Blood viscosity
Blood viscosity is the thickness or stickiness of blood. Viscosity is formally defined as the measurement of the internal resistance of a fluid to flow but can simply be thought of as the “thickness” or “stickiness” of a fluid. When blood has low viscosity, it travels quickly and without much difficulty. Viscous blood are thicker and travel more slowly. Viscosity is measured in the unit of centipoise (cp). The viscosity of water is 1 cp. Normal serum viscosity relative to water is 1.4 to 1.8 cp 1. Whole blood viscosity is the sum of plasma viscosity (mainly determined by colloid components such as albumin) plus the density and packing of all blood cells and their rigidity (see Figure 1) 2. Blood viscosity is determined by plasma viscosity, hematocrit (volume fraction of red blood cell, which constitute 99.9% of the cellular elements), red blood cell deformability, mechanical properties of red blood cells, red blood cell aggregation, and plasma viscosity.
Although plasma is mostly water, it also contains other molecules such as electrolytes, proteins (especially albumin and fibrinogen), and other macromolecules. Because of molecular interactions between these different components of plasma, it is not surprising that plasma has a higher viscosity than water. In fact, plasma at 37°C is about 1.8-times more viscous than water at the same temperature; therefore, the relative viscosity (ηr) of plasma compared to water is about 1.8 3.
The addition of formed elements to plasma (red cells, white cells, and platelets) further increases the viscosity. Of these formed elements, red cells have the greatest effect on viscosity. In the figure, the relative viscosity at 0% hematocrit (plasma without cells) is about 1.8 as shown by the y-intercept. Increasing red cell hematocrit increases relative viscosity. Note that the increase is non-linear; increased hematocrit causes a disproportionate increase in relative viscosity. Therefore, blood viscosity strongly depends on hematocrit. At a normal hematocrit of 40%, the relative viscosity of blood is about 4. Patients with an abnormal elevation in red cell hematocrit (polycythemia) have much higher blood viscosities. In fact, increasing the hematocrit from 40 to 60% (a 50% increase) increases the relative viscosity from 4 to 8 (a 100% increase). Increased viscosity increases the resistance to blood flow and thereby increases the work of the heart and impairs organ perfusion. Some patients with anemia have low hematocrits, and therefore reduced blood viscosities.
Another important factor that influences blood viscosity is temperature. Just like molasses, when blood gets cold, it becomes “thicker” and flows more slowly. Therefore, there is an inverse relationship between temperature and viscosity. Viscosity increases about 2% for each degree centigrade decrease in temperature. Normally, blood temperature does not change much in the body. However, if a person’s hand is exposed to a cold environment and the fingers become cold, the blood temperature in the fingers falls and viscosity increases, which together with sympathetic-mediated vasoconstriction decreases blood flow in the cooled region. When whole body hypothermia is induced in critical care or surgical situations, this leads to an increase in blood viscosity and impaired organ blood flow.
If clotting mechanisms are stimulated in the blood, platelet aggregation and interactions with plasma proteins occur. This leads to entrapment of red cells and clot formation, which dramatically increases blood viscosity.
Red blood cells have unique mechanical behavior, which can be discussed under the terms erythrocyte deformability and erythrocyte aggregation 4. Plasma’s viscosity is determined by water-content and macromolecular components, so these factors that affect blood viscosity are the plasma protein concentration and types of proteins in the plasma 5. Nevertheless, hematocrit has the strongest impact on whole blood viscosity. One unit increase in hematocrit can cause up to a 4% increase in blood viscosity 4. This relationship becomes increasingly sensitive as hematocrit increases. When the hematocrit rises to 60 or 70%, which it often does in polycythemia rubra vera 6, the blood viscosity can become as great as 10 times that of water, and its flow through blood vessels is greatly retarded because of increased resistance to flow 2. This will lead to decreased oxygen delivery 7. Other factors influencing blood viscosity include temperature, where an increase in temperature results in a decrease in viscosity. This is particularly important in hypothermia, where an increase in blood viscosity will cause problems with blood circulation. Because of that, blood behaves as a non-Newtonian fluid. Water behaves as a Newtonian fluid and therefore under non-turbulent conditions, its viscosity is independent of flow velocity (i.e., viscosity does not change with changes in velocity) (Figure 2). As such, the viscosity of blood varies with shear rate. Normally, shear stress-independent plasma viscosity contributes only to a tenth or less to whole blood viscosity 8. However, plasma viscosity is more important for the regulation of tissue perfusion than blood viscosity 9. Blood becomes less viscous at high shear rates like those experienced with increased flow such as during exercise or in peak-systole. Therefore, blood is a shear-thinning fluid. Contrarily, blood viscosity increases when shear rate goes down with increased vessel diameters or with low flow, such as downstream from an obstruction or in diastole. Blood viscosity also increases with increases in red cell aggregability.
