Sezary syndrome
Sezary syndrome also called Sézary syndrome, an aggressive form of a type of blood cancer called cutaneous T-cell lymphoma (CTCL), a term that collectively includes all cutaneous lymphomas arising from T lymphocytes. Sezary syndrome is named after Albert Sézary, a French dermatologist born in 1880 1. Cutaneous T-cell lymphomas occur when certain white blood cells, called T cells, become cancerous; these cancers characteristically affect the skin, causing different types of skin lesions. In Sezary syndrome, the cancerous T cells, called Sezary cells, are present in the blood, skin, and lymph nodes 2. A characteristic of Sezary cells is an abnormally shaped nucleus, described as cerebriform.
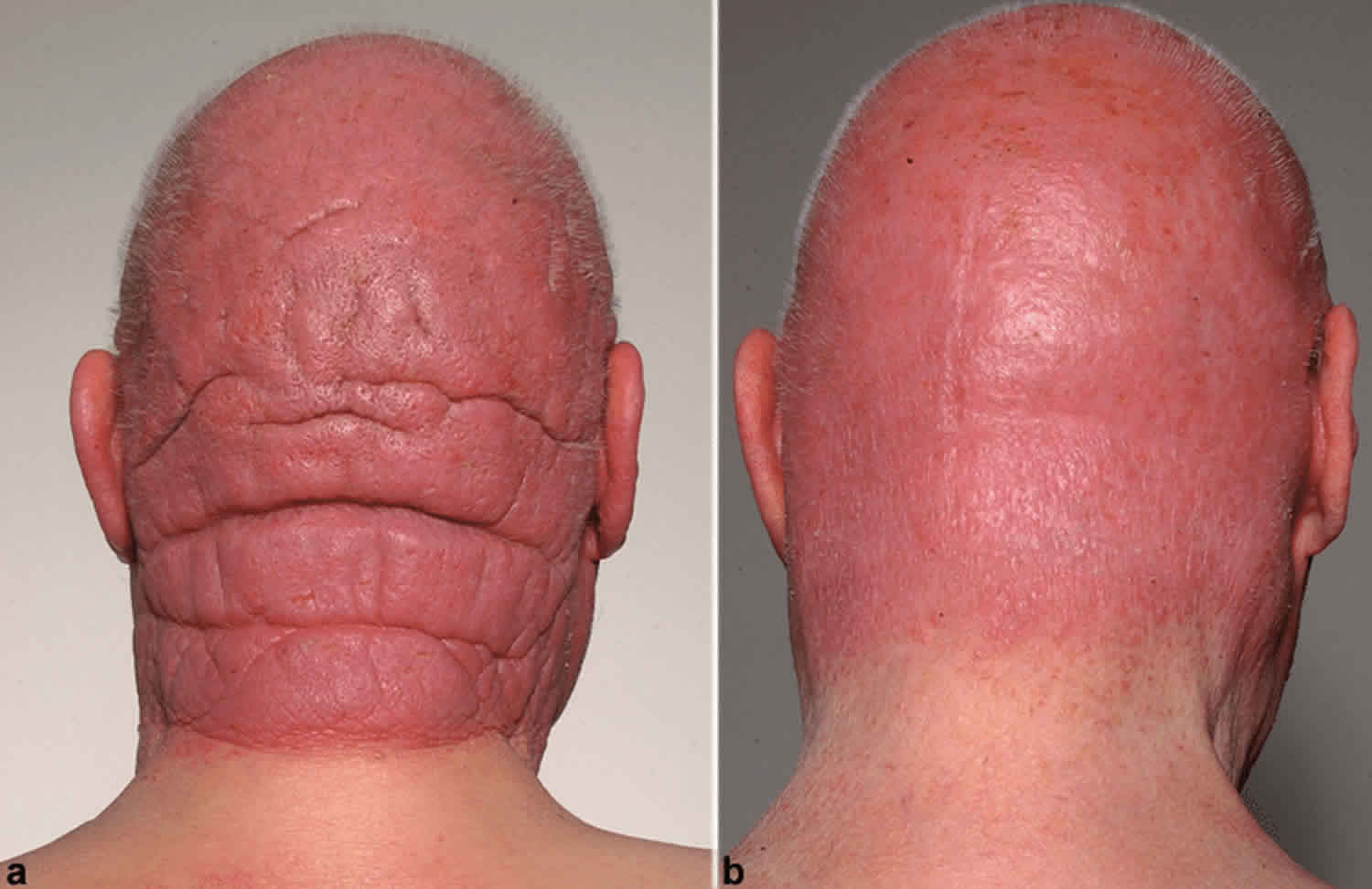
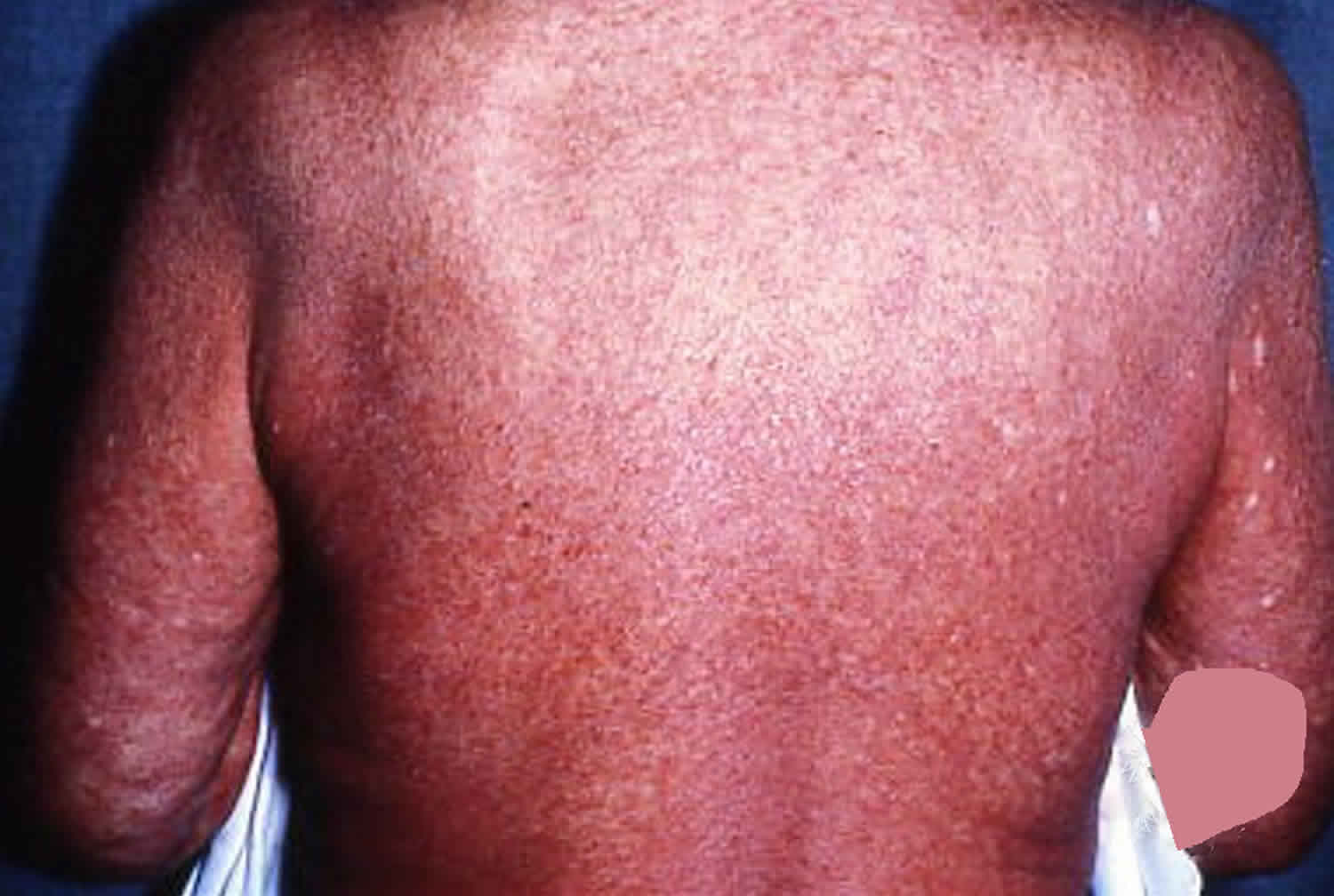
People with Sezary syndrome develop a red, severely itchy rash (erythroderma) that covers large portions of their body. Sézary cells are found in the rash. However, the skin cells themselves are not cancerous; the skin problems result when Sezary cells move from the blood into the skin. People with Sezary syndrome also have enlarged lymph nodes (lymphadenopathy). Other common signs and symptoms of this condition include hair loss (alopecia), skin swelling (edema), thickened skin on the palms of the hands and soles of the feet (palmoplantar keratoderma), abnormalities of the fingernails and toenails, and lower eyelids that turn outward (ectropion). Some people with Sezary syndrome are less able to control their body temperature than people without the condition.
