What is resistant starch ?
Resistant starch is a form of dietary fiber that resists degradation in the small intestine by gastrointestinal enzymes with potential chemopreventive and prebiotic activity 1. The American Association of Cereal Chemists in their annual meeting on 2001, define “Resistant starch is the portion of starch that is not digested in the human small intestine. Because humans differ in their ability to digest a particular resistant starch-containing material, there is no absolute distinction between the resistant starch and the non-resistant starch in a particular resistant starch-containing sample” 2.
While most starch is digested in the upper part of the gut, resistant starch resists digestion by amylases in the small intestine and so goes all the way to the large intestine unaltered 3, 4. Once in the large intestine, friendly anaerobic colonic bacteria ferment the resistant starch. This process produces gasses and short chain fatty acids (SCFAs) such as acetate, butyrate, and propionate, that help to keep the lining of the bowel healthy. Butyrate has anti-inflammatory and immunoregulatory activities and butyrate appears to exert antitumor effects by inhibiting tumor cell proliferation, inducing tumor cell differentiation and apoptosis in colorectal cancer cells 5.
Resistant starch is starch that is not easily absorbed. Different ways of cooking can create different amounts of resistant starch. For example, resistant starch is found in slightly undercooked (‘al dente’) pasta, cooked but cooled potatoes (including potato salad), cooked and cooled grains like rice, quinoa, barley and buckwheat, under-ripe bananas, beans, lentils and a product called Hi-maize used in some breads and breakfast cereals. Freekeh, a Middle Eastern grain available in some supermarkets, is another good source. In general, foods that are less highly processed contain more resistant starch. An important benefit of resistant starch is that it ferments, which produces substances that help to keep the lining of the bowel healthy.
By far the largest proportion of the resistant starch comes from grain products for all the countries studied and as expected the source of grain products varied with the type of food consumed in large amounts, such as pasta in Italy or rice in Asia.
Fiber consumption has been proposed to contribute to weight loss through several mechanisms, including the alteration of gut motility, the attenuation of nutrient absorption, and the lowering of overall caloric intake 6, 7. In addition, fiber consumption may also enhance satiety through other mechanisms, such as adding bulk and viscosity to the intestinal content and altering the secretion of gut hormones that influence metabolism and energy expenditure 8, 9. Improved satiety and reduced food intake are common theories used to describe why fiber intake may be associated with a lower body weight 10.
Growing evidence shows that many of the chronic health conditions in developed countries could be prevented or moderated by dietary changes. The most common starchy foods in the United States diet, including white bread, cakes, and noodles, consist of a large percentage of highly digestible starch. There is concern that such rapidly digested starches may contribute to chronic disease in people and animals and, because of this problem, starches that are resistant to digestive enzymes have been the focus of a growing research emphasis. Such starches, termed resistant starches 11, have been extensively reviewed in general 12 and reviewed from the standpoint of their health properties 13; increasing their content in food components 14; their health and functional properties as a food ingredient 15; and their role in gut health, potentially through butyrate production 16. The estimated daily intake of resistant starch by Americans is ∼5 g per day, much less than the minimum of 6 g of resistant starch per meal recommended for health benefits 17.
Resistant Starch Classification
Englyst et al. 11 proposed a classification system based on starch digestive rate. This system divides starches into rapidly digestible starches, slowly digestible starches, and resistant starches based on the results of in vitro digestion. Much of the research with resistant starch uses high-amylose products. Note that the FDA does not allow the term “resistant starch” on food labels; Hi-maize 260, a purified resistant starch product (Ingredion), is assayed for fiber content, and that amount can be placed on the food label. This product is often referred to as high-amylose maize resistant starch type 2 18. There are currently 5 types of resistant starch (Table 1). Substantial research has been conducted on each of the 5 types of resistant starch, and they are briefly summarized next.
Table 1. Types of resistant starches
| Designation | Description | Example | Reference |
| RSI | Physically inaccessible starch | Coarsely ground or whole-kernel grains | 19 |
| RSII | Granular starch with the B- or C-polymorph | High-amylose maize starch, raw potato, raw banana starch | 19 |
| RSIII | Retrograded starch | Cooked and cooled starchy foods | 20 |
| RSIV | Chemically modified starches | Cross-linked starch and octenyl succinate starch | 21 |
| RSV | Amylose-lipid complex | Stearic acid-complexed high-amylose starch | 22 |
Resistant starch has been classified into five basic “types” 23.
- Type 1 (resistant starch 1) is made up of starch granules surrounded by an indigestible plant matrix.
- Type 2 (resistant starch 2) occurs in its natural form such as in an uncooked potato and high amylose maize.
- Type 3 (resistant starch 3) are crystallized starches made by unique cooking and cooling processes.
- Type 4 (resistant starch 4) is a starch chemically modified by esterification, crosslinking, or transglycosylation and is not found in nature.
- Type 5 (resistant starch 5) is a starch consisting of amylose-lipid complex.
Type 1 Resistant Starch
Starch is synthesized in the endosperm of cereal grains or seeds, and starch granules are surrounded by protein matrix and cell wall material. These physical structures hinder the digestibility of starch and reduce the glycemic response 24. When cooked as whole kernels or coarsely ground seeds, the thick cell wall of legume seeds and the protein matrix in cereal grains prevent water penetration into the starch in the matrix. Therefore, the starch does not have adequate moisture to readily gelatinize and swell. Without proper swelling to expose the starch molecules, the starch is not readily susceptible to enzymatic hydrolysis. The cell wall material and the protein matrix also provide a physical barrier, preventing enzymes from reaching and hydrolyzing the starch. Examples of type I resistant starch 25 –containing foods are breads made with whole or coarsely ground kernels of grains 26 and pasta made with durum wheat by extrusion 27. Durum wheat has a high protein content and hard texture and is used for making semolina with coarse particles. Consequently, the postprandial glycemic response is substantially lower after ingesting semolina pasta compared with white bread. Residual starch that is not digested in the small intestine passes into the colon as Type 1 Resistant Starch.
Type 2 Resistant Starch
Uncooked potato starch, green banana starch, gingko starch, and high-amylose maize starch, which display the B- or C-type polymorph, are highly resistant to enzymatic hydrolysis 28 and are examples of type 2 resistant starch. However, after cooking, most of the starch, such as that in baked potato and cooked banana, becomes highly digestible as a result of starch gelatinization and loss of the B- and C-type crystallites. An exception is high-amylose starch produced by mutation of the amylose-extender (ae) gene and the gene encoding starch branching-enzyme I, which has substantially longer branch chains of intermediate components and a larger proportion of amylose 29, 30, 31. Thus, this starch displays a high gelatinization temperature, above the boiling point of water. After boiling or cooking at a temperature below its gelatinization temperature, this type of starch retains its crystalline structure and remains resistant to enzymatic hydrolysis.
Type 3 Resistant Starch
Type 3 resistant starch (RSIII) is retrograded amylose and starch 32, 33, 34. Because amylose molecules have linear structures, they have a great tendency to form double helices, particularly near refrigeration temperatures (4–5°C) and with adequate moisture content. Retrograded amylose has high gelatinization temperatures, up to 170°C, and cannot be dissociated by cooking. The gelatinization temperature of retrograded amylose, however, decreases with shortening of the amylose chain length. After starchy foods are stored, particularly in a refrigerator, amylose molecules and long branch chains of amylopectin form double helices and lose their water-binding capacity. The double helices of starch molecules do not fit into the enzymatic binding site of amylase, thus they cannot be hydrolyzed by this enzyme.
Type 4 Resistant Starch
Type 4 resistant starch (RSIV) is a chemically modified starch, formed either by cross-linking 35, 36 or by adding chemical derivatives 37. Starch with a high level of cross-linking loses the ability to swell during cooking. Consequently, the highly cross-linked starch remains in a granular form after cooking, with little enzymatic susceptibility, and cannot be hydrolyzed by amylases or fermented by microbes. Adding a chemical derivative to starch, such as octenyl succinic groups 38 or acyl groups 39, changes the structure of the starch and partially restricts the enzymatic hydrolysis of the starch molecule, resulting in resistant starch. A region of the starch without the derivative can be hydrolyzed by bacteria amylases and fermented to produce short-chain fatty acids.
Type 5 Resistant Starch
When starch interacts with lipids, amylose and long branch chains of amylopectin form single-helical complexes with fatty acids and fatty alcohols 32, 40. When the linear starch chain is in a helical-complex structure with the complexed fatty acid in the cavity of the helix, starch binding and cleavage by amylase are prevented. In addition, the amylose-lipid complex also entangles amylopectin molecules, restricting the swelling of starch granules and enzyme hydrolysis 41, 42. Because the amylose-lipid complex formation is an instant reaction and the complex can reform after cooking, type 5 resistant starch (RSV) is considered thermally stable.
It is important to recognize that starch digestibility is influenced by nonstarch components in the digest, the structure of the starch, and starch processing before digestion. The digestibility of a given starch sample is never due to a single factor as classification systems suggest; rather, the extrinsic factor with the greatest influence on digestibility is generally used to classify the starch.
The botanical role of starch is to provide plants with a stable reserve of glucose for metabolism. The digestibility of starch is an important parameter in meeting this role. The glucose reserves must be stored in a structure that is readily available to the plant, yet able to survive for long periods of time in storage organs such as seeds or tubers. The structure of starch is complex and varies widely; however, the single structural aspect with the greatest influence on digestibility is the degree and type of crystallinity within the granule. Starch with long, linear chains has a greater tendency to form crystalline structures than starch with short, highly branched chains. Because the amylose component of starch is less branched than amylopectin, high-amylose starch tends be more resistant to digestion than low-amylose starch.
