What is Sucralose
Sucralose is a synthetic organochlorine sweetener (also known as Splenda®) is an artificial sweetener, also called sugar substitute or non-nutritive sweetener, is a substance that is used instead of sucrose (table sugar) to sweeten foods and beverages. Because artificial sweeteners are many times sweeter than table sugar, smaller amounts are needed to create the same level of sweetness.
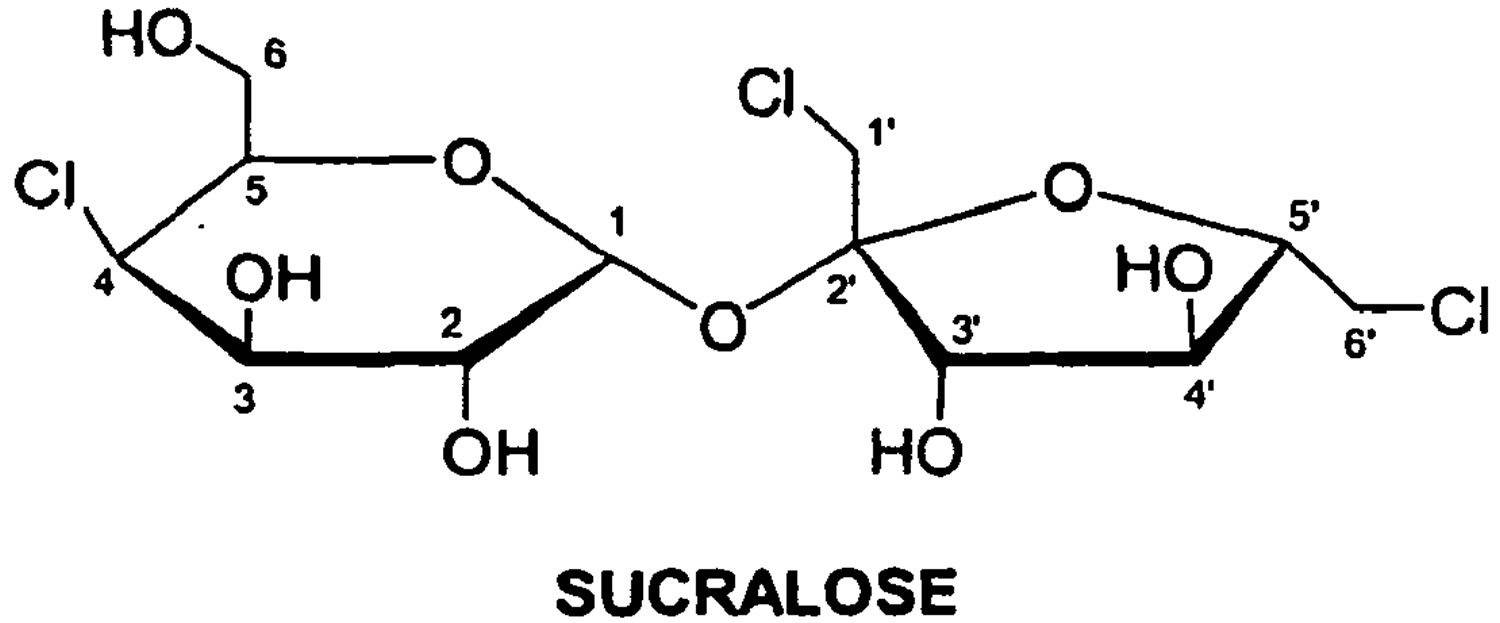
Sucralose has the chemical name 1,6-dichloro-1,6-dideoxy-[beta]-D-fructofuranosyl-4-chloro-4-deoxy-[alpha]-D-galactopyranoside 1.
Sugar substitutes are chemical or plant-based substances used to sweeten or enhance the flavor of foods and drinks. You may have heard them called “artificial sweeteners” or “non-caloric sweeteners.” They can be used as a tabletop sweetener (for example, to sweeten a glass of iced tea) and/or as an ingredient in processed foods and drinks.
Sucralose is about 600 times sweeter than sugar 2.
Sugar substitutes are regulated as food additives by the U.S. Food and Drug Administration (FDA). This means that the FDA reviews scientific evidence to be sure that a sugar substitute is safe before it can be used in foods and drinks 2.
Sucralose has been used as a general purpose sweetener that can be found in thousands of processed foods including baked goods, beverages, chewing gum, gelatins and frozen dairy desserts. Some examples include soft drinks, juices, sauces, syrups, candy, desserts, baked goods, and canned fruits. It is used in medicines, nutritional supplements, and vitamins. It is also used as a tabletop sweetener.
It is heat stable, meaning that it stays sweet even when used at high temperatures during baking, making it suitable as a sugar substitute in baked goods. Sucralose can be used in baking because it does not lose its sweet taste at high temperatures. For best results, follow the package instructions for using it in your recipes.
The range of product utilization is more extensive for sucralose than for other artificial sweeteners due to its physicochemical properties. For example, sucralose is readily soluble in ethanol, methanol, and water 3, 4, 5 and this solubility profile contributes to its versatility in both fat- and water-based food and beverage applications including alcoholic drinks. Other common artificial sweeteners such as aspartame, sodium saccharin, and acesulfame-potassium (ace-K) are only slightly or sparingly soluble in ethanol and/or methanol 6 and have more limited product applications. The Acceptable Daily Intake (ADI) level for sucralose was set at 5 mg/kg body weight per day (mg/kg/d) in the United States 7 and 15 mg/kg/d in the EU as recommended by Scientific Committee on Food of the European Commission 8, and there are no exclusions or restrictions for vulnerable population groups, including pregnant women, nursing mothers, infants, children, elderly, persons with medical conditions, and patients taking multiple medications.
What is the difference between nutritive and non-nutritive high-intensity artificial sweeteners ?
Nutritive sweeteners add caloric value to the foods that contain them, while non-nutritive sweeteners are very low in calories or contain no calories at all 2.
Nutritive sweeteners: those that contain more than 2 percent of the calories in an equivalent amount of sugar 9.
Nonnutritive sweeteners: those that contain less than 2 percent of the calories in an equivalent amount of sugar or have no calories at all. Also known as artificial sweeteners, sugar substitutes, low-calorie sweeteners, noncaloric sweeteners, or high-intensity sweeteners 9.
Food products are considered “no-calorie” if they have 5 calories or less per serving. Notice that even though the nutrition labels on sweetener packets claim to have zero calories and carbohydrate, there are a small amount calories and carbs from those added ingredients 9.
Specifically, aspartame, the only approved nutritive high-intensity sweetener, contains more than two percent of the calories in an equivalent amount of sugar, as opposed to non-nutritive sweeteners that contain less than two percent of the calories in an equivalent amount of sugar.
