Alternating hemiplegia of childhood
Alternating hemiplegia of childhood also called AHC or alternating hemiplegia syndrome is a very rare neurodevelopmental disorder with onset usually begin before the age of 18 months characterized by repeated episodes of weakness or paralysis that may affect one side of the body or the other (hemiplegia) or both sides of the body at once (quadriplegia) 1, 2, 3, 4, 5, 6, 7, 8. Additional episodic symptoms usually include intermittent abnormal eye movements or nystagmus with other oculomotor abnormalities, episodes of muscle stiffness or posturing (dystonia), respiratory and autonomic dysfunctions and in a substantial percentage of cases, seizures. Repeated sudden short term attacks of weakness or paralysis that may affect one side of the body or the other (hemiplegia) or both sides of the body at once (quadriplegia) are often preceded by precipitating factors such as environmental stress, bathing, and psychological factors 9. Some symptoms tend to occur in sequential distinctive phases and disappear with sleep 2, 3, 6, 5. The severity of alternating hemiplegia of childhood (AHC) and the specific types of episodes that occur can vary dramatically from one individual to another.
AHC is a rare disorder that was first reported in the medical literature in 1971 by doctors Simon Verret and John C. Steele 10. They described an unusual disorder in eight children who demonstrated intermittent episodes of weakness, affecting first one side of the body (hemiplegia), then the other, with onset in early childhood, including one child who manifested symptoms as early as 3 months of age.
The majority of cases of alternating hemiplegia of childhood (AHC2 or alternating hemiplegia of childhood type 2) are caused by a new change (pathogenic variant) in the ATP1A3 gene located at 19q13.2 [hg19] that is not inherited 11, 12, 13, 4. Therefore, most patients with AHC do not have a family history of the disorder. A small number of cases of AHC (alternating hemiplegia of childhood type 1 or AHC1) are caused by changes in the ATP1A2 gene, which encode two different alpha subunits of the neuronal NA+-K+ ATPase transmembrane ion pump 14, 15, 16, 13. When alternating hemiplegia of childhood does run in families, it follows an autosomal dominant pattern of inheritance 4, 13.
Mutations in the ATP1A3 gene are also found in patients with rapid-onset dystonia-parkinsonism (RDP) and cerebellar ataxia, areflexia, pes cavus, optic atrophy, and sensorineural hearing loss (CAPOS) syndrome 17, 18, 19, 20, 21. Rapid-onset dystonia-parkinsonism (RDP) is a non-dopa-responsive dystonia, with rapid onset of a few minutes to a few days before stabilization 22. Rapid-onset dystonia-parkinsonism (RDP) age at onset is between 9 months and 59 years and triggering factors are physical (e.g. exercise or childbirth) or psychological stress 22. CAPOS (cerebellar ataxia, areflexia, pes cavus, optic atrophy, and sensorineural hearing loss) syndrome is characterized by an early-childhood onset of recurrent episodes of acute ataxia associated with febrile illnesses 21. These acute episodes tend to decrease with time, but the neurologic sequelae are permanent and progressive, resulting in gait and limb ataxia and areflexia 21. Affected individuals also develop progressive visual impairment due to optic atrophy and sensorineural hearing loss beginning in childhood 21.
AHC affects males and females in equal numbers. The prevalence of AHC is estimated to occur in approximately 1 in 1,000,000 children under the age of 16 years, but this number could be underestimated due to the variability in clinical presentation and the lack of genetic analysis in the preceding epidemiologic data 23, 24. Symptoms usually become apparent within the first 18 months.
Alternating hemiplegia of childhood (AHC) underlying pathophysiological mechanisms remained poorly understood for many years, in part, because of its rarity and complex and highly variable symptoms which are not completely known 1. More research is necessary to improve early diagnosis, understand the full range of symptoms, and develop more effective treatments. Advanced molecular research has allowed a better understanding of causal genes involved and provides, at the same time, early confirmation of the diagnosis.
No specific therapy or cure exists for individuals with alternating hemiplegia of childhood. Treatment is directed toward the specific symptoms apparent in each individual. Treatment may require the coordinated efforts of a team of specialists. Pediatricians, pediatric neurologists, neurologists, ophthalmologists, and other healthcare professionals may need to systematically and comprehensively plan an affected child’s treatment. Because alternating hemiplegia of childhood is highly variable, an individualized treatment program needs to be devised for each child. The effectiveness of current therapies for alternating hemiplegia of childhood will vary greatly among affected individuals. What is effective for one person may not be effective for another.
Alternating hemiplegia of childhood treatment can be divided into acute management of the attacks and episode prevention (prophylaxis). Acute management is focused towards removing known triggers and early sleep facilitation. The use of buccal midazolam or rectal diazepam has been advocated by some authors to provide quick sedation 23. Episode prevention (prophylaxis) is focused towards avoiding known triggers and long-term drug treatment. A variety of medications have been proposed for the treatment of AHC, but calcium channel blockers are the most effective. The most common calcium channel blocker used is flunarizine in a dose of 5 to 20 mg per day 23. Flunarizine has been reported to reduce the frequency and severity of attacks, but not to completely stop them, and it is considered the drug of choice 25. Other proposed treatments include beta blockers, anticonvulsants, methysergide, amantadine, aripiprazole, and haloperidol. Antiepileptic drugs are effective in treating seizures only. Topiramate has been reported to positively influence the severity in some patients with AHC 26. A recent article reporting the use of adenosine-5′-triphosphate (ATP) orally with 2-year follow-up demonstrated promising and successful results 27. In addition, some reports supported the use of a ketogenic diet in patients with AHC 28, 29.
Alternating hemiplegia of childhood cause
In at least 78% of individuals, alternating hemiplegia of childhood is caused by a mutation in the ATP1A3 gene 30, 31, 4, 7. Very rarely, a mutation in the ATP1A2 gene is involved in alternating hemiplegia of childhood. Genes provide instructions for creating proteins that play a critical role in many functions of the body. When a mutation of a gene occurs, the protein product may be faulty, inefficient, or absent. Mutations in the ATP1A3 or ATP1A2 gene reduce the activity of the Na+/K+ ATPase (the sodium pump), impairing its ability to transport ions normally. It is unclear how a malfunctioning Na+/K+ ATPase causes the episodes of paralysis or uncontrollable movements characteristic of alternating hemiplegia of childhood.
In cases where a mutation in ATP1A3 gene is disease causing, alternating hemiplegia of childhood (AHC) almost always occurs as a new (sporadic or de novo) mutation, which means that in nearly all cases the gene mutation has occurred at the time of the formation of the egg or sperm for that child only, and no other family member will be affected 4, 7. Alternating hemiplegia of childhood is usually not inherited from or “carried” by a healthy parent, and in AHC, de novo (sporadic) mutations are more common than inherited mutations. However, when alternating hemiplegia of childhood (AHC) does run in families, it follows an autosomal dominant pattern of inheritance (where a trait is transmitted from either an affected mother or father to their child) and has been documented in at least one affected family with classic AHC, and in a number of patients with rapid-onset dystonia-parkinsonism (RDP), also due to mutations in ATP1A3 4, 13. For unknown reasons, the signs and symptoms are typically milder when AHC is found in multiple family members than when a single individual is affected.
The ATP1A3 gene provides instructions for making one part the alpha-3 subunit of a protein known as Na+/K+ ATPase or the sodium pump, that is required for the normal function of nerve cells in the brain 32. This protein uses energy from a molecule called adenosine triphosphate (ATP) to transport charged atoms (ions) into and out of cells. Specifically, Na+/K+ ATPase pumps sodium ions (Na+) out of cells and potassium ions (K+) into cells. Consequently, alternating hemiplegia of childhood (AHC) may be classified as a channelopathy, a group of disorders characterized by abnormalities in the flow of electrically charged particles known as ions (commonly calcium, sodium, and potassium) through pores in cell membranes (ion channels). These channels are involved in various functions of the body and, therefore, channelopathies can potentially cause a wide variety of symptoms.
