What is BPA
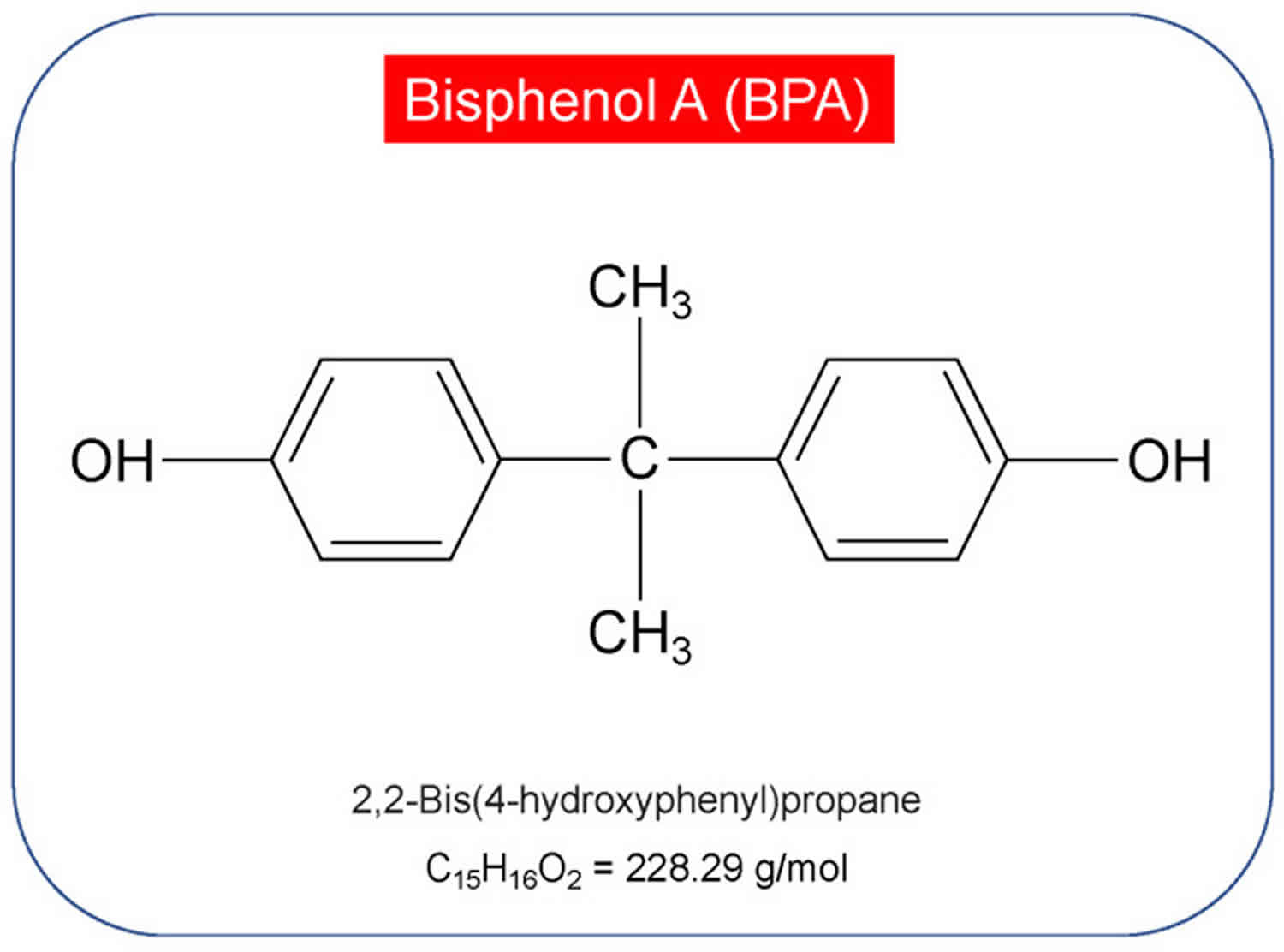
Bisphenol-A is commonly abbreviated as BPA also known as 4,4’-dihydroxy-2,2-diphenylpropane or 2,2-bis(4-hydroxyphenyl)propane [C15H16O2], is an organic compound with two hydroxyphenyl functional groups that is extensively used since 1957 in the production of polycarbonate plastics and epoxy resins for a wide range of products used daily, such as food wrappings, water bottles, drink cans, medical and electronic devices, thermal paper, shatterproof windows, eyewear, and epoxy resins that coat some metal food cans, bottle tops, and water supply pipes 1, 2, 3, 4, 5, 6. BPA has been used in food packaging since the 1960s with over 6 billion pounds produced each year and over 100 tons are estimated to be released into the atmosphere and the primary source of exposure to BPA for most people is through the diet (between 95 and 99%) 7. While air, dust, and water are other possible sources of exposure 8, BPA in food and beverages accounts for the majority of daily human exposure.
BPA is an important monomer in the production of polycarbonate plastics and epoxy resins. To protect foods and drinks from direct contact with metals, epoxy resins are frequently used in the internal coating of food and beverage containers. BPA is a building block of polycarbonate plastics, including baby bottles 9. BPA is a potential food contaminant arising from its use in reusable polycarbonate food containers such as water carboys, baby bottles and kitchen utensils. BPA is also used for some dental sealants, carbonless paper for receipts, digital media (CDs and DVDs), electronic equipment, flooring, tableware, and reusable bottles 10. A recent study reported that BPA was detectable in dust, air particles and water, making exposure widespread 11. Residues of BPA can migrate into food and beverages and be ingested by the consumer; BPA from other sources including thermal paper, cosmetics and dust can be absorbed through the skin and by inhalation.
Bisphenol A can leach into food from the protective internal epoxy resin coatings of canned foods and from consumer products such as polycarbonate tableware, food storage containers, water bottles, and baby bottles. The degree to which BPA leaches from polycarbonate bottles into liquid may depend more on the temperature of the liquid or bottle, than the age of the container. BPA can also be found in breast milk.
Bisphenol A or BPA was first synthesized in 1891 by the Russian chemist Aleksandr Pavlovich Dianin; BPA was originally named ‘Dianin’ compound 12, 13. In the 1950s, it was discovered that reaction of BPA with phosgene (carbonyl chloride, COCl2) produces a clear hard resin, known as polycarbonate 1, 14. Following this discovery, BPA has been increasingly used in a myriad of industrial and consumer applications 1, 15, 16. In the mid-1930s, Dodds et al. 17 discovered the estrogenic properties of BPA during their pursuit of a synthetic estrogen. However, these authors found that diethylstilbestrol (DES) was a far more potent estrogen using a classical estrogenic assay and concluded that the estrogenic potential of BPA was marginal 17.
BPA is often classified as an endocrine-disrupting chemical (EDC) or endocrine disruptor as it can act as a xenoestrogen, mimicking the action of estradiol (E2) on binding with the estrogen receptors ER-α and ER-β, although with a lesser affinity than 17-β-estradiol 18, 2. 17-β-estradiol acts on fertility, development, reproduction, the neurological and immunological systems, and homeostasis, both in human beings and animals 19, 20, 21. Xenoestrogens are chemicals or industrial compounds that may act as inappropriate estrogens, and/or could interfere with the actions of endogenous estrogens or interfering with estrogen signaling pathways by binding to estrogen receptors (ERs) in the human body, consequently acting as carcinogen (substance that causes cancer) 22, 23, 24.
Numerous epidemiological studies have demonstrated the correlation between exposure to BPA and the onset of chronic diseases such as obesity, type 2 diabetes and cardiovascular pathologies 25, 26, 27, 28. Furthermore, some experiments have shown that exposure to BPA causes weight increase, an alteration in the levels of glucose in the blood and resistance to insulin, as well as the development of dyslipidemias and changes in lipid metabolism 29, 30, 31. Those studies showed positive associations between exposure to BPA and the development of metabolic diseases such as obesity and type 2 diabetes 32, 33. Similarly, many studies in animals reported that exposure to BPA resulted in an intolerance to glucose, resistance to insulin and alterations in the homeostasis of glucose 34, 35. However, the mechanism of action of BPA in the organisms exposed is not absolutely clear, although many works have demonstrated that BPA induces its effects by means of the generation of free radicals and oxidative stress 36, 37. Those studies have revealed that BPA exposure could change the levels of oxidative stress indicators, and that the differences in the latter might be related to target tissues in the animals, the BPA administration route, the dose and the duration of exposure. In this sense, exposure to both high and low BPA doses could affect the homeostasis of the oxidative system in rodents, despite the sensitivity of each indicator being able to differ and long-term exposure to BPA having the capacity to cause an increase in the oxidative substances that would involve harmful health problems 38.
This endocrine-alteration capacity of BPA and the metabolic effects associated with diverse health impairments have become of increasing concern to health authorities worldwide, with the European Authority of Food Safety (EFSA) in April 2023 reducing the Tolerable Daily Intake (TDI) of BPA to the new tolerable daily intake (TDI) of BPA at 0.2 nanograms/kg body weight per day (0.2 ng or 0.2 billionths of a gram) 3. This new tolerable daily intake (TDI) is 20,000 times lower than the 2015 temporary tolerable daily intake (TDI) of 4 mcg (4 micrograms or 4 millionths of a gram) per kg body weight per day 39, 40. Furthermore, by comparing the 2023 tolerable daily intake (TDI) with estimates of dietary exposure to BPA, EFSA’s scientific experts concluded that consumers with both average and high exposure to BPA in all age groups exceeded the new TDI, indicating health concerns and potentially harmful health effects on the immune system 3, 41.
Exposure to BPA has been associated with developmental, reproductive, cardiovascular, neurological, metabolic, or immune effects, as well as oncogenic effects 2, 22. BPA can disrupt the synthesis or clearance of hormones by binding and interfering with biological receptors. BPA can also interact with key transcription factors to modulate regulation of gene expression 2.
Most regulatory agencies monitor and regulate BPA exposure in humans based on dietary exposure and aggregate exposures from water, soil, and air 22, 42. In recent years, BPA has been added to the list of banned substances in several consumer products, such as infant feeding bottles (baby bottles), spill-proof cups (sippy cups), infant formula packaging, and cosmetics 43. In the European Union (EU), BPA is classified as a substance that 3, 44:
- causes toxic effects on humans ability to reproduce (may damage infertility);
- causes serious eye damage;
- may cause respiratory irritation;
- may cause skin allergies (skin sensitizers);
- very toxic to aquatic life;
- very toxic to aquatic life with long-lasting effects.
In the United States, the use of BPA-containing epoxy resins as coatings for canned foods has recently decreased, although U.S. manufacturers have not abandoned the use of BPA for other applications, including production of countless variety of polycarbonate consumer goods 45.
Human exposure to BPA is predominantly through contaminated food and drinking water, with additional exposures from ingestion of dust, inhalation of indoor and outdoor air, and skin contact or absorption through the eye also occur 46, 47, 48, 49.
Previous studies have shown that BPA can leach from polycarbonate plastics, epoxy resins and other products in contact with foods and drinks, and as a result, routine oral ingestion of BPA is likely 50. Indeed, one risk assessment study suggested that canned food items contribute to 10-40% of the daily BPA intake 51. The use of BPA-containing products in daily life makes exposure ubiquitous, and the potential human health risks of this chemical are a major public health concern. Although numerous in vitro (test tube) and in vivo (animal) studies have been published on the effects of BPA on biological systems, there is controversy as to whether ordinary levels of exposure can have adverse effects in humans 52. However, the increasing incidence of developmental disorders is of concern, and accumulating evidence indicates that BPA has detrimental effects on neurological development. Other bisphenol analogues, used as substitutes for BPA, are also suspected of having a broad range of biological actions.
Upon entering the human body, BPA is rapidly absorbed, distributed, broken down (metabolized), and then eliminated mostly through urinary excretion 46, 48, 49, 53, 54. The absorbed BPA is broken down (metabolized) in the liver through glucuronidation or sulfonation 46, 54, 55, 56, 57, 58, 59, although oxidative metabolism by cytochrome P450 (CYP) enzymes and peroxidases can also occur 60, 61, 62. The latter leads to the formation of electrophilic or reactive species, or estrogenic metabolites 14, 60, 63, 64, 65, 66 that may bind macromolecules, such as DNA or proteins 67, 68, 69, 70, 71, 72, 73, 74. Conjugation of BPA is mainly catalyzed by the liver enzyme UDP-glucuronosyltransferases 2B15 (UGT2B15) 53, 75, 76, followed by its excretion from the body via urine 77, 78, 59. The half-life of orally absorbed BPA is less than 6 hours 54, 57, 79. Despite the rapid elimination of BPA from the body, over 90% of urine samples from the studied human populations show detectable levels of Bisphenol A and/or its metabolites 80, 81, 82. This finding supports that BPA exposure in humans is constant, recurring, and likely from multiple sources 45, 83, 84, 85. In confirmation, varying concentrations of BPA or its metabolites can be found in human urine or other body fluids over time or at different intervals within a short span of time, e.g., a single day 84, 86.
In the Fourth National Report on Human Exposure to Environmental Chemicals (Fourth Report) 87, the Centers for Disease Control and Prevention (CDC) scientists measured BPA in the urine of 2,517 participants aged six years and older who took part in CDC’s National Health and Nutrition Examination Survey (NHANES) during 2003–2004 88, 89,. By measuring BPA in urine, scientists can estimate the amount of BPA that has entered peoples’ bodies. CDC scientists found BPA in the urine of nearly all of the people tested, which indicates widespread exposure to BPA in the U.S. population 90. Finding a measurable amount of BPA in the urine does not imply that the levels of BPA cause an adverse health effect 90. Biomonitoring studies on levels of BPA provide physicians and public health officials with reference values so that they can determine whether people have been exposed to higher levels of BPA than are found in the general population. Biomonitoring data can also help scientists plan and conduct research on exposure and health effects. To date, accurate and reliable quantification of human exposure to BPA, particularly long-term exposure, remains a formidable task for population-based studies 2.
Because of the potential health risk of BPA, the chemical has been replaced by alternatives. Ironically, these BPA substitutes have also been demonstrated to have detrimental effects on human health. For example, bisphenol F (BPF) and halogenated BPA, used in products for daily life, are suspected of having toxic effects on biological systems 91.
Numerous studies on the biological effects of BPA have been published, and its potential human health hazards have been extensively summarized 92. Previous studies have linked BPA exposure to abnormalities of the reproductive system and a higher incidence of cardiovascular disease 93. Along with recent increases in the prevalence of neurobehavioral disorders 94, evidence has been accumulating that BPA can perturb nervous system development. For example, elevated gestational urinary concentrations of BPA have been correlated with adverse behavioral outcomes in children 95. However, many uncertainties remain and controversial discussions are still ongoing.
Figure 1. Bisphenol-A
Bisphenol A uses
BPA is extensively used in the production of polycarbonate plastics and epoxy resins for a wide range of products used daily, such as food wrappings, drink cans, beverage containers, compact disks (CDs), digital versatile disks (DVDs), plastic dinnerware, impact-resistant safety equipment, automobile parts, toys, electronic devices, thermal papers (e.g., cash receipts, movie tickets, and boarding passes), sports equipment, flame retardants, dental fillings or sealants, and medical devices containing polycarbonate or polysulfone plasticizers, such as contact lenses, intravenous cannulas, catheters, probes, inhalers, neonatal incubators, and hemodialysis apparatus 96, 97, 98, 99, 100, 4, 90. BPA epoxy resins are used in the protective linings of food cans, in dental sealants, and in other products.
Polycarbonate plastics made from BPA have remarkable chemical and physical properties, including excellent strength and rigidity, thermal stability, and resistance to oils and acids 14, 15, 16. BPA is used in polycarbonate plastic such as in a transparent and rigid type of plastic used to make water dispensers, food storage containers and reusable beverage bottles.
The epoxy resin of BPA has a viscous consistency, provides strong adhesion and high corrosion resistance, and is commonly used for the protective coatings and inner lining for food and beverage cans and vats 1, 15, 16. BPA monomer is also used in the manufacture of specialty plastics, such as polyester, polysulfone, polyacrylate, and polyetherimide, and as a precursor, developer, additive, or processing aid in the synthesis of other materials 22, 42, 101, 44.
How are humans exposed to BPA?
Human exposure to BPA at low levels comes from eating food or drinking water stored in containers that have BPA 90. Small children may be exposed by hand-to-mouth and direct oral (mouth) contact with materials containing BPA 90. Dental treatment with BPA-containing sealants also results in short-term exposure 90. In addition, workers who manufacture products that contain BPA can also be exposed.
It is estimated that more than 13 billion pounds of BPA have entered the global marketplace in 2021 102. Emissions from facilities producing BPA or manufacturing BPA-containing materials are substantial 103; the U.S. Environmental Protection Agency (USEPA) estimates an annual release of over one million pounds of BPA to the environment 104. BPA can be released into the environment and enter the human body at any stage during its production, or in the process of manufacture, use, or disposal of materials made from this chemical 22, 105, 106. For example, during storage, BPA can leach from the protective internal epoxy resin coating of canned foods and bottles and packaging materials into food and beverages 107, 108, 109, 110. BPA can also be released from polycarbonate plastics or food and drink containers when they are heated in a microwave or washed with harsh detergents 14, 105. Degradation of polymeric materials, such as containers or vessels, is facilitated when they hold saline, acidic, or basic compounds, resulting in the hydrolysis of ester bonds that link BPA monomers 111, 112. Additionally, BPA can leach from consumer products into surface water and soil 105, 113, 114, 115. BPA can also migrate into dust, e.g., from laminate flooring or paints, by attaching to the solid particulates present in the air 116, 117. Other sources of BPA in the environment include leachates from landfills, discharges of effluents containing BPA from municipal wastewater treatment plants, and combustion of residential waste 115, 118, 119. BPA half-life is approximately 4.5 days in water and soil, and less than one day in the air because of its low volatility 22, 113.
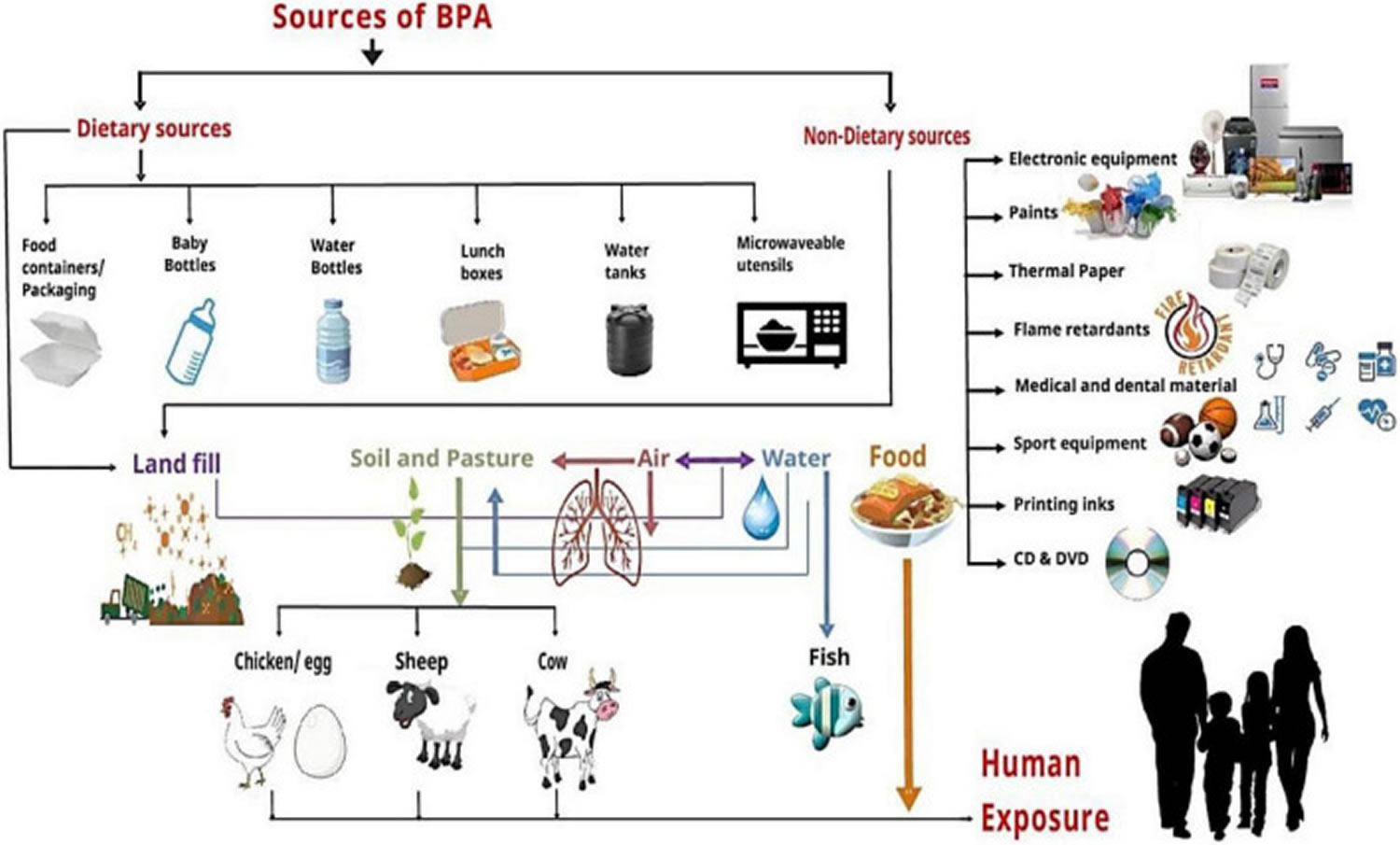
Figure 2. Sources of human exposure to BPA
Footnotes: Humans exposure to BPA via Dietary sources (food containers/packaging, baby bottles, water bottles, lunch boxes, water tanks, and microwave utensils) and Non-dietary sources (electronic equipment, paints, thermal paper, flame retardants, medical and dental materials, sports equipment, printing inks, and DVDs) contaminate landfill, soil, air, water, and food that directly or indirectly affect the human through different exposure routes.
