What is acute myeloid leukemia
Acute myeloid leukemia also called acute myelocytic leukemia, acute myelogenous leukemia, acute granulocytic leukemia, acute non-lymphocytic leukemia, or sometimes just AML, is an aggressive (fast-growing) disease in which too many myeloblasts (immature white blood cells that are not lymphoblasts) are found in the bone marrow and blood. The word “acute” in acute myelogenous leukemia denotes the disease’s rapid progression. It’s called myelogenous leukemia because it affects a group of white blood cells called the myeloid cells, which normally develop into the various types of mature blood cells, such as red blood cells, white blood cells and platelets. Acute myeloid leukemia (AML) is the most common type of acute leukemia in adults and is uncommon before the age of 45, making up about 80% of people with acute leukemia. AML is one of the most common types of leukemia in adults. Still, AML is fairly rare overall, accounting for only about 1% of all cancers. In the United States, it is estimated that 3-5 people per every 100,000 people in the general population has the disease. More than half the people diagnosed with acute myeloid leukemia are 65 years of age or older (average age of diagnosis is age 68). But AML can occur in children as well. Slightly more men than women are affected by acute myeloid leukemia (the average lifetime risk of getting AML in both sexes is about 0.5 of 1 percent), and it occurs with slightly more frequency in people of European heritage. Acute myeloid leukemia usually gets worse quickly if it is not treated. Acute myeloid leukemia starts in the bone marrow (the soft inner part of certain bones, where new blood cells are made), but most often it quickly moves into the blood, as well. Acute myeloid leukemia can sometimes spread to other parts of the body including the lymph nodes, liver, spleen, central nervous system (brain and spinal cord), and testicles. Most often, acute myeloid leukemia develops from cells that would turn into white blood cells (other than lymphocytes), but sometimes acute myeloid leukemia develops in other types of blood-forming cells.
The American Cancer Society’s estimates for leukemia in the United States for 2021 are 1:
- About 61,090 new cases of leukemia (all kinds) and 23,660 deaths from leukemia (all kinds)
- About 20,240 new cases of acute myeloid leukemia (AML). Most will be in adults.
- Acute myeloid leukemia is the second most common type of leukemia diagnosed in adults and children, but most cases occur in adults. AML makes up 31% of all adult leukemia cases.
- About 11,400 deaths from AML. Almost all will be in adults.
- Based on the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program data from 2011 to 2017, 29.5% of patients with AML were alive 5 years after diagnosis 2.
- Survival depends on several factors, including biologic features of the disease and, in particular, a patient’s age. The 5-year survival rate for people 20 and older with acute myeloid leukemia (AML) is 26%. For people younger than 20, the survival rate is 68%.
Leukemia is cancer of the white blood cells. White blood cells help your body fight infection. Your blood cells form in your bone marrow. In leukemia, however, the bone marrow produces abnormal white blood cells. These abnormal white blood cells crowd out the healthy blood cells, making it hard for blood to do its work. In acute myeloid leukemia, there are too many of a specific type of white blood cell called a myeloblast. Most often, leukemia starts in early forms of white blood cells, but some leukemias start in other blood cell types. There are several types of leukemia, which are divided based mainly on whether the leukemia is acute (fast growing) or chronic (slower growing), and whether it starts in myeloid cells or lymphoid cells.
AML is usually found in the blood and bone marrow, the spongy, red tissue in the inner part of the large bones. It can sometimes spread to other parts of the body, such as the lymph nodes, spleen, liver, brain, skin, and gums. Occasionally, acute myeloid leukemia cells can form a solid tumor called a myeloid sarcoma or chloroma that can develop anywhere in the body. This is often called extramedullary disease.
Possible risk factors for developing acute myeloid leukemia (AML) include smoking, previous chemotherapy treatment, and exposure to radiation.
Symptoms of acute myeloid leukemia include:
- Fever
- Shortness of breath
- Easy bruising or bleeding, such as frequent nosebleeds and bleeding from the gums
- Bleeding under the skin
- Weakness or feeling tired
- Weight loss or loss of appetite
- Frequent infections because people with AML do not have enough mature neutrophils
Tests that examine the blood and bone marrow diagnose acute myeloid leukemia (AML). Treatments include chemotherapy, other drugs, radiation therapy, stem cell transplants, and targeted therapy. Targeted therapy uses substances that attack cancer cells without harming normal cells. Once the leukemia is in remission, you need additional treatment to make sure that it does not come back.
Normal bone marrow, blood, and lymph tissue
To understand leukemia, it helps to know about the blood and lymph systems.
Bone marrow
Bone marrow is the soft inner part of certain bones. It is made up of blood-forming cells, fat cells, and supporting tissues. A small fraction of the blood-forming cells are blood stem cells.
Inside the bone marrow, blood stem cells develop into new blood cells. During this process, the cells become either lymphocytes (a kind of white blood cell) or other blood-forming cells, which are types of myeloid cells. Myeloid cells can develop into red blood cells, white blood cells (other than lymphocytes), or platelets. These myeloid cells are the ones that are abnormal in acute myeloid leukemia.
Types of blood cells
There are 3 main types of blood cells:
- Red blood cells (RBCs) carry oxygen from the lungs to all other tissues in the body, and take carbon dioxide back to the lungs to be removed.
- Platelets are actually cell fragments made by a type of bone marrow cell called the megakaryocyte. Platelets are important in stopping bleeding. They help plug up holes in blood vessels caused by cuts or bruises.
- White blood cells (WBCs) help the body fight infections.
There are different types of white blood cells:
- Granulocytes are mature white blood cells that develop from myeloblasts, a type of blood-forming cell in the bone marrow. Granulocytes have granules that show up as spots under the microscope. These granules contain enzymes and other substances that can destroy germs, such as bacteria. The 3 types of granulocytes – neutrophils, basophils, and eosinophils – are distinguished by the size and color of their granules.
- Monocytes are white blood cells that develop from blood-forming monoblasts in the bone marrow. After circulating in the bloodstream for about a day, monocytes enter body tissues to become macrophages, which can destroy some germs by surrounding and digesting them. Macrophages also help lymphocytes recognize germs and make antibodies to fight them.
- Lymphocytes are mature white blood cells that develop from lymphoblasts in the bone marrow. Lymphocytes are the main cells that make up lymph tissue, a major part of the immune system. Lymph tissue is found in lymph nodes, the thymus (a small organ behind the breast bone), the spleen, the tonsils and adenoids, and is scattered throughout the digestive and respiratory systems and the bone marrow. The 2 main types of lymphocytes are B cell and T cells.
Figure 1. Blood composition
Note: Blood is a complex mixture of formed elements in a liquid extracellular matrix, called blood plasma. Note that water and proteins account for 99% of the blood plasma.
Figure 2. Bone marrow anatomy
Figure 3. White blood cells development. A blood stem cell goes through several steps to become a red blood cell, platelet, or white blood cell
Figure 4. White blood cells development
Figure 5. White blood cells
Acute myeloid leukemia causes
Acute myeloid leukemia is caused by damage to the DNA (deoxyribonucleic acid) of developing cells in your bone marrow. When this happens, blood cell production goes wrong. Normally, the DNA tells the cell to grow at a set rate and to die at a set time. In acute myeloid leukemia, the mutations tell the bone marrow cell (myeloid stem cell) to continue growing and dividing. When this happens, blood cell production becomes out of control. The bone marrow produces immature cells that develop into leukemic white blood cells called myeloblasts (immature white blood cells) also referred to as an “AML cell” or “blast cell”. These abnormal cells (leukemia cells) are unable to function properly, and they can build up and crowd out healthy red blood cells, white blood cells and platelets. As a result, there are too many immature blast cells (that cannot fight infections) and not enough mature, functional red and white blood cells and platelets.
By the time AML is diagnosed, the number of healthy red blood cells, white blood cells and platelets is usually lower than normal. Having low levels of blood cells may result in infections, anemia (low red blood cell count), and excessive bleeding or bruising.
It’s not clear what causes the DNA mutations that lead to leukemia, but doctors have identified factors that increase the risk (see risk factors below).
Some genes control when your cells grow, divide to make new cells, and die at the right time:
- Genes that help cells grow, divide, or stay alive are called oncogenes.
- Genes that help keep cell division under control or make cells die at the right time are called tumor suppressor genes.
The DNA inside each cell is in long strands called chromosomes. Each time a cell divides into 2 new cells, it must make a new copy of its chromosomes. This process isn’t perfect, and errors can occur that affect genes within the chromosomes. Cancers (including acute myeloid leukemia) can be caused by mutations (changes) that turn on oncogenes or turn off tumor suppressor genes. For instance, changes in certain genes such as FLT3, c-KIT, and RAS are common in acute myeloid leukemia cells. These types of changes can stop bone marrow cells from maturing the way they normally would, or help the cells grow out of control.
Mutations in many different genes can be found in acute myeloid leukemia, but larger changes in one or more chromosomes are also common. Even though these changes involve larger pieces of DNA, their effects are still likely to be due to changes in just one or a few genes that are on that part of the chromosome. Several types of chromosome changes may be found in acute myeloid leukemia cells:
- Translocations are the most common type of chromosome change. A translocation means that a part of one chromosome breaks off and becomes attached to a different chromosome. The point at which the break occurs can affect nearby genes – for example, it can turn on oncogenes or turn off genes like RUNX1 and RARa, which would normally help blood cells to mature.
- Deletions occur when part of a chromosome is lost. This can result in the cell losing a gene that helped keep its growth in check (a tumor suppressor gene).
- Inversions occur when part of a chromosome gets turned around, so it’s now in reverse order. This can result in the loss of a gene (or genes) because the cell can no longer read its instructions (much like trying to read a book backward).
- Addition or duplication means that there is an extra chromosome or part of a chromosome. This can lead to too many copies of certain genes within the cell. This can be a problem if one or more of these genes are oncogenes.
In most cases, it’s not clear what causes the DNA mutations that lead to leukemia. Radiation, exposure to certain chemicals and some chemotherapy drugs are known risk factors for acute myeloid leukemia.
Most DNA changes related to acute myeloid leukemia occur during a person’s lifetime, rather than having been inherited before birth. Some of these acquired changes may have outside causes like radiation or cancer-causing chemicals, but in most cases the reason they occur isn’t clear. Many of these gene changes are probably just random events that sometimes happen inside a cell, without having an outside cause. They seem to happen more often as we age, which might help explain why acute myeloid leukemia usually occurs in older people.
There are many types of acute myeloid leukemia, and different cases of acute myeloid leukemia can have different gene and chromosome changes, some of which are more common than others. Doctors are trying to figure out why these changes occur and how each of them might lead to leukemia. For example, some are more common in leukemia that occurs after chemotherapy for another cancer.
Some changes seem to have more of an effect on a person’s prognosis (outlook) than others. For instance, some changes might affect how quickly the leukemia cells grow, or how likely they are to respond to treatment.
Risk factors for acute myeloid leukemia
A risk factor is something that affects your chance of getting a disease, such as cancer. Different cancers have different risk factors. Some risk factors, like smoking, can be changed. Others, like a person’s age or family history, can’t be changed.
But having a risk factor, or even several risk factors, does not always mean that a person will get the disease, and many people get cancer without having any known risk factors.
There are some known risk factors for acute myeloid leukemia (AML).
Factors that may increase your risk of acute myeloid leukemia include:
- Increasing age: The risk of acute myeloid leukemia increases with age. Acute myelogenous leukemia is most common in adults age 65 and older.
- Your sex: Men are more likely to develop acute myeloid leukemia than are women. The reason for this is not clear.
- Previous cancer treatment: People who’ve had certain types of chemotherapy and radiation therapy may have a greater risk of developing acute myeloid leukemia.
- Drugs called alkylating agents are linked to an increased risk of AML. Often a patient will get a disease called a myelodysplastic syndrome before the AML. Examples of alkylating drugs include cyclophosphamide, mechlorethamine, procarbazine, chlorambucil, melphalan, busulfan, carmustine, cisplatin, and carboplatin.
- Chemo drugs known as topoisomerase II inhibitors are also linked to AML. Acute myeloid leukemia (AML) linked to these drugs tends to occur without myelodysplastic syndrome developing first. Examples of topoisomerase II inhibitors include etoposide, teniposide, mitoxantrone, epirubicin, and doxorubicin.
- Exposure to radiation: People exposed to very high levels of radiation, such as survivors of a nuclear reactor accident, have an increased risk of developing acute myeloid leukemia. Radiation treatment for cancer has also been linked to an increased risk of AML. The risk varies based on the amount of radiation given and what area is treated. Exposure to such radiation, especially very early in life, might carry an increased risk of leukemia, but how much of a risk is not clear.
- Dangerous chemical exposure: Exposure to certain chemicals, such as benzene, is linked to a greater risk of acute myeloid leukemia. Benzene is a solvent used in the rubber industry, oil refineries, chemical plants, shoe manufacturing, and gasoline-related industries, and is also found in cigarette smoke, gasoline and motor vehicle exhaust, and some glues, cleaning products, detergents, art supplies, and paints. Some studies have linked AML risk to heavy workplace exposure to formaldehyde, but this link has not been seen in some other studies.
- Smoking: The only proven lifestyle-related risk factor for AML is smoking. Acute myeloid leukemia is linked to cigarette smoke, which contains benzene and other known cancer-causing chemicals.
