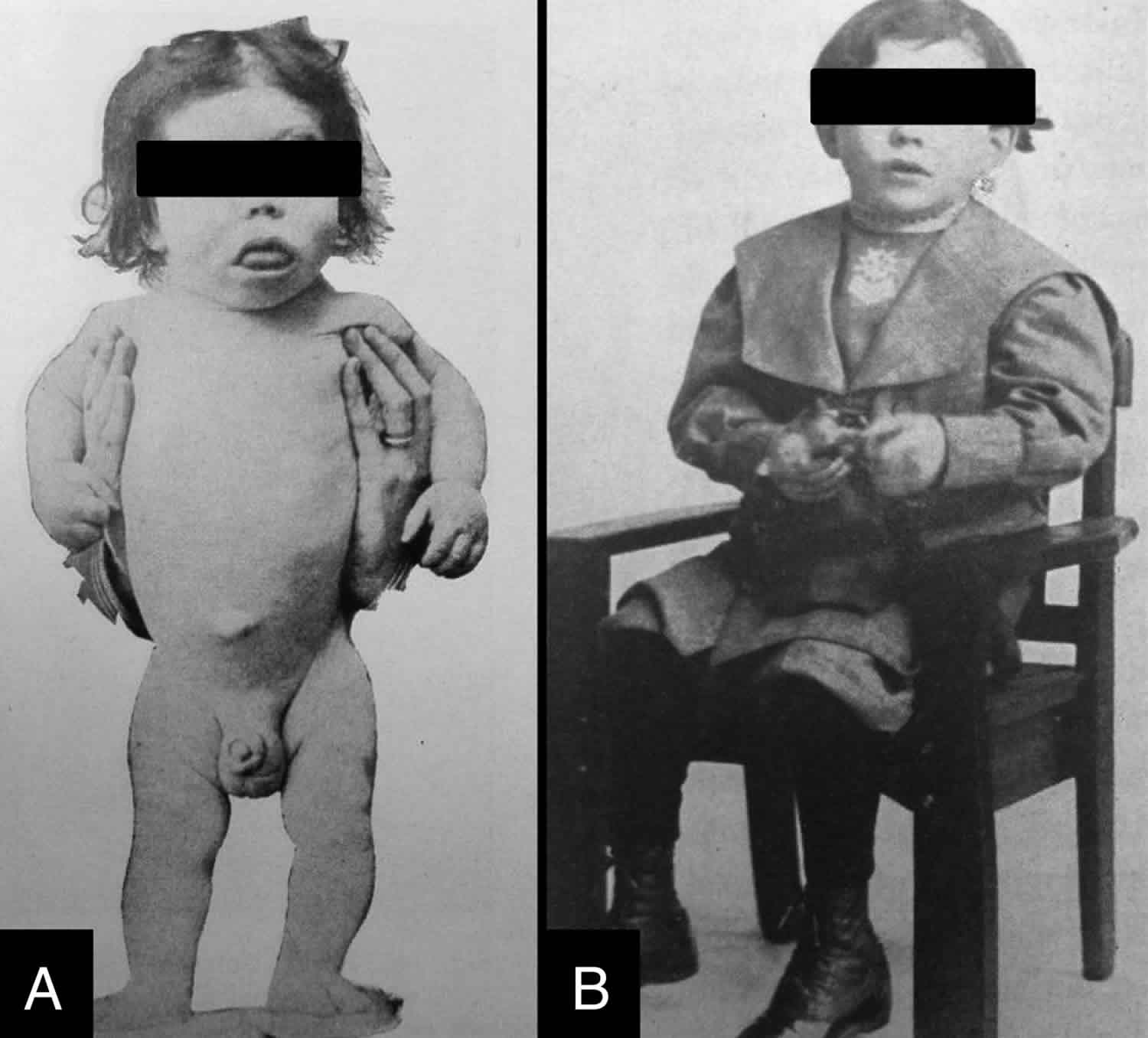
Cretinism
Cretinism is an irreversible brain damage, irreversible mental retardation and severely stunted physical growth in an infant or child due to severe iodine deficiency during pregnancy caused by inadequate thyroid hormone production (congenital hypothyroidism) 1. Cretinism is due to dietary iodine deficiency and can be prevented by correction of iodine deficiency before pregnancy. Cretinism is the most serious iodine deficiency disorder and occurs when a pregnant women is severely iodine deficient. The medical definition of cretinism involves three features: (1) an association with endemic goiter (i.e. prevalence of goiter > 5 %) and severe iodine deficiency; (2) clinical symptoms which includes some form of mental deficiency and/or defects in hearing, speech, stance, gait, hypothyroidism, and stunted growth; and (3) when iodine deficiency is corrected in the area cretinism is no longer observed 2.