Na+/K+ ATPases that include the alpha-3 subunit are primarily found in nerve cells (neurons) in the brain and are critical for their normal function. The movement of sodium and potassium ions helps regulate the electrical activity of these cells and plays an important role in the signaling process that controls muscle movement. The activity of Na+/K+ ATPase also helps regulate cell size (volume).
Additionally, Na+/K+ ATPase helps regulate a process called neurotransmitter reuptake. Neurotransmitters are chemicals that transmit signals from one neuron to another. After a neurotransmitter has had its effect, it must be removed quickly from the space between the neurons. The reuptake of neurotransmitters is carefully controlled to ensure that signals are sent and received accurately throughout the nervous system.
The spectrum of ATP1A3-related neurologic disorders includes rapid-onset dystonia-parkinsonism (RDP), alternating hemiplegia of childhood (AHC), and cerebellar ataxia, areflexia, pes cavus, optic atrophy, and sensorineural hearing loss (CAPOS) syndrome.
Because some individuals with alternating hemiplegia of childhood (AHC) do not have an identifiable mutation of the ATP1A3 gene, it is possible that mutations in other, yet to be discovered, genes may also be associated with alternating hemiplegia of childhood. Other genes which cause AHC or a disorder with similar symptoms include the CACNA1A, SLC1A3, and ATP1A2 in less than 1% of patients.
Families have noted that individuals may have “triggers” that precede a hemiplegic episode. Identified triggers for alternating hemiplegia of childhood include certain environmental situations such as extreme temperatures, crowds, irregular sleep habits, or specific odors; certain physical activities such as exercise: water exposure including bathing, swimming or showering; bright sunlight or fluorescent bulbs; certain foods such as chocolate or food dyes; certain medications; childhood illnesses and infections: and certain emotional situations such as stress, anxiety or fright. Although many different triggers have been reported, many episodes occur with no identifiable trigger.
Alternating hemiplegia of childhood inheritance pattern
Most cases of alternating hemiplegia of childhood (AHC) result from new (sporadic or de novo) mutations in the ATP1A3 or ATP1A2 gene and occur in people with no family history of the disorder in their family, which means that in nearly all cases the gene mutation has occurred at the time of the formation of the egg or sperm for that child only, and no other family member will be affected. However, alternating hemiplegia of childhood (AHC) can also run in families. When alternating hemiplegia of childhood (AHC) does run in families, it follows an autosomal dominant pattern of inheritance (where a trait is transmitted from either an affected mother or father to their child) (see Figure 1) 4, 13. For unknown reasons, the signs and symptoms are typically milder when AHC is found in multiple family members than when a single individual is affected.
In rare cases where alternating hemiplegia of childhood runs in families, it is thought that the disorder is inherited as an autosomal dominant trait. Genetic diseases are determined by the combination of genes for a particular trait that are on the chromosomes received from the father and the mother. Dominant genetic disorders occur when only a single copy of an abnormal gene is necessary for the appearance of the disease. The abnormal gene can be inherited from either parent, or can be the result of a new mutation (gene change) in the affected individual. The risk of passing the abnormal gene from affected parent to offspring is 50% for each pregnancy regardless of the sex of the resulting child.
Figure 1. Alternating hemiplegia of childhood autosomal dominant inheritance pattern
Alternating hemiplegia of childhood symptoms
Alternating hemiplegia of childhood symptoms vary with the grade of severity, type of complications, and associations with other morbidities which makes the diagnosis difficult 23, 1.
The first signs of alternating hemiplegia of childhood (AHC) may include:
- Uncontrollable eye movements (nystagmus)
- Temporary paralysis of one side of the body, leg, arm, or face (hemiplegia)
- Muscles contracting involuntarily (dystonia)
- Temporary paralysis from the neck down, including the trunk, and all four limbs (quadriplegia)
- Seizure-like episodes
Episodes have an early onset, usually in the first months of life, and are often preceded by trigger events. The first sign of AHC typically arises before one year of age, often with evidence of mild developmental delay and abnormal eye movements. Alternating hemiplegia of childhood (AHC) is characterized by short term episodes of hemiplegia which last minutes to days and affect either one or both sides of the body 23. The most frequent initial clinical signs and symptoms are muscle stiffness (tonic episode) or involuntary muscle contractions that cause repetitive or twisting movements (dystonia) with hemiplegia, quadriplegia, abnormal eye movements or nystagmus and autonomic phenomena often linked to trigger events. Short term attacks of tonic/dystonic episodes affect one side of the body, appearing with the extension of one limb.
Eye abnormalities mainly consist of nystagmus affecting only one eye, which may occur as an isolated manifestation or in association with tonic or dystonic signs 33. Brief episodes of monocular or binocular movements may occur including intermittent eye deviation, nystagmus, and dysconjugate gaze, which last for 1–3 minutes. The abnormal eye movements are most commonly unilateral and ipsilateral to the hemiplegia 34. As a general rule, infants and young children have flaccid hemiplegia, and older children are more likely to have dystonic features 23. Dystonia (involuntary muscle contractions that cause repetitive or twisting movements) may last from seconds to hours, and it is mostly one side of the body. The attack onset is abrupt which makes dystonia often mistaken for a seizure and hemiplegia for a stroke. During a single hemiplegic attack, the intensity of weakness is fluctuating. During long attacks, hemiplegia may change from one side to the other, or both sides may be affected 23. The arm is mostly weaker than the leg, and walking may not be impaired. Hemiplegia ceases during sleep and reappears on awakening but not immediately 23. Deterioration of consciousness was not associated with episodes of hemiplegia. Dystonic episodes may primarily affect the limbs on one side, causing hemidystonia, or affect the trunk, causing opisthotonic posturing. Headaches could occur at the onset of an attack but not after it. Writhing movements that suggest choreoathetosis could also be an associated feature. Mental slowing occurs early in the disease course, while mental regression and persistent neurological abnormalities could follow late in the course of the disease 35.
Autonomic phenomena such as vasomotor changes may also be observed 6, 5, 23. Incorpora et al. 36 described monozygotic twins who complained of hot water reflex seizures until 3 years each time they were immersed in a warm water bath or when hot water was sprinkled over the body eliciting irritability, unprovoked smile, head deviation, and upper-limb hypertonia to one side lasting from a few seconds to 3-4 minutes. These episodes ceased after crying. Guttural sounds and noisy breathing were observed. At 30 months, after a previous episode of an acute encephalopathy, both twins showed the typical signs of alternating hemiplegia of childhood, having numerous spontaneous episodes of dystonic movements prevalently on the right side together with ataxia, eye deviation, and autonomic disturbances.
In its classical AHC symptoms, the hemiplegic episodes are light and inconsistent at onset, but then gradually assume gradually the typical clinical manifestation 2, 3, 6, 5. Hemiplegia may start abruptly or progress over several minutes affecting one side and less frequently both sides. Episodes occur with variable frequencies and may last from a few minutes to several days. Consciousness is preserved during an attack, and the child is irritable and fretful. The arms were more severely affected than the lower limbs. During the same episode, hemiplegia may shift from one side to another. A hemiplegic event is followed by marked weakness. In association with hemiplegic episodes, tonic and dystonic movements, choreoathetosis, ataxia, nystagmus, and oculomotor abnormalities are often observed 2, 3, 23, 24, 14, 15.
