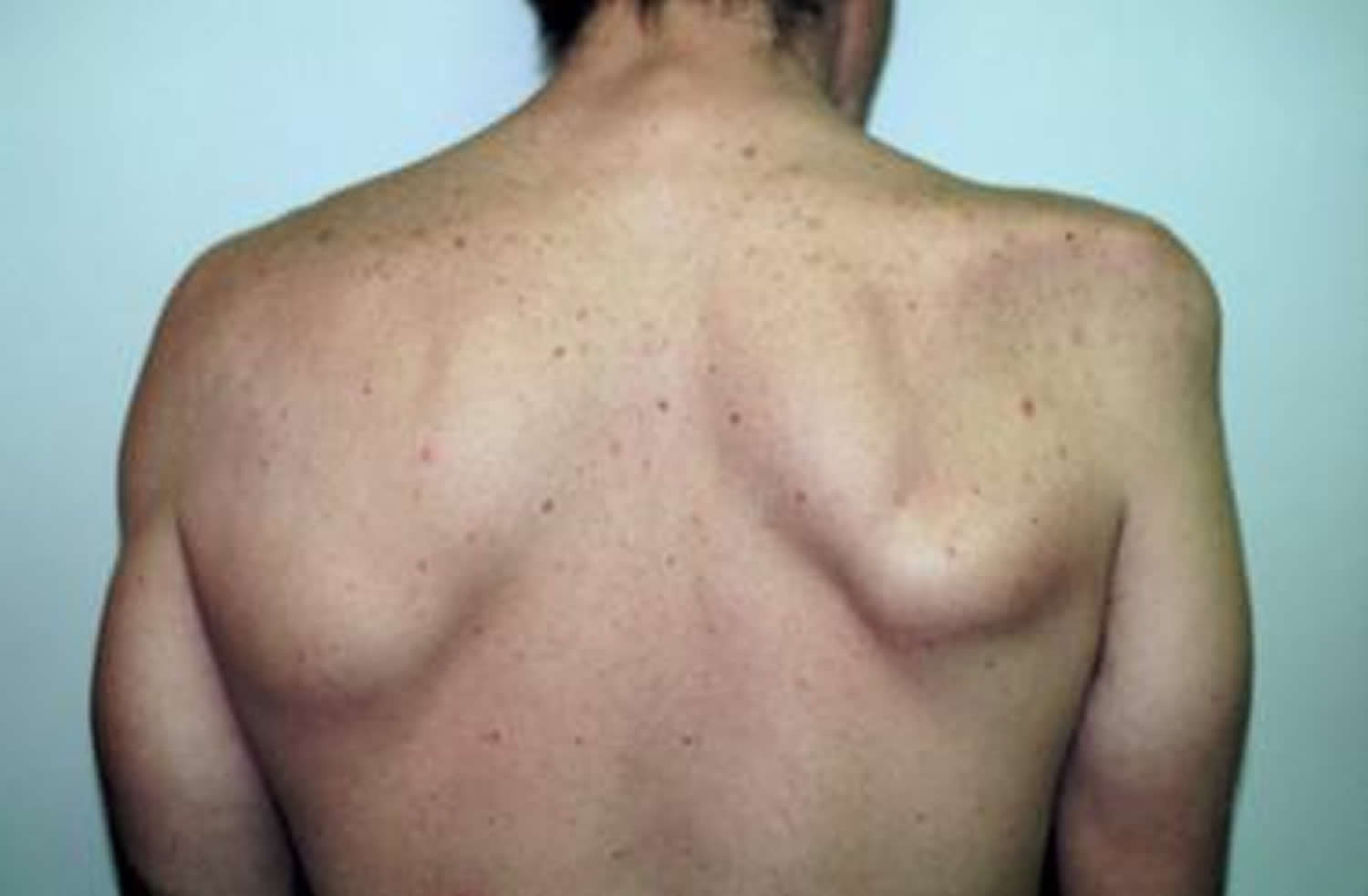
What is brachial neuritis
Idiopathic brachial neuritis also known as neuralgic amyotrophy, acute shoulder neuritis, acute brachial neuritis, idiopathic brachial plexus neuropathy or Parsonage-Turner syndrome, is a rare syndrome of unknown cause affecting mainly the lower motor neurons of the brachial plexus and/or individual nerves or nerve branches 1. Brachial neuritis has two major clinical symptoms: pain and muscle weakness from atrophy. Brachial neuritis usually is characterized by the acute onset of excruciating unilateral shoulder pain, followed by flaccid paralysis of shoulder and parascapular muscles several days later. Brachial neuritis can vary greatly in presentation and nerve involvement 2. Brachial neuritis is believed to be a multifocal, immune-mediated inflammatory process that involves the peripheral nerves. Most lesions are axonal, however those caused by demyelination usually carry a better prognosis. Motor axons are mainly affected. As a result, nerves that carry mostly motor fibers are affected to a larger degree and more commonly than mixed nerves and pure sensory nerves. Long thoracic, suprascapular, axillary, musculocutaneous nerves, anterior and posterior interosseous nerves are the most commonly affected according to a 2014 study by Ferrante et al 3. Milner 4 reports that up to 80% of patients have a unilateral onset, and 60% involves the dominant side while only 20% had a bilateral extremities onset.