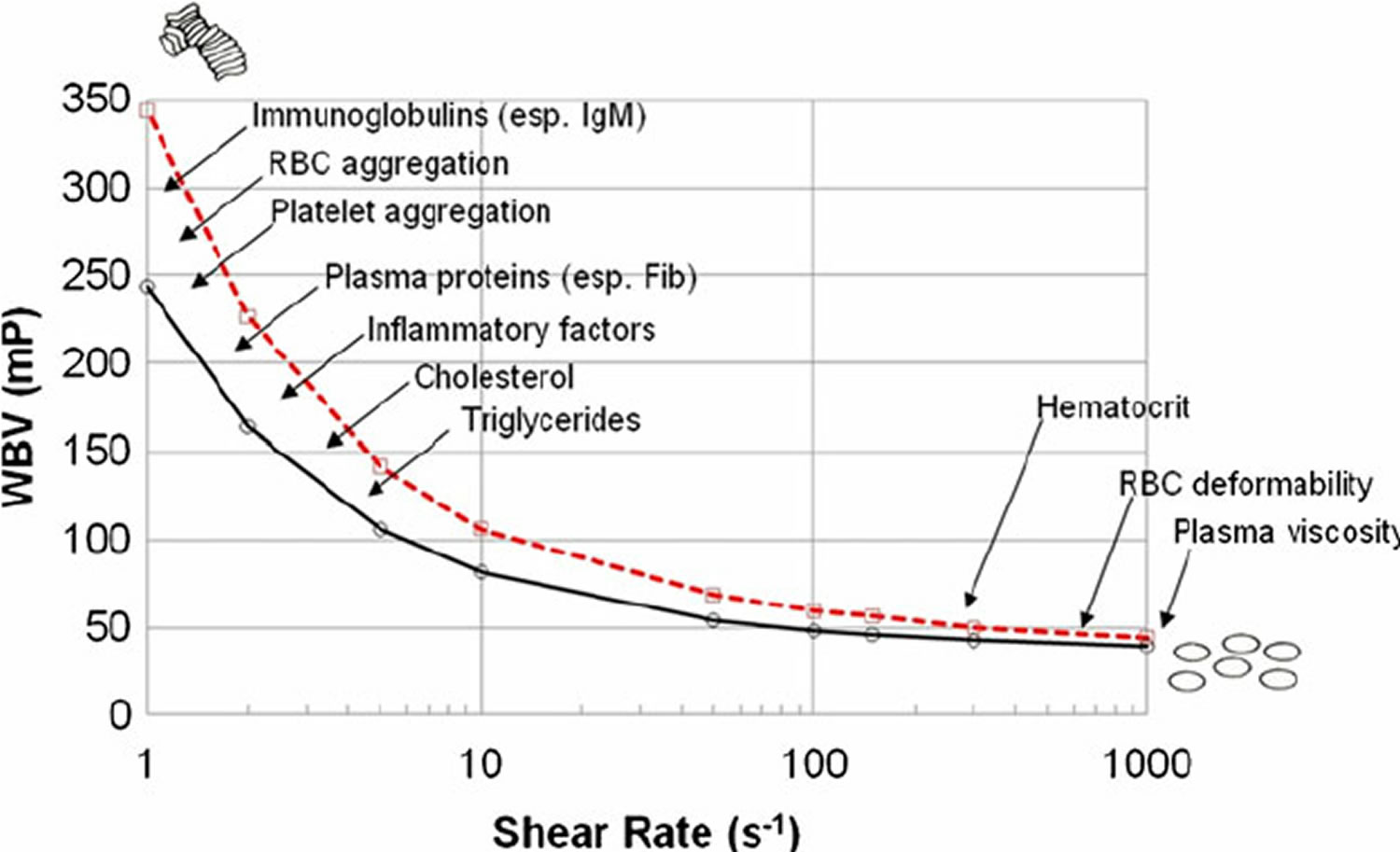
Figure 1. Whole blood viscosity
Footnote: Various determinants of whole blood viscosity. Blood viscosity curves for 2 apparently healthy males hematocrit (Hct) 45
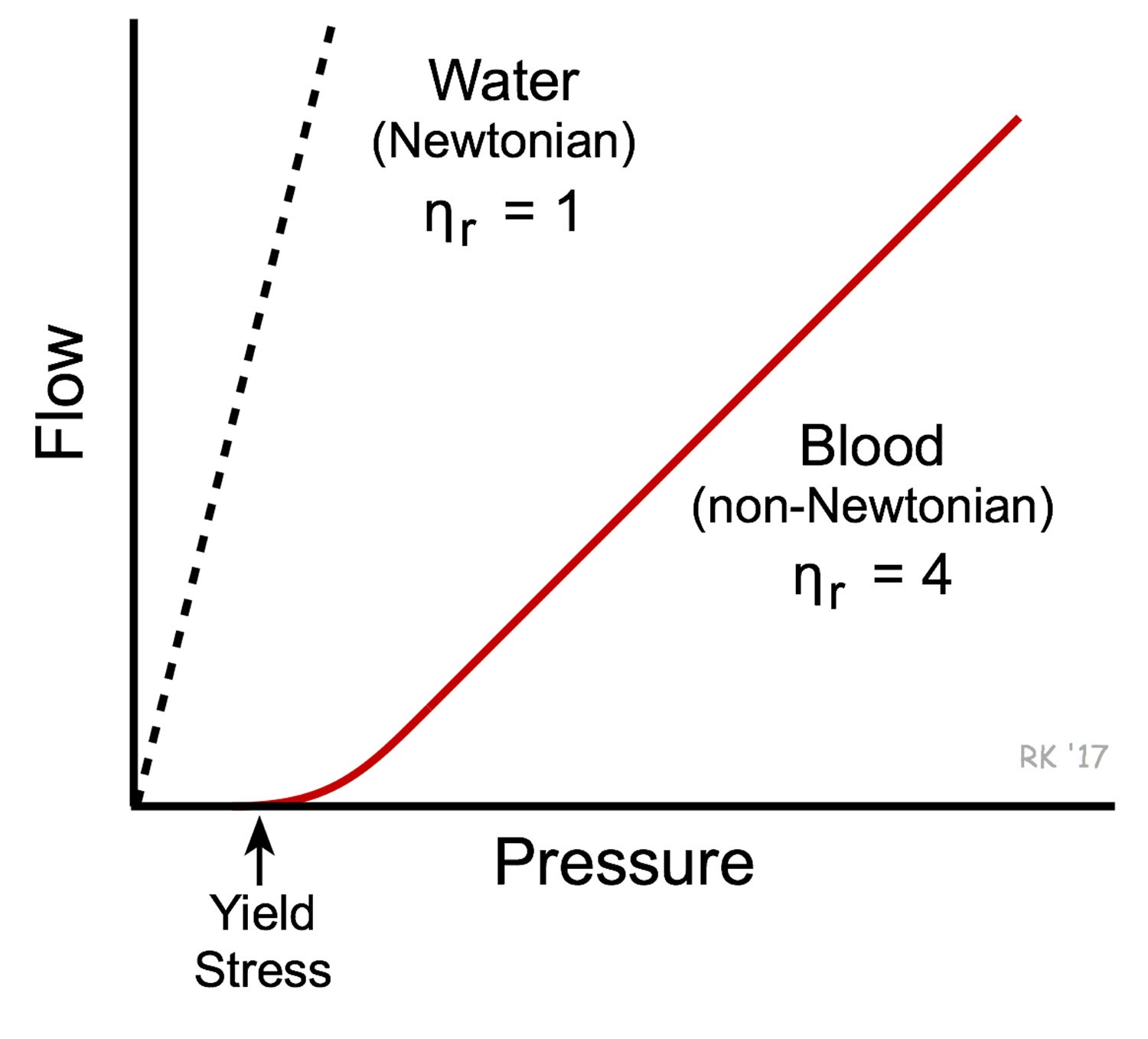
[Source 10 ]Figure 2. Blood versus water viscosity
Footnote: Water behaves as a Newtonian fluid and therefore under non-turbulent conditions, its viscosity is independent of flow velocity (i.e., viscosity does not change with changes in velocity). This is shown in the figure as a linear dashed line for the flow-pressure relationship of water. There is an inverse relationship between flow and viscosity; therefore, the greater the viscosity the smaller the slope of the flow-pressure relationship, meaning that at a given driving pressure flow will be reduced at higher viscosities. Whole blood has a much higher viscosity than water and therefore the slope of the flow-pressure relationship is less steep (see figure). Unlike water, blood is non-Newtonian because its viscosity increases at low flow velocities (e.g., during circulatory shock). Low flow states permit increased molecular interactions to occur between red cells and between plasma proteins and red cells. This can cause red cells to stick together and form chains of several cells (rouleau formation) within the microcirculation, which increases the blood viscosity. Because of the high degree of interaction between the elements of blood when it is not flowing, a driving pressure significantly greater than zero is required for stationary blood to start flowing again. This is referred to as the yield stress required to initiate flow.