One of the largest alternating hemiplegia of childhood studies was conducted by Sweney et al. 5 who reported 103 individuals (56 females and 47 males). According to these authors, the most frequent and early symptoms observed in the first 3 months of life were paroxysmal eye movements in 83% and hemiplegic episodes by 6 months in 56% of patients 5. Dystonic symptoms preceded hemiplegic episodes in 35/86 (41%) cases, and both dystonic and plegic episodes had a co-occurrence onset in 30/86 (35%) 5. The duration of dystonic and paralysis episodes was extremely variable, ranging from minutes to hours to days 5. Episodes of abnormal eye movements were reported in 96/103 (96%); nystagmus was the most frequent in 43/96 (50%) and on one side in 10/43 (23%). Intermittent cases of esotropia/exotropia or other monocular deviations were observed in 40/96 (41%) 5. Moreover, neurologic comorbidities including epilepsy were detected in 44/103 (43%), while cognitive involvement (from mild to moderate) was found in 96/96 (100%) 5. The neuropsychological evaluation performed in 41 alternating hemiplegia syndrome cases showed a high variability in the functional impairment for the cognitive, adaptive, and behavioral domains, which appeared more severe in older patients 5. Persistent ataxia was documented in 88/92 (95%) of the cases 5.
The variability in the clinical expression of paroxysmal and nonparoxysmal episodes in alternating hemiplegia of childhood patients is well known 37. Clinical signs and symptoms associated with hemiplegic events may be correlated to different gene mutations or to the influence of epigenetic factors 38. It is possible to observe an intrafamilial clinical variability as shown by Pavone et al. 39 following the clinical course and long-term outcomes of alternating hemiplegia of childhood in twin sisters. In these twins, clinical manifestations of alternating hemiplegia of childhood started in the early days of life with episodes of bath-induced abnormal ocular movements that persisted from the first months to 2 years, together with developmental delay. Preceded by episodes of acute encephalopathies, the twins at 2–3 years had paroxysmal hemiplegic events until 11 years when the hemiplegic attacks tended to regress in association with a gradual increase of migraine episodes 39. The twins did not have a similar clinical course with regard to the intensity and frequency of hemiplegic episodes, migraine attacks, and epileptic seizures, which were more pronounced in one of the twins 39. The cognitive impairment was mild in both twins. Recent diagnostic criteria were dictated by Rosewich et al. 40 and Mikaki et al. 37 who found that most alternating hemiplegia of childhood individuals show a variable degree of associated disorders such as neuropsychological abnormalities, developmental delay, intellectual disability, epileptic seizures, motor dysfunction, abnormal movements, migraine, sleep, and heart disturbances 23, 24, 14, 15.
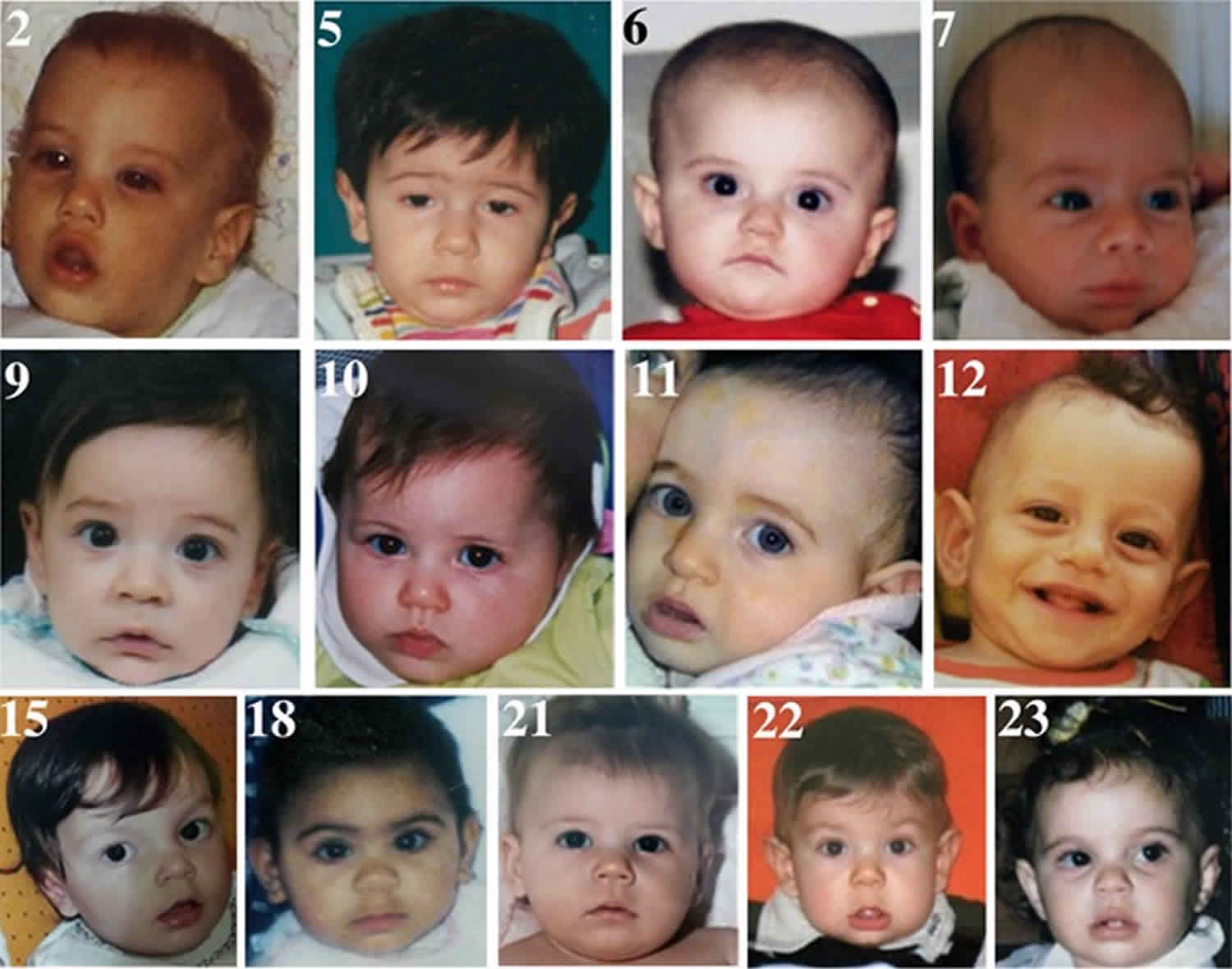
Figure 2. Alternating hemiplegia of childhood facial appearance in adults
Footnotes: Note the change in facial appearance. In some patients, there is persistence of hypotonia, thin eyebrows, and downturned mouth corners.
[Source 41 ]Paroxysmal symptoms
Paroxysmal symptoms alternating hemiplegia of childhood include an episode of uncoordinated movement which occurs occasionally and then stops.
The six categories of paroxysmal symptoms for alternating hemiplegia of childhood patients are:
Dystonia
An involuntary repetitive twisting and sustained muscle contractions. These result in abnormal movements and postures.
Hypotonia
A state of low muscle tone (the amount of tension or resistance to stretch in a muscle), often involving reduced muscle strength.
Abnormal eye movements
There are three categories of various eye movement disorders in alternating hemiplegia of childhood:
- Nystagmus – Rapid, involuntary movements on the eye. Movements may be horizontal (side-to-side), vertical (up and down), or rotary (circular).
- Esotropia – A form of strabismus in which one or both eyes turn inward. The condition can be constantly present, or occur intermittently, and can give the affected individual a “cross-eyed” appearance.
- Exotropia – A form of strabismus where the eyes have deviated outward.
Ataxia
A lack of muscle coordination which may affect speech, eye movements, the ability to swallow and eat, walking, picking up objects, and other voluntary movements.
Bulbar palsy
Bulbar palsy is the result of diseases affecting the lower cranial nerves. A speech deficit occurs due to paralysis or weakness of the muscles of articulation which are supplied by these cranial nerves.
Three types of bulbar palsy include:
- Dysarthria – difficult or unclear articulation of speech that is otherwise linguistically normal.
- Dysphagia – difficulty in swallowing and chewing food – it takes more effort than normal to transport food from the mouth to the stomach. Drooling may occur as saliva collects in the mouth and they aren’t able to swallow.