The cancerous T cells can spread to other organs in the body, including the lymph nodes, liver, spleen, and bone marrow. In addition, affected individuals have an increased risk of developing another lymphoma or other type of cancer.
Sezary syndrome most often occurs in older adults over age 60 and usually progresses rapidly; historically, affected individuals survived an average of 2 to 4 years after development of the condition, although survival has improved with newer treatments.
Sezary syndrome worldwide incidence appears to be 0.8–0.9 per million with a male to female ratio of 2:1. Sezary syndrome is not an inherited disease.
Although Sezary syndrome is sometimes referred to as a variant of another cutaneous T-cell lymphoma called mycosis fungoides, these two cancers are generally considered separate conditions.
Sezary syndrome is the second most common form of cutaneous T-cell lymphoma after mycosis fungoides, accounting for approximately 3 to 5 percent of cases of cutaneous T-cell lymphoma.
Figure 1. Sezary syndrome rash
Can Sezary syndrome be cured?
Sezary syndrome is difficult to cure. Treatment is usually palliative, with the intention of relief of symptoms and improvement in the quality of life. Patients may live many years with the disease 3.
Sezary syndrome causes
Although a small percentage of cases of Sezary syndrome are associated with human T-lymphotropic viruses type 1 and type 2, the underlying cause of most cases is currently unknown 4. In most affected individuals, the cancerous T-cells (also called Sezary cells) usually have one or more chromosomal abnormalities, such as the loss or gain of genetic material 5. These abnormalities occur during a person’s lifetime and are found only in the DNA of cancerous cells. Abnormalities have been found on most chromosomes, but some regions are more commonly affected than others. People with this condition tend to have losses of DNA from regions of chromosomes 10 and 17 or additions of DNA to regions of chromosomes 8 and 17. However, it is unclear whether these alterations play a role in Sezary syndrome, although the tendency to acquire chromosomal abnormalities (chromosomal instability) is a feature of many cancers. It can lead to genetic changes that allow cells to grow and divide uncontrollably.
The malignant T cells in the skin and blood have the following characteristics:
- CD4 positive: this indicates they are central memory T cells
- A significant percentage exhibit loss of CD26 and CD7
- Abnormal clonal Th2 cells
- Reduced Th1 (T-helper) cells
This contributes to endogenous immunosuppression.
Is Sezary syndrome inherited?
The underlying cause of Sezary syndrome is unknown in most cases. However, it generally occurs sporadically in people with no family history of the condition and is not thought to be inherited in most cases 5.
Sezary syndrome symptoms
Sezary syndrome is an aggressive form of cutaneous T-cell lymphoma which is a group of disorders that occur when T-cells (a type of white blood cell) become cancerous and affect the skin. In Sezary syndrome, specifically, the cancerous T cells are called Sezary cells and are found in the skin, lymph nodes, and blood. They can also spread to other organs in the body, including the liver, spleen, and bone marrow 6.
In Sezary syndrome, the skin all over the body is reddened (erythroderma), itchy, peeling, and painful. There may also be patches, plaques, or tumors on the skin. It is not known if Sézary syndrome is an advanced form of mycosis fungoides or a separate disease.
Although Sezary syndrome can affect people of all ages, it is most commonly diagnosed in adults over age 60. The signs and symptoms of this condition can vary but may include 6:
- A red, itchy rash that covers large portions of the body
- Enlarged lymph nodes
- Alopecia (hair loss)
- Thickened skin on the palms of the hands and soles of the feet
- Abnormalities of the fingernails and toenails
- Ectropion
- Hepatosplenomegaly (enlarged liver and spleen)
Affected people may also have an increased risk of developing another lymphoma or other type of cancer.
