Spondyloarthropathy
Spondyloarthropathy also called spondyloarthritis or seronegative spondyloarthritis, refers to a group of inflammatory rheumatic diseases that mainly attacks your spine (spondylitis) and pelvic sacroiliac joints (sacroiliitis) and in some people, the joints of their arms and legs (peripheral arthritis) 1, 2, 3, 4, 5. Spondyloarthropathy can also involve your skin, intestines and eyes. The main symptom of spondyloarthropathy in most patients is chronic low back pain (low back pain more than 3 months). This occurs most often in axial spondyloarthritis.
Spondyloarthropathy according the Assessment of SpondyloArthritis International Society (ASAS) proposed a new classification system, can be classified according to the distribution of joint involvement as being predominantly axial (axial spondyloarthritis) or peripheral (peripheral spondyloarthritis) 6, 4:
- Axial spondyloarthropathy: predominantly axial symptoms (i.e. chronic low back pain)
- axial mean it’s related to or situated in the central part of the body, encompassing the full spine and pelvis
- preradiographic or non-radiographic: no changes on radiographs (but may have MRI changes)
- radiographic: sacroiliitis on radiographs
- The most common type of spondyloarthropathy is axial spondyloarthritis (axSpA), a term that covers both ankylosing spondylitis (AS) and nonradiographic axial spondyloarthritis (nr-axSpA) where joint damage isn’t visible on X-rays. The names are confusing, but what’s important is that both mainly cause inflammation in joints in the spine and pelvis. Non-radiographic axial spondyloarthritis (nr-axSpA) and ankylosing spondylitis are two sub-types of axial spondyloarthritis. They can be thought of as two ends of the spectrum of axial spondyloarthritis. The difference is that in non-radiographic axial spondyloarthritis (nr-axSpA), the sacroiliac (SI) joints do not show definitive damage on x-rays, as seen in ankylosing spondylitis. Many people with nonradiographic axial spondyloarthritis (nr-axSpA) never go on to develop ankylosing spondylitis, and some patients with ankylosing spondylitis have only mild symptoms. There are many gradations in between that vary considerably from person to person.
- Peripheral spondyloarthropathy: only peripheral manifestations characterized by inflammatory pain and arthritis in peripheral joints and tendons other than the spine (e.g. peripheral arthritis, enthesitis, dactylitis)
Spondyloarthropathy consists of the following inflammatory rheumatic diseases 1, 2, 3, 4, 5:
- Ankylosing spondylitis. Ankylosing spondylitis is a kind of arthritis that affects the joints and ligaments of your spine. ‘Ankylosing’ means stiff and ‘spondylo’ means vertebra. About 50% to 92%% of patients with ankylosing spondylitis will be HLA-B27 (human leukocyte antigen-B27) positive 7, 8
- Psoriatic arthritis. Psoriatic arthritis is a chronic, autoimmune form of arthritis that causes joint inflammation (arthritis) and occurs with the skin condition psoriasis. Up 70% of people with psoriatic arthritis have both peripheral and spinal inflammation, but people with a type known as axial psoriatic arthritis only have arthritis in their spine.
- Reactive arthritis. Reactive arthritis is mainly triggered by an intestinal, urinary or genital tract infection, which is often treated with antibiotics. Reactive arthritis usually goes away on its own.
- Enteropathic arthritis is arthritis associated with inflammatory bowel disease like ulcerative colitis and Crohn’s disease. Like ankylosing spondylitis, enteropathic arthropathy often affects the spine but rarely causes joint destruction or disability.
- Undifferentiated spondyloarthropathy also known as undifferentiated arthritis, is defined as any arthritis of recent onset that cannot be classified according to the existing criteria for specific rheumatic disorders that lacks the clinical, serological and radiological features that would allow specific diagnosis 9. Undifferentiated spondyloarthropathy often turns out to be an early presentation of a more well-known form of arthritis with 40 to 50% of undifferentiated spondyloarthropathy patients remit spontaneously, while 30% develop rheumatoid arthritis (RA) 10, 11.
Many patients with spondyloarthritis have a first degree relative (e.g., father, mother, brother or sister) with spondyloarthritis or spondyloarthritis-related disease such as ankylosing spondylitis, psoriatic arthritis, psoriasis, Crohn’s disease, ulcerative colitis and uveitis. All forms of spondyloarthritis may ultimately develop into ankylosing spondylitis in patients with longstanding disease 12.
Many patients with spondyloarthropathy have the axial skeleton involvement and are typically negative to rheumatoid factor (RF). However, a small proportion of patients may have serum rheumatoid factor (RF) detected. Most spondyloarthropathy patients test positive for the protein product of the HLA-B27 gene.
To diagnosed spondyloarthropathy, your doctor typically will begin by going through your medical history and conducting a thorough physical exam. Tests and procedures that may be used for diagnosing spondyloarthritis include:
- Blood tests, to determine your HLA-B27 status and measure markers of inflammation (C-reactive protein [CRP], erythrocyte sedimentation rate [ESR]).
- Having the HLA-B27 gene doesn’t mean you’ll develop any of the conditions in the spondyloarthropathy family 13, 14, 15. The HLA-B27 gene alone doesn’t cause seronegative spondyloarthropathy and about 98% of people who carry it never have back pain. On the flip side, people of African descent are much less likely than others to carry HLA-B27 but can still develop inflammatory spondyloarthropathy 16.
- Imaging studies, to look for evidence of inflammation and rule out other potential causes of the patient’s symptoms. The specific type of imaging study (X-ray, ultrasound, MRI) will vary depending on the your symptoms. However, Imaging does not play a major role in differentiating between the spondyloarthropathy subtypes as imaging features are similar, especially in early disease. Exceptions to this rule are 17:
- undifferentiated spondyloarthropathy: no definite radiologic signs of sacroiliitis
- psoriatic arthritis: associated with parasyndesmophytes, a form of bony outgrowth distinct from syndesmophytes
- Also, spondylitis with bone marrow edema of the entire vertebra occurs more frequently in psoriatic arthritis.
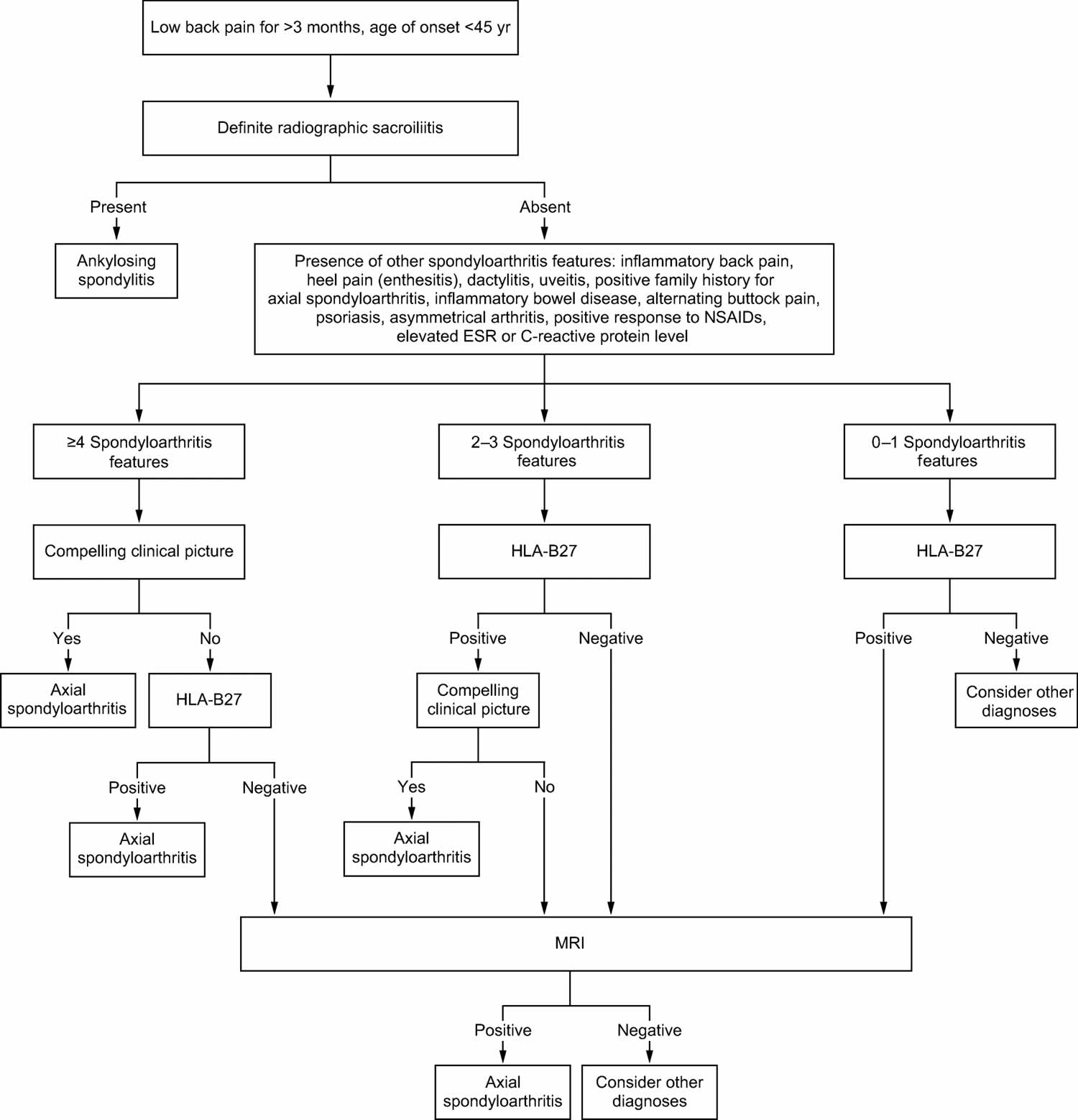
It is important to note that as there are no specific diagnostic tests for most spondyloarthropathies (see Figure 1). For this reason, it is essential to correlate the findings in history and physical examination with laboratory, radiological, and sometimes histopathological findings to establish the diagnosis.
The goal of treatment for spondyloarthropathies is to improve function, decrease pain, and reduce the risk of complications. Management should include lifestyle interventions, including an exercise program to maintain posture, strength, and range of movement. Patients should also be monitored for osteoporosis and encouraged to cease smoking.
Exercise is important for everyone with arthritis, but it’s a fundamental part of treatment if you have ankylosing spondylitis or nonradiographic axial spondyloarthritis (nr-axSpA). Exercise relieves pain better than medications and is the only way to keep your spine healthy and mobile. The American College of Rheumatology recommends that everyone with axial spondyloarthropathy get physical therapy and regularly perform exercises that “promote spine extension and mobility.” Swimming, in particular, is terrific for back health.
The first-line treatment for spine pain is nonsteroidal anti-inflammatory drugs (NSAIDs) like naproxen, ibuprofen and celecoxib. For many people with ankylosing spondylitis or nonradiographic axial spondyloarthritis (nr-axSpA), these drugs along with exercise are enough to control symptoms. They are quite effective, reducing pain, tenderness, and stiffness in 80% of patients.
If you don’t get relief from NSAIDs or have severe peripheral arthritis, you and your doctor might consider a tumor necrosis factor (TNF) blocker (anti-TNF-alpha therapy) like infliximab (Remicade), etanercept, adalimumab (Humira) or certolizumab (Cimzia). Certolizumab is the only anti-TNF-alpha therapy (TNF inhibitor) approved by the Food and Drug Administration (FDA) to treat nonradiographic axial spondyloarthritis (nr-axSpA), but doctors prescribe the others off-label based on research and clinical experience. These drugs can reduce the spinal and peripheral joint inflammation of ankylosing spondylitis and control extra-articular symptoms. Another option is an interleukin (IL)-17 blocker like secukinumab (Cosentyx) or ixekizumab (Taltz), both FDA-approved for nonradiographic axial spondyloarthritis (nr-axSpA). All of these medications, called biologics, are expensive and can have potentially serious side effects, so be sure you and your doctor weigh the pros and cons carefully.
Other medications for severe peripheral arthritis that you and your doctor might consider are called disease-modifying anti-rheumatic drugs (DMARDs), which include sulfasalazine and methotrexate. Intra-articular corticosteroid injections have a modest benefit for peripheral arthritis 18.
The treatment for psoriatic arthritis targets the joints and skin lesions. Anti-TNF-alpha therapy (TNF inhibitor) for psoriatic arthritis has been a significant advance, resulting in a significant improvement in symptoms and a delay in progression of the disease. Secukinumab, an anti-interleukin-17A monoclonal antibody, is effective at treating psoriasis and psoriatic arthritis.
The management of reactive arthritis involves assessing and treating an active infection. Chlamydia infections should be treated with antibiotics. There is no evidence to support the use of antibiotics for treatment of urogenital or enteric forms of reactive arthritis. High-dose NSAIDs are also used to treat patients with reactive arthritis. Indomethacin 50–75 mg twice daily is commonly used. Persistent reactive arthritis may respond to disease-modifying anti-rheumatic drugs (DMARDs) such as sulfasalazine, azathioprine, or methotrexate 18.
Enteropathic arthritis is also treated with NSAIDs and cyclooxygenase-2 inhibitors, but these can cause flare-ups of inflammatory bowel disease. DMARDs are beneficial for gastrointestinal and joint involvement. Anti-TNF agents can be used if traditional DMARDs fail; however, there have been rare cases of inflammatory bowel diseases precipitated by etanercept. Total colectomy (removal of the affected colon) does not improve axial involvement in inflammatory bowel disease 19.
Surgical treatment is helpful in some patients. Total hip replacement is very useful for those with hip pain and disability due to joint destruction from cartilage loss. Spinal surgery is rarely necessary, except for those with traumatic fractures (broken bones due to injury) or to correct excess flexion deformities of the neck, where the patient cannot straighten the neck.
Figure 1. Spondyloarthropathy diagnostic algorithm
Abbreviations: axSpA = axial spondyloarthritis; ESR = erythrocyte sedimentation rate; HLA-B27 = human leukocyte antigen-B27; MRI = magnetic resonance imaging
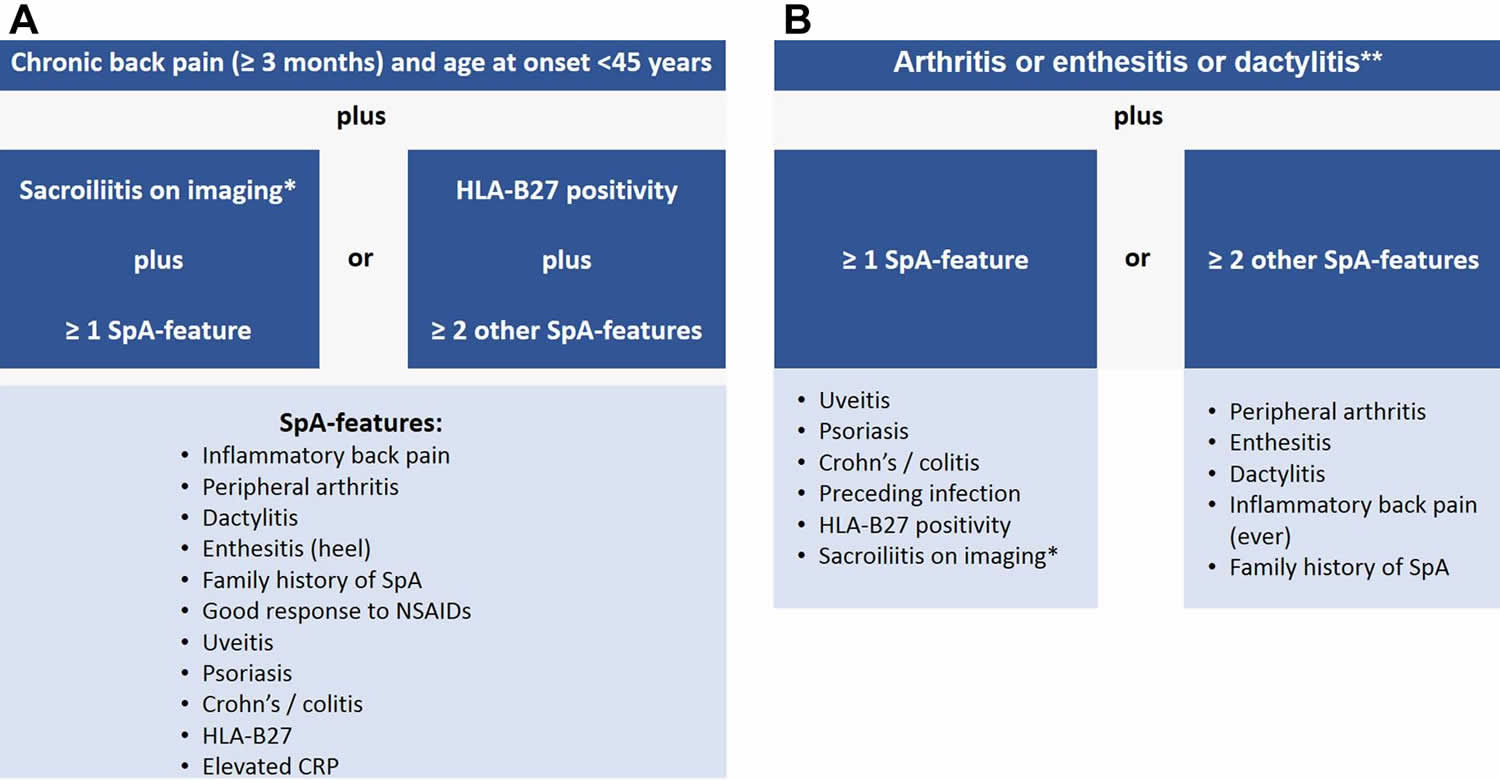
[Source 20 ]Figure 2. Spondyloarthropathy diagnostic criteria (Assessment of SpondyloArthritis International Society (ASAS) criteria)
Footnotes: Assessment of SpondyloArthritis International Society (ASAS) criteria for axial and peripheral spondyloarthritis. (A) ASAS classification criteria for axial spondyloarthritis; (B) ASAS classification criteria for peripheral spondyloarthritis. *Sacroiliitis on imaging refers to definite radiographic sacroiliitis according to the modified New York criteria or active sacroiliitis on MRI according to the ASAS definition. **Peripheral arthritis: usually predominantly lower limbs and/or asymmetric arthritis; enthesitis: clinically assessed; dactylitis: clinically assessed. SpA = spondyloarthritis.
