11.
Many infections cause retinal vasculitis. These infections include viruses like cytomegalovirus, herpes simplex, human immunodeficiency virus I 2and varicella zoster; syphilis; tuberculosis; and toxoplasmosis. If an immunosuppressed patient with systemic vasculitis develops a retinal vasculitis, an infection is statistically much more common than the underlying disease as a cause of the vasculitis 12.
Retinal vasculitis in association with uveitis is common. For example, retinal vasculitis is frequently found in association with Vogt-Koyanagi-Harada syndrome or Birdshot Choroidopathy. A primary retinal vasculitis in which the uveitis does not fit into a diagnosable pattern and in which the vasculitis predominates as the clinical manifestation is well-described, but relatively uncommon 12. In our own series of 207 patients with retinal vasculitis, 17% could be considered as having primary retinal vasculitis 12.
Behçet’s disease is the rheumatic disease most consistently associated with retinal vasculitis. Other systemic diseases that commonly cause retinal vasculitis such as sarcoidosis and multiple sclerosis are not known to cause a systemic vasculitis.
With the exception of Behçet’s disease, systemic forms of vasculitis such as granulomatosis with polyangiitis 13, eosinophilic granulomatosis with polyangiitis 14, microscopic polyangiitis, giant cell arteritis, polyarteritis nodosa, leukocytoclastic vasculitis, or primary angiitis of the central nervous system 15 cause retinal vasculitis so rarely that publications are limited to case reports or very small case series.
Table 1. Potential cause based on retinal arteriole- or venule-predominance of retinal vasculitis
| Primarily retinal arteritis | Primarily retinal phlebitis | Arteritis and phlebitis |
|---|---|---|
| Systemic lupus erythematosus | Sarcoidosis | Relapsing polychondritis* |
| Polyarteritis nodosa | Multiple sclerosis | Granulomatosis with polyangiitis * |
| Syphilis | Pars planitis | Crohn’s disease/ulcerative colitis* |
| Toxoplasmosis | Behçet’s disease | Frosted branch angiitis |
| Herpes simplex virus (HSV)/varicella zoster virus (VZV) | Birdshot chorioretinopathy | Tuberculosis |
| Idiopathic retinal vasculitis, aneurysms, and neuroretinitis (IRVAN) | Human immunodeficiency virus (HIV) associated retinal vasculitis | |
| Churg-Strauss syndrome* | Eales disease | |
| Susac syndrome |
Footnote: *These diseases are rare causes of retinal vasculitis so characterization is based on a small number of descriptions.
[Source 2 ]Retinal vasculitis pathophysiology
Despite precise clinical visualization of the retinal microvasculature, the exact pathophysiological mechanism of this condition is not clear 16. In order to understand the pathogenesis of retinal vasculitis in humans, experimental animal models have been prepared 17. The manifestations of vascular sheathing and cuffing led to the belief that retinal vasculitis results due to type III hypersensitivity reaction 18. However, there is no proven human or animal model to support this hypothesis 19. A breakdown of blood-retinal-barrier secondary to intraocular or systemic inflammation resulting in clinical features of this disease is more likely. Due to the characteristic perivascular location of the inflammation, terms such as perivasculitis and periphlebitis have been suggested to denote the underlying pathology 16.
Studies demonstrate presence of either a focal, segmental or diffuse retinal perivascular proliferation of lymphoplasmacytic infiltrates in eyes with retinal vasculitis 20. In granulomatous diseases such as sarcoidosis, there may be a collection of numerous epithelioid cells. In eyes with intermediate uveitis and retinal vasculitis secondary to lymphoma, histopathological studies demonstrate presence of lymphocytic cuffing with mural involvement of retinal veins 21. Characterization of lymphocytic cells in the perivascular region reveals predominance of CD4+ T cells as compared to CD8+ T cells or B lymphocytes 22. There is an upregulation of various inflammatory cellular markers including integrins and cell adhesion molecules 23. Increased expression of cell adhesion molecules along retinal vessels and blood-retinal-barrier cells may play an important role in inflammatory cell recruitment. The sera of patients with retinal vasculitis demonstrate an increase in the levels of type 1 interferons, mainly interferon-β. Other molecules that are up-regulated include E-selectin and s-intracellular adhesion molecules 24.
Although there is a large pathological diversity among the retinal vasculitis etiologies, the manifestations of inflammatory changes resemble in many ways. In patients with infective retinal vasculitis, culture of live organisms such as mycobacteria may be possible from various systemic foci 25. Infectious organisms may involve retinal vasculature by various mechanisms, apart from direct vascular endothelial injury. They may result in release of toxins and may up-regulate molecules such as heat-shock proteins 26. Molecular mimicry may result in aberrant activation of immunological pathways resulting in pathological manifestations 4.
