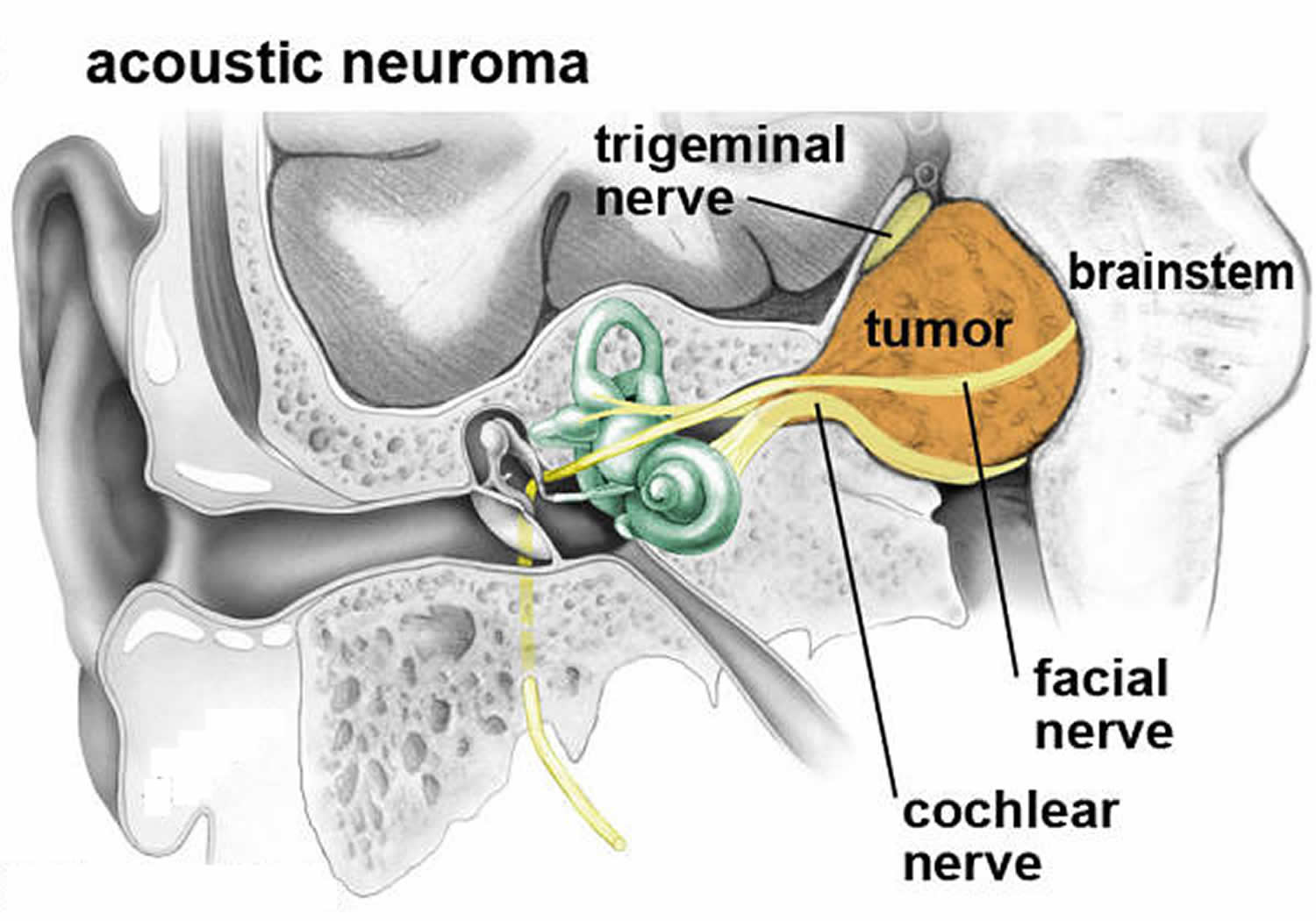
What is acoustic neuroma
Acoustic neuroma also known as vestibular schwannoma, acoustic neurinoma, acoustic neurofibroma or acoustic neurilemoma, is a rare benign (non-cancerous), usually slow-growing tumor that develops from the balance and hearing nerves supplying the inner ear – the 8th cranial nerve (also known as the acoustic nerve or vestibulocochlear nerve) consists of the vestibular and cochlear divisions, which runs from the inner ear to the brain. Branches of the vestibular nerve directly influence your balance and branches of the cochlear division influence your hearing, and pressure from an acoustic neuroma can cause hearing loss, ringing in your ear and unsteadiness. Acoustic neuroma tumor comes from an overproduction of Schwann cells–the cells that normally wrap around nerve fibers like onion skin to help support and insulate nerves. Acoustic neuromas generally originate within the internal auditory canal and may cause bony erosion as they expand. Anatomically, acoustic neuroma is the most common tumor in the cerebellopontine angle region (over 80% of tumors in the cerebellopontine angle) and surgical resection is the preferred treatment for most of the patients 1. A untreated acoustic neuroma tumor will protrude further into the brain and may eventually compromise other nerves and brainstem function. As the acoustic neuroma grows, it presses against the hearing and balance nerves (the 8th cranial nerve or vestibulocochlear nerve), usually causing unilateral (one-sided) or asymmetric hearing loss, tinnitus (ringing in the ear), and dizziness/loss of balance. As the tumor grows, it can interfere with the face sensation nerve (the 5th cranial nerve or the trigeminal nerve), causing facial numbness. Acoustic neuromas can also affect the facial nerve (the 7th cranial nerve for the muscles of the face) causing facial weakness or paralysis on the side of the tumor. If the tumor becomes large, it will eventually press against nearby brain structures (such as the brainstem and the cerebellum), becoming life-threatening. Acoustic neuromas do not spread (metastasize) to other parts of the body. The brain is not invaded by the acoustic tumor, but the tumor pushes on the brain as it enlarges.
Acoustic neuromas occur throughout the world. No ethnic groups are affected disproportionately. It is estimated that the instances of acoustic neuroma are between 1 to 3.5 in every 100,000 to 5 in every million people. Acoustic tumors constitute 6%-10% of all brain tumors and are found in roughly one of every 100,000 people per year in the United States. This translates to about 2,500-3,000 newly diagnosed acoustic tumors per year. Acoustic neuromas more common in men and between the ages of 30-60 years.
Acoustic neuromas are nearly always slow growing, do not spread, and are thought of as benign tumors. Often they have been there a long time by the time they are diagnosed. Acoustic neuromas are found most often in older people. Loss of hearing in one ear can be a sign of acoustic neuroma.
Rarely, acoustic neuromas are associated with a genetic condition called neurofibromatosis. People with neurofibromatosis are usually diagnosed at a much younger age and tumors might be on both sides of the brain (bilateral) 1. Those affected might also develop another type of brain tumor called meningiomas.
Because the acoustic neuroma grows so slowly, the symptoms are easy to miss or misinterpret. The earliest and most common symptoms of an acoustic neuroma are a gradual reduction in hearing in one ear and tinnitus, a ringing or noisy sound in the ear.
Other possible symptoms of acoustic neuroma that may occur, depending on the extent of the tumor, include: dizziness, loss of balance or clumsiness facial paralysis, numbness or tingling, headache, a feeling of something clogging the ear, or mental confusion.
Most of these tumors grow slowly, taking years before they become large enough to cause symptoms. Once diagnosed, they must be treated or carefully observed; they do not resolve on their own.
Types of acoustic neuroma
- Unilateral (one side) occurs spontaneously without any evidence of family history, accounts for 95% of acoustic neuromas.
- Bilateral (both sides) is most likely caused by a genetic condition called neurofibromatosis type 2 (NF2) which affects approximately 1 in every 40,000 people. Neurofibromatosis type 2 (NF2) is caused by a mutation in the neurofibromin 2 gene (NF2 gene) located on chromosome 22 (chromosome 22q12.2) which regulates the production of a protein known as merlin (schwannomin) that plays a role in suppressing the development of certain tumors (tumor suppressor). According to investigators, merlin (schwannomin) is related to a class of proteins (ezrin-radixin-moesin proteins) that serve to link the internal, supportive system within a cell (cytoskeleton) to proteins in cell membranes. Several different mutations of the NF2 gene have been identified in individuals with neurofibromatosis type 2 (NF2) (e.g., deletions, nonsense and frameshift mutations). Investigators suggest that different mutations of the NF2 gene (neurofibromin 2 gene) may contribute to the wide variability of symptoms and findings in affected individuals. Neurofibromatosis type 2 will be suspected if a parent/sibling/child of the patient has neurofibromatosis type 2 and acoustic neuroma occurs before age 30 or if the patient has had a meningioma, glioma, or cataract.
