Conn’s syndrome
Conn’s syndrome also called primary hyperaldosteronism or primary aldosteronism, is a condition where the adrenal glands produce too much aldosterone, a hormone that regulates blood pressure and electrolyte balance. This excess aldosterone leads to high blood pressure (hypertension), low potassium levels (hypokalemia), and an increased volume of blood in the body 1, 2, 3, 4, 5, 6, 7, 8, 9. Aldosterone normally helps your kidneys retain sodium (salt) and water, and release potassium into the urine, helping to maintain blood pressure and electrolyte balance. Excess aldosterone causes your kidneys to retain more salt than normal, which in turn increases the body’s fluid levels and blood pressure. However, in reality, most patients with primary hyperaldosteronism or Conn’s syndrome will present without low potassium levels (hypokalemia) 10. People with primary hyperaldosteronism or Conn’s syndrome may have other symptoms like headaches, muscle cramps or excessive thirst. Untreated primary aldosteronism can lead to serious complications like heart attack and kidney failure. But prompt treatment can manage the condition successfully. In 1954, J. W. Conn first reported the clinical syndrome of high blood pressure (hypertension), low potassium levels (hypokalemia), and metabolic alkalosis resulting from autonomous production of aldosterone due to an adrenal adenoma – a syndrome that continues to bear his name 11, 12. The aldosterone-producing adrenal adenoma is characterized by increased aldosterone secretion from the adrenal glands, suppressed plasma renin, hypertension, and hypokalemia 13. And now the term primary hyperaldosteronism is used to describe Conn syndrome irrespective of whether the patient has an adrenal adenoma or not 12.
Primary hyperaldosteronism or Conn’s syndrome main signs and symptoms are:
- High blood pressure or hypertension defined as systolic blood pressure (SBP) 130 mm Hg or more and/or diastolic blood pressure (DBP) of more than 80 mm Hg.
- Low blood potassium levels
- Weakness
- Muscle cramps
Primary hyperaldosteronism is the most common cause of secondary hypertension and occurs in about 6% to 20% of adult hypertensive patients, higher in patients with resistant hypertension (high blood pressure requiring three or more medications to manage) 14, 15, 12. The prevalence of 10% was noted when consecutive patients with hypertension were evaluated. However, the prevalence increased to 30% when aldosterone to renin ratio was used as a screening method in general practice. Disparity in these percentages is probably due to the use of different laboratory screening techniques, different definitions of a positive screening study indicative of primary aldosteronism, study design, and varying population ethnicity and sampling source 16. Accumulating evidence suggests that approximately 10% of hypertensive individuals (mostly sampled from specialty clinics) may have primary hyperaldosteronism 16. In patients with resistant hypertension, the addition of a mineralocorticoid antagonist has been associated with substantial efficacy in blood pressure lowering, suggesting that subclinical hyperaldosteronism may be more prevalent than recognized 17.
Anyone can develop primary aldosteronism. But primary hyperaldosteronism or Conn’s syndrome more common in people with:
- Low blood potassium levels (hypokalemia).
- High blood pressure starting before age 30.
- High blood pressure requiring three or more medications to manage (resistant hypertension).
- An adrenal tumor.
Conn’s syndrome or primary hyperaldosteronism is caused by aldosterone-producing adrenal adenomas, bilateral idiopathic adrenal hyperplasia (idiopathic hyperaldosteronism), aldosterone-producing adrenal carcinoma, and familial aldosteronism. The increased amount of aldosterone potentiates renal sodium reabsorption and water retention, and potassium excretion. The increased sodium reabsorption by the kidneys results in plasma volume expansion which is the primary initiating mechanism for hypertension. This may induce tissue inflammation and heightened sympathetic drive, with subsequent development of fibrosis in vital organs, such as heart, kidneys, and vasculature. As a result, this may lead to the development of chronic kidney disease, atrial fibrillation, stroke, ischemic heart disease and congestive heart failure 18.
Aldosterone-producing adenoma is present in 50% to 60%, and the remaining is idiopathic or bilateral adrenal hyperplasia. It is about two times more common in women than men 13.
Conn’s syndrome diagnosis can initially be confirmed with the elevated morning aldosterone to plasma renin activity ratio. If the ratio is higher than 20 to 1; then the excess aldosterone points to the adrenal gland as the primary source. The preferred treatment is adrenalectomy in those with unilateral disease. Those who are poor surgical candidates or have bilateral adrenal hyperplasia can be treated medically with mineralocorticoid antagonists as well as antihypertensive agents for further blood pressure control.
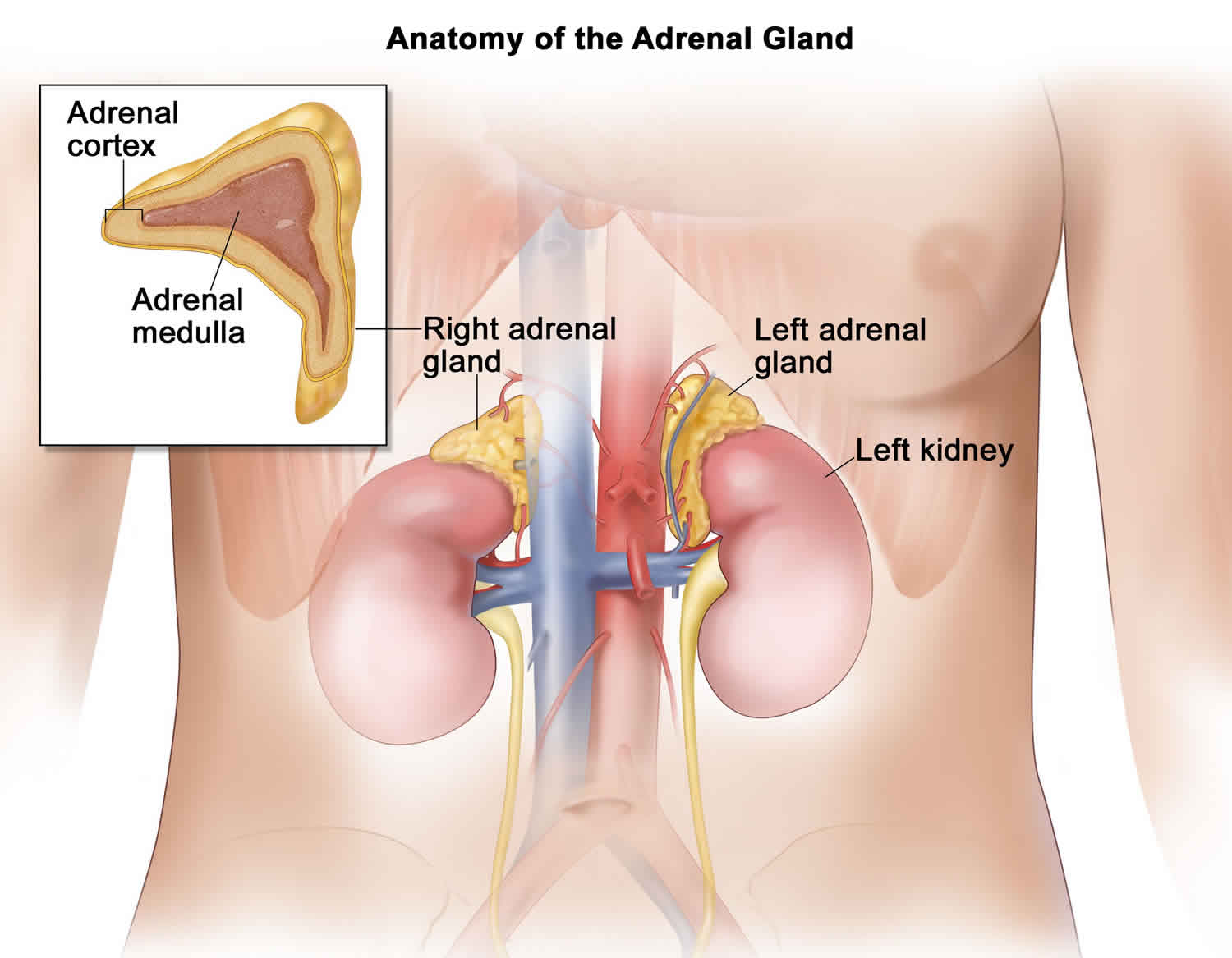
Adrenal glands
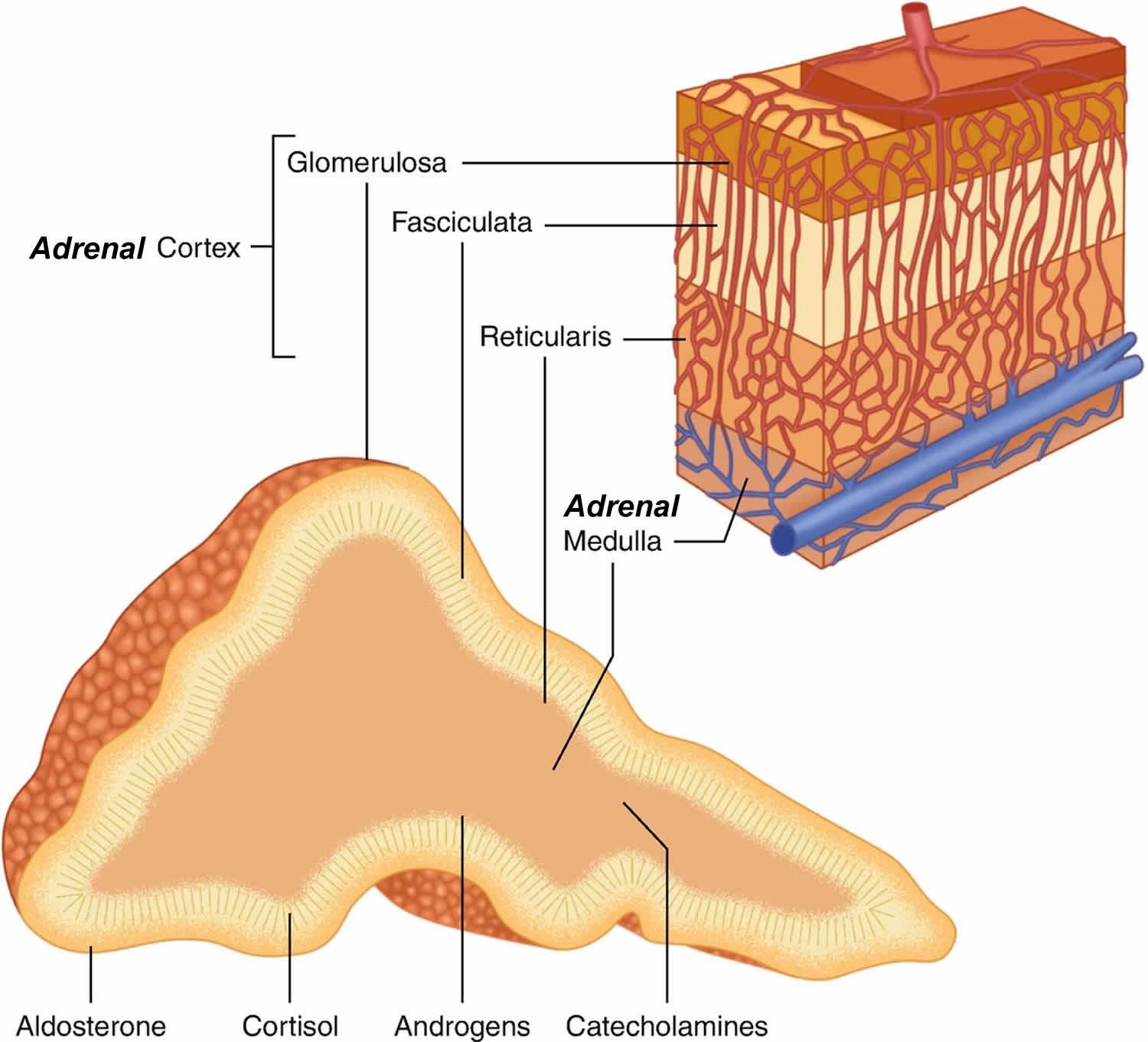
You have 2 adrenal glands, one on top of each kidney. Each adrenal gland has 2 parts. The outer part of the adrenal gland is the adrenal cortex and the inner part is the adrenal medulla.
The outer part of the adrenal gland or adrenal cortex makes certain hormones for your body. These hormones all have a similar chemical structure and are called steroids:
- Cortisol causes changes in metabolism to help the body to handle stress.
- Aldosterone also called mineralocorticoid helps your kidneys regulate the amount of salt in your blood and helps regulate blood pressure. Aldosterone is a hormone that is crucial for the retention of sodium (salt) and the elimination of potassium by your kidneys, salivary glands, sweat glands and colon. Aldosterone stimulates sodium transport across cell membranes, particularly in the distal renal tubule where sodium is exchanged for hydrogen and potassium. Secondarily, aldosterone is important in the maintenance of blood pressure and blood volume. Aldosterone also has a small effect on the metabolism of fats, carbohydrates, and proteins. Renin is an enzyme that controls aldosterone production (see the renin angiotensin aldosterone system below). Renin cleaves the blood protein angiotensinogen to form angiotensin 1 (angiotensin I), which is then converted by a second enzyme to angiotensin 2 (angiotensin II). Angiotensin 2 (angiotensin II) causes blood vessels to constrict, and it stimulates aldosterone production. Overall, this raises blood pressure and keeps sodium and potassium at normal levels.
- Adrenal androgens also called dehydroepiandrosterone or DHEA, can be converted to the sex hormones, estrogen and testosterone in other parts of the body. The amount of these hormones, however, that results from conversion of adrenal androgens is small compared to what is made in the other places in the body. In men, the testicles produce most of the androgens (male hormones). The ovaries produce most of the estrogens (female hormones) in women.
The inner part of the adrenal gland called adrenal medulla is really an extension of the nervous system. Nervous system hormones such as norepinephrine (noradrenaline), epinephrine (adrenaline), and dopamine are made in adrenal medulla. Tumors and cancers that start in the adrenal medulla include pheochromocytomas (which most often are benign) and neuroblastomas.
The outer part, the adrenal cortex, is where most adrenal tumors develop. Most adrenal cortex tumors are benign adenomas or non-cancerous tumors that don’t spread beyond the adrenal gland. These tumors are usually small (less than 4 cm) and occur in only one adrenal gland 19. Most people with adrenal adenomas have no symptoms and don’t know that they have an adrenal tumor.
Adrenocortical adenomas are categorized by whether they make hormones (functional) or not (non-functional). Most adrenal adenomas are non-functional. However, the small percentage of adrenocortical adenomas that do make hormones can cause certain medical conditions, such as:
- Cushing’s syndrome (high levels of cortisol)
- Conn’s syndrome or primary aldosteronism (high levels of aldosterone)
- Androgen and estrogen-secreting tumors
The excess hormones can cause the same symptoms as those from adrenocortical carcinomas (cancers).