Skin signs
Patients with Sezary syndrome present with diffusely red, thickened and scaly skin. Pruritus (itch) is common, often severe, and can be difficult to manage. Other features may include:
- Slowly-developing generalised induration (firmness of the skin)
- Lichenification (increased skin markings due to scratching and rubbing)
- Scaly papules due to follicular prominence
- Thickened nails
- Ectropion (drooping of lower eyelid)
- Diffuse or patchy alopecia (hair loss)
These symptoms often lead to lack of sleep, anxiety and depression.
Very rarely, Sezary syndrome has been reported to present with pruritus, Sezary cells on biopsy of normal-looking skin, and no visible rash.
Systemic involvement
Other features of Sezary syndrome include:
- Peripheral lymphadenopathy: often generalized, involving neck, armpits, groins
- Enlarged spleen
- Less commonly, liver, lungs and gastrointestinal tract can be affected.
The following malignancies are associated with Sezary syndrome:
- Hodgkin lymphoma or non-Hodgkin lymphoma
- Melanoma
- Bladder cancer
Sezary syndrome diagnosis
A diagnosis of Sezary syndrome is often suspected in people with characteristic signs and symptoms. Additional testing can then be ordered to confirm the diagnosis. This may include 2:
- A skin biopsy
- A complete blood count
- Peripheral blood smear
- Immunophenotyping
- T-cell receptor (TCR) gene rearrangement test
- Flow cytometry
Skin biopsy
If possible, select an area of indurated skin for biopsy. The removal of cells or tissues so they can be viewed under a microscope to check for signs of cancer. The doctor may remove a growth from the skin, which will be examined by a pathologist. Multiple biopsies are useful especially if morphology varies.
Features on light microscopy may include:
- Atypical lymphocytes infiltrating dermis
- Epidermotropism (cells migrating to epidermis)
- Lymphocytes with a single cerebriform nucleus (not diagnostic)
- Pautrier microabscesses, or intra-epidermal aggregates of atypical cells
- Immunohistochemistry stains positive for CD3+ and CD4+ cells
Other tests that may be done on the cells or tissue sample include the following:
- Immunophenotyping: A laboratory test that uses antibodies to identify cancer cells based on the types of antigens or markers on the surface of the cells. This test is used to help diagnose specific types of lymphoma.
- Flow cytometry: A laboratory test that measures the number of cells in a sample, the percentage of live cells in a sample, and certain characteristics of the cells, such as size, shape, and the presence of tumor (or other) markers on the cell surface. The cells from a sample of a patient’s blood, bone marrow, or other tissue are stained with a fluorescent dye, placed in a fluid, and then passed one at a time through a beam of light. The test results are based on how the cells that were stained with the fluorescent dye react to the beam of light. This test is used to help diagnose and manage certain types of cancers, such as leukemia and lymphoma. Flow cytometry can demonstrate CD4+CD7- and CD4+CD26- T cells, which are characteristic of Sezary syndrome. T-cell antigens may also be absent (CD2, CD3, CD4 and/or CD5). The CD4:CD8 ratio is > 10, but is not diagnostic.
- T-cell receptor (TCR) gene rearrangement test: A laboratory test in which cells in a sample of blood or bone marrow are checked to see if there are certain changes in the genes that make receptors on T cells (white blood cells). Testing for these gene changes can tell whether large numbers of T cells with a certain T-cell receptor are being made.
Blood tests
Sezary cells are large atypical mononuclear cells with a large cerebriform nuclei. They are found in large numbers in the peripheral blood of patients with Sezary syndrome. Smaller numbers may be found in healthy patients or those with other diseases.
- Sezary blood cell count: A procedure in which a sample of blood is viewed under a microscope to count the number of Sezary cells.
- HIV test: A test to measure the level of HIV antibodies in a sample of blood. Antibodies are made by the body when it is invaded by a foreign substance. A high level of HIV antibodies may mean the body has been infected with HIV.
Lymph node biopsy
Excisional biopsies are required to distinguish between dermatopathic lymph node changes and reactive lymph node changes.
Sezary syndrome treatment
Treatment for Sezary syndrome is determined by the stage of disease and comorbidities.