Treatment will depend on the size of your tumor. The treatment options are observation (watchful waiting), surgical removal or radiation therapy. The choice of treatment may be based on tumor size, hearing in the ear at time of diagnosis, patient age and health, and patient preference.
Figure 1. Vestibulocochlear nerve (Cranial nerve 8)

Footnote: The normal anatomy of the ear. The outer ear funnels sound to the eardrum, which vibrates three tiny bones called ossicles (malleus, incus and stapes). The spiral-shaped cochlea is filled with liquid, which moves in response to vibrations. As the fluid moves, thousands of hair cells are stimulated, sending signals along the cochlear nerve (responsible for hearing) to the brain. Attached to the cochlea are three semicircular canals positioned at right angles to each other. The three canals are able to sense head position and posture. Electrical signals from the semicircular canals are carried to the brain by the superior and inferior vestibular nerves (responsible for balance). The cochlear and vestibular nerves form a bundle inside the bony internal auditory canal. Inside the canal, the vestibulocochlear nerve lies next to the facial nerve (responsible for facial movement).
Figure 2. General structure of a neuron
Figure 3. Schwann cell and myelination
Footnote: (a) A Schwann cell of the peripheral nervous system, wrapping repeatedly around an axon to form the multilayered myelin sheath.
The myelin spirals outward away from the axon as it is laid down.
(b) An oligodendrocyte of the central nervous system (brain and spinal cord) wrapping around the axons of multiple neurons. Here, the myelin spirals inward toward the axon as it is laid down.
(c) A myelinated axon (top) and unmyelinated axon (bottom).
Figure 4. Acoustic neuroma
 Footnote: An acoustic neuroma expands out of the internal auditory canal displacing the cochlear, facial and trigeminal nerves located in the cerebellopontine angle. Eventually the tumor can compress the brainstem.
Footnote: An acoustic neuroma expands out of the internal auditory canal displacing the cochlear, facial and trigeminal nerves located in the cerebellopontine angle. Eventually the tumor can compress the brainstem.Figure 5. Acoustic neuroma
Footnote: Acoustic neuromas are classified according to their size. MRI scans and correlative illustrations of small (intracanalicular), medium and large acoustic neuromas.
Figure 6. Acoustic neuroma MRI
Acoustic neuroma causes
The exact cause of an acoustic neuroma is unknown. Most acoustic neuroma cases seem to arise for no apparent reason (spontaneously) and in about 5 percent of acoustic neuroma cases due to a mutation in the neurofibromin 2 gene (NF2 gene) located on chromosome 22 (chromosome 22q12.2) 2. Normally, the NF2 gene (neurofibromin 2 gene) regulates the production of a protein known as merlin (schwannomin) that plays a role in suppressing the development of certain tumors (tumor suppressor). According to investigators, merlin (schwannomin) is related to a class of proteins (ezrin-radixin-moesin proteins) that serve to link the internal, supportive system within a cell (cytoskeleton) to proteins in cell membranes.
What makes neurofibromin 2 gene (NF2 gene) malfunction isn’t clear, and in most cases of acoustic neuroma, there is no identifiable cause. Radiation exposure may predispose a patient to the development of acoustic neuroma as well 3. Even though mobile phone radiation has been of concern, several studies have failed to prove its causative effect on acoustic neuromas 4, 5.
An acoustic neuroma arises from a type of cell known as the Schwann cell. These cells form an insulating layer over all nerves of the peripheral nervous system (i.e., nerves outside of the central nervous system) including the eighth cranial nerve. The eighth cranial nerve is separated into two branches the cochlear branch, which transmits sound to the brain and the vestibular branch, which transmits balance information to the brain. Most acoustic neuromas occur on the vestibular portion of the eighth cranial nerve. Because acoustic neuroma tumors are made up of Schwann cells and usually occur on the vestibular portion of the eighth cranial nerve, many physicians prefer the use of the term vestibular schwannoma. However, the term acoustic neuroma is still used more often in the medical literature.
Acoustic neuroma pathogenesis
Inactivation of the NF2 tumor suppressor gene is considered a major event in the tumorigenesis of conventional schwannoma 1. A recent whole exome (DNA sequences that code for proteins) sequencing study demonstrated that 77% of acoustic neuroma show evidence of genomic inactivation of NF2 gene via loss of chromosome 22q or NF2 gene mutation 6. NF2 tumor suppressor gene inactivation is the most highly recurrent genomic alteration in acoustic neuroma 1. Biallelic inactivation can be demonstrated by exome sequencing in 45% of cases, whereas in 41% of cases only one hit either by heterozygous chromosome 22q deletion or NF2 mutation is evident. In 14% of cases no genomic hit in NF2 can be detected by exome sequencing. However, the consistent lack of the NF2 gene product merlin in the tumor cells of acoustic neuroma suggests that in cases without evidence for genetic inactivation, epigenetic mechanisms of NF2 silencing or mutational events in regions not covered by exome sequencing likely exist 7. Another recent whole exome sequencing study reports concordant results regarding NF2 alterations in acoustic neuroma 8. However, there are discrepancies between both studies regarding alterations in non-NF2 genes. While one study found ARID1A (14%), ARID1B (18%), DDR1 (11%), TSC1 (9%), TSC2 (7%), CAST (8%), ALPK2 (8%), LZTR1 (8%), and TAB3 (3%) as additional genes recurrently altered in (vestibular) schwannomas, the other study did not find recurrent somatic mutations in these genes but in CDC27 (11%) and USP8 (7%) 6. Therefore, further studies are required to clarify the role of non-NF2 gene mutations in schwannoma pathogenesis.
RNA sequencing revealed recurrent SH3PXD2A-HTRA1 fusions on chromosome 10 in about 10% of acoustic neuroma associated with a male predominance and partly co-occurring with genetic NF2 inactivation 6. Although the precise biochemical consequences of acquiring this fusion remain to be elucidated, activation of the MEK-ERK pathway seems to be involved.
Biallelic inactivation of PRKAR1A by deletion and/or mutation is considered a major event in the pathogenesis of melanotic schwannoma 9. In addition, melanotic schwannomas frequently show monosomies of chromosomes 1, 2, 17, and 22q as well as variable whole chromosomal gains 10.
Risk factors for acoustic neuroma
No specific risk factors for the development of acoustic neuromas have been identified. A variety of potential risk factors for acoustic neuroma have been studied including prior exposure to radiation to the head and neck area (as is done to treat certain cancers) or prolonged or sustained exposure to loud noises (as in an occupational setting). Research is under way to determine the specific cause and risk factors associated with an acoustic neuroma.
In a small subset of acoustic neuroma cases, acoustic neuromas occur as part of a rare disorder known as neurofibromatosis type 2 (NF2). This rare genetic disorder is usually associated with acoustic neuromas affecting both ears at once (bilateral).
Neurofibromatosis type 2
The only confirmed risk factor for acoustic neuroma is having a parent with the rare genetic disorder neurofibromatosis type 2 (NF2). But neurofibromatosis type 2 only accounts for about 5 percent of acoustic neuroma cases 11. Neurofibromatosis type 2 (NF2) is a rare disorder that affects males and females in equal numbers. All races and ethnic groups are equally affected by this disorder. Neurofibromatosis type 2 occurs with a frequency of 1 in 30,000 to 1 in 50,000 births 12.
Neurofibromatosis type 2 (NF2) is a genetic disorder caused by a mutation in the NF2 gene (neurofibromin 2 gene). The NF2 gene regulates (encodes for) the production of a protein known as merlin (schwannomin) that plays a role in suppressing the development of certain tumors (tumor suppressor). According to investigators, merlin (schwannomin) is related to a class of proteins (ezrin-radixin-moesin proteins) that serve to link the internal, supportive system within a cell (cytoskeleton) to proteins in cell membranes. Several different mutations of the NF2 gene have been identified in individuals with neurofibromatosis type 2 (e.g., deletions, nonsense and frameshift mutations). Investigators suggest that different mutations of the NF2 gene may contribute to the wide variability of symptoms and findings in affected individuals.
In some individuals with neurofibromatosis type 2, the disorder is inherited in an autosomal dominant pattern. Dominant genetic disorders occur when only a single copy of an abnormal gene is necessary to cause a particular disease. The abnormal gene can be inherited from either parent. The risk of passing the abnormal gene from affected parent to offspring is 50% for each pregnancy. This means each child of an affected parent has a 50-50 chance of inheriting it. The risk is the same for males and females.
In other individuals with neurofibromatosis type 2, there is no family history of the disease. In such cases, neurofibromatosis type 2 is caused by a new gene mutation.