Iodine deficiency disorders, which can start before birth, jeopardize children’s mental health and often their very survival. Serious iodine deficiency during pregnancy can result in stillbirth, spontaneous abortion, and congenital abnormalities such as cretinism, a grave, irreversible form of mental retardation that affects people living in iodine-deficient areas of Africa and Asia. However, of far greater significance is iodine deficiency disorder’s less visible, yet pervasive, mental impairment that reduces intellectual capacity at home, in school and at work.
Thyroid hormones are essential for normal development and growth of many target tissues, including the brain and the skeleton. Thyroid hormone action on critical genes for neurodevelopment is limited to a specific time window, and even a short period of deficiency of thyroid hormone can cause irreversible brain damage 3. During the first trimester of pregnancy fetal brain development is totally dependent on maternal thyroid function.
Iodine deficiency is now recognized by the World Health Organization (WHO) as the most common preventable cause of brain damage with in excess of 2 billion at risk from 130 countries. In 1990 it was estimated that among the 1572 million people in the world exposed to iodine deficiency (28.9 % of the then world population), 11.2 million were affected by overt cretinism, the most extreme form of mental retardation due to iodine deficiency and that another 43 million people were affected by some degree of itellectual impairment 4. Thus, iodine deficiency was a leading global cause of preventable mental impairment.
Prenatal iodine deficiency, and the resulting fetal hypothyroidism that caused cretinism, vanished from the United States and Europe in the wake of iodization campaigns 5. In the United States, iodine status has remained generally adequate in since the 1940s although studies have shown that urinary iodine levels dropped by about half between the early 1970s and the early 1990s, and most recently mild iodine deficiency has re-emerged in pregnant women. Iodine deficiency remains a major issue in other parts of the world, including parts of Europe, Africa and Asia 6. A global United Nations (UN) programme of prevention has achieved 68% household usage of iodised salt by the year 2000 compared with less than 20% prior to 1990.
Congenital hypothyroidism can be endemic, genetic, or sporadic. Endemic cretinism was especially common in areas of southern Europe around the Alps, Bangladesh, China, and Nepal. Sporadic and genetic cretinism results from abnormal development, or function of the fetal thyroid gland 7. Endemic cretinism is now included in the spectrum of the effects of iodine deficiency in a population termed the ‘iodine deficiency disorders’, which also includes a wide range of lesser degrees of cognitive defect that can be prevented by the correction of iodine deficiency. Iodine deficiency is now recognized by the World Health Organization (WHO) as the most common preventable cause of brain damage with in excess of 2 billion at risk from 130 countries. A global United Nations (UN) programme of prevention has achieved 68% household usage of iodised salt by the year 2000 compared with less than 20% prior to 1990.
Cretinism has been almost completely eliminated in developed countries by early diagnosis by newborn screening schemes and iodine supplementation programs.
In 2001, the World Health Organization (WHO), United Nations Children’s Fund (UNICEF) and International Council for Control of Iodine Deficiency Disorders (ICCIDD) developed a system for classifying iodine deficiency based upon the median urinary iodine concentration in a population 8.
Table 1. Iodine Deficiency Classification
| Iodine Deficiency | None | Mild | Moderate | Severe |
| Median urine iodine, mcg/L | >100 | 50-99 | 20-49 | < 20 |
| Goiter prevalence | < 5% | 5-20% | 20-30% | >30% |
| Neonatal thyroid-stimulating hormone (TSH), >5 IU/mL whole blood | < 3% | 3-20% | 20-40% | >40% |
| Cretinism | 0 | 0 | + | + |
Types of cretinism
There are two main types of cretinism 9. Neurological cretinism is characterized by mental retardation, deaf mutism, squint, spastic diplegia, and disorders of stance and gait. Myxoedematous or hypothyroid cretinism is less common and characterized by mental retardation (although less severe than in neurological cretinism), dwarfism, and hypothyroidism with associated physical symptoms (e.g., coarse and dry skin, husky voice, delayed sexual maturation). Some countries and regions have a higher prevalence of one type of cretinism than the other, and sometimes the symptoms of both types of cretinism can manifest in the same individual. A study of 112 cretins (neurological, myxoedematous, and mixed) living in Thailand reported that their mean IQ score was 30.8 ± 8.8 10. In addition to the presence of cretins in a community, Chen and Hetzel 9 state that mild mental retardation (IQ 50–69) is found in 5–15 % of children living in areas of endemic cretinism; these children are sometimes referred to as ‘sub-cretins’.
Both types of cretinism are due to dietary iodine deficiency and can be prevented by correction of iodine deficiency before pregnancy.