In general, there are six different treatment options available to people with Sezary syndrome. These include 7:
- Photodynamic therapy
- Radiation therapy
- Chemotherapy
- Other drug therapy (i.e. topical steriods, retinoids)
- Biologic therapy (immunotherapy)
- Targeted therapy
- Transplantation
There is no standard therapy for Sezary syndrome. Patients are prescribed topical and systemic medications. They may also receive UVB or PUVA phototherapy, and/or radiotherapy (localized superficial rays or total skin electron beam therapy).
Topical therapy for Sezary syndrome
Examples of topical therapy used to treat Sezary syndrome include:
- Emollients
- Topical corticosteroids
- Topical nitrogen mustard
- Potassium permanganate or dilute bleach baths to prevent infection
Photodynamic therapy
- Psoralen and ultraviolet A radiation (PUVA). In psoralen and ultraviolet A (PUVA) therapy, the patient receives a drug called psoralen and then ultraviolet A radiation is directed to the skin. Therapeutic trials with PUVA have shown an 80% to 90% complete remission rate with early cutaneous stages achieving the best responses. PUVA may be used in conjunction with systemic treatment 8. Continued maintenance therapy with PUVA at more protracted intervals is generally required to prolong remission duration 8. PUVA combined with interferon alpha-2a is associated with a high response rate 8.
- Narrowband ultraviolet B radiation. Single-arm and retrospective comparisons confirm narrowband ultraviolet B with 80% to 90% complete remission rates, especially for patients with early cutaneous stages 9.
- Extracorporeal photochemotherapy alone 10 or in combination with total-skin electron-beam radiation 11. Extracorporeal photopheresis is an immune-sparing therapy, the patient is given photosensitizing agent (psoralen/8-methoxsalen) and then some blood cells are taken from the body, put under a special ultraviolet A light, and put back into the body. Extracorporeal photochemotherapy may be used alone or combined with total skin electron beam radiation therapy. It is the preferred first line therapy in early stages. Response rates are improved when combined with biological response modifiers. Side effects include headache, fatigue, itch and transient hypotension (low blood pressure) with possible syncope (collapse).
Radiation therapy
Radiation therapy is a cancer treatment that uses high-energy x-rays or other types of radiation to kill cancer cells or keep them from growing. External radiation therapy uses a machine outside the body to send radiation toward the area of the body with cancer. Sometimes, total skin electron beam (TSEB) radiation therapy is used to treat mycosis fungoides and Sézary syndrome. This is a type of external radiation treatment in which a radiation therapy machine aims electrons (tiny, invisible particles) at the skin covering the whole body. External radiation therapy may also be used as palliative therapy to relieve symptoms and improve quality of life.
Ultraviolet A (UVA) radiation therapy or ultraviolet B (UVB) radiation therapy may be given using a special lamp or laser that directs radiation at the skin.
- Total skin electron beam. Electron-beam radiation of appropriate energies will penetrate only to the dermis, and thus the skin alone can be treated without systemic effects. This therapy requires a radiation therapy facility with physics support and considerable technical expertise to deliver precise dosimetry. Total skin electron beam can result in short- and long-term cutaneous toxic effects and is not widely available. This therapy can provide excellent palliation, with complete response rates of as much as 80%, and may be combined with systemic treatment. Based on the long-term survival of these early-stage patients, electron-beam radiation therapy is sometimes used with curative intent 12. Long-term disease-free survival can be achieved in patients with unilesional mycosis fungoides treated with local radiation therapy 13.
- Local electron-beam radiation or orthovoltage radiation therapy may be used to palliate areas of bulky or symptomatic skin disease 14.
Biologic therapy
Biologic therapy also called immunotherapy is a treatment that uses the patient’s immune system to fight cancer. Substances made by the body or made in a laboratory are used to boost, direct, or restore the body’s natural defenses against cancer. This type of cancer treatment is also called biotherapy or biologic therapy.
- Interferon: This treatment interferes with the division of mycosis fungoides and Sézary cells and can slow tumor growth. Interferon alpha or interferon gamma alone or in combination with topical therapy 15. A retrospective review of 198 patients with mycosis fungoides and Sezary syndrome compared time to next treatment between interferon alpha and conventional chemotherapy. Interferon alpha provided a longer time to next treatment of 8.7 months than did chemotherapy, with a time to next treatment of 3.9 months 16.
