What is arachidonic acid
Arachidonic acid is a 20 carbon chain polyunsaturated omega-6 essential fatty acid (PUFA) that is found in animal and human fat as well as in the liver, brain, and glandular organs. Arachidonic acid is present in all mammalian cells, typically esterified to membrane phospholipids (especially phosphatidylethanolamine, phosphatidylcholine, and phosphatidylinositides) and is one of the most abundant polyunsaturated fatty acids present in human tissue 1. Arachidonic acid is formed by the synthesis from dietary linoleic acid (omega-6 fatty acid) 2 and is a precursor in the biosynthesis of prostaglandins, thromboxanes, and leukotrienes, collectively known as eicosanoids 3. Arachidonic acid is a building block for molecules that can promote inflammation, blood clotting, and the constriction of blood vessels. But the body also converts arachidonic acid into molecules that calm inflammation and fight blood clots. It turns out that the body converts very little linolenic acid into arachidonic acid, even when linolenic acid is abundant in the diet. The latest nutrition guidelines call for consuming unsaturated fats like omega-6 fats in place of saturated fat. The American Heart Association along with the Institute of Medicine, recommends getting 5% to 10% of your daily calories from omega-6 fatty acids 4. For someone who usually takes in 2,000 calories a day, that translates into 11 to 22 grams. A salad dressing made with one tablespoon of safflower oil gives you 9 grams of omega-6 fatty acids; one ounce of sunflower seeds, 9 grams; one ounce of walnuts, 11 grams. Most Americans eat more omega-6 fats than omega-3 fatty acids, on average about 10 times more. A low intake of omega-3 fats is not good for cardiovascular health, so bringing the two into better balance is a good idea 4. But don’t do this by cutting back on healthy omega-6 fats. Instead, add some extra omega-3s. Nutritional scientists suggest the omega-6 fats to omega-3 fats ratio of 2:1 to 4:1, which indicates a high consumption of seafood 5. For optimal infant nutrition, the ratio of omega-6 fatty acids to omega-3 fatty acids must be not higher than 10 6. High consumption of plant oils rich in omega-6 polyunsaturated fatty acid (PUFA) and consumption of relatively low marine foods (as source of omega-3 fatty acid) increases the omega-6 fats to omega-3 fats ratio 7. According to dietary recommendations of the American Heart Association Nutrition Committee 8, it is suggested to consume fish at least two times per week or omega-3 polyunsaturated fatty acid (PUFA) 500 mg/day to prevent and reduce the risk of heart disease. The European Food Safety Authority panel recommends taking 250 mg omega-3 fatty acids per day, in contrast to the Suggested Dietary Targets in Australia recommends for adult men 610 mg omega-3 fatty acids and women 430 mg omega-3 fatty acids per day (docosahexaenoic fatty acid [DHA] + eicosapentaenoic fatty acid [EPA]) necessary to reduce the risk of cardiovascular disease 9, 10. Infant formulae should contain at least 0.2% of total fatty acids as docosahexaenoic fatty acid (DHA) and 0.35% as arachidonic acid 11.
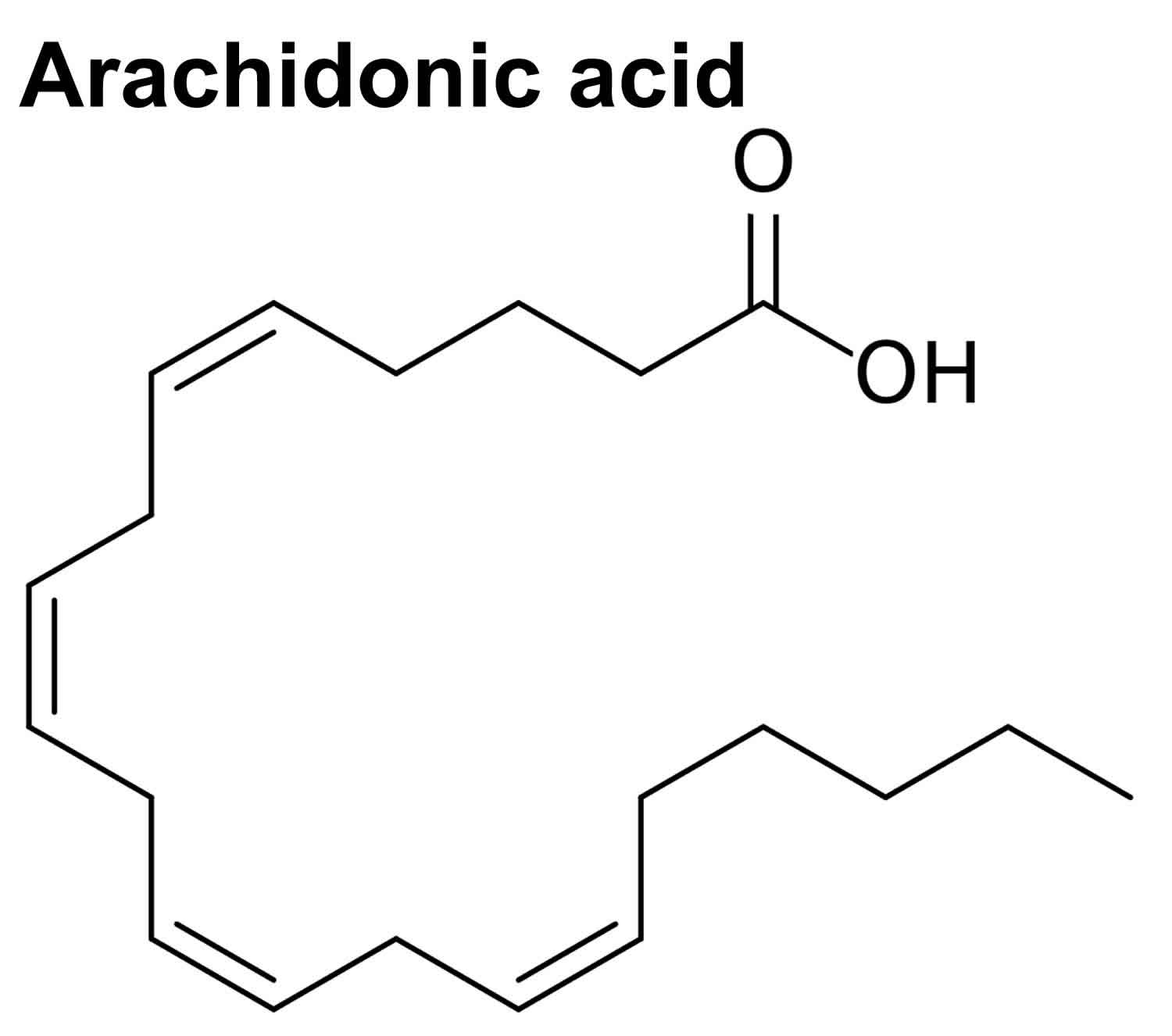
Arachidonic acid chemical formula is C20H32O2, 20:4(omega-6), where 20:4 refers to its 20 carbon atom chain with four double bonds, and (omega-6) refers to the position of the first double bond from the last, omega carbon atom 12. Arachidonic acid has an average mass of 304.467 g/mol and usually assumes a hairpin structure (Figure 1). Due to the presence of its four double bonds in the cis position (which means that all hydrogen atoms are on the same side of the double bonds), the compound has a certain degree of flexibility for interaction with proteins 13. Even at low temperature it helps in keeping the fluidity of cell membranes. The four double bonds also enable interaction with molecular oxygen giving rise to bioactive oxygenated molecules including eicosanoids (prostanoids and leukotrienes) and isoprostanes via enzymatic and non-enzymatic mechanisms, respectively 14.
Arachidonic acid is naturally found incorporated in the structural phospholipids of the cell membrane in the body or stored within lipid bodies in immune cells 15. Arachidonic acid is particularly abundant in skeletal muscle, brain, liver, spleen and retina phospholipids 16. The concentration of free arachidonic acid in the circulation is very low, owing the fact that in human plasma, albumin is highly abundant as its concentration reaches up to 35 mg/ml, which enables the binding of free fatty acids keeping their concentration below 0.1 µmol 17.
Arachidonic acid supplementation does not result in decreased docosahexaenoic fatty acid [DHA]/eicosapentaenoic fatty acid [EPA] 18. DHA/EPA composition was unchanged by 700 mg 19 or 838 mg 20 of arachidonic acid per day. In the same manner, 240 and 720 mg 21 or 1500 mg 22 of arachidonic acid per day did not change DHA/EPA composition. In contrast, it is well known that arachidonic acid composition is decreased by DHA/EPA supplementation 23. Interestingly, it is commonly observed that arachidonic acid supplementation results in large decreases in linoleic acid composition 21. It appears that the capacity for exchange or retention in the body is in the following order DHA/EPA > arachidonic acid > linoleic acid. Arachidonic acid intake from foods or supplementation is thought to have a great impact on long-chain polyunsaturated fatty acids metabolism. The continued accumulation of evidence from large and well-designed dietary surveys and clinical trials is expected to confirm this 18.
In humans, arachidonic acid plasma levels typically range from 0.1 µM to 50 µM, whereas under pathophysiological conditions, arachidonic acid plasma concentrations up to 500 µM can be found 24. Interestingly, 50 µM to 100 µM of free arachidonic acid are cytotoxic in vitro, thus it is likely that plasma arachidonic acid concentrations found in the human body exert cytotoxic and apoptosis regulatory functions. Accordingly, arachidonic acid is essential in embryogenesis and infant development, but may also be a driving factor in cardiovascular, metabolic and inflammatory diseases 25. In line with this, several studies have found an inverse relationship of plasma arachidonic acid concentrations and the risk of coronary heart disease 26. Thus, it is important to elucidate any potential influence of arachidonic acid on various aspects in human health.
Endogenous arachidonic acid generation mainly occurs via the release of arachidonic acid from cell membrane phospholipids 27. This process is catalyzed by enzymes of the phospholipase A2 (PLA2) superfamily and is induced by various cellular activation signals, including inflammation or infection driven tumor necrosis factor receptor (TNFR) and toll-like receptor 4 (TLR4) stimulation 28. Among the phospholipase A2 (PLA2) enzymes superfamily, three members contribute to eicosanoid production and are involved in distinct functions of eicosanoid metabolism. The cytosolic Ca2+ dependent PLA2 (cPLA2) alpha mainly drives free fatty acids (FFAs) production and generation of arachidonic acid, which is involved in cellular signaling. The cytosolic Ca2+ independent PLA2 (iPLA2) alpha contributes to cellular homeostasis via the synthesis of specialized pro-resolving mediators and reacylation of free arachidonic acid, and the secretory PLA2 (sPLA2) controls free arachidonic acid release and induces the local inflammatory response in a paracrine manner 29. In addition to PLA2, two other phospholipase families, namely phospholipase C (PLC) and phospholipase D (PLD), generate arachidonic acid via intermediate products such as diacylglycerol (DAG) 30. Finally, endocannabinoids serve as an endogenous source for arachidonic acid, as demonstrated by the generation of arachidonic acid from anandamide 31.
Arachidonic acid metabolites are implicated in immune surveillance, the establishment of allergy, the initiation and resolution of an inflammatory response, platelet aggregation and the coagulation cascade, glucose metabolism, neuronal signaling, female fertility, and the adaptation of mood and appetite 12. Arachidonic acid metabolites are important factors in the initiation and resolution of inflammation and have been linked to the pathophysiology of obesity, diabetes mellitus, nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH) and cardiovascular diseases 27. Interestingly, available enzyme cassettes to process arachidonic acid and G-protein coupled receptors are different for each cell and tissue type, ensuring a cell/tissue-specific regulation and function of arachidonic acid metabolism 32. The functional state of cells (e.g., activated vs. non-activated macrophages) and tissue environment impact on the synthesis of arachidonic acid derivatives, and the necessity for cell-cell interactions to produce specific eicosanoids, also termed transcellular biosynthesis, adds further complexity to arachidonic acid metabolism 33. Targeted lipidomic strategies using mass spectrometry-based technology platforms revealed that the compound effect of local lipid metabolites, and their time-dependent generation, determine the biological function of the arachidonic acid metabolome, rather than the specific concentration of a single eicosanoid at a given site and time point 34. This was also demonstrated by the partial inhibition of the immunomodulatory effects of free fatty acids by omega-3 PUFAs, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which display anti-inflammatory properties and are precursors for pro-resolving specialized pro-resolving mediators 35. Specialized pro-resolving mediators, including resolvin D1, enhance phagosome formation in macrophages and are capable of reverssing the phagocytic defect of macrophages derived from diabetic mice. They may thereby counter-balance the effects of pro-inflammatory arachidonic acid metabolites 36.
Lastly the plethora of arachidonic acid derivatives and their multitudinous implications, and the differential expression and functionality of arachidonic acid metabolites in various cells and tissues, as well as their transcellular biosynthesis, render the arachidonic acid metabolism one of the most complex regulatory systems within the human body. It is thus not surprising that many aspects of the arachidonic acid metabolome still need further investigation, despite extensive scientific progress in the field 27.
Figure 1. Arachidonic acid chemical structure (arachidonic acid hairpin conformation)
Footnote: Arachidonic acid is incorporated in phospholipids in the cells’ cytosol, adjacent to the endoplasmic reticulum membrane that is studded with the proteins necessary for phospholipid synthesis and their allocation to the diverse biological membranes 37. Of note, glycerophospholipids are composed of a glycerol backbone esterified to two hydrophobic fatty acids tails at sn- (stereospecifically numbered) 1 and 2 position and a hydrophilic head-group at sn 3 38.