Resistant Starch Health Effects
Few studies have compared types, but one recent study by Haub et al. 43 reported that cross-linked resistant starch 4 elicited a greater glucose lowering effect than the more commonly tested resistant starch 2.
In this study 44, the researchers demonstrated that the acute ingestion of a beverage containing 26.8 g resistant starch type 2 from unripe bananas (native banana starch) reduced postprandial glucose, insulin response, and subsequent voluntary energy intake. However, there was no associated effect on subjective appetite ratings.
In another study on the effects of native banana starch (resistant starch type 2) and soy milk (control) on body weight and insulin sensitivity in obese type 2 diabetics 45. Thirty patients who met the eligibility criteria were randomly assigned to two groups of fifteen subjects to receive either 24 g of native banana starch dissolved in 240 mL of water per day or 24 g/day of soy milk dissolved in the same volume of water for 4 weeks. After this period all data measures were collected, groups cross over to the alternate treatment group for an additional 4 weeks period after which final measures were taken at 8 weeks. There was no wash-out period between the interventions. All participants received both supplements, and on the two phases of the experiment they were instructed not to modify their diet and exercise habits. The study have shown that native banana starch (24 g per day during 4 weeks) significantly lowers body weight and increases insulin sensitivity 45.
A majority of human studies involving resistant starch have shown a decrease in postprandial blood glucose and insulin levels. However, it is difficult to completely understand these effects due to differences in study design and the type of resistant starch used. Behall et al. 46 found that women consuming 0.71 g, 2.57 g or 5.06 g of resistant starch had significantly lower postprandial glucose and insulin levels when compared to the control. However, this study failed to maintain an equal amount of available carbohydrate between the treatments and control. Therefore, it is difficult to determine whether the attenuation of glucose and insulin was due to the resistant starch or the fact that there was less available carbohydrate in the meal. Similarly, Reader et al. 47 reported that 7.25 g of resistant starch added to an energy bar decreased blood glucose and insulin levels in healthy adults. But, ingredients, amount of ingredients and nutrient levels were different for each treatment. A recent study by Al-Tamimi et al. 48 on Glucose and Insulin Responses in Humans. Al-Tamimi EK, Seib PA, Snyder BS, Haub MD. J Nutr Metab. 2010; 2010. https://www.ncbi.nlm.nih.gov/pubmed/20798767/)), however removed these variables by controlling for non starch ingredients and available carbohydrates. It was reported that postprandial blood glucose and insulin levels were significantly reduced with the supplementation of 30 g of resistant starch 4.
Several studies report that longer term consumption of a resistant starch may decrease fasting cholesterol and triglyceride levels. In a five week study, Behall et al. 49 found that men consuming 34% of their energy from high amylose maize, when compared to a high amylopectin carbohydrate, had significantly reduced fasting cholesterol and triglyceride levels. Resier et al. 50 reported similar results in an isocaloric and isonutrient diet with either high amylose maize or fructose. Porikos and Van Itallie 51 suggest that an interaction exists between sucrose, and therefore most likely fructose, and saturated fatty acids in turn promoting serum triglyceride levels. Interestingly, the relationship does not seem to exist for polyunsaturated fatty acids. The likely mechanism behind the ability of resistant starch to decrease cholesterol levels is an increased intestinal viscosity. However, some studies, such as Jenkins et al. 52, report conflicting data as resistant starch 2 and resistant starch 3 had no effect on serum lipid profiles. While using the same type of resistant starch, subjects were only tested for two weeks. It may be that the resistant starch requires a longer period of time to promote an effect.
Research has also been conducted which evaluates the effect of resistant starch on fat oxidation and storage. However, data between studies are contradictory with no clear conclusions. Tagliabue et al. 53 reported that resistant starch 2, obtained from raw potatoes, was able to increase fat oxidation 5 h postprandial. However, the test diet, consisting of the resistant starch 2, had significantly less gross and metabolizable energy. Therefore, it is difficult to determine if the increased fat oxidation was due to the resistant starch 2 or a decreased caloric intake. A 10 week study by Howe et al. 54 may suggest the latter. High amylose starch, compared to a high amylopectin, produced no change on fat oxidation when an isocaloric diet was consumed. Conversely, Robertson et al. 55 reported that 30 g of resistant starch 2 added to healthy subjects habitual diet resulted in a significant decrease in subcutaneous abdominal adipose tissue non-esterfied fatty acid and glycerol release. This may be a result of increased peripheral short chain fatty acid metabolism or ghrelin secretions.
Resistant Starch and Gut Bacteria
It is often recognized that the mammalian gastrointestinal (GI) tract is home to more bacterial cells than comprise the entire host. It is also well established that GI microbiota make important contributions to the health of the host, including immune system development, nutritional acquisition, and protection against infection 56. In recent years, new evidence has greatly increased our understanding of microbiota impacts on host health. For example, an altered microbiota (dysbiosis) has been associated with human diseases, such as diabetes, obesity, inflammatory bowel diseases, and colorectal cancer 56. GI microbiota have recently been shown to contribute to neurological diseases and influence host behavior 57. These insights have led to a heightened interest in the role of the diet in modulation of GI microbiota as a means to improve host health 58, 59, 60 and a new awareness of how diet can contribute to disease 56, 61.
It has long been known that diet influences the microbial communities of the GI tract. Diet-induced changes in these microbiota can have beneficial effects on the health of the host through the breakdown of dietary fibers and production of SCFAs, which are an important source of energy for the host and perform important immune modulatory roles 62, 63. Although studies to understand how different classes of resistant starch affect microbiota are limited, it is clear that high-fiber diets greatly affect the composition of mammalian microbiota 25, 64. Microbiota also reduce harmful metabolites, including bile acids, phenol, and ammonia, and influence dietary fat metabolism, which influences obesity. Changes in microbiota can occur rapidly after dietary changes. These effects can be both direct and indirect, that is, bacteria that can digest resistant starch generate energy, which provides them with a growth advantage in the gut 25. Changes in community composition can also occur from decreased pH, resulting from accumulation of SCFAs 65, 66. Other by-products of resistant starch fermentation can be used by other classes of bacteria to enhance their abundance through metabolic cross-feeding 67.
Although deep-sequencing studies of the impact of resistant starches on colonic microbiota have only recently been initiated, they should help identify the mechanisms by which specific bacterial taxa interact with the different forms of starch. The interindividual variation in response to dietary resistant starch will also be an important area of investigation for the therapeutic and preventive use of resistant starch to improve human health 68.
Another topic of intense interest is the use of prebiotics to alter colonic microbiota to benefit the health of the host 69. Although typically associated with oligosaccharides, prebiotics can represent a variety of nondigestible carbohydrates, including resistant starch 70, that impart health benefits to the host through modulation of GI bacteria (129). Because of the interactions between GI microbiota and hosts, prebiotics, including resistant starches, have the potential to correct or prevent a variety of human diseases, including obesity, diabetes, inflammatory bowel diseases, and cancer 71, 72.
Resistant Starch in Prevention of Colon Cancer
Consumption of diets with abundant fiber has long been believed to protect against colorectal cancer 73. More recently, resistant starch has received attention for potential prevention of colon cancer and inflammatory bowel diseases 60. Although studies of resistant starches and human colonic health have been limited, abstracts describing 2 recent human interventions were found. In 1, a 4-wk intervention with red meat (300 g/d) increased O6-methyl-2′-deoxyguanosine adducts and genes from the microRNA-17–92 cluster (overexpressed in colorectal cancer) in the colons of humans. However, these features were not elevated with a 4-wk intervention that included red meat plus butyrylated resistant starch (40 g/d) 74. These results suggested that resistant starch may protect the human colon against potentially damaging aspects of dietary red meat. In the second human trial, with hereditary nonpolyposis colorectal cancer gene carriers (patients with Lynch syndrome) at high risk for developing colon polyps and cancer, a diet containing 30 g/d maize starch (Novelose, Ingredion) was compared with placebo diet for 29 months. No impact on polyp or colon cancer development was observed at a 4-year follow-up 75.
In contrast to the limited number of human interventions evaluating dietary resistant starch, several studies have been done on the impacts of resistant starch and colon cancer prevention in laboratory animals. Le Leu et al. 76, 77, 78, 79 conducted extensive studies in rats treated with the colon carcinogen azoxymethane and/or fed diets high in protein to damage the colonic epithelium. Feeding high fiber or resistant starches increased fecal bulk, fecal pH, butyrate concentration, and epithelial apoptosis, and it decreased cell proliferation markers and colon carcinogenesis. When animals were fed high levels of protein, the addition of high-amylose resistant starch reduced protein fermentation products, which paralleled reduced colorectal carcinoma development. In contrast to these findings, studies with potato fiber or potato resistant starch in rats fed control protein or high-protein diets revealed no impact of dietary resistant starches on DNA damage in the colon 80. It is not clear whether the contrasting results are due to differences in experimental details, including the starches fed, or other factors. DNA damage was studied in rats fed diets with casein or soy protein and resistant starch (48% high-amylose maize starch), with attenuated DNA damage observed in rats fed high-protein diets and resistant starch 81. Butyrylated high-amylose corn starch was shown to be somewhat more effective than high-amylose corn starch in the inhibition of colon cancers in rats 82. Results from Zhao et al. 83, using a novel high-amylose starch complexed with steric acid (RSV) revealed a stunning reduction in mucin-depleted foci; however, subsequent studies found that mucin-depleted foci may not be a reliable marker for subsequent colon cancer.