Cretinism causes
Cretinism in newborn is due to severe prenatal iodine deficiency during pregnancy caused by inadequate thyroid hormone production (congenital hypothyroidism or fetal hypothyroidism). Congenital hypothyroidism can be endemic, genetic, or sporadic. Endemic cretinism is a developmental disorder that occurs in regions of severe endemic goiter. Both parents of an endemic cretin are usually goitrous, and in addition to the features of sporadic cretinism described earlier, endemic cretins often have deaf-mutism, spasticity, motor dysfunction, and abnormalities in the basal ganglia demonstrable by magnetic resonance imaging. Endemic cretinism was especially common in areas of southern Europe around the Alps, Bangladesh, China, and Nepal. Sporadic and genetic cretinism results from abnormal development, or function of the fetal thyroid gland 7.
Other factors such as the presence of goitrogens in the diet, thyroid immunity, and interactions with other trace elements such as selenium have also been postulated to have a role in the development of cretinism 11. Nonetheless, the lack of iodine in the diets of pregnant women in the first trimester appears to be a common factor in both forms of cretinism, suggesting that maternal hypothyroidism is responsible for irreversible damage to the foetal brain. In the landmark trial of 165,000 people living in an area of Papua New Guinea with severe iodine deficiency and endemic cretinism 12, families were allocated to iodine (iodised oil) or placebo (saline) and subsequent follow-up studies of the original cohort found that an injection of iodised oil before conception or in early pregnancy reduced the incidence of cretinism and improved the motor and cognitive functions of children compared with placebo treatment. Another study was undertaken in 1990 in a remote province in China with endemic cretinism 13. The effect of iodised oil given during pregnancy and to children up to 2 years of age on neurological outcomes was investigated by comparing treated children with untreated children at two years 13, and again when treated children were school-aged 14. Children of mothers given iodine earlier in pregnancy had improved cognitive outcomes compared to mothers given iodine later in pregnancy and to children treated after birth.
Iodine requirements in pregnancy
Iodine turnover, thyroidal radioiodine uptake, and balance studies suggest that the average daily requirement for iodine in nonpregnant women is 91–96 μg/d 15. The US Estimated Average Requirement (EAR) for iodine for nonpregnant, nonlactating women aged ≥14 years is 95 μg/day, and the Recommended Dietary Allowance (RDA) is 150 μg/day 15. This agrees with the World Health Organization (WHO), United Nations Children’s Fund (UNICEF), and the International Council for the Control of Iodine Deficiency Disorders (ICCIDD) Recommended Nutrient Intake for iodine of 150 μg/d for nonpregnant women 16. The iodine requirement during pregnancy 17 is sharply elevated 1) because of an increase by ≈50% in maternal thyroxine (T4) production to maintain maternal euthyroidism and to transfer thyroid hormone to the fetus; 2) because iodine needs to be transferred to the fetus for fetal thyroid hormone production, particularly in later gestation; and 3) because of a probable increase in renal iodine clearance. The US EAR is 160 μg/day for pregnancy in women aged ≥14 years, and the Recommended Dietary Allowance, set at 140% of the EAR rounded to the nearest 10 μg, is 220 μg/d 15. Recently, the WHO/UNICEF/ICCIDD increased the Recommended Nutrient Intake for iodine during pregnancy from 200 to 250 μg/day 18, but emphasized the need for more data on the level of iodine intake [and the corresponding urinary iodine (UI) concentration] that ensures maternal and newborn euthyroidism.
Cretinism prevention
For nearly all countries, the primary strategy for sustainable elimination of iodine deficiency in pregnancy remains universal salt iodization 19. However, implementation of universal salt iodization is not always feasible, which may result in insufficient access to iodized salt for women of childbearing age and pregnant women. Iodine supplementation of these groups should be considered. WHO/UNICEF/ICCIDD recommends that countries assess their salt iodization programs and then decide whether supplementation is indicated 19. Highly populated countries should use disaggregated data and categorize areas of the country according to subnational (region, province, district, etc) data. To ensure an adequate iodine supply during pregnancy, women should ideally be provided with an ample iodine intake (≥150 μg/day) for a long period of time before conception to ensure plentiful intrathyroidal iodine stores. An adequate iodine supply should continue after parturition, because the iodine requirement of a women who is fully breastfeeding her infant is likely even higher than that during pregnancy. In countries or areas where <90% of households are using iodized salt and the median urinary iodine concentration in schoolchildren is <100 μg/L, the recommendations for iodine supplementation in pregnancy and infancy are shown in Table 2.
