Hypophyseal portal system
Hypophyseal portal system also known as hypothalamo-hypophyseal portal system, is the system of blood vessels that links the hypothalamus and the anterior pituitary (adenohypophysis). Hypophyseal portal system allows endocrine communication between the hypothalamus and the anterior pituitary gland. The anterior pituitary receives releasing and inhibitory hormones in the blood. Using these the anterior pituitary is able to fulfill its function of regulating the other endocrine glands.
The anterior pituitary (adenohypophysis) is derived from embryonic ectoderm 1. The anterior pituitary (adenohypophysis) secretes five endocrine hormones from five different types of epithelial endocrine cells. The release of anterior pituitary hormones is regulated by hypothalamic hormones (releasing or inhibitory), which are synthesized in the cell bodies of neurons located in several nuclei that surround the third ventricle. These include the arcuate, the paraventricular and ventromedial nuclei and the medial preoptic and paraventricular regions. In response to neural activity, the hypothalamic hormones are released from the nerve endings into the hypophyseal portal blood and are then carried down to the anterior pituitary 2.
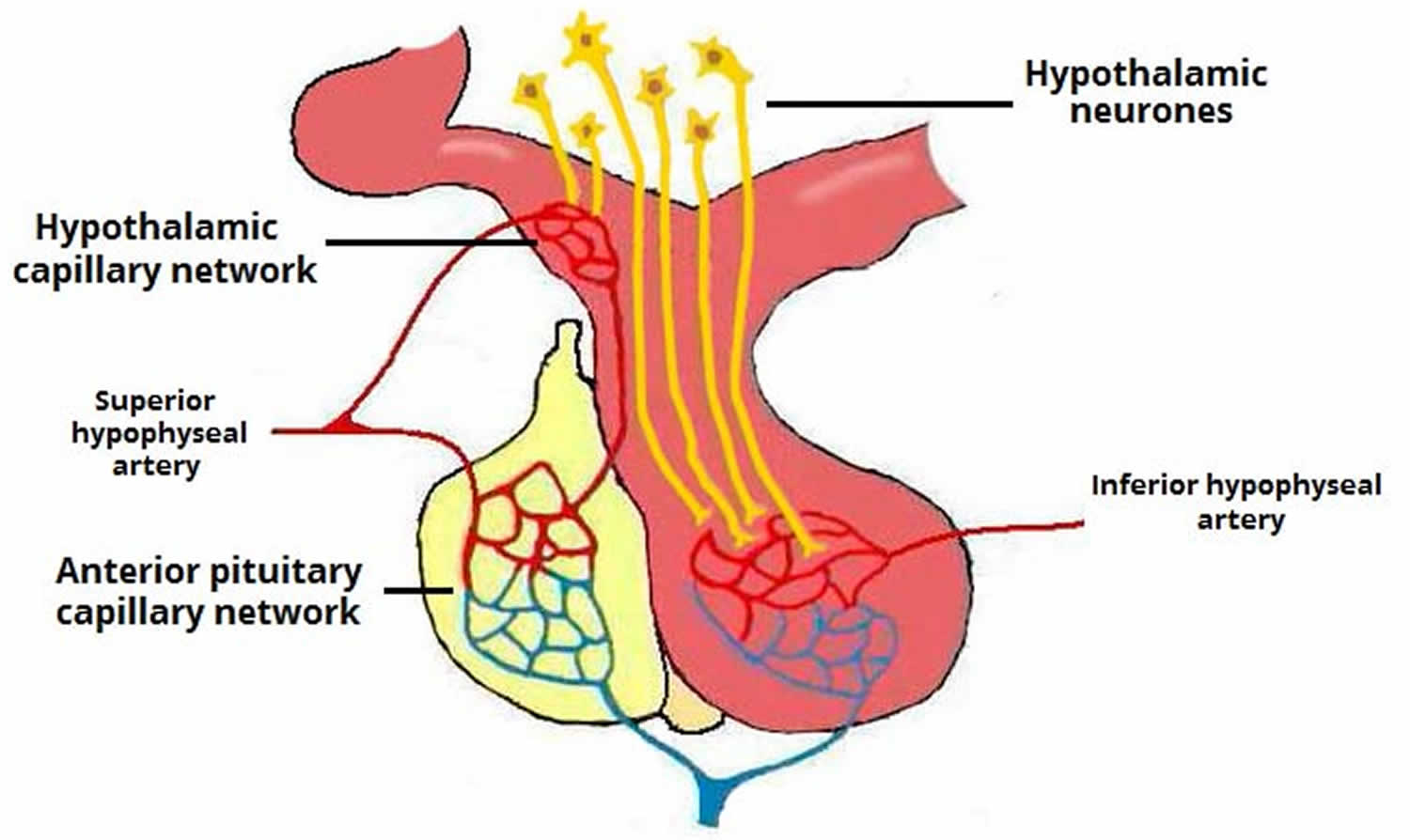