- Pseudobulbar Affect – a condition that causes uncontrollable crying and/or laughing that happens suddenly and frequently. A person having a pseudobulbar affect crying spell may cry when they don’t feel sad or when they only feel a little bit sad. Someone having a pseudobulbar affect laughing spell may laugh when they don’t feel amused or when they only feel a little bit amused.
Choreoathetosis
Ceaseless occurrence of rapid, highly complex jerky movements that appear to be well coordinated but are performed involuntarily. Choreoathetotic movements with various levels of intensity as well as ataxia, which were essentially static type, were observed in all cases reported by Aicardi et al 33. Choreoathetosis was observed in 22 (50%), ataxia in 30 (68%), and dysarthria in 29 (68%) of 44 cases reported by Mikati et al 2.
Neuropsychological disturbances
Jasien et al 42 evaluated neuropsychological abnormalities in 25 alternating hemiplegia of childhood individuals and found significant impairments in cognition, expressive and receptive language, executive function, attention, and behavior. In 20 of these patients, 10 showed attention-deficit/hyperactivity disorder (ADHD), 7 had disruptive behavior, and 3 had an anxiety disorder 42. Eight out of 25 subjects exhibited slower difficulty in comprehension, poor memory, inadequate academic performance, difficulty in math, and difficulty with self-help skills. Concerning the intellectual functioning was normal in 4/25 (16%), borderline in 3 (12%), and impaired in 18 (72%), of which in 6 (24%) were mild, in 10 (40%) moderate, and in 2 (8%) severe 42.
Intellectual disability
In 22 individuals observed by Aicardi et al. 33 5 had midly-delayed or borderline intelligence, 13 had moderate to severe cognitive difficulties, and 4 patients were not examined. In a report by Sakuragawa 6, cognitive impairment was found in 20 of 23 (87%) patients. Cognitive impairment was also observed by Mikaki et al. 2 in 40 out of 44 (90%) patients suggesting that the cognitive dysfunction correlated with the age of patients and with the age of onset of the hemiplegic attacks. This raised the doubt whether the cognitive impairment in alternating hemiplegia of childhood individuals is a primary effect of the disorder or linked to the paroxysmal episodes. Cognitive deficits of various degrees were found in 29 patients reported by Bourgeois 43: in 8 children was mild or borderline level, and in 21 moderate to severe. According to this study, the cognitive impairment was slow during the first 2 years of life and became more marked subsequently to finally reach a plateau before age 10 43. Polanowska et al. 44 performed neurological examinations in 2 adult patients and found normal or near-normal cognitive functioning with only isolated executive dysfunctions.
Epilepsy
Epileptic seizures are rarely observed at the onset of alternating hemiplegia of childhood, but during the course of the disorder, they are reported in about 50% of the cases 45, 24. In the study by Silver and Andermann 45, epileptic events were reported in 5 out of 10 (50%) patients, one of whom suffered from convulsive status epilepticus, and by Saguragawa 6 in 6 out of 9 (66%) alternating hemiplegia of childhood patients. Among 44 out of 103 (43%) alternating hemiplegia of childhood patients recorded by Sweney et al. 5, the seizures were generalized, tonic, or tonic-clonic types, and the mean age of epileptic onset attacks was reported to be around the age of 6 years. Ten children (23%) did not show further epileptic episodes until the age of 10 years or older 5. Epileptic seizures of generalized, tonic, or clonic types were reported by Saito et al 46 in 7 out of 9 (77%) alternating hemiplegia of childhood patients aged between 2 and 16 years, while ictal epileptiform discharges were detected only in 4 patients which complained of status epilepticus, repeated several times in 3 of them. Thirty-two of the 51 (62%) alternating hemiplegia of childhood patients described by Uchitel et al. 47 had epilepsy; in 18, the seizures were focal and mainly frontal. In 11 patients, seizures were generalized specifically, tonic-clonic, myoclonic, and/or absent 47. In 8 of these cases, the seizures preceded the other paroxysmal events 47. Epileptic seizures were also detected in 8/44 (19%) by Mikati et al.,[1] in 4/9 (44%) by Rosewich et al. 40 and in 2/4 (50%) by Pavone et al. 48..
Motor dysfunction
Delays in motor milestones were frequently observed. Bourgeois 43 reported in her study that independent walking in 24 subjects was achieved at an average age of 44 months, as well as poor fine motor control. The gross motor hypotonia was often observed from the onset 43. Another study conducted by Masoud et al. 49 in 23 alternating hemiplegia of childhood patients (9 males, 14 females, mean age 9 years and 4 months) was aimed to analyze gross motor, upper extremity motor control, motor speech, and dysphagia functions. According to their results, motor speech deficits were more severely affected than gross motor abnormalities measured by the Gross Motor Function Classification System (GMFCS) 49. Using Gross Motor Function Classification System (GMFCS) criteria, 20 patients were in the less than moderate category and only 3 in the moderate to severe category. Another important finding from this study was recognizing that the oropharyngeal function is the most severely affected brain domain in patients with alternating hemiplegia of childhood 49.
Migraine
It is a sign that is not commonly reported in patients with alternating hemiplegia of childhood or in family history. Migraine in family history was found in 2 out of 22 (9.1%) and in 3 cases as prodrome of the attacks in the patients reported by Sakuragawa 6. A family history of migraine in immediate family members was reported by Mikati et al 2 in 25% of the patients. A single case of migraine out of 10 was reported by Saito et al. 46 in a boy of 15 years old.
Heart dysfunction
Heart conduction abnormalities in alternating hemiplegia of childhoods have also been reported. Nakashima et al. 50 showed large fluctuations in heart rate variability, including low-and high-frequency components in a 20-year-old female while sleeping, but fluctuation rates were suppressed during paralytic attacks.
Other signs and symptoms
Paroxysmal manifestations of tonic or dystonic attacks, autonomic dysfunction such as dyspneic episodes, vasomotor changes, hyperventilation, hypoventilation/apnea, abnormal eye movements, and monocular-isolated nystagmus may often be observed in alternating hemiplegia of childhood patients 33, 43, 46.
Sleep
The clinical beneficial effect of sleep in this disorder has been widely demonstrated, but also the sleep dysfunction has been commonly reported in alternating hemiplegia of childhood subjects 33, 43. In a study by Kansagra et al. 51, 20 out of 22 alternating hemiplegia of childhood patients showed at least one type of sleep problem: 6 complained of obstructive sleep apnea syndrome, an abnormal mean overall apnea-hypopnea index, and an abnormal mean arousal index. Again, 16 had difficulty in falling asleep and staying asleep, or both, while 9 suffered from insomnia and 2 had delayed sleep-wake syndrome.
Alternating hemiplegia of childhood diagnosis
A diagnosis of alternating hemiplegia of childhood is based upon identification of characteristic symptoms, a detailed patient history, a thorough clinical evaluation and a variety of specialized tests.
Specific diagnostic criteria have been proposed for alternating hemiplegia of childhood. The seven criteria are 52:
- Onset of symptoms before 18 months;
- Repeated episodes of hemiplegia that sometimes involve both sides of the body;
- Quadriplegia that occurs as an isolated incident or as part of a hemiplegic attack;
- Relief from symptoms upon sleeping;
- Additional sudden onset attacks such as dystonia, tonic episodes, abnormal eye movements or autonomic dysfunction;
- Evidence of developmental delay or neurological abnormalities such as choreoathetosis, ataxia or cognitive disability;
- Cannot be attributed to another cause.
Clinical Testing
A diagnosis of alternating hemiplegia of childhood is primarily one of exclusion. A wide variety of specialized tests may be used to rule out other conditions. Such tests include magnetic resonance imaging (MRI), magnetic resonance angiography (MRA), and magnetic resonance spectroscopy (MRS). An MRI uses a magnetic field and radio waves to produce cross-sectional images of particular organs and bodily tissues such as brain tissue. An magnetic resonance angiography (MRA), images are produced to evaluate the blood vessels. A magnetic resonance spectroscopy (MRS) is used to detect metabolic changes in the brain and other organs.
