What is Uva Ursi
Uva ursi (Arctostaphylos uva-ursi L. Sprengel) also known as bearberry (named for the grape-like clusters of orange berries that are commonly eaten by bears), is an herbal extract derived from the fresh or dried leaves of the plant Arctostaphylos uva-ursi, a small evergreen shrub, which has been used in Native American traditional medicine since the 2nd century for treatment of urinary tract symptoms and as a diuretic. Both Arctostaphylos and uva-ursi mean “grape of the bears”, the former in Greek and the latter in Latin. Native Americans used uva ursi as a remedy for urinary tract infections (UTIs). In fact, until the discovery of sulfa drugs and antibiotics, uva ursi was a common treatment for bladder-related infections. The leaf extract of uva-ursi (Arctostaphylos or bearberry) has been approved for use for urinary tract inflammation by the German Federal Institute for Drugs and Medical Devices and is available on prescription in Germany for this indication 1. Uva ursi is reported to have diuretic, urinary antiseptic, astringent and anti-inflammatory properties 1. The Arctostaphylos uva ursi leaves extract phytochemicals include flavonoids (quercetin), iridoids, hydroquinone glycosides (mainly “arbutin”), tannins, allantoin, gallic and ellagic acids, ursolic acid, volatile oils, resins (urvone) and terpenoids. Test tube studies of uva ursi have demonstrated antibacterial activity against a variety of organisms including Escherichia coli, the most prevalent urinary pathogen 2. The active component of uva ursi is suspected to be the hydroquinone derivatives, especially arbutin and methyl arbutin which may have antiinflammatory and antiseptic activities that are excreted in the urine 3. Uva ursi has been used to treat dysuria, cystitis, urethritis, and kidney and bladder stones. It has also been recommended for inducing diuresis and to treat constipation. But uva ursi can be toxic. Hydroquinone, a component of uva ursi, can cause serious liver damage. Conventional medications that have fewer risks are available to treat urinary tract infections. While uva ursi has been used extensively in traditional medicine, there is no convincing medical evidence that it is effective in treating urinary tract infections or urinary symptoms. Limited clinical data from small studies suggest that Uva-Ursi is