Congenital insensitivity to pain
Congenital insensitivity to pain (CIP) is an extremely rare inherited condition that inhibits the ability to perceive physical pain. From birth, affected individuals never feel pain in any part of their body when injured 1. Individuals with congenital insensitivity to pain do not feel pain from any noxious stimuli, including inflammation and heat 2. People with congenital insensitivity to pain can feel the difference between sharp and dull and hot and cold, but cannot sense pain, for example, that a hot beverage is burning their tongue. This lack of pain awareness often leads to repeated injuries, accumulation of wounds, bruises, broken bones, and prevents normal healing and other health issues that may go undetected. Young children with congenital insensitivity to pain may have mouth or finger wounds due to repeated self-biting and may also experience multiple burn-related injuries. These repeated injuries often lead to a reduced life expectancy in people with congenital insensitivity to pain. Many people with congenital insensitivity to pain also have a complete loss of the sense of smell (anosmia) 3.
Congenital insensitivity to pain is considered a form of peripheral neuropathy because it affects the peripheral nervous system, which connects the brain and spinal cord to muscles and to cells that detect sensations such as touch, smell, and pain.
Congenital insensitivity to pain is a rare condition; about 20 cases have been reported in the scientific literature 3.
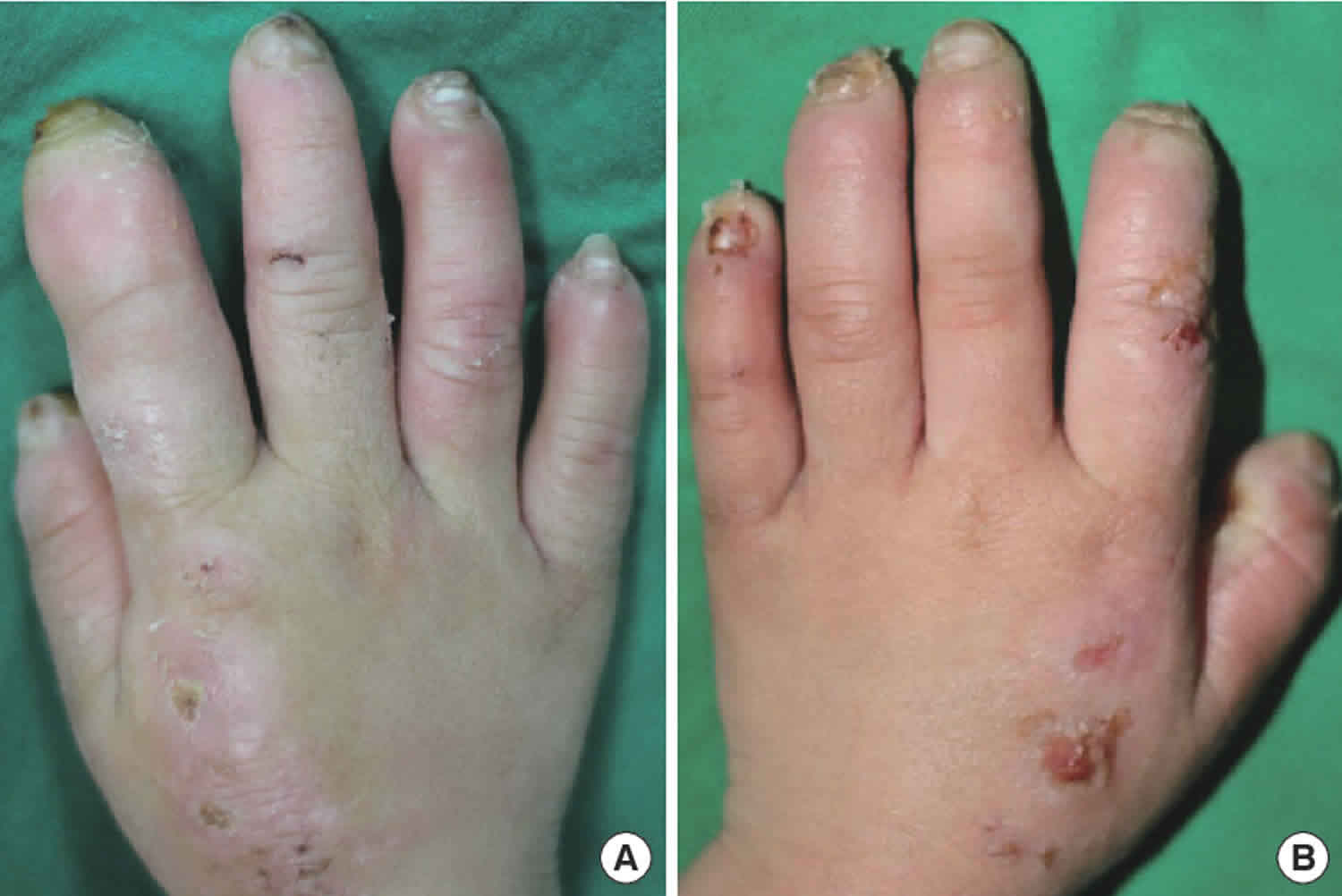
Figure 1. Congenital insensitivity to pain
Footnote: (A) Typical loss of fingertips secondary to trauma, poor wound healing, and chronic Staphylococcal aureus infections in a child with congenital insensitivity to pain; (B) Child with congenital insensitivity to pain showing loss of portions of the lower lip as a result of self-biting; (C) Example of a Charcot joint, or neuropathic joint, in an individual with congenital insensitivity to pain. The affected individual had chronic elbow dislocation, which is now permanent and results in significantly reduced arm movement; (D) Method for applying pressure to the proximal nail bed to test pain detection.
[Source 1 ]Table 1. Genes Associated with Congenital Insensitivity to Pain (CIP)
| Gene | Proportion of Affected Individuals with Mutation of This Gene | Mode of inheritance | Distinguishing Features |
|---|---|---|---|
| CLTCL1 4 | Rare | AR |
|
| NGF 5 | Rare | AR |
|
| NTRK1 6 | Common | AR |
|
| PRDM12 8 | Intermediate | AR |
|
| SCN9A 5 | Common | AR |
|
| SCN11A 3 | Rare | AD |
|
Footnotes:
1. Three individuals from a large northern Swedish family who were homozygous for the NM_002506.2:c.661C>T, (p.Arg211Trp) pathogenic variant 10. A proportion of adults who were heterozygous for the NM_002506.2:c.661C>T, (p.Arg211Trp) pathogenic variant in this family had mild or moderate problems with joint deformities but were not believed to actually be affected by congenital insensitivity to pain.
2. Pathogenic variants are typically truncating, although one missense variant and one in-frame deletion have been described 11
3. The pathogenic NM_014139.2:c.2432T>C (p.Leu811Pro) variant was found in three affected individuals and was de novo in each case. The pathogenic NM_014139.2:c.3904C>T, (p.Leu1302Phe) variant was found in an affected mother and two children. All six of these individuals had hyperhidrosis 12.
Abbreviations: AD = autosomal dominant; AR = autosomal recessive; HSAN = hereditary sensory and autonomic neuropathy; MOI = mode of inheritance
[Source 1 ]Congenital insensitivity to pain causes
Mutations in the SCN9A gene cause congenital insensitivity to pain. The SCN9A gene provides instructions for making one part (the alpha subunit) of a sodium channel called NaV1.7. Sodium channels transport positively charged sodium atoms (sodium ions) into cells and play a key role in a cell’s ability to generate and transmit electrical signals. NaV1.7 sodium channels are found in nerve cells called nociceptors that transmit pain signals to the spinal cord and brain. The NaV1.7 channel is also found in olfactory sensory neurons, which are nerve cells in the nasal cavity that transmit smell-related signals to the brain.
The SCN9A gene mutations that cause congenital insensitivity to pain result in the production of nonfunctional alpha subunits that cannot be incorporated into NaV1.7 channels. As a result, the channels cannot be formed. The absence of NaV1.7 channels impairs the transmission of pain signals from the site of injury to the brain, causing those affected to be insensitive to pain. Loss of this channel in olfactory sensory neurons likely impairs the transmission of smell-related signals to the brain, leading to anosmia.
