What is apoplexy
Apoplexy is a medical term for sudden loss of consciousness as a result of an acute vascular disturbance caused by the rupture of an intracerebral artery or its occlusion by thrombosis or embolism. The rupture of an intracerebral artery causing subarachnoid hemorrhage (SAH) may also be a cause. Apoplexy could also mean a sudden severe hemorrhage into any organ. For example, abdominal apoplexy or idiopathic spontaneous intraperitoneal hemorrhage, is a rare and often fatal condition resulting from a variety of disease processes affecting the arterial and venous abdominal vasculature 1. Adrenal apoplexy caused by massive bleeding into the adrenal gland can cause Addisonian crisis or adrenal crisis, which is a potentially life-threatening condition that results from an acute insufficiency of adrenal hormones (glucocorticoid or mineralocorticoid) and requires immediate treatment 2. Cerebellar apoplexy caused by thrombosis of the posterior inferior cerebellar artery 3.
Cerebral apoplexy
Cerebral apoplexy is more commonly known as stroke, is an acute compromise of the cerebral perfusion or vasculature or cerebrovascular accident 4. A stroke is a life-threatening medical emergency. Strokes happen when blood flow to your brain stops. Within minutes, brain cells begin to die. There are two kinds of stroke. The more common kind in the United States is called ischemic stroke, is caused by a blood clot that blocks or plugs a blood vessel in the brain. The other kind, called hemorrhagic stroke, is caused by a blood vessel that breaks and bleeds into the brain. “Mini-strokes” or transient ischemic attacks (TIAs), occur when the blood supply to the brain is briefly interrupted.
- Strokes are a medical emergency and urgent treatment is essential.
- A stroke can cause lasting brain damage, long-term disability, or even death.
- Stroke is the no. 3 cause of death in the United States. More than 140,000 people die each year from stroke in the United States.
- Stroke is the leading cause of serious, long-term disability in the United States.
- Each year, approximately 795,000 people suffer a stroke. About 600,000 of these are first attacks, and 185,000 are recurrent attacks.
- Nearly three-quarters of all strokes occur in people over the age of 65. The risk of having a stroke more than doubles each decade after the age of 55.
- Strokes can and do occur at ANY age. Nearly one fourth of strokes occur in people under the age of 65.
- Stroke death rates are higher for African-Americans than for whites, even at younger ages.
- On average, someone in the United States has a stroke every 40 seconds.
- Stroke accounted for about one of every 17 deaths in the United States in 2006. Stroke mortality for 2005 was 137,000.
- The risk of ischemic stroke in current smokers is about double that of nonsmokers after adjustment for other risk factors.
- Atrial fibrillation (AF) is an independent risk factor for stroke, increasing risk about five-fold.
- High blood pressure is the most important risk factor for stroke.
The sooner a person receives treatment for a stroke, the less damage is likely to happen.
If you suspect that you or someone else is having a stroke, call your local emergency number immediately and ask for an ambulance.
The main symptoms of stroke can be remembered with the word F.A.S.T.:
- Face – the face may have dropped on one side, the person may not be able to smile, or their mouth or eye may have dropped.
- Arms – the person with suspected stroke may not be able to lift both arms and keep them there because of weakness or numbness in one arm.
- Speech – their speech may be slurred or garbled, or the person may not be able to talk at all despite appearing to be awake.
- Time – it’s time to dial your local emergency number immediately if you see any of these signs or symptoms.
If you have any of these symptoms or if you suspect someone else is having a stroke, you must get to a hospital quickly to begin treatment. Acute stroke therapies try to stop a stroke while it is happening by quickly dissolving the blood clot or by stopping the bleeding.
Post-stroke rehabilitation helps individuals overcome disabilities that result from stroke damage. Drug therapy with blood thinners is the most common treatment for stroke.
Other symptoms and signs of stroke may include:
- Sudden numbness, paralysis or weakness of the face, arm or leg (especially on one side of the body)
- Sudden loss or blurring of vision in one or both eyes
- Dizziness
- Sudden trouble walking, dizziness, loss of balance or coordination
- Sudden confusion, trouble speaking or understanding speech
- Difficulty understanding what others are saying
- Problems with balance and co-ordination
- Difficulty swallowing (dysphagia)
- Sudden and very severe headache resulting in a blinding pain unlike anything experienced before
- Loss of consciousness
However, there may be other causes for these symptoms.
If you have any of these symptoms, you must get to a hospital quickly to begin treatment. Acute stroke therapies try to stop a stroke while it is happening by quickly dissolving the blood clot or by stopping the bleeding. Post-stroke rehabilitation helps individuals overcome disabilities that result from stroke damage. Drug therapy with blood thinners is the most common treatment for stroke.
Figure 1. Ischemic stroke
Figure 2. Hemorrhagic stroke
Figure 3. Transient ischemic attack (TIA) or mini stroke
Cerebral apoplexy causes
A stroke occurs when the blood supply to your brain is interrupted or reduced. This deprives your brain of oxygen and nutrients, which can cause your brain cells to die.
A stroke may be caused by a blocked artery (ischemic stroke) or the leaking or bursting of a blood vessel (hemorrhagic stroke). Some people may experience only a temporary disruption of blood flow to their brain (transient ischemic attack, or TIA). This causes what’s known as a mini-stroke, often lasting between a few minutes and several hours. TIAs should be treated urgently, as they’re often a warning sign you’re at risk of having a full stroke in the near future. Seek medical advice as soon as possible, even if your symptoms resolve.
There are two main causes of strokes:
- Ischemic stroke – where the blood supply is stopped because of a blood clot, accounting for 85% of all cases
- Hemorrhagic stroke– where a weakened blood vessel supplying the brain bursts
Risk factors for cerebral apoplexy
Many factors can increase your risk of a stroke. Some factors can also increase your chances of having a heart attack. Potentially treatable stroke risk factors include:
Lifestyle risk factors
- Being overweight or obese
- Physical inactivity
- Heavy or binge drinking
- Use of illicit drugs such as cocaine and methamphetamines
Medical risk factors
- High blood pressure (hypertension) — the risk of stroke begins to increase at blood pressure readings higher than 120/80 millimeters of mercury (mm Hg). Your doctor will help you decide on a target blood pressure based on your age, whether you have diabetes and other factors.
- Cigarette smoking or exposure to secondhand smoke.
- High cholesterol.
- Diabetes.
- Obstructive sleep apnea — a sleep disorder in which the oxygen level intermittently drops during the night.
- Cardiovascular disease, including heart failure, heart defects, heart infection or abnormal heart rhythm (atrial fibrillation).
Other factors associated with a higher risk of stroke include:
- Personal or family history of stroke, heart attack or transient ischemic attack.
- Being age 55 or older.
- Race — African-Americans have a higher risk of stroke than do people of other races.
- Gender — Men have a higher risk of stroke than women. Women are usually older when they have strokes, and they’re more likely to die of strokes than are men. Also, they may have some risk from some birth control pills or hormone therapies that include estrogen, as well as from pregnancy and childbirth.
Cerebral apoplexy prevention
Knowing your stroke risk factors, following your doctor’s recommendations and adopting a healthy lifestyle are the best steps you can take to prevent a stroke. If you’ve had a stroke or a transient ischemic attack (TIA), these measures may help you avoid having another stroke. The follow-up care you receive in the hospital and afterward may play a role as well.
Many stroke prevention strategies are the same as strategies to prevent heart disease. In general, healthy lifestyle recommendations include:
- Controlling high blood pressure (hypertension). One of the most important things you can do to reduce your stroke risk is to keep your blood pressure under control. If you’ve had a stroke, lowering your blood pressure can help prevent a subsequent transient ischemic attack or stroke.
Exercising, managing stress, maintaining a healthy weight, and limiting the amount of sodium and alcohol you eat and drink are all ways to keep high blood pressure in check.. In addition to recommending lifestyle changes, your doctor may prescribe medications to treat high blood pressure.
- Lowering the amount of cholesterol and saturated fat in your diet. Eating less cholesterol and fat, especially saturated fat and trans fats, may reduce the fatty deposits (plaques) in your arteries. If you can’t control your cholesterol through dietary changes alone, your doctor may prescribe a cholesterol-lowering medication.
- Quitting smoking. Smoking raises the risk of stroke for smokers and nonsmokers exposed to secondhand smoke. Quitting tobacco use reduces your risk of stroke.
- Controlling diabetes. You can manage diabetes with diet, exercise, weight control and medication.
- Maintaining a healthy weight. Being overweight contributes to other stroke risk factors, such as high blood pressure, cardiovascular disease and diabetes. Weight loss of as little as 10 pounds may lower your blood pressure and improve your cholesterol levels.
- Eating a diet rich in fruits and vegetables. A diet containing five or more daily servings of fruits or vegetables may reduce your risk of stroke. Following the Mediterranean diet, which emphasizes olive oil, fruit, nuts, vegetables and whole grains, may be helpful.
- Exercising regularly. Aerobic or “cardio” exercise reduces your risk of stroke in many ways. Exercise can lower your blood pressure, increase your level of high-density lipoprotein cholesterol, and improve the overall health of your blood vessels and heart. It also helps you lose weight, control diabetes and reduce stress. Gradually work up to 30 minutes of activity — such as walking, jogging, swimming or bicycling — on most, if not all, days of the week.
- Drinking alcohol in moderation, if at all. Alcohol can be both a risk factor and a protective measure for stroke. Heavy alcohol consumption increases your risk of high blood pressure, ischemic strokes and hemorrhagic strokes. However, drinking small to moderate amounts of alcohol, such as one drink a day, may help prevent ischemic stroke and decrease your blood’s clotting tendency. Alcohol may also interact with other drugs you’re taking. Talk to your doctor about what’s appropriate for you.
- Treating obstructive sleep apnea, if present. Your doctor may recommend an overnight oxygen assessment to screen for obstructive sleep apnea (OSA). If obstructive sleep apnea is detected, it may be treated by giving you oxygen at night or having you wear a small device in your mouth.
- Avoiding illicit drugs. Certain street drugs, such as cocaine and methamphetamines, are established risk factors for a TIA or a stroke. Cocaine reduces blood flow and can cause narrowing of arteries.
Preventive medications
If you’ve had an ischemic stroke or TIA, your doctor may recommend medications to help reduce your risk of having another stroke. These include:
- Anti-platelet drugs. Platelets are cells in your blood that initiate clots. Anti-platelet drugs make these cells less sticky and less likely to clot. The most commonly used anti-platelet medication is aspirin. Your doctor can help you determine the right dose of aspirin for you. Your doctor may also consider prescribing Aggrenox, a combination of low-dose aspirin and the anti-platelet drug dipyridamole, to reduce the risk of blood clotting. If aspirin doesn’t prevent your TIA or stroke, or if you can’t take aspirin, your doctor may instead prescribe an anti-platelet drug such as clopidogrel (Plavix).
- Anticoagulants. These drugs, which include heparin and warfarin (Coumadin), reduce blood clotting. Heparin is fast-acting and may be used over a short period of time in the hospital. Slower acting warfarin may be used over a longer term. Warfarin is a powerful blood-thinning drug, so you’ll need to take it exactly as directed and watch for side effects. Your doctor may prescribe these drugs if you have certain blood-clotting disorders, certain arterial abnormalities, an abnormal heart rhythm or other heart problems. Other newer blood thinners may be used if your TIA or stroke was caused by an abnormal heart rhythm.
Cerebral apoplexy diagnosis
To determine the most appropriate treatment for your stroke, your emergency team needs to evaluate the type of stroke you’re having and the areas of your brain affected by the stroke. They also need to rule out other possible causes of your symptoms, such as a brain tumor or a drug reaction. Your doctor may use several tests to determine your risk of stroke, including:
Physical examination. Your doctor will ask you or a family member what symptoms you’ve been having, when they started and what you were doing when they began. Your doctor then will evaluate whether these symptoms are still present. Your doctor will want to know what medications you take and whether you have experienced any head injuries. You’ll be asked about your personal and family history of heart disease, transient ischemic attack or stroke.
Your doctor will check your blood pressure and use a stethoscope to listen to your heart and to listen for a whooshing sound (bruit) over your neck (carotid) arteries, which may indicate atherosclerosis. Your doctor may also use an ophthalmoscope to check for signs of tiny cholesterol crystals or clots in the blood vessels at the back of your eyes.
