What is asterixis
Asterixis is a medical term for negative myoclonus, which is a clinical sign that describes the inability to maintain sustained posture with subsequent sudden, brief shock-like involuntary twitching or jerking of a muscle or group of muscles producing involuntary movements of various body parts and depending on which muscles you’re using, asterixis can look like flapping or tremor-like movements 1, 2, 3, 4, 5, 6, 7, 8, 9. Asterixis is a relatively frequent sign in structural central nervous system (brain and spinal cord) disorders and is a valuable sign in neurological examination. However, asterixis is not helpful in localizing intracranial pathology or in determining the underlying cause. The most common central nervous system (brain and spinal cord) cause of asterixis is ischemia or hemorrhage in the central nervous system (CNS); most frequently in the thalamus 10.
Asterixis manifests as a bilateral flapping tremor affecting various parts of the body independently and occurs at a rate of 3-5 Hz during active maintenance of posture 2. Except for the facial muscles, the tremors occur in an asymmetric fashion on either side of the body 2. The term “negative” myoclonus here doesn’t mean “bad.” Positive myoclonus is a sudden, brief muscle twitch or spasm, so positive here means “more activity.” Negative myoclonus is when a flexed muscle suddenly relaxes, so negative means “less activity.” Negative myoclonus is characterized by a brief loss of muscle tone in agonist muscles followed by a compensatory jerk of the antagonistic muscles. Asterixis, posthypoxic myoclonus and stiff man syndrome are examples of negative myoclonus 11. This negative myoclonus correlated with intermittent pauses of 50-200 milliseconds on EMG tracings in tonically active muscles 12, 13, 9.
Asterixis was first described in 1949 by James Foley and Raymond Adams 12 to describe the flapping tremor they observed with advanced hepatic encephalopathy. Two years later the same authors referred to the phenomena as a “motor disturbance—so unique that it merits a more complete description” 14. They then suggested that two abnormalities existed, one a “tremor” and the other a “lapse of posture,” which they called asterixis 14. However asterixis is not pathognomonic for liver disease, but it is a clinical sign of issues that are directly affecting your central nervous system (brain and spinal cord) 4, 1. Leavitt and Tyler 15 took the next step when they defined the electromyographic (EMG) abnormality associated with what they called metabolic tremor in hepatic encephalopathy. As with asterixis, they found brief irregular pauses or reductions in ongoing tonic EMG activity in patients with metabolic tremor 15. Later, Young and Shahani 9 were the first to describe unilateral asterixis and they classified the involuntary movements as a form of “mini-asterixis” and the pauses as negative myoclonus.
Asterixis is a clinical sign of a serious underlying disease process (e.g., certain medications, liver failure, kidney failure, heart failure, respiratory failure, electrolyte imbalances, Wilson’s disease, brain injury) that is affecting your brain and causing asterixis. Metabolic encephalopathies, especially hepatic and renal diseases, are the most common causes of bilateral asterixis. Other causes of asterixis include heart and respiratory disease, electrolyte abnormalities (e.g., hypokalemia and hypomagnesemia) and drug intoxication (e.g., phenytoin, valproate, carbamazepine, metoclopramide, lithium, ceftazidime, and barbiturates). Phenytoin can unmask latent asterixis due to unilateral lesions and asterixis due to phenytoin has also been referred to as “phenytoin flap” 1. Some antipsychotics such as lithium and clozapine and antibiotics such as ceftazidime have been rarely implicated 2. Lithium can cause asterixis at both the therapeutic and toxic plasma levels 13.
Asterixis can be either unilateral or bilateral.
- Bilateral asterixis is most commonly associated with metabolic encephalopathies, especially hepatic. The presumption in hepatic encephalopathy was that damage to brain cells due to the impaired metabolism of ammonia was predominantly related to the development of asterixis in hepatic encephalopathy 16, however, new research shows that ammonia level does not directly correlate with the development of asterixis 17, 18. Bilateral asterixis may also present in patients with cardiac and respiratory failure, uremia, electrolyte abnormalities (primarily hypoglycemia, hypokalemia, and hypomagnesemia), and drug intoxication. Phenytoin intoxication is the most commonly reported drug-induced asterixis, while other drugs implicated include benzodiazepines, barbiturates, valproate, gabapentin, carbamazepine, lithium, ceftazidime, and metoclopramide 19.
