ALCAPA heart defect
ALCAPA is short for anomalous origin of the left coronary artery from the pulmonary artery (instead of the aorta), is a rare congenital (present at birth) heart defect occurring in 1 of 300,000 births 1. ALCAPA is also known as Bland-White-Garland syndrome and accounts for 0.25%-0.5% of congenital cardiac disease 2. ALCAPA is associated with early infant mortality and sudden death in adults.
ALCAPA usually manifests as an isolated defect, but in 5% of cases it may be associated with other cardiac anomalies such as atrial septal defect, ventricular septal defect, and aortic coarctation 2.
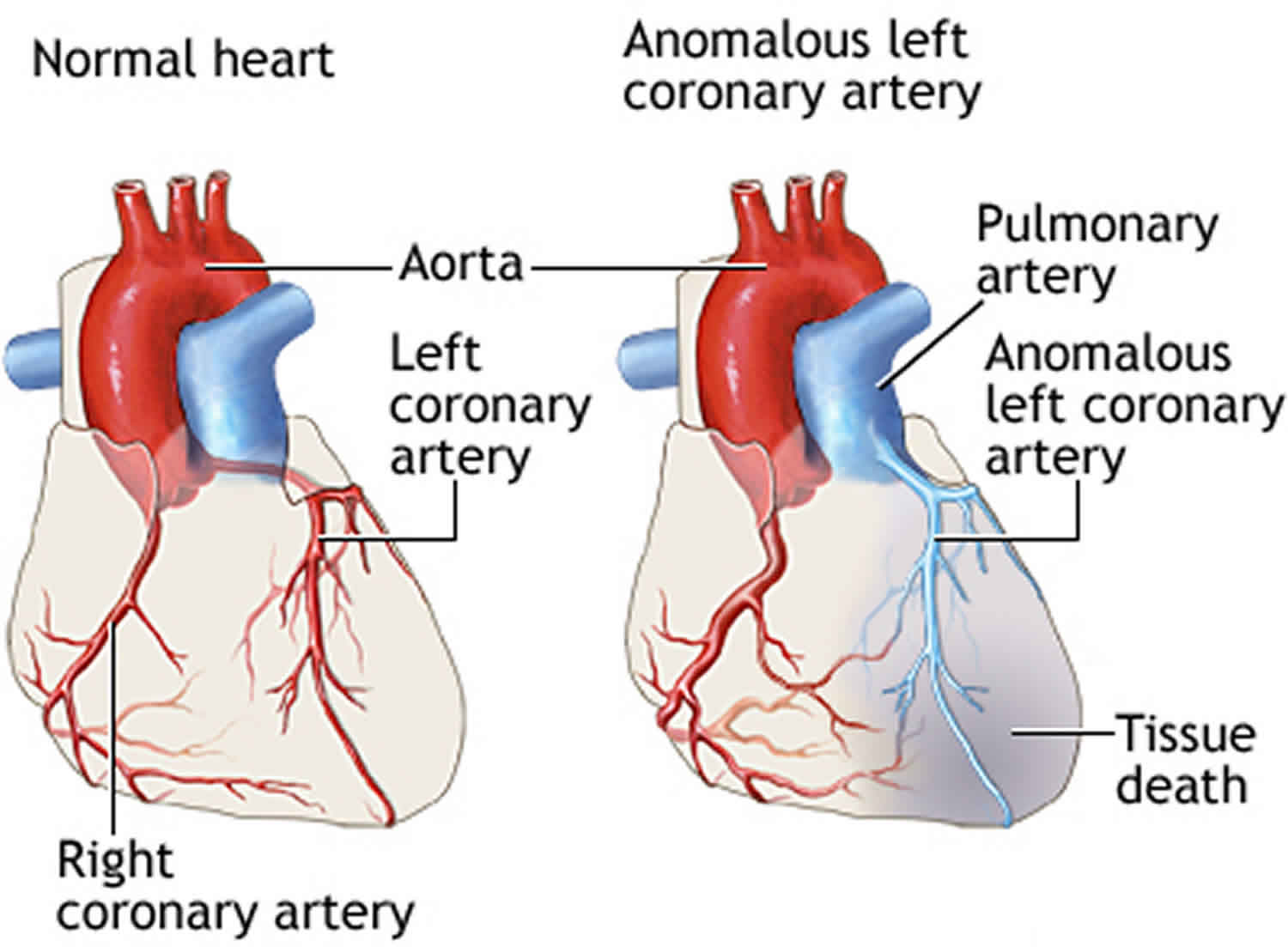
In the normal heart, the left coronary artery originates from the aorta. The left coronary artery supplies oxygen-rich blood to the heart muscle on the left side of the heart as well as the mitral valve (the heart valve between the upper and lower chambers of the heart on the left side). The aorta is the major blood vessel that takes oxygen-rich blood from the heart to the rest of the body.