Numerous hypotheses have been proposed for the potential mechanism by which colon carcinogenesis may be altered by resistant starch. The most common hypotheses focus on alteration of the water-holding capacity of the fecal stream, modification of the microbiota, and increasing SCFA production. Although the SCFA hypothesis seems to have the most enthusiastic following, theories on the impacts of dietary resistant starch on the production of SCFAs and changing the microbiota are gaining momentum 84.
SCFAs (acetate, proprionate, and butyrate) are increased in amount and concentration in many studies of resistant starch and colon health 60, 73. Butyrate, an end-product of microbial fermentation of resistant starch and the primary energy source for colonocytes, is actively transported into cells by a Na+-dependent cotransporter 85. This cell membrane transporter serves as a tumor suppressor gene and is epigenetically silenced by hypermethylation in human aberrant crypt foci (a precancerous lesion) and colorectal cancer 86. Butyrate has been of particular interest because of the role this molecule plays in colonic epithelial metabolism and differentiation and its influence on signaling pathways that regulate mucosal physiology 84, 87. In cell culture, butyrate has antitumorigenic properties, including reducing cell proliferation and inducing apoptosis of colorectal tumor cell lines 84. However, it is not clear whether the concentrations of butyrate achieved in the colon of animals and humans fed resistant starches are optimal for the suppressive effects observed in cultured cells, because the concentrations of butyrate in animals fed control diets are higher than the concentrations of butyrate needed for suppression of colon proliferation 88.
Colon cancer appears to develop as the result of dysregulation of molecular pathways that control epithelial proliferation, maturation, and apoptosis, with perturbations of both genetic and epigenetic components. In regeneration of normal mucosa, stem cells deep within the crypt undergo mitosis, with subsequent maturation and differentiation of daughter cells to mature absorptive and secretory populations of the colonic mucosa and eventual loss of aged or damaged cells through apoptosis. Multistep accumulation of mutations of genes in critical control pathways, coupled with changes in epigenetic factors, is believed to allow survival of abnormal crypt epithelial cells with the potential to undergo malignant transformation.
The molecular mechanisms by which dietary resistant starches are believed to alter the development or progression of colon cancer are incompletely understood. Potential mechanisms of action of dietary resistant starch on gene expression and mutation, DNA methylation, histone modification, and remodeling of chromatin are being studied intensively. Recently, animal studies identified both alterations in the microbiota and induction of protection against unrepaired DNA damage by dietary high-amylose maize starch and butyrylated high-amylose, with increased expression of genes involved in repair of DNA 89, which, in rapidly dividing populations of the colonic mucosa, is expected to result in fewer mutations and reduced carcinogenesis. Butyrate has been also described to exert an influence on cell proliferation and differentiation through modulation of several signal transduction pathways 90. In some colon cancer cell lines, constitutive expression of the canonical Wnt pathway, an initiating event in most colorectal cancers, is upregulated by butyrate treatment, resulting in a strong apoptotic response 91. Butyrate has also been shown to influence gene expression in the colon by modulating RNA splicing 92.
The potential for modification of epigenetic mechanisms, by their nature potentially reversible, by metabolic products of resistant starch also holds promise for dietary prevention of colorectal cancer. Butyrate is well known as an inhibitor of histone deacetylase, an enzyme that modifies wrapping of strands of DNA around nuclear histone proteins and thereby regulates gene transcription. Inhibitors of histone deacetylation, such as butyrate, have the ability to modify expression of genes that control cell cycle and apoptosis and function to suppress the development of pre-neoplastic and neoplastic phenotypes in vitro 93. Furthermore, butyrate exerts protective effects against intestinal mucosal inflammation, a component of inflammation-mediated colorectal cancer, through apoptosis of T lymphocytes and inhibition of inducible nitric oxide synthase in colonic epithelium 94.
The potential contributions to colonic homeostasis by endogenous or microbial products of resistant starch metabolism other than SCFAs are undetermined. Complex interactions between microbiota, dietary components, colonic epithelium, the immune system, and the nervous and endocrine systems are being dissected, and it is likely that mechanisms integrating these components will emerge.
Resistant Starch and Diabetes
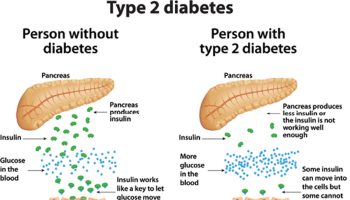
Diabetes affects 8% of the United States population and 23% of the population >60 y of age, mostly as type 2 diabetes. Both type1 and type 2 diabetes are characterized by hyperglycemia, subsequently resulting in systemic tissue toxicity. Some risk factors, including increased fasting and postprandial glucose response as well as decreased insulin sensitivity and obesity, are reversible through lifestyle modifications, which were found to be more effective than pharmacological interventions in delaying the onset of type 2 diabetes 95. One such lifestyle change is the replacement of ordinary starch in foods with resistant starch, owing to its low glycemic index. It has been reported in human studies that consuming foods, including corn porridges 96 and crackers with a high content of resistant starch resulted in lower postprandial glucose concentrations and concomitant insulin response compared with consuming foods containing ordinary starch 41, 97. In addition, consuming less digestible starches may decrease glycemic response to a subsequent meal, the “second meal effect.” Ten healthy individuals who ate high-amylose starch at breakfast showed decreased blood glucose response to a lunch containing highly digestible carbohydrate, compared with eating high-amylopectin starch at breakfast 98. Consequently, replacing ordinary dietary starch with resistant starch contributes to diabetes management. Increasing consumption of resistant starch can also aid weight management, beneficially influence body composition, or both in part because food with resistant starch has lower energy concentration and has been shown in mice to reduce body fat, an important predictor of disease 99, 100. Animal models of diabetes have also demonstrated a positive effect of dietary resistant starch, such as an improvement in glycemic control in the Goto-Kakizaki rat, a nonobese model of type 2 diabetes 101.
The potential for low glycemic index carbohydrates, such as resistant starch, to reduce diabetic complications may be related to protection of kidney function and lead to better maintenance of adequate nutritional status, particularly with respect to vitamin D. Numerous epidemiological and case-control studies have reported vitamin D insufficiency in type 1 and type 2 diabetes 102, 103, 104. However, it is unclear whether low vitamin D exposure contributed to the onset of diabetes or whether low vitamin D status was a consequence of diabetes. The major circulating form of vitamin D, 25-hydroxycholecalciferol (25D3), circulates bound to vitamin D–binding protein (DBP) until the 25D3-DBP complex is internalized through endocytosis and activated to 1,25-dihydroxycholecalciferol by the renal proximal tubule cells or reabsorbed into the blood as 25D3 (164). In both type 1 and type 2 diabetic rats, excretion of 25D3 and DBP into the urine was markedly elevated as a result of pathologies associated with reduced expression of megalin and disabled-2, which partner together to facilitate the uptake of the 25D3-DBP complex by the kidneys 105, 106. However, when cornstarch in the AIN-93G rodent diet was replaced with high-amylose maize, that is, ∼37% resistant to digestion, urinary excretion of vitamin D metabolites and DBP was virtually prevented in diabetic rats 105. Of interest was the finding that feeding diabetic rats the resistant starch had only a slight effect on attenuating fasting blood glucose concentrations, yet the renal expression of megalin and disabled-2 were normal, as confirmed in a type 2 diabetes rat model.
Resistant Starch and Obesity and Body Weight Management
Overconsumption of energy is proposed to be responsible for the obesity epidemic, and, as a consequence, new strategies are required to reduce energy intake (166). One potential dietary strategy is to increase consumption of dietary fiber, which has been associated with increased satiety and lower BMI 107, 108, 109. Dietary fiber is a diverse group of carbohydrates, and large differences exist in their physical and chemical properties. Consequently, not all sources of fiber will have the same effect on satiety or body weight 107. Resistant starches are proposed to provide many of the benefits of dietary fiber; therefore, they may aid weight management, although it has yet to be adequately demonstrated.
Accumulating evidence from rodent studies suggests that replacing rapidly digestible starch with resistant starch reduces body weight. Aziz et al. 110 found that a diet high in resistant starch reduced body weight by 40% in diet-induced obese rats. However, the diet contained 23.4% resistant starch, an amount that may not be achievable in human diets. Another study fed rats a diet containing 4%, 8%, or 16% resistant starch and found that consuming a diet with >8% resistant starch reduced adiposity compared with 0%, and for every 4% increase in resistant starch, energy intake was reduced by 9.8 kJ/d 111. Long-term studies on the effect of increasing resistant starch consumption on body weight in humans are required.
Despite the lack of long-term studies, there are several reasons to believe that consuming resistant starch could aid weight management in humans. First, because of the lower calorie content, replacing rapidly digestible starch with resistant starch reduces the energy density of the diet 112, 113. Several studies have found that reducing the energy density of the diet increases satiety and weight loss 114, 115, 116, 117.
