Best supplements and vitamins for depression
Depression also called major depressive disorder or clinical depression, is a disorder of the brain that affects about 1 in 10 U.S. adults 1. There are a variety of causes, including genetic, biological, environmental, and psychological factors. Depression can happen at any age, but it often begins in teens and young adults. Experts estimate that some 5 percent of U.S. teens have moderate to severe major depression. Depression is much more common in women. Women can also get postpartum depression after the birth of a baby. Some people get seasonal affective disorder (SAD) in the winter. Depression is one part of bipolar disorder (also called manic depression).
The symptoms and severity of depression can vary from person to person. Your mood, thoughts, physical health, and behavior all may be affected.
If you have been experiencing some of the following signs and symptoms most of the day, nearly every day, for at least two weeks, you may be suffering from depression:
- Persistent sad, anxious, or “empty” mood
- Feelings of hopelessness, or pessimism
- Irritability
- Feeling irritable‚ easily frustrated‚ or restless (this can be a common symptom among adolescents)
- Feelings of guilt, worthlessness, or helplessness
- Loss of interest or pleasure in hobbies and activities that used to be fun
- Decreased energy or fatigue
- Moving or talking more slowly
- Feeling restless or having trouble sitting still
- Difficulty concentrating, remembering, or making decisions
- Difficulty sleeping, early-morning awakening, or oversleeping and feeling tired
- Eating more or less than usual or having no appetite and/or weight changes
- Thoughts of death or suicide, or suicide attempts
- Aches or pains, headaches, cramps, or digestive problems without a clear physical cause and/or that do not ease even with treatment
- Having trouble concentrating, remembering details, or making decisions
Not everyone who is depressed experiences every symptom. Some people experience only a few symptoms while others may experience many. To be diagnosed with depression, the symptoms must be present for at least two weeks. The severity and frequency of symptoms and how long they last will vary depending on the individual and his or her particular illness. Symptoms may also vary depending on the stage of the illness.
Two common forms of depression are:
- Major depression, which includes symptoms of depression most of the time for at least 2 weeks that typically interfere with one’s ability to work, sleep, study, and eat.
- Persistent depressive disorder (dysthymia), which often includes less severe symptoms of depression that last much longer, typically for at least 2 years. A person diagnosed with persistent depressive disorder may have episodes of major depression along with periods of less severe symptoms, but symptoms must last for two years to be considered persistent depressive disorder.
Other forms of depression include:
- Postpartum depression is much more serious than the “baby blues” (relatively mild depressive and anxiety symptoms that typically clear within two weeks after delivery) that many women experience after giving birth. Women with postpartum depression experience full-blown major depression during pregnancy or after delivery (postpartum depression). The feelings of extreme sadness, anxiety, and exhaustion that accompany postpartum depression may make it difficult for these new mothers to complete daily care activities for themselves and/or for their babies.
- Psychotic depression occurs when a person has severe depression plus some form of psychosis, such as having disturbing false fixed beliefs (delusions) or hearing or seeing upsetting things that others cannot hear or see (hallucinations). The psychotic symptoms typically have a depressive “theme,” such as delusions of guilt, poverty, or illness.
- Seasonal affective disorder (SAD) is characterized by the onset of depression during the winter months, when there is less natural sunlight. This depression generally lifts during spring and summer. Winter depression, typically accompanied by social withdrawal, increased sleep, and weight gain, predictably returns every year in seasonal affective disorder.
- Bipolar disorder is different from depression, but it is included in this list is because someone with bipolar disorder experiences episodes of extremely low moods that meet the criteria for major depression (called “bipolar depression”). But a person with bipolar disorder also experiences extreme high – euphoric or irritable – moods called “mania” or a less severe form called “hypomania.”
Examples of other types of depressive disorders newly added to the diagnostic classification of DSM-5 include disruptive mood dysregulation disorder (diagnosed in children and adolescents) and premenstrual dysphoric disorder (PMDD).
Depression is usually treated with medications (antidepressants), psychotherapy or a combination of the two. If these treatments do not reduce symptoms, electroconvulsive therapy (ECT) and other brain stimulation therapies may be options to explore. DO NOT try to treat moderate or severe depression on your own.
Some people might consider supplements and vitamins such as St. John’s wort, S-Adenosyl-L-methionine (SAMe), vitamin D, vitamin B6, vitamin B12, folate (vitamin B9) and omega 3 (fish oil) for depression.
Examples of supplements that are sometimes used for depression include:
- St. John’s wort. Although this herbal supplement isn’t approved by the Food and Drug Administration (FDA) to treat depression in the U.S., it may be helpful for mild or moderate depression. St. John’s wort has been studied extensively for depression. Most studies show it works as well as antidepressants for mild-to-moderate depression. It has fewer side effects than most antidepressants. It may take 4 to 6 weeks before you see any improvement. St. John’s wort interacts with a large number of medications, including birth control pills, so check with your doctor if you are taking prescription medications. DO NOT use St. John’s wort to treat severe depression. But if you choose to use it, be careful — St. John’s wort can interfere with a number of medications, such as heart drugs, blood-thinning drugs, birth control pills, chemotherapy, HIV/AIDS medications and drugs to prevent organ rejection after a transplant. Also, avoid taking St. John’s wort while taking antidepressants because the combination can cause serious side effects.
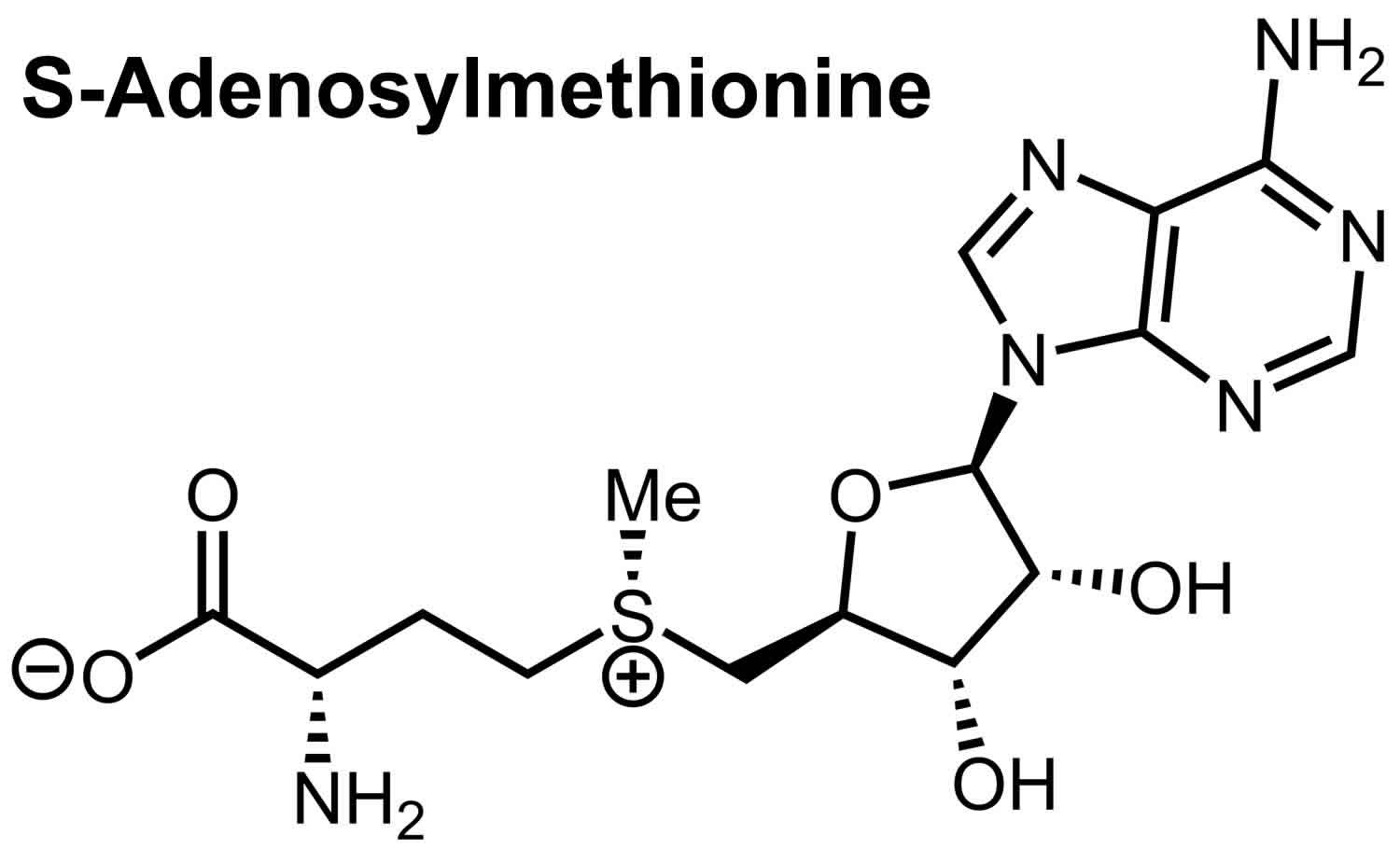
- SAMe is short for S-adenosylmethionine. Pronounced “sam-E,” this dietary supplement is a synthetic form of a chemical that occurs naturally in your body. SAMe (s-adenosyl-L-methionine) is a substance your body makes that may raise levels of the brain chemical dopamine. SAMe isn’t approved by the FDA to treat depression in the U.S. It may be helpful, but more research is needed. SAM-e has been studied for depression, but results are mixed and not all of the studies have been of good quality. Some of the studies suggest SAM-e can help relieve mild-to-moderate depression and may work faster than prescription antidepressants. If you are taking other medications for depression, talk to your doctor before taking SAMe because it may interact with them. SAM-e may trigger mania in people with bipolar disorder.
- Omega-3 fatty acids (a combination of eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]). These healthy fats are found in cold-water fish, flaxseed, flax oil, walnuts and some other foods. Omega-3 supplements are being studied as a possible treatment for depression. Omega-3 fatty acids may help relieve symptoms of depression, but evidence is mixed. While considered generally safe, in high doses, omega-3 supplements may interact with other medications. Although eating foods with omega-3 fatty acids appears to have heart-healthy benefits, more research is needed to determine if it has an effect on preventing or improving depression. Some studies suggest that fish oil, when taken with prescription antidepressants, works better than antidepressants alone. However, a review of several studies did not find any benefit. Preliminary studies suggest that one of the omega-3 fatty acids in fish oil called EPA helps relieve depression when taken with an antidepressant. Fish oil taken in high doses may increase the risk of bleeding. DO NOT take it if you also take blood thinners, such as warfarin (Coumadin), clopidogrel (Plavix), or daily aspirin.
- Saffron (Crocus satvius). Saffron extract may improve symptoms of depression, but more study is needed. One preliminary study found that it worked as well as Prozac, while another found that it worked as well as a low dose of Tofranil. Saffron can be dangerous or even life-threatening at high doses or when taken for a long time, so DO NOT take it without your doctor’s supervision. Pregnant women and people with bipolar disorder should not take saffron supplements. High doses can cause significant side effects.
- Ginkgo (Ginkgo biloba) standardized extract, 40 to 80 mg, 3 times daily, for depression. A few studies looking at gingko for treating memory problems in older adults seemed to show that it also improved symptoms of depression. One laboratory study found that gingko, when given to older rats, increased the number of serotonin-binding sites in their brains. It did not affect younger rats, so researchers thought that it might relieve depression in older adults by helping their brains respond better to serotonin. More research is needed. Gingko may increase the risk of bleeding, especially if you also take blood thinners such as warfarin (Coumadin), clopidogrel (Plavix), or aspirin. Ask your doctor before taking gingko.
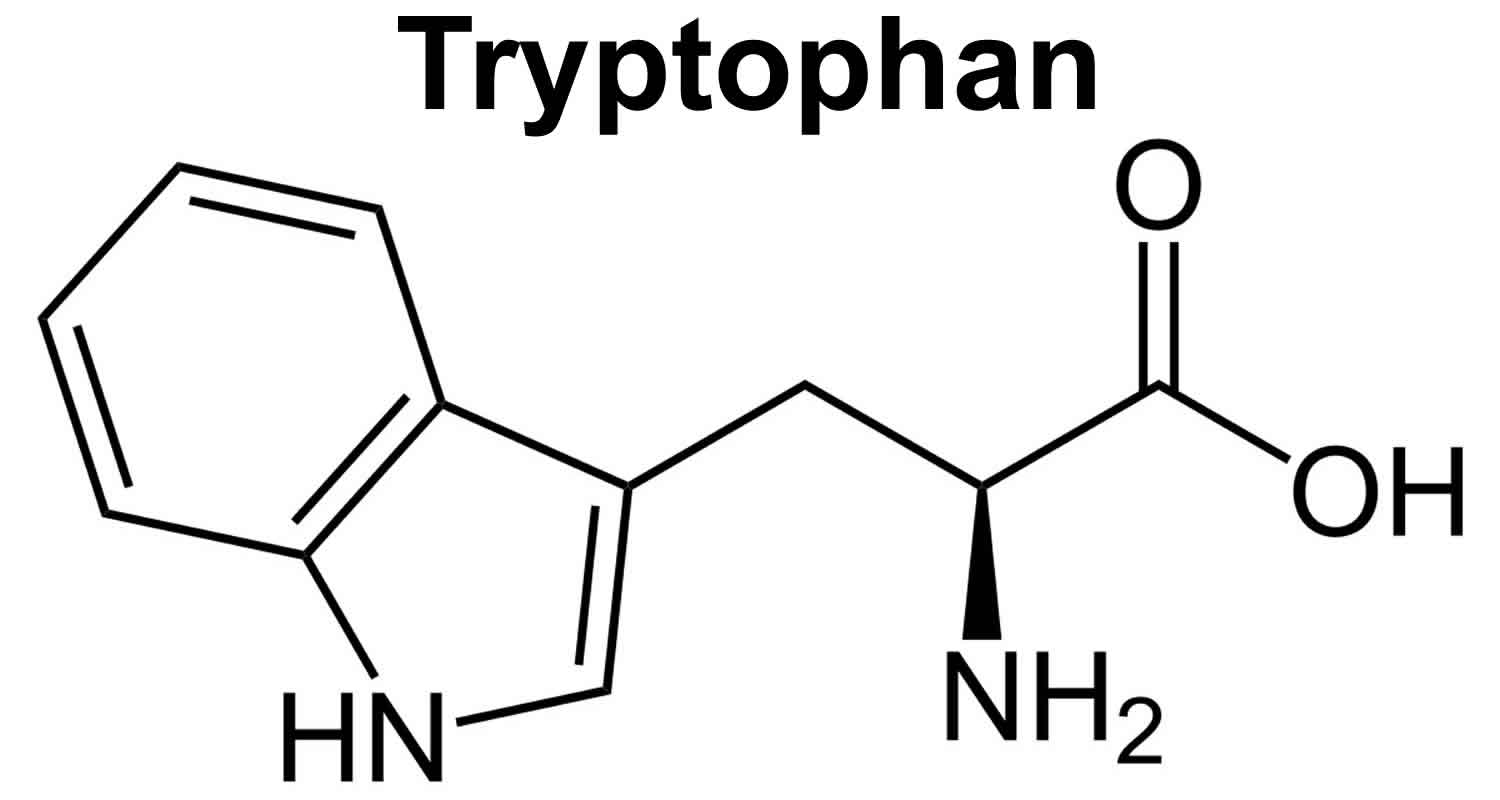
- 5-HTP also known as 5-hydroxytryptophan, is a precursor to serotonin, meaning your body changes it to serotonin (5-HT). Serotonin is commonly known as a ‘happiness hormone’ 2. Surprisingly, a decrease in serotonin (5-HT) in the brain, measured by concentrations of serotonin metabolite 5-hydroxyindoleacetic acid (5-HIAA) in cerebrospinal fluid (CSF), have not been found characteristic of depression itself, but rather of impulsivity 3, suicidality and a tendency to violence 4. Serotonin exerts its action via the serotonin-1A (5-HT1A) receptor which has been reported to play a role in both prognosis and diagnosis of depression 5 as reduced serotonin-1A (5-HT1A) receptor binding is associated with depression 6. Additionally, increased autoimmune responses to serotonin were found to correlate with successive depressive episodes 7. Serotonin-2A (5-HT2A) receptor can be found at blood platelets. The density of platelet serotonin-2A (5-HT2A) receptor tends to increase in patients with depression. However, it has been found to correlate more closely with suicidality than depression per se 8. Increased serotonin-2A (5-HT2A) receptor density could potentially serve as a marker of suicide risk (state marker of depression). 5-HTP may play a role in improving serotonin levels in your brain, a chemical that affects mood. Early studies suggest it may work like antidepressant drugs, but evidence is only preliminary and more research is needed. There is a safety concern that using 5-HTP may cause a severe neurological condition, but the link is not clear. In rare cases, contaminated 5-HTP was linked to a potentially fatal condition called eosinophilia-myalgia syndrome. Another safety concern is that 5-HTP could increase the risk of serotonin syndrome — a serious side effect — if taken with certain prescription antidepressants. Taking 5-HTP with other antidepressants can cause serotonin levels in the brain to rise to dangerous levels, a condition called serotonin syndrome. You should not take 5-HTP without your doctor’s supervision.
- Vitamin D. Vitamin D can be considered a neurosteroid, with vitamin D receptors being identified in areas involved with depression, such as the prefrontal cortex, hypothalamus, and substantia nigra 9. Vitamin D has been revealed to increase the expression of genes encoding for tyrosine hydroxylase (precursor of dopamine and norepinephrine) 9. Furthermore, a major dopamine metabolite in the striatum and accumbens has been found in methamphetamine-treated animals administered vitamin D 10. Recently, many studies have examined the relationship between vitamin D and depression symptoms, especially given the complexity of treating depression and the high prevalence of vitamin D deficiency. A systematic review summarizing the evidence from observational studies concluded that vitamin D deficiency is positively associated with depression in adults 11. However, based on these observations, it is not possible to conclude that there is a causal relationship between vitamin D and depression due to potential confounders including age, dietary intake, time spent outdoors, physical activity, smoking, alcohol use, etc 12. Many randomized controlled trials of vitamin D supplementation in depression have been reported, but their findings have been inconsistent. Although some randomized controlled trials indicate a promising effect of vitamin D supplementation on depression symptoms 13, 14, others show no such effect 15, 16.
- Vitamin B6 is a generic name for six compounds (vitamers) with vitamin B6 activity: pyridoxine, pyridoxal and pyridoxamine, which contains an amino group and their respective 5’-phosphate esters. Pyridoxal 5’ phosphate (PLP) and pyridoxamine 5’ phosphate (PMP) are the active coenzyme forms of vitamin B6 17. Vitamin B6 has been used for women with premenstrual dysphoric disorder. A few studies suggest that vitamin B6 may help relieve depression that occurs with premenstrual syndrome, although the evidence is mixed. The studies used high doses, which require a doctor’s supervision. Other studies suggest that B6 may also help with other types of depression. More research is needed.
- Studies have found that some people with depression may have low levels of folic acid, vitamin B12, or vitamin D. If you have depression, you may want to ask your doctor to check your levels. So far there is no proof that taking any of these vitamins helps relieve depression. But one study suggested that women who took folic acid supplements along with Prozac did better than those who took only Prozac.
Nutritional and dietary products aren’t monitored by the FDA the same way medications are. You can’t always be certain of what you’re getting and whether it’s safe. Also, because some herbal and dietary supplements can interfere with prescription medications or cause dangerous interactions, talk to your doctor or pharmacist before taking any supplements.
Moreover, no two people are affected the same way by depression and there is no “one-size-fits-all” for treatment. It may take some trial and error to find the treatment that works best for you.
Many people with depression benefit by making lifestyle changes, such as getting more exercise, cutting down on alcohol, giving up smoking and eating healthily.
Here are other tips that may help you or a loved one during treatment for depression:
- Try to be active and exercise. Just 30 minutes a day of walking can boost mood.
- Try to maintain a regular bedtime and wake-up time.
- Avoid using alcohol, nicotine, or drugs, including medications not prescribed for you.
- Eat regular, healthy meals.
- Set realistic goals for yourself.
- Try to spend time with other people and talk with people you trust about how you are feeling.
- Try not to isolate yourself, and let others help you.
- Expect your mood to improve gradually, not immediately.
- Do what you can as you can. Decide what must get done and what can wait.
- Postpone important decisions, such as getting married or divorced, or changing jobs until you feel better. Discuss decisions with others who know you well and have a more objective view of your situation.
- Continue to educate yourself about depression.
If someone you know is showing one or more of the following behaviors, he or she may be thinking about suicide. Don’t ignore these warning signs. Get help immediately.
- Talking about wanting to die or to kill oneself
- Looking for a way to kill oneself
- Talking about feeling hopeless or having no reason to live
- Talking about feeling trapped or in unbearable pain
- Talking about being a burden to others
- Increasing the use of alcohol or drugs
- Acting anxious or agitated; behaving recklessly
- Sleeping too little or too much
- Withdrawing or feeling isolated
- Showing rage or talking about seeking revenge
- Displaying extreme mood swings
Get Help
If you or someone you know needs help, call the National Suicide Prevention Lifeline at 1-800-273-8255. Trained crisis workers are available to talk 24 hours a day, 7 days a week.
If you think someone is in immediate danger, do not leave him or her alone—stay there and call your local emergency number.
If a loved one or friend is in danger of attempting suicide or has made an attempt:
- Make sure someone stays with that person
- Call your local emergency number immediately
- Or, if you can do so safely, take the person to the nearest hospital emergency room
- Call a suicide hotline number.
- In the U.S., call the National Suicide Prevention Lifeline at 800-273-8255. Use that same number and press “1” to reach the Veterans Crisis Line. Or call the National Hopeline Network at 1-800-784-2433
- In the UK and Ireland – call the Samaritans at 116-123
- In Australia – call Lifeline Australia at 13-11-14
- In other countries – Visit International Association for Suicide Prevention at http://www.iasp.info/resources/Crisis_Centres or Suicide.org to find a helpline in your country at http://www.suicide.org/international-suicide-hotlines.html.
Never ignore comments or concerns about suicide. Always take action to get help!
- National Mental Health Association (NMHA) 18
NMHA works to improve the mental health of all Americans through advocacy, education, research, and service 18 or go here http://www.mentalhealthamerica.net/.
- American Foundation for Suicide Prevention 19
This group is dedicated to advancing the knowledge of suicide and the ability to prevent it 19 or go here https://afsp.org/. - National Suicide Prevention Lifeline 20
National Suicide Prevention Lifeline can help prevent suicide, you can call them at 1-800-273-8255. The Lifeline provides 24/7, free and confidential support for people in distress, prevention and crisis resources for you or your loved ones, and best practices for professionals 20 or go here https://suicidepreventionlifeline.org/. - Depression and Bipolar Support Alliance 21
The Depression and Bipolar Support Alliance (DBSA) provides hope, help, support, and education to improve the lives of people who have mood disorders and manic-depressive illnesses 21 or go here http://www.dbsalliance.org
- National Alliance on Mentally Illness 22
National Alliance on Mentally Illness offers resources and help for those with a mental illness 22 or go here http://www.nami.org.
- National Institute of Mental Health 23
NIMH offers information about the symptoms, diagnosis, and treatment of mental illnesses, and supports research to help those with mental illness 23 or go here https://www.nimh.nih.gov/index.shtml.
- National Strategy for Suicide Prevention 24
National Strategy for Suicide Prevention provides information, a listing of events, and publications on suicide prevention 24 or go here http://www.mentalhealth.org/what-to-look-for/suicidal-behavior.
- National Association of Anorexia Nervosa and Associated Disorders 25
National Association of Anorexia Nervosa and Associated Disorders is a national nonprofit organization for people with eating disorders and their families. In addition to its hotline counseling, National Association of Anorexia Nervosa and Associated Disorders operates an international network of support groups and offers referrals to health care professionals who treat eating disorders 25 or go here http://www.anad.org/.
Folate (vitamin B9)
Folate also known as vitamin B9, is a water-soluble B-vitamin that is naturally present in many foods. Your body needs folate to make DNA and other genetic material. Your body also needs folate for your cells to divide and for metabolism of amino acids 26. A form of folate, called folic acid, is used in fortified foods and most dietary supplements. Some dietary supplements also contain folate in the monoglutamyl form, 5-methyl-THF (also known as L-5- MTHF, 5-MTHF, L-methylfolate or methylfolate) 27. One of the most important folate-dependent reactions is the conversion of homocysteine to methionine in the synthesis of SAMe or S-adenosyl methionine, an important methyl donor 27. Another folate-dependent reaction, the methylation of deoxyuridylate to thymidylate in the formation of DNA, is required for proper cell division. An impairment of this reaction initiates a process that can lead to megaloblastic anemia, one of the hallmarks of folate deficiency 28. People with low blood levels of folate might be more likely to have depression. In addition, they might not respond as well to antidepressant treatment as people with normal folate levels.
Folate is naturally present in a wide variety of foods, including vegetables (especially dark green leafy vegetables), fruits and fruit juices, nuts, beans, peas, seafood, eggs, dairy products, meat, poultry, and grains (see Table 2) 28. Spinach, liver, asparagus, and brussels sprouts are among the foods with the highest folate levels.
In January 1998, the U.S. Food and Drug Administration (FDA) began requiring manufacturers to add 140 mcg folic acid/100 g to enriched breads, cereals, flours, cornmeals, pastas, rice, and other grain products to reduce the risk of neural tube defects 29. Because cereals and grains are widely consumed in the United States, these products have become important contributors of folic acid to the American diet. The fortification program increased mean folic acid intakes in the United States by about 190 mcg/day 30. In April 2016, FDA approved the voluntary addition of up to 154 mcg folic acid/100 g to corn masa flour 31.
Since November 1, 1998, the Canadian government has also required the addition of 150 mcg folic acid/100 g to many grains, including enriched pasta, cornmeal, and white flour 32. Many other countries, including Costa Rica, Chile, and South Africa, have also established mandatory folic acid fortification programs 31.
Low vitamin B9 (Folate) status has been linked to depression and poor response to antidepressants in some, but not all, studies 27. The possible mechanisms are unclear but might be related to folate’s role in methylation reactions in the brain, neurotransmitter synthesis, and homocysteine metabolism 33. However, secondary factors linked to depression, such as unhealthy eating patterns and alcohol use disorder, might also contribute to the observed association between low folate status and depression 34.