Chemotherapy
Chemotherapy is a cancer treatment that uses drugs to stop the growth of cancer cells, either by killing the cells or by stopping them from dividing. When chemotherapy is taken by mouth or injected into a vein or muscle, the drugs enter the bloodstream and can reach cancer cells throughout the body (systemic chemotherapy). Sometimes the chemotherapy is topical (put on the skin in a cream, lotion, or ointment).
Chemotherapeutic agents generally demonstrate short durations of response. In a retrospective review of 198 patients with advanced-stage disease, the median time before patients required new therapy was 4 months 16. However, these comparisons may be confounded by the order in which the agents were introduced.
- Topical chemotherapy with mechlorethamine (nitrogen mustard). This form of treatment may be used palliatively or to supplement therapeutic approaches directed against nodal or visceral disease. Topical application of mechlorethamine has produced regression of cutaneous lesions, with particular efficacy in early stages of disease. The overall complete remission rate is related to skin stage; 50% to 80% of TNM classification T1 patients, 25% to 75% of T2 patients, as many as 50% of T3 patients, and 20% to 40% of T4 patients have complete responses. The overall complete remission rate in 243 patients was 64% and was related to stage; as many as 35% of stage IV patients had complete responses. Treatments are usually continued for 2 to 3 years. Continuous 5-year disease free survival may be possible in as many as 33% of T1 patients 17.
- Oral methotrexate (NCT00425555) 18.
- Pegylated liposomal doxorubicin 19.
- Fludarabine, 2-chlorodeoxyadenosine, and pentostatin are active agents for mycosis fungoides and Sezary syndrome 20.
- Single-agent chemotherapy or combination systemic chemotherapy (chlorambucil plus prednisone, mechlorethamine, cyclophosphamide, methotrexate, and combination chemotherapy) are often combined with treatment directed at the skin 16.
- Pralatrexate (folate analog) 21.
Targeted therapy
Targeted therapy is a type of treatment that uses drugs or other substances to attack cancer cells. Targeted therapies usually cause less harm to normal cells than chemotherapy or radiation therapy do.
Monoclonal antibody therapy: This treatment uses antibodies made in the laboratory from a single type of immune system cell. These antibodies can identify substances on cancer cells or normal substances that may help cancer cells grow. The antibodies attach to the substances and kill the cancer cells, block their growth, or keep them from spreading. They may be used alone or to carry drugs, toxins, or radioactive material directly to cancer cells. Monoclonal antibodies are given by infusion.
- Types of monoclonal antibodies include:
- Brentuximab vedotin 22, which contains a monoclonal antibody that binds to a protein, called CD30, found on some types of lymphoma cells. It also contains an anticancer drug that may help kill cancer cells. Two phase II trials of 58 patients with variable CD30 expression showed a 50% to 70% response rate with 50% of patients still in remission after 1 year 23.
- Mogamulizumab 24, which contains a monoclonal antibody that binds to a protein, called CCR4, found on some types of lymphoma cells. It may block this protein and help the immune system kill cancer cells. It is used to treat mycosis fungoides and Sezary syndrome that came back or did not get better after treatment with at least one systemic therapy. In a prospective randomized trial, 372 previously treated patients received either mogamulizumab, a monoclonal antibody directed against C-C chemokine receptor 4, or vorinostat, the HDACi. With a median follow-up of 17 months, the median progression-free survival favored mogamulizumab at 7.7 months versus 3.1 months for vorinostat 24. In a preliminary study such as this, no overall survival (OS) was seen.
Transplantation
Allogeneic or autologous bone marrow transplantation 25. Among these highly selected patients, the 5-year overall survival rate ranges from 30% to 50%, with a relapse-free survival rate of 15% to 25% 26.
These types of treatments produce remissions, but long-term remissions are uncommon. Treatment, therefore, is considered palliative for most patients, although major symptomatic improvement is regularly achieved. Survival in excess of 8 years, however, is common for patients with early stages of disease. All patients with mycosis fungoides and Sezary syndrome are candidates for clinical trials evaluating new approaches to treatment.
Other drug therapy
- Topical corticosteroids are used to relieve red, swollen, and inflamed skin. They are a type of steroid. Topical corticosteroids may be in a cream, lotion, or ointment.
