How to test for HIV
HIV stands for human immunodeficiency virus. It is the virus that can lead to acquired immunodeficiency syndrome, or AIDS. Unlike some other viruses, the human body cannot get rid of HIV. That means that once you have HIV, you have it for life. An estimated 1.1 million people in the United States are living with HIV, including about 162,500 people who are unaware of their status. About 1 in 7 people in the United States who have HIV don’t know they have it. Approximately 40% of new HIV infections are transmitted by people who are living with undiagnosed HIV. For those who are living with undiagnosed HIV, testing is the first step in maintaining a healthy life and reducing the spread of HIV. And the only way to know for sure whether you have HIV is to get tested. Testing associated with HIV/AIDS involves detecting HIV antigen (p24) and/or the affected person’s response to HIV (antibodies), measuring the amount of virus, or detecting the viral nucleic acid. The goals of HIV testing are to:
- Screen for and diagnose HIV infection
- Measure and monitor the amount of virus in the person’s blood (the viral load)
- Evaluate HIV’s resistance to available drug therapies
Centers for Disease Control and Prevention (CDC) recommends that everyone between the ages of 13 and 64 get tested at least once as a part of their routine health care 1. People with higher risk factors, such as more than one sex partner, other STDs, gay and bisexual men and individuals who inject drugs should be tested at least once a year.
If you’re pregnant, talk to your health care provider about getting tested for HIV and other ways to protect you and your baby from getting HIV. Ideally, all women should be screened for HIV during each pregnancy at their initial prenatal care visit or as early in pregnancy as possible. CDC also recommends a second HIV test during a woman’s third trimester for women who meet certain criteria, including: a) those who continue behaviors with a high risk for acquiring HIV, b) residing in specific high-prevalence jurisdictions, and c) receiving health care in facilities with at least 1 diagnosed HIV case per 1,000 pregnant women per year.
Preventive antiviral therapy is most effective when it is initiated early in pregnancy. However, starting antiretroviral treatment during labor and delivery, or even providing it to the newborn within hours after birth can reduce mother-to-child transmission by half 2. To maximize the benefit, it is important to obtain HIV test results for women in labor quickly in order to start antiretroviral therapy as soon as possible. CDC recommends that clinicians test for HIV any newborn whose mother’s HIV status is unknown. For those women whose HIV status is unknown at labor, CDC recommends routine, rapid HIV testing. When the mother’s HIV status is unknown prior to the onset of labor and rapid HIV testing is not done during labor, CDC recommends rapid HIV testing of the infant immediately post-partum, so that antiretroviral prophylaxis can be offered to HIV-exposed infants. When intervention begins at the intrapartum (during labor or delivery) or neonatal periods, 9% to 13% HIV transmission rates are achievable based on clinical trial and observational data. This represents a 50% reduction in HIV transmission from rates that would be expected without intervention.
You should get tested for HIV at least once a year if:
- You’re a sexually active gay or bisexual man. Some sexually active gay and bisexual men may benefit from more frequent testing (every 3 to 6 months).
- You’ve had sex with an HIV-positive partner.
- You’ve had more than one partner since your last HIV test.
- You’ve shared needles or works to inject drugs.
- You’ve exchanged sex for drugs or money.
- You have another sexually transmitted disease, hepatitis, or tuberculosis.
- You’ve had sex with anyone who has done anything listed above or with someone whose sexual history you don’t know.
You should be tested at least once a year if you keep doing any of these things. Sexually active gay and bisexual men may benefit from more frequent testing (for example, every 3 to 6 months).
Only certain body fluids; blood, semen (cum), pre-seminal fluid (pre-cum), rectal fluids, vaginal fluids, and breast milk from a person who has HIV can transmit HIV. These fluids must come in contact with a mucous membrane or damaged tissue or be directly injected into the bloodstream (from a needle or syringe) for transmission to occur. Mucous membranes are found inside the rectum, vagina, penis, and mouth. Early HIV infection often times has no symptoms. The only way to know if you are infected with HIV is to be tested. Currently, there is no effective cure that exists for HIV. However, with proper medical care, HIV can be controlled.
Before having sex for the first time with a new partner, you and your partner should talk about your sexual and drug-use history, disclose your HIV status, and consider getting tested for HIV and learning the results.
Knowing your HIV status gives you powerful information to help you take steps to keep you and your partner healthy.
- If you test positive for HIV, you can take medicine to treat HIV to stay healthy for many years and greatly reduce the chance of transmitting HIV to your sex partner.
- If you test negative for HIV, you have more prevention tools available today to prevent HIV than ever before.
- If you are pregnant for HIV, you should be tested for HIV so that you can begin treatment if you’re HIV-positive. If an HIV-positive woman is treated for HIV early in her pregnancy, the risk of transmitting HIV to her baby is very low.
What happens during an HIV test?
You will either get a blood test in a lab, or do your own test at home.
For a blood test in a lab:
A health care professional will take a blood sample from a vein in your arm, using a small needle. After the needle is inserted, a small amount of blood will be collected into a test tube or vial. You may feel a little sting when the needle goes in or out. This usually takes less than five minutes.
For at home test, you will need to get a sample of saliva from your mouth or a drop of blood from your fingertip.
The test kit will provide instructions on how to get your sample, package it, and send it to a lab.
- For a saliva test, you will use special spatula-like tool to take a swab from your mouth.
- For a fingertip antibody blood test, you will use a special tool to prick your finger and collect a sample of blood.
For more information on at-home testing, talk to your health care provider.
Will other people know my HIV test result?
If you take an anonymous test, no one but you will know the result. If you take a confidential test, your test result will be part of your medical record, but it is still protected by state and federal privacy laws.
- Anonymous testing means that nothing ties your test results to you. When you take an anonymous HIV test, you get a unique identifier that allows you to get your test results.
- Confidential testing means that your name and other identifying information will be attached to your test results. The results will go in your medical record and may be shared with your health care providers and your health insurance company. Otherwise, the results are protected by state and federal privacy laws, and they can be released only with your permission.
With confidential testing, if you test positive for HIV, the test result and your name will be reported to the state or local health department to help public health officials get better estimates of the rates of HIV in the state. The state health department will then remove all personal information about you (name, address, etc.) and share the remaining non-identifying information with CDC. CDC does not share this information with anyone else, including insurance companies.
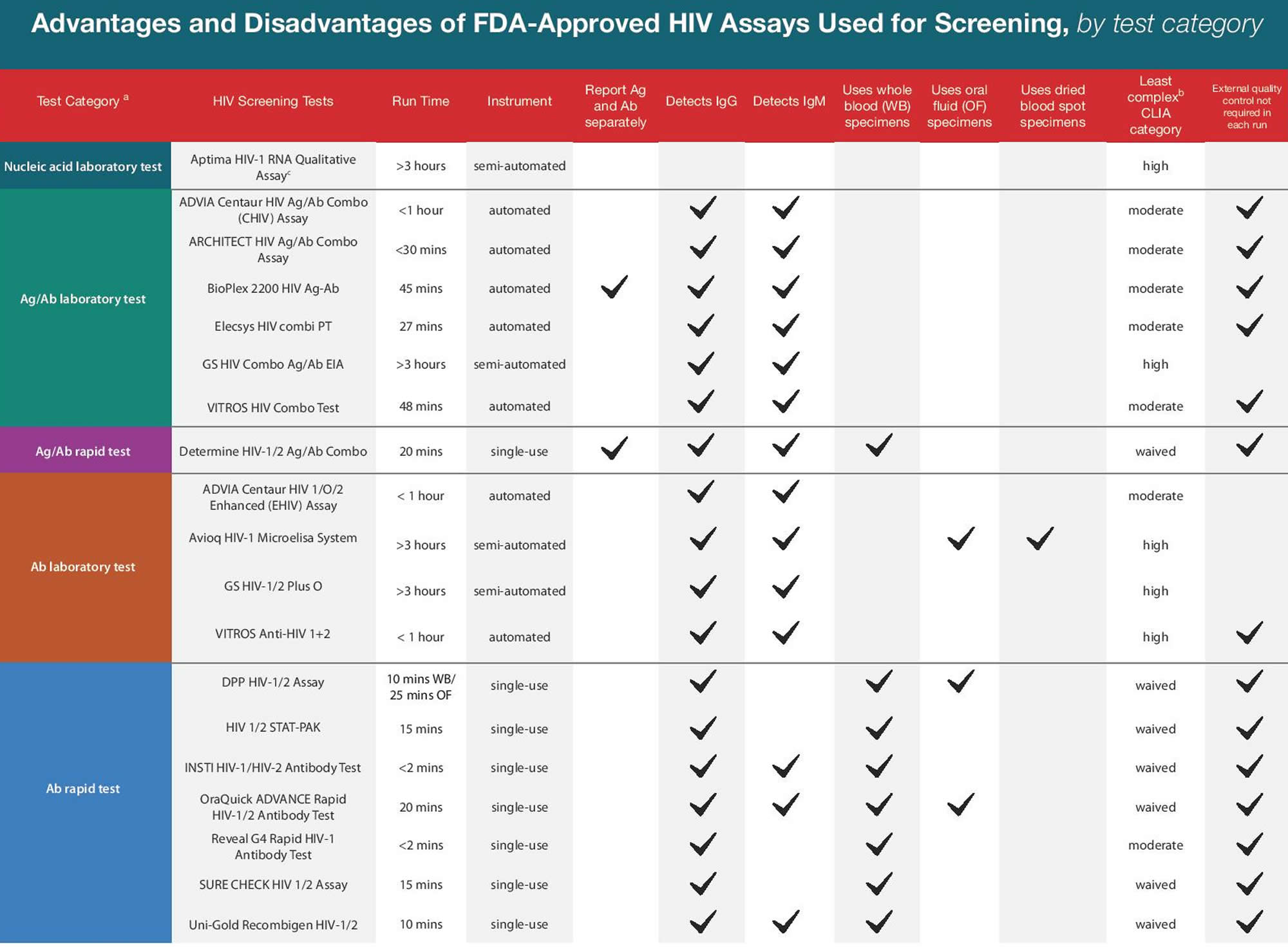
HIV test types
HIV tests are very accurate at detecting HIV, but no HIV test can detect HIV immediately after infection. How soon a test can detect infection depends upon different factors, including the type of HIV test being used. In general, nucleic acid tests (NAT) can detect HIV the soonest, followed by combination or fourth generation tests, and then antibody tests.
There are three types of HIV diagnostic tests: nucleic acid tests (NAT), antigen/antibody tests, and antibody tests.