[Source 3 ]High blood viscosity
Elevated blood viscosity is the result of either red blood cell shape deformity or a pathological increase in serum proteins, red blood cells (RBC), white blood cells (WBC), or platelets. Any pathologic elevation of the cellular components (erythrocytes, leukocytes, or platelets) or acellular components (protein) of blood can cause hyperviscosity.
Hyperviscosity syndrome is the pathological condition in which blood is “thicker” than normal and therefore flow is reduced. An increase in blood viscosity can be caused either by a deformity of the shape of red blood cells (RBCs) which causes red blood cell aggregation and decreased blood flow or by any pathological elevation of the components of blood. This includes red blood cell, white blood cell, platelets, or serum proteins.
This increase in viscosity causes sluggish blood flow, relative decreased microvascular circulation, and hypoperfusion of tissues. An increase in circulating proteins can also affect platelet aggregation and cause prolonged bleeding time. The severity of clinical symptoms is directly related to the increased levels of serum viscosity, with progressively more severe symptoms occurring as the individual patient’s serum viscosity increases. The level of viscosity at which symptoms can initially present is variable from person to person depending on the underlying physiology, but for a given patient, symptoms will typically manifest about the same level of viscosity over time.
Bleeding is the most common manifestation and typically arises from impaired platelet function resulting in oozing mucosal surfaces like epistaxis, bleeding gums, or gastrointestinal (GI) bleeding. Neurological findings can include a headache, neuropathic syndromes, generalized stupor, coma, dizziness, ataxia, hearing impairment, seizures, and stroke syndromes. These neurological manifestations are due to decreased blood flow to the central nervous system and deposition of paraproteins within the myelin sheath of peripheral nerves. Retinopathy and visual derangements such as blurred vision or double vision arise because of microvascular changes such as thrombosis or hemorrhage. The classic finding of “sausage link” or “boxcar” engorgement of retinal veins can be seen on the fundoscopic exam as well as papilledema, flame-shaped hemorrhages, or exudates. The eye examination is an important part of the physical exam because it can enable prompt diagnosis and treatment in the appropriate clinical setting. Less commonly seen are cardiopulmonary symptoms such as high output cardiac failure, shortness of breath, valvular dysfunction, or myocardial infarction. Hyperviscosity syndrome can also cause acute kidney injury, likely resulting from a relative hypoperfusion state.
Hyperviscosity syndrome is an oncologic emergency that classically presents with the triad of neurological deficits, visual changes, and mucosal bleeding 1. The most common cause of hyperviscosity syndrome is Waldenstrom macroglobulinemia and therefore, the term hyperviscosity syndrome is typically used to describe an increase in serum proteins.
High blood viscosity causes
Increased blood viscosity causes:
- Polycythemia rubra vera
- Thrombocytosis
- Dehydration
- Sickle cell disease
- Spherocytosis
- Multiple myeloma
- Waldenstrom macroglobulinemia
- Cryoglobulinemia
- Seropositive rheumatoid arthritis
- Systemic lupus erythematosus (SLE)
- Sjogren syndrome
- Castleman disease
- HIV infection
- Using recombinant versions of erythropoietin produced pharmacologically (e.g., epoetin, darbepoetin, and methoxy polyethylene glycol-epoetin beta) 11.