[Source 21 ]Who gets spondyloarthropathy?
Many spondyloarthropathy, especially ankylosing spondylitis and nonradiographic axial spondyloarthritis (nr-axSpA), strike young adults, often when they’re still in school or just starting careers and families 16. Ankylosing spondylitis tends to start in the teens and 20s and affects males two to three times more often than females although it is likely that females are underdiagnosed. Family members of affected people are at higher risk, depending partly on whether they inherited the HLA-B27 gene.
There is an uneven ethnic distribution of ankylosing spondylitis. The highest frequency appears in the far north in cultures such as Alaskan and Siberian Eskimos and Scandinavian Lapps (also called Samis), who have a higher frequency of HLA-B27. It also occurs more often in certain Native American tribes in the western U.S. and Canada. African Americans are affected less often than other races.
Based on data from the National Health and Nutrition Examination Survey (NHANES), the frequency of ankylosing spondylitis in the U.S., is 0.5 percent. The frequency for axial spondyloarthritis is 1.4 percent.
Although it was long believed that men with ankylosing spondylitis outnumbered women by three to one, it’s now known that women are underdiagnosed, especially when bone damage doesn’t show up on X-rays. Newer research suggests seronegative spondyloarthropathy is an equal opportunity offender, striking men and women equally. Black Americans are less likely to have ankylosing spondylitis than whites but tend to have more severe disease 16.
Spondyloarthropathy types
Spondyloarthropathy according the Assessment of SpondyloArthritis International Society (ASAS) proposed a new classification system, can be classified according to the distribution of joint involvement as being predominantly axial (axial spondyloarthritis) or peripheral (peripheral spondyloarthritis) 6, 4:
- Axial spondyloarthropathy: predominantly axial symptoms (i.e. chronic low back pain)
- axial mean it’s related to or situated in the central part of the body, encompassing the full spine and pelvis
- preradiographic or non-radiographic: no changes on radiographs (but may have MRI changes)
- radiographic: sacroiliitis on radiographs
- The most common type of spondyloarthropathy is axial spondyloarthritis (axSpA), a term that covers both ankylosing spondylitis (AS) and nonradiographic axial spondyloarthritis (nr-axSpA) where joint damage isn’t visible on X-rays. The names are confusing, but what’s important is that both mainly cause inflammation in joints in the spine and pelvis. Non-radiographic axial spondyloarthritis (nr-axSpA) and ankylosing spondylitis are two sub-types of axial spondyloarthritis. They can be thought of as two ends of the spectrum of axial spondyloarthritis. The difference is that in non-radiographic axial spondyloarthritis (nr-axSpA), the sacroiliac (SI) joints do not show definitive damage on x-rays, as seen in ankylosing spondylitis. Many people with nonradiographic axial spondyloarthritis (nr-axSpA) never go on to develop ankylosing spondylitis, and some patients with ankylosing spondylitis have only mild symptoms. There are many gradations in between that vary considerably from person to person.
- Peripheral spondyloarthropathy: only peripheral manifestations characterized by inflammatory pain and arthritis in peripheral joints and tendons other than the spine (e.g. peripheral arthritis, enthesitis, dactylitis)
Spondyloarthropathy consists of the following inflammatory rheumatic diseases 1, 2, 3, 4, 5:
- Ankylosing spondylitis. Ankylosing spondylitis is a kind of arthritis that affects the joints and ligaments of your spine. ‘Ankylosing’ means stiff and ‘spondylo’ means vertebra. About 50% to 92%% of patients with ankylosing spondylitis will be HLA-B27 (human leukocyte antigen-B27) positive 7, 8
- Psoriatic arthritis. Psoriatic arthritis is a chronic, autoimmune form of arthritis that causes joint inflammation (arthritis) and occurs with the skin condition psoriasis. Up 70% of people with psoriatic arthritis have both peripheral and spinal inflammation, but people with a type known as axial psoriatic arthritis only have arthritis in their spine.
- Reactive arthritis. Reactive arthritis is mainly triggered by an intestinal, urinary or genital tract infection, which is often treated with antibiotics. Reactive arthritis usually goes away on its own.
- Enteropathic arthritis is arthritis associated with inflammatory bowel disease like ulcerative colitis and Crohn’s disease. Like ankylosing spondylitis, enteropathic arthropathy often affects the spine but rarely causes joint destruction or disability.
- Undifferentiated spondyloarthropathy also known as undifferentiated arthritis, is defined as any arthritis of recent onset that cannot be classified according to the existing criteria for specific rheumatic disorders that lacks the clinical, serological and radiological features that would allow specific diagnosis 9. Undifferentiated spondyloarthropathy often turns out to be an early presentation of a more well-known form of arthritis with 40 to 50% of undifferentiated spondyloarthropathy patients remit spontaneously, while 30% develop rheumatoid arthritis (RA) 10, 11.
Axial spondyloarthropathy
The most common type of spondyloarthropathy is axial spondyloarthritis (axSpA) also called axial spondyloarthrititis, is a term that covers both ankylosing spondylitis (AS) and nonradiographic axial spondyloarthritis (nr-axSpA) where joint damage isn’t visible on X-rays. The names are confusing, but what’s important is that both mainly cause inflammation in joints in the spine and pelvis. Axial spondyloarthropathy predominantly present with axial symptoms such as low back pain (i.e. chronic low back pain) and morning stiffness. Ankylosing spondylitis and nonradiographic axial spondyloarthritis (nr-axSpA) are seen as two regions on a spectrum of inflammatory conditions rather than early and late stages of a single disease. Many people with nonradiographic axial spondyloarthritis (nr-axSpA) never go on to develop ankylosing spondylitis, and some patients with ankylosing spondylitis have only mild symptoms. There are many gradations in between that vary considerably from person to person. There is a long delay, on average 14 years, between symptoms onset and diagnosis 22.
The prevalence of axial axial spondyloarthropathy is ~1% 22. Age of onset is in the 3rd and 4th decades 21.
Assessment of SpondyloArthritis International Society (ASAS) criteria for the diagnosis of axial spondyloarthropathy 6, 4:
- ≥3 months of back pain and age of onset ≤45 years
- sacroiliitis on imaging and ≥1 clinical feature or HLA-B27 and ≥2 clinical features:
- Clinical features
- Inflammatory back pain: insidious onset; improvement with exercise but not with rest; night pain; morning back stiffness ≥30 minutes; alternating gluteal pain 2
- arthritis
- heel enthesitis
- uveitis
- dactylitis
- psoriasis
- Crohn disease
- good response to NSAIDs (absent pain) 24-48 hours after a full dose 21
- family history of axial spondyloarthropathy
- elevated CRP
- Clinical features
Imaging forms an important part of the work-up as the clinical features are somewhat non-specific 21. Radiographic evidence of axial spondyloarthropathy will be present in ~50% of patients at initial diagnosis. Some will progress to having radiographic evidence whilst others will never have radiographic evidence of axial spondyloarthropathy 22.
Plain radiograph
- Sacroiliac joint x-rays are the first-line modality. Definite radiographic sacroiliitis is characterized by the New York Criteria as bilateral grade 2 or unilateral grade 3 21.
MRI
Sacroiliac joint MRI is the second line modality. The Assessment in SpondyloArthritis International Society (ASAS) classification criteria first published in 2009 23 with a revised consensus definition of a positive MRI (active sacroiliitis) in 2016 24, utilizes imaging features of the sacroiliac joints on x-ray and MRI as one of many criteria for the classification of axial spondyloarthritis with the aim to create homogeneous populations for research 25. Its use in clinical practice has been discouraged by some authors 26. The sensitivity and specificity of the ASAS classification criteria are around 80% 26, 27, which limits its use as a diagnostic tool 26.
- The phrase “active sacroiliitis on MRI” can be used if there are features highly suggestive of inflammation. These features can be broken down into two categories 24:
- Required MRI features
- bone marrow edema (BMO) / osteitis on a T2-weighted sequence sensitive for free water (such as short tau inversion recovery (STIR) or T2FS) or bone marrow contrast enhancement on a T1-weighted sequence (such as T1FS post-Gd)*
- inflammation must be clearly present and located in a typical anatomical area (subchondral bone)
- MRI appearance must be highly suggestive of a spondyloarthropathy
- Not required MRI features – other findings related to sacroiliitis may be observed on MRI but are not required to fulfill the imaging criterion of “active sacroiliitis on MRI”:
- the sole presence of other inflammatory lesions such as synovitis, enthesitis or capsulitis without concomitant BMO is not sufficient for the definition of “active sacroiliitis on MRI”
- in the absence of MRI signs of BMO, the presence of structural lesions such as fat metaplasia, sclerosis, erosion or ankylosis does not meet the definition of “active sacroiliitis on MRI”, but can be considered chronic lesions
- Required MRI features
- *the ASAS 2016 consensus definition removed the quantitative requirement (i.e. two slices or two locations) from the required MRI features 24, 26
Ankylosing spondylitis
Ankylosing spondylitis is a type of seronegative spondyloarthropathy that causes arthritis of your spine and sacroiliac (SI) joints, although other large and small joints in your body can also become involved (e.g., shoulders, hips, ribs, heels, and small joints of the hands and feet) 28. Ankylosing spondylitis causes inflammation between your vertebrae, which are the bones that make up your spine, and in the joints between your spine and pelvis. In some people, it can affect other joints. Sometimes the eyes can become involved (known as iritis or uveitis), and — rarely — the lungs and heart can be affected. In more advanced ankylosing spondylitis cases this inflammation can lead to ankylosis (fusion) — new bone formation in the spine — causing sections of the spine to fuse in a fixed, immobile position.
Ankylosing spondylitis is more common and more severe in men. It often runs in families. The cause is unknown, but it is likely that both genes and factors in the environment playing a role.
The most common early symptoms of ankylosing spondylitis are frequent back pain and stiffness in your lower back and buttocks, which comes on gradually over the course of a few weeks or months. At first, discomfort may only be felt on one side, or alternate sides. The pain is usually dull and diffuse, rather than localized.
This pain and stiffness is usually worse in the mornings and during the night, but may be improved by a warm shower or light exercise. Also, in the early stages of ankylosing spondylitis, there may be mild fever, loss of appetite, and general discomfort. It is important to note that back pain from ankylosing spondylitis is inflammatory in nature and not mechanical.
The pain typically becomes persistent (chronic) and is felt on both sides, usually lasting for at least three months. Over the course of months or years, the stiffness and pain can spread up the spine and into the neck. Pain and tenderness spreading to the ribs, shoulder blades, hips, thighs, and heels is possible as well. Some people have symptoms that come and go. Others have severe, ongoing pain.
Ankylosing spondylitis has no cure, but medicines can relieve symptoms and may keep the disease from getting worse. Eating a healthy diet, not smoking, and exercising can also help. In rare cases, you may need surgery to straighten the spine.
Ankylosing spondylitis symptoms
The most common early symptoms of ankylosing spondylitis are frequent back pain and stiffness in your lower back and buttocks, which comes on gradually over the course of a few weeks or months. At first, discomfort may only be felt on one side, or alternate sides. The pain is usually dull and diffuse, rather than localized.
This pain and stiffness is usually worse in the mornings and during the night, but may be improved by a warm shower or light exercise. Also, in the early stages of ankylosing spondylitis, there may be mild fever, loss of appetite, and general discomfort. It is important to note that back pain from ankylosing spondylitis is inflammatory in nature and not mechanical.
The pain typically becomes persistent (chronic) and is felt on both sides, usually lasting for at least three months. Over the course of months or years, the stiffness and pain can spread up the spine and into the neck. Pain and tenderness spreading to the ribs, shoulder blades, hips, thighs, and heels is possible as well. Some people have symptoms that come and go. Others have severe, ongoing pain.
It is important to note that the course of ankylosing spondylitis varies greatly from person to person. So too can the onset of symptoms. Although symptoms usually start to appear in late adolescence or early adulthood (ages 17 to 45), symptoms can occur in children or much later in life.
Over time, ankylosing spondylitis can fuse your vertebrae together, limiting movement.
In a minority of individuals, pain does not start in the lower back, or even the neck, but in a peripheral joint such as the hip, ankle, elbow, knee, heel, or shoulder. This pain is commonly caused by enthesitis, inflammation of the site where a ligament or tendon attaches to bone. Inflammation and pain in peripheral joints is more common in children with ankylosing spondylitis. This can be confusing since, without the immediate presence of back pain, ankylosing spondylitis may look like some other form of arthritis.
Varying levels of fatigue may also result from the inflammation caused by ankylosing spondylitis as the body must expend energy to deal with the inflammation, thus causing fatigue. Also, mild to moderate anemia, which may also result from the inflammation, can contribute to an overall feeling of tiredness.
Many people with ankylosing spondylitis also experience bowel inflammation, which may be associated with Crohn’s disease or ulcerative colitis.
Ankylosing spondylitis is often accompanied by iritis or uveitis (inflammation of the eyes). About one-third of people with ankylosing spondylitis will experience inflammation of the eye at least once. Signs of iritis or uveitis are: Eye(s) becoming painful, watery, and red, blurred vision, and sensitivity to bright light.
Ankylosing spondylitis diagnosis
A rheumatologist is commonly the type of physician who will diagnose ankylosing spondylitis, since they are doctors who are specially trained in diagnosing and treating disorders that affect the joints, muscles, tendons, ligaments, connective tissue, and bones. A thorough physical exam, including X-rays, individual medical history, and a family history of ankylosing spondylitis, as well as blood work (including a test for HLA-B27) are factors in making a diagnosis.
Bamboo spine is a pathognomonic radiographic feature seen in ankylosing spondylitis that occurs as a result of vertebral body fusion by marginal syndesmophytes 29. Bamboo spine is often accompanied by fusion of the posterior vertebral elements as well. A bamboo spine typically involves the thoracolumbar and/or lumbosacral junctions and predisposes to unstable vertebral fractures and Andersson lesions (inflammatory involvement of the intervertebral discs by spondyloarthritis) 29, 30.
In a bamboo spine, the outer fibers of the annulus fibrosus of the intervertebral discs ossify, which results in the formation of marginal syndesmophytes between adjoining vertebral bodies 31. The resulting radiographic appearance, therefore, is that of thin, curved, radiopaque spicules that completely bridge adjoining vertebral bodies. There is also accompanying squaring of the anterior vertebral body margins with associated reactive sclerosis of the vertebral body margins (shiny corner sign) 31. Together these give the impression of undulating continuous lateral spinal borders on AP spinal radiographs and resemble a bamboo stem; hence the term bamboo spine.
The hallmark of ankylosing spondylitis is involvement of the sacroiliac (SI) joints. Some physicians still rely on X-ray to show erosion typical of sacroiliitis, which is inflammation of the sacroiliac joints. Using conventional X-rays to detect this involvement can be problematic because it can take seven to 10 years of disease progression for the changes in the sacroiliac (SI) joints to be serious enough to show up on conventional X-rays.
Another option is to use MRI to check for sacroiliac (SI) involvement, but MRI can be cost prohibitive in some cases.
A diagnosis of ankylosing spondylitis is based on your medical history and a physical examination. You may also have imaging or blood tests.
Ankylosing spondylitis treatment
At the present time there is no cure for ankylosing spondylitis; however, your doctor will work with you to relieve your symptoms, maintain proper posture and slow the progression of the disease. In most cases, treatment includes medication and physical therapy. Sometimes, people with severe disease need surgery to repair joint damage.
Medications
Most people with ankylosing spondylitis take medications, which may include one or more of the following:
- Over-the-counter anti-inflammatory medications to relieve pain and inflammation are commonly used to treat ankylosing spondylitis.
- Biologic medications target specific immune messages and interrupt the signal, helping to decrease or stop inflammation. These medications may be prescribed if your disease is unresponsive to other treatments.
- Janus kinase (JAK) inhibitors may also be prescribed if your disease is unresponsive to other treatments. These medications send messages to specific cells to stop inflammation from inside the cell.
- Corticosteroids can help decrease inflammation and provide some pain relief. They are usually injected into the joint. Because they are potent drugs, your doctor will determine how much and how many injections you should receive to achieve the desired benefit.
Physical therapy
Your doctor may recommend physical therapy to help:
- Relieve pain.
- Strengthen back and neck muscles.
- Improve core and abdominal muscle strength because these muscles provide support for your back.
- Improve posture.
- Maintain and improve flexibility in joints.
A physical therapist can recommend the best sleeping positions and an exercise program. Because your symptoms may worsen when inactive or at rest, it’s important to stay active and exercise regularly.
Surgery
If you have severe joint damage and you are unable to participate in your daily activities, your doctor may recommend surgery. Surgery is not for everyone. You and your doctor can discuss the options and choose what is right for you.
Your doctor will consider the following before recommending surgery:
- Your overall health.
- The condition of the affected bone or joint.
- The risks and benefits of the surgery.
Types of surgery may include joint repairs and joint replacements.
Rarely, some people may have surgery to correct or straighten the spine or repair fractures (breaks) in the vertebrae.
Non-Radiographic Axial Spondyloarthritis
Non-Radiographic Axial Spondyloarthritis (nr-axSpA) is a form of arthritis in the family of spondyloarthritis diseases. Non-radiographic axial spondyloarthritis (nr-axSpA) primarily affects your spine, but other parts of your body are also often involved. It causes inflammation in the spine and elsewhere that can lead to various symptoms including pain and stiffness.