- Unilateral asterixis is usually due to focal brain lesions in the genu and anterior portions of the internal capsule or ventrolateral thalamus 20, although there have been reports of lesions in the midbrain, parietal cortex, and frontal cortex causing unilateral asterixis 21.
Asterixis has also been said to have a prognostic value and is part of the West Haven Criteria used to grade the severity of hepatic encephalopathy as it seems to be a relatively sensitive sign of the disease but is non-specific 22, 1. Asterixis disappears with the onset of coma.
Asterixis is rarely seen in early or advanced hepatic encephalopathy. Appearance of asterixis may thus, signify worsening of encephalopathy. At the same time, a disappearance of asterixis with worsening of consciousness may also be worrisome 23. The portal-systemic encephalopathy index (PSE index) has been used in many studies to measure the efficacy of therapy for hepatic encephalopathy and combines the degree of asterixis with other variables to arrive at a score 23. Similarly, asterixis has been included as a measure of severity in various scoring models for respiratory disease 24.
Asterixis isn’t a symptom that you can self-diagnose or self-treat. Because it happens with conditions that are very serious or even deadly, you should always see a doctor if you think you have asterixis. Some symptoms mean asterixis is more serious.
If you have or a loved one has asterixis and feel confused, disoriented or show other mental changes, these symptoms need immediate medical attention. Those are signs that brain activity disruptions are more severe and could become dangerous. When asterixis affects your legs or muscles of your trunk (chest, abdomen and back), it can create serious issues. Asterixis can result in falls, which can result in injuries.
The biggest risk from not diagnosing and treating asterixis is from unmanaged or severe medical conditions that might be causing it. In addition to being life-threatening, some untreated medical conditions causing asterixis can also cause brain damage, resulting in permanent difficulties with thinking, mobility, and arm and leg use. In one study of alcoholic liver disease, asterixis was the only physical finding on admission that had a statistically significant predictive value for dying, the rate being 56% in those with asterixis, as opposed to 26% in those without it 25.
What does asterixis look like?
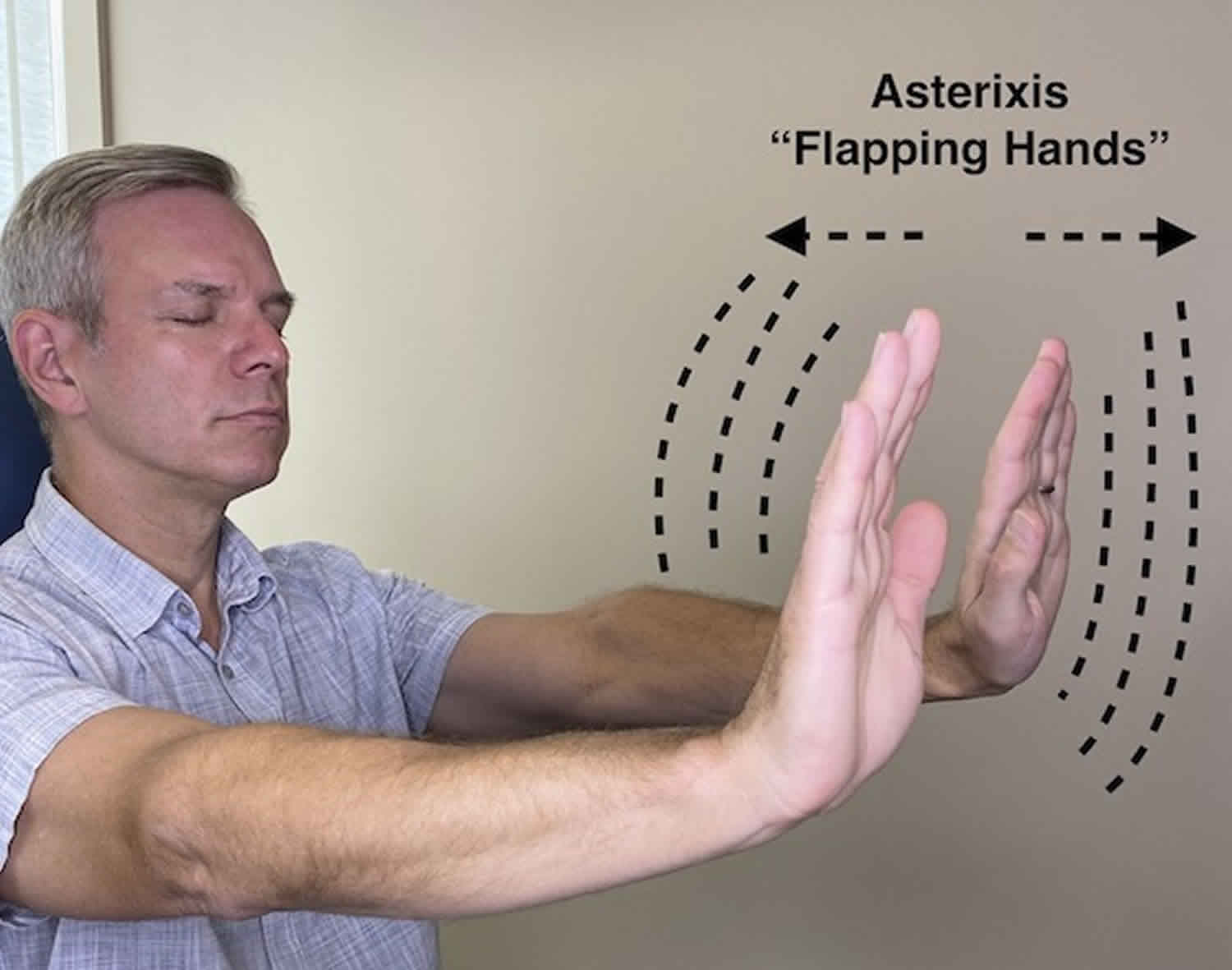
Asterixis affects muscle movements. Most of the time, it affects both sides of your body. But it may not affect both sides of your body as strongly. One side may be affected more strongly or the muscle movements may not be synchronized. It’s common for asterixis to be associated with sleepiness, mental confusion or disorientation 26. The most common way doctors will look for this is by having you hold your wrists and hands in certain ways. There are other methods to see asterixis that involve your legs and feet. But hand-related methods are the most commonly used.