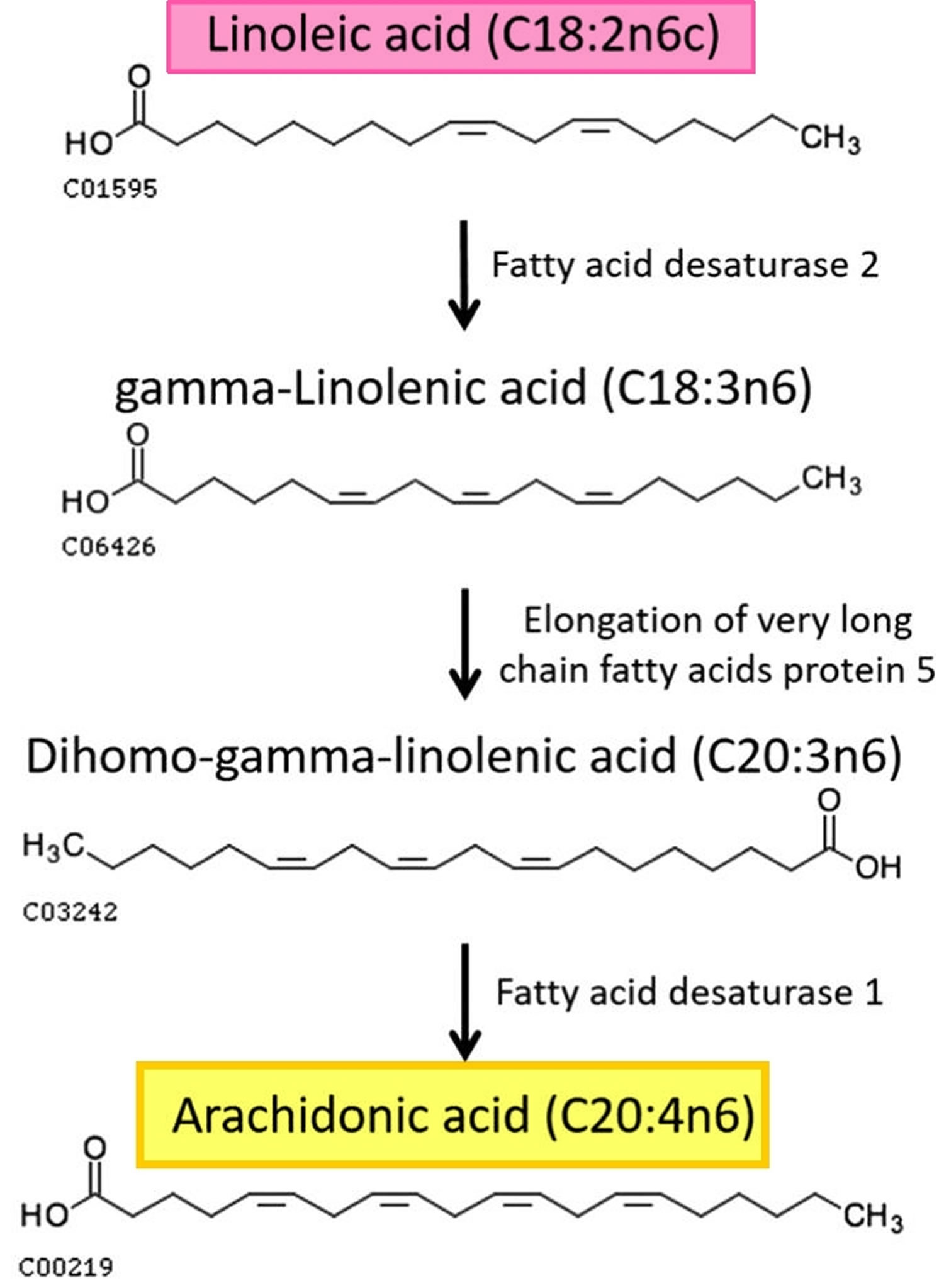
Figure 2. Formation of arachidonic acid from linoleic acid metabolism
[Source 12 ]Arachidonic acid foods
Arachidonic acid can be provided to humans by an exogenous source supplied either by the direct consumption of dietary food that contains high level of arachidonic acid, whole eggs, poultry, animal organs, salmon, tuna 39, 40, a wide range of lean meat 41 and visible meat fats 42 or through the parent molecule, linoleic acid (omega-6 fatty acid) (see Figure 2 above). Linoleic acid is considered to be an essential fatty acid since humans and some mammals lack the enzymes required for its synthesis 43. Linoleic acid is abundant in vegetable oils such as soya, corn, sunflower and safflower and also found in walnuts 44. In human body, linoleic acid is subjected to series of desaturation enzymes (delta-6 fatty acid desaturase and delta-5 fatty acid desaturase), and elongation enzymes that carry out their action in the endoplasmic reticulum (ER) membrane 45. Beside the exogenous source, endocannabinoids such as N-arachidonoyl ethanolamine (anandamide) serve as an endogenous source of arachidonic acid 12. An integrated membrane protein enzyme, fatty acid amide hydrolase, is responsible for the catalysis of anandamide into arachidonic acid and ethanolamine to eliminate the anandamide signal in the nervous system 46.
Another arachidonic acid source, that is important for herbivores and vegetarians, is linoleic acid, also an omega-6 PUFA that contains only two cis- double bonds (18:2ω-6). Linoleic acid is an essential fatty acid for animals because they cannot synthesize it, in contrast to plants, which can synthesize it from oleic acid 38. Linoleic acid is abundant in many nuts, walnuts, fatty seeds and their derived vegetable oils 7. Linoleic acid is converted in animals cells cytosol to arachidonic acid, docosatetraenoic acid (22:4ω-6) and other fatty acids by step-wise desaturation and chain elongation (see Figure 2). Linoleic acid conversion to arachidonic acid is, however, low. Linoleic acid is readily oxidized by delta 6-desaturase to gamma-linolenic acid (18:3-n6), but several factors such as aging, nutrition, smoking impair the activity of the enzyme 38. Gamma linolenic elongation step to dihomo-gamma-linolenic acid (20:3-n6) is rapid; yet, it is oxidized by delta-5 desaturase to yield arachidonic acid at a small percentage because delta-5 desaturase prefers the n-3 to n-6 fatty acids 47.
Table 1. Content of arachidonic acid and the other fatty acids per 100 g edible portion of animal foods
| Food group | Total fat (mg) | Fatty acids (mg) | |||||
| Palmitic acid | Oleic acid | Linoleic acid | Arachidonic acid | Eicosapentaenoic acid (EPA) | Docosahexaenoic acid (DHA) | ||
| Meats and poultry | |||||||
| Pork, loin, whole, lean and fat, raw | 12580 | 2720 | 5140 | 1110 | 80 | 0 | 0 |
| Pork, medium type breed, loin, lean and fat, raw | 22600 | 5600 | 9100 | 1900 | 68 | 0 | 12 |
| Chicken, broiler, thigh, meat and skin, raw | 16610 | 3511 | 5832 | 3096 | 104 | 4 | 7 |
| Chicken, broiler, thigh, meat with skin, raw | 14200 | 3300 | 5800 | 1600 | 79 | 1 | 7 |
| Beef, hip, inside (top) round steak, boneless, lean, raw | 2210 | 520 | 910 | 120 | 40 | 0 | 0 |
| Beef, inside round, lean, raw | 4300 | 890 | 1500 | 120 | 24 | 4 | 1 |
| Eggs | |||||||
| Chicken, whole, fresh or frozen, raw | 10010 | 2218 | 3810 | 1109 | 156 | 2 | 72 |
| Hen, whole, raw | 10300 | 2100 | 3500 | 1300 | 150 | 0 | 120 |
| Fishes and seafoods | |||||||
| Salmon, pink (humpback), raw | 6700 | 1044 | 1108 | 102 | 127 | 547 | 859 |
| Pink salmon, raw | 6600 | 790 | 920 | 81 | 31 | 400 | 690 |
| Flatfish (flounder or sole or plaice), raw | 1930 | 282 | 358 | 45 | 30 | 137 | 108 |
| Righteye flounder, brown sole, raw | 1300 | 150 | 140 | 10 | 50 | 100 | 72 |
| Sardine, pacific, canned in tomato sauce, drained with bones | 10450 | 1738 | 1851 | 123 | 190 | 532 | 864 |
| Sardine, Japanese pilchard, canned products, in tomato sauce | 10800 | 1900 | 1200 | 140 | 160 | 1300 | 1100 |
| Milk and dairy products | |||||||
| Cheese, cream | 34240 | 8497 | 7923 | 1032 | 50 | 0 | 0 |
| Cheese, cream | 33000 | 8700 | 6400 | 570 | 38 | 20 | 6 |
Arachidonic acid function
Arachidonic acid is an integral constituent of biological cell membrane phospholipids, conferring it with fluidity and flexibility, so necessary for the function of all cells, especially in nervous system, skeletal muscle, and immune system 38. The four double bonds of arachidonic acid predispose it to oxygenation that leads to a plethora of metabolites of considerable importance for the proper function of the immune system, promotion of allergies and inflammation, resolving of inflammation, mood, and appetite 12. Platelets, mononuclear cells, neutrophils, liver, brain and muscle have up to 25% phospholipid fatty acids as arachidonic acid 48. Arachidonic acid participates in the Lands cycle, a membrane phospholipids’ reacylation/deacylation cycle, which serves to keep the concentration of free arachidonic acid in cells at a very low level 49. Since arachidonic acid is a fundamental constituent of cell structure, it will particularly be needed for during development and growth and upon severe or widespread cell damage and injury 38.
Cell membrane fluidity
Arachidonic acid four cis double bonds endow it with mobility and flexibility conferring flexibility, fluidity and selective permeability to membranes 50. Arachidonic acid control of membrane fluidity influences the function of specific membrane proteins involved in cellular signaling 51 and plays a fundamental role in maintenance of cell and organelle integrity and vascular permeability 52. These properties might explain arachidonic acid critical role in neuron function, brain synaptic plasticity, and long-term potentiation in the hippocampus 53.
Ion channels
Non-esterified, free arachidonic acid affects neuronal excitability and synaptic transmission via acting on most voltage-gated ion (sodium [NaV], potassium [KV], calcium [CaV], and proton [HV]) channels, responsible for regulating the electric activity of excitable tissues, such as the brain, heart and muscles 38. Ion channels are large families of integral membrane proteins that form a selective pore for ions to cross the lipid bilayer, via undergoing conformational changes in response to alteration in the cell transmembrane electrical potential. These channels gate passage of specific ions and thus control the propagation of nerve impulses, muscle contraction, and hormone secretion 54. The homologous mon-, di- or tetrameric subunits of arachidonic acid-sensitive voltage-gated channels are composed of four transmembrane helices spanning the cell membrane lipid bilayer (S1-S4) making up the voltage-sensor domain, and/or 2 transmembrane segments constituting the central ion-conducting pore 55. The gating charges are situated on helix S4, a positively charged voltage sensor, which responds to changes in voltage across the membrane by inducing movements of the helix relative to the remainder of the protein or the movement of the positive charges through the membrane toward the extracellular side 55. Since S4 is in contact with the lipid bilayer, the arachidonic acid lipophilic, flexible acyl chain can position its carboxylate negative charge onto the voltage sensor, and modulate its activity, likely shifting the voltage dependency of activation via channel-activating electrostatic interactions 54.
Free arachidonic acid evoked K+ channel opening in neurons of the rat visual cortex, thus suggesting the existence of an arachidonic acid-activated type of K+ channel, which may play a critical role in modulating cortical neuronal excitability 56. Arachidonic acid was previously reported to directly activate K+ channels in gastric, pulmonary artery, and vascular smooth muscle cells, and cardiac atrial cells likely via interacting with the ion channel protein itself 57. Conversely, arachidonic acid is known to suppress the Kv4 family of voltage-dependent K+ channels, in a direct, fast, potent, and partially reversible mode 58. The activity of the large-conductance Ca2+- dependent K+ (BK) channels, which control diverse functions in the central nervous system such as sleep and neural regulation of the heart, is increased up to 4 folds by arachidonic acid, consequent to direct interaction with the channel protein 59. Conversely, arachidonic acid inhibited intermediate conductance, Ca++-activated K+ channels, which play crucial roles in agonist-mediated transepithelial Cl− secretion across airway and intestinal epithelia, via interacting with the pore-lining amino acids (aa) threonine (aa 250) and valine (aa 275) 60. Background, non-voltage-dependent two pore domain K(+) channels, which play an essential role in setting the neuronal membrane potential and potential duration are opened by arachidonic acid, and not its metabolites, provided the carboxyl end is not substituted with an alcohol or methyl ester 61. Additionally, arachidonic acid was reported to inhibit the ATP- sensitive K+ channel in cardiac myocytes almost completely, while activated the ATP-insensitive K+ channel 62.
Free, non-esterified arachidonic acid prevents ischemia-induced heart arrhythmia, a major cause of sudden cardiac death in humans, by modulating the activity of cardiac Na+ channels, the major class of ion channels that determine cardiac excitability, causing a reduction in the electrical excitability and/or automaticity of cardiac myocytes 63. Sodium channels consist of a large functional subunit and a smaller subunit, which interacts with a regulatory segment. Arachidonic acid, but not its metabolic products, was shown to voltage-dependent modulate muscle Na+ channel currents, displaying both activation and inhibitory effects depending on the depolarizing potential 64. Arachidonic acid also displayed both activation and inhibitory effects on different Cl- channels, widely distributed especially in epithelial tissues, and thus, mediate increase or block of Cl- ions permeation 65. The double bonds and hydrophilic head were recently reported to be responsible for the arachidonic acid mediated dramatic increases in proton current amplitude through the voltage-gated proton (Hv) channel. The latter lacks a pore domain but allows passage of proton through the center of each voltage sensor domain 54 and supports the rapid production of reactive oxygen species (ROS) in phagocytes through regulation of pH and membrane potential 66.
Receptors and enzymes
Exogenous or endogenously produced arachidonic acid was discovered to greatly enhance the functional activity of ligand-gated ion channels, namely the gamma-amino butyric acid receptor (GABA-R) located on the neuronal membrane, via modulating the GABA-R interaction characteristics with its ligands 67. Free arachidonic acid exposure essentially led to inhibiting the muscle and neuronal nicotinic acetylcholine receptor (nAChR), an integral membrane protein deeply embedded in the postsynaptic region, with two agonist binding sites and a central ion pore. The receptor inhibition resulted from arachidonic acid displacing lipids from their sites in the plasma membrane and direct acting as antagonist at the PUFA-protein interface 67.
