What is a brain hemorrhage
Brain hemorrhage also called intracranial hemorrhage is a life-threatening condition that encompasses four broad types of hemorrhage: epidural hemorrhage, subdural hemorrhage, subarachnoid hemorrhage, and intraparenchymal hemorrhage 1. Brain hemorrhage may be spontaneous, precipitated by an underlying vascular malformation, induced by trauma, or related to therapeutic anticoagulation. Each type of brain hemorrhage is different concerning cause, findings, prognosis, and outcome.
The anatomical location of brain hemorrhage (intracranial hemorrhage) specifies the source. In intraparenchymal hemorrhage, a ruptured arteriole is the likely culprit, underscored by contrast extravasation into the hemorrhage on computed tomography (CT) 2. Aneurysmal subarachnoid hemorrhage is due to rupture of an aneurysm in the subarachnoid space, usually near the circle of Willis. “Perimesencephalic” subarachnoid hemorrhage refers to scant subarachnoid blood around the brainstem 3 and is related to venous bleeding 4. Subdural hematomas are generally due to tearing of cortical veins, whereas epidural hematomas are typically due to arterial lacerations. Although neurotrauma is common, this review focuses on nontraumatic hemorrhage. A fistulous or malformed connection between the cerebral arteries and veins, intracranial aneurysm, or trauma may produce hemorrhage in more than one anatomical compartment.
Brain hemorrhage types
Brain hemorrhage (intracranial hemorrhage) is diagnosed by its anatomical location 5. Intraparenchymal hemorrhage (also called intracerebral hemorrhage) refers to nontraumatic bleeding into the brain parenchyma (Figure 2). Subarachnoid hemorrhage (SAH) refers to bleeding into the space between the pia and the arachnoid membranes. Nontraumatic causes of rupture include cerebral aneurysms (Figure 1), bleeding from arteriovenous malformations or tumors, cerebral amyloid angiopathy, and vasculopathies (such as vasculitis). A subdural hematoma (Figure 3) is due to bleeding between the dura and the arachnoid, whereas an epidural hematoma involves bleeding between the dura and the bone. Subdural and epidural hematomas are usually traumatic injuries.
Subarachnoid hemorrhage
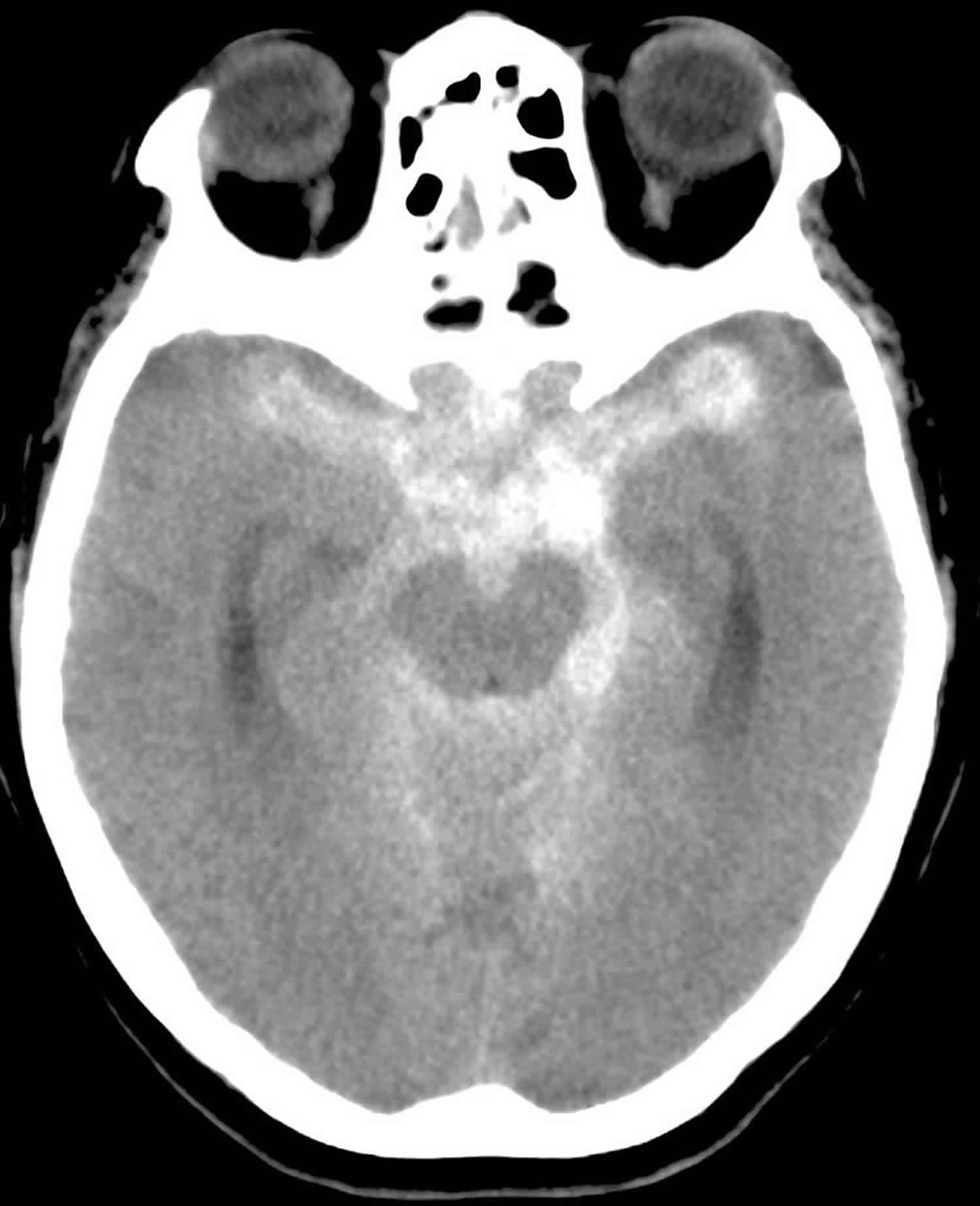
A subarachnoid hemorrhage is bleeding into the subarachnoid space 6. The area of the skull surrounding the brain (the subarachnoid space) rapidly fills with blood. A patient with subarachnoid hemorrhage may have a sudden, intense headache, neck pain, and nausea or vomiting. Sometimes this is described as the worst headache of one’s life. The sudden buildup of pressure outside the brain may also cause rapid loss of consciousness or death. Subarachnoid hemorrhage is divided into traumatic versus non-traumatic subarachnoid hemorrhage. A second categorization scheme divides subarachnoid hemorrhage into aneurysmal and non-aneurysmal subarachnoid hemorrhage. Aneurysmal subarachnoid hemorrhage occurs after rupture of a cerebral aneurysm allowing for bleeding into the subarachnoid space. Non-aneurysmal subarachnoid hemorrhage is bleeding into the subarachnoid space without identifiable aneurysms. Non-aneurysmal subarachnoid hemorrhage most commonly occurs after trauma with a blunt head injury with or without penetrating trauma or sudden acceleration changes to the head 7.
Subarachnoid hemorrhage is most often caused by abnormalities of the arteries at the base of the brain, called cerebral aneurysms. These are small areas of rounded or irregular swellings in the arteries. Where the swelling is most severe, the blood vessel wall becomes weak and prone to rupture.
Subarachnoid hemorrhage accounts for approximately 5% of all strokes and has an incidence of approximately two to 25 per 100,000 person-years for those over the age of 35 6. The incidence trends up slowly as patients age and may be very slightly more frequent in females than males (1.15:1 for the female to male ratio).
The cause of cerebral aneurysms is not known. They may develop from birth or in childhood and grow very slowly. Some people have multiple aneuryms. Subarachnoid hemorrhage can occur at any age, including in teenagers and young adults and is slightly more common in women than men.
Figure 1. Subarachnoid hemorrhage
Footnote: Subarachnoid hemorrhage on computerized tomography scan. Hyperdense (bright) signal, indicating blood, surrounds the brainstem and fills the subarachnoid space. This appearance is typical of a ruptured aneurysm.