The hand-related methods that doctors can look for asterixis include:
- Palms-out method. A healthcare provider will have you extend your arms forward. You’ll spread your fingers and hold your hands like you’re pushing against an invisible wall with the flat surfaces of your palms. That flexes muscles in your wrist. Asterixis will cause your hands to “flap” when those muscles briefly stop flexing.
- Grip method. A healthcare provider using this method will have you grip their hands with yours. They’ll ask you to squeeze tightly and hold your grip. Asterixis will cause split-second losses of grip strength that the provider can feel.
Asterixis commonly doesn’t show right away during either of these methods. Your doctor may have you hold the position for 30 seconds or more to be sure they don’t miss it.
Asterixis causes
Asterixis can happen for several reasons, but the most common ones involve certain types of organ failure. Medications and other conditions may also cause it.
Bilateral asterixis is most commonly associated with metabolic encephalopathies, especially hepatic. The presumption in hepatic encephalopathy was that damage to brain cells due to the impaired metabolism of ammonia was predominantly related to the development of asterixis in hepatic encephalopathy 16, however, new research shows that ammonia level does not directly correlate with the development of asterixis 17, 18. Bilateral asterixis may also present in patients with cardiac and respiratory failure, uremia, electrolyte abnormalities (primarily hypoglycemia, hypokalemia, and hypomagnesemia), and drug intoxication. Phenytoin intoxication is the most commonly reported drug-induced asterixis, while other drugs implicated include benzodiazepines, barbiturates, valproate, gabapentin, carbamazepine, lithium, ceftazidime, and metoclopramide 19.
Unilateral asterixis is usually due to focal brain lesions in the genu and anterior portions of the internal capsule or ventrolateral thalamus 20, although there have been reports of lesions in the midbrain, parietal cortex, and frontal cortex causing unilateral asterixis 21. A study of 45 cases with asterixis revealed ischemic or hemorrhagic disorders of the CNS to be the most frequent causes of asterixis (95.5%) and the thalamus the most frequent localization for unilateral asterixis to result (54%) 27. A good correlation was found between the presence of unilateral asterixis and structural intracranial disease 27. Unilateral asterixis has been reported in cases of cerebrovascular insult at multiple locations including the cerebellum 28, posterior thalamic-subthalamic paramedian region 29, midbrain 30, and pons 31.
