Liddle syndrome
Liddle syndrome also called pseudohyperaldosteronism is a rare inherited form of resistant high blood pressure (resistant hypertension). Resistant hypertension is a condition in which blood pressure continues to be greater than 140/90 mm Hg despite compliance with a regimen of 3 antihypertensive agents including a diuretic 1. Liddle syndrome is characterized by resistant hypertension that begins unusually early in life, often in childhood, although some affected individuals are not diagnosed until adulthood. Some people with Liddle syndrome have no additional signs or symptoms, especially in childhood. Over time, however, untreated hypertension can lead to heart disease or stroke, which may be fatal.
In addition to hypertension, affected individuals can have low levels of potassium in the blood (hypokalemia). Signs and symptoms of hypokalemia include muscle weakness or pain, fatigue, constipation, or heart palpitations. The shortage of potassium can also raise the pH of the blood, a condition known as metabolic alkalosis.
Liddle syndrome is a rare condition, although its prevalence is unknown. Liddle syndrome has been found in populations worldwide. Lin-Ping Wang et al. 2 and Liu and his team 3 studied the prevalence of Liddle syndrome. According to them, it was 0.92% to 1.52% in the Chinese population. In these studies, the study population was a group of hypokalemic patients, but Liddle syndrome can be seen in patients with normal potassium levels. Therefore, if researchers studied the incidence of Lidle syndrome in normal potassium patients with hypertension, it would likely be much higher 4. There is no gender or race predisposition.
Researchers of one study of US veterans concluded that there was approximately a 6% prevalence of symptomatic Liddle syndrome 5.
Liddle syndrome is caused by mutations in one of 3 genes (SCNN1A, SCNN1B, and SCNN1G) that encode the epithelial sodium channel (ENaC) and is inherited in an autosomal dominant manner 6, 7, 8, 9, 10. Treatment may include a low sodium diet and potassium-sparing diuretics to reduce blood pressure and normalize potassium levels. The drug of choice is amiloride. It works well in Liddle syndrome as it directly inhibits epithelial sodium channel (ENaC). Conventional anti-hypertensive therapies are not effective 11.
Liddle syndrome causes
Liddle syndrome is caused by mutations in one of 3 genes (SCNN1A, SCNN1B, and SCNN1G) that encode the epithelial sodium channel (ENaC) resulting in higher-than-normal epithelial sodium channel (ENaC) activity that is independent of aldosterone activity 6, 7, 8, 12, 9, 10. Each of these genes (SCNN1A, SCNN1B, and SCNN1G) provides instructions for making a piece (subunit) of a protein complex called the epithelial sodium channel (ENaC). These epithelial sodium channels (ENaC) are found at the surface of certain cells called epithelial cells in many tissues of the body, including the kidneys, where the channels transport sodium into cells. Over-active epithelial sodium channel (ENaC) in the distal nephrons of the kidney leads to excessive sodium reabsorption and associated electrolyte imbalances. The excess sodium retention effects blood pressure control, resulting in hypertension that is resistant to most anti-hypertensive medications. Potassium secretion in the kidney is affected, and low concentration of serum blood potassium (hypokalemia) is present in most, but not all, patients.
In the kidney, epithelial sodium channels (ENaC) open in response to signals that sodium levels in the blood are too low, which allows sodium to flow into cells. From the kidney cells, this sodium is returned to the bloodstream a process called reabsorption rather than being removed from the body in urine.
Mutations in the SCNN1A, SCNN1B, and SCNN1G gene change the structure of the respective epithelial sodium channel (ENaC) subunit. The changes alter a region of the subunit that is involved in signaling for its breakdown (degradation) when it is no longer needed. As a result of the SCNN1A, SCNN1B, and SCNN1G gene mutations, the subunit proteins are not degraded, and more epithelial sodium channels remain at the cell surface. The increase in channels at the cell surface abnormally increases the reabsorption of sodium (followed by water), which leads to hypertension. Reabsorption of sodium into the blood is linked with removal of potassium from the blood, so excess sodium reabsorption leads to hypokalemia.