Table 2. Recommendations for iodine supplementation in pregnancy and infancy in areas where <90% of the households are using iodized salt and the median urinary iodine concentration in schoolchildren is <100 μg/L.
| Women of childbearing age | Single annual oral dose of 400 mg I as iodized oil |
| or | |
| Daily oral dose of iodine as potassium iodide to meet the Recommended Nutrient Intake of 150 μg I/d | |
| Pregnant or lactating women | Single annual oral dose of 400 mg I as iodized oil |
| or | |
| Daily oral dose of iodine as potassium iodide to meet the new Recommended Nutrient Intake of 250 μg I/d | |
| Iodine supplements should not be given to women who already received iodized oil during current pregnancy or up to 3 mo before current pregnancy started |
Cretinism symptoms
Cretinism may be of two types: neurological or myxoedematous.
Neurological cretinism
The three characteristic features of neurological endemic cretinism in its fully developed form are extremely severe mental deficiency together with squint, deaf mutism, spastic diplegia and disorders of stance and gait. They usually have a goiter. The neuropathological basis of the clinical picture includes under-development of the cochlea for deafness, maldevelopment of the cerebral neocortex for mental retardation, and maldevelopment of the corpus striatum (especially putamen and globus pallidus) for the motor disorder. The cerebellum, hypothalamus, visual system, and hippocampus are relatively spared. Neurological cretinism is now thought to be predominantly caused by maternal hypothyroidism due to iodine deficiency. It may have an autosomal recessive predisposition also.
Myxedematatous cretinism
Myxedematatous cretinism or hypothyroid cretinism may present with mental retardation (although less severe than in neurological cretinism), severe growth retardation (dwarfism), incomplete maturation of the facial features, including the naso-orbital configuration, atrophy of the mandibles, puffy features (myxedema) and hypothyroidism with associated physical symptoms (e.g., thickened and dry skin, dry and decreased hair, eyelashes and eyebrows, husky voice and much delayed sexual maturation). Goiter is usually absent and the thyroid is often not palpable, suggesting thyroid atrophy. Thyroidal uptake of radioiodine is much lower than in the general population. The serum levels of T4 and T3 are extremely low, often undetectable, and TSH is dramatically high. Markedly enlarged sella turcicae have been demonstrated.
Some countries and regions have a higher prevalence of one type of cretinism than the other, and sometimes the symptoms of both types of cretinism can manifest in the same individual.
Cretinism diagnosis
Maternal urinary iodine concentration
The median urinary iodine concentration is recommended by the WHO 17 for assessing iodine intake in populations of nonpregnant and pregnant women. Daily iodine intake can be extrapolated from the urinary iodine concentration assuming 24-hour urine volumes and iodine bioavailability of 92% 15; the recommended daily iodine intake during pregnancy of 220–250 μg 16 would correspond to a median urinary iodine concentration of 135–155 μg/L during pregnancy. Pregnancy may occur in adolescence, particularly in developing countries; in a 15 y-old girl weighing ≈50 kg, a daily iodine intake of 200–250 μg would correspond to a urinary iodine concentration of ≈200 μg/L. However, during pregnancy this extrapolation of iodine intake from the urinary iodine concentration may be less valid because of an increase in renal iodine clearance 17. If renal iodine clearance increases in pregnancy, the daily iodine intake extrapolated from the urinary iodine concentration in pregnancy would be lower than that in nonpregnancy. More reference data on urinary iodine concentrations in chronically iodine-sufficient pregnant women, including trimester-specific values, would be valuable. The WHO currently recommends that a median urinary iodine concentration in a population of pregnant women of 150–249 μg/L indicates adequate iodine intake (Table 3). However, this population indicator should not be used for the purposes of individual diagnosis and treatment.
Table 1. Epidemiologic criteria for assessing iodine nutrition in a population of pregnant women based on median urinary iodine concentrations
| Median urinary iodine | Iodine intake |
| <150 μg/L | Insufficient |
| 150–249 μg/L | Adequate |
| 250–499 μg/L | More than adequate |
| ≥500 μg/L | Excessive |
Neonatal screening
The aim of neonatal screening is the earliest identification of any form of congenital hypothyroidism, but particularly those patients with severe hypothyroidism in whom disability is greatest if not treated. The identification of central congenital hypothyroidism by screening programs is under debate. Two screening strategies for the detection of congenital hypothyroidism have evolved. In the primary T4/backup TSH method, still favored in much of North America and the Netherlands, T4 is measured initially while TSH is checked on the same blood spot in those specimens in which the T4 concentration is low. In the primary TSH approach, favored in most parts of Europe and Japan, blood TSH is measured initially.