Keep in mind that just because a product is “sugar free,” it doesn’t always mean that it’s healthy. Foods and beverages that contain non-nutritive sweeteners can be included in a healthy diet, as long as the calories they save you are not added back by adding more foods as a reward later in the day, adding back calories that take you over your daily limit. The current meta-analysis 10 provides a rigorous evaluation of the scientific evidence on low-calorie sweeteners and body weight and composition. Findings from observational studies showed no association between low-calorie sweeteners intake and body weight or fat mass and a small positive association with body mass index (BMI); however, data from randomised clinical trials, which provide the highest quality of evidence for examining the potentially causal effects of low-calorie sweeteners intake, indicate that substituting low-calorie sweeteners options for their regular-calorie versions results in a modest weight loss and may be a useful dietary tool to improve compliance with weight loss or weight maintenance plans 10.
Comparison of some different sweeteners to regular sugar below:
1 packet Sugar = 11 calories + 3 grams of carbohydrate
or
1 packet Splenda (Sucralose) = 4 calories + < 1 gram of carbohydrate
or
1 packet of Sugar Twin (Aspartame) = 3 calories + < 1 gram of carbohydrate
or
1 packet of Equal (Aspartame) = 4 calories + < 1 gram of carbohydrate
or
1 teaspoon of Agave = 21 calories + 5.3 to 5.7 grams carbohydrate
or
1 teaspoon of Sugar (brown, powdered, raw, and white) and Maple syrup have 2.5 to 4.6 grams of carbohydrate per teaspoon and 10 to 18 calories.
or
1 teaspoon of Powdered Sugar = 10 calories + 2.5 grams carbohydrate
or
1 teaspoon of Maple syrup = 10 to 18 calories + 2.5 to 4.6 grams of carbohydrate
or
1 teaspoon of Honey = 21 calories + 5.3 to 5.7 grams carbohydrate
When you use a large amount of these products, it can start to add up. As with all foods, it is important not to go overboard.
[Source: American Diabetes Association. 11]Other Sugar Substitutes/Artificial Sweeteners are Approved by the FDA
The following sugar substitutes are FDA approved as food additives in the United States:
- Acesulfame K (brand names: Sunett and Sweet One)
- Advantame
- Aspartame (two brand names: Equal and Nutrasweet)
- Neotame (brand name: Newtame)
- Saccharin (two brand names: Sweet ‘N Low and Sweet Twin)
- Sucralose (brand name: Splenda)
According to the FDA, some sugar substitutes are “generally recognized as safe” (GRAS) 2. This means they do not require FDA approval because qualified experts agree the scientific evidence shows these products are safe for use in foods and drinks.
These artificial sweeteners are used by food companies to make diet drinks, baked goods, frozen desserts, candy, light yogurt and chewing gum. You can buy them to use as table top sweeteners. Add them to coffee, tea, or sprinkle them on top of fruit. Some are also available in “granular” versions which can be used in cooking and baking.
Sugar substitutes in this category include highly purified stevia extracts called “steviol glycosides” (two brand names: Pure Via and Truvia) and monk fruit extracts (two brand names: Monk Fruit in the Raw and PureLo).
Sugar alcohols are another class of sweeteners that can be used as sugar substitutes. Examples include mannitol, sorbitol, and xylitol. The FDA has determined that sugar alcohols are generally recognized as safe for use in foods and drinks.
Table 1. FDA Approved Artificial Sweeteners
| eetener | Regulatory Status | Examples of Brand Names Containing Sweetener | Multiplier of Sweetness Intensity Compared to Table Sugar (Sucrose) | Acceptable Daily Intake (ADI) milligrams per kilogram body weight per day (mg/kg bw/d) | Number of Tabletop Sweetener Packets Equivalent to ADI* |
|---|---|---|---|---|---|
| Acesulfame Potassium (Ace-K) | Approved as a sweetener and flavor enhancer in foods generally (except in meat and poultry)
| Sweet One® Sunett® | 200 x | 15 | 23 |
| Advantame | Approved as a sweetener and flavor enhancer in foods generally (except in meat and poultry)
| 20,000 x | 32.8 | 4,920 | |
| Aspartame | Approved as a sweetener and flavor enhancer in foods generally
| Nutrasweet® Equal® Sugar Twin® | 200 x | 50 | 75 |
| Neotame | Approved as a sweetener and flavor enhancer in foods generally (except in meat and poultry)
| Newtame®, | 7,000-13,000 x | 0.3 | 23 (sweetness intensity at 10,000 x sucrose) |
| Saccharin | Approved as a sweetener only in certain special dietary foods and as an additive used for certain technological purposes
| Sweet and Low® Sweet Twin® Sweet’N Low® Necta Sweet® | 200-700 x | 15 | 45 (sweetness intensity at 400 x sucrose) |
| Siraitia grosvenorii Swingle (Luo Han Guo) fruit extracts (SGFE) | SFGE containing 25%, 45% or 55% Mogroside V is the subject of GRAS notices for specific conditions of use | Nectresse® Monk Fruit in the Raw® PureLo® | 100-250 x | NS*** | ND |
| Certain high purity steviol glycosides purified from the leaves of Stevia rebaudiana (Bertoni) Bertoni | ≥95% pure glycosides Subject of GRAS notices for specific conditions of use | Truvia® PureVia® Enliten® | 200-400 x | 4** | 9 (sweetness intensity at 300 x sucrose) |
| Sucralose | Approved as a sweetener in foods generally
| Splenda® | 600 x | 5 | 23 |
Note: Table 1. FDA Approved Artificial Sweeteners
ADI indicates Acceptable Daily Intake; Joint Expert Commission on Food Additives of the World Health Organization and the Food and Agriculture Organization JECFA 12. ADI is a measure of the amount of a specific substance in food or drinking water that can be ingested over a lifetime without an appreciable health risk. Measurement is usually expressed in milligrams of sweetener per kilogram of body weight (mg/kg bw). The amount is usually set at 1/100 of the maximum level at which no adverse effect was observed in animal experiments.
* Number of Tabletop Sweetener Packets a 60 kg (132 pound) person would need to consume to reach the ADI. Calculations assume a packet of high-intensity sweetener is as sweet as two teaspoons of sugar.
**ADI established by the Joint FAO/WHO Expert Committee on Food Additives (JECFA)
*** NS means not specified. A numerical ADI may not be deemed necessary for several reasons, including evidence of the ingredient’s safety at levels well above the amounts needed to achieve the desired effect (e.g., as a sweetener) in food.
Additional Note:
Saccharin and sucralose are heat stable and are easiest to use baking and cooking. However, to keep the desirable taste, volume, color, and/or texture of a baked product, you usually will not substitute all of the sugar in a recipe for artificial sweetener. Read the package carefully for specific instructions on the best way to substitute the low-calorie sweetener for sugar in your recipes. The company’s website can also be a helpful resource for baking tips.