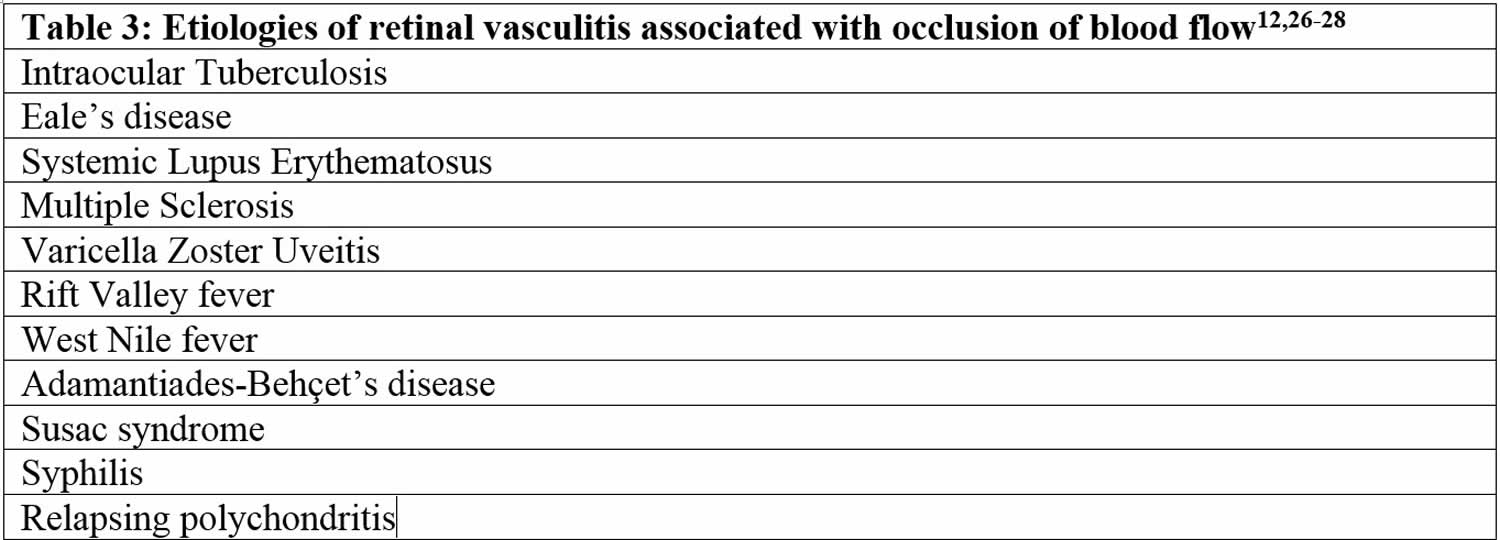
In a subset of patients with retinal vasculitis, there is occlusion of blood flow through the retinal vessels. The pathology of occlusive retinal vasculitis may be distinct from vasculitis without evidence of obliteration of blood flow. Available literature suggests that eyes with occlusive vasculitis have a poorer prognosis with a higher number of complications such as cystoid macular edema (CME), neovascularization and epiretinal membrane formation. Various etiologies associated with occlusive vasculitis are listed in Table 3 27.
Retinal vasculitis signs and symptoms
Retinal vasculitis can affect individuals with all ages and either sex. Cases of retinal vasculitis have been associated with various diseases that can affect any race or ethnicity. The patients of idiopathic retinal vasculitis, however, are typically young adults without any signs or symptoms of underlying ocular or systemic disease 7.
Retinal vasculitis symptoms
Patients with retinal vasculitis present with the following symptoms:
- Blurring of vision
- Flashes
- Floaters
- Scotoma
- Altered color vision
- Pain
- Metamorphopsia
Retinal vasculitis signs
Retinal vasculitis presents with characteristic features on clinical examination. The area of vascular involvement can be focal, diffuse or segmental. Often, retinal vasculitis may involve the veins alone 11. Retinal arteritis is more common with disease such as acute retinal necrosis and systemic vasculitidis 28.
There are various manifestations of retinal vasculitis, as listed below.
Perivascular sheathing
The classic feature of retinal vasculitis is presence of sheathing around the vessel wall. The perivascular sheathing is a collection of exudation consisting of inflammatory cells around the affected vessels. This results in appearance of a white cuff around the blood vessels. Retinal vascular sheathing is a common manifestation described with clinical entities like multiple sclerosis and Eale’s disease 29. Sarcoidosis can be associated with exuberant retinal perivascular infiltrates that give an impression of ‘candle-wax drippings’ 30.
Intraretinal infiltrates
Patches of retinitis may accompany retinal vasculitis. These are seen in individuals with Adamantiades-Behçet’s disease 31 and infectious uveitis 32. These patches of retinitis may be transient, as in Behçet’s disease, or may be accompanied by retinal necrosis. Intraretinal infiltrates can be sight-threatening and can lead to retinal atrophy, breaks and detachment.
Cotton-wool spots
Retinal vasculitis may result in micro-infarcts of the retinal nerve fiber layer that manifests as diffuse, fluffy, cotton-wool like spots in the superficial retinal surface 5. Systemic vasculitidis such as systemic lupus erythematosus 33, polyarteritis nodosa 34, Churg-Strauss syndrome 35 can be associated with retinal precapillary arteriolar occlusion resulting in cotton-wool spots. An increase in the number of cotton-wool spots may signify a flare-up of the uveitis.
Retinal necrosis
Infectious forms of uveitis associated with retinal vasculitis can be associated with necrosis of retinal layers. This is commonly seen in eyes with toxoplasmosis 36, viral infections such as varicella zoster 37 or herpes simplex 38, cytomegalovirus 39 and human T-cell lymphoma virus type 1 40. Toxoplasmosis is often associated with reactivation of the retinal lesion adjacent to a previous scar. Kyrieleis arteriolitis 41 is an accumulation of periarteriolar exudates in eyes with toxoplasmosis leading to retinal necrosis. Foci of chorioretinitis and choroiditis may be closely associated with retinal vasculitis. Association of retinal vasculitis and chorioretinal lesions in cytomegalovirus retinitis gives an appearance of pizza-pie retinopathy.
