Salmon
Atlantic salmon (Salmo salar) also known as Sea run salmon, Kelts, Black salmon or the “King of Fish,” is a species of bony fish in the family Salmonidae (salmonids). Atlantic salmon (Salmo salar) is the 3rd largest of the Salmonidae, behind Siberian Taimen (Siberian salmon or Siberian giant trout) and Pacific Chinook Salmon, growing up to a meter in length. Atlantic salmon are found in the northern Atlantic Ocean and in rivers that flow into this ocean. Most populations of Atlantic salmon (Salmo salar) fish species are anadromous, which means they live in both fresh and saltwater. Atlantic salmon have a complex life history that begins with spawning, hatching and juvenile rearing in rivers. They then migrate to saltwater to feed, grow, and mature after which the adult fish seasonally move to freshwater again to spawn 1. After spawning in freshwater, the adults, now called kelts, swim back to the ocean to possibly return to spawn again in future years. Only a tiny percent of Atlantic salmon survives from egg to adulthood. Females returning to spawn after two winters at sea lay an average of 7,500 eggs. Out of these eggs, only about 15 to 35 percent will survive to the fry stage and on average two will live long enough to spawn as adults.
Typically, an Atlantic salmon returning to U.S. waters will be 4 years old, having spent 2 years in freshwater and 2 years at sea. These fish are called “two sea winter fish,” or 2SW, and are usually 28 to 30 inches long and 8 to 12 pounds. The size of adults salmon returning to freshwater from the ocean depends on how long they lived at sea. Young salmon returning to freshwater after 1 year at sea (known as “grilse” or 1SW) are smaller than 2SW adults. Adult salmon can migrate several times to spawn—a reproductive strategy known as iteroparity—though repeat spawners are becoming increasingly rare.
While in freshwater, young Atlantic salmon—known as parr—have brown to bronze-colored bodies with dark vertical bars and red and black spots. These markings camouflage and protect them from predators. Once young salmon are ready to migrate to the ocean, their appearance changes; their vertical barring disappears and they become silvery with nearly black backs and white bellies. When adults return to freshwater to spawn, they are very bright silver. After entering the river, they will again darken to a bronze color before spawning in the fall. After spawning, adults—now called kelts—can darken further and are often referred to as black salmon. Once adults return to the ocean, they revert to their counter-shaded coloration dominated by silver.
There are three groups of Atlantic salmon: North American, European, and Baltic. These groups are found in the waters of North America, Iceland, Greenland, Europe, and Russia. Atlantic salmon spawn in the coastal rivers of northeastern North America, Iceland, Europe, and northwestern Russia. After spawning, they migrate through various portions of the North Atlantic Ocean. European and North American populations of Atlantic salmon intermix while living in the ocean, where they share summer feeding grounds off Greenland. The North American group historically ranged from northern Quebec to Newfoundland and to Long Island Sound. This group includes Canadian populations and U.S. populations. In Canada, healthy populations still exist today, however, many populations are severely depleted.
Atlantic salmon in the United States were once native to almost every coastal river northeast of the Hudson River in New York. But dams, pollution, and overfishing reduced their population size until the fisheries closed in 1948. Commercial and recreational fishing for wild sea-run Atlantic salmon is still prohibited in the United States. All Atlantic salmon in the public market is cultured and commercially grown. Currently, the only remaining wild populations of U.S. Atlantic salmon are found in a few rivers in Maine. These remaining populations comprise the Gulf of Maine distinct population segment, which is listed as endangered under the Endangered Species Act 2. Some populations in southern Canada and Europe are also declining significantly, creating concern about the status of this species globally.
The culture of Atlantic salmon is by far the most well-developed branch of marine finfish aquaculture, with this species ranking among the top ten most highly produced in global aquaculture 3. Salmon cage farming has traditionally been located at higher latitude regions, such as Chile, Canada, the Faroe Islands, Norway, Scotland and Tasmania 4. This spatial distribution is driven by existing environmental conditions in these locations, which are optimal for salmon cage farming at the sea surface, such as cold-water temperatures (8 to 14 °C), optimal biological conditions and sheltered coastlines 5. However, cold-water regions are being increasingly impacted by climate change, with ocean warming and recurrent heat wave events being recorded in recent years 6. Furthermore, disease outbreaks and parasites still pose a significant threat to the Atlantic salmon supply chain, as well as to the sustainability and profitability of sea cage farm operations 7. The threat posed by sea lice (Lepeophtheirus salmonis and Caligus elongatus) has long been recognized 8 and continues to be of particular concern, as these parasitic copepods continue to induce high levels of mortality that result in serious production shortfalls 7.
Commercial‐scale Atlantic salmon farming began in Norway in the 1960s, expedited by trials in the early 1970s that demonstrated the huge potential of family‐based breeding programmes 9. In these trials, gametes from salmon taken from approximately 40 Norwegian rivers were collected and formed the basis of the first commercial breeding programme 10. Other similar breeding programme initiatives were instigated, including the establishment of the Mowi, Rauma, Jakta and Bolaks strains in Norway 11. Together, following various crossing and international export events, these strains underpin the vast majority of global salmon aquaculture. The consolidation of breeding companies over recent years has resulted in very few but large international players that supply eggs to all the major salmon‐producing countries. These include AquaGen (Norway), Benchmark (UK; owners of both SalmoBreed and StofnFiskr), Hendrix Genetics (Netherlands; owners of Landcatch) and AquaInnovo (Chile), with further consolidation underway via a joint venture between Benchmark and AquaInnovo 12.
Due to the outcomes of domestication and selective breeding, there are both genetic and phenotypic differences between wild and farmed salmon populations 12. Escapees from salmon farms are thought to have resulted in significant introgression into wild stocks, which may impact life‐history traits and the subsequent fitness of natural populations 11. As such, approaches to prevent interbreeding of wild and farmed fish are being developed, including mass generation of triploids and gene editing to induce sterility in farmed stocks.
The diet of wild Atlantic salmon depends on their age. Young salmon eat insects, invertebrates, and plankton. The preferred diet of adult salmon is capelin. Capelin (similar in appearance to rainbow smelt) are elongated silvery fish that reach 8 to 10 inches in length.
On the other hand farmed Atlantic salmon diet relies on the supply of fish meal and fish oil from overexploited fish stocks 13. In 2013, the main suppliers of aquafeeds for farmed Atlantic salmon (EWOS, Skretting, and BioMar) included 18% fish meal and 11% fish oil in their formulations 14. Soy protein concentrate (SPC) has become an extensively used ingredient in commercial aquafeeds due to its high protein proportion and low contents of indigestible fibers and anti-nutrients, nutritional characteristics that contribute to better performance compared with plant meals 15. Furthermore, Atlantic salmon (Salmo salar) have been found to grow well on diets with moderate replacement levels of fish meal by soy protein concentrate 16. Other plant ingredients with high protein content are wheat and corn glutens, which have proven to be nutritionally valuable when combined with oilseed protein sources 17. Rendered terrestrial animal products can also be used as an alternative protein source to fish meal 18. Meals from animal by-products often present adequate amino acid profiles compared with plant protein sources, higher levels of digestible phosphorus, and competitive prices and availability in the global market. However, use of these products is not well accepted by some consumers because of health concerns in the past (e.g., bovine spongiform encephalopathy) 19. Nevertheless, the use of animal by-products in aquafeeds is widespread, and their quality has improved greatly since the 1980s with the refinement of the production methods 20. Recent work on salmonids has shown that fish meal can be replaced at high levels by poultry by-products without affecting growth performance 21. However, in contrast to plant protein sources, there is a lack of studies on the effects of these feedstuffs on the fish transcriptome.
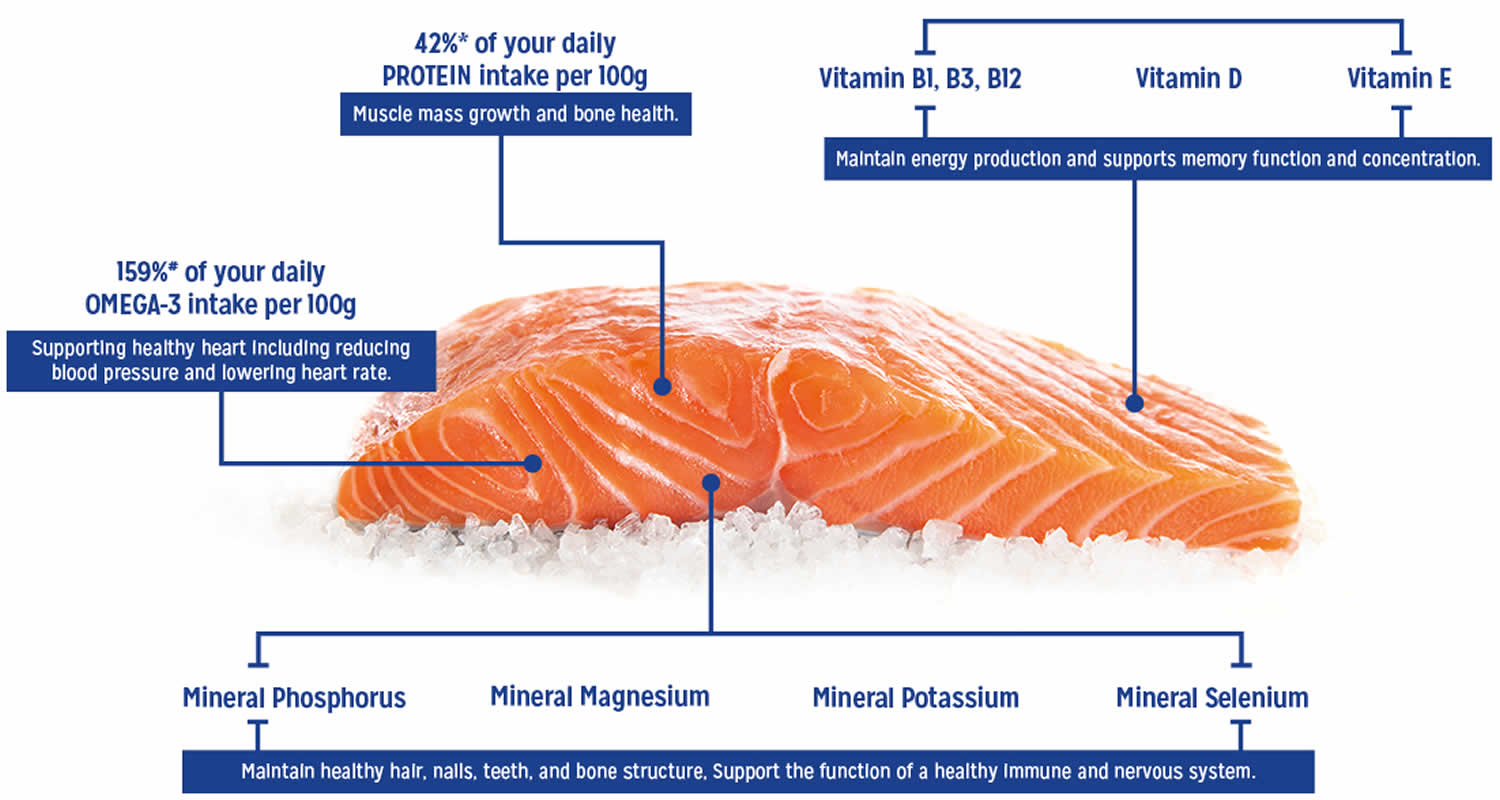
Atlantic salmon is a highly valued product due to the elevated content of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in the flesh. Besides their anti-inflammatory properties, EPA (eicosapentaenoic acid) and DHA (docosahexaenoic acid) are also essential for the proper functioning of important physiological processes in humans and fish 22. Low fish oil levels in feeds can result in reduced levels of Omega-3 fatty acids in muscle of Atlantic salmon, as salmonids have limited capacity for de novo synthesis of such long-chain omega-3 fatty acids 23. This was shown recently by Sprague et al. 24 for Scottish farmed salmon: the eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) levels in salmon flesh in 2015 had decreased to almost half the values of 2006’s production. Several solutions have been proposed to break the interdependency of the use of more sustainable oil sources and the decline of the nutritional value of Atlantic salmon. The culture of transgenic fish 25 and the use of transgenic plants 26 and new oil sources 23, have been studied. Regardless, for all of the potential paths to address the problem, a better understanding of lipid metabolism in Atlantic salmon (and fish in general) is needed.
Figure 1. Salmon
Figure 2. Essential fatty acids
Salmon nutrition
Atlantic salmon is one of the best sources of Omega-3 fatty acids found in nature due to the elevated content of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in the flesh. Omega-3 fatty acids, sometimes referred to as “n-3s,” are a group of polyunsaturated fatty acids (PUFAs) that are important for a number of functions in your body. However since your body cannot produce Omega-3 fatty acids naturally you must look to source these from what you eat. Several different Omega-3 fatty acids exist, but the majority of scientific research focuses on three: alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). ALA (alpha-linolenic acid) contains 18 carbon atoms, whereas EPA (eicosapentaenoic acid) and DHA (docosahexaenoic acid) are considered “long-chain” omega-3s because EPA contains 20 carbons and DHA contains 22 27. Polyunsaturated fatty acids (PUFAs) are frequently designated by their number of carbon atoms and double bonds. ALA (alpha-linolenic acid), for example, is known as C18:3n-3 because it has 18 carbons and 3 double bonds and is an n-3, or omega-3, fatty acid. Similarly, EPA (eicosapentaenoic acid) is known as C20:5n-3 and DHA (docosahexaenoic acid) as C22:6n-3.
ALA (alpha-linolenic acid) is found mainly in plant oils such as flaxseed, soybean, and canola oils 28. DHA (docosahexaenoic acid) and EPA (eicosapentaenoic acid) are found in fatty fish (e.g., salmon, tuna, and trout), shellfish (e.g., crab, mussels, and oysters), fish oils and krill oils, but they are originally synthesized by microalgae, not by the fish 27. When fish consume phytoplankton that consumed microalgae, they accumulate the omega-3s in their tissues 28.
The bottom line is that including seafood in your diet is healthful. Whether omega-3 supplements are beneficial is uncertain.
The 2020–2025 Dietary Guidelines for Americans states that strong evidence from mostly prospective cohort studies but also some randomized controlled trials shows that eating patterns that include seafood are associated with a reduced risk of cardiovascular disease 29. In addition, consuming about 8 ounces (less for children) per week of a variety of seafood that provides about 250 mg per day EPA and DHA is associated with fewer cardiac deaths in both healthy individuals and those with preexisting cardiovascular disease. Those who are pregnant or breastfeeding consume between 8 and 12 ounces per week of a variety of seafood from choices that are lower in mercury. These women should not consume certain types of fish, such as king mackerel, shark, swordfish, and tilefish that are high in methyl mercury, and they should limit the amount of white (albacore) tuna they consume to 6 ounces a week 30. The American Academy of Pediatrics has similar advice for breastfeeding women, recommending intakes of 200–300 mg DHA per day by consuming one to two servings of fish per week to guarantee a sufficient amount of DHA in breast milk 31.
Between 2017 and 2019, the American Heart Association (AHA) released three science advisories on omega-3s 32, 33, 34. All three advisories recommend one to two servings of seafood per week to reduce the risk of congestive heart failure, coronary heart disease, ischemic stroke, and sudden cardiac death, especially when the seafood replaces less healthy foods 34. For people with existing coronary heart disease, such as a recent myocardial infarction, the AHA recommends approximately 1 g/day EPA plus DHA, preferably from oily fish; however, supplements could also be considered under the direction of a physician 33. The American Heart Association does not recommend omega-3 supplements for people who do not have a high cardiovascular disease risk.