[Source 120 ]Main sources of human exposure to BPA
- Contamination of BPA through food exposure is the most important due to the use of BPA for manufacturing different types of plastic containers [polycarbonate (PC) and polyvinylchloride (PVC) plastics] used for food serving and exposing their direct interaction with food (see Table 1) 120. Epoxy resins are also used to manufacture food cans for inner coatings. Therefore, canned food products also play a significant role in adulterating food items. Canned food and non-canned meat and meat products are major contributors to dietary BPA exposure for all age groups. Canned food is known to be a dietary source of BPA because of the substance’s use in the lining of cans. Residual monomers of BPA compounds migrate from the can to the food product, and food consumption causes safety issues in individuals 121, 122. Food packaging materials are the primary cause of BPA accumulation in human beings. It is due to the penetration of BPA from packaging into foodstuff and beverages 123, 124.
- Bisphenol A is almost everywhere in your environment and is released from common consumer goods (see Figure 2) 125, 126, 127. BPA may enter your body through different routes like skin and oral exposure or only through inhalation 128. BPA exposure through inhalation and dermal routes accounts for less than 5% of all contact sources.
- The primary route of BPA human exposure is dietary exposure, including consumption of seafood or even freshwater fish polluted via BPA, fresh food commodities from polluted regions, ingestion of food packed in plastic and cans containers, and drinking polluted water 129.
- Bisphenol-A human exposure’s primary source is canned food items. The results from experiments indicated that canned foods had higher BPA concentrations than other food samples 130. BPA might be present in meat and meat products through contact with packaging, processing equipment or through other forms of contamination (e.g. environment, feed). Therefore, BPA exposure mainly depends on the duration and amount of canned food usage in a person’s diet regimen. In kids older than 3 years, BPA exposure’s highest mean value was approximately 69.9 ng/kg body weight/day, with an utmost value of 189 ng/kg body weight/day. In adults, 139.9 ng/kg body weight/day was the highest mean value, with the maximum contact up to 419 ng/kg body weight/day 131. Wilson and colleagues 132 estimated in a study that BPA exposure through inhalation for toddlers (1.5–5 years) was 0.23–0.42 ng/kg of body weight per day.
- The second foremost route of absorption for BPA is skin exposure 133. Direct paper contact (especially thermal paper), toys, and medical devices proportionally increase the BPA potential against the skin. Exposure to BPA in thermal paper through the skin is the second largest source of external exposure in all age groups above three years of age, ranging from 4 mcg/kg of body weight per day.
- Inhalation is the third most important route of exposure through BPA-containing vapors, mists, dust, and gases 129.
Human exposure to BPA is predominantly through contaminated food and drinking water, with additional exposures from ingestion of dust, inhalation of indoor and outdoor air, and skin contact or absorption through the eye also occur 46, 47, 48, 49.
In humans, BPA is detectable in various body fluids, such as blood, urine, saliva, sweat, breast milk, and amniotic fluid, as well as on the skin 46, 77, 134, 135, 136, 137. BPA can cross the blood–brain barrier and the placenta 14, 136, 138. Detectable levels of BPA have been found in human maternal and fetal serum and the human placenta 14, 138. BPA can also accumulate in human tissues, primarily adipose tissue, owing to its lipophilic property 119, 139, 19. The widespread presence of BPA in the human body suggests that not only dietary exposure but also non-dietary exposures through the respiratory system, integumentary system (eye and skin contact), and vertical transmission (maternofetal), as well as other routes of exposure, can have significant toxicological relevance given the toxicokinetics of this compound 48, 140, 78, 141.
Table 1. Level of BPA (g/kg) in different foods
| Food | BPA level | References |
|---|---|---|
| Cereals | 0.9–3.7 | 142 |
| Fish | 7.2–103 | 143 |
| Fruits and vegetables | 10.99–94 | 144 |
| Canned (fruits and vegetables) | 3.6–267 | 145 |
| Canned soft drinks | 0.033–3.9 | 146 |
| Milk | 1.33–175 | 147 |
Is exposure to BPA from till/cash register receipts a concern?
For population groups above three years of age thermal paper (commonly used for till/cash register receipts) was the second most important source of BPA exposure after the diet, potentially accounting for up to 75% of total exposure for adolescents. Due to uncertainties around the estimates of exposure, European Food Safety Authority’s experts consider more data are needed (especially related to BPA skin absorption and cash receipt handling habits) to perform a more refined estimate of exposure through this source.
If I am concerned, what can I do to prevent exposure to BPA?
Some animal studies suggest that infants and children may be the most vulnerable to the effects of BPA. Parents and caregivers, can make the personal choice to reduce exposures of their infants and children to BPA 148:
- Don’t microwave polycarbonate plastic food containers. Polycarbonate is strong and durable, but over time it may break down from over use at high temperatures.
- Avoid using plastic containers for hot food or drinks. Avoid microwaving plastic containers.
- Plastic containers have recycle codes on the bottom. Some, but not all, plastics that are marked with recycle codes 3 (PVC or vinyl), 6 (polystyrene foam), or 7 (not all code 7 plastic bottles contain polycarbonate and leach BPA) or “PC” (polycarbonate) may be made with BPA.
- Eat more fresh food and less canned food.
- When possible, opt for glass, porcelain or stainless steel containers, particularly for hot food or liquids.
- Breastfeed your infant if you can. For bottle-feeding, use glass bottles.
- Wash your and your children’s hands before eating or drinking. BPA can get on your hands from some items you touch, like receipts.
- Use baby bottles that are BPA free.
Bisphenol A effects on humans
The first evidence on BPA’s ability to exert biological effects was obtained in 1936 by Dowds and Lawson 149, who discovered the estrogenic properties of Bisphenol-A in vivo. In 1997, an estrogen-receptor-dependent mechanism of action for BPA was elucidated that involved estrogen receptors ERα and ERβ 150, 151, 152. Owing to BPA’s structural similarity to estradiol (i.e., major female sex hormone), BPA can interfere with steroid signaling, thereby causing reproductive health outcomes, depending on the window of exposure, dosage, duration and mode of exposure, and developmental life stage 150, 153, 154, 155, 156. The European Chemicals Agency (ECHA) has classified BPA as a reproductive toxicant and a substance of very high concern 157. The National Toxicology Program (NTP) Center for the Evaluation of Risks to Human Reproduction has stated, “The NTP has some concern for effects on the brain, behavior, and prostate gland in fetuses, infants, and children at current human exposures to bisphenol A” 42, 104, 158.
BPA is often classified as an endocrine-disrupting chemical (EDC) as it can act as a xenoestrogen 14, 159, 42. Endocrine-disrupting chemicals can mimic or antagonize endogenous hormones by interfering with their synthesis or clearance, resulting in developmental, reproductive, cardiovascular, neurological, or immune effects, metabolic disorders, or oncogenesis in both humans and animals 119, 160, 161, 162, 163, 164, 165, 166. Importantly, the endocrine system is most vulnerable to assaults by endocrine-disrupting chemicals during the prenatal and early development window, and the induced effects may persist into adulthood and be passed on to future generations 167, 168, 169, 170, 171. There is growing evidence that shows that not only endocrine-disrupting chemicals can directly affect various organ systems in humans and animals, but they can also exert transgenerational effects, presumably through placental exposure in fetus or lactational exposure in offspring 159, 168, 171.
BPA disrupts the synthesis, secretion, release, and transport of hormones by interacting with biological receptors, such as the androgen receptor (AR), thyroid hormone receptor (THR), estrogen-related receptor gamma (ERRγ), and glucocorticoid receptor (GR), as well as other nuclear and membrane estrogen receptors (ERs), such as G-protein-coupled estrogen receptor (GPER/GPR30) and estrogen-related receptor γ (ERRγ), and other nuclear receptors, e.g., constitutive androstane receptor (CAR), glucocorticoid receptor (GR), liver X receptor (LXR), peroxisome proliferator-activated receptor β/δ (PPARβ/δ), retinoic acid receptor (RAR), and retinoid X receptor (RXR) 160, 172, 173, 174, 175, 176, 177, 178. For example, BPA exhibits estrogenic, antiestrogenic, and antiandrogenic activities at multiple levels along the hypothalamus–pituitary–gonad (HPG) axis, which is a main regulator of reproductive system 155, 179.
Furthermore, BPA-induced neuroendocrine regulation may result in mental and behavioral consequences in the offspring. Higher maternal BPA levels increase the risk of developing behavior problems in preschool children 180. Prenatal BPA exposure was also strongly associated with anxiety and depression in children 181.
An alternative mode of action for BPA is its interaction with key transcription factors (TFs) to modulate regulation of gene expression 179, 182, 183, 22. Accumulating data suggest that BPA interacts with adipogenic transcription factors, such as peroxisome proliferator-activated receptors (PPARs), CCAAT-enhancer-binding proteins (C/EBPs), and nuclear factor erythroid 2-related factor 2 (Nrf2), to exert obesogenic effects 179, 182, 183. In addition, transcription factors (TFs) from homeobox gene (HOX) family and heart- and neural crest derivatives-expressed protein 2 (HAND2) are thought to play a crucial role in BPA-mediated detrimental effects 22, 179, 183.
A third mechanism of action for BPA has emerged that involves epigenetic modifications mainly aberrant DNA methylation, in a number of studies in human populations (Figure 3) 166, 184, 185, 141, 182, 186. Altogether, there is evidence that in vitro and in vivo exposures to BPA are associated with histone modifications, as well as with upregulation or downregulation of diverse miRNAs or lncRNAs, many of which are implicated in pathogenic pathways involved in various diseases, such as cancer, reproductive, neurobehavioral, cardiovascular, and metabolic diseases, and inflammation 187, 188, 189. It is prudent, however, to carefully examine the findings of these studies and interpret their results cautiously. A main concern for epigenomic studies in human populations is the epigenetic plasticity 190. Human epigenome changes dynamically according to physiologic state and pathologic conditions 191, 192, 193, 194. This is represented by the continuous shaping and reshaping of the epigenome during the developmental stage, aging, or consequent to exposure to a wide variety of chemical or physical agents attributable to lifestyle factors (e.g., smoking), occupation, medical treatments, diet, and environment, as well as various diseases and conditions 191, 195, 196, 197, 198. Therefore, associating epigenetic changes to any given exposure in human populations is tremendously challenging. In the case of BPA, this situation might be even more complicated, considering the pervasiveness of this chemical in the environment and the constant, recurring, and multiple-source exposure of humans to this compound. This is further compounded by the toxicokinetics of BPA and lack of long-term exposure biomarkers for this chemical 45, 83, 84, 85.
BPA is classified in the European Union as a substance that 3, 44:
- causes toxic effects on humans ability to reproduce (may damage infertility);
- causes serious eye damage;
- may cause respiratory irritation;
- may cause skin allergies (skin sensitizers);
- very toxic to aquatic life;
- very toxic to aquatic life with long-lasting effects.
According to the United States Environmental Protection Agency (EPA), BPA’s reference dose is 50 mcg/kg body weight per day. The European Food Safety Authority (EFSA) decreased the tolerable daily intake (TDI) dose of BPA from 50 mcg/kg body weight per day to 4 mcg/kg body weight per day in 2015 due to its harmful health effects 39, 40, 122. However, in April 2023, the European Food Safety Authority (EFSA) published a new re-evaluation of the risks to public health related to BPA in foodstuffs, significantly reducing the tolerable daily intake (TDI) set in its previous assessment in 2015 199, 3. The European Food Safety Authority (EFSA) established a new tolerable daily intake (TDI) of BPA at 0.2 nanograms/kg body weight per day (0.2 ng or 0.2 billionths of a gram) 3. This new tolerable daily intake (TDI) is 20,000 times lower than the 2015 temporary tolerable daily intake (TDI) of 4 mcg (4 micrograms or 4 millionths of a gram) per kg body weight per day 39, 40. Furthermore, by comparing the 2023 tolerable daily intake (TDI) with estimates of dietary exposure to BPA, EFSA’s scientific experts concluded that consumers with both average and high exposure to BPA in all age groups exceeded the new TDI, indicating health concerns and potentially harmful health effects on the immune system 3, 41.
Currently, an evaluation made of a group of 148 bisphenols, among which was BPA, by the European Chemicals Agency (ECHA) and the Member States of the European Union (EU), has recommended restricting 30 of them, including BPA, due to their possible hormonal and toxic effects on reproduction. In view of the above, it is important to underline that one of the characteristics of endocrine-disrupting chemicals (EDCs) is that they produce non-monotonic dose–response curves (NMDCR) in some dose ranges; a dose–response curve traces the intensity of an effect given in a range of doses examined. A non-monotonic dose–response curves (NMDCR) is characterized by a slope that changes its sign (trajectory) within the range of doses studied. Some curves are U-shaped, others are like an inverted U and, in others, the curve sign may change into multiple points throughout the dose range evaluated 200, 201, 202, 203, 204.
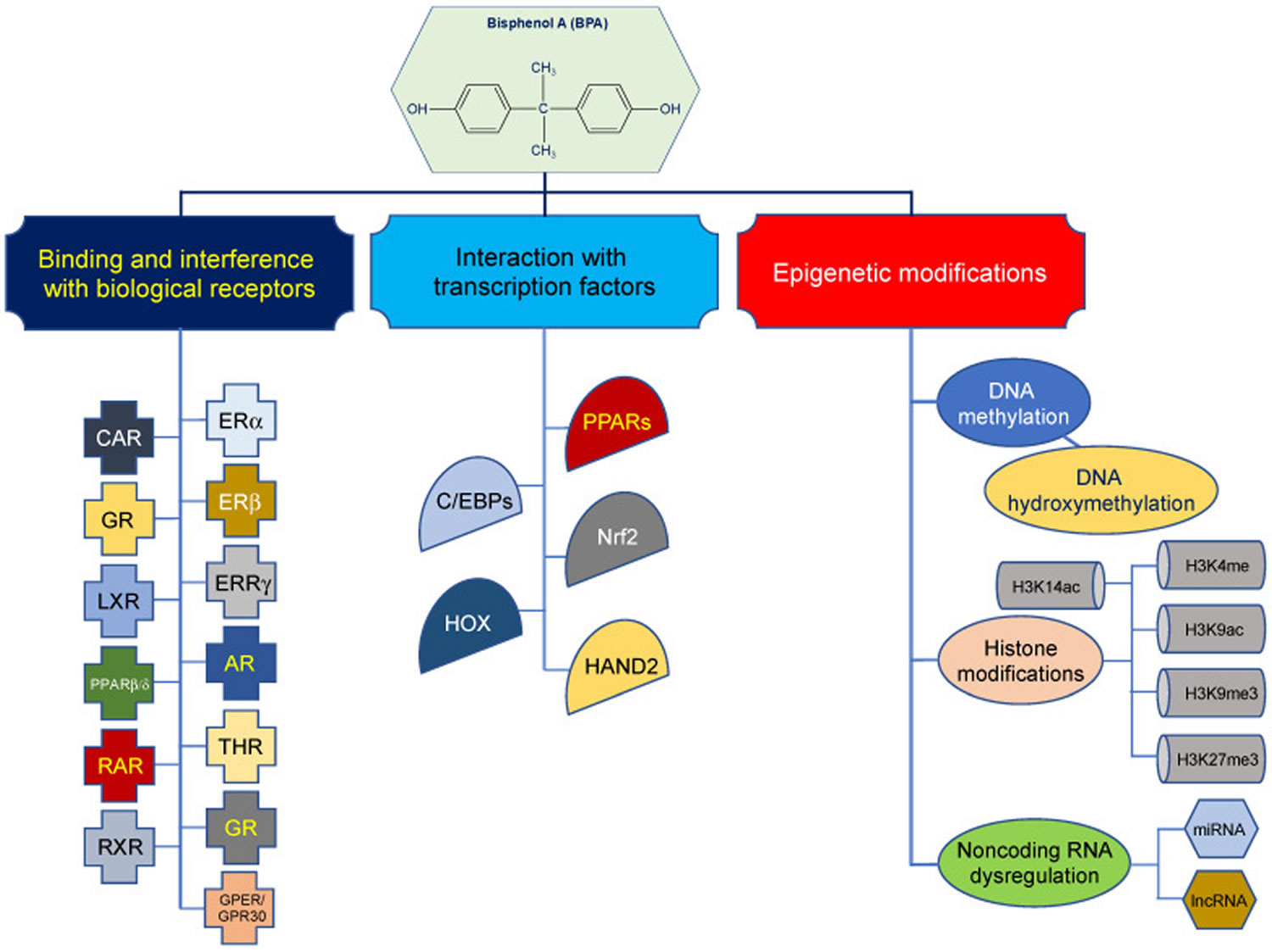
Figure 3. Bisphenol A mechanisms of action
Footnotes: Bisphenol A mechanisms of action. (1) Binding and interference with biological receptors, (2) interaction with transcription factors, and (3) epigenetic modifications. For brevity, the most investigated biological receptors, transcription factors, and histone modifications are shown. The figure is a simplified summary but not an exhaustive delineation of the molecular mechanisms of bisphenol A.
Abbreviations: AR = androgen receptor; CAR = constitutive androstane receptor; C/EBPs = CCAAT-enhancer-binding proteins; ERs = estrogen receptors; ERRγ = estrogen-related receptor γ; GPER/GPR30 = G-protein coupled estrogen receptor; GR = glucocorticoid receptor; H3K4me = histone 3 lysine 4 methylation; H3K9ac = histone 3 lysine 9 acetylation; H3K9me3 = histone 3 lysine 9 trimethylation; H3K27me3 = histone 3 lysine 27 trimethylation; H3K14ac = histone 3 lysine 14 acetylation; HAND2 = heart- and neural crest derivatives-expressed protein 2; HOX = homeobox gene family; LXR = liver X receptor; Nrf2 = nuclear factor erythroid 2-related factor 2; PARs = peroxisome proliferator-activated receptors; PPARβ/δ = peroxisome proliferator-activated receptor β/δ; RAR = retinoic acid receptor; RXR = retinoid X receptor; THR = thyroid hormone receptor.
[Source 2 ]Bisphenol A health effects
BPA can affect hormone (estrogen) concentrations. It is associated with a variety of medical disorders:
- In animals, low concentrations of BPA exposure when the fetus is developing may increase the risk for:
- Behavior changes like hyperactivity, more aggression, and learning problems
- Early puberty
- Changes in breast and prostate cells
- Increased fat cells and body weight
- Changes in the immune system
- In humans, BPA exposure when the fetus is developing may increase the risk for:
- Behavior issues (like hyperactivity and aggression)
- Later breast development during puberty
- Obesity and Diabetes
- Heart disease
- Changes in liver function
BPA as an endocrine disruptor
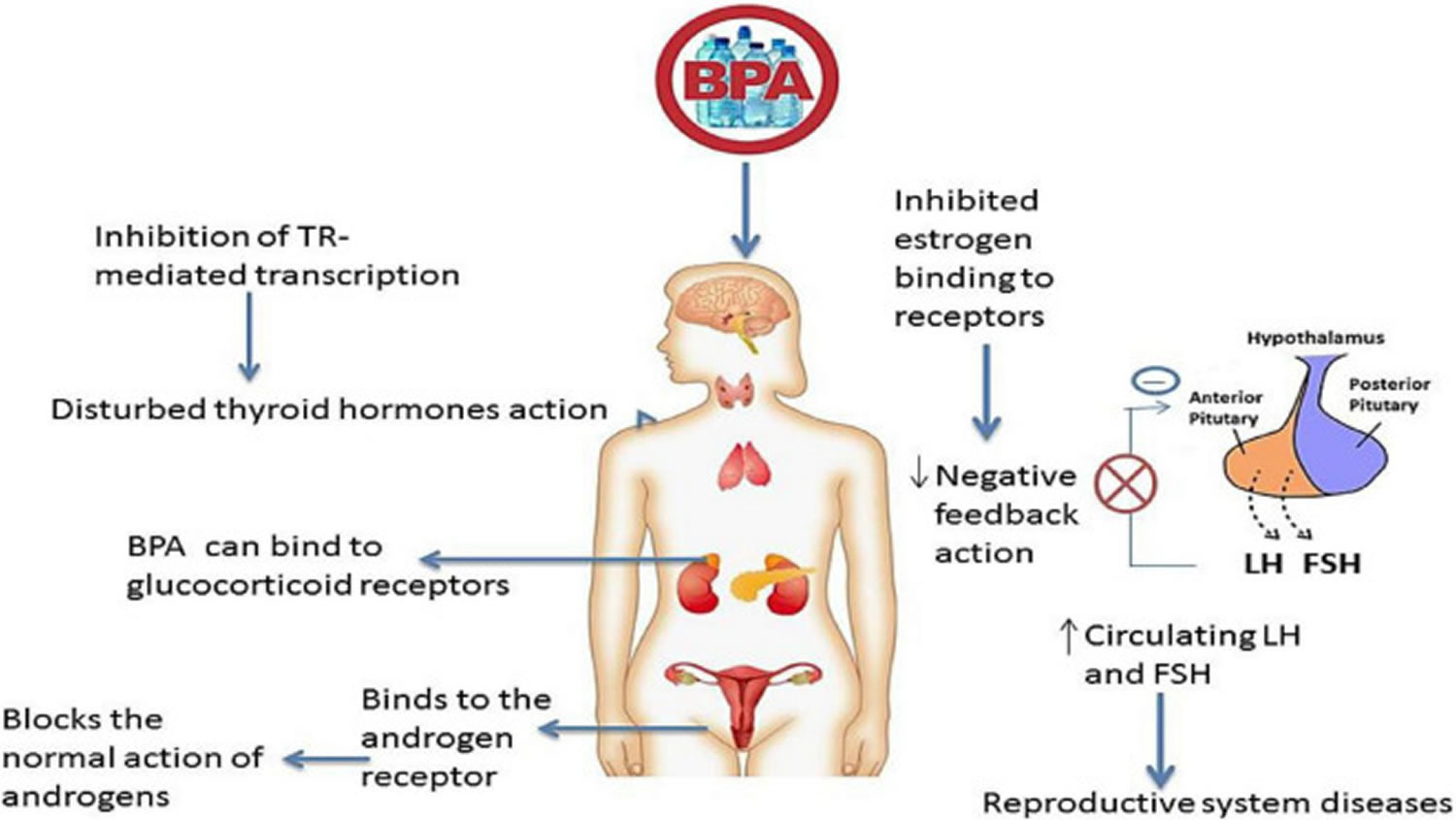
BPA is an adverse endocrine-disrupting chemical or endocrine disruptor which suppresses or alters hormonal and enzyme synthesis, secretion, release, and transportation (Table 2). BPA hinders the endocrine system’s activity by replacing endogenous hormones with transporter proteins (Figure 4). This alteration changes the free and bound hormonal concentrations present in plasma. BPA also influences the neuroendocrine function, causing a physiological interruption in the organs. Studies have shown the increased serum level of estradiol in females and reduced testosterone in males due to BPA 205, 206.