Neurofibromatosis type 2 (NF2) should be considered when an individual presents with a unilateral vestibular or other sporadic schwannoma at <30 years or meningioma at <25 years 13.
A hallmark characteristic of neurofibromatosis type 2 is the development of noncancerous acoustic neuromas on both sides of your head, as well as on other nerves. This creates the perplexing problem of the possibility of complete deafness if the tumors are left to grow unchecked. Preventing or treating the complete deafness that may befall individuals with neurofibromatosis type 2 requires complex decision making. The trend at most academic U.S. medical centers is to recommend treatment for the smallest tumor which has the best chance of preserving hearing. If this goal is successful, then treatment can also be offered for the remaining tumor. If hearing is not preserved at the initial treatment, then usually the second tumor, in the only-hearing ear, is just observed. If it shows continued growth and becomes life-threatening, or if the hearing is lost over time as the tumor grows, then treatment is undertaken. This strategy has the highest chance of preserving hearing for the longest time possible.
There are now several options to try to rehabilitate deafness in neurofibromatosis type 2 patients. Implanting the hearing part of the brainstem (auditory brainstem implant) can help restore some sound perception to these patients. Also cochlear implants can be used if the cochlear nerve is preserved following surgery. Radiosurgery may be an option although stereotactic radiosurgery may not have the effect on the neurofibromatosis type 2 patient as in patients with unilateral sporadic acoustic neuroma tumors.There are some centers using radiation therapy for neurofibromatosis type 2 with mixed results. The risk of malignant transformation after radiation is higher in this group. Recent studies have shown that these individuals may have more tumors that are resistant to radiation, due to the cell type. These cases should be handled in centers with very experienced skull base teams.
Acoustic neuroma prognosis
Acoustic neuromas are not malignant and do not spread to other parts of the body. The average growth rate of an acoustic neuroma is approximately 1.5 millimeters per year. Several studies have demonstrated that as many as 50% of tumors show little to no growth over long periods of time (5 to 10 years). No known environmental or dietary factors are known to influence the growth rate of acoustic neuromas.
However, some untreated neuromas can grow very aggressively and cause severe and permanent damage to nerves, ear and brain tissue. Hearing loss and balance problems related to the tumor may remain, even after treatment by surgery or radiation. If an acoustic neuroma is diagnosed early, when the tumour is small, treatment is more likely to preserve hearing.
Acoustic neuroma complications
An acoustic neuroma may cause a variety of permanent complications, including:
- Hearing loss
- Facial numbness and weakness
- Difficulties with balance
- Ringing in the ear
Large tumors may press on your brainstem, preventing the normal flow of fluid between your brain and spinal cord (cerebrospinal fluid). In this case, fluid can build up in your head (hydrocephalus), increasing the pressure inside your skull.
Some symptoms of an acoustic neuroma can be difficult to live with and may affect your quality of life. For example, hearing impairment may have an impact on your job and communication may be more difficult. Severe dizziness and loss of balance may also affect your job and limit the activities you can do.
Speak to your doctor or specialist if your acoustic neuroma is being monitored but you feel the symptoms are significantly affecting your daily life. There may be ways of easing your symptoms, such as using a hearing aid or painkilling medication, or you may need treatment to remove the tumor.
Recurrence
Occasionally, acoustic neuromas return after being removed. The tumors reoccur in less than five in every 100 people who have surgery to remove them.
It’s likely that you’ll need to have magnetic resonance imaging (MRI) scans over a number of years, regardless of which treatment you have.
Hydrocephalus
One of the most serious complications of acoustic neuroma is a condition called hydrocephalus.
Hydrocephalus occurs when an acoustic neuroma is very large and presses on your brainstem (the lowest part of the brain that connects to the spinal cord).
This prevents the cerebrospinal fluid (CSF) from flowing between your brain and spinal cord. The blockage can cause pressure to build up inside your skull, which in turn puts pressure on the delicate tissues in your brain.
Hydrocephalus can be treated by draining away the excess CSF. It’s important that it’s treated quickly because in severe cases it can cause brain damage. In rare cases, hydrocephalus can be fatal.
Radiosurgery and microsurgery complications
Post-treatment problems (from either surgery or radiation therapy) may include: cranial nerve deficits such facial weakness or numbness, hearing loss and dizziness. Headache, obstruction of fluid that surrounds the brain and spinal cord (cerebrospinal fluid), and/or decreased mental alertness due to blood clots or obstruction of flow of cerebrospinal fluid can also occur. Cerebrospinal fluid leakage or an infection that produces meningitis are rare complications of surgical therapy.
The facial nerve may be damaged by the acoustic neuroma or as a result of surgery. In some affected individuals, it may be necessary for the surgeon to remove portions of the facial nerve, resulting in temporary or permanent facial paralysis. The regrowth of the nerve (regeneration) and restoration of function to the muscles of the face may take up to a year. If the facial paralysis persists, a second surgery may be performed to connect the healthy portion of the facial nerve to another nerve such as the hypoglossal nerve (nerve that controls the tongue) in the neck or the nerve to masseter (nerve that helps with chewing) in the face. This may bring some improvement in function to the muscles of the face. There are a number of other surgical procedures that can aid in reanimating the face that can improve the function and appearance of the weakened side of the face.
Eye problems may develop in some individuals following surgical removal of an acoustic neuroma. Facial weakness can bring about incomplete eyelid closure on the affected side which may lead to irritation of the cornea. In rare instances, this has the potential to lead to blindness of the affected eye. The eye must be kept moist with frequent use of artificial tears, and a barrier applied during sleep, such as a moisture chamber, or taped closed. The use of an eye patch is discouraged as it may contribute to corneal damage.
Double vision (diplopia) may occur if there is pressure on the 6th cranial nerve, and there may be impairment of the muscles of the eyelids. Artificial tears or eye lubricants may be needed.
Additionally, if prolonged facial paralysis is not treated, then it is possible that food may “get lost” in the mouth on the affected side, which could contribute to dental problems.
Acoustic neuroma symptoms
Usually acoustic neuromas (vestibular schwannomas) grow very slowly and the signs and symptoms of acoustic neuroma are often subtle and may take many years to develop. This means there may not be any symptoms (asymptomatic) in the early stages when the tumor is small. However, even small tumors, depending upon their location, can cause significant symptoms or physical findings.
Many people say they notice some loss of hearing and tinnitus or ringing in the ears. Usually one ear is affected, but both ears can be involved if the person has neurofibromatosis type 2. Pressure from the tumor on adjacent nerves controlling facial muscles and sensation (facial and trigeminal nerves), nearby blood vessels, or brain structures may also cause problems.
Whether or not there are any other symptoms can depend on the size of the tumor and how hard it presses on the eighth cranial nerve. Some people with an acoustic neuroma get:
- Hearing loss in one ear (the ear affected by the tumor) is the initial symptom in approximately 90 percent of patients. Hearing loss is usually gradual (worsening over months to years)— although in some cases sudden — and occurring on only one side or more pronounced on one side. Hearing loss can also fluctuate (worsen and then improve).
- Ringing (tinnitus) in the affected ear
- Dizziness or vertigo, which is a feeling that the space around you is spinning
- Loss of balance
- Headache
- Facial weakness or paralysis, facial numbness or tingling and swallowing difficulties. Facial numbness or tingling can be constant or it may come and go (intermittent).
In some patients, acoustic neuromas may grow large enough to press against the brainstem, preventing the normal flow of cerebrospinal fluid between the brain and spinal cord. This fluid can accumulate in the skull, leading to a phenomenon called hydrocephalus, which causes pressure on the tissues of brain and results in a variety of symptoms including headaches, an impaired ability to coordinate voluntary movements (ataxia), and mental confusion. Headaches may also occur in the absence of hydrocephalus and in some rare cases may be the first sign of an acoustic neuroma. In very rare cases, an untreated acoustic neuroma may grow large enough to compress the brainstem causing life-threatening complications.
Acoustic neuroma diagnosis
Acoustic neuroma is often difficult to diagnose in the early stages because signs and symptoms may be subtle and develop gradually over time. Common symptoms such as hearing loss are also associated with many other middle and inner ear problems.
After asking questions about your symptoms, your doctor will conduct an ear exam. Your doctor may order the following tests:
- Hearing test (audiometry). In this test, conducted by a hearing specialist (audiologist), you hear sounds directed to one ear at a time. The audiologist presents a range of sounds of various tones and asks you to indicate each time you hear the sound. Each tone is repeated at faint levels to find out when you can barely hear. The audiologist may also present various words to determine your hearing ability.
- Imaging. Magnetic resonance imaging (MRI) is the preferred imaging test to confirm the presence of acoustic neuroma and can detect tumors as small as 1 to 2 millimeters in diameter. If MRI is unavailable or you can’t tolerate an MRI scan, computerized tomography (CT) may be used, but it may miss very small tumors.