Congenital insensitivity to pain inheritance pattern
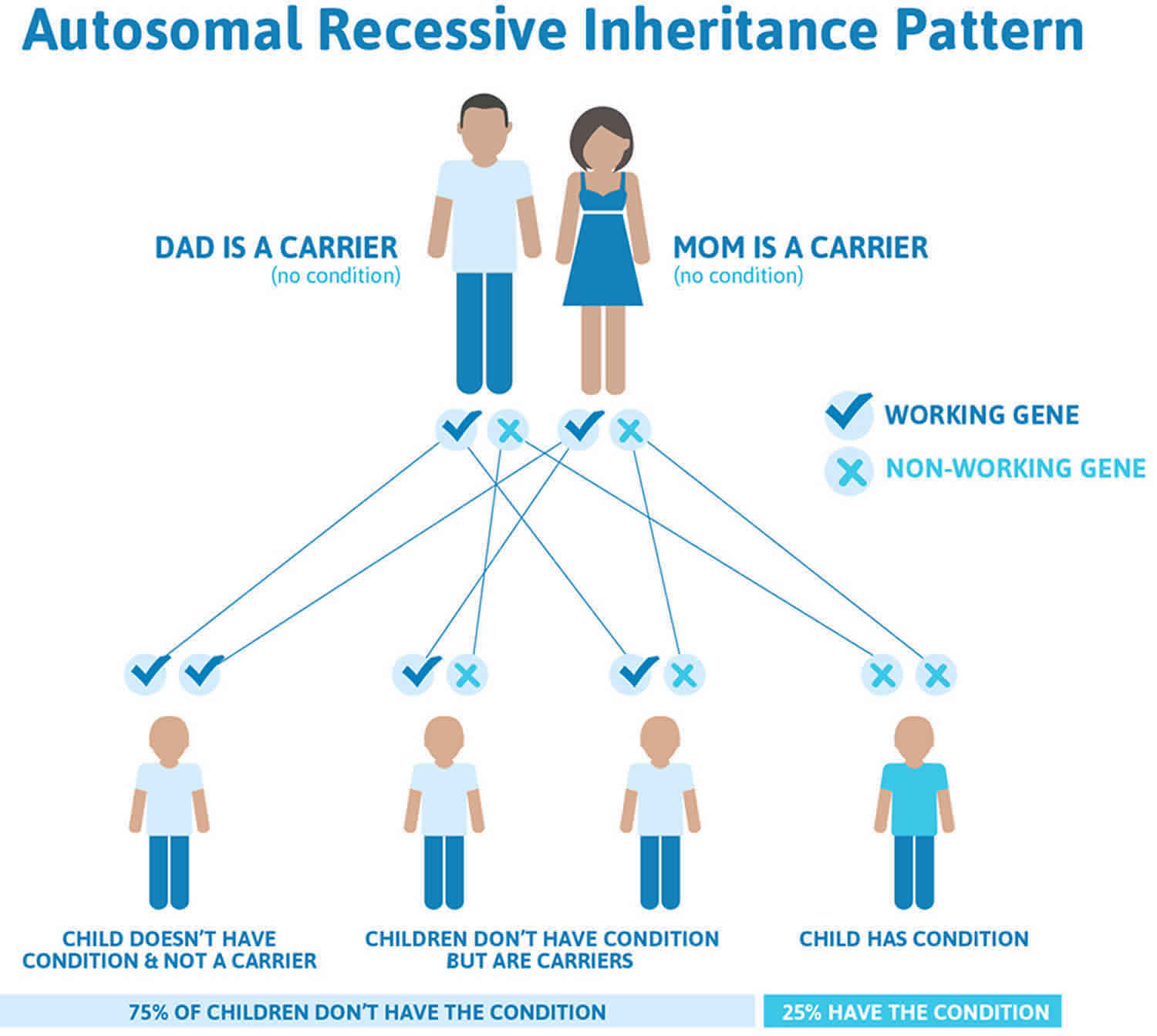
Congenital insensitivity to pain is inherited in an autosomal recessive pattern, which means both copies of the gene in each cell have mutations. The parents of an individual with an autosomal recessive condition each carry one copy of the mutated gene, but they typically do not show signs and symptoms of the condition.
It is rare to see any history of autosomal recessive conditions within a family because if someone is a carrier for one of these conditions, they would have to have a child with someone who is also a carrier for the same condition. Autosomal recessive conditions are individually pretty rare, so the chance that you and your partner are carriers for the same recessive genetic condition are likely low. Even if both partners are a carrier for the same condition, there is only a 25% chance that they will both pass down the non-working copy of the gene to the baby, thus causing a genetic condition. This chance is the same with each pregnancy, no matter how many children they have with or without the condition.
- If both partners are carriers of the same abnormal gene, they may pass on either their normal gene or their abnormal gene to their child. This occurs randomly.
- Each child of parents who both carry the same abnormal gene therefore has a 25% (1 in 4) chance of inheriting a abnormal gene from both parents and being affected by the condition.
- This also means that there is a 75% ( 3 in 4) chance that a child will not be affected by the condition. This chance remains the same in every pregnancy and is the same for boys or girls.
- There is also a 50% (2 in 4) chance that the child will inherit just one copy of the abnormal gene from a parent. If this happens, then they will be healthy carriers like their parents.
- Lastly, there is a 25% (1 in 4) chance that the child will inherit both normal copies of the gene. In this case the child will not have the condition, and will not be a carrier.
These possible outcomes occur randomly. The chance remains the same in every pregnancy and is the same for boys and girls.
Figure 2 illustrates autosomal recessive inheritance. The example below shows what happens when both dad and mum is a carrier of the abnormal gene, there is only a 25% chance that they will both pass down the abnormal gene to the baby, thus causing a genetic condition.
Figure 2. Congenital insensitivity to pain autosomal recessive inheritance pattern
People with specific questions about genetic risks or genetic testing for themselves or family members should speak with a genetics professional.
Resources for locating a genetics professional in your community are available online:
- The National Society of Genetic Counselors (https://www.findageneticcounselor.com/) offers a searchable directory of genetic counselors in the United States and Canada. You can search by location, name, area of practice/specialization, and/or ZIP Code.
- The American Board of Genetic Counseling (https://www.abgc.net/about-genetic-counseling/find-a-certified-counselor/) provides a searchable directory of certified genetic counselors worldwide. You can search by practice area, name, organization, or location.
- The Canadian Association of Genetic Counselors (https://www.cagc-accg.ca/index.php?page=225) has a searchable directory of genetic counselors in Canada. You can search by name, distance from an address, province, or services.
- The American College of Medical Genetics and Genomics (http://www.acmg.net/ACMG/Genetic_Services_Directory_Search.aspx) has a searchable database of medical genetics clinic services in the United States.
Congenital insensitivity to pain symptoms
Characteristic findings
Age-Related
- Infants and young children:
- Self-mutilating injuries of the fingers (biting off fingertips) and oral cavity such as loss of the tongue tip, injuries to the inside of the teeth/gums, and avulsion of teeth are common (see Figure 1A and 1B).
- Cuts and bruises may be present.
- Burns due to impaired temperature sensation can occur 13.
- Recurrent otitis media may be due to selectively reduced immunity to Staphylococcus aureus (see Infections) 14.
- Older individuals:
- Painless fractures and joint damage frequently occur and can lead to permanent damage.
- Bony deformities due to past fractures can occur.
- Charcot joints (neuropathic arthropathy), most commonly of the ankles, hips, and lumbar spine, are almost universal (Figure 1C).
- Charcot spine may present with progressive deformity or new motor and/or sensory deficits 15.
Eyes
- All affected individuals are at risk for corneal injuries due to absent corneal reflexes.
- Permanent corneal scarring can develop and is best assessed through a slit-lamp examination.
- Emotional tearing, as opposed to pain induced tearing, is likely to be present 14.
Infections
Apparent selectively reduced immunity to Staphylococcus aureus has been observed in some affected individuals, leading to recurrent soft tissue infections, abscesses, and osteomyelitis.
Temperature Regulation, Anhidrosis, and Hyperhidrosis
Some individuals have anhidrosis (lack of sweating), which disturbs thermoregulation and can lead to recurrent episodes of unexplained fever (see Table 1 above) 6.
Marked hyperhidrosis may be seen in those affected individuals who have a heterozygous pathogenic c.2432T>C (p.Leu811Pro) variant in SCN11A 16.