Brachial neuritis could have a very wide range of causes. More common causes that have been reported in the literature include infection, whether bacterial, parasitic or viral; Coxsackie B virus; mumps; variola major and minor; HIV; and parvovirus B19 5. Other conditions that predispose patients to develop brachial neuritis are exposure to surgery, anesthesia, hereditary factors, rheumatic diseases such as connective tissue disorders (i.e., Ehlers-Danlos Syndrome), systemic lupus erythematosus, temporal arteritis, and polyarteritis nodosa 6. Trauma to the shoulder girdle and stressful exercise are other determining factors 6. Additional sources include immunizations, including tetanus toxoid and antitoxin, diphtheria, pertussis, tetanus (DPT) vaccine, smallpox, swine flu; pregnancy and childbirth; radiation therapy; lumbar puncture; pneumoencephalogram; radiologic contrast dye administration; and allergy desensitization.
The incidence of brachial neuritis is approximately 1-2 cases per 100,000 person-years 7. Brachial neuritis occurs predominantly in males, with the male-to-female ratio for the condition ranging from 2:1 to 4:1. Brachial neuritis has been reported in individuals from age 3 months to 74 years; however, the condition’s prevalence is highest in young to middle-aged adults. Onset in childhood should be considered suggestive of hereditary brachial neuritis 8.
According to van Alfen et al. 9, early identification permits medical treatment that may repeal the severity of the clinical course.
Brachial neuritis is not a fatal condition, although the phrenic nerve may be involved 10. The risk of ‘significant’ residual disability in the involved limb after 2 years is approximately 10-20% 11. A recent survey suggested that up to half of patients are left with residual shoulder pain and decreased endurance 12.
Brachial neuritis causes
The exact cause of brachial neuritis is unknown, but brachial neuritis exists in an inherited and an idiopathic form. In the idiopathic version, the pathophysiology is unknown, but the condition is generally thought to be an immune system–mediated inflammatory reaction against nerve fibers of the brachial plexus 13. Axonopathy with subsequent Wallerian degeneration appears to predominate, but proximal conduction block has also been described in over 33% of cases in the series by Lo and Mills 14. The inherited brachial neuritis is autosomal dominant and has been linked to mutations in the SEPT9 gene on chromosome 17q 15. Septins are involved in the formation of the cytoskeleton and in cell division, but how these mutations result in brachial neuritis is unknown.
Brachial neuritis has been linked to many antecedent events or illnesses, as follows:
- Viral infection (particularly of the upper respiratory tract)
- Bacterial infection (eg, pneumonia, diphtheria, typhoid)
- Parasitic infestation
- Surgery 16
- Trauma (not related to shoulder)
- Vaccinations (eg, influenza, tetanus, diphtheria, tetanus toxoids, pertussis [DPT], smallpox, swine flu)
- Childbirth
- Miscellaneous medical investigative procedures (eg, lumbar puncture, administration of radiologic dye)
- Systemic illness (eg, polyarteritis nodosa, lymphoma, systemic lupus erythematosus, temporal arteritis, Ehlers-Danlos syndrome)
A study from England and the Netherlands found that in samples of British and Dutch patients with brachial neuritis, about 10% had hepatitis E. In the combined retrospective (British)/prospective (Dutch) study, van Eijk et al 17 found hepatitis E virus (HEV) infection in five out of 47 patients. Clinical and electrophysiologic examination indicated that the brachial neuritis was bilateral in all of the patients with hepatitis E 18.
A study by Arányi et al 7 described the ultrasonographic characteristics of brachial neuritis, finding the following abnormalities in a group of 14 patients:
- Focal or diffuse nerve or fascicle enlargement
- Incomplete nerve constriction
- Complete nerve constriction with torsion
- Fascicular entwinement
The investigators suggested that inflammation-related constriction precedes torsion, with the torsion encouraged by limb rotation 19
Using magnetic resonance imaging (MRI) to evaluate patients with brachial neuritis, a study by Sneag et al 20 suggested that instead of being associated with changes to all or part of the brachial plexus proper, the condition involves one or more mononeuropathies.