- Specialized test that evaluates your balance (electronystagmography), and a brainstem auditory evoked response (BAER).
- An electronystagmography test evaluates balance by detecting abnormal, involuntary eye movements, a condition known as nystagmus. Nystagmus may occur as a result of inner ear complications such as an acoustic neuroma.
- Brainstem auditory evoked response (BAER) checks hearing and neurological function and interaction by recording the brain’s response to certain sounds. Since an acoustic neuroma can disrupt the nerve pathway that relays sound from the ear to the brain, a positive result of a BAER exam could be caused by these tumors.
Acoustic neuroma treatment
Your acoustic neuroma treatment may vary, depending on the size and growth of the acoustic neuroma, your overall health, and if you’re experiencing symptoms. To treat acoustic neuroma, your doctor may suggest one or more of three potential treatment methods: monitoring, surgery or radiation therapy.
The options for treatment include:
- no treatment – just monitoring the tumor growth and related symptoms
- surgery – to cut out the tumor
- stereotactic radiosurgery also known as gamma knife radiosurgery – this procedure sends focused radiation to the tumor to stop it from growing.
Which treatment you have depends on the size and location or the tumor, your symptoms, your age and general health, and what you want.
Even though acoustic neuromas are not cancerous, they can cause serious, permanent damage to nerves. If you have any of the symptoms listed above, see your doctor as soon as possible.
Monitoring
If you have an acoustic neuroma that’s very small or growing very slowly, you may not need to have any immediate treatment. Instead, your condition will be carefully monitored.
Research suggests that up to three-quarters of acoustic neuromas don’t appear to be growing, so monitoring the tumor is all that’s needed 14.
Simply monitoring an acoustic neuroma is often the best option because the risks associated with surgery or radiosurgery (see below) outweigh the risk of the tumor having an adverse effect on your health.
Your doctor may recommend that you have regular imaging and hearing tests, usually every six to 12 months, to determine whether the tumor is growing and how quickly.
To help monitor your condition, you’ll need to have regular magnetic resonance imaging (MRI) scans (where a magnetic field and radio waves are used to create an image of the inside of your body). The MRI scan will be used to check the size and growth of your acoustic neuroma.
Other treatments may be considered if the tumor shows any signs of growing or if there’s a risk of it significantly affecting your health.
You may need to have an MRI scan every one to two years, although this will depend on your general health and the size of your tumor.
There are hardly any clinical parameters which reliably predict growth in a newly diagnosed acoustic neuroma. There are studies reporting age, sex, hearing loss, imbalance, initial size, tumor location, and even sidedness as predictors of future growth, but these are mostly single studies at low evidence levels which were not reproduced 15. The proportion of growing tumors at follow-up varies considerably with reported ranges of 30–70% over different periods of time, the variation most likely being due to methodological issues 16. On average, approximately 50% of tumors may be expected to grow over a 5-year period 17. Series employing quantitative measurements of acoustic neuroma growth with long-term follow-up have shown a mean maximum diameter growth of 2.9 mm/year (maximum diameter) 18. Two recent studies found that 50% of patients lost functional hearing during a 3–4 year period 19, 20. A full speech discrimination score was considered a good predictor for preservation of functional hearing 20.
Four nonrandomized studies compared outcomes from observation and stereotactic radiosurgery (SRS) showing better tumor control after stereotactic radiosurgery 21, 22. Some studies reported less hearing loss in patients with stereotactic radiosurgery, whereas in others hearing outcome and complaints were not different 15. Two studies compared either conservative management, surgery, or stereotactic radiosurgery using various quality of life questionnaires after 5–7 years of follow-up 23, 24. Both reports showed that patients with conservative management only responded more favorably in the questionnaires than those who were treated up front. In addition, hearing and facial nerve outcomes were better in observed patients, the latter only in comparison with surgery. However, in nearly all observed patients the tumors had stayed stable in size, which may represent a relevant bias but also indicates the importance of thoughtful indication to treat.
Acoustic neuroma surgery
You may need surgery to remove an acoustic neuroma, especially if the tumor is:
- Continuing to grow
- Very large
- Causing symptoms
Your surgeon may use one of several techniques for removing an acoustic neuroma, depending on the size of your tumor, hearing status and other factors.
There are various classification systems for acoustic neuroma tumor size which support decision making 25. Of those, the Koos classification system is the most commonly used (see Table 1) 26. In large acoustic neuroma (Koos grade IV), surgery is considered as the primary treatment to remove a symptomatic lesion or potentially life-threatening mass effect 26. Surgery may also be considered for smaller tumors, if cystic degeneration is present or if cure is the primary goal of treatment 27.
Table 1. The Koos grading system
| Koos Grade | Tumor Description |
|---|---|
| I | Small intracanalicular tumor |
| II | Small tumor with protrusion into the cerebellopontine angle; no contact with the brainstem |
| III | Tumor occupying the cerebellopontine cistern with no brainstem displacement |
| IV | Large tumor with brainstem and cranial nerve displacement |
The goal of surgery is to remove the tumor, preserve the facial nerve to prevent facial paralysis and preserve hearing when possible.
The probability of hearing preservation in patients with normal hearing (Gardner–Robertson class A; see Table 2) was >50–75% immediately after surgery, as well as after 2 and 5 years, and >25–50% after 10 years 28. Factors influencing preservation of serviceable hearing after microsurgery are tumor size <1 cm, presence of a distal internal auditory canal CSF fluid fundal cap, as well as good preoperative hearing function 28. The risk of persisting facial palsy is between 3% and 46% 29. It depends on tumor size and the occurrence of an immediate paresis 29. To improve the rate of functional preservation, intraoperative monitoring is mandatory for surgery of acoustic neuroma and should include somatosensoric evoked potentials and monitoring of the facial nerve comprising direct electrical stimulation and free-running electromyography. Facial motor evoked potentials are currently being evaluated 30. Intraoperative facial nerve monitoring leads to improved functional outcome and can be used to accurately predict favorable facial nerve function after surgery 30. Brainstem auditory evoked responses (BAER) should also be used when hearing preservation is attempted 28. In case of large lesions, electromyography of the lower cranial nerves is recommended.
Table 2. Gardner–Robertson scale for hearing function
| Grades | Pure Tone Audiogram (dB) | Speech Discrimination (%) |
|---|---|---|
| I | 0–30 | 70–100 |
| II | 31–50 | 50–69 |
| III | 51–90 | 5–49 |
| IV | 91–max | 1–4 |
| V | Not testable | 0 |
Surgery for an acoustic neuroma is performed under general anesthesia and involves removing the tumor through the inner ear or through a window in your skull.
The entire tumor may not be able to be completely removed in certain cases. For example, if the tumor is too close to important parts of the brain or the facial nerve.
Sometimes, surgical removal of the tumor may worsen symptoms if the hearing, balance or facial nerves are damaged during the operation.
Complications may include:
- Leakage of cerebrospinal fluid (CSF) through the wound
- Hearing loss
- Facial weakness
- Facial numbness
- Ringing in the ear
- Balance problems
- Persistent headache
- Infection of the cerebrospinal fluid (meningitis)
- Stroke or brain bleeding
Goal of surgery should be total or near-total resection, since residual tumor volume correlates with rate of recurrence. A series of 116 patients with acoustic neuroma who were treated by gross total resection, near-total resection or subtotal resection yielded recurrence rates of 3.8%, 9.4%, and 27.6%, respectively 32. The mean time to recurrence was 22 months, ranging from 6 to 143 months. In a recent study of 103 sporadic acoustic neuroma patients who underwent near-total resection or subtotal resection, those with subtotal resection experienced recurrences over 13 times more often than those treated with near-total resection 33. In a retrospective study with 111 incomplete excisions (near-total resection and subtotal resection), the 7 patients who showed evidence of tumor regrowth had all undergone subtotal resection 34. Several further series also showed a considerably greater risk of regrowth with increasing residual tumor volumes 35. For large acoustic neuroma, the lower risk of recurrence after gross total resection should be weighed against the higher risk for facial nerve dysfunction and lower rates of hearing preservation, since there seems to be a relationship between tumor volume and functional outcome 36.
For these cases, partial resection followed by stereotactic radiosurgery (see below) has become increasingly popular 37. With this combined approach, the results reported so far show superior outcome regarding facial nerve function and hearing preservation when compared with total resection, with comparable tumor control rates. However, the studies are still small and retrospective 37, 38. After intentional near-total resection or subtotal resection, a watch and scan policy is warranted as only a minority of remnants do progress; however, the risk increases with the size of the remnant 37. In cases of recurrences after radiosurgery, both reoperation and radiosurgical retreatment are possible. However, the functional risk for the facial nerve upon surgery is higher after previous irradiation, and a very meticulous, conservative dissection technique might be necessary. In acoustic neuroma recurring after surgery, radiosurgery may be used preferentially because the risk of damage to the facial nerve is lower than with a second operation 39.