Activation of eosinophils, neutrophils, and macrophages elicits powerful respiratory burst associated with reduction of molecular oxygen to superoxide via activation of the NADPH oxidase complex, which consists of five proteins residing in resting cells in the cytosol or membrane of intracellular vesicles, and in activated cells are assembled on the cell membrane 68. Generated reactive oxyegn species (ROS) induce membrane depolarization and cytoplasm pH decrease, thus restricting NADPH activity. Concentrations of arachidonic acid of 5–10 µM added to neutrophils enhanced NADPH oxidase stimulation due to arachidonic acid-mediated activation of the Hv channel, modulation of the membrane potential and pH, and efflux of the H+ ions generated together with the superoxide anion, O2− 69.
PUFA, especially arachidonic acid, are documented activators of membrane-associated, magnesium-dependent, neutral sphingomyelinases (nSMase) 70. Arachidonic acid was recently documented as activator of Schistosoma mansoni and Schistosoma haematobium tegument-associated neutral sphingomyelinase in a dose-dependent manner, eventually leading to their attrition in vitro and in vivo 71.
Cell death
Free arachidonic acid levels are kept very low in cells as uncontrolled accumulation of unesterified arachidonic acid decisively impaired cell survival via induction of apoptosis 49. The apoptotic effect was attributed to free arachidonic acid and not its metabolites as it was recorded in the absence of lipoxygenase or cyclooxygenase enzyme activity, and was speculated to be associated with oxidative stress and/or changes in membrane fluidity 72. Pompeia et al. 73 reported that the cytotoxicity of arachidonic acid is undeniable, but may well be one of its fundamental functions in vivo. arachidonic acid concentrations of 50–100 µM are cytotoxic to most cell lines in vitro. In the majority of models 1–10 µM arachidonic acid is necessary to elicit any biological response but some activities require 100–300 µM 51. This indicates that arachidonic acid apoptotic and physiological levels overlap and it is very possible that arachidonic acid cytotoxicity occurs in vivo because under some pathologic conditions, human plasma arachidonic acid levels can increase from 0.1–50 μM to 100 and up to 500 μM 73.
Arachidonic acid metabolism
Four enzymatic pathways process free arachidonic acid, resulting in the generation of a plethora of arachidonic acid derivatives with multitudinous functions (Figures 3 and 4).
First, cyclooxygenase 1 (COX1) and cyclooxygenase-2 (COX-2), also known as Prostaglandin G/H synthases, foster the production of thromboxane A2 (TXA2), prostacyclin (PGI2) and several prostaglandins (PGs) 74. Cyclooxygenase 1 (COX1) is constitutively expressed and found in all tissues, which are prone to lipopolysaccharide (LPS) induced inflammation. Thromboxane A2 (TXA2) and prostaglandins (PGs), such as prostaglandin D2 (PGD2), are metabolites of the COX1 pathway, and alter the vessel tone, mediate platelet aggregation and are implicated in immune surveillance 75. Cyclooxygenase 2 (COX2) is found in macrophages and endothelial cells, demonstrates high expression levels in the kidney and brain, and is induced by bacterial endotoxins, growth factors, hormones, and several cytokines 76. Its major metabolites prostaglandin E2 (PGE2), prostaglandin I2 (PGI2, aslo known as prostacyclin), prostaglandin D2 (PGD2) and prostaglandin F2alpha (PGF2α) adapt host immune response, vessel tone regulation, thrombus formation, pain reception, and female fertility, and are involved in neurodegeneration and cancer 77. Additionally, cyclooxygenase 3 (COX3), a splice variant of COX1, facilitates prostaglandin synthesis in the human brain and heart 78.
Second, four lipoxygenases (LOX), namely 5-LOX, 8-LOX, 12-LOX and 15-LOX, process arachidonic acid. 5-LOX produces 5-hydroperoxyicosatetraenoic acid (5-HPETE), 5-hydroxyicosatetraenoic acid (5-HETE), and 5-oxo-eicosatetraenoic acid (5-oxo-ETE), as well as various leukotrienes (LTs) 79. These metabolites affect neutrophil recruitment and diapedesis, epithelial barrier function, vascular permeability and bronchoconstriction 80. 8-LOX and 15-LOX lipoxygenases convert free arachidonic acid to 8- and 15-hydroperoxyicosatetraenoic acid (8-HPETE and 15-HPETE) and foster the production of 15-HPETE derivatives, such as 15-hydroxyicosatetraenoic acid (15-HETE), lipoxins and eoxins 81. 12-LOX metabolize arachidonic acid to 12-hydroperoxyeicosatetraenoic acid (12-HPETE), which itself serves as a precursor for 12-hydroxyeicosatetraenoic acid (12-HETE) and to hepoxilins 82. Arachidonic acid derivatives from the 8-, 15-, and 12-LOX pathways are involved in the establishment of hyperalgesia and induce the expression of fatty acid translocase 83. Lipoxins exert mainly anti-inflammatory properties. Interestingly, their synthesis is induced by the COX inhibitor aspirin, an antithrombotic agent widely used in the treatment and for secondary prevention of cardiovascular diseases 84.
Third, the cytochrome P450 (CYP450) pathway, which is mainly restricted to the liver and is well known for its function in detoxification, includes epoxygenase and ω-hydroxylase, which facilitate the production of epoxy-eicosatrienoic acids (EETs) and hydroxy-eicosatetraenoic acids (HETEs), respectively. These arachidonic acid derivatives regulate vessel constriction and dilation, hamper hyperalgesia, and inhibit COX2 expression 85.
Fourth, anandamide, an endocannabinoid, is generated from arachidonic acid via the fatty acid amide hydrolase (FAAH) in a reversible reaction 86, whereas the processing of anandamide to arachidonic acid and ethanolamine takes place when tissue damage is associated with high levels of free arachidonic acid 87. In this situation, anandamide supports tissue regeneration, and cellular proliferation via interaction with cannabinoid type 1 (CB1) receptors 88.
Finally, free arachidonic acid is also processed by non-enzymatic reactions. The four double bonds of arachidonic acid are readily oxygenated to form bioactive molecules. Thus, oxidative stress and/or exposure of arachidonic acid to reactive oxygen species (ROS) and reactive nitrogen species (RNS) result in oxidation of arachidonic acid and generate isoprostanes and nitroeicosatetraenoic acids 89. These eicosanoids were reported to inhibit COX1, whereas isoprostanes have been linked to platelet aggregation, vasoconstriction, smooth muscle cell proliferation, and cardiomyocyte hypertrophy 90. To conclude, arachidonic acid and its derivatives can enter numerous metabolic pathways that interconnect lipid metabolism with immunity. A synopsis of key enzymes, metabolites, and biological functions of the arachidonic acid metabolism is depicted in Figure 3.
The expression and activation of phospholipase A2 (PLA2) enzyme can be a response to a wide range of cellular activation signals from receptor dependent events requiring a G coupled transducing protein as Toll-like receptor 4 (TLR4), purinergic receptors and inflammation stimulation to calcium ionophores, melittin (bee venom) and tumor promoting agents [91. Three fates wait for the liberated, free functional arachidonic acid: 1) it may diffuse to other cells, 2) reincorporated into the phospholipids, or 3) metabolized.
Furthermore, the activation of PLA2 enzyme can be through the binding of tumor necrosis factor alpha (TNF-α) to its receptor, P75 and P55, inducing the release of arachidonic acid from phosphatidylcholine and phosphatidylethanolamine. Free arachidonic acid can have an important role in cell apoptosis as its accumulation that occurs as a result of arachidonyl CoA transferase inhibition, can promote the activation of sphingomyelinase, enzymes that trigger the degradation of sphingolipids (known to play an important role in cell regulation and cell cycle) to phosphocholine and ceramide 92.
Free arachidonic acid can be metabolized via enzymatic reactions. Free arachidonic acid can undergo four possible enzymatic pathways: Cyclooxygenase, Lipoxygenase, Cytochrome p450 (CYP 450) and Anandamide pathways to create bioactive oxygenated PUFA containing 20 carbon (eicosanoids) acting as local hormones and other compounds acting as signaling molecules 12. Enzymes involved in the cyclooxygenase pathway are cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) also called prostaglandin H synthase, along with downstream enzymes that mediate the production of prostaglandins (PGH2, an unstable intermediate, PGE2, PGD2 and PGF2alpha, prostacyclins (PGI2), and thromboxanes (TXA2, TXB2) 12. Lipoxygenase pathway consists of LOX-5, LOX-8, LOX-12, and LOX-15 enzymes and their products, leukotrienes (LTA4, an unstable intermediate, LTB4, LTC4, LTD4 and LTE4), lipoxins (LXA4 and LXB4 formed upon LXA4 degradation) and 8–12- 15- hydroperoxyeicosatetraenoic acid (HPETE) 12. The CYP 450 pathway involves two enzymes, CYP450 epoxygenase and CYP450 ω-hydroxylase giving rise to epoxyeicosatrienoic acid (EETs) and 20-hydroxyeicosatetraenoic acid (20-HETE) respectively. Anandamide pathway comprises the fatty acid amide hydrolase (FAAH) to produce the endocannabinoid, anandamide 93.
Arachidonic acid may additionally undergo non-enzymatic reactions. Studies proved that the exposure of carbon tetrachloride (CCL4) to rats to mimic the oxidative stress and as an induction of lipid peroxidation state in vivo, leads to the formation of PGF2-like compounds called isoprostanes and other compounds such as nitroeicosatetraenoic acids. Arachidonic acid autoxidation by reactive oxygen species (ROS) and reactive nitrogen species (RNS) are also examples of non-enzymatic oxidative metabolism 94.
Arachidonic acid metabolism and enzymes expression usually vary from cell to cell and from tissue to another according to various factors; consequently, the level and type of biosynthesized eicosanoids will differ in each case. It was reported that bone marrow macrophages differ from peritoneal macrophage responses regarding generated eicosanoids quantities and specificity. One more factor that causes this variation is the state of the cell whether it was stimulated or in resting phase. In normal cell state, eicosanoids are generated in very minute amounts and subsequent up regulation can only occur following an inflammatory stimulation 95.
The complexity of eicosanoid biosynthesis lies in the cell–cell interaction, where a donor cell has to transfer its unstable intermediate e.g. PGH2, LTA4 to another recipient cell to trigger the latter for eicosanoids biosynthesis. The single donor cell should have all the necessary enzymes to produce eicosanoids while the recipient cell has not to have all the required enzymes for arachidonic acid release. Hence, for initiation inflammation or tissue injury, at least two cells in the injured tissue must have the complete enzyme cassette to initiate eicosanoids production. Accordingly, eicosanoids are described, as mentioned above, as local hormones due to their autocrine and paracrine action. Adding to the complexity of the trans cellular interactions, the arachidonic acid intermediate metabolites are lipophilic with short half-life time (90–100 s) and require some other mechanisms to be translocated 96. These facts are in line with studies since 1985 by Dahinden et al. 97 who revealed that exogenous LTA4 stabilized by albumin is uptaken by bone-marrow mast cells to produce sulfidopeptide LTC4. Later on, it was found by Dickinson et al. 98 that a group of special proteins called fatty acid binding proteins (FABP), specific for each cell type, are responsible for increase of arachidonic acid intermediates export via their stabilization and lengthening their half-life time by up to 30 minutes at 4 °C.
Though eicosanoids have a short half-life time, they contribute to many biological activities in paracrine or autocrine manner. They have crucial dual role, regulating innate immunity and inflammation resolution through binding to G protein coupled receptors located on other cells. First, once tissues are inflamed or infected, beside pain and swelling, arachidonic acid metabolites amplify the inflammatory signals to recruit leukocytes, pro-inflammatory cytokines, and immune cells to help in pathogens resistance and clearance. Second, they balance the induced inflammatory signals by producing resolving metabolites to act as host protection, since inflammation exerts a threatening action if it is not controlled in an appropriate time manner 91. COX-1 induced eicosanoids were shown to be lethal when produced continuously. Von Moltke et al. 99 was able to prove that eicosanoids can be lethal, as COX-1-mediated prostaglandins generation were shown to be responsible for vascular leakage and mortality in mice model. Another example of homeostasis regulation, COX-1-mediated TXA2 production regulates platelets aggregation while COX-2-mediated PGI2 release inhibits platelets aggregation and promotes vasodilation. Imbalance between levels of PGI2 and TXA2 could elevate the risk of cardiovascular diseases. On these terms, physicians prescribe aspirin as a cardio protectant to inhibit COX-1 enzyme and subsequent inhibition of TXA2 91.
PLA2 is up-regulated once triggered by same receptor that leads to inflammasome activation, prompting the class switching of eicosanoids from prostaglandins to lipoxins to reprogram cells from the pro-inflammation and inflammasome activation to resolution 100. This mechanism can be enhanced by acetylated COX-2 and FLAP (5-lipoxygenase-activating protein, is a constitutively expressed protein that serves the docking of arachidonic acid with 5-LOX active site as well as the co-localization of both 5-LOX – cPLA2 to perinuclear membrane) to produce lipoxins 101.