Epithelial sodium channel (ENaC) is present in the distal colon, ducts of exocrine glands, lungs, and apical surface of the epithelial surface of distal nephron 13. Patients with Liddle syndrome have an abnormality in the epithelial sodium channels in distal nephrons due to mutations in 1 of the 3 subunits. Due to this mutation, the degradation of the sodium channels has been impaired; therefore, the quantity of these channels on the apical surface of distal nephron increases inappropriately 14. The sodium feedback inhibition system is also impaired in patients with Liddle syndrome 15. Typically, increased intracellular sodium in distal nephron cells inhibit apical epithelial sodium channels, but in patients with Liddle syndrome, they become insensitive to sodium concentration. Increased sodium channels cause increased sodium retention which results in chronic volume retention and a hypertension state and suppressed renin and aldosterone levels 16. In this population, renal biopsy showed atrophy of juxtaglomerular cells due to chronically suppressed renin and aldosterone levels.
Liddle syndrome inheritance pattern
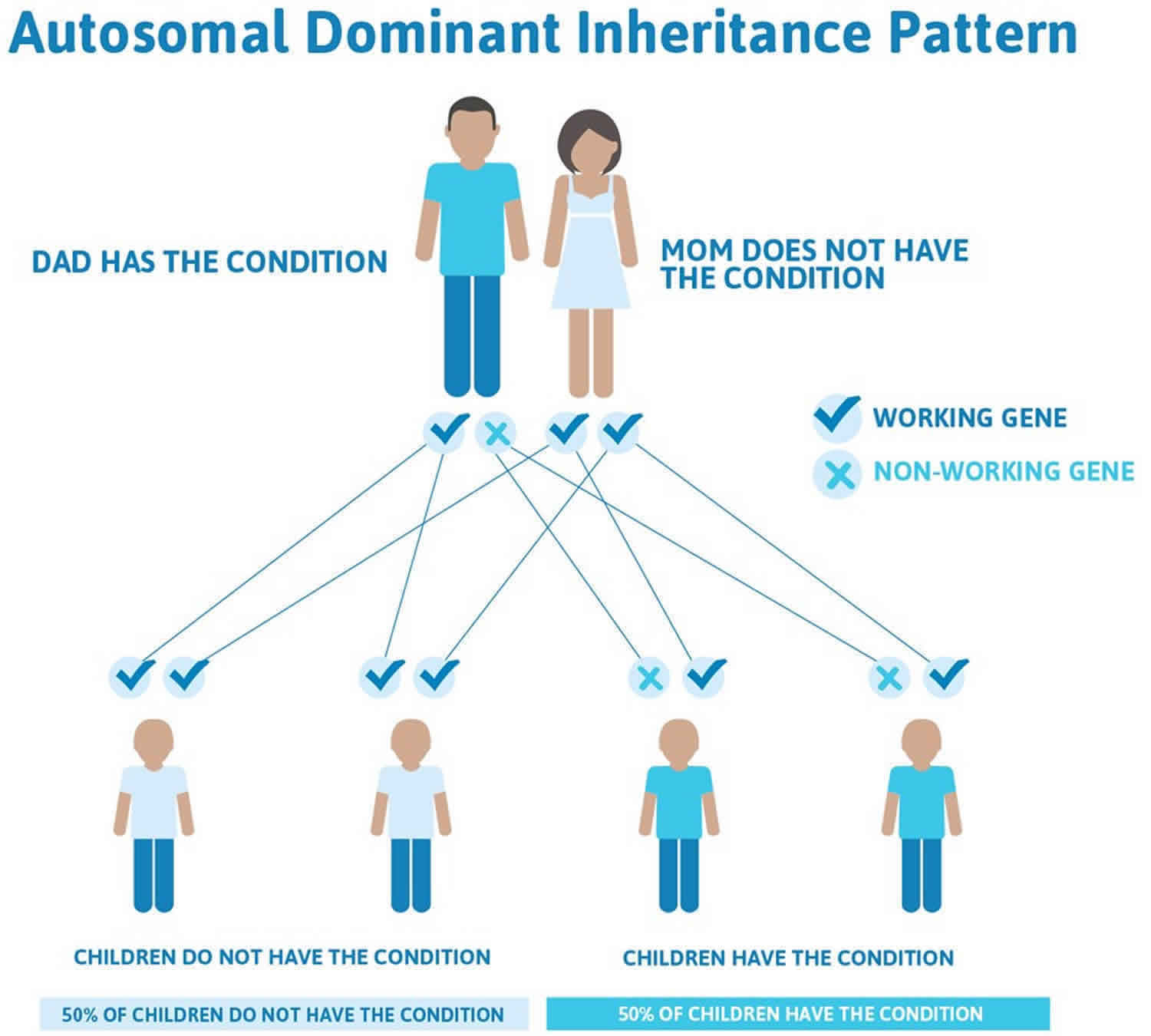
Liddle syndrome is inherited in an autosomal dominant pattern, which means one copy of the altered gene in each cell is sufficient to cause the disorder.