Table 1. Asterixis causes
| Bilateral asterixis | Unilateral asterixis |
|---|---|
| Metabolic: Liver failure, azotemia, respiratory failure | Focal brain lesions at: |
| Drugs: | Thalamus |
| Sedatives: Benzodiazepines, barbiturates | Corona radiata |
| Anticonvulsants: Phenytoin (phenytoin flap), carbamazepine, valproic acid, gabapentin | Anterior cerebral artery territory |
| Antipsychotics: Lithium | Primary motor cortex |
| Antibiotics: Ceftazidime | Parietal lobe |
| Others: Metoclopramide | Cerebellum |
| Dyselectrolytemia: Hypomagnesemia, hypokalemia | Midbrain |
| Pons | |
| Bilateral structural brain lesions |
Liver and kidney disease
Your liver and kidneys filter toxic substances out of your blood. When your liver and kidneys aren’t working properly, toxic substances can build up in your blood. Your brain is sensitive to many of those substances, so that buildup can disrupt brain function and damage brain cells.
Liver and kidney related conditions that can interfere with liver and kidney function and lead to asterixis include:
- Cirrhosis.
- Liver cancer.
- Liver disease.
- Liver failure.
- Kidney disease.
- Kidney failure.
Brain injuries
Injuries to your brain can cause brain lesions. These lesions are areas that don’t work properly because of damage or other kinds of disruptions that affect their cells. When these lesions affect certain areas responsible for muscle control, it can cause asterixis. When this happens, asterixis is usually one-sided.
Certain medications that affect your nervous system can also cause asterixis as a temporary side effect. People with reduced kidney or liver function may also be more prone to asterixis from medications.
These medications include:
- Antiseizure medications, especially phenytoin (asterixis is often called “phenytoin flap” when that drug is the cause). Other antiseizure medications like gabapentin, valproate and carbamazepine can also cause it.
- Barbiturates.
- Benzodiazepines.
- Certain antibiotics (especially cephalosporins such as cefepime and ceftazidime).
- Lithium (mood stabilizer).
- Metoclopramide (antinausea).
Other health conditions
Many other conditions can also cause or contribute to asterixis. Almost all of them have to do with changes in your blood chemistry. Examples include:
- Electrolyte imbalances, especially low blood sugar (hypoglycemia), low potassium (hypokalemia) and low magnesium (hypomagnesemia).
- Heart failure.
- Respiratory failure (especially when it causes carbon dioxide buildup in your blood).
- Wilson disease (a genetic condition that causes copper to build up, affecting your brain.
Bilateral Asterixis
Bilateral asterixis is almost always caused by toxic-metabolic encephalopathies that induce generalized cerebral dysfunction 9, especially liver and kidney related conditions, but the possibility of a focal brain lesion cannot be ruled out. Bilateral asterixis may also be seen during the recovery phase following general anesthesia, following ingestion of sedative medications, electrolyte abnormalities, advanced cardiac or respiratory disease and drug intoxication 2. The flapping tremor (liver flap) is characteristically seen in hepatic encephalopathy 2.
Unilateral Asterixis
Unilateral asterixis is most commonly due to focal structural brain lesions in the genu and the anterior portion of the internal capsule or ventrolateral thalamus 9, 32. There is a good correlation between the presence of unilateral asterixis and structural intracranial disease; nevertheless it is not helpful in localizing intracranial pathology or in determining its cause 32. Lesions in the medial frontal lobe, parietal lobe, midbrain, pons, medulla oblongata, basal ganglia, insular lesions, may also cause unilateral asterixis 2.
Pseudoasterixis
Pseudoasterixis is defined as brief, rapid, voluntary action tremors of the hands and fingers, elicited by slow flexion and extension movements of the hands at the wrists, while keeping the fingers in full hyperextension 2. Because subtle movements can trigger pseudoasterixis, it mimics asterixis, a primary disorder of muscle tone 2. However in pseudoasterixis, the patient is aware of the hand twitching in contrast to asterixis that is involuntary 33.