Here is a breakdown of the terms associated with non-radiographic axial spondyloarthritis 32:
- Axial – Relating to or situated in the central part of the body, encompassing the full spine and pelvis.
- Spondyloarthritis – Inflammatory arthritis involving the spine and peripheral joints.
- Non-Radiographic – No definitive damage seen on x-rays of spine, specifically in the sacroiliac (SI) joints.
Non-radiographic axial spondyloarthritis (nr-axSpA) is most closely associated with a highly similar condition, radiographic axial spondyloarthritis (r-axSpA), which is more commonly called ankylosing spondylitis.
Non-radiographic axial spondyloarthritis (nr-axSpA) and ankylosing spondylitis (AS) are two sub-types of axial spondyloarthritis. They can be thought of as two ends of the spectrum of axial spondyloarthritis. The difference is that in non-radiographic axial spondyloarthritis (nr-axSpA), the sacroiliac (SI) joints do not show definitive damage on x-rays, as seen in ankylosing spondylitis.
A rheumatologist will generally diagnose and treat non-radiographic axial spondyloarthritis (nr-axSpA). Unfortunately, there is no clear diagnostic test for non-radiographic axial spondyloarthritis (nr-axSpA). This means that a thorough physical exam by a knowledgeable doctor, along with a detailed patient and family history, is key to help with the diagnosis. Blood tests and imaging (x-ray or MRI) are also often helpful.
Non-radiographic axial spondyloarthritis symptoms
The first symptoms of non-radiographic axial spondyloarthritis (nr-axSpA) are often described as persistent pain and stiffness in the lower back and buttocks, which come on gradually over the course of a few weeks or months. This pain and stiffness are usually worse in the mornings and during the night, but may improve after a warm shower or light exercise. At first, discomfort may only be felt on one side, or alternate sides. The pain is usually dull and diffuse, rather than localized. Sometimes, these symptoms can be severe.
Although symptoms usually start to appear in late adolescence or early adulthood (ages 17 to 45), symptoms can occur in children or much later in life. The course of non-radiographic axial spondyloarthritis (nr-axSpA) varies greatly from person to person — no two individuals may have the exact same symptoms.
The back pain people with non-radiographic axial spondyloarthritis (nr-axSpA) experience is usually inflammatory back pain. Key characteristics of inflammatory back pain include pain and stiffness that 33:
- Start before age 45
- Develop and worsen gradually
- Persist for more than three months
- Ease with physical activity and exercise
- Get worse with immobility (especially overnight and early morning)
- Pain and stiffness in other areas of the body, such as the neck, shoulders, the ribs, hips, knees, and heels, are also common. In some individuals, pain does not start in the lower back, or even the neck, but in one of the peripheral joints mentioned above. This can be confusing since, without the immediate presence of back pain, non-radiographic axial spondyloarthritis (nr-axSpA) may look like other forms of arthritis. A person’s sex can affect the location of initial pain in non-radiographic axial spondyloarthritis (nr-axSpA). For some women, the neck and peripheral joints are often affected first, whereas in men it is more likely to be the lower back.
Other main symptoms of non-radiographic axial spondyloarthritis (nr-axSpA) may Include:
- Iritis / Uveitis – Inflammation of the eye. Symptoms often occur in one eye at a time, and may include redness, pain, sensitivity to light, and skewed vision. (Note: iritis is a medical emergency and needs immediate attention as untreated eye inflammation can lead to permanent damage and even blindness.)
- Enthesitis– Inflammation of the entheses, which is where ligaments or tendons attach to the bone. Symptoms include swelling and tenderness. Common areas impacted include the Achilles tendon at the back of the heel, the plantar fascia at the base of the heel, the rib cage, and the spine.
- Inflammatory bowel disease (IBD) – Gastrointestinal issues and bowel inflammation, which may be associated with Crohn’s disease or ulcerative colitis.
- Psoriasis – A scaly skin rash.
Fatigue is a common complaint in non-radiographic axial spondyloarthritis (nr-axSpA), and one that doesn’t often receive the attention it deserves. Fatigue can negatively impact one’s work, family or social life, ability to focus, and even emotional state.
Uncontrolled inflammation is the factor most closely associated with fatigue in non-radiographic axial spondyloarthritis (nr-axSpA). If inflammation is extensive, then the body must use energy to deal with it, producing feelings of profound, chronic tiredness. The release of certain cell messengers (cytokines) during the inflammatory process also can produce the sensation of fatigue, as well as mild to moderate anemia in some cases, which itself can further exacerbate fatigue. When inflammation is well controlled and the disease is properly managed, fatigue often lessens and energy returns.
Uncontrolled pain and stiffness can make it difficult to sleep. Besides causing fatigue, sleep deprivation can increase pain, creating a feedback loop of pain causing sleeplessness, which then causes more pain, and so on.
For all these reasons, effective pain management is crucial. Though many people with non-radiographic axial spondyloarthritis (nr-axSpA) respond well to medications alongside exercise and physical therapy, others experience more severe pain even with appropriate treatment. In these cases, it is important to work with your medical team to find appropriate solutions and design a comprehensive plan to treat the pain. Speaking with your rheumatologist about pain and fatigue is the first step.
Non-radiographic axial spondyloarthritis diagnosis
Often a rheumatologist will test the blood for the HLA-B27 gene marker, and for higher-than-normal inflammatory markers such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), which may indicate systemic inflammation. It is important to note that bloodwork is not always helpful, as not everyone with non-radiographic axial spondyloarthritis (nr-axSpA) will have the HLA-B27 gene marker, or always show elevated inflammatory markers.
There is no association between non-radiographic axial spondyloarthritis (nr-axSpA) and rheumatoid factor (RF) or antinuclear antibodies (associated with lupus).
Imaging tests such as an x-ray or MRI can be powerful tools in discovering inflammation or damage in the spine. However, those with non-radiographic axial spondyloarthritis (nr-axSpA) will not have definitive damage to the sacroiliac (SI) joints visible on plain x-rays.
MRI of the sacroiliac (SI) joints can show evidence of inflammation not visible on x-rays. Therefore, MRI can be a very important tool in diagnosing non-radiographic axial spondyloarthritis (nr-axSpA).
Non-radiographic axial spondyloarthritis treatment
Non-radiographic axial spondyloarthritis (nr-axSpA) treatment is similar to treatment for ankylosing spondylitis 34. Treatment goals should focus on relieving pain and stiffness, maintaining axial spine motion and functional ability, and preventing spinal complications. Non-pharmacologic interventions should include regular exercise, postural training, and physical therapy 35. First-line medication therapy is with long-term, daily non-steroidal anti-inflammatory drugs (NSAIDs). Should NSAIDs not provide adequate relief, they can be combined with or substituted for tumor necrosis factor inhibitors (TNF-Is) such as adalimumab, infliximab, or etanercept. Treatment with secukinumab or ixekizumab is conditionally recommended in patients with non-radiographic axial spondyloarthritis (nr-axSpA), because trials in Non-radiographic axial spondyloarthritis (nr-axSpA) have not been reported 34. Response to NSAIDs should be assessed four to six weeks after initiation and twelve weeks following initiation of tumor necrosis factor inhibitors (TNF-Is) 7. Systemic glucocorticoids are not recommended, but local steroid injections may be considered. Specialist referrals may be warranted based on the patient’s clinical picture, potential complications, and extra-articular manifestations of the disease. Rheumatologists may assist in a formal diagnosis, management, and monitoring, while dermatologists, ophthalmologists, and gastroenterologists may assist with associated non-musculoskeletal features of ankylosing spondylitis 36, 37.
Enteropathic spondyloarthropathy
Enteropathic spondyloarthropathy also known as enteropathic arthropathy or enteropathic arthritis, is a type of seronegative spondyloarthropathy that is associated with the occurrence of inflammatory bowel disease (IBD), the two best-known types of which are ulcerative colitis and Crohn’s disease. About one in five people (20% of inflammatory bowel disease patients) with Crohn’s or ulcerative colitis will develop enteropathic arthritis 38.
The most common areas affected by enteropathic arthritis are the peripheral (limb) joints and, in some cases, the entire spine can become involved, as well. Abdominal pain and, possibly, bloody diarrhea associated with inflammatory bowel disease (IBD) are also components of the disease.
A diagnosis of enteropathic arthritis is made through a complete medical examination including a history of symptoms and taking into account family history. Various tests may also be done to determine the presence of an inflammatory bowel disease and inflammatory arthritis.
Currently, there is no known cure for enteropathic arthritis but there are medications and therapies available to manage the symptoms of both the arthritis and bowel components of the disease.
Enteropathic arthritis causes
The precise causes of the enteropathic arthritis is unknown. Inflammation of the gastrointestinal tract may increase permeability, resulting in absorption of antigenic material, including bacterial antigens 39. These arthrogenic antigens may then localize in musculoskeletal tissues (including entheses and synovial membrane), thus eliciting an inflammatory response. Alternatively, an autoimmune response may be induced through molecular mimicry, in which the host’s immune response to these antigens cross reacts with self-antigens in synovial membrane and other target organs.
Of particular interest is the strong association (80%) between reactive arthritis and human leukocyte antigen (HLA)-B27, an HLA class I molecule. A potentially arthrogenic, bacterially derived antigen peptide could fit in the antigen-presenting groove of the B27 molecule, resulting in a CD8+ T-cell response 39. HLA-B27 transgenic rats develop features of enteropathic arthropathy with arthritis and gut inflammation 39.
Sacroiliitis and spondylitis are associated with HLA-B27 (40% and 60%, respectively). HLA-B27 is not associated with peripheral arthritis, with the exception of reactive arthritis.
HLA accounts only for 40% of the genetic risk for spondyloarthropathy; other polymorphisms in non-HLA genes such tumor necrosis factor (TNF) and IL-23, involved in innate immune recognition and cytokine signaling pathways, are linked with spondyloarthropathy, inflammatory bowel disease (IBD) and psoriasis 40, 41,
Enteropathic arthritis symptoms
The symptoms of enteropathic arthritis can be divided in two groups:
- Symptoms of inflammatory bowel disease (IBD)
- Arthritic symptoms in the joints and, possibly, elsewhere in your body.
- Axial arthritis (sacroiliitis and spondylitis) in inflammatory bowel disease (IBD) has the following characteristics:
- Insidious onset of low back pain, especially in younger persons
- Morning stiffness
- Exacerbated by prolonged sitting or standing
- Improved by moderate activity
- More common in Crohn disease than in ulcerative colitis 42
- Independent of gastrointestinal symptoms
- Peripheral arthritis in inflammatory bowel disease (IBD) demonstrates the following characteristics:
- Nondeforming and nonerosive
- More common in Crohn’s disease with colonic involvement than in ulcerative colitis
- May precede intestinal involvement, but usually concomitant or subsequent to bowel disease, as late as 10 years following the diagnosis
- Type 1 (pauciarticular [< 5 joints]) – Acute, self-limiting attacks, lasting less than 10 weeks; asymmetrical and affecting large joints, such as the knees, hips and shoulders; strong correlation to IBD activity, most frequently with extensive ulcerative colitis or colonic involvement in Crohn’s disease; associated with other extraintestinal manifestations of IBD 43
- Type 2 (polyarticular [>5 joints]) – Chronic, lasting months to years; more likely symmetrical, affecting small joints of the hands; independent of bowel activity 43
- Enthesitis affects the following parts of the body:
- Heel – Insertion of the Achilles tendon and plantar fascia
- Knee – Tibial tuberosity, patella
- Others – Buttocks, foot
- Extra-articular inflammatory bowel disease (IBD) demonstrates the following characteristics:
- Intestinal – Abdominal pain, weight loss, diarrhea, and hematochezia
- Skin – Pyoderma gangrenosum (in ulcerative colitis), erythema nodosum (in Crohn’s disease)
- Oral – Aphthous ulcers (in ulcerative colitis, Crohn’s disease)
- Ocular – Uveitis, anterior, nongranulomatous
- Systemic low-grade fever, secondary amyloidosis (in Crohn’s disease)
- Axial arthritis (sacroiliitis and spondylitis) in inflammatory bowel disease (IBD) has the following characteristics:
Enteropathic spondyloarthropathy complications
Complications of enteropathic arthropathy are primarily related to inflammatory bowel disease (IBD) and include the following:
- Chronic arthritis – Occasionally extra-articular involvement (uveitis)
- Secondary amyloidosis – Mainly with Crohn disease
- Toxicity of therapy
Enteropathic spondyloarthropathy diagnosis
A diagnosis of enteropathic arthritis is made through a complete medical examination including a history of symptoms and taking into account family history. Various tests may also be done to determine the presence of an inflammatory bowel disease and inflammatory arthritis.
Enteropathic spondyloarthropathy treatment
Treatment of inflammatory bowel disease (IBD), including surgery, should always be the initial strategy to induce remission of peripheral arthritis 44.
Although nonsteroidal anti-inflammatory drugs (NSAIDs) are usually recommended as first-line therapy for spondyloarthropathies, in patients with inflammatory bowel disease (IBD), these agents may exacerbate gastrointestinal symptoms 45. Selection of more cyclooxygenase-2 (COX-2)–selective NSAIDs may reduce the risk of bowel flares 46, 47. Corticosteroids may be used systemically or by local injection.
Sulfasalazine (2-3g/day) has been shown to be effective for treatment of the peripheral arthropathy associated with IBD, but not axial disease. [15] While methotrexate can be useful to treat bowel activity in Crohn disease (CD), its effect on joint disease with IBD is less certain.
Although not specifically indicated for an enteropathic arthropathy, the tumor necrosis factor (TNF) antagonists infliximab and adalimumab are indicated to treat ankylosing spondylitis and IBD, and may be effective for inflammatory bowel disease (IBD) spondyloarthropathy (including axial involvement) 48, 49, 50, 51, 52.
In a cohort of 30 patients with enteropathic arthropathy affected by active articular and gastrointestinal disease, or axial active articular inflammation, adalimumab led to sustained improvement of both articular and gastrointestinal disease activities. Significant improvement was achieved at the earliest (6-months) assessment and maintained at the 12-months follow-up 53.
Etanercept and golimumab are indicated to treat ankylosing spondylitis 54, but neither has been shown to be helpful with bowel disease, and there have been reports of new-onset inflammatory bowel disease (IBD) with these 2 agents 55.
Vedolizumab is approved for treatment of moderate to severe Crohn disease. A systematic review found evidence that it may be effective in preventing the onset of enteropathic anthropathy but there was no strong evidence for the efficacy of vedolizumab for treating existing arthritis 56.
Enteropathic spondyloarthropathy prognosis
Enteropathic spondyloarthropathy prognosis depends mainly on the prognosis of the underlying inflammatory bowel disease (IBD). Severe spinal inflammatory disease may occur, but this is rare.
Psoriatic spondyloarthropathy
Psoriatic spondyloarthropathy also known as psoriatic arthritis, is inflammatory arthritis associated with psoriasis. Psoriasis is a skin disease that causes itchy or sore patches of thick, red skin with silvery scales. You usually get them on your elbows, knees, scalp, back, face, palms and feet, but they can show up on other parts of your body. Psoriatic arthritis is usually negative for rheumatoid factor and hence classified as one of the seronegative spondyloarthropathies. Men and women are equally affected by psoriatic arthritis. The symptoms of psoriatic arthritis come and go but it is a lifelong condition that is usually progressive.
Up to 30 percent of people with psoriasis also develop psoriatic arthritis 57. In most cases (though not always), the psoriasis will precede the arthritis, sometimes by many years. When arthritis symptoms occur with psoriasis, it is called psoriatic arthritis (psoriatic spondyloarthropathy). In these cases, the joints at the end of the fingers are most commonly affected, causing inflammation and pain, but other joints like the wrists, knees, and ankles can also become involved. This is usually accompanied by symptoms in the fingernails and toenails, ranging from small pits in the nails to nearly complete destruction and crumbling as seen in reactive arthritis or fungal infections.
Patients with psoriasis who are more likely to subsequently get arthritis include those with the following characteristics 58:
- Psoriatic nail disease
- Flexural psoriasis, scalp psoriasis or post-auricular psoriasis
- Obesity
- Smoker
- Elevated C-reactive protein (CRP) at baseline.
About 20 percent of patients with psoriatic arthritis will develop spinal involvement, which is called psoriatic spondylitis 59. Inflammation of the spine can lead to complete fusion, as in ankylosing spondylitis or affect only certain areas such as the lower back or neck. Patients who are HLA-B27 positive are more likely than others to have their disease progress to the spine 59.
Psoriatic arthritis and ankylosing spondylitis are considered genetically and clinically related because both are inflammatory rheumatic diseases linked to the HLA-B27 gene 59. HLA-B27 is a powerful predisposing gene associated with several rheumatic diseases. The HLA-B27 gene itself does not cause disease, but can make people more susceptible. While a number of genes are linked to psoriatic arthritis, the highest predictive value is noted with HLA-B27.
Who gets psoriatic arthritis?
Psoriatic arthritis has an incidence of approximately 6 per 100,000 per year and a prevalence of about 1–2 per 1000 in the general population 58. Estimates of the prevalence of psoriatic arthritis among patients with psoriasis range between 4 and 30 per cent. In most patients, arthritis appears 10 years after the first signs of skin psoriasis 58. The first signs of psoriatic arthritis usually occur between the ages of 30 and 50 years of age 58. In approximately 13–17% of cases, arthritis precedes the skin disease 58.
Psoriatic arthritis causes
The exact cause of psoriatic arthritis is not known, but the main contributing factors to the development of psoriatic arthritis are a genetic predisposition, immune factors and the environment 58. In approximately 13–17% of cases, arthritis precedes the skin disease 58. Up to 40 percent of people with psoriatic arthritis have a close relative with the disease 59. If an identical twin has psoriatic arthritis, there is a 75 percent chance that the other twin will have it as well 59.
