Vitamin A
Vitamin A is name of a group of fat-soluble compounds found as preformed vitamin A (retinol and its esterified form, retinyl ester) in animal products (such as fish, liver, dairy products, and eggs) and as provitamin A carotenoids (beta-carotene, alpha-carotene and beta-cryptoxanthin) in fruit and vegetables 1, 2, 3, 4, 5. Carotenoids are pigments that give yellow, orange, and red fruits and vegetables their color. Your body is able to convert some carotenoids into vitamin A. The three active forms of vitamin A in the body are retinol, retinal, and retinoic acid 6.
There are two different types of vitamin A 7.
- Preformed vitamin A (retinol and its esterified form, retinyl ester), is found in meat (especially liver), poultry, fish, eggs and dairy products.
- Provitamin A carotenoids (beta-carotene, alpha-carotene and beta-cryptoxanthin), is found in fruits, vegetables, and other plant-based products (oily fruits and red palm oil). The most common type of provitamin A carotenoids in foods and dietary supplements is beta-carotene (β-carotene). The body converts these plant pigments into vitamin A.
The human body is not able to produce vitamin A, and therefore, you need to obtain vitamin A from your diet either as preformed vitamin A or in the form of provitamin A carotenoids 8. There are more than 50 provitamin A carotenoids, but only beta-carotene (β-carotene), alpha-carotene (α-carotene), and beta-cryptoxanthin (β-cryptoxanthin) are present in significant amounts in the human diet 9. Beta-carotene (β-carotene) is the most abundant in the diet. Beta-carotene (β-carotene) is mostly ingested through red and orange vegetables and partially through the same colored fruits and green vegetables. In Europe, carrots, spinach, and tomato products are the main contributors to beta-carotene (β-carotene) intake, while beta-cryptoxanthin (β-cryptoxanthin) is most commonly taken from various citruses and citrus juices 10. Beta-cryptoxanthin (β-cryptoxanthin), as well as other carotenoids, occurs in plants both as free and esterified with fatty acids (lauric, myristic, palmitic), and these esters contribute to total vitamin A content due to their comparable bioavailability 11. Rich sources of cryptoxanthin or its esters include, in addition to the already-mentioned citruses (satsuma mandarins, tangerines, clementines, mineolas and oranges), persimmons, chili peppers and red peppers, papaya, sea buckthorn, loquat, mango and apricots 12, 13, 14, 15. Other sources of provitamin A carotenoids also include various medicinal plants and herbs, cereals, and specific vegetable oils (see Table 2 below).
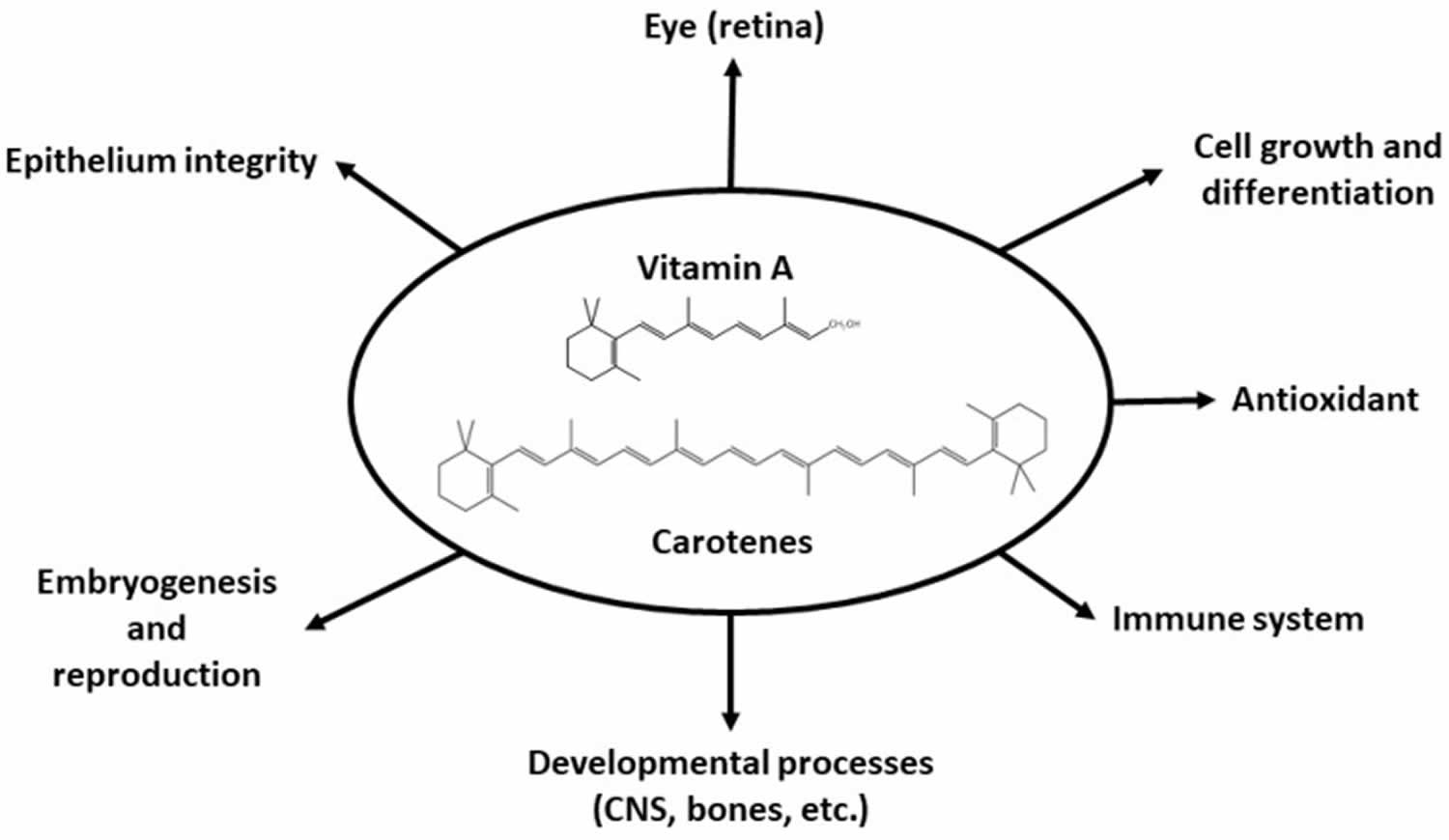
Vitamin A is important for normal vision, gene expression, the immune function, embryonic development, growth, cellular communication, and male and female reproduction 16, 17, 18. Vitamin A also helps your heart, lungs, kidneys, and other organs to work properly 19.
Vitamin A is critical for vision as an essential component of rhodopsin, a protein that absorbs light in the retinal receptors, and because it supports the normal differentiation and functioning of the conjunctival membranes and cornea 3, 4, 20. Vitamin A also supports cell growth and differentiation, playing a critical role in the normal formation and maintenance of the heart, lungs, kidneys, and other organs 3.
Both provitamin A and preformed vitamin A must be metabolized intracellularly to retinal and retinoic acid, the active forms of vitamin A, to support the vitamin’s important biological functions 3, 4. Provitamin A carotenoids are plant pigments that the body converts into vitamin A in the intestine 16. The main provitamin A carotenoids in the human diet are beta-carotene, alpha-carotene, and beta-cryptoxanthin 16. Other carotenoids in food, such as lycopene, lutein, and zeaxanthin, are not converted into vitamin A and are referred to as non-provitamin A carotenoids; they might have other important activities not involving vitamin A formation 16.
Both retinyl esters and provitamin A carotenoids are converted to retinol, which is oxidized to retinal and then to retinoic acid 3. Most of the body’s vitamin A is stored in the liver in the form of retinyl esters 16.
Normally, the liver stores 80 to 90% of the body’s vitamin A. To use vitamin A, the body releases it into the circulation bound to prealbumin (transthyretin) and retinol-binding protein (RBP). Beta-carotene and other provitamin carotenoids, contained in green leafy and yellow vegetables and deep- or bright-colored fruits, are converted to vitamin A. Carotenoids are absorbed better from vegetables when they are cooked or homogenized and served with some fat (eg, oils).
The body can convert beta-carotene into vitamin A to help meet these requirements. Although there is no Recommended Dietary Allowance (RDA) for beta-carotene, the National Institutes of Health Office of Dietary Supplements recommends eating five or more servings of fruits and vegetables per day, including dark green and leafy vegetables and deep yellow or orange fruits to get appropriate amounts of beta-carotene.
Retinol activity equivalents (RAE) were developed because provitamin A carotenoids have less vitamin A activity than preformed vitamin A; 1 microgram retinol = 3.33 IU vitamin A. Or expressed differently, 1 IU vitamin A = 0.3 mcg retinol
For dietary provitamin A carotenoids (β-carotene, α-carotene, and β-cryptoxanthin), retinol activity equivalents (RAEs) have been set at 12, 24, and 24 microgram (mcg), respectively. Using mcg RAE, the vitamin A activity of provitamin A carotenoids is half the vitamin A activity assumed when using μg retinol equivalents (μg RE) 19. This change in equivalency values is based on data demonstrating that the vitamin A activity of purified β-carotene in oil is half the activity of vitamin A, and based on recent data demonstrating that the vitamin A activity of dietary β-carotene is one-sixth, rather than one-third, the vitamin activity of purified β-carotene in oil. This change in bioconversion means that a larger amount of provitamin A carotenoids, and therefore darkly colored, carotene-rich fruits and vegetables, is needed to meet the vitamin A requirement.
Synthetic vitamin A analogs (retinoids) are being used increasingly in dermatology. The possible protective role of beta-carotene, retinol, and retinoids against some epithelial cancers is under study. However, risk of certain cancers may be increased after beta-carotene supplementation 21.
Retinol is the form of vitamin A that causes concern. In addition to getting retinol from their diets, some people may be using synthetic retinoid preparations that are chemically similar to vitamin A to treat acne, psoriasis, and other skin conditions. These retinoid preparations have been shown to have the same negative impact on bone health as dietary retinol. Use of retinoid medications in children and teens also has been linked to delays in growth.
Retinol and carotenoid levels are typically measured in plasma or serum because blood samples are easy to collect 16. However, these levels are not always reliable indicators of vitamin A status because they do not decline until vitamin A levels in the liver and other storage sites are almost depleted and because acute and chronic infections can decrease serum and plasma retinol concentrations 16. Most vitamin A is stored in the liver, so measuring vitamin A levels in the liver is the best way to assess vitamin A adequacy 16. In clinical studies, specialized research laboratories can measure liver vitamin A reserves indirectly using isotope-dilution or dose-response methods, in which plasma levels of retinol, a tracer surrogate, or both are measured over several days after the administration of vitamin A 16.
In clinical practice, plasma retinol levels alone can be used to document significant deficiency. In general, the following conversions can be used: serum retinol 1 micromole/L = 28.6 mcg/dL.
A serum or plasma retinol concentration of 20 mcg/dL (0.70 micromoles/L) or less frequently reflects moderate vitamin A deficiency, and a serum retinol level of 10 mcg/dL (0.35 micromoles/L) or less is considered an indicator of severe vitamin A deficiency 16.
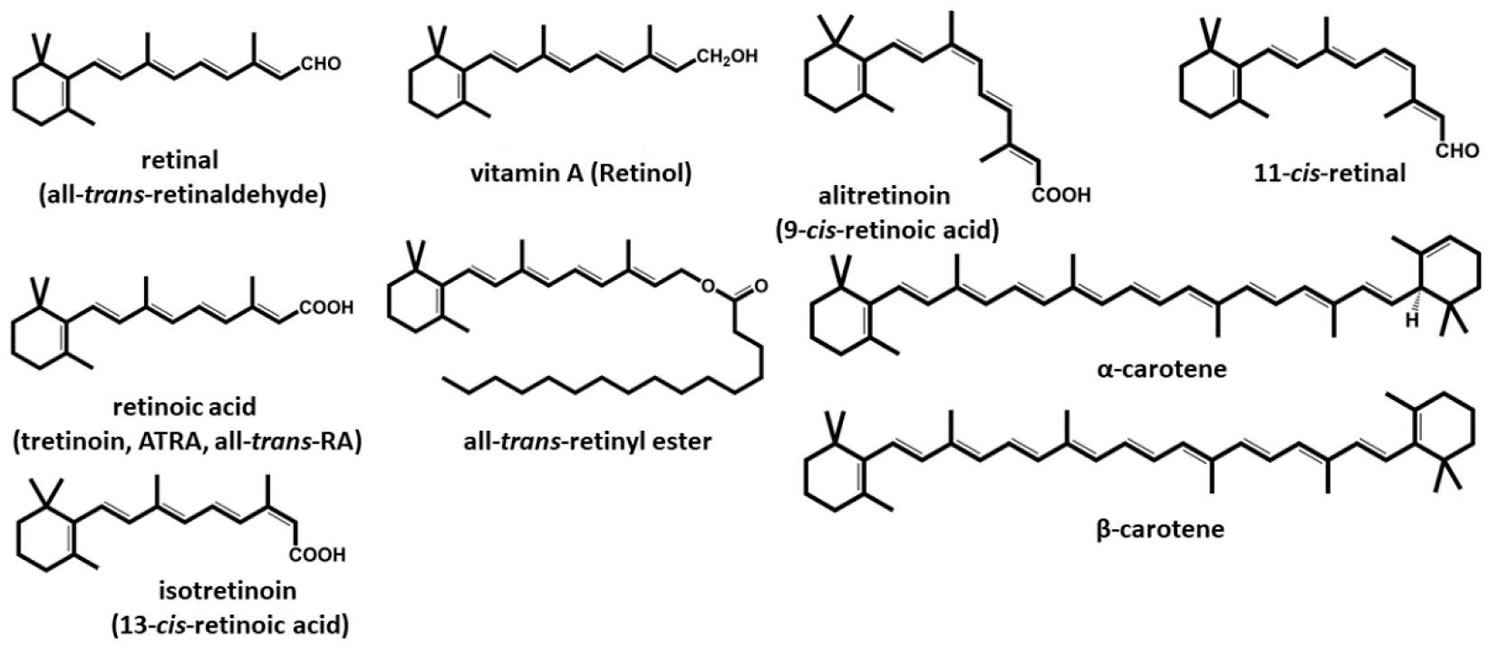
Figure 1. Vitamin A chemical structures
Footnote: Structures of vitamin A and retinoids
[Source 8 ]
Do high intakes of vitamin A increase the risk of osteoporosis?
Results from some prospective studies have suggested that long-term intakes of preformed vitamin A in excess of 1,500 mcg RAE/day (equivalent to 5,000 IU/day of vitamin A as retinol) were associated with reduced bone mineral density (BMD) and increased risk of osteoporotic fracture in older adults 22, 23, 24. However, other investigators failed to observe such detrimental effects on bone mineral density (BMD) and/or fracture risk 25, 26, 27. The recent meta-analysis of four prospective studies, including nearly 183,000 participants over 40 years of age, found that highest vs. lowest quintiles of retinol (preformed vitamin A) intake significantly increased the risk of hip fracture 28. Only excess intakes of retinol, not beta-carotene, were associated with adverse effects on bone health. Besides, the pooled analysis of four observational studies also indicated that a U-shaped relationship between circulating retinol and risk of hip fracture, suggesting that both elevated and reduced retinol concentrations in the blood were associated with an increased risk of hip fracture 28.
To date, limited experimental data have suggested that vitamin A (as all-trans-retinoic acid) may affect the development of bone-remodeling cells and stimulate bone matrix degradation (resorption) 29. Vitamin A may also interfere with the ability of vitamin D to maintain calcium balance 30. In the large Women’s Health Initiative (WHI) prospective study, the highest vs. lowest quintile of retinol intake (≥1,426 mcg/day vs. <474 mcg/day) was found to be significantly associated with increased risk of fracture only in women with the lowest vitamin D intakes (≤440 IU/day) 31.
Until supplements and fortified food are reformulated to reflect the current recommended dietary allowance (RDA) for vitamin A, it is advisable for older individuals to consume multivitamin supplements that contain no more than 2,500 IU (750 mcg) of preformed vitamin A (usually labeled vitamin A acetate or vitamin A palmitate) and no more than 2,500 IU of additional vitamin A as beta-carotene 6.
Recommended Dietary Allowance (RDA) is average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals.
Vitamin A functions
Vitamin A compounds are essential fat-soluble molecules predominantly stored in the liver in the form of retinyl esters (e.g., retinyl palmitate). When appropriate, retinyl esters are hydrolyzed to generate all-trans-retinol, which binds to retinol binding protein (RBP) before being released in the bloodstream. The all-trans-retinol/RBP complex circulates bound to the protein, transthyretin, which delivers all-trans-retinol to peripheral tissues 32. Vitamin A as retinyl esters in chylomicrons was also found to have an appreciable role in delivering vitamin A to extrahepatic tissues, especially in early life 33, 34.
Figure 2. Vitamin A physiological roles
[Source 8 ]Visual system and eyesight
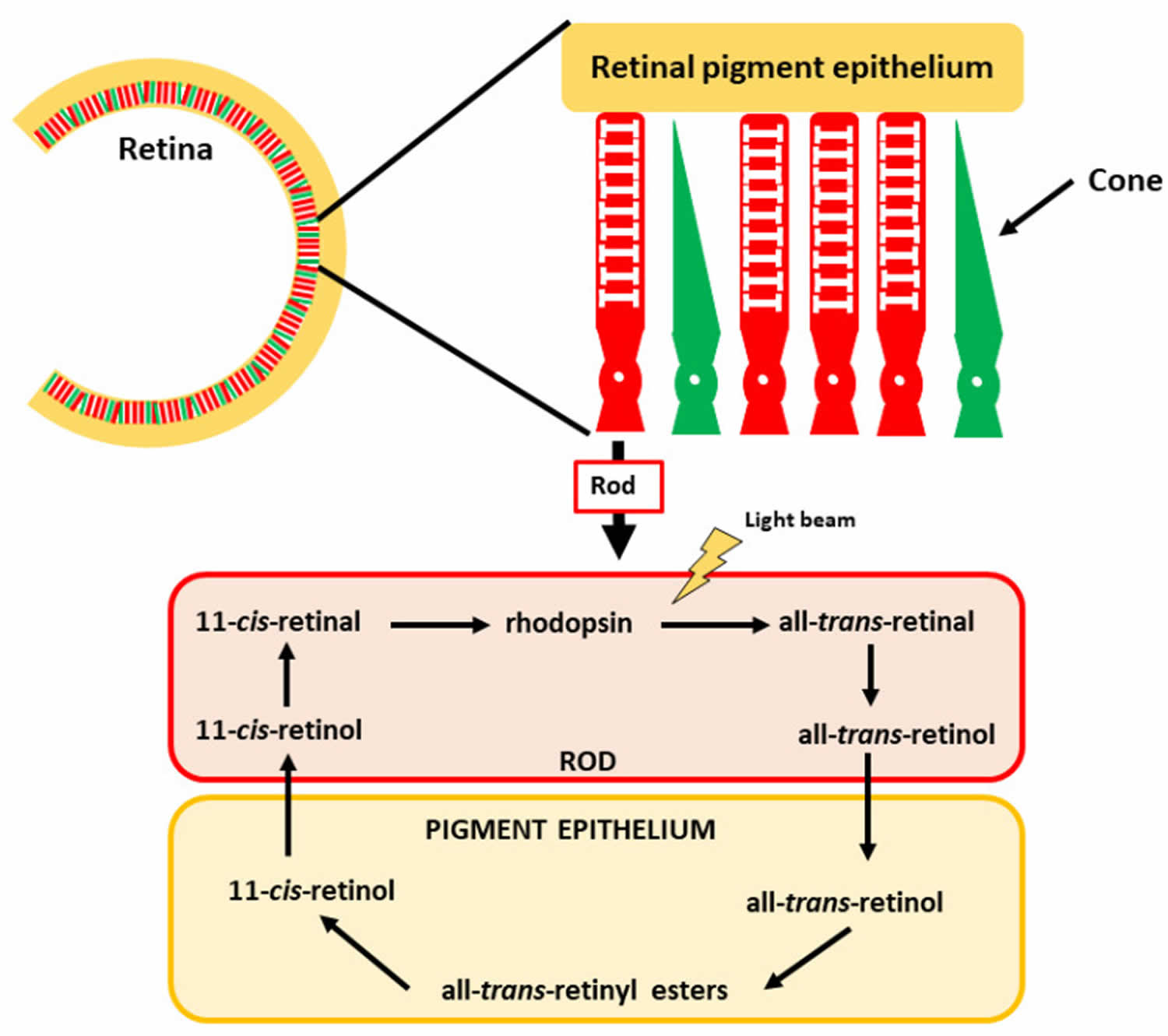
Located at the back of the eye, the retina contains two main types of light-sensitive receptor cells − known as rod and cone photoreceptor cells. Rods are sensitive to low light and hence are crucial for vision in dark situations (e.g., night vision), whereas cones are responsible for high-intensity light (color vision). Photons (particles of light) that pass through the lens are sensed by the photoreceptor cells of the retina and converted to nerve impulses (electric signals) for interpretation by the brain. All-trans-retinol is transported to the retina via the circulation and accumulates in retinal pigment epithelial (RPE) cells 35. Here, all-trans-retinol is esterified to form a retinyl ester, which can be stored. When needed, retinyl esters are broken apart (hydrolyzed) and isomerized to form 11-cis-retinol, which can be oxidized to form 11-cis-retinal. 11-cis-retinal can be shuttled across the interphotoreceptor space to the rod photoreceptor cell that is specialized for vision in low-light conditions and for detection of motion. In rod cells, 11-cis-retinal binds to a protein called opsin, a G-coupled protein receptor in the retina, to form the visual pigment rhodopsin (also known as visual purple), which is the crucial pigment for light perception 36, 37. Absorption of a photon of light catalyzes the isomerization of 11-cis-retinal to all-trans-retinal that is released from the opsin molecule. This photoisomerization triggers a cascade of events, leading to the generation of a nerve impulse conveyed by the optic nerve to the brain’s visual cortex. All-trans-retinal is converted to all-trans-retinol and transported across the interstitial space to the retinal pigment epithelial (RPE) cells, thereby completing the visual cycle. After this reaction, some all-trans-retinal can be transformed back into 11-cis-retinal, enabling recycling of this key molecule. The remaining all-trans-retinal can be transformed into retinol, which can be stored in the retinal pigment epithelial (RPE) cells to be later reused or converted into all-trans-retinal 38.
A similar cycle occurs in cone cells that contain red, green, or blue opsin proteins required for the absorption of photons from the visible light spectrum 32. Vitamin A is also essential for mammalian eye development 39. Therefore, because vitamin A is required for the normal functioning of the retina, dim-light vision, and color vision, inadequate retinol and retinal available to the retina result in impaired dark adaptation. In the severest cases of vitamin A deficiency, thinning and ulceration of the cornea leads to blindness (xerophthalmia) 40.
Figure 3. Vitamin A in vision and eyesight
Footnote: The retina comprises rod and cone photoreceptor cells, which mediate color and low light vision, respectively. The vitamin A derivative 11-cis-retinal is found in the rods, forming rhodopsin.
[Source 8 ]Regulation of gene expression
Vitamin A is an important factor in gene regulation 41, 42. This effect is exerted through interaction with nuclear receptors 8. Nuclear receptors are ligand-activated transcription factors that, upon ligand-binding, can modulate the target gene expression through direct interaction with DNA 8. Although 48 families of nuclear receptors have been described to date in humans, all members show a common structure consisting of a DNA-binding domain (DBD), a ligand-binding domain (LBD) and a hinge region that connects both structures. Ligand-binding domain (LBD) is usually a highly specific structure that recognizes the ligands for each type of receptor. These receptors can be activated both by endogenous molecules and/or xenobiotics 43.
Retinoids are known to interact with several families of nuclear receptors. In 1987, the first receptor with a high affinity for a retinoid was identified. It was named the retinoic acid receptor α (RARα) due to its ability to bind retinoic acid with a high-affinity 44, 45. This breakthrough discovery explained the mechanisms of some biological functions associated with retinoids. Since then, additional receptors have been described that interact with retinoids. These receptors are commonly called retinoid receptors. The main retinoid receptor families are RAR and retinoid X receptor (RXR), but a third retinoid-interacting nuclear receptor family has been described: RAR-related orphan receptor (ROR).
After the discovery of the first member of the retinoic acid receptor (RAR) family, RARα (NR1B1), two more RAR isoforms were successfully isolated and reported to interact with vitamin A derivates: RARβ (NR1B2) and RARγ (NR1B3) 46, 47. RARs strongly bind to retinoic acid, as well as to 9-cis-retinoic acid. The expression of these receptors is tissue-specific. RARα is widely distributed throughout the body, whereas isoform RARβ is predominantly in the brain, liver and kidneys. RARγ is highly expressed in the epidermis 48. RARs play different roles, both genomic and non-genomic. In general, they are involved in cell signaling. The non-genomic processes are mediated through phosphorylation processes 49 and the ability of RARα to regulate protein translation 50.
The second family of receptors with a high affinity for retinoids is the retinoid X receptor (RXR). This receptor represents the key for the functioning of retinoic acid receptor (RAR) and many other nuclear receptors since its presence is necessary for forming heterodimers and, subsequently, the transcriptional machinery. Retinoid X receptors (RXRs) were described for the first time several years later than RAR 51. Similar to RAR, RXR also presents three isoforms with different distribution within the body (α-NR2B1, β-NR2B2 and γ-NR2B3) 52. Whereas RXRα is mainly localized in the liver, lungs and intestines, RXRβ is ubiquitously distributed throughout the body and RXRγ is predominantly found in muscles and the brain 48. The three isoforms are important heterodimerization partners for fellow nuclear receptors, mainly from the nuclear receptor subfamily 1. The main difference between the RAR and RXR families lies in the ligand-binding domain (LBD) structure, which determines ligand specificity. ATRA (all-trans-retinoid acid) is a high-affinity ligand for RAR but can also activate RXR. However, the retinoid with the highest affinity for RXR is 9-cis-retinoic acid 48.
Although these two closely related receptor families share the same or similar ligands and have similar signaling pathways, they regulate a different set of genes.
At the molecular level, in the absence of ligands, RAR is found in the cellular nucleus forming a complex with co-repressors. In this form, RAR is inactive. Upon binding to a ligand, the complex releases co-repressors by mediating a conformational change and co-activators are recruited 53. RAR needs to heterodimerize with RXR to form the transcription machinery complex, promoting gene transcription. The heterodimer-co-activator-ligand complex recognizes specific DNA sequences for binding to the promoter region of target genes called retinoic acid-responsive elements (RARE). RXR can heterodimerize with more nuclear receptors, such as pregnane X receptor (PXR), constitutive androstane receptor (CAR) and vitamin D receptor (VDR), among others 54.
The last nuclear receptors to which retinoids can bind are RAR-related orphan receptors (RORs). As in the RAR and RXR families, the ROR family also presents three isoforms: α, β and γ, and again, in this case, their expression is tissue-specific. Therefore, RORα is mainly found in the liver and the brain, β in the retina and the brain, and γ in the liver and the testes, among other tissues 55. The main difference from the other described retinoid receptors is that they can regulate gene expression in monomers through binding to the ROR responsive elements (RORE) and hence do not need dimerization with a fellow retinoid receptor RXR 56. RORs bind to oxysterols with high specificity. However, constitutive activation of the receptor, in the absence of a ligand, has also been described 57. ATRA (all-trans-retinoid acid) is the retinoid with the highest affinity for RORβ 58.
In addition to the receptors described thus far, another family of receptors has been described that interact with retinoids, peroxisome proliferative activated receptors (PPARs). This has been documented mainly for ATRA 59. As in the case of the other retinoid receptors, this receptor family also presents three isoforms α (nuclear receptor1C1), β/δ (nuclear receptor1C2) and γ (nuclear receptor1C3) 60. To exert their regulatory activity, these receptors also heterodimerize after ligand binding with RXR to form a transcriptional complex. Highly specific ligands for these receptors are fatty acids, and they are involved in energy homeostasis, fatty acid metabolism and inflammation 61, 62. However, only isoform β/δ presents high-affinity for ATRA 63. This isoform is abundantly expressed in the skin, brain and adipose tissue. The discovery of the interaction between ATRA with PPARβ/δ explains the proliferative effect of retinoic acid in keratinocytes and its involvement in insulin sensitivity and energy homeostasis 58.
Interestingly, all-trans-retinoid acid can regulate its levels in target organs through catabolic processes, especially through interaction with the enzymes CYP26A1, CYP26B1 and CYP26C1 64. The genetic effects triggered by interactions between ATRA (all-trans-retinoid acid) and its receptors are directly involved in multiple physiological functions: cellular differentiation, tissue development, tissue regeneration, cellular apoptosis, etc. 65, 66, 67, 68. In addition, ATRA (all-trans-retinoid acid) has additional functions in gene regulation since the evidence shows it has non-coding RNA regulatory functions 69.