[Source 8 ]Intraparenchymal hemorrhage
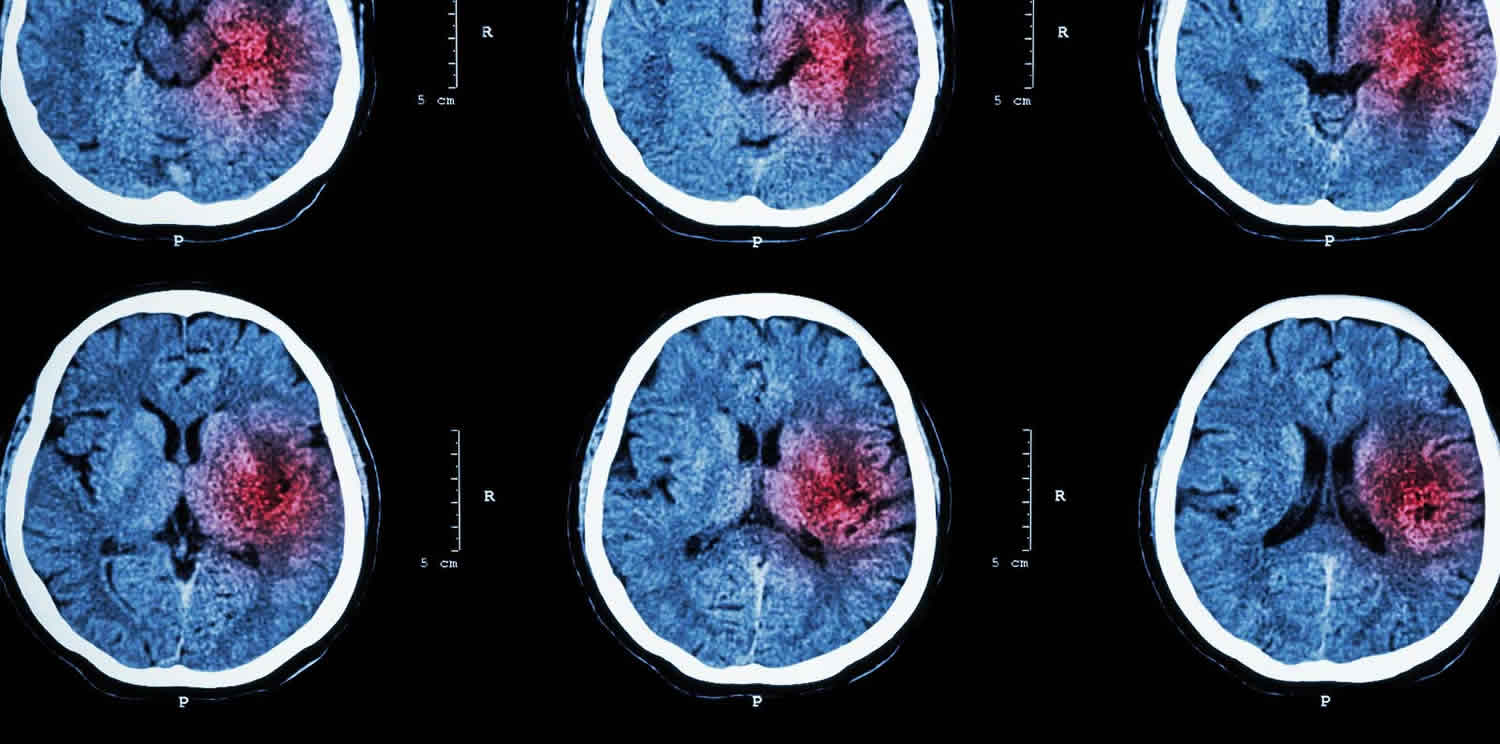
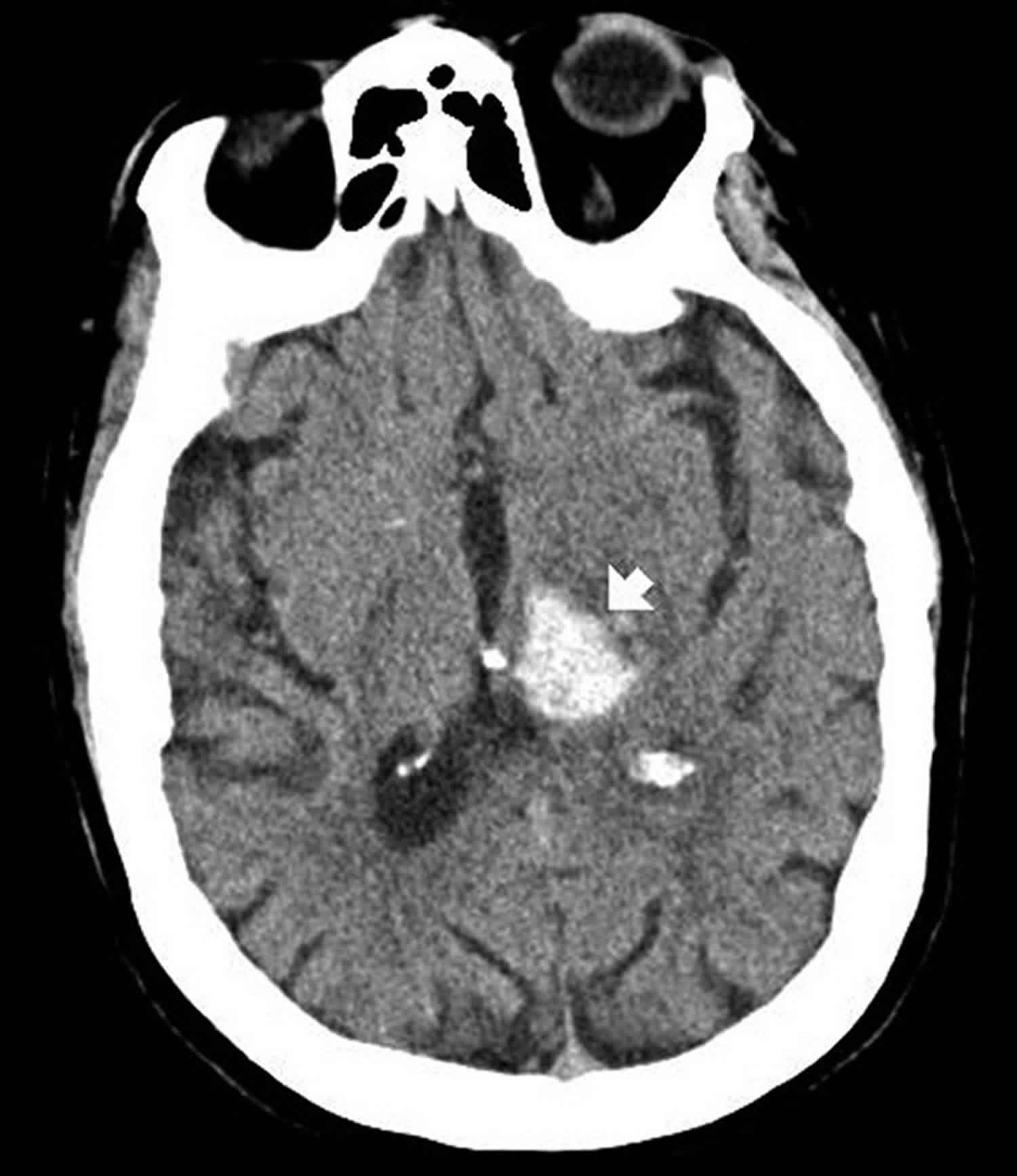
Intraparenchymal hemorrhage also called intracerebral hemorrhage, occurs when a diseased blood vessel within the brain bursts, allowing blood to leak inside the brain parenchyma proper. There are a wide variety of reasons such hemorrhage can occur including, but not limited to, hypertension, arteriovenous malformation, amyloid angiopathy, aneurysm rupture, tumor, coagulopathy, infection, vasculitis, and trauma.
The sudden increase in pressure within the brain can cause damage to the brain cells surrounding the blood. If the amount of blood increases rapidly, the sudden buildup in pressure can lead to unconsciousness or death. Intracerebral hemorrhage usually occurs in selected parts of the brain, including the basal ganglia, cerebellum, brain stem, or cortex.
The most common cause of intracerebral hemorrhage is high blood pressure (hypertension). Since high blood pressure by itself often causes no symptoms, many people with intracranial hemorrhage are not aware that they have high blood pressure, or that it needs to be treated. Less common causes of intracerebral hemorrhage include trauma, infections, tumors, blood clotting deficiencies, and abnormalities in blood vessels (such as arteriovenous malformations).
