Vitamin B12
Vitamin B12 is also known as cobalamin or cyanocobalamin (man-made form of vitamin B12), is a nutrient that helps keep your body’s nerve and blood cells healthy and helps make DNA, the genetic material in all cells. Vitamin B-12 is a water-soluble vitamin that is naturally present in some foods, added to others, and available as a dietary supplement and a prescription medication. Vitamin B12 has the largest and most complex chemical structure of all the vitamins. Vitamin B12 is unique among vitamins in that it contains a metal ion, cobalt 1, 2, 3, 4, 5. For this reason cobalamin is the term used to refer to compounds having vitamin B12 activity 1. Methylcobalamin and adenosylcobalamin (5-deoxyadenosylcobalamin) are the two forms of “active” vitamin B12 used by your body 6, 7, 8. The form of cobalamin used in most nutritional supplements and fortified foods, cyanocobalamin (man-made form of vitamin B12), is readily converted to adenosylcobalamin (5-deoxyadenosylcobalamin) and methylcobalamin in your body. In mammals, vitamin B-12 is a cofactor for only two enzymes, methionine synthase and L-methylmalonyl-coenzyme A mutase 9, 10. Methionine synthase catalyzes the conversion of homocysteine to methionine 8, 11. Methionine is required for the formation of S-adenosylmethionine, a universal methyl donor for almost 100 different substrates, including DNA, RNA, hormones, proteins, and lipids. L-methylmalonyl-CoA mutase converts L-methylmalonyl-CoA to succinyl-CoA in the degradation of propionate 4, 8, 11, an essential biochemical reaction in fat and protein metabolism. Succinyl-CoA is also required for hemoglobin synthesis.
Vitamin B12 is required for the development, myelination, and function of the central nervous system; healthy red blood cell formation; and DNA synthesis 2, 3, 4, 5, 8.
Large amounts of Vitamin B-12 seem to be nontoxic but are not recommended for regular use (ie, as a general tonic). The Recommended Dietary Allowance (RDA) for vitamin B12 is 2.4 micrograms per day (μg/day) for adolescents and adults. It is slightly higher for women who are pregnant (2.6 mcg/day) or breastfeeding (2.8 mcg/day) 1. The Food and Nutrition Board at the National Academies of Sciences, Engineering, and Medicine did not establish a Tolerable Upper Intake Level (maximum daily intake unlikely to cause adverse health effects) for vitamin B12 because of its low potential for toxicity 8. Even at large doses, vitamin B12 is generally considered to be safe because your body does not store excess amounts 12.
Vitamin B-12 also helps prevent a type of anemia called megaloblastic anemia that makes people tired and weak. Your body cannot make vitamin B12. Vitamin B-12 is synthesized only by bacteria. While present in animal products, including meats, fish, shellfish, dairy products, and eggs, it is absent in plant-based foods. People most at risk for vitamin B12 deficiency are vegans, as diets devoid of animal products will result in B12 deficiency. However, vitamin B12 issues can be caused by taking some types of stomach acid blockers. Also, some people have an autoimmune or inflammatory condition of the stomach wall that degrade the proteins that aid vitamin B12 absorption.
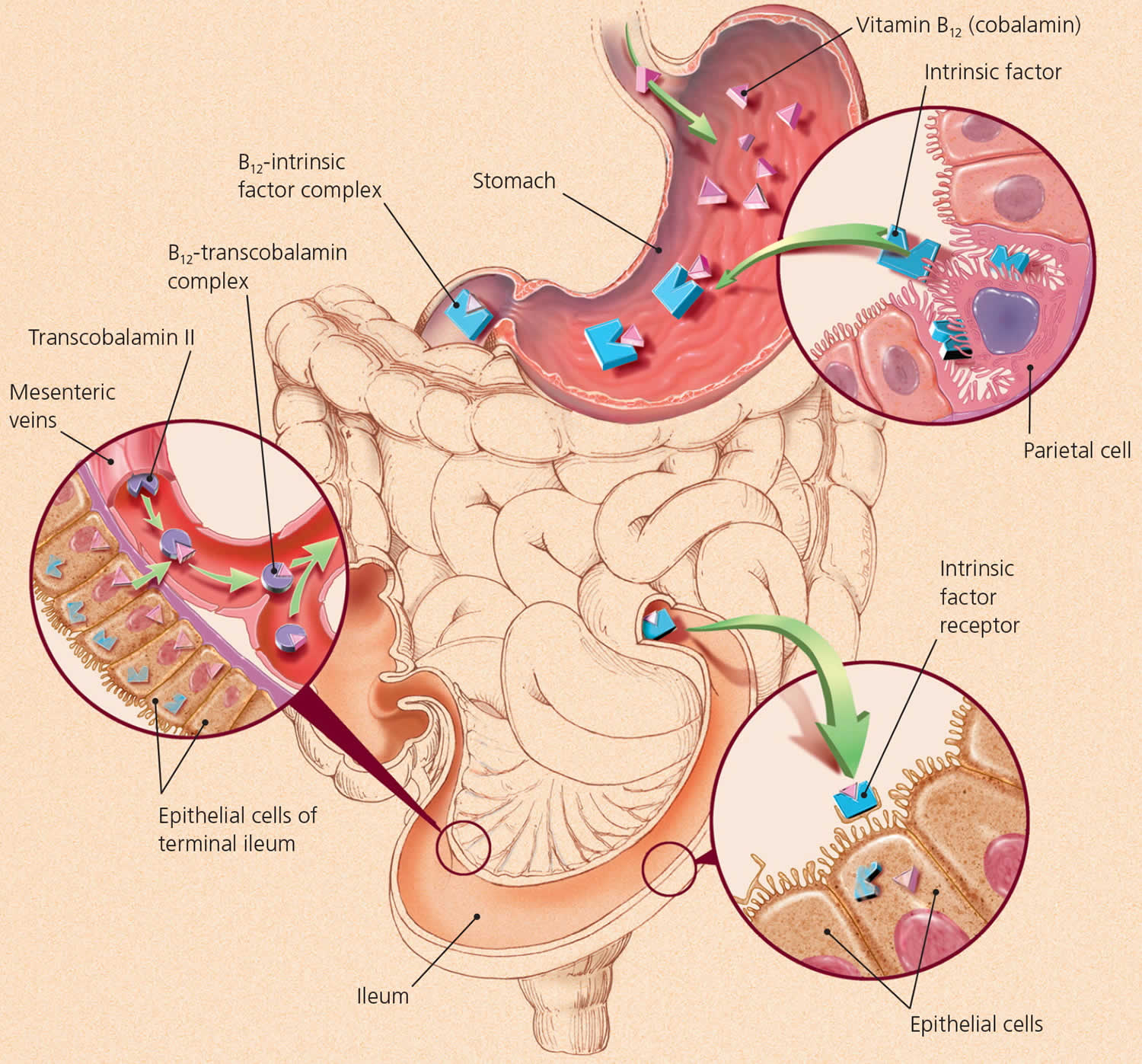
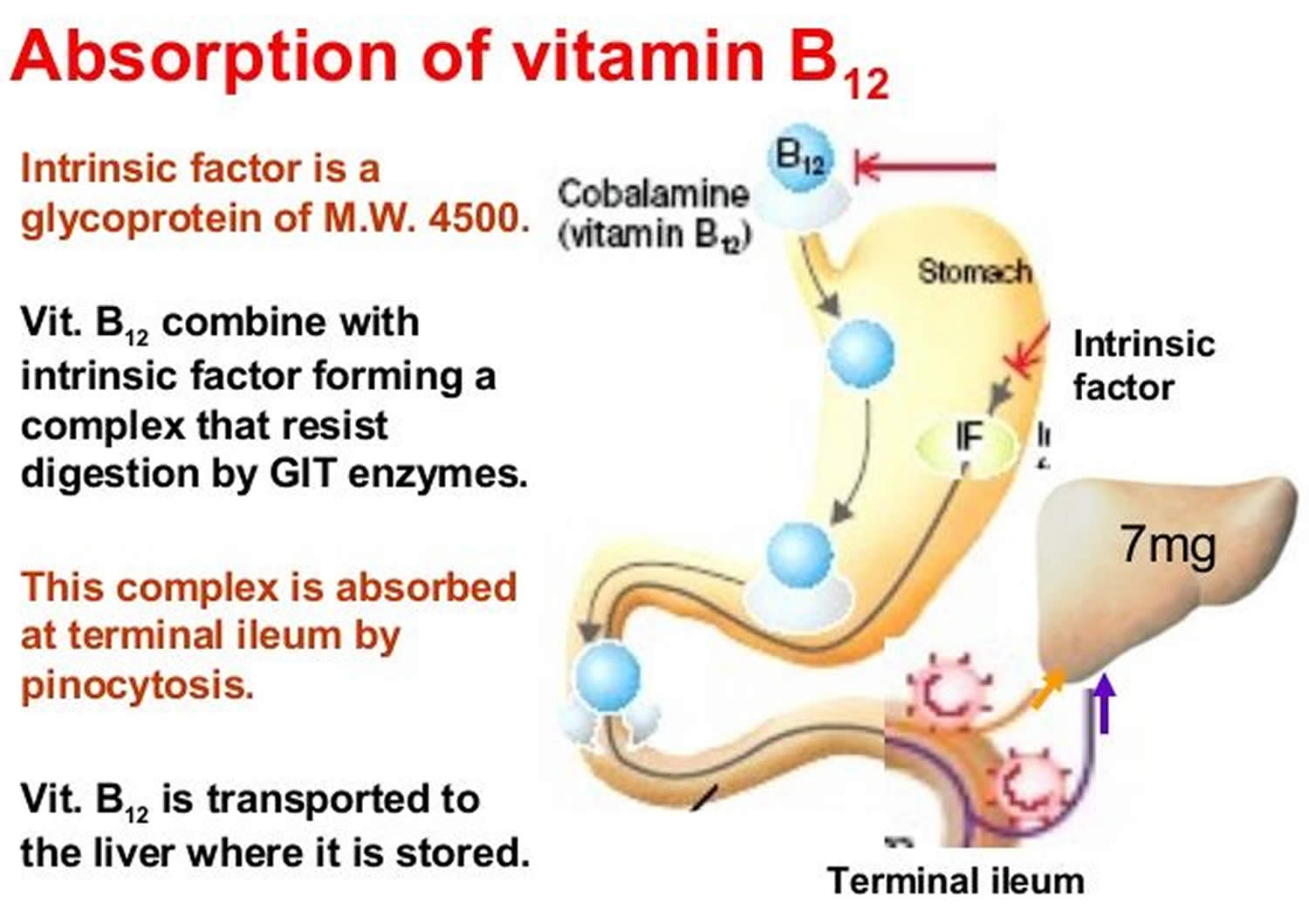
Two steps are required for your body to absorb Vitamin B-12 from food.
- First, food-bound Vitamin B-12 is released in the stomach’s acid environment (hydrochloric acid and and gastric protease in the stomach separate Vitamin B-12 from the protein to which Vitamin B-12 is attached in food) and is bound to R protein (haptocorrin) 8. Approximately 1.2% of vitamin B12 is absorbed passively without the help of intrinsic factor (IF) 13. When synthetic Vitamin B-12 is added to fortified foods and dietary supplements, it is already in free form and thus, does not require this separation step. If a patient receives the oral formulation of cobalamin at high doses, this passive absorption is sufficient to replenish vitamin B12 deficiency (a lack of vitamin B12). If intrinsic factor (IF) is present in an adequate amount, then oral cobalamin is absorbed with the help of intrinsic factor (IF). When administering cobalamin parenterally, it bypasses the intestinal barrier, absorbs quickly by diffusion, and enters into the systemic circulation 14.
- Second, pancreatic enzymes cleave this B12 complex (B12-R protein) in the small intestine. After cleavage, intrinsic factor (IF), a protein secreted by parietal cells situated in the mucosa of your stomach, binds with the free Vitamin B-12. Intrinsic factor is required for absorption of Vitamin B-12, which takes place in the terminal ileum 8, 15. Intrinsic factor (IF) binds to vitamin B12 and the complex is transported across the cell membrane bound to another glycoprotein called transcobalamin 14. Approximately 56% of a 1 mcg oral dose of Vitamin B-12 is absorbed, but absorption decreases drastically when the capacity of intrinsic factor is exceeded (at 1–2 mcg of Vitamin B-12) 16. Some people have pernicious anemia, a condition where they cannot make intrinsic factor (IF). As a result, they have trouble absorbing Vitamin B-12 from all foods and dietary supplements.
Pernicious anemia is an autoimmune disease that affects the gastric mucosa and results in gastric atrophy. This leads to the destruction of parietal cells, achlorhydria, and failure to produce intrinsic factor, resulting in Vitamin B-12 malabsorption 4, 8, 17, 18, 19. If pernicious anemia is left untreated, it causes vitamin B-12 deficiency (a lack of vitamin B12), leading to megaloblastic anemia and neurological disorders, even in the presence of adequate dietary intake of vitamin B-12. Pernicious anemia can cause fatigue, weakness, constipation, loss of appetite, and weight loss. Numbness and tingling in the hands and feet, depression, confusion, or poor memory can also occur. Symptoms of vitamin B12 deficiency can take decades to develop, and can usually only be diagnosed by a medical professional. For more details see below – Groups at Risk of Vitamin B12 deficiency.
In the blood plasma, Vitamin B-12 is bound to transcobalamins 1 and 2 20. Transcobalamin 2 is responsible for delivering Vitamin B-12 to tissues. The liver stores large amounts of Vitamin B-12. Enterohepatic reabsorption helps retain Vitamin B-12. Liver Vitamin B-12 stores can normally sustain physiologic needs for 3 to 5 years if B12 intake stops (eg, in people who become vegans) and for months to 1 year if enterohepatic reabsorption capacity is absent.
In healthy adults, vitamin B12 deficiency is uncommon, mainly because total body stores can exceed 2,500 mcg, daily turnover is slow, and dietary intake of only 2.4 mcg/day is sufficient to maintain adequate vitamin B12 status 16. In elderly individuals, vitamin B12 deficiency is more common mainly because of impaired intestinal absorption that can result in marginal to severe vitamin B12 deficiency in this population.
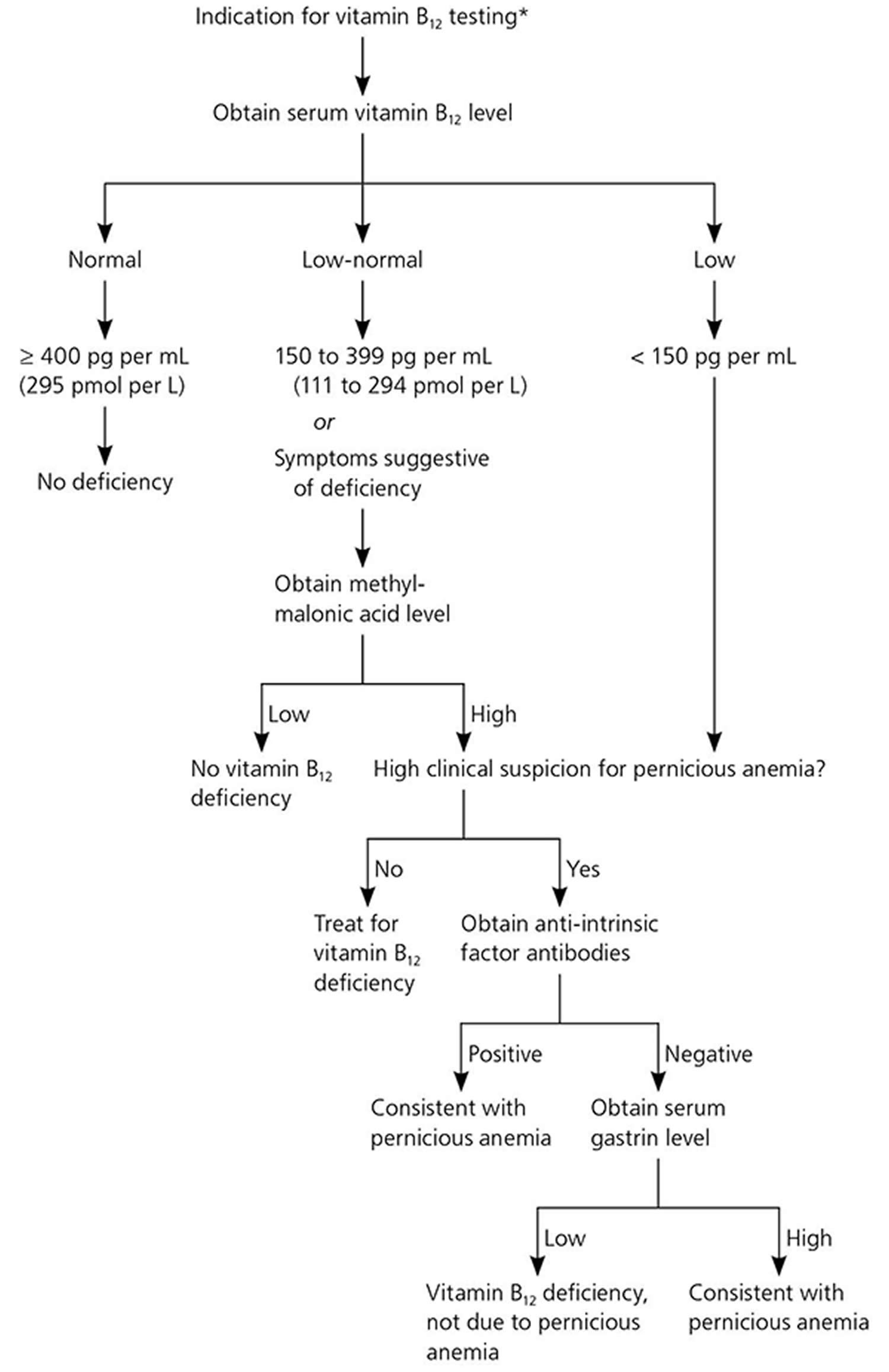
Vitamin B12 status is typically assessed by measurements of serum or plasma vitamin B12 levels 12. The cutoff between normal vitamin B12 levels and vitamin B12 deficiency varies by method and laboratory, but most laboratories define subnormal serum or plasma values as those lower than 200 or 250 pg/mL (148 or 185 pmol/L) 21. Levels of serum methylmalonic acid (MMA), a vitamin B12-associated metabolite, are the most sensitive markers of vitamin B12 status, and an methylmalonic acid (MMA) level greater than 0.271 micromol/L suggests vitamin B12 deficiency 22, 23, 24. However, MMA levels also rise with kidney failure and tend to be higher in older adults 22, 25, 26. Another marker is total plasma homocysteine levels, which rise quickly as vitamin B12 status declines; a serum homocysteine level higher than 15 micromol/L, for example, suggests vitamin B12 deficiency 27. However, this indicator has poor specificity because it is influenced by other factors, such as low folate levels and, especially, by declines in kidney function 22. Experts suggest that if a patient’s serum vitamin B12 level is less than 150 pg/ml (111 pmol/L), the patient’s serum methylmalonic acid (MMA) levels should be checked to confirm a diagnosis of vitamin B12 deficiency 23, 25.
Vitamin B12 key points
- Vitamin B12 or cobalamin plays essential roles in folate metabolism and in the synthesis of the citric acid cycle intermediate, succinyl-CoA.
- Vitamin B12 deficiency is commonly associated with chronic stomach inflammation, which may contribute to an autoimmune vitamin B12 malabsorption syndrome called pernicious anemia and to a food-bound vitamin B12 malabsorption syndrome. Impairment of vitamin B12 absorption can cause megaloblastic anemia and neurologic disorders in deficient subjects.
- Normal function of the digestive system required for food-bound vitamin B12 absorption is commonly impaired in individuals over 60 years of age, placing them at risk for vitamin B12 deficiency.
- Vitamin B12 and folate are important for homocysteine metabolism. Elevated homocysteine levels in blood are a risk factor for cardiovascular disease. Although B vitamin supplementation has been proven effective to control homocysteine levels, current data from intervention trials have not shown that lowering homocysteine levels decreases cardiovascular disease risk.
- The preservation of DNA integrity is dependent on folate and vitamin B12 availability. Poor vitamin B12 status has been linked to increased risk of breast cancer in some, but not all, observational studies. There is a need to evaluate whether supplemental vitamin B12, along with folic acid, could help reduce breast cancer incidence.
- Low maternal vitamin B12 status has been associated with an increased risk of neural tube defects, but it is not known whether vitamin B12 supplementation could help reduce the risk of neural tube defects.
- Vitamin B12 is essential for the preservation of the myelin sheath around neurons and for the synthesis of neurotransmitters. While hyperhomocysteinemia may increase the risk of cognitive impairment, it is not clear whether vitamin B12 deficiency contributes to the risk of dementia in the elderly. Although B-vitamin supplementation lowers homocysteine levels in older subjects, the long-term benefit is not yet known.
- Both depression and osteoporosis have been linked to diminished vitamin B12 status and high homocysteine levels.
- Products of animal origin constitute the primary source of vitamin B12. Older individuals and vegans are advised to use vitamin B12 fortified foods and supplements to meet their needs.
- The long-term use of certain medications, such as inhibitors of stomach acid secretion, can adversely affect vitamin B12 absorption.
Figure 1. Vitamin B12 absorption and transport
Vitamin B12 function
Vitamin B12 is required for the development, myelination, and function of the central nervous system; healthy red blood cell formation; and helps make DNA, the genetic material in all cells 28, 29. Vitamin B12 functions as a cofactor for two enzymes, methionine synthase and L-methylmalonyl-CoA mutase (see more below) 28, 21, 30. Methionine synthase catalyzes the conversion of homocysteine to the essential amino acid methionine 31, 21. Methionine is required for the formation of S-adenosylmethionine, a universal methyl donor for almost 100 different substrates, including DNA, RNA, proteins, and lipids 28, 30. L-methylmalonyl-CoA mutase converts L-methylmalonyl-CoA to succinyl-CoA in the metabolism of propionate, a short-chain fatty acid 21.
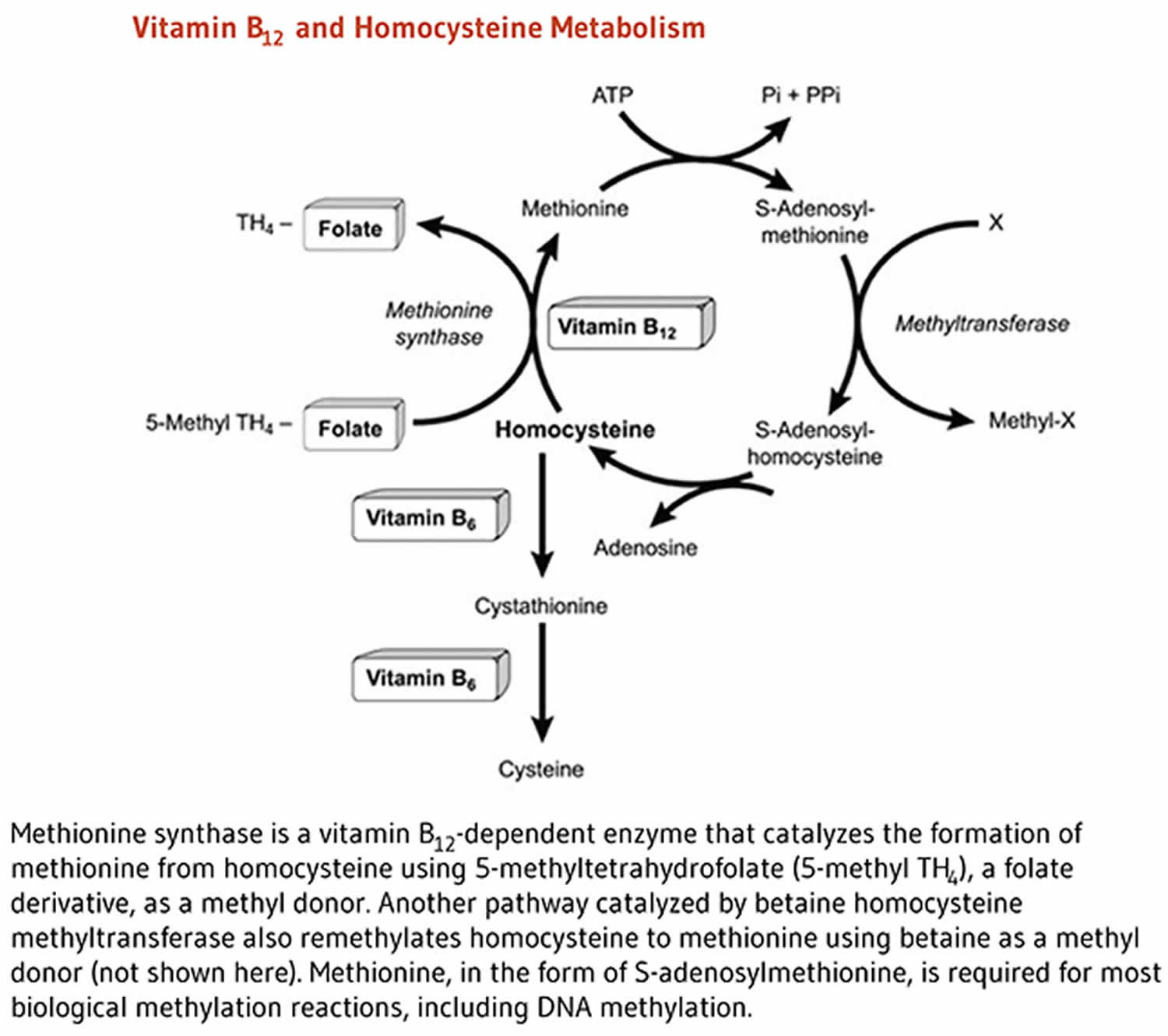
Cofactor for methionine synthase
Methylcobalamin is required for the function of the folate-dependent enzyme, methionine synthase. This enzyme is required for the synthesis of the amino acid, methionine, from homocysteine. Methionine in turn is required for the synthesis of S-adenosylmethionine, a methyl group donor used in many biological methylation reactions, including the methylation of a number of sites within DNA, RNA, and proteins 32. Aberrant methylation of DNA and proteins, which causes alterations in chromatin structure and gene expression, are a common feature of cancer cells. Inadequate function of methionine synthase can lead to an accumulation of homocysteine, which has been associated with increased risk of cardiovascular disease (Figure 2).
Figure 2. Vitamin B-12 function
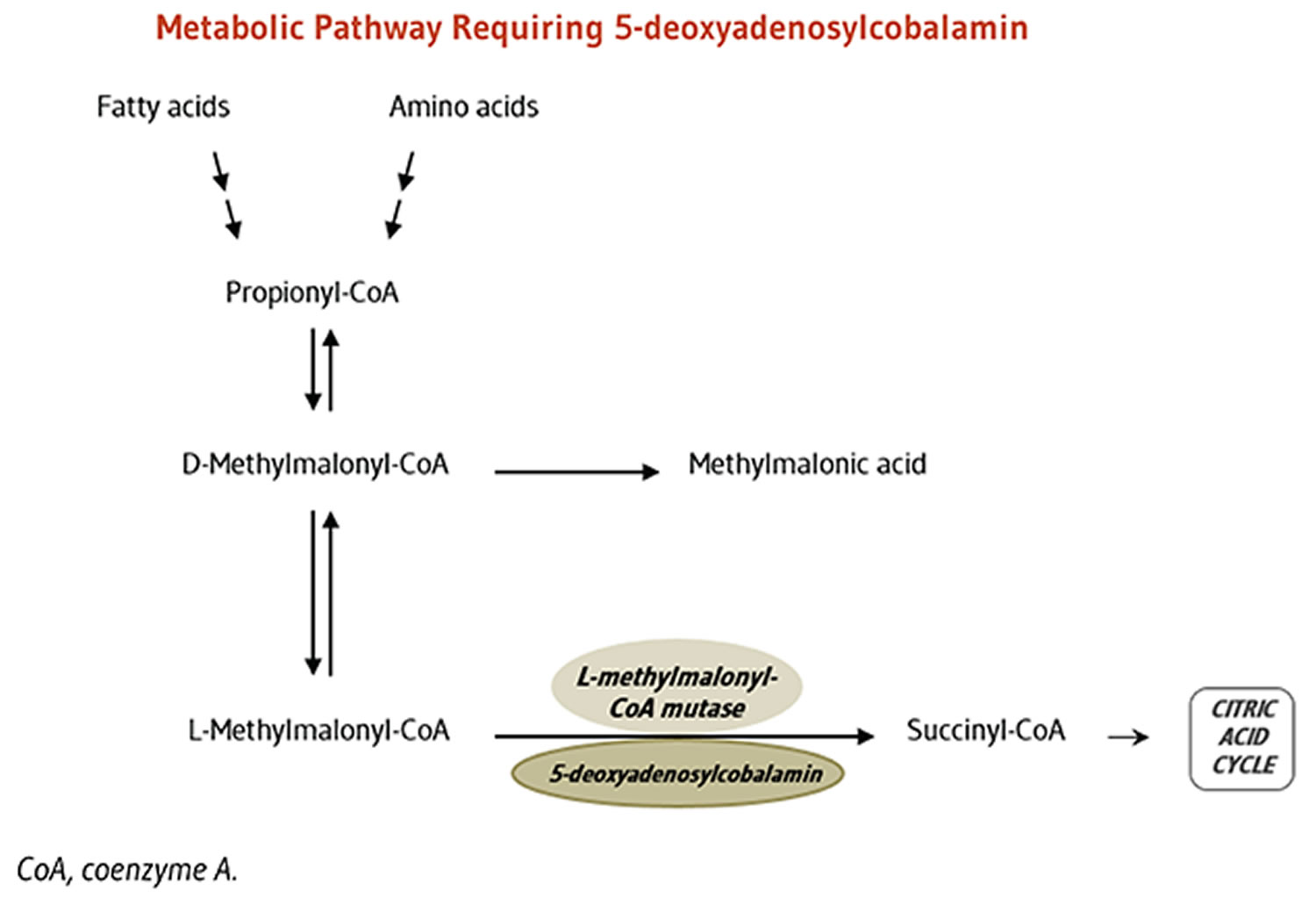
Cofactor for L-methylmalonyl-coenzyme A mutase
5-Deoxyadenosylcobalamin is required by the enzyme that catalyzes the conversion of L-methylmalonyl-coenzyme A to succinyl-coenzyme A (succinyl-CoA), which then enters the citric acid cycle (Figure 3). Succinyl-CoA plays an important role in the production of energy from lipids and proteins and is also required for the synthesis of hemoglobin, the oxygen-carrying pigment in red blood cells 32.
