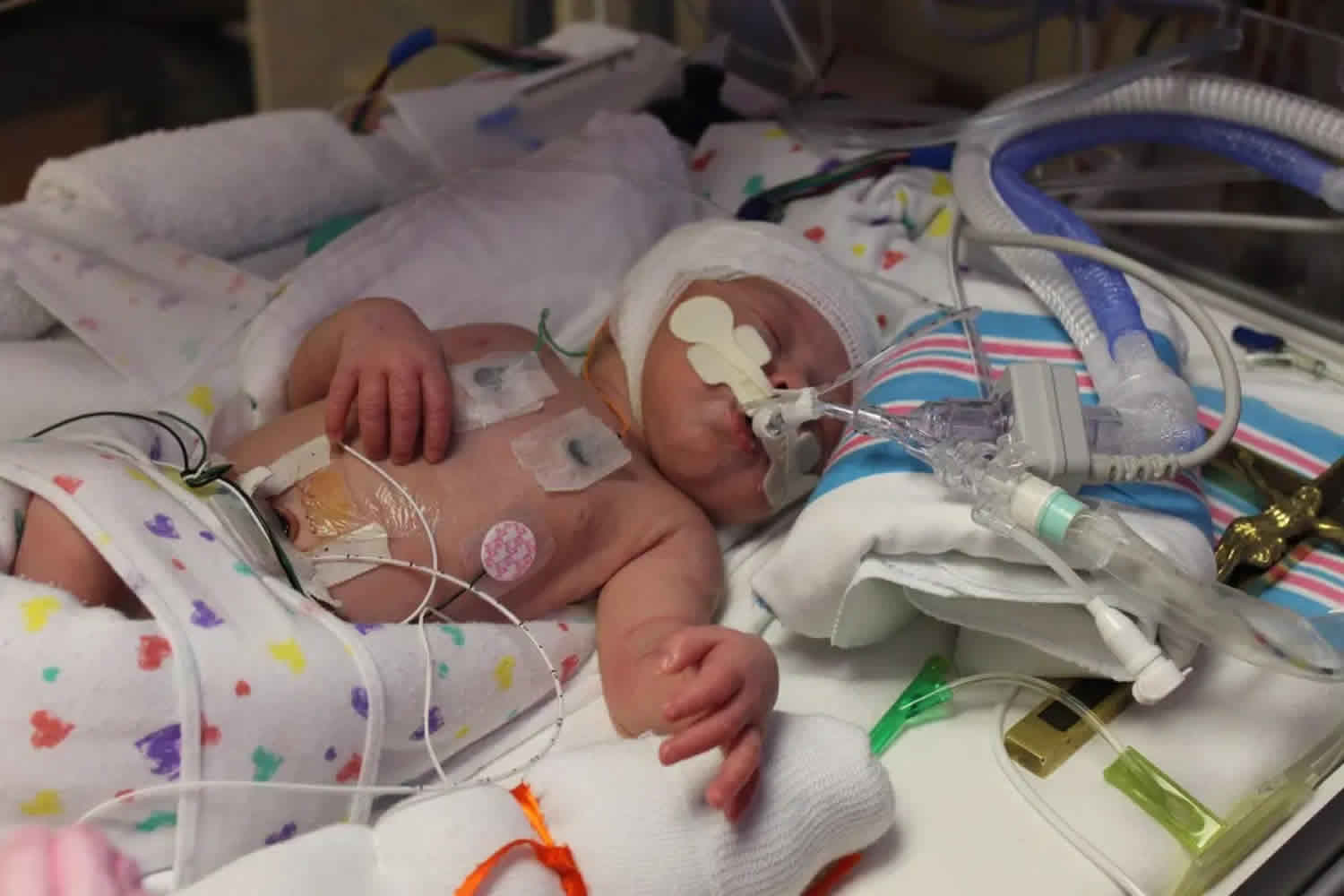
Birth asphyxia
Birth asphyxia also known as neonatal asphyxia or perinatal asphyxia is the medical condition resulting from deprivation of oxygen to a newborn infant that lasts long enough during the birth process to cause physical harm, usually to the brain. Birth asphyxia is a lack of blood flow or gas exchange to or from the fetus in the period immediately before, during, or after the birth process 1. Perinatal asphyxia can result in profound systemic and neurologic complications due decreased blood flow and/or oxygen to a fetus or infant during the peripartum period. When placental (prenatal) or pulmonary (immediate post-natal) gas exchange is compromised or ceases altogether, there is partial lack of oxygen (hypoxia) or complete lack of oxygen (anoxia) to the vital organs. This results in progressive hypoxemia (low level of oxygen in the blood) and hypercapnia (too much carbon dioxide or CO2 in the blood). If the hypoxemia is severe enough, the tissues and vital organs (muscle, liver, heart, and ultimately the brain) will develop an oxygen debt. Anaerobic glycolysis and lactic acidosis will result. Neonatal hypoxic-ischemic encephalopathy refers specifically to the neurologic complication of perinatal asphyxia 2.