Often autosomal dominant conditions can be seen in multiple generations within the family. If one looks back through their family history they notice their mother, grandfather, aunt/uncle, etc., all had the same condition. In cases where the autosomal dominant condition does run in the family, the chance for an affected person to have a child with the same condition is 50% regardless of whether it is a boy or a girl. These possible outcomes occur randomly. The chance remains the same in every pregnancy and is the same for boys and girls.
- When one parent has the abnormal gene, they will pass on either their normal gene or their abnormal gene to their child. Each of their children therefore has a 50% (1 in 2) chance of inheriting the changed gene and being affected by the condition.
- There is also a 50% (1 in 2) chance that a child will inherit the normal copy of the gene. If this happens the child will not be affected by the disorder and cannot pass it on to any of his or her children.
Figure 1 illustrates autosomal dominant inheritance. The example below shows what happens when dad has the condition, but the chances of having a child with the condition would be the same if mom had the condition.
Figure 1. Liddle syndrome autosomal dominant inheritance pattern
People with specific questions about genetic risks or genetic testing for themselves or family members should speak with a genetics professional.
Resources for locating a genetics professional in your community are available online:
- The National Society of Genetic Counselors (https://www.findageneticcounselor.com/) offers a searchable directory of genetic counselors in the United States and Canada. You can search by location, name, area of practice/specialization, and/or ZIP Code.
- The American Board of Genetic Counseling (https://www.abgc.net/about-genetic-counseling/find-a-certified-counselor/) provides a searchable directory of certified genetic counselors worldwide. You can search by practice area, name, organization, or location.
- The Canadian Association of Genetic Counselors (https://www.cagc-accg.ca/index.php?page=225) has a searchable directory of genetic counselors in Canada. You can search by name, distance from an address, province, or services.
- The American College of Medical Genetics and Genomics (http://www.acmg.net/ACMG/Genetic_Services_Directory_Search.aspx) has a searchable database of medical genetics clinic services in the United States.
Liddle syndrome symptoms
Patients with Liddle syndrome can be symptomatic or asymptomatic. Hypertension is estimated to affect about 92% of people with Liddle syndrome 6. Many Liddle syndrome patients have been observed to develop hypertension and resistant hypertension due to sodium reabsorption at the level of distal nephron at an unusually early age, between the age of 11 and 31 17, 18. Resistant hypertension in children and teenagers may alert the clinician to consider tests for Liddle syndrome. The degree of hypertension in patients with Liddle syndrome is variable, as are the downstream effects of hypertension such as damage to other organs caused by high blood pressure.
Doctors may take years or decades to diagnose it. Doctors often misdiagnose the symptoms of Liddle syndrome. Hypertension due to Liddle syndrome is sensitive to a salt-restricted diet 4. Hypertension presents with a headache, dizziness, retinopathy, chronic kidney disease, left ventricular hypertrophy, and sudden death. Due to resistant hypertension, hypokalemia, and ventricular hypertrophy, a patient can develop lethal arrhythmias which can lead to sudden death.
Untreated resistant hypertension can eventually lead to heart disease and stroke.
Hypokalemia and metabolic alkalosis happen due to excessive loss of potassium in the urine at the expense of sodium reabsorption 19. Symptoms of hypokalemia can include weakness, fatigue, muscle pain (myalgia), constipation or heart palpitations.
The incidence of hypertension and hypokalemia in patients with Liddle syndrome is about 92.4% and 71.8% respectively 20. Doctors can order genetic testing to diagnose patients with Liddle syndrome who do not have hypertension or hypokalemia. In these individuals, genetic testing is usually done because of family history.
Liddle syndrome complications
Liddle syndrome complications include hypokalemia, metabolic alkalosis, and resistant hypertension. The resistant hypertension patients can develop end-organ damage that includes myocardial infarction, transient ischemic attack/cerebrovascular accident, pulmonary edema, ventricular hypertrophy.
Liddle syndrome diagnosis
A diagnosis of Liddle syndrome is often suspected based the presence of early-onset hypertension (high blood pressure), especially in people with a family history of Liddle syndrome. Additional testing can then be ordered to confirm the diagnosis. This may include 21:
- Blood tests which can detect low levels of potassium, renin and aldosterone.