Table 1. Atlantic salmon wild and raw nutrition facts (100 g)
| Name | Amount | Unit |
|---|---|---|
| Water | 68.5 | g |
| Energy | 142 | kcal |
| Energy | 594 | kJ |
| Protein | 19.8 | g |
| Total lipid (fat) | 6.34 | g |
| Ash | 2.54 | g |
| Carbohydrate, by difference | 0 | g |
| Fiber, total dietary | 0 | g |
| Calcium, Ca | 12 | mg |
| Iron, Fe | 0.8 | mg |
| Magnesium, Mg | 29 | mg |
| Phosphorus, P | 200 | mg |
| Potassium, K | 490 | mg |
| Sodium, Na | 44 | mg |
| Zinc, Zn | 0.64 | mg |
| Copper, Cu | 0.25 | mg |
| Manganese, Mn | 0.016 | mg |
| Selenium, Se | 36.5 | µg |
| Vitamin C, total ascorbic acid | 0 | mg |
| Thiamin | 0.226 | mg |
| Riboflavin | 0.38 | mg |
| Niacin | 7.86 | mg |
| Pantothenic acid | 1.66 | mg |
| Vitamin B-6 | 0.818 | mg |
| Folate, total | 25 | µg |
| Folic acid | 0 | µg |
| Folate, food | 25 | µg |
| Folate, DFE | 25 | µg |
| Vitamin B-12 | 3.18 | µg |
| Vitamin A, RAE | 12 | µg |
| Retinol | 12 | µg |
| Vitamin A, IU | 40 | IU |
| Fatty acids, total saturated | 0.981 | g |
| SFA 14:0 | 0.137 | g |
| SFA 16:0 | 0.632 | g |
| SFA 18:0 | 0.212 | g |
| Fatty acids, total monounsaturated | 2.1 | g |
| MUFA 16:1 | 0.251 | g |
| MUFA 18:1 | 1.35 | g |
| MUFA 20:1 | 0.223 | g |
| MUFA 22:1 | 0.279 | g |
| Fatty acids, total polyunsaturated | 2.54 | g |
| PUFA 18:2 | 0.172 | g |
| PUFA 18:3 | 0.295 | g |
| PUFA 18:4 | 0.083 | g |
| PUFA 20:4 | 0.267 | g |
| PUFA 2:5 n-3 (EPA) | 0.321 | g |
| PUFA 22:5 n-3 (DPA) | 0.287 | g |
| PUFA 22:6 n-3 (DHA) | 1.12 | g |
| Cholesterol | 55 | mg |
| Tryptophan | 0.222 | g |
| Threonine | 0.87 | g |
| Isoleucine | 0.914 | g |
| Leucine | 1.61 | g |
| Lysine | 1.82 | g |
| Methionine | 0.587 | g |
| Cystine | 0.213 | g |
| Phenylalanine | 0.775 | g |
| Tyrosine | 0.67 | g |
| Valine | 1.02 | g |
| Arginine | 1.19 | g |
| Histidine | 0.584 | g |
| Alanine | 1.2 | g |
| Aspartic acid | 2.03 | g |
| Glutamic acid | 2.96 | g |
| Glycine | 0.952 | g |
| Proline | 0.702 | g |
| Serine | 0.809 | g |
Table 2. Atlantic salmon farmed and raw nutrition facts (100 g)
| Name | Amount | Unit |
|---|---|---|
| Water | 64.9 | g |
| Energy | 208 | kcal |
| Energy | 871 | kJ |
| Protein | 20.4 | g |
| Total lipid (fat) | 13.4 | g |
| Ash | 1.13 | g |
| Carbohydrate, by difference | 0 | g |
| Fiber, total dietary | 0 | g |
| Sugars, total including NLEA | 0 | g |
| Calcium, Ca | 9 | mg |
| Iron, Fe | 0.34 | mg |
| Magnesium, Mg | 27 | mg |
| Phosphorus, P | 240 | mg |
| Potassium, K | 363 | mg |
| Sodium, Na | 59 | mg |
| Zinc, Zn | 0.36 | mg |
| Copper, Cu | 0.045 | mg |
| Manganese, Mn | 0.011 | mg |
| Selenium, Se | 24 | µg |
| Vitamin C, total ascorbic acid | 3.9 | mg |
| Thiamin | 0.207 | mg |
| Riboflavin | 0.155 | mg |
| Niacin | 8.67 | mg |
| Pantothenic acid | 1.55 | mg |
| Vitamin B-6 | 0.636 | mg |
| Folate, total | 26 | µg |
| Folic acid | 0 | µg |
| Folate, food | 26 | µg |
| Folate, DFE | 26 | µg |
| Choline, total | 78.5 | mg |
| Betaine | 3 | mg |
| Vitamin B-12 | 3.23 | µg |
| Vitamin B-12, added | 0 | µg |
| Vitamin A, RAE | 58 | µg |
| Retinol | 58 | µg |
| Carotene, beta | 0 | µg |
| Carotene, alpha | 0 | µg |
| Cryptoxanthin, beta | 0 | µg |
| Vitamin A, IU | 193 | IU |
| Lycopene | 0 | µg |
| Lutein + zeaxanthin | 0 | µg |
| Vitamin E (alpha-tocopherol) | 3.55 | mg |
| Vitamin E, added | 0 | mg |
| Tocopherol, beta | 0.01 | mg |
| Tocopherol, gamma | 0.3 | mg |
| Tocopherol, delta | 0.05 | mg |
| Tocotrienol, alpha | 0.06 | mg |
| Tocotrienol, beta | 0 | mg |
| Tocotrienol, gamma | 0 | mg |
| Tocotrienol, delta | 0 | mg |
| Vitamin D (D2 + D3), International Units | 441 | IU |
| Vitamin D (D2 + D3) | 11 | µg |
| Vitamin K (phylloquinone) | 0.5 | µg |
| Vitamin K (Dihydrophylloquinone) | 0 | µg |
| Vitamin K (Menaquinone-4) | 0 | µg |
| Fatty acids, total saturated | 3.05 | g |
| SFA 4:0 | 0 | g |
| SFA 6:0 | 0 | g |
| SFA 8:0 | 0 | g |
| SFA 10:0 | 0 | g |
| SFA 12:0 | 0 | g |
| SFA 14:0 | 0.556 | g |
| SFA 15:0 | 0.046 | g |
| SFA 16:0 | 1.88 | g |
| SFA 17:0 | 0.043 | g |
| SFA 18:0 | 0.495 | g |
| SFA 20:0 | 0.022 | g |
| SFA 22:0 | 0.011 | g |
| SFA 24:0 | 0 | g |
| Fatty acids, total monounsaturated | 3.77 | g |
| MUFA 14:1 | 0 | g |
| MUFA 15:1 | 0 | g |
| MUFA 16:1 | 0.791 | g |
| MUFA 17:1 | 0 | g |
| MUFA 18:1 | 2.72 | g |
| MUFA 20:1 | 0.265 | g |
| MUFA 22:1 | 0 | g |
| Fatty acids, total polyunsaturated | 3.89 | g |
| PUFA 18:2 | 0.9 | g |
| PUFA 18:3 | 0.167 | g |
| PUFA 18:3 n-3 c,c,c (ALA) | 0.148 | g |
| PUFA 18:3 n-6 c,c,c | 0.02 | g |
| PUFA 18:4 | 0.121 | g |
| PUFA 20:2 n-6 c,c | 0.062 | g |
| PUFA 20:3 | 0.018 | g |
| PUFA 20:4 | 0.092 | g |
| PUFA 2:5 n-3 (EPA) | 0.862 | g |
| PUFA 22:5 n-3 (DPA) | 0.393 | g |
| PUFA 22:6 n-3 (DHA) | 1.1 | g |
| Cholesterol | 55 | mg |
| Tryptophan | 0.209 | g |
| Threonine | 0.86 | g |
| Isoleucine | 0.968 | g |
| Leucine | 1.62 | g |
| Lysine | 1.87 | g |
| Methionine | 0.626 | g |
| Cystine | 0.219 | g |
| Phenylalanine | 0.845 | g |
| Tyrosine | 0.759 | g |
| Valine | 1.11 | g |
| Arginine | 1.22 | g |
| Histidine | 0.549 | g |
| Alanine | 1.27 | g |
| Aspartic acid | 2.02 | g |
| Glutamic acid | 2.83 | g |
| Glycine | 0.96 | g |
| Proline | 0.721 | g |
| Serine | 0.896 | g |
| Hydroxyproline | 0.042 | g |
| Alcohol, ethyl | 0 | g |
| Caffeine | 0 | mg |
| Theobromine | 0 | mg |
Table 3. Atlantic salmon wild and cooked in dry heat nutrition facts (100 g)
| Name | Amount | Unit |
|---|---|---|
| Water | 59.6 | g |
| Energy | 182 | kcal |
| Energy | 761 | kJ |
| Protein | 25.4 | g |
| Total lipid (fat) | 8.13 | g |
| Ash | 3.26 | g |
| Carbohydrate, by difference | 0 | g |
| Fiber, total dietary | 0 | g |
| Calcium, Ca | 15 | mg |
| Iron, Fe | 1.03 | mg |
| Magnesium, Mg | 37 | mg |
| Phosphorus, P | 256 | mg |
| Potassium, K | 628 | mg |
| Sodium, Na | 56 | mg |
| Zinc, Zn | 0.82 | mg |
| Copper, Cu | 0.321 | mg |
| Manganese, Mn | 0.021 | mg |
| Selenium, Se | 46.8 | µg |
| Vitamin C, total ascorbic acid | 0 | mg |
| Thiamin | 0.275 | mg |
| Riboflavin | 0.487 | mg |
| Niacin | 10.1 | mg |
| Pantothenic acid | 1.92 | mg |
| Vitamin B-6 | 0.944 | mg |
| Folate, total | 29 | µg |
| Folic acid | 0 | µg |
| Folate, food | 29 | µg |
| Folate, DFE | 29 | µg |
| Vitamin B-12 | 3.05 | µg |
| Vitamin A, RAE | 13 | µg |
| Retinol | 13 | µg |
| Vitamin A, IU | 44 | IU |
| Fatty acids, total saturated | 1.26 | g |
| SFA 14:0 | 0.176 | g |
| SFA 16:0 | 0.81 | g |
| SFA 18:0 | 0.272 | g |
| Fatty acids, total monounsaturated | 2.7 | g |
| MUFA 16:1 | 0.322 | g |
| MUFA 18:1 | 1.73 | g |
| MUFA 20:1 | 0.286 | g |
| MUFA 22:1 | 0.358 | g |
| Fatty acids, total polyunsaturated | 3.26 | g |
| PUFA 18:2 | 0.22 | g |
| PUFA 18:3 | 0.378 | g |
| PUFA 18:4 | 0.106 | g |
| PUFA 20:4 | 0.342 | g |
| PUFA 2:5 n-3 (EPA) | 0.411 | g |
| PUFA 22:5 n-3 (DPA) | 0.368 | g |
| PUFA 22:6 n-3 (DHA) | 1.43 | g |
| Cholesterol | 71 | mg |
| Tryptophan | 0.285 | g |
| Threonine | 1.12 | g |
| Isoleucine | 1.17 | g |
| Leucine | 2.07 | g |
| Lysine | 2.34 | g |
| Methionine | 0.753 | g |
| Cystine | 0.273 | g |
| Phenylalanine | 0.993 | g |
| Tyrosine | 0.859 | g |
| Valine | 1.31 | g |
| Arginine | 1.52 | g |
| Histidine | 0.749 | g |
| Alanine | 1.54 | g |
| Aspartic acid | 2.6 | g |
| Glutamic acid | 3.8 | g |
| Glycine | 1.22 | g |
| Proline | 0.899 | g |
| Serine | 1.04 | g |
Table 4. Atlantic salmon farmed and cooked in dry heat nutrition facts (100 g)
| Name | Amount | Unit |
|---|---|---|
| Water | 64.8 | g |
| Energy | 206 | kcal |
| Energy | 861 | kJ |
| Protein | 22.1 | g |
| Total lipid (fat) | 12.4 | g |
| Ash | 1.15 | g |
| Carbohydrate, by difference | 0 | g |
| Fiber, total dietary | 0 | g |
| Sugars, total including NLEA | 0 | g |
| Calcium, Ca | 15 | mg |
| Iron, Fe | 0.34 | mg |
| Magnesium, Mg | 30 | mg |
| Phosphorus, P | 252 | mg |
| Potassium, K | 384 | mg |
| Sodium, Na | 61 | mg |
| Zinc, Zn | 0.43 | mg |
| Copper, Cu | 0.049 | mg |
| Manganese, Mn | 0.016 | mg |
| Selenium, Se | 41.4 | µg |
| Vitamin C, total ascorbic acid | 3.7 | mg |
| Thiamin | 0.34 | mg |
| Riboflavin | 0.135 | mg |
| Niacin | 8.04 | mg |
| Pantothenic acid | 1.48 | mg |
| Vitamin B-6 | 0.647 | mg |
| Folate, total | 34 | µg |
| Folic acid | 0 | µg |
| Folate, food | 34 | µg |
| Folate, DFE | 34 | µg |
| Choline, total | 90.5 | mg |
| Vitamin B-12 | 2.8 | µg |
| Vitamin B-12, added | 0 | µg |
| Vitamin A, RAE | 69 | µg |
| Retinol | 69 | µg |
| Carotene, beta | 0 | µg |
| Carotene, alpha | 0 | µg |
| Cryptoxanthin, beta | 0 | µg |
| Vitamin A, IU | 230 | IU |
| Lycopene | 0 | µg |
| Lutein + zeaxanthin | 0 | µg |
| Vitamin E (alpha-tocopherol) | 1.14 | mg |
| Vitamin E, added | 0 | mg |
| Vitamin D (D2 + D3), International Units | 526 | IU |
| Vitamin D (D2 + D3) | 13.1 | µg |
| Vitamin K (phylloquinone) | 0.1 | µg |
| Fatty acids, total saturated | 2.4 | g |
| SFA 4:0 | 0 | g |
| SFA 6:0 | 0 | g |
| SFA 8:0 | 0 | g |
| SFA 10:0 | 0.005 | g |
| SFA 12:0 | 0.013 | g |
| SFA 14:0 | 0.571 | g |
| SFA 16:0 | 1.49 | g |
| SFA 18:0 | 0.315 | g |
| Fatty acids, total monounsaturated | 4.18 | g |
| MUFA 16:1 | 0.767 | g |
| MUFA 18:1 | 2.05 | g |
| MUFA 20:1 | 1.37 | g |
| MUFA 22:1 | 0 | g |
| Fatty acids, total polyunsaturated | 4.55 | g |
| PUFA 18:2 | 0.666 | g |
| PUFA 18:3 | 0.113 | g |
| PUFA 18:4 | 0.184 | g |
| PUFA 20:4 | 1.27 | g |
| PUFA 2:5 n-3 (EPA) | 0.69 | g |
| PUFA 22:5 n-3 (DPA) | 0.17 | g |
| PUFA 22:6 n-3 (DHA) | 1.46 | g |
| Cholesterol | 63 | mg |
| Tryptophan | 0.248 | g |
| Threonine | 0.969 | g |
| Isoleucine | 1.02 | g |
| Leucine | 1.8 | g |
| Lysine | 2.03 | g |
| Methionine | 0.654 | g |
| Cystine | 0.237 | g |
| Phenylalanine | 0.863 | g |
| Tyrosine | 0.746 | g |
| Valine | 1.14 | g |
| Arginine | 1.32 | g |
| Histidine | 0.651 | g |
| Alanine | 1.34 | g |
| Aspartic acid | 2.26 | g |
| Glutamic acid | 3.3 | g |
| Glycine | 1.06 | g |
| Proline | 0.781 | g |
| Serine | 0.902 | g |
| Alcohol, ethyl | 0 | g |
| Caffeine | 0 | mg |
| Theobromine | 0 | mg |
Salmon health benefits
Omega-3 fatty acids are a group of polyunsaturated fatty acids (PUFAs) that are important for a number of functions in your body. The omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are found in seafood, such as fatty fish (e.g., salmon, tuna, and trout), shellfish (e.g., crab, mussels, and oysters), fish oils and krill oils, but they are originally synthesized by microalgae, not by the fish 27. When fish consume phytoplankton that consumed microalgae, they accumulate the omega-3s in their tissues 28. A different kind of omega-3, called alpha-linolenic acid (ALA), is found in other foods, including some plant oils (e.g., canola and soy) 28. Omega-3s are also available as dietary supplements; for example, fish oil supplements contain EPA and DHA, and flaxseed oil supplements contain ALA. Moderate evidence has emerged about the health benefits of consuming seafood. The health benefits of omega-3 dietary supplements are unclear.
Atlantic salmon is one of the best sources of omega-3 fatty acids found in nature due to the elevated content of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in the flesh. Since your body cannot produce omega-3 fatty acids naturally you must look to source these from what you eat. Several different omega-3 fatty acids exist, but the majority of scientific research focuses on three: alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). Many observational studies link higher intakes of fish and other seafood with improved health outcomes. However, it is difficult to ascertain whether the benefits are due to the omega-3 content of the seafood (which varies among species), other components in the seafood, the substitution of seafood for other less healthful foods, other healthful behaviors, or a combination of these factors. Data from randomized clinical trials are needed to shed light on these questions.
Alpha-linolenic acid (ALA) and linoleic acid are considered essential fatty acids, meaning that your body can’t make it, so you must get it from the foods and beverages you consume 39. Your body can convert some ALA into eicosapentaenoic acid (EPA) and then to docosahexaenoic acid (DHA), but the conversion (which occurs primarily in your liver) is very limited, with reported rates of less than 15% 28. Therefore, consuming EPA and DHA directly from foods and/or dietary supplements is the only practical way to increase levels of omega-3 fatty acids in your body.
Omega-3s play important roles in your body as components of the phospholipids that form the structures of cell membranes 40. DHA, in particular, is especially high in the retina, brain, and sperm 41. In addition to their structural role in cell membranes, omega-3 fatty acids (along with omega-6 fatty acids) provide energy for the body and are used to form eicosanoids. Eicosanoids are signaling molecules that have similar chemical structures to the fatty acids from which they are derived; they have wide-ranging functions in the body’s cardiovascular, pulmonary, immune, and endocrine systems 42.