Hypothalamic hormones that release or inhibit anterior pituitary hormones reach the anterior pituitary through a hypothalamic hypophyseal portal system. Usually, blood passes from the heart through an artery to a capillary to a vein and back to the heart. In a portal system, blood flows from one capillary network into a portal vein and then into a second capillary network before returning to the heart. The name of the portal system indicates the location of the second capillary network. In the hypophyseal portal system, blood flows from capillaries in the hypothalamus into portal veins that carry blood to capillaries of the anterior pituitary (see Figure 1). In other words, the hormones carried by the hypothalamo-hypophyseal portal system allow communication between the hypothalamus and anterior pituitary and establish an important link between the nervous system and the endocrine system.
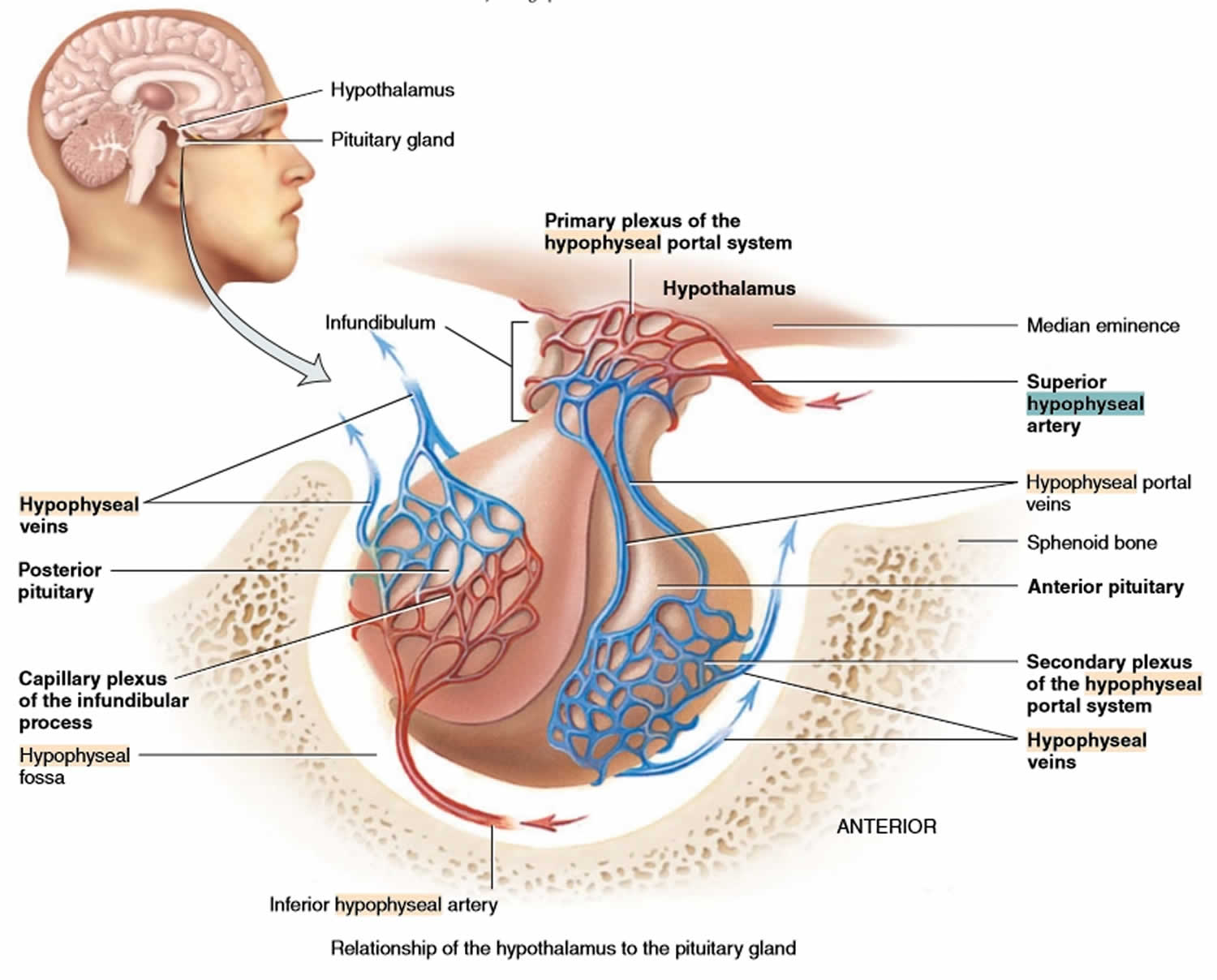
The superior hypophyseal arteries, branches from the medial aspect of the internal carotid artery just after leaving the cavernous sinus, bring blood into the hypothalamus. The superior hypophyseal arteries emerge 5-mm distal to the origin of the ophthalmic artery and then will go on to form the primary capillary network found in the median eminence. At the junction of the median eminence of the hypothalamus and the infundibulum, these arteries divide into a capillary network called the primary plexus of the hypophyseal portal system. This capillary plexus supplies blood to the pars distalis. From the primary plexus, blood drains into