- Urine tests to identify low levels of sodium (< 20 mEq or <20 mmol/L) and aldosterone.
- Genetic testing to look for a change (mutation) in the SCNN1B or SCNN1G gene.
Laboratory investigation may reveal hypokalemia and metabolic alkalosis 20. Hyperaldosteronism can also present with same features and biochemical abnormalities. Renin and aldosterone levels should be checked to differentiate between hyperaldosteronism and pseudo-hyperaldosteronism (Liddle syndrome). In patients with Liddle syndrome, renin and aldosterone levels are low in contrast to patients with hyperaldosteronism in whom aldosterone levels are high. Due to normal levels of aldosterone, spironolactone does not work for patients with Liddle syndrome 22.
Once a patient diagnosed with low-renin and low-aldosterone levels, a patient should take aldosterone for two months under the healthcare providers observation. Some conditions like glucocorticoid resistance syndrome, apparent mineralocorticoid excess syndrome, and congenital adrenal hyperplasia also respond well to this drug. If the patient does not respond to aldosterone, then the clinician should be suspicious for Liddle syndrome.
The gold standard for diagnosing Liddle syndrome is genetic sequencing of the three genes where mutations are known to be associated with Liddle syndrome: SCNN1A, SCNN1B, and SCNN1G. More than 30 different mutations in these genes have been found to cause Liddle syndrome. Most of these mutations are in SCNN1B and SCNN1G genes, and in many patients only these genes will be sequenced.
Genetic counseling is recommended prior to testing. A positive genetic test should include communication with the patient about familial decisions and outreach to potentially affected family members.
The robust response to amiloride, a potassium sparing diuretic, continues to be integral to the diagnosis of Liddle syndrome. Although other diuretics are often prescribed first for hypertension, other classes of diuretics are not appropriate as they either do not alter the activity of epithelial sodium channel (ENaC), or in the case of some other potassium sparing diuretics will not be effective due to the mechanism of Liddle syndrome.
A person diagnosed with Liddle syndrome may not develop chronic kidney disease (CKD), but it does preclude them from donating a kidney. Placement on the registry to receive a renal transplant has been effective in some patients, as the replacement of the mutated epithelial sodium channel (ENaC) with functioning epithelial sodium channel (ENaC) in the donated kidney may resolve the symptoms of Liddle syndrome.
Liddle syndrome treatment
Treatment for Liddle syndrome consists of following a low sodium diet and taking potassium-sparing diuretics, which reduce blood pressure and correct hypokalemia and metabolic alkalosis. Conventional anti-hypertensive therapies are not effective for Liddle syndrome 21. The drug of choice is amiloride. It works well in Liddle syndrome as it directly inhibits epithelial sodium channel (ENaC). Amiloride is prescribed daily with a dose ranges from 5 to 20 mg. Triamterene, another potassium-sparing diuretic, similar to amiloride can also be used to manage Liddle syndrome. A sodium-restricted diet showed a cumulative effect with these drugs 23. However, excessive sodium accumulation on the receptor makes is unavailable for the medication 24. If renal function is normal, then the incidence of hyperkalemia is very rare. Avoidance of excessive potassium in the diet is suggested along with the use of potassium-sparing diuretics. Amiloride is safe in pregnancy 25.
In a patient with Liddle syndrome the response to standard treatments for hypertension such as thiazide diuretics or ACE inhibitors may be reduced compared to other hypertensive patients, especially in the absence of treatment with amiloride. This may lead doctors to falsely believe that patients are failing to comply with prescribed medication regimens. Repeated questioning of patient compliance to medication regimens can have psychological and emotional consequences. For this reason, response to amiloride should be considered in the process of diagnosing Liddle syndrome and developing a treatment plan.
Genetic counseling is recommended for patients with Liddle syndrome and their family members.
Amiloride
Amiloride is the first-line treatment of Liddle syndrome. It targets and blocks epithelial sodium channel (ENaC) in the kidney, thereby directly countering the pathologic pathway. Other potassium sparing diuretics, like triamterene, eplerenone, spironolactone and finerenone are less effective or ineffective. Eplerenone, spironolactone and finerenone have mechanisms of action different from amiloride and triamterene which renders them ineffective in the treatment of Liddle syndrome. For example, spironolactone antagonizes binding of aldosterone to its receptor, preventing aldosterone from binding and acting in cells in the kidney. In patients with Liddle syndrome, aldosterone levels are low, and so inhibiting aldosterone’s action with spironolactone has little effect. In fact, the lack of response to spironolactone was a feature identified by Grant Liddle in his initial description of Liddle syndrome. Despite triamterene being described as the first effective treatment for Liddle syndrome, amiloride has superior efficacy to triamterene and is preferred.