In children with ALCAPA, the left coronary artery originates from the pulmonary artery. The pulmonary artery is the major blood vessel that takes oxygen-poor blood from the heart to the lungs to pick up oxygen. When this defect occurs, blood that is lacking in oxygen is carried to the heart muscle on the left side of the heart. Therefore, the heart muscle does not get enough oxygen. The tissue begins to die due to lack of oxygen. This can cause a heart attack in the baby.
A condition known as “coronary steal” further damages the heart in babies with ALCAPA. The low blood pressure in the pulmonary artery causes blood from the abnormally connected left coronary artery to flow back toward the pulmonary artery instead of toward the heart muscle. This results in less blood and oxygen to the heart muscle. This problem can also lead to a heart attack in a baby. Coronary steal develops over time in babies with ALCAPA if the condition is not treated early.
ALCAPA syndrome is one of the most common causes of myocardial ischemia and infarction in children and may also present as cardiomyopathy 3. Without treatment, approximately 90% of patients die within the first year of life 3. However, in some cases collateral blood supply from the right coronary artery may be well-established and the patients can reach to adulthood 4. In patients who live to adulthood, ALCAPA syndrome may cause myocardial infarction, left ventricular dysfunction and mitral regurgitation, malignant ventricular arrhythmias generated from myocardial scar tissue or silent myocardial ischemia, which can lead to sudden cardiac death 2.
Traditionally, ALCAPA has been diagnosed by angiography or autopsy; however, the development of cardiac computed tomography (CT) and magnetic resonance imaging (MRI) has allowed noninvasive evaluation of the coronary anatomy by direct visualization of the origin of the left coronary artery from the pulmonary artery 5.
The definitive treatment for ALCAPA is surgical intervention 6. Early diagnosis and prompt surgical intervention have excellent results and lead to gradual myocardial recovery 6.
The prognosis for patients with ALCAPA has dramatically improved as a result of both early diagnosis using echocardiography with color flow mapping and improvements in surgical techniques, including myocardial preservation.
Figure 1. ALCAPA
ALCAPA causes
ALCAPA is generally considered to be on the basis of multifactorial inheritance, similar to other congenital heart defects. Inheritance is not a factor for ALCAPA. Other congenital cardiac defects, such as patent ductus arteriosus, ventricular septal defect, tetralogy of Fallot, or coarctation of the aorta, rarely may be associated with ALCAPA. No specific association with any noncardiac anomalies is noted 7.
ALCAPA is a problem that occurs when the baby’s heart is developing early in the pregnancy. The developing blood vessel to the heart muscle does not attach correctly. In children with ALCAPA, the left coronary artery originates from the pulmonary artery. The pulmonary artery is the major blood vessel that takes oxygen-poor blood from the heart to the lungs to pick up oxygen. When this defect occurs, blood that is lacking in oxygen is carried to the heart muscle on the left side of the heart. Therefore, the heart muscle does not get enough oxygen. The tissue begins to die due to lack of oxygen. This can cause a heart attack in the baby.