Additional tests may include electroencephalogram (EEG), which measures electrical responses in the brain, and is typically used to identify epilepsy; metabolic screening to detect urine organic acids, which is indicative of certain metabolic disorders; studies of cerebrospinal fluid (CSF), which can exclude neurotransmitter deficiency disorders with similar episodic oculomotor abnormalities; erythrocyte sedimentation rates, which measures how long it takes red blood cells to settle in a test tube over a given period to detect inflammatory disorders; and hypercoagulable studies to detect disorders with a predisposition to forming blood clots.
In alternating hemiplegia of childhood individuals, the results of laboratory analysis for metabolic disorders, brain magnetic resonance imaging (MRI), angiographic MRI, CT scan, and CSF examination are normal.
Molecular genetic testing for mutations in the ATP1A3 gene is available on a clinical basis via individual targeted gene sequencing or as part of larger gene panels. Increasingly, ATP1A3 mutations are identified in the context of clinical exome sequencing.
Alternating hemiplegia of childhood treatment
No specific therapy or cure exists for individuals with alternating hemiplegia of childhood 53, 54. Treatment is directed toward the specific symptoms apparent in each individual. Treatment may require the coordinated efforts of a team of specialists. Pediatricians, pediatric neurologists, neurologists, ophthalmologists, and other healthcare professionals may need to systematically and comprehensively plan an affected child’s treatment. Because alternating hemiplegia of childhood is highly variable, an individualized treatment program needs to be devised for each child. The effectiveness of current therapies for alternating hemiplegia of childhood will vary greatly among affected individuals. What is effective for one person may not be effective for another.
Alternating hemiplegia of childhood treatment is generally focused on trying to reduce the frequency and severity of the characteristic episodes and the management of episodes when they occur. Acute treatment consists of removing the known triggers and facilitating early sleep. Triggers include psychological stress or excitement; environmental stressors (e.g., bright light, excessive heat or cold, excessive sound, crowds); water exposure (e.g., bathing, swimming); certain foods or odors (e.g., chocolate, food dyes, missed meals); excessive or atypically strenuous exercise; illness; irregular sleep (missing a nap, delayed bedtime. Avoiding triggers to the extent possible is recommended for individuals with alternating hemiplegia of childhood. In addition, long-term drug therapy may be recommended to help lessen the frequency of episodes.
A medication which has proved effective in reducing the frequency or severity of episodes in some individuals is a drug called flunarizine, a drug with calcium channel blocking properties. Flunarizine is given as a preventive (prophylactic) agent and has lessened the frequency, duration and severity of non-epileptic episodes in some individuals with alternating hemiplegia of childhood. Flunarizine is not readily available in the US. However, flunarizine is available in other countries for the treatment of migraine and other neurological symptoms. Flunarizine at a dose of 5–20 mg/day (frequently used dose 10 mg/day) 1.
Silver and Anderman 45 and Casaer 55 found that flunarizine treatment reduced the duration and severity of hemiplegic attacks, but only infrequently reduced the duration and severity of symptoms. Flunarizine was used in the treatment of alternating hemiplegia of childhood in 17 children by Bourgeois and Aicardi 56, who reported a significant decrease in the frequency of hemiplegic attacks (by more than 50%) in only one case, while 9 of the children had a significant decrease in the severity and duration of the hemoplegic attacks, with an average duration reduced from several days to a few hours. According to a large Japanese cooperative study of 23 cases of AHC, flunarizine was effective in decreasing the frequency and severity of the events in three-fourths of the cases 57. In the study by Mikaki et al 2 27 out of 44 alternating hemiplegia of childhood patients treated with flunarizine, a clinically worthy reduction in the frequency and/or severity was obtained in 21 patients (78%); flunarizine eliminated the hemiplegic attacks completely in 1 patient (4%), partially improved the attacks in 2 patients (7%), and was not effective in four patients. Sweney et al 5 obtained improvement in dystonic and hemiplegic episodes in 48 of 80 patients using flunarizine, and 21 of 55 patients using benzodiazepines. Neville and Ninan 52 suggest at the onset of each attack, the use of flunarizine, the treatment of the epileptic seizure, in attempting to avoid trigger situations, and rapid advice to sleep. In a cohort of 30 patients, Pisciotta et al 31 report the use of flunarizine as the most common treatment, which offers good results reducing duration and frequency of attacks in 50% of patients and decreased intensity in 32%, with major efficacy in younger patients.
Anti-seizure medications (anti-convulsants) are also used either alone or in combination to treat individuals with alternating hemiplegia of childhood who also have epilepsy and to prevent non-epileptic symptoms such as hemiplegia and dystonia. The effectiveness of these medications is highly variable and they are often minimally effective or ineffective. For treatment of epilepsy in AHC, there are no trials for assessing superiority of one drug over another. The efficacy of topiramate was reported in four patients by Jiang et al 58. Topiramate significantly improved the frequency and duration of hemiplegic attacks in all patients. A good response was also observed in AHC with additional symptoms, such as seizures, migraine, involuntary movements, autonomic symptoms, and impaired mental development 58. Drug-resistant epilepsy in AHC can respond to vagal nerve stimulation 47.
Van Hillegondsberg and Michaelis 59 use verapamil 6.6 mg/kg/day in 3 divided doses obtained an effective response in a 5-year-old boy with regard to frequency, severity, and duration, and after 6 months of free major debilitating episodes.
Samanta and Ramakrishnaiah 60 treated 2 patients with alternating hemiplegia of childhood with intravenous immunoglobulin (IVIG) infusion. An 8-year-old girl was treated for 4 years with periodic IVIG infusion, which resulted in free paroxysmal events during the first 16 months of treatment and a boy 2-year-old receiving IVIG infusion for 10 months remained seizure-free for 2 years since the beginning of treatment, but poor success was achieved with hemiplegic episodes.
Benzodiazepines such as diazepam have been used to reduce the duration of dystonic episodes.
Because some hemiplegic episodes have an early phase where individuals feel unwell, some researchers have recommended using certain medications to prematurely induce sleep. This can lessen the duration and severity of an episode. Such medications include buccal midazolam, chloral hydrate, melatonin, niaprazine or rectal diazepam.
Severe episodes of alternating hemiplegia of childhood can require hospitalization. In some cases, epileptic seizures can necessitate urgent medical intervention including intravenous to halt seizures or induce sleep in the setting of severe prolonged dystonia.
The various symptoms of alternating hemiplegia of childhood can affect a child’s growth and development. Episodes can disrupt daily life and impact a child’s ability to learn and participate in various activities. Proactive management of potential complications is required. A supportive team approach for children with alternating hemiplegia of childhood is of benefit and may include special education, physical therapy, and additional social, medical or vocational services. Genetic counseling may be of benefit for affected individuals and their families.
Alternating hemiplegia of childhood prognosis
Alternating hemiplegia of childhood long-term outcome was assessed by Bourgeois et al. 61 describing that the paroxysmal nystagmus is more likely to disappear before 10 years, while paroxysmal episodes of hemiplegia tend to persist at a high frequency. In addition to these, tonic, dystonic, and unilateral or bilateral paroxysms often present with high-intensity and painful sensations. Bourgeois et al 61 described that one of their patients died of bronchopneumonia after an episode of status epilepticus. Seven of the 9 patients suffered from epilepsy, developmental delay or intellectual disability, and severe language impairment 61. Besides, the gait incoordination persevered and 3 of them also showed signs of persistent hypotonia on the side mostly affected by hemiplegic attacks. They lived a normal life but needed continuous clinical assistance 61. In another work, Bourgeois evaluated on 29 alternating hemiplegia of childhood individuals a progressive reduction in intensity and frequency of the paroxysmal hemiplegic episodes while dystonic movements and choreoathetosis were constantly present 43. The data collected by Panagiotakaki et al. 24 in a large cohort of 157 patients with a median age of 20 months, ranging from 9 months to 52 years revealed that all patients experienced hemiplegic attacks, among which 86% of them had episodes of bilateral weakness, 88% had dystonic attacks, 53% had epileptic seizures, 72% developed chorea and/or dystonia, and 92% had cognitive impairment. The severity of symptoms was stable, except for abnormal eye movements and hypotonia, which regressed till disappearance into adulthood. Seven patients died due to severe plegic attacks or episodes of epileptic seizures 24.