Microsurgery
Microsurgery can be used to remove an acoustic neuroma. The surgery will be carried out under general anesthetic and the acoustic neuroma will be removed through an incision made in your skull.
Small acoustic neuromas can usually be completely removed. If you have a large tumor, a small part will occasionally be left behind to minimize the risk of damaging the facial nerve, which runs next to the acoustic nerve.
If a small part of the tumor remains, it can either be monitored with MRI scans or effectively treated using radiosurgery (see below).
- Hearing loss
After surgery to remove an acoustic neuroma, hearing in the ear affected by the tumor is almost always lost.
You may wish to discuss the possibility of having a ‘bone anchored hearing aid’ with your ENT (ear, nose and throat) surgeon, which will help divert sound from your affected ear to your good ear.
- Facial nerve damage
Surgery can occasionally damage the facial nerve (7th cranial nerve). This is because the acoustic nerve is very close to the facial nerve and large tumors are often stuck to it. Your surgeon will try not to damage your facial nerve and with large tumors will sometimes leave a small part of the tumor on the facial nerve to try to preserve it.
If your facial nerve is damaged during surgery you may find that:
- your face droops on one side (facial palsy)
- you drool saliva on the weak side of your face
- you have difficulty closing your eye on the weak side of your face
- your speech is less clear
These symptoms may improve within six to 12 months of having surgery and be helped with physiotherapy. However, it’s important to be aware that some damage to your facial nerve may be permanent.
Facial nerve damage can also affect your eyes. For example, you may find it difficult to blink or close your eye completely on the side that was operated on. As a result, your eye may dry out and you may need to use artificial tears (eye lubricant).
In cases where the tumor is small, less than one in every 100 people’s facial nerve will be badly affected after treatment.
For large tumors, around three in 10 people will have permanent, severe facial nerve weakness after surgery if a complete tumor removal is attempted. This falls to around one person in over 100 if a small part of the tumor is left on the facial nerve to preserve it.
Any minor post-surgery facial nerve weakness is likely to be temporary, although it may take several months to recover.
Recovery from surgery
Following surgery, you’ll usually need to spend up to a week in hospital to recuperate.
You should be able to return to work after about two months. The length of time it takes you to recover may depend on the size and position of the tumor that was removed. The healthcare professionals treating you will be able to advise you further.
If your acoustic neuroma was completely removed, you won’t usually need further treatment. However, you’ll continue to be monitored with MRI scans.
Radiation therapy
There are several types of radiation therapy used to treat acoustic neuroma:
- Stereotactic radiosurgery such as Gamma Knife radiosurgery, uses many tiny gamma rays to deliver a precisely targeted dose of radiation to a tumor without damaging the surrounding tissue or making an incision. The goal of stereotactic radiosurgery is to stop the growth of a tumor, preserve the facial nerve’s function and possibly preserve hearing. It may take weeks, months or years before you notice the effects of radiosurgery. Your doctor will monitor your progress with follow-up imaging studies and hearing tests.
- Stereotactic radiotherapy. Fractionated stereotactic radiotherapy (SRT) delivers a small dose of radiation to the tumor over several sessions. Fractionated stereotactic radiotherapy is done to curb the growth of the tumor without damaging surrounding brain tissue.
- Proton beam therapy. This type of radiation therapy uses high-energy beams of positively charged particles called protons. Protons are delivered to the affected area in targeted doses to treat tumors and minimize radiation exposure to the surrounding area.
Five prospective studies without randomization have revealed that stereotactic radiosurgery is superior to microsurgery for patients with acoustic neuroma <3 cm in terms of preserving facial nerve and hearing function 1. As upper limit for radiosurgery, a mass effect on the brainstem (Koos IV) is considered, but there is no clear definition by diameter or volume alone.
Several retrospective cohort studies evaluated stereotactic radiosurgery using GammaKnife with at least 100 patients, 2 years follow-up, and objective audiometric assessment. One linear accelerator series comprising over 100 patients with 2 years follow-up did not report functional hearing outcome 40. Pioneer series of stereotactic radiosurgery included patients treated with very high dose regimens. Contemporary stereotactic radiosurgery series using GammaKnife with tumor marginal doses between 12 and 14 Gray revealed 5-year tumor control rates of 90–99%, hearing preservation rates of 41–79%, facial nerve preservation rates of 95–100%, and trigeminal preservation rates of 79–99%. Numerous authors have found that the key predictor for functional hearing preservation is the quality of hearing at time of radiosurgery 41, 42. A recent review revealed a relevant decline in hearing after stereotactic radiosurgery, even in patients with normal hearing function (Gardner–Robertson grade 1) 28. The probability to preserve hearing was >75–100% after 2 years, >50–75% after 5 years, and >25–50% after 10 years. After 5 and 10 years, the rates of hearing preservation were similar to patients having microsurgery 28. However, it has to be considered that the latter data are based on selected surgical cases with a special attempt to preserve hearing.
The maximum dose at the modiolus of the cochlea has been reported to be a negative predictor for functional hearing preservation with a threshold around 4 Gray 43. However, these series comprise small retrospective cohorts of patients. Cochlear dose is likely to be one of many variables associated with hearing preservation. The recommendation is to use stereotactic radiosurgery with a dose of 11–14 Gray at the margin and 11–12 Gray when the risk of hearing loss is a critical issue 14.
There are no randomized, prospective studies comparing stereotactic radiosurgery and fractionated stereotactic radiotherapy. Six nonrandomized studies found that functional hearing preservation is similar but the rate of facial palsy and trigeminal nerve dysfunction seems higher with fractionated stereotactic radiotherapy than stereotactic radiosurgery 44, 45.
There are little data about the incidence of malignant acoustic neuroma after radiation of spontaneous, non-neurofibromatosis type 2 (NF2) acoustic neuroma. The spontaneous risk of malignancy was addressed in a large retrospective study using the SEER database. The incidence of Malignant Peripheral Nerve Sheath Tumors of the eighth cranial nerve with no history of prior radiation was 0.017 per 1 million persons/year. Compared with the incidence of benign acoustic neuroma, 1041 acoustic neuroma were present for every 1 Malignant Peripheral Nerve Sheath Tumor arising from the eighth cranial nerve. There is no evidence that spontaneous Malignant Peripheral Nerve Sheath Tumor is a feature of neurofibromatosis type 2 (NF2) as opposed to neurofibromatosis type 1 (NF1), nonetheless around half of Malignant Peripheral Nerve Sheath Tumors reported after radiation treatment are in neurofibromatosis type 2 (NF2) patients 46. This baseline rate of malignancy should be considered when estimating the risk of malignant transformation following stereotactic radiosurgery for acoustic neuroma 47. In a retrospective single center review, Pollock et al 48 did not find any radiation-induced tumors in 11,264 patient-years of follow-up after stereotactic radiosurgery. In a review by Maducdoc et al, only 8 cases with malignant transformation after surgery or stereotactic radiosurgery were found; 4 of these patients had surgery only 49.
Numerous studies have reported about transient enlargement of acoustic neuroma occurring within 3 years after radiosurgery 50, 51. This MR change observed in up to 30% of the patients is related to the therapeutic effect of stereotactic radiosurgery and is termed “pseudoprogression.” It is not a predictor of failure 50, 51. Experts recommend clinical and radiological observation within this time frame and performing annual MRI scans.
Stereotactic radiosurgery
Stereotactic radiosurgery delivers a very focused and precise dose of radiation to your acoustic neuroma. Stereotactic means locating a point (in this case the position of the tumor in your brain) using three-dimensional co-ordinates. Your doctor may recommend stereotactic radiosurgery if your tumor is small (less than 2.5 centimeters in diameter), you are an older adult or you cannot tolerate surgery for health reasons.
During stereotactic radiosurgery, the maximum amount of radiation will be aimed at your tumor without the surrounding tissue being exposed. It may be given as a single dose or delivered over several sessions. It doesn’t get rid of your tumor but aims to stop it growing further. It can only be used on small tumors or the remains of a tumor after surgery on large tumors. It’s not usually used for large tumors.
Stereotactic radiosurgery is carried out under local anesthetic, which means you’ll be conscious throughout the procedure but your scalp will be numbed. A lightweight metal frame is usually attached to the scalp and a series of scans will accurately pinpoint the position of the tumor. It can then be treated using a precise beam of radiation.