- Nucleic acid tests (NAT) look for the actual virus in the blood. Nucleic acid test (NAT) is very expensive and is not routinely used for HIV screening unless the person recently had a high-risk exposure or a possible exposure with early symptoms of HIV infection. The NAT test can give either a positive/negative result or an amount of virus present in the blood (known as an HIV viral load test). Nucleic acid testing is usually considered accurate during the early stages of infection. Nucleic acid tests (NAT) can usually tell you if you are infected with HIV 10 to 33 days after an exposure. However, it is best to get an antibody (Ab) or antigen/antibody (Ag/Ab) test at the same time to help the health care provider understand what a negative NAT means. Taking pre-exposure prophylaxis (PrEP) or post-exposure prophylaxis (PEP) may also reduce the accuracy of NAT if you have HIV.
- Antigen/antibody tests look for both HIV antibodies and antigens. Antibodies are produced by your immune system when you’re exposed to bacteria or viruses like HIV. Antigens are foreign substances that cause your immune system to activate. If you’re infected with HIV, an antigen called p24 is produced even before antibodies develop. Tests that detect both antigen and antibodies are recommended for testing done in labs and are now common in the United States. There is also a rapid antigen/antibody test available. This test can usually find HIV within 2–6 weeks of infection.
- Antibody tests (also called immunoassay) detect the presence of antibodies, proteins that a person’s body makes against HIV, not HIV itself. Most rapid tests and home tests are antibody tests. In general, antibody tests that use blood from a vein can detect HIV sooner after infection than tests done with blood from a finger prick or with oral fluid. An HIV antibody test can determine if you have HIV from 3–12 weeks after infection. That’s because it can take a few weeks or longer for your immune system to make antibodies to HIV.
- While most laboratories are now using antigen/antibody tests, laboratory-based antibody screening tests are still available. These tests require blood to be drawn from your vein into a tube and then that blood is sent to a laboratory for testing. The results may take several days to be available.
- With a rapid antibody screening test, results are ready in 30 minutes or less. These tests are used in clinical and nonclinical settings, usually with blood from a finger prick or with oral fluid.
- The oral fluid antibody self-test provides fast results. You have to swab your own mouth to collect an oral fluid sample and use a kit to test it. Results are available in 20 minutes. The manufacturer provides confidential counseling and referral to follow-up testing sites. These tests are available for purchase in stores and online. They may be used at home, or they may be used for testing in some community and clinic testing programs.
- The home collection kit involves pricking your finger to collect a blood sample, sending the sample by mail to a licensed laboratory, and then calling in for results as early as the next business day. This antibody test is anonymous. The manufacturer provides confidential counseling and referral to treatment.
Most HIV tests, including most rapid tests and home tests, are antibody tests. Antibodies are produced by your immune system when you’re exposed to viruses like HIV and bacteria. Antibody tests look for these antibodies to HIV in your blood or oral fluid. In general, antibody tests that use blood can detect HIV slightly sooner after infection than tests done with oral fluid.
If you use any type of HIV antibody test and have a positive result, you will need to take a follow-up test to confirm your results. If your first HIV test is a rapid home test and it’s positive, you will be sent to a health care provider to get follow-up testing. If your first HIV test is done in a testing lab and it’s positive, the lab will conduct the follow-up testing, usually on the same blood sample as the first test.
A combination, or fourth-generation test looks for both HIV antibodies and antigens. Antigens are foreign substances that cause your immune system to activate. The antigen is part of the virus itself and is present during acute HIV infection. If you’re infected with HIV, an antigen called p24 is produced even before antibodies develop. Combination screening tests are now recommended for testing done in labs and are becoming more common in the United States. There is now a rapid combination test available.
Nucleic acid tests (NAT) look for HIV in the blood. The NAT HIV test can give either a positive/negative result or an actual amount of virus present in the blood (known as a viral load test). This test is very expensive and not routinely used for screening individuals unless they recently had a high-risk exposure or a possible exposure with early symptoms of HIV infection.
What influence does specimen type have on HIV testing?
Laboratory-based serum or plasma assays generally offer higher sensitivities but require venipuncture, larger specimen volumes, processing, and skilled technicians. Point-of-care tests are attractive alternatives for many applications, but performance differs substantially depending on the specimen type; tests using oral transudate are significantly less sensitive than those using whole blood, and tests using whole blood are less sensitive than those using serum or plasma 3. This hierarchy is at least partially explained by the relative concentration of detectable target(s) per volume of specimen tested. Forty-five percent of whole blood is made up of cells, so additional processing to yield plasma or serum further concentrates antigens and antibodies per unit volume.
Compared with plasma, oral transudate immunoglobulin concentrations are 300-fold lower 4. Because oral antibodies rise to detectable levels later than those in blood, the window period is prolonged when oral fluid is used for testing. This was demonstrated most clearly in a study of HIV seroconverters in Nigeria, in which antibodies in oral transudate became detectable a median of 29 days after those in plasma 5.
What advantages do point-of-care HIV test have over laboratory-based HIV tests?
The introduction of the first FDA-approved rapid test in 2002 and changes in regulations permitting use of certain tests outside laboratory settings revolutionized the process of getting tested for HIV 6. Point-of-care HIV tests are portable and easy to use, and the ability to perform HIV testing on oral transudate or fingerstick whole blood eliminates the need for venipuncture and the handling, processing, or storage of blood. This can make the entire encounter self-contained, allowing for anonymous testing where permitted by law. Point-of-care HIV tests also greatly increase the likelihood that the patient receives his or her result. These tests have one key disadvantage, however: although the 8 current FDA-approved point-of-care tests have excellent and generally comparable performance characteristics, laboratory-based HIV tests have greater sensitivity, especially early after infection.
Indeed, acute HIV-1 infection remains a critical blind spot for current point-of-care HIV tests. Because acute HIV-1 infection is characterized by the presence of p24 or HIV-1 RNA in the absence of detectable anti–HIV-1 antibodies, antibody-only tests cannot diagnose acute HIV-1 infection. Multiple laboratory-based p24/IgM/IgG platforms are available, but development of a point-of-care version with comparable performance has proved more challenging. The first such test was approved by the FDA in 2013 (Determine HIV-1/2 Ag/Ab Combo; Alere, Stockport, UK). Field evaluations have demonstrated excellent sensitivity for antibody detection but variable, suboptimal sensitivity for p24 7. Studies of a newer version of this same test suggest interim improvements, with antigen sensitivity as high as 88% 8. Using plasma instead of whole blood enhances test performance, suggesting a role for this test in situations when laboratory support is available but an automated platform is not 3.
How should PrEP use influence HIV test selection?
Data are accumulating that users of tenofovir disoproxil fumarate (TDF)–based PrEP who become infected in the context of poor adherence may have delayed seroconversion on blood-based and oral point-of-care HIV tests. In the Partners PrEP Study, participants underwent point-of-care HIV testing on whole blood with both IgM/IgG and p24/IgM/IgG-sensitive tests and had samples sent for multiple laboratory-based assays every 3 months 9. Delayed seroconversion was defined as more than 100 days between the first laboratory-based evidence of infection and the first point-of-care HIV test reactivity for the participant. Compared with those on placebo, participants with any pharmacologic evidence of adherence during the seroconversion period had 7.2 times the odds of delayed seroconversion; plasma RNA levels were lower, as well, but still detectable 9. Among participants randomized to receive single-agent tenofovir disoproxil fumarate (TDF)–based PrEP in the Bangkok Tenofovir Study who subsequently became HIV infected, oral antibody reactivity trailed the first evidence of HIV (antibody or RNA) in blood by a median of 126.5 days 10. Taken together, these findings underscore the importance of using laboratory-based serum or plasma tests whenever possible in the management of PrEP users and having a low threshold for augmenting p24/IgM/IgG-sensitive assays with nucleic acid testing (NAT) among users with known or suspected poor adherence.
Testing after a HIV exposure event
HIV testing is often prompted by a defined exposure, such as a needlestick injury, condom failure, or condomless sex. Because no diagnostic test is capable of detecting infection in the eclipse period, baseline testing is obtained to rule out undiagnosed, preexisting infection in the patient. Blood-based point-of-care HIV testing should be used whenever possible, to facilitate prompt initiation of postexposure prophylaxis (if indicated) 11. Strong preference should be given to an Ag/Ab combination test over antibody-only options, and oral fluid should not be used. Follow-up testing should occur 4 to 6 weeks and 3 months after baseline, with additional testing at 6 months if the exposure event resulted in hepatitis C virus infection 12. These recommended time points reflect expert opinion rather than a strict adherence to window period durations. If the patient tests negative at the end of the window period, one can be very confident that he or she did not acquire HIV from the exposure.
Outreach HIV testing in developed countries
Mobile testing units, syringe exchange programs, and community testing events place a premium on test portability, ease of administration, flexibility in specimen type, and rapid turnaround of results, all of which make point-of-care HIV tests an obvious, frequent choice. However, longer window periods and lower sensitivities with point-of-care HIV assays compared with their laboratory-based counterparts are important trade-offs to consider. Because some outreach testing populations may be more likely to have recent HIV infection, IgM/IgG-sensitive point-of-care HIV tests (including p24/IgM/IgG-sensitive assays) should be prioritized over those capable of detecting IgG only. Whenever logistically feasible, serum or plasma should be tested instead of whole blood, and consideration should be given to sending a specimen for laboratory platform testing in addition to the point-of-care HIV test. Oral transudate should be used as a specimen only when obtaining blood (through venipuncture or fingerstick) is impractical or undesirable 13. Patients and testing clients considering oral HIV self-testing should be counseled about its longer window period compared with either blood-based point-of-care HIV tests or laboratory-based assays.
Over the counter HIV test
Currently there are only two home HIV test kits:
- Home Access HIV-1 Test System and
- OraQuick In-home HIV test.
If you buy your home test online make sure the HIV test is United States Food and Drug Administration (FDA)-approved 14.
Home Access HIV-1 Test System
The Home Access HIV-1 Test System is a home collection kit, which involves pricking your finger to collect a blood sample, sending the sample to a licensed laboratory, and then calling in for results as early as the next business day. This test is anonymous. If the test is positive, a follow-up test is performed right away, and the results include the follow-up test. The manufacturer provides confidential counseling and referral to treatment. The tests conducted on the blood sample collected at home find infection later after infection than most lab-based tests using blood from a vein, but earlier than tests conducted with oral fluid.
How reliable is the Home Access HIV-1 Test System?
Clinical studies reported to FDA showed that the sensitivity (i.e., the percentage of results that will be positive when HIV is present) was estimated to be greater than 99.9%. The specificity (i.e., the percentage of results that will be negative when HIV is not present) was also estimated to be greater than 99.9%. Results reported as positive have undergone testing using both a screening test and another test to confirm the positive result.
What about counseling?
The Home Access HIV-1 Test System has a built-in mechanism for pre-test and post-test counseling provided by the manufacturer. This counseling is anonymous and confidential. Counseling, which uses both printed material and telephone interaction, provides the user with an interpretation of the test result. Counseling also provides information on how to keep from getting infected if you are negative, and how to prevent further transmission of disease if you are infected. Counseling provides you with information about treatment options if you are infected, and can even provide referrals to doctors who treat HIV-infected individuals in your area.