A primary T4/backup TSH program will detect overt primary hypothyroidism, secondary or tertiary hypothyroidism, babies with a low serum T4 level but delayed rise in the TSH concentration, TBG deficiency and hypothyroxinemia; this approach may, however, miss subclinical hypothyroidism. A primary TSH strategy, on the other hand, will detect both overt and subclinical hypothyroidism, but will miss secondary or tertiary hypothyroidism, a delayed TSH rise, TBG deficiency and hypothyroxinemia. There are fewer false positives with a primary TSH strategy. Both programs will miss the rare infant whose T4 level on initial screening is normal but who later develops low T4 and elevated TSH concentrations. This pattern has been termed “atypical” congenital hypothyroidism or “delayed TSH” and is observed most commonly in premature babies with transient hypothyroidism or infants with less severe forms of permanent disease.
According to the European Society for Pediatric Endocrinology (ESPE) guidelines, the most sensitive test for detecting primary congenital hypothyroidism is the determination of TSH concentration that detects primary congenital hypothyroidism more effectively than primary T4 screening Primary T4 screening with confirmatory TSH testing can detect some cases of central congenital hypothyroidism, but some cases of mild congenital hypothyroidism can be missed, depending on the cutoff T4 value used.
Measurement of T4 and/or TSH is performed on an eluate of dried whole blood (DBS) collected on filter paper by skin puncture on day 1-4 of life. Primary congenital hypothyroidism screening has been shown to be effective for the testing of cord blood or the blood collected on filter paper after the age of 24 hours. Blood is applied directly to the filter paper and after drying the card is sent to the laboratory. The best time to collect blood for TSH screening is 48 to 72 hours of age. The practice of early discharge from the hospital of otherwise healthy full-term infants has resulted in a greater proportion of babies being tested before this time. For example, it has been estimated that in North America 25% or more of newborns are now discharged within 24 hours of delivery and 40% in the second 24 hours of life. Because of the neonatal TSH surge and the dynamic changes in serum T4 and T3 concentrations that occur within the first few days of life, early discharge increases the number of false positive results. It is important that in the screening laboratory the results of TSH are interpreted in relation to time of sampling.
Physicians caring for infants need to appreciate that there is always the possibility for human error in failing to identify affected infants, whichever screening program is utilized. This can occur due to poor communication, lack of receipt of requested specimens, or the failure to test an infant who is transferred between hospitals during the neonatal period. Therefore, if the diagnosis of hypothyroidism is suspected clinically, the infant should always be tested. Adult normative values, provided by many general hospital laboratories, differ from those in the newborn period and should never be employed.
Special categories of neonates with congenital hypothyroidism can be missed at screening performed at the usual time, particularly preterm babies and neonates with serious illnesses and multiple births. Drugs used in neonatal intensive care (i.e., dopamine, glucocorticoids that suppresses TSH), immaturity of hypothalamic-pituitary thyroid axis, decreased hepatic production of thyroid binding globulin, reduced transfer of maternal T4, reduced intake of iodine or excess iodine exposure, fetal blood mixing in multiple births can affect the first sample, and in many centers a second specimen is required to rule out congenital hypothyroidism. Preterm babies have a higher incidence of a unique form of hypothyroidism, characterized by a delayed elevation of TSH. These babies can later develop low T4 and elevated TSH concentrations. This pattern has been termed “atypical” congenital hypothyroidism or “delayed TSH”. Preterm babies with a birth weight of less than 1500 gr. have an incidence of congenital hypothyroidism of 1:300. Survival of even extremely premature babies (<28 weeks of gestation) is around 90% in developed countries, and the incidence of prematurity is around 11.5% in US and 11.8% worldwide. So, an increasing subpopulation of preterm babies and high-risk newborns deserves a special screening and follow up for congenital hypothyroidism.
In these categories a second specimen 2-6 weeks from the first (European Society for Paediatric Endocrinology guidelines suggested at about 15 days, or after 15 days from the first) may be indicated in a) preterm neonates with a gestational age of less than 37 weeks, b) Low Birth Weight and Very Low Birth Weight neonates, c) ill and preterm neonates admitted to neonatal intensive care unit, d) if specimen collection was within the first 24 hours of life, and e) multiple births, particularly in the case of same sex twins. The interpretation of the screening results should consider the results of a multiple sampling strategy, the age of sampling, and the maturity (GA/birth weight) of the neonate. A second screen (using a lower TSH cutoff) is able to detect the delayed elevation of TSH that occurs in these babies.
