Visual evoked potential
Visual evoked potential also called visual evoked response, is an evoked potential caused by a visual stimulus, such as an alternating checkerboard pattern on a computer screen. Visual evoked potential responses are recorded from electrodes that are placed on the back of your head at the scalp over the occipital cortex and are observed as a reading on an electroencephalogram (EEG). The light-evoked signal, small in amplitude and hidden within the normal electroencephalographic (EEG) signal, is amplified by repetitive stimulation and time-locked, signal-averaging techniques, separating it from the background EEG readings. The precise origin of the visual evoked potential signal remains unclear, but it reveals the integrity of the afferent visual pathway; damage anywhere along the path may reduce the signal. The visual evoked potential is primarily a function of central visual function, because such a large region of occipital cortex is devoted to macular projections. Thus, peripheral visual loss might be overlooked by visual evoked potential testing.
These responses usually originate from the occipital cortex, the area of the brain involved in receiving and interpreting visual signals. Visual evoked potential tests the function of your visual pathway from your retina to the occipital cortex. Visual evoked potential test measures the conduction of the visual pathways from the optic nerve, optic chiasm, and optic radiations to the occipital cortex. It is important to keep in mind that although the axons from the nasal half of the retina decussate (cross over or intersect each other) at the optic chiasm, the temporal axons do not. Therefore, retrochiasmatic lesions may not be detected by full-field checkerboard stimulation.
Visual evoked potentials are most useful for testing optic nerve function and less useful for assessing postchiasmatic disorders. In patients with retrochiasmatic lesions, MRI is a more useful test. Partial-field studies may be useful for retrochiasmatic lesions; however, they are not performed routinely in clinical settings. It is also important to note that the macula projects to the occipital pole, whereas the rest of the retina projects to the mesial calcarine cortex.
The visual evoked potential is an important test that is very good at detecting problems with the optic nerve and lesions in the anterior part of your visual pathway, before the optic nerves merge. However, it is a non-specific test and to determine the exact underlying problem in each patient, a good history and examination is also very important.
The 2 scenarios in which visual evoked potentials remain clinically useful are:
- Evaluation of the visual pathway in infants or inarticulate adults and
- Confirmation of intact visual pathways in patients suspected of nonorganic disease.
A consistently abnormal flash response in the infant or inarticulate adult reflects gross impairment. An abnormal pattern response, however, is less useful, as it may indicate damage or may be a false-negative result from inattention or the reasons just cited. Normal responses confirm intact visual pathways.
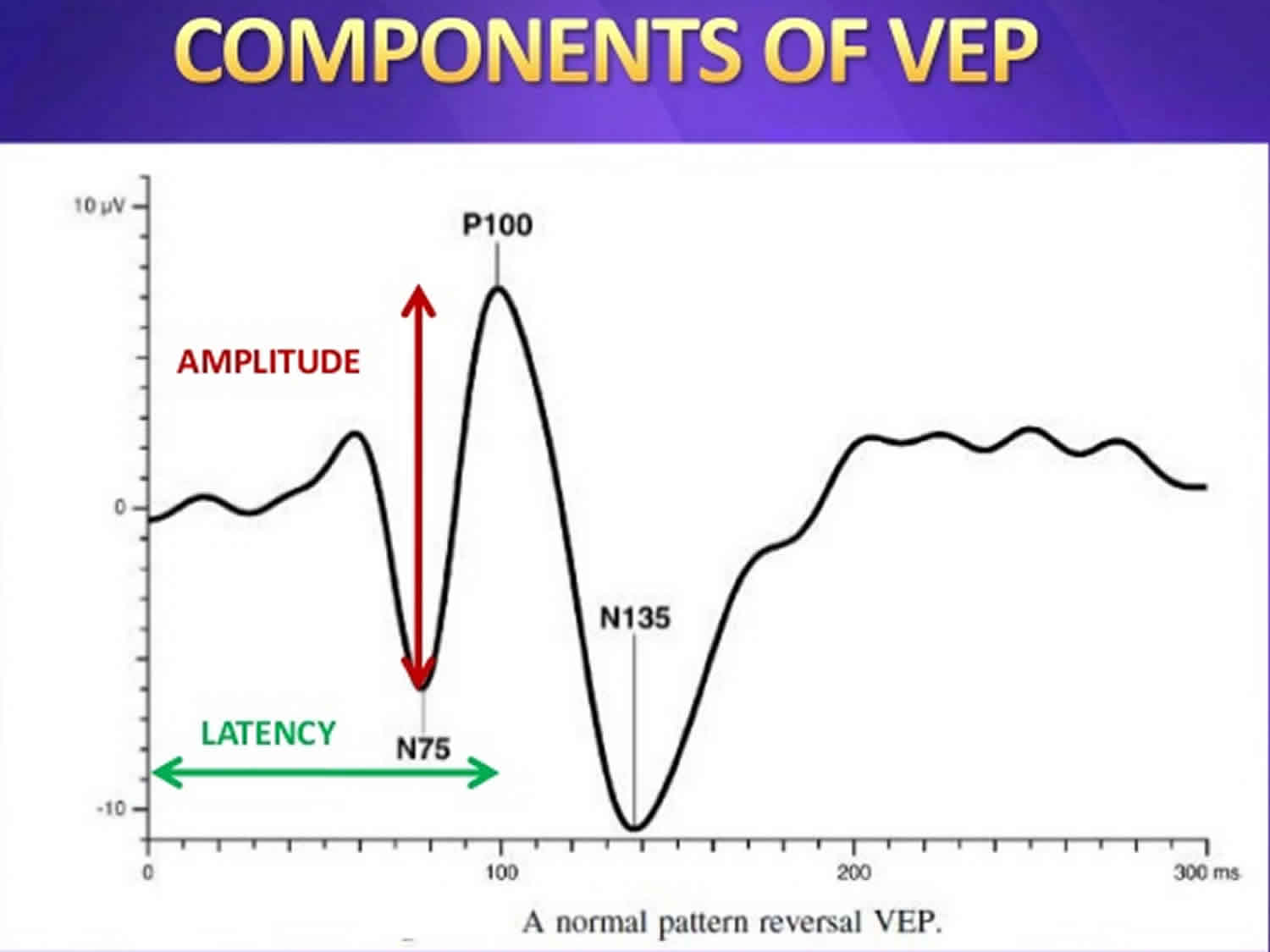
The usual waveform is an initial negative peak (N1 or N75), followed by a large positive peak (P1 or P100), followed by another negative peak (N2 or N145). Maximum value for P100 is 115 msec in patients younger than 60 years; after this age, it rises to 120 msec in women and 125 msec in men 1. Even though published norms are available in the medical literature, each individual laboratory should have its own norms to control for laboratory-to-laboratory variations in technique.
The W morphology, is most often an individual variation, though decreasing the stimulation frequency from the ubiquitous 2 Hz to 1 Hz usually converts the W shape into a conventional P100 peak. Check size and alternation rate are factors in this; the responses can be manipulated to a W or a conventional P100 response by changing these parameters. Large checks tend to produce visual evoked potentials similar to those produced by flash stimulation.
Although the visual evoked potential is very useful for detecting an anterior visual conduction disturbance, it is not specific with regard to cause. A tumor compressing the optic nerve, an ischemic disturbance, or a demyelinating disease may cause delay in the P100 (also known as P1, for its usual location at 100 msec); only additional clinical history and, often, MRI are needed to uncover the etiology.
The examiner can compare readings from each eye with standardized normal values, readings from the 2 eyes, and readings from the 2 hemispheres. Peak latencies are relatively consistent, and accurate normative data are available; amplitude data are less consistent and thus less useful. Abnormalities in the waveform result from impairment anywhere along the visual pathways, but unilateral abnormalities may reflect optic neuropathy and thus may help to reveal lesions in the absence of clear-cut fundus abnormalities. Demyelination of the optic nerve results in increased latency of the P100 waveform, without significant effect on amplitude; ischemic, compressive, and toxic damage reduce amplitude primarily, with less effect on latency.
For most clinical situations, the visual evoked potential is of limited usefulness. It is subject to numerous factors that may produce abnormal waveforms in the absence of visual pathway damage, including uncorrected refractive error, media opacity, amblyopia, fatigue, and inattention (either intentional or unintentional). In most cases, the visual evoked potential is unnecessary for the diagnosis of optic neuropathy and is less accurate for its quantification than perimetry.
A new technique being developed is the multifocal visual evoked potential (mfVEP); it is designed to detect small abnormalities in optic nerve transmission and provide topographic correlation along the visual pathway. Limited studies to date of the anterior visual pathways correlate visual field abnormalities to the abnormalities confirmed by multifocal visual evoked potential (mfVEP).
Clinical usefulness of the visual evoked potential
- The visual evoked potential is a standardized and reproducible test of optic nerve function
- It is more sensitive compared to magnetic resonance imaging (MRI) in detecting lesions affecting the visual pathway in front of the optic chiasm (area in the optic pathway where the optic nerve crosses sides)
- It is usually less costly compared to other investigations such as MRI
- If results of the visual evoked potential are negative, this can be useful in excluding certain disorders.
Where is the origin of visual evoked potential?
The generator site for visual evoked potentials is believed to be the peristriate and striate occipital cortex 1. Prolongation of P100 latency is the most common abnormality and usually represents an optic nerve dysfunction. visual evoked potential is clearly more sensitive than physical examination in detecting optic neuritis.
Ikeda et al 2 investigated current source generators (dipoles) of human visual evoked potential to pattern-onset stimuli. A visual stimulus (a checkerboard pattern) was presented for 250 msec in each of the 8 quadrants. Central and peripheral parts of each of the 4 quadrant fields were evaluated. The visual evoked potentials, consisting of initial positive-late negative waves, were recorded mainly on the occipital region contralateral to stimulated visual fields. The initial positive waves of visual evoked potential were divided into the following 2 components:
- Early component with an approximate peak latency of 70-90 msec
- Late component with an approximate peak latency of 100-120 msec
The results from these analyses of visual evoked potential indicated topographic localization of the dipoles around the calcarine fissure 2. This was comparable to the retinotopy of the human occipital lobe based on clinicopathologic studies.
In a multicenter study, Brigell et al 3 described the pattern visual evoked potential using standardized techniques, concluding that the peak latency of pattern-reversal visual evoked potential is a sensitive measure of conduction delay in the optic nerve caused by demyelination. To establish whether pattern-reversal visual evoked potential could be standardized for use as a measure in multicenter therapeutic trials for optic neuropathy or multiple sclerosis (MS), the investigators evaluated stimulus and recording variables at 4 centers.
Overall, pattern-reversal visual evoked potentials were recorded from 64 healthy subjects and 15 patients with resolved optic neuritis; the results showed equivalent latency and amplitude data from all centers, indicating that the visual evoked potential test can be standardized satisfactorily for multicenter clinical trials 3. Furthermore, the authors concluded that the N70 and P100 peak latencies and N70-P100 interocular amplitude difference were sensitive measures of resolved optic neuritis 3.
Abboud et al 4, in a study that used flash visual evoked potential to assess left-right asymmetry in the potential amplitude on the scalp in brain-damaged patients who had sustained a stroke, determined that the visual evoked potential amplitude was smaller over the ischemic hemisphere than over the intact hemisphere. This finding indicates that the left-right asymmetry in scalp visual evoked potentials of patients after brain damage may be a result of changes in the conductivity of the volume conductor, attributable to the ischemic region between the source and the electrodes.