Figure 1. Adrenal gland anatomy
Figure 2. Renin angiotensin aldosterone system
Figure 3. Renin angiotensin aldosterone system mechanism of action
Footnotes: The renin-angiotensin system (RAS) is a principal regulator of aldosterone production. The physiologic relationship between the renin-angiotensin system and aldosterone regulation is referred to as “Renin-Dependent Aldosteronism”, also referred to as “Secondary Aldosteronism”. Decreased renal-vascular perfusion resulting in decreased glomerular filtration is sensed by juxtaglomerular cells. The kidneys release Renin when there is a drop in blood pressure or a decrease in sodium chloride concentration in the tubules in the kidney. Renin cleaves the blood protein angiotensinogen to form angiotensin 1 (angiotensin I), which is then converted by angiotensin-converting enzyme (ACE) to angiotensin 2 (angiotensin II). Angiotensin 2 (angiotensin II) causes blood vessels to constrict (vasoconstriction), increases proximal tubular sodium reabsorption, and stimulates aldosterone production by increasing the transcription of aldosterone synthase. Overall, this raises blood pressure and keeps sodium and potassium at normal levels. The net effect is increased renal sodium reabsorption and intravascular volume expansion which closes the feedback loop and corrects the initial stimulus to raise renin 3.
Figure 4. Primary hyperaldosteronism diagnostic algorithm
Footnotes: (a) High-risk populations are those with a high pretest probability for having Conn’s syndrome or primary hyperaldosteronism. In the context of a suppressed renin, a plasma aldosterone >15 ng/dl essentially confirms the diagnosis of overt primary hyperaldosteronism and clinicians can proceed to localization or empiric mineralocorticoid receptor antagonist therapy. In a high-risk patient with a suppressed renin and a very low aldosterone concentration, <5 ng/dl, primary hyperaldosteronism is unlikely; however, mineralocorticoid receptor antagonist therapy should still be considered as an effective treatment for low-renin hypertension. For all other high-risk patients (suppressed renin and aldosterone 5–15 ng/dl), the diagnostic mindset should be that this is “primary hyperaldosteronism until proven otherwise.” Several options are available, including empiric initiation of mineralocorticoid receptor antagonist therapy and dietary sodium restriction, or an aldosterone suppression test (including a 24-hour urine collection to measure aldosterone and sodium excretion) to exclude the possibility of primary hyperaldosteronism or increase confidence in the diagnosis prior to pursuing localization and possible adrenalectomy.
(b) Enhanced diagnostic approach for specialist clinicians. When available, specialists may opt to test high-risk populations with a suppressed renin with a 24-hour urine collection to gain a better integrated assessment of aldosterone production. This can be performed on an ad libitum diet since many patients may already be consuming sufficient dietary sodium, or after dietary sodium loading to ensure a high-sodium balance. Ideally, the desired 24-hour urinary sodium balance should be greater than 150 mEq/24 hours (reflective of a dietary intake of ~3.5 g of sodium per day). A 24-hour urinary aldosterone excretion rate of >10 mcg in this context likely confirms primary hyperaldosteronism, whereas values between 6 and 10 mcg are suggestive of primary hyperaldosteronism pathophysiology that is likely to still respond to targeted therapy. Twenty-four-hour urinary aldosterone excretion rates less than 6 mcg suggest primary hyperaldosteronism is unlikely, but mineralocorticoid receptor antagonist therapy may still be beneficial for low-renin hypertension.
Abbreviations: MR, mineralocorticoid receptor; PA, primary aldosteronism.
[Source 5 ]Conn’s syndrome causes
The most prevalent cause of Conn’s syndrome or primary hyperaldosteronism is aldosterone-producing adrenal adenoma – a benign tumor (adenoma) on one adrenal gland that produces excess aldosterone (Parmar MS, Singh S. Conn Syndrome. [Updated 2022 Sep 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459197)), 2, 3, 12. Other causes include aldosterone-producing adrenal cancer (adrenocortical carcinoma) (1%), ectopic aldosterone secretion from the kidneys or ovaries, and bilateral adrenal hyperplasia of the zona glomerulosa also known as idiopathic hyperaldosteronism. There are familial hyperaldosteronism causes as well 20. There are other forms of hyperaldosteronism that are not familial. These conditions are caused by various problems in the adrenal glands or kidneys. In some cases, a cause for the increase in aldosterone levels cannot be found.
Familial hyperaldosteronism
Familial hyperaldosteronism is a group of inherited conditions in which the adrenal glands produce too much of the hormone aldosterone. Aldosterone helps control the amount of salt retained by your kidneys. Excess aldosterone causes your kidneys to retain more salt than normal, which in turn increases the body’s fluid levels and blood pressure. People with familial hyperaldosteronism may develop severe high blood pressure (hypertension), often early in life. Without treatment, hypertension increases the risk of strokes, heart attacks, and kidney failure.
Familial hyperaldosteronism is categorized into 3 types, distinguished by their clinical features and genetic causes.
- Familial hyperaldosteronism type 1: Familial hyperaldosteronism type 1 is glucocorticoid-remediable hyperaldosteronism that is caused by the abnormal joining together (fusion) of two similar genes called CYP11B1 and CYP11B2, which are located close together on chromosome 8. These genes provide instructions for making two enzymes that are found in the adrenal glands.
- CYP11B2 gene provides instructions for making an enzyme called aldosterone synthase previously known as corticosterone methyloxidase, which is also a member of the cytochrome P450 family of enzymes 21. Aldosterone synthase helps produce a hormone called aldosterone. The aldosterone synthase enzyme is involved in a series of three chemical reactions that produce aldosterone from other (precursor) molecules: the conversion of 11-deoxycorticosterone to corticosterone, the conversion of corticosterone to 18-hydroxycorticosterone, and the conversion of 18-hydroxycorticosterone to aldosterone. Aldosterone helps control blood pressure by maintaining proper salt and fluid levels in the body.
- CYP11B1 gene provides instructions for making an enzyme called 11-beta-hydroxylase, which is a member of the cytochrome P450 family of enzymes 22. The 11-beta-hydroxylase enzyme helps produce hormones called cortisol and corticosterone. Specifically, 11-beta-hydroxylase enzyme helps convert a molecule called 11-deoxycortisol to cortisol, and helps convert another molecule called 11-deoxycorticosterone to corticosterone. These processes are triggered by the release of a hormone called adrenocorticotropic hormone (ACTH) by the pituitary gland, located at the base of the brain.
- In familial hyperaldosteronism type 1, hypertension generally appears in childhood to early adulthood and can range from mild to severe. Familial hyperaldosteronism type 1 can be treated with steroid medications called glucocorticoids, so it is also known as glucocorticoid-remediable aldosteronism 23.
- Familial hyperaldosteronism type 2: Familial hyperaldosteronism type 2 causes are unclear. It is autosomal dominant, heterogeneous, no response to dexamethasone suppression, possibly link with a gene on chromosome 7p22 (band 11q13) and histologic findings are consistent with hyperplasia or adenomas 24.
- In familial hyperaldosteronism type 2, hypertension usually appears in early to middle adulthood and does not improve with glucocorticoid treatment 23.
- Familial hyperaldosteronism type 3: Familial hyperaldosteronism type 3 results from a mutation in the gene encoding the inwardly rectifying potassium channel Kir3.4 (KCNJ5 gene). Specific mutations in KCNJ5 gene, like those altering the G151E amino acid, are associated with a milder form of aldosteronism, compared to those with a G151R mutation that have a more severe form. The KCNJ5 gene provides instructions for making a protein that functions as a potassium channel, which means that it transports positively charged atoms (ions) of potassium (K+) into and out of cells. Potassium channels produced from the KCNJ5 gene are found in several tissues, including the adrenal glands. In adrenal glands, the flow of ions creates an electrical charge across the cell membrane, which affects the triggering of certain biochemical processes that regulate aldosterone production. Aldosterone helps control blood pressure by maintaining proper salt and fluid levels in the body. Mutations in the KCNJ5 gene likely result in the production of potassium channels that are less selective, allowing other ions (predominantly sodium) to pass as well. The abnormal ion flow results in the activation of biochemical processes (pathways) that lead to increased aldosterone production, causing the hypertension associated with familial hyperaldosteronism type 3 25.
- In most individuals with familial hyperaldosteronism type 3, the adrenal glands are enlarged up to six times their normal size 23. These affected individuals have severe hypertension that starts in childhood 23. The hypertension is difficult to treat and often results in damage to organs such as the heart and kidneys. Rarely, individuals with familial hyperaldosteronism type 3 have milder symptoms with treatable hypertension and no adrenal gland enlargement.
The prevalence of familial hyperaldosteronism is unknown. Familial hyperaldosteronism type 2 appears to be the most common variety. All types of familial hyperaldosteronism combined account for fewer than 1 out of 10 cases of hyperaldosteronism 23.
Secondary aldosteronism
Primary aldosteronism or Conn’s syndrome occurs when there’s an issue with your adrenal glands themselves producing too much aldosterone. But sometimes, other conditions can cause excess aldosterone production. When this happens, doctors call it secondary aldosteronism.
Conditions related to secondary aldosteronism include:
- Liver disease.
- Renal artery stenosis (a narrowing of the arteries that carry blood to your kidneys).
- Heart failure.
- Some types of kidney cancer.
- Pregnancy.
Conn’s syndrome symptoms
People with primary hyperaldosteronism or Conn’s syndrome usually present with high blood pressure (hypertension) and low potassium levels (hypokalemia). A systematic review conducted to assess the prevalence of primary hyperaldosteronism or Conn’s syndrome in patients with high blood pressure (hypertension) reported prevalence estimates ranging from 3.2% to 12.7% in general primary care settings and from 1% to 29.8% in specialised referral centers worldwide 26 . Left unchecked, high blood pressure (hypertension) raises your risk for complications, including heart attack and stroke, while low potassium (hypokalemia) can cause heart rhythm irregularities (arrhythmia).
Other primary hyperaldosteronism or Conn’s syndrome symptoms may include:
- Fatigue.
- Excessive thirst.
- Frequent urination (peeing more than you used to).
- Headache.
- Muscle cramps.
- Muscle weakness.
- Blurred vision.
A patient with suspected Conn’s syndrome or primary hyperaldosteronism will present with uncontrolled hypertension and will typically be young 12. These patients will require up to three antihypertensive medications including a diuretic to maintain suboptimal blood pressure control. They can also have a family history of early-onset hypertension or cerebral vascular disease at a younger age. Patients present with symptoms of severe muscle weakness, headaches, palpitations, fatigue, or muscle cramps due to symptoms related to hypokalemia (secondary to potassium wasting). Polydipsia and polyuria are present due to hypokalemia-induced nephrogenic diabetes insipidus.
Hypokalemia has been considered one of the hallmark signs in the diagnosis of primary aldosteronism; however, estimates are now that less than 37 percent of patients who have primary hyperaldosteronism will present with hypokalemia 27. Patients who have adequate sodium intake will often be more hypokalemic. Increasing sodium intake will allow more sodium delivery to the cortical collecting tubules promoting further excretion of potassium in the setting of excess aldosterone 27. Even though patients typically do not present with hypokalemia; the diagnosis should be considered in a patient with drug-resistant hypertension and hypokalemia in a patient starting a low dose of diuretic.
There are no physical exam characteristics that will lead to a diagnosis of primary hyperaldosteronism. However, due to excessive hypertension and stress on the heart, left ventricular hypertrophy can occur leading to an S4 heart sound secondary to blood trying to enter a noncompliant stiff ventricle during atrial contraction. Other findings related to longstanding hypertension can arise throughout the body affecting the heart (heart failure), kidneys (proteinuria), eyes (hypertensive retinal changes), vasculature (carotid bruits/stroke symptoms), muscle weakness, and mental status changes secondary to hypertensive encephalopathy.
Conn’s syndrome complications
If you don’t treat primary hyperaldosteronism, your blood pressure may increase to dangerous levels. It also disrupts the balance of electrolytes in your body. Electrolytes are minerals that help balance the amount of water in your body.
Long-term complications of primary aldosteronism 5, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37:
- Cardiovascular disease
- Coronary artery disease
- Heart attack
- Congestive heart failure
- Left ventricular hypertrophy
- Irregular heartbeat
- Atrial fibrillation
- Stroke
- Cardiovascular mortality
- Kidney disease
- Glomerular hyperfiltration
- Accelerated decline in glomerular filtration rate
- Kidney failure
- End-stage kidney disease
- Proteinuria
- Metabolic disease
- Type 2 diabetes 38
- Metabolic syndrome and obesity 38
- Obstructive sleep apnea (OSA) 39
- Osteoporosis and bone fractures 40
- Temporary paralysis or the inability to move.
Cardiovascular risk is associated with patients who have Conn’s syndrome because these patients will have greater mass measurements of the left ventricle as well as decreased left ventricular function when compared to patients with other types of hypertension 41. Other cardiovascular risks include stroke, atrial fibrillation, and myocardial infarction. There was a prospective study done comparing 54 patients with primary hyperaldosteronism who had treatment with either spironolactone, a mineralocorticoid receptor antagonist, or surgical resection of an adrenal adenoma. The control group was patients with primary hypertension matched for age, gender, body mass index (BMI), and duration of hypertension. The study found that before treatment, patients with primary hyperaldosteronism had a greater prevalence of cardiovascular events than those with primary hypertension. After treatment of the mineralocorticoid excess whether it be by surgical resection of adenomas or by spironolactone; there was no longer an elevated cardiovascular risk for those with primary hyperaldosteronism. The patients received follow-up for roughly seven years, and there was no significant difference between each group when it came to reaching the primary outcome of myocardial infarction, revascularization procedure, sustained arrhythmia, or stroke 42.
Metabolic syndrome is more common in those with primary hyperaldosteronism as compared to controls with similar blood pressure, sex, age, and BMI 43.
Increased aldosterone blood levels lead to increasing GFR and renal perfusion pressure. These patients will also have increased urinary albumin excretion. A study of a series of 50 patients with primary aldosteronism treated with either adrenalectomy or spironolactone was compared to those with primary hypertension on antihypertensive therapy. The results showed that at baseline, patients with primary aldosteronism had higher GFRs and albumin excretion than patients with primary hypertension. However, after a six-month follow-up of the treated primary aldosteronism patients, it was found that albuminuria was significantly decreased in addition to the reduction in the GFR when compared to the primary hypertension group 44. This research shows that with surgical resection or medical therapy in those with primary hyperaldosteronism, an underlying renal insufficiency could be present due to reversing the hyperfiltration state.
Prior stroke was found to be significantly higher in patients with primary hyperaldosteronism (12.9%) than those with primary hypertension (3.4%) in a retrospective study comparing a total of 124 patients with primary hyperaldosteronism and 465 patients with primary hypertension. The two groups had similar ages, genders, and mean blood pressure of 175/107 45.
Conn’s syndrome diagnosis
Your doctor will perform a physical exam including taking your blood pressure and asking about your symptoms. It may take 3 to 6 high blood pressure measurements at separate appointments to diagnose high blood pressure (hypertension). Home blood pressure monitoring and ambulatory blood pressure monitoring might make up some of these readings. With ambulatory blood pressure monitoring, a device takes blood pressure measurements automatically at specific times throughout the day.
Tests to help pinpoint the cause of the high blood pressure and to diagnose hyperaldosteronism might include:
- Blood tests. Blood tests are often done to check levels of aldosterone, renin, cortisol, potassium, sodium, creatinine, blood glucose, and total cholesterol and triglycerides, among others.
- A urine test (urinalysis). A urine sample can contain markers that could point to medical conditions that cause high blood pressure.