Some researchers propose that the relative intakes of omega-6s and omega-3s and the omega-6/omega-3 ratio may have important implications for the origination and development of many chronic diseases, such as cardiovascular disease and cancer 43, but the optimal ratio—if any—has not been defined 44. Others have concluded that omega-6/omega-3 ratios are too non-specific and are insensitive to individual fatty acid levels 45. Most agree that raising EPA and DHA blood levels is far more important than lowering linoleic acid or arachidonic acid levels.
Currently, most clinicians do not assess omega-3 status, but it can be done by measuring individual omega-3s in plasma or serum phospholipids and expressing them as the percentage of total phospholipid fatty acids by weight 46. Experts have not established normal ranges, but mean values for serum or plasma phospholipid EPA plus DHA among U.S. adults not taking omega-3 supplements are about 3%–4% 47. Plasma and serum fatty acid values, however, can vary substantially based on an individual’s most recent meal, so they do not reflect long-term dietary consumption 48.
The “omega-3 index” proposed by Harris and von Schacky reflects the content of EPA plus DHA in red blood cell membranes expressed as a percentage of total red blood cell fatty acids 49. The “omega-3 index” can be used as a surrogate for assessing tissue levels of EPA plus DHA 50. EPA and DHA typically comprise about 3%–5% of red blood cell fatty acids in Western populations with low fish intakes. In Japan, where fish consumption is high, erythrocyte EPA and DHA levels are about twice those of Western populations 28.
Here are things you should know about omega-3s:
- A deficiency of essential fatty acids either omega-3s or omega-6s can cause rough, scaly skin and dermatitis 40. Plasma and tissue concentrations of DHA decrease when an omega-3 fatty acid deficiency is present. However, there are no known cut-off concentrations of DHA or EPA below which functional endpoints, such as those for visual or neural function or for immune response, are impaired.
- Evidence that higher long-chain omega-3 levels are associated with a reduced risk of several chronic diseases, including coronary heart disease, suggests that many Americans could benefit from slightly higher intakes.
- Research indicates that consuming fish and other types of seafood as part of a balanced diet promotes heart health, especially when the seafood is consumed in place of less healthy foods. Fish oil and other long-chain omega-3 supplements lower triglyceride levels and might reduce the risk of some cardiovascular endpoints, especially among people with low dietary omega-3 intakes. Evidence of a protective effect for omega-3 supplementation is stronger for people with existing coronary heart disease than for healthy individuals.
- Results of studies on diets rich in seafood (fish and shellfish) and heart disease provide moderate evidence that people who eat seafood at least once a week are less likely to die of heart disease than those who rarely or never eat seafood. The Dietary Guidelines for Americans, 2015 includes a new recommendation that adults eat 8 or more ounces of a variety of seafood per week because it provides a range of nutrients, including omega-3 fatty acids. Smaller amounts are recommended for young children, and there are special recommendations for pregnant or breastfeeding women.
- Evidence suggests that seafood rich in EPA and DHA should be included in a heart-healthy diet; however, supplements of EPA and DHA have not been shown to protect against heart disease. In 2012, two groups of scientists analyzed the research on the effects of EPA/DHA supplements on heart disease risk. One group analyzed only studies in people with a history of heart disease, and the other group analyzed studies in people both with and without a history of heart disease. Neither review found strong evidence of a protective effect of the EPA/DHA supplements.
- In 2004, the FDA approved a qualified health claim for conventional foods and dietary supplements that contain EPA and DHA 30. This health claim states, “Supportive but not conclusive research shows that consumption of EPA and DHA omega-3 fatty acids may reduce the risk of coronary heart disease.” The FDA also specifies that the labels of dietary supplements should not recommend a daily intake of EPA and DHA higher than 2 g 30.
- Fish are part of a healthy eating pattern and provide key nutrients during pregnancy, breastfeeding, and/or early childhood to support a child’s brain development 30. Moderate scientific evidence shows that eating patterns relatively higher in fish but also in other foods, including vegetables, fruits, legumes, whole grains, low- or non-fat dairy, lean meats and poultry, nuts, and unsaturated vegetable oils, and lower in red and processed meats, sugar-sweetened foods and beverages, and refined grains are associated with 30:
- Fish provide iron and zinc to support children’s immune systems. Fish are a source of other nutrients like protein, vitamin B12, vitamin D, and selenium too.
- Promotion of bone health – decreases the risk for hip fractures
- Decreases in the risk of becoming overweight or obese
- Decreases in the risk for colon and rectal cancers.
- A 2012 review of the scientific literature concluded that EPA and DHA, the types of omega-3s found in seafood and fish oil, may be modestly helpful in relieving symptoms of rheumatoid arthritis. In the studies included in the review, many of the participants reported that when they were taking fish oil they had briefer morning stiffness, less joint swelling and pain, and less need for anti-inflammatory drugs to control their symptoms.
- The nutritional value of seafood is of particular importance during fetal growth and development, as well as in early infancy and childhood. Women who are pregnant or breastfeed should consume 8 to 12 ounces of seafood per week from a variety of seafood types that are low in methyl mercury as part of a healthy eating pattern and while staying within their calorie needs. Pregnant or breastfeeding women should limit the amount of white tuna (labeled as “albacore”) to no more than 6 ounces per week. They should not eat tilefish, shark, swordfish, and king mackerel because they are high in methyl mercury.
- There is ongoing research on omega-3 fatty acids and diseases of the brain and eye, but there is not enough evidence to draw conclusions about the effectiveness of omega-3s for these conditions. DHA plays important roles in the functioning of the brain and the eye. Researchers are actively investigating the possible benefits of DHA and other omega-3 fatty acids in preventing or treating a variety of brain- and eye-related conditions.
- There is conflicting evidence about whether a link might exist between the omega-3 fatty acids found in seafood and fish oil (EPA/DHA) and an increased risk of prostate cancer. Additional research on the association of omega-3 consumption and prostate cancer risk is under way.
Cardiovascular disease
Many studies show that eating fatty fish and other types of seafood as part of a healthy eating pattern helps keep your heart healthy and helps protect you from some heart problems. This interest was spurred by epidemiological research dating back to the 1970s that found low rates of heart attack (myocardial infarction) and other coronary events among Greenland Inuit and other fish-eating populations, such as those in Japan 28. Results from observational studies have been consistent with these findings, with several systematic reviews and meta-analyses showing that higher consumption of fish and higher dietary or plasma levels of omega-3s are associated with a lower risk of heart failure, coronary disease, and fatal coronary heart disease 51.
The American Heart Association (AHA) recommends eating one to two servings of seafood per week to reduce your risk of some heart problems, especially if you consume the seafood in place of less healthy foods. For people with heart disease, the AHA recommends consuming about 1 g per day EPA plus DHA, preferably from oily fish, but supplements are an option under the guidance of a healthcare provider. The AHA does not recommend omega-3 supplements for people who do not have a high risk of cardiovascular disease.
Many studies have assessed the effects of omega-3s—primarily EPA and DHA—on cardiovascular disease and cardiovascular disease risk factors, such as high blood pressure and elevated plasma lipids.
Clinical trial data from the 1989 Diet and Reinfarction Trial, the 1999 open-label GISSI-Prevenzione trial 52 and others supported the hypothesis that long-chain omega-3s offer protection from cardiovascular disease by reducing the heart’s susceptibility to arrhythmias, lowering triglyceride levels, lowering blood pressure, and decreasing platelet aggregation 53. The authors of a systematic review that included six secondary-prevention trials and one primary-prevention trial of omega-3 supplementation published between 1966 and 2005 concluded that consumption of long-chain omega-3s from fish and fish oil supplements reduces rates of all-cause mortality, cardiac death, sudden death, and stroke 53. They noted that the evidence of benefit is stronger for secondary than for primary prevention.
Results from the Japan EPA Lipid Intervention Study in 2007 54 supported the growing body of evidence that long-chain omega-3s reduce the risk of heart disease, especially in people with a history of coronary artery disease. In this study, 18,645 people with hypercholesterolemia (total cholesterol of at least 251 mg/dL) with or without coronary artery disease received either 1.8 g/day EPA plus a statin or a statin only. After a mean of 4.6 years, the EPA group had 19% fewer major coronary events than the control group. The EPA group also experienced a significant reduction in rates of unstable angina and nonfatal coronary events but not in rates of sudden cardiac death or coronary death in comparison with the control group 54.
In an analysis of the primary prevention subgroup from this study (participants with no history of coronary artery disease), EPA supplementation had no significant effects on any outcome. However, for the secondary prevention subgroup (those with a history of coronary artery disease), the EPA group had a 28% reduction in the rate of unstable angina and a 19% reduction in that of major coronary events. A separate analysis of data from this study found that the EPA supplementation did not affect total stroke incidence but did reduce the risk of recurrent stroke by 20% in patients who had previously experienced a stroke 55.
Several subsequent clinical trials, however, had largely null findings 56, 57, 58. For example, the 2012 Outcome Reduction with an Initial Glargine Intervention (ORIGIN) trial included 12,536 patients who had diabetes or a high risk of diabetes and a high risk of cardiovascular events. Supplementation with 1 g/day omega-3s (375 mg DHA and 465 mg EPA) for about 6 years significantly lowered triglyceride levels but had no effect on risk of myocardial infarction, stroke, or death from cardiovascular causes in comparison with placebo 57. Similarly, in the 2010 Alpha Omega Trial, low-dose EPA and DHA supplementation (150 mg DHA and 226 mg EPA daily, supplied in a margarine) for 40 months also failed to reduce the rate of major cardiovascular events in comparison with placebo among 4,837 older men and women who had previously experienced a myocardial infarction and were receiving antihypertensive, antithrombotic, and/or lipid-lowering medications 58.
In recent clinical trials, scientists gained additional insight into the effects of omega-3s for the primary prevention of cardiovascular disease, including in patients with diabetes. From two 2018 trials: VITamin D and OmegA-3 TriaL (VITAL) 59 and A Study of Cardiovascular Events in Diabetes (ASCEND) 60. Both trials compared the same 1 g/day omega-3 formulation (460 mg EPA and 380 mg DHA) with placebo, but in different populations. VITAL included 25,871 men aged 50 and older and women aged 55 and older with no previous heart attacks, strokes, or cancer, whereas ASCEND included 15,480 adults aged 40 or older with diabetes but no evidence of cardiovascular disease. VITAL also tested the omega-3 supplement with and without 2,000 IU/day vitamin D.
In VITAL trial, the omega-3 supplement did not significantly reduce the rate of major cardiovascular events combined (myocardial infarction, stroke, and cardiovascular mortality) after a median of 5.3 years 59. However, participants taking the omega-3 supplement did experience a statistically significant 28% reduction in total myocardial infarction rates (including a 77% reduction among African Americans and a 40% reduction among those who consumed less than 1.5 servings of fish per week). Supplement users also had significant reductions in rates of fatal myocardial infarction, total coronary heart disease, and percutaneous coronary intervention (a procedure that widens blocked or narrowed coronary arteries). No significant reductions in stroke or death rates from cardiovascular causes were observed.
ASCEND trial had similar findings 60. After a mean follow-up of 7.4 years, the omega-3 supplement did not significantly affect the risk of a serious vascular event (composite of nonfatal myocardial infarction or stroke, transient ischemic attack, and cardiovascular death, excluding intracranial hemorrhage) or revascularization. However, omega-3 supplementation did significantly reduce the risk of cardiovascular death by 19% in comparison with placebo.
The 2019 Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial (REDUCE-IT) found significant cardiovascular disease benefits with Vascepa, a high-dose, prescription form of omega-3s containing EPA in the form of icosapent ethyl (IPE), an ethyl ester 61. REDUCE-IT trial included 8,179 participants with cardiovascular disease aged 45 years or older or with diabetes and at least one other risk factor aged 50 years or older. All participants had a fasting triglyceride level of 135 to 499 mg/dL even though they were receiving statin therapy, and an LDL cholesterol level of 41 to 100 mg/dL. Patients received either 4 g/day IPE or placebo for a median of 4.9 years. Icosapent ethyl (IPE) significantly reduced rates of cardiovascular events (a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, and unstable angina) by 25%. Icosapent ethyl (IPE) also significantly reduced rates of other outcomes, including cardiovascular death by 20%, fatal or nonfatal stroke by 28%, and fatal or nonfatal myocardial infarction by 31%.
Possible reasons for conflicting findings
Dose probably plays a major role in the ability of omega-3 supplementation to confer significant benefits 62. The REDUCE-IT trial findings suggest that a high daily dose of icosapent ethyl (IPE), 4 g, is an effective adjunct to statin therapy in people with cardiovascular disease or a high risk of cardiovascular disease 59. The daily dose of 1 g used in many studies of omega-3 dietary supplements might affect some cardiovascular disease pathways 62 but has had no significant effect on the primary outcomes in several trials 60. Other factors, including the omega-3 form, study population, background dietary omega-3 intakes, and use of statins and other cardioprotective therapies might also explain the conflicting findings among studies 63.
Furthermore, the effects of long-chain omega-3s are not uniform across cardiovascular disease outcomes. Therefore, use of primary composite endpoints that combine multiple outcomes might dilute significant effects on individual components of those endpoints 63. This dilution occurred, for example, in both VITAL 59 and ASCEND 60.
A 2019 systematic review and meta-analysis of 13 trials included ASCEND, VITAL, and REDUCE-IT and a total of 127,477 participants 63. Omega-3 doses ranged from 0.376 to 4 g/day, and the mean treatment duration was 5 years. The authors concluded that long-chain omega-3 supplementation reduces the risk of myocardial infarction, coronary heart disease death, total coronary heart disease, cardiovascular disease death, and total cardiovascular disease, and the effects appear to be dose related. However, the findings showed no significant associations for risk of fatal and nonfatal stroke. The authors noted that REDUCE-IT reduced risk of stroke significantly 61, suggesting that a higher dose of omega-3s (4 g/day) might be needed to affect this outcome.
A 2020 Cochrane review of 86 randomized controlled trials published between 1968 and 2019 found that 0.5 g/day to more than 5 g/day omega-3s for 12 to 88 months in a total of 162,796 participants reduced serum triglyceride levels by about 15% and slightly decreased rates of cardiovascular mortality and coronary heart disease events 64. However, the supplements did not affect all-cause mortality, cardiovascular events, stroke, or arrhythmia. The authors of several earlier meta-analyses and systematic reviews, as well as a 2016 report from the Agency for Healthcare Research and Quality (AHRQ) 65, concluded that omega-3 supplements do not appear to significantly reduce the risk of most cardiovascular events 66. Many of these analyses, however, but not all 65, 67, did find that omega-3s reduce the risk of cardiac death.
Recommendations from the American Heart Association (AHA) and the Dietary Guidelines for Americans
Between 2017 and 2019, the American Heart Association (AHA) released three science advisories on omega-3s 32, 33, 34. All three advisories recommend one to two servings of seafood per week to reduce the risk of congestive heart failure, coronary heart disease, ischemic stroke, and sudden cardiac death, especially when the seafood replaces less healthy foods 34. For people with existing coronary heart disease, such as a recent myocardial infarction, the AHA recommends approximately 1 g/day EPA plus DHA, preferably from oily fish; however, supplements could also be considered under the direction of a physician 33. The American Heart Association does not recommend omega-3 supplements for people who do not have a high cardiovascular disease risk.
To manage high triglyceride levels, the American Heart Association concludes that 4 g/day prescription omega-3s (containing EPA plus DHA or EPA only) lower triglyceride levels when used alone or as adjuncts to other lipid-lowering medications 32. Although this finding pertains to high-dose prescription omega-3s, an earlier analysis of 58 trials also revealed a dose-response relationship between lower-dose dietary and supplemental omega-3 intakes and triglyceride levels 68. Each 1 g/day of omega-3 increase reduced triglyceride levels by 5.9 mg/dL, and the effect was stronger in people with higher baseline triglyceride levels.
The 2020–2025 Dietary Guidelines for Americans states that strong evidence from mostly prospective cohort studies but also some randomized controlled trials shows that eating patterns that include seafood are associated with a reduced risk of cardiovascular disease 29. In addition, consuming about 8 ounces (less for children) per week of a variety of seafood that provides about 250 mg per day EPA and DHA is associated with fewer cardiac deaths in both healthy individuals and those with preexisting cardiovascular disease. Those who are pregnant or breastfeeding consume between 8 and 12 ounces per week of a variety of seafood from choices that are lower in mercury. These women should not consume certain types of fish, such as king mackerel, shark, swordfish, and tilefish that are high in methyl mercury, and they should limit the amount of white (albacore) tuna they consume to 6 ounces a week 30. The American Academy of Pediatrics has similar advice for breastfeeding women, recommending intakes of 200–300 mg DHA per day by consuming one to two servings of fish per week to guarantee a sufficient amount of DHA in breast milk 31.