Intracerebral hemorrhage occurs at all ages. The average age is lower than for ischemic stroke. Less common than ischemic strokes, intracerebral hemorrhage accounts for 10% to 20% of all strokes 6. Intraparenchymal hemorrhage incidence increases for those age 55 and older with increasing incidence as age increases. There is some controversy regarding gender differences, but there may be a slight male predominance.
Figure 2. Intraparenchymal hemorrhage
Footnote: Intraparenchymal hemorrhage on computerized tomography scan. The hyperdense (bright) area represents acute bleeding (arrow). This location is suggestive of chronic hypertension, not compatible with an aneurysm, and would be highly atypical in trauma.
[Source 6 ]Subdural hemorrhage
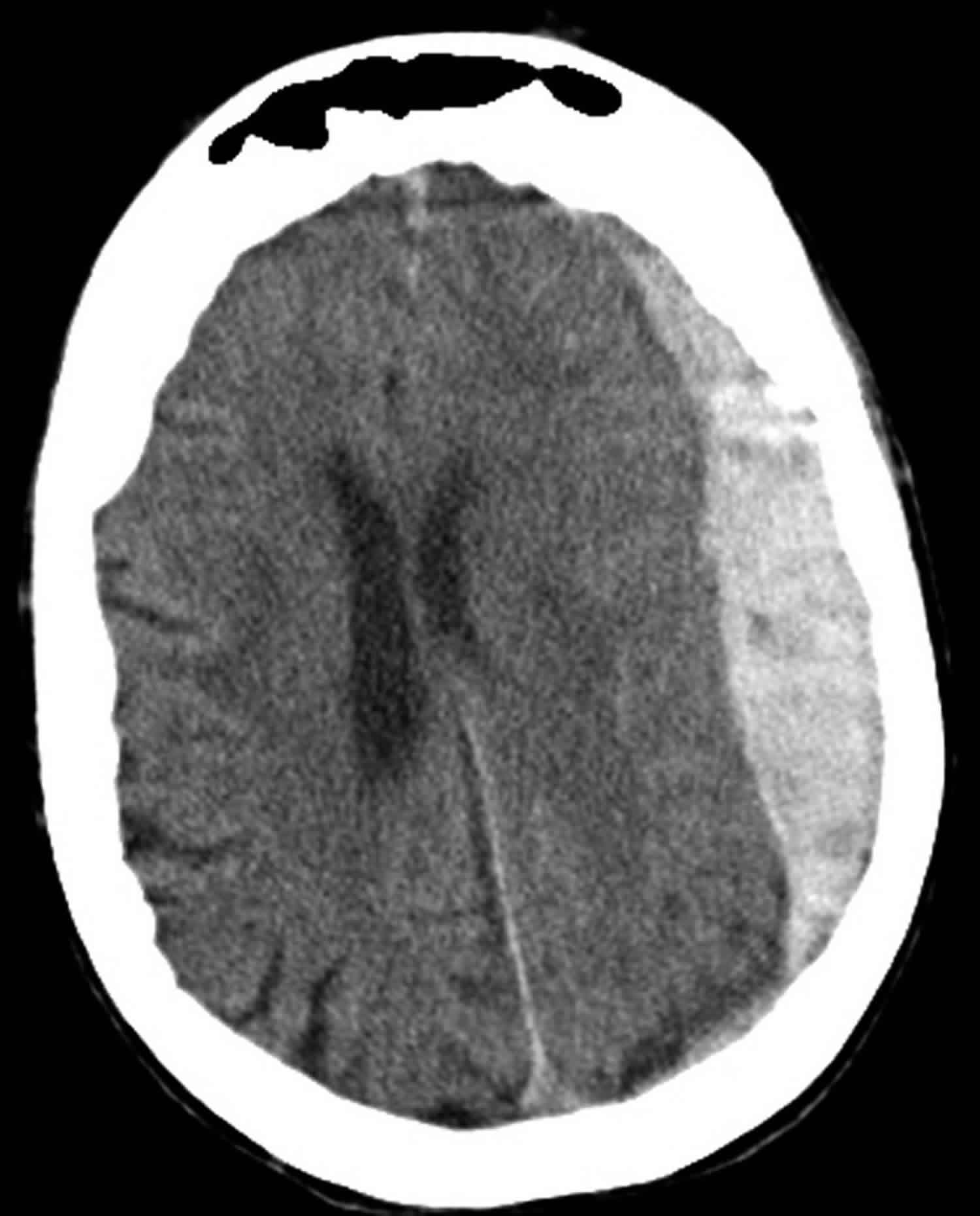
Subdural hemorrhage occurs when blood enters the subdural space which is anatomically the arachnoid space. Commonly subdural hemorrhage occurs after a vessel traversing between the brain and skull is stretched, broken or torn and begins to bleed into the subdural space. These most commonly occur after blunt head injury but may also occur after penetrating head injuries or spontaneously 9.
The incidence of subdural hematoma is estimated to be between 5% to 25% of patients with a significant head injury 6. There is an annual incidence of one to five cases per 100,000 population per year with a male to female ratio of 2:1. The incidence of subdural hematomas increases throughout life.
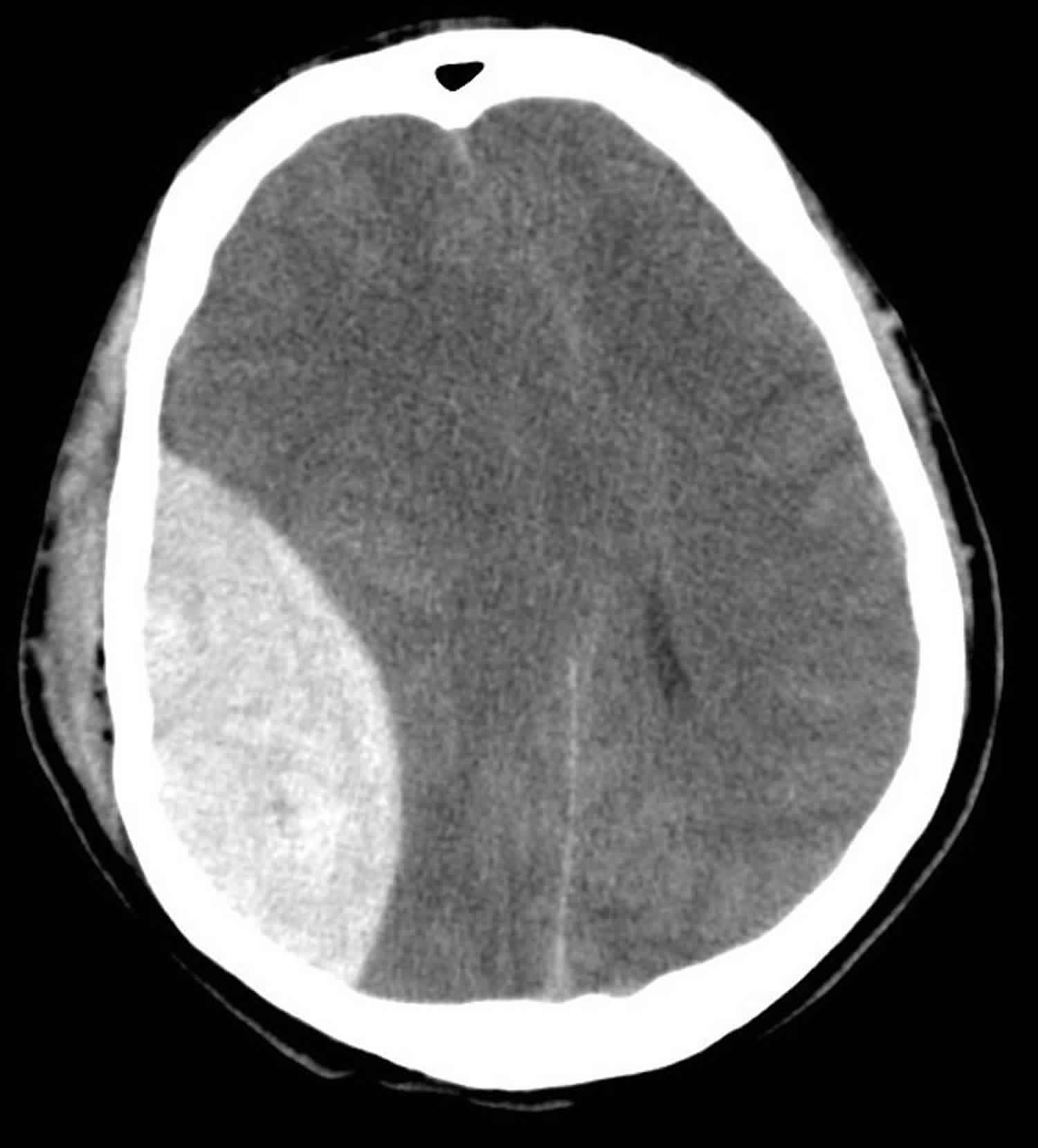
Figure 3. Subdural hemorrhage
Footnote: Subdural hemorrhage on computerized tomography scan. Hyperdense (bright) signal, indicating blood, is seen on the patient’s left. There is a midline shift to the patient’s right.