- Other blood disorders: People who’ve had another blood disorder, such as myelodysplasia, myelofibrosis, polycythemia vera or thrombocythemia, are at greater risk of developing acute myeloid leukemia. Some people who have a myelodysplastic syndrome (MDS) may develop AML. Patients with myelodysplastic syndrome (MDS) have low blood cell counts and abnormal cells in the blood and bone marrow. Myelodysplastic syndrome (MDS) can evolve over time into AML. Acute myeloid leukemia (AML) that develops after myelodysplastic syndrome (MDS) is often hard to treat. The risk of AML increases if these disorders are treated with some types of chemotherapy or radiation.
- Genetic disorders: Some syndromes that are caused by genetic mutations (abnormal changes) present at birth seem to raise the risk of acute myeloid leukemia. These include:
- Fanconi anemia
- Bloom syndrome
- Ataxia-telangiectasia
- Diamond-Blackfan anemia
- Schwachman-Diamond syndrome
- Li-Fraumeni syndrome
- Neurofibromatosis type 1
- Severe congenital neutropenia (also called Kostmann syndrome)
- Down syndrome (being born with an extra copy of chromosome 21)
- Trisomy 8 (being born with an extra copy of chromosome 8)
Many people with acute myeloid leukemia have no known risk factors, and many people who have risk factors never develop the cancer.
Having a family history
Although most cases of acute myeloid leukemia are not thought to have a strong genetic link, having a close relative (such as a parent, brother, or sister) with acute myeloid leukemia increases your risk of getting the disease.
Someone who has an identical twin who got acute myeloid leukemia before they were a year old has a very high risk of also getting acute myeloid leukemia.
Uncertain, unproven or controversial risk factors
Other factors that have been studied for a possible link to acute myeloid leukemia include:
- Exposure to electromagnetic fields (such as living near power lines)
- Workplace exposure to diesel, gasoline, and certain other chemicals and solvents
- Exposure to herbicides or pesticides
So far, none of these factors has been linked conclusively to acute myeloid leukemia. Research is being done in these areas.
Acute myeloid leukemia prevention
It’s not clear what causes most cases of acute myeloid leukemia (AML). Since most people with acute myeloid leukemia don’t have risk factors that can be changed, at the present time there is no known way to prevent most cases of acute myeloid leukemia.
Smoking is by far the most significant controllable risk factor for acute myeloid leukemia, and quitting offers the greatest chance to reduce a person’s risk of acute myeloid leukemia. Non-smokers are also much less likely than smokers to develop many other cancers, as well as heart disease, stroke, and some other diseases.
Treating some other cancers with chemotherapy or radiation may cause secondary (treatment-related) leukemias in some people. Doctors are trying to figure out how to treat these cancers without raising the risk of secondary leukemia. But for now, the obvious benefits of treating life-threatening cancers with chemotherapy and radiation must be balanced against the small chance of getting leukemia years later.
Avoiding known cancer-causing chemicals, such as benzene, might lower the risk of getting acute myeloid leukemia. But most experts agree that exposure to workplace and environmental chemicals seems to account for only a small portion of leukemias.
Acute myeloid leukemia symptoms
Acute myeloid leukemia can cause many different signs and symptoms. Some are more common with certain subtypes of acute myeloid leukemia.
General symptoms
People with acute myeloid leukemia often have several non-specific (general) symptoms. These can include:
- Weight loss
- Fatigue
- Fever
- Night sweats
- Loss of appetite
- Pale skin
- Tiredness
- Breathlessness
- A high temperature (fever)
- Excessive sweating
- Frequent infections
- Unusual and frequent bleeding, such as bleeding gums or nosebleeds
- Easily bruised skin
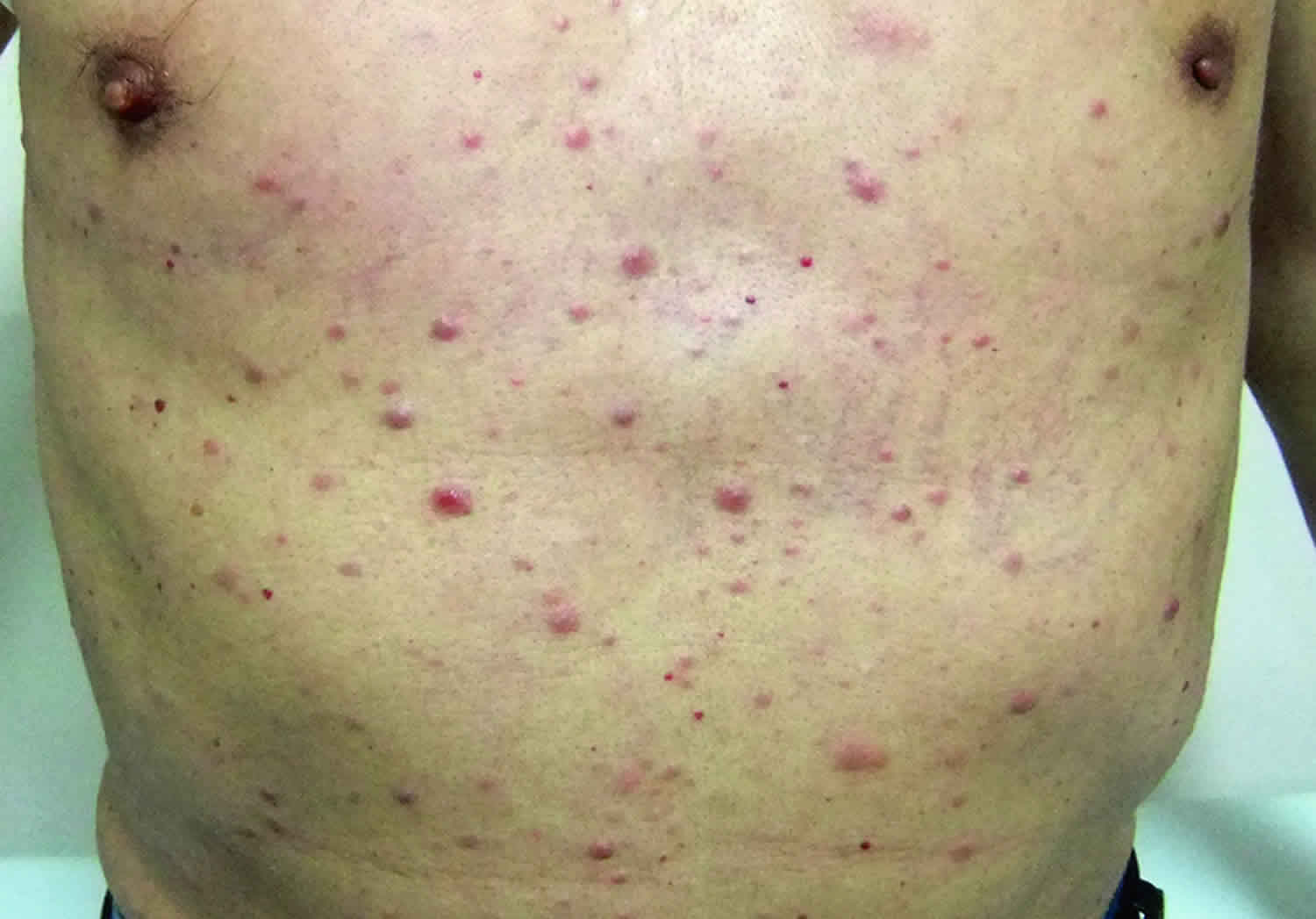
- Flat red or purple spots on the skin (petechiae)
- Bone and joint pain
- A feeling of fullness or discomfort in your tummy (abdomen), caused by swelling of the liver or spleen
These are not just symptoms of acute myeloid leukemia. More often they are caused by something other than leukemia.
In rare instances, a tumor made up of acute myeloid leukemia cells forms outside the bone marrow. This type of tumor, called a “myeloid sarcoma”, also known as “extramedullary disease,” “chloroma,” “granulocytic sarcoma,” “myeloblastoma” and “monocytoma”, can form in almost any part of the body. Typically, surgery and radiation therapy are not sufficient to treat myeloid sarcomas. They are generally treated with systemic chemotherapy regimens used for AML, even if the bone marrow and blood do not appear to be involved. “Systemic chemotherapy” is a treatment with anticancer drugs that travel through the bloodstream to cells all over the body. Treatment for myeloid sarcomas may also include allogeneic stem cell transplantation.
Symptoms caused by low numbers of blood cells
Many signs and symptoms of acute myeloid leukemia are the result of a shortage of normal blood cells, which happens when the leukemia cells crowd out the normal blood-making cells in the bone marrow. As a result, people don’t have enough normal red blood cells, white blood cells, and blood platelets. These shortages show up on blood tests, and they can also cause symptoms.
Symptoms from low red blood cell counts (anemia)
Red blood cells carry oxygen to all of the cells in the body. A shortage of red blood cells can cause:
- Tiredness (fatigue)
- Weakness
- Feeling cold
- Feeling dizzy or lightheaded
- Headaches
- Pale skin
- Shortness of breath
Symptoms from low white blood cell counts (leukopenia and neutropenia)
Infections can occur because of a shortage of normal white blood cells (leukopenia), specifically a shortage of infection-fighting white blood cells called neutrophils (a condition called neutropenia). People with acute myeloid leukemia can get infections that don’t seem to go away or may get one infection after another. Fever often goes along with the infection.
Although people with acute myeloid leukemia can have high white blood cell counts due to excess numbers of leukemia cells, these cells don’t protect against infection the way normal white blood cells do.
Symptoms from low blood platelet counts (thrombocytopenia)
Platelets normally help stop bleeding. A shortage of blood platelets (called thrombocytopenia) can lead to:
- Bruises (or small red or purple spots) on the skin
- Excess bleeding
- Frequent or severe nosebleeds
- Bleeding gums
- Heavy periods (menstrual bleeding) in women
Symptoms caused by high numbers of leukemia cells
The cancer cells in acute myeloid leukemia (called blasts) are bigger than normal white blood cells and have more trouble going through tiny blood vessels. If the blast count gets very high, these cells can clog up blood vessels and make it hard for normal red blood cells (and oxygen) to get to tissues. This is called leukostasis. Leukostasis is rare, but it is a medical emergency that needs to be treated right away. Some of the symptoms are like those seen with a stroke, and include:
- Headache
- Weakness in one side of the body
- Slurred speech
- Confusion
- Sleepiness
When blood vessels in the lungs are affected, people can have shortness of breath. Blood vessels in the eye can be affected as well, leading to blurry vision or even loss of vision.
Bleeding and clotting problems
Patients with a certain type of acute myeloid leukemia called acute promyelocytic leukemia might have problems with bleeding and blood clotting. They might have a nosebleed that won’t stop, or a cut that won’t stop oozing. They might also have calf swelling from a blood clot called a deep vein thrombosis (DVT) or chest pain and shortness of breath from a blood clot in the lung (called a pulmonary embolism or PE).
Bone or joint pain
Some people with acute myeloid leukemia have bone pain or joint pain caused by the buildup of leukemia cells in these areas.
Swelling in the abdomen
Leukemia cells may build up in the liver and spleen, making them larger. This may be noticed as a fullness or swelling of the belly. The lower ribs usually cover these organs, but when they are enlarged the doctor can feel them.
Symptoms caused by leukemia spread
Spread to the skin
If leukemia cells spread to the skin, they can cause lumps or spots that may look like common rashes. A tumor-like collection of acute myeloid leukemia cells under the skin or other parts of the body is called a chloroma, granulocytic sarcoma, or myeloid sarcoma. Rarely, acute myeloid leukemia will first appear as a chloroma, with no leukemia cells in the bone marrow.
Spread to the gums
Certain types of acute myeloid leukemia may spread to the gums, causing swelling, pain, and bleeding.
Spread to other organs
Less often, leukemia cells can spread to other organs. Spread to the brain and spinal cord can cause symptoms such as:
- Headaches
- Weakness
- Seizures
- Vomiting
- Trouble with balance
- Facial numbness
- Blurred vision
On rare occasions acute myeloid leukemia can spread to the eyes, testicles, kidneys, or other organs.
Enlarged lymph nodes
Rarely, acute myeloid leukemia can spread to lymph nodes (bean-sized collections of immune cells throughout the body), making them bigger. Affected nodes in the neck, groin, underarm areas, or above the collarbone may be felt as lumps under the skin.
Although any of the symptoms and signs above may be caused by acute myeloid leukemia, they can also be caused by other conditions. Still, if you have any of these problems, especially if they don’t go away or are getting worse, it’s important to see a doctor so the cause can be found and treated, if needed.
Acute myeloid leukaemia complications
If you have acute myeloid leukaemia (acute myeloid leukemia), you may experience a number of complications. These can be caused by the condition itself, although they can also occur as a side effect of treatment.
Weakened immune system
Having a weakened immune system – being immunocompromised – is a common complication of acute myeloid leukemia.
Even if your blood is restored to normal working order with treatment, many of the medications that are used to treat acute myeloid leukemia can temporarily weaken your immune system.
This means you’re more vulnerable to developing an infection, and any infection you develop could be more serious than usual. Complications arising from infection are the leading cause of death in people with acute myeloid leukemia. However, if treated early, nearly all infections respond to appropriate treatment.
Therefore, you may be advised to:
- take regular doses of antibiotics to prevent bacterial infections
- maintain good personal and dental hygiene
- avoid contact with anyone who’s known to have an infection – even if it’s a type of infection that you were previously immune to, such as chickenpox or measles
- check with your doctor to ensure that all of your vaccinations are up to date, although you won’t be able to have any vaccine that contains “live” viruses or bacteria, such as the shingles vaccine and MMR vaccine (against measles, mumps and rubella)
Report any possible symptoms of an infection to your treatment unit immediately because prompt treatment may be needed to prevent complications.