The issue of beneficial effects of sleep, as underlined by Aicardi et al. 33 and Bourgeois 43 has been widely demonstrated. Also confirmed by Ricci 62 in a study of 4 children, the sleep structure, duration, cycle length, REM latency, and REM and slow-wave sleep percentages yielded normal results in all children.
Alternating hemiplegia of childhood life expectancy
The long-term outcome of patients with alternating hemiplegia of childhood is generally poor due to the associated developmental delays and gradual deterioration after severe attacks. The clinical course of alternating hemiplegia of childhood is more severe in sporadic cases than in familial ones. Prognosis is greatly influenced by the age of onset, especially early occurrence of hemiplegic spells. Children with neonatal-onset manifestation usually suffer from severe developmental delay. Recurrent convulsive status epilepticus leads to deterioration of psychomotor development. In some children, motor dysfunctions caused wheelchair-dependency, but others were able to have an independent life in adulthood. As patients become older, hemiplegic spells and abnormal ocular movements become less common and hypotonia less severe 35. The episodes of hemiplegic episodes tend to decrease as the child gets older 2, 3 and in a recent study by Cordani et al. 63, epileptic seizures were found in 24/39 (62%) of AHC patients, which may have a deleterious role in the course of alternating hemiplegia of childhood, particularly if seizures are drug-resistant. There is no proof that alternating hemiplegia of childhood limits life expectancy, but patients may complain of complications, such as aspiration, which may be life-threatening 1. When present, migraine, fine and gross motor dysfunction, walking disturbances, speech, and cognitive impairment tend to persist.
- Pavone P, Pappalardo XG, Ruggieri M, Falsaperla R, Parano E. Alternating hemiplegia of childhood: a distinct clinical entity and ATP1A3-related disorders: A narrative review. Medicine (Baltimore). 2022 Aug 5;101(31):e29413. doi: 10.1097/MD.0000000000029413[↩][↩][↩][↩][↩]
- Mikati MA, Kramer U, Zupanc ML, Shanahan RJ. Alternating hemiplegia of childhood: clinical manifestations and long-term outcome. Pediatr Neurol. 2000 Aug;23(2):134-41. doi: 10.1016/s0887-8994(00)00157-0[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Incorpora G, Pavone P, Polizzi A, Cocuzza M, Privitera M, Pavone L, Ruggieri M. An 11-year follow-up study of neonatal-onset, bath-induced alternating hemiplegia of childhood in twins. J Child Neurol. 2012 May;27(5):657-62. doi: 10.1177/0883073811436249[↩][↩][↩][↩][↩]
- Alternating hemiplegia of childhood. https://rarediseases.info.nih.gov/diseases/11/alternating-hemiplegia-of-childhood[↩][↩][↩][↩][↩][↩][↩]
- Sweney MT, Silver K, Gerard-Blanluet M, Pedespan JM, Renault F, Arzimanoglou A, Schlesinger-Massart M, Lewelt AJ, Reyna SP, Swoboda KJ. Alternating hemiplegia of childhood: early characteristics and evolution of a neurodevelopmental syndrome. Pediatrics. 2009 Mar;123(3):e534-41. doi: 10.1542/peds.2008-2027[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Sakuragawa N. Alternating hemiplegia in childhood: 23 cases in Japan. Brain Dev. 1992 Sep;14(5):283-8. doi: 10.1016/s0387-7604(12)80144-6[↩][↩][↩][↩][↩][↩][↩]
- Alternating Hemiplegia of Childhood. https://rarediseases.org/rare-diseases/alternating-hemiplegia-of-childhood[↩][↩][↩]
- Alternating hemiplegia of childhood. https://medlineplus.gov/genetics/condition/alternating-hemiplegia-of-childhood[↩]
- Pavone P, Pappalardo XG, Ruggieri M, Falsaperla R, Parano E. Alternating hemiplegia of childhood: a distinct clinical entity and ATP1A3-related disorders: A narrative review. Medicine (Baltimore). 2022 Aug 5;101(31):e29413. doi: 10.1097/MD.0000000000029413). Delays in attaining developmental milestones (developmental delays), intellectual disability (cognitive impairment) and persistent issues with balance and the presence of continuous dance-like movements of limbs or facial muscles (chorea) may occur independently of episodes of paralysis, weakness or stiffness and persist between episodes ((Alternating Hemiplegia of Childhood. https://rarediseases.org/rare-diseases/alternating-hemiplegia-of-childhood[↩]
- Verret S, Steele JC. Alternating hemiplegia in childhood: a report of eight patients with complicated migraine beginning in infancy. Pediatrics. 1971 Apr;47(4):675-80.[↩]
- Rosewich H, Thiele H, Ohlenbusch A, Maschke U, Altmüller J, Frommolt P, et al. Heterozygous de-novo mutations in ATP1A3 in patients with alternating hemiplegia of childhood: a whole-exome sequencing gene-identification study. Lancet Neurol. 2012;11:764–73. doi: 10.1016/S1474-4422(12)70182-5[↩]
- Ishii A, Saito Y, Mitsui J, Ishiura H, Yoshimura J, Arai H, et al. Identification of ATP1A3 mutations by exome sequencing as the cause of alternating hemiplegia of childhood in Japanese patients. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056120[↩]
- Heinzen EL, Swoboda KJ, Hitomi Y, et al. De novo mutations in ATP1A3 cause alternating hemiplegia of childhood. Nat Genet. 2012 Sep;44(9):1030-4. doi: 10.1038/ng.2358[↩][↩][↩][↩][↩]
- Fons C, Campistol J, Panagiotakaki E, Giannotta M, Arzimanoglou A, Gobbi G, Neville B, Ebinger F, Nevšímalová S, Laan L, Casaer P, Spiel G, Ninan M, Sange G, Artuch R, Schyns T, Vavassori R, Poncelin D; ENRAH Consortium. Alternating hemiplegia of childhood: metabolic studies in the largest European series of patients. Eur J Paediatr Neurol. 2012 Jan;16(1):10-4. doi: 10.1016/j.ejpn.2011.08.006[↩][↩][↩]
- Kirshenbaum GS, Dawson N, Mullins JG, Johnston TH, Drinkhill MJ, Edwards IJ, Fox SH, Pratt JA, Brotchie JM, Roder JC, Clapcote SJ. Alternating hemiplegia of childhood-related neural and behavioural phenotypes in Na+,K+-ATPase α3 missense mutant mice. PLoS One. 2013;8(3):e60141. doi: 10.1371/journal.pone.0060141[↩][↩][↩]