If the test results are positive, what should I do?
The counselors can provide you with information about treatment options and referrals to doctors who treat HIV-infected individuals in your area.
Do I need a confirmatory test?
No, a positive result from the Home Access HIV-1 Test System means that antibodies to the HIV-1 virus are present in the blood sample submitted to the testing laboratory. The Home Access HIV-1 Test System includes confirmatory testing for HIV-1, and all confirmation testing is completed before the results are released and available to users of the test system.
How quickly will I get the results of the Home Access HIV-1 Test System?
You can anonymously call for the results approximately 7 business days (3 business days for the Express System) after shipping your specimen to the laboratory by using the unique PIN on the tear-off label included with your test kit. This label includes both the unique PIN and the toll-free number for the counseling center.
OraQuick In-Home HIV Test
The OraQuick In-Home HIV Test provides rapid results in the home. The testing procedure involves swabbing your mouth for an oral fluid sample and using a kit to test it. Results are available in 20 minutes. If you test positive, you will need a follow-up test. The manufacturer provides confidential counseling and referral to follow-up testing sites. Because the level of antibody in oral fluid is lower than it is in blood, oral fluid tests find infection later after exposure than do blood tests. Up to 1 in 12 infected people may test false-negative with this test.
The OraQuick In-Home HIV Test is a rapid self-administered over-the-counter (OTC) test. The OraQuick In-Home HIV Test kit consists of a test stick (device) to collect the specimen, a test tube (vial) to insert the test stick (device) and complete the test, testing directions, two information booklets (“HIV, Testing and Me” and “What your results mean to you”), a disposal bag and phone numbers for consumer support.
This approved test uses oral fluid to check for antibodies to HIV Type 1 and HIV Type 2, the viruses that cause AIDS. The kit is designed to allow you to take the HIV test anonymously and in private with the collection of an oral fluid sample by swabbing your upper and lower gums with the test device. After collecting the sample you insert the device into the kit’s vial which contains a developer solution, wait 20-40 minutes, and read the test result. A positive result with this test does not mean that an individual is definitely infected with HIV but rather that additional testing should be done in a medical setting to confirm the test result. Additionally, a negative test result does not mean that an individual is definitely not infected with HIV, particularly when exposure may have been within the previous three months. Again an individual should obtain a confirmatory test in a medical setting.
If the test says I’m HIV positive, what should I do?
A positive test result does not necessarily mean that you are infected with HIV. If you test positive for HIV using the OraQuick In-Home Test, you should see your healthcare provider or call the OraQuick Consumer Support Center, which has support center representatives available 24 hours a day/7 days a week to answer your questions and provide referrals to local healthcare providers for follow-up care. You will be advised to obtain confirmatory testing to confirm a positive result or inform you that the initial result was a false positive result. The test kit also contains an information booklet, “What your results mean to You,” which is designed to instruct individuals on what to do once they have obtained their test results.
Do I need a confirmatory test?
A positive test result on the OraQuick In-Home HIV Test indicates that you may be infected with HIV. Additional testing in a medical setting will either confirm a positive test result or inform you that the initial result was a false positive result.
What is a “false positive” result?
A “false positive” result occurs when an individual not infected with the HIV virus receives a test result that indicates that he or she is infected with HIV.
If the test says I’m HIV negative, what should I do?
A negative result on this test does not necessarily mean that you are not infected with HIV. The OraQuick test kit contains an information booklet, “What your results mean to You,” which is designed to instruct individuals on what to do once they have obtained their test results. The test is relatively reliable if there has been sufficient time for HIV antibodies to develop in the infected person. For the OraQuick In-Home HIV Test, that period of time, called the window period, is about three months. If you have recently been engaging in behavior that puts you at high risk for HIV infection, you should take the test again at a later time. Alternatively, you should see your health care provider who can discuss other options for HIV testing.
What is a “false negative” result?
A “false negative” result occurs when an HIV-infected individual receives a test result that incorrectly indicates that he or she is not infected with HIV.
How quickly will I get the results of the OraQuick Test?
You can read the results of the OraQuick In-Home HIV Test within 20 to 40 minutes.
HIV screening test
HIV testing is the only way for someone to know if he or she has HIV infection. Early detection and treatment of HIV infection and immune system monitoring can greatly improve long-term health. Also, if a person knows his or her HIV status, it may help change behaviors that can put that person and others at risk.
Several organizations recommend routine screening for HIV. The Centers for Disease Control and Prevention (CDC), American College of Physicians and the U.S. Preventive Services Task Force recommend that anyone between the ages of 13 and 64 (or 15 to 65 in the case of the U.S. Preventive Services Task Force) and pregnant women be screened for HIV at least once.
Certain individuals should get tested at least once to learn their status, even if they are not between the ages of 13 and 64. These include:
- People diagnosed with hepatitis, tuberculosis (TB) or a sexually transmitted disease
- People who received a blood transfusion between 1978 and 1985 or had a sexual partner who received a transfusion and later tested positive for HIV
- A healthcare worker with direct exposure to blood on the job
- Any individual who thinks they may have been exposed
HIV screening at least annually is advised for those at high risk for HIV and is recommended when an individual:
- Has had unprotected sex with more than one partner since the last HIV test
- Is a man who has had sex with another man (CDC suggests that gay or bisexual men may benefit from more frequent screening, such as every 3 to 6 months)
- Has used street drugs by injection, especially when sharing needles and/or other equipment
- Has exchanged sex for drugs or money
- Has an HIV-positive sex partner
- Has had sex with anyone who falls into one of the categories listed above or is uncertain about their sexual partner’s risk behaviors
Types of HIV screening tests
- Combination HIV antibody and antigen test — this is the recommended screening test for HIV. It is available only as a blood test. Antigen/antibody (Ag/Ab) combination (formerly fourth-generation) tests pair an IgM/IgG-sensitive antibody test with simultaneous, separate p24 antigen detection. Antigen/antibody test detects the HIV antigen called p24 plus antibodies to HIV-1 and HIV-2. (HIV-1 is the most common type found in the United States, while HIV-2 has a higher prevalence in parts of Africa.) The level of p24 antigen and the amount of virus (viral load) increase significantly soon after initial infection. Testing for p24 allows for detection of early infections, before HIV antibody is produced. About 2-8 weeks after exposure, antibodies to HIV are produced in response to the infection and remain detectable in the blood thereafter, making the antibody test useful for detecting infections weeks after exposure. By detecting both antibody and antigen, the combination test increases the likelihood that an infection is detected soon after exposure. In the lab, antigen from the patient specimen is first captured by immobilized, anti-p24 antibody on the solid phase of the assay, and then a separate, labeled anti-p24 antibody is applied, forming an “antibody sandwich.” Some of these p24/IgM/IgG-sensitive tests report a reactive result if any element is detected, whereas others yield separate results for p24, anti–HIV-1 antibodies, and anti–HIV-2 antibodies. Detecting p24 shortens the median window period down to just 18 days after infection 15.
- HIV antibody testing — all HIV antibody tests used in the U.S. detect HIV-1 and some tests have been developed that can also detect HIV-2. These tests are available as blood tests or tests of oral fluid.
- p24 antigen testing — this is used alone without the antibody test only in rare cases when there is a question about interference with an HIV antibody test.
How you can get access to HIV screening
- A blood or oral sample can be collected in a health practitioner’s office or a local clinic and sent to a laboratory for testing. Certain testing centers provide either anonymous (the name is never given) or confidential (the name is given but kept private) HIV testing and counseling. People can also contact their state, county, or city health department to find out where testing may be available.
- In these same settings, there may be a rapid test available in which results are generated in about 20 minutes.
- A home collection kit is available that allows a person to take a sample at home and then mail it to a testing center. Results are available over the phone, along with appropriate counseling.
- There is a home test for HIV test that uses an oral sample and results are available in about 20 minutes. This allows the person tested to remain anonymous and to get confidential results. The home test has two limitations: 1) testing on oral fluid is less sensitive than a blood test so the home test may miss some cases of HIV that a blood test would detect; and 2) the home test is not as accurate when it is performed at home by a lay person compared to when it is performed by a trained health professional. However, the convenience of home testing might encourage some people who might otherwise be reluctant to go to a health practitioner or clinic to learn their HIV status.
HIV testing center
To find a testing site near you, visit the National HIV and STD Testing Resources here (https://gettested.cdc.gov/).
Many testing locations are FREE and confidential. You can also buy a home testing kit at a pharmacy or online. Most HIV tests are covered by health insurance.
What is False-Negative HIV test?
When a person is infected with HIV but receives a negative test result when, in fact, an abnormal condition is actually present.
What is False-Positive HIV test?
When a person is not infected with HIV but receives a positive test result, that result is considered a false positive. Generally, HIV tests have high specificity, meaning that there are few false-positive results and most uninfected individuals are classified as uninfected by the test. If 1,000 uninfected people are tested with an HIV test and 4 have false-positive results, the HIV test’s specificity is 99.6% (996 true negative test results/1,000 HIV uninfected persons tested).
Causes of False-Positive HIV Test Results
False-positive test results can occur due to technical issues associated with the test or biological causes. Technical issues include specimen mix-up, mislabeling, improper handling, and misinterpretation of a visually read rapid test result. Biological causes include participation in an HIV vaccine study, autoimmune disorders and other medical conditions 16.
Additional Testing to Distinguish True Positive from False Positive
When a screening test is positive, additional testing is needed to determine if the positive result was accurate or whether the screening test result was falsely positive. If the screening test was a laboratory test, additional testing will generally occur using the original specimen 17.
If it was a rapid test, additional testing may occur in one of three ways: by submitting a specimen to the laboratory, by conducting a rapid test algorithm (i.e., rapid tests from different test manufacturers in sequence), or by referring the individual to a healthcare provider who can conduct additional testing 18.
If a rapid test algorithm is conducted and the initial test is reactive, but the subsequent test is not, additional testing in a laboratory is needed to rule out an early infection 19.
Impact of HIV Prevalence
HIV prevalence is the proportion of a population living with HIV infection. HIV prevalence within a population tested influences how many false-positive results there are relative to true-positive results.
High prevalence:
If you test 10,000 specimens and HIV prevalence is high (2%), 200 specimens will be from persons who are infected with HIV (true-positive) and 9,800 will be from persons who are not infected with HIV. If test specificity is 99.8%, results for approximately 20 specimens will be false-positive. In this case, of the 220 with
positive results (200 true-positives plus 20 false-positives), 91% are actually infected with HIV. The number of true positives far exceeds the number of false positives.