Conditions responsible for hyperviscosity syndrome that involve cellular components of blood include polycythemia vera, leukemia, and thrombocytosis. Sickle cell disease and spherocytosis can also contribute to hyperviscosity syndrome due to deformed red blood cells. The pathologic rise of acellular components can either be monoclonal or polyclonal. Monoclonal diseases include multiple myeloma, Waldenstrom macroglobulinemia, and cryoglobulinemia. Rheumatic conditions such as seropositive rheumatoid arthritis, systemic lupus erythematosus, and Sjogren syndrome compromise polyclonal causes of hyperviscosity syndrome as well as Castleman disease and HIV infection 12.
Hypergammaglobulinemia is the most common cause of hyperviscosity syndrome, specifically the monoclonal condition Waldenstrom macroglobulinemia. More than 30% of all Waldenstrom macroglobulinemia patients develop hyperviscosity syndrome at some point in their life because of the large star-shaped IgM pentamers that are highly viscous 1. Myelomas are the second leading cause of hyperviscosity syndrome. About 25% of hyperviscosity syndrome cases secondary to myelomas are caused by IgA, followed by IgG myelomas at less than 5% of cases.
Management of hyperviscosity syndrome consists of supportive care with intravenous fluids, plasmapheresis, and treatment of the underlying hematological condition 13.
High blood viscosity diagnosis
Laboratory evidence of high serum viscosity establishes the diagnosis. There is controversy over whether whole blood viscosity versus serum viscosity is superior, but most clinical laboratories measure the viscosity of the serum component of blood. Viscosity is measured in the unit of centipoise (cp). The viscosity of water is 1 cp. Normal serum viscosity relative to water is 1.4 to 1.8 cp. Symptoms of hyperviscosity can appear with a serum viscosity as low as 3 cp, but usually, arise when it exceeds 4 to 5 cp 14.
Further testing should include a complete blood count (CBC), full serum chemistries, coagulation profile, and urinalysis. An elevated albumin-protein gap along with significant proteinuria on routine urinalysis suggest an underlying gammopathy. Rouleaux formation on a peripheral blood smear is highly suggestive of serum stasis. Serum stasis can also lead to the malfunction of laboratory testing equipment in which lab samples cannot be analyzed. This should raise suspicion for an underlying increase in serum viscosity. Measuring quantitative immunoglobulins is not necessary to establish a diagnosis of hyperviscosity syndrome, but can help guide long-term treatment if measured before and after the intervention.
High blood viscosity treatment
Hyperviscosity syndrome is an oncological emergency, and timely treatment can prevent life-threatening complications such as thromboembolic events, myocardial infarction, and catastrophic ischemia that results in multiple organ failure. Therapy should be based on the severity of signs and symptoms rather than the calculated degree of viscosity. Most signs and symptoms are reversible with prompt treatment. Short-term management is directed at symptom control, whereas long-term management is directed at controlling the underlying hematologic condition. The mainstays of treatment include supportive therapy, plasma exchange or plasmapheresis, and chemotherapy. Dehydration can worsen hyperviscosity syndrome, and these patients are usually dehydrated. Therefore, judicious fluid administration is advised. It is considered common practice to empirically administer 1 to 2 L of normal saline when hyperviscosity syndrome is suspected. The more definitive short-term treatment is plasmapheresis. It can promptly reverse most clinical manifestations of hyperviscosity syndrome and is usually well tolerated and safe. Plasmapheresis can decrease serum viscosity by 20% to 30% and can be done on a daily basis until clinical resolution of symptoms. Patients can present with concurrent anemia or acquire dilutional anemia secondary to fluid resuscitation, and it is important to note that transfusion of packed red blood cells can increase blood viscosity. Therefore, one should wait until after plasmapheresis has reduced serum viscosity before transfusing 15.
If prompt plasmapheresis cannot be obtained, a temporizing measure that can be performed emergently is intravenous phlebotomy. This involves phlebotomizing about 1 to 2 units of the patient’s blood and concurrently replacing it with normal saline. However, this has to be performed with caution because aggressive plasma exchange can cause the elimination of clotting factors, albumin, and platelets. Phlebotomy should only be completed in the presence of severe neurological deficits like seizure or coma.