effective in preventing UTI even in high risk patients 4, 5. However, its clinical effectiveness in treating acute uncomplicated UTI and its potential to reduce antibiotic use has not yet been subject of a fully powered randomized controlled trial. A placebo-controlled trial assessing the ability of a herbal remedy, Uva-ursi, with or without advice to take ibuprofen to reduce antibiotic use for uncomplicated UTI found that there was no significant improvement in frequency symptoms between those taking Uva-ursi and placebo, nor between those taking ibuprofen and those not advised to take ibuprofen 6. Antibiotic consumption was not significantly reduced with Uva-Ursi, but was reduced with ibuprofen.
You may have a UTI if you notice these symptoms:
- Pain or burning when you urinate
- Fever, tiredness, or shakiness
- An urge to urinate often
- Pressure in your lower belly
- Urine that smells bad or looks cloudy or reddish
- Pain in your back or side below the ribs
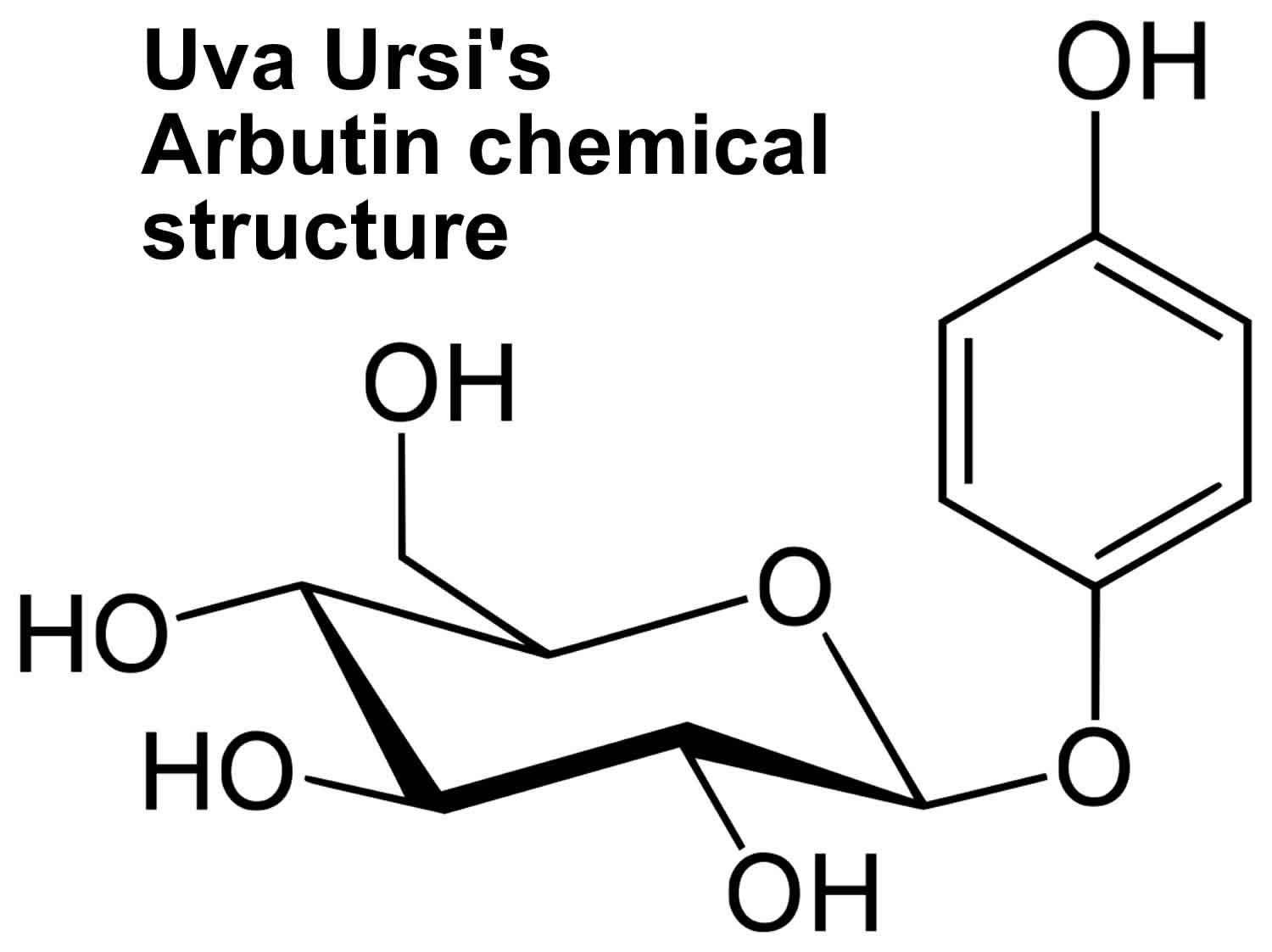
Arbutin (Figure 2), is a glucoside of hydroquinone. Its derivatives are phenolic compounds present in the bearberry leaves as dominant compounds 7 and they are also found in lingonberry leaves 8. One of the most important properties of arbutin is its widespread use as a skin-whitening agent, an action that occurs due to inhibition of the enzyme tyrosinase, the enzyme implicated in melanogenesis 9. Furthermore, another biological activity of arbutin consists in its efficacy in the treatment of various urinary tract infections due to the antibacterial, astringent, antioxidant and disinfectant effect of hydroquinone, the active metabolite of arbutin 10. This compound has a disinfectant effect on the urinary tract due to hydrolysis of hydroquinone, but only in an alkaline environment. In addition, arbutin validated its antioxidant activity through a 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical cation-scavenging assay; the results showed similar or higher antioxidant activity than hydroquinone, an efficient antioxidant. In fact, one molecule of arbutin scavenged three molecules of ABTS radical cation, while hydroquinone scavenged two molecules of the same radical cation 11. Moreover, some in vitro studies have been conducted and showed the cytotoxic effect 12 and the anti-inflammatory properties of arbutin 13.