Symptoms of an infection can include:
- a high temperature (fever)
- a headache
- aching muscles
- diarrhea
- tiredness
Bleeding
If you have acute myeloid leukemia, you’ll bleed and bruise more easily due to the low levels of platelets (clot-forming cells) in your blood. Bleeding may also be excessive.
People with advanced acute myeloid leukemia are more vulnerable to excessive bleeding inside their body, which is the second most common cause of death in people with the condition.
Serious bleeding can occur:
- inside the skull (intracranial hemorrhage) – causing symptoms such as a severe headache, stiff neck, vomiting and confusion
- inside the lungs (pulmonary hemorrhage) – causing symptoms such as coughing up blood, breathing difficulties and a bluish skin tone (cyanosis)
- inside the stomach (gastrointestinal hemorrhage) – causing symptoms such as vomiting blood and passing stools (feces) that are very dark or tar-like in color
All these types of hemorrhage should be regarded as medical emergencies. Call your local emergency services number immediately and ask for an ambulance if you think a hemorrhage is occurring.
Infertility
Many of the treatments that are used to treat acute myeloid leukemia can cause infertility. This is often temporary, but in some cases can be permanent.
People particularly at risk of permanent infertility are those who have received high doses of chemotherapy and radiotherapy in preparation for a bone marrow or stem cell transplant.
Your treatment team can give a good estimation of the risk of infertility in your specific circumstances.
It may be possible to guard against any risk of infertility before you begin your treatment. For example, men can have their sperm samples stored. Similarly, women can have eggs or fertilised embryos stored, which can then be placed back into their womb, following treatment.
However, as acute myeloid leukemia is an aggressive condition that develops rapidly, there may not always be time to do this before treatment needs to start.
Acute myeloid leukemia diagnosis
If you have signs or symptoms of acute myeloid leukemia, your doctor may recommend that you undergo diagnostic tests, including:
Blood tests
Most people with acute myeloid leukemia have too many white blood cells, not enough red blood cells and not enough platelets. The presence of blast cells — immature cells normally found in bone marrow but not circulating in the blood — is another indicator of acute myeloid leukemia.
Bone marrow test
A blood test can suggest leukemia, but it usually takes a bone marrow test to confirm the diagnosis.
Leukemia starts in the bone marrow, so checking the bone marrow for leukemia cells is a key part of testing for it. Bone marrow samples are obtained from 2 tests that are usually done at the same time:
- Bone marrow aspiration
- Bone marrow biopsy
The samples are usually taken from the back of the pelvic (hip) bone, but sometimes other bones are used instead. If only an aspiration is to be done, it may be taken from the sternum (breast bone).
For a bone marrow aspiration, you lie on a table (either on your side or on your belly). The doctor will clean the skin over the hip and then numb the area and the surface of the bone by injecting a local anesthetic. This may cause a brief stinging or burning sensation. A thin, hollow needle is then inserted into the bone, and a syringe is used to suck out a small amount of liquid bone marrow. Even with the anesthetic, most patients still have some brief pain when the marrow is removed.
A bone marrow biopsy is usually done just after the aspiration. A small piece of bone and marrow is removed with a slightly larger needle that is pushed down into the bone. This may also cause some brief pain. Once the biopsy is done, pressure will be applied to the site to help prevent bleeding.
These bone marrow tests are used to help diagnose leukemia, but they might also be repeated later to tell if the leukemia is responding to treatment.
Lumbar puncture (spinal tap)
In some cases, it may be necessary to remove some of the fluid around your spinal cord to check for leukemia cells. Your doctor can collect this fluid by inserting a small needle into the spinal canal in your lower back.
Lab tests used to diagnose and classify acute myeloid leukemia
One or more of the following lab tests may be done on the samples to diagnose acute myeloid leukemia and/or to determine the specific subtype of acute myeloid leukemia.
Complete blood count and peripheral blood smear
The complete blood count (CBC) is a test that measures the amounts of different cells in the blood, such as the red blood cells, white blood cells, and platelets. The CBC is often done along with a differential (or diff), which looks at the numbers of the different types of white blood cells. For the peripheral blood smear, a sample of blood is looked at under the microscope. Changes in the numbers and the appearance of different types of blood cells often help diagnose leukemia.
Most patients with acute myeloid leukemia have too many immature white cells in their blood, and not enough red blood cells or platelets. Many of the white blood cells may be myeloblasts (often just called blasts), which are very early forms of blood-forming cells that are not normally found in the blood. These cells don’t work like normal, mature white blood cells. These findings may suggest leukemia, but the disease usually is not diagnosed without looking at a sample of bone marrow cells.
Blood chemistry and coagulation tests
These tests measure the amounts of certain chemicals in the blood and the ability of the blood to clot. These tests are not used to diagnose leukemia, but they can help detect liver or kidney problems, abnormal levels of certain minerals in the blood, or problems with blood clotting.
Routine cell exams by microscope
Samples of blood, bone marrow, or CSF are looked at under a microscope by a pathologist (a doctor specializing in lab tests) and may be reviewed by the patient’s hematologist/oncologist (a doctor specializing in cancer and blood diseases).
The doctors will look at the size, shape, and other traits of the white blood cells in the samples to classify them into specific types.
A key element is whether the cells look mature (like normal blood cells) or immature (lacking features of normal blood cells). The most immature cells are called myeloblasts (or blasts).
The percentage of blasts in the bone marrow or blood is particularly important. Having at least 20% blasts in the marrow or blood is generally required for a diagnosis of acute myeloid leukemia. (In normal bone marrow, the blast count is 5% or less, while the blood usually doesn’t contain any blasts.) acute myeloid leukemia can also be diagnosed if the blasts are found (using another test) to have a chromosome change that occurs only in a specific type of acute myeloid leukemia, even if the blast percentage doesn’t reach 20%.
Sometimes just counting and looking at the cells isn’t enough to provide a clear diagnosis. Other lab tests may be used to confirm an acute myeloid leukemia diagnosis.
Cytochemistry
For cytochemistry tests, cells are exposed to chemical stains (dyes) that react with only some types of leukemia cells. These stains cause color changes that can be seen under a microscope, which can help the doctor determine what types of cells are present. For instance, one stain can help distinguish acute myeloid leukemia cells from acute lymphocytic leukemia (ALL) cells. The stain causes the granules of most acute myeloid leukemia cells to appear as black spots under the microscope, but it does not causeacute lymphocytic leukemia cells to change colors.
Flow cytometry and immunohistochemistry
For both flow cytometry and immunocytochemistry, samples of cells are treated with antibodies, which are proteins that stick only to certain other proteins on cells. For immunocytochemistry, the cells are then looked at under a microscope to see if the antibodies stuck to them (meaning they have these proteins), while for flow cytometry a special machine is used.
These tests are used for immunophenotyping – classifying leukemia cells according to the substances (antigens) on their surfaces. Leukemia cells can have different antigens depending on which type of cells they start in and how mature they are, and this information can be helpful in acute myeloid leukemia classification.
Chromosome tests
These tests look at the chromosomes (long strands of DNA) inside the cells. Normal human cells contain 23 pairs of chromosomes, each of which are a certain size and stain a certain way. acute myeloid leukemia cells sometimes have chromosome changes that can be seen under a microscope or found with other tests. Recognizing these changes can help identify certain types of acute myeloid leukemia and can be important in determining a patient’s outlook.
Cytogenetics: In this test, the cells are looked at under a microscope to see if the chromosomes have any abnormalities. A drawback of this test is that it usually takes about 2 to 3 weeks because the cells must grow in lab dishes for a couple of weeks before their chromosomes can be viewed.
The results of cytogenetic testing are written in a shorthand form that describes the chromosome changes:
- A translocation means parts of two chromosomes have traded places with each other. For example, if chromosomes 8 and 21 have swapped pieces, it would be written as t(8;21).
- An inversion, written as inv(16), for example, means that part of the chromosome 16 is now in reverse order but is still attached to the chromosome.
- A deletion, written as del(7) or -7, for example, indicates part of chromosome 7 has been lost.
- An addition or duplication, such as +8, for example, means that all or part of chromosome 8 has been duplicated, and too many copies of it are found within the cell.
Not all chromosome changes can be seen under a microscope. Other lab tests can often detect these changes.
Fluorescent in situ hybridization (FISH): This test looks more closely at cell DNA using special fluorescent dyes that only attach to specific genes or parts of particular chromosomes. FISH can find the chromosome changes (such as translocations) that are visible under a microscope in standard cytogenetic tests, as well as some changes too small to be seen with usual cytogenetic testing.
FISH can be used to look for changes in specific genes or parts of chromosomes. It can be used on regular blood or bone marrow samples without growing them in a lab first. This means the results are often available more quickly than with regular cytogenetic testing.
Polymerase chain reaction (PCR): This is a very sensitive test that can also find some gene and chromosome changes too small to be seen under a microscope. It is helpful in finding gene changes that are in only a few cells, making it good for finding small numbers of leukemia cells in a sample (like after treatment).
Other molecular and genetic tests
Other, newer types of lab tests can also be done on the samples to look for specific gene or other changes in the leukemia cells.
Imaging tests for acute myeloid leukemia
Imaging tests use x-rays, sound waves, magnetic fields, or radioactive particles to create pictures of the inside of the body. Leukemia doesn’t usually form tumors, so imaging tests aren’t often helpful in making the diagnosis. When imaging tests are done in people with acute myeloid leukemia, it’s most often to look for infections or other problems, rather than to look for leukemia itself. In a few cases, imaging tests may be done to help determine the extent of the disease, if it’s thought it might have spread beyond the bone marrow and blood.
X-rays
Routine chest x-rays may be done if a lung infection is suspected.
Computed tomography (CT) scan
A CT scan uses x-rays to make detailed, cross-sectional images of your body. This test can help show if any lymph nodes or organs in your body are enlarged. It isn’t usually needed to diagnose acute myeloid leukemia, but it may be done if your doctor suspects the leukemia is growing in an organ, like your spleen.
CT-guided needle biopsy: In some cases, a CT can be used to guide a biopsy needle into a suspected abnormality, such as an abscess. For this procedure, you lie on the CT scanning table while the doctor moves a biopsy needle through the skin and toward the mass. CT scans are repeated until the needle is within the mass. A sample is then removed and sent to the lab to be looked at under a microscope.
PET/CT: Some machines combine the CT scan with a PET scan (PET/CT scan). For a PET scan, glucose (a form of sugar) containing a radioactive atom is injected into the blood. Because cancer cells in the body grow rapidly, they absorb large amounts of the radioactive sugar. A special camera can then create a picture of areas of radioactivity in the body. With a PET/CT scan, the doctor can compare areas of higher radioactivity on the PET scan with the more detailed appearance of that area on the CT.
Magnetic resonance imaging (MRI) scan
Like CT scans, MRI scans make detailed images of soft tissues in the body. But MRI scans use radio waves and strong magnets instead of x-rays.
MRI scans are very helpful in looking at the brain and spinal cord, but they are not usually needed in people with acute myeloid leukemia.
Ultrasound
Ultrasound uses sound waves and their echoes to make pictures of internal organs or masses.
Ultrasound can be used to look at lymph nodes near the surface of the body or to look inside your abdomen for enlarged lymph nodes or organs such as the liver, spleen, and kidneys. (It can’t be used to look inside the chest because the ribs block the sound waves.) It is sometimes used to help guide a biopsy needle into an enlarged lymph node.
Determining your acute myeloid leukemia subtype
If your doctor determines that you have acute myeloid leukemia (AML), you may need further tests to determine the extent of the cancer and classify it into a more specific acute myeloid leukemia subtype. Your acute myeloid leukemia subtype is based on how your cells appear when examined under a microscope. Special laboratory testing also may be used to identify the specific characteristics of your cells.
Your AML subtype helps determine which treatments may be best for you. Based on your AML subtype, the doctor will decide which drugs, drug combinations and drug dosages are indicated, and will determine the appropriate duration of treatment. Doctors are studying how different types of cancer treatment affect people with different AML subtypes.
Morphology
Acute myeloid leukemia (AML) is first described by its morphology, or what the cancerous cells look like under the microscope. acute myeloid leukemia is classified by the type of normal, immature white blood cell it most closely resembles.
Most people with AML have a subtype called myeloid leukemia, which means the cancer is in the cells that normally produce neutrophils. Other patients have a type of AML called monoblastic or monocytic leukemia. In monocytic leukemia, the cells look like white blood cells called monocytes. Leukemia cells can also be a mixture of myeloblastic and monocytic cells.
Sometimes AML seems to come from cells that produce red blood cells, called erythroid, or platelets, called megakaryocytic.
Acute promyelocytic leukemia (APL) is a unique subtype of AML where the cancer cell stops maturing when the cell is at a stage called the promyelocyte or progranulocyte stage. APL is associated with a translocation between chromosomes 15 and 17 [t(15;17)].
Acute Promyelocytic Leukemia (APL)
Acute Promyelocytic Leukemia (APL) is an aggressive subtype of AML is associated with potentially life-threatening simultaneous bleeding and clotting complications. While in the past acute promyelocytic leukemia (APL) was nearly always fatal, it is now one of the most curable subtypes of AML in adults, if it is diagnosed early and treated appropriately. Acute Promyelocytic Leukemia (APL) accounts for approximately 10 percent of all AML cases and occurs primarily in middle-aged adults, although it can occur at any age. Acute Promyelocytic Leukemia (APL) can also develop after receiving chemotherapy for another disease.
