Black ginger
Black ginger also known as Kaempferia parviflora (Zingiberaceae family), “Thai ginseng”, Thai black ginger or in Thai as “Krachaidum”, is a Thai ginger species with deep purple-colored rhizomes (roots) that has traditionally been used as food and a folk medicine for more than 1000 years in Thailand 1. Kaempferia parviflora is found in tropical areas such as Malaysia, Sumatra, Borneo Island, and Thailand. Among the Hmong hill tribe, Kaempferia parviflora is widely believed to reduce perceived effort, improve physical work capacity, and prolong hill trekking 2. The dried Kaempferia parviflora root is generally pulverized and used as tea bags, while the fresh Kaempferia parviflora root is utilized to brew wine. The wine preparation is increasingly used in Thailand as a tonic and as an aphrodisiac 1. Black ginger supplement has been made into various preparations such as medicinal liquor or liquor plus honey, pills (powdered Kaempferia parviflora root with honey), capsules and tablets. Black ginger or Kaempferia parviflora has been long term used in Thai traditional medicine for treating various ailments including to cure allergy 3, anti-depressant, asthma, fatigue, weakness, impotence, gout, colic disorder, diarrhea, dysentery 4, peptic ulcer 5 and lower blood sugar in diabetes 6. In addition, it is also used as longevity promoting substance and as nerve tonic. A large number of recent studies have demonstrated the biological activities of black ginger extract (Kaempferia parviflora extract) contained numerous flavonoids 7, methoxyflavones 8, phenolic glycosides 9 and terpenoids 10, which was previously reported to possess antioxidant activity 11, cardioprotective 12, aphrodisiac 13, anticholinesterase 14, anti-inflammatory 15, anti-obesity 16 and antimutagenic 17, neuroprotective, and cognitive-enhancing properties 18. Kaempferia parviflora extract has been shown to improve physical fitness performance in clinical studies 19. The anti-oxidative activity of Kaempferia parviflora extract has been reported to exhibit antimalarial, antiviral, antimycobacterial and antibacterial 4 anti-gastric ulcer activities 20. Kaempferia parviflora extract has also exhibited antitumor activity against Hela human cervical and SKOV3 ovarian cancer cells 21.
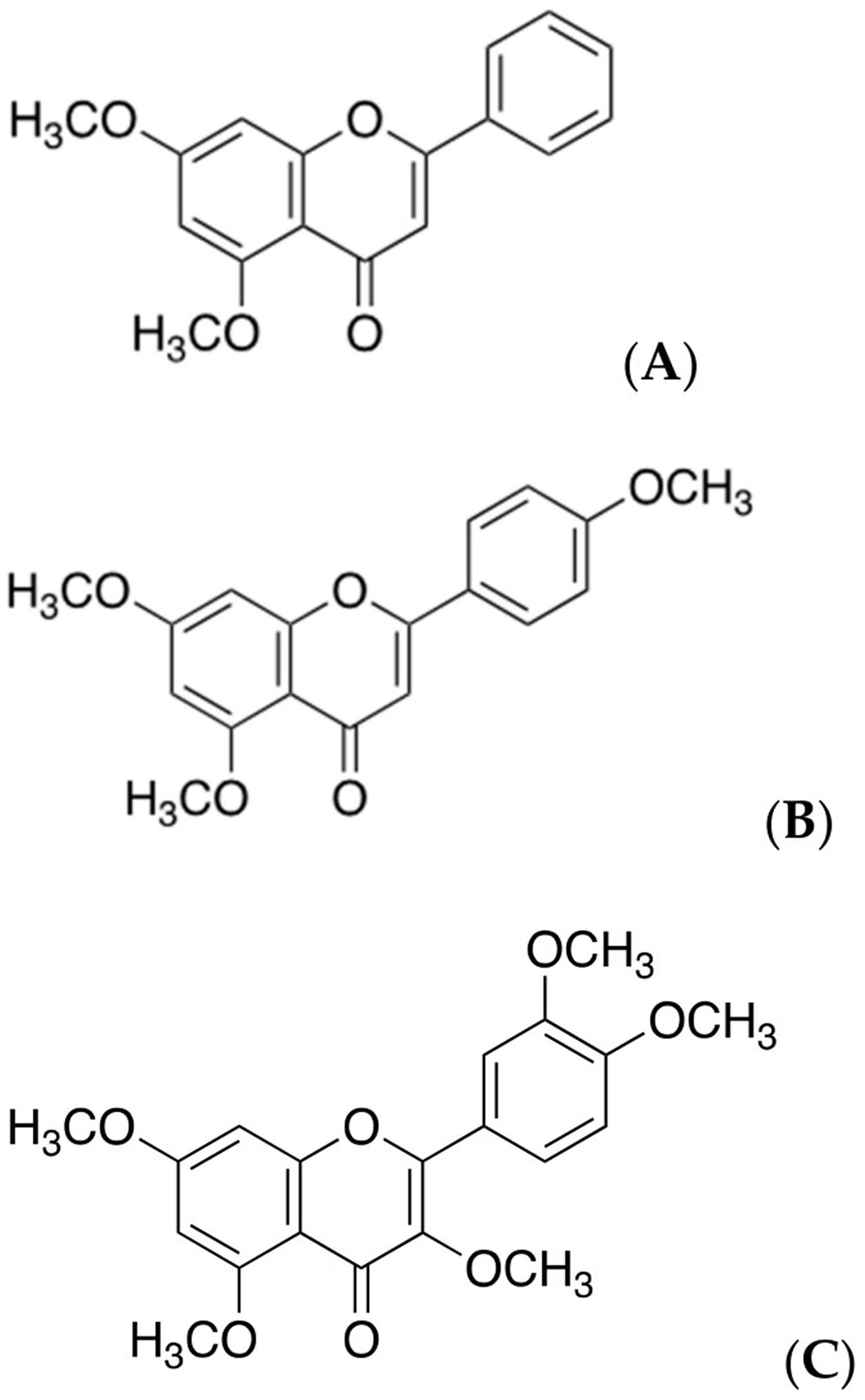
According to the previous reports by Wongsrikaew et al. 22 and Mekjaruskul et al. 23, typical high-performance liquid chromatography of Kaempferia parviflora root ethanol extract were found to have 5,7-dimethoxyflavone (DMF), 3,5,7,3′,4′-pentamethoxyflavone (PMF), and 5,7,4′-trimethoxyflavone (TMF) as major phytochemicals. These three molecules have a common structure, which comprises two methoxy groups at C-5 and C-7 of the A ring in polymethoxyflavones (Figure 2). In an in vitro study, methoxyflavone was examined for its inhibitory activities against nitric oxide production. Compound 5 (5-hydroxy-3,7,30,40-tetramethoxyflavone) exhibited the highest activity, followed by compounds 4 (5-hydroxy-7,40-dimethoxyflavone) and 3 (5-hydroxy-3,7,40-trimethoxyflavone), whereas other compounds possessed moderate or weak activity 24. In addition, more than 20 chemically identifiable constituents have been reported to have potent pharmacological effects 25. For example, flavonoids contained in Kaempferia parviflora root extract was reported to possess antioxidant activity, neuroprotective effects, and cognition-enhancing effects.21 Methoxyflavone substances in Kaempferia parviflora showed an inhibitory effect of phosphodiesterase types 5 and 6, which enhanced sexual performance 26. For antimicrobial activity, 5,7,4′-trimethoxyflavone (TMF) and 5,7,3′,4′-tetramethoxyflavone exhibited antiplasmodial activity against Plasmodium falciparum, and 3,5,7,4′-tetramethoxyflavone showed antifungal activity against Candida albicans 27. For cholinesterase inhibitory effect, Kaempferia parviflora showed the potential inhibitors toward acetylcholinesterase and butyrylcholinesterase, which may be of great interest to be considered as a treatment agent for Alzheimer’s disease 28.
Figure 1. Kaempferia parviflora bioactive compounds
Footnote: The main structure of methoxyflavone includes benzene A ring with 2 substituent groups at positions 5 and 7, an aromatic B ring with 2 substituent groups at positions 3′ and 4′, and C ring with a substituent group linking on position 3. The substituent groups might be -H, -OH, or -OCH3.