[Source 6 ]Epidural hemorrhage
An epidural hemorrhage also known as extradural hematoma, can either be arterial or venous in origin. The classical arterial epidural hematoma occurs after blunt trauma to the head, typically the temporal region. They may also occur after a penetrating head injury. There is typically a skull fracture with damage to the middle meningeal artery causing arterial bleeding into the potential epidural space. Although the middle meningeal artery is the classically described artery, any meningeal artery can lead to the arterial epidural hematoma 10.
A venous epidural hematoma occurs when there is skull fracture, and the venous bleeding from the skull fracture fills the epidural space. Venous epidural hematomas are common in pediatric patients.
Epidural hematomas are present in approximately 2% of head injury patients and account for 5% to 15% of fatal head injuries. Approximately 85% to 95% of epidural hematomas have an overlying skull fracture 6.
Figure 4. Epidural hematoma
Footnote: Extra-axial biconvex hyperdense collection associated with fracture suggestive of epidural hematoma (extradural hematoma).
[Source 11 ]Brain hemorrhage causes
Subarachnoid hemorrhage
Subarachnoid hemorrhage most commonly occurs after trauma where cortical surface vessels are injured and bleed into the subarachnoid space. Non-traumatic subarachnoid hemorrhage is most commonly due to rupture of a cerebral aneurysm. When aneurysm ruptures, blood can flow into the subarachnoid space. Other causes of subarachnoid hemorrhage include arteriovenous malformations (AVM), use of blood thinners, trauma or idiopathic causes.
Intraparenchymal hemorrhage
Non-traumatic intraparenchymal hemorrhage most often occurs secondary to hypertensive damage to cerebral blood vessels which eventually burst and bleed into the brain. Other causes include rupture of an arteriovenous malformation, rupture of an aneurysm, arteriopathy, tumor, infection, or venous outflow obstruction. Penetrating and non-penetrating trauma may also cause intraparenchymal hemorrhage.
Epidural hemorrhage
Epidural hemorrhage occurs when blood dissects into the potential space between the dura and inner table of the skull. Most commonly this occurs after a skull fracture (85% to 95% of cases). There can be damage to an arterial or venous vessel which allows blood to dissect into the potential epidural space resulting in the epidural hematoma. The most common vessel damaged it the middle meningeal artery underlying the temporoparietal region of the skull.
Subdural hemorrhage
Subdural hemorrhage has multiple causes including head trauma, coagulopathy, vascular abnormality rupture, and spontaneous. Most commonly head trauma causes motion of the brain relative to the skull which can stretch and break blood vessels traversing from the brain to the skull. If the blood vessels are damaged, they bleed into the subdural space.
Risk factors for brain hemorrhage
The most common risk factor for brain hemorrhage is hypertension 12. Antiplatelet 13 and anticoagulant medications also increase the risk of spontaneous brain hemorrhage. Cerebral amyloid angiopathy is related to build-up of amyloid proteins in arterial walls, making them more susceptible to rupture. Hemorrhages due to cerebral amyloid angiopathy are typically lobar (near the cortex), multiple, and occur in patients at least 55 years of age 14.
The rupture of an intracranial aneurysm (subarachnoid hemorrhage) may be spontaneous, precipitated by exertion, or from hypertension. About 1–2% of the adult population harbors an unruptured intracranial aneurysm 15, but only about 1% of these rupture, and how best to select patients for prophylactic aneurysm obliteration is unsettled 16. The most important modifiable risk factors are tobacco use, hypertension, and cocaine use 17; nonmodifiable risk factors include a personal history of subarachnoid hemorrhage, familial history of subarachnoid hemorrhage 18, larger aneurysm size 19, female sex 20, connective tissue disease, and older age. Arteriovenous malformations are often congenital and may become symptomatic later in life.
Brain hemorrhage signs and symptoms
Subarachnoid hemorrhage
A thunderclap headache (sudden severe headache or worst headache of life) is the classic presentation of subarachnoid hemorrhage. Other symptoms include dizziness, nausea, vomiting, diplopia, seizures, loss of consciousness, or nuchal rigidity. Physical exam findings may include focal neurologic deficits, cranial nerve palsies, nuchal rigidity, or decreased or altered consciousness.
Intraparenchymal hemorrhage
Non-traumatic intraparenchymal hemorrhages typically present with a history of sudden onset of stroke symptoms including a headache, nausea, vomiting, focal neurologic deficits, lethargy, weakness, slurred speech, syncope, vertigo, or changes in sensation.
Epidural hemorrhage
Patients with epidural hematoma report a history of a focal head injury such as blunt trauma from a hammer or baseball bat, fall or motor vehicle collision. The classic presentation of an epidural hematoma is a loss of consciousness of after the injury, followed by a lucid interval then neurologic deterioration. This classic presentation only occurs in less than 20% of patients. Other symptoms which are common include a severe headache, nausea, vomiting, lethargy, and seizure.
Subdural hemorrhage
A history of either major or minor head injury can often be found in cases of subdural hematoma. In older patients, a subdural hematoma can occur after trivial head injuries including bumping a head on a cabinet or running into a door or wall. An acute subdural can present with recent trauma, headache, nausea, vomiting, altered mental status, seizure and/or lethargy. A chronic subdural hematoma can present with a headache, nausea, vomiting, confusion, decreased consciousness, lethargy, motor deficits, aphasia, seizure or personality changes. A physical exam may demonstrate a focal motor deficit, neurologic deficits, lethargy, or altered consciousness.
Brain hemorrhage diagnosis
Subarachnoid hemorrhage
Initial evaluation includes assessing and stabilizing the airway, breathing and circulation (ABCs). Patients with subarachnoid hemorrhage can rapidly deteriorate and may need emergent intubation. Thorough neurologic examination can help identify any neurologic deficits.
The initial imaging for patients with subarachnoid hemorrhage is computed tomography (CT) head without contrast. If the patient is given contrast, this can obscure the subarachnoid hemorrhage. Acute subarachnoid hemorrhage is typically hyperdense on CT imaging. If the CT head is negative and there is still strong suspicion for subarachnoid hemorrhage a lumbar puncture should be considered. The results of the lumbar puncture may show xanthochromia. A lumbar puncture performed before 6 hours of the subarachnoid hemorrhage may fail to show xanthochromia. Additionally, lumbar puncture results may be confounded if a traumatic tap is encountered.
Identifying the cause of non-traumatic subarachnoid hemorrhage will help guide further treatment. Common workup includes either a CT angiogram (CTA) of the head and neck, magnetic resonance angiography (MRA) of the head and neck or diagnostic cerebral angiogram of the head and neck done emergently to look for an aneurysm, AVM or another source of subarachnoid hemorrhage.
Laboratory studies should also be considered including a complete blood count to check for thrombocytopenia, coagulation studies (PTT, PT/INR) to check for coagulopathy and basic metabolic panel to check for electrolyte abnormalities.
Intraparenchymal hemorrhage
Once the medical stability of the patient is ensured, CT head without contrast is the first diagnostic test most commonly performed. The imaging should be able to identify acute intraparenchymal hemorrhage as hyperdense within the parenchyma. Depending on the history, physical and imaging findings and patient an MRI brain with and without contrast should be considered as tumors within the brain may present as intraparenchymal hemorrhage. Other imaging to consider include CTA, MRA or diagnostic cerebral angiogram to look for cerebrovascular causes of the intraparenchymal hemorrhage. Evaluation should also include a complete neurologic exam to identify any neurologic deficits.