Ipata et al 5, in a study designed to assess interhemispheric visual transfer of information in humans, obtained estimates of interhemispheric transfer time that ranged from 5.77 to 12.54 msec, depending on the type of component and the location of the electrode sites. More anterior locations yielded shorter values, and overall transfer time tended to be 7 msec shorter for the N70 component than for the P100 component.
Which factors influence results of visual evoked potentials?
The usual visual evoked potentials are evoked by checkerboard stimulation. Because the cells of the visual cortex are maximally sensitive to movement at the edges, a pattern-shift method is used, with a frequency of 1-2 Hz. The size of the checks affects the amplitude of the waveform and the latency of the P100.
In addition, pupillary size, gender, and age all affect the visual evoked potential. Deterioration of visual acuity, up to 20/200, does not alter the response significantly; large checks may be required. In some studies, women have slightly shorter P100 latencies. Sedation and anesthesia abolish the visual evoked potential. Some subjects, by fixating beyond the plane of stimulation, may alter or suppress P100 altogether.
Certain drugs (eg, carbamazepine) prolong visual evoked potentials. In a study of the effects of carbamazepine and sodium valproate monotherapy on visual evoked potentials in 18 epileptic children, Yuksel et al 6 found that carbamazepine slows down central impulse conduction. Pattern-reversal visual evoked potentials were determined before administration of the antiepileptic drugs and after 1 year of therapy; the visual evoked potential amplitude showed no consistent changes after 1 year of therapy, but visual evoked potential P100 latencies were significantly prolonged after 1 year of carbamazepine therapy.
According to Trip et al 7, atrophy of the optic nerve was correlated with decreased visual evoked potential amplitude.
Sannita et al 8 evaluated the correlation between amplitude and latencies of the pattern-reversal visual evoked potential and serum glucose level in healthy volunteers. Pattern visual evoked potential and serum glucose levels were obtained at 2-hour intervals during an 8-hour experimental session. At serum glucose concentrations within the physiological range of variability (55-103 mg/dL), the P100 latency increased with increasing serum glucose level, with a 6.9% estimated latency difference between lower and higher glucose concentrations.
Visual evoked potential test
A doctor may recommend that you go for a visual evoked potential test when you are experiencing changes in your vision that can be due to problems along the pathways of certain nerves. Some of these symptoms may include:
- Loss of vision (this can be painful or non-painful);
- Double vision;
- Blurred vision;
- Flashing lights;
- Alterations in color vision; or
- Weakness of the eyes, arms or legs.
These changes are often too subtle or not easily detected on clinical examination in the doctor’s surgery. In general terms, the test is useful for detecting optic nerve problems. This nerve helps transfer signals to allow us to see, so testing the nerve allows the doctor to see how your visual system responds to light. The test is also useful because it can be used to check vision in children and adults who are unable to read eye charts.
Factors influencing visual evoked potential
The cells within the part of your brain involved with vision are most sensitive to movement at the edges of your central visual fields. If you have poor vision, this does not seem to have too much effect on the response – larger checkerboard patterns can be used. Your gender, age and the size of your pupils are other factors that can affect the visual evoked potential. If you have taken any drugs that make you drowsy, or under the influence of any anesthetic drugs, your visual evoked potential is also greatly affected.
How to prepare for a visual evoked potential test
You will be given instructions on how to prepare for the test. This will depend on where you are going to get the test done. Some things that you may need to do include:
- Washing your hair the night before, but avoiding hair chemicals, oils and lotions.
- Making sure you get plenty of sleep the night before.
- If you wear glasses, make sure you bring these along with you to the test.
- You are usually able to eat a normal meal and take your usual medications prior to the test. However any medications that may make you drowsy should be avoided.
- Arrive on time and try to relax before the test.
- On the day of the test, you should also let the technician know if you have any eye conditions such as cataracts or glaucoma as this can affect the test and should be noted in your records by the doctor.
What happens during a visual evoked potential test?
The procedure is very safe and non-invasive.
- Firstly, some wires will be glued to the top of your head to detect the brain waves.
- A technician will give you further instructions on what to do during the test. Normally, each eye will be tested separately.
- It is very important that you co-operate with the technician who conducts the test and be able to fix your vision in a certain spot. You will be asked to look at a screen similar to a television screen, with various visual patterns.
- Readings will be recorded through the wires on top of your head.
- After the procedure, the glue and wires are removed from your head. The doctor may discuss the results of the test with you after they have been analysed; otherwise the referring doctor will.
- Usually the procedure takes about 45 minutes.
A checkerboard pattern (or, less often, a flash) is used as stimulation. Responses are collected over Oz, O1, and O2 and with hemifield studies at T5 and T6 with the standard electroencephalographic (EEG) electrode placement. Monocular stimulation is used to avoid masking of a unilateral conduction abnormality. Sedation should not be used, and note should be taken of medications that the patient is taking regularly.
Testing circumstances should be standardized, including a seating distance of 70-100 cm from the monitor screen, which gives a check size of approximately 30 seconds of visual angle. The vision should be corrected to the extent possible in case of a visual problem. Pupil size and any abnormality should be noted. The P100 waveform is at its maximum in the midoccipital area. Stimulus rates of 1-2 Hz are recommended, and the filter setting should be in 1- to 200-Hz bandwidth (the outside limits are 0.2 and 300 Hz).
The recommended recording time window (ie, the sweep length) is 250 msec; 50-200 responses are to be averaged. A minimum of 2 trials should be given. The responses are averaged, and the P100 positive polarity waveform that appears in the posterior head region is analyzed. The mean latency is about 100 msec. Normative data should be assembled on a laboratory-by-laboratory basis.
Visual evoked potential pitfalls
A check size of 27 seconds of visual angle may result in normal P100 latency in a patient with cortical blindness; smaller checks (ie, ≤ 20 seconds of visual angle) should be used to demonstrate the abnormality. If cortical blindness is suspected, large checks should not be used.
In conditions such as retinal disease or refractory errors, the amplitude may be smaller and, at very small check sizes, the latency may increase. For this reason, proper refraction is of great importance.
Because the visual evoked potential measures the pathway from the retina to area 17, a normal P100 does not exclude lesions of the visual pathway beyond area 17. Consequently, the visual evoked potential may be normal in patients with the diagnosis of cortical blindness. Note that in such cases, the visual evoked potential is still useful, in that it rules out disease up to area 17 in patients with a normal response.
The usefulness of visual evoked potential is limited in malingering and hysterical visual loss. It is useful when a normal visual evoked potential is recorded, but abnormal responses are of limited diagnostic value in such cases. Baumgartner et al reported that as many as 5 of 15 healthy subjects were able to suppress their pattern visual evoked potentials 9.
Visual evoked potential side effects
There are rarely any side effects from this procedure. It is a painless procedure and apart from possible minor skin irritation from the electrodes, there are often no complications. If you have any medical conditions such as epilepsy, the visual stimuli you are exposed to is unlikely to set off seizure activity. This should however be noted with your doctor and technician before you undergo a visual evoked potential test.
After the procedure is done, patients normally return home on the same day. You should be able to drive home safely if you are feeling well after the procedure.
What does the visual evoked potential detect?
The visual evoked potential measures the time that it takes for a visual stimulus to travel from the eye to the occipital cortex. It can give the doctor an idea of whether the nerve pathways are abnormal in any way. For example, in multiple sclerosis, the insulating layer around nerve cells in the brain and spinal cord (known as the myelin sheath) can be affected. This means that it takes a longer time for electrical signals to be conducted from the eyes, resulting in an abnormal visual evoked potential. A normal visual evoked potential can be fairly sensitive in excluding a lesion of the optic nerve, along its pathways in the anterior part of the brain.
The visual evoked potential is particularly useful in detecting past optic neuritis. This refers to inflammation of the optic nerve, associated with swelling and progressive destruction of the sheath covering the nerve, and sometimes the nerve cable. As the nerve sheath is damaged, the time it takes for electrical signals to be conducted to the eyes is prolonged, resulting in an abnormal visual evoked potential. This may be seen in multiple sclerosis – one of the most common causes of optic neuritis. Abnormal visual evoked potential’s are seen in multiple sclerosis patients due to the presence of optic neuritis.
The following are less easily differentiated but may cause abnormal visual evoked potentials:
- Optic neuropathy – this can be due to damage of the optic nerve from a number of causes, including: a blockage of the nerve’s blood supply, nutritional deficiencies, or toxins. As the nerve is damaged, electrical signals do not conduct properly. Examples include diabetes in the advanced stages which can be associated with damage to the blood vessels and nerves supplying the eyes, or toxic amblyopia which is a condition of the eyes associated with decreased vision, due to a toxic reaction in part of the optic nerve.
- Tumors or lesions compressing the optic nerve – if the optic nerve is compressed, the pathway for conduction is affected and an abnormal visual evoked potential is seen.
- Glaucoma – patients who suffer from glaucoma have increased intraocular pressure (ie pressure inside the eye). This can result in damage to the optic nerve, leading to prolonged visual evoked potentials.
- Ocular hypertension (high pressure) – this refers to any situation in which the pressure in the eye is higher than normal. There are no signs of glaucoma, but patients may be at increased risk of developing glaucoma later in life.
Visual evoked potential clinical applications
With an abnormal visual evoked potential, the differential diagnostic considerations include the following 1:
- Optic neuropathy
- Optic neuritis
- Ocular hypertension
- Glaucoma [9, 10]
- Diabetes – Szabela et al found abnormal visual evoked potential in 22% of type 2 diabetics 10
- Toxic amblyopia
- Leber hereditary optic neuropathy
- Aluminum neurotoxicity 11
- Manganese intoxication 12
- Retrobulbar neuritis
- Ischemic optic neuropathy
- Multiple sclerosis (MS) 13
- Tumors compressing the optic nerve – Optic nerve gliomas, meningiomas, craniopharyngiomas, giant aneurysms, and pituitary tumors
Factors contributing to the clinical usefulness of visual evoked potential testing include the following:
- It is more sensitive than MRI or physical examination for prechiasmatic lesions
- It is an objective and reproducible test for optic nerve function
- The abnormality observed persists over long periods
- It is inexpensive as compared with MRI
Under certain circumstances, it may be helpful for positively establishing optic nerve function in patients with a subjective complaint of visual loss; a normal visual evoked potential virtually excludes an optic nerve or anterior chiasmatic lesion
In general, the visual evoked potential is preferable in cases of optic nerve and anterior chiasmatic lesions, whereas MRI is clearly superior in retrochiasmatic disease. Note that the visual evoked potential is nonspecific with regard to the underlying etiology and pathology.
Optic neuritis and neuropathy
The visual evoked potential characteristically shows an increase in P100 latency of the involved side. The use of steroids in this condition has been controversial.
Trauzettel-Klosinski et al 14 observed that oral prednisone initially had a positive effect on visual evoked potential latencies in acute optic neuritis. In this study, 48 patients with acute optic neuritis were treated with either oral methylprednisolone (100 mg/day initially with dosage reduction every 3 days; n=15 [treatment group]) or oral thiamine (100 mg/day; n=33 [control group]), 36 of them in a double-blind procedure. Oral methylprednisolone yielded a faster improvement in visual evoked potential latency in the initial phase but had no benefit after 12 weeks or 12 months.
Elvin et al 15 used Doppler ultrasonography, MRI, and visual evoked potential measurements to study abnormal optic nerve function. Visual evoked potential assessments were performed in 16 patients. Patients with impairment of visual acuity and a prolonged visual evoked potential initially had a more swollen nerve and increased flow resistance in the affected optic nerve. Statistically significant side-to-side differences were found in the optic nerve diameter and in the resistance to flow in the central retinal artery between the affected and unaffected eyes.