Some brands offer pre-made blends of sugar and low-calorie sweeteners. These blends are meant to be used in baking. They are half sugar and half low-calorie sweetener, so they have half the calories and carbohydrate as sugar. As with all low-calorie sweeteners, you will want to read the instructions for substituting these blends for sugar. For example, when replacing regular sugar with Splenda’s Sugar Blend (half-granular Splenda, half-sugar), they suggest using half as much:
1/2 cup Splenda (sucralose) Sugar Blend = 387 calories + 97 grams of carbohydrate
[Source: American Diabetes Association. Using Sugar Substitutes in the Kitchen. 13]Is sucralose safe ?
Although early studies asserted that sucralose passes through the gastrointestinal tract unchanged, subsequent analysis suggested that some of the ingested sweetener is metabolized in the gastrointestinal tract, as indicated by multiple peaks found in thin-layer chromatograms profiles of methanolic fecal extracts after oral sucralose administration. The identity and safety profile of these putative sucralose metabolites are not known at this time 14. The identity and safety profile of these putative sucralose metabolites are not known at this time 15, 16, 17. Sucralose and one of its hydrolysis products were found to be mutagenic at elevated concentrations in several testing methods. Cooking with sucralose at high temperatures was reported to generate chloropropanols, a potentially toxic class of compounds 14. Taken together, these findings indicate that sucralose is not a biologically inert compound. The thin-layer chromatograms data of Sims et al. 17 and Roberts et al. 18 appear inconsistent with the claim that sucralose is not metabolized in the gastrointestinal tract as asserted by Grice and Goldsmith 19, Molinary and Quinlan 20, and Grotz and Munro 21.
Potential toxicity from habitual sucralose ingestion. Historical in vitro genotoxicity tests found that sucralose was weakly mutagenic in a mouse lymphoma mutation assay, and that one of its hydrolysis products was weakly mutagenic in both the Ames test and mouse lymphoma mutation assay 22, 7. A subsequent comet test by Sasaki et al. 23 found that sucralose induced DNA damage in mouse gastrointestinal tract. Three independent labs showed that sucralose undergoes thermal decomposition at temperatures used in baking 24, 25, 26, 27 and heating sucralose with glycerol, the backbone of triglycerides, generated chloropropanols, a potentially toxic class of compounds 27.
The FDA approved sucralose for use in 15 food categories in 1998 and for use as a general purpose sweetener for foods in 1999 , under certain conditions of use 2. In 2004, sucralose (termed food additive E 955) was approved in the European Union (EU) in a variety of products including water- and fat-based desserts, certain alcoholic beverages, fat-based sandwich spreads, breakfast cereals, marinades, and chewing gum 28.
Sucralose safety has been extensively studied and more than 110 safety studies were reviewed by FDA in approving the use of sucralose as a general purpose sweetener for food. Before approving these sweeteners, the FDA reviewed more than 100 safety studies that were conducted on each sweetener, including studies to assess cancer risk. The results of these studies showed no evidence that these sweeteners cause cancer or pose any other threat to human health 29.
According to the National Cancer Institute (an agency of the Department of Health and Human Services), there is no evidence that sucralose and other sugar substitutes approved for use in the United States cause cancer or other serious health problems 29.
This study suggested sucralose may trigger a migraine headache 30.
Effect of sucralose on the number and relative proportions of different intestinal bacterial types
Early studies of sucralose with bacteria in culture indicated that sucralose was not utilized as a carbon source by oral bacteria 31 or by bacteria from environmental samples 32. Abou-Donia et al. 33 extended these studies to bacteria cultured from the GIT of rats that had been administered sucralose daily. An overall reduction of the existing microflora was found (≥50%) at sucralose doses that were lower than the human ADI. Beneficial bacteria including lactobacilli and bifidobacteria were disproportionately affected compared to pathogenic bacteria including enterobacteria. Further, the reduction in fecal microflora was not fully reversible even 3 mo after cessation of sucralose. Sucralose was also reported to exhibit antimicrobial activity against two oral bacterial species involved in periodontal disease 34.
Is Sucralose Bad ?
In the last several years, numerous studies in rodents reported that sucralose modulates physiological processes involved in nutrient absorption and body weight regulation via its interaction with sweet taste receptors (called T1R2/T1R3) located in enteroendocrine cells of the gastrointestinal tract 35, 36, pancreatic ß cells 37, and the hypothalamus 38.
Ren et al. 38 found that sucralose modulated the expression of the gene for the sweet taste receptor T1R2 in cells from the hypothalamus, a nutrient-sensing region of the brain, in rodents. Data showed that T1R2 expression was elevated when the hypothalamic cells were exposed to low (compared to high) extracellular glucose concentrations, and this was reversed with addition of sucralose to the low-glucose medium. That is, addition of sucralose resulted in expression levels of T1R2 that would be expected if higher extracellular glucose levels were actually present. Thus, activation of the sweet taste receptor in the brain by nonnutritive sucralose might potentially affect appetite regulation by providing an inaccurate signal regarding the actual levels of extracellular glucose in the brain 15. However, it is not yet known if the portion of sucralose that is absorbed into the systemic circulation can traverse the blood–brain barrier to reach the hypothalamus 14.
Recently, there have been significant advances in our understanding of how hormonal signals released from the gastrointestinal (GI) tract interact with circuits within the central nervous system to control appetite and energy intake 39. The gut hormones peptide YY (PYY) and glucagon-like peptide (GLP)-1 are co-secreted from intestinal enteroendocrine L-cells and released post-prandially in proportion to the amount of energy ingested 40, 41, 42. Glucagon-like peptide (GLP)-1 is an incretin hormone secreted in the gut that (1) induces glucose-dependent stimulation of insulin by the pancreas, (2) reduces glucagon secretion by the liver, (3) delays gastric emptying, and (4) increases satiety 43. Incretins are a group of metabolic hormones that stimulate a decrease in blood glucose levels. Incretins are released after eating and augment the secretion of insulin released from pancreatic beta cells of the islets of Langerhans by a blood glucose-dependent mechanism. The incretin effect of GLP-1, augmentation of insulin secretion in response to an oral glucose load, has been well characterised.
The gut hormones peptide YY (PYY) and glucagon-like peptide (GLP)-1 have both been shown to be satiety factors, reducing food intake when administered to rodents 44, 45, 46, 47, 48 and to humans 49, 50, 44, 51, 42.