Infant health and development
During pregnancy and breastfeeding, eating 8 to 12 ounces per week of fish and other seafood may improve your baby’s health. However, it is important to choose fish that are higher in EPA and DHA and lower in mercury. Examples are salmon, herring, sardines, and trout. It is not clear whether taking dietary supplements containing EPA and DHA during pregnancy or breastfeeding affects a baby’s health or development. However, some studies show that taking these supplements may slightly increase a baby’s weight at birth and the length of time the baby is in the womb, both of which may be beneficial. Breast milk contains DHA. Most commercial infant formulas also contain DHA. However, the authors of a paper published by the American Academy of Family Physicians 69 and of two Cochrane reviews (one on full-term infants and one on preterm infants) 70, 71 have concluded that the evidence is insufficient to recommend the use of infant formulas that are supplemented with these fatty acids.
Numerous studies have examined the effects of maternal seafood and omega-3 intakes on infant birth weight, length of gestation, visual and cognitive development, and other infant health outcomes. High concentrations of DHA are present in the cellular membranes of the brain and retina 40 and DHA is important for fetal growth and development. The accumulation of DHA in the retina is complete by birth, whereas accumulation in the brain continues throughout the first 2 years after birth.
Observational studies indicate that maternal consumption, during pregnancy and breastfeeding, of at least 8 ounces per week of seafood that contains DHA is associated with better infant health outcomes 29. For example, in a prospective cohort study of 341 mother–child pairs in the United States, maternal fish consumption more than twice per week compared to no weekly consumption was associated with improved visual motor skills in their children at age 3 after adjustment for covariates such as maternal age, education, maternal smoking and alcohol use during pregnancy, paternal education, and fetal growth 72. In another observational cohort study in the United Kingdom in 11,875 pregnant women who reported seafood intakes ranging from none to more than 340 g (about 12 ounces) per week, lower consumption of seafood during pregnancy was associated with an increased risk of suboptimal communication skills in the offspring at ages 6 and 18 months and suboptimal verbal IQ and prosocial behavior at age 7–8 years 73. It is not possible to establish causality, however, because all of these studies were observational.
Seafood contains varying levels of methyl mercury 30. However, results from numerous studies, including a systematic review of the literature on maternal fish intake and subsequent neurodevelopmental outcomes, show that the health benefits of consuming moderate amounts of seafood during the prenatal period outweigh the risks 74.
Several randomized controlled trials have examined whether supplementation with fish oil, EPA, and/or DHA during pregnancy and early infancy is beneficial for infant health and neurodevelopment. One of these trials examined the effects of fish oil supplementation in 2,399 pregnant women on the subsequent clinical outcomes and neurodevelopment of their children 75. Pregnant women received daily supplements of either fish oil (providing 800 mg DHA and 100 mg EPA) or placebo from less than 21 weeks’ gestation until the birth of their child. Compared to the placebo group, children of mothers who received fish oil were heavier at birth and less likely to be born very preterm (less than 34 weeks’ gestation). However, assessments of 726 of the children (all 96 preterm children and 630 randomly selected full-term children) found no differences between groups in mean cognitive composite scores or mean language composite scores at age 18 months. A follow-up study of the children at age 4 years found no differences between groups in general conceptual ability score or other assessments of cognition, language, and executive functioning 76. Another study found no benefits on visual function at age 7 years when very preterm infants (less than 33 weeks’ gestation) consumed human milk with a higher DHA concentration than normal (lactating mothers took 900 mg/day DHA supplements) for the first months of life until full term 77. In a clinical trial in 420 healthy full-term infants, those who received either DHA-enriched fish oil (250 mg DHA and 60 mg EPA) or placebo daily from birth to 6 months had similar scores on neurodevelopment assessments at 18 months 78. However, infants receiving fish oil had significantly better performance on language assessments, indicating some benefit for early communication development.
The authors of a systematic review and meta-analysis of 11 randomized controlled trials concluded that the evidence neither supports nor refutes the benefits of omega-3 supplementation during pregnancy for cognitive or visual development in infants 79. Another systematic review and meta-analysis that included two randomized controlled trials in women with a previous preterm birth found no significant differences in rates of recurrent preterm birth between women who took omega-3 supplements during pregnancy and those who did not 79. Omega-3 supplementation did, however, increase latency (time from randomization to birth) by about 2 days and mean birth weight by about 103 g.
In 2016, AHRQ 80 published a review on the effects of omega-3 fatty acids on child and maternal health. This comprehensive report evaluated the findings from 95 randomized controlled trials and 48 prospective longitudinal studies and nested case-control studies. Most studies examined the effects of fish oil supplements or other DHA and EPA combinations in pregnant or breastfeeding women or of infant formula fortified with DHA plus arachidonic acid, an omega-6. The authors concluded that, except for small beneficial effects on infant birth weight and length of gestation, omega-3 supplementation or fortification has no consistent effects on infant health outcomes 80.
Cancer prevention
Some studies suggest that people who get more omega-3s from foods and dietary supplements may have a lower risk of breast cancer and perhaps colorectal cancer. But a large clinical trial found that omega-3 supplements did not reduce the overall risk of cancer, or the risk of breast, prostate, or colorectal cancers. Overall, data from observational studies show no consistent relationship between omega-3s and overall cancer risk. Additional randomized clinical trials in progress will help clarify whether omega-3s affect cancer risk.
Researchers have hypothesized that higher intakes of omega-3s from either foods or supplements might reduce the risk of cancer due to their anti-inflammatory effects and potential to inhibit cell growth factors 81. Results from observational studies however, have been inconsistent and vary by cancer site and other factors, including gender and genetic risk. For example, some studies have shown associations between higher intakes and/or blood levels of omega-3s and a decreased risk of certain cancers, including breast 82 and colorectal cancers 83. Other studies have found no associations between omega-3s and cancer risk, and some have even found associations in the opposite direction, suggesting that omega-3s might increase the risk of certain cancers such as prostate cancer 84. The first large-scale clinical trial to examine the effects of omega-3s on the primary prevention of cancer in the general population was the newly published VITAL trial 59. This clinical trial examined the effects of omega-3 fish oil supplementation (1 g/day containing 460 mg EPA and 380 mg DHA) with or without 2,000 IU/day vitamin D for a median of 5.3 years 59. The study included 25,871 men aged 50 and older and women aged 55 and older with no previous cancer, heart attacks, or strokes. Compared with placebo, the omega-3 supplement had no significant effect on cancer incidence, cancer mortality rates, or the development of breast, prostate, or colorectal cancers.
Breast cancer
Evidence from several observational studies suggests that higher intakes of omega-3s are associated with a lower risk of breast cancer, but clinical trials are needed to confirm this finding. In the prospective Singapore Chinese Health Study of 35,298 women aged 45–74 years, those in the top three quartiles of dietary omega-3 intake had a 26% lower risk of breast cancer after an average of 5.3 years of follow-up than those in the lowest quartile 85. Similarly, among 35,016 female participants aged 50–76 years in the VITamins And Lifestyle (VITAL) Cohort, those who reported current use of fish-oil supplements had a 32% lower risk of breast cancer after a mean of 6 years than those who did not take fish oil 86.
According to a systematic review of three case-control studies and five prospective studies published in 2007–2011, evidence is increasing that higher intakes of dietary and supplemental omega-3s are associated with a lower risk of breast cancer 87. Similarly, the authors of a meta-analysis of data from 21 prospective cohort studies concluded that women with the highest dietary intakes and/or tissue levels of omega-3s had a 14% lower risk of breast cancer than those with the lowest intakes and tissue levels 82. These authors also found a dose-response relationship between higher intakes of combined omega-3s and reduced breast cancer risk. Intakes of ALA and of fish, however, had no association with differences in breast cancer risk. This finding, which could be due to varying levels of omega-3s in different fish species, warrants further investigation.
Colorectal cancer
Limited evidence from observational studies suggests that greater consumption of fish and omega-3s is associated with a reduced risk of colorectal cancer 87. The authors of a meta-analysis of 19 prospective cohort studies found no significant association between fish intake and risk of colorectal cancer overall. However, a stratified analysis showed that for participants with the highest fish consumption (those who ate fish at least seven times more often per month than those with the lowest fish consumption), the risk of colorectal cancer was 22% lower than that for the lowest fish consumers 88. Results from a more recent systematic review and meta-analysis of 22 prospective cohort studies and 19 case-control studies indicate that fish consumption is inversely associated with colorectal cancer risk. In this analysis, 21 of the studies distinguished between colon cancer and rectal cancer. The risk of rectal cancer was 21% lower for participants with the highest fish intakes (as much as one serving/day) compared to those with the lowest fish intakes (as little as none), but fish consumption had no significant association with risk of colon cancer alone 83.
Results from the VITamins And Lifestyle (VITAL) Cohort study suggest that associations between fish or omega-3 intakes and colorectal cancer risk might vary by such factors as gender and genetic risk. In this study, researchers evaluated associations between colorectal cancer risk and EPA/DHA intakes from fatty fish (salmon and fresh tuna) and fish oil supplements in 68,109 Washington residents aged 50–76 89. The amount of fatty fish consumed ranged from none to 0.8 servings per week or more. Overall, EPA and DHA intakes (from either diet or supplements) and fatty fish consumption were not associated with colorectal cancer risk, but associations varied by genetic characteristics (certain inherited genetic mutations are associated with an increased risk of colorectal cancer). For individuals in the lowest two tertiles of genetic risk, higher fatty fish consumption and higher total EPA and DHA intakes were inversely associated with colorectal cancer risk. For individuals in the highest tertile of genetic risk, higher total EPA and DHA intakes were positively associated with colorectal cancer risk. Risk also varied by gender. Among men, use of fish oil supplements reduced colorectal cancer risk by an average of 34% or more depending on the frequency and duration of use, but this effect did not occur among women. Additional research is needed to clarify possible associations between fish and omega-3 intakes and colorectal cancer risk.
Prostate cancer
Several prospective and case-control studies have investigated associations between either blood levels or intakes of omega-3s and risk of low-grade or high-grade prostate cancer. Results from these studies have been inconsistent.
A few case-control and case-cohort studies have found positive associations between blood levels of long-chain omega-3s and prostate cancer risk (particularly high-grade disease that is more advanced and more likely to spread than low-grade cancer), suggesting that omega-3s might increase prostate cancer risk. In a nested case-control analysis of men aged 55–84 years participating in the Prostate Cancer Prevention Trial, serum phospholipid levels of DHA were positively associated with risk of high-grade, but not low-grade, prostate cancer 90. Serum EPA levels, however, were not associated with risk of either grade of the disease.
Similarly, results from a case-cohort study within the Selenium and Vitamin E Cancer Prevention (SELECT) trial showed that men in the highest quartile of plasma phospholipid long-chain omega-3s had a 44% higher risk of low-grade prostate cancer and a 71% higher risk of high-grade prostate cancer than those in the lowest quartile 46. An analysis of data from the European Prospective Investigation into Cancer and Nutrition cohort also found a higher prostate cancer risk in men with higher plasma levels of omega-3s 91. Among Whites participating in the Multiethnic Cohort Study, higher levels of omega-3s in erythrocyte membranes and higher ratios of omega-3s to omega-6s were both associated with an increased risk of prostate cancer. However, the results showed no associations, even with advanced or high-grade disease, for other ethnic groups or for the population as a whole 92.
Results from other observational studies using dietary intake data suggest that higher intakes of fish and/or omega-3s reduce prostate cancer risk. Both fish and omega-3 consumption were associated with a lower risk of fatal prostate cancer in a cohort of 293,464 men participating in the National Institutes of Health (NIH)-AARP Diet and Health Study 93. In the Health Professionals Follow-up Study, a prospective cohort of over 47,000 men aged 40–75 years, those who consumed fish more than three times per week had a lower risk of metastatic prostate cancer than those who consumed fish less than twice per month 94. However, men who used fish oil supplements did not have a decreased risk of prostate cancer.
A number of systematic reviews and meta-analyses of prospective studies of the effects of fish intakes, omega-3 intakes, and omega-3 blood levels on prostate cancer risk have had inconsistent findings as well. For example, circulating levels of EPA, but not DHA, were positively associated with prostate cancer risk in a meta-analysis of 5,098 men with prostate cancer and 6,649 men without prostate cancer from seven studies 95. Another meta-analysis of 12 studies that included 4,516 men with prostate cancer and 5,728 men without prostate cancer found that high serum levels of these LC omega-3s were positively associated with high-grade disease 96. In other analyses, dietary intakes of long-chain omega-3s had no effect on prostate cancer risk 97, whereas fish consumption decreased prostate cancer mortality but had no effect on prostate cancer incidence 98. A 2015 meta-analysis found no significant associations between dietary intakes or blood levels of long-chain omega-3s and total prostate cancer risk 99. The authors noted that most dietary-intake studies included in their meta-analysis found inverse associations, whereas biomarker studies of blood levels of these fatty acids found positive associations.
Overall, the evidence to date shows no consistent relationships between prostate cancer risk or mortality and omega-3 intakes or blood levels.
Other cancers
Evidence is limited for a role of omega-3s in the prevention of cancers at other sites. For example, evidence is insufficient to determine whether omega-3s affect the risk of skin cancers, including basal-cell carcinoma, squamous-cell carcinoma, and melanoma 100. Findings from the Australian Ovarian Cancer Study suggest that there is no association between total or individual omega-3 intakes from foods and ovarian cancer risk 101.
Associations between omega-3 intakes and endometrial cancer have been mixed. Some evidence indicates that dietary intakes of EPA and DHA may provide protection from the development of endometrial cancer 102. Other evidence indicates that they decrease risk in normal-weight women but have no effect or even increase risk in overweight or obese women 103.
A systematic review and meta-analysis of 9 prospective cohort and 10 case-control studies did not find an association between fish or long-chain omega-3 intakes and risk of pancreatic cancer 104. Similarly, systematic reviews and meta-analyses have not found significant associations between fish consumption and risk of gastric or esophageal cancers 105.
Age-related macular degeneration (AMD) is a major cause of vision loss among older adults. In most cases, severe vision loss is associated with advanced AMD, which consists of either central geographic atrophy (dry AMD, the most common form) or neovascular AMD (wet AMD) 106. Based on DHA’s presence as a structural lipid in retinal cellular membranes and the beneficial effects of EPA-derived eicosanoids on retinal inflammation, neovascularization, and cell survival, researchers have suggested that these omega-3s have cytoprotective effects in the retina that may help prevent the development or progression of AMD 41. Studies suggest that people who get higher amounts of omega-3s from the foods they eat may have a lower risk of developing AMD. But once someone has AMD, taking omega-3 supplements does not keep the disease from getting worse or slow down vision loss.
Results from observational studies suggest that people who consume higher amounts of fatty fish and/or dietary long-chain omega-3s have a lower risk of developing AMD. In the cross-sectional EUREYE study of 2,275 participants aged 65 years or older, those who ate fatty fish at least once per week had a 53% lower risk of neovascular AMD than those who consumed fatty fish less often 107. Results were similar in a study in 681 elderly male twins 108 and an analysis of 38,022 healthy female health professionals 106. In the latter study, women in the highest tertiles of dietary DHA plus EPA intake (median of 330 mg/day) had a 38% lower risk of developing AMD during an average of 10 years of follow-up than those in those in the lowest tertile (median intake of 80 mg/day). Higher serum and erythrocyte membrane levels of EPA (but not DHA) have also been associated with a lower risk of neovascular AMD 109.
In the Age-Related Eye Disease (AREDS) study, a dietary supplement formulation containing 15 mg beta-carotene, 400 IU vitamin E, 500 mg vitamin C, 80 mg zinc, and 2 mg copper reduced the risk of advanced AMD in people with intermediate AMD or advanced AMD in one eye 110. Data from a nested cohort study within the AREDS population indicated that participants who reported the highest omega-3 intakes were about 30% less likely to develop central geographic atrophy and neovascular AMD than other participants 111.
These findings, combined with other epidemiological evidence, formed the basis for the Age-Related Eye Disease Study 2 (AREDS 2) clinical trial that examined whether adding 350 mg DHA and 650 mg EPA to the AREDS formulation further reduced the risk of progression to advanced AMD 112. The results showed that EPA and DHA did not provide any additional benefits after a median follow-up of 5 years. These findings are in line with those from a Cochrane review 113 that included the results from AREDS2 and the Nutritional AMD Treatment 2 study 114, a 3-year randomized clinical trial of omega-3 supplements (840 mg/day DHA and 270 mg/day EPA) in patients with early age-related maculopathy and neovascular AMD. The Cochrane review authors concluded that omega-3 supplementation for up to 5 years in people with AMD does not reduce the risk of progression to advanced AMD or of moderate to severe vision loss.