Laboratory studies should also be considered including a complete blood count to check for thrombocytopenia, coagulation studies (PTT, PT/INR) to check for coagulopathy and basic metabolic panel to check for electrolyte abnormalities.
Epidural hemorrhage
Initial evaluation includes airway, breathing, and circulation as patients can rapidly deteriorate and require intubation 21. A detailed neurologic examination helps identify neurologic deficits. With increasing intracranial pressure there may be a Cushing response (hypertension, bradycardia, and bradypnea). Emergent CT head without contrast is the imaging choice of the test due to its high sensitivity and specificity for identifying significant epidural hematomas. Historically cerebral angiography could identify the shift in cerebral blood vessels, but cerebral angiography has been supplanted by CT imaging.
Laboratory studies should also be considered including a complete blood count to check for thrombocytopenia, coagulation studies (PTT, PT/INR) to check for coagulopathy and basic metabolic panel to check for electrolyte abnormalities.
Subdural hemorrhage
After ensuring the medical stability of the patient, a detailed neurologic exam can help identify any specific neurologic deficits. Most commonly a computed tomography (CT) scan of the head without contrast is the first imaging test of choice. An acute subdural hematoma is typically hyperdense with chronic subdurals being hypodense. A subacute subdural may be isodense to the brain and more difficult to identify.
Laboratory studies should also be considered including a complete blood count to check for thrombocytopenia, coagulation studies (PTT, PT/INR) to check for coagulopathy and basic metabolic panel to check for electrolyte abnormalities.
Assessing the severity of brain hemorrhage
Assessing the severity of neurological injury is important for prioritizing diagnostic studies, guiding resuscitation, and planning interventions (e.g., a patient with a worsening examination may benefit from interventions to reduce ICP before proceeding to the angiography suite). Commonly used and well-validated scoring systems are available. Examinations should be performed off sedation: medications with short half-lives (e.g., propofol) should be discontinued (often the start of rounds is long enough), but longer lasting sedatives (e.g., benzodiazepines, barbiturates by continuous infusion) can cloud the examination for hours or days.
Glasgow Coma Scale
The Glasgow Coma Scale (GCS) 22 is a standard assessment for the level of consciousness. The three axes are eye opening (1–4 points), motor response (1–6 points), and verbal response (1–5 points). Intubated patients typically receive 1 point for verbal with a “T” noted (e.g., a score of 8T). The best possible GCS of 15 (eyes open spontaneously, follows commands, and oriented) is compatible with weakness on one side and trouble speaking, whereas the worst possible score of 3 (no eye opening, no motor response, no verbal response to pain) does not mean the patient is brain dead. The FOUR score 23 is a more recent scale that assesses pupil response to light and triggering of a mechanical ventilator, important findings in deeply comatose patients that are not part of the Glasgow Coma Scale (GCS).
NIH Stroke Scale
Competently performing the neurological examination is often frustrating for physicians who do not have comprehensive training. Designed to be a reliable, reproducible examination for trials in acute ischemic stroke, the NIH Stroke Scale (https://www.stroke.nih.gov/documents/NIH_Stroke_Scale_508C.pdf) 24 is a simplified neurological examination that covers most acute neurological symptoms: speaking and comprehension, weakness, sensory abnormalities, visual deficit, neglect, and ataxia. A normal examination has zero points, with increasing scores indicating a worse neurological deficit.
Disease-specific scores for subarachnoid hemorrhage
Disease-specific scoring systems for subarachnoid hemorrhage include the classic Hunt and Hess Scale 25. This qualitative scale is scored from 1 (no symptoms) to 5 (deeply comatose, extensor posturing). Broadly, the Hunt and Hess Scale is predictive of a variety of medical complications, vasospasm, cerebral infarction, and worse outcomes. The World Federation of Neurosurgical Societies (WFNS) Scale is similar to the Hunt and Hess Scale, but derived from the GCS, which increases interrater reliability. The Hunt and Hess, World Federation of Neurosurgical Societies, and Glasgow Coma Scale (GCS) have similar prognostic significance 26.
Predicting vasospasm is important after subarachnoid hemorrhage because vasospasm may lead to cerebral infarction. Vasospasm is generally predictable from the admission CT scan 27, with thick blood in the subarachnoid space and hemorrhage in both lateral ventricles broadly predictive of an increased risk. “Vasospasm” may refer to a narrowed vessel lumen on angiography, elevated flow velocities on transcranial Doppler (which implies a narrowed vessel lumen), new neurological symptoms (typically aphasia or weakness, usually 3–14 days after subarachnoid hemorrhage onset), or cerebral ischemia on neuroimaging referable to a narrowed vessel. Infarction on neuroimaging often does not correspond to an area of known vessel narrowing 28, however, making vasospasm an incomplete explanation for cerebral infarction after subarachnoid hemorrhage.
Disease-specific scores for brain hemorrhage
Although coma, a severe neurological deficit, and old age are predictive of mortality after both subarachnoid hemorrhage and brain hemorrhage, mortality after brain hemorrhage often has different causes. The most widely used predictive scale is the intracerebral hemorrhage [ICH] score 29, a composite of 0 (best) to 6 (worst) of hemorrhage size greater than 30 ml, age at least 80 years, intraventricular hemorrhage, cerebellar or brainstem location, and GCS. There are modified versions of the intracerebral hemorrhage score 30, and they are more similar than different. The functional outcome risk stratification scale score 31 also accounts for prehemorrhage cognitive impairment. The NIH Stroke Scale and its components assessing level of consciousness can also be used (along with age) to predict outcome after brain hemorrhage. These scores do not include physiological derangement or events after the initial assessment.
Brain hemorrhage treatment
Subarachnoid hemorrhage
Subarachnoid hemorrhage may depend if it is traumatic or non-traumatic subarachnoid hemorrhage. For traumatic subarachnoid hemorrhage, the ABCs of medicine must occur first. Early consultation with neurosurgery should be considered. If the patient is on anticoagulation or antiplatelet agents consideration should be given to reversing their effects. Care is typically conservative with close assessments of vitals and neurologic status. In obtunded patients, there may be a need for an intracranial pressure (ICP) monitor and/or external ventricular drain (EVD). Patients should be monitored for hydrocephalus or cerebral swelling. Repeat imaging can verify improvement of the traumatic subarachnoid hemorrhage. Sometimes aneurysmal rupture or incompetence of other intracranial vascular malformations can masquerade as traumatic subarachnoid hemorrhage. If there is no clear and convincing history of a traumatic origin, then a non-traumatic etiology for the subarachnoid hemorrhage should be sought.
In non-traumatic subarachnoid hemorrhage, the etiology of the hemorrhage must be ascertained and addressed. Early consultation with neurosurgery should be considered. Treatment varies depending on the etiology of the hemorrhage but can include treatment of an aneurysm or arteriovenous malformation or other etiology. Additionally, there should be a low threshold for placement of an external ventricular drain (EVD) due to the risk of hydrocephalus.
Intraparenchymal hemorrhage
Intraparenchymal hemorrhage can be life-threatening and treatment starts with the ABCs of medicine and stabilization of the patient. Blood pressure should be controlled to decrease the risk of further hemorrhage. Early consultation with neurosurgery should be considered. The treatment of intraparenchymal hemorrhage depends on the etiology of the hemorrhage. Treatment options are variable and include aggressive surgical evacuation, craniectomy, catheter-based dissolution or observation. Surgical evacuation is controversial for some forms of intraparenchymal hemorrhage. Although many intraparenchymal hemorrhages are secondary to cerebrovascular disease and hypertension, the surgeon should anticipate encountering other underlying pathology including an aneurysm, AVM, and/or tumor when evacuating an intraparenchymal hemorrhage. Sometimes evacuation of the hematoma may be more detrimental than the hematoma itself, and a craniectomy is performed instead to allow for cerebral swelling. There are a number of catheter-based systems which try to dissolve the hemorrhage. Discussion of these is beyond the scope of this article. Smaller and non-operable hemorrhages may be managed medically with control of blood pressure, reversal of anticoagulation or antiplatelet agents, and neuroprotective strategies to prevent and/or mitigate secondary cerebral injury.