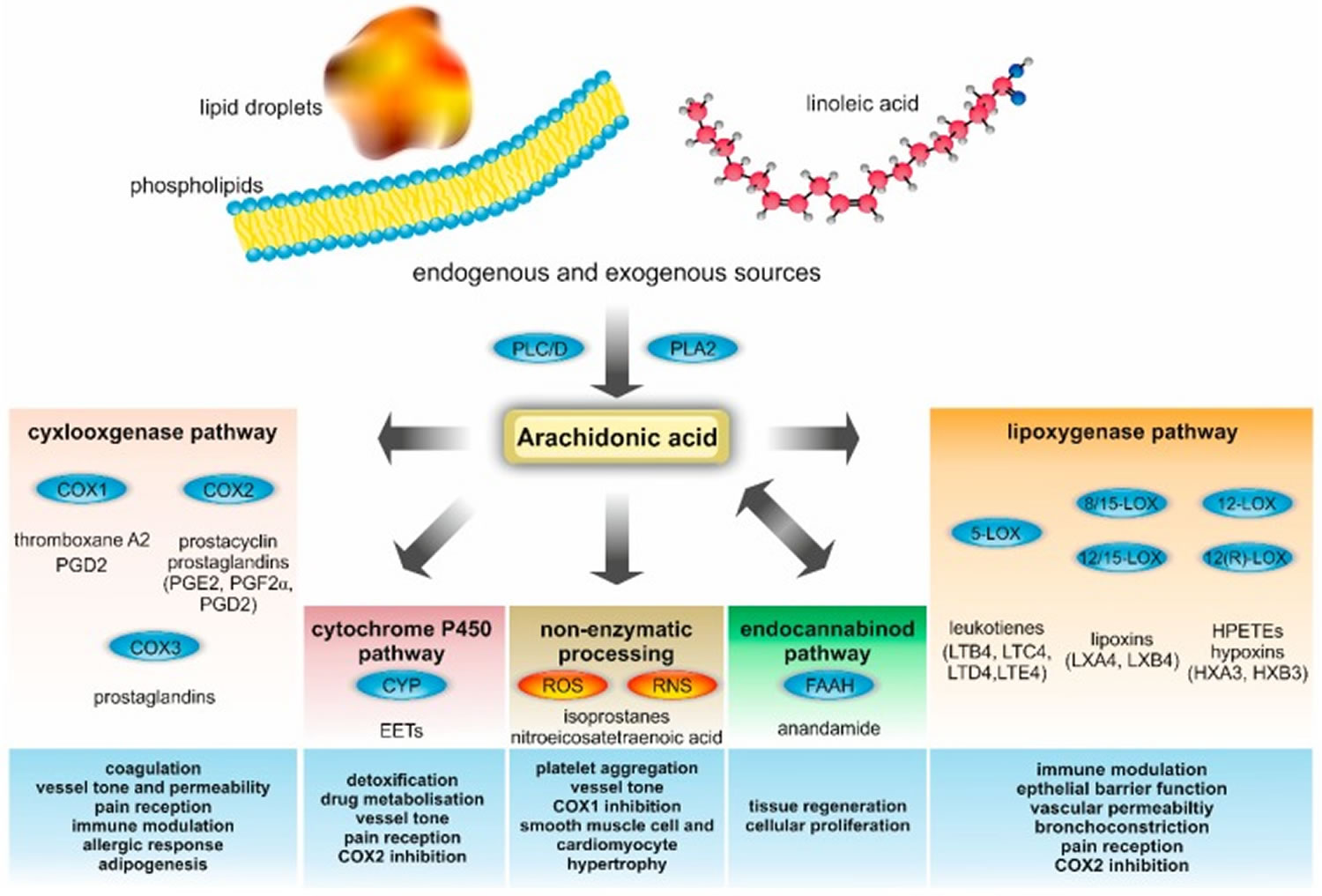
Figure 3. Arachidonic acid metabolism and their biological functions in metabolic and cardiovascular diseases
Footnote: Metabolites and enzymes involved in arachidonic acid metabolism and their biological functions in metabolic and cardiovascular diseases. Endogenous arachidonic acid is mainly derived from cell membrane phospholipids, which are processed by phospholipase A2 (PLA2), phospholipase C (PLC), and phospholipase D (PLD). Free arachidonic acid serves as a precursor for a plethora of metabolites, including prostaglandins (PGs), prostacyclin, thromboxane, HPETE, leukotrienes, lipoxins, hypoxins, anandamide, and epoxyeicosatrienoic acids (EETs). In addition to this enzymatic processing of arachidonic acid, there is also a non-enzymatic metabolization. The latter is important for the production of isoprostanes and nitroeicosatetraenoic acid. Many arachidonic acid metabolites are highly bioactive and involved in various crucial vital processes. Relevant biological functions in metabolic and cardiovascular diseases are summarized in the blue boxes at the bottom.
Abbreviations: COX = cyclooxygenase; CYP = cytochrome; ROS = Reactive oxygen species; RNS = Reactive nitrogen species; FAAH = fatty acid amide hydrolase; HX = hypoxin; LOX = lipoxygenase; LT = leukotriene; and LX = lipoxin.
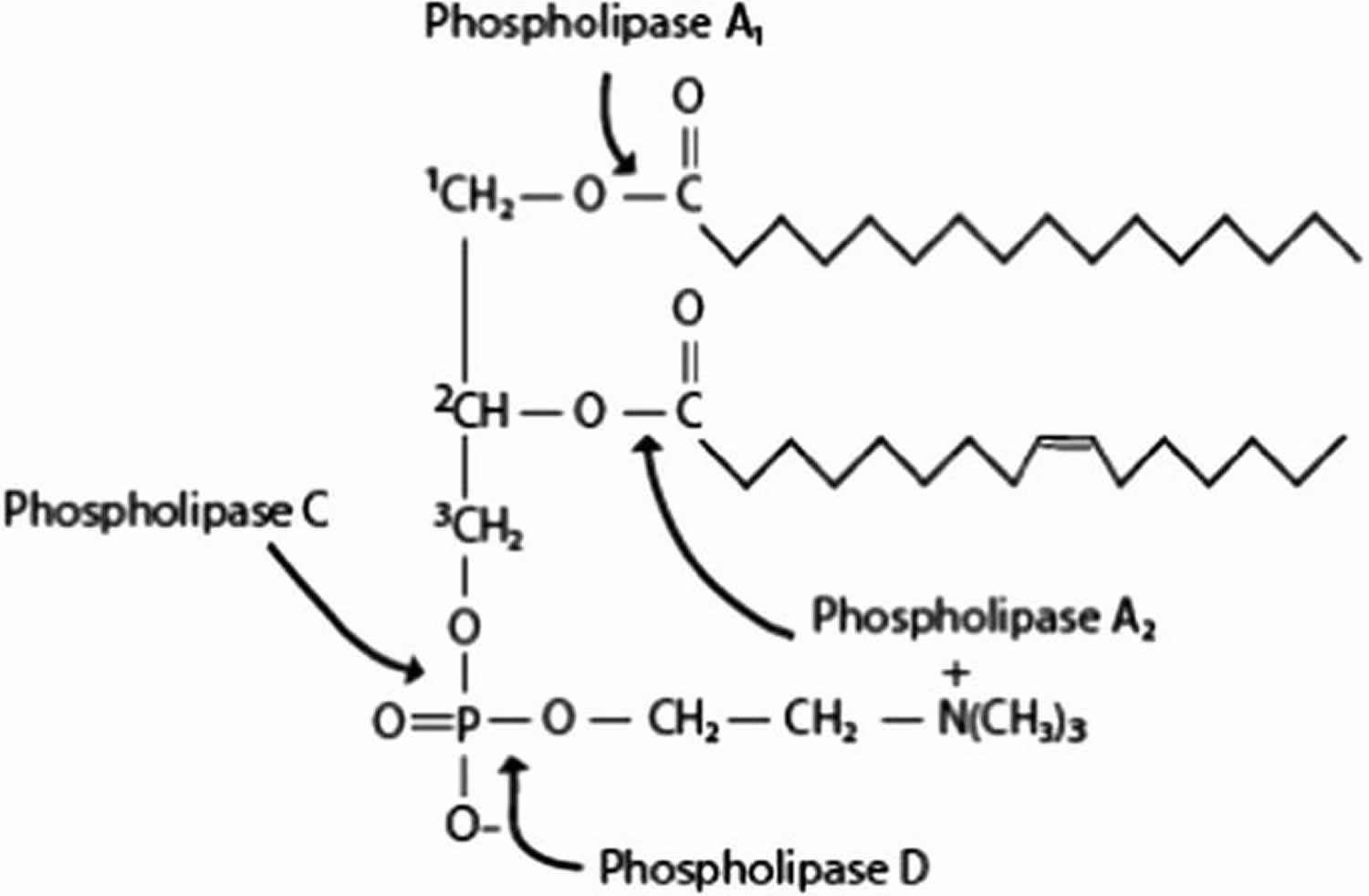
[Source 27 ]Figure 4. Arachidonic acid metabolism
Footnote: Sites of hydrolysis for each phospholipase [phospholipase A1 (PLA1), phospholipase A2 (PLA2), phospholipase C (PLC) and phospholipase D (PLD)].
[Source 12 ]Arachidonic acid benefits
Resolution of inflammation
Free arachidonic acid and its metabolites, prostaglandins (PG), namely prostaglandin F2alpha (PGF2a, also known as dinoprost), prostaglandin E2 (PGE2, also known as dinoprostone), and prostaglandin I2 (PGI2, also known as prostacyclin) display essential roles in skeletal muscle development and growth by controlling proliferation, differentiation, migration, fusion and survival of myoblasts 102. Eicosanoids (prostanoids and leukotrienes) produced from arachidonic acid tend to promote muscle growth during and after physical activity in healthy humans 38. Yet, the major action of arachidonic acid metabolites is promotion of acute inflammatory response, characterized by the production of pro-inflammatory mediators such as prostaglandin E2 (PGE2) and prostaglandin I2 (PGI2), followed by a second phase in which lipid mediators with pro-resolution activities may be generated. Resolution of inflammation is no more considered a passive process, but rather an active programmed response regulated by mediators with pro-resolving capacity, prominent among which is arachidonic acid-derived lipoxin A4 103. Lipoxin A4 stimulates cessation of neutrophil infiltration, enhances macrophage uptake of apoptotic cells in pre-clinical animal models 104, attenuates leukotriene C4-induced bronchoconstriction in asthmatic subjects, decreases eczema severity and duration and improves patients’ quality of life via inhibiting the activity of innate lymphoid cells type 2 105.
Lipoxin A4 (1 nM) was also reported to attenuate adipose inflammation, decreasing interleukin (IL)- 6 and increasing IL-10 expression in aged mice 106. Recently, lipoxin A4 encapsulated in poly-lactic-co-glycolic acid microparticles displayed considerable healing effects in topical treatment of dorsal rat skin lesions, provided interaction with its specific receptor on skin cells 104. Other arachidonic acid metabolites, notably PGE2, PGI2 and leukotriene B4 and leukotriene D4 readily promote wound healing via regulating the production of angiogenic factors and endothelial cell functions 107 and inducing stem cells’ proliferation and angiogenic potential 108. Furthermore, lipoxin A4 was reported to have anti-diabetic potential via inhibiting IL-6, tumor necrosis factor and ROS generation 109.
Newborns health
Polyunsaturated fatty acids (PUFAs), especially arachidonic acid, affect the function of numerous ion channels, the activity of various enzymes and are implicated in cell apoptosis, necrosis and death, events of critical importance during embryogenesis, thereby have significant physiological and pharmacological impact on the health of newborns 54. Arachidonic acid and docosahexaenoic acid (DHA, 22:6omega3) are important components of human milk but are lacking in cow milk and most commercial infant formula in developing countries 110. Due to its importance in development especially of the central nervous system and retina 110, the Food and Agricultural Organization (FAO)/World Health Organization (WHO) recommended that infant formula, unless specifically added, should be supplemented with arachidonic acid 111. Decreased postnatal arachidonic acid and docosahexaenoic fatty acid (DHA) blood levels in premature infants were found to be associated with neonatal morbidities, while adding DHA and arachidonic acid to preterm-infant formulas led to improved visual acuity, visual attention and cognitive development 110. The arachidonic acid levels in human milk and arachidonic acid requirements, essentiality in pre- and neonatal life and during development, and inclusion in infant formulas have recently been reviewed 112, challenged and discussed 113.
Neurological disorders
Arachidonic acid does not only influence cell membrane fluidity and the activity of ion channels, especially in the brain, it constitutes together with docosahexaenoic fatty acid (DHA) 20% of the human brain dry weight, concentrated in the neurons outer membrane and in the myelin sheath 114. Additionally, positron emission tomography was used to show that the brain of human healthy volunteers consumes arachidonic acid at a rate of 17.8 mg/d 115. Accordingly, arachidonic acid was recommended for management of central nervous system, visual and auditory damage in preterm infants via supporting neurovascular membrane integrity 116. Children with autism had lower levels of blood PUFA, especially arachidonic acid, than normal children 117 and showed notable improvement after dietary PUFA intake 118. In the elderly too, arachidonic acid supplementation improved cognitive functions 53, perhaps via increasing the proliferation of neural stem/progenitor cells or newborn neurons and general hippocampal neurogenesis 119. The charged arachidonic acid displayed beneficial effects on epileptic seizures and cardiac arrhythmia by electrostatically affecting the kV channel’s voltage sensor, thus regulating neuronal excitability 120.
Bodybuilding
In skeletal muscles, arachidonic acid has been found to make up to 15–17% of total fatty acids, thus explaining why arachidonic acid supplementation affected body composition, muscle function and power output in strength-training individuals 121. It is also possible that arachidonic acid modulates neuromuscular signaling through its incorporation into cell membranes, and/or increases neurotransmitter firing from nerve cells 122.
A 2007 clinical study examining the effects of 1,000 mg/day of arachidonic acid for 50 days found supplementation to enhance anaerobic capacity and performance in exercising men 123. Arachidonic acid supplementation during resistance-training may enhance anaerobic capacity and lessen the inflammatory response to training. However, arachidonic acid supplementation did not promote statistically greater gains in strength, muscle mass, or influence markers of muscle hypertrophy 123.
Schistosomicidal action
The first evidence relating PUFA to schistosomes came from the ability of corn oil to exposed to surface membrane antigens of Schistosoma mansoni lung-stage larvae to specific antibody binding, thus allowing serologic visualization 124. Further studies indicated that among PUFA, arachidonic acid (10 µM for 30 minutes) was the most effective in allowing specific antibody binding to otherwise hidden surface membrane antigens of Schistosoma mansoni and Schistosoma haematobium lung-stage schistosomula 125. Of importance, exposure to 20 µM arachidonic acid for 30 minutes elicited surface membrane disintegration and attrition of the schistosomula 125. Studies aiming at clarifying these observations led to identification of surface membrane sphingomyelin instrumental role in schistosome immune evasion. Controlled sphingomyelin hydrolysis by parasite tegument-associated neutral sphingomyelinase (nSMase) allows entry of nutrients but not host molecules >600 Da or antibodies. Excessive nSMase activation and consequent SM hydrolysis elicits exposure of surface membrane antigens and eventual larval death. arachidonic acid is a major nSMase activator. Accordingly, it was straightforward to predict that arachidonic acid possesses potentially potent schistosomicidal activity 126.