Acute Promyelocytic Leukemia (APL) is due to a translocation between chromosomes 15 and 17, abbreviated t(15;17). A translocation is a genetic change in which a piece of one chromosome breaks off and attaches to another chromosome. In APL, an abnormal fusion gene called PML/RARα forms as a result of the translocation. This mutated gene leads to the production of a protein that causes blood cells to get stuck in the promyelocytic stage, unable to develop into mature white blood cells. A diagnosis of APL depends upon confirmation of t(15;17) in the patient’s AML cells.
In people with acute promyelocytic leukemia (APL), immature white blood cells called promyelocytes build up in the bone marrow. The overproduction of promyelocytes leads to a shortage of normal white blood cells, red blood cells and platelets. People with acute promyelocytic leukemia (APL) are particularly susceptible to bruising and excessive bleeding. This occurs in part because of the low number of platelets in the blood and also because the leukemia cells release substances that alter the balance between bleeding and clotting.
Treatment for acute promyelocytic leukemia (APL) differs from that of the other AML subtypes. Many people with acute promyelocytic leukemia (APL) are treated with a drug called all-trans-retinoic acid (ATRA) in combination with arsenic trioxide (Trisenox) or, in high-risk cases, chemotherapy.
Cytogenetics
Acute myeloid leukemia (AML) is also classified by the cytogenetic, or chromosome, changes found in the leukemia cells. Certain chromosomal changes are closely matched with the morphology of the AML cells. More importantly, the chromosomal changes help doctors determine the best treatment options because these changes can sometimes predict how well intensive treatment will work. Chromosomal changes are commonly grouped according to the likelihood that treatment will work against the subtype of AML.
All chromosomes are numbered from 1 to 22. And, sex chromosomes are called “X” or “Y.” The letters “p” and “q” refer to the “arms” or specific areas of the chromosome. Some of the types of genetic changes found in AML include:
- A translocation, which means that a chromosome breaks off and reattaches to another chromosome
- Extra copies of a chromosome
- A deletion of a chromosome
Some of the most common chromosomal changes are grouped as follows:
- Favorable. Chromosomal changes associated with more successful treatment include abnormalities of chromosome 16 at bands p13 and q22 [t(16;16)(p13;q22), inv(16)(p13q22)] and a translocation between chromosomes 8 and 21 [t(8;21)].
- Intermediate. Changes associated with a less favorable prognosis include normal chromosomes, where no changes are found and a translocation between chromosomes 9 and 11 [t(9;11)]. Many other subtypes are considered part of this group, particularly those with 1 or more specific molecular changes. Sometimes, extra copies of chromosome 8 or trisomy 8 may be classified as intermediate risk over unfavorable (see below).
- Unfavorable. Examples of chromosomal changes that are associated with less successful treatment or with a low chance of curing the AML include extra copies of chromosomes 8 or 13 [for example, trisomy 8 (+8)], deletion of all or part of chromosomes 5 or 7, complex changes on many chromosomes, and changes to chromosome 3 at band q26.
In general, the favorable changes occur more commonly in younger patients, while the unfavorable changes are more common in people older than 60. How well treatment works still varies widely in each of these groups. Treatment is successful in the long term for 50% to 60% of patients younger than 60 with AML that is classified as favorable and for less than 10% of patients younger than 60 with AML that is classified as unfavorable. Prognosis in patients older than 60 years of age is significantly worse. How well treatment works also depends on other factors, including the number of white blood cells. It is not possible to predict exactly the likelihood of successful treatment for a person with AML.
Molecular changes
Testing for molecular changes at diagnosis helps determine a patient’s treatment options. For example, patients with changes in the NPM1 or CEBPalpha genes have a better long-term outcome, while chemotherapy does not work as well for patients with changes in the FLT3 gene. Other genetic changes linked to prognosis for people with AML include:
- RUNX1
- ASX11
- P53
- IDH1 and IDH2
Acute myeloid leukemia stages
For most types of cancer, determining the stage (extent) of the cancer is very important. The stage is based on the size of the main tumor and how far the cancer has spread. This can be helpful in predicting a person’s outlook and deciding on treatment.
Acute myeloid leukemia, on the other hand, does not usually form tumors. Acute myeloid leukemia generally is widespread throughout the bone marrow and, in some cases, has spread to other organs, such as the liver and spleen. Therefore acute myeloid leukemia is not staged like most other cancers. The outlook for a person with acute myeloid leukemia depends instead on other information, such as the subtype of acute myeloid leukemia (determined by lab tests), the patient’s age, and other lab test results.
Knowing the subtype of acute myeloid leukemia can be very important, as it sometimes affects both a patient’s outlook and the best treatment. For example, the acute promyelocytic leukemia subtype is often treated using drugs that are different from those used for other subtypes of acute myeloid leukemia. If you’re not sure which subtype of acute myeloid leukemia you have, ask your doctor about it, and about how it might affect your treatment.
Two of the main systems that have been used to classify acute myeloid leukemia into subtypes are the French-American-British (FAB) classification and the newer World Health Organization (WHO) classification.
The World Health Organization (WHO) classification is the main system used to classify AML into subtypes . It includes prognostic (predictive) factors, such as chromosomal abnormalities and genetic mutations, which are known to affect the future outcome of the cancer. These genetic factors help provide patients and their doctors with more reliable information regarding their probable outcome (prognosis), as well as how they are likely to respond to treatment.
French-American-British (FAB) classification of acute myeloid leukemia
In the 1970s, a group of French, American, and British leukemia experts divided acute myeloid leukemia into subtypes, M0 through M7, based on the type of cell the leukemia develops from and how mature the cells are. This was based largely on how the leukemia cells looked under the microscope after routine staining.
Subtypes M0 through M5 all start in immature forms of white blood cells. M6 AML starts in very immature forms of red blood cells, while M7 AML starts in immature forms of cells that make platelets.
Table 1. French-American-British (FAB) classification of acute myeloid leukemia
| FAB subtype | Name |
|---|---|
| M0 | Undifferentiated acute myeloblastic leukemia |
| M1 | Acute myeloblastic leukemia with minimal maturation |
| M2 | Acute myeloblastic leukemia with maturation |
| M3 | Acute promyelocytic leukemia (APL) |
| M4 | Acute myelomonocytic leukemia |
| M4 eos | Acute myelomonocytic leukemia with eosinophilia |
| M5 | Acute monocytic leukemia |
| M6 | Acute erythroid leukemia |
| M7 | Acute megakaryoblastic leukemia |
World Health Organization (WHO) classification of acute myeloid leukemia
The French-American-British (FAB) classification system can be useful, but it doesn’t take into account many of the factors that are now known to affect prognosis (outlook). The World Health Organization (WHO) system, most recently updated in 2016, includes some of these factors to try to better classify acute myeloid leukemia.
The WHO system divides acute myeloid leukemia into several groups:
- Acute myeloid leukemia with certain genetic abnormalities (gene or chromosome changes)
- Acute myeloid leukemia with a translocation between chromosomes 8 and 21 [t(8;21)]
- Acute myeloid leukemia with a translocation or inversion in chromosome 16 [t(16;16) or inv(16)]
- Acute promyelocytic leukemia (APL) with the PML-RARA fusion gene
- Acute myeloid leukemia with a translocation between chromosomes 9 and 11 [t(9;11)]
- Acute myeloid leukemia with a translocation between chromosomes 6 and 9 [t(6:9)]
- Acute myeloid leukemia with a translocation or inversion in chromosome 3 [t(3;3) or inv(3)]
- Acute myeloid leukemia (megakaryoblastic) with a translocation between chromosomes 1 and 22 [t(1:22)]
- Acute myeloid leukemia with the BCR-ABL1 (BCR-ABL) fusion gene*
- Acute myeloid leukemia with mutated NPM1 gene
- Acute myeloid leukemia with biallelic mutations of the CEBPA gene (that is, mutations in both copies of the gene)
- Acute myeloid leukemia with mutated RUNX1 gene.*
- NOTE: *This is still a “provisional entity,” meaning it’s not yet clear if there’s enough evidence that it’s a unique group.
- Acute myeloid leukemia with myelodysplasia-related changes
- Acute myeloid leukemia related to previous chemotherapy or radiation
- Acute myeloid leukemia not otherwise specified (This includes cases of acute myeloid leukemia that don’t fall into one of the above groups, and is similar to the FAB classification.)
- Acute myeloid leukemia with minimal differentiation (FAB M0)
- Acute myeloid leukemia without maturation (FAB M1)
- Acute myeloid leukemia with maturation (FAB M2)
- Acute myelomonocytic leukemia (FAB M4)
- Acute monoblastic/monocytic leukemia (FAB M5)
- Pure erythroid leukemia (FAB M6)
- Acute megakaryoblastic leukemia (FAB M7)
- Acute basophilic leukemia
- Acute panmyelosis with fibrosis
- Myeloid sarcoma (also known as granulocytic sarcoma or chloroma)
- Myeloid proliferations related to Down syndrome
- Undifferentiated and biphenotypic acute leukemias are not strictly acute myeloid leukemia, but are leukemias that have both lymphocytic and myeloid features. They are sometimes called mixed phenotype acute leukemias.
Prognostic factors for acute myeloid leukemia
Certain factors can affect the prognosis of patients with acute myeloid leukemia, meaning the likely outcome (a person’s prognosis) of their disease. Doctors use “prognostic factors” to help predict how a patient’s disease is likely to respond to treatment. Prognostic factors also help doctors determine a person’s risk of the leukemia coming back after treatment, and therefore if they should get more or less intensive treatment. Some prognostic factors are called “favorable risk factors” because they are associated with a lower risk of relapse after treatment. Others are called “poor/adverse risk factors” because they are associated with a higher risk of relapse after treatment.
The subtype of acute myeloid leukemia can be important in helping to determine a person’s prognosis (outlook). But other factors can also affect why some patients with acute myeloid leukemia have a better outlook than others.
Other types of cancer use numerical stages to indicate your prognosis and whether your cancer has spread, but there are no stages of acute myelogenous leukemia.
Instead, the seriousness of your condition is determined by:
- AML subtype. Chromosomal and genetic abnormalities are the most significant prognostic factors in people with AML.
- Your age. AML occurs mostly in older adults; the median age at diagnosis is 67-70 years. Acute myeloid leukemia (AML) patients are considered to be “young” if they are younger than age 60. Usually, the older the patient, the poorer the prognosis. Unfavorable genetic abnormalities increase with age. Additionally, older patients sometimes have comorbidities (other medical conditions) that can make it difficult for them to tolerate intense chemotherapy treatments.
- Your overall health
- Chromosome (cytogenetic) abnormalities
- Gene mutations
- Markers on the leukemia cells
- Results from other tests and procedures, such as the number of white blood cells found in a blood sample. A high white blood cell count ≥ 40,000/mcL at the time of diagnosis is an adverse risk factor for long-term remission.
- Status of acute myeloid leukemia after treatment. Patients who do not achieve a remission after one cycle of induction therapy have a poorer prognosis.
- Refractory AML. Patients with AML who failed to respond to the current standard treatment have a poorer prognosis.
- Relapsed AML. Patients with AML that has been treated before and relapsed (come back) have a poorer prognosis.
- Therapy-Related AML. People who received chemotherapy in the past to treat a different type of cancer may develop acute myeloid leukemia. This is known as therapy-related or treatment-related AML. In these cases, the disease is more resistant to treatment and is associated with a poorer prognosis.
- Prior blood cancer. In patients who have had a prior blood cancer, such as a myelodysplastic syndrome (MDS) or a myeloproliferative neoplasm, acute myeloid leukemia is associated
with a poorer prognosis. - Central Nervous System (CNS) Involvement and/or Extramedullary Disease. Acute myeloid leukemia (AML) can be more difficult to treat when leukemia cells have spread to the central nervous system (the area around the brain and spine) or other areas of the body outside of the bone marrow and blood. Intrathecal therapy and other potential interventions are needed to control the disease in these cases.
Chromosome (cytogenetic) abnormalities
Acute myeloid leukemia cells can have many kinds of chromosome changes, some of which can affect a person’s prognosis. Those listed below are some of the most common, but there are many others. Not all leukemias have these abnormalities. Patients whose acute myeloid leukemia doesn’t have any of these usually have an outlook that is between favorable and unfavorable.
Favorable abnormalities:
- Translocation between chromosomes 8 and 21 (seen most often in patients with M2)
- Translocation or inversion of chromosome 16
- Translocation between chromosomes 15 and 17 (seen most often in patients with M3)
Unfavorable abnormalities:
- Deletion (loss) of part of chromosome 5 or 7
- Translocation or inversion of chromosome 3
- Translocation between chromosomes 6 and 9
- Translocation between chromosomes 9 and 22
- Abnormalities of chromosome 11 (at the spot q23)
- Loss of a chromosome, so the cell has only 1 copy instead of the normal 2 (known as monosomy)
- Complex changes (those involving 3 or more chromosomes)
Gene mutations
People whose leukemia cells have certain gene mutations may have a better or worse outlook.
For instance, people with acute myeloid leukemia that has a mutation in the FLT3 gene tend to have a poorer outlook, although new drugs that target cells with this abnormal gene might lead to better outcomes. Mutations in the TP53, RUNX1, and ASXL1 genes are also linked with a worse outlook.