Recent animal studies have observed that BPA can cause developmental programmings of metabolic diseases, such as diabetes mellitus and obesity in later stages of life 207, 208, 209. BPA exposure affects glucose metabolism by disrupting pancreatic cell function and producing insulin secretion 210. BPA also affects adipocytes’ metabolic functions, leading to insulin resistance development. In an in vivo study, BPA exposure promoted insulin resistance by reducing adiponectin levels and increasing adipocytokines levels 211. Urinary BPA levels were positively linked with metabolic syndrome 161. Few epidemiological studies have found a link between BPA exposure and diabetes mellitus in patients who were not predisposed to the disease due to factors, such as age, serum cholesterol levels, or body mass index 161, 212, 213. In obese pregnant women, BPA exposure was associated with an increased risk of altered glucose metabolism 214. Furthermore, it was found that BPA shows these effects in specific trimesters, particularly in the second trimester 215. Higher urinary BPA levels were associated with childhood obesity as well as abdominal obesity 216. In addition, BPA perinatal exposure induces the development of diabetes mellitus in the offspring. BPA-induced developmental programming is thought to be due to epigenetic modifications 217.
Mental health is highly influenced by BPA, which causes sex-specific mental impairment and behavioral changes 120. Disturbed and depressive tendencies rose because “Dehydroepiandrosterone (DHEA),” a neuroactive steroid in males, is decreased, resulting in a possible pathway of the depressive-like phenotype 218.
Figure 4. Bisphenol A is an endocrine disruptor
Footnote: Bisphenol-A as an endocrine disruptor. Estrogens negatively affect the release of follicle-stimulating hormone (FSH) and luteinizing hormones (LH). BPA can inhibit the estrogen binding to its receptors at the pituitary level, resulting in high levels of follicle-stimulating hormone (FSH) and luteinizing hormones (LH) hormones in circulation. This can lead to reproductive system issues such as polycystic ovarian syndrome (PCOS). BPA acts as anti-androgen by binding with androgen and glucocorticoid receptors and affecting their action. BPA can disturb the action of the thyroid hormone by inhibiting TR-mediated transcription of T3-response genes.
[Source 120 ]Table 2. BPA effect on the endocrine system
| Specimen | Route of exposure | Health impact | References |
|---|---|---|---|
| Rats | Skin | BPA directly affects the central nervous system on exposure, primarily affecting CA3 pyramidal neurons and GABAA receptors. | 219 |
| Mice | BPA injection (125 mg/kg) | BPA exposure leads to endocrine disruption, which affects the immune system. BPA-induced steroid genesis and Nur77 gene expression in testicular Leydig cells. | 220 |
| Pregnant female mice | Oral | BPA affects the sex steroid hormone in the urogenital sinus of a fetus. Due to endocrine disruption, BPA increases estrogenic production and adversely affects the fetus’s heart, kidneys, cerebellum, and ovaries. | 221 |
| Male offspring rats | Injection of BPA (low doses) | Hyperactivity and attention deficit due to endocrine disruption. In the basolateral amygdala, the BPA accumulation results in abnormal synaptic plasticity leading to these defects. | 222 |
| Rats offspring | Oral (during gestation and lactation) | Metabolic disruption due to raised glutamate and L-α-glutamyl- L-aspartic acid ratio in the hippocampus because the 2 metabolites are involved in the malate-aspartate metabolic shuttle. Myelination, growth, glial, and neuronal development alterations due to endocrine disruption. | 223 |
BPA effect on the Reproductive system
BPA was initially explored for its estrogenic properties and was found to influence the synthesis of estrogen and testosterone 224, 225. In subsequent studies, BPA was found to interfere with sex hormone activities and associated with developmental toxicity and functional disturbances of the reproductive system (Table 3) 226. BPA is responsible for the pathogenesis of female infertility 227. BPA also distracts the function and primary development of the reproductive system (Figure 5). Recent studies reported the BPA linkage with increased levels of serum luteinizing hormone (LH), estradiol (E2), progesterone, and testosterone (T) while decreased concentrations of serum cortisol 206. A significant association between BPA and higher total testosterone (TT) concentration in serum was also reported 228.
In a study, serum BPA levels were detected (limit of assay detection: 0.5 ng/mL) in 41.8% of infertile women and 23.3% of fertile women 229. Interestingly, higher BPA exposure and levels in infertile women in a metropolitan area show evidence of a greater presence of economic activities using these chemicals and more usage in food and consumer products 229. It cannot be refuted that BPA may be detected in infertile women. In females, the BPA-induced reproductive abnormalities include increased endometrial wall thickness, the occurrence of polycystic ovary syndrome (PCOS), an increased risk of recurrent miscarriage, neonatal mortality, defective placental function, irregular cycles, and reduced primordial follicles 230, 231, 232, 233, 234, 235, 236, 237. Experimental and epidemiological studies have confirmed that BPA exposure during pregnancy affects the development and growth of offspring. Exposure to BPA in utero has been shown to affect the development of the uterus and mammary glands 238, 239. A direct association was also found between urinary BPA levels and implantation failure 240. In males, BPA can interfere with the regulation of spermatogenesis via the hypothalamic–pituitary–gonadal axis. BPA has been shown to impair male reproductive function with a reduction in sperm quality, defective ejaculation, reduced libido, and erectile dysfunction 241, 242. In experimental animals, administration of BPA significantly decreased the expression of the GnRH gene in cells of the preoptic area and circulating levels of gonadotropins and/or testosterone 243. Occupational exposure to BPA among adult males in China has been reported to be associated with changes in serum hormone levels and male sexual dysfunction 242.
Scientists observed an age-based relationship between altered endometrial wall thickness and BPA levels 244. Moreover, polycystic ovary syndrome (PCOS) patients exhibited higher BPA serum concentrations than healthy women and patients 245, 246. The researchers also detected BPA’s potential role in PCOS and adverse pregnancy outcomes like premature delivery and miscarriage 247.
Males with prolonged BPA exposure tend to have low sperm quality, sexual dysfunction, and impaired fertility 120. The amplitude of lateral head displacement (ALH), Wobble (WOB), Linearity (LIN), Mean Angular Displacement (MAD), sperm concentration, and association with BPA illustrated the fluctuated characteristics and velocity rate reduction. This array results in impaired reproductive function in males 248.
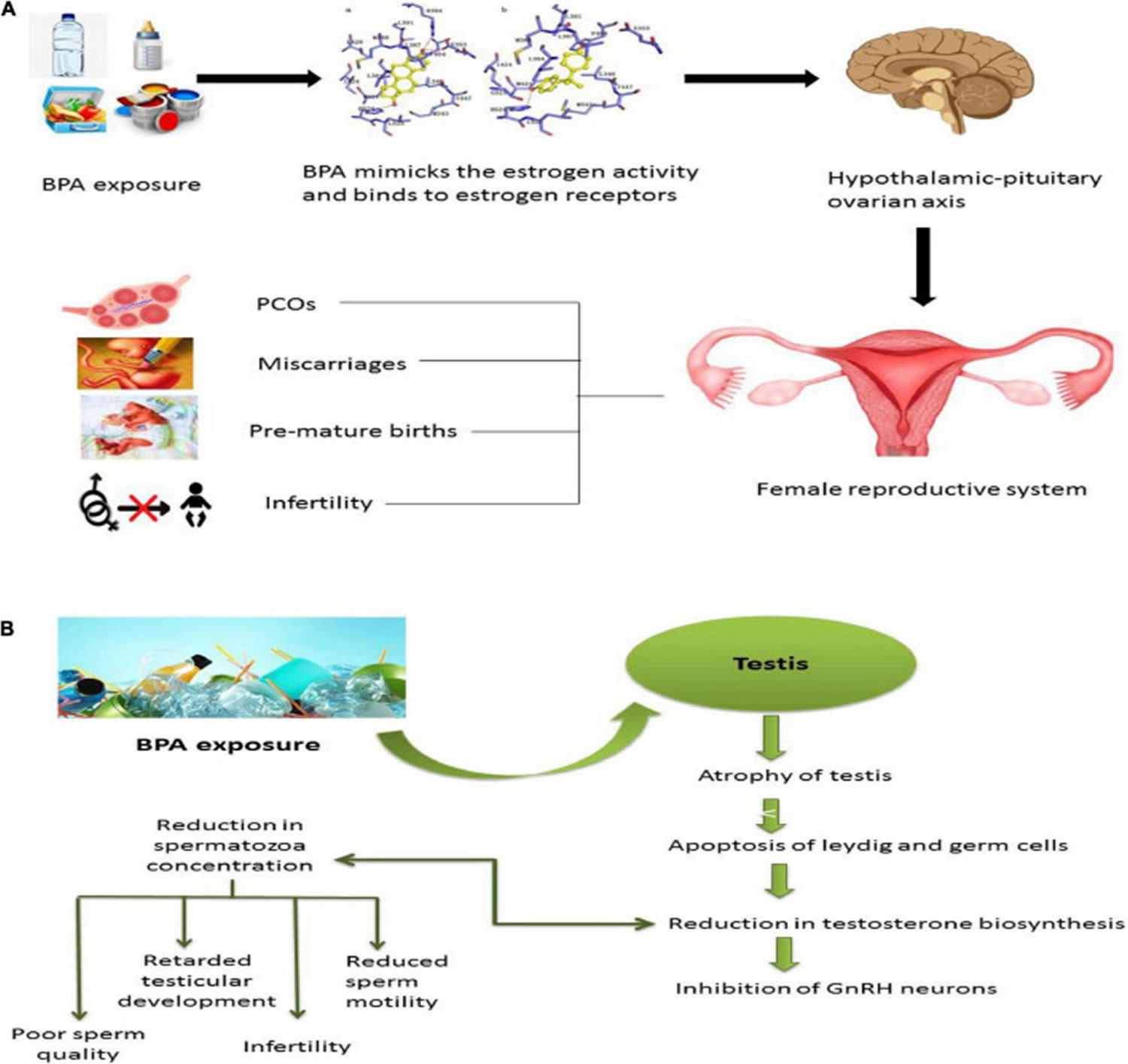
Figure 5. BPA effect on the reproductive system
Footnotes: Effect of BPA on the reproductive system. (A) When exposed to BPA, females can develop fertility-related issues as it is very similar to estrogen structure and function. It binds to estrogen receptors and causes irreversible alteration to the hypothalamic-pituitary-ovarian axis. BPA will provoke estrogen and thus increase the chances of polycystic ovarian syndrome (PCOS), delay puberty, miscarriages, endometriosis, premature births, and most of the time, BPA can cause infertility. (B) Exposure of males to bisphenol interferes with the reproductive system. BPA causes atrophy in the testis, apoptosis in Leydig cells and germ cells, and reduction in testosterone biosynthesis, which will either cause the reduction in spermatozoa reduction or inhibition of gonadotropin-releasing hormone (GnRH) neurons. It causes sperm quality and quantity alterations, retardation of testicular development, infertility, and reduction in sperm motility.
[Source 120 ]Table 3. BPA impact on the reproductive system
| Specimen | Route of exposure | Findings | References |
|---|---|---|---|
| Female Sprague– Dawley rats | Oral | Significant hormonal disorders altered the structure and functions of the ovaries and uterus. | 249 |
| KGN ovarian granulosa-like tumor cell line | In vitro | Reduction in insulin-like growth factor 1 (IGF-1) induced by FSH and aromatase expression. BPA causes a reduction in granulosa cell DNA synthesis with no changes in DNA fragmentation, showing that BPA does not encourage apoptosis. | 250 |
| Pregnant women | Oral | Creatinine-identical BPA concentrations caused a reduction in reproducibility. BPA concentration was not altered by the intake of canned fruit, fresh vegetables, fruits, or fresh and frozen fish purchased from the store. High-molecular-weight phthalate and serum tobacco smoke metabolic compound levels were significantly linked with BPA levels. | 251 |
| Males | Oral | Increased serum total testosterone, prolactin, and estradiol resulted in a reduction in the androgen index. | 252, 253 |
| Males | Serum | Sexual desire and functionality were decreased in men, followed by premature ejaculation. | 254 |
| Males | Oral | A higher level of BPA in plasma and seminal plasma has a risk of an increased infertility level. | 255 |
| Males | Serum administration | The concentration of sperm was decreased, and sperm velocity ratios were increased, followed by a reduction in sperm motility and count. | 253, 256, 257, 258 |
| Females | Oral | The level of Luteinizing hormone and progesterone was increased; hence, the risk for PCOS also increased. | 259, 260 |
BPA exposures can lead to cancer
The incidence of numerous cancer types is rising and appears to be linked with BPA (Table 4) 120. It includes breast cancer 261, ovarian cancer, uterine cancer, prostate cancer 262, 263, 264, and testicular cancer 265. The findings of the various in vivo studies on animals (i.e., mice, rats, etc.) concluded that the raised estrogenic activity depicts the carcinogenic mechanistic action of BPA 266. BPA’s activation of tumorigenesis and cancerous cell development are still under experimentation 261.
BPA stimulates cellular responses through binding to estrogen receptor (ER), although they reflect a weak affinity to each other (Figure 6) 120. The binding ability of the estrogen receptor (ER) to hold co-repressors is lost. As the regulation of co-regulators by the BPA–ER complex is disproportionate to the affinity of BPA to estrogen receptor (ER), the type and expression levels of ER-regulated targets are determinants for the tissue and cellular specificity of the BPA response. BPA can induce genomic responses at concentrations lower than the levels at which it is expected to bind to nuclear estrogen receptors (ERs) 267.
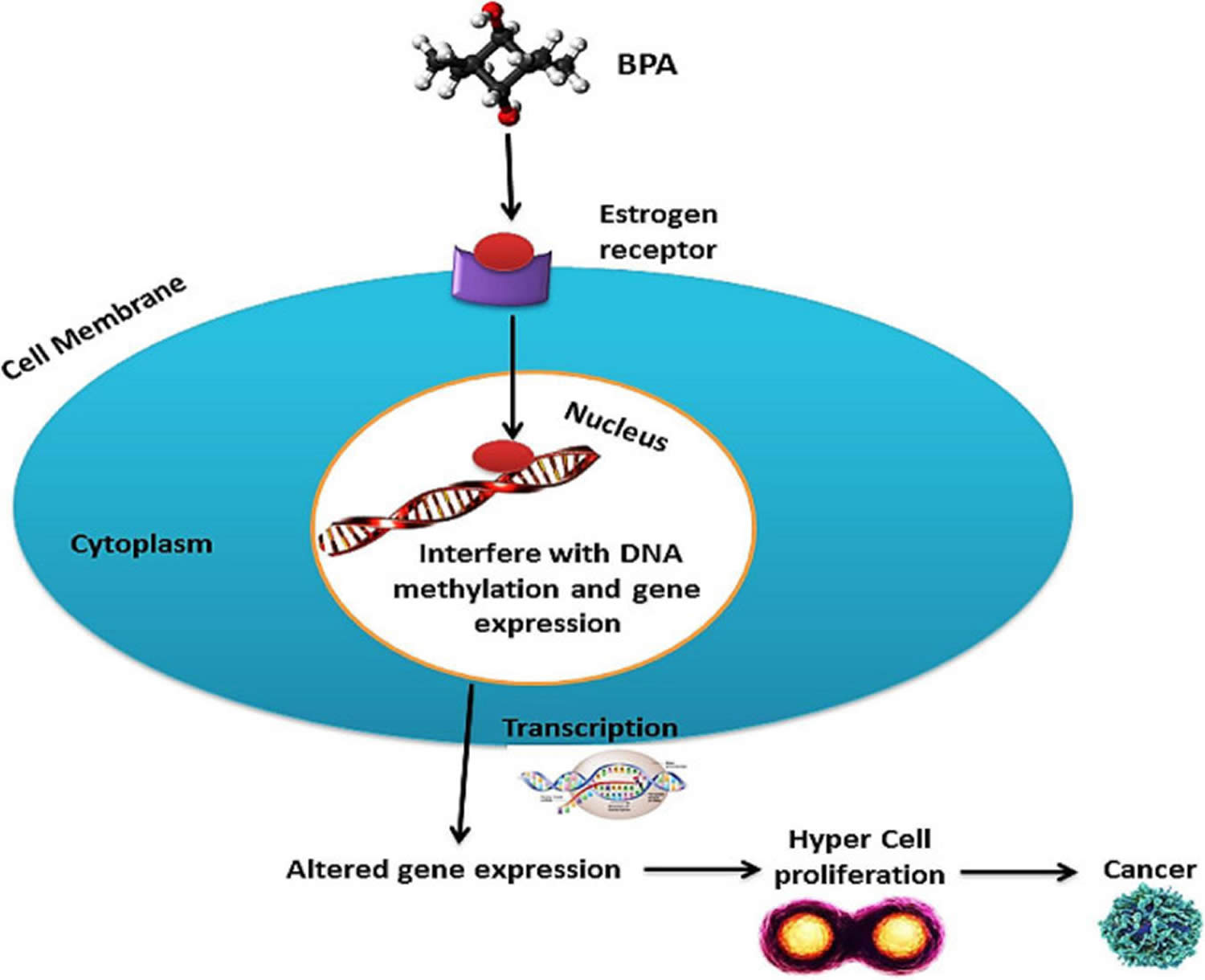
Figure 6. BPA carcinogenic activity
Footnote: BPA interacts with the estrogen receptors and interferes with DNA methylation and gene expression after entering the nucleus. Thus altered gene expression leads to cell proliferation, which may lead to cancer.
[Source 120 ]Table 4. BPA’s role in carcinogenicity
| Specimen | Route of exposure | Findings | References |
|---|---|---|---|
| Rat | Oral | The increased number of Leydig cells and proliferation was caused due to exposure to BPA during the Perinatal period. | 268 |
| Rat | Antenatal | Ductal carcinoma and ductal hyperplasias were developed due to increased BPA exposure during the perinatal period carcinoma in situ and malignant tumors. | 269 |
| Rat | Neonatal exposure | Polycystic ovary syndrome was reported due to the neonatal exposure to. | 270 |
| Mouse | Prenatal exposure | Cystadenomas, a high rate of progressive lesions of the oviduct and ovarian cysts, were observed due to Prenatal exposure to BPA. | 271 |
| Mouse | Neonatal exposure | Increased adenomyosis, cystic endometrial hyperplasia, and leiomyomas developed due to neonatal exposure to BPA. | 271 |
| Mouse | Oral | BPA exposure was studied in the renal xenograft model, and a high rate of adenocarcinoma of human progenitor cells and prostate intraepithelial neoplasia were reported. | 272 |
| Mouse | Perinatal exposure | Exposure to BPA during the perinatal period effect the offspring/infant greatly, and it leads to neoplastic lesions and hepatic pre-neoplastic. | 273 |
| Breeding C57Bl6 mice | Oral | Perinatal contact with BPA amplified the number of TEBs and the progesterone response of the mammary epithelial cells. | 274 |
| Non-human primates | Oral | BPA exposure to the fetus accelerated mammary epithelial development and a high rate of mammary buds’ density. | 275 |
BPA effect on the Immune system
Studies have revealed that oxidative stress, immune function, and inflammation are directly related to BPA exposure 120. The correlation between BPA and the induction of mitochondrial damage and cellular apoptosis resulted in systematic degradation 276, 277, 278, causing an alternation in immune cell populations and functioning of the innate and adaptive immune system owing to developmental BPA exposure (Figure 7).