Dry eye disease
Dry eye disease occurs when tears don’t provide enough moisture, causing eye discomfort and vision problems. About 14% of adults in the United States have dry eye disease, a chronic condition in which decreased tear volume and quality leads to ocular surface inflammation and damage, causing discomfort and visual impairment 115. Older women, in particular, have a higher risk of dry eye disease than other groups, possibly because of hormonal changes that affect the tear-producing glands 116. Researchers hypothesize that omega 3s—particularly EPA and DHA—might reduce the risk of dry eye disease and relieve its symptoms because of their anti-inflammatory activity, and many patients take them as adjunctive treatments to artificial tears and other medications. But a large, recent study found that the symptoms of people with dry eye disease who took fish oil supplements of 2,000 mg EPA plus 1,000 mg DHA daily for 1 year did not improve any more than those who took a placebo (a dummy pill). More research on the effects of omega-3s on dry eye disease is needed.
Some, but not all, observational studies show inverse associations between self-reported dietary consumption of omega-3s and risk of dry eye disease. For example, in a cross-sectional study of 32,470 women aged 45–84 participating in the Women’s Health Study, those in the highest quintile of total dietary omega-3 intake (mean of 1,990 mg/day) had a 17% lower risk of dry eye disease than those in the lowest quintile (mean intake of 920 mg/day) 117. The study found a similar association for DHA—women in the highest versus the lowest quintiles of DHA intake had a 12% lower risk of dry eye disease; however, the results showed no significant associations for EPA. But in another cross-sectional study of 322 postmenopausal women, total dietary omega-3 intakes were not correlated with the prevalence of dry eye disease 116.
Results from clinical trials using omega-3 supplementation, primarily EPA and DHA, have had mixed results in reducing the symptoms and signs of dry eye disease. Furthermore, there is no consensus on the optimal dose, composition, or length of omega-3 treatment for this condition 118.
The studies that have found beneficial effects from omega-3 supplementation for symptoms and signs of dry eye disease include one showing that daily supplementation with 1,000 mg omega-3s (650 mg EPA plus 350 mg DHA) for 3 months in 518 men and women (mean age about 40 years) living in northern India reduced symptoms and some signs of dry eye disease compared with placebo 119. In another clinical trial of 105 men and women, daily treatment with supplements containing 2,240 mg omega-3s (1,680 mg EPA and 560 mg DHA as re-esterified triglycerides) for 12 weeks also reduced symptoms of dry eye disease compared with placebo 120. In addition, the supplements increased tear break-up time and decreased tear osmolarity (which would be likely to reduce ocular surface damage).
However, another large, randomized, double-blind clinical trial conducted in the United States found that EPA and DHA from fish oil supplements are no better than placebo at relieving symptoms or signs of dry eye disease 115. This 12-month trial included 535 participants (about 81% female) aged 18 years or older (mean age about 58 years) with at least a 6-month history of moderate to severe dry eye disease. Among them, 349 participants received daily supplements of 3,000 mg omega-3s (2,000 mg EPA plus 1,000 mg DHA), and 186 received a placebo containing 5,000 mg olive oil. Participants could continue taking medications for dry eyes, including artificial tears and prescription anti-inflammatory eye drops, as well as omega-3 supplements as long as the total dose of EPA plus DHA was less than 1,200 mg per day. At the end of the study, symptoms were less severe than at baseline in both groups, but the results showed no significant differences between groups. Groups also showed no significant differences compared with baseline in signs of dry eye disease, including conjunctive and cornea integrity as well as tear volume and quality.
Overall, the evidence to date shows no consistent relationship between omega-3s and dry eye disease. More research is warranted to fully understand whether increased intakes of dietary or supplemental omega-3s help reduce the risk of dry eye disease and whether they are beneficial as an adjunct treatment.
Alzheimer’s disease, dementia, and cognitive function
Some, but not all, observational studies suggest that diets high in omega-3s are associated with a reduced risk of cognitive decline, Alzheimer’s disease, and dementia 121. Because DHA is an essential component of cellular membrane phospholipids in the brain, researchers hypothesize that omega-3s might protect cognitive function by helping to maintain neuronal function and cell- membrane integrity within the brain 121. This hypothesis is supported by findings from case-control studies indicating that patients with Alzheimer’s disease have lower serum levels of DHA than cognitively healthy people 122. Lower serum DHA levels are also associated with more cerebral amyloidosis (build-up of protein deposits called amyloids) in healthy older adults, whereas higher DHA is correlated with preservation of brain volume 123.
Several observational studies have examined the effects of fish, EPA, and/or DHA intakes on cognitive function in healthy older adults. In a prospective cohort study involving 210 healthy men aged 70–89, fish consumption was associated with less cognitive decline at follow-up 5 years later 124. In addition, a dose-response relationship was observed between tertiles of dietary EPA plus DHA intake and subsequent 5-year cognitive decline. Similarly, in the Rotterdam Study, a population-based prospective study of people aged 55 or older who were free from dementia at baseline, higher fish consumption among 5,386 study participants was associated with a 60% lower risk of dementia and a 70% lower risk of Alzheimer’s disease over an average of 2.1 years 125. Subsequent follow-up 6 years after baseline, however, found no associations between omega-3 intakes and incidence of dementia or Alzheimer’s disease 126. The authors suggest that the discrepancy might be explained by the short follow-up period in the first analysis and the small number of patients who developed dementia. A higher omega-3 index was associated with a greater hippocampal volume in the Women’s Health Initiative Memory Study 127 and with a larger brain volume and improved cognitive test scores in the Framingham Offspring cohort 128. A 2016 dose-response meta- analysis of 21 cohort studies found that increased intakes of fish and dietary DHA were both inversely associated with the risks of dementia and Alzheimer’s disease 129. Specifically, a 100 mg/day incremental increase in DHA intake was associated with a 14% lower risk of dementia and a 37% lower risk of Alzheimer’s disease.
Results from clinical trials, however, suggest that omega-3 supplementation does not affect cognitive function in older adults who have no cognitive impairment. In a trial in the United Kingdom, 748 cognitively healthy adults aged 70–79 years received either 500 mg DHA and 200 mg EPA or placebo daily for 24 months 130. Cognitive function did not differ significantly between the two groups, although cognitive function did not decline in either group. In the AREDS2 study, treatment with 350 mg DHA and 650 mg EPA for 5 years did not have a significant effect on cognitive function in 3,501 older adults (mean age 72.7 years) with AMD 122.
Clinical trial results also suggest that omega-3 supplementation does not benefit patients with Alzheimer’s disease, although it might help patients with mild cognitive impairment. For example, daily supplementation with 2 g DHA for 18 months did not slow the rate of cognitive decline compared to placebo in 295 participants (mean age 76 years) with mild to moderate Alzheimer’s disease 131. In the OmegaAD trial, daily supplementation with 1,700 mg DHA and 600 mg EPA for 6 months in 174 older adults with mild to moderate Alzheimer’s disease also failed to slow down the rate of cognitive decline compared to placebo 132. However, a subgroup of patients with very mild impairment experienced a significant reduction in the rate of cognitive decline. In a small trial in Malaysia, fish oil supplementation (1,290 mg DHA and 450 mg EPA daily) for 12 months improved memory—particularly short-term, working, and verbal memory—and delayed recall compared to placebo in 35 older adults with mild cognitive impairment 133.
Several systematic reviews and meta-analyses, including a Cochrane review, have assessed the effects of omega-3 supplementation on cognitive function and dementia in healthy older adults and those with Alzheimer’s disease or cognitive impairment 134, 135. Overall, the findings indicate that omega-3 supplementation does not affect cognitive function in healthy older adults or in people with Alzheimer’s disease compared to placebo. For people with mild cognitive impairment, omega-3s may improve certain aspects of cognitive function, including attention, processing speed, and immediate recall 136.
However, these findings need to be confirmed in additional clinical trials.
Rheumatoid arthritis
Rheumatoid arthritis (RA) is an autoimmune disease characterized by chronic inflammation of the joints. Rheumatoid arthritis symptoms include pain, swelling, stiffness, and functional impairments. Rheumatoid arthritis is typically treated with nonsteroidal antiinflammatory drugs (NSAIDs), corticosteroids, and disease-modifying antirheumatic drugs. Due to their antiinflammatory effects, some scientists hypothesize that omega-3s reduce some of the symptoms of rheumatoid arthritis and patients’ reliance on NSAIDs and corticosteroids. Some clinical trials have shown that taking omega-3 supplements may help manage rheumatoid arthritis when taken together with standard rheumatoid arthritis medications and other treatments. For example, people with rheumatoid arthritis who take omega-3 supplements may need less pain-relief medication, but it is not clear if the supplements reduce joint pain, swelling, or morning stiffness.
Several clinical trials, many conducted in the 1990s, have examined the use of omega-3 supplementation in patients with rheumatoid arthritis. These trials have generally shown that omega-3 supplements reduce patients’ use of antiinflammatory drugs and corticosteroids, but that they do not have consistent effects on painful and/or tender joints, joint swelling, or morning stiffness 137, 138. For example, fish oil supplementation significantly reduced NSAID use in a controlled trial in Sweden 139. In this study, 43 patients with rheumatoid arthritis received either 10 g/day fish oil (containing 1.8 g EPA and 1.2 g DHA) or placebo along with their usual rheumatoid arthritis medications. NSAID use decreased in the treatment group at 3 and 6 months, and global arthritic activity assessed by physicians improved relative to placebo at 3 months. However, patient assessments of pain, morning stiffness, and functional capacity did not differ between groups. In a 2013 clinical trial in South Korea, 81 patients with rheumatoid arthritis received either omega-3s (2.1 g EPA and 1.2 g DHA) or a sunflower oil placebo daily for 16 weeks 140. Patients were allowed to continue taking NSAIDs, glucocorticoids, and/or antirheumatic drugs throughout the study. Compared to placebo, omega-3 supplementation had no significant effects on clinical symptoms of rheumatoid arthritis, including pain and morning stiffness. In post-hoc analysis, the researchers found that the supplements reduced the amount of NSAIDs needed, but only in patients weighing more than 55 kg. In a similar study in Denmark, 51 patients received either omega-3s (2.0 g EPA and 1.2 g DHA from fish oil) or placebo daily for 12 weeks, and they continued taking rheumatoid arthritis medications 141. Compared to placebo, morning stiffness, joint tenderness, and visual pain score decreased significantly in the treatment group. However, there were no significant differences between groups in grip strength, daily activity score, or joint swelling. The amounts of NSAIDs, aspirin, and acetaminophen that patients needed did not change in either group.
Reviews and meta-analyses of studies that assessed whether fish oil and omega-3s are beneficial for rheumatoid arthritis have had inconsistent findings. Some suggest that they do not significantly affect the clinical symptoms of rheumatoid arthritis but do reduce the amounts of NSAIDs and corticosteroids that patients need 142, 137. Others indicate that omega-3s reduce joint swelling and pain, morning stiffness, and number of painful joints in addition to reducing NSAID use 138, 143. Some researchers suggest that differences in findings could be due in part to whether patient-determined use of NSAIDs is considered a measure of pain 144.
Findings to date suggest that omega-3s may be helpful as an adjunctive treatment to pharmacotherapy for reducing the symptoms of rheumatoid arthritis 144. However, more research is needed to confirm this finding.
Depression
A 2016 meta-analysis of 26 studies found a 17% lower risk of depression with higher fish intake 145. However, a 2015 Cochrane review of 26 studies found insufficient evidence to determine whether omega-3s (1,000 to 6,600 mg/day EPA, DHA, and/or other omega-3s) are beneficial for major depressive disorder in adults 146. The authors did find a small-to-modest beneficial effect on depressive symptoms, but they concluded that this effect was not clinically significant.
Inflammatory bowel disease
The authors of a systematic review of 19 randomized controlled trials concluded that the available evidence does not support the use of omega-3 supplements to treat active or inactive inflammatory bowel disease 147. Similarly, the authors of a Cochrane review concluded that, based on the evidence from two large, high-quality studies, omega-3 supplements are probably not effective for maintaining remission in people who have Crohn’s disease 148.
ADHD
A systematic review and meta-analysis of 10 studies in children with ADHD or related neurodevelopmental disorders, such as developmental coordination disorder, found no improvements with omega-3 supplementation on measures of emotional lability, oppositional behavior, conduct problems, or aggression 149. However, in subgroup analyses of only the higher-quality studies and those with strict inclusion criteria, omega-3 supplementation (60 to 1,296 mg/day EPA and/or DHA) did significantly improve parent-rated emotional lability and oppositional behavior.
Childhood allergies
A systematic review and meta-analysis of 10 prospective cohort studies and 5 randomized clinical trials on omega-3 intakes during pregnancy and outcomes of childhood allergic disease (eczema, rhino-conjunctivitis, and asthma) found inconsistent results 150. Although the authors could not draw firm conclusions due to the heterogeneity of the studies and their results, they concluded that the overall findings were “suggestive” of a protective association between higher maternal intakes of omega-3s or fish and incidence of allergic disease symptoms in the offspring. The authors of a Cochrane review that included eight LC omega-3 supplementation trials concluded that there is limited evidence to support the use of omega-3 supplements by women during pregnancy and/or lactation for reducing the risk of allergic disease in their children 151.
Cystic fibrosis
A Cochrane review of four studies of cystic fibrosis found that omega-3 supplements (300 to 5,400 mg/day EPA and/or DHA) might improve lung function and increase blood levels of essential fatty acids in people with cystic fibrosis 152. However, the authors concluded that there is not enough evidence to recommend routine use of omega-3 supplements by people with cystic fibrosis.
Best way to cook salmon
Pan-fried salmon
Ingredients:
- 4 (5-ounce) center-cut salmon fillets (about 1-inch-thick), skin on or off
- 2 tablespoons olive oil
- Salt and freshly ground black pepper
Directions:
- Bring the salmon to room temperature 10 minutes before cooking.
- Warm a large nonstick skillet with oil over medium-low heat. Season the fish with salt and pepper. Raise the heat to medium-high. Place the salmon, skin-side up in the pan. Cook until golden brown on 1 side, about 4 minutes. Turn the fish over with a spatula, and cook until it feels firm to the touch and the skin is crisp if desired, about 3 minutes more.
- The skin can be served or removed easily with a knife or spoon.
- Transfer to a plate and serve as desired.
- A trick to getting the most Omega-3 from your salmon is to eat the skin.
Crispy salmon with cherry tomatos and basil
Ingredients:
- 300g fresh Atlantic salmon (2 portions), skin on
- 6 baby new potatoes, cut in half
- 1/2 tablespoon olive oil
- sea salt for seasoning
- 1/2 punnet mixed cherry tomatoes
- 1 tablespoon balsamic vinegar or red wine vinegar
- 1/2 cup fresh basil leaves
- butter to serve
- fresh mint , to serve
- mixed lettuce
Directions:
- Cook potatoes in boiling water until tender.
- Brush both sides of salmon with oil and season skin side with salt.
- Preheat a non-stick pan or bbq to a medium heat . Cook skin side down for 7 minutes and then turn and cook for a further 7 minutes on the other side or until cooked to your liking. Remove salmon from pan and keep warm.
- Add cherry tomatoes to hot pan and toss until to slightly charred . Add vinegar to pan and toss to deglaze. Add salmon back to pan and coat with pan juices.
- Toss cooked potatoes with a little butter and mint. Serve salmon topped with cherry tomatoes and garnish with basil. Enjoy with salad.
Smoked salmon summer salad
Ingredients:
- 250g Smoked Salmon
- 6 cups kale leaf & spinach mix
- 1 avocado, sliced
- 1/2 red onion, finely sliced
- 1 pear, sliced
- 1/2 cup candied pecans
- Black pepper
- Lemon wedges, to serve
Salad dressing:
- 1/4 cup extra virgin olive oil
- 1 tbsp lemon juice
- 2 tsp honey
- 1 tsp dijon mustard
- 1 tsp wholegrain mustard
Directions:
- Combine salad dressing ingredients in a jar and place lid on tightly, shake until combined. In a large bowl add the kale and spinach and toss with salad dressing.
- Arrange kale and spinach on 4 plates, and top each plate with equal amounts of smoked salmon, avocado, red onion, pear and peacans.
- Top with cracked black pepper and a wedge of fresh lemon.
Baked salmon with herb salad
Ingredients:
- 600g fresh salmon fillets (skin off)
- 2 cups butternut pumpkin, diced
- Juice of ½ lemon
- 2 tbsp. flat parsley, finely chopped
- 2 tbsp. basil, finely chopped
- 1 garlic clove, crushed
- 1 cup cannellini beans
- 4 cups rocket
Directions:
- Cook salmon and diced pumpkin with a little olive oil in oven at 180°C for 30 minutes.
- While the salmon is cooking put the lemon juice, parsley, basil, garlic, mustard and olive oil in a food processor and blend together to make a smooth sauce.
