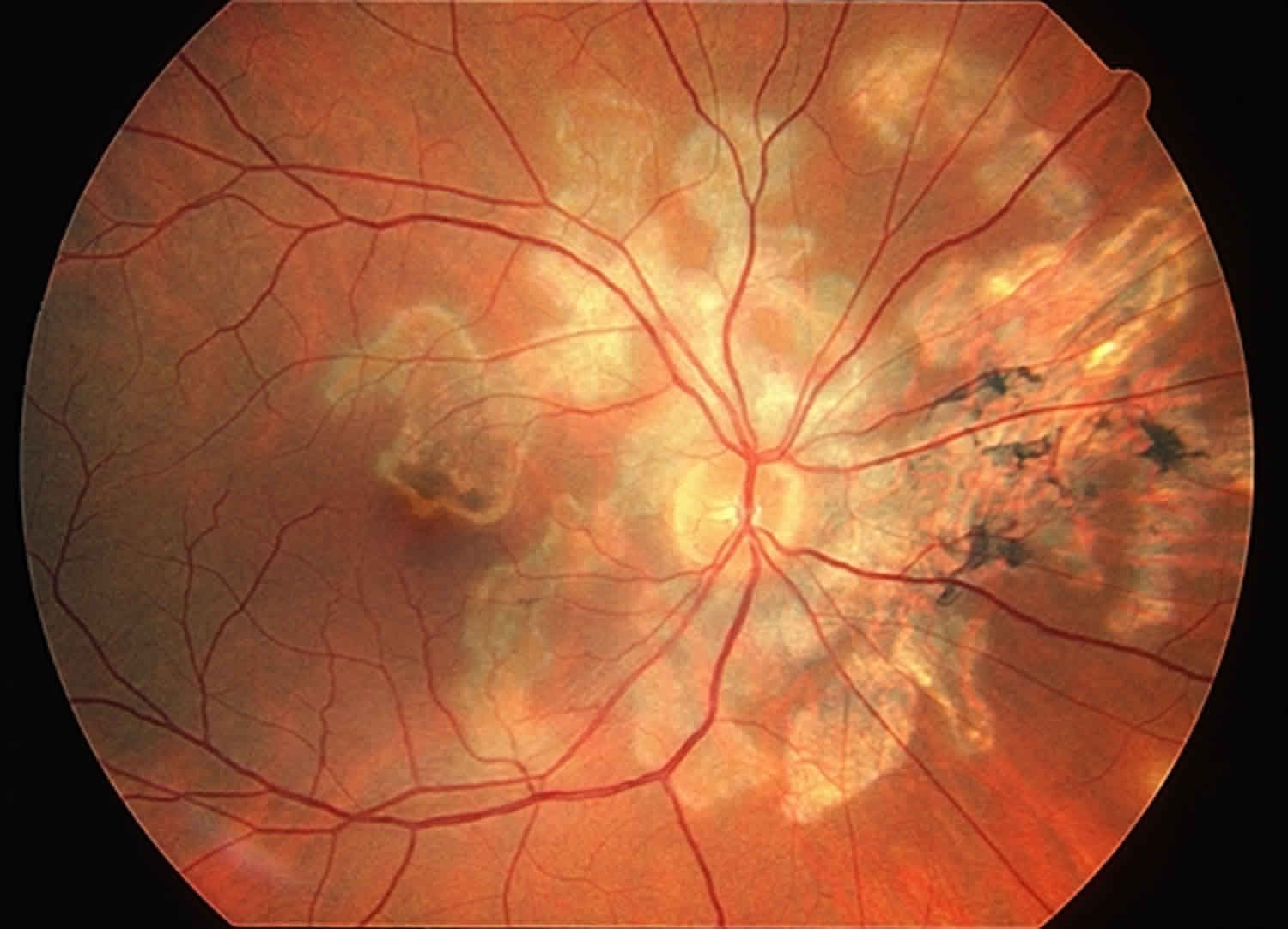
Serpiginous choroiditis
Serpiginous choroiditis also called geographic helicoid peripapillary choroidopathy, is a rare idiopathic inflammatory eye disease affecting the inner choroid and retinal pigment epithelium that typically develops between age 30 and 70 years, although it may occur in younger patients 1. Serpiginous choroiditis is a posterior uveitis displaying a geographic pattern of choroiditis that typically extends from the peripapillary area and affects the overlying retinal pigment epithelium (RPE) and the outer retina 2. Typically serpiginous choroiditis involves both eyes, is recurrent and relentlessly progressive and can cause irreversible damage to the photoreceptors with permanent vision loss if the process involves the fovea 3. Affected individuals have lesions in the eye that last from weeks to months and involve scarring of the eye tissue 4. Besides progressive foveal scarring, these patients also lose vision as a result of choroidal neovascularization. Retinal vasculitis and retinal vein occlusions may also occur 5. Though long standing remissions can be achieved through aggressive immuno-modulation, patients not receiving such therapy often suffer through multiple exacerbations and are often left with macular scarring, and significant visual loss. Recurrence of these lesions is common in serpiginous choroiditis. Vision loss may occur in one or both eyes when the macula is involved 6.
Serpiginous choroiditis is a relatively rare condition, prevalence ranging from 0.2% to 5% of all uveitis patients 7. Majority of these institute-based studies were from tertiary eye care setup and did not differentiate between classic serpiginous choroiditis and serpiginous-like choroiditis. Prevalence rates in Southeast Asian countries were found to be higher than other parts of the world 8. A possible role of infectious etiology can be implicated to explain the relative higher incidence of serpiginous choroiditis in these regions 7. However, there is uneven distribution of serpiginous choroiditis across the Southeast Asian countries and considerable regional difference exists even within the same geographical area 9. The reported prevalence of serpiginous choroiditis in India varies widely from 1.2% to 5.4% 9. However, relatively lower prevalence of serpiginous choroiditis have been reported from the other countries in Indian subcontinent 8. In addition, relative higher prevalence of serpiginous choroiditis has been reported from countries like Germany and United states in literature 10.