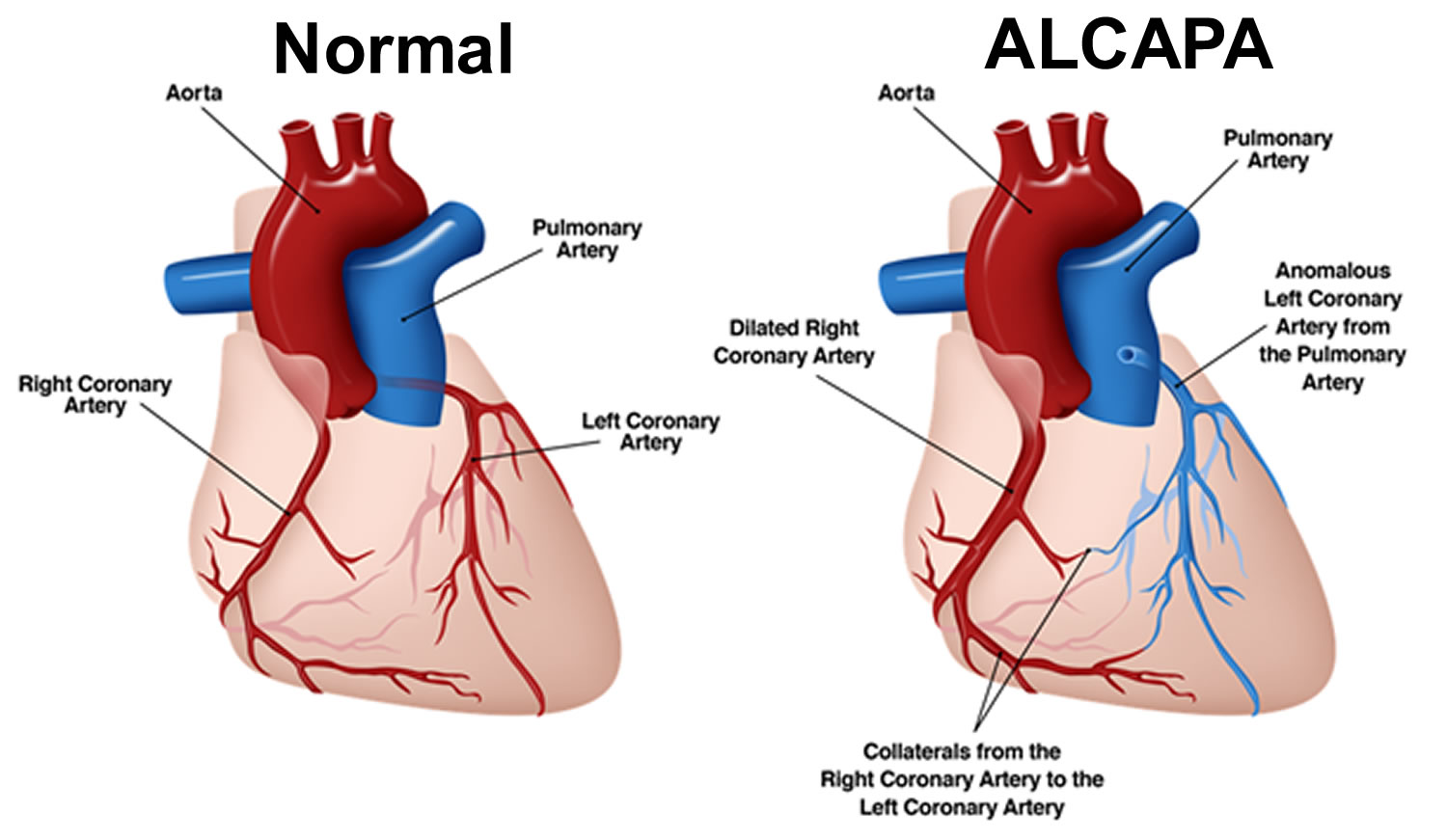
The origin of the left coronary artery from the pulmonary artery is well tolerated in fetal life because pulmonary arterial pressure equals systemic pressure, which leads to antegrade flow in the anomalous left coronary artery 2. Shortly after birth, when pulmonary arterial pressure decreases, antegrade flow and oxygen content in the left coronary artery also decrease leading to myocardial ischemia and infarction. When collateral circulation between the right and left coronary systems develops, left coronary artery flow reverses and enters the pulmonary trunk due to the low pulmonary arterial pressure. This is called “coronary steal” phenomenon, in which a left-to-right shunt leads to abnormal left ventricular perfusion 3. A “coronary steal” further damages the heart in babies with ALCAPA. Coronary steal develops over time in babies with ALCAPA if the condition is not treated early.
ALCAPA symptoms
Symptoms of ALCAPA in an infant include:
- Crying or sweating during feeding
- Irritability
- Pale skin
- Poor feeding
- Rapid breathing
- Symptoms of pain or distress in the baby (often mistaken for colic)
Symptoms usually occur in infants 1-2 months after birth because of left-to-right shunting from the higher pressure left coronary arterial system to the lower pressure pulmonary arterial system. Without treatment, approximately 90% of infants die within the first year of life 3. Death is usually due to circulatory insufficiency, myocardial infarction, or life-threatening cardiac dysrhythmias 2. However, the patients may survive into childhood and even adulthood with clinical presentations varying from symptomatic chronic mitral insufficiency or global ischemic cardiomyopathy. Factors that may lead to survival beyond infancy include the development of abundant intercoronary collateral arteries 5.
In a review of 25 infant and adult cases, Kaunitz 8 confirmed that symptom onset often coincided with closure of the ductus arteriosus 2 months after birth. He also noted in the adult cases, right coronary artery dilation and significant development of collateralization from the right coronary artery to the left coronary artery 8. This became the first distinction proposed between infants who die in the first year and those who survive to adulthood.