Figure 3. Vitamin B12 function
Vitamin B-12 Supplements
Vitamin B12 is available in multivitamin/mineral supplements, in supplements containing other B-complex vitamins, and in supplements containing only vitamin B12. Multivitamin/mineral supplements typically contain vitamin B12 at doses ranging from 5 to 25 mcg. Vitamin B12 levels are higher, generally 50–500 mcg, in supplements containing vitamin B12 with other B-complex vitamins and even higher, typically 500–1,000 mcg, in supplements containing only vitamin B12.
The most common form of vitamin B12 in dietary supplements is cyanocobalamin 33. Other forms of vitamin B12 in supplements are adenosylcobalamin, methylcobalamin, and hydroxycobalamin.
No evidence indicates that absorption rates of vitamin B12 in supplements vary by form of the vitamin. These rates are about 50% at doses (less than 1–2 mcg) that do not exceed the cobalamin-binding capacity of intrinsic factor and are substantially lower at doses well above 1–2 mcg 33. For example, absorption is only about 2% at doses of 500 mcg and 1.3% at doses of 1,000 mcg 16.
In addition to oral dietary supplements, vitamin B12 is available in sublingual preparations as tablets or lozenges. Evidence suggests no difference in efficacy between oral and sublingual forms 34.
Vitamin B12 interactions with medications
Vitamin B12 has the potential to interact with certain medications. In addition, several types of medications might adversely affect vitamin B12 levels. A few examples are provided below. Individuals taking these and other medications on a regular basis should discuss their vitamin B12 status with their healthcare providers.
- Gastric acid inhibitors: Gastric acid inhibitors include proton pump inhibitors, such as omeprazole (Prilosec®) and lansoprazole (Prevacid®), and histamine 2-receptor antagonists, such as cimetidine (Tagamet®) and ranitidine (Zantac®). These drugs are used to treat gastroesophageal reflux disease and peptic ulcer disease. They can interfere with vitamin B12 absorption from food by slowing the release of gastric acid into the stomach and thereby lead to vitamin B12 deficiency 35.
- Metformin: Metformin, an antihyperglycemic agent used as first-line treatment for prediabetes and diabetes, might reduce the absorption of vitamin B12 and significantly reduce serum vitamin B12 concentrations 35.
Vitamin B-12 Prescription Medications
Vitamin B12, in the forms of cyanocobalamin and hydroxycobalamin, can be administered parenterally, either as an intramuscular (IM) or intravenous (IV) injection, as a prescription medication, usually by intramuscular (IM) injection 21. Parenteral administration is typically used to treat vitamin B12 deficiency caused by pernicious anemia as well as other conditions (e.g., tropical sprue, pancreatic insufficiency) that result in vitamin B12 malabsorption and severe vitamin B12 deficiency 28.
Hydroxocobalamin is given parenterally, either as an intramuscular (IM) or intravenous (IV) injection 36. Cyanocobalmin administration can be via the oral, sublingual, intramuscular, or subcutaneous routes 10, 37. Cyanocobalamin use is common in the United States, whereas hydroxocobalamin is the more preferred formulation in Europe for the treatment of vitamin B12 deficiency 36. Hydroxocobalamin requires less frequent administration (every three months) compared to oral cyanocobalamin supplementation. The parenteral method of administration is particularly useful to treat vitamin B12 deficiency caused by malabsorption states in which oral administration is ineffective 14.
When treating vitamin B12 deficiency anemia with hydroxocobalamin, the dosage of each intramuscular injection of hydroxocobalamin is usually 1 mg given as a total of 5 to 10 doses administered every other day (3 times a week over two weeks) followed by every three months after that for maintenance. The duration of treatment is assessed based on the patient’s therapeutic response 38.
The recommendation is to take the following precautions when treating B12 or folate deficiency.
In a patient with folate deficiency, evaluation for coexistent vitamin B12 deficiency is necessary. If folate alone is supplemented in a vitamin B12 deficient patient, the B12 associated hematologic abnormalities may improve; however, neurological symptoms can worsen 36.
One of the neurological disorders associated with vitamin B12 deficiency is subacute combined degeneration of the cord (SCD), a condition characterized by demyelination of the dorsal and lateral columns of the spinal cord. Vitamin B12 plays a vital role in myelin production. The active form of B12 (adenosylcobalamin) serves as a cofactor in converting methylmalonyl-CoA to succinyl-CoA, an essential step in lipid synthesis. Without adenosylcobalamin, abnormal fatty acids incorporate into neuronal lipids interfering with normal myelin formation. Vitamin B12 (cobalamin) is an intracellular superoxide scavenger, which is particularly important for unmyelinated axons in the papillomacular bundle. Vitamin B12 or cobalamin deficiency may cause superoxide accumulation, which is a signal for retinal ganglion cell apoptosis, therefore causing retinal ganglion cell and axon loss 39. In 1980, Chester and colleagues 40 described segmental temporal demyelination of the retrobulbar optic nerves in monkeys with experimental vitamin B12 or cobalamin deficiency, which they postulated as the site of injury in the optic neuropathy due to vitamin B12 deficiency. They theorized that alterations in fatty acid metabolism due to cobalamin deficiency disrupted myelin formation, with secondary retinal ganglion cell loss by retrograde degeneration 40. A more recent hypothesis for the underlying mechanism takes into account the evidence that vitamin B12 or cobalamin, in addition to being an important cofactor for various enzymes, acts as an intracellular superoxide scavenger, which is particularly important for unmyelinated axons in the papillomacular bundle. As demonstrated by Chan and colleagues 41, cobalamin is an endogenous SOD mimetic. Since superoxide signals retinal ganglion cell apoptosis, superoxide accumulation due to vitamin B12 or cobalamin deficiency leads to retinal ganglion cell and axonal loss 39.
Additionally, vitamin B12 deficiency can cause abnormal DNA synthesis, potentially hindering oligodendrocyte growth, adversely affecting myelin production.
Alternatively, it is also essential to recognize that folate deficiency indirectly leads to a vitamin B12 deficient state. In the cells, folate converts to its active form methyltetrahydrofolate (MTHF). MTHF acts as a donor of methyl groups to B12 (cobalamin), forming methylcobalamin. In the absence of this methylation, methylcobalamin (active form) is not produced and is unavailable for use leading to signs and symptoms of B12 deficiency 42.
In cyanide poisoning, intravenous (IV) hydroxocobalamin should be used. The FDA approves the immediate use of an hydroxocobalamin injection kit for this purpose 43.
Vitamin B12 is also available as a prescription nasal gel spray. This formulation appears to be effective in raising vitamin B12 blood levels in adults and children 44. A small clinical study with 10 participants (mean age 81 years) found that the bioavailability of a 1,000 mcg cobalamin dose was 2% with intranasal administration, which is similar to the bioavailability of an oral dose 45.
Vitamin B12 Health Benefits
Scientists are studying Vitamin B-12 to understand how it affects health. Here are several examples of what this research has shown:
Cardiovascular disease and stroke
Cardiovascular disease is the most common cause of death in industrialized countries, such as the United States, and is on the rise in developing countries. Risk factors for cardiovascular disease include elevated low-density lipoprotein (LDL) levels, high blood pressure, low high-density lipoprotein (HDL) levels, obesity, and diabetes 46. Vitamin B12 supplements (along with other B vitamins) reduce blood levels of homocysteine, a compound linked to an increased risk of having a heart attack or stroke But despite reducing homocysteine, research shows that these vitamins don’t reduce the risk of developing cardiovascular disease or stroke 12.
Elevated homocysteine levels have also been identified as an independent risk factor for cardiovascular disease 47, 48, 49. Homocysteine is a sulfur-containing amino acid derived from methionine that is normally present in blood. Elevated homocysteine levels are thought to promote thrombogenesis, impair endothelial vasomotor function, promote lipid peroxidation, and induce vascular smooth muscle proliferation 47, 48, 50. Evidence from retrospective, cross-sectional, and prospective studies links elevated homocysteine levels with coronary heart disease and stroke 47, 51, 52, 53, 54.
The amount of homocysteine in the blood is regulated by at least three B vitamins: folate (vitamin B9), vitamin B6 (Pyridoxine), and vitamin B12 (Cobalamin) 1. In the presence of insufficient Vitamin B-12, homocysteine levels can rise due to inadequate function of methionine synthase 55. Results from several randomized controlled trials indicate that combinations of Vitamin B-12 and folic acid supplements with or without vitamin B6 (Pyridoxine) decrease homocysteine levels in people with vascular disease or diabetes and in young adult women 56, 57, 58, 59, 60, 61, 62. An early analysis of the results of 12 randomized controlled trials showed that folic acid supplementation (0.5-5 mg/day) had the greatest lowering effect on blood homocysteine levels (25% decrease); co-supplementation with folic acid and vitamin B12 (500 mcg/day) provided an additional 7% reduction (32% decrease) in blood homocysteine concentrations 63. In another study, older men and women who took a multivitamin/multimineral supplement for 8 weeks experienced a significant decrease in homocysteine levels 64. The results of a sequential supplementation trial in 53 men and women indicated that after folic acid supplementation, vitamin B12 became the major determinant of plasma homocysteine levels 65.
Although the evidence supports a role for folic acid and Vitamin B-12 supplements in lowering homocysteine levels, but results from several large prospective studies have not shown that these supplements decrease the risk of cardiovascular disease 49, 57, 58, 59, 60, 61, 62. A recent meta-analysis of data from 11 trials, including nearly 45,000 participants at risk of cardiovascular disease, showed that B-vitamin supplementation had no significant effect on risk of myocardial infarction (heart attack) or stroke, nor did it modify the risk of all-cause mortality 66. Other meta-analyses that included patients with chronic kidney disease have confirmed the lack of effect of homocysteine-lowering on risk of heart attack (myocardial infarction) and death. However, stroke risk was significantly reduced by 7%-12% with the B-vitamin supplementation 67, 68. Another meta-analysis of 12 clinical trials measuring flow-mediated vasodilation (FMD; a surrogate marker of vascular health) in response to homocysteine reduction revealed that B-vitamin supplementation was accompanied by an improved flow-mediated vasodilation in short-term <8 weeks) but not in long-term studies conducted in subjects with preexisting vascular diseases 69. Yet, some of the studies included in these meta-analyses did not use vitamin B12, and folate administration on its own has shown a protective role on vascular function and stroke risk 70. Besides, the high prevalence of malabsorption disorders and vitamin B12 deficiency in elderly individuals might warrant the use of higher doses of vitamin B12 than those used in these trials 71; in cases of malabsorption, only high-dose oral therapy or intramuscular injections can overcome vitamin B12 deficiency 16.
In the Women’s Antioxidant and Folic Acid Cardiovascular Study, women at high risk of cardiovascular disease who took daily supplements containing 1 mg Vitamin B-12, 2.5 mg folic acid, and 50 mg vitamin B6 for 7.3 years did not have a reduced risk of major cardiovascular events, despite lowered homocysteine levels 60. The Heart Outcomes Prevention Evaluation (HOPE) 2 trial, which included 5,522 patients older than 54 years with vascular disease or diabetes, found that daily treatment with 2.5 mg folic acid, 50 mg vitamin B6, and 1 mg Vitamin B-12 for an average of 5 years reduced homocysteine levels and the risk of stroke but did not reduce the risk of major cardiovascular events 58. In the Western Norway B Vitamin Intervention Trial, which included 3,096 patients undergoing coronary angiography, daily supplements of 0.4 mg Vitamin B-12 and 0.8 mg folic acid with or without 40 mg vitamin B6 for 1 year reduced homocysteine levels by 30% but did not affect total mortality or the risk of major cardiovascular events during 38 months of follow-up 61. The Norwegian Vitamin (NORVIT) trial 57 and the Vitamin Intervention for Stroke Prevention trial had similar results 62.
The American Heart Association has concluded that the available evidence is inadequate to support a role for B vitamins in reducing cardiovascular risk 49.
Dementia and Cognitive Function
Some people develop dementia as they get older. These people often have high levels of homocysteine in the blood. Observational studies have shown positive associations between elevated homocysteine levels and the incidence of both Alzheimer’s disease and dementia 72. Scientists hypothesize that elevated homocysteine levels might have a negative effect on the brain via numerous mechanisms, including cerebrovascular ischemia leading to neuronal cell death, activation of tau kinases leading to tangle deposition, and inhibition of methylation reactions 73. Vitamin B-12 (with folic acid and vitamin B6) can lower homocysteine levels, but scientists don’t know yet whether these vitamins actually help prevent or treat dementia. Vitamin B12 deficiency is a serious clinical problem, particularly common in the elderly, leading to neurological deficits and fatigue. Vitamin B12 deficiency often arises from malabsorption, in which case oral supplementation may not protect and injections may be needed 16. Vegetarians and vegans are also at higher risk.
Researchers have long been interested in the potential connection between Vitamin B-12 deficiency and dementia 48, 74. A deficiency in Vitamin B-12 causes an accumulation of homocysteine in the blood 75 and might decrease levels of substances needed to metabolize neurotransmitters 76. Observational studies show positive associations between elevated homocysteine levels and the incidence of both Alzheimer’s disease and dementia 75, 48, 77. Low Vitamin B-12 status has also been positively associated with cognitive decline 78.
Most observational studies have found correlations between low serum vitamin B12 concentrations alone or in combination with high folate concentrations and poor cognitive function 79, 80. For example, an analysis of cross-sectional 2011–2014 NHANES data on 2,420 adults aged 60 years or older found that low vitamin B12 (methylmalonic acid [MMA] greater than 0.27 micromol/L or serum vitamin B12 less than 203 pg/mL [150 pmol/L]) combined with high folic acid—unmetabolized serum folic acid greater than 0.44 mcg/L (1 nmol/L) or serum total folate higher than 32.7 mcg/L (74.1 nmol/L)—was associated an almost two to three times higher risk of cognitive impairment 79. However, a few observational studies have found no such association 81, 82. In addition, according to a systematic review of 35 prospective cohort studies in 14,325 participants aged 47 to 101 years followed for an average of 5.4 years, the evidence does not support a role for low vitamin B12 in the development of cognitive impairment or dementia 83.
Despite evidence that Vitamin B-12 lowers homocysteine levels and correlations between low Vitamin B-12 levels and cognitive decline, research has not shown that Vitamin B-12 has an independent effect on cognition 84, 85, 86, 87, 88. In one randomized, double-blind, placebo-controlled trial, 195 subjects aged 70 years or older with no or moderate cognitive impairment received 1,000 mcg Vitamin B-12, 1,000 mcg Vitamin B-12 plus 400 mcg folic acid, or placebo for 24 weeks 84. Treatment with Vitamin B-12 plus folic acid reduced homocysteine concentrations by 36%, but neither Vitamin B-12 treatment nor Vitamin B-12 plus folic acid treatment improved cognitive function.
A recent systematic review of 35 prospective cohort studies assessing the association between vitamin B12 status and cognitive deterioration in older individuals with or without dementia at baseline did not support a relationship between vitamin B12 serum concentrations and cognitive decline, dementia, or Alzheimer’s disease83. Nevertheless, studies utilizing more sensitive biomarkers of vitamin B12 status, including measures of holo-transcobalamin (holo-TC; a vitamin B12 carrier) and methylmalonic acid (MMA), showed more consistent results and a trend toward associations between poor vitamin B12 status and faster cognitive decline and risk of Alzheimer’s disease 89, 90, 91, 92, 93. Besides, it cannot be excluded that the co-occurrence of potential confounders like elevated homocysteine level and poor folate status might mitigate the true contribution of vitamin B12 status to cognitive functioning 94.
High-dose B-vitamin supplementation has been proven effective for treating hyperhomocysteinemia in elderly individuals with or without cognitive impairment. However, homocysteine-lowering trials have produced equivocal results regarding the prevention of cognitive deterioration in this population. A systematic review and meta-analysis of 18 randomized, placebo-controlled trials examining the effect of B-vitamin supplementation did not find that the decrease in homocysteine level prevented or delayed cognitive decline among older subjects 95. A more recent randomized, double-blind, placebo-controlled clinical trial in 900 older individuals at high risk of cognitive impairment found that daily supplementation of 400 mcg of folic acid and 100 mcg of vitamin B12 for two years significantly improved measures of immediate and delayed memory and slowed the rise in plasma homocysteine concentrations 96. However, supplemented subjects had no reduction in homocysteine concentrations compared to baseline, nor did they perform better in processing speed tests compared to placebo. Another two-year, randomized, placebo-controlled study in elderly adults reported that a daily regimen of 800 mcg of folic acid, 500 mcg of vitamin B12, and 20 mg of vitamin B6 significantly reduced the rate of brain atrophy compared to placebo treatment (0.5% vs. 3.7%). Interestingly, a greater benefit was seen in those with high compared to low homocysteine concentrations at baseline, suggesting the importance of lowering homocysteine levels in prevention of brain atrophy and cognitive decline 97, 98. The authors attributed the changes in homocysteine levels primarily to vitamin B12 98. The most recent randomized, double blind, placebo-controlled trial in over 2,500 individuals who suffered a stroke showed that the normalization of homocysteine concentrations by B-vitamin supplementation (2 mg of folic acid, 500 mcg of vitamin B12, and 25 mg of vitamin B6) did not improve cognitive performance or decrease incidence of cognitive decline compared to placebo 99.
Women at high risk of cardiovascular disease who participated in the Women’s Antioxidant and Folic Acid Cardiovascular Study were randomly assigned to receive daily supplements containing 1 mg Vitamin B-12, 2.5 mg folic acid and 50 mg vitamin B6, or placebo 87. After a mean of 1.2 years, B-vitamin supplementation did not affect mean cognitive change from baseline compared with placebo. However, in a subset of women with low baseline dietary intake of B vitamins, supplementation significantly slowed the rate of cognitive decline. In a trial conducted by the Alzheimer’s Disease Cooperative Study consortium that included individuals with mild-to-moderate Alzheimer’s disease, daily supplements of 1 mg Vitamin B-12, 5 mg folic acid, and 25 mg vitamin B6 for 18 months did not slow cognitive decline compared with placebo 88. Another study found similar results in 142 individuals at risk of dementia who received supplements of 2 mg folic acid and 1 mg Vitamin B-12 for 12 weeks 86.
The authors of two Cochrane reviews and a systematic review of randomized trials of the effects of B vitamins on cognitive function concluded that insufficient evidence is available to show whether Vitamin B-12 alone or in combination with vitamin B6 or folic acid has an effect on cognitive function or dementia 100, 101, 102. Additional large clinical trials of Vitamin B-12 supplementation are needed to assess whether Vitamin B-12 has a direct effect on cognitive function and dementia 75.
In general, evidence from randomized control trials does not show that vitamin B12 supplementation alone or with folic acid, vitamin B6, or both for 1 to 2 years improves cognitive function in older adults with or without dementia, mild cognitive impairment, or Alzheimer’s disease, even though supplementation lowers homocysteine levels 103. For example, an randomized control trial administered 400 mcg/day folic acid and 500 mcg/day vitamin B12 (B-vitamin group) or a placebo for 2 years to 2,919 adults aged 65 and older with homocysteine levels of 12 to 50 mcmol/L 104. Although homocysteine concentrations declined significantly more (by 5.0 mcmol/L) in the supplementation group than in the placebo group (1.3 mcmol/L), cognitive test scores did not differ between groups. A 2018 Cochrane review of vitamin and mineral supplements to maintain cognitive function in cognitively healthy people included 14 studies that compared folic acid, vitamin B12, vitamin B6, or a combination of these supplements to placebo in 27,882 participants, most of whom were aged 60 years or older 105. The supplements had little to no effect on global cognitive function when administered for up to 5 years and also appeared to have no impact when administered for 5 to 10 years.
Similarly, supplementation with vitamin B12 alone or with other B vitamins does not appear to decrease the risk or slow the progression of dementia or Alzheimer’s disease in older adults. Another 2018 Cochrane review evaluated the effects of vitamin and mineral supplements on cognitive function and dementia in people with mild cognitive impairment 106. The review included 5 trials with 879 participants that investigated B vitamin supplements (one study of folic acid only, and four trials of vitamins B6 and B12 and folic acid). Taking these B vitamins for 6 to 24 months had no apparent effect on episodic memory, executive function, speed of processing, or quality of life, although one study found a slower rate of brain atrophy over 2 years.
Additional clinical trials are needed to better understand the effects of vitamin B12 supplementation on cognitive function and cognitive decline.
Cancer
Some research shows that people with high levels of vitamin B12 have a higher risk of cancer. But other research shows that the risk of cancer is higher in people with low levels of vitamin B12 or that vitamin B12 levels don’t affect cancer risk. Therefore, the evidence for a relationship between vitamin B12 and cancer risk is mixed. More evidence is needed to understand whether vitamin B12 levels affect cancer risk.
A series of studies in young adults and older men indicated that increased levels of homocysteine and decreased levels of vitamin B12 in the blood were associated with a biomarker of chromosome breakage in white blood cells 107. In a double-blind, placebo-controlled study, the same biomarker of chromosome breakage was minimized in young adults who were supplemented with 700 mcg of folic acid and 7 mcg of vitamin B12 daily in cereal for two months 108.
Observational evidence supporting an association between higher vitamin B12 levels and increased cancer risk includes an analysis of data on 757,185 people (median age 56 years) with plasma vitamin B12 measurements 109. The results showed that the adjusted 1-year risk of cancer was 1.74 to 4.72 times higher among those with vitamin B12 levels above 813 pg/mL (600 pmol/L) than those with levels in the normal range of 203–813 pg/mL (150–600 pmol/L). An analysis by some of the same investigators of data from Danish medical registries for 25,017 people who had a cancer diagnosis between 1998 and 2014 found 1-year survival rates of 35.8% in those whose plasma cobalamin levels were higher than 1,084 pg/mL (800 pmol/L) and 69.3% in those with levels between 271 and 813 pg/mL (200–600 pmol/L) 110.
Some observational evidence also shows an association between supplements containing vitamin B12 and a higher risk of certain types of cancer. For example, an assessment of 77,118 participants aged 50 to 76 years in the Vitamins and Lifestyle cohort study found that use of at least 55 mcg/day supplemental vitamin B12 for an average of 10 years was associated with a 40% higher risk of lung cancer in men 111. However, the study found no association between supplemental vitamin B12 use and cancer risk in women.
Limited clinical trial evidence supports the finding that higher vitamin B12 intakes might increase cancer risk. In an analysis of data on 2,524 participants in the B Vitamins for the Prevention of Osteoporotic Fractures trial who were treated with supplements containing 400 mcg/day folic acid and 500 mcg/day vitamin B12 for 2 to 3 years, the risk of colorectal cancer was significantly higher, at 3.4%, in the supplementation group than in the placebo group, whose rate was 2% 112. However, high folic acid levels are potentially linked to increased risk of colorectal cancer, so the result might be due to the folic acid rather than the vitamin B12 113. Furthermore, the supplements had no significant effect on overall cancer risk.
Some observational evidence shows no association between high vitamin B12 concentrations or intakes and increased risk of certain cancers. For example, higher vitamin B12 intakes or serum concentrations were not associated with an increased risk of pancreatic cancer 114, breast cancer 115, or esophageal cancer or gastric cancer 116. Clinical trials support the lack of association between higher vitamin B12 intakes and cancer risk 117. For example, a meta-analysis of 18 randomized control trials that included 74,498 individuals found that supplements containing B vitamins, including 20 to 2,000 mcg/day vitamin B12, had little or no effect on cancer incidence, cancer deaths, or all-cause mortality during follow-up periods of 2 to 7.3 years 117.
Finally, evidence pointing to an association between lower vitamin B12 levels and a higher cancer risk includes observational data showing a risk of gastric cancer that was 5.8 times higher in male smokers with lower vitamin B12 levels (less than 394 pg/mL [291 pmol/L]) than in those with levels higher than 591 pg/mL (436 pmol/L) 118. Also, two meta-analyses found associations between lower vitamin B12 concentrations or intakes and a higher risk of colorectal cancer 119 and prostate cancer 120.
More evidence is needed to clarify whether high or low intakes of vitamin B12 influence the risk of cancer as well as the role of vitamin B12 in preventing cancer.
Breast cancer
A case-control study compared prediagnostic levels of serum folate, vitamin B6, and vitamin B12 in 195 women later diagnosed with breast cancer and 195 age-matched, cancer-free women 121. Among postmenopausal women, the association between blood levels of vitamin B12 and breast cancer suggested a threshold effect. The risk of breast cancer was more than doubled in women with serum vitamin B12 levels in the lowest quintile compared to women in the four highest quintiles 121. However, the meta-analysis of this study with three additional case-control studies found no protection associated with high compared to low vitamin B12 serum levels 115. A case-control study in Mexican women (475 cases and 1,391 controls) reported that breast cancer risk for women in the highest quartile of vitamin B12 intake (7.3-7.7 mcg/day) was 68% lower than those in the lowest quartile (2.6 mcg/day) 122. Stratification of the data revealed that the inverse association between dietary vitamin B12 intake and breast cancer risk was stronger in postmenopausal women compared to premenopausal women, though both associations were statistically significant 1. Moreover, among postmenopausal women, the apparent protection conferred by folate was only observed in women with the highest vitamin B12 quartiles of intake 122. However, more recent case-control and prospective cohort studies have reported weak to no risk reduction with vitamin B12 intakes in different populations, including Hispanic, African American and European American women 123, 124. A meta-analysis of seven case-control and seven prospective cohort studies concluded that the risk of breast cancer was not modified by high versus low vitamin B12 intakes 1. There was no joint association between folate and vitamin B12 intakes and breast cancer risk. Presently, there is little evidence to suggest a relationship between vitamin B12 status and breast cancer 1. In addition, results from observational studies are not consistently in support of an association between high dietary folate intakes and reduced risk for breast cancer. There is a need to evaluate the effect of folate and vitamin B12 supplementation in well-controlled, randomized, clinical trials, while considering various factors that modify breast cancer risk, such as menopausal status, ethnicity, and alcohol intake.
Neural tube defects
Neural tube defects may result in anencephaly or spina bifida, which are mostly fatal congenital malformations of the central nervous system. Neural tube defects arise from failure of embryonic neural tube to close, which occurs between the 21st and 28th days after conception, a time when many women are unaware of their pregnancy 125. Randomized controlled trials have demonstrated 60% to 100% reductions in neural tube defect cases when women consumed folic acid supplements in addition to a varied diet during the month before and the month after conception. Increasing evidence indicates that the homocysteine-lowering effect of folic acid plays a critical role in reducing the risk of neural tube defect 126. Homocysteine may accumulate in the blood when there is inadequate folate and/or vitamin B12 for effective functioning of the methionine synthase enzyme. Decreased vitamin B12 levels and elevated homocysteine concentrations have been found in the blood and amniotic fluid of pregnant women at high risk of neural tube defect 127. The recent meta-analysis of 12 case-control studies, including 567 mothers with current or prior neural tube defect-affected pregnancy and 1,566 unaffected mothers, showed that low maternal vitamin B12 status was associated with an increased risk of neural tube defect 128. Yet, whether vitamin B12 supplementation may be beneficial in the prevention of neural tube defect has not been evaluated 129.
Depression
Observational studies have found as many as 30% of patients hospitalized for depression are deficient in vitamin B12 130. A cross-sectional study of 700 community-living, physically disabled women over the age of 65 found that vitamin B12-deficient women were twice as likely to be severely depressed as non-deficient women 131. A population-based study in 3,884 elderly men and women with depressive disorders found that those with vitamin B12 deficiency were almost 70% more likely to experience depression than those with normal vitamin B12 status 132. The reasons for the relationship between vitamin B12 deficiency and depression are not clear but may involve a shortage in S-adenosylmethionine (SAM) 1. S-adenosylmethionine (SAM) is a methyl group donor for numerous methylation reactions in the brain, including those involved in the metabolism of neurotransmitters whose deficiency has been related to depression 133. Severe vitamin B12 deficiency in a mouse model showed dramatic alterations in the level of DNA methylation in the brain, which might lead to neurologic impairments 134. This hypothesis is supported by several studies that have shown supplementation with SAM improves depressive symptoms 135, 136, 137, 138.