Hyperpyrexia can be fatal if untreated 14.
Hypothermia can occur in cold conditions.
Development and Intellect
Development and intellect may be normal or delayed/disabled (see Table 1).
- Individuals with congenital insensitivity to pain caused by biallelic pathogenic variants in SCN9A and PRDM12 typically have normal intellect.
- Individuals with congenital insensitivity to pain caused by biallelic pathogenic variants in NTRK1 may have a variable degree of intellectual disability (see Table 1).
- Hyperactivity, impulsivity, and attention deficit are common in children with biallelic pathogenic variants in NTRK1 17.
Other
Chronic anemia of unknown cause was observed in 22/28 Israeli affected individuals with congenital insensitivity to pain and anhidrosis 18.
A few individuals have neuropathic pain, although this does not limit activities 19.
Congenital insensitivity to pain diagnosis
There are no consensus clinical diagnostic criteria for congenital insensitivity to pain. However, a diagnosis requires visible proof of lack of nociception in a conscious individual of normal intellectual ability. In those with intellectual disability congenital insensitivity to pain may be more difficult to diagnose clinically.
Clinical examination
Nociception is assessed by applying painful stimuli, which in people with normal nociception would be so difficult to bear that they would move the part of the body away from the stimulus and/or express discomfort. The authors have experience of children being incorrectly judged to have insensitivity to pain after an inadequately painful stimulus.
- The technique should not damage/scar prior to significant pain (e.g., sternal rub, which bruises before significant pain).
- Application of 5-10 kg pressure with a pen pressed onto the nail bed (the nail bed blanches for a few seconds afterward) is reliable (see Figure 1D).
Assessment of the remainder of the peripheral and central nervous system is typically normal (touch, vibration and position sense, motor functions, and deep tendon reflexes).
Supportive laboratory findings
Routine nerve conduction studies and electromyogram are typically normal 18.
Nerve biopsy is not routinely performed in clinical practice. Skin biopsy to determine intra-epidermal nerve fiber density and autonomic innervation is performed in adults in some centers.
For more information about autonomic function testing for congenital insensitivity to pain with anhidrosis please go here (https://www.ncbi.nlm.nih.gov/books/NBK481553/bin/hsan4-special-studies.pdf).
Genetic testing
Molecular genetic testing approaches can include a combination of targeted gene testing (multigene panel or single-gene testing) and genomic testing (comprehensive genomic sequencing).
Targeted gene testing requires the clinician to develop a hypothesis as to which specific gene(s) are likely to be involved, whereas genomic testing does not. Targeted testing is feasible based on phenotype in anyone older than approximately age five years (because of the difficulties of assessing subtle problems of intellectual developmental, sweating, temperature sensing, and autonomic features in infants and young children), with the exception of SCN11A.
Serial single-gene testing
The phenotype may guide the choice of which gene(s) to analyze first. Consider performing sequence analysis of:
- SCN11A first in a newborn with severe intestinal hypomotility.
- SCN9A first in an individual with normal intelligence who has anosmia.
- PRDM12 first in an individual with normal intelligence, staphylococcal infections, and hypohidrosis. Because some individuals with NTRK1-CIP and NGF-CIP have minimal learning problems, sequence analysis of these two genes should be considered next if molecular genetic testing of SCN9A and PRDM12 yield no pathogenic variants.
- NTRK1 and NGF first in an individual with evidence of learning problems or late development, staphylococcal infections, and hypohidrosis. Unexplained fever due to anhidrosis (the inability to sweat) is a characteristic and often initial feature (more so in hot climates).
If no pathogenic variant in SCN11A is found through sequence analysis OR if no or only one pathogenic variant is found through sequence analysis of the remainder of the genes listed in Table 1, gene-targeted deletion/duplication analysis should be considered.
Note: Whole-gene deletions have been reported in individuals with NGF-CIP 20 and SCN9A-CIP.
A multigene panel that includes genes for congenital insensitivity to pain and other genes of interest may be considered.
Note: The genes included in the panel and the diagnostic sensitivity of the testing used for each gene vary by laboratory and are likely to change over time. (2) Some multigene panels may include genes not associated with the condition; thus, clinicians need to determine which multigene panel is most likely to identify the genetic cause of the condition at the most reasonable cost while limiting identification of variants of uncertain significance and pathogenic variants in genes that do not explain the underlying phenotype. (3) In some laboratories, panel options may include a custom laboratory-designed panel and/or custom phenotype-focused exome analysis that includes genes specified by the clinician. (4) Methods used in a panel may include sequence analysis, deletion/duplication analysis, and/or other non-sequencing-based tests.
Comprehensive genomic testing (when available) including exome and genome sequencing may be considered. Such testing may provide or suggest a diagnosis not previously considered (e.g., mutation of a different gene or genes that results in a similar clinical presentation).
Congenital insensitivity to pain treatment
No consensus treatment or surveillance guidelines have been developed. Treatment is supportive and is best provided by specialists in pediatrics, orthopedics, dentistry, ophthalmology, and dermatology.
Individuals with congenital insensitivity to pain typically learn that others have pain and tend to respond to others’ pain normally. They often learn to simulate having pain in appropriate situations; e.g., being tackled during football.
The possibility that naloxone may temporarily relieve congenital insensitivity to pain analgesia has been suggested 21. While this medication could be of use in detecting the source of injury/illness in an affected individual, it may also expose the affected person to widespread pain from accumulated injuries.
Agents and circumstances to avoid
Avoid the following:
- Jumping, high-impact/contact sports, pastimes and jobs that involve the potential for blunt injury or severe bone and joint trauma
- The paucity of males with congenital insensitivity to pain who are older than age 20 years correlates with behaviors fueled by inability to feel pain (e.g., greater risk taking, deliberately picking fights, participation in extreme sporting events).
- Hot or cold environments; hot or cold foods, hot showers or baths; heating blankets, particularly in the perioperative period
Pregnancy management
Women with congenital insensitivity to pain are able to become pregnant and bear children normally. Obstetric staff must be made aware of the diagnosis of congenital insensitivity to pain. Labor progresses normally, but will be painless, while other senses (stretch and touch) are intact. A delay in detecting pelvic fractures in an affected woman in the postnatal period has been reported 19.
Table 2. Treatment of manifestations in individuals with congenital insensitivity to pain
| Manifestation/Concern | Treatment | Considerations/Other |
|---|---|---|
| Dental & oral lesions | Tooth extraction &/or filing (smoothing) of sharp incisal edges 22; use of a mouth guard 23 | |
| Bone fractures | Standard treatment | Treatment w/an external fixator may → potentially serious infectious complications. |
| Bone & joint deformity | Corrective osteotomy | Prolonged & intensive monitoring is necessary to avoid deformity or incomplete healing. |
| Leg length discrepancy | Shoe lift or epiphysiodesis 24 | The value of surgical intervention needs to be weighed against nonsurgical approaches incl close monitoring 25. |
| Dry eyes | Lubricating eye drops or ointments | Surgical treatment of neurotrophic keratitis has not been successful 7. |
| Longstanding infections | Wide surgical debridement | |
| Ulcerating foot lesions | Standard treatment | Appropriate footwear & periods of non-weight bearing may be appropriate. |
| Hyperthermia | Direct cooling in a bath or w/cooling blanket | Control of environmental temperatures is essential. |
| Hypothermia | Warming by a blanket | |
| Skin dryness & cracking | Topical moisturizer (lotion or cream) | Untreated dry skin can → skin infections, which ↑ the risk for serious infections (cellulitis or osteomyelitis). |
Developmental delay and intellectual disability management issues
The following information represents typical management recommendations for individuals with developmental delay / intellectual disability in the United States; standard recommendations may vary from country to country.
Developmental delay and intellectual disability educational issues may be seen in those with CTCL1-CIP, NGF-CIP, or NTRK-CIP.
- Ages 0-3 years. Referral to an early intervention program is recommended for access to occupational, physical, speech, and feeding therapy. In the US, early intervention is a federally funded program available in all states.
- Ages 3-5 years. In the US, developmental preschool through the local public school district is recommended. Before placement, an evaluation is made to determine needed services and therapies and an individualized education plan (IEP) is developed.
- Ages 5-21 years
- In the US, an individualized education plan (IEP) based on the individual’s level of function should be developed by the local public school district. Affected children are permitted to remain in the public school district until age 21.