The definitive treatment of hyperviscosity syndrome involves chemotherapy for the underlying hematologic condition. Plasmapheresis does not affect the underlying disease, so chemotherapy is often started concomitantly. A hematology/oncology consultant should administer this, and it is strongly recommended to consult with this expert as soon as hyperviscosity syndrome is identified. Since exchange therapies such as plasmapheresis and leukapheresis are the mainstay of management, these patients may require transfer to a higher level of care facility.
Low blood viscosity
Blood viscosity can be reduced by anemia, thrombocytopenia or hemodilution due to plasma volume replacement.
- Perez Rogers A, Estes M. Hyperviscosity Syndrome. [Updated 2019 Feb 18]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK518963[↩][↩][↩]
- Lenz C, Rebel A, Waschke KF, Koehler RC, Frietsch T. Blood viscosity modulates tissue perfusion: sometimes and somewhere. Transfus Altern Transfus Med. 2008;9(4):265–272. doi:10.1111/j.1778-428X.2007.00080.x https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2519874[↩][↩]
- Viscosity of Blood. https://www.cvphysiology.com/Hemodynamics/H011[↩][↩]
- Baskurt OK, Meiselman HJ (2003). “Blood rheology and hemodynamics”. Seminars in Thrombosis and Haemostasis. 29 (5): 435–450. doi:10.1055/s-2003-44551[↩][↩]
- Plasma viscosity: a forgotten variable. Clin Hemorheol Microcirc. 2008;39(1-4):243-6. https://content.iospress.com/articles/clinical-hemorheology-and-microcirculation/ch1088[↩]
- A contemporary approach to the diagnosis and management of polycythemia vera. Curr Hematol Rep. 2003 May;2(3):237-41. https://www.ncbi.nlm.nih.gov/pubmed/12901345[↩]
- Kwon O, Krishnamoorthy M, Cho YI, Sankovic JM, Banerjee RK (February 2008). “Effect of blood viscosity on oxygen transport in residual stenosed artery following angioplasty”. J Biomech Eng. 130 (1): 011003. doi:10.1115/1.2838029[↩]
- Vogel J, Kiessling I, Heinicke K, et al. Transgenic mice overexpressing erythropoietin adapt to excessive erythrocytosis by regulating blood viscosity. Blood. 2003;102:2278–84.[↩]
- Tsai AG, Friesenecker B, McCarthy M, Sakai H, Intaglietta M. Plasma viscosity regulates capillary perfusion during extreme hemodilution in hamster skin-fold model. Am J Physiol. 1998;275(6 Pt 2):H2170–80.[↩]
- Cowan, Aimee & Cho, Daniel & Rosenson, Robert. (2012). Importance of Blood Rheology in the Pathophysiology of Atherothrombosis. Cardiovascular drugs and therapy / sponsored by the International Society of Cardiovascular Pharmacotherapy. 26. 339-48. 10.1007/s10557-012-6402-4. [↩]
- Jelkmann W. Erythropoietin. Front Horm Res. 2016;47:115-27.[↩]
- Higdon ML, Atkinson CJ, Lawrence KV. Oncologic Emergencies: Recognition and Initial Management. Am Fam Physician. 2018 Jun 01;97(11):741-748.[↩]
- Georgakopoulos CD, Plotas P, Angelakis A, Kagkelaris K, Tzouvara E, Makri OE. Dexamethasone implant for immunogammopathy maculopathy associated with IgA multiple myeloma. Ther Adv Ophthalmol. 2019 Jan-Dec;11:2515841418820441[↩]
- Castillo JJ, Garcia-Sanz R, Hatjiharissi E, Kyle RA, Leleu X, McMaster M, Merlini G, Minnema MC, Morra E, Owen RG, Poulain S, Stone MJ, Tam C, Varettoni M, Dimopoulos MA, Treon SP, Kastritis E. Recommendations for the diagnosis and initial evaluation of patients with Waldenström Macroglobulinaemia: A Task Force from the 8th International Workshop on Waldenström Macroglobulinaemia. Br. J. Haematol. 2016 Oct;175(1):77-86.[↩]
- Ballestri M, Ferrari F, Magistroni R, Mariano M, Ceccherelli GB, Milanti G, De Palma M, Albertazzi A. Plasma exchange in acute and chronic hyperviscosity syndrome: a rheological approach and guidelines study. Ann. Ist. Super. Sanita. 2007;43(2):171-5.[↩]