- Blood tests. You may have several blood tests, which tell your care team how fast your blood clots, whether your blood sugar is abnormally high or low, whether critical blood chemicals are out of balance, or whether you may have an infection. Managing your blood’s clotting time and levels of sugar and other key chemicals will be part of your stroke care.
- Computerized tomography (CT) scan. A CT scan uses a series of X-rays to create a detailed image of your brain. A CT scan can show a hemorrhage, tumor, stroke and other conditions. Doctors may inject a dye into your bloodstream to view your blood vessels in your neck and brain in greater detail (computerized tomography angiography).
- Magnetic resonance imaging (MRI). An MRI uses powerful radio waves and magnets to create a detailed view of your brain. An MRI can detect brain tissue damaged by an ischemic stroke and brain hemorrhages. Your doctor may inject a dye into a blood vessel to view the arteries and veins and highlight blood flow (magnetic resonance angiography, or magnetic resonance venography).
- Carotid ultrasound. In this test, sound waves create detailed images of the inside of the carotid arteries in your neck. This test shows buildup of fatty deposits (plaques) and blood flow in your carotid arteries.
- Cerebral angiogram. In this test, your doctor inserts a thin, flexible tube (catheter) through a small incision, usually in your groin, and guides it through your major arteries and into your carotid or vertebral artery. Then your doctor injects a dye into your blood vessels to make them visible under X-ray imaging. This procedure gives a detailed view of arteries in your brain and neck.
- Echocardiogram. An echocardiogram uses sound waves to create detailed images of your heart. An echocardiogram can find a source of clots in your heart that may have traveled from your heart to your brain and caused your stroke. You may have a transesophageal echocardiogram. In this test, your doctor inserts a flexible tube with a small device (transducer) attached into your throat and down into the tube that connects the back of your mouth to your stomach (esophagus). Because your esophagus is directly behind your heart, a transesophageal echocardiogram can create clear, detailed ultrasound images of your heart and any blood clots.
Cerebral apoplexy treatment
Emergency treatment for stroke depends on whether you’re having an ischemic stroke blocking an artery — the most common kind — or a hemorrhagic stroke that involves bleeding into the brain.
Ischemic stroke
To treat an ischemic stroke, doctors must quickly restore blood flow to your brain.
Emergency treatment with medications
Therapy with clot-busting drugs must start within 3 hours if they are given into the vein — and the sooner, the better. Quick treatment not only improves your chances of survival but also may reduce complications. You may be given:
- Aspirin. Aspirin is an immediate treatment given in the emergency room to reduce the likelihood of having another stroke. Aspirin prevents blood clots from forming.
- Intravenous injection of tissue plasminogen activator (TPA). Some people can benefit from an injection of a recombinant tissue plasminogen activator (TPA), also called alteplase. An injection of TPA is usually given through a vein in the arm. This potent clot-busting drug needs to be given within 4.5 hours after stroke symptoms begin if it’s given in the vein. TPA restores blood flow by dissolving the blood clot causing your stroke, and it may help people who have had strokes recover more fully. Your doctor will consider certain risks, such as potential bleeding in the brain, to determine if TPA is appropriate for you.
Emergency procedures
Doctors sometimes treat ischemic strokes with procedures that must be performed as soon as possible, depending on features of the blood clot:
- Medications delivered directly to the brain. Doctors may insert a long, thin tube (catheter) through an artery in your groin and thread it to your brain to deliver TPA directly into the area where the stroke is occurring. The time window for this treatment is somewhat longer than for intravenous TPA but is still limited.
- Mechanical clot removal. Doctors may use a catheter to maneuver a tiny device into your brain to physically break up or grab and remove the clot.
However, recent studies suggest that for most people, delivering medication directly to the brain (intra-arterial thrombolysis) or using a device to break up or remove clots (mechanical thrombectomy) may not be beneficial. Researchers are working to determine who might benefit from this procedure.
Other procedures to decrease your risk of having another stroke
To decrease your risk of having another stroke or transient ischemic attack, your doctor may recommend a procedure to open up an artery that’s narrowed by fatty deposits (plaques). Doctors sometimes recommend the following procedures to prevent a stroke.
Options will vary depending on your situation:
- Carotid endarterectomy. In a carotid endarterectomy, a surgeon removes plaques from arteries that run along each side of your neck to your brain (carotid arteries). In this procedure, your surgeon makes an incision along the front of your neck, opens your carotid artery and removes plaques that block the carotid artery. Your surgeon then repairs the artery with stitches or a patch made from a vein or artificial material (graft). The procedure may reduce your risk of ischemic stroke. However, a carotid endarterectomy also involves risks, especially for people with heart disease or other medical conditions.
- Angioplasty and stents. In an angioplasty, a surgeon gains access to your carotid arteries most often through an artery in your groin. Here, he or she can gently and safely navigate to the carotid arteries in your neck. A balloon is then used to expand the narrowed artery. Then a stent can be inserted to support the opened artery.
Hemorrhagic stroke
Emergency treatment of hemorrhagic stroke focuses on controlling your bleeding and reducing pressure in your brain. Surgery also may be performed to help reduce future risk.
Emergency measures
If you take warfarin (Coumadin) or anti-platelet drugs such as clopidogrel (Plavix) to prevent blood clots, you may be given drugs or transfusions of blood products to counteract the blood thinners’ effects. You may also be given drugs to lower pressure in your brain (intracranial pressure), lower your blood pressure, prevent vasospasm or prevent seizures.
Once the bleeding in your brain stops, treatment usually involves supportive medical care while your body absorbs the blood. Healing is similar to what happens while a bad bruise goes away. If the area of bleeding is large, your doctor may perform surgery to remove the blood and relieve pressure on your brain.
Surgical blood vessel repair
Surgery may be used to repair blood vessel abnormalities associated with hemorrhagic strokes. Your doctor may recommend one of these procedures after a stroke or if an aneurysm or arteriovenous malformation (AVM) or other type of vascular malformation caused your hemorrhagic stroke:
- Surgical clipping. A surgeon places a tiny clamp at the base of the aneurysm, to stop blood flow to it. This clamp can keep the aneurysm from bursting, or it can prevent re-bleeding of an aneurysm that has recently hemorrhaged.
- Coiling (endovascular embolization). In this procedure, a surgeon inserts a catheter into an artery in your groin and guides it to your brain using X-ray imaging. Your surgeon then guides tiny detachable coils into the aneurysm (aneurysm coiling). The coils fill the aneurysm, which blocks blood flow into the aneurysm and causes the blood to clot.
- Surgical arteriovenous malformation (AVM) removal. Surgeons may remove a smaller AVM if it’s located in an accessible area of your brain, to eliminate the risk of rupture and lower the risk of hemorrhagic stroke. However, it’s not always possible to remove an AVM if its removal would cause too large a reduction in brain function, or if it’s large or located deep within your brain.
- Intracranial bypass. In some unique circumstances, surgical bypass of intracranial blood vessels may be an option to treat poor blood flow to a region of the brain or complex vascular lesions, such as aneurysm repair.
- Stereotactic radiosurgery. Using multiple beams of highly focused radiation, stereotactic radiosurgery is an advanced minimally invasive treatment used to repair vascular malformations.
Pituitary apoplexy
Pituitary apoplexy also known as pituitary infarction is a rare vision and life threatening disorder where bleeding occurs in the pituitary gland and/or blood flow to the pituitary gland is blocked 5, 6, 7, 8, 9, 10, 11. Apoplexy means “sudden death” usually caused by bleeding into an organ or loss of blood flow to an organ 11. The term pituitary apoplexy refers to the “sudden death” of the pituitary gland, usually caused by an acute ischemic infarction or hemorrhage. It is important to note that pituitary apoplexy may be divided into hemorrhagic or ischemic, each with unique neuroimaging findings, and some patients have elements of both 6. Pituitary apoplexy is commonly caused by bleeding inside a benign (non-cancerous) tumor of the pituitary called pituitary adenoma or pituitary neuroendocrine tumor 8, 9, 10. Pituitary adenomas are very common with more than 10,000 pituitary tumors being diagnosed each year in the United States and are often not diagnosed because these tumors are small and never cause any symptoms or health problems 12. When examining people who have died or who have had imaging tests (like MRI scans) of their head for other health problems, doctors have found that as many as 1 in 4 people may have a pituitary adenoma without knowing it 12. Pituitary tumors can occur in people of any age including in children, but they are most often found in older adults 12. The pituitary is damaged when the tumor suddenly enlarges. It either bleeds into the pituitary or blocks blood supply to the pituitary. The larger the tumor, the higher the risk for future pituitary apoplexy. When pituitary bleeding occurs in a woman during or right after childbirth, it is called Sheehan syndrome. This is a very rare condition.
Pituitary apoplexy is a rare event 10. According to recent epidemiological studies, the prevalence of pituitary apoplexy is about 6.2 cases per 100,000 in Banbury Oxfordshire, United Kingdom 13 and its incidence 0.17 episodes per 100,000 per year in Northern Finland 14. Between 2% and 12% of patients with all types of pituitary adenoma experience apoplexy and the diagnosis of pituitary tumor was unknown at time of apoplexy in more than 3 out of 4 cases 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40. If the nonfunctioning pituitary adenomas (often incidentalomas) were already known and that a decision was made to manage them conservatively, the risk of pituitary apoplexy was calculated to be between 0.2 and 0.6 events per 100 person-years in 2 metaanalyses 41, 42.
Pituitary apoplexy is characterized by a sudden onset of headache, visual symptoms and vomiting (Apoplexy Triad) with altered mental status, and hormonal dysfunction due to acute hemorrhage or infarction of a pituitary gland 6. An existing pituitary adenoma usually is present. Sudden-onset headache located behind the eyes is the most common symptom 43, 44. Several mechanisms have been theorized to explain the headache in pituitary apoplexy and include involvement of the superior division of the trigeminal nerve inside the cavernous sinus, meningeal irritation, dura-mater compression, or enlargement of sellar walls. Other symptoms include visual acuity impairment and visual field impairment from involvement of the optic nerve or chiasm, hemianopia, diplopia, ptosis, nausea and vomiting, altered mental status, and hormonal dysfunction 45, 46, 44, 47, 48, 49. Many patients complain of double vision, which is caused by extrinsic compression of one or several of the extraocular nerves traversing the cavernous sinus. The oculomotor is the nerve most commonly affected 50. Patients will have ptosis and lateral eye deviation, sometimes accompanied by pupillary dilation of the affected eye.
Pituitary apoplexy diagnosis is based on history, examination, and neuro-imaging. Radiographic features of pituitary apoplexy include enlargement of the pituitary gland, with or without bleeding 51. Macroscopic hemorrhage is common and occurs in about 85%. It shows peripheral enhancement around a non-enhancing infarcted center 51. Surrounding edema may be seen in the optic tracts and chiasm.
Pituitary apoplexy treatment can vary between conservative management to prompt resuscitation, including administration of intravenous corticosteroids to avoid Addisonian crisis and neurosurgical intervention, which generally is by the trans-sphenoidal route 7.
For patients presenting with neurological changes from pituitary tumor apoplexy, urgent surgical intervention is commonly performed for tumor resection, and optic apparatus decompression 52. Pituitary apoplexy treatment aims are to improve symptoms and relieve compression of local structures. For this purposes, surgical decompression is the most rapid way for achieving them 53, 52. It has been reported that the surgical outcomes of patients with pituitary apoplexy may be quite poor when compared to the surgical outcomes of patients operated for a pituitary tumor without pituitary apoplexy 54. Accordingly, a complete recovery of oculomotor nerve (the third cranial nerve) palsies has been described in 31–57% of patients presenting pituitary apoplexy after surgery 52, 55, 56, normalization of visual acuity in about 50% of the cases 55, 56, anterior pituitary function normalization in more than 50% of cases and complete tumor resection in most of the patients 57, 58.
Acute pituitary apoplexy can be life-threatening if its not diagnosed or treated in a timely manner. The prognosis (outlook) is good for people who is diagnosed and treated in a timely manner. When appropriately managed, visual symptoms often improve, but long-term (chronic) pituitary deficiency may remain 59.
Figure 4. Pituitary apoplexy
Footnote: Magnetic resonance image scans of a patient categorized as group 3 showing pituitary mass with low signal intensity in the T2-weighted image (A) and high signal intensity in the T1 weighted images (B) indicating acute hemorrhage.