- Discussion of transition plans including financial, vocation/employment, and medical arrangements should begin at age 12 years. Developmental pediatricians can provide assistance with transition to adulthood.
- All ages. Consultation with a developmental pediatrician is recommended to ensure the involvement of appropriate community, state, and educational agencies and to support parents in maximizing quality of life. Consideration of private supportive therapies based on the affected individual’s needs is recommended. Specific recommendations regarding type of therapy can be made by a developmental pediatrician.
In the US:
- Developmental Disabilities Administration (DDA) enrollment is recommended. DDA is a public agency that provides services and support to qualified individuals. Eligibility differs by state but is typically determined by diagnosis and/or associated cognitive/adaptive disabilities.
- Families with limited income and resources may also qualify for supplemental security income for their child with a disability.
Social and behavioral concerns
Consultation with a developmental pediatrician may be helpful in guiding parents through appropriate behavior management strategies or providing prescription medications when necessary.
Irritability, hyperactivity, impulsivity, and acting-out behaviors typically improve with age.
Prevention of primary manifestations
Table 3. Prevention of primary manifestations in individuals with congenital insensitivity to pain
| Manifestation/ Concern | Prevention | Considerations/Other |
|---|---|---|
| Injuries occurring around the home | Stair gates; soft-round edging on tables & protruding objects; guard all heating devices; close supervision of younger children in the kitchen | |
| Injuries occurring at school | Inform personnel at school of the diagnosis; seek help when an accident occurs but the child does not seem hurt. | |
| Self-inflicted injuries | Education of affected individuals about their condition. | Communicating with other families of individuals with congenital insensitivity to pain (especially affected adults) |
| Corneal abrasion | At least annual ophthalmologic evaluation; artificial tears |
|
| Staphylococcus aureus infections |
| Affects those w/NTRK1-, NGF-, CLTCL1-, & PRDM12-CIP; infections are specific for S aureus only. |
Prevention of secondary complications
Table 4. Prevention of secondary manifestations in individuals with congenital insensitivity to pain
| Manifestation/Concern | Prevention | Considerations/Other |
|---|---|---|
| Inability to use pain as an indicator in diagnosing or assessing injury severity | Laminated information letters/MediAlert bracelets | Consider providing a laminated letter confirming the diagnosis, stating the pathogenic variant(s), & giving advice on diagnosis & treatment |
| Osteomyelitis of the mandible | Early treatment of dental caries & periodontal disease | Regular dental examinations & restriction of sweets |
| Bone & joint injury due to strenuous activity when an individual has poor baseline conditioning | Activities that lead to increased strength, balance, & body awareness | Dancing (particularly ballet), swimming, cycling, & non-traumatic martial arts may be considered. |
| Inadequate sedation in postoperative period may trigger unexpected movement, causing secondary injury. | Adequate sedation during procedures | Tachycardia & hypertension in postoperative period should raise consideration of the possibility of inadequate sedation. |
| Hyper- or hypothermia | Careful monitoring of temperature during perioperative period |
Surveillance
In addition to regular evaluations by a pediatrician and dermatologist (to assess and advise on skin infections/injuries) the measures in Table 5 are recommended.
Table 5. Recommended surveillance for individuals congenital insensitivity to pain
| Manifestation/Concern | Evaluation | Frequency/Comment |
|---|---|---|
| Dental caries / Tooth damage | Dental care | Regular examinations (at least every 6 mos) |
| Early injuries | Evaluation by parents & caregivers for signs of unrecognized injury | Daily |
| Bone health | Prompt investigation & treatment of orthopedic consequences of congenital insensitivity to pain by a named orthopedic surgeon | At least yearly, or more frequently depending on bony injuries |
| Corneal damage | Ophthalmology evaluation | At least annually, or more frequently as indicated |
| Hyper- or hypothermia | Monitoring of body temperature may allow timely treatment of hyper- or hypothermia. | As needed |
Congenital insensitivity to pain life expectancy
Congenital insensitivity to pain is a rare disease with a short life expectancy, proper management could be administered in order to extend the patient’s life expectancy.
Congenital insensitivity to pain with anhidrosis
Congenital insensitivity to pain with anhidrosis (CIPA) is a rare inherited disorder whose core clinical features consist of the inability to feel pain and temperature, and decreased or absent sweating (anhidrosis) 26. CIPA or congenital insensitivity to pain with anhidrosis is also known as hereditary sensory and autonomic neuropathy type IV (HSAN IV) 27. CIPA is a rare autosomal recessive genetic disorder that is caused by the failure of nociceptive and sympathetic neuron development 28. In 1996, Indo et al 29 first identified neurotrophic tyrosine kinase receptor type 1 (NTRK1) mutations in three unrelated CIPA patients. The gene NTRK1 located on chromosome 1q21-q22 includes 17 exons and yields a 796-residue protein (tropomyosin-related kinase A [TrkA]). TrkA protein specifically binds to extracellular nerve growth factor (NGF), stimulates homodimer formation, and activates its tyrosine kinase activity, resulting in phosphorylation of specific tyrosine residues in the intracellular domain. The lack of pain sensation and the presence of anhidrosis in CIPA are caused by the absence of NGF-dependent primary afferent neurons with unmyelinated C-fibers and sympathetic postganglionic neurons, respectively 30. To date, more than 105 NTRK1 mutations have been reported in CIPA patients 31.
The signs and symptoms of CIPA appear early, usually at birth or during infancy, but with careful medical attention, affected individuals can live into adulthood. An inability to feel pain and temperature often leads to repeated severe injuries. Unintentional self-injury is common in people with CIPA, typically by biting the tongue, lips, or fingers, which may lead to spontaneous amputation of the affected area. In addition, people with CIPA heal slowly from skin and bone injuries. Repeated trauma can lead to chronic bone infections (osteomyelitis) or a condition called Charcot joints, in which the bones and tissue surrounding joints are destroyed.
Normally, sweating helps cool the body temperature. However, in people with CIPA, anhidrosis often causes recurrent, extremely high fevers (hyperpyrexia) and seizures brought on by high temperature (febrile seizures).
In addition to the characteristic features, there are other signs and symptoms of CIPA. Many affected individuals have thick, leathery skin (lichenification) on the palms of their hands or misshapen fingernails or toenails. They can also have patches on their scalp where hair does not grow (hypotrichosis). About half of people with CIPA show signs of hyperactivity or emotional instability, and many affected individuals have intellectual disability. Some people with CIPA have weak muscle tone (hypotonia) when they are young, but muscle strength and tone become more normal as they get older.
CIPA is an extremely rare condition in most populations except the Japanese and Israeli Bedouins. Of note, in 2009 the number of Japanese with CIPA was estimated at between 130 and 210 32.
In the Japanese and Israeli Bedouin populations relatively common founder pathogenic variants have been reported 33:
- Three variants – p.Phe284TrpfsTer36, p.Arg554GlyfsTer104, and p.Asp674Tyr – account for roughly 70% of pathogenic NTRK1 alleles in the Japanese.
- One variant – p.Pro621SerfsTer12 – accounts for 89% of pathogenic NTRK1 alleles among Israeli Bedouins 33.
Half of reported cases have occurred in offspring of consanguineous parents 34.
Specific carrier frequencies are not available.
The diagnosis of CIPA is suspected in infants and children with recurrent fever and biting of the tongue, lips, or fingers after eruption of the first teeth, and in older individuals with repeat traumatic injuries 35. Evaluation of sensory and autonomic functions (including pharmacologic tests) and skin and nerve biopsies were used in the past for clinical diagnosis, however, the diagnosis can now be confirmed by identification of biallelic pathogenic variants in NTRK1.
CIPA causes
Mutations in the NTRK1 gene cause CIPA. The NTRK1 gene provides instructions for making a receptor protein that attaches (binds) to another protein called NGFβ. The NTRK1 receptor is important for the survival of nerve cells (neurons).
The NTRK1 receptor is found on the surface of cells, particularly neurons that transmit pain, temperature, and touch sensations (sensory neurons). When the NGFβ protein binds to the NTRK1 receptor, signals are transmitted inside the cell that tell the cell to grow and divide, and that help it survive. Mutations in the NTRK1 gene lead to a protein that cannot transmit signals. Without the proper signaling, neurons die by a process of self-destruction called apoptosis. Loss of sensory neurons leads to the inability to feel pain in people with CIPA. In addition, people with CIPA lose the nerves leading to their sweat glands, which causes the anhidrosis seen in affected individuals.