Increased homocysteine level is another nonspecific biomarker of vitamin B12 deficiency that has been linked to depressive symptoms in the elderly 139. However, in a recent cross-sectional study conducted in 1,677 older individuals, higher vitamin B12 plasma levels, but not changes in homocysteine concentrations, were correlated with a lower prevalence of depressive symptoms 140. Few studies have examined the relationship of vitamin B12 status, homocysteine levels, and the development of depression over time. In a randomized, placebo-controlled, intervention study with over 900 older participants experiencing psychological distress, daily supplementation with folic acid (400 mcg) and vitamin B12 (100 mcg) for two years did not reduce the occurrence of symptoms of depression despite significantly improving blood folate, vitamin B12, and homocysteine levels compared to placebo 141. However, in a long-term randomized, double-blind, placebo-controlled study among sufferers of cerebrovascular accidents (stroke) at high risk of depression, daily supplementation with 2 mg of folic acid, 25 mg of vitamin B6, and 500 μg vitamin B12 significantly lowered the risk of major depressive episodes during a seven-year follow-up period compared to placebo 142. Although it cannot yet be determined whether vitamin B12 deficiency plays a causal role in depression, it may be beneficial to screen for vitamin B12 deficiency in older individuals as part of a medical evaluation for depression 1.
Osteoporosis
High homocysteine levels may affect bone remodeling by increasing bone resorption (breakdown), decreasing bone formation, and reducing bone blood flow. Another proposed mechanism involves the binding of homocysteine to the collagenous matrix of bone, which may modify collagen properties and reduce bone strength 143. Alterations of bone biomechanical properties can contribute to osteoporosis and increase the risk of fractures in the elderly. Since vitamin B12 is a determinant of homocysteine metabolism, it was suggested that the risk of osteoporotic fractures in older subjects might be enhanced by vitamin B12 deficiency. A meta-analysis of four observational studies, following a total of 7,475 older individuals for 3 to 16 years, found a weak association between an elevation in vitamin B12 of 50 picomoles/L in blood and a reduction in fracture risk 144. A randomized, placebo-controlled trial in 559 elderly individuals with low serum levels of folate and vitamin B12 and at increased risk of fracture evaluated the combined supplementation of very high doses of folic acid (5 mg/day) and vitamin B12 (1.5 mg/day) 145. The two-year study found that the supplementation improved B-vitamin status, decreased homocysteine concentrations, and reduced risk of total fractures compared to placebo 145. However, a multicenter study in 5,485 subjects with cardiovascular disease or diabetes mellitus showed that daily supplementation with folic acid (2.5 mg), vitamin B12 (1 mg), and vitamin B6 (50 mg) lowered homocysteine concentrations but had no effect on fracture risk compared to placebo 146. Another small, randomized, double-blind trial in 93 individuals with low vitamin D status found no additional benefit of B-vitamin supplementation (50 mg/day of vitamin B6, 0.5 mg/day of folic acid, and 0.5 mg/day of vitamin B12) on markers of bone health over a one-year period beyond that associated with vitamin D and calcium supplementation 147. Yet, the short length of the study did not permit a conclusion on whether the lowering of homocysteine through B-vitamin supplementation could have long-term benefits on bone strength and fracture risk 147. A large intervention study conducted in older people with no preexisting conditions is under way to evaluate the effect of B-vitamin supplementation on markers of bone health and incidence of fracture; this trial might clarify whether B vitamins could have a protective effect on bone health in the elderly population 148.
Energy and athletic performance
Due to its role in energy metabolism, vitamin B-12 is frequently promoted as an energy enhancer and an athletic performance and endurance booster. These claims are based on the fact that correcting the megaloblastic anemia caused by Vitamin B-12 deficiency should improve the associated symptoms of fatigue and weakness. However, Vitamin B-12 supplementation appears to have no beneficial effect on performance in the absence of a nutritional deficit 149, 150.
Vitamin B-12 has not been shown to cause any harm.
How much Vitamin B-12 do you need?
The amount of Vitamin B-12 you need each day depends on your age. Average daily recommended amounts for different ages are listed below in micrograms (mcg). Table 3 lists the current Recommended Dietary Allowance (RDA) for Vitamin B-12 in micrograms (mcg). For infants aged 0 to 12 months, the Food and Nutrition Board established an adequate intake (AI) for vitamin B-12 that is equivalent to the mean intake of Vitamin B-12 in healthy, breastfed infants.
- Recommended Dietary Allowance (RDA): average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals.
- Adequate Intake (AI): established when evidence is insufficient to develop an RDA and is set at a level assumed to ensure nutritional adequacy.
Table 1. Vitamin B-12 Recommended Intake
| Life Stage | Recommended Amount |
|---|---|
| Birth to 6 months | 0.4 mcg |
| Infants 7–12 months | 0.5 mcg |
| Children 1–3 years | 0.9 mcg |
| Children 4–8 years | 1.2 mcg |
| Children 9–13 years | 1.8 mcg |
| Teens 14–18 years | 2.4 mcg |
| Adults | 2.4 mcg |
| Pregnant teens and women | 2.6 mcg |
| Breastfeeding teens and women | 2.8 mcg |
What foods provide Vitamin B-12?
Vitamin B12 is found naturally in a wide variety of foods of animal origin (such as fish, meat, poultry, eggs, and dairy products) and manufacturers add it to some fortified foods (e.g., fortified breakfast cereals and fortified nutritional yeasts) 28. Plant foods have no vitamin B12 unless they are fortified 152. You can get recommended amounts of vitamin B12 by eating a variety of foods including the following:
- Fish, meat, poultry, eggs, milk, and other dairy products contain vitamin B12.
- Clams and beef liver are some of the best source of vitamin B12.
- Some breakfast cereals, nutritional yeasts, and other food products are fortified with vitamin B12.
The U.S. Department of Agriculture’s FoodData Central (https://fdc.nal.usda.gov) lists the nutrient content of many foods and provides a comprehensive list of foods containing vitamin B12 arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/VitaminB12-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/VitaminB12-Food.pdf).
The average vitamin B12 level in the breast milk of women with vitamin B12 intakes above the RDA is 0.44 mcg/L 153. The U.S. Food and Drug Administration (FDA) specifies that infant formulas sold in the United States must provide at least 0.15 mcg vitamin B12 per 100 kcal 154.
The estimated bioavailability of vitamin B12 from food varies by vitamin B12 dose because absorption decreases drastically when the capacity of intrinsic factor is exceeded (at 1–2 mcg of vitamin B12) 155. Bioavailability also varies by type of food source. For example, the bioavailability of vitamin B12 appears to be about three times higher in dairy products than in meat, fish, and poultry, and the bioavailability of vitamin B12 from dietary supplements is about 50% higher than that from food sources 156.
A variety of foods and their vitamin B12 levels per serving are listed in Table 2.
Table 2. Vitamin B12 Food Sources
| Food | Micrograms per serving | Percent DV* |
| Beef liver, cooked, pan-fried, 3 ounces | 70.7 | 2944 |
| Clams (without shells), cooked, 3 ounces | 17 | 708 |
| Tuna, bluefin, cooked, dry heat, 3 ounces | 9.3 | 385 |
| Nutritional yeast, fortified, from several brands (check label), about ¼ cup | 8.3 to 24 | 346 to 1,000 |
| Salmon, Atlantic, cooked, 3 ounces | 2.6 | 108 |
| Beef, ground, 85% lean meat/15% fat, pan-browned, 3 ounces | 2.4 | 100 |
| Milk, 2% milkfat, 1 cup | 1.3 | 54 |
| Yogurt, plain, fat free, 6-ounce container | 1 | 43 |
| Breakfast cereals, fortified with 25% of the DV for vitamin B12, 1 serving | 0.6 | 25 |
| Cheese, cheddar, 1½ ounces | 0.5 | 19 |
| Egg, whole, cooked, 1 large | 0.5 | 19 |
| Turkey, breast meat, roasted, 3 ounces | 0.3 | 14 |
| Tempeh, 1/2 cup | 0.1 | 3 |
| Banana, 1 medium | 0 | 0 |
| Bread, whole-wheat, 1 slice | 0 | 0 |
| Strawberries, raw, halved, 1/2 cup | 0 | 0 |
| Beans, kidney, boiled, 1/2 cup | 0 | 0 |
| Spinach, boiled, drained, 1/2 cup | 0 | 0 |
Footnote: *DV = Daily Value. DVs were developed by the U.S. Food and Drug Administration (FDA) to help consumers determine the level of various nutrients in a standard serving of food in relation to their approximate requirement for it. The DV for Vitamin B-12 is 6.0 mcg. However, the FDA does not require food labels to list Vitamin B-12 content unless a food has been fortified with this nutrient. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
[Source 157 ]Are you getting enough Vitamin B12?
Most people in the United States get enough Vitamin B-12 from the foods they eat. But some people have trouble absorbing Vitamin B-12 from food. As a result, Vitamin B-12 deficiency affects between 1.5% and 15% of the public. Your doctor can test your Vitamin B-12 level to see if you have a deficiency.
Certain groups may not get enough Vitamin B-12 or have trouble absorbing it:
- Many older adults, who do not have enough hydrochloric acid in their stomach to absorb the Vitamin B-12 naturally present in food. People over 50 should get most of their Vitamin B-12 from fortified foods or dietary supplements because, in most cases, their bodies can absorb Vitamin B-12 from these sources.
- People with pernicious anemia whose bodies do not make the intrinsic factor needed to absorb Vitamin B-12. Doctors usually treat pernicious anemia with Vitamin B-12 shots, although very high oral doses of Vitamin B-12 might also be effective.
- People who have had gastrointestinal surgery, such as weight loss surgery, or who have digestive disorders, such as celiac disease or Crohn’s disease. These conditions can decrease the amount of Vitamin B-12 that the body can absorb.
- Some people who eat little or no animal foods such as vegetarians and vegans. Only animal foods have Vitamin B-12 naturally. When pregnant women and women who breastfeed their babies are strict vegetarians or vegans, their babies might also not get enough Vitamin B-12.
What happens if you don’t get enough Vitamin B12?
Vitamin B-12 deficiency causes tiredness, weakness, constipation, loss of appetite, weight loss, and megaloblastic anemia. Nerve problems, such as numbness and tingling in the hands and feet, can also occur. Other symptoms of Vitamin B-12 deficiency include problems with balance, depression, confusion, dementia, poor memory, and soreness of the mouth or tongue. Vitamin B-12 deficiency can damage the nervous system even in people who don’t have anemia, so it is important to treat a deficiency as soon as possible.
In infants, signs of a Vitamin B-12 deficiency include failure to thrive, problems with movement, delays in reaching the typical developmental milestones, and megaloblastic anemia.
Large amounts of folic acid can hide a Vitamin B-12 deficiency by correcting megaloblastic anemia, a hallmark of Vitamin B-12 deficiency. But folic acid does not correct the progressive damage to the nervous system that Vitamin B-12 deficiency also causes. For this reason, healthy adults should not get more than 1,000 mcg of folic acid a day.
Vitamin B12 Deficiency
Vitamin B12 deficiency also known as cobalamin deficiency is characterized by megaloblastic anemia, fatigue, weakness, constipation, loss of appetite, and weight loss 2, 4, 158. Neurological changes, such as numbness and tingling in the hands and feet, can also occur 159, 160. Additional symptoms of Vitamin B-12 deficiency include difficulty maintaining balance, depression, confusion, dementia, poor memory, and soreness of the mouth or tongue 161. The neurological symptoms of Vitamin B-12 deficiency can occur without anemia, so early diagnosis and intervention is important to avoid irreversible damage 11. During infancy, signs of a vitamin B12 deficiency include failure to thrive, movement disorders, developmental delays, and megaloblastic anemia 162. Many of these symptoms are general and can result from a variety of medical conditions other than vitamin B-12 deficiency.
The most common cause of vitamin B12 deficiency is autoimmune pernicious anemia, a condition that carries an increased risk of gastric cancer. In pernicious anemia, absorption is impaired due to intrinsic factor deficiency arising from autoimmune destruction of parietal cells 14. Other common causes of vitamin B12 deficiency include gastrectomy, ileal resection, pancreatic insufficiency, and malabsorption syndromes including Crohn’s disease and celiac disease. Other less common causes of vitamin B12 deficiency include use of medications such as biguanides (metformin), antacids (proton pump inhibitors and H2 receptors antagonists), aminoglycoside, antibiotics and colchicines, and rarely, malabsorption due to gastrointestinal bacterial overgrowth, congenital defects (e.g. birth transcobalamin deficiency), and infestation 23. Pure nutritional deficiency is rare and usually occurs only in strict vegans 163. Because people who have difficulty absorbing vitamin B12 from food absorb free vitamin B12 normally, their vitamin B12 deficiency tends to be less severe than that of individuals with pernicious anemia, who cannot absorb either food-bound or free vitamin B12. It is recommended that vegetarians and vegans take vitamin B12 supplements to prevent vitamin B12 deficiency 164. Certain congenital conditions, such as hereditary intrinsic factor defects and congenital vitamin B12 malabsorption (Imerslund-Gräsbeck disease), can also cause severe vitamin B12 deficiency 28. In some cases, vitamin B12 deficiency can be a risk factor for cardiovascular disease 165.
Because the body stores about 1 to 5 mg vitamin B12 (or about 1,000 to 2,000 times as much as the amount typically consumed in a day), the symptoms of vitamin B12 deficiency can take several years to appear 166. The average vitamin B12 content of liver tissue is approximately 1.0 mcg/g of tissue in healthy adults 167, 168. Estimates of the average total-body vitamin B12 pool in adults range from 0.6 mg to 3.9 mg 169, 170, but most estimates are between 2 and 3 mg 171, 172, 173, 174. The highest estimate found for an individual’s total body vitamin B12 store was 11.1 mg 170. If the circulating vitamin B12 exceeds the vitamin B12 binding capacity of the blood, the excess is excreted in the urine 175. This typically occurs only after injection of vitamin B12. The highest losses of vitamin B12 ordinarily occur through the feces. Sources of fecal B12 include unabsorbed vitamin B12 from food or bile, desquamated cells, gastric and intestinal secretions, and vitamin B12 synthesized by bacteria in the colon 175. Other losses occur through the skin and metabolic reactions. Fecal 176 and urinary losses decrease when vitamin B12 stores decrease 177, 173, 178. Various studies have indicated losses of 0.1 to 0.2 percent of the vitamin B12 pool per day regardless of the size of the store, with the 0.2 percent value generally applicable to those with pernicious anemia 179, 180, 181, 173, 182, 174.
Vitamin B12 deficiency with the classic hematologic and neurologic signs and symptoms is uncommon 27. However, low or marginal vitamin B12 status (200–300 pg/mL [148–221 pmol/L]) without these symptoms is much more common, at up to 40% in Western populations, especially in those with low intakes of vitamin B12-rich foods 25. The prevalence of vitamin B12 deficiency varies by cutoff level and biomarker used. For example, among adults aged 19 and older who participated in the National Health and Nutrition Examination Survey (NHANES) between 1999 and 2004, the rate of low vitamin B12 levels in serum was 3% with a cutoff of less than 200 pg/mL (148 pmol/L) and 26% with a cutoff of less than 350 pg/mL (258 pmol/L) 183. Approximately 21% of adults older than 60 had abnormal levels of at least one vitamin B12 biomarker 183.
In the United States and the United Kingdom, the prevalence of vitamin B12 deficiency is approximately 6% in persons younger than 60 years, and nearly 20% in those older than 60 years 166. Latin American countries have a clinical or subclinical vitamin B12 deficiency rate of approximately 40% 166. The prevalence is 70% in Kenyan school children, 80% in East Indian preschool-aged children, and 70% in East Indian adults 166. Certain risk factors increase the prevalence of vitamin B12 deficiency 184. Dietary insufficiency, pernicious anemia (i.e., an autoimmune process that reduces available intrinsic factor and subsequent absorption of vitamin B12), and long-term use of metformin or acid-suppressing medications have been implicated in B12 deficiency 166, 185, 186, 187, 188, 189.
A multicenter randomized controlled trial of 390 patients with diabetes mellitus showed that those taking 850 mg of metformin three times per day had an increased risk of vitamin B12 deficiency and low vitamin B12 levels vs. placebo 188. This effect increased with duration of metformin therapy, and patients had an unclear prophylactic supplementation response 188. A case-control study that compared 25,956 patients who had vitamin B12 deficiency with 184,199 control patients found a significantly increased risk of vitamin B12 deficiency in patients who had taken proton pump inhibitors or histamine H2 blockers for at least two years 189. In light of these findings, long-term use of these medications should be periodically reassessed, particularly in patients with other risk factors for vitamin B12 deficiency 188, 189.
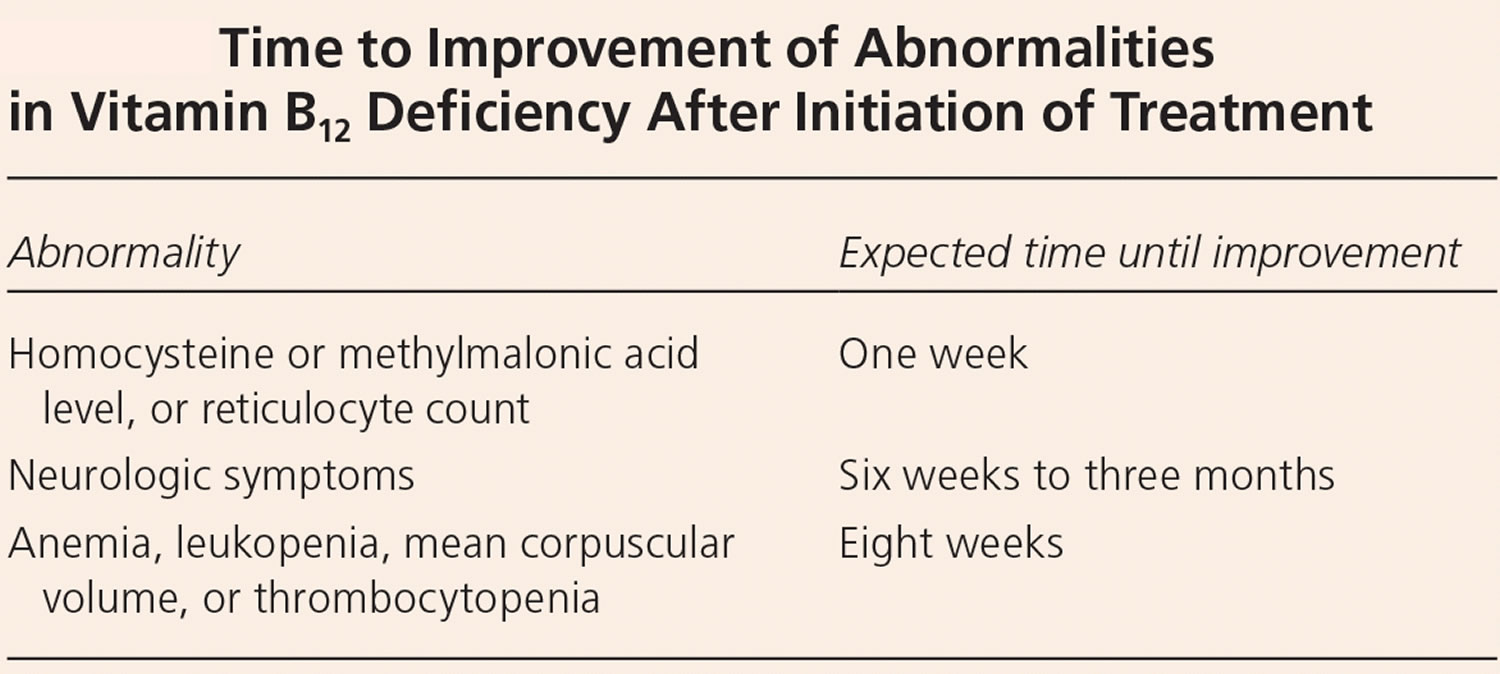
Typically, vitamin B12 deficiency is treated with intramuscular injections of cyanocobalamin or hydroxocobalamin, because this method bypasses any barriers to absorption. Hydroxocobalamin is usually the recommended option as it stays in the body for longer. Approximately 10% of the standard injectable dose of 1 mg is absorbed, which allows for rapid replacement in patients with severe deficiency or severe neurologic symptoms 28. Guidelines from the British Society for Haematology recommend injections three times per week for two weeks in patients without neurologic deficits 190. If neurologic deficits are present, injections should be given every other day for up to three weeks or until no further improvement is noted.
However, high doses of oral vitamin B12 might also be effective 12. A 2018 Cochrane review included three randomized controlled trials (RCTs) that compared very high doses (1,000–2,000 mcg) of oral with intramuscular vitamin B12 for vitamin B12 deficiency in a total of 153 participants 14. The evidence from these studies, although of low quality, showed that the ability of high oral doses of vitamin B12 supplements to normalize serum vitamin B12 was similar to that of intramuscular vitamin B12. The British Society for Haematology recommends intramuscular vitamin B12 for severe deficiency and malabsorption syndromes, whereas oral replacement may be considered for patients with asymptomatic, mild disease with no absorption or compliance concerns 190.
If vitamin B12 deficiency coexists with folate deficiency, vitamin B12 should be replaced first to prevent subacute combined degeneration of the spinal cord 166.
The British Society for Haematology does not recommend retesting vitamin B12 levels after treatment has been initiated, and no guidelines address the optimal interval for screening high-risk patients 190. In general, patients with an irreversible cause should be treated indefinitely, whereas those with a reversible cause should be treated until the deficiency is corrected and symptoms resolve 166.
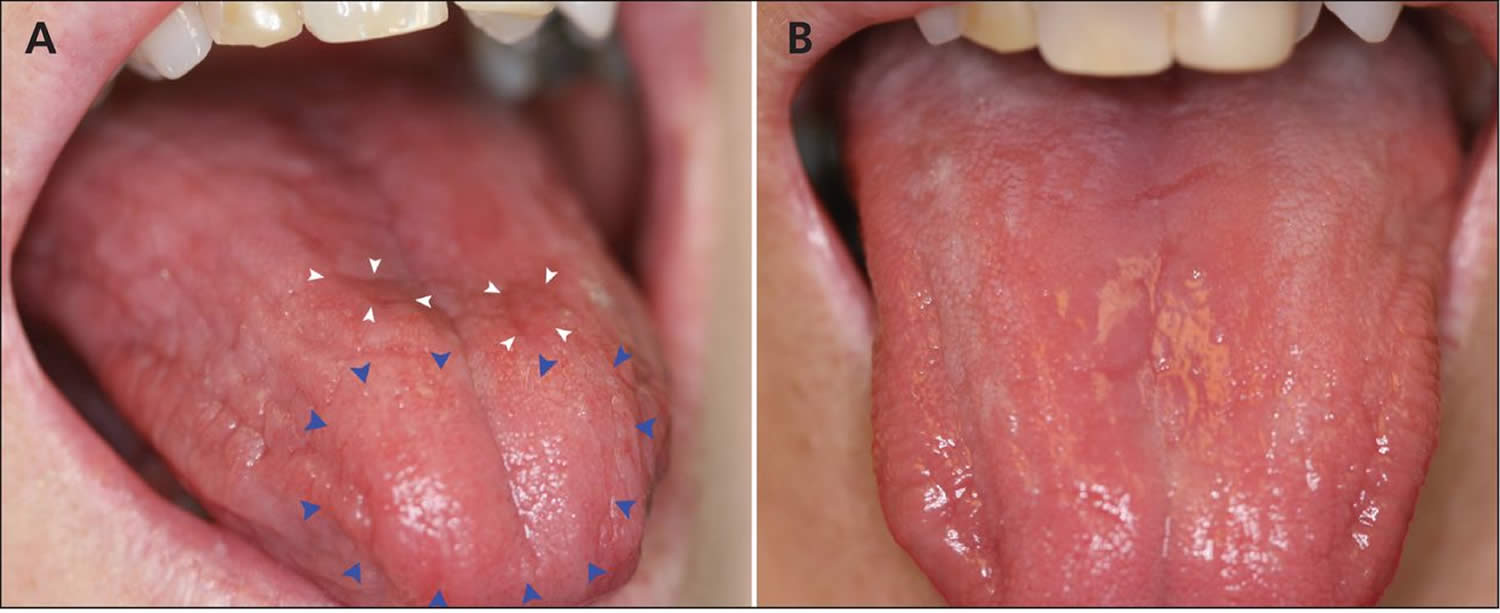
Figure 4. Glossitis secondary to vitamin B12 deficiency anemia
Footnotes: (A) Generalized dryness of the tongue of a 61-year-old woman with vitamin B12 deficiency, with atrophy (blue arrowheads) and erythematous plaques (white arrowheads). (B) Normal appearance of the tongue 3 days after the patient received a single injection of vitamin B12.
[Source 191 ]Folic Acid and Vitamin B-12 Deficiency
Large amounts of folic acid can mask the damaging effects of Vitamin B-12 deficiency by correcting the megaloblastic anemia caused by Vitamin B-12 deficiency 4, 159 without correcting the neurological damage that also occurs 2, 192. Moreover, preliminary evidence suggests that high serum folate levels might not only mask Vitamin B-12 deficiency, but could also exacerbate the anemia and worsen the cognitive symptoms associated with Vitamin B-12 deficiency 75, 193. Permanent nerve damage can occur if Vitamin B-12 deficiency is not treated. For these reasons, folic acid intake from fortified food and supplements should not exceed 1,000 mcg daily in healthy adults 159.
Vitamin B-12 Deficiency causes
Vitamin B-12 deficiency can result from:
- Inadequate intake
- Inadequate absorption
- Decreased utilization
- Use of certain drugs
Inadequate vitamin B12 intake is possible in general malnutrition, chronic alcohol abuse, and vegan or strict vegetarian diets but is otherwise unlikely. Breastfed babies of vegan mothers may develop vitamin B12 deficiency by age 4 to 6 months because in these babies, liver stores (which are normally extensive in other babies) are limited and their rapid growth rate results in high demand. Vitamin B12 malabsorption and deficiency due to inadequate dietary intake are common in the elderly. In the elderly, inadequate absorption most commonly results from decreased acid secretion in the stomach. In such cases, crystalline vitamin B-12 (such as that available in vitamin supplements) can be absorbed, but food-bound vitamin B12 is not liberated and absorbed normally.
Inadequate Vitamin B-12 absorption is the most common cause of vitamin B12 deficiency 194. Absorption of vitamin B12 from food requires normal function of the stomach, pancreas, and small intestine. Stomach acid and enzymes free vitamin B12 from food, allowing it to bind to R-protein (also known as transcobalamin-1 or haptocorrin), found in saliva and gastric fluids. In the alkaline environment of the small intestine, R-proteins are degraded by pancreatic enzymes, freeing vitamin B12 to bind to intrinsic factor (IF), a protein secreted by specialized cells in the stomach. Receptors on the surface of the ileum (final part of the small intestine) take up the IF-B12 complex only in the presence of calcium, which is supplied by the pancreas 194. Vitamin B12 can also be absorbed by passive diffusion, but this process is very inefficient—only about 1% absorption of the vitamin B12 dose is absorbed passively 9. The prevalent causes of vitamin B12 deficiency are (1) an autoimmune condition known as pernicious anemia, and (2) a disorder called food-bound vitamin B12 malabsorption. Both conditions have been associated with a chronic inflammatory disease of the stomach known as atrophic gastritis. Atrophic gastritis (an autoimmune disease characterized by the presence of antibodies directed against gastric parietal cells and intrinsic factor [IF]). Immune-mediated destruction of gastric parietal cells, gastric mucosal atrophy, hypochlorhydria, decreased IF production, subsequent vitamin B12 malabsorption, vitamin B12 deficiency and pernicious anemia (a type of megaloblastic anemia)
Inadequate absorption may occur in blind loop syndrome (with overgrowth of bacteria) or fish tapeworm infestation; in these cases, bacteria or parasites use ingested vitamin B12 so that less is available for absorption.
- Bacterial overgrowth syndromes, ileal resection or gastrointestinal diseases such as terminal ileitis, celiac disease, inflammatory bowel disease, Crohn’s disease and tropical sprue Altered absorption of the IF-vitamin B12 complex in the terminal ileum; intestinal villous atrophy and mucosal injury (celiac disease, Crohn’s disease and tropical sprue) 195
- Intestinal parasitic infestations (often accompanied by eosinophilia) caused by the protozoan Giardia lamblia or the fish tapeworm Diphyllobothrium latum 195. Vitamin B12 malabsorption through vitamin B12 trapping by the parasites
Vitamin B12 absorption may be inadequate if ileal absorptive sites are destroyed by inflammatory bowel disease or are surgically removed.