[Source 29 ]Figure 2. Chemical structures of methoxyflavones
Footnote: Chemical structures of polymethoxyflavones. Structures of (A) 5,7-methoxyflavone (DMF), (B) 5,7,4′-trimetholxyflavone (TMF), and (C) 3,5,7,3′,4′-pentamethoxyflavone (PMF).
[Source 30 ]Kaempferia parviflora benefits
A systematic review on clinical effects of Kaempferia parviflora has shown positive benefits, but it is inconclusive due to small studies included 31. Modern research technologies have demonstrated that Kaempferia parviflora can suppress body weight gain, inhibit lipid accumulation, and prevent from pathological changes resulted by insulin resistance, fatty liver, and hypertension 32. The weight gain may be obtained by the imbalance between energy expenditure and energy intake. brown adipose tissue plays a crucial role in controlling the whole-body energy expenditure and body fatness. The ethanol extract of Kaempferia parviflora at the dose of 100 mg causes a significant increase in whole-body energy expenditure by recruiting brown adipose tissue in male volunteers aged 21-29 in Japan 33.
In Mong hill tribe in Thailand, Kaempferia parviflora is believed to enhance physical work capacity and reduce perceived efforts. Kaempferia parviflora extract at the doses of 25 mg or 90 mg for 8 weeks has been demonstrated to increase physical fitness performance in 30-second chair stand test and 6 minute walk test, increase the scavenger enzymes superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px)) expression, and decrease malondialdehyde production 34. In the double-blind, placebo-controlled clinical trial, oral administration of sports nutritional supplement (Fitnox) at the single dose of 250 mg can significantly increase the levels of nitrate (NO3–) and nitrite (NO2–) in serum and saliva, leading to enhancement of overall performance and physical endurance 35. Consistently, Kaempferia parviflora extract has been found to improve physical fitness, as indicated by enhancement of grip and leg strength, balance, endurance, and locomotor activity. In addition, the daily visual analog scale (VAS) figure score, postphysical fitness test VAS fatigue score, and chronic fatigue syndrome score are found to be enhanced greatly than those in the placebo group 36. However, a randomized, double-blind, and crossover study has been demonstrated that acute administration of Kaempferia parviflora (1.35g) does not enhance exercise performance, compared with the placebo, as confirmed by repeated sprint exercise and submaximal exercise to exhaustion in college males in Thailand 37. In contrast, supplement with Kaempferia parviflora extract at 180 mg per day for 12 weeks, the soccer players are found to increase the right-hand trip strength and left-hand grip strength, compared with those in the placebo group. On the other hand, the back and leg strength, the 40-yard technical test, the sit-and reach test, the 50-metre sprint test, and the cardiorespiratory fitness test do not show any significant difference from those in the placebo group 38.
Blood circulation is closely associated with blood fluidity. It has been demonstrated that 70% methanol extract of Kaempferia parviflora significantly improves blood fluidity through activation of fibrinolysis, as indicated by elongation of euglobulin lysis time in disseminated intravascular coagulation (DIC) rat models and the fibrinolysis assays in vitro. Kaempferia parviflora methoxyflavones been involved in activation of fibrinolysis 39. In the ventricular fibrillation (VF) of swine heart model, the saline extract of Kaempferia parviflora at high doses of 100 mg/kg and 50 mg/kg is found to increase the defibrillation threshold and the upper limit of vulnerability. But it does not change ventricular fibrillation threshold. In addition, Kaempferia parviflora administration attenuates diastolic and systolic blood pressures 40. On the other hand, the extract of Kaempferia parviflora (100mg/kg) has been demonstrated to decrease cardiac functions in normal rat hearts through upregulation of cyclic guanosine monophosphate (cGMP) level and nitric oxide (NO) signaling and downregulation of Ca2+ transient 41. This is consistent with 5,7-dimethoxyflavone induced vasorelaxation through increased K+ efflux and attenuated Ca2+ influx 42.
Kaempferia parviflora is believed to benefit men’s sexual activity. However, the ethanol extract of Kaempferia parviflora at dose of 70 mg/kg does not have any effects on weights of reproductive organs but decreases mount latency, ejaculation latency, and postejaculation latency 43. On the other hand, the ethanol extract of Kaempferia parviflora has been found to increase blood flow to the testis dose-dependently 44. Phosphodiesterase type 5 (PDE-5) inhibition has become the strategy for management of erectile dysfunction. However, PDE-5 inhibitors require sexual stimulation to activate cGMP-NO and trigger erection. Thus, targeting for relaxation directly to corpus cavernosum might be a new effective approach for erectile dysfunction management. It has been demonstrated that 3,5,7,3′,4′-pentamethoxyflavone exhibits a relaxant activity on isolated human cavernosum precontrated by phenylephrine. The possible mechanism might be that 3,5,7,3′,4′-pentamethoxyflavone inhibits L-type Ca2+ channel and induces immobilization of Ca2+ from sarcoplasmic reticulum. On the other hand, 3,5,7,3′,4′-pentamethoxyflavone does not act as a calcium-activated potassium channels (KCa channels) opener, a PDE inhibitor, and a Rho-kinase inhibitor but a rather weak stimulator of NO release 22. Kaempferia parviflora extract has been demonstrated to potentially manage age-related erectile dysfunction. At the dose of 90 mg/day, Kaempferia parviflora extract significantly increases all parameters. However, it does not alter the concentration of testosterone, follicle stimulating hormone (FSH), and luteinizing hormone (LH) 45.
Table 1. Clinical effects of Kaempferia parviflora classified by outcomes
| Outcomes, Study | Sample Subgroup | Interventions | Time or Methods of Measurement | Kaempferia parviflora | Control | Summary | |||||
| Mean | SD | Mean | SD | ||||||||
| Physical or exercise performance | |||||||||||
| Maximum power output (watt) | |||||||||||
| Deema, 2007 | Endurance training groups | Kaempferia parviflora 1.35 g/day vs placebo | Baseline | 203.5 | 3.3 | 214.2 | 2.4 | Significantly increased at weeks 4 and 8 in the Kaempferia parviflora group and week 8 in the placebo group (difference from baseline) | |||
| 4 weeks | 233.7 | 2.9 | 232.1 | 1.6 | |||||||
| 8 weeks | 245 | 3.2 | 247 | 2.9 | |||||||