CIPA inheritance pattern
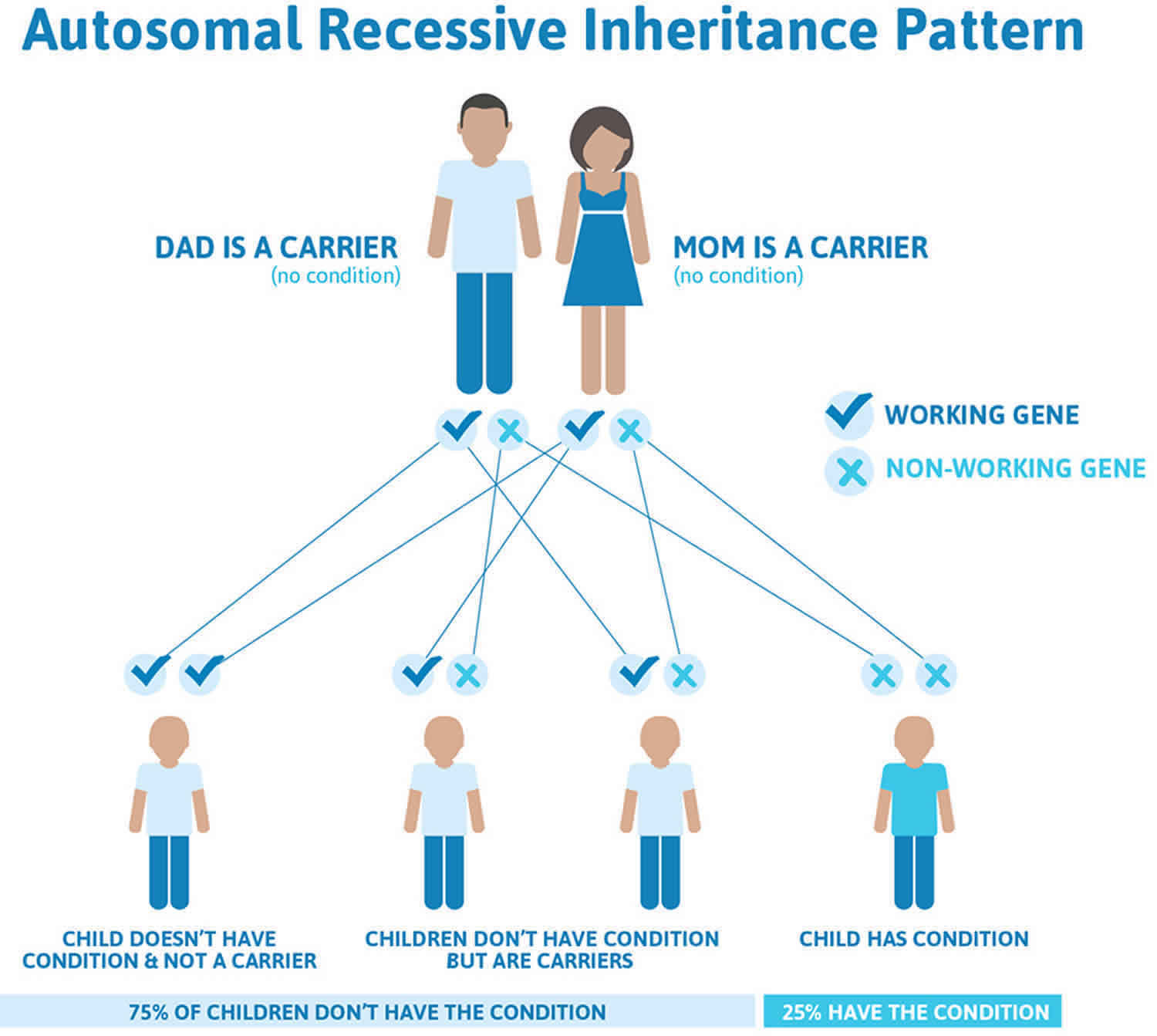
Congenital insensitivity to pain with anhidrosis or CIPA is inherited in an autosomal recessive pattern, which means both copies of the gene in each cell have mutations. The parents of an individual with an autosomal recessive condition each carry one copy of the mutated gene, but they typically do not show signs and symptoms of the condition.
It is rare to see any history of autosomal recessive conditions within a family because if someone is a carrier for one of these conditions, they would have to have a child with someone who is also a carrier for the same condition. Autosomal recessive conditions are individually pretty rare, so the chance that you and your partner are carriers for the same recessive genetic condition are likely low. Even if both partners are a carrier for the same condition, there is only a 25% chance that they will both pass down the non-working copy of the gene to the baby, thus causing a genetic condition. This chance is the same with each pregnancy, no matter how many children they have with or without the condition.
- If both partners are carriers of the same abnormal gene, they may pass on either their normal gene or their abnormal gene to their child. This occurs randomly.
- Each child of parents who both carry the same abnormal gene therefore has a 25% (1 in 4) chance of inheriting a abnormal gene from both parents and being affected by the condition.
- This also means that there is a 75% ( 3 in 4) chance that a child will not be affected by the condition. This chance remains the same in every pregnancy and is the same for boys or girls.
- There is also a 50% (2 in 4) chance that the child will inherit just one copy of the abnormal gene from a parent. If this happens, then they will be healthy carriers like their parents.
- Lastly, there is a 25% (1 in 4) chance that the child will inherit both normal copies of the gene. In this case the child will not have the condition, and will not be a carrier.
These possible outcomes occur randomly. The chance remains the same in every pregnancy and is the same for boys and girls.
Figure 3 illustrates autosomal recessive inheritance. The example below shows what happens when both dad and mum is a carrier of the abnormal gene, there is only a 25% chance that they will both pass down the abnormal gene to the baby, thus causing a genetic condition.
Figure 3. CIPA autosomal recessive inheritance pattern
People with specific questions about genetic risks or genetic testing for themselves or family members should speak with a genetics professional.
Resources for locating a genetics professional in your community are available online:
- The National Society of Genetic Counselors (https://www.findageneticcounselor.com/) offers a searchable directory of genetic counselors in the United States and Canada. You can search by location, name, area of practice/specialization, and/or ZIP Code.
- The American Board of Genetic Counseling (https://www.abgc.net/about-genetic-counseling/find-a-certified-counselor/) provides a searchable directory of certified genetic counselors worldwide. You can search by practice area, name, organization, or location.
- The Canadian Association of Genetic Counselors (https://www.cagc-accg.ca/index.php?page=225) has a searchable directory of genetic counselors in Canada. You can search by name, distance from an address, province, or services.
- The American College of Medical Genetics and Genomics (http://www.acmg.net/ACMG/Genetic_Services_Directory_Search.aspx) has a searchable database of medical genetics clinic services in the United States.
CIPA signs and symptoms
Congenital insensitivity to pain with anhidrosis (CIPA) is characterized by profound sensory loss affecting pain and temperature perception, absence of sweating (anhidrosis), and intellectual disability.
Anhidrosis
Because sweating plays an important role in maintaining normal body temperature, anhidrosis (the failure to sweat) disturbs thermoregulation in hot environmental conditions and increases susceptibility to recurrent febrile episodes 36.
Recurrent episodic fevers, usually the first clinical sign of CIPA, can begin in infancy or early childhood depending on environmental temperature 37. Recurrent febrile convulsions are also observed in some affected infants.
Occasionally, hypothermia is observed in cold environments 37.
Anhidrosis is present on the trunk and upper extremities in 100% of cases and more variable in other areas of the body 34. Although with warming the intertriginous areas of the neck, axillae, and groin can become slightly moist, no definite sweating is noted. This moisture is probably due to delayed evaporation of insensible water 37.
Insensitivity to pain
While impaired pain perception may not be apparent in early infancy, parents may recall that their infant with CIPA did not cry during venipuncture or immunizations 36.
Tongue ulcers and fingertip biting, the characteristic self-mutilation observed in infants with CIPA, begin when the primary incisors erupt, and can result in a bifid or absent tongue. Although taste buds are normal, traumatic injuries of the tongue, such as a partial loss of papillae and scar formation, may cause secondary hypogeusia or decreased taste sensation 38.