Genetic factors
As in psoriasis of the skin, many patients with psoriatic arthritis may have a familial tendency toward the condition. A twin study found that arthritis was as common in dizygotic (fraternal) twins as in monozygotic (identical) twins so unknown environmental factors may also be important. First-degree relatives of patients with psoriatic arthritis have a 50-fold increased risk of developing psoriatic arthritis compared with the general population. It is unclear whether this is due to a genetic basis of psoriasis alone, or whether there is a special genetic predisposition to arthritis as well.
Immune factors
Psoriatic arthritis occurs as a result of abnormal interaction between the immune system and the joints. People with psoriatic arthritis seem to have an overactive immune system as evidenced by raised inflammatory markers, increased antibodies and T-lymphocytes.
Activated T cells (in particular CD8+ T cells) have generally been found in the skin and joints of patients with psoriatic arthritis. Several studies have demonstrated that pro-inflammatory cytokines secreted from activated T cells (especially tumour necrosis factor alpha (TNF-α), IL-17, IL-18 and IL-23) induce proliferation and activation of synovial and epidermal fibroblasts. CD8+, IL-17+ cells, natural killer (NK) cells and innate lymphoid (ILC3) cells are present in high concentrations in the synovial fluid and skin lesions in patients with psoriatic arthritis.
Environmental factors
Environmental factors implicated in the pathogenesis of psoriatic arthritis include infections (bacterial infections such as streptococci and Borrelia burgdorferi, the cause of Lyme disease; and viral infections such as rubella), trauma (Koebner phenomenon), moving to a new house, recurrent oral ulcers, obesity and bone fractures.
An inverse association with smoking has also been reported in HLA-C*06 allele negative patients 58.
Psoriatic arthritis symptoms
The symptoms of psoriatic arthritis, which vary from person to person, can change in severity. Skin symptoms typically (but not always) appear before the joints become involved, sometimes up to 10 years before. Without treatment, many of these symptoms can lead to progressive, permanent joint damage 59.
While psoriatic arthritis is a single disease, it can have many different symptoms that affect the skin and joints, including:
- Swelling of an entire finger or toe, sometime causing a “sausage” like appearance;
- General joint pain and stiffness, especially in the morning;
- Joint swelling;
- Back pain and stiffness, primarily in the lower back, neck, and upper back;
- Reduced range of motion;
- Painful, often throbbing joints;
- Psoriatic skin lesions;
- Nail changes, including small indentations, lifting and or discoloration of the fingernails or toenails, which occur in almost all patients;
- Generalized fatigue;
- Inflammatory eye conditions that can cause redness, pain, blurred vision, and sensitivity to light. Thirty percent of PsA patients have conjunctivitis, and seven percent have iritis.
People with psoriatic arthritis usually have some skin signs eventually 58.
Psoriatic arthritis develops after skin psoriasis in approximately 75% of patients 58. Remaining patients have either a simultaneous onset of skin and joint psoriasis (10%), or joint symptoms precede any skin problem (15%). The severity of the skin disease does not predict the severity of the joint disease 58.
Plaque psoriasis is the most common form of skin psoriasis seen with psoriatic arthritis. Joint symptoms may be exacerbated by a flare in skin psoriasis but quite commonly the skin symptoms behave independently of joint symptoms. Most people with psoriatic arthritis have mild psoriasis.
Psoriatic arthritis diagnosis
Diagnosing psoriatic arthritis can be tricky, primarily because it shares similar symptoms with other diseases such as osteoarthritis, rheumatoid arthritis, and gout. Because of this, misdiagnosis can often be a problem. Early diagnosis, however, is important because long-term joint damage can be warded off better in the first few months after symptoms arise.
The diagnosis of psoriatic arthritis is based on symptoms, an examination of skin and joints and compatible X-ray findings 58. Psoriatic arthritis may present with tendinitis, enthesitis or dactylitis, rather than swollen joints.
X-ray findings that are characteristic of psoriatic arthritis include:
- Changes affecting the joints at the end of the fingers and toes
- Asymmetrical joint involvement (particularly of the sacroiliac joints)
- Erosions: Destruction of the bone and cartilage adjacent to joint spaces
- “Pencil-in-cup” deformity: results from periarticular erosions and bone resorption giving the appearance of a pencil in a cup.
- Subluxation: slipping of the alignment of the joint
- Ankylosis: fusion of bones together across the joint space
- Wispy and dense bony outgrowths around joints (‘whiskering’ and ‘spurs’, respectively)
- Increased calcium salt deposition around the insertion of ligaments and tendons into the bone and other features of enthesopathy.
- Ivory phalanx: increased radiodensity of an entire phalanx as a result of periosteal and endosteal bone formation.
MRI and ultrasound can also aid diagnosis, by identifying enthesitis, tendinitis and ligamentous inflammation.
There are no diagnostic blood tests for psoriatic arthritis but tests may be done to help confirm the diagnosis and rule out other causes.
- Markers of inflammation, ESR (erythrocyte sedimentation rate) and CRP, may reflect the severity of the inflammation in the joints.
- Rheumatoid factor is usually negative but may be positive in up to 10% of patients with psoriatic arthritis.
- Anti-CCP (anti-cyclic citrullinated peptide) is also usually negative but may be positive in up to 7% of patients with psoriatic arthritis.
- HLA-B27 testing
Classification criteria, such as CASPAR criteria, are mainly used for research purposes. Several screening questionnaires have also been developed, such as Toronto Psoriatic Arthritis Screen (ToPAS2), to help to identify patients with psoriatic arthritis.
Psoriatic arthritis treatment
Some treatments for joint psoriasis are also effective for skin psoriasis, so treatment plans may take both skin and joint disease into account 58.
The principles of psoriatic arthritis treatment include early and aggressive treatment in order to prevent joint deformity and resulting morbidity 58. The choice of treatment depends on disease manifestation (pattern of joint involvement, the severity of joint vs skin involvement, the swelling and stiffness, the extent of pain, non-articular involvement) in addition to factors regarding safety (regarding comorbidities), tolerability and patient preference 58.
Non-pharmacological management strategies are important; these include physical and occupational therapy, exercise, prescription of orthotics, and education regarding the disease and about joint protection, disease management, and the proper use of medications 58. Patients should receive assistance in weight reduction and management of cardiovascular risk factors and other comorbidities.
Those with very mild arthritis may require treatment only when their joints are painful and may stop therapy when they feel better. Non-steroidal anti-inflammatory drugs such as ibuprofen (Motrin or Advil) or naproxen (Aleve) are used as initial treatment.
If the arthritis does not respond, disease-modifying antirheumatic drugs (DMARDs) may be prescribed. These include sulfasalazine (Azulfidine), methotrexate (Rheumatrex, Trexall, Otrexup, Rasuvo), cyclosporine (Neoral, Sandimmune, Gengraf), and leflunomide. Sometimes combinations of these drugs may be used together. Azathioprine (Imuran) may help those with severe forms of psoriatic arthritis.
Other treatments include biologics, typically starting with TNF inhibitors such as adalimumab (Humira), certolizumab pegol (Cimzia), etanercept (Enbrel), golimumab (Simponi), infliximab (Remicade). Other biologics used for psoriatic arthritis include the IL-17 inhibitors secukinumab (Cosentyx) and ixekizumab (Taltz), or other classes as ustekinumab (Stelara) and abatacept (Orencia). Newer oral medications, such as tofacitinib (Xeljanz) have also been shown to be effective.
In general, a stepwise approach to pharmacological treatment is adopted 58:
- Step one
- If arthritis is mild and limited to a few joints and the skin disease is not severe, the skin is treated with topical therapies or phototherapy and the joint disease is managed with pain relief (non-steroidal anti-inflammatory drugs, heat and ice) and possibly corticosteroid injections into the joint.
- Step two
- Non-biological disease-modifying antirheumatic drugs (DMARDs) improve symptoms of pain and stiffness, but none have been shown to prevent progressive joint damage and all have the potential for serious side effects. The following medications have a beneficial effect on joint disease and psoriasis:
- Methotrexate
- Ciclosporin
- Leflunomide
- Apremilast.
- Systemic steroids may help arthritis but can often cause a flare of psoriasis on reduction in dose or discontinuation.
- Non-biological disease-modifying antirheumatic drugs (DMARDs) improve symptoms of pain and stiffness, but none have been shown to prevent progressive joint damage and all have the potential for serious side effects. The following medications have a beneficial effect on joint disease and psoriasis:
- Step three
- Biological TNF-alpha inhibitors. Biologic response modifiers licensed for use in psoriatic arthritis are:
- Adalimumab
- Etanercept
- Infliximab
- Golimumab.
- Biological TNF-alpha inhibitors. Biologic response modifiers licensed for use in psoriatic arthritis are:
- Step four
- Other agents that are under investigation or are available include:
- Abatacept: (CTLA4-Ig) a selective T-cell co-stimulation modulator
- Tofacitinib: a Janus kinase (JAK1 and 3) inhibitor
- Secukinumab, brodalumab, ixekizumab: anti-IL-17 therapies
- Ustekinumab: a human monoclonal antibody to the shared p40 subunit of IL-13 and IL-23
- Filgotinib: a JAK1 inhibitor
- Guselkumab: an anti-leukin (IL)-23 monoclonal antibody
- Upadacitinib: JAK inhibitor.
- Other agents that are under investigation or are available include:
- Step five
- In some cases, the joint disease may require orthopedic surgery.
Psoriatic arthritis prognosis
Most people with psoriatic arthritis will have ongoing problems with arthritis throughout the rest of their life. Remissions are uncommon; occurring in less than 20% of patients with less than 10% of patients having a complete remission off all medication with no signs of joint damage on X-rays. People with severe psoriatic arthritis have been reported to have a shorter lifespan than average.
Features associated with a relatively good prognosis are:
- Male sex
- Fewer joints involved
- Good functional status at presentation
- Previous remission in symptoms.
Features associated with a poor prognosis include:
- ESR > 15 mm/hr or raised CRP at presentation
- Failure of previous medication trials
- Absence of nail changes
- Joint damage (clinically or radiographically)
- HLA-B27-, -B39-, or -DQw3-positive status.
Living with psoriatic arthritis
Many people with psoriatic arthritis develop stiff joints and muscle weakness due to lack of use. Proper exercise is especially important to improve your overall health and keep your joints flexible. Walking is an excellent way to get exercise. A walking aid or shoe inserts will help to avoid undue stress on feet, ankles, or knees affected by arthritis. An exercise bike provides another good option, as well as yoga and stretching exercises to help with relaxation.
Aqua therapy can be beneficial as some people with psoriatic arthritis find it easier to move in water. Many people with psoriatic arthritis also benefit from physical and occupational therapy to strengthen muscles, protect joints from further damage, and increase flexibility.
Reactive arthritis
Reactive arthritis also known as Reiter’s syndrome, is a type of arthritis that follows a bacterial infection at a different site, commonly triggered by a bacterial infection in the digestive or urinary tract or the genitals 60. Reactive arthritis may also cause inflammation of your eyes, skin and urinary and genital systems. The knee and ankle joints are frequently affected by reactive arthritis, and many people experience pain in the sacroiliac joints in their lower back as well. Reactive arthritis is a type of seronegative spondyloarthropathy, a group of arthritis conditions that typically involve the sacroiliac joints in the lower back, and entheses (places where tendons or ligaments attach to bones). Foot pain in people with reactive arthritis is usually due to inflammation of entheses.
Reactive arthritis can have any or all of these features:
- Pain and swelling of certain joints, often the knees and/or ankles
- Swelling and pain at the heels
- Extensive swelling of the toes or fingers
- Persistent low back pain, which tends to be worse at night or in the morning
Some patients with reactive arthritis also have eye redness and irritation. Still other signs and symptoms include burning with urination and a rash on the palms or the soles of the feet.
The exact cause of reactive arthritis is unknown. However, it most often follows a bacterial infection, but the joint itself is not infected. Reactive arthritis most often occurs in men between the ages of 20 and 40, although it does sometimes affect women. Reactive arthritis may follow an infection in the urethra after unprotected sex. The most common bacteria that cause such infections is called Chlamydia trachomatis. Reactive arthritis can also follow a bacterial gastrointestinal infection (Campylobacter, Salmonella, Shigella and Yersinia) such as food poisoning. In up to one half of people thought to have reactive arthritis, there may be no infection.
Chlamydia most often transmits by sex. Chlamydia often has no symptoms but can cause a pus-like or watery discharge from the genitals. The bowel bacteria can cause diarrhea. If you develop arthritis within one month of a gastrointestinal or a genital infection – especially with a discharge – see your doctor.
Certain genes may make you more likely to get reactive arthritis. Some patients with reactive arthritis carry a gene called HLA-B27. Patients who test positive for HLA-B27 often have a more sudden and severe onset of symptoms. They also are more likely to have chronic (long-lasting) symptoms. Yet, patients who are HLA-B27 negative (do not have the gene) can still get reactive arthritis after exposure to an organism that causes it.
Reactive arthritis is rare in young children, but it may occur in teenagers. Reactive arthritis may occur in children ages 6 to 14 after Clostridium difficile gastrointestinal infections.
Reactive arthritis diagnosis is largely based on symptoms of the inducing infections and clinical features on physical exam typical of reactive arthritis. If indicated, doctors might order a test for Chlamydia infection or test for the HLA-B27 gene. The test for Chlamydia uses a urine sample or a swab of the genitals.
For most people, signs and symptoms of reactive arthritis come and go, eventually disappearing within a few weeks or up to 12 months. But the signs and symptoms of reactive arthritis may become chronic (long-lasting) in some people. Doctors tailor treatment to each individual’s symptoms, and therapy typically involves a combination of medications and exercise.
Who gets reactive arthritis?
The bacteria that cause reactive arthritis are very common and anyone can get reactive arthritis, and it occurs worldwide. In theory, anyone who becomes infected with bacterial infection in their digestive or urinary tract or the genitals might develop reactive arthritis. Yet very few people with bacterial diarrhea actually go on to have serious reactive arthritis. What remains unclear is the role of Chlamydia infection (a sexually transmitted infection) that has no symptoms. It is possible that some cases of arthritis of unknown cause are due to Chlamydia.
Certain factors can increase your risk of developing reactive arthritis, including:
- Sex. Both men and women can get reactive arthritis, but men are more likely to develop it as a result of a sexually transmitted infection. Men and women are equally affected if the condition is from a gastrointestinal infection.
- Age. It occurs most often in people between ages 20 and 40.
- Genetics. People who have a gene called HLA-B27 have a higher risk of getting reactive arthritis and of experiencing more severe and more long-lasting symptoms. But people who lack HLA-B27 can still get the condition.
- HIV infection. Having AIDS or being infected with HIV increases the risk of reactive arthritis.
Reactive arthritis tends to occur most often in men between ages 20 and 50. Some patients with reactive arthritis carry a gene called HLA-B27. Patients who test positive for HLA-B27 often have a more sudden and severe onset of symptoms. They also are more likely to have chronic (long-lasting) symptoms. Yet, patients who are HLA-B27 negative (do not have the gene) can still get reactive arthritis after exposure to an organism that causes it.
Patients with weakened immune systems due to HIV and AIDS can also develop reactive arthritis.
Reactive arthritis causes
Reactive arthritis is triggered by a bacterial infection, frequently a sexually transmitted genitals or urinary tract infection or food-borne bacterial infection, but it is separate from the infection and typically sets in after the infection has cleared. You might not be aware of the triggering infection if it causes mild symptoms or none at all. The bacteria that cause reactive arthritis are very common. The bacteria that commonly trigger reactive arthritis are Salmonella, Yersinia, Campylobacter, Shigella, and Chlamydia, but only a minority of people infected with them develop reactive arthritis. The bacteria cause arthritis by distorting your body’s defense against infections, as well as your genetic environment. How exactly each of these factors plays a role in the disease likely varies from patient to patient. Scientists do not fully understand why some people are predisposed to getting reactive arthritis.
Numerous bacteria can cause reactive arthritis. Some are transmitted sexually, and others are foodborne. The most common ones include:
Reactive arthritis isn’t contagious. However, the bacteria that cause it can be transmitted sexually or in contaminated food. Only a few of the people who are exposed to these bacteria develop reactive arthritis.
Genetics seems to partly explain susceptibility to reactive arthritis, as many affected individuals have a gene called HLA-B27. However, many people who get reactive arthritis lack the HLA-B27 genetic marker so there are other, as yet unknown, genetic and environmental contributing factors.
Risk factors for reactive arthritis
Certain factors increase your risk of reactive arthritis:
- Age. Reactive arthritis occurs most frequently in adults between the ages of 20 and 40.
- Sex. Women and men are equally likely to develop reactive arthritis in response to foodborne infections. However, men are more likely than are women to develop reactive arthritis in response to sexually transmitted bacteria.
- Hereditary factors. A specific genetic marker has been linked to reactive arthritis. But most people who have this marker never develop the condition.
Reactive arthritis prevention
Genetic factors appear to play a role in whether you’re likely to develop reactive arthritis. Though you can’t change your genetic makeup, you can reduce your exposure to the bacteria that may lead to reactive arthritis.
Store your food at proper temperatures and cook it properly. Doing these things help you avoid the many foodborne bacteria that can cause reactive arthritis, including salmonella, shigella, yersinia and campylobacter. Some sexually transmitted infections can trigger reactive arthritis. Use condoms to help lower your risk.
Reactive arthritis symptoms
Some people with reactive arthritis have mild symptoms, while others have severe symptoms that limit daily activities. The signs and symptoms of reactive arthritis typically start 1 to 6 weeks after exposure to a triggering infection of the digestive or urinary tract or genitals, but the infection has usually resolved by the time symptoms arise. The onset is typically fairly sudden, usually over the course of a few days.