Second, incorporating resistant starch into a meal may augment feelings of satiety. Although fiber intake has been associated with increased satiety, the effect of resistant starch on satiety is less clear. In rodent models, adding resistant starch to the diet increased secretion of the putative satiety hormones GLP-1 and PYY 112, 113, suggesting that it might augment satiety. The few studies that have been conducted in humans have provided mixed results. Willis et al. (177) provided participants with low-fiber muffins or muffins supplemented with resistant starch for breakfast and found that consuming the muffins containing resistant starch promoted satiety and increased the duration of satiety. Bodinham et al. 118 fed males 48 g of resistant starch across 2 separate meals and found no effect on subjective appetite, although food intake was reduced by ~1300 kJ over 24 h. Conversely, a recent study that fed participants a breakfast meal containing 25 g of resistant starch had no effect on subjective appetite or food intake over the remainder of the day 119. This study also found that plasma concentrations of GLP-1 were lower after the resistant starch meal.
Third, resistant starch may influence body weight by increasing energy expenditure or fat oxidation. It has been proposed that replacing rapidly digestible starch with resistant starch may promote fat mobilization as the result of a reduction in insulin secretion 120. However, currently, little evidence supports this hypothesis and several studies have failed to show that resistant starch increases energy expenditure or fat oxidation 121, 122, 123.
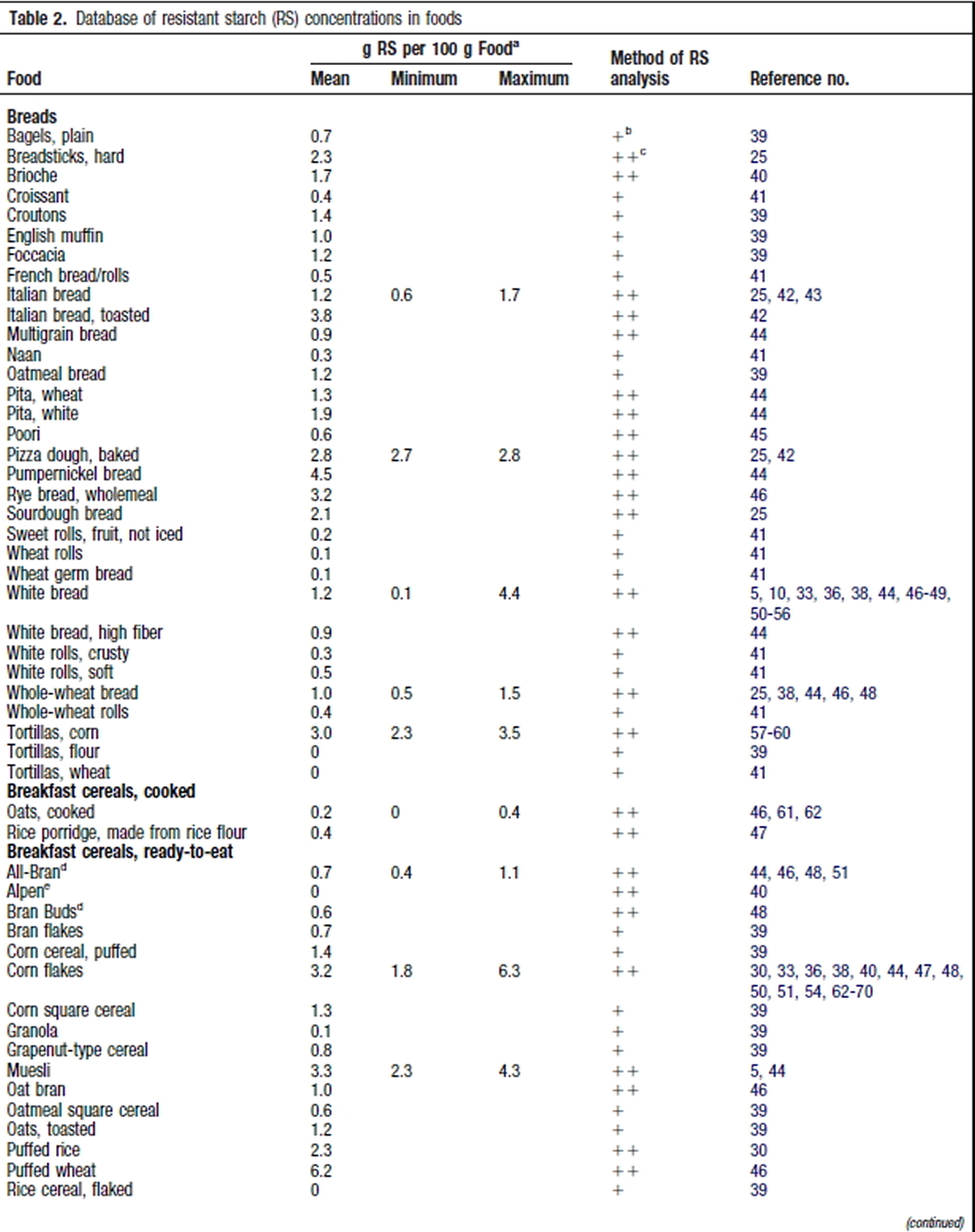
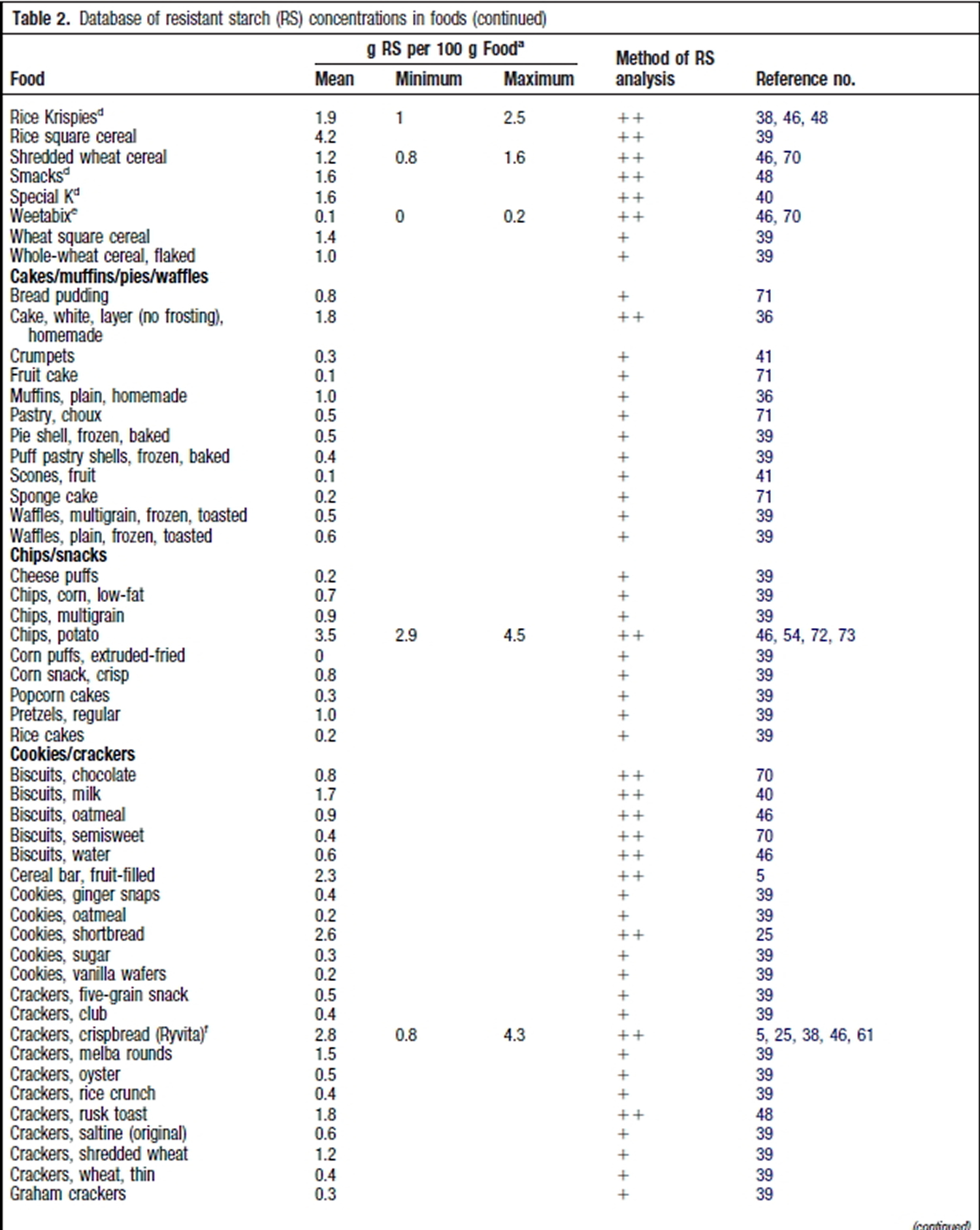
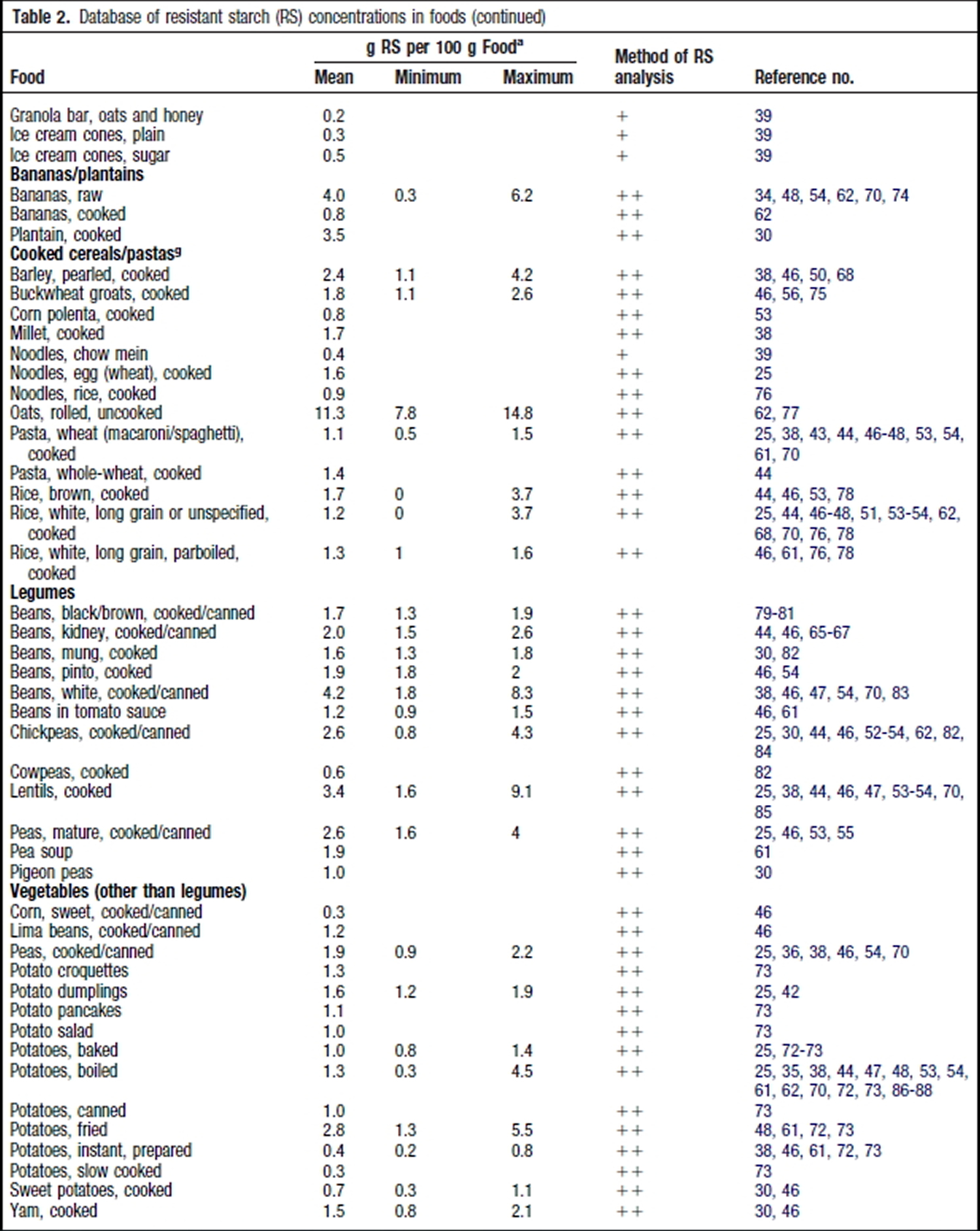
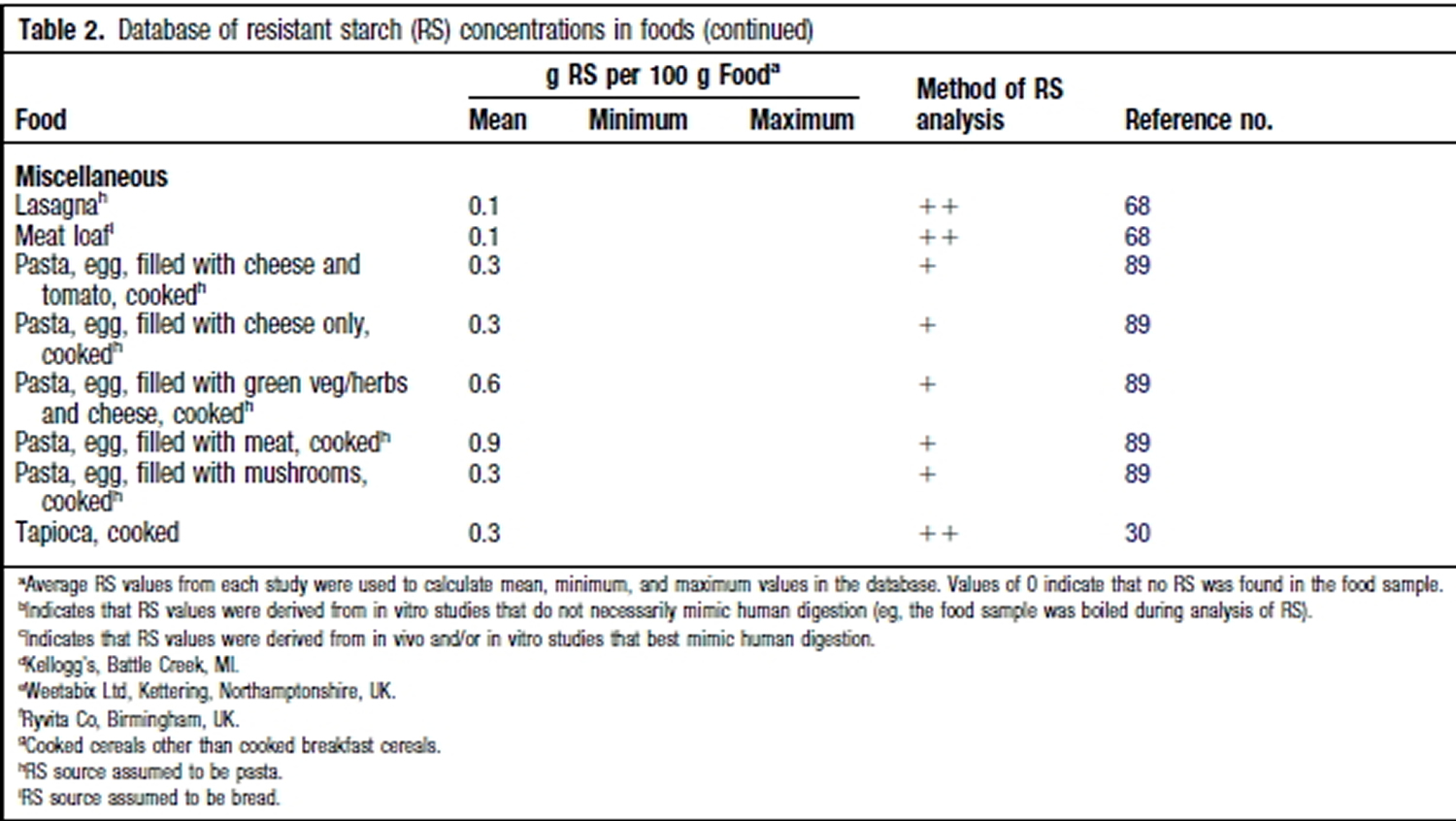
Resistant Starch Foods List
[Source: 124]- U.S. Department of Health and Human Services. Resistant Starch. https://ncit.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCI%20Thesaurus&code=C94233[↩]
- The American Association of Cereal Chemists. Resistant starch as a variable component of resistant starch-containing materials. http://www.aaccnet.org/meetings/Documents/Pre2009Abstracts/2001Abstracts/a01ma062.htm[↩]
- Resistant starch: metabolic effects and potential health benefits. Higgins JA. J AOAC Int. 2004 May-Jun; 87(3):761-8. https://www.ncbi.nlm.nih.gov/pubmed/15287677/[↩]
- Digestion of the polysaccharides of some cereal foods in the human small intestine. Englyst HN, Cummings JH. Am J Clin Nutr. 1985 Nov; 42(5):778-87. https://www.ncbi.nlm.nih.gov/pubmed/2998174/[↩]
- Brouns F, Kettlitz B, Arrigoni E. Resistant starch and “the butyrate revolution.” Trends Food Sci Technol. 2002;13:251–61[↩]
- Slavin, J.L. Dietary fiber and body weight. Nutrition 2005, 21, 411–418. https://www.ncbi.nlm.nih.gov/pubmed/15797686 [↩]
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. https://www.ncbi.nlm.nih.gov/pubmed/22254008[↩]
- Slavin, J.; Green, H. Dietary fibre and satiety. Nutr. Bull. 2007, 32, 32–42.[↩]
- Sanchez, D.; Miguel, M.; Aleixandre, A. Dietary fiber, gut peptides, and adipocytokines. J. Med. Food 2012, 15, 223–230. https://www.ncbi.nlm.nih.gov/pubmed/22181071[↩]
- Howarth, N.C.; Saltzman, E.; Roberts, S.B. Dietary fiber and weight regulation. Nutr. Rev. 2001, 59, 129–139. https://www.ncbi.nlm.nih.gov/pubmed/11396693[↩]
- Classification and measurement of nutritionally important starch fractions. Englyst HN, Kingman SM, Cummings JH. Eur J Clin Nutr. 1992 Oct; 46 Suppl 2():S33-50. https://www.ncbi.nlm.nih.gov/pubmed/1330528/[↩][↩]
- Sajilata MG, Singhal RS, Kulkarni PR. Resistant starch–A review. Compr Rev Food Sci Food Saf. 2006;5:1–17[↩]
- Nugent AP. Health properties of resistant starch. Nutr Bull. 2005;30:27–54[↩]
- Thompson DB. Strategies for the manufacture of resistant starch. Trends Food Sci Technol. 2000;11:245–53[↩]
- Fuentes-Zaragoza E, Sanchez-Zapata E, Sendra E, Sayas E, Navarro C, Fernandez-Lopez J, Perez-Alvarez JA. Resistant starch as prebiotic: A review. Starch-Stärke. 2011;63:406–15[↩]
- Brouns F, Kettlitz B, Arrigoni E. Resistant starch and “the butyrate revolution.” Trends Food Sci Technol. 2002;13:251–61 [↩]
- Resistant starch intakes in the United States. Murphy MM, Douglass JS, Birkett A. J Am Diet Assoc. 2008 Jan; 108(1):67-78. https://www.ncbi.nlm.nih.gov/pubmed/18155991/[↩]
- Keenan MJ, Zhou J, Hegsted M, et al. Role of Resistant Starch in Improving Gut Health, Adiposity, and Insulin Resistance. Advances in Nutrition. 2015;6(2):198-205. doi:10.3945/an.114.007419. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4352178/[↩]
- Englyst HN, Kingman SM, Cummings JH. Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr. 1992;46(Supp 2):S33–50. https://www.ncbi.nlm.nih.gov/pubmed/1330528[↩][↩]
- Woo KS, Seib PA. Cross-linked resistant starch: preparation and properties. Cereal Chem. 2002;79:819–25.[↩]
- Han J-A, BeMiller JN. Preparation and physical characteristics of slowly digesting modified food starches. Carbohydr Polym. 2007;67:366–74.[↩]
- Seneviratne HD, Biliaderis CG. Action of α-amylases on amylose-lipid complex superstructures. J Cereal Sci. 1991;13:129–43.[↩]
- Birt DF, Boylston T, Hendrich S, et al. Resistant Starch: Promise for Improving Human Health. Advances in Nutrition. 2013;4(6):587-601. doi:10.3945/an.113.004325. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3823506/[↩][↩]
- O’Dea K, Nestel PJ, Antonoff L. Physical factors influencing postprandial glucose and insulin responses to starch. Am J Clin Nutr. 1980;33:760–5. https://www.ncbi.nlm.nih.gov/pubmed/6987860[↩]