Biting of the fingers and ulcerated fingertips is common.
Bruises, cuts, and burns do not elicit normal reactions and are often unrecognized at the time that they occur. Accidental injuries such as falls or burns lead to multiple scars and can lead to cellulitis in the skin.
Orthopedic problems are one of the most characteristic and serious complications of CIPA 39.
Frequent orthopedic complications are:
- Multiple fractures often with hyperplastic new bone formation, avascular necrosis, and osteomyelitis
- Auto-amputation, self-mutilation (including self-inflicted soft tissue injuries)
- Leg length discrepancy
- Joint subluxation and dislocation resulting in Charcot neuroarthropathy of the feet, ankles, knees, and hips
- Septic arthritis
- Progressive scoliosis
Amputations of fingers or limbs are common as a result of these complications.
Decreased pain perception does not spare any area and even affects cranial nerves and visceral sensation 40.
Neurotrophic keratitis (degenerative disease of the corneal epithelium resulting from impaired corneal sensation) manifests initially as superficial punctate keratopathy which later can result in corneal ulceration and even perforation 41. Of note, tearing (both overflow or emotional) is normal.
Intellectual disability
Most individuals with CIPA have varying degrees of intellectual disability and show characteristic behaviors 36. Affected individuals show defects in conceptual thinking, abstract reasoning, and social behavior, as well as moderate to severe emotional disturbance 42. Some may exhibit rage. Assessments of cognitive and adaptive behavior suggest that many children with CIPA have intellectual disability (or learning disabilities) and severe attention-deficit-hyperactivity disorder (ADHD) 17.
Irritability, hyperactivity, impulsivity, and acting-out behaviors typically improve with age.
The prognosis for independent functioning varies.
Other
- Often the skin is dry with lichenification; the nails are dystrophic. Palmoplantar hyperkeratosis (thickening of the soles and the palms) appears in late infancy, often with scars and abrasions 43. Significant fissuring of the plantar skin is common. Some affected individuals develop painless deep heel ulcers that are slow to heal 44.
- Hypotonia is seen frequently in the early years, but strength and tone normalize as the individual gets older; tendon reflexes are normal 34.
- Gastrointestinal dysmotility is mild or absent.
- Vomiting is not a feature, but can be observed in some affected individuals.
- Speech is usually clear.
- Gao et al 45 have reported oral and craniofacial manifestations, including nasal malformation, submucous cleft palate, and developmental abnormalities of the teeth.
CIPA diagnosis
The diagnosis of congenital insensitivity to pain with anhidrosis (CIPA) is made clinically based on presence of the following 35:
- Impaired perception of pain and temperature, manifest in infants by biting of the tongue, lips, or fingers after the first teeth erupt; and in older individuals by repeated traumatic injuries including bruising, bone fractures and painless joint dislocations often associated with neurogenic arthropathy (Charcot joint) of the knees and ankles. There may be a history of failure to recognize burns and other injuries.
- Anhidrosis (absence of sweating), manifest as recurrent febrile episodes beginning in early infancy
- Intellectual disability.
The diagnosis of CIPA is confirmed by identification of biallelic pathogenic variants in NTRK1 (TRKA) 46. Mutation of NTRK1 accounts for all cases of properly classified CIPA.
Neurologic examination supports the diagnosis:
- Insensitivity to superficial and deep painful stimuli is confirmed when painful stimuli fail to evoke either withdrawal or emotional change [Swanson 1963]. For example, no tenderness or pain sensation is elicited even when apparently injured joints or broken bones are moved passively or actively. See also Special studies.
- Impaired temperature perception is confirmed when:
- Consistent errors are made in distinguishing between hot and cold moist substances.
- Extreme cold or heat fails to elicit the usual withdrawal response. See also Special studies.
- Visceral pain perception is also impaired.
- Impairment of the autonomic nervous system may be evident by the presence of Horner syndrome and the cold pressor test.
- Normal findings are:
- Touch, vibration and position senses
- Motor functions (unless repeated trauma has caused secondary dysfunction of motor neurons or limbs).
- Deep tendon reflexes and superficial abdominal and cremasteric reflexes. Note: No pathologic reflexes are observed.
Additional tests supporting the diagnosis of CIPA:
Skin tests demonstrating abnormalities in sweating and the lack of the axon reflex. See Special studies for more details (https://www.ncbi.nlm.nih.gov/books/NBK1769/bin/hsan4-special-studies.pdf).
Note: Skin and nerve biopsies were used to confirm the diagnosis in the past; however, molecular genetic testing is now preferred.
CIPA treatment
To establish the extent of disease and needs in an individual diagnosed with congenital insensitivity to pain with anhidrosis (CIPA) (also known as hereditary sensory and autonomic neuropathy type IV [HSAN IV]), the following evaluations are recommended:
- General physical examination for evidence of self-mutilation of tongue, lips, buccal mucosa; skin injury including infection and/or palmoplantar hyperkeratosis, bone injury including old poorly healing fractures and/or joint injury including dislocations, as well as behavioral and developmental problems
- Orthopedic consultation regarding assessment of injuries of the extremities and weight-bearing joints, including radiographs as necessary
- Dental examination to assess for auto-extraction of teeth, evidence of dental caries and/or abscess, as well as overall dental health
- Ophthalmologic examination to assess for evidence of neuropathic keratitis and its sequelae (corneal infection, ulceration, and/or perforation)
- Consultation with a clinical geneticist and/or genetic counselor
Treatment is supportive and is best provided by specialists in pediatrics, orthopedics, dentistry, ophthalmology, and dermatology.
- For anhidrosis: Monitoring body temperature helps to institute timely measures to prevent/manage hyperthermia or hypothermia.
- For insensitivity to pain: Modify as much as reasonable a child’s activities to prevent injuries. Inability to provide proper immobilization as a treatment for orthopedic injuries often delays healing; additionally, bracing and invasive orthopedic procedures increase the risk for infection. Methods used to prevent injuries to the lips, buccal mucosa, tongue, and teeth include tooth extraction, and/or filing (smoothing) of the sharp incisal edges of teeth, and/or use of a mouth guard. Skin care with moisturizers can help prevent palmar and plantar hyperkeratosis and cracking and secondary risk of infection; neurotrophic keratitis is best treated with routine care for dry eyes, prevention of corneal infection, and daily observation of the ocular surface. Interventions for behavioral, developmental, and motor delays as well as educational and social support for school-age children and adolescents are recommended.
Orthopedic
Bone fractures of weight-bearing bones and joints can lead to failure of bone union and hypertrophic osseous callous formation, as well as neurogenic arthropathy (Charcot joint) 39. Because such injuries and their sequelae are difficult to treat, the goal of orthopedic management is to prevent severe articular destruction and the need for surgical amputation.
Although the parents and caregivers of an affected child are advised to modify the child’s activities to prevent injuries, it is often very difficult due to the child’s inability to perceive pain. While protective appliances, such as braces to prevent injury to the lower limbs can be tried, they are associated with a high risk of secondary skin injury and, thus, infection.
Careful daily evaluation by parents and caregivers for early signs of otherwise unrecognized injury is important for early detection and treatment of injuries.
Appropriate footwear and periods of non-weight-bearing are important in the prevention and early treatment of ulcerating foot lesions 47.
In the treatment of various injuries the absence of pain perception makes immobilization difficult, often resulting in delayed healing. Additionally, infection is a serious potential complication of any invasive procedure, such as treatment of bone fractures with an external fixator 39.
Of note, longstanding infections require wide surgical debridement.
Bone and joint deformities can be managed by corrective osteotomy; leg length discrepancy can be managed by shoe lifts and/ or epiphysiodesis 47; however, the value of surgical intervention needs to be weighed against non-surgical approaches including close monitoring 39. Joint dislocations are best treated conservatively.
Dental
Early and routine preventative oral/dental care and timely treatment of the dental and oral conditions associated with CIPA can help reduce the characteristic oral and dental manifestations 48.
In infants, the incisal edges of newly erupted mandibular primary incisors traumatize the ventral surface of the tongue with sucking and nursing. This ulceration of the tongue can lead to bleeding and infection of the tongue, and halitosis, as well as systemic problems such as poor weight gain and failure to thrive.