There are a few treatment options for individuals with serpiginous choroiditis 1. Treatment may involve an anti-inflammatory and immune-suppressing medications, such as prednisone, or an immune system suppressing combination of prednisone, cyclosporine, and azathioprine. Additionally, the role of cyclosporine alone has been investigated. These treatments may be administered for a long period of time to prevent recurrences. A serious complication of serpiginous choroiditis is choroidal neovascularization. Laser photocoagulation or surgery may be helpful in some of these cases 6.
Figure 1. Eye anatomy
Can I inherit serpiginous choroiditis if my mother has the condition?
No familial predillection or propensity has been described 11.
My mother has been diagnosed with serpiginous choroiditis. Should my sibling and I be evaluated for this condition?
While serpiginous choroiditis does not appear to be genetic, you should share your family history with your eye doctor and have your vision evaluated periodically or if you develop any new symptoms.
Serpiginous choroiditis types
Based on the morphology and characteristics of lesions, serpiginous choroiditis can be further subdivided into the following categories: Peripapillary serpiginous choroiditis is the most common type of classic serpiginous choroiditis described in literature. Approximately 80% of the cases of classic serpiginous choroiditis reported are of peripapillary variety 12. The lesion in peripapillary classic serpiginous choroiditis is usually unifocal and occurs around the optic disc and progresses in a serpentine pattern centrifugally to involve macula. Macular serpiginous choroiditis is relatively uncommon but dreaded cause of vision loss because of early involvement of macula and higher risk of developing choroidal neovascularization (CNV) 13. Few clinical entities have been described in literature which have clinical features similar to acute posterior multifocal placoid pigment epitheliopathy (APMPPE) and serpiginous choroiditis, reflecting different ends of a disease spectrum. Golchet et al. 14 described a condition in five patients, which they called persistent placoid maculopathy (PPM), characterized by normal to mildly affected visual acuity in spite of long-standing geographic central whitish plaques involving fovea. However, other authors have reported variable disease course with poor visual outcome in patients with persistent placoid maculopathy (PPM) 15. Choroidal neovascular complications were much higher in these patients and occurred in 11 of 18 cases reported till date 16. Choroidal vasculitis leading to ischemic choroidal infarcts has been implicated in PPM 16. Relentless placoid chorioretinitis (RPC) is another term used by Jones et al. 17 to describe an unusual clinical entity resembling acute posterior multifocal placoid pigment epitheliopathy (APMPPE) and serpiginous choroiditis both clinically and angiographically with an atypical clinical course. Lesions in relentless placoid chorioretinitis are usually numerous (ranging from 50 to 100 in number) and involve posterior pole, mid-, and far-periphery predating or occurring simultaneously with macular involvement in contrast to the lesions seen in APMPPE, which are usually limited to posterior pole. Simultaneous presence of active and healed lesions scattered all over the fundus with prolonged and relapsing course was described as hallmark of RPC 17. Pigmented chorioretinal atrophy usually develops as the lesions of relentless placoid chorioretinitis heal. Because of considerable overlap between the angiographic findings 18, RPE hyperperturbations, recurrent nature of APMPPE and this subset of serpiginous choroiditis, a term “Ampiginous Choroiditis” has been used 19. Foveal involvement is relatively less in ampiginous choroiditis when compared with other subtypes of serpiginous choroiditis. However, it is not clear whether ampiginous choroiditis or relentless placoid chorioretinitis represents isolated distinct variants of serpiginous choroiditis or a clinical variant of serpiginous-like choroiditis/multifocal serpiginoid choroiditis. There are reports of ocular lesions resembling APMPPE which subsequently coalesced and healed with characteristic picture of serpiginous choroiditis 20. In a retrospective analysis of 86 patients with serpiginous choroiditis, 20 patients who presented initially with clinical picture like APMPPE had progressed to serpiginous choroiditis over a period of several months to years 20. In addition, role of an infectious etiology has been reported with these conditions. Bhuibhar and Biswas 21 isolated mycobacterial DNA from aqueous aspirate of a patient with ampiginous choroiditis, who was also tested positive for Mantoux test, interferon gamma release assay and had right hilar and paratracheal lymphadenopathy in high-resolution computerized tomography of chest. Khalifa et al. 22 reported ampiginous choroiditis in both eyes of a 17-year-old woman 3 weeks following the administration of the quadrivalent human papilloma virus vaccine.
Serpiginous-like choroiditis
serpiginous-like choroiditis is a distinct form of serpiginous choroiditis, characterized by multifocal choroidal lesions of varying shape and size which often coalesces to form diffuse choroiditis resembling serpiginous choroiditis in patients with presumed tuberculosis.[4] The term “serpigniod” and “multifocal serpigniod choroiditis” have also been used to refer these clinical entities 23. In contrast to patients with classic serpiginous choroiditis, patients with serpiginous-like choroiditis are usually from tuberculosis endemic area and more likely to have unilateral presentation, relatively younger age of presentation, multifocal lesions, located in periphery of retina, frequent sparing of the juxtapapillary region, more inflammatory reaction in vitreous and continue to show progression with development of new lesions despite effective corticosteroid therapy 24.
Serpiginous choroiditis causes
The cause of serpiginous choroiditis is unknown. Immune mediated vasculitis with subsequent occlusion of the choroidal vessels is one proposed mechanism. Although likely there is an underlying autoimmune pathogenesis, the specific trigger for this localized ocular immune process remains unknown 25. Recent studies using polymerase chain reaction (PCR) and interferon gamma release assay suggest that microorganisms may be the inciting agents 11, either through active proliferation or through induction of an immune response against the microbes. Such an immune response may also target the uvea and retina through a process of molecular mimicry. In most patients, however, diagnostic studies seeking an infectious cause remain inconclusive; thus, the intraocular inflammation is treated with corticosteroids and immunosuppresants without the use of anti-microbial agents, on the assumption that this choroidal inflammation is driven by an underlying, organ-specific, autoimmune disorder 26.