ALCAPA complications
Complications of ALCAPA include:
- Heart attack
- Heart failure
- Heart rhythm problems
- Permanent damage to the heart
ALCAPA diagnosis
Typically, ALCAPA is diagnosed during infancy. In rare cases, this defect is not diagnosed until someone is a child or adult.
During an exam, you health care provider will likely find signs of ALCAPA, including:
- Abnormal heart rhythm
- Enlarged heart
- Heart murmur (rare)
- Rapid pulse
Tests that may be ordered include:
- Echocardiogram, which is an ultrasound that views the heart structures and blood flow inside the heart
- Electrocardiogram (ECG), which measures the electrical activity of the heart
- Chest x-ray
- Multislice computed tomography (MSCT) coronary angiography
- MRI, which provides a detailed image of the heart
- Cardiac catheterization, a procedure in which a thin tube (catheter) is placed into the heart to see blood flow and take accurate measurements of blood pressure and oxygen levels
ALCAPA treatment
Surgery is needed to correct ALCAPA. Only one surgery is needed in most cases. However, the surgery will depend on the baby’s condition and the size of the involved blood vessels.
If the heart muscle supporting the mitral valve is seriously damaged from decreased oxygen, the baby may also need surgery to repair or replace the valve. The mitral valve controls blood flow between the chambers on the left side of the heart.
A heart transplant can be done in case the baby’s heart is severely damaged due to lack of oxygen.
Medicines used include:
- Water pills (diuretics )
- Drugs that make the heart muscle pump harder (inotropic agents)
- Drugs that lower the workload on the heart (beta-blockers, ACE inhibitors)
ALCAPA heart surgery
Current surgical procedures are directed at establishing revascularization by creating a two–coronary artery system via either (1) a left subclavian artery-coronary artery anastomosis, (2) a saphenous vein bypass graft, (3) Takeuchi procedure (creation of an aortopulmonary window and an intrapulmonary tunnel extending from the anomalous ostium to the window), or (4) direct reimplantation 9. By establishing a patent two–coronary artery system, most patients experience normalization of left ventricular systolic function, thereby improving long-term survival.
The need for simultaneous mitral valve reconstruction, in the presence of severe insufficiency, is controversial because spontaneous improvement of mitral valve function often occurs following surgical revascularization.
Once revascularization to a two–coronary artery system is accomplished, most patients demonstrate improved left ventricular systolic function, decreased mitral valve insufficiency, and resolution of congestive heart failure symptoms. In many cases, the classic infarct pattern on electrocardiography eventually disappears following normalization of left coronary blood flow (see the image below). Occasionally, persistent refractory mitral regurgitation will necessitate delayed mitral valve repair or replacement.
A study of 23 infants indicated that in patients with anomalous left coronary artery from the pulmonary artery (ALCAPA), aortic reimplantation of the anomalous coronary artery is an effective means of improving myocardial function but is a less effective tool for treating severe mitral valve regurgitation. In 16 infants, the anomalous artery was directly implanted into the ascending aorta, while the seven remaining patients underwent repair with a trapdoor flap or tubular extension technique. The investigators evaluated left ventricular function and degree of mitral valve regurgitation over a 10-year follow-up period. Four of the patients died early in the postoperative period (within 12 days after surgery), but improvement in myocardial function was seen in all of the remaining patients. However, of five infants diagnosed preoperatively with severe mitral valve regurgitation, only one demonstrated improvement in this condition; two patients required mitral valve replacement 10.
In another study, a comparison of coronary transfer and Takeuchi repair (intrapulmonary tunnel) revealed equal improvement of left ventricular function and resolution of mitral valve regurgitation in both groups 11. However, patients undergoing the Takeuchi technique developed significant pulmonary regurgitation, whereas those that had coronary transfer did not develop this complication 11.
Postoperative care and precautions
Diuretics, and afterload reduction may be necessary until there is significant improvement in left ventricular systolic and diastolic function with resolution of mitral valve insufficiency. These medications improve cardiac output and eliminate the preoperative symptoms of congestive heart failure.
Although unusual, there remains a risk of cardiac dysrhythmia secondary to preoperative myocardial ischemia or infarction. Monitor continuously in the immediate postoperative period 7.
The clinical status of the patient, in relation to residual congestive heart failure symptoms, determines the frequency of postoperative outpatient follow-up visits.
Most patients do not require frequent cardiac evaluation following surgical revascularization once ventricular function and mitral valve insufficiency is dramatically improved. However, follow-up reevaluation, although infrequent should be performed.