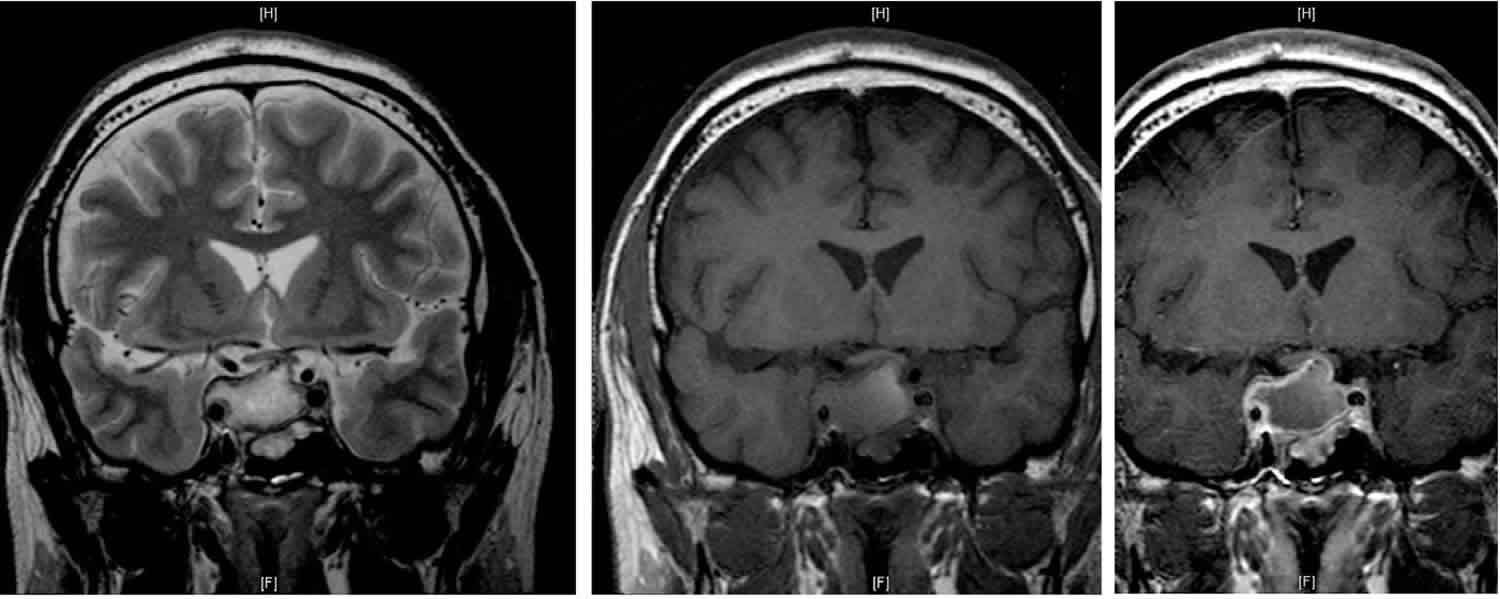
[Source 60 ]Figure 5. Pituitary apoplexy
Footnotes: 30 year old male with one month blurred vision and increasing headache. A 19 x 23 x 25 mm ovoid mass lesion is seen in the pituitary fossa with suprasellar extension (necrotic pituitary macroadenoma). The mass is not clearly separate from the anterior portion of the pituitary, which cannot be identified. A solid component is located anterolaterally (towards the left) and this component enhances. The optic chiasm is markedly compressed, draped over the superior aspect of the mass lesion. The mass is T1 and T2 hyperintense centrally with no significant enhancement post contrast. There are a few fine internal septations. No restricted diffusion within the mass. No calcifications. The remaining imaged brain, including posterior fossa structures, are normal. The white matter in the peri trigonal regions is thinned with undulation of the lateral margins of the lateral ventricles. This patient went on to have transphenoidal surgery. Final diagnosis is pituitary tumor (non-functioning gonadotroph cell adenoma).
[Source 61 ]Figure 6. Pituitary Ring Sign
Footnotes: A 55-year-old man presented with acute onset of severe headache, diplopia, nausea, and vomiting. The neuro-ophthalmologic examination showed a visual acuity with correction at the bedside of 20/50 (right eye), 20/70 (left eye), with normal color vision and confrontational visual fields. Pupils were normal without a relative afferent pupillary defect. Ocular motility, slit-lamp, and ophthalmoscopic examinations were normal. Post-contrast sagittal T1-weighted scan shows sphenoid sinus roof mucosal thickening. The horizontal arrow shows the “pituitary ring sign”; the vertical arrow shows sphenoid sinus roof mucosal thickening.
[Source 62 ]See your doctor if you have any symptoms of chronic pituitary insufficiency.
Go to the emergency room or call your local emergency number if you have symptoms of acute pituitary apoplexy, including:
- Eye muscle weakness or vision loss
- Sudden, severe headache
- Low blood pressure (which can cause fainting)
- Nausea
- Vomiting
If you develop these symptoms and you have already been diagnosed with a pituitary tumor, seek medical help right away.
What is the pituitary gland?
The pituitary is a small gland at the base of the skull, just below the brain and above the nasal passages and the fleshy back part of the roof of the mouth (known as the soft palate). The pituitary sits in a tiny bony space called the sella turcica. The nerves that connect the eyes to the brain, called the optic nerves, pass just above it. The pituitary is connected directly to part of the brain called the hypothalamus. This provides a key link between the brain and the endocrine system, a collection of glands and organs in the body that make hormones. Hormones are substances released into the blood that control how other organs work. The hypothalamus releases hormones into tiny blood vessels connected to the pituitary gland. These then cause the pituitary to make its own hormones. The pituitary is considered the “master control gland” because it makes the hormones that control the levels of other hormones made by most of the endocrine glands in the body.
The pituitary gland has 2 parts, the anterior pituitary and the posterior pituitary. Each part has distinct functions.
Figure 7. The pituitary gland location
Figure 8. Pituitary gland
Figure 9. The hypothalamus and pituitary gland (anterior and posterior) endocrine pathways and target organs
The anterior pituitary
Most pituitary tumors start in the larger, front part of the pituitary gland known as the anterior pituitary. This part of the gland makes several hormones:
- Growth hormone (GH, also known as somatotropin) promotes body growth during childhood. If a child or teen’s body makes too much growth hormone, they will grow very tall (a condition called gigantism). If an adult’s body makes too much growth hormone, the bones of the hands, feet, and face can grow larger than normal, distorting their features. This condition is called acromegaly.
Thyroid-stimulating hormone (TSH, also called thyrotropin) stimulates the thyroid gland to release thyroid hormones, which regulate body metabolism. Too much of these hormones makes you hyperactive and shaky, and too little makes you sluggish. If a pituitary tumor makes too much TSH, it can cause hyperthyroidism (an overactive thyroid gland). - Adrenocorticotropic hormone (ACTH, also known as corticotropin) causes the adrenal glands to make steroid hormones (such as cortisol). Too much ACTH from a pituitary tumor causes Cushing’s disease, the symptoms of which can include rapid weight gain and the build-up of fat in certain parts of the body, as well as high blood pressure and diabetes.
- Luteinizing hormone (LH) and follicle-stimulating hormone (FSH) are also called gonadotropins. In women their main effects are on the ovaries, where they control ovulation (the release of eggs) and the production of the hormones estrogen and progesterone. In men, LH and FSH control testosterone and sperm production in the testicles.
- Prolactin causes milk production in the female breast. Its function in men is not known. Pituitary tumors that make prolactin can cause reproductive problems, as well as milk production in both women and men.
The posterior pituitary
The smaller, back part of the pituitary gland is really an extension of brain tissue from the hypothalamus. The posterior pituitary stores and releases hormones made by the hypothalamus (vasopressin and oxytocin) into the bloodstream.
- Vasopressin (also called antidiuretic hormone, or ADH) causes the kidneys to keep water in the body, rather than losing it all in the urine. When vasopressin levels are low, a person urinates too much and becomes dehydrated. This condition is called diabetes insipidus. Vasopressin also can raise blood pressure by constricting blood vessels. It might have other functions as well.
- Oxytocin causes the uterus to contract in women during childbirth and the breasts to release milk when a woman breastfeed her baby. It might also have other functions in both men and women.
Tumors rarely start in the posterior pituitary.
Pituitary apoplexy causes
A pre-existing pituitary adenoma is usually found in cases of pituitary apoplexy 7. In the majority of the cases, the patients are unaware of the pituitary tumor 28.
Several predisposing or contributing factors for pituitary apoplexy include 64, 7:
- endocrine stimulation tests 65
- head trauma
- bromocriptine or cabergoline treatment 66, 67
- gonadotropin-releasing hormone treatment 68
- lumbar fusion in the prone position 69, 70
- pregnancy 71, 72
- pituitary irradiation 73
- anticoagulation 64
- thrombocytopenia (low platelet level) 74, 75
- erectile dysfunction medications 76
Sheehan syndrome refers to pituitary apoplexy of a nontumorous gland, presumably due to postpartum arterial spasm of arterioles supplying the anterior pituitary and its stalk. Sheehan syndrome occurs in postpartum women in which there is necrosis of the pituitary gland secondary to ischemia after significant bleeding during childbirth. It will present with adrenal insufficiency, hypothyroidism, and hypopituitarism, but rarely with visual changes. Most of the time, this entity is not included as a pituitary apoplexy as the gland did not have a pre-existing tumor, and visual symptoms are extremely rare. Sheehan syndrome is regarded as a neurologic emergency and is potentially lethal.
In 1937, Sheehan reported 11 cases of women who died in the puerperium, all of whom had necrosis of the anterior pituitary gland (adenohypophysis). Nine of the 11 cases had severe hemorrhage at delivery. The other 2 cases had no hemorrhage but were gravely ill prior to delivery. Usually, at least 1-2 liters of blood loss and hypovolemic shock are associated with a retained placenta. Sheehan syndrome occurs in 1-2% of women suffering significant postpartum hemorrhage. The clinical presentation of acute pituitary apoplexy has only been reported in the literature in a minority of patients with Sheehan syndrome. The more commonly reported scenario is a woman who develops amenorrhea years later, with a diagnosis of Sheehan syndrome being made retrospectively.
In Sheehan syndrome, lactation failure may occur as a result of prolactin deficiency, and there may be amenorrhea due to gonadotrophin deficiency. In addition, in the postpartum period, shaved pubic or axillary hair fails to regrow, and waxy skin depigmentation develops. Signs of hypothyroidism and hypoadrenalism may develop, and posterior pituitary (neurohypophysis) involvement with diabetes insipidus may occur. The less frequent involvement of the neurohypophysis probably stems from a difference in the anatomy of the vascular supply. The neurohypophysis contains an anastomotic ring of blood vessels that the adenohypophysis lacks.
The neuroimaging characteristics of Sheehan syndrome are distinctive. On MRI, the normal pituitary gland is largest in the immediate postpartum period, measuring up to 11.8 mm in height and convex in appearance. The anterior pituitary is usually hyperintense on T1-weighted images in pregnant and postpartum women when compared with controls. After delivery, the size of the pituitary gland rapidly returns to normal beyond the first week postpartum. The characteristic MRI finding in Sheehan syndrome is an enlarged pituitary gland bulging under the optic chiasm with peripheral enhancement surrounding an isointense gland; this characteristic MRI finding is called the “pituitary ring sign” 62.
Pituitary apoplexy may occur during pregnancy. Normally, the pituitary gland hypertrophies in pregnancy because of diffuse nodular hyperplasia of the prolactin secreting cells. This hypertrophy, combined with locally released factors, mediates vascular spasm and renders the pituitary more susceptible to infarction from compromised blood flow.
Okuda reported one woman with a giant pituitary adenoma who underwent triple bolus stimulation test with luteinizing hormone-releasing hormone, thyrotropin-releasing hormone (TRH), and insulin 77. The patient became stuporous, and computerized tomography (CT) scan revealed pituitary and subarachnoid hemorrhage (SAH). The investigators theorized that TRH-induced vasospasm may be a causative factor 77.
Some scientists associate pituitary apoplexy with administration of gonadotropin hormone-releasing hormone (GnRH). Corticotropin-releasing hormone (CRH) administration was associated with pituitary apoplexy in a patient with Cushing syndrome. In one study, bromocriptine therapy was associated with high T1 signal in the pituitary tumor on magnetic resonance imaging (MRI), but none of the patients studied had clinical evidence of pituitary apoplexy. Others associate pituitary apoplexy with long-term bromocriptine therapy.
Pituitary apoplexy can occur after head trauma. This probably results from shear forces applied to the pituitary stalk with contusion, hemorrhage, and infarction of the adenoma.