Atilla et al 16 found that visual evoked potential amplitude decrease was more significant in patients with ischemic optic neuropathy, whereas latency prolongation was more significant in those with optic neuritis.
Yukagawa et al 17 found delayed P100 latencies in 7 of 46 eyes in patients with uveitis due to human T-lymphotropic virus type 1 (HTLV-1).
Multiple sclerosis
The McDonald criteria have incorporated visual evoked potentials into the diagnosis of multiple sclerosis. In patients in whom an insidious neurologic progression has occurred, visual evoked potentials are recommended when MRI documents at least 4, but no more than 8, T2 lesions consistent with MS 18.
Adrenoleukodystrophy
Adrenoleukodystrophy is an X-linked metabolic disorder with very-long-chain fatty acid (VLCFA) accumulation and multifocal nervous system demyelination, often with early involvement of visual pathways. Kaplan et al 19 found that pattern-reversal visual evoked potentials were abnormal in 17% of men with adrenoleukodystrophy; no evidence indicated that reduction of VLCFA levels improved or retarded visual pathway demyelination.
Idiopathic intracranial hypertension
Kesler et al 20 used visual evoked potential studies to assess 20 patients with chronic idiopathic intracranial hypertension and found that 55% of the patients had prolonged visual evoked potentials. These latencies tended to correlate best with visual field deficits, but other clinical findings were less congruent. The prolongation of the latencies suggested demyelination as the pathogenic process occurring in these individuals.
Classic and common migraine
Shibata et al 21 recorded pattern-reversal visual evoked potential to transient checkerboard stimulus in 19 patients with migraine with visual aura (ie, classic migraine), 14 patients with migraine without aura (ie, common migraine) in the interictal period, and 43 healthy subjects. Latencies and amplitudes of pattern-reversal visual evoked potentials in each group were analyzed.
P100 amplitude was significantly higher in patients with classic migraine than in healthy subjects, whereas latencies of pattern-reversal visual evoked potentials did not differ significantly 21. No significant differences were noted in latency between the common migraine group and healthy subjects or in latencies and amplitudes of pattern-reversal visual evoked potential between the classic migraine and common migraine groups.
Zgorzalewicz 22 found prolongation of P100 and N145 latencies and reduction in amplitude in migraine patients in 1 hemisphere.
These results suggest that patients with classic migraine may have hyperexcitability in the visual pathway during interictal periods and that the increased amplitude of pattern-reversal visual evoked potentials after attacks may be due to cortical spreading depression.
Brainstem auditory evoked potential
The brainstem auditory evoked potential (BAEP), or brainstem auditory evoked response (BAER), measures the functioning of the auditory nerve and auditory pathways in the brainstem 23.
The short-latency brainstem auditory evoked potential generally is used for clinical purposes. The test can be performed with the patient under either sedation or general anesthesia. Standard broadband monaural click stimulation is used on the ear tested while a masking noise 30-40 dB lower in intensity is used on the contralateral ear. The intensity of the click should be 65-70 dB above the click perception threshold, and clicks should be repeated at a rate of about 10 Hz.
Brainstem auditory evoked potentials are useful in estimating or aiding in the assessment of hearing loss. The most commonly used method for this is evoked response audiometry. The frequency of stimulation is 50-70 Hz, and at least 3 different intensities should be used. Wave V latency shifts are used to estimate the amount of hearing loss.
In children, especially those younger than 2 years, the brainstem auditory evoked potential can be used to screen for those in whom auditory amplification might facilitate achievement of more normal speech and language development. However, some children with a normal brainstem auditory evoked potential have abnormal hearing; Kileny 24 showed middle latency abnormalities in some of these cases. Nevertheless, the role of brainstem auditory evoked potential is to identify patients who could benefit from a hearing aid. Obviously, with a normal brainstem auditory evoked potential, a hearing aid would not be useful for correcting the hearing loss.
Brainstem auditory evoked potential physiologic basis
Brainstem auditory evoked potentials predominantly activate the pathways in the brainstem that are ipsilateral to the side of click stimulation. In particular, lesions in the middle to upper pons tend to lead to ipsilateral brainstem auditory evoked potential abnormalities. The structures involved in the generation of brainstem auditory evoked potentials may be concerned more with sound localization than with hearing itself.
Whether nuclei, tracts, or both generate the peak latencies is not known. Currently, the generators are postulated to be as follows:
- Wave I – Action potential of cranial nerve (CN) VIII
- Wave II – Cochlear nucleus (and CN VIII)
- Wave III – Ipsilateral superior olivary nucleus
- Wave IV – Nucleus or axons of lateral lemniscus
- Wave V – Inferior colliculus
Factors influencing brainstem auditory evoked potentials
Factors influencing peak latencies of brainstem auditory evoked potentials include the following:
- Patient age
- Patient sex
- Auditory acuity stimulus repetition rate
- Stimulus intensity
- Signal polarity
Rarefaction (ie, movement of the earphone diaphragm away from the eardrum) produces an increase in wave I amplitude. In severe hearing loss, all waveforms may be delayed, wave I may be absent with waves II through V delayed, or all waveforms may be absent. Note that in patients with hearing loss, brainstem auditory evoked potentials still can be obtained to assess central conduction time by increasing stimulation intensity.
Kern et al 25 studied the effects of insulin-induced hypoglycemia on the auditory brainstem response (ABR) in humans. Auditory brainstem responses were examined in 30 healthy men during euglycemia and after 20 minutes and 50 minutes of steady-state hypoglycemia of 2.6 mmol/L induced with insulin. Hypoglycemia increased interpeak latencies III-V and I-V, whereas changes in the latency of wave I were not significant.
Brainstem auditory evoked potentials are very resistant to alteration by anything other than structural pathology in the brainstem auditory tracts. They are not significantly affected by barbiturate doses sufficient to render the electroencephalogram (EEG) “flat” (ie, isoelectric) or by general anesthesia, though Garcia-Larrea et al 26 reported brainstem auditory evoked potential loss with combined lidocaine and thiopental infusion.
Disorders of the peripheral vestibular system do not affect brainstem auditory evoked potential. Thus, 21 patients who had labyrinthine diseases (ie, Ménière disease, labyrinthitis, vestibular neuronitis) had no brainstem auditory evoked potential interwave latency abnormalities in terms of the limits employed for clinical neurologic purposes.
Brainstem auditory evoked potential technical aspects
An electrode is placed on each ear lobe and at Cz. The first 10 msec are averaged; 2000-4000 responses may be averaged. At least 2 separate trials should be performed. The recording montage is at least (and usually) a 2-channel montage: channel 1 is from the ipsilateral ear to the vertex, and channel 2 is from the contralateral ear to the vertex. Because of relative vertex positivity, the waveforms are recorded as upward deflections. The normal response is a series of waveforms within a 10-msec time window.
Clinically, the first 5 waves are used, with more significance placed on waves I, III, and V. Peak and interpeak latencies are measured, side-to-side differences are calculated, and wave I-V ratios may be used. Audiometry is very helpful and should be done within a reasonable interval after the brainstem auditory evoked potential study. This helps delineate any hearing loss that might influence the test results. Hearing loss in the 2000- to 4000-Hz frequency range is especially important in that it may delay the brainstem auditory evoked potential.
Recording a neonatal brainstem auditory evoked potential is technically different from recording an adult brainstem auditory evoked potential. The skin of a newborn is very sensitive; accordingly, special nonallergenic tape should be used to fix the electrode, and collodion or other irritant chemicals are to be avoided. To avoid collapse of the earlobe and obstruction of the auditory canal in premature babies, the earphone should be held slightly above the ear.
The earphone is best held by hand, and the recording preferably should be performed with the neonate asleep. This helps reduce those high-frequency EEG components that might interfere with brainstem auditory evoked potential recording. Because of the slower response, the sweep should be set at 15-20 msec and the low-frequency cutoff filter at 20-30 Hz.
Brainstem auditory evoked potential clinical utility
The most common uses of the brainstem auditory evoked potential are in multiple sclerosis (MS) and in acoustic neuroma. It is a useful screening test, though it has some limitations; magnetic resonance imaging (MRI) may be preferable when a small lesion is under consideration.
Increased I-III interpeak latency indicates a lesion from CN VIII to the superior olivary nucleus, whereas increased III-V interpeak latency suggests a lesion from the superior olivary nucleus to the inferior colliculus ipsilateral to the ear stimulated. Intraoperative monitoring during cerebellopontine angle tumor surgery may be useful in helping the surgeon preserve as much function as possible.
Cerebellopontine angle lesions (acoustic neuromas)
The brainstem auditory evoked potential may be abnormal when audiometry fails to disclose a lesion. The characteristic findings are increased I-V and increased I-III interpeak latencies ipsilateral to the lesion. Meningiomas and other cerebellopontine angle tumors may not produce any abnormalities until they are large enough to be externally compressive.
Demyelinating disease
An abnormal response may be seen with higher frequency in symptomatic patients with demyelinating disease. Sometimes, however, a positive test may be recorded in the absence of clinical brainstem symptoms.
Migraine
Zgorzalewicz 27 found significant prolongation of waves III and IV in children and adolescents with migraine, a finding that supports the idea that the brainstem contributes to the pathologic mechanism of migraine.
Multiple sclerosis
Brainstem auditory evoked potential evaluation should be considered if the clinical symptoms implicate a lesion outside the brainstem. In this case, an abnormal brainstem auditory evoked potential would further support the diagnosis of MS. If, however, the clinical sign (eg, diplopia) points to the brainstem, a brainstem auditory evoked potential abnormality is merely confirmatory. In various studies, about 20% of the patients tested for a second lesion have an abnormal brainstem auditory evoked potential, and about half of these go on to develop MS in the next 1-3 years.
Purves et al 28 reported that pattern-shift visual evoked potentials were abnormal in 45% of patients without brainstem signs, somatosensory evoked potentials (SEPs) were abnormal in 35%, and brainstem auditory evoked potentials were abnormal in 14%. When the 3 modalities were considered together, 97% of patients with definite MS, 86% of patients with probable MS, and 63% of patients with possible MS had abnormal findings on at least 1 of these tests. Similar findings were reported by Ferrer et al 29.
Kjaer 30 reported a 38% rate of abnormal brainstem auditory evoked potentials in MS patients with silent lesions, whereas 50% of these patients had an abnormal visual evoked potential and only 13% an abnormal somatosensory evoked potential (SEP). Kjaer 31 also reported 22 patients with only spinal symptoms, 55% of whom showed an abnormal brainstem auditory evoked potential.
Chiappa 32 found that the brainstem auditory evoked potential was positive in 21% of clinically unsuspected cases of MS. Most authors have concluded that of the 3 tests, brainstem auditory evoked potential yields the smallest percentage of patients; however, it still adds to the detection rate because it is abnormal in a different subset of patients.
Brainstem tumor
Bilateral prolongation of latencies and interpeak latencies may be seen. Gordon et al 33 evaluated the efficacy of auditory brainstem response as a screening test for small acoustic neuromas by assessing the diagnostic sensitivity of brainstem auditory evoked potential in these tumors. Patients with surgically proven acoustic neuromas underwent preoperative brainstem auditory evoked potential tests within 2 months of surgery. A result was considered abnormal if the interaural wave I-V latency difference exceeded 0.2 msec, the absolute wave V latency was abnormally prolonged, or waveform morphology was abnormal or absent.