All adult worms of Schistosoma mansoni and Schistosoma haematobium exposed to 2.5 mM arachidonic acid in the presence of 100% fetal calf serum showed extensive damage, disorganization, and degeneration of the tegument and the subtegumental musculature followed by death of all worms within 5 hours 127. Pure arachidonic acid and different arachidonic acid formulations elicited notable, reproducible, and safe schistosomicidal activity against larval, juvenile and adult Schistosoma mansoni and Schistosoma haematobium infection of inbred and outbred mice and hamsters 128. The ability of arachidonic acid to control infection with Schistosoma mansoni was demonstrated in Egyptian children. The chemotherapeutic activity of arachidonic acid and praziquantel was equally high in low infection settings and equally low in children with heavy infection living in high endemicity areas. The highest cure was consistently achieved in children with light or heavy infection when arachidonic acid was combined with praziquantel 129.
A breakthrough regarding the usefulness of arachidonic acid in defense against schistosomes came from the demonstration of association between resistance of the water-rat, Nectomys squamipes to repeated infection with Schistosoma mansoni and accumulation of arachidonic acid in liver cells 130. This pioneering study prompted us to examine the relation between susceptibility and resistance of rodents to Schistosoma mansoni or Schistosoma haematobium infection and arachidonic acid levels in serum and lung and liver cells before and weekly after infection. The results strongly suggested that arachidonic acid is a potent “natural” schistosomicide, and may be considered an endoschistosomicide 131.
The schistosomicidal action of arachidonic acid is based on excessive hydrolysis of parasite surface membrane sphingomyelin. Interestingly, Miltefosine, a hexa-decyl-phosphocholine, which interferes with proper biosynthesis of sphingomyelin, was recently documented as a potent schistosomicide in vitro and in vivo 132.
Anti-cancer potential
Reports decades earlier indicated that PUFA, and especially free unesterified arachidonic acid possess tumoricidal activity in vitro and in vivo 133. The most important and consistent studies documenting the tumoricidal action of PUFA, namely arachidonic acid were reported by Undurti Das and Colleagues 134, who advocated arachidonic acid as a potential anti-cancer drug 135. Thus, arachidonic acid was reported to kill tumor cells selectively in vitro via eliciting cell surface membrane lipid peroxidation, which can be blocked by vitamin E, uric acid, glutathione peroxidase and superoxide dismutase 136. Free arachidonic acid was found to inhibit the in vitro growth of human cervical carcinoma cells and methyl cholanthrene-induced sarcoma cells. Free arachidonic acid augmented the generation of superoxide anion and lipid peroxidation in the tumor cells indicating a possible correlation between the ability of unesterified PUFA to augment free radicals and their tumoricidal action 137. Moreover, free unesterified arachidonic acid, independently of its metabolites, displayed cytotoxic action on both vincristine-sensitive (KB-3-1) and resistant (KB-Ch(R)-8-5) cancer cells in vitro that appeared to be a free-radical dependent process 138. At concentrations of 100–200 µM, arachidonic acid was more effective than mesotrexate in in vitro suppression of gastric carcinoma cells, as a result of lipid peroxidation processes 139 and inhibited proliferation of human prostate cancer and human prostate epithelial cells, independently of free radicals generation 140. Arachidonic acid-mediated apoptosis of colon cancer cells appeared to be essentially due to loss of mitochondrial membrane, accumulation of ROS, and caspase-3 and caspase-9 activation 141. Accordingly, it was concluded that arachidonic acid suppresses proliferation of normal and tumor cells by a variety of mechanisms that may partly depend on the type(s) of cell(s) being tested and the way arachidonic acid is handled by the cells 138. A contradictory effect of arachidonic acid on tumorigenesis was observed in mice with a germline mutation in the adenomatous polyposis coli gene 142.
Tallima and El Ridi 143 have recently proposed that arachidonic acid may inhibit proliferation and elicit death of tumor cells via its activating impact on cell membrane-associated neutral sphingomyelinase (nSMase) and increased outer leaflet sphingomyelin hydrolysis. Disruption of the tight sphingomyelin-based hydrogen bond network around cancer cells may allow contact inhibition processes to proceed and cell proliferation to stop 71, whereby the primary sphingomyelin catabolite, ceramide released following sphingomyelin hydrolysis is a renowned secondary messenger involved in programmed cell death 144. It is of importance to recall that Miltefosine, which has been approved for the treatment of breast cancer metastasis, and is currently used for the treatment of cutaneous metastases of mammary carcinoma significantly inhibits sphingomyelin biosynthesis in human hepatoma and other tumor cells 145. The likely mechanism of action of this phospholipid analogue is inhibition of phosphatidylcholine biosynthesis, thus hindering sphingomyelin metabolism, and substantially increasing the levels of ceramide 145.
Roles in type 2 immune responses
Allergens, cysteine peptidases and numerous helminth-derived excretory-secretory products disrupt the epithelial or endothelial barriers, eliciting release of the type 2 immunity master cytokines and alarmins, TSLP (thymic stromal lymphopoietin), interleukin (IL)-25 and IL-33 126. These cytokines bind to receptors on innate lymphoid cells 2 (ILC2), tissue-resident sentinels, mainly found in the skin and at mucosal surfaces of intestine and lungs. Cytokine-receptors’ interactions result into signals that induce ILC2 recruitment, proliferation and activation. The activated ILC2 produce type 2 cytokines, principally IL-5 and IL-13, which are instrumental in the recruitment and activation of eosinophils, basophils, and mast cells 146. Major basic proteins, proteases, histamine, heparin, type 2 cytokines, and reactive ROS are not only produced inducing the various signs of inflammation, arachidonic acid is furthermore released from the activated cell membrane and oxidized to inflammatory metabolites 12. The arachidonic acid-derived metabolites are the road to generation of resolvins that help in resolving inflammation and wound and lesion healing 109. Of considerable importance is the discovery that ILC2 share with airway and gut smooth muscle cells, and/or epithelial cells, eosinophils, mast cells, macrophages, dendritic cells, and T helper 2 (Th2) lymphocytes surface membrane receptors for arachidonic acid-derived metabolites. Chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2) is a receptor for prostaglandin D2, CysLTR for cysteinyl leukotrienes D4 and E4 and ALXR for lipoxin A4. Prostaglandin D2, leukotriene D4 and E4 stimulate, while lipoxin A4 inhibits ILC-2 expansion and effector functions 147, thus, implicating arachidonic acid metabolites as secondary inducers of type 2 immune responses’ amplification, regulation and memory in airway and gut hyperresponsiveness and repair, and resistance to parasites 148.
Endocannabinoids
Endocannabinoids are so termed because they activate the same G protein-coupled, cannabinoid receptors (CB1 and CB2) as delta-9-tetrahydrocannabinol, the active component of marijuana (Cannabis sativa). Endocannabinoids are important modulators of brain reward signaling, motivational processes, emotion, stress responses, pain and energy balance 149. The endocannabinoids, N-arachidonoyl-ethanolamine and 2-arachidonoylglycerol, are arachidonic acid-derived. arachidonic acid Trans-acylase-catalyzed transfer of arachidonic acid from the sn-1 position of phospholipids to the nitrogen atom of phosphatidylethanolamine generates N-arachidonoyl-phosphatidylethanolamine (NAPE). NAPE can be hydrolyzed to arachidonylethanolamine (anandamide) in a one-step reaction catalyzed by NAPE-specific phospholipase D, or two-steps reaction catalyzed by a phospholipase C and a phosphatase. NAPE can be converted to anandamide via two further synthetic pathways 150. The importance of anandamide can be inferred from the redundancy of its precursor conversion pathways. 2-arachidonoylglycerol (2-AG) is produced from the hydrolysis of diacylglycerols (DAGs) containing arachidonate in the 2 position, catalyzed by a DAG lipase that is selective for the sn-1 position 150.
Interaction of arachidonic acid-derived endocannabinoids with their specific receptors generate signals, which control neural processes that underpin key aspects of social behavior whereby endocannabinoid signaling dysregulation is associated with social impairment related to neuropsychiatric disorders 149. Endocannabinoid-mediated signaling, especially in the brain, modulates a variety of pathophysiological processes, including appetite, pain and mood, whereby inhibition of endocannabinoid degradation is predicted to be instrumental in reducing pain and anxiety 151. Additionally, anandamide appeared to modulate human sperm motility 152 and improve renal functions and chronic inflammatory disorders of the gastrointestinal tract by regulating gut homeostasis, gastrointestinal motility, visceral sensation, and inflammation 153.
Arachidonic acid side effects
Arachidonic acid supplementation in daily doses of 1,000–1,500 mg for 50 days has been well tolerated during several clinical studies, with no significant side effects reported. All common markers of health, including kidney and liver function 154, serum lipids 155, immunity 156 and platelet aggregation 157 appear to be unaffected with this level and duration of use. Furthermore, higher concentrations of arachidonic acid in muscle tissue may be correlated with improved insulin sensitivity 158. However a study by Thomas and colleagues showed in mice study that dietary arachidonic acid could amplify beta-amyloid peptide oligomer neurotoxicity 159. Thomas and colleagues found that the administration of an arachidonic acid-enriched diet in mice for 12 weeks induced short-term memory impairment and increased deleterious effects of beta-amyloid oligomers on learning abilities 159. Arachidonic acid consumption could constitute a risk factor for Alzheimer’s disease in humans and should be taken into account in future preventive strategies 160. Arachidonic acid deleterious effect on cognitive capacity could be linked to the balance between arachidonic acid-mobilizing enzymes.
Several studies have suggested that increase of dietary arachidonic acid intake or tissue arachidonic acid amounts favors the occurrence of systemic chronic inflammation 161 and the severity of pathologies with a strong inflammatory component, such as chronic inflammatory intestinal diseases 162, rheumatoid arthritis 163 and atherosclerosis 164. This effect would be mediated by the increase of cellular free arachidonic acid and its conversion into proinflammatory eicosanoids, especially in individuals with some genetic backgrounds and in animal models 165. Furthermore, neuroinflammation is one of the pathological components of Alzheimer’s disease, and researchers in many studies have reported higher expression of enzymes using arachidonic acid as a substrate, such as cyclooxygenases and prostaglandin synthases 166 or brain production of proinflammatory eicosanoids in an Alzheimer’s disease mice model 167. However, despite the fact that neuroinflammation can induce the first synaptic dysfunction and cognitive impairment in Alzheimer’s disease, before beta-amyloid peptide accumulation and plaque formation 168, it is not known if higher arachidonic acid levels in the brain facilitate neuroinflammation. Additional experiments on inflammation kinetics in animal model and using various diets would be necessary to clarify this point.
While studies looking at arachidonic acid supplementation in sedentary subjects have failed to find changes in resting inflammatory markers in doses up to 1,500 mg daily, strength-trained subjects may respond differently. One study reported a significant reduction in resting inflammation (via marker IL-6) in young men supplementing 1,000 mg/day of arachidonic acid for 50 days in combination with resistance training 123. This suggests that rather being pro-inflammatory, supplementation of arachidonic acid while undergoing resistance training may actually improve the regulation of systemic inflammation 169.
A meta-analysis looking for associations between heart disease risk and individual fatty acids reported a significantly reduced risk of heart disease with higher levels of EPA and DHA (omega-3 fats), as well as the omega-6 arachidonic acid 170. A scientific advisory from the American Heart Association has also favorably evaluated the health impact of dietary omega-6 fats, including arachidonic acid 171. The group does not recommend limiting this essential fatty acid. In fact, the paper recommends individuals follow a diet that consists of at least 5–10% of calories coming from omega-6 fats, including arachidonic acid. It suggests dietary arachidonic acid is not a risk factor for heart disease, and may play a role in maintaining optimal metabolism and reduced heart disease risk. Maintaining sufficient intake levels of both omega-3 and omega-6 fatty acids, therefore, is recommended for optimal health.
Arachidonic acid is not carcinogenic, and studies show dietary level is not associated (positively or negatively) with risk of cancers 172, 173. Arachidonic acid remains integral to the inflammatory and cell growth process, however, which is disturbed in many types of disease including cancer. Therefore, the safety of arachidonic acid supplementation in patients suffering from cancer, inflammatory, or other diseased states is unknown, and supplementation is not recommended.