In a population study of 2,948 people aged 15 to 39 years in the United States, serum and red blood cell folate concentrations were significantly lower in individuals with major depression than in those who had never been depressed 34. An analysis of 2005-2006 National Health and Nutrition Examination Survey (NHANES) data found that higher serum concentrations of folate were associated with a lower prevalence of depression in 2,791 adults aged 20 or older 33. The association was statistically significant in females, but not in males. However, another analysis showed no associations between folate intakes from both food and dietary supplements and depression among 1,368 healthy Canadians aged 67–84 years 35. Results from a study of 52 men and women with major depressive disorder showed that only 1 of 14 participants with low serum folate levels responded to antidepressant treatment compared with 17 of 38 with normal folate levels 36.
A few studies have examined whether folate status affects the risk of depression during pregnancy or after childbirth. A systematic review of these studies had mixed results 37. One study included in the review among 709 women in Singapore found that compared with women with higher plasma folate concentrations (mean 40.4 nmol/L [17.8 ng/mL]) at 26–28 weeks’ gestation, those with lower plasma folate concentrations (mean 27.3 nmol/L [12.0 ng/mL]) had a significantly higher risk of depression during pregnancy but not after giving birth 38. Another study of 2,856 women in the United Kingdom found no significant associations between red blood cell folate levels or folate intakes from food and dietary supplements before or during pregnancy and postpartum depressive symptoms 39. More recently, a cohort study of 1,592 Chinese women found a lower prevalence of postpartum depression in women who took folic acid supplements for more than 6 months during pregnancy than in those who took them for less time 40.
Studies have had mixed results on whether folic acid supplementation might be a helpful adjuvant treatment (therapy that is given in addition to the primary or initial therapy to maximize its effectiveness) for depression when used with traditional antidepressant medications. In a clinical trial in the United Kingdom, 127 patients with major depression were randomly assigned to receive either 500 mcg folic acid or placebo in addition to 20 mg of fluoxetine daily for 10 weeks 41. Although the effects in men were not statistically significant, women who received fluoxetine plus folic acid had a significantly greater improvement in depressive symptoms than those who received fluoxetine plus placebo. Another clinical trial in the United Kingdom randomized 475 adults with moderate to severe depression who were taking antidepressant medications to either 5,000 mcg folic acid or placebo daily for 12 weeks in addition to their antidepressants 42. Measures of depression did not improve in participants taking folic acid compared with those taking placebo. The authors of a systematic review and meta-analysis of four trials of folic acid (<5,000 mcg/day in two trials; 5,000 mcg/day in two trials) in combination with fluoxetine or other antidepressants in patients with major depressive disorder concluded that less than 5,000 mcg/day folic acid might be beneficial as an adjunct to serotonin reuptake inhibitor (SSRI) therapy 43. The authors noted, however, that this conclusion was based on low-quality evidence. Another meta-analysis of four clinical trials found that 500–10,000 mcg folic acid per day for 6–12 weeks as an adjunctive treatment did not significantly affect measures of depression compared with placebo 44.
Other studies have examined the effects of methylfolate (5-methyl-THF) supplementation as an adjuvant treatment to antidepressants, and results suggest that it might have more promise than folic acid 45, 43. In a clinical trial in 148 adults with major depressive disorder, supplementation with 7,500 mcg/day methylfolate (5-methyl-THF) for 30 days followed by 15,000 mcg/day for another 30 days, both in conjunction with SSRI treatment, did not improve measures of depression compared with SSRI treatment plus placebo 46. However, in a subsequent trial with the same study design in 75 adults, supplementation with 15,000 mcg/day 5-methyl-THF plus SSRI treatment for the full 60 days did significantly improve depression compared with SSRI treatment plus placebo 46.
The authors of a systematic review and meta-analysis of three trials of methylfolate (<15,000 mcg/day in one trial, and 15,000 mcg/day in two trials) in combination with fluoxetine or other antidepressants, concluded that 15,000 mcg/day 5-methylfolate (5-methyl-THF) might be an effective adjunct to SSRI therapy in patients with major depressive disorder, although they noted that this conclusion was based on low-quality evidence 43. In addition, evidence-based guidelines from the British Association for Psychopharmacology 47 and the Canadian Network for Mood and Anxiety Treatments 46 state that methylfolate (5-methyl-THF) might be effective as an adjunct to SSRI treatment for depressive disorders.
Additional research is needed to fully understand the association between folate status and depression. Although limited evidence suggests that supplementation with certain forms and doses of folate might be a helpful adjuvant treatment (therapy that is given in addition to the primary or initial therapy to maximize its effectiveness) for depressive disorders, more research is needed to confirm these findings. In addition, many of the doses of folate used in studies of depression exceed the Tolerable Upper Intake Level (UL) and should be taken only under medical supervision. Tolerable Upper Intake Level (UL) is the Maximum daily intake unlikely to cause adverse health effects.
The amount of folate you need depends on your age. Average daily recommended amounts are listed below in micrograms (mcg) of dietary folate equivalents (DFEs). Table 1 lists the current Recommended Dietary Allowances (RDAs) for folate as mcg of dietary folate equivalents (DFEs). The the Food and Nutrition Board at the National Academies of Sciences, Engineering, and Medicine developed DFEs to reflect the higher bioavailability of folic acid than that of food folate 48. At least 85% of folic acid is estimated to be bioavailable when taken with food, whereas only about 50% of folate naturally present in food is bioavailable 49. Based on these values, the Food and Nutrition Board at the National Academies of Sciences, Engineering, and Medicine defined dietary folate equivalent (DFE) as follows:
- 1 mcg DFE = 1 mcg food folate
- 1 mcg DFE = 0.6 mcg folic acid from fortified foods or dietary supplements consumed with foods
- 1 mcg DFE = 0.5 mcg folic acid from dietary supplements taken on an empty stomach
The measure of mcg of dietary folate equivalent (DFE) is used because your body absorbs more folic acid from fortified foods and dietary supplements than folate found naturally in foods. Compared to folate found naturally in foods, you actually need less folic acid to get recommended amounts. For example, 240 mcg of folic acid and 400 mcg of folate are both equal to 400 mcg DFE. All women and teen girls who could become pregnant should consume 400 mcg of folic acid daily from supplements, fortified foods, or both in addition to the folate they get from following a healthy eating pattern.
Factors for converting mcg DFE to mcg for supplemental folate in the form of methylfolate (5-methyl-THF) have not been formally established.
For infants from birth to 12 months, the Food and Nutrition Board established an Adequate Intake (AI) for folate that is equivalent to the mean intake of folate in healthy, breastfed infants in the United States (see Table 1).
- Recommended Dietary Allowance (RDA): Average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals.
- Adequate Intake (AI): Intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an RDA.
Table 1. Recommended Dietary Allowances (RDAs) for Folate
| Age | Recommended Amount |
| Birth to 6 months | 65 mcg DFE |
| Infants 7–12 months | 80 mcg DFE |
| Children 1–3 years | 150 mcg DFE |
| Children 4–8 years | 200 mcg DFE |
| Children 9–13 years | 300 mcg DFE |
| Teens 14–18 years | 400 mcg DFE |
| Adults 19+ years | 400 mcg DFE |
| Pregnant teens and women | 600 mcg DFE |
| Breastfeeding teens and women | 500 mcg DFE |
Footnotes:
[Source 48 ]Folate is naturally present in:
- Beef liver
- Vegetables (especially asparagus, brussels sprouts, and dark green leafy vegetables such as spinach and mustard greens)
- Fruits and fruit juices (especially oranges and orange juice)
- Nuts, beans, and peas (such as peanuts, black-eyed peas, and kidney beans)
Folic acid is added to the following foods:
- Enriched bread, flour, cornmeal, pasta, and rice
- Fortified breakfast cereals
- Fortified corn masa flour (used to make corn tortillas and tamales)
The U.S. Department of Agriculture’s FoodData Central (https://fdc.nal.usda.gov) lists the nutrient content of many foods and provides a comprehensive list of foods containing folate arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/Folate-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/Folate-Food.pdf).
Table 2. Folate and Folic Acid content of selected foods
| Food | Micrograms (mcg) DFE per serving | Percent DV* |
| Beef liver, braised, 3 ounces | 215 | 54 |
| Spinach, boiled, ½ cup | 131 | 33 |
| Black-eyed peas (cowpeas), boiled, ½ cup | 105 | 26 |
| Breakfast cereals, fortified with 25% of the DV† | 100 | 25 |
| Rice, white, medium-grain, cooked, ½ cup† | 90 | 22 |
| Asparagus, boiled, 4 spears | 89 | 22 |
| Brussels sprouts, frozen, boiled, ½ cup | 78 | 20 |
| Spaghetti, cooked, enriched, ½ cup† | 74 | 19 |
| Lettuce, romaine, shredded, 1 cup | 64 | 16 |
| Avocado, raw, sliced, ½ cup | 59 | 15 |
| Spinach, raw, 1 cup | 58 | 15 |
| Broccoli, chopped, frozen, cooked, ½ cup | 52 | 13 |
| Mustard greens, chopped, frozen, boiled, ½ cup | 52 | 13 |
| Bread, white, 1 slice† | 50 | 13 |
| Green peas, frozen, boiled, ½ cup | 47 | 12 |
| Kidney beans, canned, ½ cup | 46 | 12 |
| Wheat germ, 2 tablespoons | 40 | 10 |
| Tomato juice, canned, ¾ cup | 36 | 9 |
| Crab, Dungeness, 3 ounces | 36 | 9 |
| Orange juice, ¾ cup | 35 | 9 |
| Turnip greens, frozen, boiled, ½ cup | 32 | 8 |
| Peanuts, dry roasted, 1 ounce | 27 | 7 |
| Orange, fresh, 1 small | 29 | 7 |
| Papaya, raw, cubed, ½ cup | 27 | 7 |
| Banana, 1 medium | 24 | 6 |
| Yeast, baker’s, ¼ teaspoon | 23 | 6 |
| Egg, whole, hard-boiled, 1 large | 22 | 6 |
| Cantaloupe, raw, cubed, ½ cup | 17 | 4 |
| Vegetarian baked beans, canned, ½ cup | 15 | 4 |
| Fish, halibut, cooked, 3 ounces | 12 | 3 |
| Milk, 1% fat, 1 cup | 12 | 3 |
| Ground beef, 85% lean, cooked, 3 ounces | 7 | 2 |
| Chicken breast, roasted, 3 ounces | 3 | 1 |
Footnotes: * DV = Daily Value. The FDA developed DVs to help consumers compare the nutrient contents of foods and dietary supplements within the context of a total diet. The DV for folate is 400 mcg DFE for adults and children aged 4 years and older, where mcg DFE = mcg naturally occurring folate + (1.7 x mcg folic acid). The labels must list folate content in mcg DFE per serving and if folic acid is added to the product, they must also list the amount of folic acid in mcg in parentheses. The FDA does not require food labels to list folate content unless folic acid has been added to the food. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
† Fortified with folic acid as part of the folate fortification program.
[Source 50 ]Vitamin B6
Vitamin B6 is a generic name for six compounds (vitamers) with vitamin B6 activity: pyridoxine, pyridoxal and pyridoxamine, which contains an amino group and their respective 5’-phosphate esters. Pyridoxal 5’ phosphate (PLP) and pyridoxamine 5’ phosphate (PMP) are the active coenzyme forms of vitamin B6 17. Substantial proportions of the naturally occurring pyridoxine in fruits, vegetables, and grains exist in glycosylated forms that exhibit reduced bioavailability 51. Vitamin B6 is found in a wide variety of foods 51. The richest sources of vitamin B6 include fish, beef liver and other organ meats, potatoes and other starchy vegetables, and fruit (other than citrus). In the United States, adults obtain most of their dietary vitamin B6 from fortified cereals, beef, poultry, starchy vegetables, and some non-citrus fruits 52. About 75% of vitamin B6 from a mixed diet is bioavailable 53.
Vitamin B6 deficiency is uncommon in the United States; inadequate vitamin B6 status is usually associated with low concentrations of other B-complex vitamins, such as vitamin B12 and folate 17. People who don’t get enough vitamin B6 can have a range of symptoms, including anemia, itchy rashes, dermatitis with cheilosis (scaly skin on the lips and cracks at the corners of the mouth) and a swollen tongue (glossitis) 53. Other symptoms of very low vitamin B6 levels include depression, confusion, electroencephalographic abnormalities and a weak immune system. Infants who do not get enough vitamin B6 can become irritable or develop extremely sensitive hearing or seizures 17. Individuals with borderline vitamin B6 concentrations or mild deficiency might have no deficiency signs or symptoms for months or even years. In infants, vitamin B6 deficiency causes irritability, abnormally acute hearing, and convulsive seizures 17.
End-stage renal diseases, chronic renal insufficiency, and other kidney diseases can cause vitamin B6 deficiency 51. In addition, vitamin B6 deficiency can result from malabsorption syndromes, such as celiac disease, Crohn’s disease, and ulcerative colitis. Certain genetic diseases, such as homocystinuria, can also cause vitamin B6 deficiency 17. Some medications, such as antiepileptic drugs, can lead to deficiency over time.
A systematic review without meta-analysis for vitamin B-6 as treatment for depression revealed two randomized controlled trials showing no significant effects when compared with placebo 54.
The amount of vitamin B6 you need depends on your age. Average daily recommended amounts are listed below in milligrams (mg).
Table 3. Recommended Dietary Allowances (RDAs) for Vitamin B6
| Age | Recommended Amount |
| Birth to 6 months | 0.1 mg |
| Infants 7–12 months | 0.3 mg |
| Children 1–3 years | 0.5 mg |
| Children 4–8 years | 0.6 mg |
| Children 9–13 years | 1.0 mg |
| Teens 14–18 years (boys) | 1.3 mg |
| Teens 14–18 years (girls) | 1.2 mg |
| Adults 19–50 years | 1.3 mg |
| Adults 51+ years (men) | 1.7 mg |
| Adults 51+ years (women) | 1.5 mg |
| Pregnant teens and women | 1.9 mg |
| Breastfeeding teens and women | 2.0 mg |
Vitamin B6 is found naturally in many foods and is added to other foods. You can get recommended amounts of vitamin B6 by eating a variety of foods, including the following:
- Poultry, fish, and organ meats, all rich in vitamin B6.
- Potatoes and other starchy vegetables, which are some of the major sources of vitamin B6 for Americans.
- Fruit (other than citrus), which are also among the major sources of vitamin B6 for Americans.
The U.S. Department of Agriculture’s (USDA’s) FoodData Central (https://fdc.nal.usda.gov) lists the nutrient content of many foods and provides a comprehensive list of foods containing vitamin B6 arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/VitaminB6-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/VitaminB6-Food.pdf).
Table 4. Vitamin B6 content of selected foods
| Food | Milligrams (mg) per serving | Percent DV* |
| Chickpeas, canned, 1 cup | 1.1 | 65 |
| Beef liver, pan fried, 3 ounces | 0.9 | 53 |
| Tuna, yellowfin, fresh, cooked, 3 ounces | 0.9 | 53 |
| Salmon, sockeye, cooked, 3 ounces | 0.6 | 35 |
| Chicken breast, roasted, 3 ounces | 0.5 | 29 |
| Breakfast cereals, fortified with 25% of the DV for vitamin B6 | 0.4 | 25 |
| Potatoes, boiled, 1 cup | 0.4 | 25 |
| Turkey, meat only, roasted, 3 ounces | 0.4 | 25 |
| Banana, 1 medium | 0.4 | 25 |
| Marinara (spaghetti) sauce, ready to serve, 1 cup | 0.4 | 25 |
| Ground beef, patty, 85% lean, broiled, 3 ounces | 0.3 | 18 |
| Waffles, plain, ready to heat, toasted, 1 waffle | 0.3 | 18 |
| Bulgur, cooked, 1 cup | 0.2 | 12 |
| Cottage cheese, 1% low-fat, 1 cup | 0.2 | 12 |
| Squash, winter, baked, ½ cup | 0.2 | 12 |
| Rice, white, long-grain, enriched, cooked, 1 cup | 0.1 | 6 |
| Nuts, mixed, dry-roasted, 1 ounce | 0.1 | 6 |
| Raisins, seedless, ½ cup | 0.1 | 6 |
| Onions, chopped, ½ cup | 0.1 | 6 |
| Spinach, frozen, chopped, boiled, ½ cup | 0.1 | 6 |
| Tofu, raw, firm, prepared with calcium sulfate, ½ cup | 0.1 | 6 |
| Watermelon, raw, 1 cup | 0.1 | 6 |
Footnote: *DV = Daily Value. The U.S. Food and Drug Administration (FDA) developed DVs to help consumers compare the nutrient contents of foods and dietary supplements within the context of a total diet. The DV for vitamin B6 is 1.7 mg for adults and children age 4 years and older. FDA does not require food labels to list vitamin B6 content unless vitamin B6 has been added to the food. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
[Source 50 ]Vitamin D
Vitamin D also known as “calciferol”, is fat-soluble vitamin you need for good health. Vitamin D helps your body absorb calcium, one of the main building blocks for strong bones. Together with calcium, vitamin D helps protect you from developing osteoporosis, a disease that thins and weakens your bones and makes them more likely to break. Without sufficient vitamin D, bones can become thin, brittle, or misshapen. Vitamin D sufficiency prevents rickets in children and osteomalacia in adults 55. Your body needs vitamin D for other functions too. Your muscles need it to move, and your nerves need it to carry messages between your brain and your body. Your immune system also need vitamin D to fight off invading bacteria and viruses. Many genes encoding proteins that regulate cell proliferation, differentiation, and apoptosis are modulated in part by vitamin D 56. Vitamin D is also involved in various brain processes, and vitamin D receptors are present on neurons and glia in areas of the brain thought to be involved in the pathophysiology of depression 11.
Vitamin D is naturally present in a few foods, added to others, and available as a dietary supplement. In foods and dietary supplements, vitamin D has two main forms, vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol), that differ chemically only in their side-chain structures 55. Both forms are well absorbed in the small intestine. Absorption occurs by simple passive diffusion and by a mechanism that involves intestinal membrane carrier proteins 57. The concurrent presence of fat in the gut enhances vitamin D absorption, but some vitamin D is absorbed even without dietary fat. Neither aging nor obesity alters vitamin D absorption from the gut 57.
Vitamin D is also produced by your body when ultraviolet (UV) rays from sunlight strike your skin and trigger vitamin D synthesis. Vitamin D obtained from sun exposure, foods, and supplements is biologically inert and must undergo two hydroxylations in your body for activation. The first hydroxylation, which occurs in the liver, converts vitamin D to 25-hydroxyvitamin D [25(OH)D], also known as “calcidiol.” The second hydroxylation occurs primarily in the kidney and forms the physiologically active 1,25-dihydroxyvitamin D [1,25(OH)2D], also known as “calcitriol” 58. Many tissues have vitamin D receptors, and some convert 25(OH)D to 1,25(OH)2D.
Serum concentration of 25-hydroxyvitamin D [25(OH)D] is currently the main indicator of vitamin D status. It reflects vitamin D produced endogenously and that obtained from foods and supplements 58. In serum, 25(OH)D has a fairly long circulating half-life of 15 days 58. Serum concentrations of 25-hydroxyvitamin D [25(OH)D] are reported in both nanomoles per liter (nmol/L) and nanograms per milliliter (ng/mL). One nmol/L is equal to 0.4 ng/mL, and 1 ng/mL is equal to 2.5 nmol/L.
Researchers have not definitively identified serum concentrations of 25-hydroxyvitamin D [25(OH)D] associated with vitamin D deficiency (e.g., rickets), adequacy for bone health, and overall health. After reviewing data on vitamin D needs, an expert committee of the Food and Nutrition Board at the National Academies of Sciences, Engineering, and Medicine concluded that people are at risk of vitamin D deficiency at serum 25-hydroxyvitamin D [25(OH)D] concentrations less than 30 nmol/L (12 ng/mL; see Table 6 for definitions of “deficiency” and “inadequacy”) 58. Some people are potentially at risk of inadequacy at 30 to 50 nmol/L (12–20 ng/mL). Levels of 50 nmol/L (20 ng/mL) or more are sufficient for most people. In contrast, the Endocrine Society stated that, for clinical practice, a serum 25(OH)D concentration of more than 75 nmol/L (30 ng/mL) is necessary to maximize the effect of vitamin D on calcium, bone, and muscle metabolism 59. The Food and Nutrition Board committee also noted that serum concentrations greater than 125 nmol/L (50 ng/mL) can be associated with adverse effects (Table 6).
Optimal serum concentrations of 25-hydroxyvitamin D [25(OH)D] for bone and general health have not been established because they are likely to vary by stage of life, by race and ethnicity, and with each physiological measure used 60. In addition, although 25-hydroxyvitamin D [25(OH)D] levels rise in response to increased vitamin D intake, the relationship is nonlinear 61. The amount of increase varies, for example, by baseline serum levels and duration of supplementation.
Table 5. Recommended Dietary Allowances (RDAs) for Vitamin D
| Age | Male | Female | Pregnancy | Lactation |
| 0-12 months* | 10 mcg (400 IU) | 10 mcg (400 IU) | ||
| 1–13 years | 15 mcg (600 IU) | 15 mcg (600 IU) | ||
| 14–18 years | 15 mcg (600 IU) | 15 mcg (600 IU) | 15 mcg (600 IU) | 15 mcg (600 IU) |
| 19–50 years | 15 mcg (600 IU) | 15 mcg (600 IU) | 15 mcg (600 IU) | 15 mcg (600 IU) |
| 51–70 years | 15 mcg (600 IU) | 15 mcg (600 IU) | ||
| >70 years | 20 mcg (800 IU) | 20 mcg (800 IU) |
Footnotes:
Recommended Dietary Allowance (RDA): Average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals.
*Adequate Intake (AI): Intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an RDA.
[Source 58 ]Table 6. Serum 25-Hydroxyvitamin D [25(OH)D] Concentrations and Health
| nmol/L** | ng/mL* | Health status |
|---|---|---|
| <30 | <12 | Associated with vitamin D deficiency, leading to rickets in infants and children and osteomalacia in adults |
| 30 to <50 | 12 to <20 | Generally considered inadequate for bone and overall health in healthy individuals |
| ≥50 | ≥20 | Generally considered adequate for bone and overall health in healthy individuals |
| >125 | >50 | Emerging evidence links potential adverse effects to such high levels, particularly >150 nmol/L (>60 ng/mL) |
Footnotes:
* Serum concentrations of 25(OH)D are reported in both nanomoles per liter (nmol/L) and nanograms per milliliter (ng/mL).
** 1 nmol/L = 0.4 ng/mL and 1 ng/mL = 2.5 nmol/L.
A systematic review and meta-analysis of 14 observational studies that included a total of 31,424 adults (mean age ranging from 27.5 to 77 years) found an association between deficient or low levels of 25-hydroxyvitamin D [25(OH)D] and depression 11. Clinical trials, however, do not support these findings. For example, a meta-analysis of 9 trials with a total of 4,923 adult participants diagnosed with depression or depressive symptoms found no significant reduction in symptoms after supplementation with vitamin D 62. The trials administered different amounts of vitamin D (ranging from 10 mcg [400 IU]/day to 1,000 mcg [40,000 IU]/week). They also had different study durations (5 days to 5 years), mean participant ages (range, 22 years to 75 years), and baseline 25-hydroxyvitamin D [25(OH)D] levels; furthermore, some but not all studies administered concurrent antidepressant medications.
Three trials conducted since that meta-analysis also found no effect of vitamin D supplementation on depressive symptoms. One trial included 206 adults (mean age 52 years) who were randomized to take a bolus dose of 2,500 mcg (100,000 IU) vitamin D3 followed by 500 mcg (20,000 IU)/week or a placebo for 4 months 63. Most participants had minimal or mild depression, had a low mean baseline 25(OH) level of 33.8 nmol/L (13.5 ng/mL), and were not taking antidepressants. The second trial included 155 adults aged 60–80 years who had clinically relevant depressive symptoms, no major depressive disorder, and serum 25(OH)D levels less than 50 to 70 nmol/L (20 to 28 ng/mL) depending on the season; in addition, they were not taking antidepressants 64, 65. Participants were randomized to take either 30 mcg (1,200 IU)/day vitamin D3 or a placebo for 1 year. In the VITAL trial described above, 16,657 men and women 50 years of age and older with no history of depression and 1,696 with an increased risk of recurrent depression (that had not been medically treated for the past 2 years) were randomized to take 50 mcg (2,000 IU)/day vitamin D3 (with or without fish oil) or a placebo for a median of 5.3 years 66. The groups showed no significant differences in the incidence and recurrent rates of depression, clinically relevant depressive symptoms, or changes in mood scores.
Overall, clinical trials did not find that vitamin D supplements helped prevent or treat depressive symptoms or mild depression, especially in middle-aged to older adults who were not taking prescription antidepressants. No studies have evaluated whether vitamin D supplements may benefit individuals under medical care for clinical depression who have low or deficient 25-hydroxyvitamin D [25(OH)D] levels and are taking antidepressant medication.
Table 7. Vitamin D content of selected foods
| Food | Micrograms (mcg) per serving | International Units (IU) per serving | Percent DV* |
| Cod liver oil, 1 tablespoon | 34 | 1360 | 170 |
| Trout (rainbow), farmed, cooked, 3 ounces | 16.2 | 645 | 81 |
| Salmon (sockeye), cooked, 3 ounces | 14.2 | 570 | 71 |
| Mushrooms, white, raw, sliced, exposed to UV light, ½ cup | 9.2 | 366 | 46 |
| Milk, 2% milkfat, vitamin D fortified, 1 cup | 2.9 | 120 | 15 |
| Soy, almond, and oat milks, vitamin D fortified, various brands, 1 cup | 2.5-3.6 | 100-144 | 13-18 |
| Ready-to-eat cereal, fortified with 10% of the DV for vitamin D, 1 serving | 2 | 80 | 10 |
| Sardines (Atlantic), canned in oil, drained, 2 sardines | 1.2 | 46 | 6 |
| Egg, 1 large, scrambled** | 1.1 | 44 | 6 |
| Liver, beef, braised, 3 ounces | 1 | 42 | 5 |
| Tuna fish (light), canned in water, drained, 3 ounces | 1 | 40 | 5 |
| Cheese, cheddar, 1 ounce | 0.3 | 12 | 2 |
| Mushrooms, portabella, raw, diced, ½ cup | 0.1 | 4 | 1 |
| Chicken breast, roasted, 3 ounces | 0.1 | 4 | 1 |
| Beef, ground, 90% lean, broiled, 3 ounces | 0 | 1.7 | 0 |
| Broccoli, raw, chopped, ½ cup | 0 | 0 | 0 |
| Carrots, raw, chopped, ½ cup | 0 | 0 | 0 |
| Almonds, dry roasted, 1 ounce | 0 | 0 | 0 |
| Apple, large | 0 | 0 | 0 |
| Banana, large | 0 | 0 | 0 |
| Rice, brown, long-grain, cooked, 1 cup | 0 | 0 | 0 |
| Whole wheat bread, 1 slice | 0 | 0 | 0 |
| Lentils, boiled, ½ cup | 0 | 0 | 0 |
| Sunflower seeds, roasted, ½ cup | 0 | 0 | 0 |
| Edamame, shelled, cooked, ½ cup | 0 | 0 | 0 |
Footnotes:
* DV = Daily Value. The FDA developed DVs to help consumers compare the nutrient contents of foods and dietary supplements within the context of a total diet. The DV for vitamin D is 20 mcg (800 IU) for adults and children aged 4 years and older. The labels must list vitamin D content in mcg per serving and have the option of also listing the amount in IUs in parentheses. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
** Vitamin D is in the yolk.
[Source 67 ]Omega 3 fatty acids (fish oil)
Omega-3 fatty acids are found in foods, such as fish and flaxseed, and in dietary supplements, such as fish oil 68. The three main omega-3 fatty acids are alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). Alpha-linolenic acid (ALA) is found mainly in plant oils such as flaxseed, soybean, and canola oils 69. DHA (docosahexaenoic acid) and EPA (eicosapentaenoic acid) are present in fish, fish oils, krill oils and other seafood, but they are originally synthesized by microalgae, not by the fish. When fish consume phytoplankton that consumed microalgae, they accumulate the omega-3s in their tissues 69.