- Rinse the cannellini beans and gently heat in a small saucepan until warm.
- Add half of the sauce to the beans and then toss with the rocket and add roasted pumpkin.
- Use the rest of the sauce to pour over the salmon fillets and serve.
Baked salmon with herb aioli & crisp roasted rosemary potatoes
Ingredients:
- 460g fresh salmon, skin-on
- 1kg baby potatoes, peeled & par-cooked in salted boiling water for 10 mins. then drained
- 1 tbsp olive oil
- 1 bulb garlic, unpeeled & broken into cloves
- 4 sprigs rosemary
- 1 tsp olive oil
Herb Aioli:
- ¾ cup whole egg Garlic Aioli
- 1 tsp finely chopped parsley
- 1 tsp finely chopped dill
Directions:
- Toss potatoes in olive oil then transfer onto a baking paper lined oven tray. Season generously with salt flakes and sprinkle over garlic cloves and rosemary sprigs. Bake in a preheated oven of 200°C for 40 mins.
- Brush the salmon fillets with olive oil and season with freshly ground black pepper and salt. Remove potatoes from oven and give them a good toss and turn to crisp up the underside and move them along to one end of the tray. Place the salmon fillets skin side down onto the hot oven tray with the potatoes. Return tray to oven and cook for 10 mins or until the fish is just cooked through but still lightly pink. Remove from oven.
- To serve; divide potatoes, garlic and rosemary between 4 dinner plates and arrange a fillet of salmon on each plate. Spoon a little herb aioli over the fish and serve remaining sauce in a small dish to enjoy with the crispy potatoes. Serve hot with a green salad or steamed vegetables.
Baked salmon with roast vegetables
Ingredients:
- 300g salmon fillets, skin-on
- 1 ½ tbsp olive oil
- 4 baby chat potatoes
- 3-4 small chunks pumpkin, skin-on
- 6-8 brussels sprouts, halved
- 4-6 cloves (skin-on) or 1 bulb of garlic
- ½ lemon cut into wedges
- 2-4 rosemary sprigs
- salt and freshly ground pepper
Directions:
- Preheat the oven to 180°C (fan forced).
- Place the potatoes and olive oil in an oven proof dish, season with salt and pepper and roast for 20 minutes or until starting to brown.
- Add pumpkin, brussels sprouts, lemon wedges, garlic and rosemary sprigs. Return to the oven for another 20 minutes.
- Brush the salmon with olive oil, season with salt and pepper and arrange amongst the vegetables. Return to the oven for the final 10–15 minutes.
- Remove from oven and allow to rest for a few minutes before serving. Plate salmon with the roasted vegetables, garlic and lemon wedges from the pan and steamed greens on the side.
Grilled salmon with green goddess sauce
Ingredients:
- 600g fresh salmon skin on
- 1 tsp Cumin
- 1 tsp Paprika
- 1 tbsp Extra Virgin Olive oil
Green Goddess Sauce:
- 2 Spring onion
- 1 Garlic cloves
- 1/4 cup Parsley leaves
- ¼ cup Basil
- Lemon juice half a lemon
- 1/4 cup green peas
- 1/3 cup Extra Virgin Olive oil
- Salt and pepper
Directions:
- In a high-speed blender or food processor add in the spring onions, garlic, parsley, basil, lemon juice and green peas. Blend for 2 minutes until smooth. Then slowly pour in the oil until a thick sauce form. Season with salt and pepper to taste.
- Place the salmon on a plate and coat rub cumin, paprika and olive oil over the salmon. Then heat a pan on medium heat and cook salmon skin down for 5 minutes. Then flip and cook for 2 minutes. Remove from pan.
- Serve drizzled with green goddess sauce and a side of salad and crispy potatoes.
Air fryer salmon
- Cut the salmon fillets into even sizes.
- Season each salmon fillet with garlic, paprika, salt and pepper and drizzle a little olive oil on top.
- Depending on the thickness of the salmon and the exact air fryer you have, the time could range. But on average, expect the air fryer salmon to be cooked within 7-9 minutes.
Teriyaki salmon
Ingredients:
- 530g fresh salmon
- 2 Tbsp fish sauce
- 2 Tbsp honey
- 2 Tbsp white wine vinegar
- ½ tsp sesame oil
- 4 cups spinach, steamed
Directions:
- Mix fish sauce, honey, vinegar and sesame oil together and marinate salmon portions for 30 minutes.
- Grill the salmon portions for 2-3 mins each side on a pre-heated grill, until cooked to your liking.
- Serve with spinach and enjoy.
- The Audubon Society Field Guide to North American Fishes, Whales & Dolphins. Chanticleer Press. 1983. p. 395.[↩]
- Endangered Species Act. https://www.fisheries.noaa.gov/topic/laws-policies#endangered-species-act[↩]
- FAO . The State of World Fisheries and Aquaculture 2020. Sustainability in Action. FAO; Rome, Italy: 2020.[↩]
- Calado, R., Mota, V. C., Madeira, D., & Leal, M. C. (2021). Summer Is Coming! Tackling Ocean Warming in Atlantic Salmon Cage Farming. Animals : an open access journal from MDPI, 11(6), 1800. https://doi.org/10.3390/ani11061800[↩]
- Oppedal F., Dempster T., Stien L.H. Environmental drivers of Atlantic salmon behaviour in sea-cages: A review. Aquaculture. 2011;311:1–18. doi: 10.1016/j.aquaculture.2010.11.020[↩]
- Oliver E.C.J., Burrows M.T., Donat M., Gupta A.S., Alexander L.V., Perkins-Kirkpatrick S.E., Benthuysen J.A., Hobday A.J., Holbrook N., Moore P., et al. Projected Marine Heatwaves in the 21st Century and the Potential for Ecological Impact. Front. Mar. Sci. 2019;6 doi: 10.3389/fmars.2019.00734[↩]
- Overton K., Dempster T., Oppedal F., Kristiansen T.S., Gismervik K., Stien L.H. Salmon lice treatments and salmon mortality in Norwegian aquaculture: A review. Rev. Aquac. 2019;11:1398–1417. doi: 10.1111/raq.12299[↩][↩]
- Pike AW. Sea lice–major pathogens of farmed atlantic salmon. Parasitol Today. 1989 Sep;5(9):291-7. doi: 10.1016/0169-4758(89)90020-3[↩]
- Gjedrem T. (2012) Genetic improvement for the development of efficient global aquaculture: a personal opinion review. Aquaculture 344, 12–22.[↩]
- Gjøen H.M. & Bentsen H.B. (1997) Past, present, and future of genetic improvement in salmon aquaculture. ICES Journal of Marine Science 54, 1009–14.[↩]
- Glover K.A., Solberg M.F., McGinnity P., Hindar K., Verspoor E., Coulson M.W., Hansen M.M., Araki H., Skaala O. & Svåsand F. (2017) Half a century of genetic interaction between farmed and wild Atlantic salmon: status of knowledge and unanswered questions. Fish and Fisheries 18, 890–927.[↩][↩]
- Houston, R. D., & Macqueen, D. J. (2019). Atlantic salmon (Salmo salar L.) genetics in the 21st century: taking leaps forward in aquaculture and biological understanding. Animal genetics, 50(1), 3–14. https://doi.org/10.1111/age.12748[↩][↩]
- FAO. The state of world fisheries and aquaculture 2016: contributing to food security and nutrition for all. Rome: Food and Agriculture Organization of the United Nations; 2016.[↩]
- Ytrestøyl T, Aas TS, Åsgård T. Utilisation of feed resources in production of Atlantic salmon (Salmo salar) in Norway. Aquaculture. 2015;448:365–374. doi: 10.1016/j.aquaculture.2015.06.023[↩]
- Hardy RW. Utilization of plant proteins in fish diets: effects of global demand and supplies of fishmeal. Aquac Res. 2010;41(5):770–776. doi: 10.1111/j.1365-2109.2009.02349.x[↩]
- Metochis C., Crampton V. O., Ruohonen K., Bell J. G., Adams A., Thompson K. D. The effects of increasing dietary levels of amino acid-supplemented soy protein concentrate and constant dietary supplementation of phosphorus on growth, composition and immune responses of juvenile Atlantic salmon (Salmo salar L.) Fish Physiology and Biochemistry. 2016;42(3):807–829. doi: 10.1007/s10695-015-0177-z[↩]
- Dias J, Conceição LEC, Ribeiro AR, Borges P, Valente LMP, Dinis MT. Practical diet with low fish-derived protein is able to sustain growth performance in gilthead seabream (Sparus aurata) during the grow-out phase. Aquaculture. 2009;293(3):255–262. doi: 10.1016/j.aquaculture.2009.04.042[↩]
- Naylor RL, Hardy RW, Bureau DP, Chiu A, Elliott M, Farrell AP, Forster I, Gatlin DM, Goldburg RJ, Hua K, et al. Feeding aquaculture in an era of finite resources. Proc Natl Acad Sci U S A. 2009;106(36):15103–15110. doi: 10.1073/pnas.0905235106[↩]
- Klinger D, Naylor R. Searching for solutions in aquaculture: charting a sustainable course. Annu Rev Environ Resour. 2012;37(1):247–276. doi: 10.1146/annurev-environ-021111-161531[↩]
- Bureau DP. Rendered products in fish aquaculture feeds. In: Meeker DL, Hamilton C, editors. Essential rendering: all about the animal by-product industry. Arlington: National Renderers Association; 2006. pp. 179–194.[↩]
- Hatlen B, Jakobsen JV, Crampton V, Alm M, Langmyhr E, Espe M, Hevrøy EM, Torstensen BE, Liland N, Waagbø R. Growth, feed utilization and endocrine responses in Atlantic salmon (Salmo salar) fed diets added poultry by-product meal and blood meal in combination with poultry oil. Aquac Nutr. 2015;21(5):714–725. doi: 10.1111/anu.12194[↩]
- Lorente-Cebrián S, Costa AGV, Navas-Carretero S, Zabala M, Martínez JA, Moreno-Aliaga MJ. Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: a review of the evidence. J Physiol Biochem. 2013;69(3):633–651. doi: 10.1007/s13105-013-0265-4[↩]
- Hixson SM, Parrish CC, Xue X, Wells JS, Collins SA, Anderson DM, Rise ML. Growth performance, tissue composition, and gene expression responses in Atlantic salmon (Salmo salar) fed varying levels of different lipid sources. Aquaculture. 2017;467:76–88. doi: 10.1016/j.aquaculture.2016.04.011[↩][↩]
- Sprague M, Dick JR, Tocher DR. Impact of sustainable feeds on omega-3 long-chain fatty acid levels in farmed Atlantic salmon, 2006–2015. Sci Rep. 2016;6:21892. doi: 10.1038/srep21892[↩]
- Kabeya N, Takeuchi Y, Yamamoto Y, Yazawa R, Haga Y, Satoh S, Yoshizaki G. Modification of the n-3 HUFA biosynthetic pathway by transgenesis in a marine teleost, nibe croaker. J Biotechnol. 2014;172:46–54. doi: 10.1016/j.jbiotec.2013.12.004[↩]
- Betancor MB, Sprague M, Sayanova O, Usher S, Campbell PJ, Napier JA, Caballero MJ, Tocher DR. Evaluation of a high-EPA oil from transgenic Camelina sativa in feeds for Atlantic salmon (Salmo salar L.): effects on tissue fatty acid composition, histology and gene expression. Aquaculture. 2015;444:1–12. doi: 10.1016/j.aquaculture.2015.03.020[↩]
- Omega-3 Fatty Acids. https://ods.od.nih.gov/factsheets/Omega3FattyAcids-HealthProfessional[↩][↩][↩]
- Harris WS. Omega-3 fatty acids. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:577-86.[↩][↩][↩][↩][↩][↩][↩]
- U.S. Department of Agriculture. Dietary Guidelines for Americans, 2020-2025. 9th ed. 2020. https://www.dietaryguidelines.gov/sites/default/files/2021-03/Dietary_Guidelines_for_Americans-2020-2025.pdf[↩][↩][↩]
- Advice about Eating Fish. https://www.fda.gov/food/consumers/advice-about-eating-fish[↩][↩][↩][↩][↩][↩][↩]
- Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2012 Mar;129(3):e827-41. doi: 10.1542/peds.2011-3552[↩][↩]
- Skulas-Ray AC, Wilson PWF, Harris WS, Brinton EA, Kris-Etherton PM, Richter CK, Jacobson TA, Engler MB, Miller M, Robinson JG, Blum CB, Rodriguez-Leyva D, de Ferranti SD, Welty FK; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory From the American Heart Association. Circulation. 2019 Sep 17;140(12):e673-e691. doi: 10.1161/CIR.0000000000000709[↩][↩][↩]
- Siscovick, D. S., Barringer, T. A., Fretts, A. M., Wu, J. H., Lichtenstein, A. H., Costello, R. B., Kris-Etherton, P. M., Jacobson, T. A., Engler, M. B., Alger, H. M., Appel, L. J., Mozaffarian, D., & American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology (2017). Omega-3 Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation, 135(15), e867–e884. https://doi.org/10.1161/CIR.0000000000000482[↩][↩][↩][↩]
- Rimm, E. B., Appel, L. J., Chiuve, S. E., Djoussé, L., Engler, M. B., Kris-Etherton, P. M., Mozaffarian, D., Siscovick, D. S., Lichtenstein, A. H., & American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology (2018). Seafood Long-Chain n-3 Polyunsaturated Fatty Acids and Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation, 138(1), e35–e47. https://doi.org/10.1161/CIR.0000000000000574[↩][↩][↩][↩]
- Atlantic salmon wild and raw nutrition facts. https://fdc.nal.usda.gov/fdc-app.html#/food-details/173686/nutrients[↩]
- Atlantic salmon farmed and raw nutrition facts. https://fdc.nal.usda.gov/fdc-app.html#/food-details/175167/nutrients[↩]
- Atlantic salmon wild and cooked in dry heat nutrition facts. https://fdc.nal.usda.gov/fdc-app.html#/food-details/171998/nutrients[↩]
- Atlantic salmon farmed and cooked in dry heat nutrition facts. https://fdc.nal.usda.gov/fdc-app.html#/food-details/175168/nutrients[↩]
- Jones PJH, Papamandjaris AA. Lipids: cellular metabolism. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Washington, DC: Wiley-Blackwell; 2012:132-48.[↩]
- Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). Washington, DC: National Academy Press; 2005.[↩][↩][↩]
- SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. 2005 Jan;24(1):87-138. doi: 10.1016/j.preteyeres.2004.06.002[↩][↩]
- Jones PJH, Rideout T. Lipids, sterols, and their metabolites. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2014.[↩]
- Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood). 2008 Jun;233(6):674-88. doi: 10.3181/0711-MR-311[↩]
- Wang C, Chung M, Lichtenstein A, Balk E, Kupelnick B, DeVine D, et al. Effects of omega-3 fatty acids on cardiovascular disease. Summary, evidence report/technology assessment no. 94. https://archive.ahrq.gov/downloads/pub/evidence/pdf/o3cardio/o3cardio.pdf[↩]
- Harris WS, Davidson MH. RE: Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J Natl Cancer Inst. 2014 Apr;106(4):dju019. doi: 10.1093/jnci/dju019[↩]
- Brasky, T. M., Darke, A. K., Song, X., Tangen, C. M., Goodman, P. J., Thompson, I. M., Meyskens, F. L., Jr, Goodman, G. E., Minasian, L. M., Parnes, H. L., Klein, E. A., & Kristal, A. R. (2013). Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. Journal of the National Cancer Institute, 105(15), 1132–1141. https://doi.org/10.1093/jnci/djt174[↩][↩]
- Harris WS, Sands SA, Windsor SL, Ali HA, Stevens TL, Magalski A, Porter CB, Borkon AM. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients: correlation with erythrocytes and response to supplementation. Circulation. 2004 Sep 21;110(12):1645-9. doi: 10.1161/01.CIR.0000142292.10048.B2. Epub 2004 Sep 7. Erratum in: Circulation. 2004 Nov 9;110(19):3156.[↩]
- Harris WS. Are n-3 fatty acids still cardioprotective? Curr Opin Clin Nutr Metab Care. 2013 Mar;16(2):141-9. doi: 10.1097/MCO.0b013e32835bf380[↩]
- Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med. 2004 Jul;39(1):212-20. doi: 10.1016/j.ypmed.2004.02.030[↩]
- Metcalf RG, Cleland LG, Gibson RA, Roberts-Thomson KC, Edwards JR, Sanders P, Stuklis R, James MJ, Young GD. Relation between blood and atrial fatty acids in patients undergoing cardiac bypass surgery. Am J Clin Nutr. 2010 Mar;91(3):528-34. doi: 10.3945/ajcn.2009.28302[↩]
- Del Gobbo, Liana C et al. “ω-3 Polyunsaturated Fatty Acid Biomarkers and Coronary Heart Disease: Pooling Project of 19 Cohort Studies.” JAMA internal medicine vol. 176,8 (2016): 1155-66. https://doi.org/10.1001/jamainternmed.2016.2925[↩]
- Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP, Mantini L, Marfisi RM, Mastrogiuseppe G, Mininni N, Nicolosi GL, Santini M, Schweiger C, Tavazzi L, Tognoni G, Tucci C, Valagussa F; GISSI-Prevenzione Investigators. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002 Apr 23;105(16):1897-903. doi: 10.1161/01.cir.0000014682.14181.f2[↩]
- Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, Jordan HS, Lau J. n-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr. 2006 Jul;84(1):5-17. doi: 10.1093/ajcn/84.1.5[↩][↩]
- Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K; Japan EPA lipid intervention study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007 Mar 31;369(9567):1090-8. doi: 10.1016/S0140-6736(07)60527-3. Erratum in: Lancet. 2007 Jul 21;370(9583):220.[↩][↩]
- Tanaka K, Ishikawa Y, Yokoyama M, Origasa H, Matsuzaki M, Saito Y, Matsuzawa Y, Sasaki J, Oikawa S, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K; JELIS Investigators, Japan. Reduction in the recurrence of stroke by eicosapentaenoic acid for hypercholesterolemic patients: subanalysis of the JELIS trial. Stroke. 2008 Jul;39(7):2052-8. doi: 10.1161/STROKEAHA.107.509455. Epub 2008 May 1. Erratum in: Stroke. 2008 Sep;39(9): e149.[↩]
- Risk and Prevention Study Collaborative Group, Roncaglioni MC, Tombesi M, Avanzini F, Barlera S, Caimi V, Longoni P, Marzona I, Milani V, Silletta MG, Tognoni G, Marchioli R. n-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med. 2013 May 9;368(19):1800-8. doi: 10.1056/NEJMoa1205409. Erratum in: N Engl J Med. 2013 May 30;368(22):2146.[↩]
- ORIGIN Trial Investigators, Bosch J, Gerstein HC, Dagenais GR, Díaz R, Dyal L, Jung H, Maggiono AP, Probstfield J, Ramachandran A, Riddle MC, Rydén LE, Yusuf S. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012 Jul 26;367(4):309-18. doi: 10.1056/NEJMoa1203859[↩][↩]
- Kromhout D, Giltay EJ, Geleijnse JM; Alpha Omega Trial Group. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010 Nov 18;363(21):2015-26. doi: 10.1056/NEJMoa1003603[↩][↩]
- Manson, J. E., Cook, N. R., Lee, I. M., Christen, W., Bassuk, S. S., Mora, S., Gibson, H., Albert, C. M., Gordon, D., Copeland, T., D’Agostino, D., Friedenberg, G., Ridge, C., Bubes, V., Giovannucci, E. L., Willett, W. C., Buring, J. E., & VITAL Research Group (2019). Marine n-3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. The New England journal of medicine, 380(1), 23–32. https://doi.org/10.1056/NEJMoa1811403[↩][↩][↩][↩][↩][↩]
- ASCEND Study Collaborative Group, Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, Murphy K, Aung T, Haynes R, Cox J, Murawska A, Young A, Lay M, Chen F, Sammons E, Waters E, Adler A, Bodansky J, Farmer A, McPherson R, Neil A, Simpson D, Peto R, Baigent C, Collins R, Parish S, Armitage J. Effects of n-3 Fatty Acid Supplements in Diabetes Mellitus. N Engl J Med. 2018 Oct 18;379(16):1540-1550. doi: 10.1056/NEJMoa1804989[↩][↩][↩][↩]
- Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM; REDUCE-IT Investigators. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med. 2019 Jan 3;380(1):11-22. doi: 10.1056/NEJMoa1812792[↩][↩]
- Kris-Etherton, P. M., Richter, C. K., Bowen, K. J., Skulas-Ray, A. C., Jackson, K. H., Petersen, K. S., & Harris, W. S. (2019). Recent Clinical Trials Shed New Light on the Cardiovascular Benefits of Omega-3 Fatty Acids. Methodist DeBakey cardiovascular journal, 15(3), 171–178. https://doi.org/10.14797/mdcj-15-3-171[↩][↩]
- Hu Y, Hu FB, Manson JE. Marine omega-3 supplementation and cardiovascular disease: an updated meta-analysis of 13 randomized controlled trials involving 127 477 participants. J Am Heart Assoc 2019;8:e013543.[↩][↩][↩]
- Abdelhamid, A. S., Brown, T. J., Brainard, J. S., Biswas, P., Thorpe, G. C., Moore, H. J., Deane, K. H., Summerbell, C. D., Worthington, H. V., Song, F., & Hooper, L. (2020). Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. The Cochrane database of systematic reviews, 3(3), CD003177. https://doi.org/10.1002/14651858.CD003177.pub5[↩]
- Omega-3 Fatty Acids and Cardiovascular Disease: An Updated Systematic Review. https://effectivehealthcare.ahrq.gov/products/fatty-acids-cardiovascular-disease/research[↩][↩]
- Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, Franco OH, Butterworth AS, Forouhi NG, Thompson SG, Khaw KT, Mozaffarian D, Danesh J, Di Angelantonio E. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med. 2014 Mar 18;160(6):398-406. doi: 10.7326/M13-1788. Erratum in: Ann Intern Med. 2014 May 6;160(9):658.[↩]
- Zhao YT, Chen Q, Sun YX, Li XB, Zhang P, Xu Y, Guo JH. Prevention of sudden cardiac death with omega-3 fatty acids in patients with coronary heart disease: a meta-analysis of randomized controlled trials. Ann Med. 2009;41(4):301-10. doi: 10.1080/07853890802698834[↩]
- Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011 Nov 8;58(20):2047-67. doi: 10.1016/j.jacc.2011.06.063[↩]
- O’Connor NR. Infant formula. Am Fam Physician. 2009 Apr 1;79(7):565-70. https://www.aafp.org/afp/2009/0401/p565.html[↩]
- Simmer K, Patole SK, Rao SC. Long-chain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst Rev. 2011 Dec 7;(12):CD000376. doi: 10.1002/14651858.CD000376.pub3. Update in: Cochrane Database Syst Rev. 2017 Mar 10;3:CD000376[↩]
- Schulzke SM, Patole SK, Simmer K. Long-chain polyunsaturated fatty acid supplementation in preterm infants. Cochrane Database Syst Rev. 2011 Feb 16;(2):CD000375. doi: 10.1002/14651858.CD000375.pub4. Update in: Cochrane Database Syst Rev. 2016 Dec 20;12 :CD000375[↩]
- Oken, E., Radesky, J. S., Wright, R. O., Bellinger, D. C., Amarasiriwardena, C. J., Kleinman, K. P., Hu, H., & Gillman, M. W. (2008). Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 years in a US cohort. American journal of epidemiology, 167(10), 1171–1181. https://doi.org/10.1093/aje/kwn034[↩]
- Hibbeln JR, Davis JM, Steer C, Emmett P, Rogers I, Williams C, Golding J. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet. 2007 Feb 17;369(9561):578-85. doi: 10.1016/S0140-6736(07)60277-3[↩]
- Starling, P., Charlton, K., McMahon, A. T., & Lucas, C. (2015). Fish intake during pregnancy and foetal neurodevelopment–a systematic review of the evidence. Nutrients, 7(3), 2001–2014. https://doi.org/10.3390/nu7032001[↩]
- Makrides M, Gibson RA, McPhee AJ, Yelland L, Quinlivan J, Ryan P; DOMInO Investigative Team. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: a randomized controlled trial. JAMA. 2010 Oct 20;304(15):1675-83. doi: 10.1001/jama.2010.1507[↩]
- Makrides M, Gould JF, Gawlik NR, Yelland LN, Smithers LG, Anderson PJ, Gibson RA. Four-year follow-up of children born to women in a randomized trial of prenatal DHA supplementation. JAMA. 2014 May 7;311(17):1802-4. doi: 10.1001/jama.2014.2194[↩]
- Molloy CS, Stokes S, Makrides M, Collins CT, Anderson PJ, Doyle LW. Long-term effect of high-dose supplementation with DHA on visual function at school age in children born at <33 wk gestational age: results from a follow-up of a randomized controlled trial. Am J Clin Nutr. 2016 Jan;103(1):268-75. doi: 10.3945/ajcn.115.114710[↩]
- Meldrum SJ, D’Vaz N, Simmer K, Dunstan JA, Hird K, Prescott SL. Effects of high-dose fish oil supplementation during early infancy on neurodevelopment and language: a randomised controlled trial. Br J Nutr. 2012 Oct 28;108(8):1443-54. doi: 10.1017/S0007114511006878[↩]
- Saccone G, Berghella V. Omega-3 long chain polyunsaturated fatty acids to prevent preterm birth: a systematic review and meta-analysis. Obstet Gynecol. 2015 Mar;125(3):663-672. doi: 10.1097/AOG.0000000000000668[↩][↩]
- Newberry SJ, Chung M, Booth M, Maglione M, Tang AM, C.E. OH, et al. Omega-3 fatty acids and maternal and child health: an updated systematic review. Evidence Report/Technology Assessment No. 224. (Prepared by the RAND Southern California Evidence-based Practice Center under Contract No. 290-2012-00006-I.) AHRQ Publication No. 16-E003-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2016.[↩][↩]
- Weylandt, K. H., Serini, S., Chen, Y. Q., Su, H. M., Lim, K., Cittadini, A., & Calviello, G. (2015). Omega-3 Polyunsaturated Fatty Acids: The Way Forward in Times of Mixed Evidence. BioMed research international, 2015, 143109. https://doi.org/10.1155/2015/143109[↩]
- Zheng JS, Hu XJ, Zhao YM, Yang J, Li D. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: meta-analysis of data from 21 independent prospective cohort studies. BMJ. 2013 Jun 27;346:f3706. doi: 10.1136/bmj.f3706[↩][↩]
- Wu S, Feng B, Li K, Zhu X, Liang S, Liu X, Han S, Wang B, Wu K, Miao D, Liang J, Fan D. Fish consumption and colorectal cancer risk in humans: a systematic review and meta-analysis. Am J Med. 2012 Jun;125(6):551-9.e5. doi: 10.1016/j.amjmed.2012.01.022[↩][↩]
- MacLean CH, Newberry SJ, Mojica WA, Khanna P, Issa AM, Suttorp MJ, Lim YW, Traina SB, Hilton L, Garland R, Morton SC. Effects of omega-3 fatty acids on cancer risk: a systematic review. JAMA. 2006 Jan 25;295(4):403-15. doi: 10.1001/jama.295.4.403. Erratum in: JAMA. 2006 Apr 26;295(16):1900[↩]
- Gago-Dominguez, M., Yuan, J. M., Sun, C. L., Lee, H. P., & Yu, M. C. (2003). Opposing effects of dietary n-3 and n-6 fatty acids on mammary carcinogenesis: The Singapore Chinese Health Study. British journal of cancer, 89(9), 1686–1692. https://doi.org/10.1038/sj.bjc.6601340[↩]
- Brasky, T. M., Lampe, J. W., Potter, J. D., Patterson, R. E., & White, E. (2010). Specialty supplements and breast cancer risk in the VITamins And Lifestyle (VITAL) Cohort. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 19(7), 1696–1708. https://doi.org/10.1158/1055-9965.EPI-10-0318[↩]
- Gerber M. Omega-3 fatty acids and cancers: a systematic update review of epidemiological studies. Br J Nutr. 2012 Jun;107 Suppl 2:S228-39. doi: 10.1017/S0007114512001614[↩][↩]
- Geelen A, Schouten JM, Kamphuis C, Stam BE, Burema J, Renkema JM, Bakker EJ, van’t Veer P, Kampman E. Fish consumption, n-3 fatty acids, and colorectal cancer: a meta-analysis of prospective cohort studies. Am J Epidemiol. 2007 Nov 15;166(10):1116-25. doi: 10.1093/aje/kwm197[↩]
- Kantor, E. D., Lampe, J. W., Peters, U., Vaughan, T. L., & White, E. (2014). Long-chain omega-3 polyunsaturated fatty acid intake and risk of colorectal cancer. Nutrition and cancer, 66(4), 716–727. https://doi.org/10.1080/01635581.2013.804101[↩]
- Brasky, T. M., Till, C., White, E., Neuhouser, M. L., Song, X., Goodman, P., Thompson, I. M., King, I. B., Albanes, D., & Kristal, A. R. (2011). Serum phospholipid fatty acids and prostate cancer risk: results from the prostate cancer prevention trial. American journal of epidemiology, 173(12), 1429–1439. https://doi.org/10.1093/aje/kwr027[↩]
- Dahm CC, Gorst-Rasmussen A, Crowe FL, Roswall N, Tjønneland A, Drogan D, Boeing H, Teucher B, Kaaks R, Adarakis G, Zylis D, Trichopoulou A, Fedirko V, Chajes V, Jenab M, Palli D, Pala V, Tumino R, Ricceri F, van Kranen H, Bueno-de-Mesquita HB, Quirós JR, Sánchez MJ, Luján-Barroso L, Larrañaga N, Chirlaque MD, Ardanaz E, Johansson M, Stattin P, Khaw KT, Wareham N, Wark PA, Norat T, Riboli E, Key TJ, Overvad K. Fatty acid patterns and risk of prostate cancer in a case-control study nested within the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2012 Dec;96(6):1354-61. doi: 10.3945/ajcn.112.034157[↩]
- Park, S. Y., Wilkens, L. R., Henning, S. M., Le Marchand, L., Gao, K., Goodman, M. T., Murphy, S. P., Henderson, B. E., & Kolonel, L. N. (2009). Circulating fatty acids and prostate cancer risk in a nested case-control study: the Multiethnic Cohort. Cancer causes & control : CCC, 20(2), 211–223. https://doi.org/10.1007/s10552-008-9236-4[↩]
- Bosire, C., Stampfer, M. J., Subar, A. F., Park, Y., Kirkpatrick, S. I., Chiuve, S. E., Hollenbeck, A. R., & Reedy, J. (2013). Index-based dietary patterns and the risk of prostate cancer in the NIH-AARP diet and health study. American journal of epidemiology, 177(6), 504–513. https://doi.org/10.1093/aje/kws261[↩]
- Augustsson K, Michaud DS, Rimm EB, Leitzmann MF, Stampfer MJ, Willett WC, Giovannucci E. A prospective study of intake of fish and marine fatty acids and prostate cancer. Cancer Epidemiol Biomarkers Prev. 2003 Jan;12(1):64-7.[↩]
- Crowe, F. L., Appleby, P. N., Travis, R. C., Barnett, M., Brasky, T. M., Bueno-de-Mesquita, H. B., Chajes, V., Chavarro, J. E., Chirlaque, M. D., English, D. R., Gibson, R. A., Giles, G. G., Goodman, G. E., Henning, S. M., Kaaks, R., King, I. B., Kolonel, L. N., Kristal, A. R., Neuhouser, M. L., Park, S. Y., … Endogenous Hormones, Nutritional Biomarkers and Prostate Cancer Collaborative Group (2014). Circulating fatty acids and prostate cancer risk: individual participant meta-analysis of prospective studies. Journal of the National Cancer Institute, 106(9), dju240. https://doi.org/10.1093/jnci/dju240[↩]
- Chua, M. E., Sio, M. C., Sorongon, M. C., & Morales, M. L., Jr (2013). The relevance of serum levels of long chain omega-3 polyunsaturated fatty acids and prostate cancer risk: A meta-analysis. Canadian Urological Association journal = Journal de l’Association des urologues du Canada, 7(5-6), E333–E343. https://doi.org/10.5489/cuaj.1056[↩]
- Chua, M. E., Sio, M. C., Sorongon, M. C., & Dy, J. S. (2012). Relationship of dietary intake of omega-3 and omega-6 Fatty acids with risk of prostate cancer development: a meta-analysis of prospective studies and review of literature. Prostate cancer, 2012, 826254. https://doi.org/10.1155/2012/826254[↩]
- Szymanski KM, Wheeler DC, Mucci LA. Fish consumption and prostate cancer risk: a review and meta-analysis. Am J Clin Nutr. 2010 Nov;92(5):1223-33. doi: 10.3945/ajcn.2010.29530[↩]