Similarly, BPA also decreased T regulatory (Treg) cells and up-regulated pro-inflammatory and anti-inflammatory cytokines and chemokines 120. Type 1 diabetes mellitus development in females and males could be accelerated and decline on exposure to BPA (Table 5) 279.
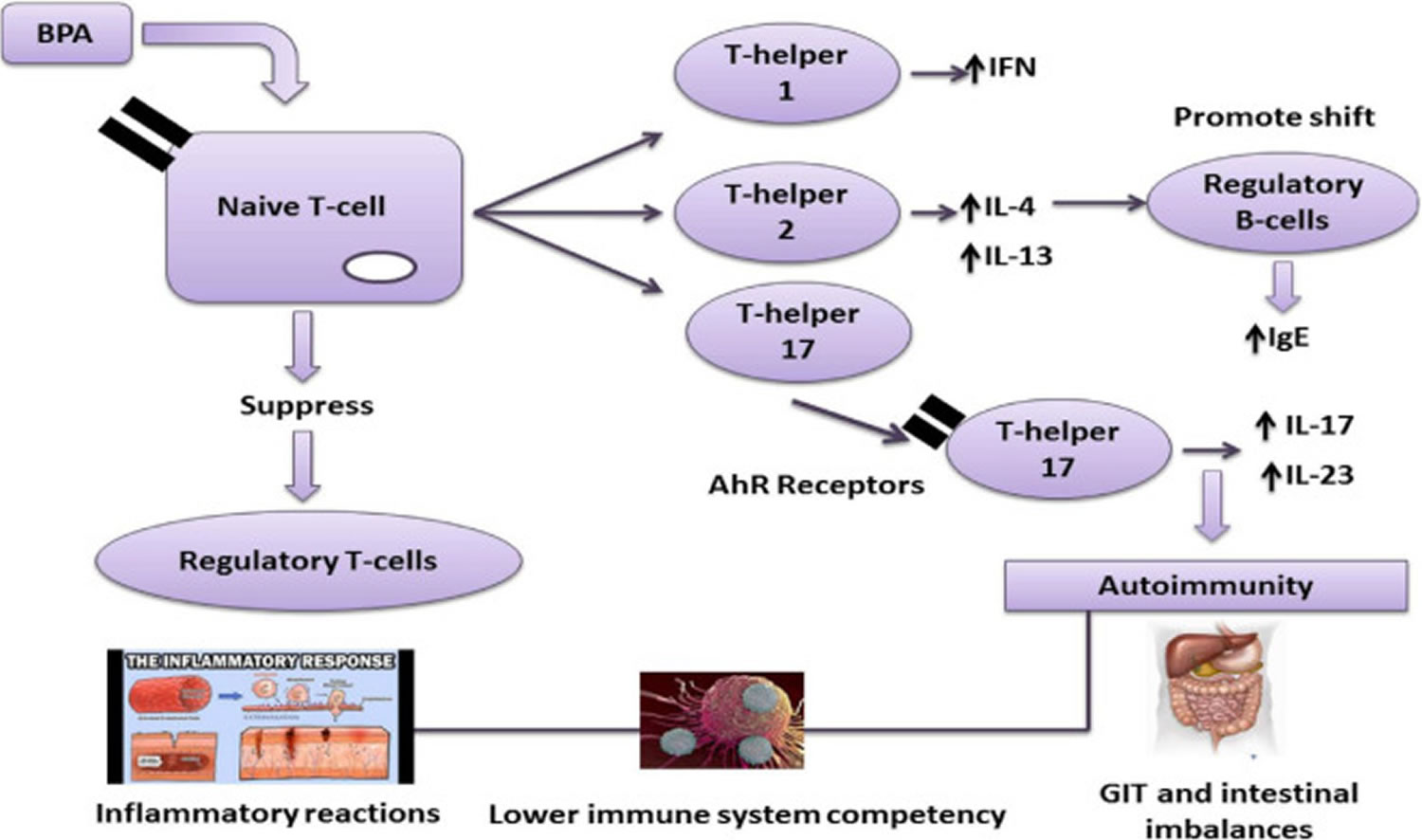
Figure 7. BPA effect on the immune system
Footnote: Effect of the BPA immune system BPA can promote autoimmunity via T-helpers 1, 2, and 17. Aryl hydrocarbon receptors (AhR) are involved in regulating immune responses, followed by the production of T-helper 17 a critical factor in T-cells in various autoimmune diseases.
[Source 120 ]Table 5. BPA effect on the immune system
| Specimen | Route of exposure | Findings | References |
|---|---|---|---|
| Pregnant women | Environmental (inhalation or dermal) | Concentrations of BPA in the mother’s urine were reciprocally associated with odds of increased IL-33/TSLP. | 280 |
| Humans | Oral and environmental | BPA can harm the immune system’s functions as assessed by CMV antibody levels and allergy or hay fever diagnosis. | 281 |
| Sprague–Dawley rats | Oral | Ten measurements out of 530 were different from vehicle controls and were primarily associated with dendritic or macrophage cell populations. BPA may have negatively affected the competency of the immune system. | 282 |
| Mice | Oral | BPA exposure via the oral route, in a given amount and for the shown contact period, has minor manipulation of features of the inflammatory response, stimulating immune-mediated diseases of the GIT. | 283 |
| Mice | Oral | Imbalances induced in intestinal and systemic immune via perinatal treatment, the appearance of inflammatory M1 macrophages in gonadal white adipose tissue with signs of aging, combined with a reduction in insulin sensitivity and an enhancement in weight gain. | 284 |
| Pregnant mice | Oral | Mother exposure to BPA modulated inborn immunity in mature offspring but did not damage the anti-viral adaptive immune response, which is dangerous for virus permission and endurance after influenza virus infection. | 285 |
| BALB/female mice | Oral | The chances of asthma increased when the mother was exposed to BPA. It might increase the airway hyperresponsiveness in their infants’ lungs as they were bare to BPA before birth and after birth via breast milk compared to those exposed to BPA after birth or not treated with BPA at all. | 286 |
| Adult zebrafish | Oral | Down-regulation in the transcription of genes involved in enzymatic antioxidant defense and impaired anxiety and fear responses. | 287 |
| Larvae of Labeo rohita | Oral | Oxidative stress and suppressed NF-κB signaling pathway leading to immunosuppression. | 288 |
| Human granulosa KGN cells | In vitro | Damage to biomacromolecules-main targets of oxidative stress was significantly increased after treatment with BPA. | 289 |
| Adult rats | Oral | BPA-induced systemic oxidative stress change ROS-induced signaling pathways in the brain. | 290 |
BPA effect on the Cardiovascular system
Prenatal BPA exposure was also linked to an increased risk of developing cardiovascular disorders and non-alcoholic liver disease in later life 291, 292, 293, 207, 294. Clinical evidence shows an association between serum and/or urinary BPA levels and cardiovascular diseases 295, 296. Increased serum BPA levels in dilated cardiomyopathic patients were also reported 297. Experimental studies confirmed that BPA exposure could adversely affect cardiac structure and function 298, 299. Furthermore, BPA exposure increases systolic blood pressure, alters heartbeat in isolated heart preparations, and blocks cardiac sodium channel receptors 300, 301, 302. BPA can also alter calcium homeostasis in the heart by stimulating estrogen receptors on the plasma membrane 303. BPA exposure was also associated with the development of atherosclerosis and peripheral arterial disease 304, 161, 305.
BPA effect on the Urinary system
Since BPA is a xenoestrogen and the kidneys possess several receptors for estrogen, BPA stimulates the estrogen receptors, leading to the proliferation of epithelial cells and also increases the volume of proximal and distal cells leading to hydronephrosis 306. BPA treatment in animals showed bladder enlargement, hypertrophy, and urinary voiding dysfunction 307. The late manifestations of voiding dysfunction were also observed in mice treated with testosterone and BPA [101]. The authors observed that in adulthood, BPA exposure is associated with lower urinary tract dysfunction 307. Interestingly, narrowing of the lumen of the prostatic urethra of mice treated with BPA testosterone was observed 307. This may be similar to prostate enlargement and bladder alterations in humans. Enlargement of the prostate and urinary bladder may decrease the average flow rate. Higher BPA levels were strongly associated with reduced glomerular filtration rate and impaired renal function 308. Chronic BPA exposure caused inflammatory infiltration, fibrosis, and tubular injury in the kidney. BPA-induced defective autophagy flux was the key mechanism behind these effects 309.
BPA effect on the Gastrointestinal System
In vitro studies have shown that BPA promotes mitochondrial dysfunction, oxidative stress, and inflammation 310, 311. BPA is well known to affect liver enzymes’ activities and promote hepatic lipid accumulation 312. High urinary BPA levels were found to be associated with non-alcoholic fatty liver disease in adults 313. BPA has been demonstrated to promote oxidative phosphorylation abnormalities in the liver mitochondria by inhibiting the first complex of the electron transport chain [108]. In several studies, BPA has been shown to pose deleterious effects on liver function and structure in both people and animals. BPA can promote hepatic steatosis in humans 314, enhance insulin resistance in HepG2 cells [110], alter its shape, and raise liver function enzymes 315. The upregulation of sterol regulatory element binding protein 1 (SREBF1) has been implicated in BPA-induced hepatic lipid accumulation in non-alcoholic fatty liver disease (NAFLD) 316.
BPA effect on the Respiratory system
It has been found that postnatal exposure to BPA is a risk factor for the development of childhood asthma 317, 318. BPA has been reported to be associated with bronchial eosinophilic inflammation or allergic sensitization 319. A research report published in 2019 showed that a doubling of BPA in a mother’s urine sample corresponded with (approximately) a 5 mL decrease in the child’s lung capacity 320. In adult murine asthma models, researchers showed an aggravating effect of BPA on eosinophil infiltration and airway inflammation as evidenced by increasing levels of Th2 cytokines and chemokines 321. The same study found that BPA affected allergic inflammation in allergic asthmatics 321. Hence, BPA exposure may have detrimental effects on the respiratory system.
BPA effect on the Nervous system
Recent research studies found that a derivative of BPA, i.e., Bisphenol F, is responsible for neuroinflammation and apoptosis of central nervous system cells, leading to abnormal neurological development in the early life stage of zebrafish 322. A disturbance in the neurotransmitter function may be responsible for the disturbance in the nervous system. One of the neurotransmitters, GABA, is responsible for maintaining the balance between the excitatory and inhibitory systems necessary for the development of a normal brain 322. Research studies on animal models have shown that prenatal exposure to BPA affects the mevalonate (MVA) pathway in rat brain fetuses 323. The MVA pathway is important for the development and function of the brain 323. Interestingly, hypothalamic exposure to BPA showed an increase in micro RNA (miRNA) miR-708-5p, which is responsible for controlling neuropeptides directly linked to obesity 324.
What does BPA free mean?
BPA free means “Not made with BPA” indicating that BPA is not used in the product or device component.
As of 2012-2013, U.S. Food and Drug Administration (FDA) regulations no longer authorize the use of BPA in baby bottles, sippy cups, and packaging for infant formula 325.
- Check labels on bottles and food containers to make sure they are BPA-free.
- Discard plastic baby bottles, infant feeding cups, water bottles, food containers, and toys that might contain BPA.
- Avoid using plastic storage containers marked with recycle codes #3 or #7, which contain BPA.
- Avoid heating plastics in the microwave, which may cause BPA and other chemicals to leach into foods.
- Avoid food from cans that might be lined with BPA plastics.
- Replace plastic products with reusable glass cookware and baby bottles. Replace plastic water bottles or coffee and tea mugs with glass or stainless steel.
- Keep plastics cool.
- Eat fewer canned goods and more fresh foods when possible.
- Goodman J.E., Peterson M.K. Encyclopedia of Toxicology. Volume 1. Elsevier Inc./Academic Press; Amsterdam, The Netherlands: 2014. Bisphenol A; pp. 514–518.[↩][↩][↩][↩]
- Besaratinia A. The State of Research and Weight of Evidence on the Epigenetic Effects of Bisphenol A. Int J Mol Sci. 2023 Apr 27;24(9):7951. doi: 10.3390/ijms24097951[↩][↩][↩][↩][↩][↩]
- EFSA CEP Panel (EFSA Panel on Food Contact Materials, Enzymes and Processing Aids), Lambré, C, Barat Baviera, JM, Bolognesi, C, Chesson, A, Cocconcelli, PS, Crebelli, R, Gott, DM, Grob, K, Lampi, E, Mengelers, M, Mortensen, A, Rivière, G, Silano, V (until 21 December 2020†), Steffensen, I-L, Tlustos, C, Vernis, L, Zorn, H, Batke, M, Bignami, M, Corsini, E, FitzGerald, R, Gundert-Remy, U, Halldorsson, T, Hart, A, Ntzani, E, Scanziani, E, Schroeder, H, Ulbrich, B, Waalkens-Berendsen, D, Woelfle, D, Al Harraq, Z, Baert, K, Carfì, M, Castoldi, AF, Croera, C and Van Loveren, H, 2023. Scientific Opinion on the re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA Journal 2023; 21(4):6857, 392 pp. https://doi.org/10.2903/j.efsa.2023.6857[↩][↩][↩][↩][↩][↩][↩][↩]
- Huang Y.Q., Wong C.K., Zheng J.S., Bouwman H., Barra R., Wahlström B., Neretin L., Wong M.H. Bisphenol A (BPA) in China: A review of sources, environmental levels, and potential human health impacts. Environ. Int. 2012;42:91–99. doi: 10.1016/j.envint.2011.04.010[↩][↩]
- Chen Z-J, Zhang K-S, Ge L-C, et al. Signals involved in the effects of bisphenol A (BPA) on proliferation and motility of Leydig cells: a comparative proteomic analysis. Toxicology Research. 2016;5(6):1573-1584. doi:10.1039/c6tx00258g. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6060681[↩]
- Vandenberg LN, Maffini MV, Sonnenschein C. et al. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2647705[↩]
- Geens T., Aerts D., Berthot C., Bourguignon J.P., Goeyens L., Lecomte P., Maghuin-Rogister G., Pironnet A.M., Pussemier L., Scippo M.L., et al. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem. Toxicol. 2012;50:3725–3740. doi: 10.1016/j.fct.2012.07.059[↩]
- Fan R., Zeng B., Liu X., Chen C., Zhuang Q., Wang Y., Hu M., Lv Y., Li J., Zhou Y., et al. Levels of bisphenol-A in different paper products in Guangzhou, China, and assessment of human exposure via dermal contact. Environ. Sci. Processes Impacts. 2015;17:667–673. doi: 10.1039/C4EM00621F[↩]
- Rubin BS. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol. 2011;127:27–34[↩]
- Biedermann S, Tschudin P, Grob K. Transfer of bisphenol A from thermal printer paper to the skin. Anal Bioanal Chem. 2010;398:571–576.[↩]
- Asimakopoulos AG, Thomaidis NS, Koupparis MA. Recent trends in biomonitoring of bisphenol A, 4-t-octylphenol, and 4-nonylphenol. Toxicol Lett. 2012;210:141–154.[↩]
- Dianin A.P. Condensation of ketones with phenols. Zhurnal Russkogo Fiziko-Khimicheskogo Obshchestva. J. Russ. Phys. Chem. Soc. St. Petersburg. 1891;23:601–611.[↩]
- Inadera H. Neurological Effects of Bisphenol A and its Analogues. Int J Med Sci. 2015 Oct 30;12(12):926-36. doi: 10.7150/ijms.13267[↩]
- Michałowicz J. Bisphenol A—Sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 2014;37:738–758. doi: 10.1016/j.etap.2014.02.003[↩][↩][↩][↩][↩][↩][↩]
- Vogel S.A. The politics of plastics: The making and unmaking of bisphenol a “safety” Am. J. Public. Health. 2009;99, Suppl. 3:S559–S566. doi: 10.2105/AJPH.2008.159228[↩][↩][↩]
- Kamrin MA. Bisphenol A: a scientific evaluation. MedGenMed. 2004 Sep 3;6(3):7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1435609[↩][↩][↩]
- Dodds EC, Lawson W. Synthetic strogenic Agents without the Phenanthrene Nucleus. Nature 137, 996 (1936). https://doi.org/10.1038/137996a0[↩][↩]
- Kim E.J., Lee D., Chung B.C., Pyo H., Lee J. Association between urinary levels of bisphenol-A and estrogen metabolism in Korean adults. Sci. Total Environ. 2014;470–471:1401–1407. doi: 10.1016/j.scitotenv.2013.07.040[↩]
- Rochester J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013;42:132–155. doi: 10.1016/j.reprotox.2013.08.008[↩][↩]
- Kabir E.R., Rahman M.S., Rahman I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015;40:241–258. doi: 10.1016/j.etap.2015.06.009[↩]
- Morgan M., Deoraj A., Felty Q., Roy D. Environmental estrogen-like endocrine disrupting chemicals and breast cancer. Mol. Cell Endocrinol. 2017;457:89–102. doi: 10.1016/j.mce.2016.10.003[↩]
- Cheng V., Guha N., Li K., et al. Proposition 65: Evidence on the Carcinogenicity of Bisphenol A (BPA), September 2022. In Reproductive and Cancer Hazard Assessment Branch, Office of Environmental Health Hazard Assessmen, California Environmental Protection Agency, Ed. California Environmental Protection Agency. https://oehha.ca.gov/media/downloads/crnr/bpahid093022.pdf[↩][↩][↩][↩][↩][↩][↩][↩]
- Anjum, Dr. Fakhsheena & Razvi, Nighat & Ghayas, Sana & Naeem, auj & Nisar, Ayesha. (2019). KNOWLEDGE AND ATTITUDE TOWARDS XENOESTROGENS. WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES. 8. 150-160. 10.20959/wjpps20198-14406.[↩]
- Roy JR, Chakraborty S, Chakraborty TR. Estrogen-like endocrine disrupting chemicals affecting puberty in humans–a review. Med Sci Monit. 2009 Jun;15(6):RA137-45. https://medscimonit.com/abstract/index/idArt/869670[↩]
- Hwang S., Lim J.E., Choi Y., Jee S.H. Bisphenol A exposure and type 2 diabetes mellitus risk: A meta-analysis. BMC Endocr. Disord. 2018;18:81. doi: 10.1186/s12902-018-0310-y[↩]
- Wu W., Li M., Liu A., Wu C., Li D., Deng Q., Zhang B., Du J., Gao X., Hong Y. Bisphenol A and the Risk of Obesity a Systematic Review with Meta-Analysis of the Epidemiological Evidence. Dose Response. 2020;18:1559325820916949. doi: 10.1177/1559325820916949[↩]
- Zhang X., Liu R. Advances in BPA-induced Oxidative Stress and Related Effects and Mechanisms in Liver, 1991-2017. Mini Rev. Med. Chem. 2020;20:432–443. doi: 10.2174/1389557518666180912105345[↩]
- Moon S., Seo M.Y., Choi K., Chang Y.S., Kim S.H., Park M.J. Urinary bisphenol A concentrations and the risk of obesity in Korean adults. Sci. Rep. 2021;11:1603. doi: 10.1038/s41598-021-80980-8[↩]
- García-Arevalo M., Alonso-Magdalena P., Rebelo Dos Santos J., Quesada I., Carneiro E.M., Nadal A. Exposure to bisphenol-A during pregnancy partially mimics the effects of a high-fat diet altering glucose homeostasis and gene expression in adult male mice. PLoS ONE. 2014;9:e100214. doi: 10.1371/journal.pone.0100214[↩]
- Marmugi A., Lasserre F., Beuzelin D., Ducheix S., Huc L., Polizzi A., Chetivaux M., Pineau T., Martin P., Guillou H., et al. Adverse effects of long-term exposure to bisphenol A during adulthood leading to hyperglycaemia and hypercholesterolemia in mice. Toxicology. 2014;325:133–143. doi: 10.1016/j.tox.2014.08.006[↩]
- Lejonklou M.H., Dunder L., Bladin E., Pettersson V., Rönn M., Lind L., Waldén T.B., Lind P.M. Effects of Low-Dose Developmental Bisphenol A Exposure on Metabolic Parameters and Gene Expression in Male and Female Fischer 344 Rat Offspring. Environ. Health Perspect. 2017;125:067018. doi: 10.1289/EHP505[↩]
- Le Magueresse-Battistoni B., Multigner L., Beausoleil C., Rousselle C. Effects of bisphenol A on metabolism and evidence of a mode of action mediated through endocrine disruption. Mol. Cell Endocrinol. 2018;475:74–91. doi: 10.1016/j.mce.2018.02.009[↩]
- Wade M., Delawder V., Reneau P., Dos Santos J.M. The effect of BPA exposure on insulin resistance and type 2 diabetes—The impact of muscle contraction. Med. Hypotheses. 2020;140:109675. doi: 10.1016/j.mehy.2020.109675[↩]
- Moon M.K., Jeong I.K., Jung O.T., Ahn H.Y., Kim H.H., Park Y.J., Jang H.C., Park K.S. Long-term oral exposure to bisphenol A induces glucose intolerance and insulin resistance. J. Endocrinol. 2015;226:35–42. doi: 10.1530/JOE-14-0714[↩]
- Oliveira K.M., Figueiredo L.S., Araujo T.R., Freitas I.N., Silva J.N., Boschero A.C., Ribeiro R.A. Prolonged bisphenol-A exposure decreases endocrine pancreatic proliferation in response to obesogenic diet in ovariectomized mice. Steroids. 2020;160:108658. doi: 10.1016/j.steroids.2020.108658[↩]
- Loganathan N., McIlwraith E.K., Belsham D.D. BPA Differentially Regulates NPY Expression in Hypothalamic Neurons Through a Mechanism Involving Oxidative Stress. Endocrinology. 2020;161:bqaa170. doi: 10.1210/endocr/bqaa170[↩]