Frosted branch angiitis
Frosted branch angiitis is a descriptive term for retinal vasculitis characterized by severe infiltration of perivascular space with lymphoplasmacytic infiltrates 42. This gives an appearance of frosted branches of a tree. This condition can be associated with lymphoproliferative diseases such as lymphoma or leukemia 43, due to accumulation of malignant cells. However, frosted branch angiitis has also been reported in systemic lupus erythematosus 44, Crohn’s disease 45, toxoplasmosis 46 and AIDS 47.
Retinal ischemia
Occlusion of retinal vasculature secondary to inflammation may result in ischemia of the retina and development of capillary non-perfusion areas. These patients may be more predisposed to develop complications arising out of retinal non-perfusion, such as neovascularization and intraocular hemorrhage. Retinal ischemia is commonly seen following tuberculosis 48 or systemic lupus erythematosus 49. Inflammatory branch retinal arteriolar occlusion may be associated with Behçet’s disease 50. Retinal vasculitis may be associated with sclerosis and attenuation of the vessel wall. This may result in development of a significant area of retinal non-perfusion. Various other complications that can result include rubeosis, tractional retinal detachment, neovascular glaucoma and recurrent vitreous hemorrhage 5.
Retinal vasculitis diagnosis
Diagnosis of retinal vasculitis is usually clinical and supported by ancillary tests including fluorescein angiography. The signs of retinal vasculitis on fundus examination are protean.
Physical examination
Examination of patients with retinal vasculitis is performed using both, slit-lamp biomicroscopy with 90 or 78-diopter lens and indirect ophthalmoscopy with either 20 or 28-diopter lens. The location of the vasculitis may be in the posterior pole or far periphery; hence, scleral indentation must be performed to carefully examine the pars plana and ora serrata.
Fluorescein angiography
Fundus fluorescein angiography is the most informative imaging modality in patients with retinal vasculitis. Fluorescein angiography is routinely used in the diagnosis, monitoring and management of patients with retinal vasculitis 51. Signs on angiography, such as vascular leakage and macular edema can help assess the activity of the disease. Leakage of dye from vascular compartment results in perivascular hyperfluorescence.
Vascular leakage
Due to inflammation and breakdown of the blood-retinal-barrier, fluorescein angiography in eyes with retinal vasculitis can demonstrate a diffuse, segmental or focal vascular leakage. In addition, there may be evidence of capillary dropout due to occlusion of blood flow. This can lead to growth of new, leaky vessels. The pattern of leakage may vary depending upon the etiology. The leakage may be limited to arterioles in cases with systemic vasculitidis or viral infections. However, venular leakage is the more common pattern of retinal vasculitis 51. Often, the vascular leakage may be peripheral. Conventional fundus cameras can capture only the central 30° or 50° field of view. Ultra-wide field imaging of the fundus allows the clinician to obtain more information compared to regular field scans. The use of ultra-wide field imaging can alter decision-making in more than 50% patients with retinal vasculitis 52.
Capillary Non-perfusion
Retinal ischemia is a feature of a subset of patients with retinal vasculitis. This presents on fluorescein angiography as areas of capillary dropout. Behçet’s disease is characterized by ischemic branch vascular occlusion 53. The areas of non-perfusion may be in the periphery or in the macula. Macular ischemia results in poorer visual outcome despite successful control of inflammation.
Retinal neovascularization
Retinal ischemia and inflammation can result in release of vascular endothelial growth factor (VEGF) that stimulates new vessel proliferation. New vessels can be found at the optic disc or elsewhere on the retina. Patients with extensive retinal ischemia and capillary non-perfusion may require scatter laser photocoagulation.
Macular edema
Patients with active retinal vasculitis may have reduced visual acuity due to presence of macular edema. Retinal thickening can be observed on fluorescein angiography as leakage of dye in the macula or presence of a diffuse hyperfluorescence increasing towards the late phase. There may be an increase in the macular leakage following laser photocoagulation performed for retinal ischemia.
Optic nerve head inflammation
Retinal vasculitis may be associated with optic nerve head hyperfluorescence. There may be staining of the optic nerve head in the late frame. This must be differentiated from optic nerve head leakage secondary to neovascularization.
Choroidal vascular flow
Choroidal vascular flow can be imaged using Indocyanine green angiography. Patients with retinal vasculitis may have an abnormal choroidal blood flow 54. Indocyanine green angiography may assist in imaging abnormal choroidal flow patterns such as choroidal neovascularization or retinochoroidal anastomosis.
Laboratory test
Appropriate laboratory investigations aid in establishing the etiology for retinal vasculitis. A tailored approach to laboratory work-up is preferred to avoid unnecessary investigations and expenses to the patient 55. The investigations must be based on systemic symptoms and signs, ocular examination and detailed history. The aim of laboratory work-up is to identify infectious, non-infectious, or immunologic causes as the treatment for each category may be different. In the absence of any laboratory positive result, malignancy should be kept in mind as one of the differential diagnosis.