A study by Ferrante and Wilbourn 21 of 281 patients with sporadic brachial neuritis found through electrodiagnostic testing that out of 379 assessable events, 174 (46%) involved a single nerve, with another 205 (54%) being multifocal. The investigators also found that most bouts of brachial neuritis purely involved motor nerves, with the second greatest number involving mostly motor nerves and the fewest involving a more even mix of sensory and motor nerves.
Brachial neuritis symptoms
Brachial neuritis has two major clinical symptoms: pain and muscle weakness from atrophy. The onset of pain in brachial neuritis is often abrupt and may follow recent illness, surgery 16, immunization, or even trauma. Up to two thirds of cases begin during the nighttime.
The patient will complain of severe pain that is sudden in onset and involves the lateral aspect of the shoulder as seen in axillary nerve involvement, scapula pain in the suprascapular nerve, the superolateral thoracic wall in long thoracic nerve, antecubital fossa in anterior interosseous nerve, and lateral arm or forearm in musculocutaneous nerve. The acute pain is self-limited and subsides after a few days to weeks. Weakness, changes in reflexes, and sensory deficits will follow. The pain usually is not positional and is worse at night, causing the patient to wake from sleep. The average time between the trigger and the symptoms is 1 to 28 days; however, 66% of patients report the trigger within seven days.
- The pain usually is localized to the right shoulder region, but it may be bilateral in 10-30% of cases.
- The pain’s intensity is very high (9+/10) and is maximal at onset. Due to the extreme pain involved, patients with brachial neuritis usually present acutely. Typically, the affected arm is supported by the uninvolved arm and is held in adduction and internal rotation.
- Usually, the pain is described as sharp or throbbing in nature.
- The pain usually is constant, but it is exacerbated by movements of the shoulder. Movements of the neck, coughing, and/or sneezing usually do not worsen the pain.
- Intense pain can last from a few hours to several weeks and requires opiate analgesia.
- Low-grade pain may persist for up to a year.
- As the pain subsides, weakness becomes apparent. In most cases of brachial neuritis, this weakness manifests within about 2 weeks of onset. Weakness is maximal at onset but can progress over 1 or more weeks. Muscle strength in affected muscles often is reduced severely (to 2 or less on the Medical Research Council [MRC] grading scale).
- A wide variety of muscles is affected, particularly those innervated by the upper trunk. The supraspinatus, infraspinatus, serratus anterior, and deltoid muscles are particularly susceptible, but many different single and multiple combinations of muscle involvement, including a pure distal form, have been reported.
- The patient may notice considerable atrophy and wasting, as well as a deep aching in the affected muscles. Atrophy of the affected muscles becomes prominent after approximately 2 weeks.
- Numbness may occur, depending on the particular nerves affected, and usually is found in the nerve distribution corresponding to maximal muscle weakness. However, numbness is rarely a prominent complaint.
- Phrenic nerve involvement occurs in up to 5% of cases and can result in significant shortness of breath 22.
- Reflexes may be reduced or absent, depending on which nerves are involved.
- Sensory loss is not prominent but may be detectable (in particular, loss of axillary nerve sensation), depending on the specific nerves affected.
Variants of brachial neuritis can present with isolated or multiple cranial neuropathies (IX, X, XI, XII) 23.
In 25-50% of patients, the medical history indicates a viral illness or vaccination that occurred days or weeks prior to the onset of symptoms. Some patients also may note recent trauma or severe exercise, surgery, infection, or immunization.
Brachial neuritis diagnosis
It is essential to diagnose brachial neuritis because it can mimic many another diagnoses, such as rotator cuff, cervical cord compression, or nerve entrapment. If brachial neuritis is mistaken for one of these conditions, the patient may undergo unnecessary surgery. Imaging is necessary to diagnose this condition, and an MRI of the shoulder with a special focus on the brachial plexus is needed to rule out musculoskeletal disorders 24. This must be discussed with the radiologist when ordering the test. Work up includes tests for Epstein Barr virus, varicella zoster, dengue, and hepatitis E. Obtain a detailed history from the patient regarding recent surgery, childbirth, infection, tetanus vaccine, influenza vaccine, and recently prescribed medications such as antivirals, antiepileptic, or botulinum toxins. Nerve conduction/electromyography (EMG) is also crucial in the evaluation of these patients. Electrophysiology studies will show different findings depending on the time of the study and the underlying pathology; for example, if demyelination is the main pathophysiology, then one will see a conduction block, demyelination, or Wallerian degeneration that may occur in varying combinations. Since brachial neuritis is an axonal disorder, nerve conduction velocities and distal latencies are usually normal. A 1996 study by Fibuch et al. 25 showed that 25% of patients had a recent viral illness before the symptoms of brachial neuritis. Ohta et al. 26 found that when emergency situations present in rural areas where there might not be an EMG or MRI machine, proper utilization of knowledge and skills such as gathering accurate history, asking the questions mentioned above, and performing physical examination can lead to the appropriate diagnosis. Newer technology using high-resolution may be helpful as well in the diagnosis of brachial neuritis 27.