- Urinary aldosterone
- Ultrasound of the kidneys. Many kidney conditions are linked to secondary hypertension. In this noninvasive test, a technician moves a small, hand-held device called a transducer over the area to be tested. The transducer sends sound waves into the body, collects the ones that bounce back and sends them to a computer. The computer then creates images of the kidneys.
- Abdominal CT scan. Adrenal glands show up well on abdominal CT scans and the location of the tumor can usually be confirmed. Abdominal CT scans also can often help determine if the tumor has spread to lymph nodes and other organs. These findings can help doctors determine if the adrenal tumor is an adenoma or a carcinoma (cancer), by looking at the following:
- Size: Tumors larger than 4 cm (1.5 inches) suggest adrenal cancer.
- Shape: Irregularly shaped adrenal tumors suggest adrenal cancer.
- Density: Low density generally indicates the presence of fat, which is suggestive of an adenoma. Density is measured using Hounsfield units (HU) on CT scan. HU less than 10 is suggestive of an adenoma.
- Abdominal magnetic resonance imaging (MRI). Like CT scans, MRI scans show detailed images of soft tissues in the body. While a CT scan is the most common form of imaging used for adrenal tumors, MRI is used in certain situations:
- To avoid exposure to radiation: MRI scans use radio waves and strong magnets instead of x-rays.
- To gather more information: MRI scans can better distinguish adrenal cancers from benign tumors.
- Scintigraphy with NP-59 (131 I-6-β-iodomethyl-19-norcholesterol) with dexamethasone suppression can be used in selected cases to help differentiate between unilateral functioning adenomas and bilateral hyperplasia, especially if adrenal vein sampling is unsuccessful or cannot be performed and the patient would otherwise be a candidate for surgical intervention 46, 47, 48. This modality can also identify laterality in primary hyperaldosteronism and may be beneficial in patients with chronic kidney disease where standard biochemical testing is more problematic 47, 48. The positive predictive value and sensitivity in this modality have been reported as about 92% 47. However, it is a difficult test and may not be available in many centers.
- The 11C-metomidate positron emission tomography (PET)-CT scanning modality with and without dexamethasone suppression is a sensitive and specific test for primary hyperaldosteronism and adrenocortical tumors 49, 50, 51, 52, 53. In addition, this scan may reliably predict the response to medical therapy for hyperaldosteronism 51.
- Gallium-68 pentixafor PET-CT scanning has demonstrated a correlation in differentiating between unilateral and bilateral hyperaldosteronism 54. In a study, the gallium-68 PET-CT scan showed a 90% correlation with adrenal vein sampling compared to only 54% with CT scans 54.
- Electrocardiogram (ECG or EKG). This painless noninvasive test records the electrical signals in the heart. This test can help determine whether a heart problem might be causing secondary hypertension. In Electrocardiogram (ECG or EKG) test, sensors (electrodes) are attached to the chest and sometimes to the limbs. The sensors connect to a computer that records the heart’s electrical signal information and displays it as waves on a monitor or on paper. This test shows how the heart is beating.
- Kidney ultrasound
- Biopsy. Imaging tests may find tumors, but often the only way to know for sure that a tumor is cancer is to remove a sample of tumor tissue to look at under the microscope. This procedure is called a biopsy. Since biopsy samples from adrenal adenomas (benign tumors) and carcinomas (cancer) can look alike under the microscope, a biopsy may not tell if an adrenal tumor is cancerous. A needle biopsy of an adrenal cancer also can spread tumor cells. For these reasons, a biopsy is generally not done before surgery if an adrenal tumor’s size and features seen on imaging tests suggest cancer. Blood tests of hormone levels combined with imaging are more useful than biopsies in diagnosing adrenal cancer. If the cancer looks like it metastasized (spread) to another part of the body such as the liver, then a needle biopsy of the metastasis may be done. If a patient is known to have an adrenal tumor and a liver biopsy shows adrenal cells are present in the liver, then the tumor is cancer. In general, a biopsy is only done in a patient with adrenal cancer when there are tumors (metastases) outside the adrenals and the doctor needs to know if they are from the adrenal cancer or are caused by some other cancer or disease. Tumors in the adrenal glands are sometimes biopsied when the patient is known to have a different type of cancer (like lung cancer) and knowing if it has spread to the adrenal glands would alter treatment.
Sometimes blood and urine tests may be done after eating a high salt diet or after receiving saline through a vein (intravenous). You may need to stop taking some medicines before the tests. Your provider will tell you what to do before the procedure.
Another procedure involves inserting a catheter into the veins of the adrenal glands. This helps check which of the two adrenal glands is making too much aldosterone. This test is important because many people have small benign tumors in the adrenal glands that do not secrete any hormones. Relying only on a CT scan may result in the wrong adrenal gland being removed.
The Endocrine Society recommends screening for specific patient presentations. Hypokalemia in a hypertensive patient is the most common clue for Conn’s syndrome or primary hyperaldosteronism. However, normal serum potassium may be present in up to 38% of patients, especially in patients with adrenal hyperplasia or familial aldosteronism 55. Patients with hypertension on triple-drug therapy and diuretic-induced hypokalemia; patients with hypertension and adrenal incidentaloma; hypertension with a family history of the early-onset cerebral vascular accident; or patients with hypertension and first-degree relatives with confirmed primary hyperaldosteronism 56.
Suspect primary aldosteronism when a patient presents with hypertension at an early age with hypokalemia and poorly controlled blood pressure despite medical therapy. The next step is to obtain a morning plasma aldosterone and renin activity. If the ratio of morning aldosterone to plasma renin activity is higher than 20 to 1; then the excess aldosterone can be attributed to the adrenal gland as the primary source. Next, any of the four confirmatory tests may follow: 1) oral sodium loading 2) saline infusion 3) fludrocortisone suppression 4) captopril challenge, should suppress aldosterone; however, in a patient with primary aldosteronism, there will be a lack of aldosterone suppression. Once primary aldosteronism is confirmed all suspected patients are recommended to undergo adrenal computed tomography scan as the initial study and to exclude possible adrenocortical carcinoma. It is then recommended for the patient to have an adrenal venous sampling 57.
The best diagnostic test involves the measurement of cortisol and aldosterone in bilateral adrenal venous effluent and a peripheral vein before and during an ACTH infusion. Cortisol will be used to evaluate the catheter placement in the adrenal veins, as levels from the two sides should be similar. When an adenoma is present, the aldosterone-to-cortisol ratio on one side is usually at least five times greater than the other indicating suppression. Bilateral hyperplasia tends to produce similar values on each side. If the study points towards a unilateral adenoma, then laparoscopic adrenalectomy is the preferred treatment. If the patient declines surgery or is not a surgical candidate, medical therapy is the recommended route. If the study points towards a bilateral cause, then medical treatment with a mineralocorticoid antagonist is warranted 58.
- Urinary potassium excretion is elevated (more than 30 mmol/day).
- Diagnosis depends on the demonstration of expanded extracellular fluid (ECF) volume (suppressed plasma renin) and non-suppressible aldosterone secretion.
- ARR (aldosterone: renin ratio) or plasma aldosterone concentration to plasma renin activity (PAC/PRA) ratio: The lack of uniform assay method and diagnostic protocols in assessing the results creates a high variability in cut-off values among various investigators. A ratio of 40 or more (20 ng/dL/h to 40 ng/dL/h) or more than 135 (68 pmol/mU to 135 pmol/mU) has a sensitivity of 73% to 93% and a specificity of 71% to 84%, indicating the need for further confirmatory studies with salt-loading (failure to lower plasma aldosterone level less than 10 ng/dL), fludrocortisone suppression test, or captopril suppression test. The Endocrine Society’s clinical practice guidelines do not specify which of these confirmatory tests should be regarded as the gold standard to confirm or exclude the diagnosis; therefore, different tests are performed by different centers.
- Once autonomous aldosterone production is established, next step is to evaluate for possible adenoma. CT scan may show an adenoma that accounts for 70% cases, but as milder forms are being recognized now, idiopathic hyperaldosteronism is the most common cause. However, CT may provide incorrect diagnosis because of the common occurrence of non-functional adrenal adenomas (incidentalomas) that may be present in 4% of the general population.
- The studies to confirm unilateral nature of adrenal hypersecretion (lateralization) either by adrenal venous sampling (invasive, difficult and possible complications) or byiodocholesterol adrenal scan have limitations.
- Plasma 18-hydroxycorticosterone is elevated in adenoma and normal in adrenal hyperplasia.
- A plasma aldosterone response to a two-hour upright position shows normal increase in adrenal hyperplasia but a paradoxical decrease in adrenal adenoma.
Once the biochemical diagnosis of primary hyperaldosteronism has been confirmed, further testing is required to determine the cause and location of the disorder. Distinguishing between aldosterone-producing adenoma, bilateral adrenal hyperplasia, and less common forms of primary hyperaldosteronism, such as glucocorticoid-remediable aldosteronism, is important. Unilateral adrenalectomy cures hypertension in 30-70% of patients with aldosterone-producing adenoma or unilateral adrenal hyperplasia, and invariably reverses hypokalemia 59. In contrast, bilateral adrenalectomy in bilateral adrenal hyperplasia cures hypertension in only <20% of patients 59. Hence, the treatment of choice is surgical in aldosterone-producing adenoma or unilateral adrenal hyperplasia, and medical therapy is generally favored in bilateral adrenal hyperplasia and glucocorticoid-remediable aldosteronism.
Biochemical characteristics can assist with the diagnosis of the various causes of primary hyperaldosteronism. Young age (<50 years old), severe hypokalemia (<3.0 mmol/L), high plasma aldosterone concentrations (> 25 ng/dl), and high urinary aldosterone excretion (>30 ug/24hr) favor the diagnosis of aldosterone-producing adenoma versus bilateral adrenal hyperplasia 60. The presence of a classical unilateral Conn’s adenoma in addition to a serum potassium < 3.5 mmol/L or estimated glomerular filtration rate > 100 mL/min/1.73 m² is nearly 100% specific for an aldosterone-producing adenoma 61. However, while sensitive or specific, these clinical tools lack validation in large cohorts, and therefore cannot be relied upon as a means to determine the underlying etiology in individual patients 62.
Patients with primary hyperaldosteronism should undergo radiographic evaluation of the adrenal glands to localize the source and define the anatomy for potential surgical approaches. Computed tomography (CT) scanning with thin-slice (3mm) spiral technique is the best radiographic procedure to visualize the adrenal glands, and serves primarily to exclude large masses that may represent adrenocortical carcinoma, which are usually more than 4 cm in size. Observation of a solitary hypodense adrenal nodule, usually < 2 cm in size, supports the diagnosis of Aprimary hyperaldosteronism. Adrenal adenomas typically are lipid-rich on CT scan (<10 HU), and have a greater than 50% washout of contrast after 10-15 minutes. However, even when biochemical features suggestive of aldosterone-producing adenoma are present, only one-third to one-half of patients have positive CT findings for a solitary adenoma 63. It is also not uncommon for both adrenal glands to be anatomically abnormal in patients with primary aldosteronism. Furthermore, it is emphasized that a radiographic abnormality does not correlate with a functional equivalent. Non-functioning adrenal ‘incidentalomas’ are not rare, especially in patients above the age of 40; these are radiographically indistinguishable from Aprimary hyperaldosteronism, and can co-exist with an Aprimary hyperaldosteronism in the ipsilateral or contralateral adrenal gland. Therefore, data suggest that adrenal anatomy determined by CT scanning may wrongly predict etiology as well as lateralization of the aldosterone source in a significant proportion of patients 64.
Adrenal vein sampling is a localization technique that is considered to be the ‘gold standard’ for distinguishing unilateral versus bilateral disease in primary hyperaldosteronism 65. Adrenal vein sampling involves sampling from the right and left adrenal veins, as well as from the inferior vena cava (IVC), for measurement of aldosterone and cortisol concentrations. Many favor performing adrenal vein sampling with adrenocorticotropin (ACTH) stimulation, which can be administered continuously or as a bolus, and may minimize stress-induced fluctuations in aldosterone secretion during the procedure as well as maximize aldosterone secretion from an Aprimary hyperaldosteronism 66. However, other studies indicate that ACTH does not significantly improve the diagnostic accuracy of the procedure, in part because it may increase secretion from the contralateral side more than from the Aprimary hyperaldosteronism and therefore blunt lateralization 67.
Multiple variables derived from adrenal vein sampling can be used to determine lateralization of aldosterone hypersecretion 68. Cortisol-corrected aldosterone ratios (A/C ratio) are determined by dividing the aldosterone concentrations from each location sampled by the cortisol concentration in the same location to correct for dilutional effects.
Hyperaldosteronism screening
Screening for hyperaldosteronism is recommended for all patients newly diagnosed with hypertension, especially those with early-onset significant hypertension, hypokalemia, resistant or intractable high blood pressure, obstructive sleep apnea, or an adrenal mass 60. The high prevalence of hyperaldosteronism among hypertensive individuals, with many cases going undiagnosed, underscores the importance of such screening 69, 60.
Screening for hyperaldosteronism typically involves a simple plasma renin and aldosterone blood test 60. A low renin level combined with an aldosterone-to-renin ratio of more than 20 or a plasma aldosterone concentration (PAC) of more than 20 ng/dL commonly indicates possible hyperaldosteronism 10, 70, 71. A ratio of 30 or more is highly suggestive of the diagnosis. Confirmatory testing, computed tomography (CT) imaging, and selective bilateral adrenal venous sampling may then be performed 10, 70, 71, 69.
Adrenal vein sampling
Adrenal vein sampling is a procedure used to determine if one or both adrenal glands are producing too much aldosterone. Adrenal vein sampling involves a skilled interventional radiologist using a catheter to access and sample blood from the adrenal veins. Adrenal vein sampling helps pinpoint the source of excess aldosterone production and is used to differentiate unilateral from bilateral pathology if radiological imaging is not helpful and the patient is potentially a candidate for adrenal surgery 70. Cortisol and aldosterone are measured in the right and left adrenal veins; cortisol is used to verify catheter placement. Bilateral hyperplasia will demonstrate similar values on either side. However, a significant variance in the aldosterone concentration, at least double, or an aldosterone-to-cortisol ratio of at least 5-fold between the 2 sides suggests unilateral disease 10, 9, 72, 73. When optional cosyntropin stimulation is administered, a 4-fold difference in adrenal vein aldosterone levels is used as the cutoff threshold 9, 72, 73.
Adrenal vein sampling is not recommended in patients aged 35 and younger with spontaneous hypokalemia, marked levels of aldosterone, and highly suspicious adrenal adenomas on CT imaging 60. Nonfunctioning adrenal adenomas are rare in such younger populations. However, adrenal vein sampling is not required even in patients aged 40 or older with marked hyperaldosteronism with a clear solitary, unilateral adrenal adenoma on CT, those who are not surgical candidates, those suspected of adrenal cancer, and those with known familial hyperaldosteronism 74.
Adrenal vein sampling is still considered the gold standard for differentiating unilateral from bilateral adrenal hyperaldosteronism 70. However, the test is complex and technically demanding, as the vessels are relatively small; therefore, the interpretation can be challenging 72, 73. The right adrenal vein, in particular, can be exceedingly challenging to cannulate. Even unilateral adrenal vein sampling can be helpful.