An associated elevation of von Willebrand factor has been reported, but its significance is unclear 27. The fact that these patients have multiple recurrent inflammatory episodes has led some to speculate that infection (possibly herpesvirus) may be the cause. Erkkila and colleagues 28 reported in a Finnish study an association of serpiginous choroiditis with a greater prevalence of HLA B7. Broekhuyse and coworkers 29 demonstrated increased immune responsiveness to retinal S antigen (arrestin) in patients with serpiginous choroiditis.
Autoimmune etiology
Auto-reactivity of circulating lymphocytes to retinal S antigen has been observed in classic serpiginous choroiditis, but not in acute posterior multifocal placoid pigment epitheliopathy (APMPPE) 30. Though both the entities affect choriocapillaris as well as retinal pigment epithelium (RPE), unlike acute posterior multifocal placoid pigment epitheliopathy (APMPPE), classic serpiginous choroiditis causes extensive structural and functional damage to the choriocapillaris, RPE, and surrounding structures. The role of retinal photoreceptor protein-mediated damage has been implicated in extensive damage to the retina by classic serpiginous choroiditis 30. Occlusion of choriocapillaris has been attributed to the etiopathogenesis of classic serpiginous choroiditis 31. Various mechanisms of choriocapillaris occlusion have been suggested in literature 32. Role of a localized immune-mediated vasculitis leading to occlusion of the choroidal vessels has been suggested by Erkkilä et al 33. King et al. 34 demonstrated elevated factor VIII/von Willebrand factor ratio in patients with classic serpiginous choroiditis and also highlighted the role of endothelial injury caused by a vasculitis-induced vasoocclusion. There are also reports of classic serpiginous choroiditis occurring in patients suffering from carcinoma 35.
Infectious etiology
Various organisms have been implicated in the pathogenesis of serpiginous choroiditis, though treatment with specific antimicrobial agents did not show any significant positive clinical results in majority of the cases.
Mycobacterium tuberculosis is the most common infectious organism implicated in etiopathogenesis of serpiginous-like choroiditis. Association between serpiginous choroiditis and presumed tuberculosis was first described by Hutchison 36. Role of the Mycobacterium tuberculosis in pathogenesis of serpiginous choroiditis was also described by Witmer in 1952, Schalegel in 1969, and Maumenee in 1970 37. Mycobacterium tuberculosis, which is believed to be sequestered in the RPE, has been implicated in eyes with panuveitis or related intraocular inflammation, including serpiginous-like choroiditis by clinicopathologic study 38. Though genomes of M. tuberculosis have been isolated from aqueous and vitreous samples of patients with serpiginous-like choroiditis 39, isolation of the bacilli in these patients remains a major challenge. In a study from North India, Bansal et al. 39 isolated mycobacterial DNA in vitreous fluid samples obtained by diagnostic pars plana vitrectomy in patients with active Mserpiginous choroiditis and latent tuberculosis by using various molecular techniques such as multitargeted polymerase chain reaction (PCR) analysis, Gene Xpert MTB/RIF assay, and the line probe assay (MTB DR plus assay). The role of autoimmunity in pathogenesis of ocular tuberculosis has been established by the isolation of autoreactive T cells in vitreous sample of patients with tubercular uveitis including multifocal serpiginoid choroiditis, which showed resistance to activation-induced cell death 40.
Viral etiology in serpiginous choroiditis has been suggested by various authors. A case of serpiginous choroiditis following herpes zoster ophthalmicus was reported by Gass 41. Using polymerase chain reaction, varicella zoster virus and herpes simplex virus have been isolated from aqueous humor of patients with serpiginous choroiditis 42. All these patients had multifocal lesions involving macula and were associated with vitritis and anterior chamber reaction. However, antiviral therapy has not been reported to have any beneficial role in treatment of serpiginous choroiditis and there are reports of disease recurrence in spite of antiviral therapy 43.
Toxoplasma gondii has also been implicated as possible etiological agent 44. Evidence of disseminated fungal infection was suggested by Pisa et al 45. They observed antibodies against Candida spp. in serum samples from four patients with serpiginous choroiditis and fungal genomes in peripheral blood were detected in four serpiginous choroiditis patients 45. However, circulating fungal DNA in serum may not be conclusive evidence of ocular fungal infection and antigen analysis has low specificity. These reports need to be interpreted with caution; many of them may have anecdotal association and positive antibody tests may merely reflect previous exposure to these organisms 46.
Serpiginous choroiditis signs and symptoms
Patients with serpiginous choroiditis can present variable vision that ranges from normal to hand movements. Patients with serpiginous choroiditis typically present with the complaints of diminution of central vision, metamorphopsia, or scotoma. Patient may remain asymptomatic until the macula is involved. Classic serpiginous choroiditis is a bilateral condition; ocular involvement is reported to be asymmetrically unilateral. Typically, anterior chamber and vitreous are usually quiet and remains clear in classic serpiginous choroiditis.
Visual field examination typically will reveal small central or paracentral scotomas. The anterior segment is usually normal, however, anterior uveitis and vitritis may occur. The optic nerve is not affected. The electroculogram and electroretinogram tend to be unchanged, although extensive disease does cause abnormal results.
Serpiginous choroiditis symptoms:
- Many individuals are asymptomatic until the fovea or para-foveal region is affected
- Painless blurring of vision with central or paracentral scotoma – patients can often draw scotoma that mimic the shape of macular lesions (Amsler grid testing is very valuable in these patients)
- Metamorphopsia due to macular involvement
- Visual field loss
- Blurred vision
Serpiginous choroiditis signs:
- Acute onset lesion are gray-yellowish discoloration of the retinal pigment epithelium with a pseudopodial configuration extending in a centrifugal manner from the optic disc. Active lesions often extend outward beyond older scars in serpentine fashion.