the anterior and posterior hypophyseal portal veins that pass down the outside of the infundibulum. It is via this system that peptides that are released at the median eminence enter the primary plexus. From that point, the peptides would be transmitted to the adenohypophysis via the hypophyseal portal veins to the secondary plexus. The portal system has fenestrated capillaries which would allow for exchange between the hypothalamus and the pituitary. The cells of the adenohypophysis express G-protein coupled receptors that bind to the peptides allowing the release of hormones from the anterior pituitary. In the anterior pituitary, the hypophyseal portal veins divide again and form another capillary network called the secondary plexus of the hypophyseal portal system. Hypophyseal veins drain blood from the anterior pituitary.
The primary and secondary capillary plexuses in the pituitary gland, plus the intervening hypophyseal portal veins, constitute the hypophyseal portal system.
Short veins from the pituitary gland drain into the neighboring dural venous sinuses.
Figure 1. Hypophyseal portal system
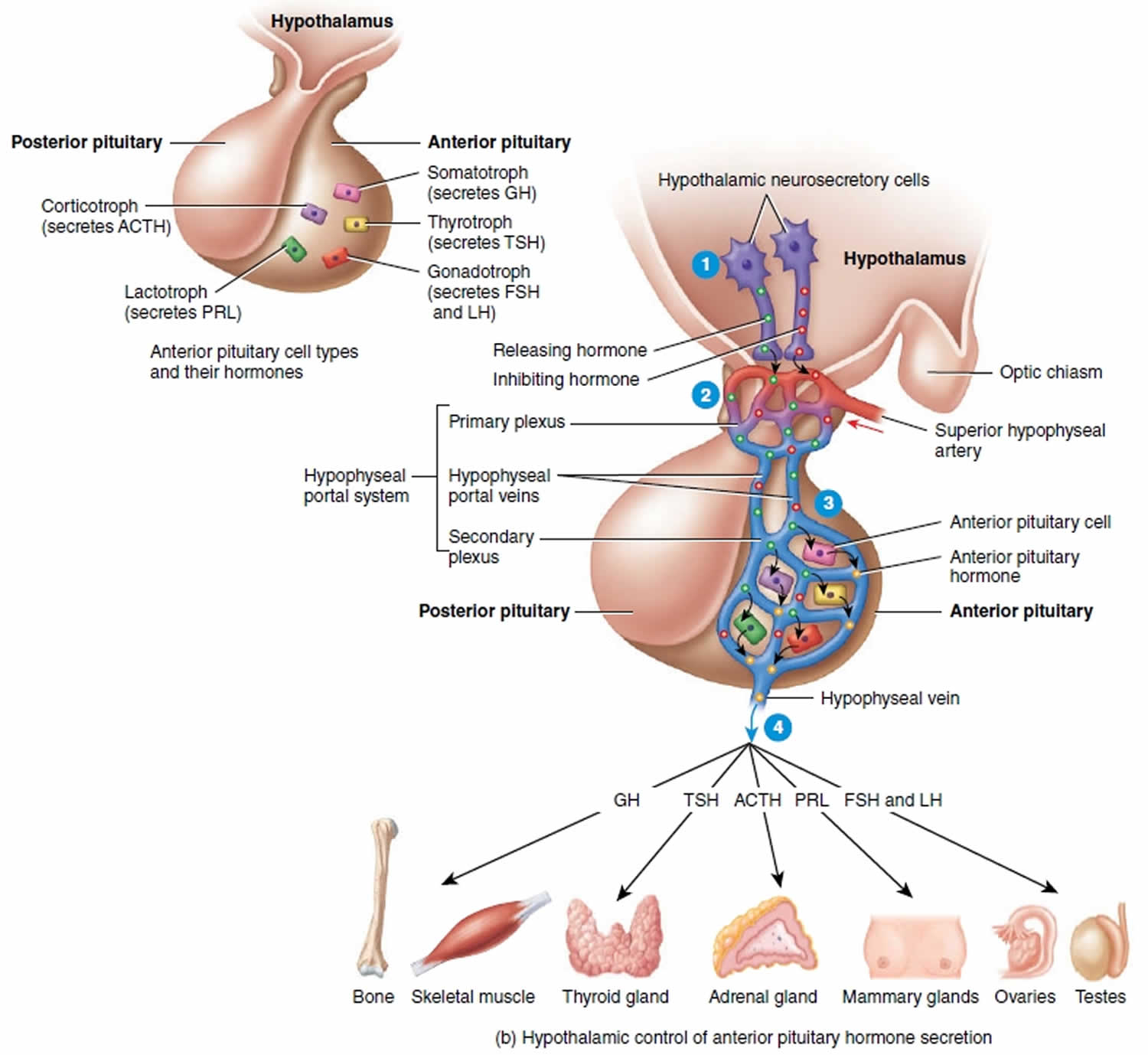
The release of hormones from the anterior pituitary gland is generally controlled by hormones from the hypothalamus. The hypothalamus exerts its control by secreting peptide hormones called releasing hormones (releasing factors), which then prompt the cells in the anterior lobe to release their hormones. The hypothalamus also secretes inhibiting hormones, which turn off the secretion of hormones by the anterior lobe when necessary. There are distinct releasing and inhibiting hormones for almost every anterior lobe hormone.
Releasing hormones made in hypothalamic neurons are secreted like neurotransmitters from the axon terminals of these neurons. In this case, neurons are serving as endocrine cells. The secreted releasing hormones enter a primary capillary plexus in the median eminence of the hypothalamus and then travel inferiorly in hypophyseal portal veins to a secondary capillary plexus in the anterior lobe. The releasing hormones leave the bloodstream and attach to the anterior lobe cells and stimulate these cells to secrete hormones (GH, LH, TSH, PRL, and ACTH). The hormones secreted from the anterior lobe cells enter the secondary plexus. From there the newly secreted anterior lobe hormones proceed into the general circulation and travel to their target organs throughout the body.
Control of anterior pituitary secretion regulation of anterior pituitary secretion by the hypothalamus occurs as follows:
- Above the optic chiasm are clusters of neurons called neurosecretory cells. They synthesize the hypothalamic releasing and inhibiting hormones in their cell bodies and package the hormones inside vesicles, which reach the axon terminals by fast axonal transport, where they are stored.