Alpha-linolenic acid (ALA) is an essential fatty acid, meaning that your body can’t make it, so you must get it from the foods and beverages you consume. Your body can convert some alpha-linolenic acid (ALA) into EPA (eicosapentaenoic acid) and then to DHA (docosahexaenoic acid), but only in very small amounts 68. Therefore, getting DHA (docosahexaenoic acid) and EPA (eicosapentaenoic acid) from foods (and dietary supplements if you take them) is the only practical way to increase levels of these omega-3 fatty acids in your body.
Omega-3s are important components of the membranes that surround each cell in your body. DHA (docosahexaenoic acid) levels are especially high in retina (eye), brain, and sperm cells. Omega-3s also provide calories to give your body energy and have many functions in your heart, blood vessels, lungs, immune system, and endocrine system (the network of hormone-producing glands).
A deficiency of omega-3s can cause rough, scaly skin and a red, swollen, itchy rash. Omega-3 deficiency is very rare in the United States. According to data from the 2011–2012 National Health and Nutrition Examination Survey (NHANES), most children and adults in the United States consume recommended amounts of omega-3s as ALA 70. Among children and teens aged 2–19 the average daily ALA intake from foods is 1.32 g for females and 1.55 g for males. In adults aged 20 and over, the average daily ALA intake from foods is 1.59 g in females and 2.06 g in males.
Consumption of DHA and EPA from foods contributes a very small amount to total daily omega-3 intakes (about 40 mg in children and teens and about 90 mg in adults) 70. Use of dietary supplements containing omega-3s also contributes to total omega-3 intakes. Fish oil is one of the most commonly used nonvitamin/nonmineral dietary supplements by U.S. adults and children 71, 72. Data from the 2012 National Health Interview Survey indicate that 7.8% of U.S. adults and 1.1% of U.S. children use supplements containing fish oil, omega-3s, and/or DHA or EPA 71, 72. According to an analysis of 2003–2008 NHANES data, use of these supplements adds about 100 mg to mean daily ALA intakes, 10 mg to mean DHA intakes, and 20 mg to mean EPA intakes in adults 73.
A deficiency of essential fatty acids—either omega-3s or omega-6s—can cause rough, scaly skin and dermatitis 74. Plasma and tissue concentrations of DHA decrease when an omega-3 fatty acid deficiency is present. However, there are no known cut-off concentrations of DHA or EPA below which functional endpoints, such as those for visual or neural function or for immune response, are impaired.
It’s uncertain whether omega-3 fatty acid supplements are helpful for depression. Although some studies have found conflicting evidence, a high-quality 2015 Cochrane review of 26 studies that included more than 1,400 people on supplementation with omega-3 fatty acids (1,000 to 6,600 mg/day EPA, DHA, and/or other omega-3s) versus placebo in patients with major depression concluded that if there is a small-to-modest beneficial effect on depressive symptoms, but it may be too small to be meaningful 75. One additional randomized controlled trial with a very low sample size showed similar effects of omega-3 fatty acids on severity and response rates in comparison with antidepressant drug treatment 76. Other analyses have suggested that if omega-3s do have an effect, EPA may be more beneficial than DHA and that omega-3s may best be used in addition to antidepressant medication rather than in place of it 77. However, all meta-analyses were based on very low quality of evidence because of limitations of the study quality, significant heterogeneity, imprecision and a possibly high risk of publication bias. Furthermore, omega-3s have not been shown to relieve symptoms of depression that occur during pregnancy or after childbirth.
A 2016 meta-analysis of 26 studies found a 17% lower risk of depression with higher fish intake 78.
Table 8. Recommended daily intake for Omega-3s
| Age | Male | Female | Pregnancy | Lactation |
| Birth to 6 months* | 0.5 g | 0.5 g | ||
| 7–12 months* | 0.5 g | 0.5 g | ||
| 1–3 years** | 0.7 g | 0.7 g | ||
| 4–8 years** | 0.9 g | 0.9 g | ||
| 9–13 years** | 1.2 g | 1.0 g | ||
| 14–18 years** | 1.6 g | 1.1 g | 1.4 g | 1.3 g |
| 19-50 years** | 1.6 g | 1.1 g | 1.4 g | 1.3 g |
| 51+ years** | 1.6 g | 1.1 g |
Footnotes:
*As total omega-3s
**As alpha-linolenic acid (ALA)
[Source 74 ]Table 9. Omega-3 content of selected foods
| Food | Grams per serving | ||
| ALA (alpha-linolenic acid) | DHA (docosahexaenoic acid) | EPA (eicosapentaenoic acid) | |
| Flaxseed oil, 1 tbsp | 7.26 | ||
| Chia seeds, 1 ounce | 5.06 | ||
| English walnuts, 1 ounce | 2.57 | ||
| Flaxseed, whole, 1 tbsp | 2.35 | ||
| Salmon, Atlantic, farmed cooked, 3 ounces | 1.24 | 0.59 | |
| Salmon, Atlantic, wild, cooked, 3 ounces | 1.22 | 0.35 | |
| Herring, Atlantic, cooked, 3 ounces* | 0.94 | 0.77 | |
| Canola oil, 1 tbsp | 1.28 | ||
| Sardines, canned in tomato sauce, drained, 3 ounces* | 0.74 | 0.45 | |
| Mackerel, Atlantic, cooked, 3 ounces* | 0.59 | 0.43 | |
| Salmon, pink, canned, drained, 3 ounces* | 0.04 | 0.63 | 0.28 |
| Soybean oil, 1 tbsp | 0.92 | ||
| Trout, rainbow, wild, cooked, 3 ounces | 0.44 | 0.4 | |
| Black walnuts, 1 ounce | 0.76 | ||
| Mayonnaise, 1 tbsp | 0.74 | ||
| Oysters, eastern, wild, cooked, 3 ounces | 0.14 | 0.23 | 0.3 |
| Sea bass, cooked, 3 ounces* | 0.47 | 0.18 | |
| Edamame, frozen, prepared, ½ cup | 0.28 | ||
| Shrimp, cooked, 3 ounces* | 0.12 | 0.12 | |
| Refried beans, canned, vegetarian, ½ cup | 0.21 | ||
| Lobster, cooked, 3 ounces* | 0.04 | 0.07 | 0.1 |
| Tuna, light, canned in water, drained, 3 ounces* | 0.17 | 0.02 | |
| Tilapia, cooked, 3 ounces* | 0.04 | 0.11 | |
| Scallops, cooked, 3 ounces* | 0.09 | 0.06 | |
| Cod, Pacific, cooked, 3 ounces* | 0.1 | 0.04 | |
| Tuna, yellowfin, cooked 3 ounces* | 0.09 | 0.01 | |
| Kidney beans, canned ½ cup | 0.1 | ||
| Baked beans, canned, vegetarian, ½ cup | 0.07 | ||
| Ground beef, 85% lean, cooked, 3 ounces** | 0.04 | ||
| Bread, whole wheat, 1 slice | 0.04 | ||
| Egg, cooked, 1 egg | 0.03 | ||
| Chicken, breast, roasted, 3 ounces | 0.02 | 0.01 | |
| Milk, low-fat (1%), 1 cup | 0.01 | ||
Footnotes:
*Except as noted, the USDA database does not specify whether fish are farmed or wild caught.
**The USDA database does not specify whether beef is grass fed or grain fed.
The U.S. Department of Agriculture’s FoodData Central (https://fdc.nal.usda.gov) lists the nutrient content of many foods and provides a comprehensive list of foods containing alpha-linolenic acid (ALA) arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/ALA-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/ALA-Food.pdf), foods containing DHA (docosahexaenoic acid) arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/DHA-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/DHA-Food.pdf), and foods containing EPA (eicosapentaenoic acid) arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/EPA-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/EPA-Food.pdf).
[Source 67 ]St John’s Wort
St. John’s wort also known as Hypericum perforatum, Klamath weed or goat weed, is a perennial flowering shrub native to Europe and Asia and was brought to the United States by European colonists. The flowers and leaves of St. John’s wort contain active ingredients such as hyperforin. The name St. John’s wort apparently refers to John the Baptist, as the plant blooms around the time of the feast of St. John the Baptist in late June (June 24). It is also said its red pigment symbolizes the blood of St. John. The word wort is an Old English term for plant. Hypericum is derived from the Greek words hyper (above) and eikon (icon or image). Historically, St. John’s wort has been used for a variety of conditions, including kidney and lung ailments, insomnia, and depression, and to aid wound healing. Currently, St. John’s wort is promoted for depression, menopausal symptoms, attention-deficit hyperactivity disorder (ADHD), somatic symptom disorder (a condition in which a person feels extreme, exaggerated anxiety about physical symptoms), obsessive-compulsive disorder, and other conditions. Topical use (applied to the skin) of St. John’s wort is promoted for various skin conditions, including wounds, bruises, and muscle pain.
There has been extensive research on the use of St. John’s wort for depression and on its interactions with medications. Several studies support the therapeutic benefit of St. John’s wort in treating mild to moderate depression 79, 80, 81, 76, 82. In fact, some research has shown St. John’s wort supplement to be as effective as several prescription antidepressants 81. In comparison with standard antidepressants, St. John’s wort showed comparable severity reductions, response, remission, and relapse rates 76. However, it’s unclear whether St. John’s wort is beneficial in the treatment of severe depression and for time periods longer than 12 weeks 83. While there may be public interest in St. John’s wort to treat depression, the U.S. Food and Drug Administration (FDA) has not approved its use as an over-the-counter or prescription medicine for depression. In research studies, taking St. John’s wort by mouth for up to 12 weeks has seemed to be safe. But because St. John’s wort interacts with many medications, it might not be safe for many people, especially those who take conventional medicines, particularly if you take any prescription drugs 84. St. John’s wort is known to affect metabolism of a number of drugs and can cause serious side effects. Combining St. John’s wort with certain antidepressants can lead to a potentially life-threatening increase of serotonin, a brain chemical targeted by antidepressants. St. John’s wort can also limit the effectiveness of many prescription medicines, including birth control pills, digoxin, some HIV drugs and cancer medications, and others 85.
St. John’s wort can also weaken the effects of many medicines, including crucially important medicines such as:
- Antidepressants
- Birth control pills
- Cyclosporine, which prevents the body from rejecting transplanted organs
- Some heart medications, including digoxin and ivabradine
- Some HIV drugs, including indinavir and nevirapine
- Some cancer medications, including irinotecan and imatinib
- Warfarin, an anticoagulant (blood thinner)
- Certain statins, including simvastatin.
Little safety information on St. John’s wort for pregnant women, during breastfeeding or in children is available, so it is especially important to talk with health experts if you are pregnant or nursing or are considering giving a dietary supplement to a child. St. John’s wort has caused birth defects in laboratory animals. Breastfeeding infants of mothers who take St. John’s wort can experience colic, drowsiness, and fussiness.
St. John’s wort may cause increased sensitivity to sunlight, especially when taken in large doses. Other side effects can include insomnia, anxiety, dry mouth, dizziness, gastrointestinal symptoms, fatigue, headache, or sexual dysfunction. Therefore, if you consider using St. John’s wort for depression, you should seek professional advise 86. Clinical studies on the use of St. John’s wort for depression have utilized liquid tinctures and standardized solid extracts (0.3% hypericin—300 mg three times a day) 87.
5 HTP
5-HTP also known as 5-hydroxytryptophan, is a chemical that your body makes from tryptophan (an essential amino acid that you get from food) 88. You can’t get 5-HTP from food. The essential amino acid tryptophan (L-tryptophan), which your body uses to make 5-HTP, can be found in turkey, chicken, milk, potatoes, pumpkin, sunflower seeds, turnip and collard greens, and seaweed. However, eating foods with tryptophan does not increase 5-HTP levels very much. After tryptophan is converted into 5-HTP, the chemical is changed into another chemical called serotonin also known as 5-hydroxytryptamine (a neurotransmitter that relays signals between brain cells). 5-HTP dietary supplements help raise serotonin levels in the brain. Since serotonin helps regulate mood and behavior, 5-HTP may have a positive effect on sleep, mood, anxiety, appetite, and pain sensation.
As a supplement, 5-HTP is commercially produced by extraction from the seeds of the African plant called Griffonia simplicifolia. In the United States 5-HTP is regulated as a food supplement. There is no U.S. Food and Drug Administration (FDA)-approved drug on the market containing 5-HTP as an active pharmaceutical ingredient 89. According to Jacobsen et al 89, no dosage form of 5-HTP has ever been formally developed as a drug and approved by a regulatory body for the treatment of a disease.
Therapeutic use of 5-HTP bypasses the conversion of tryptophan (L-tryptophan) into 5-HTP by the enzyme tryptophan hydrolase, which is the rate-limiting step in the synthesis of serotonin 88. Tryptophan hydrolase can be inhibited by numerous factors, including stress, insulin resistance, vitamin B6 deficiency, and insufficient magnesium 88. In addition, these same factors can increase the conversion of tryptophan to kynurenine via tryptophan oxygenase, making tryptophan unavailable for serotonin production.
Serotonin levels in the brain are highly dependent on levels of 5-HTP and L-tryptophan in the central nervous system (brain and spinal cord) 88. 5-HTP easily crosses the blood-brain barrier, not requiring the presence of a transport molecule. Tryptophan, on the other hand, requires use of a transport molecule to gain access to the central nervous system. Since it shares this transport molecule with several other amino acids, the presence of these competing amino acids can inhibit L-tryptophan transport into the brain.
5-HTP acts primarily by increasing levels of serotonin within the central nervous system. Other neurotransmitters and central nervous system chemicals, such as melatonin, dopamine, norepinephrine, and beta-endorphin have also been shown to increase following oral administration of 5-HTP 90.
The use of the essential amino acid L-tryptophan as a dietary supplement was discontinued in 1989 due to an outbreak of eosinophilia-myalgia syndrome (EMS) that was traced to a contaminated synthetic L-tryptophan from a single manufacturer. In 1989, the presence of a contaminant called Peak X was found in tryptophan supplements. Researchers believed that an outbreak of eosinophilic myalgia syndrome (a potentially fatal disorder that affects the skin, blood, muscles, and organs) could be traced to the contaminated tryptophan, and the FDA pulled all tryptophan supplements off the market. Since then, Peak X was also found in some 5-HTP supplements, and there have been a few reports of eosinophilic myalgia syndrome associated with taking 5-HTP. However, the level of Peak X in 5-HTP was not high enough to cause any symptoms, unless very high doses of 5-HTP were taken. Because of this concern, however, you should talk to your health care provider before taking 5-HTP, and make sure you get the supplement from a reliable manufacturer.
The primary concern regarding 5-HTP is the possibility of an eosinophilia-myalgia syndrome (EMS) similar to the illness linked to contaminated L-tryptophan. The contamination identified in certain batches of L-tryptophan has been related to production methods using bacterial fermentation and subsequent inadequate filtration. This is unlikely to occur with 5-HTP, since it is produced by extraction from plant sources. Two cases of eosinophilia-myalgia syndrome-like symptoms have been described in patients taking 5-HTP. One case reported in 1980 involved the use of very high doses (1400 mg daily) 91. Because contamination of L-tryptophan was not identified as a factor in eosinophilia-myalgia syndrome (EMS) until 1990, the product consumed by this patient was not tested for contamination. The second case involved a mother and two children who were confirmed to have taken contaminated 5-HTP 92.
5-HTP has since become a popular dietary supplement in lieu of the removal of L-tryptophan from the market. Because of its chemical and biochemical relationship to L-tryptophan, 5-HTP has been under vigilance by consumers, industry, academia and government for its safety. However, no definitive cases of toxicity have emerged despite the worldwide usage of 5-HTP for last 20 years, with the possible exception of one unresolved case of a Canadian woman. Extensive analyses of several sources of 5-HTP have shown no toxic contaminants similar to those associated with L-tryptophan, nor the presence of any other significant impurities.
5-HTP has shown promising antidepressant effects, but poor pharmacokinetics limits the therapeutic potential 93. A slow-release delivery mode would be predicted to overcome the pharmacokinetic limitations of 5-HTP, substantially enhance the pharmacological action, and transform 5-HTP into a clinically viable drug.
Native 5-HTP immediate release is a poor serotonergic antidepressant. As discussed below, effective antidepressant therapy requires sustained, minimally fluctuating serotonin (5-HT) elevation 94. A half life (T1/2) = 2 hours means that even at thrice-daily dosing 5-HTP plasma levels will fluctuate at least 5-fold at steady-state. This contrasts to the less than 0.3-fold steady-state plasma fluctuations of most SSRIs 95 (Figure 2). Furthermore, 5-HTP’s fast-onset adverse events likely results from the rapid absorption and resultant serotonin (5-HT) spikes upon administration. Co-administering a peripheral amino acid decarboxylase inhibitor (DCI) (e.g. carbidopa) with 5-HTP will modestly extend the T1/2, several-fold enhance exposure, and not affect TMax 96. Including a peripheral amino acid decarboxylase inhibitor (DCI) in a 5-HTP SR (slow release) drug could be beneficial, but could complicate formulation development, dosing, and safety.
5-HTP may help treat a wide variety of conditions related to low serotonin levels, including the following:
- Depression: Preliminary studies indicate that 5-HTP may work as well as certain antidepressant drugs to treat people with mild-to-moderate depression 97, 98, 99, 100, 101. Like the class of antidepressants known as selective serotonin reuptake inhibitors (SSRIs), which includes fluoxetine (Prozac) and sertraline (Zoloft), 5-HTP increases the levels of serotonin in the brain. One study compared the effects of 5-HTP to fluvoxamine (Luvox) in 63 people and found that those who were given 5-HTP did just as well as those who received Luvox. They also had fewer side effects than the Luvox group. However, these studies were too small to say for sure if 5-HTP works. More research is needed.
- Fibromyalgia: Research suggests that 5-HTP can improve symptoms of fibromyalgia, including pain, anxiety, morning stiffness, and fatigue. Many people with fibromyalgia have low levels of serotonin, and doctors often prescribe antidepressants. Like antidepressants, 5-HTP raises levels of serotonin in the brain. However, it does not work for all people with fibromyalgia. More studies are needed to understand its effect.
- Insomnia: In one study, people who took 5-HTP went to sleep quicker and slept more deeply than those who took a placebo. Researchers recommend 200 to 400 mg at night to stimulate serotonin, but it may take 6 to 12 weeks to be fully effective.
- Migraines and other headaches: Antidepressants are sometimes prescribed for migraine headaches. Studies suggest that high doses of 5-HTP may help people with various types of headaches, including migraines. However, the evidence is mixed, with other studies showing no effect.
- Obesity: A few small studies have investigated whether 5-HTP can help people lose weight. In one study, those who took 5-HTP ate fewer calories, although they were not trying to diet, compared to those who took placebo. Researchers believe 5-HTP led people to feel more full (satiated) after eating, so they ate less. A follow-up study, which compared 5-HTP to placebo during a diet and non-diet period, found that those who took 5-HTP lost about 2% of body weight during the non-diet period and another 3% when they dieted. Those taking placebo did not lose any weight. However, doses used in these studies were high, and many people experienced side effects such as nausea. If you are seriously overweight, see your health care provider before taking any weight-loss aid. Remember that you will need to change your eating and exercise habits to lose more than a few pounds.
Initial dosage for 5-HTP is usually 50 mg three times a day with meals. If clinical response is inadequate after two weeks, dosage may be increased to 100 mg three times a day 102. For insomnia, the dosage is usually 100-300 mg before bedtime. Because some patients may experience mild nausea when initiating treatment with 5-HTP, it is advisable to begin with 50 mg doses and titrate upward 88.
Side effects of 5-HTP are generally mild and may include nausea, heartburn, gas, feelings of fullness, and rumbling sensations in some people. At high doses, serotonin syndrome, a dangerous condition caused by too much serotonin in the body, could develop. Talk to your doctor before taking higher-than-recommended doses. People with high blood pressure or diabetes should talk to their doctor before taking 5-HTP.
People with liver disease, pregnant women, and women who are breastfeeding should not take 5-HTP.
Do NOT take 5-HTP without medical advice if you are using any of the following medications:
- An antidepressant;
- Carbidopa: Taking 5-HTP with carbidopa, a medication used to treat Parkinson disease, may cause a scleroderma-like illness. Scleroderma is a condition where the skin becomes hard, thick, and inflamed.
- Narcotic medicine;
- Tramadol: Tramadol is used for pain relief and sometimes prescribed for people with fibromyalgia, may raise serotonin levels too much if taken with 5-HTP. Serotonin syndrome has been reported in some people taking the two together.
- Cough medicine that contains dextromethorphan: Taking 5-HTP with dextromethorphan, found in cough syrups, may cause serotonin levels to increase to dangerous levels, a condition called serotonin syndrome.
- Meperidine (Demerol): Taking 5-HTP with Demerol may cause serotonin levels to increase to dangerous levels, a condition called serotonin syndrome.
- Triptans (used to treat migraines): 5-HTP can increase the risk of side effects, including serotonin syndrome, when taken with these medications:
- Naratriptan (Amerge)
- Rizatriptan (Maxalt)
- Sumatriptan (Imitrex)
- Zolmitriptan (Zomig)
Some antidepressant medications that can interact with 5-HTP include:
- SSRIs: Citalopram (Celexa), escitalopram (Lexapro), fluvoxamine (Luvox), paroxetine (Paxil), fluoxetine (Prozac), sertraline (Zoloft)
- Tricyclics: Amitriptyline (Elavil), nortriptyline (Pamelor), imipramine (Tofranil)
- Monoamine oxidase inhibitors (MAOIs): Phenelzine, (Nardil), tranylcypromine (Parnate)
- Nefazodone (Serzone)
If you take antidepressants, you should not take 5-HTP without your doctor’s supervision. These medications could combine with 5-HTP to cause serotonin syndrome, a dangerous condition involving mental changes, hot flashes, rapidly fluctuating blood pressure and heart rate, and possibly coma.
This list is not complete. Other drugs may interact with 5-HTP, including prescription and over-the-counter medicines, vitamins, and herbal products. Not all possible interactions are listed here.
Figure 1. Synthesis of 5-HTP from tryptophan
Footnotes: (A) 5-hydroxytryptamine (serotonin) metabolic pathway. Synthesis of 5-HTP from tryptophan via TPH 1 (periphery) or TPH 2 (CNS) is the rate-limiting step in 5-HT synthesis. 5-HTP is rapidly converted to 5-hydroxytryptamine (serotonin) by the ubiquitous enzyme amino acid decarboxylase. 5-hydroxytryptamine (serotonin) is metabolized to 5-HIAA, 5-hydroxytryptamine (serotonin) main metabolite, by monoamine oxidase. (B) Simplified schematic of regulatory elements of CNS 5-HTExt. Drugs interacting with each element are indicated. (C) Schematic for adjunct 5-HTP SR mechanism-of-action. Adjunct exogenous 5-HTP increases endogenous 5-hydroxytryptamine (serotonin) synthesis, increasing availability of 5-hydroxytryptamine (serotonin) for net release by concomitant SERT inhibitor treatment.
Abbreviations: 5-HT = 5-hydroxytryptamine (serotonin); 5-HTExt = extracellular 5-HT; 5-HTP = 5-hydroxytryptopan; 5-HIAA = 5-hydroxyindoleacetic acid; AADC = aromatic L-amino acid decarboxylase, also known as DOPA decarboxylase; MOA = monoamine oxidase; MOA I = monoamine oxidase inhibitor; SERT= serotonin transporter; SERT I = serotonin transporter inhibitor; SR = slow-release; TPH = tryptophan hydroxylase.
[Source 103]Figure 2. 5-HTP pharmacokinetics simulation
Footnotes: Pharmacokinetics simulation using one-compartment modeling and published human pharmacokinetic parameters for 5-HTP immediate release (IR) 96 and the canonical SSRI escitalopram 104. Even at thrice-daily dosing at 8 hour intervals, an unrealistic level of adherence in outpatients, 5-HTP plasma levels will fluctuate 5-fold between doses. In contrast, during steady-state once-daily dosing of escitalopram, plasma escitalopram levels will fluctuate only about 0.3-fold. Also shown are 5-HTP plasma levels obtained during steady-state dosing with an ideal 5-HTP slow release (SR) dosage form producing zero-order, constant, 5-HTP delivery.
[Source 103]Tryptophan
Tryptophan also known as L-tryptophan, is an essential amino acid which means your body cannot produce it, so you must get it from your diet (see Tables 10 and 11 below) 105. Some common sources of tryptophan are oats, bananas, dried prunes, milk, tuna fish, cheese, bread, chicken, turkey, peanuts, and chocolate (see Table 10). Tryptophan is an amino-acid which is probably most directly implicated in the etiopathogenesis of depression 2. Under physiological conditions, tryptophan is converted to 5-HTP (5-hydroxy-tryptophan), 5-HTP is then changed into another chemical called serotonin also known as 5-hydroxytryptamine (5-HT) a neurotransmitter that relays signals between brain cells and is a neurotransmitter essential in regulating appetite, sleep, mood, and pain 106. Tryptophan’s primary mechanism of action is its role as the metabolic precursor of the neurotransmitter serotonin. Other neurotransmitters and central nervous system (CNS) chemicals, such as melatonin, dopamine, norepinephrine, and beta-endorphin, have also been shown to increase following oral administration of tryptophan 90, 107.