Commonly performed laboratory evaluations include markers of systemic inflammation, such as erythrocyte sedimentation rate (ESR) and C-reactive protein. Based on the clinical features, infectious etiologies can be ruled out by performing specific investigations. Tuberculosis testing can be performed using Mantoux test (tuberculin skin test). The tuberculin skin test is negative in cases with sarcoidosis. Chest X-ray and Computed Tomography (CT) scan can be performed to establish the etiology in cases where the diagnosis is challenging. For diagnosis of viral and parasitic diseases, titers of antibodies or antigens can be assessed. Polymerase chain reaction has a higher sensitivity and specificity for diagnosis and can be very useful in patients with retinal vasculitis.
In patients where co-existent systemic vasculitidis is possible, investigations can include detection of anti-neutrophil cytoplasmic antibody, anti-DNA antibody, anti-nuclear antibody and rheumatoid factor among others. HLA testing can help identify diseases such as Adamantiades-Behçet’s disease (HLA-B5), birdshot chorioretinitis (HLA-A29) and systemic lupus erythematosus (HLA-DR3).
Investigations in patients without evidence of systemic or ocular disease, i.e. idiopathic retinal vasculitis, can be limited to fluorescein angiography, complete blood counts, syphilis serology, ESR, urine analysis, tuberculin and HIV testing and chest radiograph 11. It is not recommended that every test is performed for every patient diagnosed with retinal vasculitis. Rather, as mentioned, the testing panel should be based in large on the findings from the review of systems and examination of the patient.
Retinal vasculitis treatment
The treatment for retinal vasculitis depends on the underlying cause of the retinal vasculitis and its severity. In the setting of an infection, retinal vasculitis should be treated by managing the underlying infection. Retinal vasculitis secondary to anti-phospholipid antibodies might require anti-coagulation. Some forms of retinal vasculitis cause minimal disruption of visual function and do not require therapy.
Adequate control of the intraocular inflammation is sine qua none for achieving remission of retinal vasculitis. Retinal vasculitis can be recurrent and aggressive in its course requiring steroid and immunosuppressive therapy 16. Ocular complications resulting from uncontrolled retinal vasculitis can have deleterious consequences including severe visual loss. The mainstay of therapy for retinal vasculitis is medical management. Infectious etiology must be ruled out as the treatment for non-infectious disease consists of immunosuppressive therapy, which can possibly worsen intraocular infections. For example, a retinal vasculitis secondary to an infection requires treatment of the underlying infectious cause. A mild retinal vasculitis with changes mostly in the peripheral retina that minimally affect vision might be followed by observation alone with no therapeutic intervention. Severe retinal vasculitis secondary to Behcet’s disease is often treated with a monoclonal antibody directed at tumor necrosis factor (TNF) or with alpha interferon in some centers. Occlusive vasculopathy such as can occur with anti-phospholipid antibody syndrome is treated with anti-platelet medications and/or anticoagulation. In most instances, specialists will attempt therapy with local or systemic corticosteroids and an anti-metabolite such as methotrexate before advancing to biologic therapy. Corticosteroids are the most rapidly effective while anti-metabolites may take up to 3 months for benefit. Antibodies to tumor necrosis factor (TNF) have been the primary choice among biologics to treat retinal vasculitis. Anti-TNF therapy can provide rapid benefit although not usually as rapidly as corticosteroid. If this class of medication fails, the literature provides very little guidance for additional options. The possibility of a masquerade secondary to an infection or malignancy should be reassessed. The United States Food and Drug Administration (FDA) has not approved any therapies specifically for retinal vasculitis.
No study has specifically compared an anti-metabolite in combination with a TNF inhibitor to a TNF inhibitor alone in the treatment of either uveitis or retinal vasculitis. Third party payers are reluctant to approve a biologic therapy unless a patient has previously failed therapy with corticosteroid and with an anti-metabolite. Some arguments favor the combination of a biologic and an anti-metabolite: potential additive effects from different mechanisms of action and reduced likelihood to generate antibodies that neutralize the biologic. Some arguments favor the use of the biologic alone: less risk of infection, less cost, less inconvenience. In many instances, the authors add anti-TNF therapy to therapy with an anti-metabolite, and then consider gradual discontinuation of the anti-metabolite therapy if complete control of ocular inflammation has been achieved for 6 to 12 months.
Non-infectious retinal vasculitis treatment
Non-infectious retinal vasculitis is managed by systemic or local corticosteroids and steroid-sparing immunosuppressants 1. The local delivery of therapeutic agents can be done via intravitreal injections or periocular therapy, although the latter may not be sufficiently adequate for cases of severe retinal vasculitis. The choice of immunosuppressive agents must be tailored based on ocular manifestations, etiology and systemic co-morbidities. Use of advanced imaging techniques such as ultra-wide fundus photography and fluorescein angiography can aid in the management of patients with retinal vasculitis 52.
In a series of 56 patients with non-infectious uveitis, systemic prednisone (oral) was used for the treatment of two-third patients at an average dose of 27mg/day for a mean of 14 months 5. Another study from Eastern India revealed that oral steroids were used in more than 80% patients with retinal vasculitis 56. In addition, periocular and intraocular steroids such as triamcinolone have been used in vasculitis associated with pars planitis 57. However, administration of steroid-sparing immunosuppressants are recommended in cases requiring more than 10mg/day dose of oral prednisone 58.