The oral self-mutilation (i.e., the severe biting injuries [and resultant scarring] of the fingertips and/or oral soft tissues [tongue, lip, and buccal mucosa]) is found in most affected individuals. Although self-mutilation appears to decrease with age and with intellectual, social, and/or emotional development, such behaviors cannot be completely eliminated.
Methods used to prevent injuries to the lips, buccal mucosa, tongue, and teeth include tooth extraction, and/or filing (smoothing) of their sharp incisal edges 49, and/or use of a mouth guard, a protective plate of thermoplastic resin ~0.6-0.8 mm thick 48. Mouth guards must be refashioned as new teeth erupt and the jaw grows. Although use of a mouth guard is a reasonable approach, mouth guards can be difficult to prepare and/or retain.
The high rate of missing teeth and untreated carious teeth observed in individuals with CIPA suggests that dental examination and/or care is underutilized or that tooth decay may be overlooked because of the insensitivity to pain. Serious tooth decay can cause osteomyelitis that can lead to mandibular bone fracture.
Eye
Care for dry eyes, prevention of corneal infection, and daily observation of the ocular surface are crucial for maintaining good visual function 50. Of note, surgical treatment of neurotrophic keratitis has not been successful as poor outcomes of lateral tarsorrhaphy, corneal patch graft, and penetrating keratoplasty have been reported 7.
Skin
Daily care with a skin moisturizer is recommended to prevent or reduce skin cracking which can lead to bruising, and skin infections, which can progress to more significant infections such as cellulitis or osteomyelitis.
Parents or guardians should practice skin care to prevent serious infections, including daily observation of the whole skin surface and early treatment of even minor skin lesions.
Behavior
Interventions for behavioral, developmental and motor delays as well as educational and social support for school-age children and adolescents are important.
Although irritability, hyperactivity, impulsivity, and acting-out behaviors typically improve with age, medications for antipsychotic and/or attention-deficit/hyperactivity disorder (ADHD) in conjunction with behavior modification may be beneficial. The advantages and disadvantages should be weighed for each individual with CIPA.
Prevention of secondary complications
Regular dental examinations and restriction of sweets to prevent dental caries; early treatment of dental caries and periodontal disease to prevent osteomyelitis of the mandible. During and following surgical procedures, potential complications to identify and manage promptly include hyper- or hypothermia and inadequate sedation, which may trigger unexpected movement and result in secondary injuries.
Surveillance
Daily evaluation by parents and caregivers for early signs of otherwise unrecognized injury. Regular examinations by specialists in pediatrics, orthopedics, dentistry, ophthalmology, and dermatology are recommended to help prevent serious injuries and initiate early treatment. Annual follow up at a center that fosters comprehensive care and communication between the various subspecialties that are needed for optimal care.
Agents and circumstances to avoid
Hot or cold environments; hot or cold foods; hot showers or baths; jumping or high-impact activities and sports.
- Schon K, Parker A, Woods CG. Congenital Insensitivity to Pain Overview. 2018 Feb 8. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK481553[↩][↩][↩]
- Goldberg YP, MacFarlane J, MacDonald ML, Thompson J, Dube MP, Mattice M, Fraser R, Young C, Hossain S, Pape T, Payne B, Radomski C, Donaldson G, Ives E, Cox J, Younghusband HB, Green R, Duff A, Boltshauser E, Grinspan GA, Dimon JH, Sibley BG, Andria G, Toscano E, Kerdraon J, Bowsher D, Pimstone SN, Samuels ME, Sherrington R, Hayden MR. Loss-of-function mutations in the Nav1.7 gene underlie congenital indifference to pain in multiple human populations. Clin Genet. 2007;71:311–19.[↩]
- Congenital insensitivity to pain. https://ghr.nlm.nih.gov/condition/congenital-insensitivity-to-pain[↩][↩]
- Nahorski MS, Al-Gazali L, Hertecant J, Owen DJ, Borner GH, Chen YC, Benn CL, Carvalho OP, Shaikh SS, Phelan A, Robinson MS, Royle SJ, Woods CG. A novel disorder reveals clathrin heavy chain-22 is essential for human pain and touch development. Brain. 2015a;138:2147–60.[↩]
- Carvalho OP, Thornton GK, Hertecant J, Houlden H, Nicholas AK, Cox JJ, Rielly M, Al-Gazali L, Woods CG. A novel NGF mutation clarifies the molecular mechanism and extends the phenotypic spectrum of the HSAN5 neuropathy. J Med Genet. 2011;48:131–5.[↩][↩]
- Indo Y. Molecular basis of congenital insensitivity to pain with anhidrosis (CIPA): mutations and polymorphisms in TRKA (NTRK1) gene encoding the receptor tyrosine kinase for nerve growth factor. Hum Mutat. 2001;18:462–71.[↩][↩]
- Yagev R, Levy J, Shorer Z, Lifshitz T. Congenital insensitivity to pain with anhidrosis: ocular and systemic manifestations. Am J Ophthalmol. 1999;127:322–6.[↩][↩][↩]
- Zhang S, Malik Sharif S, Chen YC, Valente EM, Ahmed M, Sheridan E, Bennett C, Woods G. Clinical features for diagnosis and management of patients with PRDM12 congenital insensitivity to pain. J Med Genet. 2016;53:533–5.[↩]
- Weiss J, Pyrski M, Jacobi E, Bufe B, Willnecker V, Schick B, Zizzari P, Gossage SJ, Greer CA, Leinders-Zufall T, Woods CG, Wood JN, Zufall F. Loss-of-function mutations in sodium channel Nav1.7 cause anosmia. Nature. 2011;472:186–90.[↩]
- Einarsdottir E, Carlsson A, Minde J, Toolanen G, Svensson O, Solders G, Holmgren G, Holmberg D, Holmberg M. A mutation in the nerve growth factor beta gene (NGFB) causes loss of pain perception. Hum Mol Genet. 2004;13:799–805.[↩]
- Cox JJ, Sheynin J, Shorer Z, Reimann F, Nicholas AK, Zubovic L, Baralle M, Wraige E, Manor E, Levy J, Woods CG, Parvari R. Congenital insensitivity to pain: novel SCN9A missense and in-frame deletion mutations. Hum Mutat. 2010;31:E1670–86.[↩]
- Phatarakijnirund V, Mumm S, McAlister WH, Novack DV, Wenkert D, Clements KL, Whyte MP. Congenital insensitivity to pain: fracturing without apparent skeletal pathobiology caused by an autosomal dominant, second mutation in SCN11A encoding voltage-gated sodium channel 1.9. Bone. 2016;84:289–98.[↩]
- Cox JJ, Reimann F, Nicholas AK, Thornton G, Roberts E, Springell K, Karbani G, Jafri H, Mannan J, Raashid Y, Al-Gazali L, Hamamy H, Valente EM, Gorman S, Williams R, McHale DP, Wood JN, Gribble FM, Woods CG. An SCN9A channelopathy causes congenital inability to experience pain. Nature. 2006;444:894–8.[↩]
- Shatzky S, Moses S, Levy J, Pinsk V, Hershkovitz E, Herzog L, Shorer Z, Luder A, Parvari R. Congenital insensitivity to pain with anhidrosis (CIPA) in Israeli-Bedouins: genetic heterogeneity, novel mutations in the TRKA/NGF receptor gene, clinical findings, and results of nerve conduction studies. Am J Med Genet. 2000;92:353–60.[↩][↩][↩]
- Staudt MD, Bailey CS, Siddiqi F. Charcot spinal arthropathy in patients with congenital insensitivity to pain: a report of two cases and review of the literature. Neurosurg Rev. 2018;41:899–908.[↩]