In a research to study the effects of oral ingestion of sucralose on gut hormone response (PYY and GLP-1) and appetite in healthy normal-weight subjects 52. Ford et al. gave subjects to consume 50 ml of either water, sucralose, a non-sweet, glucose-polymer matched for sweetness with sucralose addition (50% w/v maltodextrin+0.083% sucralose) or a modified sham-feeding protocol of sucralose (0.083% w/v). Appetite ratings and plasma GLP-1, PYY, insulin and glucose were measured at regular time points for 120 min. At 120 min, energy intake at a buffet meal was measured. The results of the study showed sucralose ingestion did not increase plasma GLP-1 or PYY. Sucralose did not elicit a cephalic phase response for insulin or GLP-1. Maltodextrin ingestion significantly increased insulin and glucose compared with water. Appetite ratings and energy intake were similar for all groups. Ford et al. concluded oral ingestion of sucralose does not increase plasma GLP-1 or PYY concentrations and hence, does not reduce appetite in healthy subjects. Oral stimulation with sucralose had no effect on GLP-1, insulin or appetite 52. Both human and rodent studies demonstrated that sucralose may alter glucose, insulin, and glucagon-like peptide 1 (GLP-1) levels. Furthermore, sucralose was shown to elevate glucose and insulin levels in a small study of obese women 53, who are at increased risk for further weight gain and development of diabetes.
According to the American Heart Association news report 7th October 2014 54, a recent scientific study published in the science journal Nature 55, a study on the effects of artificial sweeteners on blood glucose homeostasis and gut microbiota. The study was conducted largely on mice and included an experiment on seven people who did not normally consume artificial sweeteners. The researchers primarily used saccharin in the experiments, however some of the experiments also included aspartame and sucralose. They found that some mice and people had a two- to four-times increase in blood sugars and changes in the types of microbes in their intestines. In summary, their results suggest that artificial sweeteners consumption in both mice and humans enhances the risk of glucose intolerance and that these adverse metabolic effects are mediated by modulation of the composition and function of the gut microbiota. Notably, several of the bacterial taxa that changed following artificial sweeteners consumption were previously associated with type 2 diabetes in humans 55. The findings counter the perception that artificial sweeteners, which are not meant to be absorbed by the digestive tract, don’t affect blood sugar or glucose tolerance – which can be a harbinger of diabetes 54. Moreover, the study’s authors, Eran Elinav and Eran Segal of the Weizmann Institute of Science in Israel, said more information and confirmation of their results are needed 55.
The American Heart Association and the American Diabetes Association reviewed the safety of artificial sweeteners in a 2012 statement and concluded they should be used “judiciously” as a way to reduce sugar intake. The findings of the American Heart Association research “at this time, there are insufficient data to determine conclusively whether the use of non-nutritive sweeteners (artificial sweeteners) to displace caloric sweeteners in beverages and foods reduces added sugars or carbohydrate intakes, or benefits appetite, energy balance, body weight, or cardiometabolic risk factors. There are some data to suggest that non-nutritive sweeteners (artificial sweeteners) may be used in a structured diet to replace sources of added sugars and that this substitution may result in modest energy intake reductions and weight loss. The impact of incorporating non-nutritive sweeteners (artificial sweeteners) and non-nutritive sweeteners-containing beverages and foods on overall diet quality should be included in assessing the overall balance of benefits and risks. Apparent from the available literature is the paucity of data from well-designed human trials exploring the potential role of non-nutritive sweeteners in achieving and maintaining a healthy body weight and minimizing cardiometabolic risk factors. The evidence reviewed suggests that when used judiciously, non-nutritive sweeteners could facilitate reductions in added sugars intake, thereby resulting in decreased total energy and weight loss/weight control, and promoting beneficial effects on related metabolic parameters. However, these potential benefits will not be fully realized if there is a compensatory increase in energy intake from other sources 56.
Sucralose and Body Weight
In a recent (published 17 July 2017) systematic review and meta-analysis of randomized controlled trials and prospective cohort studies on the effects of non-nutritive sweeteners (artificial sweeteners) and cardio-metabolic health being conducted by Dr. Azad et al. 57 found that non-nutritive sweeteners (artificial sweeteners) had no significant effect on BMI (body mass index) on participants, in fact in the included cohort studies, consumption of non-nutritive sweeteners was associated with a modest increase in BMI. In the cohort studies, consumption of non-nutritive sweeteners was associated with increases in weight and waist circumference, and higher incidence of obesity, hypertension, metabolic syndrome, type 2 diabetes and cardiovascular events 57. Theories about why artificial sweeteners might not help weight loss tend to revolve around two schools of thought, Dr. Azad said. One school holds that the sweeteners might influence dieters’ behavior in unhealthy ways. For example, a person drinking a no-calorie soda might feel free to eat calorie-laden foods, Azad noted. Artificial sweeteners also might sharpen the person’s sweet tooth, making them more likely to indulge in sugary foods. The other school holds that artificial sweeteners might influence the body itself in some as-yet-unknown way, Azad said. The artificial sweeteners could alter the way that gut microbes function in the digestion of food, or possibly change the body’s metabolism over time by sending repeated false signals that something sweet has been ingested 58. Another plausible explanation for why the study subjects gained weight and have higher incidence of obesity, hypertension, metabolic syndrome, type 2 diabetes and cardiovascular events, is that the study population involved people who are already overweight, obese, have metabolic syndrome, hypertension or suffer from type 2 diabetes 58.
Epidemiological studies in humans 59, 60, 61, 62, 63 and lab studies in animals 64, 65, 66 both suggest an association between use of artificial sweeteners and body weight gain. Epidemiological studies also demonstrated that artificial sweetener use increased the risk for metabolic syndrome, type 2 diabetes, hypertension, and cardiovascular disease 67. Most human epidemiological studies have not distinguished among the different types of artificial sweeteners (e.g., sucralose, saccharin, acesulfame-K, aspartame, neotame, stevioside, and rebaudioside) but rather treated them as a group. One exception is the Nurses’ Health Study cohort 68, which did specifically associate saccharin use with weight gain. This finding is consistent with recent animal experiments 64, 65 in which saccharin intake was related to weight gain in rats. Because synthetic organochlorine compounds and artificial sweeteners have both been associated with weight gain, and because sucralose is a member of both categories, it is important to determine its effect on mechanisms that regulate body weight.
To date, the effect of chronic oral consumption of sucralose on body weight at levels approved by the FDA and EU has not been studied prospectively in adults. In a lab setting, ingestion of sucralose tends to increase intake after a 60-min interval 69; however, it is not yet known whether this finding transfers into chronic body weight gain in free-living adult populations. In an 18-mo trial with children, participants were randomly assigned to receive an 8-oz can per day of either a noncalorically sweetened or a sugar-sweetened beverage that provided 104 kcal 70. Each can of sugar-free beverage contained 34 mg sucralose and 12 mg acesulfame-K, and the mean sucralose dosage for the sugar-free group was 1.1 mg/kg/d. The mean duration of the study was 541 d (77.3 wk), during which 477 of 641 children completed the intervention by consuming an average of 5.8 beverages per week. Measurement of urinary sucralose levels was one of several markers used to monitor compliance with the protocol. The calorie consumption from these beverages was 46,627 kcal greater for children in the sugar-sweetened group than in the sucralose-sweetened group (5.8 × 77.3 × 104). In spite of this highly significant difference in calories consumed from the beverages, the total weight gain over this 18-mo study was only 1 kg greater for children in the sugar-sweetened group compared to sucralose group. No explanation was provided to account for the small difference in weight gain given the large difference in caloric consumption from the beverages. However, one possible explanation is that the children who consumed the sugar-sweetened beverages compensated by reducing their food intake. A control group that ingested water as a comparison was not included. Another study in adolescents showed no consistent reduction of weight gain at a 2-year follow-up when their families were supplied with artificially sweetened beverages in order to reduce their consumption of sugar-sweetened sodas 71.