Disorders of the exocrine pancreas or pancreatectomy. Insufficient pancreatic enzyme activity leads to a reduction in the proteolytic degradation of haptocorrin (mediated by pancreatic proteases in the small intestine); as a consequence, vitamin B12 remains bound to haptocorrin, cannot form the IF-vitamin B12 complex and is not available for absorption by the enterocytes in the distal ileum 195.
Less common causes of inadequate vitamin B12 absorption include chronic pancreatitis, gastric bypass, partial or complete gastrectomy, gastric reduction, weight loss surgery and chronic gastritis due to Helicobacter pylori infection, malabsorption syndromes, AIDS, use of certain drugs (eg, antacids, metformin), repeated exposure to nitrous oxide (N2O), and a genetic disorder causing malabsorption in the ileum (Imerslund-Graesbeck syndrome).
- Long-term use (≥ 12 months) of drugs altering gastric acid secretion or gastric pH (e.g., proton-pump inhibitors, H2 receptor blockers and antacids) cause vitamin B-12 deficiency. These drugs reduce the production of hydrochloric acid by gastric parietal cells; as a consequence, vitamin B12 is not adequately released from the food matrix due to insufficient hydrochloric acid and low pepsin activity.
- The underlying mechanism accounting for metformin-induced vitamin B12 deficiency is not fully understood, although it may involve one or more of the following 195:
- Interference with the calcium-dependent binding of the IF-vitamin B12 complex to the cubilin receptor on enterocytes at the ileum level;
- Interaction with the cubilin endocytic receptor;
- Alteration in small intestine motility leading to small intestinal bacterial overgrowth and subsequent inhibition of IF-vitamin B12 complex absorption in the distal ileum;
- Alteration in bile acid metabolism and reabsorption;
- Increased liver accumulation of vitamin B12; and (6) Reduced IF secretion by gastric parietal cells.
- According to the British Society for Haematology guidelines for diagnosis and treatment of vitamin B12 deficiency, no recommendations can be currently given on prophylactic administration with oral vitamin B12 in patients using metformin 196. Despite the lack of definitive guidelines or recommendations on treatment of metformin-induced vitamin B12 deficiency, patients using metformin with concomitant vitamin B12 deficiency should receive cobalamin supplementation aimed to correct this deficiency and prevent the related risk of peripheral nerve damage and/or other clinical consequences 195. Importantly, prompt vitamin B12 administration should be considered, particularly in metformin-treated patients with vitamin B12 deficiency accompanied by neurologic and/or hematologic manifestations such as peripheral neuropathy and megaloblastic anemia.
- Nitrous oxide anesthesia or recreational use of nitrous oxide. Irreversible oxidation and inactivation of the coenzyme form of vitamin B12 (methylcobalamin) at the active site of the vitamin B12-dependent methionine synthase reaction, resulting in increased levels of MMA and homocysteine
Less commonly, decreased utilization of vitamin B12 or use of medications that affect vitamin B12 absorption or metabolism including the bile acid resin cholestyramine (used to treat hypercholesterolemia), colchicine (used for acute gout) and many antibiotics such as neomycin and the anti-tuberculosis drug para-aminosalicylic acid. Unlike long-term use of proton-pump inhibitors, histamine H2-receptor antagonists or metformin, the frequency or duration of use of these drugs is usually insufficient to result in clinical vitamin B12 deficiency 195. Cholestyramine can chelate IF; colchicine and antibiotics can inhibit endocytosis of the IF-vitamin B12 complex.
Lastly, inherited disorders affecting the sequential steps in the assimilation, transport and intracellular processing and metabolism of vitamin B12 (Imerslund-Gräsbeck syndrome). Reduced expression, binding activity or affinity of receptors and proteins involved in transport, intracellular processing and metabolism of vitamin B12.
Known risk factors for vitamin B12 deficiency include 197:
- Age older than 75 years
- Strict vegetarianism or a plant-based diet
- Alcohol abuse
- Atrophic gastritis
- Crohn’s disease
- Ileal resection
- Pernicious anemia
- Postgastrectomy syndrome
- Tapeworm infection
- Transcobalamin 2 deficiency
Atrophic gastritis
Atrophic gastritis is a histopathologic entity characterized by chronic inflammation of the gastric mucosa with loss of the gastric glandular cells and replacement by intestinal-type epithelium, pyloric-type glands, and fibrous tissue as a response to chronic injury 198. Atrophy of the gastric mucosa is the endpoint of chronic processes, such as chronic gastritis associated with Helicobacter pylori infection, other unidentified environmental factors, and autoimmunity directed against gastric glandular cells (autoimmune gastritis) 198. Atrophic gastritis represents the end stage of chronic gastritis, both infectious and autoimmune. In both cases, the clinical manifestations of atrophic gastritis are those of chronic gastritis, but pernicious anemia is observed specifically in patients with autoimmune gastritis and not in those with Helicobacter pylori–associated atrophic gastritis.
Atrophic gastritis is thought to affect 10%-30% of people over 60 years of age 199. Atrophic gastritis is frequently associated with the presence of autoantibodies directed toward stomach cells (see pernicious anemia) and/or infection by the bacteria, Helicobacter pylori (H. pylori) 200. Helicobacter pylori (H. pylori) infection induces chronic inflammation of the stomach, which may progress to peptic ulcer disease, atrophic gastritis, and/or gastric cancer in some individuals 201. Diminished gastric function in individuals with atrophic gastritis can result in bacterial overgrowth in the small intestine and cause food-bound vitamin B12 malabsorption. Vitamin B12 levels in serum, plasma, and gastric fluids are significantly decreased in individuals with H. pylori infection, and eradication of the bacteria has been shown to significantly improve vitamin B12 serum concentrations 202.
Pernicious anemia
Pernicious anemia is an irreversible auto-immune disease that affects the gastric mucosa and results in gastric atrophy 203, 204. This disease leads to attacks on parietal cells in the stomach, resulting in failure to produce intrinsic factor (IF) and malabsorption of dietary vitamin B12, recycled biliary vitamin B12, and free vitamin B12 205, 22. Therefore, without treatment, pernicious anemia causes vitamin B12 deficiency, even in the presence of adequate vitamin B12 intakes. Generally, it takes about 10–12 years to clinically develop symptomatic pernicious anemia, so pernicious anemia may onset with subclinical vitamin B12 deficiency 206.
Pernicious anemia has often been confused with vitamin B12 deficiency 207. Pernicious anemia denotes only vitamin B12 deficiency due to gastric atrophy and/or intrinsic factor deficiency or autoimmune gastritis 207. Pernicious anemia is considered a late stage of autoimmune gastritis.
The pathogenesis of pernicious anemia has not been clarified, but it is likely linked to the autoimmune destruction of gastric glands due to autoreactive T lymphocytes in genetically predisposed individuals 207. The role of previous Helicobacter pylori infection as a supposed but not yet definitely proven trigger of gastric autoimmunity cannot be excluded 207.
In pernicious anemia, the underlying pathogenetic mechanism is autoimmune gastritis, an organ-specific immune-mediated disorder featuring the damage of the gastric parietal cells involved in the secretion of intrinsic factor (IF) and hydrochloric acid by the gastric proton pump 208. The presence of anti-parietal cell antibodies (PCAs) directed towards the gastric proton pump (gastric H+/K+ ATPase) as well as antibodies against intrinsic factor (IF) (although in a lower percentage) are commonly associated with gastric corpus atrophy and intrinsic factor deficiency 207. Atrophic corpus gastritis is a chronic disease defined as a decrease in or loss of the original gastric glands, replaced by pseudo-pyloric or intestinal metaplasia or fibrosis 209. Gastric corpus atrophy is a necessary but insufficient condition for the onset of pernicious anemia, as gastric corpus atrophy may also take its course without pernicious anemia.
In rare cases, pernicious anemia is passed down through families. This is called congenital pernicious anemia. Babies with this type of anemia do not make enough intrinsic factor. Or they cannot properly absorb vitamin B12 in the small intestine.
Pernicious anemia is the most common cause of clinically evident vitamin B12 deficiency around the world 205, 203. The incidence of pernicious anemia in the United States is an estimated 151 per 100,000, and this condition is more common in women and in people of European ancestry 203.
An important point is that pernicious anemia may lead to potentially serious long-term complications that may be related to micronutrient deficiencies and the development of gastric neoplasms, in particular, gastric cancer and type 1 gastric neuroendocrine tumors 207. When not recognized in a timely manner or when pernicious anemia is diagnosed with delay, these complications may be potentially life-threatening and sometimes irreversible.
Vitamin B12 therapy resolves the anemia of pernicious anemia, but does not cure the atrophic gastritis, which can progress to gastric cancer 210. The incidence of gastric adenocarcinoma is 2- to 3-fold greater in patients with pernicious anemia than in the general population of the same age 211. Presently, periodic gastroscopy and/or barium studies are not advocated in patients with treated pernicious anemia who are asymptomatic, because such screening has not been demonstrated to prolong lifespan 211.
A population-based, case-control study using the Surveillance, Epidemiology, and End Results (SEER)–Medicare database found that elderly persons with pernicious anemia were not only at significantly increased risk for noncardia gastric adenocarcinoma and gastric carcinoid tumors, they were also at increased risk for the following 210:
- Tonsillar cancer
- Hypopharyngeal cancer
- Esophageal squamous cell carcinoma
- Small intestinal cancer
- Liver cancer
- Myeloma
- Acute myeloid leukemia
- Myelodysplastic syndrome
In a longitudinal study of 199 intrinsic factor antibody (IFA)–positive and 168 IFA-negative Chinese patients, Chan et al 212 found that despite a good hematologic response to therapy, both groups had an unsatisfactory neurologic response, and newly diagnosed hypothyroidism was found during follow-up. In addition, newly diagnosed cancers were also found (24 in IFA-positive patients, seven in IFA-negative patients), of which 20% were stomach cancer 212.
For the intrinsic factor antibody (IFA)-positive patients with a cancer, mean survival was 64 months; for those without a cancer, it was 129 months. Mortality was 31% in this group, in which cancer-related deaths represented 37% of the total 212. For the intrinsic factor antibody (IFA)-negative patients with a cancer, mean survival was 36 months. For those without a cancer, it was 126 months. Mortality was 21% in this group, in which cancer-related deaths represented 14% of the total.
Chan et al 212 concluded that although Chinese patients treated for pernicious anemia demonstrated a good survival period, they remained at increased risk for gastric carcinoma, and IFA-positive patients had a higher risk of developing all types of cancers and cancer-related deaths than did IFA-negative patients.
Pernicious anemia has been estimated to be present in approximately 2% of individuals over 60 years of age 213. Although anemia is often a symptom, the condition is actually the end stage of an autoimmune inflammation of the stomach known as autoimmune atrophic gastritis, resulting in destruction of stomach cells by one’s own antibodies (autoantibodies). Progressive destruction of the cells that line the stomach causes decreased secretion of acid and enzymes required to release food-bound vitamin B12. Antibodies to intrinsic factor (IF) bind to IF preventing formation of the IF-B12 complex, further inhibiting vitamin B12 absorption. About 20% of the relatives of pernicious anemia patients also have the condition, suggesting a genetic predisposition. It is also thought that H. pylori infection could be involved in initiating the autoimmune response in a subset of individuals 214. Further, co-occurrence of autoimmune atrophic gastritis with other autoimmune conditions, especially autoimmune thyroiditis and type 1 diabetes mellitus, has been reported 215.
When pernicious anemia is suspected, the first step is usually a full blood panel to test for anemia and/or macrocytosis, together with testing for cobalamin deficiency and increased levels of homocysteine and/or methylmalonic acid 207. Next, the positivity of gastric autoantibodies towards parietal cells and/or intrinsic factor is commonly assessed 207. In any case, the hematological and/or serological suspicion of pernicious anemia always needs to be confirmed by histological assessment of gastric antral and corpus biopsies obtained during gastroscopy to ascertain the presence of autoimmune gastritis 207.
Treatment of pernicious anemia generally requires injections of vitamin B12 to bypass intestinal absorption. High-dose oral supplementation is another treatment option, because consuming 1,000 μg (1 mg)/day of vitamin B12 orally should result in the absorption of about 10 μg/day (1% of dose) by passive diffusion. In fact, high-dose oral therapy is considered to be as effective as intramuscular injection 16.
Food-bound vitamin B12 malabsorption
Food-bound vitamin B12 malabsorption is defined as an impaired ability to absorb food- or protein-bound vitamin B12; individuals with this condition can fully absorb the free form 216. While the condition is the major cause of poor vitamin B12 status in the elderly population, it is usually associated with atrophic gastritis, a chronic inflammation of the lining of the stomach that ultimately results in the loss of glands in the stomach (atrophy) and decreased stomach acid production (see Atrophic gastritis). Because stomach acid is required for the release of vitamin B12 from the proteins in food, vitamin B12 absorption is diminished. Decreased stomach acid production also provides an environment conducive to the overgrowth of anaerobic bacteria in the stomach, which further interferes with vitamin B12 absorption 32. Because vitamin B12 in supplements is not bound to protein, and because intrinsic factor (IF) is still available, the absorption of supplemental vitamin B12 is not reduced as it is in pernicious anemia. Thus, individuals with food-bound vitamin B12 malabsorption do not have an increased requirement for vitamin B12; they simply need it in the crystalline form found in fortified foods and dietary supplements.
Inherited disorders of vitamin B12 absorption
Rare cases of inborn errors of vitamin B12 metabolism have been reported in the literature 194. Imerslund-Gräsbeck syndrome is an inherited vitamin B12 malabsorption syndrome that causes megaloblastic anemia and neurologic disorders of variable severity in affected subjects. Similar clinical symptoms are found in individuals with hereditary intrinsic factor deficiency (also called congenital pernicious anemia) in whom the lack of intrinsic factor (IF) results in the defective absorption of vitamin B12. Additionally, mutations affecting vitamin B12 transport in the body have been identified 217.
Other causes of vitamin B12 deficiency
Other causes of vitamin B12 deficiency include surgical resection of the stomach or portions of the small intestine where receptors for the IF-B12 complex are located. Conditions affecting the small intestine, such as malabsorption syndromes (celiac disease and tropical sprue), may also result in vitamin B12 deficiency. Because the pancreas provides critical enzymes, as well as calcium required for vitamin B12 absorption, pancreatic insufficiency may contribute to vitamin B12 deficiency. Since vitamin B12 is found only in foods of animal origin, a strict vegetarian (vegan) diet has resulted in cases of vitamin B12 deficiency. Moreover, alcoholics may experience reduced intestinal absorption of vitamin B12 9, and individuals with acquired immunodeficiency syndrome (AIDS) appear to be at increased risk of deficiency, possibly related to a failure of the IF-B12 receptor to take up the IF-B12 complex 32. Further, long-term use of acid-reducing drugs has also been implicated in vitamin B12 deficiency.
Drug interactions
A number of drugs reduce the absorption of vitamin B12. Proton-pump inhibitors (e.g., omeprazole and lansoprazole), used for therapy of Zollinger-Ellison syndrome and gastroesophageal reflux disease (GERD), markedly decrease stomach acid secretion required for the release of vitamin B12 from food but not from supplements 1. Long-term use of proton-pump inhibitors has been found to decrease blood vitamin B12 levels. However, vitamin B12 deficiency does not generally develop until after at least three years of continuous therapy 218, 219. Another class of gastric acid inhibitors known as histamine2 (H2)-receptor antagonists (e.g., cimetidine, famotidine, and ranitidine), often used to treat peptic ulcer disease, has also been found to decrease the absorption of vitamin B12 from food 1. It is not clear whether the long-term use of H2-receptor antagonists could cause overt vitamin B12 deficiency 220, 221. Individuals taking drugs that inhibit gastric acid secretion should consider taking vitamin B12 in the form of a supplement because gastric acid is not required for its absorption.
Other drugs found to inhibit vitamin B12 absorption from food include cholestyramine (a bile acid-binding resin used in the treatment of high cholesterol), chloramphenicol and neomycin (antibiotics), and colchicine (medicine for gout treatment) 1. Metformin, a medication for individuals with type 2 diabetes, was found to decrease vitamin B12 absorption by tying up free calcium required for absorption of the IF-B12 complex 195, 222. However, the clinical significance of this is unclear 223. It is not known whether calcium supplementation can reverse vitamin B12 malabsorption; therefore, calcium supplementation is not currently prescribed for the prevention or treatment of metformin-induced vitamin B12 deficiency 224. Previous reports that megadoses of vitamin C destroy vitamin B12 have not been supported 225 and may have been an artifact of the assay used to measure vitamin B12 levels 1.
Nitrous oxide, a commonly used anesthetic, oxidizes and inactivates vitamin B12, thus inhibiting both of the vitamin B12-dependent enzymes, and can produce many of the clinical features of vitamin B12 deficiency, such as megaloblastic anemia or neuropathy 1. Since nitrous oxide is commonly used for surgery in the elderly and in childbirth, some experts feel vitamin B12 deficiency should be ruled out prior to its use 199, 226.
Large doses of folic acid given to an individual with an undiagnosed vitamin B12 deficiency could correct megaloblastic anemia without correcting the underlying vitamin B12 deficiency, leaving the individual at risk of developing irreversible neurologic damage 1. For this reason, the Food and Nutrition Board of the US Institute of Medicine advises that all adults limit their intake of folic acid (supplements and fortification) to 1,000 mcg (1 mg) daily 227.
Groups at Risk of Vitamin B-12 Deficiency
The main causes of Vitamin B-12 deficiency include Vitamin B-12 malabsorption from food, pernicious anemia, postsurgical malabsorption, and dietary deficiency 228. However, in many cases, the cause of Vitamin B-12 deficiency is unknown.
The following groups are among those most likely to be Vitamin B-12 deficient.
Older adults
Depending on the definition used, between 3% and 43% of community-dwelling older adults, especially those with atrophic gastritis (chronic inflammation and thinning of your stomach), have vitamin B12 deficiency based on serum vitamin B12 levels 229, 230. The vitamin B12 deficiency rate at a cutoff of less than 211 mcg/L (156 pmol/L) at admission to a long-term care facility, according to one study, was 14%, and 38% of these older adults had levels lower than 407 pg/mL (300 pmol/L) 230.
Conditions associated with vitamin B12 deficiency include pernicious anemia, present in about 15% to 25% of older adults with vitamin B12 deficiency 231. Atrophic gastritis, an autoimmune condition affecting 2% of the general population but 8–9% of adults aged 65 and older, decreases production of intrinsic factor and secretion of hydrochloric acid in the stomach and thus decreases absorption of vitamin B12 231, 232. A third condition associated with vitamin B12 deficiency in older adults is Helicobacter pylori infection, possibly because this bacterium causes inflammation that leads to malabsorption of vitamin B12 from food 233.
Individuals with pernicious anemia
Pernicious anemia is an irreversible autoimmune disease that affects the gastric mucosa and results in gastric atrophy 203. This disease leads to attacks on parietal cells in the stomach, resulting in failure to produce intrinsic factor (IF) and malabsorption of dietary vitamin B12, recycled biliary vitamin B12, and free vitamin B12 205, 22. Therefore, without treatment, pernicious anemia causes vitamin B12 deficiency, even in the presence of adequate vitamin B12 intakes.
Pernicious anemia is the most common cause of clinically evident vitamin B12 deficiency around the world 205, 203. The incidence of pernicious anemia in the United States is an estimated 151 per 100,000, and this condition is more common in women and in people of European ancestry 203.
Individuals with gastrointestinal disorders
Individuals with stomach and small intestine disorders, such as celiac disease and Crohn’s disease, may be unable to absorb enough vitamin B12 from food to maintain healthy body stores 234. But although rates of vitamin B12 deficiency are higher in people with celiac disease than other people 235, the evidence for whether rates of vitamin B12 deficiency are higher in people with Crohn’s disease is mixed 236, 237. Vitamin B12 deficiency in people with Crohn’s disease is typically treated with intramuscular cobalamin injections, but high doses of oral cyanocobalamin therapy (e.g., 1,000 mcg/day) might be equally effective 238.
Individuals who have had gastrointestinal surgery
Surgical procedures in the gastrointestinal tract, such as for weight loss (bariatric surgery) or to remove all or part of the stomach (gastrectomy), can cause a complete or partial loss of cells that secrete hydrochloric acid and cells that secrete intrinsic factor (IF) 239, 240. Thus, these procedures reduce the amount of vitamin B12, particularly food-bound vitamin B12, that the body absorbs 239, 240. High doses (1,000 mcg/day) of oral methylcobalamin supplements appear to be as effective as hydroxycobalamin injections in normalizing vitamin B12 values in patients who have undergone Roux-en-Y gastric bypass surgery 241.
Vegetarians
Vegans who consume no animal products and vegetarians who consume some animal products (e.g., dairy products, eggs, or both) but not meat have a higher risk of developing vitamin B12 deficiency because natural food sources of vitamin B12 are limited to animal foods 242. Consumption of foods fortified with vitamin B12 (such as fortified nutritional yeasts) as well as vitamin B12 supplements can substantially reduce the risk of deficiency 242.
Infants of vegan women
Exclusively breastfed infants of women who consume no animal products might have very limited reserves of vitamin B12 and can develop vitamin B12 deficiency, sometimes very early in life 243. The infant’s vitamin B12 deficiency can be severe, especially if the mother’s vitamin B12 deficiency is severe or caused by pernicious anemia; sometimes, the mother’s own vitamin B12 deficiency is clinically mild and not recognized. Undetected and untreated vitamin B12 deficiency in infants can result in neurological damage, failure to thrive, developmental delays, and anemia 243, 244. The reasons include the small amounts of vitamin B12 in the breast milk of vegan mothers as well as the limited amounts of vitamin B12 crossing the placenta in these women during fetal development.
Vitamin B12 prevention
Because of potential interactions from prolonged medication use, healthcare providers should consider screening patients for vitamin B12 deficiency if they have been taking proton pump inhibitors or H2 blockers for more than 12 months, or metformin for more than four months 23. The average intake of vitamin B12 in the United States is 3.4 mcg per day, and the recommended dietary allowance is 2.4 mcg per day for adult men and nonpregnant women, and 2.6 mcg per day for pregnant women 8. Patients older than 50 years may not be able to adequately absorb dietary vitamin B12 and should consume food fortified with vitamin B12 8. Vegans and strict vegetarians should consume fortified cereals or supplements to prevent vitamin B12 deficiency. The American Society for Metabolic and Bariatric Surgery recommends that patients who have had weight loss surgery take 1 mg of oral vitamin B12 per day indefinitely 245.
Vitamin B12 deficiency signs and symptoms
Vitamin B12 deficiency affects multiple systems, and signs and symptoms vary in severity from mild fatigue to severe neurologic impairment 23. The substantial liver storage of vitamin B12 can delay signs and symptoms for up to 10 years after the onset of Vitamin B12 deficiency 246.
The signs and symptoms of vitamin B12 deficiency include 247:
- anaemia resulting from impaired red blood cell production
- loss of peripheral nerve function that can result in impaired sensation, movement or organ function
- visual disturbance
- memory loss
- psychiatric abnormalities
- temporary infertility in women
- vitamin B12 deficiency during pregnancy can result in fetal abnormalities, such as neural tube defects 126, 127, 128.
Vitamin B12 deficiency is generally characterized by a specific type of anemia called megaloblastic anemia. Anemia usually develops insidiously. It can cause fatigue (easily fatigued with exertion), palpitations, pale skin, weakness, constipation, loss of appetite, and weight loss 166, 185, 186, 166, 28. Megaloblastic anemia is often more severe than its symptoms indicate because its slow evolution allowing physiologic adaptation.
Skin hyperpigmentation, glossitis (swollen inflamed tongue) and infertility have also been reported 166, 185, 186. Neurologic signs and symptoms are caused by progressive demyelination and can include peripheral neuropathy, absence of normal neurologic reflexes (areflexia), and the loss of proprioception and vibratory sense. Areflexia (absence of normal neurologic reflexes) can be permanent if neuronal death occurs in the posterior and lateral spinal cord tracts 166, 185, 186, 248. Dementia-like disease, including episodes of psychosis, is possible with more severe and chronic vitamin B12 deficiency 166, 248. Clinical evaluation seems to show an inverse relationship between the severity of megaloblastic anemia and the degree of neurologic impairment 185.
Maternal vitamin B12 deficiency during pregnancy or while breastfeeding may lead to neural tube defects, developmental delay, failure to thrive, hypotonia, ataxia, and anemia 184, 249, 250, 251, 252. Women at high risk or with known deficiency should supplement with vitamin B12 during pregnancy or while breastfeeding 184, 249, 250, 251, 252.
Symptoms of vitamin B12 deficiency can take decades to develop, and can usually only be diagnosed by a medical professional 166, 23.
Vitamin B12 deficiency symptoms can include:
- Diarrhea or constipation
- Nausea
- Vomiting
- Fatigue, lack of energy, or lightheadedness when standing up or with exertion
- Loss of appetite
- Pale skin (mild jaundice)
- Shortness of breath, mostly during exercise
- Heartburn
- Swollen, red tongue or bleeding gums
General symptoms of anemia may include:
- extreme tiredness (fatigue)
- lack of energy (lethargy)
- breathlessness
- feeling faint
- headaches
- pale skin
- noticeable heartbeats (palpitations)
- hearing sounds coming from inside the body, rather than from an outside source (tinnitus)
- loss of appetite and weight loss
If you have anemia caused by a vitamin B12 deficiency, you may have other symptoms, such as 184, 253:
- a pale yellow tinge to your skin. In advanced anemia, severe pale skin with jaundice (due to hemolysis) produces a “peculiar lemon-yellow” skin color 254.
- vitiligo
- skin hyperpigmentation
- a sore and red tongue (glossitis)
- mouth ulcers
- pins and needles (paresthesia)
- changes in the way that you walk and move around (gait abnormalities)
- disturbed vision
- irritability
- depression
- changes in the way you think, feel and behave
- a decline in your mental abilities, such as memory, understanding and judgement (dementia)
- acute psychosis
- areflexia
- loss of proprioception and vibratory sense
- impaired sense of smell
If you have a low vitamin B12 level for a long time, you can have nervous system damage. Symptoms can include 255:
- Confusion
- Short-term memory loss
- Depression
- Loss of balance
- Numbness and tingling in the hands and feet
- Problems concentrating
- Irritability
- Hallucinations
- Delusions
- Optic nerve atrophy
Some of these symptoms can also happen in people who have a vitamin B12 deficiency but have not developed anemia.
Anemia usually develops insidiously. It is often more severe than its symptoms indicate because its slow evolution allows physiologic adaptation.
Occasionally, splenomegaly and hepatomegaly occur. Various gastrointestinal symptoms, including weight loss and poorly localized abdominal pain, may occur. Glossitis, usually described as burning of the tongue, is uncommon.
Neurologic symptoms develop independently from and often without hematologic abnormalities.
Subacute combined degeneration refers to degenerative changes in the nervous system due to Vitamin B12 deficiency; they affect mostly brain and spinal cord white matter, including the dorsal columns, the lateral corticospinal tracts, and the spinocerebellar tracts 256, 257. Demyelinating or axonal peripheral neuropathies can occur 258, 256.
In early stages, decreased position (proprioception) and vibratory sensation in the extremities is accompanied by mild to moderate weakness and hyporeflexia. In later stages, spasticity, extensor plantar responses, greater loss of position and vibratory sensation in the lower extremities, and ataxia emerge 259, 231. These deficits may develop in a stocking-glove distribution. Tactile, pain, and temperature sensations are usually spared but may be difficult to assess in the elderly. Areflexia can be permanent if neuronal death occurs in the posterior and lateral spinal cord tracts 248, 186.
Some patients are also irritable and mildly depressed. Dementia-like disease, including episodes of psychosis, paranoia (megaloblastic madness), poor memory, delirium, depression, confusion, and, at times, postural hypotension may occur in advanced cases 248, 166. The confusion may be difficult to differentiate from age-related dementias, such as Alzheimer disease.
Neurologic symptoms may develop independently from and often without hematologic abnormalities. Clinical evaluation seems to show an inverse relationship between the severity of megaloblastic anemia and the degree of neurologic impairment 28.