- Walker AW, Ince J, Duncan S, Webster L, Holtrop G, Ze X, Brown D, Stares M, Scott P, Bergerat A, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5:220–30. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3105703/[↩][↩][↩]
- Jenkins DJA, Wesson V, Wolever TMS, Jenkins AL, Kalmusky J, Guidici S, Csima A, Josse RG, Wong GS. Wholemeal versus wholegrain breads – proportion of whole or cracked grain and the glycemic response. BMJ. 1988;297:958–60. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1834634/[↩]
- Granfeldt Y, Bjorck I, Hagander B. On the importance of processing conditions, product thickness and egg addition for the glycemic and hormonal responses to pasta – a comparison with bread made from pasta ingredients. Eur J Clin Nutr. 1991;45:489–99. https://www.ncbi.nlm.nih.gov/pubmed/1782920[↩]
- Jane Jl. Ao Z, Duvick SA, Wiklund M, Yoo S-H, Wong K-S, Gardner C. Structures of amylopectin and starch granules: How are they synthesized? J Appl Glycosci. 2003;50:167–72[↩]
- Regina A, Bird A, Topping D, Bowden S, Freeman J, Barsby T, Kosar-Hashemi B, Li Z, Rahman S, Morell M. High-amylose wheat generated by RNA interference improves indices of large-bowel health in rats. Proc Natl Acad Sci USA. 2006;103:3546–51. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1450120/[↩]
- Li L, Jiang HX, Campbell M, Blanco M, Jane JL. Characterization of maize amylose-extender (ae) mutant starches. Part I: Relationship between resistant starch contents and molecular structures. Carbohydr Polym. 2008;74:396–404.[↩]
- Jiang H, Campbell M, Blanco M, Jane J-L. Characterization of maize amylose-extender (ae) mutant starches: Part II. Structures and properties of starch residues remaining after enzymatic hydrolysis at boiling-water temperature. Carbohydr Polym. 2010;80:1–12.[↩]
- Jane J, Robyt JF. Structure studies of amylose-v complexes and retrograded amylose by action of alpha-amylases, and a new method for preparing amylodextrins. Carbohydr Res. 1984;132:105–18. https://www.ncbi.nlm.nih.gov/pubmed/6435871[↩][↩]
- Witt T, Gidley MJ, Gilbert RG. Starch digestion mechanistic information from the time evolution of molecular size distributions. J Agric Food Chem. 2010;58:8444–52. https://www.ncbi.nlm.nih.gov/pubmed/20572670[↩]
- Sievert D, Pomeranz Y. Enzyme-resistant starch. 2. Differential scanning calorimetry studies on heat-treated starches and enzyme-resistant starch residues. Cereal Chem. 1990;67:217–21.[↩]
- Woo KS, Seib PA. Cross-linked resistant starch: preparation and properties. Cereal Chem. 2002;79:819–25[↩]
- Han J-A, BeMiller JN. Preparation and physical characteristics of slowly digesting modified food starches. Carbohydr Polym. 2007;67:366–74[↩]
- He J, Liu J, Zhang G. Slowly digestible waxy maize starch prepared by octenyl succinic anhydride esterification and heat-moisture treatment: glycemic response and mechanism. Biomacromolecules. 2008;9:175–84. https://www.ncbi.nlm.nih.gov/pubmed/18067261[↩]
- Zhang B, Huang Q, Luo F-X, Fu X, Jiang H, Jane J-l. Effects of octenylsuccinylation on the structure and properties of high-amylose maize starch. Carbohydr Polym. 2011;84:1276–81[↩]
- Annison G, Illman RJ, Topping DL. Acetylated, propionylated or butyrylated starches raise large bowel short-chain fatty acids preferentially when fed to rats. J Nutr. 2003;133:3523–8. https://www.ncbi.nlm.nih.gov/pubmed/14608068[↩]
- Ai Y, Hasjim J, Jane J-l. Effects of lipids on enzymatic hydrolysis and physical properties of starch. Carbohydr Polym. 2013;92:120–7. https://www.ncbi.nlm.nih.gov/pubmed/23218274[↩]
- Hasjim J, Lee S-O, Hendrich S, Setiawan S, Ai Y, Jane J-l. Characterization of a novel resistant-starch and its effects on postprandial plasma-glucose and insulin responses. Cereal Chem. 2010;87:257–62[↩][↩]
- Seneviratne HD, Biliaderis CG. Action of α-amylases on amylose-lipid complex superstructures. J Cereal Sci. 1991;13:129–43[↩]
- Different types of resistant starch elicit different glucose reponses in humans. Haub MD, Hubach KL, Al-Tamimi EK, Ornelas S, Seib PA. J Nutr Metab. 2010; 2010. https://www.ncbi.nlm.nih.gov/pubmed/20700404/[↩]
- Nutrients 2017, 9(7), 696; doi:10.3390/nu9070696. Acute Consumption of Resistant Starch Reduces Food Intake but Has No Effect on Appetite Ratings in Healthy Subjects. http://www.mdpi.com/2072-6643/9/7/696/htm#B10-nutrients-09-00696[↩]
- Ble-Castillo JL, Aparicio-Trápala MA, Francisco-Luria MU, et al. Effects of Native Banana Starch Supplementation on Body Weight and Insulin Sensitivity in Obese Type 2 Diabetics. International Journal of Environmental Research and Public Health. 2010;7(5):1953-1962. doi:10.3390/ijerph7051953. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2898027/[↩][↩]
- Consumption of both resistant starch and beta-glucan improves postprandial plasma glucose and insulin in women. Behall KM, Scholfield DJ, Hallfrisch JG, Liljeberg-Elmståhl HG. Diabetes Care. 2006 May; 29(5):976-81. https://www.ncbi.nlm.nih.gov/pubmed/16644623/[↩]
- Glycemic and insulinemic response of subjects with type 2 diabetes after consumption of three energy bars. Reader DM, O’Connell BS, Johnson ML, Franz M. J Am Diet Assoc. 2002 Aug; 102(8):1139-42. https://www.ncbi.nlm.nih.gov/pubmed/12171462/[↩]
- Consumption of Cross-Linked Resistant Starch (RS4(XL[↩]
- Diets containing high amylose vs amylopectin starch: effects on metabolic variables in human subjects. Behall KM, Scholfield DJ, Yuhaniak I, Canary J. Am J Clin Nutr. 1989 Feb; 49(2):337-44. https://www.ncbi.nlm.nih.gov/pubmed/2644803/[↩]