- When the neurosecretory cells of the hypothalamus are excited, nerve impulses trigger exocytosis of the vesicles. The hypothalamic hormones then diffuse into the blood of the primary plexus of the hypophyseal portal system.
- Quickly, the hypothalamic hormones are transported by the blood through the hypophyseal portal veins and into the secondary plexus. This direct route permits hypothalamic hormones to act immediately on anterior pituitary cells, before the hormones are diluted or destroyed in the general circulation. Within the secondary plexus the hypothalamic hormones diffuse out of the bloodstream and interact with anterior pituitary cells. When stimulated by the appropriate hypothalamic-releasing hormones, the anterior pituitary cells secrete hormones into the secondary plexus capillaries.
- From the secondary plexus capillaries, the anterior pituitary hormones drain into the hypophyseal veins and out into the general circulation. Anterior pituitary hormones then travel to target tissues throughout the body. Those anterior pituitary hormones that act on other endocrine glands are called tropic
hormones or tropins.
Release of anterior pituitary hormones is regulated not only by the hypothalamus but also by negative feedback. The secretory activity of three types of anterior pituitary cells (thyrotrophs, corticotrophs, and gonadotrophs) decreases when blood levels of their target gland hormones rise. For example, adrenocorticotropic hormone (ACTH) stimulates the cortex of the adrenal gland to secrete glucocorticoids, mainly cortisol. In turn, an elevated blood level of cortisol decreases secretion of both ACTH (corticotropin) and corticotropin-releasing hormone (CRH) by suppressing the activity of the anterior pituitary corticotrophs and hypothalamic neurosecretory cells.
Anterior Pituitary Hormones
Growth hormone (GH)
- Other names: somatotropic hormone or somatotropin
- Precursor cells: somatotrophs in the anterior pituitary
- Target cells: almost all tissues of the body
- Transport: 60% circulates free and 40% bound to specific growth hormone-binding proteins (GHBPs)
Mechanism of action:
Growth hormone binds to growth hormone receptors (GHRs) causing dimerization of growth hormone receptor, activation of the growth hormone receptor-associated JAK2 tyrosine kinase, and tyrosyl phosphorylation of both JAK2 and growth hormone receptor. This causes recruitment and/or activation of a variety of signaling molecules, including Manterior pituitary kinases, insulin receptor substrates, phosphatidylinositol 3′ phosphate kinase, diacylglycerol, protein kinase C, intracellular calcium, and Stat transcription factors. These signaling molecules contribute to growth hormone-induced changes in enzymatic activity, transport function, and gene expression that ultimately culminate in changes to growth and metabolism.
Regulation of growth hormone secretion:
The release of growth hormone is under dual control by the hypothalamus. Growth hormone secretion is stimulated by growth hormone-releasing hormone (GHRH) but suppressed by another hormone peptide, somatostatin (also known as growth hormone-inhibiting hormone (GHIH)). Insulin-like growth factor-1 (IGF-1) provides negative feedback for inhibiting growth hormone release from somatotrophs. Thyroid hormones (T3 and T4) up-regulate growth hormone gene expression in somatotrophs.
Physiological Functions:
Growth hormone acts almost on every type of cell. Its principal targets are bones and skeletal muscles. It has direct metabolic effects on fats, proteins and carbohydrates and indirect actions that result in skeletal growth.
- Direct Metabolic Functions: growth hormone is anabolic. It stimulates the growth of almost all tissues of the body that are capable of growing (increase in the number of cells). growth hormone also increases the rate of protein synthesis in most cells of the body and decreases the rate of glucose utilization throughout the body (diabetogenic action). Also, it increases mobilization of fatty acids from adipose tissue and increases levels of free fatty acids in the blood.
- Indirect Actions on Skeletal Growth: growth hormone stimulates the production of insulin-like growth factor-1 (IGF-1) from hepatocytes. IGF-1 mediates the growth-promoting effects of growth hormone on the skeleton. IGF-1 exerts direct actions on both cartilage and bone to stimulate growth and differentiation. These effects are crucial for growth during childhood to the end of adolescence.