Epidural hemorrhage
Treatment begins with advanced trauma life support (ATLS) including airway control, ensuring adequate ventilation and circulation 32. Intravenous (IV) access should be secured. If the patient has a Glasgow Coma Score (GCS) of 8 or less or worsening neurologic status, intubation should be performed. Immediate neurosurgical consultation should be obtained for patients with epidural hematomas as they may expand over time due to continued bleeding. Definitive treatment is an evacuation of the hematoma and stopping the bleeding source. Some smaller epidural hematomas may be managed non-surgically and watched closely for resolution.
Subdural hemorrhage
Treatment begins with ensuring adequate airway, breathing, and circulation. Intubation should be considered if the patient has a deteriorating GCS or GCS of 8 or less. Immediate neurosurgical consultation should be obtained as emergency surgery may be required to evacuate the subdural hematoma. Definitive treatment for subdural hematomas is an evacuation, but depending on the size and location some subdural hematomas may be watched for resolution.
Non-surgical management options include repeat imaging to ensure subdural stability, reversal of anticoagulation, platelet transfusions for thrombocytopenia or dysfunctional platelets, observation with frequent neurologic assessments for deterioration, and/or controlling hypertension. There is controversy about whether steroids can help stabilize the size of the subdural hematoma while giving it time to resorb or until surgical treatment.
Surgical management options include twist drill hole, burr hole(s), and craniotomy for evacuation. Data suggests that a twist drill hole has the lowest surgical complication rate with the highest recurrence rate. A craniotomy has the highest surgical complication rate with the lowest recurrence rate of the surgical options, and burr hole(s) evacuation falls somewhere between a twist drill hole and a craniotomy for complication rate and recurrence rate.
Common Principles of Management
Intracranial pressure monitoring
Emergent neurosurgical consultation should be obtained regarding the potential utility of placing a device to measure intracranial pressure. Whether such a device should be placed depends on the nature, severity, and location of the injury. If hydrocephalus is a concern, a ventricular drain will allow both measurement of intracranial pressure and drainage of cerebrospinal fluid. A parenchymal monitor permits pressure measurement of the intracranial pressure at the tip of the device. Some devices also permit measurement of brain temperature and brain oxygen tension; it is not known whether optimization of parameters other than intracranial pressure and cerebral perfusion pressure (mean arterial pressure minus intracranial pressure) leads to improved outcomes.
Elevated intracranial pressure (generally defined as >20 mm Hg) may lead to herniation and death. Interventions to reduce elevated intracranial pressure include sedation to a calm and motionless state, external ventricular drainage of cerebrospinal fluid, optimizing cerebral perfusion pressure (generally 60 to 80 mm Hg), mannitol or hypertonic saline (usually with a goal osmolality of 315–325 mOsm/L), hyperventilation, hypothermia, paralysis, and induced coma with medication (generally with barbiturates) 33.
Cerebral edema
Cerebral edema may result from cerebral ischemia, infarction, or contusion. Mannitol is commonly given for cerebral edema and signs of herniation, although repeated doses may lead to hypovolemia because mannitol is an osmotic diuretic. Hypertonic saline may be slightly more effective 34, and a bolus dose often reverses clinical signs of brain herniation and reduces intracranial pressure 35. Administration of 3% saline as a continuous infusion (0.5–1 ml/kg/h to start) is common, but there are few prospective data on which to base protocols.
Fever
Fever is common after intracranial hemorrhage. Risk factors include intraventricular blood, blood near the pituitary, damage to the anterior hypothalamus (rupture of an aneurysm of the anterior communicating artery notoriously leads to high and recurrent fever), and a ventricular drainage catheter (often placed for intraventricular hemorrhage) 36. Often, an infectious source is not found. Greater burden of fever is related to worse outcomes after intracerebral hemorrhage 37 and subarachnoid hemorrhage 38.
The routine use of acetaminophen for fever control does not improve outcomes 39. There are a variety of devices that ostensibly reduce fever, but no device has improved outcomes in a clinical trial. A circulating cool-air blanket was not effective in one study of critically ill patients in a neurological intensive care unit (ICU) 40, but an external cool-water blanket 41 and invasive catheter reduced core temperatures 42.
Enforced normothermia may lead to shivering. Increased metabolic demand from shivering can be simply and reliably measured at the bedside 43. Shivering can be ablated with sedation, but this confounds the bedside examination and may delay weaning of ventilatory support. Although some data suggest aggressive temperature control will eventually lead to improved outcomes and the expense of more ICU complications 44, it has not yet been done prospectively.
Specific management of intracerebral hemorrhage
Severe hypertension is associated with hematoma growth after the diagnostic CT scan and worse outcomes. This implies that increased arterial pressure translates into more blood extravasation into the brain; conversely, lowering blood pressure (BP) might reduce hematoma growth. A prospective randomized trial of “aggressive” versus conventional BP control found that aggressive control was associated with reduced hematoma growth 45. This study was not powered to detect a difference in clinical outcomes, but such a trial (INTERACT [Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial] 2) is now underway. The major risk (especially in patients with chronic hypertension) is that acute BP lowering might lead to reduced cerebral perfusion, cerebral ischemia, and infarction. Decreased diffusion on magnetic resonance imaging (MRI) (consistent with acute ischemia) is associated with more acute BP reduction in patients with intracerebral hemorrhage 46, and is in turn associated with increased odds of death or dependence at 3 months in multivariate models 47. The most recent guidelines on management of intracerebral hemorrhage 48 reflect the uncertainty of how best to acutely manage hypertension in this setting.
A hematoma may compress brain tissue or the outflow of cerebrospinal fluid, leading to herniation and death. Emergent neurosurgical consultation is advisable to assess the suitability of decompression. Generally agreed-on indications for surgical decompression include brainstem compression, hydrocephalus, or cerebellar hemorrhage with neurological deterioration 49. Relative indications include a cortical (as opposed to deep) or cerebellar location, a steadily worsening clinical examination, and larger hemorrhage size. When the suitability of emergent surgical decompression was not clear, a randomized trial found no benefit to emergent decompression over expectant management 49. A subpopulation that may be especially helped by surgery (cortical location and lack of ventricular hemorrhage) is eligible for enrollment in a follow-up trial (Surgical Trial in Intracerebral Haemorrhage [STICH] 2).
Warfarin use and an elevated INR are associated with hematoma growth and worse outcome in patients who present with nontraumatic intracerebral hemorrhage 50. The greater the INR, the greater the risk of hematoma growth on serial CT scans; the longer the time to normalization of the INR, the worse the clinical outcome is likely to be. It is generally agreed that anticoagulant therapy should be reversed, but the best way to do so is not known. Fresh frozen plasma (FFP) is most commonly used because of its availability and relatively low cost, and may be given in repeated doses until the INR is 1.4 or less. Prothrombin complex concentrates (PCCs) may be superior to fresh frozen plasma for warfarin-related intracerebral hemorrhage, chiefly because of faster normalization of the INR 51. A prospective, randomized trial of fresh frozen plasma versus prothrombin complex concentrate (INR Normalization in Coumadin-associated brain hemorrhage) for acute treatment is currently underway. Prothrombin complex concentrates are not a single product, but a variety of related products; before selecting one for routine use, consultation with a hematologist for the best available product and dose is advisable.
Antiplatelet therapies 52 and reduced platelet activity 53 are associated with a higher risk of hematoma growth and death. Platelet transfusion for intracerebral hemorrhage is considered experimental 49, not standard of care, and two clinical trials of platelet transfusion for acute intracerebral hemorrhage are underway 54. Desmopressin (DDAVP) reverses the effect of aspirin and improves platelet activity in other conditions 55 but has not been formally studied in patients with intracerebral hemorrhage.
Procoagulant medication is another potentially effective method to reduce hemorrhage growth and improve outcomes. One pilot study evaluated the use of aminocaproic acid for acute intracerebral hemorrhage, but early results were not promising 56. Recombinant activated factor VII led to reduced hematoma growth and better 90-day outcomes in a phase II trial 57. The phase III trial also showed reduced hematoma growth, but there was no effect on 90-day outcomes 58, and the drug was not approved by the U.S. Food and Drug Administration. There is an increased risk of thrombotic complications with factor VII compared with placebo. Procoagulant therapy is not generally used for the reversal of warfarin-related intracerebral hemorrhage, and blood products (typically plasma) would be subsequently required 49.