Reactive arthritis is characterized by inflammation of the joints, eyes, and urinary tract, but not everyone with the condition will experience all three, or they might not occur at the same time.
The main symptoms of reactive arthritis are:
- Joint pain and stiffness. The joint pain associated with reactive arthritis most commonly occurs in the knees, ankles and feet. Pain may also occur in the heels, low back or buttocks.
- Joints may become painful, red, and swollen, especially the large joints of the lower limbs, such as the knees and ankles. Morning stiffness or nighttime pain is typical. The affected joints are usually on one side of the body. There may be pain in the lower back and buttocks as well.
- Pain in the heel or foot pain is a sign of enthesitis (inflammation at a place where a tendon or ligament attaches to a bone).
- Swollen, inflamed, painful fingers or toes (dactylitis) may also occur.
- Eye inflammation. Many people who have reactive arthritis also develop eye inflammation (conjunctivitis).
- Conjunctivitis (inflammation of the transparent layer that covers the white part of the eye and lines the eyelids) and uveitis (inflammation of the middle part of the eye) can cause redness, pain, burning, itching, crusted eyelids, blurred vision, or sensitivity to light.
- Inflammation of the urinary tract. Increased frequency and discomfort during urination may occur, as can inflammation of the prostate gland or cervix.
- This symptom is more common when reactive arthritis happens after an infection of the genitals or urinary tract.
- In women, urinary tract inflammation can develop into inflammation of the cervix, fallopian tubes, vulva, or vagina.
- Increased urinary frequency and burning while urinating are signs of urinary tract inflammation.
- Enthesitis or inflammation of tendons and ligaments where they attach to bone. This happens most often in the heels and the sole of the feet.
- Swollen toes or fingers. In some cases, toes or fingers might become so swollen that they look like sausages.
- Low back pain. The pain tends to be worse at night or in the morning.
Other symptoms of reactive arthritis include:
- Skin problems. Reactive arthritis can affect skin in a variety of ways, including small ulcers in the mouth and a rash on the soles of the feet and palms of the hands. Skin rash (keratoderma blennorrhagica) consisting of reddish, raised bumps, usually on the palms or soles. The bumps may merge, forming a larger scaly rash.
- Fatigue or feeling generally unwell.
- Fever.
- Weight loss.
- Diarrhea and abdominal pain.
- In men, small, painless ulcers on the penis.
- Thickened nails.
The symptoms of reactive arthritis often clear up on their own within a few weeks or months, but they may become chronic (long-lasting) in some people.
Reactive arthritis diagnosis
Reactive arthritis diagnosis is largely based on your symptoms and clinical features on physical exam typical of reactive arthritis. During the physical exam, your doctor is likely to check your joints for swelling, warmth and tenderness, and test range of motion in your spine and affected joints. Your doctor might also check your eyes for inflammation and your skin for rashes.
If indicated, doctors might order a test for Chlamydia infection or test for the HLA-B27 gene. The test for Chlamydia uses a urine sample or a swab of the genitals. Having HLA-B27 is consistent with having reactive arthritis, but it is not definitive as people who test HLA-B27 negative can still have reactive arthritis, and not everyone who tests HLA-B27 positive has the condition.
Your doctor might recommend that a sample of your blood be tested for:
- Evidence of past or current infection
- Signs of inflammation.
- Erythrocyte sedimentation rate (sed rate) and C-reactive protein. These blood tests are measures of inflammation, but they are not specific for reactive arthritis. A positive test result can indicate any inflammatory disorder. A negative test result does not rule out reactive arthritis because these markers are usually not elevated in the chronic form of the condition.
- Antibodies associated with other types of arthritis.
- Rheumatoid factor (RF) and anticyclic citrullinated peptide (anti-CCP) antibody tests, which are associated with rheumatoid arthritis.
- Antinuclear antibody (ANA) test, which is associated with systemic lupus erythematosus.
- A genetic marker linked to reactive arthritis
Bacterial cultures
- Culturing your stool and urine specimens may reveal the presence of bacteria that frequently trigger reactive arthritis. But a negative result is not conclusive because in most cases, the infection has cleared by the time arthritic symptoms arise.
Joint fluid tests
Your doctor might use a needle to withdraw a sample of fluid from within an affected joint. This fluid will be tested for:
- White blood cell count. An increased number of white blood cells might indicate inflammation or an infection.
- Infections. Bacteria in your joint fluid might indicate septic arthritis, which can result in severe joint damage.
- Crystals. Uric acid crystals in your joint fluid might indicate gout. This very painful type of arthritis often affects the big toe.
Imaging tests
- X-rays of your low back, pelvis and joints can indicate whether you have any of the characteristic signs of reactive arthritis. X-rays can also rule out other types of arthritis. X-rays often do not pick up abnormalities until later in the course of reactive arthritis.
- Ultrasounds, computed tomography (CT), and magnetic resonance imaging (MRI). These specialized imaging techniques are especially useful for visualizing joint changes that happen in the early stages of reactive arthritis.
Reactive arthritis treatment
The goal of reactive arthritis treatment is to manage your symptoms and treat the bacterial infection that could still be present. Your doctor might use one or more of the following:
Medications
If your reactive arthritis was triggered by a bacterial infection, your doctor might prescribe an antibiotic if there is evidence of a current bacterial infection. Which antibiotic you take depends on the bacteria that are present. Signs and symptoms of reactive arthritis may be eased with:
- Over-the-counter or prescribed nonsteroidal anti-inflammatory drugs (NSAIDs) and pain medications. NSAIDs, such as indomethacin (Indocin), can relieve the inflammation, pain and swelling of reactive arthritis.
- Corticosteroids or steroids. Usually, these are injected into affected joints, but if multiple joints are involved, your doctor may prescribe them by mouth or intravenously. They are potent drugs, so doctors typically prescribe the lowest dose possible to reduce the inflammation and allow you to return to your usual activity level. You may need corticosteroid creams or eye drops for skin rashes and eye symptoms, respectively.
- Disease-modifying anti-rheumatic drugs (DMARDs). These rheumatoid arthritis drugs such as sulfasalazine (Azulfidine) or methotrexate (Trexall) to suppress the immune system on a broad level, helping to block inflammation in the joints and other tissues. Sulfasalazine may be more useful when the reactive arthritis is triggered by a gastrointestinal infection. Doctors usually only use them when anti-inflammatories and corticosteroids have not worked. Disease-modifying anti-rheumatic drugs (DMARDs) can relieve pain and stiffness for some people with reactive arthritis.
- In more severe cases, stronger immune lowering medications called “biologics” may be used such as etanercept (Enbrel) or adalimumab (Humira).
Physical therapy
Physical therapy can help ease pain and improve joint function. A physical therapist can provide you with targeted exercises for your joints and muscles for strengthening the muscles that surround a joint, providing support and improving joint flexibility.
- Strengthening exercises increase the joint’s support by developing the muscles around the affected joints. Range-of-motion exercises can increase your joints’ flexibility and reduce stiffness.
Reactive arthritis prognosis
Reactive arthritis usually develops two to four weeks after infection and typically follows a limited course, with most people recovering from its symptoms in three to 12 months.
A tendency does exist for more severe and long-term disease in patients who test positive for HLA-B27, as well as in those who have a family history of spondyloarthritis.
In about 15 to 20 percent of people with reactive arthritis, the condition recurs, sometimes brought on by reinfection. There is also a possibility of developing a chronic form of arthritis. Though the chronic arthritis brought on by reactive arthritis is usually mild, a minority of people develop a more severe form of arthritis, or spondyloarthritis.
Undifferentiated spondyloarthritis
Undifferentiated spondyloarthritis also known as undifferentiated arthritis or undifferentiated spondyloarthropathy, is defined as any spondylitis of recent onset that cannot be classified according to the existing criteria for specific spondyloarthritis (e.g., ankylosing spondylitis, psoriatic arthritis, reactive arthritis, and arthritis related to inflammatory bowel disease) that lacks the clinical, serological and radiological features that would allow specific diagnosis 9. Sometimes a doctor may make an initial diagnosis of “spondyloarthritis” or “unclassified spondyloarthritis” if certain spondylitis symptoms are present but are not distinctive enough to make a specific diagnosis. For example, an adult may have iritis, heel pain (enthesitis), and knee swelling, WITHOUT back pain, psoriasis, a recent infection, or intestinal symptoms 61. This person’s combination of disease features suggests spondyloarthritis, but they don’t neatly fit into the categories of ankylosing spondylitis, psoriatic arthritis, reactive arthritis, juvenile spondyloarthritis, or enteropathic arthritis. Over time, some people with undifferentiated spondyloarthritis will develop a more well-defined form of spondylitis such as ankylosing spondylitis, psoriatic arthritis, rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), an overlap between two or more rheumatic diseases or a self-limited syndrome of unknown cause that resolves on its own 62, 63, 64. About 40 to 50% of undifferentiated spondyloarthropathy patients remit spontaneously, while 30% develop rheumatoid arthritis (RA) and the rest into other chronic rheumatic conditions 10, 11, 65, 62.
The prevalence of undifferentiated spondyloarthritis was ranged from 0.03% to 2% in European individuals, 0.15% to 0.55% in Asian, and 0.20% to 1.3% in Northern Arctic indigenous people as reported in a recent systematic review aiming to summarize the prevalence of spondyloarthritis and its subtypes in the general population 66, 67. The frequency of undifferentiated spondyloarthritis ranges from 23% to 81% in early arthritis cohorts with most of them reporting a rate of 30% 62, 67; therefore, undifferentiated spondyloarthritis is a common diagnosis in daily rheumatology practice 68, 69, 70.
Undifferentiated spondyloarthritis is more common in females than males, and only 20 to 25 percent of people with undifferentiated spondyloarthritis are HLA-B27 positive.
Many people with undifferentiated spondyloarthritis have been told over the years that they are simply “anxious and depressed” or received a diagnosis of fibromyalgia, a chronic disorder associated with widespread muscle and soft tissue pain.
Most people with undifferentiated spondyloarthritis have one or more of the following symptoms:
- Inflammatory back pain
- Unilateral or alternating buttock pain
- Enthesitis – inflammation where a tendon or ligament attaches to the bone
- Peripheral arthritis – arthritis in the large joints of the limbs
- Arthritis of the small joints
- Swollen fingers or toes
- Heel pain
- Fatigue
- Iritis
Onset of the disease is often insidious and, even after years of inflammation, calcification of the sacroiliac joints (the joints where the spine meets the pelvis) is often absent or mild on routine X-rays.
Undifferentiated spondyloarthritis is reportedly the most difficult disease to diagnose in the spondylitis family and consequently eludes a correct diagnosis in many patients who have it. Undifferentiated spondyloarthritis is the least well known of the group and is still unrecognized by some physicians.
The therapies for undifferentiated spondyloarthritis mostly come from the experiences that have been gained in the treatment of other spondyloarthropathy, especially in ankylosing spondylitis and psoriatic arthritis. Nonsteroidal antiinflammatory drugs (NSAIDs), cyclooxygenase-2 inhibitors, and disease-modifying antirheumatic drugs (DMARDs) are the main therapeutic agents used in undifferentiated spondyloarthritis 71. Compared to ankylosing spondylitis, tumor necrosis factor-α (TNF-alpha) blockers were rarely recommended and adopted in patients with undifferentiated spondyloarthritis except the cases that exist axial involvement or have high risk of developing ankylosing spondylitis 72.
Undifferentiated spondyloarthritis symptoms
Pain may be the only complaint of people with undifferentiated spondyloarthritis. Most people with undifferentiated spondyloarthritis have one or more of the following symptoms:
- Inflammatory back pain
- Unilateral or alternating buttock pain
- Enthesitis – inflammation where a tendon or ligament attaches to the bone
- Peripheral arthritis – arthritis in the large joints of the limbs
- Arthritis of the small joints
- Swollen fingers or toes
- Heel pain
- Fatigue
- Iritis
Onset of the disease is often insidious and, even after years of inflammation, calcification of the sacroiliac joints (the joints where the spine meets the pelvis) is often absent or mild on routine X-rays.
Some people with undifferentiated spondyloarthritis later develop symptoms of the other forms of spondylitis, such as ankylosing spondylitis or psoriatic arthritis, while others will continue to have chronic, but generally not severe symptoms, and remain “undifferentiated.”
Undifferentiated spondyloarthritis diagnosis
Undifferentiated spondyloarthritis is reportedly the most difficult disease to diagnose in the seronegative spondyloarthropathy family and consequently eludes a correct diagnosis in many patients who have it 73, 74, 75. Undifferentiated spondyloarthritis is the least well known of the seronegative spondyloarthropathy group and is still unrecognized by some physicians. Sometimes a doctor may make an initial diagnosis of “spondyloarthritis” or “unclassified seronegative spondyloarthritis” if certain symptoms are present, but are not enough to make a specific diagnosis.
A person with undifferentiated spondyloarthritis may have signs and symptoms of spondylitis that do not quite fit into a specific category. For example, someone may have (or have experienced) iritis and heel pain, as well as being HLA-B27 positive, but initially not have the SI joint or spinal involvement associated with ankylosing spondylitis.
A diagnosis of undifferentiated spondyloarthritis normally comes after a physical exam, laboratory tests, and X-rays.
The differences in diagnostic criteria between ankylosing spondylitis and undifferentiated spondyloarthritis include:
- HLA-B27 Gene Test. The diagnostically helpful HLA-B27 gene seen in the majority of people with ankylosing spondylitis, is commonly absent in undifferentiated spondyloarthritis. Approximately 20-25 percent of people with undifferentiated spondyloarthritis test positive for HLA-B27.
- Only 50% of presentations have abnormal levels of acute-phase reactants (CRP, ESR), and only 10% are positive for rheumatoid factor (RF) despite many eventually declaring themselves as rheumatoid arthritis 10.
- X-rays. While X-ray of the pelvic region where the sacroiliac joint is located is essential for diagnosis in ankylosing spondylitis, subtle erosions do not always show in X-rays of those with undifferentiated spondyloarthritis.
- Inflammatory spinal pain. Symptoms of inflammatory spinal pain are the first clues of ankylosing spondylitis, which is not necessarily the case in people with undifferentiated spondyloarthritis. However, oftentimes women with any form of spondylitis feel primary pain in a different area of the body than the spine or lower back. Subsequently, a misdiagnosis of fibromyalgia is too often made in women with undifferentiated spondyloarthritis, who present with diffuse muscle aches and pains, without any measurable lab or X-ray abnormalities.
In clinical practice, rheumatologists identify undifferentiated spondyloarthritis patients based on expert opinion and experience, instead of routinely checking whether classification criteria (which were not designed as diagnostic criteria) are fulfilled 76. Hence, undifferentiated spondyloarthritis can be defined in two ways: formally as not fulfilling classification criteria for rheumatoid arthritis and pragmatically by the expertise of rheumatologists, whereby in both settings no clear alternative diagnosis should be present. For both undifferentiated spondyloarthritis populations (criteria based, expert opinion) there is little evidence on how to detect patients that will progress to rheumatoid arthritis. Likewise the value of MRI, in addition to other clinical variables, is unknown.
Undifferentiated spondyloarthritis treatment
Nonsteroidal antiinflammatory drugs (NSAIDs), cyclooxygenase-2 inhibitors, and disease-modifying antirheumatic drugs (DMARDs) are the main therapeutic agents used in undifferentiated spondyloarthritis 71. Compared to ankylosing spondylitis, tumor necrosis factor-α (TNF-alpha) blockers were rarely recommended and adopted in patients with undifferentiated spondyloarthritis except the cases that exist axial involvement or have high risk of developing ankylosing spondylitis 72.
Undifferentiated spondyloarthritis prognosis
Patients with undifferentiated spondyloarthritis generally have a good prognosis, but over time, some may develop ankylosing spondylitis or a related disease. Some experts believe that those who test positive for the HLA-B27 genetic marker are more likely to develop ankylosing spondylitis after initially being diagnosed with undifferentiated spondyloarthritis.
A minority of people with undifferentiated spondyloarthritis will have mild and intermittent symptoms requiring only symptomatic therapy, but many will have chronic, though not severe, symptoms requiring regular treatment and medication.
Juvenile spondyloarthritis
Juvenile spondyloarthritis also known as juvenile spondyloarthropathy, is the medical term for a group of childhood rheumatic diseases, which cause arthritis before the age of 16 and may span through adult life 77. Juvenile spondyloarthritis include enthesitis-related arthritis, undifferentiated spondyloarthritis, juvenile ankylosing spondylitis, psoriatic arthritis, reactive arthritis and arthritis associated with inflammatory bowel disease (enteropathic arthritis). Juvenile spondyloarthritis accounts for 15–20% of the chronic arthritis diseases in children in North America and Europe 78.
Juvenile spondyloarthritis typically causes pain and inflammation in the joints in the lower part of the body, for example, the pelvis, hips, knees and ankles. Other areas of the body can also be affected, such as the spine, eyes, skin, and bowels. Fatigue and lethargy can also occur.
In juvenile spondyloarthritis, sometimes the symptoms are episodic and unpredictable, seeming to come and go without an obvious cause over a long period of time. This cycle of disease flare up followed by remission may be repeated many times.
It is important to note that the disease progression and the severity of symptoms vary in each person. Some children may experience a mild, short-term disease, whereas others experience a severe, long-term condition.
The diagnosis of juvenile spondyloarthritis can be difficult because the symptoms are sometimes episodic and unpredictable. However, it is important to get a correct diagnosis as soon as possible in order to begin treatment. This is the role of a pediatric rheumatologist –– a physician with special training in diagnosing and treating arthritis in children. It is important to note that even in the care of the most experienced rheumatologist, a diagnosis can involve extensive testing and time.