In 2016, Pasternak et al 75 suggested the following method for cases where only unilateral adrenal vein sampling was accomplished:
- The formula, (adrenal vein aldosterone ÷ cortisol) / (inferior vena cava [IVC] aldosterone ÷ inferior vena cava [IVC] cortisol), is used to calculate the left adrenal vein (LAV)/inferior vena cava (IVC) ratio cutoff value
- Left adrenal vein (LAV)/inferior vena cava (IVC) ratio greater than 5.5 indicate unilateral hyperaldosteronism on the side tested, while results less than 0.5 predict the same but on the contralateral side.
- Left adrenal vein (LAV)/inferior vena cava (IVC) ratio between 5.5 and 0.5 are not interpretable and may indicate either bilateral or unilateral disease.
This method has an estimated specificity of 100% but only 50% sensitivity; other studies have confirmed these findings 75, 76, 77.
Adrenal venous sampling carries risks, including adrenal venous rupture, infarction, thrombus formation, bleeding, and hematoma formation 60. Therefore, the procedure should ideally be performed in centers of excellence with experience and expertise in this procedure 60. For these reasons, various noninvasive alternative diagnostic modalities to adrenal venous sampling are being explored. These tend to be cumbersome to perform with somewhat limited accuracy but may be helpful where there is experience and availability.
Laboratory findings suggestive of hyperaldosteronism
The aldosterone-to-renin ratio (ARR) or plasma aldosterone concentration to plasma renin activity (PAC/PRA) ratio has long been considered a reliable screening test for hyperaldosteronism, but results may be somewhat variable in certain situations 7, 78, 79. The levels should be drawn in the morning, and any interfering drugs should be eliminated, but this is not always safe or practical 7, 9, 80. The aldosterone-to-renin ratio (ARR) can be affected by posture, diurnal variations, electrolytes (eg, potassium levels), and several medications other than anti-hypertensives, including various antidepressants, antihistamines, dopaminergic meds, estrogens, licorice, and nonsteroidal anti-inflammatory drugs (NSAIDs) 7, 9, 80, 81, 82, 83. Other laboratory findings may demonstrate hypokalemia, mild hypernatremia, and mild hypomagnesemia, but these are not diagnostic 84, 85, 86.
Hypokalemia has historically been considered a hallmark finding closely associated with hyperaldosteronism but is now recognized as a relatively rare occurrence 7, 87, 88, 89. Therefore, the lack of hypokalemia should not be used to exclude a diagnosis of hyperaldosteronism 7. Current estimates indicate that less than 37% of those with confirmed primary hyperaldosteronism will demonstrate low serum potassium levels 90. Metabolic alkalosis is frequently observed in hyperaldosteronism due to the net loss of hydrogen ions in the urine from aldosterone-induced renal sodium retention, in which hydrogen ions are excreted in exchange for sodium 91. The historically well-documented association of hyperaldosteronism with hypokalemia has been observed in fewer than 40% of patients in published studies.
The role of the 24-hour urine collection in hyperaldosteronism is controversial, although it can detect inappropriate potassium wasting, defined as >30 mEq/day 92, 93, 94. This test can be useful in evaluating the role of extrarenal losses and diuretic abuse in hypokalemia, especially when the increase in aldosterone is barely detectable to mild. A urinary aldosterone-to-creatinine ratio has been suggested to facilitate the initial diagnosis of primary hyperaldosteronism by using an aldosterone-to-creatinine ratio of 3 ng aldosterone per 1 mg creatinine as the threshold 95. The validity of this test has not yet been established or fully verified 95.
Primary Hyperaldosteronism Diagnostic Evaluation
In primary hyperaldosteronism, plasma renin activity (PRA) is less than 1 ng/mL/hour, and the plasma renin concentration is either very low or undetectable 60. Excess aldosterone originates from the zona glomerulosa itself and not an extrinsic pathway. Therefore, a morning serum aldosterone-to-renin ratio of more than 20:1 indicates a renin-independent etiology consistent with primary hyperaldosteronism 10.
The plasma aldosterone concentration to plasma renin activity (PAC/PRA) ratio is a confirmatory test for primary hyperaldosteronism. Most studies support an elevated plasma aldosterone concentration to plasma renin activity (PAC/PRA) ratio of more than 30:1 and a plasma aldosterone concentration (PAC) of more than 20 ng/dL with a sensitivity and specificity of more than 90% as being diagnostic for primary hyperaldosteronism. However, a plasma aldosterone concentration to plasma renin activity (PAC/PRA) ratio greater than 20:1 and a plasma aldosterone concentration (PAC) of more than 15 ng/dL are reportedly sufficient to support the diagnosis 96, 97, 10.
Secondary Hyperaldosteronism Diagnostic Evaluation
In secondary hyperaldosteronism, both the plasma renin concentration and plasma renin activity (PRA) levels are increased, as renin is the primary stimulator for the excess production of aldosterone, and the serum aldosterone-to-renin ratio will, therefore, be much lower than in primary hyperaldosteronism 60. These levels are usually measured in the morning after patients have been out of bed 60. The results are more accurate when the labs are drawn at least 2 hours after the patient is out of bed and spends at least 5 minutes sitting. The serum aldosterone-to-renin ratio will be less than 20:1 60. Plasma renin activity (PRA), plasma renin concentration, and renin levels increase in secondary hyperaldosteronism, but the plasma aldosterone concentration to plasma renin activity (PAC/PRA) ratio is less than in primary hyperaldosteronism 9, 98, 96, 97.
Primary Versus Secondary Hyperaldosteronism
Serum renin levels are the most effective way to differentiate between primary and secondary hyperaldosteronism 99. Primary hyperaldosteronism always significantly suppresses renal renin production, whereas secondary hyperaldosteronism is associated with elevated serum renin concentrations 100. Secondary hyperaldosteronism tends to have higher aldosterone levels and blood pressure.
Hyperaldosteronism Confirmation
Confirmation of primary hyperaldosteronism may not be necessary in selected cases that demonstrate the combination of a markedly elevated plasma aldosterone, suppressed serum renin, hypokalemia, and resistant hypertension, although localization studies would still be needed 70, 101, 102.
Confirmatory testing for hyperaldosteronism typically includes measuring an elevated serum aldosterone level, conducting a 24-hour urinary aldosterone excretion test, or performing an aldosterone suppression test using salt-loading, captopril, or fludrocortisone, which would typically be expected to cause aldosterone suppression. All of these tests, except the captopril suppression test, may increase hypertension, induce fluid overload, and produce hypokalemia 70. No specific confirmatory test is officially preferred; however, a comparative meta-analysis suggested that the captopril challenge test may be the most feasible as it is easy to perform and considered relatively safe 70, 103.
Before performing confirmatory testing, any existing hypokalemia should be corrected 70. Negative or equivocal results from confirmatory testing in patients with positive blood screening of an aldosterone-to-renin ratio of more than 20 with low serum renin likely have mild hyperaldosteronism. They are typically treated medically with mineralocorticoid receptor antagonists (eg, spironolactone) 70.
Confirmatory tests
Oral salt-loading test
Oral salt-loading test usually involves significant dietary sodium loading and subsequent measurement of aldosterone levels 60. The oral salt-loading test consists of oral sodium loading over 3 days, usually 5000 to 6000 mg of dietary sodium daily or 90 mEq sodium tablets 60. Potassium supplements are provided to those who develop hypokalemia. After the sodium loading, a 24-hour urine aldosterone measurement is taken, with a value of >12 mcg/d commonly used to confirm primary hyperaldosteronism 60. A 24-hour urine sodium test of more than or equal to 200 mEq indicates adequate oral salt intake.
Some considerations with this test include that renal failure can complicate result interpretation by producing false negatives. Additionally, oral salt-loading test should not be administered to patients with a history of congestive heart failure, uncontrolled significant hypertension, or cardiac arrhythmias 70.
Intravenous salt-loading test
Intravenous salt-loading test is performed by administering 2 L of isotonic saline intravenously over 4 hours 60. Plasma aldosterone levels greater than 10 ng/dL following the infusion are consistent with primary hyperaldosteronism; however, the saline infusion has a false-negative rate of 30% 9. A modified version of the saline infusion test, which includes 0.5 mg of oral dexamethasone every 6 hours for 2 days, has demonstrated greater sensitivity 104. Drawbacks of this test include the need for hospitalization and potential risks such as fluid overload, worsening heart failure, hypokalemia, and increased blood pressure.
Captopril suppression test
The results of Captopril suppression test confirm primary hyperaldosteronism by evaluating the response to captopril—an angiotensin II blocker 60. In patients with primary hyperaldosteronism, aldosterone production remains elevated, while it should decrease in all other conditions 60. Patients are administered 25 to 50 mg of captopril, and serum aldosterone, renin, and cortisol levels are measured before and 1 to 2 hours after captopril administration 60.
In primary hyperaldosteronism, aldosterone is expected to decrease by at least 30%, resulting in an aldosterone-to-renin ratio of less than 30:1 60. However, serum aldosterone levels will typically remain elevated (≥8.5 ng/dL) with an aldosterone-to-renin ratio greater than 30:1 and low renin levels. The captopril suppression test is relatively safe, quick to perform as an outpatient, and inexpensive 60. Despite this, it has a relatively high rate of equivocal and false-negative results. Additionally, it is not recommended for suspected renovascular hypertension and may cause angioedema 70.
Fludrocortisone suppression test
Fludrocortisone suppression test aims to lower aldosterone levels by administering oral fludrocortisone, potassium supplementation, and salt loading 60. If aldosterone levels are not adequately suppressed after 4 days of treatment, it is considered confirmatory for primary hyperaldosteronism 105, 106. Patients receive 0.1 mg of oral fludrocortisone every 6 hours, with potassium and sodium chloride tablets taken 3 times daily with meals and a high sodium diet 60. Aldosterone and renin levels are measured after 4 days.
A serum aldosterone level of more than 6 ng/dL is considered a positive test and indicative of primary hyperaldosteronism 105, 106. However, the Fludrocortisone suppression test is labor-intensive and relatively costly. Adding a 2 mg dose of dexamethasone at midnight can enhance the reliability, sensitivity, and specificity of the fludrocortisone suppression test, improving its ability to detect milder forms of primary hyperaldosteronism 107, 108, 109, 110, 111, 112.
Negative or equivocal results from confirmatory testing in patients with positive blood screenings showing an aldosterone-to-renin ratio >20 with low serum renin suggest probable mild hyperaldosteronism 60. Such cases are generally treated medically with mineralocorticoid receptor antagonists, such as spironolactone 70.
Differentiating Unilateral From Bilateral Disease
Determining laterality is essential as it directly affects treatment and outcomes. Differentiating between adenoma and adrenal hyperplasia also aids in establishing laterality. The following findings may be helpful 60, 1:
- Serum 18-hydroxycorticosterone levels are elevated in adenomas but normal in bilateral adrenal hyperplasia 1.
- An adrenal adenoma shows a paradoxical decrease in serum aldosterone levels after 2 hours of upright positioning, whereas adrenal hyperplasia usually shows a normal or expected increase 1.
- Adrenal adenomas that autonomously overproduce aldosterone tend to be glucocorticoid-responsive, while bilateral idiopathic adrenal hyperplasia is generally not, although it may still respond somewhat to serum renin levels 113.
None of these findings are considered definitive to demonstrate laterality, which would require imaging studies or the more challenging but definitive adrenal vein sampling procedure 60.
Genetic Testing
Genetic testing is recommended for patients with primary aldosteronism who are aged 20 or younger or have a family history of the disorder. Familial hyperaldosteronism accounts for approximately 6% to 7% of all adult cases of primary hyperaldosteronism. Notably, 4 types of familial hyperaldosteronism have been described, with type 1 (glucocorticoid-responsive hyperaldosteronism) and type 3 (glucocorticoid-unresponsive hyperaldosteronism) being the most common 9. In severe cases, bilateral adrenalectomies may be necessary to manage blood pressure and severe hypokalemia, which requires lifelong glucocorticoid and mineralocorticoid replacement therapy.
Conn’s syndrome differential diagnosis
The differential diagnoses for Conn syndrome include the following:
- Essential hypertension: Essential hypertension also called idiopathic hypertension typically presents with a normal aldosterone-renin ratio (ARR) or plasma aldosterone concentration (PAC) to plasma renin activity (PRA) ratio 114
- 17-Alpha-hydroxylase deficiency: 17-Alpha-hydroxylase deficiency is a rare form of congenital adrenal hyperplasia (CAH) caused by a mutation in the CYP17A1 gene, which produces the 17α-hydroxylase enzyme 115. 17-Alpha-hydroxylase deficiency disrupts the production of cortisol and sex hormones, leading to various symptoms including hypertension, hypokalemia, and abnormal sexual development and can closely mimic hyperaldosteronism 115, 116. Patients typically present with hypogonadism and immature genitalia 116. Genetic testing may be required for a definitive diagnosis 116, 115.
- Chrétien syndrome: Chrétien syndrome is a rare disorder caused by excess secretion of proopiomelanocortin (POMC), a precursor of ACTH, from a pituitary adenoma 117. This leads to mineralocorticoid excess and adrenocortical hypertension. Symptoms include high blood pressure (hypertension), low potassium levels (hypokalemia), and metabolic alkalosis, which are similar to those seen in mineralocorticoid excess disorders 117.
- Congenital adrenal hyperplasia: Congenital adrenal hyperplasia is typically associated with a family history of 11-beta-hydroxylase or 17-alpha-hydroxylase deficiency and is characterized by low aldosterone levels 118, 115.
- Ectopic ACTH syndrome: Ectopic ACTH syndrome is a rare condition where a tumor outside the pituitary gland produces adrenocorticotropic hormone (ACTH), leading to hypercortisolism and Cushing’s syndrome 119, 120, 121, 122. The most common cause of ectopic ACTH syndrome is a neuroendocrine tumor, often in the chest, but it can also originate in the pancreas, thyroid, or other organs. Ectopic ACTH syndrome is associated with a rapid progression of hypercortisolism and can lead to significant morbidity and mortality 119, 120, 121, 122.
- Excessive licorice intake: Excessive licorice consumption can inhibits the renal conversion of cortisol to cortisone, producing a cortisol excess, which acts as a mineralocorticoid agonist simulating hyperaldosteronism 123, 124.
- Liddle syndrome: Liddle syndrome also called pseudohyperaldosteronism is a rare inherited form of high blood pressure (hypertension) characterized by early onset and often severe, salt-sensitive hypertension 125, 126. Liddle syndrome is caused by mutations in one of 3 genes (SCNN1A, SCNN1B, and SCNN1G) that encode the epithelial sodium channel (ENaC), leading to increased sodium reabsorption in the kidneys 125, 126. This, in turn, causes the body to retain too much salt and water, contributing to hypertension in childhood. Symptoms include hypertension, hypokalemia, and metabolic alkalosis, which are similar to mineralocorticoid excess disorders. Liddle syndrome, which is characterized by high urinary potassium secretion and sodium reabsorption in the renal collecting tubules despite low aldosterone levels.
- Primary glucocorticoid resistance: Primary glucocorticoid resistance also known as Chrousos syndrome, is a rare genetic disorder usually caused by mutations in the NR3C1 gene, which encodes the glucocorticoid receptor (GR) 127, 128, 129. Primary glucocorticoid resistance is characterized by a partial or complete insensitivity of tissues to glucocorticoids, primarily cortisol. This resistance leads to the hypothalamic-pituitary-adrenal (HPA) axis being overactive, resulting in increased levels of adrenocorticotropic hormone (ACTH) and cortisol and other hormones, which can manifest as hypertension, androgens, or mineralocorticoid excess 127, 128, 129.