Vitamin A and cancer
The role of retinoids in cancer has been the focus of many studies. However, no conclusive relationship has been clearly established 8. Retinoids are known to promote cell growth and tissue development through their interaction with nuclear receptors. Therefore, some studies have suggested that retinoids can be considered as cancer-promoting compounds. Although anti-cancer activity is related to the RAR transrepression of activating proteins, all-trans-retinoid acid (ATRA) has been suggested to have anti-cancer effects even though it is a receptor activator 70, 71, 72, 73. Test tube (in vitro) studies have demonstrated the effects of retinoids on apoptotic genes 74, 75 and the potential protective effect of vitamin A against some types of cancer 76, 77. This information follows a study that reported vitamin A deficiency as a factor in cancer development 78. In addition, retinol has been suggested to also be involved in regulating cell growth 73. All-trans-retinoid acid (ATRA), used in treating acute promyelocytic leukemia, promotes cell differentiation by activating transcription factors. However, it also inhibits a set of proteins involved in cell development and activates cellular apoptosis 79, 80. In addition to its differentiating effect, it has also been reported to inhibit the proliferation of lymphoma and lung, liver and solid ovarian tumors 81, 71, 82. Importantly, in addition to tretinoin and alitretinoin, other generation retinoids are already being used clinically as anti-cancer agents 83, 84.
The role of carotenoids in cancers is even more controversial, especially in the case of beta-carotene 8. Although being a well-known antioxidant with potentially positive effects in cardiovascular diseases and type 2 diabetes mellitus, several studies have reported contradictory effects of carotenoids on cancer incidence. Lower incidence of epithelial and lung cancer has been observed in various studies in individuals with a high intake of carotenoids from diets rich in fruit and vegetables 85, 86. However, some studies have reported that in a population of smokers, a higher incidence of lung cancer and mortality rate was observed after the administration of beta-carotene when compared to the control group 87, 88, 89. It is important to point out that these effects were observed with beta-carotene supplementation, not with lower carotenoid intake ingested through the diet 90. In contrast, several clinical and preclinical studies have reported carotenoids to prevent generating reactive oxygen species (ROS), to induce apoptosis in tumor cells and to prevent cancer induction 91, 92, 93.
Immune function
Vitamin A was initially coined “the anti-infective vitamin” because of its importance in the normal functioning of the immune system 94. The skin and mucosal cells, lining the airways, digestive tract, and urinary tract function as a barrier and form the body’s first line of defense against infection. Retinoic acid is produced by antigen-presenting cells (APCs), including macrophages and dendritic cells, found in these mucosal interfaces and associated lymph nodes. Retinoic acid appears to act on dendritic cells themselves to regulate their differentiation, migration, and antigen-presenting capacity. In addition, the production of retinoic acid by antigen-presenting cells (APCs) is required for the differentiation of naïve CD4 T-lymphocytes into induced regulatory T- lymphocytes (Tregs). Critical to the maintenance of mucosal integrity, the differentiation of Tregs is driven by all-trans-retinoic acid through RARα-mediated regulation of gene expression. Also, during inflammation, all-trans-retinoic acid/RARα signaling pathway promotes the conversion of naïve CD4 T-lymphocytes into effector T-lymphocytes − type 1 helper T-cells (Th1) − (rather than into Tregs) and induces the production of proinflammatory cytokines by effector T-lymphocytes in response to infection. There is also substantial evidence to suggest that retinoic acid may help prevent the development of autoimmunity 95.
Prenatal and postnatal development
Both vitamin A excess and deficiency are known to cause birth defects. Retinoid signaling begins soon after the early phase of embryonic development known as gastrulation. During fetal development, retinoic acid is critical for the development of organs, including the heart, eyes, ears, lungs, as well as other limbs and visceral organs. Vitamin A has been implicated in fetal lung maturation 32. Vitamin A status is lower in preterm newborns than in full-term infants 96. There is some evidence to suggest that vitamin A supplementation may help reduce the incidence of chronic lung disease and mortality in preterm newborns. Retinoid signaling is also involved in the expression of many proteins of the extracellular matrix (ECM; material surrounding cells), including collagen, laminin, and proteoglycans 97. Vitamin A deficiency may then result in alterations of the extracellular matrix (ECM) composition, thus disrupting organ morphology and function 97.
Red blood cell production (erythropoiesis)
Red blood cells (erythrocytes), like all blood cells, are derived from pluripotent stem cells in the bone marrow. Studies involving in vitro culture systems have suggested a role for retinoids in stem cell commitment and differentiation to the red blood cell lineage. Retinoids might also regulate apoptosis (programmed cell death) of red blood cell precursors (erythropoietic progenitor cells) 98. However, whether retinoids regulate erythropoiesis in vivo has not been established. Yet, vitamin A supplementation in vitamin A deficient-individuals has been shown to increase hemoglobin concentrations. Additionally, vitamin A appears to facilitate the mobilization of iron from storage sites to the developing red blood cell for incorporation into hemoglobin, the oxygen carrier in red blood cells 98, 99.
Nutrient interactions
Zinc deficiency
Zinc deficiency is thought to interfere with vitamin A metabolism in several ways 100: (1) zinc deficiency results in decreased synthesis of retinol-binding protein (RBP), which transports retinol through the circulation to peripheral tissues and protects the organism against potential toxicity of retinol; (2) zinc deficiency results in decreased activity of the enzyme that releases retinol from its storage form, retinyl palmitate, in the liver; and (3) zinc is required for the enzyme that converts retinol into retinal 101. The health consequences of zinc deficiency on vitamin A nutritional status in humans are yet to be defined 100.
Iron deficiency
Vitamin A deficiency often coexists with iron deficiency and may exacerbate iron deficiency anemia by altering iron metabolism 98. Vitamin A supplementation has beneficial effects on iron deficiency anemia and improves iron nutritional status among children and pregnant women 98, 99. The combination of supplemental vitamin A and iron seems to reduce anemia more effectively than either supplemental iron or vitamin A alone 102. Moreover, studies in rats have shown that iron deficiency alters plasma and liver levels of vitamin A 103, 104.
How much vitamin A do I need?
The amount of vitamin A you need depends on your age and sex. Average daily recommended amounts of preformed vitamin A and provitamin A carotenoids are listed below in micrograms (mcg) of retinol activity equivalents (RAE).
Intake recommendations for vitamin A and other nutrients are provided in the Dietary Reference Intakes (DRIs) developed by the Food and Nutrition Board (FNB) at the Institute of Medicine of the National Academies (formerly National Academy of Sciences) 105. Dietary Reference Intake (DRI) is the general term for a set of reference values used for planning and assessing nutrient intakes of healthy people. These values, which vary by age and gender, include:
- Recommended Dietary Allowance (RDA): Average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals.
- Adequate Intake (AI): Intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an RDA.
- Estimated Average Requirement (EAR): Average daily level of intake estimated to meet the requirements of 50% of healthy individuals; usually used to assess the nutrient intakes of groups of people and to plan nutritionally adequate diets for them; can also be used to assess the nutrient intakes of individuals.
- Tolerable Upper Intake Level (UL): Maximum daily intake unlikely to cause adverse health effects.
Recommended Dietary Allowances (RDAs) for vitamin A are given as mcg of retinol activity equivalents (RAE) to account for the different bioactivities of retinol and provitamin A carotenoids (see Table 1). Because the body converts all dietary sources of vitamin A into retinol, 1 mcg of physiologically available retinol is equivalent to the following amounts from dietary sources: 1 mcg of retinol, 12 mcg of beta-carotene, and 24 mcg of alpha-carotene or beta-cryptoxanthin. From dietary supplements, the body converts 2 mcg of beta-carotene to 1 mcg of retinol.
Retinol activity equivalents (RAE) were developed because provitamin A carotenoids have less vitamin A activity than preformed vitamin A; 1 mcg retinol = 3.33 IU.
Currently, vitamin A is listed on food and supplement labels in international units (IUs) even though nutrition scientists rarely use this measure. Conversion rates between mcg RAE and IU are as follows 106:
- 1 IU retinol = 0.3 mcg RAE (retinol activity equivalents)
- 1 IU beta-carotene from dietary supplements = 0.15 mcg RAE
- 1 IU beta-carotene from food = 0.05 mcg RAE
- 1 IU alpha-carotene or beta-cryptoxanthin = 0.025 mcg RAE
However, under FDA’s new labeling regulations for foods and dietary supplements that take effect by January 1, 2020 (for companies with annual sales of $10 million or more) or January 1, 2021 (for smaller companies), vitamin A will be listed only in mcg RAE and not IUs 107.
An RAE cannot be directly converted into an IU without knowing the source(s) of vitamin A. For example, the RDA of 900 mcg RAE for adolescent and adult men is equivalent to 3,000 IU if the food or supplement source is preformed vitamin A (retinol). However, this RDA is also equivalent to 6,000 IU of beta-carotene from supplements, 18,000 IU of beta-carotene from food, or 36,000 IU of alpha-carotene or beta-cryptoxanthin from food. So a mixed diet containing 900 mcg RAE provides between 3,000 and 36,000 IU of vitamin A, depending on the foods consumed.
Table 1. Recommended Dietary Allowances (RDAs) for Vitamin A
| Life Stage | Recommended Amount |
|---|---|
| Birth to 6 months* | 400 mcg RAE |
| Infants 7–12 months* | 500 mcg RAE |
| Children 1–3 years | 300 mcg RAE |
| Children 4–8 years | 400 mcg RAE |
| Children 9–13 years | 600 mcg RAE |
| Teen males 14–18 years | 900 mcg RAE |
| Teen females 14–18 years | 700 mcg RAE |
| Adult males | 900 mcg RAE |
| Adult females | 700 mcg RAE |
| Pregnant teens | 750 mcg RAE |
| Pregnant adults | 770 mcg RAE |
| Breastfeeding teens | 1,200 mcg RAE |
| Breastfeeding adults | 1,300 mcg RAE |
Footnote: * Adequate Intake (AI), equivalent to the mean intake of vitamin A in healthy, breastfed infants.
[Source 105 ]What kinds of vitamin A dietary supplements are available?
Vitamin A is available in dietary supplements, usually in the form of retinyl acetate or retinyl palmitate (preformed vitamin A), beta-carotene (provitamin A), or a combination of preformed and provitamin A 16. Most multivitamin-mineral supplements contain vitamin A. Dietary supplements that contain only vitamin A are also available.
The amounts of vitamin A in supplements vary widely, but 3,000 mcg RAE (333% of the DV) is common. This is due to the fact that the Daily Values (DV) used by the US Food and Drug Administration (FDA) for supplement labeling are based on the RDA established in 1968 rather than the most recent RDA, and multivitamin supplements typically provide 100% of the DV for most nutrients.
Multivitamins commonly have somewhat lower vitamin A amounts, often 750 to 1,050 mcg RAE (83% to 117% of the DV). Because retinol intakes of 5,000 IU/day (1,500 mcg RAE) may be associated with an increased risk of osteoporosis in older adults, some companies have reduced the retinol content in their multivitamin supplements to 2,500 IU (750 mcg RAE).
The absorption of preformed vitamin A esters from dietary supplements is 70–90%, and that of beta-carotene ranges from 8.7% to 65% 108, 109.
Vitamin A in pregnancy
Although normal fetal development requires sufficient vitamin A intake, consumption of excess preformed vitamin A (such as retinol) during early pregnancy is known to cause birth defects 110, 111. Increased maternal levels of preformed vitamin A (retinoic acid) have been shown to be associated with miscarriage and with malformations involving the central nervous and cardiac systems 112, 113. A World Health Organization (WHO) expert group consultation concluded that daily doses of below 3,000 mcg RAE/day (10,000 IU/day) or 25,000 IU (equivalent to 7500 mcg retinol) weekly after day 60 are probably safe, especially in areas where vitamin A deficiency is thought to be common 113, 114. Of note, in 2011, the World Health Organization (WHO) recommended vitamin A supplementation (up to 3,000 mcg RAE/day or 7,500 mcg RAE/week) during pregnancy in areas with high prevalence of vitamin A deficiency for the prevention of blindness 115.
In developed countries, pregnant or potentially pregnant women should monitor their intake of vitamin A from fortified food and food naturally high in preformed vitamin A (e.g., liver) and avoid taking daily multivitamin supplements that contain more than 1,500 mcg RAE (5,000 IU) of vitamin A. The UK National Institute for Health and Clinical Excellence (NICE) guidelines advise women who are not vitamin A deficient living in developed countries, owing to potential teratogenic effects, should avoid taking vitamin A supplements and liver above 700 mcg RAE/day (2,333 IU/day) 116.
There is no evidence that consumption of vitamin A from beta-carotene might increase the risk of birth defects.
The synthetic derivative of retinol, isotretinoin, is known to cause serious birth defects and should not be taken during pregnancy or if there is a possibility of becoming pregnant 117. Tretinoin (all-trans-retinoic acid), another retinol derivative, is prescribed as a topical preparation that is applied to the skin. Although percutaneous absorption of topical tretinoin is minimal, its use during pregnancy is not recommended 118.
Foods high in vitamin A
Vitamin A is found naturally in many foods and is added to some foods, such as milk and cereal. You can get recommended amounts of vitamin A by eating a variety of foods, including the following:
- Some types of fish, such as herring and salmon
- Beef liver and other organ meats (which are also high in cholesterol, so limit the amount you eat)
- Green leafy vegetables and other green, orange, and yellow vegetables, such as spinach, sweet potatoes, carrots, broccoli, and winter squash
- Fruits, including cantaloupe, mangos, and apricots
- Dairy products, such as milk and cheese
- Fortified breakfast cereals
- Eggs
Free retinol is not generally found in food. Retinyl esters (including retinyl palmitate) are the storage form of retinol in animals and thus the main precursors of retinol in food from animals. Plants contain carotenoids, some of which are precursors for vitamin A (e.g., α-carotene, β-carotene, and β-cryptoxanthin). Yellow- and orange-colored vegetables contain significant quantities of carotenoids. Green vegetables also contain carotenoids, though yellow-to-red pigments are masked by the green pigment of chlorophyll 119.
An important source of the human intake of vitamin A is the preformed version of the vitamin, which is found in foods of an animal origin. Milk and dairy products, as well as liver and its products, are the largest contributors, followed by eggs, egg products and fish 10, 16, 17. Most dietary provitamin A in the U.S. diet comes from leafy green vegetables, orange and yellow vegetables, tomato products, fruits, and some vegetable oils 16, 20, 120. Vitamin A is routinely added to some foods, including milk and margarine 16. Some ready-to-eat cereals are also fortified with vitamin A.
The amount of retinol found in the milk of individual livestock species does not differ greatly, although feed, seasonal variation and breeds do have some impact 8. In general, its concentration ranges from 20 to 80 mcg per 100 mL of whole milk. Each breed produces milk with a different amount of fat related to the amount of vitamin A in its milk. The higher the fat content in the milk, the higher the retinol content 121. The amount of retinol in dairy products depends on the amount of milk fat in the product 122. One of the richest sources of vitamin A is the livers of various livestock and poultry, which contain tens of milligrams of retinol and its retinyl esters per 100 g 8. Differences between animal species are significantly more pronounced than in the case of milk. The values found in individual studies vary considerably, but the highest vitamin A content in the liver is usually reported in pigs 123, 124.
Table 2 below lists a number of good food sources of vitamin A. The foods from animal sources in Table 2 contain primarily preformed vitamin A, the plant-based foods have provitamin A, and the foods with a mixture of ingredients from animals and plants contain both preformed vitamin A and provitamin A.
Table 2. Selected Food Sources of Vitamin A
| Food | mcg RAE per serving | Percent DV* |
|---|---|---|
| Beef liver, pan fried, 3 ounces | 6582 | 731 |
| Sweet potato, baked in skin, 1 whole | 1403 | 156 |
| Spinach, frozen, boiled, ½ cup | 573 | 64 |
| Pumpkin pie, commercially prepared, 1 piece | 488 | 54 |
| Carrots, raw, ½ cup | 459 | 51 |
| Herring, Atlantic, pickled, 3 ounces | 219 | 24 |
| Ice cream, French vanilla, soft serve, ⅔ cup | 185 | 21 |
| Milk, skim, with added vitamin A and vitamin D, 1 cup | 149 | 17 |
| Cantaloupe, raw, ½ cup | 135 | 15 |
| Cheese, ricotta, part skim, ½ cup | 133 | 15 |
| Peppers, sweet, red, raw, ½ cup | 117 | 13 |
| Mangos, raw, 1 whole | 112 | 12 |
| Breakfast cereals, fortified with 10% of the DV for vitamin A, 1 serving | 90 | 10 |
| Egg, hard boiled, 1 large | 75 | 8 |
| Black-eyed peas (cowpeas), boiled, 1 cup | 66 | 7 |
| Apricots, dried, sulfured, 5 apricots | 63 | 7 |
| Broccoli, boiled, ½ cup | 60 | 7 |
| Salmon, sockeye, cooked, 3 ounces | 59 | 7 |
| Tomato juice, canned, ¾ cup | 42 | 5 |
| Yogurt, plain, low fat, 1 cup | 32 | 4 |
| Tuna, light, canned in oil, drained solids, 3 ounces | 20 | 2 |
| Baked beans, canned, plain or vegetarian, 1 cup | 13 | 1 |
| Summer squash, all varieties, boiled, ½ cup | 10 | 1 |
| Chicken, breast meat and skin, roasted, ½ breast | 5 | 1 |
| Pistachio nuts, dry roasted, 1 ounce | 4 | 0 |
Footnote: *DV = Daily Value. U.S. Food and Drug Administration (FDA) developed Daily Values (DVs) to help consumers compare the nutrient contents of foods and dietary supplements within the context of a total diet. The Daily Value (DV) for vitamin A is 900 mcg RAE for adults and children age 4 years and older, where 1 mcg RAE = 1 mcg retinol, 2 mcg beta-carotene from supplements, 12 mcg beta-carotene from foods, 24 mcg alpha-carotene, or 24 mcg beta-cryptoxanthin 107. FDA does not require food labels to list vitamin A content unless vitamin A has been added to the food. Foods providing 20% or more of the Daily Value (DV) are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
The U.S. Department of Agriculture’s (USDA’s) FoodData Central (https://fdc.nal.usda.gov) lists the nutrient content of many foods and provides a comprehensive list of foods containing vitamin A in IUs arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/VitaminA-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/VitaminA-Food.pdf), and foods containing beta-carotene in mcg arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/VitA-betaCarotene-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/VitA-betaCarotene-Food.pdf).

Are you getting enough vitamin A?
Most people in the United States get enough vitamin A from the foods they eat, and vitamin A deficiency is rare 19. However, certain groups of people are more likely than others to have trouble getting enough vitamin A:
- Premature infants, who often have low levels of vitamin A in their first year.
- Infants, young children, pregnant women, and breastfeeding women in developing countries.
- People with cystic fibrosis.
What happens if you don’t get enough vitamin A?
Vitamin A deficiency is rare in the United States, although it is common in many developing countries. The most common symptom of vitamin A deficiency in young children and pregnant women is an eye condition called xerophthalmia. Xerophthalmia is the inability to see in low light, and it can lead to blindness if it isn’t treated.
Vitamin A benefits
Vitamin A is an antioxidant. It can come from plant or animal sources. Plant sources include colorful fruits and vegetables. Animal sources include liver and whole milk. Vitamin A is also added to foods like cereals.
Vitamin A plays a role in your:
- Vision
- Bone growth
- Reproduction 125. The vitamin A metabolite, trans retinoic acid, is essential for reproduction in both the male and female, as well as for many events in the developing embryo.
- Cell functions
- Immune system 126
Scientists are studying vitamin A to understand how it affects health. Here are some examples of what this research has shown.
Cancer
Because of the role vitamin A plays in regulating cell growth and differentiation, several studies have examined the association between vitamin A and various types of cancer. However, the relationship between serum vitamin A levels or vitamin A supplementation and cancer risk or cancer-related death is unclear. However, this does not include studies of all-trans retinoic acid, a vitamin A metabolite that is used as a drug in high doses to treat a form of leukemia 127, 128. Furthermore, in addition to tretinoin and alitretinoin, other generation retinoids are already being used clinically as anti-cancer agents 83, 84.
Several systematic reviews and meta-analyses of observational studies have shown that higher dietary intakes of retinol, carotenoids, fruits and vegetables, or a combination are associated with a lower risk of lung cancer 129, non-Hodgkin lymphoma 130, pancreatic cancer 131, oral cavity cancer 132, laryngeal cancer 132, esophageal cancer 133, ovarian cancer 134, 135, glioma 136, and bladder cancer 137. However, other observational studies have found no association between intakes of different forms of vitamin A and risk of liver cancer 138, non-Hodgkin lymphoma 139, colorectal cancer 140, prostate cancer 140 or all cancers 141.
Some clinical trial evidence suggests that supplemental vitamin A might reduce the risk of certain cancers but increase the risk of other forms of cancer, cardiovascular disease morbidity and mortality, and all-cause mortality. Examples are provided below.
The Carotene and Retinol Efficacy Trial (CARET) included 18,314 male and female current and former smokers (with at least a 20 pack-year history [equivalent to smoking 1 pack per day for 20 years or 2 packs per day for 10 years, for example] of cigarette smoking), as well as some men occupationally exposed to asbestos (who also have a higher risk of lung cancer), all aged 45–74 years. The study randomized participants to take supplements containing 30 mg beta-carotene plus 25,000 IU (7,500 mcg RAE) retinyl palmitate or a placebo daily for about 6 years to evaluate the potential effects on lung cancer risk 142. The trial was ended prematurely after a mean of 4 years, partly because the supplements were unexpectedly found to have increased lung cancer risk by 28% and death from lung cancer by 46%; the supplements (30 mg beta-carotene plus 25,000 IU (7,500 mcg RAE) retinyl palmitate) also increased the risk of all-cause mortality by 17% 142.
A subsequent study followed CARET participants for an additional 6 years after they stopped taking the study supplements 143. During this time, the differences in lung cancer risk between the intervention and placebo groups were no longer statistically significant, with one exception: women in the intervention group had a 33% higher risk of lung cancer. In a separate analysis of CARET study data, men who took the two supplements had a 35% lower risk of nonaggressive prostate cancer during the 4-year active trial, but not during the 6-year postintervention period 144. In contrast, men who took these two supplements in addition to another self-prescribed supplement (typically a multivitamin) had a 52% higher risk of aggressive prostate cancer during the active trial, but not during the postintervention period 144.
The Alpha-Tocopherol, Beta-Carotene (ATBC) Cancer Prevention Study also found that beta-carotene supplements increased the risk of lung cancer in smokers 145. In this study, 29,133 male smokers aged 50–69 years who smoked an average of 20.4 cigarettes a day for an average of 35.9 years took a supplement containing 50 mg/day alpha-tocopherol, 20 mg/day beta-carotene, both alpha-tocopherol and beta-carotene, or a placebo for 5–8 years 145. The beta-carotene supplements increased the risk of lung cancer by 18%, although they had little to no effect on the incidence of other cancers 145. The overall rate of death, primarily from lung cancer and ischemic heart disease, was 8% higher in participants who took beta-carotene. A subsequent study followed 25,563 of these participants for an additional 18 years 146. During this period, participants were no longer taking the supplements, but most continued to smoke. Participants who had taken beta-carotene in the original trial did not have a higher risk of lung cancer, but they had a 20% higher risk of death due to prostate cancer 146.
The Age-Related Eye Disease Study 2 (AREDS2) was a 5-year randomized clinical trial with 4,203 participants aged 50–85 years examining the effects on age-related macular degeneration (AMD) of a dietary supplement containing several ingredients with or without beta-carotene (15 mg [7,500 mcg RAE]) 147. No current smokers received the supplements containing beta-carotene. At the end of the trial, more lung cancers were discovered in the beta-carotene group than in the no beta-carotene group (23 vs 11 cases), and 31 of the 34 affected were former smokers. In a follow-up analysis of 3,882 of the participants 5 years after the end of AREDS2 (during which they took the AREDS2 formulation containing lutein and zeaxanthin instead of beta-carotene), the increased lung cancer risk persisted, with an 82% higher risk among participants who took the supplement containing beta-carotene during the 5-year AREDS2 trial 148.
Three other clinical trials have found no relationship between taking vitamin A or beta-carotene supplements and lung cancer incidence or mortality 149. One trial randomized 22,071 male physicians aged 40–84 years to take 50 mg beta carotene on alternate days or a placebo for 12 years 150. Eleven percent of the physicians were current smokers, and 38% were former smokers at the start of the study. The results showed no differences between the groups in number of cases of lung cancer or any malignant neoplasms or number of deaths from cancer. Another trial randomized 7,627 women (mean age 60.4 years) to take 50 mg beta-carotene on alternate days, 600 IU vitamin E on alternate days, 500 mg vitamin C daily, or a placebo for a mean of 9.4 years 151. Fifteen percent of the women were current smokers, and 41% were former smokers at the start of the study. None of the supplements had any significant effect on total cancer incidence or cancer mortality, including from lung cancer. A third trial included 29,584 healthy men and women aged 40–69 years who were living in Linxian, China, where micronutrient deficiencies are common 152. The study randomized participants to take either a placebo or one of four vitamin and mineral combinations (including one providing retinol and zinc and another providing beta carotene, vitamin E, and selenium) for 5.25 years. The investigators followed participants for an additional 10 years after they stopped taking the supplements. The nutrient doses in the supplements were equivalent to or twice as high as U.S. recommended intakes, but the study report did not provide the exact doses. During both the intervention and follow-up periods, lung cancer death rates did not differ among the five groups, even when the investigators further analyzed the results for differences by age, sex, and smoking status 152.
The Carotene and Retinol Efficacy Trial (CARET) and Alpha-Tocopherol, Beta-Carotene (ATBC) study results suggest that large supplemental doses of beta-carotene with or without retinyl palmitate have detrimental effects in current or former smokers and workers exposed to asbestos. However, the other studies described above that used similar vitamin A doses but had smaller proportions of current or former smokers do not raise this concern. Among nonsmokers, beta-carotene and vitamin A supplements do not appear to affect the risk of cancer.
Age-Related Macular Degeneration
Age-related macular degeneration (AMD), or the loss of central vision as people age, is one of the most common causes of vision loss in older people 153. Age-related macular degeneration’s causes is usually unknown, but may involve complex interactions among genetic susceptibility, environmental factors (including exposure to oxidative stress), and normal aging 153. Because of the role of oxidative stress in age-related macular degeneration (AMD) pathophysiology, supplements containing carotenoids with antioxidant functions, such as beta-carotene, lutein, and zeaxanthin, might be useful for preventing or treating this condition. Lutein and zeaxanthin (which are not precursors of vitamin A), in particular, accumulate in the retina, the tissue in the eye that is damaged by age-related macular degeneration (AMD).
The Age-Related Eye Disease Study (AREDS) trial found that participants with a high risk of developing advanced age-related macular degeneration (AMD) (i.e., those who had intermediate AMD or who had advanced AMD in one eye) had a 25% lower risk of developing advanced AMD after they took a daily supplement containing beta-carotene (15 mg [7,500 mcg RAE]), vitamin E (180 mg [400 IU] dl-alpha-tocopheryl acetate), vitamin C (500 mg), zinc (80 mg), and copper (2 mg) for 5 years than participants taking a placebo 154.
The follow-up AREDS2 study confirmed the value of this supplement in reducing the progression of AMD over a median follow-up period of 5 years 147. However, this follow-up study showed that adding lutein (10 mg) and zeaxanthin (2 mg) or omega-3 fatty acids to the formulation produced no additional benefits 147. Importantly, the follow-up study also revealed that beta-carotene was not a required ingredient; the original AREDS formulation without beta-carotene provided the same protective effect against developing advanced AMD.
In a more detailed analysis, participants with the lowest dietary intakes of lutein and zeaxanthin had a 26% lower risk of advanced AMD when they took a supplement containing these two carotenoids than those who did not take a supplement with these carotenoids 147. The risk of advanced AMD was also 18% lower in participants who took the modified AREDS supplement containing lutein and zeaxanthin but not beta-carotene than in participants who took the formulation with beta-carotene but not lutein or zeaxanthin.
A subsequent study monitored dietary intakes of several nutrients in 4,504 AREDS participants and 3,738 AREDS2 participants (mean age 71 years) for a median of 10.2 years 155. Participants in the two highest quintiles of intakes for vitamin A, beta-carotene, or lutein and zeaxanthin had a lower risk of progression to late AMD 155. For example, the risk of late AMD was 18% lower among those in the fifth quintile for vitamin A intake and 20% lower among those in the fourth quintile than among those in the first quintile 155.
At the end of the 5-year AREDS2 trial, participants were all offered the final AREDS2 formulation that included lutein and zeaxanthin in place of beta-carotene. Researchers followed up with 3,882 of these participants for an additional 5 years 148. After 10 years, participants who had taken the AREDS2 supplement with lutein and zeaxanthin had an additional 20% reduced risk of progression to late AMD compared with those who took the supplement containing beta-carotene 148. This finding confirmed the benefit of replacing beta-carotene with lutein and zeaxanthin.