- Woods CG, Babiker MO, Horrocks I, Tolmie J, Kurth I. The phenotype of congenital insensitivity to pain due to the NaV1.9 variant p.L811P. Eur J Hum Genet. 2015;23:561–3.[↩]
- Levy Erez D, Levy J, Friger M, Aharoni-Mayer Y, Cohen-Iluz M, Goldstein E. Assessment of cognitive and adaptive behaviour among individuals with congenital insensitivity to pain and anhidrosis. Dev Med Child Neurol. 2010;52:559–62.[↩][↩]
- Shatzky S, Moses S, Levy J, Pinsk V, Hershkovitz E, Herzog L, Shorer Z, Luder A, Parvari R. Congenital insensitivity to pain with anhidrosis (congenital insensitivity to painA) in Israeli-Bedouins: genetic heterogeneity, novel mutations in the TRKA/NGF receptor gene, clinical findings, and results of nerve conduction studies. Am J Med Genet. 2000;92:353–60.[↩][↩]
- Wheeler DW, Lee MC, Harrison EK, Menon DK, Woods CG. Case Report: Neuropathic pain in a patient with congenital insensitivity to pain. F1000Research. 2014;3:135.[↩][↩]
- Fitzgibbon GJ, Kingston H, Needham M, Gaunt L. Haploinsufficiency of the nerve growth factor beta gene in a 1p13 deleted female child with an insensitivity to pain. Dev Med Child Neurol. 2009;51:833–7.[↩]
- Minett MS, Pereira V, Sikandar S, Matsuyama A, Lolignier S, Kanellopoulos AH, Mancini F, Iannetti GD, Bogdanov YD, Santana Varela S, Millet Q, Baskozos G, MacAllister R, Cox JJ, Zhao J, Wood JN. Endogenous opioids contribute to insensitivity to pain in humans and mice lacking sodium channel Nav1.7. Nat Commun. 2015;6:8967[↩]
- Bodner L, Woldenberg Y, Pinsk V, Levy J. Orofacial manifestations of congenital insensitivity to pain with anhidrosis: a report of 24 cases. ASDC J Dent Child. 2002; 69:293-6, 235.[↩]
- Hutton A, McKaig S. The dental management of a child with congenital insensitivity to pain. Dent Update. 2010;37:180–2.[↩]
- Bar-On E, Weigl D, Parvari R, Katz K, Weitz R, Steinberg T. Congenital insensitivity to pain: orthopaedic manifestations. J Bone Joint Surg Br. 2002;84:252–7.[↩]
- Kim SJ, Bloom T, Sabharwal S. Leg length discrepancy in patients with slipped capital femoral epiphysis. Acta Orthop. 2013;84:271–4.[↩]
- Li N, Guo S, Wang Q, et al. Heterogeneity of clinical features and mutation analysis of NTRK1 in Han Chinese patients with congenital insensitivity to pain with anhidrosis. J Pain Res. 2019;12:453–465. Published 2019 Jan 22. doi:10.2147/JPR.S188566 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6348974[↩]
- Congenital insensitivity to pain with anhidrosis. https://ghr.nlm.nih.gov/condition/congenital-insensitivity-to-pain-with-anhidrosis[↩]
- Indo Y. NGF-dependent neurons and neurobiology of emotions and feelings: Lessons from congenital insensitivity to pain with anhidrosis. Neurosci Biobehav Rev. 2018;87:1–16.[↩]
- Indo Y, Tsuruta M, Hayashida Y, et al. Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nat Genet. 1996;13(4):485–488.[↩]
- Indo Y. Nerve growth factor, pain, itch and inflammation: lessons from congenital insensitivity to pain with anhidrosis. Expert Rev Neurother. 2010;10(11):1707–1724.[↩]
- Lv F, Xu XJ, Song YW, et al. Recurrent and novel mutations in the NTRK1 gene lead to rare congenital insensitivity to pain with anhidrosis in two Chinese patients. Clin Chim Acta. 2017;468:39–45.[↩]
- Haga N, Kubota M, Miwa Z. Epidemiology of hereditary sensory and autonomic neuropathy type IV and V in Japan. Am J Med Genet A. 2013;161A:871–4.[↩]
- Indo Y. Molecular basis of congenial insensitivity to pain with anhidrosis (CIPA): mutations and polymorphisms in TRKA (NTRK1) gene encoding the receptor tyrosine kinase for nerve growth factor. Hum Mutat. 2001;18:462–71.[↩][↩]
- Axelrod FB. Hereditary sensory and autonomic neuropathies. Familial dysautonomia and other HSANs. Clin Auton Res. 2002;12 Suppl 1:I2–14.[↩][↩][↩]
- Indo Y. Congenital Insensitivity to Pain with Anhidrosis. 2008 Aug 5 [Updated 2014 Apr 17]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1769[↩][↩]
- Indo Y. Genetics of congenital insensitivity to pain with anhidrosis (CIPA) or hereditary sensory and autonomic neuropathy type IV. Clinical, biologicial and molecular aspects of mutations in TRKA (NTRK1) gene encoding the receptor tyrosine kinase for nerve growth factor. Clin Auton Res. 2002;12 Suppl 1:I20–32.[↩][↩][↩]
- Swanson AG. Congenital insensitivity to pain with anhidrosis. Arch Neurol. 1963;8:299–306.[↩][↩][↩]
- Amano A, Akiyama S, Ikeda M, Morisaki I. Oral manifestations of hereditary sensory and autonomic neuropathy type IV. Congenital insensitivity to pain with anhidrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:425–31.[↩]
- Kim W, Guinot A, Marleix S, Chapuis M, Fraisse B, Violas P. Hereditary sensory and autonomic neuropathy type IV and orthopaedic complications. Orthop Traumatol Surg Res. 2013;99:881–5.[↩][↩][↩][↩]
- Shorer Z, Moses SW, Hershkovitz E, Pinsk V, Levy J. Neurophysioloic studies in congenital insensitivity to pain with anhidrosis. Pediatr Neurol. 2001;25:397–400.[↩]
- Mimura T, Amano S, Fukuoka S, Honda N, Arita R, Ochiai M, Yanagisawa M, Usui T, Ono K, Araki F, Yamagami S, Araie M, Awaya Y. In vivo confocal microscopy of hereditary sensory and autonomic neuropathy. Curr Eye Res. 2008;33:940–5.[↩]
- Pinsky L, DiGeorge AM. Congenital familial sensory neuropathy with anhidrosis. J Pediatr. 1966;68:1–13.[↩]
- Bonkowsky JL, Johnson J, Carey JC, Smith AG, Swoboda KJ. An infant with primary tooth loss and palmar hyperkeratosis: a novel mutation in the NTRK1 gene causing congenital insensitivity to pain with anhidrosis. Pediatrics. 2003;112:e237–41.[↩]
- Mardy S, Miura Y, Endo F, Matsuda I, Sztriha L, Frossard P, Moosa A, Ismail EA, Macaya A, Andria G, Toscano E, Gibson W, Graham GE, Indo Y. Congenital insensitivity to pain with anhidrosis: novel mutations in the TRKA (NTRK1) gene encoding a high-affinity receptor for nerve growth factor. Am J Hum Genet. 1999;64:1570–9.[↩]
- Gao L, Guo H, Ye N, Bai Y, Liu X, Yu P, Xue Y, Ma S, Wei K, Jin Y, Wen L, Xuan K. Oral and craniofacial manifestations and two novel missense mutations of the NTRK1 gene identified in the patient with congenital insensitivity to pain with anhidrosis. PLoS One. 2013;8:e66863[↩]
- Indo Y, Tsuruta M, Hayashida Y, Karim MA, Ohta K, Kawano T, Mitsubuchi H, Tonoki H, Awaya Y, Matsuda I. Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nat Genet. 1996;13:485–8.[↩]
- Bar-On E, Weigl D, Parvari R, Katz K, Weitz R, Steinberg T. Congenital insensitivity to pain. Orthopedic manifestations. J Bone Joint Surg Br. 2002;84:252–7.[↩][↩]
- Ikeda M, Kubodera T, Morisaki I, Akiyama S, Fukuta O. Oral manifestation and dental care of individuals with hereditary sensory and autonomic neuropathy with anhidrosis. In: Nihei K. Proceedings of International Symposium on Congenital Insensitivity to Pain with Anhidrosis 2003. Organizing Committee of International Symposium on Congenital Insensitivity to Pain with Anhidrosis; 2004:58-67.[↩][↩]
- Bodner L, Woldenberg Y, Pinsk V, Levy J. Orofacial manifestations of congenital insensitivity to pain with anhidrosis: a report of 24 cases. ASDC J Dent Child. 2002;69:293–6.[↩]
- Amano S, Fukuoda S, Usui T, Honda N, Ideta R, Ochiai M, Yamagami S, Araie M, Awaya Y. Ocular manifestations of congenital insensitivity to pain with anhidrosis. Am J Ophthalmol. 2006;141:472–7.[↩]