Functional magnetic resonance (fMRI) imaging studies also indicate that the human brain signals distinguish caloric sweeteners (e.g., sucrose) from noncaloric sweeteners (e.g., sucralose and saccharin) 72. However, neuroimaging studies by Rudenga and Small 73 revealed that routine use of high-potency sweeteners as a group altered brain responses to sucrose in the amygdala and insula as determined by fMRI scanning. These brain imaging studies in conjunction with epidemiological data that associate artificial sweetener use with weight gain suggest that high-potency sweeteners interfere with learned sweet–calorie relationships in humans as well as animals 67.
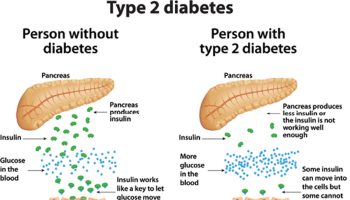
Effect of Sucralose in Patients With Diabetes
A study of patients with diabetes 74 reported no significant effect of sucralose on glycosylated hemoglobin (HbA1c) as a marker of the average plasma glucose concentrations over approximately 3 mo. Grotz et al. 74 instructed patients with diabetes to self-administer capsules of sucralose twice daily over a 3-mo period; however, data on compliance with self-administration (e.g., urinary sucralose levels) and body weight changes were not reported. Daily supervision of capsule administration, rather than self-administration, is scientifically prudent for studies of patients with diabetes because noncompliance with treatment regimens is reportedly high in this population 75. Further, no apparent information of dissolution characteristics of the capsule was provided; thus, it is unknown where sucralose was released in the GIT. Future studies of sucralose on diabetes management will require randomized trials that are carefully supervised and control for the variables involved.
Sucralose vs Aspartame
Aspartame is approved for use in food as a nutritive sweetener. Aspartame brand names include Nutrasweet®, Equal®, and Sugar Twin®. It does contain calories, aspartame, the only approved nutritive high-intensity sweetener, contains more than two percent of the calories in an equivalent amount of sugar, as opposed to non-nutritive sweeteners that contain less than two percent of the calories in an equivalent amount of sugar. But because it is about 200 times sweeter than table sugar, it is assumed that consumers are likely to use much less of it.
FDA approved aspartame in 1981 76 for uses, under certain conditions, as a tabletop sweetener, in chewing gum, cold breakfast cereals, and dry bases for certain foods (i.e., beverages, instant coffee and tea, gelatins, puddings, and fillings, and dairy products and toppings). In 1983 77, FDA approved the use of aspartame in carbonated beverages and carbonated beverage syrup bases, and in 1996, FDA approved it for use as a “general purpose sweetener” 78.
- Aspartame is not heat stable and loses its sweetness when heated, so it typically isn’t used in baked goods.
Aspartame is one of the most exhaustively studied substances in the human food supply, with more than 100 studies supporting its safety.
FDA scientists have reviewed scientific data regarding the safety of aspartame in food and concluded that it is safe for the general population under certain conditions. However, people with a rare hereditary disease known as phenylketonuria have a difficult time metabolizing phenylalanine, a component of aspartame, and should control their intake of phenylalanine from all sources, including aspartame. Labels of aspartame-containing foods and beverages must include a statement that informs individuals with phenylketonuria that the product contains phenylalanine 2.
Sucralose vs Stevia
Steviol glycosides (also referred to as Rebaudioside A, Reb-A, or rebiana) are natural constituents of the leaves of Stevia rebaudiana (Bertoni) Bertoni, a plant native to parts of South America and commonly known as Stevia. Highly purified extracts from the leaves of the Stevia rebaudiana are called “steviol glycosides.” They are non-nutritive sweeteners and are reported to be 200 to 400 times sweeter than table sugar 2.
Stevia is found in many processed foods and drinks, such as desserts, chewing gum, baked goods, candy, and yogurt. It is also used as a tabletop sweetener. Stevia can be used as a substitute for sugar when you are baking. For best results, follow the package instructions for using it in your recipes.
FDA has received many “generally recognized as safe” GRAS Notices for the use of high-purity (95% minimum purity) steviol glycosides including Rebaudioside A (also known as Reb A), Stevioside, Rebaudioside D, or steviol glycoside mixture preparations with Rebaudioside A and/or Stevioside as predominant components. This means that qualified experts agree the available scientific evidence about this sugar substitute shows it is safe for use in foods and drinks. FDA has not questioned the notifiers’ GRAS determinations for these high-purity stevia derived sweeteners under the intended conditions of use identified in the GRAS notices submitted to FDA. FDA’s response letters on such high-purity steviol glycosides are available at FDA’s GRAS Notice Inventory website 79.
- However, the use of stevia leaf and crude stevia extracts is not considered GRAS and their import into the United States is not permitted for use as sweeteners. For details, see Import Alert 45-06 80.
A lot of food manufacturers use the term “natural” to describe their products. But there is no industry-approved definition of the word, so its use is often meaningless 9. When it comes to non-nutritive sweeteners, “natural” usually refers to those that are made from a substance found in nature and/or without artificial or synthetic additives e.g. Stevia. Artificial sweeteners, on the other hand, are created in a laboratory e.g. Sucralose.
But there is no research to suggest that non-nutritive natural sweeteners are healthier or better for you in any way compared with their competitors 9.
Natural sweeteners can also refer to sweeteners with calories, such as maple syrup, molasses, barley malt and rice syrups, honey, agave nectar, coconut sugar, date sugar, and sucanat. These sweeteners are essentially less processed and arguably better for you than sugar because they contain some trace vitamins and minerals. But don’t get excited—sugar is really still sugar, and it’s far from a healthy food choice. A good goal: no more sugar from all sources than the equivalent of 6 teaspoons for women and 9 teaspoons for men each day 9.