- Vahdati H.F., Masjedi E., Hosseinzadeh H., Bedrood Z., Abnous K., Mehri S. Protective effect of crocin on bisphenol A-induced spatial learning and memory impairment in adult male rats: Role of oxidative stress and AMPA receptor. Iran. J. Basic Med. Sci. 2020;23:1146–1154. doi: 10.22038/ijbms.2020.41097.9714[↩]
- Molina-López AM, Bujalance-Reyes F, Urbano MT, Lora-Benítez A, Ayala-Soldado N, Moyano-Salvago R. Analysis of Blood Biochemistry and Pituitary-Gonadal Histology after Chronic Exposure to Bisphenol-A of Mice. Int J Environ Res Public Health. 2022 Oct 26;19(21):13894. doi: 10.3390/ijerph192113894[↩]
- Scientific opinion on bisphenol A (2015). http://www.efsa.europa.eu/sites/default/files/corporate_publications/files/factsheetbpa150121.pdf[↩][↩][↩]
- No consumer health risk from bisphenol A exposure. http://www.efsa.europa.eu/en/press/news/150121[↩][↩][↩]
- Bisphenol A in food is a health risk. https://www.efsa.europa.eu/en/news/bisphenol-food-health-risk[↩][↩]
- US EPA Bisphenol A Action Plan (CASRN 80-05-7) [CA Index Name: Phenol, 4,4′-(1-methylethylidene)bis-] 2010. https://www.epa.gov/sites/default/files/2015-09/documents/bpa_action_plan.pdf[↩][↩][↩][↩]
- Bisphenol A (BPA): Use in Food Contact Application. https://www.fda.gov/food/food-additives-petitions/bisphenol-bpa-use-food-contact-application[↩]
- Bisphenols. https://echa.europa.eu/hot-topics/bisphenols[↩][↩][↩]
- Huang R.P., Liu Z.H., Yin H., Dang Z., Wu P.X., Zhu N.W., Lin Z. Bisphenol A concentrations in human urine, human intakes across six continents, and annual trends of average intakes in adult and child populations worldwide: A thorough literature review. Sci. Total Environ. 2018;626:971–981. doi: 10.1016/j.scitotenv.2018.01.144[↩][↩][↩]
- Teeguarden J.G., Twaddle N.C., Churchwell M.I., Yang X., Fisher J.W., Seryak L.M., Doerge D.R. 24-hour human urine and serum profiles of bisphenol A following ingestion in soup: Individual pharmacokinetic data and emographics. Data Brief. 2015;4:83–86. doi: 10.1016/j.dib.2015.03.002[↩][↩][↩][↩][↩]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids) Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs: Executive summary. EFSA J. 2015;13:3978. doi: 10.2903/j.efsa.2015.3978[↩][↩]
- Liu J., Martin J.W. Prolonged Exposure to Bisphenol A from Single Dermal Contact Events. Environ. Sci. Technol. 2017;51:9940–9949. doi: 10.1021/acs.est.7b03093[↩][↩][↩][↩]
- Sasso A.F., Pirow R., Andra S.S., Church R., Nachman R.M., Linke S., Kapraun D.F., Schurman S.H., Arora M., Thayer K.A., et al. Pharmacokinetics of bisphenol A in humans following dermal administration. Environ. Int. 2020;144:106031. doi: 10.1016/j.envint.2020.106031[↩][↩][↩]
- Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009 Feb;30(1):75-95. doi: 10.1210/er.2008-0021[↩]
- von Goetz N, Wormuth M, Scheringer M, Hungerbühler K. Bisphenol a: how the most relevant exposure sources contribute to total consumer exposure. Risk Anal. 2010 Mar;30(3):473-87. doi: 10.1111/j.1539-6924.2009.01345.x[↩]
- Inadera H. Neurological Effects of Bisphenol A and its Analogues. International Journal of Medical Sciences. 2015;12(12):926-936. doi:10.7150/ijms.13267. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4661290/[↩]
- Hanioka N., Naito T., Narimatsu S. Human UDP-glucuronosyltransferase isoforms involved in bisphenol A glucuronidation. Chemosphere. 2008;74:33–36. doi: 10.1016/j.chemosphere.2008.09.053[↩][↩]
- Thayer K.A., Doerge D.R., Hunt D., Schurman S.H., Twaddle N.C., Churchwell M.I., Garantziotis S., Kissling G.E., Easterling M.R., Bucher J.R., et al. Pharmacokinetics of bisphenol A in humans following a single oral administration. Environ. Int. 2015;83:107–115. doi: 10.1016/j.envint.2015.06.008[↩][↩][↩]
- Völkel W., Colnot T., Csanády G.A., Filser J.G., Dekant W. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem. Res. Toxicol. 2002;15:1281–1287. doi: 10.1021/tx025548t[↩]
- Nishiyama T., Ogura K., Nakano H., Kaku T., Takahashi E., Ohkubo Y., Sekine K., Hiratsuka A., Kadota S., Watabe T. Sulfation of environmental estrogens by cytosolic human sulfotransferases. Drug. Metab. Pharmacokinet. 2002;17:221–228. doi: 10.2133/dmpk.17.221[↩]
- Völkel W., Bittner N., Dekant W. Quantitation of bisphenol A and bisphenol A glucuronide in biological samples by high performance liquid chromatography-tandem mass spectrometry. Drug. Metab. Dispos. 2005;33:1748–1757. doi: 10.1124/dmd.105.005454[↩][↩]
- Trdan Lušin T., Roškar R., Mrhar A. Evaluation of bisphenol A glucuronidation according to UGT1A1*28 polymorphism by a new LC-MS/MS assay. Toxicology. 2012;292:33–41. doi: 10.1016/j.tox.2011.11.015[↩]
- Landolfi A., Troisi J., Savanelli M.C., Vitale C., Barone P., Amboni M. Bisphenol A glucuronidation in patients with Parkinson’s disease. Neurotoxicology. 2017;63:90–96. doi: 10.1016/j.neuro.2017.09.008[↩][↩]
- Nakamura S., Tezuka Y., Ushiyama A., Kawashima C., Kitagawara Y., Takahashi K., Ohta S., Mashino T. Ipso substitution of bisphenol A catalyzed by microsomal cytochrome P450 and enhancement of estrogenic activity. Toxicol. Lett. 2011;203:92–95. doi: 10.1016/j.toxlet.2011.03.010[↩][↩]
- Schmidt J., Kotnik P., Trontelj J., Knez Ž., Mašič L.P. Bioactivation of bisphenol A and its analogs (BPF, BPAF, BPZ and DMBPA) in human liver microsomes. Toxicol. Vitro. 2013;27:1267–1276. doi: 10.1016/j.tiv.2013.02.016[↩]
- Ousji O., Ohlund L., Sleno L. Comprehensive In Vitro Metabolism Study of Bisphenol A Using Liquid Chromatography-High Resolution Tandem Mass Spectrometry. Chem. Res. Toxicol. 2020;33:1468–1477. doi: 10.1021/acs.chemrestox.0c00042[↩]
- Zalko D., Soto A.M., Dolo L., Dorio C., Rathahao E., Debrauwer L., Faure R., Cravedi J.P. Biotransformations of bisphenol A in a mammalian model: Answers and new questions raised by low-dose metabolic fate studies in pregnant CD1 mice. Environ. Health Perspect. 2003;111:309–319. doi: 10.1289/ehp.5603[↩]
- Jaeg J.P., Perdu E., Dolo L., Debrauwer L., Cravedi J.P., Zalko D. Characterization of new bisphenol a metabolites produced by CD1 mice liver microsomes and S9 fractions. J. Agric. Food Chem. 2004;52:4935–4942. doi: 10.1021/jf049762u[↩]
- Yoshihara S., Mizutare T., Makishima M., Suzuki N., Fujimoto N., Igarashi K., Ohta S. Potent estrogenic metabolites of bisphenol A and bisphenol B formed by rat liver S9 fraction: Their structures and estrogenic potency. Toxicol. Sci. 2004;78:50–59. doi: 10.1093/toxsci/kfh047[↩]
- Gramec Skledar D., Peterlin Mašič L. Bisphenol A and its analogs: Do their metabolites have endocrine activity? Environ. Toxicol. Pharmacol. 2016;47:182–199. doi: 10.1016/j.etap.2016.09.014[↩]
- Atkinson A., Roy D. In vitro conversion of environmental estrogenic chemical bisphenol A to DNA binding metabolite(s) Biochem. Biophys. Res. Commun. 1995;210:424–433. doi: 10.1006/bbrc.1995.1678[↩]
- Atkinson A., Roy D. In vivo DNA adduct formation by bisphenol A. Environ. Mol. Mutagen. 1995;26:60–66. doi: 10.1002/em.2850260109[↩]
- Edmonds J.S., Nomachi M., Terasaki M., Morita M., Skelton B.W., White A.H. The reaction of bisphenol A 3,4-quinone with DNA. Biochem. Biophys. Res. Commun. 2004;319:556–561. doi: 10.1016/j.bbrc.2004.05.02[↩]
- Izzotti A., Kanitz S., D’Agostini F., Camoirano A., De Flora S. Formation of adducts by bisphenol A, an endocrine disruptor, in DNA in vitro and in liver and mammary tissue of mice. Mutat. Res. 2009;679:28–32. doi: 10.1016/j.mrgentox.2009.07.011[↩]
- De Flora S., Micale R.T., La Maestra S., Izzotti A., D’Agostini F., Camoirano A., Davoli S.A., Troglio M.G., Rizzi F., Davalli P., et al. Upregulation of clusterin in prostate and DNA damage in spermatozoa from bisphenol A-treated rats and formation of DNA adducts in cultured human prostatic cells. Toxicol. Sci. 2011;122:45–51. doi: 10.1093/toxsci/kfr096[↩]
- Wu Q., Fang J., Li S., Wei J., Yang Z., Zhao H., Zhao C., Cai Z. Interaction of bisphenol A 3,4-quinone metabolite with glutathione and ribonucleosides/deoxyribonucleosides in vitro. J. Hazard. Mater. 2017;323:195–202. doi: 10.1016/j.jhazmat.2016.03.015[↩]
- Zhao H., Wei J., Xiang L., Cai Z. Mass spectrometry investigation of DNA adduct formation from bisphenol A quinone metabolite and MCF-7 cell DNA. Talanta. 2018;182:583–589. doi: 10.1016/j.talanta.2018.02.037[↩]
- Hu X., Wu J.L., Miao W., Long F., Pan H., Peng T., Yao X., Li N. Covalent Protein Modification: An Unignorable Factor for Bisphenol A-Induced Hepatotoxicity. Environ. Sci. Technol. 2022;56:9536–9545. doi: 10.1021/acs.est.2c01307[↩]
- Gramec Skledar D., Troberg J., Lavdas J., Peterlin Mašič L., Finel M. Differences in the glucuronidation of bisphenols F and S between two homologous human UGT enzymes, 1A9 and 1A10. Xenobiotica. 2015;45:511–519. doi: 10.3109/00498254.2014.999140[↩]
- Street C.M., Zhu Z., Finel M., Court M.H. Bisphenol-A glucuronidation in human liver and breast: Identification of UDP-glucuronosyltransferases (UGTs) and influence of genetic polymorphisms. Xenobiotica. 2017;47:1–10. doi: 10.3109/00498254.2016.1156784[↩]
- Genuis S.J., Beesoon S., Birkholz D., Lobo R.A. Human excretion of bisphenol A: Blood, urine, and sweat (BUS) study. J. Environ. Public Health. 2012;2012:185731. doi: 10.1155/2012/185731[↩][↩]
- Morgan M.K., Nash M., Barr D.B., Starr J.M., Scott Clifton M., Sobus J.R. Distribution, variability, and predictors of urinary bisphenol A levels in 50 North Carolina adults over a six-week monitoring period. Environ. Int. 2018;112:85–99. doi: 10.1016/j.envint.2017.12.014[↩][↩]
- Quesnot N., Bucher S., Fromenty B., Robin M.A. Modulation of metabolizing enzymes by bisphenol a in human and animal models. Chem. Res. Toxicol. 2014;27:1463–1473. doi: 10.1021/tx500087p[↩]
- Calafat A.M., Ye X., Wong L.Y., Reidy J.A., Needham L.L. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753[↩]
- Becker K., Göen T., Seiwert M., Conrad A., Pick-Fuss H., Müller J., Wittassek M., Schulz C., Kolossa-Gehring M. GerES IV: Phthalate metabolites and bisphenol A in urine of German children. Int. J. Hyg. Environ. Health. 2009;212:685–692. doi: 10.1016/j.ijheh.2009.08.002[↩]
- Gerona R.R., Pan J., Zota A.R., Schwartz J.M., Friesen M., Taylor J.A., Hunt P.A., Woodruff T.J. Direct measurement of Bisphenol A (BPA), BPA glucuronide and BPA sulfate in a diverse and low-income population of pregnant women reveals high exposure, with potential implications for previous exposure estimates: A cross-sectional study. Environ. Health. 2016;15:50. doi: 10.1186/s12940-016-0131-2[↩]
- Calafat A.M., Longnecker M.P., Koch H.M., Swan S.H., Hauser R., Goldman L.R., Lanphear B.P., Rudel R.A., Engel S.M., Teitelbaum S.L., et al. Optimal Exposure Biomarkers for Nonpersistent Chemicals in Environmental Epidemiology. Environ. Health Perspect. 2015;123:A166–A168. doi: 10.1289/ehp.1510041[↩][↩]
- Cox K.J., Porucznik C.A., Anderson D.J., Brozek E.M., Szczotka K.M., Bailey N.M., Wilkins D.G., Stanford J.B. Exposure Classification and Temporal Variability in Urinary Bisphenol A Concentrations among Couples in Utah—The HOPE Study. Environ. Health Perspect. 2016;124:498–506. doi: 10.1289/ehp.1509752[↩][↩][↩]
- Colorado-Yohar S.M., Castillo-González A.C., Sánchez-Meca J., Rubio-Aparicio M., Sánchez-Rodríguez D., Salamanca-Fernández E., Ardanaz E., Amiano P., Fernández M.F., Mendiola J., et al. Concentrations of bisphenol-A in adults from the general population: A systematic review and meta-analysis. Sci. Total Environ. 2021;775:145755. doi: 10.1016/j.scitotenv.2021.145755[↩][↩]
- Aylward L.L., Hays S.M., Zidek A. Variation in urinary spot sample, 24 h samples, and longer-term average urinary concentrations of short-lived environmental chemicals: Implications for exposure assessment and reverse dosimetry. J. Expo. Sci. Environ. Epidemiol. 2017;27:582–590. doi: 10.1038/jes.2016.54[↩]
- National Report on Human Exposure to Environmental Chemicals. https://www.cdc.gov/exposurereport[↩]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005 Apr;113(4):391-5. doi: 10.1289/ehp.7534[↩]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect. 2008 Jan;116(1):39-44. doi: 10.1289/ehp.10753[↩]
- Bisphenol A (BPA) Factsheet. https://www.cdc.gov/biomonitoring/bisphenola_factsheet.html[↩][↩][↩][↩][↩][↩]
- Delfosse V, Grimaldi M, Pons JL, Boulahtouf A, le Maire A, Cavailles V, Labesse G, Bourguet W, Balaguer P. Structural and mechanistic insights into bisphenols action provide guidelines for risk assessment and discovery of bisphenol A substitutes. Proc Natl Acad Sci U S A. 2012 Sep 11;109(37):14930-5. doi: 10.1073/pnas.1203574109[↩]
- Rubin BS. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol. 2011;127:27–34. https://www.ncbi.nlm.nih.gov/pubmed/21605673[↩]
- Melzer D, Gates P, Osborne NJ. et al. Urinary bispehnol A concentration and angiography-defined coronary artery stenosis. PLos One. 2012;7:e43378 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3419714/[↩]
- Visser SN, Danielson ML, Bitsko RH, Holbrook JR, Kogan MD, Ghandour RM, Perou R, Blumberg SJ. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003-2011. J Am Acad Child Adolesc Psychiatry. 2014 Jan;53(1):34-46.e2. doi: 10.1016/j.jaac.2013.09.001[↩]
- Harley KG, Gunier RB, Kogut K, Johnson C, Bradman A, Calafat AM, Eskenazi B. Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environ Res. 2013 Oct;126:43-50. doi: 10.1016/j.envres.2013.06.004[↩]
- Haishima Y., Hayashi Y., Yagami T., Nakamura A. Elution of bisphenol-A from hemodialyzers consisting of polycarbonate and polysulfone resins. J. Biomed. Mater. Res. 2001;58:209–215. doi: 10.1002/1097-4636(2001)58:2<209::AID-JBM1009>3.0.CO;2-7[↩]
- Fleisch A.F., Sheffield P.E., Chinn C., Edelstein B.L., Landrigan P.J. Bisphenol A and related compounds in dental materials. Pediatrics. 2010;126:760–768. doi: 10.1542/peds.2009-2693[↩]
- Ehrlich S., Calafat A.M., Humblet O., Smith T., Hauser R. Handling of thermal receipts as a source of exposure to bisphenol A. JAMA. 2014;311:859–860. doi: 10.1001/jama.2013.283735[↩]
- Buckley J.P., Kim H., Wong E., Rebholz C.M. Ultra-processed food consumption and exposure to phthalates and bisphenols in the US National Health and Nutrition Examination Survey, 2013–2014. Environ. Int. 2019;131:105057. doi: 10.1016/j.envint.2019.105057[↩]
- Iribarne-Durán L.M., Artacho-Cordón F., Peña-Caballero M., Molina-Molina J.M., Jiménez-Díaz I., Vela-Soria F., Serrano L., Hurtado J.A., Fernández M.F., Freire C., et al. Presence of Bisphenol A and Parabens in a Neonatal Intensive Care Unit: An Exploratory Study of Potential Sources of Exposure. Environ. Health Perspect. 2019;127:117004. doi: 10.1289/EHP5564[↩]
- United Nations Environment Programme (2020). An Assessment Report on Issues of Concern: Chemicals and Waste Issues Posing Risks to Human Health and the Environment – September 2020. https://wedocs.unep.org/20.500.11822/33807[↩]
- Bisphenol-A (BPA) Market – Growth, Trends, COVID-19 Impact, and Forecasts (2023-2028). https://www.researchandmarkets.com/reports/5318392/bisphenol-a-bpa-market-growth-trends-covid#rela1-5438494[↩]
- US EPA Toxics Release Inventory (TRI) Program. https://www.epa.gov/toxics-release-inventory-tri-program[↩]
- US EPA Assessing and Managing Chemicals under TSCA: Risk Management for Bisphenol A (BPA). https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/risk-management-bisphenol-bpa[↩][↩]
- Corrales J., Kristofco L.A., Steele W.B., Yates B.S., Breed C.S., Williams E.S., Brooks B.W. Global Assessment of Bisphenol A in the Environment: Review and Analysis of Its Occurrence and Bioaccumulation. Dose Response. 2015;13:1559325815598308. doi: 10.1177/1559325815598308[↩][↩][↩]
- Almeida S., Raposo A., Almeida-González M., Carrascosa C. Bisphenol A: Food Exposure and Impact on Human Health. Compr. Rev. Food Sci. Food Saf. 2018;17:1503–1517. doi: 10.1111/1541-4337.12388[↩]
- Cao X.L., Corriveau J., Popovic S. Levels of bisphenol A in canned soft drink products in Canadian markets. J. Agric. Food Chem. 2009;57:1307–1311. doi: 10.1021/jf803213g[↩]
- Carwile J.L., Luu H.T., Bassett L.S., Driscoll D.A., Yuan C., Chang J.Y., Ye X., Calafat A.M., Michels K.B. Polycarbonate bottle use and urinary bisphenol A concentrations. Environ. Health Perspect. 2009;117:1368–1372. doi: 10.1289/ehp.0900604[↩]
- Noonan G.O., Ackerman L.K., Begley T.H. Concentration of bisphenol A in highly consumed canned foods on the U. S. market. J. Agric. Food Chem. 2011;59:7178–7185. doi: 10.1021/jf201076f[↩]
- Vilarinho F., Sendón R., van der Kellen A., Vaz M.F., Sanches Silva A. Bisphenol A in food as a result of its migration from food packaging. Trends Food Sci. Technol. 2019;19:33–65. doi: 10.1016/j.tifs.2019.06.012[↩]
- Kubwabo C., Kosarac I., Stewart B., Gauthier B.R., Lalonde K., Lalonde P.J. Migration of bisphenol A from plastic baby bottles, baby bottle liners and reusable polycarbonate drinking bottles. Food Addit. Contam. Part. A Chem. Anal. Control. Expo. Risk Assess. 2009;26:928–937. doi: 10.1080/02652030802706725[↩]