On the other hand, people whose leukemia cells have changes in the NPM1 gene (and no other abnormalities) seem to have a better prognosis than people without this change. Changes in both copies of the CEBPA gene are also linked to a better outcome.
Markers on the leukemia cells
If the leukemia cells have the CD34 protein and/or the P-glycoprotein (MDR1 gene product) on their surface, it is linked to a worse outlook.
Age
Generally, people over 60 don’t do as well as younger people. Some of this may be because they are more likely to have unfavorable chromosome abnormalities. They sometimes also have other medical conditions that can make it harder for them to handle more intense chemotherapy regimens.
White blood cell count
A high white blood cell count (>100,000/mm3) at the time of diagnosis is linked to a worse outlook.
Prior blood disorder leading to acute myeloid leukemia
Having a prior blood disorder such as a myelodysplastic syndrome is linked to a worse outlook.
Acute myeloid leukemia that develops after a person is treated for another cancer is linked to a worse outlook.
Infection
Having a systemic (blood) infection when you are diagnosed is linked to a worse outlook.
Leukemia cells in the central nervous system
Leukemia that has spread to the area around the brain and spinal cord can be hard to treat, since most chemotherapy drugs can’t reach that area.
Status of acute myeloid leukemia after treatment
How well (and how quickly) the leukemia responds to treatment also affects long-term prognosis. Better initial responses have been linked with better long-term outcomes.
A remission (complete remission) is usually defined as having no evidence of disease after treatment. This means the bone marrow contains fewer than 5% blast cells, the blood cell counts are within normal limits, and there are no signs or symptoms from the leukemia. A complete molecular remission means there is no evidence of leukemia cells in the bone marrow, even when using very sensitive tests, such as PCR (polymerase chain reaction).
Minimal residual disease is a term used after treatment when leukemia cells can’t be found in the bone marrow using standard tests (such as looking at cells under a microscope), but more sensitive tests (such as flow cytometry or PCR) find evidence that there are still leukemia cells in the bone marrow.
Active disease means that either there is evidence that the leukemia is still present during treatment, or that the disease has come back after treatment (relapsed). For a patient to have relapsed, they must have more than 5% blast cells in their bone marrow.
Acute myeloid leukemia life expectancy
Generally with acute myeloid leukemia, around 20 out of 100 people (around 20%) will survive their leukemia for 5 years or more after their diagnosis.
Survival rates tell you what portion of people with the same type and stage of cancer are still alive a certain length of time (usually 5 years) after they were diagnosed. These numbers can’t tell you how long you will live, but they might help give you a better understanding about how likely it is that your treatment will be successful.
Statistics on the outlook for people with a certain type and stage of cancer are often given as 5-year survival rates, but many people live longer – often much longer – than 5 years. The 5-year survival rate is the percentage of people who live at least 5 years after being diagnosed with cancer. For example, a 5-year survival rate of 90% means that an estimated 90 out of 100 people who have that cancer are still alive 5 years after being diagnosed.
Relative survival rates are often a more accurate way to estimate the effect of cancer on survival. These rates compare people with adrenal cancer to people in the overall population. For example, if the 5-year relative survival rate for a specific type and stage of cancer is 90%, it means that people who have that cancer are, on average, about 90% as likely as people who don’t have that cancer to live for at least 5 years after being diagnosed.
But remember, the 5-year relative survival rates are estimates – your outlook can vary based on a number of factors specific to you.
The 5-year survival rate for people 20 and older with AML is 26%. For people younger than 20, the survival rate is 68%.
Note that, cancer survival rates don’t tell the whole story
Survival rates are often based on previous outcomes of large numbers of people who had the disease, but they can’t predict what will happen in any particular person’s case. There are a number of limitations to remember:
- The numbers below are among the most current available. But to get 5-year survival rates, doctors have to look at people who were treated at least 5 years ago. As treatments are improving over time, people who are now being diagnosed with adrenal cancer may have a better outlook than these statistics show.
- These statistics are based on the stage of the cancer when it was first diagnosed. They do not apply to cancers that come back later or spread, for example.
- Besides the cancer stage, many other factors can affect a person’s outlook, such as age and overall health, and how well the cancer responds to treatment.
Your doctor can tell you how these numbers may apply to you, as he or she is familiar with your situation.
Survival by age
Younger people tend to do much better than older people.
- In children aged 14 or younger, more than 65 out of 100 children (more than 65%) will survive their leukemia for 5 years or more after they are diagnosed.
- In people aged between 15 and 24, around 60 out of 100 people (around 60%) will survive their leukemia for 5 years or more after diagnosis.
- In people aged between 25 and 64, almost 40 out of 100 people (almost 40%) will survive their leukemia for 5 years or more after they are diagnosed.
- In people aged 65 or older, around 5 out of 100 people (around 5%) will survive their leukemia for 5 years or more after diagnosis.
Acute myeloid leukemia prognosis
With current treatment regimens, only a minority of acute myeloid leukemia patients are cured. Outcomes are particularly poor for older patients with low complete remission rates and there are few long-term survivors compared with the number of long-term survivors of younger patients. More than 50% of people with acute myeloid leukemia experience a return of cancer, called a relapse. If relapse occurs within the first year, then physicians may recommend that individuals participate in a clinical trial for a new treatment option. Another option is to try another round of induction therapy with a different chemotherapy regimen and new drugs. If a relapse is over a year later, then physicians may recommend an allogeneic stem cell transplant, or they may recommend repeating the chemotherapy used during induction therapy, or they may recommend both. People who experience a relapse or for whom further treatment is ineffective, may be encouraged to participate in a clinical trial.
What affects prognosis
Age
- Your age affects outlook. Younger people have a better prognosis.
Changes in genes
- Outlook is affected by changes in your genes. These are called cytogenetic tests.
- Some specific genetic abnormalities in your leukemia cells may make your leukemia harder to treat successfully.
How advanced the leukemia is
Survival is also affected by how advanced the leukemia is at diagnosis. If you have a high number of white blood cells in the blood at diagnosis, the outlook is poorer.
Changing from chronic to acute
The outcome depends on whether you had leukemia that changed (transformed) from a chronic form into an acute form. It can be more difficult to treat leukemia that has transformed, or if it has developed from a blood condition called myelodysplasia.
Secondary leukemia
It may also be harder to treat a leukemia that has developed after treatment for another cancer. This is called a secondary leukemia. It means that you developed leukemia after earlier chemotherapy damaged your bone marrow cells. This is rare, but it can happen. Secondary leukemia usually develops within 10 years of treatment for the first cancer.
How well leukemia responds to treatment
Your outlook is affected by how well the leukemia responds to treatment and how long it takes to get a remission. Remission means the leukemia is not active and doctors cannot find any sign of it. If it takes a long time to get your leukemia into remission, your leukemia may be more difficult to treat successfully.
Relapse
If acute myeloid leukemia comes back after initial treatment it is called relapsed leukemia. With relapsed acute myeloid leukemia, it is sometimes possible to get rid of all signs of the leukemia again (a second remission) with more chemotherapy.
Acute myeloid leukemia treatment
Typically, acute myeloid leukemia (AML) patients need to start treatment as soon as possible after diagnosis, because acute myeloid leukemia is an aggressive blood cancer that can be difficult to treat. However, if time allows, you may want to seek a second opinion from another doctor, as it may help you feel more confident about the recommended treatment plan. The second opinion should come from another hematologist-oncologist, preferably one who treats AML. This type of doctor will usually have the most knowledge and experience about the latest treatment options for AML.
In the past, a diagnosis of acute myeloid leukemia was generally considered a medical emergency, and treatment usually started as soon as the diagnosis was made. This often did not allow time for doctors to obtain the specific genetic profile of a patient’s leukemia prior to making treatment decisions. Preliminary research has recently found that waiting up to 7 days, in order to obtain genetic data and other laboratory test results on the AML cells, may be safe for the majority of patients. This is important in assigning patients to the best available treatment option for each patient before starting therapy.
Not everyone with AML receives the same treatment. Treatment of acute myeloid leukemia depends on several factors, including the subtype of the disease, your age, your overall health and your preferences. (The acute promyelocytic leukemia (APL) subtype of AML is treated differently.)
In general, treatment falls into two phases:
- Remission induction therapy. The purpose of the first phase of treatment is to kill the leukemia cells in your blood and bone marrow. However, remission induction usually doesn’t wipe out all of the leukemia cells, so you need further treatment to prevent the disease from returning.
- Consolidation therapy. Also called post-remission therapy, maintenance therapy or intensification, this is the second phase of treatment, which begins after leukemia is in remission. This phase of treatment is aimed at destroying the remaining leukemia cells. It’s considered crucial to decreasing the risk of relapse.
Therapies used in these phases include:
- Chemotherapy. Chemotherapy is the major form of remission induction therapy, though it can also be used for consolidation therapy. Chemotherapy uses chemicals to kill cancer cells in your body. People with acute myeloid leukemia generally stay in the hospital during chemotherapy treatments because the drugs destroy many normal blood cells in the process of killing leukemia cells. If the first cycle of chemotherapy doesn’t cause remission, it can be repeated.
- Targeted therapy. Targeted therapy uses drugs that attack specific vulnerabilities within your cancer cells. The drug midostaurin (Rydapt) stops the action of an enzyme within the leukemia cells and causes the cells to die. Midostaurin is only useful for people whose cancer cells have the FLT3 mutation. This drug is administered in pill form.
- Other drug therapy. Arsenic trioxide (Trisenox) and all-trans retinoic acid (ATRA) are anti-cancer drugs that can be used alone or in combination with chemotherapy for remission induction of a certain subtype of acute myeloid leukemia called promyelocytic leukemia. These drugs cause leukemia cells with a specific gene mutation to mature and die, or to stop dividing.
- Bone marrow transplant. A bone marrow transplant, also called a stem cell transplant, may be used for consolidation therapy. A bone marrow transplant helps re-establish healthy stem cells by replacing unhealthy bone marrow with leukemia-free stem cells that will regenerate healthy bone marrow. Prior to a bone marrow transplant, you receive very high doses of chemotherapy or radiation therapy to destroy your leukemia-producing bone marrow. Then you receive infusions of stem cells from a compatible donor (allogeneic transplant). You can also receive your own stem cells (autologous transplant) if you were previously in remission and had your healthy stem cells removed and stored for a future transplant.
- Clinical trials. Some people with leukemia choose to enroll in clinical trials to try experimental treatments or new combinations of known therapies.
Doctors often give the most intensive chemotherapy regimens to people younger than age 60. However, this age limit is just a guideline. Some older patients in good health may also benefit from intensive regimens or slightly less intensive treatments. For example, an AML patient age 63 with no other health issues may be treated as someone younger than age 60, while a person age 57 with serious health issues may be treated as someone age 60 years and older.
Pre-Treatment Tests
Before you start treatment, your doctor will perform tests to learn more about your overall health and your disease. Doctors use this information for treatment planning.
Blood chemistry profile
This blood test measures the levels of certain substances released into the blood by organs and tissues in the body. These substances include electrolytes (such as sodium, potassium and chloride), fats, proteins, glucose (blood sugar), uric acid and enzymes. The test findings indicate how well a person’s kidneys, liver and other organs are working. Although this test is not used to diagnose leukemia, if the results show that there is an abnormal amount of a particular substance in the blood, it may be a sign of disease or some other health problem. A blood chemistry profile also provides helpful information about any potential organ damage caused by leukemia cells or cancer treatments.
Human Leukocyte Antigen (HLA) typing
This blood test is done to identify certain proteins, called human leukocyte antigens (HLAs), found on the surface of most cells in the body. These proteins make up the body’s tissue type, which varies from person to person. They also play an important role in the body’s immune response to foreign substances by helping the body distinguish its own cells from foreign cells. An HLA test is done before allogeneic stem cell transplantation to find out if there is a tissue match between a potential donor and the patient receiving the transplant. While HLA typing is not used to diagnose leukemia, it is an important test for newly diagnosed AML patients if allogeneic stem cell transplantation is being considered as a treatment option.
Heart tests
Some chemotherapy drugs, such as the class of drugs called “anthracyclines,” can damage heart tissue. Because of this, your doctor may want to test your heart function before starting treatment. Examples of heart tests that may be given to AML patients include:
- Echocardiogram. In this test, a computerized image of the heart is created by bouncing sound waves off internal tissues or organs in the chest. An echocardiogram shows the size, shape and position of the heart, as well as its internal structures. It also shows if the heart is beating and pumping blood normally.
- Multigated Acquisition (MUGA) scan. For this test, patients receive a shot containing a radiotracer into a vein, and pictures of the heart are taken with a special camera. The pictures show the radiation being released by the radiotracer, making it possible to see how much blood the heart pumps with each beat.
Remission induction therapy
The first phase of treatment is called “induction.” This first phase of treatment is aimed at quickly getting rid of as many leukemia cells as possible. The initial treatment you have for acute myeloid leukemia will largely depend on whether you’re fit enough to have intensive chemotherapy, or whether treatment at a lower dosage is recommended.
Doctors often give the most intensive chemotherapy to people under the age of 60, but some older patients in good health may benefit from similar or slightly less intensive treatment.
People who are much older or are in poor health might not do well with intensive chemo.
Below are some chemotherapy drugs that are often used to treat AML:
- Cytarabine (cytosine arabinoside, ara-C; Cytosar-U) is Food and Drug Administration (FDA)-approved to be used alone or with other chemotherapy drugs to treat certain types of leukemia including AML.