Asterixis pathophysiology
Despite years of research, asterixis exact pathogenesis has not been established and remains elusive several decades since its first description 4, 1. Several theories suggest a role for the ascending activating systems associated with arousal, which is disturbed in encephalopathy and lesions of the thalamus and midbrain 34. Two types of surface electromyographic (EMG) patterns have been demonstrated in patients with encephalopathy: pure silent period (type 1) and the silent period following a brief EMG discharge (type 2) 21. These patterns are like the EMG patterns of asterixis caused by a thalamic lesion. However, the pathophysiology of thalamic asterixis has not been definitively established.
Abnormal function of diencephalic motor centers that regulate the agonist and antagonist tones has been considered to be important 35. Electrophysiological evaluation has revealed negative sharp waves in the contralateral central area, suggesting abnormal motor field activity in the cortex 36. Mini-asterixis has been proposed to be due to motor cortex involvement leading to pathologically slowed and synchronized motor cortical wave 37.
It has been postulated that fluid shifts cause swelling of Alzheimer type 2 astrocytes and metabolic derangements 13. This compromises the blood–brain barrier with upregulation of peripheral benzodiazepine receptor and production of neurosteroids. But how exactly these lead to asterixis and why this circuitry is particularly vulnerable are unclear 13.
The following pathogenic mechanisms have been suggested:
- Result of diffuse, widespread derangement of central nervous system (CNS) function 12.
- A “receptive inattentiveness to incoming information”, which could thus result from a dysfunction of the sensorimotor integration occurring in the contralateral parietal lobe and midbrain 38.
- Episodic dysfunction within neural circuits concerned with maintenance of sustained or tonic muscle contraction, due to focal, specific brain lesions or by a generalized neurochemical imbalance. The existence of a possible neural subsystem whose dysfunction could result in asterixis rather than “non-specific” central nervous system (CNS) lesions was hypothesized. Drowsiness in normal people and diffuse central nervous system lesions can also produce asterixis, perhaps by their effects on alerting or arousal mechanisms rather than by non-specific CNS actions 9.
- Electrophysiological evaluation of asterixis using Silent Period Locked Averaging (SPLA) method revealed negative sharp waves in the contralateral central area. It was suggested that asterixis is due to abnormal activity in the motor field in the cerebral cortex 39, 36.
- Recently mini-asterixis which is a part of the spectrum of the gross flapping tremor seen in hepatic encephalopathy was proposed as being due to the involvement of motor cortex causing a pathologically slowed and synchronized motor cortical wave 37.
- A failure in arm posture maintenance that is comparable to failure in leg posture control in patients with astasia 10. Astasia means inability to stand due to muscular incoordination.
The postural stability or tonic control of your arms and legs is related to multiple brainstem-spinal pathways such as the vestibulospinal, reticulospinal, or rubrospinal tracts 2. These systems are, in turn, regulated by supratentorial structures. The ventro-lateral nucleus of the thalamus is the area in which cerebello-rubral or vestibulocerebellar fibers converge 40 and is also heavily connected with the prefrontal area 41. There is evidence that the projections from the medial frontal cortex to the brainstem reticular formation may have a role in regulating muscle tone or posture 42. The occasional occurrence of bilateral asterixis and the transient nature of the symptoms suggest that the system regulating posture maintenance is not strictly unilateral. The occurrence of ipsilateral asterixis in patients with cerebellar lesions can be explained by crossing of the cerebello-rubral fibers at the superior cerebellar peduncle. Despite several postulations, the mechanism of asterixis has not yet been systematically explained.
Is asterixis preventable?
Asterixis generally isn’t preventable because it happens unpredictably. Asterixis is also not usually one of the first symptoms of a condition that can cause it.
The only form of asterixis that’s preventable is medication-related asterixis. To prevent it, take medications exactly as prescribed by your healthcare provider. If you notice any side effects, like asterixis, from a prescribed medication, call your healthcare provider right away. You should also avoid nonmedical drug use of any kind.
Asterixis diagnosis
Asterixis is typically elicited by asking the patient to hold the arm outstretched, spread the fingers, dorsiflex the wrists, keep the eyes closed and mouth open for 30 seconds if necessary and then observe for the abnormal brief downward flaps of the hands that returns rapidly to the original position 2. If not immediately apparent, the patient can be asked to keep the arms straight while the examiner gently hyperextends the patient’s wrist with a sweeping motion 2.