Of the 105 patients, 92 (87.6%) had an abnormal brainstem auditory evoked potential result, and 13 (12.4%) had completely normal waveforms and wave latencies 33. Of the 18 patients who had tumors larger than 2 cm, 12 had tumors 2.5 cm or larger and 6 had tumors between 2.1 and 2.4 cm; all 18 had abnormal brainstem auditory evoked potentials. Of the 29 patients with tumors between 1.6 and 2 cm, 25 (86%) had abnormal brainstem auditory evoked potentials. Of the 45 patients with tumors between 1 and 1.5 cm, 40 (89%) had an abnormal response. Of the 13 with tumors 9 mm or smaller, only 9 (69%) had an abnormal auditory brainstem response finding.
These data show that brainstem auditory evoked potential sensitivity decreases with decreasing tumor size 33. Therefore, MRI scanning is the preferred study in this setting because the accuracy for detection of tumors smaller than 1 cm through brainstem auditory evoked potential testing is 70%. Nevertheless, brainstem auditory evoked potential studies are useful in patients who have implanted medical devices (eg, pacemakers) that prevent MRI scanning.
Meningomyelocele
Taylor et al 34 studied brainstem auditory evoked potential and visual evoked potential in 47 infants with meningomyelocele in an effort to determine whether evoked potentials reflect early neurologic status and whether brainstem auditory evoked potentials and visual evoked potentials have prognostic value for neurologic outcome. The infants, aged 1 day to 3 months, were tested while still in hospital after the meningomyelocele repair.
Normal brainstem auditory evoked potentials were found in 41% of the patients and normal visual evoked potentials in 62%. brainstem auditory evoked potentials were abnormal in 9 infants studied who had symptomatic Arnold-Chiari malformation; visual evoked potentials were abnormal in only 55% of symptomatic infants. Visual evoked potentials did not appear to be sensitive enough to have prognostic value in these infants. Nevertheless, brainstem auditory evoked potentials were consistently abnormal in patients with symptomatic Arnold-Chiari malformation and showed a positive predictive value of 88% and an accuracy of 84% in predicting central neurologic sequelae.
Brainstem stroke
The response to brainstem auditory evoked potential studies is variable in cases of brainstem stroke; some lesions cause abnormal latencies, and some do not (eg, negative brainstem auditory evoked potential in lateral medullary syndrome).
Respiratory insufficiency after encephalitis
Schwarz et al 35 showed prolonged interpeak latencies (I-III, I-V, III-V, IV-V) and delayed absolute latencies of waves II, III, V, and I, at least on 1 side, in the brainstem auditory evoked potential. The auditory pathways are near the respiratory control centers in the brainstem; therefore, the electrophysiologic abnormalities of wave III and the IV-V complex may be a reflection of the disturbed central control of ventilation.
Outcome prediction in coma
Brainstem auditory evoked potential can be done while the patient is sedated. It can be used as a prognostic indicator. Survival is unlikely in the absence of brainstem auditory evoked potential. The brain-dead patient invariably has abnormal brainstem auditory evoked potentials—either the absence of all waveforms or the presence of wave I and the absence of all subsequent waveforms.
Brainstem auditory evoked potential and somatosensory evoked potential (SEP) studies were performed within 72 hours of admission in 127 children with severe head injury to predict the outcome of posttraumatic coma (brain death or survival). On first assessment, 50 comatose children had normal brainstem auditory evoked potential and somatosensory evoked potential (SEP); 78% survived and 22% deteriorated and died. Of the 45 who had abnormal findings; 69% improved and survived, whereas 31% deteriorated and died. All 32 children who did not have recordable brainstem auditory evoked potential and somatosensory evoked potential (SEP) died. These data suggest that brainstem auditory evoked response (BAER) is useful for predicting neurologic outcome in this setting.
Outcome prediction in perinatal asphyxia
In a study of 78 children with perinatal asphyxia who were screened with brainstem auditory evoked potentials, only 37 of the 78 developed neurodevelopmental deficits, consisting of static encephalopathy or developmental delay 36. Brainstem auditory evoked potential abnormalities were seen in 40.5% of these patients and demonstrated a specificity of 87.8%. With these findings, brainstem auditory evoked potentials may help predict adverse neurodevelopmental outcomes after perinatal asphyxia.
Childhood speech disorders
Maassen et al 37 found that children with speech and language deficit showed abnormal auditory evoked potentials in 95% of the study group.
Dementia
P300 long-latency auditory evoked potentials have been considered for using in evaluation for Alzheimer disease. In Alzheimer disease, the posterior scalp components are selectively lost. This test was also found to be strongly correlated with hypometabolism on positron emission tomography in this disease population. Although the test shows promise, its findings are considered nonspecific and nondiagnostic and are not limited to Alzheimer dementia 38.
Somatosensory evoked potentials
The actual somatosensory evoked potential is considered to be the result of summated effects of action potentials and synaptic potentials in a volume conductor 39. The short-latency somatosensory evoked potential (SLSEP) is considered to be generated from volleys traversing the large-fiber sensory system (ie, the posterior columns and medial lemnisci). Studies have shown that simply changing the size and shape of the volume conductor can create voltage differences at the surface.
Somatosensory evoked potentials physiologic basis
Large-diameter group Ia fibers and group II cutaneous afferents are primarily responsible for somatosensory evoked potentials. Because of the ease of delivery and quantification, electrical stimulation is typically used, though other types of sensory stimuli have been tried with success. When a mixed nerve is stimulated, Ia muscle afferents are activated. In the spinal cord, the dorsal columns are mainly responsible for conduction of the activity that generates the somatosensory evoked potential. In the brain, the lemniscal and thalamocortical pathways are involved. Extralemniscal pathways also may play a role.
Drews et al 40, in a study investigating the contribution of group I muscle afferent activation to the production of H reflexes and somatosensory evoked potential in humans, found that in most subjects, H reflex was correlated with somatosensory evoked potential size. If 2 identical stimuli were applied to the posterior tibial nerve with an interval of 1 sec, the second H reflex was 30% smaller than the first one. The corresponding somatosensory evoked potentials were reduced only slightly. Postactivation depression presumably results from intrinsic properties of synapses of group I muscle afferents.
In this study, electrical stimuli were applied to the tibial nerve in the popliteal fossa to study how the information is transferred from group I muscle afferents to motor neurons and to the somatosensory cortex 40. For control purposes, identical stimuli were applied to the skin. The somatosensory evoked potential evoked by skin stimulation alone had a peak latency that was 5 msec longer than the somatosensory evoked potential to transcutaneous nerve stimulation. The threshold intensity to evoke an H reflex was at least twice as high as the threshold for an somatosensory evoked potential.
The generators of median nerve somatosensory evoked potential are as follows:
- Erb point – Brachial plexus
- N11, N13 – Dorsal column, nucleus cuneatus
- P14 – Medial lemniscus
- N18 – Subcortical
- N20 – Primary sensory cortex
- P22 – Primary motor cortex
Buchner et al 41 researched the origin of P16 of the median nerve somatosensory evoked potential. After median nerve stimulation, several monophasic peaks were recorded at the scalp in the 15- to 18-msec time range. Source analysis using 3 different methods modeled a source near the center of the head with an orientation toward the activated hemisphere and a peak activity at 16 msec after the stimulus. Magnetic recordings detected no signals in this time range, which confirmed that the source had a subcortical location.
From dipole localization, the exact origin of the P16 source could not be assigned to either the subthalamic level or the thalamocortical radiation, because of the limited spatial resolution at the center of the spherical head model. An estimate of the conduction velocity of the medial lemniscus suggested a subthalamic origin 41. The P16 source was preserved in 2 patients with a lesion of the thalamocortical radiation and the ventral thalamus. Further evidence for a subthalamic location was derived from the physical mechanisms generating far-field potentials.
The generators of tibial somatosensory evoked potential are as follows:
- N22 – Dorsal gray and root entry zone at lumbosacral spine
- N29 – Nucleus gracilis
- P31 – Brainstem
- N34 – Brainstem
- P37 – Primary sensory cortex
Factors influencing somatosensory evoked potentials
- Age, height, and limb length
Vaney et al 42, in a study examining the role of physical parameters (including height, age, and upper limb length) in relation to the median nerve somatosensory evoked potential, concluded that somatosensory evoked potential studies might be affected by such parameters. The authors recorded somatosensory evoked potentials after median nerve stimulation and identified the following 3 major positive and negative peaks 42:
- P1 (16 msec), N1 (20 msec)
- P2 (28 msec), N2 (33 msec)
- P3 (43 msec), N3 (50 msec)
N1 and P1 were correlated significantly with height and limb length.
- Sleep stage
Noguchi et al 43, in a study that evaluated the changes of frontal and parietal somatosensory evoked potential in the awake state and compared them with somatosensory evoked potentials in different stages of sleep in 10 healthy adult subjects, found that frontal and parietal somatosensory evoked potential components were affected differently as sleep stages progressed. The amplitudes of frontal components were increased in sleep, whereas the amplitudes of parietal components were decreased.
The most discordant changes occurred in stages III/IV. The amplitudes for the frontal N18-P22-N30 complex and the parietal N20-P26-N32 complex increased from stage II to stages III/IV, whereas those for frontal N30-P40 and parietal N32-P40 decreased 43. P14 and frontal N18 latencies did not change significantly. The further latencies showed progressive prolongation from the awake state to slow-wave sleep.
The somatosensory evoked potential waveforms and latencies in rapid eye movement (REM) sleep were similar to those in the awake state 43. Amplitudes for frontal peaks remained slightly higher and amplitudes for parietal peaks slightly lower. Apparently both excitatory and inhibitory influences may mediate these sleep stage–related changes.
Note that significant neuropathy may be a complicating factor in acquiring somatosensory evoked potential, and the development of cortical potentials may be irregular, delayed, dispersed, and of poor amplitude. Generally, some of these difficulties can be overcome by increasing the number of samples collected.
- Water immersion
Water immersion therapy is used for cardiovascular, respiratory, and orthopedic conditions and also appears to benefit some neurologic patients, although little is known about how it affects neural activity. Sato et al 44 examined the effect of water immersion on median nerve short-latency somatosensory evoked potentials (SLSEP) and found that immersion significantly reduced short-latency somatosensory evoked potential (SLSEP) components known to originate in several cortical areas. This attenuation suggests that water immersion influences the cortical processing of somatosensory inputs.
Somatosensory evoked potential technical aspects
Stimulation techniques
Mixed nerve stimulation need not be supramaximal; a small twitch is sufficient. The duration of the stimulus is typically 200-300 msec, though some prefer longer stimuli. The repetition rate is usually 3 Hz, though some patients tolerate only 1-2 Hz. There is no change in somatosensory evoked potential until the 15-Hz stimulation frequency and no need for random stimuli (thought they may be needed to trigger off the electrocardiogram [ECG]).
Cutaneous nerve stimulation is used with the trigeminal and lateral femoral cutaneous nerves; dermatomal studies may be used to study segmental innervation disturbances. Dermatomal somatosensory evoked potentials may be technically difficult and therefore are not used routinely. Current MRI technology has largely replaced dermatomal somatosensory evoked potential studies in suspected radiculopathies and spinal cord lesions. In expert hands, however, the latter may have value in selected cases.
Recording and filtering
Surface or needle electrodes may be used. Cephalic bipolar or referential recording montages are appropriate. The general setting for filtering is 10-2500 Hz.