Juvenile spondyloarthritis symptoms
The more common symptoms of juvenile spondyloarthritis are arthritic pain, especially around the heels or toes, around the knee, and in the lower back. Frequently, the first symptom is pain at the site where ligaments and tendons attach to bone (enthesitis). Months or years later, other joints may be affected, particularly joints of the spine or sacroiliac (SI) joints – the joints at the base of the spine, where the spine meets the pelvis.
At the beginning of the disease, children often have inflamed, swollen joints like the knees and ankles, but in adults, the spine is more likely to be involved. This inflammation can cause permanent damage if left untreated.
Sometimes children with spondyloarthritis develop other symptoms as well. These include fever, psoriasis (a scaly skin rash), colitis or Crohn’s disease (inflammation of the intestines), and iritis (inflammation of the eye).
Juvenile spondyloarthritis diagnosis
The diagnosis of juvenile spondyloarthritis can be difficult because the symptoms are sometimes episodic and unpredictable. In order to make the diagnosis, the pediatric rheumatologist will commonly do a physical exam and evaluate your child’s history of symptoms as well as perform laboratory tests. X-rays are not always useful in the diagnosis of juvenile spondyloarthritis in that the changes due to spondylitis normally seen in adults are rarely present in children. Moreover, the X-rays can be difficult to interpret in teenagers since the bones are growing along the joints.
A complete physical exam is commonly performed including evaluating for arthritis; asking careful questions about, and examining for, inflammation/pain –– specifically enthesitis –– inflammation of the area where a ligament or tendon attaches to the bone. The pediatric rheumatologist may also test spinal mobility and tenderness in the sacroiliac (SI) joints (the joints at the base of the spine, where the spine meets the pelvis), as well as check movement with breathing.
A clinical history of symptoms is also important. This can include checking the family history of ankylosing spondylitis and related diseases since heredity does play a factor.
Sometimes, children with juvenile spondyloarthritis may encounter other problems such as Crohn’s disease or ulcerative colitis (inflammation of the intestine), uveitis or iritis (inflammation of the eye), or psoriasis (a scaly rash that occurs most frequently on the elbows, knees, and scalp, but can cover much of the body).
Laboratory tests
It is important to note that there is no specific laboratory test for juvenile spondyloarthritis. Blood tests show that children with juvenile spondyloarthritis do not have rheumatoid factor (RF) or antinuclear antibodies (ANA) common in other types of chronic childhood arthritis. A positive test for the HLA-B27 genetic marker often correlates with the presence of juvenile spondyloarthritis in a child with arthritis. The presence of the HLA-B27 genetic marker in spondyloarthritis varies by race, so while the HLA-B27 test is important, it is not diagnostic for juvenile spondyloarthritis. Also note that HLA-B27 is a normal gene found in 8 percent of the Caucasian population. Generally speaking, no more than 2 percent of people born with this gene will eventually develop a form of spondyloarthritis.
Juvenile spondyloarthritis treatment
The American College of Rheumatology using the Research and Development /University of California at Los Angeles (RAND/UCLA) Appropriateness Method established recommendations for the treatment of juvenile idiopathic arthritis 79. The approach was to establish “treatment groups” rather than, for example, using categories established by the juvenile idiopathic arthritis classification system. The treatment groups included children with (1) <4 joints involved (cumulative total), (2) >5 joints involved, (3) the presence of active sacroiliac arthritis, (4) systemic arthritis with active systemic features (without active arthritis), and (5) systemic arthritis with active arthritis (without active systemic features). If one considers juvenile spondyloarthritis in the context of these treatment groups, most patients would be represented in groups 1, 2, or 3 since systemic features are rarely present 80. Most children with juvenile spondyloarthritis who have active axial involvement would be in group 3, whereas children with juvenile spondyloarthritis who have only peripheral disease would be in groups 1 or 2, provided they have arthritis and not just enthesitis 80.
Features of poor prognosis and disease activity were also taken into account. Focusing on the active sacroiliitis group, the indicator of poor prognosis is radiographic damage in any joint, defined as erosions or joint space narrowing. “Low” disease activity requires normal back flexion, normal ESR or C-reactive protein (CRP), a physician global of <4/10, and a patient/parent global of <2/10. “High” disease activity requires at least two of three features; an ESR or CRP greater than twice the upper limit of normal, a physician global of >7/10, and a patient/parent global of >4/10. Those who have one or more features greater than low activity, but fewer than 2 features of high activity would be considered to have “moderate” disease activity.
Applying the juvenile idiopathic arthritis treatment recommendations to children with active sacroiliitis, a TNF inhibitor is recommended if there is high disease activity and the poor prognostic feature, provided there has been an adequate trial of nonsteroidal anti-inflammatory drugs (NSAIDs) (1-2 months) 80. In the absence of the poor prognostic feature, children with high disease activity who have had 3 months of disease-modifying antirheumatic drugs (DMARDs) such as methotrexate or sulfasalazine would proceed to a tumor necrosis factor (TNF) inhibitor. A TNF inhibitor is also recommended for patients with moderate disease activity who have had 3 months of sulfasalazine or children with low disease activity with the poor prognostic feature, provided they have had at least 6 months of sulfasalazine treatment. A TNF inhibitor would also be recommended for juvenile spondyloarthritis patients who do not have active sacroiliitis but do have active peripheral arthritis (group 1 or 2), but only after 3 to 6 months of treatment with methotrexate in addition to 1 to 2 months of prior NSAID treatment and often glucocorticoid joint injections 80.
Regular exercise helps ease joint stiffness and pain. Low-impact and joint-friendly activities like walking, swimming, biking and yoga are best, but kids with well-controlled disease can participate in just about any activity they wish, if their doctor or physical therapist approves.
Physical and occupational therapy can improve a child’s quality of life by teaching them ways to stay active and how to perform daily tasks with ease. Children with juvenile spondyloarthritis may also have trouble with balance and weaker motor skills, or the ability to move and coordinate large muscle groups. Participating in regular physical and occupational therapy can improve coordination and balance, among other things. Here are some other ways physical and occupational therapists can help a child with juvenile spondyloarthritis:
- Teach and guide them through strengthening and flexibility exercises.
- Perform body manipulation.
- Prescribe assistive devices (e.g. braces, splints).
Spondyloarthropathy causes
The exact cause of spondyloarthropathy is currently unknown. However, there is clear evidence that your genes together with environmental factors trigger proinflammatory cytokines, and these lead to an increased susceptibility to spondyloarthropathies 81. There is a close link between ankylosing spondylitis, psoriasis, and Crohn disease. This link suggests that the pathogenesis of ankylosing spondylitis involves an immune reaction within the gut or the skin that may be influenced by microbial responses 82.
Anywhere from 80% to 95% of those who have axial spondyloarthropathy carry the HLA-B27 gene, but most people with the HLA-B27 gene never get arthritis. Although HLA-B27 gene may make you more susceptible to axial spondyloarthropathy and related diseases, it needs a trigger to set it off. Several possible triggers are being investigated, including mechanical stress on the back, though no clear link has been found so far. It’s also possible that the inciting factor is dysbiosis — an imbalance of the natural microbes in the gut. Dysbiosis at the root of many chronic inflammatory diseases, including inflammatory bowel disease and rheumatoid arthritis.
Ankylosing spondylitis is hereditary. Many genes can cause it. Up to 30 of these genes have been found. The major gene that is associated with ankylosing spondylitis is HLA-B27. Almost all white people with ankylosing spondylitis are carriers of HLA-B27.
Enteropathic arthritis is a form of chronic, inflammatory arthritis. The two most common types are ulcerative colitis and Crohn’s disease. The cause of enteropathic arthritis is unclear. It may be due to bacteria that enter the bowel when inflammation damages it. People with HLA-B27 are more likely to have this form of arthritis than those without the HLA-B27 gene.
Spondyloarthropathy signs and symptoms
The main symptom of spondyloarthropathy or spondyloarthritis is low back pain that lasts longer than 3 months. However, symptoms of spondyloarthritis can vary between patients and may include axial arthritis (which affects the lower back) or peripheral arthritis (which affects the wrists, ankles, knees, and elbows):
- Longstanding low back pain. Patients generally complain of inflammatory back pain in the spine or sacroiliac joints (which connect the spine, hips, and sacrum).
- Back stiffness
- Back pain and stiffness are typically worse at night and improve with exercise
- Fatigue
- Painful swelling of joints
- Sausage-like appearance of fingers or toes
- Heel pain
- Skin and nail changes of psoriasis
- Episodes of eye inflammation (uveitis)
Because there are many things that can cause low back pain, such as from injuries and excess weight to depression and smoking 83. For many people and doctors, spondyloarthritis is low on their list because axial spondyloarthropathy accounts for only 5% of all chronic back pain 84, 85. That’s one reason spondyloarthritis can take years to diagnose. Another reason is that it may be a decade or more before bone damage shows up on X-rays. To help distinguish spondyloarthritis-related pain from other causes, watch for:
- Pain and stiffness that start gradually and last at least three months.
- Pain that feels better when you move around, exercise and stretch, and worse when you rest; with a back injury, it’s usually the opposite.
- Morning stiffness that makes it hard to get out of bed but improves as the day goes on.
- The onset of back pain is usually before the age of 45 years 81.
Peripheral arthritis can affect small and large joints in an asymmetrical pattern. Patients with spondyloarthropathy may also present with:
- Enthesitis (tenderness at where the tendons and ligaments attach to the bone) and heel pain
- Tenosynovitis (inflammation and swelling around a tendon)
- Dactylitis (swelling of the entire digit; ie, the ‘sausage digit’)
- Extra-articular manifestations (symptoms not related to the joints; eg, skin rash of psoriasis, inflammatory bowel disease, and uveitis)
- A genetic association with the HLA-B27 gene 86, 19. HLA-B27 gene is one of the most important diagnostic tests in the Caucasian population due to high sensitivity (>80% for axial axial spondyloarthropathy, lower for peripheral axial spondyloarthropathy) and specificity (∼90% in the central European population based on the estimated background prevalence of HLA-B27 of about 9%) 87, 88. However, there are substantial geographic differences in both the background population prevalence of HLA-B27 and the strength of association with spondyloarthropathies that substantially affects the diagnostic value of the test across the globe 89.
Conditions that fall under the spondyloarthropathy umbrella are surprisingly varied and symptoms can overlap. In addition to back pain, you could have:
- Psoriasis, a skin disease marked by patches of itchy red skin.
- Eye inflammation called uveitis, which affects about 30% to 40% of people with spondyloarthropathy.
- Inflammation in your gastrointestinal tract that can cause abdominal pain, unintended weight loss, and severe diarrhea.
Spondyloarthropathy complications
Other problems can occur in patients with spondyloarthritis. Complications of axial spondyloarthritis include reduced bone mineral density, fractures, neurological manifestations, and kidney disease. Low bone mineral density is commonly seen within the first 10 years of the disease.
Spondyloarthritis possible complications can include:
- Osteoporosis, which occurs in up to half of patients with ankylosing spondylitis, especially in those whose spine is fused. Osteoporosis can raise the risk of spinal fracture. Spinal fractures can result in spinal cord injuries or spinal nerve compression.
- Inflammation of part of the eye called uveitis, which occurs in about 40% of those with spondyloarthritis. Symptoms of uveitis include redness and pain of the eye. Steroid eye drops most often are effective, though severe cases may need other treatments from an ophthalmologist (eye specialist).
- Inflammation of the aortic valve in the heart, which can occur over time in patients with spondylitis. Your doctor should check your heart to make sure you do not have this problem.
- Psoriasis, a patchy skin disease, which if severe will need treatment by a dermatologist (skin doctor).
- Intestinal inflammation, which may be so severe that it requires treatment by a gastroenterologist (doctor who specializes in digestive diseases).
Rarely, glomerulopathy, immunoglobulin A nephropathy, and renal amyloidosis occur in ankylosing spondylitis 90.
Spondyloarthropathy diagnosis
Spondyloarthropathies are diagnosed according to the Assessment of Spondyloarthritis International Society (ASAS) classification criteria 3. The Assessment of Spondyloarthritis International Society (ASAS) criteria only apply to patients aged under 40 years old or those with inflammatory back pain for over 3 months. The criteria include imaging, HLA-B27 status, and the features of spondyloarthropathy.
To diagnosed spondyloarthropathy, your rheumatologis typically will begin by going through your medical history and conducting a thorough physical exam. Tests and procedures that may be used for diagnosing spondyloarthritis include:
- Blood tests, to determine your HLA-B27 status and measure markers of inflammation (C-reactive protein [CRP], erythrocyte sedimentation rate [ESR]).
- Having the HLA-B27 gene doesn’t mean you’ll develop any of the conditions in the spondyloarthropathy family 13, 14, 15. The HLA-B27 gene alone doesn’t cause seronegative spondyloarthropathy and about 98% of people who carry it never have back pain. On the flip side, people of African descent are much less likely than others to carry HLA-B27 but can still develop inflammatory spondyloarthropathy 16.
- Imaging studies, to look for evidence of inflammation and rule out other potential causes of the patient’s symptoms. The specific type of imaging study (X-ray, ultrasound, MRI) will vary depending on the your symptoms. However, Imaging does not play a major role in differentiating between the spondyloarthropathy subtypes as imaging features are similar, especially in early disease. Exceptions to this rule are 17:
- undifferentiated spondyloarthropathy: no definite radiologic signs of sacroiliitis
- psoriatic arthritis: associated with parasyndesmophytes, a form of bony outgrowth distinct from syndesmophytes
- Also, spondylitis with bone marrow edema of the entire vertebra occurs more frequently in psoriatic arthritis.
It is important to note that as there are no specific diagnostic tests for most spondyloarthropathies (see Figure 1). For this reason, it is essential to correlate the findings in history and physical examination with laboratory, radiological, and sometimes histopathological findings to establish the diagnosis.
Imaging typically includes plain radiographs and magnetic resonance imaging (MRI). Sacroiliitis (inflammation of the lower spine) on plain radiography is specific for spondyloarthropathies. It may take several years before sacroiliitis is visible on imaging.
Laboratory evaluations should include a full blood count, a complete metabolic panel, and erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP). The ESR and CRP are elevated in about 50% of patients with spondyloarthropathy. Elevated CRP is a good predictor of radiographic progression and better response to tumour necrosis factor (TNF) inhibitors 81.
Currently, HLA-B27 and C-reactive protein (CRP) are the two most commonly used serum biomarkers, and MRI scans of the sacroiliac joints are the most sensitive imaging biomarker 91, 92. The National Health and Nutrition Examination Survey (NHANES) data indicate that 6.1% of the general US population is HLA-B27 positive 93, but a prevalence of 0.5% for ankylosing spondylitis would account for only 8% of HLA-B27–positive individuals 94. Traditional radiography is an inexpensive way to identify joint damage caused by sacroiliitis, and radiographic findings can inform decisions about the need for more advanced imaging 95. However, traditional radiography is associated with intra- and inter-reader variability and poor sensitivity in axial spondyloarthropathy 92, 96, , 95, 97, 98. Alternatively, the specificity of finding inflammation of sacroiliac joints on MRI is low; relying on MRI findings for diagnosis of axial spondyloarthropathy can result in overdiagnosis or misclassification of axial spondyloarthropathy 99, 100, 101, 102. Therefore, it is necessary for biomarkers to be interpreted in the context of other clinical features that suggest a diagnosis of axial spondyloarthropathy.
Spondyloarthropathy differential diagnosis
The differential diagnoses for spondyloarthropathies is based on the distribution of the affected joints.
Axial disease
Axial spondyloarthropathy differential diagnoses to consider include:
- Mechanical back pain — this is usually brief and with a history of a specific injury
- Fibromyalgia and myalgia — there is a lack of imaging changes and a minimal response to anti-inflammatory treatment
- Vertebral compression fracture — this occurs in patients with low bone mass due to osteoporosis or previous trauma.
- Osteitis condensans ilii — this is a radiological finding that occurs in women who have had more than one child and shows sclerosis on the iliac part of the sacroiliac joint
- Familial Mediterranean fever — these patients can develop back pain, peripheral arthritis, and sacroiliitis on imaging; they can be distinguished clinically through their family history of familial Mediterranean fever 90.
Peripheral joint disease
Peripheral spondyloarthropathy differential diagnoses to consider include:
- Rheumatoid arthritis — this is symmetrical and polyarticular arthritis with radiographic changes, that is positive for rheumatoid factor and anti-citrullinated peptide antibodies
- Osteoarthritis — where symptoms worsen with activity
- Behçet syndrome — an asymmetrical, non-erosive arthritis that is most often seen in the descendants of those who lived along the ancient silk road from eastern Asia to the Mediterranean
- Crystalline arthropathy (gout) — this is typically monoarticular and intensely inflammatory arthritis that affects the feet
- Sarcoidosis — an acute polyarthritis that usually involves the soft tissue around the joints (periarthritis) in conjunction with erythema nodosum and acute uveitis 90.
Spondyloarthropathy treatment
The goal of treatment for spondyloarthropathies is to improve function, decrease pain, and reduce the risk of complications. All patients should get physical or occupational therapy and do joint-directed exercises. Management should include lifestyle interventions, including an exercise program to maintain posture, strength, and range of movement. Patients should also be monitored for osteoporosis and encouraged to cease smoking.
Exercise is important for everyone with arthritis, but it’s a fundamental part of treatment if you have ankylosing spondylitis or nonradiographic axial spondyloarthritis (nr-axSpA). Exercise relieves pain better than medications and is the only way to keep your spine healthy and mobile. The American College of Rheumatology recommends that everyone with axial spondyloarthropathy get physical therapy and regularly perform exercises that “promote spine extension and mobility.” Swimming, in particular, is terrific for back health.
The first-line treatment for spine pain is nonsteroidal anti-inflammatory drugs (NSAIDs) like naproxen, ibuprofen and celecoxib. For many people with ankylosing spondylitis or nonradiographic axial spondyloarthritis (nr-axSpA), these drugs along with exercise are enough to control symptoms. They are quite effective, reducing pain, tenderness, and stiffness in 80% of patients.