- Bassi MT, Bresolin N, Tonelli A, Nazos K, Crippa F, Baschirotto C, Zucca C, Bersano A, Dolcetta D, Boneschi FM, Barone V, Casari G. A novel mutation in the ATP1A2 gene causes alternating hemiplegia of childhood. J Med Genet. 2004 Aug;41(8):621-8. doi: 10.1136/jmg.2003.017863[↩]
- Dobyns WB, Ozelius LJ, Kramer PL, Brashear A, Farlow MR, Perry TR, et al. Rapid-onset dystonia parkinsonism. Neurology. 1993;43:2596–602. doi: 10.1212/WNL.43.12.2596[↩]
- Brashear A, DeLeon D, Bressman SB, Thyagarajan D, Farlow MR, Dobyns WB. Rapid-onset dystonia-parkinsonism in a second family. Neurology. 1997;48:1066–9. doi: 10.1212/WNL.48.4.1066[↩]
- de Carvalho Aguiar P, Sweadner KJ, Penniston JT, Zaremba J, Liu L, Caton M, et al. Mutations in the Na+/K + − ATPase alpha3 gene ATP1A3 are associated with rapid-onset dystonia parkinsonism. Neuron. 2004;43:169–75. doi: 10.1016/j.neuron.2004.06.028[↩]
- Brashear A, Dobyns WB, de Carvalho Aguiar P, Borg M, Frijns CJ, Gollamudi S, et al. The phenotypic spectrum of rapid-onset dystonia-parkinsonism (RDP) and mutations in the ATP1A3 gene. Brain. 2007;130:828–35. doi: 10.1093/brain/awl340[↩]
- Demos MK, van Karnebeek CD, Ross CJ, Adam S, Shen Y, Zhan SH, et al. A novel recurrent mutation in ATP1A3 causes CAPOS syndrome. Orphanet J Rare Dis. 2014;9:15. doi: 10.1186/1750-1172-9-15[↩][↩][↩][↩]
- Brashear A, Mink JW, Hill DF, Boggs N, McCall WV, Stacy MA, et al. ATP1A3 mutations in infants: a new rapid-onset dystonia-Parkinsonism phenotype characterized by motor delay and ataxia. Dev Med Child Neurol. 2012;54:1065–7. doi: 10.1111/j.1469-8749.2012.04421.x[↩][↩]
- Algahtani H, Ibrahim B, Shirah B, Aldarmahi A, Abdullah A. More Than a Decade of Misdiagnosis of Alternating Hemiplegia of Childhood with Catastrophic Outcome. Case Rep Med. 2017;2017:5769837. doi: 10.1155/2017/5769837[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Panagiotakaki E, De Grandis E, Stagnaro M, Heinzen EL, Fons C, Sisodiya S, de Vries B, Goubau C, Weckhuysen S, Kemlink D, Scheffer I, Lesca G, Rabilloud M, Klich A, Ramirez-Camacho A, Ulate-Campos A, Campistol J, Giannotta M, Moutard ML, Doummar D, Hubsch-Bonneaud C, Jaffer F, Cross H, Gurrieri F, Tiziano D, Nevsimalova S, Nicole S, Neville B, van den Maagdenberg AM, Mikati M, Goldstein DB, Vavassori R, Arzimanoglou A; Italian IBAHC Consortium; French AHC Consortium; International AHC Consortium. Clinical profile of patients with ATP1A3 mutations in Alternating Hemiplegia of Childhood-a study of 155 patients. Orphanet J Rare Dis. 2015 Sep 26;10:123. doi: 10.1186/s13023-015-0335-5[↩][↩][↩][↩][↩][↩]
- Neville B. G. R., Ninan M. The treatment and management of alternating hemiplegia of childhood. Developmental Medicine and Child Neurology. 2007;49(10):777–780. doi: 10.1111/j.1469-8749.2007.00777.x[↩]
- Tenney J. R., Schapiro M. B. Child Neurology: Alternating hemiplegia of childhood. Neurology. 2010;74(14):e57–e59. doi: 10.1212/WNL.0b013e3181d7d85b[↩]
- Ju J., Hirose S., Shi X.-Y., Ishii A., Hu L.-Y., Zou L.-P. Treatment with Oral ATP decreases alternating hemiplegia of childhood with de novo ATP1A3 Mutation. Orphanet Journal of Rare Diseases. 2016;11(1, article no. 55) doi: 10.1186/s13023-016-0438-7[↩]
- Ulate-Campos A., Fons C., Artuch R., et al. Alternating hemiplegia of childhood with a de novo mutation in atp1a3 and changes in SLC2A1 responsive to a ketogenic diet. Pediatric Neurology. 2014;50(4):377–379. doi: 10.1016/j.pediatrneurol.2013.11.017[↩]
- Roubergue A, Philibert B, Gautier A, Kuster A, Markowicz K, Billette de Villemeur T, Vuillaumier-Barrot S, Nicole S, Roze E, Doummar D. Excellent response to a ketogenic diet in a patient with alternating hemiplegia of childhood. JIMD Rep. 2015;15:7-12. doi: 10.1007/8904_2013_292[↩]
- Heinzen EL, Swoboda KJ, Hitomi Y, Gurrieri F, Nicole S, de Vries B, Tiziano FD, Fontaine B, Walley NM, Heavin S, Panagiotakaki E; European Alternating Hemiplegia of Childhood (AHC) Genetics Consortium; Biobanca e Registro Clinico per l’Emiplegia Alternante (I.B.AHC) Consortium; European Network for Research on Alternating Hemiplegia (ENRAH) for Small and Medium-sized Enterpriese (SMEs) Consortium; Fiori S, Abiusi E, Di Pietro L, Sweney MT, Newcomb TM, Viollet L, Huff C, Jorde LB, Reyna SP, Murphy KJ, Shianna KV, Gumbs CE, Little L, Silver K, Ptáček LJ, Haan J, Ferrari MD, Bye AM, Herkes GK, Whitelaw CM, Webb D, Lynch BJ, Uldall P, King MD, Scheffer IE, Neri G, Arzimanoglou A, van den Maagdenberg AM, Sisodiya SM, Mikati MA, Goldstein DB. De novo mutations in ATP1A3 cause alternating hemiplegia of childhood. Nat Genet. 2012 Sep;44(9):1030-4. doi: 10.1038/ng.2358[↩]
- Pisciotta L, Gherzi M, Stagnaro M, Calevo MG, Giannotta M, Vavassori MR, Veneselli E; I.B.AHC Consortium; De Grandis E. Alternating Hemiplegia of Childhood: Pharmacological treatment of 30 Italian patients. Brain Dev. 2017 Jun;39(6):521-528. doi: 10.1016/j.braindev.2017.02.001[↩][↩]
- ATP1A3 gene. https://medlineplus.gov/genetics/gene/atp1a3[↩]
- Aicardi J, Bourgeois M, Goutieres F. Alternating hemiplegia of childhood: clinical findings and diagnostic criteria. Andermann F, Aicardi J, Vigevano F, eds. In: Alternating Hemiplegia of Childhood. New York: Raven Press, 1995:3–18.[↩][↩][↩][↩][↩][↩]
- Heinzen E. L., Arzimanoglou A., Brashear A., et al. Distinct neurological disorders with ATP1A3 mutations. The Lancet Neurology. 2014;13(5):503–514. doi: 10.1016/s1474-4422(14)70011-0[↩]
- Gergont A., Kaciński M. Alternating hemiplegia of childhood: New diagnostic options. Neurologia i Neurochirurgia Polska. 2014;48(2):130–135. doi: 10.1016/j.pjnns.2013.05.003[↩][↩]
- Incorpora G, Pavone P, Ruggieri M, Cocuzza M, Mazzone L, Parano E, Privitera M. Neonatal onset of hot water reflex seizures in monozygotic twins subsequently manifesting episodes of alternating hemiplegia. Epilepsy Res. 2008 Feb;78(2-3):225-31. doi: 10.1016/j.eplepsyres.2007.08.003[↩]
- Mikati MA, Panagiotakaki E, Arzimanoglou A. Revision of the diagnostic criteria of alternating hemiplegia of childhood. Eur J Paediatr Neurol. 2021 May;32:A4-A5. doi: 10.1016/j.ejpn.2021.05.004[↩][↩]