Unlike systemic vasculitis that can be managed with colchicines and non-steroidal anti-inflammatory agents, retinal vasculitis due to causes such as Adamantiades-Behçet’s disease
requires a more aggressive approach 59. Available immunosuppressive agents include cyclosporine, azathioprine, cyclophosphamide, mycophenolate mofetil or biologic agents such as infliximab or etanercept. Cyclosporine has been used as a drug of choice in previous studies 60. In eyes with vasculitis associated with birdshot chorioretinopathy, sarcoidosis and Harada’s disease, azathioprine has been used for treatment 61. Alkylating agents such as chlorambucil and cyclophosphamide have also been used in combination with corticosteroids 5. Biologic agents have been increasingly used to achieve remission in eyes with retinal vasculitis 62. These include infliximab, tacrolimus and adalimumab, apart from other agents targeting molecules such as tumor necrosis factor (TNF) and interleukins.
Infectious retinal vasculitis must be treated with appropriate anti-microbial agents depending upon the etiology. Infectious retinal vasculitis is a heterogenous cohort with various causative organisms such as bacteria, viruses and parasites. Anti-microbial therapy, including oral and intravitreal injections in various combinations with steroids have been used for the treatment of these disease entities.
Miscellaneous therapy
Apart from immunosuppression, various therapeutic options such as pan-retinal photocoagulation have been tried in order to control retinal vasculitis 16. Cryotherapy has been used in the past to treat retinal vasculitis associated with pars planitis in the past 63. Mesenchymal stem cell therapy has been used without success in eyes with Adamantiades-Behçet’s disease to control the retinal vasculitis 64.
Disease monitoring
Patients with retinal vasculitis must be periodically examined to evaluate the extent and severity of vascular leakage 65. The disease course can be effectively monitored using fluorescein angiography. Wide-field techniques assist the clinician to assess peripheral vascular lesions and aid in guiding further therapy. Infectious retinal vasculitis can be monitored by determining the load of the causative organisms using techniques such as quantitative polymerase chain reaction 66. As retinal vasculitis may be early manifestation of generalized vasculitis of the central nervous system (CNS), one needs to remember to evaluate and monitor the CNS vasculature (i.e. employing magnetic resonance imaging with contrast) as well when indicated 67.
Retinal vasculitis prognosis
A problem inherent in studying retinal vasculitis is the heterogeneity of the disease. The prognosis, for example, of retinal vasculitis associated with sarcoidosis would presumably differ from a retinal vasculitis associated with birdshot choroidopathy. This heterogeneity is especially relevant to the infections associated with retinal vasculitis. Although tuberculosis, syphilis, toxoplasmosis, and herpes family viruses are each commonly associated with retinal vasculitis, the course for each is distinct. Accordingly, one should be extremely cautious in drawing any conclusions about vasculitis secondary to infection in this series because no single infection was associated with an adequate number of subjects to allow definitive conclusions. The heterogeneity of retinal vasculitis also applies to primary retinal vasculitis. As diagnostic sophistication improves, doctors will undoubtedly be able to define subsets of primary retinal vasculitis and the prognosis for each subset will be more meaningful than trying to determine prognosis for the aggregate group.
In a study 68 of 207 patients with retinal vasculitis, 39.4% of the eyes experienced loss of vision despite therapy, 37.4% experienced some improvement. More than 1 of 3 eyes in our series had visual acuity of 20/25 or better at baseline and were thus unlikely to experience substantial improvement, but visual acuity in 35.6% of the remaining eyes improved by at least 2 lines on the Snellen chart. In no case was improvement due to surgery (ie, cataract surgery). Cox multivariate analysis showed more likelihood to improve for patients who were nonwhite, had an ocular infection, or had worse vision at baseline. However, a ceiling effect may exist because patients with an ocular infection and patients of races other than non-Hispanic white had worse vision at baseline, potentially allowing their vision to improve to a greater degree. Direct comparisons of findings on visual outcome with previously published series are difficult because the prior studies often excluded cases with a known cause such as systemic immune-mediated disease and ocular infection.
In a longitudinal study of 52 patients with relapsing retinal vasculitis, these investigators observed visual improvement in 12 of 26 patients with retinal vasculitis alone and 10 of 26 patients with retinal vasculitis associated with a systemic inflammatory disease 69. The same group examined visual outcome of 53 patients with idiopathic retinal vasculitis and observed significantly worse visual outcome in ischemic retinal vasculitis compared with nonischemic retinal vasculitis 70. In isolated retinal vasculitis and poor visual outcome associated with macular edema and branch vein occlusion 71.