Pituitary apoplexy resulting in internal carotid artery occlusion has been reported due to the mass compressing the bilateral cavernous sinuses, resulting in obliteration of the cavernous portion of the right internal carotid artery 78.
Pituitary apoplexy during induction chemotherapy for acute myeloid leukemia has been reported by Silberstein and colleagues 79.
Pituitary apoplexy has been reported after cardiac bypass surgery by Thurtell and colleagues 80.
Brar and Garg reported a case of pituitary apoplexy in a young man who ascended to high altitude gradually, even after proper acclimatization 81.
Pituitary apoplexy has been reported in a patient with dengue fever and thrombocytopenia 82. Kruljac et al reported a patient with pituitary metastasis presenting as ischemic pituitary apoplexy after heparin-induced thrombocytopenia 83.
Weisberg warns that radiotherapy is potentially hazardous in pituitary tumors with prior hemorrhagic, necrotic, or cystic changes 84.
Some believe that pituitary apoplexy is more prevalent in patients who produce excess pituitary hormones (eg, acromegaly, Cushing syndrome), perhaps because the tumor is fueled by the hormones. Others report that most pituitary tumors that undergo apoplexy are endocrinologically silent.
Ahmed and Semple reviewed the potential complications of pituitary apoplexy, one being mechanical occlusion of the internal carotid arteries in the cavernous sinus, and the other being vasospasm 85. Both may result in brain ischemia.
Pituitary apoplexy risk factors
Pituitary apoplexy risk factors include 5, 86:
- Pituitary Tumor
- Non-functioning pituitary macroadenoma
- Certain functional tumors
- Hypertension and/or hypotension
- Surgery
- Cardiac surgery (heart lung bypass; coronary artery grafts)
- Cerebral angiography
- Major orthopedic procedures
- Drugs
- Endocrine stimulation tests (thyrotropin releasing hormone stimulation; insulin tolerance test)
- Anticoagulation therapy
- Estrogen
- Head Trauma
- Changes in intracranial pressure
- Pregnancy and Delivery (Sheehan syndrome)
- Infections
- Dengue fever
- Hypophyisitis
- Radiation therapy
- Medical treatment of a prolactinoma (especially with bromocriptine) 87.
Pituitary apoplexy pathophysiology
Pituitary apoplexy pathophysiology is not fully understood, but it is noteworthy that most cases involve patients with pituitary adenomas or, less commonly, in a nonadenomatous gland from infarction or hemorrhage 88, 89, 21, 26, 90.
The anterior pituitary gland is perfused by its portal venous system, which passes down the hypophyseal stalk. This unusual vascular supply likely contributes to frequency of pituitary apoplexy. It is more common in macroadenomas and nonfunctioning adenomas, and it rarely has been reported in microadenomas 91.
Some theorized that a gradually enlarging pituitary tumor becomes impacted at the diaphragmatic notch, compressing and distorting the hypophyseal stalk and its vascular supply 92. This deprives the anterior pituitary gland and the tumor itself of its vascular supply, apoplectically causing ischemia and subsequent necrosis.
Another theory stipulates that rapid expansion of the tumor outstrips its vascular supply, resulting in ischemia and necrosis 92. This explanation is doubtful, since most tumors that undergo apoplexy are slow growing.
Nevertheless, pituitary adenomas are prone to bleed and undergo infarction and necrosis, possibly because pituitary gland has this unique rich vascular structure and/or because pituitary tumors which have a high-energy requirement may outgrow their blood supply or because ischemia (and thus infarction) occurs after compression of infundibular or superior hypophyseal vessels against the sellar diaphragm by the expanding tumor mass with intrinsically poor vascularity 90, 93, 94.
Moreover, as recently demonstrated, pituitary tumor cells are particularly sensitive to glucose deprivation 95. In this setting, all clinical situations that acutely decrease systemic blood pressure, such as cardiac, vascular, or orthopedic surgery may decrease blood supply to the pituitary adenoma and precipitate pituitary apoplexy 92. Dynamic tests or hypoglycemia that acutely increase the metabolic needs of the tumor may also precipitate pituitary apoplexy, as well as severe vomiting/diarrhea with concomitant increased Valsalva pressure.
The inherent fragility of pituitary tumor blood vessels may also explain the hemorrhagic tendency 96. The blood vessels of pituitary adenomas show signs of incomplete maturation and poor fenestration, and their basal membranes are often ruptured 45, 97, 98. Immunohistochemical expression of vascular endothelial growth factor was found to correlate positively with the risk of pituitary hemorrhage 99. Pituitary tumor-transforming gene (PTTG), which is correlated to vascularization and expression of vascular endothelial growth factor 100, is also overexpressed in pituitary adenomas 101, 102. Fetal liver kinase 1, a vascular marker, is also expressed, particularly in nonfunctioning pituitary adenomas, particularly in older subjects 103, as is nestin, another vascular marker 104.
Whatever the mechanism, the extent of hemorrhage and necrosis will produce an increase in intrasellar pressure 21, 45, 105, 106, 107, which in turn leads to more or less pronounced compression of neighboring structures, thus explaining the broad clinical spectrum, from “classical” acute pituitary apoplexy to totally silent necrotic and/or hemorrhagic pituitary adenomas found only on pathological examination.
Pituitary apoplexy symptoms
Pituitary apoplexy usually has a short period of symptoms (acute), which can be life threatening. Pituitary apoplexy is characterized by a sudden onset of headache, visual symptoms, altered mental status, and hormonal dysfunction due to acute hemorrhage or infarction of a pituitary gland.
Pituitary apoplexy symptoms during the acute phase of apoplexy often include 5, 108:
- Severe headache (worst of your life) (95%). Sudden-onset headache located behind the eyes is the most common symptom 43, 44. Several mechanisms have been theorized to explain the headache in pituitary apoplexy and include involvement of the superior division of the trigeminal nerve inside the cavernous sinus, meningeal irritation, dura-mater compression, or enlargement of sellar walls. Other symptoms include decreased visual acuity, hemianopia, diplopia, ptosis, nausea and vomiting, altered mental status, and hormonal dysfunction.[2][28][29][30][31] Many patients complain of double vision, which is caused by extrinsic compression of one or several of the extraocular nerves. The oculomotor is the nerve most commonly affected.[23] Patients will have ptosis and lateral eye deviation, sometimes accompanied by pupillary dilation of the affected eye.
- Paralysis of the eye muscles, causing double vision (ophthalmoplegia) or problems opening an eyelid (78%)
- Loss of peripheral vision (52%) or loss of all vision in one or both eyes (64%)
- Low blood pressure (hypotension) (95%), nausea, loss of appetite, and vomiting (70%) from acute adrenal insufficiency
- Personality changes due to sudden narrowing or spasm of one of the arteries in the brain (anterior cerebral artery)
- Hemiplegia rare
- Meningismus rare
- There is one case report of stubborn hiccups in association with pituitary apoplexy 109
Less commonly, pituitary dysfunction may appear more slowly. In Sheehan syndrome, for example, the first symptom may be a failure to produce milk caused by a lack of the hormone prolactin after delivery.
As the hemorrhagic infarct resolves, over time problems with other pituitary hormones may develop, causing hypopituitarism symptoms 108:
- Growth hormone deficiency
- Adrenal insufficiency (if not already present or treated)
- Hypogonadism (body’s sex glands produce little or no hormones)
- Hypothyroidism (thyroid gland does not make enough thyroid hormone)
In rare cases, when the posterior (back part) of the pituitary is involved, symptoms may include 108:
- Failure of the uterus to contract to give birth to a baby (in women)
- Failure to produce breast milk (in women)
- Frequent urination and severe thirst (diabetes insipidus)
Pituitary apoplexy complications
Complications of untreated pituitary apoplexy can include:
- Adrenal crisis also called Addisonian crisis (condition that occurs when there is not enough cortisol, a hormone produced by the adrenal glands)
- Vision loss
- Frontal lobe herniation and chiasmal herniation have been reported 110
If other missing hormones are not replaced, symptoms of hypothyroidism and hypogonadism may develop, including infertility.
In pituitary apoplexy, the most impacting clinical problem is the lack of secretion of adrenocorticotropic hormone (ACTH), which occurs in more than two-thirds of the patients with pituitary apoplexy 7. The lack of secretion causes a cessation of cortisol secretion by the adrenal gland, which produces a variety of symptoms called “adrenal crisis” 46, 43. The patient may have nausea and vomiting, abdominal pain, bradycardia and hypotension, hypothermia, lethargy, and sometimes coma.
Pituitary apoplexy diagnosis
Your doctor will perform a physical exam and ask about your symptoms.
Tests that may be ordered include:
- Eye exams
- Magnetic resonance imaging (MRI) or computed tomographic (CT) scan of your brain
Neuroimaging
Magnetic resonance imaging (MRI) scan, as seen in Figure 1 above is the most sensitive imaging study for evaluating the pituitary gland, possibly visualizing hemorrhage not seen on CT scan 46, 50. In the first 3-5 days, hemorrhage within the sella is isointense or hypointense on T1-weighted images. On T2-weighted sequences, the blood appears hypointense. A characteristic MRI finding in ischemic (nonhemorrhagic) pituitary apoplexy is an enlarged pituitary gland bulging under the optic chiasm with peripheral enhancement surrounding a hypointense gland. Vaphiades coined the phrase “pituitary ring sign” to denote this MRI appearance (see Figure 6) 62. Vaphiades retrospectively reviewed the cranial MRIs of 3 patients with ischemic (nonhemorrhagic) pituitary apoplexy; all 3 patients displayed the “pituitary ring sign” 62. The term “pituitary ring sign” is used to describe an enlarged pituitary gland bulging under the optic chiasm, with peripheral enhancement surrounding a hypointense gland 62. This MRI appearance was first noted in 1995 by Lavallée et al. in a patient with Sheehan syndrome on a contrast-enhanced computed tomography (CT) scan and on a T1-weighted contrast-enhanced MRI scan and thought to be unique to ischaemic apoplexy in patients with Sheehan syndrome 111. However, the ring sign is not specific for pituitary infarction, because it can be seen in association craniopharyngioma 112 in addition to lymphocytic hypophysitis, pituitary abscess, and pituitary adenoma that has not undergone apoplexy 113. Lymphocytic hypophysitis is a rare inflammatory disorder of the pituitary gland, commonly manifesting late in pregnancy or during the postpartum period. It can mimic pituitary adenoma. In the majority of cases, the lymphocytic hypophysitis diagnosis is made after pituitary surgery for suspected pituitary adenoma 114.
Sphenoid sinus mucosal thickening, occurring in the setting of pituitary apoplexy, was first described by Arita et al. in 2001 115. They retrospectively evaluated 14 patients with pituitary apoplexy. The mucosa of the sphenoid sinus on MRI had thickened the compartment just beneath the sella turcica in 9 of 11 patients obtained within 7 days after the onset of apoplectic symptoms 115. Controls consisted of MR images obtained in 100 consecutive patients with pituitary adenomas but without apoplectic symptoms. Included in this group were 58 functioning and 42 non-functioning pituitary adenomas. Fifteen patients experienced thickening of the sphenoid sinus mucosa, including five with some apparent pansinusitis. The incidence of mucosal thickening of the sphenoid sinus in the patients with apoplexy was significantly greater than that in the patients without apoplexy 115. On histopathological specimens in the apoplexy patients, the thickened sphenoid sinus mucosa demonstrates a swollen sub-epithelial layer presumably responsible for the rim of MRI gadolinium enhancement 115. In 2006, Liu et al. 23 performed a retrospective review of 28 patients with pituitary apoplexy. Thickening of sphenoid sinus mucosa was present in 22 (79%) of these patients. They also noted that patients with thickened sphenoid sinus mucosa had larger tumours, a higher rate of cranial nerve deficits at presentation than those without mucosal thickening, and a higher rate of hypopituitarism and subsequent long-term hormone replacement therapy compared with those patients without thickened mucosa 23. In 2012, Agrawal et al. 116 concluded that there is a temporal association with the radiographic finding of sphenoid sinus mucosal thickening and pituitary apoplexy and that sphenoid sinus mucosal thickening may precede an apoplectic event. Each sign, “pituitary ring sign” and “sphenoid sinus mucosal thickening”, alone may exist in patients with or without pituitary apoplexy, yet presence of both signs together in the appropriate clinical context is a strong predictor of pituitary apoplexy 62. This is important because timely diagnosis treatment of pituitary apoplexy may be vision- and life-saving 62 .