- Panagiotakaki E. Alternating hemiplegia of childhood: the gap between paroxysmal manifestations and non-paroxysmal characteristics. Dev Med Child Neurol. 2019 May;61(5):506. doi: 10.1111/dmcn.14137[↩]
- Pavone P, Pappalardo XG, Incorpora G, Falsaperla R, Marino SD, Corsello G, Parano E, Ruggieri M. Long-term follow-up and novel genotype-phenotype analysis of monozygotic twins with ATP1A3 mutation in Alternating Hemiplegia of Childhood-2. Eur J Med Genet. 2020 Aug;63(8):103957. doi: 10.1016/j.ejmg.2020.103957[↩][↩][↩]
- Rosewich H, Thiele H, Ohlenbusch A, Maschke U, Altmüller J, Frommolt P, Zirn B, Ebinger F, Siemes H, Nürnberg P, Brockmann K, Gärtner J. Heterozygous de-novo mutations in ATP1A3 in patients with alternating hemiplegia of childhood: a whole-exome sequencing gene-identification study. Lancet Neurol. 2012 Sep;11(9):764-73. doi: 10.1016/S1474-4422(12)70182-5[↩][↩]
- Gurrieri, F, Tiziano, FD, Zampino, G, Neri, G. 2016. Recognizable facial features in patients with alternating hemiplegia of childhood. Am J Med Genet Part A 170A: 2698–2705. https://doi.org/10.1002/ajmg.a.37808[↩]
- Jasien JM, Bonner M, D’alli R, Prange L, Mclean M, Sachdev M, Uchitel J, Ricano J, Smith B, Mikati MA. Cognitive, adaptive, and behavioral profiles and management of alternating hemiplegia of childhood. Dev Med Child Neurol. 2019 May;61(5):547-554. doi: 10.1111/dmcn.14077[↩][↩][↩]
- Burgeous M. Alternating hemiplegia of childhood. A report of 29 cases and a review of the literature. Arzimanoglu A., Goutières F, In: Trends in Child Neurology. John Libbey Eurotext, 1996:163–8.[↩][↩][↩][↩][↩][↩][↩][↩]
- Polanowska KE, Dzieżyc K, Rosewich H, Ohlenbusch A, Seniów JB. Alternating Hemiplegia of Childhood in Two Adult Patients with a Mild Syndrome. Cogn Behav Neurol. 2018 Dec;31(4):214-219. doi: 10.1097/WNN.0000000000000178[↩]
- Silver K, Andermann F. Alternating hemiplegia of childhood: the natural history of the disorder in a group of 10 patients. Andermann F, Aicardi J, Vigevano F, eds In: Alternating Hemiplegia of Childhood. New York: Raven Press, 1995:3–18.[↩][↩][↩]
- Saito Y, Inui T, Sakakibara T, Sugai K, Sakuma H, Sasaki M. Evolution of hemiplegic attacks and epileptic seizures in alternating hemiplegia of childhood. Epilepsy Res. 2010 Aug;90(3):248-58. doi: 10.1016/j.eplepsyres.2010.05.013[↩][↩][↩]
- Uchitel J, Helseth A, Prange L, McLean M, Ghusayni R, Sachdev M, Hunanyan A, Mikati MA. The epileptology of alternating hemiplegia of childhood. Neurology. 2019 Sep 24;93(13):e1248-e1259. doi: 10.1212/WNL.0000000000008159. Epub 2019 Sep 4. Erratum in: Neurology. 2020 Oct 13;95(15):708.[↩][↩][↩][↩]
- Pavone P, Pappalardo XG, Mustafa N, Cho SY, Jin DK, Incorpora G, Falsaperla R, Marino SD, Corsello G, Parano E, Ruggieri M. Alternating Hemiplegia of Childhood: neurological comorbidities and intrafamilial variability. Ital J Pediatr. 2022 Feb 17;48(1):29. doi: 10.1186/s13052-021-01194-2[↩]
- Masoud M, Gordon K, Hall A, Jasien J, Lardinois K, Uchitel J, Mclean M, Prange L, Wuchich J, Mikati MA. Motor function domains in alternating hemiplegia of childhood. Dev Med Child Neurol. 2017 Aug;59(8):822-828. doi: 10.1111/dmcn.13443[↩][↩][↩]
- Nakashima T, Yasuda K, Kobayashi M, Wada H, Ishii A, Hirose S. Heart rate variability in a patient with alternating hemiplegia. Intractable Rare Dis Res. 2019 May;8(2):134-137. doi: 10.5582/irdr.2019.01060[↩]
- Kansagra S, Ghusayni R, Kherallah B, Gunduz T, McLean M, Prange L, Kravitz RM, Mikati MA. Polysomnography Findings and Sleep Disorders in Children With Alternating Hemiplegia of Childhood. J Clin Sleep Med. 2019 Jan 15;15(1):65-70. doi: 10.5664/jcsm.7572[↩]
- Neville BG, Ninan M. The treatment and management of alternating hemiplegia of childhood. Dev Med Child Neurol. 2007 Oct;49(10):777-80. doi: 10.1111/j.1469-8749.2007.00777.x[↩][↩]
- Samanta D. Management of Alternating Hemiplegia of Childhood: A Review. Pediatr Neurol. 2020 Feb;103:12-20. doi: 10.1016/j.pediatrneurol.2019.10.003[↩]
- Masoud M, Prange L, Wuchich J, Hunanyan A, Mikati MA. Diagnosis and Treatment of Alternating Hemiplegia of Childhood. Curr Treat Options Neurol. 2017 Feb;19(2):8. doi: 10.1007/s11940-017-0444-7[↩]
- Casaer P. Flunarizine in alternating hemiplegia in childhood. An international study in 12 children. Neuropediatrics. 1987 Nov;18(4):191-5. doi: 10.1055/s-2008-1052478[↩]
- Burgeous M, Aicardi J. The treatment of Alternating hemiplegia of childhood with flunarizine. Andermann F, Aicardi J, Vigevano F, eds. In: Alternating Hemiplegia of Childhood. New York: Raven Press, 1995:3–18.[↩]
- Sakuragawa N. The treatment of Alternating hemiplegia of childhood in Japan. Andermann F, Aicardi J, Vigevano F, eds In: Alternating Hemiplegia of Childhood. New York: Raven Press, 1995:199.[↩]
- Jiang W, Chi Z, Ma L, Du B, Shang W, Guo H, Wu W. Topiramate: a new agent for patients with alternating hemiplegia of childhood. Neuropediatrics. 2006 Aug;37(4):229-33. doi: 10.1055/s-2006-924721[↩][↩]
- Van Hillegondsberg LS, Michaelis IA. Alternating hemiplegia of childhood: First South African case report and verapamil as a possible treatment option. S Afr Med J. 2019 Feb 26;109(3):152-153. doi: 10.7196/SAMJ.2019.v109i3.13757[↩]
- Samanta D, Ramakrishnaiah R. Intravenous Immunoglobulin in the Treatment of Alternating Hemiplegia of Childhood. Clin Neuropharmacol. 2021 Jan-Feb 01;44(1):23-26. doi: 10.1097/WNF.0000000000000420[↩]
- Bourgeois M, Nevsimalova S, Aicardi J, Andermann F. Alternating hemiplegia of childhood: long-term outcome. Andermann F, Aicardi J, Vigevano F, eds. In: Alternating Hemiplegia of Childhood. New York: Raven Press, 1995:3–18.[↩][↩][↩][↩]
- Ricci S. Sleep studies of children with alternating hemiplegia of childhood. Alternating Hemiplegia of Childhood. Andermann, Aicardi, and Vigevano. 1995:95–8.[↩]
- Cordani R, Stagnaro M, Pisciotta L, Tiziano FD, Calevo MG, Nobili L; I.B.AHC Consortium; De Grandis E. Alternating Hemiplegia of Childhood: Genotype-Phenotype Correlations in a Cohort of 39 Italian Patients. Front Neurol. 2021 Apr 8;12:658451. doi: 10.3389/fneur.2021.658451[↩]