Another method to test for asterixis at the hip joint is to ask the patient to lie in a supine position and have the knees in a bent position on the bed and feet flat on the table, leaving the legs to fall to the sides. The patient is asked to relax his legs. The feet should be flat on the table and as the legs fall to the sides, watch for flapping of the legs at the hip joint. This repetitively brings the knees back together. It is also observed that gravity is required to elicit asterixis effectively 43.
Other methods of eliciting asterixis include 2:
- a) Request the patient to squeeze the doctor’s hand or the doctor’s extended fingers. Patients who are unable to maintain a posture usually are unable to maintain a steady squeeze.
- b) Have the patient squeeze a semi-inflated blood pressure cuff with instructions to maintain the reading. The readings bounce dramatically in patients with asterixis.
Once asterixis is clinically diagnosed, the laboratory investigations suggested are complete blood count, electrolytes, glucose, urea, renal function tests, liver function tests and arterial blood gases to rule out metabolic disorders associated with causing asterixis 2. A search for drug-induced asterixis should be carried out if history is suggestive of drug intoxication. Radiological investigations like computerized tomographic (CT) scan, magnetic resonance imaging (MRI) of brain in CNS lesions are very helpful in localizing the lesion especially in asterixis of vascular pathology 2. Non-vascular causes like CNS infections and tumors can also be evaluated for, in appropriate situations.
The Electromyographic (EMG) features consist of cessation of electrical activity of 35 to 200 msec in multiple muscles during which posture may be overcome by gravity or tendinous elastic forces followed by equally abrupt reactivation of motor units and restorative jerk of the affected body part 2. Ugawa et al. 39 described Silent Period Locked Averaging (SPLA) method, where backward averaging technique is used for the analysis of asterixis. This method will be useful to study the origin of asterixis and will also help to study various kinds of EMG silences that are seen in asterixis. Recently Timmerman et al 37 evaluated the cortical origin of mini-asterixis in hepatic encephalopathy by studying the hand muscle EMG recordings and brain activity recorded by magneto-encephalography (MEG) and noted that this technique could be used to distinguish different tremor syndromes.
Asterixis treatment
Asterixis is a clinical sign of an underlying disease and because there are many different causes, the treatment options vary widely 2. Your doctor will try to find the cause and treat it. Treating whatever is causing asterixis generally improves this symptom. Your healthcare provider can tell you more about possible treatments and which they recommend.
Clonazepam has been reported to improve asterixis and prevented astatic seizures in a patient who presented with viral encephalitis and bilateral insular lesions 44.
- Agarwal R, Baid R. Asterixis. J Postgrad Med. 2016 Apr-Jun;62(2):115-7. doi: 10.4103/0022-3859.180572[↩][↩][↩][↩][↩][↩]
- Gokula RM, Khasnis A. Asterixis. J Postgrad Med. 2003 Jul-Sep;49(3):272-5. https://www.jpgmonline.com/text.asp?2003/49/3/272/1148[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Ellul MA, Cross TJ, Larner AJ. Asterixis. Pract Neurol. 2017;17(1):60–62. doi: 10.1136/practneurol-2016-001393[↩]
- Zackria R, John S. Asterixis. [Updated 2023 Jul 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK535445[↩][↩][↩]