- Retinal vasculitis. https://eyewiki.org/Retinal_Vasculitis[↩][↩][↩][↩][↩]
- Rosenbaum JT, Sibley CH, Lin P. Retinal vasculitis. Curr Opin Rheumatol. 2016;28(3):228–235. doi:10.1097/BOR.0000000000000271 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4893321[↩][↩][↩][↩][↩][↩]
- Sigler EJ, Grosso A. Idiopathic Retinal Vasculitis, Aneurysms, and Neuroretinitis Syndrome. Retina. 2015 Epub ahead of print.[↩]
- Hughes EH, Dick AD. The pathology and pathogenesis of retinal vasculitis. Neuropathology and applied neurobiology. Aug 2003;29(4):325-340.[↩][↩]
- Ali A, Ku JH, Suhler EB, Choi D, Rosenbaum JT. The course of retinal vasculitis. The British journal of ophthalmology. Jun 2014;98(6):785-789.[↩][↩][↩][↩][↩][↩]
- Rosenbaum JT, Ku J, Ali A, Choi D, Suhler EB. Patients with retinal vasculitis rarely suffer from systemic vasculitis. Seminars in arthritis and rheumatism. Jun 2012;41(6):859-865.[↩]
- George RK, Walton RC, Whitcup SM, Nussenblatt RB. Primary retinal vasculitis. Systemic associations and diagnostic evaluation. Ophthalmology. Mar 1996;103(3):384-389.[↩][↩]
- Rothova A, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. The British journal of ophthalmology. Apr 1996;80(4):332-336.[↩]
- Jennette JC. Overview of the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin Exp Nephrol. 2013;17(5):603–606. doi:10.1007/s10157-013-0869-6 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4029362[↩]
- Rodriguez A, Calonge M, Pedroza-Seres M, et al. Referral patterns of uveitis in a tertiary eye care center. Archives of ophthalmology. May 1996;114(5):593-599.[↩]
- El-Asrar AM, Herbort CP, Tabbara KF. A clinical approach to the diagnosis of retinal vasculitis. International ophthalmology. Apr 2010;30(2):149-173.[↩][↩][↩]
- Rosenbaum JT, Ku J, Ali A, Choi D, Suhler EB. Patients with retinal vasculitis rarely suffer from systemic vasculitis. Semin Arthritis Rheum. 2012;41(6):859–865.[↩][↩][↩]
- Sandhu RK, Adams T, Sibley C, Suhler EB, Kim DH. Granulomatosis with Polyangiitis (Gpa) Presenting with Frosted Branch Angiitis. Retinal cases & brief reports. 2015 Epub ahead of print.[↩]
- Najem CE, Yadav R, Carlson E. Successful use of Rituximab in a patient with recalcitrant multisystemic eosinophilic granulomatosis with polyangiitis. BMJ case reports. 2015:2015.[↩]
- Rosenbaum JT, Roman-Goldstein S, Lindquist GR, Rosenbaum RB. Uveitis and central nervous system vasculitis. J. Rheumatol. 1998;25(3):593–597.[↩]
- Levy-Clarke GA, Nussenblatt R. Retinal vasculitis. International ophthalmology clinics. Spring 2005;45(2):99-113.[↩][↩][↩][↩]
- Stanford MR, Graham EM, Kasp E, Brown EC, Dumonde DC, Sanders MD. Retinal vasculitis: correlation of animal and human disease. Eye (London, England). 1987;1 ( Pt 1):69-77.[↩]
- Stubiger N, Winterhalter S, Pleyer U, Doycheva D, Zierhut M, Deuter C. [Janus-faced?: Effects and side-effects of interferon therapy in ophthalmology]. Der Ophthalmologe : Zeitschrift der Deutschen Ophthalmologischen Gesellschaft. Mar 2011;108(3):204-212.[↩]
- Paovic J, Paovic P, Vukosavljevic M. Clinical and immunological features of retinal vasculitis in systemic diseases. Vojnosanitetski pregled. Military-medical and pharmaceutical review. Dec 2009;66(12):961-965.[↩]
- Axente D. [Retinal vasculitis]. Oftalmologia (Bucharest, Romania : 1990). 2006;50(4):13-21.[↩]
- Velez G, Chan CC, Csaky KG. Fluorescein angiographic findings in primary intraocular lymphoma. Retina (Philadelphia, Pa.). Feb 2002;22(1):37-43.[↩]
- Oh HM, Yu CR, Lee Y, Chan CC, Maminishkis A, Egwuagu CE. Autoreactive memory CD4+ T lymphocytes that mediate chronic uveitis reside in the bone marrow through STAT3-dependent mechanisms. Journal of immunology (Baltimore, Md. : 1950). Sep 15 2011;187(6):3338-3346.[↩]
- Makhoul M, Dewispelaere R, Relvas LJ, et al. Characterization of retinal expression of vascular cell adhesion molecule (VCAM-1) during experimental autoimmune uveitis. Experimental eye research. Aug 2012;101:27-35.[↩]
- Lee MT, Hooper LC, Kump L, et al. Interferon-beta and adhesion molecules (E-selectin and s-intracellular adhesion molecule-1) are detected in sera from patients with retinal vasculitis and are induced in retinal vascular endothelial cells by Toll-like receptor 3 signalling. Clinical and experimental immunology. Jan 2007;147(1):71-80.[↩]
- Doycheva D, Pfannenberg C, Hetzel J, et al. Presumed tuberculosis-induced retinal vasculitis, diagnosed with positron emission tomography (18F-FDG-PET/CT), aspiration biopsy, and culture. Ocular immunology and inflammation. Jun 2010;18(3):194-199.[↩]