- Retinoids, such as bexarotene 27, are drugs related to vitamin A that can slow the growth of certain types of cancer cells. The retinoids may be taken by mouth or put on the skin.
- Lenalidomide is a drug that helps the immune system kill abnormal blood cells or cancer cells and may prevent the growth of new blood vessels that tumors need to grow 28.
- Vorinostat and romidepsin are two of the histone deacetylase (HDAC) inhibitors used to treat mycosis fungoides and Sezary syndrome 29. HDAC inhibitors cause a chemical change that stops tumor cells from dividing. A retrospective review of 198 patients with mycosis fungoides and Sézary syndrome compared time to next treatment between HDACi and conventional chemotherapy. HDACi provided a longer time to next treatment of 4.5 months than did chemotherapy, with a time to next treatment of 3.9 months 16.
Sezary syndrome prognosis
The long-term outlook (prognosis) for people with Sezary syndrome is generally poor. Sezary syndrome is difficult to cure. Treatment is usually palliative, with the intention of relief of symptoms and improvement in the quality of life. Median survival for patients with Sezary syndrome has been reported to be 2 to 4 years after development of the condition, although survival has improved with newer treatments. The disease-specific 5-year survival rate has been reported to be 24% 3. Patients usually succumb to opportunistic infections due to immune suppression.
The prognosis and treatment options depend on the following:
- The stage of the cancer.
- The type of lesion (patches, plaques, or tumors).
- The patient’s age and gender.
- Sézary syndrome. https://dermnetnz.org/topics/sezary-syndrome[↩]
- Mycosis Fungoides (Including Sézary Syndrome) Treatment (PDQ®)–Patient Version. https://www.cancer.gov/types/lymphoma/patient/mycosis-fungoides-treatment-pdq[↩][↩]
- Mycosis Fungoides (Including Sézary Syndrome) Treatment (PDQ®)–Health Professional Version. https://www.cancer.gov/types/lymphoma/hp/mycosis-fungoides-treatment-pdq[↩][↩]
- Elise A Olsen, MD; Alain H Rook, MD. UpToDate. Clinical presentation, pathologic features, and diagnosis of Sézary syndrome. May 2013[↩]
- Sézary syndrome. https://ghr.nlm.nih.gov/condition/sezary-syndrome[↩][↩]
- Cutaneous T-Cell Lymphoma. https://emedicine.medscape.com/article/2139720-overview[↩][↩]
- Mycosis Fungoides (Including Sézary Syndrome) Treatment (PDQ®)–Health Professional Version. https://www.cancer.gov/types/lymphoma/hp/mycosis-fungoides-treatment-pdq#_50[↩]
- Olsen EA, Hodak E, Anderson T, et al.: Guidelines for phototherapy of mycosis fungoides and Sézary syndrome: A consensus statement of the United States Cutaneous Lymphoma Consortium. J Am Acad Dermatol 74 (1): 27-58, 2016.[↩][↩][↩]
- Almohideb M, Walsh S, Walsh S, et al.: Bath Psoralen-ultraviolet A and Narrowband Ultraviolet B Phototherapy as Initial Therapy for Early-stage Mycosis Fungoides: A Retrospective Cohort of 267 Cases at the University of Toronto. Clin Lymphoma Myeloma Leuk 17 (9): 604-612, 2017.[↩]
- Scarisbrick JJ, Taylor P, Holtick U, et al.: U.K. consensus statement on the use of extracorporeal photopheresis for treatment of cutaneous T-cell lymphoma and chronic graft-versus-host disease. Br J Dermatol 158 (4): 659-78, 2008.[↩]
- Palareti G, Maccaferri M, Manotti C, et al.: Fibrinogen assays: a collaborative study of six different methods. C.I.S.M.E.L. Comitato Italiano per la Standardizzazione dei Metodi in Ematologia e Laboratorio. Clin Chem 37 (5): 714-9, 1991.[↩]