| No endurance training groups | Baseline | 184.1 | 2.8 | 200.8 | 4.9 | No significant effects | |||||
| 4 weeks | 190.5 | 2.1 | 197.4 | 5.3 | |||||||
| 8 weeks | 192 | 2 | 198.2 | 5.3 | |||||||
| Wasuntarawat, 2010 | Anaerobic exercise (exhaustive sprint) | Kaempferia parviflora 1.35 g/day vs placebo | Wingate 1 | 545 | 95 | 554 | 114 | Maximum power output declined (P < .05) across Wingate tests 1, 2, and 3 but there were no differences (P > .05) between Kaempferia parviflora and placebo | |||
| Wingate 2 | 499 | 99 | 495 | 109 | |||||||
| Wingate 3 | 454 | 116 | 473 | 96 | |||||||
| Mean power output (watt) | |||||||||||
| Wasuntarawat, 2010 | Anaerobic exercise (exhaustive sprint) | Kaempferia parviflora 1.35 g/day vs placebo | Wingate 1 | 417 | 65 | 416 | 65 | Mean power output declined (P < .05) across Wingate tests 1, 2, and 3 but there were no differences (P > .05) between Kaempferia parviflora and placebo | |||
| Wingate 2 | 369 | 59 | 369 | 58 | |||||||
| Wingate 3 | 323 | 61 | 334 | 57 | |||||||
| Time to finish work max test (minutes) | |||||||||||
| Deema, 2007 | Endurance training groups | Kaempferia parviflora 1.35 g/day vs placebo | Baseline | 8.2 | 0.1 | 8.5 | 0.1 | Significantly increased at weeks 4 and 8 in the Kaempferia parviflora group and week 8 in the placebo group (difference from baseline) | |||
| 4 weeks | 9.2 | 0.1 | 9.4 | 0.1 | |||||||
| 8 weeks | 9.7 | 0.1 | 9.9 | 0.1 0 | |||||||
| No endurance training groups | Baseline | 7.2 | 0.1 | 7.9 | 0.2 | No significant effects | |||||
| 4 weeks | 7.5 | 0.1 | 7.8 | 0.2 | |||||||
| 8 weeks | 7.5 | 0.1 | 7.8 | 0.1 | |||||||
| Time to exhaustion (minutes) | |||||||||||
| Wasuntarawat, 2010 | Anaerobic exercise (exhaustive sprint) | Kaempferia parviflora 1.35 g/day vs placebo | 28.3 | 12.5 | 27.6 | 11.5 | Acute ingestion of Kaempferia parviflora did not improve time to exhaustion | ||||
| Rating of perceived exertion | |||||||||||
| Wasuntarawat, 2010 | Anaerobic exercise (exhaustive sprint) | Kaempferia parviflora 1.35 g/day vs placebo | 10 min | 14 | 2 | 14 | 2 | Ratings of perceived exertion at 10 and 20 minutes and immediately after exhaustion were also not different between placebo and Kaempferia parviflora. Time to exhaustion was rated between 17 (“very hard”) and 19 (“extremely hard”) | |||
| 20 min | 17 | 2 | 17 | 2 | |||||||
| Post a | 19 | 1 | 18 | 1 | |||||||
| Percentage fatigue (%) | |||||||||||
| Wasuntarawat, 2010 | Anaerobic exercise (exhaustive sprint) | Kaempferia parviflora 1.35 g/day vs placebo | Wingate 1 | 43 | 13 | 40 | 15 | No differences in percent fatigue during each 30-second sprint were observed between placebo and Kaempferia parviflora. Percent fatigue during the third Wingate test was significantly (P < .05) greater than during the first Wingate test in both placebo and Kaempferia parviflora trials | |||
| Wingate 2 | 48 | 12 | 44 | 15 | |||||||
| Wingate 3 | 51 | 13 | 53 | 10 | |||||||
| Heart rate (BPM) | |||||||||||
| Deema, 2007 | Maximum heart rate | ||||||||||
| Endurance training groups | Kaempferia parviflora 1.35 g/day vs placebo | Baseline | 181 | 0.8 | 179.6 | 0.5 | Significantly increased at week 8 in the placebo group (difference from baseline) | ||||
| 4 weeks | 184.7 | 0.7 | 181.3 | 0.6 | |||||||
| 8 weeks | 184.6 | 0.8 | 186.6 | 0.8 | |||||||
| No endurance training groups | Baseline | 185 | 2 | 180 | 2.4 | No significant effects | |||||
| 4 weeks | 199.6 | 0.6 | 176.2 | 2.6 | |||||||
| 8 weeks | 186.4 | 1.6 | 181.2 | 1.8 | |||||||
| Wasuntarawat, 2010 | Aerobic exercise (endurance) | Kaempferia parviflora 1.35 g/day vs placebo | 10 min | 165 | 13 | 164 | 11 | Heart rate at 10 and 20 minutes and immediately after exhaustion were also not different between placebo and Kaempferia parviflora | |||
| 20 min | 174 | 10 | 172 | 9 | |||||||
| Posta | 177 | 8 | 174 | 10 | |||||||
| Lactate threshold (watt) | |||||||||||
| Deema, 2007 | Endurance training groups | Kaempferia parviflora 1.35 g/day vs placebo | Baseline | 129.6 | 1.7 | 142.5 | 1.7 | Significantly increased lactate threshold at weeks 4 and 8 in the Kaempferia parviflora group (difference from baseline) | |||
| 4 weeks | 156.8 | 2.7 | 155 | 2.3 | |||||||
| 8 weeks | 165.9 | 3.3 0 | 160 | 1.8 | |||||||
| No endurance training groups | Baseline | 120 | 4.2 | 120 | 4.2 | No significant effects from baseline | |||||
| 4 weeks | 118.80 | 5.4 | 118.80 | 6 | |||||||
| 8 weeks | 118.80 | 2.8 | 106.3 | 3.1 | |||||||
| Hand grip strength test | |||||||||||
| Wattanathorn, 2012 | Right hand (kg) | Kaempferia parviflora 25 mg/day vs placebo | Baseline | 25.06 | 3.01 | 24.53 | 2.55 | No significant effects | |||
| 4 weeks | 25 | 2.97 | 24.33 | 2.28 | |||||||
| 8 weeks | 24.86 | 3.18 | 24.33 | 2.46 | |||||||
| Kaempferia parviflora 90 mg/day vs placebo | Baseline | 23.93 | 3.3 | — | — | No significant effects | |||||
| 4 weeks | 24.6 | 3.13 | — | — | |||||||
| 8 weeks | 24.8 | 3.14 | — | — | |||||||
| Left hand (kg) | Kaempferia parviflora 25 mg/day vs placebo | Baseline | 22.06 | 1.86 | 21.06 | 1.83 | No significant effects | ||||
| 4 weeks | 21.66 | 1.5 | 21.33 | 1.58 | |||||||
| 8 weeks | 21.26 | 1.48 | 21.2 | 1.56 | |||||||
| Kaempferia parviflora 90 mg/day vs placebo | Baseline | 20.86 | 2.72 | — | — | No significant effects | |||||
| 4 weeks | 21.6 | 2.02 | — | — | |||||||
| 8 weeks | 21.6 | 1.84 | — | — | |||||||
| Promthep, 2015 | Right hand (kg/wt) | Kaempferia parviflora 180 mg/day vs placebo | Baseline | 0.65 | 0.09 | 0.63 | 0.07 | Significantly enhanced at weeks 4, 8, and 12 (difference from the placebo group in the same week) and significant difference compared with the baseline score at week 4 | |||
| 4 weeks | 0.7 | 0.09 | 0.66 | 0.07 | |||||||
| 8 weeks | 0.68 | 0.1 | 0.63 | 0.07 | |||||||
| 12 weeks | 0.65 | 0.08 | 0.62 | 0.07 | |||||||
| Left hand (kg/wt) | Kaempferia parviflora 180 mg/day vs placebo | Baseline | 0.62 | 0.08 | 0.6 | 0.08 | Significantly enhanced at week 8 (difference from the placebo group in the same week) | ||||
| 4 weeks | 0.65 | 0.1 | 0.62 | 0.07 | |||||||
| 8 weeks | 0.64 | 0.08 | 0.59 | 0.08 | |||||||
| 12 weeks | 0.61 | 0.08 | 0.57 | 0.07 | |||||||
| 30-Second chair stand test (seconds) | |||||||||||
| Wattanathorn, 2012 | Kaempferia parviflora 25 mg/day vs placebo | Baseline | 18.33 | 2.58 | 19.13 | 2.79 | No significant effects | ||||
| 4 weeks | 19 | 2.77 | 19.26 | 1.43 | |||||||
| 8 weeks | 20 | 3.11 | 18.93 | 1.7 | |||||||
| Kaempferia parviflora 90 mg/day vs placebo | Baseline | 18.6 | 2.52 | — | — | Significantly increased at week 8 compared with baseline | |||||