Individuals who have or are developing age-related macular degeneration (AMD) should talk to their health care provider about their vitamin A intakes and the supplement formulations used in the AREDS studies.
Measles
When children with vitamin A deficiency (which is rare in North America) get measles, the disease tends to be more severe. In these children, taking supplements with high doses of vitamin A can shorten the fever and diarrhea caused by measles. These supplements can also lower the risk of death in children with measles who live in developing countries where vitamin A deficiency is common.
Measles is a major cause of morbidity and mortality in children in developing countries. In 2019, measles was responsible for more than 207,500 deaths around the world, mostly in young children in low-income countries 156. About half of all measles deaths happen in Africa, but the disease is not limited to low-income countries. Vitamin A deficiency is a known risk factor for severe measles. In 2013, 11,200 deaths from measles were associated with vitamin A deficiency, and more than 95% of these deaths occurred in sub-Saharan Africa and south Asia. In a pooled analysis of randomized controlled trials within this study, vitamin A supplementation was associated with a 26% lower risk of dying from measles.
The World Health Organization recommends high oral doses (200,000 IU) of vitamin A for two days for children over age 1 with measles who live in areas with a high prevalence of vitamin A deficiency 157, 158. Recommended doses are 30,000 mcg RAE (100,000 IU) of vitamin A once for infants ages 6–11 months and 60,000 mcg RAE (200,000 IU) every 4–6 months for ages 1–5 years 157.
However, a Cochrane review that included 6 randomized controlled trials of vitamin A supplementation (15,000 mcg RAE [50,000 IU] to 60,000 mcg RAE [200,000 IU], depending on age) found that the supplementation did not affect risk of death due to measles, although it did help prevent new cases of measles 159. These randomized controlled trials assessed the value of supplementation to prevent morbidity and mortality due to measles in a total of 19,566 children aged 6 months to 5 years. The vitamin A doses used in these studies are much higher than the UL. The effectiveness of vitamin A supplementation to treat measles in countries, such as the United States, where vitamin A intakes are usually adequate is uncertain.
The body needs vitamin A to maintain the corneas and other epithelial surfaces, so the lower serum concentrations of vitamin A associated with measles, especially in people with protein-calorie malnutrition, can lead to blindness. None of the studies evaluated in a Cochrane review evaluated blindness as a primary outcome 160. However, a careful clinical investigation of 130 African children with measles revealed that half of all corneal ulcers in these children, and nearly all bilateral blindness, occurred in those with vitamin A deficiency 161.
Bronchopulmonary dysplasia in preterm infants
Preterm infants are born with inadequate body stores of vitamin A, placing them at risk of developing diseases of the eye and the respiratory and gastrointestinal tracts. About one-third of preterm infants born between 22 and 28 weeks of gestation develop bronchopulmonary dysplasia, a chronic lung disease that can be fatal or result in life-long morbidities in survivors. A few randomized controlled studies have investigated the effect of postnatal vitamin A supplementation on the incidence of bronchopulmonary dysplasia and the risk of mortality in very low birth weight infants (≤1,500 g) requiring respiratory support 162, 163, 164. In the largest, multicenter, randomized, blinded, placebo-controlled trial that enrolled 807 extremely low birth weight (ELBW; ≤1,000 g) preterm newborns, the intramuscular administration of 5,000 IU of vitamin A three times a week for four weeks significantly, though modestly, reduced the risk of bronchopulmonary dysplasia or death at 36 weeks’ postmenstrual age (gestational age plus chronological age) 163. While vitamin A supplementation was included in some neonatal programs after this trial 165, a national shortage in vitamin A supply that has affected US neonatal intensive care units since 2010 has led to a significant reduction in the use of vitamin A supplementation in premature newborns (401-1,000 g at birth) with respiratory failure 166, 167. However, a retrospective analysis of US nationwide data from 6,210 preterm infants born between 2010 and 2012 found that a reduction in vitamin A prophylaxis from 27.2% to 2.1% during the same period had no significant impact on the incidence of bronchopulmonary dysplasia or death before hospital discharge 167.
In another retrospective study, the nonrandomized use of vitamin A supplementation with inhaled nitric oxide (iNO) was found to result in a lower incidence of bronchopulmonary dysplasia (but not mortality) compared to inhaled nitric oxide (iNO) therapy alone in preterm newborns with a birth weight of 750-999 g 168. Neurodevelopment index scores at one year of age were also improved in the vitamin A group of newborns weighing 500-749 g at birth. Yet, caution is advised with the interpretation of the results, especially because the trial was not designed to assess the effect of vitamin A. In Germany, one large, multicenter, randomized study – the NeoVitaA trial – is under way to explore the effect of high-dose oral vitamin A (5,000 IU/kg/day) for 28 days on the incidence of bronchopulmonary dysplasia and mortality at 36 weeks’ postmenstrual age 169.
While high doses of vitamin A during early pregnancy can cause birth defects, vitamin A supplementation during late pregnancy may improve maternal and fetal vitamin A status 170. Although a few randomized controlled trials have failed to show an effect on maternal and neonatal mortality 171, more research is required to assess whether vitamin A supplementation during pregnancy reduces bronchopulmonary dysplasia incidence in infants.
Childhood morbidity and mortality
A recent meta-analysis of randomized controlled trials evaluating the preventive effect of vitamin A on childhood mortality indicated that vitamin A supplementation (200,000 IU every 4 or 6 months) reduced all-cause mortality by 25% (13 studies) and diarrhea-specific mortality by 30% (7 studies) in children aged 6 to 59 months. However, vitamin A administration in this age group had no preventive effect on rates of pneumonia-specific mortality (7 studies), measles-specific mortality (5 studies), or meningitis-specific mortality (3 studies). Moreover, no reduction in the risk of disease-specific mortality was found in neonates (0 to 28 days of age) and infants 1 to 6 months of age supplemented with vitamin A (67). Another meta-analysis of randomized controlled trials found no evidence of a reduction in mortality risk during infancy when either breast-feeding mothers (7 studies) or infants aged less than six months (9 studies) were supplemented with vitamin A (68).
Current WHO policy recommends vitamin A supplementation at routine vaccination contacts in children after six months of age living in regions at high risk of vitamin A deficiency. Supplementation with high doses of vitamin A − 100,000 IU (30 mg RAE) for infants 6 to 11 months of age and 200,000 IU (60 mg RAE) for children 12 to 59 months of age − is thought to provide adequate protection for up to six months (38). A recent placebo-controlled trial in Guinea-Bissau, which randomized 7,587 children (ages, 6 to 23 months old) to receive vitamin A supplementation at one vaccination contact, evaluated the co-administration of vitamin A and vaccines on child mortality (69). The study found that vitamin A supplementation had no effect on overall mortality rates, although a six-month follow-up of infants given both measles and DTP (diphtheria-tetanus-pertussis) vaccinations showed a significant reduction in mortality in girls, but not in boys (69). Although neonatal vitamin A supplementation is not currently recommended, a trial assessing the benefit of early measles vaccination − at 4.5 rather than the usual 9 months of age − found no reduction in mortality rates when children had received neonatal vitamin A supplementation (70). The recent pooled analysis of previous trials of vitamin A supplementation (VITA I-III) in Guinea-Bissau confirmed that vitamin A supplementation may interfere with vaccines. Specifically, compared to placebo, neonatal vitamin A supplementation was associated with a significant increase in mortality rates in boys (but not in girls) when children had received measles virus vaccination at 4.5 months of age rather than the usual 9 months of age (71). The timing of vitamin A interventions needs to be further examined in relation to the timing of vaccinations in order to maximize their benefits.
Disease treatment
Retinoids may be used at pharmacological doses to treat several conditions, including, acute promyelocytic leukemia, retinitis pigmentosa, and various skin diseases. It is important to note that treatment with high doses of natural or synthetic retinoids overrides the body’s own control mechanisms; therefore, retinoid therapies are associated with potential side effects and toxicities. Additionally, all of the retinoid compounds have been found to cause fetal deformations. Therefore, women who have a chance of becoming pregnant should avoid treatment with these medications. Retinoids tend to be very long acting: side effects and birth defects have been reported to occur months after discontinuing retinoid therapy 32. The retinoids discussed below are prescription drugs and should not be used without medical supervision.
Acute promyelocytic leukemia
Normal differentiation of myeloid stem cells in the bone marrow gives rise to platelets, red blood cells, and white blood cells (also called leukocytes) that are important for the immune response. Altered differentiation of myeloid cells can result in the proliferation of immature white blood cells, giving rise to leukemia. Reciprocal chromosome translocations involving the promyelocytic leukemia (PML) gene and the gene coding for retinoic acid receptor α (RARα) lead to a specific type of leukemia called acute promyelocytic leukemia (APL). The fusion protein PML/RARα represses transcription by binding to RARE in the promoter of retinoid-responsive genes involved in hematopoietic cell differentiation. Gene repression by PML/RARα is achieved by the recruitment of several chromatin modifiers, including histone deacetylases (HDACs) and DNA methyltransferases (DNMTs). Contrary to RARα wild-type receptor, PML/RARα appears to be insensitive to physiological concentrations of retinoic acid such that only treatments with high doses of all-trans-retinoic acid can restore normal differentiation and lead to significant improvements and complete remission in some acute promyelocytic leukemia patients 128.
Diseases of the skin
Both natural and synthetic retinoids have been used as pharmacologic agents to treat disorders of the skin. Acitretin is a synthetic retinoid that has been proven useful in combination treatments for psoriasis 172. Topical tretinoin (all-trans-retinoic acid) and oral isotretinoin (13-cis-retinoic acid) have been used successfully to treat mild-to-severe acne vulgaris 117, 173. Retinoids exhibit anti-inflammatory properties and regulate the proliferation and differentiation of skin epithelial cells, as well as the production of sebum. Use of pharmacological doses of retinoids (especially oral isotretinoin) by pregnant women causes birth defects and is therefore contraindicated prior to and during pregnancy.
Retinitis pigmentosa
Retinitis pigmentosa affects approximately 1.5 million people worldwide and is a leading cause of inherited blindness. Retinitis pigmentosa describes a broad spectrum of genetic disorders that result in the progressive loss of photoreceptor cells (rods and cones) in the retina of the eye 174. While at least 45 loci have been associated with retinitis pigmentosa, mutations in the rhodopsin gene (RHO), the usherin gene (USH2A), and the retinitis pigmentosa GTPase regulator gene (retinitis pigmentosaGR) account for about 30% of all retinitis pigmentosa cases 175.
Early symptoms of retinitis pigmentosa include impaired dark adaptation and night blindness, followed by the progressive loss of peripheral and central vision over time 175. The results of only one randomized controlled trial in 601 patients with common forms of retinitis pigmentosa indicated that supplementation with 15,000 IU/day of retinyl palmitate (4,500 μg RAE) significantly slowed the loss of retinal function over a period of four to six years 176. In contrast, supplementation with 400 IU/day of vitamin E (dl-α-tocopherol) modestly but significantly increased the loss of retinal function, suggesting that patients with common forms of retinitis pigmentosa may benefit from long-term vitamin A supplementation but should avoid high-dose vitamin E supplementation. Up to 12 years of follow-up in these patients did not reveal any signs of liver toxicity as a result of excess vitamin A intake 177. Because neither children younger than 18 years nor adults affected by less common forms of retinitis pigmentosa were included in the trial, no formal recommendation about vitamins A and E could be made 175. High-dose vitamin A supplementation to slow the course of retinitis pigmentosa requires medical supervision and must be discontinued if there is a possibility of pregnancy.
Vitamin A deficiency
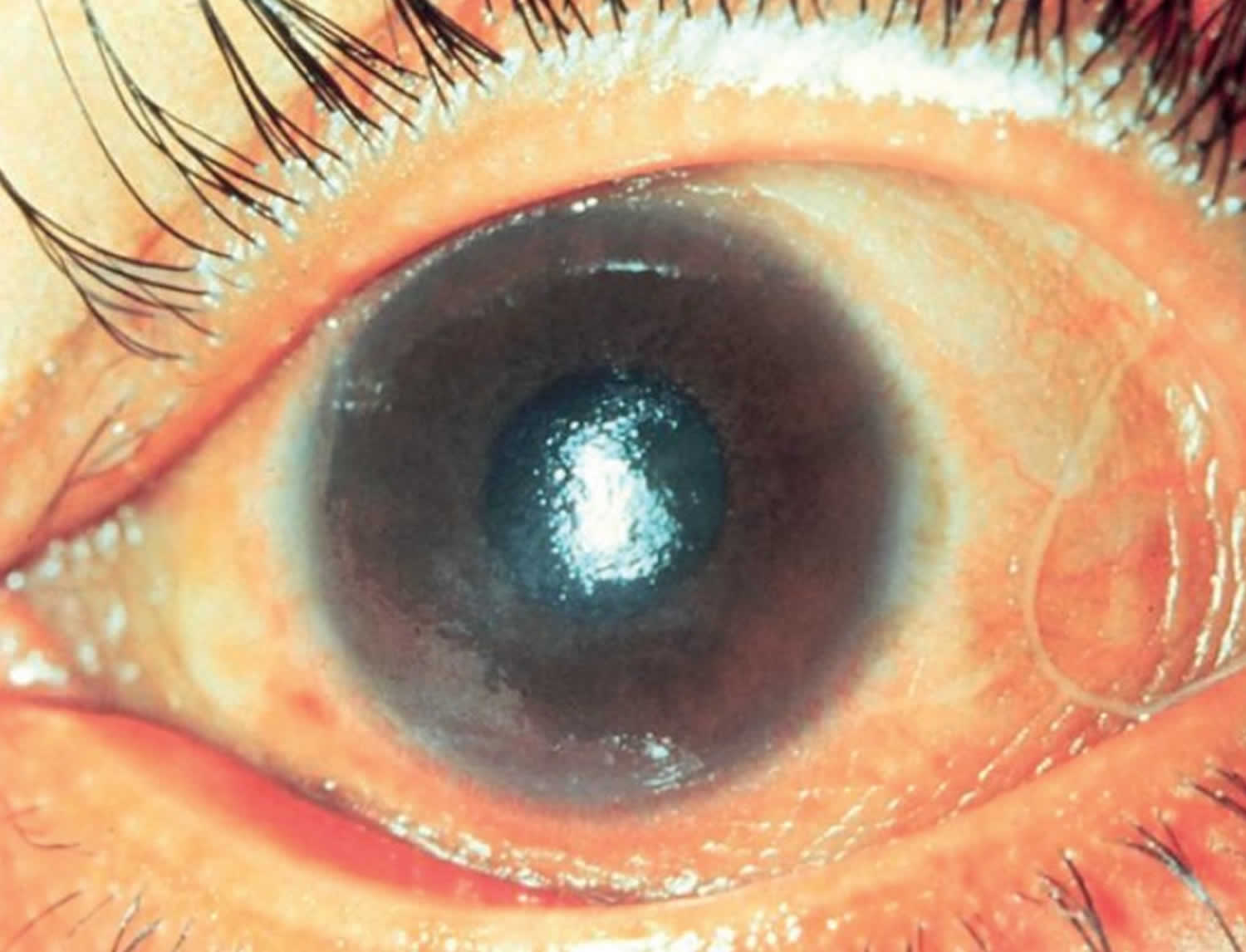
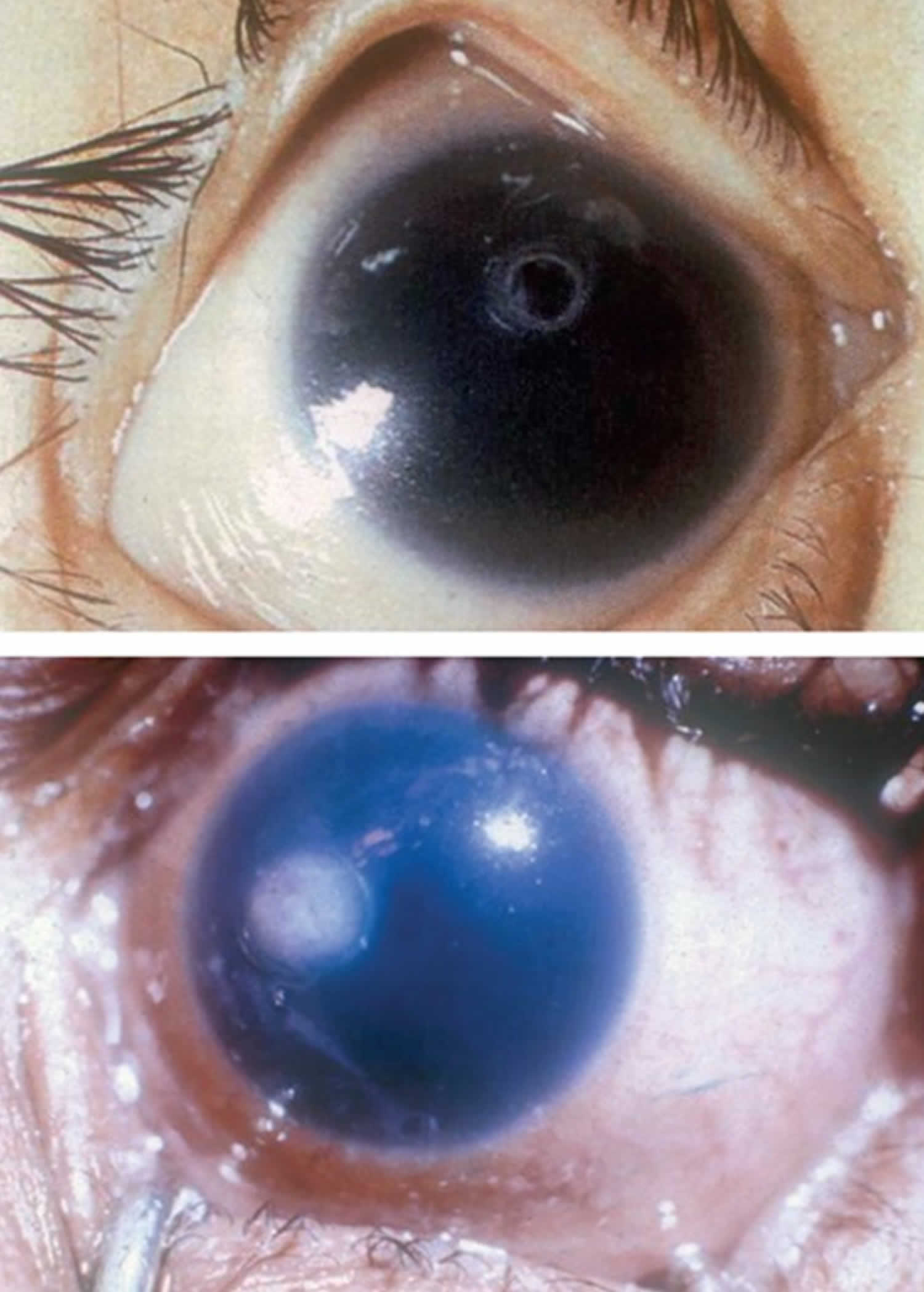
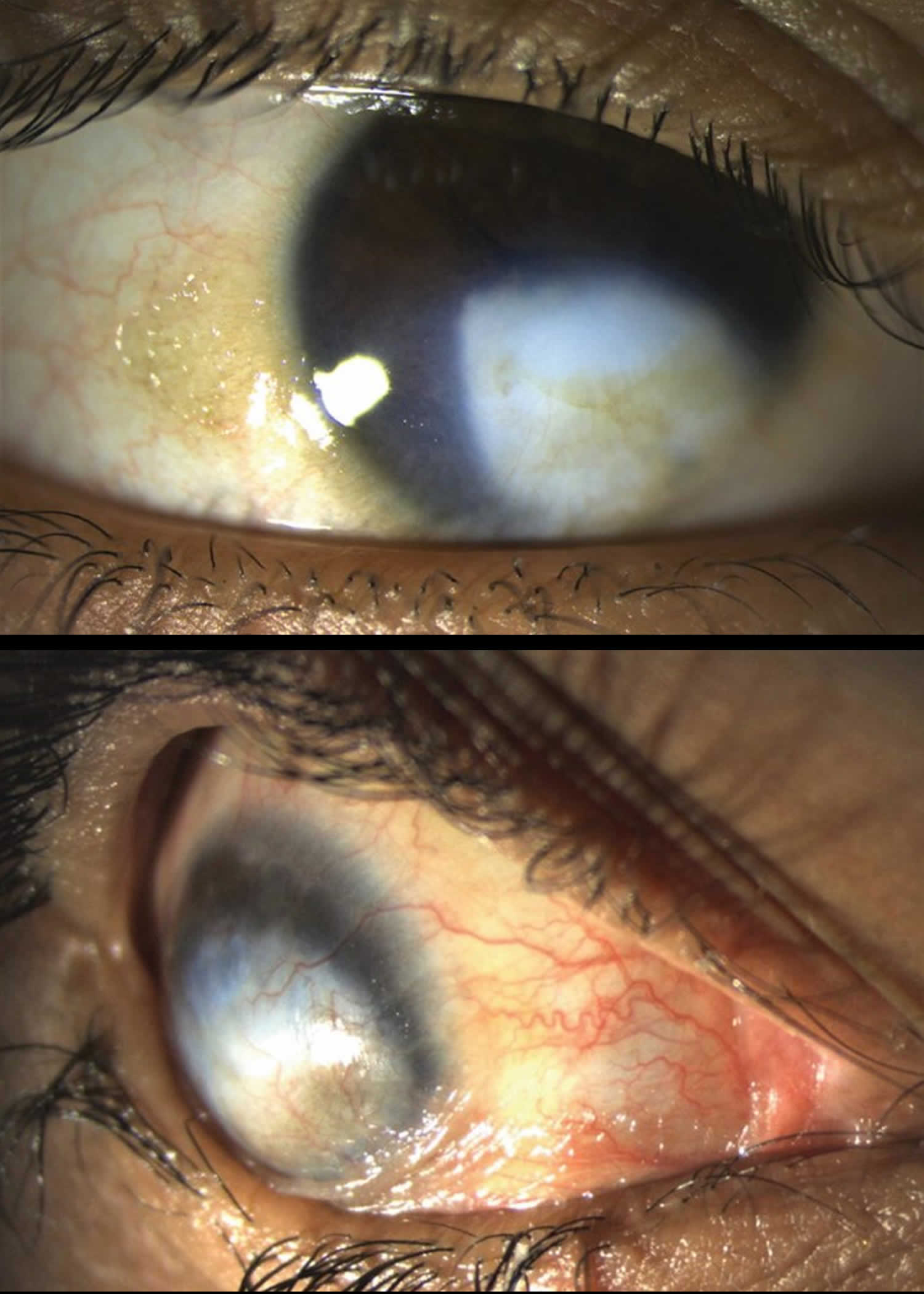
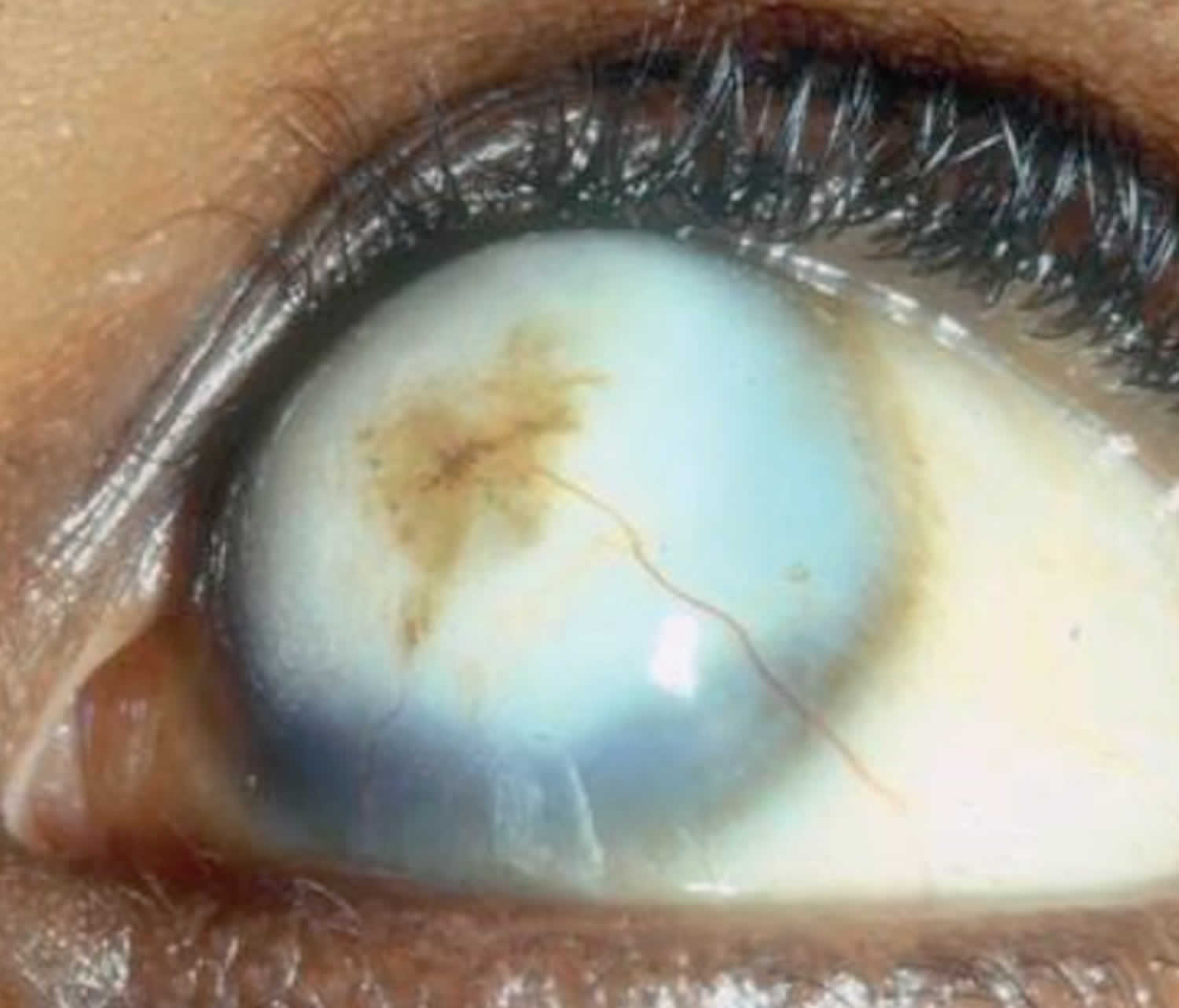
Vitamin A deficiency usually results from inadequate intakes of vitamin A from animal products (as preformed vitamin A found in fish, liver, dairy products, and eggs) and fruits, vegetables, and other plant-based products (as provitamin A carotenoids that are turned into vitamin A by your body). Vitamin A deficiency can result from inadequate intake, poor absorption of fats, or liver disorders. Vitamin A deficiency is rare in the United States, except in individuals with poor absorption of fats due to cystic fibrosis, ulcerative colitis, Crohn’s disease, weight loss surgery, short bowel syndrome, celiac disease, and, liver disease 32, 178, 179. However, vitamin A deficiency is common in many developing countries, often because residents have limited access to foods containing preformed vitamin A from animal-based food sources and they do not commonly consume available foods containing beta-carotene due to poverty or traditional diets 180, 3. A pooled analysis of population-based surveys from 138 low-income and middle-income countries found that 29% of children aged 6 months to 5 years had vitamin A deficiency in 2013 181. Vitamin A deficiency rates were highest in sub-Saharan Africa (48%) and South Asia (44%) 181. In addition, approximately 10% to 20% of pregnant people in low-income countries have vitamin A deficiency 182. Most vitamin A is stored in the liver, so measuring vitamin A levels in the liver is the best way to assess vitamin A adequacy 16. In clinical practice, plasma retinol levels alone can be used to document significant vitamin A deficiency 183. A serum or plasma retinol concentration of 0.70 μmol/L (20 mcg/dL) or less frequently reflects subclinical vitamin A deficiency, and a serum or plasma retinol level of 0.35 μmol/L (10 mcg/dL) or less is considered an indicator of severe vitamin A deficiency, where vitamin A body stores are depleted 16. Of note, the World Health Organization (WHO) considers vitamin A deficiency a public health problem when the prevalence of low serum retinol (<0.70 μmol/L) reaches 15% or more of a defined population 184. Long‐term or severe vitamin A deficiency may lead to eye lesions such as xerophthalmia (inability to see in low light) and keratomalacia (an eye disorder that involves drying and clouding of the cornea), eventually resulting in visual impairment, blindness, skin disease and growth retardation in children 185. The most common clinical sign of vitamin A deficiency is xerophthalmia, which develops after plasma retinol has been low and the eye’s vitamin A reserves have become depleted. The first sign is night blindness, or the inability to see in low light or darkness as a result of low rhodopsin levels in the retina 16, 181, 182. Xerophthalmia also affects the cornea and can eventually lead to permanent blindness; vitamin A deficiency is one of the top causes of preventable blindness in children 182.