Table 3. Clinical and laboratory findings in vitamin B12 deficiency
| General symptoms | Weight loss observed in most patients |
| Low-grade fever occurs in one third of newly diagnosed patients and promptly disappears with treatment | |
| Gastrointestinal symptoms | Smooth tongue (50% of patients) with loss of papillae. Changes in taste and loss of appetite |
| Patients may report either constipation or having several semi-solid bowel movements daily | |
| Anorexia, nausea, vomiting, heartburn, pyrosis, flatulence and a sense of fullness | |
| Brain | Altered mental status. Cognitive defects (“megaloblastic madness”): depression, mania, irritability, paranoia, delusions, lability |
| Sensory organs | Optic atrophy, anosmia, loss of taste, glossitis |
| Bone marrow | Hypercellular bone marrow |
| Increased erythroid precursors | |
| Open, immature nuclear chromatin | |
| Dyssynchrony between maturation of cytoplasm and nuclei | |
| Giant bands, metamyelocytes | |
| Karyorrhexis, dysplasia | |
| Abnormal results on flow cytometry and cytogenetic analysis | |
| Spinal cord | Myelopathy |
| Spongy degeneration | |
| Paresthesias | |
| Loss of proprioception: vibration, position, ataxic gait, limb weakness/spasticity (hyperreflexia) | |
| Positive Romberg sign | |
| Lhermitte’s sign | |
| Segmental cutaneous sensory level | |
| Autonomic nervous system | Postural hypotension |
| Incontinence | |
| Impotence | |
| Peripheral nervous system | Cutaneous sensory loss |
| Hyporeflexia symmetric weakness | |
| Paresthesias | |
| Genitourinary symptoms | Urinary retention and impaired micturition may occur because of spinal cord damage. This can predispose patients to urinary tract infections |
| Reproductive system | Infertility |
| Abnormalities in infants and children | Developmental delay or regression, permanent disability |
| The patient does not smile | |
| Feeding difficulties | |
| Hypotonia, lethargy, coma | |
| Hyperirritability, convulsions, tremors, myoclonus | |
| Microcephaly | |
| Choreoathetoid movements, peripheral blood | |
| Macrocytic red cells, macro-ovalocytes | |
| Anisocytosis, fragmented forms | |
| Hypersegmented neutrophils | |
| Leukopenia, possible immature white cells | |
| Thrombocytopenia | |
| Pancytopenia | |
| Elevated lactate dehydrogenase level (extremes possible) | |
| Elevated indirect bilirubin and aspartate aminotransferase levels | |
| Decreased haptoglobin level | |
| Elevated levels of methylmalonic acid, homocysteine, or both |
Megaloblastic anemia
Diminished activity of methionine synthase in vitamin B12 deficiency inhibits the regeneration of tetrahydrofolate (THF) and traps folate in a form that is not usable by the body, resulting in symptoms of folate deficiency even in the presence of adequate folate levels. Thus, in both folate and vitamin B12 deficiencies, folate is unavailable to participate in DNA synthesis. This impairment of DNA synthesis affects the rapidly dividing cells of the bone marrow earlier than other cells, resulting in the production of large, immature, hemoglobin-poor red blood cells. The resulting anemia is known as megaloblastic anemia and is the symptom for which the disease, pernicious anemia, was named 32. Supplementation with folic acid will provide enough usable folate to restore normal red blood cell formation. However, if vitamin B12 deficiency is the cause, it will persist despite the resolution of the anemia. Thus, megaloblastic anemia should not be treated with folic acid until the underlying cause has been determined 260.
Neurologic symptoms
The neurologic symptoms of vitamin B12 deficiency include numbness and tingling of the hands and, more commonly, the feet; difficulty walking; memory loss; disorientation; and dementia with or without mood changes. Although the progression of neurologic complications is generally gradual, such symptoms may not be reversed with treatment of vitamin B12 deficiency, especially if they have been present for a long time. The most common initial sign and symptom of vitamin B12 deficiency is burning or prickling sensation that is usually felt in the hands, arms, legs, or feet (paresthesia), present in 70% of patients with neurological symptoms 261. Paresthesias are described as tingling or numbness, and, in contrast with other neuropathies, typically start in hands or both distal extremities 261, 262. Other neurological manifestations may include subacute combined degeneration characterized by lesion of the posterior and lateral columns of the spinal cord leading to asthenia, spasticity, impaired vibratory and proprioceptive sensation with ataxia and extensor plantar responses 262; autonomic dysfunction (erectile and bladder dysfunction) 263; optic neuropathy with progression to visual loss (characterized by central and centrocecal scotomas) 264, and memory and mood involvement, up to dementia 207.
Neurologic complications are not always associated with megaloblastic anemia and are the only clinical symptom of vitamin B12 deficiency in about 25% of cases 31. Although vitamin B12 deficiency is known to damage the myelin sheath covering cranial, spinal, and peripheral nerves, the biochemical processes leading to neurological damage in vitamin B12 deficiency are not yet fully understood 265.
Gastrointestinal symptoms
Tongue soreness, appetite loss, and constipation have also been associated with vitamin B12 deficiency. The origins of these symptoms are unclear, but they may be related to the stomach inflammation underlying some cases of vitamin B12 deficiency and to the progressive destruction of the lining of the stomach 31.
Hyperhomocysteinemia
High levels of homocysteine in the blood (hyperhomocysteinemia) has been linked to heart disease and stroke 266. Hyperhomocysteinemia can be caused by a deficiency of either vitamin B12 or folate, and in human subjects mild (13–24 µM) and moderate (25–60 µM) hyperhomocysteinemia are also associated with mutations of MTHFR genes.
Vitamin B12 deficiency hyperhomocysteinemia may be associated with osteoporosis, depression, cognitive decline, and some forms of dementia in the elderly 266. More recently, vitamin B12 deficiency has been reported as common among patients with hyperhomocysteinemia and thrombosis 267, although the presence of a direct effect of vitamin B12 deficiency rather than mediated by hyperhomocysteinemia or other factors is uncertain. In fact, lifestyle-related factors, such as smoking status, body mass index (BMI), and physical activity, could interfere between hyperhomocysteinemia and the thromboembolism relationship 268. Moreover, the effect of lowering homocysteine levels in patients with intermediate (total homocysteine 30–100 µmol/L) or severe hyperhomocysteinemia (total homocysteine > 100 µmol/L) remains unknown 269. The cases described below report examples of vitamin B12 deficiency and hyperhomocysteinemia related to different causes.
A case of cerebral venous thrombosis secondary to hyperhomocysteinemia caused by vitamin B12 deficiency in a 32-year-old Indo-Aryan man who followed a strict vegetarian diet is reported by Kapur 270. The preliminary blood examination revealed macrocytic anemia with hemoglobin of 11.4 g/dL and mean corpuscular volume (MCV) of 110 fL 270. Peripheral blood film showed macrocytes and macro-ovalocytes with hypersegmented neutrophils; low serum cobalamin levels 68 pg/mL (200–600) with normal folate levels and total serum homocysteine
levels of 36 μmol/L (5.0–13.9) were observed 270. In addition to other treatments, the patient received parenteral cyanocobalamin 1000 μg once daily for seven days. Gradually, he regained sensorium, his power improved, and he was discharged on orally administered sodium valproate, warfarin, and methylcobalamin. Repeated investigations undertaken at six months after stopping anticoagulants showed normal serum cobalamin 364 pg/mL (200–600) and fasting total homocysteine levels 8.4 μmol/L. The authors conclude that hyperhomocysteinemia is an independent risk factor for cerebral venous thrombosis in patients with cobalamin deficiency, especially those who follow a strict vegetarian diet, and that hyperhomocysteinemia can be easily reversed with vitamin supplementation, cobalamin, and folic acid 270.
The cases of four Moroccan patients with acute vein thrombosis of different sites are reported by Ammouri 271. Three men and one woman of different ages (a 34-year-old man, a 60-year-old man, a 58-year-old man, and a 47-year-old woman) were selected. All patients presented low hemoglobin level (from 8.6 g/dL to 9.5 g/dL), low MCV (mean corpuscular volume), low cobalamin plasma level (about 60 pg/mL; normal >120 pg/mL), and high levels of plasma homocysteine (50 to 200 μmol/L; normal range <15 µmol/L) with normal folate plasma levels. For all, pernicious anemia and venous thrombosis secondary to hyperhomocysteinemia were evident. First, the authors speculated that normal folate levels may have contributed to the delay in the diagnosis of pernicious anemia, leading to severe hyperhomocysteinemia and the consequent development of vascular injury 271.
Hyperhomocysteinemia could lead to venous thrombosis by several pathways. For example, the toxic effect of homocysteine on the vascular endothelium and on the dotting cascade, as well procoagulant properties of homocysteine, including the decrease of antithrombin III binding to endothelial heparan sulfate, an increase of affinity between lipoprotein(a) and fibrin, induction of tissue factor activity in endothelial cells, and inhibition of inactivation of factor V by activated protein. In all patients, clinical and biological abnormalities disappeared upon vitamin B12 supplementation. The authors concluded that vitamin B12 supplements can rapidly correct hyperhomocysteinemia avoiding and preventing thrombotic events 271.
Tanaka et al. 269 reported a case of a 39-year-old man with inferior vena cava (IVC) thrombus. The analysis of risk factors of venous thromboembolism shown hyperhomocysteinemia (total homocysteine 83.1 µmol/L; normal range 5–15 µmol/L) due to an unbalanced diet with a deficiency of folic acid and vitamin B12. The patient was treated with both folic acid and vitamin B6/vitamin B12 supplementation in association with warfarin, inducing a significant resolution of thrombus after four weeks and no evidence of recurrent IVC thrombus at six months. The authors concluded that B vitamins and folic acid therapy might be effective in patients with severe hyperhomocysteinemia 269.
An interesting case of a 43-year-old man presenting with a two-week history of painless ascending sensory disturbances, suspected to be suffering from acute inflammatory polyneuropathy, is reported by Ulrich et al. 272. On clinical examination, deep tendon reflexes were preserved, muscle strength was 5/5 everywhere, and gait was ataxic. Initial laboratory assessment showed nearly normal holotranscobalamin (43 pmol/L; pmol/L normal >50 pmol/L), suggesting no vitamin B12 deficiency. Surprisingly, further investigation showed high homocysteine (48.5 µmol/L; normal <10 µmol/L), suggesting an impairment of vitamin B12-dependent metabolism leading to the diagnosis of subacute combined degeneration. The patient remembered having taken tablets containing cobalamin for three days before hospitalization. The authors concluded that holotranscobalamin can be rapidly normalized during supplementation and the analysis of methylmalonic acid (MMA) and homocysteine might help to detect B12 deficiency in patients who recently started supplementation.
A case of a 24-year-old male with unprovoked bilateral submassive pulmonary emboli with a high level of homocysteine without anemia is reported by Kovalenko et al. 273. Complete blood count showed a MCV of 104fL without anemia, and homocysteine level was 41.3 μmol/L (normal 4.0–13.7 μmol/L). Workup for macrocytosis was notable for low vitamin B12 (72 pg/mL) and folate (2.1 ng/mL) levels. After vitamin B12 supplementation, serum homocysteine levels did not decrease to normal values. The authors speculated that a poor absorption of B vitamins due to a small bowel resection two years before and excessive alcohol consumption could have impaired the results. Another case associated with alcoholism was previously described by Goette et al. 274. The authors described a rare case of a 32-year-old man with severe hyperhomocysteinemia underlying a probable cause of thromboembolic complications 274. The patient did not have a history of cardiovascular disease, but he had at least a six-month history of alcohol abuse at least six months before hospital admission. Laboratory assays showed abnormalities in liver functions, vitamin B12 (226 pg/mL; normal range 150–675 pg/mL) and folate (1.6 μg/L; normal range 1.4–11.8 μg/L) were low but within normal range, while serum homocysteine was at least 12 times higher than normal (173 μmol/L). The patient was treated with 5 mg oral folic acid and 20 mg oral vitamin B6 daily. Vitamin supplementation was then adapted and integrated with other drugs, such as weight-adapted low molecular weight heparin and L-arginine. For some patients, the authors suggested the screening for hyperhomocysteinemia in association with endothelial dysfunction markers as appropriate 274.
Elevated plasma homocysteine is involved in cognitive decline, including Alzheimer’s disease, mild cognitive impairment, and dementia, especially in elderly subjects. McCaddon 275 reported seven cases of older patients (four women aged 78 years, 84 years, 77 years and 87 years, 84 years old, and two men 71 and 75 years old). They presented with cognitive impairment and/or depression and dementia 275. Each had different vitamin B12 status with hyperhomocysteinemia. Treatment with N-acetylcysteine, together with B vitamin supplements, improves cognitive status in hyperhomocysteinemic patients. The authors concluded that it could be important to evaluate inadequate vitamin B12 and folate metabolism in subjects with cognitive diseases, underlining the importance of clinical trials to evaluate the beneficial effects of a synergistic approach to cognitively impaired hyperhomocysteinaemic patients 275.
Vitamin B12 deficiency complications
As most cases of vitamin B12 deficiency can be easily and effectively treated, complications are rare. But complications can occasionally develop, particularly if you have been vitamin B12 deficient for some time.
Anemia complications
All types of anaemia, regardless of the cause, can lead to heart and lung complications as the heart struggles to pump oxygen to the vital organs. A lack of vitamin B12 with or without anemia can cause complications.
Adults with severe anemia are at risk of developing:
- an abnormally fast heartbeat (tachycardia)
- heart failure, where the heart fails to pump enough blood around the body at the right pressure
Neurological changes
A lack of vitamin B12 can cause neurological problems, which affect your nervous system, such as:
- vision problems
- memory loss
- pins and needles
- loss of physical co-ordination (ataxia), which can affect your whole body and cause difficulty speaking or walking
- damage to parts of the nervous system (peripheral neuropathy), particularly in the legs
If neurological problems do develop, they can sometimes be irreversible.
Infertility
Vitamin B12 deficiency can sometimes lead to temporary infertility, an inability to conceive. This usually improves with appropriate vitamin B12 treatment.
Stomach cancer
If you have a vitamin B12 deficiency caused by pernicious anemia, a condition where your immune system attacks healthy cells in your stomach, your risk of developing stomach cancer is increased 207.
Neural tube defects
If you’re pregnant, not having enough vitamin B12 can increase the risk of your baby developing a serious birth defect known as a neural tube defect. The neural tube is a narrow channel that eventually forms the brain and spinal cord.
Examples of neural tube defects include:
- spina bifida – where the baby’s spine does not develop properly
- anencephaly – where a baby is born without parts of the brain and skull
- encephalocele – where a membrane or skin-covered sac containing part of the brain pushes out of a hole in the skull.
Effects of nitrous oxide
Nitrous oxide (N2O), commonly known as ‘laughing gas’, is a type of anesthetic used in dental treatments and childbirth. Using nitrous oxide can reduce the levels of vitamin B12 in your body. Nitrous oxide oxidizes and inactivates vitamin B12, thus inhibiting both of the vitamin B12-dependent enzymes, and can produce many of the clinical features of vitamin B12 deficiency, such as megaloblastic anemia or neuropathy 1. Since nitrous oxide is commonly used for surgery and childbirth, some experts feel vitamin B12 deficiency should be ruled out prior to its use 226, 199. If you are pregnant and have vitamin B12 deficiency, discuss with your doctor or midwife whether you will be able to use nitrous oxide during labor.
Vitamin B12 Deficiency diagnosis
Your health care provider will perform a physical exam. This may reveal problems with your reflexes. It is important to remember that severe neurologic disease may occur without anemia or macrocytosis. Figure 5 presents an approach to diagnosing vitamin B12 deficiency and pernicious anemia 196.
- To screen for vitamin B12 deficiency, your doctor may order blood tests to see whether you have low hemoglobin or vitamin B12 levels. Complete blood test checking for anemia and vitamin B-12 and folate levels 185, 276, 277, 278. Bone marrow suppression is common and potentially affects all cell lines, with megaloblastic anemia being most common 166, 185, 186. The resultant abnormal erythropoiesis can trigger other notable abnormal laboratory findings, such as decreased haptoglobin levels, high lactate dehydrogenase levels, and elevated reticulocyte count 166, 185, 186.
- Vitamin B12 deficiency results in impairment of the activities of vitamin B12-requiring enzymes. Impaired activity of methionine synthase results in elevated homocysteine levels, while impaired activity of L-methylmalonyl-CoA mutase results in increased levels of a metabolite of methylmalonyl-CoA called methylmalonic acid (MMA). While individuals with mild vitamin B12 deficiency may not experience symptoms, blood levels of homocysteine and/or methylmalonic acid (MMA) may be elevated 226. The Schilling test, which was once the diagnostic standard for pernicious anemia, is no longer available in the United States.
- Patients diagnosed with vitamin B12 deficiency whose history and physical examination do not suggest an obvious dietary or malabsorptive etiology should be tested for pernicious anemia with anti-intrinsic factor antibodies, particularly if other autoimmune disorders are present 166, 185, 186, 196. Patients with pernicious anemia may have hematologic findings consistent w ith normocytic anemia 166. If anti-intrinsic factor results are negative but suspicion for pernicious anemia remains, an elevated serum gastrin level is consistent with the diagnosis 185.
To date, there is no consensus about the exact definition of vitamin B12 deficiency 279. There is still a significant debate within the scientific community about the specific cut-off values that should be applied to define a low vitamin B12 status and about the definition of the best biomarker or combination of biomarkers to assess vitamin B12 status 279, 25. Varying cut-off values invariably lead to underestimating or overestimating the incidence of vitamin B12 deficiency 195. With regard to the definition of an optimal vitamin B12 status, a low vitamin B12 status (frank vitamin B12 deficiency) is generally defined as total serum vitamin B12 levels of < 148 pmol/L, with levels between 148 and 221 pmol/L being considered as “borderline” or suggestive of “marginal deficiency” 27.
In light of the above remarks, measurement of functional biomarkers of vitamin B12 status (homocysteine and methylmalonic acid [MMA]) may be useful to confirm the diagnosis of true vitamin B12 deficiency, particularly in the presence of low-normal total serum vitamin B12 levels and/or clinical suspicion of vitamin B12 deficiency 279. Therefore, total vitamin B12, its bioactive protein-bound form holotranscobalamin (HoloTC), homocysteine and methylmalonic acid (MMA) are the preferred serum biomarkers to accurately assess vitamin B12 status 25. However, it is worth noting that serum levels of homocysteine and methylmalonic acid (MMA) can be elevated even in the presence of folate deficiency, which can also be associated with macrocytic anemia and thereby confused with vitamin B12 deficiency 195. Therefore, measurement of serum folate, MMA and homocysteine levels can help to distinguish vitamin B12 deficiency from folate deficiency. Serum levels of both homocysteine and MMA are often elevated in the presence of true vitamin B12 deficiency. Conversely, homocysteine levels are elevated but MMA levels are normal in the presence of folate deficiency 280. Yet, it is also worth remembering that both homocysteine and MMA levels can be elevated in the presence of kidney disease 279.
Figure 5. Vitamin B12 deficiency diagnostic algorithm
[Source 184 ]The diagnosis of vitamin B12 deficiency is based mainly on complete blood count and vitamin B-12 and folate levels. Complete blood count usually detects megaloblastic anemia. Tissue deficiency and macrocytic indexes may precede the development of anemia. The diagnosis of vitamin B12 deficiency is based mainly on blood measurements of serum vitamin B12 level less than 200 pg/mL (148 pmol/L), complemented with second‐line tests including total homocysteine and methylmalonic acid levels, which are metabolic indicators of vitamin B12 deficiency 281. A serum vitamin B12 level less than 150 pg/mL (< 111 pmol/L) is diagnostic for vitamin B-12 deficiency 166, 185. Serum vitamin B12 levels may be artificially elevated in patients with alcoholism, liver disease, or cancer because of decreased hepatic clearance of transport proteins and resultant higher circulating levels of vitamin B12; physicians should use caution when interpreting laboratory results in these patients 282, 283. In patients with a normal or low-normal serum vitamin B12 level, complete blood count results demonstrating macrocytosis, or suspected clinical manifestations, a serum methylmalonic acid (MMA) level is an appropriate next step and is a more direct measure of vitamin B12’s physiologic activity 196, 186, 166, 185. Although not clinically validated or available for widespread use, measurement of holotranscobalamin, the metabolically active form of vitamin B12, is an emerging method of detecting deficiency 196.
The folate level is also measured because vitamin B12 deficiency must be differentiated from folate deficiency as a cause of megaloblastic anemia; folate supplementation can mask vitamin B12 deficiency and may alleviate megaloblastic anemia but allow the neurologic deficits to progress or even accelerate.
Studies have indicated that an estimated 20% of people can present with neuropsychiatric symptoms in the absence of hematological abnormalities 284.
When clinical judgment suggests vitamin B12 deficiency but the vitamin B12 level is low-normal (200 to 350 pg/mL [145 to 260 pmol/L]) or hematologic indexes are normal, other tests can be done. They include measuring the following:
- Serum methylmalonic acid (MMA) levels: An elevated MMA level supports vitamin B12 deficiency but may be due to renal failure. Methylmalonic acid (MMA) levels can also be used to monitor the response to treatment. Methylmalonic acid levels remain normal in folate deficiency.
- Homocysteine levels: Levels may be elevated with either vitamin B12 or folate deficiency.
- Less commonly, holotranscobalamin 2 (transcobalamin 2–B12 complex) content: When holotranscobalamin 2 is < 40 pg/mL (< 30 pmol/L), Vitamin B-12 is deficient.
After Vitamin B-12 deficiency is diagnosed, additional tests (eg, Schilling test) may be indicated for younger adults but usually not for the elderly. Unless dietary Vitamin B-12 is obviously inadequate, serum gastrin levels or autoantibodies to intrinsic factor may be measured; sensitivity and specificity of these tests may be poor.
- Schilling test
The Schilling test is useful only if diagnosing intrinsic factor deficiency is important, as in classic pernicious anemia. This test is not necessary for most elderly patients. The Schilling test measures absorption of free radiolabeled Vitamin B-12. Radiolabeled Vitamin B-12 is given orally, followed in 1 to 6 h by 1000 mcg (1 mg) of parenteral Vitamin B-12, which reduces uptake of radiolabeled Vitamin B-12 by the liver. Absorbed radiolabeled Vitamin B-12 is excreted in urine, which is collected for 24 h. The amount excreted is measured, and the percentage of total radiolabeled Vitamin B-12 is determined. If absorption is normal, ≥ 9% of the dose given appears in the urine. Reduced urinary excretion (< 5% if kidney function is normal) indicates inadequate Vitamin B-12 absorption. Improved absorption with the subsequent addition of intrinsic factor to radiolabeled Vitamin B-12 confirms the diagnosis of pernicious anemia.
The test is often difficult to do or interpret because of incomplete urine collection or renal insufficiency. In addition, because the Schilling test does not measure absorption of protein-bound Vitamin B-12, the test does not detect defective liberation of Vitamin B-12 from foods, which is common among the elderly. The Schilling test repletes Vitamin B-12 and can mask deficiency, so it should be done only after all other diagnostic tests and therapeutic trials.
If malabsorption is identified, the Schilling test can be repeated after a 2-wk trial of an oral antibiotic. If antibiotic therapy corrects malabsorption, the likely cause is intestinal overgrowth of bacteria (eg, blind-loop syndrome).
Vitamin B12 deficiency test
Diagnosis of Vitamin B-12 deficiency is based on complete blood count and Vitamin B-12 and folate levels. Complete blood count usually detects megaloblastic anemia. Tissue deficiency and macrocytic indexes may precede the development of anemia. A Vitamin B-12 level < 200 pg/mL (< 145 pmol/L) indicates Vitamin B-12 deficiency. The folate level is measured because Vitamin B-12 deficiency must be differentiated from folate deficiency as a cause of megaloblastic anemia; folate supplementation can mask Vitamin B-12 deficiency and may alleviate megaloblastic anemia but allow the neurologic deficits to progress or even accelerate.
When clinical judgment suggests Vitamin B-12 deficiency but the Vitamin B-12 level is low-normal (200 to 350 pg/mL [145 to 260 pmol/L]) or hematologic indexes are normal, other tests can be done. They include measuring the following:
- Serum methylmalonic acid (MMA) levels: An elevated MMA level supports Vitamin B-12 deficiency but may be due to renal failure. Methylmalonic acid (MMA) levels can also be used to monitor the response to treatment. Methylmalonic acid levels remain normal in folate deficiency.
- Homocysteine levels: Levels may be elevated with either Vitamin B-12 or folate deficiency.
- Less commonly, holotranscobalamin II (transcobalamin II–B12 complex) content: When holotranscobalamin II is < 40 pg/mL (< 30 pmol/L), Vitamin B-12 is deficient.
After Vitamin B-12 deficiency is diagnosed, additional tests (eg, Schilling test) may be indicated for younger adults but usually not for the elderly. Unless dietary Vitamin B-12 is obviously inadequate, serum gastrin levels or autoantibodies to intrinsic factor may be measured; sensitivity and specificity of these tests may be poor.
Schilling test
The Schilling test is useful only if diagnosing intrinsic factor deficiency is important, as in classic pernicious anemia. This test is not necessary for most elderly patients. The Schilling test measures absorption of free radiolabeled Vitamin B-12. Radiolabeled Vitamin B-12 is given orally, followed in 1 to 6 h by 1000 mcg (1 mg) of parenteral Vitamin B-12, which reduces uptake of radiolabeled Vitamin B-12 by the liver. Absorbed radiolabeled Vitamin B-12 is excreted in urine, which is collected for 24 h. The amount excreted is measured, and the percentage of total radiolabeled Vitamin B-12 is determined. If absorption is normal, ≥ 9% of the dose given appears in the urine. Reduced urinary excretion (< 5% if kidney function is normal) indicates inadequate Vitamin B-12 absorption. Improved absorption with the subsequent addition of intrinsic factor to radiolabeled Vitamin B-12 confirms the diagnosis of pernicious anemia.
The test is often difficult to do or interpret because of incomplete urine collection or renal insufficiency. In addition, because the Schilling test does not measure absorption of protein-bound Vitamin B-12, the test does not detect defective liberation of Vitamin B-12 from foods, which is common among the elderly. The Schilling test repletes Vitamin B-12 and can mask deficiency, so it should be done only after all other diagnostic tests and therapeutic trials.
If malabsorption is identified, the Schilling test can be repeated after a 2-week trial of an oral antibiotic. If antibiotic therapy corrects malabsorption, the likely cause is intestinal overgrowth of bacteria (eg, blind-loop syndrome).
Vitamin B12 deficiency treatment
Vitamin B12 deficiency can be treated with intramuscular injections of cyanocobalamin or hydroxocobalamin or oral vitamin B12 therapy. However, depending on the cause of the B12 deficiency, the duration and route of treatment vary. In patients who are B12 deficient due to a strict vegan diet, an oral supplement of B12 is adequate for repletion 12. Vitamin B12 1000 to 2000 mcg (1 to 2 mg) orally can be given once/day to patients who do not have severe deficiency or neurologic symptoms or signs. A 2018 Cochrane review included three randomized controlled trials (RCTs) that compared very high doses (1,000–2,000 mcg) of oral with intramuscular vitamin B12 for vitamin B12 deficiency in a total of 153 participants 14. The evidence from these studies, although of low quality, showed that the ability of high oral doses of vitamin B12 supplements to normalize serum vitamin B12 was similar to that of intramuscular vitamin B12. The British Society for Haematology recommends intramuscular vitamin B12 for severe deficiency and malabsorption syndromes, whereas oral replacement may be considered for patients with asymptomatic, mild disease with no absorption or compliance concerns 190.
For more severe deficiency, vitamin B12 1 mg IM (intramuscularly) is usually given 1 to 4 times/week for several weeks until hematologic abnormalities are corrected; then it is given once/month. Approximately 10% of the standard injectable dose of 1 mg is absorbed, which allows for rapid replacement in patients with severe deficiency or severe neurologic sy mptoms 185. Guidelines from the British Society for Haematology recommend injections three times per week for two weeks in patients without neurologic deficits 196. If neurologic deficits are present, injections should be given every other day for up to three weeks or until no further improvement is noted. Table 4 lists the usual times until improvement for abnormalities associated with vitamin B12 deficiency 16. In general, patients with an irreversible cause should be treated indefinitely, whereas those with a reversible cause should be treated until the deficiency is corrected and symptoms resolve 166. If vitamin B12 deficiency coexists with folate deficiency, vitamin B12 should be replaced first to prevent subacute combined degeneration of the spinal cord 166. The British Society for Haematology does not recommend retesting vitamin B12 levels after treatment has been initiated, and no guidelines address the optimal interval for screening high-risk patients 196.
Although hematologic abnormalities are usually corrected within 6 week (reticulocyte count should improve within 1 week), resolution of neurologic symptoms may take much longer. Neurologic symptoms that persist for months or years become irreversible. In most elderly people with vitamin B-12 deficiency and dementia, cognition does not improve after treatment.
There are 2 types of vitamin B12 injections:
- Hydroxocobalamin
- Cyanocobalamin
Typically, vitamin B12 deficiency is treated with intramuscular injections of cyanocobalamin or hydroxocobalamin, because this method bypasses any barriers to absorption. Hydroxocobalamin is usually the recommended option as it stays in the body for longer. Approximately 10% of the standard injectable dose of 1 mg is absorbed, which allows for rapid replacement in patients with severe deficiency or severe neurologic symptoms 28. Guidelines from the British Society for Haematology recommend injections three times per week for two weeks in patients without neurologic deficits 190. If neurologic deficits are present, injections should be given every other day for up to three weeks or until no further improvement is noted.
If vitamin B12 deficiency coexists with folate deficiency, vitamin B12 should be replaced first to prevent subacute combined degeneration of the spinal cord 166.
Although hematologic abnormalities are usually corrected within 6 week (reticulocyte count should improve within 1 week), resolution of neurologic symptoms may take much longer. Neurologic symptoms that persist for months or years become irreversible. In most elderly people with Vitamin B-12 deficiency and dementia, cognition does not improve after treatment. Table 6 lists the usual times until improvement for abnormalities associated with vitamin B12 deficiency 16.