Uva-ursi is widely available OTC (over-the-counter) in the UK and there is preliminary evidence suggesting that uva-ursi provides symptom relief when used in acute UTI (urinary tract infection). One study of 309 women found that those recommended to use Uvacin (an OTC preparation including uva-ursi) experienced shorter illness duration, although the numbers who subsequently reported use of the product was low (14% with advice to use versus 1% with no advice) 14. There is also some limited evidence that the prophylactic use of UVA-E, containing an aqueous/alcoholic extract of uva-ursi leaves and Taraxacum offinale (dandelion) root (n = 57) is effective for women with recurrent urinary tract infection 15. Side effects are not common but, in high doses, may include nausea, vomiting, tinnitus, shortness of breath and allergic reactions. Rare but potentially severe reactions to high doses of uva ursi may include convulsions, delirium and cardiovascular collapse.
More recently, uva ursi has been included in multiingredient dietary supplements meant for a variety of conditions such as to induce weight loss, promote wellness, prevent the effects of aging and to increase energy and stamina. Uva ursi is typically available in tablets and capsules of 150, 455 and 505 mg and is taken one to three times daily 16. It can also be prepared as tea from dried leaves or a powdered extract. Commercial products that contain uva ursi include SLIMQUICK Ultra and Animal Cuts.
Figure 1. Uva ursi plant
Footnote: Uva ursi is a trailing evergreen shrub that produces red berries and flourishes in alpine forests in many regions, including North America, Europe, the Iberian Peninsula, Siberia, and the Himalayas. It grows slowly but succeeds in places where other plants cannot, such as the walls of canyons. It has short, creeping, reddish-brown branches and pink or white bell-shaped flowers that bloom in the summer, followed by clusters of berries. Bears are said to be fond of the shiny, bright red or pink fruit, which is edible but tastes sour.
Figure 2. Uva Ursi’s Arbutin chemical structure
How long does uva ursi take to work?
Uva Ursi for uncomplicated UTI should work with 48 hours. Drinking a lot of water and urinate often to speed healing. Even without treatment, uncomplicated UTI will spontaneously resolve in about 20% of women. The likelihood that a female will develop acute pyelonephritis (kidney infection) is very small. Randomized trials have provided conflicting data regarding the occurrence of pyelonephritis in the setting of acute uncomplicated UTI managed without antibiotics 17. Whereas an increased risk for pyelonephritis was observed in some trials 18, 19, this risk was less prominent in placebo arms of other trials 20, 21.
Evidence suggests that 26% to 34% of women with lower urinary symptoms experience symptom improvement within one week in the absence of antibiotic therapy 20, 21.
About 25% of women experience UTI recurrences. Factors that indicate a poor outlook include:
- Overall health
- Advanced age
- Presence of renal calculi
- Diabetes
- Sickle cell anemia
- Presence of malignancy
- Catheterization
- Ongoing chemotherapy
Uva Ursi uses
Only the uva ursi leaves, not the berries, are used in herbal medicine. Uva ursi (Arctostaphylos uva-ursi) leaves contain arbutin, which is hydrolyzed to hydroquinone and test tube studies of uva ursi have demonstrated antibacterial activity against a variety of organisms including Escherichia coli (E. coli), the most prevalent urinary pathogen and uva-ursi is most often used as a urinary antiseptic 3. Today, uva ursi is sometimes used to treat urinary tract infections (UTIs) and cystitis (bladder inflammation). One preliminary study found that uva ursi, when combined with dandelion root and leaf, helped prevent recurrent urinary tract infections 15. Researchers believe the uva-ursi herb works best when a person’s urine is alkaline since acid destroys its antibacterial effect. Uva ursi works best at the first sign of urine infection. However, more research is needed to see if uva ursi works in humans. Furthermore, uva ursi can be toxic. Hydroquinone, a component of uva ursi, can cause serious liver damage 16. You should only take uva ursi for short periods, no longer than 5 days, under a health care provider’s supervision. You should not take a series of doses of uva ursi more than 5 times in 1 year. DO NOT take more than the recommended doses. Conventional medications that have fewer risks are available to treat urinary tract infections. Findings from a randomized controlled trial of 309 nonpregnant women suggest that similar symptom control is achieved with immediate versus delayed empiric antibiotics for acute uncomplicated cystitis 22.