- Martin, S. A., Brash, A. R., & Murphy, R. C. (2016). The discovery and early structural studies of arachidonic acid. Journal of lipid research, 57(7), 1126–1132. https://doi.org/10.1194/jlr.R068072[↩]
- Lauritzen L, Hansen HS, Jørgensen MH, Michaelsen KF. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog Lipid Res. 2001 Jan-Mar;40(1-2):1-94. doi: 10.1016/s0163-7827(00)00017-5[↩]
- Piomelli D. Eicosanoids in synaptic transmissions. Crit. Rev. Neurobiol. 1994;11:367–373.[↩]
- No need to avoid healthy omega-6 fats. https://www.health.harvard.edu/newsletter_article/no-need-to-avoid-healthy-omega-6-fats[↩][↩]
- Aleksandra A, Niveska P, Vesna V, Jasna T, Tamara P, Marija G. Milk in human nutrition: comparision of fatty acid profiles. Acta. Vet. 2009;59:569–578.[↩]
- Gerster H. Can adults adequately convert alpha-linolenic acid (18:3n-3) to eicosapentaenoic acid (20:5n-3) and docosahexaenoic acid (22:6n-3)? Int J Vitam Nutr Res. 1998;68(3):159-73.[↩]
- Abedi, E., & Sahari, M. A. (2014). Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food science & nutrition, 2(5), 443–463. https://doi.org/10.1002/fsn3.121[↩][↩]
- Kris-Etherton PM, Harris WS, Appel LJ; American Heart Association. Nutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002 Nov 19;106(21):2747-57. doi: 10.1161/01.cir.0000038493.65177.94. Erratum in: Circulation. 2003 Jan 28;107(3):512.[↩]
- Mozaffarian D, Ascherio A, Hu FB, Stampfer MJ, Willett WC, Siscovick DS, Rimm EB. Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation. 2005 Jan 18;111(2):157-64. doi: 10.1161/01.CIR.0000152099.87287.83[↩]
- Hu FB, Bronner L, Willett WC, Stampfer MJ, Rexrode KM, Albert CM, Hunter D, Manson JE. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002 Apr 10;287(14):1815-21. doi: 10.1001/jama.287.14.1815[↩]
- Kitessaa SM, Young P. Enriching milk fat with n-3 polyunsaturated fatty acids by supplementing grazing dairy cows with ruminally protected Echium oil. Anim. Feed Sci. Technol. 2011;170:35–44.[↩]
- Hanna, V. S., & Hafez, E. (2018). Synopsis of arachidonic acid metabolism: A review. Journal of advanced research, 11, 23–32. https://doi.org/10.1016/j.jare.2018.03.005[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Rich MR. Conformational analysis of arachidonic and related fatty acids using molecular dynamics simulations. Biochim Biophys Acta. 1993 Jul 28;1178(1):87-96. doi: 10.1016/0167-4889(93)90113-4[↩]
- Brash A. R. (2001). Arachidonic acid as a bioactive molecule. The Journal of clinical investigation, 107(11), 1339–1345. https://doi.org/10.1172/JCI13210[↩]
- Weller P.F. Leukocyte lipid bodies – structure and sunction ss “Eicosasomes” Trans Am Clin Climatol Assoc. 2016;127:328–340.[↩]
- Tallima H., Hadley K., El Ridi R. Praziquantel and arachidonic acid combination — An Innovative Approach to the Treatment of Schistosomiasis. In: Samie A., editor. An overview of tropical diseases. In Tech; 2015. pp. 145–172.[↩]
- Mcarthur M.J., Atshaves B.P., Frolov A., Foxworth W.D., Kier A.B., Schroeder F. Cellular uptake and intracellular trafficking of long chain fatty acids. J Lipid Res. 1999;40(8):1371–1383.[↩]
- Kawashima H. (2019). Intake of arachidonic acid-containing lipids in adult humans: dietary surveys and clinical trials. Lipids in health and disease, 18(1), 101. https://doi.org/10.1186/s12944-019-1039-y[↩][↩][↩]
- Thies F, Nebe-von-Caron G, Powell JR, Yaqoob P, Newsholme EA, Calder PC. Dietary supplementation with eicosapentaenoic acid, but not with other long-chain n-3 or n-6 polyunsaturated fatty acids, decreases natural killer cell activity in healthy subjects aged >55 y. Am J Clin Nutr. 2001;73(3):539–548.[↩]
- Kusumoto A, Ishikura Y, Kawashima H, Kiso Y, Takai S, Miyazaki M. Effects of arachidonate-enriched triacylglycerol supplementation on serum fatty acids and platelet aggregation in healthy male subjects with a fish diet. Br J Nutr. 2007;98(3):626–635.[↩]
- Kakutani S, Ishikura Y, Tateishi N, Horikawa C, Tokuda H, Kontani M, Kawashima H, Sakakibara Y, Kiso Y, Shibata H, Morita I. Supplementation of arachidonic acid-enriched oil increases arachidonic acid contents in plasma phospholipids, but does not increase their metabolites and clinical parameters in Japanese healthy elderly individuals: a randomized controlled study. Lipids Health Dis. 2011;10:241.[↩][↩]
- Nelson GJ, Schmidt PC, Bartolini G, Kelley DS, Kyle D. The effect of dietary arachidonic acid on platelet function, platelet fatty acid composition, and blood coagulation in humans. Lipids. 1997;32(4):421–425.[↩]
- Schuchardt JP, Ostermann AI, Stork L, Kutzner L, Kohrs H, Greupner T, Hahn A, Schebb NH. Effects of docosahexaenoic acid supplementation on PUFA levels in red blood cells and plasma. Prostaglandins Leukot Essent Fatty Acids. 2016;115:12–23.[↩]
- Abdelmagid S.A., Clarke S.E., Nielsen D.E., Badawi A., El-Sohemy A., Mutch D.M., Ma D.W. Comprehensive profiling of plasma fatty acid concentrations in young healthy Canadian adults. PLoS ONE. 2015;10:e0116195. doi: 10.1371/journal.pone.0116195[↩]
- Innis S.M. Impact of maternal diet on human milk composition and neurological development of infants. Am. J. Clin. Nutr. 2014;99:734S–741S. doi: 10.3945/ajcn.113.072595[↩]
- Wang L., Folsom A.R., Eckfeldt J.H. Plasma fatty acid composition and incidence of coronary heart disease in middle aged adults: The Atherosclerosis Risk in Communities (ARIC) Study. Nutr. Metab. Cardiovasc. Dis. 2003;13:256–266. doi: 10.1016/S0939-4753(03)80029-7[↩]
- Sonnweber, T., Pizzini, A., Nairz, M., Weiss, G., & Tancevski, I. (2018). Arachidonic Acid Metabolites in Cardiovascular and Metabolic Diseases. International journal of molecular sciences, 19(11), 3285. https://doi.org/10.3390/ijms19113285[↩][↩][↩][↩]
- Dennis E.A., Cao J., Hsu Y.H., Magrioti V., Kokotos G. Phospholipase A2 enzymes: Physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 2011;111:6130–6185. doi: 10.1021/cr200085w[↩]
- Miki Y., Yamamoto K., Taketomi Y., Sato H., Shimo K., Kobayashi T., Ishikawa Y., Ishii T., Nakanishi H., Ikeda K., et al. Lymphoid tissue phospholipase A2 group IID resolves contact hypersensitivity by driving antiinflammatory lipid mediators. J. Exp. Med. 2013;210:1217–1234. doi: 10.1084/jem.20121887[↩]
- Kano M. Control of synaptic function by endocannabinoid-mediated retrograde signaling. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2014;90:235–250. doi: 10.2183/pjab.90.235[↩]
- Ahn K., McKinney M.K., Cravatt B.F. Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem. Rev. 2008;108:1687–1707. doi: 10.1021/cr0782067[↩]
- Goetzl E.J., An S., Smith W.L. Specificity of expression and effects of eicosanoid mediators in normal physiology and human diseases. FASEB J. 1995;9:1051–1058. doi: 10.1096/fasebj.9.11.7649404[↩]
- Norris P.C., Reichart D., Dumlao D.S., Glass C.K., Dennis E.A. Specificity of eicosanoid production depends on the TLR-4-stimulated macrophage phenotype. J. Leukoc. Biol. 2011;90:563–574. doi: 10.1189/jlb.0311153[↩]
- Astarita G., Kendall A.C., Dennis E.A., Nicolaou A. Targeted lipidomic strategies for oxygenated metabolites of polyunsaturated fatty acids. Biochim. Biophys. Acta. 2015;1851:456–468. doi: 10.1016/j.bbalip.2014.11.012[↩]
- Serhan C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479[↩]
- Tang Y., Zhang M.J., Hellmann J., Kosuri M., Bhatnagar A., Spite M. Proresolution therapy for the treatment of delayed healing of diabetic wounds. Diabetes. 2013;62:618–627. doi: 10.2337/db12-0684[↩]
- Vance J.E. Eukaryotic lipid-biosynthetic enzymes: the same but not the same. Trends Biochem Sci. 1998;23(11):423–428.[↩]
- Tallima, H., & El Ridi, R. (2017). Arachidonic acid: Physiological roles and potential health benefits – A review. Journal of advanced research, 11, 33–41. https://doi.org/10.1016/j.jare.2017.11.004[↩][↩][↩][↩][↩][↩][↩]
- Abedi E, Sahari MA. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci Nutr. 2014 Sep;2(5):443-63. doi: 10.1002/fsn3.121[↩]
- Taber L., Chiu C.H., Whelan J. Assessment of the arachidonic acid content in foods commonly consumed in the American diet. Lipids. 1998;33(12):1151–1157.[↩]
- Sinclair A.J., Johnson L., O’Dea K., Holman R.T. Diets rich in lean beef increase arachidonic acid and long-chain ω3 polyunsaturated fatty acid levels in plasma phospholipids. Lipids. 1994;29(5):337–343.[↩]
- Li D., Ng A., Mann N.J., Sinclair A.J. Contribution of meat fat to dietary arachidonic acid. Lipids. 1998;33(4):437–440.[↩]
- Lee, J. M., Lee, H., Kang, S., & Park, W. J. (2016). Fatty Acid Desaturases, Polyunsaturated Fatty Acid Regulation, and Biotechnological Advances. Nutrients, 8(1), 23. https://doi.org/10.3390/nu8010023[↩]
- Zock PL, Blom WA, Nettleton JA, Hornstra G. Progressing Insights into the Role of Dietary Fats in the Prevention of Cardiovascular Disease. Curr Cardiol Rep. 2016 Nov;18(11):111. doi: 10.1007/s11886-016-0793-y[↩]
- Hastings N., Agaba M., Tocher D.R., Leaver M.J., Dick J.R., Sargent J.R. A vertebrate fatty acid desaturase with Delta 5 and Delta 6 activities. Proc Natl Acad Sci. 2001;98(25):14304–14309.[↩]
- Ahn K., McKinney M.K., Cravatt B.F. Enzymatic pathways that regulat endocannabinoid signaling in the nervous system. Chem Rev. 2008;108(5):1687–1707.[↩]
- Wiktorowska-Owczarek A, Berezińska M, Nowak JZ. PUFAs: Structures, Metabolism and Functions. Adv Clin Exp Med. 2015 Nov-Dec;24(6):931-41. doi: 10.17219/acem/31243[↩]
- Calder P.C. Dietary arachidonic acid: harmful, harmless or helpful? Br J Nutr. 2007;98(3):451–453.[↩]
- Pérez R, Matabosch X, Llebaria A, Balboa MA, Balsinde J. Blockade of arachidonic acid incorporation into phospholipids induces apoptosis in U937 promonocytic cells. J Lipid Res. 2006 Mar;47(3):484-91. doi: 10.1194/jlr.M500397-JLR200[↩][↩]
- Pompéia C., Lopes L.R., Miyasaka C.K., Procópio J., Sannomiya P., Curi R. Effect of fatty acids on leukocyte function. Braz J Med Biol Res. 2000;33(11):1255–1268.[↩]
- Brash AR. Arachidonic acid as a bioactive molecule. J Clin Invest. 2001 Jun;107(11):1339-45. doi: 10.1172/JCI13210[↩][↩]
- Beck R, Bertolino S, Abbot SE, Aaronson PI, Smirnov SV. Modulation of arachidonic acid release and membrane fluidity by albumin in vascular smooth muscle and endothelial cells. Circ Res. 1998 Nov 2;83(9):923-31. doi: 10.1161/01.res.83.9.923[↩]
- Tokuda H, Kontani M, Kawashima H, Akimoto K, Kusumoto A, Kiso Y, Koga Y, Shibata H. Arachidonic acid-enriched triacylglycerol improves cognitive function in elderly with low serum levels of arachidonic acid. J Oleo Sci. 2014;63(3):219-27. doi: 10.5650/jos.ess13195[↩][↩]
- Elinder F, Liin SI. Actions and Mechanisms of Polyunsaturated Fatty Acids on Voltage-Gated Ion Channels. Front Physiol. 2017 Feb 6;8:43. doi: 10.3389/fphys.2017.00043[↩][↩][↩][↩]
- Yazdi S, Stein M, Elinder F, Andersson M, Lindahl E. The Molecular Basis of Polyunsaturated Fatty Acid Interactions with the Shaker Voltage-Gated Potassium Channel. PLoS Comput Biol. 2016 Jan 11;12(1):e1004704. doi: 10.1371/journal.pcbi.1004704[↩][↩]
- Horimoto N., Nabekura J., Ogawa T. Arachidonic acid activation of potassium channels in rat visual cortex neurons. Neuroscience. 1997;77(3):661–671.[↩]
- Kirber M.T., Ordway R.W., Clapp L.H., Walsh J.V., Jr, Singer J.J. Both membrane stretch and fatty acids directly activate large conductance Ca(2+)-activated K+ channels in vascular smooth muscle cells. FEBS Lett. 1992;297(1–2):24–28.[↩]
- Kuang Q., Purhonen P., Hebert H. Structure of potassium channels. Cell Mol Life Sci. 2015;72(19):3677–3693.[↩]
- Denson D.D., Wang X., Worrell R.T., Eaton D.C. Effects of fatty acids on BK channels in GH(3) cells. Am J Physiol Cell Physiol. 2000;279(4):C1211–C1219.[↩]
- Hamilton K.L., Syme C.A., Devor D.C. Molecular localization of the inhibitory arachidonic acid binding site to the pore of hIK1. J Biol Chem. 2003;278(19):16690–16697.[↩]