- Acute lesions normally last in several weeks, followed with development of chorioretinal atrophy and retinal pigment epithelium migration along the large choroidal vasculature
- Previous inflammation is evidence with areas of atrophic retinal pigment epithelium, choriocapillaris and retina with hyperpigmentation at the adjacent to atrophic areas
- New lesions are often contiguous with the chronic lesions
- Can be complicated with choroidal neovascularization
- Mild vitritis may be present in active disease
- Skip lesions may occur
- Subretinal neovascular membranes may form
- Phlebitis may be seen in the retinal vessels as perivascular leakage on fluorescein angiography
- Fluorescein angiography of active lesions reveals early hypoflourescence, followed later by blurred hyperflourescence especially at the lesion borders
Serpiginous choroiditis complications
Choroidal neovascularization or choroidal neovascular membranes (CNVM) is the most dreaded and commonest complication associated with serpiginous choroiditis. The reported incidence of choroidal neovascularization in patients with serpiginous choroiditis ranges from 10% to 25% 47. Choroidal neovascularization typically arises close to the edge of choroidal lesions and can occur in both active or healed choroiditis. Choriocapillaritis-induced ischemia to choroid, Bruch’s membrane, and outer retina have been implicated in etiopathogenesis of choroidal neovascularization in serpiginous choroiditis. choroidal neovascularization in patients with choroiditis can be easily missed or overlooked and diagnosis of choroidal neovascularization especially occult choroidal neovascularization requires high index of suspicion 48. Classic choroidal neovascularization, which is usually characterized by early hyperfluorescence, can easily be distinguished from serpiginous choroiditis lesions which shows early hypofluorescence in fluorescein angiography. Because of its subtle or less pronounced hyperfluorescence, the diagnosis of occult choroidal neovascularization in serpiginous choroiditis poses significant challenge and may need imaging techniques such as OCT, ICG, etc 48. Subretinal fibrosis is another long-term, sight-threatening complication reported in patients with serpiginous choroiditis 20. Other less common complications include retinal vasculitis, vascular occlusions, secondary neovascularization and vitreous hemorrhage, serous retinal detachment, and cystoid macular edema 49.
Serpiginous choroiditis diagnosis
The diagnosis of serpiginous choroiditis is based on clinical findings. The fluorescein angiography is quite helpful. During the early phases the acute lesions are hypofluorescent due to blockage by edematous retinal pigment epithelium and choroidal non-perfusion. The late phase of the angiogram demonstrates leakage and staining in the active areas. Old chorioretinal scars have a mottled appearance caused by pigment clumping and atrophy, with late staining on fluorescein angiography.
Indocyanine Green angiography is useful for identification and staging of new active lesions. The area of choroidal nonperfusion seen by indocyanine green during the acute stage is generally larger than the corresponding clinically observed retinal lesions. The indocyanine green is also useful in detecting subclinical or persistent choroidal nonperfusion even when the signs of retinal activity have disappeared.
Chorioretinal findings
The typical lesion is discoid or geographic in shape often with finger-like projections. Lesions generally begin in the peripapillary area, but the macula, regrettably, may be the initial site of involvement. Acute lesions appear grayish-yellow with distinct boarders. As the lesions heal there is chorioretinal scarring with variable retinal pigment epithelium hypertrophy, and atrophy, as well as fibrosis. Choroidal neovascularization occurs in about 25% of the patients, and may lead to subretinal fluid and hemorrhage. Other findings may include retinal vasculitis, retinal detachment, retinal pigment epithelium detachment, branch vein or combined branch vein-artery occlusion and neovascularization of the optic disc or elsewhere.
Serpiginous choroiditis treatment
Acute serpiginous choroiditis is regularly treated with corticosteroids. Intravenous, oral, periocular, and intraocular steroids have all been tried for active disease. Juxtafoveal or foveal active lesions should be treated with steroids (IV followed by oral taper is often used). Some recommend peri-ocular steroid injections.
Many combinations of anti-inflammatory regimens have been prescribed for serpiginous choroiditis. Steroid sparing therapy was first suggested by Hooper and Kaplan 50, with combination azathioprine, cyclosporine and prednisone. Many experienced observers, believe that this triple therapy or other immunosuppressive regimen (e.g. cyclophosphamide) may shorten the duration of the acute lesions and prevent recurrences. There are some that recommend acyclovir based on the belief that herpes-virus may be the etiologic entity.
Considerations when deciding upon a course of treatment for active lesions include proximity to the fovea, status of the other eye, presence or absence of a choroidal neovascular membranes (CNVM), systemic disease in the patient that may be exacerbated by treatment, previous experience of the treating physician and the patient’s wishes. The efficacy of long term immunosuppression in preventing and/or minimizing recurrences has yet to be determined.
Choroidal neovascular membranes (CNVM) may be treated with laser photocoagulation for extrafoveal and juxtafoveal lesions, but evidence is still lacking. Subfoveal choroidal neovascular membrane lesions have a poor prognosis, but experimental therapies such as photodynamic therapy or proton beam irradiation may prove helpful in the future.
Corticosteroids
In patients with Cserpiginous choroiditis, reduction in visual acuity usually depends on macular involvement, and thus, it is very crucial to initiate rapid and effective treatment to preserve retinal function in this sensitive part of the eye. High-dose intravenous pulse steroids is useful in macula-threatening conditions. Recurrence of inflammation is very common and another major concern in management of patients with serpiginous choroiditis. Higher doses of corticosteroids have been proved to cause prompt resolution of inflammation but usually fail to prevent recurrence 43. Relapse of inflammation during tapering or after discontinuation of corticosteroids is common.
Immunosuppressive agents
There is no consensus as to the utility of corticosteroids used alone or in combination with immunosuppressive agents. Immunosuppressive agents such as methotrexate, azathioprine, cyclosporine, chlorambucil, or cyclophosphamide can help to attain longer period of disease inactivity and reduce the risk of potential side effects associated with high-dose systemic steroids. However, immunosuppressive agents usually take longer time to attain the desired level of therapeutic concentration of the drug and thus cannot be used to treat acute exacerbations. Immunosuppressive treatment with alkylating agents (chlorambucil and cyclophosphamide) has been found to be associated with long-term drug-free remission of classic serpiginous choroiditis 51. However, alkylating agents should be used judiciously and cautiously in these patients because of their potential life-threatening complications such as leucopenia, risk of malignancy, etc. Cyclosporine has been used in patients with classic serpiginous choroiditis with mixed results – there are reports of treatment failure and recurrence with the drug 52. A triple-agent immunosuppressive regimen consisting of cyclosporine (5 mg/kg/day initially), azathioprine (1.5 mg/kg/day), and prednisolone (1 mg/kg/day) was found to be effective in the management of serpiginous choroiditis 53.