Low prevalence:
If HIV prevalence is much lower (0.1%), only 10 of 10,000 specimens will be from persons who are infected with HIV (true-positive) and 9,990 will be from persons who are not infected with HIV. If test specificity is 99.8%, results for 20 specimens will be false-positive. In this case, of the 30 with positive results (10 true-positives plus 20 false-positives), only 33% will be actually infected and the number of false-positives will exceed the number of true-positives. A testing program in a low prevalence population that implements routine testing of everyone in the population may be testing more low-risk people, and may expect to see more false-positive than true-positive results.
HIV Diagnosis
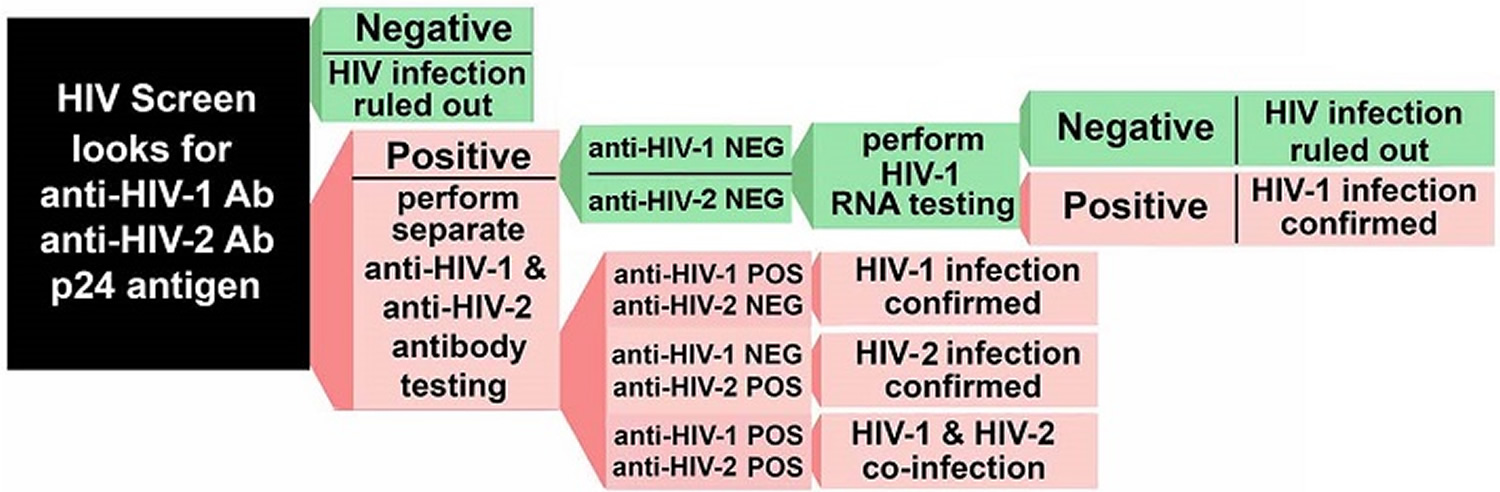
If any of the above screening tests is positive, then it must be followed by a second test to establish a diagnosis. This second test is an antibody test that is different than the first test. If the second test does not agree with the first test, then a third test is performed that detects the genetic material (RNA) of the virus.
In 2014, the CDC updated its HIV testing recommendations to include a new testing protocol, which has been accepted by the Clinical Laboratory Standards Institute:
- Screen for HIV using a combination HIV antigen/antibody test, then
- Verify a positive with a second HIV antibody test that differentiates between HIV-1 and HIV-2.
- If results between the first and second test do not agree, then the next test to perform is an HIV-1 RNA test (nucleic acid amplification test, NAAT). If the HIV-1 RNA is positive, then the test is considered positive.
Other HIV tests:
- HIV viral load testing—measures the amount of HIV in the blood; it is performed when a person is first diagnosed to help determine the status of the disease and is ordered at intervals to monitor the effectiveness of therapy.
- CD4 count—measures the number of CD4 T-cells in the blood; it is ordered when a person is first diagnosed to get a baseline assessment of the immune system and done at intervals to monitor therapy and the status of the immune system. If a person is doing well on treatment, this test may be done yearly.
- HIV genotypic resistance testing—ordered when someone is initially diagnosed to determine whether the particular strain(s) of HIV that the person has is resistant to certain antiretroviral drug therapies; also ordered when treatment is changed or when there is evidence of treatment failure.
- Phenotypic resistance testing–sometimes ordered for those who are resistant to multiple antiretroviral drugs to help guide treatment; this test evaluates whether the person’s strain(s) of HIV can be inhibited by various concentrations of antiretroviral drugs.
- Individuals who are planning to take the drug abacavir may be tested first for the gene allele, HLA-B*5701. If they are positive for it, they are at an increased risk of having a potentially severe hypersensitivity reaction and another drug should be considered.
Figure 1. HIV blood test – HIV Screening Algorithm
A number of other laboratory testing may be performed as part of overall care of an HIV-infected individual. Several tests may be done to identify and monitor the treatment of opportunistic infections, complications, and drug toxicities. Testing may also be ordered at intervals to evaluate the person’s health and organ function. Some examples include:
- Complete blood count
- Comprehensive metabolic panel
- Urinalysis
- Tests for other sexually transmitted diseases, such gonorrhea or syphilis
- Tests for other infections such as viral hepatitis or tuberculosis
Non-laboratory tests
Testing such as a chest X-ray or an imaging scan may sometimes be performed to help evaluate the person’s health status.
What if my HIV test result is Negative?
You could still have HIV. Ask your doctor about the “window period,” a period of time after a person is infected during which they won’t test positive.
A negative test result from HIV testing may mean one of two things: You don’t have HIV, or it’s too soon yet to tell.
If you were only recently exposed to HIV, you could test negative for HIV antibodies because your body hasn’t had time to create them yet. You may want to be retested for HIV antibodies in a few months or opt for one of the early-detection tests.
To stay negative, take actions to prevent HIV.
What if my HIV test result is Positive?
You may be given a follow-up test to confirm the result.
Finding out you have HIV can be scary, but you can still live a healthy life if you take action.
If you have HIV, start medical care right away. HIV treatment can keep you healthy for many years and reduce your chance of transmitting the virus to others.
Data from a National Institutes of Health sponsored trial 20 indicates there is a clear personal advantage to diagnosis soon after HIV infection and starting therapy early in the course of infection. The study further highlights the importance of routine HIV testing and the potential impact of early treatment on better health outcomes.
How soon can HIV be detected by a blood test
No HIV test can detect HIV immediately after infection. If you think you’ve been exposed to HIV in the last 72 hours, talk to your health care provider about post-exposure prophylaxis (PEP), right away.
The time between when a person may have been exposed to HIV and when a test can tell for sure whether they have HIV is called the window period. The window period varies from person to person and depends on the type of test used to detect HIV.
- A nucleic acid test (NAT) can usually tell you if you are infected with HIV 10 to 33 days after an exposure.
- An antigen/antibody test performed by a laboratory on blood from a vein can usually detect HIV infection 18 to 45 days after an exposure. Antigen/ antibody tests done with blood from a finger prick can take longer to detect HIV (18 to 90 days after an exposure). When the goal is to tell for sure that a person does not have HIV, an antigen/antibody test performed by a laboratory on blood from a vein is preferred.
- Antibody tests can usually take 23 to 90 days to reliably detect HIV infection. Most rapid tests and home tests are antibody tests. In general, antibody tests that use blood from a vein can detect HIV sooner after infection than tests done with blood from a finger prick or with oral fluid.
Ask your health care provider about the window period for the test you’re taking. If you’re using a home test, you can get that information from the materials included in the test’s package. If you get an HIV test after a potential HIV exposure and the result is negative, get tested again after the window period for the test you’re taking to be sure. If your health care provider uses an antigen/antibody test performed by a laboratory on blood from a vein you should get tested again 45 days after your most recent exposure. For other tests, you should test again at least 90 days after your most recent exposure to tell for sure if you have HIV.
If you learned you were HIV-negative the last time you were tested, you can only be sure you’re still negative if you haven’t had a potential HIV exposure since your last test. If you’re sexually active, continue to take actions to prevent HIV, like using condoms the right way every time you have sex and taking medicines to prevent HIV if you’re at high risk.
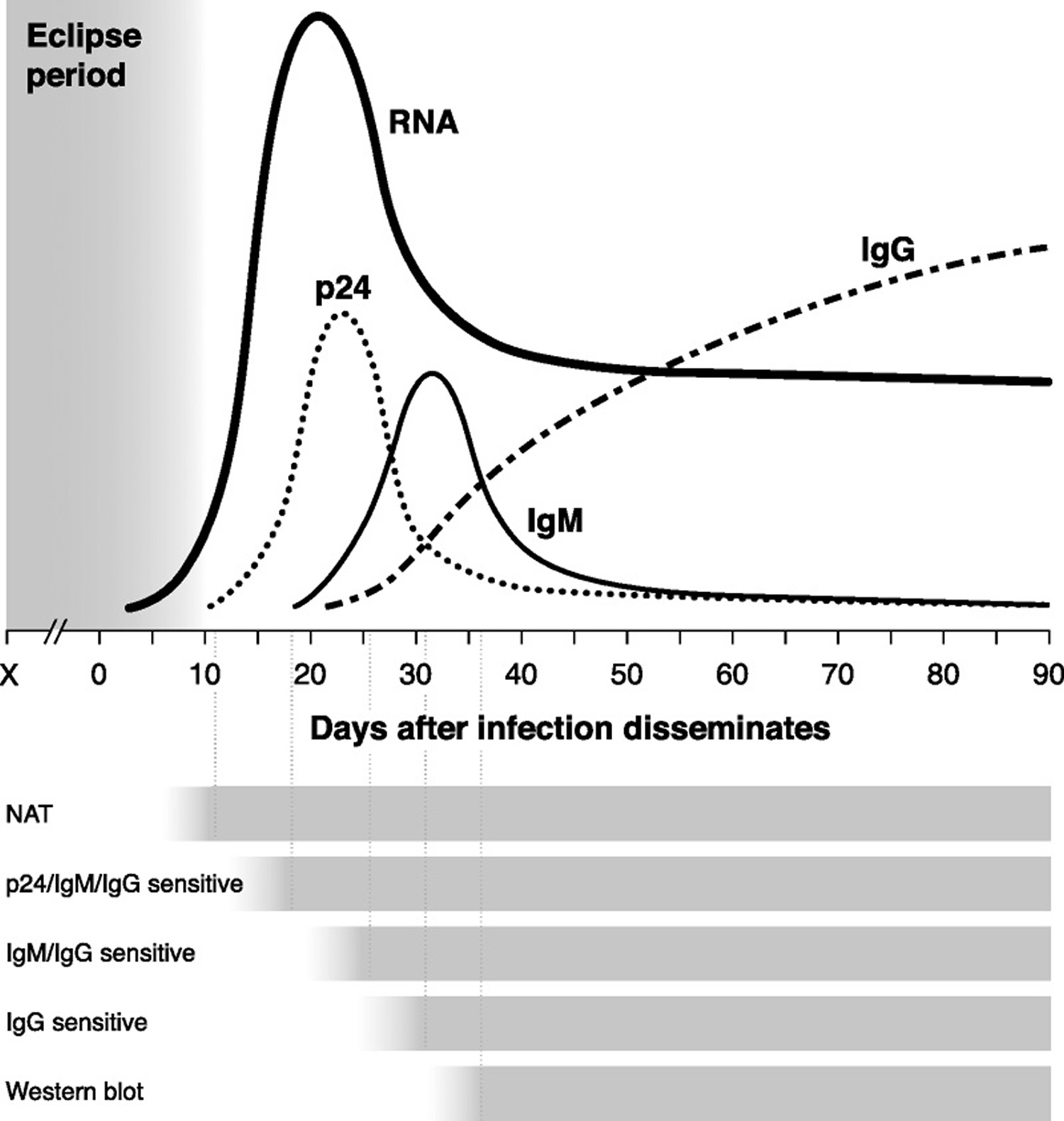
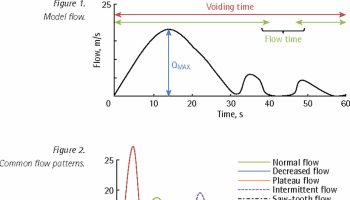
After an exposure that leads to infection, there is a variable amount of time called the eclipse period in which no existing diagnostic test is capable of detecting HIV (Figure 2). HIV RNA is the first reliable marker of infection; 50% of infected individuals have detectable plasma RNA within 12 days 15, and levels peak between 20 and 30 days 21. By day 15, the HIV-1 capsid protein p24 reaches detectable levels in the plasma 21. Antigenemia with p24 continues to rise through days 25 to 30, at which point early anti-HIV antibodies are able to complex with circulating p24; by day 50, antigen is often cleared from the bloodstream entirely 22. This short-lived detectability of p24 is therefore helpful in determining recency of infection, but it also makes its utility in diagnosis time limited.