- Poulaki V, Iliaki E, Mitsiades N, et al. Inhibition of Hsp90 attenuates inflammation in endotoxin-induced uveitis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. Jul 2007;21(9):2113-2123.[↩]
- Fuest M, Rossler G, Walter P, Plange N. [Retinal vasculitis as manifestation of multiple sclerosis]. Der Ophthalmologe : Zeitschrift der Deutschen Ophthalmologischen Gesellschaft. Sep 2014;111(9):871-875.[↩]
- Empeslidis T, Konidaris V, Brent A, Vardarinos A, Deane J. Kyrieleis plaques in herpes zoster virus-associated acute retinal necrosis: a case report. Eye (London, England). Sep 2013;27(9):1110-1112.[↩]
- Biswas J, Sharma T, Gopal L, Madhavan HN, Sulochana KN, Ramakrishnan S. Eales disease–an update. Survey of ophthalmology. May-Jun 2002;47(3):197-214.[↩]
- Caplan L, Corbett J, Goodwin J, Thomas C, Shenker D, Schatz N. Neuro-ophthalmologic signs in the angiitic form of neurosarcoidosis. Neurology. Sep 1983;33(9):1130-1135.[↩]
- Tugal-Tutkun I, Gupta V, Cunningham ET. Differential diagnosis of behcet uveitis. Ocular immunology and inflammation. Oct 2013;21(5):337-350.[↩]
- Schneider EW, Elner SG, van Kuijk FJ, et al. Chronic retinal necrosis: cytomegalovirus necrotizing retinitis associated with panretinal vasculopathy in non-HIV patients. Retina (Philadelphia, Pa.). Oct 2013;33(9):1791-1799.[↩]
- Ho TY, Chung YM, Lee AF, Tsai CY. Severe vaso-occlusive retinopathy as the primary manifestation in a patient with systemic lupus erythematosus. Journal of the Chinese Medical Association : JCMA. Jul 2008;71(7):377-380.[↩]
- Mihara M, Hayasaka S, Watanabe K, Kitagawa K, Hayasaka Y. Ocular manifestations in patients with microscopic polyangiitis. European journal of ophthalmology. Jan-Feb 2005;15(1):138-142.[↩]
- Kubal AA, Perez VL. Ocular manifestations of ANCA-associated vasculitis. Rheumatic diseases clinics of North America. Aug 2010;36(3):573-586.[↩]
- Butler NJ, Furtado JM, Winthrop KL, Smith JR. Ocular toxoplasmosis II: clinical features, pathology and management. Clinical & experimental ophthalmology. Jan-Feb 2013;41(1):95-108.[↩]
- Kirkwood BJ. Acute retinal necrosis. Insight (American Society of Ophthalmic Registered Nurses). Winter 2014;39(1):14-16.[↩]
- Roy R, Pal BP, Mathur G, Rao C, Das D, Biswas J. Acute retinal necrosis: clinical features, management and outcomes–a 10 year consecutive case series. Ocular immunology and inflammation. Jun 2014;22(3):170-174.[↩]
- Or C, Press N, Forooghian F. Acute retinal necrosis secondary to cytomegalovirus following successful treatment of cytomegalovirus anterior uveitis in an immunocompetent adult. Canadian journal of ophthalmology. Journal canadien d’ophtalmologie. Apr 2013;48(2):e18-20.[↩]
- Levy-Clarke GA, Buggage RR, Shen D, Vaughn LO, Chan CC, Davis JL. Human T-cell lymphotropic virus type-1 associated t-cell leukemia/lymphoma masquerading as necrotizing retinal vasculitis. Ophthalmology. Sep 2002;109(9):1717-1722.[↩]
- Chazalon E, Conrath J, Ridings B, Matonti F. [Kyrieleis arteritis: report of two cases and literature review]. Journal francais d’ophtalmologie. Mar 2013;36(3):191-196.[↩]
- He L, Moshfeghi DM, Wong IG. Perivascular Exudates in Frosted Branch Angiitis. Ophthalmic surgery, lasers & imaging retina. Sep 18 2014:1-4.[↩]
- Kleiner RC. Frosted branch angiitis: clinical syndrome or clinical sign? Retina (Philadelphia, Pa.). 1997;17(5):370-371.[↩]
- Quillen DA, Stathopoulos NA, Blankenship GW, Ferriss JA. Lupus associated frosted branch periphlebitis and exudative maculopathy. Retina (Philadelphia, Pa.). 1997;17(5):449-451.[↩]
- Sykes SO, Horton JC. Steroid-responsive retinal vasculitis with a frosted branch appearance in Crohns disease. Retina (Philadelphia, Pa.). 1997;17(5):451-454.[↩]
- Oh J, Huh K, Kim SW. Recurrent secondary frosted branch angiitis after toxoplasmosis vasculitis. Acta ophthalmologica Scandinavica. Feb 2005;83(1):115-117.[↩]
- Leeamornsiri S, Choopong P, Tesavibul N. Frosted branch angiitis as a result of immune recovery uveitis in a patient with cytomegalovirus retinitis. Journal of ophthalmic inflammation and infection. 2013;3(1):52.[↩]
- Sanghvi C, Bell C, Woodhead M, Hardy C, Jones N. Presumed tuberculous uveitis: diagnosis, management, and outcome. Eye (London, England). Apr 2011;25(4):475-480.[↩]
- Damato E, Chilov M, Lee R, Singh A, Harper S, Dick A. Plasma exchange and rituximab in the management of acute occlusive retinal vasculopathy secondary to systemic lupus erythematosus. Ocular immunology and inflammation. Oct 2011;19(5):379-381.[↩]