- Apparent mineralocorticoid excess: Apparent mineralocorticoid excess is a rare genetic disorder characterized by low-renin hypertension, hypokalemia, and metabolic alkalosis, resulting from impaired function of the enzyme 11β-hydroxysteroid dehydrogenase type 2 (11-β-HSD2) 130, 131, 131, 132. The 11β-hydroxysteroid dehydrogenase type 2 (11-β-HSD2) enzyme normally converts cortisol to cortisone, an inactive form, but in syndrome of apparent mineralocorticoid excess, cortisol acts on mineralocorticoid receptors, leading to sodium retention, potassium wasting, and other related symptoms. Apparent mineralocorticoid excess can be caused by congenital defects in the HSD11B2 gene, or by chronic ingestion of licorice or certain antifungal medications 130, 131, 131, 132. Genetically, apparent mineralocorticoid excess is an autosomal recessive disorder 132.
- Metabolic alkalosis
- Renal artery stenosis
- Malignant hypertension. Malignant hypertension is a severe, life-threatening form of hypertension characterized by extremely high blood pressure and damage to vital organs. Malignant hypertension is a hypertensive emergency requiring immediate medical attention. Blood pressure is typically defined as systolic blood pressure (SBP) greater than 180 mm Hg and diastolic blood pressure (DBP) greater than 120 mm Hg.
- Preeclampsia
- Gitelman syndrome
- Bartter syndrome
- Adrenal carcinoma
Conn’s syndrome treatment
Treatment for Conn’s syndrome or primary hyperaldosteronism includes laparoscopic resection for adrenal adenomas 10. This procedure will usually resolve the hypokalemia, but hypertension can persist in up to 65% of patients post adrenalectomy 10. Surgery is the preferred treatment for patients with unilateral aldosterone-producing adrenal adenoma 10. After unilateral adrenalectomy, almost all patients have the resolution of hypokalemia and moderate improvement in blood pressure control 133. In those who are unable to undergo surgery or have bilateral adrenal hyperplasia, mineralocorticoid antagonists such as spironolactone or eplerenone are an option 10. In a randomized study, the antihypertensive effects between spironolactone and eplerenone in patients with primary hyperaldosteronism were studied showing that spironolactone was more effective than eplerenone in controlling blood pressure 134. Amiloride, a sodium channel blocker, may be helpful in the treatment and other antihypertensive agents can be continued as needed to optimize blood pressure control. Spironolactone is considered the first line agent for patients who cannot undergo surgical resection.
Primary Hyperaldosteronism Management
Primary hyperaldosteronism caused by unilateral adrenal adenoma is best treated surgically 60. Robotic or laparoscopic adrenalectomy is preferred as these procedures are associated with fewer complications and a shorter hospital stay compared to open surgery 135. Complete adrenalectomy is preferred to partial adrenal gland removal due to greater efficacy and resolution of symptoms. Primary hyperaldosteronism is the most frequently encountered surgically curable cause of hypertension 136.
Preoperative spironolactone to control blood pressure for 4 to 6 weeks is recommended 60. Patients who fail to normalize their blood pressure on spironolactone preoperatively are likely to continue to be hypertensive even after surgery 60. Following surgery, about two-thirds of patients will eventually develop stable, normal blood pressure, although this may take a year 1.
Patients with hyperaldosteronism due to unilateral disease fare better long-term with surgery than with medical therapy regarding blood pressure control, maintaining serum potassium levels, and improved vascular remodeling 137. Failure of surgery to control hypertension despite the normalization of aldosterone levels suggests the following 60:
- An erroneous or inadequate initial diagnosis of unilateral hyperaldosteronism
- Underlying essential hypertension
- Vascular abnormalities or damage from chronic hyperaldosteronism
- Other unrelated causes of hypertension (eg, pheochromocytoma or renovascular disease)
Good outcomes from surgery without adequate localization occur in less than 20% of patients 138. The medical therapy of choice for nonsurgical candidates is mineralocorticoid receptor antagonists, such as spironolactone and eplerenone 139, 140, 141, 142, 143. Amiloride, a potassium-sparing diuretic, may also be used 70.
Transcatheter and percutaneous adrenal radiofrequency ablation appear to be acceptable, less invasive surgical therapies for primary unilateral hyperaldosteronism, with a clinical success rate reported at about 75% 144, 145, 146, 147, 148, 149. These procedures are currently recommended for suitable patients unwilling to have surgery or take long-term medications 144, 145, 146, 147, 148, 149. Partial adrenalectomy may be possible in some patients as it provides similar outcomes with fewer postoperative complications, but it carries a potential risk of leaving part of the abnormal aldosterone-secreting tissue behind 150.
Primary Hyperaldosteronism Secondary to Bilateral Hyperplasia
Mineralocorticoid receptor antagonists are the treatments of choice for primary hyperaldosteronism caused by bilateral hyperplasia and for patients who are not surgical candidates 60. Spironolactone or eplerenone are most commonly used 60. The selection of these agents depends on the adverse effect profile, physician experience, and individual patient characteristics.
- Spironolactone is usually the preferred medical therapy, starting at 12.5 to 25 mg daily and titrated upward every 2 weeks according to the 2016 Endocrine Society Guidelines 141. Maintenance is often reached at a daily dosage of about 100 mg of spironolactone. Gynecomastia is a significant known adverse effect of spironolactone use in men, which may occur in up to 50% of male patients who take more than 150 mg daily 142.
- Eplerenone is a more specific medication that, unlike spironolactone, does not block androgen receptors. This makes it more acceptable and preferred for long-term treatment in men as it avoids possible gynecomastia and erectile dysfunction, especially if low-dose spironolactone is not effective. Eplerenone has a relatively short half-life of about 4 hours, which is longer than spironolactone’s half-life of only 1.4 hours. However, spironolactone is more effective in controlling blood pressure 134. Eplerenone is usually started at 50 mg daily and titrated upward, up to a maximum of about 200 mg twice a day 143
Patients with bilateral or idiopathic hyperaldosteronism are typically treated medically with either spironolactone or eplerenone for blood pressure control but still tend to have higher rates of cardiovascular events than other hypertensive patients, as higher levels of serum aldosterone correspond to a greater cardiovascular risk 151. This has led to investigations into alternative therapies where standard surgery could not be utilized.
The feasibility of bilateral superselective adrenal artery embolization for treating idiopathic primary hyperaldosteronism has been demonstrated 152, 153, 154, 155, 156, 157. Early studies indicate that such selective embolization therapy produces long-term, sustained improvement in serum aldosterone levels, aldosterone: renin ratios, hypokalemia, and blood pressure with no significant side effects or adverse events after 1 year of follow-up 152, 153, 154, 155, 156, 157. This suggests that bilateral superselective adrenal artery embolization could represent a reasonable and effective alternative therapy for idiopathic primary hyperaldosteronism 152, 153, 154, 155, 156, 157.
Other Potassium-Sparing Diuretics
The clinical course ultimately dictates the drug selection, dosage, and frequency. Reports of spontaneous remission of primary hyperaldosteronism after long-term therapy with mineralocorticoid receptor antagonist medications are rare 158. Triamterene and amiloride are potassium-sparing diuretics that may have an adjunctive (add-on) role in managing aldosterone-related hypertension 60. However, amiloride is preferred as triamterene may form urinary calculi 159, 160, 161, 162, 163.
Canrenone is an active metabolite of spironolactone with similar activity but a much longer half-life (16.5 hours versus 1.4 hours) and fewer sexual adverse effects 164. Canrenone appears to have a direct beneficial myocardial effect beyond its antihypertensive actions. Although currently available in Europe, canrenone is not yet available in the United States. Medications specifically targeting aldosterone-producing adrenal CYP-11B2 cells are being developed and investigated, though this is complex due to the close similarity between CYP-11B2 and CYP-11B1 cells 165, 166, 167.
Combination therapy
Combination therapy, including medications, sodium restriction (usually <100 mEq/d), avoidance of alcohol, smoking cessation, aerobic exercise, and maintaining ideal body weight, generally yields the best results 141, 168, 169. Additional treatments, such as glucocorticoids, amiloride, and calcium channel blockers, may be used to manage hypertension and other symptoms not adequately controlled by mineralocorticoid receptor antagonists alone 70. In rare cases, surgery involving bilateral adrenalectomies may be considered for patients with hyperaldosteronism secondary to bilateral adrenal hyperplasia who are refractory to maximum medical treatment 170.
Secondary Hyperaldosteronism Management
Secondary hyperaldosteronism is best managed by addressing the underlying disease, which typically resolves the symptoms. ACE inhibitors (ACE I) and angiotensin receptor blockers are preferred for blood pressure control in these patients due to their renal protective benefits 60. In addition, salt restriction is recommended for better control of blood pressure 171.
Potassium supplements and potassium-sparing diuretics may also be used to manage secondary hyperaldosteronism 60. The treatment approach is similar to that for primary hyperaldosteronism caused by idiopathic adrenal hyperplasia 60. In cases of renal artery stenosis, surgical intervention or revascularization may be necessary to achieve optimal blood pressure control 172.
Conn’s syndrome prognosis
Few studies have suggested that the reported 10-year survival rates for hyperaldosteronism treated patients range from 90% to 95% 60. Studies show that morbidity and mortality of those with primary hyperaldosteronism are directly related to chronic elevated hypertension leading to increased risk of cardiovascular disease including coronary artery disease, stroke, and congestive heart failure secondary to left ventricular hypertrophy, although overall mortality rates do not significantly differ from those of the general population 60, 173, 36. Other studies point to the increased risk of cardiac arrhythmias secondary to persistent hypokalemia in those with primary hyperaldosteronism 117. If hypokalemia persists, it can lead to symptoms such as weakness, paralysis, constipation, and polyuria 60. Additionally, primary hyperaldosteronism and hypokalemia can impair insulin secretion, increasing the risk of developing diabetes mellitus 174, 175, 100.
Research has shown in individual studies that surgical correction by adrenalectomy leads to a better prognosis by a significant reduction in hypertension and hypokalemia when compared to those with medical therapy 176. About two-thirds of patients become normotensive after 1 year post adrenal surgery and about half of these patients remain normotensive without medication by 5 years post-surgery. Untreated hyperaldosteronism is associated with significant morbidity and mortality, largely due to uncontrolled hypertension and cardiac arrhythmias.
- Parmar MS, Singh S. Conn Syndrome. [Updated 2022 Sep 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459197[
][
][
][
][
]
- Lee D, Emblin K, Daniels R, Kallis TJ, Alallan M, Mokbel K. Transient Ischaemic Attack in a Patient With Conn Syndrome: A Case Report and Literature Review on the Importance of Identifying Secondary Hypertension. In Vivo. 2025 Jan-Feb;39(1):566-571. doi: 10.21873/invivo.13861[
][
]
- Papadopoulou-Marketou N, Vaidya A, Dluhy R, et al. Hyperaldosteronism. [Updated 2020 Aug 6]. In: Feingold KR, Ahmed SF, Anawalt B, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279065[
][
][
]
- Hundemer GL, Leung AA, Kline GA, Brown JM, Turcu AF, Vaidya A. Biomarkers to Guide Medical Therapy in Primary Aldosteronism. Endocr Rev. 2024 Jan 4;45(1):69-94. doi: 10.1210/endrev/bnad024[
]
- Vaidya A, Hundemer GL, Nanba K, Parksook WW, Brown JM. Primary Aldosteronism: State-of-the-Art Review. Am J Hypertens. 2022 Dec 8;35(12):967-988. doi: 10.1093/ajh/hpac079[
][
][
]
- Ho WY, Hsiao CC, Wu PH, Chen JY, Tu YK, Wu VC, Chen JJ. Comparison of different medical treatments for primary hyperaldosteronism: a systematic review and network meta-analysis. Ther Adv Chronic Dis. 2024 Mar 19;15:20406223241239775. doi: 10.1177/20406223241239775[