Tryptophan depletion reduces 5-HT synthesis. The intensity of depressive symptoms correlates with the level of tryptophan depletion during antidepressant treatment 108. A meta-analysis performed by Ogawa et al. 109 demonstrated reduced tryptophan levels in the plasma of patients with major depressive disorder. Decreased tryptophan could be a specific marker for major depressive disorder and bipolar disorder 110, and may play a central role in the pathophysiology of depression. It has been proven that injection of L-tryptophan modifies brain serotonin levels in rats 111. On the other hand, injection of branched-chain amino-acids (valine, leucine, isoleucine), which compete with tryptophan, causes tryptophan and serotonin depletion, and eventually, lowered mood 112. A decrease in branched chain amino-acids following antidepressant treatment correlates with clinical improvement 113.
Experimental research has shown that tryptophan can be an important determinant of mood, cognition, and behavior. Although results have been inconsistent, clinical trials have provided some initial evidence of tryptophan’s efficacy for treatment of psychiatric disorders, particularly when used in combination with other therapeutic agents. Your liver can also use tryptophan to produce niacin (vitamin B3), which is needed for energy metabolism and DNA production. In order for tryptophan in the diet to be changed into niacin, the body needs to have enough iron, riboflavin and vitamin B6 114.
The body absorbs tryptophan, converts it to 5‐HTP then forms it into serotonin (5-HT), both centrally and peripherally 115. Both tryptophan and 5‐HTP are able to penetrate the blood‐brain barrier. 5‐HTP penetrates the brain and is converted to serotonin within serotonergic neurons, which is a neurotransmitter within dopaminergic and noradrenergic neurons 115. Therefore, depressed patients administered 5‐HTP or tryptophan should experience improvement. However clinical trials in which patients have been administered tryptophan or 5‐HTP have given conflicting results and reached differing conclusions. Some reviewers have found both substances to have an antidepressant effect 116, 117. Other reviewers have found the evidence supporting use of tryptophan and 5‐HTP for depression to be weak 118, 119.
Tryptophan is a natural sedative and present in many protein-based foods and dietary proteins such as in dairy products, meats, turkey, brown rice, fish, chocolate, oats, bananas, dried dates, milk, cottage cheese, peanuts and soybeans 120. Approximately 300 mg tryptophan is available in three ounces of turkey, lamb, beef, tuna, or peanuts 121. Relative to other amino acids, small amounts are needed to have a therapeutic effect, which is fortunate because tryptophan is the least abundant amino acid in the diet 122. In 1989, the importation of L-tryptophan was banned in the United States after cases of a deadly autoimmune illness called Eosinophilia‐Myalgia Syndrome (EMS) were traced to an improperly-prepared batch of tryptophan 123. Eosinophilia‐Myalgia Syndrome (EMS) affected nearly 1 500 tryptophan users in 1989 and led to over 30 deaths. Although the tryptophan was isolated to a single Japanese factory that allowed a toxic bacterial metabolite through the purification process 124, the ban was maintained and tryptophan availability was limited to the prescription drug (Tryptan), infant formulas, and enteral feeding products. Since 1994 tryptophan has been available and marketed as a dietary supplement in the United States, while imported product remains limited by special regulations 125. The nature of the tryptophan‐EMS association has not yet been fully elucidated. It is also possible it is a chance association only, it is due to excess tryptophan itself, or it is due to a combination of the impurity and excess tryptophan 126. A similar impurity has recently been identified in 5‐HTP. The significance of this is also unknown 127.
A normal Western diet contains about 0.5 gram of tryptophan daily, of which only 2‐3% is used in central serotonin production 119. Tryptophan is transported across the blood‐brain barrier by a carrier mechanism which also transports tyrosine, pheylalanine, leucine, isoleucine, and valine. Increase in dietary tryptophan increases the amount transported across the blood‐brain barrier. Increase in the other amino acids transported by the same carrier reduces the transport of tryptophan 128.
Levels of plasma tryptophan are determined by a balance between dietary intake 129 and its removal from the plasma as a part of its essential role in protein biosynthesis 130. Aside from its role in protein formation, tryptophan is a precursor for a number of metabolites, most notably kynurenine and the neurotransmitters, serotonin and melatonin. Melatonin is a hormone that is produced by the pineal gland in human, which regulates sleep and wakefulness. Serotonin is a brain neurotransmitter, platelet clotting factor, and neurohormone found in organs throughout your body. Metabolism of tryptophan into serotonin requires nutrients such as vitamin B6, niacin, and glutathione. Niacin (also known as vitamin B3) is an important metabolite of tryptophan. It is synthesized via kynurenine and quinolinic acids, which are products of tryptophan degradation 131.
For all amino acids, including L-tryptophan, only the L isomer is used in protein synthesis 132 and can pass across the blood-brain barrier 133. In humans, tryptophan has relatively low tissue storage 134 and the overall tryptophan concentration in the body is the lowest among all amino acids 135, although only small amounts are necessary for general healthy nutrition 136. While typical intake for many individuals is approximately 900 to 1000 mg daily, the recommended daily allowance for adults is estimated to be between 250 mg/day 136 and 425 mg/day 137, which translates to a dietary intake of 3.5 to 6.0 mg/kg of body weight per day.
As with other essential amino acids, tryptophan can contribute to hepatic biosynthesis of proteins; however, tryptophan is typically incorporated into proteins at only 1–2 % of total amino acids, making it the scarcest of amino acids in dietary proteins 138. Nevertheless, if tryptophan is acutely deficient, incorporation into protein synthesis will contribute to a substantial lowering of plasma tryptophan levels 139. However, in the absence of tryptophan deficiency, the majority of consumed tryptophan is metabolized via other pathways, including for synthesis of serotonin (5-HT), melatonin and niacin (vitamin B3). Indeed, it has been estimated that only 1 % of dietary tryptophan is used for brain serotonin (5-HT) synthesis 140.
The metabolism of tryptophan for the synthesis of serotonin (5-HT) is catalysed by the rate-limiting enzyme, tryptophan hydroxylase (TPH), which converts tryptophan into 5-hydroxytryptophan (5-HTP). In turn, 5-hydroxytryptophan is decarboxylated to serotonin (5-HT) by the enzyme aromatic amino acid decarboxylase.
An important consideration for understanding effects of tryptophan administration is that only about 5% of endogenous serotonin is found in the brain; the remainder is in the gut (about 90%), principally released by enterochromaffin cells, and in peripheral tissue or in the blood, where it is taken up into blood platelets 141.
Although reduced levels of serum tryptophan have been linked to some forms of depression, depression was not relieved with intravenous tryptophan 142. Numerous studies, however, have found tryptophan depletion produced depressive relapse and even initiation of depressive symptoms in healthy subjects 143, 144. Tryptophan supplementation has been employed as a potential treatment for depression and sleep disturbance since the early 1960s 145, although a moderate-quality Cochrane review of 108 trials (including for 5-HTP) for antidepressant effects in 2002 found that only two trials were of sufficient quality to be included 146. Nevertheless, on that limited evidence, tryptophan was considered to be better than placebo in alleviating depression, at least in mild to moderately depressed people 146. Thus, tryptophan has been used pharmacologically, i.e. at daily doses sometimes well in excess of ten times the RDA (5 mg/kg) for this essential amino acid. There was early evidence for probable enhancement of brain serotonin (5-HT) function: after 50 mg/kg tryptophan (3·5 grams per 70 kg subject) was consumed in a milk drink, plasma tryptophan increased 8-fold, tryptophan in cerebrospinal fluid (CSF) increased 6-fold after 6–8 hour and the metabolite 5-hydroxyindoleacetic acid (5-HIAA) increased almost 2-fold in CSF by 8 hour, suggesting increased turnover of brain serotonin (5-HT) 147. This 2-fold increase in serotonin (5-HT) turnover was replicated in a later study of CSF 5-hydroxyindoleacetic acid (5-HIAA) changes, using 3 and 6 g tryptophan, with no further increase at the higher dose, although the level was sustained for longer, i.e. 12 vs. 8 hour 148.
Tryptophan warnings and contraindications
Case reports of serotonin syndrome have noted a connection between tryptophan used concomitantly with monoamine oxidase inhibitors 149. Serotonin syndrome is characterized by agitation, confusion, delirium, tachycardia, diaphoresis, and blood pressure fluctuations. Although no reports have been published, it is possible that tryptophan, when taken in combination with a selective serotonin reuptake inhibitor (SSRI) such as Prozac®, Paxil®, or Zoloft®, may also precipitate serotonin syndrome.
Patients with liver cirrhosis should avoid tryptophan supplementation. Cirrhotics present with reduced activity of tryptophan pyrrolase (22%), with subsequent increased free tryptophan and half-life, with decreased clearance 150. The effects of supplemental tryptophan during pregnancy are scarce. One study monitored fetal breathing activity via ultrasound over a 3.5-hour period and noted alteration in fetal breathing activity after maternal tryptophan loading (1 g) was less than observed after glucose load (100 g) 151. This, however, was a low dose of tryptophan. Further safety studies are warranted before use during pregnancy can be recommended.
Make sure you tell your doctor if you have any other medical problems, especially 152:
- Achlohydria or malabsorption (digestion problems): L-tryptophan may cause breathing problems in patients with certain types of digestion problems
- Bladder cancer: L-tryptophan may increase the risk of bladder cancer
- Cataracts: L-tryptophan may cause cataracts
- Diabetes mellitus (sugar diabetes): L-tryptophan may cause diabetes in patients with a family history of diabetes
Tryptophan Dosage
For depression: Adults can take 8 to 12 grams per day, given in 3 to 4 equally divided doses.
Evening oral doses of tryptophan as low as 250 mg have been shown to improve sleep quality, although the typical dosage range for sleep disorders is 1-3 g daily. Safe and effective dosages for other disorders range from 0.5-4 g daily, while potentially higher doses (50 mg/kg/day) have been used short term as a smoking cessation intervention.
Tryptophan side effects
Tryptophan may cause some people to become drowsy, dizzy, or less alert than they are normally. Make sure you know how you react to tryptophan before you drive, use machines, or do anything else that could be dangerous if you are dizzy or are not alert.
Tryptophan may cause dryness of the mouth. Using sugarless candy or gum, ice, or a saliva substitute may be helpful. Check with your physician or dentist if dry mouth continues for more than 2 weeks.
Avoid excessive exposure to ultraviolet light to reduce the chance of cataract formation.
Tryptophan common side effects include:
- dizziness
- drowsiness
- dry mouth
- headache
- loss of appetite
- nausea
Potential side effects at high doses (100 mg/kg/day, i.e., 7 g/150 lbs) include gastric irritation, vomiting, and head twitching 121.
Symptoms of tryptophan overdose:
- agitation
- confusion
- diarrhea
- fever
- overactive reflexes
- poor coordination
- restlessness
- shivering
- sweating
- talking or acting with excitement you cannot control
- trembling or shaking
- twitching
- vomiting
Other side effects not listed may also occur in some patients. If you notice any other effects, check with your healthcare professional.
Figure 3. Tryptophan
Table 10. Foods high in tryptophan (ordered from highest to low)
| Description | Tryptophan (g) Value Per 100 grams |
| Egg, white, dried, stabilized, glucose reduced | 1.43 |
| Egg, white, dried, powder, stabilized, glucose reduced | 1.27 |
| Egg, white, dried, flakes, stabilized, glucose reduced | 1.18 |
| Soy protein isolate | 1.12 |
| Soy protein isolate, potassium type | 1.12 |
| Seeds, sesame flour, low-fat | 1.1 |
| Egg, white, dried | 1 |
| Seaweed, spirulina, dried | 0.93 |
| Seeds, sesame flour, partially defatted | 0.88 |
| Soy protein concentrate, produced by alcohol extraction | 0.83 |
| Soy protein concentrate, produced by acid wash | 0.83 |
| Whale, beluga, meat, dried (Alaska Native) | 0.8 |
| Egg, whole, dried | 0.78 |
| Egg, whole, dried, stabilized, glucose reduced | 0.77 |
| Winged beans, mature seeds, raw | 0.76 |
| Seeds, cottonseed flour, low fat (glandless) | 0.75 |
| Tofu, dried-frozen (koyadofu) | 0.75 |
| Tofu, dried-frozen (koyadofu), prepared with calcium sulfate | 0.75 |
| Seeds, cottonseed meal, partially defatted (glandless) | 0.74 |
| Seeds, sunflower seed flour, partially defatted | 0.73 |
| Beverages, Protein powder soy based | 0.72 |
| Fish, cod, Atlantic, dried and salted | 0.7 |
| Soy flour, defatted | 0.68 |
| Seeds, sesame flour, high-fat | 0.67 |
| Soy meal, defatted, raw | 0.65 |
| Pork, fresh, variety meats and by-products, pancreas, cooked, braised | 0.62 |
| Seeds, cottonseed flour, partially defatted (glandless) | 0.62 |
| Mollusks, whelk, unspecified, cooked, moist heat | 0.62 |
| Soybeans, mature seeds, raw | 0.59 |
| Seeds, pumpkin and squash seed kernels, dried | 0.58 |
| Soybeans, mature seeds, dry roasted | 0.57 |
| Meat extender | 0.57 |
| Seeds, pumpkin and squash seed kernels, roasted, without salt | 0.57 |
| Seeds, pumpkin and squash seed kernels, roasted, with salt added | 0.57 |
| Cheese, parmesan, shredded | 0.56 |
| Cheese, mozzarella, low moisture, part-skim | 0.55 |
| Cheese, cheddar (Includes foods for USDA’s Food Distribution Program) | 0.55 |
| Game meat, elk, cooked, roasted | 0.55 |
| Leavening agents, yeast, baker’s, active dry | 0.54 |
| Parsley, freeze-dried | 0.52 |
| Cheese, mozzarella, whole milk | 0.52 |
| Soybeans, mature seeds, roasted, salted | 0.51 |
| Soybeans, mature seeds, roasted, no salt added | 0.51 |
| Milk, dry, nonfat, regular, without added vitamin A and vitamin D | 0.51 |
| Milk, dry, nonfat, regular, with added vitamin A and vitamin D | 0.51 |
| Peanut flour, defatted | 0.51 |
| Soy flour, full-fat, roasted | 0.51 |
| Soy flour, full-fat, raw | 0.5 |
| Milk, dry, nonfat, calcium reduced | 0.5 |
| Milk, dry, nonfat, instant, with added vitamin A and vitamin D | 0.49 |
| Milk, dry, nonfat, instant, without added vitamin A and vitamin D | 0.49 |
| Seeds, cottonseed kernels, roasted (glandless) | 0.49 |
| Milk, buttermilk, dried | 0.48 |
| Cheese, parmesan, hard | 0.48 |
| Spices, parsley, dried | 0.47 |
| Pork, cured, bacon, cooked, microwaved | 0.46 |
| Game meat, caribou, cooked, roasted | 0.46 |
| Seeds, chia seeds, dried | 0.44 |
| Game meat, rabbit, wild, cooked, stewed | 0.44 |
| Cheese, romano | 0.43 |
| Cheese, gruyere | 0.42 |
| Lamb, shoulder, arm, separable lean only, trimmed to 1/4″ fat, choice, cooked, braised | 0.41 |
| T.G.I. FRIDAY’S, classic sirloin steak (10 oz) | 0.41 |
| Game meat, elk, raw | 0.41 |
| CRACKER BARREL, grilled sirloin steak | 0.41 |
| Pork, ground, 96% lean / 4% fat, cooked, pan-broiled | 0.41 |
| Beef, round, top round roast, boneless, separable lean only, trimmed to 0″ fat, select, cooked, roasted | 0.41 |
| Pork, fresh, variety meats and by-products, pancreas, raw | 0.41 |
| Pork, cured, bacon, pre-sliced, cooked, pan-fried | 0.41 |
| Beef, round, eye of round roast, boneless, separable lean only, trimmed to 0″ fat, select, cooked, roasted | 0.41 |
| Beef, round, top round, separable lean only, trimmed to 0″ fat, choice, cooked, braised | 0.41 |
| Beef, round, top round, separable lean only, trimmed to 0″ fat, select, cooked, braised | 0.41 |
| Chicken, broiler or fryers, breast, skinless, boneless, meat only, cooked, braised | 0.4 |
| Goose, domesticated, meat only, cooked, roasted | 0.4 |
| Seeds, safflower seed meal, partially defatted | 0.4 |
| Game meat, goat, cooked, roasted | 0.4 |
| Beef, round, top round steak, boneless, separable lean only, trimmed to 0″ fat, all grades, cooked, grilled | 0.4 |
| Cheese, swiss | 0.4 |
| Game meat, rabbit, domesticated, composite of cuts, cooked, stewed | 0.4 |
| Beef, loin, top sirloin filet, boneless, separable lean only, trimmed to 0″ fat, select, cooked, grilled | 0.4 |
| Duck, young duckling, domesticated, White Pekin, leg, meat only, bone in, cooked without skin, braised | 0.4 |
| Beef, plate steak, boneless, inside skirt, separable lean only, trimmed to 0″ fat, select, cooked, grilled | 0.4 |
| Beef, round, top round, separable lean and fat, trimmed to 0″ fat, choice, cooked, braised | 0.4 |
| Beef, round, top round, separable lean and fat, trimmed to 0″ fat, select, cooked, braised | 0.4 |
| Lamb, Australian, imported, fresh, shoulder, arm, separable lean only, trimmed to 1/8″ fat, cooked, braised | 0.4 |
| Cereals ready-to-eat, wheat germ, toasted, plain | 0.4 |
| Lamb, New Zealand, imported, frozen, shoulder, whole (arm and blade), separable lean only, cooked, braised | 0.4 |
| Seeds, sesame butter, paste | 0.4 |
| Pork, ground, 96% lean / 4% fat, cooked, crumbles | 0.39 |
| Beef, round, top round steak, boneless, separable lean only, trimmed to 0″ fat, choice, cooked, grilled | 0.39 |
| Lamb, cubed for stew or kabob (leg and shoulder), separable lean only, trimmed to 1/4″ fat, cooked, braised | 0.39 |
| Seeds, sesame butter, tahini, from unroasted kernels (non-chemically removed seed coat) | 0.39 |
| Restaurant, family style, sirloin steak | 0.39 |
| Spices, fenugreek seed | 0.39 |
| Egg, yolk, dried | 0.39 |
| Chicken, broilers or fryers, breast, meat only, cooked, fried | 0.39 |
| Seeds, watermelon seed kernels, dried | 0.39 |
| Seeds, sesame butter, tahini, from raw and stone ground kernels | 0.39 |
| Beef, top loin filet, boneless, separable lean only, trimmed to 1/8″ fat, select, cooked, grilled | 0.39 |
| Seeds, sesame seeds, whole, dried | 0.39 |
| Beef, round, top round, separable lean and fat, trimmed to 1/8″ fat, select, cooked, braised | 0.39 |
| Beef, loin, top loin steak, boneless, lip-on, separable lean only, trimmed to 1/8″ fat, select, cooked, grilled | 0.39 |
| Chicken, stewing, light meat, meat only, cooked, stewed | 0.39 |
| Pork, fresh, loin, tenderloin, separable lean only, cooked, broiled | 0.39 |
| Beef, loin, top loin steak, boneless, lip off, separable lean only, trimmed to 0″ fat, select, cooked, grilled | 0.39 |
| Chicken, broiler or fryers, breast, skinless, boneless, meat only, cooked, grilled | 0.39 |
| Beef, round, top round, separable lean and fat, trimmed to 1/8″ fat, all grades, cooked, braised | 0.39 |
| Beef, chuck, mock tender steak, boneless, separable lean only, trimmed to 0″ fat, choice, cooked, braised | 0.39 |
| Beef, round, eye of round steak, boneless, separable lean only, trimmed to 0″ fat, select, cooked, grilled | 0.39 |
| Game meat, rabbit, domesticated, composite of cuts, cooked, roasted | 0.38 |
| Cheese, parmesan, grated | 0.38 |
| Chicken, broilers or fryers, light meat, meat only, cooked, fried | 0.38 |
| Lamb, shoulder, whole (arm and blade), separable lean only, trimmed to 1/4″ fat, choice, cooked, braised | 0.38 |
| Beef, loin, top sirloin petite roast, boneless, separable lean only, trimmed to 0″ fat, select, cooked, roasted | 0.38 |
| Beef, round, top round, separable lean and fat, trimmed to 1/8″ fat, choice, cooked, braised | 0.38 |
| Beef, chuck, mock tender steak, boneless, separable lean only, trimmed to 0″ fat, all grades, cooked, braised | 0.38 |
| Beef, rib, back ribs, bone-in, separable lean only, trimmed to 0″ fat, select, cooked, braised | 0.38 |
| Beef, round, top round roast, boneless, separable lean only, trimmed to 0″ fat, all grades, cooked, roasted | 0.38 |
| Duck, young duckling, domesticated, White Pekin, breast, meat only, boneless, cooked without skin, broiled | 0.38 |
| Game meat, boar, wild, cooked, roasted | 0.38 |
| DENNY’S, top sirloin steak | 0.38 |
| Lamb, shoulder, blade, separable lean only, trimmed to 1/4″ fat, choice, cooked, braised | 0.38 |
| Beef, chuck, mock tender steak, boneless, separable lean only, trimmed to 0″ fat, select, cooked, braised | 0.38 |
| Beef, rib eye steak, boneless, lip-on, separable lean only, trimmed to 1/8″ fat, select, cooked, grilled | 0.38 |
| Beef, rib eye steak, boneless, lip off, separable lean only, trimmed to 0″ fat, select, cooked, grilled | 0.38 |
| Beef, shank crosscuts, separable lean only, trimmed to 1/4″ fat, choice, cooked, simmered | 0.38 |
| Beef, round, eye of round roast, boneless, separable lean only, trimmed to 0″ fat, all grades, cooked, roasted | 0.38 |
| Pork, fresh, loin, tenderloin, separable lean and fat, cooked, broiled | 0.38 |
| Snacks, soy chips or crisps, salted | 0.38 |
| Fish, roe, mixed species, cooked, dry heat | 0.38 |
| Beef, rib eye roast, boneless, lip-on, separable lean only, trimmed to 1/8″ fat, select, cooked, roasted | 0.38 |
| Beef, loin, top sirloin filet, boneless, separable lean only, trimmed to 0″ fat, all grades, cooked, grilled | 0.38 |
| Pork, fresh, leg (ham), whole, separable lean only, cooked, roasted | 0.37 |
| Pork, fresh, loin, center rib (chops), boneless, separable lean only, cooked, broiled | 0.37 |
| Pork, fresh, composite of trimmed retail cuts (loin and shoulder blade), separable lean only, cooked | 0.37 |
| Beef, plate steak, boneless, outside skirt, separable lean only, trimmed to 0″ fat, select, cooked, grilled | 0.37 |
| Chicken, broilers or fryers, giblets, cooked, fried | 0.37 |
| Beef, chuck for stew, separable lean and fat, choice, cooked, braised | 0.37 |
| Turkey, retail parts, wing, meat only, cooked, roasted | 0.37 |
| Chicken, broiler or fryers, breast, skinless, boneless, meat only, with added solution, cooked, grilled | 0.37 |
| Ham and cheese spread | 0.37 |
| Seeds, sesame butter, tahini, from roasted and toasted kernels (most common type) | 0.37 |
| Veal, leg (top round), separable lean only, cooked, braised | 0.37 |
| Beef, chuck for stew, separable lean and fat, all grades, cooked, braised | 0.37 |
| Milk, dry, whole, with added vitamin D | 0.37 |
| Milk, dry, whole, without added vitamin D | 0.37 |
| Seeds, sesame seeds, whole, roasted and toasted | 0.37 |
| Seeds, sesame seed kernels, toasted, without salt added (decorticated) | 0.37 |
| Seeds, sesame meal, partially defatted | 0.37 |
| Seeds, sesame seed kernels, toasted, with salt added (decorticated) | 0.37 |
Table 11. L-tryptophan found in common foods
| L-tryptophan*(mg) | Sum of Competing Amino Acids (CAAs)** (mg) | Ratio | |
| Turkey, Skinless, Boneless, Light Meat (per pound, raw) | 410 | 9525 | 0.043 |
| Chicken, Skinless, Boneless, Light Meat (per pound, raw) | 238 | 5122 | 0.046 |
| Turkey, Skinless, Boneless, Dark Meat (per pound, raw) | 303 | 7036 | 0.043 |
| Chicken, Skinless, Boneless, Dark Meat (per pound, raw) | 256 | 5492 | 0.047 |
| Whole Milk (per quart) | 732 | 8989 | 0.081 |
| 2% Milk (per quart) | 551 | 12516 | 0.044 |
| Wheat Bread (per slice) | 19 | 317 | 0.06 |
| White Bread (per slice) | 22 | 439 | 0.05 |
| Semisweet Chocolate (per ounce) | 18 | 294 | 0.061 |
| Sweet Chocolate (per ounce) | 16 | 270 | 0.059 |
| Canned Tuna (per ounce) | 472 | 10591 | 0.045 |
| Cheddar Cheese (per ounce) | 91 | 2298 | 0.04 |
| Peanuts (per ounce) | 65 | 1574 | 0.041 |
| Oats for Oatmeal (per cup) | 147 | 2617 | 0.056 |
| Dried Prune (one) | 2 | 27 | 0.074 |
| Banana (one medium) | 11 | 237 | 0.046 |
| Apple (one medium) | 2 | 70 | 0.029 |
Footnotes:
The L-tryptophan/competing amino acid (CAA) ratio represents the relative availability of plasma L-tryptophan for crossing the blood-brain barrier and is thought to be the best indicator of brain serotonin synthesis.
*e.g. The recommended daily allowance for a 79 kg (175 lb) adult is 278 to 476 mg.
**CAAs = Isoleucine, Leucine, Phenylalanine, Tyrosine, and Valine, the five large neutral amino acids typically included in the tryptophan/competing amino acid (CAA) ratio.
SAMe (S-adenosyl methionine)
S-Adenosylmethionine is also called S-adenosyl methionine, S-adenosylmethionine, SAMe or SAM-e in the United States or ademetionine in Europe, and also often abbreviated as SAM and AdoMet, is a naturally-occurring compound found in almost every tissue and fluid in your body 155. SAM-e is not found in food. S-Adenosylmethionine is made in your body from methionine, an amino acid found in foods and ATP which serves as the major energy source for cells throughout the body. SAM-e is involved in many important processes. SAM-e plays a role in your immune system, maintains cell membranes, and helps produce and break down brain chemicals, such as serotonin, melatonin, and dopamine 156. S-Adenosylmethionine works with vitamin B12 and folate (vitamin B9). Being deficient in either vitamin B12 or folate may reduce levels of S-adenosylmethionine in your body.