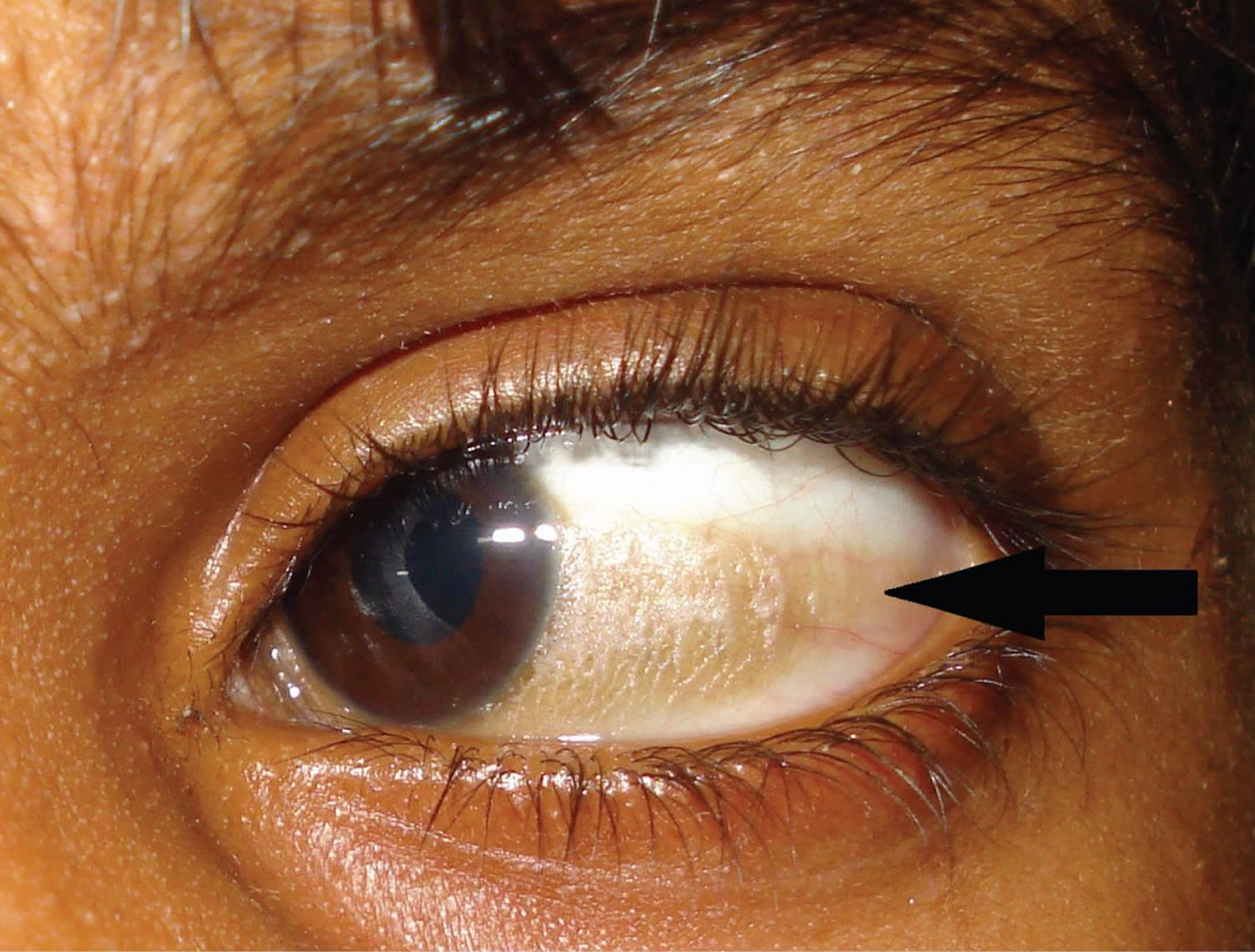
Vitamin A deficiency is one of the top causes of preventable blindness in children 186. People with vitamin A deficiency (and, often, xerophthalmia with its characteristic Bitot’s spots) tend to have low iron status, which can lead to anemia 4, 186.
The most specific clinical effect of inadequate vitamin A intake is xerophthalmia 187. It is estimated that 3 to 10 million children, mostly in developing countries, become xerophthalmic, and 250,000 to 500,000 go blind annually 188, 189. The World Health Organization 190 classified various stages of xerophthalmia to include night blindness (impaired dark adaptation due to slowed regeneration of rhodopsin), conjunctival xerosis, Bitot’s spots, corneal xerosis, corneal ulceration, and scarring, all related to vitamin A deficiency. Night blindness is the first ocular symptom to be observed with vitamin A deficiency 191, and it responds rapidly to treatment with vitamin A 192. High-dose (60 mg) vitamin A supplementation reduced the incidence of night blindness by 63 percent in Nepalese children 193. Similarly, night blindness was reduced by 50 percent in women after weekly supplementation with either 7,500 μg RE of vitamin A or β-carotene 194.
In developing countries, vitamin A deficiency and associated disorders predominantly affect children and women of reproductive age. According to the World Health Organization (WHO), 190 million preschool-aged children and 19.1 million pregnant women around the world have a serum retinol concentration below 0.70 micromoles/L 195. In these countries, low vitamin A intake is most strongly associated with health consequences during periods of high nutritional demand, such as during infancy, childhood, pregnancy, and lactation. In these countries, vitamin A deficiency typically begins during infancy, when infants do not receive adequate supplies of colostrum or breast milk 195. Chronic diarrhea also leads to excessive loss of vitamin A in young children, and vitamin A deficiency increases the risk of diarrhea 196. The most common symptom of vitamin A deficiency in young children and pregnant women is xerophthalmia. One of the early signs of xerophthalmia is night blindness, or the inability to see in low light or darkness 3. Vitamin A deficiency is one of the top causes of preventable blindness in children 195. People with vitamin A deficiency (and, often, xerophthalmia with its characteristic Bitot’s spots) tend to have low iron status, which can lead to anemia 195. Vitamin A deficiency also increases the severity and mortality risk of infections (particularly diarrhea and measles) even before the onset of xerophthalmia 195.
Vitamin A deficiency is the leading cause of preventable blindness in children worldwide:
- Impaired dark adaptation (night blindness) due to lack of the photoreceptor pigment rhodopsin.
- Xerophthalmia: dry, thickened conjunctiva and cornea
- Bitot spots: keratinized growths (metaplasia) on the conjunctivae causing hazy vision
- Keratomalacia: corneal erosions and ulceration
Vitamin A deficiency can also be recognized by its keratinizing effect on the skin and mucous membranes:
- Dry, scaly, thickened skin with prominent follicular scale (phrynoderma or follicular hyperkeratosis)
- Dry lips and thickened tongue
- Keratinisation of the urinary, gastrointestinal and respiratory tracts
Other symptoms and signs of vitamin A deficiency are:
- Impaired immunity leading to gastrointestinal and respiratory tract infections
- Growth retardation in children
Chronic vitamin A deficiency has also been associated with abnormal lung development, respiratory diseases (such as pneumonia), and an increased risk of anemia and death 197, 181, 198.
Another effect of chronic vitamin A deficiency is increased severity and mortality risk of infections (particularly measles and infection-associated diarrhea) 197. In 2013, 94,500 children in low-income and middle-income countries died of diarrhea and 11,200 died of measles as a result of vitamin A deficiency 181. More than 95% of deaths attributable to vitamin A deficiency occurred in sub-Saharan Africa and Asia, where vitamin A deficiency was responsible for 2% of all deaths in children younger than 5 years 181.
During pregnancy, vitamin A is essential for fetal organ and skeletal growth and maturation, maintenance of the maternal immune system, development of vision in the fetus, and maintenance of maternal eye health and night vision 199. Although pregnant women are susceptible to vitamin A deficiency throughout gestation, deficiency is most common in the third trimester. It is unclear whether this is due to increased demands during pregnancy from accelerated fetal development and the physiological increase in blood volume during this period 200, or to lowered serum retinol concentration due to an increase in plasma volume. In a pregnant woman with moderate vitamin A deficiency, the fetus can still obtain sufficient vitamin A to develop appropriately but at the expense of the maternal vitamin A stores 201.
Because of the role of vitamin A in maintaining the structural integrity of epithelial cells, follicular hyperkeratosis has been observed with inadequate vitamin A intake 202, 203. Men who were made vitamin A deficient under controlled conditions were then supplemented with either retinol or β-carotene, which caused the hyperkeratosis to gradually clear 203.
Vitamin A deficiency has been associated with a reduction in lymphocyte numbers, natural killer cells, and antigen-specific immunoglobulin responses 204, 205. A decrease in leukocytes and lymphoid organ weights, impaired T cell function, and decreased resistance to immunogenic tumors have been observed with inadequate vitamin A intake 206, 207. A generalized dysfunction of humoral and cell-mediated immunity is common in experimental animals and is likely to exist in humans.
In addition to xerophthalmia, vitamin A deficiency has been associated with increased risk of infectious morbidity and mortality in experimental animals and humans, especially in developing countries. A higher risk of respiratory infection and diarrhea has been reported among children with mild to moderate vitamin A deficiency 208. Mortality rates were about four times greater among children with mild xerophthalmia than those without it 209. The risk of severe morbidity and mortality decreases with vitamin A repletion. In children hospitalized with measles, case fatality 210, 211 and the severity of complications on admission were reduced when they received high doses (60 to 120 mg) of vitamin A 212, 211. In some studies, vitamin A supplementation (30 to 60 mg) has been shown to reduce the severity of diarrhea 213, 214 and Plasmodium falciparum malaria 215 in young children, but vitamin A supplementation has had little effect on the risk or severity of respiratory infections, except when associated with measles 216.
In developing countries, vitamin A supplementation has been shown to reduce the risk of mortality among young children 217, 218, 219, 220, 221, infants 216, and pregnant and postpartum women 222. Meta-analyses of the results from these and other community-based trials are consistent with a 23 to 30 percent reduction in mortality of young children beyond 6 months of age after vitamin A supplementation 223, 224, 225. The World Health Organization recommends broad-based prophylaxis in vitamin A-deficient populations. It also recommends treating children who suffer from xerophthalmia, measles, prolonged diarrhea, wasting malnutrition, and other acute infections with vitamin A 226. Furthermore, the American Academy of Pediatrics 227 recommends vitamin A supplementation for children in the United States who are hospitalized with measles.
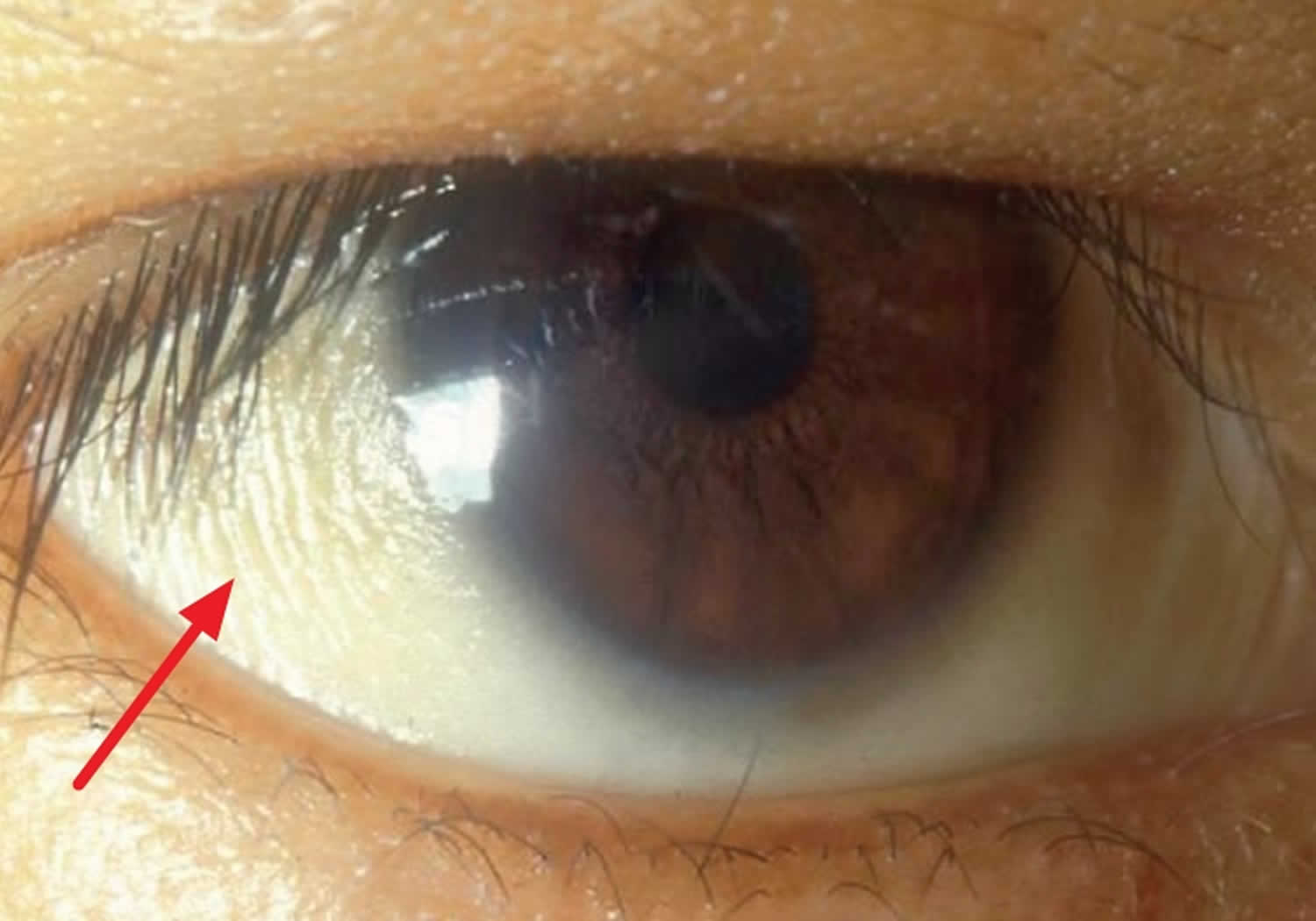
Figure 4. Bitot spots
Footnote: Left eye of an eight-year-old boy, with a large, superficial, triangular, foamy, keratinized patch in the interpalpebral region over the bulbar conjunctiva, adjacent to the temporal limbus (black arrow), suggestive of Bitot’s spots.
[Source 228 ]Figure 5. Conjunctival xerosis (dryness of the conjunctiva)
Footnote: Conjunctival xerosis (dryness of the conjunctiva) is another sign of long-standing vitamin A deficiency. Note the slight wrinkling of the temporal conjunctiva. Conjunctival xerosis can be quite difficult to detect and is therefore not a very reliable sign.
[Source 229 ]Figure 6. Corneal xerosis (drying of the cornea)
Footnote: Corneal xerosis (drying of the cornea) is a sign of sudden, acute vitamin A deficiency. The cornea becomes dry because glands in the conjunctiva no longer function normally. This leads to loss of tears and also loss of mucous, which acts as a ‘wetting agent’. The lack of mucus together with lack of tears not only leads to the dry appearance but also increases the risk of eye infection.
[Source 229 ]Figure 7. Corneal ulcer due to vitamin A deficiency
Footnote: If the acute vitamin A deficiency is not reversed as a matter of urgency, the cornea can become ulcerated and melt away. The corneal ulcer may have the appearance of a small, punched-out area in the cornea (top image), or the ulcer may have a more fluffy appearance (lower picture). In the absence of secondary infection, the eye can look surprisingly white, as in both images; however, secondary infection of the corneal ulcer is common, leading to an acutely inflamed eye.
[Source 229 ]Figure 8. Keratomalacia (corneal ulcer covering at least 1/3 of the cornea)
Footnote: The most severe form of vitamin A deficiency xerophthalmia is keratomalacia, in which more than one-third of the cornea is affected. The cornea may become edematous and thickened, and then melt away. This occurs because the structure of the collagen in the cornea is affected by a process known as necrosis. The cornea can be destroyed in just a few days. Children with keratomalacia are often malnourished, but children who previously appeared relatively healthy can also develop keratomalacia following measles infection or episodes of diarrhea; this is usually because they were vitamin A deficient and the measles infection resulted in depletion of their vitamin A stores. If you are not sure whether the child you are seeing has keratomalacia, ask about recent illness, particularly measles.
[Source 230 ]Figure 9. Corneal scarring
Footnote: The end result of corneal ulceration and keratomalacia is corneal scarring, staphylomas (forward bulging of a badly damaged cornea) or phthisis bulbi (an eye that has shrivelled up), depending on the extent of the pathology in the cornea. Most of the eye signs of vitamin A deficiency are symmetrical and bilateral, and so can lead to blindness.
[Source 229 ]Who is at risk of vitamin A deficiency?
The following groups are among those most likely to have inadequate intakes of vitamin A.
Premature infants
Preterm infants have low liver stores of vitamin A at birth, and their plasma concentrations of retinol often remain low throughout the first year of life 231, 232. Preterm infants with vitamin A deficiency have a higher risk of eye and chronic lung diseases 233, 234. However, in high-income countries, clinical vitamin A deficiency is rare in infants and occurs only in those with malabsorption disorders 235.
Infants, children, and pregnant and lactating women in low-income and middle-income countries
Pregnant women need extra vitamin A for fetal growth and tissue maintenance and to support their own metabolism 236, 237, 238. The breast milk of lactating women with adequate vitamin A intakes contains sufficient amounts of vitamin A to meet infants’ needs for the first 6 months of life239. But in people with vitamin A deficiency, the vitamin A content of breast milk is not sufficient to maintain adequate vitamin A stores in infants who are exclusively breastfed 239.
About 190 million preschool-aged children (one third of all children in this age group), mostly in Africa and Southeast Asia, have vitamin A deficiency, according to the World Health Organization 240, 181. They have a higher risk of visual impairment and of illness and death from childhood infections, such as measles and infections that cause diarrheal diseases 240, 16.
The World Health Organization estimates that 9.8 million pregnant women (15% of all pregnant women) around the world, mostly in low-income and middle-income countries, have xerophthalmia as a result of vitamin A deficiency 241.
People with cystic fibrosis
Up to 90% of people with cystic fibrosis have pancreatic insufficiency, which increases their risk of vitamin A deficiency due to difficulty absorbing fat 16, 242. Studies in Australia and the Netherlands indicate that 2% to 13% of children and adolescents with cystic fibrosis have vitamin A deficiency 243, 244. As a result, standard care for cystic fibrosis includes lifelong treatment with vitamin A (daily amounts of 750 mcg RAE to 3,000 mcg RAE, depending on age, are recommended in the United States and Australia), other fat-soluble vitamins, and pancreatic enzymes 242, 244.
Individuals with gastrointestinal disorders
Approximately one quarter of children with Crohn’s disease and ulcerative colitis have vitamin A deficiency; adults with these disorders, especially those who have had the disorder for several years, also have a higher risk of vitamin A deficiency 245, 246. Although some evidence supports the use of vitamin A supplements in people with these disorders 247, other research has found that supplementation offers no benefit 248. Some children and adults with newly diagnosed celiac disease also have vitamin A deficiency; a gluten-free diet can, but does not always, eliminate vitamin A deficiency 249, 250, 251.
Vitamin A deficiency causes
The major underlying causes of vitamin A deficiency may be summarized as follows 252, 253:
- Reduced intake of vitamin A
- Inadequate food supply. Vitamin A deficiency is endemic in areas such as southern and eastern Asia, where rice, devoid of beta-carotene, is the staple food. Xerophthalmia due to primary vitamin A deficiency is a common cause of blindness among young children in developing countries.
- Alcoholism
- Mental illness
- Dysphagia
- Vitamin A deficiency is common in prolonged protein-energy undernutrition not only because the diet is deficient but also because vitamin A storage and transport is defective.
- Impaired absorption of vitamin A
- Crohn’s disease
- Ulcerative colitis
- Celiac disease
- Pancreatic insufficiency
- Cystic fibrosis
- Bile duct obstruction
- Duodenal bypass
- Short bowel syndrome
- Chronic diarrhea
- Giardiasis
- Disordered transport of vitamin A
- Abetalipoproteinemia
- Reduced storage of vitamin A
- Liver disease and cirrhosis
- Cystic fibrosis
- Zinc deficiency
- Zinc deficiency may also be involved with the pathogenesis of secondary vitamin A deficiency. Inadequate zinc can depress the hepatic synthesis of retinol-binding protein (RBP), which is required for mobilization of retinol from the liver. In addition, zinc may play a role in the conversion of beta-carotene to retinol via the enzyme 15-15 dioxygenase 254.
Groups at risk of vitamin A deficiency
The following groups are among those most likely to have inadequate intakes of vitamin A.
Premature infants
Preterm infants have low liver stores of vitamin A at birth, and their plasma concentrations of retinol often remain low throughout the first year of life 231, 232. Preterm infants with vitamin A deficiency have a higher risk of eye and chronic lung diseases 233, 234. However, in high-income countries, clinical vitamin A deficiency is rare in infants and occurs only in those with malabsorption disorders 235.
Infants, children, and pregnant and lactating women in low-income and middle-income countries
Pregnant women need extra vitamin A for fetal growth and tissue maintenance and to support their own metabolism 236, 237, 238. The breast milk of lactating women with adequate vitamin A intakes contains sufficient amounts of vitamin A to meet infants’ needs for the first 6 months of life239. But in people with vitamin A deficiency, the vitamin A content of breast milk is not sufficient to maintain adequate vitamin A stores in infants who are exclusively breastfed 239.
About 190 million preschool-aged children (one third of all children in this age group), mostly in Africa and Southeast Asia, have vitamin A deficiency, according to the World Health Organization 240, 181. They have a higher risk of visual impairment and of illness and death from childhood infections, such as measles and infections that cause diarrheal diseases 240, 16.
The World Health Organization estimates that 9.8 million pregnant women (15% of all pregnant women) around the world, mostly in low-income and middle-income countries, have xerophthalmia as a result of vitamin A deficiency 241.
People with cystic fibrosis
Up to 90% of people with cystic fibrosis have pancreatic insufficiency, which increases their risk of vitamin A deficiency due to difficulty absorbing fat 16, 242. Studies in Australia and the Netherlands indicate that 2% to 13% of children and adolescents with cystic fibrosis have vitamin A deficiency 243, 244. As a result, standard care for cystic fibrosis includes lifelong treatment with vitamin A (daily amounts of 750 mcg RAE to 3,000 mcg RAE, depending on age, are recommended in the United States and Australia), other fat-soluble vitamins, and pancreatic enzymes 242, 244.
Individuals with gastrointestinal disorders
Approximately one quarter of children with Crohn’s disease and ulcerative colitis have vitamin A deficiency; adults with these disorders, especially those who have had the disorder for several years, also have a higher risk of vitamin A deficiency 245, 246. Although some evidence supports the use of vitamin A supplements in people with these disorders 247, other research has found that supplementation offers no benefit 248. Some children and adults with newly diagnosed celiac disease also have vitamin A deficiency; a gluten-free diet can, but does not always, eliminate vitamin A deficiency 249, 250, 251.
Vitamin A deficiency signs and symptoms
Subclinical forms of vitamin A deficiency may not cause any symptoms, but the risk of developing respiratory and diarrheal infections is increased, the growth rate is decreased, and bone development is slowed 255. Patients may have a recent history of increased infections, infertility secondary to impaired spermatogenesis, or recent spontaneous abortion secondary to impaired embryonic development 255. The patient may also report increased fatigue, as a manifestation of vitamin A deficiency anemia.
Signs and symptoms of vitamin A deficiency include the following 256:
- Bitot spots – Areas of abnormal squamous cell proliferation and keratinization of the conjunctiva can be seen in young children with vitamin A deficiency.
- Blindness – Vitamin A has a major role in phototransduction. The cone cells are responsible for the absorption of light and for color vision in bright light. The rod cells detect motion and are responsible for night vision. In the rod cells of the retina, all-trans-retinol is converted into 11-cis -retinol, which then combines with a membrane-bound protein called opsin to yield rhodopsin. [21] A similar type of reaction occurs in the cone cells of the retina to produce iodopsin. The visual pigments absorb light at different wavelengths, according to the type of cone cell they occupy. Vitamin A deficiency leads to a lack of visual pigments; this reduces the absorption of various wavelengths of light, resulting in blindness.
- Poor adaptation to darkness (nyctalopia)
- Dry skin
- Follicular hyperkeratosis (phrynoderma) secondary to blockage of hair follicles with plugs of keratin.
- Dry hair
- Itchy skin (prutitus)
- Broken fingernails
- Conjunctival xerosis
- Corneal xerosis
- Corneal ulcer
- Keratomalacia
- Corneal perforation
- Corneal scarring
- Blindness
- Other signs of vitamin A deficiency include excessive deposition of periosteal bone secondary to reduced osteoclastic activity, anemia, keratinization of mucous membranes, and impairment of the humoral and cell-mediated immune system.
In the eye, vitamin A is essential for maintenance of conjunctival and corneal epithelia as well as night vision. Vitamin A deficiency causes metaplasia and keratinization of mucus-secreting epithelium, which can cause conjunctival and corneal xerosis, corneal ulcers, keratomalacia, and corneal scarring. Rods are the retinal photoreceptor that is responsible for night vision. Rods have a singular photopigment, rhodopsin. Retinol is a vitamin A-derived cofactor that is required for the formation of rhodopsin; thus, vitamin A deficiency leads to impairment of rod function and causes nyctalopia, or night blindness due to the eye’s inability to adapt from light to dark 257.
The most common sign of severe vitamin A deficiency is an eye condition called xerophthalmia (dry eye). Xerophthalmia refers to the spectrum of eye disease that affect the conjunctiva, cornea, and retina caused by severe vitamin A deficiency. Xerophthalmia can impair your ability to see in low light (dark adaptation of the eyes), which can lead to night blindness and is an early symptom of severe vitamin A deficiency.
Xerophthalmia (dry eye) is the most common sign of vitamin A deficiency (which is nearly characteristic) results from keratinization of the eyes. It involves drying (xerosis) and thickening of the conjunctivae (conjunctival xerosis) and corneas (corneal xerosis). Superficial foamy patches composed of epithelial debris and secretions on the exposed bulbar conjunctiva (Bitot spots) develop. In advanced vitamin A deficiency, the cornea becomes hazy and can develop erosions (corneal ulcers), which can lead to its destruction (keratomalacia). Xerophthalmia can lead to blindness if it isn’t treated.
Keratinization of the skin and of the mucous membranes in the respiratory, gastrointestinal, and urinary tracts can occur. Drying, scaling, and follicular thickening of the skin and respiratory infections can result.
Immunity is generally impaired. Infection with the measles virus was found to precipitate conjunctival and corneal damage, leading to blindness in children with poor vitamin A status 258. Conversely, vitamin A deficiency can be considered a nutritionally acquired immunodeficiency disease 259. Even children who are only mildly deficient in vitamin A have a higher incidence of respiratory complications and diarrhea, as well as a higher rate of mortality from measles infection compared to children consuming sufficient vitamin A 260. Because vitamin A supplementation may decrease both the severity and incidence of measles complications in developing countries, WHO recommends that children aged at least one year receive 200,000 IU of vitamin A (60,000 mcg RAE) for two consecutive days in addition to standard treatment when children are infected with measles virus and live in areas of vitamin A deficiency 157.
The younger the patient, the more severe are the effects of vitamin A deficiency. Growth retardation and infections are common among children. Mortality rate can exceed 50% in children with severe vitamin A deficiency. Routine vitamin A supplementation with retinol capsule of 200 000 IU retinyl acetate in oil to children in endemic areas leads to a decrease of childhood mortality of 5-15% 261.
A long-term vitamin A deficiency can also lead to a higher risk of respiratory diseases (such as pneumonia) and infections (such as measles and diarrhea). It can also cause anemia (a condition in which the red blood cells do not supply enough oxygen to the body). In severe cases, not getting enough vitamin A can increase your chances of dying. A study from Bangladesh showed that almost two-thirds of children with the most severe form of xerophthalmia – known as keratomalacia (a corneal ulcer affecting more than a third of the cornea) – had died within a few months 262.
Table 3. World Health Organization (WHO) classification of vitamin A deficiency and the age groups most affected
| Grade of xerophthalmia | Peak age group (years) | Type of deficiency | Risk of death | |
|---|---|---|---|---|
| XN | Night blindness | 2–6; adult women | Longstanding. Not blinding | + |
| X1A | Conjunctival xerosis | 3–6 | Longstanding. Not blinding | + |
| X1B | Bitot’s spot | 3–6 | Longstanding. Not blinding | + |
| X2 | Corneal xerosis | 1–4 | Acute deficiency. Can be blinding | ++ |
| X3A | Corneal ulcer/ <1/3 cornea | 1–4 | Severe acute deficiency. Blinding | +++ |
| X3B | Corneal ulcer/keratomalacia ≥1/3 | 1–4 | Severe acute deficiency. Blinding | ++++ |
| XS | Corneal scarring (from X3) | >2 | Consequence of corneal ulceration | +/– |
| XF | Xerophthalmic fundus | Adults | Longstanding. Not blinding. Rare | – |
Night blindness
Night blindness or defective vision in dim light is one of the most common manifestations of vitamin A deficiency, especially in children age 2-6 or pregnant or lactating women. Although it is considered one of the earliest manifestations, children with vitamin A deficiency may develop one of the more severe signs, such as corneal ulcers, after infection or diarrhea without any of the classically early signs. Children may not be able to verbalize their symptoms, and parents need to be asked if they have noticed their children behaving differently in the dark, e.g. becoming less active or fearful. Night blindness is considered to be both a sensitive and specific indicator for serum retinol levels.