- Blood lipids, lipoproteins, apoproteins, and uric acid in men fed diets containing fructose or high-amylose cornstarch. Reiser S, Powell AS, Scholfield DJ, Panda P, Ellwood KC, Canary JJ. Am J Clin Nutr. 1989 May; 49(5):832-9. https://www.ncbi.nlm.nih.gov/pubmed/2497634/[↩]
- Diet-induced changes in serum transaminase and triglyceride levels in healthy adult men. Role of sucrose and excess calories. Porikos KP, Van Itallie TB. Am J Med. 1983 Oct; 75(4):624-30. https://www.ncbi.nlm.nih.gov/pubmed/6624769/[↩]
- Physiological effects of resistant starches on fecal bulk, short chain fatty acids, blood lipids and glycemic index. Jenkins DJ, Vuksan V, Kendall CW, Würsch P, Jeffcoat R, Waring S, Mehling CC, Vidgen E, Augustin LS, Wong E. J Am Coll Nutr. 1998 Dec; 17(6):609-16. https://www.ncbi.nlm.nih.gov/pubmed/9853541/[↩]
- The effect of raw potato starch on energy expenditure and substrate oxidation. Tagliabue A, Raben A, Heijnen ML, Deurenberg P, Pasquali E, Astrup A. Am J Clin Nutr. 1995 May; 61(5):1070-5. https://www.ncbi.nlm.nih.gov/pubmed/7733031/[↩]
- Dietary starch composition and level of energy intake alter nutrient oxidation in “carbohydrate-sensitive” men. Howe JC, Rumpler WV, Behall KM. J Nutr. 1996 Sep; 126(9):2120-9. https://www.ncbi.nlm.nih.gov/pubmed/8814200/[↩]
- Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Robertson MD, Bickerton AS, Dennis AL, Vidal H, Frayn KN. Am J Clin Nutr. 2005 Sep; 82(3):559-67. https://www.ncbi.nlm.nih.gov/pubmed/16155268/[↩]
- Sekirov I, Russell SL, Antunes CM, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. https://www.ncbi.nlm.nih.gov/pubmed/20664075[↩][↩][↩]
- Gonzalez A, Stombaugh J, Lozupone C, Turnbaugh PJ, Gordon JI, Knight R. The mind-body-microbial continuum. Dialogues Clin Neurosci. 2011;13:55–62. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3139398/[↩]
- Louis P, Scott KP, Duncan SH, Flint HJ. Understanding the effects of diet on bacterial metabolism in the large intestine. J Appl Microbiol. 2007;102:1197–208. https://www.ncbi.nlm.nih.gov/pubmed/17448155[↩]
- Bouhnik Y, Raskine L, Simoneau G, Vicaut E, Neut C, Flourié B, Brouns F, Bornet FR. The capacity of nondigestible carbohydrates to stimulate fecal bifidobacteria in healthy humans: a double-blind, randomized, placebo-controlled, parallel-group, dose-response relation study. Am J Clin Nutr. 2004;80:1658–64. https://www.ncbi.nlm.nih.gov/pubmed/15585783[↩]
- Higgins JA. Resistant starch: a promising dietary agent for the prevention/treatment of inflammatory bowel disease and bowel cancer. Curr Opin Gastroenterol. 2013;29:190–4. https://www.ncbi.nlm.nih.gov/pubmed/23385525[↩][↩][↩]
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–3. https://www.ncbi.nlm.nih.gov/pubmed/17183309[↩]
- Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62:67–72. https://www.ncbi.nlm.nih.gov/pubmed/12740060[↩]
- Cummings JH, Macfarlane GT. The control and consequences of bacterial fermentation in the human colon. J Appl Bacteriol. 1991;70:443–59. https://www.ncbi.nlm.nih.gov/pubmed/1938669[↩]
- Wu GD, Chen YY, Hoffmann C, Bittinger K, Chen Y, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3368382/[↩]
- El Oufir L, Flourié B, Bruley des Varannes S, Barry JL, Cloarec D, Bornet F, Galmiche JP. Relations between transit time, fermentation products, and hydrogen consuming flora in healthy humans. Gut. 1996;38:870–7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1383195/[↩]
- Lewis SJ, Heaton KW. Increasing butyrate concentration in the distal colon by accelerating intestinal transit. Gut. 1997;41:245–51. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1891451/[↩]
- Flint HJ, Duncan SH, Scott KP, Louis P. Interactions and competition within the microbial community of the human colon: links between diet and health. Environ Microbiol. 2007;9:1101–11. https://www.ncbi.nlm.nih.gov/pubmed/17472627[↩]
- Martínez I, Kim J, Duffy P, Schlegel V, Walter J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS ONE. 2010;5:e15046. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2993935/[↩]
- Delzenne NM, Neyrinck AM, Cani PD. Gut microbiota and metabolic disorders: how prebiotic can work? Br J Nutr. 2013;109:S81–5. https://www.ncbi.nlm.nih.gov/pubmed/23360884[↩]
- Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 2013;5:1417–35. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3705355/[↩]
- DuPont AW, DuPont HL. The intestinal microbiota and chronic disorders of the gut. Nat Rev Gastroenterol Hepatol. 2011;8:523–31. https://www.ncbi.nlm.nih.gov/pubmed/21844910[↩]
- Holmes E, Kinross J, Gibson GR, Burcelin R, Jia W, Pettersson S, Nicholson JK. Therapeutic modulation of microbiota-host metabolic interactions. Sci Transl Med. 2012;6:137rv6. https://www.ncbi.nlm.nih.gov/pubmed/22674556[↩]
- Zhang G, Hamaker BR. Cereal carbohydrates and colon health. Cereal Chem. 2010;87:331–41[↩][↩]
- Le Leu R, Conlon M, Winter J, Humphreys K, Michael M, Hu Y, Bird A, Topping D, Young G. Effect of high red meat intake and resistant starch in humans on risk factors for colorectal cancer. J Gastroenterol Hepatol. 2012;27:24–5[↩]
- Burn J, Bishop DT, Mecklin J, Macrae F, Moeslein G, Olschwang S, Bisgaard M, Ramesar R, Elliott F, Mathers J. Results of the CAPP-2-trial (aspirin and resistant starch) in HNPCC gene carriers. EJC Suppl. 2008;6:25[↩]
- Le Leu RK, Brown IL, Hu Y, Morita T, Esterman A, Young GP. Effect of dietary resistant starch and protein on colonic fermentation and intestinal tumourigenesis in rats. Carcinogenesis. 2007;28:240–5. https://www.ncbi.nlm.nih.gov/pubmed/17166881[↩]
- [↩]
- Le Leu RK, Hu Y, Young GP. Effects of resistant starch and nonstarch polysaccharides on colonic luminal environment and genotoxin-induced apoptosis in the rat. Carcinogenesis. 2002;23:713–9. https://www.ncbi.nlm.nih.gov/pubmed/12016142[↩]