Kaplun and colleagues reported the MRI evolution of pituitary changes in 2 patients with Sheehan syndrome 117. The first case initially had the pituitary ring sign, although MRI later showed an empty sella with shrinkage of the pituitary.
CT scanning generally is the initial imaging study of choice in the emergency department for patients who present with sudden-onset severe headache, visual loss, and/or ophthalmoplegia suggestive of subarachnoid hemorrhage (SAH). CT scanning can help to exclude subarachnoid hemorrhage (SAH) from an aneurysm by showing an intrasellar mass with hemorrhagic components, seen in 80% of pituitary apoplexy cases 43.
Binning and colleagues reported 6 patients with Rathke cleft cyst apoplexy presenting with the clinical and imaging features of both hemorrhagic and nonhemorrhagic pituitary apoplexy 118.
Liu et al 119 reported spontaneous partial or complete radiological disappearance of adenoma following pituitary apoplexy without the use of dopaminergic agonists (which may result in regression of pituitary adenoma).
Blood tests
Pituitary hormonal evaluation is required, as nearly 80% of patients present with a deficiency of at least one of the anterior pituitary hormones 8. The most common deficiencies are growth hormone deficit in 90% of the patients and ACTH (adrenocorticotropic hormone) deficit in 70% of them 46, 43, 120.
Blood tests will be done to check levels of:
- ACTH (adrenocorticotropic hormone)
- Cortisol
- FSH (follicle-stimulating hormone)
- Growth hormone
- LH (luteinizing hormone)
- Prolactin
- TSH (thyroid-stimulating hormone)
- Insulin-like growth factor-1 (IGF-1)
- Sodium
- Osmolarity in blood and urine
Histologic findings
Histologically, many of these tumors display hemorrhagic necrosis in their substance. This has been postulated to result from unrecognized episodes of focal hemorrhage. Bills reviewed histories of 37 patients with symptomatic pituitary apoplexy 89. By immunostaining criteria, null-cell adenomas were the most frequent tumor type found.
Pituitary apoplexy differential diagnosis
Several conditions have to be excluded as they can present with similar visual, ophthalmoplegic, and headache symptoms that occur in pituitary apoplexy. Some of the conditions will only require medical treatment, while in others, the surgical treatment is completely different.
- Rathke’s cleft cyst 121, 122, 123
- Temporal arteritis 124
- Aneurysm
- Craniopharyngioma
- Subarachnoid hemorrhage (SAH) 125
- Meningitis 126 or encephalitis
- Cavernous venous sinus thrombosis
- Basilar artery infarct
- Hypertensive encephalopathy
- Ophthalmoplegic migraine 127
- Retrobulbar neuritis
Pituitary apoplexy treatment
The key to successful management of patients with pituitary apoplexy is a team approach including critical care neurologists, neurosurgeons, neuro-ophthalmologist and endocrinologists. Together each of these specialists provide needed expertise in the management and ongoing care of these patients. Acute secondary adrenal insufficiency is seen in approximately two-thirds of patients and is the major source of mortality associated with this condition. Prompt glucocorticoid replacement is therefor mandatory and should not ne delayed for confirmatory testing. The initial management is stabilization of the hemodynamic status with IV 0.9% NaCl (sodium chloride) boluses to maintain normal tissue perfusion, and usually high dose parenteral glucocorticoids (100 mg hydrocortisone every 8 hourly intravenous). Unless significant cerebral edema is present, hydrocortisone rather than dexamethasone is favored.
Although there is a general consensus that patients with pituitary apoplexy and significant neuro-ophthalmic signs or reduced level of consciousness should have surgical decompression, there is significant controversy in the best timing for the surgical procedure due to lack of good quality outcomes data.
Management of the mass is controversial as some advocate early transsphenoidal surgical decompression in all patients, whereas others adopt a conservative approach for those patients without visual acuity or field defects and with normal consciousness 7. Emergency surgery should be reserved for patients with progressive deterioration of consciousness, hypothalamic involvement, and progressive visual worsening 7. It has been demonstrated that a significant postoperative clinical improvement is a consequence of a surgical procedure performed as soon as possible 50. Decompressive surgery can be delayed but performed within 1 week when visual acuity defects appear stable. If ophthalmoplegia is improving or stable, a conservative strategy could be considered 128, 129. Microscopic endonasal or sublabial transsphenoidal surgery is commonly used. For very large tumors and those extending over the chiasm or laterally to the temporal fossa, a craniotomy should be used to achieve complete resection. Endoscopic endonasal approaches for pituitary apoplexy are effective 48, 130, 131. Patients operated using the endoscopic approach have a similar visual outcome, but a better endocrinological outcome as the viable tumoral component can be removed from restricted areas like the cavernous sinus 132. Sometimes, an endoscopic approach can prove difficult to perform if required at late night hours as it needs the collaboration of an otolaryngologist with the neurosurgeon.
Figure 10. Pituitary apoplexy treatment
[Source 133 ]Immediate medical management
Immediate medical management of patients with pituitary apoplexy includes a careful assessment of fluids and electrolyte balance, ensure hemodynamic stability, and replacement with corticosteroids 120.
Medical treatment consists of the following:
- Medically stabilize the patient.
- Immediately evaluate electrolytes, glucose, and pituitary hormones.
- Administer high-dose corticosteroids (most patients have hypopituitarism).
- Administer appropriate endocrinologic replacement therapy alone or combined with transsphenoidal surgical decompression of the tumor.
- Avoid the “head down” position, when possible 134.
All patients should receive corticosteroids even if they do not present symptoms of adrenal crisis 7. The recommended dose is an intravenous 100–200 mg bolus of hydrocortisone. Additional administration of 50-100 mg every 6 hours should be continued. Alternatively, a continuous intravenous infusion of 2-4 mg/hour can be started after the initial bolus 43.
Surgical care
The management of pituitary apoplexy is controversial in that some advocate early transsphenoidal surgical decompression in all patients, whereas others adopt a conservative approach for selected patients (without visual acuity or field defects and with normal consciousness) 43. Children with pituitary tumor apoplexy has a more aggressive natural history in comparison to adults, and early surgery may reduce its occurrence and improve outcomes 135, 136.
Evacuation of the tumor by a neurosurgeon should be planned once the patient is medically stable, especially in the setting of altered consciousness, visual acuity, and visual field loss 43, 137, 138.
Shepard et al 139 identified 64 patients with pituitary apoplexy, 47 (73.4%) underwent intended conservative management, while 17 (26.6%) had early surgery. Tumor volumes were greater in the early surgical cohort. Among those with visual acuity and field deficits, visual outcomes were similar between both groups. Conservative management failed in 7 patients (14.9%) and they required surgery 139. Younger age, female sex, and patients with field deficits or chiasmal compression were more likely to experience unsuccessful conservative management. The authors concluded that the majority of patients with pituitary apoplexy can be successfully managed without surgical intervention assuming close neurosurgical, radiologic, and ophthalmologic follow-up is available 139.
Cavalli et al 140 retrospectively reviewed 30 patients with pituitary apoplexy; they found that 86.7% of patients presented with visual disturbances (70% acuity, 50% field, 50% diploplia), 10 (33%) patients underwent emergency surgery, and 8 underwent delayed elective surgery. At early and late follow-up, the outcome was not significantly different between groups. The authors concluded that good results are possible with conservative management in selected cases. Emergency surgery provides better visual outcomes and a tumor vertical diameter >35 mm should tip the balance in favor of surgical management in presence of visual deficit 140.
Follow up
While 80% of patients have residual hypopituitarism following pituitary apoplexy (with or without surgical decompression) some patients do not display immediate evidence of hypopituitarism. In addition, recurrent pituitary apoplexy and pituitary tumor regrowth has been reported to occur 133. MRI of the pituitary should be obtained at 3-6 month intervals until the anatomy is stable and then yearly for 5 years. A month after discharge from the hospital and recovery from the acute event, if necessary, patients are subject to repeat endocrine testing to determine if the endocrine defects persist. Repeat testing will confirm whether the patient needs to remain on life-long hormone replacement therapy.
Pituitary apoplexy prognosis
Acute pituitary apoplexy can be life-threatening if its not diagnosed or treated in a timely manner. The prognosis (outlook) is good for people who is diagnosed and treated in a timely manner. The overall mortality is 1.6% to 1.9% 7. Visual acuity, visual field defects, and ophthalmoplegia improve in the majority of the patients after both conservative and surgical decompression 59. After surgery, such improvement can be observed in the immediate postoperative period and often continues for several weeks after surgery. Visual recovery has been reported to be less likely in patients presenting with monocular or binocular blindness 7. Although visual outcome appears to be better with early intervention as compared to late 141, others found that visual deficits, resolution of oculomotor palsy, recovery from hypopituitarism, or non-neuroendocrine signs and symptoms such as headache and encephalopathy do not depend on the timing of surgery 142. A complete restoration of the oculomotor palsy usually needs 3 months, while abducens nerve palsy usually needs 6 months 50. Overall, visual improvement is seen in 75 to 85% of patients, recovery of normal vision in 38% of patients, and rectification of preoperative oculomotor palsies in 81% of patients 48.
Gross total resection and short duration of preoperative headaches are predictors for improvement in postoperative headaches 143. Hormonal replacement therapy is needed in 80% of the patients 8, 25, 142. In some cases that are treated conservatively, spontaneous remission of the tumor has occurred, and surgery is not required 144, 145. This may be caused by ischemic necrosis of the tumoral tissue 7.