When the diagnosis of Liddle syndrome is made later in life, additional anti-hypertensive medications are also needed to achieve blood pressure control. Amiloride is not time-release formulated, and it is recommended to take at similar times each day, some patients with Liddle syndrome may opt for a twice daily regimen. The treatment is life-long. Amiloride is available for custom-compounding by pharmacists. The pharmacist can prepare a dose in capsule form, without other medications or stabilizing compounds. This specialized formulation may be appropriate for ‘fine-tuning’ dose level.
While amiloride does not have sufficient data in pregnant women to be confirmed as safe, it has not been found to cause fetal harm in animal studies. Women with Liddle syndrome have continued amiloride in pregnancy without fetal or maternal complications. Blood pressure during pregnancy may be difficult to treat without amiloride, particularly in the 3rd trimester.
Low sodium diet
Limiting salt in the diet is an important component to the medical management of hypertension. In Liddle syndrome, a low salt-diet is particularly effective, especially when paired with amiloride. Because sodium retention is directly involved in the mechanism of persistent hypertension in Liddle syndrome, a low sodium diet can be helpful in achieving a goal blood pressure 26.
Liddle syndrome prognosis
With treatment, the long-term outlook (prognosis) for people with Liddle syndrome is good. However, untreated hypertension may lead to stroke, heart disease and/or kidney problems which can be fatal 21. Current studies do not address the long-term mortality of Liddle syndrome. Clinicians often under treat and misdiagnose these patients. Further studies are needed to establish morbidity and mortality in the population suffering from secondary hypertension due to Liddle syndrome.
- Mubarik A, Aeddula NR. Liddle Syndrome. [Updated 2020 Jan 13]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK536911[↩]
- Wang LP, Yang KQ, Jiang XJ, Wu HY, Zhang HM, Zou YB, Song L, Bian J, Hui RT, Liu YX, Zhou XL. Prevalence of Liddle Syndrome Among Young Hypertension Patients of Undetermined Cause in a Chinese Population. J Clin Hypertens (Greenwich). 2015 Nov;17(11):902-7.[↩]
- Liu K, Qin F, Sun X, Zhang Y, Wang J, Wu Y, Ma W, Wang W, Wu X, Qin Y, Zhang H, Zhou X, Wu H, Hui R, Zou Y, Jiang X, Song L. Analysis of the genes involved in Mendelian forms of low-renin hypertension in Chinese early-onset hypertensive patients. J. Hypertens. 2018 Mar;36(3):502-509.[↩]
- Pagani L, Diekmann Y, Sazzini M, De Fanti S, Rondinelli M, Farnetti E, Casali B, Caretto A, Novara F, Zuffardi O, Garagnani P, Mantero F, Thomas MG, Luiselli D, Rossi E. Three Reportedly Unrelated Families With Liddle Syndrome Inherited From a Common Ancestor. Hypertension. 2018 Feb;71(2):273-279.[↩][↩]
- Tapolyai M, Uysal A, Dossabhoy NR, Zsom L, Szarvas T, Lengvárszky Z, Fülöp T. High prevalence of liddle syndrome phenotype among hypertensive US Veterans in Northwest Louisiana. J Clin Hypertens (Greenwich). 2010 Nov;12(11):856-60.[↩]
- Tetti M, Monticone S, Burrello J, Matarazzo P, Veglio F, Pasini B, Jeunemaitre X, Mulatero P. Liddle Syndrome: Review of the Literature and Description of a New Case. Int J Mol Sci. 2018 Mar 11;19(3):812. doi: 10.3390/ijms19030812[↩][↩][↩]
- Mareš Š, Filipovský J, Vlková K, Pešta M, Černá V, Hrabák J, Mlíková Seidlerová J, Mayer O. A novel nonsense mutation in the β-subunit of the epithelial sodium channel causing Liddle syndrome. Blood Press. 2021 Oct;30(5):291-299. doi: 10.1080/08037051.2021.1942785[↩][↩]