The goal of stereotactic radiosurgery is to stop the growth of a tumor, preserve the facial nerve’s function and possibly preserve hearing.
Immediate side effects of stereotactic radiosurgery are rare, and you’ll usually only need to take a couple of days off work to have the treatment.
Risks of stereotactic radiosurgery include:
- Hearing loss
- Ringing in the ear
- Facial weakness or numbness
- Balance problems
- Continued tumor growth (treatment failure)
It may take weeks, months or years before the effects of radiosurgery become evident. Your doctor will monitor your progress with follow-up imaging studies and hearing tests.
Nerve damage
In some cases, stereotactic radiosurgery can cause nerve damage, although it may not be apparent until several weeks or months after treatment.
Symptoms of nerve damage can include:
- facial numbness (loss of feeling)
- facial paralysis (an inability to move part of your face)
- hearing loss
Facial paralysis affects around one in every 100 people who has stereotactic radiosurgery. It’s estimated that just under a third of people may experience hearing loss after stereotactic radiosurgery.
Stereotactic radiotherapy
Fractionated stereotactic radiotherapy (SRT) delivers a small dose of radiation to the tumor over several sessions in an effort to curb the growth of the tumor without damaging surrounding brain tissue.
Proton beam therapy
This type of radiation therapy uses high-energy beams of positively charged particles called protons that are delivered to the affected area in targeted doses to treat tumors and minimize radiation exposure to the surrounding area.
Investigational therapies
Several investigations are underway looking into the potential use of other drugs for the treatment of acoustic neuromas primarily in patients with neurofibromatosis type 2 (NF2) with promising results. These include drugs that inhibit a number of cellular mechanisms including vascular endothelial growth factor (bevacizumab and PTC299), phosphoinositide-dependent kinase-1 (OSU-03012), ERBB2 receptor (Trastuzumab), epidermal growth factor (Erlotinib and Lapatinib), and p-21 activated kinases (IPA-3).
Bevacizumab has been successfully used for patients with progressive acoustic neuroma associated with neurofibromatosis type 2 (NF2) 52. Patients experienced an improvement of hearing and objective (>20% reduced tumor volume) radiographic responses 53. In neurofibromatosis type 2 (NF2) patients with progressive acoustic neuroma, a prospective, multi-institutional, uncontrolled phase II study with 14 patients using 7.5 mg/kg bevacizumab administered every 3 weeks revealed a hearing improvement in 36% of patients, and there was no patient with a hearing decline in the trial period of 12 months 54. Volumetric assessments demonstrated a partial radiographic response of volume reduction of 20% or more in 43% (6/14 patients), making bevacizumab a potential treatment option for neurofibromatosis type 2 (NF2) patients.
Other pathways have been addressed based on preclinical or immunohistochemical target expression 55. Again, patients studied had acoustic neuroma in the context of neurofibromatosis type 2 (NF2). Neither epidermal growth factor receptor (EGFR) pathway inhibition using erlotinib nor ErbB2 (and EGFR) pathway blockade applying lapatinib has been associated with relevant radiographic responses or impact on the hearing function 56. The mechanistic target of rapamycin (mTOR) signaling cascade has also been proposed for the treatment of neurofibromatosis type 2 (NF2)-related tumors, since the mTOR pathway is considered a key driver of tumor growth in merlin (neurofibromatosis type 2 (NF2))-deficient tumors. Similarly, a small (n = 10 patients), single-institution trial study of the mTOR complex 1 inhibitor everolimus was not associated with tumor shrinkage or hearing improvement 57.
Supportive therapy
In addition to treatment to remove or stop the growth of the acoustic neuroma, your doctor may recommend supportive therapies to address symptoms or complications of an acoustic neuroma and its treatment, such as dizziness or balance problems.
Cochlear implants or other treatments may also be recommended to treat hearing loss.
Coping and support
Dealing with the possibility of hearing loss and facial paralysis and deciding which treatment would be best for you can be quite stressful. Here are some suggestions you may find helpful:
- Educate yourself about acoustic neuroma. The more you know, the better prepared you’ll be to make good choices about treatment. Besides talking to your doctor and your audiologist, you may want to talk to a counselor or social worker. Or you may find it helpful to talk to other people who’ve had an acoustic neuroma and learn more about their experiences during and after treatment.
- Maintain a strong support system. Family and friends can help you as you go through this difficult time. Sometimes, though, you may find the concern and understanding of other people with acoustic neuroma especially comforting.
Your doctor or a social worker may be able to put you in uch with a support group. Or you may find an in-person or online support group through the Acoustic Neuroma Association (https://www.anausa.org/).
- Goldbrunner, R., Weller, M., Regis, J., Lund-Johansen, M., Stavrinou, P., Reuss, D., Evans, D. G., Lefranc, F., Sallabanda, K., Falini, A., Axon, P., Sterkers, O., Fariselli, L., Wick, W., & Tonn, J. C. (2020). EANO guideline on the diagnosis and treatment of vestibular schwannoma. Neuro-oncology, 22(1), 31–45. https://doi.org/10.1093/neuonc/noz153[↩][↩][↩][↩][↩]
- Irving RM, Harada T, Moffat DA, Hardy DG, Whittaker JL, Xuereb JH, Maher ER. Somatic neurofibromatosis type 2 gene mutations and growth characteristics in vestibular schwannoma. Am J Otol. 1997 Nov; 18(6): 754-60[↩]
- Zhao F, Yang Z, Chen Y, Zhou Q, Zhang J, Liu J, Wang B, He Q, Zhang L, Yu Y, Liu P. Deregulation of the Hippo Pathway Promotes Tumor Cell Proliferation Through YAP Activity in Human Sporadic Vestibular Schwannoma. World Neurosurg. 2018 Sep;117:e269-e279. doi: 10.1016/j.wneu.2018.06.010[↩]
- Kshettry VR, Hsieh JK, Ostrom QT, Kruchko C, Barnholtz-Sloan JS. Incidence of vestibular schwannomas in the United States. J Neurooncol. 2015 Sep;124(2):223-8. doi: 10.1007/s11060-015-1827-9[↩]
- Pettersson D, Mathiesen T, Prochazka M, Bergenheim T, Florentzson R, Harder H, Nyberg G, Siesjö P, Feychting M. Long-term mobile phone use and acoustic neuroma risk. Epidemiology. 2014 Mar;25(2):233-41. doi: 10.1097/EDE.0000000000000058[↩]
- Agnihotri S, Jalali S, Wilson MR, Danesh A, Li M, Klironomos G, Krieger JR, Mansouri A, Khan O, Mamatjan Y, Landon-Brace N, Tung T, Dowar M, Li T, Bruce JP, Burrell KE, Tonge PD, Alamsahebpour A, Krischek B, Agarwalla PK, Bi WL, Dunn IF, Beroukhim R, Fehlings MG, Bril V, Pagnotta SM, Iavarone A, Pugh TJ, Aldape KD, Zadeh G. The genomic landscape of schwannoma. Nat Genet. 2016 Nov;48(11):1339-1348. doi: 10.1038/ng.3688[↩][↩][↩]
- Sainz J, Huynh DP, Figueroa K, Ragge NK, Baser ME, Pulst SM. Mutations of the neurofibromatosis type 2 gene and lack of the gene product in vestibular schwannomas. Hum Mol Genet. 1994 Jun;3(6):885-91. doi: 10.1093/hmg/3.6.885[↩]