- U.S. Food and Drug Administration. CFR – Code of Federal Regulations Title 21. Sec. 172.831 Sucralose. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=172.831[↩]
- U.S. Food and Drug Administration. Additional Information about High-Intensity Sweeteners Permitted for use in Food in the United States. https://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm397725.htm[↩][↩][↩][↩][↩][↩][↩][↩]
- Biocatalytic synthesis of disaccharide high-intensity sweeterner sucralose via a tetrachlororaffinose intermediate. Bennett C, Dordick JS, Hacking AJ, Cheetham PS. Biotechnol Bioeng. 1992 Jan 20; 39(2):211-7. https://www.ncbi.nlm.nih.gov/pubmed/18600933/[↩]
- Anderson M., Opawale F., Rao M., Delmarre D., Anyarambhatla G. Excipients for oral liquid formulations. In: Katdare A., Chaubal M. V., editors. Excipient development for pharmaceutical biotechnology, and drug delivery systems. New York, NY: Informa Healthcare USA; 2006. pp. 155–180.[↩]
- Li X., Du Z., Huang X., Yuan W., Ying H. Solubility of sucralose in different solvents from (283.15 to 333.15) K. J. Chem. Eng. Data. 2010;55:2600–2602.[↩]
- Sweeteners: state of knowledge review. Schiffman SS, Gatlin CA. Neurosci Biobehav Rev. 1993 Fall; 17(3):313-45. https://www.ncbi.nlm.nih.gov/pubmed/8272285/[↩]
- Involvement of cytochrome P450 2E1 in the (omega-1)-hydroxylation of oleic acid in human and rat liver microsomes. Adas F, Berthou F, Picart D, Lozac’h P, Beaugé F, Amet Y. J Lipid Res. 1998 Jun; 39(6):1210-9. https://www.ncbi.nlm.nih.gov/pubmed/9643352/[↩][↩]
- EU Scientific Committee on Food. Sucralose in food. https://ec.europa.eu/jrc/en/interlaboratory-comparison/sucralose-food[↩]
- American Diabetes Association. 5 Must-Know Facts About Sweeteners. http://www.diabetesforecast.org/2016/jan-feb/5-must-know-facts-about-sweeteners.html[↩][↩][↩][↩][↩][↩]
- Miller PE, Perez V. Low-calorie sweeteners and body weight and composition: a meta-analysis of randomized controlled trials and prospective cohort studies. The American Journal of Clinical Nutrition. 2014;100(3):765-777. doi:10.3945/ajcn.113.082826. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4135487/[↩][↩]
- American Diabetes Association. Not Completely Carbohydrate and Calorie-Free. http://www.diabetes.org/food-and-fitness/food/what-can-i-eat/understanding-carbohydrates/artificial-sweeteners/not-completely-carbohydrate.html[↩]
- Joint Expert Commission on Food Additives of the World Health Organization and the Food and Agriculture Organization. http://www.codexalimentarius.net/web/jecfa.jsp[↩]
- American Diabetes Association. Using Sugar Substitutes in the Kitchen. http://www.diabetes.org/food-and-fitness/food/what-can-i-eat/understanding-carbohydrates/artificial-sweeteners/using-sugar-substitutes.html[↩]
- Schiffman SS, Rother KI. Sucralose, A Synthetic Organochlorine Sweetener: Overview of Biological Issues. Journal of Toxicology and Environmental Health Part B, Critical Reviews. 2013;16(7):399-451. doi:10.1080/10937404.2013.842523. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3856475/[↩][↩][↩]
- Schiffman S. S. Rationale for further medical and health research on high-potency sweeteners. Chem. Senses. 2012;37:671–679. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3440882/[↩][↩]
- Schiffman S. S., Abou-Donia M. B. Sucralose revisited: Rebuttal of two papers about Splenda safety. Regul. Toxicol. Pharmacol. 2012;63:505–508. https://www.ncbi.nlm.nih.gov/pubmed/22579627[↩]
- Sims J., Roberts A., Daniel J. W., Renwick A. G. The metabolic fate of sucralose in rats. Food Chem. Toxicol. 2000;38(suppl. 2):S115–S121. https://www.ncbi.nlm.nih.gov/pubmed/10882824[↩][↩]
- Roberts A., Renwick A. G., Sims J., Snodin D. J. Sucralose metabolism and pharmacokinetics in man. Food Chem. Toxicol. 2000;38(suppl. 2):S31–S41. https://www.ncbi.nlm.nih.gov/pubmed/10882816[↩]
- Grice H. C., Goldsmith L. A. Sucralose—An overview of the toxicity data. Food Chem. Toxicol. 2000;38(suppl. 2):S1–S6. https://www.ncbi.nlm.nih.gov/pubmed/10882813[↩]
- Molinary S. V., Quinlan M. E. Sucralose. In: Mitchell H., editor. Sweeteners and sugar alternatives in food technology. Oxford, UK: Blackwell; 2006. pp. 130–148.[↩]
- Grotz V. L., Munro I. C. An overview of the safety of sucralose. Regul. Toxicol. Pharmacol. 2009;55:1–5. https://www.ncbi.nlm.nih.gov/pubmed/19464334[↩]
- Hawkins D. R., Wood S. W., Waller A. R., Jordan M. C. Enzyme induction studies of TGS and TGS-HP in the rat. 1987. Unpublished report from Huntingdon Research Centre, Huntingdon, U.K. Submitted to the World Health Organization by Tate & Lyle. Referenced in WHO (World Health Organization). 1989. Trichlorogalactosucrose. In Toxicological evaluation of certain food additives and contaminants WHO Food Additives Series, no. 24. Cambridge University Press, 1989, no. 655. http://www.inchem.org/documents/jecfa/jecmono/v024je05.htm[↩]
- Sasaki Y. F., Kawaguchi S., Kamaya A., Ohshita M., Kabasawa K., Iwama K., Taniguchi K., Tsuda S. The comet assay with 8 mouse organs: Results with 39 currently used food additives. Mutat. Res. 2002;519:103–119. https://www.ncbi.nlm.nih.gov/pubmed/12160896[↩]
- Hutchinson S. A. The effect of pH, temperature and reactants on the thermal and non-thermal degradation of the high-intensity sweeteners: Alitame and sucralose. 1996. PhD dissertation. New Brunswick, NJ: Rutgers University.[↩]
- Hutchinson S. A., Ho G. S., Ho C. T. Stability and degradation of the high-intensity sweeteners: Aspartame, alitame, and sucralose. Food Rev. Int. 1999;15:249–261.[↩]
- Bannach G., Almeida R. R., Lacerda L. G., Schnitzler E., Ionashiro M. Thermal stability and thermal decomposition of sucralose. Ecl. Quím. São Paulo. 2009;34:21–26.[↩]
- Rahn A., Yaylayan V. A. Thermal degradation of sucralose and its potential in generating chloropropanols in the presence of glycerol. Food Chem. 2010;118:56–61.[↩][↩]