In general, it is not recommended for patients with kidney disease to use herbal supplements. If you choose to take one, always tell your doctor, dietitian, or other health care provider. Always update the use of herbal supplements at your visits to your healthcare provider as a medication change.
The leaves of Arctostaphylos have been dried and smoked as tobacco, while leaves and berries have also been used as food.
Amarowicz and Pegg showed the antiproliferative effects of uva ursi leaves extracts against human carcinoma cell lines 23. Phenolic compounds were isolated from the crude extract of uva ursi leaves with 80% aqueous ethanol and separated in two fractions, one fraction comprised low-molecular weight phenolics and the other one, high molecular weight phenolics (tannin fraction). The data revealed that the crude extract and its two fractions have the properties to inhibit the proliferation of five human carcinoma cell lines, namely MCF-7-breast, HT-29-colon, DU-145-prostate, SK-MEL-5-skin and MDA-MB-435-skin carcinoma. The antiproliferative activity was associated with gallotanins present in the bearberry leaves 23.
Uva-ursi leaves extracts have shown beneficial effects on diuresis and electrolyte composition of urine (excretion of K⁺ and Na⁺) on mice, after administration of 5% water extract of uva ursi leaves 24. The beneficial effects on diuresis of a uva ursi extract were also exposed by Beaux et al. 25.
Uva Ursi health benefits
Through modern day scientific research in test tubes and animals, researchers have discovered that uva ursi’s ability to fight infection are due to several chemicals, including arbutin and hydroquinone. The uva ursi herb also contains tannins that have astringent effects, helping to shrink and tighten mucous membranes in the body. In turn, that helps reduce inflammation and fight infection.
Table 1. Several properties and health benefits of uva ursi
| Studied Species | Properties | Health Benefits | References |
| Uva ursi leaves | Antiproliferative | Inhibition of human carcinoma cell lines | 23 |
| Beneficial effect on diuresis and electrolyte composition | 24, 26 | ||
| Anti-microbial activity | 27, 28 |
Antimicrobial effects
The antimicrobial activity of uva ursi extract was tested by several studies 29. According to Holopainen et al. 27 the extracts of the aerial uva ursi parts have antimicrobial activity against the Gram-negative bacteria: E. coli and Proteus vulgaris, effect attributed to arbutin and metylarbutin. According to Annuk et al. 28, the aqueous extract of uva ursi leaves had antimicrobial activity against Helicobacter pylori for which tannic acid was considered responsible.
Although pharmacological research has focused primarily on arbutin, the pharmacology of the whole plant is different from that of arbutin alone. The crude uva ursi plant extracts are much more effective medicinally than the isolated constituent arbutin 30. This fact appears to be related to the activity of gallic acid, which prevents the splitting of arbutin by such enzymes as β-glucosidase contained in gut bacteria. Arbutin under-goes hydrolysis in the stomach or intestinal tract to produce hydroquinone, its aglycone, which has urinary antiseptic properties. The hydrolysis of arbutin is responsible for much of the therapeutic effect of uva ursi. By preventing the splitting of arbutin, the flavonoid components allow more arbutin to be hydrolyzed and absorbed than when arbutin is administered as an isolated component. Approximately 65% of an arbutin dosage is excreted in the urine as hydroquinone glucu-ronide or sulfate.
Arbutin alone has been reported to be an effective urinary antibiotic, but only if taken in large doses and if the urine is alkaline (once again documenting the value of whole-plant medicines). It is reported to be active against Candida albicans and Staphylococcus aureus and especially against Escherichia coli. Uva ursi also has diuretic properties.
A placebo-controlled trial assessing the ability of a herbal remedy, Uva-ursi, with or without advice to take ibuprofen to reduce antibiotic use for uncomplicated UTI found that there was no significant improvement in frequency symptoms between those taking Uva-ursi and placebo, nor between those taking ibuprofen and those not advised to take ibuprofen 6. Antibiotic consumption was not significantly reduced with Uva-Ursi, but was reduced with ibuprofen.