SAM-e is sold in the United States as a dietary supplement. S-Adenosylmethionine has been investigated most extensively for depression, osteoarthritis, and liver diseases. For all three conditions, research has not conclusively shown that SAMe is helpful.
Several studies show that SAMe helps relieve the pain of osteoarthritis. Other studies suggest that SAMe may help treat depression. The idea that SAMe might be helpful for osteoarthritis came from studies of SAMe for depression. Some of the participants in the depression studies who also had osteoarthritis said their joint symptoms improved when they took SAMe. Researchers have also examined SAMe’s use in the treatment of fibromyalgia and liver disease with mixed results. Many of the early studies used SAMe given intravenously or as an injection. Only recently have researchers been able to look at the effects of SAMe taken by mouth.
While S-adenosylmethionine antidepressant mechanism of action is not entirely clear, it is thought that its ability to function as a methyl donor increases brain levels of serotonin, dopamine, and
norepinephrine 157. Serum and cerebrospinal fluid (CSF) levels of SAM-e are reportedly low in depressed patients 158 and increases in serum SAM-e levels have been correlated with improved treatment response 159. In humans, SAMe treatment is reported to increase cerebrospinal fluid concentrations of 5‐hydroxyindole acetic acid (the main metabolite of serotonin) 160. In addition, through stimulation of phospholipid methylation, SAMe may increase the fluidity of cell membranes that is linked to an increase in β‐adrenoreceptor and muscarinic (M1) receptor density 161. Furthermore, SAMe may influence the expression of key genes in the brain affecting behaviour, memory, learning and cognition 162.
SAM-e may improve depressed mood via enhanced methylation of catecholamines and increased serotonin turnover, reuptake inhibition of norepinephrine, enhanced dopaminergic activity, decreased prolactin secretion, and increased phosphatidylcholine conversion 163. SAMe has also been reported to increase the genetic expression of brain-derived neurotrophic factor (BDNF) 164. Overall, the evidence that oral SAMe may be helpful for depression is not conclusive.
Figure 4. S-Adenosylmethionine
Some research suggests that SAMe is more effective than placebo in treating mild-to-moderate depression and is just as effective as antidepressant medications without the side effects (headaches, sleeplessness, and sexual dysfunction). In addition, antidepressants tend to take 6 to 8 weeks to begin working, while SAMe seems to begin more quickly. Researchers are not sure how SAM-e works to relieve depression. But they speculate it might increase the amount of serotonin in the brain just as some antidepressants do.
Many studies have examined injectable forms of SAMe, not oral supplements. More research is needed to determine whether SAM-e works for depression. Because serious depression is a dangerous illness, you should seek help from your doctor before taking SAMe or any supplement.
SAMe has generally shown promise as monotherapy or augmentation therapy to an antidepressant for major depressive disorder or treatment-resistant depression in open-label studies and double-blind randomized clinical trials 155, 165. However, while doses ranging from 200–3,200 mg/day of S-adenosyl methionine through different routes of administration have been used in clinical trials, SAMe’s optimal oral dose for depression is still unknown. More notably, there is little evidence regarding the best starting dose or whether sequential dose increases bring further clinical benefit.
At least 40 studies in people have evaluated S-Adenosylmethionine for depression, and many of them showed evidence of beneficial effects. However, most of these trials lasted only a few weeks, included a small number of participants, and were not of the highest scientific quality. Also, some studies used injected (intramuscular or intravenous) SAMe rather than an oral form (taken by mouth).
A high-quality Cochrane review of the effectiveness and safety of S-adenosyl methionine (SAMe) supplementation on depression severity revealed two randomized controlled trials of low risk of bias that showed no significant pooled effects for SAMe monotherapy versus placebo 166. One randomized controlled trial, also of low risk of bias, showed a significant medium short-term effect as adjunctive to standard antidepressant medication, both for depression severity. Further five randomized controlled trials, which were rated as having overall unclear risk of bias, showed similar pooled effects of SAMe monotherapy on depression severity compared with standard antidepressant medication 166. Original randomized controlled trials reported safety issues insufficiently. For all meta-analyses, the evidence was assessed as low to very low quality because of limitations of the study quality, heterogeneity, imprecision and a possibly high risk of publication bias.
Recommended doses of SAMe vary depending on the health condition being treated. Recommended daily doses of SAMe range from 200 mg to 1600 mg taken in divided doses, depending upon the condition for which it is being taken and its severity, and upon the route of administration 167. Exogenous, orally administered SAMe has a short half‐life, undergoing first‐pass effects and rapid metabolism. However, oral doses of SAMe at 1600 mg/day are significantly bioavailable and non‐toxic 168. Because SAMe is best absorbed on an empty stomach, it should be administered 30 to 60 minutes before meals or two hours after meals; people should be instructed to adhere strictly to these directions. It may also be administered parenterally, using intramuscular or intravenous routes 169.
The following list gives information on the S-adenosyl methionine (SAMe) dosages used in studies for each condition:
- Depression: 800 to 1,600 mg of SAMe per day, in 2 divided doses (morning and afternoon).
- Osteoarthritis: 600 to 1,200 mg per day in 2 to 3 divided doses.
- Fibromyalgia: A dosage of 400 mg, 2 times per day for 6 weeks.
- Alcoholic liver disease: 600 to 1,200 mg per day by mouth in divided doses for 6 months enhances liver function. For liver disease, a qualified health care provider should supervise administration of SAMe.
SAM-e safety and side effects
- Information on the long-term safety of SAMe is limited because the participants in most studies took it only for short periods of time. However, in one study of alcohol-related liver disease, participants took SAMe for 2 years; in that study, no serious side effects were reported.
- Side effects of SAMe are uncommon, and when they do occur they are dry mouth, nausea, gas, diarrhea, headache, anxiety, a feeling of elation, restlessness, and insomnia. Sweating, dizziness, and palpitations have also been reported. For this reason, you should not take SAMe at night.
- Large doses of SAMe may cause mania (abnormally elevated mood). Start at a low dose and gradually increase it. DO NOT exceed recommended doses.
- People with bipolar disorder (an illness characterized by mood swings, from depression to mania) should not take SAMe for their depressive symptoms except under the supervision of a health care provider because SAMe may worsen symptoms of mania (manic episodes) 170. In one open study, nine of 11 people with bipolar disorder experienced a switch to an ‘elevated mood state’ (hypomania, mania or euphoria) 170. Reports of induced mania and hypomania were found even in cases with no prior suggestion of bipolar disorder 171. A transient mixed manic episode with suicidal ideation was reported in a person with no previous psychiatric history on SAMe; recovery followed discontinuation 168. These findings must be interpreted with caution as bipolar 2 disorder (diagnosed by the presence of a hypomanic episode) is sometimes misdiagnosed as major depressive disorder when hypomanic episodes are overlooked.
- SAMe should not be combined with other antidepressants without first consulting your doctor.
- Although SAMe has been used to treat cholestasis during pregnancy, its safety during pregnancy has not been established.
- SAMe may decrease the effects of levodopa (L-dopa), a drug used to treat Parkinson’s disease. It’s also possible that SAMe might interact with drugs and dietary supplements that increase levels of serotonin (a chemical produced by nerve cells), such as antidepressants, L-tryptophan, and St. John’s wort.
- There’s a theoretical concern about the use of SAMe by people who are immunocompromised (such as those who are HIV-positive). Immunocompromised people are at risk for Pneumocystis carinii infection, and SAMe enhances the growth of this microorganism.
- Pregnant and breastfeeding women should not take SAMe.
- People taking SAMe may want to take a multivitamin that contains folic acid and vitamins B12 and B6.
Antidepressant medications
SAMe may interact with antidepressant medications, increasing the potential for side effects including headache, irregular or accelerated heart rate, anxiety, and restlessness, as well as the potential fatal condition called Serotonin Syndrome, mentioned above. Some experts theorize that taking SAMe increases the levels of serotonin in the brain, and many antidepressants do the same. The concern is that combining the two may increase serotonin to dangerous levels. Talk to your doctor before using SAMe if you are taking any medications for depression or anxiety.
Levodopa (L-dopa)
SAMe may reduce the effectiveness of this medication for Parkinson disease.
Medications for diabetes
SAMe may reduce levels of blood sugar and may strengthen the effect of diabetes medications, which increases the risk of hypoglycemia (low blood sugar).
Magnesium
Magnesium is an abundant mineral in your body that your body needs to stay healthy and is naturally present in many foods, added to other food products, available as a dietary supplement, and present in some medicines (such as antacids and laxatives) 172. Magnesium is important for many processes in your body, including regulating muscle and nerve function, energy production, blood sugar levels, and blood pressure and making protein, bone, and DNA. Magnesium is a cofactor in more than 300 enzyme systems that regulate diverse biochemical reactions in the body, including protein synthesis, muscle and nerve function, blood glucose control, and blood pressure regulation 173, 174, 175. Low magnesium levels don’t cause symptoms in the short term. However, chronically low magnesium levels can increase your risk of high blood pressure, heart disease, type 2 diabetes and osteoporosis. No meta-analysis of magnesium supplementation for depression was found. A recent systematic review detected no randomized controlled trials in patients with a primary diagnosis of depression 176.
Magnesium is required for energy production, oxidative phosphorylation, and glycolysis. It contributes to the structural development of bone and is required for the synthesis of DNA, RNA, and the antioxidant glutathione. Magnesium also plays a role in the active transport of calcium and potassium ions across cell membranes, a process that is important to nerve impulse conduction, muscle contraction, and normal heart rhythm 175. Many people don’t get enough magnesium in their diets. However, before you reach for a supplement, though, you should know that just a few servings of magnesium-rich foods a day can meet your need for this important nutrient.
An adult body contains approximately 25 g magnesium, with 50% to 60% present in the bones and most of the rest in soft tissues 177. Less than 1% of total magnesium is in blood serum, and these levels are kept under tight control. Normal serum magnesium concentrations range between 0.75 and 0.95 millimoles (mmol)/L 173, 178. Hypomagnesemia is defined as a serum magnesium level less than 0.75 mmol/L 179. Magnesium homeostasis is largely controlled by the kidney, which typically excretes about 120 mg magnesium into the urine each day 174. Urinary excretion is reduced when magnesium status is low 173.
Assessing magnesium status is difficult because most magnesium is inside cells or in bone 175. The most commonly used and readily available method for assessing magnesium status is measurement of serum magnesium concentration, even though serum levels have little correlation with total body magnesium levels or concentrations in specific tissues 179. Other methods for assessing magnesium status include measuring magnesium concentrations in erythrocytes, saliva, and urine; measuring ionized magnesium concentrations in blood, plasma, or serum; and conducting a magnesium-loading (or “tolerance”) test. No single method is considered satisfactory 180. Some experts 177 but not others 175 consider the tolerance test (in which urinary magnesium is measured after parenteral infusion of a dose of magnesium) to be the best method to assess magnesium status in adults. To comprehensively evaluate magnesium status, both laboratory tests and a clinical assessment might be required 179.
Table 12. Recommended Dietary Allowances (RDAs) for Magnesium
| Age | Male | Female | Pregnancy | Lactation |
| Birth to 6 months | 30 mg* | 30 mg* | ||
| 7–12 months | 75 mg* | 75 mg* | ||
| 1–3 years | 80 mg | 80 mg | ||
| 4–8 years | 130 mg | 130 mg | ||
| 9–13 years | 240 mg | 240 mg | ||
| 14–18 years | 410 mg | 360 mg | 400 mg | 360 mg |
| 19–30 years | 400 mg | 310 mg | 350 mg | 310 mg |
| 31–50 years | 420 mg | 320 mg | 360 mg | 320 mg |
| 51+ years | 420 mg | 320 mg |
Footnote: *Adequate Intake (AI)
[Source 181]Table 13. Magnesium Rich Foods
| Food | Milligrams (mg) per serving | Percent DV* |
| Almonds, dry roasted, 1 ounce | 80 | 20 |
| Spinach, boiled, ½ cup | 78 | 20 |
| Cashews, dry roasted, 1 ounce | 74 | 19 |
| Peanuts, oil roasted, ¼ cup | 63 | 16 |
| Cereal, shredded wheat, 2 large biscuits | 61 | 15 |
| Soymilk, plain or vanilla, 1 cup | 61 | 15 |
| Black beans, cooked, ½ cup | 60 | 15 |
| Edamame, shelled, cooked, ½ cup | 50 | 13 |
| Peanut butter, smooth, 2 tablespoons | 49 | 12 |
| Bread, whole wheat, 2 slices | 46 | 12 |
| Avocado, cubed, 1 cup | 44 | 11 |
| Potato, baked with skin, 3.5 ounces | 43 | 11 |
| Rice, brown, cooked, ½ cup | 42 | 11 |
| Yogurt, plain, low fat, 8 ounces | 42 | 11 |
| Breakfast cereals, fortified with 10% of the DV for magnesium | 40 | 10 |
| Oatmeal, instant, 1 packet | 36 | 9 |
| Kidney beans, canned, ½ cup | 35 | 9 |
| Banana, 1 medium | 32 | 8 |
| Salmon, Atlantic, farmed, cooked, 3 ounces | 26 | 7 |
| Milk, 1 cup | 24–27 | 6–7 |
| Halibut, cooked, 3 ounces | 24 | 6 |
| Raisins, ½ cup | 23 | 6 |
| Chicken breast, roasted, 3 ounces | 22 | 6 |
| Beef, ground, 90% lean, pan broiled, 3 ounces | 20 | 5 |
| Broccoli, chopped and cooked, ½ cup | 12 | 3 |
| Rice, white, cooked, ½ cup | 10 | 3 |
| Apple, 1 medium | 9 | 2 |
| Carrot, raw, 1 medium | 7 | 2 |
Footnote: *DV = Daily Value. DVs were developed by the U.S. Food and Drug Administration (FDA) to help consumers compare the nutrient contents of products within the context of a total diet. The DV for magnesium is 400 mg for adults and children aged 4 and older. However, the FDA does not require food labels to list magnesium content unless a food has been fortified with this nutrient. Foods providing 20% or more of the DV are considered to be high sources of a nutrient.
[Source 182]Zinc
Zinc is an essential mineral that is naturally present in some foods, added to others, and available as a dietary supplement. Zinc is also found in many cold lozenges and some over-the-counter drugs sold as cold remedies. Zinc is a nutrient that people need to stay healthy. A daily intake of zinc is required to maintain a steady state because the body has no specialized zinc storage system 183.
Zinc is found in cells throughout the body, found mainly in bones, teeth, hair, skin, liver, muscle, leukocytes, and testes 184. Zinc helps the immune system fight off invading bacteria and viruses. The body also needs zinc to make proteins and DNA, the genetic material in all cells. During pregnancy, infancy, and childhood, the body needs zinc to grow and develop properly. Zinc also helps wounds heal and is important for proper senses of taste and smell.
Zinc is involved in numerous aspects of cellular metabolism. It is required for the catalytic activity of approximately 100 enzymes 185 and it plays a role in immune function 186, protein synthesis 186, wound healing 187, DNA synthesis 185, and cell division 186. Zinc also supports normal growth and development during pregnancy, childhood, and adolescence 188 and is required for proper sense of taste and smell 189.
The effectiveness of zinc for major depression was meta-analysed by a low-quality review of three randomized controlled trials 190. It revealed a significant pooled short-term effect of medium size and low heterogeneity when zinc was taken as an adjunctive to standard antidepressant drug treatment 190. However, the available evidence had to be assessed as very low as the meta-analysis did not perform risk of bias assessments and did not report adverse events.
Most Americans get enough zinc from the foods they eat. The amount of zinc you need each day depends on your age (see Table 14). Most infants (especially those who are formula fed), children, and adults in the United States consume recommended amounts of zinc according to two national surveys, the 1988–1991 National Health and Nutrition Examination Survey 191 and the 1994 Continuing Survey of Food Intakes of Individuals 192. However, some evidence suggests that zinc intakes among older adults might be marginal. An analysis of National Health and Nutrition Examination Survey data found that 35%–45% of adults aged 60 years or older had zinc intakes below the estimated average requirement of 6.8 mg/day for elderly females and 9.4 mg/day for elderly males. When the investigators considered intakes from both food and dietary supplements, they found that 20%–25% of older adults still had inadequate zinc intakes 193.
Zinc intakes might also be low in older adults from the 2%–4% of U.S. households that are food insufficient (sometimes or often not having enough food) 194. Data from National Health and Nutrition Examination Survey indicate that adults aged 60 years or older from food-insufficient families had lower intakes of zinc and several other nutrients and were more likely to have zinc intakes below 50% of the RDA on a given day than those from food-sufficient families 195.
Certain groups of people are more likely than others to have trouble getting enough zinc:
- People who have had gastrointestinal surgery, such as weight loss surgery, or who have digestive disorders, such as ulcerative colitis or Crohn’s disease. These conditions can both decrease the amount of zinc that the body absorbs and increase the amount lost in the urine.
- Vegetarians because they do not eat meat, which is a good source of zinc. Also, the beans and grains they typically eat have compounds that keep zinc from being fully absorbed by the body. For this reason, vegetarians might need to eat as much as 50% more zinc than the recommended amounts.
- Older infants who are breastfed because breast milk does not have enough zinc for infants over 6 months of age. Older infants who do not take formula should be given foods that have zinc such as pureed meats. Formula-fed infants get enough zinc from infant formula.
- Alcoholics because alcoholic beverages decrease the amount of zinc that the body absorbs and increase the amount lost in the urine. Also, many alcoholics eat a limited amount and variety of food, so they may not get enough zinc.
- People with sickle cell disease because they might need more zinc.
Clinical zinc deficiency in humans was first described in 1961, when the consumption of diets with low zinc bioavailability due to high phytate content was associated with “adolescent nutritional dwarfism” in the Middle East 196. Since then, zinc insufficiency has been recognized by a number of experts as an important public health issue, especially in low-resource countries 197. Severe zinc deficiency is rare and caused by an inherited condition called acrodermatitis enteropathica. Acquired zinc deficiency is primarily due to malabsorption syndromes and chronic alcoholism.
Currently, there is not a sensitive and specific biomarker to detect zinc deficiency in humans. Low plasma or serum zinc concentrations are typically used as indicators of zinc status in populations and in intervention studies, but they have a number of limitations, including lack of sensitivity to detect marginal zinc deficiency, diurnal variations, and confounding by inflammation, stress, and hormones 198.
Table 14. Average daily recommended amounts for different ages are listed below in milligrams (mg)
| Life Stage | Recommended Amount |
|---|---|
| Birth to 6 months | 2 mg |
| Infants 7–12 months | 3 mg |
| Children 1–3 years | 3 mg |
| Children 4–8 years | 5 mg |
| Children 9–13 years | 8 mg |
| Teens 14–18 years (boys) | 11 mg |
| Teens 14–18 years (girls) | 9 mg |
| Adults (men) | 11 mg |
| Adults (women) | 8 mg |
| Pregnant teens | 12 mg |
| Pregnant women | 11 mg |
| Breastfeeding teens | 13 mg |
| Breastfeeding women | 12 mg |
The U.S. Department of Agriculture’s (USDA’s) Nutrient Database website (https://fdc.nal.usda.gov) lists the nutrient content of many foods and provides a comprehensive list of foods containing zinc arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/Zinc-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/Zinc-Food.pdf).
Table 15. Selected Food Sources of Zinc
| Food | Milligrams (mg) per serving | Percent DV* |
|---|---|---|
| Oysters, cooked, breaded and fried, 3 ounces | 74.0 | 493 |
| Beef chuck roast, braised, 3 ounces | 7.0 | 47 |
| Crab, Alaska king, cooked, 3 ounces | 6.5 | 43 |
| Beef patty, broiled, 3 ounces | 5.3 | 35 |
| Breakfast cereal, fortified with 25% of the DV for zinc, ¾ cup serving | 3.8 | 25 |
| Lobster, cooked, 3 ounces | 3.4 | 23 |
| Pork chop, loin, cooked, 3 ounces | 2.9 | 19 |
| Baked beans, canned, plain or vegetarian, ½ cup | 2.9 | 19 |
| Chicken, dark meat, cooked, 3 ounces | 2.4 | 16 |
| Yogurt, fruit, low fat, 8 ounces | 1.7 | 11 |
| Cashews, dry roasted, 1 ounce | 1.6 | 11 |
| Chickpeas, cooked, ½ cup | 1.3 | 9 |
| Cheese, Swiss, 1 ounce | 1.2 | 8 |
| Oatmeal, instant, plain, prepared with water, 1 packet | 1.1 | 7 |
| Milk, low-fat or non fat, 1 cup | 1.0 | 7 |
| Almonds, dry roasted, 1 ounce | 0.9 | 6 |
| Kidney beans, cooked, ½ cup | 0.9 | 6 |
| Chicken breast, roasted, skin removed, ½ breast | 0.9 | 6 |
| Cheese, cheddar or mozzarella, 1 ounce | 0.9 | 6 |
| Peas, green, frozen, cooked, ½ cup | 0.5 | 3 |
| Flounder or sole, cooked, 3 ounces | 0.3 | 2 |
Footnote: * DV = Daily Value. DVs were developed by the U.S. Food and Drug Administration to help consumers compare the nutrient contents of products within the context of a total diet. The DV for zinc is 15 mg for adults and children age 4 and older. Food labels, however, are not required to list zinc content unless a food has been fortified with this nutrient. Foods providing 20% or more of the DV are considered to be high sources of a nutrient.
[Source 199 ]Saffron
Saffron is the dried stigma (thread-like parts) of the flower Crocus sativus and was cultivated originally in Iran, Spain, Greece and India 200. Saffron is widely used as a food additive across the world, where it is valued for its coloring, flavoring and aromatizing in the production of some traditional dishes. In addition to its use as a spice, saffron has been used as a medicinal plant in traditional Iranian medicine for treatment of a wide range of disorders including depression, seizures, cognitive disorders, cancers, asthma, liver diseases, menstruation disorders and pain 201, 202. Saffron has three major active constituents including crocin (crocetin glycoside), crocetin, and safranal (Figure 5) 203. Saffron and its active constituents have demonstrated a wide range of pharmacological properties in previous experimental studies including, anti‐inflammatory, anti‐oxidative 204, anti‐hyperlipidaemic, anti‐diabetic and insulin resistance 205. With the potential hypoglycaemic and anti‐diabetic effects, saffron and its active constituents have also been found to prevent metabolic syndrome and insulin resistance in schizophrenia 206.
Saffron may also act in a manner similar to antidepressants to improve mood 202. Specifically, saffron may modulate levels of certain neurotransmitters in the brain, including serotonin, by inhibiting serotonin reuptake, thereby keeping serotonin in the brain longer 202. This mechanism of action is suggested based on findings from animal studies but additional research is needed to identify the mechanism of action through which saffron may improve mood states 202. Studies on the bioactive substances of saffron in depression indicate that the crocin acts by inhibiting the reuptake of dopamine and norepinephrine neurotransmitters, while safranal inhibits the reuptake of serotonin 207. There are in vivo studies suggesting inhibitory effects on the monoamine oxidases, MAO-A and MAO-B, enzymes responsible for the degradation of the neurotransmitters, as mentioned above, leading to an increase in their levels in the synaptic space and reducing depressive symptoms 208.
Saffron (Crocus satvius) standardized extract may help relieve depression although it is too soon to say for sure 209, 210. One preliminary study found that saffron worked as well as fluoxetine (Prozac), while another found that it worked as well as a low dose of imipramine (Tofranil). Various studies in clinical environments concluded that the daily administration of 30 mg of saffron could be useful in the management of depression when compared with imipramine or fluoxetine 211, 212, 213, 214. A trial of 66 patients with anxiety-depressive disorder compared treatment with saffron (30 mg/day) or citalopram (40 mg/day) for 6 weeks 215. The results expressed in the Hamilton depression scale (HAM-D) and the Hamilton anxiety scale (HAM-A) showed no significant difference between the use of saffron and the drug and proposed saffron to be a potentially effective and tolerable treatment for major depressive disorder with anxiety 215.
A moderate-quality meta-analysis examined the effectiveness and safety of saffron on depression severity by including five randomized controlled trials in adult patients with major depression 210. It revealed very low quality of evidence for significant greater effects versus placebo and similar effects versus antidepressant medication up to 8 weeks of treatment 210. No serious adverse events were reported, but patients receiving saffron tend to report more side effects than those receiving placebo and less side effects than those receiving antidepressant medication. Reasons for downgrading the evidence included no replication of the results (all included randomized controlled trials were conducted by the same research group), the small overall sample size and the possibly high risk of publication bias.
Saffron can be dangerous or even life-threatening at high doses or when taken for a long time, so DO NOT take it without your doctor’s supervision. Pregnant women and people with bipolar disorder should not take saffron supplements.
Figure 5. Saffron most active chemical constituents
[Source 216 ]Inositol
Inositol also known as myo-inositol, is a carbocyclic sugar that is abundant in the brain and other mammalian tissues. Myo-inositol is the precursor of inositol triphosphate, acting as an intracellular second messenger and regulating a number of hormones such as thyroid-stimulating hormone, follicle-stimulating hormone (FSH) and insulin 217. Inositol acts as a mediator of the action of insulin, and it is necessary to activate key enzymes in the metabolism of glucose. Decreased levels of myo-Inositol have been reported in (CSF) and post-mortem frontal cortex of patients with mood disorders 218. Glial cells contain a high concentration of myo-Inositol 219. Myo-Inositol contributes to glial osmoregulatory functioning 220, as well as a wide range of other structural and signaling functions. Consistently, proton magnetic resonance imaging (1H-MRS) studies of patients with major depressive disorder report reduced levels of myo-Insoitol in prefrontal and anterior cingulate cortex 221. Conversely, positive clinical outcome in major depressive disorder is associated with increased myo-Inositol levels 222. These data indicate that reduced myo-Inositol in major depressive disorder is primarily associated with depression symptoms rather than being secondary to treatment 223. Furthermore, several small trials have suggested an antidepressant effect of inositol supplementation 224. In bipolar disorder, some proton magnetic resonance imaging (1H-MRS) studies suggest frontal myo-Inositol is reduced in depressed periods and normal in euthymic periods, but these findings have been inconsistent and may be confounded by the use of mood stabilizers 225.