]
- Faconti L, Kulkarni S, Delles C, Kapil V, Lewis P, Glover M, MacDonald TM, Wilkinson IB. Diagnosis and management of primary hyperaldosteronism in patients with hypertension: a practical approach endorsed by the British and Irish Hypertension Society. J Hum Hypertens. 2024 Jan;38(1):8-18. doi: 10.1038/s41371-023-00875-1[
][
][
][
][
][
]
- Wu VC, Hu YH, Er LK, Yen RF, Chang CH, Chang YL, Lu CC, Chang CC, Lin JH, Lin YH, Wang TD, Wang CY, Tu ST, Jeff Chueh SC, Chang CC, Tseng FY, Wu KD; TAIPAI group. Case detection and diagnosis of primary aldosteronism – The consensus of Taiwan Society of Aldosteronism. J Formos Med Assoc. 2017 Dec;116(12):993-1005. doi: 10.1016/j.jfma.2017.06.004[
]
- Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF Jr. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016 May;101(5):1889-916. doi: 10.1210/jc.2015-4061[
][
][
][
][
][
][
][
]
- Cobb A, Aeddula NR. Primary Hyperaldosteronism. [Updated 2023 Dec 5]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539779[
][
][
][
][
][
][
][
][
][
]
- JEROME W. CONN, LAWRENCE H. LOUIS. Primary aldosteronism, a new clinical entity. Ann Intern Med. 1956 Jan;44(1):1-15. doi:10.7326/0003-4819-44-1-1[
]
- Cobb A, Aeddula NR. Primary Hyperaldosteronism. [Updated 2019 Apr 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539779[
][
][
][
][
]
- Parmar MS, Singh S. Conn Syndrome. [Updated 2019 Jul 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459197[
][
]
- Schirpenbach C, Reincke M. Primary aldosteronism: current knowledge and controversies in Conn’s syndrome. Nat Clin Pract Endocrinol Metab. 2007;3(3):220–227. doi: 10.1038/ncpendmet0430[
]
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018 Oct 23;138(17):e484-e594. doi: 10.1161/CIR.0000000000000596[
]
- Funder JW, Carey RM, Fardella C, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2008;93:3266-81.[
][
]
- Calhoun DA, White WB. Effectiveness of the selective aldosterone blocker, eplerenone, in patients with resistant hypertension. J Am Soc Hypertens 2008;2:462-8.[
]
- Morera J, Reznik Y. MANAGEMENT OF ENDOCRINE DISEASE: The role of confirmatory tests in the diagnosis of primary aldosteronism. Eur. J. Endocrinol. 2019 Feb 01;180(2):R45-R58.[
]
- What Is Adrenal Cancer? https://www.cancer.org/cancer/types/adrenal-cancer/about/what-is-adrenal-cortical-cancer.html[
]
- Stowasser M, Wolley M, Wu A, Gordon RD, Schewe J, Stölting G, Scholl UI. Pathogenesis of Familial Hyperaldosteronism Type II: New Concepts Involving Anion Channels. Curr. Hypertens. Rep. 2019 Apr 04;21(4):31.[
]
- CYP11B2 gene. https://medlineplus.gov/genetics/gene/cyp11b2[
]
- CYP11B1 gene. https://medlineplus.gov/genetics/gene/cyp11b1[
]
- Familial hyperaldosteronism. https://medlineplus.gov/genetics/condition/familial-hyperaldosteronism[
][
][
][
][
]
- Funder JW. The genetic basis of primary aldosteronism. Curr. Hypertens. Rep. 2012 Apr;14(2):120-4.[
]
- Dutta RK, Söderkvist P, Gimm O. Genetics of primary hyperaldosteronism. Endocr. Relat. Cancer. 2016 Oct;23(10):R437-54.[
]
- Käyser SC, Dekkers T, Groenewoud HJ, van der Wilt GJ, Carel Bakx J, van der Wel MC, Hermus AR, Lenders JW, Deinum J. Study heterogeneity and estimation of prevalence of primary aldosteronism: a systematic review and meta-regression analysis. J Clin Endocrinol Metab. 2016;101(7):2826–2835. doi: 10.1210/JC.2016-1472[
]
- Mulatero P, Stowasser M, Loh KC, Fardella CE, Gordon RD, Mosso L, Gomez-Sanchez CE, Veglio F, Young WF. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J. Clin. Endocrinol. Metab. 2004 Mar;89(3):1045-50.[
][
]
- Monticone S, Burrello J, Tizzani D, Bertello C, Viola A, Buffolo F, Gabetti L, Mengozzi G, Williams TA, Rabbia F, Veglio F, Mulatero P. Prevalence and Clinical Manifestations of Primary Aldosteronism Encountered in Primary Care Practice. J Am Coll Cardiol. 2017 Apr 11;69(14):1811-1820. doi: 10.1016/j.jacc.2017.01.052[
]
- Mulatero P, Monticone S, Bertello C, Viola A, Tizzani D, Iannaccone A, Crudo V, Burrello J, Milan A, Rabbia F, Veglio F. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab. 2013 Dec;98(12):4826-33. doi: 10.1210/jc.2013-2805[
]
- Savard S, Amar L, Plouin PF, Steichen O. Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study. Hypertension. 2013 Aug;62(2):331-6. doi: 10.1161/HYPERTENSIONAHA.113.01060[
]
- Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Incidence of Atrial Fibrillation and Mineralocorticoid Receptor Activity in Patients With Medically and Surgically Treated Primary Aldosteronism. JAMA Cardiol. 2018 Aug 1;3(8):768-774. doi: 10.1001/jamacardio.2018.2003[
]
- Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018 Jan;6(1):51-59. doi: 10.1016/S2213-8587(17)30367-4[
]
- Wu VC, Wang SM, Chang CH, Hu YH, Lin LY, Lin YH, Chueh SC, Chen L, Wu KD. Long term outcome of Aldosteronism after target treatments. Sci Rep. 2016 Sep 2;6:32103. doi: 10.1038/srep32103. Erratum in: Sci Rep. 2017 Mar 24;7:45249. doi: 10.1038/srep45249[
]
- Rossi GP, Maiolino G, Flego A, et al. PAPY Study Investigators. Adrenalectomy Lowers Incident Atrial Fibrillation in Primary Aldosteronism Patients at Long Term. Hypertension. 2018 Apr;71(4):585-591. doi: 10.1161/HYPERTENSIONAHA.117.10596[
]
- Rossi GP, Cesari M, Cuspidi C, Maiolino G, Cicala MV, Bisogni V, Mantero F, Pessina AC. Long-term control of arterial hypertension and regression of left ventricular hypertrophy with treatment of primary aldosteronism. Hypertension. 2013 Jul;62(1):62-9. doi: 10.1161/HYPERTENSIONAHA.113.01316[
]
- Ohno Y, Sone M, Inagaki N, Yamasaki T, et al. Nagahama Study; JPAS Study Group. Prevalence of Cardiovascular Disease and Its Risk Factors in Primary Aldosteronism: A Multicenter Study in Japan. Hypertension. 2018 Mar;71(3):530-537. doi: 10.1161/HYPERTENSIONAHA.117.10263[
][
]
- Campbell SE, Diaz-Arias AA, Weber KT. Fibrosis of the human heart and systemic organs in adrenal adenoma. Blood Press. 1992 Oct;1(3):149-56. doi: 10.3109/08037059209077510[
]
- Wu VC, Chueh SJ, Chen L, Chang CH, Hu YH, Lin YH, Wu KD, Yang WS; TAIPAI Study Group. Risk of new-onset diabetes mellitus in primary aldosteronism: a population study over 5 years. J Hypertens. 2017 Aug;35(8):1698-1708. doi: 10.1097/HJH.0000000000001361[
][
]
- Loh HH, Sukor N. Primary aldosteronism and obstructive sleep apnea: What do we know thus far? Front Endocrinol (Lausanne). 2022 Sep 29;13:976979. doi: 10.3389/fendo.2022.976979[
]
- Wang A, Wang Y, Liu H, Hu X, Li J, Xu H, Nie Z, Zhang L, Lyu Z. Bone and mineral metabolism in patients with primary aldosteronism: A systematic review and meta-analysis. Front Endocrinol (Lausanne). 2022 Oct 31;13:1027841. doi: 10.3389/fendo.2022.1027841[
]
- Stowasser M, Sharman J, Leano R, Gordon RD, Ward G, Cowley D, Marwick TH. Evidence for abnormal left ventricular structure and function in normotensive individuals with familial hyperaldosteronism type I. J. Clin. Endocrinol. Metab. 2005 Sep;90(9):5070-6.[
]
- Catena C, Colussi G, Nadalini E, Chiuch A, Baroselli S, Lapenna R, Sechi LA. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch. Intern. Med. 2008 Jan 14;168(1):80-5.[
]
- Hanslik G, Wallaschofski H, Dietz A, Riester A, Reincke M, Allolio B, Lang K, Quack I, Rump LC, Willenberg HS, Beuschlein F, Quinkler M, Hannemann A., participants of the German Conn’s Registry. Increased prevalence of diabetes mellitus and the metabolic syndrome in patients with primary aldosteronism of the German Conn’s Registry. Eur. J. Endocrinol. 2015 Nov;173(5):665-75.[
]
- Sechi LA, Novello M, Lapenna R, Baroselli S, Nadalini E, Colussi GL, Catena C. Long-term renal outcomes in patients with primary aldosteronism. JAMA. 2006 Jun 14;295(22):2638-45.[
]
- Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J. Am. Coll. Cardiol. 2005 Apr 19;45(8):1243-8.[
]
- Yang YH, Chang YL, Lee BC, Lu CC, Wang WT, Hu YH, Liu HW, Lin YH, Chang CC, Wu WC, Tseng FY, Lin YH, Wu VC, Hwu CM. Strategies for subtyping primary aldosteronism. J Formos Med Assoc. 2024 Mar;123 Suppl 2:S114-S124. doi: 10.1016/j.jfma.2023.05.004[
]
- Di Martino M, García Sanz I, Muñoz de Nova JL, Marín Campos C, Martínez Martín M, Domínguez Gadea L. NP-59 test for preoperative localization of primary hyperaldosteronism. Langenbecks Arch Surg. 2017 Mar;402(2):303-308. doi: 10.1007/s00423-017-1561-1[
][
][
]
- Sato T, Matsutomo N, Yamamoto T, Fukami M, Kono T. Evaluation of standardized uptake value on 131I-6β-iodomethyl-19-norcholesterol scintigraphy for diagnosis of primary aldosteronism and correspondence with adrenal venous sampling. Ann Nucl Med. 2023 Feb;37(2):89-98. doi: 10.1007/s12149-022-01805-w[
][
]
- Spyridonidis TJ, Apostolopoulos DJ. Is there a role for Nuclear Medicine in diagnosis and management of patients with primary aldosteronism? Hell J Nucl Med. 2013 May-Aug;16(2):134-9.[
]
- Puar TH, Khoo CM, Tan CJ, Tong AKT, Tan MCS, Teo AE, Ng KS, Wong KM, Reilhac A, O’Doherty J, Gomez-Sanchez CE, Kek PC, Yee S, Tan AWK, Chuah MB, Lee DHM, Wang KW, Zheng CQ, Shi L, Robins EG, Foo RSY; for the PA CURE investigators. 11C-Metomidate PET-CT versus adrenal vein sampling to subtype primary aldosteronism: a prospective clinical trial. J Hypertens. 2022 Jun 1;40(6):1179-1188. doi: 10.1097/HJH.0000000000003132[
]
- Lu CC, Chen CJ, Peng KY, Chueh JS, Chang CC, Yen RF, Wu VC; Taiwan Primary Aldosteronism Investigation (TAIPAI) Study Group. Predicting Treatment Response in Primary Aldosteronism Using 11 C-Metomidate Positron Emission Tomography. Clin Nucl Med. 2022 Nov 1;47(11):936-942. doi: 10.1097/RLU.0000000000004369[
][
]
- Wu X, Senanayake R, Goodchild E, et al. [11C]metomidate PET-CT versus adrenal vein sampling for diagnosing surgically curable primary aldosteronism: a prospective, within-patient trial. Nat Med. 2023 Jan;29(1):190-202. doi: 10.1038/s41591-022-02114-5[
]
- Maurer E, Bartsch DK. 11C-Metomidate-PET/CT versus selektive Nebennierenvenenblutentnahme zur Vorhersage des Operationserfolgs beim primären Hyperaldosteronismus : Ergebnisse der prospektiven MATCH-Studie [11C-metomidate PET-CT vs. selective adrenal vein sampling for prediction of surgical success in primary aldosteronism : Results of the prospective MATCH study]. Chirurgie (Heidelb). 2023 Oct;94(10):877-878. German. doi: 10.1007/s00104-023-01957-z[
]
- Hu J, Xu T, Shen H, Song Y, Yang J, Zhang A, Ding H, Xing N, Li Z, Qiu L, Ma L, Yang Y, Feng Z, Du Z, He W, Sun Y, Cai J, Li Q, Chen Y, Yang S; Chongqing Primary Aldosteronism Study (CONPASS) Group. Accuracy of Gallium-68 Pentixafor Positron Emission Tomography-Computed Tomography for Subtyping Diagnosis of Primary Aldosteronism. JAMA Netw Open. 2023 Feb 1;6(2):e2255609. doi: 10.1001/jamanetworkopen.2022.55609[
][
]
- Schilbach K, Junnila RK, Bidlingmaier M. Aldosterone to Renin Ratio as Screening Tool in Primary Aldosteronism. Exp. Clin. Endocrinol. Diabetes. 2019 Feb;127(2-03):84-92.[
]
- Williams TA, Reincke M. MANAGEMENT OF ENDOCRINE DISEASE: Diagnosis and management of primary aldosteronism: the Endocrine Society guideline 2016 revisited. Eur. J. Endocrinol. 2018 Jul;179(1):R19-R29.[
]
- Mattsson C, Young WF. Primary aldosteronism: diagnostic and treatment strategies. Nat Clin Pract Nephrol. 2006 Apr;2(4):198-208; quiz, 1 p following 230.[
]
- Rossi GP, Auchus RJ, Brown M, Lenders JW, Naruse M, Plouin PF, Satoh F, Young WF. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension. 2014 Jan;63(1):151-60.[
]
- Meyer A, Brabant G, Behrend M. Long-term follow-up after adrenalectomy for primary aldosteronism. World J Surg 2005;29:155-9.[
][
]
- Dominguez A, Muppidi V, Leslie SW, et al. Hyperaldosteronism. [Updated 2024 Oct 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499983[
][
][
][
][
][
][
][
][
][
][
][
][
][
][
][
][
][
][
][
][
][
][
][
][
][
][
][
][
][
][
][
][
][
][
][
][
][
]
- Kupers EM, Amar L, Raynaud A, Plouin PF, Steichen O. A clinical prediction score to diagnose unilateral primary aldosteronism. J Clin Endocrinol Metab 2012;97:3530-7.[
]
- Stowasser M. Update in primary aldosteronism. J Clin Endocrinol Metab 2009;94:3623-30.[
]
- Kempers MJ, Lenders JW, van Outheusden L, et al. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med 2009;151:329-37.[
]
- Nwariaku FE, Miller BS, Auchus R, et al. Primary hyperaldosteronism: effect of adrenal vein sampling on surgical outcome. Arch Surg 2006;141:497-502; discussion -3.[
]
- Young WF, Stanson AW, Thompson GB, Grant CS, Farley DR, van Heerden JA. Role for adrenal venous sampling in primary aldosteronism. Surgery 2004;136:1227-35.[
]
- Sechi LA, Di Fabio A, Bazzocchi M, Uzzau A, Catena C. Intrarenal hemodynamics in primary aldosteronism before and after treatment. J Clin Endocrinol Metab 2009;94:1191-7.[
]
- Rossi GP, Ganzaroli C, Miotto D, et al. Dynamic testing with high-dose adrenocorticotrophic hormone does not improve lateralization of aldosterone oversecretion in primary aldosteronism patients. J Hypertens 2006;24:371-9.[