Brachial neuritis treatment
There is no specific treatment for brachial neuritis; it is a self-limiting condition that resolves on its own 6. Initially, the severe pain must be treated either with analgesics, such as opioid medications, or neuropathic pain medications, such as tricyclic or antiepileptic agents. Corticosteroids may also help in the acute phase. Strengthening and stretching exercises are very important once the pain is under control. During the acute phase, acupuncture and transcutaneous electrical nerve stimulation (TENS) can be tried. In patients that have superimposed cervical degenerative joint disease where imaging is suggestive of nerve root-level compression, a cervical epidural injection may be helpful to distinguish between pain due to cervical radiculopathy and brachial neuritis. The patient should use the affected extremity as soon as possible and consider a shoulder stabilizer with nonsurgical management. Strengthening exercises are not recommended for completely denervated muscles, and the role of electrical stimulation is controversial but should be considered when the denervated state is for more than 4 months. As a follow-up, EMG could be done of the involved muscles which can show the extent of reinnervation 28. This bit of information can help one assess which muscles can tolerate a higher level of strengthening and at what time in treatment. For chronic patients, there is evidence that immunotherapy, such as Intravenous immunoglobulin (IVIg), may be used but this is still lacking data 29.
Brachial neuritis prognosis
If pain control, steroids, and an appropriate exercise plan with physical therapy has been prescribed, Ferrante et al reported 89% of patients recovered within 3 years, 75% within 2 years, and 36% within the first year. Recent studies show that many of these patients will continue to have pain and some functional limitation. As seen in the Van Alfen et al. 9 study, persistent pain was experienced by 30% of patients and functional limitations by 66% after a mean of 2.5 years.
- Gonzalez-Alegre P, Recober A, Kelkar P. Idiopathic brachial neuritis. Iowa Orthop J. 2002;22:81–85. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1888382[↩]
- Ortiz Torres M, Mesfin FB. Brachial Plexitis (Parsonage Turner Syndrome, Brachial Neuropathy, Brachial Radiculitis). 2018 Jan.[↩]
- Ferrante, M. A. (2004), Brachial plexopathies: Classification, causes, and consequences. Muscle Nerve, 30: 547-568. doi:10.1002/mus.20131[↩]
- Milner CS, Kannan K, Iyer VG, Thirkannad SM. Parsonage-Turner Syndrome: Clinical and Epidemiological Features From a Hand Surgeon’s Perspective. Hand (N Y). 2016 Jun;11(2):227-31.[↩]
- Feinberg JH, Radecki J. Parsonage-turner syndrome. HSS J. 2010 Sep;6(2):199-205.[↩]
- Al Khalili Y, Jain S, DeCastro A. Brachial Neuritis. [Updated 2019 Apr 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499842[↩][↩][↩]
- Brachial neuritis. https://emedicine.medscape.com/article/315811-overview[↩][↩]
- Stogbauer F, Young P, Kuhlenbaumer G, et al. Hereditary recurrent focal neuropathies: clinical and molecular features. Neurology. 2000 Feb 8. 54(3):546-51.[↩]
- van Alfen N, van Engelen BG. The clinical spectrum of neuralgic amyotrophy in 246 cases. Brain. 2006 Feb;129(Pt 2):438-50.[↩][↩]
- McEnery T, Walsh R, Burke C, McGowan A, Faul J, Cormican L. Phrenic Nerve Palsy Secondary to Parsonage-Turner Syndrome: A Diagnosis Commonly Overlooked. Lung. 2017 Apr. 195 (2):173-7.[↩]