- Le Leu RKB. Brown IL, Hu Y, Esterman A, Young GP. Suppression of azoxymethane-induced colon cancer development in rats by dietary resistant starch. Cancer Biol Ther. 2007;6:1621–6. https://www.ncbi.nlm.nih.gov/pubmed/17932462[↩]
- Paturi G, Nyanhanda T, Butts CA, Herath TD, Monro JA, Ansell J. Effects of potato fiber and potato-resistant starch on biomarkers of colonic health in rats fed diets containing red meat. J Food Sci. 2012;77:H216–23. https://www.ncbi.nlm.nih.gov/pubmed/22950602[↩]
- Toden S, Bird AR, Topping DL, Conlon MA. Differential effects of dietary whey, casein and soya on colonic DNA damage and large bowel SCFA in rats fed diets low and high in resistant starch. Br J Nutr. 2007;97:535–43. https://www.ncbi.nlm.nih.gov/pubmed/17313716[↩]
- Clarke JM, Topping DL, Bird AR, Young GP, Cobiac L. Effects of high-amylose maize starch and butyrylated high-amylose maize starch on azoxymethane-induced intestinal cancer in rats. Carcinogenesis. 2008;29:2190–4. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2577140/[↩]
- Zhao Y, Hasjim J, Li L, Jane J-L, Hendrich S, Birt DF. Inhibition of azoxymethane-induced preneoplastic lesions in the rat colon by a cooked stearic acid complexed high-amylose cornstarch. J Agric Food Chem. 2011;59:9700–8. https://www.ncbi.nlm.nih.gov/pubmed/21780846[↩]
- Fung KY, Cosgrove L, Lockett T, Head R, Topping DL. A review of the potential mechanisms for the lowering of colorectal oncogenesis by butyrate. Br J Nutr. 2012;108:820–31. https://www.ncbi.nlm.nih.gov/pubmed/22676885[↩][↩][↩]
- Gupta N, Martin PM, Prasad PD, Ganapathy V. SLC5A8 (SMCT1)-mediated transport of butyrate forms the basis for the tumor suppressive function of the transporter. Life Sci. 2006;78:2419–25. https://www.ncbi.nlm.nih.gov/pubmed/16375929[↩]
- Li H, Myeroff L, Smiraglia D, Romero MF, Pretlow TP, Kasturi L, Lutterbaugh J, Rerko RM, Casey G, Issa JP, et al. SLC5A8, a sodium transporter, is a tumor suppressor gene silenced by methylation in human colon aberrant crypt foci and cancers. Proc Natl Acad Sci USA. 2003;100:8412–7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC166243/[↩]
- Nepelska M, Cultrone A, Beguet-Crespel F, Le Roux K, Dore J, Arulampalam V, Blottiere HM. Butyrate produced by commensal bacteria potentiates phorbol esters induced AP-1 response in human intestinal epithelial cells. PLoS ONE. 2012;7:e52869. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3531367/[↩]
- Macfarlane GT, Macfarlane S. Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int. 2012;95:50–60. https://www.ncbi.nlm.nih.gov/pubmed/22468341[↩]
- Conlon MA, Kerr CA, McSweeney CS, Dunne RA, Shaw JM, Kang S, Bird AR, Morell MK, Lockett TJ, Molloy PL, et al. Resistant starches protect against colonic DNA damage and alter microbiota and gene expression in rats fed a Western diet. J Nutr. 2012;142:832–40. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3327741/[↩]
- Bordonaro M, Mariadason JM, Aslam F, Heerdt BG, Augenlicht LH. Butyrate-induced apoptotic cascade in colonic carcinoma cells: modulation of the beta-catenin-Tcf pathway and concordance with effects of sulindac and trichostatin A but not curcumin. Cell Growth Differ. 1999;10:713–20. https://www.ncbi.nlm.nih.gov/pubmed/10547075[↩]
- Lazarova DL, Bordonaro M, Carbone R, Sartorelli AC. Linear relationship between Wnt activity levels and apoptosis in colorectal carcinoma cells exposed to butyrate. Int J Cancer. 2004;110:523–31. https://www.ncbi.nlm.nih.gov/pubmed/15122584[↩]
- Bordonaro M. Crosstalk between Wnt signaling and RNA processing in colorectal cancer. J Cancer. 2013;4:96–103. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3563071/[↩]
- Le Leu RK, Hu Y, Brown IL, Young GP. Effect of high amylose maize starches on colonic fermentation and apoptotic response to DNA-damage in the colon of rats. Nutr Metab (Lond). 2009;6:11. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2656505/[↩]
- Zimmerman MA, Singh N, Martin PM, Thangaraju M, Ganapathy V, Waller JL, Shi H, Robertson KD, Munn DH, Liu K. Butyrate suppresses colonic inflammation through HDAC1-dependent Fas upregulation and Fas-mediated apoptosis of T cells. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1405–15. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3378095/[↩]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Diabetes Prevention Program Res G. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1370926/[↩]
- Alexander D. Postprandial effects of resistant starch corn porridges on blood glucose and satiety responses in non-overweight and overweight adults. Ames, IA: Iowa State University; 2012[↩]
- Kendall CWC, Esfahani A, Sanders LM, Potter SM, Vidgen E. The effect of a pre-load meal containing resistant starch on spontaneousfood intake and glucose and insulin responses. J Food Technol. 2010;8:67–73[↩]
- Brighenti F, Benini L, Del Rio D, Casiraghi C, Pellegrini N, Scazzina F, Jenkins DJA, Vantini I. Colonic fermentation of indigestible carbohydrates contributes to the second-meal effect. Am J Clin Nutr. 2006;83:817–22. https://www.ncbi.nlm.nih.gov/pubmed/16600933[↩]
- So PW, Yu WS, Kuo YT, Wasserfall C, Goldstone AP, Bell JD, Frost G. Impact of resistant starch on body fat patterning and central appetite regulation. PLoS ONE. 2007;2:e1309. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2111051/[↩]
- Behall KM, Howe JC. Resistant starch as energy. J Am Coll Nutr. 1996;15:248–54. https://www.ncbi.nlm.nih.gov/pubmed/8935440[↩]