Anti-inflammatory effects
Some early animal research is now showing that arbutin, and possibly other constituents of uva ursi, potentiate the activity of commonly prescribed anti-inflammatory drugs 30. One study found that an aqueous extract increased the inhibitory activity of dexamethasone in allergic and inflammatory models without increasing any of the side effects. Similar results have been demonstrated with isolated arbutin combined with indomethacin.
Inhibition of melanin synthesis
Uva ursi extract has been shown to inhibit the enzyme tyrosinase. This effect impairs melanin synthesis, which indicates that uva ursi extract may be effective as a whitening agent for the skin. In fact, hydroquinone is approved by the U.S. Food and Drug Administration (FDA) as an agent in over-the-counter skin products for bleaching age spots and hyperpigmentation 9.
Uva Ursi dosage
The daily dose of uva-ursi used in a randomized, double-blind, placebo-controlled trial of uva-ursi was 3600 mg (3 × 400-mg capsules) to be taken orally three times a day thus providing a total of 686 mg arbutin 3. Women participants were asked to take uva-ursi for 3 days and up to 5 days.
The study randomized a total of 382 women participants with a mean age of 43.8 years who experienced urinary symptoms for a median of 3 days across groups (uva-ursi with advice to take ibuprofen, n=102; placebo with advice to take ibuprofen, n=86; uva-ursi without advice to take ibuprofen, n=97; placebo without advice to take ibuprofen, n=97) 3. All participants received a delayed prescription for antibiotics to be used if symptoms worsened or persisted beyond 3 to 5 days.
Uva Ursi side effects
Uva ursi side effects are not common and are generally mild and may include nausea, vomiting, tinnitus, shortness of breath, irritability, insomnia and allergic reactions. Rare but potentially severe reactions to high doses of uva ursi may include convulsions, delirium and cardiovascular collapse.
Women who are pregnant or breastfeeding, and people with high blood pressure, should not take uva ursi. People who have Crohn disease, digestive problems, kidney or liver disease, or ulcers should not take uva ursi.
Uva ursi possible interactions
Uva-ursi may precipitate significant drug-drug interactions through potent cytochrome P450 isozyme inhibition 31. If you are being treated with any of the following medications, you should not use uva ursi without first talking to your health care provider.
- Lithium. It is possible that taking uva ursi may cause lithium, a drug taken to treat bipolar disorder, to build up to dangerous levels in the blood.
- Drugs and supplements that make urine more acidic. These include vitamin C, cranberry juice, orange juice, and other citrus fruits and juices.
- Nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroids. Animal studies done in Japan suggest uva ursi may increase the anti-inflammatory effects of these drugs, although it is not known whether the herb would have that effect in people.
- Iron. If you take iron supplements, take them at least 2 hours before or 2 hours after uva ursi.