Intravitreal agents
Intravitreal corticosteroid injection has been found to be a promising alternative therapeutic option as a rescue therapy in Cserpiginous choroiditis by inducing rapid remission without the systemic side effects seen with systemic immunosuppression 54. Intravitreal corticosteroid injections have been reported to be useful in the management of active serpiginous lesions, in the presence of systemic corticosteroids contraindication, and in secondary choroidal neovascularization (CNV). Long-term control of inflammation with intravitreal fluocinolone implant in a patient of classic serpiginous choroiditis was reported by Seth and Gaudio 54, but the treated eye required trabeculectomy because of persistent high intraocular pressure, refractory to medical therapy. In a retrospective case series, Miserocchi et al. 55 evaluated the safety and efficacy of intravitreal dexamethasone implant in eight eyes of seven patients with active classic serpiginous choroiditis already receiving maximal tolerated systemic immunosuppressive therapy. Intravitreal corticosteroid implant was planned in these patients because of the presence of systemic disease like uncontrolled hypertension and diabetes mellitus, gastric ulcer, cardiac disease, or osteoporosis, the severity of which contraindicated the further increase in the dose of corticosteroids in these patients despite progressing inflammation. Saatci et al. 56 reported a case of 46-year-old woman with unilateral extrafoveal choroidal neovascularization associated with an active serpiginous choroiditis, who was treated with a simultaneous intravitreal dexamethasone implant and intravitreal injection of ranibizumab. However, these reports must be interpreted and applied cautiously into the clinical practice in a tuberculosis-endemic country like India. Care should be taken to rule out serpiginous-like choroiditis before planning any intravitreal injection for the management of serpiginous choroiditis. However, intravitreal immunosuppressive like methotrexate has been administered in patients with serpiginous-like choroiditis 57.
Biologicals
Biologicals have been found to be very useful in the management of uveitic conditions refractory to other modalities of treatment. Recently, biologicals have been tried in the management of classic serpiginous choroiditis 58. Seve et al. 59 reported successful management of a 43-year-old patient with serpiginous choroiditis with infliximab who developed relapse even after treatment of multiple recurrences with intravenous methylprednisolone, intravenous cyclophosphamide, mycophenolate mofetil, cyclosporine, and oral corticosteroid. The patient was on antitubercular treatment and authors emphasized the need of prior antituberculous chemotherapy before administration of biologicals. Another case report from Spain reported the paradoxical worsening of symptoms and signs of serpiginous-like choroiditis with antitubercular medications, oral corticosteroid in a 23-year-old woman who was subsequently successfully treated with adalimumab 60. Many authors have recommended the use of biological therapy in recalcitrant cases of classic serpiginous choroiditis, where other modalities of treatment have failed and advised the need of antitubercular therapy in these patients 58. However, most of them are isolated case reports and no large scale data are available on safety of biological agents in these patients. Cordero-Coma et al 61 reported a case of presumed serpiginous-like choroiditis in a 48-year-old lady who died of disseminated tuberculosis after treatment with infliximab. The patient developed multiple relapses even after therapy with various immunosuppressive agents and her investigations, including tuberculin skin test and interferon gamma release assay, were negative 61. Extreme caution should be taken while planning anti-TNF alpha or other modalities of biological therapy in patients with classic serpiginous choroiditis, and in a tuberculosis-endemic country, the authors would suggest biological therapy as a last resource for the management of classic serpiginous choroiditis.
Treatment of serpiginous-like choroiditis or multifocal serpiginoid choroiditis
In presence of characteristic clinical lesions and suggestive history (such as contact with TB-patients, origin from TB-endemic region), treatment of serpiginous-like choroiditis is usually decided either by presumptive diagnosis such as positive tuberculin skin test and radiological evidence pulmonary involvement or definitive diagnosis such as isolation of M. tuberculosis genome in aqueous or vitreous sample of the patient. Although there is no clear-cut recommendations or guidelines, anti-tuberculosis therapy in patients with serpiginous-like choroiditis has been proven to control active inflammation as well as prevent future recurrences 62. Usually, four-drug anti-tuberculosis therapy, including isoniazid (5 mg/kg), rifampicin (450–600 mg), ethambutol (15 mg/kg), and pyrazinamide (25–30 mg/kg) first 3–4 months followed by rifampicin and isoniazid for another 9 months, are recommended. Bansal et al. 63 detected rifampicin resistance with the help of line probe assay (MTB DR plus) from vitreous samples of three patients in a series of patients with active Mserpiginous choroiditis and latent tuberculosis who initially responded poorly to anti-tuberculosis therapy. Multidrug resistance tuberculosis, in patients with Mserpiginous choroiditis or serpiginous-like choroiditis, requires high index of suspicion, especially in presence of atypical findings or poor response to therapy. Continued progression of choroiditis lesion was reported in 14% of the patients with serpiginous-like choroiditis following anti-tuberculosis therapy from a study from India 64. Paradoxical worsening of ocular lesions with anti-tuberculosis therapy is a serious concern and has reported in literature by various authors 65. It is characterized by continued progression of preexisting tuberculous lesions or the development of new lesions in a patient who initially improves with anti-tuberculosis therapy and oral steroid. In absence concomitant oral steroid therapy, Jarisch–Herxheimer reaction characterized by a strong inflammatory immunologic reaction in anterior chamber or vitreous can occur 66. Exact mechanism of paradoxical worsening with anti-tuberculosis therapy remains largely unknown. Presence of lipoarabinomannan in mycobacterial cell wall and subsequent activation of inflammatory cascade has been attributed 64. In a recently published study of 44 eyes of 29 patients with Mserpiginous choroiditis 67, 36.4% eyes showed paradoxical worsening of ocular lesions, and in 18.7% eyes, paradoxical worsening was observed in peripheral fundus. Thus, clinicians must be aware of this entity; and regular follow-up and meticulous fundus examination should be carried out in patients with serpiginous-like choroiditis while on anti-tuberculosis therapy.