- Harbour LN, Koch MS, Louis TH, Fulmer JM, Guileyardo JM. Abdominal apoplexy: two unusual cases of hemoperitoneum. Proc (Bayl Univ Med Cent). 2012;25(1):16-9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3246846/[↩]
- Hardy K, Mead B, Gill G. Adrenal apoplexy after coronary artery bypass surgery leading to Addisonian crisis. J R Soc Med. 1992;85(9):577-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1293654/pdf/jrsocmed00107-0081.pdf[↩]
- HOSSAIN K. A case of cerebellar apoplexy. Ind Med Gaz. 1946;81(1):26. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5236169/pdf/indmedgaz72707-0030b.pdf[↩]
- Khaku AS, Dulebohn SC. Stroke. [Updated 2018 Oct 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430927[↩]
- Hannoush ZC, Weiss RE. Pituitary Apoplexy. [Updated 2018 Apr 22]. In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279125[↩][↩][↩]
- Pituitary Apoplexy. https://emedicine.medscape.com/article/1198279-overview#a4[↩][↩][↩]
- Mayol Del Valle M, De Jesus O. Pituitary Apoplexy. [Updated 2023 Aug 23]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559222[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Ranabir S, Baruah MP. Pituitary apoplexy. Indian J Endocrinol Metab. 2011 Sep;15 Suppl 3(Suppl3):S188-96. doi: 10.4103/2230-8210.84862[↩][↩][↩][↩]
- Briet C, Salenave S, Chanson P. Pituitary apoplexy. Endocrinol Metab Clin North Am. 2015 Mar;44(1):199-209. https://doi.org/10.1016/j.ecl.2014.10.016[↩][↩]
- Claire Briet, Sylvie Salenave, Jean-François Bonneville, Edward R. Laws, Philippe Chanson, Pituitary Apoplexy, Endocrine Reviews, Volume 36, Issue 6, 1 December 2015, Pages 622–645, https://doi.org/10.1210/er.2015-1042[↩][↩][↩]
- Brougham M, Heusner AP, Adams RD. Acute degenerative changes in adenomas of the pituitary body–with special reference to pituitary apoplexy. J Neurosurg. 1950 Sep;7(5):421-39. doi: 10.3171/jns.1950.7.5.0421[↩][↩]
- Key Statistics About Pituitary Tumors. https://www.cancer.org/cancer/types/pituitary-tumors/about/key-statistics.html[↩][↩][↩]
- Fernandez A, Karavitaki N, Wass JA. Prevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin Endocrinol (Oxf). 2010 Mar;72(3):377-82. doi: 10.1111/j.1365-2265.2009.03667.x[↩]
- Raappana A, Koivukangas J, Ebeling T, Pirilä T. Incidence of pituitary adenomas in Northern Finland in 1992-2007. J Clin Endocrinol Metab. 2010 Sep;95(9):4268-75. doi: 10.1210/jc.2010-0537[↩]
- Wakai S, Fukushima T, Teramoto A, Sano K. Pituitary apoplexy: its incidence and clinical significance. J Neurosurg. 1981 Aug;55(2):187-93. doi: 10.3171/jns.1981.55.2.0187[↩]
- Bonicki W, Kasperlik-Załuska A, Koszewski W, Zgliczyński W, Wisławski J. Pituitary apoplexy: endocrine, surgical and oncological emergency. Incidence, clinical course and treatment with reference to 799 cases of pituitary adenomas. Acta Neurochir (Wien). 1993;120(3-4):118-22. doi: 10.1007/BF02112028[↩]
- McFadzean RM, Doyle D, Rampling R, Teasdale E, Teasdale G. Pituitary apoplexy and its effect on vision. Neurosurgery. 1991 Nov;29(5):669-75. doi: 10.1097/00006123-199111000-00005[↩]
- Ayuk J, McGregor EJ, Mitchell RD, Gittoes NJ. Acute management of pituitary apoplexy–surgery or conservative management? Clin Endocrinol (Oxf). 2004 Dec;61(6):747-52. doi: 10.1111/j.1365-2265.2004.02162.x[↩]
- Randeva HS, Schoebel J, Byrne J, Esiri M, Adams CB, Wass JA. Classical pituitary apoplexy: clinical features, management and outcome. Clin Endocrinol (Oxf). 1999 Aug;51(2):181-8. doi: 10.1046/j.1365-2265.1999.00754.x[↩]
- da Motta LA, de Mello PA, de Lacerda CM, Neto AP, da Motta LD, Filho MF. Pituitary apoplexy. Clinical course, endocrine evaluations and treatment analysis. J Neurosurg Sci. 1999 Mar;43(1):25-36.[↩]
- Verrees M, Arafah BM, Selman WR. Pituitary tumor apoplexy: characteristics, treatment, and outcomes. Neurosurg Focus. 2004 Apr 15;16(4):E6. doi: 10.3171/foc.2004.16.4.7[↩][↩][↩]
- Semple PL, Webb MK, de Villiers JC, Laws ER Jr. Pituitary apoplexy. Neurosurgery. 2005;56(1):65-72; discussion 72-3. doi: 10.1227/01.neu.0000144840.55247.38[↩]
- Liu JK, Couldwell WT. Pituitary apoplexy in the magnetic resonance imaging era: clinical significance of sphenoid sinus mucosal thickening. J Neurosurg. 2006 Jun;104(6):892-8. doi: 10.3171/jns.2006.104.6.892[↩][↩][↩]
- Dubuisson AS, Beckers A, Stevenaert A. Classical pituitary tumour apoplexy: clinical features, management and outcomes in a series of 24 patients. Clin Neurol Neurosurg. 2007 Jan;109(1):63-70. doi: 10.1016/j.clineuro.2006.01.006[↩]
- Murad-Kejbou S, Eggenberger E. Pituitary apoplexy: evaluation, management, and prognosis. Curr Opin Ophthalmol. 2009 Nov;20(6):456-61. doi: 10.1097/ICU.0b013e3283319061[↩][↩]
- Möller-Goede DL, Brändle M, Landau K, Bernays RL, Schmid C. Pituitary apoplexy: re-evaluation of risk factors for bleeding into pituitary adenomas and impact on outcome. Eur J Endocrinol. 2011 Jan;164(1):37-43. doi: 10.1530/EJE-10-0651[↩][↩]
- Turgut M, Ozsunar Y, Başak S, Güney E, Kir E, Meteoğlu I. Pituitary apoplexy: an overview of 186 cases published during the last century. Acta Neurochir (Wien). 2010 May;152(5):749-61. doi: 10.1007/s00701-009-0595-8[↩]
- Biousse V, Newman NJ, Oyesiku NM. Precipitating factors in pituitary apoplexy. J Neurol Neurosurg Psychiatry. 2001 Oct;71(4):542-5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1763528/pdf/v071p00542.pdf[↩][↩]
- Chacko AG, Chacko G, Seshadri MS, Chandy MJ. Hemorrhagic necrosis of pituitary adenomas. Neurol India. 2002 Dec;50(4):490-3.[↩]
- Chan D, Rong TC, Dalan R. Cushing’s disease presenting with pituitary apoplexy. J Clin Neurosci. 2012 Nov;19(11):1586-9. doi: 10.1016/j.jocn.2011.10.017[↩]
- Jankowski PP, Crawford JR, Khanna P, Malicki DM, Ciacci JD, Levy ML. Pituitary tumor apoplexy in adolescents. World Neurosurg. 2015 Apr;83(4):644-51. doi: 10.1016/j.wneu.2014.12.026[↩]
- Kinoshita Y, Tominaga A, Usui S, Arita K, Sugiyama K, Kurisu K. Impact of subclinical haemorrhage on the pituitary gland in patients with pituitary adenomas. Clin Endocrinol (Oxf). 2014 May;80(5):720-5. doi: 10.1111/cen.12349[↩]
- Liu ZH, Chang CN, Pai PC, Wei KC, Jung SM, Chen NY, Chuang CC. Clinical features and surgical outcome of clinical and subclinical pituitary apoplexy. J Clin Neurosci. 2010 Jun;17(6):694-9. doi: 10.1016/j.jocn.2009.11.012[↩]
- Pal A, Capatina C, Tenreiro AP, Guardiola PD, Byrne JV, Cudlip S, Karavitaki N, Wass JA. Pituitary apoplexy in non-functioning pituitary adenomas: long term follow up is important because of significant numbers of tumour recurrences. Clin Endocrinol (Oxf). 2011 Oct;75(4):501-4. doi: 10.1111/j.1365-2265.2011.04068.x[↩]
- Sarwar KN, Huda MS, Van de Velde V, Hopkins L, Luck S, Preston R, McGowan BM, Carroll PV, Powrie JK. The prevalence and natural history of pituitary hemorrhage in prolactinoma. J Clin Endocrinol Metab. 2013 Jun;98(6):2362-7. doi: 10.1210/jc.2013-1249[↩]
- Seuk JW, Kim CH, Yang MS, Cheong JH, Kim JM. Visual outcome after transsphenoidal surgery in patients with pituitary apoplexy. J Korean Neurosurg Soc. 2011 Jun;49(6):339-44. doi: 10.3340/jkns.2011.49.6.339[↩]
- Vargas G, Gonzalez B, Guinto G, Mendoza V, López-Félix B, Zepeda E, Mercado M. Pituitary apoplexy in nonfunctioning pituitary macroadenomas: a case-control study. Endocr Pract. 2014 Dec;20(12):1274-80. doi: 10.4158/EP14120.OR[↩]
- Zhang X, Zhang W, Fu LA, Cheng JX, Liu BL, Cao WD, Fei Z, Zhang JN, Liu WP, Zhen HN. Hemorrhagic pituitary macroadenoma: characteristics, endoscopic endonasal transsphenoidal surgery, and outcomes. Ann Surg Oncol. 2011 Jan;18(1):246-52. doi: 10.1245/s10434-010-1243-5[↩]
- Jho DH, Biller BM, Agarwalla PK, Swearingen B. Pituitary apoplexy: large surgical series with grading system. World Neurosurg. 2014 Nov;82(5):781-90. doi: 10.1016/j.wneu.2014.06.005[↩]
- Zhang F, Chen J, Lu Y, Ding X. Manifestation, management and outcome of subclinical pituitary adenoma apoplexy. J Clin Neurosci. 2009 Oct;16(10):1273-5. doi: 10.1016/j.jocn.2009.01.003[↩]
- Fernández-Balsells MM, Murad MH, Barwise A, Gallegos-Orozco JF, Paul A, Lane MA, Lampropulos JF, Natividad I, Perestelo-Pérez L, Ponce de León-Lovatón PG, Erwin PJ, Carey J, Montori VM. Natural history of nonfunctioning pituitary adenomas and incidentalomas: a systematic review and metaanalysis. J Clin Endocrinol Metab. 2011 Apr;96(4):905-12. doi: 10.1210/jc.2010-1054[↩]
- Sivakumar W, Chamoun R, Nguyen V, Couldwell WT. Incidental pituitary adenomas. Neurosurg Focus. 2011 Dec;31(6):E18. doi: 10.3171/2011.9.FOCUS11217[↩]
- Briet C, Salenave S, Chanson P. Pituitary apoplexy. Endocrinol Metab Clin North Am. 2015 Mar;44(1):199-209. doi: 10.1016/j.ecl.2014.10.016[↩][↩][↩][↩][↩][↩][↩][↩]
- Grzywotz A, Kleist B, Möller LC, Hans VH, Göricke S, Sure U, Müller O, Kreitschmann-Andermahr I. Pituitary apoplexy – A single center retrospective study from the neurosurgical perspective and review of the literature. Clin Neurol Neurosurg. 2017 Dec;163:39-45. doi: 10.1016/j.clineuro.2017.10.006[↩][↩][↩]
- Nawar RN, AbdelMannan D, Selman WR, Arafah BM. Pituitary tumor apoplexy: a review. J Intensive Care Med. 2008 Mar-Apr;23(2):75-90. doi: 10.1177/0885066607312992[↩][↩][↩]
- Briet C, Salenave S, Bonneville JF, Laws ER, Chanson P. Pituitary Apoplexy. Endocr Rev. 2015 Dec;36(6):622-45. doi: 10.1210/er.2015-1042[↩][↩][↩][↩]
- Wichlińska-Lubińska M, Kozera G. Pituitary apoplexy. Neurol Neurochir Pol. 2019;53(6):413-420. doi: 10.5603/PJNNS.a2019.0054[↩]
- Zoli M, Milanese L, Faustini-Fustini M, Guaraldi F, Asioli S, Zenesini C, Righi A, Frank G, Foschini MP, Sturiale C, Pasquini E, Mazzatenta D. Endoscopic Endonasal Surgery for Pituitary Apoplexy: Evidence On a 75-Case Series From a Tertiary Care Center. World Neurosurg. 2017 Oct;106:331-338. doi: 10.1016/j.wneu.2017.06.117[↩][↩][↩]
- Barkhoudarian G, Kelly DF. Pituitary Apoplexy. Neurosurg Clin N Am. 2019 Oct;30(4):457-463. doi: 10.1016/j.nec.2019.06.001[↩]
- Ricciuti R, Nocchi N, Arnaldi G, Polonara G, Luzi M. Pituitary Adenoma Apoplexy: Review of Personal Series. Asian J Neurosurg. 2018 Jul-Sep;13(3):560-564. doi: 10.4103/ajns.AJNS_344_16[↩][↩][↩][↩]