- Håvik AL, Bruland O, Myrseth E, Miletic H, Aarhus M, Knappskog PM, Lund-Johansen M. Genetic landscape of sporadic vestibular schwannoma. J Neurosurg. 2018 Mar;128(3):911-922. doi: 10.3171/2016.10.JNS161384[↩]
- Wang, L., Zehir, A., Sadowska, J., Zhou, N., Rosenblum, M., Busam, K., Agaram, N., Travis, W., Arcila, M., Dogan, S., Berger, M. F., Cheng, D. T., Ladanyi, M., Nafa, K., & Hameed, M. (2015). Consistent copy number changes and recurrent PRKAR1A mutations distinguish Melanotic Schwannomas from Melanomas: SNP-array and next generation sequencing analysis. Genes, chromosomes & cancer, 54(8), 463–471. https://doi.org/10.1002/gcc.22254[↩]
- Röhrich M, Koelsche C, Schrimpf D, Capper D, Sahm F, Kratz A, Reuss J, Hovestadt V, Jones DT, Bewerunge-Hudler M, Becker A, Weis J, Mawrin C, Mittelbronn M, Perry A, Mautner VF, Mechtersheimer G, Hartmann C, Okuducu AF, Arp M, Seiz-Rosenhagen M, Hänggi D, Heim S, Paulus W, Schittenhelm J, Ahmadi R, Herold-Mende C, Unterberg A, Pfister SM, von Deimling A, Reuss DE. Methylation-based classification of benign and malignant peripheral nerve sheath tumors. Acta Neuropathol. 2016 Jun;131(6):877-87. doi: 10.1007/s00401-016-1540-6[↩]
- Evans DG. Neurofibromatosis type 2. Handb Clin Neurol. 2015;132:87-96. doi: 10.1016/B978-0-444-62702-5.00005-6[↩]
- Evans DG, Howard E, Giblin C, Clancy T, Spencer H, Huson SM, Lalloo F. Birth incidence and prevalence of tumor-prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A. 2010 Feb;152A(2):327-32. doi: 10.1002/ajmg.a.33139[↩]
- Evans DGR, Salvador H, Chang VY, Erez A, Voss SD, Druker H, Scott HS, Tabori U. Cancer and Central Nervous System Tumor Surveillance in Pediatric Neurofibromatosis 2 and Related Disorders. Clin Cancer Res. 2017 Jun 15;23(12):e54-e61. doi: 10.1158/1078-0432.CCR-17-0590[↩]
- Germano IM, Sheehan J, Parish J, Atkins T, Asher A, Hadjipanayis CG, Burri SH, Green S, Olson JJ. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines on the Role of Radiosurgery and Radiation Therapy in the Management of Patients With Vestibular Schwannomas. Neurosurgery. 2018 Feb 1;82(2):E49-E51. doi: 10.1093/neuros/nyx515[↩][↩]
- Paldor I, Chen AS, Kaye AH. Growth rate of vestibular schwannoma. J Clin Neurosci. 2016 Oct;32:1-8. doi: 10.1016/j.jocn.2016.05.003[↩][↩]
- Varughese JK, Breivik CN, Wentzel-Larsen T, Lund-Johansen M. Growth of untreated vestibular schwannoma: a prospective study. J Neurosurg. 2012 Apr;116(4):706-12. doi: 10.3171/2011.12.JNS111662[↩]
- Hunter, J. B., Francis, D. O., O’Connell, B. P., Kabagambe, E. K., Bennett, M. L., Wanna, G. B., Rivas, A., Thompson, R. C., & Haynes, D. S. (2016). Single Institutional Experience With Observing 564 Vestibular Schwannomas: Factors Associated With Tumor Growth. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology, 37(10), 1630–1636. https://doi.org/10.1097/MAO.0000000000001219[↩]
- Sughrue ME, Yang I, Aranda D, Lobo K, Pitts LH, Cheung SW, Parsa AT. The natural history of untreated sporadic vestibular schwannomas: a comprehensive review of hearing outcomes. J Neurosurg. 2010 Jan;112(1):163-7. doi: 10.3171/2009.4.JNS08895[↩]
- Tveiten OV, Carlson ML, Goplen F, Vassbotn F, Link MJ, Lund-Johansen M. Long-term Auditory Symptoms in Patients With Sporadic Vestibular Schwannoma: An International Cross-Sectional Study. Neurosurgery. 2015 Aug;77(2):218-27; discussion 227. doi: 10.1227/NEU.0000000000000760[↩]
- Stangerup SE, Tos M, Thomsen J, Caye-Thomasen P. Hearing outcomes of vestibular schwannoma patients managed with ‘wait and scan’: predictive value of hearing level at diagnosis. J Laryngol Otol. 2010 May;124(5):490-4. doi: 10.1017/S0022215109992611[↩][↩]
- Breivik CN, Nilsen RM, Myrseth E, Pedersen PH, Varughese JK, Chaudhry AA, Lund-Johansen M. Conservative management or gamma knife radiosurgery for vestibular schwannoma: tumor growth, symptoms, and quality of life. Neurosurgery. 2013 Jul;73(1):48-56; discussion 56-7. doi: 10.1227/01.neu.0000429862.50018.b9[↩]
- Régis J, Carron R, Park MC, Soumare O, Delsanti C, Thomassin JM, Roche PH. Wait-and-see strategy compared with proactive Gamma Knife surgery in patients with intracanalicular vestibular schwannomas. J Neurosurg. 2010 Dec;113 Suppl:105-11. doi: 10.3171/2010.8.GKS101058[↩]
- Carlson ML, Tveiten OV, Driscoll CL, Goplen FK, Neff BA, Pollock BE, Tombers NM, Castner ML, Finnkirk MK, Myrseth E, Pedersen PH, Lund-Johansen M, Link MJ. Long-term quality of life in patients with vestibular schwannoma: an international multicenter cross-sectional study comparing microsurgery, stereotactic radiosurgery, observation, and nontumor controls. J Neurosurg. 2015 Apr;122(4):833-42. doi: 10.3171/2014.11.JNS14594[↩]
- Robinett ZN, Walz PC, Miles-Markley B, Moberly AC, Welling DB. Comparison of Long-term Quality-of-Life Outcomes in Vestibular Schwannoma Patients. Otolaryngol Head Neck Surg. 2014 Jun;150(6):1024-32. doi: 10.1177/0194599814524531[↩]
- Matthies C, Samii M. Management of 1000 vestibular schwannomas (acoustic neuromas): clinical presentation. Neurosurgery. 1997 Jan;40(1):1-9; discussion 9-10. doi: 10.1097/00006123-199701000-00001[↩]
- Koos WT, Day JD, Matula C, Levy DI. Neurotopographic considerations in the microsurgical treatment of small acoustic neurinomas. J Neurosurg. 1998 Mar;88(3):506-12. doi: 10.3171/jns.1998.88.3.0506[↩][↩][↩]
- Link MJ, Driscoll CL, Foote RL, Pollock BE. Radiation therapy and radiosurgery for vestibular schwannomas: indications, techniques, and results. Otolaryngol Clin North Am. 2012 Apr;45(2):353-66, viii-ix. doi: 10.1016/j.otc.2011.12.006[↩]
- Carlson ML, Vivas EX, McCracken DJ, Sweeney AD, Neff BA, Shepard NT, Olson JJ. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines on Hearing Preservation Outcomes in Patients With Sporadic Vestibular Schwannomas. Neurosurgery. 2018 Feb 1;82(2):E35-E39. doi: 10.1093/neuros/nyx511[↩][↩][↩][↩][↩]
- Morton RP, Ackerman PD, Pisansky MT, Krezalek M, Leonetti JP, Raffin MJ, Anderson DE. Prognostic factors for the incidence and recovery of delayed facial nerve palsy after vestibular schwannoma resection. J Neurosurg. 2011 Feb;114(2):375-80. doi: 10.3171/2010.5.JNS091854[↩][↩]
- Vivas EX, Carlson ML, Neff BA, Shepard NT, McCracken DJ, Sweeney AD, Olson JJ. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines on Intraoperative Cranial Nerve Monitoring in Vestibular Schwannoma Surgery. Neurosurgery. 2018 Feb 1;82(2):E44-E46. doi: 10.1093/neuros/nyx513[↩][↩]
- Gardner G, Robertson JH. Hearing preservation in unilateral acoustic neuroma surgery. Ann Otol Rhinol Laryngol. 1988 Jan-Feb;97(1):55-66. doi: 10.1177/000348948809700110[↩]
- Seol HJ, Kim CH, Park CK, Kim CH, Kim DG, Chung YS, Jung HW. Optimal extent of resection in vestibular schwannoma surgery: relationship to recurrence and facial nerve preservation. Neurol Med Chir (Tokyo). 2006 Apr;46(4):176-80; discussion 180-1. doi: 10.2176/nmc.46.176[↩]
- Jacob JT, Carlson ML, Driscoll CL, Link MJ. Volumetric analysis of tumor control following subtotal and near-total resection of vestibular schwannoma. Laryngoscope. 2016 Aug;126(8):1877-82. doi: 10.1002/lary.25779[↩]
- Chen Z, Prasad SC, Di Lella F, Medina M, Piccirillo E, Taibah A, Russo A, Yin S, Sanna M. The behavior of residual tumors and facial nerve outcomes after incomplete excision of vestibular schwannomas. J Neurosurg. 2014 Jun;120(6):1278-87. doi: 10.3171/2014.2.JNS131497[↩]