- Daunorubicin (Cerubidine) is FDA-approved to be used with other chemotherapy drugs to treat AML.
- Idarubicin (Idamycin) is FDA-approved to treat AML in combination with other chemotherapy drugs.
- Daunorubicin and cytarabine (Vyxeos) is a liposomal formulation of daunorubicin and cytarabine, which is indicated for the treatment of newly-diagnosed therapy-related AML or AML with myelodysplasia-related changes in adults and pediatric patients 1 year and older. A liposomal medication contains the active drug inside small, fat-like particles. This special fatty preparation allows more medication to reach its target (the bone marrow) and stay in the bone marrow to kill leukemia cells.
- Azacitidine (Onureg) is given by mouth and used for continued treatment of adult patients with AML who achieved first complete remission or complete remission with incomplete blood count recovery following intensive induction chemotherapy and are not able to complete intensive curative therapy.
- Azacitidine (Vidaza) and decitabine (Dacogen, Inqovi) are FDA-approved to treat myelodysplastic syndrome (MDS), another type of blood cancer. They are not FDA- approved to treat AML, but they are commonly used as an off-label treatment for AML.
Targeted therapy is a type of treatment that uses drugs or other substances to identify and attack specific types of cancer cells with less harm to normal cells. Not all cancers have the same targets. Each type of targeted therapy works a little bit differently, but they all interfere with the growth and survival of cancer cells. To find the most effective treatment, your doctor may run tests to identify the genes, proteins and other factors in your cancer cells. This helps the doctor to choose the most effective treatment for you based on the specific factors of your disease. Targeted therapy may be used alone or in combination with chemotherapy. Some types of targeted therapy include:
- FLT3 Inhibitors. Approximately one-third of AML patients have a mutation in the FLT3 gene that can increase the growth and division of AML cells. Patients with FLT3 mutations have a poor prognosis. FLT3 inhibitors are drugs that target these gene mutations. For these patients, the following targeted treatments are approved by the Food and Drug Administration (FDA):
- Midostaurin (Rydapt), taken by mouth, is for the treatment of adult patients with newly diagnosed AML that is FLT3 mutation-positive as detected by an FDA-approved test, in combination with standard cytarabine and daunorubicin induction and cytarabine consolidation.
- Gilteritinib (Xospata), taken by mouth, is for the treatment of adult patients who have relapsed or refractory AML with an FLT3 mutation as detected by an FDA-approved test.
- Other FLT3 inhibitors being studied in clinical trials for the treatment of AML include sorafenib (Nexavar), quizartinib (AC-220) and crenolanib.
- IDH Inhibitors. In some people with AML, the leukemia cells have a mutation in the IDH1 or IDH2 gene. These mutations cause cells to remain immature and divide and multiply too quickly. For these patients, the following FDA-approved targeted therapy may be used:
- Ivosidenib (Tibsovo), taken by mouth, is an IDH1 inhibitor indicated for the treatment of AML with a susceptible IDH1 mutation as detected by an FDA-approved test in:
- Adult patients with newly-diagnosed AML who are age 75 and older or who have comorbidities that preclude the use of intensive induction chemotherapy.
- Adult patients with relapsed or refractory AML.
- Enasidenib (Idhifa), a pill taken by mouth, is an IDH2 inhibitor indicated for the treatment of adult patients with relapsed or refractory AML with an IDH2 mutation as detected by an FDA-approved test.
- Ivosidenib (Tibsovo), taken by mouth, is an IDH1 inhibitor indicated for the treatment of AML with a susceptible IDH1 mutation as detected by an FDA-approved test in:
- BCL2 Inhibitors. Overexpression of the BCL2 protein allows cancer cells to evade “programmed cell death,” meaning it helps them live longer than they should. BCL2 inhibitors target the BCL2 protein. This helps restore what is called apoptosis, a process of natural cell death that is disrupted when you have cancer, restoring the body’s natural ability to tell cancer cells to die. Once this process is restored, your body can begin to kill cancer cells. With fewer cancer cells, there is room for healthy blood cells to grow in the bone marrow.
- Venetoclax (Venclexta) is an oral medicine taken by mouth each day. It is FDA-approved in combination with azacitidine, or decitabine, or low-dose cytarabine for the treatment of newly diagnosed AML in adults 75 years or older, or who have comorbidities that preclude the use of intensive induction chemotherapy. This drug binds to the leukemia cells and triggers apoptosis, a process that causes the cells to die.
- CD33 Targeted Therapy. Gemtuzumab ozogamicin (Mylotarg) is a targeted therapy linked to the chemotherapy drug calicheamicin. It binds to and then enters cells that have the CD33 protein on their surface. Once inside, it releases the toxin that kills the cells. More than 90% of AML cells have CD33 on their surface, while mature blood cells do not (and so are not as affected by the treatment). Gemtuzumab ozogamicin is FDA-approved for the treatment of newly-diagnosed CD33-positive AML in adults, as well as relapsed or refractory CD33-positive AML in adults and pediatric patients 2 years and older. This medication is injected slowly into a vein through a needle. It is given in cycles, consisting of treatment days followed by days of rest.
- Hedgehog Pathway Inhibitor. The hedgehog pathway is essential for normal embryonic development. In adults, however, abnormal activation of this pathway is thought to contribute to the development and proliferation of cancer stem cells. Research studies have shown that disruption of this pathway can decrease the number of these cancer stem cells in the bone marrow.
- Glasdegib (Daurismo), given by mouth, is FDA approved in combination with low-dose cytarabine, for the treatment of newly diagnosed AML in adult patients who are 75 and older, or who have comorbidities that preclude the use of intensive induction chemotherapy.
In younger patients, such as those under 60, induction therapy often involves treatment with 2 chemo drugs:
- Cytarabine (cytosine arabinoside, ara-C; Cytosar-U)
- An anthracycline drug such as daunorubicin (Cerubidine) or idarubicin (Idamycin)
This is sometimes called a 7 + 3 regimen, because it consists of getting cytarabine continuously for 7 days, along with short infusions of an anthracycline on each of the first 3 days.
In some situations, a third drug might be added as well to try to improve the chances of remission:
- For patients whose leukemia cells have an FLT3 gene mutation, the targeted therapy drug midostaurin (Rydapt) might be given along with chemo. This drug is taken twice daily as a pill.
- For patients whose leukemia cells have the CD33 protein, the targeted drug gemtuzumab ozogamicin (Mylotarg) might be added to chemo.
- Adding the chemo drug cladribine might be another option for some people.
Patients with poor heart function might not be able to be treated with anthracyclines, so they may be treated with another chemo drug, such as fludarabine (Fludara) or etoposide.
In rare cases where the leukemia has spread to the brain or spinal cord, chemo may also be given into the cerebrospinal fluid (CSF). Radiation therapy might be used as well.
Patients typically need to stay in the hospital during induction (and possibly for some time afterward). Induction destroys most of the normal bone marrow cells as well as the leukemia cells, so most patients develop dangerously low blood counts, and may be very ill. Most patients need antibiotics and blood product transfusions. Drugs to raise white blood cell counts (called growth factors) may also be used. Blood counts tend to stay low for a few weeks.
About a week after chemo is done, the doctor will do a bone marrow biopsy. It should show few bone marrow cells (hypocellular bone marrow) and only a small portion of blasts (making up no more than 5% of the bone marrow) for the leukemia to be considered in remission. Most people with leukemia go into remission after the first round of chemo. But if the biopsy shows that there are still leukemia cells in the bone marrow, another round of chemo may be given, either with the same drugs or with another regimen. Sometimes a stem cell transplant is recommended at this point. If it isn’t clear on the bone marrow biopsy whether the leukemia is still there, another bone marrow biopsy may be done again in about a week.
Over the next few weeks, normal bone marrow cells will return and start making new blood cells. The doctor may do other bone marrow biopsies during this time. When the blood cell counts recover, the doctor will again check cells in a bone marrow sample to see if the leukemia is in remission.
Remission induction usually does not destroy all the leukemia cells, and a small number often remain. Without post-remission therapy (consolidation), the leukemia is likely to return within several months.
Intensive chemotherapy
If you can have intensive induction chemotherapy, you’ll usually be given a combination of chemotherapy medication at a high dose to kill the cancerous cells in your blood and bone marrow.
This stage of treatment will be carried out in hospital or in a specialist center, as you’ll need very close medical and nursing supervision.
You’ll have regular blood transfusions because your blood won’t contain enough healthy blood cells.
You’ll also be vulnerable to infection, so it’s important that you’re in a clean and stable environment where your health can be carefully monitored and any infection you have can be promptly treated. You may also be prescribed antibiotics to help prevent further infection.
Depending on how well you respond to treatment, the induction phase can last from four weeks to a couple of months. You may be able to leave hospital and receive treatment on an outpatient basis if your symptoms improve.
For intensive treatment, the chemotherapy medications will be injected into a thin tube that’s inserted either into a blood vessel near your heart (central line) or into your arm (a peripherally inserted central catheter, or PICC).
In very rare cases, chemotherapy medication may also be directly administered into your cerebrospinal fluid to kill any leukaemia cells that may have spread to your nervous system. This is done using a needle that’s placed into your spine, in a similar way to a lumbar puncture.
Side effects of intensive chemotherapy for acute myeloid leukemia are common. They can include:
- nausea
- vomiting
- diarrhea
- loss of appetite
- sore mouth and mouth ulcers (mucositis)
- tiredness
- skin rashes
- hair loss
- infertility – which may be temporary or permanent (see complications of acute myeloid leukemia for more information)
Most side effects should resolve once treatment has finished. Tell a member of your care team if side effects become particularly troublesome, as there are medicines that can help you cope better with certain side effects.
Non-intensive chemotherapy
If your doctors don’t think you’re fit enough to withstand the effects of intensive chemotherapy, they may recommend non-intensive treatment. This involves using an alternative type of chemotherapy to the standard intensive therapy, which is designed more to control leukaemia rather than cure it.
The main aim of this treatment is to control the level of cancerous cells in your body and limit any symptoms you have, while reducing your risk of experiencing significant side effects of treatment.
The medications used during non-intensive chemotherapy may be given through a drip into a vein, by mouth or by injection under the skin, and can often be given on an outpatient basis.
All Trans-Retinoic Acid (ATRA)
If you have the sub-type of acute myeloid leukemia known as acute promyelocytic leukaemia (APL), you’ll usually be given capsules of a medicine called ATRA, in addition to chemotherapy.
ATRA works by changing the immature white blood cells (blast cells) into mature healthy cells, and can reduce symptoms very quickly.
Side effects of ATRA can include headaches, nausea, bone pain, and dry mouth, skin and eyes.
Assessing Treatment Response
About 2 to 3 weeks after the completion of induction therapy, blood and bone marrow tests are done to see how well your treatment is working. If no leukemic blasts are found in the bone marrow, no treatment is given for 2 to 4 weeks to give your bone marrow time to recover and make new blood cells.
When blood cell counts return to normal, your doctor will test your bone marrow again to see whether you have achieved a complete remission.
A complete remission is achieved when:
- No more than 5 percent of cells in the bone marrow are blast cells
- Blood cell counts are back to normal
- All signs and symptoms of AML are gone
If the initial treatment does not induce a remission, induction therapy can be repeated, either with the same drugs or with a new chemotherapy regimen. Patients who continue to have a high level of blasts should be considered as candidates for a clinical trial, allogeneic stem cell transplantation or drug regimens for relapsed or refractory AML.
Minimal or Measurable Residual Disease (MRD)
Even when a complete remission is achieved, some leukemia cells that cannot be seen with a microscope may still remain in the bone marrow. This is referred to as minimal residual disease (MRD), also called measurable residual disease. Patients who achieve remission after initial treatment but have minimal residual disease (measurable residual disease) are at increased risk of disease relapse. Testing for MRD can help doctors identify patients who may benefit from further treatment with intensified therapies, such as allogeneic stem cell transplantation.
It is important to get tested for minimal residual disease (MRD) after achieving remission. The tests used most commonly to detect measurable residual disease (MRD) are flow cytometry, polymerase chain reaction (PCR) and next-generation sequencing. These tests typically use samples of bone marrow cells, but in some cases blood samples can be used. They are much more sensitive and able to detect very low numbers of AML cells, compared to standard tests that can only detect AML cells that are visible with a microscope.
Consolidation therapy
If induction is successful, the next stage of treatment will be consolidation therapy to prevent a relapse. Consolidation therapy is treatment given after cancer is in remission following the initial therapy. The goal of consolidation therapy is to “consolidate” the remission by lowering the number of residual leukemia cells in your body or eliminating them entirely. This often involves receiving regular injections of chemotherapy medication that are usually given on an outpatient basis, which means that you won’t have to stay in hospital overnight. However, you may need some short stays in hospital if your symptoms suddenly get worse or if you develop an infection.
There are three basic treatment options for post-remission therapy:
- Additional intensive chemotherapy
- Stem cell transplantation
- Oral chemotherapy (as maintenance therapy)
The consolidation phase of treatment lasts several months.
It’s not always clear which treatment option is best for consolidation. Each has pros and cons. Doctors look at several factors when recommending what type of therapy a patient should get. These include:
- How many courses (cycles) of chemo it took to bring about a remission. If it took more than one, some doctors recommend that the patient get a more intensive program, which might include a stem cell transplant.
- The availability of a brother, sister, or an unrelated donor who matches the patient’s tissue type. If a close enough tissue match is found, an allogeneic (donor) stem cell transplant may be an option, especially for younger patients.