- Pituitary apoplexy. https://radiopaedia.org/articles/pituitary-apoplexy?lang=us[↩][↩]
- Ayuk J, McGregor EJ, Mitchell RD, Gittoes NJL. Acute management of pituitary apoplexy–surgery or conservative management? Clin Endocrinol (Oxf) 2004;61:747–752. doi: 10.1111/J.1365-2265.2004.02162.X[↩][↩][↩]
- Dubuisson AS, Beckers A, Stevenaert A. Classical pituitary tumour apoplexy: clinical features, management and outcomes in a series of 24 patients. Clin Neurol Neurosurg. 2007;109:63–70. doi: 10.1016/J.CLINEURO.2006.01.006[↩]
- Araujo-Castro M, Paredes I, Pérez-López C, García Feijoo P, Alvarez-Escola C, Calatayud M, Lagares A, Soledad Librizzi M, Acitores Cancela A, Rodríguez Berrocal V. Differences in clinical, hormonal, and radiological presentation and in surgical outcomes in patients presenting with and without pituitary apoplexy. A multicenter study of 245 cases. Pituitary. 2023 Apr;26(2):250-258. doi: 10.1007/s11102-023-01315-6[↩]
- Sibal L, Ball SG, Connolly V, James RA, Kane P, Kelly WF et al (2004) Pituitary apoplexy: a review of clinical presentation, management and outcome in 45 cases, vol 7. Pituitary. 10.1007/s11102-005-1050-3[↩][↩]
- Gruber A, Clayton J, Kumar S, Robertson I, Howlett T, Mansell P. Pituitary apoplexy: retrospective review of 30 patients–is surgical intervention always necessary? Br J Neurosurg. 2006;20:379–385. doi: 10.1080/02688690601046678[↩][↩]
- Nawar RN, AbdelMannan D, Selman WR, Arafah BM. Pituitary tumor apoplexy: a review. J Intensive Care Med. 2008;23:75–90. doi: 10.1177/0885066607312992[↩]
- Arbunea-Ghenoiu S, Ciubotaru GV, Dumitrascu A, Alexandrescu D, Capatina C, Poiana C Pituitary apoplexy: a retrospective study of 36 cases from a single Center. Cureus 2022;14. 10.7759/CUREUS.29769[↩]
- Giritharan S, Gnanalingham K, Kearney T. Pituitary apoplexy – bespoke patient management allows good clinical outcome. Clin Endocrinol (Oxf). 2016 Sep;85(3):415-22. doi: 10.1111/cen.13075[↩][↩]
- Kim DJ, Song YJ, Kim SJ, Park MK, Choi SS, Kim KU. Pituitary hemorrhage : classification and related factors. J Korean Neurosurg Soc. 2009;46(1):23-30. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2729820/[↩]
- Pituitary macroadenoma and periventricular leukomalacia. https://radiopaedia.org/cases/pituitary-macroadenoma-and-periventricular-leukomalacia[↩]
- Vaphiades MS. Pituitary Ring Sign Plus Sphenoid Sinus Mucosal Thickening: Neuroimaging Signs of Pituitary Apoplexy. Neuroophthalmology. 2017 Aug 9;41(6):306-309. doi: 10.1080/01658107.2017.1349807[↩][↩][↩][↩][↩][↩][↩]
- Merck Sharp & Dohme Corp. Merck Manual. Overview of the Endocrine System. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/principles-of-endocrinology/overview-of-the-endocrine-system#v27411775[↩]
- Ly S, Naman A, Chaufour-Higel B, Patey M, Arndt C, Delemer B, Litre CF. Pituitary apoplexy and rivaroxaban. Pituitary. 2017 Dec;20(6):709-710. doi: 10.1007/s11102-017-0828-4[↩][↩]
- Kuzu F, Unal M, Gul S, Bayraktaroglu T. Pituitary Apoplexy due to the Diagnostic Test in a Cushing”s Disease Patient. Turk Neurosurg. 2018;28(2):323-325. doi: 10.5137/1019-5149.JTN.16730-15.1[↩]
- Ghadirian H, Shirani M, Ghazi-Mirsaeed S, Mohebi S, Alimohamadi M. Pituitary Apoplexy during Treatment of Prolactinoma with Cabergoline. Asian J Neurosurg. 2018 Jan-Mar;13(1):93-95. doi: 10.4103/1793-5482.181130[↩]
- Aydin B, Aksu O, Asci H, Kayan M, Korkmaz H. A RARE CAUSE OF PITUITARY APOPLEXY: CABERGOLINE THERAPY. Acta Endocrinol (Buchar). 2018 Jan-Mar;14(1):113-116. doi: 10.4183/aeb.2018.113[↩]
- Keane F, Egan AM, Navin P, Brett F, Dennedy MC. Gonadotropin-releasing hormone agonist-induced pituitary apoplexy. Endocrinol Diabetes Metab Case Rep. 2016;2016:160021. doi: 10.1530/EDM-16-0021[↩]
- Joo C, Ha G, Jang Y. Pituitary apoplexy following lumbar fusion surgery in prone position: A case report. Medicine (Baltimore). 2018 May;97(19):e0676. doi: 10.1097/MD.0000000000010676[↩]
- Akakın A, Yılmaz B, Ekşi MŞ, Kılıç T. A case of pituitary apoplexy following posterior lumbar fusion surgery. J Neurosurg Spine. 2015 Nov;23(5):598-601. doi: 10.3171/2015.3.SPINE14792[↩]
- Jemel M, Kandara H, Riahi M, Gharbi R, Nagi S, Kamoun I. Gestational pituitary apoplexy: Case series and review of the literature. J Gynecol Obstet Hum Reprod. 2019 Dec;48(10):873-881. doi: 10.1016/j.jogoh.2019.05.005[↩]
- Annamalai AK, Jeyachitra G, Jeyamithra A, Ganeshkumar M, Srinivasan KG, Gurnell M. Gestational Pituitary Apoplexy. Indian J Endocrinol Metab. 2017 May-Jun;21(3):484-485. doi: 10.4103/ijem.IJEM_8_17[↩]
- Yu J, Li Y, Quan T, Li X, Peng C, Zeng J, Liang S, Huang M, He Y, Deng Y. Initial Gamma Knife radiosurgery for nonfunctioning pituitary adenomas: results from a 26-year experience. Endocrine. 2020 May;68(2):399-410. doi: 10.1007/s12020-020-02260-1[↩]
- Thomas M, Robert A, Rajole P, Robert P. A Rare Case of Pituitary Apoplexy Secondary to Dengue Fever-induced Thrombocytopenia. Cureus. 2019 Aug 5;11(8):e5323. doi: 10.7759/cureus.5323[↩]
- Balaparameswara Rao SJ, Savardekar AR, Nandeesh BN, Arivazhagan A. Management dilemmas in a rare case of pituitary apoplexy in the setting of dengue hemorrhagic fever. Surg Neurol Int. 2017 Jan 19;8:4. doi: 10.4103/2152-7806.198731[↩]
- Uneda A, Hirashita K, Yunoki M, Yoshino K, Date I. Pituitary adenoma apoplexy associated with vardenafil intake. Acta Neurochir (Wien). 2019 Jan;161(1):129-131. doi: 10.1007/s00701-018-3763-x[↩]
- Okuda O, Umezawa H, Miyaoka M. Pituitary apoplexy caused by endocrine stimulation tests: a case report. Surg Neurol. 1994 Jul;42(1):19-22. doi: 10.1016/0090-3019(94)90244-5[↩][↩]
- Chokyu I, Tsuyuguchi N, Goto T, Chokyu K, Chokyu M, Ohata K. Pituitary apoplexy causing internal carotid artery occlusion–case report. Neurol Med Chir (Tokyo). 2011;51(1):48-51. doi: 10.2176/nmc.51.48[↩]
- Silberstein L, Johnston C, Bhagat A, Tibi L, Harrison J. Pituitary apoplexy during induction chemotherapy for acute myeloid leukaemia. Br J Haematol. 2008 Oct;143(2):151. doi: 10.1111/j.1365-2141.2008.07286.x[↩]
- Thurtell MJ, Besser M, Halmagyi GM. Pituitary apoplexy causing isolated blindness after cardiac bypass surgery. Arch Ophthalmol. 2008 Apr;126(4):576-8. doi: 10.1001/archopht.126.4.576[↩]
- Brar KS, Garg MK. High altitude-induced pituitary apoplexy. Singapore Med J. 2012 Jun;53(6):e117-9. http://smj.sma.org.sg/5306/5306cr3.pdf[↩]
- Kumar V, Kataria R, Mehta VS. Dengue hemorrhagic fever: a rare cause of pituitary tumor hemorrhage and reversible vision loss. Indian J Ophthalmol. 2011 Jul-Aug;59(4):311-2. doi: 10.4103/0301-4738.82002[↩]
- Kruljac I, Cerina V, Pećina HI, Pažanin L, Matić T, Božikov V, Vrkljan M. Pituitary metastasis presenting as ischemic pituitary apoplexy following heparin-induced thrombocytopenia. Endocr Pathol. 2012 Dec;23(4):264-7. doi: 10.1007/s12022-012-9224-9[↩]
- Weisberg LA. Pituitary apoplexy. Association of degenerative change in pituitary ademona with radiotherapy and detection by cerebral computed tomography. Am J Med. 1977 Jul;63(1):109-15. doi: 10.1016/0002-9343(77)90122-x[↩]
- Ahmed SK, Semple PL. Cerebral ischaemia in pituitary apoplexy. Acta Neurochir (Wien). 2008 Nov;150(11):1193-6; discussion 1196. doi: 10.1007/s00701-008-0130-3[↩]
- Kim JP, Park BJ, Kim SB, Lim YJ. Pituitary Apoplexy due to Pituitary Adenoma Infarction. J Korean Neurosurg Soc. 2008 May;43(5):246-9. doi: 10.3340/jkns.2008.43.5.246[↩]
- Lazaro CM, Guo WY, Sami M, Hindmarsh T, Ericson K, Hulting AL, Wersäll J. Haemorrhagic pituitary tumours. Neuroradiology. 1994;36(2):111-4. doi: 10.1007/BF00588072[↩]
- Turgut, M., Seyithanoğlu, M.H., Tüzgen, S. (2014). Definition, History, Frequency, Histopathology and Pathophysiology of Pituitary Apoplexy. In: Turgut, M., Mahapatra, A., Powell, M., Muthukumar, N. (eds) Pituitary Apoplexy. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-38508-7_1[↩]
- Bills DC, Meyer FB, Laws ER Jr, Davis DH, Ebersold MJ, Scheithauer BW, Ilstrup DM, Abboud CF. A retrospective analysis of pituitary apoplexy. Neurosurgery. 1993 Oct;33(4):602-8; discussion 608-9. doi: 10.1227/00006123-199310000-00007[↩][↩]
- Rolih CA, Ober KP. Pituitary apoplexy. Endocrinol Metab Clin North Am. 1993 Jun;22(2):291-302.[↩][↩]
- Muthukumar N. Pituitary Apoplexy: A Comprehensive Review. Neurol India. 2020 May-Jun;68(Supplement):S72-S78. doi: 10.4103/0028-3886.287669[↩]
- Pituitary Apoplexy Pathophysiology. https://emedicine.medscape.com/article/1198279-overview#a5[↩][↩][↩]
- Rovit RL, Fein JM. Pituitary apoplexy: a review and reappraisal. J Neurosurg. 1972 Sep;37(3):280-8. doi: 10.3171/jns.1972.37.3.0280[↩]
- Schechter J, Goldsmith P, Wilson C, Weiner R. Morphological evidence for the presence of arteries in human prolactinomas. J Clin Endocrinol Metab. 1988 Oct;67(4):713-9. doi: 10.1210/jcem-67-4-713[↩]
- Oldfield EH, Merrill MJ. Apoplexy of pituitary adenomas: the perfect storm. J Neurosurg. 2015 Jun;122(6):1444-9. doi: 10.3171/2014.10.JNS141720[↩]
- Cardoso ER, Peterson EW. Pituitary apoplexy: a review. Neurosurgery. 1984 Mar;14(3):363-73. doi: 10.1227/00006123-198403000-00021[↩]
- Hirano A, Tomiyasu U, Zimmerman HM. The fine structure of blood vessels in chromophobe adenoma. Acta Neuropathol. 1972;22(3):200-7. doi: 10.1007/BF00684523[↩]
- Di Ieva A, Weckman A, Di Michele J, Rotondo F, Grizzi F, Kovacs K, Cusimano MD. Microvascular morphometrics of the hypophysis and pituitary tumors: from bench to operating theatre. Microvasc Res. 2013 Sep;89:7-14. doi: 10.1016/j.mvr.2013.04.009[↩]
- Lee JS, Park YS, Kwon JT, Nam TK, Lee TJ, Kim JK. Radiological apoplexy and its correlation with acute clinical presentation, angiogenesis and tumor microvascular density in pituitary adenomas. J Korean Neurosurg Soc. 2011 Oct;50(4):281-7. doi: 10.3340/jkns.2011.50.4.281[↩]
- Minematsu T, Suzuki M, Sanno N, Takekoshi S, Teramoto A, Osamura RY. PTTG overexpression is correlated with angiogenesis in human pituitary adenomas. Endocr Pathol. 2006 Summer;17(2):143-53. doi: 10.1385/ep:17:2:143[↩]