Vitamin B-12 treatment must be continued for life unless the pathophysiologic mechanism for the deficiency is corrected.
Infants of vegan mothers should receive supplemental Vitamin B-12 from birth.
In patients with a deficiency in intrinsic factor (IF), either due to pernicious anemia or gastric bypass surgery, a parenteral dose of vitamin B12 is recommended, as oral B12 will not be fully absorbed due to the lack of intrinsic factor. A dose of 1000 mcg of B12 via the intramuscular route is recommended once a month 285. In newly diagnosed patients, 1000 mcg of vitamin B12 is given intramuscularly once a week for four weeks to replenish stores before switching to once-monthly dosing 285. Studies have shown that at doses high enough to fully saturate intestinal B12 receptors, oral B12 is also effective, despite a lack of intrinsic factor 285.
In anyone at risk of developing a B12 deficiency, such as patients with Crohn’s disease or celiac disease, routine monitoring of B12 should be performed. If the severity of the disease worsens and B12 levels begin to decline, treatment is then started. However, prophylactic treatment before B12 levels fall is not indicated 286, 287, 288.
[Source 16 ]If your vitamin B12 deficiency is caused by a lack of the vitamin B12 in your diet, you may be advised to take vitamin B12 tablets every day between meals. Or you may need to have an vitamin B12 injection of hydroxocobalamin twice a year.
People who find it difficult to get enough vitamin B12 in their diets, such as those following a vegan diet, may need vitamin B12 tablets for life.
Although it’s less common, people with vitamin B12 deficiency caused by a prolonged poor diet may be advised to stop taking the tablets once their vitamin B12 levels have returned to normal and their diet has improved.
Good sources of vitamin B12 include:
- meat
- salmon and cod
- milk and other dairy products
- eggs
If you’re a vegetarian or vegan, or are looking for alternatives to meat and dairy products, there are other foods that contain vitamin B12, such as yeast extract (including Marmite), as well as some fortified breakfast cereals and soy products.
Check the nutrition labels while food shopping to see how much vitamin B12 different foods contain.
If your vitamin B12 deficiency is not caused by a lack of vitamin B12 in your diet, you’ll usually need to have an injection of hydroxocobalamin every 2 to 3 months for the rest of your life 289.
If you have had neurological symptoms that affect your nervous system, such as numbness or tingling in your hands and feet, caused by a vitamin B12 deficiency, you’ll be referred to a hematologist and may need to have vitamin B12 injections every 2 months. Your hematologist will advise on how long you need to keep taking the vitamin B12 injections.
For injections of vitamin B12 given in the UK, hydroxocobalamin is preferred to an alternative called cyanocobalamin 289. This is because hydroxocobalamin stays in the body for longer.
Monitoring your condition
It is essential for your healthcare provider to confirm vitamin B12 deficiency before starting therapy and a follow-up plan for the monitoring your response 36. To ensure your treatment is working, you may need to have further blood tests. A blood test is often carried out around 7 to 10 days after starting treatment to assess whether treatment is working. If you have severe anemia associated with vitamin B12 deficiency, your blood response should lead to a marked increase in reticulocytes (precursors of red blood cells) by one-to-two weeks 36. In mild vitamin B12 deficiency, this is less important, and follow-up should be done at two-to-three months after initiation of hydroxocobalamin 36. These measurements should include vitamin B12 levels as well as homocysteine and methylmalonic acid (MMA) levels 290. Both homocysteine and methylmalonic acid (MMA) are indicators for vitamin B12 levels and demonstrate your response to hydroxocobalamin 291. Most people who have had a vitamin B12 or folate deficiency will not need further monitoring unless their symptoms return or their treatment is ineffective 292.
Vitamin B12 deficiency prognosis
For patients who are promptly treated with vitamin B12, the prognosis is good. In general, younger patients have better outcomes compared to older individuals. The best response is obtained in people with the absence of severe neurological deficits. Therefore, it is important to start vitamin B12 treatment early. Nerve damage can be permanent if vitamin B12 treatment does not start within 6 months of symptoms. Although vitamin B12 supplementation stops progression and improves neurologic deficits in most patients with subacute combined degeneration, evidence shows complete resolution only occurs in a small percentage of them 293. A 2006 observational study evaluating 57 patients with subacute combined degeneration reported only 14% clinical resolution after B12 treatment 293. Still, the study reported that of these patients, 86% had at least some clinical improvement. Subgroup analysis revealed that the absence of sensory dermatomal deficit, negative Romberg and Babinski signs, age less than 50 years, and less than or equal to 7-segment involvement on magnetic resonance imaging correlated with complete resolution of neurologic symptoms 293. This study highlights the importance of early diagnosis and treatment of vitamin B12 deficiency, as patients with severe or prolonged neurological symptoms tend to have persistent symptoms despite treatment.
Vitamin B-12 Side Effects and Toxicity – Health Risks from Excessive Vitamin B-12
The Institute of Medicine did not establish a Tolerable Upper Intake Level (maximum daily intake unlikely to cause adverse health effects) for Vitamin B-12 because of its low potential for toxicity 31. In Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B-12, Pantothenic Acid, Biotin, and Choline, the Institute of Medicine states that “no adverse effects have been associated with excess Vitamin B-12 intake from food and supplements in healthy individuals” 159. Even at large doses, vitamin B12 is generally considered to be safe because the body does not store excess amounts.
Findings from intervention trials support these conclusions. In the NORVIT and HOPE 2 trials, Vitamin B-12 supplementation (in combination with folic acid and vitamin B6) did not cause any serious adverse events when administered at doses of 0.4 mg for 40 months (NORVIT trial) and 1.0 mg for 5 years (HOPE 2 trial) 294, 295.
- Vitamin B12. https://lpi.oregonstate.edu/mic/vitamins/vitamin-B12[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Herbert V. Vitamin B-12 in Present Knowledge in Nutrition. 17th ed. Washington, DC: International Life Sciences Institute Press, 1996.[↩][↩][↩][↩]
- Herbert V, Das K. Vitamin B-12 in Modern Nutrition in Health and Disease. 8th ed. Baltimore, MD: Williams & Wilkins, 1994.[↩][↩]
- Combs G. Vitamin B-12 in The Vitamins. New York: Academic Press, Inc., 1992.[↩][↩][↩][↩][↩][↩]
- Zittoun J, Zittoun R. Modern clinical testing strategies in cobalamin and folate deficiency. Semin Hematol. 1999 Jan;36(1):35-46.[↩][↩]
- Thakkar K, Billa G. Treatment of vitamin B12 deficiency-methylcobalamine? Cyancobalamine? Hydroxocobalamin?-clearing the confusion. Eur J Clin Nutr. 2015 Jan;69(1):1-2. doi: 10.1038/ejcn.2014.165[↩]
- Brody T. Nutritional Biochemistry. 2nd ed. San Diego: Academic Press; 1999.[↩]
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B(6), Folate, Vitamin B(12), Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academies Press; 1998. Available from: https://www.ncbi.nlm.nih.gov/books/NBK114310/ doi: 10.17226/6015[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Carmel R. Cobalamin (Vitamin B-12). In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, eds. Modern Nutrition in Health and Disease. Philadelphia: Lippincott Williams & Wilkins; 2006:482-497.[↩][↩][↩]
- Paul C, Brady DM. Comparative Bioavailability and Utilization of Particular Forms of B12 Supplements With Potential to Mitigate B12-related Genetic Polymorphisms. Integr Med (Encinitas). 2017 Feb;16(1):42-49. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5312744[↩][↩]
- Clarke R. B-vitamins and prevention of dementia. Proc Nutr Soc. 2008 Feb;67(1):75-81. doi: 10.1017/S0029665108006046[↩][↩][↩]
- Vitamin B12. https://ods.od.nih.gov/factsheets/VitaminB12-HealthProfessional[↩][↩][↩][↩][↩]
- Al Amin ASM, Gupta V. Vitamin B12 (Cobalamin) [Updated 2023 Jan 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559132[↩]
- Wang H, Li L, Qin LL, Song Y, Vidal-Alaball J, Liu TH. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency. Cochrane Database Syst Rev. 2018 Mar 15;3(3):CD004655. doi: 10.1002/14651858.CD004655.pub3[↩][↩][↩][↩][↩][↩]
- Klee GG. Cobalamin and folate evaluation: measurement of methylmalonic acid and homocysteine vs vitamin B(12) and folate. Clin Chem. 2000 Aug;46(8 Pt 2):1277-83.[↩]
- Carmel R. How I treat cobalamin (vitamin B12) deficiency. Blood. 2008 Sep 15;112(6):2214-21. doi: 10.1182/blood-2008-03-040253[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Guéant JL, Safi A, Aimone-Gastin I, Rabesona H, Bronowicki JP, Plénat F, Bigard MA, Haertlé T. Autoantibodies in pernicious anemia type I patients recognize sequence 251-256 in human intrinsic factor. Proc Assoc Am Physicians. 1997 Sep;109(5):462-9.[↩]
- Kapadia CR. Vitamin B12 in health and disease: part I–inherited disorders of function, absorption, and transport. Gastroenterologist. 1995 Dec;3(4):329-44.[↩]
- Johnson MA. If high folic acid aggravates vitamin B12 deficiency what should be done about it? Nutr Rev. 2007 Oct;65(10):451-8. doi: 10.1111/j.1753-4887.2007.tb00270.x[↩]
- Hall CA. Transcobalamins I and II as natural transport proteins of vitamin B12. J Clin Invest. 1975 Nov;56(5):1125-31. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC301974/pdf/jcinvest00173-0061.pdf[↩]
- Carmel R. Cobalamin (vitamin B12). In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2014:369-89.[↩][↩][↩][↩][↩]
- Allen LH, Miller JW, de Groot L, Rosenberg IH, Smith AD, Refsum H, Raiten DJ. Biomarkers of Nutrition for Development (BOND): Vitamin B-12 Review. J Nutr. 2018 Dec 1;148(suppl_4):1995S-2027S. doi: 10.1093/jn/nxy201[↩][↩][↩][↩][↩]
- Langan RC, Goodbred AJ. Vitamin B12 Deficiency: Recognition and Management. Am Fam Physician. 2017 Sep 15;96(6):384-389. https://www.aafp.org/pubs/afp/issues/2017/0915/p384.html[↩][↩][↩][↩][↩][↩]
- Maruvada P, Stover PJ, Mason JB, Bailey RL, Davis CD, Field MS, Finnell RH, Garza C, Green R, Gueant JL, Jacques PF, Klurfeld DM, Lamers Y, MacFarlane AJ, Miller JW, Molloy AM, O’Connor DL, Pfeiffer CM, Potischman NA, Rodricks JV, Rosenberg IH, Ross SA, Shane B, Selhub J, Stabler SP, Trasler J, Yamini S, Zappalà G. Knowledge gaps in understanding the metabolic and clinical effects of excess folates/folic acid: a summary, and perspectives, from an NIH workshop. Am J Clin Nutr. 2020 Nov 11;112(5):1390-1403. doi: 10.1093/ajcn/nqaa259[↩]
- Hannibal L, Lysne V, Bjørke-Monsen AL, Behringer S, Grünert SC, Spiekerkoetter U, Jacobsen DW, Blom HJ. Biomarkers and Algorithms for the Diagnosis of Vitamin B12 Deficiency. Front Mol Biosci. 2016 Jun 27;3:27. doi: 10.3389/fmolb.2016.00027. Erratum in: Front Mol Biosci. 2017 Aug 08;4:53.[↩][↩][↩][↩][↩]
- Mineva EM, Sternberg MR, Zhang M, Aoki Y, Storandt R, Bailey RL, Pfeiffer CM. Age-specific reference ranges are needed to interpret serum methylmalonic acid concentrations in the US population. Am J Clin Nutr. 2019 Jul 1;110(1):158-168. doi: 10.1093/ajcn/nqz045[↩]
- Green R, Allen LH, Bjørke-Monsen AL, Brito A, Guéant JL, Miller JW, Molloy AM, Nexo E, Stabler S, Toh BH, Ueland PM, Yajnik C. Vitamin B12 deficiency. Nat Rev Dis Primers. 2017 Jun 29;3:17040. doi: 10.1038/nrdp.2017.40. Erratum in: Nat Rev Dis Primers. 2017 Jul 20;3:17054.[↩][↩][↩]
- Stabler SP. Vitamin B12. In: Marriott BP, Birt DF, Stallings VA, Yates AA, eds. Present Knowledge in Nutrition. 11th ed. Washington, DC: Elsevier; 2020:257-71.[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Allen LH. Vitamin B-12. Adv Nutr. 2012 Jan;3(1):54-5. doi: 10.3945/an.111.001370[↩]
- Allen LH. Vitamin B12. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:812-20.[↩][↩]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington (DC): National Academies Press (US); 1998. Available from: https://www.ncbi.nlm.nih.gov/books/NBK114310[↩][↩][↩][↩]
- Shane B. Folic acid, vitamin B-12, and vitamin B-6. In: Stipanuk M, ed. Biochemical and Physiological Aspects of Human Nutrition. Philadelphia: W.B. Saunders Co.; 2000:483-518.[↩][↩][↩][↩][↩]
- Paul C, Brady DM. Comparative Bioavailability and Utilization of Particular Forms of B12 Supplements With Potential to Mitigate B12-related Genetic Polymorphisms. Integr Med (Encinitas). 2017 Feb;16(1):42-49.[↩][↩]
- Yazaki Y, Chow G, Mattie M. A single-center, double-blinded, randomized controlled study to evaluate the relative efficacy of sublingual and oral vitamin B-complex administration in reducing total serum homocysteine levels. J Altern Complement Med. 2006 Nov;12(9):881-5. doi: 10.1089/acm.2006.12.881[↩]
- Miller JW. Proton Pump Inhibitors, H2-Receptor Antagonists, Metformin, and Vitamin B-12 Deficiency: Clinical Implications. Adv Nutr. 2018 Jul 1;9(4):511S-518S. doi: 10.1093/advances/nmy023[↩][↩]
- Ramezanpour Ahangar E, Annamaraju P. Hydroxocobalamin. [Updated 2023 May 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557632[↩][↩][↩][↩][↩][↩]
- Vasavada A, Sanghavi DK. Cyanocobalamin. [Updated 2023 Apr 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK555964[↩]
- Skouby AP. Dosage of hydroxocobalamin for vitamin B12 deficiency. Acta Med Scand. 1970 Jul-Aug;188(1):31-6. doi: 10.1111/j.0954-6820.1970.tb08002.x[↩]
- Oliveira C. Toxic-Metabolic and Hereditary Optic Neuropathies. Continuum (Minneap Minn). 2019 Oct;25(5):1265-1288. https://journals.lww.com/continuum/Fulltext/2019/10000/Toxic_Metabolic_and_Hereditary_Optic_Neuropathies.7.aspx[↩][↩]
- Chester EM, Agamanolis DP, Harris JW, et al. Optic atrophy in experimental vitamin B12 deficiency in monkeys. Acta Neurol Scand 1980;61(1):9–26. doi:10.1111/j.1600-0404.1980.tb02991.x[↩][↩]
- Chan W, Almasieh M, Catrinescu MM, Levin LA. Cobalamin-associated superoxide scavenging in neuronal cells is a potential mechanism for vitamin B12-deprivation optic neuropathy. Am J Pathol 2018;188(1):160–172. doi:10.1016/j.ajpath.2017.08.032[↩]
- Marshall R, Milburn JM. Clinical images – a quarterly column: subacute combined degeneration of the spinal cord. Ochsner J. 2013 Summer;13(2):183-5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3684324[↩]
- Purvis MV, Rooks H, Young Lee J, Longerich S, Kahn SA. Prehospital hydroxocobalamin for inhalation injury and cyanide toxicity in the United States – analysis of a database and survey of ems providers. Ann Burns Fire Disasters. 2017 Jun 30;30(2):126-128. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5627550[↩]
- Estourgie-van Burk GF, van der Kuy PHM, de Meij TG, Benninga MA, Kneepkens CMF. Intranasal treatment of vitamin B12 deficiency in children. Eur J Pediatr. 2020 Feb;179(2):349-352. doi: 10.1007/s00431-019-03519-0[↩]
- Tillemans MP, Donders EM, Verweij SL, Van der Hoeven RT, Kalisvaart KJ. Effect of administration route on the pharmacokinetics of cobalamin in elderly patients: a randomized controlled trial. Curr Ther Res Clin Exp. 2014 Mar 20;76:21-5. doi: 10.1016/j.curtheres.2014.01.001[↩]
- National Institutes of Health. Third report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Bethesda, MD: National Cholesterol Education Program, National Heart, Lung, and Blood Institute, National Institutes of Health, September 2002. NIH Publication No. 02-5215. https://www.ncbi.nlm.nih.gov/pubmed/12485966[↩]
- Refsum H, Nurk E, Smith AD, Ueland PM, Gjesdal CG, Bjelland I, et al. The Hordaland Homocysteine Study: a community-based study of homocysteine, its determinants, and associations with disease. J Nutr 2006;136(6 Suppl):1731S-40S. https://www.ncbi.nlm.nih.gov/pubmed/16702348?dopt=Abstract[↩][↩][↩]
- Schulz RJ. Homocysteine as a biomarker for cognitive dysfunction in the elderly. Curr Opin Clin Nutr Metab Care 2007;10:718-23. https://www.ncbi.nlm.nih.gov/pubmed/18089953?dopt=Abstract[↩][↩][↩][↩]
- American Heart Association Nutrition Committee, Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation 2006;114:82-96. https://www.ncbi.nlm.nih.gov/pubmed/16785338?dopt=Abstract[↩][↩][↩]
- Malinow MR. Plasma homocyst(e)ine and arterial occlusive diseases: a mini-review. Clin Chem 1995;41:173-6. https://www.ncbi.nlm.nih.gov/pubmed/7813076%20?dopt=Abstract[↩]
- Flynn MA, Herbert V, Nolph GB, Krause G. Atherogenesis and the homocysteine-folate-cobalamin triad: do we need standardized analyses? J Am Coll Nutr 1997;16:258-67. https://www.ncbi.nlm.nih.gov/pubmed/9176833?dopt=Abstract[↩]
- Fortin LJ, Genest J Jr. Measurement of homocyst(e)ine in the prediction of arteriosclerosis. Clin Biochem 1995;28:155-62. https://www.ncbi.nlm.nih.gov/pubmed/7628074?dopt=Abstract[↩]
- Siri PW, Verhoef P, Kok FJ. Vitamins B6, B12, and folate: association with plasma total homocysteine and risk of coronary atherosclerosis. J Am Coll Nutr 1998;17:435-41. https://www.ncbi.nlm.nih.gov/pubmed/9791839?dopt=Abstract[↩]
- Ubbink JB, van der Merwe A, Delport R, Allen R H, Stabler S P, Riezler R, et al. The effect of a subnormal vitamin B6 status on homocysteine metabolism. J Clin Invest 1996;98:177-84. www.ncbi.nlm.nih.gov/pubmed/8690790?dopt=Abstract[↩]
- Clarke R. B-vitamins and prevention of dementia. Proc Nutr Soc 2008;67:75-81. Clarke R. B-vitamins and prevention of dementia. Proc Nutr Soc 2008;67:75-81.[↩]
- Lee BJ, Huang MC, Chung LJ, Cheng CH, Lin KL, Su KH, et al. Folic acid and Vitamin B-12 are more effective than vitamin B6 in lowering fasting plasma homocysteine concentration in patients with coronary artery disease. Eur J Clin Nutr 2004;58:481-7. https://www.ncbi.nlm.nih.gov/pubmed/14985687?dopt=Abstract[↩]
- Bønaa KH, Njølstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med 2006;354:1578-88. https://www.ncbi.nlm.nih.gov/pubmed/16531614?dopt=Abstract[↩][↩][↩]
- Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567-77. https://www.ncbi.nlm.nih.gov/pubmed/16531613?dopt=Abstract[↩][↩][↩]
- Clarke R, Lewington S, Sherliker P, Armitage J. Effects of B-vitamins on plasma homocysteine concentrations and on risk of cardiovascular disease and dementia. Curr Opin Clin Nutr Metab Care 2007;10:32-9. https://www.ncbi.nlm.nih.gov/pubmed/17143052?dopt=Abstract[↩][↩]
- Albert CM, Cook NR, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA.2008;299:2027-36. https://www.ncbi.nlm.nih.gov/pubmed/18460663?dopt=Abstract[↩][↩][↩]
- Ebbing M, Bleie Ø, Ueland PM, Nordrehaug JE, Nilsen DW, Vollset SE, et al. Mortality and cardiovascular events in patients treated with homocysteine-lowering B vitamins after coronary angiography: a randomized controlled trial. JAMA 2008;300:795-804. https://www.ncbi.nlm.nih.gov/pubmed/18714059?dopt=Abstract[↩][↩][↩]
- Toole JF, Malinow MR, Chambless LE, Spence JD, Pettigrew LC, Howard VJ, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA 2004;291:565-75. https://www.ncbi.nlm.nih.gov/pubmed/14762035?dopt=Abstract[↩][↩][↩]
- Collaboration HLT. Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomised trials. Homocysteine Lowering Trialists’ Collaboration. BMJ. 1998 Mar 21;316(7135):894-8.[↩]
- McKay DL, Perrone G, Rasmussen H, Dallal G, Blumberg JB. Multivitamin/mineral supplementation improves plasma B-vitamin status and homocysteine concentration in healthy older adults consuming a folate-fortified diet. J Nutr. 2000 Dec;130(12):3090-6. doi: 10.1093/jn/130.12.3090[↩]
- Quinlivan EP, McPartlin J, McNulty H, Ward M, Strain JJ, Weir DG, Scott JM. Importance of both folic acid and vitamin B12 in reduction of risk of vascular disease. Lancet. 2002 Jan 19;359(9302):227-8. doi: 10.1016/s0140-6736(02)07439-1[↩]
- Martí-Carvajal AJ, Solà I, Lathyris D, Karakitsiou DE, Simancas-Racines D. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2013 Jan 31;(1):CD006612. doi: 10.1002/14651858.CD006612.pub3. Update in: Cochrane Database Syst Rev. 2015;1:CD006612.[↩]
- Huang T, Chen Y, Yang B, Yang J, Wahlqvist ML, Li D. Meta-analysis of B vitamin supplementation on plasma homocysteine, cardiovascular and all-cause mortality. Clin Nutr. 2012 Aug;31(4):448-54. doi: 10.1016/j.clnu.2011.01.003[↩]
- Ji Y, Tan S, Xu Y, Chandra A, Shi C, Song B, Qin J, Gao Y. Vitamin B supplementation, homocysteine levels, and the risk of cerebrovascular disease: a meta-analysis. Neurology. 2013 Oct 8;81(15):1298-307. doi: 10.1212/WNL.0b013e3182a823cc[↩]
- Potter K, Hankey GJ, Green DJ, Eikelboom J, Jamrozik K, Arnolda LF. The effect of long-term homocysteine-lowering on carotid intima-media thickness and flow-mediated vasodilation in stroke patients: a randomized controlled trial and meta-analysis. BMC Cardiovasc Disord. 2008 Sep 20;8:24. doi: 10.1186/1471-2261-8-24[↩]
- Qin X, Xu M, Zhang Y, Li J, Xu X, Wang X, Xu X, Huo Y. Effect of folic acid supplementation on the progression of carotid intima-media thickness: a meta-analysis of randomized controlled trials. Atherosclerosis. 2012 Jun;222(2):307-13. doi: 10.1016/j.atherosclerosis.2011.12.007[↩]
- Spence JD. B vitamin therapy for homocysteine: renal function and vitamin B12 determine cardiovascular outcomes. Clin Chem Lab Med. 2013 Mar 1;51(3):633-7. doi: 10.1515/cclm-2012-0465[↩]
- Kim S, Choi BY, Nam JH, Kim MK, Oh DH, Yang YJ. Cognitive impairment is associated with elevated serum homocysteine levels among older adults. Eur J Nutr. 2019 Feb;58(1):399-408. doi: 10.1007/s00394-017-1604-y[↩]
- Smith AD, Refsum H. Homocysteine, B Vitamins, and Cognitive Impairment. Annu Rev Nutr. 2016 Jul 17;36:211-39. doi: 10.1146/annurev-nutr-071715-050947[↩]
- Carmel R. Megaloblastic anemias. Curr Opin Hematol 1994;1:107-12. https://www.ncbi.nlm.nih.gov/pubmed/9371268?dopt=Abstract[↩]
- Clarke R. B-vitamins and prevention of dementia. Proc Nutr Soc 2008;67:75-81. https://www.ncbi.nlm.nih.gov/pubmed/18234134?dopt=Abstract[↩][↩][↩][↩]
- Hutto BR. Folate and cobalamin in psychiatric illness. Compr Psychiatry 1997;38:305-14. https://www.ncbi.nlm.nih.gov/pubmed/9406735?dopt=Abstract[↩]
- Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med 2002;346:476-83. https://www.ncbi.nlm.nih.gov/pubmed/11844848?dopt=Abstract[↩]
- Clarke R, Birks J, Nexo E, Ueland PM, Schneede J, Scott J, et al. Low vitamin B-12 status and risk of cognitive decline in older adults. Am J Clin Nutr 2007;86:1384-91.https://www.ncbi.nlm.nih.gov/pubmed/17991650?dopt=Abstract[↩]
- Bailey RL, Jun S, Murphy L, Green R, Gahche JJ, Dwyer JT, Potischman N, McCabe GP, Miller JW. High folic acid or folate combined with low vitamin B-12 status: potential but inconsistent association with cognitive function in a nationally representative cross-sectional sample of US older adults participating in the NHANES. Am J Clin Nutr. 2020 Dec 10;112(6):1547-1557. doi: 10.1093/ajcn/nqaa239[↩][↩]
- O’Connor DMA, Laird EJ, Carey D, O’Halloran AM, Clarke R, Kenny RA, Molloy AM. Plasma concentrations of vitamin B12 and folate and global cognitive function in an older population: cross-sectional findings from The Irish Longitudinal Study on Ageing (TILDA). Br J Nutr. 2020 Sep 28;124(6):602-610. doi: 10.1017/S0007114520001427[↩]
- Lachner C, Martin C, John D, Nekkalapu S, Sasan A, Steinle N, Regenold WT. Older adult psychiatric inpatients with non-cognitive disorders should be screened for vitamin B12 deficiency. J Nutr Health Aging. 2014;18(2):209-12. doi: 10.1007/s12603-013-0378-z[↩]