- van de Langenberg R, Hanssens PE, van Overbeeke JJ, Verheul JB, Nelemans PJ, de Bondt BJ, Stokroos RJ. Management of large vestibular schwannoma. Part I. Planned subtotal resection followed by Gamma Knife surgery: radiological and clinical aspects. J Neurosurg. 2011 Nov;115(5):875-84. doi: 10.3171/2011.6.JNS101958[↩]
- Gurgel RK, Dogru S, Amdur RL, Monfared A. Facial nerve outcomes after surgery for large vestibular schwannomas: do surgical approach and extent of resection matter? Neurosurg Focus. 2012 Sep;33(3):E16. doi: 10.3171/2012.7.FOCUS12199[↩]
- Daniel, R. T., Tuleasca, C., George, M., Pralong, E., Schiappacasse, L., Zeverino, M., Maire, R., & Levivier, M. (2017). Preserving normal facial nerve function and improving hearing outcome in large vestibular schwannomas with a combined approach: planned subtotal resection followed by gamma knife radiosurgery. Acta neurochirurgica, 159(7), 1197–1211. https://doi.org/10.1007/s00701-017-3194-0[↩][↩][↩]
- Iwai Y, Ishibashi K, Watanabe Y, Uemura G, Yamanaka K. Functional Preservation After Planned Partial Resection Followed by Gamma Knife Radiosurgery for Large Vestibular Schwannomas. World Neurosurg. 2015 Aug;84(2):292-300. doi: 10.1016/j.wneu.2015.03.012[↩]
- Wise SC, Carlson ML, Tveiten ØV, Driscoll CL, Myrseth E, Lund-Johansen M, Link MJ. Surgical salvage of recurrent vestibular schwannoma following prior stereotactic radiosurgery. Laryngoscope. 2016 Nov;126(11):2580-2586. doi: 10.1002/lary.25943[↩]
- Friedman WA, Bradshaw P, Myers A, Bova FJ. Linear accelerator radiosurgery for vestibular schwannomas. J Neurosurg. 2006 Nov;105(5):657-61. doi: 10.3171/jns.2006.105.5.657[↩]
- Mousavi SH, Niranjan A, Akpinar B, Huang M, Kano H, Tonetti D, Flickinger JC, Dade Lunsford L. Hearing subclassification may predict long-term auditory outcomes after radiosurgery for vestibular schwannoma patients with good hearing. J Neurosurg. 2016 Oct;125(4):845-852. doi: 10.3171/2015.8.JNS151624. Epub 2016 Jan 8. Erratum in: J Neurosurg. 2017 Jun;126(6):2051-2052[↩]
- Akpinar B, Mousavi SH, McDowell MM, Niranjan A, Faraji AH, Flickinger JC, Lunsford LD. Early Radiosurgery Improves Hearing Preservation in Vestibular Schwannoma Patients With Normal Hearing at the Time of Diagnosis. Int J Radiat Oncol Biol Phys. 2016 Jun 1;95(2):729-34. doi: 10.1016/j.ijrobp.2016.01.019[↩]
- Linskey ME, Johnstone PA, O’Leary M, Goetsch S. Radiation exposure of normal temporal bone structures during stereotactically guided gamma knife surgery for vestibular schwannomas. J Neurosurg. 2003 Apr;98(4):800-6. doi: 10.3171/jns.2003.98.4.0800[↩]
- Persson, O., Bartek, J., Jr, Shalom, N. B., Wangerid, T., Jakola, A. S., & Förander, P. (2017). Stereotactic radiosurgery vs. fractionated radiotherapy for tumor control in vestibular schwannoma patients: a systematic review. Acta neurochirurgica, 159(6), 1013–1021. https://doi.org/10.1007/s00701-017-3164-6[↩]
- Kessel KA, Fischer H, Vogel MM, Oechsner M, Bier H, Meyer B, Combs SE. Fractionated vs. single-fraction stereotactic radiotherapy in patients with vestibular schwannoma : Hearing preservation and patients’ self-reported outcome based on an established questionnaire. Strahlenther Onkol. 2017 Mar;193(3):192-199. English. doi: 10.1007/s00066-016-1070-0. Epub 2016 Nov 1. Erratum in: Strahlenther Onkol. 2017 Feb;193(2):171.[↩]
- King AT, Rutherford SA, Hammerbeck-Ward C, Lloyd SK, Freeman SR, Pathmanaban ON, Kellett M, Obholzer R, Afridi S, Axon P, Halliday D, Parry A, Thomas OM, Laitt RD, McCabe MG, Stivaros S, Erridge S, Evans DG; English Specialist NF2 research group. Malignant Peripheral Nerve Sheath Tumors are not a Feature of Neurofibromatosis Type 2 in the Unirradiated Patient. Neurosurgery. 2018 Jul 1;83(1):38-42. doi: 10.1093/neuros/nyx368[↩]
- Carlson ML, Jacob JT, Habermann EB, Glasgow AE, Raghunathan A, Link MJ. Malignant peripheral nerve sheath tumors of the eighth cranial nerve arising without prior irradiation. J Neurosurg. 2016 Nov;125(5):1120-1129. doi: 10.3171/2015.7.JNS151056[↩]
- Pollock BE, Link MJ, Stafford SL, Parney IF, Garces YI, Foote RL. The Risk of Radiation-Induced Tumors or Malignant Transformation After Single-Fraction Intracranial Radiosurgery: Results Based on a 25-Year Experience. Int J Radiat Oncol Biol Phys. 2017 Apr 1;97(5):919-923. doi: 10.1016/j.ijrobp.2017.01.004[↩]
- Maducdoc MM, Ghavami Y, Linskey ME, Djalilian HR. Evaluation of Reported Malignant Transformation of Vestibular Schwannoma: De Novo and After Stereotactic Radiosurgery or Surgery. Otol Neurotol. 2015 Sep;36(8):1301-8. doi: 10.1097/MAO.0000000000000801[↩]
- Delsanti C, Roche PH, Thomassin JM, Régis J. Morphological changes of vestibular schwannomas after radiosurgical treatment: pitfalls and diagnosis of failure. Prog Neurol Surg. 2008;21:93-97. doi: 10.1159/000156712[↩][↩]
- Nagano O, Higuchi Y, Serizawa T, Ono J, Matsuda S, Yamakami I, Saeki N. Transient expansion of vestibular schwannoma following stereotactic radiosurgery. J Neurosurg. 2008 Nov;109(5):811-6. doi: 10.3171/JNS/2008/109/11/0811[↩][↩]
- Plotkin, S. R., Stemmer-Rachamimov, A. O., Barker, F. G., 2nd, Halpin, C., Padera, T. P., Tyrrell, A., Sorensen, A. G., Jain, R. K., & di Tomaso, E. (2009). Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. The New England journal of medicine, 361(4), 358–367. https://doi.org/10.1056/NEJMoa0902579[↩]
- Plotkin SR, Merker VL, Halpin C, Jennings D, McKenna MJ, Harris GJ, Barker FG 2nd. Bevacizumab for progressive vestibular schwannoma in neurofibromatosis type 2: a retrospective review of 31 patients. Otol Neurotol. 2012 Aug;33(6):1046-52. doi: 10.1097/MAO.0b013e31825e73f5[↩]
- Blakeley, J. O., Ye, X., Duda, D. G., Halpin, C. F., Bergner, A. L., Muzikansky, A., Merker, V. L., Gerstner, E. R., Fayad, L. M., Ahlawat, S., Jacobs, M. A., Jain, R. K., Zalewski, C., Dombi, E., Widemann, B. C., & Plotkin, S. R. (2016). Efficacy and Biomarker Study of Bevacizumab for Hearing Loss Resulting From Neurofibromatosis Type 2-Associated Vestibular Schwannomas. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 34(14), 1669–1675. https://doi.org/10.1200/JCO.2015.64.3817[↩]
- Van Gompel JJ, Agazzi S, Carlson ML, Adewumi DA, Hadjipanayis CG, Uhm JH, Olson JJ. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines on Emerging Therapies for the Treatment of Patients With Vestibular Schwannomas. Neurosurgery. 2018 Feb 1;82(2):E52-E54. doi: 10.1093/neuros/nyx516[↩]
- Karajannis, M. A., Legault, G., Hagiwara, M., Ballas, M. S., Brown, K., Nusbaum, A. O., Hochman, T., Goldberg, J. D., Koch, K. M., Golfinos, J. G., Roland, J. T., & Allen, J. C. (2012). Phase II trial of lapatinib in adult and pediatric patients with neurofibromatosis type 2 and progressive vestibular schwannomas. Neuro-oncology, 14(9), 1163–1170. https://doi.org/10.1093/neuonc/nos146[↩]
- Karajannis, M. A., Legault, G., Hagiwara, M., Giancotti, F. G., Filatov, A., Derman, A., Hochman, T., Goldberg, J. D., Vega, E., Wisoff, J. H., Golfinos, J. G., Merkelson, A., Roland, J. T., & Allen, J. C. (2014). Phase II study of everolimus in children and adults with neurofibromatosis type 2 and progressive vestibular schwannomas. Neuro-oncology, 16(2), 292–297. https://doi.org/10.1093/neuonc/not150[↩]