- Kim D. Fatty acid-sensitive two-pore domain K+ channels. Trends Pharmacol Sci. 2003 Dec;24(12):648-54. doi: 10.1016/j.tips.2003.10.008[↩]
- Kim D., Duff R.A. Regulation of K+ channels in cardiac myocytes by free fatty acids. Circ Res. 1990;67(4):1040–1046.[↩]
- Kang J.X., Leaf A. Prevention of fatal cardiac arrhythmias by polyunsaturated fatty acids. Am J Clin Nutr. 2000;71(1 Suppl):202S–207S.[↩]
- Gu H., Fang Y.J., He Y.L., Sun J., Zhu J., Mei Y.A. Modulation of muscle rNaV1.4 Na+ channel isoform by arachidonic acid and its non-metabolized analog. J Cell Physiol. 2009;219(1):173–182.[↩]
- Zhou J.J., Linsdell P. Molecular mechanism of arachidonic acid inhibition of the CFTR chloride channel. Eur J Pharmacol. 2007;563(1–3):88–91.[↩]
- Kawanabe A., Okamura Y. Effects of unsaturated fatty acids on the kinetics of voltage-gated proton channels heterologously expressed in cultured cells. J Physiol. 2016;594(3):595–610.[↩]
- Antollini SS, Barrantes FJ. Fatty Acid Regulation of Voltage- and Ligand-Gated Ion Channel Function. Front Physiol. 2016 Nov 28;7:573. doi: 10.3389/fphys.2016.00573[↩][↩]
- Pompéia C., Cury-Boaventura M.F., Curi R. Arachidonic acid triggers an oxidative burst in leukocytes. Braz J Med Biol Res. 2003;36(11):1549–1560.[↩]
- Shiose A., Sumimoto H. Arachidonic acid and phosphorylation synergistically induce a conformational change of p47phox to activate the phagocyte NADPH oxidase. J Biol Chem. 2000;275(18):13793–13801.[↩]
- Marchesini N., Hannun Y.A. Acid and neutral sphingomyelinases: roles and mechanisms of regulation. Biochem Cell Biol. 2004;82(1):27–44.[↩]
- El Ridi R., Tallima H., Migliardo F. Biochemical and biophysical methodologies open the road for effective schistosomiasis therapy and vaccination. Biochim Biophys Acta. 2017;1861(Pt B):3613–3620.[↩][↩]
- Cao Y., Pearman A.T., Zimmerman G.A., McIntyre T.M., Prescott S.M. Intracellular unesterified arachidonic acid signals apoptosis. Proc Natl Acad Sci USA. 2000;97(21):11280–11285.[↩]
- Pompeia C., Lima T., Curi R. Arachidonic acid cytotoxicity: can arachidonic acid be a physiological mediator of cell death? Cell Biochem Funct. 2003;21(2):97–104.[↩][↩]
- Smith W.L., DeWitt D.L., Garavito R.M. Cyclooxygenases: Structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145[↩]
- Taketomi Y., Ueno N., Kojima T., Sato H., Murase R., Yamamoto K., Tanaka S., Sakanaka M., Nakamura M., Nishito Y., et al. Mast cell maturation is driven via a group III phospholipase A2-prostaglandin D2-DP1 receptor paracrine axis. Nat. Immunol. 2013;14:554–563. doi: 10.1038/ni.2586[↩]
- Brock T.G., McNish R.W., Peters-Golden M. Arachidonic acid is preferentially metabolized by cyclooxygenase-2 to prostacyclin and prostaglandin E2. J. Biol. Chem. 1999;274:11660–11666. doi: 10.1074/jbc.274.17.11660[↩]
- Howe L.R., Chang S.H., Tolle K.C., Dillon R., Young L.J., Cardiff R.D., Newman R.A., Yang P., Thaler H.T., Muller W.J., et al. HER2/neu-induced mammary tumorigenesis and angiogenesis are reduced in cyclooxygenase-2 knockout mice. Cancer Res. 2005;65:10113–10119. doi: 10.1158/0008-5472.CAN-05-1524[↩]
- Chandrasekharan N.V., Dai H., Roos K.L., Evanson N.K., Tomsik J., Elton T.S., Simmons D.L. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: Cloning, structure, and expression. Proc. Natl. Acad. Sci. USA. 2002;99:13926–13931. doi: 10.1073/pnas.162468699[↩]
- Powell W.S., Rokach J. Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo-ETEs) derived from arachidonic acid. Biochim. Biophys. Acta. 2015;1851:340–355. doi: 10.1016/j.bbalip.2014.10.008[↩]
- Lammermann T., Afonso P.V., Angermann B.R., Wang J.M., Kastenmuller W., Parent C.A., Germain R.N. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498:371–375. doi: 10.1038/nature12175[↩]
- Romano M., Cianci E., Simiele F., Recchiuti A. Lipoxins and aspirin-triggered lipoxins in resolution of inflammation. Eur. J. Pharmacol. 2015;760:49–63. doi: 10.1016/j.ejphar.2015.03.083[↩]
- Feltenmark S., Gautam N., Brunnstrom A., Griffiths W., Backman L., Edenius C., Lindbom L., Bjorkholm M., Claesson H.E. Eoxins are proinflammatory arachidonic acid metabolites produced via the 15-lipoxygenase-1 pathway in human eosinophils and mast cells. Proc. Natl. Acad. Sci. USA. 2008;105:680–685. doi: 10.1073/pnas.0710127105[↩]
- Hwang S.W., Cho H., Kwak J., Lee S.Y., Kang C.J., Jung J., Cho S., Min K.H., Suh Y.G., Kim D., et al. Direct activation of capsaicin receptors by products of lipoxygenases: Endogenous capsaicin-like substances. Proc. Natl. Acad. Sci. USA. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155[↩]
- Serhan C.N. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot Essent Fatty Acids. 2005;73:141–162. doi: 10.1016/j.plefa.2005.05.002[↩]
- Fan F., Muroya Y., Roman R.J. Cytochrome P450 eicosanoids in hypertension and renal disease. Curr. Opin. Nephrol. Hypertens. 2015;24:37–46. doi: 10.1097/MNH.0000000000000088[↩]
- Ueda N., Tsuboi K., Uyama T. Metabolism of endocannabinoids and related N-acylethanolamines: Canonical and alternative pathways. FEBS J. 2013;280:1874–1894. doi: 10.1111/febs.12152[↩]
- Izzo A.A., Deutsch D.G. Unique pathway for anandamide synthesis and liver regeneration. Proc. Natl. Acad. Sci. USA. 2011;108:6339–6340. doi: 10.1073/pnas.1103566108[↩]
- Mukhopadhyay B., Cinar R., Yin S., Liu J., Tam J., Godlewski G., Harvey-White J., Mordi I., Cravatt B.F., Lotersztajn S., et al. Hyperactivation of anandamide synthesis and regulation of cell-cycle progression via cannabinoid type 1 (CB1) receptors in the regenerating liver. Proc. Natl. Acad. Sci. USA. 2011;108:6323–6328. doi: 10.1073/pnas.1017689108[↩]
- Balazy M., Poff C.D. Biological nitration of arachidonic acid. Curr. Vasc. Pharmacol. 2004;2:81–93. doi: 10.2174/1570161043476465[↩]
- Trostchansky A., Bonilla L., Thomas C.P., O’Donnell V.B., Marnett L.J., Radi R., Rubbo H. Nitroarachidonic acid, a novel peroxidase inhibitor of prostaglandin endoperoxide H synthases 1 and 2. J. Biol. Chem. 2011;286:12891–12900. doi: 10.1074/jbc.M110.154518[↩]
- Dennis E.A., Norris P.C. Eicosanoid storm in infection and inflammation. Nat Rev Immunol. 2015;15(8):511–523.[↩][↩][↩]
- Tallima H., El Ridi R. Solving the riddle of the lung-stage schistosomula paved the way to a novel remedy and an efficacious vaccine for schistosomiasis. In: El Ridi R., editor. Parasitic diseases – schistosomiasis. In Tech; 2013. pp. 179–202.[↩]
- Drenjančević I., Jukić I., Mihaljević Z., Ćosić A., Kibel A. The metabolites of arachidonic acid in microvascular function. In: Lenasi H., editor. Microcirculation Revisited – From Molecules to Clinical Practice. In Tech; 2016.[↩]
- Balazy M., Poff C.D. Biological nitration of arachidonic acid. Curr Vasc Pharmacol. 2004;2(1):81–93.[↩]
- Norris P.C., Reichart D., Dumlao D.S., Glass C.K., Dennis E.A. Specificity of eicosanoid production depends on the TLR-4-stimulated macrophage phenotype. J Leukoc Biol. 2011;90(3):563–574.[↩]
- Folco G., Folco G., Murphy R.C., Murphy R.C. Eicosanoid transcellular biosynthesis: From cell-cell interactions to in Vivo tissue responses. Pharmacol Rev. 2006;58(3):375–388.[↩]
- Dahinden C.A., Clancy R.M., Gross M., Chiller J.M., Hugli T.E. Leukotriene C4 production by murine mast cells: evidence of a role for extracellular leukotriene A4. Proc Natl Acad Sci USA. 1985;82(19):6632–6636.[↩]
- Dickinson J.S., Voelker D.R., Bernlohr D.A., Murphy R.C. Stabilization of leukotriene A 4 by epithelial fatty acid-binding Protein in the rat basophilic leukemia cell. J Biol Chem. 2004;279(9):7420–7426.[↩]
- Von Moltke J., Trinidad N.J., Moayeri M., Kintzer A.F., Wang S.B., Van Rooijen N. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature. 2012;490(7418):107–111.[↩]
- Norris P.C., Gosselin D., Reichart D., Glass C.K., Dennis E.A. Phospholipase A2 regulates eicosanoid class switching during inflammasome activation. Proc Natl Acad Sci USA. 2014;111(35):12746–12751.[↩]
- Mandal A.K., Jones P.B., Bair A.M., Christmas P., Miller D., Yamin T.D. The nuclear membrane organization of leukotriene synthesis. Proc Natl Acad Sci USA. 2008;105(51):20434–20439.[↩]
- Korotkova M., Lundberg I.E. The skeletal muscle arachidonic acid cascade in health and inflammatory disease. Nat Rev Rheumatol. 2014;10(5):295–303.[↩]
- Esser-von Bieren J. Immune-regulation and functions of eicosanoid lipid mediators. Biol Chem. 2017;398(11):1177–1191.[↩]
- Reis M.B., Pereira P.A.T., Caetano G.F., Leite M.N., Galvão A.F., Paula-Silva F.W.G. Lipoxin A4 encapsulated in PLGA microparticles accelerates wound healing of skin ulcers. PLoS ONE. 2017;12(7):e0182381.[↩][↩]
- Barnig C., Cernadas M., Dutile S., Liu X., Perrella M.A., Kazani S. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med. 2013;5(174):174ra26.[↩]
- Börgeson E., McGillicuddy F.C., Harford K.A., Corrigan N., Higgins D.F., Maderna P. Lipoxin A4 attenuates adipose inflammation. FASEB J. 2012;26(10):4287–4294.[↩]
- Pozzi A., Zent R. Regulation of endothelial cell functions by basement membrane- and arachidonic acid-derived products. Wiley Interdiscip Rev Syst Biol Med. 2009;1(2):254–272.[↩]
- Berry E., Liu Y., Chen L., Guo A.M. Eicosanoids: emerging contributors in stem cell-mediated wound healing. Prostaglandins Other Lipid Mediat. 2017;132:17–24.[↩]
- Das U.N. Is there a role for bioactive lipids in the pathobiology of diabetes mellitus? Front Endocrinol (Lausanne) 2017;8:182.[↩][↩]
- Innis S.M. Impact of maternal diet on human milk composition and neurological development of infants. Am J Clin Nutr. 2014;99(3):734S–741S.[↩][↩][↩]
- WHO and FAO joint consultation Fats and oils in human nutrition. Nutr Rev. 1995;53(7):202–205.[↩]
- Robinson D.T., Martin C.R. Fatty acid requirements for the preterm infant. Semin Fetal Neonatal Med. 2017;22(1):8–14.[↩]
- EFSA Panel on Dietetic Products Nutrition and Allergies. Scientific Opinion on the essential composition of infant and follow-on formulae. EFSA J. 2014;12(7):3760.[↩]
- Salem N., Jr, Wegher B., Mena P., Uauy R. Arachidonic and docosahexaenoic acids are biosynthesized from their 18-carbon precursors in human infants. Proc Natl Acad Sci USA. 1996;93(1):49–54.[↩]
- Rapoport S.I. Arachidonic acid and the brain. J Nutr. 2008;138(12):2515–2520.[↩]
- Crawford MA, Golfetto I, Ghebremeskel K, Min Y, Moodley T, Poston L, Phylactos A, Cunnane S, Schmidt W. The potential role for arachidonic and docosahexaenoic acids in protection against some central nervous system injuries in preterm infants. Lipids. 2003 Apr;38(4):303-15. doi: 10.1007/s11745-003-1065-1[↩]
- Meguid NA, Atta HM, Gouda AS, Khalil RO. Role of polyunsaturated fatty acids in the management of Egyptian children with autism. Clin Biochem. 2008 Sep;41(13):1044-8. doi: 10.1016/j.clinbiochem.2008.05.013[↩]
- Yui K, Koshiba M, Nakamura S, Kobayashi Y. Effects of large doses of arachidonic acid added to docosahexaenoic acid on social impairment in individuals with autism spectrum disorders: a double-blind, placebo-controlled, randomized trial. J Clin Psychopharmacol. 2012 Apr;32(2):200-6. doi: 10.1097/JCP.0b013e3182485791[↩]
- Tokuda H, Kontani M, Kawashima H, Kiso Y, Shibata H, Osumi N. Differential effect of arachidonic acid and docosahexaenoic acid on age-related decreases in hippocampal neurogenesis. Neurosci Res. 2014 Nov;88:58-66. doi: 10.1016/j.neures.2014.08.002[↩]
- Börjesson S.I., Parkkari T., Hammarström S., Elinder F. Electrostatic tuning of cellular excitability. Biophys J. 2010;98(3):396–403.[↩]
- Salem N.M., Lin Y.H., Moriguchi T., Lim S.Y., Salem N., Jr, Hibbeln J.R. Distribution of omega-6 and omega-3 polyunsaturated fatty acids in the whole rat body and 25 compartments. Prostaglandins Leukot Essent Fatty Acids. 2015;100:13–20.[↩]