Conjunctival xerosis
Conjunctival xerosis is characterized by a dull and dry appearance of the conjunctiva with slight wrinkling. It is caused by the loss of goblet cells and insufficient mucin secretion, and it can be subtle and difficult to detect clinically.
Bitot spots
Bitot spots are collections of desquamated, keratinized epithelial cells mixed with the gas-forming bacteria Corynebacterium xerosis. They appear as triangular patches of foamy, whitish, opaque deposits, typically located on the bulbar conjunctiva near the limbus at the 3 and 9 o’clock positions. They are more common temporally.
Corneal xerosis
Corneal xerosis is characterized by a dull and hazy appearance of the cornea that is caused by drying of the cornea secondary to conjunctival gland dysfunction. It may initially present with bilateral punctate corneal epithelial erosions, and it can quickly progress to the stage of corneal ulceration. Up to this stage, high-dose Vitamin A supplementation can result in the full preservation of vision.
Corneal ulceration
Corneal xerosis can lead corneal ulceration and melting if not treated urgently. Keratomalacia, the melting away of the cornea by liquefactive necrosis, is the most severe form of xerophthalmia. It can perforate and destroy the cornea in just a matter of days. A child who appears relatively healthy but develops keratomalacia should be questioned about a recent history of measles or diarrhea, which could rapidly deplete already deficient vitamin A stores.
Corneal scar
Corneal scarring due to vitamin A deficiency is often symmetric and bilateral. Other causes of corneal scarring must be ruled out.
Xerophthalmic fundus
Prolonged vitamin A deficiency can lead to structural changes in the retina. Xerophthalmic fundus changes appear as small, white, deep retinal lesions scattered throughout the posterior pole.
Vitamin A deficiency prevention
To prevent vitamin A deficiency, your diet should include dark green leafy vegetables, deep- or bright-colored fruits (eg, papayas, oranges), carrots, and yellow vegetables (eg, squash, pumpkin). Vitamin A–fortified milk and cereals, liver, egg yolks, and fish liver oils are helpful. Carotenoids are absorbed better when consumed with some dietary fat. If milk allergy is suspected in infants, they should be given adequate vitamin A in formula feedings.
In developing countries, prophylactic supplements of vitamin A palmitate in oil 200,000 IU (60,000 RAE) orally every 6 months are advised for all children between 1 and 5 yr of age; infants < 6 months can be given a one-time dose of 50,000 IU (15,000 RAE), and those aged 6 to 12 months can be given a one-time dose of 100,000 IU (30,000 RAE).
Vitamin A deficiency diagnosis
Patients presenting with clinical signs of xerophthalmia (or, in the case of a children, their parents) must be questioned on dietary, medical, and social history, including alcohol intake. Be sure to ask about any malabsorptive process, as described above, or risk factors such as living in a resource-poor country or current pregnancy or lactation. Because xerophthalmia is a manifestation of moderate-to-severe vitamin A deficiency, it is important to ask about the systemic signs of milder vitamin A deficiency, including frequent gastrointestinal and respiratory tract infections, anemia, iron deficiency, and development of xeroderma and phrynoderma (follicular hyperkeratosis often found on extensor surfaces, shoulders, and buttocks) 263.
Serum retinol levels, clinical evaluation, and response to vitamin A help diagnose vitamin A deficiency in people with symptoms, such as night blindness, or in people with diseases that impair intestinal absorption of nutrients and who are at risk of vitamin A deficiency.
Ocular findings suggest vitamin A deficiency. Dark adaptation can be impaired in other disorders (eg, zinc deficiency, retinitis pigmentosa, severe refractive errors, cataracts, diabetic retinopathy). If dark adaptation is impaired, rod scotometry and electroretinography are done to determine whether vitamin A deficiency is the cause.
Serum levels of retinol are measured. Normal range of vitamin A/retinol is 28 to 86 mcg/dL (1 to 3 mcmol/L). Vitamin A deficiency is defined as serum retinol levels of below 28 μg/dL. However, levels decrease only after the deficiency is advanced because the liver contains large stores of vitamin A. Also, decreased levels may result from acute infection, which causes retinol-binding protein and transthyretin (also called prealbumin) levels to decrease transiently.
A therapeutic trial of vitamin A may help confirm the diagnosis.
Physical exam
Aside from the ocular exam, the physical exam should include assessments for weight/body habitus, jaundice, and abdominal exam for hepatomegaly.
Blood tests
The following laboratory studies may be considered in the workup 264, 265:
- Serum vitamin A or retinol (reference range: 20-60 mcg/dL). These levels can be normal due to maintenance of circulating retinol levels by hepatic stores. A serum retinol concentration of 0.70 μmol/L (20 mcg/dL) or less frequently reflects subclinical vitamin A deficiency. A value of less than 0.70 μmol/L (20 mcg/dL) in children younger than 12 years is considered low 266. Vitamin A deficiency-related ocular symptoms have been shown to develop at serum retinol concentration less than 0.35 μmol/L (10 mcg/dL), and is considered an indicator of severe vitamin A deficiency, where vitamin A body stores are depleted 16
- Serum retinol-binding protein (RBP) (reference range: 30-75 ug/ml). A serum retinol-binding protein (RBP) study is easier to perform and less expensive than a serum retinol study, because retinol-binding protein (RBP) is a protein and can be detected by an immunologic assay. Retinol-binding protein (RBP) is also a more stable compound than retinol with respect to light and temperature. However, retinol-binding protein (RBP) levels are less accurate, because they are affected by serum protein concentrations and because types of retinol-binding protein (RBP) cannot be differentiated 267, 268, 269. The serum retinol level may be low during infection because of a transient decrease in the retinol-binding protein (RBP).
- Serum zinc (reference range: 75-120 mcg/dL). A zinc level is useful because zinc deficiency interferes with retinol-binding protein (RBP) production.
- An iron panel is useful because iron deficiency can affect the metabolism of vitamin A.
- Albumin levels are indirect measures of vitamin A levels.
- Obtain a complete blood count (CBC) with differential if anemia, infection, or sepsis is a possibility.
- An electrolyte evaluation and liver function studies should be performed to evaluate for nutritional and volume status.
Other testing
- Dark adatopmetry and night vision threshold tests 270
- Electroretinogram (ERG): Retinopathy from vitamin A deficiency is associated with decreased amplitude
- Impression cytology: conjunctival specimens can be viewed for the presence of goblet cells. A decrease in normal amount is considered an effective measurement of vitamin A deficiency 271.
- In children, radiographic films of the long bones may be useful when an evaluation is being made for bone growth and for excessive deposition of periosteal bone.
- Liver biopsy: considered the gold standard for evaluating total body vitamin A, although it is not routinely used outside of the research setting due to procedural risks.
Vitamin A deficiency treatment
The recommended vitamin A deficiency treatment regimens are described in table 5 below. Dietary deficiency of vitamin A is traditionally treated with vitamin A palmitate in oil 60,000 IU oral once/day for 2 days, followed by 4500 IU oral once/day. If vomiting or malabsorption is present or xerophthalmia is probable, a dose of 50,000 IU for infants less than 6 months of age, 100,000 IU for infants 6 to 12 months of age, or 200,000 IU for children greater than 12 months of age and adults should be given for 2 days, with a third dose at least 2 weeks later. Vitamin A deficiency is a risk factor for severe measles; treatment with vitamin A can shorten the duration of the measles and may reduce the severity of symptoms and risk of death. The World Health Organization (WHO) recommends that all children with measles in developing countries should receive 2 doses of vitamin A, (100,000 IU for children < 12 months and 200,000 IU for those >12 months) given 24 hour apart 272.
Vitamin A deficiency associated with malabsorptive or other processes is treated based on the severity of vitamin A deficiency and at the doctor’s discretion, usually involving daily treatment.
Infants born of HIV-positive mothers should receive 50,000 IU (15,000 RAE) within 48 hours of birth. Prolonged daily administration of large doses, especially to infants, must be avoided because toxicity may result.
Patients with concomitant zinc deficiency should also undergo zinc supplementation 273. If the patient’s vitamin A deficiency is from malabsorption, doctors should consider intramuscular vitamin A supplementation formulations.
For pregnant or breastfeeding women, prophylactic or therapeutic doses should not exceed 10,000 IU (3000 RAE)/day to avoid possible damage to the fetus or infant.
In regions with a high prevalence of vitamin A deficiency, the World Health Organization (WHO) recommends universal vitamin A supplementation of select populations 274. The World Health Organization (WHO) recommends a one-time dose of 100,000 IU in children 6 to 11 months of age followed by doses of 200,000 IU every 4 to 6 months up to 5 years of age 240. At-risk pregnant women should receive vitamin A supplementation at lower doses due to concern for fetotoxicity; the recommended dosing is 10,000 IU daily or 25,000 IU weekly for 12 weeks 115. The WHO no longer recommends universal supplementation for children less than 6 months of age or postpartum women 275, 276, 277.
International guidelines do not clearly outline dosing of vitamin A supplementation for asymptomatic vitamin A deficiency in resource-rich regions 274. Treatment for subclinical vitamin A deficiency includes the consumption of vitamin A rich foods, such as liver, beef, chicken, eggs, fortified milk, carrots, mangoes, sweet potatoes, and leafy green vegetables 278.
In resource-rich countries, post-weight loss surgery patients and neonates have particular dosing recommendations. Post-weight loss surgery patients are recommended to take 10,000 IU vitamin A supplementation daily and adjust as needed based on regular serum retinol level monitoring. Some weight loss surgery patients have been known to need up to 100,000 IU vitamin A supplementation daily 279. For premature infants, guidelines do not exist yet for vitamin A supplementation. Still, recent studies have shown that vitamin A supplementation of 10,000 IU every other day in very low birth weight neonates for 4 weeks has significant results, decreasing all-cause mortality by 56% and decreasing rates of oxygen requirement, sepsis, patent ductus arteriosus, and length of hospital stay 280. Vitamin A supplementation of 1,500 IU daily in extremely premature infants had a significant decrease in retinopathy of prematurity (1.6% vs. 6.9%) and a nearly 50% decrease in bronchopulmonary dysplasia 234. Vitamin A deficiency associated with other malabsorptive processes is treated on a case-by-case basis.
Treatment can be adjusted as needed based on regular serum retinol level monitoring.
Localized ocular treatment includes intense lubrication, topical retinoid acid, and management of perforation.
Table 4. Vitamin A deficiency treatment regimens
| Vitamin A dosage (IU) | |
|---|---|
| Young infants 0-5 months 1 | 50,000 IU |
| Older infants 6-11 months 1 | 100,000 IU |
| Children (males: 12 mo or more; females 12 mo to 12 y and 50 y or more) 1 | 200,000 IU |
| Women (13-49 years of age) with night blindness and/or Bitot’s spots | 10,000 every day or 25,000 every week for at least 3 months |
| Women (13-49 years of age) with active corneal lesions | 200,000 on days 1, 2, and 14 |
Footnote: 1 Schedule: severe malnutrition, day 1; measles, days 1 and 2; xerophthalmia, days 1, 2, and 14.
[Source 281 ]Vitamin A deficiency prognosis
The prognosis of vitamin A deficiency depends on the severity of the disease at treatment initiation 229. If treated promptly, patients with subclinical vitamin A deficiency have a very good prognosis without long term consequences. Treatment at any stage of severity can show improvement within a week 282.
The early ophthalmologic signs, such as night blindness, conjunctival xerosis, and Bitot spots, will resolve completely within about 2 months of vitamin A supplementation, while corneal xerosis and ulceration results in scarring that may lead to permanent vision loss despite treatment 229, 283. Irreversible conditions include punctate keratopathy, keratomalacia, and corneal perforation. At the onset of vitamin A deficiency eye signs and symptoms, patients develop an increased susceptibility to infection. In preschool children with vitamin A deficiency, the presence of ophthalmologic signs indicates increased overall mortality from gastrointestinal, pulmonary, and other vitamin A deficiency-related mucosal infections 229.
Xerophthalmia signifies a severity of vitamin A deficiency that can cause mortality from malnutrition and increased susceptibility to mucosal infections. The death rate (mortality) in children with night blindness is triple the mortality found in children with subclinical vitamin A deficiency 274. Children with both Bitot’s spots and night blindness have death rate nine times that of children with subclinical vitamin A deficiency. Nearly two-thirds of children with keratomalacia die within months 229.
Vitamin A Toxicity
High intakes of some forms, usually from supplements or certain medicines, of vitamin A can be harmful 284, 285. Acute vitamin A toxicity also referred to as hypervitaminosis A can occur from either the topical vitamin A or oral vitamin A that occurs within days to weeks after someone ingests one or a few very high doses (typically more than 100 times the Recommended Dietary Allowance [RDA]) of preformed vitamin A (retinol and retinyl ester but not carotenoids) 286, 287. Getting too much preformed vitamin A usually from supplements or certain medicines can cause dizziness, nausea, blurred vision, headaches, aching muscles, coordination problems, liver injury, jaundice, enlargement of the liver and spleen, portal hypertension and cirrhosis 288. In severe cases, cerebral spinal fluid pressure can increase, leading to drowsiness and, eventually, coma and even death 286.
High intakes of preformed vitamin A in pregnant women can also cause birth defects in their babies. Birth abnormalities include craniofacial, cardiac, and central nervous system malformations. Women who might be pregnant should not take high doses of vitamin A supplements 288. Consuming high amounts of beta-carotene or other forms of provitamin A can turn the skin yellow-orange, but this condition is harmless. High intakes of beta-carotene do not cause birth defects or the other more serious effects caused by getting too much preformed vitamin A.
Although symptoms of vitamin A toxicity may vary, headache and rash usually develop during acute or chronic toxicity.
- Acute toxicity causes increased intracranial pressure. Drowsiness, irritability, abdominal pain, nausea, and vomiting are common. Sometimes the skin subsequently peels.
- Early symptoms of chronic toxicity are sparsely distributed, coarse hair; alopecia of the eyebrows; dry, rough skin; dry eyes; and cracked lips. Later, severe headache, pseudotumor cerebri, and generalized weakness develop. Cortical hyperostosis of bone and arthralgia may occur, especially in children. Fractures may occur easily, especially in the elderly. In children, toxicity can cause pruritus, anorexia, and failure to thrive. Hepatomegaly and splenomegaly may occur.
Oral vitamin A toxicity can be acute or chronic.
- Acute oral vitamin A toxicity occurs because of the ingestion of a large amount of vitamin A over a short period of time. The typical manifestations of acute hypervitaminosis A are dry skin, cheilosis, dermatitis, joint and bone pain, headaches, and fatigue. A high proportion of patients also have liver test abnormalities, but these are typically mild. Jaundice is uncommon, but enlargement of the liver may be present. The presence of splenomegaly suggests that cirrhosis or portal hypertension was present at the time of initial presentation, as vitamin A is not stored in the spleen and does not cause splenomegaly on its own.
- In chronic oral vitamin A toxicity, intake is over a longer duration. Signs and symptoms of chronic hypervitaminosis A include low-grade fever, headache, fatigue, anorexia, intestinal disturbances, hepatosplenomegaly, anemia, hypercalcemia, subcutaneous swelling, nocturia, joint and bone pain, and skin changes such as yellowing, dryness, alopecia, and photosensitivity. Neuropsychiatric changes as a consequence of chronic hypervitaminosis A have also been reported 289. It was proposed that toxic levels of unbound retinyl esters (preformed vitamin A) can elicit neuropsychiatric effects, in-cluding depression, psychosis, and impulsivity 290.
Topical retinoids are creams, lotions and gels containing medicine derived from vitamin A. The most common adverse effect of topical retinoids is skin irritation, notably skin redness and peeling. The most severe adverse effect of systemic retinoids is teratogenicity (major birth defects following fetal exposure during pregnancy).
Chronic hypervitaminosis A (regular consumption of high doses of vitamin A) can cause dry itchy skin, painful muscles and joints, fatigue, depression, weight loss, headache, cerebral edema, enlarged liver, enlarged spleen, anemia and abnormal liver test results 286, 6. Also, symptoms of vitamin A toxicity in infants include bulging fontanels 6. Severe cases of hypervitaminosis A (overconsumption of preformed vitamin A) may result in liver damage, hemorrhage, and coma. Generally, signs of vitamin A toxicity are associated with long-term consumption of vitamin A in excess of 10 times the RDA (8,000-10,000 mcg RAE/day or 25,000-33,000 IU/day) 6. However, more research is necessary to determine if subclinical vitamin A toxicity is a concern in certain populations 291. There is evidence that some populations may be more susceptible to vitamin A toxicity at lower doses, including the elderly, chronic alcohol users, and some people with a genetic predisposition to high cholesterol 270.
Total intakes of preformed vitamin A that exceed the tolerable upper intake level (UL), as well as some retinoid medications used as topical therapies (such as isotretinoin, used to treat severe acne, and etretinate, a treatment for severe psoriasis) can cause congenital birth defects 16. These birth defects can include malformations of the eye, skull, lungs, and heart 20. Experts advise people who are or might be pregnant and those who are lactating not to take high doses (more than 3,000 mcg RAE [10,000 IU] daily) of vitamin A supplements 16.
Although carotene is converted to vitamin A in the body, excessive ingestion of beta-carotene (provitamin A) causes carotenemia, not vitamin A toxicity and is not known to be teratogenic or lead to reproductive toxicity 16. Carotenemia is usually asymptomatic but may lead to carotenosis or carotenodermia, a harmless condition in which the skin becomes yellow-orange 18. Carotenodermia or excess beta-carotene can be reversed by discontinuing beta-carotene ingestion. When taken as a supplement, beta-carotene has been associated with increased cancer risk; risk does not seem to increase when carotenoids are consumed in fruits and vegetables. The ATBC trial found that supplementation with a large amount of beta-carotene (20 mg/day), with or without 50 mg/day vitamin E, for 5–8 years increased the risk of lung cancer and mortality (mainly from lung cancer and ischemic heart disease) in male smokers 145. The CARET trial also showed that supplementation with a large amount of beta-carotene (30 mg/day) plus 7,500 mcg RAE (25,000 IU)/day retinyl palmitate for 4–8 years in current and former smokers, as well as some men occupationally exposed to asbestos, increased the risk of lung cancer and death from lung cancer 142.
The recommended daily allowance for vitamin A is 300 to 700 mcg for children and approximately 700 to 900 mcg for adults, amounts which can be provided by a normal diet 292.
Each year, in the US alone over 60,000 cases of vitamin A toxicity are reported. Unlike the water-soluble vitamins, the fat-soluble vitamins (vitamins A, D, E and K) tend to accumulate in your body. In general, people with known chronic liver diseases should avoid ingestion of more than the minimal daily requirement of vitamin A as underlying liver disease appears to increase the susceptibility to vitamin A toxicity.
Treatment of hypervitaminosis A involves simply stopping supplements (or in rare cases, foods) that contain vitamin A. Most of the signs and symptoms of hypervitaminosis A (acute retinoid toxicity, hypertriglyceridemia, skin and central nervous system symptoms) may resolve within several weeks after discontinuing from ingestion of vitamin A and instituting supportive therapy. Some of these symptoms, such as skin desquamation, remain evident for several months. The liver injury caused by high doses of vitamin A is reversible in its early stages, but may resolve only slowly with discontinuation of vitamin A ingestion and resumption of a normal diet. Usually, portal hypertension resolves within months to years after discontinuation of the vitamin A supplement. However, in some cases, the liver injury progresses to cirrhosis, even requiring transplantation 293. Patients with increased intracranial pressure may require lumbar punctures or medications such as mannitol and diuretics for therapy. However, irreversible central nervous system consequences may occur 294, 295. Eye dryness following hypervitaminosis A is managed with eye drops. Patients with hypercalcemia may require intravenous fluids and additional therapy such as calcitonin and corticosteroids 296.
Birth defects caused by vitamin A are irreversible.
What is the tolerable upper intake level of vitamin A?
Because vitamin A is fat soluble, the body stores excess vitamin A primarily in the liver, and these vitamin A levels can accumulate. In January 2001, the Food and Nutrition Board of the US Institute of Medicine set the tolerable upper intake level (UL) of vitamin A intake for adults at 3,000 mcg RAE (10,000 IU)/day of preformed vitamin A 114. The tolerable upper intake level (UL) does not apply to vitamin A derived from carotenoids (vitamin A in fruits and vegetables).
- Recommended Dietary Allowance (RDA) is the average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals.
- Retinol activity equivalents (RAE) were developed because provitamin A carotenoids have less vitamin A activity than preformed vitamin A; 1 mcg retinol = 3.33 IU.
The Food and Nutrition Board of the US Institute of Medicine has not established tolerable upper intake levels (ULs) for beta-carotene and other provitamin A carotenoids 18. However, the Food and Nutrition Board advises against the use of beta-carotene supplements for the general population, except as a provitamin A source to prevent vitamin A deficiency.
Table 5. Tolerable Upper Intake Levels (ULs) for Preformed Vitamin A
| Age | Male | Female | Pregnancy | Lactation |
|---|---|---|---|---|
| Birth to 12 months | 600 mcg | 600 mcg | ||
| 1–3 years | 600 mcg | 600 mcg | ||
| 4–8 years | 900 mcg | 900 mcg | ||
| 9–13 years | 1,700 mcg | 1,700 mcg | ||
| 14–18 years | 2,800 mcg | 2,800 mcg | 2,800 mcg | 2,800 mcg |
| 19+ years | 3,000 mcg | 3,000 mcg | 3,000 mcg | 3,000 mcg |
Footnotes: These tolerable upper intake levels (ULs) apply only to products from animal sources and supplements whose vitamin A comes entirely from retinol or its ester forms, such as retinyl palmitate. However, many dietary supplements (such as multivitamins) do not provide all of their vitamin A in retinol or its ester forms. For example, the vitamin A in some supplements consists partly or entirely of beta-carotene. In such cases, the percentage of retinol or retinyl ester in the supplement should be used to determine whether an individual’s vitamin A intake exceeds the UL. For example, a supplement whose label indicates that the product contains 3,000 mcg RAE vitamin A and that 60% of this vitamin A comes from beta-carotene (and therefore 40% comes from retinol or retinyl ester) provides 1,200 mcg RAE of preformed vitamin A. That amount is above the UL for children from birth to 8 years but below the UL for older children and adults.
[Source 120 ]Vitamin A Toxicity causes
Vitamin A is a fat-soluble vitamin that is stored in the liver. Many foods contain vitamin A, including:
- Meat, fish, and poultry
- Dairy products
- Some fruits and vegetables
Some dietary supplements also contain Vitamin A.
Vitamin A toxicity is more commonly associated with abuse of vitamin A supplements than with health intervention programs. Vitamin A toxic reactions may also be provoked by consuming liver products rich in vitamin A or excess administration of vitamin A preparations. The amount of vitamin A required to cause toxicity among individuals varies depending on age and liver function.
Acute hypervitaminosis A may occur with a single ingestion of 25,000 IU/kg or more. However, this cutoff level is decreased in individuals with heavy alcohol consumption or with kidney failure 297, 298. Signs and symptoms include nausea, vomiting, diarrhea, dizziness, lethargy, drowsiness, increased intracranial pressure, and skin changes such as erythema, pruritus, or desquamation.
Chronic hypervitaminosis A may occur with excessive ingestion of 4000 IU/kg or more daily for 6-15 months. Signs and symptoms include low-grade fever, headache, fatigue, anorexia, intestinal disturbances, hepatosplenomegaly, anemia, hypercalcemia, subcutaneous swelling, nocturia, joint and bone pain, and skin changes such as yellowing, dryness, alopecia, and photosensitivity. Neuropsychiatric changes as a consequence of chronic hypervitaminosis A have also been reported 289. It was proposed that toxic levels of unbound retinyl esters (preformed vitamin A) can elicit neuropsychiatric effects, in-cluding depression, psychosis, and impulsivity 290.
To convert International Units (IUs) to mcg RAE, use the following 299:
- 1 IU retinol = 0.3 mcg RAE (retinol activity equivalents)
- 1 IU beta-carotene from dietary supplements = 0.15 mcg RAE
- 1 IU beta-carotene from food = 0.05 mcg RAE
- 1 IU alpha-carotene or beta-cryptoxanthin = 0.025 mcg RAE
RAE can only be directly converted into IUs if the source or sources of vitamin A are known. For example, the RDA of 900 mcg RAE for adolescent and adult men is equivalent to 3,000 IU if the food or supplement source is preformed vitamin A (retinol) or if the supplement source is beta-carotene. This RDA is also equivalent to 18,000 IU beta-carotene from food or to 36,000 IU alpha-carotene or beta-cryptoxanthin from food. Therefore, a mixed diet containing 900 mcg RAE provides between 3,000 and 36,000 IU vitamin A, depending on the foods consumed.
Vitamin A is highly teratogenic if taken during pregnancy. Retinoids affect the expression of homeobox gene Hoxb-1, which regulates axial patterning of the embryo. Birth abnormalities include craniofacial, cardiac, and central nervous system malformations. Therefore, treatment with vitamin A should be avoided in pregnant patients except in areas where vitamin A deficiency is prevalent. In this circumstance, supplementation should not exceed 10,000 IU daily 296.
Vitamin A Toxicity symptoms
The signs and symptoms of hypervitaminosis A depend on the size and rapidity of the excess vitamin A intake. The symptoms of hypervitaminosis A following sudden, massive intakes of vitamin A, as with Arctic explorers who ate polar bear liver, are acute poisoning 300. The main symptoms observed in acute toxicity are nausea, irritability, reduced appetite, vomiting, blurry vision, headaches, hair loss, muscle pain, papilledema, hemorrhage, weakness, drowsiness and altered mental status 301, 302. These symptoms are quite frequent, whereas intracranial hypertension rarely appears. Idiopathic intracranial hypertension (pseudotumor cerebri), a syndrome characterized by headache, blurred vision, confusion and increased intracerebral pressure, is also being reported in patients with excessive consumption of vitamin A or by those treated with isotretinoin 303. Hypertriglyceridemia is the most common biochemical adverse effect detected after retinoid administration. This appears several weeks after the initiation of treatment. Eventually, these elevated triglyceride levels lead to liver damage, which, as a consequence, causes fibrosis and hepatic stellate cell activation, leading to possible irreversible liver damage. On the other hand, oral retinoids can cause cracked lips, headache, flushing, stomach pain, dizziness and loss of coordination 294, 304.
Sebum production is decreased as a consequence, which reduces epidermal thickness and alters the barrier function of the skin. These and other skin effects (skin dryness, pruritus, overall and fingertip fissuring), including alopecia, also appear in this type of toxicity but usually disappear upon cessation of the treatment.
In chronic vitamin A toxic patients, in addition to the acute symptoms described above, insomnia, hypothyroidism, bone destruction, anemia, fatigue, diarrhea, dry and pruritic skin, skin and mucosa desquamation, hepatosplenomegaly, liver hypertrophy, hypertension, fibrosis, sclerosis and cirrhosis can appear 305, 306.
In addition, topical administration of retinoids can be associated with significant symptoms. Topical retinoids can cause skin redness (erythema), skin peeling (secondary to the hyper-proliferation of the epidermis) and discomfort. Other adverse effects include transient hypopigmentation or hyperpigmentation, psoriasis, allergic contact dermatitis and ectropion (the eyelids turned outwards) 303, 307.
Importantly, retinoid overdose can cause the so-called “retinoic acid syndrome”. This condition manifests as acute respiratory distress with dyspnea, pleural and pericardial effusions, fever, weight gain, edema, and even multiorgan failure 308.
Hypervitaminosis A symptoms may include:
- Abnormal softening of the skull bone (in infants and children)
- Blurred vision
- Bone pain or swelling
- Bulging of the soft spot in an infant’s skull (fontanelle)
- Changes in alertness or consciousness
- Decreased appetite
- Dizziness
- Double vision (in young children)
- Drowsiness
- Hair changes, such as hair loss and oily hair
- Headache
- Irritability
- Liver damage
- Nausea
- Poor weight gain (in infants and children)
- Skin changes, such as cracking at corners of the mouth, higher sensitivity to sunlight, oily skin, peeling, itching, and yellow color to the skin
- Vision changes
- Vomiting
- Large doses of vitamin A during pregnancy can cause birth defects in the babies. Women who might be pregnant should not take high doses of vitamin A supplements.