- Ma F, Wu T, Zhao J, Ji L, Song A, Zhang M, Huang G. Plasma Homocysteine and Serum Folate and Vitamin B12 Levels in Mild Cognitive Impairment and Alzheimer’s Disease: A Case-Control Study. Nutrients. 2017 Jul 8;9(7):725. doi: 10.3390/nu9070725[↩]
- O’Leary F, Allman-Farinelli M, Samman S. Vitamin B₁₂ status, cognitive decline and dementia: a systematic review of prospective cohort studies. Br J Nutr. 2012 Dec 14;108(11):1948-61. doi: 10.1017/S0007114512004175[↩][↩]
- Eussen SJ, de Groot LC, Joosten LW, Bloo RJ, Clarke R, Ueland PM, et al. Effect of oral vitamin B-12 with or without folic acid on cognitive function in older people with mild vitamin B-12 deficiency: a randomized, placebo-controlled trial. Am J Clin Nutr 2006;84:361-70. https://www.ncbi.nlm.nih.gov/pubmed/16895884?dopt=Abstract[↩][↩]
- Hvas AM, Juul S, Lauritzen L, Nexø E, Ellegaard J. No effect of vitamin B-12 treatment on cognitive function and depression: a randomized placebo controlled study. J Affect Disord 2004;81:269-73. https://www.ncbi.nlm.nih.gov/pubmed/15337331?dopt=Abstract[↩]
- Vital Trial Collaborative Group. Effect of vitamins and aspirin on markers of platelet activation, oxidative stress and homocysteine in people at high risk of dementia. J Intern Med 2003; 254:67-75. https://www.ncbi.nlm.nih.gov/pubmed/12823643?dopt=Abstract[↩][↩]
- Kang JH, Cook N, Manson J, Buring JE, Albert CM, Grodstein F. A trial of B vitamins and cognitive function among women at high risk of cardiovascular disease. Am J Clin Nutr 2008;88:1602-10. https://www.ncbi.nlm.nih.gov/pubmed/19064521?dopt=Abstract[↩][↩]
- Aisen PS, Schneider LS, Sano M, Diaz-Arrastia R, van Dyck CH, Weiner MF, et al.; Alzheimer Disease Cooperative Study. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA 2008 ;300:1774-83. https://www.ncbi.nlm.nih.gov/pubmed/18854539?dopt=Abstract[↩][↩]
- Clarke R, Birks J, Nexo E, Ueland PM, Schneede J, Scott J, Molloy A, Evans JG. Low vitamin B-12 status and risk of cognitive decline in older adults. Am J Clin Nutr. 2007 Nov;86(5):1384-91. doi: 10.1093/ajcn/86.5.1384[↩]
- Tangney CC, Tang Y, Evans DA, Morris MC. Biochemical indicators of vitamin B12 and folate insufficiency and cognitive decline. Neurology. 2009 Jan 27;72(4):361-7. doi: 10.1212/01.wnl.0000341272.48617.b0[↩]
- Kivipelto M, Annerbo S, Hultdin J, Bäckman L, Viitanen M, Fratiglioni L, Lökk J. Homocysteine and holo-transcobalamin and the risk of dementia and Alzheimers disease: a prospective study. Eur J Neurol. 2009 Jul;16(7):808-13. doi: 10.1111/j.1468-1331.2009.02590.x[↩]
- Hooshmand B, Solomon A, Kåreholt I, Leiviskä J, Rusanen M, Ahtiluoto S, Winblad B, Laatikainen T, Soininen H, Kivipelto M. Homocysteine and holotranscobalamin and the risk of Alzheimer disease: a longitudinal study. Neurology. 2010 Oct 19;75(16):1408-14. doi: 10.1212/WNL.0b013e3181f88162[↩]
- Hooshmand B, Solomon A, Kåreholt I, Rusanen M, Hänninen T, Leiviskä J, Winblad B, Laatikainen T, Soininen H, Kivipelto M. Associations between serum homocysteine, holotranscobalamin, folate and cognition in the elderly: a longitudinal study. J Intern Med. 2012 Feb;271(2):204-12. doi: 10.1111/j.1365-2796.2011.02484.x[↩]
- Smith AD. The worldwide challenge of the dementias: a role for B vitamins and homocysteine? Food Nutr Bull. 2008 Jun;29(2 Suppl):S143-72. doi: 10.1177/15648265080292S119[↩]
- Ford AH, Almeida OP. Effect of homocysteine lowering treatment on cognitive function: a systematic review and meta-analysis of randomized controlled trials. J Alzheimers Dis. 2012;29(1):133-49. doi: 10.3233/JAD-2012-111739[↩]
- Walker JG, Batterham PJ, Mackinnon AJ, Jorm AF, Hickie I, Fenech M, Kljakovic M, Crisp D, Christensen H. Oral folic acid and vitamin B-12 supplementation to prevent cognitive decline in community-dwelling older adults with depressive symptoms–the Beyond Ageing Project: a randomized controlled trial. Am J Clin Nutr. 2012 Jan;95(1):194-203. doi: 10.3945/ajcn.110.007799. Erratum in: Am J Clin Nutr. 2012 Aug;96(2):448. Dosage error in article text.[↩]
- Smith AD, Smith SM, de Jager CA, Whitbread P, Johnston C, Agacinski G, Oulhaj A, Bradley KM, Jacoby R, Refsum H. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLoS One. 2010 Sep 8;5(9):e12244. doi: 10.1371/journal.pone.0012244[↩]
- Douaud G, Refsum H, de Jager CA, Jacoby R, Nichols TE, Smith SM, Smith AD. Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment. Proc Natl Acad Sci U S A. 2013 Jun 4;110(23):9523-8. doi: 10.1073/pnas.1301816110[↩][↩]
- Hankey GJ, Ford AH, Yi Q, Eikelboom JW, Lees KR, Chen C, Xavier D, Navarro JC, Ranawaka UK, Uddin W, Ricci S, Gommans J, Schmidt R, Almeida OP, van Bockxmeer FM; VITATOPS Trial Study Group. Effect of B vitamins and lowering homocysteine on cognitive impairment in patients with previous stroke or transient ischemic attack: a prespecified secondary analysis of a randomized, placebo-controlled trial and meta-analysis. Stroke. 2013 Aug;44(8):2232-9. doi: 10.1161/STROKEAHA.113.001886[↩]
- Balk EM, Raman G, Tatsioni A, Chung M, Lau J, Rosenberg IH. Vitamin B6, B12, and folic acid supplementation and cognitive function: a systematic review of randomized trials. Arch Intern Med 2007 Jan ;167:21-30. https://www.ncbi.nlm.nih.gov/pubmed/17210874?dopt=Abstract[↩]
- Malouf R, Areosa Sastre A. Vitamin B-12 for cognition. Cochrane Database Syst Rev 2003;(3):CD004326. https://www.ncbi.nlm.nih.gov/pubmed/12918012?dopt=Abstract[↩]
- Malouf R, Grimley Evans J. Folic acid with or without Vitamin B-12 for the prevention and treatment of health elderly and demented people. Cochrane Database Syst Rev. 2008 Oct 8;(4):CD004514. https://www.ncbi.nlm.nih.gov/pubmed/18843658?dopt=Abstract[↩]
- Zhang DM, Ye JX, Mu JS, Cui XP. Efficacy of Vitamin B Supplementation on Cognition in Elderly Patients With Cognitive-Related Diseases. J Geriatr Psychiatry Neurol. 2017 Jan;30(1):50-59. doi: 10.1177/0891988716673466[↩]
- van der Zwaluw NL, Dhonukshe-Rutten RA, van Wijngaarden JP, Brouwer-Brolsma EM, van de Rest O, In ‘t Veld PH, Enneman AW, van Dijk SC, Ham AC, Swart KM, van der Velde N, van Schoor NM, van der Cammen TJ, Uitterlinden AG, Lips P, Kessels RP, de Groot LC. Results of 2-year vitamin B treatment on cognitive performance: secondary data from an RCT. Neurology. 2014 Dec 2;83(23):2158-66. doi: 10.1212/WNL.0000000000001050[↩]
- Rutjes AW, Denton DA, Di Nisio M, Chong LY, Abraham RP, Al-Assaf AS, Anderson JL, Malik MA, Vernooij RW, Martínez G, Tabet N, McCleery J. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst Rev. 2018 Dec 17;12(12):CD011906. doi: 10.1002/14651858.CD011906.pub2[↩]
- McCleery J, Abraham RP, Denton DA, Rutjes AW, Chong LY, Al-Assaf AS, Griffith DJ, Rafeeq S, Yaman H, Malik MA, Di Nisio M, Martínez G, Vernooij RW, Tabet N. Vitamin and mineral supplementation for preventing dementia or delaying cognitive decline in people with mild cognitive impairment. Cochrane Database Syst Rev. 2018 Nov 1;11(11):CD011905. doi: 10.1002/14651858.CD011905.pub2[↩]
- Fenech M. Folate (vitamin B9) and vitamin B12 and their function in the maintenance of nuclear and mitochondrial genome integrity. Mutat Res. 2012 May 1;733(1-2):21-33. doi: 10.1016/j.mrfmmm.2011.11.003[↩]
- Fenech M. Micronucleus frequency in human lymphocytes is related to plasma vitamin B12 and homocysteine. Mutat Res. 1999 Jul 16;428(1-2):299-304. doi: 10.1016/s1383-5742(99)00056-3[↩]
- Arendt JFH, Sørensen HT, Horsfall LJ, Petersen I. Elevated Vitamin B12 Levels and Cancer Risk in UK Primary Care: A THIN Database Cohort Study. Cancer Epidemiol Biomarkers Prev. 2019 Apr;28(4):814-821. doi: 10.1158/1055-9965.EPI-17-1136[↩]
- Arendt JF, Farkas DK, Pedersen L, Nexo E, Sørensen HT. Elevated plasma vitamin B12 levels and cancer prognosis: A population-based cohort study. Cancer Epidemiol. 2016 Feb;40:158-65. doi: 10.1016/j.canep.2015.12.007[↩]
- Brasky TM, White E, Chen CL. Long-Term, Supplemental, One-Carbon Metabolism-Related Vitamin B Use in Relation to Lung Cancer Risk in the Vitamins and Lifestyle (VITAL) Cohort. J Clin Oncol. 2017 Oct 20;35(30):3440-3448. doi: 10.1200/JCO.2017.72.7735[↩]
- Oliai Araghi S, Kiefte-de Jong JC, van Dijk SC, Swart KMA, van Laarhoven HW, van Schoor NM, de Groot LCPGM, Lemmens V, Stricker BH, Uitterlinden AG, van der Velde N. Folic Acid and Vitamin B12 Supplementation and the Risk of Cancer: Long-term Follow-up of the B Vitamins for the Prevention of Osteoporotic Fractures (B-PROOF) Trial. Cancer Epidemiol Biomarkers Prev. 2019 Feb;28(2):275-282. doi: 10.1158/1055-9965.EPI-17-1198[↩]
- Ulrich CM, Potter JD. Folate supplementation: too much of a good thing? Cancer Epidemiol Biomarkers Prev. 2006 Feb;15(2):189-93. doi: 10.1158/1055-9965.EPI-152CO[↩]
- Wei DH, Mao QQ. Vitamin B6, vitamin B12 and methionine and risk of pancreatic cancer: a meta-analysis. Nutr J. 2020 Oct 4;19(1):111. doi: 10.1186/s12937-020-00628-7[↩]
- Wu W, Kang S, Zhang D. Association of vitamin B6, vitamin B12 and methionine with risk of breast cancer: a dose-response meta-analysis. Br J Cancer. 2013 Oct 1;109(7):1926-44. doi: 10.1038/bjc.2013.438[↩][↩]
- Xiao Q, Freedman ND, Ren J, Hollenbeck AR, Abnet CC, Park Y. Intakes of folate, methionine, vitamin B6, and vitamin B12 with risk of esophageal and gastric cancer in a large cohort study. Br J Cancer. 2014 Mar 4;110(5):1328-33. doi: 10.1038/bjc.2014.17[↩]
- Zhang SL, Chen TS, Ma CY, Meng YB, Zhang YF, Chen YW, Zhou YH. Effect of vitamin B supplementation on cancer incidence, death due to cancer, and total mortality: A PRISMA-compliant cumulative meta-analysis of randomized controlled trials. Medicine (Baltimore). 2016 Aug;95(31):e3485. doi: 10.1097/MD.0000000000003485[↩][↩]
- Miranti EH, Stolzenberg-Solomon R, Weinstein SJ, Selhub J, Männistö S, Taylor PR, Freedman ND, Albanes D, Abnet CC, Murphy G. Low vitamin B12 increases risk of gastric cancer: A prospective study of one-carbon metabolism nutrients and risk of upper gastrointestinal tract cancer. Int J Cancer. 2017 Sep 15;141(6):1120-1129. doi: 10.1002/ijc.30809[↩]
- Sun NH, Huang XZ, Wang SB, Li Y, Wang LY, Wang HC, Zhang CW, Zhang C, Liu HP, Wang ZN. A dose-response meta-analysis reveals an association between vitamin B12 and colorectal cancer risk. Public Health Nutr. 2016 Jun;19(8):1446-56. doi: 10.1017/S136898001500261X[↩]
- Liu Y, Wang X, Sun X, Lu S, Liu S. Vitamin intake and pancreatic cancer risk reduction: A meta-analysis of observational studies. Medicine (Baltimore). 2018 Mar;97(13):e0114. doi: 10.1097/MD.0000000000010114[↩]
- Wu K, Helzlsouer KJ, Comstock GW, Hoffman SC, Nadeau MR, Selhub J. A prospective study on folate, B12, and pyridoxal 5′-phosphate (B6) and breast cancer. Cancer Epidemiol Biomarkers Prev. 1999 Mar;8(3):209-17[↩][↩]
- Lajous M, Lazcano-Ponce E, Hernandez-Avila M, Willett W, Romieu I. Folate, vitamin B(6), and vitamin B(12) intake and the risk of breast cancer among Mexican women. Cancer Epidemiol Biomarkers Prev. 2006 Mar;15(3):443-8. doi: 10.1158/1055-9965.EPI-05-0532[↩][↩]
- Yang D, Baumgartner RN, Slattery ML, Wang C, Giuliano AR, Murtaugh MA, Risendal BC, Byers T, Baumgartner KB. Dietary intake of folate, B-vitamins and methionine and breast cancer risk among Hispanic and non-Hispanic white women. PLoS One. 2013;8(2):e54495. doi: 10.1371/journal.pone.0054495[↩]
- Bassett JK, Baglietto L, Hodge AM, Severi G, Hopper JL, English DR, Giles GG. Dietary intake of B vitamins and methionine and breast cancer risk. Cancer Causes Control. 2013 Aug;24(8):1555-63. doi: 10.1007/s10552-013-0232-y[↩]
- Eskes TK. Open or closed? A world of difference: a history of homocysteine research. Nutr Rev. 1998 Aug;56(8):236-44. doi: 10.1111/j.1753-4887.1998.tb01755.x. Erratum in: Nutr Rev 1998 Oct;56(10):313.[↩]
- Mills JL, Scott JM, Kirke PN, McPartlin JM, Conley MR, Weir DG, Molloy AM, Lee YJ. Homocysteine and neural tube defects. J Nutr. 1996 Mar;126(3):756S-760S. doi: 10.1093/jn/126.suppl_3.756S[↩][↩]
- Imbard A, Benoist JF, Blom HJ. Neural tube defects, folic acid and methylation. Int J Environ Res Public Health. 2013 Sep 17;10(9):4352-89. doi: 10.3390/ijerph10094352[↩][↩]
- Wang ZP, Shang XX, Zhao ZT. Low maternal vitamin B(12) is a risk factor for neural tube defects: a meta-analysis. J Matern Fetal Neonatal Med. 2012 Apr;25(4):389-94. doi: 10.3109/14767058.2011.580800[↩][↩]
- Dror DK, Allen LH. Interventions with vitamins B6, B12 and C in pregnancy. Paediatr Perinat Epidemiol. 2012 Jul;26 Suppl 1:55-74. doi: 10.1111/j.1365-3016.2012.01277.x[↩]
- Hutto BR. Folate and cobalamin in psychiatric illness. Compr Psychiatry. 1997 Nov-Dec;38(6):305-14. doi: 10.1016/s0010-440x(97)90925-1[↩]
- Penninx BW, Guralnik JM, Ferrucci L, Fried LP, Allen RH, Stabler SP. Vitamin B(12) deficiency and depression in physically disabled older women: epidemiologic evidence from the Women’s Health and Aging Study. Am J Psychiatry. 2000 May;157(5):715-21. doi: 10.1176/appi.ajp.157.5.715[↩]
- Tiemeier H, van Tuijl HR, Hofman A, Meijer J, Kiliaan AJ, Breteler MM. Vitamin B12, folate, and homocysteine in depression: the Rotterdam Study. Am J Psychiatry. 2002 Dec;159(12):2099-101. doi: 10.1176/appi.ajp.159.12.2099[↩]
- Mischoulon D, Fava M. Role of S-adenosyl-L-methionine in the treatment of depression: a review of the evidence. Am J Clin Nutr. 2002 Nov;76(5):1158S-61S. doi: 10.1093/ajcn/76/5.1158S[↩]
- Fernàndez-Roig S, Lai SC, Murphy MM, Fernandez-Ballart J, Quadros EV. Vitamin B12 deficiency in the brain leads to DNA hypomethylation in the TCblR/CD320 knockout mouse. Nutr Metab (Lond). 2012 May 18;9:41. doi: 10.1186/1743-7075-9-41[↩]
- Bressa GM. S-adenosyl-l-methionine (SAMe) as antidepressant: meta-analysis of clinical studies. Acta Neurol Scand Suppl. 1994;154:7-14. doi: 10.1111/j.1600-0404.1994.tb05403.x[↩]
- Bell KM, Plon L, Bunney WE Jr, Potkin SG. S-adenosylmethionine treatment of depression: a controlled clinical trial. Am J Psychiatry. 1988 Sep;145(9):1110-4. doi: 10.1176/ajp.145.9.1110[↩]
- Delle Chiaie R, Pancheri P, Scapicchio P. Efficacy and tolerability of oral and intramuscular S-adenosyl-L-methionine 1,4-butanedisulfonate (SAMe) in the treatment of major depression: comparison with imipramine in 2 multicenter studies. Am J Clin Nutr. 2002 Nov;76(5):1172S-6S. doi: 10.1093/ajcn/76/5.1172S[↩]
- Williams AL, Girard C, Jui D, Sabina A, Katz DL. S-adenosylmethionine (SAMe) as treatment for depression: a systematic review. Clin Invest Med. 2005 Jun;28(3):132-9.[↩]
- Almeida OP, McCaul K, Hankey GJ, Norman P, Jamrozik K, Flicker L. Homocysteine and depression in later life. Arch Gen Psychiatry. 2008 Nov;65(11):1286-94. doi: 10.1001/archpsyc.65.11.1286[↩]
- Moorthy D, Peter I, Scott TM, Parnell LD, Lai CQ, Crott JW, Ordovás JM, Selhub J, Griffith J, Rosenberg IH, Tucker KL, Troen AM. Status of vitamins B-12 and B-6 but not of folate, homocysteine, and the methylenetetrahydrofolate reductase C677T polymorphism are associated with impaired cognition and depression in adults. J Nutr. 2012 Aug;142(8):1554-60. doi: 10.3945/jn.112.161828[↩]
- Walker JG, Mackinnon AJ, Batterham P, Jorm AF, Hickie I, McCarthy A, Fenech M, Christensen H. Mental health literacy, folic acid and vitamin B12, and physical activity for the prevention of depression in older adults: randomised controlled trial. Br J Psychiatry. 2010 Jul;197(1):45-54. doi: 10.1192/bjp.bp.109.075291[↩]
- Almeida OP, Marsh K, Alfonso H, Flicker L, Davis TM, Hankey GJ. B-vitamins reduce the long-term risk of depression after stroke: The VITATOPS-DEP trial. Ann Neurol. 2010 Oct;68(4):503-10. doi: 10.1002/ana.22189[↩]
- Vacek TP, Kalani A, Voor MJ, Tyagi SC, Tyagi N. The role of homocysteine in bone remodeling. Clin Chem Lab Med. 2013 Mar 1;51(3):579-90. doi: 10.1515/cclm-2012-0605[↩]
- van Wijngaarden JP, Doets EL, Szczecińska A, Souverein OW, Duffy ME, Dullemeijer C, Cavelaars AE, Pietruszka B, Van’t Veer P, Brzozowska A, Dhonukshe-Rutten RA, de Groot CP. Vitamin B12, folate, homocysteine, and bone health in adults and elderly people: a systematic review with meta-analyses. J Nutr Metab. 2013;2013:486186. doi: 10.1155/2013/486186[↩]
- Sato Y, Honda Y, Iwamoto J, Kanoko T, Satoh K. Effect of folate and mecobalamin on hip fractures in patients with stroke: a randomized controlled trial. JAMA. 2005 Mar 2;293(9):1082-8. doi: 10.1001/jama.293.9.1082. Retraction in: JAMA. 2016 Jun 14;315(22):2405. Erratum in: JAMA. 2006 Jul 26;296(4):396.[↩][↩]
- Sawka AM, Ray JG, Yi Q, Josse RG, Lonn E. Randomized clinical trial of homocysteine level lowering therapy and fractures. Arch Intern Med. 2007 Oct 22;167(19):2136-9. doi: 10.1001/archinte.167.19.2136[↩]
- Herrmann W, Kirsch SH, Kruse V, Eckert R, Gräber S, Geisel J, Obeid R. One year B and D vitamins supplementation improves metabolic bone markers. Clin Chem Lab Med. 2013 Mar 1;51(3):639-47. doi: 10.1515/cclm-2012-0599[↩][↩]
- van Wijngaarden JP, Dhonukshe-Rutten RA, van Schoor NM, van der Velde N, Swart KM, Enneman AW, van Dijk SC, Brouwer-Brolsma EM, Zillikens MC, van Meurs JB, Brug J, Uitterlinden AG, Lips P, de Groot LC. Rationale and design of the B-PROOF study, a randomized controlled trial on the effect of supplemental intake of vitamin B12 and folic acid on fracture incidence. BMC Geriatr. 2011 Dec 2;11:80. doi: 10.1186/1471-2318-11-80[↩]
- Lukaski HC. Vitamin and mineral status: effects on physical performance. Nutrition 2004;20:632-44. https://www.ncbi.nlm.nih.gov/pubmed/15212745[↩]
- Jung YP, Earnest CP, Koozehchian M, Galvan E, Dalton R, Walker D, Rasmussen C, Murano PS, Greenwood M, Kreider RB. Effects of acute ingestion of a pre-workout dietary supplement with and without p-synephrine on resting energy expenditure, cognitive function and exercise performance. J Int Soc Sports Nutr. 2017 Jan 12;14:3. doi: 10.1186/s12970-016-0159-2[↩]
- National Institute of Health. Vitamin B-12. https://ods.od.nih.gov/factsheets/VitaminB12-HealthProfessional/[↩]
- Damayanti D, Jaceldo-Siegl K, Beeson WL, Fraser G, Oda K, Haddad EH. Foods and Supplements Associated with Vitamin B12 Biomarkers among Vegetarian and Non-Vegetarian Participants of the Adventist Health Study-2 (AHS-2) Calibration Study. Nutrients. 2018 Jun 4;10(6):722. doi: 10.3390/nu10060722[↩]
- Henjum S, Manger M, Hampel D, Brantsæter AL, Shahab-Ferdows S, Bastani NE, Strand TA, Refsum H, Allen LH. Vitamin B12 concentrations in milk from Norwegian women during the six first months of lactation. Eur J Clin Nutr. 2020 May;74(5):749-756. doi: 10.1038/s41430-020-0567-x[↩]
- U.S. Food and Drug Adminstration. Code of Federal Regulations Title 21 § 107 – Infant Formula. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=107.100[↩]
- Doets EL, In ‘t Veld PH, Szczecińska A, Dhonukshe-Rutten RA, Cavelaars AE, van ‘t Veer P, Brzozowska A, de Groot LC. Systematic review on daily vitamin B12 losses and bioavailability for deriving recommendations on vitamin B12 intake with the factorial approach. Ann Nutr Metab. 2013;62(4):311-22. doi: 10.1159/000346968[↩]
- Allen LH. Bioavailability of vitamin B12. Int J Vitam Nutr Res. 2010 Oct;80(4-5):330-5. doi: 10.1024/0300-9831/a000041[↩]
- U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 27. Nutrient Data Laboratory home page, 2014. https://ndb.nal.usda.gov/ndb/[↩]
- Bernard MA, Nakonezny PA, Kashner TM. The effect of Vitamin B-12 deficiency on older veterans and its relationship to health. J Am Geriatr Soc 1998;46:1199-206. https://www.ncbi.nlm.nih.gov/pubmed/9777900%20?dopt=Abstract[↩]
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B-12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academy Press, 1998.[↩][↩][↩][↩]
- Healton EB, Savage DG, Brust JC, Garrett TF, Lindenbaum J. Neurological aspects of cobalamin deficiency. Medicine 1991;70:229-44.[↩]
- Bottiglieri T. Folate, Vitamin B-12, and neuropsychiatric disorders. Nutr Rev 1996;54:382-90. https://www.ncbi.nlm.nih.gov/pubmed/9155210?dopt=Abstract[↩]
- Bjørke Monsen AL, Ueland PM. Homocysteine and methylmalonic acid in diagnosis and risk assessment from infancy to adolescence. Am J Clin Nutr. 2003 Jul;78(1):7-21. doi: 10.1093/ajcn/78.1.7[↩]
- Watanabe F, Yabuta Y, Tanioka Y, Bito T. Biologically active vitamin B12 compounds in foods for preventing deficiency among vegetarians and elderly subjects. J Agric Food Chem. 2013 Jul 17;61(28):6769-75. doi: 10.1021/jf401545z[↩]
- Pawlak R, Parrott SJ, Raj S, Cullum-Dugan D, Lucus D. How prevalent is vitamin B(12) deficiency among vegetarians? Nutr Rev. 2013 Feb;71(2):110-7. doi: 10.1111/nure.12001[↩]
- Pawlak R. Is vitamin B12 deficiency a risk factor for cardiovascular disease in vegetarians? Am J Prev Med. 2015 Jun;48(6):e11-26. doi: 10.1016/j.amepre.2015.02.00[↩]
- Hunt A, Harrington D, Robinson S. Vitamin B12 deficiency. BMJ. 2014 Sep 4;349:g5226. doi: 10.1136/bmj.g5226[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- KATO N, NARITA Y, KAMOHARA S. Liver vitamin B 12 levels in chronic liver diseases. J Vitaminol (Kyoto). 1959 Jun 10;5:134-40. doi: 10.5925/jnsv1954.5.134[↩]
- Ståhlberg KG, Radner S, Nordén A. Liver B12 in subjects with and without vitamin B12 deficiency. A quantitative and qualitative study. Scand J Haematol. 1967;4(4):312-30. doi: 10.1111/j.1600-0609.1967.tb01632.x[↩]
- Adams JF, Boddy K, Douglas AS. Interrelation of serum vitamin B 12 , total body vitamin B 12 , peripheral blood morphology and the nature of erythropoiesis. Br J Haematol. 1972 Sep;23(3):297-305. doi: 10.1111/j.1365-2141.1972.tb08876.x[↩]
- GRASBECK R, NYBERG W, REIZENSTEIN P. Biliary and fecal vit. B12 excretion in man: an isotope study. Proc Soc Exp Biol Med. 1958 Apr;97(4):780-4. doi: 10.3181/00379727-97-23879[↩][↩]
- Adams JF. The measurement of the total assayable vitamin B12 in the body. In: Heinrich HC, editor. Vitamin B12 und Intrinsic Faktor. Stuttgart, Germany: Ferdinand Enke; 1962. pp. 397–403.[↩]
- Adams JF, Tankel HI, MacEwan F. Estimation of the total body vitamin B12 in the live subject. Clin Sci. 1970 Jul;39(1):107-13. doi: 10.1042/cs0390107[↩]
- HEINRICH HC. METABOLIC BASIS OF THE DIAGNOSIS AND THERAPY OF VITAMIN B 12 DEFICIENCY. Semin Hematol. 1964 Jul;1:199-249.[↩][↩][↩]
- Reizenstein P, Ek G, Matthews CM. Vitamin B-12 kinetics in man. Implications on total-body-B-12-determinations, human requriements, and normal and pathological cellular B12 uptake. Phys Med Biol. 1966 Apr;11(2):295-306. doi: 10.1088/0031-9155/11/2/309[↩][↩]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington (DC): National Academies Press (US); 1998. 9, Vitamin B12. Available from: https://www.ncbi.nlm.nih.gov/books/NBK114302[↩][↩]
- REIZENSTEIN PG. Excretion of non-labeled vitamin B12 in man. Acta Med Scand. 1959 Nov 18;165:313-9. doi: 10.1111/j.0954-6820.1959.tb14505.x[↩]
- Adams JF. Correlation of serum and urine vitamin B12. Br Med J. 1970 Jan 17;1(5689):138-9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1699041/pdf/brmedj02270-0028.pdf[↩]
- MOLLIN DL, ROSS GI. The vitamin B12 concentrations of serum and urine of normals and of patients with megaloblastic anaemias and other diseases. J Clin Pathol. 1952 May;5(2):129-39. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1023543/pdf/jclinpath00019-0001.pdf[↩]
- Amin S, Spinks T, Ranicar A, Short MD, Hoffbrand AV. Long-term clearance of [57Co]cyanocobalamin in vegans and pernicious anaemia. Clin Sci (Lond). 1980 Jan;58(1):101-3. doi: 10.1042/cs0580101[↩]
- Boddy K, Adams JF. The long-term relationship between serum vitamin B12 and total body vitamin B12. Am J Clin Nutr. 1972 Apr;25(4):395-400. doi: 10.1093/ajcn/25.4.395[↩]
- Bozian RC, Ferguson JL, Heyssel RM, Meneely GR, Darby WJ. Evidence concerning the human requirement for vitamin B12. Use of the whole body counter for determination of absorption of vitamin B12. Am J Clin Nutr. 1963 Feb;12:117-29. doi: 10.1093/ajcn/12.2.117[↩]
- Heyssel RM, Bozian RC, Darby WJ, Bell MC. Vitamin B12 turnover in man. The assimilation of vitamin B12 from natural foodstuff by man and estimates of minimal daily dietary requirements. Am J Clin Nutr. 1966 Mar;18(3):176-84. doi: 10.1093/ajcn/18.3.176[↩]