Seizures and anticonvulsant medication
Seizures after intracerebral hemorrhage usually occur within a few days of intracerebral hemorrhage symptom onset and are associated with lobar location of hemorrhage, larger hemorrhage size, depressed mental status, and a history of epilepsy. Patients with intracerebral hemorrhage due to hepatic failure may be at particularly high risk 59. Clinical seizures should be treated with anticonvulsant medication. When subclinical seizures are suspected or a decreased level of consciousness is unexplained, continuous electroencephalography should be considered 49. Prophylactic anticonvulsant therapy is associated with more fever and worse functional outcomes 60, however, so it should not be used routinely.
Anemia and packed red blood cell transfusion
The brain is dependent on a continuous supply of nutrients, especially oxygen. Almost all oxygen in blood is bound to hemoglobin. It follows that progressive anemia, a state of reduced hemoglobin concentration in blood, will eventually lead to reduced oxygen delivery to the brain. Anemia is common in patients with intracerebral hemorrhage, and is likely to be multifactorial. Anemia is associated with worse outcomes, but the relationship is confounded by larger hematoma volume 61. Packed red blood cell transfusion is associated with reduced mortality after intracerebral hemorrhage 62, although the proximate mechanism is not clear.
Brain hemorrhage recovery
Brain hemorrhage recovery depends on the type and extent of bleed, age, other comorbidity and severity of neurological deficit at the time of admission 63.
Brain hemorrhage survival rate
Brain hemorrhage survival rate depends on the type and extent of bleed, age, other comorbidity and severity of neurological deficit at the time of admission 64.
A small amount of traumatic subarachnoid hemorrhage or small peri mesencephalic bleeds has an excellent prognosis with little if any significant long-term sequelae. A grade V aneurysmal subarachnoid, on the other hand, has a dismal prognosis, despite aggressive treatment.
Although subdural hematomas are often thought of as relatively benign entities it should be noted that the mortality in acute subdural hematomas requiring surgery is very high (50-90%), particularly in patients who are anticoagulated, and that only 20% fully recover 65.
Epidural hematoma (extradural hematoma) prognosis, even with a relatively large hematoma, is in general quite good, as long as the clot is evacuated promptly. A smaller hematoma without mass effect or swirl sign can be treated conservatively 66, sometimes resulting in calcification of the dura.
Occasionally epidural hematoma (extradural hematoma) late complications are encountered, usually relating to the injured meningeal vessel. They include:
- pseudoaneurysm
- arteriovenous fistula
- Kohli R, Chaturvedi S. Epidemiology and Clinical Manifestations of Immune Thrombocytopenia. Hamostaseologie. 2019 Mar 13[↩]
- Systematic characterization of the computed tomography angiography spot sign in primary intracerebral hemorrhage identifies patients at highest risk for hematoma expansion: the spot sign score. Delgado Almandoz JE, Yoo AJ, Stone MJ, Schaefer PW, Goldstein JN, Rosand J, Oleinik A, Lev MH, Gonzalez RG, Romero JM. Stroke. 2009 Sep; 40(9):2994-3000.[↩]
- Mayer SA, Bernardini GL, Solomon RA. Subarachnoid hemorrhage. In: Rowland LP, Pedley TA, editors. , editors Merritt’s neurology, 12th ed Philadelphia: Lippincott Williams & Wilkins; 2010[↩]
- Venous drainage in perimesencephalic hemorrhage. van der Schaaf IC, Velthuis BK, Gouw A, Rinkel GJ. Stroke. 2004 Jul; 35(7):1614-8.[↩]
- Naidech AM. Intracranial hemorrhage. Am J Respir Crit Care Med. 2011;184(9):998–1006. doi:10.1164/rccm.201103-0475CI https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3361326[↩]
- Tenny S, Thorell W. Intracranial Hemorrhage. [Updated 2019 Mar 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470242[↩][↩][↩][↩][↩][↩][↩]
- Spetzler RF, McDougall CG, Zabramski JM, Albuquerque FC, Hills NK, Nakaji P, Karis JP, Wallace RC. Ten-year analysis of saccular aneurysms in the Barrow Ruptured Aneurysm Trial. J. Neurosurg. 2019 Mar 08;:1-6[↩]
- Subarachnoid hemorrhage. https://radiopaedia.org/cases/sah[↩]
- Kanematsu R, Hanakita J, Takahashi T, Park S, Minami M. Radiologic Features and Clinical Course of Chronic Spinal Epidural Hematoma: Report of 4 Cases and Literature Review. World Neurosurg. 2018 Dec;120:82-89.[↩]
- Babu JM, Patel SA, Palumbo MA, Daniels AH. Spinal Emergencies in Primary Care Practice. Am. J. Med. 2019 Mar;132(3):300-306[↩]
- Extradural hematoma. https://radiopaedia.org/cases/extradural-haematoma[↩]
- Josephson CB, Frantzias J, Samarasekera N, Al-Shahi Salman R. The persisting burden of intracerebral haemorrhage: can effective treatments be found? PLoS Med 2010;7:e1000353[↩]
- He J, Whelton PK, Vu B, Klag M. Aspirin and the risk of hemorrhage stroke: a meta-analysis of randomized controlled trials. JAMA 1998;280:1930–1935[↩]
- Knudsen KARJ, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology 2001;56:537–539[↩]
- Wermer MJH, van der Schaaf IC, Algra A, Rinkel GJE. Risk of rupture of unruptured intracranial aneurysms in relation to patient and aneurysm characteristics: an updated meta-analysis. Stroke 2007;38:1404–1410[↩]
- Greving JP, Rinkel GJE, Buskens E, Algra A. Cost-effectiveness of preventive treatment of intracranial aneurysms. Neurology 2009;73:258–265[↩]
- Broderick JP, Viscoli CM, Brott T, Kernan WN, Brass LM, Feldmann E, Morgenstern LB, Wilterdink JL, Horwitz RI. Major risk factors for aneurysmal subarachnoid hemorrhage in the young are modifiable. Stroke 2003;34:1375–1381[↩]
- Broderick JP, Brown RD Jr, Sauerbeck L, Hornung R, Huston J III, Woo D, Anderson C, Rouleau G, Kleindorfer D, Flaherty ML, et al. ; FIA Study Investigators Greater rupture risk for familial as compared to sporadic unruptured intracranial aneurysms. Stroke 2009;40:1952–1957[↩]
- Ishibashi T, Murayama Y, Urashima M, Saguchi T, Ebara M, Arakawa H, Irie K, Takao H, Abe T. Unruptured intracranial aneurysms: incidence of rupture and risk factors. Stroke 2009;40:313–316[↩]
- Burns JD, Huston J III, Layton KF, Piepgras DG, Brown RD Jr. Intracranial aneurysm enlargement on serial magnetic resonance angiography: frequency and risk factors. Stroke 2009;40:406–411[↩]
- Marcia L, Moazzez A, Plurad DS, Putnam B, Kim DY. Utility of Repeat Head CT in Patients on Preinjury Antithrombotic Medications. Am Surg. 2018 Oct 01;84(10):1626-1629[↩]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet 1974;2:81–84[↩]
- Wijdicks EFM, Bamlet WR, Maramattom BV, Manno EM, McClelland RL. Validation of a new coma scale: the FOUR score. Ann Neurol 2005;58:585–593[↩]
- Brott T, Adams HP, Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864–870[↩]
- Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 1968;28:14–20[↩]
- Julien JS, Bandeen-Roche K, Tamargo RJ. Validation of an aneurysmal subarachnoid hemorrhage grading scale in 1532 consecutive patients. Neurosurgery 2008;63:204–211[↩]
- Claassen J, Bernardini GL, Kreiter K, Bates J, Du YE, Copeland D, Connolly ES, Mayer SA. Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage: the Fisher Scale revisited. Stroke 2001;32:2012–2020[↩]
- Rabinstein AA, Friedman JA, Weigand SD, McClelland RL, Fulgham JR, Manno EM, Atkinson JL, Wijdicks EF. Predictors of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke 2004;35:1862–1866[↩]