As with other infections, the serum antibody response starts with immunoglobulin class M (IgM) molecules, beginning to rise around day 20, peaking within another 10 to 15 days, and then shifting to a more mature immunoglobulin class G (IgG) response beginning between days 30 and 35 23.
The time from infection to the first reactive result on a given test is referred to as the window period, the length of which depends on the target being detected by a particular assay. For example, tests capable of identifying anti-HIV IgM have a shorter window period compared with those detecting only IgG. Variability in the time needed for targets to rise to detectable levels in different persons means that the window period actually reflects a distribution of time rather than a fixed length that is identical for everyone.
The CDC study 24 showed that laboratory testing using antigen/antibody (Ag/Ab) laboratory tests detects HIV infection sooner than other available tests that detect only antibodies. If a person gets a laboratory-based antigen/antibody test on blood plasma less than 45 days after a possible HIV exposure and the result is negative, follow-up testing can begin 45 days after the possible HIV exposure. For all other tests, CDC recommends testing again at least 90 days after exposure to be sure that a negative test result is accurate.
Most, but not all people will have enough HIV in their blood for a NAT (nucleic acid test) test to detect infection 7 to 28 days after infection. This is during the time when someone has acute HIV infection.
It can take 7 to 28 days for a NAT to detect HIV. Nucleic acid testing is usually considered accurate during the early stages of infection. However, it is best to get an antibody or combination test at the same time to help the doctor interpret the negative NAT. This is because a small number of people naturally decrease the amount of virus in their blood over time which can lead to an inaccurate negative NAT result. Taking pre-exposure prophylaxis (PrEP) or post exposure prophylaxis (PEP) may also reduce the accuracy of NAT if you have HIV.
Figure 2. Timeline of virologic and serologic events after HIV infection
Footnotes: The length of time between an exposure event (X) and dissemination of HIV systemically depends on the mode of transmission. The eclipse period reflects time from exposure to the first detectable marker of infection: HIV RNA in the blood. Times to reactivity for each type of diagnostic test are depicted below the graph, from the earliest (nucleic acid amplification test) to the latest (IgG-sensitive assay).
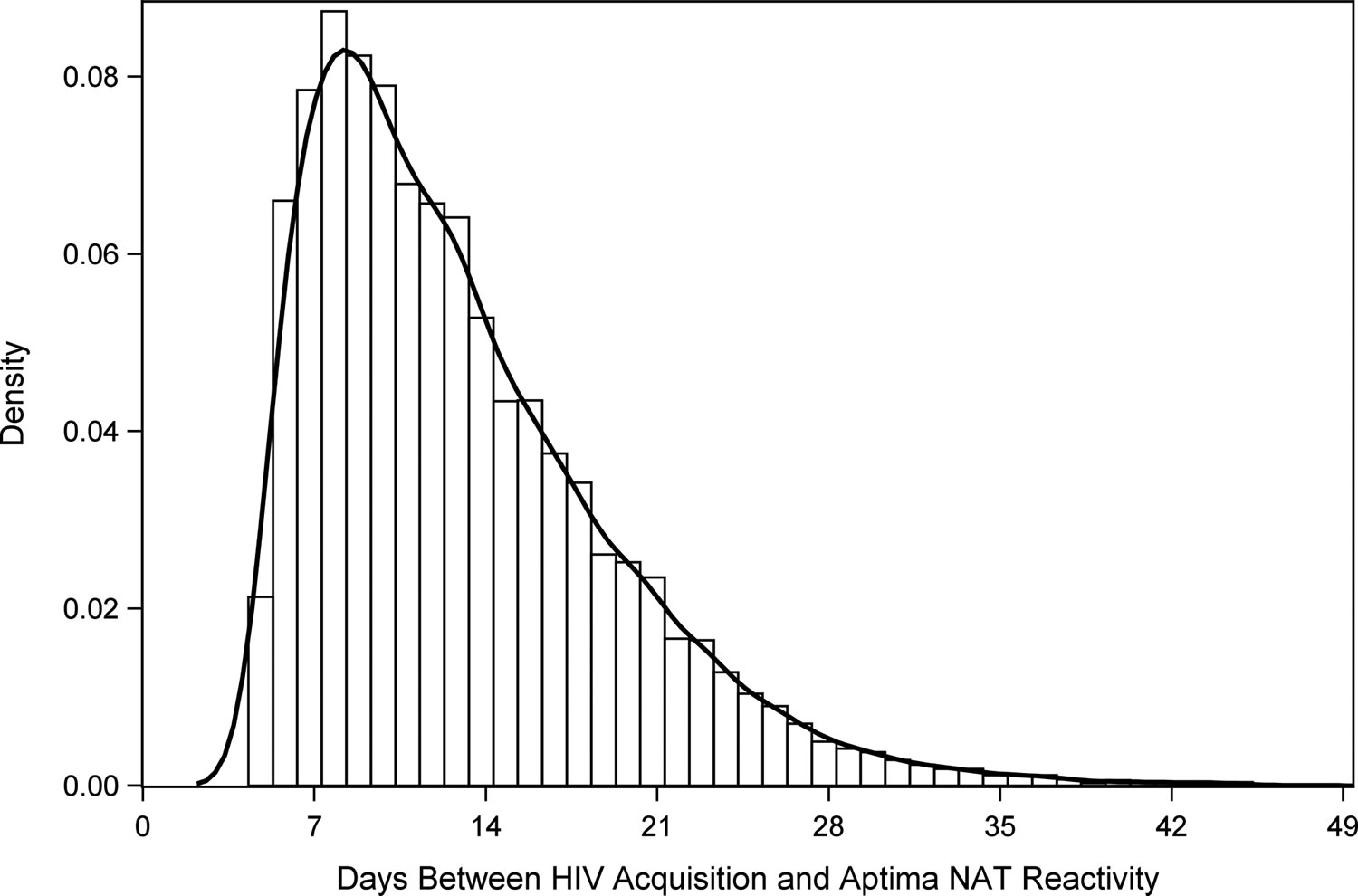
[Source 25]Figure 3. Days between HIV acquisition and Hologic Aptima NAT (nucleic acid test) reactivity. Hologic Aptima is the only nucleic acid test (NAT) currently approved by the FDA for diagnostic use in the US.
[Source 24]What is the Window Period?
The “window period” varies from person to person, and is also different depending upon the type of HIV test. Most HIV tests are antibody tests. It takes time for the body to produce enough antibodies for an HIV test to show that a person has HIV. The soonest an antibody test will detect infection is 3 weeks. Most, but not all people will develop detectable antibodies within 3 to 12 weeks of infection.
Most, but not all people will make enough antigens and antibodies for fourth generation or combination HIV tests to accurately detect infection 13 to 42 days after infection.
Most, but not all people will have enough HIV in their blood for a NAT (nucleic acid test) test to detect infection 7 to 28 days after infection. This is during the time when someone has acute HIV infection.
What you can do
Let your health care provider know if you may have been exposed to HIV. Together, you can figure out the best type of HIV test you should have. Ask your health care provider about the window period for the test you’re taking. If you’re using a home test, you can get that information from the materials included in the test’s package. If you get an HIV test within 3 months after a potential HIV exposure and the result is negative, get tested again in 3 more months to be sure.
If you think you’ve recently been exposed to HIV during sex (e.g., if the condom breaks or comes off) or through sharing needles and works to prepare drugs (e.g., cotton, cookers, water), talk to your health care provider or an emergency room doctor about taking post-exposure prophylaxis (PEP) right away. PEP must begin as soon as possible, and always within 72 hours (3 days) of a recent possible exposure.
What are HIV and AIDS?
HIV (human immunodeficiency virus) is the virus that causes AIDS (acquired immunodeficiency syndrome). HIV progressively destroys the body’s ability to fight infections and certain cancers. It weakens the immune system by infecting lymphocytes, a type of white blood cell, that normally help the body fight infections. Specific lymphocytes known as T-helper cells or CD4 cells are major targets for HIV. The virus binds to CD4 cells, enters them, replicates inside them, and eventually kills them.
Over time, the amount of HIV virus—the viral load—increases while the number of CD4 cells in the blood declines. After several years without treatment, the number of CD4 cells can drop to the point that AIDS-associated conditions and symptoms begin to appear. AIDS treatments can slow disease progression by reducing the amount of HIV in the body. This allows the body’s CD4 cells to increase or stabilize.
Currently, the Centers for Disease Control and Prevention (CDC) estimates that about 50,000 people in the U.S are newly infected with HIV each year, that 1.2 million people in the U.S. are living with HIV infection, and that nearly 13% of those with the infection are not aware of it and can pass the virus on to others. In 2012, the year with the most current statistics, nearly 14,000 people with AIDS died, and almost 660,000 have died since the beginning of the epidemic, according to the CDC.