The diagnostic criteria for neonatal hypoxic-ischemic encephalopathy are as follows:
- Metabolic acidosis with pH <7.0 (in umbilical cord or infant blood sample)
- Base Deficit -12
- APGAR score = five at 10 minutes with a continued need for resuscitation
- Presence of multiple organ-system failures
- Clinical evidence of encephalopathy: hypotonia, abnormal oculomotor or pupillary movements, weak or absent suck, apnea, hyperpnea, or clinical seizures
- Neurologic findings cannot be attributed to other cause (inborn error of metabolism, a genetic disorder, congenital neurologic disorder, medication effect).
The incidence of perinatal asphyxia is two per 1000 births in developed countries, but the rate is up to 10 times higher in developing countries where there may be limited access to maternal and neonatal care. Of those infants affected, 15-20% die in the neonatal period, and up to 25% of survivors are left with permanent neurologic deficits 3.
Some of the causes of decreased oxygen before or during the birth process may include:
- Inadequate oxygen levels in the mother’s blood due to heart or respiratory problems or lowered respirations caused by anesthesia
- Low blood pressure in the mother
- Inadequate relaxation of the uterus during labor that prevents oxygen circulation to the placenta
- Early separation of the placenta from the uterus, called placental abruption
- Compression of the umbilical cord that decreases blood flow
- Poor placenta function that may occur with high blood pressure or in post-term pregnancies, particularly those past 42 weeks
Factors that may lower oxygen in the baby after birth include:
- Severe anemia, or a low blood cell count, that limits the oxygen-carrying ability of the blood
- Low blood pressure or shock
- Respiratory problems that limit oxygen intake
- Heart or lung disease
Low oxygen levels may decrease a baby’s heart rate, blood pressure and blood flow out of the heart. This may limit the blood flow to organs and tissues, leading to improper cell function or damage. Organs typically affected by lowered oxygen include the brain, heart and blood vessels, gastrointestinal tract, lungs and kidneys.
Birth asphyxia is not an uncommon event, and because of its high morbidity and mortality, the condition is best managed by an interprofessional team.
Causes of birth asphyxia
Perinatal asphyxia can occur due to maternal hemodynamic compromise (amniotic fluid embolus), uterine conditions (uterine rupture), or placenta and umbilical cord (placental abruption, umbilical cord knot or compression) and infection. The asphyxia can occur prior to the birth or can occur immediately following birth in a compromised patient requiring resuscitation 4.
The majority of cases of perinatal asphyxia occur intrapartum, although 20% occur antepartum and other cases occur in the early post-natal period. Perinatal asphyxia can occur due to maternal events (hemorrhage, amniotic fluid embolism; hemodynamic collapse), placental events (acute abruption), uterine events (rupture), cord events (tight nuchal cord, cord prolapse/avulsion) and intrapartum infection (maternal fever in labor). A careful obstetrical and peripartum history is essential to determine the cause.
Perinatal asphyxia pathophysiology
There are three stages to brain injury in hypoxic-ischemic encephalopathy. First, there is an immediate primary neuronal injury that occurs due to interruption of oxygen and glucose to the brain. This decreases ATP and results in failure of the ATP-dependent NaK pump. Sodium enters the cell followed by water, causing cell swelling, widespread depolarization, and cell death. Cell death and lysis cause release of glutamate, an excitatory amino acid, which causes an increase in intracellular calcium and further cell death.
Following the immediate injury is a latent period of about six hours, during which reperfusion occurs, and some cells recover.