Prolactin
Precursor cells: mainly from lactotrophs in the anterior pituitary
Target cells: main target cells are mammary glands and gonads
Mechanism of action: binds to peptide hormone receptor (single transmembrane domain) to activate the JAK2-STAT intracellular signaling pathway similar to that of growth hormone
Regulation
Like growth hormone, dual hypothalamic inhibitory (from dopamine) and stimulatory hormones (prolactin-releasing hormone [PRH]) regulate prolactin secretion. The predominant hypothalamic influence is inhibitory.
Physiological functions: The main functions of prolactin are stimulating mammary gland growth and development (mammographic effect) and milk production (lactogenic effect). It also has effects on the hypothalamic-pituitary-gonadal axis and can inhibit pulsatile gonadotropin-releasing hormone (GnRH) secretion from the hypothalamus.
Follicle stimulating hormone (FSH) and luteinizing hormone (LH)
Precursor cells: gonadotrophs in the anterior pituitary
Target cells: gonads (ovaries and testes)
Mechanism of action
follicle stimulating hormone (FSH) and luteinizing hormone (LH) bind to G protein-coupled receptors to activate adenylyl cyclase enzyme, which in turn increases intracellular cAMP. cAMP activates protein kinase A (PKA) that phosphorylates intracellular proteins. These phosphorylated proteins then accomplish the final physiologic actions.
Regulation
Follicle stimulating hormone (FSH) and luteinizing hormone (LH) secretion are under the control of hypothalamic gonadotropin-releasing hormone (GnRH).
Physiological Functions: follicle stimulating hormone (FSH) and luteinizing hormone (LH) regulate the functions of the ovaries and the testes. In females, follicle stimulating hormone (FSH) stimulates growth and development of follicles in preparation for ovulation and secretion of estrogens by the mature Graafian follicle. luteinizing hormone (LH) triggers ovulation and stimulates the secretion of progesterone by the corpus luteum. In males, follicle stimulating hormone (FSH) is required for spermatogenesis, and luteinizing hormone (LH) stimulates testosterone secretion by Leydig cells.
Thyroid stimulating hormone (TSH)
Precursor cells: thyrotropes in the anterior pituitary
Target cells: thyroid follicular cells
Mechanism of action
TSH binds to the G-protein-coupled receptors on the basolateral membrane of the thyroid follicular cells. Similar to follicle stimulating hormone (FSH) and luteinizing hormone (LH), it activates the adenylyl cyclase-PKA-cAMP system to phosphorylate several proteins, which in turn achieve the final physiologic actions
Regulation
TSH secretion is under control of hypothalamic thyrotropin-releasing hormone (TRH). Also, T4 feeds back to the anterior pituitary to inhibit TSH secretion.
Physiological functions: the main function of TSH is to stimulate synthesis and secretion of thyroid hormones (tri-iodothyronine [T3] and thyroxine [T4]) from thyroid follicles. It also maintains the structural integrity of the thyroid glands.
Adrenocorticotrophic hormone (ACTH)
Precursor cells: corticotrophs in the anterior pituitary
Target cells: cells in the cortex of the adrenal glands (adrenocortical cells)
Mechanism of action
ACTH binds to its G-protein coupled receptors on the adrenocortical cells. Similar to TSH, follicle stimulating hormone (FSH), and luteinizing hormone (LH), it activates adenylyl cyclase-PKA-cAMP system to phosphorylate several proteins, which in turn achieve the final physiologic functions.
Regulation
ACTH secretion is under control of hypothalamic corticotropin-releasing hormone (CRH). It is subject to negative feedback regulation.
Physiological functions: the main function of ACTH is to stimulate secretion of adrenal cortex hormones (mainly glucocorticoids) during stress.
- El Sayed SA, Fahmy MW, Schwartz J. Physiology, Pituitary Gland. [Updated 2019 Jul 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459247[↩]
- Leonart LP, Ferreira VL, Tonin FS, Fernandez-Llimos F, Pontarolo R. Medical Treatments for Acromegaly: A Systematic Review and Network Meta-Analysis. Value Health. 2018 Jul;21(7):874-880.[↩]