Upper-extremity channels are as follows:
- Channel 1 – C3/C4 referenced to Fz
- Channel 2 – Second cervical spinous process to Erb point
- Channel 3 – Fz to contralateral cortex
Lower-extremity channels are as follows:
- Channel 1 – L1, L3 to hip
- Channel 2 – Cz- Fpz
Normal upper-limb (median nerve) short-latency somatosensory evoked potential (SLSEP)
For this test, at least 3 channels are needed, but 4 channels are generally used, as follows:
- Erb point to Fz
- Nuchal midline C2 spine electrode to Fz
- Contralateral somatosensory area scalp electrode to Fz
Extra channel – Erb point to contralateral scalp; polarity is arranged so that active electrodes, Erb point, cervical, and cortical result in an upward deflection, representing negativity, with Fz used as the reference electrode for the upper-extremity EP; this electrode location on the scalp is not inactive and may attain electronegativity at 19-20 msec after stimulation that is equal or nearly equal to that at the contralateral cortical site and abolishes N19 in Cc-Fz derivation
N/P13 deflection
Most human clinicopathologic correlations suggest that the N/P13 waveform is generated in the lower medulla, probably in the dorsal column nuclei. The negativity recorded in the Fz-Cc derivation (N19) is the difference in negativity between the 2 electrode sites and thus is a “derived” waveform. N19 generally is believed to originate in the primary sensory cortex; however, good human clinicopathologic data are available to suggest that much of N19 is generated in the thalamus.
Chiappa et al 32 and Goldie et al 45 published data on a series of patients with instructive lesions, indicating that much of the negativity after 15 msec was generated in the thalamus. Regli and Despland 46 studied 50 patients who had acute infarctions and found N19 to be preserved in small lesions confined to the postcentral gyrus but absent in large lesions involving the underlying white matter and thalamus. Epileptogenic lesions of the sensory cortex often produce augmentation of P22 but not of N19.
These data suggest that the negative deflection appearing 16-19 msec after stimulation of the median nerve at the wrist is probably generated in the thalamus. The widespread negative activity seen at 25-30 msec with lower-limb stimulation (ie, the posterior tibial nerve at the ankle) is also believed to be generated in the thalamus. The subsequent positive activity (N/P37) is probably generated in the primary sensory cortex.
When lower-limb stimulation is employed, absence of the cauda equina potential (LP) suggests the presence of a lesion at or below that level. Technical considerations (eg, muscle artifact) also may obscure the cauda equina (LP) potential.
Clinical interpretation is based on the time interval between wave peaks. Registering a good Erb point or cauda equina (LP) potential is important for allowing measurement of central conduction times. Side-to-side comparisons of latencies can be useful in clinical diagnosis. Like brainstem auditory evoked potentials (BAEPs), somatosensory evoked potentials are fairly resistant to changes by widespread influences other than structural pathology in somatosensory tracts. Short-latency somatosensory evoked potentials (SLSEP) are not significantly altered by barbiturate doses sufficient to render the electroencephalogram (EEG) isoelectric or by general anesthesia.
Yiannikas et al 47 found that both somatosensory evoked potentials and electromyography (EMG) were of limited clinical utility in patients with cervical spondylosis who presented with symptoms but without neurologic signs of root compression. In patients with clinical signs of neurologic deficit, EMG and short-latency somatosensory evoked potentials (SLSEP) may be helpful or confirmatory.
In patients with clinical evidence of myelopathy, performing short-latency somatosensory evoked potentials (SLSEP) from both upper and lower extremities may be informative and may reveal or disprove a conduction block 47. Patients with syringomyelia often have abnormal central conduction time after upper-limb stimulation. In cervical spondylosis, an abnormal latency difference may be noted between brachial plexus (EP) and lower medullary (N/P13) components after upper limb stimulation.
Le Pera et al 48 documented selective abnormality of the N13 spinal somatosensory evoked potential to dermatomal stimulation in patients with cervical monoradiculopathy. Scalp somatosensory evoked potentials to dermatomal stimulation have proved to be only partially useful in the diagnosis of monoradiculopathy, mostly in cases without motor impairment.
The aim of the study was to test the sensitivity of the spinal N13 potential in uncovering lesions of single cervical roots 48. The investigators studied 5 patients suffering from cervical monoradiculopathy by using a technique that allowed specific recording of the genuine N13, which probably is generated by dorsal horn cells.
None of the patients showed signs of muscle impairment, and needle EMG findings were always normal 48. In 4 patients, the N13 somatosensory evoked potential was absent after stimulation of the dermatome corresponding to the damaged root, whereas both the lemniscal P14 and the cortical N20 components were normal. somatosensory evoked potential recorded after stimulation of upper-limb nerves showed no abnormality in any of these patients.
Apparently, loss of N13 potential after dermatomal stimulation could be due to deafferentation of dorsal horn neurons; the N13 is particularly sensitive to initial root compression. A montage allowing recording of the genuine N13 somatosensory evoked potential, therefore, can improve the sensitivity of dermatomal somatosensory evoked potential recording in patients with suspected cervical monoradiculopathies.
Somatosensory evoked potential clinical utility
Multiple sclerosis
The somatosensory evoked potential is positive in a number of patients with multiple sclerosis (MS). Abnormalities may include prolonged latencies or lack of development of the somatosensory evoked potential. Lower-extremity studies are more often abnormal because of the longer pathway. Upper-limb short-latency somatosensory evoked potentials (SLSEP) are abnormal in about 40-60% of MS patients; lower-limb short-latency somatosensory evoked potentials (SLSEP) have an abnormality rate of about 70%, presumably because of the greater length of white matter involved. American Academy of Neurology (AAN) guidelines suggest that somatosensory evoked potentials may be useful for diagnosing clinically silent MS lesions 49.
Eisen et al 50 reported abnormal results in trigeminal nerve stimulation in 41% of patients with MS. Abnormalities of trigeminal short-latency somatosensory evoked potentials (SLSEP) have also been reported in patients who had MS, Wallenberg syndrome, cerebellopontine angle tumors, trigeminal neurinomas, or meningeal sarcoidosis with facial paralysis.
Approximately one third of short-latency somatosensory evoked potential (SLSEP) abnormalities in MS are unilateral. One fifth of the bilateral abnormalities are asymmetric. Some patients with MS show loss of N/P13 with preservation and normal latency of N19; this pattern is difficult to explain if short-latency somatosensory evoked potential (SLSEP) generator sources are assumed to be linked in series.
Comparisons of somatosensory evoked potential, visual evoked potential (VEP), and brainstem auditory evoked potential (BAEP) have found that VEP testing and somatosensory evoked potential testing are about equally sensitive for revealing clinically unsuspected lesions and that brainstem auditory evoked potential is one half to one third less sensitive. The 3 tests should be viewed as complementary.
Lumbosacral disk disease
Sitzoglou et al 51 identified clear dermatomal somatosensory evoked potential abnormalities that correlated with radiculopathy in as many as 83% of the cases studied. Electromyography (EMG) yielded positive results in about 63% of the same subjects. Thus, dermatomal somatosensory evoked potentials may complement routine electrophysiologic testing of patients with radiculopathy and may provide a sensitive noninvasive technique for defining the level of disk prolapse.
This study included 24 patients with unilateral radiculopathy, all of whom had clinical signs and symptoms of disk prolapse and positive findings on neuroradiologic testing 51. The latency and the amplitude of the first positive somatosensory evoked potential waveform were measured, and both peripheral nerve conduction studies and EMG were performed.
Tsonidis et al 52 reported on the diagnostic value of dermatomal somatosensory evoked potentials by correlating the neurophysiologic data with clinical, neuroradiologic, and operative findings in 12 patients with surgically treated lumbar disk protrusion. The retrospective study disclosed correlation of the somatosensory evoked potentials after dermatomal stimulation and surgical findings in 83% of cases.
In this study 52, the diagnostic workup included a history, a neurologic examination, routine lumbar spine films, and computed tomography (CT) and magnetic resonance imaging (MRI) of the lumbar spine, in addition to neurophysiologic investigations, especially conduction velocity studies, and standard somatosensory evoked potentials and dermatomal somatosensory evoked potentials.
Cervical syringomyelia
Patients with cervical cord syrinxes may show abnormalities in median nerve somatosensory evoked potentials, indicating a lesion in the upper cervical cord, with relative sparing of lower-limb somatosensory evoked potentials. Wagner et al 53 monitored median nerve somatosensory evoked potentials intraoperatively in 28 patients with cervical or cervicothoracic syringomyelia. Analysis was focused on somatosensory evoked potential components: N13 (spinal cord), P14 (brain stem), and N20 (cortex).
N13 was absent in about 87% of patients because of a combined effect of syringomyelia and general anesthesia, and it did not recover 53. P14 showed a significant intraoperative latency increase in 2 patients; this was irreversible in 1 patient who had a postoperative worsening of sensory function. N20 showed no significant alterations. Pure motor deficits after surgery were not predicted by somatosensory evoked potential monitoring.
Thus, intraoperative P14 recordings helped to identify, and thereby prevent, injury to the dorsal columns, whereas N13 recording did not contribute to the intraoperative monitoring of spinal cord function in syringomyelia 53.
Spondylosis
Berthier et al 54 evaluated the effects of spondylosis on somatosensory evoked potential to ascertain its potential role in the preoperative assessment of cervical myelopathy in patients with MRI abnormalities and clinical findings of either segmental spinal cord or dorsal column dysfunction; using median and tibial nerve somatosensory evoked potential, they found no clear correlation between the severity of MRI abnormalities and that of clinical presentation or somatosensory evoked potential abnormalities.
In this study 54, some patients with MRI evidence of cervical cord impingement or intramedullary T2 hyperintensity showed normal somatosensory evoked potentials, whereas 8 of 13 patients without evidence of cord narrowing or T2 signal abnormality showed abnormal somatosensory evoked potentials. This discrepancy suggests that MRI and somatosensory evoked potentials may evaluate different aspects of the disease process.
On the basis of this study, the somatosensory evoked potential does not appear to be a good measure of anatomic deficit; however, spinal cord dysfunction detected by somatosensory evoked potential may be present in patients with an unremarkable MRI image. somatosensory evoked potential recording may therefore be useful in the preoperative assessment of symptomatic patients without MRI evidence of cervical cord compression.
Lyczak et al 55 reported abnormal somatosensory evoked potentials in 56% of patients with cervical myelopathy. The tibial nerve somatosensory evoked potentials were used, and abnormal central conduction times were observed. A significant reduction of the abnormalities occurred after the operation. Abnormal somatosensory evoked potentials before the procedure correlated with the severity of myelopathy, and improvement in somatosensory evoked potentials after the procedure correlated strongly with clinical improvement.
L5/S1 radiculopathy
Dumitru and Dreyfuss 56 applied strict criteria to define a group of 20 patients with a unilateral/unilevel L5/S1 radiculopathy, concluding that the clinical utility of both segmental and dermatomal somatosensory evoked potentials is dubious in patients with known unilateral/unilevel L5 and S1 nerve root compromise.
Castello et al 57 studied the effects of lumbar nerve root decompression on somatosensory evoked potential and demonstrated a significant improvement in postoperative somatosensory evoked potential latency in patients with lateral recess stenosis.