- EMA European Medicines Agency. Assessment report on Arctostaphylos uva-ursi (l.) Spreng. folium. 2012.[↩][↩]
- Kedzia B, Wrociński T, Mrugasiewicz K, Gorecki P, Grzewińska H. Przeciwbakteryjne działanie moczu zawierajacego produkty metabolizmu arbutyny [Antibacterial action of urine containing products of arbutin metabolism]. Med Dosw Mikrobiol. 1975;27(3):305-14. Polish.[↩]
- Trill, J., Simpson, C., Webley, F., Radford, M., Stanton, L., Maishman, T., Galanopoulou, A., Flower, A., Eyles, C., Willcox, M., Hay, A., Griffiths, G., Little, P., Lewith, G., & Moore, M. (2017). Uva-ursi extract and ibuprofen as alternative treatments of adult female urinary tract infection (ATAFUTI): study protocol for a randomised controlled trial. Trials, 18(1), 421. https://doi.org/10.1186/s13063-017-2145-7[↩][↩][↩][↩]
- Albrecht J, Kreyes G. Langzeitbehandlung von Dauerkatheterpatienten: Chemoprophylaxe oder Phytotherapie? Extr urol. 1988;11(5):277–280.[↩]
- Larsson B, Jonasson A, Fianu S. Prophylactic effect of UVA-E in women with recurrent cystitis: a preliminary report. Curr Ther Res. 1993;53:441–443. doi: 10.1016/S0011-393X(05)80204-8[↩]
- Moore M, Trill J, Simpson C, Webley F, Radford M, Stanton L, Maishman T, Galanopoulou A, Flower A, Eyles C, Willcox M, Hay AD, van der Werf E, Gibbons S, Lewith G, Little P, Griffiths G. Uva-ursi extract and ibuprofen as alternative treatments for uncomplicated urinary tract infection in women (ATAFUTI): a factorial randomized trial. Clin Microbiol Infect. 2019 Aug;25(8):973-980. doi: 10.1016/j.cmi.2019.01.011[↩][↩]
- Saleem A., Harris C.S., Asim M., Cuerrier A., Martineau L., Haddad P.S., Arnason J.T. A RP-HPLC-DAD-APCI/MSD method for the characterisation of medicinal Ericaceae used by the Eeyou Istchee Cree First Nations. Phytochem. Anal. 2010;21:328–339. doi: 10.1002/pca.1203[↩]
- Ieri F., Martini S., Innocenti M., Mulinacci N. Phenolic distribution in liquid preparations of Vaccinium myrtillus L. and Vaccinium vitis idaea L. Phytochem. Anal. 2013;24:467–475. doi: 10.1002/pca.2462[↩]
- Zhu W., Gao J. The use of botanical extracts as topical skin-lightening agents for the improvement of skin pigmentation disorders. J. Invest. Derm. Symp. P. 2008;13:20–24. doi: 10.1038/jidsymp.2008.8[↩][↩]
- de Arriba S.G., Naser B., Nolte K.-U. Risk Assessment of Free Hydroquinone Derived from Arctostaphylos Uva-ursi folium Herbal Preparations. Int. J. Toxicol. 2013;32:442–453. doi: 10.1177/1091581813507721[↩]
- Tai A., Ohno A., Ito H. Isolation and Characterization of the 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) Radical Cation-Scavenging Reaction Products of Arbutin. J. Agric. Food Chem. 2016;64:7285–7290. doi: 10.1021/acs.jafc.6b02847[↩]
- Li H., Jeong Y.M., Kim S.Y., Kim M.K., Kim D.S. Arbutin inhibits TCCSUP human bladder cancer cell proliferation via up-regulation of p21. Pharmazie. 2011;66:306–309.[↩]
- Lee H.J., Kim K.W. Anti-inflammatory effects of arbutin in lipopolysaccharide-stimulated BV2 microglial cells. Inflamm. Res. 2012;61:817–825. doi: 10.1007/s00011-012-0474-2[↩]
- Little P, Moore MV, Turner S, Rumsby K, Warner G, Lowes JA, et al. Effectiveness of five different approaches in management of urinary tract infection: randomised controlled trial. BMJ. 2010;340(feb05_1):c199. doi: 10.1136/bmj.c199[↩]
- Larsson B, Jonasson A, Fianu S. Prophylactic effect of UVA-E in women with recurrent cystitis: a preliminary report. Curr Ther Res. 1993;53(4):441–3. doi: 10.1016/S0011-393X(05)80204-8[↩][↩]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Uva Ursi. [Updated 2020 Mar 28]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK556475[↩][↩]