A low-quality meta-analysis of two randomized controlled trials in patients with major depression revealed very low quality evidence for inositol as adjuvant therapy to standard antidepressants versus placebo in combination to standard antidepressants 226. A 2016 systematic review and meta-analysis of several adjunctive nutraceuticals for depression found no significant benefit over placebo for inositol 227. A 2014 meta-analysis of seven randomized controlled trials (two bipolar studies, one bipolar and major depressive disorder study, two major depressive disorder studies, and two premenstrual dysphoric disorder studies) involving 242 participants found no significant treatment effect of inositol for depressed patients 228. However, inositol showed a trend of efficacy of depressive symptoms over placebo in patients with premenstrual dysphoric disorder.
There is a lack of data on the safety and side effects of inositol. A 2014 meta-analysis of inositol for depression and anxiety disorders found that inositol marginally caused gastrointestinal upset compared with placebo 228. A 2011 European review on the safety of inositol had similar findings in that inositol induced gastrointestinal side effects such as nausea, gas and diarrhea 229.
Ginkgo biloba
Ginkgo biloba also known as Ginkgo, fossil tree, maidenhair tree, Japanese silver apricot, Baiguo or Yinhsing, is one of the oldest living tree species in the world, has a long history in traditional Chinese medicine 230. Members of the royal court were given ginkgo nuts for senility. Other historical uses for ginkgo were for asthma, bronchitis, and kidney and bladder disorders. Today, Ginkgo biloba extracts are one of the most commonly taken phytomedicines globally 231 and are often prescribed in Europe as a nootropic agent (cognitive enhancer) in old age and dementia 232. Ginkgo biloba leaves extract is also promoted as a dietary supplement for many conditions, including anxiety, allergies, dementia, eye problems, peripheral artery disease (when buildup of plaque narrows the blood vessels that carry blood to the head, organs, and limbs), tinnitus, and other health problems.
Ginkgo biloba extract contains mainly terpene trilactones, flavonol glycosides, biflavones, alkylphenols, phenolic acids, polyprenols and proanthocyanidins 233. The most prevalent of these groups are the flavonol glycosides (quercetin, catechin) 234. The terpenoids include ginkgolides and bilobalides, which represent unique components of Ginkgo biloba. Terpene trilactones, flavonol glycosides and proanthocyanidins are thought to be responsible for the pharmacological properties of Ginkgo biloba 235, whereas, ginkgolic acids are typically regarded as causing negative biological activities (e.g., cytotoxicity, mutagenicity) 236.
Ginkgotoxin, or 4′-O-methylpyridoxine, is also associated with toxicity, but is found primarily in Ginkgo biloba seeds and only in lesser amounts in the Ginkgo biloba leaves and extracts prepared from the leaves 237. In recent years, the use of fingerprinting to quantify key chemical constituents of plant-based formulations has been advocated as a quality control measure to ensure appropriate quantities of the biologically-active constituents and a lack of ginkgolic acids 233.
Ginkgo biloba is commonly available as a oral tablets, capsules or teas, but may also be available in liquid extracts. Ginkgo biloba extract is typically taken orally. Don’t eat raw or roasted Ginkgo biloba seeds, which can be poisonous. Fresh (raw) or roasted ginkgo seeds and the unprocessed ginkgo leaves can contain dangerous amounts of a toxic substance.
Ginkgo extract is made from the leaves of the Ginkgo biloba tree. The Ginkgo biloba leaves contain a complex mixture of components. The exact formulation of Ginkgo biloba extract in products available to consumers in the U.S. can vary from manufacturer to manufacturer. Ginkgo is marketed in the U.S. as a dietary supplement and the FDA regulations for dietary supplements are different from regulations for prescription or over-the-counter drugs. Federal law does not require dietary supplements to go through the same standards of premarket testing for safety or efficacy as drugs intended to treat, cure, prevent, diagnose, or mitigate disease. In contrast, in Germany and France, Ginkgo biloba extract is regulated as a prescription drug and therefore, requires registration and adherence to specified content standards. The German Commission E (the German equivalent to the U.S. Food and Drug Administration [FDA]), which evaluated the efficacy and safety of herbals licensed for prescription in Germany, approved the monograph on Ginkgo biloba extract specifying contents of 22% to 27% flavone glycosides, 5% to 7% terpene lactones (2.8% to 3.4% ginkgolides A, B, C and 2.6% to 3.2% bilobalide), and not more than 5 ppm ginkgolic acids, due to their cytotoxic and allergenic potential 238. However, Ginkgo biloba products on the marketplace in the United States and elsewhere exhibit a wide range of component concentrations 239.
In the U.S., people take Ginkgo biloba for a wide variety of health reasons, mostly to improve brain function and memory. However, clinical trials designed to assess the efficacy of Ginkgo biloba extract have not produced consistent evidence of benefit. There’s no conclusive evidence that ginkgo is helpful for any health condition.
Although some studies suggest that ginkgo may help to slightly improve memory in those with dementia, the findings have been described as unreliable. Also, other studies have had conflicting findings. In clinical trials, ginkgo biloba failed to prevent cognitive decline or dementia. Ginkgo neither helps prevent dementia or cognitive decline nor prevents Alzheimer’s-related dementia from getting worse—this is according to studies that include the long-term Ginkgo Evaluation of Memory Study, which enrolled more than 3,000 older adults. Multiple clinical trials involving thousands of patients have conclusively shown that treatment with Ginkgo biloba for up to six years does not prevent cognitive decline or dementia, including Alzheimer’s disease 240, 241, 242, 243. This is disappointing since preclinical studies indicated that Ginkgo biloba contains several components that improve brain blood flow and mitochondrial function 244, 245. Mitochondria are the “power plants” of human cells that often malfunction with age and in diseases such as Alzheimer’s disease. For various health conditions, a small amount of evidence suggests a benefit from taking ginkgo, but the overall evidence is not conclusive. These conditions include anxiety, diabetic retinopathy, glaucoma, peripheral artery disease, premenstrual syndrome (PMS), schizophrenia, and vertigo. Research seems to suggest that ginkgo doesn’t help with memory enhancement in healthy people, high blood pressure, tinnitus, multiple sclerosis, seasonal affective disorder, or the risk of having a heart attack or stroke.
Ginkgo biloba may slightly improve memory and cognitive function for people with memory problems or dementia. However, clinical guidelines are mixed. While the World Federation of Societies of Biological Psychiatry guidelines 246 suggest that a certain Ginkgo biloba extract may be used to treat dementia symptoms, the British Association for Psychopharmacology and the American Academy of Family Physician 247 concluded that its benefits are inconsistent and unreliable.
Similarly, clinical trial results are mixed. A 2009 high-quality meta-analysis concluded that the effects of Ginkgo biloba on cognitive impairment and dementia were inconsistent and unreliable 248. There are two other recent meta-analyses in dementia patients 249. In one analysis, seven studies showed that patients using ginkgo had improved scores on certain cognitive performance tests. Two studies in the same analysis using different assessments, however, did not show a statistically significant difference 249. Another meta-analysis of patients with mild cognitive impairment and Alzheimer’s disease showed that after 24 weeks of ginkgo, in combination with conventional medicine, they improved cognitive performance scores 250.
Ginkgo biloba is usually safe when properly used by healthy adults 251. Side effects of ginkgo may include headache, stomach upset, dizziness, palpitations, constipation, and allergic skin reactions. Although some clinical trials suggested ginkgo might raise the risk of stomach bleeding in older adults, a meta-analysis of numerous clinical trials found no such association 252. People with a known bleeding risk should be cautious about ginkgo possibly increasing the risk of bleeding. A 2017 review found that Ginkgo biloba has an overall low risk of drug interactions, but there may be a potential increased bleeding risk with warfarin (Coumadin) 253. Because ginkgo can dilate blood vessels, it may not be safe in patients taking medication for high blood pressure. Ginkgo taken orally may also be unsafe for children, people with diabetes, or women trying to become pregnant. It might cause early labor or extra bleeding during delivery if used near that time. Little is known about whether it’s safe to use ginkgo while breastfeeding.
In a 2013 study, rodents given ginkgo leaf extract had an increased risk of developing liver and thyroid cancer at the end of the 2-year tests. Whether these results apply to humans is unclear.
Standardized Ginkgo biloba extracts are generally safe for most people at oral doses of 120 to 240 mg per day. Although some ginkgo biloba extracts are sold over-the-counter, the EGb761® formulations tested in clinical trials are only available by prescription.
Curcumin
Curcumin also called Curcuma longa, Indian saffron or diferuloylmethane, is a natural bright yellow pigment obtained from the tubers of turmeric (Curcuma longa plant) in the ginger family, is native to Southeast Asia and is grown commercially in that region, primarily in India 254. Turmeric is used as a culinary spice and traditional medicine. Turmeric’s main active component — curcumin — is what gives a yellowish color to curry powder and is a major ingredient in curry powders common in many Indian and Asian dishes, and it is also used as a coloring for foods, fabrics and cosmetics. The extract of turmeric contains three major curcuminoids (Figure 1): Curcumin (60–70%), demethoxycurcumin (20–27%), and bisdemethoxycurcumin (10–15%) 255. The activities of turmeric are commonly attributed to curcuminoids (curcumin, demethoxycurcumin and others). The turmeric root can be dried and made into capsules, tablets, extracts, powders or teas. Or it can be made into a paste to apply to the skin. Turmeric may be unsafe for use during pregnancy in amounts greater than those commonly found in food. Little is known about whether it’s safe to use turmeric in amounts greater than those commonly found in food while breastfeeding.
Curcumin has been consumed for hundreds of years and is considered pharmacologically safe when taken at doses up to 100 mg/day 256. According to the Joint Food and Agriculture Organization/The World Health Organization (JECFA) report on food additives, the Acceptable Daily Intake Level (ADI) of curcumin is 0 to 0.1 mg/kg body weight 257. In clinical trials, curcumin was found to be safe and effective, and the US Food and Drug Administration (FDA) approved the curcumin as a “generally safe” (GRAS) compound 258.
Historically, turmeric was used in Ayurveda and other traditional Indian medical systems, as well as Eastern Asian medical systems such as traditional Chinese medicine. In India, it was traditionally used for disorders of the skin, upper respiratory tract, joints, and digestive system. Today, turmeric is promoted as a dietary supplement for a variety of conditions, including arthritis, digestive disorders, respiratory infections, allergies, liver disease, depression, and many others as antioxidant, anti-inflammatory, immune regulatory, antiviral, antifungal, antibacterial, anticancer, antidiabetic, neuro-protective, cardiovascular protective, and hepatoprotective effects 259. These properties are attributed to the key elements in the curcumin structure 260. Turmeric is currently being promoted as a dietary supplement for a variety of conditions, including arthritis, digestive disorders, respiratory infections, allergies, liver disease, depression, and many others.
For the intake of curcumin, a moderate-quality meta-analysis revealed very low quality of evidence suggesting a small but significant short-term effect of low heterogeneity on depression severity by pooling six randomized controlled trials 261. No serious adverse events were recorded. Evidence had to be downgraded due to unclear risk of selection, performance, detection, attrition and reporting bias in over the half of the included randomized controlled trials, the imprecision of the pooled effect and the possibly high risk of publication bias.
Turmeric and conventionally formulated curcumin products are probably safe when taken orally or applied to the skin in the recommended amounts. Turmeric may be unsafe for use during pregnancy in amounts greater than those commonly found in food. Little is known about whether it’s safe to use turmeric in amounts greater than those commonly found in food while breastfeeding.
Probiotics
Probiotics are supplements or foods that contain live microorganisms (in most cases, bacteria but also include yeasts) that are similar to beneficial microorganisms found in the human gut and may be beneficial to health 262. The International Scientific Association for Probiotics and Prebiotics defines “probiotics” as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” 263. In addition, products containing dead microorganisms and those made by microorganisms (such as proteins, polysaccharides, nucleotides, and peptides) are, by definition, not probiotics. Most probiotics in use today are derived either from fermented foods or from the microbes colonizing a healthy human. The human gastrointestinal tract is colonized by many microorganisms, including bacteria, archaea, viruses, fungi, and protozoa. The activity and composition of these microorganisms (collectively known as the gut microbiota, microbiome or intestinal microflora) can affect human health and disease. Priobiotics are thought to alter the microflora of the host and the live microorganisms are believed to provide health benefits when consumed 264. Products sold as probiotics include foods (such as yogurt), dietary supplements, and products that aren’t used orally, such as such as suppositories and skin creams. Although people often think of them as harmful “germs,” many microorganisms help your bodies to function properly. For example, normal intestinal bacteria digest food, destroy disease-causing microorganisms and produce vitamins. Large numbers of microorganisms live on and in our bodies. In fact, microorganisms in the human body outnumber human cells by 100 to 1. Many of the microorganisms in probiotic products are the same as or similar to the ones in your bodies 265. Although a great deal of research has been done on probiotics, much remains to be learned, because not all foods and dietary supplements labeled as “probiotics” on the market have proven health benefits 266.
Probiotics should not be confused with prebiotics. The term “prebiotics” refers to nondigestible carbohydrates that act as food for probiotics. Prebiotics are typically complex carbohydrates such as inulin and other fructo-oligosaccharides that microorganisms in the gastrointestinal tract use as metabolic fuel 267. Commonly known prebiotics are: oligofructose, inulin, galacto-oligosaccharides, lactulose and breast milk oligosaccharides. Lactulose is a synthetic disaccharide used as a drug for the treatment of constipation and hepatic encephalopathy. The prebiotic oligofructose is found naturally in many foods, such as wheat, onions, bananas, honey, garlic, and leeks. Oligofructose can also be isolated from chicory root or synthesized enzymatically from sucrose. Fermentation of oligofructose in the colon results in a large number of physiologic effects, including 268:
- Increasing the numbers of bifidobacteria in the colon
- Increasing calcium absorption
- Increasing fecal weight
- Shortening gastrointestinal transit time
- Possibly lowering blood lipid levels
The increase in colonic bifidobacteria has been assumed to benefit human health by producing compounds to inhibit potential pathogens, by reducing blood ammonia levels, and by producing vitamins and digestive enzymes 268.
Although more research is needed, there’s encouraging evidence that probiotics may help:
- Treat diarrhea, especially following treatment with certain antibiotics (e.g., prevention of antibiotic-associated diarrhea including diarrhea caused by Clostridium difficile)
- Prevent and treat vaginal yeast infections and urinary tract infections
- Treat irritable bowel syndrome
- Treat inflammatory bowel disease
- Speed treatment of certain intestinal infections
- Prevent or reduce the severity of common colds and flu
- Allergic disorders such as atopic dermatitis (eczema) and allergic rhinitis (hay fever)
- Tooth decay, periodontal disease, and other oral health problems
- Colic in infants
- Liver disease
- Prevention of necrotizing enterocolitis in very low birth weight infants.
Scientists are increasingly recognizing that the gut microbiome might influence neuropsychiatric symptoms and might be a tractable target for novel treatment options 269. A study conducted by Cryan 270 showed that disruption of the microbiome induced mice to behave in ways that mimicked human anxiety, depression, and even autism. Thus, as Cryan et al 270 noted, “that dietary treatments could be used as either [an] adjunct or sole therapy for mood disorders is not beyond the realm of possibility”. Cryan et al 271 has reported that two varieties of Bifidobacterium were more effective than escitalopram (Lexapro), an agent used to treat anxious and depressed behaviors, in a laboratory mouse strain.
The effectiveness of the supplementation with probiotics on depression severity was analysed by a moderate-quality meta-analysis of five randomized controlled trials, of which only one randomized controlled trial with overall low risk of bias was carried out on patients with major depression 272. The analysis of the randomized controlled trial revealed a significant medium but imprecise short-term effect in comparison with placebo 272. This led to overall very low quality of evidence for probiotics supplementation for depression.
- Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013 Aug 1;369(5):448-57. doi: 10.1056/NEJMra1201534[↩]
- Nobis, A., Zalewski, D., & Waszkiewicz, N. (2020). Peripheral Markers of Depression. Journal of clinical medicine, 9(12), 3793. https://doi.org/10.3390/jcm9123793[↩][↩]
- Virkkunen M., Goldman D., Nielsen D.A., Linnoila M. Low brain serotonin turnover rate (low CSF 5-HIAA) and impulsive violence. J. Psychiatry Neurosci. 1995;20:271–275.[↩]
- Lidberg L., Tuck J.R., Asberg M., Scalia-Tomba G.P., Bertilsson L. Homicide, suicide and CSF 5-HIAA. Acta Psychiatr. Scand. 1985;71:230–236. doi: 10.1111/j.1600-0447.1985.tb01279.x[↩]
- Kaufman J., DeLorenzo C., Choudhury S., Parsey R.V. The 5-HT1A receptor in major depressive disorder. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2016;26:397–410. doi: 10.1016/j.euroneuro.2015.12.039[↩]
- Wang L., Zhou C., Zhu D., Wang X., Fang L., Zhong J., Mao Q., Sun L., Gong X., Xia J., et al. Serotonin-1A receptor alterations in depression: A meta-analysis of molecular imaging studies. BMC Psychiatry. 2016;16:319. doi: 10.1186/s12888-016-1025-0[↩]
- Maes M., Ringel K., Kubera M., Berk M., Rybakowski J. Increased autoimmune activity against 5-HT: A key component of depression that is associated with inflammation and activation of cell-mediated immunity, and with severity and staging of depression. J. Affect. Disord. 2012;136:386–392. doi: 10.1016/j.jad.2011.11.016[↩]
- Mendelson S.D. The current status of the platelet 5-HT(2A) receptor in depression. J. Affect. Disord. 2000;57:13–24. doi: 10.1016/S0165-0327(99)00177-9[↩]
- Bertone-Johnson ER: Vitamin D and the occurrence of depression: causal association or circumstantial evidence? Nutr Rev 2009; 67:481–492.[↩][↩]
- Cass WA, Smith MP, Peters LE: Calcitriol protects against the dopamine- and serotonin-depleting effects of neurotoxic doses of methamphetamine. Ann N Y Acad Sci 2006; 1074:261–271.[↩]
- Anglin RE, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013 Feb;202:100-7. doi: 10.1192/bjp.bp.111.106666[↩][↩][↩]
- Bertone-Johnson E. R. (2009). Vitamin D and the occurrence of depression: causal association or circumstantial evidence?. Nutrition reviews, 67(8), 481–492. https://doi.org/10.1111/j.1753-4887.2009.00220.x[↩]
- Khoraminya N, Tehrani-Doost M, Jazayeri S, Hosseini A, Djazayery A. Therapeutic effects of vitamin D as adjunctive therapy to fluoxetine in patients with major depressive disorder. Aust N Z J Psychiatry. 2013 Mar;47(3):271-5. doi: 10.1177/0004867412465022[↩]
- Jorde R, Sneve M, Figenschau Y, Svartberg J, Waterloo K. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: randomized double blind trial. J Intern Med. 2008 Dec;264(6):599-609. doi: 10.1111/j.1365-2796.2008.02008.x[↩]
- Kjærgaard M, Waterloo K, Wang CE, Almås B, Figenschau Y, Hutchinson MS, Svartberg J, Jorde R. Effect of vitamin D supplement on depression scores in people with low levels of serum 25-hydroxyvitamin D: nested case-control study and randomised clinical trial. Br J Psychiatry. 2012 Nov;201(5):360-8. doi: 10.1192/bjp.bp.111.104349[↩]
- Yalamanchili V, Gallagher JC. Treatment with hormone therapy and calcitriol did not affect depression in older postmenopausal women: no interaction with estrogen and vitamin D receptor genotype polymorphisms. Menopause. 2012 Jun;19(6):697-703. doi: 10.1097/gme.0b013e31823bcec5[↩]
- McCormick D. Vitamin B6. In: Bowman B, Russell R, eds. Present Knowledge in Nutrition. 9th ed. Washington, DC: International Life Sciences Institute; 2006.[↩][↩][↩][↩][↩][↩]
- National Mental Health Association. http://www.mentalhealthamerica.net/[↩][↩]
- American Foundation for Suicide Prevention. http://www.afsp.org[↩][↩]
- National Suicide Prevention Lifeline. https://suicidepreventionlifeline.org/[↩][↩]
- Depression and Bipolar Support Alliance. http://www.dbsalliance.org[↩][↩]
- National Alliance on Mentally Illness https://www.nami.org/[↩][↩]
- National Institute of Mental Health. https://www.nimh.nih.gov/index.shtml[↩][↩]
- National Strategy for Suicide Prevention. http://www.mentalhealth.org/what-to-look-for/suicidal-behavior[↩][↩]
- National Association of Anorexia Nervosa and Associated Disorders. http://www.anad.org/[↩][↩]
- Bailey LB, Caudill MA. Folate. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Washington, DC: Wiley-Blackwell; 2012:321-42.[↩]
- Folate. https://ods.od.nih.gov/factsheets/Folate-HealthProfessional[↩][↩][↩]
- Carmel R. Folic acid. In: Shils M, Shike M, Ross A, Caballero B, Cousins RJ, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2005:470-81.[↩][↩]
- U.S. Food and Drug Administration. Food Standards: Amendment of Standards of Identity For Enriched Grain Products to Require Addition of Folic Acid. Federal Register 1996;61:8781-97. https://www.govinfo.gov/content/pkg/FR-1996-03-05/pdf/96-5014.pdf[↩]
- Choumenkovitch SF, Selhub J, Wilson PW, Rader JI, Rosenberg IH, Jacques PF. Folic acid intake from fortification in United States exceeds predictions. J Nutr. 2002 Sep;132(9):2792-8. doi: 10.1093/jn/132.9.2792[↩]
- Centers for Disease Control and Prevention (CDC). CDC Grand Rounds: additional opportunities to prevent neural tube defects with folic acid fortification. MMWR Morb Mortal Wkly Rep. 2010 Aug 13;59(31):980-4.[↩][↩]
- Crider KS, Bailey LB, Berry RJ. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients. 2011 Mar;3(3):370-84. doi: 10.3390/nu3030370[↩]
- Huang X, Fan Y, Han X, Huang Z, Yu M, Zhang Y, Xu Q, Li X, Wang X, Lu C, Xia Y. Association between Serum Vitamin Levels and Depression in U.S. Adults 20 Years or Older Based on National Health and Nutrition Examination Survey 2005⁻2006. Int J Environ Res Public Health. 2018 Jun 9;15(6):1215. doi: 10.3390/ijerph15061215[↩][↩]