- Yang Y, Wu C, Qu D, Xu X, Chen L, Sun Q, Zhao X. Liddle syndrome misdiagnosed as primary aldosteronism is caused by inaccurate aldosterone-rennin detection while a novel SCNN1G mutation is discovered. Blood Press. 2022 Dec;31(1):139-145. doi: 10.1080/08037051.2022.2088471[↩][↩]
- Liddle Syndrome. https://rarediseases.org/rare-diseases/liddle-syndrome[↩][↩]
- Mubarik A, Anastasopoulou C, Aeddula NR. Liddle Syndrome (Pseudohyperaldosteronism) [Updated 2024 Mar 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK536911[↩][↩]
- Liddle syndrome. https://ghr.nlm.nih.gov/condition/liddle-syndrome[↩]
- Hanukoglu I, Hanukoglu A. Epithelial sodium channel (ENaC) family: Phylogeny, structure-function, tissue distribution, and associated inherited diseases. Gene. 2016 Apr 1;579(2):95-132. doi: 10.1016/j.gene.2015.12.061[↩]
- Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994 Feb 03;367(6462):463-7.[↩]
- Lu C, Pribanic S, Debonneville A, Jiang C, Rotin D. The PY motif of ENaC, mutated in Liddle syndrome, regulates channel internalization, sorting and mobilization from subapical pool. Traffic. 2007 Sep;8(9):1246-64.[↩]
- Kellenberger S, Gautschi I, Rossier BC, Schild L. Mutations causing Liddle syndrome reduce sodium-dependent downregulation of the epithelial sodium channel in the Xenopus oocyte expression system. J. Clin. Invest. 1998 Jun 15;101(12):2741-50.[↩]
- Nakada T, Koike H, Akiya T, Katayama T, Kawamata S, Takaya K, Shigematsu H. Liddle’s syndrome, an uncommon form of hyporeninemic hypoaldosteronism: functional and histopathological studies. J. Urol. 1987 Apr;137(4):636-40.[↩]
- Cui Y, Tong A, Jiang J, Wang F, Li C. Liddle syndrome: clinical and genetic profiles. J Clin Hypertens (Greenwich). 2017 May;19(5):524-529. doi: 10.1111/jch.12949[↩]
- Liu K, Qin F, Sun X, Zhang Y, Wang J, Wu Y, Ma W, Wang W, Wu X, Qin Y, Zhang H, Zhou X, Wu H, Hui R, Zou Y, Jiang X, Song L. Analysis of the genes involved in Mendelian forms of low-renin hypertension in Chinese early-onset hypertensive patients. J Hypertens. 2018 Mar;36(3):502-509. doi: 10.1097/HJH.0000000000001556[↩]
- Unwin RJ, Luft FC, Shirley DG. Pathophysiology and management of hypokalemia: a clinical perspective. Nat Rev Nephrol. 2011 Feb;7(2):75-84.[↩]
- Monticone S, Buffolo F, Tetti M, Veglio F, Pasini B, Mulatero P. GENETICS IN ENDOCRINOLOGY: The expanding genetic horizon of primary aldosteronism. Eur. J. Endocrinol. 2018 Mar;178(3):R101-R111.[↩][↩]
- Liddle syndrome. https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=EN&Expert=526[↩][↩][↩]
- Mulatero P, Verhovez A, Morello F, Veglio F. Diagnosis and treatment of low-renin hypertension. Clin. Endocrinol. (Oxf). 2007 Sep;67(3):324-34.[↩]
- Yang KQ, Lu CX, Fan P, Zhang Y, Meng X, Dong XQ, Luo F, Liu YX, Zhang HM, Wu HY, Cai J, Zhang X, Zhou XL. Genetic screening of SCNN1B and SCNN1G genes in early-onset hypertensive patients helps to identify Liddle syndrome. Clin. Exp. Hypertens. 2018;40(2):107-111.[↩]
- Warnock DG. Liddle syndrome: an autosomal dominant form of human hypertension. Kidney Int. 1998 Jan;53(1):18-24.[↩]
- Awadalla M, Patwardhan M, Alsamsam A, Imran N. Management of Liddle Syndrome in Pregnancy: A Case Report and Literature Review. Case Rep Obstet Gynecol. 2017;2017:6279460.[↩]
- Pagani L, Diekmann Y, Sazzini M, De Fanti S, Rondinelli M, Farnetti E, Casali B, Caretto A, Novara F, Zuffardi O, Garagnani P, Mantero F, Thomas MG, Luiselli D, Rossi E. Three Reportedly Unrelated Families With Liddle Syndrome Inherited From a Common Ancestor. Hypertension. 2018 Feb;71(2):273-279. doi: 10.1161/HYPERTENSIONAHA.117.10491[↩]