ALCAPA survival rate
Without treatment, most babies with ALCAPA do not survive their first year. Children who do survive without treatment may have serious heart problems. ALCAPA left untreated, the mortality rate in the first year of life is 90% secondary to myocardial ischemia or infarction and mitral valve insufficiency leading to congestive heart failure. Sudden death may occur because of inadequate collateral circulation between the left and right coronary artery systems and/or development of arrhythmia.
With early treatment such as surgery, most babies do well and can expect a normal life. Routine follow-ups with a heart specialist (cardiologist) will be needed.
Estimated long-term survival at 20 years was recently shown to be 94.8% 12. No long-term studies of large populations of adults with corrected ALCAPA are available, but the prognosis is generally good. The late outcome after revascularization mainly depends on the extent of irreversible impaired left ventricular function and the presence of myocardial scar tissue 2.
Complications
Complications are rare. The need for future valve surgery depends on the occurrence of hemodynamic complications (eg, residual mitral valve insufficiency precipitated by permanent damage of the mitral valve architecture) following surgery.
Late complications related to coronary artery insufficiency are more likely to occur if revascularization was accomplished by any of the following:
- Surgical ligation
- Bypass grafts that may become occluded or stenotic
- Intrapulmonary tunnel technique, which may cause supravalvar pulmonary stenosis or, less commonly, become obstructed at the surgically created aortopulmonary window
Inadequate growth of the coronary anastomosis is possible, although unlikely, if surgical reimplantation of the left coronary artery was performed. This occurrence is similar to the rare reports of late coronary artery problems following the arterial switch procedure for transposition of the great vessels that also requires direct coronary transfer and reimplantation.
- Oncel G, Oncel D. Anomalous Origin of the Left Coronary Artery from the Pulmonary Artery: Diagnosis with CT Angiography. J Clin Imaging Sci. 2013;3:4. Published 2013 Jan 30. doi:10.4103/2156-7514.106618 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3625882[↩]
- Pena E, Nguyen ET, Merchant N, Dennie G. ALCAPA syndrome: Not just a pediatric disease. Radiographics. 2009;29:553–65.[↩][↩][↩][↩][↩][↩]
- Kristensen T, Kofoed KF, Helqvist S, Helvind M, Søndergaard L. Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) presenting with ventricular fibrillation in an adult: A case report. J Cardiothorac Surg. 2008;3:33.[↩][↩][↩][↩]
- Takimura CK, Nakamoto A, Hotta VT, Campos MF, Malamo M, Otsubo R. Anomalous origin of the left coronary artery from the pulmonary artery: Report of an adult case. Arq Bras Cardiol. 2002;78:309–14.[↩]
- Khanna A, Torigian DA, Ferrari VA, Bross RJ, Rosen MA. Anomalous origin of the left coronary artery from the pulmonary artery in adulthood on CT and MRI. AJR Am J Roentgenol. 2005;185:326–9.[↩][↩]
- Dodge-Khatami A, Mavroudis C, Backer CL. Anomalous origin of the left coronary artery from the pulmonary artery: Collective review of surgical therapy. Ann Thorac Surg. 2002;74:946–55.[↩][↩]
- Arciniegas E, Farooki ZQ, Hakimi M, Green EW. Management of anomalous left coronary artery from the pulmonary artery. Circulation. 1980 Aug. 62(2 Pt 2):I180-9.[↩][↩]
- Kaunitz PE. Origin of left coronary artery from pulmonary artery: Review of the literature and report of two cases. Am Heart J. 1947;33:182–206.[↩][↩]
- Erdinc M, Hosgor K, Karahan O. Repair of anomalous origin of the left coronary artery arising from right pulmonary artery with rolled-conduit-extended reimplantation in an adult. J Card Surg. 2011 Nov. 26(6):604-7.[↩]
- Kazmierczak PA, Ostrowska K, Dryzek P, et al. Repair of anomalous origin of the left coronary artery from the pulmonary artery in infants. Interact Cardiovasc Thorac Surg. 2013 Jun. 16(6):797-801.[↩]
- Neumann A, Sarikouch S, Bobylev D, et al. Long-term results after repair of anomalous origin of left coronary artery from the pulmonary artery: Takeuchi repair versus coronary transfer. Eur J Cardiothorac Surg. 2017 Feb 1. 51(2):308-15.[↩][↩]
- Lange R, Vogt M, Hörer J, Cleuziou J, Menzel A, Holper K, et al. Long-term results of repair of anomalous origin of the left coronary artery from the pulmonary artery. Ann Thorac Surg. 2007;83:1463–71.[↩]