If you don’t get relief from NSAIDs or have severe peripheral arthritis, you and your doctor might consider a tumor necrosis factor (TNF) blocker (anti-TNF-alpha therapy) like infliximab (Remicade), etanercept, adalimumab (Humira) or certolizumab (Cimzia). Certolizumab is the only anti-TNF-alpha therapy (TNF inhibitor) approved by the Food and Drug Administration (FDA) to treat nonradiographic axial spondyloarthritis (nr-axSpA), but doctors prescribe the others off-label based on research and clinical experience. These drugs can reduce the spinal and peripheral joint inflammation of ankylosing spondylitis and control extra-articular symptoms. Another option is an interleukin (IL)-17 blocker like secukinumab (Cosentyx) or ixekizumab (Taltz), both FDA-approved for nonradiographic axial spondyloarthritis (nr-axSpA). All of these medications, called biologics, are expensive and can have potentially serious side effects, so be sure you and your doctor weigh the pros and cons carefully.
TNF alpha blockers (TNF inhibitors)
- Infliximab (Remicade), which is given intravenously (by IV infusion) every 6-8 weeks at a dose of 5 mg/kg
- Etanercept (Enbrel), given by an injection of 50 mg under the skin once weekly
- Adalimumab (Humira), injected at a dose of 40 mg every other week under the skin
- Certolizumab (Cimzia), injected at a dose of 200 mg every other week or 400mg every 4 weeks under the skin
- Golimumab (Simponi), injected at a dose of 50 mg once a month under the skin
Interleukin (IL)-17 blocker
- Secukinumab (Cosentyx), injected at a dose of 150 mg every 4 weeks under the skin after a weekly loading dose for 5 weeks
However, biologic treatment may be expensive and not without side effects, including an increased risk for serious infections. Biologics can cause patients with latent tuberculosis (no symptoms) to develop an active infection. Therefore, you and your doctor should weigh the benefits and risks when considering treatment with biologics. Those with arthritis in the knees, ankles, elbows, wrists, hands and feet should try DMARD therapy before biologic treatment.
Other medications for severe peripheral arthritis that you and your doctor might consider are called disease-modifying anti-rheumatic drugs (DMARDs), which include sulfasalazine (Azulfidine) and methotrexate might be effective. These drugs relieve symptoms and may prevent damage to the joints. This class of drugs is helpful mainly in those with arthritis that also affects the joints of your arms and legs.
Injections, or shots, of corticosteroid medications into joints or tendon sheaths (the membrane around a tendon) have a modest benefit for localized (not widespread) arthritis 18.
The treatment for psoriatic arthritis targets the joints and skin lesions. Anti-TNF-alpha therapy (TNF inhibitor) for psoriatic arthritis has been a significant advance, resulting in a significant improvement in symptoms and a delay in progression of the disease. Secukinumab, an anti-interleukin-17A monoclonal antibody, is effective at treating psoriasis and psoriatic arthritis.
The management of reactive arthritis involves assessing and treating an active infection. Chlamydia infections should be treated with antibiotics. There is no evidence to support the use of antibiotics for treatment of urogenital or enteric forms of reactive arthritis. High-dose NSAIDs are also used to treat patients with reactive arthritis. Indomethacin 50–75 mg twice daily is commonly used. Persistent reactive arthritis may respond to disease-modifying anti-rheumatic drugs (DMARDs) such as sulfasalazine, azathioprine, or methotrexate 18.
Enteropathic arthritis is also treated with NSAIDs and cyclooxygenase-2 inhibitors, but these can cause flare-ups of inflammatory bowel disease. DMARDs are beneficial for gastrointestinal and joint involvement. Anti-TNF agents can be used if traditional DMARDs fail; however, there have been rare cases of inflammatory bowel diseases precipitated by etanercept. Total colectomy (removal of the affected colon) does not improve axial involvement in inflammatory bowel disease 19.
Surgical treatment is helpful in some patients. Total hip replacement is very useful for those with hip pain and disability due to joint destruction from cartilage loss. Spinal surgery is rarely necessary, except for those with traumatic fractures (broken bones due to injury) or to correct excess flexion deformities of the neck, where the patient cannot straighten the neck.
Spondyloarthropathy prognosis
The progression of ankylosing spondylitis is highly variable with the younger age of onset in ankylosing spondylitis patients being associated with poorer function outcomes but severe physical disability is uncommon 7. Most patients remain fully functional and able to work. Ankylosing spondylitis may result in the fusion of sacroiliac joints and the vertebral column, also known as a ‘bamboo spine’. Patients who have frequent episodes of iritis, hip involvement at presentation, peripheral joint involvement, and high inflammatory markers at baseline tend to have a poorer prognosis 103. Patients with the severe, long-standing ankylosing spondylitis have greater mortality compared with the general population, predominantly due to cardiovascular complications. Ankylosing spondylitis complications include 7:
- Chronic pain and disability
- Aortic regurgitation
- Pulmonary fibrosis
- Cauda equina syndrome
- Mood disorders
Psoriatic arthritis is an aggressive and debilitating disease with the potential for significant morbidity and poor quality of life in patients 104. Psoriatic arthritis requires targeted treatment with frequent monitoring and follow-up care. Complete symptomatic relief is achievable, but a significant majority of patients continue to have persistent inflammatory disease 105. Patients with uveitis will require evaluation and treatment by an ophthalmologist. Patients with psoriatic arthritis have an increased prevalence of comorbidities, including metabolic syndrome; which is a combination of obesity, diabetes mellitus, hyperlipidemia, hypertension, and cardiovascular disease 106. At least 20% of patients with psoriatic arthritis will develop severe and disabling disease. Some features are harbingers of a severe disease course and poor prognosis. These include a large number of actively inflamed joints or polyarticular presentation, elevated ESR, clinical or radiographic damage, loss of function, and diminished quality of life 107. Up to 7% of these patients may require joint surgery, with the most common being hip arthroplasty 108.
Approximately 50% of patients with reactive arthritis can expect symptoms to resolve in the first 6 months; however, 30–50% will develop chronic arthritis 109.
The prognosis of enteropathic arthritis is determined by the severity of the underlying inflammatory bowel disease. Patients with well-controlled inflammatory bowel disease rarely develop severe enteropathic arthritis 19.
Living with spondyloarthropathy
Pain, fatigue and stiffness can be continuous or off and on. Despite these symptoms, most patients with spondyloarthritis lead productive lives and have a normal lifespan, especially with the newer treatments available 110. There are things you can do to improve your health. Frequent exercise is essential to maintain joint and heart health. If you smoke, try to quit. Smoking aggravates spondyloarthritis and can speed up the rate of spinal fusion.
References- Garg N, van den Bosch F, Deodhar A. The concept of spondyloarthritis: where are we now? Best Pract Res Clin Rheumatol. 2014 Oct;28(5):663-72. doi: 10.1016/j.berh.2014.10.007
- Danve A, Deodhar A. Axial spondyloarthritis in the USA: diagnostic challenges and missed opportunities. Clin Rheumatol. 2019 Mar;38(3):625-634. doi: 10.1007/s10067-018-4397-3
- Sieper J, Rudwaleit M, Baraliakos X, Brandt J, Braun J, Burgos-Vargas R, Dougados M, Hermann KG, Landewé R, Maksymowych W, van der Heijde D. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis. 2009 Jun;68 Suppl 2:ii1-44. doi: 10.1136/ard.2008.104018
- Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, Braun J, Chou CT, Collantes-Estevez E, Dougados M, Huang F, Gu J, Khan MA, Kirazli Y, Maksymowych WP, Mielants H, Sørensen IJ, Ozgocmen S, Roussou E, Valle-Oñate R, Weber U, Wei J, Sieper J. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009 Jun;68(6):777-83. doi: 10.1136/ard.2009.108233
- Smolen JS, Schöls M, Braun J, et al. Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann Rheum Dis. 2018 Jan;77(1):3-17. doi: 10.1136/annrheumdis-2017-211734. Erratum in: Ann Rheum Dis. 2018 Mar;77(3):472.
- Rudwaleit M, van der Heijde D, Landewé R, Akkoc N, Brandt J, Chou CT, Dougados M, Huang F, Gu J, Kirazli Y, Van den Bosch F, Olivieri I, Roussou E, Scarpato S, Sørensen IJ, Valle-Oñate R, Weber U, Wei J, Sieper J. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. 2011 Jan;70(1):25-31. doi: 10.1136/ard.2010.133645
- Wenker KJ, Quint JM. Ankylosing Spondylitis. [Updated 2022 Apr 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470173
- Buchanan BK, Varacallo M. Sacroiliitis. [Updated 2022 Sep 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK448141
- Verpoort KN, van Dongen H, Allaart CF, Toes RE, Breedveld FC, Huizinga TW. Undifferentiated arthritis–disease course assessed in several inception cohorts. Clin Exp Rheumatol. 2004 Sep-Oct;22(5 Suppl 35):S12-7.
- Krabben A, Huizinga TW, van der Helm-van Mil AH. Undifferentiated arthritis characteristics and outcomes when applying the 2010 and 1987 criteria for rheumatoid arthritis. Ann Rheum Dis. 2012 Feb;71(2):238-41. doi: 10.1136/annrheumdis-2011-200205
- Thabet MM, Huizinga TW, van der Heijde DM, van der Helm-van Mil AH. The prognostic value of baseline erosions in undifferentiated arthritis. Arthritis Res Ther. 2009;11(5):R155. doi: 10.1186/ar2832
- Hermann KG, Althoff CE, Schneider U, Zühlsdorf S, Lembcke A, Hamm B, Bollow M. Spinal changes in patients with spondyloarthritis: comparison of MR imaging and radiographic appearances. Radiographics. 2005 May-Jun;25(3):559-69; discussion 569-70. doi: 10.1148/rg.253045117
- Parameswaran P, Lucke M. HLA B27 Syndromes. [Updated 2023 Mar 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK551523
- Allen RL, Bowness P, McMichael A. The role of HLA-B27 in spondyloarthritis. Immunogenetics. 1999 Nov;50(3-4):220-7. doi: 10.1007/s002510050596
- Bowness P. HLA-B27. Annu Rev Immunol. 2015;33:29-48. doi: 10.1146/annurev-immunol-032414-112110
- The Spondyloarthritis Family. https://www.arthritis.org/diseases/spondyloarthritis
- Weerakkody Y, Fahrenhorst-Jones T, Khan M, et al. Seronegative spondyloarthritis. Reference article, Radiopaedia.org https://doi.org/10.53347/rID-9160
- Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL Loscalzo J (eds). Harrison’s principles of Internal Medicine. 20th edn. New York: McGraw-Hill Education; 2018.
- Duba AS, Mathew SD. The Seronegative Spondyloarthropathies. Prim Care. 2018 Jun;45(2):271-287. doi: 10.1016/j.pop.2018.02.005
- Danve, A., Deodhar, A. Axial spondyloarthritis in the USA: diagnostic challenges and missed opportunities. Clin Rheumatol 38, 625–634 (2019). https://doi.org/10.1007/s10067-018-4397-3
- Poddubnyy D. Classification vs diagnostic criteria: the challenge of diagnosing axial spondyloarthritis. Rheumatology (Oxford). 2020 Oct 1;59(Suppl4):iv6-iv17. doi: 10.1093/rheumatology/keaa250
- Tsoi C, Griffith JF, Lee RKL, Wong PCH, Tam LS. Imaging of sacroiliitis: Current status, limitations and pitfalls. Quant Imaging Med Surg. 2019 Feb;9(2):318-335. doi: 10.21037/qims.2018.11.10
- Rudwaleit M, Jurik AG, Hermann KG, Landewé R, van der Heijde D, Baraliakos X, Marzo-Ortega H, Ostergaard M, Braun J, Sieper J. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis. 2009 Oct;68(10):1520-7. doi: 10.1136/ard.2009.110767
- Lambert RG, Bakker PA, van der Heijde D, Weber U, Rudwaleit M, Hermann KG, Sieper J, Baraliakos X, Bennett A, Braun J, Burgos-Vargas R, Dougados M, Pedersen SJ, Jurik AG, Maksymowych WP, Marzo-Ortega H, Østergaard M, Poddubnyy D, Reijnierse M, van den Bosch F, van der Horst-Bruinsma I, Landewé R. Defining active sacroiliitis on MRI for classification of axial spondyloarthritis: update by the ASAS MRI working group. Ann Rheum Dis. 2016 Nov;75(11):1958-1963. doi: 10.1136/annrheumdis-2015-208642
- Akkoc N, Khan MA. ASAS classification criteria for axial spondyloarthritis: time to modify. Clin Rheumatol. 2016 Jun;35(6):1415-23. doi: 10.1007/s10067-016-3261-6
- Diekhoff T, Lambert R, Hermann KG. MRI in axial spondyloarthritis: understanding an ‘ASAS-positive MRI’ and the ASAS classification criteria. Skeletal Radiol. 2022 Sep;51(9):1721-1730. doi: 10.1007/s00256-022-04018-4
- Sepriano A, Rubio R, Ramiro S, Landewé R, van der Heijde D. Performance of the ASAS classification criteria for axial and peripheral spondyloarthritis: a systematic literature review and meta-analysis. Ann Rheum Dis. 2017 May;76(5):886-890. doi: 10.1136/annrheumdis-2016-210747
- Overview of Ankylosing Spondylitis. https://spondylitis.org/about-spondylitis/overview-of-spondyloarthritis/ankylosing-spondylitis
- Gaillard F, Niknejad M, Fahrenhorst-Jones T, et al. Bamboo spine (ankylosing spondylitis). Reference article, Radiopaedia.org https://doi.org/10.53347/rID-962
- Saleh S, Vadera S, Feger J, et al. Andersson lesion. Reference article, Radiopaedia.org https://doi.org/10.53347/rID-14405
- Reinders A, van Wyk M. Bamboo spine – X-ray findings of ankylosing spondylitis revisited. (2012) South African Journal of Radiology. 16 (3): 111. doi:10.4102/sajr.v16i3.294
- Overview of Non-Radiographic Axial Spondyloarthritis (nr-axSpA). https://spondylitis.org/about-spondylitis/overview-of-spondyloarthritis/non-radiographic-axial-spondyloarthritis-nr-axspa
- Symptoms of Non-Radiographic Axial Spondyloarthritis. https://spondylitis.org/about-spondylitis/overview-of-spondyloarthritis/non-radiographic-axial-spondyloarthritis-nr-axspa/symptoms
- Ward, M.M., Deodhar, A., Gensler, L.S., et al. 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis. Arthritis Rheumatol, 71: 1599-1613. https://doi.org/10.1002/art.41042
- Liang H, Tian X, Liu XL, Wang SY, Dai Y, Kang L, Chen LS, Jin LF. The comparative efficacy of group- versus home-based exercise programs in patients with ankylosing spondylitis: Protocol for a meta-analysis. Medicine (Baltimore). 2018 Jul;97(29):e11540. doi: 10.1097/MD.0000000000011540
- Kivitz AJ, Wagner U, Dokoupilova E, Supronik J, Martin R, Talloczy Z, Richards HB, Porter B. Efficacy and Safety of Secukinumab 150 mg with and Without Loading Regimen in Ankylosing Spondylitis: 104-week Results from MEASURE 4 Study. Rheumatol Ther. 2018 Dec;5(2):447-462. doi: 10.1007/s40744-018-0123-5
- Proft F, Poddubnyy D. Ankylosing spondylitis and axial spondyloarthritis: recent insights and impact of new classification criteria. Ther Adv Musculoskelet Dis. 2018 Jun;10(5-6):129-139. doi: 10.1177/1759720X18773726
- Finch W. Arthritis and the gut. Postgrad Med. 1989 Aug;86(2):229-30, 233-4. doi: 10.1080/00325481.1989.11704368
- Enteropathic Arthropathies. https://emedicine.medscape.com/article/334746-overview#a6
- Vitulano C, Tedeschi V, Paladini F, Sorrentino R, Fiorillo MT. The interplay between HLA-B27 and ERAP1/ERAP2 aminopeptidases: from anti-viral protection to spondyloarthritis. Clin Exp Immunol. 2017 Dec;190(3):281-290. doi: 10.1111/cei.13020
- Generali E, Bose T, Selmi C, Voncken JW, Damoiseaux JGMC. Nature versus nurture in the spectrum of rheumatic diseases: Classification of spondyloarthritis as autoimmune or autoinflammatory. Autoimmun Rev. 2018 Sep;17(9):935-941. doi: 10.1016/j.autrev.2018.04.002
- Bourikas LA, Papadakis KA. Musculoskeletal manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2009 Dec;15(12):1915-24. doi: 10.1002/ibd.2094