Congenital hypothyroidism is defined on the basis of serum FT4 levels as severe when FT4 is <5 pmol/l, moderate when FT4 is 5 to 10 pmol/l, and mild when FT4 is 10 to 15 pmol/l, respectively. Determination of serum thyroglobulin (Tg) is useful, if below the detection threshold, to suggest athyreosis or a complete thyroglobulin synthesis defect. Measurement of thyroglobulin is most helpful when a defect in thyroglobulin synthesis or secretion is being considered. In the latter condition the serum thyroglobulin concentration is low or undetectable despite the presence of a normal or enlarged, eutopic thyroid gland. Serum thyroglobulin concentration also reflects the amount of thyroid tissue present and the degree of stimulation. For example, thyroglobulin is undetectable in most patients with thyroid agenesis, intermediate in babies with an ectopic thyroid gland, and may be elevated in patients with abnormalities of thyroid hormonogenesis not involving thyroglobulin synthesis and secretion. Considerable overlap exists, and so, the thyroglobulin value needs to be considered in association with the findings on imaging. In patients with inactivating mutations of the TSH receptor discordance between findings on thyroid imaging and the serum thyroglobulin concentration has been described in some but not all studies.
Imaging studies
Imaging studies are helpful to determine the specific etiology of congenital hypothyroidism. Both scintigraphy and ultrasound (US) should be considered in neonates with high TSH concentrations. Ideally, the association of US and scintigraphy gives the best information in a child with primary hypothyroidism. Scintigraphy shows the presence/absence (athyreosis), position (ectopic gland, in any point from the foramen caecum at the base of the tongue to the anterior mediastinum) and rough anatomic structure of the thyroid gland. US, is a useful tool in defining size and morphology of a eutopic thyroid gland, however, US alone is less effective in detecting ectopic glands. Color Doppler US improves the effectiveness of US. It is important to remember that an attempt to obtain imaging in a newborn should never delay the initiation of treatment. Scintigraphy should be carried out within 7 days of starting L-thyroxine (L-T4) treatment. Scintigraphy may be carried out with either 10-20 MBq of technetium 99m ( 99mTc) or 1-2 MBq of iodine123 (I123). Tc is more widely available, less expensive, and quicker to use than iodine123. Scintigraphy with iodine123, if available, is usually preferred because of the greater sensitivity and because, iodine123, unlike of technetium 99, is organified. Therefore, imaging with this isotope allows quantitative uptake measurements and tests for both iodine transport defects and abnormalities in thyroid oxidation. An enrichment of the tracer within the salivary gland can lead to misinterpretation, especially on lateral views, but this can be avoided by allowing the infant to feed before scintigraphy, thus empting the salivary glands and keeping the child calm under the camera. The perchlorate discharge test is considered indicative of an organification defect when a discharge of > 10% of the administered iodine123 dose occurs in a thyroid in normal position (when perchlorate is given at 2 hours).
Excess iodine intake through exposure, maternal TSH receptor blocking antibodies, inactivating mutation in the TSH receptor and in the sodium/iodide symporter (NIS), and TSH suppression from L-thyroxine treatment can interfere with the iodine123 uptake, showing no uptake in the presence of a thyroid in situ (apparent athyreosis).
Thyroid ultrasonography is performed with a high frequency linear array transducer (10-15 MHz) and allows a resolution of 0.7 to 1mm. Thyroid tissue is more echogenic than muscle and less echogenic than fat. In the case of absence of the thyroid, fat tissue can be misdiagnosed as dysplastic thyroid gland in situ. Distinguishing between thyroid hypoplasia and dysplastic non-thyroidal tissue in a newborn requires an experience and reevaluation at a later age can result in a different diagnosis.
Combining scintigraphy and thyroid ultrasound improves diagnostic accuracy and helps to address further investigations, including molecular genetic studies. Infants found to have a normal sized gland in situ in the absence of a clear diagnosis should undergo further reassessment of the thyroid axis and imaging at a later age.
Cretinism treatment
Timing of normalization of thyroid hormones is critical for brain development and therefore replacement therapy with L-thyroxine (L-T4) should be begun as soon as the diagnosis of congenital hypothyroidism is confirmed. The aims of therapy are to normalize the T4 as soon as possible, to avoid hyperthyroidism where possible, and to promote normal growth and development. The current recommendations for the initiation of treatment are thyroxine 10 to 15 μg/kg/day, crushed on a spoon and mixed in milk or water, but not put in the bottle so as to ensure full dose delivery. The tablets are sweet, and the taste is not unpleasant. The highest dose is indicated in infants with severe disease, and the lower dose in those with a mild to moderate congenital hypothyroidism. L-thyroxine tablets can be crushed and given via a small spoon, with suspension, if necessary in a few milliliters of water or breast milk or formula or juice, but care should be taken that all of the medicine has been swallowed. Thyroid hormone should not be given with substances that interfere with its absorption, such as iron, calcium, soy, or fiber. Drugs such as antacids (aluminum hydroxide) or infantile colic drops (simethicone) can interfere with L-thyroxine absorption 3. Many babies will swallow the pills whole or will chew the tablets with their gums even before they have teeth. Reliable liquid preparations are not available commercially in the US, although they have been used successfully in Europe. A brand name rather a generic formulation of L-thyroxine is recommended because they are not bioequivalent.