| 4 weeks | 19.6 | 2.13 | — | — | |||||||
| 8 weeks | 20.66 | 2.28 | — | — | |||||||
| 6-Minute Walk Test (m) | |||||||||||
| Wattanathorn, 2012 | Kaempferia parviflora 25 mg/day vs placebo | Baseline | 571.26 | 33.68 | 567.33 | 33.52 | No significant effects | ||||
| 4 weeks | 570.33 | 38.32 | 598.73 | 31.57 | |||||||
| 8 weeks | 575.53 | 36.04 | 571.26 | 32.05 | |||||||
| Kaempferia parviflora 90 mg/day vs placebo | Baseline | 572.8 | 32.65 | — | — | Significantly increased at week 8 compared with either baseline or placebo | |||||
| 4 weeks | 575.46 | 34.29 | — | — | |||||||
| 8 weeks | 601.26 | 33.7 | — | — | |||||||
| Tandem stance test (seconds) | |||||||||||
| Wattanathorn, 2012 | Opened eye | ||||||||||
| Right leg is in front | Kaempferia parviflora 25 mg/day vs Placebo | Baseline | 161.8 | 11.16 | 164.8 | 12.34 | No significant effects | ||||
| 4 weeks | 164.06 | 9.63 | 163.06 | 10.35 | |||||||
| 8 weeks | 162.26 | 8.93 | 165.06 | 9.8 | |||||||
| Kaempferia parviflora 90 mg/day vs placebo | Baseline | 164 | 10.5 | — | — | No significant effects | |||||
| 4 weeks | 166.6 | 6.81 | — | — | |||||||
| 8 weeks | 168.46 | 6.9 | — | — | |||||||
| Left leg is in front | Kaempferia parviflora 25 mg/day vs placebo | Baseline | 111.93 | 7.77 | 112.33 | 11 | No significant effects | ||||
| 4 weeks | 112.33 | 11.39 | 110.66 | 10.01 | |||||||
| 8 weeks | 111.8 | 10.16 | 109 | 10.2 | |||||||
| Kaempferia parviflora 90 mg/day vs placebo | Baseline | 108.2 | 11.32 | — | — | No significant effects | |||||
| 4 weeks | 109.33 | 13.62 | — | — | |||||||
| 8 weeks | 111.8 | 13.31 | — | — | |||||||
| Closed eye | |||||||||||
| Right leg is in front | Kaempferia parviflora 25 mg/day vs placebo | Baseline | 31.86 | 10.12 | 33.8 | 9.22 | No significant effects | ||||
| 4 weeks | 32.6 | 7.44 | 30.8 | 10.74 | |||||||
| 8 weeks | 32.73 | 7.67 | 31.66 | 10.41 | |||||||
| Kaempferia parviflora 90 mg/day vs placebo | Baseline | 31.26 | 11.09 | — | — | No significant effects | |||||
| 4 weeks | 31.86 | 9.33 | — | — | |||||||
| 8 weeks | 33.4 | 8.94 | — | — | |||||||
| Left leg is in front | Kaempferia parviflora 25 mg/day vs placebo | Baseline | 20.93 | 3.41 | 18.8 | 3.6 | No significant effects | ||||
| 4 weeks | 21.33 | 3.79 | 19.86 | 5.01 | |||||||
| 8 weeks | 21.26 | 3.19 | 21.2 | 4.57 | |||||||
| Kaempferia parviflora 90 mg/day vs placebo | Baseline | 20.46 | 4.24 | — | — | No significant effects | |||||
| 4 weeks | 21.26 | 4.58 | — | — | |||||||
| 8 weeks | 22.06 | 3.93 | — | — | |||||||
| A sit-and-reach test (cm) | |||||||||||
| Promthep, 2015 | Kaempferia parviflora 180 mg/day vs placebo | Baseline | 17.98 | 4.6 | 16.14 | 4.93 | Significant difference compared with the baseline score at week 4 (both the treatment and placebo groups) | ||||
| 4 weeks | 16.43 | 5.15 | 14.64 | 4.92 | |||||||
| 8 weeks | 16.88 | 5.19 | 14.61 | 5.24 | |||||||
| 12 weeks | 18.28 | 5.1 | 17.01 | 4.55 | |||||||
| A back-and-leg strength test (kg/wt) | |||||||||||
| Promthep, 2015 | Kaempferia parviflora 180 mg/day vs placebo | Baseline | 2.77 | 0.54 | 2.45 | 0.39 | No significant effects | ||||
| 4 weeks | 2.68 | 0.55 | 2.45 | 0.51 | |||||||
| 8 weeks | 2.77 | 0.55 | 2.44 | 0.4 | |||||||
| 12 weeks | 2.79 | 0.59 | 2.53 | 0.52 | |||||||
| A 40-yard technical test (seconds) | |||||||||||
| Promthep, 2015 | Kaempferia parviflora 180 mg/day vs placebo | Baseline | 11.61 | 0.07 | 11.99 | 0.86 | Significantly decreased at week 12 compared with the baseline | ||||
| 4 weeks | 12.06 | 1.16 | 12.34 | 1.33 | |||||||
| 8 weeks | 11.5 | 0.74 | 11.46 | 0.75 | |||||||
| 12 weeks | 10.08 | 0.47 | 10.47 | 0.9 | |||||||
| A 50-metre sprint test (seconds) | |||||||||||
| Promthep, 2015 | Kaempferia parviflora 180 mg/day vs placebo | Baseline | 6.24 | 0.31 | 6.29 | 0.37 | No significant effects in both groups. No significant differences between the groups | ||||
| 4 weeks | 6.26 | 0.31 | 6.33 | 0.49 | |||||||
| 8 weeks | 6.37 | 0.26 | 6.5 | 0.5 | |||||||
| 12 weeks | 6.33 | 0.24 | 6.47 | 0.52 | |||||||
| A cardiorespiratory fitness test VO2 max (mL/kg/min) | |||||||||||
| Promthep, 2015 | Kaempferia parviflora 180 mg/day vs placebo | Baseline | 45.09 | 9.88 | 45.09 | 9.96 | Significantly increased cardiorespiratory fitness, as indicated by VO2 max values at week 12. No significant difference between the groups | ||||
| 4 weeks | 46.95 | 7.61 | 47.85 | 10.08 | |||||||
| 8 weeks | 49.4 | 8.4 | 48.34 | 7.17 | |||||||
| 12 weeks | 51.05 | 8.4 | 47.1 | 8.45 | |||||||
| Pain indicators | |||||||||||
| Chalee, 2010 | Pain severity | Kaempferia parviflora 7% w/w vs analgesic cream | Baseline | 8.11 | 0.99 | 8.15 | 1.09 | Significantly decreased at week 4 compared with the baseline (both the treatment and placebo groups). No significant difference between the groups | |||
| 4 weeks | 6.8 | 0.76 | 6.87 | 0.65 | |||||||
| Circumference of knee joint (cm) | Baseline | 37.91 | 2.75 | 37.15 | 3 | ||||||
| 4 weeks | 37.03 | 2.56 | 36.3 | 2.64 | |||||||
| Range of motion of knee joint (range of motion) | Baseline | 110.57 | 9.45 | 109.39 | 10.66 | ||||||
| 4 weeks | 117.43 | 5.86 | 116.21 | 6.62 | |||||||
| Modified WOMAC score | Baseline | 40.31 | 6.63 | 39.97 | 6.02 | ||||||
| 4 weeks | 39.29 | 5.84 | 39 | 5.5 | |||||||
| Energy expenditure | |||||||||||
| Matsushita, 2015 | Energy expenditure change (kJ/day) | ||||||||||
| All | Kaempferia parviflora 100 mg/day vs placebo | Baseline | 6213 | 143 | 6196 | 150 | No significant in the placebo group but significant difference between the groups at 30 and 60 minutes, difference from baseline and maximal rise at 60 minutes | ||||
| 60 minutes | 6442 | 212 | NR | NR | |||||||
| High-brown adipose tissue | Kaempferia parviflora 180 mg/day vs placebo | Baseline | 6076 | 184 | 6103 | 184 | No significant in the placebo group but significant difference between the groups at 30 and 60 minutes, difference from baseline at 30, 60, 90 minutes and maximal rise at 60 minutes | ||||
| 60 minutes | 6427 | 234 | NR | NR | |||||||
| Low-brown adipose tissue | Kaempferia parviflora 180 mg/day vs placebo | Baseline | 6418 | 223 | 6334 | 261 | No significant effects | ||||
| 60 minutes | NR | NR | NR | NR | |||||||
| Erectile response | |||||||||||
| Wannanon, 2012 | Penile circumference (cm) | ||||||||||
| Resting state | Kaempferia parviflora 25 mg/day vs placebo | Baseline | 9.4 | 0.79 | 9 | 0.73 | No significant effects | ||||
| 1 month | 9 | 0.79 | 8.7 | 0.59 | |||||||
| 2 months | 9.4 | 0.79 | 8.8 | 0.59 | |||||||
| Delayb | 9.15 | 3.1 | 9.15 | 3.1 | |||||||