Treatment with anti-tuberculosis therapy alone is not sufficient for the management of serpiginous-like choroiditis. Tissue damage following strong inflammatory reaction in serpiginous-like choroiditis and the risk of paradoxical worsening with anti-tuberculosis therapy warrant high-dose corticosteroid therapy, which can be achieved through local or systemic mode of administration. Usually, oral corticosteroids (1–1.5 mg/kg/day) in tapering doses are used. There is paucity of literature on local immunosuppression in serpiginous-like choroiditis or multifocal serpiginoid choroiditis 68. In addition to bypassing potential systemic side effects, intravitreal injection of corticosteroid can prevent the risk of activation of latent tuberculosis. In addition, in conditions where the presence of an active extrapulmonary or pulmonary tuberculosis needs use of systemic immunosuppression with extreme caution, intravitreal corticosteroid can be a useful adjunct with anti-tuberculosis therapy. Jain et al.68 administered intravitreal sustained release dexamethasone implant (Ozurdex; Allergan, Irvine, CA) in nine eyes of six patients with multifocal serpigenoid choroiditis in addition to anti-tuberculosis therapy. Only one patient required additional systemic immunosuppression as appearance of newer lesions was observed following ozurdex injection. The patient also received second-line anti-tuberculosis therapy. None of the patients developed recurrence of inflammation and one eye required implant removal because of raised IOP. In a larger series of 19 eyes of 17 patients with tubercular uveitis, Agarwal et al. 69 reported successful management of choroiditis lesions with dexamethasone implant in six patients with multifocal serpiginoid choroiditis. Two eyes with paradoxical worsening showed improvement with dexamethasone implant as adjunct to oral corticosteroid. Various authors have reported the role of local immunosuppression in the management of paradoxical worsening. Julian et al. 57 have reported the efficacy of a single intravitreal injection of methotrexate (400 mg/0.1 mL) in three eyes of two patients of presumed tuberculous serpiginous-like choroiditis, where the lesions were progressing and threatening macula despite the use of antitubercular therapy. Resolution of lesions without any significant side effects was reported in these patients. The authors attributed the local immunosuppressive action of intravitreal methotrexate in controlling progressive lesions of serpiginous-like choroiditis which they thought may be because of active disease or paradoxical immune reaction to bacterial lysis 57.
Follow up
Patients must be followed carefully. A prolonged course of immunosuppression is given with a very slow taper to avoid recurrence. Amsler grid is very useful to monitor the course and activity of serpiginous choroiditis, because the scotomas correspond with the choroidal lesions. Serial photos with fluorescein angiography/indocyanine green angiography are also helpful in monitoring disease activity.
Serpiginous choroiditis prognosis
Serpiginous choroiditis is a chronic, bilateral, asymmetric, and progressive disease. Acute lesions last weeks to months then resolve with scarring and atrophy. Recurrence is the rule. The new lesions spread centrifugally contiguous to previous scars. Untreated, the prognosis is generally poor, though extent of visual loss is often unpredictable.
- Serpiginous Choroiditis. https://www.columbiaeye.org/education/digital-reference-of-ophthalmology/vitreous-retina/infectious-inflammatory-disorders/serpiginous-choroiditis%C2%A0%C2%A0[↩][↩]
- Jumper JM, McDonald R, Johnson RN, et al. Serpiginous choroiditis. In: Ryan SJ, editor. Retina. 4. 2 chapter 105. St Louis: Elsevier Mosby; 2006. pp. 1811–20.[↩]
- Abrez H, Biswas J, Sudharshan S. Clinical profile, treatment, and visual outcome of serpiginous choroiditis. Ocul Immunol Inflamm. 2007;15(4):325–35.[↩]
- Serpiginous Choroiditis. https://uveitis.org/wp-content/uploads/2017/05/serpiginous_chroiditis.pdf[↩]
- Serpiginous Choroiditis. https://webeye.ophth.uiowa.edu/eyeforum/cases/32-SerpiginousChoroiditis.htm[↩]
- White Dot Syndromes. https://emedicine.medscape.com/article/1227778-overview[↩][↩]
- Dutta Majumder P, Biswas J, Gupta A. Enigma of serpiginous choroiditis. Indian J Ophthalmol. 2019;67(3):325-333. doi:10.4103/ijo.IJO_822_18 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6407399[↩][↩]
- Rahman Z, Ahsan Z, Rahman NA, Dutta Majumder P. Pattern of uveitis in a referral hospital in Bangladesh. Ocul Immunol Inflamm. 2018;26:893–6.[↩][↩]
- Das D, Bhattacharjee H, Bhattacharyya PK, Jain L, Panicker MJ, Das K, et al. Pattern of uveitis in North East India: A tertiary eye care center study. Indian J Ophthalmol. 2009;57:144–6.[↩][↩]
- Grajewski RS, Caramoy A, Frank KF, Rubbert-Roth A, Fätkenheuer G, Kirchhof B, et al. Spectrum of uveitis in A German tertiary center: Review of 474 consecutive patients. Ocul Immunol Inflamm. 2015;23:346–52.[↩]