Intraoperative somatosensory evoked potential
The basis of intraoperative somatosensory evoked potential as a representative index of motor function is based on the fact that the vascular compromise that may cause motor dysfunction or loss also affects the lateral corticospinal tract and the dorsal spinocerebellar tract. In general terms, the regions of both motor and sensory pathways (lateral corticospinal tract and dorsolateral spinal cord and the alpha motor neurons) are served by the same vascular supply.
Intraoperative somatosensory evoked potential poses a special challenge and requires close cooperation with the operating room staff, as well as special equipment and careful attention to limit electrical noise. Careful checking of the ground is important, as is good shielding. The list of interfering factors is long and includes electrocautery equipment, nerve stimulators, and electric drills. Anesthetic agents and level of anesthesia also are interfering factors.
The following considerations should be kept in mind:
- Access to the patient is important, but the recording should not interfere with the operative procedure; a baseline recording should be done before the operation to establish the patient’s own normal value under less stressful circumstances
- The operative plan and what the surgeon expects from the monitoring must be discussed in advance; communication with the entire staff is important
- A note should be made of any preexisting diseases (eg, diabetes) that might interfere with successful somatosensory evoked potential testing
Stimulus pulse duration was evaluated by Luk et al 58, who found a duration of 0.3 msec to be the recommended choice for tibial somatosensory evoked potentials during intraoperative monitoring. Stimulus duration affected amplitude but not latency.
Somatosensory evoked potential monitoring during lumbosacral spinal stenosis is a well-known procedure. Weiss 59 found that even in routine surgical procedures, monitoring the somatosensory evoked potential along with EMG helps the surgical team to avoid neurogenic complications.
Recording motor evoked potential (MEP) and somatosensory evoked potential during thoracoabdominal aortic surgery to assess ischemia of the spinal cord has been valued by a number of authors as a means of lowering the risk of postoperative neurologic injury 60. Polo et al 61 found somatosensory evoked potential and motor evoked potential (MEP) to be useful in scoliosis surgery for assessing hypotension-related anoxic cord injury. Weigang et al 62 found somatosensory evoked potential monitoring to be useful in thoracoabdominal aortic endovascular stent grafting for prevention of spinal cord anoxia.
Arrington et al 63 used similar methods in pelvic fractures and acetabular surgery and found that combined somatosensory evoked potential and motor evoked potential (MEP) recording prevented damage to the sciatic nerve. Mills et al found that monitoring radial nerve somatosensory evoked potential was helpful in humeral nailing procedures. Schwartz et al 64 reported that monitoring ulnar somatosensory evoked potential was predictive of brachial plexus injury during surgery for correction of scoliosis. However, Deutsch et al 65 found that the false-negative rate was 9% for somatosensory evoked potential in anterior spinal surgical approaches.
Hyun et al 66 reviewed 85 cases of intraoperative monitoring using a combination of somatosensory evoked potential and MEP and found that the combination of somatosensory evoked potential and MEP had a higher sensitivity for detecting postoperative motor abnormalities than either modality alone did.
In this study 66, no postoperative neurologic abnormalities were seen when somatosensory evoked potential and MEP parameters remained stable. In 20 of the 85 cases, the MEP was abnormal, with or without somatosensory evoked potential changes. In 7 of these cases, the MEP recovered, and there were no resulting neurologic deficits. The remaining 13 patients did not have recovery of the MEP and had transient or permanent neurologic abnormalities. Four patients had somatosensory evoked potential changes with no MEP abnormalities that did not correlate to postoperative motor dysfunction.
Baba et al 67 reported the results of spinal cord EP monitoring for cervical and thoracic compressive myelopathy, finding that the preoperative EPs were not clearly helpful in predicting outcome but that early postoperative recovery of the EPs correlated with clinical improvement.
In this study 67, epidural spinal cord EPs were recorded in 95 patients undergoing surgery for cervical or thoracic compressive myelopathy. Abnormal spinal cord evoked potentials correlated significantly with the severity of spinal cord compromise and symptoms (eg, myelopathy). All of the thoracic myelopathy cases and 91% of the cervical myelopathy cases exhibited abnormal evoked potentials.
Davis et al 68 reported the use of ulnar nerve somatosensory evoked potentials to detect and prevent position-related neuropathy in the first pediatric patient in the world to undergo robotic-assisted thyroidectomy; they concluded that upper-extremity somatosensory evoked potentials should be routinely performed during this procedure. Patient positioning for this approach, as well as retraction during exposure, has the potential to result in postoperative brachial plexopathy similar to what is seen in other types of surgery.
Acute transverse myelitis
Few studies have evaluated the role of evoked potential changes in acute transverse myelitis. Misra et al assessed 10 patients who had lower-limb and upper-limb weakness by using detailed clinical, MRI, and neurophysiologic evaluation in conjunction with median and tibial somatosensory evoked potentials, upper- and lower-limb MEPs, and concentric needle EMG; they found that MRI and MEPs were useful in assessing clinical outcome but that somatosensory evoked potential played only a limited role 69.
Tuberculous myelopathy
Misra et al 70 investigated the value of somatosensory evoked potential and MEP changes in patients with Pott paraplegia and found that MEP and somatosensory evoked potential correlated with respective motor and sensory impairments, as well as with outcome.
Intracranial neoplasm
Rowed et al 71 used somatosensory evoked potential to identify the somatosensory cortex in an effort to help remove intracranial neoplasms and spare eloquent cortex. Somatosensory evoked potentials were recorded in response to contralateral median nerve stimulation from the cortical surface. Polarity reversal of short-latency somatosensory evoked potential (SLSEP) waves was used to identify the position of the central sulcus in 46 consecutive craniotomies for removal of metastases, gliomas, or meningiomas located in, near, or overlying sensorimotor cortex.
Somatosensory evoked potentials were recorded successfully in 43 of 46 cases (94%), with demonstration of polarity reversal in 42 of 43 cases (98%). somatosensory evoked potential localization led to modification of 14 of 42 procedures (33%), most frequently because of either displacement or involvement of sensorimotor cortex by tumor. Six patients (14%) developed new neurologic deficits, but none of these were attributable to incorrect identification of sensorimotor cortex. Routine use of this technique should be considered in all procedures for lesions located near the central sulcus 71.
Diabetic polyneuropathy
Somatosensory evoked potential is used in the context of diabetes mainly for purposes of confirmation, though it can also be used in selected cases when the central conduction time is needed. In general, the somatosensory evoked potential is prolonged in patients with clinically significant diabetic neuropathy. Although somatosensory evoked potential may be confirmatory, it is not used often, since routine nerve conduction studies readily yield the diagnosis in diabetic neuropathy.
Palma et al 72 studied somatosensory evoked potentials in individuals with non–insulin-dependent diabetes who had different degrees of neuropathy. The wrist–Erb point conduction velocity is decreased and the Erb point–N13 interpeak latencies are increased in patients with diabetes. The N11-N13, N13-N20, and N13-P22 interpeak latencies are within the normal range. The wrist–Erb point conduction velocity was proportional to the degree of neuropathy. The degrees of neuropathy have no influence on the EP-N13 interpeak latency.
Neurologic conditions
Giant somatosensory evoked potentials have been reported in cortical reflex myoclonus. Kofler et al 73 described enlarged cortical responses in 14 patients with progressive supranuclear palsy (PSP), attributing this finding to cortical hyperexcitability. Given that frontal lobe dementia is frequently present in patients with PSP, striatofrontal deafferentation and intracortical disinhibition may explain the increase in the size of the somatosensory evoked potential.
Ferri et al observed large-amplitude middle latency somatosensory evoked potentials in children with benign epilepsy of childhood with centrotemporal spikes. The mechanism of this is not known. An age-related decrease in amplitude and a lack of somatosensory evoked potential after age 12 years were noted. The findings may be interpreted as maturational changes.
Rinsho described a 66-year-old woman with corticobasal degeneration, cortical reflex myoclonus with related cortical spike, aphasia, clumsiness, and dystonia with rigidity. The right side was affected, and median nerve stimulation elicited a giant somatosensory evoked potential over the left scalp. He also described a 58-year-old woman with reflex myoclonus cortical myoclonic tremor and a giant cortical somatosensory evoked potential. In a study of 2 patients with corticobasal degeneration, 1 showed a giant somatosensory evoked potential and 1 did not.
Striano et al 74 studied a family with cortical tremor, myoclonus, and epilepsy and found giant somatosensory evoked potential potentials and enhanced long latency reflex I; genetic study revealed linkage on chromosome 2p.
Valeriani studied cortical myoclonus and concluded that the initial giant somatosensory evoked potential corresponded to physiologic potentials evoked in healthy subjects, whereas the late giant somatosensory evoked potential could be explained by the hyperpolarization that follows the postsynaptic excitation of the early components.
Tsuda et al 75 described Lafora body myoclonus with giant somatosensory evoked potential; positron-emission tomography revealed no increase in glucose metabolism in the somatosensory cortex.
Ugawa reported that the dipole responsible for the giant somatosensory evoked potential is localized in the sensory cortex. Some patients showed the localization for the dipole in the superior frontal gyrus in the paracentral lobule.
In a study by Schmitt et al, increased amplitude of somatosensory evoked potential was noted in all cases of ceroid lipofuscinosis.
Myoclonus and giant somatosensory evoked potential have been described in herpes simplex encephalitis. Triggs described giant somatosensory evoked potential with anterior spinal artery syndrome. The hypothesis has been advanced that this is due to lack of inhibition of the anterolateral inhibitory influences on the dorsal column medial lemniscal system.
Saitoh described giant somatosensory evoked potential in the syndrome of mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes (ie, MELAS syndrome). Lu reported dyssynergia cerebellaris myoclonica in 3 brothers in whom alcohol decreased both the myoclonus and the giant somatosensory evoked potential amplitude.
Vitamin B-12 deficiency
Both upper- and lower-limb somatosensory evoked potentials have been found to be abnormal in patients with vitamin B-12 deficiency, often showing either no components or only the peripheral EP peak. Puri et al 76 reported that serum vitamin B-12 level correlated well with the latencies of P37 and sural SNAP. On treatment, normalization of P100, MRI signal, and N20 and partial recovery of P37 latencies were seen at 6 months, 9 months, and 1 year, respectively 76.
Hyperthyroidism
Takahashi and Fujitani 77 studied median somatosensory evoked potentials in 14 patients and found that the N19-P23 amplitude was significantly higher in the patients than in the healthy control subjects.
Hypothermia
Guerit et al 78 studied the use of the somatosensory evoked potential to determine the optimal degree of hypothermia during circulatory arrest and found that the delay of somatosensory evoked potential reappearance after restoration of blood flow correlated significantly with cardiac arrest duration. They concluded that neurophysiologic monitoring of brainstem activity, as provided by somatosensory evoked potentials, enables determination of the optimal temperature for hypothermic circulatory arrest.
The investigators sequentially recorded subcortical (P14) and cortical (N20) somatosensory evoked potentials in 32 patients undergoing deep hypothermic circulatory arrest 78. Under normal hemodynamic conditions, hypothermia initially produced N20 disappearance at a mean nasopharyngeal temperature of 20.4 ± 2.6° C (range, 14.5-26.1°C) and P14 disappearance at a mean of 16.9 ± 2.0°C (range, 12.4-20.2°C). On rewarming, P14 reappeared at mean temperatures of 19.3 ± 4.0°C (range, 13.5-29.2°C) and N20 at a mean of 21.1 ± 4.1°C (range, 14.3-29.6°C) 78.