- Acute vitamin A poisoning occurs quickly, most often when an adult takes several hundred thousand international units (IUs) of vitamin A.
- Chronic vitamin A poisoning may occur over time in adults who regularly take more than 25,000 IU a day.
- Babies and children are more sensitive to vitamin A. They can become sick after taking smaller doses of vitamin A or if they swallow products that contain vitamin A, such as skin cream with retinol in it.
The symptoms of acute hypervitaminosis A are drowsiness, sluggishness, irritability or irresistible desire to sleep and severe headache and vomiting 300. During the second 24 hours, the skin of the patients began to peel around the mouth, beginning in spots and gradually spreading over larger areas. In some cases the peeling was confined to the face, but in several it was general. Lindhard 309 also described three other cases in which the skin peeled from head to foot after eating polar bear liver. The Norwegian explorer Nansen [1924] has mentioned that on two occasions he ate small amounts of bear liver without ill effects. It seems probable therefore that vitamin A toxicity only occur when large quantities are consumed.
Chronic intakes of excess vitamin A lead to increased intracranial pressure (pseudotumor cerebri), dizziness, nausea, headaches, skin irritation, pain in joints and bones, coma, and even death 3. Although hypervitaminosis A can be due to excessive dietary intakes, the condition is usually a result of consuming too much preformed vitamin A from supplements or therapeutic retinoids 4. When people consume too much vitamin A, their tissue levels take a long time to fall after they discontinue their intake, and the resulting liver damage is not always reversible.
Observational studies have suggested an association between high intakes of preformed vitamin A (more than 1,500 mcg daily—only slightly higher than the RDA), reduced bone mineral density, and increased fracture risk 310. However, the results of studies on this risk have been mixed, so the safe retinol intake level for this association is unknown.
Total intakes of preformed vitamin A that exceed the Upper Intake Level (UL) and some synthetic retinoids used as topical therapies (such as isotretinoin and etretinate) can cause congenital birth defects 3. These birth defects can include malformations of the eye, skull, lungs, and heart 20. Women who might be pregnant should not take high doses of vitamin A supplements 3.
Vitamin A Toxicity in Infants and Children
There are numerous case reports of infants, toddlers, and children who have demonstrated toxic effects due to excess vitamin A intakes for months to years. Of particular concern are intracranial (bulging fontanel) and skeletal abnormalities that can result in infants given vitamin A doses of 5,500 to 6,750 μg/day 311. The clinical presentation of vitamin A toxicity in infants and young children varies widely. The more commonly recognized signs and symptoms include skeletal abnormalities, bone tenderness and pain, increased intracranial pressure, desquamation, brittle nails, mouth fissures, alopecia, fever, headache, lethargy, irritability, weight loss, vomiting, and hepatomegaly 312. Furthermore, tolerance to excess vitamin A intake also appears to vary 313. Carpenter and coworkers 313 described two boys who developed hypervitaminosis A by age 2 years for one and by age 6 years for the other. Both were given chicken liver that supplied about 690 μg/day of vitamin A and various supplements that supplied another 135 to 750 μg/day. An older sister who had been treated similarly remained completely healthy.
How Does Vitamin A Affect Your Bones?
Vitamin A is a family of fat-soluble compounds that play an important role in vision, bone growth, reproduction, cell division, and cell differentiation. Vitamin A is important for healthy bones. However, too much vitamin A has been linked to bone loss and an increase in the risk of hip fracture. Scientists believe that excessive amounts of vitamin A trigger an increase in osteoclasts, the cells that break down bone. They also believe that too much vitamin A may interfere with vitamin D, which plays an important role in preserving bone 314.
Beta-carotene, on the other hand, is largely considered to be safe and has not been linked to adverse effects in bone or elsewhere in the body.
Vitamin A Toxicity complications
Hypervitaminosis A complications can include:
- Very high calcium level
- Failure to thrive (in infants)
- Kidney damage due to high calcium
- Liver damage
Taking too much vitamin A during pregnancy may cause birth defects. Talk to your health care provider about eating a proper diet while you are pregnant.
Consuming high amounts of beta-carotene or other forms of provitamin A can turn the skin yellow-orange, but this condition is harmless. High intakes of beta-carotene do not cause birth defects or the other more serious effects caused by getting too much preformed vitamin A.
Vitamin A Toxicity diagnosis
These tests may be done if a high vitamin A level is suspected:
- Bone x-rays
- Blood calcium test
- Cholesterol test
- Liver function test
- Blood test to check vitamin A level
Diagnosis of vitamin A toxicity is clinical. Blood vitamin levels correlate poorly with toxicity. However, if clinical diagnosis is equivocal, laboratory testing may help. In vitamin A toxicity, fasting serum retinol levels may increase from normal (28 to 86 mcg/dL [1 to 3 mcmol/L]) to > 100 mcg/dL (> 3.49 mcmol/L), sometimes to > 2000 mcg/dL (> 69.8 mcmol/L). Hypercalcemia is common.
The plasma retinol concentration is not a reliable estimate of the vitamin A requirement because of its insensitive relationship between liver concentration and there is no noninvasive marker available for the assessment of vitamin A excess 293.
Differentiating vitamin A toxicity from other disorders may be difficult. Carotenosis may also occur in severe hypothyroidism and anorexia nervosa, possibly because carotene is converted to vitamin A more slowly.
Histologically, hypervitaminosis A causes hepatocyte injury, necrosis, stellate cell hyperplasia and subsequent fibrosis resulting in perisinusoidal, pericentrilobular and periportal scarring causing sinusoidal dilatation and obstruction thusimpairing hepatic venous outflow and consequently leading to noncirrhotic portal hypertension 315.
Vitamin A Toxicity treatment
Treatment involves simply stopping supplements (or rarely, foods) that contain vitamin A. Most of the signs and symptoms of hypervitaminosis A (acute retinoid toxicity, hypertriglyceridemia, skin and central nervous system symptoms) may resolve within several weeks after discontinuing from ingestion of vitamin A and instituting supportive therapy. Some of these symptoms, such as skin desquamation, remain evident for several months. The liver injury caused by high doses of vitamin A is reversible in its early stages, but may resolve only slowly with discontinuation of vitamin A ingestion and resumption of a normal diet. Usually, portal hypertension resolves within months to years after discontinuation of the vitamin A supplement. However, in some cases, the liver injury progresses to cirrhosis, even requiring transplantation 293. Patients with increased intracranial pressure may require lumbar punctures or medications such as mannitol and diuretics for therapy. However, irreversible central nervous system consequences may occur 294, 295. Eye dryness following hypervitaminosis A is managed with eye drops. Patients with hypercalcemia may require intravenous fluids and additional therapy such as calcitonin and corticosteroids 296.
However, birth defects in the fetus of a mother who has taken megadoses of vitamin A are not reversible.
Most people fully recover.
Vitamin A Toxicity prognosis
Most people fully recover from hypervitaminosis A. The liver injury caused by high doses of vitamin A is reversible in its early stages, but may resolve only slowly with discontinuation of vitamin A ingestion and resumption of a normal diet. Usually, portal hypertension resolves within months to years after discontinuation of the vitamin A supplement. However, in some cases, the liver injury progresses to cirrhosis, even requiring transplantation 293. Patients with increased intracranial pressure may require lumbar punctures or medications such as mannitol and diuretics for therapy. Patients with hypercalcemia may require intravenous fluids and additional therapy such as calcitonin and corticosteroids 296. Birth defects caused by vitamin A are irreversible.
- D’Ambrosio D.N., Clugston R.D., Blaner W.S. Vitamin A metabolism: An update. Nutrients. 2011;3:63–103. doi: 10.3390/nu3010063[↩]
- Johnson EJ, Russell RM. Beta-Carotene. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:115-20.[↩]
- Ross CA. Vitamin A. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:778-91.[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Ross A. Vitamin A and Carotenoids. In: Shils M, Shike M, Ross A, Caballero B, Cousins R, eds. Modern Nutrition in Health and Disease. 10th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2006:351-75.[↩][↩][↩][↩][↩]
- Kam R.K., Deng Y., Chen Y., Zhao H. Retinoic acid synthesis and functions in early embryonic development. Cell. Biosci. 2012;2:11. doi: 10.1186/2045-3701-2-11[↩]
- Vitamin A. https://lpi.oregonstate.edu/mic/vitamins/vitamin-A[↩][↩][↩][↩][↩]
- National Institute of Health. Vitamin A. https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional[↩]
- Carazo A, Macáková K, Matoušová K, Krčmová LK, Protti M, Mladěnka P. Vitamin A Update: Forms, Sources, Kinetics, Detection, Function, Deficiency, Therapeutic Use and Toxicity. Nutrients. 2021 May 18;13(5):1703. doi: 10.3390/nu13051703[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Kelly M.E., Ramkumar S., Sun W., Colon Ortiz C., Kiser P.D., Golczak M., von Lintig J. The Biochemical Basis of Vitamin A Production from the Asymmetric Carotenoid β-Cryptoxanthin. ACS Chem. Biol. 2018;13:2121–2129. doi: 10.1021/acschembio.8b00290[↩]
- Beltrán-de-Miguel B., Estévez-Santiago R., Olmedilla-Alonso B. Assessment of dietary vitamin A intake (retinol, α-carotene, β-carotene, β-cryptoxanthin) and its sources in the National Survey of Dietary Intake in Spain (2009–2010) Int. J. Food Sci. Nutr. 2015;66:706–712. doi: 10.3109/09637486.2015.1077787[↩][↩]
- Ma G., Zhang L., Iida K., Madono Y., Yungyuen W., Yahata M., Yamawaki K., Kato M. Identification and quantitative analysis of β-cryptoxanthin and β-citraurin esters in Satsuma mandarin fruit during the ripening process. Food Chem. 2017;234:356–364. doi: 10.1016/j.foodchem.2017.05.015[↩]
- Maiani G., Castón M.J., Catasta G., Toti E., Cambrodón I.G., Bysted A., Granado-Lorencio F., Olmedilla-Alonso B., Knuthsen P., Valoti M., et al. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009;53(Suppl. 12):S194–S218. doi: 10.1002/mnfr.200800053[↩]
- Jiao Y., Reuss L., Wang Y. β-Cryptoxanthin: Chemistry, Occurrence, and Potential Health Benefits. Curr. Pharmacol. Rep. 2019;5:20–34. doi: 10.1007/s40495-019-00168-7[↩]
- Breithaupt D.E., Bamedi A. Carotenoid esters in vegetables and fruits: A screening with emphasis on beta-cryptoxanthin esters. J. Agric. Food Chem. 2001;49:2064–2070. doi: 10.1021/jf001276t[↩]
- Schlatterer J., Breithaupt D.E. Cryptoxanthin Structural Isomers in Oranges, Orange Juice, and Other Fruits. J. Agric. Food Chem. 2005;53:6355–6361. doi: 10.1021/jf050362w[↩]
- Blaner WS. Vitamin A and Provitamin A Carotenoids. In: Marriott BP, Birt DF, Stallings VA, Yates AA, eds. Present Knowledge in Nutrition. 11th ed. Cambridge, Massachusetts: Wiley-Blackwell; 2020:73-91.[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Ross A. Vitamin A. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2014:260-77.[↩][↩]
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academy Press; 2000. https://nap.nationalacademies.org/read/9810/chapter/1[↩][↩][↩]
- Institute of Medicine, US Panel on Micronutrients. Dietary reference intakes for vitamin A, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. National Academies Press. Washington, DC, 2001. PMID: 25057538 www.ncbi.nlm.nih.gov/pubmed/25057538.[↩][↩][↩]
- Solomons NW. Vitamin A. In: Bowman B, Russell R, eds. Present Knowledge in Nutrition. 9th ed. Washington, DC: International Life Sciences Institute; 2006:157-83.[↩][↩][↩][↩]
- Merck Sharp & Dohme Corp, Merck Manual. Vitamin A. https://www.merckmanuals.com/professional/nutritional-disorders/vitamin-deficiency,-dependency,-and-toxicity/vitamin-a#v884887[↩]
- Michaëlsson K, Lithell H, Vessby B, Melhus H. Serum retinol levels and the risk of fracture. N Engl J Med. 2003 Jan 23;348(4):287-94. doi: 10.1056/NEJMoa021171[↩]
- Feskanich D, Singh V, Willett WC, Colditz GA. Vitamin A intake and hip fractures among postmenopausal women. JAMA. 2002 Jan 2;287(1):47-54. doi: 10.1001/jama.287.1.47[↩]
- Promislow, J.H.E., Goodman-Gruen, D., Slymen, D.J. and Barrett-Connor, E. (2002), Retinol Intake and Bone Mineral Density in the Elderly: The Rancho Bernardo Study. J Bone Miner Res, 17: 1349-1358. https://doi.org/10.1359/jbmr.2002.17.8.1349[↩]
- Rejnmark L, Vestergaard P, Charles P, Hermann AP, Brot C, Eiken P, Mosekilde L. No effect of vitamin A intake on bone mineral density and fracture risk in perimenopausal women. Osteoporos Int. 2004 Nov;15(11):872-80. doi: 10.1007/s00198-004-1618-1[↩]
- Sowers MF, Wallace RB. Retinol, supplemental vitamin A and bone status. J Clin Epidemiol. 1990;43(7):693-9. doi: 10.1016/0895-4356(90)90040-v[↩]
- Ballew, C., Galuska, D. and Gillespie, C. (2001), High Serum Retinyl Esters Are Not Associated with Reduced Bone Mineral Density in the Third National Health and Nutrition Examination Survey, 1988–1994. J Bone Miner Res, 16: 2306-2312. https://doi.org/10.1359/jbmr.2001.16.12.2306[↩]
- Wu, A.-M., Huang, C.-Q., Lin, Z.-K., Tian, N.-F., Ni, W.-F., Wang, X.-Y., Xu, H.-Z. and Chi, Y.-L. (2014), The Relationship Between Vitamin A and Risk of Fracture: Meta-Analysis of Prospective Studies. J Bone Miner Res, 29: 2032-2039. https://doi.org/10.1002/jbmr.2237[↩][↩]
- Conaway HH, Henning P, Lerner UH. Vitamin a metabolism, action, and role in skeletal homeostasis. Endocr Rev. 2013 Dec;34(6):766-97. doi: 10.1210/er.2012-1071[↩]
- Johansson, S. and Melhus, H. (2001), Vitamin A Antagonizes Calcium Response to Vitamin D in Man. J Bone Miner Res, 16: 1899-1905. https://doi.org/10.1359/jbmr.2001.16.10.1899[↩]
- Caire-Juvera G, Ritenbaugh C, Wactawski-Wende J, Snetselaar LG, Chen Z. Vitamin A and retinol intakes and the risk of fractures among participants of the Women’s Health Initiative Observational Study. Am J Clin Nutr. 2009 Jan;89(1):323-30. doi: 10.3945/ajcn.2008.26451[↩]
- Ross AC. Vitamin A. In: Ross A, Caballero B, Cousins R, Tucker K, Ziegler T, eds. Modern Nutrition in Health and Disease. 11th ed: Lippincott Williams & Wilkins; 2014:260-277.[↩][↩][↩][↩][↩]
- Tan L, Green MH, Ross AC. Vitamin A kinetics in neonatal rats vs. adult rats: comparisons from model-based compartmental analysis. J Nutr. 2015 Mar;145(3):403-10. doi: 10.3945/jn.114.204065[↩]
- Tan L, Wray AE, Green MH, Ross AC. Compartmental modeling of whole-body vitamin A kinetics in unsupplemented and vitamin A-retinoic acid-supplemented neonatal rats. J Lipid Res. 2014 Aug;55(8):1738-49. doi: 10.1194/jlr.M050518[↩]
- Zhong M, Kawaguchi R, Ter-Stepanian M, Kassai M, Sun H. Vitamin A transport and the transmembrane pore in the cell-surface receptor for plasma retinol binding protein. PLoS One. 2013 Nov 1;8(11):e73838. doi: 10.1371/journal.pone.0073838[↩]
- von Lintig J. Metabolism of carotenoids and retinoids related to vision. J. Biol. Chem. 2012;287:1627–1634. doi: 10.1074/jbc.R111.303990[↩]
- Zhong M., Kawaguchi R., Kassai M., Sun H. Retina, retinol, retinal and the natural history of vitamin A as a light sensor. Nutrients. 2012;4:2069–2096. doi: 10.3390/nu4122069[↩]
- Perusek L, Maeda T. Vitamin A derivatives as treatment options for retinal degenerative diseases. Nutrients. 2013 Jul 12;5(7):2646-66. doi: 10.3390/nu5072646[↩]
- See AW, Clagett-Dame M. The temporal requirement for vitamin A in the developing eye: mechanism of action in optic fissure closure and new roles for the vitamin in regulating cell proliferation and adhesion in the embryonic retina. Dev Biol. 2009 Jan 1;325(1):94-105. doi: 10.1016/j.ydbio.2008.09.030[↩]
- Xerophthalmia. https://eyewiki.org/Xerophthalmia[↩]
- Balmer J.E., Blomhoff R. Gene expression regulation by retinoic acid. J. Lipid Res. 2002;43:1773–1808. doi: 10.1194/jlr.R100015-JLR200[↩]
- Bohn T. Carotenoids, Chronic Disease Prevention and Dietary Recommendations. Int. J. Vitam. Nutr. Res. 2017;87:121–130. doi: 10.1024/0300-9831/a000525[↩]
- di Masi A., De Marinis E., Ascenzi P., Marino M. Nuclear receptors CAR and PXR: Molecular, functional, and biomedical aspects. Mol. Aspects Med. 2009;30:297–343. doi: 10.1016/j.mam.2009.04.002[↩]
- Giguere V., Ong E.S., Segui P., Evans R.M. Identification of a receptor for the morphogen retinoic acid. Nature. 1987;330:624–629. doi: 10.1038/330624a0[↩]
- Petkovich M., Brand N.J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987;330:444–450. doi: 10.1038/330444a0[↩]
- Brand N., Petkovich M., Krust A., Chambon P., de The H., Marchio A., Tiollais P., Dejean A. Identification of a second human retinoic acid receptor. Nature. 1988;332:850–853. doi: 10.1038/332850a0[↩]
- Krust A., Kastner P., Petkovich M., Zelent A., Chambon P. A third human retinoic acid receptor, hRAR-gamma. Proc. Natl. Acad. Sci. USA. 1989;86:5310–5314. doi: 10.1073/pnas.86.14.5310[↩]
- Germain P., Chambon P., Eichele G., Evans R.M., Lazar M.A., Leid M., De Lera A.R., Lotan R., Mangelsdorf D.J., Gronemeyer H. International Union of Pharmacology. LXIII. Retinoid X receptors. Pharmacol. Rev. 2006;58:760–772. doi: 10.1124/pr.58.4.7[↩][↩][↩]
- Canon E., Cosgaya J.M., Scsucova S., Aranda A. Rapid effects of retinoic acid on CREB and ERK phosphorylation in neuronal cells. Mol. Biol. Cell. 2004;15:5583–5592. doi: 10.1091/mbc.e04-05-0439[↩]
- Poon M.M., Chen L. Retinoic acid-gated sequence-specific translational control by RARalpha. Proc. Natl. Acad. Sci. USA. 2008;105:20303–20308. doi: 10.1073/pnas.0807740105[↩]
- Mangelsdorf D.J., Ong E.S., Dyck J.A., Evans R.M. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature. 1990;345:224–229. doi: 10.1038/345224a0[↩]
- Mangelsdorf D.J., Borgmeyer U., Heyman R.A., Zhou J.Y., Ong E.S., Oro A.E., Kakizuka A., Evans R.M. Characterization of three RXR genes that mediate the action of 9-cis retinoic acid. Genes Dev. 1992;6:329–344. doi: 10.1101/gad.6.3.329[↩]
- Chebaro Y., Sirigu S., Amal I., Lutzing R., Stote R.H., Rochette-Egly C., Rochel N., Dejaegere A. Allosteric Regulation in the Ligand Binding Domain of Retinoic Acid Receptorgamma. PLoS ONE. 2017;12:e0171043. doi: 10.1371/journal.pone.0171043[↩]
- Lindh J.D., Bjorkhem-Bergman L., Eliasson E. Vitamin D and drug-metabolising enzymes. Photochem. Photobiol. Sci. 2012;11:1797–1801. doi: 10.1039/c2pp25194a[↩]
- Zhang Y., Luo X.Y., Wu D.H., Xu Y. ROR nuclear receptors: Structures, related diseases, and drug discovery. Acta Pharmacol. Sin. 2015;36:71–87. doi: 10.1038/aps.2014.120[↩]
- Jetten A.M., Kurebayashi S., Ueda E. The ROR nuclear orphan receptor subfamily: Critical regulators of multiple biological processes. Prog. Nucleic. Acid Res. Mol. Biol. 2001;69:205–247. doi: 10.1016/s0079-6603(01)69048-2[↩]
- Solt L.A., Burris T.P. Action of RORs and their ligands in (patho)physiology. Trends Endocrinol. Metab. 2012;23:619–627. doi: 10.1016/j.tem.2012.05.012[↩]
- Stehlin-Gaon C., Willmann D., Zeyer D., Sanglier S., Van Dorsselaer A., Renaud J.P., Moras D., Schule R. All-trans retinoic acid is a ligand for the orphan nuclear receptor ROR beta. Nat. Struct. Biol. 2003;10:820–825. doi: 10.1038/nsb979[↩][↩]
- Schug T.T., Berry D.C., Shaw N.S., Travis S.N., Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129:723–733. doi: 10.1016/j.cell.2007.02.050[↩]
- Chandra V., Huang P., Hamuro Y., Raghuram S., Wang Y., Burris T.P., Rastinejad F. Structure of the intact PPAR-gamma-RXR- nuclear receptor complex on DNA. Nature. 2008;456:350–356. doi: 10.1038/nature07413[↩]
- Michalik L., Auwerx J., Berger J.P., Chatterjee V.K., Glass C.K., Gonzalez F.J., Grimaldi P.A., Kadowaki T., Lazar M.A., O’Rahilly S., et al. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol. Rev. 2006;58:726–741. doi: 10.1124/pr.58.4.5[↩]
- Glatz J.F., Lagarde M. Lipid sensing and lipid sensors. Cell. Mol. Life Sci. 2007;64:2449–2451. doi: 10.1007/s00018-007-7276-7[↩]
- Berry D.C., Noy N. Is PPARbeta/delta a Retinoid Receptor? PPAR Res. 2007;2007:73256. doi: 10.1155/2007/73256[↩]
- Reijntjes S., Gale E., Maden M. Generating gradients of retinoic acid in the chick embryo: Cyp26C1 expression and a comparative analysis of the Cyp26 enzymes. Dev. Dyn. 2004;230:509–517. doi: 10.1002/dvdy.20025[↩]
- Maden M. Retinoid signalling in the development of the central nervous system. Nat. Rev. Neurosci. 2002;3:843–853. doi: 10.1038/nrn963[↩]
- Maden M. Retinoids in lung development and regeneration. Curr. Top. Dev. Biol. 2004;61:153–189. doi: 10.1016/S0070-2153(04)61007-6[↩]
- Clagett-Dame M., DeLuca H.F. The role of vitamin A in mammalian reproduction and embryonic development. Annu. Rev. Nutr. 2002;22:347–381. doi: 10.1146/annurev.nutr.22.010402.102745E[↩]
- Niles R.M. Vitamin A (retinoids) regulation of mouse melanoma growth and differentiation. J. Nutr. 2003;133:282S–286S. doi: 10.1093/jn/133.1.282S[↩]
- Cawley S., Bekiranov S., Ng H.H., Kapranov P., Sekinger E.A., Kampa D., Piccolboni A., Sementchenko V., Cheng J., Williams A.J., et al. Unbiased mapping of transcription factor binding sites along human chromosomes 21 and 22 points to widespread regulation of noncoding RNAs. Cell. 2004;116:499–509. doi: 10.1016/S0092-8674(04)00127-8[↩]
- Chen J.Y., Penco S., Ostrowski J., Balaguer P., Pons M., Starrett J.E., Reczek P., Chambon P., Gronemeyer H. RAR-specific agonist/antagonists which dissociate transactivation and AP1 transrepression inhibit anchorage-independent cell proliferation. EMBO J. 1995;14:1187–1197. doi: 10.1002/j.1460-2075.1995.tb07102.x[↩]
- Lokman N.A., Ho R., Gunasegaran K., Bonner W.M., Oehler M.K., Ricciardelli C. Anti-tumour effects of all-trans retinoid acid on serous ovarian cancer. J. Exp. Clin. Cancer Res. 2019;38:10. doi: 10.1186/s13046-018-1017-7[↩][↩]
- Huang Z., Liu Y., Qi G., Brand D., Zheng S.G. Role of Vitamin A in the Immune System. J. Clin. Med. 2018;7:258. doi: 10.3390/jcm7090258[↩]
- Altucci L., Gronemeyer H. The promise of retinoids to fight against cancer. Nat. Rev. Cancer. 2001;1:181–193. doi: 10.1038/35106036[↩][↩]
- Nagy L., Thomazy V.A., Heyman R.A., Davies P.J. Retinoid-induced apoptosis in normal and neoplastic tissues. Cell Death Differ. 1998;5:11–19. doi: 10.1038/sj.cdd.4400337[↩]
- Mrass P., Rendl M., Mildner M., Gruber F., Lengauer B., Ballaun C., Eckhart L., Tschachler E. Retinoic acid increases the expression of p53 and proapoptotic caspases and sensitizes keratinocytes to apoptosis: A possible explanation for tumor preventive action of retinoids. Cancer Res. 2004;64:6542–6548. doi: 10.1158/0008-5472.CAN-04-1129[↩]
- Maalmi H., Walter V., Jansen L., Owen R.W., Ulrich A., Schottker B., Chang-Claude J., Hoffmeister M., Brenner H. Dose-Response Relationship between Serum Retinol Levels and Survival in Patients with Colorectal Cancer: Results from the DACHS Study. Nutrients. 2018;10:510. doi: 10.3390/nu10040510[↩]
- Huang X., Gao Y., Zhi X., Ta N., Jiang H., Zheng J. Association between vitamin A, retinol and carotenoid intake and pancreatic cancer risk: Evidence from epidemiologic studies. Sci. Rep. 2016;6:38936. doi: 10.1038/srep38936[↩]
- Dawson M.I. The importance of vitamin A in nutrition. Curr. Pharm. Des. 2000;6:311–325. doi: 10.2174/1381612003401190[↩]
- Gudas L.J., Wagner J.A. Retinoids regulate stem cell differentiation. J. Cell. Physiol. 2011;226:322–330. doi: 10.1002/jcp.22417[↩]
- Costantini L., Molinari R., Farinon B., Merendino N. Retinoic Acids in the Treatment of Most Lethal Solid Cancers. J. Clin. Med. 2020;9:360. doi: 10.3390/jcm9020360[↩]
- Qu L., Tang X. Bexarotene: A promising anticancer agent. Cancer Chemother. Pharmacol. 2010;65:201–205. doi: 10.1007/s00280-009-1140-4[↩]
- Bama E.S., Grace V.M.B., Sundaram V., Jesubatham P.D. Synergistic effect of co-treatment with all-trans retinoic acid and 9-cis retinoic acid on human lung cancer cell line at molecular level. 3 Biotech. 2019;9:159. doi: 10.1007/s13205-019-1692-x[↩]