- Shibasaki H. Pathophysiology of negative myoclonus and asterixis. Adv Neurol. 1995;67:199-209.[↩]
- Tatu L, Moulin T, Martin V, Monnier G, Rumbach L. Unilateral pure thalamic asterixis: clinical, electromyographic, and topographic patterns. Neurology. 2000 Jun 27;54(12):2339-42. doi: 10.1212/wnl.54.12.2339[↩]
- Morimatsu M, Ono K, Harada A. Severe asterixis due to hypermagnesemia in chronic renal failure: a case report. Neurol Sci. 2021 Jun;42(6):2539-2542. doi: 10.1007/s10072-020-04945-x[↩]
- Myoclonus. https://www.ninds.nih.gov/health-information/disorders/myoclonus[↩]
- Young RR, Shahani BT. Asterixis: one type of negative myoclonus. Adv Neurol. 1986;43:137-56.[↩][↩][↩][↩][↩][↩]
- Kim JS. Asterixis after unilateral stroke: lesion location of 30 patients. Neurology. 2001 Feb 27;56(4):533-6. doi: 10.1212/wnl.56.4.533[↩][↩]
- Agarwal P, Frucht SJ. Myoclonus. Curr Opin Neurol. 2003 Aug;16(4):515-21. doi: 10.1097/01.wco.0000084231.82329.9c[↩]
- Adams RD, Foley JM. The neurological changes in the more common types of severe liver disease. Trans Am Neurol Assoc 1949;74:217.[↩][↩][↩]
- Bahroo LG, Shamim EA. Asterixis. In: Kompolitis K, Metman LV, editors. The Encyclopedia of Movement Disorders. Amsterdam: Elsevier; 2010. pp. 68–9.[↩][↩][↩][↩]
- Lanska DJ. Raymond D. Adams and Joseph M. Foley: Elaborating the neurologic manifestations of hepatic encephalopathy (1949-1953). J Hist Neurosci. 2021 Oct-Dec;30(4):390-404. doi: 10.1080/0964704X.2021.1891691[↩][↩]
- LEAVITT S, TYLER HR. Studies in Asterixis Part I. Arch Neurol. 1964;10(4):360–368. doi:10.1001/archneur.1964.00460160030002[↩][↩]
- Larson AM. Diagnosis and management of acute liver failure. Curr Opin Gastroenterol. 2010 May;26(3):214-21. doi: 10.1097/MOG.0b013e32833847c5[↩][↩]
- Ge PS, Runyon BA. Serum ammonia level for the evaluation of hepatic encephalopathy. JAMA. 2014 Aug 13;312(6):643-4. doi: 10.1001/jama.2014.2398[↩][↩]
- Ninan J, Feldman L. Ammonia Levels and Hepatic Encephalopathy in Patients with Known Chronic Liver Disease. J Hosp Med. 2017 Aug;12(8):659-661. doi: 10.12788/jhm.2794[↩][↩]
- Nayak R, Pandurangi A, Bhogale G, Patil N, Chate S. Asterixis (flapping tremors) as an outcome of complex psychotropic drug interaction. J Neuropsychiatry Clin Neurosci. 2012 Winter;24(1):E26-7. doi: 10.1176/appi.neuropsych.10110266[↩][↩]
- Stell R, Davis S, Carroll WM. Unilateral asterixis due to a lesion of the ventrolateral thalamus. J Neurol Neurosurg Psychiatry. 1994 Jan;57(1):116-8. doi: 10.1136/jnnp.57.1.116. Corrected and republished in: J Neurol Neurosurg Psychiatry. 1994 Jul;57(7):878-80.[↩][↩]
- Inoue M, Kojima Y, Mima T, Sawamoto N, Matsuhashi M, Fumuro T, Kinboshi M, Koganemaru S, Kanda M, Shibasaki H. Pathophysiology of unilateral asterixis due to thalamic lesion. Clin Neurophysiol. 2012 Sep;123(9):1858-64. doi: 10.1016/j.clinph.2012.01.021[↩][↩][↩]
- Ellul MA, Gholkar SA, Cross TJ. Hepatic encephalopathy due to liver cirrhosis. BMJ. 2015 Aug 11;351:h4187. doi: 10.1136/bmj.h4187[↩]
- Cordoba J. Hepatic encephalopathy: From the pathogenesis to the new treatments. ISRN Hepatology 2014. 2014:1–16.[↩][↩]
- Roche N, Chavaillon JM, Maurer C, Zureik M, Piquet J. A clinical in-hospital prognostic score for acute exacerbations of COPD. Respir Res. 2014 Aug 27;15(1):99. doi: 10.1186/s12931-014-0099-9[↩]
- Hardison WG, Lee FI. Prognosis in acute liver disease of the alcoholic patient. N Engl J Med. 1966 Jul 14;275(2):61-6. doi: 10.1056/NEJM196607142750201[↩]
- What is asterixis? https://my.clevelandclinic.org/health/symptoms/25032-asterixis[↩]