- Kahloun R, Mbarek S, Khairallah-Ksiaa I, Jelliti B, Yahia SB, Khairallah M. Branch retinal artery occlusion associated with posterior uveitis. Journal of ophthalmic inflammation and infection. 2013;3(1):16.[↩]
- Stanford MR, Verity DH. Diagnostic and therapeutic approach to patients with retinal vasculitis. International ophthalmology clinics. Spring 2000;40(2):69-83.[↩][↩]
- Leder HA, Campbell JP, Sepah YJ, et al. Ultra-wide-field retinal imaging in the management of non-infectious retinal vasculitis. Journal of ophthalmic inflammation and infection. 2013;3(1):30.[↩][↩]
- Yahia SB, Kahloun R, Jelliti B, Khairallah M. Branch retinal artery occlusion associated with Behcet disease. Ocular immunology and inflammation. Aug 2011;19(4):293-295.[↩]
- Tugal-Tutkun I, Herbort CP, Khairallah M. Scoring of dual fluorescein and ICG inflammatory angiographic signs for the grading of posterior segment inflammation (dual fluorescein and ICG angiographic scoring system for uveitis). International ophthalmology. Oct 2010;30(5):539-552.[↩]
- Majumder PD, Sudharshan S, Biswas J. Laboratory support in the diagnosis of uveitis. Indian journal of ophthalmology. Jun 2013;61(6):269-276.[↩]
- Saurabh K, Das RR, Biswas J, Kumar A. Profile of retinal vasculitis in a tertiary eye care center in Eastern India. Indian journal of ophthalmology. Jul-Aug 2011;59(4):297-301.[↩]
- Roesel M, Gutfleisch M, Heinz C, Heimes B, Zurek-Imhoff B, Heiligenhaus A. [Effect of intravitreal and orbital floor triamcinolone acetonide injection on intraocular inflammation in patients with active non-infectious uveitis]. Klinische Monatsblatter fur Augenheilkunde. Feb 2009;226(2):110-114.[↩]
- Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. American journal of ophthalmology. Oct 2000;130(4):492-513.[↩]
- Comarmond C, Wechsler B, Cacoub P, Saadoun D. Approaches to immunosuppression in Behcet’s disease. Immunotherapy. Jul 2013;5(7):743-754.[↩]
- Ozdal PC, Ortac S, Taskintuna I, Firat E. Long-term therapy with low dose cyclosporin A in ocular Behcet’s disease. Documenta ophthalmologica. Advances in ophthalmology. Nov 2002;105(3):301-312.[↩]
- Davatchi F, Sadeghi Abdollahi B, Shams H, et al. Combination of pulse cyclophosphamide and azathioprine in ocular manifestations of Behcet’s disease: longitudinal study of up to 10 years. International journal of rheumatic diseases. May 2014;17(4):444-452.[↩]
- Pasadhika S, Rosenbaum JT. Update on the use of systemic biologic agents in the treatment of noninfectious uveitis. Biologics : targets & therapy. 2014;8:67-81.[↩]
- Okinami S, Sunakawa M, Arai I, Iwaki M, Nihira M, Ogino N. Treatment of pars planitis with cryotherapy. Ophthalmologica. Journal international d’ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 1991;202(4):180-186.[↩]
- Davatchi F, Nikbin B, Shams H, Sadeghi Abdollahi B, Mohyeddin M, Shahram F. Mesenchymal stem cell therapy unable to rescue the vision from advanced Behcet’s disease retinal vasculitis: report of three patients. International journal of rheumatic diseases. Apr 2013;16(2):139-147.[↩]
- Campbell JP, Leder HA, Sepah YJ, et al. Wide-field retinal imaging in the management of noninfectious posterior uveitis. American journal of ophthalmology. Nov 2012;154(5):908-911.e902.[↩]
- Singh R, Toor P, Parchand S, Sharma K, Gupta V, Gupta A. Quantitative polymerase chain reaction for Mycobacterium tuberculosis in so-called Eales’ disease. Ocular immunology and inflammation. Jun 2012;20(3):153-157.[↩]
- Barry RJ, Nguyen QD, Lee RW, Murray PI, Denniston AK. Pharmacotherapy for uveitis: current management and emerging therapy. Clinical ophthalmology (Auckland, N.Z.). 2014;8:1891-1911.[↩]
- Ku JH, Ali A, Suhler EB, Choi D, Rosenbaum JT. Characteristics and Visual Outcome of Patients With Retinal Vasculitis. Arch Ophthalmol. 2012;130(10):1261–1266. doi:https://doi.org/10.1001/archophthalmol.2012.1596[↩]
- Stanford MR, Graham E, Kasp E, Sanders MD, Dumonde DC. A longitudinal study of clinical and immunological findings in 52 patients with relapsing retinal vasculitis. Br J Ophthalmol. 1988;72(6):442-4473390420[↩]
- Palmer HE, Stanford MR, Sanders MD, Graham EM. Visual outcome of patients with idiopathic ischaemic and non-ischaemic retinal vasculitis. Eye (Lond). 1996;10(pt 3):343-3488796160[↩]
- Graham EM, Stanford MR, Sanders MD, Kasp E, Dumonde DC. A point prevalence study of 150 patients with idiopathic retinal vasculitis, 1: diagnostic value of ophthalmological features. Br J Ophthalmol. 1989;73(9):714-7212804027[↩]