- Alexander, D. D., Bassett, J. K., Weed, D. L., Barrett, E. C., Watson, H., & Harris, W. (2015). Meta-Analysis of Long-Chain Omega-3 Polyunsaturated Fatty Acids (LCω-3PUFA) and Prostate Cancer. Nutrition and cancer, 67(4), 543–554. https://doi.org/10.1080/01635581.2015.1015745[↩]
- Noel SE, Stoneham AC, Olsen CM, Rhodes LE, Green AC. Consumption of omega-3 fatty acids and the risk of skin cancers: a systematic review and meta-analysis. Int J Cancer. 2014 Jul 1;135(1):149-56. doi: 10.1002/ijc.28630. Epub 2013 Dec 18. Erratum in: Int J Cancer. 2017 Jun 1;140(11):E15.[↩]
- Ibiebele TI, Nagle CM, Bain CJ, Webb PM. Intake of omega-3 and omega-6 fatty acids and risk of ovarian cancer. Cancer Causes Control. 2012 Nov;23(11):1775-83. doi: 10.1007/s10552-012-0053-4[↩]
- Arem, H., Neuhouser, M. L., Irwin, M. L., Cartmel, B., Lu, L., Risch, H., Mayne, S. T., & Yu, H. (2013). Omega-3 and omega-6 fatty acid intakes and endometrial cancer risk in a population-based case-control study. European journal of nutrition, 52(3), 1251–1260. https://doi.org/10.1007/s00394-012-0436-z[↩]
- Brasky, T. M., Rodabough, R. J., Liu, J., Kurta, M. L., Wise, L. A., Orchard, T. S., Cohn, D. E., Belury, M. A., White, E., Manson, J. E., & Neuhouser, M. L. (2015). Long-chain ω-3 fatty acid intake and endometrial cancer risk in the Women’s Health Initiative. The American journal of clinical nutrition, 101(4), 824–834. https://doi.org/10.3945/ajcn.114.098988[↩]
- Qin, B., Xun, P., & He, K. (2012). Fish or long-chain (n-3) PUFA intake is not associated with pancreatic cancer risk in a meta-analysis and systematic review. The Journal of nutrition, 142(6), 1067–1073. https://doi.org/10.3945/jn.111.156711[↩]
- Han YJ, Li J, Huang W, Fang Y, Xiao LN, Liao ZE. Fish consumption and risk of esophageal cancer and its subtypes: a systematic review and meta-analysis of observational studies. Eur J Clin Nutr. 2013 Feb;67(2):147-54. doi: 10.1038/ejcn.2012.213[↩]
- Christen, W. G., Schaumberg, D. A., Glynn, R. J., & Buring, J. E. (2011). Dietary ω-3 fatty acid and fish intake and incident age-related macular degeneration in women. Archives of ophthalmology (Chicago, Ill. : 1960), 129(7), 921–929. https://doi.org/10.1001/archophthalmol.2011.34[↩][↩]
- Augood C, Chakravarthy U, Young I, Vioque J, de Jong PT, Bentham G, Rahu M, Seland J, Soubrane G, Tomazzoli L, Topouzis F, Vingerling JR, Fletcher AE. Oily fish consumption, dietary docosahexaenoic acid and eicosapentaenoic acid intakes, and associations with neovascular age-related macular degeneration. Am J Clin Nutr. 2008 Aug;88(2):398-406. doi: 10.1093/ajcn/88.2.398[↩]
- Seddon JM, George S, Rosner B. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: the US Twin Study of Age-Related Macular Degeneration. Arch Ophthalmol. 2006 Jul;124(7):995-1001. doi: 10.1001/archopht.124.7.995[↩]
- Merle BM, Benlian P, Puche N, Bassols A, Delcourt C, Souied EH; Nutritional AMD Treatment 2 Study Group. Circulating omega-3 Fatty acids and neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014 Mar 28;55(3):2010-9. doi: 10.1167/iovs.14-13916[↩]
- Age-Related Eye Disease Study Research Group (2001). A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Archives of ophthalmology (Chicago, Ill. : 1960), 119(10), 1417–1436. https://doi.org/10.1001/archopht.119.10.1417[↩]
- Sangiovanni, J. P., Agrón, E., Meleth, A. D., Reed, G. F., Sperduto, R. D., Clemons, T. E., Chew, E. Y., & Age-Related Eye Disease Study Research Group (2009). {omega}-3 Long-chain polyunsaturated fatty acid intake and 12-y incidence of neovascular age-related macular degeneration and central geographic atrophy: AREDS report 30, a prospective cohort study from the Age-Related Eye Disease Study. The American journal of clinical nutrition, 90(6), 1601–1607. https://doi.org/10.3945/ajcn.2009.27594[↩]
- Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013 May 15;309(19):2005-15. doi: 10.1001/jama.2013.4997. Erratum in: JAMA. 2013 Jul 10;310(2):208.[↩]
- Lawrenson, J. G., & Evans, J. R. (2015). Omega 3 fatty acids for preventing or slowing the progression of age-related macular degeneration. The Cochrane database of systematic reviews, 2015(4), CD010015. https://doi.org/10.1002/14651858.CD010015.pub3[↩]
- Souied EH, Delcourt C, Querques G, Bassols A, Merle B, Zourdani A, Smith T, Benlian P; Nutritional AMD Treatment 2 Study Group. Oral docosahexaenoic acid in the prevention of exudative age-related macular degeneration: the Nutritional AMD Treatment 2 study. Ophthalmology. 2013 Aug;120(8):1619-31. doi: 10.1016/j.ophtha.2013.01.005[↩]
- Dry Eye Assessment and Management Study Research Group, Asbell, P. A., Maguire, M. G., Pistilli, M., Ying, G. S., Szczotka-Flynn, L. B., Hardten, D. R., Lin, M. C., & Shtein, R. M. (2018). n-3 Fatty Acid Supplementation for the Treatment of Dry Eye Disease. The New England journal of medicine, 378(18), 1681–1690. https://doi.org/10.1056/NEJMoa1709691[↩][↩]
- Ziemanski, J. F., Wolters, L. R., Jones-Jordan, L., Nichols, J. J., & Nichols, K. K. (2018). Relation Between Dietary Essential Fatty Acid Intake and Dry Eye Disease and Meibomian Gland Dysfunction in Postmenopausal Women. American journal of ophthalmology, 189, 29–40. https://doi.org/10.1016/j.ajo.2018.01.004[↩][↩]
- Miljanović, B., Trivedi, K. A., Dana, M. R., Gilbard, J. P., Buring, J. E., & Schaumberg, D. A. (2005). Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. The American journal of clinical nutrition, 82(4), 887–893. https://doi.org/10.1093/ajcn/82.4.887[↩]
- Hom MM, Asbell P, Barry B. Omegas and Dry Eye: More Knowledge, More Questions. Optom Vis Sci. 2015 Sep;92(9):948-56. doi: 10.1097/OPX.0000000000000655[↩]
- Bhargava, R., Kumar, P., Kumar, M., Mehra, N., & Mishra, A. (2013). A randomized controlled trial of omega-3 fatty acids in dry eye syndrome. International journal of ophthalmology, 6(6), 811–816. https://doi.org/10.3980/j.issn.2222-3959.2013.06.13[↩]
- Epitropoulos, A. T., Donnenfeld, E. D., Shah, Z. A., Holland, E. J., Gross, M., Faulkner, W. J., Matossian, C., Lane, S. S., Toyos, M., Bucci, F. A., Jr, & Perry, H. D. (2016). Effect of Oral Re-esterified Omega-3 Nutritional Supplementation on Dry Eyes. Cornea, 35(9), 1185–1191. https://doi.org/10.1097/ICO.0000000000000940[↩]
- Sydenham E, Dangour AD, Lim WS. Omega 3 fatty acid for the prevention of cognitive decline and dementia. Cochrane Database Syst Rev. 2012 Jun 13;(6):CD005379. doi: 10.1002/14651858.CD005379.pub3[↩][↩]
- Chew, E. Y., Clemons, T. E., Agrón, E., Launer, L. J., Grodstein, F., Bernstein, P. S., & Age-Related Eye Disease Study 2 (AREDS2) Research Group (2015). Effect of Omega-3 Fatty Acids, Lutein/Zeaxanthin, or Other Nutrient Supplementation on Cognitive Function: The AREDS2 Randomized Clinical Trial. JAMA, 314(8), 791–801. https://doi.org/10.1001/jama.2015.9677[↩][↩]
- Yassine HN, Feng Q, Azizkhanian I, Rawat V, Castor K, Fonteh AN, Harrington MG, Zheng L, Reed BR, DeCarli C, Jagust WJ, Chui HC. Association of Serum Docosahexaenoic Acid With Cerebral Amyloidosis. JAMA Neurol. 2016 Oct 1;73(10):1208-1216. doi: 10.1001/jamaneurol.2016.1924[↩]
- van Gelder BM, Tijhuis M, Kalmijn S, Kromhout D. Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly men: the Zutphen Elderly Study. Am J Clin Nutr. 2007 Apr;85(4):1142-7. doi: 10.1093/ajcn/85.4.1142[↩]
- Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol. 1997 Nov;42(5):776-82. doi: 10.1002/ana.410420514[↩]
- Engelhart MJ, Geerlings MI, Ruitenberg A, Van Swieten JC, Hofman A, Witteman JC, Breteler MM. Diet and risk of dementia: Does fat matter?: The Rotterdam Study. Neurology. 2002 Dec 24;59(12):1915-21. doi: 10.1212/01.wnl.0000038345.77753.46[↩]
- Pottala, J. V., Yaffe, K., Robinson, J. G., Espeland, M. A., Wallace, R., & Harris, W. S. (2014). Higher RBC EPA + DHA corresponds with larger total brain and hippocampal volumes: WHIMS-MRI study. Neurology, 82(5), 435–442. https://doi.org/10.1212/WNL.0000000000000080[↩]
- Tan, Z. S., Harris, W. S., Beiser, A. S., Au, R., Himali, J. J., Debette, S., Pikula, A., Decarli, C., Wolf, P. A., Vasan, R. S., Robins, S. J., & Seshadri, S. (2012). Red blood cell ω-3 fatty acid levels and markers of accelerated brain aging. Neurology, 78(9), 658–664. https://doi.org/10.1212/WNL.0b013e318249f6a9[↩]
- Zhang Y, Chen J, Qiu J, Li Y, Wang J, Jiao J. Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: a dose-response meta-analysis of 21 cohort studies. Am J Clin Nutr. 2016 Feb;103(2):330-40. doi: 10.3945/ajcn.115.124081[↩]
- Dangour AD, Allen E, Elbourne D, Fasey N, Fletcher AE, Hardy P, Holder GE, Knight R, Letley L, Richards M, Uauy R. Effect of 2-y n-3 long-chain polyunsaturated fatty acid supplementation on cognitive function in older people: a randomized, double-blind, controlled trial. Am J Clin Nutr. 2010 Jun;91(6):1725-32. doi: 10.3945/ajcn.2009.29121[↩]
- Quinn, J. F., Raman, R., Thomas, R. G., Yurko-Mauro, K., Nelson, E. B., Van Dyck, C., Galvin, J. E., Emond, J., Jack, C. R., Jr, Weiner, M., Shinto, L., & Aisen, P. S. (2010). Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA, 304(17), 1903–1911. https://doi.org/10.1001/jama.2010.1510[↩]
- Freund-Levi Y, Eriksdotter-Jönhagen M, Cederholm T, Basun H, Faxén-Irving G, Garlind A, Vedin I, Vessby B, Wahlund LO, Palmblad J. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol. 2006 Oct;63(10):1402-8. doi: 10.1001/archneur.63.10.1402[↩]
- Lee LK, Shahar S, Chin AV, Yusoff NA. Docosahexaenoic acid-concentrated fish oil supplementation in subjects with mild cognitive impairment (MCI): a 12-month randomised, double-blind, placebo-controlled trial. Psychopharmacology (Berl). 2013 Feb;225(3):605-12. doi: 10.1007/s00213-012-2848-0[↩]
- Yurko-Mauro, K., Alexander, D. D., & Van Elswyk, M. E. (2015). Docosahexaenoic acid and adult memory: a systematic review and meta-analysis. PloS one, 10(3), e0120391. https://doi.org/10.1371/journal.pone.0120391[↩]
- Jiao J, Li Q, Chu J, Zeng W, Yang M, Zhu S. Effect of n-3 PUFA supplementation on cognitive function throughout the life span from infancy to old age: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014 Dec;100(6):1422-36. doi: 10.3945/ajcn.114.095315[↩]
- Mazereeuw G, Lanctôt KL, Chau SA, Swardfager W, Herrmann N. Effects of ω-3 fatty acids on cognitive performance: a meta-analysis. Neurobiol Aging. 2012 Jul;33(7):1482.e17-29. doi: 10.1016/j.neurobiolaging.2011.12.014[↩]
- Lee YH, Bae SC, Song GG. Omega-3 polyunsaturated fatty acids and the treatment of rheumatoid arthritis: a meta-analysis. Arch Med Res. 2012 Jul;43(5):356-62. doi: 10.1016/j.arcmed.2012.06.011[↩][↩]
- Miles EA, Calder PC. Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br J Nutr. 2012 Jun;107 Suppl 2:S171-84. doi: 10.1017/S0007114512001560[↩][↩]
- Sköldstam L, Börjesson O, Kjällman A, Seiving B, Akesson B. Effect of six months of fish oil supplementation in stable rheumatoid arthritis. A double-blind, controlled study. Scand J Rheumatol. 1992;21(4):178-85. doi: 10.3109/03009749209099218[↩]
- Park Y, Lee A, Shim SC, Lee JH, Choe JY, Ahn H, Choi CB, Sung YK, Bae SC. Effect of n-3 polyunsaturated fatty acid supplementation in patients with rheumatoid arthritis: a 16-week randomized, double-blind, placebo-controlled, parallel-design multicenter study in Korea. J Nutr Biochem. 2013 Jul;24(7):1367-72. doi: 10.1016/j.jnutbio.2012.11.004[↩]
- Nielsen GL, Faarvang KL, Thomsen BS, Teglbjaerg KL, Jensen LT, Hansen TM, Lervang HH, Schmidt EB, Dyerberg J, Ernst E. The effects of dietary supplementation with n-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: a randomized, double blind trial. Eur J Clin Invest. 1992 Oct;22(10):687-91. doi: 10.1111/j.1365-2362.1992.tb01431.x[↩]
- MacLean CH, Mojica WA, Morton SC, Pencharz J, Hasenfeld Garland R, Tu W, et al. Effects of omega-3 fatty acids on lipids and glycemic control in type ii diabetes and the metabolic syndrome and on inflammatory bowel disease, rheumatoid arthritis, renal disease, systemic lupus erythematosus, and osteoporosis. Evidence Report/Technology Assessment no. 89 (prepared by Southern California/RAND Evidence-based Practice Center, under contract no. 290-02- 0003). Rockville, MD: Agency for Healthcare Research and Quality; 2004.[↩]
- Goldberg RJ, Katz J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain. 2007 May;129(1-2):210-23. doi: 10.1016/j.pain.2007.01.020[↩]
- James M, Proudman S, Cleland L. Fish oil and rheumatoid arthritis: past, present and future. Proc Nutr Soc. 2010 Aug;69(3):316-23. doi: 10.1017/S0029665110001564[↩][↩]
- Li F, Liu X, Zhang D. Fish consumption and risk of depression: a meta-analysis. J Epidemiol Community Health. 2016 Mar;70(3):299-304. doi: 10.1136/jech-2015-206278[↩]
- Appleton, K. M., Sallis, H. M., Perry, R., Ness, A. R., & Churchill, R. (2015). Omega-3 fatty acids for depression in adults. The Cochrane database of systematic reviews, 2015(11), CD004692. https://doi.org/10.1002/14651858.CD004692.pub4[↩]
- Cabré E, Mañosa M, Gassull MA. Omega-3 fatty acids and inflammatory bowel diseases – a systematic review. Br J Nutr. 2012 Jun;107 Suppl 2:S240-52. doi: 10.1017/S0007114512001626[↩]
- Lev-Tzion R, Griffiths AM, Leder O, Turner D. Omega 3 fatty acids (fish oil) for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2014 Feb 28;(2):CD006320. doi: 10.1002/14651858.CD006320.pub4[↩]
- Cooper RE, Tye C, Kuntsi J, Vassos E, Asherson P. The effect of omega-3 polyunsaturated fatty acid supplementation on emotional dysregulation, oppositional behaviour and conduct problems in ADHD: A systematic review and meta-analysis. J Affect Disord. 2016 Jan 15;190:474-482. doi: 10.1016/j.jad.2015.09.053[↩]
- Best KP, Gold M, Kennedy D, Martin J, Makrides M. Omega-3 long-chain PUFA intake during pregnancy and allergic disease outcomes in the offspring: a systematic review and meta-analysis of observational studies and randomized controlled trials. Am J Clin Nutr. 2016 Jan;103(1):128-43. doi: 10.3945/ajcn.115.111104[↩]
- Gunaratne, A. W., Makrides, M., & Collins, C. T. (2015). Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. The Cochrane database of systematic reviews, 2015(7), CD010085. https://doi.org/10.1002/14651858.CD010085.pub2[↩]
- Oliver, C., & Watson, H. (2016). Omega-3 fatty acids for cystic fibrosis. The Cochrane database of systematic reviews, 2016(1), CD002201. https://doi.org/10.1002/14651858.CD002201.pub5[↩]