When an initial dosage of 10-15 mcg/kg is used, the T4 will normalize in most infants within 1 week and the TSH will normalize within 1-month 3. Subsequent adjustments in the dosage of medication are made according to the results of thyroid function tests and the clinical picture. Often small increments or decrements of L-thyroxine (12.5 mcg) are needed. This can be accomplished by 1/2 tablet changes, by giving an alternating dosage on subsequent days, or by giving an extra tablet once a week 3.
As stated in European Society for Paediatric Endocrinology guidelines: “L-thyroxine alone is recommended as the medication of choice and should be started as soon as possible, no later than two weeks of life or immediately after confirmatory test results in infants identified in a second routine screening test. L-thyroxine should be given orally. If intravenous administration is necessary, the dose should be no more than 80% of the oral dose”. Serum or plasma free thyroxine (FT4) and TSH concentration should be determined at least 4 hours after the last L-thyroxine administration. TSH should be maintained in the age-specific reference range and free thyroxine in the upper half of the age- specific reference range. “The first follow up examination is indicated after 1-2 weeks after the start of L-thyroxine treatment and then every 2 weeks until TSH levels are completely normalized and then every 1- 3 months until 12 months of age. Between the age of one and three years, children should undergo frequent clinical and laboratory evaluations (every 2 to 4 months).” Thereafter, evaluations should be carried out every 3 to 12 months until growth is completed. “More frequent evaluations should be carried out if compliance is questioned or abnormal values are obtained. Any reduction of L-thyroxine dose should not be based on a single increase of free thyroxine concentration during treatment. “Measurements should be performed after 4-6 weeks any change in the dosage or in the L-thyroxine formulation”.
Normal short term developmental outcomes in even severely affected infants have recently been reported, with the early initiation of thyroxine at a dose of 9.5 μg/kg/day or higher and with maintenance of free tetraiodothyronine concentrations in the upper normal range during the first year 20. However, modifications in treatment recommendations may still be needed after further long term outcome analyses of screening and treatment schedules 21. Modern early discharge practices present a challenge, because the physiological surge of thyroid stimulating hormone in the first 24 hour can cause false positive results, necessitating a recall of the infant for testing if the blood spot is taken too early 22. There are logistical difficulties with screening infants after discharge or following home birth, and these special situations need a careful solution.
Re-evaluation
In hypothyroid babies in whom an organic basis was not established at birth and in whom transient disease is suspected, a trial off replacement therapy can be initiated after the age of 3 years when most thyroxine-dependent brain maturation has occurred, as shown by MRI studies. Re-evaluation is recommended if the treatment was started in a sick child (i.e. preterm), if thyroid antibodies were detectable, if no diagnostic assessment was completed, and in children who have required no increase in L-thyroxine dosage since infancy. Re-evaluation is recommended also in the case of a eutopic gland with or without goiter, if no enzyme defects have been detected, or if any other cause of transient hypothyroidism is suspected.
Re-evaluation is not necessary if venous TSH concentration has risen during the first year of life, due to either L-thyroxine underdosage or poor compliance. To perform a precise diagnosis, L-thyroxine treatment is suspended for 4-6 weeks, and biochemical testing and thyroid imaging are carried out. To establish the presence of primary hypothyroidism, without defining the cause, L-thyroxine dose may be decreased by 20-30% for 2 to 3 weeks. If TSH serum levels rise to > 10 mU/L during this period, the hypothyroidism can be confirmed.
Congenital hypothyroidism prognosis
Although all agree that the mental retardation associated with untreated congenital hypothyroidism has been largely eradicated by newborn screening, controversy persists as to whether subtle cognitive and behavioral deficits remain, particularly in the most severely affected infants. Both the initial treatment dose and early onset of treatment (before 2 weeks) are important. Time to normalization of circulating thyroid hormone levels, the initial free T4 concentration, maternal IQ, socioeconomic status, and ethnic status have also been related to outcome. The long-term problems for these babies appear to be in the areas of memory, language, fine motor, attention, and visual spatial. Inattentiveness can occur both in patients who are overtreated and those in whom treatment was initiated late or was inadequate. In addition to adequate dosage, assurance of compliance and careful long-term monitoring are essential for an optimal developmental outcome. More details about long term follow up are reported in European Society for Paediatric Endocrinology guidelines. Progressive hearing loss in congenital hypothyroidism should be recognized and corrected, because they strongly influenced the outcome.