- Dragnev K.H., Petty W.J., Shah S.J., Lewis L.D., Black C.C., Memoli V., Nugent W.C., Hermann T., Negro-Vilar A., Rigas J.R., et al. A proof-of-principle clinical trial of bexarotene in patients with non-small cell lung cancer. Clin. Cancer Res. 2007;13:1794–1800. doi: 10.1158/1078-0432.CCR-06-1836[↩][↩]
- Esteva F.J., Glaspy J., Baidas S., Laufman L., Hutchins L., Dickler M., Tripathy D., Cohen R., DeMichele A., Yocum R.C., et al. Multicenter phase II study of oral bexarotene for patients with metastatic breast cancer. J. Clin. Oncol. 2003;21:999–1006. doi: 10.1200/JCO.2003.05.068[↩][↩]
- Rousseau E.J., Davison A.J., Dunn B. Protection by beta-carotene and related compounds against oxygen-mediated cytotoxicity and genotoxicity: Implications for carcinogenesis and anticarcinogenesis. Free Radic. Biol. Med. 1992;13:407–433. doi: 10.1016/0891-5849(92)90183-H[↩]
- Monsen E.R. Dietary reference intakes for the antioxidant nutrients: Vitamin C, vitamin E, selenium, and carotenoids. J. Am. Diet. Assoc. 2000;100:637–640. doi: 10.1016/S0002-8223(00)00189-9[↩]
- Blumberg J., Block G. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study in Finland. Nutr. Rev. 1994;52:242–245. doi: 10.1111/j.1753-4887.1994.tb01430.x[↩]
- Omenn G.S., Goodman G.E., Thornquist M.D., Balmes J., Cullen M.R., Glass A., Keogh J.P., Meyskens F.L., Valanis B., Williams J.H., et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N. Engl. J. Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802[↩]
- Buring J.E., Hebert P., Hennekens C.H. The alpha-tocopherol, beta-carotene lung cancer prevention trial of vitamin E and beta-carotene: The beginning of the answers. Ann. Epidemiol. 1994;4:75. doi: 10.1016/1047-2797(94)90045-0[↩]
- Middha P., Weinstein S.J., Mannisto S., Albanes D., Mondul A.M. beta-Carotene Supplementation and Lung Cancer Incidence in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study: The Role of Tar and Nicotine. Nicotine Tob. Res. 2019;21:1045–1050. doi: 10.1093/ntr/nty115[↩]
- Nishino H., Murakosh M., Ii T., Takemura M., Kuchide M., Kanazawa M., Mou X.Y., Wada S., Masuda M., Ohsaka Y., et al. Carotenoids in cancer chemoprevention. Cancer Metastasis Rev. 2002;21:257–264. doi: 10.1023/A:1021206826750[↩]
- Koklesova L., Liskova A., Samec M., Buhrmann C., Samuel S.M., Varghese E., Ashrafizadeh M., Najafi M., Shakibaei M., Busselberg D., et al. Carotenoids in Cancer Apoptosis-The Road from Bench to Bedside and Back. Cancers. 2020;12:2425. doi: 10.3390/cancers12092425[↩]
- Park H.A., Hayden M.M., Bannerman S., Jansen J., Crowe-White K.M. Anti-Apoptotic Effects of Carotenoids in Neurodegeneration. Molecules. 2020;25:3453. doi: 10.3390/molecules25153453[↩]
- Green HN, Mellanby E. VITAMIN A AS AN ANTI-INFECTIVE AGENT. Br Med J. 1928 Oct 20;2(3537):691-6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2456524/pdf/brmedj07722-0009.pdf[↩]
- Raverdeau M, Mills KH. Modulation of T cell and innate immune responses by retinoic Acid. J Immunol. 2014 Apr 1;192(7):2953-8. doi: 10.4049/jimmunol.1303245[↩]
- Spears K, Cheney C, Zerzan J. Low plasma retinol concentrations increase the risk of developing bronchopulmonary dysplasia and long-term respiratory disability in very-low-birth-weight infants. Am J Clin Nutr. 2004 Dec;80(6):1589-94. doi: 10.1093/ajcn/80.6.1589[↩]
- Barber T, Esteban-Pretel G, Marín MP, Timoneda J. Vitamin a deficiency and alterations in the extracellular matrix. Nutrients. 2014 Nov 10;6(11):4984-5017. doi: 10.3390/nu6114984[↩][↩]
- Semba RD, Bloem MW. The anemia of vitamin A deficiency: epidemiology and pathogenesis. Eur J Clin Nutr. 2002 Apr;56(4):271-81. doi: 10.1038/sj.ejcn.1601320[↩][↩][↩][↩]
- Allen LH. Iron supplements: scientific issues concerning efficacy and implications for research and programs. J Nutr. 2002 Apr;132(4 Suppl):813S-9S. doi: 10.1093/jn/132.4.813S[↩][↩]
- Christian P, West KP Jr. Interactions between zinc and vitamin A: an update. Am J Clin Nutr. 1998 Aug;68(2 Suppl):435S-441S. doi: 10.1093/ajcn/68.2.435S[↩][↩]
- Auld DS, Bergman T. Medium- and short-chain dehydrogenase/reductase gene and protein families : The role of zinc for alcohol dehydrogenase structure and function. Cell Mol Life Sci. 2008 Dec;65(24):3961-70. doi: 10.1007/s00018-008-8593-1[↩]
- Suharno D, West CE, Muhilal, Karyadi D, Hautvast JG. Supplementation with vitamin A and iron for nutritional anaemia in pregnant women in West Java, Indonesia. Lancet. 1993 Nov 27;342(8883):1325-8. doi: 10.1016/0140-6736(93)92246-p[↩]
- Jang JT, Green JB, Beard JL, Green MH. Kinetic analysis shows that iron deficiency decreases liver vitamin A mobilization in rats. J Nutr. 2000 May;130(5):1291-6. doi: 10.1093/jn/130.5.1291[↩]
- Rosales FJ, Jang JT, Piñero DJ, Erikson KM, Beard JL, Ross AC. Iron deficiency in young rats alters the distribution of vitamin A between plasma and liver and between hepatic retinol and retinyl esters. J Nutr. 1999 Jun;129(6):1223-8. doi: 10.1093/jn/129.6.1223[↩]
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academy Press; 2001.[↩][↩]
- Otten JJ, Hellwig JP, Meyers LD, eds. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington, DC: The National Academies Press; 2006.[↩]
- Food Labeling: Revision of the Nutrition and Supplement Facts Labels and Serving Sizes of Foods That Can Reasonably Be Consumed at One Eating Occasion; Dual-Column Labeling; Updating, Modifying, and Establishing Certain Reference Amounts Customarily Consumed; Serving Size for Breath Mints; and Technical Amendments; Proposed Extension of Compliance Dates. https://www.federalregister.gov/documents/2017/10/02/2017-21019/food-labeling-revision-of-the-nutrition-and-supplement-facts-labels-and-serving-sizes-of-foods-that[↩][↩]
- Haskell MJ. The challenge to reach nutritional adequacy for vitamin A: β-carotene bioavailability and conversion–evidence in humans. Am J Clin Nutr. 2012 Nov;96(5):1193S-203S. doi: 10.3945/ajcn.112.034850[↩]
- Tanumihardjo SA, Russell RM, Stephensen CB, Gannon BM, Craft NE, Haskell MJ, Lietz G, Schulze K, Raiten DJ. Biomarkers of Nutrition for Development (BOND)-Vitamin A Review. J Nutr. 2016 Sep;146(9):1816S-48S. doi: 10.3945/jn.115.229708[↩]
- Mills JL, Simpson JL, Cunningham GC, Conley MR, Rhoads GG. Vitamin A and birth defects. Am J Obstet Gynecol. 1997 Jul;177(1):31-6. doi: 10.1016/s0002-9378(97)70434-4[↩]
- Rothman KJ, Moore LL, Singer MR, Nguyen US, Mannino S, Milunsky A. Teratogenicity of high vitamin A intake. N Engl J Med. 1995 Nov 23;333(21):1369-73. doi: 10.1056/NEJM199511233332101[↩]
- Miller RK, Hendrickx AG, Mills JL, Hummler H, Wiegand UW. Periconceptional vitamin A use: how much is teratogenic? Reprod Toxicol. 1998 Jan-Feb;12(1):75-88. doi: 10.1016/s0890-6238(97)00102-0[↩]
- World Health Organization. Safe vitamin A dosage during pregnancy and lactation. Recommendations and report of a consultation. Geneva: WHO (WHO/NUT/98), 1998. Geneva: WHO, 1998.[↩][↩]
- Food and Nutrition Board, Institute of Medicine. Vitamin A. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, D.C.: National Academy Press; 2001:65-126. https://nap.nationalacademies.org/read/10026/chapter/6[↩][↩]
- World Health Organization. (2011). Guideline : vitamin A supplementation in pregnant women. World Health Organization. https://apps.who.int/iris/handle/10665/44625[↩][↩]
- National Collaborating Centre for Women’s and Children’s Health (UK). Antenatal Care: Routine Care for the Healthy Pregnant Woman. London: RCOG Press; 2008 Mar. (NICE Clinical Guidelines, No. 62.) Available from: https://www.ncbi.nlm.nih.gov/books/NBK51886[↩]
- Orfanos CE, Zouboulis CC. Oral retinoids in the treatment of seborrhoea and acne. Dermatology. 1998;196(1):140-7. doi: 10.1159/000017848[↩][↩]
- Bozzo P, Chua-Gocheco A, Einarson A. Safety of skin care products during pregnancy. Can Fam Physician. 2011 Jun;57(6):665-7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3114665[↩]
- Groff JL. Advanced Nutrition and Human Metabolism. 2nd ed. St. Paul: West Publishing; 1995.[↩]
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academy Press; 2001. https://nap.nationalacademies.org/read/10026/chapter/1[↩][↩]
- Caprioli G., Kamgang Nzekoue F., Fiorini D., Scocco P., Trabalza-Marinucci M., Acuti G., Tardella F.M., Sagratini G., Catorci A. The effects of feeding supplementation on the nutritional quality of milk and cheese from sheep grazing on dry pasture. Int. J. Food Sci. Nutr. 2020;71:50–62. doi: 10.1080/09637486.2019.1613347[↩]
- Ollilainen V., Heinonen M., Linkola E., Varo P., Koivistoinen P. Carotenoids and Retinoids in Finnish Foods: Dairy Products and Eggs. J. Dairy Sci. 1989;72:2257–2265. doi: 10.3168/jds.S0022-0302(89)79356-5[↩]
- Álvarez R., Meléndez-Martínez A.J., Vicario I.M., Alcalde M.J. Carotenoid and Vitamin A Contents in Biological Fluids and Tissues of Animals as an Effect of the Diet: A Review. Food Rev. Int. 2015;31:319–340. doi: 10.1080/87559129.2015.1015139[↩]
- Darwish W.S., Ikenaka Y., Morshdy A.E., Eldesoky K.I., Nakayama S., Mizukawa H., Ishizuka M. β-carotene and retinol contents in the meat of herbivorous ungulates with a special reference to their public health importance. J. Vet. Med. Sci. 2016;78:351–354. doi: 10.1292/jvms.15-0287[↩]
- Nutrients. 2011 Apr; 3(4): 385–428. Published online 2011 Mar 29. doi: 10.3390/nu3040385. Vitamin A in Reproduction and Development. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3257687/[↩]
- Nat Rev Immunol. 2008 Sep; 8(9): 685–698. doi: 10.1038/nri2378. Vitamin effects on the immune system: vitamins A and D take centre stage. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2906676/[↩]
- Wang ZY, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008 Mar 1;111(5):2505-15. doi: 10.1182/blood-2007-07-102798[↩]
- Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S, Ferrara F, Fazi P, Cicconi L, Di Bona E, Specchia G, Sica S, Divona M, Levis A, Fiedler W, Cerqui E, Breccia M, Fioritoni G, Salih HR, Cazzola M, Melillo L, Carella AM, Brandts CH, Morra E, von Lilienfeld-Toal M, Hertenstein B, Wattad M, Lübbert M, Hänel M, Schmitz N, Link H, Kropp MG, Rambaldi A, La Nasa G, Luppi M, Ciceri F, Finizio O, Venditti A, Fabbiano F, Döhner K, Sauer M, Ganser A, Amadori S, Mandelli F, Döhner H, Ehninger G, Schlenk RF, Platzbecker U; Gruppo Italiano Malattie Ematologiche dell’Adulto; German-Austrian Acute Myeloid Leukemia Study Group; Study Alliance Leukemia. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013 Jul 11;369(2):111-21. doi: 10.1056/NEJMoa1300874[↩][↩]
- Rowles JL 3rd, Ranard KM, Smith JW, An R, Erdman JW Jr. Increased dietary and circulating lycopene are associated with reduced prostate cancer risk: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2017 Dec;20(4):361-377. doi: 10.1038/pcan.2017.25[↩]
- Chen F, Hu J, Liu P, Li J, Wei Z, Liu P. Carotenoid intake and risk of non-Hodgkin lymphoma: a systematic review and dose-response meta-analysis of observational studies. Ann Hematol. 2017 Jun;96(6):957-965. doi: 10.1007/s00277-016-2898-1[↩]
- Chen J, Jiang W, Shao L, Zhong D, Wu Y, Cai J. Association between intake of antioxidants and pancreatic cancer risk: a meta-analysis. Int J Food Sci Nutr. 2016 Nov;67(7):744-53. doi: 10.1080/09637486.2016.1197892[↩]
- Leoncini E, Nedovic D, Panic N, Pastorino R, Edefonti V, Boccia S. Carotenoid Intake from Natural Sources and Head and Neck Cancer: A Systematic Review and Meta-analysis of Epidemiological Studies. Cancer Epidemiol Biomarkers Prev. 2015 Jul;24(7):1003-11. doi: 10.1158/1055-9965.EPI-15-0053[↩][↩]
- Li H, He P, Lin T, Guo H, Li Y, Song Y, Wang B, Liu C, Liu L, Li J, Zhang Y, Huo Y, Zhou H, Yang Y, Ling W, Wang X, Zhang H, Xu X, Qin X. Association between plasma retinol levels and the risk of all-cause mortality in general hypertensive patients: A nested case-control study. J Clin Hypertens (Greenwich). 2020 May;22(5):906-913. doi: 10.1111/jch.13866[↩]
- Li X, Xu J. Meta-analysis of the association between dietary lycopene intake and ovarian cancer risk in postmenopausal women. Sci Rep. 2014 May 9;4:4885. doi: 10.1038/srep04885[↩]
- Wang Q, He C. Dietary vitamin A intake and the risk of ovarian cancer: a meta-analysis. Biosci Rep. 2020 Apr 30;40(4):BSR20193979. doi: 10.1042/BSR20193979[↩]
- Lv W, Zhong X, Xu L, Han W. Association between Dietary Vitamin A Intake and the Risk of Glioma: Evidence from a Meta-analysis. Nutrients. 2015 Oct 28;7(11):8897-904. doi: 10.3390/nu7115438[↩]
- Tang JE, Wang RJ, Zhong H, Yu B, Chen Y. Vitamin A and risk of bladder cancer: a meta-analysis of epidemiological studies. World J Surg Oncol. 2014 Apr 29;12:130. doi: 10.1186/1477-7819-12-130[↩]
- Leelakanok N, D’Cunha RR, Sutamtewagul G, Schweizer ML. A systematic review and meta-analysis of the association between vitamin A intake, serum vitamin A, and risk of liver cancer. Nutr Health. 2018 Jun;24(2):121-131. doi: 10.1177/0260106018777170[↩]
- Psaltopoulou T, Ntanasis-Stathopoulos I, Tsilimigras DI, Tzanninis IG, Gavriatopoulou M, Sergentanis TN. Micronutrient Intake and Risk of Hematological Malignancies in Adults: A Systematic Review and Meta-analysis of Cohort Studies. Nutr Cancer. 2018 Aug-Sep;70(6):821-839. doi: 10.1080/01635581.2018.1490444[↩]
- Wang X, Yang HH, Liu Y, Zhou Q, Chen ZH. Lycopene Consumption and Risk of Colorectal Cancer: A Meta-Analysis of Observational Studies. Nutr Cancer. 2016 Oct;68(7):1083-96. doi: 10.1080/01635581.2016.1206579[↩][↩]
- Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, Tonstad S, Vatten LJ, Riboli E, Norat T. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: a systematic review and dose-response meta-analysis of prospective studies. Am J Clin Nutr. 2018 Nov 1;108(5):1069-1091. doi: 10.1093/ajcn/nqy097[↩]
- Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, Barnhart S, Hammar S. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996 May 2;334(18):1150-5. doi: 10.1056/NEJM199605023341802[↩][↩][↩]
- Gary E. Goodman, Mark D. Thornquist, John Balmes, Mark R. Cullen, Frank L. Meyskens, Jr., Gilbert S. Omenn, Barbara Valanis, James H. Williams, Jr., The Beta-Carotene and Retinol Efficacy Trial: Incidence of Lung Cancer and Cardiovascular Disease Mortality During 6-Year Follow-up After Stopping β-Carotene and Retinol Supplements, JNCI: Journal of the National Cancer Institute, Volume 96, Issue 23, 1 December 2004, Pages 1743–1750, https://doi.org/10.1093/jnci/djh320[↩]
- Neuhouser ML, Barnett MJ, Kristal AR, Ambrosone CB, King IB, Thornquist M, Goodman GG. Dietary supplement use and prostate cancer risk in the Carotene and Retinol Efficacy Trial. Cancer Epidemiol Biomarkers Prev. 2009 Aug;18(8):2202-6. doi: 10.1158/1055-9965.EPI-09-0013[↩][↩]
- Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994 Apr 14;330(15):1029-35. doi: 10.1056/NEJM199404143301501[↩][↩][↩][↩]
- Virtamo J, Taylor PR, Kontto J, Männistö S, Utriainen M, Weinstein SJ, Huttunen J, Albanes D. Effects of α-tocopherol and β-carotene supplementation on cancer incidence and mortality: 18-year postintervention follow-up of the Alpha-tocopherol, Beta-carotene Cancer Prevention Study. Int J Cancer. 2014 Jul 1;135(1):178-85. doi: 10.1002/ijc.28641[↩][↩]
- Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013 May 15;309(19):2005-15. doi: 10.1001/jama.2013.4997. Erratum in: JAMA. 2013 Jul 10;310(2):208.[↩][↩][↩][↩]
- Chew EY, Clemons TE, Agrón E, Domalpally A, Keenan TDL, Vitale S, Weber C, Smith DC, Christen W; AREDS2 Research Group. Long-term Outcomes of Adding Lutein/Zeaxanthin and ω-3 Fatty Acids to the AREDS Supplements on Age-Related Macular Degeneration Progression: AREDS2 Report 28. JAMA Ophthalmol. 2022 Jul 1;140(7):692-698. doi: 10.1001/jamaophthalmol.2022.1640[↩][↩][↩]
- Cortés-Jofré M, Rueda JR, Asenjo-Lobos C, Madrid E, Bonfill Cosp X. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst Rev. 2020 Mar 4;3(3):CD002141. doi: 10.1002/14651858.CD002141.pub3[↩]
- Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, Belanger C, LaMotte F, Gaziano JM, Ridker PM, Willett W, Peto R. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996 May 2;334(18):1145-9. doi: 10.1056/NEJM199605023341801[↩]
- Lin J, Cook NR, Albert C, Zaharris E, Gaziano JM, Van Denburgh M, Buring JE, Manson JE. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst. 2009 Jan 7;101(1):14-23. doi: 10.1093/jnci/djn438[↩]
- Kamangar F, Qiao YL, Yu B, Sun XD, Abnet CC, Fan JH, Mark SD, Zhao P, Dawsey SM, Taylor PR. Lung cancer chemoprevention: a randomized, double-blind trial in Linxian, China. Cancer Epidemiol Biomarkers Prev. 2006 Aug;15(8):1562-4. doi: 10.1158/1055-9965.EPI-06-0316[↩][↩]
- Fleckenstein M, Keenan TDL, Guymer RH, Chakravarthy U, Schmitz-Valckenberg S, Klaver CC, Wong WT, Chew EY. Age-related macular degeneration. Nat Rev Dis Primers. 2021 May 6;7(1):31. doi: 10.1038/s41572-021-00265-2[↩][↩]
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001 Oct;119(10):1417-36. doi: 10.1001/archopht.119.10.1417. Erratum in: Arch Ophthalmol. 2008 Sep;126(9):1251.[↩]
- Agrón E, Mares J, Clemons TE, Swaroop A, Chew EY, Keenan TDL; AREDS and AREDS2 Research Groups. Dietary Nutrient Intake and Progression to Late Age-Related Macular Degeneration in the Age-Related Eye Disease Studies 1 and 2. Ophthalmology. 2021 Mar;128(3):425-442. doi: 10.1016/j.ophtha.2020.08.018[↩][↩][↩]
- Patel M, Lee AD, Redd SB, Clemmons NS, McNall RJ, Cohn AC, Gastañaduy PA. Increase in Measles Cases – United States, January 1-April 26, 2019. MMWR Morb Mortal Wkly Rep. 2019 May 3;68(17):402-404. doi: 10.15585/mmwr.mm6817e1[↩]
- World Health Organization. Guideline: Vitamin A Supplementation in Infants and Children 6-59 Months of Age. 2011. https://www.who.int/publications/i/item/9789241501767[↩][↩][↩]
- Yang HM, Mao M, Wan C. Vitamin A for treating measles in children. Cochrane Database Syst Rev 2011;2005. http://www.cochrane.org/CD001479/ARI_vitamin-a-for-measles-in-children[↩]
- Imdad A, Mayo-Wilson E, Herzer K, Bhutta ZA. Vitamin A supplementation for preventing morbidity and mortality in children from six months to five years of age. Cochrane Database Syst Rev. 2017 Mar 11;3(3):CD008524. doi: 10.1002/14651858.CD008524.pub3. Update in: Cochrane Database Syst Rev. 2022 Mar 16;3:CD008524.[↩]
- Bello S, Meremikwu MM, Ejemot-Nwadiaro RI, Oduwole O. Routine vitamin A supplementation for the prevention of blindness due to measles infection in children. Cochrane Database Syst Rev. 2011 Apr 13;(4):CD007719. doi: 10.1002/14651858.CD007719.pub2. Update in: Cochrane Database Syst Rev. 2014;1:CD007719.[↩]
- Foster A, Sommer A. Corneal ulceration, measles, and childhood blindness in Tanzania. Br J Ophthalmol. 1987 May;71(5):331-43. doi: 10.1136/bjo.71.5.331[↩]
- Ravishankar C, Nafday S, Green RS, Kamenir S, Lorber R, Stacewicz-Sapuntzakis M, Bridges ND, Holzman IR, Gelb BD. A trial of vitamin A therapy to facilitate ductal closure in premature infants. J Pediatr. 2003 Nov;143(5):644-8. doi: 10.1067/S0022-3476(03)00501-8. Erratum in: J Pediatr. 2004 Mar;144(3):412.[↩]
- Tyson JE, Wright LL, Oh W, Kennedy KA, Mele L, Ehrenkranz RA, Stoll BJ, Lemons JA, Stevenson DK, Bauer CR, Korones SB, Fanaroff AA. Vitamin A supplementation for extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med. 1999 Jun 24;340(25):1962-8. doi: 10.1056/NEJM199906243402505[↩][↩]
- Wardle SP, Hughes A, Chen S, Shaw NJ. Randomised controlled trial of oral vitamin A supplementation in preterm infants to prevent chronic lung disease. Arch Dis Child Fetal Neonatal Ed. 2001 Jan;84(1):F9-F13. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1721189/pdf/v084p000F9.pdf[↩]
- Ambalavanan N, Kennedy K, Tyson J, Carlo WA. Survey of vitamin A supplementation for extremely-low-birth-weight infants: is clinical practice consistent with the evidence? J Pediatr. 2004 Sep;145(3):304-7. doi: 10.1016/j.jpeds.2004.04.046[↩]
- Laughon MM. Vitamin A shortage and risk of bronchopulmonary dysplasia. JAMA Pediatr. 2014 Nov;168(11):995-6. doi: 10.1001/jamapediatrics.2014.1416[↩]
- Tolia VN, Murthy K, McKinley PS, Bennett MM, Clark RH. The effect of the national shortage of vitamin A on death or chronic lung disease in extremely low-birth-weight infants. JAMA Pediatr. 2014 Nov;168(11):1039-44. doi: 10.1001/jamapediatrics.2014.1353[↩][↩]
- Gadhia MM, Cutter GR, Abman SH, Kinsella JP. Effects of early inhaled nitric oxide therapy and vitamin A supplementation on the risk for bronchopulmonary dysplasia in premature newborns with respiratory failure. J Pediatr. 2014 Apr;164(4):744-8. doi: 10.1016/j.jpeds.2013.11.040[↩]
- Meyer S, Gortner L; NeoVitaA Trial Investigators. Early postnatal additional high-dose oral vitamin A supplementation versus placebo for 28 days for preventing bronchopulmonary dysplasia or death in extremely low birth weight infants. Neonatology. 2014;105(3):182-8. doi: 10.1159/000357212[↩]
- Babu TA, Sharmila V. Vitamin A supplementation in late pregnancy can decrease the incidence of bronchopulmonary dysplasia in newborns. J Matern Fetal Neonatal Med. 2010 Dec;23(12):1468-9. doi: 10.3109/14767051003678168[↩]
- Thorne-Lyman AL, Fawzi WW. Vitamin A and carotenoids during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis. Paediatr Perinat Epidemiol. 2012 Jul;26 Suppl 1(0 1):36-54. doi: 10.1111/j.1365-3016.2012.01284.x[↩]
- Booij MT, Van De Kerkhof PC. Acitretin revisited in the era of biologics. J Dermatolog Treat. 2011 Apr;22(2):86-9. doi: 10.3109/09546630903578582[↩]
- Thielitz A, Gollnick H. Topical retinoids in acne vulgaris: update on efficacy and safety. Am J Clin Dermatol. 2008;9(6):369-81. doi: 10.2165/0128071-200809060-00003[↩]
- Vishwanathan R, Johnson EJ. Eye disease. In: Erdman JJ, Macdonald I, Zeisel S, eds. Present Knowledge in Nutrition. 10th ed: John Wiley & Sons, Ltd; 2012:939-981.[↩]
- Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006 Nov 18;368(9549):1795-809. doi: 10.1016/S0140-6736(06)69740-7[↩][↩][↩]
- Berson EL, Rosner B, Sandberg MA, Hayes KC, Nicholson BW, Weigel-DiFranco C, Willett W. A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Arch Ophthalmol. 1993 Jun;111(6):761-72. doi: 10.1001/archopht.1993.01090060049022[↩]
- Sibulesky L, Hayes KC, Pronczuk A, Weigel-DiFranco C, Rosner B, Berson EL. Safety of <7500 RE (<25000 IU) vitamin A daily in adults with retinitis pigmentosa. Am J Clin Nutr. 1999 Apr;69(4):656-63. doi: 10.1093/ajcn/69.4.656[↩]
- Starck T. Advances in Corneal Research. Boston, MA: Springer; 1997. Severe corneal ulcerations and vitamin A deficiency; pp. 557–567.[↩]
- Lange AP, Moloney G, Sheldon CA, Sasaki S, Holland SP. Bilateral corneal ulceration caused by vitamin a deficiency in eosinophilic gastroenteropathy. Case Rep Ophthalmol. 2011 Sep;2(3):302-6. doi: 10.1159/000331886[↩]
- Wiseman EM, Bar-El Dadon S, Reifen R. The vicious cycle of vitamin a deficiency: A review. Crit Rev Food Sci Nutr. 2017 Nov 22;57(17):3703-3714. doi: 10.1080/10408398.2016.1160362[↩]
- Stevens GA, Bennett JE, Hennocq Q, Lu Y, De-Regil LM, Rogers L, Danaei G, Li G, White RA, Flaxman SR, Oehrle SP, Finucane MM, Guerrero R, Bhutta ZA, Then-Paulino A, Fawzi W, Black RE, Ezzati M. Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: a pooled analysis of population-based surveys. Lancet Glob Health. 2015 Sep;3(9):e528-36. doi: 10.1016/S2214-109X(15)00039-X[↩][↩][↩][↩][↩][↩][↩][↩]
- Bailey RL, West KP Jr, Black RE. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab. 2015;66 Suppl 2:22-33. doi: 10.1159/000371618[↩][↩][↩]
- Vitamin A and Carotenoids. https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional[↩]
- World Health Organization. Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations. Geneva: WHO, 2011.[↩]
- West KP. Dietary vitamin A deficiency: effects on growth, infection and mortality. Food and Nutrition Bulletin 1991;13(2):77.[↩]
- World Health Organization. Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995–2005: WHO Global Database on Vitamin A Deficiency. Geneva: World Health Organization; 2009. http://apps.who.int/iris/bitstream/10665/44110/1/9789241598019_eng.pdf[↩][↩]
- Ross AC, Tan L. Vitamin A deficiencies and excess. In: Kliegman RM, Stanton BF, St Geme JW, Schor NF, eds. Nelson Textbook of Pediatrics. 20th ed. Philadelphia, PA: Elsevier; 2016:chap 48.[↩]
- Sommer A, West KP Jr. 1996. Vitamin A Deficiency: Health, Survival, and Vision . New York: Oxford University Press.[↩]
- WHO. 1995. Global Prevalence of Vitamin A Deficiency . Micronutrient Deficiency Information System Working Paper, No. 2. Geneva: WHO.[↩]
- WHO. 1982. Control of Vitamin A Deficiency and Xerophthalmia . Technical Report Series No. 672. Geneva: WHO. https://www.ncbi.nlm.nih.gov/pubmed/6803444[↩]