- Datta, R., & Juthani-Mehta, M. (2019). Antibiotic-sparing agents for uncomplicated cystitis: uva-ursi and ibuprofen not ready for primetime. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases, 25(8), 922–924. https://doi.org/10.1016/j.cmi.2019.04.022[↩]
- Kronenberg A, Bütikofer L, Odutayo A, et al. Symptomatic treatment of uncomplicated lower urinary tract infections in the ambulatory setting: randomised, double blind trial. BMJ (Clinical research ed.) 2017;359:j4784–j4784[↩]
- Gágyor I, Bleidorn J, Kochen MM, Schmiemann G, Wegscheider K, Hummers-Pradier E. Ibuprofen versus fosfomycin for uncomplicated urinary tract infection in women: randomised controlled trial. BMJ 2015;351:h6544[↩]
- Christiaens TCM, De Meyere M, Verschraegen G, Peersman W, Heytens S, De Maeseneer JM. Randomised controlled trial of nitrofurantoin versus placebo in the treatment of uncomplicated urinary tract infection in adult women. The British journal of general practice : the journal of the Royal College of General Practitioners 2002;52(482):729–734.[↩][↩]
- Richards D, Toop L, Chambers S, Fletcher L. Response to antibiotics of women with symptoms of urinary tract infection but negative dipstick urine test results: double blind randomised controlled trial. BMJ 2005;331(7509):143.[↩][↩]
- Little P, Moore MV, Turner S, Rumsby K, Warner G, Lowes JA, Smith H, Hawke C, Leydon G, Arscott A, Turner D, Mullee M. Effectiveness of five different approaches in management of urinary tract infection: randomised controlled trial. BMJ. 2010 Feb 5;340:c199. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2817051[↩]
- Amarowicz R., Pegg R.B. Inhibition of proliferation of human carcinoma cell lines by phenolic compounds from a bearberry-leaf crude extract and its fractions. J. Funct. Foods. 2013;5:660–667. doi: 10.1016/j.jff.2013.01.009[↩][↩][↩]
- Vranješ M., Popović B.M., Štajner D., Ivetić V., Mandić A., Vranješ D. Effects of bearberry, parsley and corn silk extracts on diuresis, electrolytes composition, antioxidant capacity and histopathological features in mice kidneys. J. Funct. Foods. 2016;21:272–282. doi: 10.1016/j.jff.2015.12.016[↩][↩]
- Beaux D, Fleurentin J, Mortier F. Effect of extracts of Orthosiphon stamineus Benth, Hieracium pilosella L., Sambucus nigra L. and Arctostaphylos uva-ursi (L.) Spreng. in rats. Phytother Res. 1999 May;13(3):222-5. doi: 10.1002/(SICI)1099-1573(199905)13:3<222::AID-PTR447>3.0.CO;2-P[↩]
- Beaux D., Fleurentin J., Mortier F. Effect of extracts of Orthosiphon stamineus Benth, Hieracium pilosella L., Sambucus nigra L. and Arctostaphylos uva-ursi (L.) Spreng. in rats. Phyther. Res. 1999;13:222–225. doi: 10.1002/(SICI)1099-1573(199905)13:3<222::AID-PTR447>3.0.CO;2-P[↩]
- Holopainen M., Jahodar L., Seppänen-Laakso T., Laakso I., Kauppinen V. Antimicrobial activity of some Finnish Ericaceous plants. Acta Pharm. Fenn. 1988;97:197–202.[↩][↩]
- Annuk H., Hirmo S., Türi E., Mikelsaar M., Arak E., Wadström T. Effect on cell surface hydrophobicity and susceptibility of Helicobacter pylori to medicinal plant extracts. FEMS Microbiol. Lett. 1999;172:41–45. doi: 10.1111/j.1574-6968.1999.tb13447.x[↩][↩]
- Ștefănescu, B. E., Szabo, K., Mocan, A., & Crişan, G. (2019). Phenolic Compounds from Five Ericaceae Species Leaves and Their Related Bioavailability and Health Benefits. Molecules (Basel, Switzerland), 24(11), 2046. https://doi.org/10.3390/molecules24112046[↩]
- Uva ursi (Bearberry). Textbook of Natural Medicine (Fifth Edition), Churchill Livingstone, 2020, Pages 887-889.e1, ISBN 9780323523424 https://doi.org/10.1016/B978-0-323-43044-9.00120-5[↩][↩]
- Chauhan B, Yu C, Krantis A, Scott I, Arnason JT, Marles RJ, Foster BC. In vitro activity of uva-ursi against cytochrome P450 isoenzymes and P-glycoprotein. Can J Physiol Pharmacol. 2007 Nov;85(11):1099-107. doi: 10.1139/Y07-106[↩]