- ousins I.T., Staples C.A., Kleĉka G.M., Mackay D. A Multimedia Assessment of the Environmental Fate of Bisphenol A. Hum. Ecol. Risk Assess. Int. J. 2002;8:1107–1135. doi: 10.1080/1080-700291905846[↩]
- Cousins I.T., Staples C.A., Kleĉka G.M., Mackay D. A Multimedia Assessment of the Environmental Fate of Bisphenol A. Hum. Ecol. Risk Assess. Int. J. 2002;8:1107–1135. doi: 10.1080/1080-700291905846[↩][↩]
- Cooper J.E., Kendig E.L., Belcher S.M. Assessment of bisphenol A released from reusable plastic, aluminium and stainless steel water bottles. Chemosphere. 2011;85:943–947. doi: 10.1016/j.chemosphere.2011.06.060[↩]
- Careghini A., Mastorgio A.F., Saponaro S., Sezenna E. Bisphenol A, nonylphenols, benzophenones, and benzotriazoles in soils, groundwater, surface water, sediments, and food: A review. Environ. Sci. Pollut. Res. Int. 2015;22:5711–5741. doi: 10.1007/s11356-014-3974-5[↩][↩]
- Hanaoka T., Kawamura N., Hara K., Tsugane S. Urinary bisphenol A and plasma hormone concentrations in male workers exposed to bisphenol A diglycidyl ether and mixed organic solvents. Occup. Environ. Med. 2002;59:625–628. doi: 10.1136/oem.59.9.625[↩]
- Loganathan S.N., Kannan K. Occurrence of bisphenol A in indoor dust from two locations in the eastern United States and implications for human exposures. Arch. Environ. Contam. Toxicol. 2011;61:68–73. doi: 10.1007/s00244-010-9634-y[↩]
- Muhamad M.S., Salim M.R., Lau W.J., Yusop Z. A review on bisphenol A occurrences, health effects and treatment process via membrane technology for drinking water. Environ. Sci. Pollut. Res. Int. 2016;23:11549–11567. doi: 10.1007/s11356-016-6357-2[↩]
- Valentino R., D’Esposito V., Ariemma F., Cimmino I., Beguinot F., Formisano P. Bisphenol A environmental exposure and the detrimental effects on human metabolic health: Is it necessary to revise the risk assessment in vulnerable population? J. Endocrinol. Investig. 2016;39:259–263. doi: 10.1007/s40618-015-0336-1[↩][↩][↩]
- Manzoor MF, Tariq T, Fatima B, Sahar A, Tariq F, Munir S, Khan S, Nawaz Ranjha MMA, Sameen A, Zeng XA, Ibrahim SA. An insight into bisphenol A, food exposure and its adverse effects on health: A review. Front Nutr. 2022 Nov 3;9:1047827. doi: 10.3389/fnut.2022.1047827[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Flint S, Markle T, Thompson S, Wallace E. Bisphenol A exposure, effects, and policy: a wildlife perspective. J Environ Manag. (2012) 104:19–34. 10.1016/j.jenvman.2012.03.021[↩]
- Neufeld K, Ezell K, Grow WA. Plastic additives decrease agrin-induced acetylcholine receptor clusters and myotube formation in C2C12 skeletal muscle cell culture. CellBio. (2015) 4:12. 10.4236/cellbio.2015.41002[↩][↩]
- Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect. (2010) 118:1055–70. 10.1289/ehp.0901716[↩]
- Usman A, Ahmad M. From BPA to its analogues: is it a safe journey? Chemosphere. (2016) 158:131–42. 10.1016/j.chemosphere.2016.05.070[↩]
- Freire C, Molina-Molina J-M, Iribarne-Durán LM, Jiménez-Díaz I, Vela-Soria F, Mustieles V, et al. Concentrations of bisphenol A and parabens in socks for infants and young children in Spain and their hormone-like activities. Environ Int. (2019) 127:592–600. 10.1016/j.envint.2019.04.013[↩]
- Morgan MK, Nash M, Barr DB, Starr JM, Clifton MS, Sobus JR. Distribution, variability, and predictors of urinary bisphenol A levels in 50 North Carolina adults over a six-week monitoring period. Environ Int. (2018) 112:85–99. 10.1016/j.envint.2017.12.014[↩]
- Molina-Molina J, Jiménez-Díaz I, Fernández M, Rodriguez-Carrillo A, Peinado F, Mustieles V, et al. Determination of bisphenol A and bisphenol S concentrations and assessment of estrogen-and anti-androgen-like activities in thermal paper receipts from Brazil, France, and Spain. Environ Res. (2019) 170:406–15. 10.1016/j.envres.2018.12.046[↩]
- Bisphenol A in food is a health risk. https://www.efsa.europa.eu/en/news/bisphenol-food-health-risk. wydawnictwa.pzh.gov.pl/roczniki_pzh/download-article?id=1055[↩]
- Geens T, Aerts D, Berthot C, Bourguignon J-P, Goeyens L, Lecomte P, et al. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem Toxicol. (2012) 50:3725–40. 10.1016/j.fct.2012.07.059[↩][↩]
- Cao X-L, Perez-Locas C, Dufresne G, Clement G, Popovic S, Beraldin F, et al. Concentrations of bisphenol A in the composite food samples from the 2008 Canadian total diet study in Quebec City and dietary intake estimates. Food Addit Contam. (2011) 28:791–8. 10.1080/19440049.2010.513015[↩]
- World Health Organization. Toxicological and Health Aspects of Bisphenol A. Report of Joint FAO/WHO Expert Meeting, 2-5 November 2010 and Report of Stakeholder Meeting on Bisphenol A, 1 November 2010. Ottawa, ON: World Health Organization; (2011).[↩]
- Wilson NK, Chuang JC, Morgan MK, Lordo RA, Sheldon LS. An observational study of the potential exposures of preschool children to pentachlorophenol, bisphenol-A, and nonylphenol at home and daycare. Environ Res. (2007) 103:9–20. 10.1016/j.envres.2006.04.006[↩]
- Zalko D, Jacques C, Duplan H, Bruel S, Perdu E. Viable skin efficiently absorbs and metabolizes bisphenol A. Chemosphere. (2011) 82:424–30. 10.1016/j.chemosphere.2010.09.058[↩]
- Mendonca K., Hauser R., Calafat A.M., Arbuckle T.E., Duty S.M. Bisphenol A concentrations in maternal breast milk and infant urine. Int. Arch. Occup. Environ. Health. 2014;87:13–20. doi: 10.1007/s00420-012-0834-9[↩]
- Teeguarden J.G., Twaddle N.C., Churchwell M.I., Doerge D.R. Urine and serum biomonitoring of exposure to environmental estrogens I: Bisphenol A in pregnant women. Food Chem. Toxicol. 2016;92:129–142. doi: 10.1016/j.fct.2016.03.023[↩]
- Pinney S.E., Mesaros C.A., Snyder N.W., Busch C.M., Xiao R., Aijaz S., Ijaz N., Blair I.A., Manson J.M. Second trimester amniotic fluid bisphenol A concentration is associated with decreased birth weight in term infants. Reprod. Toxicol. 2017;67:1–9. doi: 10.1016/j.reprotox.2016.11.007[↩][↩]
- Hartle J.C., Cohen R.S., Sakamoto P., Barr D.B., Carmichael S.L. Chemical Contaminants in Raw and Pasteurized Human Milk. J. Hum. Lact. 2018;34:340–349. doi: 10.1177/0890334418759308[↩]
- Nahar M.S., Liao C., Kannan K., Harris C., Dolinoy D.C. In utero bisphenol A concentration, metabolism, and global DNA methylation across matched placenta, kidney, and liver in the human fetus. Chemosphere. 2015;124:54–60. doi: 10.1016/j.chemosphere.2014.10.071[↩][↩]
- Vandenberg L.N., Maffini M.V., Sonnenschein C., Rubin B.S., Soto A.M. Bisphenol-A and the great divide: A review of controversies in the field of endocrine disruption. Endocr. Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021[↩]
- von Goetz N., Pirow R., Hart A., Bradley E., Poças F., Arcella D., Lillegard I.T.L., Simoneau C., van Engelen J., Husoy T., et al. Including non-dietary sources into an exposure assessment of the European Food Safety Authority: The challenge of multi-sector chemicals such as Bisphenol A. Regul. Toxicol. Pharmacol. 2017;85:70–78. doi: 10.1016/j.yrtph.2017.02.004[↩]
- Mustieles V., Zhang Y., Yland J., Braun J.M., Williams P.L., Wylie B.J., Attaman J.A., Ford J.B., Azevedo A., Calafat A.M., et al. Maternal and paternal preconception exposure to phenols and preterm birth. Environ. Int. 2020;137:105523. doi: 10.1016/j.envint.2020.105523[↩][↩]
- Niu Y, Zhang J, Wu Y, Shao B. Analysis of bisphenol A and alkylphenols in cereals by automated on-line solid-phase extraction and liquid chromatography tandem mass spectrometry. J Agric Food Chem. (2012) 60:6116–22. 10.1021/jf301401k[↩]
- Munguia-Lopez E, Gerardo-Lugo S, Peralta E, Bolumen S, Soto-Valdez H. Migration of bisphenol A (BPA) from can coatings into a fatty-food simulant and tuna fish. Food Addit Contam. (2005) 22:892–8. 10.1080/02652030500163674[↩]
- Yoshida T, Horie M, Hoshino Y, Nakazawa H, Horie M, Nakazawa H. Determination of bisphenol A in canned vegetables and fruit by high performance liquid chromatography. Food Addit Contam. (2001) 18:69–75. 10.1080/026520301446412[↩]
- Cunha S, Fernandes J. Assessment of bisphenol A and bisphenol B in canned vegetables and fruits by gas chromatography–mass spectrometry after QuEChERS and dispersive liquid–liquid microextraction. Food Control. (2013) 33:549–55. 10.1016/j.foodcont.2013.03.028[↩]
- Cao X-L, Corriveau J, Popovic S. Levels of bisphenol A in canned soft drink products in Canadian markets. J Agric Food Chem. (2009) 57:1307–11. 10.1021/jf803213g[↩]
- O’Mahony J, Moloney M, Mccormack M, Nicholls IA, Mizaikoff B, Danaher M. Design and implementation of an imprinted material for the extraction of the endocrine disruptor bisphenol A from milk. J Chromatogr B. (2013) 931:164–9. 10.1016/j.jchromb.2013.05.025[↩]
- Bisphenol A (BPA). https://www.niehs.nih.gov/health/topics/agents/sya-bpa/[↩]
- Dodds E.C., Lawson W. Synthetic strogenic Agents without the Phenanthrene Nucleus. Nature. 1936;137:996. doi: 10.1038/137996a0[↩]
- Delfosse V., Grimaldi M., Pons J.L., Boulahtouf A., le Maire A., Cavailles V., Labesse G., Bourguet W., Balaguer P. Structural and mechanistic insights into bisphenols action provide guidelines for risk assessment and discovery of bisphenol A substitutes. Proc. Natl. Acad. Sci. USA. 2012;109:14930–14935. doi: 10.1073/pnas.1203574109[↩][↩]
- Kuiper G.G., Carlsson B., Grandien K., Enmark E., Häggblad J., Nilsson S., Gustafsson J.A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979[↩]
- Acconcia F., Pallottini V., Marino M. Molecular Mechanisms of Action of BPA. Dose Response. 2015;13:1559325815610582. doi: 10.1177/1559325815610582[↩]
- Welshons W.V., Nagel S.C., vom Saal F.S. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147:S56–S69. doi: 10.1210/en.2005-1159[↩]
- Wetherill Y.B., Akingbemi B.T., Kanno J., McLachlan J.A., Nadal A., Sonnenschein C., Watson C.S., Zoeller R.T., Belcher S.M. In vitro molecular mechanisms of bisphenol A action. Reprod. Toxicol. 2007;24:178–198. doi: 10.1016/j.reprotox.2007.05.010[↩]
- Cariati F., D’Uonno N., Borrillo F., Iervolino S., Galdiero G., Tomaiuolo R. Bisphenol a: An emerging threat to male fertility. Reprod. Biol. Endocrinol. 2019;17:6. doi: 10.1186/s12958-018-0447-6[↩][↩]
- Pivonello C., Muscogiuri G., Nardone A., Garifalos F., Provvisiero D.P., Verde N., de Angelis C., Conforti A., Piscopo M., Auriemma R.S., et al. Bisphenol A: An emerging threat to female fertility. Reprod. Biol. Endocrinol. 2020;18:22. doi: 10.1186/s12958-019-0558-8[↩]
- ECHA Member State Committee Unanimously Agrees that Bisphenol A Is an Endocrine Disruptor. 2017. https://echa.europa.eu/documents/10162/769b2777-19cd-9fff-33c4-54fe6d8290d5[↩]
- NTP Monograph on the Potential Human Reproductive and Developmental Effects of Bisphenol A. National Toxicology Program. National Toxicology Program-Center for the Evaluation of Risk to Human Reproduction (NTP-CHRHR) 2008. https://ntp.niehs.nih.gov/sites/default/files/ntp/ohat/bisphenol/bisphenol.pdf[↩]
- Vom Saal F.S., Vandenberg L.N. Update on the Health Effects of Bisphenol A: Overwhelming Evidence of Harm. Endocrinology. 2021;162:bqaa171. doi: 10.1210/endocr/bqaa171[↩][↩]
- Diamanti-Kandarakis E., Bourguignon J.P., Giudice L.C., Hauser R., Prins G.S., Soto A.M., Zoeller R.T., Gore A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002[↩][↩]
- Shankar A., Teppala S., Sabanayagam C. Bisphenol A and peripheral arterial disease: Results from the NHANES. Environ. Health Perspect. 2012;120:1297–1300. doi: 10.1289/ehp.1104114[↩][↩][↩][↩]
- Peretz J., Vrooman L., Ricke W.A., Hunt P.A., Ehrlich S., Hauser R., Padmanabhan V., Taylor H.S., Swan S.H., VandeVoort C.A., et al. Bisphenol a and reproductive health: Update of experimental and human evidence, 2007–2013. Environ. Health Perspect. 2014;122:775–786. doi: 10.1289/ehp.1307728[↩]
- Mustieles V., Pérez-Lobato R., Olea N., Fernández M.F. Bisphenol A: Human exposure and neurobehavior. Neurotoxicology. 2015;49:174–184. doi: 10.1016/j.neuro.2015.06.002[↩]
- Bansal A., Henao-Mejia J., Simmons R.A. Immune System: An Emerging Player in Mediating Effects of Endocrine Disruptors on Metabolic Health. Endocrinology. 2018;159:32–45. doi: 10.1210/en.2017-00882[↩]
- Hafezi S.A., Abdel-Rahman W.M. The Endocrine Disruptor Bisphenol A (BPA) Exerts a Wide Range of Effects in Carcinogenesis and Response to Therapy. Curr. Mol. Pharmacol. 2019;12:230–238. doi: 10.2174/1874467212666190306164507[↩]
- Lite C., Raja G.L., Juliet M., Sridhar V.V., Subhashree K.D., Kumar P., Chakraborty P., Arockiaraj J. In utero exposure to endocrine-disrupting chemicals, maternal factors and alterations in the epigenetic landscape underlying later-life health effects. Environ. Toxicol. Pharmacol. 2022;89:103779. doi: 10.1016/j.etap.2021.103779[↩][↩]
- Braun J.M., Hauser R. Bisphenol A and children’s health. Curr. Opin. Pediatr. 2011;23:233–239. doi: 10.1097/MOP.0b013e3283445675[↩]
- Braun J.M. Early-life exposure to EDCs: Role in childhood obesity and neurodevelopment. Nat. Rev. Endocrinol. 2017;13:161–173. doi: 10.1038/nrendo.2016.186[↩][↩]
- Lombó M., Fernández-Díez C., González-Rojo S., Herráez M.P. Genetic and epigenetic alterations induced by bisphenol A exposure during different periods of spermatogenesis: From spermatozoa to the progeny. Sci. Rep. 2019;9:18029. doi: 10.1038/s41598-019-54368-8[↩]
- Rahman M.S., Pang W.K., Ryu D.Y., Park Y.J., Pang M.G. Multigenerational and transgenerational impact of paternal bisphenol A exposure on male fertility in a mouse model. Hum. Reprod. 2020;35:1740–1752. doi: 10.1093/humrep/deaa139[↩]
- Ghassabian A., Vandenberg L., Kannan K., Trasande L. Endocrine-Disrupting Chemicals and Child Health. Annu. Rev. Pharmacol. Toxicol. 2022;62:573–594. doi: 10.1146/annurev-pharmtox-021921-093352[↩][↩]
- Rubin B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011;127:27–34. doi: 10.1016/j.jsbmb.2011.05.002[↩]
- Shanle E.K., Xu W. Endocrine disrupting chemicals targeting estrogen receptor signaling: Identification and mechanisms of action. Chem. Res. Toxicol. 2011;24:6–19. doi: 10.1021/tx100231n[↩]
- Nohynek G.J., Borgert C.J., Dietrich D., Rozman K.K. Endocrine disruption: Fact or urban legend? Toxicol. Lett. 2013;223:295–305. doi: 10.1016/j.toxlet.2013.10.022[↩]
- Chevalier N., Fénichel P. Bisphenol A: Targeting metabolic tissues. Rev. Endocr. Metab. Disord. 2015;16:299–309. doi: 10.1007/s11154-016-9333-8[↩]
- Li L., Wang Q., Zhang Y., Niu Y., Yao X., Liu H. The molecular mechanism of bisphenol A (BPA) as an endocrine disruptor by interacting with nuclear receptors: Insights from molecular dynamics (MD) simulations. PLoS ONE. 2015;10:e0120330. doi: 10.1371/journal.pone.0120330[↩]
- Wang H., Ding Z., Shi Q.M., Ge X., Wang H.X., Li M.X., Chen G., Wang Q., Ju Q., Zhang J.P., et al. Anti-androgenic mechanisms of Bisphenol A involve androgen receptor signaling pathway. Toxicology. 2017;387:10–16. doi: 10.1016/j.tox.2017.06.007[↩]
- Grimaldi M., Boulahtouf A., Toporova L., Balaguer P. Functional profiling of bisphenols for nuclear receptors. Toxicology. 2019;420:39–45. doi: 10.1016/j.tox.2019.04.003[↩]
- Cimmino I., Fiory F., Perruolo G., Miele C., Beguinot F., Formisano P., Oriente F. Potential Mechanisms of Bisphenol A (BPA) Contributing to Human Disease. Int. J. Mol. Sci. 2020;21:5761. doi: 10.3390/ijms21165761[↩][↩][↩][↩]
- Chen X., Bao H.H., Wu W.K., Yan S.Q., Sheng J., Xu Y.Y., Gu C.L., Huang K., Cao H., Su P.Y., et al. Exposure to bisphenol A during maternal pregnancy and the emotional and behavioral impact on their preschool children. Zhonghua Liu Xing Bing Xue Za Zhi = Zhonghua Liuxingbingxue Zazhi. 2018;39:188–193. doi: 10.3760/cma.j.issn.0254-6450.2018.02.010[↩]
- Perera F., Nolte E.L.R., Wang Y., Margolis A.E., Calafat A.M., Wang S., Garcia W., Hoepner L.A., Peterson B.S., Rauh V., et al. Bisphenol A exposure and symptoms of anxiety and depression among inner city children at 10-12 years of age. Environ. Res. 2016;151:195–202. doi: 10.1016/j.envres.2016.07.028[↩]
- Ma Y., Liu H., Wu J., Yuan L., Wang Y., Du X., Wang R., Marwa P.W., Petlulu P., Chen X., et al. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ. Res. 2019;176:108575. doi: 10.1016/j.envres.2019.108575[↩][↩][↩]
- Mustieles V., D’Cruz S.C., Couderq S., Rodríguez-Carrillo A., Fini J.B., Hofer T., Steffensen I.L., Dirven H., Barouki R., Olea N., et al. Bisphenol A and its analogues: A comprehensive review to identify and prioritize effect biomarkers for human biomonitoring. Environ. Int. 2020;144:105811. doi: 10.1016/j.envint.2020.105811[↩][↩][↩]
- Qin T., Zhang X., Guo T., Yang T., Gao Y., Hao W., Xiao X. Epigenetic Alteration Shaped by the Environmental Chemical Bisphenol A. Front. Genet. 2020;11:618966. doi: 10.3389/fgene.2020.618966[↩]
- Cariati F., Carbone L., Conforti A., Bagnulo F., Peluso S.R., Carotenuto C., Buonfantino C., Alviggi E., Alviggi C., Strina I. Bisphenol A-Induced Epigenetic Changes and Its Effects on the Male Reproductive System. Front. Endocrinol. 2020;11:453. doi: 10.3389/fendo.2020.00453[↩]
- Chianese R., Troisi J., Richards S., Scafuro M., Fasano S., Guida M., Pierantoni R., Meccariello R. Bisphenol A in Reproduction: Epigenetic Effects. Curr. Med. Chem. 2018;25:748–770. doi: 10.2174/0929867324666171009121001[↩]