- European Union. Directive 2003/115/EC of the European Parliament and of the Council of 22 December 2003 amending Directive 94/35/EC on sweeteners for use in foodstuffs. Off. J. Eur. Union. 2004;47(L24):65–71. http://eur-lex.europa.eu/legal-content/en/ALL/?uri=OJ:L:2004:024:TOC[↩]
- National Cancer Institute at the National Institutes of Health. Artificial Sweeteners and Cancer. https://www.cancer.gov/about-cancer/causes-prevention/risk/diet/artificial-sweeteners-fact-sheet[↩][↩]
- Patel RM, Sarma R, Grimsley E. Headache. 2006 Sep;46(8):1303-4. Popular sweetner sucralose as a migraine trigger.[↩]
- Young D. A., Bowen W. H. The influence of sucralose on bacterial metabolism. J. Dent. Res. 1990;69:1480–1484. https://www.ncbi.nlm.nih.gov/pubmed/2143512[↩]
- Labare M. P., Alexander M. Microbial cometabolism of sucralose, a chlorinated disaccharide, in environmental samples. Appl. Microbiol. Biotechnol. 1994;42:173–178. https://www.ncbi.nlm.nih.gov/pubmed/7765816[↩]
- Abou-Donia M. B., El-Masry E. M., Abdel-Rahman A. A., McLendon R. E., Schiffman S. S. Splenda alters gut microflora and increases intestinal P-glycoprotein and cytochrome P-450 in male rats. J. Toxicol. Environ. Health A. 2008;71:1415–1429. https://www.ncbi.nlm.nih.gov/pubmed/18800291[↩]
- Prashant G. M., Patil R. B., Nagaraj T., Patel V. B. The antimicrobial activity of the three commercially available intense sweeteners against common periodontal pathogens: An in vitro study. J. Contemp. Dent. Pract. 2012;13:749–752. https://www.ncbi.nlm.nih.gov/pubmed/23403996[↩]
- Jang H. J., Kokrashvili Z., Theodorakis M. J., Carlson O. D., Kim B. J., Zhou J., Kim H. H., Xu X., Chan S. L., Juhaszova M., Bernier M., Mosinger B., Margolskee R. F., Egan J. M. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc. Natl. Acad. Sci. USA. 2007;104:15069–15074. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1986614/[↩]
- Margolskee R. F., Dyer J., Kokrashvili Z., Salmon K. S. H., Ilegems E., Daly K., Maillet E. L., Ninomiya Y., Mosinger B., Shirazi-Beechey S. P. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc. Natl. Acad. Sci. USA. 2007;104:15075–15080. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1986615/[↩]
- Nakagawa Y., Nagasawa M., Yamada S., Hara A., Mogami H., Nikolaev V. O., Lohse M. J., Shigemura N., Ninomiya Y., Kojima I. Sweet taste receptor expressed in pancreatic ß-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS One. 2009;4:e5106. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2663034/[↩]
- Ren X., Zhou L., Terwilliger R., Newton S. S., de Araujo I. E. Sweet taste signaling functions as a hypothalamic glucose sensor. Front. Integr. Neurosci. 2009;3:12. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2706652/[↩][↩]
- Murphy KG, Bloom SR (2006). Gut hormones and the regulation of energy homeostasis. Nature 444, 854–859. https://www.nature.com/nature/journal/v444/n7121/full/nature05484.html[↩]
- Ghatei MA, Uttenthal LO, Christofides ND, Bryant MG, Bloom SR (1983). Molecular forms of human enteroglucagon in tissue and plasma: plasma responses to nutrient stimuli in health and in disorders of the upper gastrointestinal tract. J Clin Endocrinol Metab 57, 488–495. https://www.ncbi.nlm.nih.gov/pubmed/6874888?dopt=Abstract[↩]
- Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR (1985). Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology 89, 1070–1077. https://www.ncbi.nlm.nih.gov/pubmed/3840109?dopt=Abstract[↩]
- Le Roux CW, Batterham RL, Aylwin SJ, Patterson M, Borg CM, Wynne KJ et al. (2006). Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology 147, 3–8. https://www.ncbi.nlm.nih.gov/pubmed/16166213?dopt=Abstract[↩][↩]
- Freeman J. S. Role of the incretin pathway in the pathogenesis of type 2 diabetes mellitus. Cleve. Clin. J. Med. 2009;76(suppl. 5):S12–S19. https://www.ncbi.nlm.nih.gov/pubmed/19952298[↩]
- Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL et al. (2002). Gut hormone PYY(3-36) physiologically inhibits food intake. Nature 418, 650–654. https://www.ncbi.nlm.nih.gov/pubmed/12167864?dopt=Abstract[↩][↩]
- Challis BG, Pinnock SB, Coll AP, Carter RN, Dickson SL, O’rahilly S (2003). Acute effects of PYY3-36 on food intake and hypothalamic neuropeptide expression in the mouse. Biochem Biophys Res Commun 311, 915–919. https://www.ncbi.nlm.nih.gov/pubmed/14623268?dopt=Abstract[↩]
- Halatchev IG, Ellacott KL, Fan W, Cone RD (2004). Peptide YY3-36 inhibits food intake in mice through a melanocortin-4 receptor-independent mechanism. Endocrinology 145, 2585–2590. https://www.ncbi.nlm.nih.gov/pubmed/15016721?dopt=Abstract[↩]
- Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL (2005). Peripheral exendin-4 and peptide YY(3-36) synergistically reduce food intake through different mechanisms in mice. Endocrinology 146, 3748–3756. https://www.ncbi.nlm.nih.gov/pubmed/15932924?dopt=Abstract[↩]
- Chelikani PK, Haver AC, Reeve Jr JR, Keire DA, Reidelberger RD (2006). Daily, intermittent intravenous infusion of peptide YY(3-36) reduces daily food intake and adiposity in rats. Am J Physiol Regul Integr Comp Physiol 290, R298–R305. https://www.ncbi.nlm.nih.gov/pubmed/16210414?dopt=Abstract[↩]
- Flint A, Raben A, Astrup A, Holst JJ (1998). Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest 101, 515–520. https://www.ncbi.nlm.nih.gov/pubmed/9449682?dopt=Abstract[↩]
- Gutzwiller JP, Goke B, Drewe J, Hildebrand P, Ketterer S, Handschin D et al. (1999). Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut 44, 81–86. https://www.ncbi.nlm.nih.gov/pubmed/9862830?dopt=Abstract[↩]
- Degen L, Oesch S, Casanova M, Graf S, Ketterer S, Drewe J et al. (2005). Effect of peptide YY3-36 on food intake in humans. Gastroenterology 129, 1430–1436. https://www.ncbi.nlm.nih.gov/pubmed/16285944?dopt=Abstract[↩]