- van Alfen N, van der Werf SP, van Engelen BG. Long-term pain, fatigue, and impairment in neuralgic amyotrophy. Arch Phys Med Rehabil. 2009 Mar. 90(3):435-9.[↩]
- Cup EH, Ijspeert J, Janssen RJ, Bussemaker-Beumer C, Jacobs J, Pieterse AJ, et al. Residual complaints after neuralgic amyotrophy. Arch Phys Med Rehabil. 2013 Jan. 94(1):67-73.[↩]
- Pan YW, Wang S, Tian G, Li C, Tian W, Tian M. Typical brachial neuritis (Parsonage-Turner syndrome) with hourglass-like constrictions in the affected nerves. J Hand Surg Am. 2011 Jul. 36(7):1197-203[↩]
- Lo YL, Mills KR. Motor root conduction in neuralgic amyotrophy: evidence of proximal conduction block. J Neurol Neurosurg Psychiatry. 1999 May. 66(5):586-90.[↩]
- Laccone F, Hannibal MC, Neesen J, et al. Dysmorphic syndrome of hereditary neuralgic amyotrophy associated with a SEPT9 gene mutation–a family study. Clin Genet. 2008 Sep. 74(3):279-83.[↩]
- Park P, Lewandrowski KU, Ramnath S, et al. Brachial neuritis: an under-recognized cause of upper extremity paresis after cervical decompression surgery. Spine. 2007 Oct 15. 32(22):E640-4.[↩][↩]
- van Eijk JJ, Madden RG, van der Eijk AA, et al. Neuralgic amyotrophy and hepatitis E virus infection. Neurology. 2014 Feb 11. 82(6):498-503.[↩]
- Bazerbachi F, Haffar S, Garg SK, Lake JR. Extra-hepatic manifestations associated with hepatitis E virus infection: a comprehensive review of the literature. Gastroenterol Rep (Oxf). 2016 Feb. 4 (1):1-15.[↩]
- Aranyi Z, Csillik A, Devay K, et al. Ultrasonographic identification of nerve pathology in neuralgic amyotrophy: Enlargement, constriction, fascicular entwinement and torsion. Muscle Nerve. 2015 Feb 20.[↩]
- Sneag DB, Rancy SK, Wolfe SW, et al. Brachial plexitis or neuritis? MRI features of lesion distribution in Parsonage-Turner syndrome. Muscle Nerve. 2018 Feb 20.[↩]
- Ferrante MA, Wilbourn AJ. The Lesion Distribution among 281 Patients with Sporadic Neuralgic Amyotrophy. Muscle Nerve. 2016 Sep 28.[↩]
- Tsao BE, Ostrovskiy DA, Wilbourn AJ, et al. Phrenic neuropathy due to neuralgic amyotrophy. Neurology. 2006 May 23. 66(10):1582-4.[↩]
- Pierre PA, Laterre CE, Van den Bergh PY. Neuralgic amyotrophy with involvement of cranial nerves IX, X, XI and XII [published erratum appears in Muscle Nerve 1991 Jan;14(1):88]. Muscle Nerve. 1990 Aug. 13(8):704-7.[↩]
- Sneag DB, Rancy SK, Wolfe SW, Lee SC, Kalia V, Lee SK, Feinberg JH. Brachial plexitis or neuritis? MRI features of lesion distribution in Parsonage-Turner syndrome. Muscle Nerve. 2018 Sep;58(3):359-366. [↩]
- Fibuch EE, Mertz J, Geller B. Postoperative onset of idiopathic brachial neuritis. Anesthesiology. 1996 Feb;84(2):455-8.[↩]
- Yasunaga, H., Shiroishi, T., Ohta, K., Matsunaga, H., Ota, Y. Fascicular torsion in the median nerve within the distal third of the upper arm: three cases of nontraumatic anterior interosseous nerve palsy. J Hand Surg. 2003;28A:206–211[↩]
- Herraets IJT, Goedee HS, Telleman JA, van Asseldonk JH, Visser LH, van der Pol WL, van den Berg LH. High-resolution ultrasound in patients with Wartenberg’s migrant sensory neuritis, a case-control study. Clin Neurophysiol. 2018 Jan;129(1):232-237 [↩]
- Feinberg JH, Nguyen ET, Boachie-Adjei K, Gribbin C, Lee SK, Daluiski A, Wolfe SW. The electrodiagnostic natural history of parsonage-turner syndrome. Muscle Nerve. 2017 Oct;56(4):737-743.[↩]
- Morishima R, Nagaoka U, Nagao M, Isozaki E. Chronic Brachial Plexus Neuritis That Developed into Typical Neuralgic Amyotrophy and Positively Responded to Immunotherapy. Intern. Med. 2018 Apr 01;57(7):1021-1026.[↩]