- Río J, Montalbán J, Pujadas F, Alvarez-Sabín J, Rovira A, Codina A. Asterixis associated with anatomic cerebral lesions: a study of 45 cases. Acta Neurol Scand. 1995 May;91(5):377-81. doi: 10.1111/j.1600-0404.1995.tb07024.x[↩][↩]
- Siniscalchi A, Gallelli L, Di Benedetto O, De Sarro G. Asterixis as a presentation of cerebellar ischemic stroke. West J Emerg Med. 2012 Dec;13(6):507-8. doi: 10.5811/westjem.2012.1.6900[↩]
- Ramakrishnan S, Narayanaswamy VR. Unilateral asterixis, thalamic astasia and vertical one and half syndrome in a unilateral posterior thalamo-subthalamic paramedian infarct: An interesting case report. J Neurosci Rural Pract. 2013 Apr;4(2):220-3. doi: 10.4103/0976-3147.112775[↩]
- Kida Y, Naritomi H, Sawada T, Ogawa M, Kanako T. [Unilateral asterixis caused by midbrain hemorrhage]. Rinsho Shinkeigaku. 1987 Feb;27(2):172-6. Japanese.[↩]
- Kudo Y, Fukai M, Yamadori A. Asterixis due to pontine hemorrhage. J Neurol Neurosurg Psychiatry. 1985 May;48(5):487-9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1028342/pdf/jnnpsyc00109-0095b.pdf[↩]
- Rio, J., Montalbán, J., Pujadas, F., Alvarez-Sabín, J., Rovira, A. and Codina, A. (1995), Asterixis associated with anatomic cerebral lesions: a study of 45 cases. Acta Neurologica Scandinavica, 91: 377-381. https://doi.org/10.1111/j.1600-0404.1995.tb07024.x[↩][↩]
- Leavitt S, Tyler HR. Studies in Asterixis Part I. Arch Neurol. 1964;10(4):360–368. doi:10.1001/archneur.1964.00460160030002[↩]
- Butz M, Timmermann L, Gross J, Pollok B, Südmeyer M, Kircheis G, Häussinger D, Schnitzler A. Cortical activation associated with asterixis in manifest hepatic encephalopathy. Acta Neurol Scand. 2014 Oct;130(4):260-7. doi: 10.1111/ane.12217[↩]
- Mendizabal M, Silva MO. Images in clinical medicine. Asterixis. N Engl J Med. 2010 Aug 26;363(9):e14. doi: 10.1056/NEJMicm0911157[↩]
- Yokota T, Tsukagoshi H. Cortical activity-associated negative myoclonus. J Neurol Sci. 1992 Aug;111(1):77-81. doi: 10.1016/0022-510x(92)90115-2[↩][↩]
- Timmermann L, Gross J, Kircheis G, Häussinger D, Schnitzler A. Cortical origin of mini-asterixis in hepatic encephalopathy. Neurology. 2002 Jan 22;58(2):295-8. doi: 10.1212/wnl.58.2.295[↩][↩][↩]
- Degos JD, Verroust J, Bouchareine A, Serdaru M, Barbizet J. Asterixis in focal brain lesions. Arch Neurol. 1979 Nov;36(11):705-7. doi: 10.1001/archneur.1979.00500470075015[↩]
- Ugawa Y, Shimpo T, Mannen T. Physiological analysis of asterixis: silent period locked averaging. J Neurol Neurosurg Psychiatry. 1989 Jan;52(1):89-93. doi: 10.1136/jnnp.52.1.89[↩][↩]
- Masdeu JC, Gorelick PB. Thalamic astasia: inability to stand after unilateral thalamic lesions. Ann Neurol. 1988 Jun;23(6):596-603. doi: 10.1002/ana.410230612[↩]
- Schell GR, Strick PL. The origin of thalamic inputs to the arcuate premotor and supplementary motor areas. J Neurosci. 1984 Feb;4(2):539-60. doi: 10.1523/JNEUROSCI.04-02-00539.1984[↩]
- DeVito, J.L. and Smith, O.A., Jr. (1959), Projections from the mesial frontal cortex (supplementary motor area) to the cerebral hemispheres and brain stem of the macaca mulatta. J. Comp. Neurol., 111: 261-277. https://doi.org/10.1002/cne.901110204[↩]
- Reinfeld H, Louis S. Unilateral asterixis. Clinical significance of the sign. N Y State J Med. 1983 Feb;83(2):206-8.[↩]
- Muneta S, Yamashita Y, Fukuda H, Watanabe S, Imamura Y, Matsumoto I. Asterixis and astatic seizures in association with bilateral insular lesions in a patient with viral encephalitis. Intern Med. 1995 Aug;34(8):756-61. doi: 10.2169/internalmedicine.34.756[↩]