]
- Sacks BA, Brook OR, Brennan IM. Adrenal venous sampling: promises and pitfalls. Curr Opin Endocrinol Diabetes Obes 2013;20:180-5.[
]
- Lubitz CC, Economopoulos KP, Sy S, Johanson C, Kunzel HE, Reincke M, Gazelle GS, Weinstein MC, Gaziano TA. Cost-Effectiveness of Screening for Primary Aldosteronism and Subtype Diagnosis in the Resistant Hypertensive Patients. Circ Cardiovasc Qual Outcomes. 2015 Nov;8(6):621-30. doi: 10.1161/CIRCOUTCOMES.115.002002[
][
]
- Araujo-Castro M, Ruiz-Sánchez JG, et al. In representation of the following medical Spanish societies: Spanish Society of Endocrinology and Nutrition (SEEN), Spanish Society of Cardiology (SEC), Spanish Society of Nephrology (SEN), Spanish Society of Internal Medicine (SEMI), Spanish Radiology Society (SERAM), Spanish Society of Vascular and Interventional Radiology (SERVEI), Spanish Society of Laboratory Medicine (SEQC(ML), Spanish Society of Anatomic-Pathology, Spanish Association of Surgeons (AEC). Screening and diagnosis of primary aldosteronism. Consensus document of all the Spanish Societies involved in the management of primary aldosteronism. Endocrine. 2024 Jul;85(1):99-121. doi: 10.1007/s12020-024-03751-1[
][
][
][
][
][
][
][
][
][
][
][
][
][
]
- Harris DA, Au-Yong I, Basnyat PS, Sadler GP, Wheeler MH. Review of surgical management of aldosterone secreting tumours of the adrenal cortex. Eur J Surg Oncol. 2003 Jun;29(5):467-74. doi: 10.1016/s0748-7983(03)00051-9[
][
]
- Quencer KB. Adrenal vein sampling: technique and protocol, a systematic review. CVIR Endovasc. 2021 Apr 1;4(1):38. doi: 10.1186/s42155-021-00220-y[
][
][
]
- Kobayashi K, Alkukhun L, Rey E, Salaskar A, Acharya R. Adrenal Vein Sampling: Tips and Tricks. Radiographics. 2024 May;44(5):e230115. doi: 10.1148/rg.230115[
][
][
]
- Rossi GP, Auchus RJ, Brown M, Lenders JW, Naruse M, Plouin PF, Satoh F, Young WF Jr. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension. 2014 Jan;63(1):151-60. doi: 10.1161/HYPERTENSIONAHA.113.02097[
]
- Pasternak JD, Epelboym I, Seiser N, Wingo M, Herman M, Cowan V, Gosnell JE, Shen WT, Kerlan RK Jr, Lee JA, Duh QY, Suh I. Diagnostic utility of data from adrenal venous sampling for primary aldosteronism despite failed cannulation of the right adrenal vein. Surgery. 2016 Jan;159(1):267-73. doi: 10.1016/j.surg.2015.06.048[
][
]
- Lin L, Zhou L, Guo Y, Liu Z, Chen T, Liu Z, Wang K, Li J, Zhu Y, Ren Y. Can incomplete adrenal venous sampling data be used in predicting the subtype of primary aldosteronism? Ann Endocrinol (Paris). 2019 Nov;80(5-6):301-307. doi: 10.1016/j.ando.2019.10.001[
]
- Burrello J, Burrello A, Pieroni J, Sconfienza E, Forestiero V, Amongero M, Rossato D, Veglio F, Williams TA, Monticone S, Mulatero P. Prediction of hyperaldosteronism subtypes when adrenal vein sampling is unilaterally successful. Eur J Endocrinol. 2020 Dec;183(6):657-667. doi: 10.1530/EJE-20-0656[
]
- Stowasser M, Gordon RD. The aldosterone-renin ratio and primary aldosteronism. Mayo Clin Proc. 2002 Feb;77(2):202-3. doi: 10.4065/77.2.202-a[
]
- Veldhuizen GP, Alnazer RM, Kroon AA, de Leeuw PW. Variability of aldosterone, renin and the aldosterone-to-renin ratio in hypertensive patients without primary aldosteronism. J Hypertens. 2022 Nov 1;40(11):2256-2262. doi: 10.1097/HJH.0000000000003257[
]
- Gordon RD, Wolfe LK, Island DP, Liddle GW. A diurnal rhythm in plasma renin activity in man. J Clin Invest. 1966 Oct;45(10):1587-92. https://pmc.ncbi.nlm.nih.gov/articles/instance/292839/pdf/jcinvest00268-0079.pdf[
][
]
- Tiu SC, Choi CH, Shek CC, Ng YW, Chan FK, Ng CM, Kong AP. The use of aldosterone-renin ratio as a diagnostic test for primary hyperaldosteronism and its test characteristics under different conditions of blood sampling. J Clin Endocrinol Metab. 2005 Jan;90(1):72-8. doi: 10.1210/jc.2004-1149[
]
- Ahmed AH, Calvird M, Gordon RD, Taylor PJ, Ward G, Pimenta E, Young R, Stowasser M. Effects of two selective serotonin reuptake inhibitor antidepressants, sertraline and escitalopram, on aldosterone/renin ratio in normotensive depressed male patients. J Clin Endocrinol Metab. 2011 Apr;96(4):1039-45. doi: 10.1210/jc.2010-2603[
]
- Ahmed AH, Gordon RD, Ward G, Wolley M, McWhinney BC, Ungerer JP, Stowasser M. Effect of Combined Hormonal Replacement Therapy on the Aldosterone/Renin Ratio in Postmenopausal Women. J Clin Endocrinol Metab. 2017 Jul 1;102(7):2329-2334. doi: 10.1210/jc.2016-3851[
]
- Grasso M, Boscaro M, Scaroni C, Ceccato F. Secondary Arterial Hypertension: From Routine Clinical Practice to Evidence in Patients with Adrenal Tumor. High Blood Press Cardiovasc Prev. 2018 Dec;25(4):345-354. doi: 10.1007/s40292-018-0288-6.[
]
- Vaidya A, Mulatero P, Baudrand R, Adler GK. The Expanding Spectrum of Primary Aldosteronism: Implications for Diagnosis, Pathogenesis, and Treatment. Endocr Rev. 2018 Dec 1;39(6):1057-1088. doi: 10.1210/er.2018-00139[
]
- Seccia TM, Caroccia B, Gomez-Sanchez EP, Gomez-Sanchez CE, Rossi GP. The Biology of Normal Zona Glomerulosa and Aldosterone-Producing Adenoma: Pathological Implications. Endocr Rev. 2018 Dec 1;39(6):1029-1056. doi: 10.1210/er.2018-00060[
]
- Mosso L, Carvajal C, González A, Barraza A, Avila F, Montero J, Huete A, Gederlini A, Fardella CE. Primary aldosteronism and hypertensive disease. Hypertension. 2003 Aug;42(2):161-5. doi: 10.1161/01.HYP.0000079505.25750.11[
]
- Baguet JP, Steichen O, Mounier-Véhier C, Gosse P. SFE/SFHTA/AFCE consensus on primary aldosteronism, part 1: Epidemiology of PA, who should be screened for sporadic PA? Ann Endocrinol (Paris). 2016 Jul;77(3):187-91. doi: 10.1016/j.ando.2016.01.006[
]
- Schwartz GL, Turner ST. Screening for primary aldosteronism in essential hypertension: diagnostic accuracy of the ratio of plasma aldosterone concentration to plasma renin activity. Clin Chem. 2005 Feb;51(2):386-94. doi: 10.1373/clinchem.2004.041780[
]
- Mulatero P, Stowasser M, Loh KC, Fardella CE, Gordon RD, Mosso L, Gomez-Sanchez CE, Veglio F, Young WF Jr. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004 Mar;89(3):1045-50. doi: 10.1210/jc.2003-031337[
]
- Kassirer JP, London AM, Goldman DM, Schwartz WB. On the pathogenesis of metabolic alkalosis in hyperaldosteronism. Am J Med. 1970 Sep;49(3):306-15. doi: 10.1016/s0002-9343(70)80021-3[
]
- Blanchard A. Pathophysiologic approach in genetic hypokalemia: An update. Ann Endocrinol (Paris). 2023 Apr;84(2):298-307. doi: 10.1016/j.ando.2022.11.005[
]
- Muto S, Asano Y, Okazaki H, Kano S. Renal potassium wasting in distal renal tubular acidosis: role of aldosterone. Intern Med. 1992 Aug;31(8):1047-51. doi: 10.2169/internalmedicine.31.1047[
]
- Kaneko H, Umakoshi H, Ishihara Y, Nanba K, Tsuiki M, Kusakabe T, Satoh-Asahara N, Yasoda A, Tagami T. Reassessment of Urinary Aldosterone Measurement After Saline Infusion in Primary Aldosteronism. J Endocr Soc. 2020 Jul 22;4(9):bvaa100. doi: 10.1210/jendso/bvaa100[
]
- Wu CH, Yang YW, Hu YH, Tsai YC, Kuo KL, Lin YH, Hung SC, Wu VC, Wu KD; Taiwan Primary Aldosteronism Investigation (TAIPAI) Study Group. Comparison of 24-h urinary aldosterone level and random urinary aldosterone-to-creatinine ratio in the diagnosis of primary aldosteronism. PLoS One. 2013 Jun 28;8(6):e67417. doi: 10.1371/journal.pone.0067417[
][
]
- Vilela LAP, Almeida MQ. Diagnosis and management of primary aldosteronism. Arch Endocrinol Metab. 2017 May-Jun;61(3):305-312. doi: 10.1590/2359-3997000000274[
][
]
- Vakkalanka S, Zhao A, Samannodi M. Primary hyperaldosteronism: a case of unilateral adrenal hyperplasia with contralateral incidentaloma. BMJ Case Rep. 2016 Jul 14;2016:bcr2016216209. doi: 10.1136/bcr-2016-216209[
][
]
- Turner JM, Dmitriev M. Secondary Hypertension Overview and Workup for the Primary Care Physician. Med Clin North Am. 2023 Jul;107(4):739-747. doi: 10.1016/j.mcna.2023.03.010[
]
- Conn JW, Cohen EL, Rovner DR. Landmark article Oct 19, 1964: Suppression of plasma renin activity in primary aldosteronism. Distinguishing primary from secondary aldosteronism in hypertensive disease. By Jerome W. Conn, Edwin L. Cohen and David R. Rovner. JAMA. 1985 Jan 25;253(4):558-66. doi: 10.1001/jama.253.4.558[
]
- Adler GK, Murray GR, Turcu AF, Nian H, Yu C, Solorzano CC, Manning R, Peng D, Luther JM. Primary Aldosteronism Decreases Insulin Secretion and Increases Insulin Clearance in Humans. Hypertension. 2020 May;75(5):1251-1259. doi: 10.1161/HYPERTENSIONAHA.119.13922[
][
]
- Wrenn SM, Vaidya A, Lubitz CC. Primary aldosteronism. Gland Surg. 2020 Feb;9(1):14-24. doi: 10.21037/gs.2019.10.23[
]
- Reznik Y, Amar L, Tabarin A. SFE/SFHTA/AFCE consensus on primary aldosteronism, part 3: Confirmatory testing. Ann Endocrinol (Paris). 2016 Jul;77(3):202-7. doi: 10.1016/j.ando.2016.01.007[
]
- Wu S, Yang J, Hu J, Song Y, He W, Yang S, Luo R, Li Q. Confirmatory tests for the diagnosis of primary aldosteronism: A systematic review and meta-analysis. Clin Endocrinol (Oxf). 2019 May;90(5):641-648. doi: 10.1111/cen.13943[
]
- Piaditis GP, Kaltsas GA, Androulakis II, Gouli A, Makras P, Papadogias D, Dimitriou K, Ragkou D, Markou A, Vamvakidis K, Zografos G, Chrousos G. High prevalence of autonomous cortisol and aldosterone secretion from adrenal adenomas. Clin Endocrinol (Oxf). 2009 Dec;71(6):772-8. doi: 10.1111/j.1365-2265.2009.03551.x[
]
- Gordon RD. Primary aldosteronism. J Endocrinol Invest. 1995 Jul-Aug;18(7):495-511. doi: 10.1007/BF03349761[
][
]
- Gordon RD, Stowasser M, Klemm SA, Tunny TJ. Primary aldosteronism–some genetic, morphological, and biochemical aspects of subtypes. Steroids. 1995 Jan;60(1):35-41. doi: 10.1016/0039-128x(94)00013-3[
][
]
- Fuller PJ. Adrenal Diagnostics: An Endocrinologist’s Perspective focused on Hyperaldosteronism. Clin Biochem Rev. 2013 Nov;34(3):111-6. https://pmc.ncbi.nlm.nih.gov/articles/PMC3866948[
]
- Schmiemann G, Gebhardt K, Hummers-Pradier E, Egidi G. Prevalence of hyperaldosteronism in primary care patients with resistant hypertension. J Am Board Fam Med. 2012 Jan-Feb;25(1):98-103. doi: 10.3122/jabfm.2012.01.110099[
]
- Gouli A, Kaltsas G, Tzonou A, Markou A, Androulakis II, Ragkou D, Vamvakidis K, Zografos G, Kontogeorgos G, Chrousos GP, Piaditis G. High prevalence of autonomous aldosterone secretion among patients with essential hypertension. Eur J Clin Invest. 2011 Nov;41(11):1227-36. doi: 10.1111/j.1365-2362.2011.02531.x[
]
- Lucatello B, Benso A, Tabaro I, Capello E, Caprino MP, Marafetti L, Rossato D, Oleandri SE, Ghigo E, Maccario M. Long-term re-evaluation of primary aldosteronism after medical treatment reveals high proportion of normal mineralocorticoid secretion. Eur J Endocrinol. 2013 Mar 15;168(4):525-32. doi: 10.1530/EJE-12-0912[
]
- Markou A, Pappa T, Kaltsas G, Gouli A, Mitsakis K, Tsounas P, Prevoli A, Tsiavos V, Papanastasiou L, Zografos G, Chrousos GP, Piaditis GP. Evidence of primary aldosteronism in a predominantly female cohort of normotensive individuals: a very high odds ratio for progression into arterial hypertension. J Clin Endocrinol Metab. 2013 Apr;98(4):1409-16. doi: 10.1210/jc.2012-3353[
]
- Papanastasiou L, Markou A, Pappa T, Gouli A, Tsounas P, Fountoulakis S, Kounadi T, Tsiama V, Dasou A, Gryparis A, Samara C, Zografos G, Kaltsas G, Chrousos G, Piaditis G. Primary aldosteronism in hypertensive patients: clinical implications and target therapy. Eur J Clin Invest. 2014 Aug;44(8):697-706. doi: 10.1111/eci.12286[
]
- Stowasser M, Klemm SA, Tunny TJ, Gordon RD. Plasma aldosterone response to ACTH in subtypes of primary aldosteronism. Clin Exp Pharmacol Physiol. 1995 Jun-Jul;22(6-7):460-2. doi: 10.1111/j.1440-1681.1995.tb02044.x[
]
- Iqbal AM, Jamal SF. Essential Hypertension. [Updated 2023 Jul 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539859[
]
- Chormanski D, Sharma L, Muzio MR. 17-Hydroxylase Deficiency. [Updated 2025 Mar 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK546644[
][
][
][
]
- Akkus G. Two Cases with 17-alpha Hydroxylase Deficiency Misdiagnosed as Primary Aldosteronism. Endocr Metab Immune Disord Drug Targets. 2023;23(11):1449-1454. doi: 10.2174/1871530323666230407125523[
][
][
]
- Schiffrin EL, Chrétien M, Seidah NG, Lis M, Gutkowska J, Cantin M, Genest J. Response of human aldosteronoma cells in culture to the N-terminal glycopeptide of pro-opiomelanocortin and gamma 3-MSH. Horm Metab Res. 1983 Apr;15(4):181-4. doi: 10.1055/s-2007-1018663[
][
][
]
- Sharma L, Momodu II, Singh G. Congenital Adrenal Hyperplasia. [Updated 2025 Jan 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK448098[
]
- Guia Lopes ML, Bello C, Carvalho L, Limbert C, Sequeira Duarte J. A Rare Cause of Cushing’s Syndrome: an Adrenocorticotropic Hormone (ACTH)-Secreting Pheochromocytoma. Cureus. 2023 Apr 20;15(4):e37883. doi: 10.7759/cureus.37883[
][
]