- The possibility of collecting leukemia-free bone marrow cells from the patient. If lab tests show that a patient is in remission, collecting stem cells from the patient’s bone marrow or blood for an autologous stem cell transplant may be an option. Stem cells collected from the patient would be purged (treated in the lab to try to remove or kill any remaining leukemia cells) to lower the chances of relapse.
- The presence of one or more adverse prognostic factors, such as certain gene or chromosome changes, a very high initial white blood cell count, AML that develops from a previous blood disorder or after treatment for an earlier cancer, or spread of AML to the central nervous system. These factors might lead doctors to recommend more aggressive therapy, such as a stem cell transplant. On the other hand, for people with good prognostic factors, such as favorable gene or chromosome changes, many doctors might advise holding off on a stem cell transplant unless the disease recurs.
- The patient’s age and overall health. Older patients or those with other health problems might not be able to tolerate some of the severe side effects that can occur with high-dose chemo or stem cell transplants.
- The patient’s wishes. There are many issues relating to quality of life that need to be considered. An important issue is the higher chance of death from high-dose chemo or a stem cell transplant. This and other issues must be discussed between the patient and the doctor.
Patients with favorable risk factors are often given intensive chemotherapy with high-dose cytarabine and other drugs for their consolidation therapy. In the consolidation phase, patients generally receive multiple cycles of chemotherapy. The number of chemotherapy cycles varies from patient to patient. Patients may be hospitalized or receive post-remission therapy in an outpatient setting, depending on the clinical setting, the treatment type and other factors.
Patients with high-risk AML, based on their prognostic factors, receive more aggressive therapy, such as allogeneic stem cell transplantation, during the consolidation phase of treatment. Allogeneic stem cell transplantation is a complex treatment that can cause serious, life-threatening side effects. So, it is important to discuss the benefits and risks of this procedure with your doctor.
An important treatment decision for a patient is whether to have the stem cell transplantation after the their first remission. Often, this is when transplantation offers the best chance of preventing AML from recurring. However, it is associated with higher treatment-related morbidity and death compared to other treatment options, especially in older patients. Patients who are candidates for an allogeneic stem cell transplant should begin a search for an HLA-matched stem cell donor as soon as possible, ideally while they are receiving induction therapy.
For patients 60 years of age or older who achieve a complete remission after induction therapy but who are not able to complete intensive chemotherapy or proceed to allogeneic stem cell transplantation, the doctor may prescribe an oral formulation of azacitidine (Onureg) as maintenance therapy.
Consolidation for younger patients
For younger patients (typically those under 60), the main options for consolidation therapy are:
- Several cycles of chemo with high-dose cytarabine (ara-C) (sometimes known as HiDAC)
- Allogeneic (donor) stem cell transplant
- Autologous stem cell transplant
The best option for each person depends on the risk of the leukemia coming back after treatment, as well as other factors.
For HiDAC, cytarabine (ara-C) is given at very high doses, typically over 5 days. This is repeated about every 4 weeks, usually for a total of 3 or 4 cycles. For people who got the targeted drug midostaurin (Rydapt) during induction, this is typically continued during consolidation. Again, each round of treatment is typically given in the hospital because of the risk of serious side effects.
For patients who got chemo plus the targeted drug gemtuzumab ozogamicin (Mylotarg) for their induction therapy, a similar regimen might be used for consolidation.
Another approach after induction therapy is to give very high doses of chemo followed by either an allogeneic (from a donor) or autologous (patient’s own) stem cell transplant. Stem cell transplants have been found to reduce the risk of leukemia coming back more than standard chemo, but they are also more likely to have serious complications, including an increased risk of death from treatment.
Consolidation for patients who are older or have other health problems
Older patients or those in poor health may not be able to tolerate intensive consolidation treatment. Often, giving them more intensive therapy raises the risk of serious side effects (including treatment-related death) without providing much more of a benefit. These patients may be treated with:
- Higher-dose cytarabine (usually not quite as high as in younger patients)
- Standard-dose cytarabine, possibly along with idarubicin, daunorubicin, or mitoxantrone (For people who got the targeted drug midostaurin (Rydapt) during induction, this is typically continued during consolidation as well.)
- Non-myeloablative stem cell transplant (mini-transplant)
Another option for some people whose AML goes into remission after induction (or even after consolidation) might be treatment with oral azacitidine (Onureg).
Other treatments
Radiotherapy
Radiotherapy involves using high doses of controlled radiation to kill cancerous cells. There are two main reasons why radiotherapy is usually used to treat acute myeloid leukemia:
- to prepare the body for a bone marrow or stem cell transplant
- to treat advanced cases that have spread to the nervous system and/or brain, although this is uncommon
Side effects of radiotherapy can include hair loss, nausea and fatigue. The side effects should pass once your course of radiotherapy has been completed.
Bone marrow and stem cell transplants
If chemotherapy doesn’t work, a possible alternative treatment option is a bone marrow or stem cell transplant. For other patients who are in remission and can tolerate intensive chemotherapy, the doctor may also recommend stem cell transplantation during the consolidation phase of chemotherapy. The goal of stem cell transplantation is to cure the patient’s cancer with very high doses of chemotherapy.
Before transplantation can take place, the person receiving the stem cell transplant will need to have intensive high-dose chemotherapy and possibly radiotherapy to destroy the cells in their bone marrow. Although administering such high doses of chemotherapy drugs can kill more leukemia cells, such high doses of chemotherapy can also severely damage the stem cells in the bone marrow and cause dangerously low blood cell counts. This may result in anemia, serious infections and uncontrolled bleeding. After the intensive therapy, the patient receives an infusion of stem cells through a tube into a blood vessel (in a similar way to chemotherapy medication) to replace those destroyed by the intensive therapy. The healthy blood stem cells grow and multiply, forming new bone marrow and blood cells.
This process can put an enormous amount of strain on the body and cause significant side effects and potential complications, so you’ll usually need to stay in hospital for a few weeks.
There are two main types of stem cell transplantation:
- Allogeneic stem cell transplantation, in which a patient receives stem cells from a matched or a partially matched donor, either related or unrelated to the patient
- Autologous stem cell transplantation, in which a patient’s own stem cells are collected before chemotherapy, stored, and then returned into the patient’s body after completing chemotherapy.
Research to determine which patients are most likely to benefit from stem cell transplantation after their first complete remission is evolving. Research in this area continues to study which AML patients get the most benefit from stem cell transplant and which type of transplant is best in each situation.
Stem cell transplants are intensive treatments with real risks of serious complications, including death, and their exact role in treating AML is not always clear. Some doctors feel that if the patient is healthy enough to withstand an allogeneic transplant and a compatible donor is available, this option offers the best chance for long-term survival. Others feel that studies have not yet shown this conclusively, and that in some cases a transplant should be reserved in case the leukemia comes back after standard treatment. Still others feel that stem cell transplants should be given if the leukemia is likely to come back based on certain gene or chromosome changes.
Transplantations have better outcomes if the donor has the same tissue type as the person receiving the donation. The best candidate to provide a donation is usually a brother or sister with the same tissue type. Studies show that allogeneic stem cell transplantation may benefit patients with high-risk and intermediate-risk AML up to age 75 years and who have an HLA-matched donor.
Timing of is one of the most important factors influencing allogeneic transplant outcomes. In most cases, it is very important to start a donor search as soon as possible after an AML diagnosis. This is necessary in order to identify a suitably matched, related or unrelated donor and to plan for the best time to perform a transplant safely and successfully.
Transplantations are most successful when they’re carried out on children and young people, or older people who are otherwise in good health, and when there’s a suitable donor, such as a brother or sister.
Allogeneic Stem Cell Transplantation
Allogeneic stem cell transplantation, in which a patient receives stem cells from a matched or a partially matched donor, either related or unrelated to the patient. Allogeneic stem cell transplantation is the most common type of stem cell transplantation used to treat AML. In preparation for the transplant, patients receive a “conditioning therapy.” This consists of very high doses of chemotherapy, either with or without radiation, to kill the leukemia cells remaining in their bodies. It is also given to suppress their own immune systems, so their bodies do not reject the donor stem cells.
After the conditioning therapy, patients receive donor stem cells by IV infusion. Allogeneic stem cell transplantation uses healthy blood-forming cells from an HLA-matched donor. They can be from a family member or an unrelated person, or from a donated umbilical cord. The donated stem cells restore the bone marrow’s ability to form new blood cells.
Ideally, an allogeneic stem cell transplant will generate a new immune system for the patient that helps the body fight infections and other diseases. The new immune system also has the potential to recognize and attack any remaining cancer cells in the body. The transplanted immune cells (the graft) perceive the leukemia cells in the body as foreign and destroy them. This is called the “graft-versus-leukemia (GVL)” effect.
Compared to other treatment options, allogeneic stem cell transplantation is associated with a higher rate of side effects and mortality. However, it may be considered for patients with higher-risk AML, based on their cytogenetic and molecular test results and other prognostic factors. The decision to perform an allogeneic transplant also depends on other factors, including the patient’s age, physical fitness, comorbidities (other co-existing medical conditions) and social supports (from family members, caregivers, friends, etc), as well as the patient’s understanding of the potential benefits and risks.
One possible serious side effect of allogeneic stem cell transplantation is graft versus-host disease (GVHD). This occurs when the transplanted immune cells (the graft) from the donor identify the cells in the recipient’s body (the host) as foreign and attack them. The parts of the body most commonly damaged by graft versus-host disease (GVHD) include the skin, liver, stomach, intestines and eyes. Graft versus-host disease (GVHD) can develop within weeks after transplantation or much later. A doctor can order medications to help prevent or minimize the complications of GVHD. Most patients need to be closely monitored for GVHD for at least the first 100 days after the transplant.
Reduced-Intensity Allogeneic Stem Cell Transplantation
Reduced-Intensity Allogeneic Stem Cell Transplantation may be a treatment option for older patients who cannot tolerate the high doses of chemotherapy used in preparation for a standard allogeneic stem cell transplant. The conditioning therapy in a reduced-intensity transplant uses lower doses of chemotherapy and/or radiation. With a reduced-intensity conditioning regimen, the patient’s blood counts may not fall as low as they would with high-dose chemotherapy. Additionally, the less toxic regimens put less strain on the patient’s organs, making this regimen safer and more tolerable.
The success of reduced-intensity transplantation depends on the graft-versus-leukemia effect of the donor stem cells, rather than on high-dose treatments to kill the cancer cells. This therapy reduces the number of cancer cells, but it does not completely destroy the patient’s bone marrow. The goal is to have the donor stem cells become established in the patient’s bone marrow and produce white blood cells that will attack the patient’s remaining cancer cells. As with standard allogeneic stem cell transplantation, the risk of graft-versus-host disease (GVHD) is an important consideration and a potentially disabling side effect.
Autologous Stem Cell Transplantation
In this is a procedure in which stem cells are collected from a cancer patient before they undergo intensive chemotherapy, either with or without radiation therapy. These stem cells are stored and then put back into the patient’s blood stream after the conditioning therapy is completed.
Autologous stem cell transplantation is sometimes used for patients who do not have an HLA-matched donor. Autologous stem cell transplants are usually easier for patients to tolerate than allogeneic transplants. This is because patients receive their own stem cells (which are specially prepared for the transplant), so the risk of some complications, such as graft-versus-host disease, is lower. However, the high doses of chemotherapy can still cause serious side effects. Autologous transplants are done less frequently than allogeneic stem cell transplants for AML patients, mainly because of the lack of a graft-versus-leukemia effect and the risk of returning some leukemia cells back to the patient.
Azacitidine
Azacitidine is a possible alternative treatment for adults with acute myeloid leukemia who can’t have a stem cell transplant.
It’s recommended by the National Institute for Health and Care Excellence (NICE) for use in certain circumstances – for example, depending on the characteristics of the person’s blood and bone marrow.
Azacitidine is a chemotherapy medication that’s given by injection under the skin. It interferes with the growth of cancer cells and destroys them, and also helps bone marrow to produce normal blood cells.
Alternative medicine
No alternative treatments have been found helpful in treating acute myelogenous leukemia. But some complementary and alternative treatments may relieve the symptoms you experience due to cancer or cancer treatment.
Alternative treatments that may help relieve symptoms include:
- Acupuncture
- Aromatherapy
- Massage
- Meditation
- Relaxation exercises
Treating leukostasis
Some people with AML have very high numbers of leukemia cells in their blood when they are first diagnosed, which can cause problems with normal blood circulation. This is called leukostasis. Chemo can take a few days to lower the number of leukemia cells in the blood. In the meantime, leukapheresis (sometimes just called pheresis) might be used before chemo.
In leukapheresis, the patient’s blood is passed through a special machine that removes white blood cells (including leukemia cells) and returns the rest of the blood to the patient. Two intravenous (IV) lines are required – the blood is removed through one IV, goes through the machine, and then is returned to the patient through the other IV. Sometimes, a single large catheter is placed in a vein in the neck or under the collar bone for the pheresis, instead of using IV lines in both arms. This type of catheter is called a central venous catheter (CVC) or central line and has both IVs built in.
This treatment lowers blood counts right away. The effect is only for a short time, but it may help until the chemo has a chance to work.