- Bailey RL, Carmel R, Green R, Pfeiffer CM, Cogswell ME, Osterloh JD, Sempos CT, Yetley EA. Monitoring of vitamin B-12 nutritional status in the United States by using plasma methylmalonic acid and serum vitamin B-12. Am J Clin Nutr. 2011 Aug;94(2):552-61. doi: 10.3945/ajcn.111.015222[↩][↩]
- Langan RC, Zawistoski KJ. Update on vitamin B12 deficiency. Am Fam Physician. 2011 Jun 15;83(12):1425-30. https://www.aafp.org/pubs/afp/issues/2011/0615/p1425.html[↩][↩][↩][↩][↩]
- Stabler SP. Clinical practice. Vitamin B12 deficiency. N Engl J Med. 2013 Jan 10;368(2):149-60. doi: 10.1056/NEJMcp1113996[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Dali-Youcef N, Andrès E. An update on cobalamin deficiency in adults. QJM. 2009 Jan;102(1):17-28. doi: 10.1093/qjmed/hcn138[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Toh BH, van Driel IR, Gleeson PA. Pernicious anemia. N Engl J Med. 1997 Nov 13;337(20):1441-8. doi: 10.1056/NEJM199711133372007[↩]
- de Jager J, Kooy A, Lehert P, Wulffelé MG, van der Kolk J, Bets D, Verburg J, Donker AJ, Stehouwer CD. Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo controlled trial. BMJ. 2010 May 20;340:c2181. doi: 10.1136/bmj.c2181[↩][↩][↩][↩]
- Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013 Dec 11;310(22):2435-42. doi: 10.1001/jama.2013.280490[↩][↩][↩]
- Devalia, V., Hamilton, M.S., Molloy, A.M. and (2014), Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol, 166: 496-513. https://doi.org/10.1111/bjh.12959[↩][↩][↩][↩][↩]
- Stoopler ET, Kuperstein AS. Glossitis secondary to vitamin B12 deficiency anemia. CMAJ. 2013 Sep 3;185(12):E582. doi: 10.1503/cmaj.120970[↩]
- Chanarin I. Adverse effects of increased dietary folate. Relation to measures to reduce the incidence of neural tube defects. Clin Invest Med 1994;17:244-52. https://www.ncbi.nlm.nih.gov/pubmed/7924000?dopt=Abstract[↩]
- Johnson MA. If high folic acid aggravates Vitamin B-12 deficiency what should be done about it? Nutr Rev 2007;65:451-8. https://www.ncbi.nlm.nih.gov/pubmed/17972439?dopt=Abstract[↩]
- Kozyraki R, Cases O. Vitamin B12 absorption: mammalian physiology and acquired and inherited disorders. Biochimie. 2013 May;95(5):1002-7. doi: 10.1016/j.biochi.2012.11.004[↩][↩][↩]
- Infante M, Leoni M, Caprio M, Fabbri A. Long-term metformin therapy and vitamin B12 deficiency: An association to bear in mind. World J Diabetes. 2021 Jul 15;12(7):916-931. doi: 10.4239/wjd.v12.i7.916[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Devalia V, Hamilton MS, Molloy AM; British Committee for Standards in Haematology. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol. 2014 Aug;166(4):496-513. doi: 10.1111/bjh.12959[↩][↩][↩][↩][↩][↩][↩]
- Vitamin B-12 Associated Neurological Diseases. https://emedicine.medscape.com/article/1152670-overview[↩]
- Crafa P, Russo M, Miraglia C, et al. From Sidney to OLGA: an overview of atrophic gastritis. Acta Biomed. 2018 Dec 17. 89(8-S):93-9.[↩][↩]
- Baik HW, Russell RM. Vitamin B12 deficiency in the elderly. Annu Rev Nutr. 1999;19:357-77. doi: 10.1146/annurev.nutr.19.1.357[↩][↩][↩]
- Neumann WL, Coss E, Rugge M, Genta RM. Autoimmune atrophic gastritis–pathogenesis, pathology and management. Nat Rev Gastroenterol Hepatol. 2013 Sep;10(9):529-41. doi: 10.1038/nrgastro.2013.101[↩]
- Raza M, Bhatt H. Atrophic Gastritis. [Updated 2022 Jul 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK563275[↩]
- Lahner, E., Persechino, S. and Annibale, B. (2012), Micronutrients (Other than iron) and Helicobacter pylori Infection: A Systematic Review. Helicobacter, 17: 1-15. https://doi.org/10.1111/j.1523-5378.2011.00892.x[↩]
- Rojas Hernandez CM, Oo TH. Advances in mechanisms, diagnosis, and treatment of pernicious anemia. Discov Med. 2015 Mar;19(104):159-68. https://www.discoverymedicine.com/Cristhiam-M-Rojas-Hernandez/2015/03/advances-in-mechanisms-diagnosis-and-treatment-of-pernicious-anemia[↩][↩][↩][↩][↩][↩]
- Annibale B., Lahner E., Fave G.D. Diagnosis and management of pernicious anemia. Curr. Gastroenterol. Rep. 2011;13:518–524. doi: 10.1007/s11894-011-0225-5[↩]
- Green R, Allen LH, Bjørke-Monsen AL, Brito A, Guéant JL, Miller JW, Molloy AM, Nexo E, Stabler S, Toh BH, Ueland PM, Yajnik C. Vitamin B12 deficiency. Nat Rev Dis Primers. 2017 Jun 29;3:17040. doi: 10.1038/nrdp.2017.40. Erratum in: Nat Rev Dis Primers. 2017 Jul 20;3:17054[↩][↩][↩][↩]
- Carabotti M., Annibale B., Lahner E. Common Pitfalls in the Management of Patients with Micronutrient Deficiency: Keep in Mind the Stomach. Nutrients. 2021;13:208. doi: 10.3390/nu13010208[↩]
- Esposito G, Dottori L, Pivetta G, Ligato I, Dilaghi E, Lahner E. Pernicious Anemia: The Hematological Presentation of a Multifaceted Disorder Caused by Cobalamin Deficiency. Nutrients. 2022 Apr 17;14(8):1672. doi: 10.3390/nu14081672[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Lenti M.V., Rugge M., Lahner E., Miceli E., Toh B.H., Genta R.M., De Block C., Hershko C., Di Sabatino A. Autoimmune gastritis. Nat. Rev. Dis. Primers. 2020;6:56. doi: 10.1038/s41572-020-0187-8[↩]
- Lenti M.V., Miceli E., Cococcia S., Klersy C., Staiani M., Guglielmi F., Giuffrida P., Vanoli A., Luinetti O., De Grazia F., et al. Determinants of diagnostic delay in autoimmune atrophic gastritis. Aliment. Pharmacol. Ther. 2019;50:167–175. doi: 10.1111/apt.15317[↩]
- Murphy G, Dawsey SM, Engels EA, Ricker W, Parsons R, Etemadi A, Lin SW, Abnet CC, Freedman ND. Cancer Risk After Pernicious Anemia in the US Elderly Population. Clin Gastroenterol Hepatol. 2015 Dec;13(13):2282-9.e1-4. doi: 10.1016/j.cgh.2015.05.040[↩][↩]
- Pernicious Anemia. https://emedicine.medscape.com/article/204930-overview#a6[↩][↩]
- Chan JC, Liu HS, Kho BC, Lau TK, Li VL, Chan FH, Leong IS, Pang HK, Lee CK, Liang YS. Longitudinal study of Chinese patients with pernicious anaemia. Postgrad Med J. 2008 Dec;84(998):644-50. doi: 10.1136/pgmj.2007.06742[↩][↩][↩][↩]
- Carmel R. Megaloblastic anemias. Curr Opin Hematol. 1994 Mar;1(2):107-12.[↩]
- Banka S, Ryan K, Thomson W, Newman WG. Pernicious anemia – genetic insights. Autoimmun Rev. 2011 Jun;10(8):455-9. doi: 10.1016/j.autrev.2011.01.009[↩]
- Checchi S, Montanaro A, Ciuoli C, Brusco L, Pasqui L, Fioravanti C, Sestini F, Pacini F. Prevalence of parietal cell antibodies in a large cohort of patients with autoimmune thyroiditis. Thyroid. 2010 Dec;20(12):1385-9. doi: 10.1089/thy.2010.0041[↩]
- Ho C, Kauwell GP, Bailey LB. Practitioners’ guide to meeting the vitamin B-12 recommended dietary allowance for people aged 51 years and older. J Am Diet Assoc. 1999 Jun;99(6):725-7. doi: 10.1016/S0002-8223(99)00174-1[↩]
- Watkins D, Rosenblatt DS. Lessons in biology from patients with inborn errors of vitamin B12 metabolism. Biochimie. 2013 May;95(5):1019-22. doi: 10.1016/j.biochi.2013.01.013[↩]
- Dharmarajan TS, Kanagala MR, Murakonda P, Lebelt AS, Norkus EP. Do acid-lowering agents affect vitamin B12 status in older adults? J Am Med Dir Assoc. 2008 Mar;9(3):162-7. doi: 10.1016/j.jamda.2007.10.004[↩]
- Wilhelm SM, Rjater RG, Kale-Pradhan PB. Perils and pitfalls of long-term effects of proton pump inhibitors. Expert Rev Clin Pharmacol. 2013 Jul;6(4):443-51. doi: 10.1586/17512433.2013.811206[↩]
- Valuck RJ, Ruscin JM. A case-control study on adverse effects: H2 blocker or proton pump inhibitor use and risk of vitamin B12 deficiency in older adults. J Clin Epidemiol. 2004 Apr;57(4):422-8. doi: 10.1016/j.jclinepi.2003.08.015[↩]
- Termanini B, Gibril F, Sutliff VE, Yu F, Venzon DJ, Jensen RT. Effect of long-term gastric acid suppressive therapy on serum vitamin B12 levels in patients with Zollinger-Ellison syndrome. Am J Med. 1998 May;104(5):422-30. doi: 10.1016/s0002-9343(98)00087-4[↩]
- Bauman WA, Shaw S, Jayatilleke E, Spungen AM, Herbert V. Increased intake of calcium reverses vitamin B12 malabsorption induced by metformin. Diabetes Care. 2000 Sep;23(9):1227-31. doi: 10.2337/diacare.23.9.1227[↩]
- Obeid R. Metformin causing vitamin B12 deficiency: a guilty verdict without sufficient evidence. Diabetes Care. 2014 Feb;37(2):e22-3. doi: 10.2337/dc13-2278[↩]
- Mazokopakis EE, Starakis IK. Recommendations for diagnosis and management of metformin-induced vitamin B12 (Cbl) deficiency. Diabetes Res Clin Pract. 2012 Sep;97(3):359-67. doi: 10.1016/j.diabres.2012.06.001[↩]
- Simon JA, Hudes ES. Relation of serum ascorbic acid to serum vitamin B12, serum ferritin, and kidney stones in US adults. Arch Intern Med. 1999 Mar 22;159(6):619-24. doi: 10.1001/archinte.159.6.619[↩]
- Weir DG, Scott JM. Vitamin B12 “Cobalamin.” In: Shils M, ed. Nutrition in Health and Disease. 9th ed. Baltimore: Williams & Wilkins; 1999:447-458.[↩][↩][↩]
- Food and Nutrition Board, Institute of Medicine. Vitamin B12. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, D.C.: National Academy Press; 1998:306-356. https://nap.nationalacademies.org/read/6015/chapter/1[↩]
- Andrès E, Federici L, Affenberger S, Vidal-Alaball J, Loukili NH, Zimmer J, et al. B12 deficiency: a look beyond pernicious anemia. J Fam Pract 2007;56:537-42. https://www.ncbi.nlm.nih.gov/pubmed/17605945?dopt=Abstract[↩]
- Wong CW. Vitamin B12 deficiency in the elderly: is it worth screening? Hong Kong Med J. 2015 Apr;21(2):155-64. doi: 10.12809/hkmj144383[↩]
- Pfisterer KJ, Sharratt MT, Heckman GG, Keller HH. Vitamin B12 status in older adults living in Ontario long-term care homes: prevalence and incidence of deficiency with supplementation as a protective factor. Appl Physiol Nutr Metab. 2016 Feb;41(2):219-22. doi: 10.1139/apnm-2015-0565[↩][↩]
- Cavalcoli F, Zilli A, Conte D, Massironi S. Micronutrient deficiencies in patients with chronic atrophic autoimmune gastritis: A review. World J Gastroenterol. 2017 Jan 28;23(4):563-572. doi: 10.3748/wjg.v23.i4.563[↩][↩][↩][↩]
- WECK, M.N., STEGMAIER, C., ROTHENBACHER, D. and BRENNER, H. (2007), Epidemiology of chronic atrophic gastritis: population-based study among 9444 older adults from Germany. Alimentary Pharmacology & Therapeutics, 26: 879-887. https://doi.org/10.1111/j.1365-2036.2007.03430.x[↩]
- Kalkan, Ç., Karakaya, F., Tüzün, A., Gençtürk, Z. Bıı., and Soykan, I. (2016) Factors related to low serum vitamin B12 levels in elderly patients with non-atrophic gastritis in contrast to patients with normal vitamin B12 levels. Geriatr Gerontol Int, 16: 686– 692. doi: 10.1111/ggi.12537[↩]
- Ao M, Tsuji H, Shide K, Kosaka Y, Noda A, Inagaki N, Nakase H, Tanaka K. High prevalence of vitamin B-12 insufficiency in patients with Crohn’s disease. Asia Pac J Clin Nutr. 2017;26(6):1076-1081. doi: 10.6133/apjcn.022017.13[↩]
- Bledsoe AC, King KS, Larson JJ, Snyder M, Absah I, Choung RS, Murray JA. Micronutrient Deficiencies Are Common in Contemporary Celiac Disease Despite Lack of Overt Malabsorption Symptoms. Mayo Clin Proc. 2019 Jul;94(7):1253-1260. doi: 10.1016/j.mayocp.2018.11.036[↩]
- Mark G. Ward, MBBS, Viraj C. Kariyawasam, MBBS, Sathis B. Mogan, MBBS, Kamal V. Patel, BSc, MBBS, Maria Pantelidou, MBBS, BSc, DPMSA, Agata Sobczyńska-Malefora, MSc, PhD, François Porté, MBBS, BSc, Nyree Griffin, MBChB, MD, Simon H. C. Anderson, MD, Jeremy D. Sanderson, MD, Dominic J. Harrington, BSc, MSc, PhD, Peter M. Irving, MA, MD, Prevalence and Risk Factors for Functional Vitamin B12 Deficiency in Patients with Crohn’s Disease, Inflammatory Bowel Diseases, Volume 21, Issue 12, 1 December 2015, Pages 2839–2847, https://doi.org/10.1097/MIB.0000000000000559[↩]
- Pan Y, Liu Y, Guo H, Jabir MS, Liu X, Cui W, Li D. Associations between Folate and Vitamin B12 Levels and Inflammatory Bowel Disease: A Meta-Analysis. Nutrients. 2017 Apr 13;9(4):382. doi: 10.3390/nu9040382[↩]
- Gomollón F, Gargallo CJ, Muñoz JF, Vicente R, Lue A, Mir A, García-Alvarado M, Gracia M, García-López S. Oral Cyanocobalamin is Effective in the Treatment of Vitamin B12 Deficiency in Crohn’s Disease. Nutrients. 2017 Mar 20;9(3):308. doi: 10.3390/nu9030308[↩]
- Kornerup LS, Hvas CL, Abild CB, Richelsen B, Nexo E. Early changes in vitamin B12 uptake and biomarker status following Roux-en-Y gastric bypass and sleeve gastrectomy. Clin Nutr. 2019 Apr;38(2):906-911. doi: 10.1016/j.clnu.2018.02.007[↩][↩]
- Dogan K, Aarts EO, Koehestanie P, Betzel B, Ploeger N, de Boer H, Aufenacker TJ, van Laarhoven KJHM, Janssen IMC, Berends FJ. Optimization of vitamin suppletion after Roux-en-Y gastric bypass surgery can lower postoperative deficiencies: a randomized controlled trial. Medicine (Baltimore). 2014 Nov;93(25):e169. doi: 10.1097/MD.0000000000000169[↩][↩]
- Wendy Schijns, Jens Homan, Leah van der Meer, Ignace M Janssen, Cees J van Laarhoven, Frits J Berends, Edo O Aarts, Efficacy of oral compared with intramuscular vitamin B-12 supplementation after Roux-en-Y gastric bypass: a randomized controlled trial, The American Journal of Clinical Nutrition, Volume 108, Issue 1, July 2018, Pages 6–12, https://doi.org/10.1093/ajcn/nqy072[↩]
- Pawlak R, Lester SE, Babatunde T. The prevalence of cobalamin deficiency among vegetarians assessed by serum vitamin B12: a review of literature. Eur J Clin Nutr. 2014 May;68(5):541-8. doi: 10.1038/ejcn.2014.46. Epub 2014 Mar 26. Erratum in: Eur J Clin Nutr. 2016 Jul;70(7):866.[↩][↩]
- Dror DK, Allen LH. Vitamin B-12 in Human Milk: A Systematic Review. Adv Nutr. 2018 May 1;9(suppl_1):358S-366S. doi: 10.1093/advances/nmx019[↩][↩]
- Piccoli, GB, Clari, R, Vigotti, FN, Leone, F, Attini, R, Cabiddu, G, Mauro, G, Castelluccia, N, Colombi, N, Capizzi, I, Pani, A, Todros, T, Avagnina, P. Vegan–vegetarian diets in pregnancy: danger or panacea? A systematic narrative review. BJOG 2015; 122: 623– 633. https://doi.org/10.1111/1471-0528.13280[↩]
- Mechanick JI, Youdim A, Jones DB, Timothy Garvey W, Hurley DL, Molly McMahon M, Heinberg LJ, Kushner R, Adams TD, Shikora S, Dixon JB, Brethauer S. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient–2013 update: cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Surg Obes Relat Dis. 2013 Mar-Apr;9(2):159-91. doi: 10.1016/j.soard.2012.12.010[↩]
- Carmel R. Current concepts in cobalamin deficiency. Annu Rev Med. 2000;51:357-75. doi: 10.1146/annurev.med.51.1.357[↩]
- Active B12 assay for diagnosing vitamin B12 deficiency. https://www.nice.org.uk/advice/mib40/resources/active-b12-assay-for-diagnosing-vitamin-b12-deficiency-pdf-63499159342789[↩]
- Reynolds E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006 Nov;5(11):949-60. doi: 10.1016/S1474-4422(06)70598-1[↩][↩][↩][↩]
- Molloy AM, Kirke PN, Troendle JF, Burke H, Sutton M, Brody LC, Scott JM, Mills JL. Maternal vitamin B12 status and risk of neural tube defects in a population with high neural tube defect prevalence and no folic Acid fortification. Pediatrics. 2009 Mar;123(3):917-23. doi: 10.1542/peds.2008-1173[↩][↩]
- Dror DK, Allen LH. Effect of vitamin B12 deficiency on neurodevelopment in infants: current knowledge and possible mechanisms. Nutr Rev. 2008 May;66(5):250-5. doi: 10.1111/j.1753-4887.2008.00031.x[↩][↩]
- Centers for Disease Control and Prevention (CDC). Neurologic impairment in children associated with maternal dietary deficiency of cobalamin–Georgia, 2001. MMWR Morb Mortal Wkly Rep. 2003 Jan 31;52(4):61-4. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5204a1.htm[↩][↩]
- Hay G, Johnston C, Whitelaw A, Trygg K, Refsum H. Folate and cobalamin status in relation to breastfeeding and weaning in healthy infants. Am J Clin Nutr. 2008 Jul;88(1):105-14. doi: 10.1093/ajcn/88.1.105[↩][↩]
- How to Cite this Article: Derin, S, Koseoglu, S, Sahin, C, Sahan, M. Effect of vitamin B12 deficiency on olfactory function. Int Forum Allergy Rhinol. 2016; 6: 1051– 1055. https://doi.org/10.1002/alr.21790[↩]
- Green R. Vitamin B12 deficiency from the perspective of a practicing hematologist. Blood. 2017 May 11;129(19):2603-2611. doi: 10.1182/blood-2016-10-569186[↩]
- Pernicious anemia. https://medlineplus.gov/ency/article/000569.htm[↩]
- Hemmer B., Glocker F.X., Schumacher M., Deuschl G., Lücking C.H. Subacute combined degeneration: Clinical, electrophysiological, and magnetic resonance imaging findings. J. Neurol. Neurosurg. Psychiatry. 1998;65:822–827. doi: 10.1136/jnnp.65.6.822[↩][↩]
- Maamar M., Mezalek Z.T., Harmouche H., Adnaoui M., Aouni M., Maaouni A. Contribution of spinal MRI for unsuspected cobalamin deficiency in isolated sub-acute combined degeneration. Eur. J. Intern. Med. 2008;19:143–145. doi: 10.1016/j.ejim.2007.03.017[↩]
- Merck Sharp & Dohme Corp., Merck Manual. Vitamin B 12. https://www.merckmanuals.com/professional/nutritional-disorders/vitamin-deficiency,-dependency,-and-toxicity/vitamin-b-12[↩]
- Oo TH, Rojas-Hernandez CM. Challenging clinical presentations of pernicious anemia. Discov Med. 2017 Sep;24(131):107-115. https://www.discoverymedicine.com/Thein-H-Oo/2017/09/challenging-clinical-presentations-of-pernicious-anemia[↩]
- Herbert V. Vitamin B-12. In: Ziegler EE, Filer LJ, eds. Present Knowledge in Nutrition. 7th ed. Washington D.C.: ILSI Press; 1996:191-205.[↩]
- Healton E.B., Savage D.G., Brust J.C., Garrett T.J., Lindenbaum J. Neurologic aspects of cobalamin deficiency. Medicine. 1991;70:229–245. doi: 10.1097/00005792-199107000-00001[↩][↩]
- Gwathmey K.G., Grogan J. Nutritional neuropathies. Muscle Nerve. 2020;62:13–29. doi: 10.1002/mus.26783[↩][↩]
- Kumar N. Nutritional neuropathies. Neurol. Clin. 2007;25:209–255. doi: 10.1016/j.ncl.2006.11.001[↩]
- Briani C., Dalla Torre C., Citton V., Manara R., Pompanin S., Binotto G., Adami F. Cobalamin deficiency: Clinical picture and radiological findings. Nutrients. 2013;5:4521–4539. doi: 10.3390/nu5114521[↩]
- Scalabrino G. The multi-faceted basis of vitamin B12 (cobalamin) neurotrophism in adult central nervous system: Lessons learned from its deficiency. Prog Neurobiol. 2009 Jul;88(3):203-20. doi: 10.1016/j.pneurobio.2009.04.004[↩]
- Azzini E, Raguzzini A, Polito A. A Brief Review on Vitamin B12 Deficiency Looking at Some Case Study Reports in Adults. Int J Mol Sci. 2021 Sep 7;22(18):9694. doi: 10.3390/ijms22189694[↩][↩]
- Remacha A.F., Souto J.C., Piñana J.L., Sardà M.P., Queraltó J.M., Martí-Fàbregas J., Garcia-Moll X., Fernández C., Rodríguez Á., Cuesta J. Vitamin B12 deficiency, hyperhomocysteinemia and thrombosis: A case and control study. Int. J. Hematol. 2011;93:458–464. doi: 10.1007/s12185-011-0825-8[↩]
- Ospina-Romero M., Cannegieter S.C., Heijer M.D., Doggen C.J.M., Rosendaal F.R., Lijfering W.M. Hyperhomocysteinemia and Risk of First Venous Thrombosis: The Influence of (Unmeasured) Confounding Factors. Am. J. Epidemiol. 2018;187:1392–1400. doi: 10.1093/aje/kwy004[↩]
- Tanaka M., Taniguchi T., Saito N., Kimura T. Inferior vena cava thrombus due to hyperhomocysteinemia. J. Cardiol. Cases. 2018;18:168–170. doi: 10.1016/j.jccase.2018.07.003[↩][↩][↩]
- Kapur V., D’Cruz S., Kaur R. An uncommon presentation of hyperhomocysteinemia and vitamin B12 deficiency: A case report. J. Med. Case Rep. 2019;13:36. doi: 10.1186/s13256-019-1988-9[↩][↩][↩][↩]
- Ammouri W., Tazi Z.M., Harmouche H., Maamar M., Adnaoui M. Venous thromboembolism and hyperhomocysteinemia as first manifestation of pernicious anemia: A case series. J. Med. Case Rep. 2017;11:250. doi: 10.1186/s13256-017-1415-z[↩][↩][↩]
- Ulrich A., Muller D., Linnebank M., Tarnutzer A.A. Pitfalls in the diagnostic evaluation of subacute combined degeneration. BMJ Case Rep. 2015;2015:bcr2014208622. doi: 10.1136/bcr-2014-208622[↩]
- Kovalenko O., Kassem A.N., Jenkins M. Hyperhomocysteinemia and Pulmonary Embolism in a Young Male. Cureus. 2020;12:e7818. doi: 10.7759/cureus.7818[↩]
- Goette A., Hammwöhner M., Dierkes J., Lachmuth J., Frölich J.C., Klein H., Bode-Böger S.M. Aortic thrombus and pulmonary embolism in a patient with hyperhomocysteinemia. Nat. Clin. Pr. Neurol. 2006;3:396–399. doi: 10.1038/ncpcardio0601[↩][↩][↩]
- McCaddon A. Homocysteine and cognitive impairment; a case series in a General Practice setting. Nutr. J. 2006;5:6. doi: 10.1186/1475-2891-5-6[↩][↩][↩]
- Carmel R, Green R, Rosenblatt DS, Watkins D. Update on cobalamin, folate, and homocysteine. Hematology Am Soc Hematol Educ Program. 2003:62-81. doi: 10.1182/asheducation-2003.1.62[↩]
- Kaferle J, Strzoda CE. Evaluation of macrocytosis. Am Fam Physician. 2009 Feb 1;79(3):203-8. https://www.aafp.org/pubs/afp/issues/2009/0201/p203.html[↩]
- Stabler SP, Allen RH. Megaloblastic anemias. In: Cecil RL, Goldman L, Ausiello DA, eds. Cecil Textbook of Medicine. 22nd ed. Philadelphia, Pa.: Saunders; 2004:1050–1057.[↩]
- Obeid R, Heil SG, Verhoeven MMA, van den Heuvel EGHM, de Groot LCPGM, Eussen SJPM. Vitamin B12 Intake From Animal Foods, Biomarkers, and Health Aspects. Front Nutr. 2019 Jun 28;6:93. doi: 10.3389/fnut.2019.00093[↩][↩][↩][↩]
- Ankar A, Kumar A. Vitamin B12 Deficiency. [Updated 2022 Oct 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441923[↩]
- Oberley MJ, Yang DT. Laboratory testing for cobalamin deficiency in megaloblastic anemia. Am J Hematol. 2013 Jun;88(6):522-6. doi: 10.1002/ajh.23421[↩]
- Arendt JF, Nexo E. Cobalamin related parameters and disease patterns in patients with increased serum cobalamin levels. PLoS One. 2012;7(9):e45979. doi: 10.1371/journal.pone.0045979[↩]
- Andrès E, Serraj K, Zhu J, Vermorken AJ. The pathophysiology of elevated vitamin B12 in clinical practice. QJM. 2013 Jun;106(6):505-15. doi: 10.1093/qjmed/hct051[↩]
- Quadros EV. Advances in the understanding of cobalamin assimilation and metabolism. Br J Haematol. 2010 Jan;148(2):195-204. doi: 10.1111/j.1365-2141.2009.07937.x[↩]
- Ankar A, Kumar A. Vitamin B12 Deficiency. [Updated 2022 Oct 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441923[↩][↩][↩]
- Bromage S, Ganmaa D, Rich-Edwards JW, Rosner B, Bater J, Fawzi WW. Projected effectiveness of mandatory industrial fortification of wheat flour, milk, and edible oil with multiple micronutrients among Mongolian adults. PLoS One. 2018 Aug 2;13(8):e0201230. doi: 10.1371/journal.pone.0201230[↩]
- Homan J, Schijns W, Aarts EO, Janssen IMC, Berends FJ, de Boer H. Treatment of Vitamin and Mineral Deficiencies After Biliopancreatic Diversion With or Without Duodenal Switch: a Major Challenge. Obes Surg. 2018 Jan;28(1):234-241. doi: 10.1007/s11695-017-2841-0[↩]
- Freedberg DE, Kim LS, Yang YX. The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association. Gastroenterology. 2017 Mar;152(4):706-715. doi: 10.1053/j.gastro.2017.01.031[↩]
- BSH Guidance on B12 Replacement during the COVID-19 Pandemic, British Society for Haematology. https://b-s-h.org.uk/media/18275/bsh-guidance-b12-replacement-covid-1901052020finalv.pdf[↩][↩]
- Oh R, Brown DL. Vitamin B12 deficiency. Am Fam Physician. 2003 Mar 1;67(5):979-86. https://www.aafp.org/pubs/afp/issues/2003/0301/p979.html[↩]
- O’Leary F, Samman S. Vitamin B12 in health and disease. Nutrients. 2010 Mar;2(3):299-316. doi: 10.3390/nu2030299[↩]
- Vitamin B12 or folate deficiency anaemia treatment. https://www.nhs.uk/conditions/vitamin-b12-or-folate-deficiency-anaemia/treatment[↩]
- Vasconcelos OM, Poehm EH, McCarter RJ, Campbell WW, Quezado ZM. Potential outcome factors in subacute combined degeneration: review of observational studies. J Gen Intern Med. 2006 Oct;21(10):1063-8. doi: 10.1111/j.1525-1497.2006.00525.x[↩][↩][↩]
- Bønaa KH, Njølstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, Wang H, Nordrehaug JE, Arnesen E, Rasmussen K; NORVIT Trial Investigators. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006 Apr 13;354(15):1578-88. doi: 10.1056/NEJMoa055227[↩]
- Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, McQueen MJ, Probstfield J, Fodor G, Held C, Genest J Jr; Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006 Apr 13;354(15):1567-77. doi: 10.1056/NEJMoa060900. Epub 2006 Mar 12. Erratum in: N Engl J Med. 2006 Aug 17;355(7):746.[↩]