| Kaempferia parviflora 90 mg/day vs placebo | Baseline | 9.2 | 0.69 | 9 | 0.73 | After 1 and 2 months of treatment, significant increase in the length and width of penis when compared with the placebo treated group | |||||
| 1 month | 10.2 | 0.79 | 8.7 | 0.59 | |||||||
| 2 months | 10.15 | 0.79 | 8.8 | 0.59 | |||||||
| Delay b | 9.65 | 2.71 | 9.15 | 3.1 | |||||||
| Erection state | Kaempferia parviflora 25 mg/day vs placebo | Baseline | 10.9 | 3.87 | 11.5 | 3.87 | No significant effects | ||||
| 1 month | 10.5 | 3.68 | 10.6 | 3.1 | |||||||
| 2 months | 11.5 | 4.26 | 11.8 | 2.71 | |||||||
| Delay b | 11.7 | 3.1 | 11.7 | 2.71 | |||||||
| Kaempferia parviflora 90 mg/day vs placebo | Baseline | 10.8 | 4.26 | 11.5 | 3.87 | After 1 and 2 months of treatment, significant increase in the width when compared with the placebo treated group | |||||
| 1 month | 12.1 | 4.26 | 10.6 | 3.1 | |||||||
| 2 months | 11.9 | 4.26 | 11.8 | 2.71 | |||||||
| Delay b | 11.7 | 0.39 | 11.7 | 2.71 | |||||||
| Penile length (cm) | |||||||||||
| Resting state | Kaempferia parviflora 25 mg/day vs placebo | Baseline | 9.7 | 4.26 | 9.95 | 7.55 | No significant effects | ||||
| 1 month | 9.65 | 3.87 | 9.9 | 3.49 | |||||||
| 2 months | 10.9 | 3.87 | 10 | 3.1 | |||||||
| Delay b | 10.6 | 3.49 | 10.25 | 0.07 | |||||||
| Kaempferia parviflora 90 mg/day vs placebo | Baseline | 9.4 | 4.07 | 9.95 | 7.55 | After 1 and 2 months of treatment, significant increase in the length when compared with the placebo treated group | |||||
| 1 month | 11.25 | 3.49 | 9.9 | 3.49 | |||||||
| 2 months | 11.1 | 2.71 | 10 | 3.1 | |||||||
| Delay b | 10.65 | 3.49 | 10.25 | 0.07 | |||||||
| Erection state | Kaempferia parviflora 25 mg/day vs placebo | Baseline | 12.5 | 5.27 | 13.1 | 4.96 | No significant effects | ||||
| 1 month | 12.95 | 4.03 | 12.2 | 3.87 | |||||||
| 2 months | 13.1 | 4.34 | 12.4 | 4.18 | |||||||
| Delay b | 13.55 | 3.87 | 13.2 | 2.79 | |||||||
| Kaempferia parviflora 90 mg/day vs placebo | Baseline | 12.9 | 4.34 | 13.1 | 4.96 | After 1 and 2 months of treatment, significant increase in the length when compared with the placebo treated group | |||||
| 1 month | 13.75 | 4.34 | 12.2 | 3.87 | |||||||
| 2 months | 13.9 | 4.03 | 12.4 | 4.18 | |||||||
| Delay b | 13.5 | 4.65 | 13.2 | 2.79 | |||||||
| Latency time (mins) | |||||||||||
| Erection state | Kaempferia parviflora 25 mg/day vs placebo | Baseline | 10.9 | 13.94 | 11.6 | 12.39 | No significant effects | ||||
| 1 month | 8.6 | 12.39 | 11.7 | 11.81 | |||||||
| 2 months | 8 | 12.39 | 10 | 10.65 | |||||||
| Delay b | 7.8 | 0.59 | 10.9 | 12.78 | |||||||
| Kaempferia parviflora 90 mg/day vs placebo | Baseline | 10.4 | 9.3 | 11.6 | 12.39 | Significantly decreased the response latency to sexual erotic stimuli and still showed the significant changes during the delay period | |||||
| 1 month | 5.5 | 7.17 | 11.7 | 11.81 | |||||||
| 2 months | 5.5 | 6.58 | 10 | 10.65 | |||||||
| Delay b | 7.4 | 6.2 | 10.9 | 12.78 | |||||||
| Serum hormones concentrations | |||||||||||
| Testosterone (ng/mL) | Kaempferia parviflora 25 mg/day vs placebo | Baseline | 4.11 | 1.56 | 4.14 | 0.92 | No significant effects | ||||
| Single dose | 4.79 | 4.63 | 5.28 | 2.76 | |||||||
| 1 month | 5.11 | 3.13 | 5.92 | 5.49 | |||||||
| 2 months | 4.64 | 1.22 | 5.65 | 1.23 | |||||||
| Delay b | 5.71 | 2.38 | 5.14 | 0.92 | |||||||
| Kaempferia parviflora 90 mg/day vs placebo | Baseline | 4.11 | 1.3 | 4.14 | 0.92 | No significant effects | |||||
| Single dose | 4.89 | 2.63 | 5.28 | 2.76 | |||||||
| 1 month | 4.26 | 0.83 | 5.92 | 5.49 | |||||||
| 2 months | 5.74 | 2.6 | 5.65 | 1.23 | |||||||
| Delay b | 6.06 | 3.19 | 5.14 | 0.92 | |||||||
| FSH (IU/L) | Kaempferia parviflora 25 mg/day vs placebo | Baseline | 8.8 | 4.29 | 7.88 | 4.34 | No significant effects | ||||
| Single dose | 7.35 | 4.23 | 6.73 | 2.54 | |||||||
| 1 month | 7.23 | 5.37 | 6.52 | 5.51 | |||||||
| 2 months | 7.96 | 3.32 | 5.95 | 2.34 | |||||||
| Delay b | 8.81 | 5.12 | 5.88 | 3.34 | |||||||
| Kaempferia parviflora 90 mg/day vs placebo | Baseline | 7.53 | 2.92 | 7.88 | 4.34 | No significant effects | |||||
| Single dose | 6.14 | 2.26 | 6.73 | 2.54 | |||||||
| 1 month | 6.29 | 2.25 | 6.52 | 5.51 | |||||||
| 2 months | 6.01 | 2.79 | 5.95 | 2.34 | |||||||
| Delay b | 7.03 | 2.35 | 5.88 | 3.34 | |||||||
| LH (IU/L) | Kaempferia parviflora 25 mg/day vs placebo | Baseline | 7.25 | 5.9 | 7.14 | 5.62 | No significant effects | ||||
| Single dose | 8.12 | 2.72 | 7.59 | 3.21 | |||||||
| 1 month | 7.04 | 5.34 | 7.3 | 4.24 | |||||||
| 2 months | 8.48 | 4.64 | 7.82 | 2.34 | |||||||
| Delay b | 8.72 | 4.67 | 8.14 | 3.23 | |||||||
| Kaempferia parviflora 90 mg/day vs placebo | Baseline | 6.99 | 4.37 | 7.14 | 5.62 | No significant effects | |||||
| Single dose | 7.65 | 1.88 | 7.59 | 3.21 | |||||||
| 1 month | 8.55 | 3.64 | 7.3 | 4.24 | |||||||
| 2 months | 7.41 | 4.67 | 7.82 | 2.34 | |||||||
| Delay b | 8.82 | 4.44 | 8.14 | 3.23 | |||||||
Footnote: a Post: immediately after exercise; b Delay: 1 month after the cessation of Kaempferia parviflora administration.
Abbreviations: VO2 = oxygen consumption; ROM = range of motion; high-BAT = high brown adipose tissue; low-BAT = low brown adipose tissue; FSH = follicle-stimulating hormone; LH = luteinizing hormone.
Kaempferia parviflora on physical or exercise performance
To determine the effects of Kaempferia parviflora on physical or exercise performance, many tests were used, including maximum power output, mean power output, time to exhaustion, rating of perceived exertion, percentage fatigue, heart rate, lactate threshold, hand grip strength, 6-minute walk test, and so on (see Table 1). The main findings from 2 studies 46, 47 indicated that Kaempferia parviflora showed no acute improvement in either repeated sprint performance or endurance exercise. However, other 2 studies 34, 38 provided data of hand grip strength test, which determined the upper-body muscle strength by using a digital dynamometer. It was found that the Kaempferia parviflora 90 to 180 mg/day group significantly increased hand grip strengths of both right-hand and left-hand sides at 2 months compared with the placebo group 2. This might be explained by the increased blood flow effect of Kaempferia parviflora 48. A previous study demonstrated that Kaempferia parviflora supplementation could increase blood flow to the organs due to vasorelaxation induction 49. This partly was mediated through cyclooxygenase and nitric oxide–dependent pathways 49 and Kaempferia parviflora also showed anti-inflammatory effects 50. Therefore, the combination effect of increased blood flow and anti-inflammatory effects may facilitate muscle strength 34.