Neurologic recovery was uneventful in 23 patients; 5 patients presented with neurologic sequelae (minor or transient in 4; no recovery from anesthesia and death after 48 hours in 1), and 4 patients died during the operation 78. Of the 24 surviving patients in whom P14 disappeared when the hypothermia was deep enough to cause cardiac arrest (duration, 17-94 min), 23 had a normal neurologic outcome. By contrast, all surviving patients in whom cortical somatosensory evoked potentials disappeared at higher temperatures presented neurological sequelae.
Carotid endarterectomy monitoring
Duffy et al 79 compared the results of cerebral oximetry and somatosensory evoked potential monitoring in carotid endarterectomy. A 50% decrease in somatosensory evoked potential amplitude and a 10% decrease in regional oxygen saturation (rSO2) were considered clinically significant. Compared with somatosensory evoked potential, rSO2 had a sensitivity of 50% and a specificity of 96% 79. Clinical experience with this evolving technology is ongoing. Its role in neurovascular procedures has yet to be established.
Fiori and Parenti used 2-channel electroencephalography (EEG) and somatosensory evoked potential monitoring for selective shunting during 255 carotid endarterectomies for severe carotid stenosis; they found that computerized EEG is an easily interpretable method of monitoring and reveals rapidly developing cerebral ischemia but that severe somatosensory evoked potential changes can occur in spite of a normal EEG pattern when cerebral ischemia has a slow onset. somatosensory evoked potential monitoring is a slower recording method of but can yield a finer distinction of less severe cerebral ischemia.
Myotonic dystrophy
Patients with myotonic dystrophy have a prolonged interpeak latency between the Erb point and N/P13, indicating a sensory system involvement in this disorder.
Epidural sensory block
The existence of differential sensory block during epidural analgesia has been confirmed by some authors and disputed by others. Zaric et al 80 evaluated epidural sensory block by thermal stimulation, laser stimulation, and recording of the somatosensory evoked potential and found that recording of somatosensory evoked potential did not demonstrate significant difference between responses from the sites with the most superficial sensory block and those from the sites with the most intense sensory block.
In this study, the zone of anesthesia was smaller than the zone of any other investigated variable 80. The cranial spread of analgesia and motor block was lower than that of laser-assessed block. Partial block of laser perception and thermal perception lasted longer than analgesia and motor block. No consistent segmental or temporal differences were found between the thermal test and laser methods.
During epidural block, prolongation of latencies and reduction in amplitudes of somatosensory evoked potential produced at the most cranial analgesic dermatome did not differ significantly from those produced at the anesthetic dermatome 80. No differential block of small nerve fibers was found during epidural analgesia by thermal test and argon laser stimulation.
Postanoxic coma
Zandbergen et al 81 found short-latency somatosensory evoked potential (SLSEP) and neuron-specific enolase (NSE) to be correlated with unfavorable outcomes. Patients who were unconscious at 72 hours and had an abnormal short-latency somatosensory evoked potential (SLSEP) had poor outcomes. Even after 24 hours, the predictive value of the short-latency somatosensory evoked potential (SLSEP) was very strong.
Cardiac arrest
Wijdicks et al 82 reviewed several studies evaluating the use of somatosensory evoked potentials and prognosis after cardiac arrest and found that the absence of N20 on bilateral somatosensory evoked potentials of the median nerve was associated with negative outcome in terms of patient recovery. The false-positive rate for this finding was 0.7%. The presence of an N20 response did not correlate with a positive or negative outcome. Accordingly, the authors concluded that somatosensory evoked potentials can predict poor neurologic outcomes accurately in this population when evaluated between days 1 and 3 after cardiac arrest 82.
Stroke
Tzvetanov et al 83 attempted to answer the predictive value of median nerve somatosensory evoked potential in the early phase of stroke; the results were mixed.
Maturational changes with children
Myelination occurs earlier in the peripheral nerves than in the central pathways. Consequently, changes are noted in somatosensory evoked potentials seen as children mature. These changes are seen in the latency and morphology of waveforms. As with peripheral nerve conduction studies, the conduction velocities are decreased and do not reach adult values until age 3-4 years for peroneal nerve stimulation.
With respect to the somatosensory evoked potential waveforms, the mean Erb point latency will not change with maturation. Central conduction times are increased in infants and decrease as individuals mature. The morphology is comparable to that of cortical waveforms, which are broader in the young and decrease with age.
Finally, it is important to understand when somatosensory evoked potentials can be reliably recorded in infants. The tibial somatosensory evoked potential is absent in patients younger than 31 weeks’ gestational age but is present in 50% of newborns. The median nerve somatosensory evoked potential is reported to be present in 66-85% of term infants. Therefore, if the somatosensory evoked potential is abnormal, serial somatosensory evoked potentials would be warranted because of the variable presence of the response in this group 84.
- Clinical Utility of Evoked Potentials. https://emedicine.medscape.com/article/1137451-overview[↩][↩][↩]
- Ikeda H, Nishijo H, Miyamoto K, et al. Generators of visual evoked potentials investigated by dipole tracing in the human occipital cortex. Neuroscience. 1998 Jun. 84(3):723-39.[↩][↩]
- Brigell M, Kaufman DI, Bobak P, et al. The pattern visual evoked potential. A multicenter study using standardized techniques. Doc Ophthalmol. 1994. 86(1):65-79.[↩][↩][↩]
- Abboud S, Bar L, Rosenfeld M, Ring H, Glass I. Left-right asymmetry of visual evoked potentials in brain-damaged patients: a mathematical model and experimental results. Ann Biomed Eng. 1996 Jan-Feb. 24(1):75-86.[↩]
- Ipata A, Girelli M, Miniussi C, et al. Interhemispheric transfer of visual information in humans: the role of different callosal channels. Arch Ital Biol. 1997 Mar. 135(2):169-82.[↩]
- Yuksel A, Sarslan O, Devranoglu K. Effect of valproate and carbamazepine on visual evoked potentials in epileptic children. Acta Paediatr Jpn. 1995 Jun. 37(3):358-61.[↩]
- Trip SA, Schlottmann PG, Jones SJ. Optic nerve atrophy and retinal nerve fibre layer thinning following optic neuritis: Evidence that axonal loss is a substrate of MRI-detected atrophy. Neuroimage. 2006 Jan 26.[↩]
- Sannita WG, Fatone M, Garbarino S, et al. Effects of physiological changes of serum glucose on the pattern-VEP of healthy volunteers. Physiol Behav. 1995 Nov. 58(5):1021-6.[↩]
- Baumgartner J, Epstein CM. Voluntary alteration of visual evoked potential. Ann Neurol. 1982. 12:476.[↩]
- Szabela DA, Loba J, Palenga-Pydyn D. [The picture of visual evoked potentials in type 2 diabetes mellitus]. Klin Oczna. 2005. 107(7-9):498-501.[↩]
- Halatek T, Sinczuk-Walczak H, Rydzynski K. Prognostic significance of low serum levels of Clara cell phospholipid-binding protein in occupational aluminium neurotoxicity. J Inorg Biochem. 2005 Sep. 99(9):1904-11.[↩]
- Halatek T, Sinczuk-Walczak H, Szymczak M. Neurological and respiratory symptoms in shipyard welders exposed to manganese. Int J Occup Med Environ Health. 2005. 18(3):265-74.[↩]
- Thurtell MJ, Bala E, Yaniglos SS, Rucker JC, Peachey NS, Leigh RJ. Evaluation of optic neuropathy in multiple sclerosis using low-contrast visual evoked potentials. Neurology. 2009 Dec 1. 73(22):1849-57.[↩]
- Trauzettel-Klosinski S, Diener HC, Dietz K, et al. The effect of oral prednisolone on visual evoked potential latencies in acute optic neuritis monitored in a prospective, randomized, controlled study. Doc Ophthalmol. 1995-96. 91(2):165-79.[↩]
- Elvin A, Andersson T, Soderstrom M. Optic neuritis. Doppler ultrasonography compared with MR and correlated with visual evoked potential assessments. Acta Radiol. 1998 May. 39(3):243-8.[↩]
- Atilla H, Tekeli O, Ornek K. Pattern electroretinography and visual evoked potentials in optic nerve diseases. J Clin Neurosci. 2006 Jan. 13(1):55-9.[↩]
- Yukagawa E, Urano T, Nakahara M. Pattern-reversal Visual Evoked Potentials in Patients with Human T-lymphotropic Virus Type 1 Uveitis. Curr Eye Res. 2006. 31(1):37-42.[↩]
- Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005 Dec. 58(6):840-6.[↩]
- Kaplan PW, Tusa RJ, Shankroff J, et al. Visual evoked potentials in adrenoleukodystrophy: a trial with glycerol trioleate and Lorenzo oil. Ann Neurol. 1993 Aug. 34(2):169-74.[↩]
- Kesler A, Vakhapova V, Korczyn AD, Drory VE. Visual evoked potentials in idiopathic intracranial hypertension. Clin Neurol Neurosurg. 2009 Jun. 111(5):433-6.[↩]
- Shibata K, Osawa M, Iwata M. Pattern reversal visual evoked potentials in classic and common migraine. J Neurol Sci. 1997 Feb 12. 145(2):177-81.[↩][↩]
- Zgorzalewicz M. [Visual evoked potentials in children and school adolescents with migraine and tension-type headache. Clinical and neurophysiological correlations]. Neurol Neurochir Pol. 2005. 39(4 Suppl 1):S26-35.[↩]
- Clinical Utility of Evoked Potentials. https://emedicine.medscape.com/article/1137451-overview#a3[↩]
- Kileny P. Auditory evoked middle latency responses: current issues. Semin Hear. 1983. 4:403.[↩]
- Kern W, Kerner W, Pietrowsky R, et al. Effects of insulin and hypoglycemia on the auditory brain stem response in humans. J Neurophysiol. 1994 Aug. 72(2):678-83.[↩]
- Garcia-Larrea L, Artru F, Bertrand O, et al. Transient drug-induced abolition of BAEPs in coma. Neurology. 1988 Sep. 38(9):1487-9.[↩]
- Zgorzalewicz M. [The study of early auditory evoked potentials in primary headaches in children and adolescents and their pathogenetic implications]. Neurol Neurochir Pol. 2005. 39(4 Suppl 1):S17-25.[↩]
- Purves SJ, Low MD, Galloway J. A comparison of visual, brainstem auditory, and somatosensory evoked potentials in multiple sclerosis. Can J Neurol Sci. 1981 Feb. 8(1):15-9.[↩]