Worldwide, as many as 2.1 million people in 2013 were newly infected with HIV, 1.5 million died of AIDS-related illnesses, and 35 million people were living with HIV, according to the World Health Organization.
HIV can be spread the following ways:
- By having unprotected sex with an infected partner; the virus can enter the body through the lining of the vagina, vulva, penis, rectum, or mouth during sex. Having a sexually transmitted disease (STD) such as syphilis, genital herpes, chlamydia, gonorrhea, or bacterial vaginosis appears to make people more susceptible to and at higher risk for acquiring HIV infection during sex with infected partners.
- By sharing needles or syringes (such as with intravenous injection drug abuse), which can be contaminated with very small quantities of blood from someone infected with the virus.
- During pregnancy or birth; approximately 25% to 35% of all untreated pregnant women infected with HIV will pass the infection to their babies. HIV also can be spread to babies through the breast milk of mothers infected with the virus. If the mother is treated with antiretroviral therapy (ART) during pregnancy, she can significantly reduce the chances of passing the infection to her baby.
- Through contact with infected blood; in the U.S. today, because of screening blood for transfusion and heat-treating techniques and other treatments of blood derivatives, the risk of getting HIV from transfusions is extremely small. However, before donated blood was screened for evidence of HIV infection and before treatments were introduced to destroy HIV in some blood products, such as factor VIII and albumin, HIV was transmitted through transfusion of contaminated blood or blood components. In areas of the world where donated blood is not routinely screened or treated for HIV, there is still risk of contracting the disease through this mode of transmission.
Initially, HIV usually causes flu-like symptoms, but some people may not experience any obvious signs or symptoms. The only way to determine whether a person has been infected is through HIV testing.
A person’s HIV status, like other medical conditions and test results, is protected by the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule and cannot be shared by healthcare providers with friends, family, or employers without written permission. However, if a person tests positive for HIV, it is important that he or she tell their healthcare providers as well as all sex partners and/or anyone with whom they share needles. Counseling services are often available from the clinic or healthcare provider that performed the test and they can help to advise the individual on who needs to know.
HIV status may be shared with healthcare providers who have a “need to know” in order to treat an individual. Also, in order to determine the incidence of HIV and to provide appropriate prevention and care services, all new cases of HIV are reported to state and local health departments. As of April 2008, data from all 50 states, the District of Columbia, and 5 dependent areas (American Samoa, Guam, the Northern Mariana Islands, Puerto Rico, and the U.S. Virgin Islands) are collected using a confidential name-based reporting system.
HIV Prevention
There is currently no cure for HIV infection and no vaccine to protect against HIV, but avoiding high-risk activities such as having unprotected sex and sharing needles for injecting drugs can help to prevent its spread. Routine screening for HIV has been recommended by the Centers for Disease Control and Prevention (CDC) and several other organizations to help identify HIV infections in people who may have no signs or symptoms. The early diagnosis of HIV infection is important to prevent its transmission to others and to allow evaluation, monitoring, and early treatment of the affected person.
Treatment of HIV-infected mothers during pregnancy, precautions at birth, and avoiding breast-feeding can minimize the risk of passing the infection from mother to child. Giving the antiretroviral drug zidovudine intravenously during labor and delivery and also to the newborn twice a day by mouth for 6 weeks reduces the rate of transmission from 25-33% to about 1-2%. A combination of antiretroviral therapies is most effective at reducing the risk of HIV transmission to the baby.
Healthcare workers can protect themselves from HIV infection by following universal precautions, such as wearing gloves and avoiding needle sticks.
While there is no vaccine that prevents HIV, the CDC and the World Health Organization (WHO) recommend that individuals without HIV infection but at high risk for it consider taking pre-exposure prophylaxis (PrEP), a daily pill to help prevent infection. For people taking PrEP consistently, the risk of HIV infection was up to 92% lower compared to those who didn’t take it.
Is abstinence the only 100% effective HIV prevention?
Yes. Abstinence means not having oral, vaginal, or anal sex. An abstinent person is someone who’s never had sex or someone who’s had sex but has decided not to continue having sex for some period of time. Abstinence is the only 100% effective way to prevent HIV, other sexually transmitted diseases (STDs), and pregnancy. The longer you wait to start having oral, vaginal, or anal sex, the fewer sexual partners you are likely to have in your lifetime. Having fewer partners lowers your chances of having sex with someone who has HIV or another STD.
How can I prevent getting HIV from anal or vaginal sex?
Use condoms the right way every time you have sex, take medicines to prevent or treat HIV if appropriate, choose less risky sexual behaviors, get tested for other sexually transmitted diseases (STDs), and limit your number of sex partners. The more of these actions you take, the safer you can be.
Specifically, you can:
- Use condoms the right way every time you have sex (see How well do condoms prevent HIV?). Learn the right way to use a male condom.
- Reduce your number of sexual partners. This can lower your chances of having a sex partner who will transmit HIV to you. The more partners you have, the more likely you are to have a partner with HIV whose viral load is not suppressed or to have a sex partner with a sexually transmitted disease. Both of these factors can increase the risk of HIV transmission.
- Talk to your doctor about pre-exposure prophylaxis (PrEP), taking HIV medicines daily to prevent HIV infection, if you are at very high risk for HIV. PrEP should be considered if you are HIV-negative and in an ongoing sexual relationship with an HIV-positive partner. PrEP also should be considered if you aren’t in a mutually monogamous relationship with a partner who recently tested HIV-negative, and you are a:
- gay or bisexual man who has had anal sex without a condom or been diagnosed with an STD in the past 6 months;
- man who has sex with both men and women; or
- heterosexual man or woman who does not regularly use condoms during sex with partners of unknown HIV status who are at very high risk of HIV infection (for example, people who inject drugs or women who have bisexual male partners).
- Post-exposure prophylaxis (PEP) means taking HIV medicines after being potentially exposed to HIV to prevent becoming infected. If you’re HIV-negative or don’t know your HIV status and think you have recently been exposed to HIV during sex (for example, if the condom breaks), talk to your health care provider or an emergency room doctor about PEP right away (within 3 days). The sooner you start PEP, the better; every hour counts. If you’re prescribed PEP, you’ll need to take it once or twice daily for 28 days. Keep in mind that your chance of getting HIV is lower if your HIV-positive partner is taking medicine to treat HIV infection (called antiretroviral therapy, or ART) the right way, every day and his or her viral load remains suppressed.
- Get tested and treated for other STDs and encourage your partners to do the same. If you are sexually active, get tested at least once a year. Having other STDs increases your risk for getting or transmitting HIV. STDs can also have long-term health consequences. Find an STD testing site.
- If you’re HIV-negative and your partner is HIV-positive, encourage your partner to get and stay on treatment. If taken the right way, every day, the medicine to treat HIV (ART) reduces the amount of HIV (called “viral load”) in the blood and elsewhere in the body to very low levels. This is called “viral suppression.” Being virally suppressed is good for an HIV-positive person’s overall health and greatly reduces the chance of transmitting the virus to a partner.
- Choose less risky sexual behaviors. HIV is mainly spread by having anal or vaginal sex without a condom or without taking medicines to prevent or treat HIV.
Receptive anal sex is the riskiest type of sex for getting HIV. It’s possible for either partner—the partner inserting the penis in the anus (the top) or the partner receiving the penis (the bottom)—to get HIV, but it is much riskier for an HIV-negative partner to be the receptive partner. That’s because the lining of the rectum is thin and may allow HIV to enter the body during anal sex.
Vaginal sex also carries a risk for getting HIV, though it is less risky than receptive anal sex. Most women who get HIV get it from vaginal sex, but men can also get HIV from vaginal sex.
In general, there is little to no risk of getting or transmitting HIV from oral sex. Theoretically, transmission of HIV is possible if an HIV-positive man ejaculates in his partner’s mouth during oral sex. However, the risk is still very low, and much lower than with anal or vaginal sex. Factors that may increase the risk of transmitting HIV through oral sex are oral ulcers, bleeding gums, genital sores, and the presence of other STDs, which may or may not be visible.
Sexual activities that don’t involve contact with body fluids (semen, vaginal fluid, or blood) carry no risk of HIV transmission but may pose a risk for other STDs.
How can I prevent getting HIV from oral sex?
In general, there is little to no risk of getting or transmitting HIV from oral sex. Theoretically, transmission of HIV is possible if an HIV-positive man ejaculates in his partner’s mouth during oral sex. However, the risk is still very low, and much lower than with anal or vaginal sex.
Oral sex involves putting the mouth on the penis (fellatio), vagina (cunnilingus), or anus (anilingus). There’s little to no risk of getting or transmitting HIV through oral sex. Factors that may increase the risk of transmitting HIV through oral sex are oral ulcers, bleeding gums, genital sores, and the presence of other sexually transmitted diseases (STDs), which may or may not be visible.
While there is little to no risk of getting HIV from oral sex, using a barrier (for example, a condom, dental dam, or cut-open nonlubricated condom) can further reduce your risk of getting or transmitting HIV and protect you and your partner from some other STDs, including gonorrhea of the throat and hepatitis.
The risk is also lower if the HIV-positive partner is taking medicine to treat HIV (called antiretroviral therapy or ART), or if the HIV-negative partner is taking medicine to prevent HIV (called pre-exposure prophylaxis or PrEP). Both PrEP and ART need to be taken the right way every time in order to work.
Because your mouth may come into contact with feces or other body fluids during oral sex, it is important that you talk to a health care provider about your chances of getting hepatitis A and B. If you’ve never had hepatitis A or B, there are vaccines to prevent them. Your provider can help you decide if vaccination is right for you.
How well do condoms prevent HIV?
If you use them the right way every time you have sex, condoms are highly effective in preventing HIV infection. But it’s important to educate yourself about how to use them the right way.
Condoms can also help prevent other sexually transmitted diseases (STDs) you can get through body fluids, like gonorrhea and chlamydia. However, they provide less protection against STDs spread through skin-to-skin contact, like human papillomavirus or HPV (genital warts), genital herpes, and syphilis.
There are two main types of condoms: male and female.
Male Condoms
- A male condom is a thin layer of latex, polyurethane, polyisoprene, or natural membrane worn over the penis during sex.
- Latex condoms provide the best protection against HIV. Polyurethane (plastic) or polyisoprene (synthetic rubber) condoms are good options for people with latex allergies, but plastic ones break more often than latex ones. Natural membrane (such as lambskin) condoms have small holes in them, so they don’t block HIV and other STDs.
- Use water- or silicone-based lubricants to lower the chances that a condom will break or slip during sex. Don’t use oil-based lubricants (for example, Vaseline, shortening, mineral oil, massage oils, body lotions, and cooking oil) with latex condoms because they can weaken the condom and cause it to break. Don’t use lubricants containing nonoxynol-9. It irritates the lining of the vagina and anus and increases the risk of getting HIV.