- Oldenburg J., Fürhacker M., Hartmann C., Steinbichl P., Banaderakhshan R., Haslberger A. Different bisphenols induce non-monotonous changes in miRNA expression and LINE-1 methylation in two cell lines. Environ. Epigenet. 2021;7:dvab011. doi: 10.1093/eep/dvab011[↩]
- Cho H., Kim S.J., Park H.-W., Oh M.-J., Yu S.Y., Lee S.Y., Park C., Han J., Oh J.-H., Hwang S.Y., et al. A relationship between miRNA and gene expression in the mouse Sertoli cell line after exposure to bisphenol A. BioChip J. 2010;4:75–81. doi: 10.1007/s13206-010-4112-1[↩]
- Pang W., Lian F.Z., Leng X., Wang S.M., Li Y.B., Wang Z.Y., Li K.R., Gao Z.X., Jiang Y.G. Microarray expression profiling and co-expression network analysis of circulating LncRNAs and mRNAs associated with neurotoxicity induced by BPA. Environ. Sci. Pollut. Res. Int. 2018;25:15006–15018. doi: 10.1007/s11356-018-1678-y[↩]
- Flavahan W.A., Gaskell E., Bernstein B.E. Epigenetic plasticity and the hallmarks of cancer. Science. 2017;357:eaal2380. doi: 10.1126/science.aal2380[↩]
- Baylin S.B., Jones P.A. Epigenetic Determinants of Cancer. Cold Spring Harb. Perspect. Biol. 2016;8:a019505. doi: 10.1101/cshperspect.a019505[↩][↩]
- Feinberg A.P., Koldobskiy M.A., Gondor A. Epigenetic modulators, modifiers and mediators in cancer aetiology and progression. Nat. Rev. Genet. 2016;17:284–299. doi: 10.1038/nrg.2016.13[↩]
- Esteller M., Pandolfi P.P. The Epitranscriptome of Noncoding RNAs in Cancer. Cancer Discov. 2017;7:359–368. doi: 10.1158/2159-8290.CD-16-1292[↩]
- Greenberg M.V.C., Bourc’his D. The diverse roles of DNA methylation in mammalian development and disease. Nat. Rev. Mol. Cell. Biol. 2019;20:590–607. doi: 10.1038/s41580-019-0159-6[↩]
- Caliri A.W., Caceres A., Tommasi S., Besaratinia A. Hypomethylation of LINE-1 repeat elements and global loss of DNA hydroxymethylation in vapers and smokers. Epigenetics. 2020;15:816–829. doi: 10.1080/15592294.2020.1724401[↩]
- Tommasi S., Yoon J.I., Besaratinia A. Secondhand Smoke Induces Liver Steatosis through Deregulation of Genes Involved in Hepatic Lipid Metabolism. Int. J. Mol. Sci. 2020;21:1296. doi: 10.3390/ijms21041296[↩]
- Tommasi S., Pabustan N., Li M., Chen Y., Siegmund K.D., Besaratinia A. A novel role for vaping in mitochondrial gene dysregulation and inflammation fundamental to disease development. Sci. Rep. 2021;11:22773. doi: 10.1038/s41598-021-01965-1[↩]
- Goodman S., Chappell G., Guyton K.Z., Pogribny I.P., Rusyn I. Epigenetic alterations induced by genotoxic occupational and environmental human chemical carcinogens: An update of a systematic literature review. Mutat. Res. Rev. Mutat. Res. 2022;789:108408. doi: 10.1016/j.mrrev.2021.108408[↩]
- PLS: Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. https://www.efsa.europa.eu/en/plain-language-summary/re-evaluation-risks-public-health-related-presence-bisphenol-bpa-foodstuffs[↩]
- Birnbaum L.S., Bucher J.R., Collman G.W., Zeldin D.C., Johnson A.F., Schug T.T., Heindel J.J. Consortium-based science: The NIEHS’s multipronged, collaborative approach to assessing the health effects of bisphenol A. Environ. Health Perspect. 2012;120:1640–1644. doi: 10.1289/ehp.1205330[↩]
- Rubin B.S., Schaeberle C.M., Soto A.M. The Case for BPA as an Obesogen: Contributors to the Controversy. Front. Endocrinol. 2019;10:30. doi: 10.3389/fendo.2019.00030[↩]
- Vandenberg L.N., Colborn T., Hayes T.B., Heindel J.J., Jacobs D.R., Jr., Lee D.H., Shioda T., Soto A.M., vom Saal F.S., Welshons W.V., et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and non-monotonic dose responses. Endocr. Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050[↩]
- Vandenberg L.N., Hunt P.A., Myers J.P., Vom Saal F.S. Human exposures to bisphenol A: Mismatches between data and assumptions. Rev. Environ. Health. 2013;28:37–58. doi: 10.1515/reveh-2012-0034[↩]
- Zoeller R.T., Brown T.R., Doan L.L., Gore A.C., Skakkebaek N.E., Soto A.M., Woodruff T.J., Vom Saal F.S. Endocrine-disrupting chemicals and public health protection: A statement of principles from The Endocrine Society. Endocrinology. 2012;153:4097–4110. doi: 10.1210/en.2012-1422[↩]
- Xi W, Lee C, Yeung W, Giesy JP, Wong MH, Zhang X, et al. Effect of perinatal and postnatal bisphenol A exposure to the regulatory circuits at the hypothalamus–pituitary–gonadal axis of CD-1 mice. Reprod Toxicol. (2011) 31:409–17. 10.1016/j.reprotox.2010.12.002[↩]
- Wisniewski P, Romano RM, Kizys MM, Oliveira KC, Kasamatsu T, Giannocco G, et al. Adult exposure to bisphenol A (BPA) in Wistar rats reduces sperm quality with disruption of the hypothalamic–pituitary–testicular axis. Toxicology. (2015) 329:1–9. 10.1016/j.tox.2015.01.002[↩][↩]
- Alonso-Magdalena P., Vieira E., Soriano S., Menes L., Burks D., Quesada I., Nadal A. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ. Health Perspect. 2010;118:1243–1250. doi: 10.1289/ehp.1001993[↩][↩]
- Choi Y.J., Lee Y.A., Hong Y.C., Cho J., Lee K.S., Shin C.H., Kim B.N., Kim J.I., Park S.J., Bisgaard H., et al. Effect of prenatal bisphenol A exposure on early childhood body mass index through epigenetic influence on the insulin-like growth factor 2 receptor (IGF2R) gene. Environ. Int. 2020;143:105929. doi: 10.1016/j.envint.2020.105929[↩]
- Junge K.M., Leppert B., Jahreis S., Wissenbach D.K., Feltens R., Grützmann K., Thürmann L., Bauer T., Ishaque N., Schick M., et al. MEST mediates the impact of prenatal bisphenol A exposure on long-term body weight development. Clin. Epigenetics. 2018;10:58. doi: 10.1186/s13148-018-0478-z[↩]
- Ropero A.B., Alonso-Magdalena P., García-García E., Ripoll C., Fuentes E., Nadal A. Bisphenol-A disruption of the endocrine pancreas and blood glucose homeostasis. Int. J. Androl. 2008;31:194–200. doi: 10.1111/j.1365-2605.2007.00832.x[↩]
- Moon M.K., Jeong I.K., Oh T.J., Ahn H.Y., Kim H.H., Park Y.J., Jang H.C., Park K.S. Long-term oral exposure to bisphenol A induces glucose intolerance and insulin resistance. J. Endocrinol. 2015;226:35–42. doi: 10.1530/JOE-14-0714[↩]
- Duan Y., Yao Y., Wang B., Han L., Wang L., Sun H., Chen L. Association of urinary concentrations of bisphenols with type 2 diabetes mellitus: A case-control study. Environ. Pollut. 2018;243:1719–1726. doi: 10.1016/j.envpol.2018.09.093[↩]
- Li A.J., Xue J., Lin S., Al-Malki A.L., Al-Ghamdi M.A., Kumosani T.A., Kannan K. Urinary concentrations of environmental phenols and their association with type 2 diabetes in a population in Jeddah, Saudi Arabia. Environ. Res. 2018;166:544–552. doi: 10.1016/j.envres.2018.06.040[↩]
- Bellavia A., Cantonwine D.E., Meeker J.D., Hauser R., Seely E.W., McElrath T.F., James-Todd T. Pregnancy urinary bisphenol-A concentrations and glucose levels across BMI categories. Environ. Int. 2018;113:35–41. doi: 10.1016/j.envint.2018.01.012[↩]
- Chiu Y.H., Mínguez-Alarcón L., Ford J.B., Keller M., Seely E.W., Messerlian C., Petrozza J., Williams P.L., Ye X., Calafat A.M., et al. Trimester-Specific Urinary Bisphenol A Concentrations and Blood Glucose Levels Among Pregnant Women From a Fertility Clinic. J. Clin. Endocrinol. Metab. 2017;102:1350–1357. doi: 10.1210/jc.2017-00022[↩]
- Eng D.S., Lee J.M., Gebremariam A., Meeker J.D., Peterson K., Padmanabhan V. Bisphenol A and chronic disease risk factors in US children. Pediatrics. 2013;132:e637–e645. doi: 10.1542/peds.2013-0106[↩]
- Akash M.S.H., Sabir S., Rehman K. Bisphenol A-induced metabolic disorders: From exposure to mechanism of action. Environ. Toxicol. Pharmacol. 2020;77:103373. doi: 10.1016/j.etap.2020.103373[↩]
- Xin F, Fischer E, Krapp C, Krizman EN, Lan Y, Mesaros C, et al. Mice exposed to bisphenol A exhibit depressive-like behavior with neurotransmitter and neuroactive steroid dysfunction. Horm Behav. (2018) 102:93–104. 10.1016/j.yhbeh.2018.05.010[↩]
- Choi I-S, Cho J-H, Park E-J, Park J-W, Kim S-H, Lee M-G, et al. Multiple effects of bisphenol A, an endocrine disrupter, on GABAA receptors in acutely dissociated rat CA3 pyramidal neurons. Neurosci Res. (2007) 59:8–17. 10.1016/j.neures.2007.05.003[↩]
- Song K-H, Lee K, Choi H-S. Endocrine disrupter bisphenol a induces orphan nuclear receptor Nur77 gene expression and steroidogenesis in mouse testicular Leydig cells. Endocrinology. (2002) 143:2208–15. 10.1210/endo.143.6.8847[↩]
- Arase S, Ishii K, Igarashi K, Aisaki K, Yoshio Y, Matsushima A, et al. Endocrine disrupter bisphenol A increases in situ estrogen production in the mouse urogenital sinus. Biol Reprod. (2011) 84:734–42. 10.1095/biolreprod.110.087502[↩]
- Zhou J, Zhu X-S, Cai Z-H. The impacts of bisphenol A (BPA) on abalone (Haliotis diversicolor supertexta) embryonic development. Chemosphere. (2011) 82:443–50. 10.1016/j.chemosphere.2010.09.056[↩]
- Kunz N, Camm EJ, Somm E, Lodygensky G, Darbre S, Aubert ML, et al. Developmental and metabolic brain alterations in rats exposed to bisphenol A during gestation and lactation. Int J Dev Neurosci. (2011) 29:37–43. 10.1016/j.ijdevneu.2010.09.009[↩]
- Galloway T., Cipelli R., Guralnik J., Ferrucci L., Bandinelli S., Corsi A.M., Money C., McCormack P., Melzer D. Daily bisphenol A excretion and associations with sex hormone concentrations: Results from the InCHIANTI adult population study. Environ. Health Perspect. 2010;118:1603–1608. doi: 10.1289/ehp.100236[↩]
- Arase S., Ishii K., Igarashi K., Aisaki K., Yoshio Y., Matsushima A., Shimohigashi Y., Arima K., Kanno J., Sugimura Y. Endocrine disrupter bisphenol A increases in situ estrogen production in the mouse urogenital sinus. Biol. Reprod. 2011;84:734–742. doi: 10.1095/biolreprod.110.087502[↩]
- Sirasanagandla SR, Al-Huseini I, Sakr H, Moqadass M, Das S, Juliana N, Abu IF. Natural Products in Mitigation of Bisphenol A Toxicity: Future Therapeutic Use. Molecules. 2022 Aug 24;27(17):5384. doi: 10.3390/molecules27175384[↩]
- Pivonello C., Muscogiuri G., Nardone A., Garifalos F., Provvisiero D.P., Verde N., de Angelis C., Conforti A., Piscopo M., Auriemma R.S., et al. Bisphenol A: An emerging threat to female fertility. Reprod. Biol. Endocrinol. RBE. 2020;18:22. doi: 10.1186/s12958-019-0558-8[↩]
- Mustieles V, Ocón-Hernandez O, Mínguez-Alarcón L, Dávila-Arias C, Pérez-Lobato R, Calvente I, et al. Bisphenol A and reproductive hormones and cortisol in peripubertal boys: the INMA-Granada cohort. Sci Total Environ. (2018) 618:1046–53. 10.1016/j.scitotenv.2017.09.093[↩]
- La Rocca C., Tait S., Guerranti C., Busani L., Ciardo F., Bergamasco B., Stecca L., Perra G., Mancini F.R., Marci R., et al. Exposure to endocrine disrupters and nuclear receptor gene expression in infertile and fertile women from different Italian areas. Int. J. Environ. Res. Public Health. 2014;11:10146–10164. doi: 10.3390/ijerph111010146[↩][↩]
- Tachibana T., Wakimoto Y., Nakamuta N., Phichitraslip T., Wakitani S., Kusakabe K., Hondo E., Kiso Y. Effects of bisphenol A (BPA) on placentation and survival of the neonates in mice. J. Reprod. Dev. 2007;53:509–514. doi: 10.1262/jrd.18171[↩]
- Honma S., Suzuki A., Buchanan D.L., Katsu Y., Watanabe H., Iguchi T. Low dose effect of in utero exposure to bisphenol A and diethylstilbestrol on female mouse reproduction. Reprod. Toxicol. 2002;16:117–122. doi: 10.1016/S0890-6238(02)00006-0[↩]
- Lawson C., Gieske M., Murdoch B., Ye P., Li Y., Hassold T., Hunt P.A. Gene expression in the fetal mouse ovary is altered by exposure to low doses of bisphenol A. Biol. Reprod. 2011;84:79–86. doi: 10.1095/biolreprod.110.084814[↩]
- Rodríguez H.A., Santambrosio N., Santamaría C.G., Muñoz-de-Toro M., Luque E.H. Neonatal exposure to bisphenol A reduces the pool of primordial follicles in the rat ovary. Reprod. Toxicol. 2010;30:550–557. doi: 10.1016/j.reprotox.2010.07.008[↩]
- Sugiura-Ogasawara M., Ozaki Y., Sonta S., Makino T., Suzumori K. Exposure to bisphenol A is associated with recurrent miscarriage. Hum. Reprod. 2005;20:2325–2329. doi: 10.1093/humrep/deh888[↩]
- Mínguez-Alarcón L., Gaskins A.J., Chiu Y.H., Williams P.L., Ehrlich S., Chavarro J.E., Petrozza J.C., Ford J.B., Calafat A.M., Hauser R. Urinary bisphenol A concentrations and association with in vitro fertilization outcomes among women from a fertility clinic. Hum. Reprod. 2015;30:2120–2128. doi: 10.1093/humrep/dev183[↩]
- Vahedi M., Saeedi A., Poorbaghi S.L., Sepehrimanesh M., Fattahi M. Metabolic and endocrine effects of bisphenol A exposure in market seller women with polycystic ovary syndrome. Environ. Sci. Pollut. Res. Int. 2016;23:23546–23550. doi: 10.1007/s11356-016-7573-5[↩]
- Shen Y., Zheng Y., Jiang J., Liu Y., Luo X., Shen Z., Chen X., Wang Y., Dai Y., Zhao J., et al. Higher urinary bisphenol A concentration is associated with unexplained recurrent miscarriage risk: Evidence from a case-control study in eastern China. PLoS ONE. 2015;10:e0127886. doi: 10.1371/journal.pone.0127886[↩]
- Schönfelder G., Friedrich K., Paul M., Chahoud I. Developmental effects of prenatal exposure to bisphenol a on the uterus of rat offspring. Neoplasia. 2004;6:584–594. doi: 10.1593/neo.04217[↩]
- Markey C.M., Luque E.H., Munoz De Toro M., Sonnenschein C., Soto A.M. In utero exposure to bisphenol A alters the development and tissue organization of the mouse mammary gland. Biol. Reprod. 2001;65:1215–1223. doi: 10.1093/biolreprod/65.4.1215[↩]
- Ehrlich S., Williams P.L., Missmer S.A., Flaws J.A., Berry K.F., Calafat A.M., Ye X., Petrozza J.C., Wright D., Hauser R. Urinary bisphenol A concentrations and implantation failure among women undergoing in vitro fertilization. Environ. Health Perspect. 2012;120:978–983. doi: 10.1289/ehp.1104307[↩]
- Ji H., Miao M., Liang H., Shi H., Ruan D., Li Y., Wang J., Yuan W. Exposure of environmental Bisphenol A in relation to routine sperm parameters and sperm movement characteristics among fertile men. Sci. Rep. 2018;8:17548. doi: 10.1038/s41598-018-35787-5[↩]
- Li D., Zhou Z., Qing D., He Y., Wu T., Miao M., Wang J., Weng X., Ferber J.R., Herrinton L.J., et al. Occupational exposure to bisphenol-A (BPA) and the risk of self-reported male sexual dysfunction. Hum. Reprod. 2010;25:519–527. doi: 10.1093/humrep/dep381[↩][↩]
- Jin P., Wang X., Chang F., Bai Y., Li Y., Zhou R., Chen L. Low dose bisphenol A impairs spermatogenesis by suppressing reproductive hormone production and promoting germ cell apoptosis in adult rats. J. Biomed. Res. 2013;27:135–144. doi: 10.7555/jbr.27.20120076[↩]
- Mínguez-Alarcón L, Gaskins AJ, Chiu Y-H, Williams PL, Ehrlich S, Chavarro JE, et al. Urinary bisphenol A concentrations and association with in vitro fertilization outcomes among women from a fertility clinic. Hum Reprod. (2015) 30:2120–8. 10.1093/humrep/dev183[↩]
- Akın L, Kendirci M, Narin F, Kurtoglu S, Saraymen R, Kondolot M, et al. The endocrine disruptor bisphenol A may play a role in the aetiopathogenesis of polycystic ovary syndrome in adolescent girls. Acta Paediatr. (2015) 104:e171–7. 10.1111/apa.12885[↩]
- Vahedi M, Saeedi A, Poorbaghi SL, Sepehrimanesh M, Fattahi M. Metabolic and endocrine effects of bisphenol A exposure in market seller women with polycystic ovary syndrome. Environ Sci Pollut Res. (2016) 23:23546–50. 10.1007/s11356-016-7573-5[↩]
- Shen Y, Zheng Y, Jiang J, Liu Y, Luo X, Shen Z, et al. Higher urinary bisphenol A concentration is associated with unexplained recurrent miscarriage risk: evidence from a case-control study in eastern China. PLoS One. (2015) 10:e0127886. 10.1371/journal.pone.0127886[↩]
- Ji M, Xia J, Di J, Liu Y, Chen R, Chen Z, et al. Graphene-like boron nitride induced accelerated charge transfer for boosting the photocatalytic behavior of Bi4O5I2 towards bisphenol a removal. Chem Eng J. (2018) 331:355–63. 10.1016/j.cej.2017.08.100[↩]
- Hamdy H, Yahia D, Afifi S, Salem DA. Endocrine disruption induced by bisphenol A in young and adult female Sprague Dawley rats. Comp Clin Pathol. (2018) 27:967–74. 10.1007/s00580-018-2689-2[↩]
- Kwintkiewicz J, Nishi Y, Yanase T, Giudice LC. Peroxisome proliferator–activated receptor-γ mediates bisphenol A inhibition of FSH-stimulated IGF-1, aromatase, and estradiol in human granulosa cells. Environ Health Perspect. (2010) 118:400–6. 10.1289/ehp.0901161[↩]
- Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, et al. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics. (2011) 128:873–82. 10.1542/peds.2011-1335[↩]
- Meeker JD, Calafat AM, Hauser R. Urinary bisphenol A concentrations in relation to serum thyroid and reproductive hormone levels in men from an infertility clinic. Environ Sci Technol. (2010) 44:1458–63. 10.1021/es9028292[↩]
- Liu X, Miao M, Zhou Z, Gao E, Chen J, Wang J, et al. Exposure to bisphenol-A and reproductive hormones among male adults. Environ Toxicol Pharmacol. (2015) 39:934–41. 10.1016/j.etap.2015.03.007[↩][↩]
- Feng MJ, Wu XQ, Li J, Ding L, Wang ZQ, Shen Y, Song ZC, Wang L, Yang Q, Wang XP, Li Q, Wang JT. [Relationship between daily exposure to bisphenol A and male sexual function-a study from the reproductive center]. Zhonghua Liu Xing Bing Xue Za Zhi. 2018 Jun 10;39(6):836-840. Chinese. doi: 10.3760/cma.j.issn.0254-6450.2018.06.027[↩]