- Orchard TR, Wordsworth BP, Jewell DP. Peripheral arthropathies in inflammatory bowel disease: their articular distribution and natural history. Gut. 1998 Mar;42(3):387-91. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1727027/pdf/v042p00387.pdf
- Enteropathic Arthropathies Treatment & Management. https://emedicine.medscape.com/article/334746-treatment
- Takeuchi K, Smale S, Premchand P, Maiden L, Sherwood R, Thjodleifsson B, Bjornsson E, Bjarnason I. Prevalence and mechanism of nonsteroidal anti-inflammatory drug-induced clinical relapse in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006 Feb;4(2):196-202. doi: 10.1016/s1542-3565(05)00980-8
- Sandborn WJ, Stenson WF, Brynskov J, Lorenz RG, Steidle GM, Robbins JL, Kent JD, Bloom BJ. Safety of celecoxib in patients with ulcerative colitis in remission: a randomized, placebo-controlled, pilot study. Clin Gastroenterol Hepatol. 2006 Feb;4(2):203-11. doi: 10.1016/j.cgh.2005.12.002
- Mahadevan U, Loftus EV Jr, Tremaine WJ, Sandborn WJ. Safety of selective cyclooxygenase-2 inhibitors in inflammatory bowel disease. Am J Gastroenterol. 2002 Apr;97(4):910-4. doi: 10.1111/j.1572-0241.2002.05608.x
- Generini S, Giacomelli R, Fedi R, Fulminis A, Pignone A, Frieri G, Del Rosso A, Viscido A, Galletti B, Fazzi M, Tonelli F, Matucci-Cerinic M. Infliximab in spondyloarthropathy associated with Crohn’s disease: an open study on the efficacy of inducing and maintaining remission of musculoskeletal and gut manifestations. Ann Rheum Dis. 2004 Dec;63(12):1664-9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1754868/pdf/v063p01664.pdf
- Van Den Bosch F, Kruithof E, Baeten D, Herssens A, de Keyser F, Mielants H, Veys EM. Randomized double-blind comparison of chimeric monoclonal antibody to tumor necrosis factor alpha (infliximab) versus placebo in active spondylarthropathy. Arthritis Rheum. 2002 Mar;46(3):755-65. https://onlinelibrary.wiley.com/doi/epdf/10.1002/art.511
- Herfarth H, Obermeier F, Andus T, Rogler G, Nikolaus S, Kuehbacher T, Schreiber S. Improvement of arthritis and arthralgia after treatment with infliximab (Remicade) in a German prospective, open-label, multicenter trial in refractory Crohn’s disease. Am J Gastroenterol. 2002 Oct;97(10):2688-90. doi: 10.1111/j.1572-0241.2002.06064.x
- Kaufman I, Caspi D, Yeshurun D, Dotan I, Yaron M, Elkayam O. The effect of infliximab on extraintestinal manifestations of Crohn’s disease. Rheumatol Int. 2005 Aug;25(6):406-10. doi: 10.1007/s00296-004-0467-8
- Barrie A, Regueiro M. Biologic therapy in the management of extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2007 Nov;13(11):1424-9. doi: 10.1002/ibd.20196
- Luchetti MM, Benfaremo D, Ciccia F, Bolognini L, Ciferri M, Farinelli A, Rossini M, Mosca P, Triolo G, Gabrielli A. Adalimumab efficacy in enteropathic spondyloarthritis: A 12-mo observational multidisciplinary study. World J Gastroenterol. 2017 Oct 21;23(39):7139-7149. doi: 10.3748/wjg.v23.i39.7139
- Dougados M, van der Heijde D, Sieper J, Braun J, Citera G, Lenaerts J, van den Bosch F, Wei JC, Pedersen R, Bonin R, Jones H, Marshall L, Logeart I, Vlahos B, Bukowski JF, Maksymowych WP. Effects of Long-Term Etanercept Treatment on Clinical Outcomes and Objective Signs of Inflammation in Early Nonradiographic Axial Spondyloarthritis: 104-Week Results From a Randomized, Placebo-Controlled Study. Arthritis Care Res (Hoboken). 2017 Oct;69(10):1590-1598. https://onlinelibrary.wiley.com/doi/epdf/10.1002/acr.23276
- Fiehn C, Vay S. Induction of inflammatory bowel disease flares by golimumab: report of three patients with enteropathic spondylarthritis or ankylosing spondylitis and comorbid colitis. Arthritis Rheum. 2011 Nov;63(11):3640-1. https://onlinelibrary.wiley.com/doi/epdf/10.1002/art.30546
- Thomas Chateau, Stefanos Bonovas, Catherine Le Berre, Nicolas Mathieu, Silvio Danese, Laurent Peyrin-Biroulet, Vedolizumab Treatment in Extra-Intestinal Manifestations in Inflammatory Bowel Disease: A Systematic Review, Journal of Crohn’s and Colitis, Volume 13, Issue 12, December 2019, Pages 1569–1577, https://doi.org/10.1093/ecco-jcc/jjz095
- Højgaard P, Christensen R, Dreyer L, et al. Pain mechanisms and ultrasonic inflammatory activity as prognostic factors in patients with psoriatic arthritis: protocol for a prospective, exploratory cohort study. BMJ Open 2016; 6: e010650. doi: 10.1136/bmjopen-2015-010650
- Psoriatic arthritis. https://dermnetnz.org/topics/psoriatic-arthritis
- Overview of Psoriatic Arthritis. https://spondylitis.org/about-spondylitis/overview-of-spondyloarthritis/psoriatic-arthritis
- Reactive Arthritis. https://www.niams.nih.gov/health-topics/reactive-arthritis
- Overview of Undifferentiated Spondyloarthritis. https://spondylitis.org/about-spondylitis/overview-of-spondyloarthritis/undifferentiated-spondyloarthritis
- Olivieri I., Sarzi-Puttini P., Bugatti S., Atzeni F., d’Angelo S., Caporali R. Early treatment in early undifferentiated arthritis. Autoimmun. Rev. 2012;11:589–592. doi: 10.1016/j.autrev.2011.10.019
- Aletaha D., Smolen J.S. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA. 2018;320:1360–1372. doi: 10.1001/jama.2018.13103
- Visser K, Allaart CF, Huizinga TW. Use of methotrexate in undifferentiated arthritis. Clin Exp Rheumatol. 2010 Sep-Oct;28(5 Suppl 61):S117-21 https://www.clinexprheumatol.org/article.asp?a=4186
- van Aken J., van Dongen H., le Cessie S., Allaart C.F., Breedveld F.C., Huizinga T.W. Comparison of long term outcome of patients with rheumatoid arthritis presenting with undifferentiated arthritis or with rheumatoid arthritis: An observational cohort study. Ann. Rheum. Dis. 2006;65:20–25. doi: 10.1136/ard.2005.038471
- Stolwijk C, van Onna M, Boonen A, van Tubergen A. Global Prevalence of Spondyloarthritis: A Systematic Review and Meta-Regression Analysis. Arthritis Care Res (Hoboken). 2016 Sep;68(9):1320-31. https://onlinelibrary.wiley.com/doi/epdf/10.1002/acr.22831
- J. Zochling, J. Brandt, J. Braun, The current concept of spondyloarthritis with special emphasis on undifferentiated spondyloarthritis, Rheumatology, Volume 44, Issue 12, December 2005, Pages 1483–1491, https://doi.org/10.1093/rheumatology/kei047
- Machado P., Castrejon I., Katchamart W., et al. Multinational evidence-based recommendations on how to investigate and follow-up undifferentiated peripheral inflammatory arthritis: Integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E Initiative. Ann. Rheum. Dis. 2011;70:15–24. doi: 10.1136/ard.2010.130625
- Goldbach-Mansky R., Lee J., McCoy A., Hoxworth J., Yarboro C., Smolen J.S., Steiner G., Rosen A., Zhang C., Menard H.A., et al. Rheumatoid arthritis associated autoantibodies in patients with synovitis of recent onset. Arthritis Res. 2000;2:236–243. doi: 10.1186/ar93
- Glennås A, Kvien TK, Andrup O, Karstensen B, Munthe E. Recent onset arthritis in the elderly: a 5 year longitudinal observational study. J Rheumatol. 2000 Jan;27(1):101-8.
- Xia Q, Fan D, Yang X, Li X, Zhang X, Wang M, Xu S, Pan F. Progression rate of ankylosing spondylitis in patients with undifferentiated spondyloarthritis: A systematic review and meta-analysis. Medicine (Baltimore). 2017 Jan;96(4):e5960. doi: 10.1097/MD.0000000000005960
- Cruzat V, Cuchacovich R, Espinoza LR. Undifferentiated spondyloarthritis: recent clinical and therapeutic advances. Curr Rheumatol Rep. 2010 Oct;12(5):311-7. doi: 10.1007/s11926-010-0115-0
- Diagnosis of Undifferentiated Spondyloarthritis. https://spondylitis.org/about-spondylitis/overview-of-spondyloarthritis/undifferentiated-spondyloarthritis/diagnosis
- Combe B, Landewe R, Daien CI, et al. 2016 update of the EULAR recommendations for the management of early arthritis. Ann Rheum Dis. 2017 Jun;76(6):948-959. doi: 10.1136/annrheumdis-2016-210602
- olebatch AN, Edwards CJ, Østergaard M, van der Heijde D, Balint PV, D’Agostino MA, Forslind K, Grassi W, Haavardsholm EA, Haugeberg G, Jurik AG, Landewé RB, Naredo E, O’Connor PJ, Ostendorf B, Potocki K, Schmidt WA, Smolen JS, Sokolovic S, Watt I, Conaghan PG. EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann Rheum Dis. 2013 Jun;72(6):804-14. doi: 10.1136/annrheumdis-2012-203158
- den Hollander NK, Verstappen M, Sidhu N, van Mulligen E, Reijnierse M, van der Helm-van Mil AHM. Hand and foot MRI in contemporary undifferentiated arthritis: in which patients is MRI valuable to detect rheumatoid arthritis early? A large prospective study. Rheumatology (Oxford). 2022 Oct 6;61(10):3963-3973. doi: 10.1093/rheumatology/keac017
- Overview of Juvenile Spondyloarthritis. https://spondylitis.org/about-spondylitis/overview-of-spondyloarthritis/juvenile-spondyloarthritis
- Tse SML, Laxer RM. New advances in juvenile spondyloarthritis. Nat Rev Rheumatol. 2012;8:269–279. doi: 10.1038/nrrheum.2012.37
- Beukelman T, Patkar NM, Saag KG, Tolleson-Rinehart S, Cron RQ, DeWitt EM, Ilowite NT, Kimura Y, Laxer RM, Lovell DJ, Martini A, Rabinovich CE, Ruperto N. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res (Hoboken). 2011 Apr;63(4):465-82. doi: 10.1002/acr.20460
- Tse SM, Burgos-Vargas R, Colbert RA. Juvenile spondyloarthritis treatment recommendations. Am J Med Sci. 2012 May;343(5):367-70. doi: 10.1097/MAJ.0b013e3182514043
- Yu DT, van Tubergen A. Overview of the clinical manifestations and classification of spondyloarthritis. https://www.uptodate.com/contents/overview-of-the-clinical-manifestations-and-classification-of-spondyloarthritis
- Khan MA. Ankylosing spondylitis. Cary: Oxford University Press USA, 2009.
- Manchikanti L, Singh V, Datta S, Cohen SP, Hirsch JA (2009) Comprehensive review of epidemiology, scope, and impact of spinal pain. Pain Physician 12:E35–E70
- Underwood MR, Dawes P. Inflammatory back pain in primary care. Br J Rheumatol. 1995 Nov;34(11):1074-7. doi: 10.1093/rheumatology/34.11.1074
- Expert Panel on Musculoskeletal Imaging:; Bernard SA, Kransdorf MJ, Beaman FD, Adler RS, Amini B, Appel M, Arnold E, Cassidy RC, Greenspan BS, Lee KS, Tuite MJ, Walker EA, Ward RJ, Wessell DE, Weissman BN. ACR Appropriateness Criteria® Chronic Back Pain Suspected Sacroiliitis-Spondyloarthropathy. J Am Coll Radiol. 2017 May;14(5S):S62-S70. doi: 10.1016/j.jacr.2017.01.048
- Ehrenfeld M. Spondyloarthropathies. Best Pract Res Clin Rheumatol. 2012 Feb;26(1):135-45. doi: 10.1016/j.berh.2012.01.002
- Rudwaleit M, van der Heijde D, Khan MA, Braun J, Sieper J. How to diagnose axial spondyloarthritis early. Ann Rheum Dis. 2004 May;63(5):535-43. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1754994/pdf/v063p00535.pdf
- Braun, J., Bollow, M., Remlinger, G., Eggens, U., Rudwaleit, M., Distler, A. and Sieper, J. (1998), Prevalence of spondylarthropathies in HLA-B27 positive and negative blood donors. Arthritis & Rheumatism, 41: 58-67. https://doi.org/10.1002/1529-0131(199801)41:1<58::AID-ART8>3.0.CO;2-G
- Khan MA. HLA-B27 and its subtypes in world populations. Curr Opin Rheumatol. 1995 Jul;7(4):263-9. doi: 10.1097/00002281-199507000-00001
- Yu DT, van Tubergen A. Clinical manifestations of axial spondyloarthritis (ankylosing spondylitis and nonradiographic axial spondyloarthritis) in adults. https://www.uptodate.com/contents/clinical-manifestations-of-axial-spondyloarthritis-ankylosing-spondylitis-and-nonradiographic-axial-spondyloarthritis-in-adults
- Danve A, O’Dell J (2015) The ongoing quest for biomarkers in ankylosing spondylitis. Int J Rheum Dis 18:826–834. https://doi.org/10.1111/1756-185X.12779
- Diekhoff T, Hermann KG, Greese J, Schwenke C, Poddubnyy D, Hamm B, Sieper J (2017) Comparison of MRI with radiography for detecting structural lesions of the sacroiliac joint using CT as standard of reference: results from the SIMACT study. Ann Rheum Dis 76:1502–1508. https://doi.org/10.1136/annrheumdis-2016-210640
- Reveille JD, Hirsch R, Dillon CF, Carroll MD, Weisman MH (2012) The prevalence of HLA-B27 in the US: data from the US National Health and Nutrition Examination Survey, 2009. Arthritis Rheum 64:1407–1411. https://doi.org/10.1002/art.33503
- Reveille JD (2011) Epidemiology of spondyloarthritis in North America. Am J Med Sci 341:284–286. https://doi.org/10.1097/MAJ.0b013e31820f8c99
- Pialat JB, Di Marco L, Feydy A, Peyron C, Porta B, Himpens PH, Ltaief-Boudrigua A, Aubry S (2016) Sacroiliac joints imaging in axial spondyloarthritis. Diagn Interv Imaging 97:697–708. https://doi.org/10.1016/j.diii.2016.02.013
- Bernard SA, Kransdorf MJ, Beaman FD, Adler RS, Amini B, Appel M, Arnold E, Cassidy RC, Greenspan BS, Lee KS, Tuite MJ, Walker EA, Ward RJ, Wessell DE, Weissman BN (2017) ACR Appropriateness Criteria® chronic back pain suspected sacroiliitis-spondyloarthropathy. J Am Coll Radiol 14:S62–S70. https://doi.org/10.1016/j.jacr.2017.01.048
- Christiansen AA, Hendricks O, Kuettel D, Hørslev-Petersen K, Jurik AG, Nielsen S, Rufibach K, Loft AG, Pedersen SJ, Hermansen LT, Østergaard M, Arnbak B, Manniche C, Weber U (2017) Limited reliability of radiographic assessment of sacroiliac joints in patients with suspected early spondyloarthritis. J Rheumatol 44:70–77. https://doi.org/10.3899/jrheum.160079
- van Tubergen A, Heuft-Dorenbosch L, Schulpen G, Landewé R, Wijers R, van der Heijde D, van Engelshoven J, van der Linden S. Radiographic assessment of sacroiliitis by radiologists and rheumatologists: does training improve quality? Ann Rheum Dis. 2003 Jun;62(6):519-25. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1754576/pdf/v062p00519.pdf
- Deodhar A (2016) Sacroiliac joint magnetic resonance imaging in the diagnosis of axial spondyloarthritis: “a tiny bit of white on two consecutive slices” may be objective, but not specific. Arthritis Rheumatol 68:775–778. https://doi.org/10.1002/art.39549
- de Winter J, de Hooge M, van de Sande M, van Hoeven L, de Jong J, de Koning A, Berg IJ, Ramonda R, Baeten D, van der Heijde D, Weel A, Landewé RBM (2017) A positive MRI of the sacroiliac joints is not specific for axial spondyloarthritis but frequently occurs in healthy individuals [abstract]. Arthritis Rheumatol 69(Suppl 10):Abstract 1831
- Arnbak B, Grethe Jurik A, Horslev-Petersen K, Hendricks O, Hermansen LT, Loft AG, Østergaard M, Pedersen SJ, Zejden A, Egund N, Holst R, Manniche C, Jensen TS (2016) Associations between spondyloarthritis features and magnetic resonance imaging findings: a cross-sectional analysis of 1,020 patients with persistent low back pain. Arthritis Rheumatol 68:892–900. https://doi.org/10.1002/art.39551
- Dubreuil M, Deodhar AA (2017) Axial spondyloarthritis classification criteria: the debate continues. Curr Opin Rheumatol 29:317–322. https://doi.org/10.1097/BOR.0000000000000402
- Ankylosing spondylitis. https://bestpractice.bmj.com/topics/en-gb/366
- Tiwari V, Brent LH. Psoriatic Arthritis. [Updated 2023 Jan 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK547710
- Gladman DD, Hing EN, Schentag CT, Cook RJ. Remission in psoriatic arthritis. J Rheumatol. 2001 May;28(5):1045-8.
- Gupta S, Syrimi Z, Hughes DM, Zhao SS. Comorbidities in psoriatic arthritis: a systematic review and meta-analysis. Rheumatol Int. 2021 Feb;41(2):275-284. doi: 10.1007/s00296-020-04775-2
- Ritchlin CT, Kavanaugh A, Gladman DD, Mease PJ, Helliwell P, Boehncke WH, de Vlam K, Fiorentino D, Fitzgerald O, Gottlieb AB, McHugh NJ, Nash P, Qureshi AA, Soriano ER, Taylor WJ; Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA). Treatment recommendations for psoriatic arthritis. Ann Rheum Dis. 2009 Sep;68(9):1387-94. doi: 10.1136/ard.2008.094946
- Psoriatic arthritis. https://bestpractice.bmj.com/topics/en-us/524
- Reactive arthritis. https://bestpractice.bmj.com/topics/en-gb/597
- Spondyloarthritis. https://www.rheumatology.org/I-Am-A/Patient-Caregiver/Diseases-Conditions/Spondyloarthritis