Female Condoms
- A female condom is a thin pouch made of a synthetic latex product called nitrile. It’s designed to be worn by a woman in her vagina during sex.
- When worn in the vagina, female condoms are comparable to male condoms at preventing HIV, other STDs, and pregnancy. Some people use female condoms for anal sex. Scientists don’t currently know how well female condoms prevent HIV and other STDs when used by men or women for anal sex. But they do know that HIV can’t travel through the nitrile barrier.
- It is safe to use any kind of lubricant with nitrile female condoms.
Even if you use condoms the right way every time you have sex, there’s still a chance of getting HIV. For some individuals at high risk of getting or transmitting HIV, adding other prevention methods, like taking medicines to prevent and treat HIV, can further reduce their risk.
Can I take medicines to prevent getting HIV?
If you are at very high risk for HIV from sex or injecting drugs, taking HIV medicines daily, called pre-exposure prophylaxis (or PrEP), can greatly reduce your risk of HIV infection. You can combine additional strategies with PrEP to reduce your risk even further.
Federal guidelines recommend that PrEP be considered for people who are HIV-negative and at very high risk for HIV. This includes anyone who is in an ongoing sexual relationship with an HIV-positive partner. It also includes anyone who:
- Is not in a mutually monogamous* relationship with a partner who recently tested HIV-negative, and
- Is a
- gay or bisexual man who has had anal sex without a condom or been diagnosed with an STD in the past 6 months;
- man who has sex with both men and women; or
- heterosexual man or woman who does not regularly use condoms during sex with partners of unknown HIV status who are at substantial risk of HIV infection (for example, people who inject drugs or women who have bisexual male partners).
PrEP is also recommended for people who’ve injected drugs in the past 6 months and have shared needles or works or been in drug treatment in the past 6 months.
If you have a partner who is HIV-positive and are considering getting pregnant, talk to your doctor about PrEP. It may be an option to help protect you and your baby.
PrEP involves daily medication and regular visits to a health care provider.
Can I take medicines to prevent HIV after exposure?
Yes. Taking medicine after being potentially exposed to HIV, called post-exposure prophylaxis (or PEP), can keep you from becoming infected. But PEP must be started within 72 hours after a possible exposure.
If you think you’ve recently been exposed to HIV during sex (for example, if the condom breaks) or through sharing needles and works to prepare drugs (for example, cotton, cookers, water), talk to your health care provider or an emergency room doctor about PEP right away. The sooner you start PEP, the better; every hour counts. If you’re prescribed PEP, you’ll need to take it once or twice daily for 28 days.
Someone who is on PEP should continue to use condoms with sex partners and safe injection practices while taking PEP.
Development of AIDS
HIV initially causes an acute illness with non-specific, flu-like symptoms. During this time, the virus is present in large numbers and is carried throughout the body. About 2 to 8 weeks after exposure, the person’s immune system responds by producing antibodies against the virus. As HIV infects the immune cells called CD4 T-cells (also called helper T cells), it slowly begins to decrease their numbers. The person may be apparently healthy for a decade or more, but without treatment, HIV continues to replicate and destroy CD4 T-cells. The virus remains in places such as the brain and lymph nodes, where it will persist even during drug treatment.
The term AIDS applies to the most advanced stages of HIV infection. According to the CDC, AIDS is diagnosed when an individual’s CD4 T-cell count drops below 200 cells/mm3. AIDS is also diagnosed when an individual has HIV and an AIDS-related illness, such as tuberculosis or pneumonia caused by the microorganism Pneumocystis jirovecii (carinii). In people with AIDS, opportunistic infections are often severe and sometimes fatal because the immune system is so damaged by HIV that the body cannot fight off certain bacteria, viruses, fungi, or parasites. Those with HIV/AIDS are also at an increased risk of developing certain cancers, neurological disorders, and a variety of other conditions.
HIV and AIDS Treatment
The goals of HIV and AIDS treatment are to suppress the virus to undetectable levels and to preserve the person’s immune function and health. Suppressing viral replication prevents or inhibits HIV mutation and the development of drug resistance. It slows the progression of the disease and allows the number of CD4 T-cells to increase, improving immune function. Treatment of complications and opportunistic infections is also important, as is addressing drug therapy side effects and toxicity.
The U.S. Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents as well as WHO recommend that all individuals diagnosed with HIV receive treatment as soon as possible, including pregnant women. With advances in treatment, individuals with HIV are living longer, healthier lives. Once someone begins treatment, it is important that the person continue it for the rest of their life to help maintain health and prevent spread of HIV. Interruptions in treatment can lead to increases in the amount of virus (viral load) and can increase the risk of developing drug resistance, decrease immune function, and allow disease progression.
Drug selection
A person may be infected with drug-susceptible and/or drug-resistant strains of HIV. Testing for drug resistance is performed when a person is first diagnosed to help guide therapy. There are several classes of antiretroviral drugs used to treat HIV/AIDS. People typically take at least three drugs from two different classes in order to prevent or minimize virus replication and the emergence of drug-resistant strains. Combinations of three or more antiretroviral drugs are referred to as highly active antiretroviral therapy or HAART. There are preferred treatment regimens, but the specific drugs given must be tailored to the individual and to the strain(s) of HIV with which he or she is infected.
Drug therapies may be evaluated and changed as necessary if the person experiences treatment failure, indicating the development of resistance to one or more of the drugs the person is taking. Another reason treatment may be changed is if the individual experiences significant side effects and toxicity. This may be related to the person’s ability to absorb and metabolize the drug(s).
People with HIV/AIDS will need to work closely with their healthcare provider(s) throughout their lifetime to adjust their medications to their changing needs. Treatment of people who have developed resistance to one or more drugs or classes of drugs can become challenging. Those affected may need to consult with health practitioners who specialize in the management of “treatment-experienced” patients. New HIV/AIDS drug treatments are continually being developed and brought into clinical use.
How long does HIV test take
Regardless of the type of screening test used, a positive result will require follow-up testing to establish an HIV diagnosis. If you test positive for HIV on both the initial and follow-up testing, it means you are HIV-positive. It usually takes a few days to a few weeks to get results of an HIV test, although some rapid HIV tests can produce results in about 20 minutes.
- Nucleic acid tests (NAT) can usually tell you if you are infected with HIV 10 to 33 days after an exposure
- Antigen/antibody HIV test can usually detect HIV infection 18 to 45 days after an exposure
- Antibody HIV test can usually take 23 to 90 days to reliably detect HIV infection
Rapid HIV testing
Several rapid tests offer highly accurate information within as little as 20 minutes. These tests look for antibodies to HIV using a sample of your blood, drawn from a vein or a finger prick, or fluids collected on a treated pad that is rubbed on your upper and lower gums. A positive reaction on a rapid test requires a confirming blood test.
Home HIV testing
There are two HIV home test kits approved by the U.S. Food and Drug Administration (FDA) for home use. Both are HIV antibody tests.
The Home Access HIV-1 Test System is a home collection kit, which involves pricking the finger for a blood sample, sending the sample to a lab for testing, and then calling the lab for results as early as the next business day. If the result is positive for HIV, the lab will do a follow-up test on the same blood sample to confirm the initial HIV-positive test result.
The OraQuick In-Home HIV Test comes with a test stick and a tube with a testing solution. The test stick is used to swab the gums to get a sample of oral fluids. To get results, the test stick is inserted into the test tube. Test results are ready in 20 minutes. A positive result on this home HIV test must always be confirmed by additional HIV testing performed in a health care setting.
Home testing involves two options:
- Mailing a blood sample to a testing center and calling in for your results
- Collecting an oral fluid sample at home and using a kit to test it yourself
Both methods ensure anonymity and offer confidential counseling and referral to follow-up testing sites if your test results are positive.
Early-detection HIV testing
Some tests can detect HIV infection earlier, before antibodies are detectable in standard HIV testing. These early-detection tests evaluate your blood for genetic material from the virus or for proteins that develop within the first few weeks after infection.
Tests that detect HIV infection before you’ve developed antibodies to the virus may cost more than standard HIV testing and may not be as widely available. You will also still need standard antibody testing later to confirm results because false-positives and false-negatives are possible.
HIV test accuracy
The HIV test may not detect an infection that was contracted very recently. HIV is usually diagnosed by testing your blood or a sample of cells taken with a swab from inside your cheek for the presence of antibodies to the virus.
Some HIV tests aren’t accurate immediately after infection because it takes time for your body to produce antibodies to the virus. It can take up to three to 12 weeks for someone to make enough antibodies for an antibody test to detect HIV infection.
- Nucleic acid tests (NAT) can usually tell you if you are infected with HIV 10 to 33 days after an exposure
- Antigen/antibody HIV test can usually detect HIV infection 18 to 45 days after an exposure
- Antibody HIV test can usually take 23 to 90 days to reliably detect HIV infection
OraQuick In-Home HIV Test
As noted in the package insert, clinical studies have shown that the OraQuick In-Home HIV Test has an expected performance of approximately 92% for test sensitivity (i.e., the percentage of results that will be positive when HIV is present). This means that one false negative result would be expected out of every 12 test results in HIV infected individuals. The clinical studies also showed that the OraQuick In-Home HIV Test has an expected performance of 99.98% for test specificity (i.e., the percentage of results that will be negative when HIV is not present). This means that one false positive result would be expected out of every 5,000 test results in uninfected individuals.
It is extremely important for those who self-test using the OraQuick In-Home HIV Test to carefully read and follow all labeled directions. Even when used according to the labeled directions, there will be some false negative results and a small number of false positive results. The OraQuick test package contains step-by-step instructions, and there is also an OraQuick Consumer Support Center to assist users in the testing process.
HIV test results
What does a negative test result mean?
A negative result doesn’t necessarily mean that you don’t have HIV. That’s because of the window period— the time between when a person may have been exposed to HIV and when a test can tell for sure whether they have HIV. The window period varies from person to person and is also different depending upon the type of HIV test.
Ask your health care provider about the window period for the test you’re taking. If you’re using a home test, you can get that information from the materials included in the test’s package. If you get an HIV test after a potential HIV exposure and the result is negative, get tested again after the window period for the test you’re taking to be sure. For example, if your health care provider uses an antigen/antibody test performed by a laboratory with blood from a vein you should get tested again 45 days after your most recent exposure. For other tests, you should test again at least 90 days after your most recent exposure to tell for sure if you have HIV.
If you learned you were HIV-negative the last time you were tested, you can only be sure you’re still negative if you haven’t had a potential HIV exposure since your last test. If you’re sexually active, continue to take actions to prevent HIV, like using condoms the right way every time you have sex and taking medicines to prevent HIV if you’re at high risk.
If I have a negative result, does that mean that my partner is HIV negative also?
No. Your HIV test result reveals only your HIV status.