- Rapoport S. I. (2008). Arachidonic acid and the brain. The Journal of nutrition, 138(12), 2515–2520. https://doi.org/10.1093/jn/138.12.2515[↩]
- Roberts, M. D., Iosia, M., Kerksick, C. M., Taylor, L. W., Campbell, B., Wilborn, C. D., Harvey, T., Cooke, M., Rasmussen, C., Greenwood, M., Wilson, R., Jitomir, J., Willoughby, D., & Kreider, R. B. (2007). Effects of arachidonic acid supplementation on training adaptations in resistance-trained males. Journal of the International Society of Sports Nutrition, 4, 21. https://doi.org/10.1186/1550-2783-4-21[↩][↩][↩]
- El Ridi R., Mohamed S.H., Tallima H. Incubation of Schistosoma mansoni lung-stage schistosomula in corn oil exposes their surface membrane antigenic specificities. J Parasitol. 2003;89(5):1064–1067.[↩]
- Tallima H., Salah M., El-Ridi R. In vitro and in vivo effects of unsaturated fatty acids on Schistosoma mansoni and S. haematobium lung-stage larvae. J Parasitol. 2005;91(5):1094–1102.[↩][↩]
- Othman A., El Ridi R. Schistosomiasis. In: Bruschi F., editor. Helminth infections and their impact on global public health. Springer; Heidelberg: 2014. pp. 49–92.[↩][↩]
- El Ridi R, Aboueldahab M, Tallima H, Salah M, Mahana N, Fawzi S, Mohamed SH, Fahmy OM. In vitro and in vivo activities of arachidonic acid against Schistosoma mansoni and Schistosoma haematobium. Antimicrob Agents Chemother. 2010 Aug;54(8):3383-9. doi: 10.1128/AAC.00173-10[↩]
- El Ridi R, Tallima H, Salah M, Aboueldahab M, Fahmy OM, Al-Halbosiy MF, Mahmoud SS. Efficacy and mechanism of action of arachidonic acid in the treatment of hamsters infected with Schistosoma mansoni or Schistosoma haematobium. Int J Antimicrob Agents. 2012 Mar;39(3):232-9. doi: 10.1016/j.ijantimicag.2011.08.019[↩]
- Barakat R, Abou El-Ela NE, Sharaf S, El Sagheer O, Selim S, Tallima H, Bruins MJ, Hadley KB, El Ridi R. Efficacy and safety of arachidonic acid for treatment of school-age children in Schistosoma mansoni high-endemicity regions. Am J Trop Med Hyg. 2015 Apr;92(4):797-804. doi: 10.4269/ajtmh.14-0675[↩]
- Amaral K.B., Silva T.P., Malta K.K., Carmo L.A., Dias F.F., Almeida M.R. Natural Schistosoma mansoni infection in the wild reservoir Nectomys squamipes leads to excessive lipid droplet accumulation in hepatocytes in the absence of liver functional impairment. PLoS ONE. 2016;11(11):e0166979[↩]
- Hanna, V. S., Gawish, A., Abou El-Dahab, M., Tallima, H., & El Ridi, R. (2018). Is arachidonic acid an endoschistosomicide?. Journal of advanced research, 11, 81–89. https://doi.org/10.1016/j.jare.2018.01.005[↩]
- El-Faham M.H., Eissa M.M., Igetei J.E., Amer E.I., Liddell S., El-Azzouni M.Z. Treatment of Schistosoma mansoni with miltefosine in vitro enhances serological recognition of defined worm surface antigens. PLoS Negl Trop Dis. 2017;11(8):e0005853[↩]
- Siegel I, Liu TL, Yaghoubzadeh E, Keskey TS, Gleicher N. Cytotoxic effects of free fatty acids on ascites tumor cells. J Natl Cancer Inst. 1987 Feb;78(2):271-7.[↩]
- Zhang C, Yu H, Shen Y, Ni X, Shen S, Das UN. Polyunsaturated fatty acids trigger apoptosis of colon cancer cells through a mitochondrial pathway. Arch Med Sci. 2015 Oct 12;11(5):1081-94. doi: 10.5114/aoms.2015.54865[↩]
- Das UN. Gamma-linolenic acid, arachidonic acid, and eicosapentaenoic acid as potential anticancer drugs. Nutrition. 1990 Nov-Dec;6(6):429-34.[↩]
- Das U.N. Tumoricidal action of cis-unsaturated fatty acids and their relationship to free radicals and lipid peroxidation. Cancer Lett. 1991;56(3):235–243.[↩]
- Ramesh G., Das U.N. Effect of cis-unsaturated fatty acids on Meth-A ascitic tumour cells in vitro and in vivo. Cancer Lett. 1998;123(2):207–214.[↩]
- Das U.N., Madhavi N. Effect of polyunsaturated fatty acids on drug-sensitive and resistant tumor cells in vitro. Lipids Health Dis. 2011;10:159.[↩][↩]
- Dai J., Shen J., Pan W., Shen S., Das U.N. Effects of polyunsaturated fatty acids on the growth of gastric cancer cells in vitro. Lipids Health Dis. 2013;12:71[↩]
- Meng H., Shen Y., Shen J., Zhou F., Shen S., Das U.N. Effect of n-3 and n-6 unsaturated fatty acids on prostate cancer (PC-3) and prostate epithelial (RWPE-1) cells in vitro. Lipids Health Dis. 2013;12:160.[↩]
- Zhang C., Yu H., Shen Y., Ni X., Shen S., Das U.N. Polyunsaturated fatty acids trigger apoptosis of colon cancer cells through a mitochondrial pathway. Arch Med Sci. 2015;11(5):1081–1094.[↩]
- Petrik M.B., McEntee M.F., Chiu C.H., Whelan J. Antagonism of arachidonic acid is linked to the antitumorigenic effect of dietary eicosapentaenoic acid in Apc(Min/+) mice. J Nutr. 2000;130(5):1153–1158.[↩]
- Tallima H., Al-Halbosiy M.F., El Ridi R. Enzymatic activity and immunolocalization of Schistosoma mansoni and Schistosoma haematobium neutral sphingomyelinase. Mol Biochem Parasitol. 2011;178(1–2):23–28.[↩]
- Arenz C., Giannis A. Synthesis of the first selective irreversible inhibitor of neutral sphingomyelinase. Angew Chem Int Ed Engl. 2000;39(8):1440–1442.[↩]
- Marco C, Jiménez-López JM, Ríos-Marco P, Segovia JL, Carrasco MP. Hexadecylphosphocholine alters nonvesicular cholesterol traffic from the plasma membrane to the endoplasmic reticulum and inhibits the synthesis of sphingomyelin in HepG2 cells. Int J Biochem Cell Biol. 2009 Jun;41(6):1296-303. doi: 10.1016/j.biocel.2008.11.004[↩][↩]
- Klose C.S., Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016;17(7):765–774.[↩]
- Lund S.J., Portillo A., Cavagnero K., Baum R.E., Naji L.H., Badrani J.H. Leukotriene C4 potentiates IL-33-induced group 2 innate lymphoid cell activation and lung inflammation. J Immunol. 2017;199(3):1096–1104.[↩]
- Halim T.Y., Hwang Y.Y., Scanlon S.T., Zaghouani H., Garbi N., Fallon P.G. Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses. Nat Immunol. 2016;17(1):57–64.[↩]
- Wei D., Allsop S., Tye K., Piomelli D. Endocannabinoid signaling in the control of social behavior. Trends Neurosci. 2017;40(7):385–396.[↩][↩]
- Bisogno T., Maccarrone M. Endocannabinoid signaling and its regulation by nutrients. BioFactors. 2014;40(4):373–380.[↩][↩]
- More surprises lying ahead The endocannabinoids keep us guessing. Neuropharmacology. 2014;76(Pt B):228–234.[↩]
- Amoako A.A., Marczylo T.H., Marczylo E.L., Elson J., Willets J.M., Taylor A.H. Anandamide modulates human sperm motility: implications for men with asthenozoospermia and oligoasthenoteratozoospermia. Hum Reprod. 2013;28(8):2058–2066.[↩]
- Moradi H., Oveisi F., Khanifar E., Moreno-Sanz G., Vaziri N.D., Piomelli D. Increased renal 2-arachidonoylglycerol level is associated with improved renal function in a mouse model of acute kidney injury. Cannabis Cannabinoid Res. 2016;1(1):218–228.[↩]
- Proceedings of the International Society of Sports Nutrition (ISSN) Conference June 15–17, 2006.[↩]
- Nelson GJ, Schmidt PC, Bartolini G, Kelley DS, Phinney SD, Kyle D, Silbermann S, Schaefer EJ. The effect of dietary arachidonic acid on plasma lipoprotein distributions, apoproteins, blood lipid levels, and tissue fatty acid composition in humans. Lipids. 1997 Apr;32(4):427-33. doi: 10.1007/s11745-997-0056-6[↩]
- Kelley DS, Taylor PC, Nelson GJ, Mackey BE. Arachidonic acid supplementation enhances synthesis of eicosanoids without suppressing immune functions in young healthy men. Lipids. 1998 Feb;33(2):125-30. doi: 10.1007/s11745-998-0187-9[↩]
- Nelson GJ, Schmidt PC, Bartolini G, Kelley DS, Kyle D. The effect of dietary arachidonic acid on platelet function, platelet fatty acid composition, and blood coagulation in humans. Lipids. 1997 Apr;32(4):421-5. doi: 10.1007/s11745-997-0055-7[↩]
- Borkman M, Storlien LH, Pan DA, Jenkins AB, Chisholm DJ, Campbell LV. The relation between insulin sensitivity and the fatty-acid composition of skeletal-muscle phospholipids. N Engl J Med. 1993 Jan 28;328(4):238-44. doi: 10.1056/NEJM199301283280404[↩]
- Thomas, M. H., Paris, C., Magnien, M., Colin, J., Pelleïeux, S., Coste, F., Escanyé, M. C., Pillot, T., & Olivier, J. L. (2017). Dietary arachidonic acid increases deleterious effects of amyloid-β oligomers on learning abilities and expression of AMPA receptors: putative role of the ACSL4-cPLA2 balance. Alzheimer’s research & therapy, 9(1), 69. https://doi.org/10.1186/s13195-017-0295-1[↩][↩]
- Thomas, M. H., Paris, C., Magnien, M., Colin, J., Pelleïeux, S., Coste, F., Escanyé, M. C., Pillot, T., & Olivier, J. L. (2017). Dietary arachidonic acid increases deleterious effects of beta-amyloid oligomers on learning abilities and expression of AMPA receptors: putative role of the ACSL4-cPLA2 balance. Alzheimer’s research & therapy, 9(1), 69. https://doi.org/10.1186/s13195-017-0295-1[↩]
- Oki E, Norde MM, Carioca AAF, Ikeda RE, Souza JMP, Castro IA, Marchioni DM, Fisberg RM, Rogero MM. Interaction of SNP in the CRP gene and plasma fatty acid profile in inflammatory pattern: a cross-sectional population-based study. Nutrition. 2016;32:88–94. doi: 10.1016/j.nut.2015.07.015[↩]
- Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106:563–73. doi: 10.1038/ajg.2011.44[↩]
- Adam O, Beringer C, Kless T, Lemmen C, Adam A, Wiseman M, Adam P, Klimmek R, Forth W. Anti-inflammatory effects of a low arachidonic acid diet and fish oil in patients with rheumatoid arthritis. Rheumatol Int. 2003;23:27–36.[↩]
- Russo GL. Dietary n-6 and n-3 polyunsaturated fatty acids: from biochemistry to clinical implications in cardiovascular prevention. Biochem Pharmacol. 2009;77:937–46. doi: 10.1016/j.bcp.2008.10.020[↩]
- Dwyer JH, Allayee H, Dwyer KM, Fan J, Wu H, Mar R, Lusis AJ, Mehrabian M. Arachidonate 5-lipoxygenase promoter genotype, dietary arachidonic acid, and atherosclerosis. N Engl J Med. 2004;350:29–37. doi: 10.1056/NEJMoa025079[↩]
- Fujimi K, Noda K, Sasaki K, Wakisaka Y, Tanizaki Y, Iida M, Kiyohara Y, Kanba S, Iwaki T. Altered expression of COX-2 in subdivisions of the hippocampus during aging and in Alzheimer’s disease: the Hisayama study. Dement Geriatr Cogn Disord. 2007;23:423–31. doi: 10.1159/000101957[↩]
- Sanchez-Mejia RO, Newman JW, Toh S, Yu G-Q, Zhou Y, Halabisky B, Cissé M, Scearce-Levie K, Cheng IH, Gan L, Palop JJ, Bonventre JV, Mucke L. Phospholipase A2 reduction ameliorates cognitive deficits in a mouse model of Alzheimer’s disease. Nat Neurosci. 2008;11:1311–8. doi: 10.1038/nn.2213[↩]
- Beauquis J, Vinuesa A, Pomilio C, Pavía P, Galván V, Saravia F. Neuronal and glial alterations, increased anxiety, and cognitive impairment before hippocampal amyloid deposition in PDAPP mice, model of Alzheimer’s disease. Hippocampus. 2014;24:257–69. doi: 10.1002/hipo.22219[↩]
- Markworth JF, Mitchell CJ, D’Souza RF, Aasen KMM, Durainayagam BR, Mitchell SM, Chan AHC, Sinclair AJ, Garg M, Cameron-Smith D. Arachidonic acid supplementation modulates blood and skeletal muscle lipid profile with no effect on basal inflammation in resistance exercise trained men. Prostaglandins Leukot Essent Fatty Acids. 2018 Jan;128:74-86. doi: 10.1016/j.plefa.2017.12.003[↩]
- Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, Franco OH, Butterworth AS, Forouhi NG, Thompson SG, Khaw KT, Mozaffarian D, Danesh J, Di Angelantonio E. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med. 2014 Mar 18;160(6):398-406. doi: 10.7326/M13-1788[↩]
- Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL, Appel LJ, Engler MM, Engler MB, Sacks F. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. 2009 Feb 17;119(6):902-7. doi: 10.1161/CIRCULATIONAHA.108.191627[↩]
- Whelan J, McEntee MF. Dietary (n-6) PUFA and intestinal tumorigenesis. J Nutr. 2004 Dec;134(12 Suppl):3421S-3426S. doi: 10.1093/jn/134.12.3421S[↩]
- Astorg P. Acides gras alimentaires, cancer colorectal et cancer de la prostate: études épidémiologiques [Dietary fatty acids and colorectal and prostate cancers: epidemiological studies]. Bull Cancer. 2005 Jul;92(7):670-84. French.[↩]