Kaempferia parviflora effect on erectile dysfunction
Only one randomized controlled trial compared Kaempferia parviflora with placebo in human subjects on erectile response 51. Subjects receiving Kaempferia parviflora 25 mg/day (n = 15), or 90 mg/day (n = 15), were compared with those receiving placebo (n = 15) for 8 weeks of study period. The study found that Kaempferia parviflora at a dose of 90 mg/day exhibited a significant enhancement in all parameters (ie, response latency time to visual erotic stimuli, size and length of penis both in flaccid and erectile states) after 1 and 2 months of treatment compared with placebo 51. In addition, after 1 month and 2 months, the Kaempferia parviflora group at a dose of 90 mg/day experienced a statistically significant increase in length and width of penis both in resting state and erection state compared with the placebo group. Kaempferia parviflora showed no effects on serum hormones (ie, follicle-stimulating hormone, luteinizing hormone) see Table 1. The authors explained that the effects involved nitric oxide (NO). The experimental studies reported that Kaempferia parviflora extract could induce an increase of endothelial nitric oxide synthase and protein expression in human umbilical vein endothelial cell 52. Thus, abundance of endothelial nitric oxide synthase in endothelium of penile vasculature and sinusoidal endothelium within the corpora carvernosa might increase penile erection 53.
Kaempferia parviflora effects on pain
A study by Chalee 54 compared Kaempferia parviflora cream with analgesic cream on pain reduction in knee osteoarthritis. Pain severity, circumference of knee joint, range of motion of knee joint, and modified Western Ontario and McMaster Universities Arthritis Index score were assessed and compared within the group from baseline, or between the groups with analgesic cream. Comparing with baseline, the results in both groups showed significantly reduce pain in all indicators at 4 weeks. On the contrary, comparing with analgesic cream, Kaempferia parviflora cream showed no significant difference (Table 1). Since anti-inflammatory effect was studied only using oral administration of Kaempferia parviflora 55, the mechanism of Kaempferia parviflora cream on pain reduction was still unclear.
Kaempferia parviflora effects on energy expenditure
The effect of Kaempferia parviflora on energy expenditure has been linked to the activity of brown adipose tissue, a site of nonshivering thermogenesis 56. A previous study found that the components of Kaempferia parviflora extract activate hormone-sensitive lipase in adipocyte 57. Furthermore, 5,7-dimenthoxyflavone, the major flavonoid in Kaempferia parviflora extract, was demonstrated to have a the potent inhibitory effect on phosphodiesterase 5 (PDE5) enzyme 13. Since cGMP is a signal of hormone-sensitive lipase in adipocyte, it activates brown adipose tissue thermogenesis. Thus, it is possible that the brown adipose tissue–mediated thermogenic effect of Kaempferia parviflora extract exist via the inhibition of phosphodiesterase in brown adipose tissue. A study showed that Kaempferia parviflora extract could potentially increase whole-body energy expenditure probably through the activation of brown adipose tissue, which might benefit as an antiobesity treatment 58. A study by Matsushita et al 58 evaluated the effect of 2 doses of Kaempferia parviflora 100 mg/day or 180 mg/day on energy expenditure change compared with placebo. They found that when comparing with baseline, Kaempferia parviflora showed significant increase in energy expenditure at 30 minutes and 60 minutes in both groups. However, compared with placebo, no significant additional benefit of Kaempferia parviflora on energy expenditure was found (Table 1).
Kaempferia parviflora dosage
Till now, there is not enough scientific evidence to elucidate the optimal Kaempferia parviflora dose. Recommendation from Thai traditional medicine institute suggests the daily dose of Kaempferia parviflora is 1.2g. In addition, the powder of Kaempferia parviflora extract has been developed as a food ingredient on the market, which is standardized for containing not less than 2.5% of 5,7-dimethoxyflavone (Compound-6) and 10% of total methoxyflavones 1.
Fitnox, a sports nutritional supplement, is a unique blend of Kaempferia parviflora methoxyflavones, pomegranate peel polyphenols, and Moringa oleifera leaf saponins 29. Subchronic toxicological study shows that administration of Fitnox (at the dose of 1000 mg/kg/day for 90 days) to rats exhibits no any drug-related toxicity or mortality in either sex and no significant changes between the control and Fitnox treated groups in all parameters at the hematological, biochemistry, and histological levels 59. Another study for evaluating the toxicology of the ethanol Kaempferia parviflora extract (5, 50, and 500 mg/kg/day for 6 months) demonstrates no notable histological changes in all groups. The hematological parameters are also within the normal range in both sexes. But the body weight and the triglyceride levels at the 500 mg/kg dose rats group are lower, and the glucose and cholesterol levels are higher 60.
Kaempferia parviflora side effects
Administration with 1.35g of Kaempferia parviflora daily does not produce any adverse effects 61. Acute and chronic toxicity study has been proven that oral administration of Kaempferia parviflora does not induce any abnormal changes in body weight and histology in various visceral organs 62, 60. Toxicological study exhibits that the ethanol Kaempferia parviflora extract (at the doses of 60, 120, and 240 mg/kg for 60 days) does not induce significant changes in hemoglobin, white blood cells, or differential cell count. No any negative effects on renal and hepatic functions have been found at the tested doses 63.
An animal study of Kaempferia parviflora extract on chronic toxicity was conducted 64. They randomly divided 120 Wistar rats into 5 groups, 24 rats each (12 males and 12 females). Then, 3 treatment groups were orally administered with Kaempferia parviflora extract at doses of 5, 50, and 500 mg/kg/day for 6 months, respectively, which were equivalent to 1, 10, and 100 times that of human use, while 2 control groups were orally given distilled water and 1.0% tragacanth, respectively 64. The results showed that the histopathological study of visceral organs revealed no remarkable lesions related to the toxicity of Kaempferia parviflora extract 64. There has only been one reported case of anaphylaxis caused by black ginger in a dietary supplement 65.
- Toda, K., Hitoe, S., Takeda, S., & Shimoda, H. (2016). Black ginger extract increases physical fitness performance and muscular endurance by improving inflammation and energy metabolism. Heliyon, 2(5), e00115. https://doi.org/10.1016/j.heliyon.2016.e00115[↩][↩][↩]
- Saokaew, S., Wilairat, P., Raktanyakan, P., Dilokthornsakul, P., Dhippayom, T., Kongkaew, C., Sruamsiri, R., Chuthaputti, A., & Chaiyakunapruk, N. (2017). Clinical Effects of Krachaidum ( Kaempferia parviflora): A Systematic Review. Journal of evidence-based complementary & alternative medicine, 22(3), 413–428. https://doi.org/10.1177/2156587216669628[↩][↩]