Coping and support
Acute myelogenous leukemia is an aggressive form of cancer that typically demands quick decision-making. That leaves people with a new diagnosis faced with important decisions about a disease they may not yet understand. Here are some tips for coping:
- Learn enough to make decisions about your care. The term “leukemia” can be confusing because it refers to a group of cancers that aren’t all that similar except for the fact that they affect the bone marrow and blood. You can waste a lot of time researching information that doesn’t apply to your kind of leukemia. To avoid that, ask your doctor to write down as much information about your specific disease as possible. Then narrow your search for information accordingly.
- Write down questions for your doctor before each appointment, and look for information in your local library and on the internet. Good sources include the National Cancer Institute (https://www.cancer.gov), the American Cancer Society (https://www.cancer.org/) and the Leukemia & Lymphoma Society (https://www.lls.org/).
- Lean on family and friends. It can be difficult to talk about your diagnosis, and you’ll likely get a range of reactions when you share the news. But talking about your diagnosis can be helpful. So can the outpouring of practical help that often results.
- Take care of yourself. It’s easy to get caught up in the tests, treatments and procedures of therapy. But it’s important to take care of yourself, not just the cancer. Try to make time for yoga, cooking or other favorite diversions.
Treatment of patients Aged 60 Years and Older with Acute Myeloid Leukemia (AML)
Therapy for acute myeloid leukemia occurs more frequently in older adults; at least half of patients are older than 65 years of age when the disease is diagnosed. Treatment approaches for these patients range from standard intensive induction chemotherapy to less intensive therapies, or the best supportive care. People who are much older or are in poor health may not be able to tolerate this intense treatment. In fact, intense chemo could actually shorten their lives. Treatment of these patients is often not divided into induction and consolidation phases, but it may be given every so often as long as it seems helpful. Additionally, there are a growing number of new treatment options available for older adults.
The treatment of AML in older patients is a challenge. The higher occurrence of unfavorable cytogenetic and molecular abnormalities in the leukemia cells of many older patients makes the disease more resistant to standard chemotherapy than in younger patients. Also, as people age, they can have more difficulty tolerating more intense cancer treatments. Older patients are also more likely to have comorbidities (other medical problems), including diabetes, high blood pressure, high cholesterol, heart disease, and a history of stroke or lung disease. These comorbidities can limit treatment options. Many older patients are not offered standard treatment options with intensive chemotherapy because they are considered unlikely to survive the rigors of this treatment due to their advanced age, comorbidities and poor general level of fitness (called performance status). In some cases, intensive chemotherapy can actually shorten their lives.
The choice of therapy for older patients with acute myeloid leukemia also depends on the specific genetic profile of the leukemia cells; a patient’s genetic profile is also the best way to predict how the disease will respond to chemotherapy as some specific genetic mutations may lead to poorer outcomes. In addition, consideration needs to be given to whether patients have available support from friends and family during treatment. You should discuss your treatment goals with your doctor. The doctor should explain the risks and benefits of your different treatment options and also provide realistic expectations about the likely results of each of them.
Older patients who are physically fit and have no serious health problems may benefit from intensive treatment. Fit elderly patients may even be candidates for reduced-intensity allogeneic stem cell transplantation.
Not all patients want or can tolerate intensive therapies. Patients whose comorbidities and performance status make them poor candidates for intensive chemotherapy may still be able to participate in clinical trials. Or they may benefit from lower-intensity therapies which may relieve symptoms, improve quality of life and potentially extend survival.
Options for people who are older or are in poor health might include:
- Low-intensity chemo with a drug such as low-dose cytarabine (LDAC), azacitidine (Vidaza), or decitabine (Dacogen)
- Low-intensity chemo plus a targeted drug such as venetoclax (Venclexta) or glasdegib (Daurismo)
- A targeted drug alone, such as:
- Gemtuzumab ozogamicin (Mylotarg), if the AML cells have the CD33 protein
- Ivosidenib (Tibsovo), if the AML cells have an IDH1 gene mutation
- Enasidenib (Idhifa), if the AML cells have an IDH2 gene mutation
Some people might decide against chemo and other drugs and instead choose supportive care. This focuses on treating any symptoms or complications that arise and keeping the person as comfortable as possible.
Lower-intensity treatment strategies for induction as recommended by the National Comprehensive Cancer Network Guidelines include:
- Venetoclax (Venclexta) and azacitidine (Vidaza)
- Venetoclax and decitabine (Dacogen)
- Venetoclax and low-dose cytarabine (Cytosar-U)
- Azacitidine
- Decitabine
- Low-dose cytarabine
- Glasdegib (Daurismo)
- Gemtuzumab ozogamicin (Mylotarg) for CD33-positive AML
- Ivosidenib (Tibsovo®) for AML with an IDH1 mutation
- Enasidenib (Idhifa®) for AML with an IDH2 mutation
- Azacitidine or decitabine and sorafenib (Nexavar) for AML with an FLT3-ITD mutation
After the completion of induction therapy, blood and bone marrow tests are done to check for a remission and to look for minimal/measurable residual disease (MRD). A complete remission is still possible with lower-intensity treatments. A complete remission is achieved when:
- No more than 5 percent of cells in the bone marrow are blast cells
- Blood cell counts are back to normal
- All signs and symptoms of AML are gone
For patients who are tolerating and responding to treatment, the doctor will generally continue the treatment indefinitely as maintenance therapy. If there is no response or the cancer progresses, patients may want to consider a clinical trial or treatments for relapsed or refractory disease. Patients may also want to consider only palliative care to improve quality of life and alleviate discomfort.
Treatment of Children with Acute Myeloid Leukemia (AML)
Treatment of most children with acute myeloid leukemia (AML) is divided into 2 main phases of chemotherapy:
- Induction
- Consolidation (intensification)
Because of the intensity of treatment and the risk of serious complications, children with AML need to be treated in cancer centers or hospitals that have experience with this disease.
Induction therapy
The chemo drugs most often used to treat AML are daunorubicin (daunomycin) and cytarabine (ara-C), which are each given for several days in a row. The treatment schedule may be repeated in 10 days or 2 weeks, depending on how intense doctors want the treatment to be. A shorter time between treatments can be more effective in killing leukemia cells, but it can also cause more severe side effects.
Some children with AML may get a dose of the targeted drug gemtuzumab ozogamicin (Mylotarg) along with chemo as part of their induction treatment.
If the doctors think that the leukemia might not respond to just 2 chemo drugs alone, they may add another chemo drug such as etoposide or 6-thioguanine. Children with very high numbers of white blood cells or whose leukemia cells have certain chromosome abnormalities may fall into this group.
Treatment with these chemo drugs is repeated until the bone marrow shows no more leukemia cells. This usually occurs after 2 or 3 cycles of treatment.
Preventing relapse in the central nervous system
- Most children with AML will also get intrathecal chemotherapy (given directly into the cerebrospinal fluid, or CSF) to help prevent leukemia from relapsing in the brain or spinal cord. Radiation therapy to the brain is used less often.
Consolidation (intensification) therapy
About 85% to 90% of children with AML go into remission after induction therapy. This means no signs of leukemia are detected using standard lab tests, but it does not necessarily mean that the leukemia has been cured.
Consolidation (intensification) begins after the induction phase. The purpose is to kill any remaining leukemia cells by using more intensive treatment.
Some children have a brother or sister who would be a good stem cell donor. For these children, a stem cell transplant might be recommended once the leukemia is in remission, especially if the AML has some poorer prognostic factors. Most studies have found this improves the chance for long-term survival over chemo alone, but it is also more likely to cause serious complications. For children with good prognostic factors, some doctors may recommend just giving intensive chemotherapy, and reserving the stem cell transplant in case the AML relapses.
For most children without a good stem cell donor, consolidation consists of the chemo drug cytarabine (ara-C) in high doses. Daunorubicin may also be added. It is usually given for at least several months.
If the targeted drug gemtuzumab ozogamicin (Mylotarg) was given during induction, a dose of this drug will likely be given during this phase of treatment as well.
Intrathecal chemo (into the CSF) is usually given every 1 to 2 months for as long as intensification continues.
Maintenance chemo is not needed for children with AML (other than those with Acute Promyelocytic Leukemia [APL]).
An important part of treatment for AML is supportive care (proper nursing care, nutritional support, antibiotics, and blood transfusions). The intense treatment needed for AML usually destroys much of the bone marrow (causing severe shortages of blood cells) and can cause other serious complications. Without antibiotic treatment of infections or transfusion support, the current high remission rates would not be possible.
Refractory or recurrent AML
Less than 15% of children have refractory AML (leukemia that does not respond to initial treatment). These leukemias are often very hard to cure, and doctors may recommend a stem cell transplant if it can be done.
Generally, the outlook for a child whose AML relapses (comes back) after treatment is slightly better than if the AML never went into remission, but this depends on how long the initial remission was. In more than half of cases of relapse, the leukemia can be put into a second remission with more chemo. The chance of getting a second remission is better if the first remission lasted for at least a year, but long-term second remissions are rare without a stem cell transplant. Many different combinations of standard chemo drugs have been used in these situations, but the results have been mixed.
Another option for some children with refractory or recurrent AML is treatment with the targeted drug gemtuzumab ozogamicin (Mylotarg).
Most children whose leukemia has relapsed are good candidates for clinical trials testing new treatment regimens. The hope is that some sort of a remission can be attained so that a stem cell transplant can be considered. Some doctors may advise a stem cell transplant even if there is no remission. This can sometimes be successful.
Treatment for relapsed and refractory acute myeloid leukemia
Recurrent or relapsed AML is cancer that has come back after treatment. Most patients who receive treatment for acute myeloid leukemia achieve an initial remission. However, some patients have persistent and visibly present residual leukemia cells in the bone marrow at a level of 5% or higher, even after intensive treatment. In these cases, the disease is referred to as being “refractory” (or “refractory AML”). Approximately 30-50 percent of newly diagnosed AML patients do not achieve a complete remission with intensive induction therapy and may require additional chemotherapy. Patients who have not achieved complete remission after two cycles of intensive chemotherapy are considered to have refractory AML. For patients on targeted therapy, it may take 2 to 6 months to achieve a remission. So, it may be more difficult to define when a treatment has failed.
In other patients, despite achieving an initial remission, leukemia cells reappear in the bone marrow and normal blood cell production decreases again. This is referred to as a “relapse” of the disease (or “relapsed AML”). At the time of relapse, repeat genetic testing of the leukemia cells is considered “standard of care,” since the mutational pattern at the time of relapse may be different from when the disease was first diagnosed. This can affect treatment decisions.
Allogeneic stem cell transplantation remains the only potential curative option for patients with relapsed AML, and they must be considered fit enough to undergo the procedure. However, recent approval of several new treatments may help patients who cannot undergo a stem cell transplant to live longer with high quality of life.
Treatment options for patients with refractory or relapsed AML include:
- A clinical trial. Treatment in a clinical trial should be considered first for all patients with refractory or relapsed AML.
- Re-treatment with the same induction regimen that produced the patient’s first remission. This is an option if a relapse occurs 12 months or more after remission.
- Allogeneic stem cell transplantation. In fit patients, salvage chemotherapy can be used to induce a remission before stem cell transplantation. This is an option for patients younger than age 60 and patients older than age 60 who are physically fit.
- Targeted therapy. Some targeted therapies that may be used include:
- Gilteritinib (Xospata) for AML with an FLT3 mutation
- Low-intensity therapy (azacytidine or decitabine) plus sorafenib (Nexavar) for AML with an FLT3 mutation
- Enasidenib (Idhifa) for AML with an IDH2 mutation
- Ivosidenib (Tibsovo) for AML with an IDH1 mutation
- Gemtuzumab ozogamicin (Mylotarg) for CD33-positive AML
Research is ongoing to determine optimal drug combinations, doses and administration schedules. The drug combinations listed below are some commonly used aggressive and less aggressive treatment regimens for refractory and relapsed cases of AML.
Aggressive treatments for fit patients, suggested by the National Comprehensive Care Network Guidelines, include:
- Cladribine, cytarabine and granulocyte colony-stimulating factor (G-CSF), with or without mitoxantrone or idarubicin
- High-dose cytarabine, with or without idarubicin, or daunorubicin, or mitoxantrone
- Fludarabine, cytarabine and G-CSF, with or without idarubicin
- Etoposide and cytarabine, with or without mitoxantrone
- Clofarabine with or without cytarabine, with or without idarubicin
Less aggressive treatments, suggested by the National Comprehensive Care Network Guidelines, include:
- Hypomethylating agents (azacitidine or decitabine)
- Low-dose cytarabine
- Venetoclax plus hypomethylating agents (azacitidine or decitabine) or low-dose cytarabine
Palliative treatment
If further treatment or a clinical trial is not an option, the focus of treatment may shift to controlling symptoms caused by the leukemia, rather than trying to cure it. This is called palliative treatment or supportive care. For example, the doctor may advise less intensive chemo to try to keep the leukemia under control instead of trying to cure it.
As the leukemia grows in the bone marrow it may cause pain. It’s important that you be as comfortable as possible. Treatments that may be helpful include radiation therapy and appropriate pain-relieving medicines. If medicines such as aspirin and ibuprofen don’t help with the pain, stronger opioid medicines such as morphine are likely to be helpful. Some people may worry about taking stronger drugs for fear of being sleepy all the time or becoming addicted to them. But many people get very effective pain relief from these medicines without serious side effects.
Other common symptoms from leukemia are low blood counts and fatigue. Medicines or blood transfusions may be needed to help correct these problems. Nausea and loss of appetite can be treated with medicines and high-calorie food supplements. Infections that occur may be treated with antibiotics.
It’s very important to let your cancer care team know if you are having pain or any other symptoms so that they can be treated.