HIV is not necessarily transmitted every time you have sex. Therefore, taking an HIV test is not a way to find out if your partner is infected.
It’s important to be open with your partners and ask them to tell you their HIV status. But keep in mind that your partners may not know or may be wrong about their status, and some may not tell you if they have HIV even if they are aware of their status. Consider getting tested together so you can both know your HIV status and take steps to keep yourselves healthy.
What does a positive result mean?
A follow-up test will be conducted. If the follow-up test is also positive, it means you are living with HIV (or HIV-positive).
If you had a rapid screening test, the testing site will arrange a follow-up test to make sure the screening test result was correct. If your blood was tested in a lab, the lab will conduct a follow-up test on the same sample.
It is important that you start medical care and begin HIV treatment as soon as you are diagnosed with HIV. Antiretroviral therapy or ART (taking medicines to treat HIV infection) is recommended for all people with HIV, regardless of how long they’ve had the virus or how healthy they are. ART works by lowering the amount of virus in your body to very low levels, called viral suppression. It slows the progression of HIV and helps protect your immune system. If you are on ART and virally suppressed, you can stay healthy for many years, and greatly reduce your chance of transmitting HIV to sex partners.
If you have health insurance, your insurer is required to cover some medicines used to treat HIV. If you don’t have health insurance, or you’re unable to afford your co-pay or co-insurance amount, you may be eligible for government programs that can help through Medicaid, Medicare, the Ryan White HIV/AIDS Program, and community health centers. Your health care provider or local public health department can tell you where to get HIV treatment.
To lower your risk of transmitting HIV:
- Take medicines to treat HIV (antiretroviral therapy or ART) the right way every day. Being on ART and getting and staying virally suppressed is the most effective thing you can do to reduce the chance of transmitting HIV.
- Use condoms the right way every time you have sex.
- If your partner is HIV-negative, encourage them to talk to their health care provider to see if taking daily medicine to prevent HIV (called pre-exposure prophylaxis, or PrEP) is right for them.
- If you think your partner might have been recently exposed to HIV—for example, if the condom breaks during sex and you aren’t virally suppressed—they should talk to a health care provider as soon as possible within the next 3 days (72 hours) about taking medicines (called post-exposure prophylaxis, or PEP) to prevent getting HIV.
- Get tested and treated for STDs and encourage your partner to do the same.
Receiving a diagnosis of HIV can be a life-changing event. People can feel many emotions—sadness, hopelessness, or anger. Allied health care providers and social service providers, often available at your health care provider’s office, will have the tools to help you work through the early stages of your diagnosis and begin to manage your HIV.
Talking to others who have HIV may also be helpful. Find a local HIV support group. Learn about how other people living with HIV have handled their diagnosis.
If I test positive for HIV, does that mean I have AIDS?
No. Being HIV-positive does not mean you have AIDS. AIDS is the most advanced stage of HIV disease. HIV can lead to AIDS if a person does not get treatment or take care of their health.
It’s important to share your status with your sex partners. Whether you disclose your status to others is your decision.
Partners
It’s important to disclose your HIV status to your sex partners even if you’re uncomfortable doing it. Communicating with each other about your HIV status means you can take steps to keep both of you healthy. The more practice you have disclosing your HIV status, the easier it will become.
Many resources can help you learn ways to disclose your status to your partners. For tips on how to start the conversation with your partners, check out CDC’s Start Talking. Stop HIV (https://www.cdc.gov/actagainstaids/campaigns/starttalking/index.html).
If you’re nervous about disclosing your test result, or you have been threatened or injured by your partner, you can ask your doctor or the local health department to tell them that they might have been exposed to HIV. This is called partner notification services. Health departments do not reveal your name to your partners. They will only tell your partners that they have been exposed to HIV and should get tested.
Many states have laws that require you to tell your sexual partners if you’re HIV-positive before you have sex (anal, vaginal, or oral) or tell your drug-using partners before you share drugs or needles to inject drugs. In some states, you can be charged with a crime if you don’t tell your partner your HIV status, even if your partner doesn’t become infected.
Family and friends
In most cases, your family and friends will not know your test results or HIV status unless you tell them yourself. While telling your family that you have HIV may seem hard, you should know that disclosure has many benefits—studies have shown that people who disclose their HIV status respond better to treatment than those who don’t. And telling friends and family can provide an important source of support in managing your HIV.
If you are under 18, however, some states allow your health care provider to tell your parent(s) that you received services for HIV if they think doing so is in your best interest.
Employers
In most cases, your employer will not know your HIV status unless you tell them. But your employer does have a right to ask if you have any health conditions that would affect your ability to do your job or pose a serious risk to others. (An example might be a health care professional, like a surgeon, who does procedures where there is a risk of blood or other body fluids being exchanged.)
If you have health insurance through your employer, the insurance company cannot legally tell your employer that you have HIV. But it is possible that your employer could find out if the insurance company provides detailed information to your employer about the benefits it pays or the costs of insurance.
All people with HIV are covered under the Americans with Disabilities Act. This means that your employer cannot discriminate against you because of your HIV status as long as you can do your job.
Who will pay for my treatment if I’m HIV positive?
If you have health insurance, your insurer is required to cover some medicines used to treat HIV. If you don’t have health insurance, or you’re unable to afford your co-pay or co-insurance amount, you may be eligible for government programs that can help through Medicaid, Medicare, the Ryan White HIV/AIDS Program, and community health centers. Your health care provider or local public health department can tell you where to get HIV treatment.
- HIV Testing. https://www.cdc.gov/hiv/basics/testing.html[↩]
- Kourtis AP, Bulterys M, Nesheim SR, Lee FK. Understanding the Timing of HIV Transmission from Mother to Infant. JAMA 2001;285:(6): 709-12[↩]
- Masciotra S, Luo W, Westheimer E, et al. Performance evaluation of the FDA-approved Determine HIV-1/2 Ag/Ab Combo assay using plasma and whole blood specimens. J Clin Virol 2017.[↩][↩]
- Mortimer PP, Parry JV. Detection of antibody to HIV in saliva: A brief review. Clin Diagn Virol 1994; 2:231–243.[↩]
- Luo W, Masciotra S, Delaney KP, et al. Comparison of HIV oral fluid and plasma antibody results during early infection in a longitudinal Nigerian cohort. J Clin Virol 2013; 58 Suppl 1:e113–e118.[↩]
- Branson BM. HIV testing updates and challenges: when regulatory caution and public health imperatives collide. Curr HIV/AIDS Rep 2015; 12:117–126.[↩]
- Conway DP, Holt M, McNulty A, et al. Multi-centre evaluation of the Determine HIV Combo assay when used for point of care testing in a high risk clinic-based population. PLoS One 2014; 9:e94062.[↩]
- Fitzgerald N, Cross M, O’Shea S, et al. Diagnosing acute HIV infection at point of care: A retrospective analysis of the sensitivity and specificity of a fourth-generation point-of-care test for detection of HIV core protein p24. Sex Transm Infect 2017; 93:100–101.[↩]
- Donnell D, Ramos E, Celum C, et al. The effect of oral pre-exposure prophylaxis on the progression of HIV-1 seroconversion. AIDS 2017.[↩][↩]
- Curlin ME, Gvetadze R, Leelawiwat W, et al. Analysis of false-negative HIV rapid tests performed on oral fluid in three international clinical research studies. Clin Infect Dis 2017; 64:1663–1669. https://www.ncbi.nlm.nih.gov/pubmed/28369309[↩]
- Kuhar DT, Henderson DK, Struble KA, et al. Updated US Public Health Service Guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol 2013; 34:875–892.[↩]
- Dominguez KL, Smith DK, Thomas V, et al. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV—United States, 2016. https://stacks.cdc.gov/view/cdc/38856[↩]
- Stekler JD, O’Neal JD, Lane A, et al. Relative accuracy of serum, whole blood, and oral fluid HIV tests among Seattle men who have sex with men. J Clin Virol 2013; 58 Suppl 1:e119–e122.[↩]
- HIV Home Test Kits. https://www.fda.gov/biologicsbloodvaccines/safetyavailability/hivhometestkits/default.htm[↩]
- Delaney KP, Hanson DL, Masciotra S, et al. Time until emergence of HIV test reactivity following infection with HIV-1: Implications for interpreting test results and retesting after exposure. Clin Infect Dis 2017; 64:53–59.[↩][↩]
- Centers for Disease Control and Prevention and Association of Public Health Laboratories. Laboratory Testing for the Diagnosis of HIV Infection: Updated Recommendations. 2014. http://stacks.cdc.gov/view/cdc/23447[↩]
- Association of Public Health Laboratories. Suggested Reporting Language for the HIV Laboratory Diagnostic Testing Algorithm. 2017; https://www.aphl.org/aboutAPHL/publications/Documents/ID_2017Apr-HIV-Lab-Test-Suggested-Reporting-Language.pdf[↩]
- Centers for Disease Control and Prevention. Implementing HIV Testing in Nonclinical Settings: A Guide for HIV Testing Providers. 2016; https://www.cdc.gov/hiv/pdf/testing/CDC_HIV_Implementing_HIV_Testing_in_Nonclinical_Settings.pdf[↩]
- Centers for Disease Control and Prevention. Planning and Implementing HIV Testing Programs and Linkage Programs in Nonclinical Settings: A Guide for Program Managers. 2014; https://stacks.cdc.gov/view/cdc/23551[↩]
- Starting Antiretroviral Treatment Early Improves Outcomes for HIV-Infected Individuals. https://www.niaid.nih.gov/news-events/starting-antiretroviral-treatment-early-improves-outcomes-hiv-infected-individuals[↩]
- Busch MP, Satten GA. Time course of viremia and antibody seroconversion following human immunodeficiency virus exposure. Am J Med 1997; 102:117–124; discussion 125–116.[↩][↩]
- Henrard DR, Daar E, Farzadegan H, et al. Virologic and immunologic characterization of symptomatic and asymptomatic primary HIV-1 infection. J Acquir Immune Defic Syndr Hum Retrovirol 1995; 9:305–310.[↩]
- Cooper DA, Imrie AA, Penny R. Antibody response to human immunodeficiency virus after primary infection. J Infect Dis 1987; 155:1113–1118.[↩]
- Time Until Emergence of HIV Test Reactivity Following Infection With HIV-1: Implications for Interpreting Test Results and Retesting After Exposure, Clinical Infectious Diseases, Volume 64, Issue 1, 1 January 2017, Pages 53–59, https://doi.org/10.1093/cid/ciw666 https://academic.oup.com/cid/article/64/1/53/2194435[↩][↩]
- Busch MP, Satten GA. Time course of viremia and antibody seroconversion following human immunodeficiency virus exposure. Am J Med 1997; 102:117–124; discussion 125–116. https://www.ncbi.nlm.nih.gov/pubmed/9845513[↩]