- Dowling JE, Gibbons IR. 1961. In: Smelser GK, ed, editor. . The Structure of the Eye . New York: Academic Press.[↩]
- Sommer A. 1982. Nutritional Blindness. Xerophthalmia and Keratomalacia . New York: Oxford University Press.[↩]
- Katz J, West KP Jr, Khatry SK, Thapa MD, LeClerq SC, Pradhan EK, Pokhrel RP, Sommer A. 1995. Impact of vitamin A supplementation on prevalence and incidence of xerophthalmia in Nepal. Invest Ophthalmol Vis Sci 36:2577–2583. https://www.ncbi.nlm.nih.gov/pubmed/7499080[↩]
- Christian P, West KP Jr, Khatry SK, Katz J, LeClerq S, Pradhan EK, Shrestha SR. 1998. b. Vitamin A or beta-carotene supplementation reduces but does not eliminate maternal night blindness in Nepal. J Nutr 128:1458–1463. https://www.ncbi.nlm.nih.gov/pubmed/9732305[↩]
- World Health Organization. Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995–2005: WHO Global Database on Vitamin A Deficiency. http://apps.who.int/iris/bitstream/handle/10665/44110/9789241598019_eng.pdf?sequence=1[↩][↩][↩][↩][↩]
- Mayo-Wilson E, Imdad A, Herzer K, Yakoob MY, Bhutta ZA. Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis. BMJ 2011;343:d5094[↩]
- Boomsma DI, Martin NG, Molenaar PC. Factor and simplex models for repeated measures: application to two psychomotor measures of alcohol sensitivity in twins. Behav Genet. 1989 Jan;19(1):79-96. doi: 10.1007/BF01065885[↩][↩]
- Timoneda J, Rodríguez-Fernández L, Zaragozá R, Marín MP, Cabezuelo MT, Torres L, Viña JR, Barber T. Vitamin A Deficiency and the Lung. Nutrients. 2018 Aug 21;10(9):1132. doi: 10.3390/nu10091132[↩]
- Institute of Medicine, Food, Nutrition Board. Vitamin A. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academy Press, 2001:82–146.[↩]
- Mills JP, Terasawa E, Tanumihardjo SA. Ingestion of excessive preformed vitamin A by mothers amplifies storage of retinyl esters in early fetal livers of captive Old World monkeys. Comp Med. 2007 Oct;57(5):505-11.[↩]
- Quadro L, Hamberger L, Gottesman ME, Wang F, Colantuoni V, Blaner WS, Mendelsohn CL. Pathways of vitamin A delivery to the embryo: insights from a new tunable model of embryonic vitamin A deficiency. Endocrinology. 2005 Oct;146(10):4479-90. doi: 10.1210/en.2005-0158[↩]
- Chase HP, Kumar V, Dodds JM, Sauberlich HE, Hunter RM, Burton RS, Spalding V. 1971. Nutritional status of preschool Mexican-American migrant farm children. Am J Dis Child 122:316–324. https://www.ncbi.nlm.nih.gov/pubmed/5115528[↩]
- Sauberlich HE, Hodges HE, Wallace DL, Kolder H, Canham JE, Hood J, Raica N, Lowry LK. 1974. Vitamin A metabolism and requirements in the human studied with the use of labeled retinol. Vitam Horm 32:251–275. https://www.ncbi.nlm.nih.gov/pubmed/4617402[↩][↩]
- Cantorna MT, Nashold FE, Hayes CE. 1995. Vitamin A deficiency results in a priming environment conducive for TH1 cell development. Eur J Immunol 25:1673–1679. https://www.ncbi.nlm.nih.gov/pubmed/7614995[↩]
- Nauss KM, Newberne PM. 1985. Local and regional immune function of vitamin A-deficient rats with ocular herpes simplex virus (HSV) infections. J Nutr 115:1316–1324. https://www.ncbi.nlm.nih.gov/pubmed/3876416[↩]
- Dawson HD, Ross AC. 1999. Chronic marginal vitamin A status effects the distribution and function of T cells and natural T cells in aging Lewis rats. J Nutr 129:1782–1790. https://www.ncbi.nlm.nih.gov/pubmed/10498748[↩]
- Wiedermann U, Hanson LA, Kahu H, Dahlgren UI. 1993. Aberrant T-cell function in vitro and impaired T-cell dependent antibody response in vivo in vitamin A-deficient rats. Immunology 80:581–586. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1422259/[↩]
- Sommer A, Katz J, Tarwotjo I. 1984. Increased risk of respiratory disease and diarrhea in children with pre-existing mild vitamin A deficiency. Am J Clin Nutr 40:1090–1095. https://www.ncbi.nlm.nih.gov/pubmed/6496388[↩]
- Sommer A, Tarwotjo I, Hussaini G, Susanto D. 1983. Increased mortality in children with mild vitamin A deficiency. Lancet 2:585–588. https://www.ncbi.nlm.nih.gov/pubmed/6136744[↩]
- Barclay AJ, Foster A, Sommer A. 1987. Vitamin A supplements and mortality related to measles: A randomised clinical trial. Br Med J 294:294–296. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1245303/[↩]
- Hussey GD, Klein M. 1990. A randomized, controlled trial of vitamin A in children with severe measles. N Engl J Med 323:160–164. https://www.ncbi.nlm.nih.gov/pubmed/2194128[↩][↩]
- Coutsoudis A, Broughton M, Coovadia HM. 1991. Vitamin A supplementation reduces measles morbidity in young African children: A randomized, placebo-controlled, double-blind trial. Am J Clin Nutr 54:890–895. https://www.ncbi.nlm.nih.gov/pubmed/1951162[↩]
- Barreto ML, Santos LM, Assis AM, Araujo MP, Farenzena GG, Santos PA, Fiaccone RL. 1994. Effect of vitamin A supplementation on diarrhoea and acute lower-respiratory-tract infections in young children in Brazil. Lancet 344:228–231. https://www.ncbi.nlm.nih.gov/pubmed/7913157[↩]
- Donnen P, Dramaix M, Brasseur D, Bitwe R, Vertongen F, Hennart P. 1998. Randomized placebo-controlled clinical trial of the effect of a single high dose or daily low doses of vitamin A on the morbidity of hospitalized, malnourished children. Am J Clin Nutr 68:1254–1260. https://www.ncbi.nlm.nih.gov/pubmed/9846855[↩]
- Shankar AH, Genton B, Semba RD, Baisor M, Paino J, Tamja S, Adiguma T, Wu L, Rare L, Tielsch JM, Alpers MP, West KP Jr. 1999. Effect of vitamin A supplementation on morbidity due to Plasmodium falciparum in young children in Papua, New Guinea: A randomised trial. Lancet 354:203–209. https://www.ncbi.nlm.nih.gov/pubmed/10421302[↩]
- Humphrey JH, Agoestina T, Wu L, Usman A, Nurachim M, Subardja D, Hidayat S, Tielsch J, West KP Jr, Sommer A. 1996. Impact of neonatal vitamin A supplementation on infant morbidity and mortality. J Pediatr 128:489–496. https://www.ncbi.nlm.nih.gov/pubmed/8618182[↩][↩]
- Ghana VAST Study Team. 1993. Vitamin A supplementation in northern Ghana: Effects on clinic attendances, hospital admissions, and child mortality. Lancet 342:7–12. https://www.ncbi.nlm.nih.gov/pubmed/8100345[↩]
- Muhilal, Permeisih D, Idjradinata YR, Muherdiyantiningsih, Karyadi D. 1988. Vitamin A-fortified monosodium glutamate and health, growth, and survival of children: A controlled field trial. Am J Clin Nutr 48:1271–1276. https://www.ncbi.nlm.nih.gov/pubmed/3189216[↩]
- Rahmathullah L, Underwood BA, Thulasiraj RD, Milton RC, Ramaswamy K, Rahmathullah R, Babu G. 1990. Reduced mortality among children in southern India receiving a small weekly dose of vitamin A. N Engl J Med 323:929–935. https://www.ncbi.nlm.nih.gov/pubmed/2205798[↩]
- Sommer A, Tarwotjo I, Djunaedi E, West KP Jr, Loeden AA, Tilden R, Mele L. 1986. Impact of vitamin A supplementation on childhood mortality: A randomized controlled community trial. Lancet 1:1169–1173. https://www.ncbi.nlm.nih.gov/pubmed/2871418[↩]
- West KP Jr, Pokhrel RP, Katz J, LeClerq SC, Khatry SK, Shrestha SR, Pradhan EK, Tielsch JM, Pandey MR, Sommer A. 1991. Efficacy of vitamin A in reducing preschool child mortality in Nepal. Lancet 338:67–71. https://www.ncbi.nlm.nih.gov/pubmed/1676467[↩]
- West KP Jr, Katz J, Khatry SK, LeClerq SC, Pradhan EK, Shrestha SR, Conner PB, Dali SM, Christian P, Pokhrel RP, Sommer A. 1999. Double blind, cluster randomized trial of low dose supplementation with vitamin A or beta carotene on mortality related to pregnancy in Nepal. Br Med J 318:570–575. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC27760/[↩]
- Beaton GH, Martorell R, Aronson KJ, Edmonston B, McCabe G, Ross AC, Harvey B. 1993. Effectiveness of Vitamin A Supplementation in the Control of Young Child Morbidity and Mortality in Developing Countries . Geneva: Subcommittee on Nutrition, Administrative Committee on Coordination, World Health Organization.[↩]
- Fawzi WW, Chalmers TC, Herrera MG, Mosteller F. 1993. Vitamin A supplementation and child mortality. A meta-analysis. J Am Med Assoc 269:898–903. https://www.ncbi.nlm.nih.gov/pubmed/8426449[↩]
- Glasziou PP, Mackerras DE. 1993. Vitamin A supplementation and infectious disease: A meta-analysis. Br Med J 306:366–370. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1676417/[↩]
- WHO. 1997. Vitamin A Supplements: A Guide to Their Use in the Treatment of Vitamin A Deficiency and Xerophthalmia . Geneva: WHO.[↩]
- AAP (American Academy of Pediatrics Committee on Infectious Diseases). 1993. Vitamin A treatment of measles. Pediatrics 91:1014–1015. https://www.ncbi.nlm.nih.gov/pubmed/8474793[↩]
- Bitot spot: early marker for avoidable blindness. Siddharth Madan, Sarita Beri. CMAJ Oct 2017, 189 (40) E1264; DOI: 10.1503/cmaj.170792[↩]
- Gilbert C. The eye signs of vitamin A deficiency. Community Eye Health. 2013;26(84):66-7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3936686[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Soni, D., Sharma, B., Singh, P., Kubrey, S. (2022). Keratomalacia. In: Sharma, B., Titiyal, J.S. (eds) Corneal Emergencies. Springer, Singapore. https://doi.org/10.1007/978-981-16-5876-1_19[↩]
- Darlow BA, Graham PJ. Vitamin A supplementation to prevent mortality and short- and long-term morbidity in very low birthweight infants. Cochrane Database Syst Rev. 2011 Oct 5;(10):CD000501. doi: 10.1002/14651858.CD000501.pub3. Update in: Cochrane Database Syst Rev. 2016;8:CD000501[↩][↩]
- Schwartz, E., Zelig, R., Parker, A. and Johnson, S. (2017), Vitamin A Supplementation for the Prevention of Bronchopulmonary Dysplasia in Preterm Infants: An Update. Nutrition in Clinical Practice, 32: 346-353. https://doi.org/10.1177/0884533616673613[↩][↩]
- Rakshasbhuvankar A, Patole S, Simmer K, Pillow JJ. Enteral vitamin A for reducing severity of bronchopulmonary dysplasia in extremely preterm infants: a randomised controlled trial. BMC Pediatr. 2017 Dec 16;17(1):204. doi: 10.1186/s12887-017-0958-x[↩][↩]
- Sun H, Cheng R, Wang Z. Early vitamin A supplementation improves the outcome of retinopathy of prematurity in extermely premature infants. Retina. 2020 Jun;40(6):1176-1184. doi: 10.1097/IAE.0000000000002543[↩][↩][↩]
- Mactier H, Weaver LT. Vitamin A and preterm infants: what we know, what we don’t know, and what we need to know. Arch Dis Child Fetal Neonatal Ed. 2005 Mar;90(2):F103-8. doi: 10.1136/adc.2004.057547[↩][↩]
- McCauley ME, van den Broek N, Dou L, Othman M. Vitamin A supplementation during pregnancy for maternal and newborn outcomes. Cochrane Database Syst Rev. 2015 Oct 27;2015(10):CD008666. doi: 10.1002/14651858.CD008666.pub3[↩][↩]
- Haider BA, Sharma R, Bhutta ZA. Neonatal vitamin A supplementation for the prevention of mortality and morbidity in term neonates in low and middle income countries. Cochrane Database Syst Rev. 2017 Feb 24;2(2):CD006980. doi: 10.1002/14651858.CD006980.pub3[↩][↩]
- Ota E, da Silva Lopes K, Middleton P, Flenady V, Wariki WM, Rahman MO, Tobe-Gai R, Mori R. Antenatal interventions for preventing stillbirth, fetal loss and perinatal death: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev. 2020 Dec 18;12(12):CD009599. doi: 10.1002/14651858.CD009599.pub2[↩][↩]
- Oliveira JM, Allert R, East CE. Vitamin A supplementation for postpartum women. Cochrane Database Syst Rev. 2016 Mar 25;3(3):CD005944. doi: 10.1002/14651858.CD005944.pub3[↩][↩][↩][↩]
- World Health Organization. Guideline: vitamin A supplementation in infants and children 6-59 months of age. 25 July 2011. https://www.who.int/publications/i/item/9789241501767[↩][↩][↩][↩][↩]
- World Health Organization. Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995–2005: WHO Gobal Database on Vitamin A Deficiency. Geneva: World Health Organization; 2009.[↩][↩]
- de Vries JJ, Chang AB, Bonifant CM, Shevill E, Marchant JM. Vitamin A and beta (β)-carotene supplementation for cystic fibrosis. Cochrane Database Syst Rev. 2018 Aug 9;8(8):CD006751. doi: 10.1002/14651858.CD006751.pub5[↩][↩][↩][↩]
- Rana M, Wong-See D, Katz T, Gaskin K, Whitehead B, Jaffe A, Coakley J, Lochhead A. Fat-soluble vitamin deficiency in children and adolescents with cystic fibrosis. J Clin Pathol. 2014 Jul;67(7):605-8. doi: 10.1136/jclinpath-2013-201787[↩][↩]
- Woestenenk JW, Broos N, Stellato RK, Arets HG, van der Ent CK, Houwen RH. Vitamin A intake and serum retinol levels in children and adolescents with cystic fibrosis. Clin Nutr. 2016 Jun;35(3):654-9. doi: 10.1016/j.clnu.2015.04.010[↩][↩][↩][↩]
- Fabisiak N, Fabisiak A, Watala C, Fichna J. Fat-soluble Vitamin Deficiencies and Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. J Clin Gastroenterol. 2017 Nov/Dec;51(10):878-889. doi: 10.1097/MCG.0000000000000911[↩][↩]
- Rempel J, Grover K, El-Matary W. Micronutrient Deficiencies and Anemia in Children with Inflammatory Bowel Disease. Nutrients. 2021 Jan 15;13(1):236. doi: 10.3390/nu13010236[↩][↩]
- Santucci NR, Alkhouri RH, Baker RD, Baker SS. Vitamin and zinc status pretreatment and posttreatment in patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2014 Oct;59(4):455-7. doi: 10.1097/MPG.0000000000000477[↩][↩]
- Wright JP, Mee AS, Parfitt A, Marks IN, Burns DG, Sherman M, Tigler-Wybrandi N, Isaacs S. Vitamin A therapy in patients with Crohn’s disease. Gastroenterology. 1985 Feb;88(2):512-4. doi: 10.1016/0016-5085(85)90514-1[↩][↩]
- McGrogan L, Mackinder M, Stefanowicz F, Aroutiounova M, Catchpole A, Wadsworth J, Buchanan E, Cardigan T, Duncan H, Hansen R, Russell RK, Edwards CA, Talwar D, McGrogan P, Gerasimidis K. Micronutrient deficiencies in children with coeliac disease; a double-edged sword of both untreated disease and treatment with gluten-free diet. Clin Nutr. 2021 May;40(5):2784-2790. doi: 10.1016/j.clnu.2021.03.006[↩][↩]
- Shepherd, S.J. & Gibson, P.R (2012) Nutritional inadequacies of the gluten-free diet in both recently-diagnosed and long-term patients with coeliac disease. J Hum Nutr Diet. 26, 349–358 doi:10.1111/jhn.12018[↩][↩]
- Tokgöz Y, Terlemez S, Karul A. Fat soluble vitamin levels in children with newly diagnosed celiac disease, a case control study. BMC Pediatr. 2018 Apr 9;18(1):130. doi: 10.1186/s12887-018-1107-x[↩][↩]
- Rubino P, Mora P, Ungaro N, Gandolfi SA, Orsoni JG. Anterior Segment Findings in Vitamin A Deficiency: A Case Series. Case Rep Ophthalmol Med. 2015;2015:181267. doi: 10.1155/2015/181267[↩]
- Fernando-Langit A, Ilsen PF. Ocular manifestations of vitamin A deficiency. Clin Refract Optom. 2008;19:86–93.[↩]
- Tinley CG, Withers NJ, Sheldon CD, Quinn AG, Jackson AA. Zinc therapy for night blindness in cystic fibrosis. J Cyst Fibros. 2008 Jul;7(4):333-335. doi: 10.1016/j.jcf.2007.11.00[↩]
- Vitamin A Deficiency Clinical Presentation. https://emedicine.medscape.com/article/126004-clinical[↩][↩]
- Vitamin A Deficiency Clinical Presentation. https://emedicine.medscape.com/article/126004-clinical#b2[↩]
- Mehra D, Le PH. Physiology, Night Vision. [Updated 2022 Sep 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK545246[↩]
- Gilbert C, Awan H. Blindness in children. BMJ. 2003 Oct 4;327(7418):760-1. doi: 10.1136/bmj.327.7418.760[↩]
- Semba RD. Vitamin A and human immunodeficiency virus infection. Proc Nutr Soc. 1997 Mar;56(1B):459-69. doi: 10.1079/pns19970046[↩]
- Field CJ, Johnson IR, Schley PD. Nutrients and their role in host resistance to infection. J Leukoc Biol. 2002 Jan;71(1):16-32.[↩]
- Awasthi S, Peto R, Read S, Clark S, Pande V, Bundy D; DEVTA (Deworming and Enhanced Vitamin A) team. Vitamin A supplementation every 6 months with retinol in 1 million pre-school children in north India: DEVTA, a cluster-randomised trial. Lancet. 2013 Apr 27;381(9876):1469-77. doi: 10.1016/S0140-6736(12)62125-4[↩]
- Cohen N, Rahman H, Sprague J, Jalil MA, Leemhuis de Regt E, Mitra M. Prevalence and determinants of nutritional blindness in Bangladeshi children. World Health Stat Q. 1985;38(3):317-30. English, French.[↩]
- Chiu, M., Dillon, A., and Watson, S. (2016) Vitamin A deficiency and xerophthalmia in children of a developed country. Journal of Paediatrics and Child Health, 52: 699– 703. doi: 10.1111/jpc.13243[↩]
- Management of Bitot’s Spots. https://www.aao.org/eyenet/article/management-of-bitot-s-spots#chart[↩]
- Miller M, Humphrey J, Johnson E, Marinda E, Brookmeyer R, Katz J. Why do children become vitamin A deficient? J Nutr. 2002 Sep;132(9 Suppl):2867S-2880S. doi: 10.1093/jn/132.9.2867S[↩]
- Gomes MM, Saunders C, Ramalho A. Placenta: a possible predictor of vitamin A deficiency. Br J Nutr. 2010 May;103(9):1340-4. doi: 10.1017/S0007114509993072[↩]
- de Pee S, Dary O. Biochemical indicators of vitamin A deficiency: serum retinol and serum retinol binding protein. J Nutr. 2002 Sep;132(9 Suppl):2895S-2901S. doi: 10.1093/jn/132.9.2895S[↩]
- Weinman AR, Jorge SM, Martins AR, de Assis Md, Martinez FE, Camelo JS Jr. Assessment of vitamin A nutritional status in newborn preterm infants. Nutrition. 2007 Jun;23(6):454-60. doi: 10.1016/j.nut.2007.04.003[↩]
- Gorstein JL, Dary O, Pongtorn, Shell-Duncan B, Quick T, Wasanwisut E. Feasibility of using retinol-binding protein from capillary blood specimens to estimate serum retinol concentrations and the prevalence of vitamin A deficiency in low-resource settings. Public Health Nutr. 2008 May;11(5):513-20. doi: 10.1017/S1368980007000821[↩]
- Russell RM. The vitamin A spectrum: from deficiency to toxicity. Am J Clin Nutr. 2000 Apr;71(4):878-84. doi: 10.1093/ajcn/71.4.878[↩][↩]
- Lietman TM, Dhital SP, Dean D. Conjunctival impression cytology for vitamin A deficiency in the presence of infectious trachoma. Br J Ophthalmol. 1998 Oct;82(10):1139-42. doi: 10.1136/bjo.82.10.1139[↩]
- Measles vaccines: WHO position paper. Wkly Epidemiol Rec. 2009 Aug 28;84(35):349-60. English, French.[↩]
- Rahman MM, Wahed MA, Fuchs GJ, Baqui AH, Alvarez JO. Synergistic effect of zinc and vitamin A on the biochemical indexes of vitamin A nutrition in children. Am J Clin Nutr. 2002 Jan;75(1):92-8. doi: 10.1093/ajcn/75.1.92[↩]
- Hodge C, Taylor C. Vitamin A Deficiency. [Updated 2023 Jan 2]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK567744[↩][↩][↩]
- World Health Organization. (2011). Guideline : vitamin A supplementation in infants 1-5 months of age. World Health Organization. https://apps.who.int/iris/handle/10665/44628[↩]
- World Health Organization. (2011). Guideline : neonatal vitamin A supplementation. World Health Organization. https://apps.who.int/iris/handle/10665/44626[↩]
- World Health Organization. (2011). Guideline : vitamin A supplementation in postpartum women. World Health Organization. https://apps.who.int/iris/handle/10665/44623[↩]
- Vitamin A Deficiency Treatment & Management. https://emedicine.medscape.com/article/126004-treatment[↩]
- Via MA, Mechanick JI. Nutritional and Micronutrient Care of Bariatric Surgery Patients: Current Evidence Update. Curr Obes Rep. 2017 Sep;6(3):286-296. doi: 10.1007/s13679-017-0271-x[↩]
- Basu, S., Khanna, P., Srivastava, R. et al. Oral vitamin A supplementation in very low birth weight neonates: a randomized controlled trial. Eur J Pediatr 178, 1255–1265 (2019). https://doi.org/10.1007/s00431-019-03412-w[↩]
- Ross DA. Recommendations for Vitamin A Supplementation. The Journal of Nutrition. Volume 132, Issue 9, September 2002, Pages 2902S-2906S. https://doi.org/10.1093/jn/132.9.2902S[↩]
- Bors F, Fells P. Reversal of the complications of self-induced vitamin A deficiency. Br J Ophthalmol. 1971 Mar;55(3):210-4. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1208224/pdf/brjopthal00303-0070.pdf[↩]
- Crum AR, Srikumaran D, Woreta F. Bitot’s Spots following Bariatric Surgery: An Ocular Manifestation of a Systemic Disease. Case Rep Ophthalmol. 2017 Dec 21;8(3):581-589. doi: 10.1159/000485235[↩]
- Moise A.R., Noy N., Palczewski K., Blaner W.S. Delivery of retinoid-based therapies to target tissues. Biochemistry. 2007;46:4449–4458. doi: 10.1021/bi7003069[↩]
- Heinonen M. Food groups as the source of retinoids, carotenoids, and vitamin A in Finland. Int J Vitam Nutr Res. 1991;61(1):3-9.[↩]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Vitamin A. [Updated 2020 Nov 4]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548165[↩][↩][↩]
- U.S. National Library of Medicine, MedlinePlus. Hypervitaminosis A. https://medlineplus.gov/ency/article/000350.htm[↩]
- Vitamin A. https://ods.od.nih.gov/factsheets/VitaminA-Consumer[↩][↩]
- O’Donnell J. Polar hysteria: An expression of hypervitaminosis A.Am JTher. 2004;11(6):507–16.[↩][↩]
- Mawson A. Mefloquine use, psychosis, and violence: A retinoid toxicityhypothesis.Med Sci Monitor. 2013;19:579–83.[↩][↩]
- Penniston KL, Tanumihardjo SA. The acute and chronic toxic effects of vitamin A. Am J Clin Nutr. 2006 Feb;83(2):191-201. doi: 10.1093/ajcn/83.2.191[↩]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Vitamin A. [Updated 2013 Dec 3]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548165[↩]
- García-Muñoz P, Bernal-Bellido C, Marchal-Santiago A, et al. Liver cir-rhosis from chronic hypervitaminosis A resulting in a liver transplantation:A case report.Transpl Proc. 2019;51(1):90–1.[↩][↩][↩][↩]
- Lowe N.J., David M. New retinoids for dermatologic diseases. Uses and toxicity. Dermatol. Clin. 1988;6:539–552. doi: 10.1016/S0733-8635(18)30632-6[↩][↩][↩]
- Setty O.H., Misra U.K. Effect of heparin on vitamin A induced hyperlipidemia in rats. Int. J. Vitam. Nutr. Res. 1981;51:325–330.[↩][↩]
- Challem JJ. Teratogenicity of high vitamin A intake. N Engl J Med. 1996 May 2;334(18):1196-7.[↩][↩][↩][↩]
- Jing J., Isoherranen N., Robinson-Cohen C., Petrie I., Kestenbaum B.R., Yeung C.K. Chronic Kidney Disease Alters Vitamin A Homeostasis via Effects on Hepatic RBP4 Protein Expression and Metabolic Enzymes. Clin. Transl. Sci. 2016;9:207–215. doi: 10.1111/cts.12402[↩]
- Clugston R.D., Blaner W.S. The adverse effects of alcohol on vitamin A metabolism. Nutrients. 2012;4:356–371. doi: 10.3390/nu4050356[↩]
- Converting Units of Measure for Folate, Niacin, and Vitamins A, D, and E on the Nutrition and Supplement Facts Labels: Guidance for Industry. https://www.fda.gov/media/129863/download[↩]
- Rodahl K, Moore T. The vitamin A content and toxicity of bear and seal liver. Biochem J. 1943 Jul;37(2):166-8. doi: 10.1042/bj0370166 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1257872/pdf/biochemj00974-0009.pdf[↩][↩]
- Snodgrass S.R. Vitamin neurotoxicity. Mol. Neurobiol. 1992;6:41–73. doi: 10.1007/BF02935566[↩]
- Penniston K.L., Tanumihardjo S.A. The acute and chronic toxic effects of vitamin A. Am. J. Clin. Nutr. 2006;83:191–201. doi: 10.1093/ajcn/83.2.191[↩]
- Layton A. The use of isotretinoin in acne. Dermatoendocrinology. 2009;1:162–169. doi: 10.4161/derm.1.3.9364[↩][↩]
- Biesalski H.K. Comparative assessment of the toxicology of vitamin A and retinoids in man. Toxicology. 1989;57:117–161. doi: 10.1016/0300-483X(89)90161-3[↩]
- Genaro Pde S., Martini L.A. Vitamin A supplementation and risk of skeletal fracture. Nutr. Rev. 2004;62:65–67. doi: 10.1111/j.1753-4887.2004.tb00026.x[↩]
- Johansson S., Lind P.M., Hakansson H., Oxlund H., Orberg J., Melhus H. Subclinical hypervitaminosis A causes fragile bones in rats. Bone. 2002;31:685–689. doi: 10.1016/S8756-3282(02)00910-9[↩]
- Kuenzli S, Saurat JH. Retinoids for the treatment of psoriasis: outlook for the future. Curr Opin Investig Drugs. 2001 May;2(5):625-30.[↩]
- Patatanian E., Thompson D.F. Retinoic acid syndrome: A review. J. Clin. Pharm. Ther. 2008;33:331–338. doi: 10.1111/j.1365-2710.2008.00935.x[↩]
- Lindhard,J.[1913]. Med”.Gronland,41,461.[↩]
- Ribaya-Mercado JD, Blumberg JB. Vitamin A: is it a risk factor for osteoporosis and bone fracture? Nutr Rev. 2007 Oct;65(10):425-38. doi: 10.1111/j.1753-4887.2007.tb00268.x[↩]
- Persson B, Tunell R, Ekengren K. 1965. Chronic vitamin A intoxication during the first half year of life. Acta Paediatr Scand 54:49–60. https://www.ncbi.nlm.nih.gov/pubmed/14248225[↩]
- Bush ME, Dahms BB. 1984. Fatal hypervitaminosis A in a neonate. Arch Pathol Lab Med 108:838–842. https://www.ncbi.nlm.nih.gov/pubmed/6548125[↩]
- Carpenter TO, Pettifor JM, Russell RM, Pitha J, Mobarhan S, Ossip MS, Wainer S, Anast CS. 1987. Severe hypervitaminosis A in siblings: Evidence of variable tolerance to retinol intake. J Pediatr 111:507–512. https://www.ncbi.nlm.nih.gov/pubmed/3655980[↩][↩]
- National Institute of Arthritis and Musculoskeletal and Skin Diseases. Vitamin A and Bone Health. https://www.niams.nih.gov/Health_Info/Bone/Bone_Health/Nutrition/vitamin_a.asp[↩]
- Sy, Alexander & Kumar, Smriti & Steinberg, Jonathan & Garcia-Buitrago, Monica & Benitez, Leopoldo. (2020). Liver Damage due to Hypervitaminosis. ACG Case Reports Journal. 7. e00431. 10.14309/crj.0000000000000431[↩]