- Morris MS, Fava M, Jacques PF, Selhub J, Rosenberg IH. Depression and folate status in the US Population. Psychother Psychosom. 2003 Mar-Apr;72(2):80-7. doi: 10.1159/000068692[↩][↩]
- Gougeon L, Payette H, Morais JA, Gaudreau P, Shatenstein B, Gray-Donald K. Intakes of folate, vitamin B6 and B12 and risk of depression in community-dwelling older adults: the Quebec Longitudinal Study on Nutrition and Aging. Eur J Clin Nutr. 2016 Mar;70(3):380-5. doi: 10.1038/ejcn.2015.202[↩]
- Papakostas GI, Petersen T, Mischoulon D, Ryan JL, Nierenberg AA, Bottiglieri T, Rosenbaum JF, Alpert JE, Fava M. Serum folate, vitamin B12, and homocysteine in major depressive disorder, Part 1: predictors of clinical response in fluoxetine-resistant depression. J Clin Psychiatry. 2004 Aug;65(8):1090-5. doi: 10.4088/jcp.v65n0810[↩]
- Trujillo J, Vieira MC, Lepsch J, Rebelo F, Poston L, Pasupathy D, Kac G. A systematic review of the associations between maternal nutritional biomarkers and depression and/or anxiety during pregnancy and postpartum. J Affect Disord. 2018 May;232:185-203. doi: 10.1016/j.jad.2018.02.004[↩]
- Chong MF, Wong JX, Colega M, Chen LW, van Dam RM, Tan CS, Lim AL, Cai S, Broekman BF, Lee YS, Saw SM, Kwek K, Godfrey KM, Chong YS, Gluckman P, Meaney MJ, Chen H; GUSTO study group. Relationships of maternal folate and vitamin B12 status during pregnancy with perinatal depression: The GUSTO study. J Psychiatr Res. 2014 Aug;55:110-6. doi: 10.1016/j.jpsychires.2014.04.006[↩]
- Blunden, C. H., Inskip, H. M., Robinson, S. M., Cooper, C., Godfrey, K. M., & Kendrick, T. R. (2012). Postpartum depressive symptoms: the B-vitamin link. Mental health in family medicine, 9(1), 5–13. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3487611[↩]
- Yan, J., Liu, Y., Cao, L., Zheng, Y., Li, W., & Huang, G. (2017). Association between Duration of Folic Acid Supplementation during Pregnancy and Risk of Postpartum Depression. Nutrients, 9(11), 1206. https://doi.org/10.3390/nu9111206[↩]
- Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: a randomised, placebo controlled trial. J Affect Disord. 2000 Nov;60(2):121-30. doi: 10.1016/s0165-0327(00)00153-1[↩]
- Bedson E, Bell D, Carr D, Carter B, Hughes D, Jorgensen A, Lewis H, Lloyd K, McCaddon A, Moat S, Pink J, Pirmohamed M, Roberts S, Russell I, Sylvestre Y, Tranter R, Whitaker R, Wilkinson C, Williams N. Folate Augmentation of Treatment–Evaluation for Depression (FolATED): randomised trial and economic evaluation. Health Technol Assess. 2014 Jul;18(48):vii-viii, 1-159. doi: 10.3310/hta18480[↩]
- Roberts E, Carter B, Young AH. Caveat emptor: Folate in unipolar depressive illness, a systematic review and meta-analysis. J Psychopharmacol. 2018 Apr;32(4):377-384. doi: 10.1177/0269881118756060[↩][↩][↩]
- Sarris J, Murphy J, Mischoulon D, Papakostas GI, Fava M, Berk M, Ng CH. Adjunctive Nutraceuticals for Depression: A Systematic Review and Meta-Analyses. Am J Psychiatry. 2016 Jun 1;173(6):575-87. doi: 10.1176/appi.ajp.2016.15091228[↩]
- Ravindran, A. V., Balneaves, L. G., Faulkner, G., Ortiz, A., McIntosh, D., Morehouse, R. L., Ravindran, L., Yatham, L. N., Kennedy, S. H., Lam, R. W., MacQueen, G. M., Milev, R. V., Parikh, S. V., & CANMAT Depression Work Group (2016). Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 5. Complementary and Alternative Medicine Treatments. Canadian journal of psychiatry. Revue canadienne de psychiatrie, 61(9), 576–587. https://doi.org/10.1177/0706743716660290[↩]
- Papakostas GI, Shelton RC, Zajecka JM, Etemad B, Rickels K, Clain A, Baer L, Dalton ED, Sacco GR, Schoenfeld D, Pencina M, Meisner A, Bottiglieri T, Nelson E, Mischoulon D, Alpert JE, Barbee JG, Zisook S, Fava M. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am J Psychiatry. 2012 Dec;169(12):1267-74. doi: 10.1176/appi.ajp.2012.11071114[↩][↩][↩]
- Cleare A, Pariante CM, Young AH, Anderson IM, Christmas D, Cowen PJ, Dickens C, Ferrier IN, Geddes J, Gilbody S, Haddad PM, Katona C, Lewis G, Malizia A, McAllister-Williams RH, Ramchandani P, Scott J, Taylor D, Uher R; Members of the Consensus Meeting. Evidence-based guidelines for treating depressive disorders with antidepressants: A revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol. 2015 May;29(5):459-525. doi: 10.1177/0269881115581093[↩]
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academy Press; 1998.[↩][↩]
- Bailey LB, Caudill MA. Folate. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Washington, DC: Wiley-Blackwell; 2012:321-42[↩]
- U.S. Department of Agriculture, Agricultural Research Service. FoodData Central. https://fdc.nal.usda.gov[↩][↩]
- Mackey A, Davis S, Gregory J. Vitamin B6. In: Shils M, Shike M, Ross A, Caballero B, Cousins R, eds. Modern Nutrition in Health and Disease. 10th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2005.[↩][↩][↩]
- Subar AF, Krebs-Smith SM, Cook A, Kahle LL. Dietary sources of nutrients among US adults, 1989 to 1991. J Am Diet Assoc. 1998 May;98(5):537-47. doi: 10.1016/S0002-8223(98)00122-9[↩]
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academy Press; 1998. https://www.nap.edu/catalog/6015/dietary-reference-intakes-for-thiamin-riboflavin-niacin-vitamin-b6-folate-vitamin-b12-pantothenic-acid-biotin-and-choline[↩][↩]
- Williams AL, Cotter A, Sabina A, Girard C, Goodman J, Katz DL. The role for vitamin B-6 as treatment for depression: a systematic review. Fam Pract. 2005 Oct;22(5):532-7. doi: 10.1093/fampra/cmi040[↩]
- Vitamin D. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional[↩][↩]
- Jones G. Vitamin D. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease, 11th ed. Philadelphia: Lippincott Williams & Wilkins, 2014.[↩]
- Silva MC, Furlanetto TW. Intestinal absorption of vitamin D: a systematic review. Nutr Rev. 2018 Jan 1;76(1):60-76. doi: 10.1093/nutrit/nux034[↩][↩]
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academy Press, 2010.[↩][↩][↩][↩][↩]
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011 Jul;96(7):1911-30. doi: 10.1210/jc.2011-0385. Epub 2011 Jun 6. Erratum in: J Clin Endocrinol Metab. 2011 Dec;96(12):3908.[↩]
- Brown LL, Cohen B, Tabor D, Zappalà G, Maruvada P, Coates PM. The vitamin D paradox in Black Americans: a systems-based approach to investigating clinical practice, research, and public health – expert panel meeting report. BMC Proc. 2018 May 9;12(Suppl 6):6. doi: 10.1186/s12919-018-0102-4[↩]
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academy Press, 2010[↩][↩]
- Gowda U, Mutowo MP, Smith BJ, Wluka AE, Renzaho AM. Vitamin D supplementation to reduce depression in adults: meta-analysis of randomized controlled trials. Nutrition. 2015 Mar;31(3):421-9. doi: 10.1016/j.nut.2014.06.017[↩]
- Jorde, R., & Kubiak, J. (2018). No improvement in depressive symptoms by vitamin D supplementation: results from a randomised controlled trial. Journal of nutritional science, 7, e30. https://doi.org/10.1017/jns.2018.19[↩]
- Jorde R, Grimnes G. Vitamin D: no cure for depression. Am J Clin Nutr. 2019 Nov 1;110(5):1043-1044. doi: 10.1093/ajcn/nqz186[↩]
- de Koning, E. J., Lips, P., Penninx, B., Elders, P., Heijboer, A. C., den Heijer, M., Bet, P. M., van Marwijk, H., & van Schoor, N. M. (2019). Vitamin D supplementation for the prevention of depression and poor physical function in older persons: the D-Vitaal study, a randomized clinical trial. The American journal of clinical nutrition, 110(5), 1119–1130. https://doi.org/10.1093/ajcn/nqz141[↩]
- Okereke, O. I., Reynolds, C. F., 3rd, Mischoulon, D., Chang, G., Vyas, C. M., Cook, N. R., Weinberg, A., Bubes, V., Copeland, T., Friedenberg, G., Lee, I. M., Buring, J. E., & Manson, J. E. (2020). Effect of Long-term Vitamin D3 Supplementation vs Placebo on Risk of Depression or Clinically Relevant Depressive Symptoms and on Change in Mood Scores: A Randomized Clinical Trial. JAMA, 324(5), 471–480. https://doi.org/10.1001/jama.2020.10224[↩]
- U.S. Department of Agriculture, Agricultural Research Service. FoodData Central. https://fdc.nal.usda.gov/index.html[↩][↩]
- Omega-3 Fatty Acids. https://ods.od.nih.gov/factsheets/Omega3FattyAcids-Consumer[↩][↩]
- Harris WS. Omega-3 fatty acids. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:577-86.[↩][↩]
- U.S. Department of Agriculture, Agricultural Research Service. What We Eat in America NHANES 2017-2018. https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/1718/wweia_2017_2018_data.pdf[↩][↩]
- Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report. 2015 Feb 10;(79):1-16.[↩][↩]
- Black LI, Clarke TC, Barnes PM, Stussman BJ, Nahin RL. Use of complementary health approaches among children aged 4-17 years in the United States: National Health Interview Survey, 2007-2012. Natl Health Stat Report. 2015 Feb 10;(78):1-19.[↩][↩]
- Papanikolaou Y, Brooks J, Reider C, Fulgoni VL 3rd. U.S. adults are not meeting recommended levels for fish and omega-3 fatty acid intake: results of an analysis using observational data from NHANES 2003-2008. Nutr J. 2014 Apr 2;13:31. doi: 10.1186/1475-2891-13-31. Erratum in: Nutr J. 2014;13:64.[↩]
- Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). Washington, DC: National Academy Press; 2005.[↩][↩]
- Appleton KM, Sallis HM, Perry R, Ness AR, Churchill R. Omega-3 fatty acids for depression in adults. Cochrane Database Syst Rev. 2015 Nov 5;2015(11):CD004692. doi: 10.1002/14651858.CD004692.pub4 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5321518[↩]
- Haller, H., Anheyer, D., Cramer, H., & Dobos, G. (2019). Complementary therapies for clinical depression: an overview of systematic reviews. BMJ open, 9(8), e028527. https://doi.org/10.1136/bmjopen-2018-028527[↩][↩][↩]
- Sublette, M. E., Ellis, S. P., Geant, A. L., & Mann, J. J. (2011). Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. The Journal of clinical psychiatry, 72(12), 1577–1584. https://doi.org/10.4088/JCP.10m06634[↩]
- Li F, Liu X, Zhang D. Fish consumption and risk of depression: a meta-analysis. J Epidemiol Community Health. 2016 Mar;70(3):299-304. doi: 10.1136/jech-2015-206278[↩]
- Ng QX, Venkatanarayanan N, Ho CY. Clinical use of Hypericum perforatum (St John’s wort) in depression: A meta-analysis. J Affect Disord. 2017 Mar 1;210:211-221. doi: 10.1016/j.jad.2016.12.048[↩]
- Apaydin, E. A., Maher, A. R., Shanman, R., Booth, M. S., Miles, J. N., Sorbero, M. E., & Hempel, S. (2016). A systematic review of St. John’s wort for major depressive disorder. Systematic reviews, 5(1), 148. https://doi.org/10.1186/s13643-016-0325-2[↩]
- Linde, K., Berner, M. M., & Kriston, L. (2008). St John’s wort for major depression. The Cochrane database of systematic reviews, 2008(4), CD000448. https://doi.org/10.1002/14651858.CD000448.pub3[↩][↩]
- Maher, A. R., Hempel, S., Apaydin, E., Shanman, R. M., Booth, M., Miles, J. N., & Sorbero, M. E. (2016). St. John’s Wort for Major Depressive Disorder: A Systematic Review. Rand health quarterly, 5(4), 12. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5158227[↩]
- St. John’s Wort https://www.nccih.nih.gov/health/st-johns-wort[↩]
- Mills, E., Montori, V. M., Wu, P., Gallicano, K., Clarke, M., & Guyatt, G. (2004). Interaction of St John’s wort with conventional drugs: systematic review of clinical trials. BMJ (Clinical research ed.), 329(7456), 27–30. https://doi.org/10.1136/bmj.329.7456.27[↩]
- 5 Things To Know About St. John’s Wort and Depression. https://www.nccih.nih.gov/health/tips/things-to-know-about-st-johns-wort-and-depression[↩]
- DGPPN, BÄK, KBV . Clinical practice guideline for unipolar depression [S3-Leitlinie/Nationale VersorgungsLeitlinie Unipolare Depression – Langfassung, 2 Auflage. Version 5], 2015.[↩]
- St. John’s Wort (Hypericum perforatum):Clinical Effects on Depression and Other Conditions. Alt Med Rev 1998;3(1):18-26. https://altmedrev.com/wp-content/uploads/2019/02/v3-1-18.pdf[↩]
- 5-Hydroxytryptophan. Alternative Medicine Review Volume 3, Number 3, 1998, pages 224-226. https://altmedrev.com/wp-content/uploads/2019/02/v3-3-224.pdf[↩][↩][↩][↩][↩]
- Jacobsen, J., Krystal, A. D., Krishnan, K., & Caron, M. G. (2016). Adjunctive 5-Hydroxytryptophan Slow-Release for Treatment-Resistant Depression: Clinical and Preclinical Rationale. Trends in pharmacological sciences, 37(11), 933–944. https://doi.org/10.1016/j.tips.2016.09.001[↩][↩]
- den Boer JA, Westenberg HG. Behavioral, neuroendocrine, and biochemical effects of 5-hydroxytryptophan administration in panic disorder. Psychiatry Res 1990;31:267-278.[↩][↩]
- Sternberg EM, Van Woert MH, Young SN, et al. Development of a scleroderma-like illness during therapy with L-5-hydroxytryptophan and carbidopa. N Engl J Med 1980;303:782-787.[↩]
- Michelson D, Page SW, Casey R, et al. An eosinophilia-myalgia syndrome related disorder associated with exposure to L-5-hydroxytryptophan. J Rheumatol 1994;21:2261-2265.[↩]
- Jacobsen JPR, Krystal AD, Krishnan KRR, Caron MG. Adjunctive 5-hydroxytryptophan slow-release for treatment-resistant depression: Clinical and pre-clinical rationale. Trends in pharmacological sciences. 2016;37(11):933-944. doi:10.1016/j.tips.2016.09.001 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5728156[↩]
- Haddad PM. Antidepressant discontinuation syndromes. Drug Saf. 2001;24:183–197.[↩]
- van Harten J. Clinical pharmacokinetics of selective serotonin reuptake inhibitors. Clin Pharmacokinet. 1993;24:203–220.[↩]
- Gijsman HJ, et al. Placebo-controlled comparison of three dose-regimens of 5-hydroxytryptophan challenge test in healthy volunteers. J Clin Psychopharmacol. 2002;22:183–189.[↩][↩]
- Angst J, Woggon B, Schoepf J. The treatment of depression with L-5-hydroxytryptophan versus imipramine. Results of two open and one double-blind study. Arch Psychiatr Nervenkr. 1977;224(2):175-186.[↩]
- Cauffield JS, Forbes HJ. Dietary supplements used in the treatment of depression, anxiety, and sleep disorders. Lippincotts Prim Care Pract. 1999;3(3):290-304.[↩]
- Shaw K, Turner J, Del Mar C. Are tryptophan and 5-hydroxytryptophan effective treatments for depression? A meta-analysis. Aust N Z J Psychiatry. 2002;36(4):488-491.[↩]
- Van Praag HM. Management of depression with serotonin precursors. Biol Psychiatry. 1981;16(3):291-310.[↩]
- Zmilacher K, Battegay R, Gastpar M. L-5-hydroxytryptophan alone and in combination with a peripheral decarboxylase inhibitor in the treatment of depression. Neuropsychobiology. 1988;20(1):28-33.[↩]
- Birdsall TC. 5-Hydroxytryptophan: a clinically-effective serotonin precursor. Altern Med Rev. 1998;3(4):271-280.[↩]
- Jacobsen JPR, Krystal AD, Krishnan KRR, Caron MG. Adjunctive 5-hydroxytryptophan slow-release for treatment-resistant depression: Clinical and pre-clinical rationale. Trends in pharmacological sciences. 2016;37(11):933-944. doi:10.1016/j.tips.2016.09.001 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5728156/[↩][↩]
- Nilausen DO, et al. The perception and pharmacokinetics of a 20-mg dose of escitalopram orodispersible tablets in a relative bioavailability study in healthy men. Clin Ther. 2011;33:1492–1502.[↩]
- Gibson, E. (2018). Tryptophan supplementation and serotonin function: Genetic variations in behavioural effects. Proceedings of the Nutrition Society, 77(2), 174-188. doi:10.1017/S0029665117004451[↩]
- Effects of tryptophan loading on human cognition, mood, and sleep. Silber BY, Schmitt JA. Neurosci Biobehav Rev. 2010 Mar; 34(3):387-407. https://www.ncbi.nlm.nih.gov/pubmed/19715722[↩]
- van Praag HM, Lemus C. Monoamine precursors in the treatment of psychiatric disorders. In: Wurtman RJ, Wurtman JJ, eds. Nutrition and the Brain. New York, NY: Raven Press; 1986:89-139.[↩]
- Capuron L., Ravaud A., Neveu P.J., Miller A.H., Maes M., Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol. Psychiatry. 2002;7:468–473. doi: 10.1038/sj.mp.4000995[↩]
- Ogawa S., Fujii T., Koga N., Hori H., Teraishi T., Hattori K., Noda T., Higuchi T., Motohashi N., Kunugi H. Plasma L-tryptophan concentration in major depressive disorder: New data and meta-analysis. J. Clin. Psychiatry. 2014;75:e906–e915. doi: 10.4088/JCP.13r08908[↩]
- rand S.J., Möller M., Harvey B.H. A review of biomarkers in mood and psychotic disorders: A dissection of clinical vs. preclinical correlates. Curr. Neuropharmacol. 2015;13:324–368. doi: 10.2174/1570159X13666150307004545[↩]
- Fernstrom J.D., Wurtman R.J. Brain serotonin content: Physiological dependence on plasma tryptophan levels. Science. 1971;173:149–152. doi: 10.1126/science.173.3992.149[↩]
- Young S.N. The effect of raising and lowering tryptophan levels on human mood and social behaviour. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013;368:20110375. doi: 10.1098/rstb.2011.0375[↩]
- Kaddurah-Daouk R., Bogdanov M.B., Wikoff W.R., Zhu H., Boyle S.H., Churchill E., Wang Z., Rush A.J., Krishnan R.R., Pickering E., et al. Pharmacometabolomic mapping of early biochemical changes induced by sertraline and placebo. Transl. Psychiatry. 2013;3:e223. doi: 10.1038/tp.2012.142[↩]
- Nagai R, Taniguchi N. Amino acids and proteins. In: Baynes JW, Dominiczak MH, eds. Medical Biochemistry. 5th ed. Philadelphia, PA: Elsevier; 2019:chap 2.[↩]
- Lader M, Herrington R. Drug treatment in psychiatry ‐ psychotropic drugs. In: Praag H, Lader H, Rafaelsen O, Sachar E editor(s). Handbook of Biological Psychiatry. Vol. 5, New York, NY: Marcel Dekker, 1981.[↩][↩]
- Praag H. Management of depression with serotonin precursors. Biological Psychiatry 1981;16(3):291‐310.[↩]
- Gelenberg A, Gibson C, Wojcik J. Neurotransmitter precursors for the treatment of depression. Psychopharmacology Bulletin 1982;18(1):7‐18.[↩]
- D’Elia G, Hanson L, Raotma H. L‐tryptophan and 5‐hydroxytryptophan in the treatment of depression, a review. Acta Psychiatrica Scandinavica 1978;57(3):239‐52.[↩]
- Beckmann H, Kaspers S. Serotonin precursors as an antidepressant: an overview. [Serotonin‐Vorstufen als Antidepressiva: eine Ubersicht.]. Fortschritte Neurologie und Psychiatrie 1983;51:176‐82.[↩][↩]
- Friedman M., Levin C.E. Nutritional and medicinal aspects of d-amino acids. Amino Acids. 2012;42:1553–1582. doi: 10.1007/s00726-011-0915-1[↩]
- Marz RB. Medical Nutrition from Marz. 2nd ed. Portland, OR: Omni-Press; 1999:95-98.[↩][↩]
- Young LS, Stoll S. Proteins and amino acids. In: Matarese LE, Gottschlich MM, eds. Contemporary Nutrition Support Practice. 2nd ed. New York, NY: Saunders; 2003:94-104.[↩]
- Kilbourne EM, Philen RM, Kamb ML, Falk H. Tryptophan produced by Showa Denko and epidemic eosinophilia-myalgia syndrome. J Rheumatol Suppl 1996;46:81-88.[↩]
- Toyo’oka T, Yamazaki T, Tanimoto T, Sato K, Sato M, Toyoda M, et al. Characterization of contaminants in EMS‐associated L‐tryptophan samples by high‐performance liquid chromatography. Chemical and Pharmaceutical Bulletin 1991;39(3):820‐2.[↩]
- Fernstrom JD. Effects and side effects associated with the non-nutritional use of tryptophan by humans. J Nutr. 2012 Dec;142(12):2236S-2244S. doi: 10.3945/jn.111.157065[↩]
- Horowitz R, Daniels S. Bias or biology: evaluating the epidemiologic studies of L‐tryptophan and the eosinophilia‐myalgia syndrome. Journal of Rheumatology 1996;46(Suppl 1):81‐8.[↩]
- Michelson D, Page S, Casey R, Trucksess M, Love L, Milstien S, et al. An eosinophilia‐myalgia syndrome related disorder associated with exposure to L‐5‐hydroxytryptophan. Journal of Rheumatology 1994;21(12):2261‐5.[↩]
- Wurtman R, Hefti F, Melamed E. Precursor control of neurotransmitter synthesis. Pharmacological Reviews 1981;32(4):315‐35.[↩]
- Young V.R., Hussein M.A., Murray E., Scrimshaw N.S. Plasma tryptophan response curve and its relation to tryptophan requirements in young adult men. J. Nutr. 1971;101:45–59.[↩]
- Richard D.M., Dawes M.A., Mathias C.W., Acheson A., Hill-Kapturczak N., Dougherty D.M. l-tryptophan: Basic metabolic functions, behavioral research and therapeutic indications. Int. J. Tryptophan Res. IJTR. 2009;2:45–60.[↩]
- L-Tryptophan. http://www.hmdb.ca/metabolites/HMDB0000929[↩]
- Stryer L. Biochemistry. 4th ed. New York: WH Freeman and Company; 1995.[↩]
- Pardridge WM, Oldendorf WH. Kinetic analysis of blood-brain barrier transport of amino acids. Biochim Biophys Acta. 1975;401:128–36.[↩]
- Reilly JG, McTavish SFB, Young AH. Rapid depletion of plasma tryptophan: A review of studies and experimental methodology. J Psychopharmacol. 1997;11:381–92.[↩]
- Young LS, Stoll S. Proteins and amino acids. In: Matarese LE, Gottschlich MM, editors. Contemporary Nutrition Support Practice. 2nd ed. Vol. 1. New York: Saunders; 2003. pp. 94–104.[↩]
- Rambali B, Van Andel E, Schenk G, et al. The contribution of cocoa additive to cigarette smoking addiction. RIVM report 650270002/2002. The National Institute for Public Health and the Environment; Netherlands: 2002.[↩][↩]
- Young VR. Adult amino acid requirements: The case for a major revision in current recommendations. J Nutr. 1994;124:1517S–23S.[↩]
- Fernstrom MH, Fernstrom JD. Brain tryptophan concentrations and serotonin synthesis remain responsive to food consumption after the ingestion of sequential meals. Am J Clin Nutr. 1995 Feb;61(2):312-9. doi: 10.1093/ajcn/61.2.312[↩]
- van Donkelaar EL, Blokland A, Ferrington L, Kelly PA, Steinbusch HW, Prickaerts J. Mechanism of acute tryptophan depletion: is it only serotonin? Mol Psychiatry. 2011 Jul;16(7):695-713. doi: 10.1038/mp.2011.9[↩]
- Richard, DM, Dawes, MA, Mathias, CW et al. (2009) L-Tryptophan: basic metabolic functions, behavioral research and therapeutic indications. Int J Tryptophan Res 2, 45–60. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2908021[↩]
- Jenkins, T. A., Nguyen, J. C., Polglaze, K. E., & Bertrand, P. P. (2016). Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients, 8(1), 56. https://doi.org/10.3390/nu8010056[↩]
- Cowen PJ, Parry-Billings M, Newsholme EA. Decreased plasma tryptophan levels in major depression. J Affect Disord 1989;16:27-31.[↩]
- Clark L, Roiser JP, Cools R, et al. Stop signal response inhibition is not modulated by tryptophan depletion or the serotonin transporter polymorphism in healthy volunteers: implications for the 5-HT theory of impulsivity. Psychopharmacology (Berl) 2005;182:570-578.[↩]
- Praschak-Rieder N, Wilson AA, Hussey D, et al. Effects of tryptophan depletion on the serotonin transporter in healthy humans. Biol Psychiatry 2005;58:825-830.[↩]
- Thomson, J, Rankin, H, Ashcroft, GW et al. (1982) The treatment of depression in general practice: a comparison of L-tryptophan, amitriptyline, and a combination of L-tryptophan and amitriptyline with placebo. Psychol Med 12, 741–751.[↩]
- Shaw KA, Turner J, Del Mar C, et al. . Tryptophan and 5-hydroxytryptophan for depression. Cochrane Database Syst Rev 2002;12 10.1002/14651858.CD003198[↩][↩]
- Eccleston D, Ashcroft GW, Crawford TB. Effect of tryptophan administration on 5HIAA in cerebrospinal fluid in man. J Neurol Neurosurg Psychiatry. 1970 Apr;33(2):269-72. doi: 10.1136/jnnp.33.2.269[↩]
- Young SN, Gauthier S. Effect of tryptophan administration on tryptophan, 5-hydroxyindoleacetic acid and indoleacetic acid in human lumbar and cisternal cerebrospinal fluid. J Neurol Neurosurg Psychiatry. 1981 Apr;44(4):323-8. doi: 10.1136/jnnp.44.4.323[↩]
- Price WA, Zimmer B, Kucas P. Serotonin syndrome: a case report. J Clin Pharmacol 1986;26:77-78.[↩]
- Rossle M, Herz R, Klein B, Gerok W. Tryptophan metabolism in liver diseases: a pharmacokinetic and enzymatic study. Klin Wochenschr 1986;64:590-594. [Article in German][↩]
- Devoe LD, Castillo RA, Searle NS, Searle JR. Maternal dietary substrates and human fetal biophysical activity. I. The effects of tryptophan and glucose on fetal breathing movements. Am J Obstet Gynecol 1986;155:135-139.[↩]
- Tryptophan (Oral Route). https://www.mayoclinic.org/drugs-supplements/tryptophan-oral-route/before-using/drg-20064453[↩]
- United States Department of Agriculture Agricultural Research Service. USDA Food Composition Databases. https://ndb.nal.usda.gov/ndb/search/list[↩]
- Richard D.M., Dawes M.A., Mathias C.W., Acheson A., Hill-Kapturczak N., Dougherty D.M. l-tryptophan: Basic metabolic functions, behavioral research and therapeutic indications. Int. J. Tryptophan Res. IJTR. 2009;2:45–60 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2908021/[↩]