- Ford HE, Peters V, Martin NM, Sleeth ML, Ghatei MA, Frost GS, Bloom SR. Eur J Clin Nutr. 2011 Apr;65(4):508-13. doi: 10.1038/ejcn.2010.291. Epub 2011 Jan 19. Effects of oral ingestion of sucralose on gut hormone response and appetite in healthy normal-weight subjects. https://www.nature.com/ejcn/journal/v65/n4/full/ejcn2010291a.html[↩][↩]
- Pepino M. Y., Tiemann C. D., Patterson B. W., Wice B. M., Klein S. Sucralose affects glycemic and hormonal responses to an oral glucose load. Diabetes Care. 2013;36:2530–2535. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3747933/[↩]
- American Heart Association. Artificial sweeteners may increase blood sugar. http://news.heart.org/artificial-sweeteners-may-increase-blood-sugar/[↩][↩]
- Nature doi:10.1038/nature13793. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. https://www.nature.com/nature/journal/vaop/ncurrent/pdf/nature13793.pdf[↩][↩][↩]
- A Scientific Statement From the American Heart Association and the American Diabetes Association. Nonnutritive Sweeteners: Current Use and Health Perspectives. http://circ.ahajournals.org/content/circulationaha/126/4/509.full.pdf[↩]
- Azad MB, Abou-Setta AM, Chauhan BF, et al. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ : Canadian Medical Association Journal. 2017;189(28):E929-E939. doi:10.1503/cmaj.161390. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5515645/[↩][↩]
- U.S. National Library of Medicine. Medline Plus. Could Artificial Sweeteners Raise Your Odds for Obesity ?. https://medlineplus.gov/news/fullstory_167249.html[↩][↩]
- Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Fowler SP, Williams K, Resendez RG, Hunt KJ, Hazuda HP, Stern MP. Obesity (Silver Spring). 2008 Aug; 16(8):1894-900. https://www.ncbi.nlm.nih.gov/pubmed/18535548/[↩]
- Artificial sweetener use and one-year weight change among women. Stellman SD, Garfinkel L. Prev Med. 1986 Mar; 15(2):195-202. https://www.ncbi.nlm.nih.gov/pubmed/3714671/[↩]
- Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, D’Agostino RB, Gaziano JM, Vasan RS. Circulation. 2007 Jul 31; 116(5):480-8. https://www.ncbi.nlm.nih.gov/pubmed/17646581/[↩]
- Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Lutsey PL, Steffen LM, Stevens J. Circulation. 2008 Feb 12; 117(6):754-61. https://www.ncbi.nlm.nih.gov/pubmed/18212291/[↩]
- Gain weight by “going diet?” Artificial sweeteners and the neurobiology of sugar cravings: Neuroscience 2010. Yang Q. Yale J Biol Med. 2010 Jun; 83(2):101-8. https://www.ncbi.nlm.nih.gov/pubmed/20589192/[↩]
- A role for sweet taste: calorie predictive relations in energy regulation by rats. Swithers SE, Davidson TL. Behav Neurosci. 2008 Feb; 122(1):161-73. https://www.ncbi.nlm.nih.gov/pubmed/18298259/[↩][↩]
- General and persistent effects of high-intensity sweeteners on body weight gain and caloric compensation in rats. Swithers SE, Baker CR, Davidson TL. Behav Neurosci. 2009 Aug; 123(4):772-80. https://www.ncbi.nlm.nih.gov/pubmed/19634935/[↩][↩]
- High-intensity sweeteners and energy balance. Swithers SE, Martin AA, Davidson TL. Physiol Behav. 2010 Apr 26; 100(1):55-62. https://www.ncbi.nlm.nih.gov/pubmed/20060008/[↩]
- Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Swithers SE. Trends Endocrinol Metab. 2013 Sep; 24(9):431-41. https://www.ncbi.nlm.nih.gov/pubmed/23850261/[↩][↩]
- Patterns of weight change and their relation to diet in a cohort of healthy women. Colditz GA, Willett WC, Stampfer MJ, London SJ, Segal MR, Speizer FE. Am J Clin Nutr. 1990 Jun; 51(6):1100-5. https://www.ncbi.nlm.nih.gov/pubmed/2349925/[↩]
- Inverse association between the effect of carbohydrates on blood glucose and subsequent short-term food intake in young men. Anderson GH, Catherine NL, Woodend DM, Wolever TM. Am J Clin Nutr. 2002 Nov; 76(5):1023-30. https://www.ncbi.nlm.nih.gov/pubmed/12399274/[↩]
- A trial of sugar-free or sugar-sweetened beverages and body weight in children. de Ruyter JC, Olthof MR, Seidell JC, Katan MB. N Engl J Med. 2012 Oct 11; 367(15):1397-406. https://www.ncbi.nlm.nih.gov/pubmed/22998340/[↩]
- A randomized trial of sugar-sweetened beverages and adolescent body weight. Ebbeling CB, Feldman HA, Chomitz VR, Antonelli TA, Gortmaker SL, Osganian SK, Ludwig DS. N Engl J Med. 2012 Oct 11; 367(15):1407-16. https://www.ncbi.nlm.nih.gov/pubmed/22998339/[↩]
- Sucrose activates human taste pathways differently from artificial sweetener. Frank GK, Oberndorfer TA, Simmons AN, Paulus MP, Fudge JL, Yang TT, Kaye WH. Neuroimage. 2008 Feb 15; 39(4):1559-69. https://www.ncbi.nlm.nih.gov/pubmed/18096409/[↩]
- Amygdala response to sucrose consumption is inversely related to artificial sweetener use. Rudenga KJ, Small DM. Appetite. 2012 Apr; 58(2):504-7. https://www.ncbi.nlm.nih.gov/pubmed/22178008/[↩]
- Lack of effect of sucralose on glucose homeostasis in subjects with type 2 diabetes. Grotz VL, Henry RR, McGill JB, Prince MJ, Shamoon H, Trout JR, Pi-Sunyer FX. J Am Diet Assoc. 2003 Dec; 103(12):1607-12. https://www.ncbi.nlm.nih.gov/pubmed/14647086/[↩][↩]
- World Health Organization. Obesity and overweight 2016. Updated June 2016. http://www.who.int/mediacentre/factsheets/fs311/en/[↩]
- U.S. Food and Drug Administration. Aspartame; Commissioner’s Final Decision. Friday 24 July, 1981. https://www.fda.gov/downloads/Food/IngredientsPackagingLabeling/FoodAdditivesIngredients/UCM404380.pdf[↩]
- U.S. Food and Drug Administration. Aspartame. https://www.fda.gov/downloads/Food/IngredientsPackagingLabeling/FoodAdditivesIngredients/UCM404332.pdf[↩]
- U.S. Food and Drug Administration. Aspartame. https://www.gpo.gov/fdsys/pkg/FR-1996-06-28/pdf/96-16522.pdf[↩]
- U.S. Food and Drug Administration. GRAS Notices. https://www.accessdata.fda.gov/scripts/fdcc/?set=GRASNotices[↩]
- U.S. Food and Drug Administration. Import Alert 45-06. https://www.accessdata.fda.gov/cms_ia/importalert_119.html[↩]