Late secondary neuronal injury occurs over the next 24-48 hours as reperfusion results in blood flow to and from damaged areas, spreading toxic neurotransmitters and widening the area of brain affected.
Birth asphyxia stages
Perinatal asphyxia can result in systemic effects, including neurologic insult, respiratory distress and pulmonary hypertension, and liver, myocardial, and renal dysfunction. Depending on the severity and timing of the hypoxic insult, a neonate with hypoxic-ischemic encephalopathy due to perinatal asphyxia can demonstrate a variety of neurologic findings. Using the Sarnat staging for encephalopathy can be useful.
- Sarnat Stage 1, the least severe stage, there is generalized sympathetic tone and the neonate may be hyper-alert with prolonged periods of wakefulness, mydriasis and increased deep tendon reflexes.
- Sarnat Stage 2, the neonate may be lethargic or obtunded, with decreased tone, strong distal flexion, and generalized parasympathetic tone with miosis, bradycardia and increased secretions. Seizures are common in Sarnat Stage 2.
- Sarnat Stage 3, the most severe, is characterized by a profoundly decreased level of consciousness, flaccid tone, decreased deep tendon reflexes and very abnormal EEG. Clinical seizures are less common in Sarnat Stage 3 due to the profound injury in the brain preventing the propagation of clinical seizures.
Symptoms of birth asphyxia
Each baby may experience symptoms of birth asphyxia differently. However, the following are the most common symptoms.
Before delivery, symptoms may include:
- Abnormal heart rate or rhythm
- An increased acid level in a baby’s blood
At birth, symptoms may include:
- Bluish or pale skin color
- Low heart rate
- Weak muscle tone and reflexes
- Weak cry
- Gasping or weak breathing
- Meconium — the first stool passed by the baby — in the amniotic fluid, which can block small airways and interfere with breathing
Birth asphyxia diagnosis
The following test are used to diagnose birth asphyxia:
- Severe acid levels — pH less than 7.00 — in the arterial blood of the umbilical cord.
- Apgar score of zero to three for longer than five minutes. The Apgar test is used just after birth to evaluate a newborn’s color, heartbeat, reflexes, muscle tone and respiration.
- Neurological problems, such as seizures, coma and poor muscle tone.
- Respiratory distress, low blood pressure, or other signs of low blood flow to the kidneys or intestines.
Problems with a baby’s circulatory, digestive and respiratory systems may also suggest that a baby has birth asphyxia.
A chest radiograph may determine the need for intubation and/or need for exogenous surfactant therapy. An arterial blood gas is useful in diagnosing respiratory versus metabolic acidosis and degree of hypoxemia. Liver damage can be determined by serum transaminase levels and coagulation factors. Troponin and CK-MB can be useful in determining myocardial insult and creatinine and blood urea nitrogen can ascertain the extent of renal dysfunction. Physiologically stressed infants rapidly deplete glucose stores and can develop profound hypoglycemia. Frequent blood glucose checks during the critical period of resuscitation are recommended 5.
Birth asphyxia treatment
Birth asphyxia is a complex condition that can be difficult to predict or prevent. Prompt treatment is important to minimize the damaging effects of decreased oxygen to the baby.
Specific treatment for birth asphyxia is based on:
- The baby’s age, overall health and medical history
- Severity of the baby’s condition
- The baby’s tolerance for specific medications, procedures or therapies
- Expectations for the course of the condition
Treatment may include:
- Giving the mother extra oxygen before delivery
- Emergency delivery or Caesarean section
- Assisted ventilation and medications to support the baby’s breathing and blood pressure
- Extracorporeal membrane oxygenation (ECMO). An extracorporeal membrane oxygenation (ECMO) machine may be used for babies who are experiencing serious heart or lung failure. The machine delivers oxygen to the baby’s brain and body as temporary support. It works by draining the baby’s blood into an artificial lung where oxygen is added and carbon dioxide is removed, then pumping the blood back into the child.