- Sharma A, Gerbarg P, Bottiglieri T, Massoumi L, Carpenter LL, Lavretsky H, Muskin PR, Brown RP, Mischoulon D; as Work Group of the American Psychiatric Association Council on Research. S-Adenosylmethionine (SAMe) for Neuropsychiatric Disorders: A Clinician-Oriented Review of Research. J Clin Psychiatry. 2017 Jun;78(6):e656-e667. doi: 10.4088/JCP.16r11113[↩][↩]
- Baldessarini RJ. Neuropharmacology of S-adenosyl-L-methionine. Am J Med 1987;83:95-103.[↩]
- Otero-Losada ME, Rubio MC. Acute changes in 5-HT metabolism after S-adenosyl-L-methionine administration. Gen Pharmacol. 1989;20(4):403-6. doi: 10.1016/0306-3623(89)90186-9[↩]
- Lakhan SE, Vieira KF. Nutritional therapies for mental disorders. Nutr J 2008;7:2. doi:10.1186/1475-2891-7-2[↩]
- Bell KM, Potkin SG, Carreon D, Plon L. S-adenosylmethionine blood levels in major depression: changes with drug treatment. Acta Neurol Scand Suppl 1994;154:15-18.[↩]
- Agnoli A, Andreoli V, Casacchia M, Cerbo R. Effect of s-adenosyl-l-methionine (SAMe) upon depressive symptoms. J Psychiatr Res. 1976;13(1):43-54. doi: 10.1016/0022-3956(76)90008-x[↩]
- Bottiglieri T. S-Adenosyl-L-methionine (SAMe): from the bench to the bedside–molecular basis of a pleiotrophic molecule. Am J Clin Nutr. 2002 Nov;76(5):1151S-7S. doi: 10.1093/ajcn/76/5.1151S[↩]
- Sugden C. One-carbon metabolism in psychiatric illness. Nutr Res Rev. 2006 Jun;19(1):117-36. doi: 10.1079/NRR2006119[↩]
- Papakostas GI. Evidence for S-adenosyl-L-methionine (SAM-e) for the treatment of major depressive disorder. J Clin Psychiatry. 2009;70 Suppl 5:18-22. doi: 10.4088/JCP.8157su1c.04[↩]
- Li Q, Cui J, Fang C, Zhang X, Li L. S-adenosylmethionine Administration Attenuates Low Brain-Derived Neurotrophic Factor Expression Induced by Chronic Cerebrovascular Hypoperfusion or Beta Amyloid Treatment. Neurosci Bull. 2016 Apr;32(2):153-61. doi: 10.1007/s12264-016-0023-z[↩]
- De Berardis D, Marini S, Serroni N, Rapini G, Iasevoli F, Valchera A, Signorelli M, Aguglia E, Perna G, Salone A, Di Iorio G, Martinotti G, Di Giannantonio M. S-Adenosyl-L-Methionine augmentation in patients with stage II treatment-resistant major depressive disorder: an open label, fixed dose, single-blind study. ScientificWorldJournal. 2013 May 12;2013:204649. doi: 10.1155/2013/204649[↩]
- Galizia, I., Oldani, L., Macritchie, K., Amari, E., Dougall, D., Jones, T. N., Lam, R. W., Massei, G. J., Yatham, L. N., & Young, A. H. (2016). S-adenosyl methionine (SAMe) for depression in adults. The Cochrane database of systematic reviews, 10(10), CD011286. https://doi.org/10.1002/14651858.CD011286.pub2[↩][↩]
- Delle Chiaie R, Pancheri P, Scapicchio P. Efficacy and tolerability of oral and intramuscular S-adenosyl-L-methionine 1,4-butanedisulfonate (SAMe) in the treatment of major depression: comparison with imipramine in 2 multicenter studies. Am J Clin Nutr. 2002 Nov;76(5):1172S-6S. doi: 10.1093/ajcn/76/5.1172S[↩]
- Gören JL, Stoll AL, Damico KE, Sarmiento IA, Cohen BM. Bioavailability and lack of toxicity of S-adenosyl-L-methionine (SAMe) in humans. Pharmacotherapy. 2004 Nov;24(11):1501-7. doi: 10.1592/phco.24.16.1501.50943[↩][↩]
- Williams AL, Girard C, Jui D, Sabina A, Katz DL. S-adenosylmethionine (SAMe) as treatment for depression: a systematic review. Clin Invest Med. 2005 Jun;28(3):132-9.[↩]
- Carney MW, Chary TK, Bottiglieri T, Reynolds EH. The switch mechanism and the bipolar/unipolar dichotomy. Br J Psychiatry. 1989 Jan;154:48-51. doi: 10.1192/bjp.154.1.48[↩][↩]
- Kagan BL, Sultzer DL, Rosenlicht N, Gerner RH. Oral S-adenosylmethionine in depression: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 1990 May;147(5):591-5. doi: 10.1176/ajp.147.5.591[↩]
- National Institutes of Health. Magnesium. https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/[↩]
- Institute of Medicine (IOM). Food and Nutrition Board. Dietary Reference Intakes: Calcium, Phosphorus, Magnesium, Vitamin D and Fluoride. Washington, DC: National Academy Press, 1997.[↩][↩][↩]
- Rude RK. Magnesium. In: Coates PM, Betz JM, Blackman MR, Cragg GM, Levine M, Moss J, White JD, eds. Encyclopedia of Dietary Supplements. 2nd ed. New York, NY: Informa Healthcare; 2010:527-37.[↩][↩]
- Rude RK. Magnesium. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, Mass: Lippincott Williams & Wilkins; 2012:159-75.[↩][↩][↩][↩]
- Derom ML, Sayón-Orea C, Martínez-Ortega JM, Martínez-González MA. Magnesium and depression: a systematic review. Nutr Neurosci. 2013 Sep;16(5):191-206. doi: 10.1179/1476830512Y.0000000044[↩]
- Volpe SL. Magnesium. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Ames, Iowa; John Wiley & Sons, 2012:459-74.[↩][↩]
- Elin RJ. Assessment of magnesium status for diagnosis and therapy. Magnes Res 2010;23:1-5. https://www.ncbi.nlm.nih.gov/pubmed/20736141?dopt=Abstract[↩]
- Gibson, RS. Principles of Nutritional Assessment, 2nd ed. New York, NY: Oxford University Press, 2005.[↩][↩][↩]
- Witkowski M, Hubert J, Mazur A. Methods of assessment of magnesium status in humans: a systematic review. Magnesium Res 2011;24:163-80. https://www.ncbi.nlm.nih.gov/pubmed/22064327?dopt=Abstract[↩]
- Institute of Medicine (IOM). Food and Nutrition Board. Dietary Reference Intakes: Calcium, Phosphorus, Magnesium, Vitamin D and Fluoride. Washington, DC: National Academy Press, 1997. https://www.nap.edu/read/5776/chapter/1[↩]
- United States Department of Agriculture Agricultural Research Service. USDA Food Composition Databases. https://ndb.nal.usda.gov/ndb/[↩]
- Rink L, Gabriel P. Zinc and the immune system. Proc Nutr Soc. 2000 Nov;59(4):541-52. doi: 10.1017/s0029665100000781[↩]
- Merck Sharp & Dohme Corp., Merck Manual. Zinc. https://www.merckmanuals.com/professional/nutritional-disorders/mineral-deficiency-and-toxicity/zinc[↩]
- Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc (2001) https://doi.org/10.17226/10026[↩][↩]
- Prasad AS. Zinc: an overview. Nutrition. 1995 Jan-Feb;11(1 Suppl):93-9.[↩][↩][↩]
- Heyneman CA. Zinc deficiency and taste disorders. Ann Pharmacother. 1996 Feb;30(2):186-7. doi: 10.1177/106002809603000215[↩]
- Maret W, Sandstead HH. Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol. 2006;20(1):3-18. doi: 10.1016/j.jtemb.2006.01.006[↩]
- Prasad AS, Beck FW, Grabowski SM, Kaplan J, Mathog RH. Zinc deficiency: changes in cytokine production and T-cell subpopulations in patients with head and neck cancer and in noncancer subjects. Proc Assoc Am Physicians. 1997 Jan;109(1):68-77.[↩]
- Schefft C, Kilarski LL, Bschor T, Köhler S. Efficacy of adding nutritional supplements in unipolar depression: A systematic review and meta-analysis. Eur Neuropsychopharmacol. 2017 Nov;27(11):1090-1109. doi: 10.1016/j.euroneuro.2017.07.004[↩][↩]
- Alaimo K, McDowell MA, Briefel RR, et al. Dietary intake of vitamins, minerals, and fiber of persons ages 2 months and over in the United States: Third National Health and Nutrition Examination Survey, Phase 1, 1986-91. https://www.cdc.gov/nchs/data/ad/ad258.pdf[↩]
- Interagency Board for Nutrition Monitoring and Related Research. Third Report on Nutrition Monitoring in the United States. Washington, DC: U.S. Government Printing Office, 1995.[↩]
- Ervin RB, Kennedy-Stephenson J. Mineral intakes of elderly adult supplement and non-supplement users in the third national health and nutrition examination survey. J Nutr 2002;132:3422-7. https://www.ncbi.nlm.nih.gov/pubmed/12421862?dopt=Abstract[↩]
- Ribar DS, Hamrick KS. Dynamics of Poverty and Food Sufficiency. Food Assistance and Nutrition Report Number 36, 2003. Washington, DC: U.S. Department of Agriculture, Economic Research Service. https://www.ers.usda.gov/publications/pub-details/?pubid=46766[↩]
- Dixon LB, Winkleby MA, Radimer KL. Dietary intakes and serum nutrients differ between adults from food-insufficient and food-sufficient families: Third National Health and Nutrition Examination Survey, 1988-1994. J Nutr 2001;131:1232-46. https://www.ncbi.nlm.nih.gov/pubmed/11285332?dopt=Abstract[↩]
- PRASAD AS, HALSTED JA, NADIMI M. Syndrome of iron deficiency anemia, hepatosplenomegaly, hypogonadism, dwarfism and geophagia. Am J Med. 1961 Oct;31:532-46. doi: 10.1016/0002-9343(61)90137-1[↩]
- Prasad AS. Impact of the discovery of human zinc deficiency on health. J Trace Elem Med Biol. 2014 Oct;28(4):357-63. doi: 10.1016/j.jtemb.2014.09.002[↩]
- Krebs NF. Update on zinc deficiency and excess in clinical pediatric practice. Ann Nutr Metab. 2013;62 Suppl 1:19-29. doi: 10.1159/000348261[↩]
- U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 27. Nutrient Data Laboratory home page, 2014. https://ndb.nal.usda.gov/ndb/[↩]
- Hosseinzadeh H, Motamedshariaty V, Hadizadeh F. Antidepressant effect of kaempferol, a constituent of saffron (Crocus sativus) petal, in mice and rats. Pharmacologyonline. 2007;2:367–370.[↩]
- Kianbakht S, Hashem Dabaghian F. Anti‐obesity and Anorectic Effects of Saffron and its Constituent Crocin in Obese Wistar Rat. Journal of Medicinal Plants 2015;14:25‐33.[↩]
- Ulbricht C, Conquer J, Costa D, Hollands W, Iannuzzi C, Isaac R, Jordan JK, Ledesma N, Ostroff C, Serrano JM, Shaffer MD, Varghese M. An evidence-based systematic review of saffron (Crocus sativus) by the Natural Standard Research Collaboration. J Diet Suppl. 2011 Mar;8(1):58-114. doi: 10.3109/19390211.2011.547666[↩][↩][↩][↩]
- Kianbakht S, Hajiaghaee R. Anti‐hyperglycemic effects of saffron and its active constituents, crocin and safranal, in alloxan‐induced diabetic rats. Journal of Medicinal Plants 2011;10(39):82‐9.[↩]
- Kamalipour M, Akhondzadeh S. Cardiovascular effects of saffron: an evidence‐based review. Journal of Tehran Heart Center 2011;6:59‐61.[↩]
- Mashmoul M, Azlan A, Khaza’ai H, Yusof BNM, Noor SM. Saffron: a natural potent antioxidant as a promising anti‐obesity drug. Antioxidants 2013;2:293‐308.[↩]
- Fadai F, Mousavi B, Ashtari Z, Alibeigi N, Farhang S, Hashempour S, et al. Saffron aqueous extract prevents metabolic syndrome in patients with schizophrenia on olanzapine treatment: a randomized triple blind placebo controlled study. Pharmacopsychotherapy 2014;47:156‐61.[↩]
- José Bagur, M., Alonso Salinas, G. L., Jiménez-Monreal, A. M., Chaouqi, S., Llorens, S., Martínez-Tomé, M., & Alonso, G. L. (2017). Saffron: An Old Medicinal Plant and a Potential Novel Functional Food. Molecules (Basel, Switzerland), 23(1), 30. https://doi.org/10.3390/molecules23010030[↩]
- De Monte C., Carradori S., Chimenti P., Secci D., Mannina L., Alcaro F., Petzer A., N’Da C.I., Gidaro M.C., Costa G., et al. New insights into the biological properties of Crocus sativus L.: Chemical modifications, human monoamine oxidases inhibition and molecular modeling studies. Eur. J. Med. Chem. 2014;82:164–171. doi: 10.1016/j.ejmech.2014.05.048[↩]
- Hausenblas, H. A., Heekin, K., Mutchie, H. L., & Anton, S. (2015). A systematic review of randomized controlled trials examining the effectiveness of saffron (Crocus sativus L.) on psychological and behavioral outcomes. Journal of integrative medicine, 13(4), 231–240. https://doi.org/10.1016/S2095-4964(15)60176-5[↩]
- Hausenblas, H. A., Saha, D., Dubyak, P. J., & Anton, S. D. (2013). Saffron (Crocus sativus L.) and major depressive disorder: a meta-analysis of randomized clinical trials. Journal of integrative medicine, 11(6), 377–383. https://doi.org/10.3736/jintegrmed2013056[↩][↩][↩]
- Akhondzadeh S., Fallah-Pour H., Afkham K., Jamshidi A.H., Khalighi-Cigaroudi F. Comparison of Crocus sativus L. and imipramine in the treatment of mild to moderate depression: A pilot double-blind randomized trial [ISRCTN45683816] BMC Complement. Altern. Med. 2004;4 doi: 10.1186/1472-6882-4-12[↩]
- Akhondzadeh S., Tahmacebi-Pour N., Noorbala A.A., Amini H., Fallah-Pour H., Jamshidi A.H., Khani M. Crocus sativus L. in the treatment of mild to moderate depression: A double-blind, randomized and placebo-controlled trial. Phytother. Res. 2005;19:148–151. doi: 10.1002/ptr.1647[↩]
- Noorbala A.A., Akhondzadeh S., Tahmacebi-Pour N., Jamshidi A.H. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: A double-blind, randomized pilot trial. J. Ethnopharmacol. 2005;97:281–284. doi: 10.1016/j.jep.2004.11.004[↩]
- Shahmansouri N., Farokhnia M., Abbasi S.-H., Kassaian S.E., Noorbala Tafti A.A., Gougol A., Yekehtaz H., Forghani S., Mahmoodian M., Saroukhani S., et al. A randomized, double-blind, clinical trial comparing the efficacy and safety of Crocus sativus L. with fluoxetine for improving mild to moderate depression in post percutaneous coronary intervention patients. J. Affect. Disord. 2014;155:216–222. doi: 10.1016/j.jad.2013.11.003[↩]
- Ghajar A., Neishabouri S.M., Velayati N., Jahangard L., Matinnia N., Haghighi M., Ghaleiha A., Afarideh M., Salimi S., Meysamie A., et al. Crocus sativus L. versus Citalopram in the Treatment of Major Depressive Disorder with Anxious Distress: A Double-Blind, Controlled Clinical Trial. Pharmacopsychiatry. 2016;50:152–160. doi: 10.1055/s-0042-116159[↩][↩]
- Zare M, Bazrafshan A, Malekpour Afshar R, Mazloomi SM. Saffron (adjunct) for people with schizophrenia who have antipsychotic‐induced metabolic syndrome. Cochrane Database of Systematic Reviews 2018, Issue 3. Art. No.: CD012950. DOI: 10.1002/14651858.CD012950[↩]
- Bizzarri M, Fuso A, Dinicola S, Cucina A, Bevilacqua A. Pharmacodynamics and pharmacokinetics of inositol(s) in health and disease. Expert Opin Drug Metab Toxicol. 2016 Oct;12(10):1181-96. doi: 10.1080/17425255.2016.1206887[↩]
- Shimon H, Agam G, Belmaker RH, Hyde TM, Kleinman JE (1997). Reduced frontal cortex inositol levels in postmortem brain of suicide victims and patients with bipolar disorder. Am J Psychiatry 154: 1148–1150.[↩]
- Griffin JL, Bollard M, Nicholson JK, Bhakoo K (2002). Spectral profiles of cultured neuronal and glial cells derived from HRMAS (1)H NMR spectroscopy. NMR Biomed 15: 375–384.[↩]
- Fisher SK, Novak JE, Agranoff BW (2002). Inositol and higher inositol phosphates in neural tissues: homeostasis, metabolism and functional significance. J Neurochem 82: 736–754.[↩]
- Chen LP, Dai HY, Dai ZZ, Xu CT, Wu RH (2014). Anterior cingulate cortex and cerebellar hemisphere neurometabolite changes in depression treatment: A 1H magnetic resonance spectroscopy study. Psychiatry Clin Neurosci 68: 357–364.[↩]
- Zheng H, Zhang L, Li L, Liu P, Gao J, Liu X et al (2010). High-frequency rTMS treatment increases left prefrontal myo-inositol in young patients with treatment-resistant depression. Prog Neuropsychopharmacol Biol Psychiatry 34: 1189–1195.[↩]
- Chiappelli, J., Rowland, L. M., Wijtenburg, S. A., Muellerklein, F., Tagamets, M., McMahon, R. P., Gaston, F., Kochunov, P., & Hong, L. E. (2015). Evaluation of Myo-Inositol as a Potential Biomarker for Depression in Schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 40(9), 2157–2164. https://doi.org/10.1038/npp.2015.57[↩]
- Levine J (1997). Controlled trials of inositol in psychiatry. Eur Neuropsychopharmacol 7: 147–155.[↩]
- Silverstone PH, McGrath BM, Kim H (2005). Bipolar disorder and myo-inositol: a review of the magnetic resonance spectroscopy findings. Bipolar Disord 7: 1–10.[↩]
- Mukai T, Kishi T, Matsuda Y, Iwata N. A meta-analysis of inositol for depression and anxiety disorders. Hum Psychopharmacol. 2014 Jan;29(1):55-63. doi: 10.1002/hup.2369[↩]
- Sarris J, Murphy J, Mischoulon D, Papakostas GI, Fava M, Berk M, Ng CH. Adjunctive Nutraceuticals for Depression: A Systematic Review and Meta-Analyses. Am J Psychiatry. 2016 Jun 1;173(6):575-87. https://doi.org/10.1176/appi.ajp.2016.15091228[↩]
- Mukai T, Kishi T, Matsuda Y, Iwata N. A meta-analysis of inositol for depression and anxiety disorders. Hum Psychopharmacol. 2014 Jan;29(1):55-63. https://doi.org/10.1002/hup.2369[↩][↩]
- Carlomagno G, Unfer V. Inositol safety: clinical evidences. Eur Rev Med Pharmacol Sci. 2011 Aug;15(8):931-6.[↩]
- Zhou, Z., and Zheng, S. (2003). The missing link in Ginkgo evolution. Nature 423, 821-822.[↩]
- Ernst E. The risk-benefit profile of commonly used herbal therapies: Ginkgo, St. John’s wort, ginseng, echinacea, saw palmetto, and kava. Annals of Internal Medicine. 2002;136(1):42–53[↩]
- Kennedy DO, Wightman EL. Herbal extracts and phytochemicals: plant secondary metabolites and the enhancement of human brain function. Advances in Nutrition. 2011;2:32–50 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3042794/[↩]
- van Beek, T.A., and Montoro, P. (2009). Chemical analysis and quality control of Ginkgo biloba leaves, extracts, and phytopharmaceuticals. J. Chromatogr. A 1216, 2002-2032.[↩][↩]
- Brondino N, De Silvestri A, Re S, et al. A Systematic Review and Meta-Analysis of Ginkgo biloba in Neuropsychiatric Disorders: From Ancient Tradition to Modern-Day Medicine. Evidence-based Complementary and Alternative Medicine : eCAM. 2013;2013:915691. doi:10.1155/2013/915691. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3679686/[↩]
- Kleijnen J, Knipschild P. Ginkgo biloba. The Lancet. 1992;340(8828):1136–1139[↩]
- Westendorf, J., and Regan, J. (2000). Induction of DNA strand-breaks in primary rat hepatocytes by ginkgolic acids. Pharmazie 55, 864-865.[↩]
- Arenz, A., Klein, M., Fiehe, K., Gross, J., Drewke, C., Hemscheidt, T., and Leistner, E. (1996). Occurrence of neurotoxic 4′-O-methylpyridoxine in Ginkgo biloba leaves, Ginkgo medications and Japanese Ginkgo food. Planta Med. 62, 548-551.[↩]
- Kressmann, S., Müller, W.E., and Blume, H.H. (2002). Pharmaceutical quality of different Ginkgo biloba brands. J. Pharm. Pharmacol. 54, 661-669.[↩]
- Gawron-Gzella, A., Marek, P., Chanaj, J., and Matlawska, I. (2010). Comparative analysis of pharmaceuticals and dietary supplements containing extracts from the leaves of Ginkgo biloba L. Acta Pol. Pharm. 67, 335-343.[↩]
- DeKosky ST, Williamson JD, Fitzpatrick AL, Kronmal RA, Ives DG, Saxton JA, Lopez OL, Burke G, Carlson MC, Fried LP, Kuller LH, Robbins JA, Tracy RP, Woolard NF, Dunn L, Snitz BE, Nahin RL, Furberg CD; Ginkgo Evaluation of Memory (GEM) Study Investigators. Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA. 2008 Nov 19;300(19):2253-62. doi: 10.1001/jama.2008.683. Erratum in: JAMA. 2008 Dec 17;300(23):2730[↩]
- Dodge HH, Zitzelberger T, Oken BS, Howieson D, Kaye J. A randomized placebo-controlled trial of Ginkgo biloba for the prevention of cognitive decline. Neurology. 2008 May 6;70(19 Pt 2):1809-17. doi: 10.1212/01.wnl.0000303814.13509.db[↩]
- Snitz BE, O’Meara ES, Carlson MC, Arnold AM, Ives DG, Rapp SR, Saxton J, Lopez OL, Dunn LO, Sink KM, DeKosky ST; Ginkgo Evaluation of Memory (GEM) Study Investigators. Ginkgo biloba for preventing cognitive decline in older adults: a randomized trial. JAMA. 2009 Dec 23;302(24):2663-70. doi: 10.1001/jama.2009.1913[↩]
- Vellas B, Coley N, Ousset PJ, Berrut G, Dartigues JF, Dubois B, Grandjean H, Pasquier F, Piette F, Robert P, Touchon J, Garnier P, Mathiex-Fortunet H, Andrieu S; GuidAge Study Group. Long-term use of standardised Ginkgo biloba extract for the prevention of Alzheimer’s disease (GuidAge): a randomised placebo-controlled trial. Lancet Neurol. 2012 Oct;11(10):851-9. doi: 10.1016/S1474-4422(12)70206-5[↩]
- Mahadevan S, Park Y. Multifaceted therapeutic benefits of Ginkgo biloba L.: chemistry, efficacy, safety, and uses. J Food Sci. 2008 Jan;73(1):R14-9. doi: 10.1111/j.1750-3841.2007.00597.x[↩]
- Eckert A. Mitochondrial effects of Ginkgo biloba extract. Int Psychogeriatr. 2012 Aug;24 Suppl 1:S18-20. doi: 10.1017/S1041610212000531[↩]
- World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for the Biological Treatment of Alzheimer ’ s disease and other dementias. The World Journal of Biological Psychiatry, 2011; 12: 2–32. https://www.wfsbp.org/fileadmin/user_upload/Treatment_Guidelines/WFSBP_Treatment_Guidelines_Dementia.pdf[↩]
- Treatment of Alzheimer Disease. Am Fam Physician. 2011 Jun 15;83(12):1403-1412. https://www.aafp.org/afp/2011/0615/p1403.html[↩]
- Birks J, Grimley Evans J. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst Rev. 2009 Jan 21;(1):CD003120. doi: 10.1002/14651858.CD003120[↩]
- Hashiguchi M, Ohta Y, Shimizu M, Maruyama J, Mochizuki M. Meta-analysis of the efficacy and safety of Ginkgo biloba extract for the treatment of dementia. J Pharm Health Care Sci. 2015 Apr 10;1:14. doi: 10.1186/s40780-015-0014-7[↩][↩]
- Yang G, Wang Y, Sun J, Zhang K, Liu J. Ginkgo Biloba for Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Curr Top Med Chem. 2016;16(5):520-8. doi: 10.2174/1568026615666150813143520[↩]
- Kellermann AJ, Kloft C. Is there a risk of bleeding associated with standardized Ginkgo biloba extract therapy? A systematic review and meta-analysis. Pharmacotherapy. 2011 May;31(5):490-502. doi: 10.1592/phco.31.5.490[↩]
- Heinonen T, Gaus W. Cross matching observations on toxicological and clinical data for the assessment of tolerability and safety of Ginkgo biloba leaf extract. Toxicology. 2015 Jan 2;327:95-115. doi: 10.1016/j.tox.2014.10.013[↩]
- Common Herbal Dietary Supplement–Drug Interactions. Am Fam Physician. 2017 Jul 15;96(2):101-107. https://www.aafp.org/afp/2017/0715/p101.html[↩]
- Alibeiki, F., Jafari, N., Karimi, M., & Peeri Dogaheh, H. (2017). Potent anti-cancer effects of less polar Curcumin analogues on gastric adenocarcinoma and esophageal squamous cell carcinoma cells. Scientific reports, 7(1), 2559. https://doi.org/10.1038/s41598-017-02666-4[↩]
- Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA. The Essential Medicinal Chemistry of Curcumin. J Med Chem. 2017 Mar 9;60(5):1620-1637. doi: 10.1021/acs.jmedchem.6b00975[↩]
- Zlotogorski, A.; Dayan, A.; Dayan, D.; Chaushu, G.; Salo, T.; Vered, M. (2013). Nutraceuticals as new treatment approaches for oral cancer -I: Curcumin. Oral Oncology 49: 187-191.[↩]
- WHO FOOD ADDITIVES SERIES: 52. CURCUMIN. https://apps.who.int/iris/bitstream/handle/10665/42849/WHO_TRS_922.pdf;jsessionid=0712A5359CCD928747F01055C86D8E11?sequence=1[↩]
- https://www.fda.gov/media/132575/download[↩]
- Cao J, Wang T, Wang M. Investigation of the anti-cataractogenic mechanisms of curcumin through in vivo and in vitro studies. BMC Ophthalmol. 2018 Feb 17;18(1):48. doi: 10.1186/s12886-018-0711-8[↩]
- Aggarwal, B. B., Deb, L., & Prasad, S. (2014). Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses. Molecules (Basel, Switzerland), 20(1), 185–205. https://doi.org/10.3390/molecules20010185[↩]
- Ng QX, Koh SSH, Chan HW, Ho CYX. Clinical Use of Curcumin in Depression: A Meta-Analysis. J Am Med Dir Assoc. 2017 Jun 1;18(6):503-508. doi: 10.1016/j.jamda.2016.12.071[↩]
- National Center for Complementary and Integrative Health. Probiotics. https://nccih.nih.gov/health/probiotics [↩]
- Hill, C., Guarner, F., Reid, G. et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11, 506–514 (2014). https://doi.org/10.1038[↩]
- Clin Perinatol. 2013 Mar; 40(1): 11–25. Published online 2013 Jan 17. doi: 10.1016/j.clp.2012.12.002. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3575601/[↩]
- The National Institute of Health, Medline Plus. The Basics of Probiotics. https://medlineplus.gov/magazine/issues/winter16/articles/winter16pg22.html[↩]
- Probiotics. https://ods.od.nih.gov/factsheets/Probiotics-HealthProfessional[↩]
- Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, Verbeke K, Reid G. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017 Aug;14(8):491-502. doi: 10.1038/nrgastro.2017.75[↩]
- Probiotics and prebiotics. https://www.worldgastroenterology.org/guidelines/global-guidelines/probiotics-and-prebiotics/probiotics-and-prebiotics-english[↩][↩]
- Evrensel A., Ceylan M.E. The Gut-Brain Axis: The Missing Link in Depression. Clin. Psychopharmacol. Neurosci. 2015;13:239–244. doi: 10.9758/cpn.2015.13.3.239[↩]
- Borre YE, Moloney RD, Clarke G, Dinan TG, Cryan JF. The impact of microbiota on brain and behavior: mechanisms & therapeutic potential. Adv Exp Med Biol. 2014;817:373-403. doi: 10.1007/978-1-4939-0897-4_17[↩][↩]
- Savignac HM, Kiely B, Dinan TG, Cryan JF. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol Motil. 2014 Nov;26(11):1615-27. doi: 10.1111/nmo.12427[↩]
- Huang, R., Wang, K., & Hu, J. (2016). Effect of Probiotics on Depression: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients, 8(8), 483. https://doi.org/10.3390/nu8080483[↩][↩]