- Shen L, Keenan MJ, Reggio A, Williams C, Martin RJ. Dietary-resistant starch improves maternal glycemic control in Goto-Kakizaki rat. Mol Nutr Food Res. 2011;55:1499–508. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4826617/[↩]
- Mutlu A, Mutlu GY, Ozsu E, Cizmecioglu FM, Hatun S. Vitamin D deficiency in children and adolescents with type 1 diabetes. J Clin Res Pediatr Endocrinol. 2011;3:179–83. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3245490/[↩]
- McGill AT, Stewart JM, Lithander FE, Strik CM, Poppitt SD. Relationships of low serum vitamin D3 with anthropometry and markers of the metabolic syndrome and diabetes in overweight and obesity. Nutr J. 2008;7:4. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2265738/[↩]
- Lee JI, Oh SJ, Ha WC, Kwon HS, Sohn TS, Son HS, Cha BY. Serum 25-hydroxyvitamin D concentration and arterial stiffness among type 2 diabetes. Diabetes Res Clin Pract. 2012;95:42–7. https://www.ncbi.nlm.nih.gov/pubmed/21963093[↩]
- Smazal AL, Borcherding NC, Anderegg AS, Whitley EM, Schalinske KL, Rowling MJ. Dietary resistant starch prevents urinary excretion of 25-hydroxycholecalciferol and vitamin D-binding protein in type 1 diabetic rats. J Nutr. 2013;143:1123–8. https://www.ncbi.nlm.nih.gov/pubmed/23677864[↩][↩]
- Anderson RL, Ternes SB, Strand KA, Rowling MJ. Vitamin D homeostasis is compromised due to increased urinary excretion of the 25-hydroxycholecalciferol-vitamin D-binding protein complex in the Zucker diabetic fatty rat. Am J Physiol Endocrinol Metab. 2010;299:E959–67. https://www.ncbi.nlm.nih.gov/pubmed/20876762[↩]
- Wanders AJ, van den Borne J, de Graaf C, Hulshof T, Jonathan MC, Kristensen M, Mars M, Schols HA, Feskens EJM. Effects of dietary fibre on subjective appetite, energy intake and body weight: a systematic review of randomized controlled trials. Obes Rev. 2011;12:724–39. https://www.ncbi.nlm.nih.gov/pubmed/21676152[↩][↩]
- Du H, van der A DL, Boshuizen HC, Forouhi NG, Wareham NJ, Halkjaer J, Tjonneland A, Overvad K, Jakobsen MU, Boeing H, et al. Dietary fiber and subsequent changes in body weight and waist circumference in European men and women. Am J Clin Nutr. 2010;91:329–36. https://www.ncbi.nlm.nih.gov/pubmed/20016015[↩]
- Slavin JL. Dietary fiber and body weight. Nutrition. 2005;21:411–8. https://www.ncbi.nlm.nih.gov/pubmed/15797686[↩]
- Aziz AA, Kenney LS, Goulet B, Abdel-Aal ES. Dietary starch type affects body weight and glycemic control in freely fed but not energy-restricted obese rats. J Nutr. 2009;139:1881–9. https://www.ncbi.nlm.nih.gov/pubmed/19692526[↩]
- Belobrajdic DP, King RA, Christophersen CT, Bird AR. Dietary resistant starch dose-dependently reduces adiposity in obesity-prone and obesity-resistant male rats. Nutr Metab (L0nd). 2012;9(1):93. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3541085/[↩]
- Keenan MJ, Zhou J, McCutcheon KL, Raggio AM, Bateman HG, Todd E, Jones CK, Tulley RT, Melton S, Martin RJ, et al. Effects of resistant starch, a non-digestible fermentable fiber, on reducing body fat. Obesity (Silver Spring). 2006;14:1523–34. https://www.ncbi.nlm.nih.gov/pubmed/17030963[↩][↩]
- Zhou J, Martin RJ, Tulley RT, Raggio AM, McCutcheon KL, Shen L, Danna SC, Tripathy S, Hegsted M, Keenan MJ. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am J Physiol Endocrinol Metab. 2008;295:E1160–6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2584810/[↩][↩]
- Rolls BJ, Roe LS, Meengs JS. Salad and satiety: energy density and portion size of a first-course salad affect energy intake at lunch. J Am Diet Assoc. 2004;104:1570–6. https://www.ncbi.nlm.nih.gov/pubmed/15389416[↩]
- Yao M, Roberts SB. Dietary energy density and weight regulation. Nutr Rev. 2001;59:247–58. https://www.ncbi.nlm.nih.gov/pubmed/11518179[↩]
- Rolls BJ, Bell EA, Thorwart ML. Water incorporated into a food but not served with a food decreases energy intake in lean women. Am J Clin Nutr. 1999;70:448–55. https://www.ncbi.nlm.nih.gov/pubmed/10500012[↩]
- Blatt AD, Williams RA, Roe LS, Rolls BJ. Effects of energy content and energy density of pre-portioned entrees on energy intake. Obesity (Silver Spring). 2012;20:2010–8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3374070/[↩]
- Bodinham CL, Frost GS, Robertson MD. Acute ingestion of resistant starch reduces food intake in healthy adults. Br J Nutr. 2010;103:917–22. https://www.ncbi.nlm.nih.gov/pubmed/19857367[↩]
- Klosterbuer AS, Thomas W, Slavin JL. Resistant starch and pullulan reduce postprandial glucose, insulin, and GLP-1, but have no effect on satiety in healthy humans. J Agric Food Chem. 2012;60:11928–34. https://www.ncbi.nlm.nih.gov/pubmed/23136915[↩]
- Tapsell LC. Diet and metabolic syndrome: where does resistant starch fit in? J AOAC Int. 2004;87:756–60. https://www.ncbi.nlm.nih.gov/pubmed/15287676[↩]
- Tagliabue A, Raben A, Heijnen ML, Deurenberg P, Pasquali E, Astrup A. The effect of raw potato starch on energy-expenditure and substrate oxidation. Am J Clin Nutr. 1995;61:1070–5. https://www.ncbi.nlm.nih.gov/pubmed/7733031[↩]
- Ranganathan S, Champ M, Pechard C, Blanchard P, Nguyen M, Colonna P, Krempf M. Comparative-study of the acute effects of resistant starch and dietary-fibers on metabolic indexes in men. Am J Clin Nutr. 1994;59:879–83. https://www.ncbi.nlm.nih.gov/pubmed/8147333[↩]
- Howe JC, Rumpler WV, Behall KM. Dietary starch composition and level of energy intake alter nutrient oxidation in ”carbohydrate-sensitive’’ men. J Nutr. 1996;126:2120–9. https://www.ncbi.nlm.nih.gov/pubmed/8814200[↩]
- J Am Diet Assoc. 2008;108:67-78. Resistant Starch Intakes in the United States. http://www.sciencedirect.com/science/article/pii/S0002822307019323[↩]