- Filippella M, Galland F, Kujas M, Young J, Faggiano A, Lombardi G, Colao A, Meduri G, Chanson P. Pituitary tumour transforming gene (PTTG) expression correlates with the proliferative activity and recurrence status of pituitary adenomas: a clinical and immunohistochemical study. Clin Endocrinol (Oxf). 2006 Oct;65(4):536-43. doi: 10.1111/j.1365-2265.2006.02630.x[↩]
- Vlotides G, Eigler T, Melmed S. Pituitary tumor-transforming gene: physiology and implications for tumorigenesis. Endocr Rev. 2007 Apr;28(2):165-86. doi: 10.1210/er.2006-0042[↩]
- Niveiro M, Aranda FI, Peiró G, Alenda C, Picó A. Immunohistochemical analysis of tumor angiogenic factors in human pituitary adenomas. Hum Pathol. 2005 Oct;36(10):1090-5. doi: 10.1016/j.humpath.2005.07.015[↩]
- Perez-Millan MI, Berner SI, Luque GM, De Bonis C, Sevlever G, Becu-Villalobos D, Cristina C. Enhanced nestin expression and small blood vessels in human pituitary adenomas. Pituitary. 2013 Sep;16(3):303-10. doi: 10.1007/s11102-012-0421-9[↩]
- Arafah BM, Harrington JF, Madhoun ZT, Selman WR. Improvement of pituitary function after surgical decompression for pituitary tumor apoplexy. J Clin Endocrinol Metab. 1990 Aug;71(2):323-8. doi: 10.1210/jcem-71-2-323[↩]
- Kruse A, Astrup J, Cold GE, Hansen HH. Pressure and blood flow in pituitary adenomas measured during transsphenoidal surgery. Br J Neurosurg. 1992;6(4):333-41. doi: 10.3109/02688699209023792. Erratum in: Br J Neurosurg 1992;6(5):511[↩]
- Zayour DH, Selman WR, Arafah BM. Extreme elevation of intrasellar pressure in patients with pituitary tumor apoplexy: relation to pituitary function. J Clin Endocrinol Metab. 2004 Nov;89(11):5649-54. doi: 10.1210/jc.2004-0884[↩]
- Pituitary apoplexy. https://medlineplus.gov/ency/article/001167.htm[↩][↩][↩]
- Simsek Bagir G, Civi S, Kardes O, Kayaselcuk F, Ertorer ME. Stubborn hiccups as a sign of massive apoplexy in a naive acromegaly patient with pituitary macroadenoma. Endocrinol Diabetes Metab Case Rep. 2017 May 18;2017:17-0044. doi: 10.1530/EDM-17-0044[↩]
- Pineyro MM, Furtenbach P, Lima R, Wajskopf S, Sgarbi N, Pisabarro R. Brain and Optic Chiasm Herniation into Sella after Pituitary Tumor Apoplexy. Front Endocrinol (Lausanne). 2017 Aug 7;8:192. doi: 10.3389/fendo.2017.00192[↩]
- Lavallée G, Morcos R, Palardy J, Aubé M, Gilbert D. MR of nonhemorrhagic postpartum pituitary apoplexy. AJNR Am J Neuroradiol. 1995 Oct;16(9):1939-41. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8338223/pdf/8693999.pdf[↩]
- Rogg JM, Tung GA, Anderson G, Cortez S. Pituitary apoplexy: early detection with diffusion-weighted MR imaging. AJNR Am J Neuroradiol. 2002 Aug;23(7):1240-5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8185738[↩]
- Arita K, Tominaga A, Sugiyama K, Eguchi K, Iida K, Sumida M, Migita K, Kurisu K. Natural course of incidentally found nonfunctioning pituitary adenoma, with special reference to pituitary apoplexy during follow-up examination. J Neurosurg. 2006 Jun;104(6):884-91. doi: 10.3171/jns.2006.104.6.884[↩]
- Flanagan DE, Ibrahim AE, Ellison DW, Armitage M, Gawne-Cain M, Lees PD. Inflammatory hypophysitis – the spectrum of disease. Acta Neurochir (Wien). 2002 Jan;144(1):47-56. doi: 10.1007/s701-002-8273-5[↩]
- Arita K, Kurisu K, Tominaga A, Sugiyama K, Ikawa F, Yoshioka H, Sumida M, Kanou Y, Yajin K, Ogawa R. Thickening of sphenoid sinus mucosa during the acute stage of pituitary apoplexy. J Neurosurg. 2001 Nov;95(5):897-901. doi: 10.3171/jns.2001.95.5.0897[↩][↩][↩][↩]
- Agrawal B, Dziurzynski K, Salamat MS, Baskaya M. The temporal association of sphenoid sinus mucosal thickening on MR imaging with pituitary apoplexy. Turk Neurosurg. 2012;22(6):785-90. doi: 10.5137/1019-5149.JTN.4273-11.1[↩]
- Kaplun J, Fratila C, Ferenczi A, Yang WC, Lantos G, Fleckman AM, Schubart UK. Sequential pituitary MR imaging in Sheehan syndrome: report of 2 cases. AJNR Am J Neuroradiol. 2008 May;29(5):941-3. doi: 10.3174/ajnr.A1016[↩]
- Binning MJ, Liu JK, Gannon J, Osborn AG, Couldwell WT. Hemorrhagic and nonhemorrhagic Rathke cleft cysts mimicking pituitary apoplexy. J Neurosurg. 2008 Jan;108(1):3-8. doi: 10.3171/JNS/2008/108/01/0003[↩]
- Liu S, Wang X, Liu YH, Mao Q. Spontaneous disappearance of the pituitary macroadenoma after apoplexy: a case report and review of the literature. Neurol India. 2012 Sep-Oct;60(5):530-2. doi: 10.4103/0028-3886.103211[↩]
- Veldhuis JD, Hammond JM. Endocrine function after spontaneous infarction of the human pituitary: report, review, and reappraisal. Endocr Rev. 1980 Winter;1(1):100-7. doi: 10.1210/edrv-1-1-100[↩][↩]
- Martinez Santos J, Hannay M, Olar A, Eskandari R. Rathke’s Cleft Cyst Apoplexy in Two Teenage Sisters. Pediatr Neurosurg. 2019;54(6):428-435. doi: 10.1159/000503112[↩]
- Jung HN, Kim ST, Kong DS, Suh SI, Ryoo I. Rathke Cleft Cysts with Apoplexy-Like Symptoms: Clinicoradiologic Comparisons with Pituitary Adenomas with Apoplexy. World Neurosurg. 2020 Oct;142:e1-e9. doi: 10.1016/j.wneu.2020.03.086[↩]
- Schooner L, Wedemeyer MA, Bonney PA, Lin M, Hurth K, Mathew A, Liu CJ, Shiroishi M, Carmichael JD, Weiss MH, Zada G. Hemorrhagic Presentation of Rathke Cleft Cysts: A Surgical Case Series. Oper Neurosurg (Hagerstown). 2020 May 1;18(5):470-479. doi: 10.1093/ons/opz239[↩]
- Pedro B, Patrícia T, Aldomiro F. Pituitary Apoplexy May Be Mistaken for Temporal Arteritis. Eur J Case Rep Intern Med. 2019 Oct 16;6(11):001261. doi: 10.12890/2019_001261[↩]
- Choudhury M, Eligar V, DeLloyd A, Davies JS. A case of pituitary apoplexy masquerading as subarachnoid hemorrhage. Clin Case Rep. 2016 Jan 22;4(3):255-7. doi: 10.1002/ccr3.488[↩]
- Law-Ye B, Pyatigorskaya N, Leclercq D. Pituitary Apoplexy Mimicking Bacterial Meningitis with Intracranial Hypertension. World Neurosurg. 2017 Jan;97:748.e3-748.e5. doi: 10.1016/j.wneu.2016.10.032[↩]
- Shabas D, Sheikh HU, Gilad R. Pituitary Apoplexy Presenting as Status Migrainosus. Headache. 2017 Apr;57(4):641-642. doi: 10.1111/head.13046[↩]
- Almeida JP, Sanchez MM, Karekezi C, Warsi N, Fernández-Gajardo R, Panwar J, Mansouri A, Suppiah S, Nassiri F, Nejad R, Kucharczyk W, Ridout R, Joaquim AF, Gentili F, Zadeh G. Pituitary Apoplexy: Results of Surgical and Conservative Management Clinical Series and Review of the Literature. World Neurosurg. 2019 Oct;130:e988-e999. doi: 10.1016/j.wneu.2019.07.055[↩]
- Seo Y, Kim YH, Dho YS, Kim JH, Kim JW, Park CK, Kim DG. The Outcomes of Pituitary Apoplexy with Conservative Treatment: Experiences at a Single Institution. World Neurosurg. 2018 Jul;115:e703-e710. doi: 10.1016/j.wneu.2018.04.139[↩]
- Pangal DJ, Chesney K, Memel Z, Bonney PA, Strickland BA, Carmichael J, Shiroishi M, Jason Liu CS, Zada G. Pituitary Apoplexy Case Series: Outcomes After Endoscopic Endonasal Transsphenoidal Surgery at a Single Tertiary Center. World Neurosurg. 2020 May;137:e366-e372. doi: 10.1016/j.wneu.2020.01.204[↩]
- Zhan R, Li X, Li X. Endoscopic Endonasal Transsphenoidal Approach for Apoplectic Pituitary Tumor: Surgical Outcomes and Complications in 45 Patients. J Neurol Surg B Skull Base. 2016 Feb;77(1):54-60. doi: 10.1055/s-0035-1560046[↩]
- Teixeira JC, Lavrador J, Simão D, Miguéns J. Pituitary Apoplexy: Should Endoscopic Surgery Be the Gold Standard? World Neurosurg. 2018 Mar;111:e495-e499. doi: 10.1016/j.wneu.2017.12.103[↩]
- Hannoush ZC, Weiss RE. Pituitary Apoplexy. [Updated 2018 Apr 22]. In: De Groot LJ, Chrousos G, Dungan K, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279125[↩][↩]
- Kiyofuji S, Perry A, Graffeo CS, Giannini C, Link MJ. The dangers of the “Head Down” position in patients with untreated pituitary macroadenomas: case series and review of literature. Pituitary. 2018 Jun;21(3):231-237. doi: 10.1007/s11102-017-0851-5[↩]
- Culpin E, Crank M, Igra M, Connolly DJA, Dimitri P, Mirza S, Sinha S. Pituitary tumour apoplexy within prolactinomas in children: a more aggressive condition? Pituitary. 2018 Oct;21(5):474-479. doi: 10.1007/s11102-018-0900-8[↩]
- Zhang N, Zhou P, Meng Y, Ye F, Jiang S. A retrospective review of 34 cases of pediatric pituitary adenoma. Childs Nerv Syst. 2017 Nov;33(11):1961-1967. doi: 10.1007/s00381-017-3538-3[↩]
- Thomason K, Macleod K, Eyres KS. Hyponatraemia after orthopaedic surgery – a case of pituitary apoplexy. Ann R Coll Surg Engl. 2009 Apr;91(3):W3-5. doi: 10.1308/147870809X400912[↩]
- Muthukumar N, Rossette D, Soundaram M, Senthilbabu S, Badrinarayanan T. Blindness following pituitary apoplexy: timing of surgery and neuro-ophthalmic outcome. J Clin Neurosci. 2008 Aug. 15(8):873-9.[↩]
- Shepard MJ, Snyder MH, Soldozy S, Ampie LL, Morales-Valero SF, Jane JA. Radiological and clinical outcomes of pituitary apoplexy: comparison of conservative management versus early surgical intervention. J Neurosurg. 2021 Apr 30;135(5):1310-1318. doi: 10.3171/2020.9.JNS202899[↩][↩][↩]
- Cavalli A, Martin A, Connolly DJ, Mirza S, Sinha S. Pituitary apoplexy: how to define safe boundaries of conservative management? Early and long-term outcomes from a single UK tertiary neurosurgical unit. Br J Neurosurg. 2021 Jun;35(3):334-340. doi: 10.1080/02688697.2020.1812523[↩][↩]
- Abdulbaki A, Kanaan I. The impact of surgical timing on visual outcome in pituitary apoplexy: Literature review and case illustration. Surg Neurol Int. 2017 Feb 6;8:16. doi: 10.4103/2152-7806.199557[↩]
- Rutkowski MJ, Kunwar S, Blevins L, Aghi MK. Surgical intervention for pituitary apoplexy: an analysis of functional outcomes. J Neurosurg. 2018 Aug;129(2):417-424. doi: 10.3171/2017.2.JNS1784[↩][↩]
- Suri H, Dougherty C. Presentation and Management of Headache in Pituitary Apoplexy. Curr Pain Headache Rep. 2019 Jul 29;23(9):61. doi: 10.1007/s11916-019-0798-5[↩]
- Eichberg DG, Di L, Shah AH, Kaye WA, Komotar RJ. Spontaneous preoperative pituitary adenoma resolution following apoplexy: a case presentation and literature review. Br J Neurosurg. 2020 Oct;34(5):502-507. doi: 10.1080/02688697.2018.1529737[↩]
- Souteiro P, Belo S, Carvalho D. A rare case of spontaneous Cushing disease remission induced by pituitary apoplexy. J Endocrinol Invest. 2017 May;40(5):555-556. doi: 10.1007/s40618-017-0645-7[↩]