- Kobayashi, S.; Kato, T.; Azuma, T.; Kikuzaki, H.; Abe, K. Anti-allergenic activity of polymethoxyflavones from Kaempferia parviflora. J. Funct. Foods 2015, 13, 100–107.[↩]
- Yenjai, C.; Prasanphen, K.; Daodee, S.; Wongpanich, V.; Kittakoop, P. Bioactive flavonoids from Kaempferia parviflora. Fitoterapia 2004, 75, 89–92.[↩][↩]
- Rujjanawate, C.; Kanjanapothi, D.; Amornlerdpison, D.; Pojanagaroon, S. Anti-gastric ulcer effect of Kaempferia parviflora. J. Ethnopharmacol. 2005, 102, 120–122.[↩]
- Akase, T.; Shimada, T.; Terabayashi, S.; Ikeya, Y.; Sanada, H.; Aburada, M. Antiobesity effects of Kaempferia parviflora in spontaneously obese type II diabetic mice. J. Nat. Med. 2011, 65, 73–80.[↩]
- Sutthanut K, Sripanidkulchai B, Yenjai C, Jay M. Simultaneous identification and quantitation of 11 flavonoid constituents in Kaempferia parviflora by gas chromatography. J Chromatogr A. 2007 Mar 2;1143(1-2):227-33. doi: 10.1016/j.chroma.2007.01.033[↩]
- Ochiai, W.; Kobayashi, H.; Kitaoka, S.; Kashiwada, M.; Koyama, Y.; Nakaishi, S.; Nagai, T.; Aburada, M.; Sugiyama, K. Effect of the active ingredient of Kaempferia parviflora, 5, 7-dimethoxyflavone, on the pharmacokinetics of midazolam. J. Nat. Med. 2018, 72, 607–614.[↩]
- Chaipech, S.; Morikawa, T.; Ninomiya, K.; Yoshikawa, M.; Pongpiriyadacha, Y.; Hayakawa, T.; Muraoka, O. Structures of two new phenolic glycosides, kaempferiaosides A and B, and hepatoprotective constituents from the rhizomes of Kaempferia parviflora. Chem. Pharm. Bull. 2012, 60, 62–69.[↩]
- Pripdeevech, P.; Pitija, K.; Rujjanawate, C.; Pojanagaroon, S.; Kittakoop, P.; Wongpornchai, S. Adaptogenic-active components from Kaempferia parviflora rhizomes. Food Chem. 2012, 132, 1150–1155.[↩]
- Thao, N.P.; Luyen, B.T.T.; Lee, S.H.; Jang, H.D.; Kim, Y.H. Anti-osteoporotic and antioxidant activities by rhizomes of Kaempferia parviflora wall. Ex Baker. Nat. Prod. Sci. 2016, 22, 13–19.[↩]
- Malakul W, Ingkaninan K, Sawasdee P, Woodman OL. The ethanolic extract of Kaempferia parviflora reduces ischaemic injury in rat isolated hearts. J Ethnopharmacol. 2011;137:184–191.[↩]
- Temkitthawon P, Hinds TR, Beavo JA, et al. Kaempferia parviflora, a plant used in traditional medicine to enhance sexual performance contains large amounts of low affinity PDE5 inhibitors. J Ethnopharmacol. 2011;137:1437–1441.[↩][↩]
- Sawasdee, P.; Sabphon, C.; Sitthiwongwanit, D.; Kokpol, U. Anticholinesterase activity of 7-methoxyflavones isolated from Kaempferia parviflora. Phytother. Res. 2009, 23, 1792–1794.[↩]
- Tewtrakul, S.; Subhadhirasakul, S.; Karalai, C.; Ponglimanont, C.; Cheenpracha, S. Anti-inflammatory effects of compounds from Kaempferia parviflora and Boesenbergia pandurata. Food Chem. 2009, 115, 534–538.[↩]
- Yoshino, S.; Awa, R.; Miyake, Y.; Fukuhara, I.; Sato, H.; Ashino, T.; Tomita, S.; Kuwahara, H. Daily intake of Kaempferia parviflora extract decreases abdominal fat in overweight and preobese subjects: A randomized, double-blind, placebo-controlled clinical study. Diabetes Metab. Syndr. Obes. 2018, 11, 447–458.[↩]
- Azuma, T.; Kayano, S.-I.; Matsumura, Y.; Konishi, Y.; Tanaka, Y.; Kikuzaki, H. Antimutagenic and α-glucosidase inhibitory effects of constituents from Kaempferia parviflora. Food Chem. 2011, 125, 471–475.[↩]
- Spencer JP. Flavonoids and brain health: multiple effects underpinned by common mechanisms. Genes Nutr. 2009 Dec;4(4):243-50. doi: 10.1007/s12263-009-0136-3[↩]
- Wattanathorn J, Muchimapura S, Tong-Un T, Saenghong N, Thukhum-Mee W, Sripanidkulchai B. Positive Modulation Effect of 8-Week Consumption of Kaempferia parviflora on Health-Related Physical Fitness and Oxidative Status in Healthy Elderly Volunteers. Evid Based Complement Alternat Med. 2012;2012:732816. doi: 10.1155/2012/732816[↩]
- Rujjanawate C, Kanjanapothi D, Amornlerdpison D, Pojanagaroon S. Anti-gastric ulcer effect of Kaempferia parviflora. J Ethnopharmacol. 2005 Oct 31;102(1):120-2. doi: 10.1016/j.jep.2005.03.035[↩]
- Paramee, S., Sookkhee, S., Sakonwasun, C., Na Takuathung, M., Mungkornasawakul, P., Nimlamool, W., & Potikanond, S. (2018). Anti-cancer effects of Kaempferia parviflora on ovarian cancer SKOV3 cells. BMC complementary and alternative medicine, 18(1), 178. https://doi.org/10.1186/s12906-018-2241-6[↩]
- Jansakul C., Tachanaparuksa K., Mulvany M. J., Sukpondma Y. Relaxant mechanisms of 3, 5, 7, 3′, 4′-pentamethoxyflavone on isolated human cavernosum. European Journal of Pharmacology. 2012;691(1-3):235–244. doi: 10.1016/j.ejphar.2012.07.019[↩][↩]
- Plaingam W., Sangsuthum S., Angkhasirisap W., Tencomnao T. Kaempferia parviflora rhizome extract and Myristica fragrans volatile oil increase the levels of monoamine neurotransmitters and impact the proteomic profiles in the rat hippocampus: Mechanistic insights into their neuroprotective effects. Journal of Traditional and Complementary Medicine. 2017;7(4):538–552. doi: 10.1016/j.jtcme.2017.01.002[↩]
- Tewtrakul S, Subhadhirasakul S, Karalai C, Ponglimanont C, Cheenpracha S. Anti-inflammatory effects of compounds from Kaempferia parviflora and Boesenbergia pandurata. Food Chem. 2009;115:534–538.[↩]
- Chuthaputti A. Krachai Dam: a champion herbal product. J Thai Traditional Alter Med. 2013;11:4–16.[↩]
- Temkitthawon P, Hinds TR, Beavo JA, Viyoch J, Suwanborirux K, Pongamornkul W, Sawasdee P, Ingkaninan K. Kaempferia parviflora, a plant used in traditional medicine to enhance sexual performance contains large amounts of low affinity PDE5 inhibitors. J Ethnopharmacol. 2011 Oct 11;137(3):1437-41. doi: 10.1016/j.jep.2011.08.025[↩]
- Yenjai C, Prasanphen K, Daodee S, Wongpanich V, Kittakoop P. Bioactive flavonoids from Kaempferia parviflora. Fitoterapia. 2004 Jan;75(1):89-92. doi: 10.1016/j.fitote.2003.08.017[↩]
- Sawasdee P, Sabphon C, Sitthiwongwanit D, Kokpol U. Anticholinesterase activity of 7-methoxyflavones isolated from Kaempferia parviflora. Phytother Res. 2009 Dec;23(12):1792-4. doi: 10.1002/ptr.2858[↩]
- Chen, D., Li, H., Li, W., Feng, S., & Deng, D. (2018). Kaempferia parviflora and Its Methoxyflavones: Chemistry and Biological Activities. Evidence-based complementary and alternative medicine : eCAM, 2018, 4057456. https://doi.org/10.1155/2018/4057456[↩][↩]
- Lee, J. H., Ahn, N. H., Choi, S. B., Kwon, Y., & Yang, S. H. (2021). Natural Products Targeting Amyloid Beta in Alzheimer’s Disease. International journal of molecular sciences, 22(5), 2341. https://doi.org/10.3390/ijms22052341[↩]
- Saokaew S., Wilairat P., Raktanyakan P., et al. Clinical Effects of Krachaidum (Kaempferia parviflora): A Systematic Review. Evidence-Based Complementary and Alternative Medicine. 2017;22(3):413–428. doi: 10.1177/2156587216669628[↩]
- Shimada T., Horikawa T., Ikeya Y., et al. Preventive effect of Kaempferia parviflora ethyl acetate extract and its major components polymethoxyflavonoid on metabolic diseases. Fitoterapia. 2011;82(8):1272–1278. doi: 10.1016/j.fitote.2011.08.018[↩]
- Matsushita M., Yoneshiro T., Aita S., et al. Kaempferia parviflora extract increases whole-body energy expenditure in humans: Roles of brown adipose tissue. Journal of Nutritional Science and Vitaminology. 2015;61(1):79–83. doi: 10.3177/jnsv.61.79[↩]