- Den Hond E, Tournaye H, De Sutter P, Ombelet W, Baeyens W, Covaci A, et al. Human exposure to endocrine disrupting chemicals and fertility: a case–control study in male subfertility patients. Environ Int. (2015) 84:154–60. 10.1016/j.envint.2015.07.017[↩]
- Hart RJ, Doherty DA, Keelan JA, Minaee NS, Thorstensen EB, Dickinson JE, et al. The impact of antenatal bisphenol A exposure on male reproductive function at 20–22 years of age. Reprod Biomed Online. (2018) 36:340–7. 10.1016/j.rbmo.2017.11.009[↩]
- Tian Y, Zhou X, Miao M, Li D-K, Wang Z, Li R, et al. Association of bisphenol A exposure with LINE-1 hydroxymethylation in human semen. Int J Environ Res Public Health. (2018) 15:1770. 10.3390/ijerph15081770[↩]
- Radwan M, Wielgomas B, Dziewirska E, Radwan P, Kałużny P, Klimowska A, et al. Urinary bisphenol A levels and male fertility. Am J Mens Health. (2018) 12:2144–51. 10.1177/1557988318799163[↩]
- Adoamnei E, Mendiola J, Vela-Soria F, Fernández MF, Olea N, Jørgensen N, et al. Urinary bisphenol A concentrations are associated with reproductive parameters in young men. Environ Res. (2018) 161:122–8. 10.1016/j.envres.2017.11.002[↩]
- Pollack AZ, Mumford SL, Krall JR, Carmichael AE, Sjaarda LA, Perkins NJ, et al. Exposure to bisphenol A, chlorophenols, benzophenones, and parabens in relation to reproductive hormones in healthy women: a chemical mixture approach. Environ Int. (2018) 120:137–44. 10.1016/j.envint.2018.07.028[↩]
- Song H, Zhang T, Yang P, Li M, Yang Y, Wang Y, et al. Low doses of bisphenol A stimulate the proliferation of breast cancer cells via ERK1/2/ERRγ signals. Toxicol Vitro. (2015) 30:521–8. 10.1016/j.tiv.2015.09.009[↩][↩]
- Maffini MV, Rubin BS, Sonnenschein C, Soto AM. Endocrine disruptors and reproductive health: the case of bisphenol-A. Mol Cell Endocrinol. (2006) 254:179–86. 10.1016/j.mce.2006.04.033[↩]
- Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci USA. (2007) 104:13056–61. 10.1073/pnas.0703739104[↩]
- Doherty LF, Bromer JG, Zhou Y, Aldad TS, Taylor HS. In utero exposure to diethylstilbestrol (DES) or bisphenol-A (BPA) increases EZH2 expression in the mammary gland: an epigenetic mechanism linking endocrine disruptors to breast cancer. Horm Cancer. (2010) 1:146–55. 10.1007/s12672-010-0015-9[↩]
- Fenichel P, Chevalier N, Brucker-Davis F. Bisphenol A: an endocrine and metabolic disruptor. Ann Endocrinol. (2013) 74:211–20. 10.1016/j.ando.2013.04.002[↩]
- Rubin BS. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol. (2011) 127:27–34. 10.1016/j.jsbmb.2011.05.002[↩]
- Welshons WV, Nagel SC, Vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. (2006) 147:s56–69. 10.1210/en.2005-1159[↩]
- Nanjappa MK, Simon L, Akingbemi BT. The industrial chemical bisphenol A (BPA) interferes with proliferative activity and development of steroidogenic capacity in rat Leydig cells. Biol Reprod. (2012) 86:1–12. 10.1095/biolreprod.111.095349[↩]
- Acevedo N, Davis B, Schaeberle CM, Sonnenschein C, Soto AM. Perinatally administered bisphenol a as a potential mammary gland carcinogen in rats. Environ Health Perspect. (2013) 121:1040–6. 10.1289/ehp.1306734[↩]
- Fernández M, Bourguignon N, Lux-Lantos V, Libertun C. Neonatal exposure to bisphenol A and reproductive and endocrine alterations resembling the polycystic ovarian syndrome in adult rats. Environ Health Perspect. (2010) 118:1217–22. 10.1289/ehp.0901257[↩]
- Newbold RR, Jefferson WN, Padilla-Banks E. Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod Toxicol. (2007) 24:253–8. 10.1016/j.reprotox.2007.07.006[↩][↩]
- Prins GS, Hu W-Y, Shi G-B, Hu D-P, Majumdar S, Li G, et al. Bisphenol A promotes human prostate stem-progenitor cell self-renewal and increases in vivo carcinogenesis in human prostate epithelium. Endocrinology. (2014) 155:805–17. 10.1210/en.2013-1955[↩]
- Weinhouse C, Anderson OS, Bergin IL, Vandenbergh DJ, Gyekis JP, Dingman MA, et al. Dose-dependent incidence of hepatic tumors in adult mice following perinatal exposure to bisphenol A. Environ Health Perspect. (2014) 122:485–91. 10.1289/ehp.1307449[↩]
- Ayyanan A, Laribi O, Schuepbach-Mallepell S, Schrick C, Gutierrez M, Tanos T, et al. Perinatal exposure to bisphenol a increases adult mammary gland progesterone response and cell number. Mol Endocrinol. (2011) 25:1915–23. 10.1210/me.2011-1129[↩]
- Tharp AP, Maffini MV, Hunt PA, Vandevoort CA, Sonnenschein C, Soto AM. Bisphenol A alters the development of the rhesus monkey mammary gland. Proc Natl Acad Sci USA. (2012) 109:8190–5. 10.1073/pnas.1120488109[↩]
- Moon MK, Kim MJ, Jung IK, Koo YD, Ann HY, Lee KJ, et al. Bisphenol A impairs mitochondrial function in the liver at doses below the no observed adverse effect level. J Korean Med Sci. (2012) 27:644–52. 10.3346/jkms.2012.27.6.644[↩]
- Xu Y, Ai J, Zhang H. The mechanism of degradation of bisphenol A using the magnetically separable CuFe2O4/peroxymonosulfate heterogeneous oxidation process. J Hazard Mater. (2016) 309:87–96. 10.1016/j.jhazmat.2016.01.023[↩]
- Park JC, Lee M-C, Yoon D-S, Han J, Kim M, Hwang U-K, et al. Effects of bisphenol A and its analogs bisphenol F and S on life parameters, antioxidant system, and response of defensome in the marine rotifer Brachionus koreanus. Aqu Toxicol. (2018) 199:21–9. 10.1016/j.aquatox.2018.03.024[↩]
- Xu X, Zong S, Chen W, Liu D. Comparative study of bisphenol A degradation via heterogeneously catalyzed H2O2 and persulfate: reactivity, products, stability and mechanism. Chem Eng J. (2019) 369:470–9. 10.1016/j.cej.2019.03.099[↩]
- Ashley-Martin J, Dodds L, Levy AR, Platt RW, Marshall JS, Arbuckle TE. Prenatal exposure to phthalates, bisphenol A and perfluoroalkyl substances and cord blood levels of IgE, TSLP and IL-33. Environ Res. (2015) 140:360–8. 10.1016/j.envres.2015.04.010[↩]
- Rees Clayton EM, Todd M, Dowd JB, Aiello AE. The impact of bisphenol A and triclosan on immune parameters in the US population, NHANES 2003–2006. Environ Health Perspect. (2011) 119:390–6. 10.1289/ehp.1002883[↩]
- Li J, Bach A, Crawford RB, Phadnis-Moghe AS, Chen W, D’ingillo S, et al. CLARITY-BPA: effects of chronic bisphenol A exposure on the immune system: part 2–characterization of lymphoproliferative and immune effector responses by splenic leukocytes. Toxicology. (2018) 396:54–67. 10.1016/j.tox.2018.02.004[↩]
- Roy A, Gaylo A, Cao W, Saubermann LJ, Lawrence BP. Neither direct nor developmental exposure to bisphenol A alters the severity of experimental inflammatory colitis in mice. J Immunotoxicol. (2013) 10:334–40. 10.3109/1547691X.2012.747231[↩]
- Malaisé Y, Menard S, Cartier C, Gaultier E, Lasserre F, Lencina C, et al. Gut dysbiosis and impairment of immune system homeostasis in perinatally-exposed mice to bisphenol A precede obese phenotype development. Sci Rep. (2017) 7:14472. 10.1038/s41598-017-15196-w[↩]
- Roy A, Bauer SM, Lawrence BP. Developmental exposure to bisphenol A modulates innate but not adaptive immune responses to influenza A virus infection. PLoS One. (2012) 7:e38448. 10.1371/journal.pone.0038448[↩]
- Nakajima Y, Goldblum RM, Midoro-Horiuti T. Fetal exposure to bisphenol A as a risk factor for the development of childhood asthma: an animal model study. Environ Health. (2012) 11:8. 10.1186/1476-069X-11-8[↩]
- Salahinejad A, Attaran A, Naderi M, Meuthen D, Niyogi S, Chivers DP. Chronic exposure to bisphenol S induces oxidative stress, abnormal anxiety, and fear responses in adult zebrafish (Danio rerio). Sci Total Environ. (2021) 750:141633. 10.1016/j.scitotenv.2020.141633[↩]
- Faheem M, Adeel M, Khaliq S, Lone KP. Bisphenol-A induced antioxidants imbalance and cytokines alteration leading to immune suppression during larval development of Labeo rohita. Environ Sci Pollut Res. (2020) 27:26800–9. 10.1007/s11356-020-08959-y[↩]
- Huang M, Liu S, Fu L, Jiang X, Yang M. Bisphenol A and its analogues bisphenol S, bisphenol F and bisphenol AF induce oxidative stress and biomacromolecular damage in human granulosa KGN cells. Chemosphere. (2020) 253:126707. 10.1016/j.chemosphere.2020.126707[↩]
- Kobayashi K, Liu Y, Ichikawa H, Takemura S, Minamiyama Y. Effects of bisphenol A on oxidative stress in the rat brain. Antioxidants. (2020) 9:240. 10.3390/antiox9030240[↩]
- Sirasanagandla S.R., Al-Huseini I., Sofin R.G.S., Das S. Perinatal exposure to Bisphenol A and developmental programming of the cardiovascular changes in the offspring. Curr. Med. Chem. 2022;29:4235–4250. doi: 10.2174/0929867328666211206111835[↩]
- Wei J., Lin Y., Li Y., Ying C., Chen J., Song L., Zhou Z., Lv Z., Xia W., Chen X., et al. Perinatal exposure to bisphenol A at reference dose predisposes offspring to metabolic syndrome in adult rats on a high-fat diet. Endocrinology. 2011;152:3049–3061. doi: 10.1210/en.2011-0045[↩]
- Ma Y., Xia W., Wang D.Q., Wan Y.J., Xu B., Chen X., Li Y.Y., Xu S.Q. Hepatic DNA methylation modifications in early development of rats resulting from perinatal BPA exposure contribute to insulin resistance in adulthood. Diabetologia. 2013;56:2059–2067. doi: 10.1007/s00125-013-2944-7[↩]
- Miyawaki J., Sakayama K., Kato H., Yamamoto H., Masuno H. Perinatal and postnatal exposure to bisphenol a increases adipose tissue mass and serum cholesterol level in mice. J. Atheroscler. Thromb. 2007;14:245–252. doi: 10.5551/jat.E486[↩]
- Lang I.A., Galloway T.S., Scarlett A., Henley W.E., Depledge M., Wallace R.B., Melzer D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300:1303–1310. doi: 10.1001/jama.300.11.1303[↩]
- Melzer D., Gates P., Osborne N.J., Henley W.E., Cipelli R., Young A., Money C., McCormack P., Schofield P., Mosedale D., et al. Urinary bisphenol a concentration and angiography-defined coronary artery stenosis. PLoS ONE. 2012;7:e43378. doi: 10.1371/annotation/5f293018-48a3-40ae-96b7-04438d1d9cb9[↩]
- Xiong Q., Liu X., Shen Y., Yu P., Chen S., Hu J., Yu J., Li J., Wang H.S., Cheng X., et al. Elevated serum Bisphenol A level in patients with dilated cardiomyopathy. Int. J. Environ. Res. Public Health. 2015;12:5329–5337. doi: 10.3390/ijerph120505329[↩]
- Patel B.B., Raad M., Sebag I.A., Chalifour L.E. Lifelong exposure to bisphenol a alters cardiac structure/function, protein expression, and DNA methylation in adult mice. Toxicol. Sci. Off. J. Soc. Toxicol. 2013;133:174–185. doi: 10.1093/toxsci/kft026[↩]
- Posnack N.G., Jaimes R., 3rd, Asfour H., Swift L.M., Wengrowski A.M., Sarvazyan N., Kay M.W. Bisphenol A exposure and cardiac electrical conduction in excised rat hearts. Environ. Health Perspect. 2014;122:384–390. doi: 10.1289/ehp.1206157[↩]
- Cagampang F.R., Torrens C., Anthony F.W., Hanson M.A. Developmental exposure to bisphenol A leads to cardiometabolic dysfunction in adult mouse offspring. J. Dev. Orig. Health Dis. 2012;3:287–292. doi: 10.1017/S2040174412000153[↩]
- Yan S., Chen Y., Dong M., Song W., Belcher S.M., Wang H.S. Bisphenol A and 17β-estradiol promote arrhythmia in the female heart via alteration of calcium handling. PLoS ONE. 2011;6:e25455. doi: 10.1371/journal.pone.0025455[↩]
- O’Reilly A.O., Eberhardt E., Weidner C., Alzheimer C., Wallace B.A., Lampert A. Bisphenol A binds to the local anesthetic receptor site to block the human cardiac sodium channel. PLoS ONE. 2012;7:e41667. doi: 10.1371/journal.pone.0041667[↩]
- Belcher S.M., Chen Y., Yan S., Wang H.S. Rapid estrogen receptor-mediated mechanisms determine the sexually dimorphic sensitivity of ventricular myocytes to 17β-estradiol and the environmental endocrine disruptor bisphenol A. Endocrinology. 2012;153:712–720. doi: 10.1210/en.2011-1772[↩]
- Lind P.M., Lind L. Circulating levels of bisphenol A and phthalates are related to carotid atherosclerosis in the elderly. Atherosclerosis. 2011;218:207–213. doi: 10.1016/j.atherosclerosis.2011.05.001[↩]
- Sui Y., Park S.H., Helsley R.N., Sunkara M., Gonzalez F.J., Morris A.J., Zhou C. Bisphenol A increases atherosclerosis in pregnane X receptor-humanized ApoE deficient mice. J. Am. Heart Assoc. 2014;3:e000492. doi: 10.1161/JAHA.113.000492[↩]
- Poormoosavi S.M., Najafzadehvarzi H., Behmanesh M.A., Amirgholami R. Protective effects of Asparagus officinalis extract against Bisphenol A- induced toxicity in Wistar rats. Toxicol. Rep. 2018;5:427–433. doi: 10.1016/j.toxrep.2018.02.010[↩]
- Nicholson T.M., Nguyen J.L., Leverson G.E., Taylor J.A., Vom Saal F.S., Wood R.W., Ricke W.A. Endocrine disruptor bisphenol A is implicated in urinary voiding dysfunction in male mice. Am. J. Physiol. Ren. Physiol. 2018;315:F1208–F1216. doi: 10.1152/ajprenal.00582.2017[↩][↩][↩]
- Kataria A., Trasande L., Trachtman H. The effects of environmental chemicals on renal function. Nat. Rev Nephrol. 2015;11:610–625. doi: 10.1038/nrneph.2015.94[↩]
- Priego A.R., Parra E.G., Mas S., Morgado-Pascual J.L., Ruiz-Ortega M., Rayego-Mateos S. Bisphenol A Modulates Autophagy and Exacerbates Chronic Kidney Damage in Mice. Int. J. Mol. Sci. 2021;22:7189. doi: 10.3390/ijms22137189[↩]
- Bosch-Panadero E., Mas S., Civantos E., Abaigar P., Camarero V., Ruiz-Priego A., Ortiz A., Egido J., González-Parra E. Bisphenol A is an exogenous toxin that promotes mitochondrial injury and death in tubular cells. Environ. Toxicol. 2018;33:325–332. doi: 10.1002/tox.22519[↩]
- Gassman N.R. Induction of oxidative stress by bisphenol A and its pleiotropic effects. Environ. Mol. Mutagenesis. 2017;58:60–71. doi: 10.1002/em.22072[↩]
- Lee M.R., Park H., Bae S., Lim Y.H., Kim J.H., Cho S.H., Hong Y.C. Urinary bisphenol A concentrations are associated with abnormal liver function in the elderly: A repeated panel study. J. Epidemiol. Community Health. 2014;68:312–317. doi: 10.1136/jech-2013-202548[↩]
- Kim D., Yoo E.R., Li A.A., Cholankeril G., Tighe S.P., Kim W., Harrison S.A., Ahmed A. Elevated urinary bisphenol A levels are associated with non-alcoholic fatty liver disease among adults in the United States. Liver Int. Off. J. Int. Assoc. Study Liver. 2019;39:1335–1342. doi: 10.1111/liv.14110[↩]
- Mahdavinia M., Alizadeh S., Raesi Vanani A., Dehghani M.A., Shirani M., Alipour M., Shahmohammadi H.A., Rafiei Asl S. Effects of quercetin on bisphenol A-induced mitochondrial toxicity in rat liver. Iran. J. Basic Med. Sci. 2019;22:499–505. doi: 10.22038/ijbms.2019.32486.7952[↩]
- Geng S., Wang S., Zhu W., Xie C., Li X., Wu J., Zhu J., Jiang Y., Yang X., Li Y., et al. Curcumin attenuates BPA-induced insulin resistance in HepG2 cells through suppression of JNK/p38 pathways. Toxicol. Lett. 2017;272:75–83. doi: 10.1016/j.toxlet.2017.03.011[↩]
- Lin Y., Ding D., Huang Q., Liu Q., Lu H., Lu Y., Chi Y., Sun X., Ye G., Zhu H., et al. Downregulation of miR-192 causes hepatic steatosis and lipid accumulation by inducing SREBF1: Novel mechanism for bisphenol A-triggered non-alcoholic fatty liver disease. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids. 2017;1862:869–882. doi: 10.1016/j.bbalip.2017.05.001[↩]
- Wu M., Wang S., Weng Q., Chen H., Shen J., Li Z., Wu Y., Zhao Y., Li M., Wu Y., et al. Prenatal and postnatal exposure to Bisphenol A and Asthma: A systemic review and meta-analysis. J. Thorac. Dis. 2021;13:1684–1696. doi: 10.21037/jtd-20-1550[↩]
- Abellan A, Mensink-Bout SM, Garcia-Esteban R, Beneito A, Chatzi L, Duarte-Salles T, Fernandez MF, Garcia-Aymerich J, Granum B, Iñiguez C, Jaddoe VWV, Kannan K, Lertxundi A, Lopez-Espinosa MJ, Philippat C, Sakhi AK, Santos S, Siroux V, Sunyer J, Trasande L, Vafeiadi M, Vela-Soria F, Yang TC, Zabaleta C, Vrijheid M, Duijts L, Casas M. In utero exposure to bisphenols and asthma, wheeze, and lung function in school-age children: a prospective meta-analysis of 8 European birth cohorts. Environ Int. 2022 Apr;162:107178. doi: 10.1016/j.envint.2022.107178[↩]
- Midoro-Horiuti T., Tiwari R., Watson C.S., Goldblum R.M. Maternal bisphenol a exposure promotes the development of experimental asthma in mouse pups. Environ. Health Perspect. 2010;118:273–277. doi: 10.1289/ehp.0901259[↩]
- Exposure to BPA in the womb linked to wheezing and poorer lung function in children. https://www.sciencedaily.com/releases/2019/10/191001094205.htm[↩]
- He M., Ichinose T., Yoshida S., Takano H., Nishikawa M., Shibamoto T., Sun G. Exposure to bisphenol A enhanced lung eosinophilia in adult male mice. Allergy Asthma Clin. Immunol. Off. J. Can. Soc. Allergy Clin. Immunol. 2016;12:16. doi: 10.1186/s13223-016-0122-4[↩][↩]
- Gu J., Guo M., Yin X., Huang C., Qian L., Zhou L., Wang Z., Wang L., Shi L., Ji G. A systematic comparison of neurotoxicity of bisphenol A and its derivatives in zebrafish. Sci. Total Environ. 2022;805:150210. doi: 10.1016/j.scitotenv.2021.150210[↩][↩]
- Tonini C., Segatto M., Gagliardi S., Bertoli S., Leone A., Barberio L., Mandalà M., Pallottini V. Maternal Dietary Exposure to Low-Dose Bisphenol A Affects Metabolic and Signaling Pathways in the Brain of Rat Fetuses. Nutrients. 2020;12:1448. doi: 10.3390/nu12051448[↩][↩]
- McIlwraith E.K., Lieu C.V., Belsham D.D. Bisphenol A induces miR-708-5p through an ER stress-mediated mechanism altering neuronatin and neuropeptide Y expression in hypothalamic neuronal models. Mol. Cell. Endocrinol. 2022;539:111480. doi: 10.1016/j.mce.2021.111480[↩]
- Bisphenol A (BPA). https://www.toxtown.nlm.nih.gov/chemicals-and-contaminants/bisphenol-a-bpa[↩]