Therapeutic hypothermia is the treatment for neonatal hypoxic-ischemic encephalopathy 1. Following the immediate primary neuronal injury, during which there is an interruption of oxygen and glucose to the brain, there is a latent period of up to 6 hours before a secondary phase of injury occurs as the injured areas are reperfused, and damaged cells lyse, releasing toxic neurotransmitters. The goal of therapeutic hypothermia is to intervene during the latent period and minimize damage from the secondary neuronal injury. Therapeutic hypothermia, when started within six hours of injury decreases mortality and severe disability from 62% to 48% and increases survival with the normal outcome from 24% to 40% with a number needed to treat of six to seven. Whole body cooling appears to be more effective at decreasing death than selective head cooling, but both modalities are effective at decreasing severe disability and a combined outcome of death and severe disability. Infants with moderate encephalopathy (Sarnat Stage 2) benefit most from therapeutic hypothermia. Importantly, cooling does not appear to decrease death at the cost of more severely neurologic impairment in survivors. Side effects associated with therapeutic hypothermia include peripheral vasoconstriction, diuresis, cardiac dysfunction, arrhythmias, coagulopathy, thrombocytopenia, leukocyte dysfunction, pulmonary hypertension and sclerema (calcium deposits in the skin). Therapeutic hypothermia can be delivered safely with specialized equipment and monitoring in sophisticated medical centers 6.
The treatment of respiratory distress, pulmonary hypertension, coagulopathy and myocardial dysfunction is supportive. Infants with respiratory distress and pulmonary hypertension may require intubation, surfactant, oxygen and inhaled nitric oxide. Coagulopathy is treated with the prudent use of blood products to maintain oxygen-carrying capacity and coagulation. Myocardial dysfunction may result in a need for vasopressors. Renal dysfunction may result in oliguria or anuria; therefore, use of crystalloid fluid and blood products should be cautious.
Birth asphyxia prognosis
The long-term prognosis of birth asphyxia infants has been difficult to assess. In the short term, the condition is reported to have a mortality in excess of 30%, with the majority of deaths occurring within the first few days after birth. Those infants who survive are often left with mild to severe neurological deficits, and they also end up dying from aspiration or systemic infections. Long-term survivors have been found to have disabling cerebral palsy, inadequate mental development or low psychomotor scores, seizures, blindness, and severe hearing impairment. The management of these infants, in the long run, is complex and prohibitively expensive 7.
- Gillam-Krakauer M, Gowen Jr CW. Birth Asphyxia. [Updated 2020 Apr 23]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430782[↩][↩]
- Sugiura-Ogasawara M, Ebara T, Yamada Y, Shoji N, Matsuki T, Kano H, Kurihara T, Omori T, Tomizawa M, Miyata M, Kamijima M, Saitoh S., Japan Environment, Children’s Study (JECS) Group. Adverse pregnancy and perinatal outcome in patients with recurrent pregnancy loss: Multiple imputation analyses with propensity score adjustment applied to a large-scale birth cohort of the Japan Environment and Children’s Study. Am. J. Reprod. Immunol. 2019 Jan;81(1):e13072.[↩]
- Odd D, Heep A, Luyt K, Draycott T. Hypoxic-ischemic brain injury: Planned delivery before intrapartum events. J Neonatal Perinatal Med. 2017;10(4):347-353.[↩]
- Viaroli F, Cheung PY, O’Reilly M, Polglase GR, Pichler G, Schmölzer GM. Reducing Brain Injury of Preterm Infants in the Delivery Room. Front Pediatr. 2018;6:290. Published 2018 Oct 16. doi:10.3389/fped.2018.00290 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6198082[↩]
- Salas J, Tekes A, Hwang M, Northington FJ, Huisman TAGM. Head Ultrasound in Neonatal Hypoxic-Ischemic Injury and Its Mimickers for Clinicians: A Review of the Patterns of Injury and the Evolution of Findings Over Time. Neonatology. 2018;114(3):185-197.[↩]
- Kebaya LMN, Kiruja J, Maina M, Kimani S, Kerubo C, McArthur A, Munn Z, Ayieko P. Basic newborn resuscitation guidelines for healthcare providers in Maragua District Hospital: a best practice implementation project. JBI Database System Rev Implement Rep. 2018 Jul;16(7):1564-1581.[↩]
- Riley C, Spies LA, Prater L, Garner SL. Improving Neonatal Outcomes Through Global Professional Development. Adv Neonatal Care. 2019 Feb;19(1):56-64.[↩]