- Nazari Khanamiri H, Rao NA. Serpiginous choroiditis and infectious multifocal serpiginoid choroiditis. Surv Ophthalmol. 2013;58(3):203-232. doi:10.1016/j.survophthal.2012.08.008 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3631461[↩][↩]
- Nazari Khanamiri H, Rao NA. Serpiginous choroiditis and infectious multifocal serpiginoid choroiditis. Surv Ophthalmol. 2013;58:203–32.[↩]
- Mansour AM, Jampol LM, Packo KH, Hrisomalos NF. Macular serpiginous choroiditis. Retina. 1988;8:125–31.[↩]
- Golchet PR, Jampol LM, Wilson D, Yannuzzi LA, Ober M, Stroh E, et al. Persistent placoid maculopathy: A new clinical entity. Trans Am Ophthalmol Soc. 2006;104:108–20.[↩]
- Nika M, Kalyani PS, Jayasundera KT, Comer GM. Pathogenesis of persistent placoid maculopathy: A multimodal imaging analysis. Retina. 2015;35:1531–9.[↩]
- Lenassi E, Kojovic M, Jaki Mekjavić P, Sega S, Vidovič Valentinčič N. Persistent placoid maculopathy complicated by cerebral vasculitis. J Neuroophthalmol. 2017;37:273–5.[↩][↩]
- Jones BE, Jampol LM, Yannuzzi LA, Tittl M, Johnson MW, Han DP, et al. Relentless placoid chorioretinitis: A new entity or an unusual variant of serpiginous chorioretinitis? Arch Ophthalmol. 2000;118:931–8.[↩][↩]
- Jyotirmay B, Jafferji SS, Sudharshan S, Kalpana B. Clinical profile, treatment, and visual outcome of ampiginous choroiditis. Ocul Immunol Inflamm. 2010;18:46–51.[↩]
- Lim WK, Buggage RR, Nussenblatt RB. Serpiginous choroiditis. Surv Ophthalmol. 2005;50:231–44.[↩]
- Gupta V, Agarwal A, Gupta A, Bambery P, Narang S. Clinical characteristics of serpiginous choroidopathy in North India. Am J Ophthalmol. 2002;134:47–56.[↩][↩][↩]
- Bhuibhar SS, Biswas J. Nested PCR-positive tubercular ampiginous choroiditis: A case report. Ocul Immunol Inflamm. 2012;20:303–5.[↩]
- Khalifa YM, Monahan PM, Acharya NR. Ampiginous choroiditis following quadrivalent human papilloma virus vaccine. Br J Ophthalmol. 2010;94:137–9.[↩]
- Vasconcelos-Santos DV, Rao PK, Davies JB, Sohn EH, Rao NA. Clinical features of tuberculous serpiginouslike choroiditis in contrast to classic serpiginous choroiditis. Arch Ophthalmol. 2010;128:853–8.[↩]
- Gupta A, Bansal R, Gupta V, Sharma A, Bambery P. Ocular signs predictive of tubercular uveitis. Am J Ophthalmol. 2010;149:562–70.[↩]
- Lim WK, Buggage RR, Nussenblatt RB. Serpiginous choroiditis. Surv Ophthalmol. 2005;50(3):231–44.[↩]
- Sahin OG. Long-term cyclophosphamide treatment in a case with serpiginous choroiditis. Case Report Ophthalmol. 2010;1(2):71–6.[↩]
- King DG, Grizzard WS, Sever RJ, Espinosa L: Serpiginous Choroidopathy Associated with Elevated Factor VIII-von Willebrand Factor Antigen. Retina 1990; 10:97[↩]
- Erkkila H, Laatikainen L, Jokinen E: Immunological Studies on Serpiginous Choroiditis. Graefe’s Arch Clin Exp Ophthalmol. 1982; 219:131-134[↩]
- Broekhuyse RM, Van Herck M, Pinckers AJLG, et al: Immune Responsiveness to Retinal S-Antigen and Opsin in Serpiginous Choroiditis and Other Retinal Diseases. Doc Ophthalmol . 1988; 69:83-93[↩]
- Broekhuyse RM, van Herck M, Pinckers AJ, Winkens HJ, van Vugt AH, Ryckaert S, et al. Immune responsiveness to retinal S-antigen and opsin in serpiginous choroiditis and other retinal diseases. Doc Ophthalmol. 1988;69:83–93.[↩][↩]
- Laatikainen L, Erkkilä H. A follow-up study on serpiginous choroiditis. Acta Ophthalmol (Copenh) 1981;59:707–18.[↩]
- Hayreh SS, Baines JA. Occlusion of the posterior ciliary artery. II. Chorio-retinal lesions. Br J Ophthalmol. 1972;56:736–53.[↩]
- Erkkilä H, Laatikainen L, Jokinen E. Immunological studies on serpiginous choroiditis. Graefes Arch Clin Exp Ophthalmol. 1982;219:131–4.[↩]
- King DG, Grizzard WS, Sever RJ, Espinoza L. Serpiginous choroidopathy associated with elevated factor VIII-von Willebrand factor antigen. Retina. 1990;10:97–101.[↩]
- Jordano Pérez JJ, Córdoba Lorenzo M, Ruiz Lomas C, Márquez Báez FJ, Ortega Ortiz A. Serpiginous choroiditis in a patient with uterine cervix carcinoma. Arch Soc Esp Oftalmol. 2012;87:86–9.[↩]
- Hutchinson J. Serpiginous choroiditis in scrofulous subjects: choroidal lupus. Arch Surg (Lond) 1900;11:126–35.[↩]
- Maumenee AE. Clinical entities in “uveitis”. An approach to the study of intraocular inflammation. Am J Ophthalmol. 1970;69:1–27.[↩]
- Rao NA, Saraswathy S, Smith RE. Tuberculous uveitis: Distribution of mycobacterium tuberculosis in the retinal pigment epithelium. Arch Ophthalmol. 2006;124:1777–9.[↩]
- Bansal R, Gupta A, Gupta V, Dogra MR, Sharma A, Bambery P, et al. Tubercular serpiginous-like choroiditis presenting as multifocal serpiginoid choroiditis. Ophthalmology. 2012;119:2334–42.[↩][↩]
- Tagirasa R, Parmar S, Barik MR, Devadas S, Basu S. Autoreactive T cells in immunopathogenesis of TB-associated uveitis. Invest Ophthalmol Vis Sci. 2017;58:5682–91.[↩]
- Gass J. St. Louis: C.V. Mosby Co; 1997. Stereoscopic Atlas of Macular Diseases: A Funduscopic and Angiographic Presentation; pp. 158–65.[↩]