- Wattanathorn, J., Muchimapura, S., Tong-Un, T., Saenghong, N., Thukhum-Mee, W., & Sripanidkulchai, B. (2012). Positive Modulation Effect of 8-Week Consumption of Kaempferia parviflora on Health-Related Physical Fitness and Oxidative Status in Healthy Elderly Volunteers. Evidence-based complementary and alternative medicine : eCAM, 2012, 732816. https://doi.org/10.1155/2012/732816[↩][↩][↩]
- Jacob J., Gopi S., Divya C. A Randomized Single Dose Parallel Study on Enhancement of Nitric Oxide in Serum and Saliva with the Use of Natural Sports Supplement in Healthy Adults. Journal of Dietary Supplements. 2018;15(2):161–172. doi: 10.1080/19390211.2017.1331944[↩]
- Toda K. Enhancement of physical fitness by black ginger extract rich in polymethoxyflavones: a double-blind randomized crossover trial. Integrative Molecular Medicine. 2016;3(2):628–634. doi: 10.15761/IMM.1000215[↩]
- Wasuntarawat C., Pengnet S., Walaikavinan N., et al. No effect of acute ingestion of Thai ginseng (Kaempferia parviflora) on sprint and endurance exercise performance in humans. Journal of Sports Sciences. 2010;28(11):1243–1250. doi: 10.1080/02640414.2010.506221[↩]
- Promthep, K., Eungpinichpong, W., Sripanidkulchai, B., & Chatchawan, U. (2015). Effect of Kaempferia parviflora Extract on Physical Fitness of Soccer Players: A Randomized Double-Blind Placebo-Controlled Trial. Medical science monitor basic research, 21, 100–108. https://doi.org/10.12659/MSMBR.894301[↩][↩]
- Murata K, Deguchi T, Fujita T, Matsuda H. Improvement in blood fluidity by Kaempferia parviflora rhizome. J Nat Med. 2013 Oct;67(4):719-24. doi: 10.1007/s11418-012-0729-9[↩]
- Weerateerangkul P., Surinkaew S., Chattipakorn S. C., Chattipakorn N. Effects of Kaempferia parviflora Wall. Ex. baker on electrophysiology of the swine hearts. Indian Journal of Medical Research. 2013;137(1):156–163.[↩]
- Weerateerangkul P., Palee S., Chinda K., Chattipakorn S. C., Chattipakorn N. Effects of Kaempferia parviflora Wall. Ex. baker and sildenafil citrate on cGMP level, cardiac function, and intracellular Ca2+ regulation in rat hearts. Journal of Cardiovascular Pharmacology. 2012;60(3):299–309. doi: 10.1097/FJC.0b013e3182609a52[↩]
- Tep-Areenan P, Sawasdee P, Randall M. Possible mechanisms of vasorelaxation for 5,7-dimethoxyflavone from Kaempferia parviflora in the rat aorta. Phytother Res. 2010 Oct;24(10):1520-5. doi: 10.1002/ptr.3164[↩]
- Chaturapanich G., Chaiyakul S., Verawatnapakul V., Yimlamai T., Pholpramool C. Enhancement of aphrodisiac activity in male rats by ethanol extract of Kaempferia parviflora and exercise training. Andrologia. 2012;44(supplement 1):323–328. doi: 10.1111/j.1439-0272.2011.01184.x[↩]
- Chaturapanich G., Chaiyakul S., Verawatnapakul V., Pholpramool C. Effects of Kaempferia parviflora extracts on reproductive parameters and spermatic blood flow in male rats. Reproduction. 2008;136(4):515–522. doi: 10.1530/REP-08-0069[↩]
- Wannanon P., Wattanathorn J., Tong-Un T., et al. Efficacy assessment of Kaempferia Parviflora for the management of Erectile Dysfunction. Online Journal of Biological Sciences. 2012;12(4):149–155. doi: 10.3844/ojbsci.2012.149.155[↩]
- Deema P. Effect of Kaempferia parviflora and Endurance Training on Lactate Threshold in Humans. Phitsanulok, Thailand: Naresuan University; 2007.[↩]
- Wasuntarawat C, Pengnet S, Walaikavinan N, et al. No effect of acute ingestion of Thai ginseng (Kaempferia parviflora) on sprint and endurance exercise performance in humans. J Sports Sci. 2010;28:1243–1250.[↩]
- Chaturapanich G, Chaiyakul S, Verawatnapakul V, Pholpramool C. Effects of Kaempferia parviflora extracts on reproductive parameters and spermatic blood flow in male rats. Reproduction. 2008 Oct;136(4):515-22. doi: 10.1530/REP-08-0069[↩]
- Tep-Areenan P, Ingkaninan K, Randall MD. Mechanisms of Kaempferia parviflora extract (KPE)-induced vasorelaxation in the rat aorta. Asian Biomed. 2010;4:103–111.[↩][↩]
- Sae-Wong C, Matsuda H, Tewtrakul S, et al. Suppressive effects of methoxyflavonoids isolated from Kaempferia parviflora on inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells. J Ethnopharmacol. 2011;136:488–495.[↩]
- Wannanon P, Wattanathorn J, Tong-Un T, et al. Efficacy assessment of Kaempferia parviflora for the management of erectile dysfunction. Online J Biol Sci. 2012;12:149–155.[↩][↩]
- Guohua H, Yanhua L, Rengang M, Dongzhi W, Zhengzhi M, Hua Z. Aphrodisiac properties of Allium tuberosum seeds extract. J Ethnopharmacol. 2009;122:579–582.[↩]
- Tajuddin Ahmad S, Latif A, Qasmi IA, Amin KM. An experimental study of sexual function improving effect of Myristica fragrans Houtt. (nutmeg). BMC Complement Altern Med. 2005;5:16.[↩]
- Chalee S. Study of Efficacy and Anti-Inflammatory Effect of K. parviflora Extract Cream in Osteoarthritis of Knee. Khon Kaen, Thailand: Khon Kaen University; 2010.[↩]
- Horigome S, Yoshida I, Ito S, et al. Inhibitory effects of Kaempferia parviflora extract on monocyte adhesion and cellular reactive oxygen species production in human umbilical vein endothelial cells [published online December 24, 2015]. Eur J Nutr. doi:10.1007/s00394-015-1141-5[↩]
- Yoshino S, Kim M, Awa R, Kuwahara H, Kano Y, Kawada T. Kaempferia parviflora extract increases energy consumption through activation of BAT in mice. Food Sci Nutr. 2014;2:634–637.[↩]
- Okabe Y, Shimada T, Horikawa T, et al. Suppression of adipocyte hypertrophy by polymethoxyflavonoids isolated from Kaempferia parviflora. Phytomedicine. 2014;21:800–806.[↩]
- Matsushita M, Yoneshiro T, Aita S, Kamiya T, Kusaba N, Yamaguchi K, Takagaki K, Kameya T, Sugie H, Saito M. Kaempferia parviflora extract increases whole-body energy expenditure in humans: roles of brown adipose tissue. J Nutr Sci Vitaminol (Tokyo). 2015;61(1):79-83. doi: 10.3177/jnsv.61.79[↩][↩]
- Jacob J., Amalraj A., Divya C., Janadri S., Manjunatha P., Gopi S. Oral toxicity study of sports nutritional powder in Wistar rats: A 90 day repeated dose study. Toxicology Reports. 2018;5:497–503. doi: 10.1016/j.toxrep.2018.04.001[↩]
- Chivapat S., Chavalittumrong P., Attawish A., Rungsipipat A. Chronic toxicity study of Kaempferia parviflora wall ex. Extract. Thai Journal of Veterinary Medicine. 2010;40(4):377–383.[↩][↩]
- Saokaew S, Wilairat P, Raktanyakan P, Dilokthornsakul P, Dhippayom T, Kongkaew C, Sruamsiri R, Chuthaputti A, Chaiyakunapruk N. Clinical Effects of Krachaidum ( Kaempferia parviflora): A Systematic Review. J Evid Based Complementary Altern Med. 2017 Jul;22(3):413-428. doi: 10.1177/2156587216669628[↩]
- Zhang M., Pan D. R., Zhou F. BP neural network extraction process by orthogonal beautiful azalea flavonoids. Journal of Xinyang Normal University. 2011;2:261–264.[↩]
- Sudwan P., Saenphet K., Saenphet S., Suwansirikul S. Effect of Kaempferia parviflora Wall. ex. Baker on sexual activity of male rats and its toxicity. The Southeast Asian Journal of Tropical Medicine and Public Health. 2006;37:210–215.[↩]
- Chivapat S, Chavalittumrong P, Attawish A, Rungsipipat A. Chronic toxicity study of Kaempferia parviflora Wall ex. extract. Thai J Vet Med. 2010;40:377–383.[↩][↩][↩]
- Hayashi, E., Sowa‐Osako, J., Fukai, K., Natsumi, A., Yagami, A., Sato, N., Shimojo, N., Nakamura, M., Matsunaga, K. and Tsuruta, D. (2019), Case of anaphylaxis caused by black ginger in a dietary supplement. J Dermatol, 46: e56-e57. https://doi.org/10.1111/1346-8138.14592[↩]