- Ferrer S, Jimenez P, Mellado L, Thieck E. [Clinical correlations and evoked potentials in 29 cases of definitive multiple sclerosis]. Rev Med Chil. 1993 Oct. 121(10):1154-60.[↩]
- Kjaer M. Evoked potentials in the diagnosis of multiple sclerosis. Electroencephalogr Clin Neurophysiol Suppl. 1987. 39:291-6.[↩]
- Kjaer M. Brain stem auditory and visual evoked potentials in multiple sclerosis. Acta Neurol Scand. 1980 Jul. 62(1):14-9.[↩]
- Chiappa KH. Pattern shift visual, brainstem auditory, and short-latency somatosensory evoked potentials in multiple sclerosis. Neurology. 1980 Jul. 30(7 Pt 2):110-23.[↩][↩]
- Gordon ML, Cohen NL. Efficacy of auditory brainstem response as a screening test for small acoustic neuromas. Am J Otol. 1995 Mar. 16(2):136-9.[↩][↩][↩]
- Taylor MJ, Boor R, Keenan NK, et al. Brainstem auditory and visual evoked potentials in infants with myelomeningocele. Brain Dev. 1996 Mar-Apr. 18(2):99-104.[↩]
- Schwarz G, Litscher G, Rumpl E, et al. Brainstem auditory evoked potentials in respiratory insufficiency following encephalitis. Int J Neurosci. 1996 Feb. 84(1-4):35-44.[↩]
- Jiang ZD, Liu XY, Shi BP, Lin L, Bu CF, Wilkinson AR. Brainstem auditory outcomes and correlation with neurodevelopment after perinatal asphyxia. Pediatr Neurol. 2008 Sep. 39(3):189-95.[↩]
- Maassen B, Pasman J, Nijland L. Clinical use of AEVP- and AERP-measures in childhood speech disorders. Clin Linguist Phon. 2006 Apr-May. 20(2-3):125-34.[↩]
- Nuwer M. Assessment of digital EEG, quantitative EEG, and EEG brain mapping: report of the American Academy of Neurology and the American Clinical Neurophysiology Society. Neurology. 1997 Jul. 49(1):277-92.[↩]
- Clinical Utility of Evoked Potentials. https://emedicine.medscape.com/article/1137451-overview#a4[↩]
- Drews H, Gerilovsky L, Studer LM, et al. Contribution of a Ia muscle afferent activation to the rise of H reflexes and somatosensory evoked potentials in man. Somatosens Mot Res. 1998. 15(2):109-17.[↩][↩]
- Buchner H, Waberski TD, Fuchs M, et al. Origin of P16 median nerve SEP component identified by dipole source analysis–subthalamic or within the thalamo-cortical radiation?. Exp Brain Res. 1995. 104(3):511-8.[↩][↩]
- Vaney N, Gupta S, Aggarwal S, et al. Median nerve somatosensory evoked potentials correlation with physical parameters. Indian J Physiol Pharmacol. 1996 Apr. 40(2):175-9.[↩][↩]
- Noguchi Y, Yamada T, Yeh M. Dissociated changes of frontal and parietal somatosensory evoked potentials in sleep. Neurology. 1995 Jan. 45(1):154-60.[↩][↩][↩]
- Sato D, Yamashiro K, Onishi H, Shimoyama Y, Yoshida T, Maruyama A. The effect of water immersion on short-latency somatosensory evoked potentials in human. BMC Neurosci. 2012 Jan 24. 13(1):13.[↩]
- Goldie WD, Chiappa KH, Young RR, et al. Brainstem auditory and short-latency somatosensory evoked responses in brain death. Neurology. 1981 Mar. 31(3):248-56.[↩]
- Regli F, Despland PA. Usefulness of short-latency SEPs in 50 cases with cerebrovascular lesions. Presented at 34th Annual Meeting, American Academy of Neurology, Washington DC. April 25-May 1. 1982.[↩]
- Yiannikas C, Shahani BT, Young RR. Short-latency somatosensory-evoked potentials from radial, median, ulnar, and peroneal nerve stimulation in the assessment of cervical spondylosis. Comparison with conventional electromyography. Arch Neurol. 1986 Dec. 43(12):1264-71.[↩][↩]
- Le Pera D, Valeriani M, Tonali P, et al. Selective abnormality of the N13 spinal SEP to dermatomal stimulation in patients with cervical monoradiculopathy. Neurophysiol Clin. 1998 Jun. 28(3):221-9.[↩][↩][↩]
- [Guideline] Gronseth GS, Ashman EJ. Practice parameter: the usefulness of evoked potentials in identifying clinically silent lesions in patients with suspected multiple sclerosis (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000 May 9. 54(9):1720-5.[↩]
- Eisen A, Purves S, Hoirch M. Central nervous system amplification: its potential in the diagnosis of early multiple sclerosis. Neurology. 1982 Apr. 32(4):359-64.[↩]
- Sitzoglou K, Fotiou F, Tsiptsios I, et al. Dermatomal SEPs–a complementary study in evaluating patients with lumbosacral disc prolapse. Int J Psychophysiol. 1997 Apr. 25(3):221-6. [↩][↩]
- Tsonidis C, Tsitsopoulos P, Sitzoglou K, et al. Comparison of dermatomal SEPs and operative findings in lumbar disc disease. Int J Psychophysiol. 1996 Dec. 24(3):267-70.[↩][↩]
- Wagner W, Perneczky A, Maurer JC. Intraoperative monitoring of median nerve somatosensory evoked potentials in cervical syringomyelia: analysis of 28 cases. Minim Invasive Neurosurg. 1995 Mar. 38(1):27-31.[↩][↩][↩]
- Berthier E, Turjman F, Mauguiere F. Diagnostic utility of somatosensory evoked potentials (SEPs) in presurgical assessment of cervical spondylotic myelopathy. Neurophysiol Clin. 1996. 26(5):300-10.[↩][↩]
- Lyczak P, Zabielski W. [Correlations between clinical course and investigation results of central conduction time of somatosensory potentials evoked from tibial nerves in patients with cervical myelopathy treated by surgery]. Pol Merkuriusz Lek. 2005 Aug. 19(110):162-5.[↩]
- Dumitru D, Dreyfuss P. Dermatomal/segmental somatosensory evoked potential evaluation of L5/S1 unilateral/unilevel radiculopathies. Muscle Nerve. 1996 Apr. 19(4):442-9.[↩]
- Castello PH, Place HM, Hemler DE, et al. Quantification of lumbar nerve root decompression using somatosensory-evoked potentials. J Spinal Disord. 1995 Dec. 8(6):444-50.[↩]
- Luk KD, Hu Y, Lu WW. Effect of stimulus pulse duration on intraoperative somatosensory evoked potential (SEP) monitoring. J Spinal Disord. 2001 Jun. 14(3):247-51.[↩]
- Weiss DS. Spinal cord and nerve root monitoring during surgical treatment of lumbar stenosis. Clin Orthop. 2001 Mar. (384):82-100.[↩]
- Shinzawa M, Yoshitani K, Minatoya K, Irie T, Ogino H, Ohnishi Y. Changes of motor evoked potentials during descending thoracic and thoracoabdominal aortic surgery with deep hypothermic circulatory arrest. J Anesth. 2011 Dec 27.[↩]
- Polo A, Tercedor A, Paniagua-Soto J. [Neurophysiological monitoring during scoliosis surgery using controlled hypotension]. Rev Esp Anestesiol Reanim. 2000 Oct. 47(8):367-70.[↩]
- Weigang E, Hartert M, Siegenthaler MP. Neurophysiological monitoring during thoracoabdominal aortic endovascular stent graft implantation. Eur J Cardiothorac Surg. 2006 Mar. 29(3):392-6.[↩]
- Arrington ED, Hochschild DP, Steinagle TJ. Monitoring of somatosensory and motor evoked potentials during open reduction and internal fixation of pelvis and acetabular fractures. Orthopedics. 2000 Oct. 23(10):1081-3.[↩]
- Schwartz DM, Drummond DS, Hahn M. Prevention of positional brachial plexopathy during surgical correction of scoliosis. J Spinal Disord. 2000 Apr. 13(2):178-82.[↩]
- Deutsch H, Arginteanu M, Manhart K, et al. Somatosensory evoked potential monitoring in anterior thoracic vertebrectomy. J Neurosurg. 2000 Apr. 92(2 Suppl):155-61.[↩]
- Hyun SJ, Rhim SC, Kang JK, Hong SH, Park BR. Combined motor- and somatosensory-evoked potential monitoring for spine and spinal cord surgery: correlation of clinical and neurophysiological data in 85 consecutive procedures. Spinal Cord. 2009 Aug. 47(8):616-22. [↩][↩]
- Baba H, Maezawa Y, Imura S, et al. Spinal cord evoked potential monitoring for cervical and thoracic compressive myelopathy. Paraplegia. 1996 Feb. 34(2):100-6.[↩][↩]
- Davis SF, Abdel Khalek M, Giles J, Fox C, Lirette L, Kandil E. Detection and prevention of impending brachial plexus injury secondary to arm positioning using ulnar nerve somatosensory evoked potentials during transaxillary approach for thyroid lobectomy. Am J Electroneurodiagnostic Technol. 2011 Dec. 51(4):274-9.[↩]
- Misra UK, Kalita J, Kumar S. A clinical, MRI and neurophysiological study of acute transverse myelitis. J Neurol Sci. 1996 Jun. 138(1-2):150-6.[↩]
- Misra UK, Kalita J. Somatosensory and motor evoked potential changes in patients with Pott”s paraplegia. Spinal Cord. 1996 May. 34(5):272-6.[↩]
- Rowed DW, Houlden DA, Basavakumar DG. Somatosensory evoked potential identification of sensorimotor cortex in removal of intracranial neoplasms. Can J Neurol Sci. 1997 May. 24(2):116-20.[↩][↩]
- Palma V, Serra LL, Armentano V, et al. Somatosensory evoked potentials in non-insulin-dependent diabetics with different degrees of neuropathy. Diabetes Res Clin Pract. 1994 Sep. 25(2):91-6.[↩]
- Kofler M, Muller J, Reggiani L. Somatosensory evoked potentials in progressive supranuclear palsy. J Neurol Sci. 2000 Oct 1. 179(S 1-2):85-91.[↩]
- Striano P, Madia F, Minetti C. Electroclinical and genetic findings in a family with cortical tremor, myoclonus, and epilepsy. Epilepsia. 2005 Dec. 46(12):1993-5.[↩]
- Tsuda H, Katsumi Y, Nakamura M. [Cerebral blood flow and metabolism in Lafora disease]. Rinsho Shinkeigaku. 1995 Feb. 35(2):175-9.[↩]
- Puri V, Chaudhry N, Goel S. Vitamin B12 deficiency: a clinical and electrophysiological profile. Electromyogr Clin Neurophysiol. 2005 Jul-Aug. 45(5):273-84.[↩][↩]
- Takahashi K, Fujitani Y. Somatosensory and visual evoked potentials in hyperthyroidism. Electroencephalogr Clin Neurophysiol. 1970 Dec. 29(6):551-6.[↩]
- Guerit JM, Verhelst R, Rubay J, et al. The use of somatosensory evoked potentials to determine the optimal degree of hypothermia during circulatory arrest. J Card Surg. 1994 Sep. 9(5):596-603.[↩][↩][↩][↩]
- Duffy CM, Manninen PH, Chan A, et al. Comparison of cerebral oximeter and evoked potential monitoring in carotid endarterectomy. Can J Anaesth. 1997 Oct. 44(10):1077-81.[↩][↩]
- Zaric D, Hallgren S, Leissner L, et al. Evaluation of epidural sensory block by thermal stimulation, laser stimulation, and recording of somatosensory evoked potentials. Reg Anesth. 1996 Mar-Apr. 21(2):124-38.[↩][↩][↩]
- Zandbergen EG, Hijdra A, Koelman JH. Prediction of poor outcome within the first 3 days of postanoxic coma. Neurology. 2006. 66(1):62-68.[↩]
- Wijdicks EF, Hijdra A, Young GB, Bassetti CL, Wiebe S. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006 Jul 25. 67(2):203-10.[↩][↩]
- Tzvetanov P, Rousseff RT. Predictive value of median-SSEP in early phase of stroke: a comparison in supratentorial infarction and hemorrhage. Clin Neurol Neurosurg. 2005 Oct. 107(6):475-81.[↩]
- Chiappa KH. Evoked Potentials in Clinical Medicine. 2nd ed. New York: Raven Press. 1990. 37-171, 196-197.[↩]