- Navi D, Riaz N, Levin YS, et al.: The Stanford University experience with conventional-dose, total skin electron-beam therapy in the treatment of generalized patch or plaque (T2) and tumor (T3) mycosis fungoides. Arch Dermatol 147 (5): 561-7, 2011.[↩]
- Micaily B, Miyamoto C, Kantor G, et al.: Radiotherapy for unilesional mycosis fungoides. Int J Radiat Oncol Biol Phys 42 (2): 361-4, 1998.[↩]
- Thomas TO, Agrawal P, Guitart J, et al.: Outcome of patients treated with a single-fraction dose of palliative radiation for cutaneous T-cell lymphoma. Int J Radiat Oncol Biol Phys 85 (3): 747-53, 2013.[↩]
- Olsen EA, Bunn PA: Interferon in the treatment of cutaneous T-cell lymphoma. Hematol Oncol Clin North Am 9 (5): 1089-107, 1995.[↩]
- Hughes CF, Khot A, McCormack C, et al.: Lack of durable disease control with chemotherapy for mycosis fungoides and Sézary syndrome: a comparative study of systemic therapy. Blood 125 (1): 71-81, 2015.[↩][↩][↩][↩]
- Lessin SR, Duvic M, Guitart J, et al.: Topical chemotherapy in cutaneous T-cell lymphoma: positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol 149 (1): 25-32, 2013.[↩]
- Zackheim HS, Kashani-Sabet M, McMillan A: Low-dose methotrexate to treat mycosis fungoides: a retrospective study in 69 patients. J Am Acad Dermatol 49 (5): 873-8, 2003.[↩]
- Dummer R, Quaglino P, Becker JC, et al.: Prospective international multicenter phase II trial of intravenous pegylated liposomal doxorubicin monochemotherapy in patients with stage IIB, IVA, or IVB advanced mycosis fungoides: final results from EORTC 21012. J Clin Oncol 30 (33): 4091-7, 2012.[↩]
- Kurzrock R, Pilat S, Duvic M: Pentostatin therapy of T-cell lymphomas with cutaneous manifestations. J Clin Oncol 17 (10): 3117-21, 1999.[↩]
- Talpur R, Thompson A, Gangar P, et al.: Pralatrexate alone or in combination with bexarotene: long-term tolerability in relapsed/refractory mycosis fungoides. Clin Lymphoma Myeloma Leuk 14 (4): 297-304, 2014.[↩]
- Kim YH, Tavallaee M, Sundram U, et al.: Phase II Investigator-Initiated Study of Brentuximab Vedotin in Mycosis Fungoides and Sézary Syndrome With Variable CD30 Expression Level: A Multi-Institution Collaborative Project. J Clin Oncol 33 (32): 3750-8, 2015.[↩]
- Duvic M, Tetzlaff MT, Gangar P, et al.: Results of a Phase II Trial of Brentuximab Vedotin for CD30+ Cutaneous T-Cell Lymphoma and Lymphomatoid Papulosis. J Clin Oncol 33 (32): 3759-65, 2015.[↩]
- Kim YH, Bagot M, Pinter-Brown L, et al.: Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol 19 (9): 1192-1204, 2018.[↩][↩]
- Duarte RF, Boumendil A, Onida F, et al.: Long-term outcome of allogeneic hematopoietic cell transplantation for patients with mycosis fungoides and Sézary syndrome: a European society for blood and marrow transplantation lymphoma working party extended analysis. J Clin Oncol 32 (29): 3347-8, 2014.[↩]
- Lechowicz MJ, Lazarus HM, Carreras J, et al.: Allogeneic hematopoietic cell transplantation for mycosis fungoides and Sezary syndrome. Bone Marrow Transplant 49 (11): 1360-5, 2014.[↩]
- Heald P, Mehlmauer M, Martin AG, et al.: Topical bexarotene therapy for patients with refractory or persistent early-stage cutaneous T-cell lymphoma: results of the phase III clinical trial. J Am Acad Dermatol 49 (5): 801-15, 2003.[↩]
- Querfeld C, Rosen ST, Guitart J, et al.: Results of an open-label multicenter phase 2 trial of lenalidomide monotherapy in refractory mycosis fungoides and Sézary syndrome. Blood 123 (8): 1159-66, 2014.[↩]
- Duvic M, Dummer R, Becker JC, et al.: Panobinostat activity in both bexarotene-exposed and -naïve patients with refractory cutaneous T-cell lymphoma: results of a phase II trial. Eur J Cancer 49 (2): 386-94, 2013.[↩]