- Hemphill JC, III, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001;32:891–897[↩]
- Weimar C, Benemann J, Diener H-C. Development and validation of the Essen Intracerebral Haemorrhage Score. J Neurol Neurosurg Psychiatry 2006;77:601–605[↩]
- Rost NS, Smith EE, Chang Y, Snider RW, Chanderraj R, Schwab K, FitzMaurice E, Wendell L, Goldstein JN, Greenberg SM, et al. ; Prediction of functional outcome in patients with primary intracerebral hemorrhage: the FUNC score. Stroke 2008;39:2304–2309[↩]
- Acciarresi M, Altavilla R, Mosconi MG, Caso V. Management of intracranial haemorrhage, unruptured aneurysms and arteriovenous malformations during and after pregnancy. Curr. Opin. Neurol. 2019 Feb;32(1):36-42[↩]
- Dennis LJ, Mayer SA. Diagnosis and management of increased intracranial pressure. Neurol India 2001;49:S37–S50[↩]
- Kamel H, Navi BB, Nakagawa K, Hemphill JC, III, Ko NU. Hypertonic saline versus mannitol for the treatment of elevated intracranial pressure: a meta-analysis of randomized clinical trials. Crit Care Med 2011;39:554–559[↩]
- Koenig MA, Bryan M, Lewin JL, III, Mirski MA, Geocadin RG, Stevens RD. Reversal of transtentorial herniation with hypertonic saline. Neurology 2008;70:1023–1029[↩]
- Commichau C, Scarmeas N, Mayer SA. Risk factors for fever in the neurologic intensive care unit. Neurology 2003;60:837–841[↩]
- Schwarz S, Hafner K, Aschoff A, Schwab S. Incidence and prognostic significance of fever following intracerebral hemorrhage. Neurology 2000;54:354–361[↩]
- Todd MM, Hindman BJ, Clarke WR, Torner JC, Weeks JB, Bayman EO, Shi Q, Spofford CM. Perioperative fever and outcome in surgical patients with aneurysmal subarachnoid hemorrhage. Neurosurgery 2009;64:897–908, discussion 908[↩]
- den Hertog HM, van der Worp HB, van Gemert HM, Algra A, Kappelle LJ, van Gijn J, Koudstaal PJ, Dippel DW. The Paracetamol (Acetaminophen) in Stroke (PAIS) trial: a multicentre, randomised, placebo-controlled, phase III trial. Lancet Neurol 2009;8:434–440[↩]
- Mayer S, Commichau C, Scarmeas N, Presciutti M, Bates J, Copeland D. Clinical trial of an air-circulating cooling blanket for fever control in critically ill neurologic patients. Neurology 2001;56:292–298[↩]
- Mayer SA, Kowalski RG, Presciutti M, Ostapkovich ND, McGann E, Fitzsimmons BF, Yavagal DR, Du YE, Naidech AM, Janjua NA, et al. Clinical trial of a novel surface cooling system for fever control in neurocritical care patients. Crit Care Med 2004;32:2508–2515[↩]
- Diringer MN. Treatment of fever in the neurologic intensive care unit with a catheter-based heat exchange system. Crit Care Med 2004;32:559–564[↩]
- Badjatia N, Strongilis E, Gordon E, Prescutti M, Fernandez L, Fernandez A, Buitrago M, Schmidt JM, Ostapkovich ND, Mayer SA. Metabolic impact of shivering during therapeutic temperature modulation: the Bedside Shivering Assessment Scale. Stroke 2008;39:3242–3247[↩]
- Badjatia N, Fernandez L, Schmidt JM, Lee K, Claassen J, Connolly ES, Mayer SA. Impact of induced normothermia on outcome after subarachnoid hemorrhage: a case–control study. Neurosurgery 2010;66:696–700; discussion 700–691[↩]
- Anderson C, Huang Y, Wang J, Arima H, Neal B, Peng B, Heeley E, Skulina C, Parsons M, Kim J, et al. Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT): a randomised pilot trial. Lancet Neurol 2008;7:391–399[↩]
- Prabhakaran S, Gupta R, Ouyang B, John S, Temes RE, Mohammad Y, Lee VH, Bleck TP. Acute brain infarcts after spontaneous intracerebral hemorrhage: a diffusion-weighted imaging study. Stroke 2010;41:89–94[↩]
- Garg RK, Liebling SM, Maas MB, Nemeth AJ, Russell EJ, Naidech AM. Blood pressure reduction, decreased diffusion on MRI, and outcomes after intracerebral hemorrhage. Stroke. In press.[↩]
- Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, Karimi A, Shaw MD, Barer DH; STICH Investigators Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet 2005;365:387–397[↩]
- Morgenstern LB, Hemphill JC, III, Anderson C, Becker K, Broderick JP, Connolly ES, Jr, Greenberg SM, Huang JN, Macdonald RL, Messe SR, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2010;41:2108–2129[↩][↩][↩][↩][↩]
- Flibotte J, Hagan N, O’Donnell J, Greenberg S, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology 2004;63:1059–1064[↩]
- Huttner HB, Schellinger PD, Hartmann M, Kohrmann M, Juettler E, Wikner J, Mueller S, Meyding-Lamade U, Strobl R, Mansmann U, et al. Hematoma growth and outcome in treated neurocritical care patients with intracerebral hemorrhage related to oral anticoagulant therapy: comparison of acute treatment strategies using vitamin K, fresh frozen plasma, and prothrombin complex concentrates. Stroke 2006;37:1465–1470[↩]
- Saloheimo P, Ahonen M, Juvela S, Pyhtinen J, Savolainen E, Hillborm M. Regular aspirin use preceding the onset of primary intracerebral hemorrhage is an independent predictor for death. Stroke 2006;37:129–133[↩]
- Thompson BB, Bejot Y, Caso V, Castillo J, Christensen H, Flaherty ML, Foerch C, Ghandehari K, Giroud M, Greenberg SM, et al. Prior antiplatelet therapy and outcome following intracerebral hemorrhage: a systematic review. Neurology 2010;75:1333–1342[↩]
- de Gans K, de Haan RJ, Majoie CB, Koopman MM, Brand A, Dijkgraaf MG, Vermeulen M, Roos YB. PATCH: platelet transfusion in cerebral haemorrhage: study protocol for a multicentre, randomised, controlled trial. BMC Neurol 2010;10:19[↩]
- Franchini M. The use of desmopressin as a hemostatic agent: a concise review. Am J Hematol 2007;82:731–735[↩]
- Piriyawat P, Morgenstern LB, Yawn DH, Hall CE, Grotta JC. Treatment of acute intracerebral hemorrhage with ε-aminocaproic acid: a pilot study. Neurocrit Care 2004;1:47–51[↩]
- Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer M, Skolnick BE, Steiner T. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2005;352:777–785[↩]
- Mayer SA, Brun NC, Broderick J, Davis S, Diringer MN, Skolnick BE, Steiner T. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2008;358:2127–2137[↩]
- Bhatia V, Batra Y, Acharya SK. Prophylactic phenytoin does not improve cerebral edema or survival in acute liver failure—a controlled clinical trial. J Hepatol 2004;41:89–96[↩]
- Naidech AM, Garg RK, Liebling S, Levasseur K, Macken MP, Schuele SU, Batjer HH. Anticonvulsant use and outcomes after intracerebral hemorrhage. Stroke 2009;40:3810–3815[↩]
- Kumar MA, Rost NS, Snider RW, Chanderraj R, Greenberg SM, Smith EE, Rosand J. Anemia and hematoma volume in acute intracerebral hemorrhage. Crit Care Med 2009;37:1442–1447[↩]
- Sheth KN, Gilson AJ, Chang Y, Kumar MA, Rahman RM, Rost NS, Schwab K, Cortellini L, Goldstein JN, Smith EE, Greenberg SM, Rosand J. Packed red blood cell transfusion and decreased mortality in intracerebral hemorrhage. Neurosurgery 2011;68:1286–1292[↩]
- Yi HJ, Sung JH, Lee MH, Lee DH. Experience of the New FlowGate2 Device as a Balloon Guide Catheter for Ischemic Stroke Intervention. World Neurosurg. 2019 Mar 06[↩]
- Kim CH, Kim SE, Jeon JP. Meta-Analysis of Endovascular Treatment for Acute M2 Occlusion. J Korean Neurosurg Soc. 2019 Mar;62(2):193-200[↩]
- Jallo J, Loftus CM. Neurotrauma and Critical Care of the Brain. Thieme Medical Pub. (2009) ISBN:1604060328[↩]
- Sullivan TP, Jarvik JG, Cohen WA. Follow-up of conservatively managed epidural hematomas: implications for timing of repeat CT. AJNR Am J Neuroradiol. 1999;20 (1): 107-13.[↩]