- Priya K, Madhavan HN, Reiser BJ, Biswas J, Saptagirish R, Narayana KM, et al. Association of herpesviruses in the aqueous humor of patients with serpiginous choroiditis: A polymerase chain reaction-based study. Ocul Immunol Inflamm. 2002;10:253–61.[↩]
- Christmas NJ, Oh KT, Oh DM, Folk JC. Long-term follow-up of patients with serpinginous choroiditis. Retina. 2002;22:550–6.[↩][↩]
- Mahendradas P, Kamath G, Mahalakshmi B, Shetty KB. Serpiginous choroiditis-like picture due to ocular toxoplasmosis. Ocul Immunol Inflamm. 2007;15:127–30.[↩]
- Pisa D, Ramos M, García P, Escoto R, Barraquer R, Molina S, et al. Fungal infection in patients with serpiginous choroiditis or acute zonal occult outer retinopathy. J Clin Microbiol. 2008;46:130–5.[↩][↩]
- Baarsma GS, Deutman AF. Serpiginous (geographic) choroiditis. Doc Ophthalmol Adv Ophthalmol. 1976;40:269–85.[↩]
- Kuo IC, Cunningham ET., Jr Ocular neovascularization in patients with uveitis. Int Ophthalmol Clin. 2000;40:111–26.[↩]
- Bansal R, Bansal P, Gupta A, Gupta V, Dogra MR, Singh R, et al. Diagnostic challenges in inflammatory choroidal neovascular membranes. Ocul Immunol Inflamm. 2017;25:554–62.[↩][↩]
- Gupta V, Gupta A, Rao NA. Intraocular tuberculosis – An update. Surv Ophthalmol. 2007;52:561–87.[↩]
- Hooper PL, Kaplan HJ: Triple Agent Immunosuppression in Geographic Helicoid Choroidopathy. Ophthalmology. 1991; 98:944-952[↩]
- Venkatesh P, Gogia V, Gupta S, Tayade A, Shilpy N, Shah BM, et al. Pulse cyclophosphamide therapy in the management of patients with macular serpiginous choroidopathy. Indian J Ophthalmol. 2015;63:318–22.[↩]
- Akpek EK, Baltatzis S, Yang J, Foster CS. Long-term immunosuppressive treatment of serpiginous choroiditis. Ocul Immunol Inflamm. 2001;9:153–67.[↩]
- Venkatesh P, Tayade A, Gogia V, Gupta S, Shah BM, Vohra R. Short-term intensive immunosuppression: A randomized, three-arm study of intravenous pulse methylprednisolone and cyclophosphamide in macular serpiginous choroiditis. Ocul Immunol Inflamm. 2018;26:469–76.[↩]
- Seth RK, Gaudio PA. Treatment of serpiginous choroiditis with intravitreous fluocinolone acetonide implant. Ocul Immunol Inflamm. 2008;16:103–5.[↩][↩]
- Miserocchi E, Berchicci L, Iuliano L, Modorati G, Bandello F. Dexamethasone intravitreal implant in serpiginous choroiditis. Br J Ophthalmol. 2017;101:327–32.[↩]
- Saatci AO, Ayhan Z, Engin Durmaz C, Takes O. Simultaneous single dexamethasone implant and ranibizumab injection in a case with active serpiginous choroiditis and choroidal neovascular membrane. Case Rep Ophthalmol. 2015;6:408–14.[↩]
- Julian K, Langner-Wegscheider BJ, Haas A, De Smet MD. Intravitreal methotrexate in the management of presumed tuberculous serpiginous-like choroiditis. Retina. 2013;33:1943–8.[↩][↩][↩]
- Chinchurreta Capote A, Requena Jiménez JM, Lorenzo Soto M, Romero Gómez C, García de Lucas MD. Effectiveness of adalimumab for refractory serpiginous choroiditis. Ocul Immunol Inflamm. 2014;22:405–8.[↩][↩]
- Seve P, Mennesson E, Grange JD, Broussolle C, Kodjikian L. Infliximab in serpiginous choroiditis. Acta Ophthalmol. 2010;88:e342–3.[↩]
- Llorenç V, Molins B, Rey A, Mesquida M, Adán A. Adalimumab in serpiginous choroiditis. Ocul Immunol Inflamm. 2013;21:237–40.[↩]
- Cordero-Coma M, Benito MF, Hernández AM, Antolín SC, Ruíz JM. Serpiginous choroiditis. Ophthalmology. 2008;115:1633. 1633.e1-2.[↩][↩]
- Oray M, Zakiev Z, Çaǧatay T, Tuǧal-Tutkun İ. Treatment results in serpiginous choroiditis and multifocal serpiginoid choroiditis associated with latent tuberculosis. Turk J Ophthalmol. 2017;47:89–93.[↩]
- Bansal R, Sharma K, Gupta A, Sharma A, Singh MP, Gupta V, et al. Detection of Mycobacterium tuberculosis genome in vitreous fluid of eyes with multifocal serpiginoid choroiditis. Ophthalmology. 2015;122:840–50.[↩]
- Gupta V, Bansal R, Gupta A. Continuous progression of tubercular serpiginous-like choroiditis after initiating antituberculosis treatment. Am J Ophthalmol. 2011;152:857–63.[↩][↩]
- Ganesh SK, Ali BS. Paradoxical worsening of a case of TB subretinal abscess with serpiginous-like choroiditis following the initiation of antitubercular therapy. Indian J Ophthalmol. 2017;65:761–4.[↩]
- Cheung CM, Chee SP. Jarisch-herxheimer reaction: Paradoxical worsening of tuberculosis chorioretinitis following initiation of antituberculous therapy. Eye (Lond) 2009;23:1472–3.[↩]
- Aggarwal K, Agarwal A, Deokar A, Singh R, Bansal R, Sharma A, et al. Ultra-wide field imaging in paradoxical worsening of tubercular multifocal serpiginoid choroiditis after the initiation of anti-tubercular therapy. Ocul Immunol Inflamm. 2017:1–6. doi: 10.1080/09273948.2017.1373829. [Epub: ahead of print].[↩]
- Jain L, Panda KG, Basu S. Clinical outcomes of adjunctive sustained-release intravitreal dexamethasone implants in tuberculosis-associated multifocal serpigenoid choroiditis. Ocul Immunol Inflamm. 2018;26:877–83.[↩][↩]
- Agarwal A, Handa S, Aggarwal K, Sharma M, Singh R, Sharma A, et al. The role of dexamethasone implant in the management of tubercular uveitis. Ocul Immunol Inflamm. 2018;26:884–92.[↩]