- Birtolo MF, Grossrubatscher EM, Antonini S, Loli P, Mazziotti G, Lania AG, Chiodini I. Preoperative management of patients with ectopic Cushing’s syndrome caused by ACTH-secreting pheochromocytoma: a case series and review of the literature. J Endocrinol Invest. 2023 Oct;46(10):1983-1994. doi: 10.1007/s40618-023-02105-4[
][
]
- Kankoç A, Şatır Türk M, Özkan D, Sayan M, Çelik A. Typical Carcinoid Tumor Cases Causing Ectopic ACTH Syndrome: Dramatic Response to Surgery. Port J Card Thorac Vasc Surg. 2023 Apr 4;30(1):53-56. doi: 10.48729/pjctvs.258[
][
]
- Golounina OO, Belaya ZE, Rozhinskaya LY, Pikunov MY, Markovich AA, Dzeranova LK, Marova EI, Kuznetsov NS, Fadeev VV, Melnichenko GA, Dedov II. [Survival predictors in patients with ectopic acth syndrome]. Probl Endokrinol (Mosk). 2022 Aug 15;68(6):30-42. Russian. doi: 10.14341/probl13144[
][
]
- Sabbadin C, Armanini D. Syndromes that Mimic an Excess of Mineralocorticoids. High Blood Press Cardiovasc Prev. 2016 Sep;23(3):231-5. doi: 10.1007/s40292-016-0160-5[
]
- Bangert K, Kluger MA, Kluge S, Janneck M. Life-Threatening Complications of Excessive Licorice Consumption. Dtsch Arztebl Int. 2021 Dec 27;118(51-52):890-891. doi: 10.3238/arztebl.m2021.0390[
]
- Liddle Syndrome. https://rarediseases.org/rare-diseases/liddle-syndrome[
][
]
- Mubarik A, Anastasopoulou C, Aeddula NR. Liddle Syndrome (Pseudohyperaldosteronism) [Updated 2024 Mar 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK536911[
][
]
- Kino T, Nicolaides NC, Charmandari E, et al. Primary Generalized Glucocorticoid Resistance Syndrome. [Updated 2024 May 19]. In: Feingold KR, Ahmed SF, Anawalt B, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK278930[
][
]
- Huang H, Wang W. Molecular mechanisms of glucocorticoid resistance. Eur J Clin Invest. 2023 Feb;53(2):e13901. doi: 10.1111/eci.13901[
][
]
- Nicolaides NC, Charmandari E. Primary Generalized Glucocorticoid Resistance and Hypersensitivity Syndromes: A 2021 Update. Int J Mol Sci. 2021 Oct 7;22(19):10839. doi: 10.3390/ijms221910839[
][
]
- Apparent mineralocorticoid excess syndromes (including chronic licorice ingestion). https://www.uptodate.com/contents/apparent-mineralocorticoid-excess-syndromes-including-chronic-licorice-ingestion[
][
]
- Lu YT, Zhang D, Zhang QY, Zhou ZM, Yang KQ, Zhou XL, Peng F. Apparent mineralocorticoid excess: comprehensive overview of molecular genetics. J Transl Med. 2022 Nov 3;20(1):500. doi: 10.1186/s12967-022-03698-9[
][
][
][
]
- Al-Harbi T, Al-Shaikh A. Apparent mineralocorticoid excess syndrome: report of one family with three affected children. J Pediatr Endocrinol Metab. 2012;25(11-12):1083-8. doi: 10.1515/jpem-2012-0113[
][
][
]
- Milsom SR, Espiner EA, Nicholls MG, Gwynne J, Perry EG. The blood pressure response to unilateral adrenalectomy in primary aldosteronism. Q. J. Med. 1986 Dec;61(236):1141-51.[
]
- Parthasarathy HK, Ménard J, White WB, Young WF Jr, Williams GH, Williams B, Ruilope LM, McInnes GT, Connell JM, MacDonald TM. A double-blind, randomized study comparing the antihypertensive effect of eplerenone and spironolactone in patients with hypertension and evidence of primary aldosteronism. J Hypertens. 2011 May;29(5):980-90. doi: 10.1097/HJH.0b013e3283455ca5[
][
]
- Aronova A, Fahey TJ III, Zarnegar R. Management of hypertension in primary aldosteronism. World J Cardiol. 2014 May 26;6(5):227-33. doi: 10.4330/wjc.v6.i5.227[
]
- Bruedgam D, Adolf C, Schneider H, Schwarzlmueller P, Mueller L, Handgriff L, Bidlingmaier M, Kunz S, Zimmermann P, Deniz S, Williams TA, Beuschlein F, Reincke M, Heinrich DA. Postoperative ACTH-stimulated aldosterone predicts biochemical outcome in primary aldosteronism. Eur J Endocrinol. 2023 Dec 6;189(6):611-618. doi: 10.1093/ejendo/lvad159[
]
- Chen ZW, Liao CW, Pan CT, Tsai CH, Chang YY, Chang CC, Lee BC, Chiu YW, Huang WC, Lai TS, Lu CC, Chueh JS, Wu VC, Hung CS, Lin YH; TAIPAI Study Group. Reversal of arterial stiffness in medically and surgically treated unilateral primary aldosteronism. J Hypertens. 2024 Mar 1;42(3):538-545. doi: 10.1097/HJH.0000000000003631[
]
- Rossi GP, Rossitto G, Amar L, et al. Clinical Outcomes of 1625 Patients With Primary Aldosteronism Subtyped With Adrenal Vein Sampling. Hypertension. 2019 Oct;74(4):800-808. doi: 10.1161/HYPERTENSIONAHA.119.13463[
]
- Veselý J. Spironolakton v léčbě hypertenze: opomíjená molekula [Spironolactone in the treatment of hypertension: a neglected molecule]. Vnitr Lek. 2018 Fall;64(7-8):815-820. Czech.[
]
- Rossi GP, Mulatero P, Satoh F. 10 good reasons why adrenal vein sampling is the preferred method for referring primary aldosteronism patients for adrenalectomy. J Hypertens. 2019 Mar;37(3):603-611. doi: 10.1097/HJH.0000000000001939[
]
- Williams TA, Reincke M. MANAGEMENT OF ENDOCRINE DISEASE: Diagnosis and management of primary aldosteronism: the Endocrine Society guideline 2016 revisited. Eur J Endocrinol. 2018 Jul;179(1):R19-R29. doi: 10.1530/EJE-17-0990[
][
][
]
- Patibandla S, Heaton J, Kyaw H. Spironolactone. [Updated 2023 Jul 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554421[
][
]
- Hughes JC, Cassagnol M. Eplerenone. [Updated 2023 May 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK553100[
][
]
- Yang S, Wang G, Li N, Zhu Q. The outcomes of transcatheter adrenal ablation in patients with primary aldosteronism: a systematic review and meta-analysis. BMC Endocr Disord. 2023 May 8;23(1):103. doi: 10.1186/s12902-023-01356-9[
][
]
- Sacks BA, Sacks AC, Faintuch S. Radiofrequency ablation treatment for aldosterone-producing adenomas. Curr Opin Endocrinol Diabetes Obes. 2017 Jun;24(3):169-173. doi: 10.1097/MED.0000000000000329[
][
]
- Yang R, Xu L, Lian H, Gan W, Guo H. Retroperitoneoscopic-guided cool-tip radiofrequency ablation of adrenocortical aldosteronoma. J Endourol. 2014 Oct;28(10):1208-14. doi: 10.1089/end.2013.0635[
][
]
- Bouhanick B, Delchier MC, Lagarde S, Boulestreau R, Conil C, Gosse P, Rousseau H, Lepage B, Olivier P, Papadopoulos P, Trillaud H, Cremer A; for the ADERADHTA group. Radiofrequency ablation for adenoma in patients with primary aldosteronism and hypertension: ADERADHTA, a pilot study. J Hypertens. 2021 Apr 1;39(4):759-765. doi: 10.1097/HJH.0000000000002708[
][
]
- Oguro S, Ota H, Yanagaki S, Kawabata M, Kamada H, Omata K, Tezuka Y, Ono Y, Morimoto R, Satoh F, Toyama H, Tanimoto K, Konno D, Yamauchi M, Niwa Y, Miyamoto H, Mori K, Tanaka T, Ishihata H, Takase K. Transvenous Radiofrequency Catheter Ablation for an Aldosterone-Producing Tumor of the Left Adrenal Gland: A First in Human Case Report. Cardiovasc Intervent Radiol. 2023 Dec;46(12):1666-1673. doi: 10.1007/s00270-023-03584-x[
][
]
- Oguro S, Morimoto R, Seiji K, Ota H, Kinoshita T, Kawabata M, Ono Y, Omata K, Tezuka Y, Satoh F, Ito S, Moriya N, Matsui S, Nishikawa T, Omura M, Nakai K, Nakatsuka S, Kurihara I, Miyashita K, Koda W, Minami T, Takeda Y, Kometani M, Oki Y, Oishi T, Ushio T, Goshima S, Takase K. Safety and feasibility of radiofrequency ablation using bipolar electrodes for aldosterone-producing adenoma: a multicentric prospective clinical study. Sci Rep. 2022 Aug 18;12(1):14090. doi: 10.1038/s41598-022-18136-5[
][
]
- Xiang H, Zhang T, Song W, Yang D, Zhu X. Adrenalectomy for primary aldosteronism and its related surgical characteristics. Front Endocrinol (Lausanne). 2024 Jun 20;15:1416287. doi: 10.3389/fendo.2024.1416287[
]
- Samnani S, Cenzer I, Kline GA, Lee SJ, Hundemer GL, McClurg C, Pasieka JL, Boscardin WJ, Ronksley PE, Leung AA. Time to Benefit of Surgery vs Targeted Medical Therapy for Patients With Primary Aldosteronism: A Meta-analysis. J Clin Endocrinol Metab. 2024 Feb 20;109(3):e1280-e1289. doi: 10.1210/clinem/dgad654[
]
- Mao M, Feng R, Khan NA, Tao L, Tang P, Zhao Y, Chen J, Li X, Zhao H, Shi Q, Wang L, Lyu F, Asghar MA, He Y, Chang J, Xiang R. Safety and efficacy of bilateral superselective adrenal arterial embolization for treatment of idiopathic hyperaldosteronism: a prospective single-center study. BMC Surg. 2024 Aug 24;24(1):242. doi: 10.1186/s12893-024-02530-z[
][
][
]
- Dong H, Zou Y, He J, Deng Y, Chen Y, Song L, Xu B, Gao R, Jiang X. Superselective adrenal arterial embolization for idiopathic hyperaldosteronism: 12-month results from a proof-of-principle trial. Catheter Cardiovasc Interv. 2021 May 1;97 Suppl 2:976-981. doi: 10.1002/ccd.29554[
][
][
]
- Zhou Y, Liu Q, Wang X, Wan J, Liu S, Luo T, He P, Hou J, Pu J, Wang D, Liang D, Yang Y, Wang P. Adrenal Ablation Versus Mineralocorticoid Receptor Antagonism for the Treatment of Primary Aldosteronism: A Single-Center Prospective Cohort Study. Am J Hypertens. 2022 Dec 8;35(12):1014-1023. doi: 10.1093/ajh/hpac105[
][
][
]
- Nakamura T, Hayashi K. Accumulating evidence suggests the potential of selective adrenal artery embolization as a standard treatment for primary aldosteronism. Hypertens Res. 2024 Jun;47(6):1744-1746. doi: 10.1038/s41440-024-01656-0[
][
][
]
- Yokota K. Adrenal arterial embolization: a possible new treatment for patients with primary aldosteronism. Hypertens Res. 2024 Feb;47(2):358-360. doi: 10.1038/s41440-023-01456-y[
][
][
]
- Zhou Y, Wang X, Hou J, Wan J, Yang Y, Liu S, Luo T, Liu Q, Xue Q, Wang P. A controlled trial of percutaneous adrenal arterial embolization for hypertension in patients with idiopathic hyperaldosteronism. Hypertens Res. 2024 Feb;47(2):311-321. doi: 10.1038/s41440-023-01420-w[
][
][
]
- Mai X, Kometani M, Yoneda T. Spontaneous Remission of Primary Aldosteronism with Mineralocorticoid Receptor Antagonist Therapy: A Review. Int J Mol Sci. 2022 Nov 10;23(22):13821. doi: 10.3390/ijms232213821[
]
- Matlaga BR, Shah OD, Assimos DG. Drug-induced urinary calculi. Rev Urol. 2003 Fall;5(4):227-31. https://pmc.ncbi.nlm.nih.gov/articles/PMC1508366[
]
- Daudon M, Jungers P. Drug-induced renal calculi: epidemiology, prevention and management. Drugs. 2004;64(3):245-75. doi: 10.2165/00003495-200464030-00003[
]
- Carr MC, Prien EL Jr, Babayan RK. Triamterene nephrolithiasis: renewed attention is warranted. J Urol. 1990 Dec;144(6):1339-40. doi: 10.1016/s0022-5347(17)39734-3[
]
- Dooley DP, Callsen ME, Geiling JA. Triamterene nephrolithiasis. Mil Med. 1989 Mar;154(3):126-7.[
]
- Niyazov R, Sharman T. Triamterene. [Updated 2023 May 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557650[
]
- Armanini D, Sabbadin C, Donà G, Clari G, Bordin L. Aldosterone receptor blockers spironolactone and canrenone: two multivalent drugs. Expert Opin Pharmacother. 2014 May;15(7):909-12. doi: 10.1517/14656566.2014.896901[
]
- Cerny MA. Progress towards clinically useful aldosterone synthase inhibitors. Curr Top Med Chem. 2013;13(12):1385-401. doi: 10.2174/1568026611313120003[
]
- Bernhardt R. The potential of targeting CYP11B. Expert Opin Ther Targets. 2016 Aug;20(8):923-34. doi: 10.1517/14728222.2016.1151873[
]
- Liu W, Li Z, Chu S, Ma X, Wang X, Jiang M, Bai G. Atractylenolide-I covalently binds to CYP11B2, selectively inhibits aldosterone synthesis, and improves hyperaldosteronism. Acta Pharm Sin B. 2022 Jan;12(1):135-148. doi: 10.1016/j.apsb.2021.09.013[
]
- Funder JW. Primary Aldosteronism: The Next Five Years. Horm Metab Res. 2017 Dec;49(12):977-983. doi: 10.1055/s-0043-119802[
]
- Kline GA, Prebtani APH, Leung AA, Schiffrin EL. Primary aldosteronism: a common cause of resistant hypertension. CMAJ. 2017 Jun 5;189(22):E773-E778. doi: 10.1503/cmaj.161486[
]
- Marín-Martínez L, Ríos-Vergara AJ, Kyriakos G, Álvarez-Martín MC, Hernández-Alonso E. Bilateral Adrenalectomy in a Patient With Refractory Primary Aldosteronism Due to Adrenal Hyperplasia. Cureus. 2022 Apr 19;14(4):e24267. doi: 10.7759/cureus.24267[
]
- Zhou L, Jiang Y, Zhang C, Su T, Jiang L, Zhou W, Zhong X, Wu L, Wang W. Effects of a low-sodium diet in patients with idiopathic hyperaldosteronism: a randomized controlled trial. Front Endocrinol (Lausanne). 2023 Apr 19;14:1124479. doi: 10.3389/fendo.2023.1124479[
]
- Bokhari MR, Bokhari SRA. Renal Artery Stenosis. [Updated 2023 Jul 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430718[
]
- Bernini G, Galetta F, Franzoni F, Bardini M, Taurino C, Bernardini M, Ghiadoni L, Bernini M, Santoro G, Salvetti A. Arterial stiffness, intima-media thickness and carotid artery fibrosis in patients with primary aldosteronism. J Hypertens. 2008 Dec;26(12):2399-405. doi: 10.1097/HJH.0b013e32831286fd[
]
- Moustaki M, Paschou SA, Vakali EC, Vryonidou A. Secondary diabetes mellitus due to primary aldosteronism. Endocrine. 2023 Jan;79(1):17-30. doi: 10.1007/s12020-022-03168-8[
]
- Luther JM. Effects of aldosterone on insulin sensitivity and secretion. Steroids. 2014 Dec;91:54-60. doi: 10.1016/j.steroids.2014.08.016[
]
- Miyake Y, Tanaka K, Nishikawa T, Naruse M, Takayanagi R, Sasano H, Takeda Y, Shibata H, Sone M, Satoh F, Yamada M, Ueshiba H, Katabami T, Iwasaki Y, Tanaka H, Tanahashi Y, Suzuki S, Hasegawa T, Katsumata N, Tajima T, Yanase T. Prognosis of primary aldosteronism in Japan: results from a nationwide epidemiological study. Endocr J. 2014;61(1):35-40. doi: 10.1507/endocrj.ej13-0353[
]