- Srivastav A, Maisnam I, Dutta D, Ghosh S, Mukhopadhyay S, Chowdhury S. Cretinism revisited. Indian J Endocrinol Metab. 2012;16(Suppl 2):S336–S337. doi:10.4103/2230-8210.104081 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3603067[↩]
- World Health Organization. (2001). Assessment of iodine deficiency disorders and monitoring their elimination : a guide for programme managers, 2nd ed. World Health Organization. https://apps.who.int/iris/handle/10665/61278[↩]
- Segni M. Congenital Hypothyroidism. [Updated 2019 Aug 11]. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279004[↩][↩][↩][↩]
- WHO, UNICEF, and ICCIDD. 1994. Indicators for assessing Iodine Deficiency Disorders and their control through salt iodization. Geneva: WHO publ. WHO/NUT/94.6. 1-55 pp.[↩]
- Kimball OP. History of the prevention of endemic goitre. Bull World Health Organ. 1953;9(2):241–248[↩]
- Iodine Deficiency. https://www.thyroid.org/iodine-deficiency[↩]
- Chen ZP, Hetzel BS. Cretinism revisited. Best Pract Res Clin Endocrinol Metab. 2010;24:39–50.[↩][↩]
- [Guideline] WHO. Guideline: fortification of food-grade salt with iodine for the prevention and control of iodine deficiency disorders. Geneva: World Health Organization; 2014.[↩]
- Chen ZP, Hetzel BS. Cretinism revisited. Best Pract Res Clin Endocrinol Metab. 2010;24(1):39–50. doi:10.1016/j.beem.2009.08.014 https://doi.org/10.1016/j.beem.2009.08.014[↩][↩]
- Rajatanavin R, Chailurkit L, Winichakoon P, et al. Endemic cretinism in Thailand: a multidisciplinary survey. Eur J Endocrinol. 1997;137(4):349–355. doi:10.1530/eje.0.1370349[↩]
- Zimmermann MB. Iodine deficiency in pregnancy and the effects of maternal iodine supplementation on the offspring: a review. Am J Clin Nutr. 2009;89(2):668S–72S. doi:10.3945/ajcn.2008.26811C[↩]
- Pharoah POD, Buttfield IH & Hetzel BS. Neurological damage to the fetus resulting from severe iodine deficiency during pregnancy. Lancet 1971; 1: 308–310.[↩]
- Timing of Vulnerability of the Brain to Iodine Deficiency in Endemic Cretinism. N Engl J Med 1994; 331:1739-1744 DOI: 10.1056/NEJM199412293312603[↩][↩]
- O’Donnell KJ, Abdul Rakeman M, Dou ZH, Cao XY, ZengYM, DeLong N, Brenner G, Tai M, Dong W! DeLong GR. (2002) Effects of iodine supplementation during pregnancy on child growth and development at school age. Dev Med Child Neurol M 76-81.[↩]
- Institute of Medicine, Academy of Sciences. Iodine. In: Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. Washington, DC: National Academy Press, 2001:258–89.[↩][↩][↩][↩][↩]
- World Health Organization. United Nations Children’s Fund & International Council for the Control of Iodine Deficiency Disorders. Assessment of iodine deficiency disorders and monitoring their elimination. 2nd ed. Geneva, Switzerland: WHO, 2007.[↩][↩]
- Glinoer D. The regulation of thyroid function during normal pregnancy: importance of the iodine nutrition status. Best Pract Res Clin Endocrinol Metab 2004;18:133–52.[↩][↩][↩]
- World Health Organization. United Nations Children’s Fund & International Council for the Control of Iodine Deficiency Disorders. Assessment of iodine deficiency disorders and monitoring their elimination. 3rd ed. Geneva, Switzerland: WHO, 2007. https://apps.who.int/iris/bitstream/handle/10665/43781/9789241595827_eng.pdf[↩]
- WHO/UNICEF. Reaching optimal iodine nutrition in pregnant and lactating women and young children. Joint Statement of the World Health Organization and the United Nations Children’s Fund. Geneva, Switzerland: World Health Organization, 2007.[↩][↩][↩]
- Bongers-Schokking JJ, Koot HM, Wiersma D, Verkerk PH, de Muinck Keizer-Schrama SM. Influence of timing and dose of thyroid hormone replacement on development in infants with congenital hypothyroidism. J Pediatr. 2000;136:292–7.[↩]
- Van Vliet G. Treatment of congenital hypothyroidism. Lancet. 2001;358:86–7.[↩]
- Salisbury S. Cretinism: The past, present and future of diagnosis and cure. Paediatr Child Health. 2003;8(2):105–106. doi:10.1093/pch/8.2.105 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2791432[↩]